User login
Recurrent Bacterial Meningitis
Recurrent bacterial meningitis (RBM), particularly when caused by Streptococcus pneumoniae, warrants an aggressive and thorough evaluation to exclude transdural communication. We present an unusual case of RBM as a late manifestation of a traumatic head injury sustained 10 years prior and describe presentation, etiology, diagnosis, and treatment options for RBM.
Case Report
A middle‐aged woman with type 2 diabetes mellitus, hypertension, and a prior history of S. pneumoniae meningitis 1 year earlier, presented to an outside hospital with complaints of fever, headache, and change in mental status. Materials for basic laboratory tests and blood cultures were drawn in the Emergency Department; these showed diabetic ketoacidosis. Computed tomography (CT) scan of the head was negative and a lumbar puncture (LP) was attempted, but was unsuccessful. The patient was started on intravenous insulin drip, vancomycin, and ceftriaxone and was transported to our facility via Life‐Flight. She also developed acute respiratory failure requiring mechanical ventilation.
After arrival, the patient had a normal repeat CT scan of her head and a successful LP. Cerebrospinal fluid (CSF) revealed 9064 white blood cells (WBCs)/mm3 with 77% neutrophils and 9% lymphocytes, protein concentration of 275 mg/dL, and glucose of 93 mg/dL. CSF culture and Gram stain were negative, while 1 blood culture drawn at the outside hospital grew penicillin‐resistant S. pneumoniae (MIC 2 g/mL). WBC count was 9660/mm3 with 45% band forms. Bacterial meningitis was confirmed and the patient was continued on intravenous antibiotics and insulin drip. Additional laboratory studies revealed normal complement levels and a negative human immunodeficiency virus (HIV) 1 and HIV 2 antibody screen. The patient was extubated in 48 hours. and was treated with a total of 2 weeks of ceftriaxone and vancomycin for penicillin‐resistant S. pneumoniae meningitis.
The patient had an uneventful full recovery and was discharged from the hospital with neurosurgery follow‐up. The neurosurgeon ordered a CT scan of the facial bones, which revealed an irregular calcification in the right frontal sinus adjacent to the cribriform plate and thinning of the posterior wall of the sinus. Upon requestioning at a subsequent neurosurgical appointment, the patient recalled being an unrestrained passenger and striking her head against the windshield in a motor vehicle accident (MVA) approximately 10 years ago. Ever since the MVA, she noticed intermittent postnasal discharge while recumbent. However, she never sought a medical opinion and denied complaints of anterior rhinorrhea.
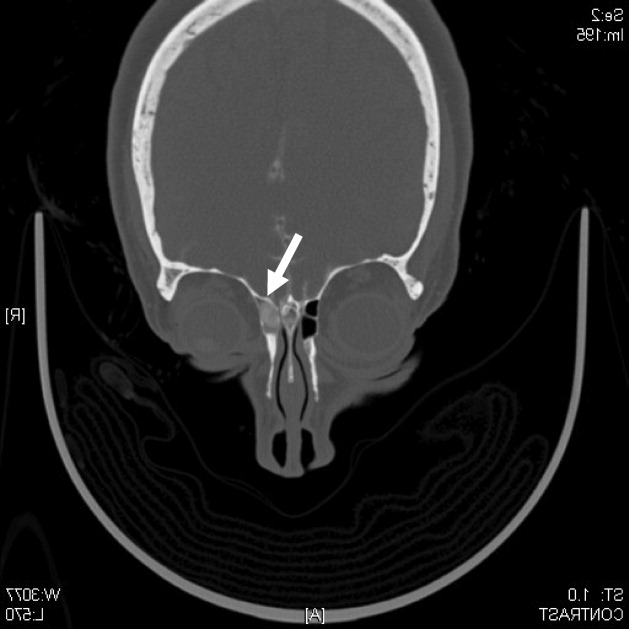
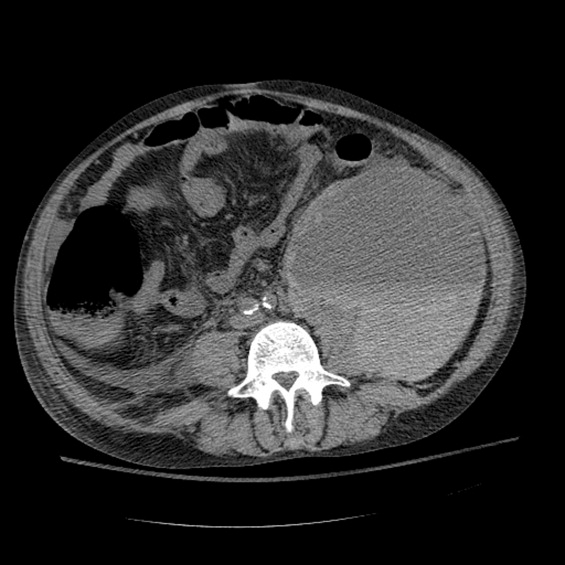
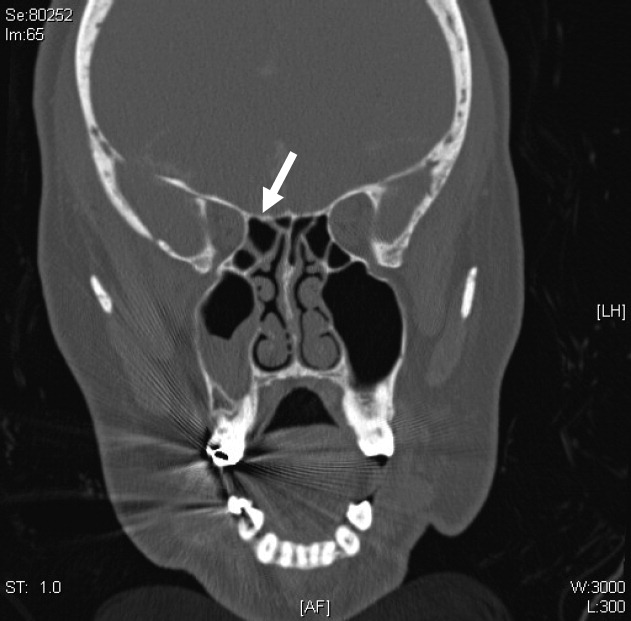
A CT cisternography confirmed the presence of CSF leakage with contrast accumulation via a defect in the right paramedian cribriform plate. Contrast opacification was seen in the fovea ethmoidalis extending into the right frontal sinus (Figure 1). The patient subsequently underwent transnasal endoscopic CSF leak repair (Figure 2). The postoperative cisternogram did not reveal the transdural communication. However a follow‐up cisternogram performed 3 months later demonstrated a recurrent CSF leak.

The patient was rehospitalized with grand‐mal seizures and a third episode of S. pneumoniae meningitis, this time with a penicillin‐sensitive strain. She was treated with a 2‐week course of ceftriaxone and also received heptavalent pneumococcal vaccine to supplement the 23‐valent pneumococcal vaccine. Two weeks after the hospital discharge, the patient underwent successful bifrontal transcranial repair. Currently, she has been disease‐free for 3 months and is followed closely by neurosurgery as an outpatient.
Discussion
After immune deficiency is ruled out, it is essential to evaluate for transdural communication between the subarachnoid space and the base of the skull resulting in a CSF leak as a cause for RBM. Meningitis secondary to a CSF leak is most commonly caused by S. pneumoniae, followed by Neisseria meningitidis and Haemophilus influenzae.1 Complement and immunoglobulin subclass defects may also predispose to RBM.2, 3
A recent case series by Adriani et al.4 suggested that as many as 77% of patients with RBM have an identifiable risk factor such as a remote head injury or CSF leakage. CSF rhinorrhea is most often secondary to trauma, occurring in approximately 1% to 3% of all blunt head injuries.2 Accidental falls, MVAs, altercations, and gunshot wounds are also commonly responsible.3 Nontraumatic CSF leaks are very rare but may be secondary to spontaneous, congenital, or iatrogenic etiologies.1, 3 Spontaneous CSF leaks could also occur due to violent sneezing or coughing.1, 3 Congenital defects include weakened preformed pathways, failure of germ layer closures, and bone imperfections.1, 3 Infrequently, CSF leak can be a complication of intracranial, otologic, nasal, or paranasal sinus surgeries.1, 3 Other rarer etiologies include intracranial tumors and hydrocephalus.1, 3
Bacterial meningitis due to traumatic CSF leak can present within 24 hours to as long as several decades after the development of the leak.2, 3 Along with the classic symptoms and signs of meningitis, including fever, headache, neck stiffness, change in sensorium, seizures, and vomiting, patients may also present with CSF rhinorrhea, CSF otorrhea, hearing impairment, or cranial injury residua.3, 5 It is important to note that CSF rhinorrhea and otorrhea are not always present in cases of chronic, posttraumatic CSF leaks.
The visualization of a fracture or bony dehiscence is very difficult but critical for identification and surgical repair. Frontal and ethmoid sinuses and cribriform plate are common fracture sites.1, 3, 5 CSF leakage may be from the anterior, middle, or posterior compartments, eventually ending in the nasal cavity.1, 3, 5 Various imaging modalities, including contrast cisternogram, high‐resolution CT, fluorescein nasal endoscopy, and magnetic resonance imaging (MRI) have been advocated for diagnosing the source of CSF leak with variable sensitivity and specificity.6 High‐resolution CT helps in identifying surgical anatomy and bony defects whereas contrast cisternography is confirmatory when the CSF leak is active.1, 6 Protein electrophoresis demonstrating 2 electrophoretically separate transferrin bands confirms CSF.7
In patients with persistent CSF rhinorrhea, there is a 19% overall risk of meningitis with an annual incidence of 0.3 meningitis episodes per year.8 The risk of meningitis is the greatest in the first year following the onset of a CSF leak.8 Generally, patients with posttraumatic CSF leak lasting more than 7 to 10 days need surgical repair to decrease their risk of bacterial meningitis.1, 3, 5 Endoscopic surgical correction with a success rate of 90% is an effective treatment for CSF leak and involves placement of a temporary lumbar drain in addition to endonasal duraplasty performed with the aid of microscope and/or nasoscope.9 Complicated anterior cranial base fractures are not as amenable to endoscopic repair and may require a combined intracranial extradural and intradural approach or a transcranial approach.10
Information on prophylactic antibiotics and vaccination is still evolving. Currently available evidence from randomized control trials does not support prophylactic antibiotic use in patients with basilar skull fracture with or without the evidence of CSF leakage.11 A meta‐analysis of 4 randomized controlled trials of patients with acute basilar skull fracture showed no significant difference between the antibiotic prophylaxis groups and control groups with respect to reduction of the frequency of meningitis, the need for surgical correction, meningitis‐related mortality, and all‐cause mortality.11 Direct invasion of the meninges by nasopharyngeal bacteria bypassing the circulating serum antibodies may limit the potential effectiveness of vaccination in preventing RBM.12 However, vaccination is generally recommended in patients with complement or immunoglobulin deficiency or after splenectomy.4
- ,.Cerebrospinal fluid rhinorrhea and recurrent meningitis.Clin Infect Dis.1993;17:364–368.
- ,,, et al.Late manifestations of traumatic lesions of the anterior skull base.Skull Base Surg.1997;7(2):77–83.
- ,.Posttraumatic bacterial meningitis.Ann Intern Med.1970;72:869–874.
- ,,, et al.Community‐acquired recurrent bacterial meningitis in adults.Clin Infect Dis.2007;45:e46–e51.
- .Meningitis following trauma to the head and face.JAMA.1960;173:1818–1822.
- ,,.Post‐traumatic cerebrospinal fluid rhinorrhea: modern high‐definition computed tomography is all that is required for the effective demonstration of the site of leakage.Clin Radiol.1994;49:100–103.
- ,,.Identification of CSF leakage by immunofixation.Arch Otolarygol.1979;105:447–448.
- ,,.Risk of meningitis with cerebrospinal fluid rhinorrhea.Ann Otol Rhinol Laryngol.2007;116 (12):902–905.
- ,,.Endoscopic management of cerebrospinal fluid rhinorrhea.Laryngoscope.2004;114(10):1833–1837.
- ,,, et al.Surgical management of anterior cranial base fractures with cerebrospinal fluid fistulae: a single‐institution experience.Neurosurgery.2008;62:463–471.
- ,,.Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures.Cochrane Database Syst Rev.2006;25(1):CD004884.
- ,,.Recurrent streptococcus pneumoniae meningitis.J Trop Pediatr.2002;48:249–250.
Recurrent bacterial meningitis (RBM), particularly when caused by Streptococcus pneumoniae, warrants an aggressive and thorough evaluation to exclude transdural communication. We present an unusual case of RBM as a late manifestation of a traumatic head injury sustained 10 years prior and describe presentation, etiology, diagnosis, and treatment options for RBM.
Case Report
A middle‐aged woman with type 2 diabetes mellitus, hypertension, and a prior history of S. pneumoniae meningitis 1 year earlier, presented to an outside hospital with complaints of fever, headache, and change in mental status. Materials for basic laboratory tests and blood cultures were drawn in the Emergency Department; these showed diabetic ketoacidosis. Computed tomography (CT) scan of the head was negative and a lumbar puncture (LP) was attempted, but was unsuccessful. The patient was started on intravenous insulin drip, vancomycin, and ceftriaxone and was transported to our facility via Life‐Flight. She also developed acute respiratory failure requiring mechanical ventilation.
After arrival, the patient had a normal repeat CT scan of her head and a successful LP. Cerebrospinal fluid (CSF) revealed 9064 white blood cells (WBCs)/mm3 with 77% neutrophils and 9% lymphocytes, protein concentration of 275 mg/dL, and glucose of 93 mg/dL. CSF culture and Gram stain were negative, while 1 blood culture drawn at the outside hospital grew penicillin‐resistant S. pneumoniae (MIC 2 g/mL). WBC count was 9660/mm3 with 45% band forms. Bacterial meningitis was confirmed and the patient was continued on intravenous antibiotics and insulin drip. Additional laboratory studies revealed normal complement levels and a negative human immunodeficiency virus (HIV) 1 and HIV 2 antibody screen. The patient was extubated in 48 hours. and was treated with a total of 2 weeks of ceftriaxone and vancomycin for penicillin‐resistant S. pneumoniae meningitis.
The patient had an uneventful full recovery and was discharged from the hospital with neurosurgery follow‐up. The neurosurgeon ordered a CT scan of the facial bones, which revealed an irregular calcification in the right frontal sinus adjacent to the cribriform plate and thinning of the posterior wall of the sinus. Upon requestioning at a subsequent neurosurgical appointment, the patient recalled being an unrestrained passenger and striking her head against the windshield in a motor vehicle accident (MVA) approximately 10 years ago. Ever since the MVA, she noticed intermittent postnasal discharge while recumbent. However, she never sought a medical opinion and denied complaints of anterior rhinorrhea.
A CT cisternography confirmed the presence of CSF leakage with contrast accumulation via a defect in the right paramedian cribriform plate. Contrast opacification was seen in the fovea ethmoidalis extending into the right frontal sinus (Figure 1). The patient subsequently underwent transnasal endoscopic CSF leak repair (Figure 2). The postoperative cisternogram did not reveal the transdural communication. However a follow‐up cisternogram performed 3 months later demonstrated a recurrent CSF leak.


The patient was rehospitalized with grand‐mal seizures and a third episode of S. pneumoniae meningitis, this time with a penicillin‐sensitive strain. She was treated with a 2‐week course of ceftriaxone and also received heptavalent pneumococcal vaccine to supplement the 23‐valent pneumococcal vaccine. Two weeks after the hospital discharge, the patient underwent successful bifrontal transcranial repair. Currently, she has been disease‐free for 3 months and is followed closely by neurosurgery as an outpatient.
Discussion
After immune deficiency is ruled out, it is essential to evaluate for transdural communication between the subarachnoid space and the base of the skull resulting in a CSF leak as a cause for RBM. Meningitis secondary to a CSF leak is most commonly caused by S. pneumoniae, followed by Neisseria meningitidis and Haemophilus influenzae.1 Complement and immunoglobulin subclass defects may also predispose to RBM.2, 3
A recent case series by Adriani et al.4 suggested that as many as 77% of patients with RBM have an identifiable risk factor such as a remote head injury or CSF leakage. CSF rhinorrhea is most often secondary to trauma, occurring in approximately 1% to 3% of all blunt head injuries.2 Accidental falls, MVAs, altercations, and gunshot wounds are also commonly responsible.3 Nontraumatic CSF leaks are very rare but may be secondary to spontaneous, congenital, or iatrogenic etiologies.1, 3 Spontaneous CSF leaks could also occur due to violent sneezing or coughing.1, 3 Congenital defects include weakened preformed pathways, failure of germ layer closures, and bone imperfections.1, 3 Infrequently, CSF leak can be a complication of intracranial, otologic, nasal, or paranasal sinus surgeries.1, 3 Other rarer etiologies include intracranial tumors and hydrocephalus.1, 3
Bacterial meningitis due to traumatic CSF leak can present within 24 hours to as long as several decades after the development of the leak.2, 3 Along with the classic symptoms and signs of meningitis, including fever, headache, neck stiffness, change in sensorium, seizures, and vomiting, patients may also present with CSF rhinorrhea, CSF otorrhea, hearing impairment, or cranial injury residua.3, 5 It is important to note that CSF rhinorrhea and otorrhea are not always present in cases of chronic, posttraumatic CSF leaks.
The visualization of a fracture or bony dehiscence is very difficult but critical for identification and surgical repair. Frontal and ethmoid sinuses and cribriform plate are common fracture sites.1, 3, 5 CSF leakage may be from the anterior, middle, or posterior compartments, eventually ending in the nasal cavity.1, 3, 5 Various imaging modalities, including contrast cisternogram, high‐resolution CT, fluorescein nasal endoscopy, and magnetic resonance imaging (MRI) have been advocated for diagnosing the source of CSF leak with variable sensitivity and specificity.6 High‐resolution CT helps in identifying surgical anatomy and bony defects whereas contrast cisternography is confirmatory when the CSF leak is active.1, 6 Protein electrophoresis demonstrating 2 electrophoretically separate transferrin bands confirms CSF.7
In patients with persistent CSF rhinorrhea, there is a 19% overall risk of meningitis with an annual incidence of 0.3 meningitis episodes per year.8 The risk of meningitis is the greatest in the first year following the onset of a CSF leak.8 Generally, patients with posttraumatic CSF leak lasting more than 7 to 10 days need surgical repair to decrease their risk of bacterial meningitis.1, 3, 5 Endoscopic surgical correction with a success rate of 90% is an effective treatment for CSF leak and involves placement of a temporary lumbar drain in addition to endonasal duraplasty performed with the aid of microscope and/or nasoscope.9 Complicated anterior cranial base fractures are not as amenable to endoscopic repair and may require a combined intracranial extradural and intradural approach or a transcranial approach.10
Information on prophylactic antibiotics and vaccination is still evolving. Currently available evidence from randomized control trials does not support prophylactic antibiotic use in patients with basilar skull fracture with or without the evidence of CSF leakage.11 A meta‐analysis of 4 randomized controlled trials of patients with acute basilar skull fracture showed no significant difference between the antibiotic prophylaxis groups and control groups with respect to reduction of the frequency of meningitis, the need for surgical correction, meningitis‐related mortality, and all‐cause mortality.11 Direct invasion of the meninges by nasopharyngeal bacteria bypassing the circulating serum antibodies may limit the potential effectiveness of vaccination in preventing RBM.12 However, vaccination is generally recommended in patients with complement or immunoglobulin deficiency or after splenectomy.4
Recurrent bacterial meningitis (RBM), particularly when caused by Streptococcus pneumoniae, warrants an aggressive and thorough evaluation to exclude transdural communication. We present an unusual case of RBM as a late manifestation of a traumatic head injury sustained 10 years prior and describe presentation, etiology, diagnosis, and treatment options for RBM.
Case Report
A middle‐aged woman with type 2 diabetes mellitus, hypertension, and a prior history of S. pneumoniae meningitis 1 year earlier, presented to an outside hospital with complaints of fever, headache, and change in mental status. Materials for basic laboratory tests and blood cultures were drawn in the Emergency Department; these showed diabetic ketoacidosis. Computed tomography (CT) scan of the head was negative and a lumbar puncture (LP) was attempted, but was unsuccessful. The patient was started on intravenous insulin drip, vancomycin, and ceftriaxone and was transported to our facility via Life‐Flight. She also developed acute respiratory failure requiring mechanical ventilation.
After arrival, the patient had a normal repeat CT scan of her head and a successful LP. Cerebrospinal fluid (CSF) revealed 9064 white blood cells (WBCs)/mm3 with 77% neutrophils and 9% lymphocytes, protein concentration of 275 mg/dL, and glucose of 93 mg/dL. CSF culture and Gram stain were negative, while 1 blood culture drawn at the outside hospital grew penicillin‐resistant S. pneumoniae (MIC 2 g/mL). WBC count was 9660/mm3 with 45% band forms. Bacterial meningitis was confirmed and the patient was continued on intravenous antibiotics and insulin drip. Additional laboratory studies revealed normal complement levels and a negative human immunodeficiency virus (HIV) 1 and HIV 2 antibody screen. The patient was extubated in 48 hours. and was treated with a total of 2 weeks of ceftriaxone and vancomycin for penicillin‐resistant S. pneumoniae meningitis.
The patient had an uneventful full recovery and was discharged from the hospital with neurosurgery follow‐up. The neurosurgeon ordered a CT scan of the facial bones, which revealed an irregular calcification in the right frontal sinus adjacent to the cribriform plate and thinning of the posterior wall of the sinus. Upon requestioning at a subsequent neurosurgical appointment, the patient recalled being an unrestrained passenger and striking her head against the windshield in a motor vehicle accident (MVA) approximately 10 years ago. Ever since the MVA, she noticed intermittent postnasal discharge while recumbent. However, she never sought a medical opinion and denied complaints of anterior rhinorrhea.
A CT cisternography confirmed the presence of CSF leakage with contrast accumulation via a defect in the right paramedian cribriform plate. Contrast opacification was seen in the fovea ethmoidalis extending into the right frontal sinus (Figure 1). The patient subsequently underwent transnasal endoscopic CSF leak repair (Figure 2). The postoperative cisternogram did not reveal the transdural communication. However a follow‐up cisternogram performed 3 months later demonstrated a recurrent CSF leak.


The patient was rehospitalized with grand‐mal seizures and a third episode of S. pneumoniae meningitis, this time with a penicillin‐sensitive strain. She was treated with a 2‐week course of ceftriaxone and also received heptavalent pneumococcal vaccine to supplement the 23‐valent pneumococcal vaccine. Two weeks after the hospital discharge, the patient underwent successful bifrontal transcranial repair. Currently, she has been disease‐free for 3 months and is followed closely by neurosurgery as an outpatient.
Discussion
After immune deficiency is ruled out, it is essential to evaluate for transdural communication between the subarachnoid space and the base of the skull resulting in a CSF leak as a cause for RBM. Meningitis secondary to a CSF leak is most commonly caused by S. pneumoniae, followed by Neisseria meningitidis and Haemophilus influenzae.1 Complement and immunoglobulin subclass defects may also predispose to RBM.2, 3
A recent case series by Adriani et al.4 suggested that as many as 77% of patients with RBM have an identifiable risk factor such as a remote head injury or CSF leakage. CSF rhinorrhea is most often secondary to trauma, occurring in approximately 1% to 3% of all blunt head injuries.2 Accidental falls, MVAs, altercations, and gunshot wounds are also commonly responsible.3 Nontraumatic CSF leaks are very rare but may be secondary to spontaneous, congenital, or iatrogenic etiologies.1, 3 Spontaneous CSF leaks could also occur due to violent sneezing or coughing.1, 3 Congenital defects include weakened preformed pathways, failure of germ layer closures, and bone imperfections.1, 3 Infrequently, CSF leak can be a complication of intracranial, otologic, nasal, or paranasal sinus surgeries.1, 3 Other rarer etiologies include intracranial tumors and hydrocephalus.1, 3
Bacterial meningitis due to traumatic CSF leak can present within 24 hours to as long as several decades after the development of the leak.2, 3 Along with the classic symptoms and signs of meningitis, including fever, headache, neck stiffness, change in sensorium, seizures, and vomiting, patients may also present with CSF rhinorrhea, CSF otorrhea, hearing impairment, or cranial injury residua.3, 5 It is important to note that CSF rhinorrhea and otorrhea are not always present in cases of chronic, posttraumatic CSF leaks.
The visualization of a fracture or bony dehiscence is very difficult but critical for identification and surgical repair. Frontal and ethmoid sinuses and cribriform plate are common fracture sites.1, 3, 5 CSF leakage may be from the anterior, middle, or posterior compartments, eventually ending in the nasal cavity.1, 3, 5 Various imaging modalities, including contrast cisternogram, high‐resolution CT, fluorescein nasal endoscopy, and magnetic resonance imaging (MRI) have been advocated for diagnosing the source of CSF leak with variable sensitivity and specificity.6 High‐resolution CT helps in identifying surgical anatomy and bony defects whereas contrast cisternography is confirmatory when the CSF leak is active.1, 6 Protein electrophoresis demonstrating 2 electrophoretically separate transferrin bands confirms CSF.7
In patients with persistent CSF rhinorrhea, there is a 19% overall risk of meningitis with an annual incidence of 0.3 meningitis episodes per year.8 The risk of meningitis is the greatest in the first year following the onset of a CSF leak.8 Generally, patients with posttraumatic CSF leak lasting more than 7 to 10 days need surgical repair to decrease their risk of bacterial meningitis.1, 3, 5 Endoscopic surgical correction with a success rate of 90% is an effective treatment for CSF leak and involves placement of a temporary lumbar drain in addition to endonasal duraplasty performed with the aid of microscope and/or nasoscope.9 Complicated anterior cranial base fractures are not as amenable to endoscopic repair and may require a combined intracranial extradural and intradural approach or a transcranial approach.10
Information on prophylactic antibiotics and vaccination is still evolving. Currently available evidence from randomized control trials does not support prophylactic antibiotic use in patients with basilar skull fracture with or without the evidence of CSF leakage.11 A meta‐analysis of 4 randomized controlled trials of patients with acute basilar skull fracture showed no significant difference between the antibiotic prophylaxis groups and control groups with respect to reduction of the frequency of meningitis, the need for surgical correction, meningitis‐related mortality, and all‐cause mortality.11 Direct invasion of the meninges by nasopharyngeal bacteria bypassing the circulating serum antibodies may limit the potential effectiveness of vaccination in preventing RBM.12 However, vaccination is generally recommended in patients with complement or immunoglobulin deficiency or after splenectomy.4
- ,.Cerebrospinal fluid rhinorrhea and recurrent meningitis.Clin Infect Dis.1993;17:364–368.
- ,,, et al.Late manifestations of traumatic lesions of the anterior skull base.Skull Base Surg.1997;7(2):77–83.
- ,.Posttraumatic bacterial meningitis.Ann Intern Med.1970;72:869–874.
- ,,, et al.Community‐acquired recurrent bacterial meningitis in adults.Clin Infect Dis.2007;45:e46–e51.
- .Meningitis following trauma to the head and face.JAMA.1960;173:1818–1822.
- ,,.Post‐traumatic cerebrospinal fluid rhinorrhea: modern high‐definition computed tomography is all that is required for the effective demonstration of the site of leakage.Clin Radiol.1994;49:100–103.
- ,,.Identification of CSF leakage by immunofixation.Arch Otolarygol.1979;105:447–448.
- ,,.Risk of meningitis with cerebrospinal fluid rhinorrhea.Ann Otol Rhinol Laryngol.2007;116 (12):902–905.
- ,,.Endoscopic management of cerebrospinal fluid rhinorrhea.Laryngoscope.2004;114(10):1833–1837.
- ,,, et al.Surgical management of anterior cranial base fractures with cerebrospinal fluid fistulae: a single‐institution experience.Neurosurgery.2008;62:463–471.
- ,,.Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures.Cochrane Database Syst Rev.2006;25(1):CD004884.
- ,,.Recurrent streptococcus pneumoniae meningitis.J Trop Pediatr.2002;48:249–250.
- ,.Cerebrospinal fluid rhinorrhea and recurrent meningitis.Clin Infect Dis.1993;17:364–368.
- ,,, et al.Late manifestations of traumatic lesions of the anterior skull base.Skull Base Surg.1997;7(2):77–83.
- ,.Posttraumatic bacterial meningitis.Ann Intern Med.1970;72:869–874.
- ,,, et al.Community‐acquired recurrent bacterial meningitis in adults.Clin Infect Dis.2007;45:e46–e51.
- .Meningitis following trauma to the head and face.JAMA.1960;173:1818–1822.
- ,,.Post‐traumatic cerebrospinal fluid rhinorrhea: modern high‐definition computed tomography is all that is required for the effective demonstration of the site of leakage.Clin Radiol.1994;49:100–103.
- ,,.Identification of CSF leakage by immunofixation.Arch Otolarygol.1979;105:447–448.
- ,,.Risk of meningitis with cerebrospinal fluid rhinorrhea.Ann Otol Rhinol Laryngol.2007;116 (12):902–905.
- ,,.Endoscopic management of cerebrospinal fluid rhinorrhea.Laryngoscope.2004;114(10):1833–1837.
- ,,, et al.Surgical management of anterior cranial base fractures with cerebrospinal fluid fistulae: a single‐institution experience.Neurosurgery.2008;62:463–471.
- ,,.Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures.Cochrane Database Syst Rev.2006;25(1):CD004884.
- ,,.Recurrent streptococcus pneumoniae meningitis.J Trop Pediatr.2002;48:249–250.
Postcards from Our Students
During their junior medicine rotation, our students are asked to post to Blackboard (an online student forum) an anonymous essay about an issue of professionalism or ethics, either inspiring or troubling. In many ways, these vignettes are like postcards, written by visitors describing foreign cultures and norms. They represent a way for the students to debrief, but also provide an opportunity for us, as faculty, to reflect upon the way we practice and teach medicine. Many postingslike postcards from exotic or historic placesare inspiring stories of residents and faculty extending themselves for their patients. Unfortunately, unlike typical postcards, there are also essays that are troubling or provoking and challenge us to consider how we could improve the professional and ethical environment on our teams.
In order to begin a learning process with our faculty and housestaff, we have presented a number of these anonymous essays at both faculty and housestaff Department of Medicine conferences as well as our monthly hospital Ethics conference. The goal of these conferences was to gather as a moral community to reflect on our students' experience and consider ways in which our day to day practice as attendings could be informed by what they tell us. In addition, the junior medicine site directors have a session each quarter with their junior students to review some of the most significant issues brought up by their essays.
Practically, these vignettes and conferences serve three main purposes:
-
Raising Awareness: Many professional issues noted by our students occur under the radar. Attendings are often unaware of the issues of professionalism and/or ethics confronting our students and housestaff.
-
Exploring Attitudes: Some attending may underemphasize the importance of specific issues of professionalism and/or ethics. Open discussions at faculty or resident conferences create opportunities for individuals to reflect upon their own reactions and for the group to create a norm.
-
Sharing Skills: It is difficult to learn the practice of professionalism and ethics from a book. Skill in this area is gained primarily by experience. Conferences provide an excellent forum for seasoned physicians to share wisdom with less experienced physicians. In addition, important teaching points can be made: Students should not deliver bad news alone. Errors should be disclosed.
Following are 3 of the essays we presented, along with brief commentaries. At the end, we provide practical suggestions for individual attendings to improve the professional climate on their teams.
The Hospital Didn't Wait
Code. On 12, the surgical wards floor. Elise sprinted to the stairwell, dashed up to 12, and ran to the corner room as fast as she could. She could see the room before she got there. Instinctively, she started reviewing the steps she had memorized so many months ago. But when she finally arrived at the patient's bathroom, her thought process came to a jolting halt as she came upon the gruesome scene.
The 76‐year‐old patient had hanged himself with the cinching rope from his garment bag, and now dangled suspended from a high towel rack against the wall. Nurses from the floor started to file in, and without losing a beat Elise barked commands. Together they brought the man's body down to the floor, laid him on his back, and stripped off his hospital gown. Elise was in charge; deliberately but forcefully, she ordered a nurse to retrieve a defibrillator, and had another resident check for a pulse. There was none. Anesthesiology was here. Quickly and expertly, they shoved a plastic tube down his throat and began ventilation. The nurse placed on the electrodes between chest compressions then called to clear the body. Airway stepped back. The chest pumper stepped back. The body lurched forward as the defibrillator issued a long beep and discharged. Still no pulse. The cycle repeated.
Finally, Elise called a stop. Time of death, 19:37. By now there were about 20 people crammed in the patient room, all of whom had a separate role during the code. Some stayed behind, while the rest left to return to their interrupted work. The medical student didn't know what to think as he returned to the team room. His jaw was sorehad he been clenching it the whole time?and as he brought his hand up to rub his face, he saw that his knuckles were bloody. Somehow he had scraped them during the code. As he logged back into the computer to finish off his evening notes, he knew that he wouldn't have time to reflect until hours later when he returned home. Codes happened all the time. There was still work to be done in the hospital, and the hospital didn't wait.
The room had already been assigned to a patient waiting in the Emergency Department downstairs. That patient would be here in a few minutes. The hospital didn't wait.
When we presented this case in our conferences, there was universal agreement that such a traumatic event merits, even demands, team debriefing and processing. But in the real life aftermath of this traumatic event, the take‐home message for the medical student was that the hospital didn't wait for such discussions. We know this is not unique to our institution. In a study of 32 medical students who were asked to reflect on their most memorable patient death,1 debriefing sessions were rare and many students felt inadequately supported. While experienced clinicians may be accustomed to seeing patients die, students are new to the culture of the hospital, and have not had the chance to develop the defense mechanisms necessary to cope with this sort of experience. Angoff2 writes, As medical educators, we ought to ask our students how they are coping with long hours, fatigue, illness, suffering, and death. We ought to model and commend compassion and react to the deep feelings of our students in the same way we would teach them to react to the deep feelings of their patients.
I Told a Man Today That He Had Brain Cancer
The resident, intern, and I were huddled together in our team room when the report came back on the computer. New 3.5 2.3 1.7 cm contrast‐enhancing lesion seen anterior to genu of corpus callosum. Concerning for metastatic focus vs. lymphoma. Advise follow‐up. It wasn't unexpected but we had nevertheless been hoping for better.
The three of us went into his room and I was waiting to see how my resident would deliver the bad news, but she didn't. She simply said that we were continuing to do imaging studies and that a neurology team would be in touch. There were probably several reasons why she didn't tell him: not enough time, not her responsibility, or maybe she was just uncomfortable with it. Whatever the case, we left the room with my patient still oblivious to the awful mass now tangled in his head.
If my resident was taking a pass on this conversation, I knew it fell to me he needed to hear it from his primary team. I came back after rounds alone, sat down next to his bed, and told him that his MRI results had come back, and that I had unfortunate news.
I told him that the images showed that his lung cancer had spread to his brain.
I paused to give him a chance to let it sink in. He turned away and looked up at the ceiling.
Where is it? How big is it?
What now?
Reflecting on this case, our audiences were disturbed that a student would attempt this difficult conversation alone, while recognizing that the student clearly felt a sense of responsibility and desire to help his patient by sharing important information. We talked about how students may erroneously pick up a message that the team member who has spent the most time with a patient is the most obvious choice to have difficult conversations. We also noted that, unfortunately, sometimes students are directly asked by their team to shoulder this responsibility on their own. In this painful account, there is no mention of preparation, supervision, or support for the student before or after the encounter. The student perceived (rightly or wrongly) that the team leaders lacked comfort or skill to deliver the bad news, and stepped in. It is possible that the attending lacked the skill and ability to model an interaction, but more likely the deficit was in awareness and attitude. It is unlikely the attending knew that the student had this conversation alone. One of the major reasons we present these vignettes is to make attendings and housestaff more aware of issues that occur under their radar so that they can take preventative action. However, once the resident or attending found out that the student had this conversation alone, the student should be pulled aside for a 1:1 discussion. At the end of the day, the student should know that it was inappropriate to attempt this conversation alone
Rosenbaum3 reviewed a number of strategies to teach the skill of delivering bad news, from lecture and small group discussions to role play and standardized patients. When asked, students cited role‐modeling as the best way to learn how to deliver bad news.4 Observation of a veteran clinician provides a firm foundation for learning; but that is not enough. Unfortunately, we know from the literature (and our student vignettes suggest) that students and residents are unprepared to carry out these conversations properly, either because of misguided attitudes, lack of experience, or inadequate training.57 We conceptualize engaging in difficult conversations as a procedure, demanding a skill set. Mere observation of an expert executing this procedure is only a beginning. With any other skill, from successful completion of a lumbar puncture to initiating cardiopulmonary resuscitation (CPR), a student would never conclude that knowing the patient the best sufficiently credentials the student to undertake these procedures. We maintain that a difficult conversationbe it breaking bad news, discussing end‐of‐life care preferences, code status discussions, or prognosisis a clinical intervention, like any other procedure in medicine. If performed with skill and caution, it can bring about a stronger therapeutic relationship and increased support for the patient; if performed clumsily, it can lead to unintended adverse outcomes, including misunderstanding, mistrust, anxiety, and anger.
A Decimal Point Got Misplaced
On palliative care, I had a 90 year‐old man with end stage lung CA that presented to the ED with increasing SOB. The resident decided that giving him some morphine would be a good solution but was worried that too much would push him over the edge. He was thin; his O2 sats weren't that good After some discussion it was decided that 2.5 mg should be the starting amount. Unfortunately, when the note was written a decimal point got misplaced and he got 25 mg as a first dose. He ended up very sedated for most of the day but his breathing was ok.
The mistake was not discussed with the patient or the patient's family. While it did not cause any lasting harm, I wondered if telling the patient/patient's family that an error had been made would have been more ethically sound.
When we presented this case in our conferences, there was little controversy about whether the error should have been disclosed. The discussion did provide reinforcement for doing a simple but difficult task. Our analysis is that the nondiscussion of this error reflects a deficit in attitude and possibly skill. The team was aware of the error, but the resident and attending did not take the opportunity to disclose an error. They should have. We do not know whether the attending or resident felt unprepared to discuss this or were simply unimpressed with the adverse event. We do get the sense that the student did not feel comfortable raising the issue with the team. As such, it was a missed opportunity to seek help from any number of hospital resources and find encouragement to take on difficult encounters.
Much has been written about apologies.810 Disclosing errors and apologizing is the ethical standard, and many of our institutions have made it policy. Yet in the moment, it is embarrassing, anxiety provoking, and our concern about litigation looms large. Learning to do the right thing begins, perhaps with lectures and standardized patients, but only when students see it modeled by our housestaff and faculty, does it take root for good.
Our housestaff are quite good at managing medical issues, but they may still need help in creating the appropriate environment for professional learning and growth. This is 1 of the most important contributions an attending can make. We have emphasized that faculty have an important role to play in the area of professional development, reinforcing the rudimentary information preclinical students are presented with in the classroom and processing experiences residents are exposed to on a regular basis. If the hospital doesn't wait, then it is the attending physician's job to create the space and time for trainees to think about what is happening and ask if it could have been done better.
A number of seasoned clinical teachers have written about ways to improve teaching on the wards.11 Below, we will add to that discussion by considering practical ways to enhance learning about professionalism and ethics (see Table 1). Note should be made that while we focus on specific behaviors and activities, underlying all is the importance of availability, presence, and intention. Like all good teaching, these activities require planning and effort.
| Attending Activity | Examples |
|---|---|
| Creating an Open Climate | |
| Breaking Communication Barriers | Setting aside time for introductions and team building exercises at the beginning of a rotation, with attending participating equally with residents and students |
| Emphasizing attending availability to discuss or review problems of any kind | |
| Setting Clear Expectations | Emphasizing the importance of patient‐clinician or family‐clinician communication from the outset |
| Devoting some attending rounds to Difficult Conversations (e.g., breaking bad news or code status discussions) | |
| Explicitly stating that no ethical question is a stupid question and providing positive feedback for raising such questions for the team | |
| Regular Check‐ins | Establishing team communication rounds: 10 minutes every day to review a good, bad, or awkward interaction from the past day (e.g., family meeting, DNR discussion) |
| Setting aside time on rounds or during attending teaching sessions to explore the team's or an individual's emotional responses to a patient's death or deterioration | |
| Writing exercises that focus on our reactions to challenging situations that are shared with the group | |
| Supervision and Modeling | |
| Planning | Clarifying an agenda and practicing key phrases for a family meeting with the resident prior to meeting the family |
| Anticipating which patients may require a code status discussion and discussing a game plan on rounds | |
| Modeling | Students observe the attending facilitate a family meeting |
| Residents observe the attending apologizing for an error, no matter how small | |
| Attending thinks about an interpersonal conflict out loud and models asking patient‐relations for help | |
| Debriefing | Reviewing a family meeting with and giving feedback to the resident who facilitated |
| Reviewing a challenging code status discussion as a team |
Creating an Open Climate
The medical team, of which the attending, residents, and students are all a part, should not only be a unit that provides excellent medical care to its patients, but should also create a culture of continuous learning and improvement. As such, it is important to create a safe atmosphere where teachers are invested in the growth of their learners and learners feel free to question the prevailing logic and practice, including issues of professionalism and ethics. As Malcolm Gladwell12 describes in Outliers, Korean Air jets were crashing because subordinates were afraid to question their superiors. Once that culture changed, Korean Air safety improved dramatically. Similarly, breaking down some of the hierarchical barriers should improve the culture of a medical team. We typically make an effort to get to know our students and residents on a more personal basis: where they are from, who is in their family, what was their major, what are their interests outside of medicine, and what has been surprising to them in their training so far. Whether we set aside time when we first meet or e‐mail our questions before the first day, we aim for this to be 1 of the first team activities. We also share our own stories, making clear that the attending is part of the team, and not just an evaluating supervisor.
Vignette 1 describes the student's trauma of witnessing a code and the inability to process the event with anyone afterward. Failed resuscitation attempts are the most dramatic examples, but even expected deaths, nonfatal adverse events, and conflict between patients and providers may be traumatic for new trainees inexperienced with the reality of medicine. Attendings should be aware of these potentially traumatic events and make time to check in with the team members about how they are dealing with their emotions. Taking time on attending rounds, for example, allows the attending to not only model reflective practice and self‐care, but also elevates team support to a place traditionally reserved for discussions about diagnosis and treatment.
Supervision and Modeling
Vignettes 2 and 3 center around challenging communication tasks that require special training, including instruction, modeling, feedback, and practice. Unfortunately, as some of our student accounts document, many teaching opportunities are missed. As attendings, our duties include being aware of these opportunities, and being prepared to model competent patientor familydoctor interactions. Emphasizing the importance of the doctor‐patient relationship is in fact one of the key skills of an effective attending role model.13
When opportunities arise for any potentially difficult conversation, we make every effort to identify the issue, prebrief with the team about how to conduct the discussion, and either offer to model the conversation or be present to observe and provide feedback and debriefing afterwards. For example, by asking about all DNR discussions had with our patients, we gain insight into the skill level of our housestaff. As important, the housestaff understand that we believe that these conversations are vital to review during formal rounds, with the same attention we give to chest pain and electrocardiograms (ECGs).
Two key skills that develop with experience are the ability to know the limits of one's knowledge and to know when to ask for help. We try to be open about naming those limits and thinking about the other members of the larger healthcare team that may provide insight, skill, and expertise. We are used to doing this with medical questions (eg, asking the gastroenterology consult team to locate a source of bleeding). Asking our risk management, patient‐relations, or ethics services to assist with a difficult communication task or conflict with a family is no different, and often something the housestaff may not readily do.
We are grateful to our students and their postcards for the snapshots of our local medical culture. While we are gratified to read of excellent role modeling, we are also disappointed to read of situations which have left our students confused, demoralized and cynical. But if these exercises are to reach their full potential, they should tell us about where we would like to go, in addition to where we have been. We believe that our conferences have stimulated our faculty and housestaff to reflect on the professionalism lessons they are teaching. Reading the student postings has definitely affected our approach to teaching professionalism. They reinforce what every parent and educator knows: when it comes to teaching professionalism, communication and ethics, what matters most is the behavior of the teacher. Our words mean little if our actions do not live out what we espouse.
Acknowledgements
We are grateful for Michael Chan and his classmates from the NUFSM class of 2010 for their thoughtful essays. David Neely, Director of Undergraduate Education, Department of Medicine, Eytan Szmuilowicz, Palliative Medicine. Kathy Neely, Chairman of NMH Ethics Committee. Co‐director of Patient, Physician and Society.
- .This is just too awful; I just can't believe I experienced that.Acad Med.2005;80(7):634–640.
- .A piece of my mind.JAMA.2001;286(9):1017–1018.
- .Teaching medical students and residents skills for delivering bad news: a review of strategies.Acad Med.2004;79(2):107–117.
- .Third‐year medical students' experiences with dying patients during the internal medicine clerkship: a qualitative study of the informal curriculum.Acad Med.2005;80(7):641–647.
- ,,.How do medical residents discuss resuscitation with patients? Official journal of the Society for Research and Education in Primary Care Internal Medicine.J Gen Intern Med.1995;10(8):436–442.
- ,,.See one, do one, teach one? House staff experience discussing do‐not‐resuscitate orders.Arch Intern Med.1996;156(12):1285–1289.
- ,,,.Residents' end‐of‐life decision making with adult hospitalized patients: a review of the literature.Acad Med.2005:80(7)622–633.
- .Physician error and disclosure.Clin Obstet Gynecol.2008;51(4):700–708.
- .Revealing medical errors to your patients.Chest.2009;133:1064–1065.
- .Apology in medical practice.JAMA.2006;296:1401–1404.
- .What if Osler were one of us? Inpatient teaching today.J Gen Intern Med.1997;12(Suppl 2):S41–S48.
- Malcolm Gladwell.Outliers.New York:Little, Brown, Co.,2008: p.177–223.
- ,,,,.Attributes of excellent attending‐physician role models.N Engl J Med.1998;339(27):1986–1993.
During their junior medicine rotation, our students are asked to post to Blackboard (an online student forum) an anonymous essay about an issue of professionalism or ethics, either inspiring or troubling. In many ways, these vignettes are like postcards, written by visitors describing foreign cultures and norms. They represent a way for the students to debrief, but also provide an opportunity for us, as faculty, to reflect upon the way we practice and teach medicine. Many postingslike postcards from exotic or historic placesare inspiring stories of residents and faculty extending themselves for their patients. Unfortunately, unlike typical postcards, there are also essays that are troubling or provoking and challenge us to consider how we could improve the professional and ethical environment on our teams.
In order to begin a learning process with our faculty and housestaff, we have presented a number of these anonymous essays at both faculty and housestaff Department of Medicine conferences as well as our monthly hospital Ethics conference. The goal of these conferences was to gather as a moral community to reflect on our students' experience and consider ways in which our day to day practice as attendings could be informed by what they tell us. In addition, the junior medicine site directors have a session each quarter with their junior students to review some of the most significant issues brought up by their essays.
Practically, these vignettes and conferences serve three main purposes:
-
Raising Awareness: Many professional issues noted by our students occur under the radar. Attendings are often unaware of the issues of professionalism and/or ethics confronting our students and housestaff.
-
Exploring Attitudes: Some attending may underemphasize the importance of specific issues of professionalism and/or ethics. Open discussions at faculty or resident conferences create opportunities for individuals to reflect upon their own reactions and for the group to create a norm.
-
Sharing Skills: It is difficult to learn the practice of professionalism and ethics from a book. Skill in this area is gained primarily by experience. Conferences provide an excellent forum for seasoned physicians to share wisdom with less experienced physicians. In addition, important teaching points can be made: Students should not deliver bad news alone. Errors should be disclosed.
Following are 3 of the essays we presented, along with brief commentaries. At the end, we provide practical suggestions for individual attendings to improve the professional climate on their teams.
The Hospital Didn't Wait
Code. On 12, the surgical wards floor. Elise sprinted to the stairwell, dashed up to 12, and ran to the corner room as fast as she could. She could see the room before she got there. Instinctively, she started reviewing the steps she had memorized so many months ago. But when she finally arrived at the patient's bathroom, her thought process came to a jolting halt as she came upon the gruesome scene.
The 76‐year‐old patient had hanged himself with the cinching rope from his garment bag, and now dangled suspended from a high towel rack against the wall. Nurses from the floor started to file in, and without losing a beat Elise barked commands. Together they brought the man's body down to the floor, laid him on his back, and stripped off his hospital gown. Elise was in charge; deliberately but forcefully, she ordered a nurse to retrieve a defibrillator, and had another resident check for a pulse. There was none. Anesthesiology was here. Quickly and expertly, they shoved a plastic tube down his throat and began ventilation. The nurse placed on the electrodes between chest compressions then called to clear the body. Airway stepped back. The chest pumper stepped back. The body lurched forward as the defibrillator issued a long beep and discharged. Still no pulse. The cycle repeated.
Finally, Elise called a stop. Time of death, 19:37. By now there were about 20 people crammed in the patient room, all of whom had a separate role during the code. Some stayed behind, while the rest left to return to their interrupted work. The medical student didn't know what to think as he returned to the team room. His jaw was sorehad he been clenching it the whole time?and as he brought his hand up to rub his face, he saw that his knuckles were bloody. Somehow he had scraped them during the code. As he logged back into the computer to finish off his evening notes, he knew that he wouldn't have time to reflect until hours later when he returned home. Codes happened all the time. There was still work to be done in the hospital, and the hospital didn't wait.
The room had already been assigned to a patient waiting in the Emergency Department downstairs. That patient would be here in a few minutes. The hospital didn't wait.
When we presented this case in our conferences, there was universal agreement that such a traumatic event merits, even demands, team debriefing and processing. But in the real life aftermath of this traumatic event, the take‐home message for the medical student was that the hospital didn't wait for such discussions. We know this is not unique to our institution. In a study of 32 medical students who were asked to reflect on their most memorable patient death,1 debriefing sessions were rare and many students felt inadequately supported. While experienced clinicians may be accustomed to seeing patients die, students are new to the culture of the hospital, and have not had the chance to develop the defense mechanisms necessary to cope with this sort of experience. Angoff2 writes, As medical educators, we ought to ask our students how they are coping with long hours, fatigue, illness, suffering, and death. We ought to model and commend compassion and react to the deep feelings of our students in the same way we would teach them to react to the deep feelings of their patients.
I Told a Man Today That He Had Brain Cancer
The resident, intern, and I were huddled together in our team room when the report came back on the computer. New 3.5 2.3 1.7 cm contrast‐enhancing lesion seen anterior to genu of corpus callosum. Concerning for metastatic focus vs. lymphoma. Advise follow‐up. It wasn't unexpected but we had nevertheless been hoping for better.
The three of us went into his room and I was waiting to see how my resident would deliver the bad news, but she didn't. She simply said that we were continuing to do imaging studies and that a neurology team would be in touch. There were probably several reasons why she didn't tell him: not enough time, not her responsibility, or maybe she was just uncomfortable with it. Whatever the case, we left the room with my patient still oblivious to the awful mass now tangled in his head.
If my resident was taking a pass on this conversation, I knew it fell to me he needed to hear it from his primary team. I came back after rounds alone, sat down next to his bed, and told him that his MRI results had come back, and that I had unfortunate news.
I told him that the images showed that his lung cancer had spread to his brain.
I paused to give him a chance to let it sink in. He turned away and looked up at the ceiling.
Where is it? How big is it?
What now?
Reflecting on this case, our audiences were disturbed that a student would attempt this difficult conversation alone, while recognizing that the student clearly felt a sense of responsibility and desire to help his patient by sharing important information. We talked about how students may erroneously pick up a message that the team member who has spent the most time with a patient is the most obvious choice to have difficult conversations. We also noted that, unfortunately, sometimes students are directly asked by their team to shoulder this responsibility on their own. In this painful account, there is no mention of preparation, supervision, or support for the student before or after the encounter. The student perceived (rightly or wrongly) that the team leaders lacked comfort or skill to deliver the bad news, and stepped in. It is possible that the attending lacked the skill and ability to model an interaction, but more likely the deficit was in awareness and attitude. It is unlikely the attending knew that the student had this conversation alone. One of the major reasons we present these vignettes is to make attendings and housestaff more aware of issues that occur under their radar so that they can take preventative action. However, once the resident or attending found out that the student had this conversation alone, the student should be pulled aside for a 1:1 discussion. At the end of the day, the student should know that it was inappropriate to attempt this conversation alone
Rosenbaum3 reviewed a number of strategies to teach the skill of delivering bad news, from lecture and small group discussions to role play and standardized patients. When asked, students cited role‐modeling as the best way to learn how to deliver bad news.4 Observation of a veteran clinician provides a firm foundation for learning; but that is not enough. Unfortunately, we know from the literature (and our student vignettes suggest) that students and residents are unprepared to carry out these conversations properly, either because of misguided attitudes, lack of experience, or inadequate training.57 We conceptualize engaging in difficult conversations as a procedure, demanding a skill set. Mere observation of an expert executing this procedure is only a beginning. With any other skill, from successful completion of a lumbar puncture to initiating cardiopulmonary resuscitation (CPR), a student would never conclude that knowing the patient the best sufficiently credentials the student to undertake these procedures. We maintain that a difficult conversationbe it breaking bad news, discussing end‐of‐life care preferences, code status discussions, or prognosisis a clinical intervention, like any other procedure in medicine. If performed with skill and caution, it can bring about a stronger therapeutic relationship and increased support for the patient; if performed clumsily, it can lead to unintended adverse outcomes, including misunderstanding, mistrust, anxiety, and anger.
A Decimal Point Got Misplaced
On palliative care, I had a 90 year‐old man with end stage lung CA that presented to the ED with increasing SOB. The resident decided that giving him some morphine would be a good solution but was worried that too much would push him over the edge. He was thin; his O2 sats weren't that good After some discussion it was decided that 2.5 mg should be the starting amount. Unfortunately, when the note was written a decimal point got misplaced and he got 25 mg as a first dose. He ended up very sedated for most of the day but his breathing was ok.
The mistake was not discussed with the patient or the patient's family. While it did not cause any lasting harm, I wondered if telling the patient/patient's family that an error had been made would have been more ethically sound.
When we presented this case in our conferences, there was little controversy about whether the error should have been disclosed. The discussion did provide reinforcement for doing a simple but difficult task. Our analysis is that the nondiscussion of this error reflects a deficit in attitude and possibly skill. The team was aware of the error, but the resident and attending did not take the opportunity to disclose an error. They should have. We do not know whether the attending or resident felt unprepared to discuss this or were simply unimpressed with the adverse event. We do get the sense that the student did not feel comfortable raising the issue with the team. As such, it was a missed opportunity to seek help from any number of hospital resources and find encouragement to take on difficult encounters.
Much has been written about apologies.810 Disclosing errors and apologizing is the ethical standard, and many of our institutions have made it policy. Yet in the moment, it is embarrassing, anxiety provoking, and our concern about litigation looms large. Learning to do the right thing begins, perhaps with lectures and standardized patients, but only when students see it modeled by our housestaff and faculty, does it take root for good.
Our housestaff are quite good at managing medical issues, but they may still need help in creating the appropriate environment for professional learning and growth. This is 1 of the most important contributions an attending can make. We have emphasized that faculty have an important role to play in the area of professional development, reinforcing the rudimentary information preclinical students are presented with in the classroom and processing experiences residents are exposed to on a regular basis. If the hospital doesn't wait, then it is the attending physician's job to create the space and time for trainees to think about what is happening and ask if it could have been done better.
A number of seasoned clinical teachers have written about ways to improve teaching on the wards.11 Below, we will add to that discussion by considering practical ways to enhance learning about professionalism and ethics (see Table 1). Note should be made that while we focus on specific behaviors and activities, underlying all is the importance of availability, presence, and intention. Like all good teaching, these activities require planning and effort.
| Attending Activity | Examples |
|---|---|
| Creating an Open Climate | |
| Breaking Communication Barriers | Setting aside time for introductions and team building exercises at the beginning of a rotation, with attending participating equally with residents and students |
| Emphasizing attending availability to discuss or review problems of any kind | |
| Setting Clear Expectations | Emphasizing the importance of patient‐clinician or family‐clinician communication from the outset |
| Devoting some attending rounds to Difficult Conversations (e.g., breaking bad news or code status discussions) | |
| Explicitly stating that no ethical question is a stupid question and providing positive feedback for raising such questions for the team | |
| Regular Check‐ins | Establishing team communication rounds: 10 minutes every day to review a good, bad, or awkward interaction from the past day (e.g., family meeting, DNR discussion) |
| Setting aside time on rounds or during attending teaching sessions to explore the team's or an individual's emotional responses to a patient's death or deterioration | |
| Writing exercises that focus on our reactions to challenging situations that are shared with the group | |
| Supervision and Modeling | |
| Planning | Clarifying an agenda and practicing key phrases for a family meeting with the resident prior to meeting the family |
| Anticipating which patients may require a code status discussion and discussing a game plan on rounds | |
| Modeling | Students observe the attending facilitate a family meeting |
| Residents observe the attending apologizing for an error, no matter how small | |
| Attending thinks about an interpersonal conflict out loud and models asking patient‐relations for help | |
| Debriefing | Reviewing a family meeting with and giving feedback to the resident who facilitated |
| Reviewing a challenging code status discussion as a team |
Creating an Open Climate
The medical team, of which the attending, residents, and students are all a part, should not only be a unit that provides excellent medical care to its patients, but should also create a culture of continuous learning and improvement. As such, it is important to create a safe atmosphere where teachers are invested in the growth of their learners and learners feel free to question the prevailing logic and practice, including issues of professionalism and ethics. As Malcolm Gladwell12 describes in Outliers, Korean Air jets were crashing because subordinates were afraid to question their superiors. Once that culture changed, Korean Air safety improved dramatically. Similarly, breaking down some of the hierarchical barriers should improve the culture of a medical team. We typically make an effort to get to know our students and residents on a more personal basis: where they are from, who is in their family, what was their major, what are their interests outside of medicine, and what has been surprising to them in their training so far. Whether we set aside time when we first meet or e‐mail our questions before the first day, we aim for this to be 1 of the first team activities. We also share our own stories, making clear that the attending is part of the team, and not just an evaluating supervisor.
Vignette 1 describes the student's trauma of witnessing a code and the inability to process the event with anyone afterward. Failed resuscitation attempts are the most dramatic examples, but even expected deaths, nonfatal adverse events, and conflict between patients and providers may be traumatic for new trainees inexperienced with the reality of medicine. Attendings should be aware of these potentially traumatic events and make time to check in with the team members about how they are dealing with their emotions. Taking time on attending rounds, for example, allows the attending to not only model reflective practice and self‐care, but also elevates team support to a place traditionally reserved for discussions about diagnosis and treatment.
Supervision and Modeling
Vignettes 2 and 3 center around challenging communication tasks that require special training, including instruction, modeling, feedback, and practice. Unfortunately, as some of our student accounts document, many teaching opportunities are missed. As attendings, our duties include being aware of these opportunities, and being prepared to model competent patientor familydoctor interactions. Emphasizing the importance of the doctor‐patient relationship is in fact one of the key skills of an effective attending role model.13
When opportunities arise for any potentially difficult conversation, we make every effort to identify the issue, prebrief with the team about how to conduct the discussion, and either offer to model the conversation or be present to observe and provide feedback and debriefing afterwards. For example, by asking about all DNR discussions had with our patients, we gain insight into the skill level of our housestaff. As important, the housestaff understand that we believe that these conversations are vital to review during formal rounds, with the same attention we give to chest pain and electrocardiograms (ECGs).
Two key skills that develop with experience are the ability to know the limits of one's knowledge and to know when to ask for help. We try to be open about naming those limits and thinking about the other members of the larger healthcare team that may provide insight, skill, and expertise. We are used to doing this with medical questions (eg, asking the gastroenterology consult team to locate a source of bleeding). Asking our risk management, patient‐relations, or ethics services to assist with a difficult communication task or conflict with a family is no different, and often something the housestaff may not readily do.
We are grateful to our students and their postcards for the snapshots of our local medical culture. While we are gratified to read of excellent role modeling, we are also disappointed to read of situations which have left our students confused, demoralized and cynical. But if these exercises are to reach their full potential, they should tell us about where we would like to go, in addition to where we have been. We believe that our conferences have stimulated our faculty and housestaff to reflect on the professionalism lessons they are teaching. Reading the student postings has definitely affected our approach to teaching professionalism. They reinforce what every parent and educator knows: when it comes to teaching professionalism, communication and ethics, what matters most is the behavior of the teacher. Our words mean little if our actions do not live out what we espouse.
Acknowledgements
We are grateful for Michael Chan and his classmates from the NUFSM class of 2010 for their thoughtful essays. David Neely, Director of Undergraduate Education, Department of Medicine, Eytan Szmuilowicz, Palliative Medicine. Kathy Neely, Chairman of NMH Ethics Committee. Co‐director of Patient, Physician and Society.
During their junior medicine rotation, our students are asked to post to Blackboard (an online student forum) an anonymous essay about an issue of professionalism or ethics, either inspiring or troubling. In many ways, these vignettes are like postcards, written by visitors describing foreign cultures and norms. They represent a way for the students to debrief, but also provide an opportunity for us, as faculty, to reflect upon the way we practice and teach medicine. Many postingslike postcards from exotic or historic placesare inspiring stories of residents and faculty extending themselves for their patients. Unfortunately, unlike typical postcards, there are also essays that are troubling or provoking and challenge us to consider how we could improve the professional and ethical environment on our teams.
In order to begin a learning process with our faculty and housestaff, we have presented a number of these anonymous essays at both faculty and housestaff Department of Medicine conferences as well as our monthly hospital Ethics conference. The goal of these conferences was to gather as a moral community to reflect on our students' experience and consider ways in which our day to day practice as attendings could be informed by what they tell us. In addition, the junior medicine site directors have a session each quarter with their junior students to review some of the most significant issues brought up by their essays.
Practically, these vignettes and conferences serve three main purposes:
-
Raising Awareness: Many professional issues noted by our students occur under the radar. Attendings are often unaware of the issues of professionalism and/or ethics confronting our students and housestaff.
-
Exploring Attitudes: Some attending may underemphasize the importance of specific issues of professionalism and/or ethics. Open discussions at faculty or resident conferences create opportunities for individuals to reflect upon their own reactions and for the group to create a norm.
-
Sharing Skills: It is difficult to learn the practice of professionalism and ethics from a book. Skill in this area is gained primarily by experience. Conferences provide an excellent forum for seasoned physicians to share wisdom with less experienced physicians. In addition, important teaching points can be made: Students should not deliver bad news alone. Errors should be disclosed.
Following are 3 of the essays we presented, along with brief commentaries. At the end, we provide practical suggestions for individual attendings to improve the professional climate on their teams.
The Hospital Didn't Wait
Code. On 12, the surgical wards floor. Elise sprinted to the stairwell, dashed up to 12, and ran to the corner room as fast as she could. She could see the room before she got there. Instinctively, she started reviewing the steps she had memorized so many months ago. But when she finally arrived at the patient's bathroom, her thought process came to a jolting halt as she came upon the gruesome scene.
The 76‐year‐old patient had hanged himself with the cinching rope from his garment bag, and now dangled suspended from a high towel rack against the wall. Nurses from the floor started to file in, and without losing a beat Elise barked commands. Together they brought the man's body down to the floor, laid him on his back, and stripped off his hospital gown. Elise was in charge; deliberately but forcefully, she ordered a nurse to retrieve a defibrillator, and had another resident check for a pulse. There was none. Anesthesiology was here. Quickly and expertly, they shoved a plastic tube down his throat and began ventilation. The nurse placed on the electrodes between chest compressions then called to clear the body. Airway stepped back. The chest pumper stepped back. The body lurched forward as the defibrillator issued a long beep and discharged. Still no pulse. The cycle repeated.
Finally, Elise called a stop. Time of death, 19:37. By now there were about 20 people crammed in the patient room, all of whom had a separate role during the code. Some stayed behind, while the rest left to return to their interrupted work. The medical student didn't know what to think as he returned to the team room. His jaw was sorehad he been clenching it the whole time?and as he brought his hand up to rub his face, he saw that his knuckles were bloody. Somehow he had scraped them during the code. As he logged back into the computer to finish off his evening notes, he knew that he wouldn't have time to reflect until hours later when he returned home. Codes happened all the time. There was still work to be done in the hospital, and the hospital didn't wait.
The room had already been assigned to a patient waiting in the Emergency Department downstairs. That patient would be here in a few minutes. The hospital didn't wait.
When we presented this case in our conferences, there was universal agreement that such a traumatic event merits, even demands, team debriefing and processing. But in the real life aftermath of this traumatic event, the take‐home message for the medical student was that the hospital didn't wait for such discussions. We know this is not unique to our institution. In a study of 32 medical students who were asked to reflect on their most memorable patient death,1 debriefing sessions were rare and many students felt inadequately supported. While experienced clinicians may be accustomed to seeing patients die, students are new to the culture of the hospital, and have not had the chance to develop the defense mechanisms necessary to cope with this sort of experience. Angoff2 writes, As medical educators, we ought to ask our students how they are coping with long hours, fatigue, illness, suffering, and death. We ought to model and commend compassion and react to the deep feelings of our students in the same way we would teach them to react to the deep feelings of their patients.
I Told a Man Today That He Had Brain Cancer
The resident, intern, and I were huddled together in our team room when the report came back on the computer. New 3.5 2.3 1.7 cm contrast‐enhancing lesion seen anterior to genu of corpus callosum. Concerning for metastatic focus vs. lymphoma. Advise follow‐up. It wasn't unexpected but we had nevertheless been hoping for better.
The three of us went into his room and I was waiting to see how my resident would deliver the bad news, but she didn't. She simply said that we were continuing to do imaging studies and that a neurology team would be in touch. There were probably several reasons why she didn't tell him: not enough time, not her responsibility, or maybe she was just uncomfortable with it. Whatever the case, we left the room with my patient still oblivious to the awful mass now tangled in his head.
If my resident was taking a pass on this conversation, I knew it fell to me he needed to hear it from his primary team. I came back after rounds alone, sat down next to his bed, and told him that his MRI results had come back, and that I had unfortunate news.
I told him that the images showed that his lung cancer had spread to his brain.
I paused to give him a chance to let it sink in. He turned away and looked up at the ceiling.
Where is it? How big is it?
What now?
Reflecting on this case, our audiences were disturbed that a student would attempt this difficult conversation alone, while recognizing that the student clearly felt a sense of responsibility and desire to help his patient by sharing important information. We talked about how students may erroneously pick up a message that the team member who has spent the most time with a patient is the most obvious choice to have difficult conversations. We also noted that, unfortunately, sometimes students are directly asked by their team to shoulder this responsibility on their own. In this painful account, there is no mention of preparation, supervision, or support for the student before or after the encounter. The student perceived (rightly or wrongly) that the team leaders lacked comfort or skill to deliver the bad news, and stepped in. It is possible that the attending lacked the skill and ability to model an interaction, but more likely the deficit was in awareness and attitude. It is unlikely the attending knew that the student had this conversation alone. One of the major reasons we present these vignettes is to make attendings and housestaff more aware of issues that occur under their radar so that they can take preventative action. However, once the resident or attending found out that the student had this conversation alone, the student should be pulled aside for a 1:1 discussion. At the end of the day, the student should know that it was inappropriate to attempt this conversation alone
Rosenbaum3 reviewed a number of strategies to teach the skill of delivering bad news, from lecture and small group discussions to role play and standardized patients. When asked, students cited role‐modeling as the best way to learn how to deliver bad news.4 Observation of a veteran clinician provides a firm foundation for learning; but that is not enough. Unfortunately, we know from the literature (and our student vignettes suggest) that students and residents are unprepared to carry out these conversations properly, either because of misguided attitudes, lack of experience, or inadequate training.57 We conceptualize engaging in difficult conversations as a procedure, demanding a skill set. Mere observation of an expert executing this procedure is only a beginning. With any other skill, from successful completion of a lumbar puncture to initiating cardiopulmonary resuscitation (CPR), a student would never conclude that knowing the patient the best sufficiently credentials the student to undertake these procedures. We maintain that a difficult conversationbe it breaking bad news, discussing end‐of‐life care preferences, code status discussions, or prognosisis a clinical intervention, like any other procedure in medicine. If performed with skill and caution, it can bring about a stronger therapeutic relationship and increased support for the patient; if performed clumsily, it can lead to unintended adverse outcomes, including misunderstanding, mistrust, anxiety, and anger.
A Decimal Point Got Misplaced
On palliative care, I had a 90 year‐old man with end stage lung CA that presented to the ED with increasing SOB. The resident decided that giving him some morphine would be a good solution but was worried that too much would push him over the edge. He was thin; his O2 sats weren't that good After some discussion it was decided that 2.5 mg should be the starting amount. Unfortunately, when the note was written a decimal point got misplaced and he got 25 mg as a first dose. He ended up very sedated for most of the day but his breathing was ok.
The mistake was not discussed with the patient or the patient's family. While it did not cause any lasting harm, I wondered if telling the patient/patient's family that an error had been made would have been more ethically sound.
When we presented this case in our conferences, there was little controversy about whether the error should have been disclosed. The discussion did provide reinforcement for doing a simple but difficult task. Our analysis is that the nondiscussion of this error reflects a deficit in attitude and possibly skill. The team was aware of the error, but the resident and attending did not take the opportunity to disclose an error. They should have. We do not know whether the attending or resident felt unprepared to discuss this or were simply unimpressed with the adverse event. We do get the sense that the student did not feel comfortable raising the issue with the team. As such, it was a missed opportunity to seek help from any number of hospital resources and find encouragement to take on difficult encounters.
Much has been written about apologies.810 Disclosing errors and apologizing is the ethical standard, and many of our institutions have made it policy. Yet in the moment, it is embarrassing, anxiety provoking, and our concern about litigation looms large. Learning to do the right thing begins, perhaps with lectures and standardized patients, but only when students see it modeled by our housestaff and faculty, does it take root for good.
Our housestaff are quite good at managing medical issues, but they may still need help in creating the appropriate environment for professional learning and growth. This is 1 of the most important contributions an attending can make. We have emphasized that faculty have an important role to play in the area of professional development, reinforcing the rudimentary information preclinical students are presented with in the classroom and processing experiences residents are exposed to on a regular basis. If the hospital doesn't wait, then it is the attending physician's job to create the space and time for trainees to think about what is happening and ask if it could have been done better.
A number of seasoned clinical teachers have written about ways to improve teaching on the wards.11 Below, we will add to that discussion by considering practical ways to enhance learning about professionalism and ethics (see Table 1). Note should be made that while we focus on specific behaviors and activities, underlying all is the importance of availability, presence, and intention. Like all good teaching, these activities require planning and effort.
| Attending Activity | Examples |
|---|---|
| Creating an Open Climate | |
| Breaking Communication Barriers | Setting aside time for introductions and team building exercises at the beginning of a rotation, with attending participating equally with residents and students |
| Emphasizing attending availability to discuss or review problems of any kind | |
| Setting Clear Expectations | Emphasizing the importance of patient‐clinician or family‐clinician communication from the outset |
| Devoting some attending rounds to Difficult Conversations (e.g., breaking bad news or code status discussions) | |
| Explicitly stating that no ethical question is a stupid question and providing positive feedback for raising such questions for the team | |
| Regular Check‐ins | Establishing team communication rounds: 10 minutes every day to review a good, bad, or awkward interaction from the past day (e.g., family meeting, DNR discussion) |
| Setting aside time on rounds or during attending teaching sessions to explore the team's or an individual's emotional responses to a patient's death or deterioration | |
| Writing exercises that focus on our reactions to challenging situations that are shared with the group | |
| Supervision and Modeling | |
| Planning | Clarifying an agenda and practicing key phrases for a family meeting with the resident prior to meeting the family |
| Anticipating which patients may require a code status discussion and discussing a game plan on rounds | |
| Modeling | Students observe the attending facilitate a family meeting |
| Residents observe the attending apologizing for an error, no matter how small | |
| Attending thinks about an interpersonal conflict out loud and models asking patient‐relations for help | |
| Debriefing | Reviewing a family meeting with and giving feedback to the resident who facilitated |
| Reviewing a challenging code status discussion as a team |
Creating an Open Climate
The medical team, of which the attending, residents, and students are all a part, should not only be a unit that provides excellent medical care to its patients, but should also create a culture of continuous learning and improvement. As such, it is important to create a safe atmosphere where teachers are invested in the growth of their learners and learners feel free to question the prevailing logic and practice, including issues of professionalism and ethics. As Malcolm Gladwell12 describes in Outliers, Korean Air jets were crashing because subordinates were afraid to question their superiors. Once that culture changed, Korean Air safety improved dramatically. Similarly, breaking down some of the hierarchical barriers should improve the culture of a medical team. We typically make an effort to get to know our students and residents on a more personal basis: where they are from, who is in their family, what was their major, what are their interests outside of medicine, and what has been surprising to them in their training so far. Whether we set aside time when we first meet or e‐mail our questions before the first day, we aim for this to be 1 of the first team activities. We also share our own stories, making clear that the attending is part of the team, and not just an evaluating supervisor.
Vignette 1 describes the student's trauma of witnessing a code and the inability to process the event with anyone afterward. Failed resuscitation attempts are the most dramatic examples, but even expected deaths, nonfatal adverse events, and conflict between patients and providers may be traumatic for new trainees inexperienced with the reality of medicine. Attendings should be aware of these potentially traumatic events and make time to check in with the team members about how they are dealing with their emotions. Taking time on attending rounds, for example, allows the attending to not only model reflective practice and self‐care, but also elevates team support to a place traditionally reserved for discussions about diagnosis and treatment.
Supervision and Modeling
Vignettes 2 and 3 center around challenging communication tasks that require special training, including instruction, modeling, feedback, and practice. Unfortunately, as some of our student accounts document, many teaching opportunities are missed. As attendings, our duties include being aware of these opportunities, and being prepared to model competent patientor familydoctor interactions. Emphasizing the importance of the doctor‐patient relationship is in fact one of the key skills of an effective attending role model.13
When opportunities arise for any potentially difficult conversation, we make every effort to identify the issue, prebrief with the team about how to conduct the discussion, and either offer to model the conversation or be present to observe and provide feedback and debriefing afterwards. For example, by asking about all DNR discussions had with our patients, we gain insight into the skill level of our housestaff. As important, the housestaff understand that we believe that these conversations are vital to review during formal rounds, with the same attention we give to chest pain and electrocardiograms (ECGs).
Two key skills that develop with experience are the ability to know the limits of one's knowledge and to know when to ask for help. We try to be open about naming those limits and thinking about the other members of the larger healthcare team that may provide insight, skill, and expertise. We are used to doing this with medical questions (eg, asking the gastroenterology consult team to locate a source of bleeding). Asking our risk management, patient‐relations, or ethics services to assist with a difficult communication task or conflict with a family is no different, and often something the housestaff may not readily do.
We are grateful to our students and their postcards for the snapshots of our local medical culture. While we are gratified to read of excellent role modeling, we are also disappointed to read of situations which have left our students confused, demoralized and cynical. But if these exercises are to reach their full potential, they should tell us about where we would like to go, in addition to where we have been. We believe that our conferences have stimulated our faculty and housestaff to reflect on the professionalism lessons they are teaching. Reading the student postings has definitely affected our approach to teaching professionalism. They reinforce what every parent and educator knows: when it comes to teaching professionalism, communication and ethics, what matters most is the behavior of the teacher. Our words mean little if our actions do not live out what we espouse.
Acknowledgements
We are grateful for Michael Chan and his classmates from the NUFSM class of 2010 for their thoughtful essays. David Neely, Director of Undergraduate Education, Department of Medicine, Eytan Szmuilowicz, Palliative Medicine. Kathy Neely, Chairman of NMH Ethics Committee. Co‐director of Patient, Physician and Society.
- .This is just too awful; I just can't believe I experienced that.Acad Med.2005;80(7):634–640.
- .A piece of my mind.JAMA.2001;286(9):1017–1018.
- .Teaching medical students and residents skills for delivering bad news: a review of strategies.Acad Med.2004;79(2):107–117.
- .Third‐year medical students' experiences with dying patients during the internal medicine clerkship: a qualitative study of the informal curriculum.Acad Med.2005;80(7):641–647.
- ,,.How do medical residents discuss resuscitation with patients? Official journal of the Society for Research and Education in Primary Care Internal Medicine.J Gen Intern Med.1995;10(8):436–442.
- ,,.See one, do one, teach one? House staff experience discussing do‐not‐resuscitate orders.Arch Intern Med.1996;156(12):1285–1289.
- ,,,.Residents' end‐of‐life decision making with adult hospitalized patients: a review of the literature.Acad Med.2005:80(7)622–633.
- .Physician error and disclosure.Clin Obstet Gynecol.2008;51(4):700–708.
- .Revealing medical errors to your patients.Chest.2009;133:1064–1065.
- .Apology in medical practice.JAMA.2006;296:1401–1404.
- .What if Osler were one of us? Inpatient teaching today.J Gen Intern Med.1997;12(Suppl 2):S41–S48.
- Malcolm Gladwell.Outliers.New York:Little, Brown, Co.,2008: p.177–223.
- ,,,,.Attributes of excellent attending‐physician role models.N Engl J Med.1998;339(27):1986–1993.
- .This is just too awful; I just can't believe I experienced that.Acad Med.2005;80(7):634–640.
- .A piece of my mind.JAMA.2001;286(9):1017–1018.
- .Teaching medical students and residents skills for delivering bad news: a review of strategies.Acad Med.2004;79(2):107–117.
- .Third‐year medical students' experiences with dying patients during the internal medicine clerkship: a qualitative study of the informal curriculum.Acad Med.2005;80(7):641–647.
- ,,.How do medical residents discuss resuscitation with patients? Official journal of the Society for Research and Education in Primary Care Internal Medicine.J Gen Intern Med.1995;10(8):436–442.
- ,,.See one, do one, teach one? House staff experience discussing do‐not‐resuscitate orders.Arch Intern Med.1996;156(12):1285–1289.
- ,,,.Residents' end‐of‐life decision making with adult hospitalized patients: a review of the literature.Acad Med.2005:80(7)622–633.
- .Physician error and disclosure.Clin Obstet Gynecol.2008;51(4):700–708.
- .Revealing medical errors to your patients.Chest.2009;133:1064–1065.
- .Apology in medical practice.JAMA.2006;296:1401–1404.
- .What if Osler were one of us? Inpatient teaching today.J Gen Intern Med.1997;12(Suppl 2):S41–S48.
- Malcolm Gladwell.Outliers.New York:Little, Brown, Co.,2008: p.177–223.
- ,,,,.Attributes of excellent attending‐physician role models.N Engl J Med.1998;339(27):1986–1993.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www.blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www.blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www.blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Severe babesiosis
An 85‐year‐old male from rural southern New Jersey with history of innumerable tick bites over many years was admitted for fever of unknown origin. On hospital day 2, his hemoglobin dropped from 11.3 g/dL to 7.5 g/dL, with an associated elevated indirect bilirubin and lactate dehydrogenase. Blood smear showed numerous intracellular and extracellular trophozoites, with approximately 10% to 30% of red cells infected, consistent with severe babesiosis. Given his high parasitemia, new hypoxia, lethargy, and advanced age, treatment was initiated with intravenous antibiotics and red cell exchange transfusion.
Babesiosis should be considered on the differential diagnosis of hemolytic anemia in patients that live in or have traveled to endemic areas, especially with history of tick bites. The most common appearance on blood smear is round to oval rings with pale blue cytoplasm and a red‐staining nucleus (Fig. 1). Exoerythrocytic parasites or the pathognomonic Maltese Cross tetrad forms (not present in our patient's smear) help to differentiate from falciparum malaria.
The patient's parasite burden and clinical status markedly improved with treatment and he was discharged home. 0
An 85‐year‐old male from rural southern New Jersey with history of innumerable tick bites over many years was admitted for fever of unknown origin. On hospital day 2, his hemoglobin dropped from 11.3 g/dL to 7.5 g/dL, with an associated elevated indirect bilirubin and lactate dehydrogenase. Blood smear showed numerous intracellular and extracellular trophozoites, with approximately 10% to 30% of red cells infected, consistent with severe babesiosis. Given his high parasitemia, new hypoxia, lethargy, and advanced age, treatment was initiated with intravenous antibiotics and red cell exchange transfusion.
Babesiosis should be considered on the differential diagnosis of hemolytic anemia in patients that live in or have traveled to endemic areas, especially with history of tick bites. The most common appearance on blood smear is round to oval rings with pale blue cytoplasm and a red‐staining nucleus (Fig. 1). Exoerythrocytic parasites or the pathognomonic Maltese Cross tetrad forms (not present in our patient's smear) help to differentiate from falciparum malaria.
The patient's parasite burden and clinical status markedly improved with treatment and he was discharged home. 0

An 85‐year‐old male from rural southern New Jersey with history of innumerable tick bites over many years was admitted for fever of unknown origin. On hospital day 2, his hemoglobin dropped from 11.3 g/dL to 7.5 g/dL, with an associated elevated indirect bilirubin and lactate dehydrogenase. Blood smear showed numerous intracellular and extracellular trophozoites, with approximately 10% to 30% of red cells infected, consistent with severe babesiosis. Given his high parasitemia, new hypoxia, lethargy, and advanced age, treatment was initiated with intravenous antibiotics and red cell exchange transfusion.
Babesiosis should be considered on the differential diagnosis of hemolytic anemia in patients that live in or have traveled to endemic areas, especially with history of tick bites. The most common appearance on blood smear is round to oval rings with pale blue cytoplasm and a red‐staining nucleus (Fig. 1). Exoerythrocytic parasites or the pathognomonic Maltese Cross tetrad forms (not present in our patient's smear) help to differentiate from falciparum malaria.
The patient's parasite burden and clinical status markedly improved with treatment and he was discharged home. 0

Quality of Hospital Communication
It is well established that patients have difficulty understanding written health materials,1 medical terminology,2, 3 and other aspects of provider‐patient communication.4, 5 Such difficulties in communication can be magnified at transitions of care like hospital discharge.6 Patients often receive a large amount of information in a short period of time at discharge, and this information may be delivered in a way that is not straightforward or standardized.7, 8 When asked, patients commonly report a poor understanding of important self‐care instructions such as how to take medications upon returning home.9, 10 One study even showed that more than half of patients did not recall anyone providing instructions about how they should care for themselves after hospitalization.11 Poor medication management after hospital discharge contributes to adverse events,1215 inadequate disease control,16 and in the setting of cardiovascular disease, higher mortality.17, 18 Most adverse events after hospital discharge could be prevented or ameliorated through relatively simple means, including better communication among patients and providers.6, 1416, 1921 Greater attention to communication and care transitions could also reduce the number of unplanned rehospitalizations in the United States.22
Patients' health literacy is an important factor in effective health communication, yet little research has examined the role of health literacy in care transitions. Health literacy is defined as the extent to which an individual is able to obtain, process and understand basic health information and services needed to make appropriate health decisions.23, 24 Low health literacy is a prevalent problem in the United States, affecting approximately 40% of adults.25 Research has shown that low health literacy is associated with low self‐efficacy26 and less interaction in physician‐patient encounters,27 which in combination with physicians' use of complex medical language,28 may contribute to poor physician‐patient communication. Patients with low health literacy also have greater difficulty understanding prescription drug labels,29 limited knowledge of disease self‐management skills,30 a higher incidence of hospitalization,31 and higher mortality rates.3234
In order to elucidate the relationship between patient‐provider communication and health literacy in the hospital setting, we analyzed patients' ratings of their communication experience during their hospitalization. We report patients' perceptions of the clarity of communication and how this may vary by level of health literacy and other important patient characteristics.
Methods
Setting and Participants
Patients admitted to the general medical wards at Grady Memorial Hospital were recruited for participation. Grady Memorial Hospital is a public, urban teaching hospital located in Atlanta, GA. It serves a primarily low income, African American population, many of whom lack health insurance. Approximately 30% to 50% of patients at this hospital have inadequate health literacy skills.35
The present study was conducted as preliminary research for a randomized controlled trial to improve post‐discharge medication adherence among patients with acute coronary syndromes (ACS). The criteria for the present study mirrored those of the planned trial. Patients were eligible for the current study if they were admitted with suspected ACS and evidence of myocardial ischemia.36 Exclusion criteria included lack of cooperation/refusal to participate, unintelligible speech (eg, dysarthria), lack of English fluency (determined subjectively by interviewer), delirium (determined by lack of orientation to person, place, and time), severe hearing impairment (determined subjectively by interviewer), visual acuity worse than 20/60 (per pocket vision screening card), acute psychotic illness (per admission history), police custody, age younger than 18 years, no regular telephone number, administration of all medications by a caregiver, and not taking prescription medications in the 6 months before admission.
Data Collection and Measures
Enrollment occurred between August 2005 and April 2006, after approval was obtained from both the Emory University Institutional Review Board (IRB) and Grady Research Oversight Committee. Interested and willing participants provided written informed consent and subsequently completed an interviewer‐assisted questionnaire prior to hospital discharge to collect information regarding demographics and cardiovascular risk factors. To ensure that answers were not confounded by participants' inability to read the questionnaire text, all questions were read to participants by study interviewers, with the exception of the health literacy assessmentthe Rapid Estimate of Adult Literacy in Medicine (REALM).37 The REALM classifies a patient's literacy according to the number of medical terms from a list that the patient pronounces correctly. It correlates highly with other assessments of literacy and health literacy.38 Cognitive function was measured using the Mini‐Mental State Examination (MMSE).39
Research staff contacted patients by telephone approximately 2 weeks after hospital discharge to complete a survey which included the Interpersonal Processes of Care in Diverse Populations Questionnaire (IPC).40 The IPC is a validated, self‐report questionnaire with high internal consistency reliability. It was developed and normalized among ethnically diverse populations of low socioeconomic status. Items on the IPC originally referred to communication during the last 6 months in the outpatient clinic; they were reworded to refer to the recent hospitalization only. The research assistant administered 8 of 12 domains of the IPC that were most pertinent to rating the quality and clarity of patient communication with hospital physicians.41 Four other IPC domains that pertained to interpersonal style (eg, friendliness, emotional support) were not administered to minimize response burden. Each domain was comprised of 2 to 7 items, and responses were given on a 5‐point Likert scale. The 8 domains and sample items were as follows: (1) General clarity (eg, Did the doctors use medical words that you did not understand?); (2) Elicitation of and responsiveness to patient problems, concerns, and expectations (eg, Did the doctors listen carefully to what you had to say?); (3) Explanations of condition, progress, and prognosis (eg, Did the doctors make sure you understand your health problem?); (4) Explanations of processes of care (eg, Did the doctors explain why a test was being done?); (5) Explanations of self‐care (eg, Did the doctors tell you what you could do to take care of yourself at home?); (6) Empowerment (eg, Did the doctors make you feel that following your treatment plan would make a difference in your health?); (7) Decision‐making: responsiveness to patient preferences regarding decisions (eg, Did the doctors try to involve you or include you in decisions about your treatment?); and (8) Consideration of patient's desire and ability to comply with recommendations (eg, Did the doctors understand the kinds of problems you might have in doing the recommended treatment?).
Statistical Analysis
Patient characteristics were summarized using frequency, mean, and standard deviation measures. Nondichotomous measures were recategorized into dichotomous variables as follows: age (less than 55 years vs. 55 years or older), race (black vs. white or other), marital status (married or living with someone vs. living alone), education (less than high school vs. high school graduate), employment status (employed full/part time vs. unemployed/retired), MMSE score (cognitively impaired [MMSE score 24] vs. no significant cognitive impairment [MMSE score >24]),39 and health literacy score (inadequate [REALM score 0 to 44] vs. marginal or adequate [REALM score 45‐66]).38 Dichotomous variables were summarized using frequencies.
Scores for each individual IPC question ranged from 1 to 5 with lower scores indicating better communication, except for questions in the domain of general clarity where higher scores indicated better communication. Then, for each of the 8 domains, scores of the individual IPC questions within that domain were averaged.
Bivariate analyses were conducted for each of the 8 IPC domains, by level of health literacy and other relevant patient characteristics, using the independent samples t‐test. Multivariable linear regression models were then constructed to examine the independent association of health literacy with each of the 8 IPC domains, while controlling for other patient characteristics that were also found to be associated with IPC domain scores. Bivariate analyses were also conducted for each of the 27 individual IPC items, to gain an understanding of which items might be driving the overall effect. A 2‐sided P < 0.05 was considered statistically significant. All analyses were performed using SPSS 15 for Windows (SPSS, Chicago, IL).
Results
Patient Characteristics
A total of 109 eligible patients were approached, 100 agreed to participate and were enrolled in the hospital, and 84 of them completed the follow‐up interview by telephone to comprise the sample for this study (Table 1). Most of the 84 participants were under the age of 55 (54%), male (58%), African American (88%), unemployed (79%), lived alone (73%), and had completed high school (62%). Age ranged from 24 to 80 years, REALM score ranged from 0 to 66, and MMSE ranged from 12 to 30. A large proportion (44%) had inadequate health literacy skills, and 50% had cognitive impairment. Patients with inadequate health literacy were more likely to have not finished high school and to suffer cognitive impairment, P < 0.01 for each comparison.
| Characteristic | n (%) |
|---|---|
| Age | |
| <55 years | 45 (54) |
| 55 years | 39 (46) |
| Gender | |
| Male | 49 (58) |
| Female | 35 (42) |
| Race | |
| Black | 74 (88) |
| White or other | 10 (12) |
| Marital status | |
| Married or living with someone | 23 (27) |
| Living alone | 61 (73) |
| Education | |
| Did not complete high school | 32 (38) |
| High school graduate | 52 (62) |
| Employment status | |
| Employed (full/part time) | 18 (21) |
| Not employed | 66 (79) |
| Mini‐Mental State Exam | |
| Cognition impaired | 42 (50) |
| Cognition not impaired | 42 (50) |
| Health literacy | |
| Inadequate | 37 (44) |
| Marginal or adequate | 47 (56) |
Hospital Communication Ratings by IPC Domains
Overall, patients' ratings of hospital communication were positive, with most IPC domain score means lying in the favorable half of the Likert scale (Table 2). The domains with the best communication ratings were responsiveness to patient concerns (mean = 1.68), explanations of condition and prognosis (mean = 1.75), and empowerment (mean 1.76). The domain of worst performance was consideration of patients' desire and ability to comply with recommendations (mean = 3.15).
| IPC Domain | Total (n = 84), Mean (SD) | Patients with Inadequate Literacy (n = 37), Mean (SD) | Patients with Marginal or Adequate Literacy (n = 47), Mean (SD) | P Value | |
|---|---|---|---|---|---|
| |||||
| 1 | General clarity* | 3.66 (1.00) | 3.36 (1.14) | 3.89 (0.74) | 0.02 |
| 2 | Responsiveness to patient concerns | 1.68 (0.68) | 1.86 (0.76) | 1.53 (0.58) | 0.03 |
| 3 | Explanations of condition and prognosis | 1.75 (0.87) | 1.93 (0.99) | 1.61 (0.74) | 0.09 |
| 4 | Explanations of processes of care | 2.01 (0.86) | 2.22 (0.96) | 1.84 (0.74) | 0.04 |
| 5 | Explanations of self‐care | 2.37 (1.04) | 2.42 (1.20) | 2.33 (0.90) | 0.71 |
| 6 | Empowerment | 1.76 (1.03) | 1.85 (1.27) | 1.69 (0.81) | 0.51 |
| 7 | Decision‐making | 2.34 (0.78) | 2.34 (0.80) | 2.34 (0.77) | 1.00 |
| 8 | Consideration of patients' desire and ability to comply with recommendations | 3.15 (1.19) | 3.24 (1.16) | 3.07 (1.23) | 0.54 |
In bivariate analyses that compared IPC domains by patients' level of health literacy, several differences emerged. Patients with inadequate health literacy skills gave significantly worse ratings to the quality of communication on the domains of general clarity (mean = 3.36 vs. 3.89 for patients with marginal or adequate health literacy, P = 0.02), Responsiveness to patient concerns (mean = 1.86 vs. 1.53, P = 0.03), and Explanations of processes of care (mean = 2.22 vs. 1.84, P = 0.04). On a fourth domain, Explanations of condition and prognosis, a nonsignificant trend was present (mean = 1.93 vs. 1.61, P = 0.09).
Fewer significant relationships were found between other patient characteristics and IPC domain scores. Patients who were age 55 or older provided worse ratings on explanations of self‐care (mean = 2.74 vs. 2.05 for patients under the age of 55, P = 0.003). Lower ratings on the domain of general clarity, which indicated unclear communication, were found among patients who had not graduated from high school (mean = 3.31 vs. 3.88 for high school graduates, P = 0.02) or who had cognitive impairment (mean = 3.39 vs. 3.93 for patients without impaired cognition, P = 0.01). No significant differences were present by gender or race.
Based on these bivariate relationships, terms for inadequate health literacy, age 55, Cognitive impairment, and high school graduation were entered into multivariable models that predicted scores on each of the 8 IPC domains. Inadequate health literacy was independently associated with Responsiveness to patient concerns ( = 0.512, P = 0.007) and Explanations of processes of care ( = 0.548, P = 0.023); a nonsignificant trend was present for consideration of patients' desire and ability to comply with recommendations ( = 0.582, P = 0.09). The association of age with explanations of self‐care remained after adjustment for the other variables ( = 0.705, P = 0.002). None of the patient characteristics was independently associated with ratings of general clarity.
IPC Item Responses
Examination of responses on the individual IPC items revealed the specific areas of difficulty in communication as rated by patients (Table 3). In the domain of general clarity, patients with inadequate literacy provided poorer ratings on the item pertaining to use of medical terminology (mean = 2.92 vs. 3.68 for patients with marginal or adequate literacy, P = 0.004). Regarding Responsiveness to patient concerns, differences by literacy were present in the item that pertained to patients being given enough time to say what they thought was important (mean = 2.27 vs. 1.51, P = 0.003). On the domain of explanations of processes of care, the item rated differently by patients with inadequate literacy referred to feeling confused about their care because doctors did not explain things well (mean = 2.51 vs. 1.83, P = 0.02).
Discussion
We used a validated instrument, the IPC,40 to examine patients' ratings of the quality and clarity of hospital‐based communication. Overall, patients provided favorable ratings in many domains, including those pertaining to Responsiveness to patient concerns and Explanations of condition and prognosis. Clinicians' consideration of patients' desire and ability to comply with recommendations was rated least favorably overall. This represents an important area for improvement, particularly when considering the prevalence of nonadherence to medical therapy after hospital discharge, which may be as high as 50%.9, 42 Nonadherence after hospital discharge contributes to avoidable emergency department visits,43 hospital readmissions,44 and higher mortality.18, 45 The results of this study suggest that hospital physicians should give greater consideration to patients' preferences and problems that they may have in following the treatment recommendations.16 Future research will determine the extent to which this may enhance post‐discharge adherence.
Another important finding is that patients with inadequate health literacy rated hospital‐based communication less favorably than did patients with marginal or adequate literacy. In bivariate analyses, this effect was seen on several domains, including general clarity, Responsiveness to patient concerns, and explanations of processes of care. The latter 2 relationships persisted after adjustment for age, cognitive impairment, and educational attainment. To our knowledge, this is the first study which examines the effect of health literacy on patients' ratings of hospital‐based communication.
The majority of the literature on health communication and health literacy focuses on the outpatient setting.34, 46 However, the quality and clarity of patient‐provider communication in the hospital is also critically important. Ineffective communication in the hospital contributes to poor care transitions and post‐discharge complications. Patients commonly leave the hospital with a poor understanding of what transpired (eg, diagnoses, treatment provided, major test results) and inadequate knowledge about the self‐care activities that they must perform upon returning home (eg, medication management, physical activity, follow‐up appointments).911 Poor communication is often cited as the main underlying and remediable factor behind medical errors, adverse events, and the readmissions that commonly occur after hospital discharge.6, 16, 20 The results of this study provide complementary evidence, showing that patients often feel they have experienced suboptimal communication in the hospital setting. These findings highlight an opportunity for improvement in care transitions and patient safety, particularly among patients with inadequate health literacy.
In outpatient research that utilized the IPC, Schillinger et al.41 found that patients with inadequate functional health literacy reported significantly worse communication on the domains of general clarity, explanations of processes of care, and Explanations of condition and prognosis. Subsequent analyses by Sudore et al.47 demonstrated that patients with inadequate or marginal health literacy more often reported that physicians did not give them enough time to say what they thought was important, did not explain processes of care well, and did not ask about problems in following the recommended treatment (Table 3, IPC items 3, 12, and 26, respectively). Our findings were very similar. These relatively consistent results across studies and populations strengthen the conclusion that patients with inadequate health literacy feel their physicians do not communicate as effectively in these areas.
| IPC Items | Overall (n = 84), Mean (SD) | Inadequate Literacy (n = 37), Mean (SD) | Marginal or Adequate Literacy (n = 47), Mean (SD) | P Value |
|---|---|---|---|---|
| ||||
| General clarity* | ||||
| 1. Did the doctors use medical words you did not understand? | 3.35 (1.14) | 2.92 (1.40) | 3.68 (0.73) | 0.004 |
| 2. Did you have trouble understanding your doctors because they spoke too fast? | 3.98 (1.06) | 3.81 (1.13) | 4.11 (1.01) | 0.21 |
| Responsiveness to patient concerns | ||||
| 3. Did the doctors give you enough time to say what you thought was important? | 1.85 (1.14) | 2.27 (1.28) | 1.51 (0.88) | 0.003 |
| 4. Did the doctors listen carefully to what you had to say? | 1.62 (0.88) | 1.76 (1.04) | 1.51 (0.72) | 0.22 |
| 5. Did the doctors ignore what you told them? | 1.70 (0.92) | 1.81 (1.09) | 1.62 (0.77) | 0.38 |
| 6. Did the doctors take your concerns seriously? | 1.55 (0.92) | 1.65 (0.98) | 1.47 (0.88) | 0.38 |
| Explanations of condition and prognosis | ||||
| 7. Did the doctors give you enough information about your health problems? | 1.88 (1.11) | 2.11 (1.27) | 1.70 (0.95) | 0.11 |
| 8. Did the doctors make sure you understand your health problems? | 1.62 (0.88) | 1.76 (0.98) | 1.51 (0.78) | 0.22 |
| Explanations of processes of care | ||||
| 9. Did the doctors explain why a test was being done? | 1.70 (1.10) | 1.89 (1.24) | 1.55 (0.95) | 0.16 |
| 10. Did the doctors explain how the test was done? | 2.20 (1.35) | 2.27 (1.39) | 2.15 (1.34) | 0.69 |
| 11. Did the doctors tell you what they were doing as they examined you? | 1.99 (1.20) | 2.22 (1.34) | 1.81 (1.06) | 0.13 |
| 12. Did you feel confused about what was going on with your medical care because doctors did not explain things well? | 2.13 (1.23) | 2.51 (1.47) | 1.83 (0.92) | 0.02 |
| Explanations of self‐care | ||||
| 13. Did the doctors tell you what you could do to take care of yourself at home? | 1.67 (1.09) | 1.81 (1.29) | 1.55 (0.90) | 0.31 |
| 14. Did the doctors tell you how to pay attention to your symptoms and when to call the doctor? | 2.01 (1.38) | 2.19 (1.60) | 1.87 (1.17) | 0.32 |
| 15. Did the doctors clearly explain how to take the medicine (that is when, how much and for how long)? | 1.88 (1.36) | 2.00 (1.53) | 1.79 (1.22) | 0.48 |
| 16. Did the doctors go over all the medicines you are taking? | 2.39 (1.55) | 2.51 (1.74) | 2.30 (1.40) | 0.54 |
| 17. Did the doctors give you written instruction about how to take the medicine (other than what was on the container)? | 3.29 (1.70) | 3.05 (1.75) | 3.48 (1.66) | 0.26 |
| 18. Did the doctors tell you the reason for taking each medicine? | 2.05 (1.43) | 2.24 (1.64) | 1.89 (1.24) | 0.29 |
| 19. Did the doctors tell you about side effects you might get from your medicine? | 3.32 (1.64) | 3.11 (1.73) | 3.49 (1.56) | 0.29 |
| Empowerment | ||||
| 20. Did doctors make you feel that following your treatment plan would make a difference in your health? | 1.75 (1.07) | 1.89 (1.27) | 1.64 (0.90) | 0.31 |
| 21. Did the doctors make you feel that your everyday activities such as your diet and lifestyle would make a difference in your health? | 1.77 (1.21) | 1.81 (1.41) | 1.74 (1.03) | 0.81 |
| Decision‐making | ||||
| 22. Did the doctors try to involve you or include you in decisions about your treatment? | 2.43 (1.55) | 2.30 (1.49) | 2.53 (1.60) | 0.49 |
| 23. Did the doctors ask how you felt about different treatments? | 3.08 (1.58) | 2.89 (1.66) | 3.23 (1.51) | 0.33 |
| 24. Did the doctors make decision without taking your preferences and opinions into account? | 2.23 (1.35) | 2.34 (1.55) | 2.15 (1.20) | 0.54 |
| 25. Did you feel pressured by doctors in the hospital to have a treatment you were not sure you wanted? | 1.60 (0.97) | 1.81 (1.18) | 1.43 (0.74) | 0.09 |
| Consideration of patients' desire and ability to comply with recommendations | ||||
| 26. Did the doctors ask if you might have any problems actually doing the recommended treatment (for example taking the medication correctly)? | 3.82 (1.47) | 4.08 (1.40) | 3.62 (1.51) | 0.15 |
| 27. Did the doctors understand the kinds of problems you might have in doing the recommended treatment? | 2.43 (1.44) | 2.26 (1.52) | 2.57 (1.38) | 0.34 |
Importantly, the differences in patient responses by literacy category were driven by a few IPC items. These items pertained to physicians' use of medical terminology, the amount of time they gave patients to express their concerns, and how well they explained the patients' medical care. Training physicians to improve their communication skills in these specific areas may improve their ability to communicate effectively with patients who have limited literacy skills. Indeed, published recommendations on how to improve the clarity of verbal communication emphasize just a few major areas, including limiting the amount of medical terminology used, effectively encouraging patients to ask questions and express their concerns, and asking patients to teach‐back key points to make sure the physician has provided adequate explanation.4851 The present study provides some evidence for those recommendations, which for the most part, have been based on clinical experience and expert opinion.
There remains a need for professional education about health literacy and techniques to improve communication with patients who may have limited literacy skills. Many experts advocate clear verbal communication with all patients, so‐called Universal Precautions.52 Although 10 years have passed since the American Medical Association (AMA) called for more work in this area,53 few curricula have been described in the literature.48, 5456 The extent to which health literacy curricula have been implemented in medical schools and other professional schools is unknown. The impact of such training on the communication skills of health care providers and patient outcomes is also unclear.
The strengths of this study include a relatively good response rate and use of a validated measure to grade the quality of physician‐patient communication. This measure, the IPC, has been used previously in the context of health literacy.41 Nevertheless, certain limitations should be acknowledged. First, the study was performed at a single teaching hospital, where patients had a high prevalence of inadequate health literacy. The findings may not generalize to other institutions that serve a different patient population or to nonacademic programs. Second, communication was assessed by patient report, rather than by recording patient‐provider discussions for rating by independent observers. While patient report is inherently more subjective, patients' own perceptions about the effectiveness of health communication are arguably more important than those of independent raters, and thus, the data source may not represent a true limitation. Third, patient responses were obtained approximately 2 weeks after hospital discharge, and accordingly, they are subject to recall bias, which may be greater among those with cognitive impairment. Finally, patients were directed to rate the communication of the overall group of physicians who took care of them in the hospital. Given the academic setting, patients typically received care from a team that included medical students, interns, a resident, and an attending physician. We were not able to determine whether patients' ratings were influenced by a specific member of the team, nor how ratings may have been influenced by certain characteristics of that team member (eg, year of training, prior communication skills training, race or gender concordance, etc).
In summary, by surveying patients soon after an acute care hospitalization, we determined that certain areas held room for improvement, such as consideration of patients' desire and ability to comply with treatment recommendations. Patients with inadequate health literacy reported lower quality physician‐patient communication on several domains. They expressed particular concern about physicians' use of medical terminology, not getting enough time to express their concerns, and not receiving clear enough explanations about the medical care. Efforts are needed to improve physicians' communication skills in these areas. Such training should be evaluated to determine if it has a beneficial effect on physician communication skills and patient outcomes.
- ,,,,.The gap between patient reading comprehension and the readability of patient education materials.J Fam Pract.1990;31:533–538.
- .Differences between patients' and doctors' interpretation of some common medical terms.BMJ.1970;1:286–289.
- ,,.Patient understanding of commonly used medical vocabulary.J Fam Pract.1987;25:176–178.
- ,,,,,.Improving education for patients with low literacy skills.Am Fam Physician.1996;53:205–211.
- ,,,.Doctor‐patient communication: a review of the literature.Soc Sci Med.1995;40:903–918.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,.The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process.J Patient Saf.2007;3(2):97–106.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80(8):991–994.
- ,,.Hospital discharge information and older patients: do they get what they need?J Hosp Med.2007;2(5):291–296.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust NZ J Med.1999;29(2):220–227.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- ,,, et al.Adherence to medications by patients after acute coronary syndromes.Ann Pharmacother.2005;39(11):1792–1797.
- ,,, et al.Impact of medication therapy discontinuation on mortality after myocardial infarction.Arch Intern Med.2006;166(17):1842–1847.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,,,.Impact of patient communication problems on the risk of preventable adverse events in acute care settings.CMAJ.2008;178(12):1555–1562.
- ,,.Communication gaps and readmissions to hospital for patients aged 75 years and older: observational study.Qual Saf Health Care.2008;17(1):71–75.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- Institute of Medicine.Health Literacy. A Prescription to End Confusion.Washington, DC:National Academies Press;2004.
- ,,,.Current Bibliographies in Medicine: Health Literacy.Bethesda, MD:National Library of Medicine;2000.
- ,,. National Assessment of Adult Literacy (NAAL). A first look at the literacy of America's adults in the 21st century. Available at: http://nces.ed.gov/naal. Accessed January2010.
- ,,, et al.The health care experience of patients with low literacy.Arch Fam Med.1996;5:329–334.
- ,,,.Patient literacy and question‐asking behavior in the medical encounter: a mixed‐methods analysis.J Gen Intern Med.2007;22(6):782–786.
- ,,,.Babel babble: physicians' use of unclarified medical jargon with patients.Am J Health Behav.2007;31(Suppl 1):S85–S95.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,.Relationship of functional health literacy to patients' knowledge of their chronic disease: a study of patients with hypertension and diabetes.Arch Intern Med.1998;158(2):166–172.
- ,,,.Health literacy and the risk of hospital admission.J Gen Intern Med.1998;13:791–798.
- ,,, et al.Limited literacy and mortality in the elderly: The Health, Aging, and Body Composition Study.J Gen Intern Med.2006;21(8):806–812.
- ,,,,,.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167(14):1503–1509.
- ,,,,.Literacy and health outcomes: a systematic review of the literature.J Gen Intern Med.2004;19(12):1129–1139.
- ,,, et al.Inadequate functional health literacy among patients at two public hospitals.JAMA.1995;274(21):1677–1682.
- ,,, et al.ACC/AHA 2002 guideline update for the management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction–summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina).J Am Coll Cardiol.2002;40(7):1366–1374.
- ,,, et al.Rapid assessment of literacy levels of adult primary care patients.Fam Med.1991;23(6):433–435.
- ,,,.Literacy testing in health care research. In: Schwartzberg JG, VanGeest JB, Wang CC, eds.Understanding Health Literacy.Chicago:American Medical Association;2005:157–179.
- ,,.“Mini‐Mental State”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,, et al.Interpersonal processes of care in diverse populations.Milbank Q.1999;77:305–339.
- ,,,,.Functional health literacy and the quality of physician‐patient communication among diabetes patients.Patient Educ Couns.2004;52(3):315–323.
- ,,,.Frequency and predictors of prescription‐related issues after hospital discharge.J Hosp Med.2008;3(1):12–19.
- ,,,,.Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure.Am J Health Syst Pharm.2004;61(19):2043–2049.
- ,,,,,.Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills.Clin Pharmacol Ther.2009;85(6):651–658.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113(24):2803–2809.
- ,,.Patient‐physician communication: a descriptive summary of the literature.Patient Educ Couns.1988;12:99–119.
- ,,,,,.Unraveling the relationship between literacy, language proficiency, and patient‐physician communication.Patient Educ Couns.2009;75(3):398–402.
- ,.Teaching about health literacy and clear communication.J Gen Intern Med.2006;21:888–890.
- .Health Literacy: A Manual for Clinicians.Chicago, IL:American Medical Association;2003.
- ,.Communicating with patients who cannot read.N Engl J Med.1997;337:272–274.
- ,,,.The role of health literacy in patient‐physician communication.Fam Med.2002;34(5):383–389.
- ,,,,,.Health literacy: universal precautions needed.J Allied Health.2004;33(2):150–155.
- American Medical Association Council on Scientific Affairs.Health literacy.JAMA.1999;281:552–557.
- ,,.Teaching medical students about health literacy: 2 Chicago initiatives.Am J Health Behav.2007;31Suppl 1:S111–S114.
- ,,,,,.Development and implementation of a health literacy training program for medical residents.Med Educ Online.2006;11(13):1–8.
- ,.The use of standardized patients to teach low‐literacy communication skills.Am J Health Behav.2007;31Suppl 1:S105–S110.
It is well established that patients have difficulty understanding written health materials,1 medical terminology,2, 3 and other aspects of provider‐patient communication.4, 5 Such difficulties in communication can be magnified at transitions of care like hospital discharge.6 Patients often receive a large amount of information in a short period of time at discharge, and this information may be delivered in a way that is not straightforward or standardized.7, 8 When asked, patients commonly report a poor understanding of important self‐care instructions such as how to take medications upon returning home.9, 10 One study even showed that more than half of patients did not recall anyone providing instructions about how they should care for themselves after hospitalization.11 Poor medication management after hospital discharge contributes to adverse events,1215 inadequate disease control,16 and in the setting of cardiovascular disease, higher mortality.17, 18 Most adverse events after hospital discharge could be prevented or ameliorated through relatively simple means, including better communication among patients and providers.6, 1416, 1921 Greater attention to communication and care transitions could also reduce the number of unplanned rehospitalizations in the United States.22
Patients' health literacy is an important factor in effective health communication, yet little research has examined the role of health literacy in care transitions. Health literacy is defined as the extent to which an individual is able to obtain, process and understand basic health information and services needed to make appropriate health decisions.23, 24 Low health literacy is a prevalent problem in the United States, affecting approximately 40% of adults.25 Research has shown that low health literacy is associated with low self‐efficacy26 and less interaction in physician‐patient encounters,27 which in combination with physicians' use of complex medical language,28 may contribute to poor physician‐patient communication. Patients with low health literacy also have greater difficulty understanding prescription drug labels,29 limited knowledge of disease self‐management skills,30 a higher incidence of hospitalization,31 and higher mortality rates.3234
In order to elucidate the relationship between patient‐provider communication and health literacy in the hospital setting, we analyzed patients' ratings of their communication experience during their hospitalization. We report patients' perceptions of the clarity of communication and how this may vary by level of health literacy and other important patient characteristics.
Methods
Setting and Participants
Patients admitted to the general medical wards at Grady Memorial Hospital were recruited for participation. Grady Memorial Hospital is a public, urban teaching hospital located in Atlanta, GA. It serves a primarily low income, African American population, many of whom lack health insurance. Approximately 30% to 50% of patients at this hospital have inadequate health literacy skills.35
The present study was conducted as preliminary research for a randomized controlled trial to improve post‐discharge medication adherence among patients with acute coronary syndromes (ACS). The criteria for the present study mirrored those of the planned trial. Patients were eligible for the current study if they were admitted with suspected ACS and evidence of myocardial ischemia.36 Exclusion criteria included lack of cooperation/refusal to participate, unintelligible speech (eg, dysarthria), lack of English fluency (determined subjectively by interviewer), delirium (determined by lack of orientation to person, place, and time), severe hearing impairment (determined subjectively by interviewer), visual acuity worse than 20/60 (per pocket vision screening card), acute psychotic illness (per admission history), police custody, age younger than 18 years, no regular telephone number, administration of all medications by a caregiver, and not taking prescription medications in the 6 months before admission.
Data Collection and Measures
Enrollment occurred between August 2005 and April 2006, after approval was obtained from both the Emory University Institutional Review Board (IRB) and Grady Research Oversight Committee. Interested and willing participants provided written informed consent and subsequently completed an interviewer‐assisted questionnaire prior to hospital discharge to collect information regarding demographics and cardiovascular risk factors. To ensure that answers were not confounded by participants' inability to read the questionnaire text, all questions were read to participants by study interviewers, with the exception of the health literacy assessmentthe Rapid Estimate of Adult Literacy in Medicine (REALM).37 The REALM classifies a patient's literacy according to the number of medical terms from a list that the patient pronounces correctly. It correlates highly with other assessments of literacy and health literacy.38 Cognitive function was measured using the Mini‐Mental State Examination (MMSE).39
Research staff contacted patients by telephone approximately 2 weeks after hospital discharge to complete a survey which included the Interpersonal Processes of Care in Diverse Populations Questionnaire (IPC).40 The IPC is a validated, self‐report questionnaire with high internal consistency reliability. It was developed and normalized among ethnically diverse populations of low socioeconomic status. Items on the IPC originally referred to communication during the last 6 months in the outpatient clinic; they were reworded to refer to the recent hospitalization only. The research assistant administered 8 of 12 domains of the IPC that were most pertinent to rating the quality and clarity of patient communication with hospital physicians.41 Four other IPC domains that pertained to interpersonal style (eg, friendliness, emotional support) were not administered to minimize response burden. Each domain was comprised of 2 to 7 items, and responses were given on a 5‐point Likert scale. The 8 domains and sample items were as follows: (1) General clarity (eg, Did the doctors use medical words that you did not understand?); (2) Elicitation of and responsiveness to patient problems, concerns, and expectations (eg, Did the doctors listen carefully to what you had to say?); (3) Explanations of condition, progress, and prognosis (eg, Did the doctors make sure you understand your health problem?); (4) Explanations of processes of care (eg, Did the doctors explain why a test was being done?); (5) Explanations of self‐care (eg, Did the doctors tell you what you could do to take care of yourself at home?); (6) Empowerment (eg, Did the doctors make you feel that following your treatment plan would make a difference in your health?); (7) Decision‐making: responsiveness to patient preferences regarding decisions (eg, Did the doctors try to involve you or include you in decisions about your treatment?); and (8) Consideration of patient's desire and ability to comply with recommendations (eg, Did the doctors understand the kinds of problems you might have in doing the recommended treatment?).
Statistical Analysis
Patient characteristics were summarized using frequency, mean, and standard deviation measures. Nondichotomous measures were recategorized into dichotomous variables as follows: age (less than 55 years vs. 55 years or older), race (black vs. white or other), marital status (married or living with someone vs. living alone), education (less than high school vs. high school graduate), employment status (employed full/part time vs. unemployed/retired), MMSE score (cognitively impaired [MMSE score 24] vs. no significant cognitive impairment [MMSE score >24]),39 and health literacy score (inadequate [REALM score 0 to 44] vs. marginal or adequate [REALM score 45‐66]).38 Dichotomous variables were summarized using frequencies.
Scores for each individual IPC question ranged from 1 to 5 with lower scores indicating better communication, except for questions in the domain of general clarity where higher scores indicated better communication. Then, for each of the 8 domains, scores of the individual IPC questions within that domain were averaged.
Bivariate analyses were conducted for each of the 8 IPC domains, by level of health literacy and other relevant patient characteristics, using the independent samples t‐test. Multivariable linear regression models were then constructed to examine the independent association of health literacy with each of the 8 IPC domains, while controlling for other patient characteristics that were also found to be associated with IPC domain scores. Bivariate analyses were also conducted for each of the 27 individual IPC items, to gain an understanding of which items might be driving the overall effect. A 2‐sided P < 0.05 was considered statistically significant. All analyses were performed using SPSS 15 for Windows (SPSS, Chicago, IL).
Results
Patient Characteristics
A total of 109 eligible patients were approached, 100 agreed to participate and were enrolled in the hospital, and 84 of them completed the follow‐up interview by telephone to comprise the sample for this study (Table 1). Most of the 84 participants were under the age of 55 (54%), male (58%), African American (88%), unemployed (79%), lived alone (73%), and had completed high school (62%). Age ranged from 24 to 80 years, REALM score ranged from 0 to 66, and MMSE ranged from 12 to 30. A large proportion (44%) had inadequate health literacy skills, and 50% had cognitive impairment. Patients with inadequate health literacy were more likely to have not finished high school and to suffer cognitive impairment, P < 0.01 for each comparison.
| Characteristic | n (%) |
|---|---|
| Age | |
| <55 years | 45 (54) |
| 55 years | 39 (46) |
| Gender | |
| Male | 49 (58) |
| Female | 35 (42) |
| Race | |
| Black | 74 (88) |
| White or other | 10 (12) |
| Marital status | |
| Married or living with someone | 23 (27) |
| Living alone | 61 (73) |
| Education | |
| Did not complete high school | 32 (38) |
| High school graduate | 52 (62) |
| Employment status | |
| Employed (full/part time) | 18 (21) |
| Not employed | 66 (79) |
| Mini‐Mental State Exam | |
| Cognition impaired | 42 (50) |
| Cognition not impaired | 42 (50) |
| Health literacy | |
| Inadequate | 37 (44) |
| Marginal or adequate | 47 (56) |
Hospital Communication Ratings by IPC Domains
Overall, patients' ratings of hospital communication were positive, with most IPC domain score means lying in the favorable half of the Likert scale (Table 2). The domains with the best communication ratings were responsiveness to patient concerns (mean = 1.68), explanations of condition and prognosis (mean = 1.75), and empowerment (mean 1.76). The domain of worst performance was consideration of patients' desire and ability to comply with recommendations (mean = 3.15).
| IPC Domain | Total (n = 84), Mean (SD) | Patients with Inadequate Literacy (n = 37), Mean (SD) | Patients with Marginal or Adequate Literacy (n = 47), Mean (SD) | P Value | |
|---|---|---|---|---|---|
| |||||
| 1 | General clarity* | 3.66 (1.00) | 3.36 (1.14) | 3.89 (0.74) | 0.02 |
| 2 | Responsiveness to patient concerns | 1.68 (0.68) | 1.86 (0.76) | 1.53 (0.58) | 0.03 |
| 3 | Explanations of condition and prognosis | 1.75 (0.87) | 1.93 (0.99) | 1.61 (0.74) | 0.09 |
| 4 | Explanations of processes of care | 2.01 (0.86) | 2.22 (0.96) | 1.84 (0.74) | 0.04 |
| 5 | Explanations of self‐care | 2.37 (1.04) | 2.42 (1.20) | 2.33 (0.90) | 0.71 |
| 6 | Empowerment | 1.76 (1.03) | 1.85 (1.27) | 1.69 (0.81) | 0.51 |
| 7 | Decision‐making | 2.34 (0.78) | 2.34 (0.80) | 2.34 (0.77) | 1.00 |
| 8 | Consideration of patients' desire and ability to comply with recommendations | 3.15 (1.19) | 3.24 (1.16) | 3.07 (1.23) | 0.54 |
In bivariate analyses that compared IPC domains by patients' level of health literacy, several differences emerged. Patients with inadequate health literacy skills gave significantly worse ratings to the quality of communication on the domains of general clarity (mean = 3.36 vs. 3.89 for patients with marginal or adequate health literacy, P = 0.02), Responsiveness to patient concerns (mean = 1.86 vs. 1.53, P = 0.03), and Explanations of processes of care (mean = 2.22 vs. 1.84, P = 0.04). On a fourth domain, Explanations of condition and prognosis, a nonsignificant trend was present (mean = 1.93 vs. 1.61, P = 0.09).
Fewer significant relationships were found between other patient characteristics and IPC domain scores. Patients who were age 55 or older provided worse ratings on explanations of self‐care (mean = 2.74 vs. 2.05 for patients under the age of 55, P = 0.003). Lower ratings on the domain of general clarity, which indicated unclear communication, were found among patients who had not graduated from high school (mean = 3.31 vs. 3.88 for high school graduates, P = 0.02) or who had cognitive impairment (mean = 3.39 vs. 3.93 for patients without impaired cognition, P = 0.01). No significant differences were present by gender or race.
Based on these bivariate relationships, terms for inadequate health literacy, age 55, Cognitive impairment, and high school graduation were entered into multivariable models that predicted scores on each of the 8 IPC domains. Inadequate health literacy was independently associated with Responsiveness to patient concerns ( = 0.512, P = 0.007) and Explanations of processes of care ( = 0.548, P = 0.023); a nonsignificant trend was present for consideration of patients' desire and ability to comply with recommendations ( = 0.582, P = 0.09). The association of age with explanations of self‐care remained after adjustment for the other variables ( = 0.705, P = 0.002). None of the patient characteristics was independently associated with ratings of general clarity.
IPC Item Responses
Examination of responses on the individual IPC items revealed the specific areas of difficulty in communication as rated by patients (Table 3). In the domain of general clarity, patients with inadequate literacy provided poorer ratings on the item pertaining to use of medical terminology (mean = 2.92 vs. 3.68 for patients with marginal or adequate literacy, P = 0.004). Regarding Responsiveness to patient concerns, differences by literacy were present in the item that pertained to patients being given enough time to say what they thought was important (mean = 2.27 vs. 1.51, P = 0.003). On the domain of explanations of processes of care, the item rated differently by patients with inadequate literacy referred to feeling confused about their care because doctors did not explain things well (mean = 2.51 vs. 1.83, P = 0.02).
Discussion
We used a validated instrument, the IPC,40 to examine patients' ratings of the quality and clarity of hospital‐based communication. Overall, patients provided favorable ratings in many domains, including those pertaining to Responsiveness to patient concerns and Explanations of condition and prognosis. Clinicians' consideration of patients' desire and ability to comply with recommendations was rated least favorably overall. This represents an important area for improvement, particularly when considering the prevalence of nonadherence to medical therapy after hospital discharge, which may be as high as 50%.9, 42 Nonadherence after hospital discharge contributes to avoidable emergency department visits,43 hospital readmissions,44 and higher mortality.18, 45 The results of this study suggest that hospital physicians should give greater consideration to patients' preferences and problems that they may have in following the treatment recommendations.16 Future research will determine the extent to which this may enhance post‐discharge adherence.
Another important finding is that patients with inadequate health literacy rated hospital‐based communication less favorably than did patients with marginal or adequate literacy. In bivariate analyses, this effect was seen on several domains, including general clarity, Responsiveness to patient concerns, and explanations of processes of care. The latter 2 relationships persisted after adjustment for age, cognitive impairment, and educational attainment. To our knowledge, this is the first study which examines the effect of health literacy on patients' ratings of hospital‐based communication.
The majority of the literature on health communication and health literacy focuses on the outpatient setting.34, 46 However, the quality and clarity of patient‐provider communication in the hospital is also critically important. Ineffective communication in the hospital contributes to poor care transitions and post‐discharge complications. Patients commonly leave the hospital with a poor understanding of what transpired (eg, diagnoses, treatment provided, major test results) and inadequate knowledge about the self‐care activities that they must perform upon returning home (eg, medication management, physical activity, follow‐up appointments).911 Poor communication is often cited as the main underlying and remediable factor behind medical errors, adverse events, and the readmissions that commonly occur after hospital discharge.6, 16, 20 The results of this study provide complementary evidence, showing that patients often feel they have experienced suboptimal communication in the hospital setting. These findings highlight an opportunity for improvement in care transitions and patient safety, particularly among patients with inadequate health literacy.
In outpatient research that utilized the IPC, Schillinger et al.41 found that patients with inadequate functional health literacy reported significantly worse communication on the domains of general clarity, explanations of processes of care, and Explanations of condition and prognosis. Subsequent analyses by Sudore et al.47 demonstrated that patients with inadequate or marginal health literacy more often reported that physicians did not give them enough time to say what they thought was important, did not explain processes of care well, and did not ask about problems in following the recommended treatment (Table 3, IPC items 3, 12, and 26, respectively). Our findings were very similar. These relatively consistent results across studies and populations strengthen the conclusion that patients with inadequate health literacy feel their physicians do not communicate as effectively in these areas.
| IPC Items | Overall (n = 84), Mean (SD) | Inadequate Literacy (n = 37), Mean (SD) | Marginal or Adequate Literacy (n = 47), Mean (SD) | P Value |
|---|---|---|---|---|
| ||||
| General clarity* | ||||
| 1. Did the doctors use medical words you did not understand? | 3.35 (1.14) | 2.92 (1.40) | 3.68 (0.73) | 0.004 |
| 2. Did you have trouble understanding your doctors because they spoke too fast? | 3.98 (1.06) | 3.81 (1.13) | 4.11 (1.01) | 0.21 |
| Responsiveness to patient concerns | ||||
| 3. Did the doctors give you enough time to say what you thought was important? | 1.85 (1.14) | 2.27 (1.28) | 1.51 (0.88) | 0.003 |
| 4. Did the doctors listen carefully to what you had to say? | 1.62 (0.88) | 1.76 (1.04) | 1.51 (0.72) | 0.22 |
| 5. Did the doctors ignore what you told them? | 1.70 (0.92) | 1.81 (1.09) | 1.62 (0.77) | 0.38 |
| 6. Did the doctors take your concerns seriously? | 1.55 (0.92) | 1.65 (0.98) | 1.47 (0.88) | 0.38 |
| Explanations of condition and prognosis | ||||
| 7. Did the doctors give you enough information about your health problems? | 1.88 (1.11) | 2.11 (1.27) | 1.70 (0.95) | 0.11 |
| 8. Did the doctors make sure you understand your health problems? | 1.62 (0.88) | 1.76 (0.98) | 1.51 (0.78) | 0.22 |
| Explanations of processes of care | ||||
| 9. Did the doctors explain why a test was being done? | 1.70 (1.10) | 1.89 (1.24) | 1.55 (0.95) | 0.16 |
| 10. Did the doctors explain how the test was done? | 2.20 (1.35) | 2.27 (1.39) | 2.15 (1.34) | 0.69 |
| 11. Did the doctors tell you what they were doing as they examined you? | 1.99 (1.20) | 2.22 (1.34) | 1.81 (1.06) | 0.13 |
| 12. Did you feel confused about what was going on with your medical care because doctors did not explain things well? | 2.13 (1.23) | 2.51 (1.47) | 1.83 (0.92) | 0.02 |
| Explanations of self‐care | ||||
| 13. Did the doctors tell you what you could do to take care of yourself at home? | 1.67 (1.09) | 1.81 (1.29) | 1.55 (0.90) | 0.31 |
| 14. Did the doctors tell you how to pay attention to your symptoms and when to call the doctor? | 2.01 (1.38) | 2.19 (1.60) | 1.87 (1.17) | 0.32 |
| 15. Did the doctors clearly explain how to take the medicine (that is when, how much and for how long)? | 1.88 (1.36) | 2.00 (1.53) | 1.79 (1.22) | 0.48 |
| 16. Did the doctors go over all the medicines you are taking? | 2.39 (1.55) | 2.51 (1.74) | 2.30 (1.40) | 0.54 |
| 17. Did the doctors give you written instruction about how to take the medicine (other than what was on the container)? | 3.29 (1.70) | 3.05 (1.75) | 3.48 (1.66) | 0.26 |
| 18. Did the doctors tell you the reason for taking each medicine? | 2.05 (1.43) | 2.24 (1.64) | 1.89 (1.24) | 0.29 |
| 19. Did the doctors tell you about side effects you might get from your medicine? | 3.32 (1.64) | 3.11 (1.73) | 3.49 (1.56) | 0.29 |
| Empowerment | ||||
| 20. Did doctors make you feel that following your treatment plan would make a difference in your health? | 1.75 (1.07) | 1.89 (1.27) | 1.64 (0.90) | 0.31 |
| 21. Did the doctors make you feel that your everyday activities such as your diet and lifestyle would make a difference in your health? | 1.77 (1.21) | 1.81 (1.41) | 1.74 (1.03) | 0.81 |
| Decision‐making | ||||
| 22. Did the doctors try to involve you or include you in decisions about your treatment? | 2.43 (1.55) | 2.30 (1.49) | 2.53 (1.60) | 0.49 |
| 23. Did the doctors ask how you felt about different treatments? | 3.08 (1.58) | 2.89 (1.66) | 3.23 (1.51) | 0.33 |
| 24. Did the doctors make decision without taking your preferences and opinions into account? | 2.23 (1.35) | 2.34 (1.55) | 2.15 (1.20) | 0.54 |
| 25. Did you feel pressured by doctors in the hospital to have a treatment you were not sure you wanted? | 1.60 (0.97) | 1.81 (1.18) | 1.43 (0.74) | 0.09 |
| Consideration of patients' desire and ability to comply with recommendations | ||||
| 26. Did the doctors ask if you might have any problems actually doing the recommended treatment (for example taking the medication correctly)? | 3.82 (1.47) | 4.08 (1.40) | 3.62 (1.51) | 0.15 |
| 27. Did the doctors understand the kinds of problems you might have in doing the recommended treatment? | 2.43 (1.44) | 2.26 (1.52) | 2.57 (1.38) | 0.34 |
Importantly, the differences in patient responses by literacy category were driven by a few IPC items. These items pertained to physicians' use of medical terminology, the amount of time they gave patients to express their concerns, and how well they explained the patients' medical care. Training physicians to improve their communication skills in these specific areas may improve their ability to communicate effectively with patients who have limited literacy skills. Indeed, published recommendations on how to improve the clarity of verbal communication emphasize just a few major areas, including limiting the amount of medical terminology used, effectively encouraging patients to ask questions and express their concerns, and asking patients to teach‐back key points to make sure the physician has provided adequate explanation.4851 The present study provides some evidence for those recommendations, which for the most part, have been based on clinical experience and expert opinion.
There remains a need for professional education about health literacy and techniques to improve communication with patients who may have limited literacy skills. Many experts advocate clear verbal communication with all patients, so‐called Universal Precautions.52 Although 10 years have passed since the American Medical Association (AMA) called for more work in this area,53 few curricula have been described in the literature.48, 5456 The extent to which health literacy curricula have been implemented in medical schools and other professional schools is unknown. The impact of such training on the communication skills of health care providers and patient outcomes is also unclear.
The strengths of this study include a relatively good response rate and use of a validated measure to grade the quality of physician‐patient communication. This measure, the IPC, has been used previously in the context of health literacy.41 Nevertheless, certain limitations should be acknowledged. First, the study was performed at a single teaching hospital, where patients had a high prevalence of inadequate health literacy. The findings may not generalize to other institutions that serve a different patient population or to nonacademic programs. Second, communication was assessed by patient report, rather than by recording patient‐provider discussions for rating by independent observers. While patient report is inherently more subjective, patients' own perceptions about the effectiveness of health communication are arguably more important than those of independent raters, and thus, the data source may not represent a true limitation. Third, patient responses were obtained approximately 2 weeks after hospital discharge, and accordingly, they are subject to recall bias, which may be greater among those with cognitive impairment. Finally, patients were directed to rate the communication of the overall group of physicians who took care of them in the hospital. Given the academic setting, patients typically received care from a team that included medical students, interns, a resident, and an attending physician. We were not able to determine whether patients' ratings were influenced by a specific member of the team, nor how ratings may have been influenced by certain characteristics of that team member (eg, year of training, prior communication skills training, race or gender concordance, etc).
In summary, by surveying patients soon after an acute care hospitalization, we determined that certain areas held room for improvement, such as consideration of patients' desire and ability to comply with treatment recommendations. Patients with inadequate health literacy reported lower quality physician‐patient communication on several domains. They expressed particular concern about physicians' use of medical terminology, not getting enough time to express their concerns, and not receiving clear enough explanations about the medical care. Efforts are needed to improve physicians' communication skills in these areas. Such training should be evaluated to determine if it has a beneficial effect on physician communication skills and patient outcomes.
It is well established that patients have difficulty understanding written health materials,1 medical terminology,2, 3 and other aspects of provider‐patient communication.4, 5 Such difficulties in communication can be magnified at transitions of care like hospital discharge.6 Patients often receive a large amount of information in a short period of time at discharge, and this information may be delivered in a way that is not straightforward or standardized.7, 8 When asked, patients commonly report a poor understanding of important self‐care instructions such as how to take medications upon returning home.9, 10 One study even showed that more than half of patients did not recall anyone providing instructions about how they should care for themselves after hospitalization.11 Poor medication management after hospital discharge contributes to adverse events,1215 inadequate disease control,16 and in the setting of cardiovascular disease, higher mortality.17, 18 Most adverse events after hospital discharge could be prevented or ameliorated through relatively simple means, including better communication among patients and providers.6, 1416, 1921 Greater attention to communication and care transitions could also reduce the number of unplanned rehospitalizations in the United States.22
Patients' health literacy is an important factor in effective health communication, yet little research has examined the role of health literacy in care transitions. Health literacy is defined as the extent to which an individual is able to obtain, process and understand basic health information and services needed to make appropriate health decisions.23, 24 Low health literacy is a prevalent problem in the United States, affecting approximately 40% of adults.25 Research has shown that low health literacy is associated with low self‐efficacy26 and less interaction in physician‐patient encounters,27 which in combination with physicians' use of complex medical language,28 may contribute to poor physician‐patient communication. Patients with low health literacy also have greater difficulty understanding prescription drug labels,29 limited knowledge of disease self‐management skills,30 a higher incidence of hospitalization,31 and higher mortality rates.3234
In order to elucidate the relationship between patient‐provider communication and health literacy in the hospital setting, we analyzed patients' ratings of their communication experience during their hospitalization. We report patients' perceptions of the clarity of communication and how this may vary by level of health literacy and other important patient characteristics.
Methods
Setting and Participants
Patients admitted to the general medical wards at Grady Memorial Hospital were recruited for participation. Grady Memorial Hospital is a public, urban teaching hospital located in Atlanta, GA. It serves a primarily low income, African American population, many of whom lack health insurance. Approximately 30% to 50% of patients at this hospital have inadequate health literacy skills.35
The present study was conducted as preliminary research for a randomized controlled trial to improve post‐discharge medication adherence among patients with acute coronary syndromes (ACS). The criteria for the present study mirrored those of the planned trial. Patients were eligible for the current study if they were admitted with suspected ACS and evidence of myocardial ischemia.36 Exclusion criteria included lack of cooperation/refusal to participate, unintelligible speech (eg, dysarthria), lack of English fluency (determined subjectively by interviewer), delirium (determined by lack of orientation to person, place, and time), severe hearing impairment (determined subjectively by interviewer), visual acuity worse than 20/60 (per pocket vision screening card), acute psychotic illness (per admission history), police custody, age younger than 18 years, no regular telephone number, administration of all medications by a caregiver, and not taking prescription medications in the 6 months before admission.
Data Collection and Measures
Enrollment occurred between August 2005 and April 2006, after approval was obtained from both the Emory University Institutional Review Board (IRB) and Grady Research Oversight Committee. Interested and willing participants provided written informed consent and subsequently completed an interviewer‐assisted questionnaire prior to hospital discharge to collect information regarding demographics and cardiovascular risk factors. To ensure that answers were not confounded by participants' inability to read the questionnaire text, all questions were read to participants by study interviewers, with the exception of the health literacy assessmentthe Rapid Estimate of Adult Literacy in Medicine (REALM).37 The REALM classifies a patient's literacy according to the number of medical terms from a list that the patient pronounces correctly. It correlates highly with other assessments of literacy and health literacy.38 Cognitive function was measured using the Mini‐Mental State Examination (MMSE).39
Research staff contacted patients by telephone approximately 2 weeks after hospital discharge to complete a survey which included the Interpersonal Processes of Care in Diverse Populations Questionnaire (IPC).40 The IPC is a validated, self‐report questionnaire with high internal consistency reliability. It was developed and normalized among ethnically diverse populations of low socioeconomic status. Items on the IPC originally referred to communication during the last 6 months in the outpatient clinic; they were reworded to refer to the recent hospitalization only. The research assistant administered 8 of 12 domains of the IPC that were most pertinent to rating the quality and clarity of patient communication with hospital physicians.41 Four other IPC domains that pertained to interpersonal style (eg, friendliness, emotional support) were not administered to minimize response burden. Each domain was comprised of 2 to 7 items, and responses were given on a 5‐point Likert scale. The 8 domains and sample items were as follows: (1) General clarity (eg, Did the doctors use medical words that you did not understand?); (2) Elicitation of and responsiveness to patient problems, concerns, and expectations (eg, Did the doctors listen carefully to what you had to say?); (3) Explanations of condition, progress, and prognosis (eg, Did the doctors make sure you understand your health problem?); (4) Explanations of processes of care (eg, Did the doctors explain why a test was being done?); (5) Explanations of self‐care (eg, Did the doctors tell you what you could do to take care of yourself at home?); (6) Empowerment (eg, Did the doctors make you feel that following your treatment plan would make a difference in your health?); (7) Decision‐making: responsiveness to patient preferences regarding decisions (eg, Did the doctors try to involve you or include you in decisions about your treatment?); and (8) Consideration of patient's desire and ability to comply with recommendations (eg, Did the doctors understand the kinds of problems you might have in doing the recommended treatment?).
Statistical Analysis
Patient characteristics were summarized using frequency, mean, and standard deviation measures. Nondichotomous measures were recategorized into dichotomous variables as follows: age (less than 55 years vs. 55 years or older), race (black vs. white or other), marital status (married or living with someone vs. living alone), education (less than high school vs. high school graduate), employment status (employed full/part time vs. unemployed/retired), MMSE score (cognitively impaired [MMSE score 24] vs. no significant cognitive impairment [MMSE score >24]),39 and health literacy score (inadequate [REALM score 0 to 44] vs. marginal or adequate [REALM score 45‐66]).38 Dichotomous variables were summarized using frequencies.
Scores for each individual IPC question ranged from 1 to 5 with lower scores indicating better communication, except for questions in the domain of general clarity where higher scores indicated better communication. Then, for each of the 8 domains, scores of the individual IPC questions within that domain were averaged.
Bivariate analyses were conducted for each of the 8 IPC domains, by level of health literacy and other relevant patient characteristics, using the independent samples t‐test. Multivariable linear regression models were then constructed to examine the independent association of health literacy with each of the 8 IPC domains, while controlling for other patient characteristics that were also found to be associated with IPC domain scores. Bivariate analyses were also conducted for each of the 27 individual IPC items, to gain an understanding of which items might be driving the overall effect. A 2‐sided P < 0.05 was considered statistically significant. All analyses were performed using SPSS 15 for Windows (SPSS, Chicago, IL).
Results
Patient Characteristics
A total of 109 eligible patients were approached, 100 agreed to participate and were enrolled in the hospital, and 84 of them completed the follow‐up interview by telephone to comprise the sample for this study (Table 1). Most of the 84 participants were under the age of 55 (54%), male (58%), African American (88%), unemployed (79%), lived alone (73%), and had completed high school (62%). Age ranged from 24 to 80 years, REALM score ranged from 0 to 66, and MMSE ranged from 12 to 30. A large proportion (44%) had inadequate health literacy skills, and 50% had cognitive impairment. Patients with inadequate health literacy were more likely to have not finished high school and to suffer cognitive impairment, P < 0.01 for each comparison.
| Characteristic | n (%) |
|---|---|
| Age | |
| <55 years | 45 (54) |
| 55 years | 39 (46) |
| Gender | |
| Male | 49 (58) |
| Female | 35 (42) |
| Race | |
| Black | 74 (88) |
| White or other | 10 (12) |
| Marital status | |
| Married or living with someone | 23 (27) |
| Living alone | 61 (73) |
| Education | |
| Did not complete high school | 32 (38) |
| High school graduate | 52 (62) |
| Employment status | |
| Employed (full/part time) | 18 (21) |
| Not employed | 66 (79) |
| Mini‐Mental State Exam | |
| Cognition impaired | 42 (50) |
| Cognition not impaired | 42 (50) |
| Health literacy | |
| Inadequate | 37 (44) |
| Marginal or adequate | 47 (56) |
Hospital Communication Ratings by IPC Domains
Overall, patients' ratings of hospital communication were positive, with most IPC domain score means lying in the favorable half of the Likert scale (Table 2). The domains with the best communication ratings were responsiveness to patient concerns (mean = 1.68), explanations of condition and prognosis (mean = 1.75), and empowerment (mean 1.76). The domain of worst performance was consideration of patients' desire and ability to comply with recommendations (mean = 3.15).
| IPC Domain | Total (n = 84), Mean (SD) | Patients with Inadequate Literacy (n = 37), Mean (SD) | Patients with Marginal or Adequate Literacy (n = 47), Mean (SD) | P Value | |
|---|---|---|---|---|---|
| |||||
| 1 | General clarity* | 3.66 (1.00) | 3.36 (1.14) | 3.89 (0.74) | 0.02 |
| 2 | Responsiveness to patient concerns | 1.68 (0.68) | 1.86 (0.76) | 1.53 (0.58) | 0.03 |
| 3 | Explanations of condition and prognosis | 1.75 (0.87) | 1.93 (0.99) | 1.61 (0.74) | 0.09 |
| 4 | Explanations of processes of care | 2.01 (0.86) | 2.22 (0.96) | 1.84 (0.74) | 0.04 |
| 5 | Explanations of self‐care | 2.37 (1.04) | 2.42 (1.20) | 2.33 (0.90) | 0.71 |
| 6 | Empowerment | 1.76 (1.03) | 1.85 (1.27) | 1.69 (0.81) | 0.51 |
| 7 | Decision‐making | 2.34 (0.78) | 2.34 (0.80) | 2.34 (0.77) | 1.00 |
| 8 | Consideration of patients' desire and ability to comply with recommendations | 3.15 (1.19) | 3.24 (1.16) | 3.07 (1.23) | 0.54 |
In bivariate analyses that compared IPC domains by patients' level of health literacy, several differences emerged. Patients with inadequate health literacy skills gave significantly worse ratings to the quality of communication on the domains of general clarity (mean = 3.36 vs. 3.89 for patients with marginal or adequate health literacy, P = 0.02), Responsiveness to patient concerns (mean = 1.86 vs. 1.53, P = 0.03), and Explanations of processes of care (mean = 2.22 vs. 1.84, P = 0.04). On a fourth domain, Explanations of condition and prognosis, a nonsignificant trend was present (mean = 1.93 vs. 1.61, P = 0.09).
Fewer significant relationships were found between other patient characteristics and IPC domain scores. Patients who were age 55 or older provided worse ratings on explanations of self‐care (mean = 2.74 vs. 2.05 for patients under the age of 55, P = 0.003). Lower ratings on the domain of general clarity, which indicated unclear communication, were found among patients who had not graduated from high school (mean = 3.31 vs. 3.88 for high school graduates, P = 0.02) or who had cognitive impairment (mean = 3.39 vs. 3.93 for patients without impaired cognition, P = 0.01). No significant differences were present by gender or race.
Based on these bivariate relationships, terms for inadequate health literacy, age 55, Cognitive impairment, and high school graduation were entered into multivariable models that predicted scores on each of the 8 IPC domains. Inadequate health literacy was independently associated with Responsiveness to patient concerns ( = 0.512, P = 0.007) and Explanations of processes of care ( = 0.548, P = 0.023); a nonsignificant trend was present for consideration of patients' desire and ability to comply with recommendations ( = 0.582, P = 0.09). The association of age with explanations of self‐care remained after adjustment for the other variables ( = 0.705, P = 0.002). None of the patient characteristics was independently associated with ratings of general clarity.
IPC Item Responses
Examination of responses on the individual IPC items revealed the specific areas of difficulty in communication as rated by patients (Table 3). In the domain of general clarity, patients with inadequate literacy provided poorer ratings on the item pertaining to use of medical terminology (mean = 2.92 vs. 3.68 for patients with marginal or adequate literacy, P = 0.004). Regarding Responsiveness to patient concerns, differences by literacy were present in the item that pertained to patients being given enough time to say what they thought was important (mean = 2.27 vs. 1.51, P = 0.003). On the domain of explanations of processes of care, the item rated differently by patients with inadequate literacy referred to feeling confused about their care because doctors did not explain things well (mean = 2.51 vs. 1.83, P = 0.02).
Discussion
We used a validated instrument, the IPC,40 to examine patients' ratings of the quality and clarity of hospital‐based communication. Overall, patients provided favorable ratings in many domains, including those pertaining to Responsiveness to patient concerns and Explanations of condition and prognosis. Clinicians' consideration of patients' desire and ability to comply with recommendations was rated least favorably overall. This represents an important area for improvement, particularly when considering the prevalence of nonadherence to medical therapy after hospital discharge, which may be as high as 50%.9, 42 Nonadherence after hospital discharge contributes to avoidable emergency department visits,43 hospital readmissions,44 and higher mortality.18, 45 The results of this study suggest that hospital physicians should give greater consideration to patients' preferences and problems that they may have in following the treatment recommendations.16 Future research will determine the extent to which this may enhance post‐discharge adherence.
Another important finding is that patients with inadequate health literacy rated hospital‐based communication less favorably than did patients with marginal or adequate literacy. In bivariate analyses, this effect was seen on several domains, including general clarity, Responsiveness to patient concerns, and explanations of processes of care. The latter 2 relationships persisted after adjustment for age, cognitive impairment, and educational attainment. To our knowledge, this is the first study which examines the effect of health literacy on patients' ratings of hospital‐based communication.
The majority of the literature on health communication and health literacy focuses on the outpatient setting.34, 46 However, the quality and clarity of patient‐provider communication in the hospital is also critically important. Ineffective communication in the hospital contributes to poor care transitions and post‐discharge complications. Patients commonly leave the hospital with a poor understanding of what transpired (eg, diagnoses, treatment provided, major test results) and inadequate knowledge about the self‐care activities that they must perform upon returning home (eg, medication management, physical activity, follow‐up appointments).911 Poor communication is often cited as the main underlying and remediable factor behind medical errors, adverse events, and the readmissions that commonly occur after hospital discharge.6, 16, 20 The results of this study provide complementary evidence, showing that patients often feel they have experienced suboptimal communication in the hospital setting. These findings highlight an opportunity for improvement in care transitions and patient safety, particularly among patients with inadequate health literacy.
In outpatient research that utilized the IPC, Schillinger et al.41 found that patients with inadequate functional health literacy reported significantly worse communication on the domains of general clarity, explanations of processes of care, and Explanations of condition and prognosis. Subsequent analyses by Sudore et al.47 demonstrated that patients with inadequate or marginal health literacy more often reported that physicians did not give them enough time to say what they thought was important, did not explain processes of care well, and did not ask about problems in following the recommended treatment (Table 3, IPC items 3, 12, and 26, respectively). Our findings were very similar. These relatively consistent results across studies and populations strengthen the conclusion that patients with inadequate health literacy feel their physicians do not communicate as effectively in these areas.
| IPC Items | Overall (n = 84), Mean (SD) | Inadequate Literacy (n = 37), Mean (SD) | Marginal or Adequate Literacy (n = 47), Mean (SD) | P Value |
|---|---|---|---|---|
| ||||
| General clarity* | ||||
| 1. Did the doctors use medical words you did not understand? | 3.35 (1.14) | 2.92 (1.40) | 3.68 (0.73) | 0.004 |
| 2. Did you have trouble understanding your doctors because they spoke too fast? | 3.98 (1.06) | 3.81 (1.13) | 4.11 (1.01) | 0.21 |
| Responsiveness to patient concerns | ||||
| 3. Did the doctors give you enough time to say what you thought was important? | 1.85 (1.14) | 2.27 (1.28) | 1.51 (0.88) | 0.003 |
| 4. Did the doctors listen carefully to what you had to say? | 1.62 (0.88) | 1.76 (1.04) | 1.51 (0.72) | 0.22 |
| 5. Did the doctors ignore what you told them? | 1.70 (0.92) | 1.81 (1.09) | 1.62 (0.77) | 0.38 |
| 6. Did the doctors take your concerns seriously? | 1.55 (0.92) | 1.65 (0.98) | 1.47 (0.88) | 0.38 |
| Explanations of condition and prognosis | ||||
| 7. Did the doctors give you enough information about your health problems? | 1.88 (1.11) | 2.11 (1.27) | 1.70 (0.95) | 0.11 |
| 8. Did the doctors make sure you understand your health problems? | 1.62 (0.88) | 1.76 (0.98) | 1.51 (0.78) | 0.22 |
| Explanations of processes of care | ||||
| 9. Did the doctors explain why a test was being done? | 1.70 (1.10) | 1.89 (1.24) | 1.55 (0.95) | 0.16 |
| 10. Did the doctors explain how the test was done? | 2.20 (1.35) | 2.27 (1.39) | 2.15 (1.34) | 0.69 |
| 11. Did the doctors tell you what they were doing as they examined you? | 1.99 (1.20) | 2.22 (1.34) | 1.81 (1.06) | 0.13 |
| 12. Did you feel confused about what was going on with your medical care because doctors did not explain things well? | 2.13 (1.23) | 2.51 (1.47) | 1.83 (0.92) | 0.02 |
| Explanations of self‐care | ||||
| 13. Did the doctors tell you what you could do to take care of yourself at home? | 1.67 (1.09) | 1.81 (1.29) | 1.55 (0.90) | 0.31 |
| 14. Did the doctors tell you how to pay attention to your symptoms and when to call the doctor? | 2.01 (1.38) | 2.19 (1.60) | 1.87 (1.17) | 0.32 |
| 15. Did the doctors clearly explain how to take the medicine (that is when, how much and for how long)? | 1.88 (1.36) | 2.00 (1.53) | 1.79 (1.22) | 0.48 |
| 16. Did the doctors go over all the medicines you are taking? | 2.39 (1.55) | 2.51 (1.74) | 2.30 (1.40) | 0.54 |
| 17. Did the doctors give you written instruction about how to take the medicine (other than what was on the container)? | 3.29 (1.70) | 3.05 (1.75) | 3.48 (1.66) | 0.26 |
| 18. Did the doctors tell you the reason for taking each medicine? | 2.05 (1.43) | 2.24 (1.64) | 1.89 (1.24) | 0.29 |
| 19. Did the doctors tell you about side effects you might get from your medicine? | 3.32 (1.64) | 3.11 (1.73) | 3.49 (1.56) | 0.29 |
| Empowerment | ||||
| 20. Did doctors make you feel that following your treatment plan would make a difference in your health? | 1.75 (1.07) | 1.89 (1.27) | 1.64 (0.90) | 0.31 |
| 21. Did the doctors make you feel that your everyday activities such as your diet and lifestyle would make a difference in your health? | 1.77 (1.21) | 1.81 (1.41) | 1.74 (1.03) | 0.81 |
| Decision‐making | ||||
| 22. Did the doctors try to involve you or include you in decisions about your treatment? | 2.43 (1.55) | 2.30 (1.49) | 2.53 (1.60) | 0.49 |
| 23. Did the doctors ask how you felt about different treatments? | 3.08 (1.58) | 2.89 (1.66) | 3.23 (1.51) | 0.33 |
| 24. Did the doctors make decision without taking your preferences and opinions into account? | 2.23 (1.35) | 2.34 (1.55) | 2.15 (1.20) | 0.54 |
| 25. Did you feel pressured by doctors in the hospital to have a treatment you were not sure you wanted? | 1.60 (0.97) | 1.81 (1.18) | 1.43 (0.74) | 0.09 |
| Consideration of patients' desire and ability to comply with recommendations | ||||
| 26. Did the doctors ask if you might have any problems actually doing the recommended treatment (for example taking the medication correctly)? | 3.82 (1.47) | 4.08 (1.40) | 3.62 (1.51) | 0.15 |
| 27. Did the doctors understand the kinds of problems you might have in doing the recommended treatment? | 2.43 (1.44) | 2.26 (1.52) | 2.57 (1.38) | 0.34 |
Importantly, the differences in patient responses by literacy category were driven by a few IPC items. These items pertained to physicians' use of medical terminology, the amount of time they gave patients to express their concerns, and how well they explained the patients' medical care. Training physicians to improve their communication skills in these specific areas may improve their ability to communicate effectively with patients who have limited literacy skills. Indeed, published recommendations on how to improve the clarity of verbal communication emphasize just a few major areas, including limiting the amount of medical terminology used, effectively encouraging patients to ask questions and express their concerns, and asking patients to teach‐back key points to make sure the physician has provided adequate explanation.4851 The present study provides some evidence for those recommendations, which for the most part, have been based on clinical experience and expert opinion.
There remains a need for professional education about health literacy and techniques to improve communication with patients who may have limited literacy skills. Many experts advocate clear verbal communication with all patients, so‐called Universal Precautions.52 Although 10 years have passed since the American Medical Association (AMA) called for more work in this area,53 few curricula have been described in the literature.48, 5456 The extent to which health literacy curricula have been implemented in medical schools and other professional schools is unknown. The impact of such training on the communication skills of health care providers and patient outcomes is also unclear.
The strengths of this study include a relatively good response rate and use of a validated measure to grade the quality of physician‐patient communication. This measure, the IPC, has been used previously in the context of health literacy.41 Nevertheless, certain limitations should be acknowledged. First, the study was performed at a single teaching hospital, where patients had a high prevalence of inadequate health literacy. The findings may not generalize to other institutions that serve a different patient population or to nonacademic programs. Second, communication was assessed by patient report, rather than by recording patient‐provider discussions for rating by independent observers. While patient report is inherently more subjective, patients' own perceptions about the effectiveness of health communication are arguably more important than those of independent raters, and thus, the data source may not represent a true limitation. Third, patient responses were obtained approximately 2 weeks after hospital discharge, and accordingly, they are subject to recall bias, which may be greater among those with cognitive impairment. Finally, patients were directed to rate the communication of the overall group of physicians who took care of them in the hospital. Given the academic setting, patients typically received care from a team that included medical students, interns, a resident, and an attending physician. We were not able to determine whether patients' ratings were influenced by a specific member of the team, nor how ratings may have been influenced by certain characteristics of that team member (eg, year of training, prior communication skills training, race or gender concordance, etc).
In summary, by surveying patients soon after an acute care hospitalization, we determined that certain areas held room for improvement, such as consideration of patients' desire and ability to comply with treatment recommendations. Patients with inadequate health literacy reported lower quality physician‐patient communication on several domains. They expressed particular concern about physicians' use of medical terminology, not getting enough time to express their concerns, and not receiving clear enough explanations about the medical care. Efforts are needed to improve physicians' communication skills in these areas. Such training should be evaluated to determine if it has a beneficial effect on physician communication skills and patient outcomes.
- ,,,,.The gap between patient reading comprehension and the readability of patient education materials.J Fam Pract.1990;31:533–538.
- .Differences between patients' and doctors' interpretation of some common medical terms.BMJ.1970;1:286–289.
- ,,.Patient understanding of commonly used medical vocabulary.J Fam Pract.1987;25:176–178.
- ,,,,,.Improving education for patients with low literacy skills.Am Fam Physician.1996;53:205–211.
- ,,,.Doctor‐patient communication: a review of the literature.Soc Sci Med.1995;40:903–918.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,.The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process.J Patient Saf.2007;3(2):97–106.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80(8):991–994.
- ,,.Hospital discharge information and older patients: do they get what they need?J Hosp Med.2007;2(5):291–296.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust NZ J Med.1999;29(2):220–227.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- ,,, et al.Adherence to medications by patients after acute coronary syndromes.Ann Pharmacother.2005;39(11):1792–1797.
- ,,, et al.Impact of medication therapy discontinuation on mortality after myocardial infarction.Arch Intern Med.2006;166(17):1842–1847.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,,,.Impact of patient communication problems on the risk of preventable adverse events in acute care settings.CMAJ.2008;178(12):1555–1562.
- ,,.Communication gaps and readmissions to hospital for patients aged 75 years and older: observational study.Qual Saf Health Care.2008;17(1):71–75.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- Institute of Medicine.Health Literacy. A Prescription to End Confusion.Washington, DC:National Academies Press;2004.
- ,,,.Current Bibliographies in Medicine: Health Literacy.Bethesda, MD:National Library of Medicine;2000.
- ,,. National Assessment of Adult Literacy (NAAL). A first look at the literacy of America's adults in the 21st century. Available at: http://nces.ed.gov/naal. Accessed January2010.
- ,,, et al.The health care experience of patients with low literacy.Arch Fam Med.1996;5:329–334.
- ,,,.Patient literacy and question‐asking behavior in the medical encounter: a mixed‐methods analysis.J Gen Intern Med.2007;22(6):782–786.
- ,,,.Babel babble: physicians' use of unclarified medical jargon with patients.Am J Health Behav.2007;31(Suppl 1):S85–S95.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,.Relationship of functional health literacy to patients' knowledge of their chronic disease: a study of patients with hypertension and diabetes.Arch Intern Med.1998;158(2):166–172.
- ,,,.Health literacy and the risk of hospital admission.J Gen Intern Med.1998;13:791–798.
- ,,, et al.Limited literacy and mortality in the elderly: The Health, Aging, and Body Composition Study.J Gen Intern Med.2006;21(8):806–812.
- ,,,,,.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167(14):1503–1509.
- ,,,,.Literacy and health outcomes: a systematic review of the literature.J Gen Intern Med.2004;19(12):1129–1139.
- ,,, et al.Inadequate functional health literacy among patients at two public hospitals.JAMA.1995;274(21):1677–1682.
- ,,, et al.ACC/AHA 2002 guideline update for the management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction–summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina).J Am Coll Cardiol.2002;40(7):1366–1374.
- ,,, et al.Rapid assessment of literacy levels of adult primary care patients.Fam Med.1991;23(6):433–435.
- ,,,.Literacy testing in health care research. In: Schwartzberg JG, VanGeest JB, Wang CC, eds.Understanding Health Literacy.Chicago:American Medical Association;2005:157–179.
- ,,.“Mini‐Mental State”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,, et al.Interpersonal processes of care in diverse populations.Milbank Q.1999;77:305–339.
- ,,,,.Functional health literacy and the quality of physician‐patient communication among diabetes patients.Patient Educ Couns.2004;52(3):315–323.
- ,,,.Frequency and predictors of prescription‐related issues after hospital discharge.J Hosp Med.2008;3(1):12–19.
- ,,,,.Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure.Am J Health Syst Pharm.2004;61(19):2043–2049.
- ,,,,,.Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills.Clin Pharmacol Ther.2009;85(6):651–658.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113(24):2803–2809.
- ,,.Patient‐physician communication: a descriptive summary of the literature.Patient Educ Couns.1988;12:99–119.
- ,,,,,.Unraveling the relationship between literacy, language proficiency, and patient‐physician communication.Patient Educ Couns.2009;75(3):398–402.
- ,.Teaching about health literacy and clear communication.J Gen Intern Med.2006;21:888–890.
- .Health Literacy: A Manual for Clinicians.Chicago, IL:American Medical Association;2003.
- ,.Communicating with patients who cannot read.N Engl J Med.1997;337:272–274.
- ,,,.The role of health literacy in patient‐physician communication.Fam Med.2002;34(5):383–389.
- ,,,,,.Health literacy: universal precautions needed.J Allied Health.2004;33(2):150–155.
- American Medical Association Council on Scientific Affairs.Health literacy.JAMA.1999;281:552–557.
- ,,.Teaching medical students about health literacy: 2 Chicago initiatives.Am J Health Behav.2007;31Suppl 1:S111–S114.
- ,,,,,.Development and implementation of a health literacy training program for medical residents.Med Educ Online.2006;11(13):1–8.
- ,.The use of standardized patients to teach low‐literacy communication skills.Am J Health Behav.2007;31Suppl 1:S105–S110.
- ,,,,.The gap between patient reading comprehension and the readability of patient education materials.J Fam Pract.1990;31:533–538.
- .Differences between patients' and doctors' interpretation of some common medical terms.BMJ.1970;1:286–289.
- ,,.Patient understanding of commonly used medical vocabulary.J Fam Pract.1987;25:176–178.
- ,,,,,.Improving education for patients with low literacy skills.Am Fam Physician.1996;53:205–211.
- ,,,.Doctor‐patient communication: a review of the literature.Soc Sci Med.1995;40:903–918.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,.The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process.J Patient Saf.2007;3(2):97–106.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80(8):991–994.
- ,,.Hospital discharge information and older patients: do they get what they need?J Hosp Med.2007;2(5):291–296.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust NZ J Med.1999;29(2):220–227.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- ,,, et al.Adherence to medications by patients after acute coronary syndromes.Ann Pharmacother.2005;39(11):1792–1797.
- ,,, et al.Impact of medication therapy discontinuation on mortality after myocardial infarction.Arch Intern Med.2006;166(17):1842–1847.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,,,.Impact of patient communication problems on the risk of preventable adverse events in acute care settings.CMAJ.2008;178(12):1555–1562.
- ,,.Communication gaps and readmissions to hospital for patients aged 75 years and older: observational study.Qual Saf Health Care.2008;17(1):71–75.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- Institute of Medicine.Health Literacy. A Prescription to End Confusion.Washington, DC:National Academies Press;2004.
- ,,,.Current Bibliographies in Medicine: Health Literacy.Bethesda, MD:National Library of Medicine;2000.
- ,,. National Assessment of Adult Literacy (NAAL). A first look at the literacy of America's adults in the 21st century. Available at: http://nces.ed.gov/naal. Accessed January2010.
- ,,, et al.The health care experience of patients with low literacy.Arch Fam Med.1996;5:329–334.
- ,,,.Patient literacy and question‐asking behavior in the medical encounter: a mixed‐methods analysis.J Gen Intern Med.2007;22(6):782–786.
- ,,,.Babel babble: physicians' use of unclarified medical jargon with patients.Am J Health Behav.2007;31(Suppl 1):S85–S95.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,.Relationship of functional health literacy to patients' knowledge of their chronic disease: a study of patients with hypertension and diabetes.Arch Intern Med.1998;158(2):166–172.
- ,,,.Health literacy and the risk of hospital admission.J Gen Intern Med.1998;13:791–798.
- ,,, et al.Limited literacy and mortality in the elderly: The Health, Aging, and Body Composition Study.J Gen Intern Med.2006;21(8):806–812.
- ,,,,,.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167(14):1503–1509.
- ,,,,.Literacy and health outcomes: a systematic review of the literature.J Gen Intern Med.2004;19(12):1129–1139.
- ,,, et al.Inadequate functional health literacy among patients at two public hospitals.JAMA.1995;274(21):1677–1682.
- ,,, et al.ACC/AHA 2002 guideline update for the management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction–summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina).J Am Coll Cardiol.2002;40(7):1366–1374.
- ,,, et al.Rapid assessment of literacy levels of adult primary care patients.Fam Med.1991;23(6):433–435.
- ,,,.Literacy testing in health care research. In: Schwartzberg JG, VanGeest JB, Wang CC, eds.Understanding Health Literacy.Chicago:American Medical Association;2005:157–179.
- ,,.“Mini‐Mental State”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,, et al.Interpersonal processes of care in diverse populations.Milbank Q.1999;77:305–339.
- ,,,,.Functional health literacy and the quality of physician‐patient communication among diabetes patients.Patient Educ Couns.2004;52(3):315–323.
- ,,,.Frequency and predictors of prescription‐related issues after hospital discharge.J Hosp Med.2008;3(1):12–19.
- ,,,,.Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure.Am J Health Syst Pharm.2004;61(19):2043–2049.
- ,,,,,.Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills.Clin Pharmacol Ther.2009;85(6):651–658.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113(24):2803–2809.
- ,,.Patient‐physician communication: a descriptive summary of the literature.Patient Educ Couns.1988;12:99–119.
- ,,,,,.Unraveling the relationship between literacy, language proficiency, and patient‐physician communication.Patient Educ Couns.2009;75(3):398–402.
- ,.Teaching about health literacy and clear communication.J Gen Intern Med.2006;21:888–890.
- .Health Literacy: A Manual for Clinicians.Chicago, IL:American Medical Association;2003.
- ,.Communicating with patients who cannot read.N Engl J Med.1997;337:272–274.
- ,,,.The role of health literacy in patient‐physician communication.Fam Med.2002;34(5):383–389.
- ,,,,,.Health literacy: universal precautions needed.J Allied Health.2004;33(2):150–155.
- American Medical Association Council on Scientific Affairs.Health literacy.JAMA.1999;281:552–557.
- ,,.Teaching medical students about health literacy: 2 Chicago initiatives.Am J Health Behav.2007;31Suppl 1:S111–S114.
- ,,,,,.Development and implementation of a health literacy training program for medical residents.Med Educ Online.2006;11(13):1–8.
- ,.The use of standardized patients to teach low‐literacy communication skills.Am J Health Behav.2007;31Suppl 1:S105–S110.
Copyright © 2010 Society of Hospital Medicine
Hospitalists' Awareness of Patient Charges
Hospitalists have been suggested to offer rational and efficient medical care through specialized knowledge about inpatient care services.1 The goal that hospitalists will use care resources more efficiently presumes hospitalists' accurate knowledge about charges and costs.
Data regarding physicians' awareness of care charges and its impact upon care is limited. An international meta‐analysis of clinicians' awareness of pharmaceutical prices demonstrated poor accuracy of physicians' estimates of charges, but effects of increasing their knowledge remained unexamined.2 Continuous exposure to education and alerts about charges have been demonstrated to diminish physicians' unnecessary use of specific laboratory assays in a single teaching hospital, in a pediatric emergency department, and an outpatient primary care system; test use declined when physicians were alerted to test charges at the point‐of‐care without negative impact upon clinical outcomes; when the notices ceased, utilization climbed back towards baseline levels. Specific to inpatient care, a single‐center study evaluated the impact of price‐alerts upon laboratory and imaging use, but showed no effects.36
Applicability of these existing data for contemporary hospitalists are limited, and most data were collected before hospitalists developed as an organized focus of practice. A review of existing literature revealed no published data demonstrating hospitalists' higher expert awareness of charges generated by inpatient care. Published comparisons of the care expense generated by hospitalists' care versus that of general internists or academic teams have shown minimal and inconsistent effects.79 Those data showing reduced costs from hospitalists were associated with small length‐of‐stay reductions, rather than more expert resource utilization.7 We measured the accuracy and precision of hospitalist's estimates of charges associated with services commonly used in inpatient care.
Setting
Two community‐based private, academic‐affiliated hospitals operated by a not‐for‐profit health system in Washington State, comprising together 895 inpatient beds. The questionnaire instrument was approved by the governing Institutional Review Board (IRB).
Methods
A list of true charges for 14 services, procedures, tests, and physician charges commonly ordered by adult medicine hospitalists was acquired directly from the responsible departments of a multi‐hospital system operated by a single non‐profit entity using a unified chargemaster. Specifically, we acquired the charge that a hypothetical self‐paying patient would receive for each service, excluding any adjustments exercised by other payer sources. The list of charges was reported to the organization's financial officers for affirmation. Physician charges were standardized to geographically‐adjusted Medicare charges obtained directly from the American Medical Assocation's online Common Procedural Terminology tool.10 A cross‐section of hospitalists (n = 25) was surveyed from a private hospitalist group and an academically‐affiliated hospitalist service. Hospitalists included US and international medical graduates, new‐graduates from residency training and clinicians with a range of prior experiences in academic centers, government hospital systems, and private primary care. Respondents were asked to estimate to the nearest dollar the billing charge that a hypothetical self‐pay patient would receive for each care item. Direct data collection was arranged through the groups' medical directors and occurred in the hospitalist groups' regular business meetings. The design gathered no data on individual characteristics of respondents such as domestic or international education, sex, age, or time in practice, nor from which practice‐group a given respondent originated, to affirm to participants that their responses could not be used to imply performance measures or quality profiles.
Findings
Hospitalists tended to rank the expense of items in essentially the correct order, as reflected by the rough trend of rising estimates compared to true‐charges (Figure 1). Of note, we did not ask hospitalists to discern the different charges of 2 appropriate competing clinical choices, but asked for estimated prices of diverse services. The range of respondents' estimates about each item was broad. The mean‐value of hospitalists' estimates for each care item was less revealing than the range and diversity of estimates about each care item. Accuracy of hospitalists' estimates of charges was poor. Only 10.8% of hospitalists' estimates were within 10% of the actual unadjusted charge, 17.8% within 20% of that charge, and 24.8% were within a 30% margin of accuracy. Summary results are presented (Table 1). Pearson's r correlation value between the unadjusted charges and the estimates made by hospitalists was 0.548, a coefficient of determination equal to 0.300. Thus, the true charges list we obtained had only a low‐grade association with hospitalists' estimates (Figure 1). Hospitalists' estimates about the charges of relatively‐expensive items (abdominal computed tomography [CT]) overlapped with their estimates about the least‐expensive items (such as a urine culture ). Inter‐hospitalist agreement about charges associated with each care item was also low; estimates for each care item charge varied over logarithmic orders of magnitude.
| Care Service | Unadjusted Charge, USD$ | Mean Estimate, USD$ | Minimum Estimate, USD$ | Maximum Estimate, USD$ | % of Estimates Within 10% Accuracy | % of Estimates Within 20% Accuracy | % of Estimates Within 30% Accuracy |
|---|---|---|---|---|---|---|---|
| |||||||
| Complete blood count | 30 | 73 | 10 | 440 | 16 | 20 | 20 |
| Complete metabolic panel | 37 | 135 | 15 | 1200 | 4 | 16 | 16 |
| Urinalysis with microscopy | 37 | 53 | 15 | 105 | 12 | 20 | 24 |
| Urine culture | 26 | 77 | 20 | 200 | 4 | 16 | 20 |
| Ward bed, charge per night | 744 | 998 | 300 | 3000 | 20 | 20 | 20 |
| ICU Bed, charge per night | 1107 | 2018 | 750 | 6000 | 8 | 12 | 12 |
| Chest x‐ray | 271 | 169 | 60 | 700 | 12 | 16 | 24 |
| CT scan, abdomen | 2204 | 803 | 150 | 1800 | 0 | 4 | 4 |
| Methylpredisolone 125 mg IV dose | 26.63 | 63 | 3 | 200 | 4 | 20 | 24 |
| Levofloxacin 500 mg IV dose | 105.41 | 114 | 10 | 500 | 24 | 28 | 36 |
| Levofloxacin 500 mg oral dose | 29.78 | 25 | 4 | 70 | 12 | 12 | 20 |
| Admission services (CPT code 99223) | 169.56 | 225 | 100 | 700 | 8 | 36 | 52 |
| Inpatient care services (CPT code 99232) | 62.47 | 110 | 40 | 400 | 12 | 28 | 48 |
| Central venous catheter placement (CPT 36569) | 286.04 | 338 | 50 | 1200 | 8 | 16 | 28 |
| Average % correct | 10.8 | 17.8 | 24.8 | ||||
Discussion
To date, hospitalist programs have shown little impact upon care costs when compared with other inpatient care staffing models. One limiting factor may be the opacity of medical care pricing. Patients have been demonstrated to have little access to knowledge of what care will cost them and complex barriers prevent them from gaining pricing information.11, 12 Hospitalists may be conjectured to serve as expert sources on the costs and values of medical care services on behalf of inpatients, but our observations suggest that hospitalists' actual knowledge of patient‐charges is lacking. The opacity of US medical prices to patients appears to extend to hospital‐care physicians as well. We observe that no widespread mechanism exists by which hospitalists would be well‐positioned to become informed about the actual charges their patients receive. Unadjusted chargemaster lists are generally restricted information, and would be difficult to access outside of participation in the charge‐notifications used in the existing studies cited above.
The inquiry was specifically limited to how closely hospitalists' estimates of the unadjusted charges for some commonly‐ordered items compare to the actual unadjusted chargemaster at their own institutions. We did not assess the hospitalists' perceptions about the accuracy of their estimates, nor the impact of specific hospitalist characteristics upon accuracy. Our sample's representation of the larger national population of hospital physicians is not established, but engenders no expectation that these clinicians' charge‐awareness is substantively different from that of hospitalists in most other institutions. It is not known what specific clinician or practice‐setting characteristics will direct charge‐awareness, or will influence the impact of charge‐awareness upon clinical practices.
The range of estimates different hospitalists made about the same care items in the same facilities was very broad, which argues that respondents did not estimate charges based upon a different knowledge base of which the investigators were unaware. This is important because our use of unadjusted charges to self‐pay patients as true prices is necessarily somewhat arbitrary. Chargemaster price may not reflect the institution's cost of performing the service, the different prices paid for a single service by different payer sources, nor reflect services' true value based upon outcomes. However, recognizing that these actual prices are somewhat artificial, the use of these prices suffices for the current inquiry, and does not negate our findings of hospitalists' low accuracy and low agreement. Also noteworthy, an unadjusted chargemaster can often represent the charges received by those uninsured US patients for whom payer‐source adjustment is inaccessible, and informs downstream accounting such as the value of unpaid care a hospital delivers annually.
The most immediate matter for examination among hospitalists is what effects increased charge‐awareness may exert upon clinical decisions and practice processes. It appears that the premise that hospitalists' exercise expert knowledge of costs is likely not valid; but it is unknown whether accurate charge‐awareness among hospitalists will improve cost‐reductions by hospitalists. In some payer‐arrangements, accurate charge‐awareness might engender reduced care quantity, rather than increased efficiency or quality. The impact of upgrading hospitalists' knowledge about the charges and costs they generate, and the most effective method to do so, is worthy of investigation; based upon this initial data we encourage and are undertaking a larger‐scale study and exploration of the effects of enhanced hospitalist charge‐awareness.
Conclusion
Hospitalists have low awareness of the charges associated with inpatient care. The opacity of hospital care pricing to patient populations extends also to hospitalist physicians. Hospitalists likely do not improve cost‐efficiency through expert knowledge of services' costs to patients. Education and reminder systems to apprise hospitalists of charges should be examined as possible tools to optimize the use of inpatient care resources.
- ,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–201.
- ,,.Physician awareness of drug cost: a systematic review.PLoS Med.2007;4(9):e283.
- ,,,,.The effect of price information on test‐ordering behavior and patient outcomes in a pediatric emergency department.Pediatrics.1999;103(4):877–882.
- ,,.The effect on test ordering of informing physicians of the charges for outpatient diagnostic tests.N Engl J Med1990;322:1499–1504.
- ,,,.Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy.Postgrad Med J.2006;82:823–829.
- ,,, et al.Tanasijevic does the computerized display of charges affect inpatient ancillary test utilization?Arch Intern Med.1997;157(21):2501–2508.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the united states: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22:662–667.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357(25):2589–2600.
- American Medical Association, CPT and RVU Search utility. Available at: https://catalog.ama‐assn.org/Catalog/cpt/cpt_search.jsp. Accessed December 2009.
- ,.Does price tranparency improve market efficiency? Implications of empirical evidence in other markets for the health sector. 2007, Congressional Research Service Report RL34101. Available at: http://ftp.fas.org/sgp/crs/secrecy/RL34101. Accessed December2009.
- .The pricing of US hospital services.Health Aff.2006;25(1):57–69.
Hospitalists have been suggested to offer rational and efficient medical care through specialized knowledge about inpatient care services.1 The goal that hospitalists will use care resources more efficiently presumes hospitalists' accurate knowledge about charges and costs.
Data regarding physicians' awareness of care charges and its impact upon care is limited. An international meta‐analysis of clinicians' awareness of pharmaceutical prices demonstrated poor accuracy of physicians' estimates of charges, but effects of increasing their knowledge remained unexamined.2 Continuous exposure to education and alerts about charges have been demonstrated to diminish physicians' unnecessary use of specific laboratory assays in a single teaching hospital, in a pediatric emergency department, and an outpatient primary care system; test use declined when physicians were alerted to test charges at the point‐of‐care without negative impact upon clinical outcomes; when the notices ceased, utilization climbed back towards baseline levels. Specific to inpatient care, a single‐center study evaluated the impact of price‐alerts upon laboratory and imaging use, but showed no effects.36
Applicability of these existing data for contemporary hospitalists are limited, and most data were collected before hospitalists developed as an organized focus of practice. A review of existing literature revealed no published data demonstrating hospitalists' higher expert awareness of charges generated by inpatient care. Published comparisons of the care expense generated by hospitalists' care versus that of general internists or academic teams have shown minimal and inconsistent effects.79 Those data showing reduced costs from hospitalists were associated with small length‐of‐stay reductions, rather than more expert resource utilization.7 We measured the accuracy and precision of hospitalist's estimates of charges associated with services commonly used in inpatient care.
Setting
Two community‐based private, academic‐affiliated hospitals operated by a not‐for‐profit health system in Washington State, comprising together 895 inpatient beds. The questionnaire instrument was approved by the governing Institutional Review Board (IRB).
Methods
A list of true charges for 14 services, procedures, tests, and physician charges commonly ordered by adult medicine hospitalists was acquired directly from the responsible departments of a multi‐hospital system operated by a single non‐profit entity using a unified chargemaster. Specifically, we acquired the charge that a hypothetical self‐paying patient would receive for each service, excluding any adjustments exercised by other payer sources. The list of charges was reported to the organization's financial officers for affirmation. Physician charges were standardized to geographically‐adjusted Medicare charges obtained directly from the American Medical Assocation's online Common Procedural Terminology tool.10 A cross‐section of hospitalists (n = 25) was surveyed from a private hospitalist group and an academically‐affiliated hospitalist service. Hospitalists included US and international medical graduates, new‐graduates from residency training and clinicians with a range of prior experiences in academic centers, government hospital systems, and private primary care. Respondents were asked to estimate to the nearest dollar the billing charge that a hypothetical self‐pay patient would receive for each care item. Direct data collection was arranged through the groups' medical directors and occurred in the hospitalist groups' regular business meetings. The design gathered no data on individual characteristics of respondents such as domestic or international education, sex, age, or time in practice, nor from which practice‐group a given respondent originated, to affirm to participants that their responses could not be used to imply performance measures or quality profiles.
Findings
Hospitalists tended to rank the expense of items in essentially the correct order, as reflected by the rough trend of rising estimates compared to true‐charges (Figure 1). Of note, we did not ask hospitalists to discern the different charges of 2 appropriate competing clinical choices, but asked for estimated prices of diverse services. The range of respondents' estimates about each item was broad. The mean‐value of hospitalists' estimates for each care item was less revealing than the range and diversity of estimates about each care item. Accuracy of hospitalists' estimates of charges was poor. Only 10.8% of hospitalists' estimates were within 10% of the actual unadjusted charge, 17.8% within 20% of that charge, and 24.8% were within a 30% margin of accuracy. Summary results are presented (Table 1). Pearson's r correlation value between the unadjusted charges and the estimates made by hospitalists was 0.548, a coefficient of determination equal to 0.300. Thus, the true charges list we obtained had only a low‐grade association with hospitalists' estimates (Figure 1). Hospitalists' estimates about the charges of relatively‐expensive items (abdominal computed tomography [CT]) overlapped with their estimates about the least‐expensive items (such as a urine culture ). Inter‐hospitalist agreement about charges associated with each care item was also low; estimates for each care item charge varied over logarithmic orders of magnitude.
| Care Service | Unadjusted Charge, USD$ | Mean Estimate, USD$ | Minimum Estimate, USD$ | Maximum Estimate, USD$ | % of Estimates Within 10% Accuracy | % of Estimates Within 20% Accuracy | % of Estimates Within 30% Accuracy |
|---|---|---|---|---|---|---|---|
| |||||||
| Complete blood count | 30 | 73 | 10 | 440 | 16 | 20 | 20 |
| Complete metabolic panel | 37 | 135 | 15 | 1200 | 4 | 16 | 16 |
| Urinalysis with microscopy | 37 | 53 | 15 | 105 | 12 | 20 | 24 |
| Urine culture | 26 | 77 | 20 | 200 | 4 | 16 | 20 |
| Ward bed, charge per night | 744 | 998 | 300 | 3000 | 20 | 20 | 20 |
| ICU Bed, charge per night | 1107 | 2018 | 750 | 6000 | 8 | 12 | 12 |
| Chest x‐ray | 271 | 169 | 60 | 700 | 12 | 16 | 24 |
| CT scan, abdomen | 2204 | 803 | 150 | 1800 | 0 | 4 | 4 |
| Methylpredisolone 125 mg IV dose | 26.63 | 63 | 3 | 200 | 4 | 20 | 24 |
| Levofloxacin 500 mg IV dose | 105.41 | 114 | 10 | 500 | 24 | 28 | 36 |
| Levofloxacin 500 mg oral dose | 29.78 | 25 | 4 | 70 | 12 | 12 | 20 |
| Admission services (CPT code 99223) | 169.56 | 225 | 100 | 700 | 8 | 36 | 52 |
| Inpatient care services (CPT code 99232) | 62.47 | 110 | 40 | 400 | 12 | 28 | 48 |
| Central venous catheter placement (CPT 36569) | 286.04 | 338 | 50 | 1200 | 8 | 16 | 28 |
| Average % correct | 10.8 | 17.8 | 24.8 | ||||

Discussion
To date, hospitalist programs have shown little impact upon care costs when compared with other inpatient care staffing models. One limiting factor may be the opacity of medical care pricing. Patients have been demonstrated to have little access to knowledge of what care will cost them and complex barriers prevent them from gaining pricing information.11, 12 Hospitalists may be conjectured to serve as expert sources on the costs and values of medical care services on behalf of inpatients, but our observations suggest that hospitalists' actual knowledge of patient‐charges is lacking. The opacity of US medical prices to patients appears to extend to hospital‐care physicians as well. We observe that no widespread mechanism exists by which hospitalists would be well‐positioned to become informed about the actual charges their patients receive. Unadjusted chargemaster lists are generally restricted information, and would be difficult to access outside of participation in the charge‐notifications used in the existing studies cited above.
The inquiry was specifically limited to how closely hospitalists' estimates of the unadjusted charges for some commonly‐ordered items compare to the actual unadjusted chargemaster at their own institutions. We did not assess the hospitalists' perceptions about the accuracy of their estimates, nor the impact of specific hospitalist characteristics upon accuracy. Our sample's representation of the larger national population of hospital physicians is not established, but engenders no expectation that these clinicians' charge‐awareness is substantively different from that of hospitalists in most other institutions. It is not known what specific clinician or practice‐setting characteristics will direct charge‐awareness, or will influence the impact of charge‐awareness upon clinical practices.
The range of estimates different hospitalists made about the same care items in the same facilities was very broad, which argues that respondents did not estimate charges based upon a different knowledge base of which the investigators were unaware. This is important because our use of unadjusted charges to self‐pay patients as true prices is necessarily somewhat arbitrary. Chargemaster price may not reflect the institution's cost of performing the service, the different prices paid for a single service by different payer sources, nor reflect services' true value based upon outcomes. However, recognizing that these actual prices are somewhat artificial, the use of these prices suffices for the current inquiry, and does not negate our findings of hospitalists' low accuracy and low agreement. Also noteworthy, an unadjusted chargemaster can often represent the charges received by those uninsured US patients for whom payer‐source adjustment is inaccessible, and informs downstream accounting such as the value of unpaid care a hospital delivers annually.
The most immediate matter for examination among hospitalists is what effects increased charge‐awareness may exert upon clinical decisions and practice processes. It appears that the premise that hospitalists' exercise expert knowledge of costs is likely not valid; but it is unknown whether accurate charge‐awareness among hospitalists will improve cost‐reductions by hospitalists. In some payer‐arrangements, accurate charge‐awareness might engender reduced care quantity, rather than increased efficiency or quality. The impact of upgrading hospitalists' knowledge about the charges and costs they generate, and the most effective method to do so, is worthy of investigation; based upon this initial data we encourage and are undertaking a larger‐scale study and exploration of the effects of enhanced hospitalist charge‐awareness.
Conclusion
Hospitalists have low awareness of the charges associated with inpatient care. The opacity of hospital care pricing to patient populations extends also to hospitalist physicians. Hospitalists likely do not improve cost‐efficiency through expert knowledge of services' costs to patients. Education and reminder systems to apprise hospitalists of charges should be examined as possible tools to optimize the use of inpatient care resources.
Hospitalists have been suggested to offer rational and efficient medical care through specialized knowledge about inpatient care services.1 The goal that hospitalists will use care resources more efficiently presumes hospitalists' accurate knowledge about charges and costs.
Data regarding physicians' awareness of care charges and its impact upon care is limited. An international meta‐analysis of clinicians' awareness of pharmaceutical prices demonstrated poor accuracy of physicians' estimates of charges, but effects of increasing their knowledge remained unexamined.2 Continuous exposure to education and alerts about charges have been demonstrated to diminish physicians' unnecessary use of specific laboratory assays in a single teaching hospital, in a pediatric emergency department, and an outpatient primary care system; test use declined when physicians were alerted to test charges at the point‐of‐care without negative impact upon clinical outcomes; when the notices ceased, utilization climbed back towards baseline levels. Specific to inpatient care, a single‐center study evaluated the impact of price‐alerts upon laboratory and imaging use, but showed no effects.36
Applicability of these existing data for contemporary hospitalists are limited, and most data were collected before hospitalists developed as an organized focus of practice. A review of existing literature revealed no published data demonstrating hospitalists' higher expert awareness of charges generated by inpatient care. Published comparisons of the care expense generated by hospitalists' care versus that of general internists or academic teams have shown minimal and inconsistent effects.79 Those data showing reduced costs from hospitalists were associated with small length‐of‐stay reductions, rather than more expert resource utilization.7 We measured the accuracy and precision of hospitalist's estimates of charges associated with services commonly used in inpatient care.
Setting
Two community‐based private, academic‐affiliated hospitals operated by a not‐for‐profit health system in Washington State, comprising together 895 inpatient beds. The questionnaire instrument was approved by the governing Institutional Review Board (IRB).
Methods
A list of true charges for 14 services, procedures, tests, and physician charges commonly ordered by adult medicine hospitalists was acquired directly from the responsible departments of a multi‐hospital system operated by a single non‐profit entity using a unified chargemaster. Specifically, we acquired the charge that a hypothetical self‐paying patient would receive for each service, excluding any adjustments exercised by other payer sources. The list of charges was reported to the organization's financial officers for affirmation. Physician charges were standardized to geographically‐adjusted Medicare charges obtained directly from the American Medical Assocation's online Common Procedural Terminology tool.10 A cross‐section of hospitalists (n = 25) was surveyed from a private hospitalist group and an academically‐affiliated hospitalist service. Hospitalists included US and international medical graduates, new‐graduates from residency training and clinicians with a range of prior experiences in academic centers, government hospital systems, and private primary care. Respondents were asked to estimate to the nearest dollar the billing charge that a hypothetical self‐pay patient would receive for each care item. Direct data collection was arranged through the groups' medical directors and occurred in the hospitalist groups' regular business meetings. The design gathered no data on individual characteristics of respondents such as domestic or international education, sex, age, or time in practice, nor from which practice‐group a given respondent originated, to affirm to participants that their responses could not be used to imply performance measures or quality profiles.
Findings
Hospitalists tended to rank the expense of items in essentially the correct order, as reflected by the rough trend of rising estimates compared to true‐charges (Figure 1). Of note, we did not ask hospitalists to discern the different charges of 2 appropriate competing clinical choices, but asked for estimated prices of diverse services. The range of respondents' estimates about each item was broad. The mean‐value of hospitalists' estimates for each care item was less revealing than the range and diversity of estimates about each care item. Accuracy of hospitalists' estimates of charges was poor. Only 10.8% of hospitalists' estimates were within 10% of the actual unadjusted charge, 17.8% within 20% of that charge, and 24.8% were within a 30% margin of accuracy. Summary results are presented (Table 1). Pearson's r correlation value between the unadjusted charges and the estimates made by hospitalists was 0.548, a coefficient of determination equal to 0.300. Thus, the true charges list we obtained had only a low‐grade association with hospitalists' estimates (Figure 1). Hospitalists' estimates about the charges of relatively‐expensive items (abdominal computed tomography [CT]) overlapped with their estimates about the least‐expensive items (such as a urine culture ). Inter‐hospitalist agreement about charges associated with each care item was also low; estimates for each care item charge varied over logarithmic orders of magnitude.
| Care Service | Unadjusted Charge, USD$ | Mean Estimate, USD$ | Minimum Estimate, USD$ | Maximum Estimate, USD$ | % of Estimates Within 10% Accuracy | % of Estimates Within 20% Accuracy | % of Estimates Within 30% Accuracy |
|---|---|---|---|---|---|---|---|
| |||||||
| Complete blood count | 30 | 73 | 10 | 440 | 16 | 20 | 20 |
| Complete metabolic panel | 37 | 135 | 15 | 1200 | 4 | 16 | 16 |
| Urinalysis with microscopy | 37 | 53 | 15 | 105 | 12 | 20 | 24 |
| Urine culture | 26 | 77 | 20 | 200 | 4 | 16 | 20 |
| Ward bed, charge per night | 744 | 998 | 300 | 3000 | 20 | 20 | 20 |
| ICU Bed, charge per night | 1107 | 2018 | 750 | 6000 | 8 | 12 | 12 |
| Chest x‐ray | 271 | 169 | 60 | 700 | 12 | 16 | 24 |
| CT scan, abdomen | 2204 | 803 | 150 | 1800 | 0 | 4 | 4 |
| Methylpredisolone 125 mg IV dose | 26.63 | 63 | 3 | 200 | 4 | 20 | 24 |
| Levofloxacin 500 mg IV dose | 105.41 | 114 | 10 | 500 | 24 | 28 | 36 |
| Levofloxacin 500 mg oral dose | 29.78 | 25 | 4 | 70 | 12 | 12 | 20 |
| Admission services (CPT code 99223) | 169.56 | 225 | 100 | 700 | 8 | 36 | 52 |
| Inpatient care services (CPT code 99232) | 62.47 | 110 | 40 | 400 | 12 | 28 | 48 |
| Central venous catheter placement (CPT 36569) | 286.04 | 338 | 50 | 1200 | 8 | 16 | 28 |
| Average % correct | 10.8 | 17.8 | 24.8 | ||||

Discussion
To date, hospitalist programs have shown little impact upon care costs when compared with other inpatient care staffing models. One limiting factor may be the opacity of medical care pricing. Patients have been demonstrated to have little access to knowledge of what care will cost them and complex barriers prevent them from gaining pricing information.11, 12 Hospitalists may be conjectured to serve as expert sources on the costs and values of medical care services on behalf of inpatients, but our observations suggest that hospitalists' actual knowledge of patient‐charges is lacking. The opacity of US medical prices to patients appears to extend to hospital‐care physicians as well. We observe that no widespread mechanism exists by which hospitalists would be well‐positioned to become informed about the actual charges their patients receive. Unadjusted chargemaster lists are generally restricted information, and would be difficult to access outside of participation in the charge‐notifications used in the existing studies cited above.
The inquiry was specifically limited to how closely hospitalists' estimates of the unadjusted charges for some commonly‐ordered items compare to the actual unadjusted chargemaster at their own institutions. We did not assess the hospitalists' perceptions about the accuracy of their estimates, nor the impact of specific hospitalist characteristics upon accuracy. Our sample's representation of the larger national population of hospital physicians is not established, but engenders no expectation that these clinicians' charge‐awareness is substantively different from that of hospitalists in most other institutions. It is not known what specific clinician or practice‐setting characteristics will direct charge‐awareness, or will influence the impact of charge‐awareness upon clinical practices.
The range of estimates different hospitalists made about the same care items in the same facilities was very broad, which argues that respondents did not estimate charges based upon a different knowledge base of which the investigators were unaware. This is important because our use of unadjusted charges to self‐pay patients as true prices is necessarily somewhat arbitrary. Chargemaster price may not reflect the institution's cost of performing the service, the different prices paid for a single service by different payer sources, nor reflect services' true value based upon outcomes. However, recognizing that these actual prices are somewhat artificial, the use of these prices suffices for the current inquiry, and does not negate our findings of hospitalists' low accuracy and low agreement. Also noteworthy, an unadjusted chargemaster can often represent the charges received by those uninsured US patients for whom payer‐source adjustment is inaccessible, and informs downstream accounting such as the value of unpaid care a hospital delivers annually.
The most immediate matter for examination among hospitalists is what effects increased charge‐awareness may exert upon clinical decisions and practice processes. It appears that the premise that hospitalists' exercise expert knowledge of costs is likely not valid; but it is unknown whether accurate charge‐awareness among hospitalists will improve cost‐reductions by hospitalists. In some payer‐arrangements, accurate charge‐awareness might engender reduced care quantity, rather than increased efficiency or quality. The impact of upgrading hospitalists' knowledge about the charges and costs they generate, and the most effective method to do so, is worthy of investigation; based upon this initial data we encourage and are undertaking a larger‐scale study and exploration of the effects of enhanced hospitalist charge‐awareness.
Conclusion
Hospitalists have low awareness of the charges associated with inpatient care. The opacity of hospital care pricing to patient populations extends also to hospitalist physicians. Hospitalists likely do not improve cost‐efficiency through expert knowledge of services' costs to patients. Education and reminder systems to apprise hospitalists of charges should be examined as possible tools to optimize the use of inpatient care resources.
- ,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–201.
- ,,.Physician awareness of drug cost: a systematic review.PLoS Med.2007;4(9):e283.
- ,,,,.The effect of price information on test‐ordering behavior and patient outcomes in a pediatric emergency department.Pediatrics.1999;103(4):877–882.
- ,,.The effect on test ordering of informing physicians of the charges for outpatient diagnostic tests.N Engl J Med1990;322:1499–1504.
- ,,,.Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy.Postgrad Med J.2006;82:823–829.
- ,,, et al.Tanasijevic does the computerized display of charges affect inpatient ancillary test utilization?Arch Intern Med.1997;157(21):2501–2508.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the united states: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22:662–667.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357(25):2589–2600.
- American Medical Association, CPT and RVU Search utility. Available at: https://catalog.ama‐assn.org/Catalog/cpt/cpt_search.jsp. Accessed December 2009.
- ,.Does price tranparency improve market efficiency? Implications of empirical evidence in other markets for the health sector. 2007, Congressional Research Service Report RL34101. Available at: http://ftp.fas.org/sgp/crs/secrecy/RL34101. Accessed December2009.
- .The pricing of US hospital services.Health Aff.2006;25(1):57–69.
- ,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–201.
- ,,.Physician awareness of drug cost: a systematic review.PLoS Med.2007;4(9):e283.
- ,,,,.The effect of price information on test‐ordering behavior and patient outcomes in a pediatric emergency department.Pediatrics.1999;103(4):877–882.
- ,,.The effect on test ordering of informing physicians of the charges for outpatient diagnostic tests.N Engl J Med1990;322:1499–1504.
- ,,,.Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy.Postgrad Med J.2006;82:823–829.
- ,,, et al.Tanasijevic does the computerized display of charges affect inpatient ancillary test utilization?Arch Intern Med.1997;157(21):2501–2508.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the united states: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22:662–667.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357(25):2589–2600.
- American Medical Association, CPT and RVU Search utility. Available at: https://catalog.ama‐assn.org/Catalog/cpt/cpt_search.jsp. Accessed December 2009.
- ,.Does price tranparency improve market efficiency? Implications of empirical evidence in other markets for the health sector. 2007, Congressional Research Service Report RL34101. Available at: http://ftp.fas.org/sgp/crs/secrecy/RL34101. Accessed December2009.
- .The pricing of US hospital services.Health Aff.2006;25(1):57–69.
A Novel Approach to Physician Shortages
The demand for physician talent is intensifying as the US healthcare system confronts an unprecedented confluence of demographic pressures. Not only will 78 million retiring baby‐boomers require significant healthcare resources, but tens of thousands of practicing physicians will themselves reach retirement age within the next decade.1 At the same time, factors like the large increase in the percentage of female physicians (who are more likely to work part time), the growth of nonpractice opportunities for MDs, and generational demands for greater worklife balance are creating a major supply‐demand mismatch within the physician workforce.2 In this demographic atmosphere, the ability to recruit and retain physician leaders confers tremendous value to healthcare enterprisesboth public and private. Recruiting and retaining strategies already weigh heavily in the most palpable shortage areas, like primary care, but the system faces widespread unmet demand for a variety of specialist and generalist practitioners.36
This article does not address the public policy implications of the upcoming physician shortage, recognition of which will lead to the largest increase in new medical school slots in decades.7 Rather, we set out to illustrate how successful nonmedical businesses are embracing a thoughtful, systematic approach to retaining talent, based on the philosophy that keeping and engaging valued employees is more efficient than recruiting and orienting replacements. We posit that the innovations used by progressive companies could apply to recruitment and retention challenges confronting medicine.
How Industry Approaches the Talent Vacuum: Talent Facilitation
Leaders outside of medicine have long acknowledged that changing demographics and a global economy are driving unprecedented employee turnover.8 In confronting a talent vacuum, forward‐thinking managers have prioritized retaining key talent (rather than hiring anew) in planning for the future.9, 10 Doing so begins with attempts to understand the relationship between workers and the workplace, with a particular emphasis on appreciating workers' priorities. Indeed, new executive positions with titles like Chief Learning Officer and Chief Experience Officer are appearing as companies realize a need for focused expertise beyond traditional human resource departments. These companies understand that offering higher salaries is not the only retention strategyand often not even the most effective one.
The Four Actions of Talent Facilitation
The talent facilitation process centers on four actions: attract, engage, develop, and retain. None of these actions can stand alone, and all should be present, to some degree, at all stages of a worker's tenure. To attract or engage an employee or practice partner is not a 1‐time hook, but a constant and dynamic process.
Importantly, the concepts addressed here are not specific to 1 type of corporate system, size, or management level. Although the early business focus had been on upper‐level and executive talent within large corporate settings, there is an increasing recognition that a dedicated talent strategy is useful wherever recruiting and retaining talented people is important (and where is it not?).
The ideas presented here may seem most applicable to leaders of large physician corporations, hospital‐owned physician groups, or large integrated healthcare systems (such as Kaiser) that employ physicians. However, we also believe that the ideas apply across‐the‐board in medicine, including entities such as small, private, physician‐owned groups. We argue that regardless of the exact practice structure, a limited pool of resources must be dedicated to the attraction and retention of talented partners or employees, or to the cost of replacing those people if they pursue other opportunities (or the cost of inefficient and disengaged physicians). While an integrated health system may have the resources and scale to hire a Chief Experience Officer, we do not anticipate that a 5‐partner private practice would. Rather, we point to examples to illustrate the talent facilitation paradigm as a tool to systematically frame the allocation of those resources. Undoubtedly, the specific shape of a thoughtful talent facilitation effort will vary when applied in a large urban academic medical center vs. an integrated healthcare system vs. a small physician private practice, but the basic principles remain the same.
Attract
Increasingly, companies approach talented prospects with dedicated marketing campaigns to convey the value of a work environment.11 Silicon Valley employee lounges with free massages and foosball tables are the iconic example of attraction, but the concept runs deeper. Today's workers seek access to state‐of‐the‐art ideas and technology and often want to be part of a larger vision. Many seek opportunities to integrate their own professional and personal aspirations into a particular job description.
Hospital executives have long recognized the importance of attracting physicians to their facility (after all, the physicians draw patients and thus generate revenue). The traditional approach has surrounded perks, from comfortable doctor's lounges to the latest in surgical technology. But, the stakes seem greater now than before, and successful talent facilitation strategies are going beyond the tried and true.
Clearly, physicians seek financially stable practice settings with historical success. But they may also seek evidence of a defined strategic plan focused on more than mere profitability. Physicians may gravitate to practice environments that endorse progressive movements like the No One Dies Alone campaign.12 Similarly, recognition of movements beyond healthcarea commitment to Leadership in Energy and Environmental Design (LEED) (Green Building Rating System; US Green Building Council [USGBC];
The current recruitment campaign of California's prison healthcare system offers an unlikely source of inspiration. The prison system was placed in receivership to address a shortage of competent physician staff and other inadequacies. A central feature of the campaign is an attractive starting salary and good benefits. But, the campaign does not rely on money alone. For example, the campaign's website (

Engage
The corporate tool being employed at this stage is a strategy called on‐boarding, which emphasizes a streamlined integration of newcomers with existing workers and culture, and prioritizes aligning organizational roles with a worker's specific skills and interests. On‐boarding also emphasizes the value of early and frequent provision of constructive feedback from same‐level peers or managers with advanced coaching skills.
Many companies use formal survey tools to measure employee engagement and regularly evaluate the proficiency of system leaders in the ability to engage their employees. An engineering firm executive recently told us (P.K., C.K.) that he performs detailed and frequent on‐the‐job interviews, even with company veterans. The primary goal of these interviews is to ensure that engineers spend at least 85% of their time on work that: (1) they find interesting and (2) allows for the application of their best skills. Wherever possible, traditional job descriptions are altered to achieve this. Inevitably, there is still work (15% in this particular corporate vision) that no one prefers but needs to get done, but this process of active recalibration minimizes this fraction to the degree possible.
Even within a small physician practice group, one can imagine how a strategic approach of inviting and acknowledging individual physician's professional goals and particular talents may challenge the long‐held belief that everyone within a group enjoys and must do the exact same job. Once these goals and talents are articulated, groups may find that allowing for more customized roles within the practice enhances professional satisfaction.
Social networking, collaboration, and sharing of best practices are staples of engaging companies. The Cisco and Qualcomm companies, for example, utilize elaborate e‐networks (rough corporate equivalents to Facebook) to foster collegial interaction within and across traditional hierarchical boundaries so that managers and executives directly engage the ideas of employees at every level.14 The premise is logical: engaged employees will be more likely to contribute innovative ideas which, when listened to, are more likely to engage employees.
Most physicians will recognize the traditional resident report as a model for engagement. Beyond its educational value, interaction with program leadership, social bonding, collaborative effort, and exploration of best practices add tremendous value. Many companies would jump at the chance to engrain a similar cultural staple. Enhancing this type of interaction in a postresidency setting may promote engagement in a given system, especially if it facilitates interactions between physicians and senior hospital leaders. Absent these types of interactions, ensuring regular provision of peer review and/or constructive feedback can help systematically enhance 2‐way communication and enhance engagement.
Develop
Talent development relies on mentorship reflecting a genuine interest in an individual's future. Development strategies include pairing formal annual talent reviews or (in the case of practice partners) formal peer review with strategic development plans. Effective development strategy may include transparent succession planning so that individuals are aware they are being groomed for future roles.
A well‐known adage suggests, People quit the manager or administrator, not the job. Development in this sense relies on presenting new opportunities and knowing that people flourish when allowed to explore multiple paths forward. In many companies, the role of Chief Experience or Chief Learning Officers is to enhance development planning. Consider how career coaching of young hospitalists could transform an infinitely portable and volatile commodity job, prone to burnout, into an engaged specialist of sorts with immense value to a hospital. Hospitalists have already demonstrated their potential as quality improvement leaders.15 Imagine if hospital leadership enlisted a young hospitalist in a relevant quality improvement task force, such as one working on preventing falls. With appropriate support, the physician could obtain skills for quality improvement evaluation that would not only enhance his or her engagement with the hospital system but also provide a valuable analysis for the hospital.
As an example of development strategy within a small practice setting, consider the following real‐life anecdote: a group of 4 physicians recently completed a long and expensive recruitment of a new partner. The new partner, intrigued by the local hospital's surgical robot technology, sought the support of her partners (who are not currently using the technology) to partake in an expensive robotics training program. The partners decided not to provide the financial support. The new partner subsequently left the group for a nearby practice opportunity that would provide for the training, and the group was faced with the loss of a partner (one‐fourth of the practice!) and the cost of repeating the recruiting process. A preemptive evaluation of the value of investing in the development of the new partner and enhancing that partner's professional development may have proven wise despite the significant up‐front costs. In this case the manager the new partner quit was the inflexibility of the practice trajectory.
Retain
The economic incentive to retain talented workers is not subtle. If it was, companies would not be funneling resources into Chief Experience Officers. Likewise, the estimated cost for a medical practice to replace an individual physician is as at least $250,000.16, 17
In retention, as in attraction, salary is only part of the equation.18 People want fair and competitive compensation, and may leave if they are not getting it, but they will not stay (and will not stay engaged) only for a salary bump. Retention is enhanced when workers can advance according to skills and talent, rather than mere tenure. An effective retention policy responds to people's desire to incorporate individual professional goals into their work and allows for people to customize their career rather than simply occupy a job class. Effective retention policy respects worklife balance and recognizes that this balance might look different for 2 people with the same job. It may take the form of positive reinforcement (rather than subtle disdain) for using vacation time or allowing for participation in international service projects. Many literally feel that they need to quit their job in order to take time off or explore other interests.
Worklife balance has been a longstanding issue in medicine, and innovative augmentation strategies may well help retain top talent. Today's successful medical school applicants not only show aptitude in the classroom, they often have many well‐developed nonmedical skills. No one can expect that medical training will somehow convince them to leave everything else behind. Moreover, today's residency graduates, already with Generation Y sensibilities, have completed their entire training under the auspices of the Accreditation Council for Graduate Medical Education (ACGME) duty hours regulations, which has made residents far more comfortable with shift work and defined hours.
At the other end of the generation spectrum, as large numbers of physicians ready for retirement, effective talent facilitation strategies may evaluate how to reoffer medicine as a valid option for senior physicians who still wish to work. Retaining these physicians will require an appreciation of their lifestyle goals, as they will likely find continuing a traditional practice role untenable. A recent survey of orthopedic surgeons 50 years of age or older found that having a part‐time option was a common reason they continued practicing, and that the option to work part‐time would have the most impact on keeping these surgeons working past age 65 years.19 Those working part‐time were doing so in a wide range of practice arrangements including private practice. However, one‐third of those surveyed said a part‐time option was not available to them. Clearly, in an environment of workforce shortages, physician‐leaders must begin to think about worklife balance not only for new doctors but for those considering retirement.
Critics will point out the financial drawbacks in the provision of worklife balance. But the cost may pale in comparison to the cost of replacing physician leaders. Moreover, engaged physicians are more likely to add value in the form of intangible capital such as patient satisfaction and practice innovation. As such, we argue that effective retention strategy in medicine is likely to be cost‐effective, even if it requires significant new up‐front resources.
Lessons From Industry
Doctors frequently assume that the challenges and obstacles confronted in healthcare are unique to medicine. But, for every phrase like When I started practice, I decided how long office visits were, not the insurance company, or Young doctors just don't want to work as hard, there is a parallel utterance in the greater business world. Luckily, there are now examples of the healthcare world learning lessons from business. For example, innovators in medical quality improvement found value in the experience of other industries.20 Airlines and automakers have long honed systems for error prevention and possess expertise that may curb errors in the hospital.2123
We suspect that the ideas and practice of talent facilitation have already made their way into some medical settings. A Google search reveals multiple opportunities for hospital‐based talent managers, and websites advertise the availability of talent consultants ready to lend their expertise to the medical world. In the arena of academic medicine, the University of California, San Francisco (UCSF) Division of Hospital Medicine put some of the ideas of talent facilitation into practice over the past year, in part in response to an increasingly competitive market for academic hospitalists.24 Leaders introduced a formal faculty development program that links junior faculty with mentors and facilitates early and frequent feedback across hierarchical boundaries.25 These more intentional mentoring efforts were accompanied by a seminar series aimed at the needs of new faculty members, a research incubator program, divisional grand rounds, and other web‐based and in‐person forums for sharing best practices and innovations. Less formal social events have also been promoted. Importantly, these sweeping strategies seek to encompass the needs of both teaching and nonteaching hospitalists within UCSF.26
Clearly, an academic hospitalist group with 45 faculty physicians has unique characteristics that inform the specifics of its talent facilitation strategy. The interventions discussed above are meant to represent examples of the types of strategies that may be utilized by physician groups once a decision is made to focus on talent management. Undoubtedly, the shape of such efforts will vary in diverse practice settings, but physician leaders have much to gain through further exploration of where these core principles already exist within medicine and where they may be more effectively deployed. By examining how multinational businesses are systematically applying the concepts of talent facilitation to address a global talent shortage, the doctoring profession might again take an outside hint to help inform its future.
- Long Term Care: Aging Baby Boom Generation Will Increase Demand and Burden on Federal and State Budgets. United States General Accounting Office Testimony before the Special Committee on Aging, US Senate. Hearing Before the Special Committee on Aging of the US Senate,2002. Available at:http://www.gao.gov/new.items/d02544t.pdf. Accessed July 2009.
- ,.Physician workforce shortages: implications and issues for academic health centers and policymakers.Acad Med.2006;81(9):782–787.
- .New York moves to tackle shortage of primary‐care doctors.Lancet.2008;371(9615):801–802.
- ,.The US dermatology workforce: a specialty remains in shortage.J Am Acad Dermatol.2008;59(5):741–745.
- ,.Challenges and opportunities for recruiting a new generation of neurosurgeons.Neurosurgery.2007;61(6):1314–1319.
- ,.The developing crisis in the national general surgery workforce.J Am Coll Surg.2008;206(5):790–795.
- Medical School Enrollment Plans: Analysis of the 2007 AAMC Survey. Publication of the Association of American Medical Colleges, Center for Workforce Studies, April2008. Available at:http://www.aamc.org/workforce. Accessed July 2009.
- It's 2008: Do You Know Where Your Talent Is? Why acquisition and retention strategies don't work. Part 1 of a Deloitte Research Series on Talent Management.2008. Available at: http://www.deloitte.com/dtt/cda/content/UKConsulting_TalentMgtResearchReport.pdf. Accessed August 2009.
- ,,.The race for talent: retaining and engaging workers in the 21st century.Hum Resour Plann.2004;27(3):12–25.
- Expecting sales growth, CEOs cite worker retention as critical to success. March 1,2004. Available at:http://www.barometersurveys.com/production/barsurv.nsf/89343582e94adb6185256b84006c8ffe/9672ab2f54cf99f885256e5500768232?OpenDocument. Accessed July 2009.
- Jet Blue announces aviation university gateway program for pilot candidates: airline partners with Embry‐Riddle Aeronautical University, University of North Dakota, and Cape Air to fill pilot pipeline. January 30, 2008. Available at:http://investor.jetblue.com/phoenix.zhtml?c=131045287(4):487–494.
- ,,.A review of physician turnover: rates, causes, and consequences.Am J Med Qual.2004;19(2):56–66.
- ,,.The impact on revenue of physician turnover: an assessment model and experience in a large healthcare center.J Med Pract Manage.2006;21(6):351–355.
- ,,.Employee motivation: a powerful new model.Harv Bus Rev.2008;86(7–8):78,84,160.
- ,,.Work satisfaction and retirement plans of orthopaedic surgeons 50 years of age or older.Clin Orthop Relat Res.2008;466(1):231–238.
- ,,,.The long road to patient safety: a status report on patient safety systems.JAMA.2005(22);294:2858–2865.
- .Error reduction through team leadership: what surgeons can learn from the airline industry.Clin Neurosurg.2007;54:195–199.
- ,.Applying the Toyota production system: using a patient safety alert system to reduce error.Jt Comm J Qual Patient Saf.2007;33(7):376–386.
- ,,,.Improving Papanikolaou test quality and reducing medical errors by using Toyota production system methods.Am J Obstet Gynecol.2006;194(1):57–64.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction.2006; 1–45. Available at: http://www.hospitalmedicine.org. Accessed July 2009.
- UCSF Department of Medicine, Division of Hospital Medicine, Faculty Development. Available at: http://hospsrvr.ucsf.edu/cme/fds.html. Accessed July 2009.
- ,,, et al. Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–245.
The demand for physician talent is intensifying as the US healthcare system confronts an unprecedented confluence of demographic pressures. Not only will 78 million retiring baby‐boomers require significant healthcare resources, but tens of thousands of practicing physicians will themselves reach retirement age within the next decade.1 At the same time, factors like the large increase in the percentage of female physicians (who are more likely to work part time), the growth of nonpractice opportunities for MDs, and generational demands for greater worklife balance are creating a major supply‐demand mismatch within the physician workforce.2 In this demographic atmosphere, the ability to recruit and retain physician leaders confers tremendous value to healthcare enterprisesboth public and private. Recruiting and retaining strategies already weigh heavily in the most palpable shortage areas, like primary care, but the system faces widespread unmet demand for a variety of specialist and generalist practitioners.36
This article does not address the public policy implications of the upcoming physician shortage, recognition of which will lead to the largest increase in new medical school slots in decades.7 Rather, we set out to illustrate how successful nonmedical businesses are embracing a thoughtful, systematic approach to retaining talent, based on the philosophy that keeping and engaging valued employees is more efficient than recruiting and orienting replacements. We posit that the innovations used by progressive companies could apply to recruitment and retention challenges confronting medicine.
How Industry Approaches the Talent Vacuum: Talent Facilitation
Leaders outside of medicine have long acknowledged that changing demographics and a global economy are driving unprecedented employee turnover.8 In confronting a talent vacuum, forward‐thinking managers have prioritized retaining key talent (rather than hiring anew) in planning for the future.9, 10 Doing so begins with attempts to understand the relationship between workers and the workplace, with a particular emphasis on appreciating workers' priorities. Indeed, new executive positions with titles like Chief Learning Officer and Chief Experience Officer are appearing as companies realize a need for focused expertise beyond traditional human resource departments. These companies understand that offering higher salaries is not the only retention strategyand often not even the most effective one.
The Four Actions of Talent Facilitation
The talent facilitation process centers on four actions: attract, engage, develop, and retain. None of these actions can stand alone, and all should be present, to some degree, at all stages of a worker's tenure. To attract or engage an employee or practice partner is not a 1‐time hook, but a constant and dynamic process.
Importantly, the concepts addressed here are not specific to 1 type of corporate system, size, or management level. Although the early business focus had been on upper‐level and executive talent within large corporate settings, there is an increasing recognition that a dedicated talent strategy is useful wherever recruiting and retaining talented people is important (and where is it not?).
The ideas presented here may seem most applicable to leaders of large physician corporations, hospital‐owned physician groups, or large integrated healthcare systems (such as Kaiser) that employ physicians. However, we also believe that the ideas apply across‐the‐board in medicine, including entities such as small, private, physician‐owned groups. We argue that regardless of the exact practice structure, a limited pool of resources must be dedicated to the attraction and retention of talented partners or employees, or to the cost of replacing those people if they pursue other opportunities (or the cost of inefficient and disengaged physicians). While an integrated health system may have the resources and scale to hire a Chief Experience Officer, we do not anticipate that a 5‐partner private practice would. Rather, we point to examples to illustrate the talent facilitation paradigm as a tool to systematically frame the allocation of those resources. Undoubtedly, the specific shape of a thoughtful talent facilitation effort will vary when applied in a large urban academic medical center vs. an integrated healthcare system vs. a small physician private practice, but the basic principles remain the same.
Attract
Increasingly, companies approach talented prospects with dedicated marketing campaigns to convey the value of a work environment.11 Silicon Valley employee lounges with free massages and foosball tables are the iconic example of attraction, but the concept runs deeper. Today's workers seek access to state‐of‐the‐art ideas and technology and often want to be part of a larger vision. Many seek opportunities to integrate their own professional and personal aspirations into a particular job description.
Hospital executives have long recognized the importance of attracting physicians to their facility (after all, the physicians draw patients and thus generate revenue). The traditional approach has surrounded perks, from comfortable doctor's lounges to the latest in surgical technology. But, the stakes seem greater now than before, and successful talent facilitation strategies are going beyond the tried and true.
Clearly, physicians seek financially stable practice settings with historical success. But they may also seek evidence of a defined strategic plan focused on more than mere profitability. Physicians may gravitate to practice environments that endorse progressive movements like the No One Dies Alone campaign.12 Similarly, recognition of movements beyond healthcarea commitment to Leadership in Energy and Environmental Design (LEED) (Green Building Rating System; US Green Building Council [USGBC];
The current recruitment campaign of California's prison healthcare system offers an unlikely source of inspiration. The prison system was placed in receivership to address a shortage of competent physician staff and other inadequacies. A central feature of the campaign is an attractive starting salary and good benefits. But, the campaign does not rely on money alone. For example, the campaign's website (

Engage
The corporate tool being employed at this stage is a strategy called on‐boarding, which emphasizes a streamlined integration of newcomers with existing workers and culture, and prioritizes aligning organizational roles with a worker's specific skills and interests. On‐boarding also emphasizes the value of early and frequent provision of constructive feedback from same‐level peers or managers with advanced coaching skills.
Many companies use formal survey tools to measure employee engagement and regularly evaluate the proficiency of system leaders in the ability to engage their employees. An engineering firm executive recently told us (P.K., C.K.) that he performs detailed and frequent on‐the‐job interviews, even with company veterans. The primary goal of these interviews is to ensure that engineers spend at least 85% of their time on work that: (1) they find interesting and (2) allows for the application of their best skills. Wherever possible, traditional job descriptions are altered to achieve this. Inevitably, there is still work (15% in this particular corporate vision) that no one prefers but needs to get done, but this process of active recalibration minimizes this fraction to the degree possible.
Even within a small physician practice group, one can imagine how a strategic approach of inviting and acknowledging individual physician's professional goals and particular talents may challenge the long‐held belief that everyone within a group enjoys and must do the exact same job. Once these goals and talents are articulated, groups may find that allowing for more customized roles within the practice enhances professional satisfaction.
Social networking, collaboration, and sharing of best practices are staples of engaging companies. The Cisco and Qualcomm companies, for example, utilize elaborate e‐networks (rough corporate equivalents to Facebook) to foster collegial interaction within and across traditional hierarchical boundaries so that managers and executives directly engage the ideas of employees at every level.14 The premise is logical: engaged employees will be more likely to contribute innovative ideas which, when listened to, are more likely to engage employees.
Most physicians will recognize the traditional resident report as a model for engagement. Beyond its educational value, interaction with program leadership, social bonding, collaborative effort, and exploration of best practices add tremendous value. Many companies would jump at the chance to engrain a similar cultural staple. Enhancing this type of interaction in a postresidency setting may promote engagement in a given system, especially if it facilitates interactions between physicians and senior hospital leaders. Absent these types of interactions, ensuring regular provision of peer review and/or constructive feedback can help systematically enhance 2‐way communication and enhance engagement.
Develop
Talent development relies on mentorship reflecting a genuine interest in an individual's future. Development strategies include pairing formal annual talent reviews or (in the case of practice partners) formal peer review with strategic development plans. Effective development strategy may include transparent succession planning so that individuals are aware they are being groomed for future roles.
A well‐known adage suggests, People quit the manager or administrator, not the job. Development in this sense relies on presenting new opportunities and knowing that people flourish when allowed to explore multiple paths forward. In many companies, the role of Chief Experience or Chief Learning Officers is to enhance development planning. Consider how career coaching of young hospitalists could transform an infinitely portable and volatile commodity job, prone to burnout, into an engaged specialist of sorts with immense value to a hospital. Hospitalists have already demonstrated their potential as quality improvement leaders.15 Imagine if hospital leadership enlisted a young hospitalist in a relevant quality improvement task force, such as one working on preventing falls. With appropriate support, the physician could obtain skills for quality improvement evaluation that would not only enhance his or her engagement with the hospital system but also provide a valuable analysis for the hospital.
As an example of development strategy within a small practice setting, consider the following real‐life anecdote: a group of 4 physicians recently completed a long and expensive recruitment of a new partner. The new partner, intrigued by the local hospital's surgical robot technology, sought the support of her partners (who are not currently using the technology) to partake in an expensive robotics training program. The partners decided not to provide the financial support. The new partner subsequently left the group for a nearby practice opportunity that would provide for the training, and the group was faced with the loss of a partner (one‐fourth of the practice!) and the cost of repeating the recruiting process. A preemptive evaluation of the value of investing in the development of the new partner and enhancing that partner's professional development may have proven wise despite the significant up‐front costs. In this case the manager the new partner quit was the inflexibility of the practice trajectory.
Retain
The economic incentive to retain talented workers is not subtle. If it was, companies would not be funneling resources into Chief Experience Officers. Likewise, the estimated cost for a medical practice to replace an individual physician is as at least $250,000.16, 17
In retention, as in attraction, salary is only part of the equation.18 People want fair and competitive compensation, and may leave if they are not getting it, but they will not stay (and will not stay engaged) only for a salary bump. Retention is enhanced when workers can advance according to skills and talent, rather than mere tenure. An effective retention policy responds to people's desire to incorporate individual professional goals into their work and allows for people to customize their career rather than simply occupy a job class. Effective retention policy respects worklife balance and recognizes that this balance might look different for 2 people with the same job. It may take the form of positive reinforcement (rather than subtle disdain) for using vacation time or allowing for participation in international service projects. Many literally feel that they need to quit their job in order to take time off or explore other interests.
Worklife balance has been a longstanding issue in medicine, and innovative augmentation strategies may well help retain top talent. Today's successful medical school applicants not only show aptitude in the classroom, they often have many well‐developed nonmedical skills. No one can expect that medical training will somehow convince them to leave everything else behind. Moreover, today's residency graduates, already with Generation Y sensibilities, have completed their entire training under the auspices of the Accreditation Council for Graduate Medical Education (ACGME) duty hours regulations, which has made residents far more comfortable with shift work and defined hours.
At the other end of the generation spectrum, as large numbers of physicians ready for retirement, effective talent facilitation strategies may evaluate how to reoffer medicine as a valid option for senior physicians who still wish to work. Retaining these physicians will require an appreciation of their lifestyle goals, as they will likely find continuing a traditional practice role untenable. A recent survey of orthopedic surgeons 50 years of age or older found that having a part‐time option was a common reason they continued practicing, and that the option to work part‐time would have the most impact on keeping these surgeons working past age 65 years.19 Those working part‐time were doing so in a wide range of practice arrangements including private practice. However, one‐third of those surveyed said a part‐time option was not available to them. Clearly, in an environment of workforce shortages, physician‐leaders must begin to think about worklife balance not only for new doctors but for those considering retirement.
Critics will point out the financial drawbacks in the provision of worklife balance. But the cost may pale in comparison to the cost of replacing physician leaders. Moreover, engaged physicians are more likely to add value in the form of intangible capital such as patient satisfaction and practice innovation. As such, we argue that effective retention strategy in medicine is likely to be cost‐effective, even if it requires significant new up‐front resources.
Lessons From Industry
Doctors frequently assume that the challenges and obstacles confronted in healthcare are unique to medicine. But, for every phrase like When I started practice, I decided how long office visits were, not the insurance company, or Young doctors just don't want to work as hard, there is a parallel utterance in the greater business world. Luckily, there are now examples of the healthcare world learning lessons from business. For example, innovators in medical quality improvement found value in the experience of other industries.20 Airlines and automakers have long honed systems for error prevention and possess expertise that may curb errors in the hospital.2123
We suspect that the ideas and practice of talent facilitation have already made their way into some medical settings. A Google search reveals multiple opportunities for hospital‐based talent managers, and websites advertise the availability of talent consultants ready to lend their expertise to the medical world. In the arena of academic medicine, the University of California, San Francisco (UCSF) Division of Hospital Medicine put some of the ideas of talent facilitation into practice over the past year, in part in response to an increasingly competitive market for academic hospitalists.24 Leaders introduced a formal faculty development program that links junior faculty with mentors and facilitates early and frequent feedback across hierarchical boundaries.25 These more intentional mentoring efforts were accompanied by a seminar series aimed at the needs of new faculty members, a research incubator program, divisional grand rounds, and other web‐based and in‐person forums for sharing best practices and innovations. Less formal social events have also been promoted. Importantly, these sweeping strategies seek to encompass the needs of both teaching and nonteaching hospitalists within UCSF.26
Clearly, an academic hospitalist group with 45 faculty physicians has unique characteristics that inform the specifics of its talent facilitation strategy. The interventions discussed above are meant to represent examples of the types of strategies that may be utilized by physician groups once a decision is made to focus on talent management. Undoubtedly, the shape of such efforts will vary in diverse practice settings, but physician leaders have much to gain through further exploration of where these core principles already exist within medicine and where they may be more effectively deployed. By examining how multinational businesses are systematically applying the concepts of talent facilitation to address a global talent shortage, the doctoring profession might again take an outside hint to help inform its future.
The demand for physician talent is intensifying as the US healthcare system confronts an unprecedented confluence of demographic pressures. Not only will 78 million retiring baby‐boomers require significant healthcare resources, but tens of thousands of practicing physicians will themselves reach retirement age within the next decade.1 At the same time, factors like the large increase in the percentage of female physicians (who are more likely to work part time), the growth of nonpractice opportunities for MDs, and generational demands for greater worklife balance are creating a major supply‐demand mismatch within the physician workforce.2 In this demographic atmosphere, the ability to recruit and retain physician leaders confers tremendous value to healthcare enterprisesboth public and private. Recruiting and retaining strategies already weigh heavily in the most palpable shortage areas, like primary care, but the system faces widespread unmet demand for a variety of specialist and generalist practitioners.36
This article does not address the public policy implications of the upcoming physician shortage, recognition of which will lead to the largest increase in new medical school slots in decades.7 Rather, we set out to illustrate how successful nonmedical businesses are embracing a thoughtful, systematic approach to retaining talent, based on the philosophy that keeping and engaging valued employees is more efficient than recruiting and orienting replacements. We posit that the innovations used by progressive companies could apply to recruitment and retention challenges confronting medicine.
How Industry Approaches the Talent Vacuum: Talent Facilitation
Leaders outside of medicine have long acknowledged that changing demographics and a global economy are driving unprecedented employee turnover.8 In confronting a talent vacuum, forward‐thinking managers have prioritized retaining key talent (rather than hiring anew) in planning for the future.9, 10 Doing so begins with attempts to understand the relationship between workers and the workplace, with a particular emphasis on appreciating workers' priorities. Indeed, new executive positions with titles like Chief Learning Officer and Chief Experience Officer are appearing as companies realize a need for focused expertise beyond traditional human resource departments. These companies understand that offering higher salaries is not the only retention strategyand often not even the most effective one.
The Four Actions of Talent Facilitation
The talent facilitation process centers on four actions: attract, engage, develop, and retain. None of these actions can stand alone, and all should be present, to some degree, at all stages of a worker's tenure. To attract or engage an employee or practice partner is not a 1‐time hook, but a constant and dynamic process.
Importantly, the concepts addressed here are not specific to 1 type of corporate system, size, or management level. Although the early business focus had been on upper‐level and executive talent within large corporate settings, there is an increasing recognition that a dedicated talent strategy is useful wherever recruiting and retaining talented people is important (and where is it not?).
The ideas presented here may seem most applicable to leaders of large physician corporations, hospital‐owned physician groups, or large integrated healthcare systems (such as Kaiser) that employ physicians. However, we also believe that the ideas apply across‐the‐board in medicine, including entities such as small, private, physician‐owned groups. We argue that regardless of the exact practice structure, a limited pool of resources must be dedicated to the attraction and retention of talented partners or employees, or to the cost of replacing those people if they pursue other opportunities (or the cost of inefficient and disengaged physicians). While an integrated health system may have the resources and scale to hire a Chief Experience Officer, we do not anticipate that a 5‐partner private practice would. Rather, we point to examples to illustrate the talent facilitation paradigm as a tool to systematically frame the allocation of those resources. Undoubtedly, the specific shape of a thoughtful talent facilitation effort will vary when applied in a large urban academic medical center vs. an integrated healthcare system vs. a small physician private practice, but the basic principles remain the same.
Attract
Increasingly, companies approach talented prospects with dedicated marketing campaigns to convey the value of a work environment.11 Silicon Valley employee lounges with free massages and foosball tables are the iconic example of attraction, but the concept runs deeper. Today's workers seek access to state‐of‐the‐art ideas and technology and often want to be part of a larger vision. Many seek opportunities to integrate their own professional and personal aspirations into a particular job description.
Hospital executives have long recognized the importance of attracting physicians to their facility (after all, the physicians draw patients and thus generate revenue). The traditional approach has surrounded perks, from comfortable doctor's lounges to the latest in surgical technology. But, the stakes seem greater now than before, and successful talent facilitation strategies are going beyond the tried and true.
Clearly, physicians seek financially stable practice settings with historical success. But they may also seek evidence of a defined strategic plan focused on more than mere profitability. Physicians may gravitate to practice environments that endorse progressive movements like the No One Dies Alone campaign.12 Similarly, recognition of movements beyond healthcarea commitment to Leadership in Energy and Environmental Design (LEED) (Green Building Rating System; US Green Building Council [USGBC];
The current recruitment campaign of California's prison healthcare system offers an unlikely source of inspiration. The prison system was placed in receivership to address a shortage of competent physician staff and other inadequacies. A central feature of the campaign is an attractive starting salary and good benefits. But, the campaign does not rely on money alone. For example, the campaign's website (

Engage
The corporate tool being employed at this stage is a strategy called on‐boarding, which emphasizes a streamlined integration of newcomers with existing workers and culture, and prioritizes aligning organizational roles with a worker's specific skills and interests. On‐boarding also emphasizes the value of early and frequent provision of constructive feedback from same‐level peers or managers with advanced coaching skills.
Many companies use formal survey tools to measure employee engagement and regularly evaluate the proficiency of system leaders in the ability to engage their employees. An engineering firm executive recently told us (P.K., C.K.) that he performs detailed and frequent on‐the‐job interviews, even with company veterans. The primary goal of these interviews is to ensure that engineers spend at least 85% of their time on work that: (1) they find interesting and (2) allows for the application of their best skills. Wherever possible, traditional job descriptions are altered to achieve this. Inevitably, there is still work (15% in this particular corporate vision) that no one prefers but needs to get done, but this process of active recalibration minimizes this fraction to the degree possible.
Even within a small physician practice group, one can imagine how a strategic approach of inviting and acknowledging individual physician's professional goals and particular talents may challenge the long‐held belief that everyone within a group enjoys and must do the exact same job. Once these goals and talents are articulated, groups may find that allowing for more customized roles within the practice enhances professional satisfaction.
Social networking, collaboration, and sharing of best practices are staples of engaging companies. The Cisco and Qualcomm companies, for example, utilize elaborate e‐networks (rough corporate equivalents to Facebook) to foster collegial interaction within and across traditional hierarchical boundaries so that managers and executives directly engage the ideas of employees at every level.14 The premise is logical: engaged employees will be more likely to contribute innovative ideas which, when listened to, are more likely to engage employees.
Most physicians will recognize the traditional resident report as a model for engagement. Beyond its educational value, interaction with program leadership, social bonding, collaborative effort, and exploration of best practices add tremendous value. Many companies would jump at the chance to engrain a similar cultural staple. Enhancing this type of interaction in a postresidency setting may promote engagement in a given system, especially if it facilitates interactions between physicians and senior hospital leaders. Absent these types of interactions, ensuring regular provision of peer review and/or constructive feedback can help systematically enhance 2‐way communication and enhance engagement.
Develop
Talent development relies on mentorship reflecting a genuine interest in an individual's future. Development strategies include pairing formal annual talent reviews or (in the case of practice partners) formal peer review with strategic development plans. Effective development strategy may include transparent succession planning so that individuals are aware they are being groomed for future roles.
A well‐known adage suggests, People quit the manager or administrator, not the job. Development in this sense relies on presenting new opportunities and knowing that people flourish when allowed to explore multiple paths forward. In many companies, the role of Chief Experience or Chief Learning Officers is to enhance development planning. Consider how career coaching of young hospitalists could transform an infinitely portable and volatile commodity job, prone to burnout, into an engaged specialist of sorts with immense value to a hospital. Hospitalists have already demonstrated their potential as quality improvement leaders.15 Imagine if hospital leadership enlisted a young hospitalist in a relevant quality improvement task force, such as one working on preventing falls. With appropriate support, the physician could obtain skills for quality improvement evaluation that would not only enhance his or her engagement with the hospital system but also provide a valuable analysis for the hospital.
As an example of development strategy within a small practice setting, consider the following real‐life anecdote: a group of 4 physicians recently completed a long and expensive recruitment of a new partner. The new partner, intrigued by the local hospital's surgical robot technology, sought the support of her partners (who are not currently using the technology) to partake in an expensive robotics training program. The partners decided not to provide the financial support. The new partner subsequently left the group for a nearby practice opportunity that would provide for the training, and the group was faced with the loss of a partner (one‐fourth of the practice!) and the cost of repeating the recruiting process. A preemptive evaluation of the value of investing in the development of the new partner and enhancing that partner's professional development may have proven wise despite the significant up‐front costs. In this case the manager the new partner quit was the inflexibility of the practice trajectory.
Retain
The economic incentive to retain talented workers is not subtle. If it was, companies would not be funneling resources into Chief Experience Officers. Likewise, the estimated cost for a medical practice to replace an individual physician is as at least $250,000.16, 17
In retention, as in attraction, salary is only part of the equation.18 People want fair and competitive compensation, and may leave if they are not getting it, but they will not stay (and will not stay engaged) only for a salary bump. Retention is enhanced when workers can advance according to skills and talent, rather than mere tenure. An effective retention policy responds to people's desire to incorporate individual professional goals into their work and allows for people to customize their career rather than simply occupy a job class. Effective retention policy respects worklife balance and recognizes that this balance might look different for 2 people with the same job. It may take the form of positive reinforcement (rather than subtle disdain) for using vacation time or allowing for participation in international service projects. Many literally feel that they need to quit their job in order to take time off or explore other interests.
Worklife balance has been a longstanding issue in medicine, and innovative augmentation strategies may well help retain top talent. Today's successful medical school applicants not only show aptitude in the classroom, they often have many well‐developed nonmedical skills. No one can expect that medical training will somehow convince them to leave everything else behind. Moreover, today's residency graduates, already with Generation Y sensibilities, have completed their entire training under the auspices of the Accreditation Council for Graduate Medical Education (ACGME) duty hours regulations, which has made residents far more comfortable with shift work and defined hours.
At the other end of the generation spectrum, as large numbers of physicians ready for retirement, effective talent facilitation strategies may evaluate how to reoffer medicine as a valid option for senior physicians who still wish to work. Retaining these physicians will require an appreciation of their lifestyle goals, as they will likely find continuing a traditional practice role untenable. A recent survey of orthopedic surgeons 50 years of age or older found that having a part‐time option was a common reason they continued practicing, and that the option to work part‐time would have the most impact on keeping these surgeons working past age 65 years.19 Those working part‐time were doing so in a wide range of practice arrangements including private practice. However, one‐third of those surveyed said a part‐time option was not available to them. Clearly, in an environment of workforce shortages, physician‐leaders must begin to think about worklife balance not only for new doctors but for those considering retirement.
Critics will point out the financial drawbacks in the provision of worklife balance. But the cost may pale in comparison to the cost of replacing physician leaders. Moreover, engaged physicians are more likely to add value in the form of intangible capital such as patient satisfaction and practice innovation. As such, we argue that effective retention strategy in medicine is likely to be cost‐effective, even if it requires significant new up‐front resources.
Lessons From Industry
Doctors frequently assume that the challenges and obstacles confronted in healthcare are unique to medicine. But, for every phrase like When I started practice, I decided how long office visits were, not the insurance company, or Young doctors just don't want to work as hard, there is a parallel utterance in the greater business world. Luckily, there are now examples of the healthcare world learning lessons from business. For example, innovators in medical quality improvement found value in the experience of other industries.20 Airlines and automakers have long honed systems for error prevention and possess expertise that may curb errors in the hospital.2123
We suspect that the ideas and practice of talent facilitation have already made their way into some medical settings. A Google search reveals multiple opportunities for hospital‐based talent managers, and websites advertise the availability of talent consultants ready to lend their expertise to the medical world. In the arena of academic medicine, the University of California, San Francisco (UCSF) Division of Hospital Medicine put some of the ideas of talent facilitation into practice over the past year, in part in response to an increasingly competitive market for academic hospitalists.24 Leaders introduced a formal faculty development program that links junior faculty with mentors and facilitates early and frequent feedback across hierarchical boundaries.25 These more intentional mentoring efforts were accompanied by a seminar series aimed at the needs of new faculty members, a research incubator program, divisional grand rounds, and other web‐based and in‐person forums for sharing best practices and innovations. Less formal social events have also been promoted. Importantly, these sweeping strategies seek to encompass the needs of both teaching and nonteaching hospitalists within UCSF.26
Clearly, an academic hospitalist group with 45 faculty physicians has unique characteristics that inform the specifics of its talent facilitation strategy. The interventions discussed above are meant to represent examples of the types of strategies that may be utilized by physician groups once a decision is made to focus on talent management. Undoubtedly, the shape of such efforts will vary in diverse practice settings, but physician leaders have much to gain through further exploration of where these core principles already exist within medicine and where they may be more effectively deployed. By examining how multinational businesses are systematically applying the concepts of talent facilitation to address a global talent shortage, the doctoring profession might again take an outside hint to help inform its future.
- Long Term Care: Aging Baby Boom Generation Will Increase Demand and Burden on Federal and State Budgets. United States General Accounting Office Testimony before the Special Committee on Aging, US Senate. Hearing Before the Special Committee on Aging of the US Senate,2002. Available at:http://www.gao.gov/new.items/d02544t.pdf. Accessed July 2009.
- ,.Physician workforce shortages: implications and issues for academic health centers and policymakers.Acad Med.2006;81(9):782–787.
- .New York moves to tackle shortage of primary‐care doctors.Lancet.2008;371(9615):801–802.
- ,.The US dermatology workforce: a specialty remains in shortage.J Am Acad Dermatol.2008;59(5):741–745.
- ,.Challenges and opportunities for recruiting a new generation of neurosurgeons.Neurosurgery.2007;61(6):1314–1319.
- ,.The developing crisis in the national general surgery workforce.J Am Coll Surg.2008;206(5):790–795.
- Medical School Enrollment Plans: Analysis of the 2007 AAMC Survey. Publication of the Association of American Medical Colleges, Center for Workforce Studies, April2008. Available at:http://www.aamc.org/workforce. Accessed July 2009.
- It's 2008: Do You Know Where Your Talent Is? Why acquisition and retention strategies don't work. Part 1 of a Deloitte Research Series on Talent Management.2008. Available at: http://www.deloitte.com/dtt/cda/content/UKConsulting_TalentMgtResearchReport.pdf. Accessed August 2009.
- ,,.The race for talent: retaining and engaging workers in the 21st century.Hum Resour Plann.2004;27(3):12–25.
- Expecting sales growth, CEOs cite worker retention as critical to success. March 1,2004. Available at:http://www.barometersurveys.com/production/barsurv.nsf/89343582e94adb6185256b84006c8ffe/9672ab2f54cf99f885256e5500768232?OpenDocument. Accessed July 2009.
- Jet Blue announces aviation university gateway program for pilot candidates: airline partners with Embry‐Riddle Aeronautical University, University of North Dakota, and Cape Air to fill pilot pipeline. January 30, 2008. Available at:http://investor.jetblue.com/phoenix.zhtml?c=131045287(4):487–494.
- ,,.A review of physician turnover: rates, causes, and consequences.Am J Med Qual.2004;19(2):56–66.
- ,,.The impact on revenue of physician turnover: an assessment model and experience in a large healthcare center.J Med Pract Manage.2006;21(6):351–355.
- ,,.Employee motivation: a powerful new model.Harv Bus Rev.2008;86(7–8):78,84,160.
- ,,.Work satisfaction and retirement plans of orthopaedic surgeons 50 years of age or older.Clin Orthop Relat Res.2008;466(1):231–238.
- ,,,.The long road to patient safety: a status report on patient safety systems.JAMA.2005(22);294:2858–2865.
- .Error reduction through team leadership: what surgeons can learn from the airline industry.Clin Neurosurg.2007;54:195–199.
- ,.Applying the Toyota production system: using a patient safety alert system to reduce error.Jt Comm J Qual Patient Saf.2007;33(7):376–386.
- ,,,.Improving Papanikolaou test quality and reducing medical errors by using Toyota production system methods.Am J Obstet Gynecol.2006;194(1):57–64.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction.2006; 1–45. Available at: http://www.hospitalmedicine.org. Accessed July 2009.
- UCSF Department of Medicine, Division of Hospital Medicine, Faculty Development. Available at: http://hospsrvr.ucsf.edu/cme/fds.html. Accessed July 2009.
- ,,, et al. Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–245.
- Long Term Care: Aging Baby Boom Generation Will Increase Demand and Burden on Federal and State Budgets. United States General Accounting Office Testimony before the Special Committee on Aging, US Senate. Hearing Before the Special Committee on Aging of the US Senate,2002. Available at:http://www.gao.gov/new.items/d02544t.pdf. Accessed July 2009.
- ,.Physician workforce shortages: implications and issues for academic health centers and policymakers.Acad Med.2006;81(9):782–787.
- .New York moves to tackle shortage of primary‐care doctors.Lancet.2008;371(9615):801–802.
- ,.The US dermatology workforce: a specialty remains in shortage.J Am Acad Dermatol.2008;59(5):741–745.
- ,.Challenges and opportunities for recruiting a new generation of neurosurgeons.Neurosurgery.2007;61(6):1314–1319.
- ,.The developing crisis in the national general surgery workforce.J Am Coll Surg.2008;206(5):790–795.
- Medical School Enrollment Plans: Analysis of the 2007 AAMC Survey. Publication of the Association of American Medical Colleges, Center for Workforce Studies, April2008. Available at:http://www.aamc.org/workforce. Accessed July 2009.
- It's 2008: Do You Know Where Your Talent Is? Why acquisition and retention strategies don't work. Part 1 of a Deloitte Research Series on Talent Management.2008. Available at: http://www.deloitte.com/dtt/cda/content/UKConsulting_TalentMgtResearchReport.pdf. Accessed August 2009.
- ,,.The race for talent: retaining and engaging workers in the 21st century.Hum Resour Plann.2004;27(3):12–25.
- Expecting sales growth, CEOs cite worker retention as critical to success. March 1,2004. Available at:http://www.barometersurveys.com/production/barsurv.nsf/89343582e94adb6185256b84006c8ffe/9672ab2f54cf99f885256e5500768232?OpenDocument. Accessed July 2009.
- Jet Blue announces aviation university gateway program for pilot candidates: airline partners with Embry‐Riddle Aeronautical University, University of North Dakota, and Cape Air to fill pilot pipeline. January 30, 2008. Available at:http://investor.jetblue.com/phoenix.zhtml?c=131045287(4):487–494.
- ,,.A review of physician turnover: rates, causes, and consequences.Am J Med Qual.2004;19(2):56–66.
- ,,.The impact on revenue of physician turnover: an assessment model and experience in a large healthcare center.J Med Pract Manage.2006;21(6):351–355.
- ,,.Employee motivation: a powerful new model.Harv Bus Rev.2008;86(7–8):78,84,160.
- ,,.Work satisfaction and retirement plans of orthopaedic surgeons 50 years of age or older.Clin Orthop Relat Res.2008;466(1):231–238.
- ,,,.The long road to patient safety: a status report on patient safety systems.JAMA.2005(22);294:2858–2865.
- .Error reduction through team leadership: what surgeons can learn from the airline industry.Clin Neurosurg.2007;54:195–199.
- ,.Applying the Toyota production system: using a patient safety alert system to reduce error.Jt Comm J Qual Patient Saf.2007;33(7):376–386.
- ,,,.Improving Papanikolaou test quality and reducing medical errors by using Toyota production system methods.Am J Obstet Gynecol.2006;194(1):57–64.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction.2006; 1–45. Available at: http://www.hospitalmedicine.org. Accessed July 2009.
- UCSF Department of Medicine, Division of Hospital Medicine, Faculty Development. Available at: http://hospsrvr.ucsf.edu/cme/fds.html. Accessed July 2009.
- ,,, et al. Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–245.
Macrolides and Quinolones for AECOPD
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).
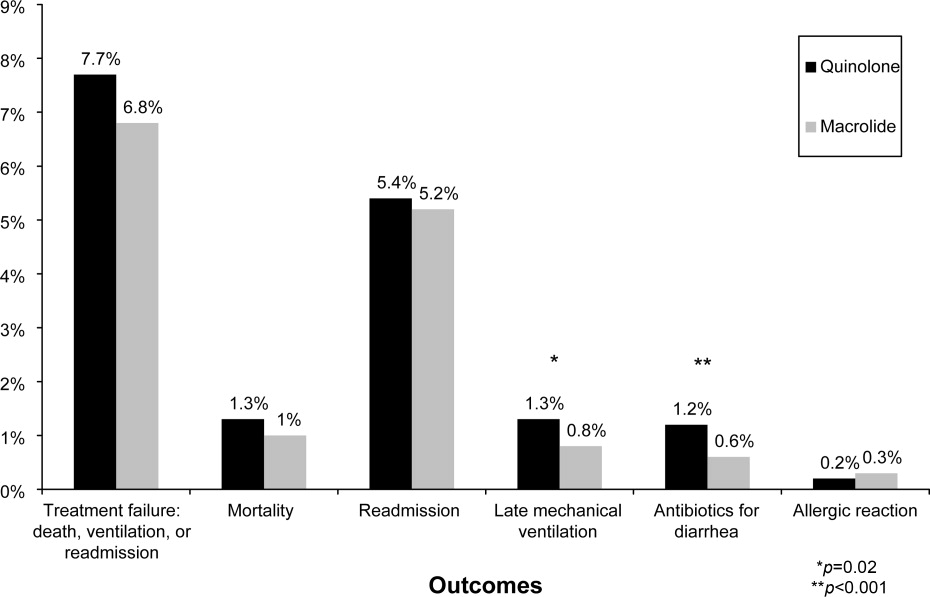
| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).

| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).

| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
Copyright © 2010 Society of Hospital Medicine
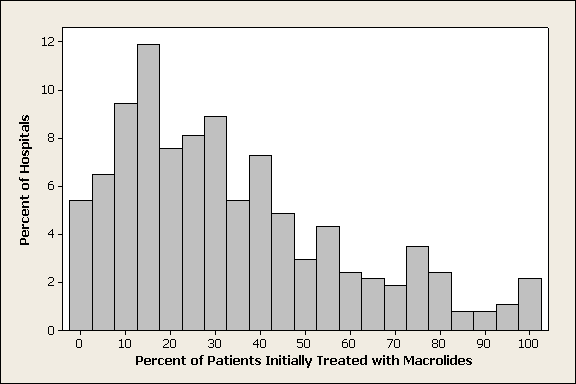
Evaluation of Hemostasis
A previously healthy 25‐year‐old Guatemalan man presented to the emergency department with 1 day of fever, nausea, vomiting, and right lower quadrant abdominal pain. A computed tomography (CT) scan revealed acute appendicitis. The patient underwent an uncomplicated laparoscopic appendectomy and was discharged in stable condition after 48 hours.
Five days after the operation he returned to the emergency department with abdominal pain, nausea, vomiting, and lightheadedness. He was tachycardic, and his hemoglobin was 9.5 g/dL (normal, 13.3‐17.7 g/dL), decreased from 14.4 g/dL prior to his appendectomy. A CT scan showed intraperitoneal blood with active extravasation of contrast at the site of the appendectomy.
Additional laboratory testing revealed an activated partial thromboplastin time (aPTT) of 52 seconds (normal, 37 seconds) and protime (also prothrombin time [PT]) of 14 seconds (normal, 14.1 seconds). The platelet count was 449,000/L (normal, 150‐400,000/L) and the fibrinogen level was 337 mg/dL (normal, 170‐440 mg/dL). Crystalloid and packed red blood cells were administered. Since further laboratory evaluation of the prolonged aPTT was not immediately available, the patient was empirically treated with fresh frozen plasma (FFP), cryoprecipitate, and Factor VIII/von Willebrand factor concentrate. At laparotomy, bleeding was observed at the previous operative site, and 2 L of intraperitoneal blood was evacuated.
The next morning, Factor VIII and Factor IX (FIX) activities and the ristocetin cofactor study performed on specimens obtained immediately prior to the second operation were normal, but the FIX activity was 5% of normal. The diagnosis of FXI deficiency was made and 2 to 3 units of FFP (the amount necessary to maintain the patient's measured FXI activity near 20% of normal) were transfused daily. Nine days of FFP infusions were required to achieve complete wound hemostasis. The patient had no further bleeding episodes after discharge.
Upon further interviewing, the patient revealed that 2 months prior he sustained a small laceration on his arm that bled for a long time and that his brother had experienced prolonged bleeding after a dental extraction.
Commentary
Routine performance of preprocedural laboratory testing, and complete reliance on the results as a means of excluding a propensity to bleeding, may not only lead to excessive testing and delayed procedures, but also provides false reassurance because normal routine laboratory studies cannot be used to exclude some bleeding disorders (Table 1).
| Von Willebrand disease |
| Mild hemophilia A (Factor VIII deficiency) |
| Mild hemophilia B (Factor IX deficiency) |
| Mild hemophilia C (Factor XI deficiency) |
| Qualitative platelet disorders (congenital or acquired) |
| Factor XIII deficiency |
| Disorders of fibrinolysis (eg, antiplasmin deficiency, plasminogen activator inhibitor type 1 deficiency) |
| Disorders of the vasculature or integument (hereditary hemorrhagic telangiectasia, Ehlers‐Danlos syndrome) |
Most studies evaluating routine laboratory testing of hemostatic variables prior to invasive procedures come from patients undergoing elective general surgery. A 1988 study concluded that there is no benefit in the routine preoperative use of the PT, aPTT, platelet count, and bleeding time in the absence of clinical evidence of a hemostatic defect, as assessed by a patient questionnaire and a thorough physical examination.1 A subsequent European, prospective, multicenter study confirmed that abnormalities of preoperative laboratory screening in the absence of a history of bleeding or clinical abnormality were not associated with worse surgical morbidity or mortality, compared to patients with normal screening laboratory studies.2 A recent systematic review has also confirmed the poor positive predictive value of screening tests when used in isolation, and recommended a history‐based and physical exam‐based approach.3 Questionnaires have been shown to be particularly important tools for eliciting clinically significant bleeding disorders that may require revision of the surgical plan.1,4
FXI is a serine protease whose activity is crucial for robust fibrin clot formation and inhibition of fibrinolysis at sites of vascular injury.5 FXI deficiency is an autosomal recessive disorder with an incidence of 1 per 1,000,000 in the general population, with a significantly higher incidence in the Ashkenazi Jewish population. While the risk of spontaneous hemorrhage is typically low, life‐threatening bleeding may occur after surgery or trauma. The severity of the measured FXI level deficiency does not always correlate with risk of bleeding. Periprocedural prophylaxis and treatment of bleeding aim to replace FXI to the low‐normal range by administering FXI concentrate, (not available in the United States) or FFP. Antifibrinolytic agents such as tranexamic acid or ϵ‐aminocaproic acid may be used adjunctively in cases of mucosal bleeding.5
In this case, preoperative screening, either using a questionnaire or careful history‐taking, would have identified the patient's personal and family history of bleeding and prompted appropriate preoperative coagulation testing, which could have exposed the hemostatic defect, allowing for modification of the perioperative medical plan.
In summary, preoperative bleeding evaluations should be performed routinely and should begin with a careful history (use of a questionnaire may be considered) and physical examination. Excessive bleeding after prior surgery, trauma, dental extractions, parturition, or circumcision; bleeding tendency in family members; current use of medications that may increase bleeding risk (such as anticoagulants or aspirin); and physical signs associated with bleeding should be assessed. If clinical details fail to expose a potential bleeding disorder, it is safe and cost‐effective1 to proceed with surgery without performing additional laboratory testing. In contrast, any abnormality on the clinical assessment should trigger preoperative laboratory analysis of basic hemostatic parameters, which may prompt further testing or hematology consultation.
- ,,.A prospective evaluation of the efficacy of preoperative coagulation testing.Ann Surg.1988;208(5):554–557.
- ,,,,.A prospective multicenter evaluation of preoperative hemostatic screening tests. The French Associations for Surgical Research.Am J Surg.1995;170(1):19–23.
- ,,,.Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures.Br J Haematol.2008;140:496–504.
- ,,, et al.A practical concept for preoperative identification of patients with impaired primary hemostasis.Clin Appl Thrombosis Haemost.2004;10(3):195–204.
- ,.Factor XI deficiency.Haemophilia.2008;14(6):1183–1189.
A previously healthy 25‐year‐old Guatemalan man presented to the emergency department with 1 day of fever, nausea, vomiting, and right lower quadrant abdominal pain. A computed tomography (CT) scan revealed acute appendicitis. The patient underwent an uncomplicated laparoscopic appendectomy and was discharged in stable condition after 48 hours.
Five days after the operation he returned to the emergency department with abdominal pain, nausea, vomiting, and lightheadedness. He was tachycardic, and his hemoglobin was 9.5 g/dL (normal, 13.3‐17.7 g/dL), decreased from 14.4 g/dL prior to his appendectomy. A CT scan showed intraperitoneal blood with active extravasation of contrast at the site of the appendectomy.
Additional laboratory testing revealed an activated partial thromboplastin time (aPTT) of 52 seconds (normal, 37 seconds) and protime (also prothrombin time [PT]) of 14 seconds (normal, 14.1 seconds). The platelet count was 449,000/L (normal, 150‐400,000/L) and the fibrinogen level was 337 mg/dL (normal, 170‐440 mg/dL). Crystalloid and packed red blood cells were administered. Since further laboratory evaluation of the prolonged aPTT was not immediately available, the patient was empirically treated with fresh frozen plasma (FFP), cryoprecipitate, and Factor VIII/von Willebrand factor concentrate. At laparotomy, bleeding was observed at the previous operative site, and 2 L of intraperitoneal blood was evacuated.
The next morning, Factor VIII and Factor IX (FIX) activities and the ristocetin cofactor study performed on specimens obtained immediately prior to the second operation were normal, but the FIX activity was 5% of normal. The diagnosis of FXI deficiency was made and 2 to 3 units of FFP (the amount necessary to maintain the patient's measured FXI activity near 20% of normal) were transfused daily. Nine days of FFP infusions were required to achieve complete wound hemostasis. The patient had no further bleeding episodes after discharge.
Upon further interviewing, the patient revealed that 2 months prior he sustained a small laceration on his arm that bled for a long time and that his brother had experienced prolonged bleeding after a dental extraction.
Commentary
Routine performance of preprocedural laboratory testing, and complete reliance on the results as a means of excluding a propensity to bleeding, may not only lead to excessive testing and delayed procedures, but also provides false reassurance because normal routine laboratory studies cannot be used to exclude some bleeding disorders (Table 1).
| Von Willebrand disease |
| Mild hemophilia A (Factor VIII deficiency) |
| Mild hemophilia B (Factor IX deficiency) |
| Mild hemophilia C (Factor XI deficiency) |
| Qualitative platelet disorders (congenital or acquired) |
| Factor XIII deficiency |
| Disorders of fibrinolysis (eg, antiplasmin deficiency, plasminogen activator inhibitor type 1 deficiency) |
| Disorders of the vasculature or integument (hereditary hemorrhagic telangiectasia, Ehlers‐Danlos syndrome) |
Most studies evaluating routine laboratory testing of hemostatic variables prior to invasive procedures come from patients undergoing elective general surgery. A 1988 study concluded that there is no benefit in the routine preoperative use of the PT, aPTT, platelet count, and bleeding time in the absence of clinical evidence of a hemostatic defect, as assessed by a patient questionnaire and a thorough physical examination.1 A subsequent European, prospective, multicenter study confirmed that abnormalities of preoperative laboratory screening in the absence of a history of bleeding or clinical abnormality were not associated with worse surgical morbidity or mortality, compared to patients with normal screening laboratory studies.2 A recent systematic review has also confirmed the poor positive predictive value of screening tests when used in isolation, and recommended a history‐based and physical exam‐based approach.3 Questionnaires have been shown to be particularly important tools for eliciting clinically significant bleeding disorders that may require revision of the surgical plan.1,4
FXI is a serine protease whose activity is crucial for robust fibrin clot formation and inhibition of fibrinolysis at sites of vascular injury.5 FXI deficiency is an autosomal recessive disorder with an incidence of 1 per 1,000,000 in the general population, with a significantly higher incidence in the Ashkenazi Jewish population. While the risk of spontaneous hemorrhage is typically low, life‐threatening bleeding may occur after surgery or trauma. The severity of the measured FXI level deficiency does not always correlate with risk of bleeding. Periprocedural prophylaxis and treatment of bleeding aim to replace FXI to the low‐normal range by administering FXI concentrate, (not available in the United States) or FFP. Antifibrinolytic agents such as tranexamic acid or ϵ‐aminocaproic acid may be used adjunctively in cases of mucosal bleeding.5
In this case, preoperative screening, either using a questionnaire or careful history‐taking, would have identified the patient's personal and family history of bleeding and prompted appropriate preoperative coagulation testing, which could have exposed the hemostatic defect, allowing for modification of the perioperative medical plan.
In summary, preoperative bleeding evaluations should be performed routinely and should begin with a careful history (use of a questionnaire may be considered) and physical examination. Excessive bleeding after prior surgery, trauma, dental extractions, parturition, or circumcision; bleeding tendency in family members; current use of medications that may increase bleeding risk (such as anticoagulants or aspirin); and physical signs associated with bleeding should be assessed. If clinical details fail to expose a potential bleeding disorder, it is safe and cost‐effective1 to proceed with surgery without performing additional laboratory testing. In contrast, any abnormality on the clinical assessment should trigger preoperative laboratory analysis of basic hemostatic parameters, which may prompt further testing or hematology consultation.
A previously healthy 25‐year‐old Guatemalan man presented to the emergency department with 1 day of fever, nausea, vomiting, and right lower quadrant abdominal pain. A computed tomography (CT) scan revealed acute appendicitis. The patient underwent an uncomplicated laparoscopic appendectomy and was discharged in stable condition after 48 hours.
Five days after the operation he returned to the emergency department with abdominal pain, nausea, vomiting, and lightheadedness. He was tachycardic, and his hemoglobin was 9.5 g/dL (normal, 13.3‐17.7 g/dL), decreased from 14.4 g/dL prior to his appendectomy. A CT scan showed intraperitoneal blood with active extravasation of contrast at the site of the appendectomy.
Additional laboratory testing revealed an activated partial thromboplastin time (aPTT) of 52 seconds (normal, 37 seconds) and protime (also prothrombin time [PT]) of 14 seconds (normal, 14.1 seconds). The platelet count was 449,000/L (normal, 150‐400,000/L) and the fibrinogen level was 337 mg/dL (normal, 170‐440 mg/dL). Crystalloid and packed red blood cells were administered. Since further laboratory evaluation of the prolonged aPTT was not immediately available, the patient was empirically treated with fresh frozen plasma (FFP), cryoprecipitate, and Factor VIII/von Willebrand factor concentrate. At laparotomy, bleeding was observed at the previous operative site, and 2 L of intraperitoneal blood was evacuated.
The next morning, Factor VIII and Factor IX (FIX) activities and the ristocetin cofactor study performed on specimens obtained immediately prior to the second operation were normal, but the FIX activity was 5% of normal. The diagnosis of FXI deficiency was made and 2 to 3 units of FFP (the amount necessary to maintain the patient's measured FXI activity near 20% of normal) were transfused daily. Nine days of FFP infusions were required to achieve complete wound hemostasis. The patient had no further bleeding episodes after discharge.
Upon further interviewing, the patient revealed that 2 months prior he sustained a small laceration on his arm that bled for a long time and that his brother had experienced prolonged bleeding after a dental extraction.
Commentary
Routine performance of preprocedural laboratory testing, and complete reliance on the results as a means of excluding a propensity to bleeding, may not only lead to excessive testing and delayed procedures, but also provides false reassurance because normal routine laboratory studies cannot be used to exclude some bleeding disorders (Table 1).
| Von Willebrand disease |
| Mild hemophilia A (Factor VIII deficiency) |
| Mild hemophilia B (Factor IX deficiency) |
| Mild hemophilia C (Factor XI deficiency) |
| Qualitative platelet disorders (congenital or acquired) |
| Factor XIII deficiency |
| Disorders of fibrinolysis (eg, antiplasmin deficiency, plasminogen activator inhibitor type 1 deficiency) |
| Disorders of the vasculature or integument (hereditary hemorrhagic telangiectasia, Ehlers‐Danlos syndrome) |
Most studies evaluating routine laboratory testing of hemostatic variables prior to invasive procedures come from patients undergoing elective general surgery. A 1988 study concluded that there is no benefit in the routine preoperative use of the PT, aPTT, platelet count, and bleeding time in the absence of clinical evidence of a hemostatic defect, as assessed by a patient questionnaire and a thorough physical examination.1 A subsequent European, prospective, multicenter study confirmed that abnormalities of preoperative laboratory screening in the absence of a history of bleeding or clinical abnormality were not associated with worse surgical morbidity or mortality, compared to patients with normal screening laboratory studies.2 A recent systematic review has also confirmed the poor positive predictive value of screening tests when used in isolation, and recommended a history‐based and physical exam‐based approach.3 Questionnaires have been shown to be particularly important tools for eliciting clinically significant bleeding disorders that may require revision of the surgical plan.1,4
FXI is a serine protease whose activity is crucial for robust fibrin clot formation and inhibition of fibrinolysis at sites of vascular injury.5 FXI deficiency is an autosomal recessive disorder with an incidence of 1 per 1,000,000 in the general population, with a significantly higher incidence in the Ashkenazi Jewish population. While the risk of spontaneous hemorrhage is typically low, life‐threatening bleeding may occur after surgery or trauma. The severity of the measured FXI level deficiency does not always correlate with risk of bleeding. Periprocedural prophylaxis and treatment of bleeding aim to replace FXI to the low‐normal range by administering FXI concentrate, (not available in the United States) or FFP. Antifibrinolytic agents such as tranexamic acid or ϵ‐aminocaproic acid may be used adjunctively in cases of mucosal bleeding.5
In this case, preoperative screening, either using a questionnaire or careful history‐taking, would have identified the patient's personal and family history of bleeding and prompted appropriate preoperative coagulation testing, which could have exposed the hemostatic defect, allowing for modification of the perioperative medical plan.
In summary, preoperative bleeding evaluations should be performed routinely and should begin with a careful history (use of a questionnaire may be considered) and physical examination. Excessive bleeding after prior surgery, trauma, dental extractions, parturition, or circumcision; bleeding tendency in family members; current use of medications that may increase bleeding risk (such as anticoagulants or aspirin); and physical signs associated with bleeding should be assessed. If clinical details fail to expose a potential bleeding disorder, it is safe and cost‐effective1 to proceed with surgery without performing additional laboratory testing. In contrast, any abnormality on the clinical assessment should trigger preoperative laboratory analysis of basic hemostatic parameters, which may prompt further testing or hematology consultation.
- ,,.A prospective evaluation of the efficacy of preoperative coagulation testing.Ann Surg.1988;208(5):554–557.
- ,,,,.A prospective multicenter evaluation of preoperative hemostatic screening tests. The French Associations for Surgical Research.Am J Surg.1995;170(1):19–23.
- ,,,.Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures.Br J Haematol.2008;140:496–504.
- ,,, et al.A practical concept for preoperative identification of patients with impaired primary hemostasis.Clin Appl Thrombosis Haemost.2004;10(3):195–204.
- ,.Factor XI deficiency.Haemophilia.2008;14(6):1183–1189.
- ,,.A prospective evaluation of the efficacy of preoperative coagulation testing.Ann Surg.1988;208(5):554–557.
- ,,,,.A prospective multicenter evaluation of preoperative hemostatic screening tests. The French Associations for Surgical Research.Am J Surg.1995;170(1):19–23.
- ,,,.Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures.Br J Haematol.2008;140:496–504.
- ,,, et al.A practical concept for preoperative identification of patients with impaired primary hemostasis.Clin Appl Thrombosis Haemost.2004;10(3):195–204.
- ,.Factor XI deficiency.Haemophilia.2008;14(6):1183–1189.
Spontaneous Retroperitoneal Hematoma
A 56‐year‐old male presented to the emergency department with a 2‐week history of increasing abdominal girth, nausea, vomiting, and lower extremity edema. His girlfriend had also noted a yellow tinge to his skin and eyes. His past medical history was significant for bipolar disorder, alcohol‐related seizures, and pneumonia. He had no allergies and denied medications prior to admission. Family history was negative for liver disease and social history was notable for ongoing tobacco use and alcohol dependence. He was afebrile with stable vital signs. Physical examination demonstrated an alert gentleman whose answers to questions required occasional factual correction by his partner. His abdomen was distended and nontender with prominent vasculature and shifting dullness. Lower extremity edema was symmetric and bilateral, rated as 2+. Scattered spider angiomata and a fine bilateral hand tremor without asterixis were also noted. Initial laboratory data demonstrated a white blood cell count of 13,900/L, hematocrit 37%, and platelet count 176,000/L. His sodium was 130 mg/dL, blood urea nitrogen (BUN) 1 mg/dL, and creatinine 0.7 mg/dL. International normalized ratio (INR) was 1.8, aspartate aminotransferase (AST) was 117 U/L, alanine aminotransferase (ALT) 33 U/L, alkaline phosphatase 191 U/L, total bilirubin 9.2 mg/dL, total protein 7.0 g/dL, and albumin 1.9 g/dL. Abdominal ultrasound revealed a diffusely hyperechoic liver with a large amount of ascites.
The patient was admitted with the diagnoses of presumed alcoholic hepatitis and end‐stage liver disease. Model for End‐Stage Liver Disease (MELD) score was 21 and discriminant function 16.8. Paracentesis demonstrated a serum‐ascites albumin gradient of >1.1 and no evidence of spontaneous bacterial peritonitis. Diuresis was initiated. He was placed on unfractionated heparin at a dose of 5000 units every 8 hours for deep venous thrombosis (DVT) prophylaxis. By hospital day 3, the patient's laboratory values had improved, yet his stay was prolonged by alcohol withdrawal requiring benzodiazepines, altered mental status presumed secondary to hepatic encephalopathy, acute renal failure, aspiration pneumonia, and persistent unexplained leukocytosis. He required medical restraints during this time given confusion and propensity to ambulate without assistance, yet sustained no falls or other known trauma in care delivered during this time.
Between days 14 and 17, the patient's hematocrit fell from 36% to 30%; vital signs remained stable. He underwent an uncomplicated, ultrasound‐guided therapeutic paracentesis, which yielded 1.4 L of straw‐colored fluid on the afternoon of day 17; the procedure was attempted only on the right side. On the morning of day 18, the patient's blood pressure dropped to 78/55 mmHg with a pulse of 123 beats per minute; he became pale and unresponsive. Physical examination was notable for somnolence and a tender, warm left flank mass, contralateral to his paracentesis site. No flank or periumbilical ecchymoses were identified. Complete blood count demonstrated a white blood count (WBC) of 22,970/L, hematocrit 16%, and platelet count 104,000/L. INR was 2.0, unchanged from the last check on day 10. Partial thromboplastin time was 41 seconds and fibrinogen was 293 mg/dL (normal 150‐400 mg/dL). Peripheral blood smear was negative for red cell fragments. Blood chemistries revealed a sodium of 134 mg/dL, bicarbonate 20 mEq/L, anion gap 7, BUN 24 mg/dL, and creatinine 1.6 mg/dL (up from 1.0 mg/dL the previous day). His venous lactate level was 4.6 mmol/L and arterial blood gas sampling on room air demonstrated a pH of 7.35, partial pressure of carbon dioxide (pCO2) 29 mmHg, partial pressure of oxygen (pCO2) 54 mmHg, and bicarbonate 15 mEq/L. A femoral introducer was placed for volume resuscitation and the patient was urgently transfused with packed red blood cells (PRBCs) and fresh‐frozen plasma (FFP) to correct his coagulopathy. Computed tomography of the abdomen revealed a large left retroperitoneal hematoma measuring 15 15 22 cm3 (Figure 1). Despite transfusion, his hematocrit continued to fall. Urgent angiography was performed, upon which he was found to have active bleeding from the left L3‐L5 lumbar arteries. These were successfully embolized. He required PRBCs and FFP transfusion only once following this procedure. Given a transient decrease in his urine output, his bladder pressures were followed closely for evidence of abdominal compartment syndrome, which did not develop. He was transferred from the intensive care unit (ICU) to the floor on day 20, where his physical exam and hematocrit remained stable and his delirium slowly cleared. He was ultimately discharged to a skilled nursing facility on day 33.
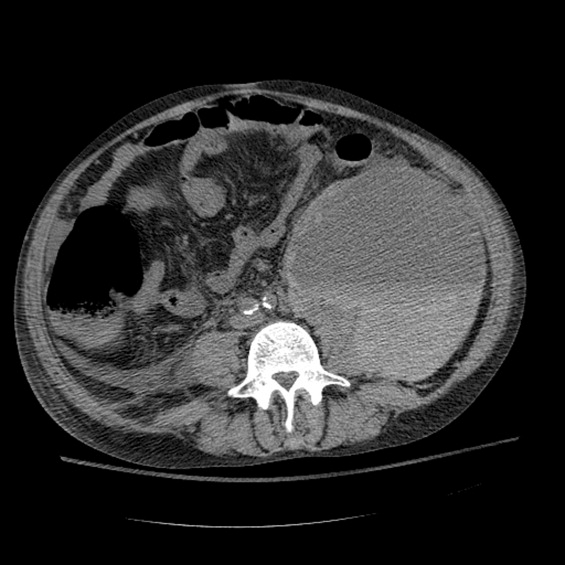
Discussion
Spontaneous retroperitoneal hematoma is a well‐recognized entity that may present with the classic triad of abdominal or groin pain, palpable abdominal or flank mass, and shock or lower extremity motor or sensory changes due to femoral nerve compression.1 Although classically described as skin findings associated with retroperitoneal hemorrhage, Cullen and Grey‐Turner signs are relatively late findings that may not develop in all patients.
Retroperitoneal hemorrhage is well‐recognized as a result of iatrogenic anticoagulation,1 but has been reported less commonly as a result of coagulopathy related to cirrhosis. Di Bisceglie and Richart2 describe 2 patients with MELD scores of 29 and 25, respectively, who developed spontaneous retroperitoneal and rectus muscle hemorrhage. Both had evidence of associated disseminated intravascular coagulation (DIC) and died. Even less common is spontaneous lumbar artery rupture, occurring rarely in the absence of trauma or instrumentation. One reported bleed developed in the context of systemic anticoagulation for a mechanical valve.3 Halak et al.4 relate a case in which the only known risk factor was chronic renal disease. Hama et al.5 describe a patient with a history of alcoholic liver cirrhosis and INR of 2.3 whose lumbar artery rupture was successfully managed with transcatheter arterial embolization.
It is difficult to ascertain the effect of prophylactic anticoagulation in development of this particular hemorrhage. Retroperitoneal bleeding is a very rare complication of pharmacologic prophylaxis for DVT reported with both low‐molecular weight and unfractionated heparins.6 There may be additive risk of prophylaxis in a cirrhotic patient with baseline elevated INR and thrombocytopenia, particularly in the context of renal failure.
Options in the management of spontaneous retroperitoneal hematoma include transarterial embolization, percutaneous decompression, and open surgery. Nonoperative management of these bleeds when possible may be preferable in cirrhotic patients, as their baseline liver disease renders them higher‐risk candidates for surgery. There are no randomized trials comparing these approaches.1
In summary, we report the case of a 56‐year‐old man with end‐stage liver disease and associated coagulopathy without evidence of DIC who survived to discharge with intravascular management of a spontaneous retroperitoneal hemorrhage from the lumbar arteries. To our knowledge, this is the second reported case of spontaneous retroperitoneal hemorrhage in a cirrhotic in which the lumbar arteries were implicated and the first in which multiple arteries were found to be bleeding simultaneously. Any hospitalized patient who develops abdominal pain, flank pain, or hemodynamic instability in the context of coagulopathy, regardless of cause, warrants evaluation for retroperitoneal bleed. Appropriate early management includes immediate resuscitation, intensive monitoring, urgent imaging, and surgical and interventional radiology consultation in order to prevent a fatal outcome.
- ,,,.Management of spontaneous and iatrogenic retroperitoneal haemorrhage: conservative management, endovascular intervention or open surgery?Int J Clin Pract.2007;62:1604–1613.
- ,.Spontaneous retroperitoneal and rectus muscle hemorrhage as a potentially lethal complication of cirrhosis.Liver Int.2006;26:1291–1293.
- ,,.Spontaneous rupture of a lumbar artery. A rare etiology of retroperitoneal hematoma.Urologe A.2003;42:840–844.
- ,,,,.Spontaneous ruptured lumbar artery in a chronic renal failure patient.Eur J Vasc Endovasc Surg.2001;21:569–571.
- ,,.Spontaneous rupture of the lumbar artery.Intern Med.2004;43:759.
- ,,.The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis.Arch Surg.2006;141:790–799.
A 56‐year‐old male presented to the emergency department with a 2‐week history of increasing abdominal girth, nausea, vomiting, and lower extremity edema. His girlfriend had also noted a yellow tinge to his skin and eyes. His past medical history was significant for bipolar disorder, alcohol‐related seizures, and pneumonia. He had no allergies and denied medications prior to admission. Family history was negative for liver disease and social history was notable for ongoing tobacco use and alcohol dependence. He was afebrile with stable vital signs. Physical examination demonstrated an alert gentleman whose answers to questions required occasional factual correction by his partner. His abdomen was distended and nontender with prominent vasculature and shifting dullness. Lower extremity edema was symmetric and bilateral, rated as 2+. Scattered spider angiomata and a fine bilateral hand tremor without asterixis were also noted. Initial laboratory data demonstrated a white blood cell count of 13,900/L, hematocrit 37%, and platelet count 176,000/L. His sodium was 130 mg/dL, blood urea nitrogen (BUN) 1 mg/dL, and creatinine 0.7 mg/dL. International normalized ratio (INR) was 1.8, aspartate aminotransferase (AST) was 117 U/L, alanine aminotransferase (ALT) 33 U/L, alkaline phosphatase 191 U/L, total bilirubin 9.2 mg/dL, total protein 7.0 g/dL, and albumin 1.9 g/dL. Abdominal ultrasound revealed a diffusely hyperechoic liver with a large amount of ascites.
The patient was admitted with the diagnoses of presumed alcoholic hepatitis and end‐stage liver disease. Model for End‐Stage Liver Disease (MELD) score was 21 and discriminant function 16.8. Paracentesis demonstrated a serum‐ascites albumin gradient of >1.1 and no evidence of spontaneous bacterial peritonitis. Diuresis was initiated. He was placed on unfractionated heparin at a dose of 5000 units every 8 hours for deep venous thrombosis (DVT) prophylaxis. By hospital day 3, the patient's laboratory values had improved, yet his stay was prolonged by alcohol withdrawal requiring benzodiazepines, altered mental status presumed secondary to hepatic encephalopathy, acute renal failure, aspiration pneumonia, and persistent unexplained leukocytosis. He required medical restraints during this time given confusion and propensity to ambulate without assistance, yet sustained no falls or other known trauma in care delivered during this time.
Between days 14 and 17, the patient's hematocrit fell from 36% to 30%; vital signs remained stable. He underwent an uncomplicated, ultrasound‐guided therapeutic paracentesis, which yielded 1.4 L of straw‐colored fluid on the afternoon of day 17; the procedure was attempted only on the right side. On the morning of day 18, the patient's blood pressure dropped to 78/55 mmHg with a pulse of 123 beats per minute; he became pale and unresponsive. Physical examination was notable for somnolence and a tender, warm left flank mass, contralateral to his paracentesis site. No flank or periumbilical ecchymoses were identified. Complete blood count demonstrated a white blood count (WBC) of 22,970/L, hematocrit 16%, and platelet count 104,000/L. INR was 2.0, unchanged from the last check on day 10. Partial thromboplastin time was 41 seconds and fibrinogen was 293 mg/dL (normal 150‐400 mg/dL). Peripheral blood smear was negative for red cell fragments. Blood chemistries revealed a sodium of 134 mg/dL, bicarbonate 20 mEq/L, anion gap 7, BUN 24 mg/dL, and creatinine 1.6 mg/dL (up from 1.0 mg/dL the previous day). His venous lactate level was 4.6 mmol/L and arterial blood gas sampling on room air demonstrated a pH of 7.35, partial pressure of carbon dioxide (pCO2) 29 mmHg, partial pressure of oxygen (pCO2) 54 mmHg, and bicarbonate 15 mEq/L. A femoral introducer was placed for volume resuscitation and the patient was urgently transfused with packed red blood cells (PRBCs) and fresh‐frozen plasma (FFP) to correct his coagulopathy. Computed tomography of the abdomen revealed a large left retroperitoneal hematoma measuring 15 15 22 cm3 (Figure 1). Despite transfusion, his hematocrit continued to fall. Urgent angiography was performed, upon which he was found to have active bleeding from the left L3‐L5 lumbar arteries. These were successfully embolized. He required PRBCs and FFP transfusion only once following this procedure. Given a transient decrease in his urine output, his bladder pressures were followed closely for evidence of abdominal compartment syndrome, which did not develop. He was transferred from the intensive care unit (ICU) to the floor on day 20, where his physical exam and hematocrit remained stable and his delirium slowly cleared. He was ultimately discharged to a skilled nursing facility on day 33.

Discussion
Spontaneous retroperitoneal hematoma is a well‐recognized entity that may present with the classic triad of abdominal or groin pain, palpable abdominal or flank mass, and shock or lower extremity motor or sensory changes due to femoral nerve compression.1 Although classically described as skin findings associated with retroperitoneal hemorrhage, Cullen and Grey‐Turner signs are relatively late findings that may not develop in all patients.
Retroperitoneal hemorrhage is well‐recognized as a result of iatrogenic anticoagulation,1 but has been reported less commonly as a result of coagulopathy related to cirrhosis. Di Bisceglie and Richart2 describe 2 patients with MELD scores of 29 and 25, respectively, who developed spontaneous retroperitoneal and rectus muscle hemorrhage. Both had evidence of associated disseminated intravascular coagulation (DIC) and died. Even less common is spontaneous lumbar artery rupture, occurring rarely in the absence of trauma or instrumentation. One reported bleed developed in the context of systemic anticoagulation for a mechanical valve.3 Halak et al.4 relate a case in which the only known risk factor was chronic renal disease. Hama et al.5 describe a patient with a history of alcoholic liver cirrhosis and INR of 2.3 whose lumbar artery rupture was successfully managed with transcatheter arterial embolization.
It is difficult to ascertain the effect of prophylactic anticoagulation in development of this particular hemorrhage. Retroperitoneal bleeding is a very rare complication of pharmacologic prophylaxis for DVT reported with both low‐molecular weight and unfractionated heparins.6 There may be additive risk of prophylaxis in a cirrhotic patient with baseline elevated INR and thrombocytopenia, particularly in the context of renal failure.
Options in the management of spontaneous retroperitoneal hematoma include transarterial embolization, percutaneous decompression, and open surgery. Nonoperative management of these bleeds when possible may be preferable in cirrhotic patients, as their baseline liver disease renders them higher‐risk candidates for surgery. There are no randomized trials comparing these approaches.1
In summary, we report the case of a 56‐year‐old man with end‐stage liver disease and associated coagulopathy without evidence of DIC who survived to discharge with intravascular management of a spontaneous retroperitoneal hemorrhage from the lumbar arteries. To our knowledge, this is the second reported case of spontaneous retroperitoneal hemorrhage in a cirrhotic in which the lumbar arteries were implicated and the first in which multiple arteries were found to be bleeding simultaneously. Any hospitalized patient who develops abdominal pain, flank pain, or hemodynamic instability in the context of coagulopathy, regardless of cause, warrants evaluation for retroperitoneal bleed. Appropriate early management includes immediate resuscitation, intensive monitoring, urgent imaging, and surgical and interventional radiology consultation in order to prevent a fatal outcome.
A 56‐year‐old male presented to the emergency department with a 2‐week history of increasing abdominal girth, nausea, vomiting, and lower extremity edema. His girlfriend had also noted a yellow tinge to his skin and eyes. His past medical history was significant for bipolar disorder, alcohol‐related seizures, and pneumonia. He had no allergies and denied medications prior to admission. Family history was negative for liver disease and social history was notable for ongoing tobacco use and alcohol dependence. He was afebrile with stable vital signs. Physical examination demonstrated an alert gentleman whose answers to questions required occasional factual correction by his partner. His abdomen was distended and nontender with prominent vasculature and shifting dullness. Lower extremity edema was symmetric and bilateral, rated as 2+. Scattered spider angiomata and a fine bilateral hand tremor without asterixis were also noted. Initial laboratory data demonstrated a white blood cell count of 13,900/L, hematocrit 37%, and platelet count 176,000/L. His sodium was 130 mg/dL, blood urea nitrogen (BUN) 1 mg/dL, and creatinine 0.7 mg/dL. International normalized ratio (INR) was 1.8, aspartate aminotransferase (AST) was 117 U/L, alanine aminotransferase (ALT) 33 U/L, alkaline phosphatase 191 U/L, total bilirubin 9.2 mg/dL, total protein 7.0 g/dL, and albumin 1.9 g/dL. Abdominal ultrasound revealed a diffusely hyperechoic liver with a large amount of ascites.
The patient was admitted with the diagnoses of presumed alcoholic hepatitis and end‐stage liver disease. Model for End‐Stage Liver Disease (MELD) score was 21 and discriminant function 16.8. Paracentesis demonstrated a serum‐ascites albumin gradient of >1.1 and no evidence of spontaneous bacterial peritonitis. Diuresis was initiated. He was placed on unfractionated heparin at a dose of 5000 units every 8 hours for deep venous thrombosis (DVT) prophylaxis. By hospital day 3, the patient's laboratory values had improved, yet his stay was prolonged by alcohol withdrawal requiring benzodiazepines, altered mental status presumed secondary to hepatic encephalopathy, acute renal failure, aspiration pneumonia, and persistent unexplained leukocytosis. He required medical restraints during this time given confusion and propensity to ambulate without assistance, yet sustained no falls or other known trauma in care delivered during this time.
Between days 14 and 17, the patient's hematocrit fell from 36% to 30%; vital signs remained stable. He underwent an uncomplicated, ultrasound‐guided therapeutic paracentesis, which yielded 1.4 L of straw‐colored fluid on the afternoon of day 17; the procedure was attempted only on the right side. On the morning of day 18, the patient's blood pressure dropped to 78/55 mmHg with a pulse of 123 beats per minute; he became pale and unresponsive. Physical examination was notable for somnolence and a tender, warm left flank mass, contralateral to his paracentesis site. No flank or periumbilical ecchymoses were identified. Complete blood count demonstrated a white blood count (WBC) of 22,970/L, hematocrit 16%, and platelet count 104,000/L. INR was 2.0, unchanged from the last check on day 10. Partial thromboplastin time was 41 seconds and fibrinogen was 293 mg/dL (normal 150‐400 mg/dL). Peripheral blood smear was negative for red cell fragments. Blood chemistries revealed a sodium of 134 mg/dL, bicarbonate 20 mEq/L, anion gap 7, BUN 24 mg/dL, and creatinine 1.6 mg/dL (up from 1.0 mg/dL the previous day). His venous lactate level was 4.6 mmol/L and arterial blood gas sampling on room air demonstrated a pH of 7.35, partial pressure of carbon dioxide (pCO2) 29 mmHg, partial pressure of oxygen (pCO2) 54 mmHg, and bicarbonate 15 mEq/L. A femoral introducer was placed for volume resuscitation and the patient was urgently transfused with packed red blood cells (PRBCs) and fresh‐frozen plasma (FFP) to correct his coagulopathy. Computed tomography of the abdomen revealed a large left retroperitoneal hematoma measuring 15 15 22 cm3 (Figure 1). Despite transfusion, his hematocrit continued to fall. Urgent angiography was performed, upon which he was found to have active bleeding from the left L3‐L5 lumbar arteries. These were successfully embolized. He required PRBCs and FFP transfusion only once following this procedure. Given a transient decrease in his urine output, his bladder pressures were followed closely for evidence of abdominal compartment syndrome, which did not develop. He was transferred from the intensive care unit (ICU) to the floor on day 20, where his physical exam and hematocrit remained stable and his delirium slowly cleared. He was ultimately discharged to a skilled nursing facility on day 33.

Discussion
Spontaneous retroperitoneal hematoma is a well‐recognized entity that may present with the classic triad of abdominal or groin pain, palpable abdominal or flank mass, and shock or lower extremity motor or sensory changes due to femoral nerve compression.1 Although classically described as skin findings associated with retroperitoneal hemorrhage, Cullen and Grey‐Turner signs are relatively late findings that may not develop in all patients.
Retroperitoneal hemorrhage is well‐recognized as a result of iatrogenic anticoagulation,1 but has been reported less commonly as a result of coagulopathy related to cirrhosis. Di Bisceglie and Richart2 describe 2 patients with MELD scores of 29 and 25, respectively, who developed spontaneous retroperitoneal and rectus muscle hemorrhage. Both had evidence of associated disseminated intravascular coagulation (DIC) and died. Even less common is spontaneous lumbar artery rupture, occurring rarely in the absence of trauma or instrumentation. One reported bleed developed in the context of systemic anticoagulation for a mechanical valve.3 Halak et al.4 relate a case in which the only known risk factor was chronic renal disease. Hama et al.5 describe a patient with a history of alcoholic liver cirrhosis and INR of 2.3 whose lumbar artery rupture was successfully managed with transcatheter arterial embolization.
It is difficult to ascertain the effect of prophylactic anticoagulation in development of this particular hemorrhage. Retroperitoneal bleeding is a very rare complication of pharmacologic prophylaxis for DVT reported with both low‐molecular weight and unfractionated heparins.6 There may be additive risk of prophylaxis in a cirrhotic patient with baseline elevated INR and thrombocytopenia, particularly in the context of renal failure.
Options in the management of spontaneous retroperitoneal hematoma include transarterial embolization, percutaneous decompression, and open surgery. Nonoperative management of these bleeds when possible may be preferable in cirrhotic patients, as their baseline liver disease renders them higher‐risk candidates for surgery. There are no randomized trials comparing these approaches.1
In summary, we report the case of a 56‐year‐old man with end‐stage liver disease and associated coagulopathy without evidence of DIC who survived to discharge with intravascular management of a spontaneous retroperitoneal hemorrhage from the lumbar arteries. To our knowledge, this is the second reported case of spontaneous retroperitoneal hemorrhage in a cirrhotic in which the lumbar arteries were implicated and the first in which multiple arteries were found to be bleeding simultaneously. Any hospitalized patient who develops abdominal pain, flank pain, or hemodynamic instability in the context of coagulopathy, regardless of cause, warrants evaluation for retroperitoneal bleed. Appropriate early management includes immediate resuscitation, intensive monitoring, urgent imaging, and surgical and interventional radiology consultation in order to prevent a fatal outcome.
- ,,,.Management of spontaneous and iatrogenic retroperitoneal haemorrhage: conservative management, endovascular intervention or open surgery?Int J Clin Pract.2007;62:1604–1613.
- ,.Spontaneous retroperitoneal and rectus muscle hemorrhage as a potentially lethal complication of cirrhosis.Liver Int.2006;26:1291–1293.
- ,,.Spontaneous rupture of a lumbar artery. A rare etiology of retroperitoneal hematoma.Urologe A.2003;42:840–844.
- ,,,,.Spontaneous ruptured lumbar artery in a chronic renal failure patient.Eur J Vasc Endovasc Surg.2001;21:569–571.
- ,,.Spontaneous rupture of the lumbar artery.Intern Med.2004;43:759.
- ,,.The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis.Arch Surg.2006;141:790–799.
- ,,,.Management of spontaneous and iatrogenic retroperitoneal haemorrhage: conservative management, endovascular intervention or open surgery?Int J Clin Pract.2007;62:1604–1613.
- ,.Spontaneous retroperitoneal and rectus muscle hemorrhage as a potentially lethal complication of cirrhosis.Liver Int.2006;26:1291–1293.
- ,,.Spontaneous rupture of a lumbar artery. A rare etiology of retroperitoneal hematoma.Urologe A.2003;42:840–844.
- ,,,,.Spontaneous ruptured lumbar artery in a chronic renal failure patient.Eur J Vasc Endovasc Surg.2001;21:569–571.
- ,,.Spontaneous rupture of the lumbar artery.Intern Med.2004;43:759.
- ,,.The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis.Arch Surg.2006;141:790–799.