User login
Hospitalist Time‐Motion
The hospitalist model of care has experienced dramatic growth. In 2003 it was estimated that there were 8000 US hospitalists, a number projected to ultimately reach more than 19 000.1, 2 This rapid growth has largely been driven by improvements in clinical efficiency as a result of hospitalist programs. There is a substantial body of evidence showing that hospitalists reduce length of stay and inpatient costs.3 Despite the rapid growth and proven benefit to clinical efficiency, no studies have evaluated the type and frequency of activities that hospitalists perform during routine work. Although the use of hospitalists improves clinical efficiency for the hospital, relatively little is known about how the hospital can improve efficiency for the hospitalist.
Our institution greatly expanded our hospitalist program in June 2003 to create a resident‐uncovered hospitalist service. The impetus for this change was the need to comply with newly revised Accreditation Council for Graduate Medicine Education (ACGME) program requirements regarding resident duty hours. Many teaching hospitals have implemented similar resident‐uncovered hospitalist services.4 Inefficiencies in their work activities quickly became apparent to our hospitalists. Furthermore, our hospitalists believed that they frequently performed simultaneous activities and that they were excessively interrupted by pages.
To evaluate the type and frequency of activities that the hospitalists performed during routine work, we performed a time‐motion study of hospitalist physicians on the resident‐uncovered hospitalist service. Our goal was to identify areas for systems improvements and activities that were better suited for nonphysician providers and to quantify the time spent multitasking and the frequency of paging interruptions.
METHODS
Northwestern Memorial Hospital (NMH) is a 753‐bed hospital in Chicago, Illinois. NMH is the primary teaching hospital affiliated with the Feinberg School of Medicine of Northwestern University. There are 2 general medicine services at NMH: a traditional resident‐covered ward service and the resident‐uncovered hospitalist service. Patients are admitted to one of these 2 services on the basis of, in order of importance, capacity of the services, preference of the outpatient physician, and potential educational value of the admission. Patients admitted to the hospitalist service are preferentially given beds on specific wards intended for hospitalist service patients. Fourth‐year medical students are frequently paired with hospitalists during their medicine subinternship.
The resident‐uncovered hospitalist service comprises 5 daytime hospitalists on duty at a time. The hospitalists are on service for 7 consecutive days, usually followed by 7 consecutive days off. Hospitalists pick up new patients from the night float hospitalist each morning. Daytime admitting duties rotate on a daily basis. One hospitalist accepts new admissions each morning from 7:00 AM until noon. Two hospitalists accept admissions from noon until 5:00 PM. One hospitalist accepts admissions from 5:00 PM until 9:00 PM. One hospitalist is free from accepting new admissions each day. All daytime hospitalists begin the workday at 7:00 AM and leave when their duties are completed for the day. One night float hospitalist is on duty each night of the week. The night float hospitalist performs admissions and all cross cover activities from 7:00 PM until 7:00 AM.
We first conducted a pilot study to help identify specific activities that our hospitalists routinely perform. Broad categories and subcategories of activities were created based on the results of our pilot study, and a published time‐motion study performed on emergency medicine physicians5 (Table 1). Once activities were defined and codes established, our research assistant unobtrusively shadowed hospitalist physicians for periods lasting 3‐5 hours. The observation periods were distributed in order to sample all activities that a daytime hospitalist would perform throughout a typical week. Observation periods included 2 morning admitting periods, 4 morning nonadmitting periods, 4 afternoon admitting periods, 4 afternoon nonadmitting periods, and 2 admitting periods from 5:00 PM to 9:00 PM. Activities were recorded on a standardized data collection form in 1‐minute intervals. When multiple activities were performed at the same time, all activities were recorded in the same 1‐minute interval. Incoming pages were recorded as well. To minimize the possibility that observation would affect hospitalist behavior, the research assistant was instructed not to initiate conversation with the hospitalists.
| Direct patient care |
| Taking initial history and physical exam |
| Seeing patient in follow‐up visit |
| Going over discharge instructions |
| Family meetings |
| Indirect patient care |
| Reviewing test results and medical records |
| Documentation |
| Documenting history and physical, daily notes, filling out discharge instructions, writing out prescriptions |
| Communication |
| Taking report from night float, taking admission report, face‐to‐face discussion, initiating and returning pages |
| Orders |
| Writing/emnputting orders, calling radiology |
| Professional development |
| Going to conferences, grand rounds, etc |
| Reading articles, textbooks, online references |
| Education |
| Teaching during work rounds |
| Didactic sessions with subintern |
| Travel |
| Walking, taking elevator, etc |
| Personal |
| Lunch, washroom break, etc. |
The data collection forms were manually abstracted and minutes tallied for each category and subcategory, for which summary statistics were converted to percentage of total minutes.
RESULTS
Ten hospitalists were shadowed by a single research assistant for a total of 4467 minutes. Seven hospitalists were male and 3 were female. The hospitalists were a mean age of 31 1.6 years of age and had been practicing as a hospitalist for a mean of 2.1 1.0 years. The hospitalists saw an average of 9.4 4.0 patients on the days they were shadowed by the research assistant. Because simultaneous activities were recorded, a total of 5557 minutes of activities were recorded.
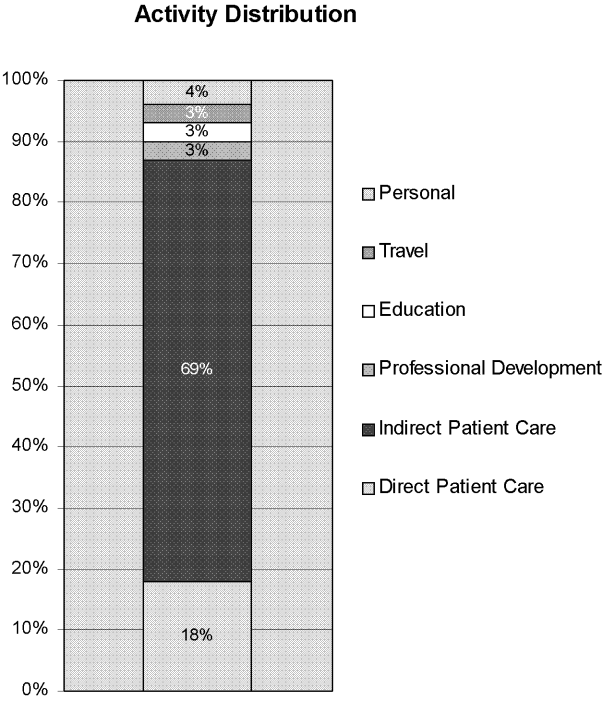
The distribution of total minutes recorded in each activity category is shown in Figure 1. Hospitalists spent 18% of their time doing direct patient care, 69% on indirect patient care, 4% on personal activities, and 3% each on professional development, education, and travel.
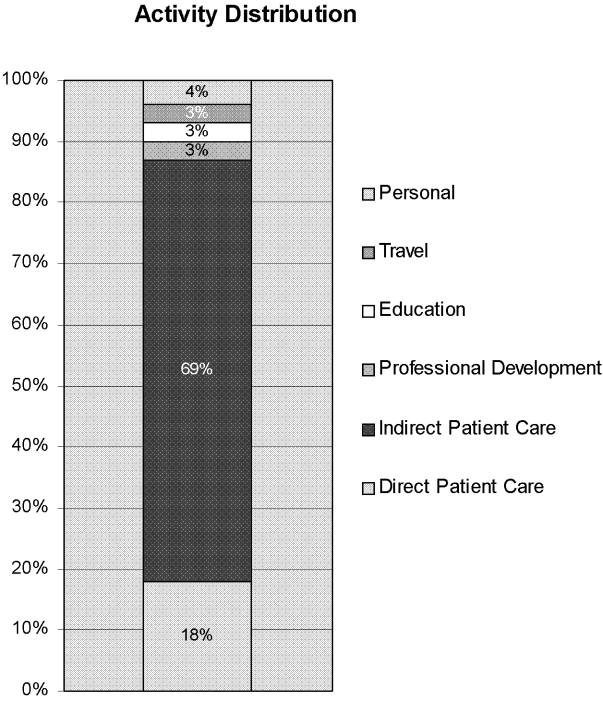
Of the time hospitalists directly cared for patients, 18% was spent obtaining histories and performing physical examinations on new patients, 53% seeing patients in follow‐up visits, 16% going over discharge instructions, and 13% in family meetings (Figure 2). Of the time hospitalists spent doing indirect patient care, 37% was taken up by documentation, 21% by reviewing results, 7% by orders, and 35% by communication (Figure 2).
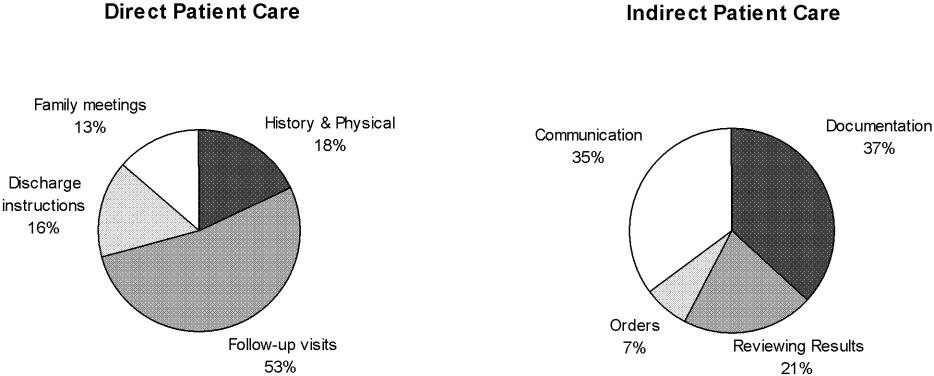
As just explained, communication accounted for 35% of indirect patient care activities; it also accounted for 24% of the total activity minutes. The time spent by hospitalists on communication was further broken down as 23% paging other physicians, 31% returning pages, 34% in face‐to‐face communication, 5% taking report on new admissions, 4% on sign‐out to the night float hospitalist, and 3% receiving sign‐out from the night float hospitalist.
Multitasking, performing more than 1 activity at the same time, was done 21% of the time. Hospitalists received an average of 3.4 1.5 pages per hour, and 7% of total activity time was spent returning pages. Other forms of interruption were not evaluated.
DISCUSSION
Our study had several important findings. First, hospitalists spent most of their time on indirect patient care activities and relatively little time on direct patient care. Time‐motion studies of nonhospitalist physicians have reported similar findings.5, 6 A considerable amount of hospitalist time was spent on documentation. This finding also has been reported in studies of nonhospitalist physicians.5, 7
A unique finding in our study was the large amount of time, 24% of total minutes, spent on communication. A study of emergency medicine physicians by Hollingsworth found that 13% of their time was spent on communication activities.5 The large amount of time spent on communication in our study underscores the need for hospitalists to have outstanding communication skills and systems that support efficient communication. Hospitalists spent 6% of their total time paging other physicians and 7% returning pages. Improvements in the efficiency of paging communication could greatly reduce the amount of time communicating by page. Our paging system provides unidirectional alphanumeric paging. In an effort to improve the efficiency of paging, we have asked nurses and consultants to include FYI and callback in the text of the page so it is clear whether the person who has paged the hospitalist needs to be called back. This simple solution to help reduce the number of unnecessary callbacks has previously been proposed by others.8
Another part of solving this problem is adopting the use of 2‐way pagers instead of alphanumeric pagers. Two‐way paging can increase the efficiency of communication even further. For example, a nurse sends a hospitalist a page that asks if the previous diet orders for a patient just returned from a procedure can be resumed. This hospitalist is on another floor in another patient's room. Rather than spending time leaving the other patient's room, finding a phone, calling the floor, waiting for an answer, and then waiting on hold, the hospitalist simply texts a 1‐word answer, Yes, in the 2‐way paging system. In addition to the time occupied by paging activities, hospitalists spent a large amount of time in face‐to‐face communication (8% of total activity time). On the one hand, having hospitalists discuss patient care with consultants and nurses in person on an ongoing basis throughout the day may improve clinical efficiency. On the other hand, the constant potential for interruption may be problematic. Similarly, 2‐way paging could facilitate communication to such a degree that it could actually increase the frequency of interruptions. Research on improvements in communications systems, interventions to improve communication skills, and team‐based care is warranted in order to evaluate the impact on hospitalist workflow.
An important finding in our study was that multitasking and paging interruptions were common. Although this may come as no surprise to practicing hospitalists, the distraction caused by interruptions and multitasking is an important potential cause of medical errors.911 A thorough examination of the types of activities performed simultaneously and whether they contributed to medical error was beyond the scope of our study. Some activities, such as documenting a note on a patient while reviewing the patient's lab results, are concordant (ie, conducted for the same patient) and therefore may be unlikely to contribute to medical error. Other combinations of activities, such as returning a page about one patient while documenting a note on a different patient or having face‐to‐face communication about one patient while entering an order on another patient, are discordant. Discordant activities may contribute to medical error. Further research of the effect of hospitalist multitasking and interruption on medical error is warranted and should be conducted within the framework of concordant versus discordant activities.
We had hoped to find activities that could be performed by non‐physician providers. No high impact activities were discovered that would be better suited for a non‐physician provider in this study. Clerical tasks, such as calling for radiology orders or obtaining medical records, amounted to a small percentage of hospitalist time (less than 1% combined). We did identify several activities in which automation or process improvement would be helpful. Hospitalists spent 5% of time on the combined activity of documenting discharge instructions and writing out prescriptions. Our institution is in the process of implementing an electronic medical record and computerized physician order entry. We are currently working on an automated process to generate printed discharge instructions and prescriptions. This has the potential not only to improve efficiency, but also to eliminate medication errors, as care is transitioned to the outpatient setting.
Our study had several limitations. First, our findings reflect the experience at one institution. Hospitalist practices vary widely in their staffing and scheduling models as well as in their organizational support. The amount of time that hospitalists spend on activities may differ between practices and between individual hospitalists in the same practice. Another limitation to our study pertains to the workflow of our hospitalists and the locations of their patients. As discussed earlier, patients were assigned to a hospitalist according to time of admission, not location of admission. Because of this, the hospitalists were caring for patients on as many as 5 wards. Although travel time amounted to only 3% of total minutes, it is possible that communication time could have been reduced if patients were distributed to hospitalists on the basis of patient location rather than time of admission of patient. For example, physicians and nurses might spend less time communicating in person compared to communicating via unidirectional paging, which frequently requires waiting for a callback. Finally, our study only observed activities performed by the daytime hospitalists at our hospital. The distribution and types of activities performed by nighttime hospitalists may be somewhat different.
Our study may serve as a model for hospitalist time‐motion studies in other settings. Our findings are of particular importance to resident‐uncovered hospitalist programs in academic hospitals, a setting in which operational inefficiencies may be abundant as house staff members have been poorly positioned in the hospital organization to lobby for process change. We hope that our study is a precursor to research evaluating modifications to the environments and systems in which hospitalists work. Such modifications have the potential to improve productivity and work conditions and promote career satisfaction.
Acknowledgements
We thank Patricia Georgas for shadowing the hospitalists and collecting the data in this study.
- Society of Hospital Medicine. Available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=FAQs106:441–445.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;4:392–393.
- ,,,,.How do physicians and nurses spend their time in the Emergency Department?Ann Emerg Med.1998;31:97–91.
- ,,,,.A time‐motion study of the activities of attending physicians in an Internal Medicine and Internal Medicine‐Pediatrics resident continuity clinic.Acad Med.2000;75:1138–1143.
- ,,,.Work interrupted: a comparison of workplace interruptions in emergency departments and primary care offices.Ann Emerg Med.2001;38:146–151.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,,,.Emergency department workplace interruptions: are emergency physicians “interrupt‐driven” and “multitasking”?Acad Emerg Med.2000;7:1239–243.
- ,,.Understanding medical error and improving patient safety in the inpatient setting.Med Clin N Am.2002;86:847–867.
- ,,,.Sharps‐related injuries in health care workers: a case‐crossover study.Am J Med.2003;114:687–694.
The hospitalist model of care has experienced dramatic growth. In 2003 it was estimated that there were 8000 US hospitalists, a number projected to ultimately reach more than 19 000.1, 2 This rapid growth has largely been driven by improvements in clinical efficiency as a result of hospitalist programs. There is a substantial body of evidence showing that hospitalists reduce length of stay and inpatient costs.3 Despite the rapid growth and proven benefit to clinical efficiency, no studies have evaluated the type and frequency of activities that hospitalists perform during routine work. Although the use of hospitalists improves clinical efficiency for the hospital, relatively little is known about how the hospital can improve efficiency for the hospitalist.
Our institution greatly expanded our hospitalist program in June 2003 to create a resident‐uncovered hospitalist service. The impetus for this change was the need to comply with newly revised Accreditation Council for Graduate Medicine Education (ACGME) program requirements regarding resident duty hours. Many teaching hospitals have implemented similar resident‐uncovered hospitalist services.4 Inefficiencies in their work activities quickly became apparent to our hospitalists. Furthermore, our hospitalists believed that they frequently performed simultaneous activities and that they were excessively interrupted by pages.
To evaluate the type and frequency of activities that the hospitalists performed during routine work, we performed a time‐motion study of hospitalist physicians on the resident‐uncovered hospitalist service. Our goal was to identify areas for systems improvements and activities that were better suited for nonphysician providers and to quantify the time spent multitasking and the frequency of paging interruptions.
METHODS
Northwestern Memorial Hospital (NMH) is a 753‐bed hospital in Chicago, Illinois. NMH is the primary teaching hospital affiliated with the Feinberg School of Medicine of Northwestern University. There are 2 general medicine services at NMH: a traditional resident‐covered ward service and the resident‐uncovered hospitalist service. Patients are admitted to one of these 2 services on the basis of, in order of importance, capacity of the services, preference of the outpatient physician, and potential educational value of the admission. Patients admitted to the hospitalist service are preferentially given beds on specific wards intended for hospitalist service patients. Fourth‐year medical students are frequently paired with hospitalists during their medicine subinternship.
The resident‐uncovered hospitalist service comprises 5 daytime hospitalists on duty at a time. The hospitalists are on service for 7 consecutive days, usually followed by 7 consecutive days off. Hospitalists pick up new patients from the night float hospitalist each morning. Daytime admitting duties rotate on a daily basis. One hospitalist accepts new admissions each morning from 7:00 AM until noon. Two hospitalists accept admissions from noon until 5:00 PM. One hospitalist accepts admissions from 5:00 PM until 9:00 PM. One hospitalist is free from accepting new admissions each day. All daytime hospitalists begin the workday at 7:00 AM and leave when their duties are completed for the day. One night float hospitalist is on duty each night of the week. The night float hospitalist performs admissions and all cross cover activities from 7:00 PM until 7:00 AM.
We first conducted a pilot study to help identify specific activities that our hospitalists routinely perform. Broad categories and subcategories of activities were created based on the results of our pilot study, and a published time‐motion study performed on emergency medicine physicians5 (Table 1). Once activities were defined and codes established, our research assistant unobtrusively shadowed hospitalist physicians for periods lasting 3‐5 hours. The observation periods were distributed in order to sample all activities that a daytime hospitalist would perform throughout a typical week. Observation periods included 2 morning admitting periods, 4 morning nonadmitting periods, 4 afternoon admitting periods, 4 afternoon nonadmitting periods, and 2 admitting periods from 5:00 PM to 9:00 PM. Activities were recorded on a standardized data collection form in 1‐minute intervals. When multiple activities were performed at the same time, all activities were recorded in the same 1‐minute interval. Incoming pages were recorded as well. To minimize the possibility that observation would affect hospitalist behavior, the research assistant was instructed not to initiate conversation with the hospitalists.
| Direct patient care |
| Taking initial history and physical exam |
| Seeing patient in follow‐up visit |
| Going over discharge instructions |
| Family meetings |
| Indirect patient care |
| Reviewing test results and medical records |
| Documentation |
| Documenting history and physical, daily notes, filling out discharge instructions, writing out prescriptions |
| Communication |
| Taking report from night float, taking admission report, face‐to‐face discussion, initiating and returning pages |
| Orders |
| Writing/emnputting orders, calling radiology |
| Professional development |
| Going to conferences, grand rounds, etc |
| Reading articles, textbooks, online references |
| Education |
| Teaching during work rounds |
| Didactic sessions with subintern |
| Travel |
| Walking, taking elevator, etc |
| Personal |
| Lunch, washroom break, etc. |
The data collection forms were manually abstracted and minutes tallied for each category and subcategory, for which summary statistics were converted to percentage of total minutes.
RESULTS
Ten hospitalists were shadowed by a single research assistant for a total of 4467 minutes. Seven hospitalists were male and 3 were female. The hospitalists were a mean age of 31 1.6 years of age and had been practicing as a hospitalist for a mean of 2.1 1.0 years. The hospitalists saw an average of 9.4 4.0 patients on the days they were shadowed by the research assistant. Because simultaneous activities were recorded, a total of 5557 minutes of activities were recorded.
The distribution of total minutes recorded in each activity category is shown in Figure 1. Hospitalists spent 18% of their time doing direct patient care, 69% on indirect patient care, 4% on personal activities, and 3% each on professional development, education, and travel.

Of the time hospitalists directly cared for patients, 18% was spent obtaining histories and performing physical examinations on new patients, 53% seeing patients in follow‐up visits, 16% going over discharge instructions, and 13% in family meetings (Figure 2). Of the time hospitalists spent doing indirect patient care, 37% was taken up by documentation, 21% by reviewing results, 7% by orders, and 35% by communication (Figure 2).

As just explained, communication accounted for 35% of indirect patient care activities; it also accounted for 24% of the total activity minutes. The time spent by hospitalists on communication was further broken down as 23% paging other physicians, 31% returning pages, 34% in face‐to‐face communication, 5% taking report on new admissions, 4% on sign‐out to the night float hospitalist, and 3% receiving sign‐out from the night float hospitalist.
Multitasking, performing more than 1 activity at the same time, was done 21% of the time. Hospitalists received an average of 3.4 1.5 pages per hour, and 7% of total activity time was spent returning pages. Other forms of interruption were not evaluated.
DISCUSSION
Our study had several important findings. First, hospitalists spent most of their time on indirect patient care activities and relatively little time on direct patient care. Time‐motion studies of nonhospitalist physicians have reported similar findings.5, 6 A considerable amount of hospitalist time was spent on documentation. This finding also has been reported in studies of nonhospitalist physicians.5, 7
A unique finding in our study was the large amount of time, 24% of total minutes, spent on communication. A study of emergency medicine physicians by Hollingsworth found that 13% of their time was spent on communication activities.5 The large amount of time spent on communication in our study underscores the need for hospitalists to have outstanding communication skills and systems that support efficient communication. Hospitalists spent 6% of their total time paging other physicians and 7% returning pages. Improvements in the efficiency of paging communication could greatly reduce the amount of time communicating by page. Our paging system provides unidirectional alphanumeric paging. In an effort to improve the efficiency of paging, we have asked nurses and consultants to include FYI and callback in the text of the page so it is clear whether the person who has paged the hospitalist needs to be called back. This simple solution to help reduce the number of unnecessary callbacks has previously been proposed by others.8
Another part of solving this problem is adopting the use of 2‐way pagers instead of alphanumeric pagers. Two‐way paging can increase the efficiency of communication even further. For example, a nurse sends a hospitalist a page that asks if the previous diet orders for a patient just returned from a procedure can be resumed. This hospitalist is on another floor in another patient's room. Rather than spending time leaving the other patient's room, finding a phone, calling the floor, waiting for an answer, and then waiting on hold, the hospitalist simply texts a 1‐word answer, Yes, in the 2‐way paging system. In addition to the time occupied by paging activities, hospitalists spent a large amount of time in face‐to‐face communication (8% of total activity time). On the one hand, having hospitalists discuss patient care with consultants and nurses in person on an ongoing basis throughout the day may improve clinical efficiency. On the other hand, the constant potential for interruption may be problematic. Similarly, 2‐way paging could facilitate communication to such a degree that it could actually increase the frequency of interruptions. Research on improvements in communications systems, interventions to improve communication skills, and team‐based care is warranted in order to evaluate the impact on hospitalist workflow.
An important finding in our study was that multitasking and paging interruptions were common. Although this may come as no surprise to practicing hospitalists, the distraction caused by interruptions and multitasking is an important potential cause of medical errors.911 A thorough examination of the types of activities performed simultaneously and whether they contributed to medical error was beyond the scope of our study. Some activities, such as documenting a note on a patient while reviewing the patient's lab results, are concordant (ie, conducted for the same patient) and therefore may be unlikely to contribute to medical error. Other combinations of activities, such as returning a page about one patient while documenting a note on a different patient or having face‐to‐face communication about one patient while entering an order on another patient, are discordant. Discordant activities may contribute to medical error. Further research of the effect of hospitalist multitasking and interruption on medical error is warranted and should be conducted within the framework of concordant versus discordant activities.
We had hoped to find activities that could be performed by non‐physician providers. No high impact activities were discovered that would be better suited for a non‐physician provider in this study. Clerical tasks, such as calling for radiology orders or obtaining medical records, amounted to a small percentage of hospitalist time (less than 1% combined). We did identify several activities in which automation or process improvement would be helpful. Hospitalists spent 5% of time on the combined activity of documenting discharge instructions and writing out prescriptions. Our institution is in the process of implementing an electronic medical record and computerized physician order entry. We are currently working on an automated process to generate printed discharge instructions and prescriptions. This has the potential not only to improve efficiency, but also to eliminate medication errors, as care is transitioned to the outpatient setting.
Our study had several limitations. First, our findings reflect the experience at one institution. Hospitalist practices vary widely in their staffing and scheduling models as well as in their organizational support. The amount of time that hospitalists spend on activities may differ between practices and between individual hospitalists in the same practice. Another limitation to our study pertains to the workflow of our hospitalists and the locations of their patients. As discussed earlier, patients were assigned to a hospitalist according to time of admission, not location of admission. Because of this, the hospitalists were caring for patients on as many as 5 wards. Although travel time amounted to only 3% of total minutes, it is possible that communication time could have been reduced if patients were distributed to hospitalists on the basis of patient location rather than time of admission of patient. For example, physicians and nurses might spend less time communicating in person compared to communicating via unidirectional paging, which frequently requires waiting for a callback. Finally, our study only observed activities performed by the daytime hospitalists at our hospital. The distribution and types of activities performed by nighttime hospitalists may be somewhat different.
Our study may serve as a model for hospitalist time‐motion studies in other settings. Our findings are of particular importance to resident‐uncovered hospitalist programs in academic hospitals, a setting in which operational inefficiencies may be abundant as house staff members have been poorly positioned in the hospital organization to lobby for process change. We hope that our study is a precursor to research evaluating modifications to the environments and systems in which hospitalists work. Such modifications have the potential to improve productivity and work conditions and promote career satisfaction.
Acknowledgements
We thank Patricia Georgas for shadowing the hospitalists and collecting the data in this study.
The hospitalist model of care has experienced dramatic growth. In 2003 it was estimated that there were 8000 US hospitalists, a number projected to ultimately reach more than 19 000.1, 2 This rapid growth has largely been driven by improvements in clinical efficiency as a result of hospitalist programs. There is a substantial body of evidence showing that hospitalists reduce length of stay and inpatient costs.3 Despite the rapid growth and proven benefit to clinical efficiency, no studies have evaluated the type and frequency of activities that hospitalists perform during routine work. Although the use of hospitalists improves clinical efficiency for the hospital, relatively little is known about how the hospital can improve efficiency for the hospitalist.
Our institution greatly expanded our hospitalist program in June 2003 to create a resident‐uncovered hospitalist service. The impetus for this change was the need to comply with newly revised Accreditation Council for Graduate Medicine Education (ACGME) program requirements regarding resident duty hours. Many teaching hospitals have implemented similar resident‐uncovered hospitalist services.4 Inefficiencies in their work activities quickly became apparent to our hospitalists. Furthermore, our hospitalists believed that they frequently performed simultaneous activities and that they were excessively interrupted by pages.
To evaluate the type and frequency of activities that the hospitalists performed during routine work, we performed a time‐motion study of hospitalist physicians on the resident‐uncovered hospitalist service. Our goal was to identify areas for systems improvements and activities that were better suited for nonphysician providers and to quantify the time spent multitasking and the frequency of paging interruptions.
METHODS
Northwestern Memorial Hospital (NMH) is a 753‐bed hospital in Chicago, Illinois. NMH is the primary teaching hospital affiliated with the Feinberg School of Medicine of Northwestern University. There are 2 general medicine services at NMH: a traditional resident‐covered ward service and the resident‐uncovered hospitalist service. Patients are admitted to one of these 2 services on the basis of, in order of importance, capacity of the services, preference of the outpatient physician, and potential educational value of the admission. Patients admitted to the hospitalist service are preferentially given beds on specific wards intended for hospitalist service patients. Fourth‐year medical students are frequently paired with hospitalists during their medicine subinternship.
The resident‐uncovered hospitalist service comprises 5 daytime hospitalists on duty at a time. The hospitalists are on service for 7 consecutive days, usually followed by 7 consecutive days off. Hospitalists pick up new patients from the night float hospitalist each morning. Daytime admitting duties rotate on a daily basis. One hospitalist accepts new admissions each morning from 7:00 AM until noon. Two hospitalists accept admissions from noon until 5:00 PM. One hospitalist accepts admissions from 5:00 PM until 9:00 PM. One hospitalist is free from accepting new admissions each day. All daytime hospitalists begin the workday at 7:00 AM and leave when their duties are completed for the day. One night float hospitalist is on duty each night of the week. The night float hospitalist performs admissions and all cross cover activities from 7:00 PM until 7:00 AM.
We first conducted a pilot study to help identify specific activities that our hospitalists routinely perform. Broad categories and subcategories of activities were created based on the results of our pilot study, and a published time‐motion study performed on emergency medicine physicians5 (Table 1). Once activities were defined and codes established, our research assistant unobtrusively shadowed hospitalist physicians for periods lasting 3‐5 hours. The observation periods were distributed in order to sample all activities that a daytime hospitalist would perform throughout a typical week. Observation periods included 2 morning admitting periods, 4 morning nonadmitting periods, 4 afternoon admitting periods, 4 afternoon nonadmitting periods, and 2 admitting periods from 5:00 PM to 9:00 PM. Activities were recorded on a standardized data collection form in 1‐minute intervals. When multiple activities were performed at the same time, all activities were recorded in the same 1‐minute interval. Incoming pages were recorded as well. To minimize the possibility that observation would affect hospitalist behavior, the research assistant was instructed not to initiate conversation with the hospitalists.
| Direct patient care |
| Taking initial history and physical exam |
| Seeing patient in follow‐up visit |
| Going over discharge instructions |
| Family meetings |
| Indirect patient care |
| Reviewing test results and medical records |
| Documentation |
| Documenting history and physical, daily notes, filling out discharge instructions, writing out prescriptions |
| Communication |
| Taking report from night float, taking admission report, face‐to‐face discussion, initiating and returning pages |
| Orders |
| Writing/emnputting orders, calling radiology |
| Professional development |
| Going to conferences, grand rounds, etc |
| Reading articles, textbooks, online references |
| Education |
| Teaching during work rounds |
| Didactic sessions with subintern |
| Travel |
| Walking, taking elevator, etc |
| Personal |
| Lunch, washroom break, etc. |
The data collection forms were manually abstracted and minutes tallied for each category and subcategory, for which summary statistics were converted to percentage of total minutes.
RESULTS
Ten hospitalists were shadowed by a single research assistant for a total of 4467 minutes. Seven hospitalists were male and 3 were female. The hospitalists were a mean age of 31 1.6 years of age and had been practicing as a hospitalist for a mean of 2.1 1.0 years. The hospitalists saw an average of 9.4 4.0 patients on the days they were shadowed by the research assistant. Because simultaneous activities were recorded, a total of 5557 minutes of activities were recorded.
The distribution of total minutes recorded in each activity category is shown in Figure 1. Hospitalists spent 18% of their time doing direct patient care, 69% on indirect patient care, 4% on personal activities, and 3% each on professional development, education, and travel.

Of the time hospitalists directly cared for patients, 18% was spent obtaining histories and performing physical examinations on new patients, 53% seeing patients in follow‐up visits, 16% going over discharge instructions, and 13% in family meetings (Figure 2). Of the time hospitalists spent doing indirect patient care, 37% was taken up by documentation, 21% by reviewing results, 7% by orders, and 35% by communication (Figure 2).

As just explained, communication accounted for 35% of indirect patient care activities; it also accounted for 24% of the total activity minutes. The time spent by hospitalists on communication was further broken down as 23% paging other physicians, 31% returning pages, 34% in face‐to‐face communication, 5% taking report on new admissions, 4% on sign‐out to the night float hospitalist, and 3% receiving sign‐out from the night float hospitalist.
Multitasking, performing more than 1 activity at the same time, was done 21% of the time. Hospitalists received an average of 3.4 1.5 pages per hour, and 7% of total activity time was spent returning pages. Other forms of interruption were not evaluated.
DISCUSSION
Our study had several important findings. First, hospitalists spent most of their time on indirect patient care activities and relatively little time on direct patient care. Time‐motion studies of nonhospitalist physicians have reported similar findings.5, 6 A considerable amount of hospitalist time was spent on documentation. This finding also has been reported in studies of nonhospitalist physicians.5, 7
A unique finding in our study was the large amount of time, 24% of total minutes, spent on communication. A study of emergency medicine physicians by Hollingsworth found that 13% of their time was spent on communication activities.5 The large amount of time spent on communication in our study underscores the need for hospitalists to have outstanding communication skills and systems that support efficient communication. Hospitalists spent 6% of their total time paging other physicians and 7% returning pages. Improvements in the efficiency of paging communication could greatly reduce the amount of time communicating by page. Our paging system provides unidirectional alphanumeric paging. In an effort to improve the efficiency of paging, we have asked nurses and consultants to include FYI and callback in the text of the page so it is clear whether the person who has paged the hospitalist needs to be called back. This simple solution to help reduce the number of unnecessary callbacks has previously been proposed by others.8
Another part of solving this problem is adopting the use of 2‐way pagers instead of alphanumeric pagers. Two‐way paging can increase the efficiency of communication even further. For example, a nurse sends a hospitalist a page that asks if the previous diet orders for a patient just returned from a procedure can be resumed. This hospitalist is on another floor in another patient's room. Rather than spending time leaving the other patient's room, finding a phone, calling the floor, waiting for an answer, and then waiting on hold, the hospitalist simply texts a 1‐word answer, Yes, in the 2‐way paging system. In addition to the time occupied by paging activities, hospitalists spent a large amount of time in face‐to‐face communication (8% of total activity time). On the one hand, having hospitalists discuss patient care with consultants and nurses in person on an ongoing basis throughout the day may improve clinical efficiency. On the other hand, the constant potential for interruption may be problematic. Similarly, 2‐way paging could facilitate communication to such a degree that it could actually increase the frequency of interruptions. Research on improvements in communications systems, interventions to improve communication skills, and team‐based care is warranted in order to evaluate the impact on hospitalist workflow.
An important finding in our study was that multitasking and paging interruptions were common. Although this may come as no surprise to practicing hospitalists, the distraction caused by interruptions and multitasking is an important potential cause of medical errors.911 A thorough examination of the types of activities performed simultaneously and whether they contributed to medical error was beyond the scope of our study. Some activities, such as documenting a note on a patient while reviewing the patient's lab results, are concordant (ie, conducted for the same patient) and therefore may be unlikely to contribute to medical error. Other combinations of activities, such as returning a page about one patient while documenting a note on a different patient or having face‐to‐face communication about one patient while entering an order on another patient, are discordant. Discordant activities may contribute to medical error. Further research of the effect of hospitalist multitasking and interruption on medical error is warranted and should be conducted within the framework of concordant versus discordant activities.
We had hoped to find activities that could be performed by non‐physician providers. No high impact activities were discovered that would be better suited for a non‐physician provider in this study. Clerical tasks, such as calling for radiology orders or obtaining medical records, amounted to a small percentage of hospitalist time (less than 1% combined). We did identify several activities in which automation or process improvement would be helpful. Hospitalists spent 5% of time on the combined activity of documenting discharge instructions and writing out prescriptions. Our institution is in the process of implementing an electronic medical record and computerized physician order entry. We are currently working on an automated process to generate printed discharge instructions and prescriptions. This has the potential not only to improve efficiency, but also to eliminate medication errors, as care is transitioned to the outpatient setting.
Our study had several limitations. First, our findings reflect the experience at one institution. Hospitalist practices vary widely in their staffing and scheduling models as well as in their organizational support. The amount of time that hospitalists spend on activities may differ between practices and between individual hospitalists in the same practice. Another limitation to our study pertains to the workflow of our hospitalists and the locations of their patients. As discussed earlier, patients were assigned to a hospitalist according to time of admission, not location of admission. Because of this, the hospitalists were caring for patients on as many as 5 wards. Although travel time amounted to only 3% of total minutes, it is possible that communication time could have been reduced if patients were distributed to hospitalists on the basis of patient location rather than time of admission of patient. For example, physicians and nurses might spend less time communicating in person compared to communicating via unidirectional paging, which frequently requires waiting for a callback. Finally, our study only observed activities performed by the daytime hospitalists at our hospital. The distribution and types of activities performed by nighttime hospitalists may be somewhat different.
Our study may serve as a model for hospitalist time‐motion studies in other settings. Our findings are of particular importance to resident‐uncovered hospitalist programs in academic hospitals, a setting in which operational inefficiencies may be abundant as house staff members have been poorly positioned in the hospital organization to lobby for process change. We hope that our study is a precursor to research evaluating modifications to the environments and systems in which hospitalists work. Such modifications have the potential to improve productivity and work conditions and promote career satisfaction.
Acknowledgements
We thank Patricia Georgas for shadowing the hospitalists and collecting the data in this study.
- Society of Hospital Medicine. Available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=FAQs106:441–445.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;4:392–393.
- ,,,,.How do physicians and nurses spend their time in the Emergency Department?Ann Emerg Med.1998;31:97–91.
- ,,,,.A time‐motion study of the activities of attending physicians in an Internal Medicine and Internal Medicine‐Pediatrics resident continuity clinic.Acad Med.2000;75:1138–1143.
- ,,,.Work interrupted: a comparison of workplace interruptions in emergency departments and primary care offices.Ann Emerg Med.2001;38:146–151.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,,,.Emergency department workplace interruptions: are emergency physicians “interrupt‐driven” and “multitasking”?Acad Emerg Med.2000;7:1239–243.
- ,,.Understanding medical error and improving patient safety in the inpatient setting.Med Clin N Am.2002;86:847–867.
- ,,,.Sharps‐related injuries in health care workers: a case‐crossover study.Am J Med.2003;114:687–694.
- Society of Hospital Medicine. Available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=FAQs106:441–445.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;4:392–393.
- ,,,,.How do physicians and nurses spend their time in the Emergency Department?Ann Emerg Med.1998;31:97–91.
- ,,,,.A time‐motion study of the activities of attending physicians in an Internal Medicine and Internal Medicine‐Pediatrics resident continuity clinic.Acad Med.2000;75:1138–1143.
- ,,,.Work interrupted: a comparison of workplace interruptions in emergency departments and primary care offices.Ann Emerg Med.2001;38:146–151.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,,,.Emergency department workplace interruptions: are emergency physicians “interrupt‐driven” and “multitasking”?Acad Emerg Med.2000;7:1239–243.
- ,,.Understanding medical error and improving patient safety in the inpatient setting.Med Clin N Am.2002;86:847–867.
- ,,,.Sharps‐related injuries in health care workers: a case‐crossover study.Am J Med.2003;114:687–694.
Copyright © 2006 Society of Hospital Medicine
Acute Aortic Dissection
Aortic dissection is an uncommon but highly lethal disease with an incidence of approximately 2,000 cases per year in the United States.1 It is often mistaken for less serious pathology. In one series, aortic dissection was missed in 38% of patients at presentation, with 28% of patients first diagnosed at autopsy.2 Early recognition and management are crucial. If untreated, the mortality rate for acute aortic dissection increases by approximately 1% per hour over the first 48 hours and may reach 70% at 1 week. As many as 90% of untreated patients who suffer aortic dissection die within 3 months of presentation.3, 4 Generally, cardiothoracic surgeons or cardiologists experienced with managing aortic dissection should direct patient evaluation and treatment. Hospitalists, however, are increasingly assuming responsibility for the initial triage and management of patients with acute chest pain syndromes and therefore must be able to rapidly identify aortic dissection, initiate supportive therapy, and refer patients to appropriate specialty care.
PATHOPHYSIOLOGY
Aortic dissection occurs when layers of the aortic wall separate because of infiltration of high‐pressure arterial blood. The proximate causes are elevated shear stress across the aortic lumen in the setting of a concomitant defect in the aortic media. Shear stress is caused by the rapid increase in luminal pressure per unit of time (dP/dt) that results from cardiac systole. As the aorta traverses away from the heart, an increasing proportion of the kinetic energy of left ventricular systole is stored in the aortic wall as potential energy, which facilitates anterograde propagation of cardiac output during diastole. This conversion of kinetic to potential energy also attenuates shear stress. As the proximal aorta is subject to the steepest fluctuations in pressure, it is at the highest risk of dissection. Degeneration of the aortic media is part of the normal aging process but is accelerated in persons with a bicuspid aortic valve, Turner's syndrome, inflammatory arteritis, or inherited diseases of collagen formation.
Once the aortic intima is compromised, blood dissects longitudinally through the aortic media and propagates proximally or distally, creating a false lumen that may communicate with the true lumen of the aorta. Blood may flow through the true lumen, the false lumen, or both. Propagation of the dissection causes much of the morbidity associated with aortic dissection by disrupting blood flow across branch vessels or by directly compromising the pericardium or aortic valve. Over time, the dissection may traverse the entire aortic wall, causing aortic rupture and exsanguination.
CLASSIFICATION
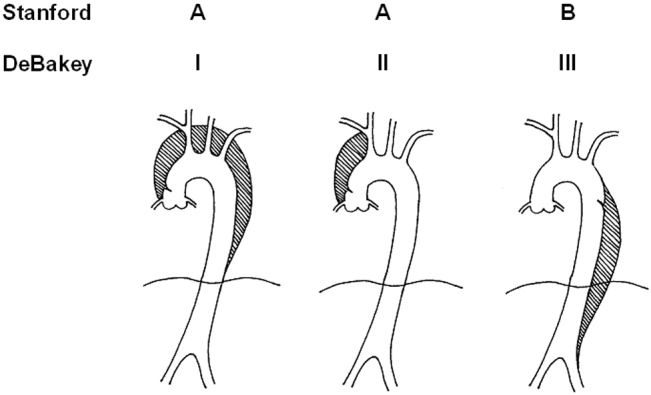
Acute aortic dissection is classified as any aortic dissection diagnosed within 2 weeks of the onset of symptoms, which is the period of highest risk of mortality. Patients who survive more than 2 weeks without treatment are considered to have chronic dissection. Aortic dissections are further classified according to their anatomic location. The fundamental distinction is whether the dissection is proximal (involving the aortic root or ascending aorta) or distal (below the left subclavian artery). The Stanford and DeBakey classification systems are the classification systems most commonly used (Figure 1).
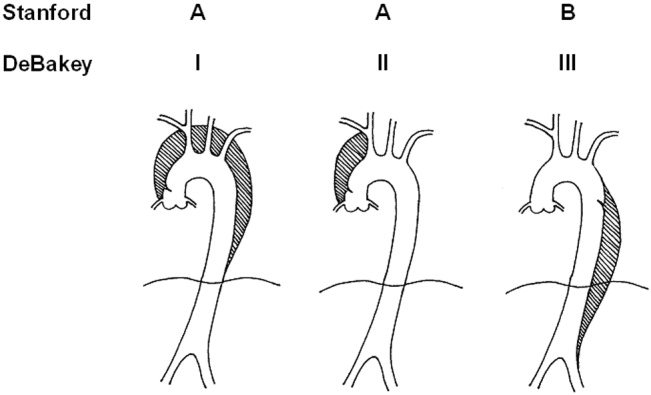
Some variants of aortic dissection are not described in either the Stanford or DeBakey systems. Aortic intramural hematomas (IMH) are caused by intramural hemorrhage of the vasa vasorum without an identifiable intimal tear.57 Penetrating atherosclerotic ulcers (PAUs) are focal defects in the aortic wall with surrounding hematoma but no longitudinal dissection across tissue planes, typically resulting from advanced atherosclerotic disease.8 The pathophysiologic distinctions between IMH, PAU, and classic aortic dissection remain somewhat controversial. Both IMH and PAU may progress to aortic aneurysm formation, frank dissection, or aortic rupture, suggesting that these entities represent a spectrum of diseases with broad overlap (Table 1).9, 10
| Acuity | |
| Acute 2 weeks after onset | |
| Chronic: >2 weeks after onset | |
| Anatomic location: | |
| Ascending aorta: | Stanford Type A, Debakey Type II |
| Ascending and descending aorta: | Stanford Type A, Debakey Type I |
| Descending aorta: | Stanford Type B, Debakey Type III |
| Pathophysiology: | |
| Class 1: Classical aortic dissection with initimal flap between true and false lumen | |
| Class 2: Aortic intramural hematoma without identifiable intimal flap | |
| Class 3: Intimal tear without hematoma (limited dissection) | |
| Class 4: Atherosclerotic plaque rupture with aortic penetrating ulcer | |
| Class 5: Iatrogenic or traumatic aortic dissection (intra‐aortic catherterization, high‐speed deceleration injury, blunt chest trauma) | |
EPIDEMIOLOGY
Aortic dissection is a rare disease, with an estimated incidence of approximately 5‐30 cases per 1 million people per year.1114 Fewer than 0.5% of patients presenting to an emergency department with chest or back pain suffer from aortic dissection.15 Two thirds of patients are male, with an average age at presentation of approximately 65 years. A history of systemic hypertension, found in up to 72% of patients, is by far the most common risk factor.2, 14, 16 Atherosclerosis, a history of prior cardiac surgery, and known aortic aneurysm are other major risk factors.14 The epidemiology of aortic dissection is substantially different in young patients (40 years of age). Hypertension and atherosclerosis become significantly less common, as other risk factors, such as Marfan syndrome, take precedence17 (Table 2). Other risk factors for aortic dissection include:
-
Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos): In the International Registry of Acute Aortic Dissection (IRAD), the largest prospective analysis of aortic dissection to date, 50% of the young patients presenting with aortic dissection had Marfan syndrome.17
-
Bicuspid aortic valve (BAV): Individuals with BAV are 5‐18 times more likely to suffer aortic dissection than those with a trileaflet valve.18, 19 In one survey, 52% of asymptomatic young men with BAV were found to have aortic root dilatation, a frequent precursor of dissection.20 Vascular tissue in individuals with BAV has been found to have increased levels of matrix metalloproteinases, which may degrade elastic matrix components and accelerate medial necrosis.21
-
Aortic coarctation: Aortic coarctation is associated with upper extremity hypertension, BAV and aortic dilatation, all of which predispose to aortic dissection.
-
Turner syndrome: Aortic root dilatation with or without dissection has been incidentally noted in 6%‐9% of patients with Turner syndrome.22, 23
-
Strenuous exercise: Multiple case reports have associated aortic dissection with high‐intensity weightlifting. Many affected individuals were subsequently found to have at least one other risk factor, including hypertension, anabolic steroid abuse, and cocaine abuse.2426
-
Large vessel arteritis: Large vessel arteritides, specifically giant cell arteritis, Takayasu's disease, and tertiary syphilis have long been associated with aortic dilatation and dissection.
-
Cocaine and methamphetamine ingestion: Sympathomimetic drugs cause rapid increases in heart rate and blood pressure, markedly increasing aortic intraluminal shear stress. Furthermore, cocaine is thought to be directly toxic to vascular endothelium and may accelerate medial necrosis.2730
-
Third trimester pregnancy, especially in patients with diseases of collagen31; The significance of pregnancy has recently been called into question by data from the IRAD trial. Of 346 enrolled women with aortic dissection, only 2 were pregnant, suggesting that the previously held association of pregnancy with aortic dissection may be an artifact of selective reporting.1
-
Blunt chest trauma or high‐speed deceleration injury.
-
Iatrogenic injury, typically from intra‐aortic catheterization.
| Hypertension |
| Atherosclerotic disease |
| History of cardiac surgery |
| Aortic aneurysm |
| Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos) |
| Bicuspid aortic valve (BAV) |
| Aortic coarctation |
| Turner syndrome |
| Strenuous exercise |
| Large vessel arteritis: giant cell, Takayasu's, syphilis |
| Cocaine and methamphetamine ingestion |
| Third‐trimester pregnancy |
| Blunt chest trauma or high‐speed deceleration injury |
| Iatrogenic injury, typically from intra‐aortic catheterization |
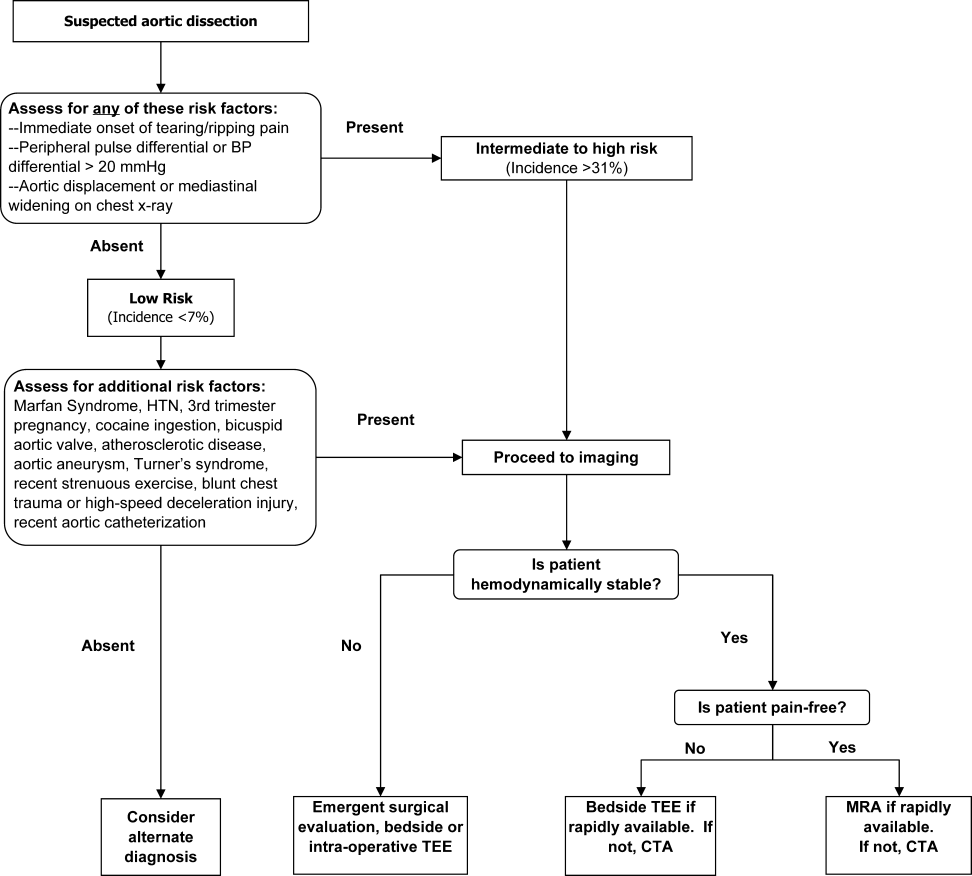
INITIAL EVALUATION
The differential diagnosis for acute aortic dissection includes acute coronary syndrome, pulmonary embolus, pneumothorax, pneumonia, musculoskeletal pain, acute cholecystitis, esophageal spasm or rupture, acute pancreatitis, and acute pericarditis. Acute aortic dissections are rarely asymptomatic; in fact, the absence of sudden‐onset chest pain decreases the likelihood of dissection (negative LR 0.3).32 In the IRAD trial, approximately 95% of patients with aortic dissection complained of pain in the chest, back, or abdomen, with 90% characterizing their pain as either severe or the worst ever and 64% describing it as sharp.14 Although the presence of tearing or ripping chest or back pain suggests aortic dissection (positive LR 1.2‐10.8), its absence does not reliably exclude this diagnosis.32 The wide variability in the presentation of aortic dissection increases the challenge of establishing a diagnosis. Clinical findings depend largely on the anatomical location of the dissection and may include pulse deficits, neurologic deficits, hypotension, hypertension, and end‐organ ischemia. Women who develop aortic dissection are generally older and present later than men. Their symptoms are less typical and are likely to be confounded by altered mental status.1 A diagnosis of aortic dissection should be strongly considered for patients presenting with acute chest or back pain and otherwise unexplained aortic insufficiency, focal neurologic deficits, pulse deficits, or end‐organ injury (Table 3).
| Hypotension or shock due to: |
| a. Hemopericardium and pericardial tamponade |
| b. Acute aortic insufficiency due to dilatation of the aortic annulus |
| c. Aortic rupture |
| d. Lactic acidosis |
| e. Spinal shock |
| Acute myocardial ischemia/emnfarction due to coronary ostial occlusion |
| Pericardial friction rub due to hemopericardium |
| Syncope |
| Pleural effusion or frank hemothorax |
| Acute renal failure due to dissection across renal arteries |
| Mesenteric ischemia due to dissection across intra‐abdominal arteries |
| Neurologic deficits: |
| a. Stroke due to occlusion of arch vessels |
| b. Limb weakness |
| c. Spinal cord deficits due to cord ischemia |
| d. Horner syndrome due to compression of superior sympathetic ganglion. |
| e. Hoarseness due to compression of left recurrent laryngeal nerve |
Electrocardiogram: Electrocardiographic abnormalities are commonly seen in aortic dissection and may include ST‐segment or T‐wave abnormalities or left ventricular hypertrophy.14 Proximal aortic dissections may compromise coronary artery perfusion, generating electrocardiogram (ECG) findings compatible with acute myocardial infarction, which may lead the clinician to diagnose and treat myocardial infarction while missing the underlying diagnosis.33 In a recent survey, 9 of 44 patients (21%) presenting with acute aortic dissection were initially diagnosed with acute coronary syndrome and anticoagulated, with 2 deaths.34 ECGs must therefore be interpreted with extreme caution in aortic dissection.
Chest x‐ray: In the emergency department, chest radiography is a mainstay of the evaluation of acute chest pain. Unfortunately, plain‐film radiography has limited utility for diagnosing aortic dissection.35 In the IRAD trial, mediastinal widening (>8 cm) and abnormal aortic contour, the classic radiographic findings in aortic dissection, were present in only 50%‐60% of cases. Twelve percent of patients had a completely normal chest x‐ray.14 A pooled analysis of previous studies demonstrated that the sensitivity of widened mediastinum and abnormal aortic contour was 65% and 71%, respectively.32 Nonspecific radiographic findings, most notably pleural effusion, were common.36 Thus, if the index of suspicion for aortic dissection is elevated, a confirmatory study must be obtained (Figure 2).
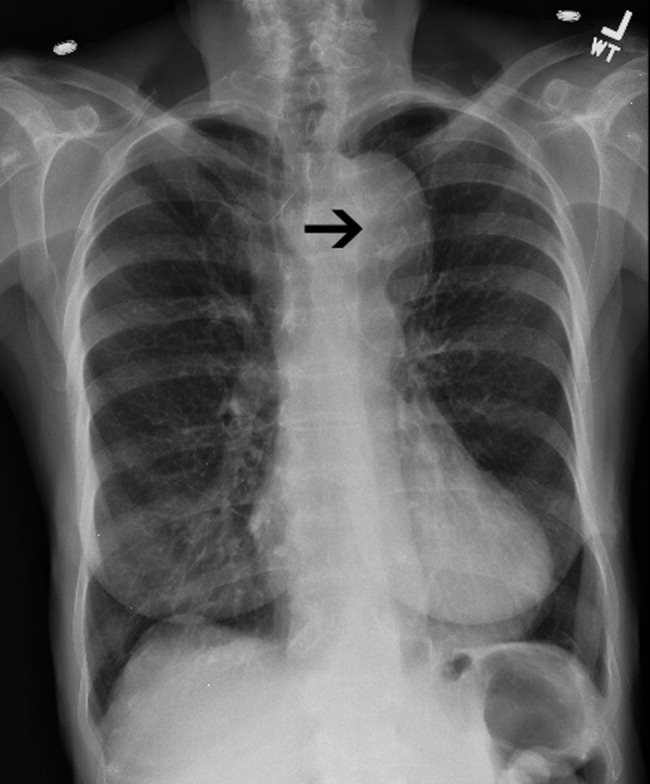
Clinical Prediction Tool
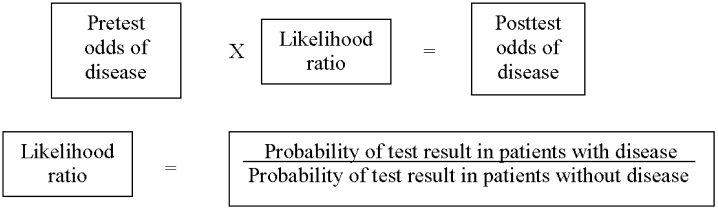
Three clinical features were demonstrated to be effective in identifying aortic dissection in patients presenting with acute chest or back pain: immediate onset of tearing or ripping chest pain, mediastinal widening or aortic enlargement/displacement observed on chest x‐ray, and arm pulse or blood pressure differential exceeding 20 mm Hg. When all 3 findings were absent, dissection was unlikely (7% probability, negative LR 0.07 [CI 0.03‐0.17]). If either chest pain or radiographic findings were present, the likelihood was intermediate (31%‐39% probability). With any other combination of findings, dissection was likely (83‐100% probability). This prediction tool effectively identified 96% of all patients who presented to an emergency department with acute aortic dissection.15 However, 4% of patients categorized as low risk were ultimately diagnosed with aortic dissection. Given the exceptionally high mortality resulting from a missed diagnosis, a 4% false‐negative rate is unacceptably high. Thus, the absence of any of the aforementioned findings should not dissuade the clinician from obtaining a confirmatory imaging study if the pretest probability for acute aortic dissection is elevated.
CONFIRMATORY IMAGING STUDIES
The ideal confirmatory imaging modality should identify aortic dissection with high sensitivity and specificity. It should also identify the entry and exit points of the dissection and provide information about the extent of compromise of the aortic valve, pericardium, and great vessels. Four imaging modalities sufficiently meet these criteria in order to be considered diagnostically useful.
Aortography: Previously the gold standard for diagnosing aortic dissection, aortography is no longer a first‐line imaging modality. The sensitivity and specificity of aortography are at best equivalent and probably inferior to less invasive imaging modalities.37, 38 False negatives may occur if both the true and false lumens opacify equally with contrast, or if the false lumen is sufficiently thrombosed to preclude any instillation of contrast. Aortography cannot identify aortic intramural hematomas, is invasive and highly operator dependent, requires nephrotoxic contrast, and generally takes longer to obtain than other modalities.39
Aortography uniquely offers excellent visualization of the coronary arteries and great vessels and is preferred when such information is necessary. Percutaneous aortic endovascular stent grafting has been recently employed to repair distal aortic dissections.4043 As a result, aortography is gaining new life as a therapeutic modality.
CT angiography: Spiral CT angiography (CTA) is the most commonly used modality for diagnosing aortic dissection.44 It is emergently available at most hospitals, and images can be obtained in minutes. Sensitivity and specificity may approach 100%, and CTA may be more sensitive than MRA or TEE in evaluating arch vessel involvement.4547 Like conventional angiography, CTA requires administration of nephrotoxic contrast. It frequently cannot visualize the entry and exit sites (intimal flaps) of a dissection and provides limited information about the coronary arteries and no information about the competency of the aortic valve.48, 49 Thus, if aortic dissection is identified by CTA, a second study may be needed to provide further diagnostic information and to guide surgical intervention (Figures 3 and 4).

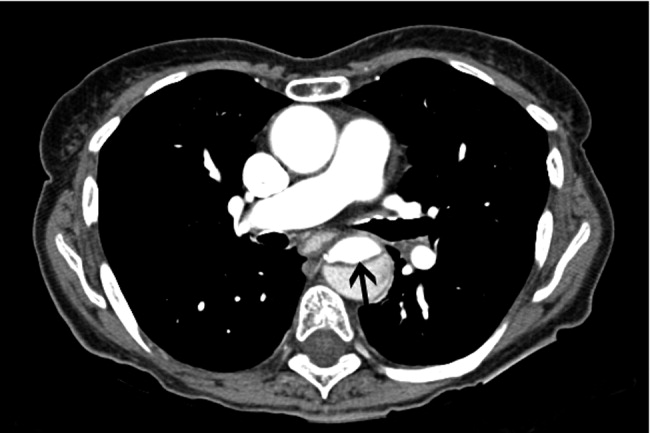
Magnetic Resonance Angiography: Magnetic resonance angiography (MRA) offers excellent noninvasive evaluation of the thoracic aorta. Sensitivity and specificity are probably superior to spiral CTA, and MRA generally identifies the location of the intimal tear and provides some functional information about the aortic valve.44, 50, 51 MRA is not emergently available at many hospitals. Scanning is time intensive, requiring the patient to remain motionless and relatively inaccessible for up to an hour. Furthermore, patient claustrophobia and the presence of implanted devices such as pacemakers or ferromagnetic foreign bodies may preclude MRA.
Transesophageal echocardiography: The sensitivity and specificity of transesophageal echocardiography (TEE) are also excellenton a par with CTA and MRA. In addition to providing excellent visualization of the thoracic aorta, TEE provides superb images of the pericardium and detailed assessment of aortic valve function.52 It also is extremely effective at visualizing the aortic intimal flap.44, 49, 53 A significant advantage of TEE is its portability, allowing rapid diagnosis at the bedside. For this reason, it is particularly useful for evaluation of patients who are hemodynamically unstable and are suspected to have an aortic dissection. Because of the anatomic relationship of the aorta with the esophagus and the trachea, TEE more effectively identifies proximal than distal dissections.43 TEE is also somewhat invasive, usually requires patient sedation, and is highly operator dependent, requiring the availability of an experienced and technically skilled operator (Figure 5).
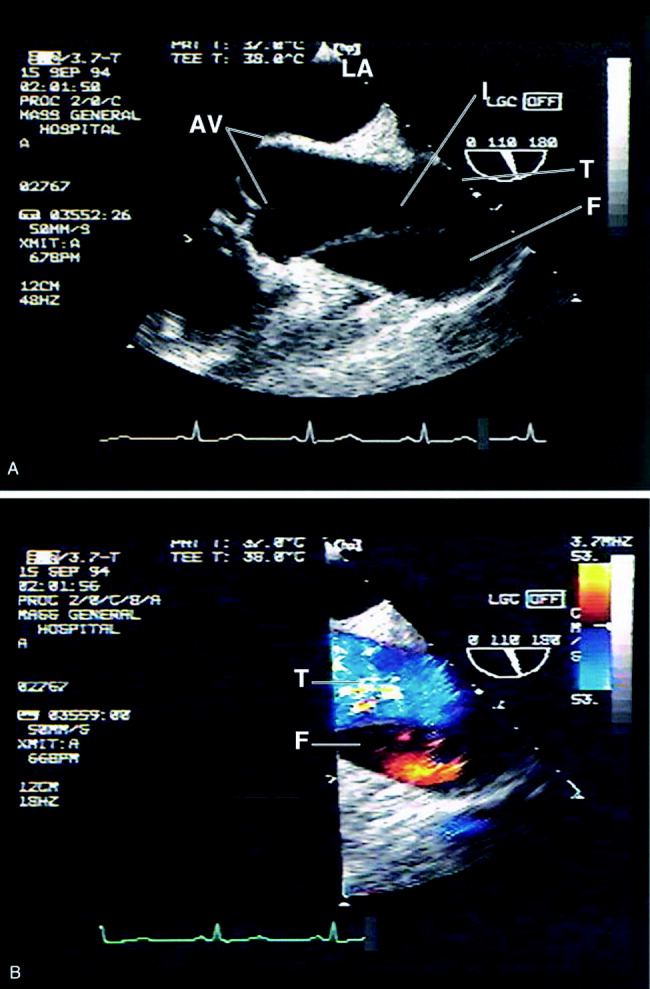
Transthoracic echocardiography: Although it is an excellent tool for the evaluation of many aspects of cardiac anatomy and function, surface echocardiography can reliably visualize only limited portions of the ascending and descending aorta.54, 55 As a consequence, it is neither sensitive nor specific enough to diagnose aortic dissection. Transthoracic echocardiography (TTE) does, however, play a role in rapidly assessing patients at the bedside for aortic valve or pericardial compromise when these complications are suspected.
Recommendations
CTA, MRA, and TEE are all highly sensitive and specific modalities for diagnosing aortic dissection. Therefore, the condition of the patient, the information needed, and the resources and expertise immediately available should drive the choice of study. MRA is considered the gold standard diagnostic study and is the preferred modality for hemodynamically stable patients with suspected aortic dissection. Because of slow data acquisition and the inaccessibility of patients in the scanner, it is generally unsuited for unstable patients, including those with ongoing pain. Bedside TEE is an excellent choice for patients who are too unstable for MRA but is less effective at visualizing distal dissections. Arch aortography is generally reserved for the confirmation of questionable diagnoses or to image specific branch arteries (Tables 4 and 5).
| Overall | Proximal | Distal | |
|---|---|---|---|
| |||
| TEE | 88% | 90% | 80% |
| CTA | 93% | 93% | 93% |
| MRA | 100% | 100% | 100% |
| Aortogram | 87% | 87% | 87% |
| TEE | CTA | MRA | Aortography | |
|---|---|---|---|---|
| ||||
| Sensitivity | ++ | ++ | +++ | ++ |
| Specificity | +++ | ++ | +++ | ++ |
| Classification | +++ | ++ | ++ | + |
| Intimal flap | +++ | ‐ | ++ | + |
| Aortic regurgitation | +++ | ++ | ++ | |
| Pericardial effusion | +++ | ++ | ++ | |
| Branch vessel involvement | + | ++ | ++ | +++ |
| Coronary artery involvement | ++ | + | + | +++ |
Most trials comparing CTA, MRA, and TEE were performed in the early 1990s. Computed tomography has evolved significantly over the intervening decade, and some of the diagnostic limitations previously ascribed to CTA, such as the inability to generate 3‐D reconstructed images, no longer exist. Furthermore, CT angiography is widely available and is gaining increasing acceptance as a first‐line imaging modality for patients with noncardiac chest pain.48 Medical centers that maintain round‐the‐clock CT capability may have limited or delayed access to TEE, MRA, or aortography. Given the potential for rapid and dramatic patient deterioration, it is imperative that a diagnosis be established quickly when aortic dissection is suspected. Thus, when the choice is obtaining an immediate CTA or a delayed TEE or MRA, CTA is generally the better choice (Figure 6).
MANAGEMENT
Acute Management:
Approximately half of all patients who present with acute aortic dissection are acutely hypertensive.14 Hypertensive aortic dissection is a hypertensive emergency that mandates immediate decrease in blood pressure to the lowest level that maintains organ perfusion. As a rule, short‐acting, parenteral, titratable antihypertensive agents should be used (Table 6). Intravenous beta‐adrenergic blockers are the mainstay of acute and chronic therapy. Their negative inotropic and chronotropic effects decrease shear stress across the aortic lumen and decrease the likelihood of dissection propagation and aortic dilatation.56, 57 Parenteral vasodilators (eg, nitroprusside and nitroglycerin) should be initiated if beta‐blockers prove insufficient for lowering blood pressure. They should never be used alone, as they may cause reflex tachycardia and consequently may increase intraluminal shear stress. The use of opiates for analgesia and benzodiazepines for anxiolysis further decreases blood pressure by controlling the severe pain and anxiety often associated with acute dissection.
| Name | Mechanism | Dose | Cautions/contraindications |
|---|---|---|---|
| Esmolol | Cardioselective beta‐1 blocker | Load: 500 g/kg IV | Asthma or bronchospasm |
| Drip: 50 g kg1 min1 IV. | Bradycardia | ||
| Increase by increments of 50 g/min | 2nd‐ or 3rd‐degree AV block | ||
| Cocaine or methamphetamine abuse | |||
| Labetalol | Nonselective beta 1,2 blocker | Load: 20 mg IV | Asthma or bronchospasm |
| Selective alpha‐1 blocker | Drip: 2 mg/min IV | Bradycardia | |
| 2nd or 3rd degree AV block | |||
| Cocaine or methamphetamine abuse | |||
| Enalaprilat | ACE inhibitor | 0.625‐1.25 mg IV q 6 hours. | Angioedema |
| Max dose: 5 mg q 6 hours. | Pregnancy | ||
| Renal artery stenosis | |||
| Severe renal insufficiency | |||
| Nitroprusside | Direct arterial vasodilator | Begin at 0.3 g kg1 min1 IV. | May cause reflex tachycardia |
| Max dose 10 g kg1 min1 | Cyanide/thiocyanate toxicityespecially in renal or hepatic insufficiency | ||
| Nitroglycerin | Vascular smooth muscle relaxation | 5‐200 g/min IV | Decreases preloadcontraindicated in tamponade or other preload‐dependent states |
| Concomitant use of sildenafil or similar agents |
Hypotension or shock, which develop in 15%‐30% of patients with acute aortic dissection, are ominous findings that frequently portends impending hemodynamic collapse.14, 58 Patients who develop hypotension are at a fivefold increased risk of death (55.0% vs. 10.3%) and are at markedly increased risk of developing neurologic deficits, as well as myocardial, mesenteric, and limb ischemia. Hypotension may result from pump failure (due to acute aortic insufficiency, pericardial tamponade, or myocardial ischemia), aortic rupture, systemic lactic acidosis, or spinal shock. Bedside transthoracic echocardiography may be particularly useful for the evaluation of hypotensive patients, as it can be used to quickly and noninvasively determine the integrity of the aortic valve and pericardium. Although hypotension may transiently respond to volume resuscitation, all hypotensive patients with aortic dissection, regardless of type, should be immediately referred for emergent surgical evaluation. Pericardiocentesis in the setting of pericardial tamponade remains controversial; a small study suggested that decompression of the pericardial sac may hasten hemodynamic collapse by accelerating blood loss.59
Facilities that do not maintain urgent cardiopulmonary bypass capability should emergently transport patients with aortic dissection to a facility that provides a higher level of care. Transfer should not be delayed to confirm a questionable diagnosis. Proximal aortic dissection frequently compromises the pericardium, aortic valve, and arch vessels, and therefore emergent surgical repair is indicated. When treated medically, proximal dissection carries a dismal 60% in‐hospital mortality rate.14, 60 In contrast, distal aortic dissection is generally treated medically, with surgical intervention generally reserved for patients with an expanding aortic aneurysm, elevated risk of aortic rupture, refractory hypertension, intractable pain, visceral hypoperfusion, and limb ischemia or paresis.11, 61, 62 Individual branch vessel occlusion may be effectively ameliorated with conventional arterial stenting or balloon fenestration.
Endovascular stent grafting has been used successfully in lieu of surgery for patients with acute or chronic distal (type B) aortic dissections.39, 4042, 63 The stent graft is deployed across the proximal intimal tear, obliterating the false lumen and facilitating aortic healing. Early studies suggested that endovascular stent grafting may be safer and more efficacious than conventional surgical repair of distal dissection.41 A recent meta‐analysis of published trials of endovascular aortic stenting found procedural success rates exceeding 95% and a major complication rate of 11%. Thirty‐day mortality was approximately 5%, with 6‐, 12‐ and 24‐month mortality rates plateauing at 10%. Centers with high patient volume had fewer complications and much lower acute mortality rates.14, 64 These medium‐term outcomes compare favorably with conventional therapy. Endovascular stenting has not been prospectively compared against conventional therapy in randomized trials, and it therefore remains unclear who should be referred for endovascular stenting instead of conventional therapy.
Long‐term Management
Survivors of aortic dissection, especially those with diseases of collagen, have a systemic disease that predisposes them to further aortic and great vessel events. Almost one third of survivors of acute aortic dissection will develop dissection propagation or aortic rupture or will require aortic surgery within 5 years of presentation.41, 60 Young patients who present for aortic dissection should be screened for Marfan syndrome according to the Gent nosology.65 To reduce shear stress to the aortic lumen, all patients should be treated with beta‐blockers for life, with blood pressure targeted to be below 135/80.60, 66 Patients who do not tolerate beta blockade may benefit from treatment with diltiazem or verapamil. Progression to aortic aneurysm is common, and patients should undergo serial imaging of the aorta at 1, 3, 6, and 12 months after discharge and annually thereafter. Dilatation of the proximal aorta to >5.0 cm and of the distal aorta to >6.0 cm should prompt referral for surgical or possibly endovascular repair.41, 67 Although supporting data are limited, it is generally accepted that patients should moderate their physical activity to avoid extremes of tachycardia and blood pressure elevation. Sports that involve high speed or sudden deceleration, such as ice hockey, downhill skiing, and football, should be strictly avoided. Patients should be warned to seek immediate medical attention if they develop recurrent chest or back pain or focal neurologic deficits.
PROGNOSIS
Despite significant medical and surgical advances, aortic dissection remains exceptionally lethal. Patients with proximal dissections are more likely to die than those with distal dissections. Using data from the IRAD trial, Mehta et al determined that age 70 years (OR, 1.70), abrupt onset of chest pain (OR 2.60), hypotension/shock/tamponade (OR, 2.97), renal failure (OR, 4.77), pulse deficit (OR, 2.03), and abnormal ECG (OR, 1.77) were independent determinants of death.59 Medical treatment of proximal dissection is generally reserved for patients too ill, unstable, or frail to undergo surgery. In contrast, most patients with distal dissection are managed medically, with surgery generally reserved for those with acute complications. Hence, patients with proximal dissections who are managed medically and those with distal dissections who are managed surgically have the worst outcomes. Outcomes for women are worse than those for men, which is probably attributable to several factors. Women dissect at an older age, present later after the onset of symptoms, and are more likely to have confounding symptoms that may delay timely diagnosis1 (Table 7).
| Proximal (DeBakey I, II; Stanford A) | Distal (DeBakey III; Stanford B) | |||
|---|---|---|---|---|
| Surgical | Medical | Surgical | Medical | |
| In‐hospital mortality | 26% | 58% | 31% | 11% |
| Average | 35% | 15% | ||
CONCLUSION
Aortic dissection is a rare and acutely life‐threatening cause of acute chest and back pain. Delays in diagnosis and misdiagnoses are common, frequently with catastrophic consequences. The key to diagnosis is maintaining a high index of suspicion for dissection, especially in patients who present with acute severe chest, back, or abdominal pain in the setting of unexplained acute pulse deficits, neurologic deficits, or acute end‐organ injury. Three clinical findings have been shown to be diagnostically useful: immediate onset of tearing or ripping chest or back pain, mediastinal widening or abnormal aortic contour on chest radiograph, and peripheral pulse deficits or variable pulse pressure (>20 mm Hg). If all 3 findings are absent, acute aortic dissection is unlikely. The presence of any of these findings should prompt further workup. A normal chest radiograph does not rule out aortic dissection. Only TEE, CT, and MR angiography are sufficiently specific to rule out dissection. Aortography is rarely used as a first‐line diagnostic tool but may be useful as a confirmatory test or to provide additional anatomic information. Patients who present with proximal aortic dissection or with any aortic dissection with concomitant hypotension are at exceptionally high risk of death and should be immediately referred for surgical evaluation. Beta‐blockers are the mainstay of acute and chronic therapy of aortic dissection. Survivors of aortic dissection are at a markedly elevated risk for further aortic events and should be followed vigilantly posthospitalization.
- ,,, et al.Gender‐related differences in acute aortic dissection.Circulation.2004;109:3014–3021.
- ,,, et al.Clinical features and differential diagnosis of aortic dissection: Experience with 236 cases.Mayo Clin Proc.1993;68:642–651.
- ,.The natural history of thoracic aortic aneurysm disease: an overview.J Card Surg.1997;12(suppl):270–278.
- ,,.Dissecting aneurysms of the aorta: a review of 505 cases.Medicine.1958;37:217–279.
- ,,,.Intimal tear without hematoma; an important variant of aortic dissection that can elude current imaging techniques.Circulation.1999;99:1331–1336.
- ,,, et al.Intramural hemorrhage of the thoracic aorta: diagnostic and therapeutic implications.Circulation.1995;92:1465–1472.
- ,.Acute aortic dissection and its variants; towards a common diagnostic and therapeutic approach.Circulation.1995;92:1376–1378.
- ,,.The penetrating aortic ulcer: pathologic manifestations, diagnosis and management.Mayo Clinic Proc.1988;63:718–725.
- ,,, et al.Acute intramural hematoma of the aorta—a mystery in evolution.Circulation.2005;111:1063–1070.
- ,.Intramural hematoma in acute aortic syndrome; more than one variant of dissection?Circulation.2002;106:284–285.
- ,,,,,,.Acute aortic dissection: population‐based incidence compared with degenerative aortic aneurysm rupture.Mayo Clin Proc.2004;79(2):176–180.
- ,,,,,,.Epidemiology and clinicopathology of aortic dissection.Chest.2000;117:1271–1278.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD).JAMA.2000;283:897–903.
- ,,.Clinical prediction of acute aortic dissection.Arch Intern Med.2000;160:2977–2982.
- ,.Risk factors for aortic dissection: a necropsy study of 161 cases.Am J Cardiol.1984;53:849–855.
- ,,, et al.Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD).J Am Coll Cardiol.2004;43:665–669.
- ,,,.Association of aortic dilatation with regurgitant, stenotic and functionally normal bicuspid aortic valves.J Am Coll Cardiol.1992;19:283–288.
- .Clinical significance of the bicuspid aortic valve.Heart.2000;83:81–85.
- ,,,.Aortic root dilatation in young men with normally functioning bicuspid aortic valves.Heart.1999;82:19–22.
- ,,, et al.Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation.J Thorac Cardiovasc Surg.2003;126:797–806.
- ,,.Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome.Pediatrics.1998;102(1):e12.
- ,,, et al.Aortic dilatation, dissection and rupture in patients with Turner syndrome.J Pediatr.1986;109:820–826.
- ,.Weight lifting and type II aortic dissection. A case report.J Sports Med Phys Fitness.2004;44:424–427.
- ,,.Recreational weight lifting and aortic dissection: case report.J Vasc Surg.1993;17:774–776.
- ,,,,,,.Ascending aortic dissection in weight lifters with cystic medial degeneration.Ann Thoracic Surg.1990;49:638–642.
- ,,,,.Acute aortic dissection related to crack cocaine.Circulation.2002;105:1592–1595.
- ,.Thoracic aortic dissection secondary to crack cocaine ingestion.Am J Emerg Med.1997;15:507–509.
- ,.Cocaine‐associated dissection of the thoracic aorta.J Emerg Med.1992;10:723–727.
- ,.Methamphetamine as a risk factor for acute aortic dissection.J Forensic Sci.1999;44(1):23–26.
- ,,,,,.Arterial dissections associated with pregnancy.J Vasc Surg.1995;21:515–520.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,,,.Fatal haemostatic complications due to thrombolytic therapy in patients falsely diagnosed as acute myocardial infarction.Eur Heart J.1992;13:840–843.
- ,,,,.The inadvertent administration of anticoagulants to ED patients ultimately diagnosed with thoracic aortic dissection.Am J Emerg Med.2005;23:439–442..
- ,,, et al.Chest radiography for the diagnosis of acute aortic syndrome.Am J Med.2004;116(2):73–77.
- ,,, et al.Clinical significance of pleural effusion in acute aortic dissection.Chest.2002;121:825–830.
- ,,,.Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography.J Am Coll Cardiol.1995;25:1393–1401.
- ,.Digital subtraction angiography of aortic dissection.Am J Roentgenol.1983;141:157–161.
- ,,, et al.Spectrum of conditions initially suggesting acute aortic dissection but with negative aortograms.Am J Cardiol.1986;57:322–326.
- ,,, et al.Nonsurgical reconstruction of thoracic aortic dissection by stent‐graft placement.N Engl J Med.1999;340:1539–1545.
- ,,, et al.Endovascular stent‐graft placement for the treatment of aortic dissection.N Engl J Med.1999;340:1546–1552.
- ,.Aortic dissection: New frontiers in diagnosis and management. Part II: Therapeutic management and follow‐up.Circulation.2003;108:772–778.
- ,,,,,.Aortic dissection: percutaneous management of ischemic complications with endovascular stents and balloon fenestration.J Vasc Surg.1996;23:241–251.
- ,,, et al.Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD).Am J Cardiol.2002;89:1235–1238.
- ,,, et al.Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging.Radiology.1996;199:347–352.
- ,,,.Assessment of the thoracic aorta by spiral CT.AJR Am J Roengenol.1992;158:1127–1130.
- ,Radiologic evaluation of aortic dissection.Radiology.1991;180:297–305.
- ,,,,,.Computed tomography of thoracic aortic dissection: accuracy and pitfalls.J Comput Assist Tomogr.1986;10:211–215.
- ,,.Spiral CT in acute non‐cardiac chest pain.Clin Radiol.1999;54(1):38–45.
- ,,, et al.Diagnosis of thoracic aortic dissection. Magnetic resonance imaging versus transesophageal echocardiography.Circulation.1992;85:434–447.
- ,,,,.Comparison of conventional and transesophageal echocardiography with magnetic resonance imaging for anatomical mapping of thoracic aortic dissection: a dual noninvasive imaging study with anatomical and/or angiographic validation.Int J Card Imaging.1994;10:1–14.
- ,.Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: part I. Aortic dissection, aortic intramural hematoma and penetrating atherosclerotic ulcer of the aorta.Chest.1999;116:1772–1779.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328:1–9.
- ,,,,,.Echocardiography in diagnosis of aortic dissection.Lancet.1989;1:457–461.
- ,,,,.A prospectus on the prevention of aortic rupture in the Marfan syndrome with data on survivorship without treatment.Johns Hopkins Med J.1971;129:123–129.
- ,,,.Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome.N Engl J Med.1994;330:1335–1341.
- ,,, et al.Clinical characteristics of hypotension in patients with acute aortic dissection.Am J Cardiol.2005;95:48–52.
- ,,.Cardiac tamponade complicating proximal aortic dissection: is pericardiocentesis harmful?Circulation.1994;90:2375–2379.
- ,,, et al.Predicting in‐hospital mortality in acute type A aortic dissection.Circulation.2002;105:200–206.
- ,,, et al.Diagnosis and management of aortic dissection: task force report of the European Society of Cardiology.Eur Heart J.2001;22:1642–1681.
- ,,,,,.Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the international registry of aortic dissection.Circulation.2003;108(suppl II):II‐312–II‐317.
- ,,, et al.Endovascular stent‐graft treatment of aortic dissection: determinants of post‐interventional outcome.Eur Heart J.2005;26:489–497.
- ,,, et al.Endovascular stent‐graft placement in aortic dissection: a meta‐analysis [advance access online].Eur Heart J.2005. Published online October 14, 2005.
- ,,,,.Revised diagnostic criteria for the Marfan syndrome.Am J Med Genet.1996;62:417–426.
- ,,,.Treatment of dissecting aneurysms of the aorta without surgery.J Thorac Cardiovasc Surg.1965;50:364–373.
- ,,,,,.Unoperated aortic aneurysms: a survey of 170 patients.Ann Thorac Surg.1995;59:1204–1209.
Aortic dissection is an uncommon but highly lethal disease with an incidence of approximately 2,000 cases per year in the United States.1 It is often mistaken for less serious pathology. In one series, aortic dissection was missed in 38% of patients at presentation, with 28% of patients first diagnosed at autopsy.2 Early recognition and management are crucial. If untreated, the mortality rate for acute aortic dissection increases by approximately 1% per hour over the first 48 hours and may reach 70% at 1 week. As many as 90% of untreated patients who suffer aortic dissection die within 3 months of presentation.3, 4 Generally, cardiothoracic surgeons or cardiologists experienced with managing aortic dissection should direct patient evaluation and treatment. Hospitalists, however, are increasingly assuming responsibility for the initial triage and management of patients with acute chest pain syndromes and therefore must be able to rapidly identify aortic dissection, initiate supportive therapy, and refer patients to appropriate specialty care.
PATHOPHYSIOLOGY
Aortic dissection occurs when layers of the aortic wall separate because of infiltration of high‐pressure arterial blood. The proximate causes are elevated shear stress across the aortic lumen in the setting of a concomitant defect in the aortic media. Shear stress is caused by the rapid increase in luminal pressure per unit of time (dP/dt) that results from cardiac systole. As the aorta traverses away from the heart, an increasing proportion of the kinetic energy of left ventricular systole is stored in the aortic wall as potential energy, which facilitates anterograde propagation of cardiac output during diastole. This conversion of kinetic to potential energy also attenuates shear stress. As the proximal aorta is subject to the steepest fluctuations in pressure, it is at the highest risk of dissection. Degeneration of the aortic media is part of the normal aging process but is accelerated in persons with a bicuspid aortic valve, Turner's syndrome, inflammatory arteritis, or inherited diseases of collagen formation.
Once the aortic intima is compromised, blood dissects longitudinally through the aortic media and propagates proximally or distally, creating a false lumen that may communicate with the true lumen of the aorta. Blood may flow through the true lumen, the false lumen, or both. Propagation of the dissection causes much of the morbidity associated with aortic dissection by disrupting blood flow across branch vessels or by directly compromising the pericardium or aortic valve. Over time, the dissection may traverse the entire aortic wall, causing aortic rupture and exsanguination.
CLASSIFICATION
Acute aortic dissection is classified as any aortic dissection diagnosed within 2 weeks of the onset of symptoms, which is the period of highest risk of mortality. Patients who survive more than 2 weeks without treatment are considered to have chronic dissection. Aortic dissections are further classified according to their anatomic location. The fundamental distinction is whether the dissection is proximal (involving the aortic root or ascending aorta) or distal (below the left subclavian artery). The Stanford and DeBakey classification systems are the classification systems most commonly used (Figure 1).

Some variants of aortic dissection are not described in either the Stanford or DeBakey systems. Aortic intramural hematomas (IMH) are caused by intramural hemorrhage of the vasa vasorum without an identifiable intimal tear.57 Penetrating atherosclerotic ulcers (PAUs) are focal defects in the aortic wall with surrounding hematoma but no longitudinal dissection across tissue planes, typically resulting from advanced atherosclerotic disease.8 The pathophysiologic distinctions between IMH, PAU, and classic aortic dissection remain somewhat controversial. Both IMH and PAU may progress to aortic aneurysm formation, frank dissection, or aortic rupture, suggesting that these entities represent a spectrum of diseases with broad overlap (Table 1).9, 10
| Acuity | |
| Acute 2 weeks after onset | |
| Chronic: >2 weeks after onset | |
| Anatomic location: | |
| Ascending aorta: | Stanford Type A, Debakey Type II |
| Ascending and descending aorta: | Stanford Type A, Debakey Type I |
| Descending aorta: | Stanford Type B, Debakey Type III |
| Pathophysiology: | |
| Class 1: Classical aortic dissection with initimal flap between true and false lumen | |
| Class 2: Aortic intramural hematoma without identifiable intimal flap | |
| Class 3: Intimal tear without hematoma (limited dissection) | |
| Class 4: Atherosclerotic plaque rupture with aortic penetrating ulcer | |
| Class 5: Iatrogenic or traumatic aortic dissection (intra‐aortic catherterization, high‐speed deceleration injury, blunt chest trauma) | |
EPIDEMIOLOGY
Aortic dissection is a rare disease, with an estimated incidence of approximately 5‐30 cases per 1 million people per year.1114 Fewer than 0.5% of patients presenting to an emergency department with chest or back pain suffer from aortic dissection.15 Two thirds of patients are male, with an average age at presentation of approximately 65 years. A history of systemic hypertension, found in up to 72% of patients, is by far the most common risk factor.2, 14, 16 Atherosclerosis, a history of prior cardiac surgery, and known aortic aneurysm are other major risk factors.14 The epidemiology of aortic dissection is substantially different in young patients (40 years of age). Hypertension and atherosclerosis become significantly less common, as other risk factors, such as Marfan syndrome, take precedence17 (Table 2). Other risk factors for aortic dissection include:
-
Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos): In the International Registry of Acute Aortic Dissection (IRAD), the largest prospective analysis of aortic dissection to date, 50% of the young patients presenting with aortic dissection had Marfan syndrome.17
-
Bicuspid aortic valve (BAV): Individuals with BAV are 5‐18 times more likely to suffer aortic dissection than those with a trileaflet valve.18, 19 In one survey, 52% of asymptomatic young men with BAV were found to have aortic root dilatation, a frequent precursor of dissection.20 Vascular tissue in individuals with BAV has been found to have increased levels of matrix metalloproteinases, which may degrade elastic matrix components and accelerate medial necrosis.21
-
Aortic coarctation: Aortic coarctation is associated with upper extremity hypertension, BAV and aortic dilatation, all of which predispose to aortic dissection.
-
Turner syndrome: Aortic root dilatation with or without dissection has been incidentally noted in 6%‐9% of patients with Turner syndrome.22, 23
-
Strenuous exercise: Multiple case reports have associated aortic dissection with high‐intensity weightlifting. Many affected individuals were subsequently found to have at least one other risk factor, including hypertension, anabolic steroid abuse, and cocaine abuse.2426
-
Large vessel arteritis: Large vessel arteritides, specifically giant cell arteritis, Takayasu's disease, and tertiary syphilis have long been associated with aortic dilatation and dissection.
-
Cocaine and methamphetamine ingestion: Sympathomimetic drugs cause rapid increases in heart rate and blood pressure, markedly increasing aortic intraluminal shear stress. Furthermore, cocaine is thought to be directly toxic to vascular endothelium and may accelerate medial necrosis.2730
-
Third trimester pregnancy, especially in patients with diseases of collagen31; The significance of pregnancy has recently been called into question by data from the IRAD trial. Of 346 enrolled women with aortic dissection, only 2 were pregnant, suggesting that the previously held association of pregnancy with aortic dissection may be an artifact of selective reporting.1
-
Blunt chest trauma or high‐speed deceleration injury.
-
Iatrogenic injury, typically from intra‐aortic catheterization.
| Hypertension |
| Atherosclerotic disease |
| History of cardiac surgery |
| Aortic aneurysm |
| Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos) |
| Bicuspid aortic valve (BAV) |
| Aortic coarctation |
| Turner syndrome |
| Strenuous exercise |
| Large vessel arteritis: giant cell, Takayasu's, syphilis |
| Cocaine and methamphetamine ingestion |
| Third‐trimester pregnancy |
| Blunt chest trauma or high‐speed deceleration injury |
| Iatrogenic injury, typically from intra‐aortic catheterization |
INITIAL EVALUATION
The differential diagnosis for acute aortic dissection includes acute coronary syndrome, pulmonary embolus, pneumothorax, pneumonia, musculoskeletal pain, acute cholecystitis, esophageal spasm or rupture, acute pancreatitis, and acute pericarditis. Acute aortic dissections are rarely asymptomatic; in fact, the absence of sudden‐onset chest pain decreases the likelihood of dissection (negative LR 0.3).32 In the IRAD trial, approximately 95% of patients with aortic dissection complained of pain in the chest, back, or abdomen, with 90% characterizing their pain as either severe or the worst ever and 64% describing it as sharp.14 Although the presence of tearing or ripping chest or back pain suggests aortic dissection (positive LR 1.2‐10.8), its absence does not reliably exclude this diagnosis.32 The wide variability in the presentation of aortic dissection increases the challenge of establishing a diagnosis. Clinical findings depend largely on the anatomical location of the dissection and may include pulse deficits, neurologic deficits, hypotension, hypertension, and end‐organ ischemia. Women who develop aortic dissection are generally older and present later than men. Their symptoms are less typical and are likely to be confounded by altered mental status.1 A diagnosis of aortic dissection should be strongly considered for patients presenting with acute chest or back pain and otherwise unexplained aortic insufficiency, focal neurologic deficits, pulse deficits, or end‐organ injury (Table 3).
| Hypotension or shock due to: |
| a. Hemopericardium and pericardial tamponade |
| b. Acute aortic insufficiency due to dilatation of the aortic annulus |
| c. Aortic rupture |
| d. Lactic acidosis |
| e. Spinal shock |
| Acute myocardial ischemia/emnfarction due to coronary ostial occlusion |
| Pericardial friction rub due to hemopericardium |
| Syncope |
| Pleural effusion or frank hemothorax |
| Acute renal failure due to dissection across renal arteries |
| Mesenteric ischemia due to dissection across intra‐abdominal arteries |
| Neurologic deficits: |
| a. Stroke due to occlusion of arch vessels |
| b. Limb weakness |
| c. Spinal cord deficits due to cord ischemia |
| d. Horner syndrome due to compression of superior sympathetic ganglion. |
| e. Hoarseness due to compression of left recurrent laryngeal nerve |
Electrocardiogram: Electrocardiographic abnormalities are commonly seen in aortic dissection and may include ST‐segment or T‐wave abnormalities or left ventricular hypertrophy.14 Proximal aortic dissections may compromise coronary artery perfusion, generating electrocardiogram (ECG) findings compatible with acute myocardial infarction, which may lead the clinician to diagnose and treat myocardial infarction while missing the underlying diagnosis.33 In a recent survey, 9 of 44 patients (21%) presenting with acute aortic dissection were initially diagnosed with acute coronary syndrome and anticoagulated, with 2 deaths.34 ECGs must therefore be interpreted with extreme caution in aortic dissection.
Chest x‐ray: In the emergency department, chest radiography is a mainstay of the evaluation of acute chest pain. Unfortunately, plain‐film radiography has limited utility for diagnosing aortic dissection.35 In the IRAD trial, mediastinal widening (>8 cm) and abnormal aortic contour, the classic radiographic findings in aortic dissection, were present in only 50%‐60% of cases. Twelve percent of patients had a completely normal chest x‐ray.14 A pooled analysis of previous studies demonstrated that the sensitivity of widened mediastinum and abnormal aortic contour was 65% and 71%, respectively.32 Nonspecific radiographic findings, most notably pleural effusion, were common.36 Thus, if the index of suspicion for aortic dissection is elevated, a confirmatory study must be obtained (Figure 2).

Clinical Prediction Tool
Three clinical features were demonstrated to be effective in identifying aortic dissection in patients presenting with acute chest or back pain: immediate onset of tearing or ripping chest pain, mediastinal widening or aortic enlargement/displacement observed on chest x‐ray, and arm pulse or blood pressure differential exceeding 20 mm Hg. When all 3 findings were absent, dissection was unlikely (7% probability, negative LR 0.07 [CI 0.03‐0.17]). If either chest pain or radiographic findings were present, the likelihood was intermediate (31%‐39% probability). With any other combination of findings, dissection was likely (83‐100% probability). This prediction tool effectively identified 96% of all patients who presented to an emergency department with acute aortic dissection.15 However, 4% of patients categorized as low risk were ultimately diagnosed with aortic dissection. Given the exceptionally high mortality resulting from a missed diagnosis, a 4% false‐negative rate is unacceptably high. Thus, the absence of any of the aforementioned findings should not dissuade the clinician from obtaining a confirmatory imaging study if the pretest probability for acute aortic dissection is elevated.
CONFIRMATORY IMAGING STUDIES
The ideal confirmatory imaging modality should identify aortic dissection with high sensitivity and specificity. It should also identify the entry and exit points of the dissection and provide information about the extent of compromise of the aortic valve, pericardium, and great vessels. Four imaging modalities sufficiently meet these criteria in order to be considered diagnostically useful.
Aortography: Previously the gold standard for diagnosing aortic dissection, aortography is no longer a first‐line imaging modality. The sensitivity and specificity of aortography are at best equivalent and probably inferior to less invasive imaging modalities.37, 38 False negatives may occur if both the true and false lumens opacify equally with contrast, or if the false lumen is sufficiently thrombosed to preclude any instillation of contrast. Aortography cannot identify aortic intramural hematomas, is invasive and highly operator dependent, requires nephrotoxic contrast, and generally takes longer to obtain than other modalities.39
Aortography uniquely offers excellent visualization of the coronary arteries and great vessels and is preferred when such information is necessary. Percutaneous aortic endovascular stent grafting has been recently employed to repair distal aortic dissections.4043 As a result, aortography is gaining new life as a therapeutic modality.
CT angiography: Spiral CT angiography (CTA) is the most commonly used modality for diagnosing aortic dissection.44 It is emergently available at most hospitals, and images can be obtained in minutes. Sensitivity and specificity may approach 100%, and CTA may be more sensitive than MRA or TEE in evaluating arch vessel involvement.4547 Like conventional angiography, CTA requires administration of nephrotoxic contrast. It frequently cannot visualize the entry and exit sites (intimal flaps) of a dissection and provides limited information about the coronary arteries and no information about the competency of the aortic valve.48, 49 Thus, if aortic dissection is identified by CTA, a second study may be needed to provide further diagnostic information and to guide surgical intervention (Figures 3 and 4).


Magnetic Resonance Angiography: Magnetic resonance angiography (MRA) offers excellent noninvasive evaluation of the thoracic aorta. Sensitivity and specificity are probably superior to spiral CTA, and MRA generally identifies the location of the intimal tear and provides some functional information about the aortic valve.44, 50, 51 MRA is not emergently available at many hospitals. Scanning is time intensive, requiring the patient to remain motionless and relatively inaccessible for up to an hour. Furthermore, patient claustrophobia and the presence of implanted devices such as pacemakers or ferromagnetic foreign bodies may preclude MRA.
Transesophageal echocardiography: The sensitivity and specificity of transesophageal echocardiography (TEE) are also excellenton a par with CTA and MRA. In addition to providing excellent visualization of the thoracic aorta, TEE provides superb images of the pericardium and detailed assessment of aortic valve function.52 It also is extremely effective at visualizing the aortic intimal flap.44, 49, 53 A significant advantage of TEE is its portability, allowing rapid diagnosis at the bedside. For this reason, it is particularly useful for evaluation of patients who are hemodynamically unstable and are suspected to have an aortic dissection. Because of the anatomic relationship of the aorta with the esophagus and the trachea, TEE more effectively identifies proximal than distal dissections.43 TEE is also somewhat invasive, usually requires patient sedation, and is highly operator dependent, requiring the availability of an experienced and technically skilled operator (Figure 5).

Transthoracic echocardiography: Although it is an excellent tool for the evaluation of many aspects of cardiac anatomy and function, surface echocardiography can reliably visualize only limited portions of the ascending and descending aorta.54, 55 As a consequence, it is neither sensitive nor specific enough to diagnose aortic dissection. Transthoracic echocardiography (TTE) does, however, play a role in rapidly assessing patients at the bedside for aortic valve or pericardial compromise when these complications are suspected.
Recommendations
CTA, MRA, and TEE are all highly sensitive and specific modalities for diagnosing aortic dissection. Therefore, the condition of the patient, the information needed, and the resources and expertise immediately available should drive the choice of study. MRA is considered the gold standard diagnostic study and is the preferred modality for hemodynamically stable patients with suspected aortic dissection. Because of slow data acquisition and the inaccessibility of patients in the scanner, it is generally unsuited for unstable patients, including those with ongoing pain. Bedside TEE is an excellent choice for patients who are too unstable for MRA but is less effective at visualizing distal dissections. Arch aortography is generally reserved for the confirmation of questionable diagnoses or to image specific branch arteries (Tables 4 and 5).
| Overall | Proximal | Distal | |
|---|---|---|---|
| |||
| TEE | 88% | 90% | 80% |
| CTA | 93% | 93% | 93% |
| MRA | 100% | 100% | 100% |
| Aortogram | 87% | 87% | 87% |
| TEE | CTA | MRA | Aortography | |
|---|---|---|---|---|
| ||||
| Sensitivity | ++ | ++ | +++ | ++ |
| Specificity | +++ | ++ | +++ | ++ |
| Classification | +++ | ++ | ++ | + |
| Intimal flap | +++ | ‐ | ++ | + |
| Aortic regurgitation | +++ | ++ | ++ | |
| Pericardial effusion | +++ | ++ | ++ | |
| Branch vessel involvement | + | ++ | ++ | +++ |
| Coronary artery involvement | ++ | + | + | +++ |
Most trials comparing CTA, MRA, and TEE were performed in the early 1990s. Computed tomography has evolved significantly over the intervening decade, and some of the diagnostic limitations previously ascribed to CTA, such as the inability to generate 3‐D reconstructed images, no longer exist. Furthermore, CT angiography is widely available and is gaining increasing acceptance as a first‐line imaging modality for patients with noncardiac chest pain.48 Medical centers that maintain round‐the‐clock CT capability may have limited or delayed access to TEE, MRA, or aortography. Given the potential for rapid and dramatic patient deterioration, it is imperative that a diagnosis be established quickly when aortic dissection is suspected. Thus, when the choice is obtaining an immediate CTA or a delayed TEE or MRA, CTA is generally the better choice (Figure 6).

MANAGEMENT
Acute Management:
Approximately half of all patients who present with acute aortic dissection are acutely hypertensive.14 Hypertensive aortic dissection is a hypertensive emergency that mandates immediate decrease in blood pressure to the lowest level that maintains organ perfusion. As a rule, short‐acting, parenteral, titratable antihypertensive agents should be used (Table 6). Intravenous beta‐adrenergic blockers are the mainstay of acute and chronic therapy. Their negative inotropic and chronotropic effects decrease shear stress across the aortic lumen and decrease the likelihood of dissection propagation and aortic dilatation.56, 57 Parenteral vasodilators (eg, nitroprusside and nitroglycerin) should be initiated if beta‐blockers prove insufficient for lowering blood pressure. They should never be used alone, as they may cause reflex tachycardia and consequently may increase intraluminal shear stress. The use of opiates for analgesia and benzodiazepines for anxiolysis further decreases blood pressure by controlling the severe pain and anxiety often associated with acute dissection.
| Name | Mechanism | Dose | Cautions/contraindications |
|---|---|---|---|
| Esmolol | Cardioselective beta‐1 blocker | Load: 500 g/kg IV | Asthma or bronchospasm |
| Drip: 50 g kg1 min1 IV. | Bradycardia | ||
| Increase by increments of 50 g/min | 2nd‐ or 3rd‐degree AV block | ||
| Cocaine or methamphetamine abuse | |||
| Labetalol | Nonselective beta 1,2 blocker | Load: 20 mg IV | Asthma or bronchospasm |
| Selective alpha‐1 blocker | Drip: 2 mg/min IV | Bradycardia | |
| 2nd or 3rd degree AV block | |||
| Cocaine or methamphetamine abuse | |||
| Enalaprilat | ACE inhibitor | 0.625‐1.25 mg IV q 6 hours. | Angioedema |
| Max dose: 5 mg q 6 hours. | Pregnancy | ||
| Renal artery stenosis | |||
| Severe renal insufficiency | |||
| Nitroprusside | Direct arterial vasodilator | Begin at 0.3 g kg1 min1 IV. | May cause reflex tachycardia |
| Max dose 10 g kg1 min1 | Cyanide/thiocyanate toxicityespecially in renal or hepatic insufficiency | ||
| Nitroglycerin | Vascular smooth muscle relaxation | 5‐200 g/min IV | Decreases preloadcontraindicated in tamponade or other preload‐dependent states |
| Concomitant use of sildenafil or similar agents |
Hypotension or shock, which develop in 15%‐30% of patients with acute aortic dissection, are ominous findings that frequently portends impending hemodynamic collapse.14, 58 Patients who develop hypotension are at a fivefold increased risk of death (55.0% vs. 10.3%) and are at markedly increased risk of developing neurologic deficits, as well as myocardial, mesenteric, and limb ischemia. Hypotension may result from pump failure (due to acute aortic insufficiency, pericardial tamponade, or myocardial ischemia), aortic rupture, systemic lactic acidosis, or spinal shock. Bedside transthoracic echocardiography may be particularly useful for the evaluation of hypotensive patients, as it can be used to quickly and noninvasively determine the integrity of the aortic valve and pericardium. Although hypotension may transiently respond to volume resuscitation, all hypotensive patients with aortic dissection, regardless of type, should be immediately referred for emergent surgical evaluation. Pericardiocentesis in the setting of pericardial tamponade remains controversial; a small study suggested that decompression of the pericardial sac may hasten hemodynamic collapse by accelerating blood loss.59
Facilities that do not maintain urgent cardiopulmonary bypass capability should emergently transport patients with aortic dissection to a facility that provides a higher level of care. Transfer should not be delayed to confirm a questionable diagnosis. Proximal aortic dissection frequently compromises the pericardium, aortic valve, and arch vessels, and therefore emergent surgical repair is indicated. When treated medically, proximal dissection carries a dismal 60% in‐hospital mortality rate.14, 60 In contrast, distal aortic dissection is generally treated medically, with surgical intervention generally reserved for patients with an expanding aortic aneurysm, elevated risk of aortic rupture, refractory hypertension, intractable pain, visceral hypoperfusion, and limb ischemia or paresis.11, 61, 62 Individual branch vessel occlusion may be effectively ameliorated with conventional arterial stenting or balloon fenestration.
Endovascular stent grafting has been used successfully in lieu of surgery for patients with acute or chronic distal (type B) aortic dissections.39, 4042, 63 The stent graft is deployed across the proximal intimal tear, obliterating the false lumen and facilitating aortic healing. Early studies suggested that endovascular stent grafting may be safer and more efficacious than conventional surgical repair of distal dissection.41 A recent meta‐analysis of published trials of endovascular aortic stenting found procedural success rates exceeding 95% and a major complication rate of 11%. Thirty‐day mortality was approximately 5%, with 6‐, 12‐ and 24‐month mortality rates plateauing at 10%. Centers with high patient volume had fewer complications and much lower acute mortality rates.14, 64 These medium‐term outcomes compare favorably with conventional therapy. Endovascular stenting has not been prospectively compared against conventional therapy in randomized trials, and it therefore remains unclear who should be referred for endovascular stenting instead of conventional therapy.
Long‐term Management
Survivors of aortic dissection, especially those with diseases of collagen, have a systemic disease that predisposes them to further aortic and great vessel events. Almost one third of survivors of acute aortic dissection will develop dissection propagation or aortic rupture or will require aortic surgery within 5 years of presentation.41, 60 Young patients who present for aortic dissection should be screened for Marfan syndrome according to the Gent nosology.65 To reduce shear stress to the aortic lumen, all patients should be treated with beta‐blockers for life, with blood pressure targeted to be below 135/80.60, 66 Patients who do not tolerate beta blockade may benefit from treatment with diltiazem or verapamil. Progression to aortic aneurysm is common, and patients should undergo serial imaging of the aorta at 1, 3, 6, and 12 months after discharge and annually thereafter. Dilatation of the proximal aorta to >5.0 cm and of the distal aorta to >6.0 cm should prompt referral for surgical or possibly endovascular repair.41, 67 Although supporting data are limited, it is generally accepted that patients should moderate their physical activity to avoid extremes of tachycardia and blood pressure elevation. Sports that involve high speed or sudden deceleration, such as ice hockey, downhill skiing, and football, should be strictly avoided. Patients should be warned to seek immediate medical attention if they develop recurrent chest or back pain or focal neurologic deficits.
PROGNOSIS
Despite significant medical and surgical advances, aortic dissection remains exceptionally lethal. Patients with proximal dissections are more likely to die than those with distal dissections. Using data from the IRAD trial, Mehta et al determined that age 70 years (OR, 1.70), abrupt onset of chest pain (OR 2.60), hypotension/shock/tamponade (OR, 2.97), renal failure (OR, 4.77), pulse deficit (OR, 2.03), and abnormal ECG (OR, 1.77) were independent determinants of death.59 Medical treatment of proximal dissection is generally reserved for patients too ill, unstable, or frail to undergo surgery. In contrast, most patients with distal dissection are managed medically, with surgery generally reserved for those with acute complications. Hence, patients with proximal dissections who are managed medically and those with distal dissections who are managed surgically have the worst outcomes. Outcomes for women are worse than those for men, which is probably attributable to several factors. Women dissect at an older age, present later after the onset of symptoms, and are more likely to have confounding symptoms that may delay timely diagnosis1 (Table 7).
| Proximal (DeBakey I, II; Stanford A) | Distal (DeBakey III; Stanford B) | |||
|---|---|---|---|---|
| Surgical | Medical | Surgical | Medical | |
| In‐hospital mortality | 26% | 58% | 31% | 11% |
| Average | 35% | 15% | ||
CONCLUSION
Aortic dissection is a rare and acutely life‐threatening cause of acute chest and back pain. Delays in diagnosis and misdiagnoses are common, frequently with catastrophic consequences. The key to diagnosis is maintaining a high index of suspicion for dissection, especially in patients who present with acute severe chest, back, or abdominal pain in the setting of unexplained acute pulse deficits, neurologic deficits, or acute end‐organ injury. Three clinical findings have been shown to be diagnostically useful: immediate onset of tearing or ripping chest or back pain, mediastinal widening or abnormal aortic contour on chest radiograph, and peripheral pulse deficits or variable pulse pressure (>20 mm Hg). If all 3 findings are absent, acute aortic dissection is unlikely. The presence of any of these findings should prompt further workup. A normal chest radiograph does not rule out aortic dissection. Only TEE, CT, and MR angiography are sufficiently specific to rule out dissection. Aortography is rarely used as a first‐line diagnostic tool but may be useful as a confirmatory test or to provide additional anatomic information. Patients who present with proximal aortic dissection or with any aortic dissection with concomitant hypotension are at exceptionally high risk of death and should be immediately referred for surgical evaluation. Beta‐blockers are the mainstay of acute and chronic therapy of aortic dissection. Survivors of aortic dissection are at a markedly elevated risk for further aortic events and should be followed vigilantly posthospitalization.
Aortic dissection is an uncommon but highly lethal disease with an incidence of approximately 2,000 cases per year in the United States.1 It is often mistaken for less serious pathology. In one series, aortic dissection was missed in 38% of patients at presentation, with 28% of patients first diagnosed at autopsy.2 Early recognition and management are crucial. If untreated, the mortality rate for acute aortic dissection increases by approximately 1% per hour over the first 48 hours and may reach 70% at 1 week. As many as 90% of untreated patients who suffer aortic dissection die within 3 months of presentation.3, 4 Generally, cardiothoracic surgeons or cardiologists experienced with managing aortic dissection should direct patient evaluation and treatment. Hospitalists, however, are increasingly assuming responsibility for the initial triage and management of patients with acute chest pain syndromes and therefore must be able to rapidly identify aortic dissection, initiate supportive therapy, and refer patients to appropriate specialty care.
PATHOPHYSIOLOGY
Aortic dissection occurs when layers of the aortic wall separate because of infiltration of high‐pressure arterial blood. The proximate causes are elevated shear stress across the aortic lumen in the setting of a concomitant defect in the aortic media. Shear stress is caused by the rapid increase in luminal pressure per unit of time (dP/dt) that results from cardiac systole. As the aorta traverses away from the heart, an increasing proportion of the kinetic energy of left ventricular systole is stored in the aortic wall as potential energy, which facilitates anterograde propagation of cardiac output during diastole. This conversion of kinetic to potential energy also attenuates shear stress. As the proximal aorta is subject to the steepest fluctuations in pressure, it is at the highest risk of dissection. Degeneration of the aortic media is part of the normal aging process but is accelerated in persons with a bicuspid aortic valve, Turner's syndrome, inflammatory arteritis, or inherited diseases of collagen formation.
Once the aortic intima is compromised, blood dissects longitudinally through the aortic media and propagates proximally or distally, creating a false lumen that may communicate with the true lumen of the aorta. Blood may flow through the true lumen, the false lumen, or both. Propagation of the dissection causes much of the morbidity associated with aortic dissection by disrupting blood flow across branch vessels or by directly compromising the pericardium or aortic valve. Over time, the dissection may traverse the entire aortic wall, causing aortic rupture and exsanguination.
CLASSIFICATION
Acute aortic dissection is classified as any aortic dissection diagnosed within 2 weeks of the onset of symptoms, which is the period of highest risk of mortality. Patients who survive more than 2 weeks without treatment are considered to have chronic dissection. Aortic dissections are further classified according to their anatomic location. The fundamental distinction is whether the dissection is proximal (involving the aortic root or ascending aorta) or distal (below the left subclavian artery). The Stanford and DeBakey classification systems are the classification systems most commonly used (Figure 1).

Some variants of aortic dissection are not described in either the Stanford or DeBakey systems. Aortic intramural hematomas (IMH) are caused by intramural hemorrhage of the vasa vasorum without an identifiable intimal tear.57 Penetrating atherosclerotic ulcers (PAUs) are focal defects in the aortic wall with surrounding hematoma but no longitudinal dissection across tissue planes, typically resulting from advanced atherosclerotic disease.8 The pathophysiologic distinctions between IMH, PAU, and classic aortic dissection remain somewhat controversial. Both IMH and PAU may progress to aortic aneurysm formation, frank dissection, or aortic rupture, suggesting that these entities represent a spectrum of diseases with broad overlap (Table 1).9, 10
| Acuity | |
| Acute 2 weeks after onset | |
| Chronic: >2 weeks after onset | |
| Anatomic location: | |
| Ascending aorta: | Stanford Type A, Debakey Type II |
| Ascending and descending aorta: | Stanford Type A, Debakey Type I |
| Descending aorta: | Stanford Type B, Debakey Type III |
| Pathophysiology: | |
| Class 1: Classical aortic dissection with initimal flap between true and false lumen | |
| Class 2: Aortic intramural hematoma without identifiable intimal flap | |
| Class 3: Intimal tear without hematoma (limited dissection) | |
| Class 4: Atherosclerotic plaque rupture with aortic penetrating ulcer | |
| Class 5: Iatrogenic or traumatic aortic dissection (intra‐aortic catherterization, high‐speed deceleration injury, blunt chest trauma) | |
EPIDEMIOLOGY
Aortic dissection is a rare disease, with an estimated incidence of approximately 5‐30 cases per 1 million people per year.1114 Fewer than 0.5% of patients presenting to an emergency department with chest or back pain suffer from aortic dissection.15 Two thirds of patients are male, with an average age at presentation of approximately 65 years. A history of systemic hypertension, found in up to 72% of patients, is by far the most common risk factor.2, 14, 16 Atherosclerosis, a history of prior cardiac surgery, and known aortic aneurysm are other major risk factors.14 The epidemiology of aortic dissection is substantially different in young patients (40 years of age). Hypertension and atherosclerosis become significantly less common, as other risk factors, such as Marfan syndrome, take precedence17 (Table 2). Other risk factors for aortic dissection include:
-
Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos): In the International Registry of Acute Aortic Dissection (IRAD), the largest prospective analysis of aortic dissection to date, 50% of the young patients presenting with aortic dissection had Marfan syndrome.17
-
Bicuspid aortic valve (BAV): Individuals with BAV are 5‐18 times more likely to suffer aortic dissection than those with a trileaflet valve.18, 19 In one survey, 52% of asymptomatic young men with BAV were found to have aortic root dilatation, a frequent precursor of dissection.20 Vascular tissue in individuals with BAV has been found to have increased levels of matrix metalloproteinases, which may degrade elastic matrix components and accelerate medial necrosis.21
-
Aortic coarctation: Aortic coarctation is associated with upper extremity hypertension, BAV and aortic dilatation, all of which predispose to aortic dissection.
-
Turner syndrome: Aortic root dilatation with or without dissection has been incidentally noted in 6%‐9% of patients with Turner syndrome.22, 23
-
Strenuous exercise: Multiple case reports have associated aortic dissection with high‐intensity weightlifting. Many affected individuals were subsequently found to have at least one other risk factor, including hypertension, anabolic steroid abuse, and cocaine abuse.2426
-
Large vessel arteritis: Large vessel arteritides, specifically giant cell arteritis, Takayasu's disease, and tertiary syphilis have long been associated with aortic dilatation and dissection.
-
Cocaine and methamphetamine ingestion: Sympathomimetic drugs cause rapid increases in heart rate and blood pressure, markedly increasing aortic intraluminal shear stress. Furthermore, cocaine is thought to be directly toxic to vascular endothelium and may accelerate medial necrosis.2730
-
Third trimester pregnancy, especially in patients with diseases of collagen31; The significance of pregnancy has recently been called into question by data from the IRAD trial. Of 346 enrolled women with aortic dissection, only 2 were pregnant, suggesting that the previously held association of pregnancy with aortic dissection may be an artifact of selective reporting.1
-
Blunt chest trauma or high‐speed deceleration injury.
-
Iatrogenic injury, typically from intra‐aortic catheterization.
| Hypertension |
| Atherosclerotic disease |
| History of cardiac surgery |
| Aortic aneurysm |
| Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos) |
| Bicuspid aortic valve (BAV) |
| Aortic coarctation |
| Turner syndrome |
| Strenuous exercise |
| Large vessel arteritis: giant cell, Takayasu's, syphilis |
| Cocaine and methamphetamine ingestion |
| Third‐trimester pregnancy |
| Blunt chest trauma or high‐speed deceleration injury |
| Iatrogenic injury, typically from intra‐aortic catheterization |
INITIAL EVALUATION
The differential diagnosis for acute aortic dissection includes acute coronary syndrome, pulmonary embolus, pneumothorax, pneumonia, musculoskeletal pain, acute cholecystitis, esophageal spasm or rupture, acute pancreatitis, and acute pericarditis. Acute aortic dissections are rarely asymptomatic; in fact, the absence of sudden‐onset chest pain decreases the likelihood of dissection (negative LR 0.3).32 In the IRAD trial, approximately 95% of patients with aortic dissection complained of pain in the chest, back, or abdomen, with 90% characterizing their pain as either severe or the worst ever and 64% describing it as sharp.14 Although the presence of tearing or ripping chest or back pain suggests aortic dissection (positive LR 1.2‐10.8), its absence does not reliably exclude this diagnosis.32 The wide variability in the presentation of aortic dissection increases the challenge of establishing a diagnosis. Clinical findings depend largely on the anatomical location of the dissection and may include pulse deficits, neurologic deficits, hypotension, hypertension, and end‐organ ischemia. Women who develop aortic dissection are generally older and present later than men. Their symptoms are less typical and are likely to be confounded by altered mental status.1 A diagnosis of aortic dissection should be strongly considered for patients presenting with acute chest or back pain and otherwise unexplained aortic insufficiency, focal neurologic deficits, pulse deficits, or end‐organ injury (Table 3).
| Hypotension or shock due to: |
| a. Hemopericardium and pericardial tamponade |
| b. Acute aortic insufficiency due to dilatation of the aortic annulus |
| c. Aortic rupture |
| d. Lactic acidosis |
| e. Spinal shock |
| Acute myocardial ischemia/emnfarction due to coronary ostial occlusion |
| Pericardial friction rub due to hemopericardium |
| Syncope |
| Pleural effusion or frank hemothorax |
| Acute renal failure due to dissection across renal arteries |
| Mesenteric ischemia due to dissection across intra‐abdominal arteries |
| Neurologic deficits: |
| a. Stroke due to occlusion of arch vessels |
| b. Limb weakness |
| c. Spinal cord deficits due to cord ischemia |
| d. Horner syndrome due to compression of superior sympathetic ganglion. |
| e. Hoarseness due to compression of left recurrent laryngeal nerve |
Electrocardiogram: Electrocardiographic abnormalities are commonly seen in aortic dissection and may include ST‐segment or T‐wave abnormalities or left ventricular hypertrophy.14 Proximal aortic dissections may compromise coronary artery perfusion, generating electrocardiogram (ECG) findings compatible with acute myocardial infarction, which may lead the clinician to diagnose and treat myocardial infarction while missing the underlying diagnosis.33 In a recent survey, 9 of 44 patients (21%) presenting with acute aortic dissection were initially diagnosed with acute coronary syndrome and anticoagulated, with 2 deaths.34 ECGs must therefore be interpreted with extreme caution in aortic dissection.
Chest x‐ray: In the emergency department, chest radiography is a mainstay of the evaluation of acute chest pain. Unfortunately, plain‐film radiography has limited utility for diagnosing aortic dissection.35 In the IRAD trial, mediastinal widening (>8 cm) and abnormal aortic contour, the classic radiographic findings in aortic dissection, were present in only 50%‐60% of cases. Twelve percent of patients had a completely normal chest x‐ray.14 A pooled analysis of previous studies demonstrated that the sensitivity of widened mediastinum and abnormal aortic contour was 65% and 71%, respectively.32 Nonspecific radiographic findings, most notably pleural effusion, were common.36 Thus, if the index of suspicion for aortic dissection is elevated, a confirmatory study must be obtained (Figure 2).

Clinical Prediction Tool
Three clinical features were demonstrated to be effective in identifying aortic dissection in patients presenting with acute chest or back pain: immediate onset of tearing or ripping chest pain, mediastinal widening or aortic enlargement/displacement observed on chest x‐ray, and arm pulse or blood pressure differential exceeding 20 mm Hg. When all 3 findings were absent, dissection was unlikely (7% probability, negative LR 0.07 [CI 0.03‐0.17]). If either chest pain or radiographic findings were present, the likelihood was intermediate (31%‐39% probability). With any other combination of findings, dissection was likely (83‐100% probability). This prediction tool effectively identified 96% of all patients who presented to an emergency department with acute aortic dissection.15 However, 4% of patients categorized as low risk were ultimately diagnosed with aortic dissection. Given the exceptionally high mortality resulting from a missed diagnosis, a 4% false‐negative rate is unacceptably high. Thus, the absence of any of the aforementioned findings should not dissuade the clinician from obtaining a confirmatory imaging study if the pretest probability for acute aortic dissection is elevated.
CONFIRMATORY IMAGING STUDIES
The ideal confirmatory imaging modality should identify aortic dissection with high sensitivity and specificity. It should also identify the entry and exit points of the dissection and provide information about the extent of compromise of the aortic valve, pericardium, and great vessels. Four imaging modalities sufficiently meet these criteria in order to be considered diagnostically useful.
Aortography: Previously the gold standard for diagnosing aortic dissection, aortography is no longer a first‐line imaging modality. The sensitivity and specificity of aortography are at best equivalent and probably inferior to less invasive imaging modalities.37, 38 False negatives may occur if both the true and false lumens opacify equally with contrast, or if the false lumen is sufficiently thrombosed to preclude any instillation of contrast. Aortography cannot identify aortic intramural hematomas, is invasive and highly operator dependent, requires nephrotoxic contrast, and generally takes longer to obtain than other modalities.39
Aortography uniquely offers excellent visualization of the coronary arteries and great vessels and is preferred when such information is necessary. Percutaneous aortic endovascular stent grafting has been recently employed to repair distal aortic dissections.4043 As a result, aortography is gaining new life as a therapeutic modality.
CT angiography: Spiral CT angiography (CTA) is the most commonly used modality for diagnosing aortic dissection.44 It is emergently available at most hospitals, and images can be obtained in minutes. Sensitivity and specificity may approach 100%, and CTA may be more sensitive than MRA or TEE in evaluating arch vessel involvement.4547 Like conventional angiography, CTA requires administration of nephrotoxic contrast. It frequently cannot visualize the entry and exit sites (intimal flaps) of a dissection and provides limited information about the coronary arteries and no information about the competency of the aortic valve.48, 49 Thus, if aortic dissection is identified by CTA, a second study may be needed to provide further diagnostic information and to guide surgical intervention (Figures 3 and 4).


Magnetic Resonance Angiography: Magnetic resonance angiography (MRA) offers excellent noninvasive evaluation of the thoracic aorta. Sensitivity and specificity are probably superior to spiral CTA, and MRA generally identifies the location of the intimal tear and provides some functional information about the aortic valve.44, 50, 51 MRA is not emergently available at many hospitals. Scanning is time intensive, requiring the patient to remain motionless and relatively inaccessible for up to an hour. Furthermore, patient claustrophobia and the presence of implanted devices such as pacemakers or ferromagnetic foreign bodies may preclude MRA.
Transesophageal echocardiography: The sensitivity and specificity of transesophageal echocardiography (TEE) are also excellenton a par with CTA and MRA. In addition to providing excellent visualization of the thoracic aorta, TEE provides superb images of the pericardium and detailed assessment of aortic valve function.52 It also is extremely effective at visualizing the aortic intimal flap.44, 49, 53 A significant advantage of TEE is its portability, allowing rapid diagnosis at the bedside. For this reason, it is particularly useful for evaluation of patients who are hemodynamically unstable and are suspected to have an aortic dissection. Because of the anatomic relationship of the aorta with the esophagus and the trachea, TEE more effectively identifies proximal than distal dissections.43 TEE is also somewhat invasive, usually requires patient sedation, and is highly operator dependent, requiring the availability of an experienced and technically skilled operator (Figure 5).

Transthoracic echocardiography: Although it is an excellent tool for the evaluation of many aspects of cardiac anatomy and function, surface echocardiography can reliably visualize only limited portions of the ascending and descending aorta.54, 55 As a consequence, it is neither sensitive nor specific enough to diagnose aortic dissection. Transthoracic echocardiography (TTE) does, however, play a role in rapidly assessing patients at the bedside for aortic valve or pericardial compromise when these complications are suspected.
Recommendations
CTA, MRA, and TEE are all highly sensitive and specific modalities for diagnosing aortic dissection. Therefore, the condition of the patient, the information needed, and the resources and expertise immediately available should drive the choice of study. MRA is considered the gold standard diagnostic study and is the preferred modality for hemodynamically stable patients with suspected aortic dissection. Because of slow data acquisition and the inaccessibility of patients in the scanner, it is generally unsuited for unstable patients, including those with ongoing pain. Bedside TEE is an excellent choice for patients who are too unstable for MRA but is less effective at visualizing distal dissections. Arch aortography is generally reserved for the confirmation of questionable diagnoses or to image specific branch arteries (Tables 4 and 5).
| Overall | Proximal | Distal | |
|---|---|---|---|
| |||
| TEE | 88% | 90% | 80% |
| CTA | 93% | 93% | 93% |
| MRA | 100% | 100% | 100% |
| Aortogram | 87% | 87% | 87% |
| TEE | CTA | MRA | Aortography | |
|---|---|---|---|---|
| ||||
| Sensitivity | ++ | ++ | +++ | ++ |
| Specificity | +++ | ++ | +++ | ++ |
| Classification | +++ | ++ | ++ | + |
| Intimal flap | +++ | ‐ | ++ | + |
| Aortic regurgitation | +++ | ++ | ++ | |
| Pericardial effusion | +++ | ++ | ++ | |
| Branch vessel involvement | + | ++ | ++ | +++ |
| Coronary artery involvement | ++ | + | + | +++ |
Most trials comparing CTA, MRA, and TEE were performed in the early 1990s. Computed tomography has evolved significantly over the intervening decade, and some of the diagnostic limitations previously ascribed to CTA, such as the inability to generate 3‐D reconstructed images, no longer exist. Furthermore, CT angiography is widely available and is gaining increasing acceptance as a first‐line imaging modality for patients with noncardiac chest pain.48 Medical centers that maintain round‐the‐clock CT capability may have limited or delayed access to TEE, MRA, or aortography. Given the potential for rapid and dramatic patient deterioration, it is imperative that a diagnosis be established quickly when aortic dissection is suspected. Thus, when the choice is obtaining an immediate CTA or a delayed TEE or MRA, CTA is generally the better choice (Figure 6).

MANAGEMENT
Acute Management:
Approximately half of all patients who present with acute aortic dissection are acutely hypertensive.14 Hypertensive aortic dissection is a hypertensive emergency that mandates immediate decrease in blood pressure to the lowest level that maintains organ perfusion. As a rule, short‐acting, parenteral, titratable antihypertensive agents should be used (Table 6). Intravenous beta‐adrenergic blockers are the mainstay of acute and chronic therapy. Their negative inotropic and chronotropic effects decrease shear stress across the aortic lumen and decrease the likelihood of dissection propagation and aortic dilatation.56, 57 Parenteral vasodilators (eg, nitroprusside and nitroglycerin) should be initiated if beta‐blockers prove insufficient for lowering blood pressure. They should never be used alone, as they may cause reflex tachycardia and consequently may increase intraluminal shear stress. The use of opiates for analgesia and benzodiazepines for anxiolysis further decreases blood pressure by controlling the severe pain and anxiety often associated with acute dissection.
| Name | Mechanism | Dose | Cautions/contraindications |
|---|---|---|---|
| Esmolol | Cardioselective beta‐1 blocker | Load: 500 g/kg IV | Asthma or bronchospasm |
| Drip: 50 g kg1 min1 IV. | Bradycardia | ||
| Increase by increments of 50 g/min | 2nd‐ or 3rd‐degree AV block | ||
| Cocaine or methamphetamine abuse | |||
| Labetalol | Nonselective beta 1,2 blocker | Load: 20 mg IV | Asthma or bronchospasm |
| Selective alpha‐1 blocker | Drip: 2 mg/min IV | Bradycardia | |
| 2nd or 3rd degree AV block | |||
| Cocaine or methamphetamine abuse | |||
| Enalaprilat | ACE inhibitor | 0.625‐1.25 mg IV q 6 hours. | Angioedema |
| Max dose: 5 mg q 6 hours. | Pregnancy | ||
| Renal artery stenosis | |||
| Severe renal insufficiency | |||
| Nitroprusside | Direct arterial vasodilator | Begin at 0.3 g kg1 min1 IV. | May cause reflex tachycardia |
| Max dose 10 g kg1 min1 | Cyanide/thiocyanate toxicityespecially in renal or hepatic insufficiency | ||
| Nitroglycerin | Vascular smooth muscle relaxation | 5‐200 g/min IV | Decreases preloadcontraindicated in tamponade or other preload‐dependent states |
| Concomitant use of sildenafil or similar agents |
Hypotension or shock, which develop in 15%‐30% of patients with acute aortic dissection, are ominous findings that frequently portends impending hemodynamic collapse.14, 58 Patients who develop hypotension are at a fivefold increased risk of death (55.0% vs. 10.3%) and are at markedly increased risk of developing neurologic deficits, as well as myocardial, mesenteric, and limb ischemia. Hypotension may result from pump failure (due to acute aortic insufficiency, pericardial tamponade, or myocardial ischemia), aortic rupture, systemic lactic acidosis, or spinal shock. Bedside transthoracic echocardiography may be particularly useful for the evaluation of hypotensive patients, as it can be used to quickly and noninvasively determine the integrity of the aortic valve and pericardium. Although hypotension may transiently respond to volume resuscitation, all hypotensive patients with aortic dissection, regardless of type, should be immediately referred for emergent surgical evaluation. Pericardiocentesis in the setting of pericardial tamponade remains controversial; a small study suggested that decompression of the pericardial sac may hasten hemodynamic collapse by accelerating blood loss.59
Facilities that do not maintain urgent cardiopulmonary bypass capability should emergently transport patients with aortic dissection to a facility that provides a higher level of care. Transfer should not be delayed to confirm a questionable diagnosis. Proximal aortic dissection frequently compromises the pericardium, aortic valve, and arch vessels, and therefore emergent surgical repair is indicated. When treated medically, proximal dissection carries a dismal 60% in‐hospital mortality rate.14, 60 In contrast, distal aortic dissection is generally treated medically, with surgical intervention generally reserved for patients with an expanding aortic aneurysm, elevated risk of aortic rupture, refractory hypertension, intractable pain, visceral hypoperfusion, and limb ischemia or paresis.11, 61, 62 Individual branch vessel occlusion may be effectively ameliorated with conventional arterial stenting or balloon fenestration.
Endovascular stent grafting has been used successfully in lieu of surgery for patients with acute or chronic distal (type B) aortic dissections.39, 4042, 63 The stent graft is deployed across the proximal intimal tear, obliterating the false lumen and facilitating aortic healing. Early studies suggested that endovascular stent grafting may be safer and more efficacious than conventional surgical repair of distal dissection.41 A recent meta‐analysis of published trials of endovascular aortic stenting found procedural success rates exceeding 95% and a major complication rate of 11%. Thirty‐day mortality was approximately 5%, with 6‐, 12‐ and 24‐month mortality rates plateauing at 10%. Centers with high patient volume had fewer complications and much lower acute mortality rates.14, 64 These medium‐term outcomes compare favorably with conventional therapy. Endovascular stenting has not been prospectively compared against conventional therapy in randomized trials, and it therefore remains unclear who should be referred for endovascular stenting instead of conventional therapy.
Long‐term Management
Survivors of aortic dissection, especially those with diseases of collagen, have a systemic disease that predisposes them to further aortic and great vessel events. Almost one third of survivors of acute aortic dissection will develop dissection propagation or aortic rupture or will require aortic surgery within 5 years of presentation.41, 60 Young patients who present for aortic dissection should be screened for Marfan syndrome according to the Gent nosology.65 To reduce shear stress to the aortic lumen, all patients should be treated with beta‐blockers for life, with blood pressure targeted to be below 135/80.60, 66 Patients who do not tolerate beta blockade may benefit from treatment with diltiazem or verapamil. Progression to aortic aneurysm is common, and patients should undergo serial imaging of the aorta at 1, 3, 6, and 12 months after discharge and annually thereafter. Dilatation of the proximal aorta to >5.0 cm and of the distal aorta to >6.0 cm should prompt referral for surgical or possibly endovascular repair.41, 67 Although supporting data are limited, it is generally accepted that patients should moderate their physical activity to avoid extremes of tachycardia and blood pressure elevation. Sports that involve high speed or sudden deceleration, such as ice hockey, downhill skiing, and football, should be strictly avoided. Patients should be warned to seek immediate medical attention if they develop recurrent chest or back pain or focal neurologic deficits.
PROGNOSIS
Despite significant medical and surgical advances, aortic dissection remains exceptionally lethal. Patients with proximal dissections are more likely to die than those with distal dissections. Using data from the IRAD trial, Mehta et al determined that age 70 years (OR, 1.70), abrupt onset of chest pain (OR 2.60), hypotension/shock/tamponade (OR, 2.97), renal failure (OR, 4.77), pulse deficit (OR, 2.03), and abnormal ECG (OR, 1.77) were independent determinants of death.59 Medical treatment of proximal dissection is generally reserved for patients too ill, unstable, or frail to undergo surgery. In contrast, most patients with distal dissection are managed medically, with surgery generally reserved for those with acute complications. Hence, patients with proximal dissections who are managed medically and those with distal dissections who are managed surgically have the worst outcomes. Outcomes for women are worse than those for men, which is probably attributable to several factors. Women dissect at an older age, present later after the onset of symptoms, and are more likely to have confounding symptoms that may delay timely diagnosis1 (Table 7).
| Proximal (DeBakey I, II; Stanford A) | Distal (DeBakey III; Stanford B) | |||
|---|---|---|---|---|
| Surgical | Medical | Surgical | Medical | |
| In‐hospital mortality | 26% | 58% | 31% | 11% |
| Average | 35% | 15% | ||
CONCLUSION
Aortic dissection is a rare and acutely life‐threatening cause of acute chest and back pain. Delays in diagnosis and misdiagnoses are common, frequently with catastrophic consequences. The key to diagnosis is maintaining a high index of suspicion for dissection, especially in patients who present with acute severe chest, back, or abdominal pain in the setting of unexplained acute pulse deficits, neurologic deficits, or acute end‐organ injury. Three clinical findings have been shown to be diagnostically useful: immediate onset of tearing or ripping chest or back pain, mediastinal widening or abnormal aortic contour on chest radiograph, and peripheral pulse deficits or variable pulse pressure (>20 mm Hg). If all 3 findings are absent, acute aortic dissection is unlikely. The presence of any of these findings should prompt further workup. A normal chest radiograph does not rule out aortic dissection. Only TEE, CT, and MR angiography are sufficiently specific to rule out dissection. Aortography is rarely used as a first‐line diagnostic tool but may be useful as a confirmatory test or to provide additional anatomic information. Patients who present with proximal aortic dissection or with any aortic dissection with concomitant hypotension are at exceptionally high risk of death and should be immediately referred for surgical evaluation. Beta‐blockers are the mainstay of acute and chronic therapy of aortic dissection. Survivors of aortic dissection are at a markedly elevated risk for further aortic events and should be followed vigilantly posthospitalization.
- ,,, et al.Gender‐related differences in acute aortic dissection.Circulation.2004;109:3014–3021.
- ,,, et al.Clinical features and differential diagnosis of aortic dissection: Experience with 236 cases.Mayo Clin Proc.1993;68:642–651.
- ,.The natural history of thoracic aortic aneurysm disease: an overview.J Card Surg.1997;12(suppl):270–278.
- ,,.Dissecting aneurysms of the aorta: a review of 505 cases.Medicine.1958;37:217–279.
- ,,,.Intimal tear without hematoma; an important variant of aortic dissection that can elude current imaging techniques.Circulation.1999;99:1331–1336.
- ,,, et al.Intramural hemorrhage of the thoracic aorta: diagnostic and therapeutic implications.Circulation.1995;92:1465–1472.
- ,.Acute aortic dissection and its variants; towards a common diagnostic and therapeutic approach.Circulation.1995;92:1376–1378.
- ,,.The penetrating aortic ulcer: pathologic manifestations, diagnosis and management.Mayo Clinic Proc.1988;63:718–725.
- ,,, et al.Acute intramural hematoma of the aorta—a mystery in evolution.Circulation.2005;111:1063–1070.
- ,.Intramural hematoma in acute aortic syndrome; more than one variant of dissection?Circulation.2002;106:284–285.
- ,,,,,,.Acute aortic dissection: population‐based incidence compared with degenerative aortic aneurysm rupture.Mayo Clin Proc.2004;79(2):176–180.
- ,,,,,,.Epidemiology and clinicopathology of aortic dissection.Chest.2000;117:1271–1278.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD).JAMA.2000;283:897–903.
- ,,.Clinical prediction of acute aortic dissection.Arch Intern Med.2000;160:2977–2982.
- ,.Risk factors for aortic dissection: a necropsy study of 161 cases.Am J Cardiol.1984;53:849–855.
- ,,, et al.Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD).J Am Coll Cardiol.2004;43:665–669.
- ,,,.Association of aortic dilatation with regurgitant, stenotic and functionally normal bicuspid aortic valves.J Am Coll Cardiol.1992;19:283–288.
- .Clinical significance of the bicuspid aortic valve.Heart.2000;83:81–85.
- ,,,.Aortic root dilatation in young men with normally functioning bicuspid aortic valves.Heart.1999;82:19–22.
- ,,, et al.Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation.J Thorac Cardiovasc Surg.2003;126:797–806.
- ,,.Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome.Pediatrics.1998;102(1):e12.
- ,,, et al.Aortic dilatation, dissection and rupture in patients with Turner syndrome.J Pediatr.1986;109:820–826.
- ,.Weight lifting and type II aortic dissection. A case report.J Sports Med Phys Fitness.2004;44:424–427.
- ,,.Recreational weight lifting and aortic dissection: case report.J Vasc Surg.1993;17:774–776.
- ,,,,,,.Ascending aortic dissection in weight lifters with cystic medial degeneration.Ann Thoracic Surg.1990;49:638–642.
- ,,,,.Acute aortic dissection related to crack cocaine.Circulation.2002;105:1592–1595.
- ,.Thoracic aortic dissection secondary to crack cocaine ingestion.Am J Emerg Med.1997;15:507–509.
- ,.Cocaine‐associated dissection of the thoracic aorta.J Emerg Med.1992;10:723–727.
- ,.Methamphetamine as a risk factor for acute aortic dissection.J Forensic Sci.1999;44(1):23–26.
- ,,,,,.Arterial dissections associated with pregnancy.J Vasc Surg.1995;21:515–520.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,,,.Fatal haemostatic complications due to thrombolytic therapy in patients falsely diagnosed as acute myocardial infarction.Eur Heart J.1992;13:840–843.
- ,,,,.The inadvertent administration of anticoagulants to ED patients ultimately diagnosed with thoracic aortic dissection.Am J Emerg Med.2005;23:439–442..
- ,,, et al.Chest radiography for the diagnosis of acute aortic syndrome.Am J Med.2004;116(2):73–77.
- ,,, et al.Clinical significance of pleural effusion in acute aortic dissection.Chest.2002;121:825–830.
- ,,,.Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography.J Am Coll Cardiol.1995;25:1393–1401.
- ,.Digital subtraction angiography of aortic dissection.Am J Roentgenol.1983;141:157–161.
- ,,, et al.Spectrum of conditions initially suggesting acute aortic dissection but with negative aortograms.Am J Cardiol.1986;57:322–326.
- ,,, et al.Nonsurgical reconstruction of thoracic aortic dissection by stent‐graft placement.N Engl J Med.1999;340:1539–1545.
- ,,, et al.Endovascular stent‐graft placement for the treatment of aortic dissection.N Engl J Med.1999;340:1546–1552.
- ,.Aortic dissection: New frontiers in diagnosis and management. Part II: Therapeutic management and follow‐up.Circulation.2003;108:772–778.
- ,,,,,.Aortic dissection: percutaneous management of ischemic complications with endovascular stents and balloon fenestration.J Vasc Surg.1996;23:241–251.
- ,,, et al.Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD).Am J Cardiol.2002;89:1235–1238.
- ,,, et al.Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging.Radiology.1996;199:347–352.
- ,,,.Assessment of the thoracic aorta by spiral CT.AJR Am J Roengenol.1992;158:1127–1130.
- ,Radiologic evaluation of aortic dissection.Radiology.1991;180:297–305.
- ,,,,,.Computed tomography of thoracic aortic dissection: accuracy and pitfalls.J Comput Assist Tomogr.1986;10:211–215.
- ,,.Spiral CT in acute non‐cardiac chest pain.Clin Radiol.1999;54(1):38–45.
- ,,, et al.Diagnosis of thoracic aortic dissection. Magnetic resonance imaging versus transesophageal echocardiography.Circulation.1992;85:434–447.
- ,,,,.Comparison of conventional and transesophageal echocardiography with magnetic resonance imaging for anatomical mapping of thoracic aortic dissection: a dual noninvasive imaging study with anatomical and/or angiographic validation.Int J Card Imaging.1994;10:1–14.
- ,.Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: part I. Aortic dissection, aortic intramural hematoma and penetrating atherosclerotic ulcer of the aorta.Chest.1999;116:1772–1779.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328:1–9.
- ,,,,,.Echocardiography in diagnosis of aortic dissection.Lancet.1989;1:457–461.
- ,,,,.A prospectus on the prevention of aortic rupture in the Marfan syndrome with data on survivorship without treatment.Johns Hopkins Med J.1971;129:123–129.
- ,,,.Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome.N Engl J Med.1994;330:1335–1341.
- ,,, et al.Clinical characteristics of hypotension in patients with acute aortic dissection.Am J Cardiol.2005;95:48–52.
- ,,.Cardiac tamponade complicating proximal aortic dissection: is pericardiocentesis harmful?Circulation.1994;90:2375–2379.
- ,,, et al.Predicting in‐hospital mortality in acute type A aortic dissection.Circulation.2002;105:200–206.
- ,,, et al.Diagnosis and management of aortic dissection: task force report of the European Society of Cardiology.Eur Heart J.2001;22:1642–1681.
- ,,,,,.Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the international registry of aortic dissection.Circulation.2003;108(suppl II):II‐312–II‐317.
- ,,, et al.Endovascular stent‐graft treatment of aortic dissection: determinants of post‐interventional outcome.Eur Heart J.2005;26:489–497.
- ,,, et al.Endovascular stent‐graft placement in aortic dissection: a meta‐analysis [advance access online].Eur Heart J.2005. Published online October 14, 2005.
- ,,,,.Revised diagnostic criteria for the Marfan syndrome.Am J Med Genet.1996;62:417–426.
- ,,,.Treatment of dissecting aneurysms of the aorta without surgery.J Thorac Cardiovasc Surg.1965;50:364–373.
- ,,,,,.Unoperated aortic aneurysms: a survey of 170 patients.Ann Thorac Surg.1995;59:1204–1209.
- ,,, et al.Gender‐related differences in acute aortic dissection.Circulation.2004;109:3014–3021.
- ,,, et al.Clinical features and differential diagnosis of aortic dissection: Experience with 236 cases.Mayo Clin Proc.1993;68:642–651.
- ,.The natural history of thoracic aortic aneurysm disease: an overview.J Card Surg.1997;12(suppl):270–278.
- ,,.Dissecting aneurysms of the aorta: a review of 505 cases.Medicine.1958;37:217–279.
- ,,,.Intimal tear without hematoma; an important variant of aortic dissection that can elude current imaging techniques.Circulation.1999;99:1331–1336.
- ,,, et al.Intramural hemorrhage of the thoracic aorta: diagnostic and therapeutic implications.Circulation.1995;92:1465–1472.
- ,.Acute aortic dissection and its variants; towards a common diagnostic and therapeutic approach.Circulation.1995;92:1376–1378.
- ,,.The penetrating aortic ulcer: pathologic manifestations, diagnosis and management.Mayo Clinic Proc.1988;63:718–725.
- ,,, et al.Acute intramural hematoma of the aorta—a mystery in evolution.Circulation.2005;111:1063–1070.
- ,.Intramural hematoma in acute aortic syndrome; more than one variant of dissection?Circulation.2002;106:284–285.
- ,,,,,,.Acute aortic dissection: population‐based incidence compared with degenerative aortic aneurysm rupture.Mayo Clin Proc.2004;79(2):176–180.
- ,,,,,,.Epidemiology and clinicopathology of aortic dissection.Chest.2000;117:1271–1278.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD).JAMA.2000;283:897–903.
- ,,.Clinical prediction of acute aortic dissection.Arch Intern Med.2000;160:2977–2982.
- ,.Risk factors for aortic dissection: a necropsy study of 161 cases.Am J Cardiol.1984;53:849–855.
- ,,, et al.Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD).J Am Coll Cardiol.2004;43:665–669.
- ,,,.Association of aortic dilatation with regurgitant, stenotic and functionally normal bicuspid aortic valves.J Am Coll Cardiol.1992;19:283–288.
- .Clinical significance of the bicuspid aortic valve.Heart.2000;83:81–85.
- ,,,.Aortic root dilatation in young men with normally functioning bicuspid aortic valves.Heart.1999;82:19–22.
- ,,, et al.Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation.J Thorac Cardiovasc Surg.2003;126:797–806.
- ,,.Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome.Pediatrics.1998;102(1):e12.
- ,,, et al.Aortic dilatation, dissection and rupture in patients with Turner syndrome.J Pediatr.1986;109:820–826.
- ,.Weight lifting and type II aortic dissection. A case report.J Sports Med Phys Fitness.2004;44:424–427.
- ,,.Recreational weight lifting and aortic dissection: case report.J Vasc Surg.1993;17:774–776.
- ,,,,,,.Ascending aortic dissection in weight lifters with cystic medial degeneration.Ann Thoracic Surg.1990;49:638–642.
- ,,,,.Acute aortic dissection related to crack cocaine.Circulation.2002;105:1592–1595.
- ,.Thoracic aortic dissection secondary to crack cocaine ingestion.Am J Emerg Med.1997;15:507–509.
- ,.Cocaine‐associated dissection of the thoracic aorta.J Emerg Med.1992;10:723–727.
- ,.Methamphetamine as a risk factor for acute aortic dissection.J Forensic Sci.1999;44(1):23–26.
- ,,,,,.Arterial dissections associated with pregnancy.J Vasc Surg.1995;21:515–520.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,,,.Fatal haemostatic complications due to thrombolytic therapy in patients falsely diagnosed as acute myocardial infarction.Eur Heart J.1992;13:840–843.
- ,,,,.The inadvertent administration of anticoagulants to ED patients ultimately diagnosed with thoracic aortic dissection.Am J Emerg Med.2005;23:439–442..
- ,,, et al.Chest radiography for the diagnosis of acute aortic syndrome.Am J Med.2004;116(2):73–77.
- ,,, et al.Clinical significance of pleural effusion in acute aortic dissection.Chest.2002;121:825–830.
- ,,,.Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography.J Am Coll Cardiol.1995;25:1393–1401.
- ,.Digital subtraction angiography of aortic dissection.Am J Roentgenol.1983;141:157–161.
- ,,, et al.Spectrum of conditions initially suggesting acute aortic dissection but with negative aortograms.Am J Cardiol.1986;57:322–326.
- ,,, et al.Nonsurgical reconstruction of thoracic aortic dissection by stent‐graft placement.N Engl J Med.1999;340:1539–1545.
- ,,, et al.Endovascular stent‐graft placement for the treatment of aortic dissection.N Engl J Med.1999;340:1546–1552.
- ,.Aortic dissection: New frontiers in diagnosis and management. Part II: Therapeutic management and follow‐up.Circulation.2003;108:772–778.
- ,,,,,.Aortic dissection: percutaneous management of ischemic complications with endovascular stents and balloon fenestration.J Vasc Surg.1996;23:241–251.
- ,,, et al.Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD).Am J Cardiol.2002;89:1235–1238.
- ,,, et al.Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging.Radiology.1996;199:347–352.
- ,,,.Assessment of the thoracic aorta by spiral CT.AJR Am J Roengenol.1992;158:1127–1130.
- ,Radiologic evaluation of aortic dissection.Radiology.1991;180:297–305.
- ,,,,,.Computed tomography of thoracic aortic dissection: accuracy and pitfalls.J Comput Assist Tomogr.1986;10:211–215.
- ,,.Spiral CT in acute non‐cardiac chest pain.Clin Radiol.1999;54(1):38–45.
- ,,, et al.Diagnosis of thoracic aortic dissection. Magnetic resonance imaging versus transesophageal echocardiography.Circulation.1992;85:434–447.
- ,,,,.Comparison of conventional and transesophageal echocardiography with magnetic resonance imaging for anatomical mapping of thoracic aortic dissection: a dual noninvasive imaging study with anatomical and/or angiographic validation.Int J Card Imaging.1994;10:1–14.
- ,.Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: part I. Aortic dissection, aortic intramural hematoma and penetrating atherosclerotic ulcer of the aorta.Chest.1999;116:1772–1779.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328:1–9.
- ,,,,,.Echocardiography in diagnosis of aortic dissection.Lancet.1989;1:457–461.
- ,,,,.A prospectus on the prevention of aortic rupture in the Marfan syndrome with data on survivorship without treatment.Johns Hopkins Med J.1971;129:123–129.
- ,,,.Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome.N Engl J Med.1994;330:1335–1341.
- ,,, et al.Clinical characteristics of hypotension in patients with acute aortic dissection.Am J Cardiol.2005;95:48–52.
- ,,.Cardiac tamponade complicating proximal aortic dissection: is pericardiocentesis harmful?Circulation.1994;90:2375–2379.
- ,,, et al.Predicting in‐hospital mortality in acute type A aortic dissection.Circulation.2002;105:200–206.
- ,,, et al.Diagnosis and management of aortic dissection: task force report of the European Society of Cardiology.Eur Heart J.2001;22:1642–1681.
- ,,,,,.Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the international registry of aortic dissection.Circulation.2003;108(suppl II):II‐312–II‐317.
- ,,, et al.Endovascular stent‐graft treatment of aortic dissection: determinants of post‐interventional outcome.Eur Heart J.2005;26:489–497.
- ,,, et al.Endovascular stent‐graft placement in aortic dissection: a meta‐analysis [advance access online].Eur Heart J.2005. Published online October 14, 2005.
- ,,,,.Revised diagnostic criteria for the Marfan syndrome.Am J Med Genet.1996;62:417–426.
- ,,,.Treatment of dissecting aneurysms of the aorta without surgery.J Thorac Cardiovasc Surg.1965;50:364–373.
- ,,,,,.Unoperated aortic aneurysms: a survey of 170 patients.Ann Thorac Surg.1995;59:1204–1209.
View from the Hospital Bed
Six years ago, at the age of 76, I suffered a type B aortic dissection while lifting weights. At the time, I managed a busy architecture practice, jogged a 9‐minute mile, and had never experienced a serious illness. My only medication was a baby aspirin every morning. The dissection was diagnosed promptly and treated medically with a stay of less than a week in the hospital. My son Eric, a hospitalist, immediately flew in from Denver and stayed with me throughout my hospitalization. Within days of my discharge, however, I began experiencing a series of mild discomforts. I had difficulty sleeping, mild indigestion, and a burning sensation in my thighs after walking relatively short distances. My primary care physician and cardiologist didn't seem concerned about these symptoms, and as they were initially mild, I accepted them as the residual effects of sleep deprivation, hospital food, and muscle atrophy. Nobody recognized that my dissection had propagated, effectively cutting off blood flow below my diaphragm.
To my good fortune, Eric had previously scheduled a second visit long before I had become ill. I vividly recall him walking into our house in the early evening, taking one look at me, and saying, I don't like what I see. My most notable memory of the return ambulance trip was how cold my feet were. I had never experienced that intense a sensation of cold before. I recall arriving at a very busy, crowded ER and Eric aggressively trying to get priority attention. The next thing I remember was waking up in the ICU the next afternoon. My kidneys had failed, and my intestines were not getting blood flow. By then, I had become too unstable to undergo aortic surgery. As a last‐ditch effort, an interventional radiologist tried to open my aorta using four large biliary stents, none of which deployed properly. Then a vascular surgeon suggested performing an axillo‐bifemoral bypass, which is much less invasive than aortic surgery, in order to restore blood flow to my kidneys and intestines. It saved my life.
There are many things about the hospital environment that elevate anxiety and vulnerability, and perhaps that is inevitable. The concentration of sick people is depressing. I had several roommates, each much younger than I, with prognoses that appeared far less favorable than mine. I listened to their doctors, some with bedside manners so clinical that they bordered on insensitivity. In one instance, when there was clearly a communication barrier, I saw a patient and family become bewildered and overwhelmed. Watching this unfold only heightened my own sense of vulnerability.
When it became evident that I was not going to die, my emotions ranged from elation at having beaten the odds to outright fear. I had come to the hospital healthy, fit, and independent. Now a host of new concerns emerged. What would my limitations be, and to what kind of lifestyle could I look forward? Would I be self‐sufficient, or would I become a burden to my family? Perhaps these were ungrateful responses to having just dodged the bullet, but I take no responsibility for my subconscious. Nights were the worst, especially when sleep was elusive. My days were filled with tests and visitors, but I had ample time to court my anxieties after dark. As an artillery reconnaissance officer during World War II, I had known fear, but this was different. Fear during combat was shared by all and functionally accommodated by most. It became part of a common bond. Maybe because of our youth and inexperience, we only worried about being killeda singular event, and then it was all over. Thoughts of permanent disability and its consequences never crossed our minds.
My physical recovery was far more rapid than my psychological and emotional recovery. My physicians told me that they had never encountered a case like mine and that we were in uncharted waters. Although I appreciated their candor, this was less than reassuring. Furthermore, the mild symptoms I had experienced during my re‐dissection sensitized me to every new little pain, twinge, or discomfort. How was I to differentiate relevant and significant new symptoms from hypochondria? It took a very long time to recover my sense of well‐being. The support of my wife and family was invaluable, but ultimately this is something one must sort out for oneself. I recognized that I could not face the rest of my life with fear and anxiety. I tried professional counseling without noticeable benefit. Eventually, I learned to analyze each concern as it surfaced, recognize it for what it was, and put it in perspective. The passage of time and daily meditation also contributed to my emotional healing. Today, some of the old ghosts still emerge from the shadows, but now they have no substance and rapidly disappear.
I attribute my survival and recovery over the past six years to the marvels of modern medicine (accompanied by some miscues and imperfections), the forceful advocacy of a loving wife and a physician son who were always there at critical moments, and a significant dose of pure good luck. As I write this, a few days before my 82nd birthday, I remain actively engaged in my practice, still work out, albeit more prudently than before, and walk a brisk 16‐minute mile. I will forever be grateful for the professionalism and dedication of the health care personnel I encountered and for the astonishing technology and infrastructure that saved my life.
Six years ago, at the age of 76, I suffered a type B aortic dissection while lifting weights. At the time, I managed a busy architecture practice, jogged a 9‐minute mile, and had never experienced a serious illness. My only medication was a baby aspirin every morning. The dissection was diagnosed promptly and treated medically with a stay of less than a week in the hospital. My son Eric, a hospitalist, immediately flew in from Denver and stayed with me throughout my hospitalization. Within days of my discharge, however, I began experiencing a series of mild discomforts. I had difficulty sleeping, mild indigestion, and a burning sensation in my thighs after walking relatively short distances. My primary care physician and cardiologist didn't seem concerned about these symptoms, and as they were initially mild, I accepted them as the residual effects of sleep deprivation, hospital food, and muscle atrophy. Nobody recognized that my dissection had propagated, effectively cutting off blood flow below my diaphragm.
To my good fortune, Eric had previously scheduled a second visit long before I had become ill. I vividly recall him walking into our house in the early evening, taking one look at me, and saying, I don't like what I see. My most notable memory of the return ambulance trip was how cold my feet were. I had never experienced that intense a sensation of cold before. I recall arriving at a very busy, crowded ER and Eric aggressively trying to get priority attention. The next thing I remember was waking up in the ICU the next afternoon. My kidneys had failed, and my intestines were not getting blood flow. By then, I had become too unstable to undergo aortic surgery. As a last‐ditch effort, an interventional radiologist tried to open my aorta using four large biliary stents, none of which deployed properly. Then a vascular surgeon suggested performing an axillo‐bifemoral bypass, which is much less invasive than aortic surgery, in order to restore blood flow to my kidneys and intestines. It saved my life.
There are many things about the hospital environment that elevate anxiety and vulnerability, and perhaps that is inevitable. The concentration of sick people is depressing. I had several roommates, each much younger than I, with prognoses that appeared far less favorable than mine. I listened to their doctors, some with bedside manners so clinical that they bordered on insensitivity. In one instance, when there was clearly a communication barrier, I saw a patient and family become bewildered and overwhelmed. Watching this unfold only heightened my own sense of vulnerability.
When it became evident that I was not going to die, my emotions ranged from elation at having beaten the odds to outright fear. I had come to the hospital healthy, fit, and independent. Now a host of new concerns emerged. What would my limitations be, and to what kind of lifestyle could I look forward? Would I be self‐sufficient, or would I become a burden to my family? Perhaps these were ungrateful responses to having just dodged the bullet, but I take no responsibility for my subconscious. Nights were the worst, especially when sleep was elusive. My days were filled with tests and visitors, but I had ample time to court my anxieties after dark. As an artillery reconnaissance officer during World War II, I had known fear, but this was different. Fear during combat was shared by all and functionally accommodated by most. It became part of a common bond. Maybe because of our youth and inexperience, we only worried about being killeda singular event, and then it was all over. Thoughts of permanent disability and its consequences never crossed our minds.
My physical recovery was far more rapid than my psychological and emotional recovery. My physicians told me that they had never encountered a case like mine and that we were in uncharted waters. Although I appreciated their candor, this was less than reassuring. Furthermore, the mild symptoms I had experienced during my re‐dissection sensitized me to every new little pain, twinge, or discomfort. How was I to differentiate relevant and significant new symptoms from hypochondria? It took a very long time to recover my sense of well‐being. The support of my wife and family was invaluable, but ultimately this is something one must sort out for oneself. I recognized that I could not face the rest of my life with fear and anxiety. I tried professional counseling without noticeable benefit. Eventually, I learned to analyze each concern as it surfaced, recognize it for what it was, and put it in perspective. The passage of time and daily meditation also contributed to my emotional healing. Today, some of the old ghosts still emerge from the shadows, but now they have no substance and rapidly disappear.
I attribute my survival and recovery over the past six years to the marvels of modern medicine (accompanied by some miscues and imperfections), the forceful advocacy of a loving wife and a physician son who were always there at critical moments, and a significant dose of pure good luck. As I write this, a few days before my 82nd birthday, I remain actively engaged in my practice, still work out, albeit more prudently than before, and walk a brisk 16‐minute mile. I will forever be grateful for the professionalism and dedication of the health care personnel I encountered and for the astonishing technology and infrastructure that saved my life.
Six years ago, at the age of 76, I suffered a type B aortic dissection while lifting weights. At the time, I managed a busy architecture practice, jogged a 9‐minute mile, and had never experienced a serious illness. My only medication was a baby aspirin every morning. The dissection was diagnosed promptly and treated medically with a stay of less than a week in the hospital. My son Eric, a hospitalist, immediately flew in from Denver and stayed with me throughout my hospitalization. Within days of my discharge, however, I began experiencing a series of mild discomforts. I had difficulty sleeping, mild indigestion, and a burning sensation in my thighs after walking relatively short distances. My primary care physician and cardiologist didn't seem concerned about these symptoms, and as they were initially mild, I accepted them as the residual effects of sleep deprivation, hospital food, and muscle atrophy. Nobody recognized that my dissection had propagated, effectively cutting off blood flow below my diaphragm.
To my good fortune, Eric had previously scheduled a second visit long before I had become ill. I vividly recall him walking into our house in the early evening, taking one look at me, and saying, I don't like what I see. My most notable memory of the return ambulance trip was how cold my feet were. I had never experienced that intense a sensation of cold before. I recall arriving at a very busy, crowded ER and Eric aggressively trying to get priority attention. The next thing I remember was waking up in the ICU the next afternoon. My kidneys had failed, and my intestines were not getting blood flow. By then, I had become too unstable to undergo aortic surgery. As a last‐ditch effort, an interventional radiologist tried to open my aorta using four large biliary stents, none of which deployed properly. Then a vascular surgeon suggested performing an axillo‐bifemoral bypass, which is much less invasive than aortic surgery, in order to restore blood flow to my kidneys and intestines. It saved my life.
There are many things about the hospital environment that elevate anxiety and vulnerability, and perhaps that is inevitable. The concentration of sick people is depressing. I had several roommates, each much younger than I, with prognoses that appeared far less favorable than mine. I listened to their doctors, some with bedside manners so clinical that they bordered on insensitivity. In one instance, when there was clearly a communication barrier, I saw a patient and family become bewildered and overwhelmed. Watching this unfold only heightened my own sense of vulnerability.
When it became evident that I was not going to die, my emotions ranged from elation at having beaten the odds to outright fear. I had come to the hospital healthy, fit, and independent. Now a host of new concerns emerged. What would my limitations be, and to what kind of lifestyle could I look forward? Would I be self‐sufficient, or would I become a burden to my family? Perhaps these were ungrateful responses to having just dodged the bullet, but I take no responsibility for my subconscious. Nights were the worst, especially when sleep was elusive. My days were filled with tests and visitors, but I had ample time to court my anxieties after dark. As an artillery reconnaissance officer during World War II, I had known fear, but this was different. Fear during combat was shared by all and functionally accommodated by most. It became part of a common bond. Maybe because of our youth and inexperience, we only worried about being killeda singular event, and then it was all over. Thoughts of permanent disability and its consequences never crossed our minds.
My physical recovery was far more rapid than my psychological and emotional recovery. My physicians told me that they had never encountered a case like mine and that we were in uncharted waters. Although I appreciated their candor, this was less than reassuring. Furthermore, the mild symptoms I had experienced during my re‐dissection sensitized me to every new little pain, twinge, or discomfort. How was I to differentiate relevant and significant new symptoms from hypochondria? It took a very long time to recover my sense of well‐being. The support of my wife and family was invaluable, but ultimately this is something one must sort out for oneself. I recognized that I could not face the rest of my life with fear and anxiety. I tried professional counseling without noticeable benefit. Eventually, I learned to analyze each concern as it surfaced, recognize it for what it was, and put it in perspective. The passage of time and daily meditation also contributed to my emotional healing. Today, some of the old ghosts still emerge from the shadows, but now they have no substance and rapidly disappear.
I attribute my survival and recovery over the past six years to the marvels of modern medicine (accompanied by some miscues and imperfections), the forceful advocacy of a loving wife and a physician son who were always there at critical moments, and a significant dose of pure good luck. As I write this, a few days before my 82nd birthday, I remain actively engaged in my practice, still work out, albeit more prudently than before, and walk a brisk 16‐minute mile. I will forever be grateful for the professionalism and dedication of the health care personnel I encountered and for the astonishing technology and infrastructure that saved my life.
Impact of CT on PE Diagnosis
Spiral computed tomographic pulmonary angiography (CTPA) is a common first‐line test for the evaluation of suspected pulmonary embolism (PE). At our institution CTPA became the initial diagnostic study in 83% of patients with suspected PE within 3 years of the introduction of CT,1 and by 2001 CTPA had become the most common diagnostic test performed nationwide in patients diagnosed with PE.2 Most scans are interpreted as either positive or negative for pulmonary embolism, providing clinicians with a greater sense of diagnostic certainty than with the probabilistic results of lung scintigraphy. Initial studies of CTPA supported this appearance of diagnostic certainty, reporting sensitivity and specificity of greater than 90%,3, 4 but several subsequent studies have failed to reproduce these results.57 Newer multidetector CT scans are believed to be more accurate than earlier single‐detector CT,8 but true estimates of CTPA test characteristics will not be known until publication of the forthcoming PIOPED II study.9
Even without these data, CT‐based diagnostic algorithms have already appeared.1014 These algorithms generally focus on minimizing the false‐negative rate through use of serial testing (involving combinations of serum D‐dimer, lower‐extremity ultrasound, and CTPA). A recent meta‐analysis demonstrated that negative CTPA is highly accurate at ruling out PE, with test characteristics similar to conventional pulmonary angiography.15 Another meta‐analysis found that the 3‐month rate of subsequent venous thromboembolism after negative CTPA was 1.4% (95% CI 1.1%‐1.8%),16 supporting the strategy of withholding anticoagulants after negative CTPA in combination with other tests. However, use of serial testing to establish the diagnosis of PE and initiate anticoagulation has not been systematically evaluated or recommended, even for patients with a low pretest probability of PE.17
To assess the potential impact of these algorithms on the diagnosis of PE in clinical practice, we analyzed the clinical presentation and treatment of a cohort of patients at our institution who underwent CTPA for suspected PE.1 We calculated a range of posttest probabilities for pulmonary embolism for these patients, given the pretest probabilities, test results, and estimates of CTPA test characteristics. We then compared the treatment decisions of clinicians to the posttest probabilities of PE in order to establish the potential frequency of false‐positive and false‐negative diagnoses and to determine if patients were treated appropriately based on these estimates.
METHODS
Sites and Subjects
Details of the sites, subjects, and methods used to collect patient‐level data in this analysis have been previously published.1 The study was performed at Moffitt‐Long Hospital and San Francisco General Hospital, teaching hospitals affiliated with the University of California San Francisco School of Medicine. At both sites, single‐detector CT scans were available 24 hours a day throughout the study period and were read by attending radiologists who specialized in thoracic imaging. We excluded patients whose CTPA was not completed as the initial test in the evaluation of suspected PE, those who underwent testing for any indication other than suspected acute PE, and those with incomplete medical records or technically inadequate CTPA.
We randomly selected 345 patients who underwent CTPA between January 1, 1998, and December 31, 2000, from the Radiology Department databases. One investigator (R.L.T.) then abstracted charts of all patients. For each subject, we collected data about history and clinical presentation, diagnostic impressions of the treating clinicians, treatments administered both before and after diagnostic testing, CTPA result, results of other diagnostic tests for PE, and final clinical diagnosis. During the study period, there were no institution‐ or department‐specific guidelines or decision aids available for the diagnosis of PE. Ventilation‐perfusion scan, lower extremity ultrasound, and pulmonary angiography were available, but highly sensitive D‐dimer assays were not in use. The study was approved by the Institutional Review Boards of both sites, and requirement for written informed consent from patients was waived.
Estimates of Pretest Probabilities of Pulmonary Embolism and CTPA Test Characteristics
Several prediction rules1820 generate clinical pretest probabilities for patients with suspected PE. We used the Wells score18 to assign a pretest probability of low, moderate, or high to each patient on the basis of the following clinical variables: leg swelling, hemoptysis, tachycardia, history of recent immobilization, history of prior DVT or PE, active malignancy, and lack of a more likely alternative diagnosis. We chose this rule as (unlike other prediction rules such as the Geneva rule20) the Wells score has been validated for hospitalized patients with suspected PE and does not require arterial blood gas measurements. The prevalence of PE reported in the evaluation of the Wells score was 3.4%, 27.8%, and 78.3% for low, moderate, and high pretest probabilities, respectively.18
As in our previous study,1 we assumed CTPA to be 90% sensitive and 95% specific based on published estimates.3, 17 These values correspond to a positive likelihood ratio of 18 and a negative likelihood ratio of 0.1.21 We chose these values as a best‐case estimate of the test characteristics of CTPA, although other studies have found less impressive results.7 Using these pretest probabilities and likelihood ratios, we then used Bayes' theorem (Figure 1) to calculate the range of expected posttest probabilities of pulmonary embolism.
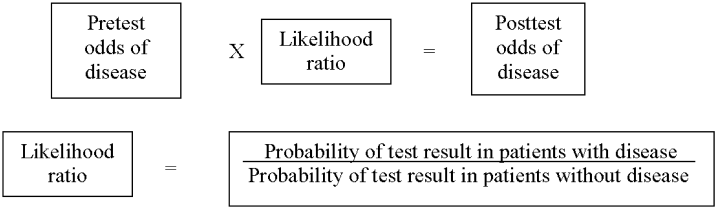
Calculation of Posttest Probabilities and Comparison to Treatment Outcomes
For each pretest probability category, we used the posttest probabilities calculated above to determine the number of true‐positive pulmonary emboli, as follows:
RESULTS
Patient Characteristics
After excluding 23 patients receiving anticoagulants for other indications prior to CTPA, the study cohort included 322 patients (57.7% female), with an average age of 58.6 years, of whom 20.5% had cancer and 4.5% had a prior history of thromboembolic disease. Scans were primarily ordered by the medicine service (47.7% of cases) and emergency department (22.9%). CTPA was the initial test for 9% of patients evaluated for suspected acute PE during the first 6 months of the study period, increasing to 83% by the end of 2000.1 The overall pretest probability distribution remained the same throughout the entire study period.1
Test Results and Treatment Decisions
Most patients in our cohort had a low (n = 184, 57.1%) or a moderate (n = 101, 31.4%) pretest probability of PE (Table 1). The likelihood of a positive CTPA increased as the pretest probability increased, but even among patients with high clinical risk, only 35.1% had positive CT scans. In total, scans were positive in 57 patients and negative in 265 patients. Clinicians treated 55 patients with a positive CTPA (96.5%); none of these patients underwent additional testing for DVT or PE after the imaging study. Among patients with a negative CTPA, 254 (95.8%) were not treated; none of the patients in whom anticoagulation was withheld underwent further testing, whereas the other 11 patients were treated on the basis of other tests (5 high‐probability ventilation‐perfusion scans, 3 positive leg ultrasounds, and 3 for unclear reasons). Overall, 66 patients (20.5%) were treated for pulmonary embolism.
| Pretest probability of PE (number of CTPA performed) | Low (N = 184) | Moderate (N = 101) | High (N = 37) | Total (N = 322) |
|---|---|---|---|---|
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| CTPA negative for PE (% of pretest probability group) | 162 (88.0%) | 79 (78.2%) | 24 (64.9%) | 265 (82.3%) |
| Patients with positive CT subsequently treated for PE (% of pretest probability group) | 21 (11.4%) | 21 (20.8%) | 13 (35.1%) | 55 (17.1%) |
| Patients treated for PE despite negative CT (% of pretest probability group) | 5 (2.7%) | 3 (3.0%) | 3 (8.1%) | 11 (3.4%) |
| Total patients treated for PE (% of pretest probability group) | 26 (14.1%) | 24 (23.8%) | 16 (43.2%) | 66 (20.5%) |
Literature‐Derived Estimates of Posttest Probabilities of Pulmonary Embolism
Patients who have a low pretest probability of PE and a positive CTPA have a posttest probability of 41.6% under our estimate of CTPA test characteristics. Patients with moderate pretest probability have a posttest probability of 87.4% and patients with a high pretest probability will have a 98.5% probability of embolism with a positive scan. The traditional treatment threshold for PE is a posttest probability of 90%.22
Observed Versus Expected PE Rates and Subsequent Treatment
Only 9 of the 22 patients (41%) with a low pretest probability and a positive CTPA likely represent true‐positive emboli. However, clinicians chose to treat 21 of the 22 patients with this combination of pretest probability and imaging findings. Thus, 12 emboli would be considered possible false‐positive diagnoses. Similarly, in the moderate pretest probability group, 2 of 21 patients with moderate pretest probability and 0 of 13 patients with high pretest probability treated for PE had a possibly false‐positive diagnosis. Thus, in total, 25.4% (14 of 55) patients treated for PE had a possible false‐positive diagnosis of pulmonary embolism and may have been unnecessarily administered anticoagulants (Table 2). All patients who potentially had a false‐positive PE had either a low or moderate pretest probability of PE; in fact, the majority (57.1%) of patients with a low pretest probability of PE who were subsequently treated for PE likely had a false‐positive diagnosis.
| Pretest probability | ||||
|---|---|---|---|---|
| Low (n = 184) | Moderate (n = 101) | High (n = 37) | Total (n = 322) | |
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| Patients with positive CTPA treated for pulmonary embolism (n, % treated in risk group) | 21 (95.4%) | 21 (95.4%) | 13 (100%) | 55 (96.5%) |
| Calculated number and rate of probable true‐positive evaluations | ||||
| Number of true‐positive PE (n, % treated in risk group) | 9 (42.9%) | 19 (90.5%) | 13 (100%) | 41 (74.6%) |
| Calculated number and rate of possible false‐positive evaluations | ||||
| Number of possible false‐positive PE (n, % in risk group with unexpected PE) | 12 (58.1%) | 2 (9.5%) | 0 | 14 (25.4%) |
Clinicians were more likely to overtreat a patient with a possible false‐positive CT scan than to withhold treatment from a patient with a possible false‐negative diagnosis. Using the same estimates of CTPA test characteristics, the incidence of possible false‐negative diagnosis of PE was 1.6% (4 possible false‐negative diagnoses among 254 patients with negative CTPA results who were not treated for PE.) All these patients had a high pretest probability of PE.
DISCUSSION
Physicians at our institution regarded CTPA results as definitive, anticoagulating 96.5% of patients with a positive CT and withholding treatment in 95.8% of patients with a negative scan. This practice pattern may result in unnecessary anticoagulation of many patients with a low pretest probability of PE who may have had false‐positive CTPA findings. In contrast, the rate of possible false‐negative diagnosis of PE was low, consistent with the results of several other studies.16
The use of CTPA is likely to increase because of the publication of multiple algorithms advocating that CTPA be the chief imaging study used in the diagnosis of PE.1014 These algorithms recommend serial testing on patients with a negative CTPA in order to minimize the false‐negative rate, but they do not require systematic follow‐up in patients with a positive scan, even if the pretest probability was low. In management trials, this approach resulted in a low false‐negative rate (1.0%‐1.8% at 3‐month follow‐up).1114 However, the rate of major bleeding in patients treated for PE was 3.2%‐6.0% at 3 months,1214 illustrating the potential risk of anticoagulating patients who may have false‐positive diagnoses. Furthermore, premature diagnostic closure after a CTPA positive for PE may result in additional morbidity as a result of missing the true diagnosis.
One potential explanation for the large number of potential false‐positive emboli seen in low‐risk patients is that it is difficult to accurately diagnose distal pulmonary emboli with CTPA. The interrater reliability of CTPA for diagnosis of subsegmental PE is suboptimal,23 and the clinical significance of these emboli remains uncertain.24 Thus, many emboli found in patients with low pretest probability actually may have been subsegmental PE that would not have been diagnosed by another radiologist. As CTPA is more accurate for diagnosing central PE,25 clinicians should consider reviewing positive scans with the interpreting radiologist, especially when the pretest probability was low and the filling defects identified are in distal vessels.
Our results may also illustrate that clinicians have a lower treatment threshold when presented with apparently definitive evidence of pulmonary embolism. Previous proposals on the appropriate treatment threshold for PE, which used Bayesian decision‐making methods similar to ours,22 incorporated PIOPED26 data on the pretest probability of pulmonary embolism, the test characteristics of ventilation‐perfusion scans, and the clinical outcomes of patients in each test result/pretest probability category. However, there is no corresponding data for CTPA, as its test characteristics are still uncertain, and long‐term clinical outcomes have not been documented for patients treated (or not treated) on the basis of CT results.
Our study had several limitations. First, charting bias potentially was introduced by our using a retrospective method of collecting data for calculating pretest probabilities. To address this potential bias, we collected data from the entire medical record, including information available at and preceding the time of the CT scan. We believe this method was effective, as the range of pretest probabilities and the prevalence of PE in our study were very similar to those seen in a number of prospective studies.1820, 26, 27 Although other risk indices exist, the Wells score has been shown to have predictive powers equal to other algorithms and to clinicians; implicit assessments.28, 29 In our cohort, 35.1% of patients with a high pretest probability were diagnosed with PE; although this was lower than that in the initial Wells cohort,18 it was very similar to a subsequent validation study using the Wells algorithm, in which the prevalence of PE in patients with high pretest probability was 37.5%.27 Plasma D‐dimer testing is not routinely used at our hospitals, but it is a component of some CTPA‐based diagnostic algorithms.1114 Although use of D‐dimer testing may have led to fewer scans in patients with negative D‐dimer test results and low pretest probability,30 the high false‐positive rate for D‐dimer assays31 makes it difficult to predict the effect of widespread D‐dimer use on the overall pretest probability distribution. Using our assumptions about CT test characteristics, a pretest probability of more than 30% is required to generate a posttest probability of PE of at least 90% (the traditional treatment threshold for anticoagulant therapy22) with a positive scan. Extensive D‐dimer use would be unlikely to cause such a shift in the distribution of pretest probabilities.
Finally, CT technology has continued to advance, and many institutions now use 64‐slice scanners32 in contrast to the single‐slice scanners in use at the time our data were collected. Our assumptions were that CTPA has a positive likelihood ratio of 18.0 and a negative likelihood ratio of 0.1 (corresponding to a sensitivity of 90% and a specificity of 95%), although many studies of single‐detector CTPA found less impressive values.5, 7 Multidetector CT is thought to be more accurate than was earlier technology, but the true diagnostic performance of multidetector CT is not yet known. However, our findings pertain primarily to clinicians' responses to test results, so even if newer scanners are more accurate, Bayesian analysis will still be required in order to appropriately treat patients. A recent meta‐analysis of diagnostic strategies for PE found CTPA to have a positive likelihood ratio of 24.1, but even using this higher value, patients with a low pretest probability and positive CTPA still have a posttest probability of PE below the traditional treatment threshold.33 As most patients undergoing evaluation for suspected PE have a low pretest probability,17 a substantial number of false‐positive diagnoses of PE may still occur, even with a more accurate diagnostic test.
CT pulmonary angiography has become the first‐line test for pulmonary embolism at our institution, a situation likely mirrored elsewhere. CTPA is safe and rapid and offers the advantage of revealing ancillary lung findings that may be clinically significant.12 Although the test is an important addition to a clinician's diagnostic armamentarium, Bayesian analysis must be used to interpret its results, especially when CTPA is used as the first‐line diagnostic test. Our data raise the troubling concern that reliance on CTPA as the sole diagnostic test for suspected pulmonary embolism may result in a large number of patients with false‐positive CT scans receiving anticoagulation treatment.
- ,,,,.The impact of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- ,,.Trends in the use of diagnostic imaging in patients hospitalized with acute pulmonary embolism.Am J Cardiol.2004;93:1316–1317.
- ,,,.Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single‐breath‐old technique—comparison with pulmonary angiography.Radiology.1992;185:381–387.
- ,,, et al.Pulmonary embolism: validation of spiral CT angiography in 149 patients.Radiology.1996;201:467–470.
- ,,,,.Lung scintigraphy and helical computed tomography for the diagnosis of pulmonary embolism: a meta‐analysis.Clin Appl Thromb Hemost.2001;7(2):87–92.
- ,,,.The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism.Arch Intern Med.2000;160(3):293–298.
- ,,.Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review.Ann Intern Med.2000;132(3):227–232.
- ,,, et al.Suspected acute pulmonary embolism: evaluation with multi‐detector row CT versus digital subtraction pulmonary arteriography.Radiology.2004;233:806–815.
- ,,,.Overview of Prospective Investigation of Pulmonary Embolism Diagnosis II.Semin Nucl Med.2002;32(3):173–182.
- ,,, et al.Management of suspected pulmonary embolism (PE) by D‐dimer and multi‐slice computed tomography in outpatients: an outcome study.J Thromb Haemost.2005;3:1926–1932.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,, et al.Single‐detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: a multicenter clinical management study of 510 patients.Ann Intern Med.2003;138:307–314.
- ,,, et al.Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study.Lancet.2002;260:1914–1920.
- ,,, et al.Diagnosing pulmonary embolism in outpatients with clinical assessment, D‐dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study.Am J Med.2004;116:291–299.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review.JAMA.2005;293:2012–2017.
- ,,,.Meta‐analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,.Clinical Practice: The evaluation of suspected pulmonary embolism.N Engl J Med.2003;349:1247–1256.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,.A structured clinical model for predicting the probability of pulmonary embolism.Am J Med.2003;114(3):173–179.
- ,,,,.Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161(1):92–97.
- ,,,.Interpretation of diagnostic tests and strategies for their use in quantitative decision making. In:Diagnostic strategies for common medical problems.Philadelphia, PA:American College of Physicians,1999.
- ,,,.Strategy for diagnosis of patients with suspected acute pulmonary embolism.Chest.1993;103:1553–1559.
- ,,, et al.Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement.Eur Radiol.2003;13:823–829.
- ,.Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy.Chest.1995;108:978–981.
- ,,, et al.Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism.Ann Intern Med.2001;135(2):88–97.
- Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED).The PIOPED Investigators.JAMA.1990;263:2753–2759.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d‐dimer.Ann Intern Med.2001;135(2):98–107.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,, et al.Does this patient have pulmonary embolism?JAMA.2003;290:2849–2858.
- ,,,,.Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review.Ann Intern Med.2003;138:941–951.
- ,,, et al.D‐dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review.Ann Intern Med.2004;140:589–602.
- .Multislice computed tomography for pulmonary embolism—a technological marvel.N Engl J Med2005;352(17):1812–4.
- ,,,,,.Systematic review and meta‐analysis of strategies for the diagnosis of suspected pulmonary embolism.Br Med J.2005;331:259.
Spiral computed tomographic pulmonary angiography (CTPA) is a common first‐line test for the evaluation of suspected pulmonary embolism (PE). At our institution CTPA became the initial diagnostic study in 83% of patients with suspected PE within 3 years of the introduction of CT,1 and by 2001 CTPA had become the most common diagnostic test performed nationwide in patients diagnosed with PE.2 Most scans are interpreted as either positive or negative for pulmonary embolism, providing clinicians with a greater sense of diagnostic certainty than with the probabilistic results of lung scintigraphy. Initial studies of CTPA supported this appearance of diagnostic certainty, reporting sensitivity and specificity of greater than 90%,3, 4 but several subsequent studies have failed to reproduce these results.57 Newer multidetector CT scans are believed to be more accurate than earlier single‐detector CT,8 but true estimates of CTPA test characteristics will not be known until publication of the forthcoming PIOPED II study.9
Even without these data, CT‐based diagnostic algorithms have already appeared.1014 These algorithms generally focus on minimizing the false‐negative rate through use of serial testing (involving combinations of serum D‐dimer, lower‐extremity ultrasound, and CTPA). A recent meta‐analysis demonstrated that negative CTPA is highly accurate at ruling out PE, with test characteristics similar to conventional pulmonary angiography.15 Another meta‐analysis found that the 3‐month rate of subsequent venous thromboembolism after negative CTPA was 1.4% (95% CI 1.1%‐1.8%),16 supporting the strategy of withholding anticoagulants after negative CTPA in combination with other tests. However, use of serial testing to establish the diagnosis of PE and initiate anticoagulation has not been systematically evaluated or recommended, even for patients with a low pretest probability of PE.17
To assess the potential impact of these algorithms on the diagnosis of PE in clinical practice, we analyzed the clinical presentation and treatment of a cohort of patients at our institution who underwent CTPA for suspected PE.1 We calculated a range of posttest probabilities for pulmonary embolism for these patients, given the pretest probabilities, test results, and estimates of CTPA test characteristics. We then compared the treatment decisions of clinicians to the posttest probabilities of PE in order to establish the potential frequency of false‐positive and false‐negative diagnoses and to determine if patients were treated appropriately based on these estimates.
METHODS
Sites and Subjects
Details of the sites, subjects, and methods used to collect patient‐level data in this analysis have been previously published.1 The study was performed at Moffitt‐Long Hospital and San Francisco General Hospital, teaching hospitals affiliated with the University of California San Francisco School of Medicine. At both sites, single‐detector CT scans were available 24 hours a day throughout the study period and were read by attending radiologists who specialized in thoracic imaging. We excluded patients whose CTPA was not completed as the initial test in the evaluation of suspected PE, those who underwent testing for any indication other than suspected acute PE, and those with incomplete medical records or technically inadequate CTPA.
We randomly selected 345 patients who underwent CTPA between January 1, 1998, and December 31, 2000, from the Radiology Department databases. One investigator (R.L.T.) then abstracted charts of all patients. For each subject, we collected data about history and clinical presentation, diagnostic impressions of the treating clinicians, treatments administered both before and after diagnostic testing, CTPA result, results of other diagnostic tests for PE, and final clinical diagnosis. During the study period, there were no institution‐ or department‐specific guidelines or decision aids available for the diagnosis of PE. Ventilation‐perfusion scan, lower extremity ultrasound, and pulmonary angiography were available, but highly sensitive D‐dimer assays were not in use. The study was approved by the Institutional Review Boards of both sites, and requirement for written informed consent from patients was waived.
Estimates of Pretest Probabilities of Pulmonary Embolism and CTPA Test Characteristics
Several prediction rules1820 generate clinical pretest probabilities for patients with suspected PE. We used the Wells score18 to assign a pretest probability of low, moderate, or high to each patient on the basis of the following clinical variables: leg swelling, hemoptysis, tachycardia, history of recent immobilization, history of prior DVT or PE, active malignancy, and lack of a more likely alternative diagnosis. We chose this rule as (unlike other prediction rules such as the Geneva rule20) the Wells score has been validated for hospitalized patients with suspected PE and does not require arterial blood gas measurements. The prevalence of PE reported in the evaluation of the Wells score was 3.4%, 27.8%, and 78.3% for low, moderate, and high pretest probabilities, respectively.18
As in our previous study,1 we assumed CTPA to be 90% sensitive and 95% specific based on published estimates.3, 17 These values correspond to a positive likelihood ratio of 18 and a negative likelihood ratio of 0.1.21 We chose these values as a best‐case estimate of the test characteristics of CTPA, although other studies have found less impressive results.7 Using these pretest probabilities and likelihood ratios, we then used Bayes' theorem (Figure 1) to calculate the range of expected posttest probabilities of pulmonary embolism.

Calculation of Posttest Probabilities and Comparison to Treatment Outcomes
For each pretest probability category, we used the posttest probabilities calculated above to determine the number of true‐positive pulmonary emboli, as follows:
RESULTS
Patient Characteristics
After excluding 23 patients receiving anticoagulants for other indications prior to CTPA, the study cohort included 322 patients (57.7% female), with an average age of 58.6 years, of whom 20.5% had cancer and 4.5% had a prior history of thromboembolic disease. Scans were primarily ordered by the medicine service (47.7% of cases) and emergency department (22.9%). CTPA was the initial test for 9% of patients evaluated for suspected acute PE during the first 6 months of the study period, increasing to 83% by the end of 2000.1 The overall pretest probability distribution remained the same throughout the entire study period.1
Test Results and Treatment Decisions
Most patients in our cohort had a low (n = 184, 57.1%) or a moderate (n = 101, 31.4%) pretest probability of PE (Table 1). The likelihood of a positive CTPA increased as the pretest probability increased, but even among patients with high clinical risk, only 35.1% had positive CT scans. In total, scans were positive in 57 patients and negative in 265 patients. Clinicians treated 55 patients with a positive CTPA (96.5%); none of these patients underwent additional testing for DVT or PE after the imaging study. Among patients with a negative CTPA, 254 (95.8%) were not treated; none of the patients in whom anticoagulation was withheld underwent further testing, whereas the other 11 patients were treated on the basis of other tests (5 high‐probability ventilation‐perfusion scans, 3 positive leg ultrasounds, and 3 for unclear reasons). Overall, 66 patients (20.5%) were treated for pulmonary embolism.
| Pretest probability of PE (number of CTPA performed) | Low (N = 184) | Moderate (N = 101) | High (N = 37) | Total (N = 322) |
|---|---|---|---|---|
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| CTPA negative for PE (% of pretest probability group) | 162 (88.0%) | 79 (78.2%) | 24 (64.9%) | 265 (82.3%) |
| Patients with positive CT subsequently treated for PE (% of pretest probability group) | 21 (11.4%) | 21 (20.8%) | 13 (35.1%) | 55 (17.1%) |
| Patients treated for PE despite negative CT (% of pretest probability group) | 5 (2.7%) | 3 (3.0%) | 3 (8.1%) | 11 (3.4%) |
| Total patients treated for PE (% of pretest probability group) | 26 (14.1%) | 24 (23.8%) | 16 (43.2%) | 66 (20.5%) |
Literature‐Derived Estimates of Posttest Probabilities of Pulmonary Embolism
Patients who have a low pretest probability of PE and a positive CTPA have a posttest probability of 41.6% under our estimate of CTPA test characteristics. Patients with moderate pretest probability have a posttest probability of 87.4% and patients with a high pretest probability will have a 98.5% probability of embolism with a positive scan. The traditional treatment threshold for PE is a posttest probability of 90%.22
Observed Versus Expected PE Rates and Subsequent Treatment
Only 9 of the 22 patients (41%) with a low pretest probability and a positive CTPA likely represent true‐positive emboli. However, clinicians chose to treat 21 of the 22 patients with this combination of pretest probability and imaging findings. Thus, 12 emboli would be considered possible false‐positive diagnoses. Similarly, in the moderate pretest probability group, 2 of 21 patients with moderate pretest probability and 0 of 13 patients with high pretest probability treated for PE had a possibly false‐positive diagnosis. Thus, in total, 25.4% (14 of 55) patients treated for PE had a possible false‐positive diagnosis of pulmonary embolism and may have been unnecessarily administered anticoagulants (Table 2). All patients who potentially had a false‐positive PE had either a low or moderate pretest probability of PE; in fact, the majority (57.1%) of patients with a low pretest probability of PE who were subsequently treated for PE likely had a false‐positive diagnosis.
| Pretest probability | ||||
|---|---|---|---|---|
| Low (n = 184) | Moderate (n = 101) | High (n = 37) | Total (n = 322) | |
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| Patients with positive CTPA treated for pulmonary embolism (n, % treated in risk group) | 21 (95.4%) | 21 (95.4%) | 13 (100%) | 55 (96.5%) |
| Calculated number and rate of probable true‐positive evaluations | ||||
| Number of true‐positive PE (n, % treated in risk group) | 9 (42.9%) | 19 (90.5%) | 13 (100%) | 41 (74.6%) |
| Calculated number and rate of possible false‐positive evaluations | ||||
| Number of possible false‐positive PE (n, % in risk group with unexpected PE) | 12 (58.1%) | 2 (9.5%) | 0 | 14 (25.4%) |
Clinicians were more likely to overtreat a patient with a possible false‐positive CT scan than to withhold treatment from a patient with a possible false‐negative diagnosis. Using the same estimates of CTPA test characteristics, the incidence of possible false‐negative diagnosis of PE was 1.6% (4 possible false‐negative diagnoses among 254 patients with negative CTPA results who were not treated for PE.) All these patients had a high pretest probability of PE.
DISCUSSION
Physicians at our institution regarded CTPA results as definitive, anticoagulating 96.5% of patients with a positive CT and withholding treatment in 95.8% of patients with a negative scan. This practice pattern may result in unnecessary anticoagulation of many patients with a low pretest probability of PE who may have had false‐positive CTPA findings. In contrast, the rate of possible false‐negative diagnosis of PE was low, consistent with the results of several other studies.16
The use of CTPA is likely to increase because of the publication of multiple algorithms advocating that CTPA be the chief imaging study used in the diagnosis of PE.1014 These algorithms recommend serial testing on patients with a negative CTPA in order to minimize the false‐negative rate, but they do not require systematic follow‐up in patients with a positive scan, even if the pretest probability was low. In management trials, this approach resulted in a low false‐negative rate (1.0%‐1.8% at 3‐month follow‐up).1114 However, the rate of major bleeding in patients treated for PE was 3.2%‐6.0% at 3 months,1214 illustrating the potential risk of anticoagulating patients who may have false‐positive diagnoses. Furthermore, premature diagnostic closure after a CTPA positive for PE may result in additional morbidity as a result of missing the true diagnosis.
One potential explanation for the large number of potential false‐positive emboli seen in low‐risk patients is that it is difficult to accurately diagnose distal pulmonary emboli with CTPA. The interrater reliability of CTPA for diagnosis of subsegmental PE is suboptimal,23 and the clinical significance of these emboli remains uncertain.24 Thus, many emboli found in patients with low pretest probability actually may have been subsegmental PE that would not have been diagnosed by another radiologist. As CTPA is more accurate for diagnosing central PE,25 clinicians should consider reviewing positive scans with the interpreting radiologist, especially when the pretest probability was low and the filling defects identified are in distal vessels.
Our results may also illustrate that clinicians have a lower treatment threshold when presented with apparently definitive evidence of pulmonary embolism. Previous proposals on the appropriate treatment threshold for PE, which used Bayesian decision‐making methods similar to ours,22 incorporated PIOPED26 data on the pretest probability of pulmonary embolism, the test characteristics of ventilation‐perfusion scans, and the clinical outcomes of patients in each test result/pretest probability category. However, there is no corresponding data for CTPA, as its test characteristics are still uncertain, and long‐term clinical outcomes have not been documented for patients treated (or not treated) on the basis of CT results.
Our study had several limitations. First, charting bias potentially was introduced by our using a retrospective method of collecting data for calculating pretest probabilities. To address this potential bias, we collected data from the entire medical record, including information available at and preceding the time of the CT scan. We believe this method was effective, as the range of pretest probabilities and the prevalence of PE in our study were very similar to those seen in a number of prospective studies.1820, 26, 27 Although other risk indices exist, the Wells score has been shown to have predictive powers equal to other algorithms and to clinicians; implicit assessments.28, 29 In our cohort, 35.1% of patients with a high pretest probability were diagnosed with PE; although this was lower than that in the initial Wells cohort,18 it was very similar to a subsequent validation study using the Wells algorithm, in which the prevalence of PE in patients with high pretest probability was 37.5%.27 Plasma D‐dimer testing is not routinely used at our hospitals, but it is a component of some CTPA‐based diagnostic algorithms.1114 Although use of D‐dimer testing may have led to fewer scans in patients with negative D‐dimer test results and low pretest probability,30 the high false‐positive rate for D‐dimer assays31 makes it difficult to predict the effect of widespread D‐dimer use on the overall pretest probability distribution. Using our assumptions about CT test characteristics, a pretest probability of more than 30% is required to generate a posttest probability of PE of at least 90% (the traditional treatment threshold for anticoagulant therapy22) with a positive scan. Extensive D‐dimer use would be unlikely to cause such a shift in the distribution of pretest probabilities.
Finally, CT technology has continued to advance, and many institutions now use 64‐slice scanners32 in contrast to the single‐slice scanners in use at the time our data were collected. Our assumptions were that CTPA has a positive likelihood ratio of 18.0 and a negative likelihood ratio of 0.1 (corresponding to a sensitivity of 90% and a specificity of 95%), although many studies of single‐detector CTPA found less impressive values.5, 7 Multidetector CT is thought to be more accurate than was earlier technology, but the true diagnostic performance of multidetector CT is not yet known. However, our findings pertain primarily to clinicians' responses to test results, so even if newer scanners are more accurate, Bayesian analysis will still be required in order to appropriately treat patients. A recent meta‐analysis of diagnostic strategies for PE found CTPA to have a positive likelihood ratio of 24.1, but even using this higher value, patients with a low pretest probability and positive CTPA still have a posttest probability of PE below the traditional treatment threshold.33 As most patients undergoing evaluation for suspected PE have a low pretest probability,17 a substantial number of false‐positive diagnoses of PE may still occur, even with a more accurate diagnostic test.
CT pulmonary angiography has become the first‐line test for pulmonary embolism at our institution, a situation likely mirrored elsewhere. CTPA is safe and rapid and offers the advantage of revealing ancillary lung findings that may be clinically significant.12 Although the test is an important addition to a clinician's diagnostic armamentarium, Bayesian analysis must be used to interpret its results, especially when CTPA is used as the first‐line diagnostic test. Our data raise the troubling concern that reliance on CTPA as the sole diagnostic test for suspected pulmonary embolism may result in a large number of patients with false‐positive CT scans receiving anticoagulation treatment.
Spiral computed tomographic pulmonary angiography (CTPA) is a common first‐line test for the evaluation of suspected pulmonary embolism (PE). At our institution CTPA became the initial diagnostic study in 83% of patients with suspected PE within 3 years of the introduction of CT,1 and by 2001 CTPA had become the most common diagnostic test performed nationwide in patients diagnosed with PE.2 Most scans are interpreted as either positive or negative for pulmonary embolism, providing clinicians with a greater sense of diagnostic certainty than with the probabilistic results of lung scintigraphy. Initial studies of CTPA supported this appearance of diagnostic certainty, reporting sensitivity and specificity of greater than 90%,3, 4 but several subsequent studies have failed to reproduce these results.57 Newer multidetector CT scans are believed to be more accurate than earlier single‐detector CT,8 but true estimates of CTPA test characteristics will not be known until publication of the forthcoming PIOPED II study.9
Even without these data, CT‐based diagnostic algorithms have already appeared.1014 These algorithms generally focus on minimizing the false‐negative rate through use of serial testing (involving combinations of serum D‐dimer, lower‐extremity ultrasound, and CTPA). A recent meta‐analysis demonstrated that negative CTPA is highly accurate at ruling out PE, with test characteristics similar to conventional pulmonary angiography.15 Another meta‐analysis found that the 3‐month rate of subsequent venous thromboembolism after negative CTPA was 1.4% (95% CI 1.1%‐1.8%),16 supporting the strategy of withholding anticoagulants after negative CTPA in combination with other tests. However, use of serial testing to establish the diagnosis of PE and initiate anticoagulation has not been systematically evaluated or recommended, even for patients with a low pretest probability of PE.17
To assess the potential impact of these algorithms on the diagnosis of PE in clinical practice, we analyzed the clinical presentation and treatment of a cohort of patients at our institution who underwent CTPA for suspected PE.1 We calculated a range of posttest probabilities for pulmonary embolism for these patients, given the pretest probabilities, test results, and estimates of CTPA test characteristics. We then compared the treatment decisions of clinicians to the posttest probabilities of PE in order to establish the potential frequency of false‐positive and false‐negative diagnoses and to determine if patients were treated appropriately based on these estimates.
METHODS
Sites and Subjects
Details of the sites, subjects, and methods used to collect patient‐level data in this analysis have been previously published.1 The study was performed at Moffitt‐Long Hospital and San Francisco General Hospital, teaching hospitals affiliated with the University of California San Francisco School of Medicine. At both sites, single‐detector CT scans were available 24 hours a day throughout the study period and were read by attending radiologists who specialized in thoracic imaging. We excluded patients whose CTPA was not completed as the initial test in the evaluation of suspected PE, those who underwent testing for any indication other than suspected acute PE, and those with incomplete medical records or technically inadequate CTPA.
We randomly selected 345 patients who underwent CTPA between January 1, 1998, and December 31, 2000, from the Radiology Department databases. One investigator (R.L.T.) then abstracted charts of all patients. For each subject, we collected data about history and clinical presentation, diagnostic impressions of the treating clinicians, treatments administered both before and after diagnostic testing, CTPA result, results of other diagnostic tests for PE, and final clinical diagnosis. During the study period, there were no institution‐ or department‐specific guidelines or decision aids available for the diagnosis of PE. Ventilation‐perfusion scan, lower extremity ultrasound, and pulmonary angiography were available, but highly sensitive D‐dimer assays were not in use. The study was approved by the Institutional Review Boards of both sites, and requirement for written informed consent from patients was waived.
Estimates of Pretest Probabilities of Pulmonary Embolism and CTPA Test Characteristics
Several prediction rules1820 generate clinical pretest probabilities for patients with suspected PE. We used the Wells score18 to assign a pretest probability of low, moderate, or high to each patient on the basis of the following clinical variables: leg swelling, hemoptysis, tachycardia, history of recent immobilization, history of prior DVT or PE, active malignancy, and lack of a more likely alternative diagnosis. We chose this rule as (unlike other prediction rules such as the Geneva rule20) the Wells score has been validated for hospitalized patients with suspected PE and does not require arterial blood gas measurements. The prevalence of PE reported in the evaluation of the Wells score was 3.4%, 27.8%, and 78.3% for low, moderate, and high pretest probabilities, respectively.18
As in our previous study,1 we assumed CTPA to be 90% sensitive and 95% specific based on published estimates.3, 17 These values correspond to a positive likelihood ratio of 18 and a negative likelihood ratio of 0.1.21 We chose these values as a best‐case estimate of the test characteristics of CTPA, although other studies have found less impressive results.7 Using these pretest probabilities and likelihood ratios, we then used Bayes' theorem (Figure 1) to calculate the range of expected posttest probabilities of pulmonary embolism.

Calculation of Posttest Probabilities and Comparison to Treatment Outcomes
For each pretest probability category, we used the posttest probabilities calculated above to determine the number of true‐positive pulmonary emboli, as follows:
RESULTS
Patient Characteristics
After excluding 23 patients receiving anticoagulants for other indications prior to CTPA, the study cohort included 322 patients (57.7% female), with an average age of 58.6 years, of whom 20.5% had cancer and 4.5% had a prior history of thromboembolic disease. Scans were primarily ordered by the medicine service (47.7% of cases) and emergency department (22.9%). CTPA was the initial test for 9% of patients evaluated for suspected acute PE during the first 6 months of the study period, increasing to 83% by the end of 2000.1 The overall pretest probability distribution remained the same throughout the entire study period.1
Test Results and Treatment Decisions
Most patients in our cohort had a low (n = 184, 57.1%) or a moderate (n = 101, 31.4%) pretest probability of PE (Table 1). The likelihood of a positive CTPA increased as the pretest probability increased, but even among patients with high clinical risk, only 35.1% had positive CT scans. In total, scans were positive in 57 patients and negative in 265 patients. Clinicians treated 55 patients with a positive CTPA (96.5%); none of these patients underwent additional testing for DVT or PE after the imaging study. Among patients with a negative CTPA, 254 (95.8%) were not treated; none of the patients in whom anticoagulation was withheld underwent further testing, whereas the other 11 patients were treated on the basis of other tests (5 high‐probability ventilation‐perfusion scans, 3 positive leg ultrasounds, and 3 for unclear reasons). Overall, 66 patients (20.5%) were treated for pulmonary embolism.
| Pretest probability of PE (number of CTPA performed) | Low (N = 184) | Moderate (N = 101) | High (N = 37) | Total (N = 322) |
|---|---|---|---|---|
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| CTPA negative for PE (% of pretest probability group) | 162 (88.0%) | 79 (78.2%) | 24 (64.9%) | 265 (82.3%) |
| Patients with positive CT subsequently treated for PE (% of pretest probability group) | 21 (11.4%) | 21 (20.8%) | 13 (35.1%) | 55 (17.1%) |
| Patients treated for PE despite negative CT (% of pretest probability group) | 5 (2.7%) | 3 (3.0%) | 3 (8.1%) | 11 (3.4%) |
| Total patients treated for PE (% of pretest probability group) | 26 (14.1%) | 24 (23.8%) | 16 (43.2%) | 66 (20.5%) |
Literature‐Derived Estimates of Posttest Probabilities of Pulmonary Embolism
Patients who have a low pretest probability of PE and a positive CTPA have a posttest probability of 41.6% under our estimate of CTPA test characteristics. Patients with moderate pretest probability have a posttest probability of 87.4% and patients with a high pretest probability will have a 98.5% probability of embolism with a positive scan. The traditional treatment threshold for PE is a posttest probability of 90%.22
Observed Versus Expected PE Rates and Subsequent Treatment
Only 9 of the 22 patients (41%) with a low pretest probability and a positive CTPA likely represent true‐positive emboli. However, clinicians chose to treat 21 of the 22 patients with this combination of pretest probability and imaging findings. Thus, 12 emboli would be considered possible false‐positive diagnoses. Similarly, in the moderate pretest probability group, 2 of 21 patients with moderate pretest probability and 0 of 13 patients with high pretest probability treated for PE had a possibly false‐positive diagnosis. Thus, in total, 25.4% (14 of 55) patients treated for PE had a possible false‐positive diagnosis of pulmonary embolism and may have been unnecessarily administered anticoagulants (Table 2). All patients who potentially had a false‐positive PE had either a low or moderate pretest probability of PE; in fact, the majority (57.1%) of patients with a low pretest probability of PE who were subsequently treated for PE likely had a false‐positive diagnosis.
| Pretest probability | ||||
|---|---|---|---|---|
| Low (n = 184) | Moderate (n = 101) | High (n = 37) | Total (n = 322) | |
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| Patients with positive CTPA treated for pulmonary embolism (n, % treated in risk group) | 21 (95.4%) | 21 (95.4%) | 13 (100%) | 55 (96.5%) |
| Calculated number and rate of probable true‐positive evaluations | ||||
| Number of true‐positive PE (n, % treated in risk group) | 9 (42.9%) | 19 (90.5%) | 13 (100%) | 41 (74.6%) |
| Calculated number and rate of possible false‐positive evaluations | ||||
| Number of possible false‐positive PE (n, % in risk group with unexpected PE) | 12 (58.1%) | 2 (9.5%) | 0 | 14 (25.4%) |
Clinicians were more likely to overtreat a patient with a possible false‐positive CT scan than to withhold treatment from a patient with a possible false‐negative diagnosis. Using the same estimates of CTPA test characteristics, the incidence of possible false‐negative diagnosis of PE was 1.6% (4 possible false‐negative diagnoses among 254 patients with negative CTPA results who were not treated for PE.) All these patients had a high pretest probability of PE.
DISCUSSION
Physicians at our institution regarded CTPA results as definitive, anticoagulating 96.5% of patients with a positive CT and withholding treatment in 95.8% of patients with a negative scan. This practice pattern may result in unnecessary anticoagulation of many patients with a low pretest probability of PE who may have had false‐positive CTPA findings. In contrast, the rate of possible false‐negative diagnosis of PE was low, consistent with the results of several other studies.16
The use of CTPA is likely to increase because of the publication of multiple algorithms advocating that CTPA be the chief imaging study used in the diagnosis of PE.1014 These algorithms recommend serial testing on patients with a negative CTPA in order to minimize the false‐negative rate, but they do not require systematic follow‐up in patients with a positive scan, even if the pretest probability was low. In management trials, this approach resulted in a low false‐negative rate (1.0%‐1.8% at 3‐month follow‐up).1114 However, the rate of major bleeding in patients treated for PE was 3.2%‐6.0% at 3 months,1214 illustrating the potential risk of anticoagulating patients who may have false‐positive diagnoses. Furthermore, premature diagnostic closure after a CTPA positive for PE may result in additional morbidity as a result of missing the true diagnosis.
One potential explanation for the large number of potential false‐positive emboli seen in low‐risk patients is that it is difficult to accurately diagnose distal pulmonary emboli with CTPA. The interrater reliability of CTPA for diagnosis of subsegmental PE is suboptimal,23 and the clinical significance of these emboli remains uncertain.24 Thus, many emboli found in patients with low pretest probability actually may have been subsegmental PE that would not have been diagnosed by another radiologist. As CTPA is more accurate for diagnosing central PE,25 clinicians should consider reviewing positive scans with the interpreting radiologist, especially when the pretest probability was low and the filling defects identified are in distal vessels.
Our results may also illustrate that clinicians have a lower treatment threshold when presented with apparently definitive evidence of pulmonary embolism. Previous proposals on the appropriate treatment threshold for PE, which used Bayesian decision‐making methods similar to ours,22 incorporated PIOPED26 data on the pretest probability of pulmonary embolism, the test characteristics of ventilation‐perfusion scans, and the clinical outcomes of patients in each test result/pretest probability category. However, there is no corresponding data for CTPA, as its test characteristics are still uncertain, and long‐term clinical outcomes have not been documented for patients treated (or not treated) on the basis of CT results.
Our study had several limitations. First, charting bias potentially was introduced by our using a retrospective method of collecting data for calculating pretest probabilities. To address this potential bias, we collected data from the entire medical record, including information available at and preceding the time of the CT scan. We believe this method was effective, as the range of pretest probabilities and the prevalence of PE in our study were very similar to those seen in a number of prospective studies.1820, 26, 27 Although other risk indices exist, the Wells score has been shown to have predictive powers equal to other algorithms and to clinicians; implicit assessments.28, 29 In our cohort, 35.1% of patients with a high pretest probability were diagnosed with PE; although this was lower than that in the initial Wells cohort,18 it was very similar to a subsequent validation study using the Wells algorithm, in which the prevalence of PE in patients with high pretest probability was 37.5%.27 Plasma D‐dimer testing is not routinely used at our hospitals, but it is a component of some CTPA‐based diagnostic algorithms.1114 Although use of D‐dimer testing may have led to fewer scans in patients with negative D‐dimer test results and low pretest probability,30 the high false‐positive rate for D‐dimer assays31 makes it difficult to predict the effect of widespread D‐dimer use on the overall pretest probability distribution. Using our assumptions about CT test characteristics, a pretest probability of more than 30% is required to generate a posttest probability of PE of at least 90% (the traditional treatment threshold for anticoagulant therapy22) with a positive scan. Extensive D‐dimer use would be unlikely to cause such a shift in the distribution of pretest probabilities.
Finally, CT technology has continued to advance, and many institutions now use 64‐slice scanners32 in contrast to the single‐slice scanners in use at the time our data were collected. Our assumptions were that CTPA has a positive likelihood ratio of 18.0 and a negative likelihood ratio of 0.1 (corresponding to a sensitivity of 90% and a specificity of 95%), although many studies of single‐detector CTPA found less impressive values.5, 7 Multidetector CT is thought to be more accurate than was earlier technology, but the true diagnostic performance of multidetector CT is not yet known. However, our findings pertain primarily to clinicians' responses to test results, so even if newer scanners are more accurate, Bayesian analysis will still be required in order to appropriately treat patients. A recent meta‐analysis of diagnostic strategies for PE found CTPA to have a positive likelihood ratio of 24.1, but even using this higher value, patients with a low pretest probability and positive CTPA still have a posttest probability of PE below the traditional treatment threshold.33 As most patients undergoing evaluation for suspected PE have a low pretest probability,17 a substantial number of false‐positive diagnoses of PE may still occur, even with a more accurate diagnostic test.
CT pulmonary angiography has become the first‐line test for pulmonary embolism at our institution, a situation likely mirrored elsewhere. CTPA is safe and rapid and offers the advantage of revealing ancillary lung findings that may be clinically significant.12 Although the test is an important addition to a clinician's diagnostic armamentarium, Bayesian analysis must be used to interpret its results, especially when CTPA is used as the first‐line diagnostic test. Our data raise the troubling concern that reliance on CTPA as the sole diagnostic test for suspected pulmonary embolism may result in a large number of patients with false‐positive CT scans receiving anticoagulation treatment.
- ,,,,.The impact of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- ,,.Trends in the use of diagnostic imaging in patients hospitalized with acute pulmonary embolism.Am J Cardiol.2004;93:1316–1317.
- ,,,.Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single‐breath‐old technique—comparison with pulmonary angiography.Radiology.1992;185:381–387.
- ,,, et al.Pulmonary embolism: validation of spiral CT angiography in 149 patients.Radiology.1996;201:467–470.
- ,,,,.Lung scintigraphy and helical computed tomography for the diagnosis of pulmonary embolism: a meta‐analysis.Clin Appl Thromb Hemost.2001;7(2):87–92.
- ,,,.The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism.Arch Intern Med.2000;160(3):293–298.
- ,,.Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review.Ann Intern Med.2000;132(3):227–232.
- ,,, et al.Suspected acute pulmonary embolism: evaluation with multi‐detector row CT versus digital subtraction pulmonary arteriography.Radiology.2004;233:806–815.
- ,,,.Overview of Prospective Investigation of Pulmonary Embolism Diagnosis II.Semin Nucl Med.2002;32(3):173–182.
- ,,, et al.Management of suspected pulmonary embolism (PE) by D‐dimer and multi‐slice computed tomography in outpatients: an outcome study.J Thromb Haemost.2005;3:1926–1932.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,, et al.Single‐detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: a multicenter clinical management study of 510 patients.Ann Intern Med.2003;138:307–314.
- ,,, et al.Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study.Lancet.2002;260:1914–1920.
- ,,, et al.Diagnosing pulmonary embolism in outpatients with clinical assessment, D‐dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study.Am J Med.2004;116:291–299.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review.JAMA.2005;293:2012–2017.
- ,,,.Meta‐analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,.Clinical Practice: The evaluation of suspected pulmonary embolism.N Engl J Med.2003;349:1247–1256.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,.A structured clinical model for predicting the probability of pulmonary embolism.Am J Med.2003;114(3):173–179.
- ,,,,.Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161(1):92–97.
- ,,,.Interpretation of diagnostic tests and strategies for their use in quantitative decision making. In:Diagnostic strategies for common medical problems.Philadelphia, PA:American College of Physicians,1999.
- ,,,.Strategy for diagnosis of patients with suspected acute pulmonary embolism.Chest.1993;103:1553–1559.
- ,,, et al.Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement.Eur Radiol.2003;13:823–829.
- ,.Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy.Chest.1995;108:978–981.
- ,,, et al.Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism.Ann Intern Med.2001;135(2):88–97.
- Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED).The PIOPED Investigators.JAMA.1990;263:2753–2759.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d‐dimer.Ann Intern Med.2001;135(2):98–107.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,, et al.Does this patient have pulmonary embolism?JAMA.2003;290:2849–2858.
- ,,,,.Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review.Ann Intern Med.2003;138:941–951.
- ,,, et al.D‐dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review.Ann Intern Med.2004;140:589–602.
- .Multislice computed tomography for pulmonary embolism—a technological marvel.N Engl J Med2005;352(17):1812–4.
- ,,,,,.Systematic review and meta‐analysis of strategies for the diagnosis of suspected pulmonary embolism.Br Med J.2005;331:259.
- ,,,,.The impact of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- ,,.Trends in the use of diagnostic imaging in patients hospitalized with acute pulmonary embolism.Am J Cardiol.2004;93:1316–1317.
- ,,,.Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single‐breath‐old technique—comparison with pulmonary angiography.Radiology.1992;185:381–387.
- ,,, et al.Pulmonary embolism: validation of spiral CT angiography in 149 patients.Radiology.1996;201:467–470.
- ,,,,.Lung scintigraphy and helical computed tomography for the diagnosis of pulmonary embolism: a meta‐analysis.Clin Appl Thromb Hemost.2001;7(2):87–92.
- ,,,.The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism.Arch Intern Med.2000;160(3):293–298.
- ,,.Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review.Ann Intern Med.2000;132(3):227–232.
- ,,, et al.Suspected acute pulmonary embolism: evaluation with multi‐detector row CT versus digital subtraction pulmonary arteriography.Radiology.2004;233:806–815.
- ,,,.Overview of Prospective Investigation of Pulmonary Embolism Diagnosis II.Semin Nucl Med.2002;32(3):173–182.
- ,,, et al.Management of suspected pulmonary embolism (PE) by D‐dimer and multi‐slice computed tomography in outpatients: an outcome study.J Thromb Haemost.2005;3:1926–1932.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,, et al.Single‐detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: a multicenter clinical management study of 510 patients.Ann Intern Med.2003;138:307–314.
- ,,, et al.Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study.Lancet.2002;260:1914–1920.
- ,,, et al.Diagnosing pulmonary embolism in outpatients with clinical assessment, D‐dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study.Am J Med.2004;116:291–299.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review.JAMA.2005;293:2012–2017.
- ,,,.Meta‐analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,.Clinical Practice: The evaluation of suspected pulmonary embolism.N Engl J Med.2003;349:1247–1256.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,.A structured clinical model for predicting the probability of pulmonary embolism.Am J Med.2003;114(3):173–179.
- ,,,,.Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161(1):92–97.
- ,,,.Interpretation of diagnostic tests and strategies for their use in quantitative decision making. In:Diagnostic strategies for common medical problems.Philadelphia, PA:American College of Physicians,1999.
- ,,,.Strategy for diagnosis of patients with suspected acute pulmonary embolism.Chest.1993;103:1553–1559.
- ,,, et al.Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement.Eur Radiol.2003;13:823–829.
- ,.Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy.Chest.1995;108:978–981.
- ,,, et al.Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism.Ann Intern Med.2001;135(2):88–97.
- Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED).The PIOPED Investigators.JAMA.1990;263:2753–2759.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d‐dimer.Ann Intern Med.2001;135(2):98–107.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,, et al.Does this patient have pulmonary embolism?JAMA.2003;290:2849–2858.
- ,,,,.Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review.Ann Intern Med.2003;138:941–951.
- ,,, et al.D‐dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review.Ann Intern Med.2004;140:589–602.
- .Multislice computed tomography for pulmonary embolism—a technological marvel.N Engl J Med2005;352(17):1812–4.
- ,,,,,.Systematic review and meta‐analysis of strategies for the diagnosis of suspected pulmonary embolism.Br Med J.2005;331:259.
Copyright © 2006 Society of Hospital Medicine
Editorial
I recently performed a PubMed search for hospitalists, which returned 561 citations, yet a second search for pediatric hospitalists produced only 37 citations. Growing up in Boston as a sports fan, my memories are filled with images that parallel these findings. One particularly vivid memory is of a Patriots game years ago. During that game, a very dynamic member of the opposing team was caught on camera picking up a phone on the sideline and telling the caller to call out the National Guard because we are killing the Patriots.
Now, pediatric hospital medicine is hardly being killed, and admittedly there were several methodological flaws in how I collected my data. However, this gap in number of publications must shrink if pediatric hospital medicine is to thrive. Like both hospital medicine and emergency medicine before it, pediatric hospital medicine must demonstrate what makes the field distinct and unique if is to be truly recognized as a medical subspecialty. The surest way to succeed in this endeavor is through the dissemination of information via peer‐reviewed journals such as the Journal of Hospital Medicine, potentially an ideal home for us.
It is important to note that dissemination of information is not limited to publication of original research. Pediatric hospital medicine is primarily a clinical field, and as such, practitioners may be spending 80%‐90% of their time (or more) caring for patients. This obviously does not leave much time for other academic pursuits. That being said, sharing many kinds of information can promote excellence in the care of hospitalized pediatric patients. Here are some types of articles that may prove useful.
-
Writing that integrates, rather than discovers, new knowledge
-
Review articles addressing the diagnosis and treatment of clinical conditions
-
Illustrative case reports or series drawn from clinical practice
-
Descriptions of best practice
-
QI/QA programs
-
Patient safety initiatives
-
Use of decision support or other information technology tools
-
Strategies to maintain physician wellness and career longevity
-
Creation of educational curricula or competency assessment methods
-
Leadership and professional development
This suggestion to share information of many types is not meant to downplay the importance of original research. As pediatric hospital medicine grows, its research component must grow as well in order to continually define and redefine the field itself, especially with regard to collaborative studies. In the future, it will no longer be acceptable for pediatric hospital programs to be practicing in isolation, without regard for nationally recognized and published benchmarks or other measures of quality. However, I believe that it is equally important for individuals to have outlets for these other forms of scholarship. Both the Society of Hospital Medicine and the Journal of Hospital Medicine are committed to the growth and development of pediatric hospital medicine. We encourage pediatric hospitalists to submit manuscripts and to become reviewers. You can do both at
I recently performed a PubMed search for hospitalists, which returned 561 citations, yet a second search for pediatric hospitalists produced only 37 citations. Growing up in Boston as a sports fan, my memories are filled with images that parallel these findings. One particularly vivid memory is of a Patriots game years ago. During that game, a very dynamic member of the opposing team was caught on camera picking up a phone on the sideline and telling the caller to call out the National Guard because we are killing the Patriots.
Now, pediatric hospital medicine is hardly being killed, and admittedly there were several methodological flaws in how I collected my data. However, this gap in number of publications must shrink if pediatric hospital medicine is to thrive. Like both hospital medicine and emergency medicine before it, pediatric hospital medicine must demonstrate what makes the field distinct and unique if is to be truly recognized as a medical subspecialty. The surest way to succeed in this endeavor is through the dissemination of information via peer‐reviewed journals such as the Journal of Hospital Medicine, potentially an ideal home for us.
It is important to note that dissemination of information is not limited to publication of original research. Pediatric hospital medicine is primarily a clinical field, and as such, practitioners may be spending 80%‐90% of their time (or more) caring for patients. This obviously does not leave much time for other academic pursuits. That being said, sharing many kinds of information can promote excellence in the care of hospitalized pediatric patients. Here are some types of articles that may prove useful.
-
Writing that integrates, rather than discovers, new knowledge
-
Review articles addressing the diagnosis and treatment of clinical conditions
-
Illustrative case reports or series drawn from clinical practice
-
Descriptions of best practice
-
QI/QA programs
-
Patient safety initiatives
-
Use of decision support or other information technology tools
-
Strategies to maintain physician wellness and career longevity
-
Creation of educational curricula or competency assessment methods
-
Leadership and professional development
This suggestion to share information of many types is not meant to downplay the importance of original research. As pediatric hospital medicine grows, its research component must grow as well in order to continually define and redefine the field itself, especially with regard to collaborative studies. In the future, it will no longer be acceptable for pediatric hospital programs to be practicing in isolation, without regard for nationally recognized and published benchmarks or other measures of quality. However, I believe that it is equally important for individuals to have outlets for these other forms of scholarship. Both the Society of Hospital Medicine and the Journal of Hospital Medicine are committed to the growth and development of pediatric hospital medicine. We encourage pediatric hospitalists to submit manuscripts and to become reviewers. You can do both at
I recently performed a PubMed search for hospitalists, which returned 561 citations, yet a second search for pediatric hospitalists produced only 37 citations. Growing up in Boston as a sports fan, my memories are filled with images that parallel these findings. One particularly vivid memory is of a Patriots game years ago. During that game, a very dynamic member of the opposing team was caught on camera picking up a phone on the sideline and telling the caller to call out the National Guard because we are killing the Patriots.
Now, pediatric hospital medicine is hardly being killed, and admittedly there were several methodological flaws in how I collected my data. However, this gap in number of publications must shrink if pediatric hospital medicine is to thrive. Like both hospital medicine and emergency medicine before it, pediatric hospital medicine must demonstrate what makes the field distinct and unique if is to be truly recognized as a medical subspecialty. The surest way to succeed in this endeavor is through the dissemination of information via peer‐reviewed journals such as the Journal of Hospital Medicine, potentially an ideal home for us.
It is important to note that dissemination of information is not limited to publication of original research. Pediatric hospital medicine is primarily a clinical field, and as such, practitioners may be spending 80%‐90% of their time (or more) caring for patients. This obviously does not leave much time for other academic pursuits. That being said, sharing many kinds of information can promote excellence in the care of hospitalized pediatric patients. Here are some types of articles that may prove useful.
-
Writing that integrates, rather than discovers, new knowledge
-
Review articles addressing the diagnosis and treatment of clinical conditions
-
Illustrative case reports or series drawn from clinical practice
-
Descriptions of best practice
-
QI/QA programs
-
Patient safety initiatives
-
Use of decision support or other information technology tools
-
Strategies to maintain physician wellness and career longevity
-
Creation of educational curricula or competency assessment methods
-
Leadership and professional development
This suggestion to share information of many types is not meant to downplay the importance of original research. As pediatric hospital medicine grows, its research component must grow as well in order to continually define and redefine the field itself, especially with regard to collaborative studies. In the future, it will no longer be acceptable for pediatric hospital programs to be practicing in isolation, without regard for nationally recognized and published benchmarks or other measures of quality. However, I believe that it is equally important for individuals to have outlets for these other forms of scholarship. Both the Society of Hospital Medicine and the Journal of Hospital Medicine are committed to the growth and development of pediatric hospital medicine. We encourage pediatric hospitalists to submit manuscripts and to become reviewers. You can do both at
Status of US Hospital Medicine Groups
The term hospitalist was coined in 1996 in an article1 that appeared in the New England Journal of Medicine. Robert M. Wachter, MD, and Lee Goldman, MD, of the University of California, San Francisco, defined hospitalists as hospital‐based physicians who take responsibility for managing medical inpatients. Hospitalists were described as having responsibility for seeing unassigned hospital patients and being available for in‐hospital consultations. Several years later, the Society of Hospital Medicine posted the definition of a hospitalist as someone whose primary professional focus is the medical care of hospitalized patientsin patient care, education, research, and administrative activities.
In January 2002, Wachter and Goldman published a follow‐up article,2 The Hospitalist Movement 5 Years Later, in the Journal of the American Medical Association. This formal review of 19 published studies analyzed the impact of hospital medicine groups on financial and clinical outcomes. Wachter and Goldman concluded, Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction. These studies indicate an average reduction of cost per stay of 13.4% and an average reduction in length of stay of 16.6%.
The evolution of the hospitalist movement has been fast paced and extensive. Given the recent pace of growth, a scholarly analysis estimated that the mature hospitalist workforce in the United States will eventually total 20,000, making it the equivalent of the cardiology specialty.3 Beyond sheer growth, medical literature has demonstrated positive effects of the hospitalist model on patient quality outcomes, including readmission rates, postoperative complications, and mortality.47
In addition to peer‐reviewed medical literature, there is anecdotal evidence about the growth and effects of the hospitalist movement:
The Society of Hospital Medicine (SHM), the hospitalist professional society, estimated that in 2003 there were 8000 physicians practicing as hospitalists in the United States.8
Twelve of the country's top 15 hospitals have hospital medicine groups.8
As hospital medicine groups have proliferated, 4 major employment models have evolved. Hospitalists can be employees of: 1) a hospital or a hospital subsidiary; 2) a multispecialty or primary care physician group; 3) a medical group (local or national) of independent hospitalists; or 4) a university or medical school. However, there is little published data on the prevalence of each of these hospitalist employment models, nationally or by type of hospital.
To better understand the extent and nature of the hospitalist movement, the American Hospital Association (AHA) utilized its 2003 Annual Survey to gather data on hospital medicine groups in the United States
DATA AND METHODS
The data for our analysis came from the 2003 AHA Annual Survey. Conducted since 1946, this survey is the principal data collection mechanism of the American Hospital Association and is a basic source of data on hospitals in the United States about the availability of services, utilization, personnel, finances, and governance. Its main purpose is to provide a cross‐sectional view of hospitals and hospital performance over time. In the 2003 survey, a series of items were added about hospitalists including whether hospitals had hospital medicine groups, the number of hospitalists operating in such groups, and the employment model used.
The study population for this analysis was limited to US community hospitals (n = 4895). Community hospitals are defined as all adult and pediatric nonfederal, short‐term general, and specialty hospitals whose facilities and services are available to the public. Excluded from the analysis were all federal hospitals, long‐term care hospitals, and psychiatric hospitals.
Imputation of Missing Data
In the 2003 survey, 77% of the 4895 US community hospitals answered the question on specific use of hospitalists. To get a complete picture of the number of groups and hospitalists, we imputed data for the nonresponding hospitals.
We performed logistic regression analysis of data from the responding hospitals to estimate the number of nonresponding hospitals that had a group and the number of hospitalists in these groups. The dependent variable in the regression was whether a hospital had a group, and the independent variables included hospital characteristics for which data were available for all US hospitals, both survey respondents and nonrespondents. The results of the regression analysis were then applied to the data for each nonresponding hospital to estimate its probability of having a group. These probabilities were summed over the various nonresponding hospitals to estimate the total number of nonresponding hospitals that had groups.
To impute the number of hospitalists in the nonresponding set of hospitals, the additional number of groups was stratified into the 9 US Census Divisions. On the basis of reported data, the average number of hospitalists per group was calculated at the Census Division level. The per‐group value was then applied to the number of additional groups, and the result was added to the total number of reported hospitalists. The Census Division values were then summed to produce the national total. To produce results for all other control groupings, the national total was then apportioned across the categories according to percentage of hospitalists by category on the basis of the reported data.
Analytical Plan
In analyzing the hospitalist movement across the country, we realized there are 2 dimensions of diffusion, which can be characterized as breadth and depth. In the present study:
The measure of breadth is the percentage of hospital medicine groups in a given group of hospitals. In the Results section, this measure is sometimes referred to as penetration.
The measure of depth is the number of hospitalists for each average daily census (ADC) of 100 patients. For instance, for a hospital with an average daily census of 100 that has 4 hospitalists, that measure is 4. To compute this metric for a given category of hospitals (eg, major teaching hospitals), the numerator is the number of hospitalists and the denominator is the ADC at hospitals that have hospital medicine groups. The metric reflects the in‐hospital impact of hospital medicine groups at their hospitals.
Using these 2 measures, it is possible to differentiate between a group of hospitals that has many hospital medicine groups but each group has a minimal impact at the hospital versus a group of hospitals that has few hospital medicine groups but each group has a major impact at the hospital.
The analysis also characterizes the employment status of hospitalists by comparing the proportion of hospitals in each of the employment models by category of hospital.
RESULTS
Diffusion and Impact
Overall, the penetration of hospital medicine groups across the 4895 hospitals in the United States is 29% and the in‐hospital impact at hospitals with hospital medicine groups is 3.93 hospitalists per 100 ADC. The average hospital medicine group has 7.9 hospitalists at a hospital with an ADC of 200.6.
Geographic Categories (Tables 1A and 2A)
The Northeast (46%) and the Pacific (40%) divisions have the greatest penetration of hospital medicine groups. The West North Central Division (16%) has the lowest penetration of hospital medicine groups. Hospital medicine groups in the West South Central Division average 11.1 hospitalists, which partially explains why this region has the greatest in‐hospital impact (6.24 hospitalists per 100 ADC). At the other end of the spectrum are the Middle Atlantic and East South Central divisions with (2.42 and 2.83 hospitalists per 100 ADC, respectively.
| Category | Hospitals | Hospital medicine groups | Hospitals with hospital medicine groups (%) |
|---|---|---|---|
| |||
| Region | |||
| 1: Northeast | 203 | 94 | 46% |
| 2: Mid‐Atlantic | 486 | 172 | 35% |
| 3: South‐Atlantic | 731 | 272 | 37% |
| 4: East North Central | 732 | 209 | 29% |
| 5: East South Central | 427 | 92 | 22% |
| 6: West North Central | 675 | 106 | 16% |
| 7: West South Central | 737 | 164 | 22% |
| 8: Mountain | 348 | 83 | 24% |
| 9: Pacific | 556 | 223 | 40% |
| Rural/urban | |||
| Rural | 2166 | 235 | 11% |
| Small urban | 1285 | 488 | 38% |
| Large urban | 1444 | 692 | 48% |
| Total | 4895 | 1415 | 29% |
| Category | Groups (hospitals) | Hospitalists | Hospitalists per group | Hospitalists per 100 census |
|---|---|---|---|---|
| ||||
| Region | ||||
| 1: Northeast | 94 | 669 | 7.1 | 3.62 |
| 2: Mid‐Atlantic | 172 | 1133 | 6.6 | 2.42 |
| 3: South Atlantic | 272 | 1933 | 7.1 | 3.21 |
| 4: East North Central | 209 | 2087 | 10.0 | 4.65 |
| 5: East South Central | 92 | 433 | 4.7 | 2.83 |
| 6: West North Central | 106 | 887 | 8.4 | 4.37 |
| 7: West South Central | 164 | 1828 | 11.1 | 6.24 |
| 8: Mountain | 83 | 644 | 7.8 | 4.43 |
| 9: Pacific | 223 | 1546 | 6.9 | 4.56 |
| Rural/urban | ||||
| Rural | 235 | 893 | 3.8 | 4.85 |
| Small urban | 488 | 3236 | 6.6 | 3.03 |
| Large urban | 692 | 7030 | 10.2 | 4.43 |
| Total | 1415 | 11 159 | 7.9 | 3.93 |
There are more hospital medicine groups in urban locations. The penetration of hospital medicine groups is 48% at hospitals in large metropolitan locations (ie, with a population of more than 1 million), 38% at hospitals in small metropolitan locations, and 11% at hospitals in rural areas. However, rural hospitals have a relatively high in‐hospital impact (4.85 hospitalists per 100 ADC), explained by an average group size of 3.8 and an average ADC of 78.4.
Hospital Size, Control/Ownership, and Teaching Status (Tables 1B and 2B)
The penetration of hospital medicine groups increases as the size of the hospital increases. Six percent of hospitals with 6‐24 beds have groups, whereas 71% of hospitals with 500+ beds have groups. Among hospitals with 200 or more beds, 55% have hospital medicine groups compared to 19% of hospitals with fewer than 200 beds. As would be expected, larger hospitals have larger hospital medicine groups: hospitals with 6‐24 beds average 2.1 hospitalists, whereas hospitals with 500+ beds average 14.2 hospitalists. However, hospitalists have a proportionately greater impact at smaller hospitals. Their greatest impact is at hospitals with 6‐24 beds (46.34 hospitalists per 100 ADC); their smallest impact is at hospitals with 500+ beds (2.47 hospitalists per 100 ADC).
| Category | Hospitals | Hospital medicine groups | Hospitals with hospital medicine groups (%) |
|---|---|---|---|
| |||
| Size | |||
| 6‐24 beds | 327 | 18 | 6% |
| 25‐49 beds | 965 | 88 | 9% |
| 50‐99 beds | 1031 | 168 | 16% |
| 100‐199 beds | 1168 | 372 | 32% |
| 200‐299 beds | 624 | 287 | 46% |
| 300‐399 beds | 349 | 183 | 52% |
| 400‐499 beds | 172 | 116 | 67% |
| 500+ beds | 259 | 183 | 71% |
| Control | |||
| Government | 1121 | 161 | 14% |
| Not for profit | 2984 | 1032 | 35% |
| For profit | 790 | 222 | 28% |
| Teaching status | |||
| Nonteaching | 3800 | 823 | 22% |
| Other teaching | 779 | 382 | 49% |
| Major teaching | 316 | 210 | 66% |
| Total | 4895 | 1415 | 29% |
| Category | Groups (hospitals) | Hospitalists | Hospitalists per group | Hospitalists per 100 census |
|---|---|---|---|---|
| ||||
| Size | ||||
| 6‐24 beds | 18 | 38 | 2.1 | 46.34 |
| 25‐49 beds | 88 | 260 | 3.0 | 17.94 |
| 50‐99 beds | 168 | 885 | 5.3 | 12.75 |
| 100‐199 beds | 372 | 1757 | 4.7 | 5.29 |
| 200‐299 beds | 287 | 2308 | 8.0 | 4.72 |
| 300‐399 beds | 183 | 1,553 | 8.5 | 3.29 |
| 400‐499 beds | 116 | 1751 | 15.1 | 4.35 |
| 500+ beds | 183 | 2,607 | 14.2 | 2.47 |
| Control | ||||
| Government | 161 | 1,674 | 10.4 | 5.85 |
| Not for profit | 1032 | 8,481 | 8.2 | 3.64 |
| For profit | 222 | 1,004 | 4.5 | 4.47 |
| Teaching Status | ||||
| Nonteaching | 823 | 4,910 | 6.0 | 4.85 |
| Other teaching | 382 | 2,678 | 7.0 | 3.25 |
| Major teaching | 210 | 3,571 | 17.0 | 3.57 |
| Total | 1415 | 11 159 | 7.9 | 3.93 |
Of the 3 categories of control, government groups have the lowest penetration of hospital medicine groups (14%). However, the hospital medicine groups at these government‐controlled hospitals are large (10.4 hospitalists), and they have a significant in‐hospital impact on care at these hospitals (5.85 hospitalists per 100 ADC). Not‐for‐profit hospitals have the highest penetration of hospital medicine groups (35%), whereas hospital medicine groups at for‐profit hospitals have the lowest average size (4.5 hospitalists).
There appears to be a relationship between teaching status and the likelihood that a hospital has a hospital medicine group. The penetration of hospital medicine groups is 66% at major teaching hospitals, 49% at other teaching hospitals, and 22% at nonteaching hospitals. However, nonteaching hospitals have a relatively high in‐hospital impact (4.85 hospitalists per 100 ADC). This is explained by their having an average group size of 6.0, but an average ADC of only 123.0 (compared to 477.0 for major teaching hospitals and 215.7 for other teaching hospitals).
Employment Models
The results of the analysis of hospitalist employment models (data not shown) can be summarized as follows:
Employees of hospitals: This employment model averaged 33% of all groups, with an average size of 9.8 hospitalists. The employees of hospital model was more prevalent in the Mid‐Atlantic (56%), New England (49%), and West North Central (45%) regions and in rural hospitals (45%). The East South Central (16%) and West South Central (12%) regions and for‐profit hospitals (20%) had fewer hospital employee groups.
Employees of medical groups: This employment model averaged 29% of all groups, with an average of 7.4 hospitalists. More hospitals in the East South Central (35%) and New England (34%) regions had this employment model. Fewer hospitals in the Mid‐Atlantic (18%) and West North Central (18%) regions and rural (18%) hospitals had medical group‐based groups.
Employees of independent hospitalist groups: This employment group averaged 25% of all groups and had the smallest mean number of hospitalists (6.9). This employment model was more prevalent in for‐profit hospitals (43%) and was less prevalent in the New England (9%) and Mid‐Atlantic (11%) regions and in major teaching hospitals (11%) and government hospitals (19%).
CONCLUSIONS
Hospital medicine groups appear to have become part of the mainstream delivery of health care. With more than 11 000 hospitalists, the specialty is equivalent in size to the gastroenterology medical specialty.9 Fifty‐five percent of hospitals with more than 200 beds have hospital medicine groups. Furthermore, it appears that the growth of the hospitalist movement has not peaked. It is likely that the number of hospitals with hospital medicine groups will increase and that existing hospital medicine groups will continue to add hospitalists.
No one employment model of hospital medicine group appears to dominate the health care landscape. We expect that there will continue to be diversity among the organizations that choose to establish hospital medicine groups.
In light of this growth and diversity, hospital medicine groups appear to be valued by a wide range of stakeholders in the health care industry. The potential benefits provided by hospitalists include financial savings, improved throughput efficiency, improved quality and safety, improved medical education, and better provider satisfaction.
Despite this success story, the hospitalist movement has maintained a relatively low profile among consumers and some segments of the health care industry. This is likely to change. As the hospital medicine specialty gains recognition, hospitalists will receive increased scrutiny and attention. This emerging specialty will need to be able to clearly define its role and document its performance in the constantly changing health care industry.
ADDENDUM
Subsequent to the acceptance of this manuscript, the authors received results of the 2004 Annual Survey of the American Hospital Association. Some highlights of the new data and comparisons to the 2003 results are as follows:
The penetration of hospitals with hospital medicine groups grew from 29% to 34% (for hospitals with 200+ beds, the penetration grew from 55% to 63%)
An estimated 1,661 hospitals have hospital medicine groups (an increase of 17% from 2003)
The average size of a hospital medicine group decreased from 7.9 physicians to 7.5 physicians (a decrease of 5%)
It is estimated that there are 12,504 hospitalists in the U.S. (an increase of 12% from 2003)
Hospital medicine groups remain equally distributed among the three employment models: employees of hospitals 30%, employees of medical groups 29%, employees of independent hospitalist groups 29%
These updated results indicate strong hospitalist growth over the one year period and continued diversity among hospital medicine programs, reinforcing the conclusions of the manuscript.
APPENDIX
AHA Annual Survey Overview
Conducted since 1946, the AHA Annual Survey is the principal data collection mechanism of the American Hospital Association and is a basic source of data on hospitals in the United States. Its main purpose is to provide a cross‐sectional view of the hospital field each year and to make it possible to monitor hospital performance over time. The information that it gathers from a universe of approximately 5700 hospitals concerns primarily the availability of services, utilization, personnel, finances, and governance. Newly added to the 2003 survey were the following questions regarding hospitalists: Do hospitalists provide care for patients in your hospital? YES □ NO □
Hospitalist is defined as a physician whose primary professional focus is the care of hospitalized medical patients (through clinical, education, administrative and research activity).
If yes, please report the number of full time and part time hospitalists?
Full‐time ______
Part‐time ______
Full‐time equivalent (FTE) is the total number of hours worked by all employees over the full (12 month) reporting period divided by the normal number of hours worked by a full‐time employee for that same period. For example, if your hospital considers a normal workweek for a full‐time employee to be 40 hours, a total of 2080 hours would be worked over a full year (52 weeks). If the total number of hours worked by all employees on the payroll is 208 000, then the number of FTEs is 100 (employees). The FTE calculation for a specific occupational category such as registered nurses is exactly the same. The calculation for each occupational category should be based on the number of hours worked by staff employed in that specific category.
If yes, please select the category below that best describes the employment model for your hospitalists:
□ Independent provider group
□ Employed by your hospital
□ Employed by a physician group
□ Employed by a university or school program
□ Other
It is the results from these questions that are the subject of this analysis and the manuscript.
- ,.The emerging role of “hospitalists” in the American health care system.N Eng J Med.1996;335:514–517.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,,.The potential size of the hospitalist workforce in the United StatesAm J Med.1999;106:441–445.
- .Implementation of a hospitalist service at a community hospital: evolution of service utilization, costs, and patient outcomes [abstract]. National Association of Inpatient Physicians, 3rd Annual Meeting. Philadelphia, Penn, April 11‐12,2000.
- ,,,,,.Decreased length of stay, costs, and mortality in a randomized trial of academic hospitalists [abstract]. National Association of Inpatient Physicians, 4th Annual Meeting, Atlanta, GA, March 27‐28,2001.
- ,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,.Program description: a hospitalist run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163:1477–1480.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. July 2003. Available at: http://www.hospitalmedicine.org/presentation/apps/indlist/intro.asp?flag=18. Accessed February2005.
- American Medical Association.Physician characteristics and distribution in the US, 2004.Chicago, Ill:American Medical Association,2004.
The term hospitalist was coined in 1996 in an article1 that appeared in the New England Journal of Medicine. Robert M. Wachter, MD, and Lee Goldman, MD, of the University of California, San Francisco, defined hospitalists as hospital‐based physicians who take responsibility for managing medical inpatients. Hospitalists were described as having responsibility for seeing unassigned hospital patients and being available for in‐hospital consultations. Several years later, the Society of Hospital Medicine posted the definition of a hospitalist as someone whose primary professional focus is the medical care of hospitalized patientsin patient care, education, research, and administrative activities.
In January 2002, Wachter and Goldman published a follow‐up article,2 The Hospitalist Movement 5 Years Later, in the Journal of the American Medical Association. This formal review of 19 published studies analyzed the impact of hospital medicine groups on financial and clinical outcomes. Wachter and Goldman concluded, Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction. These studies indicate an average reduction of cost per stay of 13.4% and an average reduction in length of stay of 16.6%.
The evolution of the hospitalist movement has been fast paced and extensive. Given the recent pace of growth, a scholarly analysis estimated that the mature hospitalist workforce in the United States will eventually total 20,000, making it the equivalent of the cardiology specialty.3 Beyond sheer growth, medical literature has demonstrated positive effects of the hospitalist model on patient quality outcomes, including readmission rates, postoperative complications, and mortality.47
In addition to peer‐reviewed medical literature, there is anecdotal evidence about the growth and effects of the hospitalist movement:
The Society of Hospital Medicine (SHM), the hospitalist professional society, estimated that in 2003 there were 8000 physicians practicing as hospitalists in the United States.8
Twelve of the country's top 15 hospitals have hospital medicine groups.8
As hospital medicine groups have proliferated, 4 major employment models have evolved. Hospitalists can be employees of: 1) a hospital or a hospital subsidiary; 2) a multispecialty or primary care physician group; 3) a medical group (local or national) of independent hospitalists; or 4) a university or medical school. However, there is little published data on the prevalence of each of these hospitalist employment models, nationally or by type of hospital.
To better understand the extent and nature of the hospitalist movement, the American Hospital Association (AHA) utilized its 2003 Annual Survey to gather data on hospital medicine groups in the United States
DATA AND METHODS
The data for our analysis came from the 2003 AHA Annual Survey. Conducted since 1946, this survey is the principal data collection mechanism of the American Hospital Association and is a basic source of data on hospitals in the United States about the availability of services, utilization, personnel, finances, and governance. Its main purpose is to provide a cross‐sectional view of hospitals and hospital performance over time. In the 2003 survey, a series of items were added about hospitalists including whether hospitals had hospital medicine groups, the number of hospitalists operating in such groups, and the employment model used.
The study population for this analysis was limited to US community hospitals (n = 4895). Community hospitals are defined as all adult and pediatric nonfederal, short‐term general, and specialty hospitals whose facilities and services are available to the public. Excluded from the analysis were all federal hospitals, long‐term care hospitals, and psychiatric hospitals.
Imputation of Missing Data
In the 2003 survey, 77% of the 4895 US community hospitals answered the question on specific use of hospitalists. To get a complete picture of the number of groups and hospitalists, we imputed data for the nonresponding hospitals.
We performed logistic regression analysis of data from the responding hospitals to estimate the number of nonresponding hospitals that had a group and the number of hospitalists in these groups. The dependent variable in the regression was whether a hospital had a group, and the independent variables included hospital characteristics for which data were available for all US hospitals, both survey respondents and nonrespondents. The results of the regression analysis were then applied to the data for each nonresponding hospital to estimate its probability of having a group. These probabilities were summed over the various nonresponding hospitals to estimate the total number of nonresponding hospitals that had groups.
To impute the number of hospitalists in the nonresponding set of hospitals, the additional number of groups was stratified into the 9 US Census Divisions. On the basis of reported data, the average number of hospitalists per group was calculated at the Census Division level. The per‐group value was then applied to the number of additional groups, and the result was added to the total number of reported hospitalists. The Census Division values were then summed to produce the national total. To produce results for all other control groupings, the national total was then apportioned across the categories according to percentage of hospitalists by category on the basis of the reported data.
Analytical Plan
In analyzing the hospitalist movement across the country, we realized there are 2 dimensions of diffusion, which can be characterized as breadth and depth. In the present study:
The measure of breadth is the percentage of hospital medicine groups in a given group of hospitals. In the Results section, this measure is sometimes referred to as penetration.
The measure of depth is the number of hospitalists for each average daily census (ADC) of 100 patients. For instance, for a hospital with an average daily census of 100 that has 4 hospitalists, that measure is 4. To compute this metric for a given category of hospitals (eg, major teaching hospitals), the numerator is the number of hospitalists and the denominator is the ADC at hospitals that have hospital medicine groups. The metric reflects the in‐hospital impact of hospital medicine groups at their hospitals.
Using these 2 measures, it is possible to differentiate between a group of hospitals that has many hospital medicine groups but each group has a minimal impact at the hospital versus a group of hospitals that has few hospital medicine groups but each group has a major impact at the hospital.
The analysis also characterizes the employment status of hospitalists by comparing the proportion of hospitals in each of the employment models by category of hospital.
RESULTS
Diffusion and Impact
Overall, the penetration of hospital medicine groups across the 4895 hospitals in the United States is 29% and the in‐hospital impact at hospitals with hospital medicine groups is 3.93 hospitalists per 100 ADC. The average hospital medicine group has 7.9 hospitalists at a hospital with an ADC of 200.6.
Geographic Categories (Tables 1A and 2A)
The Northeast (46%) and the Pacific (40%) divisions have the greatest penetration of hospital medicine groups. The West North Central Division (16%) has the lowest penetration of hospital medicine groups. Hospital medicine groups in the West South Central Division average 11.1 hospitalists, which partially explains why this region has the greatest in‐hospital impact (6.24 hospitalists per 100 ADC). At the other end of the spectrum are the Middle Atlantic and East South Central divisions with (2.42 and 2.83 hospitalists per 100 ADC, respectively.
| Category | Hospitals | Hospital medicine groups | Hospitals with hospital medicine groups (%) |
|---|---|---|---|
| |||
| Region | |||
| 1: Northeast | 203 | 94 | 46% |
| 2: Mid‐Atlantic | 486 | 172 | 35% |
| 3: South‐Atlantic | 731 | 272 | 37% |
| 4: East North Central | 732 | 209 | 29% |
| 5: East South Central | 427 | 92 | 22% |
| 6: West North Central | 675 | 106 | 16% |
| 7: West South Central | 737 | 164 | 22% |
| 8: Mountain | 348 | 83 | 24% |
| 9: Pacific | 556 | 223 | 40% |
| Rural/urban | |||
| Rural | 2166 | 235 | 11% |
| Small urban | 1285 | 488 | 38% |
| Large urban | 1444 | 692 | 48% |
| Total | 4895 | 1415 | 29% |
| Category | Groups (hospitals) | Hospitalists | Hospitalists per group | Hospitalists per 100 census |
|---|---|---|---|---|
| ||||
| Region | ||||
| 1: Northeast | 94 | 669 | 7.1 | 3.62 |
| 2: Mid‐Atlantic | 172 | 1133 | 6.6 | 2.42 |
| 3: South Atlantic | 272 | 1933 | 7.1 | 3.21 |
| 4: East North Central | 209 | 2087 | 10.0 | 4.65 |
| 5: East South Central | 92 | 433 | 4.7 | 2.83 |
| 6: West North Central | 106 | 887 | 8.4 | 4.37 |
| 7: West South Central | 164 | 1828 | 11.1 | 6.24 |
| 8: Mountain | 83 | 644 | 7.8 | 4.43 |
| 9: Pacific | 223 | 1546 | 6.9 | 4.56 |
| Rural/urban | ||||
| Rural | 235 | 893 | 3.8 | 4.85 |
| Small urban | 488 | 3236 | 6.6 | 3.03 |
| Large urban | 692 | 7030 | 10.2 | 4.43 |
| Total | 1415 | 11 159 | 7.9 | 3.93 |
There are more hospital medicine groups in urban locations. The penetration of hospital medicine groups is 48% at hospitals in large metropolitan locations (ie, with a population of more than 1 million), 38% at hospitals in small metropolitan locations, and 11% at hospitals in rural areas. However, rural hospitals have a relatively high in‐hospital impact (4.85 hospitalists per 100 ADC), explained by an average group size of 3.8 and an average ADC of 78.4.
Hospital Size, Control/Ownership, and Teaching Status (Tables 1B and 2B)
The penetration of hospital medicine groups increases as the size of the hospital increases. Six percent of hospitals with 6‐24 beds have groups, whereas 71% of hospitals with 500+ beds have groups. Among hospitals with 200 or more beds, 55% have hospital medicine groups compared to 19% of hospitals with fewer than 200 beds. As would be expected, larger hospitals have larger hospital medicine groups: hospitals with 6‐24 beds average 2.1 hospitalists, whereas hospitals with 500+ beds average 14.2 hospitalists. However, hospitalists have a proportionately greater impact at smaller hospitals. Their greatest impact is at hospitals with 6‐24 beds (46.34 hospitalists per 100 ADC); their smallest impact is at hospitals with 500+ beds (2.47 hospitalists per 100 ADC).
| Category | Hospitals | Hospital medicine groups | Hospitals with hospital medicine groups (%) |
|---|---|---|---|
| |||
| Size | |||
| 6‐24 beds | 327 | 18 | 6% |
| 25‐49 beds | 965 | 88 | 9% |
| 50‐99 beds | 1031 | 168 | 16% |
| 100‐199 beds | 1168 | 372 | 32% |
| 200‐299 beds | 624 | 287 | 46% |
| 300‐399 beds | 349 | 183 | 52% |
| 400‐499 beds | 172 | 116 | 67% |
| 500+ beds | 259 | 183 | 71% |
| Control | |||
| Government | 1121 | 161 | 14% |
| Not for profit | 2984 | 1032 | 35% |
| For profit | 790 | 222 | 28% |
| Teaching status | |||
| Nonteaching | 3800 | 823 | 22% |
| Other teaching | 779 | 382 | 49% |
| Major teaching | 316 | 210 | 66% |
| Total | 4895 | 1415 | 29% |
| Category | Groups (hospitals) | Hospitalists | Hospitalists per group | Hospitalists per 100 census |
|---|---|---|---|---|
| ||||
| Size | ||||
| 6‐24 beds | 18 | 38 | 2.1 | 46.34 |
| 25‐49 beds | 88 | 260 | 3.0 | 17.94 |
| 50‐99 beds | 168 | 885 | 5.3 | 12.75 |
| 100‐199 beds | 372 | 1757 | 4.7 | 5.29 |
| 200‐299 beds | 287 | 2308 | 8.0 | 4.72 |
| 300‐399 beds | 183 | 1,553 | 8.5 | 3.29 |
| 400‐499 beds | 116 | 1751 | 15.1 | 4.35 |
| 500+ beds | 183 | 2,607 | 14.2 | 2.47 |
| Control | ||||
| Government | 161 | 1,674 | 10.4 | 5.85 |
| Not for profit | 1032 | 8,481 | 8.2 | 3.64 |
| For profit | 222 | 1,004 | 4.5 | 4.47 |
| Teaching Status | ||||
| Nonteaching | 823 | 4,910 | 6.0 | 4.85 |
| Other teaching | 382 | 2,678 | 7.0 | 3.25 |
| Major teaching | 210 | 3,571 | 17.0 | 3.57 |
| Total | 1415 | 11 159 | 7.9 | 3.93 |
Of the 3 categories of control, government groups have the lowest penetration of hospital medicine groups (14%). However, the hospital medicine groups at these government‐controlled hospitals are large (10.4 hospitalists), and they have a significant in‐hospital impact on care at these hospitals (5.85 hospitalists per 100 ADC). Not‐for‐profit hospitals have the highest penetration of hospital medicine groups (35%), whereas hospital medicine groups at for‐profit hospitals have the lowest average size (4.5 hospitalists).
There appears to be a relationship between teaching status and the likelihood that a hospital has a hospital medicine group. The penetration of hospital medicine groups is 66% at major teaching hospitals, 49% at other teaching hospitals, and 22% at nonteaching hospitals. However, nonteaching hospitals have a relatively high in‐hospital impact (4.85 hospitalists per 100 ADC). This is explained by their having an average group size of 6.0, but an average ADC of only 123.0 (compared to 477.0 for major teaching hospitals and 215.7 for other teaching hospitals).
Employment Models
The results of the analysis of hospitalist employment models (data not shown) can be summarized as follows:
Employees of hospitals: This employment model averaged 33% of all groups, with an average size of 9.8 hospitalists. The employees of hospital model was more prevalent in the Mid‐Atlantic (56%), New England (49%), and West North Central (45%) regions and in rural hospitals (45%). The East South Central (16%) and West South Central (12%) regions and for‐profit hospitals (20%) had fewer hospital employee groups.
Employees of medical groups: This employment model averaged 29% of all groups, with an average of 7.4 hospitalists. More hospitals in the East South Central (35%) and New England (34%) regions had this employment model. Fewer hospitals in the Mid‐Atlantic (18%) and West North Central (18%) regions and rural (18%) hospitals had medical group‐based groups.
Employees of independent hospitalist groups: This employment group averaged 25% of all groups and had the smallest mean number of hospitalists (6.9). This employment model was more prevalent in for‐profit hospitals (43%) and was less prevalent in the New England (9%) and Mid‐Atlantic (11%) regions and in major teaching hospitals (11%) and government hospitals (19%).
CONCLUSIONS
Hospital medicine groups appear to have become part of the mainstream delivery of health care. With more than 11 000 hospitalists, the specialty is equivalent in size to the gastroenterology medical specialty.9 Fifty‐five percent of hospitals with more than 200 beds have hospital medicine groups. Furthermore, it appears that the growth of the hospitalist movement has not peaked. It is likely that the number of hospitals with hospital medicine groups will increase and that existing hospital medicine groups will continue to add hospitalists.
No one employment model of hospital medicine group appears to dominate the health care landscape. We expect that there will continue to be diversity among the organizations that choose to establish hospital medicine groups.
In light of this growth and diversity, hospital medicine groups appear to be valued by a wide range of stakeholders in the health care industry. The potential benefits provided by hospitalists include financial savings, improved throughput efficiency, improved quality and safety, improved medical education, and better provider satisfaction.
Despite this success story, the hospitalist movement has maintained a relatively low profile among consumers and some segments of the health care industry. This is likely to change. As the hospital medicine specialty gains recognition, hospitalists will receive increased scrutiny and attention. This emerging specialty will need to be able to clearly define its role and document its performance in the constantly changing health care industry.
ADDENDUM
Subsequent to the acceptance of this manuscript, the authors received results of the 2004 Annual Survey of the American Hospital Association. Some highlights of the new data and comparisons to the 2003 results are as follows:
The penetration of hospitals with hospital medicine groups grew from 29% to 34% (for hospitals with 200+ beds, the penetration grew from 55% to 63%)
An estimated 1,661 hospitals have hospital medicine groups (an increase of 17% from 2003)
The average size of a hospital medicine group decreased from 7.9 physicians to 7.5 physicians (a decrease of 5%)
It is estimated that there are 12,504 hospitalists in the U.S. (an increase of 12% from 2003)
Hospital medicine groups remain equally distributed among the three employment models: employees of hospitals 30%, employees of medical groups 29%, employees of independent hospitalist groups 29%
These updated results indicate strong hospitalist growth over the one year period and continued diversity among hospital medicine programs, reinforcing the conclusions of the manuscript.
APPENDIX
AHA Annual Survey Overview
Conducted since 1946, the AHA Annual Survey is the principal data collection mechanism of the American Hospital Association and is a basic source of data on hospitals in the United States. Its main purpose is to provide a cross‐sectional view of the hospital field each year and to make it possible to monitor hospital performance over time. The information that it gathers from a universe of approximately 5700 hospitals concerns primarily the availability of services, utilization, personnel, finances, and governance. Newly added to the 2003 survey were the following questions regarding hospitalists: Do hospitalists provide care for patients in your hospital? YES □ NO □
Hospitalist is defined as a physician whose primary professional focus is the care of hospitalized medical patients (through clinical, education, administrative and research activity).
If yes, please report the number of full time and part time hospitalists?
Full‐time ______
Part‐time ______
Full‐time equivalent (FTE) is the total number of hours worked by all employees over the full (12 month) reporting period divided by the normal number of hours worked by a full‐time employee for that same period. For example, if your hospital considers a normal workweek for a full‐time employee to be 40 hours, a total of 2080 hours would be worked over a full year (52 weeks). If the total number of hours worked by all employees on the payroll is 208 000, then the number of FTEs is 100 (employees). The FTE calculation for a specific occupational category such as registered nurses is exactly the same. The calculation for each occupational category should be based on the number of hours worked by staff employed in that specific category.
If yes, please select the category below that best describes the employment model for your hospitalists:
□ Independent provider group
□ Employed by your hospital
□ Employed by a physician group
□ Employed by a university or school program
□ Other
It is the results from these questions that are the subject of this analysis and the manuscript.
The term hospitalist was coined in 1996 in an article1 that appeared in the New England Journal of Medicine. Robert M. Wachter, MD, and Lee Goldman, MD, of the University of California, San Francisco, defined hospitalists as hospital‐based physicians who take responsibility for managing medical inpatients. Hospitalists were described as having responsibility for seeing unassigned hospital patients and being available for in‐hospital consultations. Several years later, the Society of Hospital Medicine posted the definition of a hospitalist as someone whose primary professional focus is the medical care of hospitalized patientsin patient care, education, research, and administrative activities.
In January 2002, Wachter and Goldman published a follow‐up article,2 The Hospitalist Movement 5 Years Later, in the Journal of the American Medical Association. This formal review of 19 published studies analyzed the impact of hospital medicine groups on financial and clinical outcomes. Wachter and Goldman concluded, Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction. These studies indicate an average reduction of cost per stay of 13.4% and an average reduction in length of stay of 16.6%.
The evolution of the hospitalist movement has been fast paced and extensive. Given the recent pace of growth, a scholarly analysis estimated that the mature hospitalist workforce in the United States will eventually total 20,000, making it the equivalent of the cardiology specialty.3 Beyond sheer growth, medical literature has demonstrated positive effects of the hospitalist model on patient quality outcomes, including readmission rates, postoperative complications, and mortality.47
In addition to peer‐reviewed medical literature, there is anecdotal evidence about the growth and effects of the hospitalist movement:
The Society of Hospital Medicine (SHM), the hospitalist professional society, estimated that in 2003 there were 8000 physicians practicing as hospitalists in the United States.8
Twelve of the country's top 15 hospitals have hospital medicine groups.8
As hospital medicine groups have proliferated, 4 major employment models have evolved. Hospitalists can be employees of: 1) a hospital or a hospital subsidiary; 2) a multispecialty or primary care physician group; 3) a medical group (local or national) of independent hospitalists; or 4) a university or medical school. However, there is little published data on the prevalence of each of these hospitalist employment models, nationally or by type of hospital.
To better understand the extent and nature of the hospitalist movement, the American Hospital Association (AHA) utilized its 2003 Annual Survey to gather data on hospital medicine groups in the United States
DATA AND METHODS
The data for our analysis came from the 2003 AHA Annual Survey. Conducted since 1946, this survey is the principal data collection mechanism of the American Hospital Association and is a basic source of data on hospitals in the United States about the availability of services, utilization, personnel, finances, and governance. Its main purpose is to provide a cross‐sectional view of hospitals and hospital performance over time. In the 2003 survey, a series of items were added about hospitalists including whether hospitals had hospital medicine groups, the number of hospitalists operating in such groups, and the employment model used.
The study population for this analysis was limited to US community hospitals (n = 4895). Community hospitals are defined as all adult and pediatric nonfederal, short‐term general, and specialty hospitals whose facilities and services are available to the public. Excluded from the analysis were all federal hospitals, long‐term care hospitals, and psychiatric hospitals.
Imputation of Missing Data
In the 2003 survey, 77% of the 4895 US community hospitals answered the question on specific use of hospitalists. To get a complete picture of the number of groups and hospitalists, we imputed data for the nonresponding hospitals.
We performed logistic regression analysis of data from the responding hospitals to estimate the number of nonresponding hospitals that had a group and the number of hospitalists in these groups. The dependent variable in the regression was whether a hospital had a group, and the independent variables included hospital characteristics for which data were available for all US hospitals, both survey respondents and nonrespondents. The results of the regression analysis were then applied to the data for each nonresponding hospital to estimate its probability of having a group. These probabilities were summed over the various nonresponding hospitals to estimate the total number of nonresponding hospitals that had groups.
To impute the number of hospitalists in the nonresponding set of hospitals, the additional number of groups was stratified into the 9 US Census Divisions. On the basis of reported data, the average number of hospitalists per group was calculated at the Census Division level. The per‐group value was then applied to the number of additional groups, and the result was added to the total number of reported hospitalists. The Census Division values were then summed to produce the national total. To produce results for all other control groupings, the national total was then apportioned across the categories according to percentage of hospitalists by category on the basis of the reported data.
Analytical Plan
In analyzing the hospitalist movement across the country, we realized there are 2 dimensions of diffusion, which can be characterized as breadth and depth. In the present study:
The measure of breadth is the percentage of hospital medicine groups in a given group of hospitals. In the Results section, this measure is sometimes referred to as penetration.
The measure of depth is the number of hospitalists for each average daily census (ADC) of 100 patients. For instance, for a hospital with an average daily census of 100 that has 4 hospitalists, that measure is 4. To compute this metric for a given category of hospitals (eg, major teaching hospitals), the numerator is the number of hospitalists and the denominator is the ADC at hospitals that have hospital medicine groups. The metric reflects the in‐hospital impact of hospital medicine groups at their hospitals.
Using these 2 measures, it is possible to differentiate between a group of hospitals that has many hospital medicine groups but each group has a minimal impact at the hospital versus a group of hospitals that has few hospital medicine groups but each group has a major impact at the hospital.
The analysis also characterizes the employment status of hospitalists by comparing the proportion of hospitals in each of the employment models by category of hospital.
RESULTS
Diffusion and Impact
Overall, the penetration of hospital medicine groups across the 4895 hospitals in the United States is 29% and the in‐hospital impact at hospitals with hospital medicine groups is 3.93 hospitalists per 100 ADC. The average hospital medicine group has 7.9 hospitalists at a hospital with an ADC of 200.6.
Geographic Categories (Tables 1A and 2A)
The Northeast (46%) and the Pacific (40%) divisions have the greatest penetration of hospital medicine groups. The West North Central Division (16%) has the lowest penetration of hospital medicine groups. Hospital medicine groups in the West South Central Division average 11.1 hospitalists, which partially explains why this region has the greatest in‐hospital impact (6.24 hospitalists per 100 ADC). At the other end of the spectrum are the Middle Atlantic and East South Central divisions with (2.42 and 2.83 hospitalists per 100 ADC, respectively.
| Category | Hospitals | Hospital medicine groups | Hospitals with hospital medicine groups (%) |
|---|---|---|---|
| |||
| Region | |||
| 1: Northeast | 203 | 94 | 46% |
| 2: Mid‐Atlantic | 486 | 172 | 35% |
| 3: South‐Atlantic | 731 | 272 | 37% |
| 4: East North Central | 732 | 209 | 29% |
| 5: East South Central | 427 | 92 | 22% |
| 6: West North Central | 675 | 106 | 16% |
| 7: West South Central | 737 | 164 | 22% |
| 8: Mountain | 348 | 83 | 24% |
| 9: Pacific | 556 | 223 | 40% |
| Rural/urban | |||
| Rural | 2166 | 235 | 11% |
| Small urban | 1285 | 488 | 38% |
| Large urban | 1444 | 692 | 48% |
| Total | 4895 | 1415 | 29% |
| Category | Groups (hospitals) | Hospitalists | Hospitalists per group | Hospitalists per 100 census |
|---|---|---|---|---|
| ||||
| Region | ||||
| 1: Northeast | 94 | 669 | 7.1 | 3.62 |
| 2: Mid‐Atlantic | 172 | 1133 | 6.6 | 2.42 |
| 3: South Atlantic | 272 | 1933 | 7.1 | 3.21 |
| 4: East North Central | 209 | 2087 | 10.0 | 4.65 |
| 5: East South Central | 92 | 433 | 4.7 | 2.83 |
| 6: West North Central | 106 | 887 | 8.4 | 4.37 |
| 7: West South Central | 164 | 1828 | 11.1 | 6.24 |
| 8: Mountain | 83 | 644 | 7.8 | 4.43 |
| 9: Pacific | 223 | 1546 | 6.9 | 4.56 |
| Rural/urban | ||||
| Rural | 235 | 893 | 3.8 | 4.85 |
| Small urban | 488 | 3236 | 6.6 | 3.03 |
| Large urban | 692 | 7030 | 10.2 | 4.43 |
| Total | 1415 | 11 159 | 7.9 | 3.93 |
There are more hospital medicine groups in urban locations. The penetration of hospital medicine groups is 48% at hospitals in large metropolitan locations (ie, with a population of more than 1 million), 38% at hospitals in small metropolitan locations, and 11% at hospitals in rural areas. However, rural hospitals have a relatively high in‐hospital impact (4.85 hospitalists per 100 ADC), explained by an average group size of 3.8 and an average ADC of 78.4.
Hospital Size, Control/Ownership, and Teaching Status (Tables 1B and 2B)
The penetration of hospital medicine groups increases as the size of the hospital increases. Six percent of hospitals with 6‐24 beds have groups, whereas 71% of hospitals with 500+ beds have groups. Among hospitals with 200 or more beds, 55% have hospital medicine groups compared to 19% of hospitals with fewer than 200 beds. As would be expected, larger hospitals have larger hospital medicine groups: hospitals with 6‐24 beds average 2.1 hospitalists, whereas hospitals with 500+ beds average 14.2 hospitalists. However, hospitalists have a proportionately greater impact at smaller hospitals. Their greatest impact is at hospitals with 6‐24 beds (46.34 hospitalists per 100 ADC); their smallest impact is at hospitals with 500+ beds (2.47 hospitalists per 100 ADC).
| Category | Hospitals | Hospital medicine groups | Hospitals with hospital medicine groups (%) |
|---|---|---|---|
| |||
| Size | |||
| 6‐24 beds | 327 | 18 | 6% |
| 25‐49 beds | 965 | 88 | 9% |
| 50‐99 beds | 1031 | 168 | 16% |
| 100‐199 beds | 1168 | 372 | 32% |
| 200‐299 beds | 624 | 287 | 46% |
| 300‐399 beds | 349 | 183 | 52% |
| 400‐499 beds | 172 | 116 | 67% |
| 500+ beds | 259 | 183 | 71% |
| Control | |||
| Government | 1121 | 161 | 14% |
| Not for profit | 2984 | 1032 | 35% |
| For profit | 790 | 222 | 28% |
| Teaching status | |||
| Nonteaching | 3800 | 823 | 22% |
| Other teaching | 779 | 382 | 49% |
| Major teaching | 316 | 210 | 66% |
| Total | 4895 | 1415 | 29% |
| Category | Groups (hospitals) | Hospitalists | Hospitalists per group | Hospitalists per 100 census |
|---|---|---|---|---|
| ||||
| Size | ||||
| 6‐24 beds | 18 | 38 | 2.1 | 46.34 |
| 25‐49 beds | 88 | 260 | 3.0 | 17.94 |
| 50‐99 beds | 168 | 885 | 5.3 | 12.75 |
| 100‐199 beds | 372 | 1757 | 4.7 | 5.29 |
| 200‐299 beds | 287 | 2308 | 8.0 | 4.72 |
| 300‐399 beds | 183 | 1,553 | 8.5 | 3.29 |
| 400‐499 beds | 116 | 1751 | 15.1 | 4.35 |
| 500+ beds | 183 | 2,607 | 14.2 | 2.47 |
| Control | ||||
| Government | 161 | 1,674 | 10.4 | 5.85 |
| Not for profit | 1032 | 8,481 | 8.2 | 3.64 |
| For profit | 222 | 1,004 | 4.5 | 4.47 |
| Teaching Status | ||||
| Nonteaching | 823 | 4,910 | 6.0 | 4.85 |
| Other teaching | 382 | 2,678 | 7.0 | 3.25 |
| Major teaching | 210 | 3,571 | 17.0 | 3.57 |
| Total | 1415 | 11 159 | 7.9 | 3.93 |
Of the 3 categories of control, government groups have the lowest penetration of hospital medicine groups (14%). However, the hospital medicine groups at these government‐controlled hospitals are large (10.4 hospitalists), and they have a significant in‐hospital impact on care at these hospitals (5.85 hospitalists per 100 ADC). Not‐for‐profit hospitals have the highest penetration of hospital medicine groups (35%), whereas hospital medicine groups at for‐profit hospitals have the lowest average size (4.5 hospitalists).
There appears to be a relationship between teaching status and the likelihood that a hospital has a hospital medicine group. The penetration of hospital medicine groups is 66% at major teaching hospitals, 49% at other teaching hospitals, and 22% at nonteaching hospitals. However, nonteaching hospitals have a relatively high in‐hospital impact (4.85 hospitalists per 100 ADC). This is explained by their having an average group size of 6.0, but an average ADC of only 123.0 (compared to 477.0 for major teaching hospitals and 215.7 for other teaching hospitals).
Employment Models
The results of the analysis of hospitalist employment models (data not shown) can be summarized as follows:
Employees of hospitals: This employment model averaged 33% of all groups, with an average size of 9.8 hospitalists. The employees of hospital model was more prevalent in the Mid‐Atlantic (56%), New England (49%), and West North Central (45%) regions and in rural hospitals (45%). The East South Central (16%) and West South Central (12%) regions and for‐profit hospitals (20%) had fewer hospital employee groups.
Employees of medical groups: This employment model averaged 29% of all groups, with an average of 7.4 hospitalists. More hospitals in the East South Central (35%) and New England (34%) regions had this employment model. Fewer hospitals in the Mid‐Atlantic (18%) and West North Central (18%) regions and rural (18%) hospitals had medical group‐based groups.
Employees of independent hospitalist groups: This employment group averaged 25% of all groups and had the smallest mean number of hospitalists (6.9). This employment model was more prevalent in for‐profit hospitals (43%) and was less prevalent in the New England (9%) and Mid‐Atlantic (11%) regions and in major teaching hospitals (11%) and government hospitals (19%).
CONCLUSIONS
Hospital medicine groups appear to have become part of the mainstream delivery of health care. With more than 11 000 hospitalists, the specialty is equivalent in size to the gastroenterology medical specialty.9 Fifty‐five percent of hospitals with more than 200 beds have hospital medicine groups. Furthermore, it appears that the growth of the hospitalist movement has not peaked. It is likely that the number of hospitals with hospital medicine groups will increase and that existing hospital medicine groups will continue to add hospitalists.
No one employment model of hospital medicine group appears to dominate the health care landscape. We expect that there will continue to be diversity among the organizations that choose to establish hospital medicine groups.
In light of this growth and diversity, hospital medicine groups appear to be valued by a wide range of stakeholders in the health care industry. The potential benefits provided by hospitalists include financial savings, improved throughput efficiency, improved quality and safety, improved medical education, and better provider satisfaction.
Despite this success story, the hospitalist movement has maintained a relatively low profile among consumers and some segments of the health care industry. This is likely to change. As the hospital medicine specialty gains recognition, hospitalists will receive increased scrutiny and attention. This emerging specialty will need to be able to clearly define its role and document its performance in the constantly changing health care industry.
ADDENDUM
Subsequent to the acceptance of this manuscript, the authors received results of the 2004 Annual Survey of the American Hospital Association. Some highlights of the new data and comparisons to the 2003 results are as follows:
The penetration of hospitals with hospital medicine groups grew from 29% to 34% (for hospitals with 200+ beds, the penetration grew from 55% to 63%)
An estimated 1,661 hospitals have hospital medicine groups (an increase of 17% from 2003)
The average size of a hospital medicine group decreased from 7.9 physicians to 7.5 physicians (a decrease of 5%)
It is estimated that there are 12,504 hospitalists in the U.S. (an increase of 12% from 2003)
Hospital medicine groups remain equally distributed among the three employment models: employees of hospitals 30%, employees of medical groups 29%, employees of independent hospitalist groups 29%
These updated results indicate strong hospitalist growth over the one year period and continued diversity among hospital medicine programs, reinforcing the conclusions of the manuscript.
APPENDIX
AHA Annual Survey Overview
Conducted since 1946, the AHA Annual Survey is the principal data collection mechanism of the American Hospital Association and is a basic source of data on hospitals in the United States. Its main purpose is to provide a cross‐sectional view of the hospital field each year and to make it possible to monitor hospital performance over time. The information that it gathers from a universe of approximately 5700 hospitals concerns primarily the availability of services, utilization, personnel, finances, and governance. Newly added to the 2003 survey were the following questions regarding hospitalists: Do hospitalists provide care for patients in your hospital? YES □ NO □
Hospitalist is defined as a physician whose primary professional focus is the care of hospitalized medical patients (through clinical, education, administrative and research activity).
If yes, please report the number of full time and part time hospitalists?
Full‐time ______
Part‐time ______
Full‐time equivalent (FTE) is the total number of hours worked by all employees over the full (12 month) reporting period divided by the normal number of hours worked by a full‐time employee for that same period. For example, if your hospital considers a normal workweek for a full‐time employee to be 40 hours, a total of 2080 hours would be worked over a full year (52 weeks). If the total number of hours worked by all employees on the payroll is 208 000, then the number of FTEs is 100 (employees). The FTE calculation for a specific occupational category such as registered nurses is exactly the same. The calculation for each occupational category should be based on the number of hours worked by staff employed in that specific category.
If yes, please select the category below that best describes the employment model for your hospitalists:
□ Independent provider group
□ Employed by your hospital
□ Employed by a physician group
□ Employed by a university or school program
□ Other
It is the results from these questions that are the subject of this analysis and the manuscript.
- ,.The emerging role of “hospitalists” in the American health care system.N Eng J Med.1996;335:514–517.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,,.The potential size of the hospitalist workforce in the United StatesAm J Med.1999;106:441–445.
- .Implementation of a hospitalist service at a community hospital: evolution of service utilization, costs, and patient outcomes [abstract]. National Association of Inpatient Physicians, 3rd Annual Meeting. Philadelphia, Penn, April 11‐12,2000.
- ,,,,,.Decreased length of stay, costs, and mortality in a randomized trial of academic hospitalists [abstract]. National Association of Inpatient Physicians, 4th Annual Meeting, Atlanta, GA, March 27‐28,2001.
- ,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,.Program description: a hospitalist run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163:1477–1480.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. July 2003. Available at: http://www.hospitalmedicine.org/presentation/apps/indlist/intro.asp?flag=18. Accessed February2005.
- American Medical Association.Physician characteristics and distribution in the US, 2004.Chicago, Ill:American Medical Association,2004.
- ,.The emerging role of “hospitalists” in the American health care system.N Eng J Med.1996;335:514–517.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,,.The potential size of the hospitalist workforce in the United StatesAm J Med.1999;106:441–445.
- .Implementation of a hospitalist service at a community hospital: evolution of service utilization, costs, and patient outcomes [abstract]. National Association of Inpatient Physicians, 3rd Annual Meeting. Philadelphia, Penn, April 11‐12,2000.
- ,,,,,.Decreased length of stay, costs, and mortality in a randomized trial of academic hospitalists [abstract]. National Association of Inpatient Physicians, 4th Annual Meeting, Atlanta, GA, March 27‐28,2001.
- ,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,.Program description: a hospitalist run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163:1477–1480.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. July 2003. Available at: http://www.hospitalmedicine.org/presentation/apps/indlist/intro.asp?flag=18. Accessed February2005.
- American Medical Association.Physician characteristics and distribution in the US, 2004.Chicago, Ill:American Medical Association,2004.
Copyright © 2006 Society of Hospital Medicine
Of Lizards and Leeches
We are proud to practice medicine in the modern era: the 21st-century heirs to Hippocrates. Along the way we have abandoned a materia medica of bizarre and unusual therapies like mummy powder and eye of newt. Our pharmaceuticals are lined up in bottles and bags with clearly marked expiration dates. It’s a far cry from the witches in Macbeth, standing around the fire chanting:
Round about the cauldron go;
In the poison’d entrails throw.
Toad, that under cold stone
Days and nights has thirty-one
Swelter’d venom sleeping got,
Boil thou first i’ the charmed pot.
Double, double toil and trouble;
Fire burn and cauldron bubble.
Or have things really changed? We would shake our heads at the remedies of Shakespeare’s son-in-law, Dr. John Hall, who used spider webs, poultry larynx, and animal excreta as part of his materia medica. But wait: Perhaps we should think twice before condemning him. Perhaps we are less different then we think. We just use similar products that have been sanitized.
Some modern medicines retain their strong biotherapeutic flavor. The field of organotherapy led to the extraction of active elements from the glands of mammals and eventually insulin, thyroid extract, growth hormone, testosterone, and adrenaline. The modern forms of these drugs are just a few steps removed from their origins, but somehow don’t strike one as unusual. Read on to see how we use such non-mammalian biotherapeutic exotica as lizard spit, salmon sperm, and leech saliva as part of the most modern pharmaceutical armamentarium.
Lizards
How unlikely would it seem, but all too true, that the newest weapon in the fight against diabetes is derived from lizard spit? The lizards in question are Heloderma horridum and Heloderma suspectum (aka the Mexican beaded lizard and the Gila monster).
This strange tale begins in the Bronx, N.Y., not renowned (aside from the Bronx Zoo) as a home for Sonoran lizards. Cockroaches and rats may be the dominant fauna there. Dr. John Eng, an endocrinologist, was hunting for new hormones. In the venom of the beaded lizard he discovered a vasoactive hormone he named exendin-3. In the venom of the Gila monster he found the less vasoactive exendin-4, which seemed to have an interesting effect on beta cells.
Dr. John Eng eventually patented exendin-4, and now we have the newest drug on the market for the treatment of diabetes. The first of class of incretin mimetics, synthetic exendin-4, is also known as exanatide and marketed as Byetta. Administered as a twice-daily injection, exanatide stimulates beta cells, via a specific receptor, to secrete insulin in a glucose-dependent fashion, suppresses glucagon overproduction, slows gastric emptying, and improves satiety. The net result is that most patients experience improvement of glucose control and weight loss. The most common side effects are nausea, which tends to be moderate, self limited, and a result of hypoglycemia. As with any new drug, side effects may still be determined over time. As of yet there have been no reports of reptilian metamorphosis
Salmon
The sperm of salmon is worth mentioning here as a bridge between diabetes and the treatment of coagulation disorders. An important step in the biotherapy of insulin depended on salmon sperm. Salmon sperm contains protamines, which are small arginine-rich nuclear proteins that stabilize DNA. Salmon sperm was used because it is more easily obtained than some mammalian alternatives.
When we write prescriptions for NPH insulin, how often do we contemplate what those initials represent? The acronym stands for neutral protamine Hagedorn. In 1923 Hans Christian Hagedorn (a Danish physician, 1888-1971) and August Steenberg Krogh (a Nobel-prize winning physiologist, 1874-1949) obtained the rights from Sir Frederick Grant Banting (1891-1941) and Charles Best (1899-1941), who had first isolated insulin, and formed a company called Nordisk Insulinlaboratorium to produce insulin for Scandinavians. Krogh’s wife, Marie, was diabetic.
Ten years later Hagedorn and Jensen discovered that injection of insulin would have a prolonged effect if mixed with protamine-rich salmon sperm. The necessity of a pH of 7 for activation made the handling of insulin difficult. Zinc was added to the mix as a stabilizer. By 1946, an easier-to-use crystallized form was developed, and it was marketed by 1950 as NPH insulin.
When a patient is overdosed with heparin, excessive bleeding can be a problem. Protamine sulfate is a valuable medication used for reversal of heparin. Protamine is a strongly basic substance that combines with the strongly acidic heparin to form a stable complex. The protamine-heparin complex is not an anticoagulant; protamine causes a dissociation of the heparin-antithrombin III complex, resulting in loss of heparin’s anticoagulant activity. Given too quickly it may cause hypotension or anaphylaxis and may cause allergic reactions to patients with fish hypersensitivity.
Leeches
From the anticoagulant effect of salmon sperm, we move to the world of Annelida. More than any other creature, the leech stands out as the epitome of biotherapy. Its name alone, Hirudo medicinalis, emphasizes its medical nature. Used by many ancient societies, the leech reached its zenith in mid-19th century France. Leeches were the fashion, women’s dresses were decorated with faux leeches, and cosmetics were applied to give that “healthy pale look” sometimes attained by being bled with leeches.
In 1833 more than 40 million leeches were imported into France. However, the leech’s days were numbered. The biggest blow was when Pierre Louis made his name as the father of medical statistics by proving leeches led to a worse outcome in treating pneumonia. The death of the leech was the birth of evidence-based medicine.
But all is not lost for the leech lover. The use of the leech as an anticoagulant was recognized in 1884. In its modern chemical form, recombinant leech saliva marketed under names such as lepirudin, is indicated for coronary thrombolysis, unstable angina hemodialysis, heparin-induced thrombocytopenia, and DVT prophylaxis. Recombinant hirudin, a man-made chemical similar to leech saliva, is manufactured in large quantities and is much easier to obtain than “milking” leeches. The mechanism of action is direct inhibition of thrombin. Leeches are making a comeback in the treatment of skin grafts, however. A mechanical leech has also been designed.
The Future
The argument for the protection of our planet’s biodiversity could not be more obvious. A new treatment for diabetes comes from Gila monsters. What novel substances lurk in the ever-shrinking rain forests? Whether from lizard or leech, the day of biotherapy is not yet done. Despite all this, I’m not cornering the market on synthetic eye of newt. TH
Jamie Newman, MD, FACP, is the physician editor of The Hospitalist, consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
We are proud to practice medicine in the modern era: the 21st-century heirs to Hippocrates. Along the way we have abandoned a materia medica of bizarre and unusual therapies like mummy powder and eye of newt. Our pharmaceuticals are lined up in bottles and bags with clearly marked expiration dates. It’s a far cry from the witches in Macbeth, standing around the fire chanting:
Round about the cauldron go;
In the poison’d entrails throw.
Toad, that under cold stone
Days and nights has thirty-one
Swelter’d venom sleeping got,
Boil thou first i’ the charmed pot.
Double, double toil and trouble;
Fire burn and cauldron bubble.
Or have things really changed? We would shake our heads at the remedies of Shakespeare’s son-in-law, Dr. John Hall, who used spider webs, poultry larynx, and animal excreta as part of his materia medica. But wait: Perhaps we should think twice before condemning him. Perhaps we are less different then we think. We just use similar products that have been sanitized.
Some modern medicines retain their strong biotherapeutic flavor. The field of organotherapy led to the extraction of active elements from the glands of mammals and eventually insulin, thyroid extract, growth hormone, testosterone, and adrenaline. The modern forms of these drugs are just a few steps removed from their origins, but somehow don’t strike one as unusual. Read on to see how we use such non-mammalian biotherapeutic exotica as lizard spit, salmon sperm, and leech saliva as part of the most modern pharmaceutical armamentarium.
Lizards
How unlikely would it seem, but all too true, that the newest weapon in the fight against diabetes is derived from lizard spit? The lizards in question are Heloderma horridum and Heloderma suspectum (aka the Mexican beaded lizard and the Gila monster).
This strange tale begins in the Bronx, N.Y., not renowned (aside from the Bronx Zoo) as a home for Sonoran lizards. Cockroaches and rats may be the dominant fauna there. Dr. John Eng, an endocrinologist, was hunting for new hormones. In the venom of the beaded lizard he discovered a vasoactive hormone he named exendin-3. In the venom of the Gila monster he found the less vasoactive exendin-4, which seemed to have an interesting effect on beta cells.
Dr. John Eng eventually patented exendin-4, and now we have the newest drug on the market for the treatment of diabetes. The first of class of incretin mimetics, synthetic exendin-4, is also known as exanatide and marketed as Byetta. Administered as a twice-daily injection, exanatide stimulates beta cells, via a specific receptor, to secrete insulin in a glucose-dependent fashion, suppresses glucagon overproduction, slows gastric emptying, and improves satiety. The net result is that most patients experience improvement of glucose control and weight loss. The most common side effects are nausea, which tends to be moderate, self limited, and a result of hypoglycemia. As with any new drug, side effects may still be determined over time. As of yet there have been no reports of reptilian metamorphosis
Salmon
The sperm of salmon is worth mentioning here as a bridge between diabetes and the treatment of coagulation disorders. An important step in the biotherapy of insulin depended on salmon sperm. Salmon sperm contains protamines, which are small arginine-rich nuclear proteins that stabilize DNA. Salmon sperm was used because it is more easily obtained than some mammalian alternatives.
When we write prescriptions for NPH insulin, how often do we contemplate what those initials represent? The acronym stands for neutral protamine Hagedorn. In 1923 Hans Christian Hagedorn (a Danish physician, 1888-1971) and August Steenberg Krogh (a Nobel-prize winning physiologist, 1874-1949) obtained the rights from Sir Frederick Grant Banting (1891-1941) and Charles Best (1899-1941), who had first isolated insulin, and formed a company called Nordisk Insulinlaboratorium to produce insulin for Scandinavians. Krogh’s wife, Marie, was diabetic.
Ten years later Hagedorn and Jensen discovered that injection of insulin would have a prolonged effect if mixed with protamine-rich salmon sperm. The necessity of a pH of 7 for activation made the handling of insulin difficult. Zinc was added to the mix as a stabilizer. By 1946, an easier-to-use crystallized form was developed, and it was marketed by 1950 as NPH insulin.
When a patient is overdosed with heparin, excessive bleeding can be a problem. Protamine sulfate is a valuable medication used for reversal of heparin. Protamine is a strongly basic substance that combines with the strongly acidic heparin to form a stable complex. The protamine-heparin complex is not an anticoagulant; protamine causes a dissociation of the heparin-antithrombin III complex, resulting in loss of heparin’s anticoagulant activity. Given too quickly it may cause hypotension or anaphylaxis and may cause allergic reactions to patients with fish hypersensitivity.
Leeches
From the anticoagulant effect of salmon sperm, we move to the world of Annelida. More than any other creature, the leech stands out as the epitome of biotherapy. Its name alone, Hirudo medicinalis, emphasizes its medical nature. Used by many ancient societies, the leech reached its zenith in mid-19th century France. Leeches were the fashion, women’s dresses were decorated with faux leeches, and cosmetics were applied to give that “healthy pale look” sometimes attained by being bled with leeches.
In 1833 more than 40 million leeches were imported into France. However, the leech’s days were numbered. The biggest blow was when Pierre Louis made his name as the father of medical statistics by proving leeches led to a worse outcome in treating pneumonia. The death of the leech was the birth of evidence-based medicine.
But all is not lost for the leech lover. The use of the leech as an anticoagulant was recognized in 1884. In its modern chemical form, recombinant leech saliva marketed under names such as lepirudin, is indicated for coronary thrombolysis, unstable angina hemodialysis, heparin-induced thrombocytopenia, and DVT prophylaxis. Recombinant hirudin, a man-made chemical similar to leech saliva, is manufactured in large quantities and is much easier to obtain than “milking” leeches. The mechanism of action is direct inhibition of thrombin. Leeches are making a comeback in the treatment of skin grafts, however. A mechanical leech has also been designed.
The Future
The argument for the protection of our planet’s biodiversity could not be more obvious. A new treatment for diabetes comes from Gila monsters. What novel substances lurk in the ever-shrinking rain forests? Whether from lizard or leech, the day of biotherapy is not yet done. Despite all this, I’m not cornering the market on synthetic eye of newt. TH
Jamie Newman, MD, FACP, is the physician editor of The Hospitalist, consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
We are proud to practice medicine in the modern era: the 21st-century heirs to Hippocrates. Along the way we have abandoned a materia medica of bizarre and unusual therapies like mummy powder and eye of newt. Our pharmaceuticals are lined up in bottles and bags with clearly marked expiration dates. It’s a far cry from the witches in Macbeth, standing around the fire chanting:
Round about the cauldron go;
In the poison’d entrails throw.
Toad, that under cold stone
Days and nights has thirty-one
Swelter’d venom sleeping got,
Boil thou first i’ the charmed pot.
Double, double toil and trouble;
Fire burn and cauldron bubble.
Or have things really changed? We would shake our heads at the remedies of Shakespeare’s son-in-law, Dr. John Hall, who used spider webs, poultry larynx, and animal excreta as part of his materia medica. But wait: Perhaps we should think twice before condemning him. Perhaps we are less different then we think. We just use similar products that have been sanitized.
Some modern medicines retain their strong biotherapeutic flavor. The field of organotherapy led to the extraction of active elements from the glands of mammals and eventually insulin, thyroid extract, growth hormone, testosterone, and adrenaline. The modern forms of these drugs are just a few steps removed from their origins, but somehow don’t strike one as unusual. Read on to see how we use such non-mammalian biotherapeutic exotica as lizard spit, salmon sperm, and leech saliva as part of the most modern pharmaceutical armamentarium.
Lizards
How unlikely would it seem, but all too true, that the newest weapon in the fight against diabetes is derived from lizard spit? The lizards in question are Heloderma horridum and Heloderma suspectum (aka the Mexican beaded lizard and the Gila monster).
This strange tale begins in the Bronx, N.Y., not renowned (aside from the Bronx Zoo) as a home for Sonoran lizards. Cockroaches and rats may be the dominant fauna there. Dr. John Eng, an endocrinologist, was hunting for new hormones. In the venom of the beaded lizard he discovered a vasoactive hormone he named exendin-3. In the venom of the Gila monster he found the less vasoactive exendin-4, which seemed to have an interesting effect on beta cells.
Dr. John Eng eventually patented exendin-4, and now we have the newest drug on the market for the treatment of diabetes. The first of class of incretin mimetics, synthetic exendin-4, is also known as exanatide and marketed as Byetta. Administered as a twice-daily injection, exanatide stimulates beta cells, via a specific receptor, to secrete insulin in a glucose-dependent fashion, suppresses glucagon overproduction, slows gastric emptying, and improves satiety. The net result is that most patients experience improvement of glucose control and weight loss. The most common side effects are nausea, which tends to be moderate, self limited, and a result of hypoglycemia. As with any new drug, side effects may still be determined over time. As of yet there have been no reports of reptilian metamorphosis
Salmon
The sperm of salmon is worth mentioning here as a bridge between diabetes and the treatment of coagulation disorders. An important step in the biotherapy of insulin depended on salmon sperm. Salmon sperm contains protamines, which are small arginine-rich nuclear proteins that stabilize DNA. Salmon sperm was used because it is more easily obtained than some mammalian alternatives.
When we write prescriptions for NPH insulin, how often do we contemplate what those initials represent? The acronym stands for neutral protamine Hagedorn. In 1923 Hans Christian Hagedorn (a Danish physician, 1888-1971) and August Steenberg Krogh (a Nobel-prize winning physiologist, 1874-1949) obtained the rights from Sir Frederick Grant Banting (1891-1941) and Charles Best (1899-1941), who had first isolated insulin, and formed a company called Nordisk Insulinlaboratorium to produce insulin for Scandinavians. Krogh’s wife, Marie, was diabetic.
Ten years later Hagedorn and Jensen discovered that injection of insulin would have a prolonged effect if mixed with protamine-rich salmon sperm. The necessity of a pH of 7 for activation made the handling of insulin difficult. Zinc was added to the mix as a stabilizer. By 1946, an easier-to-use crystallized form was developed, and it was marketed by 1950 as NPH insulin.
When a patient is overdosed with heparin, excessive bleeding can be a problem. Protamine sulfate is a valuable medication used for reversal of heparin. Protamine is a strongly basic substance that combines with the strongly acidic heparin to form a stable complex. The protamine-heparin complex is not an anticoagulant; protamine causes a dissociation of the heparin-antithrombin III complex, resulting in loss of heparin’s anticoagulant activity. Given too quickly it may cause hypotension or anaphylaxis and may cause allergic reactions to patients with fish hypersensitivity.
Leeches
From the anticoagulant effect of salmon sperm, we move to the world of Annelida. More than any other creature, the leech stands out as the epitome of biotherapy. Its name alone, Hirudo medicinalis, emphasizes its medical nature. Used by many ancient societies, the leech reached its zenith in mid-19th century France. Leeches were the fashion, women’s dresses were decorated with faux leeches, and cosmetics were applied to give that “healthy pale look” sometimes attained by being bled with leeches.
In 1833 more than 40 million leeches were imported into France. However, the leech’s days were numbered. The biggest blow was when Pierre Louis made his name as the father of medical statistics by proving leeches led to a worse outcome in treating pneumonia. The death of the leech was the birth of evidence-based medicine.
But all is not lost for the leech lover. The use of the leech as an anticoagulant was recognized in 1884. In its modern chemical form, recombinant leech saliva marketed under names such as lepirudin, is indicated for coronary thrombolysis, unstable angina hemodialysis, heparin-induced thrombocytopenia, and DVT prophylaxis. Recombinant hirudin, a man-made chemical similar to leech saliva, is manufactured in large quantities and is much easier to obtain than “milking” leeches. The mechanism of action is direct inhibition of thrombin. Leeches are making a comeback in the treatment of skin grafts, however. A mechanical leech has also been designed.
The Future
The argument for the protection of our planet’s biodiversity could not be more obvious. A new treatment for diabetes comes from Gila monsters. What novel substances lurk in the ever-shrinking rain forests? Whether from lizard or leech, the day of biotherapy is not yet done. Despite all this, I’m not cornering the market on synthetic eye of newt. TH
Jamie Newman, MD, FACP, is the physician editor of The Hospitalist, consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
Invaluable Assistants
Editors’ note: “Alliances” is a series written about the relationships that hospitalists have with members of the clinical care team—from the team members’ points of view. Each installment of “Alliances” provides valuable, revealing feedback that hospitalists can use to continually improve their intrateam relationships and, ultimately, patient care.
Several months ago, a patient with decompensated end-stage liver disease was admitted to the Internal Medicine Hospitalist Service at the University of Texas Medical Branch in Galveston and required a paracentesis. One of the new hospitalist faculty members was taken aback when the physician assistant (PA) on the service volunteered to do the procedure. “He was surprised,” says Karen Kislingbury, PA-C, a member of SHM’s Non-Physician Provider Task Force and a PA with the Internal Medicine Hospitalist Service, “that the scope of practice for the physician assistant included [performing] procedures.”
PAs are not new to the hospital setting, and their inclusion as physician extenders to increase patient access to care will likely increase in the current regulatory environment—especially state-mandated staff/patient ratios and resident work hour limitations. The efficacy of utilizing physician extenders to improve patient care and outcomes has been validated in studies over the past two decades. A recent Journal of Trauma study found statistically significant reductions in floor, ICU, and overall hospital lengths of stay after incorporating physician extenders into their trauma service.1
However, hospitalists unfamiliar with PAs may not understand their colleagues’ roles and scope of practice. As her anecdote illustrated, Kislingbury notes that “although PAs aren’t new to the healthcare delivery system, and physicians have been utilizing us for a long time, our partnership in the unique setting of hospital medicine is kind of new.”
Kislingbury’s colleague Ryan Genzink, PA-C, who works with Hospitalists of West Michigan, a private hospitalists-only group that subcontracts hospitalist services to Spectrum Health of Grand Rapids, Mich., agrees with her assessment.
“There are more and more PAs and [nurse practitioners] working in hospital medicine, and I think there is a lot of curiosity and some apprehension on the part of people who have not worked with these non-physician providers,” says Genzink.
Genzink, also a member of SHM’s Non-Physician Provider Task Force, speculates that the apprehension of physicians who have not worked with PAs may be due to a misunderstanding of the PA’s role. “They’re either underestimating or overestimating exactly what a PA can do or what they are getting when they hire a PA,” he says.
A Short History of the Profession
PA programs officially began in the mid-1960s at Duke University Medical School (Durham, N.C.). Eugene Stead, MD, is credited with developing the concept of the physician assistant as a health professional who would work with physician supervision to extend patient access to care, according to the American Academy of Physician Assistants.
For the first class of PAs in 1965, Dr. Stead selected Navy corpsmen who had received medical training and experience during their service in Vietnam. The curriculum was based on Dr. Stead’s knowledge of fast-track training of physicians during World War II. From this early program, the profession has evolved to more than 130 programs that now adhere to rigorous national accreditation standards set forth by the independent Accreditation Review Commission on Education for the Physician Assistant (ARC-PA). The ARC-PA is sponsored by the American Medical Association and the American College of Surgeons, among many other professional medical organizations (www.aapa.org/geninfo1.html).
Scope of Practice
Prerequisites to PA programs include two years of college courses in basic and behavioral science, as well as prior experience in healthcare. According to a report generated by the Association of Physician Assistant Programs, most PA students have earned a bachelor’s degree and have an average of 38 months of healthcare experience before being admitted to a PA program.2
The first year of PA education comprises a didactic curriculum with coursework in anatomy, physiology, biochemistry, pharmacology, physical diagnosis, pathophysiology, microbiology, clinical laboratory sciences, behavioral sciences, and medical ethics. In the second year, students receive hands-on clinical training through a series of rotations—typically in family and internal medicine, obstetrics and gynecology, pediatrics, general surgery, emergency medicine, and psychiatry. By the time they graduate (typical PA programs last an average of 26 months), PAs will have completed more than 2,000 hours of supervised clinical practice.
PAs work in all areas of medicine. Although hospital bylaws and state regulations often stipulate the PA’s scope of practice, the major determinant of duties is the supervising physician. The relationship between supervising physician and PA, says Kislingbury, is a collaborative one. Duties are “defined on an individual basis, and they are determined based on our [PAs’] experience, the physicians’ experience with us, and then the nuances of the system and the hospital itself.
“The PA who is hired should know what his or her scope of practice is,” she continues. “By the time they have graduated and obtained their license, they should know what their state allows them to do.”
For instance, according to the American Academy of Physician Assistants, 48 of the 50 states, plus the District of Columbia and [the U.S. Territory of] Guam, authorize PAs to prescribe medications. In California, PA prescriptions are referred to as “written prescription transmittal orders.”
“For the most part,” says Genzink, “the supervising physician determines what the PA is capable of doing, within the guidelines of state law.”
Within Genzink’s hospital medicine group (with which he has been affiliated for five years) the physician and PA roles are very similar.
“We see the same type of patients in a team approach. For instance, it’s not uncommon for one of us to order a test early in the day, and then, when results come back, the other person may be discharging that patient or prescribing other treatments, if necessary,” he explains. “In general, the physicians take care of the more complicated patients, while PAs take care of more routine patients.”
Genzink’s group experience aligns with findings of a 1998 University of Pittsburgh School of Nursing Study, which evaluated provider roles and patient outcomes in an acute care setting.3 Compared with acute care nurse practitioners and PAs, residents in that study tended to care for patients who were older and sicker.
Genzink reports that in his group initial histories and physicals, as well as the consultations, are performed exclusively by the PAs and then the physician takes over for treatment. “Based on the acuity of the patient,” says Genzink, “the physician may be right down there to see the patient immediately.”
Areas for Improvement?
Although the two PAs interviewed for this article report positive experiences working with hospitalists, they admit that some physicians continue to hold misperceptions about the PA’s role in caring for patients.
Kislingbury says that hospitalists could improve their delegation of duties to the PAs and recognize their scope of practice. She admits that delegation duties can be improved through gaining experience. “Although the PA profession has been around for a while, there are a surprising number of institutions that do not utilize physician assistants on the wards in routine rounds and bedside-type care,” says Kislingbury.
“I think some of the problems develop when they [physicians] hire a PA and expect to get a physician—and they [don’t],” says Genzink. “The easiest way for me to explain the role is to compare it to a teaching model. All physicians have been through residency programs. They understand the hierarchy that involves training and teaching residents. PAs come out of school ‘green,’ with the assumption that training will go on at the workplace. So, if a physician takes the same stance toward a new PA as they would toward an intern, that is a pretty close comparison.
“You begin by letting PAs or interns do a few simple things, and as they master those, you teach them more,” he continues. “And then, hopefully, over time they’ve been able to master everything that the physician is able to master. [Employing a PA] is a significant investment. And, it takes time. Sometimes, that process can be very easy, depending on the person. Sometimes it can be very slow, and I think that’s sometimes where some of the frustration may come in.”
Genzink adds that in his hospital medicine group, the physicians are familiar with the idea that part of their job as supervising physicians is to train new PAs.
Kislingbury points that out that PAs can also play a role in informing the physician team members about the range of cases they are allowed to treat, thus furthering the collaboration between PAs and hospitalists: “It is merely a matter of educating the team members about what we can and cannot do.”
Accordingly, the SHM Non-Physician Provider Task Force was formed to provide a resource to hospitalists who work with PAs and have questions about scope of practice, reimbursement, and other issues as they pertain to PAs and nurse practitioners. (Visit www.hospitalmedicine.org for more information.) The Task Force is a resource for non-physicians providers, too, offering educational opportunities at SHM meetings, more visibility with the specialty, and a voice for advocacy.
The Positives of the Collaboration
While the PAs report that hospitalists could improve in communicating about their practice roles with PAs, “There are so many things that hospitalists do right!” says Kislingbury. Calling the experience of working with hospitalists a privilege, she says that “where it is a true partnership, we are treated as equals, we are given the responsibility that our experience will allow, and we are truly team members.
“Hospitalists are geared into the efficiencies of the system and the nuances of the hospital. These are subtleties that come with practicing in an area for a long period of time, not just coming in for a month and then leaving and returning,” she says. “Hospitalists know the daily ins and outs, and it is really a pleasure to learn from them.”
Prior to his affiliation with Hospitalists of West Michigan, Genzink was employed directly by a hospital in Grand Rapids. The physicians with whom he now works have been hospitalists almost exclusively throughout their medical careers. “One of the main benefits they offer is availability, simply because we [the group’s practice members] are in the hospital 24/7,” he says. “They also have more experience in dealing with more complex issues, just as do the PAs that are working in our system.”
What about the notion that PAs and nurse practitioners are more skilled or practiced with patient and family communications? One study by Rudy, et al. found that nurse practitioners and PAs were more likely than residents to discuss patients with bedside nurses and to interact with patients’ families.3 Genzink does not find this to be the case in his group’s practice.
“That presumption [that PAs are more communicative with families and patients] may have come about simply because as the demands on hospitalists continue to grow and the workload increases, adding the PA to the team means there are more people to do things like that [handle family communications],” he says. “Certainly, in our group, the PAs do lots of patient education, and we talk to patients about end-of-life issues and other difficult matters as well. But that is not delegated to them; in our group, both the PAs and the physicians participate equally in patient and family communication.”
Daily Learning
Hospitalists and PAs also complement each other in the interdisciplinary care team because, “as a general rule, hospitalists love to teach,” says Kislingbury. “They don’t forget that just because you have your PA degree your learning does not stop there. The PA profession is almost like on-the-job training. You are allowed to choose the specialty that you want, and you gain your experience when you enter that [arena], as opposed to an internship or residency, where you first gain experience and then enter the specialty. We so appreciate the ability of the hospitalist to teach because we are learning while doing, on a day-to-day basis. It’s invaluable to have their teaching.” TH
Gretchen Henkel also writes about dealing with difficult families in this issue.
Resources
- Christmas AB, Reynolds J, Hodges S, et al. Physician extenders impact trauma systems. J Trauma. 2005 May;58(5):917-920.
- Nineteenth Annual Report on Physician Assistant Educational Programs in the United States, 2002-2203. Alexandria, Va. Association of Physician Assistant Programs.
- Rudy EB, Davidson LJ, Daly B, et al. Care activities and outcomes of patients cared for by acute care nurse practitioners, physician assistants, and resident physicians: a comparison. Am J Crit Care. 1998 Jul;7(4):267-281.
Editors’ note: “Alliances” is a series written about the relationships that hospitalists have with members of the clinical care team—from the team members’ points of view. Each installment of “Alliances” provides valuable, revealing feedback that hospitalists can use to continually improve their intrateam relationships and, ultimately, patient care.
Several months ago, a patient with decompensated end-stage liver disease was admitted to the Internal Medicine Hospitalist Service at the University of Texas Medical Branch in Galveston and required a paracentesis. One of the new hospitalist faculty members was taken aback when the physician assistant (PA) on the service volunteered to do the procedure. “He was surprised,” says Karen Kislingbury, PA-C, a member of SHM’s Non-Physician Provider Task Force and a PA with the Internal Medicine Hospitalist Service, “that the scope of practice for the physician assistant included [performing] procedures.”
PAs are not new to the hospital setting, and their inclusion as physician extenders to increase patient access to care will likely increase in the current regulatory environment—especially state-mandated staff/patient ratios and resident work hour limitations. The efficacy of utilizing physician extenders to improve patient care and outcomes has been validated in studies over the past two decades. A recent Journal of Trauma study found statistically significant reductions in floor, ICU, and overall hospital lengths of stay after incorporating physician extenders into their trauma service.1
However, hospitalists unfamiliar with PAs may not understand their colleagues’ roles and scope of practice. As her anecdote illustrated, Kislingbury notes that “although PAs aren’t new to the healthcare delivery system, and physicians have been utilizing us for a long time, our partnership in the unique setting of hospital medicine is kind of new.”
Kislingbury’s colleague Ryan Genzink, PA-C, who works with Hospitalists of West Michigan, a private hospitalists-only group that subcontracts hospitalist services to Spectrum Health of Grand Rapids, Mich., agrees with her assessment.
“There are more and more PAs and [nurse practitioners] working in hospital medicine, and I think there is a lot of curiosity and some apprehension on the part of people who have not worked with these non-physician providers,” says Genzink.
Genzink, also a member of SHM’s Non-Physician Provider Task Force, speculates that the apprehension of physicians who have not worked with PAs may be due to a misunderstanding of the PA’s role. “They’re either underestimating or overestimating exactly what a PA can do or what they are getting when they hire a PA,” he says.
A Short History of the Profession
PA programs officially began in the mid-1960s at Duke University Medical School (Durham, N.C.). Eugene Stead, MD, is credited with developing the concept of the physician assistant as a health professional who would work with physician supervision to extend patient access to care, according to the American Academy of Physician Assistants.
For the first class of PAs in 1965, Dr. Stead selected Navy corpsmen who had received medical training and experience during their service in Vietnam. The curriculum was based on Dr. Stead’s knowledge of fast-track training of physicians during World War II. From this early program, the profession has evolved to more than 130 programs that now adhere to rigorous national accreditation standards set forth by the independent Accreditation Review Commission on Education for the Physician Assistant (ARC-PA). The ARC-PA is sponsored by the American Medical Association and the American College of Surgeons, among many other professional medical organizations (www.aapa.org/geninfo1.html).
Scope of Practice
Prerequisites to PA programs include two years of college courses in basic and behavioral science, as well as prior experience in healthcare. According to a report generated by the Association of Physician Assistant Programs, most PA students have earned a bachelor’s degree and have an average of 38 months of healthcare experience before being admitted to a PA program.2
The first year of PA education comprises a didactic curriculum with coursework in anatomy, physiology, biochemistry, pharmacology, physical diagnosis, pathophysiology, microbiology, clinical laboratory sciences, behavioral sciences, and medical ethics. In the second year, students receive hands-on clinical training through a series of rotations—typically in family and internal medicine, obstetrics and gynecology, pediatrics, general surgery, emergency medicine, and psychiatry. By the time they graduate (typical PA programs last an average of 26 months), PAs will have completed more than 2,000 hours of supervised clinical practice.
PAs work in all areas of medicine. Although hospital bylaws and state regulations often stipulate the PA’s scope of practice, the major determinant of duties is the supervising physician. The relationship between supervising physician and PA, says Kislingbury, is a collaborative one. Duties are “defined on an individual basis, and they are determined based on our [PAs’] experience, the physicians’ experience with us, and then the nuances of the system and the hospital itself.
“The PA who is hired should know what his or her scope of practice is,” she continues. “By the time they have graduated and obtained their license, they should know what their state allows them to do.”
For instance, according to the American Academy of Physician Assistants, 48 of the 50 states, plus the District of Columbia and [the U.S. Territory of] Guam, authorize PAs to prescribe medications. In California, PA prescriptions are referred to as “written prescription transmittal orders.”
“For the most part,” says Genzink, “the supervising physician determines what the PA is capable of doing, within the guidelines of state law.”
Within Genzink’s hospital medicine group (with which he has been affiliated for five years) the physician and PA roles are very similar.
“We see the same type of patients in a team approach. For instance, it’s not uncommon for one of us to order a test early in the day, and then, when results come back, the other person may be discharging that patient or prescribing other treatments, if necessary,” he explains. “In general, the physicians take care of the more complicated patients, while PAs take care of more routine patients.”
Genzink’s group experience aligns with findings of a 1998 University of Pittsburgh School of Nursing Study, which evaluated provider roles and patient outcomes in an acute care setting.3 Compared with acute care nurse practitioners and PAs, residents in that study tended to care for patients who were older and sicker.
Genzink reports that in his group initial histories and physicals, as well as the consultations, are performed exclusively by the PAs and then the physician takes over for treatment. “Based on the acuity of the patient,” says Genzink, “the physician may be right down there to see the patient immediately.”
Areas for Improvement?
Although the two PAs interviewed for this article report positive experiences working with hospitalists, they admit that some physicians continue to hold misperceptions about the PA’s role in caring for patients.
Kislingbury says that hospitalists could improve their delegation of duties to the PAs and recognize their scope of practice. She admits that delegation duties can be improved through gaining experience. “Although the PA profession has been around for a while, there are a surprising number of institutions that do not utilize physician assistants on the wards in routine rounds and bedside-type care,” says Kislingbury.
“I think some of the problems develop when they [physicians] hire a PA and expect to get a physician—and they [don’t],” says Genzink. “The easiest way for me to explain the role is to compare it to a teaching model. All physicians have been through residency programs. They understand the hierarchy that involves training and teaching residents. PAs come out of school ‘green,’ with the assumption that training will go on at the workplace. So, if a physician takes the same stance toward a new PA as they would toward an intern, that is a pretty close comparison.
“You begin by letting PAs or interns do a few simple things, and as they master those, you teach them more,” he continues. “And then, hopefully, over time they’ve been able to master everything that the physician is able to master. [Employing a PA] is a significant investment. And, it takes time. Sometimes, that process can be very easy, depending on the person. Sometimes it can be very slow, and I think that’s sometimes where some of the frustration may come in.”
Genzink adds that in his hospital medicine group, the physicians are familiar with the idea that part of their job as supervising physicians is to train new PAs.
Kislingbury points that out that PAs can also play a role in informing the physician team members about the range of cases they are allowed to treat, thus furthering the collaboration between PAs and hospitalists: “It is merely a matter of educating the team members about what we can and cannot do.”
Accordingly, the SHM Non-Physician Provider Task Force was formed to provide a resource to hospitalists who work with PAs and have questions about scope of practice, reimbursement, and other issues as they pertain to PAs and nurse practitioners. (Visit www.hospitalmedicine.org for more information.) The Task Force is a resource for non-physicians providers, too, offering educational opportunities at SHM meetings, more visibility with the specialty, and a voice for advocacy.
The Positives of the Collaboration
While the PAs report that hospitalists could improve in communicating about their practice roles with PAs, “There are so many things that hospitalists do right!” says Kislingbury. Calling the experience of working with hospitalists a privilege, she says that “where it is a true partnership, we are treated as equals, we are given the responsibility that our experience will allow, and we are truly team members.
“Hospitalists are geared into the efficiencies of the system and the nuances of the hospital. These are subtleties that come with practicing in an area for a long period of time, not just coming in for a month and then leaving and returning,” she says. “Hospitalists know the daily ins and outs, and it is really a pleasure to learn from them.”
Prior to his affiliation with Hospitalists of West Michigan, Genzink was employed directly by a hospital in Grand Rapids. The physicians with whom he now works have been hospitalists almost exclusively throughout their medical careers. “One of the main benefits they offer is availability, simply because we [the group’s practice members] are in the hospital 24/7,” he says. “They also have more experience in dealing with more complex issues, just as do the PAs that are working in our system.”
What about the notion that PAs and nurse practitioners are more skilled or practiced with patient and family communications? One study by Rudy, et al. found that nurse practitioners and PAs were more likely than residents to discuss patients with bedside nurses and to interact with patients’ families.3 Genzink does not find this to be the case in his group’s practice.
“That presumption [that PAs are more communicative with families and patients] may have come about simply because as the demands on hospitalists continue to grow and the workload increases, adding the PA to the team means there are more people to do things like that [handle family communications],” he says. “Certainly, in our group, the PAs do lots of patient education, and we talk to patients about end-of-life issues and other difficult matters as well. But that is not delegated to them; in our group, both the PAs and the physicians participate equally in patient and family communication.”
Daily Learning
Hospitalists and PAs also complement each other in the interdisciplinary care team because, “as a general rule, hospitalists love to teach,” says Kislingbury. “They don’t forget that just because you have your PA degree your learning does not stop there. The PA profession is almost like on-the-job training. You are allowed to choose the specialty that you want, and you gain your experience when you enter that [arena], as opposed to an internship or residency, where you first gain experience and then enter the specialty. We so appreciate the ability of the hospitalist to teach because we are learning while doing, on a day-to-day basis. It’s invaluable to have their teaching.” TH
Gretchen Henkel also writes about dealing with difficult families in this issue.
Resources
- Christmas AB, Reynolds J, Hodges S, et al. Physician extenders impact trauma systems. J Trauma. 2005 May;58(5):917-920.
- Nineteenth Annual Report on Physician Assistant Educational Programs in the United States, 2002-2203. Alexandria, Va. Association of Physician Assistant Programs.
- Rudy EB, Davidson LJ, Daly B, et al. Care activities and outcomes of patients cared for by acute care nurse practitioners, physician assistants, and resident physicians: a comparison. Am J Crit Care. 1998 Jul;7(4):267-281.
Editors’ note: “Alliances” is a series written about the relationships that hospitalists have with members of the clinical care team—from the team members’ points of view. Each installment of “Alliances” provides valuable, revealing feedback that hospitalists can use to continually improve their intrateam relationships and, ultimately, patient care.
Several months ago, a patient with decompensated end-stage liver disease was admitted to the Internal Medicine Hospitalist Service at the University of Texas Medical Branch in Galveston and required a paracentesis. One of the new hospitalist faculty members was taken aback when the physician assistant (PA) on the service volunteered to do the procedure. “He was surprised,” says Karen Kislingbury, PA-C, a member of SHM’s Non-Physician Provider Task Force and a PA with the Internal Medicine Hospitalist Service, “that the scope of practice for the physician assistant included [performing] procedures.”
PAs are not new to the hospital setting, and their inclusion as physician extenders to increase patient access to care will likely increase in the current regulatory environment—especially state-mandated staff/patient ratios and resident work hour limitations. The efficacy of utilizing physician extenders to improve patient care and outcomes has been validated in studies over the past two decades. A recent Journal of Trauma study found statistically significant reductions in floor, ICU, and overall hospital lengths of stay after incorporating physician extenders into their trauma service.1
However, hospitalists unfamiliar with PAs may not understand their colleagues’ roles and scope of practice. As her anecdote illustrated, Kislingbury notes that “although PAs aren’t new to the healthcare delivery system, and physicians have been utilizing us for a long time, our partnership in the unique setting of hospital medicine is kind of new.”
Kislingbury’s colleague Ryan Genzink, PA-C, who works with Hospitalists of West Michigan, a private hospitalists-only group that subcontracts hospitalist services to Spectrum Health of Grand Rapids, Mich., agrees with her assessment.
“There are more and more PAs and [nurse practitioners] working in hospital medicine, and I think there is a lot of curiosity and some apprehension on the part of people who have not worked with these non-physician providers,” says Genzink.
Genzink, also a member of SHM’s Non-Physician Provider Task Force, speculates that the apprehension of physicians who have not worked with PAs may be due to a misunderstanding of the PA’s role. “They’re either underestimating or overestimating exactly what a PA can do or what they are getting when they hire a PA,” he says.
A Short History of the Profession
PA programs officially began in the mid-1960s at Duke University Medical School (Durham, N.C.). Eugene Stead, MD, is credited with developing the concept of the physician assistant as a health professional who would work with physician supervision to extend patient access to care, according to the American Academy of Physician Assistants.
For the first class of PAs in 1965, Dr. Stead selected Navy corpsmen who had received medical training and experience during their service in Vietnam. The curriculum was based on Dr. Stead’s knowledge of fast-track training of physicians during World War II. From this early program, the profession has evolved to more than 130 programs that now adhere to rigorous national accreditation standards set forth by the independent Accreditation Review Commission on Education for the Physician Assistant (ARC-PA). The ARC-PA is sponsored by the American Medical Association and the American College of Surgeons, among many other professional medical organizations (www.aapa.org/geninfo1.html).
Scope of Practice
Prerequisites to PA programs include two years of college courses in basic and behavioral science, as well as prior experience in healthcare. According to a report generated by the Association of Physician Assistant Programs, most PA students have earned a bachelor’s degree and have an average of 38 months of healthcare experience before being admitted to a PA program.2
The first year of PA education comprises a didactic curriculum with coursework in anatomy, physiology, biochemistry, pharmacology, physical diagnosis, pathophysiology, microbiology, clinical laboratory sciences, behavioral sciences, and medical ethics. In the second year, students receive hands-on clinical training through a series of rotations—typically in family and internal medicine, obstetrics and gynecology, pediatrics, general surgery, emergency medicine, and psychiatry. By the time they graduate (typical PA programs last an average of 26 months), PAs will have completed more than 2,000 hours of supervised clinical practice.
PAs work in all areas of medicine. Although hospital bylaws and state regulations often stipulate the PA’s scope of practice, the major determinant of duties is the supervising physician. The relationship between supervising physician and PA, says Kislingbury, is a collaborative one. Duties are “defined on an individual basis, and they are determined based on our [PAs’] experience, the physicians’ experience with us, and then the nuances of the system and the hospital itself.
“The PA who is hired should know what his or her scope of practice is,” she continues. “By the time they have graduated and obtained their license, they should know what their state allows them to do.”
For instance, according to the American Academy of Physician Assistants, 48 of the 50 states, plus the District of Columbia and [the U.S. Territory of] Guam, authorize PAs to prescribe medications. In California, PA prescriptions are referred to as “written prescription transmittal orders.”
“For the most part,” says Genzink, “the supervising physician determines what the PA is capable of doing, within the guidelines of state law.”
Within Genzink’s hospital medicine group (with which he has been affiliated for five years) the physician and PA roles are very similar.
“We see the same type of patients in a team approach. For instance, it’s not uncommon for one of us to order a test early in the day, and then, when results come back, the other person may be discharging that patient or prescribing other treatments, if necessary,” he explains. “In general, the physicians take care of the more complicated patients, while PAs take care of more routine patients.”
Genzink’s group experience aligns with findings of a 1998 University of Pittsburgh School of Nursing Study, which evaluated provider roles and patient outcomes in an acute care setting.3 Compared with acute care nurse practitioners and PAs, residents in that study tended to care for patients who were older and sicker.
Genzink reports that in his group initial histories and physicals, as well as the consultations, are performed exclusively by the PAs and then the physician takes over for treatment. “Based on the acuity of the patient,” says Genzink, “the physician may be right down there to see the patient immediately.”
Areas for Improvement?
Although the two PAs interviewed for this article report positive experiences working with hospitalists, they admit that some physicians continue to hold misperceptions about the PA’s role in caring for patients.
Kislingbury says that hospitalists could improve their delegation of duties to the PAs and recognize their scope of practice. She admits that delegation duties can be improved through gaining experience. “Although the PA profession has been around for a while, there are a surprising number of institutions that do not utilize physician assistants on the wards in routine rounds and bedside-type care,” says Kislingbury.
“I think some of the problems develop when they [physicians] hire a PA and expect to get a physician—and they [don’t],” says Genzink. “The easiest way for me to explain the role is to compare it to a teaching model. All physicians have been through residency programs. They understand the hierarchy that involves training and teaching residents. PAs come out of school ‘green,’ with the assumption that training will go on at the workplace. So, if a physician takes the same stance toward a new PA as they would toward an intern, that is a pretty close comparison.
“You begin by letting PAs or interns do a few simple things, and as they master those, you teach them more,” he continues. “And then, hopefully, over time they’ve been able to master everything that the physician is able to master. [Employing a PA] is a significant investment. And, it takes time. Sometimes, that process can be very easy, depending on the person. Sometimes it can be very slow, and I think that’s sometimes where some of the frustration may come in.”
Genzink adds that in his hospital medicine group, the physicians are familiar with the idea that part of their job as supervising physicians is to train new PAs.
Kislingbury points that out that PAs can also play a role in informing the physician team members about the range of cases they are allowed to treat, thus furthering the collaboration between PAs and hospitalists: “It is merely a matter of educating the team members about what we can and cannot do.”
Accordingly, the SHM Non-Physician Provider Task Force was formed to provide a resource to hospitalists who work with PAs and have questions about scope of practice, reimbursement, and other issues as they pertain to PAs and nurse practitioners. (Visit www.hospitalmedicine.org for more information.) The Task Force is a resource for non-physicians providers, too, offering educational opportunities at SHM meetings, more visibility with the specialty, and a voice for advocacy.
The Positives of the Collaboration
While the PAs report that hospitalists could improve in communicating about their practice roles with PAs, “There are so many things that hospitalists do right!” says Kislingbury. Calling the experience of working with hospitalists a privilege, she says that “where it is a true partnership, we are treated as equals, we are given the responsibility that our experience will allow, and we are truly team members.
“Hospitalists are geared into the efficiencies of the system and the nuances of the hospital. These are subtleties that come with practicing in an area for a long period of time, not just coming in for a month and then leaving and returning,” she says. “Hospitalists know the daily ins and outs, and it is really a pleasure to learn from them.”
Prior to his affiliation with Hospitalists of West Michigan, Genzink was employed directly by a hospital in Grand Rapids. The physicians with whom he now works have been hospitalists almost exclusively throughout their medical careers. “One of the main benefits they offer is availability, simply because we [the group’s practice members] are in the hospital 24/7,” he says. “They also have more experience in dealing with more complex issues, just as do the PAs that are working in our system.”
What about the notion that PAs and nurse practitioners are more skilled or practiced with patient and family communications? One study by Rudy, et al. found that nurse practitioners and PAs were more likely than residents to discuss patients with bedside nurses and to interact with patients’ families.3 Genzink does not find this to be the case in his group’s practice.
“That presumption [that PAs are more communicative with families and patients] may have come about simply because as the demands on hospitalists continue to grow and the workload increases, adding the PA to the team means there are more people to do things like that [handle family communications],” he says. “Certainly, in our group, the PAs do lots of patient education, and we talk to patients about end-of-life issues and other difficult matters as well. But that is not delegated to them; in our group, both the PAs and the physicians participate equally in patient and family communication.”
Daily Learning
Hospitalists and PAs also complement each other in the interdisciplinary care team because, “as a general rule, hospitalists love to teach,” says Kislingbury. “They don’t forget that just because you have your PA degree your learning does not stop there. The PA profession is almost like on-the-job training. You are allowed to choose the specialty that you want, and you gain your experience when you enter that [arena], as opposed to an internship or residency, where you first gain experience and then enter the specialty. We so appreciate the ability of the hospitalist to teach because we are learning while doing, on a day-to-day basis. It’s invaluable to have their teaching.” TH
Gretchen Henkel also writes about dealing with difficult families in this issue.
Resources
- Christmas AB, Reynolds J, Hodges S, et al. Physician extenders impact trauma systems. J Trauma. 2005 May;58(5):917-920.
- Nineteenth Annual Report on Physician Assistant Educational Programs in the United States, 2002-2203. Alexandria, Va. Association of Physician Assistant Programs.
- Rudy EB, Davidson LJ, Daly B, et al. Care activities and outcomes of patients cared for by acute care nurse practitioners, physician assistants, and resident physicians: a comparison. Am J Crit Care. 1998 Jul;7(4):267-281.
Medical Errors, Appropriate Dress for Physicians, Blood Cultures for Pneumonia Pts, and More
Rothschild JM, Landrigan CP, Cronin JW, et al. The critical care safety study: the incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33:1694-1700.
Background: Critically ill patients require complex, immediate, high-intensity care, potentially placing them at increased risk of iatrogenic injury. The frequency and nature of adverse events and errors in the modern ICU have not been clearly defined.
Methods: Harvard researchers conducted a prospective, one-year, observational study of a MICU and a CCU at a tertiary care medical center. Adverse events and medical errors were identified by a four-pronged approach: direct 24-hour observation of interns, voluntary incident reporting, a computerized adverse drug event monitoring system, and chart abstraction. Two physicians independently assessed the type, severity, and preventability of the incidents.
Results: A total of 391 patients comprising 1,490 patient-days were observed and included. Twenty percent of all patients suffered an adverse event, 45% of which were preventable and 13% of which were felt to be life-threatening. There were 223 serious errors (those that caused harm or had the potential to cause harm) observed of which 11% were life threatening. Medication adverse events and medication errors accounted for a large proportion of the incidents during the study. Slips and lapses in care were much more common than rule-based (such as using the wrong protocol) or knowledge-based mistakes.
Discussion: Since the Institute of Medicine report in 1999, there has been an increasing focus on patient safety in the inpatient setting. Based on the results of this study and others, it appears the high-intensity, fast-paced nature of critical care places patients at substantial risk for iatrogenic injury. Up to 20% of patients admitted to the ICU in this study suffered an adverse event or a medical error, which translates into 0.8 adverse events and 1.5 serious medical errors per day in a 10-bed ICU.
Because failure to carry out intended plans (usually secondary to slips and lapses on the part of healthcare providers) was the most common cause of adverse events and errors, the authors address possible solutions. They propose employing computerized-order entry, clinical pharmacists in the ICU, closed ICU staffing, “smart” intravenous pumps, and improved teamwork and communication among healthcare providers. Hospitalists often manage critically ill patients and should be aware of the high risk of medical errors and should consider implementing specific systems changes to mitigate the risk.
The Value of Obtaining Blood Cultures in Pneumonia Pts
Kennedy M, Bates DW, Wright SB, et al. Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005 Nov;46(5):393-400.
Background: Previous observational studies in patients hospitalized with community-acquired pneumonia (CAP) have shown obtaining blood cultures may have a mortality benefit. This practice has become expert guideline-recommended, the standard of care, as well as a quality marker in the management of CAP. Several recent studies have questioned the utility and cost-effectiveness of this practice.
Methods: Harvard researchers performed a prospective, observational, cohort study of adults admitted to an urban university medical center. Researchers identified patients who had all of the following: clinical CAP, radiographic CAP, and blood cultures at admission. Blood cultures were classified as positive, negative, or contaminated based on previously established criteria. Data were collected on antimicrobial sensitivities, empiric antibiotic choices, and antibiotic changes.
Results: In one year, 414 patients with clinical and radiographic CAP had blood cultures at the time of admission. Twenty-nine of 414 (7%) of patients had true bacteremia while 25 of 414 (6%) had contaminants. Antibiotic therapy was altered in response to blood culture results in 15 of 414 patients (3.6%), of which 11 (2.7%) had therapy narrowed and four (1.0%) had therapy broadened. Of the 11 patients with bacteremia whose therapy was not changed, culture results supported narrowing therapy in eight cases but this was not done.
Discussion: This well done prospective observational study adds to a growing body of evidence questioning the utility of routine blood cultures on all patients hospitalized with CAP. The argument traditionally has been made that blood cultures allow clinicians to narrow or broaden antibiotics based on sensitivities. Yet, empiric therapy was broadened in response to bacteremia in only a small fraction of patients (1%) and in only 11 of 19 patients was therapy appropriately narrowed based on the blood cultures. The study did not measure the impact of blood cultures on clinical outcomes, but these striking results reveal that routine blood cultures rarely alter our management of hospitalized patients with CAP.
Further, many have argued obtaining routine blood cultures in CAP can have negative consequences. Blood cultures are relatively costly and time intensive, contaminated blood cultures can lead to repeated testing and increased length of stay, and delays in obtaining blood cultures can delay antibiotic administration, another important quality marker in CAP. For now, it remains the standard of care to obtain blood cultures in these patients, but hospitalists should be aware of the limitations of this practice and consider focusing on other clinical interventions and quality measures in CAP.
A Review Study: A Dyspneic Emergency Patient
Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005 Oct 19;294:944-1956.
Background: Distinguishing CHF from non-cardiac causes of dyspnea is a major challenge for hospitalists and emergency physicians, particularly in patients with a prior history of cardiac disease. Traditionally, clinicians have relied on the history, physical examination, and basic tests (chest X-ray and electrocardiogram) to diagnose CHF, but rapid B-type natriuretic peptide (BNP) testing is now widely incorporated as well.
A previous article in the Rational Clinical Examination series (Can the clinical examination diagnose left-sided heart failure in adults? JAMA. 1997;277(21):1712-1719) found that systolic dysfunction was moderately well predicted by an abnormal apical impulse on physical examination, radiographic cardiomegaly or venous redistribution, or electrocardiographic q waves or left bundle branch block.
Methods: In this review, the authors update and extend previous findings by also assessing the utility of serum BNP testing. The authors identified articles evaluating the diagnostic accuracy of the clinical exam and laboratory testing in diagnosing CHF in patients presenting to the emergency department with undifferentiated dyspnea. The “gold standard” was a clinical diagnosis of CHF made by the treating clinicians after an appropriate diagnostic workup. Summary likelihood ratios (LRs) were calculated using meta-analytic methodology.
Results/discussion: The authors determined that several findings increase the probability of CHF. A prior history of CHF (LR 5.8, CI 4.1-8.0) or myocardial infarction (LR 3.1, 95% CI 2.0-4.9), symptoms of paroxysmal nocturnal dyspnea (LR 2.6, 95% CI 1.5-4.5) and orthopnea (LR 2.2, 95% CI 1.2-3.9) were the most predictive historical factors. On physical examination, the presence of an S3 (LR 11, 95% CI 4.9-25), jugular venous distension (5.1, 95% CI 3.2-7.9), lung rales (LR 2.8, 95% CI 1.9-4.1), and peripheral edema (2.3, 95% CI 1.5-3.7) increased the probability of CHF. In interpreting these results, it is helpful to remember that a likelihood ratio of 2 increases the post-test probability by about 15%, and an LR of 5 increases the post-test probability by about 30%. Thus, a prior history of CHF and presence of an S3 or jugular venous distension are the most useful findings. Interestingly, clinician’s gestalt was equally predictive (LR 4.4, 95% CI 1.8-10.0.)
The most useful radiographic findings were venous congestion (LR 12.0, 95% CI 6.8-21) and the presence of cardiomegaly (LR 3.3; 95% CI 2.4-4.7). The single most predictive ECG finding was atrial fibrillation (LR 3.8; 95% CI 2.7-8.8); any abnormality on ECG had an LR of 2.2 (95% CI 1.6-3.1). Serum BNP levels were not more predictive of CHF than the history or physical examination; a BNP of >250 was associated with an LR of 4.6 (95% CI 2.6-8.0).
Few findings markedly decreased the probability of CHF. Here, it is helpful to remember that an LR of 0.5 decreases the post-test probability by about 15%, and an LR of 0.2 decreases the post-test probability by about 30%. With these in mind, the absence of cardiomegaly on CXR significantly changes the post-test probability (LR 0.33; 95% CI 0.23-0.48). A serum BNP level of less than 100pg/ml strongly argues against CHF, with an LR of 0.11 (95% CI 0.07-0.16); this finding lowers the post-test probability of CHF by about 45% compared to the pre-test probability.
In summary, the most useful findings for ruling in CHF in dyspneic emergency department patients were clinical gestalt, a prior history of CHF, findings of an S3 or jugular venous distension, and radiographic findings of venous congestion or cardiomegaly. Absence of radiographic cardiomegaly and a BNP of less than 100pg/ml argue against CHF. These must be interpreted in the context of the clinical pre-test probability of CHF, as none of the findings had likelihood ratios sufficient to be diagnostic of CHF when used individually.
What Should I Wear Today?
Rehman SU, Nietert PJ, Cope DW, Kilpatrick AO. What to wear today? Effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005 Nov; 118(11): 1279-1286.
Background: This study addresses the prototypical everyday clinical dilemma: What should I wear to work?
Methods: Patients and visitors to an outpatient Veterans Affairs internal medicine clinic in South Carolina were shown photographs of male and female physicians in four different styles of dress:
- Professional (male physician wearing white coat with tie, female physician wearing white coat with tailored skirt or trousers);
- Business (suit and tie for male, tailored trouser or skirt for female);
- Surgical (surgical scrubs for both male and female): and
- Casual (jeans and t-shirt or short skirt).
The study was randomized so that male and female respondents viewed photographs of either male or female physicians. Respondents were asked to report how strongly they felt about the importance of their physician’s appearance, and their preference for each style of dress; specifically, respondents were asked which physician was the most trustworthy, which physician they felt most comfortable with for routine examinations and emergencies, and which physician they felt most comfortable discussing psychological, sexual, and social problems with.
Results: Respondents overwhelmingly preferred professional attire for all questions: 76.3% felt most comfortable with a professionally dressed physician for all encounters, with surgical scrubs a distant second (10.2%), ahead of business dress (8.8%). Respondents were also significantly more willing to discuss psychological, sexual, and social problems with a professionally dressed physician. Even for care in an emergency situation, respondents still expressed a significant preference for professional attire over scrubs.
In a logistic regression model, patients who were older, African-American, and had less than a high school education were significantly more likely to prefer professional attire. Interestingly, female respondents who viewed photographs of female physicians placed significantly greater emphasis on physician’s attire than did male respondents.
Discussion: The study is clearly subject to caveats, chiefly that it was conducted at a single VA clinic and that only one aspect of the physician-patient encounter was addressed. Undoubtedly, patient’s preferences were influenced by the popular portrayal of physicians on TV shows. Nevertheless, given that hospitalists typically see older patients with whom they are not familiar, the initial clinical encounter may indeed by influenced by something as simple as wearing a white coat.
UA by Nephrologist Versus Hospital-Based Clinical Labs
Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis. 2005 Nov;46(5):820-829.
Background: Distinguishing the correct cause of acute renal failure is a frequent clinical dilemma for hospitalists, particularly diagnosing acute tubular necrosis (ATN), which is the most common cause of in-hospital acute renal failure. Although urinalysis with microscopy is the first test ordered on noting an abnormal serum creatinine, most hospitalists rely on the results generated by a laboratory technician. Anecdotally, many nephrologists have noted significant differences between urinalysis results performed by technicians and results found by nephrologists.
Methods: This study enrolled 26 patients hospitalized with acute renal failure on whom nephrology consultation was obtained. Urinalysis was performed both by laboratory personnel and a nephrologist (nephrologist A) who was blinded to the patient’s clinical information. Both sets of urinalysis results were independently used by nephrologist A and a second nephrologist (nephrologist B) to arrive at a clinical diagnosis for the patient, without having access to any other clinical information. These diagnoses were compared to the final diagnosis determined by the consulting nephrology service, who themselves did not have access to the diagnosis of either nephrologist A or B.
Results: The influence of having a nephrologist perform and interpret the urinalysis was striking. Nephrologist A was able to correctly diagnose 92.3% of cases based solely on his interpretation of the urinalysis. However, when given only the laboratory report of the urinalysis, both nephrologists were unable to diagnose most cases (23.1% for nephrologist A and 19.2% for nephrologist B). The major difference appeared to be in nephrologist A’s ability to find renal tubular epithelial (RTE) cells and RTE casts, which are pathognomonic of ATN. RTE cells and granular casts were frequently misinterpreted as squamous epithelial cells by laboratory personnel. This was particularly important as 81% of patients in the study had ATN as the primary cause of renal failure. Acanthocytes (dysmorphic red blood cells) were also missed by laboratory personnel in all six patients who were subsequently diagnosed with glomerulonephritis; nephrologist A correctly noted acanthocytes in five of these patients, and arrived at the correct diagnosis in all six patients.
Discussion: Microscopic evaluation of urine sediment has become a lost art among physicians, especially since passage of the Clinical Laboratory Improvement Amendments (CLIA) in 1988, which mandated that only CLIA-certified personnel could perform most laboratory tests. While it is probably unrealistic to call for training in microscopic urinalysis for all physicians, hospitalists in particular would benefit from such training, and at the very least should be mindful that laboratory urinalysis results may miss subtle findings that can be invaluable in diagnosing acute renal failure. This study points out the need for greater oversight and training of laboratory personnel, and serves as a reminder to clinicians that laboratory results should not be considered the gold standard. TH
Rothschild JM, Landrigan CP, Cronin JW, et al. The critical care safety study: the incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33:1694-1700.
Background: Critically ill patients require complex, immediate, high-intensity care, potentially placing them at increased risk of iatrogenic injury. The frequency and nature of adverse events and errors in the modern ICU have not been clearly defined.
Methods: Harvard researchers conducted a prospective, one-year, observational study of a MICU and a CCU at a tertiary care medical center. Adverse events and medical errors were identified by a four-pronged approach: direct 24-hour observation of interns, voluntary incident reporting, a computerized adverse drug event monitoring system, and chart abstraction. Two physicians independently assessed the type, severity, and preventability of the incidents.
Results: A total of 391 patients comprising 1,490 patient-days were observed and included. Twenty percent of all patients suffered an adverse event, 45% of which were preventable and 13% of which were felt to be life-threatening. There were 223 serious errors (those that caused harm or had the potential to cause harm) observed of which 11% were life threatening. Medication adverse events and medication errors accounted for a large proportion of the incidents during the study. Slips and lapses in care were much more common than rule-based (such as using the wrong protocol) or knowledge-based mistakes.
Discussion: Since the Institute of Medicine report in 1999, there has been an increasing focus on patient safety in the inpatient setting. Based on the results of this study and others, it appears the high-intensity, fast-paced nature of critical care places patients at substantial risk for iatrogenic injury. Up to 20% of patients admitted to the ICU in this study suffered an adverse event or a medical error, which translates into 0.8 adverse events and 1.5 serious medical errors per day in a 10-bed ICU.
Because failure to carry out intended plans (usually secondary to slips and lapses on the part of healthcare providers) was the most common cause of adverse events and errors, the authors address possible solutions. They propose employing computerized-order entry, clinical pharmacists in the ICU, closed ICU staffing, “smart” intravenous pumps, and improved teamwork and communication among healthcare providers. Hospitalists often manage critically ill patients and should be aware of the high risk of medical errors and should consider implementing specific systems changes to mitigate the risk.
The Value of Obtaining Blood Cultures in Pneumonia Pts
Kennedy M, Bates DW, Wright SB, et al. Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005 Nov;46(5):393-400.
Background: Previous observational studies in patients hospitalized with community-acquired pneumonia (CAP) have shown obtaining blood cultures may have a mortality benefit. This practice has become expert guideline-recommended, the standard of care, as well as a quality marker in the management of CAP. Several recent studies have questioned the utility and cost-effectiveness of this practice.
Methods: Harvard researchers performed a prospective, observational, cohort study of adults admitted to an urban university medical center. Researchers identified patients who had all of the following: clinical CAP, radiographic CAP, and blood cultures at admission. Blood cultures were classified as positive, negative, or contaminated based on previously established criteria. Data were collected on antimicrobial sensitivities, empiric antibiotic choices, and antibiotic changes.
Results: In one year, 414 patients with clinical and radiographic CAP had blood cultures at the time of admission. Twenty-nine of 414 (7%) of patients had true bacteremia while 25 of 414 (6%) had contaminants. Antibiotic therapy was altered in response to blood culture results in 15 of 414 patients (3.6%), of which 11 (2.7%) had therapy narrowed and four (1.0%) had therapy broadened. Of the 11 patients with bacteremia whose therapy was not changed, culture results supported narrowing therapy in eight cases but this was not done.
Discussion: This well done prospective observational study adds to a growing body of evidence questioning the utility of routine blood cultures on all patients hospitalized with CAP. The argument traditionally has been made that blood cultures allow clinicians to narrow or broaden antibiotics based on sensitivities. Yet, empiric therapy was broadened in response to bacteremia in only a small fraction of patients (1%) and in only 11 of 19 patients was therapy appropriately narrowed based on the blood cultures. The study did not measure the impact of blood cultures on clinical outcomes, but these striking results reveal that routine blood cultures rarely alter our management of hospitalized patients with CAP.
Further, many have argued obtaining routine blood cultures in CAP can have negative consequences. Blood cultures are relatively costly and time intensive, contaminated blood cultures can lead to repeated testing and increased length of stay, and delays in obtaining blood cultures can delay antibiotic administration, another important quality marker in CAP. For now, it remains the standard of care to obtain blood cultures in these patients, but hospitalists should be aware of the limitations of this practice and consider focusing on other clinical interventions and quality measures in CAP.
A Review Study: A Dyspneic Emergency Patient
Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005 Oct 19;294:944-1956.
Background: Distinguishing CHF from non-cardiac causes of dyspnea is a major challenge for hospitalists and emergency physicians, particularly in patients with a prior history of cardiac disease. Traditionally, clinicians have relied on the history, physical examination, and basic tests (chest X-ray and electrocardiogram) to diagnose CHF, but rapid B-type natriuretic peptide (BNP) testing is now widely incorporated as well.
A previous article in the Rational Clinical Examination series (Can the clinical examination diagnose left-sided heart failure in adults? JAMA. 1997;277(21):1712-1719) found that systolic dysfunction was moderately well predicted by an abnormal apical impulse on physical examination, radiographic cardiomegaly or venous redistribution, or electrocardiographic q waves or left bundle branch block.
Methods: In this review, the authors update and extend previous findings by also assessing the utility of serum BNP testing. The authors identified articles evaluating the diagnostic accuracy of the clinical exam and laboratory testing in diagnosing CHF in patients presenting to the emergency department with undifferentiated dyspnea. The “gold standard” was a clinical diagnosis of CHF made by the treating clinicians after an appropriate diagnostic workup. Summary likelihood ratios (LRs) were calculated using meta-analytic methodology.
Results/discussion: The authors determined that several findings increase the probability of CHF. A prior history of CHF (LR 5.8, CI 4.1-8.0) or myocardial infarction (LR 3.1, 95% CI 2.0-4.9), symptoms of paroxysmal nocturnal dyspnea (LR 2.6, 95% CI 1.5-4.5) and orthopnea (LR 2.2, 95% CI 1.2-3.9) were the most predictive historical factors. On physical examination, the presence of an S3 (LR 11, 95% CI 4.9-25), jugular venous distension (5.1, 95% CI 3.2-7.9), lung rales (LR 2.8, 95% CI 1.9-4.1), and peripheral edema (2.3, 95% CI 1.5-3.7) increased the probability of CHF. In interpreting these results, it is helpful to remember that a likelihood ratio of 2 increases the post-test probability by about 15%, and an LR of 5 increases the post-test probability by about 30%. Thus, a prior history of CHF and presence of an S3 or jugular venous distension are the most useful findings. Interestingly, clinician’s gestalt was equally predictive (LR 4.4, 95% CI 1.8-10.0.)
The most useful radiographic findings were venous congestion (LR 12.0, 95% CI 6.8-21) and the presence of cardiomegaly (LR 3.3; 95% CI 2.4-4.7). The single most predictive ECG finding was atrial fibrillation (LR 3.8; 95% CI 2.7-8.8); any abnormality on ECG had an LR of 2.2 (95% CI 1.6-3.1). Serum BNP levels were not more predictive of CHF than the history or physical examination; a BNP of >250 was associated with an LR of 4.6 (95% CI 2.6-8.0).
Few findings markedly decreased the probability of CHF. Here, it is helpful to remember that an LR of 0.5 decreases the post-test probability by about 15%, and an LR of 0.2 decreases the post-test probability by about 30%. With these in mind, the absence of cardiomegaly on CXR significantly changes the post-test probability (LR 0.33; 95% CI 0.23-0.48). A serum BNP level of less than 100pg/ml strongly argues against CHF, with an LR of 0.11 (95% CI 0.07-0.16); this finding lowers the post-test probability of CHF by about 45% compared to the pre-test probability.
In summary, the most useful findings for ruling in CHF in dyspneic emergency department patients were clinical gestalt, a prior history of CHF, findings of an S3 or jugular venous distension, and radiographic findings of venous congestion or cardiomegaly. Absence of radiographic cardiomegaly and a BNP of less than 100pg/ml argue against CHF. These must be interpreted in the context of the clinical pre-test probability of CHF, as none of the findings had likelihood ratios sufficient to be diagnostic of CHF when used individually.
What Should I Wear Today?
Rehman SU, Nietert PJ, Cope DW, Kilpatrick AO. What to wear today? Effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005 Nov; 118(11): 1279-1286.
Background: This study addresses the prototypical everyday clinical dilemma: What should I wear to work?
Methods: Patients and visitors to an outpatient Veterans Affairs internal medicine clinic in South Carolina were shown photographs of male and female physicians in four different styles of dress:
- Professional (male physician wearing white coat with tie, female physician wearing white coat with tailored skirt or trousers);
- Business (suit and tie for male, tailored trouser or skirt for female);
- Surgical (surgical scrubs for both male and female): and
- Casual (jeans and t-shirt or short skirt).
The study was randomized so that male and female respondents viewed photographs of either male or female physicians. Respondents were asked to report how strongly they felt about the importance of their physician’s appearance, and their preference for each style of dress; specifically, respondents were asked which physician was the most trustworthy, which physician they felt most comfortable with for routine examinations and emergencies, and which physician they felt most comfortable discussing psychological, sexual, and social problems with.
Results: Respondents overwhelmingly preferred professional attire for all questions: 76.3% felt most comfortable with a professionally dressed physician for all encounters, with surgical scrubs a distant second (10.2%), ahead of business dress (8.8%). Respondents were also significantly more willing to discuss psychological, sexual, and social problems with a professionally dressed physician. Even for care in an emergency situation, respondents still expressed a significant preference for professional attire over scrubs.
In a logistic regression model, patients who were older, African-American, and had less than a high school education were significantly more likely to prefer professional attire. Interestingly, female respondents who viewed photographs of female physicians placed significantly greater emphasis on physician’s attire than did male respondents.
Discussion: The study is clearly subject to caveats, chiefly that it was conducted at a single VA clinic and that only one aspect of the physician-patient encounter was addressed. Undoubtedly, patient’s preferences were influenced by the popular portrayal of physicians on TV shows. Nevertheless, given that hospitalists typically see older patients with whom they are not familiar, the initial clinical encounter may indeed by influenced by something as simple as wearing a white coat.
UA by Nephrologist Versus Hospital-Based Clinical Labs
Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis. 2005 Nov;46(5):820-829.
Background: Distinguishing the correct cause of acute renal failure is a frequent clinical dilemma for hospitalists, particularly diagnosing acute tubular necrosis (ATN), which is the most common cause of in-hospital acute renal failure. Although urinalysis with microscopy is the first test ordered on noting an abnormal serum creatinine, most hospitalists rely on the results generated by a laboratory technician. Anecdotally, many nephrologists have noted significant differences between urinalysis results performed by technicians and results found by nephrologists.
Methods: This study enrolled 26 patients hospitalized with acute renal failure on whom nephrology consultation was obtained. Urinalysis was performed both by laboratory personnel and a nephrologist (nephrologist A) who was blinded to the patient’s clinical information. Both sets of urinalysis results were independently used by nephrologist A and a second nephrologist (nephrologist B) to arrive at a clinical diagnosis for the patient, without having access to any other clinical information. These diagnoses were compared to the final diagnosis determined by the consulting nephrology service, who themselves did not have access to the diagnosis of either nephrologist A or B.
Results: The influence of having a nephrologist perform and interpret the urinalysis was striking. Nephrologist A was able to correctly diagnose 92.3% of cases based solely on his interpretation of the urinalysis. However, when given only the laboratory report of the urinalysis, both nephrologists were unable to diagnose most cases (23.1% for nephrologist A and 19.2% for nephrologist B). The major difference appeared to be in nephrologist A’s ability to find renal tubular epithelial (RTE) cells and RTE casts, which are pathognomonic of ATN. RTE cells and granular casts were frequently misinterpreted as squamous epithelial cells by laboratory personnel. This was particularly important as 81% of patients in the study had ATN as the primary cause of renal failure. Acanthocytes (dysmorphic red blood cells) were also missed by laboratory personnel in all six patients who were subsequently diagnosed with glomerulonephritis; nephrologist A correctly noted acanthocytes in five of these patients, and arrived at the correct diagnosis in all six patients.
Discussion: Microscopic evaluation of urine sediment has become a lost art among physicians, especially since passage of the Clinical Laboratory Improvement Amendments (CLIA) in 1988, which mandated that only CLIA-certified personnel could perform most laboratory tests. While it is probably unrealistic to call for training in microscopic urinalysis for all physicians, hospitalists in particular would benefit from such training, and at the very least should be mindful that laboratory urinalysis results may miss subtle findings that can be invaluable in diagnosing acute renal failure. This study points out the need for greater oversight and training of laboratory personnel, and serves as a reminder to clinicians that laboratory results should not be considered the gold standard. TH
Rothschild JM, Landrigan CP, Cronin JW, et al. The critical care safety study: the incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33:1694-1700.
Background: Critically ill patients require complex, immediate, high-intensity care, potentially placing them at increased risk of iatrogenic injury. The frequency and nature of adverse events and errors in the modern ICU have not been clearly defined.
Methods: Harvard researchers conducted a prospective, one-year, observational study of a MICU and a CCU at a tertiary care medical center. Adverse events and medical errors were identified by a four-pronged approach: direct 24-hour observation of interns, voluntary incident reporting, a computerized adverse drug event monitoring system, and chart abstraction. Two physicians independently assessed the type, severity, and preventability of the incidents.
Results: A total of 391 patients comprising 1,490 patient-days were observed and included. Twenty percent of all patients suffered an adverse event, 45% of which were preventable and 13% of which were felt to be life-threatening. There were 223 serious errors (those that caused harm or had the potential to cause harm) observed of which 11% were life threatening. Medication adverse events and medication errors accounted for a large proportion of the incidents during the study. Slips and lapses in care were much more common than rule-based (such as using the wrong protocol) or knowledge-based mistakes.
Discussion: Since the Institute of Medicine report in 1999, there has been an increasing focus on patient safety in the inpatient setting. Based on the results of this study and others, it appears the high-intensity, fast-paced nature of critical care places patients at substantial risk for iatrogenic injury. Up to 20% of patients admitted to the ICU in this study suffered an adverse event or a medical error, which translates into 0.8 adverse events and 1.5 serious medical errors per day in a 10-bed ICU.
Because failure to carry out intended plans (usually secondary to slips and lapses on the part of healthcare providers) was the most common cause of adverse events and errors, the authors address possible solutions. They propose employing computerized-order entry, clinical pharmacists in the ICU, closed ICU staffing, “smart” intravenous pumps, and improved teamwork and communication among healthcare providers. Hospitalists often manage critically ill patients and should be aware of the high risk of medical errors and should consider implementing specific systems changes to mitigate the risk.
The Value of Obtaining Blood Cultures in Pneumonia Pts
Kennedy M, Bates DW, Wright SB, et al. Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005 Nov;46(5):393-400.
Background: Previous observational studies in patients hospitalized with community-acquired pneumonia (CAP) have shown obtaining blood cultures may have a mortality benefit. This practice has become expert guideline-recommended, the standard of care, as well as a quality marker in the management of CAP. Several recent studies have questioned the utility and cost-effectiveness of this practice.
Methods: Harvard researchers performed a prospective, observational, cohort study of adults admitted to an urban university medical center. Researchers identified patients who had all of the following: clinical CAP, radiographic CAP, and blood cultures at admission. Blood cultures were classified as positive, negative, or contaminated based on previously established criteria. Data were collected on antimicrobial sensitivities, empiric antibiotic choices, and antibiotic changes.
Results: In one year, 414 patients with clinical and radiographic CAP had blood cultures at the time of admission. Twenty-nine of 414 (7%) of patients had true bacteremia while 25 of 414 (6%) had contaminants. Antibiotic therapy was altered in response to blood culture results in 15 of 414 patients (3.6%), of which 11 (2.7%) had therapy narrowed and four (1.0%) had therapy broadened. Of the 11 patients with bacteremia whose therapy was not changed, culture results supported narrowing therapy in eight cases but this was not done.
Discussion: This well done prospective observational study adds to a growing body of evidence questioning the utility of routine blood cultures on all patients hospitalized with CAP. The argument traditionally has been made that blood cultures allow clinicians to narrow or broaden antibiotics based on sensitivities. Yet, empiric therapy was broadened in response to bacteremia in only a small fraction of patients (1%) and in only 11 of 19 patients was therapy appropriately narrowed based on the blood cultures. The study did not measure the impact of blood cultures on clinical outcomes, but these striking results reveal that routine blood cultures rarely alter our management of hospitalized patients with CAP.
Further, many have argued obtaining routine blood cultures in CAP can have negative consequences. Blood cultures are relatively costly and time intensive, contaminated blood cultures can lead to repeated testing and increased length of stay, and delays in obtaining blood cultures can delay antibiotic administration, another important quality marker in CAP. For now, it remains the standard of care to obtain blood cultures in these patients, but hospitalists should be aware of the limitations of this practice and consider focusing on other clinical interventions and quality measures in CAP.
A Review Study: A Dyspneic Emergency Patient
Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005 Oct 19;294:944-1956.
Background: Distinguishing CHF from non-cardiac causes of dyspnea is a major challenge for hospitalists and emergency physicians, particularly in patients with a prior history of cardiac disease. Traditionally, clinicians have relied on the history, physical examination, and basic tests (chest X-ray and electrocardiogram) to diagnose CHF, but rapid B-type natriuretic peptide (BNP) testing is now widely incorporated as well.
A previous article in the Rational Clinical Examination series (Can the clinical examination diagnose left-sided heart failure in adults? JAMA. 1997;277(21):1712-1719) found that systolic dysfunction was moderately well predicted by an abnormal apical impulse on physical examination, radiographic cardiomegaly or venous redistribution, or electrocardiographic q waves or left bundle branch block.
Methods: In this review, the authors update and extend previous findings by also assessing the utility of serum BNP testing. The authors identified articles evaluating the diagnostic accuracy of the clinical exam and laboratory testing in diagnosing CHF in patients presenting to the emergency department with undifferentiated dyspnea. The “gold standard” was a clinical diagnosis of CHF made by the treating clinicians after an appropriate diagnostic workup. Summary likelihood ratios (LRs) were calculated using meta-analytic methodology.
Results/discussion: The authors determined that several findings increase the probability of CHF. A prior history of CHF (LR 5.8, CI 4.1-8.0) or myocardial infarction (LR 3.1, 95% CI 2.0-4.9), symptoms of paroxysmal nocturnal dyspnea (LR 2.6, 95% CI 1.5-4.5) and orthopnea (LR 2.2, 95% CI 1.2-3.9) were the most predictive historical factors. On physical examination, the presence of an S3 (LR 11, 95% CI 4.9-25), jugular venous distension (5.1, 95% CI 3.2-7.9), lung rales (LR 2.8, 95% CI 1.9-4.1), and peripheral edema (2.3, 95% CI 1.5-3.7) increased the probability of CHF. In interpreting these results, it is helpful to remember that a likelihood ratio of 2 increases the post-test probability by about 15%, and an LR of 5 increases the post-test probability by about 30%. Thus, a prior history of CHF and presence of an S3 or jugular venous distension are the most useful findings. Interestingly, clinician’s gestalt was equally predictive (LR 4.4, 95% CI 1.8-10.0.)
The most useful radiographic findings were venous congestion (LR 12.0, 95% CI 6.8-21) and the presence of cardiomegaly (LR 3.3; 95% CI 2.4-4.7). The single most predictive ECG finding was atrial fibrillation (LR 3.8; 95% CI 2.7-8.8); any abnormality on ECG had an LR of 2.2 (95% CI 1.6-3.1). Serum BNP levels were not more predictive of CHF than the history or physical examination; a BNP of >250 was associated with an LR of 4.6 (95% CI 2.6-8.0).
Few findings markedly decreased the probability of CHF. Here, it is helpful to remember that an LR of 0.5 decreases the post-test probability by about 15%, and an LR of 0.2 decreases the post-test probability by about 30%. With these in mind, the absence of cardiomegaly on CXR significantly changes the post-test probability (LR 0.33; 95% CI 0.23-0.48). A serum BNP level of less than 100pg/ml strongly argues against CHF, with an LR of 0.11 (95% CI 0.07-0.16); this finding lowers the post-test probability of CHF by about 45% compared to the pre-test probability.
In summary, the most useful findings for ruling in CHF in dyspneic emergency department patients were clinical gestalt, a prior history of CHF, findings of an S3 or jugular venous distension, and radiographic findings of venous congestion or cardiomegaly. Absence of radiographic cardiomegaly and a BNP of less than 100pg/ml argue against CHF. These must be interpreted in the context of the clinical pre-test probability of CHF, as none of the findings had likelihood ratios sufficient to be diagnostic of CHF when used individually.
What Should I Wear Today?
Rehman SU, Nietert PJ, Cope DW, Kilpatrick AO. What to wear today? Effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005 Nov; 118(11): 1279-1286.
Background: This study addresses the prototypical everyday clinical dilemma: What should I wear to work?
Methods: Patients and visitors to an outpatient Veterans Affairs internal medicine clinic in South Carolina were shown photographs of male and female physicians in four different styles of dress:
- Professional (male physician wearing white coat with tie, female physician wearing white coat with tailored skirt or trousers);
- Business (suit and tie for male, tailored trouser or skirt for female);
- Surgical (surgical scrubs for both male and female): and
- Casual (jeans and t-shirt or short skirt).
The study was randomized so that male and female respondents viewed photographs of either male or female physicians. Respondents were asked to report how strongly they felt about the importance of their physician’s appearance, and their preference for each style of dress; specifically, respondents were asked which physician was the most trustworthy, which physician they felt most comfortable with for routine examinations and emergencies, and which physician they felt most comfortable discussing psychological, sexual, and social problems with.
Results: Respondents overwhelmingly preferred professional attire for all questions: 76.3% felt most comfortable with a professionally dressed physician for all encounters, with surgical scrubs a distant second (10.2%), ahead of business dress (8.8%). Respondents were also significantly more willing to discuss psychological, sexual, and social problems with a professionally dressed physician. Even for care in an emergency situation, respondents still expressed a significant preference for professional attire over scrubs.
In a logistic regression model, patients who were older, African-American, and had less than a high school education were significantly more likely to prefer professional attire. Interestingly, female respondents who viewed photographs of female physicians placed significantly greater emphasis on physician’s attire than did male respondents.
Discussion: The study is clearly subject to caveats, chiefly that it was conducted at a single VA clinic and that only one aspect of the physician-patient encounter was addressed. Undoubtedly, patient’s preferences were influenced by the popular portrayal of physicians on TV shows. Nevertheless, given that hospitalists typically see older patients with whom they are not familiar, the initial clinical encounter may indeed by influenced by something as simple as wearing a white coat.
UA by Nephrologist Versus Hospital-Based Clinical Labs
Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis. 2005 Nov;46(5):820-829.
Background: Distinguishing the correct cause of acute renal failure is a frequent clinical dilemma for hospitalists, particularly diagnosing acute tubular necrosis (ATN), which is the most common cause of in-hospital acute renal failure. Although urinalysis with microscopy is the first test ordered on noting an abnormal serum creatinine, most hospitalists rely on the results generated by a laboratory technician. Anecdotally, many nephrologists have noted significant differences between urinalysis results performed by technicians and results found by nephrologists.
Methods: This study enrolled 26 patients hospitalized with acute renal failure on whom nephrology consultation was obtained. Urinalysis was performed both by laboratory personnel and a nephrologist (nephrologist A) who was blinded to the patient’s clinical information. Both sets of urinalysis results were independently used by nephrologist A and a second nephrologist (nephrologist B) to arrive at a clinical diagnosis for the patient, without having access to any other clinical information. These diagnoses were compared to the final diagnosis determined by the consulting nephrology service, who themselves did not have access to the diagnosis of either nephrologist A or B.
Results: The influence of having a nephrologist perform and interpret the urinalysis was striking. Nephrologist A was able to correctly diagnose 92.3% of cases based solely on his interpretation of the urinalysis. However, when given only the laboratory report of the urinalysis, both nephrologists were unable to diagnose most cases (23.1% for nephrologist A and 19.2% for nephrologist B). The major difference appeared to be in nephrologist A’s ability to find renal tubular epithelial (RTE) cells and RTE casts, which are pathognomonic of ATN. RTE cells and granular casts were frequently misinterpreted as squamous epithelial cells by laboratory personnel. This was particularly important as 81% of patients in the study had ATN as the primary cause of renal failure. Acanthocytes (dysmorphic red blood cells) were also missed by laboratory personnel in all six patients who were subsequently diagnosed with glomerulonephritis; nephrologist A correctly noted acanthocytes in five of these patients, and arrived at the correct diagnosis in all six patients.
Discussion: Microscopic evaluation of urine sediment has become a lost art among physicians, especially since passage of the Clinical Laboratory Improvement Amendments (CLIA) in 1988, which mandated that only CLIA-certified personnel could perform most laboratory tests. While it is probably unrealistic to call for training in microscopic urinalysis for all physicians, hospitalists in particular would benefit from such training, and at the very least should be mindful that laboratory urinalysis results may miss subtle findings that can be invaluable in diagnosing acute renal failure. This study points out the need for greater oversight and training of laboratory personnel, and serves as a reminder to clinicians that laboratory results should not be considered the gold standard. TH
An Internist in Iraq
Editors’ note: During 2006 we will publish coverage of hospital practices in other countries. This and the article on Africa (p. 26) are the third and fourth articles in that effort.
The sound of helicopters, the sight of concrete blast barriers and of sandbags, and the smell of smoke were the first impressions I had upon arriving at Balad Air Base, Iraq. I am a military physician used to working in a clean, safe, predictable hospital environment when I arrived in Iraq on my first deployment to a combat zone. Few military doctors arrive at Balad with extensive training in combat medicine even though that is our primary mission. Through teamwork and the varied talents of different backgrounds, we provide excellent care to American, coalition, and Iraqi patients. An internist by training and practice, I share my experiences as a member of that combat medicine team.
At one time the Iraqi Air Force Academy, Balad Air Base is approximately 40 miles north of Baghdad near the Tigris River in the heart of the “Sunni Triangle.” The Air Force Theater Hospital (AFTH)—one of several expeditionary hospitals in the Iraqi theater—is located at Balad Air Base. Although Air Force in name, the hospital is truly a joint mission, with medical staff from both the U.S Army and Air Force working side by side. The hospital is robust in capability, but is not permanent in nature.
Hospital Structure
The hospital functions out of a multitude of large tents joined in tandem. Although climate controlled, the tents provide only a minimal barrier to dust and noise, and keeping the area clean and speaking in normal tones is a constant struggle. Like hospitals in the United States, there are distinct units within the AFTH: an emergency department, operating rooms, an ICU, a general medicine and surgical ward unit, a pharmacy, a clinical laboratory, and a radiology section.
The Patients
Patients arrive at AFTH either directly from the field or, after initial triage and stabilization, or from smaller treatment facilities. AFTH is primarily a trauma center, and the majority of patients arrive via helicopter given the need for rapid movement and the danger inherent in vehicular transport. The sound of helicopter rotors is omnipresent at AFTH. The proximity of the landing pad to the hospital results in one of the impressions of Balad that I will not soon forget: that of the conversation-deafening and air-reverberating arrival of new patients.
The majority of patients who arrive at AFTH have sustained some type of combat-related injury, usually gunshot or improvised-explosive device (IED) wounds. These patients are initially assessed by emergency medicine physicians and surgeons. Many of the patients go immediately to the operating room for wound management, and those who require a higher level of care (either pre- or post-operatively) are moved to the ICU.
As an internist, my role is as a member of the ICU team of physicians that cares for these critically ill patients. The physicians who comprise the ICU team have different backgrounds, including general surgery, internal medicine, anesthesiology, emergency medicine, and subspecialties (currently a general internist, a medicine intensivist, a cardiologist, and an infectious disease doctor).
The goal of the ICU team is to provide for continuity of care of these critically ill patients during their ICU stay and to ensure that other AFTH staff members—most notably surgeons—can concentrate on new patients as they arrive. In addition to caring for critically ill trauma patients, my fellow internists and I also function much as we do at home: evaluating and admitting patients from the emergency department whose conditions are traditionally managed by internal medicine, including acute coronary syndromes, diabetic ketoacidosis, syncope, and gastrointestinal bleeding, to name a recent few.
How I Spend My Time
Apart from the caring for the occasional internal medicine patient, I spend the majority of my time working outside of the usual realm of the internist. In the noisy combat hospital, conventional internal medicine patient evaluations are impossible. The history is often limited by the patient’s physical condition and, for many of the Iraqi soldiers, a language barrier. Physical exams are done more with sight and touch than with a stethoscope. The past medical and surgical history is uncertain. The knowledge and skills required to care for these trauma patients are also a departure from routine internal medicine practice.
Fortunately, I discovered that, although little used since residency, my ability to manage ventilators and to perform invasive procedures was quick to return and was immediately put into practice. I have learned aspects of critical care as practiced in the theater hospital ICU that I was unfamiliar with initially—such as the intricacies of post-operative and trauma care—on the job. I have become familiar with dressings, drains, and the concepts of resuscitation and of “secondary survey.” I have acquired a working knowledge of the various types of surgical procedures performed, and the subsequent care required thereof, in trauma patients. I have become familiar with treating elevated intracranial pressure in patients who have had craniotomies for penetrating brain injuries, with monitoring airway pressures and oxygenation in patients with blast-related pulmonary contusions, with following bladder pressures and serial exams in patients with abdominal trauma, and with managing chest tubes in patients with penetrating thoracic injuries.
I have even overcome a reluctance shared by many in internal medicine and have learned to look under surgical bandages—a feat that may undermine the truth that gives rise to the joke about hiding something from internists. Perhaps the most important concept I have learned in caring for combat trauma patients in the ICU is vigilance.
The primary survey, completed by the emergency medicine and trauma surgeons, usually discovers and addresses the large or obvious wounds that bring patients to our facility. When the patients arrive in the ICU after having their initial resuscitation and “damage control” operative intervention, it falls to the intensive care physician to both continue resuscitation and to look for as yet undiagnosed or delayed injury presentations. This constitutes the secondary survey and is an ongoing process. Patients often arrive in the ICU still recovering from their injuries; they require close attention to physiologic parameters such as temperature, heart rate, arterial pressure, and urine output. Their laboratory measurements, including oxygenation and ventilation, acid-base status (e.g., a reliance on the base excess, a tool more familiar to those in surgery than in medicine), hemoglobin concentration, and indices of coagulation, require constant attention.
In addition, patients often come to the ICU with vascular lines that were placed in the field under less than sterile conditions and require replacement. While major wounds have usually been addressed, minor wounds (such as missed fragments of shrapnel and subtle vascular injuries) or delayed presentations (including blast injuries and compartment syndromes) must be identified in the ICU and mandate constant awareness.
Specific Challenges
There are challenges, both personally and professionally, to working in a combat zone. Like everyone here, I am away from family and home for an extended time. Although fairly secure, one’s personal safety from ongoing mortar attacks is also an emotional burden. The hours are long and the recreational opportunities are limited on base. Traveling off base is strictly limited for obvious security reasons and most hospital personnel spend their entire tour in Iraq within the confines of the base perimeter.
Professionally, the biggest challenge to working at the AFTH is our location and resultant long supply train. Almost every item needed to stock a modern hospital comes not from the local economy but from outside the country and must be either flown or trucked in. This logistic trail requires constant attention to efficiency and inventory and when supplies are out or equipment is down, sometimes we must resort to ingenuity. We try to do the best we can for every individual yet, akin to the concept of military triage, we must use our medical resources with the utilitarian philosophy of “the greatest good for the greatest number.”
Final Thoughts
Practicing medicine at AFTH has been, for me, the opportunity of a lifetime. I work with very talented people, learn an amazing amount, and—most importantly—help care for our men and women in uniform. Although we practice medicine much differently from the way we do at home, the adjustment to the combat hospital is facilitated by the close teamwork among physicians here. This is exemplified in the ICU, where twice daily ICU rounds are led by a surgical intensivist and are attended by general surgeons, surgical subspecialists, and the ICU team, including internists and medicine subspecialists. In how many medical facilities do surgeons and internists, caring for the same patients, perform bedside rounds together as a matter of routine?
I believe this sense of teamwork exists in the combat zone for several reasons, including necessity, in which efficient use of time and manpower is critical, and of fluidity, in which the constant mixing and turnover of hospital staff prevents departmental barriers from developing. Perhaps the most important reason teamwork flourishes at AFTH is the overarching sense of mission in an austere environment. We are at Balad Air Base primarily to care for wounded American and Iraqi military members, and that responsibility under these conditions requires a resourceful and collaborative approach to the practice of medicine. TH
The views expressed herein are those of the author and do not represent the opinions of the U.S. Government, the U.S. Department of Defense, or the U.S. Air Force.
Editors’ note: During 2006 we will publish coverage of hospital practices in other countries. This and the article on Africa (p. 26) are the third and fourth articles in that effort.
The sound of helicopters, the sight of concrete blast barriers and of sandbags, and the smell of smoke were the first impressions I had upon arriving at Balad Air Base, Iraq. I am a military physician used to working in a clean, safe, predictable hospital environment when I arrived in Iraq on my first deployment to a combat zone. Few military doctors arrive at Balad with extensive training in combat medicine even though that is our primary mission. Through teamwork and the varied talents of different backgrounds, we provide excellent care to American, coalition, and Iraqi patients. An internist by training and practice, I share my experiences as a member of that combat medicine team.
At one time the Iraqi Air Force Academy, Balad Air Base is approximately 40 miles north of Baghdad near the Tigris River in the heart of the “Sunni Triangle.” The Air Force Theater Hospital (AFTH)—one of several expeditionary hospitals in the Iraqi theater—is located at Balad Air Base. Although Air Force in name, the hospital is truly a joint mission, with medical staff from both the U.S Army and Air Force working side by side. The hospital is robust in capability, but is not permanent in nature.
Hospital Structure
The hospital functions out of a multitude of large tents joined in tandem. Although climate controlled, the tents provide only a minimal barrier to dust and noise, and keeping the area clean and speaking in normal tones is a constant struggle. Like hospitals in the United States, there are distinct units within the AFTH: an emergency department, operating rooms, an ICU, a general medicine and surgical ward unit, a pharmacy, a clinical laboratory, and a radiology section.
The Patients
Patients arrive at AFTH either directly from the field or, after initial triage and stabilization, or from smaller treatment facilities. AFTH is primarily a trauma center, and the majority of patients arrive via helicopter given the need for rapid movement and the danger inherent in vehicular transport. The sound of helicopter rotors is omnipresent at AFTH. The proximity of the landing pad to the hospital results in one of the impressions of Balad that I will not soon forget: that of the conversation-deafening and air-reverberating arrival of new patients.
The majority of patients who arrive at AFTH have sustained some type of combat-related injury, usually gunshot or improvised-explosive device (IED) wounds. These patients are initially assessed by emergency medicine physicians and surgeons. Many of the patients go immediately to the operating room for wound management, and those who require a higher level of care (either pre- or post-operatively) are moved to the ICU.
As an internist, my role is as a member of the ICU team of physicians that cares for these critically ill patients. The physicians who comprise the ICU team have different backgrounds, including general surgery, internal medicine, anesthesiology, emergency medicine, and subspecialties (currently a general internist, a medicine intensivist, a cardiologist, and an infectious disease doctor).
The goal of the ICU team is to provide for continuity of care of these critically ill patients during their ICU stay and to ensure that other AFTH staff members—most notably surgeons—can concentrate on new patients as they arrive. In addition to caring for critically ill trauma patients, my fellow internists and I also function much as we do at home: evaluating and admitting patients from the emergency department whose conditions are traditionally managed by internal medicine, including acute coronary syndromes, diabetic ketoacidosis, syncope, and gastrointestinal bleeding, to name a recent few.
How I Spend My Time
Apart from the caring for the occasional internal medicine patient, I spend the majority of my time working outside of the usual realm of the internist. In the noisy combat hospital, conventional internal medicine patient evaluations are impossible. The history is often limited by the patient’s physical condition and, for many of the Iraqi soldiers, a language barrier. Physical exams are done more with sight and touch than with a stethoscope. The past medical and surgical history is uncertain. The knowledge and skills required to care for these trauma patients are also a departure from routine internal medicine practice.
Fortunately, I discovered that, although little used since residency, my ability to manage ventilators and to perform invasive procedures was quick to return and was immediately put into practice. I have learned aspects of critical care as practiced in the theater hospital ICU that I was unfamiliar with initially—such as the intricacies of post-operative and trauma care—on the job. I have become familiar with dressings, drains, and the concepts of resuscitation and of “secondary survey.” I have acquired a working knowledge of the various types of surgical procedures performed, and the subsequent care required thereof, in trauma patients. I have become familiar with treating elevated intracranial pressure in patients who have had craniotomies for penetrating brain injuries, with monitoring airway pressures and oxygenation in patients with blast-related pulmonary contusions, with following bladder pressures and serial exams in patients with abdominal trauma, and with managing chest tubes in patients with penetrating thoracic injuries.
I have even overcome a reluctance shared by many in internal medicine and have learned to look under surgical bandages—a feat that may undermine the truth that gives rise to the joke about hiding something from internists. Perhaps the most important concept I have learned in caring for combat trauma patients in the ICU is vigilance.
The primary survey, completed by the emergency medicine and trauma surgeons, usually discovers and addresses the large or obvious wounds that bring patients to our facility. When the patients arrive in the ICU after having their initial resuscitation and “damage control” operative intervention, it falls to the intensive care physician to both continue resuscitation and to look for as yet undiagnosed or delayed injury presentations. This constitutes the secondary survey and is an ongoing process. Patients often arrive in the ICU still recovering from their injuries; they require close attention to physiologic parameters such as temperature, heart rate, arterial pressure, and urine output. Their laboratory measurements, including oxygenation and ventilation, acid-base status (e.g., a reliance on the base excess, a tool more familiar to those in surgery than in medicine), hemoglobin concentration, and indices of coagulation, require constant attention.
In addition, patients often come to the ICU with vascular lines that were placed in the field under less than sterile conditions and require replacement. While major wounds have usually been addressed, minor wounds (such as missed fragments of shrapnel and subtle vascular injuries) or delayed presentations (including blast injuries and compartment syndromes) must be identified in the ICU and mandate constant awareness.
Specific Challenges
There are challenges, both personally and professionally, to working in a combat zone. Like everyone here, I am away from family and home for an extended time. Although fairly secure, one’s personal safety from ongoing mortar attacks is also an emotional burden. The hours are long and the recreational opportunities are limited on base. Traveling off base is strictly limited for obvious security reasons and most hospital personnel spend their entire tour in Iraq within the confines of the base perimeter.
Professionally, the biggest challenge to working at the AFTH is our location and resultant long supply train. Almost every item needed to stock a modern hospital comes not from the local economy but from outside the country and must be either flown or trucked in. This logistic trail requires constant attention to efficiency and inventory and when supplies are out or equipment is down, sometimes we must resort to ingenuity. We try to do the best we can for every individual yet, akin to the concept of military triage, we must use our medical resources with the utilitarian philosophy of “the greatest good for the greatest number.”
Final Thoughts
Practicing medicine at AFTH has been, for me, the opportunity of a lifetime. I work with very talented people, learn an amazing amount, and—most importantly—help care for our men and women in uniform. Although we practice medicine much differently from the way we do at home, the adjustment to the combat hospital is facilitated by the close teamwork among physicians here. This is exemplified in the ICU, where twice daily ICU rounds are led by a surgical intensivist and are attended by general surgeons, surgical subspecialists, and the ICU team, including internists and medicine subspecialists. In how many medical facilities do surgeons and internists, caring for the same patients, perform bedside rounds together as a matter of routine?
I believe this sense of teamwork exists in the combat zone for several reasons, including necessity, in which efficient use of time and manpower is critical, and of fluidity, in which the constant mixing and turnover of hospital staff prevents departmental barriers from developing. Perhaps the most important reason teamwork flourishes at AFTH is the overarching sense of mission in an austere environment. We are at Balad Air Base primarily to care for wounded American and Iraqi military members, and that responsibility under these conditions requires a resourceful and collaborative approach to the practice of medicine. TH
The views expressed herein are those of the author and do not represent the opinions of the U.S. Government, the U.S. Department of Defense, or the U.S. Air Force.
Editors’ note: During 2006 we will publish coverage of hospital practices in other countries. This and the article on Africa (p. 26) are the third and fourth articles in that effort.
The sound of helicopters, the sight of concrete blast barriers and of sandbags, and the smell of smoke were the first impressions I had upon arriving at Balad Air Base, Iraq. I am a military physician used to working in a clean, safe, predictable hospital environment when I arrived in Iraq on my first deployment to a combat zone. Few military doctors arrive at Balad with extensive training in combat medicine even though that is our primary mission. Through teamwork and the varied talents of different backgrounds, we provide excellent care to American, coalition, and Iraqi patients. An internist by training and practice, I share my experiences as a member of that combat medicine team.
At one time the Iraqi Air Force Academy, Balad Air Base is approximately 40 miles north of Baghdad near the Tigris River in the heart of the “Sunni Triangle.” The Air Force Theater Hospital (AFTH)—one of several expeditionary hospitals in the Iraqi theater—is located at Balad Air Base. Although Air Force in name, the hospital is truly a joint mission, with medical staff from both the U.S Army and Air Force working side by side. The hospital is robust in capability, but is not permanent in nature.
Hospital Structure
The hospital functions out of a multitude of large tents joined in tandem. Although climate controlled, the tents provide only a minimal barrier to dust and noise, and keeping the area clean and speaking in normal tones is a constant struggle. Like hospitals in the United States, there are distinct units within the AFTH: an emergency department, operating rooms, an ICU, a general medicine and surgical ward unit, a pharmacy, a clinical laboratory, and a radiology section.
The Patients
Patients arrive at AFTH either directly from the field or, after initial triage and stabilization, or from smaller treatment facilities. AFTH is primarily a trauma center, and the majority of patients arrive via helicopter given the need for rapid movement and the danger inherent in vehicular transport. The sound of helicopter rotors is omnipresent at AFTH. The proximity of the landing pad to the hospital results in one of the impressions of Balad that I will not soon forget: that of the conversation-deafening and air-reverberating arrival of new patients.
The majority of patients who arrive at AFTH have sustained some type of combat-related injury, usually gunshot or improvised-explosive device (IED) wounds. These patients are initially assessed by emergency medicine physicians and surgeons. Many of the patients go immediately to the operating room for wound management, and those who require a higher level of care (either pre- or post-operatively) are moved to the ICU.
As an internist, my role is as a member of the ICU team of physicians that cares for these critically ill patients. The physicians who comprise the ICU team have different backgrounds, including general surgery, internal medicine, anesthesiology, emergency medicine, and subspecialties (currently a general internist, a medicine intensivist, a cardiologist, and an infectious disease doctor).
The goal of the ICU team is to provide for continuity of care of these critically ill patients during their ICU stay and to ensure that other AFTH staff members—most notably surgeons—can concentrate on new patients as they arrive. In addition to caring for critically ill trauma patients, my fellow internists and I also function much as we do at home: evaluating and admitting patients from the emergency department whose conditions are traditionally managed by internal medicine, including acute coronary syndromes, diabetic ketoacidosis, syncope, and gastrointestinal bleeding, to name a recent few.
How I Spend My Time
Apart from the caring for the occasional internal medicine patient, I spend the majority of my time working outside of the usual realm of the internist. In the noisy combat hospital, conventional internal medicine patient evaluations are impossible. The history is often limited by the patient’s physical condition and, for many of the Iraqi soldiers, a language barrier. Physical exams are done more with sight and touch than with a stethoscope. The past medical and surgical history is uncertain. The knowledge and skills required to care for these trauma patients are also a departure from routine internal medicine practice.
Fortunately, I discovered that, although little used since residency, my ability to manage ventilators and to perform invasive procedures was quick to return and was immediately put into practice. I have learned aspects of critical care as practiced in the theater hospital ICU that I was unfamiliar with initially—such as the intricacies of post-operative and trauma care—on the job. I have become familiar with dressings, drains, and the concepts of resuscitation and of “secondary survey.” I have acquired a working knowledge of the various types of surgical procedures performed, and the subsequent care required thereof, in trauma patients. I have become familiar with treating elevated intracranial pressure in patients who have had craniotomies for penetrating brain injuries, with monitoring airway pressures and oxygenation in patients with blast-related pulmonary contusions, with following bladder pressures and serial exams in patients with abdominal trauma, and with managing chest tubes in patients with penetrating thoracic injuries.
I have even overcome a reluctance shared by many in internal medicine and have learned to look under surgical bandages—a feat that may undermine the truth that gives rise to the joke about hiding something from internists. Perhaps the most important concept I have learned in caring for combat trauma patients in the ICU is vigilance.
The primary survey, completed by the emergency medicine and trauma surgeons, usually discovers and addresses the large or obvious wounds that bring patients to our facility. When the patients arrive in the ICU after having their initial resuscitation and “damage control” operative intervention, it falls to the intensive care physician to both continue resuscitation and to look for as yet undiagnosed or delayed injury presentations. This constitutes the secondary survey and is an ongoing process. Patients often arrive in the ICU still recovering from their injuries; they require close attention to physiologic parameters such as temperature, heart rate, arterial pressure, and urine output. Their laboratory measurements, including oxygenation and ventilation, acid-base status (e.g., a reliance on the base excess, a tool more familiar to those in surgery than in medicine), hemoglobin concentration, and indices of coagulation, require constant attention.
In addition, patients often come to the ICU with vascular lines that were placed in the field under less than sterile conditions and require replacement. While major wounds have usually been addressed, minor wounds (such as missed fragments of shrapnel and subtle vascular injuries) or delayed presentations (including blast injuries and compartment syndromes) must be identified in the ICU and mandate constant awareness.
Specific Challenges
There are challenges, both personally and professionally, to working in a combat zone. Like everyone here, I am away from family and home for an extended time. Although fairly secure, one’s personal safety from ongoing mortar attacks is also an emotional burden. The hours are long and the recreational opportunities are limited on base. Traveling off base is strictly limited for obvious security reasons and most hospital personnel spend their entire tour in Iraq within the confines of the base perimeter.
Professionally, the biggest challenge to working at the AFTH is our location and resultant long supply train. Almost every item needed to stock a modern hospital comes not from the local economy but from outside the country and must be either flown or trucked in. This logistic trail requires constant attention to efficiency and inventory and when supplies are out or equipment is down, sometimes we must resort to ingenuity. We try to do the best we can for every individual yet, akin to the concept of military triage, we must use our medical resources with the utilitarian philosophy of “the greatest good for the greatest number.”
Final Thoughts
Practicing medicine at AFTH has been, for me, the opportunity of a lifetime. I work with very talented people, learn an amazing amount, and—most importantly—help care for our men and women in uniform. Although we practice medicine much differently from the way we do at home, the adjustment to the combat hospital is facilitated by the close teamwork among physicians here. This is exemplified in the ICU, where twice daily ICU rounds are led by a surgical intensivist and are attended by general surgeons, surgical subspecialists, and the ICU team, including internists and medicine subspecialists. In how many medical facilities do surgeons and internists, caring for the same patients, perform bedside rounds together as a matter of routine?
I believe this sense of teamwork exists in the combat zone for several reasons, including necessity, in which efficient use of time and manpower is critical, and of fluidity, in which the constant mixing and turnover of hospital staff prevents departmental barriers from developing. Perhaps the most important reason teamwork flourishes at AFTH is the overarching sense of mission in an austere environment. We are at Balad Air Base primarily to care for wounded American and Iraqi military members, and that responsibility under these conditions requires a resourceful and collaborative approach to the practice of medicine. TH
The views expressed herein are those of the author and do not represent the opinions of the U.S. Government, the U.S. Department of Defense, or the U.S. Air Force.