User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
The physician as leader
Physicians are placed in positions of leadership by the medical team, by the community, and by society, particularly during times of crisis such as the COVID pandemic. They are looked to by the media at times of health care news such as the overturning of Roe v. Wade.1 In a 2015 survey of resident physicians, two-thirds agreed that a formalized leadership curriculum would help them become better supervisors and clinicians.2 While all physicians are viewed as leaders, the concept of leadership is rarely, if ever, described or developed as a part of medical training. This month’s column will provide insights into defining leadership as a physician in the medical and administrative settings.
Benefits of effective leadership
Physicians, whether they are clinicians, researchers, administrators, or teachers, are expected to oversee and engage their teams. A report by the Institute of Medicine recommended that academic health centers “develop leaders at all levels who can manage the organizational and system changes necessary to improve health through innovation in health professions education, patient care, and research.”3 Hospitals with higher-rated management practices and more highly rated boards of directors have been shown to deliver higher-quality care and better clinical outcomes, including lower mortality.
To illustrate, the clinicians at the Mayo Clinic annually rate their supervisors on a Leader Index, a simple 12-question survey of five leadership domains: truthfulness, transparency, character, capability, and partnership. All supervisors were physicians and scientists. Their findings revealed that for each one-point increase in composite leadership score, there was a 3.3% decrease in the likelihood of burnout and a 9.0% increase in the likelihood of satisfaction in the physicians supervised.4
Interprofessional teamwork and engagement are vital skills for a leader to create a successful team. Enhanced management practices have also been associated with higher patient approval ratings and better financial performance. Effective leadership additionally affects physician well-being, with stronger leadership associated with less physician burnout and higher satisfaction.5
Leadership styles enhance quality measures in health care.6 The most effective leadership styles are ones in which the staff feels they are part of a team, are engaged, and are mentored.7 While leadership styles can vary, the common theme is staff engagement. An authoritative style leader is one who mobilizes the team toward a vision, that is, “Come with me.” An affiliative style leader creates harmony and builds emotional bonds where “people come first.” Democratic leaders forge a consensus through staff participation by asking, “What do you think?” Finally, a leader who uses a coaching style helps staff to identify their strengths and weaknesses and work toward improvement. These leadership behaviors are in contradistinction to the unsuccessful coercive leader who demands immediate compliance, that is, “Do what I tell you.”
Five fundamental leadership principles are shown in Table 1.8
Effective leaders have an open (growth) mindset, unwavering attention to diversity, equity, and inclusion, and to building relationships and trust; they practice effective communication and listening, focus on results, and cocreate support structures.
A growth mindset is the belief that one’s abilities are not innate but can improve through effort and learning.9
Emotional intelligence
A survey of business senior managers rated the qualities found in the most outstanding leaders. Using objective criteria like profitability the study psychologists interviewed the highest-rated leaders to compare their capabilities. While intellects and cognitive skills were important, the results showed that emotional intelligence (EI) was twice as important as technical skills and IQ.10 As an example, in a 1996 study, when senior managers had an optimal level of EI, their division’s yearly earnings were 20% higher than estimated.11
EI is a leadership competency that deals with the ability to understand and manage your own emotions and your interactions with others.10 At the Cleveland Clinic, EI is exemplified by the acronym HEART, whereby the team strives to improve the patient experience, mainly when an error occurs. The health care team is using EI by showing its the ability to Hear, Empathize, Apologize, Reply, and Thank. When an untoward event occurs, the physician, as the leader of the team, must lead by example when communicating with staff and patients. EI consists of five components (Table 2).13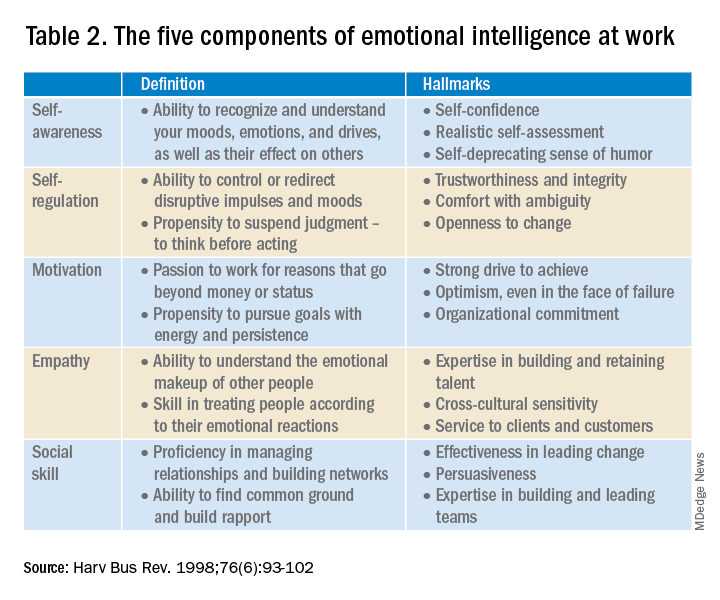
- Self-awareness is insight by which you can improve. Maintaining a journal of your daily thoughts may assist with this as well as simply pausing to pay attention during times of heightened emotions.
- Self-regulation shows control, that is, behaving according to your values, and being accountable and calm when challenged.
- Purpose, knowing your “why,” produces motivation and helps maintain optimism.
- Empathy shows the ability to understand the emotions of other people.
- Social skill is the ability to establish mutually rewarding relationships.
Given all the above benefits, it is no surprise that companies are actively trying use artificial intelligence to improve EI.12
Learning to be a leader
In medical school, students are expected to develop skills to handle and resolve conflicts, learn to share leadership, take mutual responsibility, and monitor their own performance.13 Although training of young physicians in leadership is not unprecedented, a systemic review revealed a lack of analytic studies to evaluate the effectiveness of the teaching methods.14 During undergraduate medical education, standard curricula and methods of instruction on leadership are not established, resulting in variable outcomes.
The Association of American Medical Colleges offers a curriculum, “Preparing Medical Students to Be Physician Leaders: A Leadership Training Program for Students Designed and Led by Students.”15 The objectives of this training are to help students identify their “personal style of leadership, recognize strengths and weaknesses, utilize effective communication strategies, appropriately delegate team member responsibilities, and provide constructive feedback to help improve team function.”
Take-home points
Following the completion of formal medical education, physicians are thrust into leadership roles. The key to being an effective leader is using EI to mentor the team and make staff feel connected to the team’s meaning and purpose, so they feel valued.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Carsen S and Xia C. McGill J Med. 2006 Jan;9(1):1-2.
2. Jardine D et al. J Grad Med Educ. 2015;7(2):307-9.
3. Institute of Medicine. Acad Emerg Med. July 2004;11(7):802-6.
4. Shanafelt TD et al. Mayo Clin Proc. April 2015;90(4):432-40.
5. Rotenstein LS et al. Harv Bus Rev. Oct. 17, 2018.
6. Sfantou SF. Healthcare 2017;5(4):73.
7. Goleman D. Harv Bus Rev. March-April 2000.
8. Collins-Nakai R. McGill J Med [Internet]. 2020 Dec. 1 [cited 2023 Mar. 28];9(1).
9. Dweck C. Harv Bus Rev. Jan. 13, 2016.
10. Goleman D. Harv Bus Rev. 1998 Nov-Dec;76(6):93-102..
11. Goleman D et al. Primal leadership: Realizing the power of emotional intelligence. Boston: Harvard Business School Publishing, 2002.12. Limon D and Plaster B. Harv Bus Rev. Jan. 25, 2022.
13. Chen T-Y. Tzu Chi Med J. Apr–Jun 2018;30(2):66-70.
14. Kumar B et al. BMC Med Educ. 2020;20:175.
15. Richards K et al. Med Ed Portal. Dec. 13 2019.
Physicians are placed in positions of leadership by the medical team, by the community, and by society, particularly during times of crisis such as the COVID pandemic. They are looked to by the media at times of health care news such as the overturning of Roe v. Wade.1 In a 2015 survey of resident physicians, two-thirds agreed that a formalized leadership curriculum would help them become better supervisors and clinicians.2 While all physicians are viewed as leaders, the concept of leadership is rarely, if ever, described or developed as a part of medical training. This month’s column will provide insights into defining leadership as a physician in the medical and administrative settings.
Benefits of effective leadership
Physicians, whether they are clinicians, researchers, administrators, or teachers, are expected to oversee and engage their teams. A report by the Institute of Medicine recommended that academic health centers “develop leaders at all levels who can manage the organizational and system changes necessary to improve health through innovation in health professions education, patient care, and research.”3 Hospitals with higher-rated management practices and more highly rated boards of directors have been shown to deliver higher-quality care and better clinical outcomes, including lower mortality.
To illustrate, the clinicians at the Mayo Clinic annually rate their supervisors on a Leader Index, a simple 12-question survey of five leadership domains: truthfulness, transparency, character, capability, and partnership. All supervisors were physicians and scientists. Their findings revealed that for each one-point increase in composite leadership score, there was a 3.3% decrease in the likelihood of burnout and a 9.0% increase in the likelihood of satisfaction in the physicians supervised.4
Interprofessional teamwork and engagement are vital skills for a leader to create a successful team. Enhanced management practices have also been associated with higher patient approval ratings and better financial performance. Effective leadership additionally affects physician well-being, with stronger leadership associated with less physician burnout and higher satisfaction.5
Leadership styles enhance quality measures in health care.6 The most effective leadership styles are ones in which the staff feels they are part of a team, are engaged, and are mentored.7 While leadership styles can vary, the common theme is staff engagement. An authoritative style leader is one who mobilizes the team toward a vision, that is, “Come with me.” An affiliative style leader creates harmony and builds emotional bonds where “people come first.” Democratic leaders forge a consensus through staff participation by asking, “What do you think?” Finally, a leader who uses a coaching style helps staff to identify their strengths and weaknesses and work toward improvement. These leadership behaviors are in contradistinction to the unsuccessful coercive leader who demands immediate compliance, that is, “Do what I tell you.”
Five fundamental leadership principles are shown in Table 1.8
Effective leaders have an open (growth) mindset, unwavering attention to diversity, equity, and inclusion, and to building relationships and trust; they practice effective communication and listening, focus on results, and cocreate support structures.
A growth mindset is the belief that one’s abilities are not innate but can improve through effort and learning.9
Emotional intelligence
A survey of business senior managers rated the qualities found in the most outstanding leaders. Using objective criteria like profitability the study psychologists interviewed the highest-rated leaders to compare their capabilities. While intellects and cognitive skills were important, the results showed that emotional intelligence (EI) was twice as important as technical skills and IQ.10 As an example, in a 1996 study, when senior managers had an optimal level of EI, their division’s yearly earnings were 20% higher than estimated.11
EI is a leadership competency that deals with the ability to understand and manage your own emotions and your interactions with others.10 At the Cleveland Clinic, EI is exemplified by the acronym HEART, whereby the team strives to improve the patient experience, mainly when an error occurs. The health care team is using EI by showing its the ability to Hear, Empathize, Apologize, Reply, and Thank. When an untoward event occurs, the physician, as the leader of the team, must lead by example when communicating with staff and patients. EI consists of five components (Table 2).13
- Self-awareness is insight by which you can improve. Maintaining a journal of your daily thoughts may assist with this as well as simply pausing to pay attention during times of heightened emotions.
- Self-regulation shows control, that is, behaving according to your values, and being accountable and calm when challenged.
- Purpose, knowing your “why,” produces motivation and helps maintain optimism.
- Empathy shows the ability to understand the emotions of other people.
- Social skill is the ability to establish mutually rewarding relationships.
Given all the above benefits, it is no surprise that companies are actively trying use artificial intelligence to improve EI.12
Learning to be a leader
In medical school, students are expected to develop skills to handle and resolve conflicts, learn to share leadership, take mutual responsibility, and monitor their own performance.13 Although training of young physicians in leadership is not unprecedented, a systemic review revealed a lack of analytic studies to evaluate the effectiveness of the teaching methods.14 During undergraduate medical education, standard curricula and methods of instruction on leadership are not established, resulting in variable outcomes.
The Association of American Medical Colleges offers a curriculum, “Preparing Medical Students to Be Physician Leaders: A Leadership Training Program for Students Designed and Led by Students.”15 The objectives of this training are to help students identify their “personal style of leadership, recognize strengths and weaknesses, utilize effective communication strategies, appropriately delegate team member responsibilities, and provide constructive feedback to help improve team function.”
Take-home points
Following the completion of formal medical education, physicians are thrust into leadership roles. The key to being an effective leader is using EI to mentor the team and make staff feel connected to the team’s meaning and purpose, so they feel valued.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Carsen S and Xia C. McGill J Med. 2006 Jan;9(1):1-2.
2. Jardine D et al. J Grad Med Educ. 2015;7(2):307-9.
3. Institute of Medicine. Acad Emerg Med. July 2004;11(7):802-6.
4. Shanafelt TD et al. Mayo Clin Proc. April 2015;90(4):432-40.
5. Rotenstein LS et al. Harv Bus Rev. Oct. 17, 2018.
6. Sfantou SF. Healthcare 2017;5(4):73.
7. Goleman D. Harv Bus Rev. March-April 2000.
8. Collins-Nakai R. McGill J Med [Internet]. 2020 Dec. 1 [cited 2023 Mar. 28];9(1).
9. Dweck C. Harv Bus Rev. Jan. 13, 2016.
10. Goleman D. Harv Bus Rev. 1998 Nov-Dec;76(6):93-102..
11. Goleman D et al. Primal leadership: Realizing the power of emotional intelligence. Boston: Harvard Business School Publishing, 2002.12. Limon D and Plaster B. Harv Bus Rev. Jan. 25, 2022.
13. Chen T-Y. Tzu Chi Med J. Apr–Jun 2018;30(2):66-70.
14. Kumar B et al. BMC Med Educ. 2020;20:175.
15. Richards K et al. Med Ed Portal. Dec. 13 2019.
Physicians are placed in positions of leadership by the medical team, by the community, and by society, particularly during times of crisis such as the COVID pandemic. They are looked to by the media at times of health care news such as the overturning of Roe v. Wade.1 In a 2015 survey of resident physicians, two-thirds agreed that a formalized leadership curriculum would help them become better supervisors and clinicians.2 While all physicians are viewed as leaders, the concept of leadership is rarely, if ever, described or developed as a part of medical training. This month’s column will provide insights into defining leadership as a physician in the medical and administrative settings.
Benefits of effective leadership
Physicians, whether they are clinicians, researchers, administrators, or teachers, are expected to oversee and engage their teams. A report by the Institute of Medicine recommended that academic health centers “develop leaders at all levels who can manage the organizational and system changes necessary to improve health through innovation in health professions education, patient care, and research.”3 Hospitals with higher-rated management practices and more highly rated boards of directors have been shown to deliver higher-quality care and better clinical outcomes, including lower mortality.
To illustrate, the clinicians at the Mayo Clinic annually rate their supervisors on a Leader Index, a simple 12-question survey of five leadership domains: truthfulness, transparency, character, capability, and partnership. All supervisors were physicians and scientists. Their findings revealed that for each one-point increase in composite leadership score, there was a 3.3% decrease in the likelihood of burnout and a 9.0% increase in the likelihood of satisfaction in the physicians supervised.4
Interprofessional teamwork and engagement are vital skills for a leader to create a successful team. Enhanced management practices have also been associated with higher patient approval ratings and better financial performance. Effective leadership additionally affects physician well-being, with stronger leadership associated with less physician burnout and higher satisfaction.5
Leadership styles enhance quality measures in health care.6 The most effective leadership styles are ones in which the staff feels they are part of a team, are engaged, and are mentored.7 While leadership styles can vary, the common theme is staff engagement. An authoritative style leader is one who mobilizes the team toward a vision, that is, “Come with me.” An affiliative style leader creates harmony and builds emotional bonds where “people come first.” Democratic leaders forge a consensus through staff participation by asking, “What do you think?” Finally, a leader who uses a coaching style helps staff to identify their strengths and weaknesses and work toward improvement. These leadership behaviors are in contradistinction to the unsuccessful coercive leader who demands immediate compliance, that is, “Do what I tell you.”
Five fundamental leadership principles are shown in Table 1.8
Effective leaders have an open (growth) mindset, unwavering attention to diversity, equity, and inclusion, and to building relationships and trust; they practice effective communication and listening, focus on results, and cocreate support structures.
A growth mindset is the belief that one’s abilities are not innate but can improve through effort and learning.9
Emotional intelligence
A survey of business senior managers rated the qualities found in the most outstanding leaders. Using objective criteria like profitability the study psychologists interviewed the highest-rated leaders to compare their capabilities. While intellects and cognitive skills were important, the results showed that emotional intelligence (EI) was twice as important as technical skills and IQ.10 As an example, in a 1996 study, when senior managers had an optimal level of EI, their division’s yearly earnings were 20% higher than estimated.11
EI is a leadership competency that deals with the ability to understand and manage your own emotions and your interactions with others.10 At the Cleveland Clinic, EI is exemplified by the acronym HEART, whereby the team strives to improve the patient experience, mainly when an error occurs. The health care team is using EI by showing its the ability to Hear, Empathize, Apologize, Reply, and Thank. When an untoward event occurs, the physician, as the leader of the team, must lead by example when communicating with staff and patients. EI consists of five components (Table 2).13
- Self-awareness is insight by which you can improve. Maintaining a journal of your daily thoughts may assist with this as well as simply pausing to pay attention during times of heightened emotions.
- Self-regulation shows control, that is, behaving according to your values, and being accountable and calm when challenged.
- Purpose, knowing your “why,” produces motivation and helps maintain optimism.
- Empathy shows the ability to understand the emotions of other people.
- Social skill is the ability to establish mutually rewarding relationships.
Given all the above benefits, it is no surprise that companies are actively trying use artificial intelligence to improve EI.12
Learning to be a leader
In medical school, students are expected to develop skills to handle and resolve conflicts, learn to share leadership, take mutual responsibility, and monitor their own performance.13 Although training of young physicians in leadership is not unprecedented, a systemic review revealed a lack of analytic studies to evaluate the effectiveness of the teaching methods.14 During undergraduate medical education, standard curricula and methods of instruction on leadership are not established, resulting in variable outcomes.
The Association of American Medical Colleges offers a curriculum, “Preparing Medical Students to Be Physician Leaders: A Leadership Training Program for Students Designed and Led by Students.”15 The objectives of this training are to help students identify their “personal style of leadership, recognize strengths and weaknesses, utilize effective communication strategies, appropriately delegate team member responsibilities, and provide constructive feedback to help improve team function.”
Take-home points
Following the completion of formal medical education, physicians are thrust into leadership roles. The key to being an effective leader is using EI to mentor the team and make staff feel connected to the team’s meaning and purpose, so they feel valued.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Carsen S and Xia C. McGill J Med. 2006 Jan;9(1):1-2.
2. Jardine D et al. J Grad Med Educ. 2015;7(2):307-9.
3. Institute of Medicine. Acad Emerg Med. July 2004;11(7):802-6.
4. Shanafelt TD et al. Mayo Clin Proc. April 2015;90(4):432-40.
5. Rotenstein LS et al. Harv Bus Rev. Oct. 17, 2018.
6. Sfantou SF. Healthcare 2017;5(4):73.
7. Goleman D. Harv Bus Rev. March-April 2000.
8. Collins-Nakai R. McGill J Med [Internet]. 2020 Dec. 1 [cited 2023 Mar. 28];9(1).
9. Dweck C. Harv Bus Rev. Jan. 13, 2016.
10. Goleman D. Harv Bus Rev. 1998 Nov-Dec;76(6):93-102..
11. Goleman D et al. Primal leadership: Realizing the power of emotional intelligence. Boston: Harvard Business School Publishing, 2002.12. Limon D and Plaster B. Harv Bus Rev. Jan. 25, 2022.
13. Chen T-Y. Tzu Chi Med J. Apr–Jun 2018;30(2):66-70.
14. Kumar B et al. BMC Med Educ. 2020;20:175.
15. Richards K et al. Med Ed Portal. Dec. 13 2019.
‘Excess’ deaths surging, but why?
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.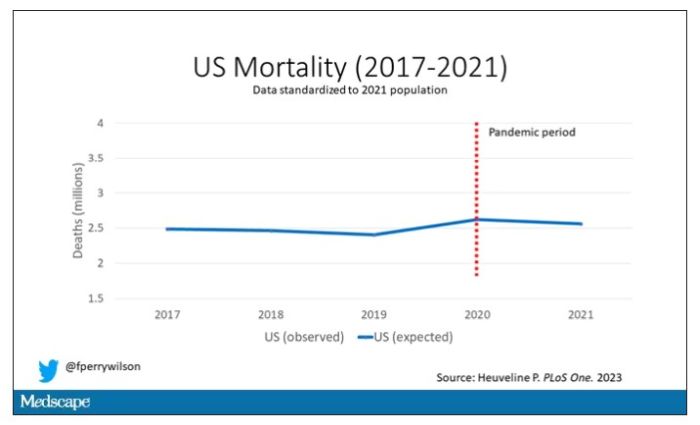
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.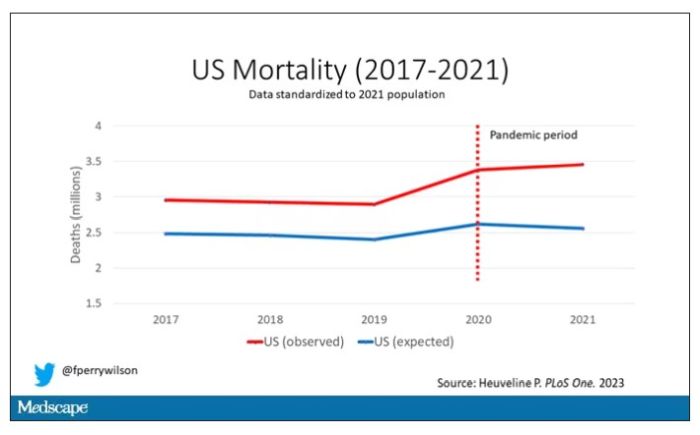
Highlighted here in green, then, is the excess mortality over time in the United States.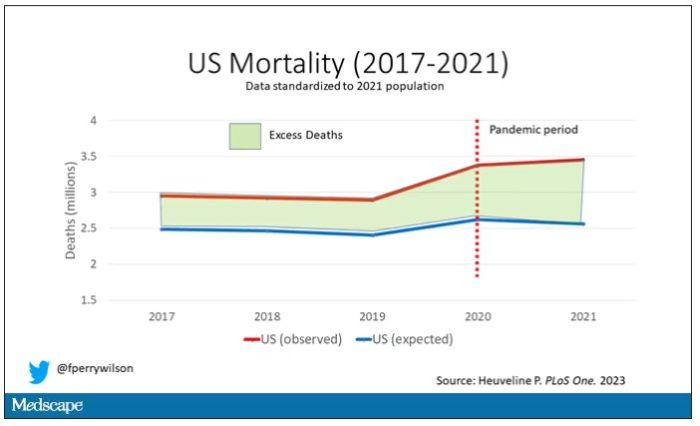
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
Spotting STIs: Vaginal swabs work best
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
FDA panels vote to modify isotretinoin iPLEDGE REMS
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
Sweaty treatment for social anxiety could pass the sniff test
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
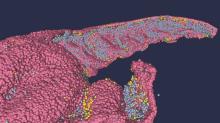
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Surgical management of borderline ovarian tumors, part 1
Borderline ovarian tumors (BOTs) are estimated to comprise 10%-15% of all epithelial tumors of the ovary. They are characterized by their behavior, which falls somewhere between benign ovarian masses and frank carcinomas. They have cytologic characteristics suggesting malignancy, such as higher cellular proliferation and more variable nuclear atypia, but, unlike carcinomas, they lack destructive stromal invasion. For decades after their recognition by the International Federation of Gynecology and Obstetrics in 1971, these tumors were classified as being of low malignant potential (and subsequently referred to as LMP tumors of the ovary). Beginning with the 2014 World Health Organization classification, the recommended terminology is now borderline tumor of the ovary.
The primary treatment for BOTs is surgery. With a mean age at diagnosis in the fifth decade, many patients with BOTs desire ovarian preservation to maintain fertility and/or prevent surgical menopause. This raises multiple questions regarding the use of fertility-sparing surgery for BOTs: What types of procedures are safe and should be offered? For those patients who undergo fertility-sparing surgery initially, is additional surgery indicated after completion of childbearing or at an age closer to natural menopause? What should this completion surgery include?
Ovarian-sparing surgery
The diagnosis of a BOT is frequently only confirmed after the decision for ovarian conservation has been made. What should be considered before electing to proceed with ovarian cystectomy instead of unilateral salpingo-oophorectomy (USO)?
Is the risk of recurrence higher with cystectomy versus oophorectomy?
Yes. The risk of recurrence of BOT appears to be higher after cystectomy than it is after oophorectomy. There is a large range reported in the literature, with the risk of recurrence after cystectomy described as between 12% and 58%. Most studies report recurrences between 25% and 35% of patients who undergo cystectomy. In contrast, the risk of recurrence after USO is often reported to be approximately 10%. Higher risk of recurrence after cystectomy is speculated to be due to leaving some BOT at the time of initial surgery.
Multiple meta-analyses have found an increased risk of recurrence after cystectomy. The risk of recurrence after unilateral cystectomy was 19.4%, compared with 9.1% after USO, in 2,145 patients included in a 2017 meta-analysis.1 Similarly, a 2021 meta-analysis found a significantly higher rate of BOT recurrence in patients who underwent unilateral or bilateral cystectomy compared with USO (odds ratio, 2.02; 95% confidence interval, 1.59-2.57).2
Does the higher recurrence risk translate into a difference in long-term outcomes?
No. Despite an increased risk of recurrence after cystectomy, ovarian-sparing surgery does not appear to alter patients’ survival. The pooled mortality estimate was 1.6% for those undergoing fertility-sparing surgery (95% CI, 0.011-0.023), compared with 2.0% for those undergoing radical surgery (95% CI, 0.014-0.029), in a 2015 meta-analysis of over 5,100 patients. The analysis included studies in which patients underwent unilateral cystectomy, bilateral cystectomy, USO, or USO plus contralateral cystectomy. The low mortality rate did not allow for comparison between the different types of fertility-sparing surgeries.3
Do we accept a higher risk of recurrence with ovarian sparing surgery to improve fertility?
Data are mixed. When we examine studies describing fertility rates after conservative surgery, there are significant limitations to interpreting the data available. Some studies do not differentiate among patients who underwent fertility-sparing surgery, or between those who had cystectomy versus USO. Other studies do not report the number of patients who tried to achieve pregnancy after surgery. Conception rates are reported to be as high as 88.2%, which was in 116 patients who were able to be reached after fertility-sparing surgery (retained at least one ovary). Of the 51 patients who tried to conceive, 45 were successful.4
Multiple studies and meta-analyses have shown no difference in postoperative pregnancy rates when comparing oophorectomy to cystectomy. For instance, in a 2021 meta-analysis, there was no significant difference noted in pregnancy rates between patients who underwent USO versus cystectomy (OR, 0.92; 95% CI, 0.60-1.42).
There are some data that support improved postoperative pregnancy rates in more conservative surgery, especially in the setting of bilateral BOT. In a small study of 32 patients who had laparoscopic staging for bilateral BOTs, patients were randomized to unilateral oophorectomy plus contralateral cystectomy or to bilateral cystectomy, which was referred to as ultraconservative surgery. The time to first recurrence was shorter in the ultraconservative group (although this lost significance when regression analysis was performed), but the time to first live birth was shorter and the relative chance of having a baby was higher in the bilateral cystectomy group.5
Ovarian-sparing procedures should be offered to patients in the setting of BOT. With ovarian-sparing surgery, it is important to counsel patients about the increased risk of recurrence and need for long-term follow-up. Pregnancy rates are generally good after fertility-sparing surgery. Surgery to conserve both ovaries does not seem to improve pregnancy rates in the setting of unilateral BOTs.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Jiao X et al. Int J Gynecol Cancer. 2017 Nov;27(9):1833-41.
2. Wang P and Fang L. World J Surg Oncol. 2021 Apr 21;19(1):132.
3. Vasconcelos I and de Sousa Mendes M. Eur J Cancer. 2015 Mar;51(5):620-31.
4. Song T et al. Int J Gynecol Cancer. 2011 May;21(4):640-6.
5. Palomba S et al. Hum Reprod. 2010 Aug;25(8):1966-72.
Borderline ovarian tumors (BOTs) are estimated to comprise 10%-15% of all epithelial tumors of the ovary. They are characterized by their behavior, which falls somewhere between benign ovarian masses and frank carcinomas. They have cytologic characteristics suggesting malignancy, such as higher cellular proliferation and more variable nuclear atypia, but, unlike carcinomas, they lack destructive stromal invasion. For decades after their recognition by the International Federation of Gynecology and Obstetrics in 1971, these tumors were classified as being of low malignant potential (and subsequently referred to as LMP tumors of the ovary). Beginning with the 2014 World Health Organization classification, the recommended terminology is now borderline tumor of the ovary.
The primary treatment for BOTs is surgery. With a mean age at diagnosis in the fifth decade, many patients with BOTs desire ovarian preservation to maintain fertility and/or prevent surgical menopause. This raises multiple questions regarding the use of fertility-sparing surgery for BOTs: What types of procedures are safe and should be offered? For those patients who undergo fertility-sparing surgery initially, is additional surgery indicated after completion of childbearing or at an age closer to natural menopause? What should this completion surgery include?
Ovarian-sparing surgery
The diagnosis of a BOT is frequently only confirmed after the decision for ovarian conservation has been made. What should be considered before electing to proceed with ovarian cystectomy instead of unilateral salpingo-oophorectomy (USO)?
Is the risk of recurrence higher with cystectomy versus oophorectomy?
Yes. The risk of recurrence of BOT appears to be higher after cystectomy than it is after oophorectomy. There is a large range reported in the literature, with the risk of recurrence after cystectomy described as between 12% and 58%. Most studies report recurrences between 25% and 35% of patients who undergo cystectomy. In contrast, the risk of recurrence after USO is often reported to be approximately 10%. Higher risk of recurrence after cystectomy is speculated to be due to leaving some BOT at the time of initial surgery.
Multiple meta-analyses have found an increased risk of recurrence after cystectomy. The risk of recurrence after unilateral cystectomy was 19.4%, compared with 9.1% after USO, in 2,145 patients included in a 2017 meta-analysis.1 Similarly, a 2021 meta-analysis found a significantly higher rate of BOT recurrence in patients who underwent unilateral or bilateral cystectomy compared with USO (odds ratio, 2.02; 95% confidence interval, 1.59-2.57).2
Does the higher recurrence risk translate into a difference in long-term outcomes?
No. Despite an increased risk of recurrence after cystectomy, ovarian-sparing surgery does not appear to alter patients’ survival. The pooled mortality estimate was 1.6% for those undergoing fertility-sparing surgery (95% CI, 0.011-0.023), compared with 2.0% for those undergoing radical surgery (95% CI, 0.014-0.029), in a 2015 meta-analysis of over 5,100 patients. The analysis included studies in which patients underwent unilateral cystectomy, bilateral cystectomy, USO, or USO plus contralateral cystectomy. The low mortality rate did not allow for comparison between the different types of fertility-sparing surgeries.3
Do we accept a higher risk of recurrence with ovarian sparing surgery to improve fertility?
Data are mixed. When we examine studies describing fertility rates after conservative surgery, there are significant limitations to interpreting the data available. Some studies do not differentiate among patients who underwent fertility-sparing surgery, or between those who had cystectomy versus USO. Other studies do not report the number of patients who tried to achieve pregnancy after surgery. Conception rates are reported to be as high as 88.2%, which was in 116 patients who were able to be reached after fertility-sparing surgery (retained at least one ovary). Of the 51 patients who tried to conceive, 45 were successful.4
Multiple studies and meta-analyses have shown no difference in postoperative pregnancy rates when comparing oophorectomy to cystectomy. For instance, in a 2021 meta-analysis, there was no significant difference noted in pregnancy rates between patients who underwent USO versus cystectomy (OR, 0.92; 95% CI, 0.60-1.42).
There are some data that support improved postoperative pregnancy rates in more conservative surgery, especially in the setting of bilateral BOT. In a small study of 32 patients who had laparoscopic staging for bilateral BOTs, patients were randomized to unilateral oophorectomy plus contralateral cystectomy or to bilateral cystectomy, which was referred to as ultraconservative surgery. The time to first recurrence was shorter in the ultraconservative group (although this lost significance when regression analysis was performed), but the time to first live birth was shorter and the relative chance of having a baby was higher in the bilateral cystectomy group.5
Ovarian-sparing procedures should be offered to patients in the setting of BOT. With ovarian-sparing surgery, it is important to counsel patients about the increased risk of recurrence and need for long-term follow-up. Pregnancy rates are generally good after fertility-sparing surgery. Surgery to conserve both ovaries does not seem to improve pregnancy rates in the setting of unilateral BOTs.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Jiao X et al. Int J Gynecol Cancer. 2017 Nov;27(9):1833-41.
2. Wang P and Fang L. World J Surg Oncol. 2021 Apr 21;19(1):132.
3. Vasconcelos I and de Sousa Mendes M. Eur J Cancer. 2015 Mar;51(5):620-31.
4. Song T et al. Int J Gynecol Cancer. 2011 May;21(4):640-6.
5. Palomba S et al. Hum Reprod. 2010 Aug;25(8):1966-72.
Borderline ovarian tumors (BOTs) are estimated to comprise 10%-15% of all epithelial tumors of the ovary. They are characterized by their behavior, which falls somewhere between benign ovarian masses and frank carcinomas. They have cytologic characteristics suggesting malignancy, such as higher cellular proliferation and more variable nuclear atypia, but, unlike carcinomas, they lack destructive stromal invasion. For decades after their recognition by the International Federation of Gynecology and Obstetrics in 1971, these tumors were classified as being of low malignant potential (and subsequently referred to as LMP tumors of the ovary). Beginning with the 2014 World Health Organization classification, the recommended terminology is now borderline tumor of the ovary.
The primary treatment for BOTs is surgery. With a mean age at diagnosis in the fifth decade, many patients with BOTs desire ovarian preservation to maintain fertility and/or prevent surgical menopause. This raises multiple questions regarding the use of fertility-sparing surgery for BOTs: What types of procedures are safe and should be offered? For those patients who undergo fertility-sparing surgery initially, is additional surgery indicated after completion of childbearing or at an age closer to natural menopause? What should this completion surgery include?
Ovarian-sparing surgery
The diagnosis of a BOT is frequently only confirmed after the decision for ovarian conservation has been made. What should be considered before electing to proceed with ovarian cystectomy instead of unilateral salpingo-oophorectomy (USO)?
Is the risk of recurrence higher with cystectomy versus oophorectomy?
Yes. The risk of recurrence of BOT appears to be higher after cystectomy than it is after oophorectomy. There is a large range reported in the literature, with the risk of recurrence after cystectomy described as between 12% and 58%. Most studies report recurrences between 25% and 35% of patients who undergo cystectomy. In contrast, the risk of recurrence after USO is often reported to be approximately 10%. Higher risk of recurrence after cystectomy is speculated to be due to leaving some BOT at the time of initial surgery.
Multiple meta-analyses have found an increased risk of recurrence after cystectomy. The risk of recurrence after unilateral cystectomy was 19.4%, compared with 9.1% after USO, in 2,145 patients included in a 2017 meta-analysis.1 Similarly, a 2021 meta-analysis found a significantly higher rate of BOT recurrence in patients who underwent unilateral or bilateral cystectomy compared with USO (odds ratio, 2.02; 95% confidence interval, 1.59-2.57).2
Does the higher recurrence risk translate into a difference in long-term outcomes?
No. Despite an increased risk of recurrence after cystectomy, ovarian-sparing surgery does not appear to alter patients’ survival. The pooled mortality estimate was 1.6% for those undergoing fertility-sparing surgery (95% CI, 0.011-0.023), compared with 2.0% for those undergoing radical surgery (95% CI, 0.014-0.029), in a 2015 meta-analysis of over 5,100 patients. The analysis included studies in which patients underwent unilateral cystectomy, bilateral cystectomy, USO, or USO plus contralateral cystectomy. The low mortality rate did not allow for comparison between the different types of fertility-sparing surgeries.3
Do we accept a higher risk of recurrence with ovarian sparing surgery to improve fertility?
Data are mixed. When we examine studies describing fertility rates after conservative surgery, there are significant limitations to interpreting the data available. Some studies do not differentiate among patients who underwent fertility-sparing surgery, or between those who had cystectomy versus USO. Other studies do not report the number of patients who tried to achieve pregnancy after surgery. Conception rates are reported to be as high as 88.2%, which was in 116 patients who were able to be reached after fertility-sparing surgery (retained at least one ovary). Of the 51 patients who tried to conceive, 45 were successful.4
Multiple studies and meta-analyses have shown no difference in postoperative pregnancy rates when comparing oophorectomy to cystectomy. For instance, in a 2021 meta-analysis, there was no significant difference noted in pregnancy rates between patients who underwent USO versus cystectomy (OR, 0.92; 95% CI, 0.60-1.42).
There are some data that support improved postoperative pregnancy rates in more conservative surgery, especially in the setting of bilateral BOT. In a small study of 32 patients who had laparoscopic staging for bilateral BOTs, patients were randomized to unilateral oophorectomy plus contralateral cystectomy or to bilateral cystectomy, which was referred to as ultraconservative surgery. The time to first recurrence was shorter in the ultraconservative group (although this lost significance when regression analysis was performed), but the time to first live birth was shorter and the relative chance of having a baby was higher in the bilateral cystectomy group.5
Ovarian-sparing procedures should be offered to patients in the setting of BOT. With ovarian-sparing surgery, it is important to counsel patients about the increased risk of recurrence and need for long-term follow-up. Pregnancy rates are generally good after fertility-sparing surgery. Surgery to conserve both ovaries does not seem to improve pregnancy rates in the setting of unilateral BOTs.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Jiao X et al. Int J Gynecol Cancer. 2017 Nov;27(9):1833-41.
2. Wang P and Fang L. World J Surg Oncol. 2021 Apr 21;19(1):132.
3. Vasconcelos I and de Sousa Mendes M. Eur J Cancer. 2015 Mar;51(5):620-31.
4. Song T et al. Int J Gynecol Cancer. 2011 May;21(4):640-6.
5. Palomba S et al. Hum Reprod. 2010 Aug;25(8):1966-72.
FDA approves OTC naloxone, but will cost be a barrier?
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Breast conservation safe even with multiple-site tumors
BOSTON – , as new data show a low risk of recurrence at 5 years when they are treated with breast-conserving therapy and radiation.
“[The study] proves the oncologic safety of breast conservation in women with two or three sites of disease, making this a very reasonable option for (previously reluctant) surgeons to present to patients,” first author Kari Rosenkranz, MD, an associate professor at Dartmouth Health in Norwich, Vt., said in an interview.
The findings were presented here at the International Conference on Surgical Cancer Care (SSO 2023), and were published online in the Journal of Clinical Oncology.
Commenting on the study, Hiram S. Cody III, MD, an attending surgeon and professor of surgery at Weill Cornell Medicine, Memorial Sloan Kettering Cancer Center, in New York, said the findings provide valuable new evidence on the issue.
“This is an important study confirming that breast conservation is feasible and safe for women with multiple ipsilateral breast cancers, with excellent results comparable to those for women with unifocal (single site) disease,” he said in an interview.
Although there have been as many as seven previous randomized trials that have shown identical outcomes in survival and local control of disease with breast-conserving therapy versus mastectomy, all those studies excluded patients with more than one site of disease.
At present, many surgeons and guidelines continue to recommend mastectomy for women with multiple-site tumors, based on older data that showed higher recurrence rates.
That is why the new study is so important, Dr. Cody explained. “Here, we see in a prospective trial that breast-conserving therapy is feasible for those with more than one site of disease as well, with high survival and very low rates of local recurrence,” he emphasized.
Dr. Cody noted that “the ideal candidate would be a woman with relatively small tumor size and a breast large enough that the multiple excisions could be performed with a good cosmetic result.”
“We have followed this approach for some time and hope that with the publication of these results more surgeons will recommend this approach for suitable patients,” he said.
The new results were also highlighted in a press release from Mayo Clinic highlighting the Journal of Clinical Oncology publication. Lead author of the article, surgical oncologist Judy Boughey, MD, from the Mayo Clinic in Rochester, Minn., commented: “I am excited about these findings because it will empower patients and the multidisciplinary care teams caring for patients to be thinking about this option for women who may want to preserve their breast.”
This study showed the rate of cancer local recurrence was 3.1%, she noted. This is an excellent outcome and is similar to the local recurrence rate for patients with a single tumor in a breast who had breast-conserving therapy, Dr. Boughey said.
Historically, women with multiple tumors in one breast have been advised to have a mastectomy. Now, patients can be offered a less invasive option with faster recovery, resulting in better patient satisfaction and cosmetic outcomes, she added.
Study details
This study, known as the ACOSOG (Alliance) Z11102 trial, was a phase 2 trial conducted in 204 patients enrolled between 2012 and 2016 who had two or three sites of biopsy-proven breast cancer (each site less rhan 5 cm in size, with cN0 or cN1 disease).
These patients were a median age of 61 years, and 83.5% were ER-positive/HER2-negative, 11.5% were HER2-positive, 5.0% were ER-negative/HER2-negative, and 77.5% were node-negative.
All patients were treated with breast conservation surgery, including lumpectomy resected to negative margins, followed by whole breast radiation with a cavity boost to all lumpectomy beds.
With a median follow-up of 66.4 months, six patients developed local recurrence, with five of the recurrences occurring in the ipsilateral breast and one in the chest wall.
For the primary endpoint, the six recurrences represented an estimated cumulative incidence of local recurrence of 3.1% (95% CI, 1.3-6.4), well below the cutoff of 8% that was determined to be the acceptable 5-year local recurrence rate based on historic recurrence rates for unifocal disease, Dr. Rosenkranz explained.
There were no cases of synchronous local and distant recurrences, six contralateral breast cancers, and three new primary nonbreast cancers. Eight patients died, including one related to breast cancer.
There were no significant associations between risk of local recurrence and factors including patient age, number of sites of preoperative biopsy-proven breast cancer, HER2 status, and pathologic T and N category.
In terms of secondary endpoints, 14 patients (7.1%) converted to mastectomy because of positive margins, while 67.6% achieved margin-negative excision in a single operation.
Regarding cosmesis, 70.6% of patients reported good or excellent cosmetic outcomes at 2 years.
In terms of adherence, the whole breast radiation therapy protocol was feasible in most patients.
Of note, among patients without a breast preoperative MRI, the 5-year rate of local recurrence was significantly higher, at 22.6% (n = 14) at 5 years, compared with 1.7% among the 180 patients who did have a preoperative MRI (P = .002). However, Dr. Rosenkranz said these differences should be interpreted with caution.
“We may look at these data and think we should consider preoperative breast MRI in patients who do have known multiple ipsilateral breast cancer, although I think this cohort was certainly much too small to draw definitive conclusions, and this was not a planned secondary endpoint of the trial,” she said during her presentation.
Most prefer breast conservation, when possible
Overall, the findings are important considering the array of known benefits of breast conservation over mastectomy, Dr. Rosenkranz concluded.
“The reason this is so important is that we know that patients who undergo breast conservation report improved quality of life, self-esteem, and body image, and therefore it’s incumbent on us as surgeons to expand the indications for breast conservation where we can,” she told the audience.
Speaking with this news organization, she added that the decision-making around breast conservation versus mastectomy can be complicated, and some women do opt for mastectomy because of a variety of factors; therefore, “tailoring therapy to the individual goals and priorities in addition to the disease characteristics is critical.”
That said, she added that “the majority of patients who are eligible for breast conservation do prefer this option.”
Dr. Rosenkranz and Dr. Cody have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BOSTON – , as new data show a low risk of recurrence at 5 years when they are treated with breast-conserving therapy and radiation.
“[The study] proves the oncologic safety of breast conservation in women with two or three sites of disease, making this a very reasonable option for (previously reluctant) surgeons to present to patients,” first author Kari Rosenkranz, MD, an associate professor at Dartmouth Health in Norwich, Vt., said in an interview.
The findings were presented here at the International Conference on Surgical Cancer Care (SSO 2023), and were published online in the Journal of Clinical Oncology.
Commenting on the study, Hiram S. Cody III, MD, an attending surgeon and professor of surgery at Weill Cornell Medicine, Memorial Sloan Kettering Cancer Center, in New York, said the findings provide valuable new evidence on the issue.
“This is an important study confirming that breast conservation is feasible and safe for women with multiple ipsilateral breast cancers, with excellent results comparable to those for women with unifocal (single site) disease,” he said in an interview.
Although there have been as many as seven previous randomized trials that have shown identical outcomes in survival and local control of disease with breast-conserving therapy versus mastectomy, all those studies excluded patients with more than one site of disease.
At present, many surgeons and guidelines continue to recommend mastectomy for women with multiple-site tumors, based on older data that showed higher recurrence rates.
That is why the new study is so important, Dr. Cody explained. “Here, we see in a prospective trial that breast-conserving therapy is feasible for those with more than one site of disease as well, with high survival and very low rates of local recurrence,” he emphasized.
Dr. Cody noted that “the ideal candidate would be a woman with relatively small tumor size and a breast large enough that the multiple excisions could be performed with a good cosmetic result.”
“We have followed this approach for some time and hope that with the publication of these results more surgeons will recommend this approach for suitable patients,” he said.
The new results were also highlighted in a press release from Mayo Clinic highlighting the Journal of Clinical Oncology publication. Lead author of the article, surgical oncologist Judy Boughey, MD, from the Mayo Clinic in Rochester, Minn., commented: “I am excited about these findings because it will empower patients and the multidisciplinary care teams caring for patients to be thinking about this option for women who may want to preserve their breast.”
This study showed the rate of cancer local recurrence was 3.1%, she noted. This is an excellent outcome and is similar to the local recurrence rate for patients with a single tumor in a breast who had breast-conserving therapy, Dr. Boughey said.
Historically, women with multiple tumors in one breast have been advised to have a mastectomy. Now, patients can be offered a less invasive option with faster recovery, resulting in better patient satisfaction and cosmetic outcomes, she added.
Study details
This study, known as the ACOSOG (Alliance) Z11102 trial, was a phase 2 trial conducted in 204 patients enrolled between 2012 and 2016 who had two or three sites of biopsy-proven breast cancer (each site less rhan 5 cm in size, with cN0 or cN1 disease).
These patients were a median age of 61 years, and 83.5% were ER-positive/HER2-negative, 11.5% were HER2-positive, 5.0% were ER-negative/HER2-negative, and 77.5% were node-negative.
All patients were treated with breast conservation surgery, including lumpectomy resected to negative margins, followed by whole breast radiation with a cavity boost to all lumpectomy beds.
With a median follow-up of 66.4 months, six patients developed local recurrence, with five of the recurrences occurring in the ipsilateral breast and one in the chest wall.
For the primary endpoint, the six recurrences represented an estimated cumulative incidence of local recurrence of 3.1% (95% CI, 1.3-6.4), well below the cutoff of 8% that was determined to be the acceptable 5-year local recurrence rate based on historic recurrence rates for unifocal disease, Dr. Rosenkranz explained.
There were no cases of synchronous local and distant recurrences, six contralateral breast cancers, and three new primary nonbreast cancers. Eight patients died, including one related to breast cancer.
There were no significant associations between risk of local recurrence and factors including patient age, number of sites of preoperative biopsy-proven breast cancer, HER2 status, and pathologic T and N category.
In terms of secondary endpoints, 14 patients (7.1%) converted to mastectomy because of positive margins, while 67.6% achieved margin-negative excision in a single operation.
Regarding cosmesis, 70.6% of patients reported good or excellent cosmetic outcomes at 2 years.
In terms of adherence, the whole breast radiation therapy protocol was feasible in most patients.
Of note, among patients without a breast preoperative MRI, the 5-year rate of local recurrence was significantly higher, at 22.6% (n = 14) at 5 years, compared with 1.7% among the 180 patients who did have a preoperative MRI (P = .002). However, Dr. Rosenkranz said these differences should be interpreted with caution.
“We may look at these data and think we should consider preoperative breast MRI in patients who do have known multiple ipsilateral breast cancer, although I think this cohort was certainly much too small to draw definitive conclusions, and this was not a planned secondary endpoint of the trial,” she said during her presentation.
Most prefer breast conservation, when possible
Overall, the findings are important considering the array of known benefits of breast conservation over mastectomy, Dr. Rosenkranz concluded.
“The reason this is so important is that we know that patients who undergo breast conservation report improved quality of life, self-esteem, and body image, and therefore it’s incumbent on us as surgeons to expand the indications for breast conservation where we can,” she told the audience.
Speaking with this news organization, she added that the decision-making around breast conservation versus mastectomy can be complicated, and some women do opt for mastectomy because of a variety of factors; therefore, “tailoring therapy to the individual goals and priorities in addition to the disease characteristics is critical.”
That said, she added that “the majority of patients who are eligible for breast conservation do prefer this option.”
Dr. Rosenkranz and Dr. Cody have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BOSTON – , as new data show a low risk of recurrence at 5 years when they are treated with breast-conserving therapy and radiation.
“[The study] proves the oncologic safety of breast conservation in women with two or three sites of disease, making this a very reasonable option for (previously reluctant) surgeons to present to patients,” first author Kari Rosenkranz, MD, an associate professor at Dartmouth Health in Norwich, Vt., said in an interview.
The findings were presented here at the International Conference on Surgical Cancer Care (SSO 2023), and were published online in the Journal of Clinical Oncology.
Commenting on the study, Hiram S. Cody III, MD, an attending surgeon and professor of surgery at Weill Cornell Medicine, Memorial Sloan Kettering Cancer Center, in New York, said the findings provide valuable new evidence on the issue.
“This is an important study confirming that breast conservation is feasible and safe for women with multiple ipsilateral breast cancers, with excellent results comparable to those for women with unifocal (single site) disease,” he said in an interview.
Although there have been as many as seven previous randomized trials that have shown identical outcomes in survival and local control of disease with breast-conserving therapy versus mastectomy, all those studies excluded patients with more than one site of disease.
At present, many surgeons and guidelines continue to recommend mastectomy for women with multiple-site tumors, based on older data that showed higher recurrence rates.
That is why the new study is so important, Dr. Cody explained. “Here, we see in a prospective trial that breast-conserving therapy is feasible for those with more than one site of disease as well, with high survival and very low rates of local recurrence,” he emphasized.
Dr. Cody noted that “the ideal candidate would be a woman with relatively small tumor size and a breast large enough that the multiple excisions could be performed with a good cosmetic result.”
“We have followed this approach for some time and hope that with the publication of these results more surgeons will recommend this approach for suitable patients,” he said.
The new results were also highlighted in a press release from Mayo Clinic highlighting the Journal of Clinical Oncology publication. Lead author of the article, surgical oncologist Judy Boughey, MD, from the Mayo Clinic in Rochester, Minn., commented: “I am excited about these findings because it will empower patients and the multidisciplinary care teams caring for patients to be thinking about this option for women who may want to preserve their breast.”
This study showed the rate of cancer local recurrence was 3.1%, she noted. This is an excellent outcome and is similar to the local recurrence rate for patients with a single tumor in a breast who had breast-conserving therapy, Dr. Boughey said.
Historically, women with multiple tumors in one breast have been advised to have a mastectomy. Now, patients can be offered a less invasive option with faster recovery, resulting in better patient satisfaction and cosmetic outcomes, she added.
Study details
This study, known as the ACOSOG (Alliance) Z11102 trial, was a phase 2 trial conducted in 204 patients enrolled between 2012 and 2016 who had two or three sites of biopsy-proven breast cancer (each site less rhan 5 cm in size, with cN0 or cN1 disease).
These patients were a median age of 61 years, and 83.5% were ER-positive/HER2-negative, 11.5% were HER2-positive, 5.0% were ER-negative/HER2-negative, and 77.5% were node-negative.
All patients were treated with breast conservation surgery, including lumpectomy resected to negative margins, followed by whole breast radiation with a cavity boost to all lumpectomy beds.
With a median follow-up of 66.4 months, six patients developed local recurrence, with five of the recurrences occurring in the ipsilateral breast and one in the chest wall.
For the primary endpoint, the six recurrences represented an estimated cumulative incidence of local recurrence of 3.1% (95% CI, 1.3-6.4), well below the cutoff of 8% that was determined to be the acceptable 5-year local recurrence rate based on historic recurrence rates for unifocal disease, Dr. Rosenkranz explained.
There were no cases of synchronous local and distant recurrences, six contralateral breast cancers, and three new primary nonbreast cancers. Eight patients died, including one related to breast cancer.
There were no significant associations between risk of local recurrence and factors including patient age, number of sites of preoperative biopsy-proven breast cancer, HER2 status, and pathologic T and N category.
In terms of secondary endpoints, 14 patients (7.1%) converted to mastectomy because of positive margins, while 67.6% achieved margin-negative excision in a single operation.
Regarding cosmesis, 70.6% of patients reported good or excellent cosmetic outcomes at 2 years.
In terms of adherence, the whole breast radiation therapy protocol was feasible in most patients.
Of note, among patients without a breast preoperative MRI, the 5-year rate of local recurrence was significantly higher, at 22.6% (n = 14) at 5 years, compared with 1.7% among the 180 patients who did have a preoperative MRI (P = .002). However, Dr. Rosenkranz said these differences should be interpreted with caution.
“We may look at these data and think we should consider preoperative breast MRI in patients who do have known multiple ipsilateral breast cancer, although I think this cohort was certainly much too small to draw definitive conclusions, and this was not a planned secondary endpoint of the trial,” she said during her presentation.
Most prefer breast conservation, when possible
Overall, the findings are important considering the array of known benefits of breast conservation over mastectomy, Dr. Rosenkranz concluded.
“The reason this is so important is that we know that patients who undergo breast conservation report improved quality of life, self-esteem, and body image, and therefore it’s incumbent on us as surgeons to expand the indications for breast conservation where we can,” she told the audience.
Speaking with this news organization, she added that the decision-making around breast conservation versus mastectomy can be complicated, and some women do opt for mastectomy because of a variety of factors; therefore, “tailoring therapy to the individual goals and priorities in addition to the disease characteristics is critical.”
That said, she added that “the majority of patients who are eligible for breast conservation do prefer this option.”
Dr. Rosenkranz and Dr. Cody have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SSO 2023
FDA Advisory panels consider easing isotretinoin requirements
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
Advances in the treatment of fetal demise in the second and third trimester
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.
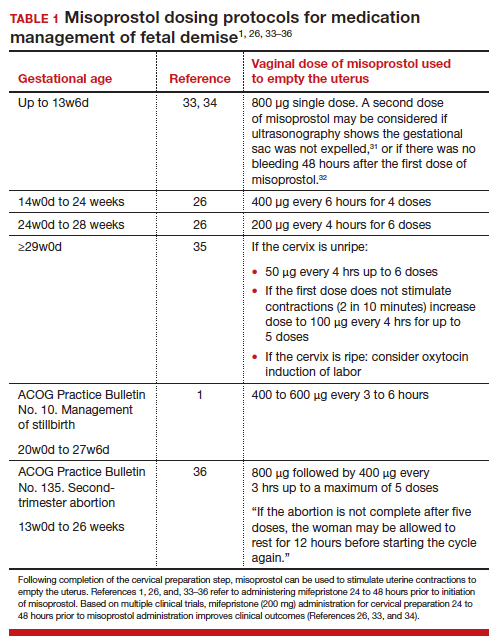
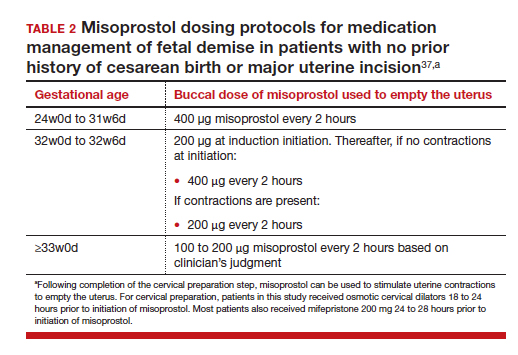

For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.