User login
Race, Sex Affect Congenital Heart Surgery Outcomes
FT. LAUDERDALE, FLA. – Sex and race appear to play a role in outcomes following congenital heart surgery in children and adolescents, according to a new analysis of data from almost 21,000 patients.
Black patients had significantly greater rates of mortality and complications and a significantly longer length of postoperative stay than other races, while female patients had a significantly shorter length of stay than males, Dr. Daniel J. DiBardino reported at the annual meeting of the Society of Thoracic Surgeons.
"The analysis of demographic and clinical data from nearly 21,000 patients in the congenital heart surgery database revealed important associations between gender, race, and outcome," said Dr. DiBardino, who is a cardiac surgeon at the Blair E. Batson Children’s Hospital in Jackson, Miss.
Dr. DiBardino’s study was chosen as a 2011 Richard E. Clark Paper by the Society of Thoracic Surgeons.
The researchers used data from the Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD). Patients were included in the analysis if they were less than 18 years of age and had undergone cardiac surgery between 2007 and 2009.
Exclusion criteria included centers with more than 15% of data missing for key variables and centers with very small samples (less than five cases).
Data collection included demographics (age, sex, weight, and race) and preoperative data (noncardiac/genetic abnormalities and STS-defined risk factors). Race was classified as white, black, Hispanic, and other.
Operations were classified by STAT Mortality category, which is "a complexity stratification tool based on empiric data from 80,000 cases in STS and EACTS (European Association for Cardio-Thoracic Surgery) databases," said Dr. DiBardino.
The researchers looked at hospital mortality, postoperative length of stay, and complications. Multivariable analyses included dichotomous variables (mortality, complications) and a continuous variable (postoperative length of stay). Models were adjusted for age, weight, noncardiac/genetic abnormalities, any other STS preoperative risk factor, and STAT Mortality category.
In all, 20,399 patients were included from 49 centers. Of these, 54% were male. In terms of race, 55% were white, 17% were black, 16% were Hispanic, and 12% were other.
Based on unadjusted outcomes, there were no differences between the sexes for in-hospital mortality or complications. However, females had significantly shorter postoperative stays. In terms of race, white patients had significantly lower mortality, shorter length of stay, and fewer complications than any of the other racial groups.
In the adjusted multivariate analysis, there was no difference for mortality between the sexes. However, black patients had a significantly greater mortality risk with an odds ratio of 1.67.
Females did have a significantly shorter mean length of stay – 0.8 fewer days. In terms of race, black patients had a significantly longer mean length of stay by 2.4 hospital days, compared with white patients. Hispanic patients also had a significantly longer mean length of stay by almost 1 hospital day.
There was no difference between the sexes in terms of the occurrence of complications. In terms of race, "black patients experienced significantly more complications than other races with an odds ratio of 1.15," according to Dr. DiBardino.
The study is unique with the respect to the use of multivariable models. The researchers measured the association of sex and race with outcomes within each center and then combined the results, in order to mitigate the potential center effects.
"Our results cannot be explained by the possibility that patients of certain races might be disproportionately treated at centers with poorer outcomes in general."
The evaluation of complex relationships between clinical variables and socioeconomic and other factors affecting health care remains a significant challenge.
Since some pertinent socioeconomic data are not collected in the STS-CHSD, an analysis of a linked data set, which capitalizes on the strengths of both the CHSD and those of an administrative claims data set may be the next logical step, said Dr. DiBardino.
Dr. DiBardino and his coinvestigators reported that they have no relevant disclosures.
FT. LAUDERDALE, FLA. – Sex and race appear to play a role in outcomes following congenital heart surgery in children and adolescents, according to a new analysis of data from almost 21,000 patients.
Black patients had significantly greater rates of mortality and complications and a significantly longer length of postoperative stay than other races, while female patients had a significantly shorter length of stay than males, Dr. Daniel J. DiBardino reported at the annual meeting of the Society of Thoracic Surgeons.
"The analysis of demographic and clinical data from nearly 21,000 patients in the congenital heart surgery database revealed important associations between gender, race, and outcome," said Dr. DiBardino, who is a cardiac surgeon at the Blair E. Batson Children’s Hospital in Jackson, Miss.
Dr. DiBardino’s study was chosen as a 2011 Richard E. Clark Paper by the Society of Thoracic Surgeons.
The researchers used data from the Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD). Patients were included in the analysis if they were less than 18 years of age and had undergone cardiac surgery between 2007 and 2009.
Exclusion criteria included centers with more than 15% of data missing for key variables and centers with very small samples (less than five cases).
Data collection included demographics (age, sex, weight, and race) and preoperative data (noncardiac/genetic abnormalities and STS-defined risk factors). Race was classified as white, black, Hispanic, and other.
Operations were classified by STAT Mortality category, which is "a complexity stratification tool based on empiric data from 80,000 cases in STS and EACTS (European Association for Cardio-Thoracic Surgery) databases," said Dr. DiBardino.
The researchers looked at hospital mortality, postoperative length of stay, and complications. Multivariable analyses included dichotomous variables (mortality, complications) and a continuous variable (postoperative length of stay). Models were adjusted for age, weight, noncardiac/genetic abnormalities, any other STS preoperative risk factor, and STAT Mortality category.
In all, 20,399 patients were included from 49 centers. Of these, 54% were male. In terms of race, 55% were white, 17% were black, 16% were Hispanic, and 12% were other.
Based on unadjusted outcomes, there were no differences between the sexes for in-hospital mortality or complications. However, females had significantly shorter postoperative stays. In terms of race, white patients had significantly lower mortality, shorter length of stay, and fewer complications than any of the other racial groups.
In the adjusted multivariate analysis, there was no difference for mortality between the sexes. However, black patients had a significantly greater mortality risk with an odds ratio of 1.67.
Females did have a significantly shorter mean length of stay – 0.8 fewer days. In terms of race, black patients had a significantly longer mean length of stay by 2.4 hospital days, compared with white patients. Hispanic patients also had a significantly longer mean length of stay by almost 1 hospital day.
There was no difference between the sexes in terms of the occurrence of complications. In terms of race, "black patients experienced significantly more complications than other races with an odds ratio of 1.15," according to Dr. DiBardino.
The study is unique with the respect to the use of multivariable models. The researchers measured the association of sex and race with outcomes within each center and then combined the results, in order to mitigate the potential center effects.
"Our results cannot be explained by the possibility that patients of certain races might be disproportionately treated at centers with poorer outcomes in general."
The evaluation of complex relationships between clinical variables and socioeconomic and other factors affecting health care remains a significant challenge.
Since some pertinent socioeconomic data are not collected in the STS-CHSD, an analysis of a linked data set, which capitalizes on the strengths of both the CHSD and those of an administrative claims data set may be the next logical step, said Dr. DiBardino.
Dr. DiBardino and his coinvestigators reported that they have no relevant disclosures.
FT. LAUDERDALE, FLA. – Sex and race appear to play a role in outcomes following congenital heart surgery in children and adolescents, according to a new analysis of data from almost 21,000 patients.
Black patients had significantly greater rates of mortality and complications and a significantly longer length of postoperative stay than other races, while female patients had a significantly shorter length of stay than males, Dr. Daniel J. DiBardino reported at the annual meeting of the Society of Thoracic Surgeons.
"The analysis of demographic and clinical data from nearly 21,000 patients in the congenital heart surgery database revealed important associations between gender, race, and outcome," said Dr. DiBardino, who is a cardiac surgeon at the Blair E. Batson Children’s Hospital in Jackson, Miss.
Dr. DiBardino’s study was chosen as a 2011 Richard E. Clark Paper by the Society of Thoracic Surgeons.
The researchers used data from the Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD). Patients were included in the analysis if they were less than 18 years of age and had undergone cardiac surgery between 2007 and 2009.
Exclusion criteria included centers with more than 15% of data missing for key variables and centers with very small samples (less than five cases).
Data collection included demographics (age, sex, weight, and race) and preoperative data (noncardiac/genetic abnormalities and STS-defined risk factors). Race was classified as white, black, Hispanic, and other.
Operations were classified by STAT Mortality category, which is "a complexity stratification tool based on empiric data from 80,000 cases in STS and EACTS (European Association for Cardio-Thoracic Surgery) databases," said Dr. DiBardino.
The researchers looked at hospital mortality, postoperative length of stay, and complications. Multivariable analyses included dichotomous variables (mortality, complications) and a continuous variable (postoperative length of stay). Models were adjusted for age, weight, noncardiac/genetic abnormalities, any other STS preoperative risk factor, and STAT Mortality category.
In all, 20,399 patients were included from 49 centers. Of these, 54% were male. In terms of race, 55% were white, 17% were black, 16% were Hispanic, and 12% were other.
Based on unadjusted outcomes, there were no differences between the sexes for in-hospital mortality or complications. However, females had significantly shorter postoperative stays. In terms of race, white patients had significantly lower mortality, shorter length of stay, and fewer complications than any of the other racial groups.
In the adjusted multivariate analysis, there was no difference for mortality between the sexes. However, black patients had a significantly greater mortality risk with an odds ratio of 1.67.
Females did have a significantly shorter mean length of stay – 0.8 fewer days. In terms of race, black patients had a significantly longer mean length of stay by 2.4 hospital days, compared with white patients. Hispanic patients also had a significantly longer mean length of stay by almost 1 hospital day.
There was no difference between the sexes in terms of the occurrence of complications. In terms of race, "black patients experienced significantly more complications than other races with an odds ratio of 1.15," according to Dr. DiBardino.
The study is unique with the respect to the use of multivariable models. The researchers measured the association of sex and race with outcomes within each center and then combined the results, in order to mitigate the potential center effects.
"Our results cannot be explained by the possibility that patients of certain races might be disproportionately treated at centers with poorer outcomes in general."
The evaluation of complex relationships between clinical variables and socioeconomic and other factors affecting health care remains a significant challenge.
Since some pertinent socioeconomic data are not collected in the STS-CHSD, an analysis of a linked data set, which capitalizes on the strengths of both the CHSD and those of an administrative claims data set may be the next logical step, said Dr. DiBardino.
Dr. DiBardino and his coinvestigators reported that they have no relevant disclosures.
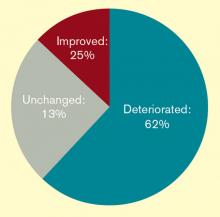
Major Finding: In adjusted multivariate analyses, black patients had a significantly greater mortality risk (67%), a significantly longer mean length of stay by 2.4 hospital days, and a significantly greater risk of complications (15%). Female patients had a significantly shorter mean length of stay – 0.8 fewer days.
Data Source: The retrospective review included 20,399 patients younger than 18 years from 49 centers, collected in the Society of Thoracic Surgeons Congenital Heart Surgery Database.
Disclosures: Dr. DiBardino and his coinvestigators reported that they have no relevant disclosures.
Surgical Coaching: A Timely Idea?
The role of a coach is to provide objective and constructive feedback on what he or she observes, helping the practitioner to recognize what is successful and what can be improved. Coaches do not judge or instruct; instead, they guide and facilitate. They act as collaborators and partners to assist in developing a better understanding of their own performance, and they help them to use their experience, knowledge, and abilities to provide the best care possible (Nursing Standard 2009;23:48-55). The focus should always be on the surgeon and not what the coach would do in a similar situation.
Coaching can be valuable for surgeons at all stages of their career (J. Am. Coll. Surg. 2012;214:115-24). It is easy to imagine the role of a coach in smoothing the increasingly jarring transition from training to independent practice. But experience in other areas suggests that established practitioners can also benefit.
As one develops expertise, actions become more automated and more experienced practitioners spend less time examining their approaches and actions (Fitts, P.M.; Posner, M.I.; Human Performance. Brooks/Cole Publishing Co.: Belmont, Calif., 1967; Work 2006;26:93-6). A coach can serve as a catalyst to jump-start introspection and further practice improvement.
The Importance of Adult Learning Theory
Until recently, medical education has not encompassed the proven principles by which adults learn. In 2007, Boonyasai and colleagues developed a list of adult learning principles based on major educational theories that could be applied in medicine (JAMA 2007;298:1023-37):
- Enabling adult learners to be active participants.
- Providing content relating to the learner’s current experiences.
- Assessing learners’ needs and tailoring teaching to their past experience.
- Allowing learners to identify and pursue their own learning goals.
- Allowing learners to practice their learning.
- Supporting learners during self-directed learning.
- Providing feedback to learners.
- Facilitating learner self-reflection.
- Role-modeling behaviors.
A coaching program would almost by definition include at least the first eight principles, so this list is likely to be an effective approach for improving performance.
What Makes a Good Coach?
The best athletic coaches were not always the standout athletes. They did, however, almost always participate in the sport they coach at a very high level. This is because the characteristics of a good athletic coach to do not necessarily parallel the characteristics of a good athlete, but an intimate knowledge of the skill set is critical.
Similarly, the most experienced and skilled surgeons will not necessarily make the best coaches, but a surgical coach by definition must be a surgeon. A surgical coach must develop an easy rapport and a trusting relationship with each surgeon. The coach must be empathetic and tactful, but also flexible – able to ask probing questions and make constructive comments (Consult. Psychol. J. Pract. Res.;2001;53:240-50). The best surgical coaches are likely to be experienced, thoughtful, inquisitive, nonjudgmental, and well respected by their colleagues.
The coach described by Atul Gawande in "Personal Best," his article on surgical coaching, embodied all of those qualities and excelled as a surgical coach (Gawande A. Personal Best. Top Athletes and Singers Have Coaches. Should You? New Yorker Oct. 3, 2011). When we questioned him about his deftness in this new role, he credited the light hand (socially) that he developed from years of intraoperative consults.
Coaches need time and flexibility in their schedule. For this reason, surgeons who are nearing retirement or who are newly retired may be good candidates to serve as coaches. Many of these surgeons are likely to have the experience and respect required for surgical coaching. The key is to ensure that they also have the flexibility, openness, and lack of judgment.
Another potential pool of coaches may be surgeons interested in a more flexible lifestyle for personal reasons, such as childrearing or caregiving for an ill or elderly family member. Surgical coaching can provide a way to remain engaged in surgery and continue to contribute to the field without the same demands as a busy surgical practice.
Some Basic Principles
Jim Knight has developed a paradigm that he terms "partnership learning" to coach teachers (Knight, J. Instructional Coaching: A Partnership Approach to Improving Instruction. Corwin Press: Thousand Oaks, Calif., 2007). He contrasts this with the "dominator approach" upon which most traditional professional development is based – for example, the situation in which a person gives a PowerPoint presentation to convey an "expert opinion" to a roomful of people. Sound familiar?
Instead, Mr. Knight advocates the use of core principles that will foster a partnership, the cornerstone of coaching (Knight, J. Partnership Learning. University of Kansas Center for Research on Learning: Lawrence, Kan., 2002). Here are some ways they could apply to surgical coaching:
- Equality – The opinions and approaches of the surgeon and the surgical coach are equally valuable.
- Choice – At a minimum, the surgeon should be allowed to choose the specific case and setting for each coaching session.
- Voice and dialogue – The surgeon should feel free to speak openly. Coaches should listen more than they talk. The coach should not control or dominate the interaction, but rather engage in a dialogue.
- Reflection – "Reflection on action" after an operation is likely to be more effective than "reflection in action" in the operating room so that the surgeon can concentrate fully on dissecting his or her own performance. In addition, coaching sessions can take place in a private, confidential setting away from patients and other providers. The use of video as a "thinking device" to prompt open dialogue holds significant promise.
- Praxis – Surgeons should be encouraged to explicitly think about how they will apply insights from the coaching session to their clinical practice.
Three other points deserve mention. Confidentiality and trust are critical, especially as surgeons acclimate to the idea of working with a coach. Additionally, the coaching style should be individualized and adapted to each surgeon throughout a coaching session. Such adaptability is an important characteristic of a successful coach. Finally, coaches should not have administrative oversight for the surgeon they are coaching. This is to ensure that the content of coaching sessions remain focused on performance improvement and not on performance evaluations or career development.
Will It Work?
There are very little empirical data on coaching in any discipline. What does exist tends to be exploratory and qualitative. However, Cornett and Knight describe several randomized trials, and a review in the field of education suggests that coaching will be successful (Cornett, J.; Knight, J. Research on Coaching:Approaches and Perspectives, 2009;192-216).
Researchers found that only 10% of teachers used a new skill in the classroom when they were provided with a verbal description. After modeling, practice, and feedback were added, the rate of adoption increased to 19%. It was only with the addition of peer coaching that an astounding 95% of teachers utilized the new skill. (Bush, R. N. Effective Staff Development in Making Our Schools More Effective: Proceedings of Three State Conferences. Far West Laboratories: San Francisco, 1984).
Other studies demonstrated that coaching increased skill transfer from 15% to 75%, compared with traditional approaches to professional development. Even more striking was the fact that these skills were still being used 6 months later. If we are even half as successful with coaching in surgery, results will be orders of magnitude better than any previous attempts at intraoperative performance improvement.
How Do We Move Forward?
The American College of Surgeons Division of Education – with its dedication to improving quality, safety, and education – is in a particularly strong position to develop surgical coaching and is exploring potential programs with us in Wisconsin and with others. Other surgical societies, including local and regional organizations, offer another opportunity to develop coaching programs. The state chapters of the American Academy of Pediatrics instituted a quality improvement initiative that included team coaching, and found several advantages to this approach over a national one (J. Contin. Educ. Health Profess. 2008;28:131-9).
Trust in, familiarity with, and participation in local/regional societies or state chapters is likely to increase acceptance and participation by practicing surgeons. The infrastructure of a regional society allows for participation across all practice settings – not just in large hospitals where a coach may be locally available – yet it is small enough to afford some level of familiarity, trust, and respect for the coach.
This type of cross-institutional collaboration may seem counterintuitive in light of the traditional competitive relationships of neighboring institutions; however, the success of programs such as the Surgical Care and Outcomes Assessment Program (SCOAP) in Washington State and the Michigan Surgical Collaboratives (MSQC and MSBC) suggests that as a discipline we are ready to work together to improve the quality and safety of surgical care.
Given the current paucity of data, we must continue to study any new programs or interventions, but surgical coaching seems like an idea whose time has come.
Acknowledgments
I would like to thank Atul Gawande, Yue-Yung Hu, Robert Osteen, and Michael Zinner for conversations and research that helped me formulate these ideas.☐
Dr. Greenberg is an associate professor of surgery, and director of the Wisconsin Surgical Outcomes Research Program at the University of Wisconsin, Madison.
The role of a coach is to provide objective and constructive feedback on what he or she observes, helping the practitioner to recognize what is successful and what can be improved. Coaches do not judge or instruct; instead, they guide and facilitate. They act as collaborators and partners to assist in developing a better understanding of their own performance, and they help them to use their experience, knowledge, and abilities to provide the best care possible (Nursing Standard 2009;23:48-55). The focus should always be on the surgeon and not what the coach would do in a similar situation.
Coaching can be valuable for surgeons at all stages of their career (J. Am. Coll. Surg. 2012;214:115-24). It is easy to imagine the role of a coach in smoothing the increasingly jarring transition from training to independent practice. But experience in other areas suggests that established practitioners can also benefit.
As one develops expertise, actions become more automated and more experienced practitioners spend less time examining their approaches and actions (Fitts, P.M.; Posner, M.I.; Human Performance. Brooks/Cole Publishing Co.: Belmont, Calif., 1967; Work 2006;26:93-6). A coach can serve as a catalyst to jump-start introspection and further practice improvement.
The Importance of Adult Learning Theory
Until recently, medical education has not encompassed the proven principles by which adults learn. In 2007, Boonyasai and colleagues developed a list of adult learning principles based on major educational theories that could be applied in medicine (JAMA 2007;298:1023-37):
- Enabling adult learners to be active participants.
- Providing content relating to the learner’s current experiences.
- Assessing learners’ needs and tailoring teaching to their past experience.
- Allowing learners to identify and pursue their own learning goals.
- Allowing learners to practice their learning.
- Supporting learners during self-directed learning.
- Providing feedback to learners.
- Facilitating learner self-reflection.
- Role-modeling behaviors.
A coaching program would almost by definition include at least the first eight principles, so this list is likely to be an effective approach for improving performance.
What Makes a Good Coach?
The best athletic coaches were not always the standout athletes. They did, however, almost always participate in the sport they coach at a very high level. This is because the characteristics of a good athletic coach to do not necessarily parallel the characteristics of a good athlete, but an intimate knowledge of the skill set is critical.
Similarly, the most experienced and skilled surgeons will not necessarily make the best coaches, but a surgical coach by definition must be a surgeon. A surgical coach must develop an easy rapport and a trusting relationship with each surgeon. The coach must be empathetic and tactful, but also flexible – able to ask probing questions and make constructive comments (Consult. Psychol. J. Pract. Res.;2001;53:240-50). The best surgical coaches are likely to be experienced, thoughtful, inquisitive, nonjudgmental, and well respected by their colleagues.
The coach described by Atul Gawande in "Personal Best," his article on surgical coaching, embodied all of those qualities and excelled as a surgical coach (Gawande A. Personal Best. Top Athletes and Singers Have Coaches. Should You? New Yorker Oct. 3, 2011). When we questioned him about his deftness in this new role, he credited the light hand (socially) that he developed from years of intraoperative consults.
Coaches need time and flexibility in their schedule. For this reason, surgeons who are nearing retirement or who are newly retired may be good candidates to serve as coaches. Many of these surgeons are likely to have the experience and respect required for surgical coaching. The key is to ensure that they also have the flexibility, openness, and lack of judgment.
Another potential pool of coaches may be surgeons interested in a more flexible lifestyle for personal reasons, such as childrearing or caregiving for an ill or elderly family member. Surgical coaching can provide a way to remain engaged in surgery and continue to contribute to the field without the same demands as a busy surgical practice.
Some Basic Principles
Jim Knight has developed a paradigm that he terms "partnership learning" to coach teachers (Knight, J. Instructional Coaching: A Partnership Approach to Improving Instruction. Corwin Press: Thousand Oaks, Calif., 2007). He contrasts this with the "dominator approach" upon which most traditional professional development is based – for example, the situation in which a person gives a PowerPoint presentation to convey an "expert opinion" to a roomful of people. Sound familiar?
Instead, Mr. Knight advocates the use of core principles that will foster a partnership, the cornerstone of coaching (Knight, J. Partnership Learning. University of Kansas Center for Research on Learning: Lawrence, Kan., 2002). Here are some ways they could apply to surgical coaching:
- Equality – The opinions and approaches of the surgeon and the surgical coach are equally valuable.
- Choice – At a minimum, the surgeon should be allowed to choose the specific case and setting for each coaching session.
- Voice and dialogue – The surgeon should feel free to speak openly. Coaches should listen more than they talk. The coach should not control or dominate the interaction, but rather engage in a dialogue.
- Reflection – "Reflection on action" after an operation is likely to be more effective than "reflection in action" in the operating room so that the surgeon can concentrate fully on dissecting his or her own performance. In addition, coaching sessions can take place in a private, confidential setting away from patients and other providers. The use of video as a "thinking device" to prompt open dialogue holds significant promise.
- Praxis – Surgeons should be encouraged to explicitly think about how they will apply insights from the coaching session to their clinical practice.
Three other points deserve mention. Confidentiality and trust are critical, especially as surgeons acclimate to the idea of working with a coach. Additionally, the coaching style should be individualized and adapted to each surgeon throughout a coaching session. Such adaptability is an important characteristic of a successful coach. Finally, coaches should not have administrative oversight for the surgeon they are coaching. This is to ensure that the content of coaching sessions remain focused on performance improvement and not on performance evaluations or career development.
Will It Work?
There are very little empirical data on coaching in any discipline. What does exist tends to be exploratory and qualitative. However, Cornett and Knight describe several randomized trials, and a review in the field of education suggests that coaching will be successful (Cornett, J.; Knight, J. Research on Coaching:Approaches and Perspectives, 2009;192-216).
Researchers found that only 10% of teachers used a new skill in the classroom when they were provided with a verbal description. After modeling, practice, and feedback were added, the rate of adoption increased to 19%. It was only with the addition of peer coaching that an astounding 95% of teachers utilized the new skill. (Bush, R. N. Effective Staff Development in Making Our Schools More Effective: Proceedings of Three State Conferences. Far West Laboratories: San Francisco, 1984).
Other studies demonstrated that coaching increased skill transfer from 15% to 75%, compared with traditional approaches to professional development. Even more striking was the fact that these skills were still being used 6 months later. If we are even half as successful with coaching in surgery, results will be orders of magnitude better than any previous attempts at intraoperative performance improvement.
How Do We Move Forward?
The American College of Surgeons Division of Education – with its dedication to improving quality, safety, and education – is in a particularly strong position to develop surgical coaching and is exploring potential programs with us in Wisconsin and with others. Other surgical societies, including local and regional organizations, offer another opportunity to develop coaching programs. The state chapters of the American Academy of Pediatrics instituted a quality improvement initiative that included team coaching, and found several advantages to this approach over a national one (J. Contin. Educ. Health Profess. 2008;28:131-9).
Trust in, familiarity with, and participation in local/regional societies or state chapters is likely to increase acceptance and participation by practicing surgeons. The infrastructure of a regional society allows for participation across all practice settings – not just in large hospitals where a coach may be locally available – yet it is small enough to afford some level of familiarity, trust, and respect for the coach.
This type of cross-institutional collaboration may seem counterintuitive in light of the traditional competitive relationships of neighboring institutions; however, the success of programs such as the Surgical Care and Outcomes Assessment Program (SCOAP) in Washington State and the Michigan Surgical Collaboratives (MSQC and MSBC) suggests that as a discipline we are ready to work together to improve the quality and safety of surgical care.
Given the current paucity of data, we must continue to study any new programs or interventions, but surgical coaching seems like an idea whose time has come.
Acknowledgments
I would like to thank Atul Gawande, Yue-Yung Hu, Robert Osteen, and Michael Zinner for conversations and research that helped me formulate these ideas.☐
Dr. Greenberg is an associate professor of surgery, and director of the Wisconsin Surgical Outcomes Research Program at the University of Wisconsin, Madison.
The role of a coach is to provide objective and constructive feedback on what he or she observes, helping the practitioner to recognize what is successful and what can be improved. Coaches do not judge or instruct; instead, they guide and facilitate. They act as collaborators and partners to assist in developing a better understanding of their own performance, and they help them to use their experience, knowledge, and abilities to provide the best care possible (Nursing Standard 2009;23:48-55). The focus should always be on the surgeon and not what the coach would do in a similar situation.
Coaching can be valuable for surgeons at all stages of their career (J. Am. Coll. Surg. 2012;214:115-24). It is easy to imagine the role of a coach in smoothing the increasingly jarring transition from training to independent practice. But experience in other areas suggests that established practitioners can also benefit.
As one develops expertise, actions become more automated and more experienced practitioners spend less time examining their approaches and actions (Fitts, P.M.; Posner, M.I.; Human Performance. Brooks/Cole Publishing Co.: Belmont, Calif., 1967; Work 2006;26:93-6). A coach can serve as a catalyst to jump-start introspection and further practice improvement.
The Importance of Adult Learning Theory
Until recently, medical education has not encompassed the proven principles by which adults learn. In 2007, Boonyasai and colleagues developed a list of adult learning principles based on major educational theories that could be applied in medicine (JAMA 2007;298:1023-37):
- Enabling adult learners to be active participants.
- Providing content relating to the learner’s current experiences.
- Assessing learners’ needs and tailoring teaching to their past experience.
- Allowing learners to identify and pursue their own learning goals.
- Allowing learners to practice their learning.
- Supporting learners during self-directed learning.
- Providing feedback to learners.
- Facilitating learner self-reflection.
- Role-modeling behaviors.
A coaching program would almost by definition include at least the first eight principles, so this list is likely to be an effective approach for improving performance.
What Makes a Good Coach?
The best athletic coaches were not always the standout athletes. They did, however, almost always participate in the sport they coach at a very high level. This is because the characteristics of a good athletic coach to do not necessarily parallel the characteristics of a good athlete, but an intimate knowledge of the skill set is critical.
Similarly, the most experienced and skilled surgeons will not necessarily make the best coaches, but a surgical coach by definition must be a surgeon. A surgical coach must develop an easy rapport and a trusting relationship with each surgeon. The coach must be empathetic and tactful, but also flexible – able to ask probing questions and make constructive comments (Consult. Psychol. J. Pract. Res.;2001;53:240-50). The best surgical coaches are likely to be experienced, thoughtful, inquisitive, nonjudgmental, and well respected by their colleagues.
The coach described by Atul Gawande in "Personal Best," his article on surgical coaching, embodied all of those qualities and excelled as a surgical coach (Gawande A. Personal Best. Top Athletes and Singers Have Coaches. Should You? New Yorker Oct. 3, 2011). When we questioned him about his deftness in this new role, he credited the light hand (socially) that he developed from years of intraoperative consults.
Coaches need time and flexibility in their schedule. For this reason, surgeons who are nearing retirement or who are newly retired may be good candidates to serve as coaches. Many of these surgeons are likely to have the experience and respect required for surgical coaching. The key is to ensure that they also have the flexibility, openness, and lack of judgment.
Another potential pool of coaches may be surgeons interested in a more flexible lifestyle for personal reasons, such as childrearing or caregiving for an ill or elderly family member. Surgical coaching can provide a way to remain engaged in surgery and continue to contribute to the field without the same demands as a busy surgical practice.
Some Basic Principles
Jim Knight has developed a paradigm that he terms "partnership learning" to coach teachers (Knight, J. Instructional Coaching: A Partnership Approach to Improving Instruction. Corwin Press: Thousand Oaks, Calif., 2007). He contrasts this with the "dominator approach" upon which most traditional professional development is based – for example, the situation in which a person gives a PowerPoint presentation to convey an "expert opinion" to a roomful of people. Sound familiar?
Instead, Mr. Knight advocates the use of core principles that will foster a partnership, the cornerstone of coaching (Knight, J. Partnership Learning. University of Kansas Center for Research on Learning: Lawrence, Kan., 2002). Here are some ways they could apply to surgical coaching:
- Equality – The opinions and approaches of the surgeon and the surgical coach are equally valuable.
- Choice – At a minimum, the surgeon should be allowed to choose the specific case and setting for each coaching session.
- Voice and dialogue – The surgeon should feel free to speak openly. Coaches should listen more than they talk. The coach should not control or dominate the interaction, but rather engage in a dialogue.
- Reflection – "Reflection on action" after an operation is likely to be more effective than "reflection in action" in the operating room so that the surgeon can concentrate fully on dissecting his or her own performance. In addition, coaching sessions can take place in a private, confidential setting away from patients and other providers. The use of video as a "thinking device" to prompt open dialogue holds significant promise.
- Praxis – Surgeons should be encouraged to explicitly think about how they will apply insights from the coaching session to their clinical practice.
Three other points deserve mention. Confidentiality and trust are critical, especially as surgeons acclimate to the idea of working with a coach. Additionally, the coaching style should be individualized and adapted to each surgeon throughout a coaching session. Such adaptability is an important characteristic of a successful coach. Finally, coaches should not have administrative oversight for the surgeon they are coaching. This is to ensure that the content of coaching sessions remain focused on performance improvement and not on performance evaluations or career development.
Will It Work?
There are very little empirical data on coaching in any discipline. What does exist tends to be exploratory and qualitative. However, Cornett and Knight describe several randomized trials, and a review in the field of education suggests that coaching will be successful (Cornett, J.; Knight, J. Research on Coaching:Approaches and Perspectives, 2009;192-216).
Researchers found that only 10% of teachers used a new skill in the classroom when they were provided with a verbal description. After modeling, practice, and feedback were added, the rate of adoption increased to 19%. It was only with the addition of peer coaching that an astounding 95% of teachers utilized the new skill. (Bush, R. N. Effective Staff Development in Making Our Schools More Effective: Proceedings of Three State Conferences. Far West Laboratories: San Francisco, 1984).
Other studies demonstrated that coaching increased skill transfer from 15% to 75%, compared with traditional approaches to professional development. Even more striking was the fact that these skills were still being used 6 months later. If we are even half as successful with coaching in surgery, results will be orders of magnitude better than any previous attempts at intraoperative performance improvement.
How Do We Move Forward?
The American College of Surgeons Division of Education – with its dedication to improving quality, safety, and education – is in a particularly strong position to develop surgical coaching and is exploring potential programs with us in Wisconsin and with others. Other surgical societies, including local and regional organizations, offer another opportunity to develop coaching programs. The state chapters of the American Academy of Pediatrics instituted a quality improvement initiative that included team coaching, and found several advantages to this approach over a national one (J. Contin. Educ. Health Profess. 2008;28:131-9).
Trust in, familiarity with, and participation in local/regional societies or state chapters is likely to increase acceptance and participation by practicing surgeons. The infrastructure of a regional society allows for participation across all practice settings – not just in large hospitals where a coach may be locally available – yet it is small enough to afford some level of familiarity, trust, and respect for the coach.
This type of cross-institutional collaboration may seem counterintuitive in light of the traditional competitive relationships of neighboring institutions; however, the success of programs such as the Surgical Care and Outcomes Assessment Program (SCOAP) in Washington State and the Michigan Surgical Collaboratives (MSQC and MSBC) suggests that as a discipline we are ready to work together to improve the quality and safety of surgical care.
Given the current paucity of data, we must continue to study any new programs or interventions, but surgical coaching seems like an idea whose time has come.
Acknowledgments
I would like to thank Atul Gawande, Yue-Yung Hu, Robert Osteen, and Michael Zinner for conversations and research that helped me formulate these ideas.☐
Dr. Greenberg is an associate professor of surgery, and director of the Wisconsin Surgical Outcomes Research Program at the University of Wisconsin, Madison.
Some Online Resources
AATS Resident Resources: www.aats.org/TSR/index.html
CTSNET Residents Section: www.ctsnet.org/sections/residents
Thoracic Surgery Directors Association: www.tsda.org
Thoracic Surgery News: www.thoracicsurgerynews.com
Thoracic Surgery Residents Association: www.tsranet.org
Thoracic Surgery Foundation for Research and Education: www.tsfre.org
AATS Resident Resources: www.aats.org/TSR/index.html
CTSNET Residents Section: www.ctsnet.org/sections/residents
Thoracic Surgery Directors Association: www.tsda.org
Thoracic Surgery News: www.thoracicsurgerynews.com
Thoracic Surgery Residents Association: www.tsranet.org
Thoracic Surgery Foundation for Research and Education: www.tsfre.org
AATS Resident Resources: www.aats.org/TSR/index.html
CTSNET Residents Section: www.ctsnet.org/sections/residents
Thoracic Surgery Directors Association: www.tsda.org
Thoracic Surgery News: www.thoracicsurgerynews.com
Thoracic Surgery Residents Association: www.tsranet.org
Thoracic Surgery Foundation for Research and Education: www.tsfre.org
Training, Leadership, Commitment Integral to HM Improving Stroke Care
Stroke specialists like to say that “time is brain.” With an emphatic focus on those first few critical hours, however, it’s sometimes easy to overlook the vital role that hospitalists play in the days, weeks, and months that follow.
A recent study in The Neurohospitalist suggests that compared to community-based neurologists, practitioners of neurohospital medicine can reduce the length of stay for patients with ischemic stroke.1 A separate study, however, suggests that similar success might have come at a price for their less-specialized hospitalist counterparts.2 Among stroke patients, the latter study found that while the HM model is also associated with a reduced length of stay, it is associated with increased discharges to inpatient rehabilitation centers instead of to home, and higher readmission rates.
In sum, the evidence raises questions about whether rank-and-file hospitalists are adequately equipped to deal with a disease that is a core competency for the profession and ranks among the top sources of adult disability in the United States, at an estimated cost of $34.3 billion in 2008.3
“I think there’s been a mismatch between the training of the average hospitalist and then the expectations for the amount of neurological care they end up delivering once in practice,” says David Likosky, MD, SFHM, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash. “When surveyed, it’s been shown that hospitalists feel that care of stroke is one of the areas with which they’re least comfortable once they get out into practice.” Over the past decade, several studies have reinforced the notion of a training deficit.4,5
Demographic trends suggest that getting up to speed will be imperative, however. “One alarming thing we’re seeing is strokes among individuals that are not in the elderly group, and that group seems to be increasing at an alarming rate,” says Daniel T. Lackland, PhD, professor of epidemiology and neurosciences at the Medical University of South Carolina in Charleston. Hospitals are seeing more ischemic stroke patients in their 40s and 50s, likely a reflection of risk factors such as hypertension, diabetes, and hyperlipidemia that are occurring earlier in life. And because those patients are younger, the aftermath of a stroke could linger for decades.
Although the stroke mortality rate is declining in the U.S., statistics find that about 14% of all patients diagnosed with an initial stroke will have a second one within a year, placing continued strain on a healthcare system already stretched thin.6 Hospitalists, Dr. Lackland says, have an “ideal” opportunity to help build up and improve that system, potentially yielding significant cost savings along with the dramatic improvement in quality of life. Making the most of that opportunity, though, will require a solid understanding of multiple trends that are quickly transforming stroke care delivery.
Time Is of the Essence
Kevin Barrett, MD, MSc, assistant professor of neurology and stroke telemedicine director at the Mayo Clinic in Jacksonville, Fla., says hospitals are focusing more and more on a metric known as “door-to-needle time.” The goal is to treat at least half of incoming ischemic stroke patients with intravenous tissue-type plasminogen activator (IV tPA) within the first 60 minutes after onset of symptoms.
The American Heart Association/American Stroke Association has reinforced the message with its Get With the Guidelines Stroke Program. A recent analysis suggested the program has led to more timely tPA administration and, in turn, better patient outcomes (the program is funded in part through the Bristol-Myers Squib/Sanofi Pharmaceutical Partnership).7
At the same time, clinical research has widened the window for IV tPA delivery from three hours to 4.5 hours for certain patients after the onset of symptoms. Dr. Barrett says “strong evidence” from the European Cooperative Acute Stroke Study III has convinced most clinicians, and the FDA is expected to follow suit in officially approving the extension.8 As more stroke centers become certified, the use of IV tPA has increased accordingly.
Patients who have missed the time window or are not good candidates for IV tPA can still be aided by interarterial tPA at the site of the clot up to six hours after the onset of symptoms. Dr. Likosky says the treatment option should be of particular interest to hospitalists, given that strokes can occur post-operatively and in other patients who cannot receive IV tPA because of bleeding risk.
—Karim Godamunne, MD, MBA, SFHM, medical director, Eagle Hospital Physicians, Roswell, Ga.
For up to eight hours after the onset of symptoms, mechanical clot removal techniques have shown continued efficacy at revascularizing affected areas, with some newer options also offering greater promise of improving patient outcomes. Even with the prospects of declining complication rates, however, “evaluating and initiating treatment in a timely fashion is still going to be one of the most important predictors of outcome,” Dr. Barrett says.
After the initial intervention, hospitalists often are the go-to providers for anticipating and preventing common post-stroke complications, such as aspiration pneumonia, VTE from immobilization, and other infections. The proper use of anti-platelet agents and high-dose statins, also falling solidly within the HM realm, can pay big dividends if used consistently.
Meanwhile, newer studies and clinical observations are widening the scope of considerations that should be on every hospitalist’s radar. Here are a few cited by stroke experts:
Permissive hypertension. After an ischemic stroke, the benefit of permissive hypertension is still widely misunderstood. Perhaps counterintuitively, high blood pressure after a stroke can help protect the area of the brain that is damaged but not yet dead, sometimes called the penumbra. “I highlight this because I think it’s a common mistake, that internists are very used to high blood pressure being a bad thing,” says Andrew Josephson, MD, associate professor of clinical neurology and director of the neurohospitalist program at the University of California at San Francisco (UCSF) Medical Center. “And in general, it is; it’s a cause of stroke. But once somebody has a stroke, in the acute period, it’s important to allow the blood pressure to be high.”
Atrial fibrillation. The accepted role of atrial fibrillation in stroke is evolving. Research suggests that the common but often preventable arrhythmia is an important cause of stroke in about 15% to 20% of cases.9 By the time of hospital discharge, however, Dr. Josephson says physicians haven’t established a cause in about 1 in 4 cases. For these “cryptogenic strokes,” he says, doctors have long suspected that atrial fibrillation not picked up during the initial EKG or by the monitoring with cardiac telemetry could be a major cause.
Recent observations suggest that a longer monitoring period of up to 30 days may uncover atrial fibrillation in a sizable fraction of those patients, highlighting the importance of keeping a close eye on stroke patients both in the hospital and beyond. “It’s very important to identify, because atrial fibrillation changes what we do for folks to prevent a second stroke,” Dr. Josephson explains. Instead of anti-platelet medicine like aspirin, patients with atrial fibrillation often receive anticoagulants like warfarin, or the more recently approved dabigatran and rivaroxaban.
Transient ischemic attack. Improvements in imaging techniques like MRI have likewise begun to shift how stroke patients are treated. For example, Dr. Likosky says, medicine is moving away from a time-based definition of transient ischemic attack (TIA), in which symptoms resolve within 24 hours, to a tissue-based definition. Recent MRI imaging has uncovered evidence of a new infarction in more than half of patients initially diagnosed with TIA.10
“If they do have an infarction on their scan, even if they had symptoms that only lasted for five minutes, that’s a stroke,” Dr. Josephson says. And even a true TIA, he says, represents “a kind of stroke where you got really lucky and you’re not left with deficits, but the risk is still very high.” Accordingly, more patients with TIA are being admitted to the hospital to receive a full workup and preventive treatment. “We think that by evaluating these people urgently, we can reduce the risk of having a stroke by maybe 75% over a three-month time period,” Dr. Josephson says.
Hemorrhagic stroke. To date, the vast majority of patients with hemorrhagic stroke (which accounts for only 13% of all stroke cases) have been managed by neurosurgeons and neurologists. But here, too, Dr. Likosky says the picture could be changing. Recent findings that surgical treatment of intracranial hemorrhaging might not benefit many patients could shift the care paradigm toward a medical management strategy that involves more hospitalists.
Innovations Aplenty
The increasing complexity of stroke care and uneven distribution of resources and expertise have helped fuel several important innovations in delivery, most notably telestroke and neurohospital medicine. Both are being driven, in part, by an increased awareness of time-sensitive interventions and a frequent lack of on-site neurologists at smaller and more rural facilities. If telestroke programs are expanding the reach of neurologists, neurohospitalists are helping to fill the gaps in inpatient stroke care.
Amid the changes, one element is proving a necessary constant: a team approach that relies heavily on the HM emphasis on quality metrics, intensive monitoring, and careful coordination. Who better to lead the charge than hospitalists, says Mary E. Jensen, MD, professor of radiology and neurosurgery at the University of Virginia in Charlottesville. “They’re the ones who are in the hospital, and when these patients go bad, they go bad fast,” she says.
More broadly, Dr. Jensen says, hospitalists should get in on the ground floor when their facility seeks certification as a primary or a comprehensive stroke center. “And they need to make sure that the hospital isn’t just trying to get the sexy elements—the guy with the cath or the gal with the cath who can pull the clot out—but that they have a complete program that involves the care of the patient after they’ve had the procedure done,” she says.
As healthcare reform efforts are making clear, the responsibility doesn’t end after discharge, either. The Affordable Care Act includes a hospital readmission reduction program that will kick in this October, with penalties for hospitals posting unacceptably high 30-day readmission rates. Amy Kind, MD, PhD, assistant professor of medicine in the Division of Geriatrics at the University of Wisconsin School of Medicine and Public Health in Madison, is convinced that a key contributor to high rehospitalization rates among stroke patients may be the woefully incomplete nature of discharge communication.

—Mary E. Jensen, MD, professor of radiology and neurosurgery, University of Virginia, Charlottesville
Dr. Kind, for example, has found a disturbing pattern in communication regarding issues like dysphagia, a common complication among stroke patients and an important risk factor for pneumonia. Countering the risk usually requires such measures as putting patients on a special diet or elevating the head of their bed. “We looked at the quality of the communication of that information in discharge summaries, and it’s just abysmal. It’s absolutely abysmal,” she says. Without clear directives to providers in the next setting of care, such as a skilled-nursing facility, patients could be erroneously put back on a regular diet and aspirate, sending them right back to the hospital.
As one potential solution, Dr. Kind’s team is developing a multidisciplinary stroke discharge summary tool that automatically imports elements like speech-language pathology and dietary recommendations. Although most discharge communication may focus on more visible issues and interventions, Dr. Kind argues that some of the “bread and butter” concerns might ultimately prove just as important for long-term patient outcomes.
Karim Godamunne, MD, MBA, SFHM, vice president of clinical systems integration and medical director of Eagle Hospital Physicians in Atlanta, sees telemedicine as another potential tool to help reach patients after discharge, especially those who haven’t received follow-up care from a primary-care physician (PCP). “We need to be the champions at our hospitals for improving care processes, and we need to work in partnership with the nurses and the other professionals,” Dr. Godamunne says. “As a group, we can really make a difference, and stroke is one of those areas in which we can truly contribute.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Freeman WD, Dawson SB, Raper C, Thiemann K, et al. Neurohospitalists reduce length of stay for patients with ischemic stroke. The Neurohospitalist. 2011;1(2): 67-70.
- Howrey BT, Kuo Y-F, Goodwin JS. Association of care by hospitalists on discharge destination and 30-day outcomes after acute ischemic stroke. Medical Care. 2011;49(8): 701-707.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
- Glasheen JJ, Epstein KR, Siegal E, Kutner JS, Prochazka AV. The spectrum of community-based hospitalist practice: a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001; 111(3):247-254.
- Dickerson LM, Carek PJ, Quattlebaum RG. Prevention of recurrent ischemic stroke. Am Fam Physician. 2007; 76(3):382-388.
- Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750-758.
- Hacke W, Kaste M, Bluhmki E, Brozman M, et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e91.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
Stroke specialists like to say that “time is brain.” With an emphatic focus on those first few critical hours, however, it’s sometimes easy to overlook the vital role that hospitalists play in the days, weeks, and months that follow.
A recent study in The Neurohospitalist suggests that compared to community-based neurologists, practitioners of neurohospital medicine can reduce the length of stay for patients with ischemic stroke.1 A separate study, however, suggests that similar success might have come at a price for their less-specialized hospitalist counterparts.2 Among stroke patients, the latter study found that while the HM model is also associated with a reduced length of stay, it is associated with increased discharges to inpatient rehabilitation centers instead of to home, and higher readmission rates.
In sum, the evidence raises questions about whether rank-and-file hospitalists are adequately equipped to deal with a disease that is a core competency for the profession and ranks among the top sources of adult disability in the United States, at an estimated cost of $34.3 billion in 2008.3
“I think there’s been a mismatch between the training of the average hospitalist and then the expectations for the amount of neurological care they end up delivering once in practice,” says David Likosky, MD, SFHM, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash. “When surveyed, it’s been shown that hospitalists feel that care of stroke is one of the areas with which they’re least comfortable once they get out into practice.” Over the past decade, several studies have reinforced the notion of a training deficit.4,5
Demographic trends suggest that getting up to speed will be imperative, however. “One alarming thing we’re seeing is strokes among individuals that are not in the elderly group, and that group seems to be increasing at an alarming rate,” says Daniel T. Lackland, PhD, professor of epidemiology and neurosciences at the Medical University of South Carolina in Charleston. Hospitals are seeing more ischemic stroke patients in their 40s and 50s, likely a reflection of risk factors such as hypertension, diabetes, and hyperlipidemia that are occurring earlier in life. And because those patients are younger, the aftermath of a stroke could linger for decades.
Although the stroke mortality rate is declining in the U.S., statistics find that about 14% of all patients diagnosed with an initial stroke will have a second one within a year, placing continued strain on a healthcare system already stretched thin.6 Hospitalists, Dr. Lackland says, have an “ideal” opportunity to help build up and improve that system, potentially yielding significant cost savings along with the dramatic improvement in quality of life. Making the most of that opportunity, though, will require a solid understanding of multiple trends that are quickly transforming stroke care delivery.
Time Is of the Essence
Kevin Barrett, MD, MSc, assistant professor of neurology and stroke telemedicine director at the Mayo Clinic in Jacksonville, Fla., says hospitals are focusing more and more on a metric known as “door-to-needle time.” The goal is to treat at least half of incoming ischemic stroke patients with intravenous tissue-type plasminogen activator (IV tPA) within the first 60 minutes after onset of symptoms.
The American Heart Association/American Stroke Association has reinforced the message with its Get With the Guidelines Stroke Program. A recent analysis suggested the program has led to more timely tPA administration and, in turn, better patient outcomes (the program is funded in part through the Bristol-Myers Squib/Sanofi Pharmaceutical Partnership).7
At the same time, clinical research has widened the window for IV tPA delivery from three hours to 4.5 hours for certain patients after the onset of symptoms. Dr. Barrett says “strong evidence” from the European Cooperative Acute Stroke Study III has convinced most clinicians, and the FDA is expected to follow suit in officially approving the extension.8 As more stroke centers become certified, the use of IV tPA has increased accordingly.
Patients who have missed the time window or are not good candidates for IV tPA can still be aided by interarterial tPA at the site of the clot up to six hours after the onset of symptoms. Dr. Likosky says the treatment option should be of particular interest to hospitalists, given that strokes can occur post-operatively and in other patients who cannot receive IV tPA because of bleeding risk.
—Karim Godamunne, MD, MBA, SFHM, medical director, Eagle Hospital Physicians, Roswell, Ga.
For up to eight hours after the onset of symptoms, mechanical clot removal techniques have shown continued efficacy at revascularizing affected areas, with some newer options also offering greater promise of improving patient outcomes. Even with the prospects of declining complication rates, however, “evaluating and initiating treatment in a timely fashion is still going to be one of the most important predictors of outcome,” Dr. Barrett says.
After the initial intervention, hospitalists often are the go-to providers for anticipating and preventing common post-stroke complications, such as aspiration pneumonia, VTE from immobilization, and other infections. The proper use of anti-platelet agents and high-dose statins, also falling solidly within the HM realm, can pay big dividends if used consistently.
Meanwhile, newer studies and clinical observations are widening the scope of considerations that should be on every hospitalist’s radar. Here are a few cited by stroke experts:
Permissive hypertension. After an ischemic stroke, the benefit of permissive hypertension is still widely misunderstood. Perhaps counterintuitively, high blood pressure after a stroke can help protect the area of the brain that is damaged but not yet dead, sometimes called the penumbra. “I highlight this because I think it’s a common mistake, that internists are very used to high blood pressure being a bad thing,” says Andrew Josephson, MD, associate professor of clinical neurology and director of the neurohospitalist program at the University of California at San Francisco (UCSF) Medical Center. “And in general, it is; it’s a cause of stroke. But once somebody has a stroke, in the acute period, it’s important to allow the blood pressure to be high.”
Atrial fibrillation. The accepted role of atrial fibrillation in stroke is evolving. Research suggests that the common but often preventable arrhythmia is an important cause of stroke in about 15% to 20% of cases.9 By the time of hospital discharge, however, Dr. Josephson says physicians haven’t established a cause in about 1 in 4 cases. For these “cryptogenic strokes,” he says, doctors have long suspected that atrial fibrillation not picked up during the initial EKG or by the monitoring with cardiac telemetry could be a major cause.
Recent observations suggest that a longer monitoring period of up to 30 days may uncover atrial fibrillation in a sizable fraction of those patients, highlighting the importance of keeping a close eye on stroke patients both in the hospital and beyond. “It’s very important to identify, because atrial fibrillation changes what we do for folks to prevent a second stroke,” Dr. Josephson explains. Instead of anti-platelet medicine like aspirin, patients with atrial fibrillation often receive anticoagulants like warfarin, or the more recently approved dabigatran and rivaroxaban.
Transient ischemic attack. Improvements in imaging techniques like MRI have likewise begun to shift how stroke patients are treated. For example, Dr. Likosky says, medicine is moving away from a time-based definition of transient ischemic attack (TIA), in which symptoms resolve within 24 hours, to a tissue-based definition. Recent MRI imaging has uncovered evidence of a new infarction in more than half of patients initially diagnosed with TIA.10
“If they do have an infarction on their scan, even if they had symptoms that only lasted for five minutes, that’s a stroke,” Dr. Josephson says. And even a true TIA, he says, represents “a kind of stroke where you got really lucky and you’re not left with deficits, but the risk is still very high.” Accordingly, more patients with TIA are being admitted to the hospital to receive a full workup and preventive treatment. “We think that by evaluating these people urgently, we can reduce the risk of having a stroke by maybe 75% over a three-month time period,” Dr. Josephson says.
Hemorrhagic stroke. To date, the vast majority of patients with hemorrhagic stroke (which accounts for only 13% of all stroke cases) have been managed by neurosurgeons and neurologists. But here, too, Dr. Likosky says the picture could be changing. Recent findings that surgical treatment of intracranial hemorrhaging might not benefit many patients could shift the care paradigm toward a medical management strategy that involves more hospitalists.
Innovations Aplenty
The increasing complexity of stroke care and uneven distribution of resources and expertise have helped fuel several important innovations in delivery, most notably telestroke and neurohospital medicine. Both are being driven, in part, by an increased awareness of time-sensitive interventions and a frequent lack of on-site neurologists at smaller and more rural facilities. If telestroke programs are expanding the reach of neurologists, neurohospitalists are helping to fill the gaps in inpatient stroke care.
Amid the changes, one element is proving a necessary constant: a team approach that relies heavily on the HM emphasis on quality metrics, intensive monitoring, and careful coordination. Who better to lead the charge than hospitalists, says Mary E. Jensen, MD, professor of radiology and neurosurgery at the University of Virginia in Charlottesville. “They’re the ones who are in the hospital, and when these patients go bad, they go bad fast,” she says.
More broadly, Dr. Jensen says, hospitalists should get in on the ground floor when their facility seeks certification as a primary or a comprehensive stroke center. “And they need to make sure that the hospital isn’t just trying to get the sexy elements—the guy with the cath or the gal with the cath who can pull the clot out—but that they have a complete program that involves the care of the patient after they’ve had the procedure done,” she says.
As healthcare reform efforts are making clear, the responsibility doesn’t end after discharge, either. The Affordable Care Act includes a hospital readmission reduction program that will kick in this October, with penalties for hospitals posting unacceptably high 30-day readmission rates. Amy Kind, MD, PhD, assistant professor of medicine in the Division of Geriatrics at the University of Wisconsin School of Medicine and Public Health in Madison, is convinced that a key contributor to high rehospitalization rates among stroke patients may be the woefully incomplete nature of discharge communication.

—Mary E. Jensen, MD, professor of radiology and neurosurgery, University of Virginia, Charlottesville
Dr. Kind, for example, has found a disturbing pattern in communication regarding issues like dysphagia, a common complication among stroke patients and an important risk factor for pneumonia. Countering the risk usually requires such measures as putting patients on a special diet or elevating the head of their bed. “We looked at the quality of the communication of that information in discharge summaries, and it’s just abysmal. It’s absolutely abysmal,” she says. Without clear directives to providers in the next setting of care, such as a skilled-nursing facility, patients could be erroneously put back on a regular diet and aspirate, sending them right back to the hospital.
As one potential solution, Dr. Kind’s team is developing a multidisciplinary stroke discharge summary tool that automatically imports elements like speech-language pathology and dietary recommendations. Although most discharge communication may focus on more visible issues and interventions, Dr. Kind argues that some of the “bread and butter” concerns might ultimately prove just as important for long-term patient outcomes.
Karim Godamunne, MD, MBA, SFHM, vice president of clinical systems integration and medical director of Eagle Hospital Physicians in Atlanta, sees telemedicine as another potential tool to help reach patients after discharge, especially those who haven’t received follow-up care from a primary-care physician (PCP). “We need to be the champions at our hospitals for improving care processes, and we need to work in partnership with the nurses and the other professionals,” Dr. Godamunne says. “As a group, we can really make a difference, and stroke is one of those areas in which we can truly contribute.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Freeman WD, Dawson SB, Raper C, Thiemann K, et al. Neurohospitalists reduce length of stay for patients with ischemic stroke. The Neurohospitalist. 2011;1(2): 67-70.
- Howrey BT, Kuo Y-F, Goodwin JS. Association of care by hospitalists on discharge destination and 30-day outcomes after acute ischemic stroke. Medical Care. 2011;49(8): 701-707.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
- Glasheen JJ, Epstein KR, Siegal E, Kutner JS, Prochazka AV. The spectrum of community-based hospitalist practice: a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001; 111(3):247-254.
- Dickerson LM, Carek PJ, Quattlebaum RG. Prevention of recurrent ischemic stroke. Am Fam Physician. 2007; 76(3):382-388.
- Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750-758.
- Hacke W, Kaste M, Bluhmki E, Brozman M, et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e91.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
Stroke specialists like to say that “time is brain.” With an emphatic focus on those first few critical hours, however, it’s sometimes easy to overlook the vital role that hospitalists play in the days, weeks, and months that follow.
A recent study in The Neurohospitalist suggests that compared to community-based neurologists, practitioners of neurohospital medicine can reduce the length of stay for patients with ischemic stroke.1 A separate study, however, suggests that similar success might have come at a price for their less-specialized hospitalist counterparts.2 Among stroke patients, the latter study found that while the HM model is also associated with a reduced length of stay, it is associated with increased discharges to inpatient rehabilitation centers instead of to home, and higher readmission rates.
In sum, the evidence raises questions about whether rank-and-file hospitalists are adequately equipped to deal with a disease that is a core competency for the profession and ranks among the top sources of adult disability in the United States, at an estimated cost of $34.3 billion in 2008.3
“I think there’s been a mismatch between the training of the average hospitalist and then the expectations for the amount of neurological care they end up delivering once in practice,” says David Likosky, MD, SFHM, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash. “When surveyed, it’s been shown that hospitalists feel that care of stroke is one of the areas with which they’re least comfortable once they get out into practice.” Over the past decade, several studies have reinforced the notion of a training deficit.4,5
Demographic trends suggest that getting up to speed will be imperative, however. “One alarming thing we’re seeing is strokes among individuals that are not in the elderly group, and that group seems to be increasing at an alarming rate,” says Daniel T. Lackland, PhD, professor of epidemiology and neurosciences at the Medical University of South Carolina in Charleston. Hospitals are seeing more ischemic stroke patients in their 40s and 50s, likely a reflection of risk factors such as hypertension, diabetes, and hyperlipidemia that are occurring earlier in life. And because those patients are younger, the aftermath of a stroke could linger for decades.
Although the stroke mortality rate is declining in the U.S., statistics find that about 14% of all patients diagnosed with an initial stroke will have a second one within a year, placing continued strain on a healthcare system already stretched thin.6 Hospitalists, Dr. Lackland says, have an “ideal” opportunity to help build up and improve that system, potentially yielding significant cost savings along with the dramatic improvement in quality of life. Making the most of that opportunity, though, will require a solid understanding of multiple trends that are quickly transforming stroke care delivery.
Time Is of the Essence
Kevin Barrett, MD, MSc, assistant professor of neurology and stroke telemedicine director at the Mayo Clinic in Jacksonville, Fla., says hospitals are focusing more and more on a metric known as “door-to-needle time.” The goal is to treat at least half of incoming ischemic stroke patients with intravenous tissue-type plasminogen activator (IV tPA) within the first 60 minutes after onset of symptoms.
The American Heart Association/American Stroke Association has reinforced the message with its Get With the Guidelines Stroke Program. A recent analysis suggested the program has led to more timely tPA administration and, in turn, better patient outcomes (the program is funded in part through the Bristol-Myers Squib/Sanofi Pharmaceutical Partnership).7
At the same time, clinical research has widened the window for IV tPA delivery from three hours to 4.5 hours for certain patients after the onset of symptoms. Dr. Barrett says “strong evidence” from the European Cooperative Acute Stroke Study III has convinced most clinicians, and the FDA is expected to follow suit in officially approving the extension.8 As more stroke centers become certified, the use of IV tPA has increased accordingly.
Patients who have missed the time window or are not good candidates for IV tPA can still be aided by interarterial tPA at the site of the clot up to six hours after the onset of symptoms. Dr. Likosky says the treatment option should be of particular interest to hospitalists, given that strokes can occur post-operatively and in other patients who cannot receive IV tPA because of bleeding risk.
—Karim Godamunne, MD, MBA, SFHM, medical director, Eagle Hospital Physicians, Roswell, Ga.
For up to eight hours after the onset of symptoms, mechanical clot removal techniques have shown continued efficacy at revascularizing affected areas, with some newer options also offering greater promise of improving patient outcomes. Even with the prospects of declining complication rates, however, “evaluating and initiating treatment in a timely fashion is still going to be one of the most important predictors of outcome,” Dr. Barrett says.
After the initial intervention, hospitalists often are the go-to providers for anticipating and preventing common post-stroke complications, such as aspiration pneumonia, VTE from immobilization, and other infections. The proper use of anti-platelet agents and high-dose statins, also falling solidly within the HM realm, can pay big dividends if used consistently.
Meanwhile, newer studies and clinical observations are widening the scope of considerations that should be on every hospitalist’s radar. Here are a few cited by stroke experts:
Permissive hypertension. After an ischemic stroke, the benefit of permissive hypertension is still widely misunderstood. Perhaps counterintuitively, high blood pressure after a stroke can help protect the area of the brain that is damaged but not yet dead, sometimes called the penumbra. “I highlight this because I think it’s a common mistake, that internists are very used to high blood pressure being a bad thing,” says Andrew Josephson, MD, associate professor of clinical neurology and director of the neurohospitalist program at the University of California at San Francisco (UCSF) Medical Center. “And in general, it is; it’s a cause of stroke. But once somebody has a stroke, in the acute period, it’s important to allow the blood pressure to be high.”
Atrial fibrillation. The accepted role of atrial fibrillation in stroke is evolving. Research suggests that the common but often preventable arrhythmia is an important cause of stroke in about 15% to 20% of cases.9 By the time of hospital discharge, however, Dr. Josephson says physicians haven’t established a cause in about 1 in 4 cases. For these “cryptogenic strokes,” he says, doctors have long suspected that atrial fibrillation not picked up during the initial EKG or by the monitoring with cardiac telemetry could be a major cause.
Recent observations suggest that a longer monitoring period of up to 30 days may uncover atrial fibrillation in a sizable fraction of those patients, highlighting the importance of keeping a close eye on stroke patients both in the hospital and beyond. “It’s very important to identify, because atrial fibrillation changes what we do for folks to prevent a second stroke,” Dr. Josephson explains. Instead of anti-platelet medicine like aspirin, patients with atrial fibrillation often receive anticoagulants like warfarin, or the more recently approved dabigatran and rivaroxaban.
Transient ischemic attack. Improvements in imaging techniques like MRI have likewise begun to shift how stroke patients are treated. For example, Dr. Likosky says, medicine is moving away from a time-based definition of transient ischemic attack (TIA), in which symptoms resolve within 24 hours, to a tissue-based definition. Recent MRI imaging has uncovered evidence of a new infarction in more than half of patients initially diagnosed with TIA.10
“If they do have an infarction on their scan, even if they had symptoms that only lasted for five minutes, that’s a stroke,” Dr. Josephson says. And even a true TIA, he says, represents “a kind of stroke where you got really lucky and you’re not left with deficits, but the risk is still very high.” Accordingly, more patients with TIA are being admitted to the hospital to receive a full workup and preventive treatment. “We think that by evaluating these people urgently, we can reduce the risk of having a stroke by maybe 75% over a three-month time period,” Dr. Josephson says.
Hemorrhagic stroke. To date, the vast majority of patients with hemorrhagic stroke (which accounts for only 13% of all stroke cases) have been managed by neurosurgeons and neurologists. But here, too, Dr. Likosky says the picture could be changing. Recent findings that surgical treatment of intracranial hemorrhaging might not benefit many patients could shift the care paradigm toward a medical management strategy that involves more hospitalists.
Innovations Aplenty
The increasing complexity of stroke care and uneven distribution of resources and expertise have helped fuel several important innovations in delivery, most notably telestroke and neurohospital medicine. Both are being driven, in part, by an increased awareness of time-sensitive interventions and a frequent lack of on-site neurologists at smaller and more rural facilities. If telestroke programs are expanding the reach of neurologists, neurohospitalists are helping to fill the gaps in inpatient stroke care.
Amid the changes, one element is proving a necessary constant: a team approach that relies heavily on the HM emphasis on quality metrics, intensive monitoring, and careful coordination. Who better to lead the charge than hospitalists, says Mary E. Jensen, MD, professor of radiology and neurosurgery at the University of Virginia in Charlottesville. “They’re the ones who are in the hospital, and when these patients go bad, they go bad fast,” she says.
More broadly, Dr. Jensen says, hospitalists should get in on the ground floor when their facility seeks certification as a primary or a comprehensive stroke center. “And they need to make sure that the hospital isn’t just trying to get the sexy elements—the guy with the cath or the gal with the cath who can pull the clot out—but that they have a complete program that involves the care of the patient after they’ve had the procedure done,” she says.
As healthcare reform efforts are making clear, the responsibility doesn’t end after discharge, either. The Affordable Care Act includes a hospital readmission reduction program that will kick in this October, with penalties for hospitals posting unacceptably high 30-day readmission rates. Amy Kind, MD, PhD, assistant professor of medicine in the Division of Geriatrics at the University of Wisconsin School of Medicine and Public Health in Madison, is convinced that a key contributor to high rehospitalization rates among stroke patients may be the woefully incomplete nature of discharge communication.

—Mary E. Jensen, MD, professor of radiology and neurosurgery, University of Virginia, Charlottesville
Dr. Kind, for example, has found a disturbing pattern in communication regarding issues like dysphagia, a common complication among stroke patients and an important risk factor for pneumonia. Countering the risk usually requires such measures as putting patients on a special diet or elevating the head of their bed. “We looked at the quality of the communication of that information in discharge summaries, and it’s just abysmal. It’s absolutely abysmal,” she says. Without clear directives to providers in the next setting of care, such as a skilled-nursing facility, patients could be erroneously put back on a regular diet and aspirate, sending them right back to the hospital.
As one potential solution, Dr. Kind’s team is developing a multidisciplinary stroke discharge summary tool that automatically imports elements like speech-language pathology and dietary recommendations. Although most discharge communication may focus on more visible issues and interventions, Dr. Kind argues that some of the “bread and butter” concerns might ultimately prove just as important for long-term patient outcomes.
Karim Godamunne, MD, MBA, SFHM, vice president of clinical systems integration and medical director of Eagle Hospital Physicians in Atlanta, sees telemedicine as another potential tool to help reach patients after discharge, especially those who haven’t received follow-up care from a primary-care physician (PCP). “We need to be the champions at our hospitals for improving care processes, and we need to work in partnership with the nurses and the other professionals,” Dr. Godamunne says. “As a group, we can really make a difference, and stroke is one of those areas in which we can truly contribute.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Freeman WD, Dawson SB, Raper C, Thiemann K, et al. Neurohospitalists reduce length of stay for patients with ischemic stroke. The Neurohospitalist. 2011;1(2): 67-70.
- Howrey BT, Kuo Y-F, Goodwin JS. Association of care by hospitalists on discharge destination and 30-day outcomes after acute ischemic stroke. Medical Care. 2011;49(8): 701-707.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
- Glasheen JJ, Epstein KR, Siegal E, Kutner JS, Prochazka AV. The spectrum of community-based hospitalist practice: a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001; 111(3):247-254.
- Dickerson LM, Carek PJ, Quattlebaum RG. Prevention of recurrent ischemic stroke. Am Fam Physician. 2007; 76(3):382-388.
- Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750-758.
- Hacke W, Kaste M, Bluhmki E, Brozman M, et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e91.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
Is Hospitalist Proficiency in Bedside Procedures in Decline?
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
What Is the Role of Steroids in Septic Shock?
The Case
An 81-year-old woman with diabetes mellitus presents with a three-day history of fever, chills, left-side flank pain, and dysuria. Her blood pressure upon presentation is 75/45 mm/Hg, her heart rate is 120 beats per minute, and she has a temperature of 103.1°`F and a respiratory rate of 22 breaths/minute. On physical examination, she is an ill-appearing elderly woman, with dry oral mucosa and left costo-vertebral angle tenderness. Lab work shows leukocytosis of 18,000 mg/dL with 88% polymorphonuclear leukocyte (PMN), the urine analysis is consistent with a urinary tract infection, and a chemistry panel reveals elevated BUN and creatinine levels of 52 mg/dL and 2.4 mg/dL, respectively. In the emergency department, she is given a bolus of 2 liters normal saline, but her blood pressure remains 78/49 mm/Hg. She is then started on broad-spectrum antibiotics and a norepinephrine drip, and is admitted to the ICU.
What role would steroids add to her management?
Background
Sepsis is the clinical syndrome defined by the presence of systemic inflammatory response syndrome (SIRS) in the setting of an infection. SIRS is defined by the presence of at least two of the following: fever or hypothermia; leukocytosis, leukopenia, or bandemia; heart rate >90 bpm; or tachypnea or hypocapnia.
When acute organ dysfunction, such as acute renal failure, altered mental status, or acute lung injury (hypoxemia), is present, sepsis is classified as severe.
Septic shock is a state of sepsis associated with acute circulatory collapse characterized by persistent arterial hypotension (defined as a systolic blood pressure <90 mmHg, a mean arterial pressure <60 mmHg, or a reduction in systolic blood pressure of >40 mmHg from baseline) despite fluid resuscitation attempts.1
The incidence and mortality due to sepsis and septic shock is directly related to the age of the patient, many of whom require ICU hospitalization.2 Clinically, this portends a great challenge, as the incidence of sepsis is likely to increase as the U.S. population ages.
Initial management of a patient with sepsis/septic shock is goal-directed therapy, which consists of early administration of broad-spectrum antibiotics, crystalloid or colloid fluid resuscitation, and use of vasopressor support to improve hemodynamics and maintain a mean arterial
pressure ≥65 mmHg. Patients with acute lung injury may also require prompt ventilator support.
The role of steroids in sepsis is controversial.
Review of Steroids
Steroids have long been known for their anti-inflammatory properties. From the 1950s to the 1980s, high-dose steroids (methylprednisolone 30mg/kg and dexamethasone 3 mg/kg to 6 mg/kg in divided doses) were used in the management of sepsis. This was based on a study by Schumer that showed steroids reduced mortality to 10% from 38%.3
Later, Sprung and colleagues demonstrated reversal of shock and improved short-term survival with high-dose steroids in patients with sepsis, but subsequent prospective randomized trials did not support this beneficial effect of high-dose steroids.4-6 In fact, two meta-analyses in 1995 concluded that high-dose steroids are ineffective and potentially harmful, and associated with higher mortality, secondary infections, and renal and hepatic dysfunction.7,8 Thereafter, the use of high-dose steroids fell into disfavor.
In the early 2000s, there was an emergence in the use of low-dose steroids in patients with sepsis. This was based on various trials showing the benefit of the use of low-dose steroids in the reversal of septic shock without significant side effects, discussed further below.
Pathophysiology
Steroids improve hemodynamic parameters. In an animal model, Hinshaw and colleagues induced septic shock in adrenalectomized dogs by infusing lethal doses of E. coli. The untreated dogs died within hours, whereas the dogs treated with antibiotics and steroids had a complete recovery from shock, and survived more than 100 hours.9
During sepsis, endotoxins induce nitric oxide synthase, which produces relaxation of vascular smooth muscle tone, with resultant hypotension and reduced contractility response to norepinephrine.10 Corticosteroids prevent induction of nitric oxide synthase and enhance the vaso-active response to catecholamines through the glucocorticoid receptors. In vascular endothelial cells, glucocorticoids also inhibit serum phospholipase A2, reducing the production of vasodilators, such as prostacyclin and prostaglandin E1.11,12
Steroids reduce inflammation. Sepsis is driven by a systemic inflammatory response, in which components of the outer-cell membrane of both gram-positive and gram-negative bacteria and endotoxins induce the production of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-alpha) and interleukin-1 (IL-1).13 These cytokines have a direct toxic effect on various tissues. In addition, inflammatory cytokines suppress adrenal response to adrenocorticotropic hormone (ACTH), which results in decreased endogenous cortisol production, and compete with glucocorticoids for their receptors, inducing resistance to the action of steroids at the tissue level.14
In healthy volunteers challenged with bacterial endotoxins, low-dose steroids (~10 mg of prednisolone) suppress the release of proinflammatory cytokines and prevent the activation of endothelial cells and neutrophils.15 Steroids also inhibit the release of toxic enzymes, such as lysozymes and superoxides from neutrophils.16,17
Data Supporting Steroid Use in Septic Shock
Mortality data. Two major studies evaluated the effect of low-dose steroids in patients with septic shock. Annane and colleagues conducted a placebo-controlled, blinded trial and divided the study population into “responders” and “nonresponders” based on their response to ACTH stimulation test. Within the “nonresponder” group, steroids reduced the risk of mortality by 16% (63% mortality in the placebo group and 53% mortality in the corticosteroid group, P=0.02).16 Steroids also significantly reduced ICU mortality (70% versus 58%), hospital mortality (72% versus 61%) and one-year mortality (77% versus 68%) compared with placebo. No statistically significant difference in mortality between steroids and placebo was seen in the “responder” group.16
The CORTICUS trial, a multicenter, randomized, double-blind, placebo-controlled trial, showed no significant difference in 28-day mortality between those treated with corticosteroids (39.2%) and those receiving a placebo (36%, P=0.069).17 There was also no significant difference in either hospital or ICU mortality in this study.
A recent meta-analysis demonstrated no significant effect of corticosteroid treatment on 28-day mortality, ICU mortality, or hospital mortality in septic shock. However, subanalysis of trials using a prolonged course (>5 days) of low-dose steroids (300 mg of hydrocortisone or equivalent) showed a significant reduction in 28-day all-cause mortality (P=0.02) and hospital mortality (P =0.05), and a decrease in ICU length of stay.18
Reversal of shock. Various studies have shown a decrease in the time necessary to reverse septic shock with the use of low-dose steroids. Annane and colleagues showed the median time to vasopressor therapy withdrawal was seven days in the group treated with steroids versus nine days in the placebo group (P=0.01).16 The CORTICUS study demonstrated significantly shorter times to reversal of shock in the group treated with hydrocortisone compared with the placebo group—3.3 days versus 5.8 days (P<0.001).17 In a smaller study, 68% of hydrocortisone-treated patients achieved seven-day shock reversal compared with 21% in the placebo group, a difference of 47% in the rate of reversal of shock.18
Guidelines for the Use of Steroids in Septic Shock
In which sepsis patients should I use steroids? The large clinical trials that found a benefit to low-dose steroids included patients with a systolic blood pressure <90 mm/Hg for more than one hour, despite aggressive fluid and vasopressor therapy. Based on these and other smaller trials, the Surviving Sepsis Campaign recommends the addition of IV steroids to those patients with septic shock who don’t respond to adequate fluid and vasopressor resuscitation.19
Should I obtain ACTH stimulation test in these patients? Although the Annane study showed that “nonresponders”—those who did not achieve ≥9 mcg/dL increase in cortisol level after 30 to 60 minutes of ACTH administration—were more likely to benefit from steroids, the overall trial population appeared to benefit regardless of the ACTH response.16
Furthermore, the CORTICUS study showed no difference between the corticotropin responder and nonresponder group. Also, most cortisol immunoassays measure total cortisol (free and protein-bound), whereas the free cortisol level is the more clinically relevant measurement. Hence, current guidelines from the American College of Critical Care Medicine and Surviving Sepsis Campaign do not recommend performing an ACTH stimulation test prior to administration of steroids.20
What type of steroids should I use, and at what dose? Current guidelines recommend IV hydrocortisone in a dose of 200 mg/day to 300 mg/day given as 50 mg every six hours or 100 mg every eight hours for at least seven days before tapering. Alternatively, IV hydrocortisone can be given as a bolus of 100 mg followed by a continuous infusion at 10 mg/hr (240 mg/day). Hydrocortisone at this dose has intrinsic mineralocorticoid activity, obviating the need for adding fludrocortisone. Fludrocortisone may otherwise be added as 50 mcg daily, if using a corticosteroid without significant mineralocorticoid activity. Patients with septic shock should not be treated with dexamethasone, which causes immediate and prolonged suppression of the hypothalamic-pituitary-adrenal axis.21
Do I need to taper off the steroids? It is recommended to wean the steroids after seven or more days of use, when vasopressors are no longer required. Keh and colleagues noted a 30% recurrence of shock in patients when the steroids were not tapered.22 There was also evidence of immunologic rebound after abrupt cessation of steroids, with an increase in inflammatory markers.23 The taper should decrease the dose every two or three days in small steps.
What potential side effects should I be concerned about? Overall, the use of higher dose corticosteroids is associated with significant potential side effects, including a worsening of the underlying infection, new infection, hyperglycemia, hypernatremia, and gastrointestinal bleeding. In a meta-analysis of nine clinical trials with high-dose corticosteroids (a starting dose of ~30mg/kg/day of methylprednisolone), Cronin and colleagues found a trend toward increased mortality due to secondary infections (relative risk 1.70; 95% confidence interval, 0.70 to 4.12).24 A recent meta-analysis of 15 trials found low-dose corticosteroids reduced ICU mortality and increased the proportion of shock reversal by Day 7 and by Day 28 without increasing the rate of gastroduodenal bleeding, super-infection, or hyperglycemia.25
Back to the Case
Our patient was admitted to the medical ICU. After obtaining urine and blood cultures, she was started on IV levofloxacin. She remained hypotensive despite IV fluids and IV norepinephrine. She was started on IV hydrocortisone 50 mg every six hours. Over the next 48 hours, her hemodynamic parameters improved. Urine and blood cultures came back positive for E. coli. Her BUN and creatinine decreased to 24 mg/dL and 1.4 mg/dL, respectively.
Later, she was weaned off norepinephrine and transferred out of the ICU. On hospital Day 7, a slow taper of her hydrocortisone initiated, and antibiotics were switched to oral levofloxacin. She was later discharged home in stable condition.
Bottom Line
In patients with septic shock that is unresponsive to IV fluid resuscitation and vasopressors, the addition of low-dose corticosteroids is relatively safe and can improve rate of reversal of shock, reduce time to reversal of shock, decrease ICU length of stay, and potentially lower mortality.
Drs. Gandhi and Asudani are health science assistant professors of medicine in the Division of Hospital Medicine at the University of California at San Diego.
References
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530-538.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184:333-341.
- Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137-1143.
- The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317:659-665.
- Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653-658.
- Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294-1303.
- Cronin L, Cook DJ, Cartlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;24:1430-1439.
- Hinshaw LB, Beller BK, Chang AC, et al. Corticosteroid/antibiotic treatment of adrenalectomized dogs challenged with lethal E. coli. Circ Shock. 1985;16:265-277.
- Rees DD, Cellek S, Palmer RM, Moncada S. Dexamethasone prevents the induction of NO synthase and the associated effects on vascular tone, an insight into endotoxin shock. BioChem BioPhy Res Comm. 1990;173:541-547.
- Axelrod L. Inhibition of prostacyclin production mediates permissive effect of glucocorticoids on vascular tone. Lancet. 1983;1:904-906.
- Annane D, Bellissant E, Sebille V, et al. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenaline reserve. Br J Clin Pharmacol. 1991;46:589-597.
- DeKruif MD, Lemaire LL, Giebelen IA, et al. Prednisolone dose dependently influences inflammation and coagulation during human endotoxemia. J Immunol. 2007;178:1845-1851.
- Goldstein IM, Roos D, Weissman, G et al. Influence of corticosteroids on human polymorphonuclear leukocyte function in vitro. Inflammation. 1976;1:305-316.
- Briegel J, Kellerman W, Forst H, et al. Low-dose hydrocortisone infusion attenuates the SIRS. The Phospolipase A2 Study Group. Clin Invest. 1994;72:782-787.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisones on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy in patients with septic shock. N Engl J Med. 2008;358:111-124.
- Annane D, Bellissant E, Bollaert P. Corticosteroids in the treatment of severe sepsis and septic shock in adults. A systematic review. JAMA. 2009;301:2362-2375.
- Dellinger DP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17-60.
- Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937-1949.
- Reincke M, Allolio B, Würth G, et al. The hypothalamic-pituitary-adrenal axis in critical illness: Response to dexamethasone and corticotropin-releasing hormone. J Clin Endocrinol Metab. 1993;77:151-156
- Keh D, Weber-Carstens S, Ahlers O. Adjunctive therapies in severe sepsis and septic shock: current place of steroids. Curr Infect Dis Rep. 2008;10:354-361.
- Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low dose” hydrocortisone in septic shock: a double blind study, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512-520.
- Cronin L, Cook DJ, Carlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23;1430-1439.
- Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for treating severe sepsis and septic shock. Cochrane Database Syst Rev. 2004;1:CD002243.
The Case
An 81-year-old woman with diabetes mellitus presents with a three-day history of fever, chills, left-side flank pain, and dysuria. Her blood pressure upon presentation is 75/45 mm/Hg, her heart rate is 120 beats per minute, and she has a temperature of 103.1°`F and a respiratory rate of 22 breaths/minute. On physical examination, she is an ill-appearing elderly woman, with dry oral mucosa and left costo-vertebral angle tenderness. Lab work shows leukocytosis of 18,000 mg/dL with 88% polymorphonuclear leukocyte (PMN), the urine analysis is consistent with a urinary tract infection, and a chemistry panel reveals elevated BUN and creatinine levels of 52 mg/dL and 2.4 mg/dL, respectively. In the emergency department, she is given a bolus of 2 liters normal saline, but her blood pressure remains 78/49 mm/Hg. She is then started on broad-spectrum antibiotics and a norepinephrine drip, and is admitted to the ICU.
What role would steroids add to her management?
Background
Sepsis is the clinical syndrome defined by the presence of systemic inflammatory response syndrome (SIRS) in the setting of an infection. SIRS is defined by the presence of at least two of the following: fever or hypothermia; leukocytosis, leukopenia, or bandemia; heart rate >90 bpm; or tachypnea or hypocapnia.
When acute organ dysfunction, such as acute renal failure, altered mental status, or acute lung injury (hypoxemia), is present, sepsis is classified as severe.
Septic shock is a state of sepsis associated with acute circulatory collapse characterized by persistent arterial hypotension (defined as a systolic blood pressure <90 mmHg, a mean arterial pressure <60 mmHg, or a reduction in systolic blood pressure of >40 mmHg from baseline) despite fluid resuscitation attempts.1
The incidence and mortality due to sepsis and septic shock is directly related to the age of the patient, many of whom require ICU hospitalization.2 Clinically, this portends a great challenge, as the incidence of sepsis is likely to increase as the U.S. population ages.
Initial management of a patient with sepsis/septic shock is goal-directed therapy, which consists of early administration of broad-spectrum antibiotics, crystalloid or colloid fluid resuscitation, and use of vasopressor support to improve hemodynamics and maintain a mean arterial
pressure ≥65 mmHg. Patients with acute lung injury may also require prompt ventilator support.
The role of steroids in sepsis is controversial.
Review of Steroids
Steroids have long been known for their anti-inflammatory properties. From the 1950s to the 1980s, high-dose steroids (methylprednisolone 30mg/kg and dexamethasone 3 mg/kg to 6 mg/kg in divided doses) were used in the management of sepsis. This was based on a study by Schumer that showed steroids reduced mortality to 10% from 38%.3
Later, Sprung and colleagues demonstrated reversal of shock and improved short-term survival with high-dose steroids in patients with sepsis, but subsequent prospective randomized trials did not support this beneficial effect of high-dose steroids.4-6 In fact, two meta-analyses in 1995 concluded that high-dose steroids are ineffective and potentially harmful, and associated with higher mortality, secondary infections, and renal and hepatic dysfunction.7,8 Thereafter, the use of high-dose steroids fell into disfavor.
In the early 2000s, there was an emergence in the use of low-dose steroids in patients with sepsis. This was based on various trials showing the benefit of the use of low-dose steroids in the reversal of septic shock without significant side effects, discussed further below.
Pathophysiology
Steroids improve hemodynamic parameters. In an animal model, Hinshaw and colleagues induced septic shock in adrenalectomized dogs by infusing lethal doses of E. coli. The untreated dogs died within hours, whereas the dogs treated with antibiotics and steroids had a complete recovery from shock, and survived more than 100 hours.9
During sepsis, endotoxins induce nitric oxide synthase, which produces relaxation of vascular smooth muscle tone, with resultant hypotension and reduced contractility response to norepinephrine.10 Corticosteroids prevent induction of nitric oxide synthase and enhance the vaso-active response to catecholamines through the glucocorticoid receptors. In vascular endothelial cells, glucocorticoids also inhibit serum phospholipase A2, reducing the production of vasodilators, such as prostacyclin and prostaglandin E1.11,12
Steroids reduce inflammation. Sepsis is driven by a systemic inflammatory response, in which components of the outer-cell membrane of both gram-positive and gram-negative bacteria and endotoxins induce the production of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-alpha) and interleukin-1 (IL-1).13 These cytokines have a direct toxic effect on various tissues. In addition, inflammatory cytokines suppress adrenal response to adrenocorticotropic hormone (ACTH), which results in decreased endogenous cortisol production, and compete with glucocorticoids for their receptors, inducing resistance to the action of steroids at the tissue level.14
In healthy volunteers challenged with bacterial endotoxins, low-dose steroids (~10 mg of prednisolone) suppress the release of proinflammatory cytokines and prevent the activation of endothelial cells and neutrophils.15 Steroids also inhibit the release of toxic enzymes, such as lysozymes and superoxides from neutrophils.16,17
Data Supporting Steroid Use in Septic Shock
Mortality data. Two major studies evaluated the effect of low-dose steroids in patients with septic shock. Annane and colleagues conducted a placebo-controlled, blinded trial and divided the study population into “responders” and “nonresponders” based on their response to ACTH stimulation test. Within the “nonresponder” group, steroids reduced the risk of mortality by 16% (63% mortality in the placebo group and 53% mortality in the corticosteroid group, P=0.02).16 Steroids also significantly reduced ICU mortality (70% versus 58%), hospital mortality (72% versus 61%) and one-year mortality (77% versus 68%) compared with placebo. No statistically significant difference in mortality between steroids and placebo was seen in the “responder” group.16
The CORTICUS trial, a multicenter, randomized, double-blind, placebo-controlled trial, showed no significant difference in 28-day mortality between those treated with corticosteroids (39.2%) and those receiving a placebo (36%, P=0.069).17 There was also no significant difference in either hospital or ICU mortality in this study.
A recent meta-analysis demonstrated no significant effect of corticosteroid treatment on 28-day mortality, ICU mortality, or hospital mortality in septic shock. However, subanalysis of trials using a prolonged course (>5 days) of low-dose steroids (300 mg of hydrocortisone or equivalent) showed a significant reduction in 28-day all-cause mortality (P=0.02) and hospital mortality (P =0.05), and a decrease in ICU length of stay.18
Reversal of shock. Various studies have shown a decrease in the time necessary to reverse septic shock with the use of low-dose steroids. Annane and colleagues showed the median time to vasopressor therapy withdrawal was seven days in the group treated with steroids versus nine days in the placebo group (P=0.01).16 The CORTICUS study demonstrated significantly shorter times to reversal of shock in the group treated with hydrocortisone compared with the placebo group—3.3 days versus 5.8 days (P<0.001).17 In a smaller study, 68% of hydrocortisone-treated patients achieved seven-day shock reversal compared with 21% in the placebo group, a difference of 47% in the rate of reversal of shock.18
Guidelines for the Use of Steroids in Septic Shock
In which sepsis patients should I use steroids? The large clinical trials that found a benefit to low-dose steroids included patients with a systolic blood pressure <90 mm/Hg for more than one hour, despite aggressive fluid and vasopressor therapy. Based on these and other smaller trials, the Surviving Sepsis Campaign recommends the addition of IV steroids to those patients with septic shock who don’t respond to adequate fluid and vasopressor resuscitation.19
Should I obtain ACTH stimulation test in these patients? Although the Annane study showed that “nonresponders”—those who did not achieve ≥9 mcg/dL increase in cortisol level after 30 to 60 minutes of ACTH administration—were more likely to benefit from steroids, the overall trial population appeared to benefit regardless of the ACTH response.16
Furthermore, the CORTICUS study showed no difference between the corticotropin responder and nonresponder group. Also, most cortisol immunoassays measure total cortisol (free and protein-bound), whereas the free cortisol level is the more clinically relevant measurement. Hence, current guidelines from the American College of Critical Care Medicine and Surviving Sepsis Campaign do not recommend performing an ACTH stimulation test prior to administration of steroids.20
What type of steroids should I use, and at what dose? Current guidelines recommend IV hydrocortisone in a dose of 200 mg/day to 300 mg/day given as 50 mg every six hours or 100 mg every eight hours for at least seven days before tapering. Alternatively, IV hydrocortisone can be given as a bolus of 100 mg followed by a continuous infusion at 10 mg/hr (240 mg/day). Hydrocortisone at this dose has intrinsic mineralocorticoid activity, obviating the need for adding fludrocortisone. Fludrocortisone may otherwise be added as 50 mcg daily, if using a corticosteroid without significant mineralocorticoid activity. Patients with septic shock should not be treated with dexamethasone, which causes immediate and prolonged suppression of the hypothalamic-pituitary-adrenal axis.21
Do I need to taper off the steroids? It is recommended to wean the steroids after seven or more days of use, when vasopressors are no longer required. Keh and colleagues noted a 30% recurrence of shock in patients when the steroids were not tapered.22 There was also evidence of immunologic rebound after abrupt cessation of steroids, with an increase in inflammatory markers.23 The taper should decrease the dose every two or three days in small steps.
What potential side effects should I be concerned about? Overall, the use of higher dose corticosteroids is associated with significant potential side effects, including a worsening of the underlying infection, new infection, hyperglycemia, hypernatremia, and gastrointestinal bleeding. In a meta-analysis of nine clinical trials with high-dose corticosteroids (a starting dose of ~30mg/kg/day of methylprednisolone), Cronin and colleagues found a trend toward increased mortality due to secondary infections (relative risk 1.70; 95% confidence interval, 0.70 to 4.12).24 A recent meta-analysis of 15 trials found low-dose corticosteroids reduced ICU mortality and increased the proportion of shock reversal by Day 7 and by Day 28 without increasing the rate of gastroduodenal bleeding, super-infection, or hyperglycemia.25
Back to the Case
Our patient was admitted to the medical ICU. After obtaining urine and blood cultures, she was started on IV levofloxacin. She remained hypotensive despite IV fluids and IV norepinephrine. She was started on IV hydrocortisone 50 mg every six hours. Over the next 48 hours, her hemodynamic parameters improved. Urine and blood cultures came back positive for E. coli. Her BUN and creatinine decreased to 24 mg/dL and 1.4 mg/dL, respectively.
Later, she was weaned off norepinephrine and transferred out of the ICU. On hospital Day 7, a slow taper of her hydrocortisone initiated, and antibiotics were switched to oral levofloxacin. She was later discharged home in stable condition.
Bottom Line
In patients with septic shock that is unresponsive to IV fluid resuscitation and vasopressors, the addition of low-dose corticosteroids is relatively safe and can improve rate of reversal of shock, reduce time to reversal of shock, decrease ICU length of stay, and potentially lower mortality.
Drs. Gandhi and Asudani are health science assistant professors of medicine in the Division of Hospital Medicine at the University of California at San Diego.
References
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530-538.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184:333-341.
- Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137-1143.
- The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317:659-665.
- Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653-658.
- Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294-1303.
- Cronin L, Cook DJ, Cartlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;24:1430-1439.
- Hinshaw LB, Beller BK, Chang AC, et al. Corticosteroid/antibiotic treatment of adrenalectomized dogs challenged with lethal E. coli. Circ Shock. 1985;16:265-277.
- Rees DD, Cellek S, Palmer RM, Moncada S. Dexamethasone prevents the induction of NO synthase and the associated effects on vascular tone, an insight into endotoxin shock. BioChem BioPhy Res Comm. 1990;173:541-547.
- Axelrod L. Inhibition of prostacyclin production mediates permissive effect of glucocorticoids on vascular tone. Lancet. 1983;1:904-906.
- Annane D, Bellissant E, Sebille V, et al. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenaline reserve. Br J Clin Pharmacol. 1991;46:589-597.
- DeKruif MD, Lemaire LL, Giebelen IA, et al. Prednisolone dose dependently influences inflammation and coagulation during human endotoxemia. J Immunol. 2007;178:1845-1851.
- Goldstein IM, Roos D, Weissman, G et al. Influence of corticosteroids on human polymorphonuclear leukocyte function in vitro. Inflammation. 1976;1:305-316.
- Briegel J, Kellerman W, Forst H, et al. Low-dose hydrocortisone infusion attenuates the SIRS. The Phospolipase A2 Study Group. Clin Invest. 1994;72:782-787.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisones on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy in patients with septic shock. N Engl J Med. 2008;358:111-124.
- Annane D, Bellissant E, Bollaert P. Corticosteroids in the treatment of severe sepsis and septic shock in adults. A systematic review. JAMA. 2009;301:2362-2375.
- Dellinger DP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17-60.
- Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937-1949.
- Reincke M, Allolio B, Würth G, et al. The hypothalamic-pituitary-adrenal axis in critical illness: Response to dexamethasone and corticotropin-releasing hormone. J Clin Endocrinol Metab. 1993;77:151-156
- Keh D, Weber-Carstens S, Ahlers O. Adjunctive therapies in severe sepsis and septic shock: current place of steroids. Curr Infect Dis Rep. 2008;10:354-361.
- Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low dose” hydrocortisone in septic shock: a double blind study, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512-520.
- Cronin L, Cook DJ, Carlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23;1430-1439.
- Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for treating severe sepsis and septic shock. Cochrane Database Syst Rev. 2004;1:CD002243.
The Case
An 81-year-old woman with diabetes mellitus presents with a three-day history of fever, chills, left-side flank pain, and dysuria. Her blood pressure upon presentation is 75/45 mm/Hg, her heart rate is 120 beats per minute, and she has a temperature of 103.1°`F and a respiratory rate of 22 breaths/minute. On physical examination, she is an ill-appearing elderly woman, with dry oral mucosa and left costo-vertebral angle tenderness. Lab work shows leukocytosis of 18,000 mg/dL with 88% polymorphonuclear leukocyte (PMN), the urine analysis is consistent with a urinary tract infection, and a chemistry panel reveals elevated BUN and creatinine levels of 52 mg/dL and 2.4 mg/dL, respectively. In the emergency department, she is given a bolus of 2 liters normal saline, but her blood pressure remains 78/49 mm/Hg. She is then started on broad-spectrum antibiotics and a norepinephrine drip, and is admitted to the ICU.
What role would steroids add to her management?
Background
Sepsis is the clinical syndrome defined by the presence of systemic inflammatory response syndrome (SIRS) in the setting of an infection. SIRS is defined by the presence of at least two of the following: fever or hypothermia; leukocytosis, leukopenia, or bandemia; heart rate >90 bpm; or tachypnea or hypocapnia.
When acute organ dysfunction, such as acute renal failure, altered mental status, or acute lung injury (hypoxemia), is present, sepsis is classified as severe.
Septic shock is a state of sepsis associated with acute circulatory collapse characterized by persistent arterial hypotension (defined as a systolic blood pressure <90 mmHg, a mean arterial pressure <60 mmHg, or a reduction in systolic blood pressure of >40 mmHg from baseline) despite fluid resuscitation attempts.1
The incidence and mortality due to sepsis and septic shock is directly related to the age of the patient, many of whom require ICU hospitalization.2 Clinically, this portends a great challenge, as the incidence of sepsis is likely to increase as the U.S. population ages.
Initial management of a patient with sepsis/septic shock is goal-directed therapy, which consists of early administration of broad-spectrum antibiotics, crystalloid or colloid fluid resuscitation, and use of vasopressor support to improve hemodynamics and maintain a mean arterial
pressure ≥65 mmHg. Patients with acute lung injury may also require prompt ventilator support.
The role of steroids in sepsis is controversial.
Review of Steroids
Steroids have long been known for their anti-inflammatory properties. From the 1950s to the 1980s, high-dose steroids (methylprednisolone 30mg/kg and dexamethasone 3 mg/kg to 6 mg/kg in divided doses) were used in the management of sepsis. This was based on a study by Schumer that showed steroids reduced mortality to 10% from 38%.3
Later, Sprung and colleagues demonstrated reversal of shock and improved short-term survival with high-dose steroids in patients with sepsis, but subsequent prospective randomized trials did not support this beneficial effect of high-dose steroids.4-6 In fact, two meta-analyses in 1995 concluded that high-dose steroids are ineffective and potentially harmful, and associated with higher mortality, secondary infections, and renal and hepatic dysfunction.7,8 Thereafter, the use of high-dose steroids fell into disfavor.
In the early 2000s, there was an emergence in the use of low-dose steroids in patients with sepsis. This was based on various trials showing the benefit of the use of low-dose steroids in the reversal of septic shock without significant side effects, discussed further below.
Pathophysiology
Steroids improve hemodynamic parameters. In an animal model, Hinshaw and colleagues induced septic shock in adrenalectomized dogs by infusing lethal doses of E. coli. The untreated dogs died within hours, whereas the dogs treated with antibiotics and steroids had a complete recovery from shock, and survived more than 100 hours.9
During sepsis, endotoxins induce nitric oxide synthase, which produces relaxation of vascular smooth muscle tone, with resultant hypotension and reduced contractility response to norepinephrine.10 Corticosteroids prevent induction of nitric oxide synthase and enhance the vaso-active response to catecholamines through the glucocorticoid receptors. In vascular endothelial cells, glucocorticoids also inhibit serum phospholipase A2, reducing the production of vasodilators, such as prostacyclin and prostaglandin E1.11,12
Steroids reduce inflammation. Sepsis is driven by a systemic inflammatory response, in which components of the outer-cell membrane of both gram-positive and gram-negative bacteria and endotoxins induce the production of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-alpha) and interleukin-1 (IL-1).13 These cytokines have a direct toxic effect on various tissues. In addition, inflammatory cytokines suppress adrenal response to adrenocorticotropic hormone (ACTH), which results in decreased endogenous cortisol production, and compete with glucocorticoids for their receptors, inducing resistance to the action of steroids at the tissue level.14
In healthy volunteers challenged with bacterial endotoxins, low-dose steroids (~10 mg of prednisolone) suppress the release of proinflammatory cytokines and prevent the activation of endothelial cells and neutrophils.15 Steroids also inhibit the release of toxic enzymes, such as lysozymes and superoxides from neutrophils.16,17
Data Supporting Steroid Use in Septic Shock
Mortality data. Two major studies evaluated the effect of low-dose steroids in patients with septic shock. Annane and colleagues conducted a placebo-controlled, blinded trial and divided the study population into “responders” and “nonresponders” based on their response to ACTH stimulation test. Within the “nonresponder” group, steroids reduced the risk of mortality by 16% (63% mortality in the placebo group and 53% mortality in the corticosteroid group, P=0.02).16 Steroids also significantly reduced ICU mortality (70% versus 58%), hospital mortality (72% versus 61%) and one-year mortality (77% versus 68%) compared with placebo. No statistically significant difference in mortality between steroids and placebo was seen in the “responder” group.16
The CORTICUS trial, a multicenter, randomized, double-blind, placebo-controlled trial, showed no significant difference in 28-day mortality between those treated with corticosteroids (39.2%) and those receiving a placebo (36%, P=0.069).17 There was also no significant difference in either hospital or ICU mortality in this study.
A recent meta-analysis demonstrated no significant effect of corticosteroid treatment on 28-day mortality, ICU mortality, or hospital mortality in septic shock. However, subanalysis of trials using a prolonged course (>5 days) of low-dose steroids (300 mg of hydrocortisone or equivalent) showed a significant reduction in 28-day all-cause mortality (P=0.02) and hospital mortality (P =0.05), and a decrease in ICU length of stay.18
Reversal of shock. Various studies have shown a decrease in the time necessary to reverse septic shock with the use of low-dose steroids. Annane and colleagues showed the median time to vasopressor therapy withdrawal was seven days in the group treated with steroids versus nine days in the placebo group (P=0.01).16 The CORTICUS study demonstrated significantly shorter times to reversal of shock in the group treated with hydrocortisone compared with the placebo group—3.3 days versus 5.8 days (P<0.001).17 In a smaller study, 68% of hydrocortisone-treated patients achieved seven-day shock reversal compared with 21% in the placebo group, a difference of 47% in the rate of reversal of shock.18
Guidelines for the Use of Steroids in Septic Shock
In which sepsis patients should I use steroids? The large clinical trials that found a benefit to low-dose steroids included patients with a systolic blood pressure <90 mm/Hg for more than one hour, despite aggressive fluid and vasopressor therapy. Based on these and other smaller trials, the Surviving Sepsis Campaign recommends the addition of IV steroids to those patients with septic shock who don’t respond to adequate fluid and vasopressor resuscitation.19
Should I obtain ACTH stimulation test in these patients? Although the Annane study showed that “nonresponders”—those who did not achieve ≥9 mcg/dL increase in cortisol level after 30 to 60 minutes of ACTH administration—were more likely to benefit from steroids, the overall trial population appeared to benefit regardless of the ACTH response.16
Furthermore, the CORTICUS study showed no difference between the corticotropin responder and nonresponder group. Also, most cortisol immunoassays measure total cortisol (free and protein-bound), whereas the free cortisol level is the more clinically relevant measurement. Hence, current guidelines from the American College of Critical Care Medicine and Surviving Sepsis Campaign do not recommend performing an ACTH stimulation test prior to administration of steroids.20
What type of steroids should I use, and at what dose? Current guidelines recommend IV hydrocortisone in a dose of 200 mg/day to 300 mg/day given as 50 mg every six hours or 100 mg every eight hours for at least seven days before tapering. Alternatively, IV hydrocortisone can be given as a bolus of 100 mg followed by a continuous infusion at 10 mg/hr (240 mg/day). Hydrocortisone at this dose has intrinsic mineralocorticoid activity, obviating the need for adding fludrocortisone. Fludrocortisone may otherwise be added as 50 mcg daily, if using a corticosteroid without significant mineralocorticoid activity. Patients with septic shock should not be treated with dexamethasone, which causes immediate and prolonged suppression of the hypothalamic-pituitary-adrenal axis.21
Do I need to taper off the steroids? It is recommended to wean the steroids after seven or more days of use, when vasopressors are no longer required. Keh and colleagues noted a 30% recurrence of shock in patients when the steroids were not tapered.22 There was also evidence of immunologic rebound after abrupt cessation of steroids, with an increase in inflammatory markers.23 The taper should decrease the dose every two or three days in small steps.
What potential side effects should I be concerned about? Overall, the use of higher dose corticosteroids is associated with significant potential side effects, including a worsening of the underlying infection, new infection, hyperglycemia, hypernatremia, and gastrointestinal bleeding. In a meta-analysis of nine clinical trials with high-dose corticosteroids (a starting dose of ~30mg/kg/day of methylprednisolone), Cronin and colleagues found a trend toward increased mortality due to secondary infections (relative risk 1.70; 95% confidence interval, 0.70 to 4.12).24 A recent meta-analysis of 15 trials found low-dose corticosteroids reduced ICU mortality and increased the proportion of shock reversal by Day 7 and by Day 28 without increasing the rate of gastroduodenal bleeding, super-infection, or hyperglycemia.25
Back to the Case
Our patient was admitted to the medical ICU. After obtaining urine and blood cultures, she was started on IV levofloxacin. She remained hypotensive despite IV fluids and IV norepinephrine. She was started on IV hydrocortisone 50 mg every six hours. Over the next 48 hours, her hemodynamic parameters improved. Urine and blood cultures came back positive for E. coli. Her BUN and creatinine decreased to 24 mg/dL and 1.4 mg/dL, respectively.
Later, she was weaned off norepinephrine and transferred out of the ICU. On hospital Day 7, a slow taper of her hydrocortisone initiated, and antibiotics were switched to oral levofloxacin. She was later discharged home in stable condition.
Bottom Line
In patients with septic shock that is unresponsive to IV fluid resuscitation and vasopressors, the addition of low-dose corticosteroids is relatively safe and can improve rate of reversal of shock, reduce time to reversal of shock, decrease ICU length of stay, and potentially lower mortality.
Drs. Gandhi and Asudani are health science assistant professors of medicine in the Division of Hospital Medicine at the University of California at San Diego.
References
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530-538.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184:333-341.
- Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137-1143.
- The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317:659-665.
- Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653-658.
- Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294-1303.
- Cronin L, Cook DJ, Cartlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;24:1430-1439.
- Hinshaw LB, Beller BK, Chang AC, et al. Corticosteroid/antibiotic treatment of adrenalectomized dogs challenged with lethal E. coli. Circ Shock. 1985;16:265-277.
- Rees DD, Cellek S, Palmer RM, Moncada S. Dexamethasone prevents the induction of NO synthase and the associated effects on vascular tone, an insight into endotoxin shock. BioChem BioPhy Res Comm. 1990;173:541-547.
- Axelrod L. Inhibition of prostacyclin production mediates permissive effect of glucocorticoids on vascular tone. Lancet. 1983;1:904-906.
- Annane D, Bellissant E, Sebille V, et al. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenaline reserve. Br J Clin Pharmacol. 1991;46:589-597.
- DeKruif MD, Lemaire LL, Giebelen IA, et al. Prednisolone dose dependently influences inflammation and coagulation during human endotoxemia. J Immunol. 2007;178:1845-1851.
- Goldstein IM, Roos D, Weissman, G et al. Influence of corticosteroids on human polymorphonuclear leukocyte function in vitro. Inflammation. 1976;1:305-316.
- Briegel J, Kellerman W, Forst H, et al. Low-dose hydrocortisone infusion attenuates the SIRS. The Phospolipase A2 Study Group. Clin Invest. 1994;72:782-787.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisones on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy in patients with septic shock. N Engl J Med. 2008;358:111-124.
- Annane D, Bellissant E, Bollaert P. Corticosteroids in the treatment of severe sepsis and septic shock in adults. A systematic review. JAMA. 2009;301:2362-2375.
- Dellinger DP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17-60.
- Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937-1949.
- Reincke M, Allolio B, Würth G, et al. The hypothalamic-pituitary-adrenal axis in critical illness: Response to dexamethasone and corticotropin-releasing hormone. J Clin Endocrinol Metab. 1993;77:151-156
- Keh D, Weber-Carstens S, Ahlers O. Adjunctive therapies in severe sepsis and septic shock: current place of steroids. Curr Infect Dis Rep. 2008;10:354-361.
- Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low dose” hydrocortisone in septic shock: a double blind study, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512-520.
- Cronin L, Cook DJ, Carlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23;1430-1439.
- Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for treating severe sepsis and septic shock. Cochrane Database Syst Rev. 2004;1:CD002243.
ITL: Physician Reviews of HM-Relevant Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Initial trophic feedings effective for patients with acute lung injury
- IM vs. IV benzodiazepines in status epilepticus
- CDI risk following antibiotic cessation
- Acid suppression associated with increased complications in CDI patients
- Perioperative statins and cardiac events in surgical patients
- Enoxaparin vs. unfractionated heparin during PCI
- Optimal serum potassium levels for AMI patients
- PPIs superior to H2-blockers for lowering UGI bleeding following ACS and STEMI
Initial Lower-Volume Enteral Feeding Better Tolerated, but Has No Mortality Benefit
Clinical question: In mechanically ventilated patients with acute lung injury, do initial lower-volume enteral feedings (trophic feedings) improve clinical outcomes when compared with full enteral feedings?
Background: Malnutrition in critically ill patients is associated with poor outcomes, but conflicting data exist regarding the best timing, amount, and formulation of enteral nutrition to initiate. Initiation of lower-volume enteral feeding with periodic assessment of gastric residual volume is common practice, but the effects of this practice are unknown.
Study design: Multicenter randomized controlled open-label study.
Setting: Forty-four hospitals in the National Heart, Lung, and Blood Institute (NHLBI) Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network.
Synopsis: One thousand patients with acute lung injury receiving mechanical ventilation for longer than 72 hours were randomized using a Web-based system to receive either trophic or full enteric feedings for the first six days of mechanical ventilation. After the sixth day, a full enteric feeding protocol was used in all patients. All analyses were by intention-to-treat.
There was no significant difference between the trophic and full enteral feeding groups with regard to the primary outcome of ventilator-free days through Day 28 (14.9% vs. 15.0%, P=0.89). There were also no significant differences between groups in secondary outcomes, which included 60-day mortality, ICU-free days, organ-failure-free days, or the incidence of new infections. However, gastrointestinal intolerances occurred less often in the trophic feeding group, and these patients received fewer anti-diarrheal and prokinetic agents. The full feeding group gained 2.1 liters of fluid by Day 7, but this fluid gain did not cause significant differences in measures of circulatory or pulmonary physiology.
Limitations include open-label study design and inclusion of only critically ill adult medical patients with acute lung injury.
Bottom line: Initial lower-volume tube feedings in mechanically ventilated patients with acute lung injury did not improve clinical outcomes compared with full enteral feedings, but they were associated with fewer instances of gastrointestinal intolerance.
Citation: National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795-803.
Intramuscular Benzodiazepines as Good as IV Benzodiazepines in Status Epilepticus
Clinical question: Is intramuscular (IM) midazolam noninferior to intravenous lorazepam in patients in status epilepticus?
Background: Studies have shown that IV benzodiazepines, particularly lorazepam, are effective for patients in status epilepticus. Studies have not evaluated IM benzodiazepines. However, many emergency medical service (EMS) agencies use IM midazolam because IM administration is easier than IV administration, and midazolam has a longer nonrefrigerated shelf life than lorazepam does.
Study design: Randomized, double-blinded clinical trial.
Setting: Thirty-three EMS agencies across the United States.
Synopsis: Based on the 893 adults and children in status epilepticus included in this double-blind study, the researchers found IM midazolam to be noninferior to, and in fact superior to, IV lorazepam for treating seizures prior to arrival at EDs. Specifically, they found 10% more (95% CI 4.0% to 16.1%; P<0.001 for noninferiority and P<0.001 for superiority) seizure-free patients arriving at EDs when IM midazolam was administered. Seizures ceased in patients given IM midazolam in less time on average than it took for paramedics to administer IV lorazepam.
Hospitalists might be less inclined than EMS personnel to use IM midazolam because their patients have established IV access and refrigerated drugs are readily available. However, since IM midazolam was superior to IV lorazepam in this prehospital study, a similar trial in hospitalized patients is warranted. In subsequent trials, it would be useful to administer both midazolam and lorazepam via IM and IV routes, and IM injections by an autoinjector could be compared with the conventional manner.
Bottom line: In the prehospital setting, IM midazolam is at least as good as IV lorazepam in treating status epilepticus in children and adults.
Citation: Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591-600.
Clostridium Difficile Infection Risk Remains High at Least Three Months after Antibiotics Are Stopped
Clinical question: How long are patients at higher risk for Clostridium difficile infection (CDI) after completing antibiotics?
Background: Studies have shown that patients given antibiotics are at higher risk for CDI than those who are not, particularly if they take multiple antibiotics at high doses for a prolonged period of time. However, it is not known how long a patient remains at high risk for CDI after completing antibiotic therapy.
Study design: Case-control study.
Setting: Nine hospitals in Netherlands.
Synopsis: The study compared 337 hospitalized patients who had CDI with 337 nondiarrheal controls and 227 non-CDI diarrheal controls. The study showed a seven- to tenfold increased risk for CDI during antibiotic treatment and in the 30 days following cessation of antibiotics. A 2.7-fold increased risk of CDI was seen in the one- to three-month period after antibiotic treatment was stopped.
Because researchers only obtained information about antibiotic use in the three months preceding the onset of diarrhea, it is unknown if CDI risk remains elevated longer than three months after antibiotics are stopped. Also of note, the enzyme immunoassays used in this study to diagnose C. diff had sensitivities of between 60% and 85%.
As hospitalists, this information can be used to advise patients about their continued risk for CDI after cessation of antibiotics. Further studies are needed to determine the risk after three months.
Bottom line: Patients are at highest risk for CDI up to one month after stopping antibiotics but continue to be at higher risk for at least two additional months.
Citation: Hensgens MPN, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67:742-748.
Acid Suppression and Poor Outcomes in C. Diff Patients
Clinical question: What are potential modifiable risk factors associated with increased complications and mortality in patients hospitalized with C. diff infection (CDI)?
Background: CDI is a growing cause of morbidity and mortality in hospitalized patients. Evidence is growing for the association of acid suppression, among other modifiable risk factors, with complications and mortality in patients with CDI.
Study design: Retrospective case review.
Setting: Naval medical center.
Synopsis: A laboratory, medical record, and pharmacy database query found 485 patients with CDI. Complications of CDI were defined as ICU admission, surgery, and megacolon. Factors significantly associated with CDI complications and mortality were admission for CDI, corticosteroid use >5 mg per day, age ≥80 years, and prescription acid suppression (including H2-blockers and proton-pump inhibitors).
The latter two risk factors were associated with mortality alone. In multivariable regression, the odds of mortality among patients on acid suppression was more than four times the odds of those not on acid suppression (OR 4.74, 95% CI, 1.57 to 14.36).
Although this is a retrospective cohort study and cannot prove a causal relationship, the data add to a growing body of evidence supporting the risk of CDI complications and mortality for those on acid suppression.
Bottom line: In hospitalized patients with CDI, acid suppression is associated with increased complications and mortality and should be discontinued in this population when possible.
Citation: Morrison RH, Hall NS, Said M, et al. Risk factors associated with complications and mortality in patients with Clostridium difficile infection. Clin Infect Dis. 2011;53:1173-1178.
Perioperative Statins Reduce Cardiac Events for Surgical Patients
Clinical question: Does perioperative statin use improve cardiac outcomes (death, myocardial infarction, atrial fibrillation, and ICU and hospital lengths of stay) in statin-naive patients undergoing cardiac or non-cardiac surgery?
Background: Statins have been hypothesized to reduce perioperative cardiac complications because they reduce vascular and systemic inflammation caused by surgery, and several meta-analyses have demonstrated their efficacy. To date, no meta-analyses have specifically evaluated the benefits of perioperative statins in non-cardiac surgery from randomized controlled trials.
Study design: Systematic review of the literature and meta-analysis.
Setting: Fifteen randomized controlled trials of hospitalized surgical patients.
Synopsis: A systematic review examined the effects of statins on a variety of perioperative cardiac outcomes (death, myocardial infarction, atrial fibrillation, and ICU and hospital lengths of stay); 11 of the 15 patients were undergoing cardiac surgery.
Perioperative statins decreased the risk of atrial fibrillation in patients undergoing cardiac surgery (RR 0.56; 95% CI 0.45-0.69; number needed to treat [NNT], 6). In both cardiac and non-cardiac surgical patients, statins reduced the risk of myocardial infarction (RR 0.53; 95% CI 0.38 to 0.74; NNT 23). Statin treatment also reduced the mean length of hospital stay (in days, mean difference -0.32; 95% CI, -0.53 to -0.11) but did not reduce the length of ICU stay (mean difference -0.08; 95% CI, -0.25 to 0.10). Risk of death was not reduced with statin treatment (RR 0.62; 95% CI, 0.34 to 1.14).
Bottom line: Perioperative statins reduce the risk of postoperative atrial fibrillation in cardiac surgical patients, the risk of postoperative MI in cardiac and non-cardiac surgical patients, and the mean length of hospital stay.
Citation: Chopra V, Wesorick DH, Sussman JB, et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta-analysis. Arch Surg. 2012;147:181-188.
Enoxaparin Safe and Effective during Percutaneous Coronary Intervention
Clinical question: Is enoxaparin safe and efficacious compared with unfractionated heparin during percutaneous coronary intervention (PCI)?
Background: Despite problems with the use of unfractionated heparin during PCI, current guidelines give it a Class 1 recommendation for PCI in ST-elevation myocardial infarction (MI). There is growing evidence that enoxaparin can provide predictable, effective anticoagulation during PCI. Although several trials have examined this issue, none have been sufficiently powered to evaluate mortality.
Study design: Systematic review and meta-analysis.
Setting: Twenty-three trials or registries of patients undergoing PCI.
Synopsis: A systematic review found 23 trials representing 30,966 patients that examined the effects of enoxaparin versus unfractionated heparin on risk of mortality, MI, complications of MI, and major bleeding. Of these, 10,243 (33.1%) patients underwent primary PCI for ST elevation MI, 8,750 (28.2%) underwent PCI after fibrinolysis, and 11,973 (38.7%) patients had either scheduled PCI or PCI for non-ST elevation acute coronary syndrome.
Of all the patients, 13,943 (45%) received enoxaparin and 17,023 (55%) received unfractionated heparin. Enoxaparin was associated with significant reductions in all-cause mortality (RR 0.66; 95% CI, 0.57 to 0.76), composite of death or MI (RR 0.68; 95% CI, 0.57 to 0.81), and complications of MI (RR 0.75; 95% CI, 0.6 to 0.85). For patients who received primary PCI for ST elevation MI, enoxaparin reduced the risk of complications of MI by 44% (RR 0.56; 95% CI, 0.42 to 0.76). Enoxaparin also reduced the risk of major bleeding (RR 0.80, 95% CI, 0.68 to 0.95) with even more striking results for the 14 studies that compared intravenous enoxaparin to unfractionated heparin (RR 0.66; 95% CI, 0.52 to 0.83).
Bottom line: Enoxaparin is safe and efficacious when used during PCI. Patients who receive enoxaparin during PCI have reduced risk for death, MI, complications of MI, and major bleeding when compared with patients who receive unfractionated heparin.
Citation: Silvain J, Beygui F, Barthelmy O, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012;344:e553.
Serum Potassium Levels and Mortality in Acute Myocardial Infarction
Clinical question: What is the relationship between serum potassium levels and mortality in acute myocardial infarction (AMI) patients?
Background: Several smaller studies in the pre-beta-blocker and pre-reperfusion era recommended maintaining serum potassium levels between 4.0 mEq/L and 5.0 mEq/L in AMI patients. However, current studies examining the relationship between potassium levels and mortality in AMI patients are lacking.
Study design: Retrospective cohort study.
Setting: Multicenter study involving 67 hospitals in the U.S.
Synopsis: Using the Cerner Health Facts database, which included 38,689 patients with biomarker-confirmed AMI, this study showed there was a U-shaped relationship between mean post-admission serum potassium level and in-hospital mortality.
Compared with the reference group of 3.5 mEq/L to less than 4.0 mEq/L (mortality rate 4.8%; 95% CI, 4.4% to 5.2%), mortality was comparable for those with mean post-admission potassium of 4.0 mEq/L to less than 4.5 mEq/L (5.0%; 95% CI, 4.7% to 5.3%). Mortality was twice as great for potassium of 4.5 mEq/L to less than 5.0 mEq/L (10.0%; 95% CI, 9.1% to 10.9%), and even greater for higher potassium strata. Similarly, mortality rates were higher for potassium levels of less than 3.5 mEq/L. Rates of ventricular fibrillation or cardiac arrest were higher among patients with potassium levels of less than 3.0 mEq/L or more than 5.0 mEq/L.
Bottom line: For inpatients with AMI, serum potassium levels of 3.5 mEq/L to 4.5 mEq/L should be maintained for the best outcomes. Repletion of serum potassium to levels greater than 4.5 mEq/L is associated with increased mortality and should be avoided.
Citation: Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157-164.
Proton-Pump Inhibitors Better than H2-Blockers in ACS and STEMI
Clinical question: Are proton-pump inhibitors (PPIs) better than H2-blockers at preventing UGI bleeding in patients after acute coronary syndrome (ACS) or ST-elevation myocardial infarction (STEMI)?
Background: It is not definitively known if PPIs are the same or better than H2-blockers in preventing UGI bleeding in patients on high-risk medications (aspirin, clopidogrel, and anticoagulants) after ACS or STEMI.
Study design: Randomized double-blinded controlled trial.
Setting: Single hospital.
Synopsis: Patients with ACS or STEMI were treated with aspirin, clopidogrel, and enoxaparin or thrombolytics. They were then randomized to either esomeprazole 20 mg or famotidine 40 mg, both administered nightly. They were followed throughout their hospital stay and were then followed between four and 52 weeks after discharge (mean duration: 19 weeks for esomeprazole, 18 weeks for famotidine). The primary end point was time to a composite outcome, consisting of UGI bleeding, obstruction, or perforation. Overall, 313 patients were randomized (164 to esomeprazole and 149 to famotidine).
The treatment groups were equivalent in baseline characteristics, and compliance in both groups was excellent (>98%). The primary endpoint occurred in three patients in the esomeprazole group and in 12 patients in the famotidine group (hazard ratio 0.21; 95% CI, 0.06 to 0.75; P=0.008).
Bottom line: PPIs are superior to H2-blockers in reducing UGI bleeding in patients on high-risk medications (aspirin, clopidogrel, and enoxaparin or thrombolytics) after ACS and STEMI. This confirms the recommendations of the 2010 ACCF/ACG/AHA Expert Consensus that PPIs should be used in those on dual antiplatelet therapy on anticoagulants.
Citation: Ng FH, Tunggal P, Chu WM, et al. Esomeprazole compared with famotidine in the prevention of upper gastrointestinal bleeding in patients with acute coronary syndrome or myocardial infarction. Am J Gastroenterol. 2012;107:389-396.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Initial trophic feedings effective for patients with acute lung injury
- IM vs. IV benzodiazepines in status epilepticus
- CDI risk following antibiotic cessation
- Acid suppression associated with increased complications in CDI patients
- Perioperative statins and cardiac events in surgical patients
- Enoxaparin vs. unfractionated heparin during PCI
- Optimal serum potassium levels for AMI patients
- PPIs superior to H2-blockers for lowering UGI bleeding following ACS and STEMI
Initial Lower-Volume Enteral Feeding Better Tolerated, but Has No Mortality Benefit
Clinical question: In mechanically ventilated patients with acute lung injury, do initial lower-volume enteral feedings (trophic feedings) improve clinical outcomes when compared with full enteral feedings?
Background: Malnutrition in critically ill patients is associated with poor outcomes, but conflicting data exist regarding the best timing, amount, and formulation of enteral nutrition to initiate. Initiation of lower-volume enteral feeding with periodic assessment of gastric residual volume is common practice, but the effects of this practice are unknown.
Study design: Multicenter randomized controlled open-label study.
Setting: Forty-four hospitals in the National Heart, Lung, and Blood Institute (NHLBI) Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network.
Synopsis: One thousand patients with acute lung injury receiving mechanical ventilation for longer than 72 hours were randomized using a Web-based system to receive either trophic or full enteric feedings for the first six days of mechanical ventilation. After the sixth day, a full enteric feeding protocol was used in all patients. All analyses were by intention-to-treat.
There was no significant difference between the trophic and full enteral feeding groups with regard to the primary outcome of ventilator-free days through Day 28 (14.9% vs. 15.0%, P=0.89). There were also no significant differences between groups in secondary outcomes, which included 60-day mortality, ICU-free days, organ-failure-free days, or the incidence of new infections. However, gastrointestinal intolerances occurred less often in the trophic feeding group, and these patients received fewer anti-diarrheal and prokinetic agents. The full feeding group gained 2.1 liters of fluid by Day 7, but this fluid gain did not cause significant differences in measures of circulatory or pulmonary physiology.
Limitations include open-label study design and inclusion of only critically ill adult medical patients with acute lung injury.
Bottom line: Initial lower-volume tube feedings in mechanically ventilated patients with acute lung injury did not improve clinical outcomes compared with full enteral feedings, but they were associated with fewer instances of gastrointestinal intolerance.
Citation: National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795-803.
Intramuscular Benzodiazepines as Good as IV Benzodiazepines in Status Epilepticus
Clinical question: Is intramuscular (IM) midazolam noninferior to intravenous lorazepam in patients in status epilepticus?
Background: Studies have shown that IV benzodiazepines, particularly lorazepam, are effective for patients in status epilepticus. Studies have not evaluated IM benzodiazepines. However, many emergency medical service (EMS) agencies use IM midazolam because IM administration is easier than IV administration, and midazolam has a longer nonrefrigerated shelf life than lorazepam does.
Study design: Randomized, double-blinded clinical trial.
Setting: Thirty-three EMS agencies across the United States.
Synopsis: Based on the 893 adults and children in status epilepticus included in this double-blind study, the researchers found IM midazolam to be noninferior to, and in fact superior to, IV lorazepam for treating seizures prior to arrival at EDs. Specifically, they found 10% more (95% CI 4.0% to 16.1%; P<0.001 for noninferiority and P<0.001 for superiority) seizure-free patients arriving at EDs when IM midazolam was administered. Seizures ceased in patients given IM midazolam in less time on average than it took for paramedics to administer IV lorazepam.
Hospitalists might be less inclined than EMS personnel to use IM midazolam because their patients have established IV access and refrigerated drugs are readily available. However, since IM midazolam was superior to IV lorazepam in this prehospital study, a similar trial in hospitalized patients is warranted. In subsequent trials, it would be useful to administer both midazolam and lorazepam via IM and IV routes, and IM injections by an autoinjector could be compared with the conventional manner.
Bottom line: In the prehospital setting, IM midazolam is at least as good as IV lorazepam in treating status epilepticus in children and adults.
Citation: Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591-600.
Clostridium Difficile Infection Risk Remains High at Least Three Months after Antibiotics Are Stopped
Clinical question: How long are patients at higher risk for Clostridium difficile infection (CDI) after completing antibiotics?
Background: Studies have shown that patients given antibiotics are at higher risk for CDI than those who are not, particularly if they take multiple antibiotics at high doses for a prolonged period of time. However, it is not known how long a patient remains at high risk for CDI after completing antibiotic therapy.
Study design: Case-control study.
Setting: Nine hospitals in Netherlands.
Synopsis: The study compared 337 hospitalized patients who had CDI with 337 nondiarrheal controls and 227 non-CDI diarrheal controls. The study showed a seven- to tenfold increased risk for CDI during antibiotic treatment and in the 30 days following cessation of antibiotics. A 2.7-fold increased risk of CDI was seen in the one- to three-month period after antibiotic treatment was stopped.
Because researchers only obtained information about antibiotic use in the three months preceding the onset of diarrhea, it is unknown if CDI risk remains elevated longer than three months after antibiotics are stopped. Also of note, the enzyme immunoassays used in this study to diagnose C. diff had sensitivities of between 60% and 85%.
As hospitalists, this information can be used to advise patients about their continued risk for CDI after cessation of antibiotics. Further studies are needed to determine the risk after three months.
Bottom line: Patients are at highest risk for CDI up to one month after stopping antibiotics but continue to be at higher risk for at least two additional months.
Citation: Hensgens MPN, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67:742-748.
Acid Suppression and Poor Outcomes in C. Diff Patients
Clinical question: What are potential modifiable risk factors associated with increased complications and mortality in patients hospitalized with C. diff infection (CDI)?
Background: CDI is a growing cause of morbidity and mortality in hospitalized patients. Evidence is growing for the association of acid suppression, among other modifiable risk factors, with complications and mortality in patients with CDI.
Study design: Retrospective case review.
Setting: Naval medical center.
Synopsis: A laboratory, medical record, and pharmacy database query found 485 patients with CDI. Complications of CDI were defined as ICU admission, surgery, and megacolon. Factors significantly associated with CDI complications and mortality were admission for CDI, corticosteroid use >5 mg per day, age ≥80 years, and prescription acid suppression (including H2-blockers and proton-pump inhibitors).
The latter two risk factors were associated with mortality alone. In multivariable regression, the odds of mortality among patients on acid suppression was more than four times the odds of those not on acid suppression (OR 4.74, 95% CI, 1.57 to 14.36).
Although this is a retrospective cohort study and cannot prove a causal relationship, the data add to a growing body of evidence supporting the risk of CDI complications and mortality for those on acid suppression.
Bottom line: In hospitalized patients with CDI, acid suppression is associated with increased complications and mortality and should be discontinued in this population when possible.
Citation: Morrison RH, Hall NS, Said M, et al. Risk factors associated with complications and mortality in patients with Clostridium difficile infection. Clin Infect Dis. 2011;53:1173-1178.
Perioperative Statins Reduce Cardiac Events for Surgical Patients
Clinical question: Does perioperative statin use improve cardiac outcomes (death, myocardial infarction, atrial fibrillation, and ICU and hospital lengths of stay) in statin-naive patients undergoing cardiac or non-cardiac surgery?
Background: Statins have been hypothesized to reduce perioperative cardiac complications because they reduce vascular and systemic inflammation caused by surgery, and several meta-analyses have demonstrated their efficacy. To date, no meta-analyses have specifically evaluated the benefits of perioperative statins in non-cardiac surgery from randomized controlled trials.
Study design: Systematic review of the literature and meta-analysis.
Setting: Fifteen randomized controlled trials of hospitalized surgical patients.
Synopsis: A systematic review examined the effects of statins on a variety of perioperative cardiac outcomes (death, myocardial infarction, atrial fibrillation, and ICU and hospital lengths of stay); 11 of the 15 patients were undergoing cardiac surgery.
Perioperative statins decreased the risk of atrial fibrillation in patients undergoing cardiac surgery (RR 0.56; 95% CI 0.45-0.69; number needed to treat [NNT], 6). In both cardiac and non-cardiac surgical patients, statins reduced the risk of myocardial infarction (RR 0.53; 95% CI 0.38 to 0.74; NNT 23). Statin treatment also reduced the mean length of hospital stay (in days, mean difference -0.32; 95% CI, -0.53 to -0.11) but did not reduce the length of ICU stay (mean difference -0.08; 95% CI, -0.25 to 0.10). Risk of death was not reduced with statin treatment (RR 0.62; 95% CI, 0.34 to 1.14).
Bottom line: Perioperative statins reduce the risk of postoperative atrial fibrillation in cardiac surgical patients, the risk of postoperative MI in cardiac and non-cardiac surgical patients, and the mean length of hospital stay.
Citation: Chopra V, Wesorick DH, Sussman JB, et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta-analysis. Arch Surg. 2012;147:181-188.
Enoxaparin Safe and Effective during Percutaneous Coronary Intervention
Clinical question: Is enoxaparin safe and efficacious compared with unfractionated heparin during percutaneous coronary intervention (PCI)?
Background: Despite problems with the use of unfractionated heparin during PCI, current guidelines give it a Class 1 recommendation for PCI in ST-elevation myocardial infarction (MI). There is growing evidence that enoxaparin can provide predictable, effective anticoagulation during PCI. Although several trials have examined this issue, none have been sufficiently powered to evaluate mortality.
Study design: Systematic review and meta-analysis.
Setting: Twenty-three trials or registries of patients undergoing PCI.
Synopsis: A systematic review found 23 trials representing 30,966 patients that examined the effects of enoxaparin versus unfractionated heparin on risk of mortality, MI, complications of MI, and major bleeding. Of these, 10,243 (33.1%) patients underwent primary PCI for ST elevation MI, 8,750 (28.2%) underwent PCI after fibrinolysis, and 11,973 (38.7%) patients had either scheduled PCI or PCI for non-ST elevation acute coronary syndrome.
Of all the patients, 13,943 (45%) received enoxaparin and 17,023 (55%) received unfractionated heparin. Enoxaparin was associated with significant reductions in all-cause mortality (RR 0.66; 95% CI, 0.57 to 0.76), composite of death or MI (RR 0.68; 95% CI, 0.57 to 0.81), and complications of MI (RR 0.75; 95% CI, 0.6 to 0.85). For patients who received primary PCI for ST elevation MI, enoxaparin reduced the risk of complications of MI by 44% (RR 0.56; 95% CI, 0.42 to 0.76). Enoxaparin also reduced the risk of major bleeding (RR 0.80, 95% CI, 0.68 to 0.95) with even more striking results for the 14 studies that compared intravenous enoxaparin to unfractionated heparin (RR 0.66; 95% CI, 0.52 to 0.83).
Bottom line: Enoxaparin is safe and efficacious when used during PCI. Patients who receive enoxaparin during PCI have reduced risk for death, MI, complications of MI, and major bleeding when compared with patients who receive unfractionated heparin.
Citation: Silvain J, Beygui F, Barthelmy O, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012;344:e553.
Serum Potassium Levels and Mortality in Acute Myocardial Infarction
Clinical question: What is the relationship between serum potassium levels and mortality in acute myocardial infarction (AMI) patients?
Background: Several smaller studies in the pre-beta-blocker and pre-reperfusion era recommended maintaining serum potassium levels between 4.0 mEq/L and 5.0 mEq/L in AMI patients. However, current studies examining the relationship between potassium levels and mortality in AMI patients are lacking.
Study design: Retrospective cohort study.
Setting: Multicenter study involving 67 hospitals in the U.S.
Synopsis: Using the Cerner Health Facts database, which included 38,689 patients with biomarker-confirmed AMI, this study showed there was a U-shaped relationship between mean post-admission serum potassium level and in-hospital mortality.
Compared with the reference group of 3.5 mEq/L to less than 4.0 mEq/L (mortality rate 4.8%; 95% CI, 4.4% to 5.2%), mortality was comparable for those with mean post-admission potassium of 4.0 mEq/L to less than 4.5 mEq/L (5.0%; 95% CI, 4.7% to 5.3%). Mortality was twice as great for potassium of 4.5 mEq/L to less than 5.0 mEq/L (10.0%; 95% CI, 9.1% to 10.9%), and even greater for higher potassium strata. Similarly, mortality rates were higher for potassium levels of less than 3.5 mEq/L. Rates of ventricular fibrillation or cardiac arrest were higher among patients with potassium levels of less than 3.0 mEq/L or more than 5.0 mEq/L.
Bottom line: For inpatients with AMI, serum potassium levels of 3.5 mEq/L to 4.5 mEq/L should be maintained for the best outcomes. Repletion of serum potassium to levels greater than 4.5 mEq/L is associated with increased mortality and should be avoided.
Citation: Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157-164.
Proton-Pump Inhibitors Better than H2-Blockers in ACS and STEMI
Clinical question: Are proton-pump inhibitors (PPIs) better than H2-blockers at preventing UGI bleeding in patients after acute coronary syndrome (ACS) or ST-elevation myocardial infarction (STEMI)?
Background: It is not definitively known if PPIs are the same or better than H2-blockers in preventing UGI bleeding in patients on high-risk medications (aspirin, clopidogrel, and anticoagulants) after ACS or STEMI.
Study design: Randomized double-blinded controlled trial.
Setting: Single hospital.
Synopsis: Patients with ACS or STEMI were treated with aspirin, clopidogrel, and enoxaparin or thrombolytics. They were then randomized to either esomeprazole 20 mg or famotidine 40 mg, both administered nightly. They were followed throughout their hospital stay and were then followed between four and 52 weeks after discharge (mean duration: 19 weeks for esomeprazole, 18 weeks for famotidine). The primary end point was time to a composite outcome, consisting of UGI bleeding, obstruction, or perforation. Overall, 313 patients were randomized (164 to esomeprazole and 149 to famotidine).
The treatment groups were equivalent in baseline characteristics, and compliance in both groups was excellent (>98%). The primary endpoint occurred in three patients in the esomeprazole group and in 12 patients in the famotidine group (hazard ratio 0.21; 95% CI, 0.06 to 0.75; P=0.008).
Bottom line: PPIs are superior to H2-blockers in reducing UGI bleeding in patients on high-risk medications (aspirin, clopidogrel, and enoxaparin or thrombolytics) after ACS and STEMI. This confirms the recommendations of the 2010 ACCF/ACG/AHA Expert Consensus that PPIs should be used in those on dual antiplatelet therapy on anticoagulants.
Citation: Ng FH, Tunggal P, Chu WM, et al. Esomeprazole compared with famotidine in the prevention of upper gastrointestinal bleeding in patients with acute coronary syndrome or myocardial infarction. Am J Gastroenterol. 2012;107:389-396.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Initial trophic feedings effective for patients with acute lung injury
- IM vs. IV benzodiazepines in status epilepticus
- CDI risk following antibiotic cessation
- Acid suppression associated with increased complications in CDI patients
- Perioperative statins and cardiac events in surgical patients
- Enoxaparin vs. unfractionated heparin during PCI
- Optimal serum potassium levels for AMI patients
- PPIs superior to H2-blockers for lowering UGI bleeding following ACS and STEMI
Initial Lower-Volume Enteral Feeding Better Tolerated, but Has No Mortality Benefit
Clinical question: In mechanically ventilated patients with acute lung injury, do initial lower-volume enteral feedings (trophic feedings) improve clinical outcomes when compared with full enteral feedings?
Background: Malnutrition in critically ill patients is associated with poor outcomes, but conflicting data exist regarding the best timing, amount, and formulation of enteral nutrition to initiate. Initiation of lower-volume enteral feeding with periodic assessment of gastric residual volume is common practice, but the effects of this practice are unknown.
Study design: Multicenter randomized controlled open-label study.
Setting: Forty-four hospitals in the National Heart, Lung, and Blood Institute (NHLBI) Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network.
Synopsis: One thousand patients with acute lung injury receiving mechanical ventilation for longer than 72 hours were randomized using a Web-based system to receive either trophic or full enteric feedings for the first six days of mechanical ventilation. After the sixth day, a full enteric feeding protocol was used in all patients. All analyses were by intention-to-treat.
There was no significant difference between the trophic and full enteral feeding groups with regard to the primary outcome of ventilator-free days through Day 28 (14.9% vs. 15.0%, P=0.89). There were also no significant differences between groups in secondary outcomes, which included 60-day mortality, ICU-free days, organ-failure-free days, or the incidence of new infections. However, gastrointestinal intolerances occurred less often in the trophic feeding group, and these patients received fewer anti-diarrheal and prokinetic agents. The full feeding group gained 2.1 liters of fluid by Day 7, but this fluid gain did not cause significant differences in measures of circulatory or pulmonary physiology.
Limitations include open-label study design and inclusion of only critically ill adult medical patients with acute lung injury.
Bottom line: Initial lower-volume tube feedings in mechanically ventilated patients with acute lung injury did not improve clinical outcomes compared with full enteral feedings, but they were associated with fewer instances of gastrointestinal intolerance.
Citation: National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795-803.
Intramuscular Benzodiazepines as Good as IV Benzodiazepines in Status Epilepticus
Clinical question: Is intramuscular (IM) midazolam noninferior to intravenous lorazepam in patients in status epilepticus?
Background: Studies have shown that IV benzodiazepines, particularly lorazepam, are effective for patients in status epilepticus. Studies have not evaluated IM benzodiazepines. However, many emergency medical service (EMS) agencies use IM midazolam because IM administration is easier than IV administration, and midazolam has a longer nonrefrigerated shelf life than lorazepam does.
Study design: Randomized, double-blinded clinical trial.
Setting: Thirty-three EMS agencies across the United States.
Synopsis: Based on the 893 adults and children in status epilepticus included in this double-blind study, the researchers found IM midazolam to be noninferior to, and in fact superior to, IV lorazepam for treating seizures prior to arrival at EDs. Specifically, they found 10% more (95% CI 4.0% to 16.1%; P<0.001 for noninferiority and P<0.001 for superiority) seizure-free patients arriving at EDs when IM midazolam was administered. Seizures ceased in patients given IM midazolam in less time on average than it took for paramedics to administer IV lorazepam.
Hospitalists might be less inclined than EMS personnel to use IM midazolam because their patients have established IV access and refrigerated drugs are readily available. However, since IM midazolam was superior to IV lorazepam in this prehospital study, a similar trial in hospitalized patients is warranted. In subsequent trials, it would be useful to administer both midazolam and lorazepam via IM and IV routes, and IM injections by an autoinjector could be compared with the conventional manner.
Bottom line: In the prehospital setting, IM midazolam is at least as good as IV lorazepam in treating status epilepticus in children and adults.
Citation: Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591-600.
Clostridium Difficile Infection Risk Remains High at Least Three Months after Antibiotics Are Stopped
Clinical question: How long are patients at higher risk for Clostridium difficile infection (CDI) after completing antibiotics?
Background: Studies have shown that patients given antibiotics are at higher risk for CDI than those who are not, particularly if they take multiple antibiotics at high doses for a prolonged period of time. However, it is not known how long a patient remains at high risk for CDI after completing antibiotic therapy.
Study design: Case-control study.
Setting: Nine hospitals in Netherlands.
Synopsis: The study compared 337 hospitalized patients who had CDI with 337 nondiarrheal controls and 227 non-CDI diarrheal controls. The study showed a seven- to tenfold increased risk for CDI during antibiotic treatment and in the 30 days following cessation of antibiotics. A 2.7-fold increased risk of CDI was seen in the one- to three-month period after antibiotic treatment was stopped.
Because researchers only obtained information about antibiotic use in the three months preceding the onset of diarrhea, it is unknown if CDI risk remains elevated longer than three months after antibiotics are stopped. Also of note, the enzyme immunoassays used in this study to diagnose C. diff had sensitivities of between 60% and 85%.
As hospitalists, this information can be used to advise patients about their continued risk for CDI after cessation of antibiotics. Further studies are needed to determine the risk after three months.
Bottom line: Patients are at highest risk for CDI up to one month after stopping antibiotics but continue to be at higher risk for at least two additional months.
Citation: Hensgens MPN, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67:742-748.
Acid Suppression and Poor Outcomes in C. Diff Patients
Clinical question: What are potential modifiable risk factors associated with increased complications and mortality in patients hospitalized with C. diff infection (CDI)?
Background: CDI is a growing cause of morbidity and mortality in hospitalized patients. Evidence is growing for the association of acid suppression, among other modifiable risk factors, with complications and mortality in patients with CDI.
Study design: Retrospective case review.
Setting: Naval medical center.
Synopsis: A laboratory, medical record, and pharmacy database query found 485 patients with CDI. Complications of CDI were defined as ICU admission, surgery, and megacolon. Factors significantly associated with CDI complications and mortality were admission for CDI, corticosteroid use >5 mg per day, age ≥80 years, and prescription acid suppression (including H2-blockers and proton-pump inhibitors).
The latter two risk factors were associated with mortality alone. In multivariable regression, the odds of mortality among patients on acid suppression was more than four times the odds of those not on acid suppression (OR 4.74, 95% CI, 1.57 to 14.36).
Although this is a retrospective cohort study and cannot prove a causal relationship, the data add to a growing body of evidence supporting the risk of CDI complications and mortality for those on acid suppression.
Bottom line: In hospitalized patients with CDI, acid suppression is associated with increased complications and mortality and should be discontinued in this population when possible.
Citation: Morrison RH, Hall NS, Said M, et al. Risk factors associated with complications and mortality in patients with Clostridium difficile infection. Clin Infect Dis. 2011;53:1173-1178.
Perioperative Statins Reduce Cardiac Events for Surgical Patients
Clinical question: Does perioperative statin use improve cardiac outcomes (death, myocardial infarction, atrial fibrillation, and ICU and hospital lengths of stay) in statin-naive patients undergoing cardiac or non-cardiac surgery?
Background: Statins have been hypothesized to reduce perioperative cardiac complications because they reduce vascular and systemic inflammation caused by surgery, and several meta-analyses have demonstrated their efficacy. To date, no meta-analyses have specifically evaluated the benefits of perioperative statins in non-cardiac surgery from randomized controlled trials.
Study design: Systematic review of the literature and meta-analysis.
Setting: Fifteen randomized controlled trials of hospitalized surgical patients.
Synopsis: A systematic review examined the effects of statins on a variety of perioperative cardiac outcomes (death, myocardial infarction, atrial fibrillation, and ICU and hospital lengths of stay); 11 of the 15 patients were undergoing cardiac surgery.
Perioperative statins decreased the risk of atrial fibrillation in patients undergoing cardiac surgery (RR 0.56; 95% CI 0.45-0.69; number needed to treat [NNT], 6). In both cardiac and non-cardiac surgical patients, statins reduced the risk of myocardial infarction (RR 0.53; 95% CI 0.38 to 0.74; NNT 23). Statin treatment also reduced the mean length of hospital stay (in days, mean difference -0.32; 95% CI, -0.53 to -0.11) but did not reduce the length of ICU stay (mean difference -0.08; 95% CI, -0.25 to 0.10). Risk of death was not reduced with statin treatment (RR 0.62; 95% CI, 0.34 to 1.14).
Bottom line: Perioperative statins reduce the risk of postoperative atrial fibrillation in cardiac surgical patients, the risk of postoperative MI in cardiac and non-cardiac surgical patients, and the mean length of hospital stay.
Citation: Chopra V, Wesorick DH, Sussman JB, et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta-analysis. Arch Surg. 2012;147:181-188.
Enoxaparin Safe and Effective during Percutaneous Coronary Intervention
Clinical question: Is enoxaparin safe and efficacious compared with unfractionated heparin during percutaneous coronary intervention (PCI)?
Background: Despite problems with the use of unfractionated heparin during PCI, current guidelines give it a Class 1 recommendation for PCI in ST-elevation myocardial infarction (MI). There is growing evidence that enoxaparin can provide predictable, effective anticoagulation during PCI. Although several trials have examined this issue, none have been sufficiently powered to evaluate mortality.
Study design: Systematic review and meta-analysis.
Setting: Twenty-three trials or registries of patients undergoing PCI.
Synopsis: A systematic review found 23 trials representing 30,966 patients that examined the effects of enoxaparin versus unfractionated heparin on risk of mortality, MI, complications of MI, and major bleeding. Of these, 10,243 (33.1%) patients underwent primary PCI for ST elevation MI, 8,750 (28.2%) underwent PCI after fibrinolysis, and 11,973 (38.7%) patients had either scheduled PCI or PCI for non-ST elevation acute coronary syndrome.
Of all the patients, 13,943 (45%) received enoxaparin and 17,023 (55%) received unfractionated heparin. Enoxaparin was associated with significant reductions in all-cause mortality (RR 0.66; 95% CI, 0.57 to 0.76), composite of death or MI (RR 0.68; 95% CI, 0.57 to 0.81), and complications of MI (RR 0.75; 95% CI, 0.6 to 0.85). For patients who received primary PCI for ST elevation MI, enoxaparin reduced the risk of complications of MI by 44% (RR 0.56; 95% CI, 0.42 to 0.76). Enoxaparin also reduced the risk of major bleeding (RR 0.80, 95% CI, 0.68 to 0.95) with even more striking results for the 14 studies that compared intravenous enoxaparin to unfractionated heparin (RR 0.66; 95% CI, 0.52 to 0.83).
Bottom line: Enoxaparin is safe and efficacious when used during PCI. Patients who receive enoxaparin during PCI have reduced risk for death, MI, complications of MI, and major bleeding when compared with patients who receive unfractionated heparin.
Citation: Silvain J, Beygui F, Barthelmy O, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012;344:e553.
Serum Potassium Levels and Mortality in Acute Myocardial Infarction
Clinical question: What is the relationship between serum potassium levels and mortality in acute myocardial infarction (AMI) patients?
Background: Several smaller studies in the pre-beta-blocker and pre-reperfusion era recommended maintaining serum potassium levels between 4.0 mEq/L and 5.0 mEq/L in AMI patients. However, current studies examining the relationship between potassium levels and mortality in AMI patients are lacking.
Study design: Retrospective cohort study.
Setting: Multicenter study involving 67 hospitals in the U.S.
Synopsis: Using the Cerner Health Facts database, which included 38,689 patients with biomarker-confirmed AMI, this study showed there was a U-shaped relationship between mean post-admission serum potassium level and in-hospital mortality.
Compared with the reference group of 3.5 mEq/L to less than 4.0 mEq/L (mortality rate 4.8%; 95% CI, 4.4% to 5.2%), mortality was comparable for those with mean post-admission potassium of 4.0 mEq/L to less than 4.5 mEq/L (5.0%; 95% CI, 4.7% to 5.3%). Mortality was twice as great for potassium of 4.5 mEq/L to less than 5.0 mEq/L (10.0%; 95% CI, 9.1% to 10.9%), and even greater for higher potassium strata. Similarly, mortality rates were higher for potassium levels of less than 3.5 mEq/L. Rates of ventricular fibrillation or cardiac arrest were higher among patients with potassium levels of less than 3.0 mEq/L or more than 5.0 mEq/L.
Bottom line: For inpatients with AMI, serum potassium levels of 3.5 mEq/L to 4.5 mEq/L should be maintained for the best outcomes. Repletion of serum potassium to levels greater than 4.5 mEq/L is associated with increased mortality and should be avoided.
Citation: Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157-164.
Proton-Pump Inhibitors Better than H2-Blockers in ACS and STEMI
Clinical question: Are proton-pump inhibitors (PPIs) better than H2-blockers at preventing UGI bleeding in patients after acute coronary syndrome (ACS) or ST-elevation myocardial infarction (STEMI)?
Background: It is not definitively known if PPIs are the same or better than H2-blockers in preventing UGI bleeding in patients on high-risk medications (aspirin, clopidogrel, and anticoagulants) after ACS or STEMI.
Study design: Randomized double-blinded controlled trial.
Setting: Single hospital.
Synopsis: Patients with ACS or STEMI were treated with aspirin, clopidogrel, and enoxaparin or thrombolytics. They were then randomized to either esomeprazole 20 mg or famotidine 40 mg, both administered nightly. They were followed throughout their hospital stay and were then followed between four and 52 weeks after discharge (mean duration: 19 weeks for esomeprazole, 18 weeks for famotidine). The primary end point was time to a composite outcome, consisting of UGI bleeding, obstruction, or perforation. Overall, 313 patients were randomized (164 to esomeprazole and 149 to famotidine).
The treatment groups were equivalent in baseline characteristics, and compliance in both groups was excellent (>98%). The primary endpoint occurred in three patients in the esomeprazole group and in 12 patients in the famotidine group (hazard ratio 0.21; 95% CI, 0.06 to 0.75; P=0.008).
Bottom line: PPIs are superior to H2-blockers in reducing UGI bleeding in patients on high-risk medications (aspirin, clopidogrel, and enoxaparin or thrombolytics) after ACS and STEMI. This confirms the recommendations of the 2010 ACCF/ACG/AHA Expert Consensus that PPIs should be used in those on dual antiplatelet therapy on anticoagulants.
Citation: Ng FH, Tunggal P, Chu WM, et al. Esomeprazole compared with famotidine in the prevention of upper gastrointestinal bleeding in patients with acute coronary syndrome or myocardial infarction. Am J Gastroenterol. 2012;107:389-396.
Empiric Acyclovir Recommended for Potential Neonatal Herpes Simplex Virus Infection
Clinical question: What is the association between delayed acyclovir therapy and death in neonates with herpes simplex virus (HSV) infection?
Background: Neonatal HSV infection may result in significant morbidity and mortality if untreated. Because symptoms upon presentation may be nonspecific and testing may take several days, clinicians must often decide whether to initiate empiric therapy with acyclovir. However, this may also have consequences related to increased costs of care and the side effects of acyclovir.
Study design: Multicenter retrospective cohort study.
Setting: Forty-one freestanding tertiary-care children’s hospitals.
Synopsis: The Pediatric Health Information System (PHIS) database was used to identify 1,086 infants <28 days of age with a discharge diagnosis of HSV from 2003 to 2009 who received their first dose of intravenous acyclovir within the first seven days of admission. Delayed acyclovir was defined as administration after hospital Day One. Propensity scores and multivariate logistic regression analysis were used to attempt to account for patient (e.g. severity of illness) and hospital confounders. The mortality rate was 9.5% (95% confidence interval [CI]: 6.3%-13.82%) for delayed therapy compared with 6.6% (95% CI: 5.0%-8.5%) for early therapy.
Limitations of this study include the inability to identify all potential patient-level confounders that might have led clinicians to initiate early acyclovir therapy. Although the authors attempted to account for variables that might have defined sicker patients, such as intubation or vasoactive agent infusion, they could not identify patients with just skin, eye, or mouth disease from this database, nor could they identify specific clinical concerns, such as hypothermia. Thus, while the authors conclude that empiric acyclovir is prudent if HSV testing is performed, more specific clinical indications cannot be gleaned from this review.
Bottom line: Delayed initiation of acyclovir increases mortality in neonatal HSV infection.
Citation: Shah SS, Aronson PL, Mohamad Z, Lorch SA. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics. 2011;128(6):1153-1160.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Clinical question: What is the association between delayed acyclovir therapy and death in neonates with herpes simplex virus (HSV) infection?
Background: Neonatal HSV infection may result in significant morbidity and mortality if untreated. Because symptoms upon presentation may be nonspecific and testing may take several days, clinicians must often decide whether to initiate empiric therapy with acyclovir. However, this may also have consequences related to increased costs of care and the side effects of acyclovir.
Study design: Multicenter retrospective cohort study.
Setting: Forty-one freestanding tertiary-care children’s hospitals.
Synopsis: The Pediatric Health Information System (PHIS) database was used to identify 1,086 infants <28 days of age with a discharge diagnosis of HSV from 2003 to 2009 who received their first dose of intravenous acyclovir within the first seven days of admission. Delayed acyclovir was defined as administration after hospital Day One. Propensity scores and multivariate logistic regression analysis were used to attempt to account for patient (e.g. severity of illness) and hospital confounders. The mortality rate was 9.5% (95% confidence interval [CI]: 6.3%-13.82%) for delayed therapy compared with 6.6% (95% CI: 5.0%-8.5%) for early therapy.
Limitations of this study include the inability to identify all potential patient-level confounders that might have led clinicians to initiate early acyclovir therapy. Although the authors attempted to account for variables that might have defined sicker patients, such as intubation or vasoactive agent infusion, they could not identify patients with just skin, eye, or mouth disease from this database, nor could they identify specific clinical concerns, such as hypothermia. Thus, while the authors conclude that empiric acyclovir is prudent if HSV testing is performed, more specific clinical indications cannot be gleaned from this review.
Bottom line: Delayed initiation of acyclovir increases mortality in neonatal HSV infection.
Citation: Shah SS, Aronson PL, Mohamad Z, Lorch SA. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics. 2011;128(6):1153-1160.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Clinical question: What is the association between delayed acyclovir therapy and death in neonates with herpes simplex virus (HSV) infection?
Background: Neonatal HSV infection may result in significant morbidity and mortality if untreated. Because symptoms upon presentation may be nonspecific and testing may take several days, clinicians must often decide whether to initiate empiric therapy with acyclovir. However, this may also have consequences related to increased costs of care and the side effects of acyclovir.
Study design: Multicenter retrospective cohort study.
Setting: Forty-one freestanding tertiary-care children’s hospitals.
Synopsis: The Pediatric Health Information System (PHIS) database was used to identify 1,086 infants <28 days of age with a discharge diagnosis of HSV from 2003 to 2009 who received their first dose of intravenous acyclovir within the first seven days of admission. Delayed acyclovir was defined as administration after hospital Day One. Propensity scores and multivariate logistic regression analysis were used to attempt to account for patient (e.g. severity of illness) and hospital confounders. The mortality rate was 9.5% (95% confidence interval [CI]: 6.3%-13.82%) for delayed therapy compared with 6.6% (95% CI: 5.0%-8.5%) for early therapy.
Limitations of this study include the inability to identify all potential patient-level confounders that might have led clinicians to initiate early acyclovir therapy. Although the authors attempted to account for variables that might have defined sicker patients, such as intubation or vasoactive agent infusion, they could not identify patients with just skin, eye, or mouth disease from this database, nor could they identify specific clinical concerns, such as hypothermia. Thus, while the authors conclude that empiric acyclovir is prudent if HSV testing is performed, more specific clinical indications cannot be gleaned from this review.
Bottom line: Delayed initiation of acyclovir increases mortality in neonatal HSV infection.
Citation: Shah SS, Aronson PL, Mohamad Z, Lorch SA. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics. 2011;128(6):1153-1160.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Project BOOST Helps California Hospital Improve Care
Soon, hospitals with unnecessary 30-day readmissions will be penalized. Some proactive hospitals are already tackling care transitions to reduce readmissions, improve patient care, and reduce costs.
Lodi Memorial Hospital in Northern California is one such hospital. The 214-bed facility has made preventing readmissions a key strategic goal for improving care, especially for vulnerable, frail elderly patients.
Lodi executives say readmissions are a challenge at the hospital because many different specialties and house staff are involved in the discharge process.
With many options from which to choose, Lodi selected SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions). Project BOOST, unlike other programs, focuses on first identifying system deficiencies, then building cohesive multidisciplinary teams.
“If you layer a clinical intervention onto a broken system,” says BOOST mentor Stephanie Rennke, MD, “success isn’t likely. Project BOOST helps the team map current processes, find deficiencies, assess resources, and redefine a culture of safety.
“BOOST starts with an assessment of how the system functions, and identifies its strengths and weaknesses so you can focus your efforts on critical areas for improvement and tailor the BOOST intervention to fit the unique dynamics of an institution,” she says.
Search for a Solution
No two institutions are exactly the same, especially when analyzing patient-safety culture. Project BOOST is customized to the needs of each BOOST site, which has proven useful to Lodi, as it found it had different needs than other hospitals.
“We chose Project BOOST because it seemed workable and implementable, while offering a less expensive and less complicated solution than competitors’ products,” says Valerie Cronin, Lodi’s director of utilization.
The BOOST mentor, key to this customization, provided Lodi with the reassurance that comes with working directly with someone who has faced similar challenges—not just theoretically, but also on the floor—and was willing to work with Lodi to develop practical solutions. The mentor provided reassurance, support, and perspective as Lodi walked through the BOOST implementation process.
Keys to Success
—Valerie Cronin, director of utilization, Lodi Memorial Hospital, California
Team-focused care, clear communication, and administrative support were keys to successful BOOST implementation at Lodi, says Cronin.
The house staff was overwhelmed, and adding a quality-improvement (QI) project to implement and manage might have seemed like an impossible challenge. By developing multidisciplinary teams, however, Lodi was able to distribute the tasks of implementation and began to recognize the value and benefit of Project BOOST, which already had strong support from hospital executives.
A 14-member multidisciplinary team was formed to oversee the Project BOOST implementation. The team then was divided into sub-groups to work on the main components of Project BOOST: Passport to Care Form, Target Assessment Tool, the Teachback process, and Follow-Up Phone Calls. The sub-groups’ main objectives were to ensure that BOOST effectively changed processes and work practices for a stronger and safer discharge process.
To support these teams and foster communication laterally among healthcare providers and vertically with hospital administration, Lodi established a structured meeting format, delegated task assignments for accountability, and appointed an implementation champion.
Capitalizing on the experience of its BOOST mentor, the Lodi multidisciplinary teams mapped out the process to assess threats to the system and opportunities for improvement, and began moving forward with implementable solutions for sustainable change.
“Evaluating the whole discharge process allowed us to see the gaps and discrepancies in the discharge process,” Cronin says. “Each discipline had their own set of procedures and materials, which proved lacking and inconsistent for our patients. It was an essential and eye-opening experience for Lodi to make change.”
One of the biggest revelations was learning how broken the discharge process was for the nurses. When Lodi looked at its current process of using case managers to handle high-risk patients and leaving the remaining patient discharges to the nurses, they found that the process was not strong enough to support patient load.
Knowledge Is Power
Cronin says Lodi has standardized its patient educational materials and started the patient education process as soon as patients are admitted. This step optimizes a patient’s understanding of diagnoses and care instructions when the time comes for discharge.
Through the Project BOOST assessment, Lodi ascertained that many of its patients were being discharged to skilled-nursing facilities, which heightens the complexity of post-care and introduces the potential for increased risks. Lodi is now working to better communicate with the skilled-nursing facilities using its Project BOOST training to streamline the discharge process.
The implementation of the Teach Back communication strategy has been critical in increasing patient knowledge and adherence to care instructions, official say. Based on the success of Project BOOST implementation, Teach Back has been incorporated into mandatory nurse training throughout the hospital.
Improved Care
More than 80 patients were discharged through the BOOST process in the first 90 days of implementation. Lodi is already experiencing the benefits of Project BOOST organizationally and
expects to see the financial impacts soon through a lower 30-day readmission rate.
“Lodi has experienced a shift in patient safety culture with improved communication through a team approach to care,” Cronin says. “Lodi expects high success through Project BOOST with the goal of implementing Project BOOST across all disease states and every discipline using Teachback.
“Project BOOST has been the ideal program to align with our strategic organizational goals to improve transitions of care and re-create the patient safety culture to make systemwide sustainable change,” she says.
For more information about Project BOOST, visit www.hospitalmedicine.org/boost.
Jacqui Petock, marketing project manager
Soon, hospitals with unnecessary 30-day readmissions will be penalized. Some proactive hospitals are already tackling care transitions to reduce readmissions, improve patient care, and reduce costs.
Lodi Memorial Hospital in Northern California is one such hospital. The 214-bed facility has made preventing readmissions a key strategic goal for improving care, especially for vulnerable, frail elderly patients.
Lodi executives say readmissions are a challenge at the hospital because many different specialties and house staff are involved in the discharge process.
With many options from which to choose, Lodi selected SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions). Project BOOST, unlike other programs, focuses on first identifying system deficiencies, then building cohesive multidisciplinary teams.
“If you layer a clinical intervention onto a broken system,” says BOOST mentor Stephanie Rennke, MD, “success isn’t likely. Project BOOST helps the team map current processes, find deficiencies, assess resources, and redefine a culture of safety.
“BOOST starts with an assessment of how the system functions, and identifies its strengths and weaknesses so you can focus your efforts on critical areas for improvement and tailor the BOOST intervention to fit the unique dynamics of an institution,” she says.
Search for a Solution
No two institutions are exactly the same, especially when analyzing patient-safety culture. Project BOOST is customized to the needs of each BOOST site, which has proven useful to Lodi, as it found it had different needs than other hospitals.
“We chose Project BOOST because it seemed workable and implementable, while offering a less expensive and less complicated solution than competitors’ products,” says Valerie Cronin, Lodi’s director of utilization.
The BOOST mentor, key to this customization, provided Lodi with the reassurance that comes with working directly with someone who has faced similar challenges—not just theoretically, but also on the floor—and was willing to work with Lodi to develop practical solutions. The mentor provided reassurance, support, and perspective as Lodi walked through the BOOST implementation process.
Keys to Success
—Valerie Cronin, director of utilization, Lodi Memorial Hospital, California
Team-focused care, clear communication, and administrative support were keys to successful BOOST implementation at Lodi, says Cronin.
The house staff was overwhelmed, and adding a quality-improvement (QI) project to implement and manage might have seemed like an impossible challenge. By developing multidisciplinary teams, however, Lodi was able to distribute the tasks of implementation and began to recognize the value and benefit of Project BOOST, which already had strong support from hospital executives.
A 14-member multidisciplinary team was formed to oversee the Project BOOST implementation. The team then was divided into sub-groups to work on the main components of Project BOOST: Passport to Care Form, Target Assessment Tool, the Teachback process, and Follow-Up Phone Calls. The sub-groups’ main objectives were to ensure that BOOST effectively changed processes and work practices for a stronger and safer discharge process.
To support these teams and foster communication laterally among healthcare providers and vertically with hospital administration, Lodi established a structured meeting format, delegated task assignments for accountability, and appointed an implementation champion.
Capitalizing on the experience of its BOOST mentor, the Lodi multidisciplinary teams mapped out the process to assess threats to the system and opportunities for improvement, and began moving forward with implementable solutions for sustainable change.
“Evaluating the whole discharge process allowed us to see the gaps and discrepancies in the discharge process,” Cronin says. “Each discipline had their own set of procedures and materials, which proved lacking and inconsistent for our patients. It was an essential and eye-opening experience for Lodi to make change.”
One of the biggest revelations was learning how broken the discharge process was for the nurses. When Lodi looked at its current process of using case managers to handle high-risk patients and leaving the remaining patient discharges to the nurses, they found that the process was not strong enough to support patient load.
Knowledge Is Power
Cronin says Lodi has standardized its patient educational materials and started the patient education process as soon as patients are admitted. This step optimizes a patient’s understanding of diagnoses and care instructions when the time comes for discharge.
Through the Project BOOST assessment, Lodi ascertained that many of its patients were being discharged to skilled-nursing facilities, which heightens the complexity of post-care and introduces the potential for increased risks. Lodi is now working to better communicate with the skilled-nursing facilities using its Project BOOST training to streamline the discharge process.
The implementation of the Teach Back communication strategy has been critical in increasing patient knowledge and adherence to care instructions, official say. Based on the success of Project BOOST implementation, Teach Back has been incorporated into mandatory nurse training throughout the hospital.
Improved Care
More than 80 patients were discharged through the BOOST process in the first 90 days of implementation. Lodi is already experiencing the benefits of Project BOOST organizationally and
expects to see the financial impacts soon through a lower 30-day readmission rate.
“Lodi has experienced a shift in patient safety culture with improved communication through a team approach to care,” Cronin says. “Lodi expects high success through Project BOOST with the goal of implementing Project BOOST across all disease states and every discipline using Teachback.
“Project BOOST has been the ideal program to align with our strategic organizational goals to improve transitions of care and re-create the patient safety culture to make systemwide sustainable change,” she says.
For more information about Project BOOST, visit www.hospitalmedicine.org/boost.
Jacqui Petock, marketing project manager
Soon, hospitals with unnecessary 30-day readmissions will be penalized. Some proactive hospitals are already tackling care transitions to reduce readmissions, improve patient care, and reduce costs.
Lodi Memorial Hospital in Northern California is one such hospital. The 214-bed facility has made preventing readmissions a key strategic goal for improving care, especially for vulnerable, frail elderly patients.
Lodi executives say readmissions are a challenge at the hospital because many different specialties and house staff are involved in the discharge process.
With many options from which to choose, Lodi selected SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions). Project BOOST, unlike other programs, focuses on first identifying system deficiencies, then building cohesive multidisciplinary teams.
“If you layer a clinical intervention onto a broken system,” says BOOST mentor Stephanie Rennke, MD, “success isn’t likely. Project BOOST helps the team map current processes, find deficiencies, assess resources, and redefine a culture of safety.
“BOOST starts with an assessment of how the system functions, and identifies its strengths and weaknesses so you can focus your efforts on critical areas for improvement and tailor the BOOST intervention to fit the unique dynamics of an institution,” she says.
Search for a Solution
No two institutions are exactly the same, especially when analyzing patient-safety culture. Project BOOST is customized to the needs of each BOOST site, which has proven useful to Lodi, as it found it had different needs than other hospitals.
“We chose Project BOOST because it seemed workable and implementable, while offering a less expensive and less complicated solution than competitors’ products,” says Valerie Cronin, Lodi’s director of utilization.
The BOOST mentor, key to this customization, provided Lodi with the reassurance that comes with working directly with someone who has faced similar challenges—not just theoretically, but also on the floor—and was willing to work with Lodi to develop practical solutions. The mentor provided reassurance, support, and perspective as Lodi walked through the BOOST implementation process.
Keys to Success
—Valerie Cronin, director of utilization, Lodi Memorial Hospital, California
Team-focused care, clear communication, and administrative support were keys to successful BOOST implementation at Lodi, says Cronin.
The house staff was overwhelmed, and adding a quality-improvement (QI) project to implement and manage might have seemed like an impossible challenge. By developing multidisciplinary teams, however, Lodi was able to distribute the tasks of implementation and began to recognize the value and benefit of Project BOOST, which already had strong support from hospital executives.
A 14-member multidisciplinary team was formed to oversee the Project BOOST implementation. The team then was divided into sub-groups to work on the main components of Project BOOST: Passport to Care Form, Target Assessment Tool, the Teachback process, and Follow-Up Phone Calls. The sub-groups’ main objectives were to ensure that BOOST effectively changed processes and work practices for a stronger and safer discharge process.
To support these teams and foster communication laterally among healthcare providers and vertically with hospital administration, Lodi established a structured meeting format, delegated task assignments for accountability, and appointed an implementation champion.
Capitalizing on the experience of its BOOST mentor, the Lodi multidisciplinary teams mapped out the process to assess threats to the system and opportunities for improvement, and began moving forward with implementable solutions for sustainable change.
“Evaluating the whole discharge process allowed us to see the gaps and discrepancies in the discharge process,” Cronin says. “Each discipline had their own set of procedures and materials, which proved lacking and inconsistent for our patients. It was an essential and eye-opening experience for Lodi to make change.”
One of the biggest revelations was learning how broken the discharge process was for the nurses. When Lodi looked at its current process of using case managers to handle high-risk patients and leaving the remaining patient discharges to the nurses, they found that the process was not strong enough to support patient load.
Knowledge Is Power
Cronin says Lodi has standardized its patient educational materials and started the patient education process as soon as patients are admitted. This step optimizes a patient’s understanding of diagnoses and care instructions when the time comes for discharge.
Through the Project BOOST assessment, Lodi ascertained that many of its patients were being discharged to skilled-nursing facilities, which heightens the complexity of post-care and introduces the potential for increased risks. Lodi is now working to better communicate with the skilled-nursing facilities using its Project BOOST training to streamline the discharge process.
The implementation of the Teach Back communication strategy has been critical in increasing patient knowledge and adherence to care instructions, official say. Based on the success of Project BOOST implementation, Teach Back has been incorporated into mandatory nurse training throughout the hospital.
Improved Care
More than 80 patients were discharged through the BOOST process in the first 90 days of implementation. Lodi is already experiencing the benefits of Project BOOST organizationally and
expects to see the financial impacts soon through a lower 30-day readmission rate.
“Lodi has experienced a shift in patient safety culture with improved communication through a team approach to care,” Cronin says. “Lodi expects high success through Project BOOST with the goal of implementing Project BOOST across all disease states and every discipline using Teachback.
“Project BOOST has been the ideal program to align with our strategic organizational goals to improve transitions of care and re-create the patient safety culture to make systemwide sustainable change,” she says.
For more information about Project BOOST, visit www.hospitalmedicine.org/boost.
Jacqui Petock, marketing project manager
More in the Affordable Care Act than the Mandate
As America weighs the value of healthcare and health reform, many moving parts of the Patient Protection and Affordable Care Act (ACA) will continue to reshape the healthcare system. And hospitalists continue to be part of the reshaping.
The full title of the bill should serve as a reminder to hospitalists of the dual purposes of the ACA: patient protection and cost control. The bulk of popular consciousness about the ACA focused around the favored positions (no pre-existing conditions and children remaining on parents’ plans until age 26) or around points of contention (the individual mandate and the Medicaid expansion).
The lesser-mentioned provisions centering around cost, quality, and payment reform have the potential to substantively reframe the conversation, particularly from the provider perspective. Hospitalists have a unique expertise that positions them to be critical in shaping these programs and regulations.
Two initiatives aim at moving the traditional fee-for-service models of payment for health services toward pay-for-performance. Both the hospital value-based purchasing and physician value-based payment modifier use quality measures to link the performance of care with payments. Hospitals and associated groups are in the process of working with the Centers for Medicare & Medicaid Services (CMS) as the value-based purchasing program moves forward.
The physician value-based modifier will combine quality measures and reports, similar to the 2010 Quality and Resource Use Reports piloted this year in Nebraska, Iowa, Kansas, and Missouri, with a to-be-determined payment adjustment. These reports represent a major step toward Medicare providing large-scale feedback to providers on healthcare quality and costs. When the modifier is enforced, a percentage of payments will reflect quality scores, making the feedback from hospitalists important in creating meaningful and useful measures.
Concurrently, the ACA spurred the development of additional quality-improvement (QI) programs. Quality efforts ranging from reducing hospital-acquired infections, stemming preventable complications, meaningful use of electronic health records (EHRs), and reducing readmissions round out a complement of programs that strive to create safer, more cost-effective care for patients. For example, the Partnership for Patients is a collaborative program between providers, patient groups, and the government that catalyzes care improvement in hospitals. Hospitalists have been QI leaders at the front lines in their hospitals for years, affording an informed perspective on policy development.
The ACA is a constellation of programs and initiatives that seek to test new systems for care delivery and payment reform while providing access and quality protections for patients. Even without the ACA, reforms in quality and payment models are necessary and will occur.
Through SHM, hospitalists actively are sharing their experiences with policymakers in an effort to create responsive and reflective programs. It is precisely this expertise with hospitalized patients that affords hospitalists a ground- and systems-level perspective on healthcare. With so many reforms taking place, it is vital that hospitalists remain connected and informed about these issues and engage the opportunities for policy leadership and feedback.
To get involved with SHM’s advocacy efforts, visit www.hospitalmedicine.org/advocacy.
As America weighs the value of healthcare and health reform, many moving parts of the Patient Protection and Affordable Care Act (ACA) will continue to reshape the healthcare system. And hospitalists continue to be part of the reshaping.
The full title of the bill should serve as a reminder to hospitalists of the dual purposes of the ACA: patient protection and cost control. The bulk of popular consciousness about the ACA focused around the favored positions (no pre-existing conditions and children remaining on parents’ plans until age 26) or around points of contention (the individual mandate and the Medicaid expansion).
The lesser-mentioned provisions centering around cost, quality, and payment reform have the potential to substantively reframe the conversation, particularly from the provider perspective. Hospitalists have a unique expertise that positions them to be critical in shaping these programs and regulations.
Two initiatives aim at moving the traditional fee-for-service models of payment for health services toward pay-for-performance. Both the hospital value-based purchasing and physician value-based payment modifier use quality measures to link the performance of care with payments. Hospitals and associated groups are in the process of working with the Centers for Medicare & Medicaid Services (CMS) as the value-based purchasing program moves forward.
The physician value-based modifier will combine quality measures and reports, similar to the 2010 Quality and Resource Use Reports piloted this year in Nebraska, Iowa, Kansas, and Missouri, with a to-be-determined payment adjustment. These reports represent a major step toward Medicare providing large-scale feedback to providers on healthcare quality and costs. When the modifier is enforced, a percentage of payments will reflect quality scores, making the feedback from hospitalists important in creating meaningful and useful measures.
Concurrently, the ACA spurred the development of additional quality-improvement (QI) programs. Quality efforts ranging from reducing hospital-acquired infections, stemming preventable complications, meaningful use of electronic health records (EHRs), and reducing readmissions round out a complement of programs that strive to create safer, more cost-effective care for patients. For example, the Partnership for Patients is a collaborative program between providers, patient groups, and the government that catalyzes care improvement in hospitals. Hospitalists have been QI leaders at the front lines in their hospitals for years, affording an informed perspective on policy development.
The ACA is a constellation of programs and initiatives that seek to test new systems for care delivery and payment reform while providing access and quality protections for patients. Even without the ACA, reforms in quality and payment models are necessary and will occur.
Through SHM, hospitalists actively are sharing their experiences with policymakers in an effort to create responsive and reflective programs. It is precisely this expertise with hospitalized patients that affords hospitalists a ground- and systems-level perspective on healthcare. With so many reforms taking place, it is vital that hospitalists remain connected and informed about these issues and engage the opportunities for policy leadership and feedback.
To get involved with SHM’s advocacy efforts, visit www.hospitalmedicine.org/advocacy.
As America weighs the value of healthcare and health reform, many moving parts of the Patient Protection and Affordable Care Act (ACA) will continue to reshape the healthcare system. And hospitalists continue to be part of the reshaping.
The full title of the bill should serve as a reminder to hospitalists of the dual purposes of the ACA: patient protection and cost control. The bulk of popular consciousness about the ACA focused around the favored positions (no pre-existing conditions and children remaining on parents’ plans until age 26) or around points of contention (the individual mandate and the Medicaid expansion).
The lesser-mentioned provisions centering around cost, quality, and payment reform have the potential to substantively reframe the conversation, particularly from the provider perspective. Hospitalists have a unique expertise that positions them to be critical in shaping these programs and regulations.
Two initiatives aim at moving the traditional fee-for-service models of payment for health services toward pay-for-performance. Both the hospital value-based purchasing and physician value-based payment modifier use quality measures to link the performance of care with payments. Hospitals and associated groups are in the process of working with the Centers for Medicare & Medicaid Services (CMS) as the value-based purchasing program moves forward.
The physician value-based modifier will combine quality measures and reports, similar to the 2010 Quality and Resource Use Reports piloted this year in Nebraska, Iowa, Kansas, and Missouri, with a to-be-determined payment adjustment. These reports represent a major step toward Medicare providing large-scale feedback to providers on healthcare quality and costs. When the modifier is enforced, a percentage of payments will reflect quality scores, making the feedback from hospitalists important in creating meaningful and useful measures.
Concurrently, the ACA spurred the development of additional quality-improvement (QI) programs. Quality efforts ranging from reducing hospital-acquired infections, stemming preventable complications, meaningful use of electronic health records (EHRs), and reducing readmissions round out a complement of programs that strive to create safer, more cost-effective care for patients. For example, the Partnership for Patients is a collaborative program between providers, patient groups, and the government that catalyzes care improvement in hospitals. Hospitalists have been QI leaders at the front lines in their hospitals for years, affording an informed perspective on policy development.
The ACA is a constellation of programs and initiatives that seek to test new systems for care delivery and payment reform while providing access and quality protections for patients. Even without the ACA, reforms in quality and payment models are necessary and will occur.
Through SHM, hospitalists actively are sharing their experiences with policymakers in an effort to create responsive and reflective programs. It is precisely this expertise with hospitalized patients that affords hospitalists a ground- and systems-level perspective on healthcare. With so many reforms taking place, it is vital that hospitalists remain connected and informed about these issues and engage the opportunities for policy leadership and feedback.
To get involved with SHM’s advocacy efforts, visit www.hospitalmedicine.org/advocacy.