User login
U.S. syphilis cases reach 70-year high
Cases of the sexually transmitted disease syphilis soared in 2021 to the highest total in more than 70 years, a new report says.
Earlier in 2023, the Centers for Disease Control and Prevention issued preliminary projections that syphilis rates had made a startling jump from 2020 to 2021. But now that health officials have finalized all of the 2021 data, the increase is worse than what was announced back in March.
In just a 1-year period, from 2020 to 2021, cases increased by 32%, to 176,713, according to newly finalized data from the CDC. That is the highest total number of syphilis cases the U.S. has seen since 1950.
The total number of STD cases in the U.S. in 2021 was 2.5 million, including 1.6 million cases of chlamydia, which was up 4% over the year prior.
A CDC official labeled the situation an epidemic.
“The reasons for the ongoing increases are multifaceted – and so are the solutions,” said Leandro Mena, MD, MPH, director of the CDC’s STD prevention division, in a statement. “It will take many of us working together to effectively use new and existing tools to increase access to quality sexual health care services for more people and to encourage ongoing innovation and prioritization of STI prevention and treatment in this country.”
Syphilis causes sores and rashes and, left untreated over a long period of time, can cause severe problems in organs, the brain, and the nervous system. Untreated congenital syphilis can lead to stillbirth. The treatment for syphilis is antibiotics.
The CDC called a 32% increase from 2020 to 2021 of congenital syphilis cases “alarming,” reporting that it resulted in 220 stillbirths and infant deaths in 2021.
The rise in STDs during the pandemic has been attributed to decreased attention and resources devoted to sexual health. Opioid use is also considered a contributing factor.
A version of this article first appeared on WebMD.com.
Cases of the sexually transmitted disease syphilis soared in 2021 to the highest total in more than 70 years, a new report says.
Earlier in 2023, the Centers for Disease Control and Prevention issued preliminary projections that syphilis rates had made a startling jump from 2020 to 2021. But now that health officials have finalized all of the 2021 data, the increase is worse than what was announced back in March.
In just a 1-year period, from 2020 to 2021, cases increased by 32%, to 176,713, according to newly finalized data from the CDC. That is the highest total number of syphilis cases the U.S. has seen since 1950.
The total number of STD cases in the U.S. in 2021 was 2.5 million, including 1.6 million cases of chlamydia, which was up 4% over the year prior.
A CDC official labeled the situation an epidemic.
“The reasons for the ongoing increases are multifaceted – and so are the solutions,” said Leandro Mena, MD, MPH, director of the CDC’s STD prevention division, in a statement. “It will take many of us working together to effectively use new and existing tools to increase access to quality sexual health care services for more people and to encourage ongoing innovation and prioritization of STI prevention and treatment in this country.”
Syphilis causes sores and rashes and, left untreated over a long period of time, can cause severe problems in organs, the brain, and the nervous system. Untreated congenital syphilis can lead to stillbirth. The treatment for syphilis is antibiotics.
The CDC called a 32% increase from 2020 to 2021 of congenital syphilis cases “alarming,” reporting that it resulted in 220 stillbirths and infant deaths in 2021.
The rise in STDs during the pandemic has been attributed to decreased attention and resources devoted to sexual health. Opioid use is also considered a contributing factor.
A version of this article first appeared on WebMD.com.
Cases of the sexually transmitted disease syphilis soared in 2021 to the highest total in more than 70 years, a new report says.
Earlier in 2023, the Centers for Disease Control and Prevention issued preliminary projections that syphilis rates had made a startling jump from 2020 to 2021. But now that health officials have finalized all of the 2021 data, the increase is worse than what was announced back in March.
In just a 1-year period, from 2020 to 2021, cases increased by 32%, to 176,713, according to newly finalized data from the CDC. That is the highest total number of syphilis cases the U.S. has seen since 1950.
The total number of STD cases in the U.S. in 2021 was 2.5 million, including 1.6 million cases of chlamydia, which was up 4% over the year prior.
A CDC official labeled the situation an epidemic.
“The reasons for the ongoing increases are multifaceted – and so are the solutions,” said Leandro Mena, MD, MPH, director of the CDC’s STD prevention division, in a statement. “It will take many of us working together to effectively use new and existing tools to increase access to quality sexual health care services for more people and to encourage ongoing innovation and prioritization of STI prevention and treatment in this country.”
Syphilis causes sores and rashes and, left untreated over a long period of time, can cause severe problems in organs, the brain, and the nervous system. Untreated congenital syphilis can lead to stillbirth. The treatment for syphilis is antibiotics.
The CDC called a 32% increase from 2020 to 2021 of congenital syphilis cases “alarming,” reporting that it resulted in 220 stillbirths and infant deaths in 2021.
The rise in STDs during the pandemic has been attributed to decreased attention and resources devoted to sexual health. Opioid use is also considered a contributing factor.
A version of this article first appeared on WebMD.com.
Training the future cardiac intensivist to meet the demands of the modern cardiovascular ICU
PULMONARY VASCULAR & CARDIOVASCULAR NETWORK
Cardiovascular Medicine & Surgery Section
Over the recent decades, the cardiovascular intensive care unit (CICU) has seen a significant transformation. Not only has the acuity of cardiac conditions evolved, but the prevalence of noncardiac critical illness has multiplied (Yuriditsky E, et al. ATS Sch. 2022;3[4]):522).
(CCM) (Morrow DA, et al. Circulation. 2012;126:1408).
However, fewer than 15% of modern CICUs are staffed by physicians dual-boarded in cardiology and CCM; most believe that CCM training is necessary to effectively practice in the CICU (van Diepen S, et al. Circ Cardiovasc Qual Outcomes. 2017;10:e003864).
How best do we develop future cardiac intensivists to manage complex decompensated cardiovascular disease with compounded medical critical illness?
Multiple training pathways leading to board eligibility and dual certification have been outlined (Geller BJ, et al. J Am Coll Cardiol. 2018;72:1171). A commonly elected path requires the completion of a 1-year CCM fellowship following a 3-year general cardiology fellowship.
As few programs exist, limited guidance is available surrounding CCM fellowship design for the cardiologist; however, proposed curricula have been published (Yuriditsky E, et al. ATS Sch. 2022;3[4]:522).
Developing such programs requires collaboration between cardiologists and intensivists to secure funding, develop infrastructure, obtain accreditation, and to recruit candidates.
Having completed dual training, I not only saw my skillset flourish, but the partnership between CCM and cardiology strengthen. As interest in this field grows, we eagerly await to see program adaptation and innovative curriculum design.
Eugene Yuriditsky, MD
Section Fellow-in-Training
PULMONARY VASCULAR & CARDIOVASCULAR NETWORK
Cardiovascular Medicine & Surgery Section
Over the recent decades, the cardiovascular intensive care unit (CICU) has seen a significant transformation. Not only has the acuity of cardiac conditions evolved, but the prevalence of noncardiac critical illness has multiplied (Yuriditsky E, et al. ATS Sch. 2022;3[4]):522).
(CCM) (Morrow DA, et al. Circulation. 2012;126:1408).
However, fewer than 15% of modern CICUs are staffed by physicians dual-boarded in cardiology and CCM; most believe that CCM training is necessary to effectively practice in the CICU (van Diepen S, et al. Circ Cardiovasc Qual Outcomes. 2017;10:e003864).
How best do we develop future cardiac intensivists to manage complex decompensated cardiovascular disease with compounded medical critical illness?
Multiple training pathways leading to board eligibility and dual certification have been outlined (Geller BJ, et al. J Am Coll Cardiol. 2018;72:1171). A commonly elected path requires the completion of a 1-year CCM fellowship following a 3-year general cardiology fellowship.
As few programs exist, limited guidance is available surrounding CCM fellowship design for the cardiologist; however, proposed curricula have been published (Yuriditsky E, et al. ATS Sch. 2022;3[4]:522).
Developing such programs requires collaboration between cardiologists and intensivists to secure funding, develop infrastructure, obtain accreditation, and to recruit candidates.
Having completed dual training, I not only saw my skillset flourish, but the partnership between CCM and cardiology strengthen. As interest in this field grows, we eagerly await to see program adaptation and innovative curriculum design.
Eugene Yuriditsky, MD
Section Fellow-in-Training
PULMONARY VASCULAR & CARDIOVASCULAR NETWORK
Cardiovascular Medicine & Surgery Section
Over the recent decades, the cardiovascular intensive care unit (CICU) has seen a significant transformation. Not only has the acuity of cardiac conditions evolved, but the prevalence of noncardiac critical illness has multiplied (Yuriditsky E, et al. ATS Sch. 2022;3[4]):522).
(CCM) (Morrow DA, et al. Circulation. 2012;126:1408).
However, fewer than 15% of modern CICUs are staffed by physicians dual-boarded in cardiology and CCM; most believe that CCM training is necessary to effectively practice in the CICU (van Diepen S, et al. Circ Cardiovasc Qual Outcomes. 2017;10:e003864).
How best do we develop future cardiac intensivists to manage complex decompensated cardiovascular disease with compounded medical critical illness?
Multiple training pathways leading to board eligibility and dual certification have been outlined (Geller BJ, et al. J Am Coll Cardiol. 2018;72:1171). A commonly elected path requires the completion of a 1-year CCM fellowship following a 3-year general cardiology fellowship.
As few programs exist, limited guidance is available surrounding CCM fellowship design for the cardiologist; however, proposed curricula have been published (Yuriditsky E, et al. ATS Sch. 2022;3[4]:522).
Developing such programs requires collaboration between cardiologists and intensivists to secure funding, develop infrastructure, obtain accreditation, and to recruit candidates.
Having completed dual training, I not only saw my skillset flourish, but the partnership between CCM and cardiology strengthen. As interest in this field grows, we eagerly await to see program adaptation and innovative curriculum design.
Eugene Yuriditsky, MD
Section Fellow-in-Training
Cocaine damage can be misdiagnosed as nasal vasculitis
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
FROM RHEUMATOLOGY ADVANCES IN PRACTICE
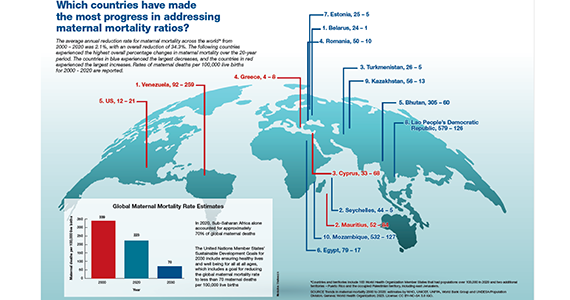
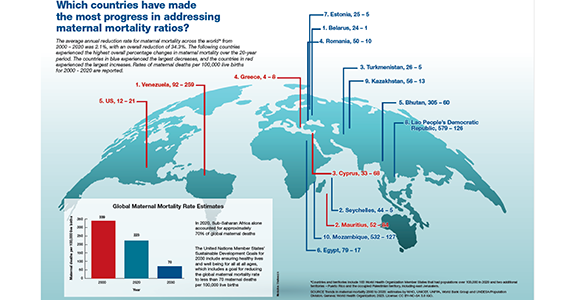
Which countries have made the most progress in addressing maternal mortality ratios?


Is azithromycin prophylaxis appropriate for vaginal delivery in low- and middle-resource populations?
Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
EXPERT COMMENTARY
Maternal peripartum infection is 1 of the top 5 causes of maternal death, accounting for about 10% of cases of maternal mortality. Cesarean delivery (CD), of course, is the most important risk factor for puerperal infection. However, even vaginal delivery, particularly in low- to middle-resource countries, where deliveries often occur under less-than-optimal conditions, may be associated with a surprisingly high frequency of both maternal and neonatal infections. The beneficial effect of prophylactic antibiotics for CD is well established. An important remaining question is whether similar benefit can be achieved with prophylaxis for women planning to have a vaginal birth.
In 2017, Oluwalana and colleagues conducted a prospective, randomized, double-blind, placebo-controlled trial of a single 2-g oral dose of azithromycin in Gambian women undergoing labor.1 During the 8 weeks after delivery, maternal infections were lower in the azithromycin group, 3.6% versus 9.2% (relative risk [RR], 0.40; 95% confidence interval [CI], 0.22–0.71; P=.002). Infections also were lower in the newborns, 18.1% versus 23.8% (RR, 0.76; 95% CI, 0.58–0.99; P=.052), delivered to mothers who received azithromycin. The greatest impact on neonatal infections was the reduced frequency of skin infections.
In 2021, Subramaniam and colleagues evaluated the effect of a single dose of oral azithromycin with, or without, amoxicillin on the prevalence of peripartum infection in laboring women in Cameroon.2 Patients and their newborns were followed for 6 weeks after delivery. Unlike the previous investigation, the authors were unable to show a protective effect of prophylaxis on either maternal or neonatal infection.
Against this backdrop, Tita and colleagues conducted a remarkably large, well-designed, randomized, placebo-controlled study of azithromycin prophylaxis in women at 8 different sites in 7 low- or middle-income countries (the A-PLUS investigation).3
Details of the study
The investigators randomly assigned 29,278 patients at or beyond 28 weeks’ gestation to receive either a 2-g oral dose of azithromycin or placebo during labor. This particular drug was chosen because it is readily available, inexpensive, well tolerated, and has a broad range of activity against many important pelvic pathogens, including genital mycoplasmas. Some patients also received other antibiotics, for example, for group B streptococcal (GBS) prophylaxis or for CD prophylaxis if abdominal delivery was indicated.
The 2 primary outcomes were a composite of maternal sepsis or death and a composite of stillbirth or neonatal death or sepsis within 4 weeks of delivery. Secondary outcomes included individual components of the primary outcomes.
Results. The results of the investigation were compelling, and the data safety monitoring committee recommended stopping the trial early because of clear maternal benefit. The groups were well balanced with respect to important characteristics, such as incidence of CD, receipt of other prophylactic antibiotics, and median time between randomization and delivery.
The incidence of maternal sepsis or death was lower in the azithromycin group (1.6% vs 2.4%; RR, 0.67; 95% CI, 0.56–0.79; P<.001). The key effect was on the frequency of maternal sepsis because the incidence of maternal death was very low in both groups, 0.1%. With respect to secondary outcomes, prophylaxis was effective in reducing the frequency of endometritis (RR, 0.66; 95% CI, 0.55–0.79) and perineal and incisional infection (RR, 0.71; 95% CI, 0.56–0.85).
There was no difference in the frequency of neonatal sepsis or death. There also was no difference in the frequency of adverse drug effects in either group. Of note, more cases of neonatal pyloric stenosis were observed in the azithromycin group, but the overall incidence was lower than the expected background rate. This possible “signal” is important because this effect has been noted with increased frequency in neonates who received this antibiotic. ●
I believe that Tita and colleagues are quite correct in concluding that the simple, inexpensive intervention of azithromycin prophylaxis should be used routinely in patient populations similar to those included in this investigation and that the intervention can be invaluable in advancing the World Health Organization’s campaign to reduce the rate of maternal mortality in low- and middleresource nations.
What is not clear, however, is whether this same intervention would be effective in high-resource countries in which the level of skill of the obstetric providers is higher and more uniform; deliveries occur under more optimal sanitary conditions; treatment and prophylaxis for infections such as gonorrhea, chlamydia, chorioamnionitis, and GBS is more consistent; and early neonatal care is more robust. A similar large trial in wellresourced nations would indeed be welcome, particularly if the trial also addressed the possibility of an adverse effect on the neonatal microbiome if a policy of nearly universal antibiotic prophylaxis was adopted.
In the interim, we should focus our attention on the key interventions that are of proven value in decreasing the risk of peripartum maternal and neonatal infection:
- consistently screening for GBS colonization and administering intrapartum antibiotic prophylaxis to patients who test positive
- consistently screening for gonococcal and chlamydia infection in the antepartum period and treating infected patients with appropriate antibiotics
- minimizing the number of internal vaginal examinations during labor, particularly following rupture of membranes
- promptly identifying patients with chorioamnionitis and treating with antibiotics that specifically target GBS and Escherichia coli, the 2 most likely causes of neonatal sepsis, pneumonia, and meningitis
- administering preoperative prophylactic antibiotics (cefazolin plus azithromycin) to women who require CD.
PATRICK DUFF, MD
- Oluwalana C, Camara B, Bottomley C, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double-blind trial. Pediatrics. 2017;139:e20162281. doi:10.1542/peds.2016-2281.
- Subramaniam A, Ye Y, Mbah R, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol. 2021;138:703-713. doi:10.1097/AOG.0000000000004565.
- Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.

Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
EXPERT COMMENTARY
Maternal peripartum infection is 1 of the top 5 causes of maternal death, accounting for about 10% of cases of maternal mortality. Cesarean delivery (CD), of course, is the most important risk factor for puerperal infection. However, even vaginal delivery, particularly in low- to middle-resource countries, where deliveries often occur under less-than-optimal conditions, may be associated with a surprisingly high frequency of both maternal and neonatal infections. The beneficial effect of prophylactic antibiotics for CD is well established. An important remaining question is whether similar benefit can be achieved with prophylaxis for women planning to have a vaginal birth.
In 2017, Oluwalana and colleagues conducted a prospective, randomized, double-blind, placebo-controlled trial of a single 2-g oral dose of azithromycin in Gambian women undergoing labor.1 During the 8 weeks after delivery, maternal infections were lower in the azithromycin group, 3.6% versus 9.2% (relative risk [RR], 0.40; 95% confidence interval [CI], 0.22–0.71; P=.002). Infections also were lower in the newborns, 18.1% versus 23.8% (RR, 0.76; 95% CI, 0.58–0.99; P=.052), delivered to mothers who received azithromycin. The greatest impact on neonatal infections was the reduced frequency of skin infections.
In 2021, Subramaniam and colleagues evaluated the effect of a single dose of oral azithromycin with, or without, amoxicillin on the prevalence of peripartum infection in laboring women in Cameroon.2 Patients and their newborns were followed for 6 weeks after delivery. Unlike the previous investigation, the authors were unable to show a protective effect of prophylaxis on either maternal or neonatal infection.
Against this backdrop, Tita and colleagues conducted a remarkably large, well-designed, randomized, placebo-controlled study of azithromycin prophylaxis in women at 8 different sites in 7 low- or middle-income countries (the A-PLUS investigation).3
Details of the study
The investigators randomly assigned 29,278 patients at or beyond 28 weeks’ gestation to receive either a 2-g oral dose of azithromycin or placebo during labor. This particular drug was chosen because it is readily available, inexpensive, well tolerated, and has a broad range of activity against many important pelvic pathogens, including genital mycoplasmas. Some patients also received other antibiotics, for example, for group B streptococcal (GBS) prophylaxis or for CD prophylaxis if abdominal delivery was indicated.
The 2 primary outcomes were a composite of maternal sepsis or death and a composite of stillbirth or neonatal death or sepsis within 4 weeks of delivery. Secondary outcomes included individual components of the primary outcomes.
Results. The results of the investigation were compelling, and the data safety monitoring committee recommended stopping the trial early because of clear maternal benefit. The groups were well balanced with respect to important characteristics, such as incidence of CD, receipt of other prophylactic antibiotics, and median time between randomization and delivery.
The incidence of maternal sepsis or death was lower in the azithromycin group (1.6% vs 2.4%; RR, 0.67; 95% CI, 0.56–0.79; P<.001). The key effect was on the frequency of maternal sepsis because the incidence of maternal death was very low in both groups, 0.1%. With respect to secondary outcomes, prophylaxis was effective in reducing the frequency of endometritis (RR, 0.66; 95% CI, 0.55–0.79) and perineal and incisional infection (RR, 0.71; 95% CI, 0.56–0.85).
There was no difference in the frequency of neonatal sepsis or death. There also was no difference in the frequency of adverse drug effects in either group. Of note, more cases of neonatal pyloric stenosis were observed in the azithromycin group, but the overall incidence was lower than the expected background rate. This possible “signal” is important because this effect has been noted with increased frequency in neonates who received this antibiotic. ●
I believe that Tita and colleagues are quite correct in concluding that the simple, inexpensive intervention of azithromycin prophylaxis should be used routinely in patient populations similar to those included in this investigation and that the intervention can be invaluable in advancing the World Health Organization’s campaign to reduce the rate of maternal mortality in low- and middleresource nations.
What is not clear, however, is whether this same intervention would be effective in high-resource countries in which the level of skill of the obstetric providers is higher and more uniform; deliveries occur under more optimal sanitary conditions; treatment and prophylaxis for infections such as gonorrhea, chlamydia, chorioamnionitis, and GBS is more consistent; and early neonatal care is more robust. A similar large trial in wellresourced nations would indeed be welcome, particularly if the trial also addressed the possibility of an adverse effect on the neonatal microbiome if a policy of nearly universal antibiotic prophylaxis was adopted.
In the interim, we should focus our attention on the key interventions that are of proven value in decreasing the risk of peripartum maternal and neonatal infection:
- consistently screening for GBS colonization and administering intrapartum antibiotic prophylaxis to patients who test positive
- consistently screening for gonococcal and chlamydia infection in the antepartum period and treating infected patients with appropriate antibiotics
- minimizing the number of internal vaginal examinations during labor, particularly following rupture of membranes
- promptly identifying patients with chorioamnionitis and treating with antibiotics that specifically target GBS and Escherichia coli, the 2 most likely causes of neonatal sepsis, pneumonia, and meningitis
- administering preoperative prophylactic antibiotics (cefazolin plus azithromycin) to women who require CD.
PATRICK DUFF, MD

Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
EXPERT COMMENTARY
Maternal peripartum infection is 1 of the top 5 causes of maternal death, accounting for about 10% of cases of maternal mortality. Cesarean delivery (CD), of course, is the most important risk factor for puerperal infection. However, even vaginal delivery, particularly in low- to middle-resource countries, where deliveries often occur under less-than-optimal conditions, may be associated with a surprisingly high frequency of both maternal and neonatal infections. The beneficial effect of prophylactic antibiotics for CD is well established. An important remaining question is whether similar benefit can be achieved with prophylaxis for women planning to have a vaginal birth.
In 2017, Oluwalana and colleagues conducted a prospective, randomized, double-blind, placebo-controlled trial of a single 2-g oral dose of azithromycin in Gambian women undergoing labor.1 During the 8 weeks after delivery, maternal infections were lower in the azithromycin group, 3.6% versus 9.2% (relative risk [RR], 0.40; 95% confidence interval [CI], 0.22–0.71; P=.002). Infections also were lower in the newborns, 18.1% versus 23.8% (RR, 0.76; 95% CI, 0.58–0.99; P=.052), delivered to mothers who received azithromycin. The greatest impact on neonatal infections was the reduced frequency of skin infections.
In 2021, Subramaniam and colleagues evaluated the effect of a single dose of oral azithromycin with, or without, amoxicillin on the prevalence of peripartum infection in laboring women in Cameroon.2 Patients and their newborns were followed for 6 weeks after delivery. Unlike the previous investigation, the authors were unable to show a protective effect of prophylaxis on either maternal or neonatal infection.
Against this backdrop, Tita and colleagues conducted a remarkably large, well-designed, randomized, placebo-controlled study of azithromycin prophylaxis in women at 8 different sites in 7 low- or middle-income countries (the A-PLUS investigation).3
Details of the study
The investigators randomly assigned 29,278 patients at or beyond 28 weeks’ gestation to receive either a 2-g oral dose of azithromycin or placebo during labor. This particular drug was chosen because it is readily available, inexpensive, well tolerated, and has a broad range of activity against many important pelvic pathogens, including genital mycoplasmas. Some patients also received other antibiotics, for example, for group B streptococcal (GBS) prophylaxis or for CD prophylaxis if abdominal delivery was indicated.
The 2 primary outcomes were a composite of maternal sepsis or death and a composite of stillbirth or neonatal death or sepsis within 4 weeks of delivery. Secondary outcomes included individual components of the primary outcomes.
Results. The results of the investigation were compelling, and the data safety monitoring committee recommended stopping the trial early because of clear maternal benefit. The groups were well balanced with respect to important characteristics, such as incidence of CD, receipt of other prophylactic antibiotics, and median time between randomization and delivery.
The incidence of maternal sepsis or death was lower in the azithromycin group (1.6% vs 2.4%; RR, 0.67; 95% CI, 0.56–0.79; P<.001). The key effect was on the frequency of maternal sepsis because the incidence of maternal death was very low in both groups, 0.1%. With respect to secondary outcomes, prophylaxis was effective in reducing the frequency of endometritis (RR, 0.66; 95% CI, 0.55–0.79) and perineal and incisional infection (RR, 0.71; 95% CI, 0.56–0.85).
There was no difference in the frequency of neonatal sepsis or death. There also was no difference in the frequency of adverse drug effects in either group. Of note, more cases of neonatal pyloric stenosis were observed in the azithromycin group, but the overall incidence was lower than the expected background rate. This possible “signal” is important because this effect has been noted with increased frequency in neonates who received this antibiotic. ●
I believe that Tita and colleagues are quite correct in concluding that the simple, inexpensive intervention of azithromycin prophylaxis should be used routinely in patient populations similar to those included in this investigation and that the intervention can be invaluable in advancing the World Health Organization’s campaign to reduce the rate of maternal mortality in low- and middleresource nations.
What is not clear, however, is whether this same intervention would be effective in high-resource countries in which the level of skill of the obstetric providers is higher and more uniform; deliveries occur under more optimal sanitary conditions; treatment and prophylaxis for infections such as gonorrhea, chlamydia, chorioamnionitis, and GBS is more consistent; and early neonatal care is more robust. A similar large trial in wellresourced nations would indeed be welcome, particularly if the trial also addressed the possibility of an adverse effect on the neonatal microbiome if a policy of nearly universal antibiotic prophylaxis was adopted.
In the interim, we should focus our attention on the key interventions that are of proven value in decreasing the risk of peripartum maternal and neonatal infection:
- consistently screening for GBS colonization and administering intrapartum antibiotic prophylaxis to patients who test positive
- consistently screening for gonococcal and chlamydia infection in the antepartum period and treating infected patients with appropriate antibiotics
- minimizing the number of internal vaginal examinations during labor, particularly following rupture of membranes
- promptly identifying patients with chorioamnionitis and treating with antibiotics that specifically target GBS and Escherichia coli, the 2 most likely causes of neonatal sepsis, pneumonia, and meningitis
- administering preoperative prophylactic antibiotics (cefazolin plus azithromycin) to women who require CD.
PATRICK DUFF, MD
- Oluwalana C, Camara B, Bottomley C, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double-blind trial. Pediatrics. 2017;139:e20162281. doi:10.1542/peds.2016-2281.
- Subramaniam A, Ye Y, Mbah R, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol. 2021;138:703-713. doi:10.1097/AOG.0000000000004565.
- Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
- Oluwalana C, Camara B, Bottomley C, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double-blind trial. Pediatrics. 2017;139:e20162281. doi:10.1542/peds.2016-2281.
- Subramaniam A, Ye Y, Mbah R, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol. 2021;138:703-713. doi:10.1097/AOG.0000000000004565.
- Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
What is the most effective management of first trimester miscarriage?
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
Study compares noninvasive treatments of cutaneous neurofibromas
PHOENIX – after only one treatment, according to preliminary results of an ongoing prospective trial that compared several treatment modalities.
“Neurofibromatosis type 1 is the most common single-gene disease of mankind, but there is so much we have yet to learn about it,” study author Patricia Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. Dr. Richey also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston, and is working with R. Rox Anderson, MD, director of the Wellman Center, on this project. In his words, she said, “the lack of better treatments for cNF is a ‘problem worth solving.’ ”
“The accepted and widely available treatments for cNF result in scars and hypopigmentation. Our treatments do not,” she added. Since the epidermis overlying cNF is normal, “there is no reason to use nonselective or surgical methods and destroy a perfectly good epidermis when you don’t need to.”
Four treatments vs. controls
For the study, Dr. Richey and colleagues enrolled 19 adults with a total of 307 cNFs measuring 2-4 mm in size to receive one of four treatments: electrocautery with an insulated radiofrequency needle; 755-nm alexandrite laser with negative pressure (8-mm spot size, 100 J/cm2 fluence, 3-ms pulse duration); 980-nm diode laser (delivered via 8-mm sapphire skin-contact window), and intratumoral injection of 10 mg/mL deoxycholic acid at a volume approximately equal to that of the tumor. The average age of the participants was 49 years and 15 were female.
The investigators applied 5% lidocaine/prilocaine for 40 minutes to treatment sites before randomizing the tumors to treatment or to the control arm (no treatment). They compared safety, tolerability (including pain scores), and efficacy of each modality as measured by the change in cNF volume/height via three-dimensional imaging and clinical improvement via physician assessment at 6 months. All 19 participants have completed the 6-month assessment.
All modalities reduced or eliminated some of the cNFs by 6 months after treatment, with statistically significant reductions in height and volume across all four treatments. A wide variation of responses was observed. Specifically, the mean tumor volume changes for each modality, compared with controls, were –33.4% versus –5.1% among those treated with the 755-nm alexandrite laser; –24.9% versus –9.2% among those treated with the 980-nm diode laser, –23.3% versus –0.8% among those treated with insulated-needle radiofrequency coagulation, and –29.4% versus –3.7% among those treated with deoxycholic acid.
The variation in responses “may be due to histologic diversity of cNF or may indicate a need for more fine-tuned dosimetry, or a combination,” Dr. Richey said. “Our future trials will address this. We will also be treating all skin types in our upcoming trials.”
No adverse events categorized as higher than grade 2 occurred in any of the treatment groups, and no signs of regrowth or growth stimulation have been observed to date.
Tolerability of treatments
As for general tolerability, the 980-nm laser treatment caused moderate to severe pain; the alexandrite laser caused mild pain; insulated-needle radiofrequency coagulation caused mild pain, though more than deoxycholic acid injections or alexandrite laser, and pain associated with the deoxycholic acid injections was minimal.
When residual neurofibroma tumor was present histologically, its appearance was similar to that of untreated tumors in controls. There was no evidence of atypia, mitosis, or tumor inflammation, and mild fibrosis was present at the sites of prior tumor.
“It was surprising that all four modalities did work to some extent,” Dr. Richey said, noting that the lack of ulceration with deoxycholic acid injection “was pleasantly surprising.” Treatment with the 980-nm diode laser “was a bit more painful than we anticipated.”
The positive results of this trial has raised “more questions for us to answer. We have three additional trials in the works to fine tune these treatments and optimize dose/delivery, with the end goal of treating younger people.”
Dr. Richey said that she was “amazed” by how motivated the enrollees were to participate in the trial, noting that many patients with cNF undergo general anesthesia to have dozens of tumors surgically removed at once. “They pay $10,000-$20,000 on average out of pocket, as this surgery is considered cosmetic,” she said.
“This very important study could lead to effective, relatively noninvasive, therapy for small neurofibromas,” said Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., who was not involved with the study and was asked to comment on the results.
“Remarkably, all four treatments worked to varying degrees, but of all the treatments, the selective alexandrite laser appeared to achieve the best results. Further study will be needed to see just how effective these treatments are, and to determine the best and safest treatment parameters. Given how common this autosomal dominant disease is, and how disfiguring neurofibromas become as they enlarge, a well-tolerated noninvasive nonsurgical treatment with limited side effects is highly sought after.”
The study, which was named the best clinical abstract at the meeting, was supported by the Neurofibromatosis Therapeutic Acceleration Program. Dr. Anderson is supported in part as the Lancer Endowed Chair in Dermatology at MGH. Dr. Dover reported having no relevant disclosures.
PHOENIX – after only one treatment, according to preliminary results of an ongoing prospective trial that compared several treatment modalities.
“Neurofibromatosis type 1 is the most common single-gene disease of mankind, but there is so much we have yet to learn about it,” study author Patricia Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. Dr. Richey also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston, and is working with R. Rox Anderson, MD, director of the Wellman Center, on this project. In his words, she said, “the lack of better treatments for cNF is a ‘problem worth solving.’ ”
“The accepted and widely available treatments for cNF result in scars and hypopigmentation. Our treatments do not,” she added. Since the epidermis overlying cNF is normal, “there is no reason to use nonselective or surgical methods and destroy a perfectly good epidermis when you don’t need to.”
Four treatments vs. controls
For the study, Dr. Richey and colleagues enrolled 19 adults with a total of 307 cNFs measuring 2-4 mm in size to receive one of four treatments: electrocautery with an insulated radiofrequency needle; 755-nm alexandrite laser with negative pressure (8-mm spot size, 100 J/cm2 fluence, 3-ms pulse duration); 980-nm diode laser (delivered via 8-mm sapphire skin-contact window), and intratumoral injection of 10 mg/mL deoxycholic acid at a volume approximately equal to that of the tumor. The average age of the participants was 49 years and 15 were female.
The investigators applied 5% lidocaine/prilocaine for 40 minutes to treatment sites before randomizing the tumors to treatment or to the control arm (no treatment). They compared safety, tolerability (including pain scores), and efficacy of each modality as measured by the change in cNF volume/height via three-dimensional imaging and clinical improvement via physician assessment at 6 months. All 19 participants have completed the 6-month assessment.
All modalities reduced or eliminated some of the cNFs by 6 months after treatment, with statistically significant reductions in height and volume across all four treatments. A wide variation of responses was observed. Specifically, the mean tumor volume changes for each modality, compared with controls, were –33.4% versus –5.1% among those treated with the 755-nm alexandrite laser; –24.9% versus –9.2% among those treated with the 980-nm diode laser, –23.3% versus –0.8% among those treated with insulated-needle radiofrequency coagulation, and –29.4% versus –3.7% among those treated with deoxycholic acid.
The variation in responses “may be due to histologic diversity of cNF or may indicate a need for more fine-tuned dosimetry, or a combination,” Dr. Richey said. “Our future trials will address this. We will also be treating all skin types in our upcoming trials.”
No adverse events categorized as higher than grade 2 occurred in any of the treatment groups, and no signs of regrowth or growth stimulation have been observed to date.
Tolerability of treatments
As for general tolerability, the 980-nm laser treatment caused moderate to severe pain; the alexandrite laser caused mild pain; insulated-needle radiofrequency coagulation caused mild pain, though more than deoxycholic acid injections or alexandrite laser, and pain associated with the deoxycholic acid injections was minimal.
When residual neurofibroma tumor was present histologically, its appearance was similar to that of untreated tumors in controls. There was no evidence of atypia, mitosis, or tumor inflammation, and mild fibrosis was present at the sites of prior tumor.
“It was surprising that all four modalities did work to some extent,” Dr. Richey said, noting that the lack of ulceration with deoxycholic acid injection “was pleasantly surprising.” Treatment with the 980-nm diode laser “was a bit more painful than we anticipated.”
The positive results of this trial has raised “more questions for us to answer. We have three additional trials in the works to fine tune these treatments and optimize dose/delivery, with the end goal of treating younger people.”
Dr. Richey said that she was “amazed” by how motivated the enrollees were to participate in the trial, noting that many patients with cNF undergo general anesthesia to have dozens of tumors surgically removed at once. “They pay $10,000-$20,000 on average out of pocket, as this surgery is considered cosmetic,” she said.
“This very important study could lead to effective, relatively noninvasive, therapy for small neurofibromas,” said Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., who was not involved with the study and was asked to comment on the results.
“Remarkably, all four treatments worked to varying degrees, but of all the treatments, the selective alexandrite laser appeared to achieve the best results. Further study will be needed to see just how effective these treatments are, and to determine the best and safest treatment parameters. Given how common this autosomal dominant disease is, and how disfiguring neurofibromas become as they enlarge, a well-tolerated noninvasive nonsurgical treatment with limited side effects is highly sought after.”
The study, which was named the best clinical abstract at the meeting, was supported by the Neurofibromatosis Therapeutic Acceleration Program. Dr. Anderson is supported in part as the Lancer Endowed Chair in Dermatology at MGH. Dr. Dover reported having no relevant disclosures.
PHOENIX – after only one treatment, according to preliminary results of an ongoing prospective trial that compared several treatment modalities.
“Neurofibromatosis type 1 is the most common single-gene disease of mankind, but there is so much we have yet to learn about it,” study author Patricia Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. Dr. Richey also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston, and is working with R. Rox Anderson, MD, director of the Wellman Center, on this project. In his words, she said, “the lack of better treatments for cNF is a ‘problem worth solving.’ ”
“The accepted and widely available treatments for cNF result in scars and hypopigmentation. Our treatments do not,” she added. Since the epidermis overlying cNF is normal, “there is no reason to use nonselective or surgical methods and destroy a perfectly good epidermis when you don’t need to.”
Four treatments vs. controls
For the study, Dr. Richey and colleagues enrolled 19 adults with a total of 307 cNFs measuring 2-4 mm in size to receive one of four treatments: electrocautery with an insulated radiofrequency needle; 755-nm alexandrite laser with negative pressure (8-mm spot size, 100 J/cm2 fluence, 3-ms pulse duration); 980-nm diode laser (delivered via 8-mm sapphire skin-contact window), and intratumoral injection of 10 mg/mL deoxycholic acid at a volume approximately equal to that of the tumor. The average age of the participants was 49 years and 15 were female.
The investigators applied 5% lidocaine/prilocaine for 40 minutes to treatment sites before randomizing the tumors to treatment or to the control arm (no treatment). They compared safety, tolerability (including pain scores), and efficacy of each modality as measured by the change in cNF volume/height via three-dimensional imaging and clinical improvement via physician assessment at 6 months. All 19 participants have completed the 6-month assessment.
All modalities reduced or eliminated some of the cNFs by 6 months after treatment, with statistically significant reductions in height and volume across all four treatments. A wide variation of responses was observed. Specifically, the mean tumor volume changes for each modality, compared with controls, were –33.4% versus –5.1% among those treated with the 755-nm alexandrite laser; –24.9% versus –9.2% among those treated with the 980-nm diode laser, –23.3% versus –0.8% among those treated with insulated-needle radiofrequency coagulation, and –29.4% versus –3.7% among those treated with deoxycholic acid.
The variation in responses “may be due to histologic diversity of cNF or may indicate a need for more fine-tuned dosimetry, or a combination,” Dr. Richey said. “Our future trials will address this. We will also be treating all skin types in our upcoming trials.”
No adverse events categorized as higher than grade 2 occurred in any of the treatment groups, and no signs of regrowth or growth stimulation have been observed to date.
Tolerability of treatments
As for general tolerability, the 980-nm laser treatment caused moderate to severe pain; the alexandrite laser caused mild pain; insulated-needle radiofrequency coagulation caused mild pain, though more than deoxycholic acid injections or alexandrite laser, and pain associated with the deoxycholic acid injections was minimal.
When residual neurofibroma tumor was present histologically, its appearance was similar to that of untreated tumors in controls. There was no evidence of atypia, mitosis, or tumor inflammation, and mild fibrosis was present at the sites of prior tumor.
“It was surprising that all four modalities did work to some extent,” Dr. Richey said, noting that the lack of ulceration with deoxycholic acid injection “was pleasantly surprising.” Treatment with the 980-nm diode laser “was a bit more painful than we anticipated.”
The positive results of this trial has raised “more questions for us to answer. We have three additional trials in the works to fine tune these treatments and optimize dose/delivery, with the end goal of treating younger people.”
Dr. Richey said that she was “amazed” by how motivated the enrollees were to participate in the trial, noting that many patients with cNF undergo general anesthesia to have dozens of tumors surgically removed at once. “They pay $10,000-$20,000 on average out of pocket, as this surgery is considered cosmetic,” she said.
“This very important study could lead to effective, relatively noninvasive, therapy for small neurofibromas,” said Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., who was not involved with the study and was asked to comment on the results.
“Remarkably, all four treatments worked to varying degrees, but of all the treatments, the selective alexandrite laser appeared to achieve the best results. Further study will be needed to see just how effective these treatments are, and to determine the best and safest treatment parameters. Given how common this autosomal dominant disease is, and how disfiguring neurofibromas become as they enlarge, a well-tolerated noninvasive nonsurgical treatment with limited side effects is highly sought after.”
The study, which was named the best clinical abstract at the meeting, was supported by the Neurofibromatosis Therapeutic Acceleration Program. Dr. Anderson is supported in part as the Lancer Endowed Chair in Dermatology at MGH. Dr. Dover reported having no relevant disclosures.
AT ASLMS 2023
You’ve quit smoking with vaping. Now what?
This article is part of a series from Medscape on vaping.
Every day, Sonia Sharma, PA, meets people like Natalie H., who is trying to quit vaping.
Natalie, a member of the nicotine addiction support group at the University of California San Francisco’s Fontana Tobacco Treatment Center, switched from traditional cigarettes to vaping but found the electronic version just as addictive and eventually decided to quit using nicotine completely.
“I went from being an occasional cigarette smoker, a few a month, to a daily vaper,” said Natalie, who preferred not to give her last name to protect her privacy. “Vaping made my nicotine addiction worse, not better.”
“We have people tell us they vape before their feet hit the ground in the morning,” said Ms. Sharma, who coleads Natalie’s support group at UCSF. Ms. Sharma has met individuals who had smoked four to five cigarettes a day, switched to e-cigarettes to quit smoking, then vaped the equivalent of a pack a day. Others had switched to vapes to quit but ended up both vaping and smoking again. And others picked up vaping without ever smoking. They want to quit, she said, but are not sure how.
Researchers from the National Institutes of Health in 2020 reported that 5.6 million adults in the United States vaped. A little over 57% of people said they started using e-cigarettes to quit smoking traditional cigarettes. Another study in 2021 based on survey data found that about 60% of e-cigarette users wanted to quit their vaping habit.
Vaping has been marketed as a way to help people kick their smoking habit. Research is inconclusive on this claim. But unlike cessation tools like nicotine gums or lozenges, using vapes for cessation is uncharted territory. Vapers lack guidance for how to use the devices to quit, and they have even less direction on what to do if they develop an addiction to the vapes themselves.
A new addiction?
Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery in New Jersey, said she has witnessed a higher level of nicotine addiction in the vapers with whom she has worked.
“When someone takes a hit from a vaping device, it doesn’t generate the burn it would from traditional tobacco,” Ms. Hanna said. “This causes people to take a deeper pull, and when they take a deeper pull, they establish a higher level of nicotine dependence over time.”
A 2019 study of nearly 900 people published in the New England Journal of Medicine found that smokers who used vapes for cessation were twice as likely to have quit smoking cigarettes as those who used other nicotine replacement therapy. However, 80% of people who switched to vaping were using e-cigarettes a year after they tried to quit smoking.
Given that potential for addiction, Nancy Rigotti, MD, director of Massachusetts General Hospital’s Tobacco Research and Treatment Center in Boston, said patients must use vapes “properly” for cessation. That means giving up smoking completely and quitting vapes as soon as patients are sure they will not go back to smoking tobacco.
“We are going to need to help these people to stop vaping,” said Dr. Rigotti, who is working with Achieve Life Sciences, a pharmaceutical company developing a prescription drug to treat nicotine addiction from vapes and cigarettes.
And many nicotine users who have tried vaping to quit smoking end up becoming dual users.
“It’s important to stress that health benefits [of switching to vaping] only occur if the switch to vapes is complete and permanent. So far, that appears difficult to do for most people who smoke, and in my anecdotal experience it has not worked,” said J. Taylor Hays, MD, the former medical director of Mayo Clinic’s Nicotine Dependence Center in Rochester, Minn.
Besides challenges in communicating the current evidence, no established method exists to help vapers quit, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There are some experimental methods like using social interventions, counseling, and some educational campaigns,” Dr. Nargis said. “[Little] progress has been done in terms of clinical interventions.”
Unlike cessation products such as gum or a nicotine patch, which have clear recommendations for duration of use, similar guidelines don’t exist for vapes, in part because the U.S. Food and Drug Administration hasn’t yet granted approval of vapes as cessation products.
Alex Clark, the CEO of Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping, said people could vape for longer and still benefit from making the switch from traditional cigarettes.
“The most important thing is that people start replacing cigarettes with a smoke-free product and continue until they’ve completely switched,” said Mr. Clark, whose group accepts donations from the e-cigarette industry. “Following switching, people are encouraged to continue with the product for as long as they feel necessary.”
But 2013 guidelines from the FDA advised makers of nicotine-replacement therapies – including gums, patches, and lozenges – to include labeling that advises users to complete treatment. According to the agency, if a person feels like they “need to use [the NRT product] for a longer period to keep from smoking, talk to your health care provider.”
Dr. Hays, who is now an emeritus professor at the Mayo Clinic, said he would not recommend patients try vaping as a cessation device given the availability of more proven techniques such as patches and gums. If a patient insists, vaping could be considered under the medical guidance of a cessation professional. He also advised people purchase products only from large tobacco companies that are likely to have “reasonable quality control.” Hundreds of vaping devices are on the market, and they are not all equivalent, he said.
But when an e-cigarette user wants to quit vaping, guidance might boil down to using traditional tobacco cessation methods like gums and lozenges because few tools exist to help people with a vaping-specific addiction.
The long-term health outcomes of vaping are also unclear, and decades will pass before scientists are able to make conclusions, according to Thomas Eissenberg, PhD, codirector of Virginia Commonwealth University’s Center for the Study of Tobacco Products in Richmond.
“I don’t think anyone knows what the long-term effects of heated propylene glycol and vegetable glycerin and flavors intended as food ingredients are, especially when these compounds are inhaled hundreds of times a day, week after week, year after year,” Dr. Eissenberg said.
Dr. Rigotti reported that she receives no funding from the tobacco or e-cigarette industry. She is working with Achieve Life Sciences to develop a tool for vaping cessation. Dr. Eissenberg, Ms. Hanna, Dr. Hays, Dr. Nargis, and Ms. Sharma reported no funding from the tobacco or e-cigarette industry.
A version of this article first appeared on Medscape.com.
This article is part of a series from Medscape on vaping.
Every day, Sonia Sharma, PA, meets people like Natalie H., who is trying to quit vaping.
Natalie, a member of the nicotine addiction support group at the University of California San Francisco’s Fontana Tobacco Treatment Center, switched from traditional cigarettes to vaping but found the electronic version just as addictive and eventually decided to quit using nicotine completely.
“I went from being an occasional cigarette smoker, a few a month, to a daily vaper,” said Natalie, who preferred not to give her last name to protect her privacy. “Vaping made my nicotine addiction worse, not better.”
“We have people tell us they vape before their feet hit the ground in the morning,” said Ms. Sharma, who coleads Natalie’s support group at UCSF. Ms. Sharma has met individuals who had smoked four to five cigarettes a day, switched to e-cigarettes to quit smoking, then vaped the equivalent of a pack a day. Others had switched to vapes to quit but ended up both vaping and smoking again. And others picked up vaping without ever smoking. They want to quit, she said, but are not sure how.
Researchers from the National Institutes of Health in 2020 reported that 5.6 million adults in the United States vaped. A little over 57% of people said they started using e-cigarettes to quit smoking traditional cigarettes. Another study in 2021 based on survey data found that about 60% of e-cigarette users wanted to quit their vaping habit.
Vaping has been marketed as a way to help people kick their smoking habit. Research is inconclusive on this claim. But unlike cessation tools like nicotine gums or lozenges, using vapes for cessation is uncharted territory. Vapers lack guidance for how to use the devices to quit, and they have even less direction on what to do if they develop an addiction to the vapes themselves.
A new addiction?
Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery in New Jersey, said she has witnessed a higher level of nicotine addiction in the vapers with whom she has worked.
“When someone takes a hit from a vaping device, it doesn’t generate the burn it would from traditional tobacco,” Ms. Hanna said. “This causes people to take a deeper pull, and when they take a deeper pull, they establish a higher level of nicotine dependence over time.”
A 2019 study of nearly 900 people published in the New England Journal of Medicine found that smokers who used vapes for cessation were twice as likely to have quit smoking cigarettes as those who used other nicotine replacement therapy. However, 80% of people who switched to vaping were using e-cigarettes a year after they tried to quit smoking.
Given that potential for addiction, Nancy Rigotti, MD, director of Massachusetts General Hospital’s Tobacco Research and Treatment Center in Boston, said patients must use vapes “properly” for cessation. That means giving up smoking completely and quitting vapes as soon as patients are sure they will not go back to smoking tobacco.
“We are going to need to help these people to stop vaping,” said Dr. Rigotti, who is working with Achieve Life Sciences, a pharmaceutical company developing a prescription drug to treat nicotine addiction from vapes and cigarettes.
And many nicotine users who have tried vaping to quit smoking end up becoming dual users.
“It’s important to stress that health benefits [of switching to vaping] only occur if the switch to vapes is complete and permanent. So far, that appears difficult to do for most people who smoke, and in my anecdotal experience it has not worked,” said J. Taylor Hays, MD, the former medical director of Mayo Clinic’s Nicotine Dependence Center in Rochester, Minn.
Besides challenges in communicating the current evidence, no established method exists to help vapers quit, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There are some experimental methods like using social interventions, counseling, and some educational campaigns,” Dr. Nargis said. “[Little] progress has been done in terms of clinical interventions.”
Unlike cessation products such as gum or a nicotine patch, which have clear recommendations for duration of use, similar guidelines don’t exist for vapes, in part because the U.S. Food and Drug Administration hasn’t yet granted approval of vapes as cessation products.
Alex Clark, the CEO of Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping, said people could vape for longer and still benefit from making the switch from traditional cigarettes.
“The most important thing is that people start replacing cigarettes with a smoke-free product and continue until they’ve completely switched,” said Mr. Clark, whose group accepts donations from the e-cigarette industry. “Following switching, people are encouraged to continue with the product for as long as they feel necessary.”
But 2013 guidelines from the FDA advised makers of nicotine-replacement therapies – including gums, patches, and lozenges – to include labeling that advises users to complete treatment. According to the agency, if a person feels like they “need to use [the NRT product] for a longer period to keep from smoking, talk to your health care provider.”
Dr. Hays, who is now an emeritus professor at the Mayo Clinic, said he would not recommend patients try vaping as a cessation device given the availability of more proven techniques such as patches and gums. If a patient insists, vaping could be considered under the medical guidance of a cessation professional. He also advised people purchase products only from large tobacco companies that are likely to have “reasonable quality control.” Hundreds of vaping devices are on the market, and they are not all equivalent, he said.
But when an e-cigarette user wants to quit vaping, guidance might boil down to using traditional tobacco cessation methods like gums and lozenges because few tools exist to help people with a vaping-specific addiction.
The long-term health outcomes of vaping are also unclear, and decades will pass before scientists are able to make conclusions, according to Thomas Eissenberg, PhD, codirector of Virginia Commonwealth University’s Center for the Study of Tobacco Products in Richmond.
“I don’t think anyone knows what the long-term effects of heated propylene glycol and vegetable glycerin and flavors intended as food ingredients are, especially when these compounds are inhaled hundreds of times a day, week after week, year after year,” Dr. Eissenberg said.
Dr. Rigotti reported that she receives no funding from the tobacco or e-cigarette industry. She is working with Achieve Life Sciences to develop a tool for vaping cessation. Dr. Eissenberg, Ms. Hanna, Dr. Hays, Dr. Nargis, and Ms. Sharma reported no funding from the tobacco or e-cigarette industry.
A version of this article first appeared on Medscape.com.
This article is part of a series from Medscape on vaping.
Every day, Sonia Sharma, PA, meets people like Natalie H., who is trying to quit vaping.
Natalie, a member of the nicotine addiction support group at the University of California San Francisco’s Fontana Tobacco Treatment Center, switched from traditional cigarettes to vaping but found the electronic version just as addictive and eventually decided to quit using nicotine completely.
“I went from being an occasional cigarette smoker, a few a month, to a daily vaper,” said Natalie, who preferred not to give her last name to protect her privacy. “Vaping made my nicotine addiction worse, not better.”
“We have people tell us they vape before their feet hit the ground in the morning,” said Ms. Sharma, who coleads Natalie’s support group at UCSF. Ms. Sharma has met individuals who had smoked four to five cigarettes a day, switched to e-cigarettes to quit smoking, then vaped the equivalent of a pack a day. Others had switched to vapes to quit but ended up both vaping and smoking again. And others picked up vaping without ever smoking. They want to quit, she said, but are not sure how.
Researchers from the National Institutes of Health in 2020 reported that 5.6 million adults in the United States vaped. A little over 57% of people said they started using e-cigarettes to quit smoking traditional cigarettes. Another study in 2021 based on survey data found that about 60% of e-cigarette users wanted to quit their vaping habit.
Vaping has been marketed as a way to help people kick their smoking habit. Research is inconclusive on this claim. But unlike cessation tools like nicotine gums or lozenges, using vapes for cessation is uncharted territory. Vapers lack guidance for how to use the devices to quit, and they have even less direction on what to do if they develop an addiction to the vapes themselves.
A new addiction?
Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery in New Jersey, said she has witnessed a higher level of nicotine addiction in the vapers with whom she has worked.
“When someone takes a hit from a vaping device, it doesn’t generate the burn it would from traditional tobacco,” Ms. Hanna said. “This causes people to take a deeper pull, and when they take a deeper pull, they establish a higher level of nicotine dependence over time.”
A 2019 study of nearly 900 people published in the New England Journal of Medicine found that smokers who used vapes for cessation were twice as likely to have quit smoking cigarettes as those who used other nicotine replacement therapy. However, 80% of people who switched to vaping were using e-cigarettes a year after they tried to quit smoking.
Given that potential for addiction, Nancy Rigotti, MD, director of Massachusetts General Hospital’s Tobacco Research and Treatment Center in Boston, said patients must use vapes “properly” for cessation. That means giving up smoking completely and quitting vapes as soon as patients are sure they will not go back to smoking tobacco.
“We are going to need to help these people to stop vaping,” said Dr. Rigotti, who is working with Achieve Life Sciences, a pharmaceutical company developing a prescription drug to treat nicotine addiction from vapes and cigarettes.
And many nicotine users who have tried vaping to quit smoking end up becoming dual users.
“It’s important to stress that health benefits [of switching to vaping] only occur if the switch to vapes is complete and permanent. So far, that appears difficult to do for most people who smoke, and in my anecdotal experience it has not worked,” said J. Taylor Hays, MD, the former medical director of Mayo Clinic’s Nicotine Dependence Center in Rochester, Minn.
Besides challenges in communicating the current evidence, no established method exists to help vapers quit, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There are some experimental methods like using social interventions, counseling, and some educational campaigns,” Dr. Nargis said. “[Little] progress has been done in terms of clinical interventions.”
Unlike cessation products such as gum or a nicotine patch, which have clear recommendations for duration of use, similar guidelines don’t exist for vapes, in part because the U.S. Food and Drug Administration hasn’t yet granted approval of vapes as cessation products.
Alex Clark, the CEO of Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping, said people could vape for longer and still benefit from making the switch from traditional cigarettes.
“The most important thing is that people start replacing cigarettes with a smoke-free product and continue until they’ve completely switched,” said Mr. Clark, whose group accepts donations from the e-cigarette industry. “Following switching, people are encouraged to continue with the product for as long as they feel necessary.”
But 2013 guidelines from the FDA advised makers of nicotine-replacement therapies – including gums, patches, and lozenges – to include labeling that advises users to complete treatment. According to the agency, if a person feels like they “need to use [the NRT product] for a longer period to keep from smoking, talk to your health care provider.”
Dr. Hays, who is now an emeritus professor at the Mayo Clinic, said he would not recommend patients try vaping as a cessation device given the availability of more proven techniques such as patches and gums. If a patient insists, vaping could be considered under the medical guidance of a cessation professional. He also advised people purchase products only from large tobacco companies that are likely to have “reasonable quality control.” Hundreds of vaping devices are on the market, and they are not all equivalent, he said.
But when an e-cigarette user wants to quit vaping, guidance might boil down to using traditional tobacco cessation methods like gums and lozenges because few tools exist to help people with a vaping-specific addiction.
The long-term health outcomes of vaping are also unclear, and decades will pass before scientists are able to make conclusions, according to Thomas Eissenberg, PhD, codirector of Virginia Commonwealth University’s Center for the Study of Tobacco Products in Richmond.
“I don’t think anyone knows what the long-term effects of heated propylene glycol and vegetable glycerin and flavors intended as food ingredients are, especially when these compounds are inhaled hundreds of times a day, week after week, year after year,” Dr. Eissenberg said.
Dr. Rigotti reported that she receives no funding from the tobacco or e-cigarette industry. She is working with Achieve Life Sciences to develop a tool for vaping cessation. Dr. Eissenberg, Ms. Hanna, Dr. Hays, Dr. Nargis, and Ms. Sharma reported no funding from the tobacco or e-cigarette industry.
A version of this article first appeared on Medscape.com.
Insurers refusing MRI for women at high risk for breast cancer
Women harboring BRCA1/2 gene mutations are at high risk for breast cancer, and thus it’s recommended they undergo annual breast MRI screening in addition to mammogram screening.
However, some women are finding that their insurer is refusing to cover the cost of the MRI.
A new study exploring this issue was presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“Despite guidelines supporting annual breast MRI for screening in patients with gBRCA1/2, insurance denials were present in 11% of patients,” said lead author Sushmita Gordhandas, MD, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “In a high-resource setting, up to 14% of patients who were denied coverage did not undergo recommended MRI screening.”
She also pointed out that the rate of denials was rising. “Compared to 2020, there were significantly more denials, and denials on appeal, in 2021,” Dr. Gordhandas said. “This suggested worsening barriers and added burden on health care systems.”
The addition of MRI to mammography is a standard recommendation for women with BRCA mutations, she pointed out, as it has been shown improve detection of early disease and decrease interval cancer development.
An expert not involved in the study noted that the recommendation for annual MRI screening in women at high risk for breast cancer is “substantiated by many publications, including multiple prospective clinical trials.”
Linda Moy, MD, a radiologist at NYU Langone’s Perlmutter Cancer Center and professor of radiology at NYU Grossman School of Medicine, both in New York, noted that the American Cancer Society’s Guidelines for screening breast MRI recommends annual breast MRI in women with a lifetime risk of greater than 20% – which includes women who are BRCA carriers – and recommends the screening begins at age 30.
“The lifetime breast cancer risk is 72% among BRCA1 and 69% among BRCA2 carriers,” she said, adding that the “American College of Radiology also recommends for BRCA carriers to undergo annual screening MRI at age 30.”
The National Comprehensive Cancer Network recommends that women at high risk for breast cancer undergo a mammogram and breast MRI every year starting at age 25 to 40, depending on the type of gene mutation, noted Dr. Gordhandas. “These guidelines are consistent with those from American College of Obstetricians and Gynecologists, the American Cancer Society, and the American College of Radiology.”
Denials increased over time
For the study, Dr. Gordhandas and colleagues looked at the frequency of insurance denials for indicated breast MRI screening in women with germline BRCA1/2 pathogenic variants, and also looked at recent trends in denials over time.
The cohort comprised 682 women with BRCA1/2 gene mutations who were followed in a specialized high-risk breast cancer clinic, and who had breast MRIs ordered from 2020 to 2021. They were then cross-referenced with a database of insurance denials. Radiology records were also accessed to determine if screening breast MRIs had been performed in 2020 and 2021, and rates of MRI denials and results after appeals were determined. The rates between the 2 years were then compared.
The team found that overall, 73 women (11%) had an MRI denied. The median age of women who received a denial was 38 years, whereas those who had it approved was 44 years. “Patients with denials were significantly younger and more likely to be in the Medicaid population,” said Dr. Gordhandas.
In 2020, 29 breast MRIs (5%) were denied, and on appeal, 8 (28%) were denied and 21 (72%) approved. The number of denials rose in 2021 but approvals remained the same; 45 breast MRIs were denied (8%); on appeal, 23 (51%) were denied, and 22 (49%) approved.
Thus, noted the authors, there were significantly more denials in 2021 as compared with 2020 (P = .044), and the denials in 2021 denials were statistically more likely to be denied on appeal (P = .045).
Among the women whose coverage was denied, four (14%) in 2020 and five (11%) in 2021 did not have an MRI screening performed. And within this group, 17 women (2.5%) received a diagnosis of cancer; 12 (1.8%) had invasive carcinoma, and 5 (0.7%) had ductal carcinoma in situ (DCIS). One patient with DCIS had an MRI denial prior to receiving her diagnosis.
“The top reasons given for denials were that they were outside the approved time frame, authorization on file for a similar study, and that the clinician failed to show medical necessity,” she explained.
Additional data are needed to establish a trend. “We are working to increase the approval time frame, which is currently 45 days, and provide resources for the patient to deal with denials,” Dr. Gordhandas added. “We also have to advocate for updates to [U.S. Preventive Services Task Force] screening recommendations in high-risk patients.”
Dr. Gordhandas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women harboring BRCA1/2 gene mutations are at high risk for breast cancer, and thus it’s recommended they undergo annual breast MRI screening in addition to mammogram screening.
However, some women are finding that their insurer is refusing to cover the cost of the MRI.
A new study exploring this issue was presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“Despite guidelines supporting annual breast MRI for screening in patients with gBRCA1/2, insurance denials were present in 11% of patients,” said lead author Sushmita Gordhandas, MD, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “In a high-resource setting, up to 14% of patients who were denied coverage did not undergo recommended MRI screening.”
She also pointed out that the rate of denials was rising. “Compared to 2020, there were significantly more denials, and denials on appeal, in 2021,” Dr. Gordhandas said. “This suggested worsening barriers and added burden on health care systems.”
The addition of MRI to mammography is a standard recommendation for women with BRCA mutations, she pointed out, as it has been shown improve detection of early disease and decrease interval cancer development.
An expert not involved in the study noted that the recommendation for annual MRI screening in women at high risk for breast cancer is “substantiated by many publications, including multiple prospective clinical trials.”
Linda Moy, MD, a radiologist at NYU Langone’s Perlmutter Cancer Center and professor of radiology at NYU Grossman School of Medicine, both in New York, noted that the American Cancer Society’s Guidelines for screening breast MRI recommends annual breast MRI in women with a lifetime risk of greater than 20% – which includes women who are BRCA carriers – and recommends the screening begins at age 30.
“The lifetime breast cancer risk is 72% among BRCA1 and 69% among BRCA2 carriers,” she said, adding that the “American College of Radiology also recommends for BRCA carriers to undergo annual screening MRI at age 30.”
The National Comprehensive Cancer Network recommends that women at high risk for breast cancer undergo a mammogram and breast MRI every year starting at age 25 to 40, depending on the type of gene mutation, noted Dr. Gordhandas. “These guidelines are consistent with those from American College of Obstetricians and Gynecologists, the American Cancer Society, and the American College of Radiology.”
Denials increased over time
For the study, Dr. Gordhandas and colleagues looked at the frequency of insurance denials for indicated breast MRI screening in women with germline BRCA1/2 pathogenic variants, and also looked at recent trends in denials over time.
The cohort comprised 682 women with BRCA1/2 gene mutations who were followed in a specialized high-risk breast cancer clinic, and who had breast MRIs ordered from 2020 to 2021. They were then cross-referenced with a database of insurance denials. Radiology records were also accessed to determine if screening breast MRIs had been performed in 2020 and 2021, and rates of MRI denials and results after appeals were determined. The rates between the 2 years were then compared.
The team found that overall, 73 women (11%) had an MRI denied. The median age of women who received a denial was 38 years, whereas those who had it approved was 44 years. “Patients with denials were significantly younger and more likely to be in the Medicaid population,” said Dr. Gordhandas.
In 2020, 29 breast MRIs (5%) were denied, and on appeal, 8 (28%) were denied and 21 (72%) approved. The number of denials rose in 2021 but approvals remained the same; 45 breast MRIs were denied (8%); on appeal, 23 (51%) were denied, and 22 (49%) approved.
Thus, noted the authors, there were significantly more denials in 2021 as compared with 2020 (P = .044), and the denials in 2021 denials were statistically more likely to be denied on appeal (P = .045).
Among the women whose coverage was denied, four (14%) in 2020 and five (11%) in 2021 did not have an MRI screening performed. And within this group, 17 women (2.5%) received a diagnosis of cancer; 12 (1.8%) had invasive carcinoma, and 5 (0.7%) had ductal carcinoma in situ (DCIS). One patient with DCIS had an MRI denial prior to receiving her diagnosis.
“The top reasons given for denials were that they were outside the approved time frame, authorization on file for a similar study, and that the clinician failed to show medical necessity,” she explained.
Additional data are needed to establish a trend. “We are working to increase the approval time frame, which is currently 45 days, and provide resources for the patient to deal with denials,” Dr. Gordhandas added. “We also have to advocate for updates to [U.S. Preventive Services Task Force] screening recommendations in high-risk patients.”
Dr. Gordhandas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women harboring BRCA1/2 gene mutations are at high risk for breast cancer, and thus it’s recommended they undergo annual breast MRI screening in addition to mammogram screening.
However, some women are finding that their insurer is refusing to cover the cost of the MRI.
A new study exploring this issue was presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“Despite guidelines supporting annual breast MRI for screening in patients with gBRCA1/2, insurance denials were present in 11% of patients,” said lead author Sushmita Gordhandas, MD, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “In a high-resource setting, up to 14% of patients who were denied coverage did not undergo recommended MRI screening.”
She also pointed out that the rate of denials was rising. “Compared to 2020, there were significantly more denials, and denials on appeal, in 2021,” Dr. Gordhandas said. “This suggested worsening barriers and added burden on health care systems.”
The addition of MRI to mammography is a standard recommendation for women with BRCA mutations, she pointed out, as it has been shown improve detection of early disease and decrease interval cancer development.
An expert not involved in the study noted that the recommendation for annual MRI screening in women at high risk for breast cancer is “substantiated by many publications, including multiple prospective clinical trials.”
Linda Moy, MD, a radiologist at NYU Langone’s Perlmutter Cancer Center and professor of radiology at NYU Grossman School of Medicine, both in New York, noted that the American Cancer Society’s Guidelines for screening breast MRI recommends annual breast MRI in women with a lifetime risk of greater than 20% – which includes women who are BRCA carriers – and recommends the screening begins at age 30.
“The lifetime breast cancer risk is 72% among BRCA1 and 69% among BRCA2 carriers,” she said, adding that the “American College of Radiology also recommends for BRCA carriers to undergo annual screening MRI at age 30.”
The National Comprehensive Cancer Network recommends that women at high risk for breast cancer undergo a mammogram and breast MRI every year starting at age 25 to 40, depending on the type of gene mutation, noted Dr. Gordhandas. “These guidelines are consistent with those from American College of Obstetricians and Gynecologists, the American Cancer Society, and the American College of Radiology.”
Denials increased over time
For the study, Dr. Gordhandas and colleagues looked at the frequency of insurance denials for indicated breast MRI screening in women with germline BRCA1/2 pathogenic variants, and also looked at recent trends in denials over time.
The cohort comprised 682 women with BRCA1/2 gene mutations who were followed in a specialized high-risk breast cancer clinic, and who had breast MRIs ordered from 2020 to 2021. They were then cross-referenced with a database of insurance denials. Radiology records were also accessed to determine if screening breast MRIs had been performed in 2020 and 2021, and rates of MRI denials and results after appeals were determined. The rates between the 2 years were then compared.
The team found that overall, 73 women (11%) had an MRI denied. The median age of women who received a denial was 38 years, whereas those who had it approved was 44 years. “Patients with denials were significantly younger and more likely to be in the Medicaid population,” said Dr. Gordhandas.
In 2020, 29 breast MRIs (5%) were denied, and on appeal, 8 (28%) were denied and 21 (72%) approved. The number of denials rose in 2021 but approvals remained the same; 45 breast MRIs were denied (8%); on appeal, 23 (51%) were denied, and 22 (49%) approved.
Thus, noted the authors, there were significantly more denials in 2021 as compared with 2020 (P = .044), and the denials in 2021 denials were statistically more likely to be denied on appeal (P = .045).
Among the women whose coverage was denied, four (14%) in 2020 and five (11%) in 2021 did not have an MRI screening performed. And within this group, 17 women (2.5%) received a diagnosis of cancer; 12 (1.8%) had invasive carcinoma, and 5 (0.7%) had ductal carcinoma in situ (DCIS). One patient with DCIS had an MRI denial prior to receiving her diagnosis.
“The top reasons given for denials were that they were outside the approved time frame, authorization on file for a similar study, and that the clinician failed to show medical necessity,” she explained.
Additional data are needed to establish a trend. “We are working to increase the approval time frame, which is currently 45 days, and provide resources for the patient to deal with denials,” Dr. Gordhandas added. “We also have to advocate for updates to [U.S. Preventive Services Task Force] screening recommendations in high-risk patients.”
Dr. Gordhandas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SGO 2023
What will vaping lead to? Emerging research shows damage, and addiction
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.