User login
Managing Physician Performance in Hospital Medicine
Joel Barker describes leadership as “…the ability to take people where they otherwise would not go.” In other words, leadership is about creating change in something that exists today. Management, on the other hand, may be considered a series of steps to ensure that things happen the desired and consistent way. Although this article is not of scope sufficient to explore the differences between management and leadership, it will address a domain in which the 2 intimately intersect. Managing others relies upon many foundations of leadership, such as establishing the group’s vision and setting key strategic goals. In like manner, successful leadership in stimulating change is dependent on the effective management of personnel to ensure that the culture, work habits, outcomes, and behaviors are consistent with the change efforts. This article will focus on the management of physicians in hospital medicine groups. The 8 steps outlined are applicable regardless of employer type, group size, or mission. Almost all of the skills necessary to effectively implement a performance management system can be learned and are best practiced on a regular basis. Furthermore, there are many existing resources for further education and development in these areas based on one’s current level of competency.
The author wishes to acknowledge the faculty of the American College of Physician Executives for their work in assembling many of the concepts found in this article. The course “Managing Physician Performance in Organizations” serves to underscore an integrated model of performance management and explores some of the theoretical bases of human behavior not included here.
Defining Your Group
Before you can manage performance, you must know the parameters by which the group is defined. The prerequisites for performance management include salient statements of mission, vision, and values. The mission defines the purpose for the group being in place and usually reflects the interests of the hospital(s) or medical group affiliated with or actually employing the hospital medicine group. The mission statement should be able to answer the questions “Why does our hospital medicine group exist? What purpose does it serve? In very broad terms, what scope of services do we provide?” The vision is a concise summary of what the group would like to be or achieve in the future, and it may relate to growth, range of services, outcomes, or other dimensions. Most often the vision is the leader’s platform for change in order to articulate the rationale for creating a better future. Values are those characteristics that guide decision making and provide guidance for everyone’s expected behavior and conduct in the group. Values can be thought of as the “lens” through which the vision is carried out and the mission upheld.
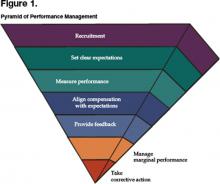
From the mission, vision, and values come strategies for achieving successful change and the more specific goals that the group is to attain. In some cases the group may have undertaken a formal strategic planning process that rendered a series of goals, objectives, and/or programs to be carried out in the immediate to intermediate term. We now reach the vital area in which a well structured and supported performance management system can play a pivotal role in ensuring the successful implementation of strategic thinking. Until now, the thought and planning process had focused on the right thing to do. From here, the focus becomes doing things right. Once you have completely answered the questions above and have a confident sense of where your group is heading and why, then the steps that follow will enable you to stack the deck in favor of achieving the level of performance you desire. Note that each step is embedded in action. Figure 1 represents the pyramid of performance management, a prioritized approach to managing others.
Recruiting the Right People
Not everyone has the luxury of personally hiring each physician in their group, much less having a surplus of candidates that are outstanding in every dimension. The reality in 2005 is that there continues to be demand for hospitalists far exceeding the available supply. This “seller’s market” (i.e., a hospitalist “sells” his or her services to an employer) represents a challenging dynamic for new or growing hospital medicine groups attempting to recruit the top candidates. It gets even worse when you consider hospital medicine as a new specialty, often finding itself in hospitals where the medical staff are skeptical or apprehensive in accepting the new group, and one bad hire can undermine the group’s chances of success. Furthermore, there may not be adequate experience or expertise in recruiting new physicians or correctly identifying those who would be a proper fit for the group. So how does one go about recruiting the right people?
Planning begins with having defined the group in terms of the mission and values. Knowing the vision and specific strategies to be employed lends insight into what type of individual would best fit with the needs and culture of the group. It is important to list the desired qualities on paper and plan for assessing each one, knowing that there is no perfect candidate and these characteristics must therefore be prioritized. Remember, what makes a good hospitalist in your group does not mean they will be good somewhere else; be sure you define very clearly what exactly “good” means. At the same time, it is also critical to outline the selling points of potentially joining your group in terms of 3 areas: the practice itself, compensation, and location.
The next step consists of preparing a slate of candidates for interviews. There are many methods of finding (i.e., sourcing) strong candidates, one of the best of which is to ask members of your current group or other trusted colleagues for referrals. If you are interested in filling a position with a more specific skill set such as information technology, palliative care, or clinical teaching, then a “make or buy” decision needs to be made to either recruit for the individual already in possession of such credentials or to hire more generically and then train accordingly. Once candidates are identified, a deliberate process of reviewing their written materials and interviewing them by telephone will determine the appropriateness of an in person interview. Speaking with references can occur at any time, and some advocate for this to occur prior to bringing a candidate for formal interview, as another mechanism of screening and to focus interview questions on site. The formal interview itself should be well structured and enable your key stakeholders to meet with the candidate and submit an immediate assessment. The shorter the turnaround time to extend an offer, the more decisive and committed to the candidate you will appear. Likewise, if you have a diverse composition of interviewers who weigh in with their perspectives, then there should be little to delay a hiring decision.
There are 3 additional points to remember when looking to hire an additional hospitalist into your group. First, it is estimated that 70% of physicians who leave a job do so because of spousal discontent. To mitigate this possibility, invite the spouse to accompany the candidate to the interview location, and assemble a parallel agenda for him or her.. Do not consider yourself on a “best behavior” basis during courtship alone; you need to continue nurturing the candidate and family well into the first year of employment to ensure a good transition. Second, be realistic about your expectations. There is no perfect candidate, so you must prioritize those qualities you want most from them. If you wait for perfection, the delay will cause you to overlook many very good physicians. Finally, take another look at the performance management pyramid. The reason the area for recruitment is so large is because of the disproportionate amount of time that one should invest in recruitment processes. Hiring the right people up front will make the rest of the steps far easier and minimize the likelihood of your being drawn into the nadir of the pyramid.
Setting Clear Expectations
Do you have a job description? When you read it, does it adequately describe what is expected of your hospitalists? Do you have an orientation for new members to your group? How long does it last? Is additional training offered? Are there outcomes that you expect from this training? And once you have oriented, trained, and offered a job description, does the actual work environment support or negate your efforts―i.e., does culture trump your formal process?
The cycle of setting clear expectations about work performance begins during the recruitment phase. Being absolutely forthcoming about what it is like to work in your group and what you expect from each and every member is paramount to allow both you and the candidate to determine a good fit. Once the physician has joined your group, orientation and training should hardly be a 1-, 2- or 3-day exercise. These are continuous and ongoing processes, given our rapidly changing practice environment. In fact, change is one of the only reliable characteristics of what we do, and extending the welcome “The job you take today is unlikely to be the job you will have next year” is hardly inappropriate. Be mindful that setting clear expectations with all of your hospitalists is the bedrock of a functional performance management system. Defining expectations alone will often improve performance, vis-à-vis the Hawthorne effect.
Expectations should always be depersonalized and focus on behavior. Behavior itself may be regarded in 2 distinct domains: those behaviors that are observed, and those outcomes that are measurable. Examples of observable behaviors include interpersonal interactions with nurses and consultants, pager response times, and attendance at monthly team meetings. Measurable outcomes include work RVU productivity, patient satisfaction, readmission rates, and compliance with coding and documentation guidelines. There are many ways to organize dimensions of performance that you may expect from your physicians―the 6 aims of quality (safe, timely, effective, efficient, equitable, and patient centered, as outlined in the IOM report Crossing the Quality Chasm), maintenance of a healthy workplace, citizenship, relationships with others, etc.―yet the key is to define and communicate them, then check often for understanding.
Measuring Actual Performance
Be the first to admit “the numbers are wrong,” and you will save hearing it from many others. There are many inherent problems in measuring actual performance, and the data may never be perfect. As an exercise, try assigning individual readmission rates within your group, and you will find that because of handoffs within the group and lack of precision in identifying who actually discharged the patient, there will be many arguments over whether the data is valid. However, in most circumstances, if the data is flawed, it still may serve a strong purpose to highlight the relative variation within the group. Searching for quantifiable systemic data and being transparent about the limitations of the data will be an exercise worth undertaking. In like manner, behavioral observation data are potentially fraught with conflict if the data are focused on judgment of character traits (I believe this hospitalist has a good bedside manner) rather than on observable behaviors (This hospitalist always/sometimes/never comes to meetings on time). Measures are best when they are objective, relevant to the position, and interpretable. Remember: All measures are flawed; some are useful.
Aligning Compensation With Expectations
Conventional wisdom states that people will do more of what they are incentivized to do. The corollary to this is to be sure what you incentivize is actually what you want. For the group that is trying to improve individual productivity and reduce length of stay, providing financial rewards for work RVU’s alone may result in less assertiveness in managing timely discharges and bickering over who picks up the 11 p.m. vs. 2 a.m. overnight admission the following morning. Ultimately, compensation must be intimately linked with the mission of the group, and tremendous care must be taken in determining the construct of any system. Although it is well beyond the scope of this article to detail the many considerations of designing a compensation system, one must understand that it is only one component―and not the most important component―of a performance management program.
Here are a few points to consider as you integrate your compensation system into the rest of the steps in the pyramid:
- A straight salary with or without a “guaranteed” bonus is unlikely to reward or motivate any new behaviors.
- For a performance-based compensation plan to have sufficient impact, at least 20%–30% of compensation must be tied to performance.
- Consider having both group and individual measures as part of your plan to engender a sense of teamwork and collective effort in performing well.
- Limit the number of variables in the plan to 3–5; otherwise, measures are too diluted to carry meaningful weight.
- Perform a local market comparison for benchmarking your goal median compensation; often administrative staff are more willing to share this information with other administrative staff if the understanding is that all market results will be shared.
- The process of constructing or evolving your plan, being inclusive of members of your group as well as any group sponsors, ends up being far more valuable than the final plan itself.
Providing Regular Feedback
Have you ever had a complaint that sounded like “I get way too much feedback around here?” Probably not. More likely is the case that your hospitalists wonder how they stand in terms of being compared to others and to themselves over time. The creed “no news must be good news” is hardly supportive of promoting top performance. Feedback itself can be highly influential and reflects the expectations explained by the group leader. Expectations not measured or fed back to the individual hospitalists will be expectations soon forgotten or ignored, because they may be felt not to matter.
Effective feedback is both formal and informal. The annual performance review is a common example of the former, but it is in no way meant to be the only feedback a hospitalist should receive, nor is it the most powerful. The annual review should be well structured, can outline longer term goals and ideas for self-improvement, and may serve in some key administrative functions like compensation and promotion. Informal, regular feedback, however, may serve you much better in driving performance, because it is timelier, more relevant to daily work, and more specific to the individual. Individuals also respond much more constructively to positive feedback, and some experts believe the ratio of positive to negative feedback should be on the order of 9 to 1. Be sure that feedback is done in a coaching manner and focuses on the behavior (You may try sitting down when you talk with patients as a way of making them feel more at ease) rather than on the person themselves (You’re really not a good communicator).
Managing Marginal Performance
Marginal performance can be defined as a physician whose observed behaviors or measured outcomes are at significant variance from what is expected. This pattern takes place over time and happens in spite of having in place all the other elements of a performance management system. Consider the “clock puncher” who rarely helps out the rest of the team on busy days and never shows up to group meetings or committees. Or the “tortoise” that has wonderful staff relations but chronically arrives at work late and repeatedly forgets to submit inpatient charges. Then there’s the “hothead” who is clinically adept and has high patient satisfaction but loses his or her temper with nursing and is pervasively confrontational with consultants. The steps to be taken in these and other cases like them include ensuring adequate documentation, reaching an agreement with the individual in recognizing that there is a problem, generating options for causality, negotiating a contract for improvement, and then letting future behavior determine the consequences.
Taking Corrective Action
Sometimes you simply cannot fix everything, and you need to be easy on yourself for having reached the point where the situation is no longer remediable in spite of your best efforts. In the end, everyone will be better off. When physician conduct becomes detrimental to patient safety, staff safety or quality patient care; is disruptive to the organization; or is otherwise chronically aberrant, then it is time to take adverse action. Since there are many pitfalls that have HR and legal implications, it is advisable to consult with relevant personnel to avoid problems with inadequate documentation and the potential need to report actions to state agencies and the National Practitioner Data Bank (per the Healthcare Quality Improvement Act of 1986).
Resources
- Ury W, Fisher R. Getting to Yes: Negotiating Agreement Without Giving In. 2nd ed. New York: Penguin Books; 1991.
- Reinertsen J. Physicians as leaders in the improvement of health care systems. Ann Intern Med. 1998;128:833-8.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- American College of Physician Executives. Managing Physician Performance in Organizations. Ongoing courses available at www.acpe.org.
Joel Barker describes leadership as “…the ability to take people where they otherwise would not go.” In other words, leadership is about creating change in something that exists today. Management, on the other hand, may be considered a series of steps to ensure that things happen the desired and consistent way. Although this article is not of scope sufficient to explore the differences between management and leadership, it will address a domain in which the 2 intimately intersect. Managing others relies upon many foundations of leadership, such as establishing the group’s vision and setting key strategic goals. In like manner, successful leadership in stimulating change is dependent on the effective management of personnel to ensure that the culture, work habits, outcomes, and behaviors are consistent with the change efforts. This article will focus on the management of physicians in hospital medicine groups. The 8 steps outlined are applicable regardless of employer type, group size, or mission. Almost all of the skills necessary to effectively implement a performance management system can be learned and are best practiced on a regular basis. Furthermore, there are many existing resources for further education and development in these areas based on one’s current level of competency.
The author wishes to acknowledge the faculty of the American College of Physician Executives for their work in assembling many of the concepts found in this article. The course “Managing Physician Performance in Organizations” serves to underscore an integrated model of performance management and explores some of the theoretical bases of human behavior not included here.
Defining Your Group
Before you can manage performance, you must know the parameters by which the group is defined. The prerequisites for performance management include salient statements of mission, vision, and values. The mission defines the purpose for the group being in place and usually reflects the interests of the hospital(s) or medical group affiliated with or actually employing the hospital medicine group. The mission statement should be able to answer the questions “Why does our hospital medicine group exist? What purpose does it serve? In very broad terms, what scope of services do we provide?” The vision is a concise summary of what the group would like to be or achieve in the future, and it may relate to growth, range of services, outcomes, or other dimensions. Most often the vision is the leader’s platform for change in order to articulate the rationale for creating a better future. Values are those characteristics that guide decision making and provide guidance for everyone’s expected behavior and conduct in the group. Values can be thought of as the “lens” through which the vision is carried out and the mission upheld.
From the mission, vision, and values come strategies for achieving successful change and the more specific goals that the group is to attain. In some cases the group may have undertaken a formal strategic planning process that rendered a series of goals, objectives, and/or programs to be carried out in the immediate to intermediate term. We now reach the vital area in which a well structured and supported performance management system can play a pivotal role in ensuring the successful implementation of strategic thinking. Until now, the thought and planning process had focused on the right thing to do. From here, the focus becomes doing things right. Once you have completely answered the questions above and have a confident sense of where your group is heading and why, then the steps that follow will enable you to stack the deck in favor of achieving the level of performance you desire. Note that each step is embedded in action. Figure 1 represents the pyramid of performance management, a prioritized approach to managing others.
Recruiting the Right People
Not everyone has the luxury of personally hiring each physician in their group, much less having a surplus of candidates that are outstanding in every dimension. The reality in 2005 is that there continues to be demand for hospitalists far exceeding the available supply. This “seller’s market” (i.e., a hospitalist “sells” his or her services to an employer) represents a challenging dynamic for new or growing hospital medicine groups attempting to recruit the top candidates. It gets even worse when you consider hospital medicine as a new specialty, often finding itself in hospitals where the medical staff are skeptical or apprehensive in accepting the new group, and one bad hire can undermine the group’s chances of success. Furthermore, there may not be adequate experience or expertise in recruiting new physicians or correctly identifying those who would be a proper fit for the group. So how does one go about recruiting the right people?
Planning begins with having defined the group in terms of the mission and values. Knowing the vision and specific strategies to be employed lends insight into what type of individual would best fit with the needs and culture of the group. It is important to list the desired qualities on paper and plan for assessing each one, knowing that there is no perfect candidate and these characteristics must therefore be prioritized. Remember, what makes a good hospitalist in your group does not mean they will be good somewhere else; be sure you define very clearly what exactly “good” means. At the same time, it is also critical to outline the selling points of potentially joining your group in terms of 3 areas: the practice itself, compensation, and location.
The next step consists of preparing a slate of candidates for interviews. There are many methods of finding (i.e., sourcing) strong candidates, one of the best of which is to ask members of your current group or other trusted colleagues for referrals. If you are interested in filling a position with a more specific skill set such as information technology, palliative care, or clinical teaching, then a “make or buy” decision needs to be made to either recruit for the individual already in possession of such credentials or to hire more generically and then train accordingly. Once candidates are identified, a deliberate process of reviewing their written materials and interviewing them by telephone will determine the appropriateness of an in person interview. Speaking with references can occur at any time, and some advocate for this to occur prior to bringing a candidate for formal interview, as another mechanism of screening and to focus interview questions on site. The formal interview itself should be well structured and enable your key stakeholders to meet with the candidate and submit an immediate assessment. The shorter the turnaround time to extend an offer, the more decisive and committed to the candidate you will appear. Likewise, if you have a diverse composition of interviewers who weigh in with their perspectives, then there should be little to delay a hiring decision.
There are 3 additional points to remember when looking to hire an additional hospitalist into your group. First, it is estimated that 70% of physicians who leave a job do so because of spousal discontent. To mitigate this possibility, invite the spouse to accompany the candidate to the interview location, and assemble a parallel agenda for him or her.. Do not consider yourself on a “best behavior” basis during courtship alone; you need to continue nurturing the candidate and family well into the first year of employment to ensure a good transition. Second, be realistic about your expectations. There is no perfect candidate, so you must prioritize those qualities you want most from them. If you wait for perfection, the delay will cause you to overlook many very good physicians. Finally, take another look at the performance management pyramid. The reason the area for recruitment is so large is because of the disproportionate amount of time that one should invest in recruitment processes. Hiring the right people up front will make the rest of the steps far easier and minimize the likelihood of your being drawn into the nadir of the pyramid.
Setting Clear Expectations
Do you have a job description? When you read it, does it adequately describe what is expected of your hospitalists? Do you have an orientation for new members to your group? How long does it last? Is additional training offered? Are there outcomes that you expect from this training? And once you have oriented, trained, and offered a job description, does the actual work environment support or negate your efforts―i.e., does culture trump your formal process?
The cycle of setting clear expectations about work performance begins during the recruitment phase. Being absolutely forthcoming about what it is like to work in your group and what you expect from each and every member is paramount to allow both you and the candidate to determine a good fit. Once the physician has joined your group, orientation and training should hardly be a 1-, 2- or 3-day exercise. These are continuous and ongoing processes, given our rapidly changing practice environment. In fact, change is one of the only reliable characteristics of what we do, and extending the welcome “The job you take today is unlikely to be the job you will have next year” is hardly inappropriate. Be mindful that setting clear expectations with all of your hospitalists is the bedrock of a functional performance management system. Defining expectations alone will often improve performance, vis-à-vis the Hawthorne effect.
Expectations should always be depersonalized and focus on behavior. Behavior itself may be regarded in 2 distinct domains: those behaviors that are observed, and those outcomes that are measurable. Examples of observable behaviors include interpersonal interactions with nurses and consultants, pager response times, and attendance at monthly team meetings. Measurable outcomes include work RVU productivity, patient satisfaction, readmission rates, and compliance with coding and documentation guidelines. There are many ways to organize dimensions of performance that you may expect from your physicians―the 6 aims of quality (safe, timely, effective, efficient, equitable, and patient centered, as outlined in the IOM report Crossing the Quality Chasm), maintenance of a healthy workplace, citizenship, relationships with others, etc.―yet the key is to define and communicate them, then check often for understanding.
Measuring Actual Performance
Be the first to admit “the numbers are wrong,” and you will save hearing it from many others. There are many inherent problems in measuring actual performance, and the data may never be perfect. As an exercise, try assigning individual readmission rates within your group, and you will find that because of handoffs within the group and lack of precision in identifying who actually discharged the patient, there will be many arguments over whether the data is valid. However, in most circumstances, if the data is flawed, it still may serve a strong purpose to highlight the relative variation within the group. Searching for quantifiable systemic data and being transparent about the limitations of the data will be an exercise worth undertaking. In like manner, behavioral observation data are potentially fraught with conflict if the data are focused on judgment of character traits (I believe this hospitalist has a good bedside manner) rather than on observable behaviors (This hospitalist always/sometimes/never comes to meetings on time). Measures are best when they are objective, relevant to the position, and interpretable. Remember: All measures are flawed; some are useful.
Aligning Compensation With Expectations
Conventional wisdom states that people will do more of what they are incentivized to do. The corollary to this is to be sure what you incentivize is actually what you want. For the group that is trying to improve individual productivity and reduce length of stay, providing financial rewards for work RVU’s alone may result in less assertiveness in managing timely discharges and bickering over who picks up the 11 p.m. vs. 2 a.m. overnight admission the following morning. Ultimately, compensation must be intimately linked with the mission of the group, and tremendous care must be taken in determining the construct of any system. Although it is well beyond the scope of this article to detail the many considerations of designing a compensation system, one must understand that it is only one component―and not the most important component―of a performance management program.
Here are a few points to consider as you integrate your compensation system into the rest of the steps in the pyramid:
- A straight salary with or without a “guaranteed” bonus is unlikely to reward or motivate any new behaviors.
- For a performance-based compensation plan to have sufficient impact, at least 20%–30% of compensation must be tied to performance.
- Consider having both group and individual measures as part of your plan to engender a sense of teamwork and collective effort in performing well.
- Limit the number of variables in the plan to 3–5; otherwise, measures are too diluted to carry meaningful weight.
- Perform a local market comparison for benchmarking your goal median compensation; often administrative staff are more willing to share this information with other administrative staff if the understanding is that all market results will be shared.
- The process of constructing or evolving your plan, being inclusive of members of your group as well as any group sponsors, ends up being far more valuable than the final plan itself.
Providing Regular Feedback
Have you ever had a complaint that sounded like “I get way too much feedback around here?” Probably not. More likely is the case that your hospitalists wonder how they stand in terms of being compared to others and to themselves over time. The creed “no news must be good news” is hardly supportive of promoting top performance. Feedback itself can be highly influential and reflects the expectations explained by the group leader. Expectations not measured or fed back to the individual hospitalists will be expectations soon forgotten or ignored, because they may be felt not to matter.
Effective feedback is both formal and informal. The annual performance review is a common example of the former, but it is in no way meant to be the only feedback a hospitalist should receive, nor is it the most powerful. The annual review should be well structured, can outline longer term goals and ideas for self-improvement, and may serve in some key administrative functions like compensation and promotion. Informal, regular feedback, however, may serve you much better in driving performance, because it is timelier, more relevant to daily work, and more specific to the individual. Individuals also respond much more constructively to positive feedback, and some experts believe the ratio of positive to negative feedback should be on the order of 9 to 1. Be sure that feedback is done in a coaching manner and focuses on the behavior (You may try sitting down when you talk with patients as a way of making them feel more at ease) rather than on the person themselves (You’re really not a good communicator).
Managing Marginal Performance
Marginal performance can be defined as a physician whose observed behaviors or measured outcomes are at significant variance from what is expected. This pattern takes place over time and happens in spite of having in place all the other elements of a performance management system. Consider the “clock puncher” who rarely helps out the rest of the team on busy days and never shows up to group meetings or committees. Or the “tortoise” that has wonderful staff relations but chronically arrives at work late and repeatedly forgets to submit inpatient charges. Then there’s the “hothead” who is clinically adept and has high patient satisfaction but loses his or her temper with nursing and is pervasively confrontational with consultants. The steps to be taken in these and other cases like them include ensuring adequate documentation, reaching an agreement with the individual in recognizing that there is a problem, generating options for causality, negotiating a contract for improvement, and then letting future behavior determine the consequences.
Taking Corrective Action
Sometimes you simply cannot fix everything, and you need to be easy on yourself for having reached the point where the situation is no longer remediable in spite of your best efforts. In the end, everyone will be better off. When physician conduct becomes detrimental to patient safety, staff safety or quality patient care; is disruptive to the organization; or is otherwise chronically aberrant, then it is time to take adverse action. Since there are many pitfalls that have HR and legal implications, it is advisable to consult with relevant personnel to avoid problems with inadequate documentation and the potential need to report actions to state agencies and the National Practitioner Data Bank (per the Healthcare Quality Improvement Act of 1986).
Resources
- Ury W, Fisher R. Getting to Yes: Negotiating Agreement Without Giving In. 2nd ed. New York: Penguin Books; 1991.
- Reinertsen J. Physicians as leaders in the improvement of health care systems. Ann Intern Med. 1998;128:833-8.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- American College of Physician Executives. Managing Physician Performance in Organizations. Ongoing courses available at www.acpe.org.
Joel Barker describes leadership as “…the ability to take people where they otherwise would not go.” In other words, leadership is about creating change in something that exists today. Management, on the other hand, may be considered a series of steps to ensure that things happen the desired and consistent way. Although this article is not of scope sufficient to explore the differences between management and leadership, it will address a domain in which the 2 intimately intersect. Managing others relies upon many foundations of leadership, such as establishing the group’s vision and setting key strategic goals. In like manner, successful leadership in stimulating change is dependent on the effective management of personnel to ensure that the culture, work habits, outcomes, and behaviors are consistent with the change efforts. This article will focus on the management of physicians in hospital medicine groups. The 8 steps outlined are applicable regardless of employer type, group size, or mission. Almost all of the skills necessary to effectively implement a performance management system can be learned and are best practiced on a regular basis. Furthermore, there are many existing resources for further education and development in these areas based on one’s current level of competency.
The author wishes to acknowledge the faculty of the American College of Physician Executives for their work in assembling many of the concepts found in this article. The course “Managing Physician Performance in Organizations” serves to underscore an integrated model of performance management and explores some of the theoretical bases of human behavior not included here.
Defining Your Group
Before you can manage performance, you must know the parameters by which the group is defined. The prerequisites for performance management include salient statements of mission, vision, and values. The mission defines the purpose for the group being in place and usually reflects the interests of the hospital(s) or medical group affiliated with or actually employing the hospital medicine group. The mission statement should be able to answer the questions “Why does our hospital medicine group exist? What purpose does it serve? In very broad terms, what scope of services do we provide?” The vision is a concise summary of what the group would like to be or achieve in the future, and it may relate to growth, range of services, outcomes, or other dimensions. Most often the vision is the leader’s platform for change in order to articulate the rationale for creating a better future. Values are those characteristics that guide decision making and provide guidance for everyone’s expected behavior and conduct in the group. Values can be thought of as the “lens” through which the vision is carried out and the mission upheld.
From the mission, vision, and values come strategies for achieving successful change and the more specific goals that the group is to attain. In some cases the group may have undertaken a formal strategic planning process that rendered a series of goals, objectives, and/or programs to be carried out in the immediate to intermediate term. We now reach the vital area in which a well structured and supported performance management system can play a pivotal role in ensuring the successful implementation of strategic thinking. Until now, the thought and planning process had focused on the right thing to do. From here, the focus becomes doing things right. Once you have completely answered the questions above and have a confident sense of where your group is heading and why, then the steps that follow will enable you to stack the deck in favor of achieving the level of performance you desire. Note that each step is embedded in action. Figure 1 represents the pyramid of performance management, a prioritized approach to managing others.
Recruiting the Right People
Not everyone has the luxury of personally hiring each physician in their group, much less having a surplus of candidates that are outstanding in every dimension. The reality in 2005 is that there continues to be demand for hospitalists far exceeding the available supply. This “seller’s market” (i.e., a hospitalist “sells” his or her services to an employer) represents a challenging dynamic for new or growing hospital medicine groups attempting to recruit the top candidates. It gets even worse when you consider hospital medicine as a new specialty, often finding itself in hospitals where the medical staff are skeptical or apprehensive in accepting the new group, and one bad hire can undermine the group’s chances of success. Furthermore, there may not be adequate experience or expertise in recruiting new physicians or correctly identifying those who would be a proper fit for the group. So how does one go about recruiting the right people?
Planning begins with having defined the group in terms of the mission and values. Knowing the vision and specific strategies to be employed lends insight into what type of individual would best fit with the needs and culture of the group. It is important to list the desired qualities on paper and plan for assessing each one, knowing that there is no perfect candidate and these characteristics must therefore be prioritized. Remember, what makes a good hospitalist in your group does not mean they will be good somewhere else; be sure you define very clearly what exactly “good” means. At the same time, it is also critical to outline the selling points of potentially joining your group in terms of 3 areas: the practice itself, compensation, and location.
The next step consists of preparing a slate of candidates for interviews. There are many methods of finding (i.e., sourcing) strong candidates, one of the best of which is to ask members of your current group or other trusted colleagues for referrals. If you are interested in filling a position with a more specific skill set such as information technology, palliative care, or clinical teaching, then a “make or buy” decision needs to be made to either recruit for the individual already in possession of such credentials or to hire more generically and then train accordingly. Once candidates are identified, a deliberate process of reviewing their written materials and interviewing them by telephone will determine the appropriateness of an in person interview. Speaking with references can occur at any time, and some advocate for this to occur prior to bringing a candidate for formal interview, as another mechanism of screening and to focus interview questions on site. The formal interview itself should be well structured and enable your key stakeholders to meet with the candidate and submit an immediate assessment. The shorter the turnaround time to extend an offer, the more decisive and committed to the candidate you will appear. Likewise, if you have a diverse composition of interviewers who weigh in with their perspectives, then there should be little to delay a hiring decision.
There are 3 additional points to remember when looking to hire an additional hospitalist into your group. First, it is estimated that 70% of physicians who leave a job do so because of spousal discontent. To mitigate this possibility, invite the spouse to accompany the candidate to the interview location, and assemble a parallel agenda for him or her.. Do not consider yourself on a “best behavior” basis during courtship alone; you need to continue nurturing the candidate and family well into the first year of employment to ensure a good transition. Second, be realistic about your expectations. There is no perfect candidate, so you must prioritize those qualities you want most from them. If you wait for perfection, the delay will cause you to overlook many very good physicians. Finally, take another look at the performance management pyramid. The reason the area for recruitment is so large is because of the disproportionate amount of time that one should invest in recruitment processes. Hiring the right people up front will make the rest of the steps far easier and minimize the likelihood of your being drawn into the nadir of the pyramid.
Setting Clear Expectations
Do you have a job description? When you read it, does it adequately describe what is expected of your hospitalists? Do you have an orientation for new members to your group? How long does it last? Is additional training offered? Are there outcomes that you expect from this training? And once you have oriented, trained, and offered a job description, does the actual work environment support or negate your efforts―i.e., does culture trump your formal process?
The cycle of setting clear expectations about work performance begins during the recruitment phase. Being absolutely forthcoming about what it is like to work in your group and what you expect from each and every member is paramount to allow both you and the candidate to determine a good fit. Once the physician has joined your group, orientation and training should hardly be a 1-, 2- or 3-day exercise. These are continuous and ongoing processes, given our rapidly changing practice environment. In fact, change is one of the only reliable characteristics of what we do, and extending the welcome “The job you take today is unlikely to be the job you will have next year” is hardly inappropriate. Be mindful that setting clear expectations with all of your hospitalists is the bedrock of a functional performance management system. Defining expectations alone will often improve performance, vis-à-vis the Hawthorne effect.
Expectations should always be depersonalized and focus on behavior. Behavior itself may be regarded in 2 distinct domains: those behaviors that are observed, and those outcomes that are measurable. Examples of observable behaviors include interpersonal interactions with nurses and consultants, pager response times, and attendance at monthly team meetings. Measurable outcomes include work RVU productivity, patient satisfaction, readmission rates, and compliance with coding and documentation guidelines. There are many ways to organize dimensions of performance that you may expect from your physicians―the 6 aims of quality (safe, timely, effective, efficient, equitable, and patient centered, as outlined in the IOM report Crossing the Quality Chasm), maintenance of a healthy workplace, citizenship, relationships with others, etc.―yet the key is to define and communicate them, then check often for understanding.
Measuring Actual Performance
Be the first to admit “the numbers are wrong,” and you will save hearing it from many others. There are many inherent problems in measuring actual performance, and the data may never be perfect. As an exercise, try assigning individual readmission rates within your group, and you will find that because of handoffs within the group and lack of precision in identifying who actually discharged the patient, there will be many arguments over whether the data is valid. However, in most circumstances, if the data is flawed, it still may serve a strong purpose to highlight the relative variation within the group. Searching for quantifiable systemic data and being transparent about the limitations of the data will be an exercise worth undertaking. In like manner, behavioral observation data are potentially fraught with conflict if the data are focused on judgment of character traits (I believe this hospitalist has a good bedside manner) rather than on observable behaviors (This hospitalist always/sometimes/never comes to meetings on time). Measures are best when they are objective, relevant to the position, and interpretable. Remember: All measures are flawed; some are useful.
Aligning Compensation With Expectations
Conventional wisdom states that people will do more of what they are incentivized to do. The corollary to this is to be sure what you incentivize is actually what you want. For the group that is trying to improve individual productivity and reduce length of stay, providing financial rewards for work RVU’s alone may result in less assertiveness in managing timely discharges and bickering over who picks up the 11 p.m. vs. 2 a.m. overnight admission the following morning. Ultimately, compensation must be intimately linked with the mission of the group, and tremendous care must be taken in determining the construct of any system. Although it is well beyond the scope of this article to detail the many considerations of designing a compensation system, one must understand that it is only one component―and not the most important component―of a performance management program.
Here are a few points to consider as you integrate your compensation system into the rest of the steps in the pyramid:
- A straight salary with or without a “guaranteed” bonus is unlikely to reward or motivate any new behaviors.
- For a performance-based compensation plan to have sufficient impact, at least 20%–30% of compensation must be tied to performance.
- Consider having both group and individual measures as part of your plan to engender a sense of teamwork and collective effort in performing well.
- Limit the number of variables in the plan to 3–5; otherwise, measures are too diluted to carry meaningful weight.
- Perform a local market comparison for benchmarking your goal median compensation; often administrative staff are more willing to share this information with other administrative staff if the understanding is that all market results will be shared.
- The process of constructing or evolving your plan, being inclusive of members of your group as well as any group sponsors, ends up being far more valuable than the final plan itself.
Providing Regular Feedback
Have you ever had a complaint that sounded like “I get way too much feedback around here?” Probably not. More likely is the case that your hospitalists wonder how they stand in terms of being compared to others and to themselves over time. The creed “no news must be good news” is hardly supportive of promoting top performance. Feedback itself can be highly influential and reflects the expectations explained by the group leader. Expectations not measured or fed back to the individual hospitalists will be expectations soon forgotten or ignored, because they may be felt not to matter.
Effective feedback is both formal and informal. The annual performance review is a common example of the former, but it is in no way meant to be the only feedback a hospitalist should receive, nor is it the most powerful. The annual review should be well structured, can outline longer term goals and ideas for self-improvement, and may serve in some key administrative functions like compensation and promotion. Informal, regular feedback, however, may serve you much better in driving performance, because it is timelier, more relevant to daily work, and more specific to the individual. Individuals also respond much more constructively to positive feedback, and some experts believe the ratio of positive to negative feedback should be on the order of 9 to 1. Be sure that feedback is done in a coaching manner and focuses on the behavior (You may try sitting down when you talk with patients as a way of making them feel more at ease) rather than on the person themselves (You’re really not a good communicator).
Managing Marginal Performance
Marginal performance can be defined as a physician whose observed behaviors or measured outcomes are at significant variance from what is expected. This pattern takes place over time and happens in spite of having in place all the other elements of a performance management system. Consider the “clock puncher” who rarely helps out the rest of the team on busy days and never shows up to group meetings or committees. Or the “tortoise” that has wonderful staff relations but chronically arrives at work late and repeatedly forgets to submit inpatient charges. Then there’s the “hothead” who is clinically adept and has high patient satisfaction but loses his or her temper with nursing and is pervasively confrontational with consultants. The steps to be taken in these and other cases like them include ensuring adequate documentation, reaching an agreement with the individual in recognizing that there is a problem, generating options for causality, negotiating a contract for improvement, and then letting future behavior determine the consequences.
Taking Corrective Action
Sometimes you simply cannot fix everything, and you need to be easy on yourself for having reached the point where the situation is no longer remediable in spite of your best efforts. In the end, everyone will be better off. When physician conduct becomes detrimental to patient safety, staff safety or quality patient care; is disruptive to the organization; or is otherwise chronically aberrant, then it is time to take adverse action. Since there are many pitfalls that have HR and legal implications, it is advisable to consult with relevant personnel to avoid problems with inadequate documentation and the potential need to report actions to state agencies and the National Practitioner Data Bank (per the Healthcare Quality Improvement Act of 1986).
Resources
- Ury W, Fisher R. Getting to Yes: Negotiating Agreement Without Giving In. 2nd ed. New York: Penguin Books; 1991.
- Reinertsen J. Physicians as leaders in the improvement of health care systems. Ann Intern Med. 1998;128:833-8.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- American College of Physician Executives. Managing Physician Performance in Organizations. Ongoing courses available at www.acpe.org.
Four Physicians Presented SHM's 2005 National Awards of Excellence
SHM presented its 2005 national awards of excellence to four hospitalists whose work and research have contributed significantly to hospital medicine and to the betterment of hospital care across America. The award winners, who were recognized at the SHM annual meeting in Chicago, included:
- Sunil Kripalani, MD, MSc, assistant professor, Division of General Medicine, Emory University School of Medicine, and attending physician and assistant director for research, Hospitalist Program, Grady Memorial Hospital, both in Atlanta, GA– recipient of Young Investigator Award.
- Shaun Frost, MD, FACP, assistant professor of Medicine, University of Minnesota Medical School, and hospitalist, HealthPartners Medical Group and Clinics, Regions Hospital, St Paul, MN– recipient of Clinical Excellence Award.
- Joseph Ming Wah Li, MD, hospitalist and director of the Hospital Medicine section, Beth Israel Deaconess Medical Center, Boston, MA– recipient of Outstanding Service in Hospital Medicine Award.
- Jeff Wiese, MD, associate professor of medicine, associate chairman of medicine, director of the Internal Medicine Residency Program, Tulane University Health Sciences Center, and chief of medicine, Medical Center of Louisiana at New Orleans and Charity Hospital, New Orleans, LA– recipient of Excellence in Teaching Award.
Dr. Kripalani has established himself as one of the leading investigators in the field of patient literacy and its impact on health outcomes. He has been the recipient of more than $1 million in grant funding, including a prestigious K23 Patient Oriented Research Career Development Award from the National Institutes of Health (NIH) to examine the relationship between health literacy and medication adherence after hospital discharge. He is currently the principal investigator on a randomized trial of two low literacy interventions designed to improve medication adherence among patients with coronary heart disease, funded by the American Heart Association. In addition, through a Pfizer Health Literacy Scholar Award, he has established a training program to improve physician communication with low literacy patients.
Dr. Kripalani has authored over 20 scientific and educational publications, including articles in the Journal of the American Medical Association, Journal of General Internal Medicine, and American Journal of Preventive Medicine. He serves as a reviewer for several prominent medical journals and has reviewed grants for the NIH. Dr. Kripalani has lectured at the Centers for Disease Control and Prevention, Georgia Hospital Association, SHM, and Society of General Internal Medicine (SGIM), where he coordinates the health literacy interest group. He is also serving as an associate editor of the upcoming book, Hospital Medicine Secrets, and coeditor of an upcoming special issue on health literacy for the Journal of General Internal Medicine.
In addition to these activities, Dr. Kripalani has proven himself a dedicated champion of SHM, contributing substantial time to research efforts at SHM, including the SHM Research Committee, Continuity of Care Task Force, Abstract Committee, Advisory Board Young Hospitalists Section, and the research section of SHM’s The Hospitalist publication.
After graduating summa cum laude from Rice University in 1993 with a BA in Psychology, Dr. Kripalani received an MD with honors from Baylor College of Medicine in 1997. He completed his residency in Internal Medicine at Emory University in Atlanta in 2000, where he also completed one of the nation’s first Hospital Medicine Fellowships, including a Master of Science in Clinical Research.
Dr. Frost has dedicated himself to the advancement of clinical knowledge through clinical teaching and scientific publication. He is a member of the Regions Hospital Palliative Care Service and Patient Safety Committee, was a lead participant in a “Lean” implementation team on inpatient testing results, and was selected as the leafter of Regions Hospital “Best Care, Best Experience” work team on provider support. He is also currently participating in the development and implementation of inpatient “Prepared Practice Teams,” a model of multidisciplinary rounding to enhance communication among physicians, nurses, case managers, social workers, and pharmacists.
A teaching faculty member of the University of Minnesota Medical School, he is highly regarded by residents and medical students, and has been instrumental in developing curricula in perioperative medicine for residents to improve the systems of surgical care through education.
Dr. Frost is a frequent lecturer on topics ranging from perioperative medicine to venous thromboembolism and has been published in: Annals of Internal Medicine, JAMA, Medical Clinics of North America, Mayo Clinic Proceedings, Cleveland Clinic Journal of Medicine, and The Hospitalist. He currently is lead investigator for a trial on preoperative medication administration.
Dr. Frost has demonstrated consistent leadership within SHM. He is regarded as the definitive resource in local chapter development due to his work in the SHM Lake Erie Chapter, where he was founder and president. He also is credited with establishing the very first formal chapter of SHM. His vision for the future of chapter activities – including community service and a national recognition program – resulted in a Membership Committee task force on chapter development. As a leader in the Midwest SHM region, Dr. Frost was named a Councilor to the SHM Midwest Council. His outstanding performance led to his assuming the chair of the Council in 2004. Dr. Frost is also recognized as a subject matter expert in biomedical ethics, serving consecutive terms on the Ethics Committee as well.
Dr. Frost earned his MD at the University of Texas Southwestern Medical School in Dallas as an AOA graduate, and completed his residency in Internal Medicine there. From 1998 through 2004, as a hospitalist at Cleveland Clinic Foundation, he was a contributor to the development, maturation, and operation of its hospital medicine model of care.
Dr. Li was the first hospitalist at the Beth Israel Deaconess Hospital Medicine Program in 1998. There he helped define the role of an academic hospitalist through clinical work, teaching, and service on countless committees and hospital initiatives. He quickly distinguished himself and was made associate chief of the HCA/ACOVE medical teaching
firm and, more recently, director of the BIDMC Hospital Medicine Program. A key focus for Dr. Li was broadening the Hospital Medicine Program at BIDMC. Under his guidance, the program grew to eleven hospitalists that account for over 50% of all general medicine admissions and over 50% of teaching attending months on the medical service. He also developed a system that allowed staff to provide 24/7 seamless coverage and created a website of referring physicians. He initiated new clinical programs and working arrangements for the hospitalist team, and helped institute a program to staff a local hospital with Beth Israel Deaconess hospitalists.
Dr. Li’s advocacy for Hospital Medicine did not stop at the doors of BIDMC, however. He was a co-developer of the first Harvard Medical School CME course on the emerging role of hospital medicine, and was the cofounder of the Boston Area Hospitalists and the SHM Northeast Regional Chapter of hospitalists. A charter member of SHM, he co-directed the first SHM annual northeastern regional meeting in 2001. He currently is a member of the SHM Education Committee, Annual Meeting Committee, and Membership Committee Task Force.
A nationally recognized expert in hospital medicine, Dr. Li lectures extensively and has testified on hearings dealing with mandatory hospitalist programs. He has published numerous articles in Critical Pathways in Cardiology, WebMD, Infectious Diseases in Clinical Practice, Current Opinion in Pulmonary Medicine, and Medscape.com, to name a few.
After earning his MD from the University of Oklahoma in Oklahoma City in 1994, Dr. Li did his residency at New England Deaconess Hospital before becoming chief medical resident at Beth Israel Deaconess Medical Center.
Dr. Wiese has received 21 awards for teaching over the last five years, including six from the University of California at San Francisco, where he started his career in 1998 as a clinical instructor. Since joining Tulane University in 2000, he has earned 16 teaching awards, including the prestigious all Tulane Faculty of the Year Award (twice) and the Virginia Furrow Award for Innovation in Medical Education. On the clinical wards, he has twice won Attending of the Year honors, and his Professor Rounds are routinely rated among the best.
Dr. Wiese designed numerous innovative curriculums. As a result of his clinical diagnosis innovations, the Clinical Diagnosis scores at Tulane increased from the 46th percentile to the 80th and 82nd percentile, with 10% of the 2004 class scoring in the top percentile in the nation. As a result of his restructuring of core curriculum to emphasize rational, evidenced based medical decision making, Tulane’s internal medicine program recently went the highest on its match list in the past 20 years. And through Dr. Wiese’s pyramid mentor system, Tulane Internal Medicine presented more regional and national presentations than any residency program in the country.
Dr. Wiese has written over 50 articles, books, or book chapters, is assistant editor for two educational textbooks and a reviewer for six national journals, has authored two textbooks, and is on the editorial board for a monthly publication. As an active SHM member, he has served on the Education Committee, Southern SHM Committee, and was program director for SHM’s Intensive Care Pre-course.
Dr. Wiese received his MD from Johns Hopkins School of Medicine in 1995. He completed his residency and chief residency training in Internal Medicine at the University of California at San Francisco, where he also completed a fellowship in General Internal Medicine with a focus on Hospitalist Medicine. He joined Tulane in 2000, after being recruited to start a hospitalist system at the Medical Center of Louisiana at New Orleans (Charity Hospital). His hospitalist proposal was accepted by the state and hospital administration, helping to provide funding to hospitalists at Charity.
Please join us in congratulating all of this year’s outstanding award winners.
SHM presented its 2005 national awards of excellence to four hospitalists whose work and research have contributed significantly to hospital medicine and to the betterment of hospital care across America. The award winners, who were recognized at the SHM annual meeting in Chicago, included:
- Sunil Kripalani, MD, MSc, assistant professor, Division of General Medicine, Emory University School of Medicine, and attending physician and assistant director for research, Hospitalist Program, Grady Memorial Hospital, both in Atlanta, GA– recipient of Young Investigator Award.
- Shaun Frost, MD, FACP, assistant professor of Medicine, University of Minnesota Medical School, and hospitalist, HealthPartners Medical Group and Clinics, Regions Hospital, St Paul, MN– recipient of Clinical Excellence Award.
- Joseph Ming Wah Li, MD, hospitalist and director of the Hospital Medicine section, Beth Israel Deaconess Medical Center, Boston, MA– recipient of Outstanding Service in Hospital Medicine Award.
- Jeff Wiese, MD, associate professor of medicine, associate chairman of medicine, director of the Internal Medicine Residency Program, Tulane University Health Sciences Center, and chief of medicine, Medical Center of Louisiana at New Orleans and Charity Hospital, New Orleans, LA– recipient of Excellence in Teaching Award.
Dr. Kripalani has established himself as one of the leading investigators in the field of patient literacy and its impact on health outcomes. He has been the recipient of more than $1 million in grant funding, including a prestigious K23 Patient Oriented Research Career Development Award from the National Institutes of Health (NIH) to examine the relationship between health literacy and medication adherence after hospital discharge. He is currently the principal investigator on a randomized trial of two low literacy interventions designed to improve medication adherence among patients with coronary heart disease, funded by the American Heart Association. In addition, through a Pfizer Health Literacy Scholar Award, he has established a training program to improve physician communication with low literacy patients.
Dr. Kripalani has authored over 20 scientific and educational publications, including articles in the Journal of the American Medical Association, Journal of General Internal Medicine, and American Journal of Preventive Medicine. He serves as a reviewer for several prominent medical journals and has reviewed grants for the NIH. Dr. Kripalani has lectured at the Centers for Disease Control and Prevention, Georgia Hospital Association, SHM, and Society of General Internal Medicine (SGIM), where he coordinates the health literacy interest group. He is also serving as an associate editor of the upcoming book, Hospital Medicine Secrets, and coeditor of an upcoming special issue on health literacy for the Journal of General Internal Medicine.
In addition to these activities, Dr. Kripalani has proven himself a dedicated champion of SHM, contributing substantial time to research efforts at SHM, including the SHM Research Committee, Continuity of Care Task Force, Abstract Committee, Advisory Board Young Hospitalists Section, and the research section of SHM’s The Hospitalist publication.
After graduating summa cum laude from Rice University in 1993 with a BA in Psychology, Dr. Kripalani received an MD with honors from Baylor College of Medicine in 1997. He completed his residency in Internal Medicine at Emory University in Atlanta in 2000, where he also completed one of the nation’s first Hospital Medicine Fellowships, including a Master of Science in Clinical Research.
Dr. Frost has dedicated himself to the advancement of clinical knowledge through clinical teaching and scientific publication. He is a member of the Regions Hospital Palliative Care Service and Patient Safety Committee, was a lead participant in a “Lean” implementation team on inpatient testing results, and was selected as the leafter of Regions Hospital “Best Care, Best Experience” work team on provider support. He is also currently participating in the development and implementation of inpatient “Prepared Practice Teams,” a model of multidisciplinary rounding to enhance communication among physicians, nurses, case managers, social workers, and pharmacists.
A teaching faculty member of the University of Minnesota Medical School, he is highly regarded by residents and medical students, and has been instrumental in developing curricula in perioperative medicine for residents to improve the systems of surgical care through education.
Dr. Frost is a frequent lecturer on topics ranging from perioperative medicine to venous thromboembolism and has been published in: Annals of Internal Medicine, JAMA, Medical Clinics of North America, Mayo Clinic Proceedings, Cleveland Clinic Journal of Medicine, and The Hospitalist. He currently is lead investigator for a trial on preoperative medication administration.
Dr. Frost has demonstrated consistent leadership within SHM. He is regarded as the definitive resource in local chapter development due to his work in the SHM Lake Erie Chapter, where he was founder and president. He also is credited with establishing the very first formal chapter of SHM. His vision for the future of chapter activities – including community service and a national recognition program – resulted in a Membership Committee task force on chapter development. As a leader in the Midwest SHM region, Dr. Frost was named a Councilor to the SHM Midwest Council. His outstanding performance led to his assuming the chair of the Council in 2004. Dr. Frost is also recognized as a subject matter expert in biomedical ethics, serving consecutive terms on the Ethics Committee as well.
Dr. Frost earned his MD at the University of Texas Southwestern Medical School in Dallas as an AOA graduate, and completed his residency in Internal Medicine there. From 1998 through 2004, as a hospitalist at Cleveland Clinic Foundation, he was a contributor to the development, maturation, and operation of its hospital medicine model of care.
Dr. Li was the first hospitalist at the Beth Israel Deaconess Hospital Medicine Program in 1998. There he helped define the role of an academic hospitalist through clinical work, teaching, and service on countless committees and hospital initiatives. He quickly distinguished himself and was made associate chief of the HCA/ACOVE medical teaching
firm and, more recently, director of the BIDMC Hospital Medicine Program. A key focus for Dr. Li was broadening the Hospital Medicine Program at BIDMC. Under his guidance, the program grew to eleven hospitalists that account for over 50% of all general medicine admissions and over 50% of teaching attending months on the medical service. He also developed a system that allowed staff to provide 24/7 seamless coverage and created a website of referring physicians. He initiated new clinical programs and working arrangements for the hospitalist team, and helped institute a program to staff a local hospital with Beth Israel Deaconess hospitalists.
Dr. Li’s advocacy for Hospital Medicine did not stop at the doors of BIDMC, however. He was a co-developer of the first Harvard Medical School CME course on the emerging role of hospital medicine, and was the cofounder of the Boston Area Hospitalists and the SHM Northeast Regional Chapter of hospitalists. A charter member of SHM, he co-directed the first SHM annual northeastern regional meeting in 2001. He currently is a member of the SHM Education Committee, Annual Meeting Committee, and Membership Committee Task Force.
A nationally recognized expert in hospital medicine, Dr. Li lectures extensively and has testified on hearings dealing with mandatory hospitalist programs. He has published numerous articles in Critical Pathways in Cardiology, WebMD, Infectious Diseases in Clinical Practice, Current Opinion in Pulmonary Medicine, and Medscape.com, to name a few.
After earning his MD from the University of Oklahoma in Oklahoma City in 1994, Dr. Li did his residency at New England Deaconess Hospital before becoming chief medical resident at Beth Israel Deaconess Medical Center.
Dr. Wiese has received 21 awards for teaching over the last five years, including six from the University of California at San Francisco, where he started his career in 1998 as a clinical instructor. Since joining Tulane University in 2000, he has earned 16 teaching awards, including the prestigious all Tulane Faculty of the Year Award (twice) and the Virginia Furrow Award for Innovation in Medical Education. On the clinical wards, he has twice won Attending of the Year honors, and his Professor Rounds are routinely rated among the best.
Dr. Wiese designed numerous innovative curriculums. As a result of his clinical diagnosis innovations, the Clinical Diagnosis scores at Tulane increased from the 46th percentile to the 80th and 82nd percentile, with 10% of the 2004 class scoring in the top percentile in the nation. As a result of his restructuring of core curriculum to emphasize rational, evidenced based medical decision making, Tulane’s internal medicine program recently went the highest on its match list in the past 20 years. And through Dr. Wiese’s pyramid mentor system, Tulane Internal Medicine presented more regional and national presentations than any residency program in the country.
Dr. Wiese has written over 50 articles, books, or book chapters, is assistant editor for two educational textbooks and a reviewer for six national journals, has authored two textbooks, and is on the editorial board for a monthly publication. As an active SHM member, he has served on the Education Committee, Southern SHM Committee, and was program director for SHM’s Intensive Care Pre-course.
Dr. Wiese received his MD from Johns Hopkins School of Medicine in 1995. He completed his residency and chief residency training in Internal Medicine at the University of California at San Francisco, where he also completed a fellowship in General Internal Medicine with a focus on Hospitalist Medicine. He joined Tulane in 2000, after being recruited to start a hospitalist system at the Medical Center of Louisiana at New Orleans (Charity Hospital). His hospitalist proposal was accepted by the state and hospital administration, helping to provide funding to hospitalists at Charity.
Please join us in congratulating all of this year’s outstanding award winners.
SHM presented its 2005 national awards of excellence to four hospitalists whose work and research have contributed significantly to hospital medicine and to the betterment of hospital care across America. The award winners, who were recognized at the SHM annual meeting in Chicago, included:
- Sunil Kripalani, MD, MSc, assistant professor, Division of General Medicine, Emory University School of Medicine, and attending physician and assistant director for research, Hospitalist Program, Grady Memorial Hospital, both in Atlanta, GA– recipient of Young Investigator Award.
- Shaun Frost, MD, FACP, assistant professor of Medicine, University of Minnesota Medical School, and hospitalist, HealthPartners Medical Group and Clinics, Regions Hospital, St Paul, MN– recipient of Clinical Excellence Award.
- Joseph Ming Wah Li, MD, hospitalist and director of the Hospital Medicine section, Beth Israel Deaconess Medical Center, Boston, MA– recipient of Outstanding Service in Hospital Medicine Award.
- Jeff Wiese, MD, associate professor of medicine, associate chairman of medicine, director of the Internal Medicine Residency Program, Tulane University Health Sciences Center, and chief of medicine, Medical Center of Louisiana at New Orleans and Charity Hospital, New Orleans, LA– recipient of Excellence in Teaching Award.
Dr. Kripalani has established himself as one of the leading investigators in the field of patient literacy and its impact on health outcomes. He has been the recipient of more than $1 million in grant funding, including a prestigious K23 Patient Oriented Research Career Development Award from the National Institutes of Health (NIH) to examine the relationship between health literacy and medication adherence after hospital discharge. He is currently the principal investigator on a randomized trial of two low literacy interventions designed to improve medication adherence among patients with coronary heart disease, funded by the American Heart Association. In addition, through a Pfizer Health Literacy Scholar Award, he has established a training program to improve physician communication with low literacy patients.
Dr. Kripalani has authored over 20 scientific and educational publications, including articles in the Journal of the American Medical Association, Journal of General Internal Medicine, and American Journal of Preventive Medicine. He serves as a reviewer for several prominent medical journals and has reviewed grants for the NIH. Dr. Kripalani has lectured at the Centers for Disease Control and Prevention, Georgia Hospital Association, SHM, and Society of General Internal Medicine (SGIM), where he coordinates the health literacy interest group. He is also serving as an associate editor of the upcoming book, Hospital Medicine Secrets, and coeditor of an upcoming special issue on health literacy for the Journal of General Internal Medicine.
In addition to these activities, Dr. Kripalani has proven himself a dedicated champion of SHM, contributing substantial time to research efforts at SHM, including the SHM Research Committee, Continuity of Care Task Force, Abstract Committee, Advisory Board Young Hospitalists Section, and the research section of SHM’s The Hospitalist publication.
After graduating summa cum laude from Rice University in 1993 with a BA in Psychology, Dr. Kripalani received an MD with honors from Baylor College of Medicine in 1997. He completed his residency in Internal Medicine at Emory University in Atlanta in 2000, where he also completed one of the nation’s first Hospital Medicine Fellowships, including a Master of Science in Clinical Research.
Dr. Frost has dedicated himself to the advancement of clinical knowledge through clinical teaching and scientific publication. He is a member of the Regions Hospital Palliative Care Service and Patient Safety Committee, was a lead participant in a “Lean” implementation team on inpatient testing results, and was selected as the leafter of Regions Hospital “Best Care, Best Experience” work team on provider support. He is also currently participating in the development and implementation of inpatient “Prepared Practice Teams,” a model of multidisciplinary rounding to enhance communication among physicians, nurses, case managers, social workers, and pharmacists.
A teaching faculty member of the University of Minnesota Medical School, he is highly regarded by residents and medical students, and has been instrumental in developing curricula in perioperative medicine for residents to improve the systems of surgical care through education.
Dr. Frost is a frequent lecturer on topics ranging from perioperative medicine to venous thromboembolism and has been published in: Annals of Internal Medicine, JAMA, Medical Clinics of North America, Mayo Clinic Proceedings, Cleveland Clinic Journal of Medicine, and The Hospitalist. He currently is lead investigator for a trial on preoperative medication administration.
Dr. Frost has demonstrated consistent leadership within SHM. He is regarded as the definitive resource in local chapter development due to his work in the SHM Lake Erie Chapter, where he was founder and president. He also is credited with establishing the very first formal chapter of SHM. His vision for the future of chapter activities – including community service and a national recognition program – resulted in a Membership Committee task force on chapter development. As a leader in the Midwest SHM region, Dr. Frost was named a Councilor to the SHM Midwest Council. His outstanding performance led to his assuming the chair of the Council in 2004. Dr. Frost is also recognized as a subject matter expert in biomedical ethics, serving consecutive terms on the Ethics Committee as well.
Dr. Frost earned his MD at the University of Texas Southwestern Medical School in Dallas as an AOA graduate, and completed his residency in Internal Medicine there. From 1998 through 2004, as a hospitalist at Cleveland Clinic Foundation, he was a contributor to the development, maturation, and operation of its hospital medicine model of care.
Dr. Li was the first hospitalist at the Beth Israel Deaconess Hospital Medicine Program in 1998. There he helped define the role of an academic hospitalist through clinical work, teaching, and service on countless committees and hospital initiatives. He quickly distinguished himself and was made associate chief of the HCA/ACOVE medical teaching
firm and, more recently, director of the BIDMC Hospital Medicine Program. A key focus for Dr. Li was broadening the Hospital Medicine Program at BIDMC. Under his guidance, the program grew to eleven hospitalists that account for over 50% of all general medicine admissions and over 50% of teaching attending months on the medical service. He also developed a system that allowed staff to provide 24/7 seamless coverage and created a website of referring physicians. He initiated new clinical programs and working arrangements for the hospitalist team, and helped institute a program to staff a local hospital with Beth Israel Deaconess hospitalists.
Dr. Li’s advocacy for Hospital Medicine did not stop at the doors of BIDMC, however. He was a co-developer of the first Harvard Medical School CME course on the emerging role of hospital medicine, and was the cofounder of the Boston Area Hospitalists and the SHM Northeast Regional Chapter of hospitalists. A charter member of SHM, he co-directed the first SHM annual northeastern regional meeting in 2001. He currently is a member of the SHM Education Committee, Annual Meeting Committee, and Membership Committee Task Force.
A nationally recognized expert in hospital medicine, Dr. Li lectures extensively and has testified on hearings dealing with mandatory hospitalist programs. He has published numerous articles in Critical Pathways in Cardiology, WebMD, Infectious Diseases in Clinical Practice, Current Opinion in Pulmonary Medicine, and Medscape.com, to name a few.
After earning his MD from the University of Oklahoma in Oklahoma City in 1994, Dr. Li did his residency at New England Deaconess Hospital before becoming chief medical resident at Beth Israel Deaconess Medical Center.
Dr. Wiese has received 21 awards for teaching over the last five years, including six from the University of California at San Francisco, where he started his career in 1998 as a clinical instructor. Since joining Tulane University in 2000, he has earned 16 teaching awards, including the prestigious all Tulane Faculty of the Year Award (twice) and the Virginia Furrow Award for Innovation in Medical Education. On the clinical wards, he has twice won Attending of the Year honors, and his Professor Rounds are routinely rated among the best.
Dr. Wiese designed numerous innovative curriculums. As a result of his clinical diagnosis innovations, the Clinical Diagnosis scores at Tulane increased from the 46th percentile to the 80th and 82nd percentile, with 10% of the 2004 class scoring in the top percentile in the nation. As a result of his restructuring of core curriculum to emphasize rational, evidenced based medical decision making, Tulane’s internal medicine program recently went the highest on its match list in the past 20 years. And through Dr. Wiese’s pyramid mentor system, Tulane Internal Medicine presented more regional and national presentations than any residency program in the country.
Dr. Wiese has written over 50 articles, books, or book chapters, is assistant editor for two educational textbooks and a reviewer for six national journals, has authored two textbooks, and is on the editorial board for a monthly publication. As an active SHM member, he has served on the Education Committee, Southern SHM Committee, and was program director for SHM’s Intensive Care Pre-course.
Dr. Wiese received his MD from Johns Hopkins School of Medicine in 1995. He completed his residency and chief residency training in Internal Medicine at the University of California at San Francisco, where he also completed a fellowship in General Internal Medicine with a focus on Hospitalist Medicine. He joined Tulane in 2000, after being recruited to start a hospitalist system at the Medical Center of Louisiana at New Orleans (Charity Hospital). His hospitalist proposal was accepted by the state and hospital administration, helping to provide funding to hospitalists at Charity.
Please join us in congratulating all of this year’s outstanding award winners.
SHM Inducts New Officers at Annual Meeting
Steven Pantilat, MD, assumed the role of SHM’s new president at the 2005 Annual Meeting, along with a slate of other newly elected officers, including: Mary Jo Gorman, MD, MBA, president elect, William Atchley, MD, FACP, treasurer, and Lisa Kettering, MD, FACP, secretary. “These are exciting times of growth for SHM and hospital medicine,” said Jeanne Huddleston, MD, SHM’s immediate past president. “So we’re thrilled to bring on a team of extraordinary leaders who have long demonstrated their commitment to our organization’s goals and to the hospital medicine movement.”
“I’m extremely pleased to have this opportunity to lead SHM at this critical juncture,” said new President Dr. Pantilat. “Hospitalists are leading breakthrough initiatives around the country in areas such as patient safety, hospital leadership, and quality of care. But there are other important areas where we can make a difference. My goals for SHM this year are twofold. First, I plan to promote research in hospital medicine to discover how best to improve the quality of care for hospitalized patients. Second, I have appointed a Palliative Care Task force to examine how hospitalists can improve the care of patients with serious and life threatening illnesses.”
Dr. Pantilat is an associate professor of clinical medicine in the Department of Medicine at the University of California, San Francisco. He also is a hospitalist attending on the medical service and is the founding director of both the Palliative Care Consult Service and the Comfort Care Suites, a 2-bed inpatient palliative care unit at UCSF. Dr. Pantilat is a full-time faculty member in the Program in Medical Ethics at UCSF, a faculty scholar of the Soros Foundation Project on Death in America and a recipient of a research career development award from the National Institute on Aging. Dr. Pantilat is also the director of the UCSF Palliative Care leadership Center, which trains teams from hospitals across the country to develop and implement palliative care services in their own institutions.
In addition to his research on improving palliative care, Dr. Pantilat teaches palliative care at UCSF and is coeditor of an end of life care series in the Journal of the American Medical Association (JAMA) titled “Perspectives on Care at the Close of Life.”
A charter member of SHM, Dr. Pantilat has served in numerous leadership positions through the years, including as first chair of the SHM Ethics Committee, member of the Board of Directors and treasurer.
SHM President-elect Mary Jo Gorman, MD, MBA, is chief medical officer for IPC The Hospitalist Company, a private practice hospital medicine company. There she works with more than 300 physicians in developing programs and strategies that enhance clinical performance and drive the delivery system towards more efficient care and greater patient satisfaction. She also oversees IPC’s physician training, mentoring and retention programs, as well as IPC’s call center nurses, healthcare services and
clinical studies.
Dr. Gorman has been a practicing hospitalist since 1997, when she founded the first hospital medicine practice in St. Louis, MO. Her original group merged into IPC in January 1999 and since that time has grown to become the dominant hospital medicine group in the city. Dr. Gorman is a charter member of SHM and has served on multiple committees, including chairman of the Public Policy Committee.
New Treasurer William Atchley, MD is the director of the hospital medicine service at Sentara Careplex Hospital in Hampton, VA. He has been a practicing hospitalist since 1995, when he founded the hospital medicine practice for Sentara Medical Group in Norfolk, VA. The program grew to provide coverage to three local hospitals. He also helped to create the Division of Hospital Medicine in Sentara Medical Group. In 2002 he led Sentara Medical Group to start the hospital medicine service at Sentara Careplex Hospital. At that time he founded Peninsula Inpatient Medicine Specialists, which now has eight hospitalists.
Dr. Atchley is a charter member of SHM and had previously served as secretary since 2003. He also serves the organization as chair of the Awards Committee and a member of the Finance Committee and the Southern Regional Council. He previously served on the Benchmarks and Compensation Task Force, Membership Committee and the Annual Session Planning Committee.
Secretary Lisa Kettering, MD, FACP, is associate director of Inpatient Services for the Department of Graduate Medical Education, Internal Medicine, at Exempla Saint Joseph Hospital in Denver. She also serves as director of the Evidence Based Medicine Curriculum for the Exempla Saint Joseph Hospital Internal Medicine residency program and is an assistant clinical professor in the Department of Internal Medicine at the University of Colorado School of Medicine.
Dr. Kettering is a charter member of SHM and was elected to the board in 2003. She has also served as chair of the Membership Committee from 20032005, and course director for the 6th Annual Meeting. She has served on the Awards Committee, Nominations Committee, Annual Meeting Planning Committee, and the Education Committee. She currently is a member of the Western Regional Council and is president of the Rocky Mountain Chapter of SHM.
Please join us in congratulating all the new officers.
Steven Pantilat, MD, assumed the role of SHM’s new president at the 2005 Annual Meeting, along with a slate of other newly elected officers, including: Mary Jo Gorman, MD, MBA, president elect, William Atchley, MD, FACP, treasurer, and Lisa Kettering, MD, FACP, secretary. “These are exciting times of growth for SHM and hospital medicine,” said Jeanne Huddleston, MD, SHM’s immediate past president. “So we’re thrilled to bring on a team of extraordinary leaders who have long demonstrated their commitment to our organization’s goals and to the hospital medicine movement.”
“I’m extremely pleased to have this opportunity to lead SHM at this critical juncture,” said new President Dr. Pantilat. “Hospitalists are leading breakthrough initiatives around the country in areas such as patient safety, hospital leadership, and quality of care. But there are other important areas where we can make a difference. My goals for SHM this year are twofold. First, I plan to promote research in hospital medicine to discover how best to improve the quality of care for hospitalized patients. Second, I have appointed a Palliative Care Task force to examine how hospitalists can improve the care of patients with serious and life threatening illnesses.”
Dr. Pantilat is an associate professor of clinical medicine in the Department of Medicine at the University of California, San Francisco. He also is a hospitalist attending on the medical service and is the founding director of both the Palliative Care Consult Service and the Comfort Care Suites, a 2-bed inpatient palliative care unit at UCSF. Dr. Pantilat is a full-time faculty member in the Program in Medical Ethics at UCSF, a faculty scholar of the Soros Foundation Project on Death in America and a recipient of a research career development award from the National Institute on Aging. Dr. Pantilat is also the director of the UCSF Palliative Care leadership Center, which trains teams from hospitals across the country to develop and implement palliative care services in their own institutions.
In addition to his research on improving palliative care, Dr. Pantilat teaches palliative care at UCSF and is coeditor of an end of life care series in the Journal of the American Medical Association (JAMA) titled “Perspectives on Care at the Close of Life.”
A charter member of SHM, Dr. Pantilat has served in numerous leadership positions through the years, including as first chair of the SHM Ethics Committee, member of the Board of Directors and treasurer.
SHM President-elect Mary Jo Gorman, MD, MBA, is chief medical officer for IPC The Hospitalist Company, a private practice hospital medicine company. There she works with more than 300 physicians in developing programs and strategies that enhance clinical performance and drive the delivery system towards more efficient care and greater patient satisfaction. She also oversees IPC’s physician training, mentoring and retention programs, as well as IPC’s call center nurses, healthcare services and
clinical studies.
Dr. Gorman has been a practicing hospitalist since 1997, when she founded the first hospital medicine practice in St. Louis, MO. Her original group merged into IPC in January 1999 and since that time has grown to become the dominant hospital medicine group in the city. Dr. Gorman is a charter member of SHM and has served on multiple committees, including chairman of the Public Policy Committee.
New Treasurer William Atchley, MD is the director of the hospital medicine service at Sentara Careplex Hospital in Hampton, VA. He has been a practicing hospitalist since 1995, when he founded the hospital medicine practice for Sentara Medical Group in Norfolk, VA. The program grew to provide coverage to three local hospitals. He also helped to create the Division of Hospital Medicine in Sentara Medical Group. In 2002 he led Sentara Medical Group to start the hospital medicine service at Sentara Careplex Hospital. At that time he founded Peninsula Inpatient Medicine Specialists, which now has eight hospitalists.
Dr. Atchley is a charter member of SHM and had previously served as secretary since 2003. He also serves the organization as chair of the Awards Committee and a member of the Finance Committee and the Southern Regional Council. He previously served on the Benchmarks and Compensation Task Force, Membership Committee and the Annual Session Planning Committee.
Secretary Lisa Kettering, MD, FACP, is associate director of Inpatient Services for the Department of Graduate Medical Education, Internal Medicine, at Exempla Saint Joseph Hospital in Denver. She also serves as director of the Evidence Based Medicine Curriculum for the Exempla Saint Joseph Hospital Internal Medicine residency program and is an assistant clinical professor in the Department of Internal Medicine at the University of Colorado School of Medicine.
Dr. Kettering is a charter member of SHM and was elected to the board in 2003. She has also served as chair of the Membership Committee from 20032005, and course director for the 6th Annual Meeting. She has served on the Awards Committee, Nominations Committee, Annual Meeting Planning Committee, and the Education Committee. She currently is a member of the Western Regional Council and is president of the Rocky Mountain Chapter of SHM.
Please join us in congratulating all the new officers.
Steven Pantilat, MD, assumed the role of SHM’s new president at the 2005 Annual Meeting, along with a slate of other newly elected officers, including: Mary Jo Gorman, MD, MBA, president elect, William Atchley, MD, FACP, treasurer, and Lisa Kettering, MD, FACP, secretary. “These are exciting times of growth for SHM and hospital medicine,” said Jeanne Huddleston, MD, SHM’s immediate past president. “So we’re thrilled to bring on a team of extraordinary leaders who have long demonstrated their commitment to our organization’s goals and to the hospital medicine movement.”
“I’m extremely pleased to have this opportunity to lead SHM at this critical juncture,” said new President Dr. Pantilat. “Hospitalists are leading breakthrough initiatives around the country in areas such as patient safety, hospital leadership, and quality of care. But there are other important areas where we can make a difference. My goals for SHM this year are twofold. First, I plan to promote research in hospital medicine to discover how best to improve the quality of care for hospitalized patients. Second, I have appointed a Palliative Care Task force to examine how hospitalists can improve the care of patients with serious and life threatening illnesses.”
Dr. Pantilat is an associate professor of clinical medicine in the Department of Medicine at the University of California, San Francisco. He also is a hospitalist attending on the medical service and is the founding director of both the Palliative Care Consult Service and the Comfort Care Suites, a 2-bed inpatient palliative care unit at UCSF. Dr. Pantilat is a full-time faculty member in the Program in Medical Ethics at UCSF, a faculty scholar of the Soros Foundation Project on Death in America and a recipient of a research career development award from the National Institute on Aging. Dr. Pantilat is also the director of the UCSF Palliative Care leadership Center, which trains teams from hospitals across the country to develop and implement palliative care services in their own institutions.
In addition to his research on improving palliative care, Dr. Pantilat teaches palliative care at UCSF and is coeditor of an end of life care series in the Journal of the American Medical Association (JAMA) titled “Perspectives on Care at the Close of Life.”
A charter member of SHM, Dr. Pantilat has served in numerous leadership positions through the years, including as first chair of the SHM Ethics Committee, member of the Board of Directors and treasurer.
SHM President-elect Mary Jo Gorman, MD, MBA, is chief medical officer for IPC The Hospitalist Company, a private practice hospital medicine company. There she works with more than 300 physicians in developing programs and strategies that enhance clinical performance and drive the delivery system towards more efficient care and greater patient satisfaction. She also oversees IPC’s physician training, mentoring and retention programs, as well as IPC’s call center nurses, healthcare services and
clinical studies.
Dr. Gorman has been a practicing hospitalist since 1997, when she founded the first hospital medicine practice in St. Louis, MO. Her original group merged into IPC in January 1999 and since that time has grown to become the dominant hospital medicine group in the city. Dr. Gorman is a charter member of SHM and has served on multiple committees, including chairman of the Public Policy Committee.
New Treasurer William Atchley, MD is the director of the hospital medicine service at Sentara Careplex Hospital in Hampton, VA. He has been a practicing hospitalist since 1995, when he founded the hospital medicine practice for Sentara Medical Group in Norfolk, VA. The program grew to provide coverage to three local hospitals. He also helped to create the Division of Hospital Medicine in Sentara Medical Group. In 2002 he led Sentara Medical Group to start the hospital medicine service at Sentara Careplex Hospital. At that time he founded Peninsula Inpatient Medicine Specialists, which now has eight hospitalists.
Dr. Atchley is a charter member of SHM and had previously served as secretary since 2003. He also serves the organization as chair of the Awards Committee and a member of the Finance Committee and the Southern Regional Council. He previously served on the Benchmarks and Compensation Task Force, Membership Committee and the Annual Session Planning Committee.
Secretary Lisa Kettering, MD, FACP, is associate director of Inpatient Services for the Department of Graduate Medical Education, Internal Medicine, at Exempla Saint Joseph Hospital in Denver. She also serves as director of the Evidence Based Medicine Curriculum for the Exempla Saint Joseph Hospital Internal Medicine residency program and is an assistant clinical professor in the Department of Internal Medicine at the University of Colorado School of Medicine.
Dr. Kettering is a charter member of SHM and was elected to the board in 2003. She has also served as chair of the Membership Committee from 20032005, and course director for the 6th Annual Meeting. She has served on the Awards Committee, Nominations Committee, Annual Meeting Planning Committee, and the Education Committee. She currently is a member of the Western Regional Council and is president of the Rocky Mountain Chapter of SHM.
Please join us in congratulating all the new officers.
Diabetic retinopathy: Treating systemic conditions aggressively can save sight
Intimate partner violence
Imatinib for Kaposi Sarcoma: A Mixed Blessing
Liability when patients die after treatment
Can a psychiatrist legally and safely prescribe medication to reduce pain and, if so, when? How can a psychiatrist avoid a negligence charge if the patient commits suicide after discharge?
This article offers answers to those questions.
Methadone prescription for pain blamed for overdose death
Richmond (VA) Circuit Court
The patient had been receiving psychiatric treatment for approximately 1 year and also sought care for chronic pain during that time. The psychiatrist prescribed a pain medication and advised the patient to find a physician specializing in pain management, which the patient did.
Later, the patient and her husband told the psychiatrist during an emergency visit that no other physician was willing to treat her pain and requested pain medication. The psychiatrist viewed this request as possible drug-seeking behavior but considered the incident a crisis. She gave the patient a 2-week prescription of methadone for both pain and withdrawal.
Five days later, the patient’s husband found her dead; her autopsy showed a high level of methadone and two other medications.
The plaintiff’s estate claimed that the psychiatrist was negligent and that the patient died from methadone intoxication. The defense argued that the prescription was appropriate, and that amitriptyline, which the patient also had been taking, caused the sudden cardiac arrest that led to her death.
- The jury found for the defense.
Dr. Grant’s observations
A physician can prescribe any medication for a legitimate purpose. When prescribing outside your psychiatric expertise—such as medication for this patient’s chronic pain—the following recommendations can help you prevent a negligence claim:
• Document your physical examination. Assess the physical and psychological aspects of a pain condition before treating it. Then document the condition and the rationale behind your treatment choice based on the medical assessment.
If you are uncomfortable examining and diagnosing a medical condition, avoid prescribing pain medication. Instead, refer the patient to a physician specializing in pain management.
• If prescribing pain medication, document the type, location, and severity of pain. Also document your discussion of pain management options with the patient, and ask about previous pain-reduction interventions.
• Assess type, quantity, and frequency of prescription drug use as well as illicit drug and alcohol use. Order urine and serum toxicology tests if you suspect or need to document substance abuse.
As in this case, refer patients with chronic pain to their primary care physicians or to another specialist for appropriate pain management. Pain reduction may require psychological and behavioral interventions (such as cognitivebehavioral therapy, relaxation therapy, hypnosis, biofeedback, stress management, educating patients and their families about pain management) as well as physical therapy, anesthetic treatments, or surgical evaluation.1
• Assessing pain in the ER. A different level of chronic pain assessment may be necessary in the emergency room, and the law recognizes that resources—such as information from other providers—are limited in the ER.2 In this case, the patient reported that no one was willing to treat her, and the psychiatrist feared she was seeking a prescription for illicit use. In such cases, consider contacting the patient’s previous pain specialist or hospitalizing the patient if you fear he or she will go into withdrawal.
Plaintiff: Premature discharge caused alcohol-related suicide by drowning
Lucas County (OH) Common Pleas Court
The patient, age 41, had a longstanding, treatment-refractory alcohol use disorder.
He was admitted to the hospital after he was dismissed from a halfway house; upon admission, his blood alcohol level was 0.41%.
When assessed by a psychiatrist several days later, the patient showed suicidal behavior. The psychiatrist evaluated him three additional times. After the final visit, the patient renounced suicide, and the psychiatrist decided that he had improved. The patient’s discharge was planned—with aftercare housing and outpatient program particiption arranged—and he left the hospital in a taxi.
Three days later, the patient was found dead in a creek. An autopsy showed that the patient died by drowning and that his blood alcohol level was 0.32%. The death was ruled a suicide secondary to excessive alcohol consumption.
The plaintiff—the patient’s estate —charged that the psychiatrist was negligent in discharging the patient from the hospital and claimed that lack of a post-discharge recovery plan made the suicide likely.
The defense argued that the patient’s history of suicide attempts was known and that a discharge plan—which included housing and participation in an outpatient program—was in place before he was discharged.
- The jury found for the defense.
Dr. Grant’s observations
Many factors associated with managed care—such as cost-containment policies that shorten hospital stays, shorter visits that limit opportunity to develop a therapeutic alliance with patients, and limited ability to communicate with patients—have increased the risk of malpractice suits alleging premature discharge of patients who later kill themselves.3
To avoid such a suit:4
• Document the patient’s risk factors for suicide as well as specific suicidal thoughts and methods expressed, extent of planning and action taken toward a suicide attempt, access to means, and response to prior therapeutic interventions.
• Explain in your notes why specific risk factors were ruled out. This supports the conclusion that you properly assessed the patient.
• Obtain a proper history of the patient’s current illness. Understanding how a patient’s substance use is affecting his mood may influence plans for care after discharge.
• Do not rely solely on a patient’s statements about suicidality. Document information from other sources (old records, previous providers, or family members) and note that you tried to contact collateral sources or get permission to talk with the patient’s family
• Arrange outpatient services that focus on substance addiction (for example, support groups such as Alcoholics Anonymous [see], and therapy with an addictions specialist). Schedule timely visits for therapy and medication management. A medical follow-up may be needed if health concerns are associated with a mental health issue. A patient may need to be placed in a sober house or residential facility if he cannot stay sober on his own.
1. Bronheim HE, Fulop G, Kunkel EJ, et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. Psychosomatics 1998;39:S8-S30.
2. Gutheil TG, Appelbaum PS. Clinical Handbook of Psychiatry and the Law (3rd ed). Philadelphia: Lippincott Williams & Wilkins, 2000.
3. Simon RI. Psychiatrists’ duties in discharging sicker and potentially violent inpatients in the managed care era. Psychiatr Serv 1998;49:62-7.
4. Simpson S, Stacy M. Avoiding the malpractice snare: documenting suicide risk assessment. J Psychiatr Pract 2004;10:185-9.
Can a psychiatrist legally and safely prescribe medication to reduce pain and, if so, when? How can a psychiatrist avoid a negligence charge if the patient commits suicide after discharge?
This article offers answers to those questions.
Methadone prescription for pain blamed for overdose death
Richmond (VA) Circuit Court
The patient had been receiving psychiatric treatment for approximately 1 year and also sought care for chronic pain during that time. The psychiatrist prescribed a pain medication and advised the patient to find a physician specializing in pain management, which the patient did.
Later, the patient and her husband told the psychiatrist during an emergency visit that no other physician was willing to treat her pain and requested pain medication. The psychiatrist viewed this request as possible drug-seeking behavior but considered the incident a crisis. She gave the patient a 2-week prescription of methadone for both pain and withdrawal.
Five days later, the patient’s husband found her dead; her autopsy showed a high level of methadone and two other medications.
The plaintiff’s estate claimed that the psychiatrist was negligent and that the patient died from methadone intoxication. The defense argued that the prescription was appropriate, and that amitriptyline, which the patient also had been taking, caused the sudden cardiac arrest that led to her death.
- The jury found for the defense.
Dr. Grant’s observations
A physician can prescribe any medication for a legitimate purpose. When prescribing outside your psychiatric expertise—such as medication for this patient’s chronic pain—the following recommendations can help you prevent a negligence claim:
• Document your physical examination. Assess the physical and psychological aspects of a pain condition before treating it. Then document the condition and the rationale behind your treatment choice based on the medical assessment.
If you are uncomfortable examining and diagnosing a medical condition, avoid prescribing pain medication. Instead, refer the patient to a physician specializing in pain management.
• If prescribing pain medication, document the type, location, and severity of pain. Also document your discussion of pain management options with the patient, and ask about previous pain-reduction interventions.
• Assess type, quantity, and frequency of prescription drug use as well as illicit drug and alcohol use. Order urine and serum toxicology tests if you suspect or need to document substance abuse.
As in this case, refer patients with chronic pain to their primary care physicians or to another specialist for appropriate pain management. Pain reduction may require psychological and behavioral interventions (such as cognitivebehavioral therapy, relaxation therapy, hypnosis, biofeedback, stress management, educating patients and their families about pain management) as well as physical therapy, anesthetic treatments, or surgical evaluation.1
• Assessing pain in the ER. A different level of chronic pain assessment may be necessary in the emergency room, and the law recognizes that resources—such as information from other providers—are limited in the ER.2 In this case, the patient reported that no one was willing to treat her, and the psychiatrist feared she was seeking a prescription for illicit use. In such cases, consider contacting the patient’s previous pain specialist or hospitalizing the patient if you fear he or she will go into withdrawal.
Plaintiff: Premature discharge caused alcohol-related suicide by drowning
Lucas County (OH) Common Pleas Court
The patient, age 41, had a longstanding, treatment-refractory alcohol use disorder.
He was admitted to the hospital after he was dismissed from a halfway house; upon admission, his blood alcohol level was 0.41%.
When assessed by a psychiatrist several days later, the patient showed suicidal behavior. The psychiatrist evaluated him three additional times. After the final visit, the patient renounced suicide, and the psychiatrist decided that he had improved. The patient’s discharge was planned—with aftercare housing and outpatient program particiption arranged—and he left the hospital in a taxi.
Three days later, the patient was found dead in a creek. An autopsy showed that the patient died by drowning and that his blood alcohol level was 0.32%. The death was ruled a suicide secondary to excessive alcohol consumption.
The plaintiff—the patient’s estate —charged that the psychiatrist was negligent in discharging the patient from the hospital and claimed that lack of a post-discharge recovery plan made the suicide likely.
The defense argued that the patient’s history of suicide attempts was known and that a discharge plan—which included housing and participation in an outpatient program—was in place before he was discharged.
- The jury found for the defense.
Dr. Grant’s observations
Many factors associated with managed care—such as cost-containment policies that shorten hospital stays, shorter visits that limit opportunity to develop a therapeutic alliance with patients, and limited ability to communicate with patients—have increased the risk of malpractice suits alleging premature discharge of patients who later kill themselves.3
To avoid such a suit:4
• Document the patient’s risk factors for suicide as well as specific suicidal thoughts and methods expressed, extent of planning and action taken toward a suicide attempt, access to means, and response to prior therapeutic interventions.
• Explain in your notes why specific risk factors were ruled out. This supports the conclusion that you properly assessed the patient.
• Obtain a proper history of the patient’s current illness. Understanding how a patient’s substance use is affecting his mood may influence plans for care after discharge.
• Do not rely solely on a patient’s statements about suicidality. Document information from other sources (old records, previous providers, or family members) and note that you tried to contact collateral sources or get permission to talk with the patient’s family
• Arrange outpatient services that focus on substance addiction (for example, support groups such as Alcoholics Anonymous [see], and therapy with an addictions specialist). Schedule timely visits for therapy and medication management. A medical follow-up may be needed if health concerns are associated with a mental health issue. A patient may need to be placed in a sober house or residential facility if he cannot stay sober on his own.
Can a psychiatrist legally and safely prescribe medication to reduce pain and, if so, when? How can a psychiatrist avoid a negligence charge if the patient commits suicide after discharge?
This article offers answers to those questions.
Methadone prescription for pain blamed for overdose death
Richmond (VA) Circuit Court
The patient had been receiving psychiatric treatment for approximately 1 year and also sought care for chronic pain during that time. The psychiatrist prescribed a pain medication and advised the patient to find a physician specializing in pain management, which the patient did.
Later, the patient and her husband told the psychiatrist during an emergency visit that no other physician was willing to treat her pain and requested pain medication. The psychiatrist viewed this request as possible drug-seeking behavior but considered the incident a crisis. She gave the patient a 2-week prescription of methadone for both pain and withdrawal.
Five days later, the patient’s husband found her dead; her autopsy showed a high level of methadone and two other medications.
The plaintiff’s estate claimed that the psychiatrist was negligent and that the patient died from methadone intoxication. The defense argued that the prescription was appropriate, and that amitriptyline, which the patient also had been taking, caused the sudden cardiac arrest that led to her death.
- The jury found for the defense.
Dr. Grant’s observations
A physician can prescribe any medication for a legitimate purpose. When prescribing outside your psychiatric expertise—such as medication for this patient’s chronic pain—the following recommendations can help you prevent a negligence claim:
• Document your physical examination. Assess the physical and psychological aspects of a pain condition before treating it. Then document the condition and the rationale behind your treatment choice based on the medical assessment.
If you are uncomfortable examining and diagnosing a medical condition, avoid prescribing pain medication. Instead, refer the patient to a physician specializing in pain management.
• If prescribing pain medication, document the type, location, and severity of pain. Also document your discussion of pain management options with the patient, and ask about previous pain-reduction interventions.
• Assess type, quantity, and frequency of prescription drug use as well as illicit drug and alcohol use. Order urine and serum toxicology tests if you suspect or need to document substance abuse.
As in this case, refer patients with chronic pain to their primary care physicians or to another specialist for appropriate pain management. Pain reduction may require psychological and behavioral interventions (such as cognitivebehavioral therapy, relaxation therapy, hypnosis, biofeedback, stress management, educating patients and their families about pain management) as well as physical therapy, anesthetic treatments, or surgical evaluation.1
• Assessing pain in the ER. A different level of chronic pain assessment may be necessary in the emergency room, and the law recognizes that resources—such as information from other providers—are limited in the ER.2 In this case, the patient reported that no one was willing to treat her, and the psychiatrist feared she was seeking a prescription for illicit use. In such cases, consider contacting the patient’s previous pain specialist or hospitalizing the patient if you fear he or she will go into withdrawal.
Plaintiff: Premature discharge caused alcohol-related suicide by drowning
Lucas County (OH) Common Pleas Court
The patient, age 41, had a longstanding, treatment-refractory alcohol use disorder.
He was admitted to the hospital after he was dismissed from a halfway house; upon admission, his blood alcohol level was 0.41%.
When assessed by a psychiatrist several days later, the patient showed suicidal behavior. The psychiatrist evaluated him three additional times. After the final visit, the patient renounced suicide, and the psychiatrist decided that he had improved. The patient’s discharge was planned—with aftercare housing and outpatient program particiption arranged—and he left the hospital in a taxi.
Three days later, the patient was found dead in a creek. An autopsy showed that the patient died by drowning and that his blood alcohol level was 0.32%. The death was ruled a suicide secondary to excessive alcohol consumption.
The plaintiff—the patient’s estate —charged that the psychiatrist was negligent in discharging the patient from the hospital and claimed that lack of a post-discharge recovery plan made the suicide likely.
The defense argued that the patient’s history of suicide attempts was known and that a discharge plan—which included housing and participation in an outpatient program—was in place before he was discharged.
- The jury found for the defense.
Dr. Grant’s observations
Many factors associated with managed care—such as cost-containment policies that shorten hospital stays, shorter visits that limit opportunity to develop a therapeutic alliance with patients, and limited ability to communicate with patients—have increased the risk of malpractice suits alleging premature discharge of patients who later kill themselves.3
To avoid such a suit:4
• Document the patient’s risk factors for suicide as well as specific suicidal thoughts and methods expressed, extent of planning and action taken toward a suicide attempt, access to means, and response to prior therapeutic interventions.
• Explain in your notes why specific risk factors were ruled out. This supports the conclusion that you properly assessed the patient.
• Obtain a proper history of the patient’s current illness. Understanding how a patient’s substance use is affecting his mood may influence plans for care after discharge.
• Do not rely solely on a patient’s statements about suicidality. Document information from other sources (old records, previous providers, or family members) and note that you tried to contact collateral sources or get permission to talk with the patient’s family
• Arrange outpatient services that focus on substance addiction (for example, support groups such as Alcoholics Anonymous [see], and therapy with an addictions specialist). Schedule timely visits for therapy and medication management. A medical follow-up may be needed if health concerns are associated with a mental health issue. A patient may need to be placed in a sober house or residential facility if he cannot stay sober on his own.
1. Bronheim HE, Fulop G, Kunkel EJ, et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. Psychosomatics 1998;39:S8-S30.
2. Gutheil TG, Appelbaum PS. Clinical Handbook of Psychiatry and the Law (3rd ed). Philadelphia: Lippincott Williams & Wilkins, 2000.
3. Simon RI. Psychiatrists’ duties in discharging sicker and potentially violent inpatients in the managed care era. Psychiatr Serv 1998;49:62-7.
4. Simpson S, Stacy M. Avoiding the malpractice snare: documenting suicide risk assessment. J Psychiatr Pract 2004;10:185-9.
1. Bronheim HE, Fulop G, Kunkel EJ, et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. Psychosomatics 1998;39:S8-S30.
2. Gutheil TG, Appelbaum PS. Clinical Handbook of Psychiatry and the Law (3rd ed). Philadelphia: Lippincott Williams & Wilkins, 2000.
3. Simon RI. Psychiatrists’ duties in discharging sicker and potentially violent inpatients in the managed care era. Psychiatr Serv 1998;49:62-7.
4. Simpson S, Stacy M. Avoiding the malpractice snare: documenting suicide risk assessment. J Psychiatr Pract 2004;10:185-9.
ADHD or bipolar disorder? Age-specific manic symptoms are key
Knowing what to look for can help you differentiate between pediatric bipolar disorder and attention-deficit/hyperactivity disorder (ADHD):
- Bipolar disorder is a problem with mood. Children with bipolar mania are elated and/or irritable and experience mood states that appear uncontrollable.
- ADHD is a problem with cognitive functioning, including attention, distractibility, and energy level.
Mood and cognitive symptoms may overlap,1,2 but recognizing manic features is the key to distinguishing between these disorders—even when they co-occur.
We offer tips from our experience and a recent clinical trial to help you sort out the core symptoms that point to bipolar mania.
BIPOLAR CORE SYMPTOMS
Pediatric bipolar disorder is relatively rare, but children with it can experience substantial impairment and developmental delay. Intervening early with effective treatment3 can improve their quality of life, function, and prognosis.
Diagnostic criteria for type I bipolar disorder require at least one manic episode and are the same for all ages. Many clinicians and researchers have advocated adapting DSM-IV criteria for children, but we believe separate adult and pediatric criteria would confuse discussions about the same phenomena. We do agree that symptoms should be evaluated in a developmentally appropriate context, as mania can present differently across the ages (Table 1).
Mania in children and young adolescents tends to present with rapid cycling and a primarily irritable mood.4 Older adolescents and adults may present with more-distinct mood changes, with a primarily euphoric mood. Euphoric mania is less common in adults than previously thought. Forty percent to 60% of adults with bipolar disorder experience a chronic course, rather than more-discrete mood episodes.
A manic episode is an abnormally and persistently elevated (euphoria) or irritable mood that lasts at least 1 week. To satisfy DSM-IV-TR diagnostic criteria for a manic episode:
- patients with euphoria require three additional symptoms
- those who are irritable (and not euphoric) require another four symptoms.5
These symptoms must significantly impair several areas of functioning and not be caused by other mental or physical illness, including substance use or abuse. When depressive symptoms occur in the same week as mania, the mixed mania modifier is used.
Table 1
Diagnostic features of bipolar mania in adolescents vs adults
| Feature | Prepubertal and early adolescent | Older adolescent and adult |
|---|---|---|
| Initial episode | Mixed presentations predominate | Mania is more balanced between mixed and euphoric |
| Episode type | More consistently ill | Persistent/distinct episodes |
| Primary mood | Irritable | Euphoric |
| Duration | Chronic, continuous course | Weeks |
| Inter-episode functioning | Less distinct episodes | May return to baseline or deteriorate over time |
| Reality testing | Delusions (grandiosity) is common; hallucinations | More variable |
Disruptive and aggressive behavior are common and are what usually prompts parents to bring children to psychiatrists. These behaviors are not diagnostic of mania, however, and aggression has many other causes.
The threshold between a variant of normal and pathologic disruptive behavior can be difficult to establish and varies from culture to culture. Some families, for example, would allow a child to tell the parents what to do, whereas other families consider this a serious boundary violation.
Prolonged rages have been used as a proxy for mood swings. Although we agree that rages lasting >15 minutes and out-of-proportion to the circumstances may signal bipolar disorder, they are not diagnostic.
Other symptoms. Psychotic symptoms (hallucinations, delusions, disorganization) can occur in youths with bipolar disorder. Evaluation often reveals impaired social and cognitive development. Keep in mind that a child’s developmental level can affect symptom expression.
ADHD CORE SYMPTOMS
Children with ADHD often present with hyperactive, uncontrollable behaviors and academic failure. To meet DSM-IV-TR diagnostic criteria, they must show symptoms before age 7. Primary symptoms may be inattention, hyperactivity and impulsivity, or both.
ADHD is a disorder of attention and the cognitive skills related to attention, rather than a mood disorder. Children with ADHD show substantially impaired function in at least two settings (such as at home and in school), and—unlike bipolar disorder—their symptoms are persistent rather than episodic.
DIFFERENTIATING BY SYMPTOMS
When differentiating between ADHD and bipolar disorder in children, remain focused on both diagnoses’ core symptoms.
Euphoria, or elation, is a key distinguishing factor in bipolar disorder.6 Although all children are at times giddy or silly in appropriate environments—such as during slumber parties—consider a threshold of appropriateness when making a bipolar diagnosis. Families perceive the giddiness, inappropriate laughter, and elevated mood of children with mania as disturbing and inappropriate, not funny or endearing. They are often annoyed and concerned.
Children with primary ADHD do not show inappropriately elevated mood. In fact, their failures often make these children dysphoric.
Irritability is common in children with psychiatric illnesses. Manic youngsters can be very irritable most of the time. Families describe “walking on eggshells” because of these children’s touchiness. Unpredictable triggers set off explosive, prolonged tantrums that may be associated with aggression, and their mood swings are almost constant.
Children with ADHD can be irritable, but their irritability is less severe and intense than that seen in bipolar disorder. Stimulant medication “wear-off” can cause irritability in ADHD, so consider this possibility if symptoms occur mostly in the evening.
Grandiosity can be confusing to evaluate in children but is often a core symptom in bipolar disorder. All children sometimes say self-inflating things, but those with pathologic grandiosity cross the threshold into the dysfunctional belief that they are better, stronger, smarter, or more talented than others.
For example, a 7-year-old patient insisted he was the world’s best chess player and could beat anyone, including Russian chess masters. When the therapist asked him about chess, he did not know the names of the pieces or how they moved. Yet despite facing these contradictory facts, he continued to insist that he was the best.
Children with grandiosity may act inappropriately on their beliefs, such as by telling adults what to do or engaging in risky, daredevil acts with no concern for their safety or the law.
Children with ADHD are not usually grandiose. Instead, they often become demoralized and develop poor self-esteem from negative feedback about their behavior.
Decreased need for sleep is the hallmark symptom of mania that is absent in other psychiatric disorders. A true decreased need for sleep is only indicated in someone sleeping less than his or her usual cumulative hours each day, without fatigue or recuperative sleep.
Children with bipolar disorder may need 1 or more hours less sleep or deny needing sleep at all. Use age-appropriate amounts of sleep as a standard. A school age child usually averages 9 to 11 hours of sleep per night. If the patient is getting only 6 hours and is not tired, this would be a decreased need for sleep. A 24-hour sleep history can easily assess decreased need for sleep (Box).
Determine daytime fatigue by self-report or observation by parents or teachers. Then ascertain if there are periods of days with less fatigue. Many bipolar youth have a nearly continuous decreased need for sleep.
Children with ADHD often have difficulty settling at night, which delays their falling asleep. The sleep history will likely show that—once asleep—they sleep well for an appropriate amount of time or are fatigued during the day.
Decreased need for sleep is a hallmark symptom of bipolar mania. A 24-hour sleep history may help determine if a child’s irregular sleep patterns signal ADHD or bipolar disorder.
To perform the sleep history, collect time in bed and time asleep over several days, or ask the patient,
- On a typical night, not your best, not your worst:”
- When do you go to bed?
- How long does it take to fall asleep?
- Once asleep, do you wake up?
- How long are you awake?
- When do you arise in the morning?
- What is the total amount of time you are asleep?
- Do you take naps?
- Are you rested or fatigued during the day?
Pressured speech, or the need to talk excessively, is a relatively straightforward symptom. Children experiencing mania often speak so quickly and excessively that others cannot understand or interrupt them. Flight of ideas and racing thoughts are reflected in their speech.
By contrast, rapid speech by children with ADHD is related to hyperactivity. They speak too fast and often become distracted from the topic.
Racing thoughts. Children with bipolar disorder may report that their thoughts come so quickly they cannot get them out fast enough. The idea that their thoughts “need a stop-sign” suggests racing thoughts, a core bipolar symptom. Their speech can be unintelligible, with rapid changes in thought patterns, flight of ideas, and sentence fragments. Children with ADHD are energetic and quick but do not report racing thoughts.
LESS-HELPFUL SYMPTOMS
Distractibility—a core symptom of both inattentive ADHD and manic episodes—does not help differentiate the two diagnoses. The high comorbidity of ADHD with bipolar disorder increases the likelihood that the child will be easily distracted.
Multi-tasking. Increased goal-directed activity is often associated with the high energy of children with mania. They have more energy than most people, are always on the go, and engage in multiple projects or activities that may be markedly creative or unrealistic. This increased energy—combined with other hallmark manic symptoms—can lead to high-risk behaviors.
Hyperactivity in ADHD can appear similar to agitation in bipolar disorder. In both disorders, children may engage in many tasks—not finishing any of them—or appear to move quickly from one task to another.
High-risk behaviors. Parents often report their children with bipolar disorder have tried to jump from moving vehicles, “fly” off of roofs, and jump their bicycles or skateboards over impossible distances. These children behave as if the laws of nature do not apply to them. Children with ADHD behave impulsively but are not always “daredevils.” Their activities appear more impulsive and feature high activity in inappropriate situations, rather than distinctly high-risk activities.
RESPONSE TO THERAPY
Our group7 showed that pediatric manic and ADHD symptoms respond differently to mood-stabilizer treatment (Table 2).
We first used open-label divalproex sodium to treat manic symptoms in 40 children ages 6 to 17 with bipolar I or II disorder and concurrent ADHD. Serum valproic acid levels averaged 82 μg/mL. Manic symptoms improved in 80% of patients, whereas ADHD symptoms improved in <10%. Most children’s symptoms still met severity criteria for ADHD.
In a subsequent double-blind, crossover trial, we compared the effects of mixed amphetamine salts (MAS) or placebo on ADHD symptoms in 30 children whose manic symptoms stabilized on divalproex. MAS showed a significant, independent effect on ADHD symptoms One patient’s manic symptoms recurred during stimulant therapy and subsided with MAS discontinuation.
In this trial, mania symptoms responded to divalproex, whereas ADHD symptoms did not. MAS treatment showed a specific effect on ADHD symptoms of inattention, impulsivity, and hyperactivity. The shared symptoms of mania and ADHD (impulsivity and hyperactivity) decreased with divalproex to some extent.
Table 2
Pediatric mania and ADHD
respond differently to mood-stabilizer therapy
| Children with bipolar I or II disorder and concurrent ADHD | Treatment with divalproex, 8 weeks (N = 40)a | Subjects enter double-blind, crossover treatment with MAS and placebo, 2 weeks each (N = 30) | |
|---|---|---|---|
| Manic symptomsb | 32 of 40 (80%) improved; significant (P <0.0001) | MAS | Placebo |
| No significant change in manic symptoms (P = 0.17) | |||
| ADHD symptomsc | 3 of 40 (7.5%) improved; not significant (P = 0.96) | 26 of 30 (87%) improved | 3 of 30 (10%) improved |
| CGI scores improved 1.9 points more on MAS than on placebo, a significant difference (P <0.0001) | |||
| a Average divalproex blood levels = 82 μg/mL | |||
| b Manic symptom improvement defined as >50% decrease in baseline Young Mania Rating Scale scores | |||
| c ADHD symptom improvement defined as Clinical Global Impression (CGI)–Improvement scores of 1 or 2 | |||
| MAS: Mixed amphetamine salts | |||
| Source: Reference 7 | |||
WHEN MANIA/ADHD CO-OCCUR
ADHD and bipolar disorder symptoms overlap to a great extent, and the disorders can co-occur:
- Up to 20% of children diagnosed with ADHD also meet bipolar criteria.
- Two-thirds of children with bipolar disorder may also meet criteria for ADHD, with reports ranging from 29% to 98%.1,2
When trying to differentiate ADHD and bipolar disorder in children, consider the core symptoms of each diagnosis (Table 3).
Table 3
Core symptoms: Pediatric bipolar disorder vs ADHD
| Symptom | Bipolar disorder | ADHD |
|---|---|---|
| Euphoria/giddiness | Excessive | Appropriate to situations |
| Irritability | Severe and intense, often accompanied by tantrums | Occasional, may be caused by medication “wear-off” |
| Self-esteem | Grandiose | Demoralized |
| Sleep patterns | Decreased need for sleep | Difficulty settling at night |
| Speech patterns | Pressured, fragmented, with flight of ideas | Energetic and quick |
| Thought processes | Racing thoughts | Patients do not report racing thoughts |
| Psychosis can occur at times | ||
| Attention | Distractible | Distractible |
| Activity level | High energy, on-the-go, multiple projects, creative | Hyperactive, multiple projects |
| High-risk behaviors | Impulsive | |
| Disruptive behaviors | Can become aggressive | Intrusive and active |
Related resources
- Geller B, DelBello MP (eds). Bipolar disorder in childhood and early adolescence. New York: Guilford Press, 2003.
- Papolos DF, Papolos J. Bipolar child: The definitive and reassuring guide to childhood’s most misunderstood disorder. New York: Broadway Books, 2002.
- Fristad MA, Goldberg Arnold JS. Raising a moody child: How to cope with depression and bipolar disorder. New York: Guilford Press, 2003.
- Child and Adolescent Bipolar Foundation. Available at www.cabf.org. Accessed March 4, 2005.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Scheffer receives research support from and is a speaker for Abbott Laboratories.
Dr. Apps reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
2. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35(8):997-1008.
3. Patel NC, Sallee FR. What’s the best treatment for ADHD/bipolar mania? Current Psychiatry 2005;3(3):27-37.
4. Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry 2004;61:459-67.
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., rev). Washington, DC: American Psychiatric Association, 2000.
6. Geller B, Williams M, Zimerman B, et al. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord 1998;51(2):81-91.
7. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
Knowing what to look for can help you differentiate between pediatric bipolar disorder and attention-deficit/hyperactivity disorder (ADHD):
- Bipolar disorder is a problem with mood. Children with bipolar mania are elated and/or irritable and experience mood states that appear uncontrollable.
- ADHD is a problem with cognitive functioning, including attention, distractibility, and energy level.
Mood and cognitive symptoms may overlap,1,2 but recognizing manic features is the key to distinguishing between these disorders—even when they co-occur.
We offer tips from our experience and a recent clinical trial to help you sort out the core symptoms that point to bipolar mania.
BIPOLAR CORE SYMPTOMS
Pediatric bipolar disorder is relatively rare, but children with it can experience substantial impairment and developmental delay. Intervening early with effective treatment3 can improve their quality of life, function, and prognosis.
Diagnostic criteria for type I bipolar disorder require at least one manic episode and are the same for all ages. Many clinicians and researchers have advocated adapting DSM-IV criteria for children, but we believe separate adult and pediatric criteria would confuse discussions about the same phenomena. We do agree that symptoms should be evaluated in a developmentally appropriate context, as mania can present differently across the ages (Table 1).
Mania in children and young adolescents tends to present with rapid cycling and a primarily irritable mood.4 Older adolescents and adults may present with more-distinct mood changes, with a primarily euphoric mood. Euphoric mania is less common in adults than previously thought. Forty percent to 60% of adults with bipolar disorder experience a chronic course, rather than more-discrete mood episodes.
A manic episode is an abnormally and persistently elevated (euphoria) or irritable mood that lasts at least 1 week. To satisfy DSM-IV-TR diagnostic criteria for a manic episode:
- patients with euphoria require three additional symptoms
- those who are irritable (and not euphoric) require another four symptoms.5
These symptoms must significantly impair several areas of functioning and not be caused by other mental or physical illness, including substance use or abuse. When depressive symptoms occur in the same week as mania, the mixed mania modifier is used.
Table 1
Diagnostic features of bipolar mania in adolescents vs adults
| Feature | Prepubertal and early adolescent | Older adolescent and adult |
|---|---|---|
| Initial episode | Mixed presentations predominate | Mania is more balanced between mixed and euphoric |
| Episode type | More consistently ill | Persistent/distinct episodes |
| Primary mood | Irritable | Euphoric |
| Duration | Chronic, continuous course | Weeks |
| Inter-episode functioning | Less distinct episodes | May return to baseline or deteriorate over time |
| Reality testing | Delusions (grandiosity) is common; hallucinations | More variable |
Disruptive and aggressive behavior are common and are what usually prompts parents to bring children to psychiatrists. These behaviors are not diagnostic of mania, however, and aggression has many other causes.
The threshold between a variant of normal and pathologic disruptive behavior can be difficult to establish and varies from culture to culture. Some families, for example, would allow a child to tell the parents what to do, whereas other families consider this a serious boundary violation.
Prolonged rages have been used as a proxy for mood swings. Although we agree that rages lasting >15 minutes and out-of-proportion to the circumstances may signal bipolar disorder, they are not diagnostic.
Other symptoms. Psychotic symptoms (hallucinations, delusions, disorganization) can occur in youths with bipolar disorder. Evaluation often reveals impaired social and cognitive development. Keep in mind that a child’s developmental level can affect symptom expression.
ADHD CORE SYMPTOMS
Children with ADHD often present with hyperactive, uncontrollable behaviors and academic failure. To meet DSM-IV-TR diagnostic criteria, they must show symptoms before age 7. Primary symptoms may be inattention, hyperactivity and impulsivity, or both.
ADHD is a disorder of attention and the cognitive skills related to attention, rather than a mood disorder. Children with ADHD show substantially impaired function in at least two settings (such as at home and in school), and—unlike bipolar disorder—their symptoms are persistent rather than episodic.
DIFFERENTIATING BY SYMPTOMS
When differentiating between ADHD and bipolar disorder in children, remain focused on both diagnoses’ core symptoms.
Euphoria, or elation, is a key distinguishing factor in bipolar disorder.6 Although all children are at times giddy or silly in appropriate environments—such as during slumber parties—consider a threshold of appropriateness when making a bipolar diagnosis. Families perceive the giddiness, inappropriate laughter, and elevated mood of children with mania as disturbing and inappropriate, not funny or endearing. They are often annoyed and concerned.
Children with primary ADHD do not show inappropriately elevated mood. In fact, their failures often make these children dysphoric.
Irritability is common in children with psychiatric illnesses. Manic youngsters can be very irritable most of the time. Families describe “walking on eggshells” because of these children’s touchiness. Unpredictable triggers set off explosive, prolonged tantrums that may be associated with aggression, and their mood swings are almost constant.
Children with ADHD can be irritable, but their irritability is less severe and intense than that seen in bipolar disorder. Stimulant medication “wear-off” can cause irritability in ADHD, so consider this possibility if symptoms occur mostly in the evening.
Grandiosity can be confusing to evaluate in children but is often a core symptom in bipolar disorder. All children sometimes say self-inflating things, but those with pathologic grandiosity cross the threshold into the dysfunctional belief that they are better, stronger, smarter, or more talented than others.
For example, a 7-year-old patient insisted he was the world’s best chess player and could beat anyone, including Russian chess masters. When the therapist asked him about chess, he did not know the names of the pieces or how they moved. Yet despite facing these contradictory facts, he continued to insist that he was the best.
Children with grandiosity may act inappropriately on their beliefs, such as by telling adults what to do or engaging in risky, daredevil acts with no concern for their safety or the law.
Children with ADHD are not usually grandiose. Instead, they often become demoralized and develop poor self-esteem from negative feedback about their behavior.
Decreased need for sleep is the hallmark symptom of mania that is absent in other psychiatric disorders. A true decreased need for sleep is only indicated in someone sleeping less than his or her usual cumulative hours each day, without fatigue or recuperative sleep.
Children with bipolar disorder may need 1 or more hours less sleep or deny needing sleep at all. Use age-appropriate amounts of sleep as a standard. A school age child usually averages 9 to 11 hours of sleep per night. If the patient is getting only 6 hours and is not tired, this would be a decreased need for sleep. A 24-hour sleep history can easily assess decreased need for sleep (Box).
Determine daytime fatigue by self-report or observation by parents or teachers. Then ascertain if there are periods of days with less fatigue. Many bipolar youth have a nearly continuous decreased need for sleep.
Children with ADHD often have difficulty settling at night, which delays their falling asleep. The sleep history will likely show that—once asleep—they sleep well for an appropriate amount of time or are fatigued during the day.
Decreased need for sleep is a hallmark symptom of bipolar mania. A 24-hour sleep history may help determine if a child’s irregular sleep patterns signal ADHD or bipolar disorder.
To perform the sleep history, collect time in bed and time asleep over several days, or ask the patient,
- On a typical night, not your best, not your worst:”
- When do you go to bed?
- How long does it take to fall asleep?
- Once asleep, do you wake up?
- How long are you awake?
- When do you arise in the morning?
- What is the total amount of time you are asleep?
- Do you take naps?
- Are you rested or fatigued during the day?
Pressured speech, or the need to talk excessively, is a relatively straightforward symptom. Children experiencing mania often speak so quickly and excessively that others cannot understand or interrupt them. Flight of ideas and racing thoughts are reflected in their speech.
By contrast, rapid speech by children with ADHD is related to hyperactivity. They speak too fast and often become distracted from the topic.
Racing thoughts. Children with bipolar disorder may report that their thoughts come so quickly they cannot get them out fast enough. The idea that their thoughts “need a stop-sign” suggests racing thoughts, a core bipolar symptom. Their speech can be unintelligible, with rapid changes in thought patterns, flight of ideas, and sentence fragments. Children with ADHD are energetic and quick but do not report racing thoughts.
LESS-HELPFUL SYMPTOMS
Distractibility—a core symptom of both inattentive ADHD and manic episodes—does not help differentiate the two diagnoses. The high comorbidity of ADHD with bipolar disorder increases the likelihood that the child will be easily distracted.
Multi-tasking. Increased goal-directed activity is often associated with the high energy of children with mania. They have more energy than most people, are always on the go, and engage in multiple projects or activities that may be markedly creative or unrealistic. This increased energy—combined with other hallmark manic symptoms—can lead to high-risk behaviors.
Hyperactivity in ADHD can appear similar to agitation in bipolar disorder. In both disorders, children may engage in many tasks—not finishing any of them—or appear to move quickly from one task to another.
High-risk behaviors. Parents often report their children with bipolar disorder have tried to jump from moving vehicles, “fly” off of roofs, and jump their bicycles or skateboards over impossible distances. These children behave as if the laws of nature do not apply to them. Children with ADHD behave impulsively but are not always “daredevils.” Their activities appear more impulsive and feature high activity in inappropriate situations, rather than distinctly high-risk activities.
RESPONSE TO THERAPY
Our group7 showed that pediatric manic and ADHD symptoms respond differently to mood-stabilizer treatment (Table 2).
We first used open-label divalproex sodium to treat manic symptoms in 40 children ages 6 to 17 with bipolar I or II disorder and concurrent ADHD. Serum valproic acid levels averaged 82 μg/mL. Manic symptoms improved in 80% of patients, whereas ADHD symptoms improved in <10%. Most children’s symptoms still met severity criteria for ADHD.
In a subsequent double-blind, crossover trial, we compared the effects of mixed amphetamine salts (MAS) or placebo on ADHD symptoms in 30 children whose manic symptoms stabilized on divalproex. MAS showed a significant, independent effect on ADHD symptoms One patient’s manic symptoms recurred during stimulant therapy and subsided with MAS discontinuation.
In this trial, mania symptoms responded to divalproex, whereas ADHD symptoms did not. MAS treatment showed a specific effect on ADHD symptoms of inattention, impulsivity, and hyperactivity. The shared symptoms of mania and ADHD (impulsivity and hyperactivity) decreased with divalproex to some extent.
Table 2
Pediatric mania and ADHD
respond differently to mood-stabilizer therapy
| Children with bipolar I or II disorder and concurrent ADHD | Treatment with divalproex, 8 weeks (N = 40)a | Subjects enter double-blind, crossover treatment with MAS and placebo, 2 weeks each (N = 30) | |
|---|---|---|---|
| Manic symptomsb | 32 of 40 (80%) improved; significant (P <0.0001) | MAS | Placebo |
| No significant change in manic symptoms (P = 0.17) | |||
| ADHD symptomsc | 3 of 40 (7.5%) improved; not significant (P = 0.96) | 26 of 30 (87%) improved | 3 of 30 (10%) improved |
| CGI scores improved 1.9 points more on MAS than on placebo, a significant difference (P <0.0001) | |||
| a Average divalproex blood levels = 82 μg/mL | |||
| b Manic symptom improvement defined as >50% decrease in baseline Young Mania Rating Scale scores | |||
| c ADHD symptom improvement defined as Clinical Global Impression (CGI)–Improvement scores of 1 or 2 | |||
| MAS: Mixed amphetamine salts | |||
| Source: Reference 7 | |||
WHEN MANIA/ADHD CO-OCCUR
ADHD and bipolar disorder symptoms overlap to a great extent, and the disorders can co-occur:
- Up to 20% of children diagnosed with ADHD also meet bipolar criteria.
- Two-thirds of children with bipolar disorder may also meet criteria for ADHD, with reports ranging from 29% to 98%.1,2
When trying to differentiate ADHD and bipolar disorder in children, consider the core symptoms of each diagnosis (Table 3).
Table 3
Core symptoms: Pediatric bipolar disorder vs ADHD
| Symptom | Bipolar disorder | ADHD |
|---|---|---|
| Euphoria/giddiness | Excessive | Appropriate to situations |
| Irritability | Severe and intense, often accompanied by tantrums | Occasional, may be caused by medication “wear-off” |
| Self-esteem | Grandiose | Demoralized |
| Sleep patterns | Decreased need for sleep | Difficulty settling at night |
| Speech patterns | Pressured, fragmented, with flight of ideas | Energetic and quick |
| Thought processes | Racing thoughts | Patients do not report racing thoughts |
| Psychosis can occur at times | ||
| Attention | Distractible | Distractible |
| Activity level | High energy, on-the-go, multiple projects, creative | Hyperactive, multiple projects |
| High-risk behaviors | Impulsive | |
| Disruptive behaviors | Can become aggressive | Intrusive and active |
Related resources
- Geller B, DelBello MP (eds). Bipolar disorder in childhood and early adolescence. New York: Guilford Press, 2003.
- Papolos DF, Papolos J. Bipolar child: The definitive and reassuring guide to childhood’s most misunderstood disorder. New York: Broadway Books, 2002.
- Fristad MA, Goldberg Arnold JS. Raising a moody child: How to cope with depression and bipolar disorder. New York: Guilford Press, 2003.
- Child and Adolescent Bipolar Foundation. Available at www.cabf.org. Accessed March 4, 2005.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Scheffer receives research support from and is a speaker for Abbott Laboratories.
Dr. Apps reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Knowing what to look for can help you differentiate between pediatric bipolar disorder and attention-deficit/hyperactivity disorder (ADHD):
- Bipolar disorder is a problem with mood. Children with bipolar mania are elated and/or irritable and experience mood states that appear uncontrollable.
- ADHD is a problem with cognitive functioning, including attention, distractibility, and energy level.
Mood and cognitive symptoms may overlap,1,2 but recognizing manic features is the key to distinguishing between these disorders—even when they co-occur.
We offer tips from our experience and a recent clinical trial to help you sort out the core symptoms that point to bipolar mania.
BIPOLAR CORE SYMPTOMS
Pediatric bipolar disorder is relatively rare, but children with it can experience substantial impairment and developmental delay. Intervening early with effective treatment3 can improve their quality of life, function, and prognosis.
Diagnostic criteria for type I bipolar disorder require at least one manic episode and are the same for all ages. Many clinicians and researchers have advocated adapting DSM-IV criteria for children, but we believe separate adult and pediatric criteria would confuse discussions about the same phenomena. We do agree that symptoms should be evaluated in a developmentally appropriate context, as mania can present differently across the ages (Table 1).
Mania in children and young adolescents tends to present with rapid cycling and a primarily irritable mood.4 Older adolescents and adults may present with more-distinct mood changes, with a primarily euphoric mood. Euphoric mania is less common in adults than previously thought. Forty percent to 60% of adults with bipolar disorder experience a chronic course, rather than more-discrete mood episodes.
A manic episode is an abnormally and persistently elevated (euphoria) or irritable mood that lasts at least 1 week. To satisfy DSM-IV-TR diagnostic criteria for a manic episode:
- patients with euphoria require three additional symptoms
- those who are irritable (and not euphoric) require another four symptoms.5
These symptoms must significantly impair several areas of functioning and not be caused by other mental or physical illness, including substance use or abuse. When depressive symptoms occur in the same week as mania, the mixed mania modifier is used.
Table 1
Diagnostic features of bipolar mania in adolescents vs adults
| Feature | Prepubertal and early adolescent | Older adolescent and adult |
|---|---|---|
| Initial episode | Mixed presentations predominate | Mania is more balanced between mixed and euphoric |
| Episode type | More consistently ill | Persistent/distinct episodes |
| Primary mood | Irritable | Euphoric |
| Duration | Chronic, continuous course | Weeks |
| Inter-episode functioning | Less distinct episodes | May return to baseline or deteriorate over time |
| Reality testing | Delusions (grandiosity) is common; hallucinations | More variable |
Disruptive and aggressive behavior are common and are what usually prompts parents to bring children to psychiatrists. These behaviors are not diagnostic of mania, however, and aggression has many other causes.
The threshold between a variant of normal and pathologic disruptive behavior can be difficult to establish and varies from culture to culture. Some families, for example, would allow a child to tell the parents what to do, whereas other families consider this a serious boundary violation.
Prolonged rages have been used as a proxy for mood swings. Although we agree that rages lasting >15 minutes and out-of-proportion to the circumstances may signal bipolar disorder, they are not diagnostic.
Other symptoms. Psychotic symptoms (hallucinations, delusions, disorganization) can occur in youths with bipolar disorder. Evaluation often reveals impaired social and cognitive development. Keep in mind that a child’s developmental level can affect symptom expression.
ADHD CORE SYMPTOMS
Children with ADHD often present with hyperactive, uncontrollable behaviors and academic failure. To meet DSM-IV-TR diagnostic criteria, they must show symptoms before age 7. Primary symptoms may be inattention, hyperactivity and impulsivity, or both.
ADHD is a disorder of attention and the cognitive skills related to attention, rather than a mood disorder. Children with ADHD show substantially impaired function in at least two settings (such as at home and in school), and—unlike bipolar disorder—their symptoms are persistent rather than episodic.
DIFFERENTIATING BY SYMPTOMS
When differentiating between ADHD and bipolar disorder in children, remain focused on both diagnoses’ core symptoms.
Euphoria, or elation, is a key distinguishing factor in bipolar disorder.6 Although all children are at times giddy or silly in appropriate environments—such as during slumber parties—consider a threshold of appropriateness when making a bipolar diagnosis. Families perceive the giddiness, inappropriate laughter, and elevated mood of children with mania as disturbing and inappropriate, not funny or endearing. They are often annoyed and concerned.
Children with primary ADHD do not show inappropriately elevated mood. In fact, their failures often make these children dysphoric.
Irritability is common in children with psychiatric illnesses. Manic youngsters can be very irritable most of the time. Families describe “walking on eggshells” because of these children’s touchiness. Unpredictable triggers set off explosive, prolonged tantrums that may be associated with aggression, and their mood swings are almost constant.
Children with ADHD can be irritable, but their irritability is less severe and intense than that seen in bipolar disorder. Stimulant medication “wear-off” can cause irritability in ADHD, so consider this possibility if symptoms occur mostly in the evening.
Grandiosity can be confusing to evaluate in children but is often a core symptom in bipolar disorder. All children sometimes say self-inflating things, but those with pathologic grandiosity cross the threshold into the dysfunctional belief that they are better, stronger, smarter, or more talented than others.
For example, a 7-year-old patient insisted he was the world’s best chess player and could beat anyone, including Russian chess masters. When the therapist asked him about chess, he did not know the names of the pieces or how they moved. Yet despite facing these contradictory facts, he continued to insist that he was the best.
Children with grandiosity may act inappropriately on their beliefs, such as by telling adults what to do or engaging in risky, daredevil acts with no concern for their safety or the law.
Children with ADHD are not usually grandiose. Instead, they often become demoralized and develop poor self-esteem from negative feedback about their behavior.
Decreased need for sleep is the hallmark symptom of mania that is absent in other psychiatric disorders. A true decreased need for sleep is only indicated in someone sleeping less than his or her usual cumulative hours each day, without fatigue or recuperative sleep.
Children with bipolar disorder may need 1 or more hours less sleep or deny needing sleep at all. Use age-appropriate amounts of sleep as a standard. A school age child usually averages 9 to 11 hours of sleep per night. If the patient is getting only 6 hours and is not tired, this would be a decreased need for sleep. A 24-hour sleep history can easily assess decreased need for sleep (Box).
Determine daytime fatigue by self-report or observation by parents or teachers. Then ascertain if there are periods of days with less fatigue. Many bipolar youth have a nearly continuous decreased need for sleep.
Children with ADHD often have difficulty settling at night, which delays their falling asleep. The sleep history will likely show that—once asleep—they sleep well for an appropriate amount of time or are fatigued during the day.
Decreased need for sleep is a hallmark symptom of bipolar mania. A 24-hour sleep history may help determine if a child’s irregular sleep patterns signal ADHD or bipolar disorder.
To perform the sleep history, collect time in bed and time asleep over several days, or ask the patient,
- On a typical night, not your best, not your worst:”
- When do you go to bed?
- How long does it take to fall asleep?
- Once asleep, do you wake up?
- How long are you awake?
- When do you arise in the morning?
- What is the total amount of time you are asleep?
- Do you take naps?
- Are you rested or fatigued during the day?
Pressured speech, or the need to talk excessively, is a relatively straightforward symptom. Children experiencing mania often speak so quickly and excessively that others cannot understand or interrupt them. Flight of ideas and racing thoughts are reflected in their speech.
By contrast, rapid speech by children with ADHD is related to hyperactivity. They speak too fast and often become distracted from the topic.
Racing thoughts. Children with bipolar disorder may report that their thoughts come so quickly they cannot get them out fast enough. The idea that their thoughts “need a stop-sign” suggests racing thoughts, a core bipolar symptom. Their speech can be unintelligible, with rapid changes in thought patterns, flight of ideas, and sentence fragments. Children with ADHD are energetic and quick but do not report racing thoughts.
LESS-HELPFUL SYMPTOMS
Distractibility—a core symptom of both inattentive ADHD and manic episodes—does not help differentiate the two diagnoses. The high comorbidity of ADHD with bipolar disorder increases the likelihood that the child will be easily distracted.
Multi-tasking. Increased goal-directed activity is often associated with the high energy of children with mania. They have more energy than most people, are always on the go, and engage in multiple projects or activities that may be markedly creative or unrealistic. This increased energy—combined with other hallmark manic symptoms—can lead to high-risk behaviors.
Hyperactivity in ADHD can appear similar to agitation in bipolar disorder. In both disorders, children may engage in many tasks—not finishing any of them—or appear to move quickly from one task to another.
High-risk behaviors. Parents often report their children with bipolar disorder have tried to jump from moving vehicles, “fly” off of roofs, and jump their bicycles or skateboards over impossible distances. These children behave as if the laws of nature do not apply to them. Children with ADHD behave impulsively but are not always “daredevils.” Their activities appear more impulsive and feature high activity in inappropriate situations, rather than distinctly high-risk activities.
RESPONSE TO THERAPY
Our group7 showed that pediatric manic and ADHD symptoms respond differently to mood-stabilizer treatment (Table 2).
We first used open-label divalproex sodium to treat manic symptoms in 40 children ages 6 to 17 with bipolar I or II disorder and concurrent ADHD. Serum valproic acid levels averaged 82 μg/mL. Manic symptoms improved in 80% of patients, whereas ADHD symptoms improved in <10%. Most children’s symptoms still met severity criteria for ADHD.
In a subsequent double-blind, crossover trial, we compared the effects of mixed amphetamine salts (MAS) or placebo on ADHD symptoms in 30 children whose manic symptoms stabilized on divalproex. MAS showed a significant, independent effect on ADHD symptoms One patient’s manic symptoms recurred during stimulant therapy and subsided with MAS discontinuation.
In this trial, mania symptoms responded to divalproex, whereas ADHD symptoms did not. MAS treatment showed a specific effect on ADHD symptoms of inattention, impulsivity, and hyperactivity. The shared symptoms of mania and ADHD (impulsivity and hyperactivity) decreased with divalproex to some extent.
Table 2
Pediatric mania and ADHD
respond differently to mood-stabilizer therapy
| Children with bipolar I or II disorder and concurrent ADHD | Treatment with divalproex, 8 weeks (N = 40)a | Subjects enter double-blind, crossover treatment with MAS and placebo, 2 weeks each (N = 30) | |
|---|---|---|---|
| Manic symptomsb | 32 of 40 (80%) improved; significant (P <0.0001) | MAS | Placebo |
| No significant change in manic symptoms (P = 0.17) | |||
| ADHD symptomsc | 3 of 40 (7.5%) improved; not significant (P = 0.96) | 26 of 30 (87%) improved | 3 of 30 (10%) improved |
| CGI scores improved 1.9 points more on MAS than on placebo, a significant difference (P <0.0001) | |||
| a Average divalproex blood levels = 82 μg/mL | |||
| b Manic symptom improvement defined as >50% decrease in baseline Young Mania Rating Scale scores | |||
| c ADHD symptom improvement defined as Clinical Global Impression (CGI)–Improvement scores of 1 or 2 | |||
| MAS: Mixed amphetamine salts | |||
| Source: Reference 7 | |||
WHEN MANIA/ADHD CO-OCCUR
ADHD and bipolar disorder symptoms overlap to a great extent, and the disorders can co-occur:
- Up to 20% of children diagnosed with ADHD also meet bipolar criteria.
- Two-thirds of children with bipolar disorder may also meet criteria for ADHD, with reports ranging from 29% to 98%.1,2
When trying to differentiate ADHD and bipolar disorder in children, consider the core symptoms of each diagnosis (Table 3).
Table 3
Core symptoms: Pediatric bipolar disorder vs ADHD
| Symptom | Bipolar disorder | ADHD |
|---|---|---|
| Euphoria/giddiness | Excessive | Appropriate to situations |
| Irritability | Severe and intense, often accompanied by tantrums | Occasional, may be caused by medication “wear-off” |
| Self-esteem | Grandiose | Demoralized |
| Sleep patterns | Decreased need for sleep | Difficulty settling at night |
| Speech patterns | Pressured, fragmented, with flight of ideas | Energetic and quick |
| Thought processes | Racing thoughts | Patients do not report racing thoughts |
| Psychosis can occur at times | ||
| Attention | Distractible | Distractible |
| Activity level | High energy, on-the-go, multiple projects, creative | Hyperactive, multiple projects |
| High-risk behaviors | Impulsive | |
| Disruptive behaviors | Can become aggressive | Intrusive and active |
Related resources
- Geller B, DelBello MP (eds). Bipolar disorder in childhood and early adolescence. New York: Guilford Press, 2003.
- Papolos DF, Papolos J. Bipolar child: The definitive and reassuring guide to childhood’s most misunderstood disorder. New York: Broadway Books, 2002.
- Fristad MA, Goldberg Arnold JS. Raising a moody child: How to cope with depression and bipolar disorder. New York: Guilford Press, 2003.
- Child and Adolescent Bipolar Foundation. Available at www.cabf.org. Accessed March 4, 2005.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Scheffer receives research support from and is a speaker for Abbott Laboratories.
Dr. Apps reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
2. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35(8):997-1008.
3. Patel NC, Sallee FR. What’s the best treatment for ADHD/bipolar mania? Current Psychiatry 2005;3(3):27-37.
4. Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry 2004;61:459-67.
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., rev). Washington, DC: American Psychiatric Association, 2000.
6. Geller B, Williams M, Zimerman B, et al. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord 1998;51(2):81-91.
7. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
1. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
2. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35(8):997-1008.
3. Patel NC, Sallee FR. What’s the best treatment for ADHD/bipolar mania? Current Psychiatry 2005;3(3):27-37.
4. Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry 2004;61:459-67.
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., rev). Washington, DC: American Psychiatric Association, 2000.
6. Geller B, Williams M, Zimerman B, et al. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord 1998;51(2):81-91.
7. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
Sarcoidosis on Black Skin 'Can Really Fool You'
NEW ORLEANS — Because of its myriad presentations on black skin, sarcoidosis can be called “the great imitator,” Rebat Halder, M.D., said at the annual meeting of the American Academy of Dermatology.
“The appearance on the skin can have many different morphologies,” said Dr. Halder, chair of the department of dermatology at Howard University, Washington.
“It can really fool you: The lesions can be macular, papular, ichthyosiform, nodular, ulcerative, vesicular, annular, or it can simply present as areas of hypopigmentation with no apparent inflammation.”
Sarcoidosis, characterized by noncaseating epithelioid granulomas that may affect any organ system, is uncommon in any group of patients. However, it is about 16 times more common in blacks than in whites, with an incidence of 35-65/100,000 among blacks. Black women in their fourth decade are most commonly affected, with an incidence of about 100/100,000, Dr. Halder said in an interview.
The etiology of sarcoidosis is unknown, although familial clustering has been observed.
For unknown reasons, sarcoidosis is often more aggressive and difficult to treat in blacks. They have a higher rate of relapse, are more likely to experience multiorgan involvement, and have a slightly higher mortality rate than whites.
About 90% of patients have lung involvement, usually fibrosis due to granulomatous lesions.
Skin lesions appear in about 35% of patients. Other affected organs are the eyes, liver, heart, central nervous system, and spleen.
Any suspicious lesions should be biopsied. Typically, histology will show characteristic noncaseating granulomas. Since so many patients have lung involvement, a chest x-ray is imperative, Dr. Halder said. “If you suspect a skin lesion is sarcoidosis, you must search for the disease elsewhere in the body.”
Papular sarcoidosis consists of red-brown papules usually occurring on the face, around the eyes, nose, mouth, and nape of neck.
The papules can be larger, or quite fine, with the skin assuming a sandpaperlike texture, Dr. Halder said.
Lupus pernio lesions are red or purple indurated plaques usually occurring around the nose. These lesions can affect the nasal cartilage or bone and upper respiratory system as well.
Plaquelike sarcoidosis can occur anywhere on the skin, but is most common on the back of the neck and on the arms and legs.
This condition is characterized by round or oval, red-brown to purple, infiltrated plaques.
Ulcerative sarcoidosis can be especially debilitating, especially when it occurs on the palms of the hands or soles of the feet.
These lesions are small but can become quite deep, Dr. Halder commented.
Vesicular sarcoidosis can occur anywhere on the skin and can easily be confused with other blistering diseases or with skin infections.
Hypopigmented sarcoidosis is especially difficult to recognize, he said. “This presents only as areas of the skin without pigment. You would have to consider sarcoidosis along with hypopigmented cutaneous T-cell lymphoma, vitiligo, or tinea versicolor. Again, biopsy is crucial to diagnosis.”
Treatment for skin lesions usually consists of topical or intralesional steroids.
Extensive or recalcitrant disease requires more aggressive treatment, which includes drugs such as oral steroids, methotrexate, allopurinol, thalidomide, or oral retinoids.
There has been some success with infliximab as well, Dr. Halder said.
Adjunctive phototherapy is useful for hypopigmented sarcoidosis, the investigator said.
NEW ORLEANS — Because of its myriad presentations on black skin, sarcoidosis can be called “the great imitator,” Rebat Halder, M.D., said at the annual meeting of the American Academy of Dermatology.
“The appearance on the skin can have many different morphologies,” said Dr. Halder, chair of the department of dermatology at Howard University, Washington.
“It can really fool you: The lesions can be macular, papular, ichthyosiform, nodular, ulcerative, vesicular, annular, or it can simply present as areas of hypopigmentation with no apparent inflammation.”
Sarcoidosis, characterized by noncaseating epithelioid granulomas that may affect any organ system, is uncommon in any group of patients. However, it is about 16 times more common in blacks than in whites, with an incidence of 35-65/100,000 among blacks. Black women in their fourth decade are most commonly affected, with an incidence of about 100/100,000, Dr. Halder said in an interview.
The etiology of sarcoidosis is unknown, although familial clustering has been observed.
For unknown reasons, sarcoidosis is often more aggressive and difficult to treat in blacks. They have a higher rate of relapse, are more likely to experience multiorgan involvement, and have a slightly higher mortality rate than whites.
About 90% of patients have lung involvement, usually fibrosis due to granulomatous lesions.
Skin lesions appear in about 35% of patients. Other affected organs are the eyes, liver, heart, central nervous system, and spleen.
Any suspicious lesions should be biopsied. Typically, histology will show characteristic noncaseating granulomas. Since so many patients have lung involvement, a chest x-ray is imperative, Dr. Halder said. “If you suspect a skin lesion is sarcoidosis, you must search for the disease elsewhere in the body.”
Papular sarcoidosis consists of red-brown papules usually occurring on the face, around the eyes, nose, mouth, and nape of neck.
The papules can be larger, or quite fine, with the skin assuming a sandpaperlike texture, Dr. Halder said.
Lupus pernio lesions are red or purple indurated plaques usually occurring around the nose. These lesions can affect the nasal cartilage or bone and upper respiratory system as well.
Plaquelike sarcoidosis can occur anywhere on the skin, but is most common on the back of the neck and on the arms and legs.
This condition is characterized by round or oval, red-brown to purple, infiltrated plaques.
Ulcerative sarcoidosis can be especially debilitating, especially when it occurs on the palms of the hands or soles of the feet.
These lesions are small but can become quite deep, Dr. Halder commented.
Vesicular sarcoidosis can occur anywhere on the skin and can easily be confused with other blistering diseases or with skin infections.
Hypopigmented sarcoidosis is especially difficult to recognize, he said. “This presents only as areas of the skin without pigment. You would have to consider sarcoidosis along with hypopigmented cutaneous T-cell lymphoma, vitiligo, or tinea versicolor. Again, biopsy is crucial to diagnosis.”
Treatment for skin lesions usually consists of topical or intralesional steroids.
Extensive or recalcitrant disease requires more aggressive treatment, which includes drugs such as oral steroids, methotrexate, allopurinol, thalidomide, or oral retinoids.
There has been some success with infliximab as well, Dr. Halder said.
Adjunctive phototherapy is useful for hypopigmented sarcoidosis, the investigator said.
NEW ORLEANS — Because of its myriad presentations on black skin, sarcoidosis can be called “the great imitator,” Rebat Halder, M.D., said at the annual meeting of the American Academy of Dermatology.
“The appearance on the skin can have many different morphologies,” said Dr. Halder, chair of the department of dermatology at Howard University, Washington.
“It can really fool you: The lesions can be macular, papular, ichthyosiform, nodular, ulcerative, vesicular, annular, or it can simply present as areas of hypopigmentation with no apparent inflammation.”
Sarcoidosis, characterized by noncaseating epithelioid granulomas that may affect any organ system, is uncommon in any group of patients. However, it is about 16 times more common in blacks than in whites, with an incidence of 35-65/100,000 among blacks. Black women in their fourth decade are most commonly affected, with an incidence of about 100/100,000, Dr. Halder said in an interview.
The etiology of sarcoidosis is unknown, although familial clustering has been observed.
For unknown reasons, sarcoidosis is often more aggressive and difficult to treat in blacks. They have a higher rate of relapse, are more likely to experience multiorgan involvement, and have a slightly higher mortality rate than whites.
About 90% of patients have lung involvement, usually fibrosis due to granulomatous lesions.
Skin lesions appear in about 35% of patients. Other affected organs are the eyes, liver, heart, central nervous system, and spleen.
Any suspicious lesions should be biopsied. Typically, histology will show characteristic noncaseating granulomas. Since so many patients have lung involvement, a chest x-ray is imperative, Dr. Halder said. “If you suspect a skin lesion is sarcoidosis, you must search for the disease elsewhere in the body.”
Papular sarcoidosis consists of red-brown papules usually occurring on the face, around the eyes, nose, mouth, and nape of neck.
The papules can be larger, or quite fine, with the skin assuming a sandpaperlike texture, Dr. Halder said.
Lupus pernio lesions are red or purple indurated plaques usually occurring around the nose. These lesions can affect the nasal cartilage or bone and upper respiratory system as well.
Plaquelike sarcoidosis can occur anywhere on the skin, but is most common on the back of the neck and on the arms and legs.
This condition is characterized by round or oval, red-brown to purple, infiltrated plaques.
Ulcerative sarcoidosis can be especially debilitating, especially when it occurs on the palms of the hands or soles of the feet.
These lesions are small but can become quite deep, Dr. Halder commented.
Vesicular sarcoidosis can occur anywhere on the skin and can easily be confused with other blistering diseases or with skin infections.
Hypopigmented sarcoidosis is especially difficult to recognize, he said. “This presents only as areas of the skin without pigment. You would have to consider sarcoidosis along with hypopigmented cutaneous T-cell lymphoma, vitiligo, or tinea versicolor. Again, biopsy is crucial to diagnosis.”
Treatment for skin lesions usually consists of topical or intralesional steroids.
Extensive or recalcitrant disease requires more aggressive treatment, which includes drugs such as oral steroids, methotrexate, allopurinol, thalidomide, or oral retinoids.
There has been some success with infliximab as well, Dr. Halder said.
Adjunctive phototherapy is useful for hypopigmented sarcoidosis, the investigator said.
Weight Loss Possible in SLE Patients on Steroids
LONDON — Significant weight loss is possible in obese patients with systemic lupus erythematosus being treated with corticosteroids, Siaw Ing Yeo, M.D., said at the Sixth European Lupus Meeting.
This was demonstrated in a study that compared simple calorie restriction with a low-glycemic-index diet. In this type of diet, patients avoid refined carbohydrates, consuming only low-glycemic-index complex carbohydrates—those that have little immediate effect on blood glucose—and higher amounts of protein and fat.
The study randomly assigned 23 women aged 18-65 years whose body mass index was 25 or greater and who were on a stable dose of prednisolone of 5-20 mg/day to one of the two diets for 6 weeks.
There were significant weight reductions in both groups, with a mean weight loss of 2.44 kg in the low-calorie group and a mean of 4 kg in the low-glycemic-index group, Dr. Yeo said in a poster session at the meeting, sponsored by the British Society for Rheumatology.
Fasting LDL, HDL, triglycerides, glucose, insulin, C-reactive protein, fibrinogen, and homocysteine levels did not alter significantly on either diet. Fasting urate levels showed a trend toward improvement in the low-calorie group and remained unchanged in the low-glycemic-index diet, said Dr. Yeo of the lupus research unit at the Rayne Institute, St. Thomas' Hospital, London.
Constipation was reported by 50% of patients in the low-glycemic-index group, while increased bowel frequency and bloating were reported by 25% of those in the low-calorie group.
A surprising additional finding was that patients in both groups reported significant improvements on the fatigue severity score. Finally, neither diet was associated with a disease flare, she said.
LONDON — Significant weight loss is possible in obese patients with systemic lupus erythematosus being treated with corticosteroids, Siaw Ing Yeo, M.D., said at the Sixth European Lupus Meeting.
This was demonstrated in a study that compared simple calorie restriction with a low-glycemic-index diet. In this type of diet, patients avoid refined carbohydrates, consuming only low-glycemic-index complex carbohydrates—those that have little immediate effect on blood glucose—and higher amounts of protein and fat.
The study randomly assigned 23 women aged 18-65 years whose body mass index was 25 or greater and who were on a stable dose of prednisolone of 5-20 mg/day to one of the two diets for 6 weeks.
There were significant weight reductions in both groups, with a mean weight loss of 2.44 kg in the low-calorie group and a mean of 4 kg in the low-glycemic-index group, Dr. Yeo said in a poster session at the meeting, sponsored by the British Society for Rheumatology.
Fasting LDL, HDL, triglycerides, glucose, insulin, C-reactive protein, fibrinogen, and homocysteine levels did not alter significantly on either diet. Fasting urate levels showed a trend toward improvement in the low-calorie group and remained unchanged in the low-glycemic-index diet, said Dr. Yeo of the lupus research unit at the Rayne Institute, St. Thomas' Hospital, London.
Constipation was reported by 50% of patients in the low-glycemic-index group, while increased bowel frequency and bloating were reported by 25% of those in the low-calorie group.
A surprising additional finding was that patients in both groups reported significant improvements on the fatigue severity score. Finally, neither diet was associated with a disease flare, she said.
LONDON — Significant weight loss is possible in obese patients with systemic lupus erythematosus being treated with corticosteroids, Siaw Ing Yeo, M.D., said at the Sixth European Lupus Meeting.
This was demonstrated in a study that compared simple calorie restriction with a low-glycemic-index diet. In this type of diet, patients avoid refined carbohydrates, consuming only low-glycemic-index complex carbohydrates—those that have little immediate effect on blood glucose—and higher amounts of protein and fat.
The study randomly assigned 23 women aged 18-65 years whose body mass index was 25 or greater and who were on a stable dose of prednisolone of 5-20 mg/day to one of the two diets for 6 weeks.
There were significant weight reductions in both groups, with a mean weight loss of 2.44 kg in the low-calorie group and a mean of 4 kg in the low-glycemic-index group, Dr. Yeo said in a poster session at the meeting, sponsored by the British Society for Rheumatology.
Fasting LDL, HDL, triglycerides, glucose, insulin, C-reactive protein, fibrinogen, and homocysteine levels did not alter significantly on either diet. Fasting urate levels showed a trend toward improvement in the low-calorie group and remained unchanged in the low-glycemic-index diet, said Dr. Yeo of the lupus research unit at the Rayne Institute, St. Thomas' Hospital, London.
Constipation was reported by 50% of patients in the low-glycemic-index group, while increased bowel frequency and bloating were reported by 25% of those in the low-calorie group.
A surprising additional finding was that patients in both groups reported significant improvements on the fatigue severity score. Finally, neither diet was associated with a disease flare, she said.