User login
Flu season’s almost here: Are you ready?
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
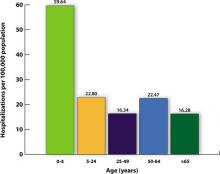
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
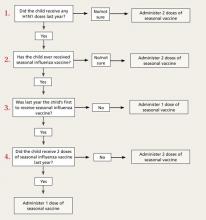
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
Should you restrain yourself from ordering restraints?
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
Hallucinogen sequelae
I appreciated “The woman who saw the light” (Current Psychiatry, July 2010, p. 44-48) in which Dr. R. Andrew Sewell et al describe a 30-year-old woman with schizoaffective disorder and a 7-year history of visual disturbances, including “flashing lights.” The authors’ differential diagnosis did not include the possibility of visual disturbance secondary to atypical anti-psychotic serotonergic antagonism. Photopsia and similar phenomena are not uncommon with 5HT antagonist antidepressants, such as nefazodone.1 They also are well-known sequelae of lysergic acid diethylamide (LSD), a complex serotonin antagonist/agonist, and would be included under the DSM-IV-TR diagnosis hallucinogen persisting perceptual disorder (HPPD).2 Risperidone, a 5HT2-blocking atypical, and selective serotonin reuptake inhibitors may worsen HPPD effects.3,4 Visual disturbance with risperidone also has been reported in a patient with no LSD exposure.5 Dr. Sewell’s patient was treated sequentially with aripiprazole and olanzapine. Both have 5HT blocking properties.
I wonder if the patient has a history of hallucinogen or LSD exposure, or whether her visual symptoms might be related to the use of atypical anti-psychotics combined with sertraline. It would be interesting to see if her symptoms abated with use of a first-generation antipsychotic.
Charles Krasnow, MD
Adjunct clinical assistant professor of psychiatry
University of Michigan Medical School
Ann Arbor, MI
The authors respond
We agree with Dr. Krasnow that HPPD belongs within our differential diagnosis for photopsia and regret omitting it from our article. We consider this to be unlikely, however, because she had no prior LSD use, a history of well-formed visual hallucinations not characteristic of HPPD, and no other characteristic symptoms of HPPD (palinopsia, afterimages, illusory movement, etc.).
In addition, she tolerated olanzapine well, and there is anecdotal evidence and 1 case report to suggest that olanzapine exacerbates HPPD.1
HPPD typically is considered a rare sequela of LSD use, although even more rarely it may be caused by other drugs. Common visual disturbances attributed to HPPD are recurrent geometric hallucinations, perception of peripheral movement, colored flashes, intensified colors, palinopsia, positive afterimages, haloes around objects, macropsia, and micropsia occurring spontaneously in individuals with no prior psychopathology. These disturbances can be intermittent or continuous, slowly reversible or irreversible, but are severe, intrusive, and cause functional debility. Sufferers retain insight that these phenomena are the consequence of LSD use and usually seek psychiatric help.
HPPD may be diagnosed by the presence of an identifiable trigger, prodromal symptoms, and presentation onset; by the characteristics of the perceptual disturbances, their frequency, duration, intensity, and course; and by the accompanying negative affect and preserved insight.2
This LSD-induced persistence of visual imagery after the image is removed from the visual field is thought to result from dysfunction of serotonergic cortical inhibitory interneurons with GABAergic outputs that normally suppress visual processors.3 Clonazepam often is helpful.2
R. Andrew Sewell, MD
VA Connecticut Healthcare/Yale University
School of Medicine
New Haven, CT
David Kozin
McLean Hospital/Harvard Medical School
Belmont, MA
Miles G. Cunningham, MD, PhD
McLean Hospital/Harvard Medical School
Belmont, MA
1. Espiard ML, Lecardeur L, Abadie P, et al. Hallucinogen persisting perception disorder after psilocybin consumption: a case study. Eur Psychiatry. 2005;20:458-460.
2. Lerner AG, Gelkopf M, Skladman I, et al. Clonazepam treatment of lysergic acid diethylamide-induced hallucinogen persisting perception disorder with anxiety features. Int Clin Psychopharmacol. 2003;18:101-105.
3. Abraham HD, Aldridge AM. Adverse consequences of lysergic acid diethylamide. Addiction. 1993;88:1327-1334.
I appreciated “The woman who saw the light” (Current Psychiatry, July 2010, p. 44-48) in which Dr. R. Andrew Sewell et al describe a 30-year-old woman with schizoaffective disorder and a 7-year history of visual disturbances, including “flashing lights.” The authors’ differential diagnosis did not include the possibility of visual disturbance secondary to atypical anti-psychotic serotonergic antagonism. Photopsia and similar phenomena are not uncommon with 5HT antagonist antidepressants, such as nefazodone.1 They also are well-known sequelae of lysergic acid diethylamide (LSD), a complex serotonin antagonist/agonist, and would be included under the DSM-IV-TR diagnosis hallucinogen persisting perceptual disorder (HPPD).2 Risperidone, a 5HT2-blocking atypical, and selective serotonin reuptake inhibitors may worsen HPPD effects.3,4 Visual disturbance with risperidone also has been reported in a patient with no LSD exposure.5 Dr. Sewell’s patient was treated sequentially with aripiprazole and olanzapine. Both have 5HT blocking properties.
I wonder if the patient has a history of hallucinogen or LSD exposure, or whether her visual symptoms might be related to the use of atypical anti-psychotics combined with sertraline. It would be interesting to see if her symptoms abated with use of a first-generation antipsychotic.
Charles Krasnow, MD
Adjunct clinical assistant professor of psychiatry
University of Michigan Medical School
Ann Arbor, MI
The authors respond
We agree with Dr. Krasnow that HPPD belongs within our differential diagnosis for photopsia and regret omitting it from our article. We consider this to be unlikely, however, because she had no prior LSD use, a history of well-formed visual hallucinations not characteristic of HPPD, and no other characteristic symptoms of HPPD (palinopsia, afterimages, illusory movement, etc.).
In addition, she tolerated olanzapine well, and there is anecdotal evidence and 1 case report to suggest that olanzapine exacerbates HPPD.1
HPPD typically is considered a rare sequela of LSD use, although even more rarely it may be caused by other drugs. Common visual disturbances attributed to HPPD are recurrent geometric hallucinations, perception of peripheral movement, colored flashes, intensified colors, palinopsia, positive afterimages, haloes around objects, macropsia, and micropsia occurring spontaneously in individuals with no prior psychopathology. These disturbances can be intermittent or continuous, slowly reversible or irreversible, but are severe, intrusive, and cause functional debility. Sufferers retain insight that these phenomena are the consequence of LSD use and usually seek psychiatric help.
HPPD may be diagnosed by the presence of an identifiable trigger, prodromal symptoms, and presentation onset; by the characteristics of the perceptual disturbances, their frequency, duration, intensity, and course; and by the accompanying negative affect and preserved insight.2
This LSD-induced persistence of visual imagery after the image is removed from the visual field is thought to result from dysfunction of serotonergic cortical inhibitory interneurons with GABAergic outputs that normally suppress visual processors.3 Clonazepam often is helpful.2
R. Andrew Sewell, MD
VA Connecticut Healthcare/Yale University
School of Medicine
New Haven, CT
David Kozin
McLean Hospital/Harvard Medical School
Belmont, MA
Miles G. Cunningham, MD, PhD
McLean Hospital/Harvard Medical School
Belmont, MA
I appreciated “The woman who saw the light” (Current Psychiatry, July 2010, p. 44-48) in which Dr. R. Andrew Sewell et al describe a 30-year-old woman with schizoaffective disorder and a 7-year history of visual disturbances, including “flashing lights.” The authors’ differential diagnosis did not include the possibility of visual disturbance secondary to atypical anti-psychotic serotonergic antagonism. Photopsia and similar phenomena are not uncommon with 5HT antagonist antidepressants, such as nefazodone.1 They also are well-known sequelae of lysergic acid diethylamide (LSD), a complex serotonin antagonist/agonist, and would be included under the DSM-IV-TR diagnosis hallucinogen persisting perceptual disorder (HPPD).2 Risperidone, a 5HT2-blocking atypical, and selective serotonin reuptake inhibitors may worsen HPPD effects.3,4 Visual disturbance with risperidone also has been reported in a patient with no LSD exposure.5 Dr. Sewell’s patient was treated sequentially with aripiprazole and olanzapine. Both have 5HT blocking properties.
I wonder if the patient has a history of hallucinogen or LSD exposure, or whether her visual symptoms might be related to the use of atypical anti-psychotics combined with sertraline. It would be interesting to see if her symptoms abated with use of a first-generation antipsychotic.
Charles Krasnow, MD
Adjunct clinical assistant professor of psychiatry
University of Michigan Medical School
Ann Arbor, MI
The authors respond
We agree with Dr. Krasnow that HPPD belongs within our differential diagnosis for photopsia and regret omitting it from our article. We consider this to be unlikely, however, because she had no prior LSD use, a history of well-formed visual hallucinations not characteristic of HPPD, and no other characteristic symptoms of HPPD (palinopsia, afterimages, illusory movement, etc.).
In addition, she tolerated olanzapine well, and there is anecdotal evidence and 1 case report to suggest that olanzapine exacerbates HPPD.1
HPPD typically is considered a rare sequela of LSD use, although even more rarely it may be caused by other drugs. Common visual disturbances attributed to HPPD are recurrent geometric hallucinations, perception of peripheral movement, colored flashes, intensified colors, palinopsia, positive afterimages, haloes around objects, macropsia, and micropsia occurring spontaneously in individuals with no prior psychopathology. These disturbances can be intermittent or continuous, slowly reversible or irreversible, but are severe, intrusive, and cause functional debility. Sufferers retain insight that these phenomena are the consequence of LSD use and usually seek psychiatric help.
HPPD may be diagnosed by the presence of an identifiable trigger, prodromal symptoms, and presentation onset; by the characteristics of the perceptual disturbances, their frequency, duration, intensity, and course; and by the accompanying negative affect and preserved insight.2
This LSD-induced persistence of visual imagery after the image is removed from the visual field is thought to result from dysfunction of serotonergic cortical inhibitory interneurons with GABAergic outputs that normally suppress visual processors.3 Clonazepam often is helpful.2
R. Andrew Sewell, MD
VA Connecticut Healthcare/Yale University
School of Medicine
New Haven, CT
David Kozin
McLean Hospital/Harvard Medical School
Belmont, MA
Miles G. Cunningham, MD, PhD
McLean Hospital/Harvard Medical School
Belmont, MA
1. Espiard ML, Lecardeur L, Abadie P, et al. Hallucinogen persisting perception disorder after psilocybin consumption: a case study. Eur Psychiatry. 2005;20:458-460.
2. Lerner AG, Gelkopf M, Skladman I, et al. Clonazepam treatment of lysergic acid diethylamide-induced hallucinogen persisting perception disorder with anxiety features. Int Clin Psychopharmacol. 2003;18:101-105.
3. Abraham HD, Aldridge AM. Adverse consequences of lysergic acid diethylamide. Addiction. 1993;88:1327-1334.
1. Espiard ML, Lecardeur L, Abadie P, et al. Hallucinogen persisting perception disorder after psilocybin consumption: a case study. Eur Psychiatry. 2005;20:458-460.
2. Lerner AG, Gelkopf M, Skladman I, et al. Clonazepam treatment of lysergic acid diethylamide-induced hallucinogen persisting perception disorder with anxiety features. Int Clin Psychopharmacol. 2003;18:101-105.
3. Abraham HD, Aldridge AM. Adverse consequences of lysergic acid diethylamide. Addiction. 1993;88:1327-1334.
The Child With Food Allergy
Pediatricians play an integral role in the initial diagnosis and management of children with a suspected food allergy. History, some useful laboratory tests, and careful counseling can go a long way to identify these sometimes challenging patients.
A considerable amount of anxiety often surrounds food allergy concerns, and this should be addressed (or at least acknowledged) with patients and their families.
Start with history, the most important diagnostic factor: Ask children and parents about specific symptoms, their timing, and foods the child has ingested. If the child experienced anaphylaxis, then include medication and insect stings in your history taking. The good news for primary care is that 90% of food allergies are caused by a few foods: milk, egg, wheat, soy, peanut, fish, shellfish, and nuts. Other triggers are relatively rare.
Allergy skin testing and/or specific immunoglobulin E (IgE) blood tests can support your diagnosis. However, these findings need to be correlated with history because false-positive results occur frequently. It is important to realize that the level of specific IgE to a food is not correlated with the severity of a reaction, but instead is correlated with the likelihood of having a single reaction.
Determine if the child's symptoms truly suggest an allergic reaction or instead point to a non–food-related cause. Psychological conditions such bulimia, anorexia, or factitious disorder can mimic a food allergy, for example.
Your differential diagnosis also includes structural abnormalities of the GI tract; cystic fibrosis with chronic diarrhea from pancreatic insufficiency; and illness caused by contaminants and additives such as flavorings, dyes, preservatives, or infectious organisms. Also check for exposure to pharmacologic contaminants such as caffeine or tyramine in certain foods.
Rule out lactose intolerance and other disaccharidase deficiencies (especially if the symptoms are limited to the GI tract) and a non-IgE reaction called food protein-induced enterocolitis syndrome (FPIES). An FPIES diagnosis is based on clinical presentation and symptoms because allergy skin testing and specific IgE assays are not helpful.
Also consider gastroesophageal reflux. Children whose symptoms do not improve with proton pump inhibitors might have eosinophilic esophagitis. A food allergen sometimes triggers this condition, and consultation with a gastroenterologist and a biopsy are the best clinical strategies.
It is appropriate for a general pediatrician to treat children with a food allergy when the source of the reaction is easily identifiable, when their symptoms are consistent with an allergic reaction, and when their condition is non–life threatening (and supported by specific IgE test results, if obtained).
Avoidance of the culprit allergen is essential to management. Stress the importance of reading all food labels. Self-injectable epinephrine (such as Mylan Inc.'s EpiPen or EpiPen Jr.) is another essential component. Instruct patients on how and when to use epinephrine, including what to do when anaphylaxis starts in school or in a day care setting. In some cases, it may be appropriate to suggest that the patient wear a medical alert bracelet or necklace.
Education is probably the most important factor in management. Talk to patients and families about prognosis, cross-reactive allergens, and the nutritional needs of patients with multiple food allergies. Keep in mind that if the list of foods to avoid is extensive, this may interfere with normal growth and development. A dietician can help educate families not only on what foods to avoid, but on what foods are encouraged.
Make sure parents are comfortable with your treatment plan. If you are confident in your identification of the culprit food, you can implement a food-elimination diet based on details from the history. Prescribe the appropriate dose of epinephrine and outline an anaphylaxis plan (easily found on the Web site www.foodallergy.org
Pediatricians can order in vitro specific IgE blood tests such as Phadia AB's Pharmacia CAP or UniCAP. These are helpful when performed by a reliable laboratory and may obviate the need for the skin testing of some patients for some foods. These tests are relatively good predictors of peanut, milk, and egg allergies.
In contrast, total IgE and complete blood count assays generally are not helpful. Performance of specific IgE blood tests to foods that are known to be clinically tolerated should be avoided; this just leads to confusion by all parties. In addition, avoid testing for specific IgG to foods because this strategy is not helpful for diagnosis. IgG is a measure of exposure only, and therefore positive results are not uncommon.
Most commonly, I see food-allergic patients post diagnosis to explain the results of previous tests and to develop an ongoing plan for avoidance, which includes strategies in the case of future accidental exposure.
If tolerance is suspected, I discuss with children and parents when to consider a food challenge. Such a protocol is probably best performed at a specialist's office, particularly if a more comprehensive, double-blind, placebo-controlled challenge is warranted. Food challenges require significant time and resources, including advance preparations in case anaphylaxis occurs.
Also consider referral to a specialist when a food culprit is not easily identified; when there is disparity between diagnostic test findings and patient history; and when the patient and family require more comprehensive education.
Pediatricians play an integral role in the initial diagnosis and management of children with a suspected food allergy. History, some useful laboratory tests, and careful counseling can go a long way to identify these sometimes challenging patients.
A considerable amount of anxiety often surrounds food allergy concerns, and this should be addressed (or at least acknowledged) with patients and their families.
Start with history, the most important diagnostic factor: Ask children and parents about specific symptoms, their timing, and foods the child has ingested. If the child experienced anaphylaxis, then include medication and insect stings in your history taking. The good news for primary care is that 90% of food allergies are caused by a few foods: milk, egg, wheat, soy, peanut, fish, shellfish, and nuts. Other triggers are relatively rare.
Allergy skin testing and/or specific immunoglobulin E (IgE) blood tests can support your diagnosis. However, these findings need to be correlated with history because false-positive results occur frequently. It is important to realize that the level of specific IgE to a food is not correlated with the severity of a reaction, but instead is correlated with the likelihood of having a single reaction.
Determine if the child's symptoms truly suggest an allergic reaction or instead point to a non–food-related cause. Psychological conditions such bulimia, anorexia, or factitious disorder can mimic a food allergy, for example.
Your differential diagnosis also includes structural abnormalities of the GI tract; cystic fibrosis with chronic diarrhea from pancreatic insufficiency; and illness caused by contaminants and additives such as flavorings, dyes, preservatives, or infectious organisms. Also check for exposure to pharmacologic contaminants such as caffeine or tyramine in certain foods.
Rule out lactose intolerance and other disaccharidase deficiencies (especially if the symptoms are limited to the GI tract) and a non-IgE reaction called food protein-induced enterocolitis syndrome (FPIES). An FPIES diagnosis is based on clinical presentation and symptoms because allergy skin testing and specific IgE assays are not helpful.
Also consider gastroesophageal reflux. Children whose symptoms do not improve with proton pump inhibitors might have eosinophilic esophagitis. A food allergen sometimes triggers this condition, and consultation with a gastroenterologist and a biopsy are the best clinical strategies.
It is appropriate for a general pediatrician to treat children with a food allergy when the source of the reaction is easily identifiable, when their symptoms are consistent with an allergic reaction, and when their condition is non–life threatening (and supported by specific IgE test results, if obtained).
Avoidance of the culprit allergen is essential to management. Stress the importance of reading all food labels. Self-injectable epinephrine (such as Mylan Inc.'s EpiPen or EpiPen Jr.) is another essential component. Instruct patients on how and when to use epinephrine, including what to do when anaphylaxis starts in school or in a day care setting. In some cases, it may be appropriate to suggest that the patient wear a medical alert bracelet or necklace.
Education is probably the most important factor in management. Talk to patients and families about prognosis, cross-reactive allergens, and the nutritional needs of patients with multiple food allergies. Keep in mind that if the list of foods to avoid is extensive, this may interfere with normal growth and development. A dietician can help educate families not only on what foods to avoid, but on what foods are encouraged.
Make sure parents are comfortable with your treatment plan. If you are confident in your identification of the culprit food, you can implement a food-elimination diet based on details from the history. Prescribe the appropriate dose of epinephrine and outline an anaphylaxis plan (easily found on the Web site www.foodallergy.org
Pediatricians can order in vitro specific IgE blood tests such as Phadia AB's Pharmacia CAP or UniCAP. These are helpful when performed by a reliable laboratory and may obviate the need for the skin testing of some patients for some foods. These tests are relatively good predictors of peanut, milk, and egg allergies.
In contrast, total IgE and complete blood count assays generally are not helpful. Performance of specific IgE blood tests to foods that are known to be clinically tolerated should be avoided; this just leads to confusion by all parties. In addition, avoid testing for specific IgG to foods because this strategy is not helpful for diagnosis. IgG is a measure of exposure only, and therefore positive results are not uncommon.
Most commonly, I see food-allergic patients post diagnosis to explain the results of previous tests and to develop an ongoing plan for avoidance, which includes strategies in the case of future accidental exposure.
If tolerance is suspected, I discuss with children and parents when to consider a food challenge. Such a protocol is probably best performed at a specialist's office, particularly if a more comprehensive, double-blind, placebo-controlled challenge is warranted. Food challenges require significant time and resources, including advance preparations in case anaphylaxis occurs.
Also consider referral to a specialist when a food culprit is not easily identified; when there is disparity between diagnostic test findings and patient history; and when the patient and family require more comprehensive education.
Pediatricians play an integral role in the initial diagnosis and management of children with a suspected food allergy. History, some useful laboratory tests, and careful counseling can go a long way to identify these sometimes challenging patients.
A considerable amount of anxiety often surrounds food allergy concerns, and this should be addressed (or at least acknowledged) with patients and their families.
Start with history, the most important diagnostic factor: Ask children and parents about specific symptoms, their timing, and foods the child has ingested. If the child experienced anaphylaxis, then include medication and insect stings in your history taking. The good news for primary care is that 90% of food allergies are caused by a few foods: milk, egg, wheat, soy, peanut, fish, shellfish, and nuts. Other triggers are relatively rare.
Allergy skin testing and/or specific immunoglobulin E (IgE) blood tests can support your diagnosis. However, these findings need to be correlated with history because false-positive results occur frequently. It is important to realize that the level of specific IgE to a food is not correlated with the severity of a reaction, but instead is correlated with the likelihood of having a single reaction.
Determine if the child's symptoms truly suggest an allergic reaction or instead point to a non–food-related cause. Psychological conditions such bulimia, anorexia, or factitious disorder can mimic a food allergy, for example.
Your differential diagnosis also includes structural abnormalities of the GI tract; cystic fibrosis with chronic diarrhea from pancreatic insufficiency; and illness caused by contaminants and additives such as flavorings, dyes, preservatives, or infectious organisms. Also check for exposure to pharmacologic contaminants such as caffeine or tyramine in certain foods.
Rule out lactose intolerance and other disaccharidase deficiencies (especially if the symptoms are limited to the GI tract) and a non-IgE reaction called food protein-induced enterocolitis syndrome (FPIES). An FPIES diagnosis is based on clinical presentation and symptoms because allergy skin testing and specific IgE assays are not helpful.
Also consider gastroesophageal reflux. Children whose symptoms do not improve with proton pump inhibitors might have eosinophilic esophagitis. A food allergen sometimes triggers this condition, and consultation with a gastroenterologist and a biopsy are the best clinical strategies.
It is appropriate for a general pediatrician to treat children with a food allergy when the source of the reaction is easily identifiable, when their symptoms are consistent with an allergic reaction, and when their condition is non–life threatening (and supported by specific IgE test results, if obtained).
Avoidance of the culprit allergen is essential to management. Stress the importance of reading all food labels. Self-injectable epinephrine (such as Mylan Inc.'s EpiPen or EpiPen Jr.) is another essential component. Instruct patients on how and when to use epinephrine, including what to do when anaphylaxis starts in school or in a day care setting. In some cases, it may be appropriate to suggest that the patient wear a medical alert bracelet or necklace.
Education is probably the most important factor in management. Talk to patients and families about prognosis, cross-reactive allergens, and the nutritional needs of patients with multiple food allergies. Keep in mind that if the list of foods to avoid is extensive, this may interfere with normal growth and development. A dietician can help educate families not only on what foods to avoid, but on what foods are encouraged.
Make sure parents are comfortable with your treatment plan. If you are confident in your identification of the culprit food, you can implement a food-elimination diet based on details from the history. Prescribe the appropriate dose of epinephrine and outline an anaphylaxis plan (easily found on the Web site www.foodallergy.org
Pediatricians can order in vitro specific IgE blood tests such as Phadia AB's Pharmacia CAP or UniCAP. These are helpful when performed by a reliable laboratory and may obviate the need for the skin testing of some patients for some foods. These tests are relatively good predictors of peanut, milk, and egg allergies.
In contrast, total IgE and complete blood count assays generally are not helpful. Performance of specific IgE blood tests to foods that are known to be clinically tolerated should be avoided; this just leads to confusion by all parties. In addition, avoid testing for specific IgG to foods because this strategy is not helpful for diagnosis. IgG is a measure of exposure only, and therefore positive results are not uncommon.
Most commonly, I see food-allergic patients post diagnosis to explain the results of previous tests and to develop an ongoing plan for avoidance, which includes strategies in the case of future accidental exposure.
If tolerance is suspected, I discuss with children and parents when to consider a food challenge. Such a protocol is probably best performed at a specialist's office, particularly if a more comprehensive, double-blind, placebo-controlled challenge is warranted. Food challenges require significant time and resources, including advance preparations in case anaphylaxis occurs.
Also consider referral to a specialist when a food culprit is not easily identified; when there is disparity between diagnostic test findings and patient history; and when the patient and family require more comprehensive education.
Antipsychotics Spur Rapid Metabolic Changes
NEW ORLEANS — Worrisome and clinically measurable metabolic changes can be seen in just 12 weeks among children and adolescents who received antipsychotic medications in a National Institutes of Health–sponsored study, prompting serious concern among clinicians who learned of the results at the annual meeting.
The results struck at the heart of a troubling dichotomy: an explosion of prescriptions of antipsychotic medications for children, but little evidence in real-world practice that young patients are being routinely screened for metabolic changes that have the potential to shorten life expectancy.
The ongoing Metabolic Effects of Antipsychotics in Children study has already enrolled more than 140 children aged 7–18 years who had been slated to be placed on antipsychotics in the community. Investigators closely monitored changes over a 3-month period in body fat using dual-energy x-ray absorptiometry (DXA) and insulin sensitivity using gold standard methods. They also trackeding clinically available measures such as body mass index (BMI) percentile, and plasma glucose and lipids.
Body fat percentages rose in “not all, but certainly the majority of these children and youth,” said Dr. John W. Newcomer, professor of psychiatry and medicine and director of the center for clinical studies at Washington University in St. Louis.
Mean increases were highly variable among children and adolescents taking antipsychotic medications, but have averaged almost 3 kilos, or 6.5 pounds, “of body fat, not just weight,” in just 12 weeks, he said.
Some variance was seen in mean percent body fat accrual depending on which antipsychotic medication the children and adolescents received in the randomized open-label study.
However, box plots revealed “substantial overlap” in the results, showing that each individual child's metabolic response to a given drug is somewhat unpredictable.
“You can find kids who take any one of these medications and potentially get a substantial increase in body fat, and you can also find kids who take any one of these agents who actually have very little change in body fat, although some medications are associated with a higher risk of substantial increase,” Dr. Newcomer said.
Increases in BMI percentiles were “substantial” as well, and closely paralleled more sophisticated measures of body fat, such as DXA.
“The good news is, it's pretty easy to track the changes in adiposity,” said Dr. Newcomer in an interview following the meeting.
“We used very fancy and expensive measures of body fat, but what pediatricians have in the front of every kid's chart (the BMI percentage table) does a darned good job of not only lining up where the child is at the baseline screen, but also in tracking changes over time.”
In a similar vein, the study found that simple blood cholesterol profiles—especially triglycerides and HDL—did a “halfway decent job” of estimating insulin sensitivity at baseline and then tracking changes through the early months of therapy, Dr. Newcomer added.
“The point is … don't wait a year to check the labs,” he said. “Don't not look.”
What is troubling to many is the fact that many clinicians indeed are not looking.
A Medicaid claims data study published earlier this year found that glucose screening was performed in just 31.6% and lipid testing in just 13.4% of 5,370 children aged 6–17 years prescribed antipsychotic drugs from July 1, 2004, to June 30, 2006 (Arch. Pediatr. Adolesc. Med. 2010;164:344–51).
Dr. Newcomer, a coauthor on the Medicaid claims research, said a growing number of “very eye-opening studies” about the enduring impact of childhood metabolic dysregulation and obesity should make clinicians weigh risks and consequences carefully when choosing drugs to prescribe for childhood schizophrenia, and perhaps even more so for use in disruptive behavior disorders and other nonpsychotic diagnoses.
“I have certainly learned that there are children at the end of the road of clinical options who are either not going to be in school or [are] unable to participate without some heroic treatment measures, such as low-dose antipsychotic treatment, to help them to re-engage in education,” he commented.
At the same time, relatively brief pharmacologic interventions for children who do not have schizophrenia or bipolar disorder should leave “a metabolic footprint … as modest as possible,” he said.
The Washington University study extended body weight findings from the nonrandomized SATIETY study published last year (JAMA 2009;302:1765–73), in which 272 4- to 19-year-olds prescribed antipsychotic drugs gained from a mean 4.4 kg (aripiprazole) to 8.5 kg (olanzapine) in a median of just 10.8 weeks on medication.
At the APA scientific session where interim data were released from the MEAC study, one audience member rose to call the findings “catastrophic.”
“What you're showing us is very, very scary,” he told Dr. Newcomer, who replied that the metabolic impacts of other classes of drugs widely used in children, including benzodiazepines and high-dose antidepressants, are also potentially concerning.
“We're having this policy debate under a streetlamp as though second-generation antipsychotics are the only drugs that cause weight gain,” Dr. Newcomer said. “Let's not kid ourselves.”
One alternative raised at the session was intensive behavioral modification, such as a yearlong, school-based program for disruptive children described by Dr. Jacob Venter of Wellesley, Mass., and his colleagues at the same APA scientific session.
Dr. Newcomer pointed to the University of Arizona behavioral study as an example of how nonpharmacologic interventions can produce “some good results,” even among children with severe behavioral dysregulation.
“The problem is, I don't know about your town, but in St. Louis, there is a 6-month waiting list to see a child psychiatrist,” he told the audience.
By the time they can be seen, “these families are in great distress and sometimes aren't terribly interested in taking those referrals for behavioral treatments, either because they already tried some therapy or because they seek rapid change,” he said.
Families want the quick responses they associate with medication, and when a trial of behavioral modification is suggested as a starting place, “we can't give it away.”
As for trying to reduce prescribing of antipsychotic medications to children, particularly among those who do not have symptoms consistent with bipolar disorder or schizophrenia, Dr. Newcomer, who also chairs Missouri's Drug Utilization Review Board, was somewhat skeptical about the potential to substantially reduce that clinical practice.
“Like it or not, that horse is out of the barn. The clinical benefits can be obvious to parents, children, and their doctors, so there will continue to be interest in this therapeutic approach, even as we fully elaborate the risks. This is happening all over the country. The rates of prescriptions are going up. The off-label use is tremendous, suggesting a lot of unmet need,” he said.
Indeed, a series of studies conducted by a team led by Dr. Mark Olfson at Columbia University, New York, has found that prescribing of antipsychotic medications by psychiatrists and primary care physicians has skyrocketed in the United States since the mid-1990s, with treatment of disruptive behavior disorders, including attention-deficit/hyperactivity disorder, playing a significant role in the increase.
In one example, Dr. Olfson reported that antipsychotic use by 2- to 5-year-olds covered by private insurance rose from 0.78/1,000 to 1.59/1,000 from 1999 to 2007.
Less than half of the children in the study had received a mental health assessment, a psychotherapy visit, or a consultation with a psychiatrist.
Antipsychotic medication was prescribed in more than 1.2 million outpatient office visits by children in 2002, up from 201,000 in 1993, Dr. Olfson reported (Arch. Gen. Psychiatry 2006;63:679–85). Diagnoses of disruptive behavior disorders (37.8%), mood disorders (31.8%), pervasive developmental disorders or mental retardation (17.3%), and psychotic disorders (14.2%) accounted for most of those visits.
Dr. Newcomer disclosed that he has served as a consultant to several pharmaceutical companies but reported no financial conflicts of interest relevant to his study.
NEW ORLEANS — Worrisome and clinically measurable metabolic changes can be seen in just 12 weeks among children and adolescents who received antipsychotic medications in a National Institutes of Health–sponsored study, prompting serious concern among clinicians who learned of the results at the annual meeting.
The results struck at the heart of a troubling dichotomy: an explosion of prescriptions of antipsychotic medications for children, but little evidence in real-world practice that young patients are being routinely screened for metabolic changes that have the potential to shorten life expectancy.
The ongoing Metabolic Effects of Antipsychotics in Children study has already enrolled more than 140 children aged 7–18 years who had been slated to be placed on antipsychotics in the community. Investigators closely monitored changes over a 3-month period in body fat using dual-energy x-ray absorptiometry (DXA) and insulin sensitivity using gold standard methods. They also trackeding clinically available measures such as body mass index (BMI) percentile, and plasma glucose and lipids.
Body fat percentages rose in “not all, but certainly the majority of these children and youth,” said Dr. John W. Newcomer, professor of psychiatry and medicine and director of the center for clinical studies at Washington University in St. Louis.
Mean increases were highly variable among children and adolescents taking antipsychotic medications, but have averaged almost 3 kilos, or 6.5 pounds, “of body fat, not just weight,” in just 12 weeks, he said.
Some variance was seen in mean percent body fat accrual depending on which antipsychotic medication the children and adolescents received in the randomized open-label study.
However, box plots revealed “substantial overlap” in the results, showing that each individual child's metabolic response to a given drug is somewhat unpredictable.
“You can find kids who take any one of these medications and potentially get a substantial increase in body fat, and you can also find kids who take any one of these agents who actually have very little change in body fat, although some medications are associated with a higher risk of substantial increase,” Dr. Newcomer said.
Increases in BMI percentiles were “substantial” as well, and closely paralleled more sophisticated measures of body fat, such as DXA.
“The good news is, it's pretty easy to track the changes in adiposity,” said Dr. Newcomer in an interview following the meeting.
“We used very fancy and expensive measures of body fat, but what pediatricians have in the front of every kid's chart (the BMI percentage table) does a darned good job of not only lining up where the child is at the baseline screen, but also in tracking changes over time.”
In a similar vein, the study found that simple blood cholesterol profiles—especially triglycerides and HDL—did a “halfway decent job” of estimating insulin sensitivity at baseline and then tracking changes through the early months of therapy, Dr. Newcomer added.
“The point is … don't wait a year to check the labs,” he said. “Don't not look.”
What is troubling to many is the fact that many clinicians indeed are not looking.
A Medicaid claims data study published earlier this year found that glucose screening was performed in just 31.6% and lipid testing in just 13.4% of 5,370 children aged 6–17 years prescribed antipsychotic drugs from July 1, 2004, to June 30, 2006 (Arch. Pediatr. Adolesc. Med. 2010;164:344–51).
Dr. Newcomer, a coauthor on the Medicaid claims research, said a growing number of “very eye-opening studies” about the enduring impact of childhood metabolic dysregulation and obesity should make clinicians weigh risks and consequences carefully when choosing drugs to prescribe for childhood schizophrenia, and perhaps even more so for use in disruptive behavior disorders and other nonpsychotic diagnoses.
“I have certainly learned that there are children at the end of the road of clinical options who are either not going to be in school or [are] unable to participate without some heroic treatment measures, such as low-dose antipsychotic treatment, to help them to re-engage in education,” he commented.
At the same time, relatively brief pharmacologic interventions for children who do not have schizophrenia or bipolar disorder should leave “a metabolic footprint … as modest as possible,” he said.
The Washington University study extended body weight findings from the nonrandomized SATIETY study published last year (JAMA 2009;302:1765–73), in which 272 4- to 19-year-olds prescribed antipsychotic drugs gained from a mean 4.4 kg (aripiprazole) to 8.5 kg (olanzapine) in a median of just 10.8 weeks on medication.
At the APA scientific session where interim data were released from the MEAC study, one audience member rose to call the findings “catastrophic.”
“What you're showing us is very, very scary,” he told Dr. Newcomer, who replied that the metabolic impacts of other classes of drugs widely used in children, including benzodiazepines and high-dose antidepressants, are also potentially concerning.
“We're having this policy debate under a streetlamp as though second-generation antipsychotics are the only drugs that cause weight gain,” Dr. Newcomer said. “Let's not kid ourselves.”
One alternative raised at the session was intensive behavioral modification, such as a yearlong, school-based program for disruptive children described by Dr. Jacob Venter of Wellesley, Mass., and his colleagues at the same APA scientific session.
Dr. Newcomer pointed to the University of Arizona behavioral study as an example of how nonpharmacologic interventions can produce “some good results,” even among children with severe behavioral dysregulation.
“The problem is, I don't know about your town, but in St. Louis, there is a 6-month waiting list to see a child psychiatrist,” he told the audience.
By the time they can be seen, “these families are in great distress and sometimes aren't terribly interested in taking those referrals for behavioral treatments, either because they already tried some therapy or because they seek rapid change,” he said.
Families want the quick responses they associate with medication, and when a trial of behavioral modification is suggested as a starting place, “we can't give it away.”
As for trying to reduce prescribing of antipsychotic medications to children, particularly among those who do not have symptoms consistent with bipolar disorder or schizophrenia, Dr. Newcomer, who also chairs Missouri's Drug Utilization Review Board, was somewhat skeptical about the potential to substantially reduce that clinical practice.
“Like it or not, that horse is out of the barn. The clinical benefits can be obvious to parents, children, and their doctors, so there will continue to be interest in this therapeutic approach, even as we fully elaborate the risks. This is happening all over the country. The rates of prescriptions are going up. The off-label use is tremendous, suggesting a lot of unmet need,” he said.
Indeed, a series of studies conducted by a team led by Dr. Mark Olfson at Columbia University, New York, has found that prescribing of antipsychotic medications by psychiatrists and primary care physicians has skyrocketed in the United States since the mid-1990s, with treatment of disruptive behavior disorders, including attention-deficit/hyperactivity disorder, playing a significant role in the increase.
In one example, Dr. Olfson reported that antipsychotic use by 2- to 5-year-olds covered by private insurance rose from 0.78/1,000 to 1.59/1,000 from 1999 to 2007.
Less than half of the children in the study had received a mental health assessment, a psychotherapy visit, or a consultation with a psychiatrist.
Antipsychotic medication was prescribed in more than 1.2 million outpatient office visits by children in 2002, up from 201,000 in 1993, Dr. Olfson reported (Arch. Gen. Psychiatry 2006;63:679–85). Diagnoses of disruptive behavior disorders (37.8%), mood disorders (31.8%), pervasive developmental disorders or mental retardation (17.3%), and psychotic disorders (14.2%) accounted for most of those visits.
Dr. Newcomer disclosed that he has served as a consultant to several pharmaceutical companies but reported no financial conflicts of interest relevant to his study.
NEW ORLEANS — Worrisome and clinically measurable metabolic changes can be seen in just 12 weeks among children and adolescents who received antipsychotic medications in a National Institutes of Health–sponsored study, prompting serious concern among clinicians who learned of the results at the annual meeting.
The results struck at the heart of a troubling dichotomy: an explosion of prescriptions of antipsychotic medications for children, but little evidence in real-world practice that young patients are being routinely screened for metabolic changes that have the potential to shorten life expectancy.
The ongoing Metabolic Effects of Antipsychotics in Children study has already enrolled more than 140 children aged 7–18 years who had been slated to be placed on antipsychotics in the community. Investigators closely monitored changes over a 3-month period in body fat using dual-energy x-ray absorptiometry (DXA) and insulin sensitivity using gold standard methods. They also trackeding clinically available measures such as body mass index (BMI) percentile, and plasma glucose and lipids.
Body fat percentages rose in “not all, but certainly the majority of these children and youth,” said Dr. John W. Newcomer, professor of psychiatry and medicine and director of the center for clinical studies at Washington University in St. Louis.
Mean increases were highly variable among children and adolescents taking antipsychotic medications, but have averaged almost 3 kilos, or 6.5 pounds, “of body fat, not just weight,” in just 12 weeks, he said.
Some variance was seen in mean percent body fat accrual depending on which antipsychotic medication the children and adolescents received in the randomized open-label study.
However, box plots revealed “substantial overlap” in the results, showing that each individual child's metabolic response to a given drug is somewhat unpredictable.
“You can find kids who take any one of these medications and potentially get a substantial increase in body fat, and you can also find kids who take any one of these agents who actually have very little change in body fat, although some medications are associated with a higher risk of substantial increase,” Dr. Newcomer said.
Increases in BMI percentiles were “substantial” as well, and closely paralleled more sophisticated measures of body fat, such as DXA.
“The good news is, it's pretty easy to track the changes in adiposity,” said Dr. Newcomer in an interview following the meeting.
“We used very fancy and expensive measures of body fat, but what pediatricians have in the front of every kid's chart (the BMI percentage table) does a darned good job of not only lining up where the child is at the baseline screen, but also in tracking changes over time.”
In a similar vein, the study found that simple blood cholesterol profiles—especially triglycerides and HDL—did a “halfway decent job” of estimating insulin sensitivity at baseline and then tracking changes through the early months of therapy, Dr. Newcomer added.
“The point is … don't wait a year to check the labs,” he said. “Don't not look.”
What is troubling to many is the fact that many clinicians indeed are not looking.
A Medicaid claims data study published earlier this year found that glucose screening was performed in just 31.6% and lipid testing in just 13.4% of 5,370 children aged 6–17 years prescribed antipsychotic drugs from July 1, 2004, to June 30, 2006 (Arch. Pediatr. Adolesc. Med. 2010;164:344–51).
Dr. Newcomer, a coauthor on the Medicaid claims research, said a growing number of “very eye-opening studies” about the enduring impact of childhood metabolic dysregulation and obesity should make clinicians weigh risks and consequences carefully when choosing drugs to prescribe for childhood schizophrenia, and perhaps even more so for use in disruptive behavior disorders and other nonpsychotic diagnoses.
“I have certainly learned that there are children at the end of the road of clinical options who are either not going to be in school or [are] unable to participate without some heroic treatment measures, such as low-dose antipsychotic treatment, to help them to re-engage in education,” he commented.
At the same time, relatively brief pharmacologic interventions for children who do not have schizophrenia or bipolar disorder should leave “a metabolic footprint … as modest as possible,” he said.
The Washington University study extended body weight findings from the nonrandomized SATIETY study published last year (JAMA 2009;302:1765–73), in which 272 4- to 19-year-olds prescribed antipsychotic drugs gained from a mean 4.4 kg (aripiprazole) to 8.5 kg (olanzapine) in a median of just 10.8 weeks on medication.
At the APA scientific session where interim data were released from the MEAC study, one audience member rose to call the findings “catastrophic.”
“What you're showing us is very, very scary,” he told Dr. Newcomer, who replied that the metabolic impacts of other classes of drugs widely used in children, including benzodiazepines and high-dose antidepressants, are also potentially concerning.
“We're having this policy debate under a streetlamp as though second-generation antipsychotics are the only drugs that cause weight gain,” Dr. Newcomer said. “Let's not kid ourselves.”
One alternative raised at the session was intensive behavioral modification, such as a yearlong, school-based program for disruptive children described by Dr. Jacob Venter of Wellesley, Mass., and his colleagues at the same APA scientific session.
Dr. Newcomer pointed to the University of Arizona behavioral study as an example of how nonpharmacologic interventions can produce “some good results,” even among children with severe behavioral dysregulation.
“The problem is, I don't know about your town, but in St. Louis, there is a 6-month waiting list to see a child psychiatrist,” he told the audience.
By the time they can be seen, “these families are in great distress and sometimes aren't terribly interested in taking those referrals for behavioral treatments, either because they already tried some therapy or because they seek rapid change,” he said.
Families want the quick responses they associate with medication, and when a trial of behavioral modification is suggested as a starting place, “we can't give it away.”
As for trying to reduce prescribing of antipsychotic medications to children, particularly among those who do not have symptoms consistent with bipolar disorder or schizophrenia, Dr. Newcomer, who also chairs Missouri's Drug Utilization Review Board, was somewhat skeptical about the potential to substantially reduce that clinical practice.
“Like it or not, that horse is out of the barn. The clinical benefits can be obvious to parents, children, and their doctors, so there will continue to be interest in this therapeutic approach, even as we fully elaborate the risks. This is happening all over the country. The rates of prescriptions are going up. The off-label use is tremendous, suggesting a lot of unmet need,” he said.
Indeed, a series of studies conducted by a team led by Dr. Mark Olfson at Columbia University, New York, has found that prescribing of antipsychotic medications by psychiatrists and primary care physicians has skyrocketed in the United States since the mid-1990s, with treatment of disruptive behavior disorders, including attention-deficit/hyperactivity disorder, playing a significant role in the increase.
In one example, Dr. Olfson reported that antipsychotic use by 2- to 5-year-olds covered by private insurance rose from 0.78/1,000 to 1.59/1,000 from 1999 to 2007.
Less than half of the children in the study had received a mental health assessment, a psychotherapy visit, or a consultation with a psychiatrist.
Antipsychotic medication was prescribed in more than 1.2 million outpatient office visits by children in 2002, up from 201,000 in 1993, Dr. Olfson reported (Arch. Gen. Psychiatry 2006;63:679–85). Diagnoses of disruptive behavior disorders (37.8%), mood disorders (31.8%), pervasive developmental disorders or mental retardation (17.3%), and psychotic disorders (14.2%) accounted for most of those visits.
Dr. Newcomer disclosed that he has served as a consultant to several pharmaceutical companies but reported no financial conflicts of interest relevant to his study.
Help Parents Change Style for Raising Teens
www.CHADIS.com[email protected]
Adolescents are often the most intimidating of our patients. Let's face it: Most of us chose pediatrics because we like little kids. If a 15-minute office visit with a sullen teenager can be so difficult, imagine living with one 24/7. Actually, many of us won't have to imagine—we ourselves are the parents of adolescents, and we know just how challenging that can be.
Despite our own feelings of inadequacy, we can help parents make the transition from raising the innocent younger child to guiding the testy teen into adulthood. A failure to make that transition in parenting style can contribute greatly to a suboptimal outcome.
But your guidance needs to start early. When a parent comes into the office demanding that you administer a drug test or a pregnancy test, you have probably missed the window for effective action. The horse is well out of the barn.
The time to start is earlier—much earlier. All of parenting involves the balancing act between supporting dependency and promoting independence. When people first become parents, they are consumed with accepting the huge dependency of their baby. As the child gets older, parents must allow the child more independence for things to go smoothly.
But adolescence is a time when that balancing act requires truly skilled acrobatics. Teens and their parents need to negotiate the “Four I's” of adolescent development: Initiative, Individuation, Independence, and Intimacy.
Adolescents clearly need to take the initiative in their activities, including when they do their chores and how they manage homework. If parents get in the way and try to structure all of that, they're going to get a lot of pushback.
In terms of individuation—discovering who they are—teenagers are highly sensitive to the standards of peers. They're more interested in what their peers think they should do than what their parents think they should do. On one level, this includes how many ear piercings they have and how they dress. But on a broader level, they need to think their parents are wrong about most things in order to feel “like their own person.” Offering an opinion can be beneficial in giving the adolescent something to counter, but ideally save consequences for more substantial failings. In terms of independence, teenagers are better educated by learning from the consequences of their own actions when those actions are not harmful to their futures.
And in terms of intimacy, teens want and need privacy for their budding relationships. Parents need to learn how to be available to talk about relationships, but not ask too many questions.
Different teens move through these changes at different times. And on top of that, the transition may not always go in one direction. A teen may want to be very independent in choosing her clothes. But the same teen may want a lot of parental help on getting her homework done and on handling peer situations. That's part of what makes parenting adolescents so difficult.
Parents need to gradually release control and let their teens exert more independence. But the key word in that sentence is “gradually,” and parents need to be alert for signs that the child is not ready or has not yet earned that freedom.
Let's say the parents have allowed their 13-year-old to have a cell phone. Let's say that a few weeks later, the child hurls the phone against the wall in anger, shattering it beyond repair. Some parents might be tempted to say: “That's it. I'm not buying you another cell phone until you're in college,” but that is unlikely to be the most educational solution. The time frame should be measured in days or weeks, not in months or years. If consequences are too severe, kids tend to write their parents off completely and feel they have been written off.
Instead, the parents should give the teen a clear path to re-earning the privilege, negotiating the terms. Maybe he has to contribute 80% of his allowance and do some extra chores until the phone is paid for. Showing that they're reasonable and willing to negotiate is a model of adult behavior, and it's also their key to success.
The older the child, the more important it is to negotiate what the rules are to be, and also what exceptions there might be. It's fine if there's a general rule that they can't stay out after 11 o'clock. But if a special event comes along that starts at 10 o'clock and won't end until 2 a.m., it's best to be flexible about the curfew this one time. When teens and parents negotiate one-time exceptions as needed, there is structure but rebellion or sneaking is not brought out.
Negotiation is important. A 30-year longitudinal study from the University of California, Berkeley, demonstrated that parents who managed to negotiate the rules with their children had more harmonious relationships with them later (New Dir. Child Adolesc. Dev. 2005;108:61–9). Often a dynamic arises in families where the parents are so generally annoyed with their teen that they reflexively answer, “No!” to any request. That can be really counterproductive when it comes to parenting adolescents. The first response should be: “Yes, if at all possible. Let's talk about it.”
I recommend that parents explicitly discuss the request using the following six points in deciding with the adolescent on their request. Posting these on the refrigerator and making discussing them a routine lets the teen know they are being taken seriously, slows the reflex to say “no,” and may help install them as a mantra in the teen's brain for future decision making:
Six Guides for Decision Making
1. Is it safe?
2. Is it legal?
3. Does it conflict with responsibilities?
4. Does it meet a developmental need?
5. Does it interfere with others?
6. Could it harm his/her development?
Anyone who's read “The Catcher in the Rye” (New York: Little, Brown and Co., 1951) by J.D. Salinger knows that teenagers are especially sensitive to hypocrisy. Parents often talk about the importance of being a moral person, but the teen is aware that they're cheating on their income taxes. They will reject their parents' moral code if they see them being hypocritical.
Clearly, the best way for the parent to encourage their offspring to uphold good moral standards is to actually live those standards 24/7. But almost everyone fails to live up to those standards from time to time, and if they're parents of an adolescent, the teen is sure to be right there when they do. Adolescents appreciate and learn from honesty when that happens. The parent could admit, “Yes, I know I said that you should never curse another driver, but I was so angry that I forgot my own rule.”
In these days of one- and two-child families, where parents often depend on their own children for friendship and companionship, it can be especially devastating to hear a teen say: “I hate you. You're the worst parents ever.” When that happens—and it's almost certain to happen, since it's the rare child who never utters such a sentiment—the parent's best response is not to rise to the bait of an angry teenager. They don't really mean it. And if the parent shows too much visible distress, or starts to punish them for saying those things, there won't be as much opportunity to recover. A simple “I am sorry you feel that way right now. I can see that you are really angry about [my decision, your curfew, what I said].”
And when the teen notices that the parent has not reacted to such provocation, that in itself is a valuable life lesson. The next time a street tough tosses off an insult, he'll be more likely to simply shrug his shoulders and walk away. For additional information on dealing with adolescents, the American Academy of Pediatrics maintains a particularly good collection of resources for parents at www.healthychildren.org
www.CHADIS.com[email protected]
Adolescents are often the most intimidating of our patients. Let's face it: Most of us chose pediatrics because we like little kids. If a 15-minute office visit with a sullen teenager can be so difficult, imagine living with one 24/7. Actually, many of us won't have to imagine—we ourselves are the parents of adolescents, and we know just how challenging that can be.
Despite our own feelings of inadequacy, we can help parents make the transition from raising the innocent younger child to guiding the testy teen into adulthood. A failure to make that transition in parenting style can contribute greatly to a suboptimal outcome.
But your guidance needs to start early. When a parent comes into the office demanding that you administer a drug test or a pregnancy test, you have probably missed the window for effective action. The horse is well out of the barn.
The time to start is earlier—much earlier. All of parenting involves the balancing act between supporting dependency and promoting independence. When people first become parents, they are consumed with accepting the huge dependency of their baby. As the child gets older, parents must allow the child more independence for things to go smoothly.
But adolescence is a time when that balancing act requires truly skilled acrobatics. Teens and their parents need to negotiate the “Four I's” of adolescent development: Initiative, Individuation, Independence, and Intimacy.
Adolescents clearly need to take the initiative in their activities, including when they do their chores and how they manage homework. If parents get in the way and try to structure all of that, they're going to get a lot of pushback.
In terms of individuation—discovering who they are—teenagers are highly sensitive to the standards of peers. They're more interested in what their peers think they should do than what their parents think they should do. On one level, this includes how many ear piercings they have and how they dress. But on a broader level, they need to think their parents are wrong about most things in order to feel “like their own person.” Offering an opinion can be beneficial in giving the adolescent something to counter, but ideally save consequences for more substantial failings. In terms of independence, teenagers are better educated by learning from the consequences of their own actions when those actions are not harmful to their futures.
And in terms of intimacy, teens want and need privacy for their budding relationships. Parents need to learn how to be available to talk about relationships, but not ask too many questions.
Different teens move through these changes at different times. And on top of that, the transition may not always go in one direction. A teen may want to be very independent in choosing her clothes. But the same teen may want a lot of parental help on getting her homework done and on handling peer situations. That's part of what makes parenting adolescents so difficult.
Parents need to gradually release control and let their teens exert more independence. But the key word in that sentence is “gradually,” and parents need to be alert for signs that the child is not ready or has not yet earned that freedom.
Let's say the parents have allowed their 13-year-old to have a cell phone. Let's say that a few weeks later, the child hurls the phone against the wall in anger, shattering it beyond repair. Some parents might be tempted to say: “That's it. I'm not buying you another cell phone until you're in college,” but that is unlikely to be the most educational solution. The time frame should be measured in days or weeks, not in months or years. If consequences are too severe, kids tend to write their parents off completely and feel they have been written off.
Instead, the parents should give the teen a clear path to re-earning the privilege, negotiating the terms. Maybe he has to contribute 80% of his allowance and do some extra chores until the phone is paid for. Showing that they're reasonable and willing to negotiate is a model of adult behavior, and it's also their key to success.
The older the child, the more important it is to negotiate what the rules are to be, and also what exceptions there might be. It's fine if there's a general rule that they can't stay out after 11 o'clock. But if a special event comes along that starts at 10 o'clock and won't end until 2 a.m., it's best to be flexible about the curfew this one time. When teens and parents negotiate one-time exceptions as needed, there is structure but rebellion or sneaking is not brought out.
Negotiation is important. A 30-year longitudinal study from the University of California, Berkeley, demonstrated that parents who managed to negotiate the rules with their children had more harmonious relationships with them later (New Dir. Child Adolesc. Dev. 2005;108:61–9). Often a dynamic arises in families where the parents are so generally annoyed with their teen that they reflexively answer, “No!” to any request. That can be really counterproductive when it comes to parenting adolescents. The first response should be: “Yes, if at all possible. Let's talk about it.”
I recommend that parents explicitly discuss the request using the following six points in deciding with the adolescent on their request. Posting these on the refrigerator and making discussing them a routine lets the teen know they are being taken seriously, slows the reflex to say “no,” and may help install them as a mantra in the teen's brain for future decision making:
Six Guides for Decision Making
1. Is it safe?
2. Is it legal?
3. Does it conflict with responsibilities?
4. Does it meet a developmental need?
5. Does it interfere with others?
6. Could it harm his/her development?
Anyone who's read “The Catcher in the Rye” (New York: Little, Brown and Co., 1951) by J.D. Salinger knows that teenagers are especially sensitive to hypocrisy. Parents often talk about the importance of being a moral person, but the teen is aware that they're cheating on their income taxes. They will reject their parents' moral code if they see them being hypocritical.
Clearly, the best way for the parent to encourage their offspring to uphold good moral standards is to actually live those standards 24/7. But almost everyone fails to live up to those standards from time to time, and if they're parents of an adolescent, the teen is sure to be right there when they do. Adolescents appreciate and learn from honesty when that happens. The parent could admit, “Yes, I know I said that you should never curse another driver, but I was so angry that I forgot my own rule.”
In these days of one- and two-child families, where parents often depend on their own children for friendship and companionship, it can be especially devastating to hear a teen say: “I hate you. You're the worst parents ever.” When that happens—and it's almost certain to happen, since it's the rare child who never utters such a sentiment—the parent's best response is not to rise to the bait of an angry teenager. They don't really mean it. And if the parent shows too much visible distress, or starts to punish them for saying those things, there won't be as much opportunity to recover. A simple “I am sorry you feel that way right now. I can see that you are really angry about [my decision, your curfew, what I said].”
And when the teen notices that the parent has not reacted to such provocation, that in itself is a valuable life lesson. The next time a street tough tosses off an insult, he'll be more likely to simply shrug his shoulders and walk away. For additional information on dealing with adolescents, the American Academy of Pediatrics maintains a particularly good collection of resources for parents at www.healthychildren.org
www.CHADIS.com[email protected]
Adolescents are often the most intimidating of our patients. Let's face it: Most of us chose pediatrics because we like little kids. If a 15-minute office visit with a sullen teenager can be so difficult, imagine living with one 24/7. Actually, many of us won't have to imagine—we ourselves are the parents of adolescents, and we know just how challenging that can be.
Despite our own feelings of inadequacy, we can help parents make the transition from raising the innocent younger child to guiding the testy teen into adulthood. A failure to make that transition in parenting style can contribute greatly to a suboptimal outcome.
But your guidance needs to start early. When a parent comes into the office demanding that you administer a drug test or a pregnancy test, you have probably missed the window for effective action. The horse is well out of the barn.
The time to start is earlier—much earlier. All of parenting involves the balancing act between supporting dependency and promoting independence. When people first become parents, they are consumed with accepting the huge dependency of their baby. As the child gets older, parents must allow the child more independence for things to go smoothly.
But adolescence is a time when that balancing act requires truly skilled acrobatics. Teens and their parents need to negotiate the “Four I's” of adolescent development: Initiative, Individuation, Independence, and Intimacy.
Adolescents clearly need to take the initiative in their activities, including when they do their chores and how they manage homework. If parents get in the way and try to structure all of that, they're going to get a lot of pushback.
In terms of individuation—discovering who they are—teenagers are highly sensitive to the standards of peers. They're more interested in what their peers think they should do than what their parents think they should do. On one level, this includes how many ear piercings they have and how they dress. But on a broader level, they need to think their parents are wrong about most things in order to feel “like their own person.” Offering an opinion can be beneficial in giving the adolescent something to counter, but ideally save consequences for more substantial failings. In terms of independence, teenagers are better educated by learning from the consequences of their own actions when those actions are not harmful to their futures.
And in terms of intimacy, teens want and need privacy for their budding relationships. Parents need to learn how to be available to talk about relationships, but not ask too many questions.
Different teens move through these changes at different times. And on top of that, the transition may not always go in one direction. A teen may want to be very independent in choosing her clothes. But the same teen may want a lot of parental help on getting her homework done and on handling peer situations. That's part of what makes parenting adolescents so difficult.
Parents need to gradually release control and let their teens exert more independence. But the key word in that sentence is “gradually,” and parents need to be alert for signs that the child is not ready or has not yet earned that freedom.
Let's say the parents have allowed their 13-year-old to have a cell phone. Let's say that a few weeks later, the child hurls the phone against the wall in anger, shattering it beyond repair. Some parents might be tempted to say: “That's it. I'm not buying you another cell phone until you're in college,” but that is unlikely to be the most educational solution. The time frame should be measured in days or weeks, not in months or years. If consequences are too severe, kids tend to write their parents off completely and feel they have been written off.
Instead, the parents should give the teen a clear path to re-earning the privilege, negotiating the terms. Maybe he has to contribute 80% of his allowance and do some extra chores until the phone is paid for. Showing that they're reasonable and willing to negotiate is a model of adult behavior, and it's also their key to success.
The older the child, the more important it is to negotiate what the rules are to be, and also what exceptions there might be. It's fine if there's a general rule that they can't stay out after 11 o'clock. But if a special event comes along that starts at 10 o'clock and won't end until 2 a.m., it's best to be flexible about the curfew this one time. When teens and parents negotiate one-time exceptions as needed, there is structure but rebellion or sneaking is not brought out.
Negotiation is important. A 30-year longitudinal study from the University of California, Berkeley, demonstrated that parents who managed to negotiate the rules with their children had more harmonious relationships with them later (New Dir. Child Adolesc. Dev. 2005;108:61–9). Often a dynamic arises in families where the parents are so generally annoyed with their teen that they reflexively answer, “No!” to any request. That can be really counterproductive when it comes to parenting adolescents. The first response should be: “Yes, if at all possible. Let's talk about it.”
I recommend that parents explicitly discuss the request using the following six points in deciding with the adolescent on their request. Posting these on the refrigerator and making discussing them a routine lets the teen know they are being taken seriously, slows the reflex to say “no,” and may help install them as a mantra in the teen's brain for future decision making:
Six Guides for Decision Making
1. Is it safe?
2. Is it legal?
3. Does it conflict with responsibilities?
4. Does it meet a developmental need?
5. Does it interfere with others?
6. Could it harm his/her development?
Anyone who's read “The Catcher in the Rye” (New York: Little, Brown and Co., 1951) by J.D. Salinger knows that teenagers are especially sensitive to hypocrisy. Parents often talk about the importance of being a moral person, but the teen is aware that they're cheating on their income taxes. They will reject their parents' moral code if they see them being hypocritical.
Clearly, the best way for the parent to encourage their offspring to uphold good moral standards is to actually live those standards 24/7. But almost everyone fails to live up to those standards from time to time, and if they're parents of an adolescent, the teen is sure to be right there when they do. Adolescents appreciate and learn from honesty when that happens. The parent could admit, “Yes, I know I said that you should never curse another driver, but I was so angry that I forgot my own rule.”
In these days of one- and two-child families, where parents often depend on their own children for friendship and companionship, it can be especially devastating to hear a teen say: “I hate you. You're the worst parents ever.” When that happens—and it's almost certain to happen, since it's the rare child who never utters such a sentiment—the parent's best response is not to rise to the bait of an angry teenager. They don't really mean it. And if the parent shows too much visible distress, or starts to punish them for saying those things, there won't be as much opportunity to recover. A simple “I am sorry you feel that way right now. I can see that you are really angry about [my decision, your curfew, what I said].”
And when the teen notices that the parent has not reacted to such provocation, that in itself is a valuable life lesson. The next time a street tough tosses off an insult, he'll be more likely to simply shrug his shoulders and walk away. For additional information on dealing with adolescents, the American Academy of Pediatrics maintains a particularly good collection of resources for parents at www.healthychildren.org
Concussion Rates Are Rising in Younger Athletes Aged 8-13 Years
Approximately 40% of emergency department visits for sports-related concussions in young athletes occurred in children aged 8–13 years, based on data from concussion-related ED visits in the United States between 2001 and 2005.
There are two main concerns about sports-related concussion in younger children, compared with college athletes and adults, lead author Dr. Lisa L. Bakhos said in an interview. Dr. Bakhos conducted the study while she was a teaching fellow at Brown University in Providence, R.I. (Pediatrics 2010 Aug. 30 [doi:10.1542/peds.2009–3101]).
"First, many parents, coaches, teachers, and other adults feel that because these athletes are so young, they could not possibly get seriously hurt. As we have seen time and time again, this is, of course, not the case," said Dr. Bakhos, who is currently an emergency physician at the Jersey Shore University Medical Center in Neptune, N.J.
In addition, more data have surfaced about cognitive deficits in older children after concussion, she said, "which leads to conjecture that younger children would suffer the same — if not more — deficits long term."
However, the link between sports-related concussion and cognitive deficits needs further study, she commented.
The American Academy of Pediatrics has just released a new clinical report, "Sport-Related Concussion in Children and Adolescents" to aid in this effort (Pediatrics 2010 Aug. 30 [doi:10.1542/peds. 2010–2005]).
To get a better picture of the scope of sports-related concussion in young athletes, Dr. Bakhos and her colleagues reviewed data from the NEISS (National Electronic Injury Surveillance System) from 1997 through 2007, and from the NEISS-AIP (All-Injury Program) from 2001 through 2005.
The NEISS system allows researchers to investigate injury- and product-related ED visits.
In 2001–2005, approximately half of all ED visits for concussion across older and younger age groups were related to sports, including 58% of visits in children aged 8–13 years and 46% of visits in those aged 14–19 years.
Put another way, approximately 4 in 1,000 children aged 8–13 years and 6 in 1,000 of those aged 14–19 years went to the ED for a sports-related concussion.
During the 10-year period of 1997–2007, ED visits for the most popular organized team sports (football, ice hockey, soccer, basketball, and baseball) doubled in 8- to 13-year-olds and increased by more than 200% in 14- to 19-year-olds.
"The take-home message for pediatricians is, take concussion seriously even in the very young athlete," said Dr. Bakhos. "Children with concussion should be followed just as closely as a child with a sprained ankle or a broken bone. Return-to-play guidelines should be followed closely and stressed to parents."
"We as pediatricians should also stress to parents the importance of concussion prevention in sport as well, mostly [by] the use of helmets at all times," she emphasized.
The study was limited by the exclusion of sports-related concussions that were treated in non-ED settings, and by the underreporting of sports-related concussions by young athletes, their parents, and their coaches, the researchers noted.
But the rise in sports-related concussions in younger and older children suggests the need for more research and guidance in preventing and treating these injuries, they added.
To help clinicians manage sports-related concussions in young athletes, the AAP published a new clinical report that "outlines the current state of knowledge on pediatric and adolescent sport-related concussions," wrote lead authors Dr. Mark E. Halstead and Dr. Kevin D. Walter, on behalf of the AAP's Council on Sports Medicine and Fitness. It includes the SCAT 2 (Sport Concussion Assessment Tool 2), a standardized method of evaluating concussion in athletes aged 10 and older.
The report outlines recommendations regarding sports-related concussion, including the following:
▸ Stay off the field. Even if symptoms subside, young athletes should never return to play on the same day they have a concussion. Younger athletes need more recovery time and a more conservative approach than do college or professional athletes.
▸ See a doctor. Any children or adolescents who suffer concussions during sports should be medically cleared by a physician before they return to activity.
▸ Rest mind and body. All young athletes should refrain from physical activity until they are asymptomatic at rest and when active. Rest includes mental as well as physical rest.
Some evidence suggests that cognitive exertion — including doing homework, watching TV, and playing video games — can exacerbate symptoms post concussion.
In the last few years, several states have passed laws requiring educational materials about sports-related concussion for school-aged athletes, coaches, and parents. The state laws were a consideration, but the AAP began working on the report before the first law was passed, said Dr. Halstead, director of the sports concussion program in the department of orthopedics at Washington University in St. Louis.
"We felt there was a need to address specifically the [pediatric] athlete and address all the recent research that has been published on this topic," he said in an interview.
"The recommendations presented aren't significantly different from other recent documents published, but these were primarily published in sports medicine journals, which many pediatricians do not review.
"We wanted to bring these recommendations to the forefront to the pediatric community, and expand upon the details provided in previous documents published.
"We have highlighted some of the new research on neuroimaging, balance assessments, long-term complications, education, and neuropsychological testing," Dr. Halstead said.
Dr. Walter added, "I think it is also important to recognize that because we have learned more about concussion diagnosis, treatment, and complications, the treatment that coaches and parents received when they had a concussion themselves at a young age is likely different than today."
Many parents and coaches don't think concussion is a big deal because they had one when they were younger and they "toughed it out" and "are fine now," said Dr. Walter, program director of pediatric and adolescent sports medicine at Children's Hospital of Wisconsin in Milwaukee.
The authors acknowledged the lack of published baseline neuropsychological data on children younger than 12 years, and noted that assessment by a neuropsychologist might be helpful for children who have had more than one concussion, or whose postconcussive symptoms persist for several months.
I'm not surprised by the increase in reports of concussions in young athletes. And because not every kid with a concussion goes to the emergency department, there are even more injuries occurring that are not being reported.
I think greater awareness and better diagnosis are the main reasons why the number of sports-related concussions is rising. Until 10 years ago, the medical literature focused only on concussions that involved loss of consciousness. But what we have learned in the past decade is that the subtleties of this injury are absolutely critical for diagnosis.
For example, loss of consciousness is actually less predictive than loss of memory. (I published a paper in 2003 showing that amnesia or memory loss around the time of the concussion is 10 times more predictive than a loss of consciousness.) Changes in the way we define the injury are driving the rise in reported concussions in young athletes.
As we continue to peel the onion on concussion, we realize that it is an extremely complex injury, and that there are more problems in those who are injured—particularly kids. Also, we now have animal models that help show what happens in the brain after a concussion. This knowledge base has accumulated at warp speed over the last 10 years, and with that has come better recognition, better management, and better understanding of the injury, as well as more concern.
Most importantly, neurocognitive testing is becoming more widely used as a way to assess sports-related concussion, and it is the key to why there is so much attention now being paid to the injury: We now have a way to measure it by collecting baseline data. The sensitivity and specificity of such tests are impressive.
One of the keys to improving the management of pediatric concussion is to get knowledge related to this injury, as well as its many assessment tools, into pediatric offices. Clinics are available around the United States to help pediatricians who want to incorporate neurocognitive testing into their practices. The American Academy of Pediatrics' report by Dr. Halstead and Dr. Walter lists several assessment tools, and it includes other valuable, relevant information about managing sports-related concussions in young athletes.
MICHAEL COLLINS, PH.D., is the assistant director of the sports medicine concussion program at the University of Pittsburgh Medical Center. He also coauthored the Centers for Disease Control and Prevention's “Heads Up: Brain Injury in Your Practice” tool kit for physicians.
I'm not surprised by the increase in reports of concussions in young athletes. And because not every kid with a concussion goes to the emergency department, there are even more injuries occurring that are not being reported.
I think greater awareness and better diagnosis are the main reasons why the number of sports-related concussions is rising. Until 10 years ago, the medical literature focused only on concussions that involved loss of consciousness. But what we have learned in the past decade is that the subtleties of this injury are absolutely critical for diagnosis.
For example, loss of consciousness is actually less predictive than loss of memory. (I published a paper in 2003 showing that amnesia or memory loss around the time of the concussion is 10 times more predictive than a loss of consciousness.) Changes in the way we define the injury are driving the rise in reported concussions in young athletes.
As we continue to peel the onion on concussion, we realize that it is an extremely complex injury, and that there are more problems in those who are injured—particularly kids. Also, we now have animal models that help show what happens in the brain after a concussion. This knowledge base has accumulated at warp speed over the last 10 years, and with that has come better recognition, better management, and better understanding of the injury, as well as more concern.
Most importantly, neurocognitive testing is becoming more widely used as a way to assess sports-related concussion, and it is the key to why there is so much attention now being paid to the injury: We now have a way to measure it by collecting baseline data. The sensitivity and specificity of such tests are impressive.
One of the keys to improving the management of pediatric concussion is to get knowledge related to this injury, as well as its many assessment tools, into pediatric offices. Clinics are available around the United States to help pediatricians who want to incorporate neurocognitive testing into their practices. The American Academy of Pediatrics' report by Dr. Halstead and Dr. Walter lists several assessment tools, and it includes other valuable, relevant information about managing sports-related concussions in young athletes.
MICHAEL COLLINS, PH.D., is the assistant director of the sports medicine concussion program at the University of Pittsburgh Medical Center. He also coauthored the Centers for Disease Control and Prevention's “Heads Up: Brain Injury in Your Practice” tool kit for physicians.
I'm not surprised by the increase in reports of concussions in young athletes. And because not every kid with a concussion goes to the emergency department, there are even more injuries occurring that are not being reported.
I think greater awareness and better diagnosis are the main reasons why the number of sports-related concussions is rising. Until 10 years ago, the medical literature focused only on concussions that involved loss of consciousness. But what we have learned in the past decade is that the subtleties of this injury are absolutely critical for diagnosis.
For example, loss of consciousness is actually less predictive than loss of memory. (I published a paper in 2003 showing that amnesia or memory loss around the time of the concussion is 10 times more predictive than a loss of consciousness.) Changes in the way we define the injury are driving the rise in reported concussions in young athletes.
As we continue to peel the onion on concussion, we realize that it is an extremely complex injury, and that there are more problems in those who are injured—particularly kids. Also, we now have animal models that help show what happens in the brain after a concussion. This knowledge base has accumulated at warp speed over the last 10 years, and with that has come better recognition, better management, and better understanding of the injury, as well as more concern.
Most importantly, neurocognitive testing is becoming more widely used as a way to assess sports-related concussion, and it is the key to why there is so much attention now being paid to the injury: We now have a way to measure it by collecting baseline data. The sensitivity and specificity of such tests are impressive.
One of the keys to improving the management of pediatric concussion is to get knowledge related to this injury, as well as its many assessment tools, into pediatric offices. Clinics are available around the United States to help pediatricians who want to incorporate neurocognitive testing into their practices. The American Academy of Pediatrics' report by Dr. Halstead and Dr. Walter lists several assessment tools, and it includes other valuable, relevant information about managing sports-related concussions in young athletes.
MICHAEL COLLINS, PH.D., is the assistant director of the sports medicine concussion program at the University of Pittsburgh Medical Center. He also coauthored the Centers for Disease Control and Prevention's “Heads Up: Brain Injury in Your Practice” tool kit for physicians.
Approximately 40% of emergency department visits for sports-related concussions in young athletes occurred in children aged 8–13 years, based on data from concussion-related ED visits in the United States between 2001 and 2005.
There are two main concerns about sports-related concussion in younger children, compared with college athletes and adults, lead author Dr. Lisa L. Bakhos said in an interview. Dr. Bakhos conducted the study while she was a teaching fellow at Brown University in Providence, R.I. (Pediatrics 2010 Aug. 30 [doi:10.1542/peds.2009–3101]).
"First, many parents, coaches, teachers, and other adults feel that because these athletes are so young, they could not possibly get seriously hurt. As we have seen time and time again, this is, of course, not the case," said Dr. Bakhos, who is currently an emergency physician at the Jersey Shore University Medical Center in Neptune, N.J.
In addition, more data have surfaced about cognitive deficits in older children after concussion, she said, "which leads to conjecture that younger children would suffer the same — if not more — deficits long term."
However, the link between sports-related concussion and cognitive deficits needs further study, she commented.
The American Academy of Pediatrics has just released a new clinical report, "Sport-Related Concussion in Children and Adolescents" to aid in this effort (Pediatrics 2010 Aug. 30 [doi:10.1542/peds. 2010–2005]).
To get a better picture of the scope of sports-related concussion in young athletes, Dr. Bakhos and her colleagues reviewed data from the NEISS (National Electronic Injury Surveillance System) from 1997 through 2007, and from the NEISS-AIP (All-Injury Program) from 2001 through 2005.
The NEISS system allows researchers to investigate injury- and product-related ED visits.
In 2001–2005, approximately half of all ED visits for concussion across older and younger age groups were related to sports, including 58% of visits in children aged 8–13 years and 46% of visits in those aged 14–19 years.
Put another way, approximately 4 in 1,000 children aged 8–13 years and 6 in 1,000 of those aged 14–19 years went to the ED for a sports-related concussion.
During the 10-year period of 1997–2007, ED visits for the most popular organized team sports (football, ice hockey, soccer, basketball, and baseball) doubled in 8- to 13-year-olds and increased by more than 200% in 14- to 19-year-olds.
"The take-home message for pediatricians is, take concussion seriously even in the very young athlete," said Dr. Bakhos. "Children with concussion should be followed just as closely as a child with a sprained ankle or a broken bone. Return-to-play guidelines should be followed closely and stressed to parents."
"We as pediatricians should also stress to parents the importance of concussion prevention in sport as well, mostly [by] the use of helmets at all times," she emphasized.
The study was limited by the exclusion of sports-related concussions that were treated in non-ED settings, and by the underreporting of sports-related concussions by young athletes, their parents, and their coaches, the researchers noted.
But the rise in sports-related concussions in younger and older children suggests the need for more research and guidance in preventing and treating these injuries, they added.
To help clinicians manage sports-related concussions in young athletes, the AAP published a new clinical report that "outlines the current state of knowledge on pediatric and adolescent sport-related concussions," wrote lead authors Dr. Mark E. Halstead and Dr. Kevin D. Walter, on behalf of the AAP's Council on Sports Medicine and Fitness. It includes the SCAT 2 (Sport Concussion Assessment Tool 2), a standardized method of evaluating concussion in athletes aged 10 and older.
The report outlines recommendations regarding sports-related concussion, including the following:
▸ Stay off the field. Even if symptoms subside, young athletes should never return to play on the same day they have a concussion. Younger athletes need more recovery time and a more conservative approach than do college or professional athletes.
▸ See a doctor. Any children or adolescents who suffer concussions during sports should be medically cleared by a physician before they return to activity.
▸ Rest mind and body. All young athletes should refrain from physical activity until they are asymptomatic at rest and when active. Rest includes mental as well as physical rest.
Some evidence suggests that cognitive exertion — including doing homework, watching TV, and playing video games — can exacerbate symptoms post concussion.
In the last few years, several states have passed laws requiring educational materials about sports-related concussion for school-aged athletes, coaches, and parents. The state laws were a consideration, but the AAP began working on the report before the first law was passed, said Dr. Halstead, director of the sports concussion program in the department of orthopedics at Washington University in St. Louis.
"We felt there was a need to address specifically the [pediatric] athlete and address all the recent research that has been published on this topic," he said in an interview.
"The recommendations presented aren't significantly different from other recent documents published, but these were primarily published in sports medicine journals, which many pediatricians do not review.
"We wanted to bring these recommendations to the forefront to the pediatric community, and expand upon the details provided in previous documents published.
"We have highlighted some of the new research on neuroimaging, balance assessments, long-term complications, education, and neuropsychological testing," Dr. Halstead said.
Dr. Walter added, "I think it is also important to recognize that because we have learned more about concussion diagnosis, treatment, and complications, the treatment that coaches and parents received when they had a concussion themselves at a young age is likely different than today."
Many parents and coaches don't think concussion is a big deal because they had one when they were younger and they "toughed it out" and "are fine now," said Dr. Walter, program director of pediatric and adolescent sports medicine at Children's Hospital of Wisconsin in Milwaukee.
The authors acknowledged the lack of published baseline neuropsychological data on children younger than 12 years, and noted that assessment by a neuropsychologist might be helpful for children who have had more than one concussion, or whose postconcussive symptoms persist for several months.
Approximately 40% of emergency department visits for sports-related concussions in young athletes occurred in children aged 8–13 years, based on data from concussion-related ED visits in the United States between 2001 and 2005.
There are two main concerns about sports-related concussion in younger children, compared with college athletes and adults, lead author Dr. Lisa L. Bakhos said in an interview. Dr. Bakhos conducted the study while she was a teaching fellow at Brown University in Providence, R.I. (Pediatrics 2010 Aug. 30 [doi:10.1542/peds.2009–3101]).
"First, many parents, coaches, teachers, and other adults feel that because these athletes are so young, they could not possibly get seriously hurt. As we have seen time and time again, this is, of course, not the case," said Dr. Bakhos, who is currently an emergency physician at the Jersey Shore University Medical Center in Neptune, N.J.
In addition, more data have surfaced about cognitive deficits in older children after concussion, she said, "which leads to conjecture that younger children would suffer the same — if not more — deficits long term."
However, the link between sports-related concussion and cognitive deficits needs further study, she commented.
The American Academy of Pediatrics has just released a new clinical report, "Sport-Related Concussion in Children and Adolescents" to aid in this effort (Pediatrics 2010 Aug. 30 [doi:10.1542/peds. 2010–2005]).
To get a better picture of the scope of sports-related concussion in young athletes, Dr. Bakhos and her colleagues reviewed data from the NEISS (National Electronic Injury Surveillance System) from 1997 through 2007, and from the NEISS-AIP (All-Injury Program) from 2001 through 2005.
The NEISS system allows researchers to investigate injury- and product-related ED visits.
In 2001–2005, approximately half of all ED visits for concussion across older and younger age groups were related to sports, including 58% of visits in children aged 8–13 years and 46% of visits in those aged 14–19 years.
Put another way, approximately 4 in 1,000 children aged 8–13 years and 6 in 1,000 of those aged 14–19 years went to the ED for a sports-related concussion.
During the 10-year period of 1997–2007, ED visits for the most popular organized team sports (football, ice hockey, soccer, basketball, and baseball) doubled in 8- to 13-year-olds and increased by more than 200% in 14- to 19-year-olds.
"The take-home message for pediatricians is, take concussion seriously even in the very young athlete," said Dr. Bakhos. "Children with concussion should be followed just as closely as a child with a sprained ankle or a broken bone. Return-to-play guidelines should be followed closely and stressed to parents."
"We as pediatricians should also stress to parents the importance of concussion prevention in sport as well, mostly [by] the use of helmets at all times," she emphasized.
The study was limited by the exclusion of sports-related concussions that were treated in non-ED settings, and by the underreporting of sports-related concussions by young athletes, their parents, and their coaches, the researchers noted.
But the rise in sports-related concussions in younger and older children suggests the need for more research and guidance in preventing and treating these injuries, they added.
To help clinicians manage sports-related concussions in young athletes, the AAP published a new clinical report that "outlines the current state of knowledge on pediatric and adolescent sport-related concussions," wrote lead authors Dr. Mark E. Halstead and Dr. Kevin D. Walter, on behalf of the AAP's Council on Sports Medicine and Fitness. It includes the SCAT 2 (Sport Concussion Assessment Tool 2), a standardized method of evaluating concussion in athletes aged 10 and older.
The report outlines recommendations regarding sports-related concussion, including the following:
▸ Stay off the field. Even if symptoms subside, young athletes should never return to play on the same day they have a concussion. Younger athletes need more recovery time and a more conservative approach than do college or professional athletes.
▸ See a doctor. Any children or adolescents who suffer concussions during sports should be medically cleared by a physician before they return to activity.
▸ Rest mind and body. All young athletes should refrain from physical activity until they are asymptomatic at rest and when active. Rest includes mental as well as physical rest.
Some evidence suggests that cognitive exertion — including doing homework, watching TV, and playing video games — can exacerbate symptoms post concussion.
In the last few years, several states have passed laws requiring educational materials about sports-related concussion for school-aged athletes, coaches, and parents. The state laws were a consideration, but the AAP began working on the report before the first law was passed, said Dr. Halstead, director of the sports concussion program in the department of orthopedics at Washington University in St. Louis.
"We felt there was a need to address specifically the [pediatric] athlete and address all the recent research that has been published on this topic," he said in an interview.
"The recommendations presented aren't significantly different from other recent documents published, but these were primarily published in sports medicine journals, which many pediatricians do not review.
"We wanted to bring these recommendations to the forefront to the pediatric community, and expand upon the details provided in previous documents published.
"We have highlighted some of the new research on neuroimaging, balance assessments, long-term complications, education, and neuropsychological testing," Dr. Halstead said.
Dr. Walter added, "I think it is also important to recognize that because we have learned more about concussion diagnosis, treatment, and complications, the treatment that coaches and parents received when they had a concussion themselves at a young age is likely different than today."
Many parents and coaches don't think concussion is a big deal because they had one when they were younger and they "toughed it out" and "are fine now," said Dr. Walter, program director of pediatric and adolescent sports medicine at Children's Hospital of Wisconsin in Milwaukee.
The authors acknowledged the lack of published baseline neuropsychological data on children younger than 12 years, and noted that assessment by a neuropsychologist might be helpful for children who have had more than one concussion, or whose postconcussive symptoms persist for several months.
Aggressive Negotiations
Hospitalists are in position to take a leading role in the prevention of catheter-related bloodstream infections (CRBSIs), according to a spokeswoman for the Association for Professionals in Infection Control and Epidemiology (APIC).
The APIC perspective is timely, as the group released a survey this summer that found hospitals continue to struggle with preventable hospital-associated infections (HAIs). Half the survey respondents said their institutions struggle with CRBSIs and blame lack of time, resources, and a lack of administrative initiative as “hindering their ability to combat these infections more aggressively.”
The push also comes as a new Centers for Medicare and Medicaid Services rule means that, beginning next year, central-line-associated bloodstream infections (CLABSIs) will be reported and posted on a CDC website. The public disclosure of such preventable infections should motivate physicians to more aggressively address the problem, according to the APIC.
“Hospitalists need to be the champions,” says Sharon Jacobs, RN, MS, CIC, manager of infection prevention and control at St. Clair Hospital in Pittsburgh.
To that end, Jacobs offers some tips on how hospitalists and others can help stem the tide of the estimated 80,000 patients a year who develop CRBSIs. They include:
- Hand hygiene: The use of gloves during procedures does not mean physicians should forgo washing their hands.
- Large drapes: Most vendors now include drapes in their line kits, but for those that might not, consider using the largest drape available to cover and protect as much of the patient as possible. Consider creating a cart to store all applicable equipment.
- Focus on care continuum: Insertion of a line is the first step. Make sure the line is properly maintained as long as it remains in the patient. Consider replacing lines hastily inserted in the ED or other departments. Remove all lines as quickly as clinically efficient.
Another key, Jacobs says, is to create a collaborative environment where hospitalists, intensivists, nurses, and others will feel encouraged to point out improvements instead of feeling chastised for pointing out potential errors.
“The mindset is changing,” she adds. “It doesn’t take any longer to follow these procedures than it does to put a line in without them.”
Hospitalists are in position to take a leading role in the prevention of catheter-related bloodstream infections (CRBSIs), according to a spokeswoman for the Association for Professionals in Infection Control and Epidemiology (APIC).
The APIC perspective is timely, as the group released a survey this summer that found hospitals continue to struggle with preventable hospital-associated infections (HAIs). Half the survey respondents said their institutions struggle with CRBSIs and blame lack of time, resources, and a lack of administrative initiative as “hindering their ability to combat these infections more aggressively.”
The push also comes as a new Centers for Medicare and Medicaid Services rule means that, beginning next year, central-line-associated bloodstream infections (CLABSIs) will be reported and posted on a CDC website. The public disclosure of such preventable infections should motivate physicians to more aggressively address the problem, according to the APIC.
“Hospitalists need to be the champions,” says Sharon Jacobs, RN, MS, CIC, manager of infection prevention and control at St. Clair Hospital in Pittsburgh.
To that end, Jacobs offers some tips on how hospitalists and others can help stem the tide of the estimated 80,000 patients a year who develop CRBSIs. They include:
- Hand hygiene: The use of gloves during procedures does not mean physicians should forgo washing their hands.
- Large drapes: Most vendors now include drapes in their line kits, but for those that might not, consider using the largest drape available to cover and protect as much of the patient as possible. Consider creating a cart to store all applicable equipment.
- Focus on care continuum: Insertion of a line is the first step. Make sure the line is properly maintained as long as it remains in the patient. Consider replacing lines hastily inserted in the ED or other departments. Remove all lines as quickly as clinically efficient.
Another key, Jacobs says, is to create a collaborative environment where hospitalists, intensivists, nurses, and others will feel encouraged to point out improvements instead of feeling chastised for pointing out potential errors.
“The mindset is changing,” she adds. “It doesn’t take any longer to follow these procedures than it does to put a line in without them.”
Hospitalists are in position to take a leading role in the prevention of catheter-related bloodstream infections (CRBSIs), according to a spokeswoman for the Association for Professionals in Infection Control and Epidemiology (APIC).
The APIC perspective is timely, as the group released a survey this summer that found hospitals continue to struggle with preventable hospital-associated infections (HAIs). Half the survey respondents said their institutions struggle with CRBSIs and blame lack of time, resources, and a lack of administrative initiative as “hindering their ability to combat these infections more aggressively.”
The push also comes as a new Centers for Medicare and Medicaid Services rule means that, beginning next year, central-line-associated bloodstream infections (CLABSIs) will be reported and posted on a CDC website. The public disclosure of such preventable infections should motivate physicians to more aggressively address the problem, according to the APIC.
“Hospitalists need to be the champions,” says Sharon Jacobs, RN, MS, CIC, manager of infection prevention and control at St. Clair Hospital in Pittsburgh.
To that end, Jacobs offers some tips on how hospitalists and others can help stem the tide of the estimated 80,000 patients a year who develop CRBSIs. They include:
- Hand hygiene: The use of gloves during procedures does not mean physicians should forgo washing their hands.
- Large drapes: Most vendors now include drapes in their line kits, but for those that might not, consider using the largest drape available to cover and protect as much of the patient as possible. Consider creating a cart to store all applicable equipment.
- Focus on care continuum: Insertion of a line is the first step. Make sure the line is properly maintained as long as it remains in the patient. Consider replacing lines hastily inserted in the ED or other departments. Remove all lines as quickly as clinically efficient.
Another key, Jacobs says, is to create a collaborative environment where hospitalists, intensivists, nurses, and others will feel encouraged to point out improvements instead of feeling chastised for pointing out potential errors.
“The mindset is changing,” she adds. “It doesn’t take any longer to follow these procedures than it does to put a line in without them.”
In the Literature: Research You Need to Know
Clinical question: Is there a clinical benefit to continuing dual antiplatelet therapy for more than 12 months after drug-eluting stent placement?
Background: Current guidelines recommend dual antiplatelet therapy for at least 12 months after the placement of a drug-eluting stent. However, no randomized trials have addressed the effects of dual therapy beyond 12 months.
Study design: Randomized, open-label trial.
Setting: Twenty-two cardiac centers in South Korea.
Synopsis: Investigators looked at 2,701 patients, all of whom had undergone drug-eluting stent placement followed by dual therapy with aspirin and clopidogrel for at least 12 months with no major cardiac, cerebrovascular, or bleeding events during that time. Patients were randomized to continue aspirin plus clopidogrel or aspirin alone. The median therapy was 19 months.
Dual therapy led to no significant difference in the primary outcome of myocardial infarction or death from cardiac causes compared with aspirin alone (1.8% vs. 1.2%), nor in any secondary outcomes.
This study has several limitations, including an open-label design. An unexpectedly low event rate dilutes the power of the study to detect clinically important treatment effects.
Bottom line: This study showed no benefit to continuing clopidogrel for more than 12 months after drug-eluting stent placement in addition to aspirin; however, it was significantly underpowered.
Citation: Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362(15):1374-1382.
Reviewed for TH eWire by Robert Chang, MD, Anita Hart, MD, Hae-won Kim, MD, Robert Paretti, MD, Helena Pasieka, MD, and Matt Smitherman, MD, University of Michigan, Ann Arbor.
For more physician reviews of HM-related research, visit our website.
Clinical question: Is there a clinical benefit to continuing dual antiplatelet therapy for more than 12 months after drug-eluting stent placement?
Background: Current guidelines recommend dual antiplatelet therapy for at least 12 months after the placement of a drug-eluting stent. However, no randomized trials have addressed the effects of dual therapy beyond 12 months.
Study design: Randomized, open-label trial.
Setting: Twenty-two cardiac centers in South Korea.
Synopsis: Investigators looked at 2,701 patients, all of whom had undergone drug-eluting stent placement followed by dual therapy with aspirin and clopidogrel for at least 12 months with no major cardiac, cerebrovascular, or bleeding events during that time. Patients were randomized to continue aspirin plus clopidogrel or aspirin alone. The median therapy was 19 months.
Dual therapy led to no significant difference in the primary outcome of myocardial infarction or death from cardiac causes compared with aspirin alone (1.8% vs. 1.2%), nor in any secondary outcomes.
This study has several limitations, including an open-label design. An unexpectedly low event rate dilutes the power of the study to detect clinically important treatment effects.
Bottom line: This study showed no benefit to continuing clopidogrel for more than 12 months after drug-eluting stent placement in addition to aspirin; however, it was significantly underpowered.
Citation: Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362(15):1374-1382.
Reviewed for TH eWire by Robert Chang, MD, Anita Hart, MD, Hae-won Kim, MD, Robert Paretti, MD, Helena Pasieka, MD, and Matt Smitherman, MD, University of Michigan, Ann Arbor.
For more physician reviews of HM-related research, visit our website.
Clinical question: Is there a clinical benefit to continuing dual antiplatelet therapy for more than 12 months after drug-eluting stent placement?
Background: Current guidelines recommend dual antiplatelet therapy for at least 12 months after the placement of a drug-eluting stent. However, no randomized trials have addressed the effects of dual therapy beyond 12 months.
Study design: Randomized, open-label trial.
Setting: Twenty-two cardiac centers in South Korea.
Synopsis: Investigators looked at 2,701 patients, all of whom had undergone drug-eluting stent placement followed by dual therapy with aspirin and clopidogrel for at least 12 months with no major cardiac, cerebrovascular, or bleeding events during that time. Patients were randomized to continue aspirin plus clopidogrel or aspirin alone. The median therapy was 19 months.
Dual therapy led to no significant difference in the primary outcome of myocardial infarction or death from cardiac causes compared with aspirin alone (1.8% vs. 1.2%), nor in any secondary outcomes.
This study has several limitations, including an open-label design. An unexpectedly low event rate dilutes the power of the study to detect clinically important treatment effects.
Bottom line: This study showed no benefit to continuing clopidogrel for more than 12 months after drug-eluting stent placement in addition to aspirin; however, it was significantly underpowered.
Citation: Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362(15):1374-1382.
Reviewed for TH eWire by Robert Chang, MD, Anita Hart, MD, Hae-won Kim, MD, Robert Paretti, MD, Helena Pasieka, MD, and Matt Smitherman, MD, University of Michigan, Ann Arbor.
For more physician reviews of HM-related research, visit our website.
Short Title/Panesar
After another round of epinephrine, I started chest compressionsagain. Warm sweat seeped down my neck and back. In just 10 minutes, the 24‐bed pediatric intensive care unit (PICU) had become much smaller and more confined. Everyone funneled into this one room, this one bed. Beyond it was a blur of color and sound. I forced my strength onto Ricky, my 17‐year‐old patient with muscular dystrophy and end‐stage heart failure. He was admitted 1 week ago with respiratory distress, and he had only gotten worse since then.
Ricky's body, now cool and pale, was a blob of relaxed skin and loose bone beneath me. I straightened my arms, jutted the heel of my left hand over my right and pushed onto his chest. I pushed hard and fast, like I was taught; a dumb robotic motion again and again, trying to keep good position and form. I stared up at the monitor between every few compressions, looking at all the waveforms, anticipating, as if something on the screen was going to pop up suddenly and say EVERYTHING IS GOING TO BE OKAY. But it didn't.
I glanced beyond the bedside. There was a flurry of people bumping each other, asking for things, telling things, giving and receiving things. All of them were moving, but really had no place to go. This was the place. And in the far corner of the room, stood Mom and Dad.
In between the sink and recliner chair filled with clothes and books, they were the only people that stood still. Swollen feet planted in white socks. Their shoulders sagged.
I love you, I love you, Ricky. No, no, no. Mom and Dad kept on saying between sobs. I wondered if he could hear them. I wished he could hear them. Then I wished they would stop saying anything at all. I felt a rib give under my hands.
From the corner of my left eye, I saw Dad holding Mom upright by the wall. She wore red‐rimmed eyes and wet cheeks and took puttering breaths. Dad squeezed tissues in his hand, then into his eyes and nose and then back into his fist. They kept saying the words over and over, like they knew no other. I pushed harder and faster, but he didn't turn any pinker. Damn it. Damn it. There was plenty of noise, but above it all, I heard their voices. When I was told to hold compressions to check for a pulse, I stood still with my hands at my sides. I felt unnecessary. My precious little contribution to the commotion interrupted.
We all looked up at the monitor.
Stop, please stop. That's it, dad said, somewhere in the infinite pause. Mom still mumbled no over and over again. I turned to her and listened. I watched her pursed mouth and I imagined what it was Dad felt as he held her. Her body shook a Morse code into him, telling him it was time to give up. That it was over. That she couldn't take watching me pound away on her son's chest anymore. That it was okay to let Ricky rest. All the words that she couldn't find, or have the coordination to say, Dad translated for her.
He held her with a desperate grip, for a few moments longer. Maybe, the harder he squeezed, the more life he could push out of her, out of himself, and that effervescent pulse would find its way to their son's heart. But maybe all he could sense was her quiet internal whisper. And they told us again, as I remembered, just like all the soft conversations we had before in the back of the room, while Ricky slept, sedated on narcotics.
I put my fingers over his radial artery and closed my eyes.
Don't let Ricky feel pain, she said. It was the day we intubated him, only hours after he had been admitted.
Do what you can do, just don't Dad trailed off. He stared up at the ceiling and sighed. We listened to the gasp and hiss of the ventilator for a few more moments in silence.
We can try what you say, but no pain. We should know when to quitfor Ricky. Okay? Mom said, waiting for the tears as her nose moistened. She stared up at me.
Okay, I said. I nodded and stared back.
Dad squeezed her arm again, wrapped his around hers and massaged her. She had started to shake.
No pulse. I opened my eyes.
I stared at Ricky's face. His eyelids were half open, his lips were blue. No change on the monitor. I motioned to start compressions again.
Okay, Dad said.
Okay, stop! He's had enough. His dry lips and wet face moved and voiced the end. The room froze.
My muscles relaxed and I splayed my fingers wide, my way of showing that I was letting up. I watched myself lean back, unbelieving, and looked at the screen again. The rhythm drifted from pulseless electrical activity to asystole.
Mom and Dad simultaneously shut their eyes as if they saw something that we didn't and couldn't bear to see anymore. They opened them, looked at Ricky and slowly, as everyone stared, moved to the foot of the bed and began to rub his bare feet.
In subtle efficiency, the room was transformed. We turned off the monitors, we pushed out the carts and equipment, we picked up after ourselves, we dimmed the lights, we pulled the curtains and we left Mom and Dad with their son. Slow, deliberate whispers and motion now, it sounded empty without the background of rhythmic mechanical sounds. No dings, bleeps or rings; no pistons, suctions or pumps; only the occasional sound of tissue being ripped out of a box.
We sat with the family an hour later, in the conference room, to sign papers, for autopsy, for death certificates, for funeral preparations. Mom and Dad didn't know how to answer, and they drifted together to consider how they wanted Ricky's body cared for. Burial? Cremation?
I don't know, Mom said as she tried to fold the shredded tissues in her hand. He had a spinal rod placed his back years ago for his scoliosis. A titanium rod in his spine. Would that even burn? she asked.
We stared at each other, confused and unknowing.
We never thought of that, she said. She began to think out loud. If he were cremated, I don't think I'd want his spinal rod as a reminder. She cocked her head back. What a weird keepsake! She laughed, Where would I keep it? Above the fireplace? How odd. She rolled her eyes, and started to cry again.
After another round of epinephrine, I started chest compressionsagain. Warm sweat seeped down my neck and back. In just 10 minutes, the 24‐bed pediatric intensive care unit (PICU) had become much smaller and more confined. Everyone funneled into this one room, this one bed. Beyond it was a blur of color and sound. I forced my strength onto Ricky, my 17‐year‐old patient with muscular dystrophy and end‐stage heart failure. He was admitted 1 week ago with respiratory distress, and he had only gotten worse since then.
Ricky's body, now cool and pale, was a blob of relaxed skin and loose bone beneath me. I straightened my arms, jutted the heel of my left hand over my right and pushed onto his chest. I pushed hard and fast, like I was taught; a dumb robotic motion again and again, trying to keep good position and form. I stared up at the monitor between every few compressions, looking at all the waveforms, anticipating, as if something on the screen was going to pop up suddenly and say EVERYTHING IS GOING TO BE OKAY. But it didn't.
I glanced beyond the bedside. There was a flurry of people bumping each other, asking for things, telling things, giving and receiving things. All of them were moving, but really had no place to go. This was the place. And in the far corner of the room, stood Mom and Dad.
In between the sink and recliner chair filled with clothes and books, they were the only people that stood still. Swollen feet planted in white socks. Their shoulders sagged.
I love you, I love you, Ricky. No, no, no. Mom and Dad kept on saying between sobs. I wondered if he could hear them. I wished he could hear them. Then I wished they would stop saying anything at all. I felt a rib give under my hands.
From the corner of my left eye, I saw Dad holding Mom upright by the wall. She wore red‐rimmed eyes and wet cheeks and took puttering breaths. Dad squeezed tissues in his hand, then into his eyes and nose and then back into his fist. They kept saying the words over and over, like they knew no other. I pushed harder and faster, but he didn't turn any pinker. Damn it. Damn it. There was plenty of noise, but above it all, I heard their voices. When I was told to hold compressions to check for a pulse, I stood still with my hands at my sides. I felt unnecessary. My precious little contribution to the commotion interrupted.
We all looked up at the monitor.
Stop, please stop. That's it, dad said, somewhere in the infinite pause. Mom still mumbled no over and over again. I turned to her and listened. I watched her pursed mouth and I imagined what it was Dad felt as he held her. Her body shook a Morse code into him, telling him it was time to give up. That it was over. That she couldn't take watching me pound away on her son's chest anymore. That it was okay to let Ricky rest. All the words that she couldn't find, or have the coordination to say, Dad translated for her.
He held her with a desperate grip, for a few moments longer. Maybe, the harder he squeezed, the more life he could push out of her, out of himself, and that effervescent pulse would find its way to their son's heart. But maybe all he could sense was her quiet internal whisper. And they told us again, as I remembered, just like all the soft conversations we had before in the back of the room, while Ricky slept, sedated on narcotics.
I put my fingers over his radial artery and closed my eyes.
Don't let Ricky feel pain, she said. It was the day we intubated him, only hours after he had been admitted.
Do what you can do, just don't Dad trailed off. He stared up at the ceiling and sighed. We listened to the gasp and hiss of the ventilator for a few more moments in silence.
We can try what you say, but no pain. We should know when to quitfor Ricky. Okay? Mom said, waiting for the tears as her nose moistened. She stared up at me.
Okay, I said. I nodded and stared back.
Dad squeezed her arm again, wrapped his around hers and massaged her. She had started to shake.
No pulse. I opened my eyes.
I stared at Ricky's face. His eyelids were half open, his lips were blue. No change on the monitor. I motioned to start compressions again.
Okay, Dad said.
Okay, stop! He's had enough. His dry lips and wet face moved and voiced the end. The room froze.
My muscles relaxed and I splayed my fingers wide, my way of showing that I was letting up. I watched myself lean back, unbelieving, and looked at the screen again. The rhythm drifted from pulseless electrical activity to asystole.
Mom and Dad simultaneously shut their eyes as if they saw something that we didn't and couldn't bear to see anymore. They opened them, looked at Ricky and slowly, as everyone stared, moved to the foot of the bed and began to rub his bare feet.
In subtle efficiency, the room was transformed. We turned off the monitors, we pushed out the carts and equipment, we picked up after ourselves, we dimmed the lights, we pulled the curtains and we left Mom and Dad with their son. Slow, deliberate whispers and motion now, it sounded empty without the background of rhythmic mechanical sounds. No dings, bleeps or rings; no pistons, suctions or pumps; only the occasional sound of tissue being ripped out of a box.
We sat with the family an hour later, in the conference room, to sign papers, for autopsy, for death certificates, for funeral preparations. Mom and Dad didn't know how to answer, and they drifted together to consider how they wanted Ricky's body cared for. Burial? Cremation?
I don't know, Mom said as she tried to fold the shredded tissues in her hand. He had a spinal rod placed his back years ago for his scoliosis. A titanium rod in his spine. Would that even burn? she asked.
We stared at each other, confused and unknowing.
We never thought of that, she said. She began to think out loud. If he were cremated, I don't think I'd want his spinal rod as a reminder. She cocked her head back. What a weird keepsake! She laughed, Where would I keep it? Above the fireplace? How odd. She rolled her eyes, and started to cry again.
After another round of epinephrine, I started chest compressionsagain. Warm sweat seeped down my neck and back. In just 10 minutes, the 24‐bed pediatric intensive care unit (PICU) had become much smaller and more confined. Everyone funneled into this one room, this one bed. Beyond it was a blur of color and sound. I forced my strength onto Ricky, my 17‐year‐old patient with muscular dystrophy and end‐stage heart failure. He was admitted 1 week ago with respiratory distress, and he had only gotten worse since then.
Ricky's body, now cool and pale, was a blob of relaxed skin and loose bone beneath me. I straightened my arms, jutted the heel of my left hand over my right and pushed onto his chest. I pushed hard and fast, like I was taught; a dumb robotic motion again and again, trying to keep good position and form. I stared up at the monitor between every few compressions, looking at all the waveforms, anticipating, as if something on the screen was going to pop up suddenly and say EVERYTHING IS GOING TO BE OKAY. But it didn't.
I glanced beyond the bedside. There was a flurry of people bumping each other, asking for things, telling things, giving and receiving things. All of them were moving, but really had no place to go. This was the place. And in the far corner of the room, stood Mom and Dad.
In between the sink and recliner chair filled with clothes and books, they were the only people that stood still. Swollen feet planted in white socks. Their shoulders sagged.
I love you, I love you, Ricky. No, no, no. Mom and Dad kept on saying between sobs. I wondered if he could hear them. I wished he could hear them. Then I wished they would stop saying anything at all. I felt a rib give under my hands.
From the corner of my left eye, I saw Dad holding Mom upright by the wall. She wore red‐rimmed eyes and wet cheeks and took puttering breaths. Dad squeezed tissues in his hand, then into his eyes and nose and then back into his fist. They kept saying the words over and over, like they knew no other. I pushed harder and faster, but he didn't turn any pinker. Damn it. Damn it. There was plenty of noise, but above it all, I heard their voices. When I was told to hold compressions to check for a pulse, I stood still with my hands at my sides. I felt unnecessary. My precious little contribution to the commotion interrupted.
We all looked up at the monitor.
Stop, please stop. That's it, dad said, somewhere in the infinite pause. Mom still mumbled no over and over again. I turned to her and listened. I watched her pursed mouth and I imagined what it was Dad felt as he held her. Her body shook a Morse code into him, telling him it was time to give up. That it was over. That she couldn't take watching me pound away on her son's chest anymore. That it was okay to let Ricky rest. All the words that she couldn't find, or have the coordination to say, Dad translated for her.
He held her with a desperate grip, for a few moments longer. Maybe, the harder he squeezed, the more life he could push out of her, out of himself, and that effervescent pulse would find its way to their son's heart. But maybe all he could sense was her quiet internal whisper. And they told us again, as I remembered, just like all the soft conversations we had before in the back of the room, while Ricky slept, sedated on narcotics.
I put my fingers over his radial artery and closed my eyes.
Don't let Ricky feel pain, she said. It was the day we intubated him, only hours after he had been admitted.
Do what you can do, just don't Dad trailed off. He stared up at the ceiling and sighed. We listened to the gasp and hiss of the ventilator for a few more moments in silence.
We can try what you say, but no pain. We should know when to quitfor Ricky. Okay? Mom said, waiting for the tears as her nose moistened. She stared up at me.
Okay, I said. I nodded and stared back.
Dad squeezed her arm again, wrapped his around hers and massaged her. She had started to shake.
No pulse. I opened my eyes.
I stared at Ricky's face. His eyelids were half open, his lips were blue. No change on the monitor. I motioned to start compressions again.
Okay, Dad said.
Okay, stop! He's had enough. His dry lips and wet face moved and voiced the end. The room froze.
My muscles relaxed and I splayed my fingers wide, my way of showing that I was letting up. I watched myself lean back, unbelieving, and looked at the screen again. The rhythm drifted from pulseless electrical activity to asystole.
Mom and Dad simultaneously shut their eyes as if they saw something that we didn't and couldn't bear to see anymore. They opened them, looked at Ricky and slowly, as everyone stared, moved to the foot of the bed and began to rub his bare feet.
In subtle efficiency, the room was transformed. We turned off the monitors, we pushed out the carts and equipment, we picked up after ourselves, we dimmed the lights, we pulled the curtains and we left Mom and Dad with their son. Slow, deliberate whispers and motion now, it sounded empty without the background of rhythmic mechanical sounds. No dings, bleeps or rings; no pistons, suctions or pumps; only the occasional sound of tissue being ripped out of a box.
We sat with the family an hour later, in the conference room, to sign papers, for autopsy, for death certificates, for funeral preparations. Mom and Dad didn't know how to answer, and they drifted together to consider how they wanted Ricky's body cared for. Burial? Cremation?
I don't know, Mom said as she tried to fold the shredded tissues in her hand. He had a spinal rod placed his back years ago for his scoliosis. A titanium rod in his spine. Would that even burn? she asked.
We stared at each other, confused and unknowing.
We never thought of that, she said. She began to think out loud. If he were cremated, I don't think I'd want his spinal rod as a reminder. She cocked her head back. What a weird keepsake! She laughed, Where would I keep it? Above the fireplace? How odd. She rolled her eyes, and started to cry again.