User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
COVID led to rise in pregnancy-related deaths: New research
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.
New state bill could protect docs prescribing abortion pills to out-of-state patients
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
Life’s Essential 8: Higher scores extend health span
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report on
This study leveraged the UK Biobank and included more than 135,000 U.K. adults with a mean age of 55. The AHA metric was defined as including the following lifestyle behavioral factors:
- Not smoking.
- Regular physical activity.
- Healthy weight.
- Healthy diet.
- Healthy sleep (defined as an average of 7-9 hours nightly).
- Blood pressure in a healthy range.
- Blood glucose in a healthy range.
- Non-HDL cholesterol in a healthy range.
This study was just published in JAMA Internal Medicine. I’d like to acknowledge that I’m a coauthor of this study, along with my colleagues at Tulane.
We divided the study population into three groups: those with low, moderate, and high scores on the Life’s Essential 8 metric – low, moderate, and high cardiovascular health. Overall, the average life expectancy free of chronic disease was estimated to be age 50, with 25 additional years in men and 30 additional years in women.
We saw large differences across the Life’s Essential 8 metric group. Men with high cardiovascular health scores tended to have an additional 7 years of life expectancy free of chronic disease, compared with those who had poorer scores. In women, the difference was about 9.5 years between high scores and lower scores. Also, the number of years lived with chronic disease was compressed in those with high cardiovascular health scores. They tended to have fewer years living with those chronic diseases but more years living free of chronic diseases.
We were interested in how these results might differ by socioeconomic status, educational level, and income level, as well as the Townsend deprivation index. We were intrigued by the finding that the gain in life expectancy free of chronic disease was very similar across all socioeconomic strata – those with lower education and lower income gained as much in terms of chronic disease–free life expectancy as those who were in the higher socioeconomic strata.
Overall, the findings make a compelling case for the importance of lifestyle factors in extending health span and years free of chronic disease. It can be motivating to tell our patients that a healthy lifestyle not only extends life expectancy but also extends years of health free of chronic disease.
Nonetheless, we do have many disparities in life expectancy and health span. So it will be very important to population health to narrow those health disparities through education about the importance of lifestyle factors, more research on implementation of lifestyle factors and behaviors, and public policy to make a healthy lifestyle both affordable and accessible to all people across all of these socioeconomic groups.
Thank you so much for your attention.
JoAnn E. Manson, MD, DrPH, is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report on
This study leveraged the UK Biobank and included more than 135,000 U.K. adults with a mean age of 55. The AHA metric was defined as including the following lifestyle behavioral factors:
- Not smoking.
- Regular physical activity.
- Healthy weight.
- Healthy diet.
- Healthy sleep (defined as an average of 7-9 hours nightly).
- Blood pressure in a healthy range.
- Blood glucose in a healthy range.
- Non-HDL cholesterol in a healthy range.
This study was just published in JAMA Internal Medicine. I’d like to acknowledge that I’m a coauthor of this study, along with my colleagues at Tulane.
We divided the study population into three groups: those with low, moderate, and high scores on the Life’s Essential 8 metric – low, moderate, and high cardiovascular health. Overall, the average life expectancy free of chronic disease was estimated to be age 50, with 25 additional years in men and 30 additional years in women.
We saw large differences across the Life’s Essential 8 metric group. Men with high cardiovascular health scores tended to have an additional 7 years of life expectancy free of chronic disease, compared with those who had poorer scores. In women, the difference was about 9.5 years between high scores and lower scores. Also, the number of years lived with chronic disease was compressed in those with high cardiovascular health scores. They tended to have fewer years living with those chronic diseases but more years living free of chronic diseases.
We were interested in how these results might differ by socioeconomic status, educational level, and income level, as well as the Townsend deprivation index. We were intrigued by the finding that the gain in life expectancy free of chronic disease was very similar across all socioeconomic strata – those with lower education and lower income gained as much in terms of chronic disease–free life expectancy as those who were in the higher socioeconomic strata.
Overall, the findings make a compelling case for the importance of lifestyle factors in extending health span and years free of chronic disease. It can be motivating to tell our patients that a healthy lifestyle not only extends life expectancy but also extends years of health free of chronic disease.
Nonetheless, we do have many disparities in life expectancy and health span. So it will be very important to population health to narrow those health disparities through education about the importance of lifestyle factors, more research on implementation of lifestyle factors and behaviors, and public policy to make a healthy lifestyle both affordable and accessible to all people across all of these socioeconomic groups.
Thank you so much for your attention.
JoAnn E. Manson, MD, DrPH, is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report on
This study leveraged the UK Biobank and included more than 135,000 U.K. adults with a mean age of 55. The AHA metric was defined as including the following lifestyle behavioral factors:
- Not smoking.
- Regular physical activity.
- Healthy weight.
- Healthy diet.
- Healthy sleep (defined as an average of 7-9 hours nightly).
- Blood pressure in a healthy range.
- Blood glucose in a healthy range.
- Non-HDL cholesterol in a healthy range.
This study was just published in JAMA Internal Medicine. I’d like to acknowledge that I’m a coauthor of this study, along with my colleagues at Tulane.
We divided the study population into three groups: those with low, moderate, and high scores on the Life’s Essential 8 metric – low, moderate, and high cardiovascular health. Overall, the average life expectancy free of chronic disease was estimated to be age 50, with 25 additional years in men and 30 additional years in women.
We saw large differences across the Life’s Essential 8 metric group. Men with high cardiovascular health scores tended to have an additional 7 years of life expectancy free of chronic disease, compared with those who had poorer scores. In women, the difference was about 9.5 years between high scores and lower scores. Also, the number of years lived with chronic disease was compressed in those with high cardiovascular health scores. They tended to have fewer years living with those chronic diseases but more years living free of chronic diseases.
We were interested in how these results might differ by socioeconomic status, educational level, and income level, as well as the Townsend deprivation index. We were intrigued by the finding that the gain in life expectancy free of chronic disease was very similar across all socioeconomic strata – those with lower education and lower income gained as much in terms of chronic disease–free life expectancy as those who were in the higher socioeconomic strata.
Overall, the findings make a compelling case for the importance of lifestyle factors in extending health span and years free of chronic disease. It can be motivating to tell our patients that a healthy lifestyle not only extends life expectancy but also extends years of health free of chronic disease.
Nonetheless, we do have many disparities in life expectancy and health span. So it will be very important to population health to narrow those health disparities through education about the importance of lifestyle factors, more research on implementation of lifestyle factors and behaviors, and public policy to make a healthy lifestyle both affordable and accessible to all people across all of these socioeconomic groups.
Thank you so much for your attention.
JoAnn E. Manson, MD, DrPH, is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
Forceps may help moms with obesity avoid cesareans
Among patients who undergo forceps-assisted vaginal delivery, obesity does not appear to be associated with increased risk for complications such as injuries to the anal sphincter or the need for their babies to be admitted to the neonatal intensive care unit, researchers have found.
But obesity does appear to increase the chances that when physicians attempt operative vaginal delivery with either forceps or a vacuum, patients will wind up undergoing cesarean delivery, another study found.
Taken together, the new data may help inform physicians’ decisions about when to consider operative vaginal delivery as an alternative to emergency cesarean births.
A prospective study showed that failed operative vaginal delivery – that is, a cesarean delivery after an attempted operative vaginal delivery – occurred for 10.1% of patients with obesity and 4.2% of those without obesity.
Researchers presented the findings at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“We want to really try to reduce the rate of C-sections and primary cesarean deliveries. One of the ways to do that is to attempt operative vaginal delivery,” said Marissa Platner, MD, assistant professor of maternal-fetal medicine at Emory University School of Medicine, Atlanta, who was not involved in the new research.
Data on how obesity influences risks with operative vaginal delivery have been limited and mixed, the researchers said.
To examine how often attempted operative vaginal delivery fails in patients with obesity, Jennifer Grasch, MD, a maternal-fetal medicine fellow at the Ohio State University Wexner Medical Center, Columbus, and her colleagues conducted a secondary analysis of data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which included more than 10,000 participants.
“We know that cesarean sections among people with obesity are associated with increased complications, such as higher rates of infection and wound complications, than for people with lower BMI,” Dr. Grasch said. “Operative vaginal delivery can be an alternative to cesarean delivery in some situations, so we were interested in whether attempted operative vaginal delivery was also associated with higher rates of complications in individuals with obesity than those without obesity.”
The researchers focused on 791 patients with an attempted operative vaginal delivery. About 40% had a BMI of 30 or greater. Clinicians used a vacuum in approximately 60% of the attempts.
After an attempted vacuum-assisted delivery, neonatal morbidity was more common for infants whose mothers had obesity than for those whose mothers did not (32.7% vs. 22.3%; adjusted odds ratio, 1.61 [1.07-2.43]). Neonatal morbidity did not differ by obesity status following forceps-attempted delivery. Other adverse outcomes, including measures of maternal morbidity, did not significantly differ by obesity status, according to the researchers.
Choice may come down to experience
Several factors influence whether a clinician chooses forceps- or vacuum-assisted delivery or cesarean delivery, “but one of the most important is experience,” Dr. Grasch said. “Complication rates with both forms of operative vaginal delivery are low, yet there has been a trend toward lower rates of both in the last few decades.”
Elizabeth Cochrane, MD, a maternal-fetal medicine fellow at Mount Sinai Hospital, New York, and her colleagues investigated the relationship between obesity and adverse outcomes among patients with forceps-assisted vaginal deliveries.
The researchers analyzed data from 897 patients who underwent a forceps-assisted vaginal delivery between 2017 and 2021; 29% had a BMI of 30 or greater.
Injuries to the anal sphincter – which can lead to fecal incontinence – occurred in 18.7% of patients without obesity and in 17.7% of those with obesity. Admission to the neonatal intensive care unit occurred in 11.5% of patients without obesity and in 12.3% of patients with obesity. The differences were not statistically significant.
The bottom line: For forceps-assisted vaginal delivery, “obesity does not appear to be associated with increased rates” of adverse outcomes for mothers or newborns, the researchers concluded.
Reassuring data
The study by Dr. Cochrane’s group “provides helpful information for providers to be reassured when they are performing forceps deliveries” for patients with obesity, Dr. Platner said.
Rates of obesity have risen in the United States, and physicians often wonder whether a patient with obesity could be a candidate for forceps-assisted delivery, Dr. Cochrane said. In 2019, 29% of women had obesity before becoming pregnant.
“It all really comes down to how comfortable the provider is in that skill set and also the overall clinical scenario,” she said. “Sometimes an operative delivery with forceps or a vacuum can be the fastest way to deliver a baby when there is acute concern for maternal decompensation or fetal decompensation.”
The alternative is an emergency cesarean delivery. Given that those operations can be riskier and more difficult for patients with higher BMIs, a forceps-assisted delivery may be “an interesting alternative to emergency caesarean sections, as long as it is in an appropriate clinical setting with providers who feel very confident and comfortable using those devices,” Dr. Cochrane said.
A version of this article first appeared on Medscape.com.
Among patients who undergo forceps-assisted vaginal delivery, obesity does not appear to be associated with increased risk for complications such as injuries to the anal sphincter or the need for their babies to be admitted to the neonatal intensive care unit, researchers have found.
But obesity does appear to increase the chances that when physicians attempt operative vaginal delivery with either forceps or a vacuum, patients will wind up undergoing cesarean delivery, another study found.
Taken together, the new data may help inform physicians’ decisions about when to consider operative vaginal delivery as an alternative to emergency cesarean births.
A prospective study showed that failed operative vaginal delivery – that is, a cesarean delivery after an attempted operative vaginal delivery – occurred for 10.1% of patients with obesity and 4.2% of those without obesity.
Researchers presented the findings at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“We want to really try to reduce the rate of C-sections and primary cesarean deliveries. One of the ways to do that is to attempt operative vaginal delivery,” said Marissa Platner, MD, assistant professor of maternal-fetal medicine at Emory University School of Medicine, Atlanta, who was not involved in the new research.
Data on how obesity influences risks with operative vaginal delivery have been limited and mixed, the researchers said.
To examine how often attempted operative vaginal delivery fails in patients with obesity, Jennifer Grasch, MD, a maternal-fetal medicine fellow at the Ohio State University Wexner Medical Center, Columbus, and her colleagues conducted a secondary analysis of data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which included more than 10,000 participants.
“We know that cesarean sections among people with obesity are associated with increased complications, such as higher rates of infection and wound complications, than for people with lower BMI,” Dr. Grasch said. “Operative vaginal delivery can be an alternative to cesarean delivery in some situations, so we were interested in whether attempted operative vaginal delivery was also associated with higher rates of complications in individuals with obesity than those without obesity.”
The researchers focused on 791 patients with an attempted operative vaginal delivery. About 40% had a BMI of 30 or greater. Clinicians used a vacuum in approximately 60% of the attempts.
After an attempted vacuum-assisted delivery, neonatal morbidity was more common for infants whose mothers had obesity than for those whose mothers did not (32.7% vs. 22.3%; adjusted odds ratio, 1.61 [1.07-2.43]). Neonatal morbidity did not differ by obesity status following forceps-attempted delivery. Other adverse outcomes, including measures of maternal morbidity, did not significantly differ by obesity status, according to the researchers.
Choice may come down to experience
Several factors influence whether a clinician chooses forceps- or vacuum-assisted delivery or cesarean delivery, “but one of the most important is experience,” Dr. Grasch said. “Complication rates with both forms of operative vaginal delivery are low, yet there has been a trend toward lower rates of both in the last few decades.”
Elizabeth Cochrane, MD, a maternal-fetal medicine fellow at Mount Sinai Hospital, New York, and her colleagues investigated the relationship between obesity and adverse outcomes among patients with forceps-assisted vaginal deliveries.
The researchers analyzed data from 897 patients who underwent a forceps-assisted vaginal delivery between 2017 and 2021; 29% had a BMI of 30 or greater.
Injuries to the anal sphincter – which can lead to fecal incontinence – occurred in 18.7% of patients without obesity and in 17.7% of those with obesity. Admission to the neonatal intensive care unit occurred in 11.5% of patients without obesity and in 12.3% of patients with obesity. The differences were not statistically significant.
The bottom line: For forceps-assisted vaginal delivery, “obesity does not appear to be associated with increased rates” of adverse outcomes for mothers or newborns, the researchers concluded.
Reassuring data
The study by Dr. Cochrane’s group “provides helpful information for providers to be reassured when they are performing forceps deliveries” for patients with obesity, Dr. Platner said.
Rates of obesity have risen in the United States, and physicians often wonder whether a patient with obesity could be a candidate for forceps-assisted delivery, Dr. Cochrane said. In 2019, 29% of women had obesity before becoming pregnant.
“It all really comes down to how comfortable the provider is in that skill set and also the overall clinical scenario,” she said. “Sometimes an operative delivery with forceps or a vacuum can be the fastest way to deliver a baby when there is acute concern for maternal decompensation or fetal decompensation.”
The alternative is an emergency cesarean delivery. Given that those operations can be riskier and more difficult for patients with higher BMIs, a forceps-assisted delivery may be “an interesting alternative to emergency caesarean sections, as long as it is in an appropriate clinical setting with providers who feel very confident and comfortable using those devices,” Dr. Cochrane said.
A version of this article first appeared on Medscape.com.
Among patients who undergo forceps-assisted vaginal delivery, obesity does not appear to be associated with increased risk for complications such as injuries to the anal sphincter or the need for their babies to be admitted to the neonatal intensive care unit, researchers have found.
But obesity does appear to increase the chances that when physicians attempt operative vaginal delivery with either forceps or a vacuum, patients will wind up undergoing cesarean delivery, another study found.
Taken together, the new data may help inform physicians’ decisions about when to consider operative vaginal delivery as an alternative to emergency cesarean births.
A prospective study showed that failed operative vaginal delivery – that is, a cesarean delivery after an attempted operative vaginal delivery – occurred for 10.1% of patients with obesity and 4.2% of those without obesity.
Researchers presented the findings at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“We want to really try to reduce the rate of C-sections and primary cesarean deliveries. One of the ways to do that is to attempt operative vaginal delivery,” said Marissa Platner, MD, assistant professor of maternal-fetal medicine at Emory University School of Medicine, Atlanta, who was not involved in the new research.
Data on how obesity influences risks with operative vaginal delivery have been limited and mixed, the researchers said.
To examine how often attempted operative vaginal delivery fails in patients with obesity, Jennifer Grasch, MD, a maternal-fetal medicine fellow at the Ohio State University Wexner Medical Center, Columbus, and her colleagues conducted a secondary analysis of data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which included more than 10,000 participants.
“We know that cesarean sections among people with obesity are associated with increased complications, such as higher rates of infection and wound complications, than for people with lower BMI,” Dr. Grasch said. “Operative vaginal delivery can be an alternative to cesarean delivery in some situations, so we were interested in whether attempted operative vaginal delivery was also associated with higher rates of complications in individuals with obesity than those without obesity.”
The researchers focused on 791 patients with an attempted operative vaginal delivery. About 40% had a BMI of 30 or greater. Clinicians used a vacuum in approximately 60% of the attempts.
After an attempted vacuum-assisted delivery, neonatal morbidity was more common for infants whose mothers had obesity than for those whose mothers did not (32.7% vs. 22.3%; adjusted odds ratio, 1.61 [1.07-2.43]). Neonatal morbidity did not differ by obesity status following forceps-attempted delivery. Other adverse outcomes, including measures of maternal morbidity, did not significantly differ by obesity status, according to the researchers.
Choice may come down to experience
Several factors influence whether a clinician chooses forceps- or vacuum-assisted delivery or cesarean delivery, “but one of the most important is experience,” Dr. Grasch said. “Complication rates with both forms of operative vaginal delivery are low, yet there has been a trend toward lower rates of both in the last few decades.”
Elizabeth Cochrane, MD, a maternal-fetal medicine fellow at Mount Sinai Hospital, New York, and her colleagues investigated the relationship between obesity and adverse outcomes among patients with forceps-assisted vaginal deliveries.
The researchers analyzed data from 897 patients who underwent a forceps-assisted vaginal delivery between 2017 and 2021; 29% had a BMI of 30 or greater.
Injuries to the anal sphincter – which can lead to fecal incontinence – occurred in 18.7% of patients without obesity and in 17.7% of those with obesity. Admission to the neonatal intensive care unit occurred in 11.5% of patients without obesity and in 12.3% of patients with obesity. The differences were not statistically significant.
The bottom line: For forceps-assisted vaginal delivery, “obesity does not appear to be associated with increased rates” of adverse outcomes for mothers or newborns, the researchers concluded.
Reassuring data
The study by Dr. Cochrane’s group “provides helpful information for providers to be reassured when they are performing forceps deliveries” for patients with obesity, Dr. Platner said.
Rates of obesity have risen in the United States, and physicians often wonder whether a patient with obesity could be a candidate for forceps-assisted delivery, Dr. Cochrane said. In 2019, 29% of women had obesity before becoming pregnant.
“It all really comes down to how comfortable the provider is in that skill set and also the overall clinical scenario,” she said. “Sometimes an operative delivery with forceps or a vacuum can be the fastest way to deliver a baby when there is acute concern for maternal decompensation or fetal decompensation.”
The alternative is an emergency cesarean delivery. Given that those operations can be riskier and more difficult for patients with higher BMIs, a forceps-assisted delivery may be “an interesting alternative to emergency caesarean sections, as long as it is in an appropriate clinical setting with providers who feel very confident and comfortable using those devices,” Dr. Cochrane said.
A version of this article first appeared on Medscape.com.
FROM SMFM 2023
Luxe vacations, private jets: Medical device maker, surgeon to pay $46 million penalty in kickback scheme
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
Multi-cancer early detection liquid biopsy testing: A predictive genetic test not quite ready for prime time
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?
The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
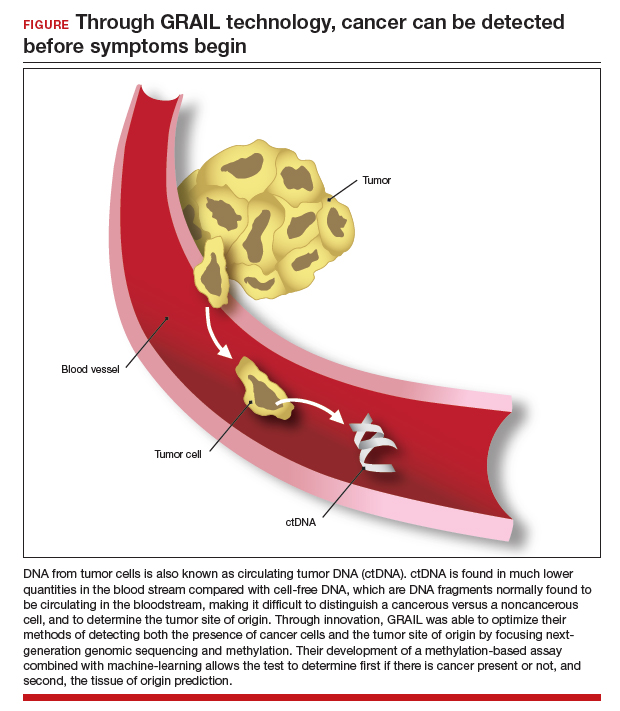
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
Product updates and reviews
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort
The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
CarePostRoe.com: Study seeks to document poor quality medical care due to new abortion bans
In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
Osteoporosis drugs may extend life after fracture
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Home-based HPV cervical cancer screening ‘cost effective’
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN


