User login
Venous Thromboembolism and Weight Changes in Veteran Patients Using Megestrol Acetate as an Appetite Stimulant
New and Noteworthy Information—October
Transient ischemic attack (TIA) is linked with a substantial risk of disability, researchers reported in the September 13 online Stroke. The 510 consecutive patients prospectively enrolled in the study had minor stroke or TIA, were not previously disabled, and had a CT or CT angiography completed within 24 hours of symptom onset. After assessing disability 90 days following the event, the investigators found that 15% of patients had a disabled outcome. Those who experienced recurrent strokes were more likely to be disabled—53% of patients with recurrent strokes were disabled, compared with 12% of those who did not have a recurrent stroke. “In terms of absolute numbers, most patients have disability as a result of their presenting event; however, recurrent events have the largest relative impact on outcome,” the study authors concluded.
Persons with high plasma glucose levels that are still within the normal range are more likely to have atrophy of brain structures associated with neurodegenerative processes, according to a study published in the September 4 Neurology. Investigators used MRI scans to assess hippocampal and amygdalar volumes in a sample of 266 cognitively healthy persons ages 60 to 64 who did not have type 2 diabetes. Results showed that plasma glucose levels were significantly linked with hippocampal and amygdalar atrophy. After controlling for age, sex, BMI, hypertension, alcohol, and smoking, the researchers found that plasma glucose levels accounted for a 6% to 10% change in volume. “These findings suggest that even in the subclinical range and in the absence of diabetes, monitoring and management of plasma glucose levels could have an impact on cerebral health,” the study authors wrote.
The FDA has approved once-a-day tablet Aubagio (teriflunomide) for treatment of adults with relapsing forms of multiple sclerosis (MS). During a clinical trial, patients taking teriflunomide had a relapse rate that was 30% lower than that of patients taking placebo. The most common side effects observed during clinical trials were diarrhea, abnormal liver tests, nausea, and hair loss, and physicians should conduct blood tests to check patients’ liver function before the drug is prescribed as well as periodically during treatment, researchers said. In addition, because of a risk of fetal harm, women of childbearing age must have a negative pregnancy test before beginning teriflunomide and should use birth control throughout treatment. Teriflunomide is the second oral treatment therapy for MS to be approved in the United States.
Patients who appear likely to have sporadic Creutzfeldt-Jakob disease may benefit from CSF 14-3-3 assays to clarify the diagnosis, researchers reported in the online September 19 Neurology. In a systematic literature review, the investigators identified articles from 1995 to January 1, 2011, that involved patients who had CSF analysis for protein 14-3-3. Based on data from 1,849 patients, the researchers determined that assays for CSF 14-3-3 are probably moderately accurate in diagnosing Creutzfeldt-Jakob—the assays had a sensitivity of 92%, specificity of 80%, likelihood ratio of 4.7, and negative likelihood ratio of 0.10. The study authors recommend CSF 14-3-3 assays “for patients who have rapidly progressive dementia and are strongly suspected of having sporadic Creutzfeldt-Jakob and for whom diagnosis remains uncertain (pretest probably of between 20% and 90%).”
Children with migraine and children with tension-type headaches are significantly more likely to have behavioral and emotional symptoms, and the frequency of headaches affects the likelihood of these symptoms, according to a study published in the online September 17 Cephalagia. After examining a sample of 1,856 children ages 5 to 11, investigators found that those with migraine were significantly more likely to experience abnormalities in somatic, anxiety-depressive, social, attention, internalizing, and total score domains of the Child Behavior Checklist. Children with tension-type headaches had a lower rate of abnormalities than children with migraine, but those with tension-type headaches still had significantly more abnormalities than controls. Children with headaches are more likely to have internalizing symptoms than externalizing symptoms such as rule breaking and aggressivity, the researchers found.
Heavy alcohol intake is associated with experiencing intracerebral hemorrhage at a younger age, according to a study published in the September 11 Neurology. Researchers prospectively followed 562 adults with spontaneous intracerebral hemorrhage and recorded information about their alcohol intake. A total of 137 patients were heavy alcohol drinkers, and these patients were more likely to be younger (median age, 60), to have a history of ischemic heart disease, and to be smokers. Furthermore, heavy alcohol drinkers had significantly lower platelet counts and prothrombin ratio. The investigators noted that although heavy alcohol intake is associated with intracerebral hemorrhage at a younger age, “the underlying vasculopathy remains unexplored in these patients. Indirect markers suggest small-vessel disease at an early stage that might be enhanced by moderate hemostatic disorders,” the authors concluded.
Long-term use of ginkgo biloba extract does not prevent the onset of Alzheimer’s disease in older patients, according to a study published in the online September 5 Lancet Neurology. Researchers enrolled 2,854 participants in a parallel-group, double-blind clinical trial in which 1,406 persons were randomized to receive ginkgo biloba extract and 1,414 persons were randomized to placebo. After five years of follow-up, 61 participants taking ginkgo biloba were diagnosed with probable Alzheimer’s disease, while 73 participants in the placebo group received a diagnosis of probable Alzheimer’s disease, though the risk was not proportional over time. The incidence of adverse events, as well as hemorrhagic or cardiovascular events, did not differ between groups. “Long-term use of standardized ginkgo biloba extract in this trial did not reduce the risk of progression to Alzheimer’s disease compared with placebo,” the researchers concluded.
The 13.3–mg/24 h dosage strength of the Exelon Patch (rivastigmine transdermal system) has been approved by the FDA for treatment of mild to moderate Alzheimer’s disease. Approval was based on the performance of the 13.3–mg/24 h dosage in the 48-week, double-blind phase of the OPTIMA study, which analyzed patients with mild to moderate Alzheimer’s disease who met predefined functional and cognitive decline criteria for the 9.5–mg/24 h dose. Compared with patients taking the 9.5–mg/24 h dose, patients taking the 13.3–mg/24 h dose showed statistically significant improvement in overall function. In addition, the overall safety profile of the 13.3–mg/24 h dose was the same as that of the lower dose, and fewer patients on the 13.3–mg/24 h dose needed to discontinue treatment than patients on the 9.5–mg/24 h dose.
The FDA has approved Nucynta (tapentadol) for management of neuropathic pain associated with diabetic peripheral neuropathy when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. According to preclinical studies, the drug is a centrally acting synthetic analgesic, though the exact mechanism of action is unknown. In two randomized-withdrawal, placebo-controlled phase III trials, researchers studied patients who had at least a one-point reduction in pain intensity during three weeks of treatment and then continued for an additional 12 weeks on the same dose, which was titrated to balance individual tolerability and efficacy. These patients had significantly better pain control than those who switched to placebo. The most common adverse events associated with the drug were nausea, constipation, vomiting, dizziness, headache, and somnolence, but tapentadol was generally well tolerated.
A mouse model of abnormal adult-generated granule cells (DGCs) has provided the first direct evidence that abnormal DGCs are linked to seizures, researchers reported in the September 20 Neuron. To isolate the effects of the abnormal cells, investigators used a transgenic mouse model to selectively delete PTEN from DGCs generated after birth. As a result of PTEN deletion, the mammalian target of rapamycin pathway was hyperactivated, which produced abnormal DGCs that resembled those in epilepsy. “Strikingly, animals in which PTEN was deleted from 9% or more of the DGC population developed spontaneous seizures in about four weeks, confirming that abnormal DGCs, which are present in both animals and humans with epilepsy, are capable of causing the disease,” the researchers stated.
Patients with multiple sclerosis (MS) who receive gingko biloba 120 mg twice a day do not show improved cognitive performance, researchers reported in the September 18 Neurology. The investigators compared the performance of two groups of patients with MS who scored 1 SD or more below the mean on one of four neuropsychologic tests. Sixty-one patients received 120 mg of ginkgo biloba twice a day for 12 weeks, and 59 patients received placebo. The researchers evaluated participants’ cognitive performance following treatment and found no statistically significant difference in scores between the two groups. Furthermore, no significant adverse events related to gingko biloba treatment occurred, according to the study authors. Overall, the investigators concluded that gingko biloba does not improve cognitive function in patients with MS.
The Solitaire Flow Restoration device performs substantially better than the Merci Retrieval System in treating acute ischemic stroke, according to a study published in the online August 24 Lancet. In a randomized, parallel-group, noninferiority trial, the efficacy and safety of the Solitaire device, a self-expanding stent retriever designed to quickly restore blood flow, was compared with the efficacy and safety of the standard Merci Retrieval system. The 58 patients in the Solitaire group achieved the primary efficacy outcome 61% of the time, compared with 24% of patients in the Merci group, investigators said. Furthermore, patients in the Solitaire group had lower 90-day mortality than patients in the Merci group (17 versus 38). “The Solitaire device might be a future treatment of choice for endovascular recanalization in acute ischemic stroke,” the researchers concluded.
—Lauren LeBano
Transient ischemic attack (TIA) is linked with a substantial risk of disability, researchers reported in the September 13 online Stroke. The 510 consecutive patients prospectively enrolled in the study had minor stroke or TIA, were not previously disabled, and had a CT or CT angiography completed within 24 hours of symptom onset. After assessing disability 90 days following the event, the investigators found that 15% of patients had a disabled outcome. Those who experienced recurrent strokes were more likely to be disabled—53% of patients with recurrent strokes were disabled, compared with 12% of those who did not have a recurrent stroke. “In terms of absolute numbers, most patients have disability as a result of their presenting event; however, recurrent events have the largest relative impact on outcome,” the study authors concluded.
Persons with high plasma glucose levels that are still within the normal range are more likely to have atrophy of brain structures associated with neurodegenerative processes, according to a study published in the September 4 Neurology. Investigators used MRI scans to assess hippocampal and amygdalar volumes in a sample of 266 cognitively healthy persons ages 60 to 64 who did not have type 2 diabetes. Results showed that plasma glucose levels were significantly linked with hippocampal and amygdalar atrophy. After controlling for age, sex, BMI, hypertension, alcohol, and smoking, the researchers found that plasma glucose levels accounted for a 6% to 10% change in volume. “These findings suggest that even in the subclinical range and in the absence of diabetes, monitoring and management of plasma glucose levels could have an impact on cerebral health,” the study authors wrote.
The FDA has approved once-a-day tablet Aubagio (teriflunomide) for treatment of adults with relapsing forms of multiple sclerosis (MS). During a clinical trial, patients taking teriflunomide had a relapse rate that was 30% lower than that of patients taking placebo. The most common side effects observed during clinical trials were diarrhea, abnormal liver tests, nausea, and hair loss, and physicians should conduct blood tests to check patients’ liver function before the drug is prescribed as well as periodically during treatment, researchers said. In addition, because of a risk of fetal harm, women of childbearing age must have a negative pregnancy test before beginning teriflunomide and should use birth control throughout treatment. Teriflunomide is the second oral treatment therapy for MS to be approved in the United States.
Patients who appear likely to have sporadic Creutzfeldt-Jakob disease may benefit from CSF 14-3-3 assays to clarify the diagnosis, researchers reported in the online September 19 Neurology. In a systematic literature review, the investigators identified articles from 1995 to January 1, 2011, that involved patients who had CSF analysis for protein 14-3-3. Based on data from 1,849 patients, the researchers determined that assays for CSF 14-3-3 are probably moderately accurate in diagnosing Creutzfeldt-Jakob—the assays had a sensitivity of 92%, specificity of 80%, likelihood ratio of 4.7, and negative likelihood ratio of 0.10. The study authors recommend CSF 14-3-3 assays “for patients who have rapidly progressive dementia and are strongly suspected of having sporadic Creutzfeldt-Jakob and for whom diagnosis remains uncertain (pretest probably of between 20% and 90%).”
Children with migraine and children with tension-type headaches are significantly more likely to have behavioral and emotional symptoms, and the frequency of headaches affects the likelihood of these symptoms, according to a study published in the online September 17 Cephalagia. After examining a sample of 1,856 children ages 5 to 11, investigators found that those with migraine were significantly more likely to experience abnormalities in somatic, anxiety-depressive, social, attention, internalizing, and total score domains of the Child Behavior Checklist. Children with tension-type headaches had a lower rate of abnormalities than children with migraine, but those with tension-type headaches still had significantly more abnormalities than controls. Children with headaches are more likely to have internalizing symptoms than externalizing symptoms such as rule breaking and aggressivity, the researchers found.
Heavy alcohol intake is associated with experiencing intracerebral hemorrhage at a younger age, according to a study published in the September 11 Neurology. Researchers prospectively followed 562 adults with spontaneous intracerebral hemorrhage and recorded information about their alcohol intake. A total of 137 patients were heavy alcohol drinkers, and these patients were more likely to be younger (median age, 60), to have a history of ischemic heart disease, and to be smokers. Furthermore, heavy alcohol drinkers had significantly lower platelet counts and prothrombin ratio. The investigators noted that although heavy alcohol intake is associated with intracerebral hemorrhage at a younger age, “the underlying vasculopathy remains unexplored in these patients. Indirect markers suggest small-vessel disease at an early stage that might be enhanced by moderate hemostatic disorders,” the authors concluded.
Long-term use of ginkgo biloba extract does not prevent the onset of Alzheimer’s disease in older patients, according to a study published in the online September 5 Lancet Neurology. Researchers enrolled 2,854 participants in a parallel-group, double-blind clinical trial in which 1,406 persons were randomized to receive ginkgo biloba extract and 1,414 persons were randomized to placebo. After five years of follow-up, 61 participants taking ginkgo biloba were diagnosed with probable Alzheimer’s disease, while 73 participants in the placebo group received a diagnosis of probable Alzheimer’s disease, though the risk was not proportional over time. The incidence of adverse events, as well as hemorrhagic or cardiovascular events, did not differ between groups. “Long-term use of standardized ginkgo biloba extract in this trial did not reduce the risk of progression to Alzheimer’s disease compared with placebo,” the researchers concluded.
The 13.3–mg/24 h dosage strength of the Exelon Patch (rivastigmine transdermal system) has been approved by the FDA for treatment of mild to moderate Alzheimer’s disease. Approval was based on the performance of the 13.3–mg/24 h dosage in the 48-week, double-blind phase of the OPTIMA study, which analyzed patients with mild to moderate Alzheimer’s disease who met predefined functional and cognitive decline criteria for the 9.5–mg/24 h dose. Compared with patients taking the 9.5–mg/24 h dose, patients taking the 13.3–mg/24 h dose showed statistically significant improvement in overall function. In addition, the overall safety profile of the 13.3–mg/24 h dose was the same as that of the lower dose, and fewer patients on the 13.3–mg/24 h dose needed to discontinue treatment than patients on the 9.5–mg/24 h dose.
The FDA has approved Nucynta (tapentadol) for management of neuropathic pain associated with diabetic peripheral neuropathy when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. According to preclinical studies, the drug is a centrally acting synthetic analgesic, though the exact mechanism of action is unknown. In two randomized-withdrawal, placebo-controlled phase III trials, researchers studied patients who had at least a one-point reduction in pain intensity during three weeks of treatment and then continued for an additional 12 weeks on the same dose, which was titrated to balance individual tolerability and efficacy. These patients had significantly better pain control than those who switched to placebo. The most common adverse events associated with the drug were nausea, constipation, vomiting, dizziness, headache, and somnolence, but tapentadol was generally well tolerated.
A mouse model of abnormal adult-generated granule cells (DGCs) has provided the first direct evidence that abnormal DGCs are linked to seizures, researchers reported in the September 20 Neuron. To isolate the effects of the abnormal cells, investigators used a transgenic mouse model to selectively delete PTEN from DGCs generated after birth. As a result of PTEN deletion, the mammalian target of rapamycin pathway was hyperactivated, which produced abnormal DGCs that resembled those in epilepsy. “Strikingly, animals in which PTEN was deleted from 9% or more of the DGC population developed spontaneous seizures in about four weeks, confirming that abnormal DGCs, which are present in both animals and humans with epilepsy, are capable of causing the disease,” the researchers stated.
Patients with multiple sclerosis (MS) who receive gingko biloba 120 mg twice a day do not show improved cognitive performance, researchers reported in the September 18 Neurology. The investigators compared the performance of two groups of patients with MS who scored 1 SD or more below the mean on one of four neuropsychologic tests. Sixty-one patients received 120 mg of ginkgo biloba twice a day for 12 weeks, and 59 patients received placebo. The researchers evaluated participants’ cognitive performance following treatment and found no statistically significant difference in scores between the two groups. Furthermore, no significant adverse events related to gingko biloba treatment occurred, according to the study authors. Overall, the investigators concluded that gingko biloba does not improve cognitive function in patients with MS.
The Solitaire Flow Restoration device performs substantially better than the Merci Retrieval System in treating acute ischemic stroke, according to a study published in the online August 24 Lancet. In a randomized, parallel-group, noninferiority trial, the efficacy and safety of the Solitaire device, a self-expanding stent retriever designed to quickly restore blood flow, was compared with the efficacy and safety of the standard Merci Retrieval system. The 58 patients in the Solitaire group achieved the primary efficacy outcome 61% of the time, compared with 24% of patients in the Merci group, investigators said. Furthermore, patients in the Solitaire group had lower 90-day mortality than patients in the Merci group (17 versus 38). “The Solitaire device might be a future treatment of choice for endovascular recanalization in acute ischemic stroke,” the researchers concluded.
—Lauren LeBano
Transient ischemic attack (TIA) is linked with a substantial risk of disability, researchers reported in the September 13 online Stroke. The 510 consecutive patients prospectively enrolled in the study had minor stroke or TIA, were not previously disabled, and had a CT or CT angiography completed within 24 hours of symptom onset. After assessing disability 90 days following the event, the investigators found that 15% of patients had a disabled outcome. Those who experienced recurrent strokes were more likely to be disabled—53% of patients with recurrent strokes were disabled, compared with 12% of those who did not have a recurrent stroke. “In terms of absolute numbers, most patients have disability as a result of their presenting event; however, recurrent events have the largest relative impact on outcome,” the study authors concluded.
Persons with high plasma glucose levels that are still within the normal range are more likely to have atrophy of brain structures associated with neurodegenerative processes, according to a study published in the September 4 Neurology. Investigators used MRI scans to assess hippocampal and amygdalar volumes in a sample of 266 cognitively healthy persons ages 60 to 64 who did not have type 2 diabetes. Results showed that plasma glucose levels were significantly linked with hippocampal and amygdalar atrophy. After controlling for age, sex, BMI, hypertension, alcohol, and smoking, the researchers found that plasma glucose levels accounted for a 6% to 10% change in volume. “These findings suggest that even in the subclinical range and in the absence of diabetes, monitoring and management of plasma glucose levels could have an impact on cerebral health,” the study authors wrote.
The FDA has approved once-a-day tablet Aubagio (teriflunomide) for treatment of adults with relapsing forms of multiple sclerosis (MS). During a clinical trial, patients taking teriflunomide had a relapse rate that was 30% lower than that of patients taking placebo. The most common side effects observed during clinical trials were diarrhea, abnormal liver tests, nausea, and hair loss, and physicians should conduct blood tests to check patients’ liver function before the drug is prescribed as well as periodically during treatment, researchers said. In addition, because of a risk of fetal harm, women of childbearing age must have a negative pregnancy test before beginning teriflunomide and should use birth control throughout treatment. Teriflunomide is the second oral treatment therapy for MS to be approved in the United States.
Patients who appear likely to have sporadic Creutzfeldt-Jakob disease may benefit from CSF 14-3-3 assays to clarify the diagnosis, researchers reported in the online September 19 Neurology. In a systematic literature review, the investigators identified articles from 1995 to January 1, 2011, that involved patients who had CSF analysis for protein 14-3-3. Based on data from 1,849 patients, the researchers determined that assays for CSF 14-3-3 are probably moderately accurate in diagnosing Creutzfeldt-Jakob—the assays had a sensitivity of 92%, specificity of 80%, likelihood ratio of 4.7, and negative likelihood ratio of 0.10. The study authors recommend CSF 14-3-3 assays “for patients who have rapidly progressive dementia and are strongly suspected of having sporadic Creutzfeldt-Jakob and for whom diagnosis remains uncertain (pretest probably of between 20% and 90%).”
Children with migraine and children with tension-type headaches are significantly more likely to have behavioral and emotional symptoms, and the frequency of headaches affects the likelihood of these symptoms, according to a study published in the online September 17 Cephalagia. After examining a sample of 1,856 children ages 5 to 11, investigators found that those with migraine were significantly more likely to experience abnormalities in somatic, anxiety-depressive, social, attention, internalizing, and total score domains of the Child Behavior Checklist. Children with tension-type headaches had a lower rate of abnormalities than children with migraine, but those with tension-type headaches still had significantly more abnormalities than controls. Children with headaches are more likely to have internalizing symptoms than externalizing symptoms such as rule breaking and aggressivity, the researchers found.
Heavy alcohol intake is associated with experiencing intracerebral hemorrhage at a younger age, according to a study published in the September 11 Neurology. Researchers prospectively followed 562 adults with spontaneous intracerebral hemorrhage and recorded information about their alcohol intake. A total of 137 patients were heavy alcohol drinkers, and these patients were more likely to be younger (median age, 60), to have a history of ischemic heart disease, and to be smokers. Furthermore, heavy alcohol drinkers had significantly lower platelet counts and prothrombin ratio. The investigators noted that although heavy alcohol intake is associated with intracerebral hemorrhage at a younger age, “the underlying vasculopathy remains unexplored in these patients. Indirect markers suggest small-vessel disease at an early stage that might be enhanced by moderate hemostatic disorders,” the authors concluded.
Long-term use of ginkgo biloba extract does not prevent the onset of Alzheimer’s disease in older patients, according to a study published in the online September 5 Lancet Neurology. Researchers enrolled 2,854 participants in a parallel-group, double-blind clinical trial in which 1,406 persons were randomized to receive ginkgo biloba extract and 1,414 persons were randomized to placebo. After five years of follow-up, 61 participants taking ginkgo biloba were diagnosed with probable Alzheimer’s disease, while 73 participants in the placebo group received a diagnosis of probable Alzheimer’s disease, though the risk was not proportional over time. The incidence of adverse events, as well as hemorrhagic or cardiovascular events, did not differ between groups. “Long-term use of standardized ginkgo biloba extract in this trial did not reduce the risk of progression to Alzheimer’s disease compared with placebo,” the researchers concluded.
The 13.3–mg/24 h dosage strength of the Exelon Patch (rivastigmine transdermal system) has been approved by the FDA for treatment of mild to moderate Alzheimer’s disease. Approval was based on the performance of the 13.3–mg/24 h dosage in the 48-week, double-blind phase of the OPTIMA study, which analyzed patients with mild to moderate Alzheimer’s disease who met predefined functional and cognitive decline criteria for the 9.5–mg/24 h dose. Compared with patients taking the 9.5–mg/24 h dose, patients taking the 13.3–mg/24 h dose showed statistically significant improvement in overall function. In addition, the overall safety profile of the 13.3–mg/24 h dose was the same as that of the lower dose, and fewer patients on the 13.3–mg/24 h dose needed to discontinue treatment than patients on the 9.5–mg/24 h dose.
The FDA has approved Nucynta (tapentadol) for management of neuropathic pain associated with diabetic peripheral neuropathy when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. According to preclinical studies, the drug is a centrally acting synthetic analgesic, though the exact mechanism of action is unknown. In two randomized-withdrawal, placebo-controlled phase III trials, researchers studied patients who had at least a one-point reduction in pain intensity during three weeks of treatment and then continued for an additional 12 weeks on the same dose, which was titrated to balance individual tolerability and efficacy. These patients had significantly better pain control than those who switched to placebo. The most common adverse events associated with the drug were nausea, constipation, vomiting, dizziness, headache, and somnolence, but tapentadol was generally well tolerated.
A mouse model of abnormal adult-generated granule cells (DGCs) has provided the first direct evidence that abnormal DGCs are linked to seizures, researchers reported in the September 20 Neuron. To isolate the effects of the abnormal cells, investigators used a transgenic mouse model to selectively delete PTEN from DGCs generated after birth. As a result of PTEN deletion, the mammalian target of rapamycin pathway was hyperactivated, which produced abnormal DGCs that resembled those in epilepsy. “Strikingly, animals in which PTEN was deleted from 9% or more of the DGC population developed spontaneous seizures in about four weeks, confirming that abnormal DGCs, which are present in both animals and humans with epilepsy, are capable of causing the disease,” the researchers stated.
Patients with multiple sclerosis (MS) who receive gingko biloba 120 mg twice a day do not show improved cognitive performance, researchers reported in the September 18 Neurology. The investigators compared the performance of two groups of patients with MS who scored 1 SD or more below the mean on one of four neuropsychologic tests. Sixty-one patients received 120 mg of ginkgo biloba twice a day for 12 weeks, and 59 patients received placebo. The researchers evaluated participants’ cognitive performance following treatment and found no statistically significant difference in scores between the two groups. Furthermore, no significant adverse events related to gingko biloba treatment occurred, according to the study authors. Overall, the investigators concluded that gingko biloba does not improve cognitive function in patients with MS.
The Solitaire Flow Restoration device performs substantially better than the Merci Retrieval System in treating acute ischemic stroke, according to a study published in the online August 24 Lancet. In a randomized, parallel-group, noninferiority trial, the efficacy and safety of the Solitaire device, a self-expanding stent retriever designed to quickly restore blood flow, was compared with the efficacy and safety of the standard Merci Retrieval system. The 58 patients in the Solitaire group achieved the primary efficacy outcome 61% of the time, compared with 24% of patients in the Merci group, investigators said. Furthermore, patients in the Solitaire group had lower 90-day mortality than patients in the Merci group (17 versus 38). “The Solitaire device might be a future treatment of choice for endovascular recanalization in acute ischemic stroke,” the researchers concluded.
—Lauren LeBano
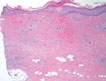
Livedoid Vasculopathy
What's Eating You? Bedbugs Revisited (Cimex lectularius)
Menkes Syndrome Presenting as Possible Child Abuse
Stalked by a ‘patient’
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
Hyperpigmentation and atrophy
A 35-year-old woman sought care at our clinic for a plaque on her upper left arm. She said that it had started 3 months earlier as a small indentation, but had recently became larger and hyperpigmented. The lesion was not pruritic or painful, and she had no associated weakness or systemic symptoms. The patient denied any insect bites, instrumentation, topical ointments, or trauma to the area.
Physical examination revealed a 3.5 × 2.5 cm area of hyperpigmentation on the posterior aspect of the left arm, overlying the musculotendinous junction of the lateral head of the triceps (FIGURE 1). The lesion had an irregular border and a central region approximately 1 cm in diameter associated with a nontender subcutaneous mass that felt tethered to the skin. There was significant thinning of the subcutaneous fat beneath the hyperpigmentation relative to the normal surrounding skin. The patient had normal triceps function and a normal distal neurovascular exam.
Concerned about malignancy, we ordered computed tomography (CT) and magnetic resonance imaging (MRI) of the left arm. Both demonstrated a nonspecific density in the subcutaneous tissue, and focal indentation and architectural distortion of the skin and subcutaneous tissue in the area in question. In addition, the MRI showed skin tethering extending to the superficial myofascial layer of the posterior triceps muscle and a small superficial blood vessel (FIGURE 2).
We referred the patient to Plastic Surgery for tissue diagnosis. A punch biopsy revealed dense dermal sclerosis that could be consistent with a morphea-like process. As malignancy could not be excluded, the patient was then referred to a dermatologist to determine the need for excision.
FIGURE 1
A 3.5 × 2.5 cm lesion on the left posterior arm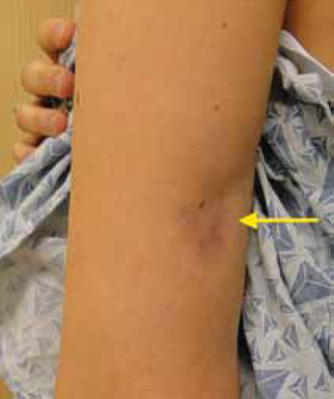
FIGURE 2
MRI reveals skin tethering to superficial myofascial layer
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophy due to a steroid injection
During further discussion with the patient, she revealed that she had received a steroid injection 4 to 5 months before the lesion appeared. Although we felt reasonably certain that the steroid injection was the cause of the lesion, the patient had the lesion excised.
The most striking pathologic findings in the excised specimen were seen in the subcutaneous tissue; there was extensive fat necrosis, with abundant amorphous eosinophilic and amphophilic debris replacing, and interspersed between, adipocytes (FIGURE 3). Extensive lipomembranous changes were also seen. The constellation of pathologic findings was nonspecific.
FIGURE 3
Variation in adipocyte size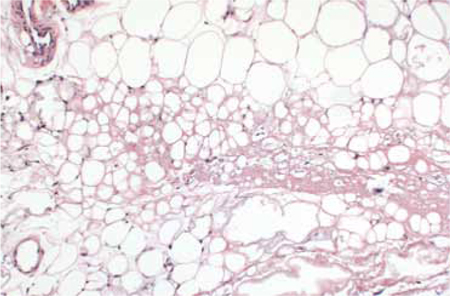
Low magnification of the excised specimen shows variation of adipocyte size, with some fat cells being replaced by amorphous eosinophilic and amphophilic material.
When treatment does harm
Steroid injections are commonly used to treat dermatologic and musculoskeletal conditions such as keloids, alopecia areata, neuromas, and inflamed bursas. However, these injections can have long-lasting dermatologic consequences such as altered pigmentation, dermal and fat atrophy, hypo- and hyperpigmentation, and telangiectasias.1-13 Localized lipodystrophy, the loss of subcutaneous fat in a localized area, can also be a result of steroid injection, as well as the injection of other drugs such as insulin or antibiotics.14
Morphea-like change, which we saw in our patient, is less common, but has also been described in the literature.1,2 Morphea presents with a single or several circumscribed indurated patches or plaques, usually with hypo- or hyperpigmentation.15
The timing of cutaneous changes due to steroid injection is variable. Case reports describe changes in pigmentation and atrophy beginning several weeks to several months after injection.9,10,12,13 This delay may occur because depot steroid preparations can remain in the skin for prolonged periods; one study demonstrated that small amounts persisted for more than a year after injection.2
Lesion with unclear etiology? Focus on the history
Because the cutaneous changes that steroids can induce are varied and nonspecific, it is important to carefully elicit any history of steroid injections when working up a patient for a cutaneous lesion of unclear etiology. Additional workup of neoplastic, infiltrative, vascular, and less commonly, infectious causes should be conducted if the etiology of such a lesion cannot be explained.
Although lesions from steroid injections are not usually evaluated with CT or MRI imaging, one study involving 2 volunteers suggested that pulsed ultrasound may be helpful in determining the long-term changes in skin thickness from steroid injection.16 Thus, it appears that radiological studies have little role in the diagnosis of steroid-induced skin changes, but may be used to raise or lower suspicion for other etiologies of cutaneous change when the diagnosis is unclear.
Healing comes in time, and sometimes, with saline
Cutaneous atrophy caused by steroid injections may resolve spontaneously within one to 2 years, or may persist.7,10,13,16,17 Treatment of persistent atrophy with normal saline infiltration has been used, and appears to be safe, tolerable, and relatively effective.17
Preventive steps to keep in mind
Attention to risk may reduce the likelihood and severity of cutaneous damage. Insoluble preparations should be used only for deep injections into joints, bursae, or muscles, and care should be taken not to track the steroid into the more superficial tissues.
More soluble preparations should be used for superficial structures.10,12 In addition, the lowest effective concentration of steroid preparation should be used, and it should not be mixed with vasoconstrictors like epinephrine.10 The anatomical location of the injection also plays a role in the extent and duration of change.10 For instance, injections into more superficial structures (eg, skin, tendons) could produce cutaneous changes that are more obvious than injections into deeper structures (eg, joints, bursae).10
Our patient
As noted earlier, our patient had the lesion excised. At follow-up one week later, she continued to progress well clinically.
CORRESPONDENCE Tia Kostas, MD, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115; [email protected]
1. Holt PJ, Marks R, Waddington E. ‘Pseudomorphoea’: a side effect of subcutaneous corticosteroid injection. Br J Dermatol. 1975;92:689-691.
2. Joshi R. Incidental finding of skin deposits of corticosteroids without associated granulomatous inflammation: report of three cases. Indian J Dermatol Venereol Leprol. 2008;74:44-46.
3. Reddy PD, Zelicof SB, Ruotolo C, et al. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995;(317):185-187.
4. Stapczynski JS. Localized depigmentation after steroid injection of a ganglion cyst on the hand. Ann Emerg Med. 1991;20:807-809.
5. Okere K, Jones MC. A case of skin hypopigmentation secondary to a corticosteroid injection. South Med J. 2006;99:1393-1394.
6. Basadonna PT, Rucco V, Gasparini D, et al. Plantar fat pad atrophy after corticosteroid injection for an interdigital neuroma: a case report. Am J Phys Med Rehabil. 1999;78:283-285.
7. DiStefano V, Nixon JE. Steroid-induced skin changes following local injection. Clin Orthop Relat Res. 1972;87:254-256.
8. Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. Report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
9. Gallardo MJ, Johnson DA. Cutaneous hypopigmentation following a posterior sub-tenon triamcinolone injection. Am J Ophthalmol. 2004;137:779-780.
10. Jacobs MB. Local subcutaneous atrophy after corticosteroid injection. Postgrad Med. 1986;80:159-160.
11. Louis DS, Hankin FM, Eckenrode JF. Cutaneous atrophy after corticosteroid injection. Am Fam Physician. 1986;33:183-186.
12. Lund IM, Donde R, Knudsen EA. Persistent local cutaneous atrophy following corticosteroid injection for tendinitis. Rheumatol Rehabil. 1979;18:91-93.
13. Schetman D, Hambrick GW, Jr, Wilson CE. Cutaneous changes following local injection of triamcinolone. Arch Dermatol. 1963;88:820-828.
14. Myers SA, Sheedy MP. Lipodystrophy. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:586–590.
15. Falanga V, Killoran CE. Morphea. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:543–546.
16. Gomez EC, Berman B, Miller DL. Ultrasonic assessment of cutaneous atrophy caused by intradermal corticosteroids. J Dermatol Surg Oncol. 1982;8:1071-1074.
17. Shumaker PR, Rao J, Goldman MP. Treatment of local, persistent cutaneous atrophy following corticosteroid injection with normal saline infiltration. Dermatol Surg. 2005;31:1340-1343.
A 35-year-old woman sought care at our clinic for a plaque on her upper left arm. She said that it had started 3 months earlier as a small indentation, but had recently became larger and hyperpigmented. The lesion was not pruritic or painful, and she had no associated weakness or systemic symptoms. The patient denied any insect bites, instrumentation, topical ointments, or trauma to the area.
Physical examination revealed a 3.5 × 2.5 cm area of hyperpigmentation on the posterior aspect of the left arm, overlying the musculotendinous junction of the lateral head of the triceps (FIGURE 1). The lesion had an irregular border and a central region approximately 1 cm in diameter associated with a nontender subcutaneous mass that felt tethered to the skin. There was significant thinning of the subcutaneous fat beneath the hyperpigmentation relative to the normal surrounding skin. The patient had normal triceps function and a normal distal neurovascular exam.
Concerned about malignancy, we ordered computed tomography (CT) and magnetic resonance imaging (MRI) of the left arm. Both demonstrated a nonspecific density in the subcutaneous tissue, and focal indentation and architectural distortion of the skin and subcutaneous tissue in the area in question. In addition, the MRI showed skin tethering extending to the superficial myofascial layer of the posterior triceps muscle and a small superficial blood vessel (FIGURE 2).
We referred the patient to Plastic Surgery for tissue diagnosis. A punch biopsy revealed dense dermal sclerosis that could be consistent with a morphea-like process. As malignancy could not be excluded, the patient was then referred to a dermatologist to determine the need for excision.
FIGURE 1
A 3.5 × 2.5 cm lesion on the left posterior arm
FIGURE 2
MRI reveals skin tethering to superficial myofascial layer
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophy due to a steroid injection
During further discussion with the patient, she revealed that she had received a steroid injection 4 to 5 months before the lesion appeared. Although we felt reasonably certain that the steroid injection was the cause of the lesion, the patient had the lesion excised.
The most striking pathologic findings in the excised specimen were seen in the subcutaneous tissue; there was extensive fat necrosis, with abundant amorphous eosinophilic and amphophilic debris replacing, and interspersed between, adipocytes (FIGURE 3). Extensive lipomembranous changes were also seen. The constellation of pathologic findings was nonspecific.
FIGURE 3
Variation in adipocyte size
Low magnification of the excised specimen shows variation of adipocyte size, with some fat cells being replaced by amorphous eosinophilic and amphophilic material.
When treatment does harm
Steroid injections are commonly used to treat dermatologic and musculoskeletal conditions such as keloids, alopecia areata, neuromas, and inflamed bursas. However, these injections can have long-lasting dermatologic consequences such as altered pigmentation, dermal and fat atrophy, hypo- and hyperpigmentation, and telangiectasias.1-13 Localized lipodystrophy, the loss of subcutaneous fat in a localized area, can also be a result of steroid injection, as well as the injection of other drugs such as insulin or antibiotics.14
Morphea-like change, which we saw in our patient, is less common, but has also been described in the literature.1,2 Morphea presents with a single or several circumscribed indurated patches or plaques, usually with hypo- or hyperpigmentation.15
The timing of cutaneous changes due to steroid injection is variable. Case reports describe changes in pigmentation and atrophy beginning several weeks to several months after injection.9,10,12,13 This delay may occur because depot steroid preparations can remain in the skin for prolonged periods; one study demonstrated that small amounts persisted for more than a year after injection.2
Lesion with unclear etiology? Focus on the history
Because the cutaneous changes that steroids can induce are varied and nonspecific, it is important to carefully elicit any history of steroid injections when working up a patient for a cutaneous lesion of unclear etiology. Additional workup of neoplastic, infiltrative, vascular, and less commonly, infectious causes should be conducted if the etiology of such a lesion cannot be explained.
Although lesions from steroid injections are not usually evaluated with CT or MRI imaging, one study involving 2 volunteers suggested that pulsed ultrasound may be helpful in determining the long-term changes in skin thickness from steroid injection.16 Thus, it appears that radiological studies have little role in the diagnosis of steroid-induced skin changes, but may be used to raise or lower suspicion for other etiologies of cutaneous change when the diagnosis is unclear.
Healing comes in time, and sometimes, with saline
Cutaneous atrophy caused by steroid injections may resolve spontaneously within one to 2 years, or may persist.7,10,13,16,17 Treatment of persistent atrophy with normal saline infiltration has been used, and appears to be safe, tolerable, and relatively effective.17
Preventive steps to keep in mind
Attention to risk may reduce the likelihood and severity of cutaneous damage. Insoluble preparations should be used only for deep injections into joints, bursae, or muscles, and care should be taken not to track the steroid into the more superficial tissues.
More soluble preparations should be used for superficial structures.10,12 In addition, the lowest effective concentration of steroid preparation should be used, and it should not be mixed with vasoconstrictors like epinephrine.10 The anatomical location of the injection also plays a role in the extent and duration of change.10 For instance, injections into more superficial structures (eg, skin, tendons) could produce cutaneous changes that are more obvious than injections into deeper structures (eg, joints, bursae).10
Our patient
As noted earlier, our patient had the lesion excised. At follow-up one week later, she continued to progress well clinically.
CORRESPONDENCE Tia Kostas, MD, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115; [email protected]
A 35-year-old woman sought care at our clinic for a plaque on her upper left arm. She said that it had started 3 months earlier as a small indentation, but had recently became larger and hyperpigmented. The lesion was not pruritic or painful, and she had no associated weakness or systemic symptoms. The patient denied any insect bites, instrumentation, topical ointments, or trauma to the area.
Physical examination revealed a 3.5 × 2.5 cm area of hyperpigmentation on the posterior aspect of the left arm, overlying the musculotendinous junction of the lateral head of the triceps (FIGURE 1). The lesion had an irregular border and a central region approximately 1 cm in diameter associated with a nontender subcutaneous mass that felt tethered to the skin. There was significant thinning of the subcutaneous fat beneath the hyperpigmentation relative to the normal surrounding skin. The patient had normal triceps function and a normal distal neurovascular exam.
Concerned about malignancy, we ordered computed tomography (CT) and magnetic resonance imaging (MRI) of the left arm. Both demonstrated a nonspecific density in the subcutaneous tissue, and focal indentation and architectural distortion of the skin and subcutaneous tissue in the area in question. In addition, the MRI showed skin tethering extending to the superficial myofascial layer of the posterior triceps muscle and a small superficial blood vessel (FIGURE 2).
We referred the patient to Plastic Surgery for tissue diagnosis. A punch biopsy revealed dense dermal sclerosis that could be consistent with a morphea-like process. As malignancy could not be excluded, the patient was then referred to a dermatologist to determine the need for excision.
FIGURE 1
A 3.5 × 2.5 cm lesion on the left posterior arm
FIGURE 2
MRI reveals skin tethering to superficial myofascial layer
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophy due to a steroid injection
During further discussion with the patient, she revealed that she had received a steroid injection 4 to 5 months before the lesion appeared. Although we felt reasonably certain that the steroid injection was the cause of the lesion, the patient had the lesion excised.
The most striking pathologic findings in the excised specimen were seen in the subcutaneous tissue; there was extensive fat necrosis, with abundant amorphous eosinophilic and amphophilic debris replacing, and interspersed between, adipocytes (FIGURE 3). Extensive lipomembranous changes were also seen. The constellation of pathologic findings was nonspecific.
FIGURE 3
Variation in adipocyte size
Low magnification of the excised specimen shows variation of adipocyte size, with some fat cells being replaced by amorphous eosinophilic and amphophilic material.
When treatment does harm
Steroid injections are commonly used to treat dermatologic and musculoskeletal conditions such as keloids, alopecia areata, neuromas, and inflamed bursas. However, these injections can have long-lasting dermatologic consequences such as altered pigmentation, dermal and fat atrophy, hypo- and hyperpigmentation, and telangiectasias.1-13 Localized lipodystrophy, the loss of subcutaneous fat in a localized area, can also be a result of steroid injection, as well as the injection of other drugs such as insulin or antibiotics.14
Morphea-like change, which we saw in our patient, is less common, but has also been described in the literature.1,2 Morphea presents with a single or several circumscribed indurated patches or plaques, usually with hypo- or hyperpigmentation.15
The timing of cutaneous changes due to steroid injection is variable. Case reports describe changes in pigmentation and atrophy beginning several weeks to several months after injection.9,10,12,13 This delay may occur because depot steroid preparations can remain in the skin for prolonged periods; one study demonstrated that small amounts persisted for more than a year after injection.2
Lesion with unclear etiology? Focus on the history
Because the cutaneous changes that steroids can induce are varied and nonspecific, it is important to carefully elicit any history of steroid injections when working up a patient for a cutaneous lesion of unclear etiology. Additional workup of neoplastic, infiltrative, vascular, and less commonly, infectious causes should be conducted if the etiology of such a lesion cannot be explained.
Although lesions from steroid injections are not usually evaluated with CT or MRI imaging, one study involving 2 volunteers suggested that pulsed ultrasound may be helpful in determining the long-term changes in skin thickness from steroid injection.16 Thus, it appears that radiological studies have little role in the diagnosis of steroid-induced skin changes, but may be used to raise or lower suspicion for other etiologies of cutaneous change when the diagnosis is unclear.
Healing comes in time, and sometimes, with saline
Cutaneous atrophy caused by steroid injections may resolve spontaneously within one to 2 years, or may persist.7,10,13,16,17 Treatment of persistent atrophy with normal saline infiltration has been used, and appears to be safe, tolerable, and relatively effective.17
Preventive steps to keep in mind
Attention to risk may reduce the likelihood and severity of cutaneous damage. Insoluble preparations should be used only for deep injections into joints, bursae, or muscles, and care should be taken not to track the steroid into the more superficial tissues.
More soluble preparations should be used for superficial structures.10,12 In addition, the lowest effective concentration of steroid preparation should be used, and it should not be mixed with vasoconstrictors like epinephrine.10 The anatomical location of the injection also plays a role in the extent and duration of change.10 For instance, injections into more superficial structures (eg, skin, tendons) could produce cutaneous changes that are more obvious than injections into deeper structures (eg, joints, bursae).10
Our patient
As noted earlier, our patient had the lesion excised. At follow-up one week later, she continued to progress well clinically.
CORRESPONDENCE Tia Kostas, MD, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115; [email protected]
1. Holt PJ, Marks R, Waddington E. ‘Pseudomorphoea’: a side effect of subcutaneous corticosteroid injection. Br J Dermatol. 1975;92:689-691.
2. Joshi R. Incidental finding of skin deposits of corticosteroids without associated granulomatous inflammation: report of three cases. Indian J Dermatol Venereol Leprol. 2008;74:44-46.
3. Reddy PD, Zelicof SB, Ruotolo C, et al. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995;(317):185-187.
4. Stapczynski JS. Localized depigmentation after steroid injection of a ganglion cyst on the hand. Ann Emerg Med. 1991;20:807-809.
5. Okere K, Jones MC. A case of skin hypopigmentation secondary to a corticosteroid injection. South Med J. 2006;99:1393-1394.
6. Basadonna PT, Rucco V, Gasparini D, et al. Plantar fat pad atrophy after corticosteroid injection for an interdigital neuroma: a case report. Am J Phys Med Rehabil. 1999;78:283-285.
7. DiStefano V, Nixon JE. Steroid-induced skin changes following local injection. Clin Orthop Relat Res. 1972;87:254-256.
8. Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. Report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
9. Gallardo MJ, Johnson DA. Cutaneous hypopigmentation following a posterior sub-tenon triamcinolone injection. Am J Ophthalmol. 2004;137:779-780.
10. Jacobs MB. Local subcutaneous atrophy after corticosteroid injection. Postgrad Med. 1986;80:159-160.
11. Louis DS, Hankin FM, Eckenrode JF. Cutaneous atrophy after corticosteroid injection. Am Fam Physician. 1986;33:183-186.
12. Lund IM, Donde R, Knudsen EA. Persistent local cutaneous atrophy following corticosteroid injection for tendinitis. Rheumatol Rehabil. 1979;18:91-93.
13. Schetman D, Hambrick GW, Jr, Wilson CE. Cutaneous changes following local injection of triamcinolone. Arch Dermatol. 1963;88:820-828.
14. Myers SA, Sheedy MP. Lipodystrophy. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:586–590.
15. Falanga V, Killoran CE. Morphea. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:543–546.
16. Gomez EC, Berman B, Miller DL. Ultrasonic assessment of cutaneous atrophy caused by intradermal corticosteroids. J Dermatol Surg Oncol. 1982;8:1071-1074.
17. Shumaker PR, Rao J, Goldman MP. Treatment of local, persistent cutaneous atrophy following corticosteroid injection with normal saline infiltration. Dermatol Surg. 2005;31:1340-1343.
1. Holt PJ, Marks R, Waddington E. ‘Pseudomorphoea’: a side effect of subcutaneous corticosteroid injection. Br J Dermatol. 1975;92:689-691.
2. Joshi R. Incidental finding of skin deposits of corticosteroids without associated granulomatous inflammation: report of three cases. Indian J Dermatol Venereol Leprol. 2008;74:44-46.
3. Reddy PD, Zelicof SB, Ruotolo C, et al. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995;(317):185-187.
4. Stapczynski JS. Localized depigmentation after steroid injection of a ganglion cyst on the hand. Ann Emerg Med. 1991;20:807-809.
5. Okere K, Jones MC. A case of skin hypopigmentation secondary to a corticosteroid injection. South Med J. 2006;99:1393-1394.
6. Basadonna PT, Rucco V, Gasparini D, et al. Plantar fat pad atrophy after corticosteroid injection for an interdigital neuroma: a case report. Am J Phys Med Rehabil. 1999;78:283-285.
7. DiStefano V, Nixon JE. Steroid-induced skin changes following local injection. Clin Orthop Relat Res. 1972;87:254-256.
8. Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. Report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
9. Gallardo MJ, Johnson DA. Cutaneous hypopigmentation following a posterior sub-tenon triamcinolone injection. Am J Ophthalmol. 2004;137:779-780.
10. Jacobs MB. Local subcutaneous atrophy after corticosteroid injection. Postgrad Med. 1986;80:159-160.
11. Louis DS, Hankin FM, Eckenrode JF. Cutaneous atrophy after corticosteroid injection. Am Fam Physician. 1986;33:183-186.
12. Lund IM, Donde R, Knudsen EA. Persistent local cutaneous atrophy following corticosteroid injection for tendinitis. Rheumatol Rehabil. 1979;18:91-93.
13. Schetman D, Hambrick GW, Jr, Wilson CE. Cutaneous changes following local injection of triamcinolone. Arch Dermatol. 1963;88:820-828.
14. Myers SA, Sheedy MP. Lipodystrophy. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:586–590.
15. Falanga V, Killoran CE. Morphea. In: Wolf KG, Lowell A, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies, Inc; 2003:543–546.
16. Gomez EC, Berman B, Miller DL. Ultrasonic assessment of cutaneous atrophy caused by intradermal corticosteroids. J Dermatol Surg Oncol. 1982;8:1071-1074.
17. Shumaker PR, Rao J, Goldman MP. Treatment of local, persistent cutaneous atrophy following corticosteroid injection with normal saline infiltration. Dermatol Surg. 2005;31:1340-1343.
Can calcium supplements cause serious adverse effects in healthy people?
Yes, according to studies with some limitations. Calcium supplements with or without vitamin D increase the risk of myocardial infarction (MI), with numbers needed to harm (NNH) over 5 years of 69 to 240 (strength of recommendation [SOR]: B, meta-analyses of randomized controlled trials [RCTs] that evaluated a predominantly older female population and were limited by study designs).
Calcium supplements with or without vitamin D may increase the risk of stroke, with an NNH over 5 years of 283 (SOR: B, meta-analyses of RCTs).
Calcium supplementation, but not a diet rich in calcium, also increases the risk of renal calculi, with an NNH over 7 years of 272 (SOR: B, RCT and a cohort study, which also evaluated a predominantly older female population).
Evidence summary
A meta-analysis of 11 randomized, double-blinded placebo-controlled studies assessed the relationship between calcium supplements and the risk of cardiovascular events.1 A total of 20,071 predominantly female patients (83%) with a mean age of 72 years (range, 51-77 years) received ≥500 mg elemental calcium per day for at least 1 year. Median follow-up was 3.6 to 4 years. Five studies provided individual patient data and all 11 provided trial-level data.
In the 5 studies contributing patient data, women taking calcium supplements had an increased incidence of MI (hazard ratio [HR]=1.31; 95% confidence interval [CI], 1.02-1.67; P=.035) with an NNH of 69 over 5 years of calcium supplementation. The trial-level data, from 11 trials with 11,921 patients, also showed an increased incidence of MI in women taking calcium (relative risk [RR]=1.27; 95% CI, 1.01-1.59; P=.038). Neither the patient data nor the trial-level data demonstrated a significant increase in strokes.
Limitations of this meta-analysis include the fact that none of the trials was designed to address the risk of cardiovascular disease; in addition, some studies assessed outcomes by patient self-report, raising the possibility of information bias.
Some studies also show an increased stroke risk
The Women’s Health Initiative (WHI) study initially reported no increase in cardiovascular risk among women who received calcium and vitamin D supplements, but it didn’t take into account whether women were already taking calcium or vitamin D at the time of randomization.2 Re-analysis of the 16,718 women (mean age 62.9 years) randomized to calcium and vitamin D and not taking calcium supplements before the study found a statistically significant increase in the risk of MI or revascularization (HR=1.16; 95% CI, 1.01-1.34; P=.04).3
A meta-analysis of these findings and 2 additional RCTs (88% of subjects were female) comparing calcium and vitamin D supplementation with placebo found an increased risk of MI or stroke (RR=1.16; 95% CI, 1.02-1.32; P=.02).
Another meta-analysis that examined the WHI data and 5 placebo-controlled studies of calcium or calcium and vitamin D supplementation (82% of subjects were female) found an increased risk of MI, with NNHs over 5 years of 240 for MI (RR=1.26; 95% CI, 1.07-1.47; P=.005), 283 for stroke (RR=1.19; 95% CI, 1.02-1.39; P=.03), and 178 for the composite of MI or stroke (RR=1.17; 95% CI, 1.05-1.31; P=.005).3 The number needed to treat with calcium (with or without vitamin D) for 5 years to prevent one fracture was 302. The conclusions of this study were limited by post hoc and subgroup analyses.4
These studies did not address dietary sources rich in calcium. Dietary calcium results in lower peak serum levels than supplementary calcium, with less potential for adverse effects.3
Supplemental, but not dietary, calcium raises the risk of kidney stones
To assess the risk of renal calculi, the WHI randomized 36,282 postmenopausal women to calcium with vitamin D or placebo. Calcium and vitamin D increased the risk of renal calculi (HR=1.17; 95% CI, 1.02-1.34), with an NNH of 272 over 7 years.5
In a prospective cohort study of 91,731 women with 12-year follow-up, supplementary calcium was associated with an increased risk of kidney stone formation (RR=1.2; 95% CI, 1.02-1.41), whereas high dietary calcium was linked to a lower risk.6
Recommendations
The Institute of Medicine’s (IOM’s) recommended dietary allowance for calcium from diet plus supplements is 1000 mg a day for women until 50 years of age and no more than 1200 mg a day for women older than 50 years. The IOM advocates a maximum calcium intake of 2000 mg a day for women in both age groups because of the increased risk of kidney stones.7
1. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691.-
2. Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846-854.
3. Bolland M, Grey A, Avenell A, et al. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040.-
4. Abrahamsen B, Sahota O. Do calcium plus vitamin D supplements increase cardiovascular risk? BMJ. 2011;342:d2080.-
5. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
6. Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497-504.
7. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Report brief, November 2010. Washington, DC: Institute of Medicine; 2001. Available at: www.iom.edu/~/ media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20 Calcium%202010%20Report%20Brief.pdf. Access September 14, 2012.
Yes, according to studies with some limitations. Calcium supplements with or without vitamin D increase the risk of myocardial infarction (MI), with numbers needed to harm (NNH) over 5 years of 69 to 240 (strength of recommendation [SOR]: B, meta-analyses of randomized controlled trials [RCTs] that evaluated a predominantly older female population and were limited by study designs).
Calcium supplements with or without vitamin D may increase the risk of stroke, with an NNH over 5 years of 283 (SOR: B, meta-analyses of RCTs).
Calcium supplementation, but not a diet rich in calcium, also increases the risk of renal calculi, with an NNH over 7 years of 272 (SOR: B, RCT and a cohort study, which also evaluated a predominantly older female population).
Evidence summary
A meta-analysis of 11 randomized, double-blinded placebo-controlled studies assessed the relationship between calcium supplements and the risk of cardiovascular events.1 A total of 20,071 predominantly female patients (83%) with a mean age of 72 years (range, 51-77 years) received ≥500 mg elemental calcium per day for at least 1 year. Median follow-up was 3.6 to 4 years. Five studies provided individual patient data and all 11 provided trial-level data.
In the 5 studies contributing patient data, women taking calcium supplements had an increased incidence of MI (hazard ratio [HR]=1.31; 95% confidence interval [CI], 1.02-1.67; P=.035) with an NNH of 69 over 5 years of calcium supplementation. The trial-level data, from 11 trials with 11,921 patients, also showed an increased incidence of MI in women taking calcium (relative risk [RR]=1.27; 95% CI, 1.01-1.59; P=.038). Neither the patient data nor the trial-level data demonstrated a significant increase in strokes.
Limitations of this meta-analysis include the fact that none of the trials was designed to address the risk of cardiovascular disease; in addition, some studies assessed outcomes by patient self-report, raising the possibility of information bias.
Some studies also show an increased stroke risk
The Women’s Health Initiative (WHI) study initially reported no increase in cardiovascular risk among women who received calcium and vitamin D supplements, but it didn’t take into account whether women were already taking calcium or vitamin D at the time of randomization.2 Re-analysis of the 16,718 women (mean age 62.9 years) randomized to calcium and vitamin D and not taking calcium supplements before the study found a statistically significant increase in the risk of MI or revascularization (HR=1.16; 95% CI, 1.01-1.34; P=.04).3
A meta-analysis of these findings and 2 additional RCTs (88% of subjects were female) comparing calcium and vitamin D supplementation with placebo found an increased risk of MI or stroke (RR=1.16; 95% CI, 1.02-1.32; P=.02).
Another meta-analysis that examined the WHI data and 5 placebo-controlled studies of calcium or calcium and vitamin D supplementation (82% of subjects were female) found an increased risk of MI, with NNHs over 5 years of 240 for MI (RR=1.26; 95% CI, 1.07-1.47; P=.005), 283 for stroke (RR=1.19; 95% CI, 1.02-1.39; P=.03), and 178 for the composite of MI or stroke (RR=1.17; 95% CI, 1.05-1.31; P=.005).3 The number needed to treat with calcium (with or without vitamin D) for 5 years to prevent one fracture was 302. The conclusions of this study were limited by post hoc and subgroup analyses.4
These studies did not address dietary sources rich in calcium. Dietary calcium results in lower peak serum levels than supplementary calcium, with less potential for adverse effects.3
Supplemental, but not dietary, calcium raises the risk of kidney stones
To assess the risk of renal calculi, the WHI randomized 36,282 postmenopausal women to calcium with vitamin D or placebo. Calcium and vitamin D increased the risk of renal calculi (HR=1.17; 95% CI, 1.02-1.34), with an NNH of 272 over 7 years.5
In a prospective cohort study of 91,731 women with 12-year follow-up, supplementary calcium was associated with an increased risk of kidney stone formation (RR=1.2; 95% CI, 1.02-1.41), whereas high dietary calcium was linked to a lower risk.6
Recommendations
The Institute of Medicine’s (IOM’s) recommended dietary allowance for calcium from diet plus supplements is 1000 mg a day for women until 50 years of age and no more than 1200 mg a day for women older than 50 years. The IOM advocates a maximum calcium intake of 2000 mg a day for women in both age groups because of the increased risk of kidney stones.7
Yes, according to studies with some limitations. Calcium supplements with or without vitamin D increase the risk of myocardial infarction (MI), with numbers needed to harm (NNH) over 5 years of 69 to 240 (strength of recommendation [SOR]: B, meta-analyses of randomized controlled trials [RCTs] that evaluated a predominantly older female population and were limited by study designs).
Calcium supplements with or without vitamin D may increase the risk of stroke, with an NNH over 5 years of 283 (SOR: B, meta-analyses of RCTs).
Calcium supplementation, but not a diet rich in calcium, also increases the risk of renal calculi, with an NNH over 7 years of 272 (SOR: B, RCT and a cohort study, which also evaluated a predominantly older female population).
Evidence summary
A meta-analysis of 11 randomized, double-blinded placebo-controlled studies assessed the relationship between calcium supplements and the risk of cardiovascular events.1 A total of 20,071 predominantly female patients (83%) with a mean age of 72 years (range, 51-77 years) received ≥500 mg elemental calcium per day for at least 1 year. Median follow-up was 3.6 to 4 years. Five studies provided individual patient data and all 11 provided trial-level data.
In the 5 studies contributing patient data, women taking calcium supplements had an increased incidence of MI (hazard ratio [HR]=1.31; 95% confidence interval [CI], 1.02-1.67; P=.035) with an NNH of 69 over 5 years of calcium supplementation. The trial-level data, from 11 trials with 11,921 patients, also showed an increased incidence of MI in women taking calcium (relative risk [RR]=1.27; 95% CI, 1.01-1.59; P=.038). Neither the patient data nor the trial-level data demonstrated a significant increase in strokes.
Limitations of this meta-analysis include the fact that none of the trials was designed to address the risk of cardiovascular disease; in addition, some studies assessed outcomes by patient self-report, raising the possibility of information bias.
Some studies also show an increased stroke risk
The Women’s Health Initiative (WHI) study initially reported no increase in cardiovascular risk among women who received calcium and vitamin D supplements, but it didn’t take into account whether women were already taking calcium or vitamin D at the time of randomization.2 Re-analysis of the 16,718 women (mean age 62.9 years) randomized to calcium and vitamin D and not taking calcium supplements before the study found a statistically significant increase in the risk of MI or revascularization (HR=1.16; 95% CI, 1.01-1.34; P=.04).3
A meta-analysis of these findings and 2 additional RCTs (88% of subjects were female) comparing calcium and vitamin D supplementation with placebo found an increased risk of MI or stroke (RR=1.16; 95% CI, 1.02-1.32; P=.02).
Another meta-analysis that examined the WHI data and 5 placebo-controlled studies of calcium or calcium and vitamin D supplementation (82% of subjects were female) found an increased risk of MI, with NNHs over 5 years of 240 for MI (RR=1.26; 95% CI, 1.07-1.47; P=.005), 283 for stroke (RR=1.19; 95% CI, 1.02-1.39; P=.03), and 178 for the composite of MI or stroke (RR=1.17; 95% CI, 1.05-1.31; P=.005).3 The number needed to treat with calcium (with or without vitamin D) for 5 years to prevent one fracture was 302. The conclusions of this study were limited by post hoc and subgroup analyses.4
These studies did not address dietary sources rich in calcium. Dietary calcium results in lower peak serum levels than supplementary calcium, with less potential for adverse effects.3
Supplemental, but not dietary, calcium raises the risk of kidney stones
To assess the risk of renal calculi, the WHI randomized 36,282 postmenopausal women to calcium with vitamin D or placebo. Calcium and vitamin D increased the risk of renal calculi (HR=1.17; 95% CI, 1.02-1.34), with an NNH of 272 over 7 years.5
In a prospective cohort study of 91,731 women with 12-year follow-up, supplementary calcium was associated with an increased risk of kidney stone formation (RR=1.2; 95% CI, 1.02-1.41), whereas high dietary calcium was linked to a lower risk.6
Recommendations
The Institute of Medicine’s (IOM’s) recommended dietary allowance for calcium from diet plus supplements is 1000 mg a day for women until 50 years of age and no more than 1200 mg a day for women older than 50 years. The IOM advocates a maximum calcium intake of 2000 mg a day for women in both age groups because of the increased risk of kidney stones.7
1. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691.-
2. Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846-854.
3. Bolland M, Grey A, Avenell A, et al. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040.-
4. Abrahamsen B, Sahota O. Do calcium plus vitamin D supplements increase cardiovascular risk? BMJ. 2011;342:d2080.-
5. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
6. Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497-504.
7. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Report brief, November 2010. Washington, DC: Institute of Medicine; 2001. Available at: www.iom.edu/~/ media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20 Calcium%202010%20Report%20Brief.pdf. Access September 14, 2012.
1. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691.-
2. Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846-854.
3. Bolland M, Grey A, Avenell A, et al. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040.-
4. Abrahamsen B, Sahota O. Do calcium plus vitamin D supplements increase cardiovascular risk? BMJ. 2011;342:d2080.-
5. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
6. Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497-504.
7. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Report brief, November 2010. Washington, DC: Institute of Medicine; 2001. Available at: www.iom.edu/~/ media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20 Calcium%202010%20Report%20Brief.pdf. Access September 14, 2012.
Evidence-based answers from the Family Physicians Inquiries Network
Preventive coding can be a snap
Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Download Medicare Guide
Guide to Billing the Medicare Annual Exam
Melanie Witt, RN, CPC, COBGC, MA (October 2012)
Coding and billing for the care provided at a well-woman visit can be uncomplicated if you know the right codes for the right program. Here, I present information for straightforward preventive care. (I am assuming the patient has not also presented with a significant problem at the same visit.)
First, a patient who is not Medicare-eligible should have the annual well-woman exam billed using the CPT preventive medicine codes. There are some private insurers, however, that will only accept HCPCS codes for an annual gyn exam. These special codes are:
S0610 Annual gynecological examination, new patient
S0612 Annual gynecological examination, established patient
S0613 Annual gynecological examination; clinical breast examination without pelvic evaluation
Notably, Aetna Cigna, and United Healthcare require these codes for a gyn exam, but many BC/BS programs, for whom these codes were originally created, are now reverting to the CPT preventive medicine codes for all preventive care.
The CPT preventive codes are grouped by age and require an age- and gender-appropriate history, examination, and counseling/anticipatory guidance. The Medicare E/M documentation guidelines do not apply to preventive services, and a head-to-toe examination is also not required. CPT recognizes ACOG as an authoritative body to make recommendations for the expected preventive service for women, and if such a service is provided and documented, the preventive codes are to be reported.
The chart below summarizes the CPT preventive codes by patient status and age in comparison to ACOG age groupings.
| New Patient Preventive Medicine Code | |||
|---|---|---|---|
| New patient codes include an initial comprehensive preventive medicine evaluation and management of an individual including an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures | |||
| ACOG: 13–18 years 99384 (12–17 years) 99385 (18–39 years) | ACOG: 19–39 years 99385 (18–39 years) | ACOG: 40–64 years 99386 (40–64 years) | ACOG: 65 years and older 99387 (65 years and older) |
| Established Patient Preventive Medicine Codes | |||
| Established patient codes include periodic comprehensive preventive medicine reevaluation and management of an individual including an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures | |||
| ACOG: 13–18 years 99394 (12–17 years) 99395 (18–39 years) | ACOG: 19–39 years 99395 (18–39 years) | ACOG: 40–64 years 99396 (40–64 years) | ACOG: 65 years and older 99397 (65 years and older) |
The main code
The appropriate diagnostic link for the CPT preventive gyn annual well-woman exam is V72.31, whether or not a Pap specimen is collected. The collection of the Pap specimen is included in the preventive service, as is counseling regarding birth control, or general questions about preventing problems, including hormone replacement therapy.
If a pelvic examination is not performed, say because the patient is young and not sexually active, but an examination of other areas is carried out, the same preventive codes are reported, but the diagnosis code changes to V70.0, general health exam.
What about Medicare?
Coding. Medicare requirements are somewhat different. First, Medicare covers only a small portion of the preventive service; that is, they cover a physical examination of the genital organs and breasts and the collection and conveyance of a Pap specimen to the lab in the covered year only. Think of the complete preventive service as described in CPT as a pie—Medicare pays for 2 slices of that pie in a covered year. The two codes for these services are:
G0101 (Cervical or vaginal cancer screening; pelvic and clinical breast examination)
Q0091 (Screening Papanicolaou smear; obtaining, preparing, and conveyance of cervical or vaginal smear to laboratory)
If the patient is at low risk for developing cervical or vaginal cancer, the screening pelvic exam and Pap collection are paid every 2 years. If the woman is at high risk, Medicare will cover this portion of the encounter every year. The high-risk criteria must be re-documented every year and must include one of the following:
- Early onset of sexual activity (under age 16)
- Multiple sexual partners (five or more in a lifetime)
- History of a sexually transmitted disease (including HIV infection)
- Fewer than three negative Pap smears within the previous 7 years
- Diethylstilbestrol (DES)-exposed daughters of women who took DES during pregnancy.
If the Medicare-eligible patient is still of childbearing age, she is also considered high-risk if she has had an examination that indicated the presence of cervical or vaginal cancer or other genital abnormalities during any of the preceding 3 years. Note that these criteria do not include a history of breast cancer or a past history of cancer more than 3 years ago.
Billing. Because Medicare is paying only for a portion of the preventive service, you will need to subtract the Medicare allowable for codes G0101 and Q0091 from your normal fee for the preventive service.
- Example: If your usual fee for 99397 is $200, and the Medicare allowable for both the G and Q service is $82, you will charge the patient for the noncovered parts of the service at the rate of $118, and you will bill Medicare for their share of $82. You will collect from all sources the $200 for the preventive service. Remember, however, to get the patient to sign an ABN with regard to the Medicare part of the service. This will ensure that, if denied by Medicare, the patient will be held fully responsible for the denied amount.
The Medicare modifier is –GA (add it to codes G0101 and Q0091). Diagnostic coding is V72.31 (because a pelvic exam is performed). This code may also be linked to the collection code. For a high-risk patient, use code V15.89 (rather than V72.31). This code must be linked to the G and Q codes.
“Guide to Billing the Medicare Annual Exam” is a detailed Medicare checklist offered by the author that includes all billing scenarios for a Medicare patient. Click here to download a PDF.
Ms. Witt can be contacted directly at [email protected] should you have additional questions regarding coding and billing for preventive services.
We want to hear from you! Tell us what you think.
Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Download Medicare Guide
Guide to Billing the Medicare Annual Exam
Melanie Witt, RN, CPC, COBGC, MA (October 2012)
Coding and billing for the care provided at a well-woman visit can be uncomplicated if you know the right codes for the right program. Here, I present information for straightforward preventive care. (I am assuming the patient has not also presented with a significant problem at the same visit.)
First, a patient who is not Medicare-eligible should have the annual well-woman exam billed using the CPT preventive medicine codes. There are some private insurers, however, that will only accept HCPCS codes for an annual gyn exam. These special codes are:
S0610 Annual gynecological examination, new patient
S0612 Annual gynecological examination, established patient
S0613 Annual gynecological examination; clinical breast examination without pelvic evaluation
Notably, Aetna Cigna, and United Healthcare require these codes for a gyn exam, but many BC/BS programs, for whom these codes were originally created, are now reverting to the CPT preventive medicine codes for all preventive care.
The CPT preventive codes are grouped by age and require an age- and gender-appropriate history, examination, and counseling/anticipatory guidance. The Medicare E/M documentation guidelines do not apply to preventive services, and a head-to-toe examination is also not required. CPT recognizes ACOG as an authoritative body to make recommendations for the expected preventive service for women, and if such a service is provided and documented, the preventive codes are to be reported.
The chart below summarizes the CPT preventive codes by patient status and age in comparison to ACOG age groupings.
| New Patient Preventive Medicine Code | |||
|---|---|---|---|
| New patient codes include an initial comprehensive preventive medicine evaluation and management of an individual including an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures | |||
| ACOG: 13–18 years 99384 (12–17 years) 99385 (18–39 years) | ACOG: 19–39 years 99385 (18–39 years) | ACOG: 40–64 years 99386 (40–64 years) | ACOG: 65 years and older 99387 (65 years and older) |
| Established Patient Preventive Medicine Codes | |||
| Established patient codes include periodic comprehensive preventive medicine reevaluation and management of an individual including an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures | |||
| ACOG: 13–18 years 99394 (12–17 years) 99395 (18–39 years) | ACOG: 19–39 years 99395 (18–39 years) | ACOG: 40–64 years 99396 (40–64 years) | ACOG: 65 years and older 99397 (65 years and older) |
The main code
The appropriate diagnostic link for the CPT preventive gyn annual well-woman exam is V72.31, whether or not a Pap specimen is collected. The collection of the Pap specimen is included in the preventive service, as is counseling regarding birth control, or general questions about preventing problems, including hormone replacement therapy.
If a pelvic examination is not performed, say because the patient is young and not sexually active, but an examination of other areas is carried out, the same preventive codes are reported, but the diagnosis code changes to V70.0, general health exam.
What about Medicare?
Coding. Medicare requirements are somewhat different. First, Medicare covers only a small portion of the preventive service; that is, they cover a physical examination of the genital organs and breasts and the collection and conveyance of a Pap specimen to the lab in the covered year only. Think of the complete preventive service as described in CPT as a pie—Medicare pays for 2 slices of that pie in a covered year. The two codes for these services are:
G0101 (Cervical or vaginal cancer screening; pelvic and clinical breast examination)
Q0091 (Screening Papanicolaou smear; obtaining, preparing, and conveyance of cervical or vaginal smear to laboratory)
If the patient is at low risk for developing cervical or vaginal cancer, the screening pelvic exam and Pap collection are paid every 2 years. If the woman is at high risk, Medicare will cover this portion of the encounter every year. The high-risk criteria must be re-documented every year and must include one of the following:
- Early onset of sexual activity (under age 16)
- Multiple sexual partners (five or more in a lifetime)
- History of a sexually transmitted disease (including HIV infection)
- Fewer than three negative Pap smears within the previous 7 years
- Diethylstilbestrol (DES)-exposed daughters of women who took DES during pregnancy.
If the Medicare-eligible patient is still of childbearing age, she is also considered high-risk if she has had an examination that indicated the presence of cervical or vaginal cancer or other genital abnormalities during any of the preceding 3 years. Note that these criteria do not include a history of breast cancer or a past history of cancer more than 3 years ago.
Billing. Because Medicare is paying only for a portion of the preventive service, you will need to subtract the Medicare allowable for codes G0101 and Q0091 from your normal fee for the preventive service.
- Example: If your usual fee for 99397 is $200, and the Medicare allowable for both the G and Q service is $82, you will charge the patient for the noncovered parts of the service at the rate of $118, and you will bill Medicare for their share of $82. You will collect from all sources the $200 for the preventive service. Remember, however, to get the patient to sign an ABN with regard to the Medicare part of the service. This will ensure that, if denied by Medicare, the patient will be held fully responsible for the denied amount.
The Medicare modifier is –GA (add it to codes G0101 and Q0091). Diagnostic coding is V72.31 (because a pelvic exam is performed). This code may also be linked to the collection code. For a high-risk patient, use code V15.89 (rather than V72.31). This code must be linked to the G and Q codes.
“Guide to Billing the Medicare Annual Exam” is a detailed Medicare checklist offered by the author that includes all billing scenarios for a Medicare patient. Click here to download a PDF.
Ms. Witt can be contacted directly at [email protected] should you have additional questions regarding coding and billing for preventive services.
We want to hear from you! Tell us what you think.
Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Download Medicare Guide
Guide to Billing the Medicare Annual Exam
Melanie Witt, RN, CPC, COBGC, MA (October 2012)
Coding and billing for the care provided at a well-woman visit can be uncomplicated if you know the right codes for the right program. Here, I present information for straightforward preventive care. (I am assuming the patient has not also presented with a significant problem at the same visit.)
First, a patient who is not Medicare-eligible should have the annual well-woman exam billed using the CPT preventive medicine codes. There are some private insurers, however, that will only accept HCPCS codes for an annual gyn exam. These special codes are:
S0610 Annual gynecological examination, new patient
S0612 Annual gynecological examination, established patient
S0613 Annual gynecological examination; clinical breast examination without pelvic evaluation
Notably, Aetna Cigna, and United Healthcare require these codes for a gyn exam, but many BC/BS programs, for whom these codes were originally created, are now reverting to the CPT preventive medicine codes for all preventive care.
The CPT preventive codes are grouped by age and require an age- and gender-appropriate history, examination, and counseling/anticipatory guidance. The Medicare E/M documentation guidelines do not apply to preventive services, and a head-to-toe examination is also not required. CPT recognizes ACOG as an authoritative body to make recommendations for the expected preventive service for women, and if such a service is provided and documented, the preventive codes are to be reported.
The chart below summarizes the CPT preventive codes by patient status and age in comparison to ACOG age groupings.
| New Patient Preventive Medicine Code | |||
|---|---|---|---|
| New patient codes include an initial comprehensive preventive medicine evaluation and management of an individual including an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures | |||
| ACOG: 13–18 years 99384 (12–17 years) 99385 (18–39 years) | ACOG: 19–39 years 99385 (18–39 years) | ACOG: 40–64 years 99386 (40–64 years) | ACOG: 65 years and older 99387 (65 years and older) |
| Established Patient Preventive Medicine Codes | |||
| Established patient codes include periodic comprehensive preventive medicine reevaluation and management of an individual including an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures | |||
| ACOG: 13–18 years 99394 (12–17 years) 99395 (18–39 years) | ACOG: 19–39 years 99395 (18–39 years) | ACOG: 40–64 years 99396 (40–64 years) | ACOG: 65 years and older 99397 (65 years and older) |
The main code
The appropriate diagnostic link for the CPT preventive gyn annual well-woman exam is V72.31, whether or not a Pap specimen is collected. The collection of the Pap specimen is included in the preventive service, as is counseling regarding birth control, or general questions about preventing problems, including hormone replacement therapy.
If a pelvic examination is not performed, say because the patient is young and not sexually active, but an examination of other areas is carried out, the same preventive codes are reported, but the diagnosis code changes to V70.0, general health exam.
What about Medicare?
Coding. Medicare requirements are somewhat different. First, Medicare covers only a small portion of the preventive service; that is, they cover a physical examination of the genital organs and breasts and the collection and conveyance of a Pap specimen to the lab in the covered year only. Think of the complete preventive service as described in CPT as a pie—Medicare pays for 2 slices of that pie in a covered year. The two codes for these services are:
G0101 (Cervical or vaginal cancer screening; pelvic and clinical breast examination)
Q0091 (Screening Papanicolaou smear; obtaining, preparing, and conveyance of cervical or vaginal smear to laboratory)
If the patient is at low risk for developing cervical or vaginal cancer, the screening pelvic exam and Pap collection are paid every 2 years. If the woman is at high risk, Medicare will cover this portion of the encounter every year. The high-risk criteria must be re-documented every year and must include one of the following:
- Early onset of sexual activity (under age 16)
- Multiple sexual partners (five or more in a lifetime)
- History of a sexually transmitted disease (including HIV infection)
- Fewer than three negative Pap smears within the previous 7 years
- Diethylstilbestrol (DES)-exposed daughters of women who took DES during pregnancy.
If the Medicare-eligible patient is still of childbearing age, she is also considered high-risk if she has had an examination that indicated the presence of cervical or vaginal cancer or other genital abnormalities during any of the preceding 3 years. Note that these criteria do not include a history of breast cancer or a past history of cancer more than 3 years ago.
Billing. Because Medicare is paying only for a portion of the preventive service, you will need to subtract the Medicare allowable for codes G0101 and Q0091 from your normal fee for the preventive service.
- Example: If your usual fee for 99397 is $200, and the Medicare allowable for both the G and Q service is $82, you will charge the patient for the noncovered parts of the service at the rate of $118, and you will bill Medicare for their share of $82. You will collect from all sources the $200 for the preventive service. Remember, however, to get the patient to sign an ABN with regard to the Medicare part of the service. This will ensure that, if denied by Medicare, the patient will be held fully responsible for the denied amount.
The Medicare modifier is –GA (add it to codes G0101 and Q0091). Diagnostic coding is V72.31 (because a pelvic exam is performed). This code may also be linked to the collection code. For a high-risk patient, use code V15.89 (rather than V72.31). This code must be linked to the G and Q codes.
“Guide to Billing the Medicare Annual Exam” is a detailed Medicare checklist offered by the author that includes all billing scenarios for a Medicare patient. Click here to download a PDF.
Ms. Witt can be contacted directly at [email protected] should you have additional questions regarding coding and billing for preventive services.
We want to hear from you! Tell us what you think.
UPDATE: PELVIC FLOOR DYSFUNCTION
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.
When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.

When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.

When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-