User login
Quality Commitment
Ehab Hanna, MD, MBBch, FHM, understands better than most why hospitalists are frustrated with all the grand plans to utilize information technology (IT) for streamlining admissions, medical reconciliations, and the discharge process. As assistant chief medical information officer at Eastern Maine Medical Center in Bangor, he spends half his time scouting and assessing the value of new IT platforms and the other half as a front-line hospitalist.
Dr. Hanna and his colleagues are frustrated that software systems promise to deliver electronic medical records (EMR) but freeze up too often, take too long to download files, or can’t handle the functions for which they were developed. But he also knows the future of healthcare hinges on IT as much as anything else—and that done correctly, and probably expensively, it can be a savior.
“Every time we want to come up with a quality-initiative project, we want to ask, ‘What can IT do for us?’ ” Dr. Hanna says. He also acknowledges that “it’s all types of money, whether it be resources, funding, or people to implement [the system]. And there’s physician resistance to it.”
The link between quality and cost is paramount to healthcare and HM. As evidence, the keynote theme of HM09 in Chicago was quality improvement (QI)—defining it, making it a priority, setting up analytical metrics to measure it, and the most difficult step: implementing it. QI projects vary in size, shape, and scope. On one end of the spectrum: hand-washing compliance systems and simple programs to increase the prescription of pneumococcal vaccines. On the laborious and expensive end: EMR system integration with ambulatory care and pharmacy.
Industry leaders agree QI projects must include measurable goals and incentives for success. The flip side is that failure to reach those goals has to include a level of accountability.
One thing is for sure: The choice to focus on patient safety no longer is a choice, it’s a mandate. Patient-safety advocates are barking louder than ever, and the public and politicians are taking note. Medicare reimbursements are increasingly tied to performance measures, a trend that is likely to accelerate in light of recent news that Medicare will sink into the red in just eight years. Many expect that threshold to keep moving closer, too. President Obama has pledged to push major healthcare reform legislation—including a focus on EMR—through Congress. He wants to sign it into law by Labor Day.
On the other hand, there still is a relatively small sample of data on the effectiveness of pay-for-performance contracting in relation to overall patient health. There is a recurring call from many outside the HM field for more independent, empirical data that can pinpoint the quantifiable value of hospitalists. Discussions based on those values could satisfy group leaders, hospital administrators, and government regulators who still use the tried-and-true HM formula: value equals quality divided by cost.
“I see about as many challenges in QI as I do opportunities,” says SHM President Scott Flanders, MD, FHM, director of the hospitalist program at the University of Michigan Health System in Ann Arbor. “Is the horse before the cart? We have spent a lot of time and effort putting in place … programs before understanding the clinical effect.”
Can IT be EZ?
Dr. Hanna says hospitalists would embrace new IT initiatives immediately if they were easier to use. Many hospitalists are frustrated that in their pocket sits a handy, portable device that works in real time as a computer, a phone, a CD player, a GPS tracking device, and a scheduling secretary, yet they can’t use their E&M coding system without encountering constant hiccups, interruptions that take valuable time out of an already crowded 12-hour shift.
“Why isn’t it working like the iPhone?” is a complaint Dr. Hanna says he hears all the time.
One answer is that a number of medical software programs are limited in nature and don’t automatically network well with other systems already in place. User errors and other problems crop up regularly; plus, there isn’t a repository for people to measure different systems against each other. Some SHM leaders are considering a plan to create an online resource for IT vendors, but the society is leery of making recommendations because of potential conflicts of interest.
The Joint Commission, which accredits and certifies more than 15,000 healthcare organizations and programs, has been asked—by Dr. Hanna, among others—if it intends to regulate EMR vendors, which would let hospitalists know which systems are most reliable and useful. Commission President Mark Chassin, MD, MPP, MPH, said after his HM09 keynote address that his agency has no intention of doing so.
—Scott Flanders, MD, FHM, SHM president
“We don’t see [that] as a good message for us to give,” Dr. Chassin said.
IT also can be used in creative ways to spur patient-safety improvements. As hospitalists struggle to increase compliance with hand-hygiene standards, several hospitals have resorted to using video cameras above sinks to track whose hands are clean and whose hands are not clean, Robert Wachter, MD, FHM, said during his plenary address to conclude HM09. Real-time tracking runs through a software program and is displayed on a small, LED screen that hangs from the ceiling. Positive results are praised, while low rates of compliance result in pages to HM and hospital leaders to address hygiene issues. Dr. Wachter, professor and associate chairman of the department of medicine at the University of California at San Francisco Medical Center, chief of the division of hospital medicine, former SHM president and author of the blog “Wachter’s World” (www.wachtersworld), said UCSF hospital executives are considering the video system.
Dr. Wachter also says hospitalists can’t lament the rise of QI and the stricter standards that are attached. Nor can HM complain too loudly about the burdens QI places on them. As hospitalists argue that their value is partly defined by their contributions to hospital quality, they have to expect to be accountable to the claim. “We have positioned ourselves as being leaders in quality and safety,” he says. “This is not going out and being branded. We bring this in. We often say we have two sick patients; one is in front of us, the other is our organization.”
Pay for Performance?
Another largely unanswered question is how valuable pay-for-performance will be in improving patient care, safety, and satisfaction. While most HM contracts include additional compensation based on volume, bonus pay often ignores such measurable outcomes as readmission rates and length of stay.
“Does incentivizing healthcare quality actually improve health?” asks Susan Freeman, MD, chief medical officer at Temple University Hospital in Philadelphia. “It’s really hard to measure things that don’t happen—the myocardial infarction that didn’t happen, the stroke that didn’t happen.”
Active pay-for-performance programs numbered 160 in November 2007, four times the number of programs four years earlier.1 Yet as the concept catches on, the average physician incentive was 2% or less, and it seems to play a small role in how care was delivered.2
A more recent look at the issue concludes that if insurance companies and private employers worked with hospitals and physicians, attitudes toward pay for performance might change. “The amount of incentives available to physicians strongly affected their rate of participation,” say the authors of a May study in the American Journal of Managed Care. “Our analysis suggests that all stakeholders—health plans, physicians, and patients—would benefit from health plans collaborating on their pay-for-performance efforts to maximize physician participation.”3
Russell Holman, MD, FHM, chief operating officer of Cogent Healthcare, says another pay-for-performance concern is that improved quality is not always something that translates quickly into bottom-line savings. In today’s economic environment, in which every dollar spent has to be justified, it can actually be tougher to sell the upfront costs and long-term savings associated with investment in QI projects. “Improving quality is going to take a long time to see the advantages to the healthcare system,” says Dr. Holman, a former SHM president. “It is delayed gratification.”
Is HM Ready?
Dr. Holman says the pay-for-performance solution might end up as more of a hybrid of two reimbursement models, incentive-based pay and the controversial notion of billing bundling. Most hospitalists and HM groups are not entirely comfortable with the bundling idea, in which the hospital receives a lump-sum reimbursement for all services performed for a patient, then shells out payment to the surgeons, hospitalists, nurses, etc. The concept of hospitals as payment intermediaries adds an extra layer of bureaucracy, but the bundling concept appears to be gaining momentum.
While QI projects are the trendy way to measure value, Dr. Chassin points out that physicians need more detailed thresholds to exceed. Such measures as prescribing beta-blockers after myocardial infarction were good first-generation concepts, but better care requires better benchmarks, he says. Dr. Wachter echoes the sentiment, telling HM09 attendees that relying on past successes in QI won’t help hospitalists demonstrate their value or answer the “What have you done for me lately?” demand from hospital administrators.
Dr. Chassin compares healthcare to other high-pressure industries that do much better at controlling mistakes. Some estimates show nearly 100,000 people a year die from medical errors. Why? Many high-risk industries—airlines, nuclear energy, mining—have better quality and safety processes to protect against routine errors that continually plague healthcare (e.g., hospital-acquired infections, operations on the wrong patient).
The responsibility to get better doesn’t belong to one medical group, either. Dr. Chassin says researchers, the Centers for Medicare and Medicaid Services, and trade groups like SHM have to work collaboratively to design benchmarks that can be measured quantitatively. Once those measuring sticks are in place, though, Dr. Chassin believes hospitalists are the first responders who can best identify and solve problems.
“You have to understand the causes of the problems you’re trying to fix,” he says. “Hospitalists are on the front lines.” TH
Richard Quinn is a freelance writer based in New Jersey.
References
- Baker G, Delbanco S. Pay for performance: national perspective. 2006 longitudinal survey results with 2007 market updates. MedVantage Web site. Available at www.medvantage.com/Pdf/2006 NationalP4PStudy.pdf. Accessed May 17, 2009.
- Pearson SD, Schneider EC, Kleinman KP, Colin KL, Singer JA. The impact of pay-for-performance on health care quality in Massachusetts, 2001-2003. Health Aff (Millwood). 2008;27(4):1167-1176.
- De Brantes FS, D’Andrea BG. Physicians respond to pay-for-performance incentives: larger incentives yield greater participation. Am J Manag Care. 2009;15(5):305-310.
Ehab Hanna, MD, MBBch, FHM, understands better than most why hospitalists are frustrated with all the grand plans to utilize information technology (IT) for streamlining admissions, medical reconciliations, and the discharge process. As assistant chief medical information officer at Eastern Maine Medical Center in Bangor, he spends half his time scouting and assessing the value of new IT platforms and the other half as a front-line hospitalist.
Dr. Hanna and his colleagues are frustrated that software systems promise to deliver electronic medical records (EMR) but freeze up too often, take too long to download files, or can’t handle the functions for which they were developed. But he also knows the future of healthcare hinges on IT as much as anything else—and that done correctly, and probably expensively, it can be a savior.
“Every time we want to come up with a quality-initiative project, we want to ask, ‘What can IT do for us?’ ” Dr. Hanna says. He also acknowledges that “it’s all types of money, whether it be resources, funding, or people to implement [the system]. And there’s physician resistance to it.”
The link between quality and cost is paramount to healthcare and HM. As evidence, the keynote theme of HM09 in Chicago was quality improvement (QI)—defining it, making it a priority, setting up analytical metrics to measure it, and the most difficult step: implementing it. QI projects vary in size, shape, and scope. On one end of the spectrum: hand-washing compliance systems and simple programs to increase the prescription of pneumococcal vaccines. On the laborious and expensive end: EMR system integration with ambulatory care and pharmacy.
Industry leaders agree QI projects must include measurable goals and incentives for success. The flip side is that failure to reach those goals has to include a level of accountability.
One thing is for sure: The choice to focus on patient safety no longer is a choice, it’s a mandate. Patient-safety advocates are barking louder than ever, and the public and politicians are taking note. Medicare reimbursements are increasingly tied to performance measures, a trend that is likely to accelerate in light of recent news that Medicare will sink into the red in just eight years. Many expect that threshold to keep moving closer, too. President Obama has pledged to push major healthcare reform legislation—including a focus on EMR—through Congress. He wants to sign it into law by Labor Day.
On the other hand, there still is a relatively small sample of data on the effectiveness of pay-for-performance contracting in relation to overall patient health. There is a recurring call from many outside the HM field for more independent, empirical data that can pinpoint the quantifiable value of hospitalists. Discussions based on those values could satisfy group leaders, hospital administrators, and government regulators who still use the tried-and-true HM formula: value equals quality divided by cost.
“I see about as many challenges in QI as I do opportunities,” says SHM President Scott Flanders, MD, FHM, director of the hospitalist program at the University of Michigan Health System in Ann Arbor. “Is the horse before the cart? We have spent a lot of time and effort putting in place … programs before understanding the clinical effect.”
Can IT be EZ?
Dr. Hanna says hospitalists would embrace new IT initiatives immediately if they were easier to use. Many hospitalists are frustrated that in their pocket sits a handy, portable device that works in real time as a computer, a phone, a CD player, a GPS tracking device, and a scheduling secretary, yet they can’t use their E&M coding system without encountering constant hiccups, interruptions that take valuable time out of an already crowded 12-hour shift.
“Why isn’t it working like the iPhone?” is a complaint Dr. Hanna says he hears all the time.
One answer is that a number of medical software programs are limited in nature and don’t automatically network well with other systems already in place. User errors and other problems crop up regularly; plus, there isn’t a repository for people to measure different systems against each other. Some SHM leaders are considering a plan to create an online resource for IT vendors, but the society is leery of making recommendations because of potential conflicts of interest.
The Joint Commission, which accredits and certifies more than 15,000 healthcare organizations and programs, has been asked—by Dr. Hanna, among others—if it intends to regulate EMR vendors, which would let hospitalists know which systems are most reliable and useful. Commission President Mark Chassin, MD, MPP, MPH, said after his HM09 keynote address that his agency has no intention of doing so.
—Scott Flanders, MD, FHM, SHM president
“We don’t see [that] as a good message for us to give,” Dr. Chassin said.
IT also can be used in creative ways to spur patient-safety improvements. As hospitalists struggle to increase compliance with hand-hygiene standards, several hospitals have resorted to using video cameras above sinks to track whose hands are clean and whose hands are not clean, Robert Wachter, MD, FHM, said during his plenary address to conclude HM09. Real-time tracking runs through a software program and is displayed on a small, LED screen that hangs from the ceiling. Positive results are praised, while low rates of compliance result in pages to HM and hospital leaders to address hygiene issues. Dr. Wachter, professor and associate chairman of the department of medicine at the University of California at San Francisco Medical Center, chief of the division of hospital medicine, former SHM president and author of the blog “Wachter’s World” (www.wachtersworld), said UCSF hospital executives are considering the video system.
Dr. Wachter also says hospitalists can’t lament the rise of QI and the stricter standards that are attached. Nor can HM complain too loudly about the burdens QI places on them. As hospitalists argue that their value is partly defined by their contributions to hospital quality, they have to expect to be accountable to the claim. “We have positioned ourselves as being leaders in quality and safety,” he says. “This is not going out and being branded. We bring this in. We often say we have two sick patients; one is in front of us, the other is our organization.”
Pay for Performance?
Another largely unanswered question is how valuable pay-for-performance will be in improving patient care, safety, and satisfaction. While most HM contracts include additional compensation based on volume, bonus pay often ignores such measurable outcomes as readmission rates and length of stay.
“Does incentivizing healthcare quality actually improve health?” asks Susan Freeman, MD, chief medical officer at Temple University Hospital in Philadelphia. “It’s really hard to measure things that don’t happen—the myocardial infarction that didn’t happen, the stroke that didn’t happen.”
Active pay-for-performance programs numbered 160 in November 2007, four times the number of programs four years earlier.1 Yet as the concept catches on, the average physician incentive was 2% or less, and it seems to play a small role in how care was delivered.2
A more recent look at the issue concludes that if insurance companies and private employers worked with hospitals and physicians, attitudes toward pay for performance might change. “The amount of incentives available to physicians strongly affected their rate of participation,” say the authors of a May study in the American Journal of Managed Care. “Our analysis suggests that all stakeholders—health plans, physicians, and patients—would benefit from health plans collaborating on their pay-for-performance efforts to maximize physician participation.”3
Russell Holman, MD, FHM, chief operating officer of Cogent Healthcare, says another pay-for-performance concern is that improved quality is not always something that translates quickly into bottom-line savings. In today’s economic environment, in which every dollar spent has to be justified, it can actually be tougher to sell the upfront costs and long-term savings associated with investment in QI projects. “Improving quality is going to take a long time to see the advantages to the healthcare system,” says Dr. Holman, a former SHM president. “It is delayed gratification.”
Is HM Ready?
Dr. Holman says the pay-for-performance solution might end up as more of a hybrid of two reimbursement models, incentive-based pay and the controversial notion of billing bundling. Most hospitalists and HM groups are not entirely comfortable with the bundling idea, in which the hospital receives a lump-sum reimbursement for all services performed for a patient, then shells out payment to the surgeons, hospitalists, nurses, etc. The concept of hospitals as payment intermediaries adds an extra layer of bureaucracy, but the bundling concept appears to be gaining momentum.
While QI projects are the trendy way to measure value, Dr. Chassin points out that physicians need more detailed thresholds to exceed. Such measures as prescribing beta-blockers after myocardial infarction were good first-generation concepts, but better care requires better benchmarks, he says. Dr. Wachter echoes the sentiment, telling HM09 attendees that relying on past successes in QI won’t help hospitalists demonstrate their value or answer the “What have you done for me lately?” demand from hospital administrators.
Dr. Chassin compares healthcare to other high-pressure industries that do much better at controlling mistakes. Some estimates show nearly 100,000 people a year die from medical errors. Why? Many high-risk industries—airlines, nuclear energy, mining—have better quality and safety processes to protect against routine errors that continually plague healthcare (e.g., hospital-acquired infections, operations on the wrong patient).
The responsibility to get better doesn’t belong to one medical group, either. Dr. Chassin says researchers, the Centers for Medicare and Medicaid Services, and trade groups like SHM have to work collaboratively to design benchmarks that can be measured quantitatively. Once those measuring sticks are in place, though, Dr. Chassin believes hospitalists are the first responders who can best identify and solve problems.
“You have to understand the causes of the problems you’re trying to fix,” he says. “Hospitalists are on the front lines.” TH
Richard Quinn is a freelance writer based in New Jersey.
References
- Baker G, Delbanco S. Pay for performance: national perspective. 2006 longitudinal survey results with 2007 market updates. MedVantage Web site. Available at www.medvantage.com/Pdf/2006 NationalP4PStudy.pdf. Accessed May 17, 2009.
- Pearson SD, Schneider EC, Kleinman KP, Colin KL, Singer JA. The impact of pay-for-performance on health care quality in Massachusetts, 2001-2003. Health Aff (Millwood). 2008;27(4):1167-1176.
- De Brantes FS, D’Andrea BG. Physicians respond to pay-for-performance incentives: larger incentives yield greater participation. Am J Manag Care. 2009;15(5):305-310.
Ehab Hanna, MD, MBBch, FHM, understands better than most why hospitalists are frustrated with all the grand plans to utilize information technology (IT) for streamlining admissions, medical reconciliations, and the discharge process. As assistant chief medical information officer at Eastern Maine Medical Center in Bangor, he spends half his time scouting and assessing the value of new IT platforms and the other half as a front-line hospitalist.
Dr. Hanna and his colleagues are frustrated that software systems promise to deliver electronic medical records (EMR) but freeze up too often, take too long to download files, or can’t handle the functions for which they were developed. But he also knows the future of healthcare hinges on IT as much as anything else—and that done correctly, and probably expensively, it can be a savior.
“Every time we want to come up with a quality-initiative project, we want to ask, ‘What can IT do for us?’ ” Dr. Hanna says. He also acknowledges that “it’s all types of money, whether it be resources, funding, or people to implement [the system]. And there’s physician resistance to it.”
The link between quality and cost is paramount to healthcare and HM. As evidence, the keynote theme of HM09 in Chicago was quality improvement (QI)—defining it, making it a priority, setting up analytical metrics to measure it, and the most difficult step: implementing it. QI projects vary in size, shape, and scope. On one end of the spectrum: hand-washing compliance systems and simple programs to increase the prescription of pneumococcal vaccines. On the laborious and expensive end: EMR system integration with ambulatory care and pharmacy.
Industry leaders agree QI projects must include measurable goals and incentives for success. The flip side is that failure to reach those goals has to include a level of accountability.
One thing is for sure: The choice to focus on patient safety no longer is a choice, it’s a mandate. Patient-safety advocates are barking louder than ever, and the public and politicians are taking note. Medicare reimbursements are increasingly tied to performance measures, a trend that is likely to accelerate in light of recent news that Medicare will sink into the red in just eight years. Many expect that threshold to keep moving closer, too. President Obama has pledged to push major healthcare reform legislation—including a focus on EMR—through Congress. He wants to sign it into law by Labor Day.
On the other hand, there still is a relatively small sample of data on the effectiveness of pay-for-performance contracting in relation to overall patient health. There is a recurring call from many outside the HM field for more independent, empirical data that can pinpoint the quantifiable value of hospitalists. Discussions based on those values could satisfy group leaders, hospital administrators, and government regulators who still use the tried-and-true HM formula: value equals quality divided by cost.
“I see about as many challenges in QI as I do opportunities,” says SHM President Scott Flanders, MD, FHM, director of the hospitalist program at the University of Michigan Health System in Ann Arbor. “Is the horse before the cart? We have spent a lot of time and effort putting in place … programs before understanding the clinical effect.”
Can IT be EZ?
Dr. Hanna says hospitalists would embrace new IT initiatives immediately if they were easier to use. Many hospitalists are frustrated that in their pocket sits a handy, portable device that works in real time as a computer, a phone, a CD player, a GPS tracking device, and a scheduling secretary, yet they can’t use their E&M coding system without encountering constant hiccups, interruptions that take valuable time out of an already crowded 12-hour shift.
“Why isn’t it working like the iPhone?” is a complaint Dr. Hanna says he hears all the time.
One answer is that a number of medical software programs are limited in nature and don’t automatically network well with other systems already in place. User errors and other problems crop up regularly; plus, there isn’t a repository for people to measure different systems against each other. Some SHM leaders are considering a plan to create an online resource for IT vendors, but the society is leery of making recommendations because of potential conflicts of interest.
The Joint Commission, which accredits and certifies more than 15,000 healthcare organizations and programs, has been asked—by Dr. Hanna, among others—if it intends to regulate EMR vendors, which would let hospitalists know which systems are most reliable and useful. Commission President Mark Chassin, MD, MPP, MPH, said after his HM09 keynote address that his agency has no intention of doing so.
—Scott Flanders, MD, FHM, SHM president
“We don’t see [that] as a good message for us to give,” Dr. Chassin said.
IT also can be used in creative ways to spur patient-safety improvements. As hospitalists struggle to increase compliance with hand-hygiene standards, several hospitals have resorted to using video cameras above sinks to track whose hands are clean and whose hands are not clean, Robert Wachter, MD, FHM, said during his plenary address to conclude HM09. Real-time tracking runs through a software program and is displayed on a small, LED screen that hangs from the ceiling. Positive results are praised, while low rates of compliance result in pages to HM and hospital leaders to address hygiene issues. Dr. Wachter, professor and associate chairman of the department of medicine at the University of California at San Francisco Medical Center, chief of the division of hospital medicine, former SHM president and author of the blog “Wachter’s World” (www.wachtersworld), said UCSF hospital executives are considering the video system.
Dr. Wachter also says hospitalists can’t lament the rise of QI and the stricter standards that are attached. Nor can HM complain too loudly about the burdens QI places on them. As hospitalists argue that their value is partly defined by their contributions to hospital quality, they have to expect to be accountable to the claim. “We have positioned ourselves as being leaders in quality and safety,” he says. “This is not going out and being branded. We bring this in. We often say we have two sick patients; one is in front of us, the other is our organization.”
Pay for Performance?
Another largely unanswered question is how valuable pay-for-performance will be in improving patient care, safety, and satisfaction. While most HM contracts include additional compensation based on volume, bonus pay often ignores such measurable outcomes as readmission rates and length of stay.
“Does incentivizing healthcare quality actually improve health?” asks Susan Freeman, MD, chief medical officer at Temple University Hospital in Philadelphia. “It’s really hard to measure things that don’t happen—the myocardial infarction that didn’t happen, the stroke that didn’t happen.”
Active pay-for-performance programs numbered 160 in November 2007, four times the number of programs four years earlier.1 Yet as the concept catches on, the average physician incentive was 2% or less, and it seems to play a small role in how care was delivered.2
A more recent look at the issue concludes that if insurance companies and private employers worked with hospitals and physicians, attitudes toward pay for performance might change. “The amount of incentives available to physicians strongly affected their rate of participation,” say the authors of a May study in the American Journal of Managed Care. “Our analysis suggests that all stakeholders—health plans, physicians, and patients—would benefit from health plans collaborating on their pay-for-performance efforts to maximize physician participation.”3
Russell Holman, MD, FHM, chief operating officer of Cogent Healthcare, says another pay-for-performance concern is that improved quality is not always something that translates quickly into bottom-line savings. In today’s economic environment, in which every dollar spent has to be justified, it can actually be tougher to sell the upfront costs and long-term savings associated with investment in QI projects. “Improving quality is going to take a long time to see the advantages to the healthcare system,” says Dr. Holman, a former SHM president. “It is delayed gratification.”
Is HM Ready?
Dr. Holman says the pay-for-performance solution might end up as more of a hybrid of two reimbursement models, incentive-based pay and the controversial notion of billing bundling. Most hospitalists and HM groups are not entirely comfortable with the bundling idea, in which the hospital receives a lump-sum reimbursement for all services performed for a patient, then shells out payment to the surgeons, hospitalists, nurses, etc. The concept of hospitals as payment intermediaries adds an extra layer of bureaucracy, but the bundling concept appears to be gaining momentum.
While QI projects are the trendy way to measure value, Dr. Chassin points out that physicians need more detailed thresholds to exceed. Such measures as prescribing beta-blockers after myocardial infarction were good first-generation concepts, but better care requires better benchmarks, he says. Dr. Wachter echoes the sentiment, telling HM09 attendees that relying on past successes in QI won’t help hospitalists demonstrate their value or answer the “What have you done for me lately?” demand from hospital administrators.
Dr. Chassin compares healthcare to other high-pressure industries that do much better at controlling mistakes. Some estimates show nearly 100,000 people a year die from medical errors. Why? Many high-risk industries—airlines, nuclear energy, mining—have better quality and safety processes to protect against routine errors that continually plague healthcare (e.g., hospital-acquired infections, operations on the wrong patient).
The responsibility to get better doesn’t belong to one medical group, either. Dr. Chassin says researchers, the Centers for Medicare and Medicaid Services, and trade groups like SHM have to work collaboratively to design benchmarks that can be measured quantitatively. Once those measuring sticks are in place, though, Dr. Chassin believes hospitalists are the first responders who can best identify and solve problems.
“You have to understand the causes of the problems you’re trying to fix,” he says. “Hospitalists are on the front lines.” TH
Richard Quinn is a freelance writer based in New Jersey.
References
- Baker G, Delbanco S. Pay for performance: national perspective. 2006 longitudinal survey results with 2007 market updates. MedVantage Web site. Available at www.medvantage.com/Pdf/2006 NationalP4PStudy.pdf. Accessed May 17, 2009.
- Pearson SD, Schneider EC, Kleinman KP, Colin KL, Singer JA. The impact of pay-for-performance on health care quality in Massachusetts, 2001-2003. Health Aff (Millwood). 2008;27(4):1167-1176.
- De Brantes FS, D’Andrea BG. Physicians respond to pay-for-performance incentives: larger incentives yield greater participation. Am J Manag Care. 2009;15(5):305-310.
Beware the Doughnut Hole
Judy Zerzan, MD, MPH, was at a loss. A Health and Aging Policy Fellow and hospitalist at the University of Colorado Denver, Dr. Zerzan was examining a Medicare patient who was admitted to the hospital with an apparent urinary tract infection. But the same patient had been released from the hospital only five days earlier with an antibiotic prescription to treat an oddly similar infection.
The confusion only increased when lab cultures revealed an E. coli infection. It should have been sensitive to most antibiotics, especially to the brand-name moxifloxacin the patient said she had been taking since her last hospital stay.
Several days into the patient’s rehospitalization, a medical student finally cracked the case. “The medical student went in and started talking to her some more and learned that she didn’t fill the prescription because under her [insurance] plan, it was going to be something like $50, and she felt like she couldn’t afford it,” Dr. Zerzan recalls. “She felt really embarrassed about it, so she didn’t want to tell us that she didn’t fill it.”
The medical team sent the woman home with a $4 generic prescription. “She was much relieved,” Dr. Zerzan says. More importantly, the patient did not return to the hospital for a third stay.
Hospitalists might not see a $46 difference in drug pricing as a core consideration of patient care. But with many seniors enrolled in Medicare Part D’s prescription drug plan falling through holes in the partially privatized safety net, many agree that far more must be done to ensure that financial stress doesn’t lead to medical misfortune.
“It is an ethical issue,” says Stephen Soumerai, ScD, director of the Drug Policy Research Program and a professor in the Department of Ambulatory Care and Prevention at Harvard University. “ … It’s easy to target those people who are the most vulnerable in our society and, therefore, it is an opportunity to try to find ways for them to lower their problems of economic access to medicine.”
Soumerai calls cost-related medication nonadherence a matter of distributive justice. In a study published last year in the Journal of the American Medical Association, he and 10 co-authors found that even after enrolling in Medicare Part D, the sickest beneficiaries were just as likely to skip medications they couldn’t afford.1
Identify the Coverage Gap
Medicare’s prescription drug plan, initiated in 2006, has provided coverage for many beneficiaries who previously went without. But it has been widely criticized as unnecessarily confusing by both doctors and patients—and particularly expensive for beneficiaries unlucky enough to fall into its notorious gap in coverage, dubbed the “doughnut hole” (see “Medicare Part D: The Basics,” right).
That economic burden can be exacerbated by medical illiteracy, ignorance, and misinformation. Recent Consumer Reports polls suggest as few as 4% of patients discuss drug prices with their doctors; almost half of Americans have reservations or misgivings about lower-cost generic drugs.
As focal points in the coordination of patient care, hospitalists are better positioned than most to help steer the most vulnerable away from the douhgnut hole while helping hospitals equitably distribute limited resources. But hospitalists often are completely unaware of a patient’s plight. “My research interest is prescription drug coverage, so I think I’m more keyed into it, but certainly even I don’t generally think about, when discharging a patient, if something is going to be on their Part D formulary or not, or what sort of prior-authorization hoops their primary-care doc may have to go through,” Dr. Zerzan says. “And I think that’s generally true of my colleagues as well.”
With tight schedules, a limited personal history with patients, disparities in local resources, and a litany of cost-control measures established by insurers, even hospitalists in the know concede that helping Medicare patients manage drug costs can be an exercise in frustration. As a result, patients are often left with medications that require higher cost-sharing or aren’t even on a plan’s formulary, forcing them to pay out-of-pocket for a prescription. Adding insult to injury, any money spent on a medication not covered by a Part D plan doesn’t count toward getting a patient out of the doughnut hole.
The Extra Mile
Jocelyne Watrous, a Medicare beneficiary consultant at the Willimantic, Conn.-based Center for Medicare Advocacy, says drug affordability while in the doughnut hole is a hardship for many. But so are specialty drug copays that range from 25% to 33% of the total price (see Table 1, below). For expensive prescriptions, Watrous says, hospitalists can get the name of a patient’s Part D formulary from the membership card and check for restrictions, such as prior authorization, quantity limits, or step therapy. “This is a terrible burden to place on physicians and their staffs, but nothing is worse than having the patient come back to the hospital via the ER because they could not get the drug prescribed by their doctor,” she says. “Best to square it all away before discharge, if possible.”
Brandon Koretz, MD, an associate clinical professor of geriatric medicine at the University of California at Los Angeles, says keeping track of differences among the dozens of Part D plans isn’t feasible. “Oftentimes, what happens is, you find yourself writing a prescription for what you think is a reasonable and cost-effective treatment, only to find out that drug A is not on insurance company B’s formulary, but drug C is,” Dr. Koretz says.
And if doctors are confused by the array of Part D formularies, hospitalists wonder, how can geriatric patients be expected to navigate the system, especially given the not-insignificant number with cognitive impairments?
Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C., compares the consumer paralysis created by the proliferation of prescription drug plans to walking by a store display featuring 20 brands of jam. “You’re kind of awed by it, but you don’t buy anything because you’re kind of intimidated,” he says. Only instead of 20 varieties, the typical local government agency offered 48 competing Part D plans in November 2006, with some boasting more than 70. In a new study sponsored by the Henry J. Kaiser Family Foundation, Massachusetts Institute of Technology economist Jonathan Gruber found that when seniors made a decision, only 6% to 9% chose the least expensive plan, while the remaining seniors paid an extra $360 to $520 annually (www.kff.org/medicare/7864.cfm).
All too often, economic problems are simply transferred to other providers. Nursing home pharmacists have complained to Dr. Zerzan about doctors switching seniors’ medications to hospital formulary drugs that aren’t covered under Part D, requiring pharmacists to switch the drugs back again. “From their standpoint, it takes a ton of work because hospitalists don’t know what’s on their formulary,” she says.
Miscommunication can wreak havoc in other ways. Dr. Zerzan recalls how her parents were hosting her grandmother in Oregon when she fell and broke her pelvis. At the hospital, she arrived with two overlapping medication lists from her primary-care physician, cardiologist, and rheumatologist in California. The lists contained several combination pills that essentially duplicated her cholesterol and blood pressure medications. Instead of conducting a medication review, the hospital left the lists intact and added its own, sending her grandmother home with a “fistful of prescriptions.” Unsurprisingly, her blood pressure dipped dangerously. “She actually ended up going back into the hospital briefly to sort out her medications because it was such a mess,” Dr. Zerzan says.
Two-Way Conversation
Hospitalists generally don’t have the benefit of a longstanding relationship with their patients, says Ashley Beard, MD, PhD, a pharmaceutical policy research fellow in the Department of Ambulatory Care and Prevention at Harvard University. “And they’re dealing with people who are at their most vulnerable and least able to communicate effectively about what is going on in their lives.”
The virtual impossibility of knowing Part D formularies for every patient, she says, only increases the importance of effective bedside discussions and open-ended questions. “I think that communicating about costs really has been, and continues to be, a taboo subject in direct patient encounters, even though it is widely talked about in the research and popular press,” she says.
Likewise, hospitalists can intervene during transition planning, Dr. Beard says, when “the goal of the hospitalist is to stabilize the patient to be able to go out into the community and then have community follow-up care, preferably by a primary-care physician.” For people who don’t seek care regularly, she says, part of that stabilization can be a medication review that eliminates nonessential or harmful drugs and alleviates a patient’s financial burden.
Christine Lum Lung, MD, medical director of the independent Northern Colorado Hospitalists group, says a proactive discharge-planning department can be a huge help in coordinating such transitions of care. Her privately run group, affiliated with two private nonprofit hospitals in Loveland and Fort Collins, works closely with a “very active and involved” department that regularly meets with patients to assess financial issues. “Then they will approach us oftentimes with any concerns or issues with the discharge plan and medication,” she says.
Real-Time Solutions
Patient advocates have proposed a combination of other incentives to encourage better coordination among healthcare providers, including penalties for preventable rehospitalizations and a faster rollout of e-prescribing and electronic databases. Many hospitalists are particularly enthusiastic about the potential of electronic health records to assist them and their patients, though researchers like Soumerai are far less convinced about the merits of such a billion-dollar investment.
In the meantime, Dr. Lum Lung points to other low-cost solutions. At every workstation, her hospitals have posted details of the $4-a-month generic prescription programs offered by retailers like Target and Walmart. “I think it is probably prudent for us to be cognizant of that for everybody,” she says, “regardless of their insurance or payor source.”
For a recently discharged patient, one hospital Dr. Lum Lung works with proactively asked a pharmacist to run a few antibiotics through the patient’s insurance formulary to help pick the most cost-effective one. If a patient can’t afford the drug, the discharge-planning department can look into local drug assistance programs or the hospital’s voucher system, which allows medications to be filled by an in-house pharmacy. “We don’t want to—especially right now—make somebody have to make a choice between making their mortgage or rent payment or paying for a very expensive medication,” she says.
With so much information coming at them at once, hospitalists say, patients may need to be monitored once they get home. And with limited medical resources, physicians must constantly ask themselves whether they’re using the most appropriate and least expensive medications for every patient. “The hospitalists, in particular, are the natural leaders for this kind of thinking,” Dr. Koretz says. “Thinking about medical problems not as isolated, patient-specific problems, but rather as problems of systems of care, and processes of care.” TH
Bryn Nelson is a freelance medical writer based in Seattle.
Reference
- Madden JM, Graves AJ, Zhang F, et al. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299(16):1922-1928.
Judy Zerzan, MD, MPH, was at a loss. A Health and Aging Policy Fellow and hospitalist at the University of Colorado Denver, Dr. Zerzan was examining a Medicare patient who was admitted to the hospital with an apparent urinary tract infection. But the same patient had been released from the hospital only five days earlier with an antibiotic prescription to treat an oddly similar infection.
The confusion only increased when lab cultures revealed an E. coli infection. It should have been sensitive to most antibiotics, especially to the brand-name moxifloxacin the patient said she had been taking since her last hospital stay.
Several days into the patient’s rehospitalization, a medical student finally cracked the case. “The medical student went in and started talking to her some more and learned that she didn’t fill the prescription because under her [insurance] plan, it was going to be something like $50, and she felt like she couldn’t afford it,” Dr. Zerzan recalls. “She felt really embarrassed about it, so she didn’t want to tell us that she didn’t fill it.”
The medical team sent the woman home with a $4 generic prescription. “She was much relieved,” Dr. Zerzan says. More importantly, the patient did not return to the hospital for a third stay.
Hospitalists might not see a $46 difference in drug pricing as a core consideration of patient care. But with many seniors enrolled in Medicare Part D’s prescription drug plan falling through holes in the partially privatized safety net, many agree that far more must be done to ensure that financial stress doesn’t lead to medical misfortune.
“It is an ethical issue,” says Stephen Soumerai, ScD, director of the Drug Policy Research Program and a professor in the Department of Ambulatory Care and Prevention at Harvard University. “ … It’s easy to target those people who are the most vulnerable in our society and, therefore, it is an opportunity to try to find ways for them to lower their problems of economic access to medicine.”
Soumerai calls cost-related medication nonadherence a matter of distributive justice. In a study published last year in the Journal of the American Medical Association, he and 10 co-authors found that even after enrolling in Medicare Part D, the sickest beneficiaries were just as likely to skip medications they couldn’t afford.1
Identify the Coverage Gap
Medicare’s prescription drug plan, initiated in 2006, has provided coverage for many beneficiaries who previously went without. But it has been widely criticized as unnecessarily confusing by both doctors and patients—and particularly expensive for beneficiaries unlucky enough to fall into its notorious gap in coverage, dubbed the “doughnut hole” (see “Medicare Part D: The Basics,” right).
That economic burden can be exacerbated by medical illiteracy, ignorance, and misinformation. Recent Consumer Reports polls suggest as few as 4% of patients discuss drug prices with their doctors; almost half of Americans have reservations or misgivings about lower-cost generic drugs.
As focal points in the coordination of patient care, hospitalists are better positioned than most to help steer the most vulnerable away from the douhgnut hole while helping hospitals equitably distribute limited resources. But hospitalists often are completely unaware of a patient’s plight. “My research interest is prescription drug coverage, so I think I’m more keyed into it, but certainly even I don’t generally think about, when discharging a patient, if something is going to be on their Part D formulary or not, or what sort of prior-authorization hoops their primary-care doc may have to go through,” Dr. Zerzan says. “And I think that’s generally true of my colleagues as well.”
With tight schedules, a limited personal history with patients, disparities in local resources, and a litany of cost-control measures established by insurers, even hospitalists in the know concede that helping Medicare patients manage drug costs can be an exercise in frustration. As a result, patients are often left with medications that require higher cost-sharing or aren’t even on a plan’s formulary, forcing them to pay out-of-pocket for a prescription. Adding insult to injury, any money spent on a medication not covered by a Part D plan doesn’t count toward getting a patient out of the doughnut hole.
The Extra Mile
Jocelyne Watrous, a Medicare beneficiary consultant at the Willimantic, Conn.-based Center for Medicare Advocacy, says drug affordability while in the doughnut hole is a hardship for many. But so are specialty drug copays that range from 25% to 33% of the total price (see Table 1, below). For expensive prescriptions, Watrous says, hospitalists can get the name of a patient’s Part D formulary from the membership card and check for restrictions, such as prior authorization, quantity limits, or step therapy. “This is a terrible burden to place on physicians and their staffs, but nothing is worse than having the patient come back to the hospital via the ER because they could not get the drug prescribed by their doctor,” she says. “Best to square it all away before discharge, if possible.”
Brandon Koretz, MD, an associate clinical professor of geriatric medicine at the University of California at Los Angeles, says keeping track of differences among the dozens of Part D plans isn’t feasible. “Oftentimes, what happens is, you find yourself writing a prescription for what you think is a reasonable and cost-effective treatment, only to find out that drug A is not on insurance company B’s formulary, but drug C is,” Dr. Koretz says.
And if doctors are confused by the array of Part D formularies, hospitalists wonder, how can geriatric patients be expected to navigate the system, especially given the not-insignificant number with cognitive impairments?
Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C., compares the consumer paralysis created by the proliferation of prescription drug plans to walking by a store display featuring 20 brands of jam. “You’re kind of awed by it, but you don’t buy anything because you’re kind of intimidated,” he says. Only instead of 20 varieties, the typical local government agency offered 48 competing Part D plans in November 2006, with some boasting more than 70. In a new study sponsored by the Henry J. Kaiser Family Foundation, Massachusetts Institute of Technology economist Jonathan Gruber found that when seniors made a decision, only 6% to 9% chose the least expensive plan, while the remaining seniors paid an extra $360 to $520 annually (www.kff.org/medicare/7864.cfm).
All too often, economic problems are simply transferred to other providers. Nursing home pharmacists have complained to Dr. Zerzan about doctors switching seniors’ medications to hospital formulary drugs that aren’t covered under Part D, requiring pharmacists to switch the drugs back again. “From their standpoint, it takes a ton of work because hospitalists don’t know what’s on their formulary,” she says.
Miscommunication can wreak havoc in other ways. Dr. Zerzan recalls how her parents were hosting her grandmother in Oregon when she fell and broke her pelvis. At the hospital, she arrived with two overlapping medication lists from her primary-care physician, cardiologist, and rheumatologist in California. The lists contained several combination pills that essentially duplicated her cholesterol and blood pressure medications. Instead of conducting a medication review, the hospital left the lists intact and added its own, sending her grandmother home with a “fistful of prescriptions.” Unsurprisingly, her blood pressure dipped dangerously. “She actually ended up going back into the hospital briefly to sort out her medications because it was such a mess,” Dr. Zerzan says.
Two-Way Conversation
Hospitalists generally don’t have the benefit of a longstanding relationship with their patients, says Ashley Beard, MD, PhD, a pharmaceutical policy research fellow in the Department of Ambulatory Care and Prevention at Harvard University. “And they’re dealing with people who are at their most vulnerable and least able to communicate effectively about what is going on in their lives.”
The virtual impossibility of knowing Part D formularies for every patient, she says, only increases the importance of effective bedside discussions and open-ended questions. “I think that communicating about costs really has been, and continues to be, a taboo subject in direct patient encounters, even though it is widely talked about in the research and popular press,” she says.
Likewise, hospitalists can intervene during transition planning, Dr. Beard says, when “the goal of the hospitalist is to stabilize the patient to be able to go out into the community and then have community follow-up care, preferably by a primary-care physician.” For people who don’t seek care regularly, she says, part of that stabilization can be a medication review that eliminates nonessential or harmful drugs and alleviates a patient’s financial burden.
Christine Lum Lung, MD, medical director of the independent Northern Colorado Hospitalists group, says a proactive discharge-planning department can be a huge help in coordinating such transitions of care. Her privately run group, affiliated with two private nonprofit hospitals in Loveland and Fort Collins, works closely with a “very active and involved” department that regularly meets with patients to assess financial issues. “Then they will approach us oftentimes with any concerns or issues with the discharge plan and medication,” she says.
Real-Time Solutions
Patient advocates have proposed a combination of other incentives to encourage better coordination among healthcare providers, including penalties for preventable rehospitalizations and a faster rollout of e-prescribing and electronic databases. Many hospitalists are particularly enthusiastic about the potential of electronic health records to assist them and their patients, though researchers like Soumerai are far less convinced about the merits of such a billion-dollar investment.
In the meantime, Dr. Lum Lung points to other low-cost solutions. At every workstation, her hospitals have posted details of the $4-a-month generic prescription programs offered by retailers like Target and Walmart. “I think it is probably prudent for us to be cognizant of that for everybody,” she says, “regardless of their insurance or payor source.”
For a recently discharged patient, one hospital Dr. Lum Lung works with proactively asked a pharmacist to run a few antibiotics through the patient’s insurance formulary to help pick the most cost-effective one. If a patient can’t afford the drug, the discharge-planning department can look into local drug assistance programs or the hospital’s voucher system, which allows medications to be filled by an in-house pharmacy. “We don’t want to—especially right now—make somebody have to make a choice between making their mortgage or rent payment or paying for a very expensive medication,” she says.
With so much information coming at them at once, hospitalists say, patients may need to be monitored once they get home. And with limited medical resources, physicians must constantly ask themselves whether they’re using the most appropriate and least expensive medications for every patient. “The hospitalists, in particular, are the natural leaders for this kind of thinking,” Dr. Koretz says. “Thinking about medical problems not as isolated, patient-specific problems, but rather as problems of systems of care, and processes of care.” TH
Bryn Nelson is a freelance medical writer based in Seattle.
Reference
- Madden JM, Graves AJ, Zhang F, et al. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299(16):1922-1928.
Judy Zerzan, MD, MPH, was at a loss. A Health and Aging Policy Fellow and hospitalist at the University of Colorado Denver, Dr. Zerzan was examining a Medicare patient who was admitted to the hospital with an apparent urinary tract infection. But the same patient had been released from the hospital only five days earlier with an antibiotic prescription to treat an oddly similar infection.
The confusion only increased when lab cultures revealed an E. coli infection. It should have been sensitive to most antibiotics, especially to the brand-name moxifloxacin the patient said she had been taking since her last hospital stay.
Several days into the patient’s rehospitalization, a medical student finally cracked the case. “The medical student went in and started talking to her some more and learned that she didn’t fill the prescription because under her [insurance] plan, it was going to be something like $50, and she felt like she couldn’t afford it,” Dr. Zerzan recalls. “She felt really embarrassed about it, so she didn’t want to tell us that she didn’t fill it.”
The medical team sent the woman home with a $4 generic prescription. “She was much relieved,” Dr. Zerzan says. More importantly, the patient did not return to the hospital for a third stay.
Hospitalists might not see a $46 difference in drug pricing as a core consideration of patient care. But with many seniors enrolled in Medicare Part D’s prescription drug plan falling through holes in the partially privatized safety net, many agree that far more must be done to ensure that financial stress doesn’t lead to medical misfortune.
“It is an ethical issue,” says Stephen Soumerai, ScD, director of the Drug Policy Research Program and a professor in the Department of Ambulatory Care and Prevention at Harvard University. “ … It’s easy to target those people who are the most vulnerable in our society and, therefore, it is an opportunity to try to find ways for them to lower their problems of economic access to medicine.”
Soumerai calls cost-related medication nonadherence a matter of distributive justice. In a study published last year in the Journal of the American Medical Association, he and 10 co-authors found that even after enrolling in Medicare Part D, the sickest beneficiaries were just as likely to skip medications they couldn’t afford.1
Identify the Coverage Gap
Medicare’s prescription drug plan, initiated in 2006, has provided coverage for many beneficiaries who previously went without. But it has been widely criticized as unnecessarily confusing by both doctors and patients—and particularly expensive for beneficiaries unlucky enough to fall into its notorious gap in coverage, dubbed the “doughnut hole” (see “Medicare Part D: The Basics,” right).
That economic burden can be exacerbated by medical illiteracy, ignorance, and misinformation. Recent Consumer Reports polls suggest as few as 4% of patients discuss drug prices with their doctors; almost half of Americans have reservations or misgivings about lower-cost generic drugs.
As focal points in the coordination of patient care, hospitalists are better positioned than most to help steer the most vulnerable away from the douhgnut hole while helping hospitals equitably distribute limited resources. But hospitalists often are completely unaware of a patient’s plight. “My research interest is prescription drug coverage, so I think I’m more keyed into it, but certainly even I don’t generally think about, when discharging a patient, if something is going to be on their Part D formulary or not, or what sort of prior-authorization hoops their primary-care doc may have to go through,” Dr. Zerzan says. “And I think that’s generally true of my colleagues as well.”
With tight schedules, a limited personal history with patients, disparities in local resources, and a litany of cost-control measures established by insurers, even hospitalists in the know concede that helping Medicare patients manage drug costs can be an exercise in frustration. As a result, patients are often left with medications that require higher cost-sharing or aren’t even on a plan’s formulary, forcing them to pay out-of-pocket for a prescription. Adding insult to injury, any money spent on a medication not covered by a Part D plan doesn’t count toward getting a patient out of the doughnut hole.
The Extra Mile
Jocelyne Watrous, a Medicare beneficiary consultant at the Willimantic, Conn.-based Center for Medicare Advocacy, says drug affordability while in the doughnut hole is a hardship for many. But so are specialty drug copays that range from 25% to 33% of the total price (see Table 1, below). For expensive prescriptions, Watrous says, hospitalists can get the name of a patient’s Part D formulary from the membership card and check for restrictions, such as prior authorization, quantity limits, or step therapy. “This is a terrible burden to place on physicians and their staffs, but nothing is worse than having the patient come back to the hospital via the ER because they could not get the drug prescribed by their doctor,” she says. “Best to square it all away before discharge, if possible.”
Brandon Koretz, MD, an associate clinical professor of geriatric medicine at the University of California at Los Angeles, says keeping track of differences among the dozens of Part D plans isn’t feasible. “Oftentimes, what happens is, you find yourself writing a prescription for what you think is a reasonable and cost-effective treatment, only to find out that drug A is not on insurance company B’s formulary, but drug C is,” Dr. Koretz says.
And if doctors are confused by the array of Part D formularies, hospitalists wonder, how can geriatric patients be expected to navigate the system, especially given the not-insignificant number with cognitive impairments?
Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C., compares the consumer paralysis created by the proliferation of prescription drug plans to walking by a store display featuring 20 brands of jam. “You’re kind of awed by it, but you don’t buy anything because you’re kind of intimidated,” he says. Only instead of 20 varieties, the typical local government agency offered 48 competing Part D plans in November 2006, with some boasting more than 70. In a new study sponsored by the Henry J. Kaiser Family Foundation, Massachusetts Institute of Technology economist Jonathan Gruber found that when seniors made a decision, only 6% to 9% chose the least expensive plan, while the remaining seniors paid an extra $360 to $520 annually (www.kff.org/medicare/7864.cfm).
All too often, economic problems are simply transferred to other providers. Nursing home pharmacists have complained to Dr. Zerzan about doctors switching seniors’ medications to hospital formulary drugs that aren’t covered under Part D, requiring pharmacists to switch the drugs back again. “From their standpoint, it takes a ton of work because hospitalists don’t know what’s on their formulary,” she says.
Miscommunication can wreak havoc in other ways. Dr. Zerzan recalls how her parents were hosting her grandmother in Oregon when she fell and broke her pelvis. At the hospital, she arrived with two overlapping medication lists from her primary-care physician, cardiologist, and rheumatologist in California. The lists contained several combination pills that essentially duplicated her cholesterol and blood pressure medications. Instead of conducting a medication review, the hospital left the lists intact and added its own, sending her grandmother home with a “fistful of prescriptions.” Unsurprisingly, her blood pressure dipped dangerously. “She actually ended up going back into the hospital briefly to sort out her medications because it was such a mess,” Dr. Zerzan says.
Two-Way Conversation
Hospitalists generally don’t have the benefit of a longstanding relationship with their patients, says Ashley Beard, MD, PhD, a pharmaceutical policy research fellow in the Department of Ambulatory Care and Prevention at Harvard University. “And they’re dealing with people who are at their most vulnerable and least able to communicate effectively about what is going on in their lives.”
The virtual impossibility of knowing Part D formularies for every patient, she says, only increases the importance of effective bedside discussions and open-ended questions. “I think that communicating about costs really has been, and continues to be, a taboo subject in direct patient encounters, even though it is widely talked about in the research and popular press,” she says.
Likewise, hospitalists can intervene during transition planning, Dr. Beard says, when “the goal of the hospitalist is to stabilize the patient to be able to go out into the community and then have community follow-up care, preferably by a primary-care physician.” For people who don’t seek care regularly, she says, part of that stabilization can be a medication review that eliminates nonessential or harmful drugs and alleviates a patient’s financial burden.
Christine Lum Lung, MD, medical director of the independent Northern Colorado Hospitalists group, says a proactive discharge-planning department can be a huge help in coordinating such transitions of care. Her privately run group, affiliated with two private nonprofit hospitals in Loveland and Fort Collins, works closely with a “very active and involved” department that regularly meets with patients to assess financial issues. “Then they will approach us oftentimes with any concerns or issues with the discharge plan and medication,” she says.
Real-Time Solutions
Patient advocates have proposed a combination of other incentives to encourage better coordination among healthcare providers, including penalties for preventable rehospitalizations and a faster rollout of e-prescribing and electronic databases. Many hospitalists are particularly enthusiastic about the potential of electronic health records to assist them and their patients, though researchers like Soumerai are far less convinced about the merits of such a billion-dollar investment.
In the meantime, Dr. Lum Lung points to other low-cost solutions. At every workstation, her hospitals have posted details of the $4-a-month generic prescription programs offered by retailers like Target and Walmart. “I think it is probably prudent for us to be cognizant of that for everybody,” she says, “regardless of their insurance or payor source.”
For a recently discharged patient, one hospital Dr. Lum Lung works with proactively asked a pharmacist to run a few antibiotics through the patient’s insurance formulary to help pick the most cost-effective one. If a patient can’t afford the drug, the discharge-planning department can look into local drug assistance programs or the hospital’s voucher system, which allows medications to be filled by an in-house pharmacy. “We don’t want to—especially right now—make somebody have to make a choice between making their mortgage or rent payment or paying for a very expensive medication,” she says.
With so much information coming at them at once, hospitalists say, patients may need to be monitored once they get home. And with limited medical resources, physicians must constantly ask themselves whether they’re using the most appropriate and least expensive medications for every patient. “The hospitalists, in particular, are the natural leaders for this kind of thinking,” Dr. Koretz says. “Thinking about medical problems not as isolated, patient-specific problems, but rather as problems of systems of care, and processes of care.” TH
Bryn Nelson is a freelance medical writer based in Seattle.
Reference
- Madden JM, Graves AJ, Zhang F, et al. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299(16):1922-1928.
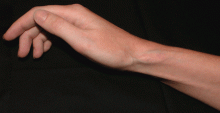
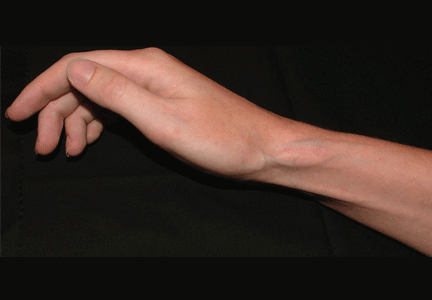
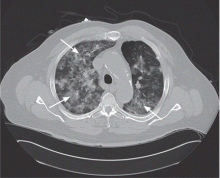
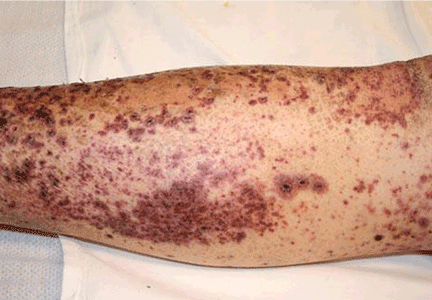
Soft tissue atrophy after corticosteroid injection
A 27-year-old woman presents with pain and tenderness over her right radial styloid. Examination reveals tenderness to palpation and a positive Finkelstein test, and her condition is diagnosed as de Quervain tenosynovitis. She is referred for occupational therapy and for a corticosteroid injection.
Q: On the basis of the skin findings, which corticosteroid injection was most likely used?
- Triamcinolone hexacetonide (Aristospan)
- Dexamethasone sodium phosphate (Decadron)
- Betamethasone sodium phosphate and betamethasone acetate (Celestone Soluspan)
- Triamcinolone acetonide (Kenalog-40)
A: Both triamcinolone hexacetonide and triamcinolone acetonide are correct, as they are the least soluble of the agents listed.
ADVERSE EFFECTS OF STEROID INJECTIONS
Soft tissue atrophy and local depigmentation are possible adverse effects of any steroid injection, particularly when given at a superficial site.1,2 Although these are rare, with an estimated risk of less than 1%, patients still need to be told about these potential side effects.3 In addition, these adverse effects of injection may be prevented by applying pressure with gauze over the injection site as the needle is withdrawn to prevent leakage of corticosteroid along the needle track.3
Soft tissue atrophy generally appears in 1 to 4 months and resolves 6 to 30 months later. 4 Patients with darker skin are at greater risk of depigmentation.
The cause of the pigment changes is not fully understood but may be related either to the steroid or to the constituents of the vehicle in which the steroid is suspended.5
CHOOSING THE APPROPRIATE STEROID PREPARATION
Although soft tissue (fat) atrophy and local depigmentation are possible with any steroid preparation injected into soft tissue, the risk can be modulated by using a corticosteroid agent with appropriate solubility. A less soluble agent such as triamcinolone acetonide or hexacetonide is preferred for intra-articular injections of deep structures, such as the knee, elbow, or shoulder. A more soluble agent, such as betamethasone sodium phosphate and acetate or dexamethasone sodium phosphate, is preferred for soft tissue injections of bursae, tendon sheaths, metacarpophalangeal joints, proximal phalangeal joints, and the carpal tunnel.
OTHER POSSIBLE COMPLICATIONS
Other potential complications of corticosteroid injection include pain, bleeding, infection (risk 1 in 40,000), flushing, post-injection flare (< 1%), nerve damage, tendon weakening, and rarely, tendon rupture. In cases of tendonitis, it is very important to ensure that the drug is injected into the tendon sheath and not the tendon. A general rule for tendon sheath injection is to not inject if resistance is met.
PATIENT UNWILLING TO RECEIVE MORE INJECTIONS
This patient’s symptoms persist, with a painful right wrist, perhaps due to refractory de Quervain tenosynovitis, nerve damage, or tendinosis. In time, the tenosynovitis and atrophy may improve, but she is reluctant to receive any more injections, as she was not forewarned about the possibility of atrophy.
- Saunders S, Longworth S. Injection Techniques in Orthopaedics and Sports Medicine. 3rd ed. London: Elsevier; 2006.
- Cardone DA, Tallia AF. Joint and soft tissue injection. Am Fam Physician 2002; 66:283–288.
- Gray RG, Gottlieb NL. Intra-articular corticosteroids: an updated assessment. Clin Orthop Relat Res 1983; 177:235–263.
- Cassidy JT, Bole GG. Cutaneous atrophy secondary to intra-articular corticosteroid administration. Ann Intern Med 1966; 65:1008–1018.
- Newman RJ. Local skin depigmentation due to corticosteroid injection. Br Med J (Clin Res Ed) 1984; 288:1725–1726.
A 27-year-old woman presents with pain and tenderness over her right radial styloid. Examination reveals tenderness to palpation and a positive Finkelstein test, and her condition is diagnosed as de Quervain tenosynovitis. She is referred for occupational therapy and for a corticosteroid injection.
Q: On the basis of the skin findings, which corticosteroid injection was most likely used?
- Triamcinolone hexacetonide (Aristospan)
- Dexamethasone sodium phosphate (Decadron)
- Betamethasone sodium phosphate and betamethasone acetate (Celestone Soluspan)
- Triamcinolone acetonide (Kenalog-40)
A: Both triamcinolone hexacetonide and triamcinolone acetonide are correct, as they are the least soluble of the agents listed.
ADVERSE EFFECTS OF STEROID INJECTIONS
Soft tissue atrophy and local depigmentation are possible adverse effects of any steroid injection, particularly when given at a superficial site.1,2 Although these are rare, with an estimated risk of less than 1%, patients still need to be told about these potential side effects.3 In addition, these adverse effects of injection may be prevented by applying pressure with gauze over the injection site as the needle is withdrawn to prevent leakage of corticosteroid along the needle track.3
Soft tissue atrophy generally appears in 1 to 4 months and resolves 6 to 30 months later. 4 Patients with darker skin are at greater risk of depigmentation.
The cause of the pigment changes is not fully understood but may be related either to the steroid or to the constituents of the vehicle in which the steroid is suspended.5
CHOOSING THE APPROPRIATE STEROID PREPARATION
Although soft tissue (fat) atrophy and local depigmentation are possible with any steroid preparation injected into soft tissue, the risk can be modulated by using a corticosteroid agent with appropriate solubility. A less soluble agent such as triamcinolone acetonide or hexacetonide is preferred for intra-articular injections of deep structures, such as the knee, elbow, or shoulder. A more soluble agent, such as betamethasone sodium phosphate and acetate or dexamethasone sodium phosphate, is preferred for soft tissue injections of bursae, tendon sheaths, metacarpophalangeal joints, proximal phalangeal joints, and the carpal tunnel.
OTHER POSSIBLE COMPLICATIONS
Other potential complications of corticosteroid injection include pain, bleeding, infection (risk 1 in 40,000), flushing, post-injection flare (< 1%), nerve damage, tendon weakening, and rarely, tendon rupture. In cases of tendonitis, it is very important to ensure that the drug is injected into the tendon sheath and not the tendon. A general rule for tendon sheath injection is to not inject if resistance is met.
PATIENT UNWILLING TO RECEIVE MORE INJECTIONS
This patient’s symptoms persist, with a painful right wrist, perhaps due to refractory de Quervain tenosynovitis, nerve damage, or tendinosis. In time, the tenosynovitis and atrophy may improve, but she is reluctant to receive any more injections, as she was not forewarned about the possibility of atrophy.
A 27-year-old woman presents with pain and tenderness over her right radial styloid. Examination reveals tenderness to palpation and a positive Finkelstein test, and her condition is diagnosed as de Quervain tenosynovitis. She is referred for occupational therapy and for a corticosteroid injection.
Q: On the basis of the skin findings, which corticosteroid injection was most likely used?
- Triamcinolone hexacetonide (Aristospan)
- Dexamethasone sodium phosphate (Decadron)
- Betamethasone sodium phosphate and betamethasone acetate (Celestone Soluspan)
- Triamcinolone acetonide (Kenalog-40)
A: Both triamcinolone hexacetonide and triamcinolone acetonide are correct, as they are the least soluble of the agents listed.
ADVERSE EFFECTS OF STEROID INJECTIONS
Soft tissue atrophy and local depigmentation are possible adverse effects of any steroid injection, particularly when given at a superficial site.1,2 Although these are rare, with an estimated risk of less than 1%, patients still need to be told about these potential side effects.3 In addition, these adverse effects of injection may be prevented by applying pressure with gauze over the injection site as the needle is withdrawn to prevent leakage of corticosteroid along the needle track.3
Soft tissue atrophy generally appears in 1 to 4 months and resolves 6 to 30 months later. 4 Patients with darker skin are at greater risk of depigmentation.
The cause of the pigment changes is not fully understood but may be related either to the steroid or to the constituents of the vehicle in which the steroid is suspended.5
CHOOSING THE APPROPRIATE STEROID PREPARATION
Although soft tissue (fat) atrophy and local depigmentation are possible with any steroid preparation injected into soft tissue, the risk can be modulated by using a corticosteroid agent with appropriate solubility. A less soluble agent such as triamcinolone acetonide or hexacetonide is preferred for intra-articular injections of deep structures, such as the knee, elbow, or shoulder. A more soluble agent, such as betamethasone sodium phosphate and acetate or dexamethasone sodium phosphate, is preferred for soft tissue injections of bursae, tendon sheaths, metacarpophalangeal joints, proximal phalangeal joints, and the carpal tunnel.
OTHER POSSIBLE COMPLICATIONS
Other potential complications of corticosteroid injection include pain, bleeding, infection (risk 1 in 40,000), flushing, post-injection flare (< 1%), nerve damage, tendon weakening, and rarely, tendon rupture. In cases of tendonitis, it is very important to ensure that the drug is injected into the tendon sheath and not the tendon. A general rule for tendon sheath injection is to not inject if resistance is met.
PATIENT UNWILLING TO RECEIVE MORE INJECTIONS
This patient’s symptoms persist, with a painful right wrist, perhaps due to refractory de Quervain tenosynovitis, nerve damage, or tendinosis. In time, the tenosynovitis and atrophy may improve, but she is reluctant to receive any more injections, as she was not forewarned about the possibility of atrophy.
- Saunders S, Longworth S. Injection Techniques in Orthopaedics and Sports Medicine. 3rd ed. London: Elsevier; 2006.
- Cardone DA, Tallia AF. Joint and soft tissue injection. Am Fam Physician 2002; 66:283–288.
- Gray RG, Gottlieb NL. Intra-articular corticosteroids: an updated assessment. Clin Orthop Relat Res 1983; 177:235–263.
- Cassidy JT, Bole GG. Cutaneous atrophy secondary to intra-articular corticosteroid administration. Ann Intern Med 1966; 65:1008–1018.
- Newman RJ. Local skin depigmentation due to corticosteroid injection. Br Med J (Clin Res Ed) 1984; 288:1725–1726.
- Saunders S, Longworth S. Injection Techniques in Orthopaedics and Sports Medicine. 3rd ed. London: Elsevier; 2006.
- Cardone DA, Tallia AF. Joint and soft tissue injection. Am Fam Physician 2002; 66:283–288.
- Gray RG, Gottlieb NL. Intra-articular corticosteroids: an updated assessment. Clin Orthop Relat Res 1983; 177:235–263.
- Cassidy JT, Bole GG. Cutaneous atrophy secondary to intra-articular corticosteroid administration. Ann Intern Med 1966; 65:1008–1018.
- Newman RJ. Local skin depigmentation due to corticosteroid injection. Br Med J (Clin Res Ed) 1984; 288:1725–1726.
Is telemetry overused? Is it as helpful as thought?
Telemetry—from the Greek words tele (remote) and metron (measure)—for cardiac monitoring was developed in the mid-1960s by Spacelabs Medical for use in spaceflight.1 The system was later adopted in hospitals to detect life-threatening arrhythmias.
Guidelines for the use of telemetry were published in 1991 by the American College of Cardiology (ACC)2 in response to concerns raised by its increasing use in noncritical care settings during the 30 years after its introduction to clinical medicine. The latest revision of the guidelines was published in 2004 by the American Heart Association (AHA).3
However, the guidelines are based largely on expert opinion and on research data in electrocardiography. Few clinical trials of telemetry have been published, and they were either retrospective or nonrandomized. In fact, there were no published randomized trials at the time the 2004 guidelines were written. Moreover, very few of these studies evaluated the impact of cardiac telemetry monitoring on physician management decisions.
We reviewed the literature to find out how cardiac telemetry is being used in clinical practice and how it might be used more selectively. The literature search was performed using Ovid MEDLINE (1996 to present) and PubMed Central using the key search terms “cardiac monitoring,” “telemetry monitoring,” “telemetry,” and “inpatient.” References from articles identified using Ovid MEDLINE (1996 to present) and PubMed Central that were relevant to our review were also included.
THREE CLASSES OF RISK
- Class II consists of patients for whom cardiac monitoring may be of benefit in some cases but is not essential for all (Table 2).
PATIENTS AT LOW RISK DO NOT BENEFIT
Telemetry monitoring has become an essential and commonly used clinical tool in most hospital systems. However, physicians do not seem to be using the risk stratification guidelines routinely or appropriately. The result is that many patients are being monitored needlessly, because telemetric monitoring neither affects how patients at low risk are managed nor improves their clinical outcomes.
Saleem et al4 reported that, of 105 patients at low risk who presented with chest pain and were admitted to a telemetry unit, none experienced a cardiac event or arrhythmia warranting changes in management while in the hospital.
Durairaj et al5 conducted a prospective cohort study of 1,033 patients admitted consecutively from an emergency department to an inpatient telemetry unit from July 1998 to January 1999. Patients were initially stratified according to a prediction model proposed by Goldman et al6 into groups at high, moderate, low, and very low risk. The risk groups were substratified according to the presence or absence of chest pain. The outcomes measured were transfer to an intensive care unit and a major cardiac complication, which included acute myocardial infarction, cardiac arrest, ventricular fibrillation, temporary pacemaker implantation, cardiogenic shock, emergency cardioversion, use of an intraaortic balloon assist device, intubation, and recurrent ischemic pain requiring coronary revascularization within 72 hours after admission or requiring cardiac catheterization followed by coronary revascularization before discharge from the hospital. The subgroup of patients who were classified as being at very low risk and who did not have chest pain (n = 318) did not experience any major cardiac complication.
Sivaram and colleagues7 studied the role of telemetric monitoring in the management of patients with class I, II, and III indications for telemetric monitoring outside of critical care units. The class was assigned at the time of discharge for the purpose of the study. A total of 297 telemetry events were noted during the study, but only 12 (4%) of the events led to changes in patient management: a change in medication in 8 patients, cardioversion for unstable atrial flutter in 1 patient, insertion of a pacemaker for sinus pause in 1, and electrophysiology studies in 2 patients.
Estrada et al8 examined the clinical outcomes of 2,240 patients admitted to a non-intensive care unit. The physicians perceived telemetric monitoring as helpful in 283 (12.6%) of the patients. However, data obtained from telemetry monitoring directly affected management decisions in only 156 patients (7% of the original study population). The researchers concluded that physicians may overestimate the role of telemetry in guiding patient management.
Hollander et al9 examined the outcomes of 261 patients admitted because of chest pain who had normal or nonspecific findings on electrocardiography on presentation. Only 4 patients (1.5%) experienced arrhythmias. The authors concluded that the policy of admitting patients at low risk to monitored beds should be reevaluated.
Snider et al10 showed that patients presenting with atypical chest pain and normal electrocardiographic findings were at low risk of arrhythmias and did not benefit from telemetric monitoring.
Schull and Redelmeier11 performed a 5-year observational study in which they reviewed all telemetry admissions (N = 8,932) to a tertiary care facility. Twenty patients experienced cardiac arrest during the study period, but telemetric monitoring was in use at the time in only 16 of the 20. Furthermore, the telemetry monitors signalled the onset of cardiac arrest in only 9 of these 16 patients. Three of the patients whose hearts stopped beating survived until discharge: two in whom telemetry actually signalled the onset of cardiac arrest and one in whom it did not.
TELEMETRY CAN GIVE FALSE-POSITIVE ALARMS
Inappropriate use of telemetric monitoring increases the chance of artifacts or false-positive rhythms being misinterpreted as dysrhythmias and can potentially lead to errors in management.
Cases have been reported of patients undergoing invasive procedures because of artifacts seen during telemetric monitoring. Knight et al12 described 12 patients who underwent unnecessary diagnostic or therapeutic interventions as a result of misdiagnosis of artifacts as ventricular tachycardia.
We did not discover in our review any data correlating the frequency of false-positive telemetric monitoring findings to management errors. On the other hand, it is also not possible to discern from these studies how often cardiac telemetric monitoring reaffirmed the clinical impression and facilitated ongoing therapy.
TELEMETRY IS EXPENSIVE
Telemetry requires specialized equipment and trained personnel, making it both costly and labor-intensive. The additional costs and cost-effectiveness of telemetry remain uncertain. Studies of its medical costs have found wide variations across different hospital systems. Sivaram et al,7 in an observational study published in 1998, estimated the cost per patient at $683. At our hospital, the current cost of telemetric monitoring is at least $1,400 per patient per 24 hours.
Whatever the true cost, inappropriate use of telemetry creates a financial burden on the health care system and adds to unnecessary costs incurred by patients.
POTENTIAL BARRIERS TO APPROPRIATE USE OF TELEMETRY
A number of factors contribute to the inappropriate use of telemetry. Possible causes for its overuse may be a lack of awareness of the ACC and AHA guidelines, nonadherence to the guidelines, or a combination of factors.
Even when physicians are aware of these guidelines, adherence may be suboptimal for a variety of reasons (reviewed by Mehta13). Adams et al14 revealed that most studies evaluating adherence were biased by overreporting, since the levels of adherence were self-reported.
OUR RECOMMENDATIONS
To improve on the appropriate use of telemetry, we recommend that several strategies be implemented.
Current guidelines for in-hospital cardiac monitoring need to be updated, particularly since the recommendations were based on evidence that is several decades old. Also, medical care has improved since the publication of the last guidelines, justifying an update in the guidelines.
Guidelines for cardiac monitoring should be incorporated into the curriculum for physician education to increase awareness of the guidelines. Hospitals should ensure that the emergency medicine staff is educated with regard to ensuring appropriateness of admissions to telemetry units.
Finally, the implementation of predictive models similar to that developed by Goldman et al6 and implemented in the study by Durairaj5 could help to ensure that cardiac telemetry is reserved for patients who will benefit from it the most.
- NASA Scientific and Technical Information (STI). Space-proven medical monitor: the total patient care package. Health and medicine. Originating technology/NASA contribution. Spinoff 2006. www.sti.nasa.gov/Textonly/tto/Spinoff2006/hm_2.html. Accessed 1/2009.
- Jaffe AS, Atkins JM, Field JM, et al. Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. J Am Coll Cardiol 1991; 18:1431–1433.
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004; 110:2721–2746.
- Saleem MA, McClung JA, Aronow WS, Kannam H. Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in-hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci 2005; 60:605–606.
- Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: a prospective cohort study of risk stratification and outcomes. Am J Med 2001; 110:7–11.
- Goldman A, Cook EF, Johnson PA, et al. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334:1498–1504.
- Sivaram CA, Summers JH, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998; 21:503–505.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995; 76:960–965.
- Hollander JE, Valentine SM, McCuskey CF, Brogan GX. Are monitored telemetry beds necessary for patients with nontraumatic chest pain and normal or nonspecific electrocardiograms? Am J Cardiol 1997; 79:1110–1111.
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122:517–523.
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7:647–652.
- Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med 1999; 341:1270–1274.
- Mehta NB. The doctors’ challenge: How can we follow guidelines better? Cleve Clin J Med 2004; 71:81–85.
- Adams AS, Soumerai SB, Lomas J, Ross-Degnan D. Evidence of self-reporting bias in assessing adherence to guidelines. Int J Quality Health Care 1999; 11:187–192.
Telemetry—from the Greek words tele (remote) and metron (measure)—for cardiac monitoring was developed in the mid-1960s by Spacelabs Medical for use in spaceflight.1 The system was later adopted in hospitals to detect life-threatening arrhythmias.
Guidelines for the use of telemetry were published in 1991 by the American College of Cardiology (ACC)2 in response to concerns raised by its increasing use in noncritical care settings during the 30 years after its introduction to clinical medicine. The latest revision of the guidelines was published in 2004 by the American Heart Association (AHA).3
However, the guidelines are based largely on expert opinion and on research data in electrocardiography. Few clinical trials of telemetry have been published, and they were either retrospective or nonrandomized. In fact, there were no published randomized trials at the time the 2004 guidelines were written. Moreover, very few of these studies evaluated the impact of cardiac telemetry monitoring on physician management decisions.
We reviewed the literature to find out how cardiac telemetry is being used in clinical practice and how it might be used more selectively. The literature search was performed using Ovid MEDLINE (1996 to present) and PubMed Central using the key search terms “cardiac monitoring,” “telemetry monitoring,” “telemetry,” and “inpatient.” References from articles identified using Ovid MEDLINE (1996 to present) and PubMed Central that were relevant to our review were also included.
THREE CLASSES OF RISK
- Class II consists of patients for whom cardiac monitoring may be of benefit in some cases but is not essential for all (Table 2).
PATIENTS AT LOW RISK DO NOT BENEFIT
Telemetry monitoring has become an essential and commonly used clinical tool in most hospital systems. However, physicians do not seem to be using the risk stratification guidelines routinely or appropriately. The result is that many patients are being monitored needlessly, because telemetric monitoring neither affects how patients at low risk are managed nor improves their clinical outcomes.
Saleem et al4 reported that, of 105 patients at low risk who presented with chest pain and were admitted to a telemetry unit, none experienced a cardiac event or arrhythmia warranting changes in management while in the hospital.
Durairaj et al5 conducted a prospective cohort study of 1,033 patients admitted consecutively from an emergency department to an inpatient telemetry unit from July 1998 to January 1999. Patients were initially stratified according to a prediction model proposed by Goldman et al6 into groups at high, moderate, low, and very low risk. The risk groups were substratified according to the presence or absence of chest pain. The outcomes measured were transfer to an intensive care unit and a major cardiac complication, which included acute myocardial infarction, cardiac arrest, ventricular fibrillation, temporary pacemaker implantation, cardiogenic shock, emergency cardioversion, use of an intraaortic balloon assist device, intubation, and recurrent ischemic pain requiring coronary revascularization within 72 hours after admission or requiring cardiac catheterization followed by coronary revascularization before discharge from the hospital. The subgroup of patients who were classified as being at very low risk and who did not have chest pain (n = 318) did not experience any major cardiac complication.
Sivaram and colleagues7 studied the role of telemetric monitoring in the management of patients with class I, II, and III indications for telemetric monitoring outside of critical care units. The class was assigned at the time of discharge for the purpose of the study. A total of 297 telemetry events were noted during the study, but only 12 (4%) of the events led to changes in patient management: a change in medication in 8 patients, cardioversion for unstable atrial flutter in 1 patient, insertion of a pacemaker for sinus pause in 1, and electrophysiology studies in 2 patients.
Estrada et al8 examined the clinical outcomes of 2,240 patients admitted to a non-intensive care unit. The physicians perceived telemetric monitoring as helpful in 283 (12.6%) of the patients. However, data obtained from telemetry monitoring directly affected management decisions in only 156 patients (7% of the original study population). The researchers concluded that physicians may overestimate the role of telemetry in guiding patient management.
Hollander et al9 examined the outcomes of 261 patients admitted because of chest pain who had normal or nonspecific findings on electrocardiography on presentation. Only 4 patients (1.5%) experienced arrhythmias. The authors concluded that the policy of admitting patients at low risk to monitored beds should be reevaluated.
Snider et al10 showed that patients presenting with atypical chest pain and normal electrocardiographic findings were at low risk of arrhythmias and did not benefit from telemetric monitoring.
Schull and Redelmeier11 performed a 5-year observational study in which they reviewed all telemetry admissions (N = 8,932) to a tertiary care facility. Twenty patients experienced cardiac arrest during the study period, but telemetric monitoring was in use at the time in only 16 of the 20. Furthermore, the telemetry monitors signalled the onset of cardiac arrest in only 9 of these 16 patients. Three of the patients whose hearts stopped beating survived until discharge: two in whom telemetry actually signalled the onset of cardiac arrest and one in whom it did not.
TELEMETRY CAN GIVE FALSE-POSITIVE ALARMS
Inappropriate use of telemetric monitoring increases the chance of artifacts or false-positive rhythms being misinterpreted as dysrhythmias and can potentially lead to errors in management.
Cases have been reported of patients undergoing invasive procedures because of artifacts seen during telemetric monitoring. Knight et al12 described 12 patients who underwent unnecessary diagnostic or therapeutic interventions as a result of misdiagnosis of artifacts as ventricular tachycardia.
We did not discover in our review any data correlating the frequency of false-positive telemetric monitoring findings to management errors. On the other hand, it is also not possible to discern from these studies how often cardiac telemetric monitoring reaffirmed the clinical impression and facilitated ongoing therapy.
TELEMETRY IS EXPENSIVE
Telemetry requires specialized equipment and trained personnel, making it both costly and labor-intensive. The additional costs and cost-effectiveness of telemetry remain uncertain. Studies of its medical costs have found wide variations across different hospital systems. Sivaram et al,7 in an observational study published in 1998, estimated the cost per patient at $683. At our hospital, the current cost of telemetric monitoring is at least $1,400 per patient per 24 hours.
Whatever the true cost, inappropriate use of telemetry creates a financial burden on the health care system and adds to unnecessary costs incurred by patients.
POTENTIAL BARRIERS TO APPROPRIATE USE OF TELEMETRY
A number of factors contribute to the inappropriate use of telemetry. Possible causes for its overuse may be a lack of awareness of the ACC and AHA guidelines, nonadherence to the guidelines, or a combination of factors.
Even when physicians are aware of these guidelines, adherence may be suboptimal for a variety of reasons (reviewed by Mehta13). Adams et al14 revealed that most studies evaluating adherence were biased by overreporting, since the levels of adherence were self-reported.
OUR RECOMMENDATIONS
To improve on the appropriate use of telemetry, we recommend that several strategies be implemented.
Current guidelines for in-hospital cardiac monitoring need to be updated, particularly since the recommendations were based on evidence that is several decades old. Also, medical care has improved since the publication of the last guidelines, justifying an update in the guidelines.
Guidelines for cardiac monitoring should be incorporated into the curriculum for physician education to increase awareness of the guidelines. Hospitals should ensure that the emergency medicine staff is educated with regard to ensuring appropriateness of admissions to telemetry units.
Finally, the implementation of predictive models similar to that developed by Goldman et al6 and implemented in the study by Durairaj5 could help to ensure that cardiac telemetry is reserved for patients who will benefit from it the most.
Telemetry—from the Greek words tele (remote) and metron (measure)—for cardiac monitoring was developed in the mid-1960s by Spacelabs Medical for use in spaceflight.1 The system was later adopted in hospitals to detect life-threatening arrhythmias.
Guidelines for the use of telemetry were published in 1991 by the American College of Cardiology (ACC)2 in response to concerns raised by its increasing use in noncritical care settings during the 30 years after its introduction to clinical medicine. The latest revision of the guidelines was published in 2004 by the American Heart Association (AHA).3
However, the guidelines are based largely on expert opinion and on research data in electrocardiography. Few clinical trials of telemetry have been published, and they were either retrospective or nonrandomized. In fact, there were no published randomized trials at the time the 2004 guidelines were written. Moreover, very few of these studies evaluated the impact of cardiac telemetry monitoring on physician management decisions.
We reviewed the literature to find out how cardiac telemetry is being used in clinical practice and how it might be used more selectively. The literature search was performed using Ovid MEDLINE (1996 to present) and PubMed Central using the key search terms “cardiac monitoring,” “telemetry monitoring,” “telemetry,” and “inpatient.” References from articles identified using Ovid MEDLINE (1996 to present) and PubMed Central that were relevant to our review were also included.
THREE CLASSES OF RISK
- Class II consists of patients for whom cardiac monitoring may be of benefit in some cases but is not essential for all (Table 2).
PATIENTS AT LOW RISK DO NOT BENEFIT
Telemetry monitoring has become an essential and commonly used clinical tool in most hospital systems. However, physicians do not seem to be using the risk stratification guidelines routinely or appropriately. The result is that many patients are being monitored needlessly, because telemetric monitoring neither affects how patients at low risk are managed nor improves their clinical outcomes.
Saleem et al4 reported that, of 105 patients at low risk who presented with chest pain and were admitted to a telemetry unit, none experienced a cardiac event or arrhythmia warranting changes in management while in the hospital.
Durairaj et al5 conducted a prospective cohort study of 1,033 patients admitted consecutively from an emergency department to an inpatient telemetry unit from July 1998 to January 1999. Patients were initially stratified according to a prediction model proposed by Goldman et al6 into groups at high, moderate, low, and very low risk. The risk groups were substratified according to the presence or absence of chest pain. The outcomes measured were transfer to an intensive care unit and a major cardiac complication, which included acute myocardial infarction, cardiac arrest, ventricular fibrillation, temporary pacemaker implantation, cardiogenic shock, emergency cardioversion, use of an intraaortic balloon assist device, intubation, and recurrent ischemic pain requiring coronary revascularization within 72 hours after admission or requiring cardiac catheterization followed by coronary revascularization before discharge from the hospital. The subgroup of patients who were classified as being at very low risk and who did not have chest pain (n = 318) did not experience any major cardiac complication.
Sivaram and colleagues7 studied the role of telemetric monitoring in the management of patients with class I, II, and III indications for telemetric monitoring outside of critical care units. The class was assigned at the time of discharge for the purpose of the study. A total of 297 telemetry events were noted during the study, but only 12 (4%) of the events led to changes in patient management: a change in medication in 8 patients, cardioversion for unstable atrial flutter in 1 patient, insertion of a pacemaker for sinus pause in 1, and electrophysiology studies in 2 patients.
Estrada et al8 examined the clinical outcomes of 2,240 patients admitted to a non-intensive care unit. The physicians perceived telemetric monitoring as helpful in 283 (12.6%) of the patients. However, data obtained from telemetry monitoring directly affected management decisions in only 156 patients (7% of the original study population). The researchers concluded that physicians may overestimate the role of telemetry in guiding patient management.
Hollander et al9 examined the outcomes of 261 patients admitted because of chest pain who had normal or nonspecific findings on electrocardiography on presentation. Only 4 patients (1.5%) experienced arrhythmias. The authors concluded that the policy of admitting patients at low risk to monitored beds should be reevaluated.
Snider et al10 showed that patients presenting with atypical chest pain and normal electrocardiographic findings were at low risk of arrhythmias and did not benefit from telemetric monitoring.
Schull and Redelmeier11 performed a 5-year observational study in which they reviewed all telemetry admissions (N = 8,932) to a tertiary care facility. Twenty patients experienced cardiac arrest during the study period, but telemetric monitoring was in use at the time in only 16 of the 20. Furthermore, the telemetry monitors signalled the onset of cardiac arrest in only 9 of these 16 patients. Three of the patients whose hearts stopped beating survived until discharge: two in whom telemetry actually signalled the onset of cardiac arrest and one in whom it did not.
TELEMETRY CAN GIVE FALSE-POSITIVE ALARMS
Inappropriate use of telemetric monitoring increases the chance of artifacts or false-positive rhythms being misinterpreted as dysrhythmias and can potentially lead to errors in management.
Cases have been reported of patients undergoing invasive procedures because of artifacts seen during telemetric monitoring. Knight et al12 described 12 patients who underwent unnecessary diagnostic or therapeutic interventions as a result of misdiagnosis of artifacts as ventricular tachycardia.
We did not discover in our review any data correlating the frequency of false-positive telemetric monitoring findings to management errors. On the other hand, it is also not possible to discern from these studies how often cardiac telemetric monitoring reaffirmed the clinical impression and facilitated ongoing therapy.
TELEMETRY IS EXPENSIVE
Telemetry requires specialized equipment and trained personnel, making it both costly and labor-intensive. The additional costs and cost-effectiveness of telemetry remain uncertain. Studies of its medical costs have found wide variations across different hospital systems. Sivaram et al,7 in an observational study published in 1998, estimated the cost per patient at $683. At our hospital, the current cost of telemetric monitoring is at least $1,400 per patient per 24 hours.
Whatever the true cost, inappropriate use of telemetry creates a financial burden on the health care system and adds to unnecessary costs incurred by patients.
POTENTIAL BARRIERS TO APPROPRIATE USE OF TELEMETRY
A number of factors contribute to the inappropriate use of telemetry. Possible causes for its overuse may be a lack of awareness of the ACC and AHA guidelines, nonadherence to the guidelines, or a combination of factors.
Even when physicians are aware of these guidelines, adherence may be suboptimal for a variety of reasons (reviewed by Mehta13). Adams et al14 revealed that most studies evaluating adherence were biased by overreporting, since the levels of adherence were self-reported.
OUR RECOMMENDATIONS
To improve on the appropriate use of telemetry, we recommend that several strategies be implemented.
Current guidelines for in-hospital cardiac monitoring need to be updated, particularly since the recommendations were based on evidence that is several decades old. Also, medical care has improved since the publication of the last guidelines, justifying an update in the guidelines.
Guidelines for cardiac monitoring should be incorporated into the curriculum for physician education to increase awareness of the guidelines. Hospitals should ensure that the emergency medicine staff is educated with regard to ensuring appropriateness of admissions to telemetry units.
Finally, the implementation of predictive models similar to that developed by Goldman et al6 and implemented in the study by Durairaj5 could help to ensure that cardiac telemetry is reserved for patients who will benefit from it the most.
- NASA Scientific and Technical Information (STI). Space-proven medical monitor: the total patient care package. Health and medicine. Originating technology/NASA contribution. Spinoff 2006. www.sti.nasa.gov/Textonly/tto/Spinoff2006/hm_2.html. Accessed 1/2009.
- Jaffe AS, Atkins JM, Field JM, et al. Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. J Am Coll Cardiol 1991; 18:1431–1433.
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004; 110:2721–2746.
- Saleem MA, McClung JA, Aronow WS, Kannam H. Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in-hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci 2005; 60:605–606.
- Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: a prospective cohort study of risk stratification and outcomes. Am J Med 2001; 110:7–11.
- Goldman A, Cook EF, Johnson PA, et al. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334:1498–1504.
- Sivaram CA, Summers JH, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998; 21:503–505.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995; 76:960–965.
- Hollander JE, Valentine SM, McCuskey CF, Brogan GX. Are monitored telemetry beds necessary for patients with nontraumatic chest pain and normal or nonspecific electrocardiograms? Am J Cardiol 1997; 79:1110–1111.
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122:517–523.
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7:647–652.
- Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med 1999; 341:1270–1274.
- Mehta NB. The doctors’ challenge: How can we follow guidelines better? Cleve Clin J Med 2004; 71:81–85.
- Adams AS, Soumerai SB, Lomas J, Ross-Degnan D. Evidence of self-reporting bias in assessing adherence to guidelines. Int J Quality Health Care 1999; 11:187–192.
- NASA Scientific and Technical Information (STI). Space-proven medical monitor: the total patient care package. Health and medicine. Originating technology/NASA contribution. Spinoff 2006. www.sti.nasa.gov/Textonly/tto/Spinoff2006/hm_2.html. Accessed 1/2009.
- Jaffe AS, Atkins JM, Field JM, et al. Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. J Am Coll Cardiol 1991; 18:1431–1433.
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004; 110:2721–2746.
- Saleem MA, McClung JA, Aronow WS, Kannam H. Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in-hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci 2005; 60:605–606.
- Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: a prospective cohort study of risk stratification and outcomes. Am J Med 2001; 110:7–11.
- Goldman A, Cook EF, Johnson PA, et al. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334:1498–1504.
- Sivaram CA, Summers JH, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998; 21:503–505.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995; 76:960–965.
- Hollander JE, Valentine SM, McCuskey CF, Brogan GX. Are monitored telemetry beds necessary for patients with nontraumatic chest pain and normal or nonspecific electrocardiograms? Am J Cardiol 1997; 79:1110–1111.
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122:517–523.
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7:647–652.
- Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med 1999; 341:1270–1274.
- Mehta NB. The doctors’ challenge: How can we follow guidelines better? Cleve Clin J Med 2004; 71:81–85.
- Adams AS, Soumerai SB, Lomas J, Ross-Degnan D. Evidence of self-reporting bias in assessing adherence to guidelines. Int J Quality Health Care 1999; 11:187–192.
KEY POINTS
- Guidelines from the American College of Cardiology (1991) and American Heart Association (2004) divide patients into three risk classes for whom telemetry is, may be, or is not indicated.
- Few studies have addressed whether telemetry is beneficial in clinical practice.
- The available evidence suggests that telemetry infrequently influences physician management decisions for patients at low risk, although it may in a relatively small subset at high risk.
- Inappropriate use of telemetry is associated with unnecessary testing and treatment and higher cost of care.
- Better risk-assessment and selection strategies are needed to identify patients for whom telemetry monitoring will be most beneficial.
A 72-year-old man with a purpuric rash
A 72-year-old man whose medical history includes diabetes mellitus, hypertension, coronary artery disease, aortic valve replacement, atrial fibrillation, and chronic obstructive pulmonary disease was in his usual state of health until 2 weeks ago, when he developed a purpuric rash on his legs. His physician started him on prednisone 40 mg daily for the rash; however, 1 week later he presented to a hospital emergency room when his family found him confused and diaphoretic.
In the emergency room, he was found to be hypoglycemic, with a serum glucose level of 40 mg/dL, which was promptly treated. His mental status improved partially. In the hospital, the rash worsened and progressed upwards to his trunk and upper extremities. He was transferred to our institution for further workup and management.
A review of systems reveals occasional epistaxis in the summer, recent fatigue, cough, and shortness of breath on exertion. His medications at the time of transfer include warfarin (Coumadin), amlodipine (Norvasc), insulin, ipratropium and albuterol (Combivent) inhalers, and prednisone 40 mg daily. He has not undergone surgery recently.
PHYSICAL EXAMINATION
He is alert and oriented to person but not to time and place.
Vital signs. Oral temperature 101.1°F (38.4°C), pulse rate 108, blood pressure 108/79 mm/Hg, respiratory rate 22, oxygen saturation 93% by pulse oximetry on room air, weight 94 kg (207 lb).
Head, eyes, ears, nose, and throat. No pallor or icterus, pupils are equally reactive, nasal mucosa not inflamed or ulcerated, mucous membranes moist, no sinus tenderness.
Neck. No jugular venous distention and no cervical lymphadenopathy.
Cardiovascular. Tachycardia, irregularly irregular rhythm, prosthetic valve sounds, no murmurs, rubs, or gallops.
Respiratory. Bibasal crackles (right side more than the left). No wheezing.
Abdomen. Soft, nontender, nondistended, no palpable organomegaly, bowel sounds normal.
Extremities. No edema, good peripheral pulses.
Neurologic. No focal deficits noted.
Lymphatic. No enlarged lymph nodes.
Musculoskeletal. Traumatic right second distal interphalangeal amputation. Otherwise, no joint abnormality or restriction of movement.
Initial laboratory values:
- White blood cell count 15.78 × 109/L (normal 4.5–11.0)
- Absolute neutrophil count 13.3 × 109/L (4.0–11.0)
- Hemoglobin 13.3 g/dL (13.5–17.5)
- Platelet count 133 × 109/L (150–400)
- International normalized ratio (INR) 1.8
- Sodium 136 mmol/L (135–146)
- Potassium 4.6 mmol/L (3.5–5.0)
- Blood urea nitrogen 31 mg/dL (10–25)
- Creatinine 1.6 mg/dL (0.70–1.40)
- Glucose 62 mg/dL (65–100)
- Bicarbonate 23 mmol/L (23–32)
- Albumin 2.5 g/dL (3.5–5.0)
- Total protein 4.6 g/dL (6.0–8.4)
- Bilirubin 1.2 mg/dL (0.0–1.5)
- Aspartate aminotransferase 41 U/L (7–40)
- Alanine aminotransferase 74 U/L (5–50)
- Alkaline phosphatase 55 U/L (40–150)
- C-reactive protein 9.9 mg/dL (0.0–1.0).
Other studies
Electrocardiography shows atrial fibrillation and left ventricular hypertrophy, but no acute changes.
Computed tomography (CT) of the head shows no evidence of hemorrhage or infarction.
Blood cultures are sent at the time of hospital admission.
WHICH TEST IS NEXT?
1. Which is the most appropriate next step for this patient?
- Urinalysis
- CT of the chest
- Echocardiography
- Skin biopsy
The rash in Figure 1 is palpable purpura, which strongly suggests small-vessel cutaneous vasculitis, a condition that can occur in a broad range of settings. An underlying cause is identified in over 70% of cases. Cutaneous vasculitis may herald a primary small-vessel systemic vasculitis such as Wegener granulomatosis, microscopic polyangiitis, or Henoch-Schönlein purpura. It can also be secondary to a spectrum of underlying triggers or diseases that include medications, infections, malignancies such as lymphoproliferative disorders, cryoglobulinemia secondary to hepatitis C viral infection, and connective tissue diseases such as rheumatoid arthritis and systemic lupus erythematosus.
Infective endocarditis is associated with a secondary form of vasculitis and is a strong possibility in this patient, who has a prosthetic aortic valve, fever, and a high white blood cell count.
Thrombocytopenia should also prompt an assessment for any drugs the patient is taking that affect platelet function. However, thrombocytopenia typically results in nonpalpable purpura.
Idiopathic isolated cutaneous vasculitis, in which no underlying cause for the cutaneous vasculitis can be identified, is the diagnosis in less than 30% of cases.
A vasculitic disease process can involve multiple sites, which may be asymptomatic on presentation. Identifying these sites is important, not only to establish the diagnosis, but also to detect potentially life-threatening complications early.
Thus, in this patient, urinalysis should be done promptly to check for active sediment consisting of red cell casts, which would suggest renal involvement (glomerulonephritis). Also, a rising blood pressure and creatinine would point to renal involvement and warrant more aggressive initial therapy.
Chest radiography should be done to rule out pulmonary infiltrates, septic emboli, nodules, or cavities that could represent vasculitic or infectious involvement of the lungs. CT of the chest may be needed to further characterize abnormalities on chest radiography.
Echocardiography should certainly be pursued as part of the workup for endocarditis, but urinalysis is of the utmost importance in this patient at this point.
More diagnostic information is needed before considering skin biopsy.
Clues from the urinalysis and chest radiography
Our patient’s sedimentation rate is 24 mm/hour. The urinalysis is strongly positive for blood and a moderate amount of protein but negative for leukocyte esterase and nitrite. The urine sediment contains numerous red blood cell casts and 6 to 10 white blood cells per high-power field.
Pending return of blood cultures, ceftriaxone (Rocephin) and azithromycin (Zithromax) are started to treat possible community-acquired pneumonia. Vancomycin (Vancocin) is empirically added, given the possibility of prosthetic valve endocarditis. Gram stain on blood cultures shows gram-positive cocci.
Ground-glass attenuation implies focal or diffuse opacification of the lung that does not obscure the vascular structures, is not associated with air bronchograms, and appears as a “veil” across the lung parenchyma.1 It can be seen in acute diseases such as infection (including pneumonia from atypical bacteria, viruses, acid-fast bacilli, and Pneumocystis jiroveci), pulmonary hemorrhage of any cause, acute viral, eosinophilic, and interstitial pneumonias, and hypersensitivity pneumonitis. It can also be seen in chronic disease states such as interstitial lung disease, bronchoalveolar carcinoma, alveolar proteinosis, and sarcoidosis.
WHICH TEST WOULD NOT HELP?
2. Which of the following tests is least likely to help in the diagnostic evaluation at this point?
- Transthoracic echocardiography
- Transesophageal echocardiography
- Bronchoscopy
- Cystoscopy
In this case, the least likely to help is cystoscopy.
This patient’s vasculitic-appearing rash, ground-glass pulmonary infiltrates, and impaired renal function with red cell casts suggest a pulmonary-renal syndrome, and with this constellation of features, a systemic vasculitis is very likely. Therefore, the focus of the evaluation should be on any evidence to support a diagnosis of vasculitis, as well as other possible causes.
In a patient with diabetes, an artificial heart valve, and fever, the possibility of infection, especially endocarditis, remains high. Transthoracic echocardiography is warranted, and if it is negative for vegetations, transesophageal echocardiography would be a reasonable next option.
Bronchoscopy is warranted to determine if the infiltrates represent pulmonary hemorrhage, which can be seen in certain types of vasculitic and systemic disorders.
The finding of red cell casts in the urine indicates glomerulonephritis, and therefore the kidneys are the likely source of the urinary red blood cells, making cystoscopy of no utility in this current, acute setting.
Case continued: His condition worsens
Transthoracic echocardiography reveals a well-seated mechanical prosthetic aortic valve, trivial aortic regurgitation, a peak gradient of 23 mm Hg and a mean gradient of 12 mm Hg (normal values for his prosthetic valve), and no valvular vegetations. Transesophageal echocardiography confirms the absence of vegetations.
His oxygen requirement increases, and analysis of arterial blood gases reveals a pH of 7.37, Pco2 49 mm Hg (normal range 35–45), Po2 102 mm Hg (normal 80–100), and bicarbonate 28 mmol/L while breathing 100% supplemental oxygen by a nonrebreather face mask. He is taken to the medical intensive care unit for intubation and mechanical ventilation. Bronchoscopy performed while he is intubated confirms diffuse alveolar hemorrhage. Pulse intravenous methylprednisolone (Solu-Medrol) therapy is started.
DIFFUSE ALVEOLAR HEMORRHAGE
3. Which of the following is not true about diffuse alveolar hemorrhage?
- Its onset is usually abrupt or of short duration
- It is always associated with hemoptysis
- Radiography most commonly shows new patchy or diffuse alveolar opacities
- Pulmonary function testing shows increased diffusing capacity of the lung for carbon monoxide (Dlco)
Hemoptysis is absent at presentation in as many as 33% of patients.
The onset of diffuse alveolar hemorrhage is usually abrupt or of short duration, with initial symptoms of cough, fever, and dyspnea. Some patients, such as ours, can present with severe acute respiratory distress syndrome requiring mechanical ventilation. Radiography most often shows new patchy alveolar opacities, and CT may reveal a ground-glass appearance. On pulmonary function testing, the Dlco is high, owing to the hemoglobin within the alveoli.
ACUTE GLOMERULONEPHRITIS PLUS PULMONARY HEMORRHAGE EQUALS…?
4. Which disease could have manifestations consistent with acute glomerulonephritis and pulmonary hemorrhage?
- Antiglomerular basement membrane disease
- Wegener granulomatosis
- Microscopic polyangiitis
- Systemic lupus erythematosus
All of these are possible.
The combined presentation of acute glomerulonephritis and pulmonary hemorrhage (also called pulmonary-renal syndrome) is usually seen in antiglomerular basement membrane disease (Goodpasture syndrome) and small-vessel systemic vasculitides such as Wegener granulomatosis and microscopic polyangiitis.2,3 It can also be seen in patients with systemic lupus erythematosus.
Antiglomerular basement membrane disease
In antiglomerular basement membrane disease, circulating antibodies are directed towards an antigen intrinsic to the glomerular basement membrane, typically leading to acute glomerulonephritis associated with crescent formation. It may present as acute renal failure in which urinalysis shows proteinuria with sediment characterized by red cell casts. Pulmonary involvement, usually alveolar hemorrhage, is present in approximately 60% to 70% of cases.
The diagnosis requires demonstration of antiglomerular basement membrane antibodies in either the serum or the kidney. Renal biopsy is usually recommended because the accuracy of serum assays is variable.
A key histologic feature of the renal lesion in antiglomerular basement membrane disease is crescentic glomerulonephritis in which immunofluorescence microscopy demonstrates the virtually pathognomonic finding of linear deposition of immunoglobulin G along the glomerular capillaries.
The treatment of choice for antiglomerular basement membrane disease is plasmapheresis and immunosuppression with a combination of glucocorticoids and cyclophosphamide (Cytoxan). If the disease is high on the differential diagnosis, empiric plasmapheresis should be started while waiting for diagnostic studies, because the prognosis of untreated glomerulonephritis is poor.
Wegener granulomatosis
Wegener granulomatosis is a systemic vasculitis of the medium and small arteries, arterioles, and venules that classically involves the upper and lower respiratory tracts and the kidneys. Patients may present with persistent rhinorrhea and epistaxis, cough with chest radiographs showing nodules, fixed infiltrates, or cavities, and abnormal urinary sediment with microscopic hematuria with or without red cell casts.
From 75% to 90% of patients with active Wegener granulomatosis are positive for antineutrophil cytoplasmic antibody (ANCA). In 60% to 80% of cases, ANCA is directed against proteinase 3 (PR3), which produces a cytoplasmic standing pattern by immunofluorescence (cANCA), while 5% to 20% have ANCA directed against myeloper-oxidase, which produces a perinuclear staining pattern (pANCA). A small number of patients with Wegener granulomatosis are ANCA-negative.
The diagnosis is usually confirmed by tissue biopsy at the site of active disease, which shows necrotizing vasculitis with granulomatous inflammation. The renal lesion is typically that of a focal, segmental, necrotizing glomerulonephritis that has few to no immune complexes (pauci-immune glomerulonephritis).
The treatment of severe disease involves a combination of cyclophosphamide and glucocorticoids initially to achieve remission followed by maintenance therapy with methotrexate or azathioprine (Imuran).
Microscopic polyangiitis
Microscopic polyangiitis is a systemic vasculitis of the capillaries, venules, and arterioles, with little or no immune complex deposition. Nearly all patients have renal involvement, and 10% to 30% have lung involvement. In those with lung involvement, diffuse alveolar hemorrhage is the most common manifestation.
On histopathologic study, microscopic polyangiitis differs from Wegener granulomatosis in that it does not have granuloma formation. However, the renal lesion is that of a pauci-immune glomerulonephritis and is identical to that seen in Wegener granulomatosis. From 70% to 85% of patients with microscopic polyangiitis are ANCA-positive, and most of these have pANCA.
The management of active severe microscopic polyangiitis is identical to that of Wegener granulomatosis.
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease characterized by tissue-binding autoantibody and immune-complex-mediated organ damage. It can involve multiple organ systems, and the diagnosis is based on characteristic clinical features and autoantibodies. The sensitivity of antinuclear antibody for lupus is close to 100%, which makes it a good screening tool. Antibodies to dsDNA and Smith antigen have high specificity for lupus.
About 75% of patients have renal involvement at some point in their disease course. The different types of renal disease in systemic lupus are usually differentiated with a renal biopsy, with immune-complex-mediated glomerular diseases being the most common.
The most common pulmonary manifestation is pleuritis with or without pleural effusion. Life-threatening pulmonary manifestations include pulmonary hemorrhage and interstitial inflammation leading to fibrosis.
Lupus has great clinical variability and the treatment approach is based on the organ manifestations, disease activity, and severity.
CASE CONTINUED: ARRIVING AT THE DIAGNOSIS
We start our patient on cyclophosphamide 175 mg daily in view of possible Wegener granulomatosis.
Even though purpura is extremely rare in primary antiglomerular basement membrane disease, this patient has life-threatening pulmonary hemorrhage, a complication seen in over 50% of these patients. Therefore, plasmapheresis is started empirically.
On the second day of cyclophosphamide treatment, tests for ANCA, glomerular basement membrane antibody, and antinuclear antibody are reported as negative, and complement levels are normal. Bronchoalveolar lavage shows no infection. Follow-up blood cultures are negative.
To summarize the findings so far, this patient has a purpuric skin rash, active urine sediment with red cell casts indicating glomerulonephritis, acute renal failure, and severe pulmonary hemorrhage requiring mechanical ventilation. Although one set of blood cultures showed gram-positive cocci, no source of infection, particularly endocarditis, could be identified.
Antiglomerular basement membrane disease would still be high on the list of suspected diagnoses, given his diffuse alveolar hemorrhage. As mentioned earlier, renal biopsy is imperative to making a diagnosis, because serologic tests have variable accuracy. And making the correct diagnosis has therapeutic implications.
Renal biopsy is performed and shows immune-complex mesangiopathic glomerulonephritis with positive immunofluorescent staining in the mesangium for IgA. Only one glomerulus shows fibrinoid necrosis.
Skin biopsy results obtained earlier showed positive direct immunofluorescence for IgA. Both renal and skin biopsies suggested Henoch-Schönlein purpura.
IgA deposition in the kidney and skin has been associated with liver cirrhosis, celiac disease, and infections with agents such as human immunodeficiency virus, cytomegalovirus, Haemophilus parainfluenzae, and Staphylococcus aureus. In a Japanese study,4 renal biopsy specimens from 116 patients with IgA nephropathy and from 122 patients with other types of kidney disease were examined for the presence of S aureus antigen in the glomeruli. Although antigen was not detected in non-IgA disease, 68% of specimens from patients with IgA nephropathy had S aureus cell envelope antigen together with IgA antibody in the glomeruli. However, no single antigen has been consistently identified, so it seems more probable that the development of IgA deposition in kidneys is a consequence of aberrant IgA immune response rather than the antigen itself.
HENOCH-SCHÖNLEIN PURPURA
Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is characterized by the tissue deposition of IgA-containing immune complexes. It is predominantly a disease of children but it can be seen in adults. A UK study found the prevalence to be 20 per 100,000 children, with the highest prevalence between ages 4 and 7 (70 per 100,000).5
The four cardinal clinical features of Henoch-Schönlein purpura are purpuric rash, abdominal pain, arthralgia, and renal involvement. Almost all patients have purpuric rash at some point in their disease course. Arthralgia with or without arthritis is typically migratory, oligoarticular, and nondeforming, usually affecting the large joints of the lower extremities; involvement of the upper extremities is less common.
Skin biopsy typically shows leukocytoclastic vasculitis in postcapillary venules with IgA deposition, and these findings are pathognomonic of Henoch-Schönlein purpura.
Gastrointestinal involvement can range from mild symptoms such as nausea, vomiting, abdominal pain, and paralytic ileus to severe disease such as gastrointestinal hemorrhage, bowel infarction, bowel perforation, and intussusception.
Renal involvement is common and is important, as it can in rare cases progress to end-stage renal disease. The urinalysis usually shows mild proteinuria with active sediment with red cell casts. Most patients have relatively mild disease, characterized by asymptomatic hematuria with a normal or slightly elevated creatinine. However, severe involvement may occur, with nephrotic syndrome, hypertension, and acute renal failure.
Different presentation in adults vs children
Adults with Henoch-Schönlein purpura only rarely present with bowel intussusception, whereas some studies have found that adults are more likely than children to develop significant renal involvement, including end-stage renal disease.6,7
There is a general but not absolute correlation between the severity of clinical manifestations and the findings on renal biopsy. A poor prognosis (significant proteinuria, hypertension, renal insufficiency, or end-stage renal disease) is associated with crescent formation involving more than 50% of the glomeruli.8
Our current understanding of the longterm outcome of the renal disease in Henoch-Schönlein purpura is primarily derived from studies in children. In one study, complete recovery occurred in 94% of children and 89% of adults.7 A long-term study of 250 adults with Henoch-Schönlein purpura and renal involvement of sufficient severity to require biopsy reported that, at a median follow up of 15 years, 11% had become dialysis-dependent and 13% had severe renal failure (creatinine clearance < 30 mL/min).6 Recurrence is common, occurring in approximately one-third of patients, more likely in those with nephritis.8
The diagnosis of Henoch-Schönlein purpura is typically made on the basis of key clinical features. In patients such as ours who have an atypical presentation, biopsy of affected skin and renal biopsy can be essential in the diagnosis. Diffuse alveolar hemorrhage is exceedingly rare in Henoch-Schönlein purpura but can be seen, as in our patient.9,10 In this setting, the findings of IgA deposits in skin and renal biopsy specimens, together with the absence of other clinical, serologic, or histologic features of other more-common potential causes, secured the diagnosis in this patient.
Henoch-Schönlein purpura is usually self-limited and requires no specific therapy. Evidence suggests that glucocorticoids enhance the rate of resolution of the arthritis and abdominal pain but do not appear to prevent recurrent disease or lessen the likelihood of progression of renal disease.8 Patients with severe renal involvement with renal function impairment may benefit from pulse intravenous corticosteroid therapy (methylprednisolone 250–1,000 mg per day for 3 days), followed by oral steroids for 3 months.11
In anecdotal reports, renal function improved in 19 of 21 children with Henoch-Schönlein purpura and severe crescentic nephritis.12 Studies have evaluated cyclophosphamide13 and plasmapheresis,14 but their role remains uncertain. Renal transplantation is an option in patients who progress to end-stage renal disease.
OUR CASE CONTINUED
In our patient, plasmapheresis was discontinued. As the pulmonary hemorrhage had developed during treatment with prednisone, we decided to continue cyclophosphamide, given the life-threatening nature of his disease. His pulmonary status improved and he was extubated.
During his initial hospital stay, he was taking heparin for anticoagulation therapy. However, given the life-threatening diffuse alveolar hemorrhage, heparin was stopped during the course of his stay in the intensive care unit. Once he was stable and was transferred out of the intensive care unit, heparin was resumed, and his anticoagulation therapy was bridged to warfarin just before discharge. He was eventually discharged on a tapering dose of oral prednisone and cyclophosphamide for 3 months, after which he was switched to azathioprine for maintenance therapy. He was doing well 6 months later, with a serum creatinine level of 1.6 mg/dL, no red cell casts in the urine, and no rash.
TAKE-HOME POINT
In any case of suspected vasculitis that presents with skin disease, it is essential to look for other sites with potentially life-threatening involvement. Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is not always benign and can be associated with serious complications such as renal failure, gastrointestinal events, and, very rarely, diffuse alveolar hemorrhage.
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997; 169:355–367.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002; 39:42–47.
- Koyama A, Sharmin S, Sakurai H, et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 2004; 66:121–132.
- Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002; 360:1197–1202.
- Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002; 13:1271–1278.
- Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M, Gonzalez-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum 1997; 40:859–864.
- Saulsbury FT. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore) 1999; 78:395–409.
- Nadrous HF, Yu AC, Specks U, Ryu JH. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc 2004; 79:1151–1157.
- Vats KR, Vats A, Kim Y, Dassenko D, Sinaiko AR. Henoch-Schönlein purpura and pulmonary hemorrhage: a report and literature review. Pediatr Nephrol 1999; 13:530–534.
- Niaudet P, Habib R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol 1998; 12:238–243.
- Bergstein J, Leiser J, Andreoli SP. Response of crescentic Henoch-Schöenlein purpura nephritis to corticosteroid and azathioprine therapy. Clin Nephrol 1998; 49:9–14.
- Tarshish P, Bernstein J, Edelmann CM. Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 2004; 19:51–56.
- Hattori M, Ito K, Konomoto T, Kawaguchi H, Yoshioka T, Khono M. Plasmapheresis as the sole therapy for rapidly progressive Henoch-Schönlein purpura nephritis in children. Am J Kidney Dis 1999; 33:427–433.
A 72-year-old man whose medical history includes diabetes mellitus, hypertension, coronary artery disease, aortic valve replacement, atrial fibrillation, and chronic obstructive pulmonary disease was in his usual state of health until 2 weeks ago, when he developed a purpuric rash on his legs. His physician started him on prednisone 40 mg daily for the rash; however, 1 week later he presented to a hospital emergency room when his family found him confused and diaphoretic.
In the emergency room, he was found to be hypoglycemic, with a serum glucose level of 40 mg/dL, which was promptly treated. His mental status improved partially. In the hospital, the rash worsened and progressed upwards to his trunk and upper extremities. He was transferred to our institution for further workup and management.
A review of systems reveals occasional epistaxis in the summer, recent fatigue, cough, and shortness of breath on exertion. His medications at the time of transfer include warfarin (Coumadin), amlodipine (Norvasc), insulin, ipratropium and albuterol (Combivent) inhalers, and prednisone 40 mg daily. He has not undergone surgery recently.
PHYSICAL EXAMINATION
He is alert and oriented to person but not to time and place.
Vital signs. Oral temperature 101.1°F (38.4°C), pulse rate 108, blood pressure 108/79 mm/Hg, respiratory rate 22, oxygen saturation 93% by pulse oximetry on room air, weight 94 kg (207 lb).
Head, eyes, ears, nose, and throat. No pallor or icterus, pupils are equally reactive, nasal mucosa not inflamed or ulcerated, mucous membranes moist, no sinus tenderness.
Neck. No jugular venous distention and no cervical lymphadenopathy.
Cardiovascular. Tachycardia, irregularly irregular rhythm, prosthetic valve sounds, no murmurs, rubs, or gallops.
Respiratory. Bibasal crackles (right side more than the left). No wheezing.
Abdomen. Soft, nontender, nondistended, no palpable organomegaly, bowel sounds normal.
Extremities. No edema, good peripheral pulses.
Neurologic. No focal deficits noted.
Lymphatic. No enlarged lymph nodes.
Musculoskeletal. Traumatic right second distal interphalangeal amputation. Otherwise, no joint abnormality or restriction of movement.
Initial laboratory values:
- White blood cell count 15.78 × 109/L (normal 4.5–11.0)
- Absolute neutrophil count 13.3 × 109/L (4.0–11.0)
- Hemoglobin 13.3 g/dL (13.5–17.5)
- Platelet count 133 × 109/L (150–400)
- International normalized ratio (INR) 1.8
- Sodium 136 mmol/L (135–146)
- Potassium 4.6 mmol/L (3.5–5.0)
- Blood urea nitrogen 31 mg/dL (10–25)
- Creatinine 1.6 mg/dL (0.70–1.40)
- Glucose 62 mg/dL (65–100)
- Bicarbonate 23 mmol/L (23–32)
- Albumin 2.5 g/dL (3.5–5.0)
- Total protein 4.6 g/dL (6.0–8.4)
- Bilirubin 1.2 mg/dL (0.0–1.5)
- Aspartate aminotransferase 41 U/L (7–40)
- Alanine aminotransferase 74 U/L (5–50)
- Alkaline phosphatase 55 U/L (40–150)
- C-reactive protein 9.9 mg/dL (0.0–1.0).
Other studies
Electrocardiography shows atrial fibrillation and left ventricular hypertrophy, but no acute changes.
Computed tomography (CT) of the head shows no evidence of hemorrhage or infarction.
Blood cultures are sent at the time of hospital admission.
WHICH TEST IS NEXT?
1. Which is the most appropriate next step for this patient?
- Urinalysis
- CT of the chest
- Echocardiography
- Skin biopsy
The rash in Figure 1 is palpable purpura, which strongly suggests small-vessel cutaneous vasculitis, a condition that can occur in a broad range of settings. An underlying cause is identified in over 70% of cases. Cutaneous vasculitis may herald a primary small-vessel systemic vasculitis such as Wegener granulomatosis, microscopic polyangiitis, or Henoch-Schönlein purpura. It can also be secondary to a spectrum of underlying triggers or diseases that include medications, infections, malignancies such as lymphoproliferative disorders, cryoglobulinemia secondary to hepatitis C viral infection, and connective tissue diseases such as rheumatoid arthritis and systemic lupus erythematosus.
Infective endocarditis is associated with a secondary form of vasculitis and is a strong possibility in this patient, who has a prosthetic aortic valve, fever, and a high white blood cell count.
Thrombocytopenia should also prompt an assessment for any drugs the patient is taking that affect platelet function. However, thrombocytopenia typically results in nonpalpable purpura.
Idiopathic isolated cutaneous vasculitis, in which no underlying cause for the cutaneous vasculitis can be identified, is the diagnosis in less than 30% of cases.
A vasculitic disease process can involve multiple sites, which may be asymptomatic on presentation. Identifying these sites is important, not only to establish the diagnosis, but also to detect potentially life-threatening complications early.
Thus, in this patient, urinalysis should be done promptly to check for active sediment consisting of red cell casts, which would suggest renal involvement (glomerulonephritis). Also, a rising blood pressure and creatinine would point to renal involvement and warrant more aggressive initial therapy.
Chest radiography should be done to rule out pulmonary infiltrates, septic emboli, nodules, or cavities that could represent vasculitic or infectious involvement of the lungs. CT of the chest may be needed to further characterize abnormalities on chest radiography.
Echocardiography should certainly be pursued as part of the workup for endocarditis, but urinalysis is of the utmost importance in this patient at this point.
More diagnostic information is needed before considering skin biopsy.
Clues from the urinalysis and chest radiography
Our patient’s sedimentation rate is 24 mm/hour. The urinalysis is strongly positive for blood and a moderate amount of protein but negative for leukocyte esterase and nitrite. The urine sediment contains numerous red blood cell casts and 6 to 10 white blood cells per high-power field.
Pending return of blood cultures, ceftriaxone (Rocephin) and azithromycin (Zithromax) are started to treat possible community-acquired pneumonia. Vancomycin (Vancocin) is empirically added, given the possibility of prosthetic valve endocarditis. Gram stain on blood cultures shows gram-positive cocci.
Ground-glass attenuation implies focal or diffuse opacification of the lung that does not obscure the vascular structures, is not associated with air bronchograms, and appears as a “veil” across the lung parenchyma.1 It can be seen in acute diseases such as infection (including pneumonia from atypical bacteria, viruses, acid-fast bacilli, and Pneumocystis jiroveci), pulmonary hemorrhage of any cause, acute viral, eosinophilic, and interstitial pneumonias, and hypersensitivity pneumonitis. It can also be seen in chronic disease states such as interstitial lung disease, bronchoalveolar carcinoma, alveolar proteinosis, and sarcoidosis.
WHICH TEST WOULD NOT HELP?
2. Which of the following tests is least likely to help in the diagnostic evaluation at this point?
- Transthoracic echocardiography
- Transesophageal echocardiography
- Bronchoscopy
- Cystoscopy
In this case, the least likely to help is cystoscopy.
This patient’s vasculitic-appearing rash, ground-glass pulmonary infiltrates, and impaired renal function with red cell casts suggest a pulmonary-renal syndrome, and with this constellation of features, a systemic vasculitis is very likely. Therefore, the focus of the evaluation should be on any evidence to support a diagnosis of vasculitis, as well as other possible causes.
In a patient with diabetes, an artificial heart valve, and fever, the possibility of infection, especially endocarditis, remains high. Transthoracic echocardiography is warranted, and if it is negative for vegetations, transesophageal echocardiography would be a reasonable next option.
Bronchoscopy is warranted to determine if the infiltrates represent pulmonary hemorrhage, which can be seen in certain types of vasculitic and systemic disorders.
The finding of red cell casts in the urine indicates glomerulonephritis, and therefore the kidneys are the likely source of the urinary red blood cells, making cystoscopy of no utility in this current, acute setting.
Case continued: His condition worsens
Transthoracic echocardiography reveals a well-seated mechanical prosthetic aortic valve, trivial aortic regurgitation, a peak gradient of 23 mm Hg and a mean gradient of 12 mm Hg (normal values for his prosthetic valve), and no valvular vegetations. Transesophageal echocardiography confirms the absence of vegetations.
His oxygen requirement increases, and analysis of arterial blood gases reveals a pH of 7.37, Pco2 49 mm Hg (normal range 35–45), Po2 102 mm Hg (normal 80–100), and bicarbonate 28 mmol/L while breathing 100% supplemental oxygen by a nonrebreather face mask. He is taken to the medical intensive care unit for intubation and mechanical ventilation. Bronchoscopy performed while he is intubated confirms diffuse alveolar hemorrhage. Pulse intravenous methylprednisolone (Solu-Medrol) therapy is started.
DIFFUSE ALVEOLAR HEMORRHAGE
3. Which of the following is not true about diffuse alveolar hemorrhage?
- Its onset is usually abrupt or of short duration
- It is always associated with hemoptysis
- Radiography most commonly shows new patchy or diffuse alveolar opacities
- Pulmonary function testing shows increased diffusing capacity of the lung for carbon monoxide (Dlco)
Hemoptysis is absent at presentation in as many as 33% of patients.
The onset of diffuse alveolar hemorrhage is usually abrupt or of short duration, with initial symptoms of cough, fever, and dyspnea. Some patients, such as ours, can present with severe acute respiratory distress syndrome requiring mechanical ventilation. Radiography most often shows new patchy alveolar opacities, and CT may reveal a ground-glass appearance. On pulmonary function testing, the Dlco is high, owing to the hemoglobin within the alveoli.
ACUTE GLOMERULONEPHRITIS PLUS PULMONARY HEMORRHAGE EQUALS…?
4. Which disease could have manifestations consistent with acute glomerulonephritis and pulmonary hemorrhage?
- Antiglomerular basement membrane disease
- Wegener granulomatosis
- Microscopic polyangiitis
- Systemic lupus erythematosus
All of these are possible.
The combined presentation of acute glomerulonephritis and pulmonary hemorrhage (also called pulmonary-renal syndrome) is usually seen in antiglomerular basement membrane disease (Goodpasture syndrome) and small-vessel systemic vasculitides such as Wegener granulomatosis and microscopic polyangiitis.2,3 It can also be seen in patients with systemic lupus erythematosus.
Antiglomerular basement membrane disease
In antiglomerular basement membrane disease, circulating antibodies are directed towards an antigen intrinsic to the glomerular basement membrane, typically leading to acute glomerulonephritis associated with crescent formation. It may present as acute renal failure in which urinalysis shows proteinuria with sediment characterized by red cell casts. Pulmonary involvement, usually alveolar hemorrhage, is present in approximately 60% to 70% of cases.
The diagnosis requires demonstration of antiglomerular basement membrane antibodies in either the serum or the kidney. Renal biopsy is usually recommended because the accuracy of serum assays is variable.
A key histologic feature of the renal lesion in antiglomerular basement membrane disease is crescentic glomerulonephritis in which immunofluorescence microscopy demonstrates the virtually pathognomonic finding of linear deposition of immunoglobulin G along the glomerular capillaries.
The treatment of choice for antiglomerular basement membrane disease is plasmapheresis and immunosuppression with a combination of glucocorticoids and cyclophosphamide (Cytoxan). If the disease is high on the differential diagnosis, empiric plasmapheresis should be started while waiting for diagnostic studies, because the prognosis of untreated glomerulonephritis is poor.
Wegener granulomatosis
Wegener granulomatosis is a systemic vasculitis of the medium and small arteries, arterioles, and venules that classically involves the upper and lower respiratory tracts and the kidneys. Patients may present with persistent rhinorrhea and epistaxis, cough with chest radiographs showing nodules, fixed infiltrates, or cavities, and abnormal urinary sediment with microscopic hematuria with or without red cell casts.
From 75% to 90% of patients with active Wegener granulomatosis are positive for antineutrophil cytoplasmic antibody (ANCA). In 60% to 80% of cases, ANCA is directed against proteinase 3 (PR3), which produces a cytoplasmic standing pattern by immunofluorescence (cANCA), while 5% to 20% have ANCA directed against myeloper-oxidase, which produces a perinuclear staining pattern (pANCA). A small number of patients with Wegener granulomatosis are ANCA-negative.
The diagnosis is usually confirmed by tissue biopsy at the site of active disease, which shows necrotizing vasculitis with granulomatous inflammation. The renal lesion is typically that of a focal, segmental, necrotizing glomerulonephritis that has few to no immune complexes (pauci-immune glomerulonephritis).
The treatment of severe disease involves a combination of cyclophosphamide and glucocorticoids initially to achieve remission followed by maintenance therapy with methotrexate or azathioprine (Imuran).
Microscopic polyangiitis
Microscopic polyangiitis is a systemic vasculitis of the capillaries, venules, and arterioles, with little or no immune complex deposition. Nearly all patients have renal involvement, and 10% to 30% have lung involvement. In those with lung involvement, diffuse alveolar hemorrhage is the most common manifestation.
On histopathologic study, microscopic polyangiitis differs from Wegener granulomatosis in that it does not have granuloma formation. However, the renal lesion is that of a pauci-immune glomerulonephritis and is identical to that seen in Wegener granulomatosis. From 70% to 85% of patients with microscopic polyangiitis are ANCA-positive, and most of these have pANCA.
The management of active severe microscopic polyangiitis is identical to that of Wegener granulomatosis.
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease characterized by tissue-binding autoantibody and immune-complex-mediated organ damage. It can involve multiple organ systems, and the diagnosis is based on characteristic clinical features and autoantibodies. The sensitivity of antinuclear antibody for lupus is close to 100%, which makes it a good screening tool. Antibodies to dsDNA and Smith antigen have high specificity for lupus.
About 75% of patients have renal involvement at some point in their disease course. The different types of renal disease in systemic lupus are usually differentiated with a renal biopsy, with immune-complex-mediated glomerular diseases being the most common.
The most common pulmonary manifestation is pleuritis with or without pleural effusion. Life-threatening pulmonary manifestations include pulmonary hemorrhage and interstitial inflammation leading to fibrosis.
Lupus has great clinical variability and the treatment approach is based on the organ manifestations, disease activity, and severity.
CASE CONTINUED: ARRIVING AT THE DIAGNOSIS
We start our patient on cyclophosphamide 175 mg daily in view of possible Wegener granulomatosis.
Even though purpura is extremely rare in primary antiglomerular basement membrane disease, this patient has life-threatening pulmonary hemorrhage, a complication seen in over 50% of these patients. Therefore, plasmapheresis is started empirically.
On the second day of cyclophosphamide treatment, tests for ANCA, glomerular basement membrane antibody, and antinuclear antibody are reported as negative, and complement levels are normal. Bronchoalveolar lavage shows no infection. Follow-up blood cultures are negative.
To summarize the findings so far, this patient has a purpuric skin rash, active urine sediment with red cell casts indicating glomerulonephritis, acute renal failure, and severe pulmonary hemorrhage requiring mechanical ventilation. Although one set of blood cultures showed gram-positive cocci, no source of infection, particularly endocarditis, could be identified.
Antiglomerular basement membrane disease would still be high on the list of suspected diagnoses, given his diffuse alveolar hemorrhage. As mentioned earlier, renal biopsy is imperative to making a diagnosis, because serologic tests have variable accuracy. And making the correct diagnosis has therapeutic implications.
Renal biopsy is performed and shows immune-complex mesangiopathic glomerulonephritis with positive immunofluorescent staining in the mesangium for IgA. Only one glomerulus shows fibrinoid necrosis.
Skin biopsy results obtained earlier showed positive direct immunofluorescence for IgA. Both renal and skin biopsies suggested Henoch-Schönlein purpura.
IgA deposition in the kidney and skin has been associated with liver cirrhosis, celiac disease, and infections with agents such as human immunodeficiency virus, cytomegalovirus, Haemophilus parainfluenzae, and Staphylococcus aureus. In a Japanese study,4 renal biopsy specimens from 116 patients with IgA nephropathy and from 122 patients with other types of kidney disease were examined for the presence of S aureus antigen in the glomeruli. Although antigen was not detected in non-IgA disease, 68% of specimens from patients with IgA nephropathy had S aureus cell envelope antigen together with IgA antibody in the glomeruli. However, no single antigen has been consistently identified, so it seems more probable that the development of IgA deposition in kidneys is a consequence of aberrant IgA immune response rather than the antigen itself.
HENOCH-SCHÖNLEIN PURPURA
Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is characterized by the tissue deposition of IgA-containing immune complexes. It is predominantly a disease of children but it can be seen in adults. A UK study found the prevalence to be 20 per 100,000 children, with the highest prevalence between ages 4 and 7 (70 per 100,000).5
The four cardinal clinical features of Henoch-Schönlein purpura are purpuric rash, abdominal pain, arthralgia, and renal involvement. Almost all patients have purpuric rash at some point in their disease course. Arthralgia with or without arthritis is typically migratory, oligoarticular, and nondeforming, usually affecting the large joints of the lower extremities; involvement of the upper extremities is less common.
Skin biopsy typically shows leukocytoclastic vasculitis in postcapillary venules with IgA deposition, and these findings are pathognomonic of Henoch-Schönlein purpura.
Gastrointestinal involvement can range from mild symptoms such as nausea, vomiting, abdominal pain, and paralytic ileus to severe disease such as gastrointestinal hemorrhage, bowel infarction, bowel perforation, and intussusception.
Renal involvement is common and is important, as it can in rare cases progress to end-stage renal disease. The urinalysis usually shows mild proteinuria with active sediment with red cell casts. Most patients have relatively mild disease, characterized by asymptomatic hematuria with a normal or slightly elevated creatinine. However, severe involvement may occur, with nephrotic syndrome, hypertension, and acute renal failure.
Different presentation in adults vs children
Adults with Henoch-Schönlein purpura only rarely present with bowel intussusception, whereas some studies have found that adults are more likely than children to develop significant renal involvement, including end-stage renal disease.6,7
There is a general but not absolute correlation between the severity of clinical manifestations and the findings on renal biopsy. A poor prognosis (significant proteinuria, hypertension, renal insufficiency, or end-stage renal disease) is associated with crescent formation involving more than 50% of the glomeruli.8
Our current understanding of the longterm outcome of the renal disease in Henoch-Schönlein purpura is primarily derived from studies in children. In one study, complete recovery occurred in 94% of children and 89% of adults.7 A long-term study of 250 adults with Henoch-Schönlein purpura and renal involvement of sufficient severity to require biopsy reported that, at a median follow up of 15 years, 11% had become dialysis-dependent and 13% had severe renal failure (creatinine clearance < 30 mL/min).6 Recurrence is common, occurring in approximately one-third of patients, more likely in those with nephritis.8
The diagnosis of Henoch-Schönlein purpura is typically made on the basis of key clinical features. In patients such as ours who have an atypical presentation, biopsy of affected skin and renal biopsy can be essential in the diagnosis. Diffuse alveolar hemorrhage is exceedingly rare in Henoch-Schönlein purpura but can be seen, as in our patient.9,10 In this setting, the findings of IgA deposits in skin and renal biopsy specimens, together with the absence of other clinical, serologic, or histologic features of other more-common potential causes, secured the diagnosis in this patient.
Henoch-Schönlein purpura is usually self-limited and requires no specific therapy. Evidence suggests that glucocorticoids enhance the rate of resolution of the arthritis and abdominal pain but do not appear to prevent recurrent disease or lessen the likelihood of progression of renal disease.8 Patients with severe renal involvement with renal function impairment may benefit from pulse intravenous corticosteroid therapy (methylprednisolone 250–1,000 mg per day for 3 days), followed by oral steroids for 3 months.11
In anecdotal reports, renal function improved in 19 of 21 children with Henoch-Schönlein purpura and severe crescentic nephritis.12 Studies have evaluated cyclophosphamide13 and plasmapheresis,14 but their role remains uncertain. Renal transplantation is an option in patients who progress to end-stage renal disease.
OUR CASE CONTINUED
In our patient, plasmapheresis was discontinued. As the pulmonary hemorrhage had developed during treatment with prednisone, we decided to continue cyclophosphamide, given the life-threatening nature of his disease. His pulmonary status improved and he was extubated.
During his initial hospital stay, he was taking heparin for anticoagulation therapy. However, given the life-threatening diffuse alveolar hemorrhage, heparin was stopped during the course of his stay in the intensive care unit. Once he was stable and was transferred out of the intensive care unit, heparin was resumed, and his anticoagulation therapy was bridged to warfarin just before discharge. He was eventually discharged on a tapering dose of oral prednisone and cyclophosphamide for 3 months, after which he was switched to azathioprine for maintenance therapy. He was doing well 6 months later, with a serum creatinine level of 1.6 mg/dL, no red cell casts in the urine, and no rash.
TAKE-HOME POINT
In any case of suspected vasculitis that presents with skin disease, it is essential to look for other sites with potentially life-threatening involvement. Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is not always benign and can be associated with serious complications such as renal failure, gastrointestinal events, and, very rarely, diffuse alveolar hemorrhage.
A 72-year-old man whose medical history includes diabetes mellitus, hypertension, coronary artery disease, aortic valve replacement, atrial fibrillation, and chronic obstructive pulmonary disease was in his usual state of health until 2 weeks ago, when he developed a purpuric rash on his legs. His physician started him on prednisone 40 mg daily for the rash; however, 1 week later he presented to a hospital emergency room when his family found him confused and diaphoretic.
In the emergency room, he was found to be hypoglycemic, with a serum glucose level of 40 mg/dL, which was promptly treated. His mental status improved partially. In the hospital, the rash worsened and progressed upwards to his trunk and upper extremities. He was transferred to our institution for further workup and management.
A review of systems reveals occasional epistaxis in the summer, recent fatigue, cough, and shortness of breath on exertion. His medications at the time of transfer include warfarin (Coumadin), amlodipine (Norvasc), insulin, ipratropium and albuterol (Combivent) inhalers, and prednisone 40 mg daily. He has not undergone surgery recently.
PHYSICAL EXAMINATION
He is alert and oriented to person but not to time and place.
Vital signs. Oral temperature 101.1°F (38.4°C), pulse rate 108, blood pressure 108/79 mm/Hg, respiratory rate 22, oxygen saturation 93% by pulse oximetry on room air, weight 94 kg (207 lb).
Head, eyes, ears, nose, and throat. No pallor or icterus, pupils are equally reactive, nasal mucosa not inflamed or ulcerated, mucous membranes moist, no sinus tenderness.
Neck. No jugular venous distention and no cervical lymphadenopathy.
Cardiovascular. Tachycardia, irregularly irregular rhythm, prosthetic valve sounds, no murmurs, rubs, or gallops.
Respiratory. Bibasal crackles (right side more than the left). No wheezing.
Abdomen. Soft, nontender, nondistended, no palpable organomegaly, bowel sounds normal.
Extremities. No edema, good peripheral pulses.
Neurologic. No focal deficits noted.
Lymphatic. No enlarged lymph nodes.
Musculoskeletal. Traumatic right second distal interphalangeal amputation. Otherwise, no joint abnormality or restriction of movement.
Initial laboratory values:
- White blood cell count 15.78 × 109/L (normal 4.5–11.0)
- Absolute neutrophil count 13.3 × 109/L (4.0–11.0)
- Hemoglobin 13.3 g/dL (13.5–17.5)
- Platelet count 133 × 109/L (150–400)
- International normalized ratio (INR) 1.8
- Sodium 136 mmol/L (135–146)
- Potassium 4.6 mmol/L (3.5–5.0)
- Blood urea nitrogen 31 mg/dL (10–25)
- Creatinine 1.6 mg/dL (0.70–1.40)
- Glucose 62 mg/dL (65–100)
- Bicarbonate 23 mmol/L (23–32)
- Albumin 2.5 g/dL (3.5–5.0)
- Total protein 4.6 g/dL (6.0–8.4)
- Bilirubin 1.2 mg/dL (0.0–1.5)
- Aspartate aminotransferase 41 U/L (7–40)
- Alanine aminotransferase 74 U/L (5–50)
- Alkaline phosphatase 55 U/L (40–150)
- C-reactive protein 9.9 mg/dL (0.0–1.0).
Other studies
Electrocardiography shows atrial fibrillation and left ventricular hypertrophy, but no acute changes.
Computed tomography (CT) of the head shows no evidence of hemorrhage or infarction.
Blood cultures are sent at the time of hospital admission.
WHICH TEST IS NEXT?
1. Which is the most appropriate next step for this patient?
- Urinalysis
- CT of the chest
- Echocardiography
- Skin biopsy
The rash in Figure 1 is palpable purpura, which strongly suggests small-vessel cutaneous vasculitis, a condition that can occur in a broad range of settings. An underlying cause is identified in over 70% of cases. Cutaneous vasculitis may herald a primary small-vessel systemic vasculitis such as Wegener granulomatosis, microscopic polyangiitis, or Henoch-Schönlein purpura. It can also be secondary to a spectrum of underlying triggers or diseases that include medications, infections, malignancies such as lymphoproliferative disorders, cryoglobulinemia secondary to hepatitis C viral infection, and connective tissue diseases such as rheumatoid arthritis and systemic lupus erythematosus.
Infective endocarditis is associated with a secondary form of vasculitis and is a strong possibility in this patient, who has a prosthetic aortic valve, fever, and a high white blood cell count.
Thrombocytopenia should also prompt an assessment for any drugs the patient is taking that affect platelet function. However, thrombocytopenia typically results in nonpalpable purpura.
Idiopathic isolated cutaneous vasculitis, in which no underlying cause for the cutaneous vasculitis can be identified, is the diagnosis in less than 30% of cases.
A vasculitic disease process can involve multiple sites, which may be asymptomatic on presentation. Identifying these sites is important, not only to establish the diagnosis, but also to detect potentially life-threatening complications early.
Thus, in this patient, urinalysis should be done promptly to check for active sediment consisting of red cell casts, which would suggest renal involvement (glomerulonephritis). Also, a rising blood pressure and creatinine would point to renal involvement and warrant more aggressive initial therapy.
Chest radiography should be done to rule out pulmonary infiltrates, septic emboli, nodules, or cavities that could represent vasculitic or infectious involvement of the lungs. CT of the chest may be needed to further characterize abnormalities on chest radiography.
Echocardiography should certainly be pursued as part of the workup for endocarditis, but urinalysis is of the utmost importance in this patient at this point.
More diagnostic information is needed before considering skin biopsy.
Clues from the urinalysis and chest radiography
Our patient’s sedimentation rate is 24 mm/hour. The urinalysis is strongly positive for blood and a moderate amount of protein but negative for leukocyte esterase and nitrite. The urine sediment contains numerous red blood cell casts and 6 to 10 white blood cells per high-power field.
Pending return of blood cultures, ceftriaxone (Rocephin) and azithromycin (Zithromax) are started to treat possible community-acquired pneumonia. Vancomycin (Vancocin) is empirically added, given the possibility of prosthetic valve endocarditis. Gram stain on blood cultures shows gram-positive cocci.
Ground-glass attenuation implies focal or diffuse opacification of the lung that does not obscure the vascular structures, is not associated with air bronchograms, and appears as a “veil” across the lung parenchyma.1 It can be seen in acute diseases such as infection (including pneumonia from atypical bacteria, viruses, acid-fast bacilli, and Pneumocystis jiroveci), pulmonary hemorrhage of any cause, acute viral, eosinophilic, and interstitial pneumonias, and hypersensitivity pneumonitis. It can also be seen in chronic disease states such as interstitial lung disease, bronchoalveolar carcinoma, alveolar proteinosis, and sarcoidosis.
WHICH TEST WOULD NOT HELP?
2. Which of the following tests is least likely to help in the diagnostic evaluation at this point?
- Transthoracic echocardiography
- Transesophageal echocardiography
- Bronchoscopy
- Cystoscopy
In this case, the least likely to help is cystoscopy.
This patient’s vasculitic-appearing rash, ground-glass pulmonary infiltrates, and impaired renal function with red cell casts suggest a pulmonary-renal syndrome, and with this constellation of features, a systemic vasculitis is very likely. Therefore, the focus of the evaluation should be on any evidence to support a diagnosis of vasculitis, as well as other possible causes.
In a patient with diabetes, an artificial heart valve, and fever, the possibility of infection, especially endocarditis, remains high. Transthoracic echocardiography is warranted, and if it is negative for vegetations, transesophageal echocardiography would be a reasonable next option.
Bronchoscopy is warranted to determine if the infiltrates represent pulmonary hemorrhage, which can be seen in certain types of vasculitic and systemic disorders.
The finding of red cell casts in the urine indicates glomerulonephritis, and therefore the kidneys are the likely source of the urinary red blood cells, making cystoscopy of no utility in this current, acute setting.
Case continued: His condition worsens
Transthoracic echocardiography reveals a well-seated mechanical prosthetic aortic valve, trivial aortic regurgitation, a peak gradient of 23 mm Hg and a mean gradient of 12 mm Hg (normal values for his prosthetic valve), and no valvular vegetations. Transesophageal echocardiography confirms the absence of vegetations.
His oxygen requirement increases, and analysis of arterial blood gases reveals a pH of 7.37, Pco2 49 mm Hg (normal range 35–45), Po2 102 mm Hg (normal 80–100), and bicarbonate 28 mmol/L while breathing 100% supplemental oxygen by a nonrebreather face mask. He is taken to the medical intensive care unit for intubation and mechanical ventilation. Bronchoscopy performed while he is intubated confirms diffuse alveolar hemorrhage. Pulse intravenous methylprednisolone (Solu-Medrol) therapy is started.
DIFFUSE ALVEOLAR HEMORRHAGE
3. Which of the following is not true about diffuse alveolar hemorrhage?
- Its onset is usually abrupt or of short duration
- It is always associated with hemoptysis
- Radiography most commonly shows new patchy or diffuse alveolar opacities
- Pulmonary function testing shows increased diffusing capacity of the lung for carbon monoxide (Dlco)
Hemoptysis is absent at presentation in as many as 33% of patients.
The onset of diffuse alveolar hemorrhage is usually abrupt or of short duration, with initial symptoms of cough, fever, and dyspnea. Some patients, such as ours, can present with severe acute respiratory distress syndrome requiring mechanical ventilation. Radiography most often shows new patchy alveolar opacities, and CT may reveal a ground-glass appearance. On pulmonary function testing, the Dlco is high, owing to the hemoglobin within the alveoli.
ACUTE GLOMERULONEPHRITIS PLUS PULMONARY HEMORRHAGE EQUALS…?
4. Which disease could have manifestations consistent with acute glomerulonephritis and pulmonary hemorrhage?
- Antiglomerular basement membrane disease
- Wegener granulomatosis
- Microscopic polyangiitis
- Systemic lupus erythematosus
All of these are possible.
The combined presentation of acute glomerulonephritis and pulmonary hemorrhage (also called pulmonary-renal syndrome) is usually seen in antiglomerular basement membrane disease (Goodpasture syndrome) and small-vessel systemic vasculitides such as Wegener granulomatosis and microscopic polyangiitis.2,3 It can also be seen in patients with systemic lupus erythematosus.
Antiglomerular basement membrane disease
In antiglomerular basement membrane disease, circulating antibodies are directed towards an antigen intrinsic to the glomerular basement membrane, typically leading to acute glomerulonephritis associated with crescent formation. It may present as acute renal failure in which urinalysis shows proteinuria with sediment characterized by red cell casts. Pulmonary involvement, usually alveolar hemorrhage, is present in approximately 60% to 70% of cases.
The diagnosis requires demonstration of antiglomerular basement membrane antibodies in either the serum or the kidney. Renal biopsy is usually recommended because the accuracy of serum assays is variable.
A key histologic feature of the renal lesion in antiglomerular basement membrane disease is crescentic glomerulonephritis in which immunofluorescence microscopy demonstrates the virtually pathognomonic finding of linear deposition of immunoglobulin G along the glomerular capillaries.
The treatment of choice for antiglomerular basement membrane disease is plasmapheresis and immunosuppression with a combination of glucocorticoids and cyclophosphamide (Cytoxan). If the disease is high on the differential diagnosis, empiric plasmapheresis should be started while waiting for diagnostic studies, because the prognosis of untreated glomerulonephritis is poor.
Wegener granulomatosis
Wegener granulomatosis is a systemic vasculitis of the medium and small arteries, arterioles, and venules that classically involves the upper and lower respiratory tracts and the kidneys. Patients may present with persistent rhinorrhea and epistaxis, cough with chest radiographs showing nodules, fixed infiltrates, or cavities, and abnormal urinary sediment with microscopic hematuria with or without red cell casts.
From 75% to 90% of patients with active Wegener granulomatosis are positive for antineutrophil cytoplasmic antibody (ANCA). In 60% to 80% of cases, ANCA is directed against proteinase 3 (PR3), which produces a cytoplasmic standing pattern by immunofluorescence (cANCA), while 5% to 20% have ANCA directed against myeloper-oxidase, which produces a perinuclear staining pattern (pANCA). A small number of patients with Wegener granulomatosis are ANCA-negative.
The diagnosis is usually confirmed by tissue biopsy at the site of active disease, which shows necrotizing vasculitis with granulomatous inflammation. The renal lesion is typically that of a focal, segmental, necrotizing glomerulonephritis that has few to no immune complexes (pauci-immune glomerulonephritis).
The treatment of severe disease involves a combination of cyclophosphamide and glucocorticoids initially to achieve remission followed by maintenance therapy with methotrexate or azathioprine (Imuran).
Microscopic polyangiitis
Microscopic polyangiitis is a systemic vasculitis of the capillaries, venules, and arterioles, with little or no immune complex deposition. Nearly all patients have renal involvement, and 10% to 30% have lung involvement. In those with lung involvement, diffuse alveolar hemorrhage is the most common manifestation.
On histopathologic study, microscopic polyangiitis differs from Wegener granulomatosis in that it does not have granuloma formation. However, the renal lesion is that of a pauci-immune glomerulonephritis and is identical to that seen in Wegener granulomatosis. From 70% to 85% of patients with microscopic polyangiitis are ANCA-positive, and most of these have pANCA.
The management of active severe microscopic polyangiitis is identical to that of Wegener granulomatosis.
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease characterized by tissue-binding autoantibody and immune-complex-mediated organ damage. It can involve multiple organ systems, and the diagnosis is based on characteristic clinical features and autoantibodies. The sensitivity of antinuclear antibody for lupus is close to 100%, which makes it a good screening tool. Antibodies to dsDNA and Smith antigen have high specificity for lupus.
About 75% of patients have renal involvement at some point in their disease course. The different types of renal disease in systemic lupus are usually differentiated with a renal biopsy, with immune-complex-mediated glomerular diseases being the most common.
The most common pulmonary manifestation is pleuritis with or without pleural effusion. Life-threatening pulmonary manifestations include pulmonary hemorrhage and interstitial inflammation leading to fibrosis.
Lupus has great clinical variability and the treatment approach is based on the organ manifestations, disease activity, and severity.
CASE CONTINUED: ARRIVING AT THE DIAGNOSIS
We start our patient on cyclophosphamide 175 mg daily in view of possible Wegener granulomatosis.
Even though purpura is extremely rare in primary antiglomerular basement membrane disease, this patient has life-threatening pulmonary hemorrhage, a complication seen in over 50% of these patients. Therefore, plasmapheresis is started empirically.
On the second day of cyclophosphamide treatment, tests for ANCA, glomerular basement membrane antibody, and antinuclear antibody are reported as negative, and complement levels are normal. Bronchoalveolar lavage shows no infection. Follow-up blood cultures are negative.
To summarize the findings so far, this patient has a purpuric skin rash, active urine sediment with red cell casts indicating glomerulonephritis, acute renal failure, and severe pulmonary hemorrhage requiring mechanical ventilation. Although one set of blood cultures showed gram-positive cocci, no source of infection, particularly endocarditis, could be identified.
Antiglomerular basement membrane disease would still be high on the list of suspected diagnoses, given his diffuse alveolar hemorrhage. As mentioned earlier, renal biopsy is imperative to making a diagnosis, because serologic tests have variable accuracy. And making the correct diagnosis has therapeutic implications.
Renal biopsy is performed and shows immune-complex mesangiopathic glomerulonephritis with positive immunofluorescent staining in the mesangium for IgA. Only one glomerulus shows fibrinoid necrosis.
Skin biopsy results obtained earlier showed positive direct immunofluorescence for IgA. Both renal and skin biopsies suggested Henoch-Schönlein purpura.
IgA deposition in the kidney and skin has been associated with liver cirrhosis, celiac disease, and infections with agents such as human immunodeficiency virus, cytomegalovirus, Haemophilus parainfluenzae, and Staphylococcus aureus. In a Japanese study,4 renal biopsy specimens from 116 patients with IgA nephropathy and from 122 patients with other types of kidney disease were examined for the presence of S aureus antigen in the glomeruli. Although antigen was not detected in non-IgA disease, 68% of specimens from patients with IgA nephropathy had S aureus cell envelope antigen together with IgA antibody in the glomeruli. However, no single antigen has been consistently identified, so it seems more probable that the development of IgA deposition in kidneys is a consequence of aberrant IgA immune response rather than the antigen itself.
HENOCH-SCHÖNLEIN PURPURA
Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is characterized by the tissue deposition of IgA-containing immune complexes. It is predominantly a disease of children but it can be seen in adults. A UK study found the prevalence to be 20 per 100,000 children, with the highest prevalence between ages 4 and 7 (70 per 100,000).5
The four cardinal clinical features of Henoch-Schönlein purpura are purpuric rash, abdominal pain, arthralgia, and renal involvement. Almost all patients have purpuric rash at some point in their disease course. Arthralgia with or without arthritis is typically migratory, oligoarticular, and nondeforming, usually affecting the large joints of the lower extremities; involvement of the upper extremities is less common.
Skin biopsy typically shows leukocytoclastic vasculitis in postcapillary venules with IgA deposition, and these findings are pathognomonic of Henoch-Schönlein purpura.
Gastrointestinal involvement can range from mild symptoms such as nausea, vomiting, abdominal pain, and paralytic ileus to severe disease such as gastrointestinal hemorrhage, bowel infarction, bowel perforation, and intussusception.
Renal involvement is common and is important, as it can in rare cases progress to end-stage renal disease. The urinalysis usually shows mild proteinuria with active sediment with red cell casts. Most patients have relatively mild disease, characterized by asymptomatic hematuria with a normal or slightly elevated creatinine. However, severe involvement may occur, with nephrotic syndrome, hypertension, and acute renal failure.
Different presentation in adults vs children
Adults with Henoch-Schönlein purpura only rarely present with bowel intussusception, whereas some studies have found that adults are more likely than children to develop significant renal involvement, including end-stage renal disease.6,7
There is a general but not absolute correlation between the severity of clinical manifestations and the findings on renal biopsy. A poor prognosis (significant proteinuria, hypertension, renal insufficiency, or end-stage renal disease) is associated with crescent formation involving more than 50% of the glomeruli.8
Our current understanding of the longterm outcome of the renal disease in Henoch-Schönlein purpura is primarily derived from studies in children. In one study, complete recovery occurred in 94% of children and 89% of adults.7 A long-term study of 250 adults with Henoch-Schönlein purpura and renal involvement of sufficient severity to require biopsy reported that, at a median follow up of 15 years, 11% had become dialysis-dependent and 13% had severe renal failure (creatinine clearance < 30 mL/min).6 Recurrence is common, occurring in approximately one-third of patients, more likely in those with nephritis.8
The diagnosis of Henoch-Schönlein purpura is typically made on the basis of key clinical features. In patients such as ours who have an atypical presentation, biopsy of affected skin and renal biopsy can be essential in the diagnosis. Diffuse alveolar hemorrhage is exceedingly rare in Henoch-Schönlein purpura but can be seen, as in our patient.9,10 In this setting, the findings of IgA deposits in skin and renal biopsy specimens, together with the absence of other clinical, serologic, or histologic features of other more-common potential causes, secured the diagnosis in this patient.
Henoch-Schönlein purpura is usually self-limited and requires no specific therapy. Evidence suggests that glucocorticoids enhance the rate of resolution of the arthritis and abdominal pain but do not appear to prevent recurrent disease or lessen the likelihood of progression of renal disease.8 Patients with severe renal involvement with renal function impairment may benefit from pulse intravenous corticosteroid therapy (methylprednisolone 250–1,000 mg per day for 3 days), followed by oral steroids for 3 months.11
In anecdotal reports, renal function improved in 19 of 21 children with Henoch-Schönlein purpura and severe crescentic nephritis.12 Studies have evaluated cyclophosphamide13 and plasmapheresis,14 but their role remains uncertain. Renal transplantation is an option in patients who progress to end-stage renal disease.
OUR CASE CONTINUED
In our patient, plasmapheresis was discontinued. As the pulmonary hemorrhage had developed during treatment with prednisone, we decided to continue cyclophosphamide, given the life-threatening nature of his disease. His pulmonary status improved and he was extubated.
During his initial hospital stay, he was taking heparin for anticoagulation therapy. However, given the life-threatening diffuse alveolar hemorrhage, heparin was stopped during the course of his stay in the intensive care unit. Once he was stable and was transferred out of the intensive care unit, heparin was resumed, and his anticoagulation therapy was bridged to warfarin just before discharge. He was eventually discharged on a tapering dose of oral prednisone and cyclophosphamide for 3 months, after which he was switched to azathioprine for maintenance therapy. He was doing well 6 months later, with a serum creatinine level of 1.6 mg/dL, no red cell casts in the urine, and no rash.
TAKE-HOME POINT
In any case of suspected vasculitis that presents with skin disease, it is essential to look for other sites with potentially life-threatening involvement. Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is not always benign and can be associated with serious complications such as renal failure, gastrointestinal events, and, very rarely, diffuse alveolar hemorrhage.
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997; 169:355–367.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002; 39:42–47.
- Koyama A, Sharmin S, Sakurai H, et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 2004; 66:121–132.
- Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002; 360:1197–1202.
- Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002; 13:1271–1278.
- Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M, Gonzalez-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum 1997; 40:859–864.
- Saulsbury FT. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore) 1999; 78:395–409.
- Nadrous HF, Yu AC, Specks U, Ryu JH. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc 2004; 79:1151–1157.
- Vats KR, Vats A, Kim Y, Dassenko D, Sinaiko AR. Henoch-Schönlein purpura and pulmonary hemorrhage: a report and literature review. Pediatr Nephrol 1999; 13:530–534.
- Niaudet P, Habib R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol 1998; 12:238–243.
- Bergstein J, Leiser J, Andreoli SP. Response of crescentic Henoch-Schöenlein purpura nephritis to corticosteroid and azathioprine therapy. Clin Nephrol 1998; 49:9–14.
- Tarshish P, Bernstein J, Edelmann CM. Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 2004; 19:51–56.
- Hattori M, Ito K, Konomoto T, Kawaguchi H, Yoshioka T, Khono M. Plasmapheresis as the sole therapy for rapidly progressive Henoch-Schönlein purpura nephritis in children. Am J Kidney Dis 1999; 33:427–433.
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997; 169:355–367.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002; 39:42–47.
- Koyama A, Sharmin S, Sakurai H, et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 2004; 66:121–132.
- Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002; 360:1197–1202.
- Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002; 13:1271–1278.
- Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M, Gonzalez-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum 1997; 40:859–864.
- Saulsbury FT. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore) 1999; 78:395–409.
- Nadrous HF, Yu AC, Specks U, Ryu JH. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc 2004; 79:1151–1157.
- Vats KR, Vats A, Kim Y, Dassenko D, Sinaiko AR. Henoch-Schönlein purpura and pulmonary hemorrhage: a report and literature review. Pediatr Nephrol 1999; 13:530–534.
- Niaudet P, Habib R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol 1998; 12:238–243.
- Bergstein J, Leiser J, Andreoli SP. Response of crescentic Henoch-Schöenlein purpura nephritis to corticosteroid and azathioprine therapy. Clin Nephrol 1998; 49:9–14.
- Tarshish P, Bernstein J, Edelmann CM. Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 2004; 19:51–56.
- Hattori M, Ito K, Konomoto T, Kawaguchi H, Yoshioka T, Khono M. Plasmapheresis as the sole therapy for rapidly progressive Henoch-Schönlein purpura nephritis in children. Am J Kidney Dis 1999; 33:427–433.
New developments in the diagnosis of fibromyalgia syndrome: Say goodbye to tender points?
A relatively new diagnostic tool, the Symptom Intensity Scale, is an easy, quick way to assess both regional pain and fatigue in a patient. It can be used to establish the diagnosis of fibromyalgia syndrome and measure its severity in daily clinical practice without the need to count tender points. It can also be used to detect fibromyalgia as a comorbidity in other clinical illnesses; by uncovering fibromyalgia, the questionnaire serves as a surrogate measure of depression, anxiety, other serious personality disorders, previous or ongoing abuse, and, when fatigue is the dominant symptom, a consideration of obstructive sleep apnea—all part of the pathoetiology of fibromyalgia in that individual.
This manuscript reviews previous criteria and definitions by which fibromyalgia syndrome was recognized, describes how the new questionnaire was developed, and discusses its implications. It is not meant as a review of the pathogenesis or treatment of fibromyalgia or when to send the patient to the rheumatologist. Each of those topics requires lengthy and complex discussions, which are beyond the scope of this paper.
A COMMON, MULTIFACTORIAL DISEASE
The pathoetiology of fibromyalgia syndrome is rooted in disordered sleep, increased stress, and abnormal neurosensory processing, with secondary endocrine and autonomic dysfunction in those who are genetically predisposed.1–4 Because fibromyalgia is multifactorial, it is best understood from the perspective of an inclusive biopsychosocial model rather than a limited biomedical model.5 Its characteristic signs and symptoms are best understood as emanating from a physiologic state, called central sensitization syndrome, in which the nervous system overresponds to stimuli.1,3 This anomalous state of heightened nervous system response is not confined to the peripheral nervous system, but is also present in the autonomic and central nervous systems.3,4
Fibromyalgia syndrome is common, affecting 0.5% to 5% of the general population,6 and is either the second or third most common diagnosis in a rheumatology practice. Importantly for internists, a diagnosis of fibromyalgia syndrome should be made in 10% to 15% of primary care patients.7 The high prevalence alone demands diagnostic recognition.
KNOWN IN HISTORY AND LITERATURE
Although the designation fibromyalgia syndrome is new, the illness has been with us for as long as we’ve been us. In fact, the word rheumatology may have its origin in fibromyalgia syndrome. Galen (about 180 ad) blamed the symptoms of diffuse pain on the “rheuma,” which has been interpreted as “a great fluxion which races [from the center?] to various parts of the body, and goes from one to another.”8 (Is this the origin of blood-letting as a treatment for diseases?) In 1592, the French physician Guillaume de Baillou introduced the term rheumatism to describe both muscle and joint pain.9
Literature also knows fibromyalgia syndrome. Hans Christian Andersen described a supersensitive princess for whom a pea beneath many mattresses was sufficient to ruin her sleep. In The Fall of the House of Usher, Edgar Allan Poe described Roderick Usher as having an “acute bodily illness and mental disorder that oppressed him.” Usher would wear garments of only soft texture because rough cloth was painful. Light hurt his eyes, forcing him to keep the curtains drawn. Although he had previously played and enjoyed violin music, he could no longer tolerate the sound of the violin. In fact, he suffered such hyperacusis that he could hear his sister moving in her grave many floors below. Other stories by Poe such as Rats in the Wall and The Tell-Tale Heart give more evidence that he was well acquainted with the symptoms of central sensitization syndrome.
HOW THE DEFINITION HAS EVOLVED
To recognize fibromyalgia we need an accurate definition, which has evolved over the years. If we don’t know where we’ve been, it is difficult to understand where we are now or how we got here.
Gowers,10 in 1904, was the first to describe diffuse pain as “fibrositis.” He believed that the pain was due to proliferation or inflammation (or both) of subcutaneous and fibrous tissue, a histopathology that has not been satisfactorily demonstrated to this date. Unfortunately for our purposes, his paper was a descriptive essay that made no attempt at codification. In fact, attempts to clinically define and classify fibromyalgia syndrome have been relatively recent.
Hench11 proposed the first clinical definition in 1976, and it probably did more harm than good. His criteria were two: pain, and no physiologic explanation. The diagnosis was therefore made by ruling out everything else rather than by ruling it in by clinical criteria. Consequently, the diagnosing physician had to investigate the symptom or symptoms by ordering potentially limitless testing, which all had to be normal before the diagnosis could be entertained. I continue to see this phenomenon today as new patients with classic fibromyalgia syndrome arrive carrying reports of normal magnetic imaging of the entire body and serologic testing—a “connective tissue disease workup.”
Counting tender points
Smythe (1979)12 was the first to define and classify fibromyalgia syndrome as a rule-in diagnosis. Smythe’s criteria included tender points in at least 12 of 14 anatomic locations using 4 kg of pressure. In practice, the pressure is approximate—the nail bed blanches in a normotensive examiner with a force of 4 kg. He also described four necessary signs and symptoms: diffuse pain of at least 3 months’ duration, disturbed sleep, skin-roll tenderness at the upper trapezius border, and normal results on laboratory tests. He and Moldofsky13 also found a relationship between disordered slow-wave sleep and the symptoms of fibromyalgia syndrome.
Yunus et al (1981)14 compared signs and symptoms in 50 patients with fibromyalgia syndrome and 50 healthy controls to develop criteria for the disease. Of the resulting criteria, two were mandatory: diffuse pain of at least 3 months’ duration and lack of other obvious causes. The definition also required tenderness in at least 5 of 40 tender points and outlined 10 minor criteria.
Signs and factors that modulate fibromyalgia syndrome and that were derived from these minor criteria are still clinically important today. Factors that aggravate pain include cold or humid weather, fatigue, sedentary state, anxiety, and overactivity. Relieving factors include a hot shower, physical activity, warm dry weather, and massage.
The American College of Rheumatology (1990). Understanding that at any one time approximately 15% of the general population experiences widespread pain,15 a committee of the American College of Rheumatology (ACR) set out to differentiate patients with fibromyalgia syndrome from those with less severe widespread pain. The committee compared signs and symptoms in 293 patients deemed by experts to have fibromyalgia syndrome and 265 control patients matched for age, sex, and concomitant rheumatic disorders. 16
The symptom of widespread pain of at least 3 months’ duration and tenderness in at least 11 of 18 points became the ACR’s diagnostic criteria and provided a sensitivity of 88% and a specificity of 81% compared with the experts’ opinion as the gold standard test.
Low specificity is one of the recognized problems with the ACR criteria: 19% of patients with at least 11 tender points did not have fibromyalgia syndrome. In addition, tender points don’t correlate well with some measures of illness activity, such as the Fibromyalgia Impact Questionnaire.17
Is the tender-point count a good measure?
The best argument for continuing to count tender points as part of the clinical evaluation is that it is a measure of severity. Higher numbers of tender points indicate greater psychological distress and greater severity and frequency of other, closely related fibromyalgia symptoms.18,19 Nearly everyone in the general population has at least a few tender points.16 In fibromyalgia syndrome, the tender-point count is a good status surrogate, a measure of the state of the illness.
But should a state/status measure be used as an illness trait and a criterion for diagnosis? I believe not. Consider, as an analogy, the use of the erythrocyte sedimentation rate in patients with rheumatoid arthritis. An elevated sedimentation rate may indicate increased systemic inflammation, but it is a measure of the status of rheumatoid arthritis, not a trait of this disease. This is why I believe that most rheumatologists would disagree with using some value of the erythrocyte sedimentation rate as a criterion for the diagnosis of rheumatoid arthritis and, by analogy, the tender-point count as a criterion for the diagnosis of fibromyalgia syndrome. Also, the number of tender points, a surrogate for diffuse pain, does not fully capture the essence of the illness, in which accompanying fatigue is often severe and nearly always present.20
A CONTEMPORARY DEFINITION AND ITS VALIDATION
As the concept of fibromyalgia syndrome evolved, a movement away from tender points took hold.21
The Manchester criteria22 used a pain diagram to establish the diagnosis, in which the patient indicated the areas of pain on a simple drawing, obviating the need for tender points. It showed good agreement with the ACR criteria, and in fact identified patients with more severe symptoms.
The London Fibromyalgia Epidemiology Study Screening Questionnaire,23 designed as an epidemiologic tool to estimate the prevalence of the syndrome, was the first test to specifically include both pain and fatigue.
White et al,24 in a very important subsequent study, showed that higher fatigue scores differentiated patients with widespread pain and only a few tender points (7–10) from those with more tender points. This report helped to set the stage for the Symptom Intensity Scale.
What the Symptom Intensity Scale measures
- The Regional Pain Scale score, which is the number of anatomic areas—out of a possible 19—in which the patient feels pain
- A fatigue visual analogue scale score, in which the patient makes a mark somewhere along a 10-cm line to indicate how tired he or she feels. Subsequently, the clinician measures the position of the mark from the left end of the line with a ruler.
How the Symptom Intensity Scale was developed
Wolfe (2003)25 mailed a survey to 12,799 patients who had rheumatoid arthritis, osteoarthritis, or fibromyalgia syndrome. The questionnaire asked respondents if they had pain in 38 articular and nonarticular anatomic regions and to complete a 10-cm fatigue visual analogue scale. He observed that pain in a subset of 19 primarily nonarticular sites differentiated fibromyalgia syndrome from the other two diseases. Calling the number of painful areas of these 19 sites the Regional Pain Scale, he analyzed this measure using Mokken analysis and Rasch analysis to ensure that the questionnaire was statistically valid.
Wolfe also showed that a score of at least 8 points on the Regional Pain Scale, combined with a score of at least 6 cm on the fatigue visual analogue scale, provided the best diagnostic precision consistent with a diagnosis of fibromyalgia syndrome. The combination of these two measures became known as the Survey Criteria.
Katz, Wolfe, and Michaud (2006)26 next compared the diagnostic precision of the Survey Criteria, the ACR criteria, and a physician’s clinical diagnosis. The clinicians made their clinical diagnosis by “considering the long-term patient-clinician experience [including] factors related to pain, tenderness, fatigue, sleep disturbance, comorbidity, and psychosocial variables,” or as I call it, the company fibromyalgia syndrome keeps (Table 2).7,14,16,20 The Survey Criteria (8 points or higher on the Regional Pain Scale plus 6 cm or higher on the fatigue visual analogue scale) showed a roughly 75% concordance among all three definitions in 206 patients with fibromyalgia syndrome. In a cohort with clinically diagnosed fibromyalgia syndrome, a Regional Pain Scale score of 8 or more had a sensitivity of 83.2%, a specificity of 87.6%, and a percent correct of 85.4%. The authors reported that a score of 6 cm or more on the fatigue visual analogue scale “was also at the optimum level” for diagnosing fibromyalgia, but they did not provide more information.
Wolfe and Rasker (2006),27 using these data, devised the Symptom Intensity Scale, the score of which is calculated as the fatigue visual analogue scale score plus half the Regional Pain Scale score, all divided by 2. The scale is therefore a continuous variable rather than a categorical one, and scores can range from 0 to 9.75.
The authors gave the questionnaire to 25,417 patients who had various rheumatic diseases and found that a score of 5.75 or higher differentiated fibromyalgia syndrome from other rheumatic diseases, identifying 95% of patients who would satisfy the Survey Criteria for fibromyalgia.
In addition, they found a linear relationship between the Symptom Intensity Scale score and key symptoms of fibromyalgia syndrome. Of even greater importance, the Symptom Intensity Scale score showed closer association with general health than scores on the Health Assessment Questionnaire, a 27-question patient activity scale, the Arthritis Impact Measurement Scale, or the Short Form-36. It also proved to correlate with mood, probability of having diabetes, need for hospitalization, history of or relative time to myocardial infarction, number of comorbidities, rate of disability, and risk of early death (relative risk 1.12, 95% confidence interval 1.10–1.14). The Symptom Intensity Scale is therefore a diagnostic tool as well as a simple measure of general health among all rheumatic disease patients.
IMPLICATIONS OF THE SYMPTOM INTENSITY SCALE
Three arguments provide a strong rationale for using the Symptom Intensity Scale in the outpatient clinic to investigate the biopsychosocial aspects of illness in our patients:
- It is a simple way to measure overall health
- It can uncover comorbid depression
- It can detect fibromyalgia syndrome in patients who have other diseases.
It measures overall health
Unlike instruments intended for a particular disease such as the Disease Activity Score, which measures disease severity only in rheumatoid arthritis, the Symptom Intensity Scale score can also be used as a measure of global health (or disease severity), and the Survey Criteria (8 or more on the Regional Pain Score and 6 or more on the fatigue visual analogue scale) can be used to establish diagnosis. In fact, instruments like the Disease Activity Score essentially ignore biopsychosocial issues that are captured by the Symptom Intensity Scale.27
By detecting fibromyalgia syndrome in our patients, we identify people with symptoms of pain and distress that do not easily fit the prevalent model of organic disease. Measures like the Disease Activity Score are specifically suited as end points in controlled efficacy trials, but if these are the only measures physicians use to estimate a patient’s health in the clinic, they do so at their own and their patient’s peril.27
Because the continuous Symptom Intensity Scale score strongly correlates with patient-perceived pain, depression, and general health, it is an ideal instrument for outpatient evaluation. It complements a complete patient history and physical examination by measuring biopsychosocial factors.
It uncovers comorbid depression
Rheumatologists do a woeful job of recognizing and diagnosing depression in patients with rheumatoid arthritis. Sleath et al28 found that patients with rheumatoid arthritis who were diagnosed with depression in the office were always the ones who initiated the discussion of that diagnosis. Their doctors did not elicit it.
Middleton et al29 found that patients with concomitant fibromyalgia syndrome and systemic lupus erythematosus (SLE) had higher depression scores than did SLE patients without fibromyalgia syndrome. Moussavi et al,30 writing for the World Health Organization about the findings of a 60-country survey, concluded: “The comorbid state of depression incrementally worsens health compared with depression alone, with any of the chronic diseases alone, and with any combination of chronic diseases without depression.”30
Worse health implies earlier death. Ang et al31 reported that a higher average 4-year depression scale score conferred a hazard ratio of 1.35 (P < .001) for earlier death among 1,290 rheumatoid arthritis patients followed for 12 years.
By using a test like the Symptom Intensity Scale to detect fibromyalgia syndrome alone or to detect it in patients with other diseases, we implicitly recognize the high likelihood of simultaneous depression. Recognition and treatment of depression will improve overall health.
It can detect fibromyalgia syndrome in patients with other diseases
Not surprisingly, distress-related fibromyalgia syndrome is more common in patients with chronic rheumatic or arthritic diseases, with a frequency ranging from 5% in osteoarthritis to 47% in Sjögren syndrome.1 When present, fibromyalgia syndrome changes the features of the other disease.
Wolfe and Michaud32 used the Survey Criteria to evaluate 11,866 rheumatoid arthritis patients and found that 17.1% of them also had fibromyalgia syndrome, and those that did had higher levels of pain, greater global severity, higher scores on the Health Assessment Questionnaire and Short Form-36 mental component, and more disability than those without fibromyalgia syndrome.
Urrows et al33 found that the mean tender-joint count correlated with the mean tenderpoint count in 67 patients with rheumatoid arthritis followed for 75 days. Comorbid fibromyalgia syndrome rendered joints more tender, so that an examiner using the tender-joint count as a major indicator of disease severity might overestimate severity and excessively treat a rheumatoid arthritis patient with unrecognized concurrent fibromyalgia syndrome. Because comorbid fibromyalgia syndrome can inflate Health Assessment Questionnaire scores and subjective pain scale scores in rheumatoid arthritis, more appropriate investigation and management decisions should follow recognition.
Concurrent fibromyalgia syndrome can also be troublesome in SLE. Patients with fibromyalgia syndrome had greater disability than patients without fibromyalgia syndrome despite having no worse SLE damage scores.29 Comorbid fibromyalgia syndrome in SLE has also been shown to diminish quality of life as measured by the Short Form-36.34
Fibromyalgia syndrome also has the potential to confound the diagnosis of concomitant diseases. Wolfe et al35 found that 22.1% of 458 patients with SLE also had fibromyalgia syndrome using the Symptom Intensity Scale criteria. At the time of referral to a rheumatologist, patients who met the criteria for fibromyalgia syndrome were more likely to have self-reported a diagnosis of SLE than were patients for whom SLE had been previously physician-confirmed. The authors warned that fibromyalgia syndrome could intrude into the precision of the diagnosis if only a positive antinuclear antibody test and “soft” SLE criteria were used for diagnosis. If we are unaware of fibromyalgia syndrome, spurious diagnoses may ensue.
BOTTOM LINE
I use the Symptom Intensity Scale as part of routine evaluation in my office. Most patients can complete it with no instruction in 2 minutes or less. I believe it should be used in the clinic to confirm a diagnosis of fibromyalgia syndrome in patients with chronic diffuse pain at rest and to identify comorbid distress in patients with other diseases. This test complements a careful patient history and physical examination, and through its symptom and general health correlations facilitates characterization of our patients’ illnesses in line with the biopsychosocial model.
Since the Symptom Intensity Scale has been shown to be an accurate surrogate measure for general health, depression, disability, and death, fibromyalgia syndrome diagnosed using this instrument implies that this illness is not just centrally mediated pain, but that it carries increased medical risk. It can also be used as a research tool to measure the prevalence of fibromyalgia syndrome in other diseases.
Although the Symptom Intensity Scale is not yet recognized by the ACR as part (or the whole) of the classification criteria for fibromyalgia syndrome, it has already been shown in published studies to be a valid research tool, and it will very likely be the cornerstone of the new criteria.
Goodbye to tender points? Get used to it.
- Wilke WS. The clinical utility of fibromyalgia. J Clin Rheumatol 1999; 5:97–103.
- Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum 2002; 46:845–847.
- Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 2008; 9:417–422.
- Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses 2007; 69:517–525.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977; 196:129–136.
- Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol 2003; 17:547–561.
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38:19–28.
- Lagier R. Nosology versus pathology, two approaches to rheumatic diseases illustrated by Alfred Baring Garrod and Jean-Martin Charcot. Rheumatology (Oxford) 2001; 40:467–471.
- Ruhman W. The earliest book on rheumatism. Br J Rheumatol 1940; 2:140–162.
- Gowers WR. A lecture on lumbago: its lessons and analogues. Br Med J 1904; 1:117–121.
- Hench PK. Nonarticular rheumatism. Arthritis Rheum 1976; 19(suppl):1081–1088.
- Smythe HA. “Fibrositis” as a disorder of pain modulation. Clin Rheum Dis 1979; 5:823–832.
- Smythe HA, Moldofsky H. Two contributions to understanding of the “fibrositis” syndrome. Bull Rheum Dis 1977–1978; 28:928–931.
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched controls. Semin Arthritis Rheum 1981; 11:151–171.
- Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol 1993; 20:710–713.
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33:160–172.
- McVeigh JG, Finch MB, Hurley DA, Basford JR, Sim J, Baxter GD. Tender point count and total myalgic score in fibromyalgia: changes over a 28-day period. Rheumatol Int 2007; 27:1011–1018.
- Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ 1994; 309:696–699.
- Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis 1997; 56:268–271.
- Mease PJ, Arnold LM, Crofford LJ, et al. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum 2008; 59:952–960.
- Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol 2007; 34:914–922.
- Macfarlane GJ, Croft PR, Schollum J, Silman AJ. Widespread pain: is an improved classification possible? J Rheumatol 1996; 23:1628–1632.
- White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol 1999; 26:880–884.
- White KP, Speechly M, Harth M, Osbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol 1999; 26:1577–1585.
- Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatolol 2003; 30:369–378.
- Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis. a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum 2006; 54:169–176.
- Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol 2006; 33:2291–2299.
- Sleath B, Chewning B, De Vellis BM, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum 2008; 59:186–191.
- Middleton GD, McFarlin JE, Lippski PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum 1994; 37:1181–1188.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1013–1019.
- Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemograghic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 2004; 31:695–700.
- Urrows S, Affleck G, Tennen H, Higgins P. Unique clinical and psychological correlates of fibromyalgia tender points and joint tenderness in rheumatoid arthritis. Arthritis Rheum 1994; 37:1513–1520.
- Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol 1997; 24:2145–2148.
- Wolfe F, Petri M, Alarcon GS, et al. Fibromyalgia, systemic lupus erythematosus (SLE) and evaluation of SLE activity. J Rheumatol 2009; 36:.27–33.
A relatively new diagnostic tool, the Symptom Intensity Scale, is an easy, quick way to assess both regional pain and fatigue in a patient. It can be used to establish the diagnosis of fibromyalgia syndrome and measure its severity in daily clinical practice without the need to count tender points. It can also be used to detect fibromyalgia as a comorbidity in other clinical illnesses; by uncovering fibromyalgia, the questionnaire serves as a surrogate measure of depression, anxiety, other serious personality disorders, previous or ongoing abuse, and, when fatigue is the dominant symptom, a consideration of obstructive sleep apnea—all part of the pathoetiology of fibromyalgia in that individual.
This manuscript reviews previous criteria and definitions by which fibromyalgia syndrome was recognized, describes how the new questionnaire was developed, and discusses its implications. It is not meant as a review of the pathogenesis or treatment of fibromyalgia or when to send the patient to the rheumatologist. Each of those topics requires lengthy and complex discussions, which are beyond the scope of this paper.
A COMMON, MULTIFACTORIAL DISEASE
The pathoetiology of fibromyalgia syndrome is rooted in disordered sleep, increased stress, and abnormal neurosensory processing, with secondary endocrine and autonomic dysfunction in those who are genetically predisposed.1–4 Because fibromyalgia is multifactorial, it is best understood from the perspective of an inclusive biopsychosocial model rather than a limited biomedical model.5 Its characteristic signs and symptoms are best understood as emanating from a physiologic state, called central sensitization syndrome, in which the nervous system overresponds to stimuli.1,3 This anomalous state of heightened nervous system response is not confined to the peripheral nervous system, but is also present in the autonomic and central nervous systems.3,4
Fibromyalgia syndrome is common, affecting 0.5% to 5% of the general population,6 and is either the second or third most common diagnosis in a rheumatology practice. Importantly for internists, a diagnosis of fibromyalgia syndrome should be made in 10% to 15% of primary care patients.7 The high prevalence alone demands diagnostic recognition.
KNOWN IN HISTORY AND LITERATURE
Although the designation fibromyalgia syndrome is new, the illness has been with us for as long as we’ve been us. In fact, the word rheumatology may have its origin in fibromyalgia syndrome. Galen (about 180 ad) blamed the symptoms of diffuse pain on the “rheuma,” which has been interpreted as “a great fluxion which races [from the center?] to various parts of the body, and goes from one to another.”8 (Is this the origin of blood-letting as a treatment for diseases?) In 1592, the French physician Guillaume de Baillou introduced the term rheumatism to describe both muscle and joint pain.9
Literature also knows fibromyalgia syndrome. Hans Christian Andersen described a supersensitive princess for whom a pea beneath many mattresses was sufficient to ruin her sleep. In The Fall of the House of Usher, Edgar Allan Poe described Roderick Usher as having an “acute bodily illness and mental disorder that oppressed him.” Usher would wear garments of only soft texture because rough cloth was painful. Light hurt his eyes, forcing him to keep the curtains drawn. Although he had previously played and enjoyed violin music, he could no longer tolerate the sound of the violin. In fact, he suffered such hyperacusis that he could hear his sister moving in her grave many floors below. Other stories by Poe such as Rats in the Wall and The Tell-Tale Heart give more evidence that he was well acquainted with the symptoms of central sensitization syndrome.
HOW THE DEFINITION HAS EVOLVED
To recognize fibromyalgia we need an accurate definition, which has evolved over the years. If we don’t know where we’ve been, it is difficult to understand where we are now or how we got here.
Gowers,10 in 1904, was the first to describe diffuse pain as “fibrositis.” He believed that the pain was due to proliferation or inflammation (or both) of subcutaneous and fibrous tissue, a histopathology that has not been satisfactorily demonstrated to this date. Unfortunately for our purposes, his paper was a descriptive essay that made no attempt at codification. In fact, attempts to clinically define and classify fibromyalgia syndrome have been relatively recent.
Hench11 proposed the first clinical definition in 1976, and it probably did more harm than good. His criteria were two: pain, and no physiologic explanation. The diagnosis was therefore made by ruling out everything else rather than by ruling it in by clinical criteria. Consequently, the diagnosing physician had to investigate the symptom or symptoms by ordering potentially limitless testing, which all had to be normal before the diagnosis could be entertained. I continue to see this phenomenon today as new patients with classic fibromyalgia syndrome arrive carrying reports of normal magnetic imaging of the entire body and serologic testing—a “connective tissue disease workup.”
Counting tender points
Smythe (1979)12 was the first to define and classify fibromyalgia syndrome as a rule-in diagnosis. Smythe’s criteria included tender points in at least 12 of 14 anatomic locations using 4 kg of pressure. In practice, the pressure is approximate—the nail bed blanches in a normotensive examiner with a force of 4 kg. He also described four necessary signs and symptoms: diffuse pain of at least 3 months’ duration, disturbed sleep, skin-roll tenderness at the upper trapezius border, and normal results on laboratory tests. He and Moldofsky13 also found a relationship between disordered slow-wave sleep and the symptoms of fibromyalgia syndrome.
Yunus et al (1981)14 compared signs and symptoms in 50 patients with fibromyalgia syndrome and 50 healthy controls to develop criteria for the disease. Of the resulting criteria, two were mandatory: diffuse pain of at least 3 months’ duration and lack of other obvious causes. The definition also required tenderness in at least 5 of 40 tender points and outlined 10 minor criteria.
Signs and factors that modulate fibromyalgia syndrome and that were derived from these minor criteria are still clinically important today. Factors that aggravate pain include cold or humid weather, fatigue, sedentary state, anxiety, and overactivity. Relieving factors include a hot shower, physical activity, warm dry weather, and massage.
The American College of Rheumatology (1990). Understanding that at any one time approximately 15% of the general population experiences widespread pain,15 a committee of the American College of Rheumatology (ACR) set out to differentiate patients with fibromyalgia syndrome from those with less severe widespread pain. The committee compared signs and symptoms in 293 patients deemed by experts to have fibromyalgia syndrome and 265 control patients matched for age, sex, and concomitant rheumatic disorders. 16
The symptom of widespread pain of at least 3 months’ duration and tenderness in at least 11 of 18 points became the ACR’s diagnostic criteria and provided a sensitivity of 88% and a specificity of 81% compared with the experts’ opinion as the gold standard test.
Low specificity is one of the recognized problems with the ACR criteria: 19% of patients with at least 11 tender points did not have fibromyalgia syndrome. In addition, tender points don’t correlate well with some measures of illness activity, such as the Fibromyalgia Impact Questionnaire.17
Is the tender-point count a good measure?
The best argument for continuing to count tender points as part of the clinical evaluation is that it is a measure of severity. Higher numbers of tender points indicate greater psychological distress and greater severity and frequency of other, closely related fibromyalgia symptoms.18,19 Nearly everyone in the general population has at least a few tender points.16 In fibromyalgia syndrome, the tender-point count is a good status surrogate, a measure of the state of the illness.
But should a state/status measure be used as an illness trait and a criterion for diagnosis? I believe not. Consider, as an analogy, the use of the erythrocyte sedimentation rate in patients with rheumatoid arthritis. An elevated sedimentation rate may indicate increased systemic inflammation, but it is a measure of the status of rheumatoid arthritis, not a trait of this disease. This is why I believe that most rheumatologists would disagree with using some value of the erythrocyte sedimentation rate as a criterion for the diagnosis of rheumatoid arthritis and, by analogy, the tender-point count as a criterion for the diagnosis of fibromyalgia syndrome. Also, the number of tender points, a surrogate for diffuse pain, does not fully capture the essence of the illness, in which accompanying fatigue is often severe and nearly always present.20
A CONTEMPORARY DEFINITION AND ITS VALIDATION
As the concept of fibromyalgia syndrome evolved, a movement away from tender points took hold.21
The Manchester criteria22 used a pain diagram to establish the diagnosis, in which the patient indicated the areas of pain on a simple drawing, obviating the need for tender points. It showed good agreement with the ACR criteria, and in fact identified patients with more severe symptoms.
The London Fibromyalgia Epidemiology Study Screening Questionnaire,23 designed as an epidemiologic tool to estimate the prevalence of the syndrome, was the first test to specifically include both pain and fatigue.
White et al,24 in a very important subsequent study, showed that higher fatigue scores differentiated patients with widespread pain and only a few tender points (7–10) from those with more tender points. This report helped to set the stage for the Symptom Intensity Scale.
What the Symptom Intensity Scale measures
- The Regional Pain Scale score, which is the number of anatomic areas—out of a possible 19—in which the patient feels pain
- A fatigue visual analogue scale score, in which the patient makes a mark somewhere along a 10-cm line to indicate how tired he or she feels. Subsequently, the clinician measures the position of the mark from the left end of the line with a ruler.
How the Symptom Intensity Scale was developed
Wolfe (2003)25 mailed a survey to 12,799 patients who had rheumatoid arthritis, osteoarthritis, or fibromyalgia syndrome. The questionnaire asked respondents if they had pain in 38 articular and nonarticular anatomic regions and to complete a 10-cm fatigue visual analogue scale. He observed that pain in a subset of 19 primarily nonarticular sites differentiated fibromyalgia syndrome from the other two diseases. Calling the number of painful areas of these 19 sites the Regional Pain Scale, he analyzed this measure using Mokken analysis and Rasch analysis to ensure that the questionnaire was statistically valid.
Wolfe also showed that a score of at least 8 points on the Regional Pain Scale, combined with a score of at least 6 cm on the fatigue visual analogue scale, provided the best diagnostic precision consistent with a diagnosis of fibromyalgia syndrome. The combination of these two measures became known as the Survey Criteria.
Katz, Wolfe, and Michaud (2006)26 next compared the diagnostic precision of the Survey Criteria, the ACR criteria, and a physician’s clinical diagnosis. The clinicians made their clinical diagnosis by “considering the long-term patient-clinician experience [including] factors related to pain, tenderness, fatigue, sleep disturbance, comorbidity, and psychosocial variables,” or as I call it, the company fibromyalgia syndrome keeps (Table 2).7,14,16,20 The Survey Criteria (8 points or higher on the Regional Pain Scale plus 6 cm or higher on the fatigue visual analogue scale) showed a roughly 75% concordance among all three definitions in 206 patients with fibromyalgia syndrome. In a cohort with clinically diagnosed fibromyalgia syndrome, a Regional Pain Scale score of 8 or more had a sensitivity of 83.2%, a specificity of 87.6%, and a percent correct of 85.4%. The authors reported that a score of 6 cm or more on the fatigue visual analogue scale “was also at the optimum level” for diagnosing fibromyalgia, but they did not provide more information.
Wolfe and Rasker (2006),27 using these data, devised the Symptom Intensity Scale, the score of which is calculated as the fatigue visual analogue scale score plus half the Regional Pain Scale score, all divided by 2. The scale is therefore a continuous variable rather than a categorical one, and scores can range from 0 to 9.75.
The authors gave the questionnaire to 25,417 patients who had various rheumatic diseases and found that a score of 5.75 or higher differentiated fibromyalgia syndrome from other rheumatic diseases, identifying 95% of patients who would satisfy the Survey Criteria for fibromyalgia.
In addition, they found a linear relationship between the Symptom Intensity Scale score and key symptoms of fibromyalgia syndrome. Of even greater importance, the Symptom Intensity Scale score showed closer association with general health than scores on the Health Assessment Questionnaire, a 27-question patient activity scale, the Arthritis Impact Measurement Scale, or the Short Form-36. It also proved to correlate with mood, probability of having diabetes, need for hospitalization, history of or relative time to myocardial infarction, number of comorbidities, rate of disability, and risk of early death (relative risk 1.12, 95% confidence interval 1.10–1.14). The Symptom Intensity Scale is therefore a diagnostic tool as well as a simple measure of general health among all rheumatic disease patients.
IMPLICATIONS OF THE SYMPTOM INTENSITY SCALE
Three arguments provide a strong rationale for using the Symptom Intensity Scale in the outpatient clinic to investigate the biopsychosocial aspects of illness in our patients:
- It is a simple way to measure overall health
- It can uncover comorbid depression
- It can detect fibromyalgia syndrome in patients who have other diseases.
It measures overall health
Unlike instruments intended for a particular disease such as the Disease Activity Score, which measures disease severity only in rheumatoid arthritis, the Symptom Intensity Scale score can also be used as a measure of global health (or disease severity), and the Survey Criteria (8 or more on the Regional Pain Score and 6 or more on the fatigue visual analogue scale) can be used to establish diagnosis. In fact, instruments like the Disease Activity Score essentially ignore biopsychosocial issues that are captured by the Symptom Intensity Scale.27
By detecting fibromyalgia syndrome in our patients, we identify people with symptoms of pain and distress that do not easily fit the prevalent model of organic disease. Measures like the Disease Activity Score are specifically suited as end points in controlled efficacy trials, but if these are the only measures physicians use to estimate a patient’s health in the clinic, they do so at their own and their patient’s peril.27
Because the continuous Symptom Intensity Scale score strongly correlates with patient-perceived pain, depression, and general health, it is an ideal instrument for outpatient evaluation. It complements a complete patient history and physical examination by measuring biopsychosocial factors.
It uncovers comorbid depression
Rheumatologists do a woeful job of recognizing and diagnosing depression in patients with rheumatoid arthritis. Sleath et al28 found that patients with rheumatoid arthritis who were diagnosed with depression in the office were always the ones who initiated the discussion of that diagnosis. Their doctors did not elicit it.
Middleton et al29 found that patients with concomitant fibromyalgia syndrome and systemic lupus erythematosus (SLE) had higher depression scores than did SLE patients without fibromyalgia syndrome. Moussavi et al,30 writing for the World Health Organization about the findings of a 60-country survey, concluded: “The comorbid state of depression incrementally worsens health compared with depression alone, with any of the chronic diseases alone, and with any combination of chronic diseases without depression.”30
Worse health implies earlier death. Ang et al31 reported that a higher average 4-year depression scale score conferred a hazard ratio of 1.35 (P < .001) for earlier death among 1,290 rheumatoid arthritis patients followed for 12 years.
By using a test like the Symptom Intensity Scale to detect fibromyalgia syndrome alone or to detect it in patients with other diseases, we implicitly recognize the high likelihood of simultaneous depression. Recognition and treatment of depression will improve overall health.
It can detect fibromyalgia syndrome in patients with other diseases
Not surprisingly, distress-related fibromyalgia syndrome is more common in patients with chronic rheumatic or arthritic diseases, with a frequency ranging from 5% in osteoarthritis to 47% in Sjögren syndrome.1 When present, fibromyalgia syndrome changes the features of the other disease.
Wolfe and Michaud32 used the Survey Criteria to evaluate 11,866 rheumatoid arthritis patients and found that 17.1% of them also had fibromyalgia syndrome, and those that did had higher levels of pain, greater global severity, higher scores on the Health Assessment Questionnaire and Short Form-36 mental component, and more disability than those without fibromyalgia syndrome.
Urrows et al33 found that the mean tender-joint count correlated with the mean tenderpoint count in 67 patients with rheumatoid arthritis followed for 75 days. Comorbid fibromyalgia syndrome rendered joints more tender, so that an examiner using the tender-joint count as a major indicator of disease severity might overestimate severity and excessively treat a rheumatoid arthritis patient with unrecognized concurrent fibromyalgia syndrome. Because comorbid fibromyalgia syndrome can inflate Health Assessment Questionnaire scores and subjective pain scale scores in rheumatoid arthritis, more appropriate investigation and management decisions should follow recognition.
Concurrent fibromyalgia syndrome can also be troublesome in SLE. Patients with fibromyalgia syndrome had greater disability than patients without fibromyalgia syndrome despite having no worse SLE damage scores.29 Comorbid fibromyalgia syndrome in SLE has also been shown to diminish quality of life as measured by the Short Form-36.34
Fibromyalgia syndrome also has the potential to confound the diagnosis of concomitant diseases. Wolfe et al35 found that 22.1% of 458 patients with SLE also had fibromyalgia syndrome using the Symptom Intensity Scale criteria. At the time of referral to a rheumatologist, patients who met the criteria for fibromyalgia syndrome were more likely to have self-reported a diagnosis of SLE than were patients for whom SLE had been previously physician-confirmed. The authors warned that fibromyalgia syndrome could intrude into the precision of the diagnosis if only a positive antinuclear antibody test and “soft” SLE criteria were used for diagnosis. If we are unaware of fibromyalgia syndrome, spurious diagnoses may ensue.
BOTTOM LINE
I use the Symptom Intensity Scale as part of routine evaluation in my office. Most patients can complete it with no instruction in 2 minutes or less. I believe it should be used in the clinic to confirm a diagnosis of fibromyalgia syndrome in patients with chronic diffuse pain at rest and to identify comorbid distress in patients with other diseases. This test complements a careful patient history and physical examination, and through its symptom and general health correlations facilitates characterization of our patients’ illnesses in line with the biopsychosocial model.
Since the Symptom Intensity Scale has been shown to be an accurate surrogate measure for general health, depression, disability, and death, fibromyalgia syndrome diagnosed using this instrument implies that this illness is not just centrally mediated pain, but that it carries increased medical risk. It can also be used as a research tool to measure the prevalence of fibromyalgia syndrome in other diseases.
Although the Symptom Intensity Scale is not yet recognized by the ACR as part (or the whole) of the classification criteria for fibromyalgia syndrome, it has already been shown in published studies to be a valid research tool, and it will very likely be the cornerstone of the new criteria.
Goodbye to tender points? Get used to it.
A relatively new diagnostic tool, the Symptom Intensity Scale, is an easy, quick way to assess both regional pain and fatigue in a patient. It can be used to establish the diagnosis of fibromyalgia syndrome and measure its severity in daily clinical practice without the need to count tender points. It can also be used to detect fibromyalgia as a comorbidity in other clinical illnesses; by uncovering fibromyalgia, the questionnaire serves as a surrogate measure of depression, anxiety, other serious personality disorders, previous or ongoing abuse, and, when fatigue is the dominant symptom, a consideration of obstructive sleep apnea—all part of the pathoetiology of fibromyalgia in that individual.
This manuscript reviews previous criteria and definitions by which fibromyalgia syndrome was recognized, describes how the new questionnaire was developed, and discusses its implications. It is not meant as a review of the pathogenesis or treatment of fibromyalgia or when to send the patient to the rheumatologist. Each of those topics requires lengthy and complex discussions, which are beyond the scope of this paper.
A COMMON, MULTIFACTORIAL DISEASE
The pathoetiology of fibromyalgia syndrome is rooted in disordered sleep, increased stress, and abnormal neurosensory processing, with secondary endocrine and autonomic dysfunction in those who are genetically predisposed.1–4 Because fibromyalgia is multifactorial, it is best understood from the perspective of an inclusive biopsychosocial model rather than a limited biomedical model.5 Its characteristic signs and symptoms are best understood as emanating from a physiologic state, called central sensitization syndrome, in which the nervous system overresponds to stimuli.1,3 This anomalous state of heightened nervous system response is not confined to the peripheral nervous system, but is also present in the autonomic and central nervous systems.3,4
Fibromyalgia syndrome is common, affecting 0.5% to 5% of the general population,6 and is either the second or third most common diagnosis in a rheumatology practice. Importantly for internists, a diagnosis of fibromyalgia syndrome should be made in 10% to 15% of primary care patients.7 The high prevalence alone demands diagnostic recognition.
KNOWN IN HISTORY AND LITERATURE
Although the designation fibromyalgia syndrome is new, the illness has been with us for as long as we’ve been us. In fact, the word rheumatology may have its origin in fibromyalgia syndrome. Galen (about 180 ad) blamed the symptoms of diffuse pain on the “rheuma,” which has been interpreted as “a great fluxion which races [from the center?] to various parts of the body, and goes from one to another.”8 (Is this the origin of blood-letting as a treatment for diseases?) In 1592, the French physician Guillaume de Baillou introduced the term rheumatism to describe both muscle and joint pain.9
Literature also knows fibromyalgia syndrome. Hans Christian Andersen described a supersensitive princess for whom a pea beneath many mattresses was sufficient to ruin her sleep. In The Fall of the House of Usher, Edgar Allan Poe described Roderick Usher as having an “acute bodily illness and mental disorder that oppressed him.” Usher would wear garments of only soft texture because rough cloth was painful. Light hurt his eyes, forcing him to keep the curtains drawn. Although he had previously played and enjoyed violin music, he could no longer tolerate the sound of the violin. In fact, he suffered such hyperacusis that he could hear his sister moving in her grave many floors below. Other stories by Poe such as Rats in the Wall and The Tell-Tale Heart give more evidence that he was well acquainted with the symptoms of central sensitization syndrome.
HOW THE DEFINITION HAS EVOLVED
To recognize fibromyalgia we need an accurate definition, which has evolved over the years. If we don’t know where we’ve been, it is difficult to understand where we are now or how we got here.
Gowers,10 in 1904, was the first to describe diffuse pain as “fibrositis.” He believed that the pain was due to proliferation or inflammation (or both) of subcutaneous and fibrous tissue, a histopathology that has not been satisfactorily demonstrated to this date. Unfortunately for our purposes, his paper was a descriptive essay that made no attempt at codification. In fact, attempts to clinically define and classify fibromyalgia syndrome have been relatively recent.
Hench11 proposed the first clinical definition in 1976, and it probably did more harm than good. His criteria were two: pain, and no physiologic explanation. The diagnosis was therefore made by ruling out everything else rather than by ruling it in by clinical criteria. Consequently, the diagnosing physician had to investigate the symptom or symptoms by ordering potentially limitless testing, which all had to be normal before the diagnosis could be entertained. I continue to see this phenomenon today as new patients with classic fibromyalgia syndrome arrive carrying reports of normal magnetic imaging of the entire body and serologic testing—a “connective tissue disease workup.”
Counting tender points
Smythe (1979)12 was the first to define and classify fibromyalgia syndrome as a rule-in diagnosis. Smythe’s criteria included tender points in at least 12 of 14 anatomic locations using 4 kg of pressure. In practice, the pressure is approximate—the nail bed blanches in a normotensive examiner with a force of 4 kg. He also described four necessary signs and symptoms: diffuse pain of at least 3 months’ duration, disturbed sleep, skin-roll tenderness at the upper trapezius border, and normal results on laboratory tests. He and Moldofsky13 also found a relationship between disordered slow-wave sleep and the symptoms of fibromyalgia syndrome.
Yunus et al (1981)14 compared signs and symptoms in 50 patients with fibromyalgia syndrome and 50 healthy controls to develop criteria for the disease. Of the resulting criteria, two were mandatory: diffuse pain of at least 3 months’ duration and lack of other obvious causes. The definition also required tenderness in at least 5 of 40 tender points and outlined 10 minor criteria.
Signs and factors that modulate fibromyalgia syndrome and that were derived from these minor criteria are still clinically important today. Factors that aggravate pain include cold or humid weather, fatigue, sedentary state, anxiety, and overactivity. Relieving factors include a hot shower, physical activity, warm dry weather, and massage.
The American College of Rheumatology (1990). Understanding that at any one time approximately 15% of the general population experiences widespread pain,15 a committee of the American College of Rheumatology (ACR) set out to differentiate patients with fibromyalgia syndrome from those with less severe widespread pain. The committee compared signs and symptoms in 293 patients deemed by experts to have fibromyalgia syndrome and 265 control patients matched for age, sex, and concomitant rheumatic disorders. 16
The symptom of widespread pain of at least 3 months’ duration and tenderness in at least 11 of 18 points became the ACR’s diagnostic criteria and provided a sensitivity of 88% and a specificity of 81% compared with the experts’ opinion as the gold standard test.
Low specificity is one of the recognized problems with the ACR criteria: 19% of patients with at least 11 tender points did not have fibromyalgia syndrome. In addition, tender points don’t correlate well with some measures of illness activity, such as the Fibromyalgia Impact Questionnaire.17
Is the tender-point count a good measure?
The best argument for continuing to count tender points as part of the clinical evaluation is that it is a measure of severity. Higher numbers of tender points indicate greater psychological distress and greater severity and frequency of other, closely related fibromyalgia symptoms.18,19 Nearly everyone in the general population has at least a few tender points.16 In fibromyalgia syndrome, the tender-point count is a good status surrogate, a measure of the state of the illness.
But should a state/status measure be used as an illness trait and a criterion for diagnosis? I believe not. Consider, as an analogy, the use of the erythrocyte sedimentation rate in patients with rheumatoid arthritis. An elevated sedimentation rate may indicate increased systemic inflammation, but it is a measure of the status of rheumatoid arthritis, not a trait of this disease. This is why I believe that most rheumatologists would disagree with using some value of the erythrocyte sedimentation rate as a criterion for the diagnosis of rheumatoid arthritis and, by analogy, the tender-point count as a criterion for the diagnosis of fibromyalgia syndrome. Also, the number of tender points, a surrogate for diffuse pain, does not fully capture the essence of the illness, in which accompanying fatigue is often severe and nearly always present.20
A CONTEMPORARY DEFINITION AND ITS VALIDATION
As the concept of fibromyalgia syndrome evolved, a movement away from tender points took hold.21
The Manchester criteria22 used a pain diagram to establish the diagnosis, in which the patient indicated the areas of pain on a simple drawing, obviating the need for tender points. It showed good agreement with the ACR criteria, and in fact identified patients with more severe symptoms.
The London Fibromyalgia Epidemiology Study Screening Questionnaire,23 designed as an epidemiologic tool to estimate the prevalence of the syndrome, was the first test to specifically include both pain and fatigue.
White et al,24 in a very important subsequent study, showed that higher fatigue scores differentiated patients with widespread pain and only a few tender points (7–10) from those with more tender points. This report helped to set the stage for the Symptom Intensity Scale.
What the Symptom Intensity Scale measures
- The Regional Pain Scale score, which is the number of anatomic areas—out of a possible 19—in which the patient feels pain
- A fatigue visual analogue scale score, in which the patient makes a mark somewhere along a 10-cm line to indicate how tired he or she feels. Subsequently, the clinician measures the position of the mark from the left end of the line with a ruler.
How the Symptom Intensity Scale was developed
Wolfe (2003)25 mailed a survey to 12,799 patients who had rheumatoid arthritis, osteoarthritis, or fibromyalgia syndrome. The questionnaire asked respondents if they had pain in 38 articular and nonarticular anatomic regions and to complete a 10-cm fatigue visual analogue scale. He observed that pain in a subset of 19 primarily nonarticular sites differentiated fibromyalgia syndrome from the other two diseases. Calling the number of painful areas of these 19 sites the Regional Pain Scale, he analyzed this measure using Mokken analysis and Rasch analysis to ensure that the questionnaire was statistically valid.
Wolfe also showed that a score of at least 8 points on the Regional Pain Scale, combined with a score of at least 6 cm on the fatigue visual analogue scale, provided the best diagnostic precision consistent with a diagnosis of fibromyalgia syndrome. The combination of these two measures became known as the Survey Criteria.
Katz, Wolfe, and Michaud (2006)26 next compared the diagnostic precision of the Survey Criteria, the ACR criteria, and a physician’s clinical diagnosis. The clinicians made their clinical diagnosis by “considering the long-term patient-clinician experience [including] factors related to pain, tenderness, fatigue, sleep disturbance, comorbidity, and psychosocial variables,” or as I call it, the company fibromyalgia syndrome keeps (Table 2).7,14,16,20 The Survey Criteria (8 points or higher on the Regional Pain Scale plus 6 cm or higher on the fatigue visual analogue scale) showed a roughly 75% concordance among all three definitions in 206 patients with fibromyalgia syndrome. In a cohort with clinically diagnosed fibromyalgia syndrome, a Regional Pain Scale score of 8 or more had a sensitivity of 83.2%, a specificity of 87.6%, and a percent correct of 85.4%. The authors reported that a score of 6 cm or more on the fatigue visual analogue scale “was also at the optimum level” for diagnosing fibromyalgia, but they did not provide more information.
Wolfe and Rasker (2006),27 using these data, devised the Symptom Intensity Scale, the score of which is calculated as the fatigue visual analogue scale score plus half the Regional Pain Scale score, all divided by 2. The scale is therefore a continuous variable rather than a categorical one, and scores can range from 0 to 9.75.
The authors gave the questionnaire to 25,417 patients who had various rheumatic diseases and found that a score of 5.75 or higher differentiated fibromyalgia syndrome from other rheumatic diseases, identifying 95% of patients who would satisfy the Survey Criteria for fibromyalgia.
In addition, they found a linear relationship between the Symptom Intensity Scale score and key symptoms of fibromyalgia syndrome. Of even greater importance, the Symptom Intensity Scale score showed closer association with general health than scores on the Health Assessment Questionnaire, a 27-question patient activity scale, the Arthritis Impact Measurement Scale, or the Short Form-36. It also proved to correlate with mood, probability of having diabetes, need for hospitalization, history of or relative time to myocardial infarction, number of comorbidities, rate of disability, and risk of early death (relative risk 1.12, 95% confidence interval 1.10–1.14). The Symptom Intensity Scale is therefore a diagnostic tool as well as a simple measure of general health among all rheumatic disease patients.
IMPLICATIONS OF THE SYMPTOM INTENSITY SCALE
Three arguments provide a strong rationale for using the Symptom Intensity Scale in the outpatient clinic to investigate the biopsychosocial aspects of illness in our patients:
- It is a simple way to measure overall health
- It can uncover comorbid depression
- It can detect fibromyalgia syndrome in patients who have other diseases.
It measures overall health
Unlike instruments intended for a particular disease such as the Disease Activity Score, which measures disease severity only in rheumatoid arthritis, the Symptom Intensity Scale score can also be used as a measure of global health (or disease severity), and the Survey Criteria (8 or more on the Regional Pain Score and 6 or more on the fatigue visual analogue scale) can be used to establish diagnosis. In fact, instruments like the Disease Activity Score essentially ignore biopsychosocial issues that are captured by the Symptom Intensity Scale.27
By detecting fibromyalgia syndrome in our patients, we identify people with symptoms of pain and distress that do not easily fit the prevalent model of organic disease. Measures like the Disease Activity Score are specifically suited as end points in controlled efficacy trials, but if these are the only measures physicians use to estimate a patient’s health in the clinic, they do so at their own and their patient’s peril.27
Because the continuous Symptom Intensity Scale score strongly correlates with patient-perceived pain, depression, and general health, it is an ideal instrument for outpatient evaluation. It complements a complete patient history and physical examination by measuring biopsychosocial factors.
It uncovers comorbid depression
Rheumatologists do a woeful job of recognizing and diagnosing depression in patients with rheumatoid arthritis. Sleath et al28 found that patients with rheumatoid arthritis who were diagnosed with depression in the office were always the ones who initiated the discussion of that diagnosis. Their doctors did not elicit it.
Middleton et al29 found that patients with concomitant fibromyalgia syndrome and systemic lupus erythematosus (SLE) had higher depression scores than did SLE patients without fibromyalgia syndrome. Moussavi et al,30 writing for the World Health Organization about the findings of a 60-country survey, concluded: “The comorbid state of depression incrementally worsens health compared with depression alone, with any of the chronic diseases alone, and with any combination of chronic diseases without depression.”30
Worse health implies earlier death. Ang et al31 reported that a higher average 4-year depression scale score conferred a hazard ratio of 1.35 (P < .001) for earlier death among 1,290 rheumatoid arthritis patients followed for 12 years.
By using a test like the Symptom Intensity Scale to detect fibromyalgia syndrome alone or to detect it in patients with other diseases, we implicitly recognize the high likelihood of simultaneous depression. Recognition and treatment of depression will improve overall health.
It can detect fibromyalgia syndrome in patients with other diseases
Not surprisingly, distress-related fibromyalgia syndrome is more common in patients with chronic rheumatic or arthritic diseases, with a frequency ranging from 5% in osteoarthritis to 47% in Sjögren syndrome.1 When present, fibromyalgia syndrome changes the features of the other disease.
Wolfe and Michaud32 used the Survey Criteria to evaluate 11,866 rheumatoid arthritis patients and found that 17.1% of them also had fibromyalgia syndrome, and those that did had higher levels of pain, greater global severity, higher scores on the Health Assessment Questionnaire and Short Form-36 mental component, and more disability than those without fibromyalgia syndrome.
Urrows et al33 found that the mean tender-joint count correlated with the mean tenderpoint count in 67 patients with rheumatoid arthritis followed for 75 days. Comorbid fibromyalgia syndrome rendered joints more tender, so that an examiner using the tender-joint count as a major indicator of disease severity might overestimate severity and excessively treat a rheumatoid arthritis patient with unrecognized concurrent fibromyalgia syndrome. Because comorbid fibromyalgia syndrome can inflate Health Assessment Questionnaire scores and subjective pain scale scores in rheumatoid arthritis, more appropriate investigation and management decisions should follow recognition.
Concurrent fibromyalgia syndrome can also be troublesome in SLE. Patients with fibromyalgia syndrome had greater disability than patients without fibromyalgia syndrome despite having no worse SLE damage scores.29 Comorbid fibromyalgia syndrome in SLE has also been shown to diminish quality of life as measured by the Short Form-36.34
Fibromyalgia syndrome also has the potential to confound the diagnosis of concomitant diseases. Wolfe et al35 found that 22.1% of 458 patients with SLE also had fibromyalgia syndrome using the Symptom Intensity Scale criteria. At the time of referral to a rheumatologist, patients who met the criteria for fibromyalgia syndrome were more likely to have self-reported a diagnosis of SLE than were patients for whom SLE had been previously physician-confirmed. The authors warned that fibromyalgia syndrome could intrude into the precision of the diagnosis if only a positive antinuclear antibody test and “soft” SLE criteria were used for diagnosis. If we are unaware of fibromyalgia syndrome, spurious diagnoses may ensue.
BOTTOM LINE
I use the Symptom Intensity Scale as part of routine evaluation in my office. Most patients can complete it with no instruction in 2 minutes or less. I believe it should be used in the clinic to confirm a diagnosis of fibromyalgia syndrome in patients with chronic diffuse pain at rest and to identify comorbid distress in patients with other diseases. This test complements a careful patient history and physical examination, and through its symptom and general health correlations facilitates characterization of our patients’ illnesses in line with the biopsychosocial model.
Since the Symptom Intensity Scale has been shown to be an accurate surrogate measure for general health, depression, disability, and death, fibromyalgia syndrome diagnosed using this instrument implies that this illness is not just centrally mediated pain, but that it carries increased medical risk. It can also be used as a research tool to measure the prevalence of fibromyalgia syndrome in other diseases.
Although the Symptom Intensity Scale is not yet recognized by the ACR as part (or the whole) of the classification criteria for fibromyalgia syndrome, it has already been shown in published studies to be a valid research tool, and it will very likely be the cornerstone of the new criteria.
Goodbye to tender points? Get used to it.
- Wilke WS. The clinical utility of fibromyalgia. J Clin Rheumatol 1999; 5:97–103.
- Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum 2002; 46:845–847.
- Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 2008; 9:417–422.
- Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses 2007; 69:517–525.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977; 196:129–136.
- Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol 2003; 17:547–561.
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38:19–28.
- Lagier R. Nosology versus pathology, two approaches to rheumatic diseases illustrated by Alfred Baring Garrod and Jean-Martin Charcot. Rheumatology (Oxford) 2001; 40:467–471.
- Ruhman W. The earliest book on rheumatism. Br J Rheumatol 1940; 2:140–162.
- Gowers WR. A lecture on lumbago: its lessons and analogues. Br Med J 1904; 1:117–121.
- Hench PK. Nonarticular rheumatism. Arthritis Rheum 1976; 19(suppl):1081–1088.
- Smythe HA. “Fibrositis” as a disorder of pain modulation. Clin Rheum Dis 1979; 5:823–832.
- Smythe HA, Moldofsky H. Two contributions to understanding of the “fibrositis” syndrome. Bull Rheum Dis 1977–1978; 28:928–931.
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched controls. Semin Arthritis Rheum 1981; 11:151–171.
- Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol 1993; 20:710–713.
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33:160–172.
- McVeigh JG, Finch MB, Hurley DA, Basford JR, Sim J, Baxter GD. Tender point count and total myalgic score in fibromyalgia: changes over a 28-day period. Rheumatol Int 2007; 27:1011–1018.
- Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ 1994; 309:696–699.
- Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis 1997; 56:268–271.
- Mease PJ, Arnold LM, Crofford LJ, et al. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum 2008; 59:952–960.
- Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol 2007; 34:914–922.
- Macfarlane GJ, Croft PR, Schollum J, Silman AJ. Widespread pain: is an improved classification possible? J Rheumatol 1996; 23:1628–1632.
- White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol 1999; 26:880–884.
- White KP, Speechly M, Harth M, Osbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol 1999; 26:1577–1585.
- Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatolol 2003; 30:369–378.
- Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis. a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum 2006; 54:169–176.
- Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol 2006; 33:2291–2299.
- Sleath B, Chewning B, De Vellis BM, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum 2008; 59:186–191.
- Middleton GD, McFarlin JE, Lippski PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum 1994; 37:1181–1188.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1013–1019.
- Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemograghic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 2004; 31:695–700.
- Urrows S, Affleck G, Tennen H, Higgins P. Unique clinical and psychological correlates of fibromyalgia tender points and joint tenderness in rheumatoid arthritis. Arthritis Rheum 1994; 37:1513–1520.
- Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol 1997; 24:2145–2148.
- Wolfe F, Petri M, Alarcon GS, et al. Fibromyalgia, systemic lupus erythematosus (SLE) and evaluation of SLE activity. J Rheumatol 2009; 36:.27–33.
- Wilke WS. The clinical utility of fibromyalgia. J Clin Rheumatol 1999; 5:97–103.
- Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum 2002; 46:845–847.
- Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 2008; 9:417–422.
- Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses 2007; 69:517–525.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977; 196:129–136.
- Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol 2003; 17:547–561.
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38:19–28.
- Lagier R. Nosology versus pathology, two approaches to rheumatic diseases illustrated by Alfred Baring Garrod and Jean-Martin Charcot. Rheumatology (Oxford) 2001; 40:467–471.
- Ruhman W. The earliest book on rheumatism. Br J Rheumatol 1940; 2:140–162.
- Gowers WR. A lecture on lumbago: its lessons and analogues. Br Med J 1904; 1:117–121.
- Hench PK. Nonarticular rheumatism. Arthritis Rheum 1976; 19(suppl):1081–1088.
- Smythe HA. “Fibrositis” as a disorder of pain modulation. Clin Rheum Dis 1979; 5:823–832.
- Smythe HA, Moldofsky H. Two contributions to understanding of the “fibrositis” syndrome. Bull Rheum Dis 1977–1978; 28:928–931.
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched controls. Semin Arthritis Rheum 1981; 11:151–171.
- Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol 1993; 20:710–713.
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33:160–172.
- McVeigh JG, Finch MB, Hurley DA, Basford JR, Sim J, Baxter GD. Tender point count and total myalgic score in fibromyalgia: changes over a 28-day period. Rheumatol Int 2007; 27:1011–1018.
- Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ 1994; 309:696–699.
- Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis 1997; 56:268–271.
- Mease PJ, Arnold LM, Crofford LJ, et al. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum 2008; 59:952–960.
- Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol 2007; 34:914–922.
- Macfarlane GJ, Croft PR, Schollum J, Silman AJ. Widespread pain: is an improved classification possible? J Rheumatol 1996; 23:1628–1632.
- White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol 1999; 26:880–884.
- White KP, Speechly M, Harth M, Osbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol 1999; 26:1577–1585.
- Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatolol 2003; 30:369–378.
- Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis. a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum 2006; 54:169–176.
- Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol 2006; 33:2291–2299.
- Sleath B, Chewning B, De Vellis BM, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum 2008; 59:186–191.
- Middleton GD, McFarlin JE, Lippski PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum 1994; 37:1181–1188.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1013–1019.
- Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemograghic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 2004; 31:695–700.
- Urrows S, Affleck G, Tennen H, Higgins P. Unique clinical and psychological correlates of fibromyalgia tender points and joint tenderness in rheumatoid arthritis. Arthritis Rheum 1994; 37:1513–1520.
- Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol 1997; 24:2145–2148.
- Wolfe F, Petri M, Alarcon GS, et al. Fibromyalgia, systemic lupus erythematosus (SLE) and evaluation of SLE activity. J Rheumatol 2009; 36:.27–33.
KEY POINTS
- The Symptom Intensity Scale questionnaire consists of two parts: a list of 19 anatomic areas in which the patient is asked if he or she feels pain (the total number of yes answers being the Regional Pain Scale score), and a visual analogue scale for fatigue.
- According to the Survey Criteria, a diagnosis of fibromyalgia can be entertained if the Regional Pain Scale score is 8 points or higher and the fatigue visual analogue scale score is 6 cm or higher.
- The number of tender points, a surrogate for diffuse pain, does not fully capture the essence of fibromyalgia syndrome, in which accompanying fatigue is often severe and nearly always present.
- The Symptom Intensity Scale is an accurate surrogate measure for general health, depression, disability, and death. Fibromyalgia syndrome diagnosed with this instrument implies that this illness carries increased medical risk.
Problems with Using Women's Cancer Screening Rates to Measure Performance
Grand Rounds: Boy, 10, With Knee Pain
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.
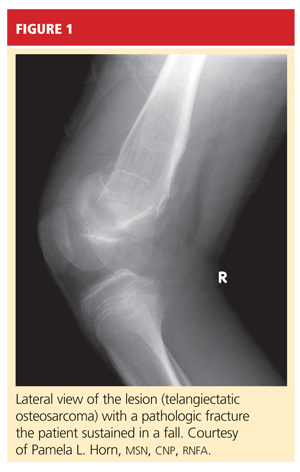
His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).
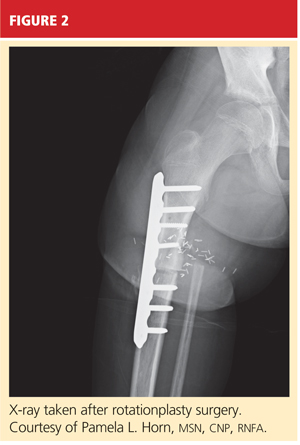
The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13
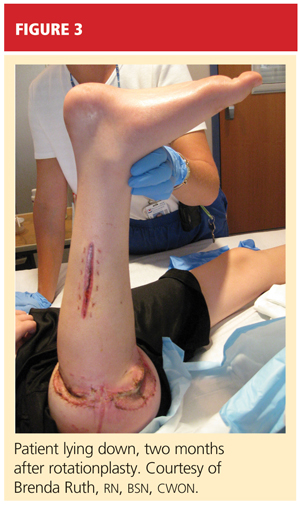
The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.
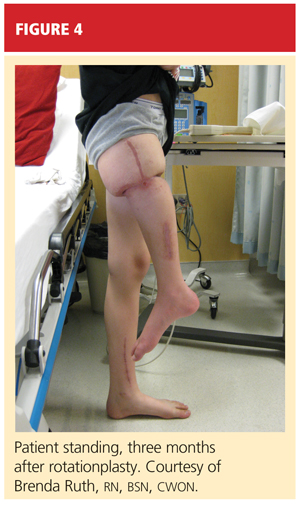
At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.

His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).

The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13

The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.

At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.

His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).

The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13

The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.

At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.