User login
Educational Impact of Smartphones
Medical residents are rapidly adopting smartphones. Recent statistics revealed that 85% of medical providers currently own a smartphone, and the majority use it in their clinical work.[1] Smartphone capabilities that include the use of text messaging, e‐mail, and mobile phone functions in the clinical setting may improve efficiency and quality of care by reducing the response time for urgent issues.[2] There is, however, increasing recognition that healthcare information technology can create unintended negative consequences. For example, studies have suggested that healthcare information technologies, such as the computerized physician order entry, may actually increase errors by creating new work, changing clinical workflow, and altering communication patterns.[3, 4, 5]
Smartphone use for clinical communication can have unintended consequences by increasing interruptions, reducing interprofessional relationships, and widening the gap between what nurses and physicians perceive as urgent clinical problems.[6] However, no studies have evaluated the impact of smartphones on the educational experience of medical trainees. Although previous studies have described the use of smartphones by trainees for rapid access to electronic medical resources,[7, 8, 9] we did not identify in our literature review any previous studies on the impact of using the smartphone's primary functionas a communication deviceon the educational experience of residents and medical students. Therefore, our study aimed to examine the impact of using smartphones for clinical communication on medical education.
METHODS
Design
The design of the study was qualitative research methodology using interview data, ethnographic data, and content analysis of text‐based messages.
Setting
From June 2009 to September 2010, we conducted a multisite evaluation study on general internal medicine (GIM) wards at 5 large academic teaching hospitals in the city of Toronto, Canada at St. Michael's Hospital, Sunnybrook Health Sciences Centre, Toronto General Hospital, Toronto Western Hospital, and Mount Sinai Hospital. Each hospital has clinical teaching units consisting typically of 4 medical teams. Each team includes 1 attending physician, 1 senior resident, 2 or more junior residents, and 2 to 4 medical students. Each hospital had 2 to 4 GIM wards in different geographic locations.
Communication Systems
To make it easier for nurses and other health professionals to communicate with the physician teams, all sites centralized communication to 1 team member, who acts as the single point of contact on behalf of their assigned team in the communication of patient‐related issues. We facilitated this communication through a shared device (either a pager or a smartphone). The senior resident typically carried the shared device during the day and the on‐call junior resident at night and on the weekends. Two hospitals provided smartphones to all residents, whereas a third site provided smartphones only to the senior residents. The standard processes of communication required that physicians respond to all calls and text messages. At the 3 sites with institutional smartphones, nurses could send text messages with patient information using a Web‐based system. We encrypted data sent to institutional smartphones to protect patient information.
Data Collection
Using a mixed‐methods ethnographic approach, we collected data using semistructured interviews, ethnographic observations, and content analysis of text messages. The original larger study focused primarily on examining the overall clinical impact of smartphone use.[10] For our current study, we analyzed the data with a focus on evaluating the impact of smartphones on the educational experience of medical trainees on the GIM teaching service. The respective institutions' research ethics boards approved the study.
Interviews
We conducted semistructured interviews with residents, medical students, attending physicians, and other clinicians across all of the sites to examine how clinicians perceived the impact of smartphones on medical education. We used a purposeful sampling strategy where we interviewed different groups of healthcare professionals who we suspected would represent different viewpoints on the use of smartphones for clinical communication. To obtain diverse perspectives, we snowball sampled by asking interviewees to suggest colleagues with differing views to participate in the interviews. The interview guide consisted of open‐ended questions with additional probes to elicit more detailed information from these frontline clinicians who initiate and receive communication. One of the study investigators (V.L.) conducted interviews that varied from 15 to 45 minutes in duration. We recorded, transcribed verbatim, and analyzed the interviews using NVivo software (QSR International, Doncaster, Victoria, Australia). We added additional questions iteratively as themes emerged from the initial interviews. One of the study investigators (V.L.) encouraged participants to speak freely, to raise issues that they perceived to be important, and to support their responses with examples.
Observations
We observed the communication processes in the hospitals by conducting a work‐shadowing approach that followed individual residents in their work environments. These observations included 1‐on‐1 supervision encounters involving attending staff, medical students, and other residents, and informal and formal teaching rounds. The observation periods included the usual working day (from 8 am to 6 pm) as well as the busiest times on call, typically from 6 pm until 11 pm. We sampled different residents for different time periods. We adopted a nonparticipatory observation technique where we observed all interruptions, communication interactions, and patterns from a distance. We defined workflow interruptions as an intrusion of an unplanned and unscheduled task, causing a discontinuation of tasks, a noticeable break, or task switch behaviour.[11] Data collection included timing of events and writing field notes. One of the study investigators (V.L.) performed all the work‐shadowing observations.
E‐mail
To study the volume and content of messages, we collected e‐mail communications between January 2009 and June 2009 from consenting residents at the 2 hospitals that provided smartphones to all GIM residents. E‐mail information included the sender, the receiver, the time of message, and the message content. To look at usage, we calculated the average number of e‐mails sent and received. To assess interruptions on formal teaching sessions, we paid particular attention to e‐mails received and sent during protected educational timeweekdays from 8 am to 9 am (morning report) and 12 pm to 1 pm (noon rounds). We randomly sampled 20% of all e‐mails sent between residents for content analysis and organized content related to medical education into thematic categories.
Analysis
We used a deductive approach to analyze the interview transcripts by applying a conceptual framework that assessed the educational impact of patient safety interventions.[12] This framework identified 5 educational domains (learning, teaching, supervision, assessment, and feedback). Three study investigators mapped interview data, work‐shadowing data, and e‐mail content to themes (V.L., B.W., and R.W.), and grouped data that did not translate into themes into new categories. We then triangulated the data to develop themes of the educational impact of smartphone communication by both perceived use and actual use, and subsequently constructed a framework of how smartphone communication affected education.
RESULTS
We conducted 124 semistructured interviews with residents, medical students, attending physicians, and other clinicians across all the sites to examine how clinicians perceived the impact of smartphones on medical education. We work‐shadowed 40 individual residents for a total of 196 hours (Table 1). We analyzed the 13,714 e‐mails sent from or received to 34 residents. To analyze e‐mail content, we reviewed 1179 e‐mails sent among residents.
| Methods | Sites | |||||
|---|---|---|---|---|---|---|
| St. Michael's Hospital | Sunnybrook Health Sciences Centre | Toronto General Hospital | Toronto Western Hospital | Mount Sinai Hospital | All Hospitals | |
| ||||||
| Work‐shadowing residents | ||||||
| Hours | 60 hours | 35 hours | 57 hours 55 minutes | 27 hours 46 minutes | 15 hours | 196 hours |
| No. of residents | 12 | 7 | 12 | 6 | 3 | 40 |
| Interviews with clinicians | ||||||
| Physicians | 10 | 5 | 13 | 5 | 33 | |
| Medical students | 5 | 4 | 1 | 1 | 11 | |
| Nurses | 9 | 11 | 15 | 14 | 49 | |
| Other health professionsa | 7 | 10 | 8 | 6 | 31 | |
| Total | 31 | 30 | 37 | 26 | 124 | |
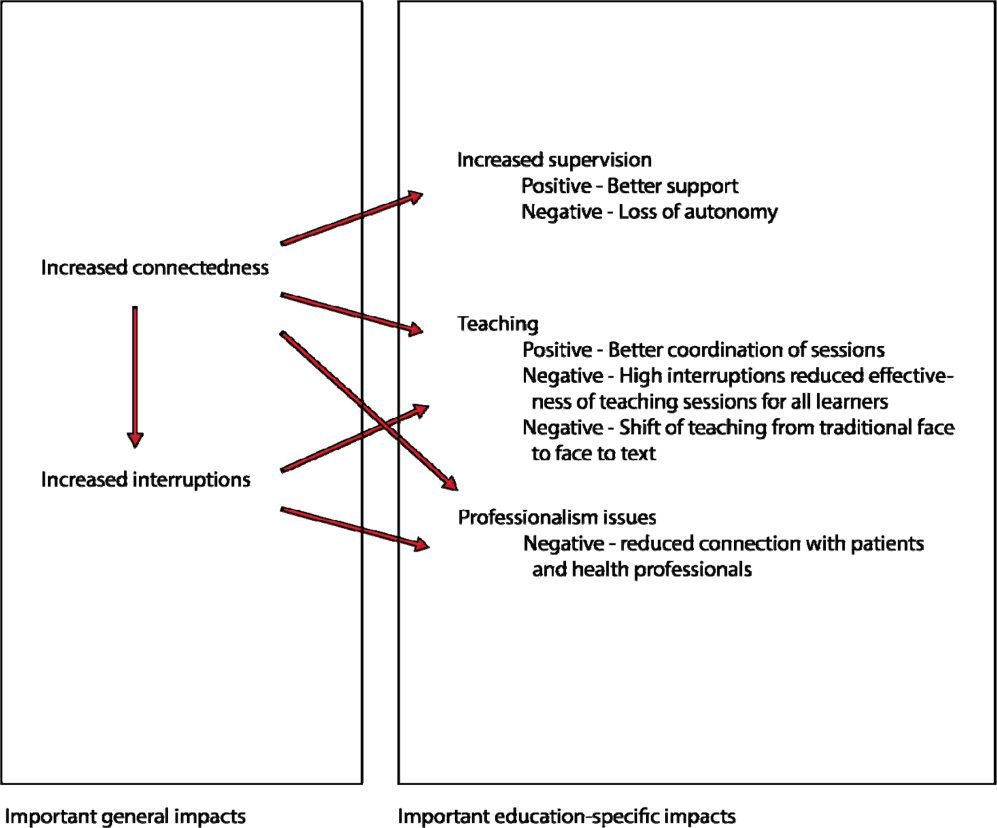
We found that 2 key characteristics of smartphone use for clinical communication, namely an increase in connectedness leading to an increase in interruptions, impacted 3 educational domains: teaching, supervision, and professionalism (Figure 1).

Increased Connectedness
As a communication device, smartphones increase the ability to receive and respond to messages through voice, e‐mail, and text messaging. Not surprisingly, with the improved ability and mobility to communicate, medical trainees perceived being more connected with their team members, who included other residents, medical students, and attending staff as well as with other clinical services and professions. These smartphone communication activities appeared to be pervasive, occurring on the wards, at the bedside, while in transit, and in teaching sessions (Box 1: increased connectedness).
Box
Increased connectedness
I've used the Blackberry system and it's nice to be able to quickly text each other little messages especially for meeting times because then you don't have to page them and wait by the phone. So that's been great for in the team. (Interview Resident 3)
It's incredibly useful for when you're paging somebody else. Often times I'll be consulting with another physician on a patient and I'll say This is my BlackBerry. Call me back after you've seen the patient' or Call me back when you have a plan' or, you know, whatever. So that's extremely valuable which we never had with pages and no one would ever page you for that because it was too much of a pain. (Interview Resident 1)
My personal experience has been that if you need to speak to a more senior individual it's much easier to contact them via the BlackBerry. (Interview Medical Student 1)
At 7:25 pm, MD11 returns to the patient's room and continues examining her. While in the patient's room, I could see her talking on the BlackBerrys. I asked her later what calls she had while in the room. It turns out she had 3 phone calls and 2 texts. Two of the calls were from the radiation oncologists and 1 call from the pathologist. She also received 1 text on the Team BlackBerry and 1 text on the Senior's BlackBerry from the pharmacist. (Field Notes, Work-shadowing MD11)
Interruptions
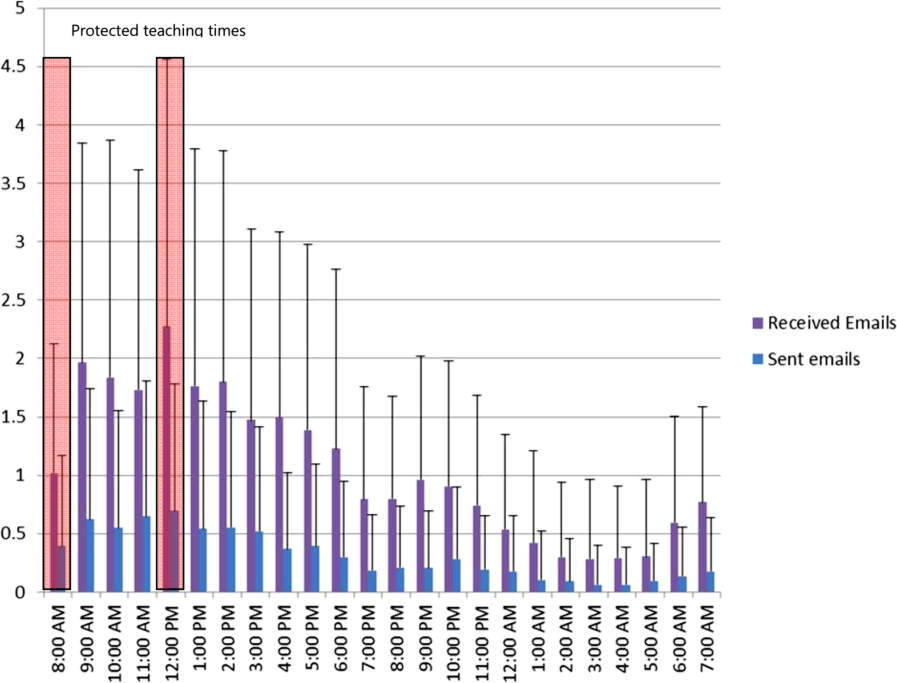
The increased connectedness caused by smartphone use led residents to perceive an increase in the frequency of interruptions. The multitude of communication and contact options made available by smartphones to health providers created an expansive network of connected individuals who were in constant communication with each other. Instead of the difficulties associated with numeric paging and waiting for a response, nurses typically found it easier to call directly or send a text message to residents' smartphones. From the e‐mail analysis, residents received, on a daily basis, on average 25.7 e‐mails, (median, 20; interquartile range [IQR]: 1428) to the team smartphone and sent 7.5 e‐mails (median, 6; IQR: 410). During protected educational time, each resident received an average of 1.0 e‐mail (median, 1; IQR: 01) between 8 am and 9 am and an average of 2.3 e‐mails (median, 2; IQR: 13) during 12 pm to 1 pm (Figure 2). Each of these communication events, whether a phone call, e‐mail, or text‐message, led to an interruption (Box 2). Given that smartphones made it easier for nurses to contact residents, some residents attributed the increase in interruptions to a reduction in the threshold for nurses to communicate.
Box
Increased interruptions
The only negative I can think of is just the incredible number of communications that you get, you know, text messages and e‐mails and everything else. So just the, the number can sometimes be overwhelming. (Interview Resident 1)
Some of [the nurses] rely a little bit more on the BlackBerry so that they will tend to call you a bit more frequently for things that maybe sometimes they should try to find answer for themselves (Interview Resident 2)
And now with the option of being able to, if you really needed to, call them and talk to them directly, I think that kind of improves communication. They're easier to find. (Interview Nurse 4)
Supervision
Smartphone communication appeared to positively impact trainee supervision. Increased connectedness between team members allowed junior trainees to have access and rapidly communicate with a more experienced clinician, which provided them with greater support. Residents found smartphones particularly useful in situations where they felt uncomfortable or where they did not feel competent. Some of these instances related to procedural competence, with residents feeling more comfortable knowing they have rapid access to support (Box 3: increased support).
Box
Supervision
Increased support
It makes me feel more comfortable in the sense that I can instantly make a call or a text and have a question answered if I need an answer. Or if it were an emergency having the ability to talk on the phone and be talked through an emergency situation, or a procedure for example like if you were in a remote area or the physician was in a remote area and you were in hospital and you would need some of that guidance or counselling, there's no substitution. (Interview Medical Student 1)
I'm ready can u dblchk [sic] that I landmarked correctly. (Email from Junior to Senior)
MD3 returns to the patient's room to do a paracentesis with [junior resident]. He calls on his BB to [senior resident] to inform her that they are starting and then hangs up. [Senior resident] arrives at the patient's room. (Field Notes, Workshadowing MD3)
Decreased autonomy
The difference with the Blackberry is they're more likely to say By the way, this happened. Should I do this?' And I write back Yes', No.' If they didn't have that contact like I said they probably would have done something and then because they're making a decision on their own they could very easily have spent the time to research whatever to figure whether that was the right thing to do before doing it. Now they have an outlet where they can pass an idea off of me and then have me make, it's easier for me to make a decision for them. So that can negatively impact education. (Interview Attending 1)
What do I do for a high phosphate?(Email from Junior to Senior)
Hey Pt X's k is 5.5. Was going to shift her. What do u think? (Email from Junior to Senior)
You probably saw the hb 92. Let's give prbc asap while he's on HD.(Email from Staff to Residents on the team)
hb‐ hemoglobin, prbc packed red blood cells, HD ‐ hemodialysis
Hi. Just checking the bloodwork. What is happening to ms X? [sic] Creatinine rising still. Is a foley in? Urology reconsulted? (Email from Attending Staff to Junior Resident)
On the other hand, supervisors perceived that the easy rapid access afforded by smartphone use lowered the threshold for trainees to contact them. In some instances, these attending physicians felt that their trainees would text them for advice when they could have looked up the information themselves. As a result, the increased reliance on the attending physician's input prior to committing to a management plan decreased the trainee's autonomy and independent decision making (Box 3: decreased autonomy). In addition to trainee requests for increased staff involvement, smartphone use made it easier for attending physicians to initiate text messages to their residents as well. In some instances, staff physicians adopted a more hands‐on approach by directing their residents on how to manage their patients. It is unclear if trainees perceived this taking over of care as negatively influencing their education.
Teaching
Medical teams also frequently used smartphones to communicate the location and timing of educational rounds. We observed instances where residents communicated updated information relating to scheduled rounds, as well as for informing team members about spontaneous teaching sessions (Box 4: communicating rounds). Despite this initial benefit, staff physicians worried that interruptions resulting from smartphone use during educational sessions lowered the effectiveness of these sessions for all learners by creating a fragmented learning experience (Box 4: fragmented learning). Our data indicated that residents carrying the team smartphones received and sent a high number of e‐mails throughout the day, which continued at a similar rate during the protected educational time (Figure 2). Additionally, some of the teaching experiences that traditionally would occur in a face‐to‐face manner appeared to have migrated to text‐based interactions. It is unclear whether trainees perceive these text‐based interactions as more or less effective teaching encounters (Box 4: text‐based teaching).
Box
Teaching
Communicating rounds
One is that they can more efficiently communicate about the timing and location of education rounds in case they forget or sort of as an organizer for them (Interview Attending 3)
Physical Exam rounds is at 1:00 outside the morning report room. K. has kindly volunteered! If you miss us then the exam will be on the 3rd floor in room X. Pt X. See you there (Email from chief medical resident to trainees)
Fragmented learning
Because Blackberry is there, it's something that is potentially time occupying and can take the attention away from things and this is true of any Blackberries. People who have Blackberries they always look at their Blackberries so, you know, there are times when I'm sitting face to face with people and residents are looking at their Blackberries. So it's another way that they can be distracted. (Interview Attending 1).
I've seen that be an issue. I've certainly seen them losing concentration during a teaching session because they're being Blackberried, getting Blackberry messages. (Interview Attending 3)
2:06 Team meeting with Attending in a conference room.
2:29 Team BlackBerry (BB) beeps. Senior glances at BB. She dials a number on the Team BB. Speaks on the Team BB and turns to [Junior resident] to inform her that the family is here. She returns to the caller. Senior then hangs up and resumes to her teaching.
2:35 Attending's BB rings. She takes a look and ends the BB call.
2:39 Senior's BB rings. Senior picks up and talks about a patient's case and condition. Senior turns to [junior resident] and asks a question. Team members resume talking among themselves.
2:46 Senior hangs up on the phone call.
2:49 Team discusses another patient's condition/case.
2:57 Junior resident uses her BB to text.
3:02 Team BB beeps. It is a message about a patient's case.
3:05 Meeting ends. (Field Note excerpts, Work‐shadowing MD6)
Text‐based teaching
The resident would get very frustrated with how many questions we have once we've started. Like if three different medical students or four different medical students or four different places all texting him with, oh by the way, what does this stand for?, and he's responding to each of them individually then he has to answer it four different times as opposed to just in person when he can get us all together in a group and it's actually a learning experience. If questions are answered in an email, it's not really helpful for the rest of us. (Interview, medical student SB1)
That would be a great unifying diagnosis, but there may be some underlying element of ROH/NASH also I would hold off on A/C as we do not know if he has varices. Will need to review noncontrast CT ?HCC. Thx (Email from Consulting Staff to Junior)
A/C anticoagulation, CT computed tomography, HCC‐ hepatocellular carcinoma, NASH non‐alcoholic steatohepatitis, ROH alcohol
Professionalism
Our data revealed that smartphone interruptions occurred during teaching rounds and interactions with patients and with other clinical staff. Often these interruptions involved messages or phone calls pertaining to clinical concerns or tasks that nurses communicated to the residents via their smartphone (Box 5). Yet, by responding to these interruptions and initiating communications on their smartphones during patient care encounters and formal teaching sessions, trainees were perceived by other clinicians who were in attendance with them as being rude or disrespectful. Attending staff also tended to role model similar smartphone behaviors. Although we did not specifically work‐shadow attending staff, we did observe frequent usage of their personal smartphones during their interactions with residents.
Box
Professionalism
I don't like it when I see them checking messages when you're trying to talk to them. I think you're losing some of that communication sort of polite behaviour that maybe we knew a little bit more before all this texting and Blackberry. (Interview Allied Health 5)
I think that the etiquette of the Blackberry can be offensive, could be offensive especially with some of our older patients (Interview Allied Health 6)
Senior walks out of the patient's room while typing on the BlackBerry. She finishes typing and returns to the room at 5:36. Senior looks at her BlackBerry and starts typing inside the room in front of the patient. She paused to look at the patient and the resident doing the procedure [paracentesis]. She resumes texting again and walks out of the room at 5:38. Another resident walks out and Senior speaks with the resident. Senior returns to the room and speaks with the patient. She asks the patient if he has ever gotten a successful tap before. Senior looks at her BlackBerry and starts typing. (Field NotesWork‐shadowing MD2)
I think it is almost completely negative in terms of its medical education [Any positive] factors are grossly outweighed by the significant disruptions to their ability to concentrate and participate in the educational session. And I think almost to some extent it's an implicit permission that gets granted to the house staff to disrupt their own teaching experience and disrupt others around them because everybody is doing it because everybody is being Blackberried. So it almost becomes the new social norm and while that may be a new social norm I'm not sure that that's a good thing How big is the negative impact? That's much harder to say. It's probably not a big impact on top of the endless other disruptions in the day to teaching, but it is measurable because it's a new factor so it's observable by me on top of all the other factors which have been there for years. (Interview Attending 3)
2:10‐Attending goes to the whiteboard to teach research methods to the team. Spotted Medical student#1 looking at his IPhone and typing.
2:15‐Med student#1 using the calculator function on his IPhone.
2:20‐Attending glances at his BB quickly.
2:28‐Attending resumes discussion of the patients' cases (Field notes, Work‐shadowing MD7).
DISCUSSION
The educational impacts of smartphone use for communication appear to center on increased connectedness of medical trainees and increased interruptions, which have positive and negative impacts in the areas of teaching, supervision, and professionalism. Smartphone communication provided potential educational benefits through (1) safer supervision with rapid access to help and (2) easier coordination of teaching sessions. Threats to the educational experience included (1) a high level of interruptions to both teachers and learners, which may reduce the effectiveness of formal and informal teaching; (2) replacement of face‐to‐face teaching with texting; (3) a potential erosion of autonomy and independence due to easy access to supervisors and easy ability for supervisors to take over; and (4) professionalism issues with difficulties balancing between clinical service demands and communication during patient and interprofessional encounters.
This study is the first to describe the intersection of clinical communication with smartphones and medical education. A recent study found that residents reported high use of smartphones during rounds for patient care as well as personal issues.[13] We have previously described the perceived impacts of smartphones on clinical communication, which included improved efficiency but concerns for increased interruptions and threats to professionalism.[6] We also observed that sites that used smartphones had increased interruptions compared to those with just pagers.[10] We have also described the content of e‐mail messages between clinicians and found that all e‐mails from nurses to physicians involved clinical care, but e‐mail exchanges between physicians were split between clinical care (60.4%), coordination within the team (53.5%), medical education (9.4%), and social communication (3.9%).[14] This study adds to the literature by focusing on the impacts of smartphone use to medical education and describing the perceived and observed impacts. This study provides a further example of how healthcare information technology can cause unintended consequences on medical education and appear to relate to the linked impacts of increased connectivity and the increased interruptions.[3] In essence, the trainee becomes more global, less local. Being more global translates to increased connections with people separated in physical space. Yet, this increased global connectedness resulted in the trainee being less local, with attention diverted elsewhere, taking away from the quality of patient interactions and interactions with other interprofessional team members. It also reduces the effectiveness of educational sessions for all participants. Although the level of supervision and autonomy are independently related, being too connected to supervisors may lower trainee autonomy by reducing independent thinking around patient issues.[15] It may also move teaching and learning from face‐to‐face conversations to text‐based messages. Although there have been existing tensions between service delivery and medical education, increased connectedness may tilt the balance toward the demands of service delivery and efficiency optimization at the expense of the educational experience. Finally, smartphone use appeared to create an internal tension among trainees, who have to juggle balancing professional behaviors and expectations in their dual role as learner and care provider; it would be educationally unprofessional to interrupt a teaching session and respond to a text message. However, failing to respond to a nurse who has sent a message and is expecting a response would be clinically unprofessional.
To address these threats, we advocate improving systems and processes to reduce interruptions and provide education on the tensions created by increased connectedness. Smarter communication systems could limit interrupting messages to urgent messages and queue nonurgent messages.[16] They could also inform senders about protected educational time. Even more sophisticated systems could inform the sender on the status of the receiver. For example, systems could indicate if they are available or if they are busy in an educational session or an important meeting with a patient and their family. Processes to reduce interruptions include interprofessional consensus on what constitutes an urgent issue and giving explicit permission to learners to ignore their smartphones during educational sessions except for critical communications purposes. Finally, education around smartphone communication for both learners and teachers may help minimize threats to learner autonomy, to face‐to‐face teaching, and to professionalism.[17]
Our study has several limitations. We derived this information from a general study of the impact of smartphones on clinical communication. Our study can be seen as hypothesis generating, and further research is warranted to validate these findings. There may be limits to generalizability as all sites adopted similar communication processes that included centralizing communications to make it easier for senders to reach a responsible physician.
In conclusion, we have provided a summary of the impact of rapidly emerging information technology on the educational experience of medical trainees and identified both positive and negative impacts. Of note, the negative impacts appear to be related to being more global and less local and high interruptions. Further research is required to confirm these unintended consequences as well as to develop solutions to address them. Educators should be aware of these findings and the need to develop curriculum to address and manage the negative impacts of smartphone use in the clinical training environment.
Acknowledgments
Disclosure: Nothing to report.
- , . Smartphone app use among medical providers in ACGME training programs. J Med Syst. 2012;36:3135–3139.
- , , , et al. The use of smartphones for clinical communication on internal medicine wards. J Hosp Med. 2010;5:553–559.
- , , , , , . Anticipating and addressing the unintended consequences of health IT and policy: a report from the AMIA 2009 Health Policy Meeting. J Am Med Inform Assoc. 2011;18(1):82–90.
- , , , , . Types of unintended consequences related to computerized provider order entry. J Am Med Inform Assoc. 2006;13(5):547–556.
- , , , . “e‐Iatrogenesis”: the most critical unintended consequence of CPOE and other HIT. J Am Med Inform Assoc. 2007;14(3):387–388.
- , , , et al. An evaluation of the use of smartphones to communicate between clinicians: a mixed‐methods study. J Med Internet Res. 2011;13(3):e59.
- . Smartphones in clinical practice, medical education, and research. Arch Intern Med. 2011;171(14):1294–1296.
- , , , . Use of handheld computers in medical education. A systematic review. J Gen Intern Med. 2006;21(5):531–537.
- , , , . A review and a framework of handheld computer adoption in healthcare. Int J Med Inform. 2005;74(5):409–422.
- , , , et al. The intended and unintended consequences of communication systems on General Internal Medicine inpatient care delivery: a prospective observational case study of five teaching hospitals [published online ahead of print January 25, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2012‐001160.
- , , , , . Hospital doctors' workflow interruptions and activities: an observation study. BMJ Qual Saf. 2011;20(6):491–497.
- , , , et al. Computerised provider order entry and residency education in an academic medical centre. Med Educ. 2012;46:795–806.
- , , , . Smartphone use during inpatient attending rounds: Prevalence, patterns and potential for distraction. J Hosp Med. 2012;7(8):595–599.
- , , , et al. Understanding interprofessional communication: a content analysis of email communications between doctors and nurses. Applied Clinical Informatics. 2012;3:38–51.
- , , , . Preserving professional credibility: grounded theory study of medical trainees' requests for clinical support. BMJ. 2009;338:b128.
- , , , , . Beyond paging: building a web‐based communication tool for nurses and physicians. J Gen Intern Med. 2009;24(1):105–110.
- , . Distracted doctoring: smartphones before patients? CMAJ. 2012;184:1440.
Medical residents are rapidly adopting smartphones. Recent statistics revealed that 85% of medical providers currently own a smartphone, and the majority use it in their clinical work.[1] Smartphone capabilities that include the use of text messaging, e‐mail, and mobile phone functions in the clinical setting may improve efficiency and quality of care by reducing the response time for urgent issues.[2] There is, however, increasing recognition that healthcare information technology can create unintended negative consequences. For example, studies have suggested that healthcare information technologies, such as the computerized physician order entry, may actually increase errors by creating new work, changing clinical workflow, and altering communication patterns.[3, 4, 5]
Smartphone use for clinical communication can have unintended consequences by increasing interruptions, reducing interprofessional relationships, and widening the gap between what nurses and physicians perceive as urgent clinical problems.[6] However, no studies have evaluated the impact of smartphones on the educational experience of medical trainees. Although previous studies have described the use of smartphones by trainees for rapid access to electronic medical resources,[7, 8, 9] we did not identify in our literature review any previous studies on the impact of using the smartphone's primary functionas a communication deviceon the educational experience of residents and medical students. Therefore, our study aimed to examine the impact of using smartphones for clinical communication on medical education.
METHODS
Design
The design of the study was qualitative research methodology using interview data, ethnographic data, and content analysis of text‐based messages.
Setting
From June 2009 to September 2010, we conducted a multisite evaluation study on general internal medicine (GIM) wards at 5 large academic teaching hospitals in the city of Toronto, Canada at St. Michael's Hospital, Sunnybrook Health Sciences Centre, Toronto General Hospital, Toronto Western Hospital, and Mount Sinai Hospital. Each hospital has clinical teaching units consisting typically of 4 medical teams. Each team includes 1 attending physician, 1 senior resident, 2 or more junior residents, and 2 to 4 medical students. Each hospital had 2 to 4 GIM wards in different geographic locations.
Communication Systems
To make it easier for nurses and other health professionals to communicate with the physician teams, all sites centralized communication to 1 team member, who acts as the single point of contact on behalf of their assigned team in the communication of patient‐related issues. We facilitated this communication through a shared device (either a pager or a smartphone). The senior resident typically carried the shared device during the day and the on‐call junior resident at night and on the weekends. Two hospitals provided smartphones to all residents, whereas a third site provided smartphones only to the senior residents. The standard processes of communication required that physicians respond to all calls and text messages. At the 3 sites with institutional smartphones, nurses could send text messages with patient information using a Web‐based system. We encrypted data sent to institutional smartphones to protect patient information.
Data Collection
Using a mixed‐methods ethnographic approach, we collected data using semistructured interviews, ethnographic observations, and content analysis of text messages. The original larger study focused primarily on examining the overall clinical impact of smartphone use.[10] For our current study, we analyzed the data with a focus on evaluating the impact of smartphones on the educational experience of medical trainees on the GIM teaching service. The respective institutions' research ethics boards approved the study.
Interviews
We conducted semistructured interviews with residents, medical students, attending physicians, and other clinicians across all of the sites to examine how clinicians perceived the impact of smartphones on medical education. We used a purposeful sampling strategy where we interviewed different groups of healthcare professionals who we suspected would represent different viewpoints on the use of smartphones for clinical communication. To obtain diverse perspectives, we snowball sampled by asking interviewees to suggest colleagues with differing views to participate in the interviews. The interview guide consisted of open‐ended questions with additional probes to elicit more detailed information from these frontline clinicians who initiate and receive communication. One of the study investigators (V.L.) conducted interviews that varied from 15 to 45 minutes in duration. We recorded, transcribed verbatim, and analyzed the interviews using NVivo software (QSR International, Doncaster, Victoria, Australia). We added additional questions iteratively as themes emerged from the initial interviews. One of the study investigators (V.L.) encouraged participants to speak freely, to raise issues that they perceived to be important, and to support their responses with examples.
Observations
We observed the communication processes in the hospitals by conducting a work‐shadowing approach that followed individual residents in their work environments. These observations included 1‐on‐1 supervision encounters involving attending staff, medical students, and other residents, and informal and formal teaching rounds. The observation periods included the usual working day (from 8 am to 6 pm) as well as the busiest times on call, typically from 6 pm until 11 pm. We sampled different residents for different time periods. We adopted a nonparticipatory observation technique where we observed all interruptions, communication interactions, and patterns from a distance. We defined workflow interruptions as an intrusion of an unplanned and unscheduled task, causing a discontinuation of tasks, a noticeable break, or task switch behaviour.[11] Data collection included timing of events and writing field notes. One of the study investigators (V.L.) performed all the work‐shadowing observations.
E‐mail
To study the volume and content of messages, we collected e‐mail communications between January 2009 and June 2009 from consenting residents at the 2 hospitals that provided smartphones to all GIM residents. E‐mail information included the sender, the receiver, the time of message, and the message content. To look at usage, we calculated the average number of e‐mails sent and received. To assess interruptions on formal teaching sessions, we paid particular attention to e‐mails received and sent during protected educational timeweekdays from 8 am to 9 am (morning report) and 12 pm to 1 pm (noon rounds). We randomly sampled 20% of all e‐mails sent between residents for content analysis and organized content related to medical education into thematic categories.
Analysis
We used a deductive approach to analyze the interview transcripts by applying a conceptual framework that assessed the educational impact of patient safety interventions.[12] This framework identified 5 educational domains (learning, teaching, supervision, assessment, and feedback). Three study investigators mapped interview data, work‐shadowing data, and e‐mail content to themes (V.L., B.W., and R.W.), and grouped data that did not translate into themes into new categories. We then triangulated the data to develop themes of the educational impact of smartphone communication by both perceived use and actual use, and subsequently constructed a framework of how smartphone communication affected education.
RESULTS
We conducted 124 semistructured interviews with residents, medical students, attending physicians, and other clinicians across all the sites to examine how clinicians perceived the impact of smartphones on medical education. We work‐shadowed 40 individual residents for a total of 196 hours (Table 1). We analyzed the 13,714 e‐mails sent from or received to 34 residents. To analyze e‐mail content, we reviewed 1179 e‐mails sent among residents.
| Methods | Sites | |||||
|---|---|---|---|---|---|---|
| St. Michael's Hospital | Sunnybrook Health Sciences Centre | Toronto General Hospital | Toronto Western Hospital | Mount Sinai Hospital | All Hospitals | |
| ||||||
| Work‐shadowing residents | ||||||
| Hours | 60 hours | 35 hours | 57 hours 55 minutes | 27 hours 46 minutes | 15 hours | 196 hours |
| No. of residents | 12 | 7 | 12 | 6 | 3 | 40 |
| Interviews with clinicians | ||||||
| Physicians | 10 | 5 | 13 | 5 | 33 | |
| Medical students | 5 | 4 | 1 | 1 | 11 | |
| Nurses | 9 | 11 | 15 | 14 | 49 | |
| Other health professionsa | 7 | 10 | 8 | 6 | 31 | |
| Total | 31 | 30 | 37 | 26 | 124 | |
We found that 2 key characteristics of smartphone use for clinical communication, namely an increase in connectedness leading to an increase in interruptions, impacted 3 educational domains: teaching, supervision, and professionalism (Figure 1).

Increased Connectedness
As a communication device, smartphones increase the ability to receive and respond to messages through voice, e‐mail, and text messaging. Not surprisingly, with the improved ability and mobility to communicate, medical trainees perceived being more connected with their team members, who included other residents, medical students, and attending staff as well as with other clinical services and professions. These smartphone communication activities appeared to be pervasive, occurring on the wards, at the bedside, while in transit, and in teaching sessions (Box 1: increased connectedness).
Box
Increased connectedness
I've used the Blackberry system and it's nice to be able to quickly text each other little messages especially for meeting times because then you don't have to page them and wait by the phone. So that's been great for in the team. (Interview Resident 3)
It's incredibly useful for when you're paging somebody else. Often times I'll be consulting with another physician on a patient and I'll say This is my BlackBerry. Call me back after you've seen the patient' or Call me back when you have a plan' or, you know, whatever. So that's extremely valuable which we never had with pages and no one would ever page you for that because it was too much of a pain. (Interview Resident 1)
My personal experience has been that if you need to speak to a more senior individual it's much easier to contact them via the BlackBerry. (Interview Medical Student 1)
At 7:25 pm, MD11 returns to the patient's room and continues examining her. While in the patient's room, I could see her talking on the BlackBerrys. I asked her later what calls she had while in the room. It turns out she had 3 phone calls and 2 texts. Two of the calls were from the radiation oncologists and 1 call from the pathologist. She also received 1 text on the Team BlackBerry and 1 text on the Senior's BlackBerry from the pharmacist. (Field Notes, Work-shadowing MD11)
Interruptions
The increased connectedness caused by smartphone use led residents to perceive an increase in the frequency of interruptions. The multitude of communication and contact options made available by smartphones to health providers created an expansive network of connected individuals who were in constant communication with each other. Instead of the difficulties associated with numeric paging and waiting for a response, nurses typically found it easier to call directly or send a text message to residents' smartphones. From the e‐mail analysis, residents received, on a daily basis, on average 25.7 e‐mails, (median, 20; interquartile range [IQR]: 1428) to the team smartphone and sent 7.5 e‐mails (median, 6; IQR: 410). During protected educational time, each resident received an average of 1.0 e‐mail (median, 1; IQR: 01) between 8 am and 9 am and an average of 2.3 e‐mails (median, 2; IQR: 13) during 12 pm to 1 pm (Figure 2). Each of these communication events, whether a phone call, e‐mail, or text‐message, led to an interruption (Box 2). Given that smartphones made it easier for nurses to contact residents, some residents attributed the increase in interruptions to a reduction in the threshold for nurses to communicate.
Box
Increased interruptions
The only negative I can think of is just the incredible number of communications that you get, you know, text messages and e‐mails and everything else. So just the, the number can sometimes be overwhelming. (Interview Resident 1)
Some of [the nurses] rely a little bit more on the BlackBerry so that they will tend to call you a bit more frequently for things that maybe sometimes they should try to find answer for themselves (Interview Resident 2)
And now with the option of being able to, if you really needed to, call them and talk to them directly, I think that kind of improves communication. They're easier to find. (Interview Nurse 4)

Supervision
Smartphone communication appeared to positively impact trainee supervision. Increased connectedness between team members allowed junior trainees to have access and rapidly communicate with a more experienced clinician, which provided them with greater support. Residents found smartphones particularly useful in situations where they felt uncomfortable or where they did not feel competent. Some of these instances related to procedural competence, with residents feeling more comfortable knowing they have rapid access to support (Box 3: increased support).
Box
Supervision
Increased support
It makes me feel more comfortable in the sense that I can instantly make a call or a text and have a question answered if I need an answer. Or if it were an emergency having the ability to talk on the phone and be talked through an emergency situation, or a procedure for example like if you were in a remote area or the physician was in a remote area and you were in hospital and you would need some of that guidance or counselling, there's no substitution. (Interview Medical Student 1)
I'm ready can u dblchk [sic] that I landmarked correctly. (Email from Junior to Senior)
MD3 returns to the patient's room to do a paracentesis with [junior resident]. He calls on his BB to [senior resident] to inform her that they are starting and then hangs up. [Senior resident] arrives at the patient's room. (Field Notes, Workshadowing MD3)
Decreased autonomy
The difference with the Blackberry is they're more likely to say By the way, this happened. Should I do this?' And I write back Yes', No.' If they didn't have that contact like I said they probably would have done something and then because they're making a decision on their own they could very easily have spent the time to research whatever to figure whether that was the right thing to do before doing it. Now they have an outlet where they can pass an idea off of me and then have me make, it's easier for me to make a decision for them. So that can negatively impact education. (Interview Attending 1)
What do I do for a high phosphate?(Email from Junior to Senior)
Hey Pt X's k is 5.5. Was going to shift her. What do u think? (Email from Junior to Senior)
You probably saw the hb 92. Let's give prbc asap while he's on HD.(Email from Staff to Residents on the team)
hb‐ hemoglobin, prbc packed red blood cells, HD ‐ hemodialysis
Hi. Just checking the bloodwork. What is happening to ms X? [sic] Creatinine rising still. Is a foley in? Urology reconsulted? (Email from Attending Staff to Junior Resident)
On the other hand, supervisors perceived that the easy rapid access afforded by smartphone use lowered the threshold for trainees to contact them. In some instances, these attending physicians felt that their trainees would text them for advice when they could have looked up the information themselves. As a result, the increased reliance on the attending physician's input prior to committing to a management plan decreased the trainee's autonomy and independent decision making (Box 3: decreased autonomy). In addition to trainee requests for increased staff involvement, smartphone use made it easier for attending physicians to initiate text messages to their residents as well. In some instances, staff physicians adopted a more hands‐on approach by directing their residents on how to manage their patients. It is unclear if trainees perceived this taking over of care as negatively influencing their education.
Teaching
Medical teams also frequently used smartphones to communicate the location and timing of educational rounds. We observed instances where residents communicated updated information relating to scheduled rounds, as well as for informing team members about spontaneous teaching sessions (Box 4: communicating rounds). Despite this initial benefit, staff physicians worried that interruptions resulting from smartphone use during educational sessions lowered the effectiveness of these sessions for all learners by creating a fragmented learning experience (Box 4: fragmented learning). Our data indicated that residents carrying the team smartphones received and sent a high number of e‐mails throughout the day, which continued at a similar rate during the protected educational time (Figure 2). Additionally, some of the teaching experiences that traditionally would occur in a face‐to‐face manner appeared to have migrated to text‐based interactions. It is unclear whether trainees perceive these text‐based interactions as more or less effective teaching encounters (Box 4: text‐based teaching).
Box
Teaching
Communicating rounds
One is that they can more efficiently communicate about the timing and location of education rounds in case they forget or sort of as an organizer for them (Interview Attending 3)
Physical Exam rounds is at 1:00 outside the morning report room. K. has kindly volunteered! If you miss us then the exam will be on the 3rd floor in room X. Pt X. See you there (Email from chief medical resident to trainees)
Fragmented learning
Because Blackberry is there, it's something that is potentially time occupying and can take the attention away from things and this is true of any Blackberries. People who have Blackberries they always look at their Blackberries so, you know, there are times when I'm sitting face to face with people and residents are looking at their Blackberries. So it's another way that they can be distracted. (Interview Attending 1).
I've seen that be an issue. I've certainly seen them losing concentration during a teaching session because they're being Blackberried, getting Blackberry messages. (Interview Attending 3)
2:06 Team meeting with Attending in a conference room.
2:29 Team BlackBerry (BB) beeps. Senior glances at BB. She dials a number on the Team BB. Speaks on the Team BB and turns to [Junior resident] to inform her that the family is here. She returns to the caller. Senior then hangs up and resumes to her teaching.
2:35 Attending's BB rings. She takes a look and ends the BB call.
2:39 Senior's BB rings. Senior picks up and talks about a patient's case and condition. Senior turns to [junior resident] and asks a question. Team members resume talking among themselves.
2:46 Senior hangs up on the phone call.
2:49 Team discusses another patient's condition/case.
2:57 Junior resident uses her BB to text.
3:02 Team BB beeps. It is a message about a patient's case.
3:05 Meeting ends. (Field Note excerpts, Work‐shadowing MD6)
Text‐based teaching
The resident would get very frustrated with how many questions we have once we've started. Like if three different medical students or four different medical students or four different places all texting him with, oh by the way, what does this stand for?, and he's responding to each of them individually then he has to answer it four different times as opposed to just in person when he can get us all together in a group and it's actually a learning experience. If questions are answered in an email, it's not really helpful for the rest of us. (Interview, medical student SB1)
That would be a great unifying diagnosis, but there may be some underlying element of ROH/NASH also I would hold off on A/C as we do not know if he has varices. Will need to review noncontrast CT ?HCC. Thx (Email from Consulting Staff to Junior)
A/C anticoagulation, CT computed tomography, HCC‐ hepatocellular carcinoma, NASH non‐alcoholic steatohepatitis, ROH alcohol
Professionalism
Our data revealed that smartphone interruptions occurred during teaching rounds and interactions with patients and with other clinical staff. Often these interruptions involved messages or phone calls pertaining to clinical concerns or tasks that nurses communicated to the residents via their smartphone (Box 5). Yet, by responding to these interruptions and initiating communications on their smartphones during patient care encounters and formal teaching sessions, trainees were perceived by other clinicians who were in attendance with them as being rude or disrespectful. Attending staff also tended to role model similar smartphone behaviors. Although we did not specifically work‐shadow attending staff, we did observe frequent usage of their personal smartphones during their interactions with residents.
Box
Professionalism
I don't like it when I see them checking messages when you're trying to talk to them. I think you're losing some of that communication sort of polite behaviour that maybe we knew a little bit more before all this texting and Blackberry. (Interview Allied Health 5)
I think that the etiquette of the Blackberry can be offensive, could be offensive especially with some of our older patients (Interview Allied Health 6)
Senior walks out of the patient's room while typing on the BlackBerry. She finishes typing and returns to the room at 5:36. Senior looks at her BlackBerry and starts typing inside the room in front of the patient. She paused to look at the patient and the resident doing the procedure [paracentesis]. She resumes texting again and walks out of the room at 5:38. Another resident walks out and Senior speaks with the resident. Senior returns to the room and speaks with the patient. She asks the patient if he has ever gotten a successful tap before. Senior looks at her BlackBerry and starts typing. (Field NotesWork‐shadowing MD2)
I think it is almost completely negative in terms of its medical education [Any positive] factors are grossly outweighed by the significant disruptions to their ability to concentrate and participate in the educational session. And I think almost to some extent it's an implicit permission that gets granted to the house staff to disrupt their own teaching experience and disrupt others around them because everybody is doing it because everybody is being Blackberried. So it almost becomes the new social norm and while that may be a new social norm I'm not sure that that's a good thing How big is the negative impact? That's much harder to say. It's probably not a big impact on top of the endless other disruptions in the day to teaching, but it is measurable because it's a new factor so it's observable by me on top of all the other factors which have been there for years. (Interview Attending 3)
2:10‐Attending goes to the whiteboard to teach research methods to the team. Spotted Medical student#1 looking at his IPhone and typing.
2:15‐Med student#1 using the calculator function on his IPhone.
2:20‐Attending glances at his BB quickly.
2:28‐Attending resumes discussion of the patients' cases (Field notes, Work‐shadowing MD7).
DISCUSSION
The educational impacts of smartphone use for communication appear to center on increased connectedness of medical trainees and increased interruptions, which have positive and negative impacts in the areas of teaching, supervision, and professionalism. Smartphone communication provided potential educational benefits through (1) safer supervision with rapid access to help and (2) easier coordination of teaching sessions. Threats to the educational experience included (1) a high level of interruptions to both teachers and learners, which may reduce the effectiveness of formal and informal teaching; (2) replacement of face‐to‐face teaching with texting; (3) a potential erosion of autonomy and independence due to easy access to supervisors and easy ability for supervisors to take over; and (4) professionalism issues with difficulties balancing between clinical service demands and communication during patient and interprofessional encounters.
This study is the first to describe the intersection of clinical communication with smartphones and medical education. A recent study found that residents reported high use of smartphones during rounds for patient care as well as personal issues.[13] We have previously described the perceived impacts of smartphones on clinical communication, which included improved efficiency but concerns for increased interruptions and threats to professionalism.[6] We also observed that sites that used smartphones had increased interruptions compared to those with just pagers.[10] We have also described the content of e‐mail messages between clinicians and found that all e‐mails from nurses to physicians involved clinical care, but e‐mail exchanges between physicians were split between clinical care (60.4%), coordination within the team (53.5%), medical education (9.4%), and social communication (3.9%).[14] This study adds to the literature by focusing on the impacts of smartphone use to medical education and describing the perceived and observed impacts. This study provides a further example of how healthcare information technology can cause unintended consequences on medical education and appear to relate to the linked impacts of increased connectivity and the increased interruptions.[3] In essence, the trainee becomes more global, less local. Being more global translates to increased connections with people separated in physical space. Yet, this increased global connectedness resulted in the trainee being less local, with attention diverted elsewhere, taking away from the quality of patient interactions and interactions with other interprofessional team members. It also reduces the effectiveness of educational sessions for all participants. Although the level of supervision and autonomy are independently related, being too connected to supervisors may lower trainee autonomy by reducing independent thinking around patient issues.[15] It may also move teaching and learning from face‐to‐face conversations to text‐based messages. Although there have been existing tensions between service delivery and medical education, increased connectedness may tilt the balance toward the demands of service delivery and efficiency optimization at the expense of the educational experience. Finally, smartphone use appeared to create an internal tension among trainees, who have to juggle balancing professional behaviors and expectations in their dual role as learner and care provider; it would be educationally unprofessional to interrupt a teaching session and respond to a text message. However, failing to respond to a nurse who has sent a message and is expecting a response would be clinically unprofessional.
To address these threats, we advocate improving systems and processes to reduce interruptions and provide education on the tensions created by increased connectedness. Smarter communication systems could limit interrupting messages to urgent messages and queue nonurgent messages.[16] They could also inform senders about protected educational time. Even more sophisticated systems could inform the sender on the status of the receiver. For example, systems could indicate if they are available or if they are busy in an educational session or an important meeting with a patient and their family. Processes to reduce interruptions include interprofessional consensus on what constitutes an urgent issue and giving explicit permission to learners to ignore their smartphones during educational sessions except for critical communications purposes. Finally, education around smartphone communication for both learners and teachers may help minimize threats to learner autonomy, to face‐to‐face teaching, and to professionalism.[17]
Our study has several limitations. We derived this information from a general study of the impact of smartphones on clinical communication. Our study can be seen as hypothesis generating, and further research is warranted to validate these findings. There may be limits to generalizability as all sites adopted similar communication processes that included centralizing communications to make it easier for senders to reach a responsible physician.
In conclusion, we have provided a summary of the impact of rapidly emerging information technology on the educational experience of medical trainees and identified both positive and negative impacts. Of note, the negative impacts appear to be related to being more global and less local and high interruptions. Further research is required to confirm these unintended consequences as well as to develop solutions to address them. Educators should be aware of these findings and the need to develop curriculum to address and manage the negative impacts of smartphone use in the clinical training environment.
Acknowledgments
Disclosure: Nothing to report.
Medical residents are rapidly adopting smartphones. Recent statistics revealed that 85% of medical providers currently own a smartphone, and the majority use it in their clinical work.[1] Smartphone capabilities that include the use of text messaging, e‐mail, and mobile phone functions in the clinical setting may improve efficiency and quality of care by reducing the response time for urgent issues.[2] There is, however, increasing recognition that healthcare information technology can create unintended negative consequences. For example, studies have suggested that healthcare information technologies, such as the computerized physician order entry, may actually increase errors by creating new work, changing clinical workflow, and altering communication patterns.[3, 4, 5]
Smartphone use for clinical communication can have unintended consequences by increasing interruptions, reducing interprofessional relationships, and widening the gap between what nurses and physicians perceive as urgent clinical problems.[6] However, no studies have evaluated the impact of smartphones on the educational experience of medical trainees. Although previous studies have described the use of smartphones by trainees for rapid access to electronic medical resources,[7, 8, 9] we did not identify in our literature review any previous studies on the impact of using the smartphone's primary functionas a communication deviceon the educational experience of residents and medical students. Therefore, our study aimed to examine the impact of using smartphones for clinical communication on medical education.
METHODS
Design
The design of the study was qualitative research methodology using interview data, ethnographic data, and content analysis of text‐based messages.
Setting
From June 2009 to September 2010, we conducted a multisite evaluation study on general internal medicine (GIM) wards at 5 large academic teaching hospitals in the city of Toronto, Canada at St. Michael's Hospital, Sunnybrook Health Sciences Centre, Toronto General Hospital, Toronto Western Hospital, and Mount Sinai Hospital. Each hospital has clinical teaching units consisting typically of 4 medical teams. Each team includes 1 attending physician, 1 senior resident, 2 or more junior residents, and 2 to 4 medical students. Each hospital had 2 to 4 GIM wards in different geographic locations.
Communication Systems
To make it easier for nurses and other health professionals to communicate with the physician teams, all sites centralized communication to 1 team member, who acts as the single point of contact on behalf of their assigned team in the communication of patient‐related issues. We facilitated this communication through a shared device (either a pager or a smartphone). The senior resident typically carried the shared device during the day and the on‐call junior resident at night and on the weekends. Two hospitals provided smartphones to all residents, whereas a third site provided smartphones only to the senior residents. The standard processes of communication required that physicians respond to all calls and text messages. At the 3 sites with institutional smartphones, nurses could send text messages with patient information using a Web‐based system. We encrypted data sent to institutional smartphones to protect patient information.
Data Collection
Using a mixed‐methods ethnographic approach, we collected data using semistructured interviews, ethnographic observations, and content analysis of text messages. The original larger study focused primarily on examining the overall clinical impact of smartphone use.[10] For our current study, we analyzed the data with a focus on evaluating the impact of smartphones on the educational experience of medical trainees on the GIM teaching service. The respective institutions' research ethics boards approved the study.
Interviews
We conducted semistructured interviews with residents, medical students, attending physicians, and other clinicians across all of the sites to examine how clinicians perceived the impact of smartphones on medical education. We used a purposeful sampling strategy where we interviewed different groups of healthcare professionals who we suspected would represent different viewpoints on the use of smartphones for clinical communication. To obtain diverse perspectives, we snowball sampled by asking interviewees to suggest colleagues with differing views to participate in the interviews. The interview guide consisted of open‐ended questions with additional probes to elicit more detailed information from these frontline clinicians who initiate and receive communication. One of the study investigators (V.L.) conducted interviews that varied from 15 to 45 minutes in duration. We recorded, transcribed verbatim, and analyzed the interviews using NVivo software (QSR International, Doncaster, Victoria, Australia). We added additional questions iteratively as themes emerged from the initial interviews. One of the study investigators (V.L.) encouraged participants to speak freely, to raise issues that they perceived to be important, and to support their responses with examples.
Observations
We observed the communication processes in the hospitals by conducting a work‐shadowing approach that followed individual residents in their work environments. These observations included 1‐on‐1 supervision encounters involving attending staff, medical students, and other residents, and informal and formal teaching rounds. The observation periods included the usual working day (from 8 am to 6 pm) as well as the busiest times on call, typically from 6 pm until 11 pm. We sampled different residents for different time periods. We adopted a nonparticipatory observation technique where we observed all interruptions, communication interactions, and patterns from a distance. We defined workflow interruptions as an intrusion of an unplanned and unscheduled task, causing a discontinuation of tasks, a noticeable break, or task switch behaviour.[11] Data collection included timing of events and writing field notes. One of the study investigators (V.L.) performed all the work‐shadowing observations.
E‐mail
To study the volume and content of messages, we collected e‐mail communications between January 2009 and June 2009 from consenting residents at the 2 hospitals that provided smartphones to all GIM residents. E‐mail information included the sender, the receiver, the time of message, and the message content. To look at usage, we calculated the average number of e‐mails sent and received. To assess interruptions on formal teaching sessions, we paid particular attention to e‐mails received and sent during protected educational timeweekdays from 8 am to 9 am (morning report) and 12 pm to 1 pm (noon rounds). We randomly sampled 20% of all e‐mails sent between residents for content analysis and organized content related to medical education into thematic categories.
Analysis
We used a deductive approach to analyze the interview transcripts by applying a conceptual framework that assessed the educational impact of patient safety interventions.[12] This framework identified 5 educational domains (learning, teaching, supervision, assessment, and feedback). Three study investigators mapped interview data, work‐shadowing data, and e‐mail content to themes (V.L., B.W., and R.W.), and grouped data that did not translate into themes into new categories. We then triangulated the data to develop themes of the educational impact of smartphone communication by both perceived use and actual use, and subsequently constructed a framework of how smartphone communication affected education.
RESULTS
We conducted 124 semistructured interviews with residents, medical students, attending physicians, and other clinicians across all the sites to examine how clinicians perceived the impact of smartphones on medical education. We work‐shadowed 40 individual residents for a total of 196 hours (Table 1). We analyzed the 13,714 e‐mails sent from or received to 34 residents. To analyze e‐mail content, we reviewed 1179 e‐mails sent among residents.
| Methods | Sites | |||||
|---|---|---|---|---|---|---|
| St. Michael's Hospital | Sunnybrook Health Sciences Centre | Toronto General Hospital | Toronto Western Hospital | Mount Sinai Hospital | All Hospitals | |
| ||||||
| Work‐shadowing residents | ||||||
| Hours | 60 hours | 35 hours | 57 hours 55 minutes | 27 hours 46 minutes | 15 hours | 196 hours |
| No. of residents | 12 | 7 | 12 | 6 | 3 | 40 |
| Interviews with clinicians | ||||||
| Physicians | 10 | 5 | 13 | 5 | 33 | |
| Medical students | 5 | 4 | 1 | 1 | 11 | |
| Nurses | 9 | 11 | 15 | 14 | 49 | |
| Other health professionsa | 7 | 10 | 8 | 6 | 31 | |
| Total | 31 | 30 | 37 | 26 | 124 | |
We found that 2 key characteristics of smartphone use for clinical communication, namely an increase in connectedness leading to an increase in interruptions, impacted 3 educational domains: teaching, supervision, and professionalism (Figure 1).

Increased Connectedness
As a communication device, smartphones increase the ability to receive and respond to messages through voice, e‐mail, and text messaging. Not surprisingly, with the improved ability and mobility to communicate, medical trainees perceived being more connected with their team members, who included other residents, medical students, and attending staff as well as with other clinical services and professions. These smartphone communication activities appeared to be pervasive, occurring on the wards, at the bedside, while in transit, and in teaching sessions (Box 1: increased connectedness).
Box
Increased connectedness
I've used the Blackberry system and it's nice to be able to quickly text each other little messages especially for meeting times because then you don't have to page them and wait by the phone. So that's been great for in the team. (Interview Resident 3)
It's incredibly useful for when you're paging somebody else. Often times I'll be consulting with another physician on a patient and I'll say This is my BlackBerry. Call me back after you've seen the patient' or Call me back when you have a plan' or, you know, whatever. So that's extremely valuable which we never had with pages and no one would ever page you for that because it was too much of a pain. (Interview Resident 1)
My personal experience has been that if you need to speak to a more senior individual it's much easier to contact them via the BlackBerry. (Interview Medical Student 1)
At 7:25 pm, MD11 returns to the patient's room and continues examining her. While in the patient's room, I could see her talking on the BlackBerrys. I asked her later what calls she had while in the room. It turns out she had 3 phone calls and 2 texts. Two of the calls were from the radiation oncologists and 1 call from the pathologist. She also received 1 text on the Team BlackBerry and 1 text on the Senior's BlackBerry from the pharmacist. (Field Notes, Work-shadowing MD11)
Interruptions
The increased connectedness caused by smartphone use led residents to perceive an increase in the frequency of interruptions. The multitude of communication and contact options made available by smartphones to health providers created an expansive network of connected individuals who were in constant communication with each other. Instead of the difficulties associated with numeric paging and waiting for a response, nurses typically found it easier to call directly or send a text message to residents' smartphones. From the e‐mail analysis, residents received, on a daily basis, on average 25.7 e‐mails, (median, 20; interquartile range [IQR]: 1428) to the team smartphone and sent 7.5 e‐mails (median, 6; IQR: 410). During protected educational time, each resident received an average of 1.0 e‐mail (median, 1; IQR: 01) between 8 am and 9 am and an average of 2.3 e‐mails (median, 2; IQR: 13) during 12 pm to 1 pm (Figure 2). Each of these communication events, whether a phone call, e‐mail, or text‐message, led to an interruption (Box 2). Given that smartphones made it easier for nurses to contact residents, some residents attributed the increase in interruptions to a reduction in the threshold for nurses to communicate.
Box
Increased interruptions
The only negative I can think of is just the incredible number of communications that you get, you know, text messages and e‐mails and everything else. So just the, the number can sometimes be overwhelming. (Interview Resident 1)
Some of [the nurses] rely a little bit more on the BlackBerry so that they will tend to call you a bit more frequently for things that maybe sometimes they should try to find answer for themselves (Interview Resident 2)
And now with the option of being able to, if you really needed to, call them and talk to them directly, I think that kind of improves communication. They're easier to find. (Interview Nurse 4)

Supervision
Smartphone communication appeared to positively impact trainee supervision. Increased connectedness between team members allowed junior trainees to have access and rapidly communicate with a more experienced clinician, which provided them with greater support. Residents found smartphones particularly useful in situations where they felt uncomfortable or where they did not feel competent. Some of these instances related to procedural competence, with residents feeling more comfortable knowing they have rapid access to support (Box 3: increased support).
Box
Supervision
Increased support
It makes me feel more comfortable in the sense that I can instantly make a call or a text and have a question answered if I need an answer. Or if it were an emergency having the ability to talk on the phone and be talked through an emergency situation, or a procedure for example like if you were in a remote area or the physician was in a remote area and you were in hospital and you would need some of that guidance or counselling, there's no substitution. (Interview Medical Student 1)
I'm ready can u dblchk [sic] that I landmarked correctly. (Email from Junior to Senior)
MD3 returns to the patient's room to do a paracentesis with [junior resident]. He calls on his BB to [senior resident] to inform her that they are starting and then hangs up. [Senior resident] arrives at the patient's room. (Field Notes, Workshadowing MD3)
Decreased autonomy
The difference with the Blackberry is they're more likely to say By the way, this happened. Should I do this?' And I write back Yes', No.' If they didn't have that contact like I said they probably would have done something and then because they're making a decision on their own they could very easily have spent the time to research whatever to figure whether that was the right thing to do before doing it. Now they have an outlet where they can pass an idea off of me and then have me make, it's easier for me to make a decision for them. So that can negatively impact education. (Interview Attending 1)
What do I do for a high phosphate?(Email from Junior to Senior)
Hey Pt X's k is 5.5. Was going to shift her. What do u think? (Email from Junior to Senior)
You probably saw the hb 92. Let's give prbc asap while he's on HD.(Email from Staff to Residents on the team)
hb‐ hemoglobin, prbc packed red blood cells, HD ‐ hemodialysis
Hi. Just checking the bloodwork. What is happening to ms X? [sic] Creatinine rising still. Is a foley in? Urology reconsulted? (Email from Attending Staff to Junior Resident)
On the other hand, supervisors perceived that the easy rapid access afforded by smartphone use lowered the threshold for trainees to contact them. In some instances, these attending physicians felt that their trainees would text them for advice when they could have looked up the information themselves. As a result, the increased reliance on the attending physician's input prior to committing to a management plan decreased the trainee's autonomy and independent decision making (Box 3: decreased autonomy). In addition to trainee requests for increased staff involvement, smartphone use made it easier for attending physicians to initiate text messages to their residents as well. In some instances, staff physicians adopted a more hands‐on approach by directing their residents on how to manage their patients. It is unclear if trainees perceived this taking over of care as negatively influencing their education.
Teaching
Medical teams also frequently used smartphones to communicate the location and timing of educational rounds. We observed instances where residents communicated updated information relating to scheduled rounds, as well as for informing team members about spontaneous teaching sessions (Box 4: communicating rounds). Despite this initial benefit, staff physicians worried that interruptions resulting from smartphone use during educational sessions lowered the effectiveness of these sessions for all learners by creating a fragmented learning experience (Box 4: fragmented learning). Our data indicated that residents carrying the team smartphones received and sent a high number of e‐mails throughout the day, which continued at a similar rate during the protected educational time (Figure 2). Additionally, some of the teaching experiences that traditionally would occur in a face‐to‐face manner appeared to have migrated to text‐based interactions. It is unclear whether trainees perceive these text‐based interactions as more or less effective teaching encounters (Box 4: text‐based teaching).
Box
Teaching
Communicating rounds
One is that they can more efficiently communicate about the timing and location of education rounds in case they forget or sort of as an organizer for them (Interview Attending 3)
Physical Exam rounds is at 1:00 outside the morning report room. K. has kindly volunteered! If you miss us then the exam will be on the 3rd floor in room X. Pt X. See you there (Email from chief medical resident to trainees)
Fragmented learning
Because Blackberry is there, it's something that is potentially time occupying and can take the attention away from things and this is true of any Blackberries. People who have Blackberries they always look at their Blackberries so, you know, there are times when I'm sitting face to face with people and residents are looking at their Blackberries. So it's another way that they can be distracted. (Interview Attending 1).
I've seen that be an issue. I've certainly seen them losing concentration during a teaching session because they're being Blackberried, getting Blackberry messages. (Interview Attending 3)
2:06 Team meeting with Attending in a conference room.
2:29 Team BlackBerry (BB) beeps. Senior glances at BB. She dials a number on the Team BB. Speaks on the Team BB and turns to [Junior resident] to inform her that the family is here. She returns to the caller. Senior then hangs up and resumes to her teaching.
2:35 Attending's BB rings. She takes a look and ends the BB call.
2:39 Senior's BB rings. Senior picks up and talks about a patient's case and condition. Senior turns to [junior resident] and asks a question. Team members resume talking among themselves.
2:46 Senior hangs up on the phone call.
2:49 Team discusses another patient's condition/case.
2:57 Junior resident uses her BB to text.
3:02 Team BB beeps. It is a message about a patient's case.
3:05 Meeting ends. (Field Note excerpts, Work‐shadowing MD6)
Text‐based teaching
The resident would get very frustrated with how many questions we have once we've started. Like if three different medical students or four different medical students or four different places all texting him with, oh by the way, what does this stand for?, and he's responding to each of them individually then he has to answer it four different times as opposed to just in person when he can get us all together in a group and it's actually a learning experience. If questions are answered in an email, it's not really helpful for the rest of us. (Interview, medical student SB1)
That would be a great unifying diagnosis, but there may be some underlying element of ROH/NASH also I would hold off on A/C as we do not know if he has varices. Will need to review noncontrast CT ?HCC. Thx (Email from Consulting Staff to Junior)
A/C anticoagulation, CT computed tomography, HCC‐ hepatocellular carcinoma, NASH non‐alcoholic steatohepatitis, ROH alcohol
Professionalism
Our data revealed that smartphone interruptions occurred during teaching rounds and interactions with patients and with other clinical staff. Often these interruptions involved messages or phone calls pertaining to clinical concerns or tasks that nurses communicated to the residents via their smartphone (Box 5). Yet, by responding to these interruptions and initiating communications on their smartphones during patient care encounters and formal teaching sessions, trainees were perceived by other clinicians who were in attendance with them as being rude or disrespectful. Attending staff also tended to role model similar smartphone behaviors. Although we did not specifically work‐shadow attending staff, we did observe frequent usage of their personal smartphones during their interactions with residents.
Box
Professionalism
I don't like it when I see them checking messages when you're trying to talk to them. I think you're losing some of that communication sort of polite behaviour that maybe we knew a little bit more before all this texting and Blackberry. (Interview Allied Health 5)
I think that the etiquette of the Blackberry can be offensive, could be offensive especially with some of our older patients (Interview Allied Health 6)
Senior walks out of the patient's room while typing on the BlackBerry. She finishes typing and returns to the room at 5:36. Senior looks at her BlackBerry and starts typing inside the room in front of the patient. She paused to look at the patient and the resident doing the procedure [paracentesis]. She resumes texting again and walks out of the room at 5:38. Another resident walks out and Senior speaks with the resident. Senior returns to the room and speaks with the patient. She asks the patient if he has ever gotten a successful tap before. Senior looks at her BlackBerry and starts typing. (Field NotesWork‐shadowing MD2)
I think it is almost completely negative in terms of its medical education [Any positive] factors are grossly outweighed by the significant disruptions to their ability to concentrate and participate in the educational session. And I think almost to some extent it's an implicit permission that gets granted to the house staff to disrupt their own teaching experience and disrupt others around them because everybody is doing it because everybody is being Blackberried. So it almost becomes the new social norm and while that may be a new social norm I'm not sure that that's a good thing How big is the negative impact? That's much harder to say. It's probably not a big impact on top of the endless other disruptions in the day to teaching, but it is measurable because it's a new factor so it's observable by me on top of all the other factors which have been there for years. (Interview Attending 3)
2:10‐Attending goes to the whiteboard to teach research methods to the team. Spotted Medical student#1 looking at his IPhone and typing.
2:15‐Med student#1 using the calculator function on his IPhone.
2:20‐Attending glances at his BB quickly.
2:28‐Attending resumes discussion of the patients' cases (Field notes, Work‐shadowing MD7).
DISCUSSION
The educational impacts of smartphone use for communication appear to center on increased connectedness of medical trainees and increased interruptions, which have positive and negative impacts in the areas of teaching, supervision, and professionalism. Smartphone communication provided potential educational benefits through (1) safer supervision with rapid access to help and (2) easier coordination of teaching sessions. Threats to the educational experience included (1) a high level of interruptions to both teachers and learners, which may reduce the effectiveness of formal and informal teaching; (2) replacement of face‐to‐face teaching with texting; (3) a potential erosion of autonomy and independence due to easy access to supervisors and easy ability for supervisors to take over; and (4) professionalism issues with difficulties balancing between clinical service demands and communication during patient and interprofessional encounters.
This study is the first to describe the intersection of clinical communication with smartphones and medical education. A recent study found that residents reported high use of smartphones during rounds for patient care as well as personal issues.[13] We have previously described the perceived impacts of smartphones on clinical communication, which included improved efficiency but concerns for increased interruptions and threats to professionalism.[6] We also observed that sites that used smartphones had increased interruptions compared to those with just pagers.[10] We have also described the content of e‐mail messages between clinicians and found that all e‐mails from nurses to physicians involved clinical care, but e‐mail exchanges between physicians were split between clinical care (60.4%), coordination within the team (53.5%), medical education (9.4%), and social communication (3.9%).[14] This study adds to the literature by focusing on the impacts of smartphone use to medical education and describing the perceived and observed impacts. This study provides a further example of how healthcare information technology can cause unintended consequences on medical education and appear to relate to the linked impacts of increased connectivity and the increased interruptions.[3] In essence, the trainee becomes more global, less local. Being more global translates to increased connections with people separated in physical space. Yet, this increased global connectedness resulted in the trainee being less local, with attention diverted elsewhere, taking away from the quality of patient interactions and interactions with other interprofessional team members. It also reduces the effectiveness of educational sessions for all participants. Although the level of supervision and autonomy are independently related, being too connected to supervisors may lower trainee autonomy by reducing independent thinking around patient issues.[15] It may also move teaching and learning from face‐to‐face conversations to text‐based messages. Although there have been existing tensions between service delivery and medical education, increased connectedness may tilt the balance toward the demands of service delivery and efficiency optimization at the expense of the educational experience. Finally, smartphone use appeared to create an internal tension among trainees, who have to juggle balancing professional behaviors and expectations in their dual role as learner and care provider; it would be educationally unprofessional to interrupt a teaching session and respond to a text message. However, failing to respond to a nurse who has sent a message and is expecting a response would be clinically unprofessional.
To address these threats, we advocate improving systems and processes to reduce interruptions and provide education on the tensions created by increased connectedness. Smarter communication systems could limit interrupting messages to urgent messages and queue nonurgent messages.[16] They could also inform senders about protected educational time. Even more sophisticated systems could inform the sender on the status of the receiver. For example, systems could indicate if they are available or if they are busy in an educational session or an important meeting with a patient and their family. Processes to reduce interruptions include interprofessional consensus on what constitutes an urgent issue and giving explicit permission to learners to ignore their smartphones during educational sessions except for critical communications purposes. Finally, education around smartphone communication for both learners and teachers may help minimize threats to learner autonomy, to face‐to‐face teaching, and to professionalism.[17]
Our study has several limitations. We derived this information from a general study of the impact of smartphones on clinical communication. Our study can be seen as hypothesis generating, and further research is warranted to validate these findings. There may be limits to generalizability as all sites adopted similar communication processes that included centralizing communications to make it easier for senders to reach a responsible physician.
In conclusion, we have provided a summary of the impact of rapidly emerging information technology on the educational experience of medical trainees and identified both positive and negative impacts. Of note, the negative impacts appear to be related to being more global and less local and high interruptions. Further research is required to confirm these unintended consequences as well as to develop solutions to address them. Educators should be aware of these findings and the need to develop curriculum to address and manage the negative impacts of smartphone use in the clinical training environment.
Acknowledgments
Disclosure: Nothing to report.
- , . Smartphone app use among medical providers in ACGME training programs. J Med Syst. 2012;36:3135–3139.
- , , , et al. The use of smartphones for clinical communication on internal medicine wards. J Hosp Med. 2010;5:553–559.
- , , , , , . Anticipating and addressing the unintended consequences of health IT and policy: a report from the AMIA 2009 Health Policy Meeting. J Am Med Inform Assoc. 2011;18(1):82–90.
- , , , , . Types of unintended consequences related to computerized provider order entry. J Am Med Inform Assoc. 2006;13(5):547–556.
- , , , . “e‐Iatrogenesis”: the most critical unintended consequence of CPOE and other HIT. J Am Med Inform Assoc. 2007;14(3):387–388.
- , , , et al. An evaluation of the use of smartphones to communicate between clinicians: a mixed‐methods study. J Med Internet Res. 2011;13(3):e59.
- . Smartphones in clinical practice, medical education, and research. Arch Intern Med. 2011;171(14):1294–1296.
- , , , . Use of handheld computers in medical education. A systematic review. J Gen Intern Med. 2006;21(5):531–537.
- , , , . A review and a framework of handheld computer adoption in healthcare. Int J Med Inform. 2005;74(5):409–422.
- , , , et al. The intended and unintended consequences of communication systems on General Internal Medicine inpatient care delivery: a prospective observational case study of five teaching hospitals [published online ahead of print January 25, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2012‐001160.
- , , , , . Hospital doctors' workflow interruptions and activities: an observation study. BMJ Qual Saf. 2011;20(6):491–497.
- , , , et al. Computerised provider order entry and residency education in an academic medical centre. Med Educ. 2012;46:795–806.
- , , , . Smartphone use during inpatient attending rounds: Prevalence, patterns and potential for distraction. J Hosp Med. 2012;7(8):595–599.
- , , , et al. Understanding interprofessional communication: a content analysis of email communications between doctors and nurses. Applied Clinical Informatics. 2012;3:38–51.
- , , , . Preserving professional credibility: grounded theory study of medical trainees' requests for clinical support. BMJ. 2009;338:b128.
- , , , , . Beyond paging: building a web‐based communication tool for nurses and physicians. J Gen Intern Med. 2009;24(1):105–110.
- , . Distracted doctoring: smartphones before patients? CMAJ. 2012;184:1440.
- , . Smartphone app use among medical providers in ACGME training programs. J Med Syst. 2012;36:3135–3139.
- , , , et al. The use of smartphones for clinical communication on internal medicine wards. J Hosp Med. 2010;5:553–559.
- , , , , , . Anticipating and addressing the unintended consequences of health IT and policy: a report from the AMIA 2009 Health Policy Meeting. J Am Med Inform Assoc. 2011;18(1):82–90.
- , , , , . Types of unintended consequences related to computerized provider order entry. J Am Med Inform Assoc. 2006;13(5):547–556.
- , , , . “e‐Iatrogenesis”: the most critical unintended consequence of CPOE and other HIT. J Am Med Inform Assoc. 2007;14(3):387–388.
- , , , et al. An evaluation of the use of smartphones to communicate between clinicians: a mixed‐methods study. J Med Internet Res. 2011;13(3):e59.
- . Smartphones in clinical practice, medical education, and research. Arch Intern Med. 2011;171(14):1294–1296.
- , , , . Use of handheld computers in medical education. A systematic review. J Gen Intern Med. 2006;21(5):531–537.
- , , , . A review and a framework of handheld computer adoption in healthcare. Int J Med Inform. 2005;74(5):409–422.
- , , , et al. The intended and unintended consequences of communication systems on General Internal Medicine inpatient care delivery: a prospective observational case study of five teaching hospitals [published online ahead of print January 25, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2012‐001160.
- , , , , . Hospital doctors' workflow interruptions and activities: an observation study. BMJ Qual Saf. 2011;20(6):491–497.
- , , , et al. Computerised provider order entry and residency education in an academic medical centre. Med Educ. 2012;46:795–806.
- , , , . Smartphone use during inpatient attending rounds: Prevalence, patterns and potential for distraction. J Hosp Med. 2012;7(8):595–599.
- , , , et al. Understanding interprofessional communication: a content analysis of email communications between doctors and nurses. Applied Clinical Informatics. 2012;3:38–51.
- , , , . Preserving professional credibility: grounded theory study of medical trainees' requests for clinical support. BMJ. 2009;338:b128.
- , , , , . Beyond paging: building a web‐based communication tool for nurses and physicians. J Gen Intern Med. 2009;24(1):105–110.
- , . Distracted doctoring: smartphones before patients? CMAJ. 2012;184:1440.
© 2013 Society of Hospital Medicine
Impact of pretreatment PET on disease control and treatment decisions in locoregionally advanced esophageal cancer patients treated with chemoradiotherapy
Most patients with esophageal cancer are diagnosed with locoregionally advanced disease at presentation, with an overall 5-year survival rate of 19%.1 Clinical trials have failed to specify the optimal treatment regimen; however, a multimodal approach to therapy is considered the standard of care for patients with locoregionally advanced disease.2 Most often, patients are treated with chemoradiotherapy with or without subsequent esophagectomy. Curative-intent interventions for advanced esophageal cancer are necessarily aggressive and may be associated with significant morbidity and even treatment-related mortality. Appropriate selection of patients for intervention is necessary so that those who are most likely to benefit can initiate curative-intent therapy, whereas those who are unlikely to benefit from intervention may be appropriately initiated on less toxic palliative-intent treatment. The use of positron emission tomography (PET) in esophageal cancer staging has improved the ability to detect distant disease at diagnosis,3-7 an important factor in determining the appropriate treatment regimen and prognosis…
To read the full article, click on the PDF icon at the top of this introduction.
Most patients with esophageal cancer are diagnosed with locoregionally advanced disease at presentation, with an overall 5-year survival rate of 19%.1 Clinical trials have failed to specify the optimal treatment regimen; however, a multimodal approach to therapy is considered the standard of care for patients with locoregionally advanced disease.2 Most often, patients are treated with chemoradiotherapy with or without subsequent esophagectomy. Curative-intent interventions for advanced esophageal cancer are necessarily aggressive and may be associated with significant morbidity and even treatment-related mortality. Appropriate selection of patients for intervention is necessary so that those who are most likely to benefit can initiate curative-intent therapy, whereas those who are unlikely to benefit from intervention may be appropriately initiated on less toxic palliative-intent treatment. The use of positron emission tomography (PET) in esophageal cancer staging has improved the ability to detect distant disease at diagnosis,3-7 an important factor in determining the appropriate treatment regimen and prognosis…
To read the full article, click on the PDF icon at the top of this introduction.
Most patients with esophageal cancer are diagnosed with locoregionally advanced disease at presentation, with an overall 5-year survival rate of 19%.1 Clinical trials have failed to specify the optimal treatment regimen; however, a multimodal approach to therapy is considered the standard of care for patients with locoregionally advanced disease.2 Most often, patients are treated with chemoradiotherapy with or without subsequent esophagectomy. Curative-intent interventions for advanced esophageal cancer are necessarily aggressive and may be associated with significant morbidity and even treatment-related mortality. Appropriate selection of patients for intervention is necessary so that those who are most likely to benefit can initiate curative-intent therapy, whereas those who are unlikely to benefit from intervention may be appropriately initiated on less toxic palliative-intent treatment. The use of positron emission tomography (PET) in esophageal cancer staging has improved the ability to detect distant disease at diagnosis,3-7 an important factor in determining the appropriate treatment regimen and prognosis…
To read the full article, click on the PDF icon at the top of this introduction.
Investigational noninterferon HCV treatment effective across patient groups
ORLANDO – Both 12- and 24-week regimens of an investigational interferon-free therapy for hepatitis C were associated with high sustained virological response rates at 12 weeks post treatment in a phase II study of patients with hepatitis C virus genotype 1 who had baseline characteristics associated with poor response to interferon-based therapies.
The findings of this subgroup analysis of the randomized, open-label, multicenter Aviator trial demonstrate that the high response rates recently reported for the entire cohort also apply to patients with older age, black race, Hispanic/Latino ethnicity, interleukin (IL)-28B non-cc genotype, and higher body mass index (BMI), Dr. Frederick Nunes reported at the annual Digestive Disease Week.
The Aviator trial assessed the safety and efficacy of various dosing regimens and combinations of three AbbVie investigational direct-acting antivirals (DAAs) with or without ribavirin, including the potent hepatitis C virus (HCV) protease inhibitor ABT-450 dosed with 100 mg of ritonavir (ABT-450/r, dosed at 100 or 150 mg daily), the NS5A inhibitor ABT-267 (25 mg daily), and the non-nucleoside NS5B inhibitor ABT-333 (400 mg twice daily). A total of 247 patients were included in the subanalysis, Dr. Nunes reported at the meeting.
The regimen that included all three investigational drugs and ribavirin, known as 3 DAA/RBV, was associated with sustained virological response rates of 99% and 93% at 12 weeks in treatment-naive patients and previous peg-interferon/ribavirin (peg-IFN/RBV) null responders, respectively, said Dr. Nunes, who is clinical associate professor of medicine in the University of Pennsylvania Health System, and section chief of gastroenterology at Pennsylvania Hospital, Philadelphia.
In the current analysis, high sustained virological response rates at 12 weeks (SVR12) were also achieved in the 247 patients with chronic HCV genotype 1, including 159 treatment-naive and 88 previous peg-IFN/RBV null responders, who were assigned to 12 or 24 weeks of 3 DAA/RBV. The virological response rates were 99% and 93% for the treatment-naive 12- and 24-week groups, respectively, and 93% and 98% for the null responder 12- and 24-week groups.
The high responses occurred regardless of treatment duration, age, race, ethnicity, BMI, Homeostatic Model of Assessment–Insulin Resistance (HOMA-IR), IL-28B host genotype, or baseline viral load. The rates did not differ significantly on any comparison made in treatment-naive patients or previous null responders, he said.
In the treatment-naive patients, no breakthroughs occurred, although 1% of those in the 12-week treatment arm relapsed, and 3% of the 24-week treatment group relapsed.
In the null responder group, no relapses occurred, but breakthroughs occurred in 7% of those in the 12-week treatment arm, and in 2% of the 24-week treatment arm, he said.
Treatment was safe and generally well tolerated. Four patients discontinued treatment due to drug-related adverse events, most commonly fatigue (in 32.7% and 23.9% of treatment-naive and null responders, respectively) and headache (31.4% and 30.7%, respectively).
One patient had a serious adverse event (arthralgia) considered to be possibly related to the study drug regimen.
Because ribavirin was included in the treatment regimens, it is impossible to tease out whether the adverse events were associated with that drug or with the investigational DAAs, Dr. Nunes noted.
Patients included in the study were noncirrhotic adults aged 18-70 years with a BMI between 18 and 38 kg/m2, and HCV genotype 1.
"The overall efficacy was excellent irrespective of the baseline grouping. So black race, Hispanic/Latino ethnicity, age greater than 50, BMI over 30, male gender, HOMA-IR greater than 3, IL-28B non-cc genotype, and viral load greater than 7 logs all had very high SVR12 response rates," Dr. Nunes said.
The safety and efficacy of this interferon-free 3 DAA/RBV therapy will be further explored in phase III studies, he said.
Dr. Nunes has received grant or research support from Merck, Abbott Laboratories, and Roche Pharma AG.
ORLANDO – Both 12- and 24-week regimens of an investigational interferon-free therapy for hepatitis C were associated with high sustained virological response rates at 12 weeks post treatment in a phase II study of patients with hepatitis C virus genotype 1 who had baseline characteristics associated with poor response to interferon-based therapies.
The findings of this subgroup analysis of the randomized, open-label, multicenter Aviator trial demonstrate that the high response rates recently reported for the entire cohort also apply to patients with older age, black race, Hispanic/Latino ethnicity, interleukin (IL)-28B non-cc genotype, and higher body mass index (BMI), Dr. Frederick Nunes reported at the annual Digestive Disease Week.
The Aviator trial assessed the safety and efficacy of various dosing regimens and combinations of three AbbVie investigational direct-acting antivirals (DAAs) with or without ribavirin, including the potent hepatitis C virus (HCV) protease inhibitor ABT-450 dosed with 100 mg of ritonavir (ABT-450/r, dosed at 100 or 150 mg daily), the NS5A inhibitor ABT-267 (25 mg daily), and the non-nucleoside NS5B inhibitor ABT-333 (400 mg twice daily). A total of 247 patients were included in the subanalysis, Dr. Nunes reported at the meeting.
The regimen that included all three investigational drugs and ribavirin, known as 3 DAA/RBV, was associated with sustained virological response rates of 99% and 93% at 12 weeks in treatment-naive patients and previous peg-interferon/ribavirin (peg-IFN/RBV) null responders, respectively, said Dr. Nunes, who is clinical associate professor of medicine in the University of Pennsylvania Health System, and section chief of gastroenterology at Pennsylvania Hospital, Philadelphia.
In the current analysis, high sustained virological response rates at 12 weeks (SVR12) were also achieved in the 247 patients with chronic HCV genotype 1, including 159 treatment-naive and 88 previous peg-IFN/RBV null responders, who were assigned to 12 or 24 weeks of 3 DAA/RBV. The virological response rates were 99% and 93% for the treatment-naive 12- and 24-week groups, respectively, and 93% and 98% for the null responder 12- and 24-week groups.
The high responses occurred regardless of treatment duration, age, race, ethnicity, BMI, Homeostatic Model of Assessment–Insulin Resistance (HOMA-IR), IL-28B host genotype, or baseline viral load. The rates did not differ significantly on any comparison made in treatment-naive patients or previous null responders, he said.
In the treatment-naive patients, no breakthroughs occurred, although 1% of those in the 12-week treatment arm relapsed, and 3% of the 24-week treatment group relapsed.
In the null responder group, no relapses occurred, but breakthroughs occurred in 7% of those in the 12-week treatment arm, and in 2% of the 24-week treatment arm, he said.
Treatment was safe and generally well tolerated. Four patients discontinued treatment due to drug-related adverse events, most commonly fatigue (in 32.7% and 23.9% of treatment-naive and null responders, respectively) and headache (31.4% and 30.7%, respectively).
One patient had a serious adverse event (arthralgia) considered to be possibly related to the study drug regimen.
Because ribavirin was included in the treatment regimens, it is impossible to tease out whether the adverse events were associated with that drug or with the investigational DAAs, Dr. Nunes noted.
Patients included in the study were noncirrhotic adults aged 18-70 years with a BMI between 18 and 38 kg/m2, and HCV genotype 1.
"The overall efficacy was excellent irrespective of the baseline grouping. So black race, Hispanic/Latino ethnicity, age greater than 50, BMI over 30, male gender, HOMA-IR greater than 3, IL-28B non-cc genotype, and viral load greater than 7 logs all had very high SVR12 response rates," Dr. Nunes said.
The safety and efficacy of this interferon-free 3 DAA/RBV therapy will be further explored in phase III studies, he said.
Dr. Nunes has received grant or research support from Merck, Abbott Laboratories, and Roche Pharma AG.
ORLANDO – Both 12- and 24-week regimens of an investigational interferon-free therapy for hepatitis C were associated with high sustained virological response rates at 12 weeks post treatment in a phase II study of patients with hepatitis C virus genotype 1 who had baseline characteristics associated with poor response to interferon-based therapies.
The findings of this subgroup analysis of the randomized, open-label, multicenter Aviator trial demonstrate that the high response rates recently reported for the entire cohort also apply to patients with older age, black race, Hispanic/Latino ethnicity, interleukin (IL)-28B non-cc genotype, and higher body mass index (BMI), Dr. Frederick Nunes reported at the annual Digestive Disease Week.
The Aviator trial assessed the safety and efficacy of various dosing regimens and combinations of three AbbVie investigational direct-acting antivirals (DAAs) with or without ribavirin, including the potent hepatitis C virus (HCV) protease inhibitor ABT-450 dosed with 100 mg of ritonavir (ABT-450/r, dosed at 100 or 150 mg daily), the NS5A inhibitor ABT-267 (25 mg daily), and the non-nucleoside NS5B inhibitor ABT-333 (400 mg twice daily). A total of 247 patients were included in the subanalysis, Dr. Nunes reported at the meeting.
The regimen that included all three investigational drugs and ribavirin, known as 3 DAA/RBV, was associated with sustained virological response rates of 99% and 93% at 12 weeks in treatment-naive patients and previous peg-interferon/ribavirin (peg-IFN/RBV) null responders, respectively, said Dr. Nunes, who is clinical associate professor of medicine in the University of Pennsylvania Health System, and section chief of gastroenterology at Pennsylvania Hospital, Philadelphia.
In the current analysis, high sustained virological response rates at 12 weeks (SVR12) were also achieved in the 247 patients with chronic HCV genotype 1, including 159 treatment-naive and 88 previous peg-IFN/RBV null responders, who were assigned to 12 or 24 weeks of 3 DAA/RBV. The virological response rates were 99% and 93% for the treatment-naive 12- and 24-week groups, respectively, and 93% and 98% for the null responder 12- and 24-week groups.
The high responses occurred regardless of treatment duration, age, race, ethnicity, BMI, Homeostatic Model of Assessment–Insulin Resistance (HOMA-IR), IL-28B host genotype, or baseline viral load. The rates did not differ significantly on any comparison made in treatment-naive patients or previous null responders, he said.
In the treatment-naive patients, no breakthroughs occurred, although 1% of those in the 12-week treatment arm relapsed, and 3% of the 24-week treatment group relapsed.
In the null responder group, no relapses occurred, but breakthroughs occurred in 7% of those in the 12-week treatment arm, and in 2% of the 24-week treatment arm, he said.
Treatment was safe and generally well tolerated. Four patients discontinued treatment due to drug-related adverse events, most commonly fatigue (in 32.7% and 23.9% of treatment-naive and null responders, respectively) and headache (31.4% and 30.7%, respectively).
One patient had a serious adverse event (arthralgia) considered to be possibly related to the study drug regimen.
Because ribavirin was included in the treatment regimens, it is impossible to tease out whether the adverse events were associated with that drug or with the investigational DAAs, Dr. Nunes noted.
Patients included in the study were noncirrhotic adults aged 18-70 years with a BMI between 18 and 38 kg/m2, and HCV genotype 1.
"The overall efficacy was excellent irrespective of the baseline grouping. So black race, Hispanic/Latino ethnicity, age greater than 50, BMI over 30, male gender, HOMA-IR greater than 3, IL-28B non-cc genotype, and viral load greater than 7 logs all had very high SVR12 response rates," Dr. Nunes said.
The safety and efficacy of this interferon-free 3 DAA/RBV therapy will be further explored in phase III studies, he said.
Dr. Nunes has received grant or research support from Merck, Abbott Laboratories, and Roche Pharma AG.
AT DDW 2013
Major finding: An investigational interferon-free therapy is effective in patients at risk for poor response to interferon-based therapy.
Data source: A randomized, open-label, multicenter trial (Aviator trial).
Disclosures: Dr. Nunes has received grant or research support from Merck, Abbott Laboratories, and Roche Pharma AG.
Predicting life expectancy in patients with advanced incurable cancer: a review
ABSTRACT
Oncologists frequently face the difficult task of estimating prognosis in patients with incurable malignancies. Their prediction of prognosis informs decision-making ranging from recommendations of cancer treatments to hospice enrollment. Unfortunately, physicians’ estimates of prognosis are often inaccurate and overly optimistic. Further, physicians often fail to disclose their prognosis estimates, despite patient wishes to the contrary. Several studies have examined patient factors that might improve physicians’ prognostic accuracy, including performance status, clinical symptoms and laboratory values. Prognostic models have been developed and validated but, to date, none are able to provide accurate estimates throughout the spectrum of advanced illness. This review examines tools utilized to predict life expectancy for patients with advanced, incurable cancer.
*For a PDF of the full article, click on the link to the left of this introduction.
ABSTRACT
Oncologists frequently face the difficult task of estimating prognosis in patients with incurable malignancies. Their prediction of prognosis informs decision-making ranging from recommendations of cancer treatments to hospice enrollment. Unfortunately, physicians’ estimates of prognosis are often inaccurate and overly optimistic. Further, physicians often fail to disclose their prognosis estimates, despite patient wishes to the contrary. Several studies have examined patient factors that might improve physicians’ prognostic accuracy, including performance status, clinical symptoms and laboratory values. Prognostic models have been developed and validated but, to date, none are able to provide accurate estimates throughout the spectrum of advanced illness. This review examines tools utilized to predict life expectancy for patients with advanced, incurable cancer.
*For a PDF of the full article, click on the link to the left of this introduction.
ABSTRACT
Oncologists frequently face the difficult task of estimating prognosis in patients with incurable malignancies. Their prediction of prognosis informs decision-making ranging from recommendations of cancer treatments to hospice enrollment. Unfortunately, physicians’ estimates of prognosis are often inaccurate and overly optimistic. Further, physicians often fail to disclose their prognosis estimates, despite patient wishes to the contrary. Several studies have examined patient factors that might improve physicians’ prognostic accuracy, including performance status, clinical symptoms and laboratory values. Prognostic models have been developed and validated but, to date, none are able to provide accurate estimates throughout the spectrum of advanced illness. This review examines tools utilized to predict life expectancy for patients with advanced, incurable cancer.
*For a PDF of the full article, click on the link to the left of this introduction.
Utilization of radiotherapy services by a palliative care unit: pattern and implication
Background The role of radiotherapy in palliation is well recognized. Analyzing referrals from an inpatient palliative care unit to the radiation oncology service may help in planning palliative care services and educational programs.
Objective To determine the pattern and rate of referrals from a PCU to the RO service at a tertiary oncology facility in Saudi Arabia.
Methods Referrals from the PCU to the RO service were prospectively identified over the period beginning November 27, 2007 and ending March 9, 2011. The appropriateness of referrals was determined by 2 radiation oncologists.
Results Of the 635 cancer admissions to the PCU, 25 (3.9%) referrals to RO were made, and 32 sites were irradiated. All patients had a poor performance status (ECOG 3). The most common areas irradiated were vertebrae (40.6%), pelvis (18.7%) and other bony structures (28.1%). Pain control was the most frequent reason for referral (87.5%). Only one referral was regarded by the RO service as inappropriate, indicating that 96% of the referrals were appropriate. The mean time lapse between referral and starting radiation was 4 3.6 days. A total of 75% of the patients died in the PCU within a median of 30 days post radiotherapy.
Conclusion The small minority of patients in the PCU referred for radiotherapy were deemed appropriate referrals by the radiation oncologists despite their poor performance status and limited time remaining. When planning a PCU with similar admission criteria, the availability of a radiotherapy facility in close proximity may not be a priority.
Click on the PDF icon at the top of this introduction to read the full article.
Background The role of radiotherapy in palliation is well recognized. Analyzing referrals from an inpatient palliative care unit to the radiation oncology service may help in planning palliative care services and educational programs.
Objective To determine the pattern and rate of referrals from a PCU to the RO service at a tertiary oncology facility in Saudi Arabia.
Methods Referrals from the PCU to the RO service were prospectively identified over the period beginning November 27, 2007 and ending March 9, 2011. The appropriateness of referrals was determined by 2 radiation oncologists.
Results Of the 635 cancer admissions to the PCU, 25 (3.9%) referrals to RO were made, and 32 sites were irradiated. All patients had a poor performance status (ECOG 3). The most common areas irradiated were vertebrae (40.6%), pelvis (18.7%) and other bony structures (28.1%). Pain control was the most frequent reason for referral (87.5%). Only one referral was regarded by the RO service as inappropriate, indicating that 96% of the referrals were appropriate. The mean time lapse between referral and starting radiation was 4 3.6 days. A total of 75% of the patients died in the PCU within a median of 30 days post radiotherapy.
Conclusion The small minority of patients in the PCU referred for radiotherapy were deemed appropriate referrals by the radiation oncologists despite their poor performance status and limited time remaining. When planning a PCU with similar admission criteria, the availability of a radiotherapy facility in close proximity may not be a priority.
Click on the PDF icon at the top of this introduction to read the full article.
Background The role of radiotherapy in palliation is well recognized. Analyzing referrals from an inpatient palliative care unit to the radiation oncology service may help in planning palliative care services and educational programs.
Objective To determine the pattern and rate of referrals from a PCU to the RO service at a tertiary oncology facility in Saudi Arabia.
Methods Referrals from the PCU to the RO service were prospectively identified over the period beginning November 27, 2007 and ending March 9, 2011. The appropriateness of referrals was determined by 2 radiation oncologists.
Results Of the 635 cancer admissions to the PCU, 25 (3.9%) referrals to RO were made, and 32 sites were irradiated. All patients had a poor performance status (ECOG 3). The most common areas irradiated were vertebrae (40.6%), pelvis (18.7%) and other bony structures (28.1%). Pain control was the most frequent reason for referral (87.5%). Only one referral was regarded by the RO service as inappropriate, indicating that 96% of the referrals were appropriate. The mean time lapse between referral and starting radiation was 4 3.6 days. A total of 75% of the patients died in the PCU within a median of 30 days post radiotherapy.
Conclusion The small minority of patients in the PCU referred for radiotherapy were deemed appropriate referrals by the radiation oncologists despite their poor performance status and limited time remaining. When planning a PCU with similar admission criteria, the availability of a radiotherapy facility in close proximity may not be a priority.
Click on the PDF icon at the top of this introduction to read the full article.
Canada alters blood donor policy for MSM

Canada is lifting the lifetime ban on blood donations from men who have sex with men (MSM).
The country’s new policy will allow MSM to donate blood if they have remained celibate for 5 years. The policy is set to take effect July 22.
Health Canada approved this change based on a request from Canadian Blood Services and Héma-Québec submitted last December.
The lifetime ban on MSM blood donation was insituted in the 1980s after thousands of Canadians were infected with HIV through blood transfusions.
In the US, the lifetime ban is still in place. But the UK, Australia, and other countries have adopted policies alowing MSM to donate if they have refrained from sexual activity for a certain period of time.
Supporters of the lifetime ban on MSM have said such a policy helps ensure a safe blood supply. But critics have said banning MSM is discriminatory, and the policy lacks scientific evidence to support it.
“We recognize that many people will feel that this change does not go far enough, but, given the history of the blood system in Canada, we see this as a first and prudent step forward on this policy,” said Dana Devine, Vice President of Medical, Scientific, and Research Affairs at Canadian Blood Services.
“It’s the right thing to do, and we are committed to regular review of this policy as additional data emerge and new technologies are implemented.”
Under the new policy, potential male blood donors will be asked whether they have had sex with a man in the past 5 years rather than “even once, since 1977,” which is how the policy has read until now.
Canadian Blood Services has been pursuing data to inform a policy change on MSM for several years. In September 2011, the organization’s board of directors passed a motion committing to re-examine this policy, with a view to reduce the lifetime exclusion to no less than 5 years and no longer than 10 years.
After conducting risk analyses and consulting with scientific experts, as well as patient and community groups, Canadian Blood Services and Héma-Québec submitted a policy request to Health Canada in December 2012.
Health Canada approved the policy change with the stipulation that blood operators must closely monitor and report the potential impacts of this change in terms of transmissible disease rates back to the regulator. ![]()

Canada is lifting the lifetime ban on blood donations from men who have sex with men (MSM).
The country’s new policy will allow MSM to donate blood if they have remained celibate for 5 years. The policy is set to take effect July 22.
Health Canada approved this change based on a request from Canadian Blood Services and Héma-Québec submitted last December.
The lifetime ban on MSM blood donation was insituted in the 1980s after thousands of Canadians were infected with HIV through blood transfusions.
In the US, the lifetime ban is still in place. But the UK, Australia, and other countries have adopted policies alowing MSM to donate if they have refrained from sexual activity for a certain period of time.
Supporters of the lifetime ban on MSM have said such a policy helps ensure a safe blood supply. But critics have said banning MSM is discriminatory, and the policy lacks scientific evidence to support it.
“We recognize that many people will feel that this change does not go far enough, but, given the history of the blood system in Canada, we see this as a first and prudent step forward on this policy,” said Dana Devine, Vice President of Medical, Scientific, and Research Affairs at Canadian Blood Services.
“It’s the right thing to do, and we are committed to regular review of this policy as additional data emerge and new technologies are implemented.”
Under the new policy, potential male blood donors will be asked whether they have had sex with a man in the past 5 years rather than “even once, since 1977,” which is how the policy has read until now.
Canadian Blood Services has been pursuing data to inform a policy change on MSM for several years. In September 2011, the organization’s board of directors passed a motion committing to re-examine this policy, with a view to reduce the lifetime exclusion to no less than 5 years and no longer than 10 years.
After conducting risk analyses and consulting with scientific experts, as well as patient and community groups, Canadian Blood Services and Héma-Québec submitted a policy request to Health Canada in December 2012.
Health Canada approved the policy change with the stipulation that blood operators must closely monitor and report the potential impacts of this change in terms of transmissible disease rates back to the regulator. ![]()

Canada is lifting the lifetime ban on blood donations from men who have sex with men (MSM).
The country’s new policy will allow MSM to donate blood if they have remained celibate for 5 years. The policy is set to take effect July 22.
Health Canada approved this change based on a request from Canadian Blood Services and Héma-Québec submitted last December.
The lifetime ban on MSM blood donation was insituted in the 1980s after thousands of Canadians were infected with HIV through blood transfusions.
In the US, the lifetime ban is still in place. But the UK, Australia, and other countries have adopted policies alowing MSM to donate if they have refrained from sexual activity for a certain period of time.
Supporters of the lifetime ban on MSM have said such a policy helps ensure a safe blood supply. But critics have said banning MSM is discriminatory, and the policy lacks scientific evidence to support it.
“We recognize that many people will feel that this change does not go far enough, but, given the history of the blood system in Canada, we see this as a first and prudent step forward on this policy,” said Dana Devine, Vice President of Medical, Scientific, and Research Affairs at Canadian Blood Services.
“It’s the right thing to do, and we are committed to regular review of this policy as additional data emerge and new technologies are implemented.”
Under the new policy, potential male blood donors will be asked whether they have had sex with a man in the past 5 years rather than “even once, since 1977,” which is how the policy has read until now.
Canadian Blood Services has been pursuing data to inform a policy change on MSM for several years. In September 2011, the organization’s board of directors passed a motion committing to re-examine this policy, with a view to reduce the lifetime exclusion to no less than 5 years and no longer than 10 years.
After conducting risk analyses and consulting with scientific experts, as well as patient and community groups, Canadian Blood Services and Héma-Québec submitted a policy request to Health Canada in December 2012.
Health Canada approved the policy change with the stipulation that blood operators must closely monitor and report the potential impacts of this change in terms of transmissible disease rates back to the regulator. ![]()
Measuring priority symptoms in advanced bladder cancer: development and initial validation of a brief symptom index
Background Improved measurement of clinically meaningful symptoms is needed in advanced bladder cancer.
Objective This study developed and examined the initial reliability and validity of a new measure of advanced bladder cancer specific symptoms, the NCCN-FACT Bladder Symptom Index-18 (NFBlSI-18), which assesses the symptoms perceived as most important by patients and oncology clinical experts.
Methods A total of 31 individuals with advanced bladder cancer rated the importance of 28 symptoms. In addition, 10 oncology clinical experts rated symptoms as treatment- or disease-related. Patient-rated symptoms were reconciled with published clinicians’ symptom priorities, producing the NFBlSI-18. Participants completed measures of quality of life (QoL) and performance status to examine initial validity.
Results An 18-item symptom index for advanced bladder cancer included 3 subscales: disease-related symptoms, treatment side effects, and general function/well-being. Lower scores indicate greater symptom burden. Preliminary reliability reveals good internal consistency for the full NFBlSI-18 ( 0.83). The NFBlSI-18 was significantly associated with QOL criteria and performance status, in the expected direction.
Limitations Limitations include the cross-sectional design and the relatively low reliability of the disease-related symptoms subscale.
Conclusion The NFBlSI-18 demonstrates preliminary evidence as a valid brief measure of the most important symptoms of advanced bladder cancer, as rated by both patients and oncology clinical experts. The NFBlSI-18 should have greater acceptability to regulatory authorities than previously developed questionnaires.
*Click on the PDF icon at the top of this introduction to read the full article.
Background Improved measurement of clinically meaningful symptoms is needed in advanced bladder cancer.
Objective This study developed and examined the initial reliability and validity of a new measure of advanced bladder cancer specific symptoms, the NCCN-FACT Bladder Symptom Index-18 (NFBlSI-18), which assesses the symptoms perceived as most important by patients and oncology clinical experts.
Methods A total of 31 individuals with advanced bladder cancer rated the importance of 28 symptoms. In addition, 10 oncology clinical experts rated symptoms as treatment- or disease-related. Patient-rated symptoms were reconciled with published clinicians’ symptom priorities, producing the NFBlSI-18. Participants completed measures of quality of life (QoL) and performance status to examine initial validity.
Results An 18-item symptom index for advanced bladder cancer included 3 subscales: disease-related symptoms, treatment side effects, and general function/well-being. Lower scores indicate greater symptom burden. Preliminary reliability reveals good internal consistency for the full NFBlSI-18 ( 0.83). The NFBlSI-18 was significantly associated with QOL criteria and performance status, in the expected direction.
Limitations Limitations include the cross-sectional design and the relatively low reliability of the disease-related symptoms subscale.
Conclusion The NFBlSI-18 demonstrates preliminary evidence as a valid brief measure of the most important symptoms of advanced bladder cancer, as rated by both patients and oncology clinical experts. The NFBlSI-18 should have greater acceptability to regulatory authorities than previously developed questionnaires.
*Click on the PDF icon at the top of this introduction to read the full article.
Background Improved measurement of clinically meaningful symptoms is needed in advanced bladder cancer.
Objective This study developed and examined the initial reliability and validity of a new measure of advanced bladder cancer specific symptoms, the NCCN-FACT Bladder Symptom Index-18 (NFBlSI-18), which assesses the symptoms perceived as most important by patients and oncology clinical experts.
Methods A total of 31 individuals with advanced bladder cancer rated the importance of 28 symptoms. In addition, 10 oncology clinical experts rated symptoms as treatment- or disease-related. Patient-rated symptoms were reconciled with published clinicians’ symptom priorities, producing the NFBlSI-18. Participants completed measures of quality of life (QoL) and performance status to examine initial validity.
Results An 18-item symptom index for advanced bladder cancer included 3 subscales: disease-related symptoms, treatment side effects, and general function/well-being. Lower scores indicate greater symptom burden. Preliminary reliability reveals good internal consistency for the full NFBlSI-18 ( 0.83). The NFBlSI-18 was significantly associated with QOL criteria and performance status, in the expected direction.
Limitations Limitations include the cross-sectional design and the relatively low reliability of the disease-related symptoms subscale.
Conclusion The NFBlSI-18 demonstrates preliminary evidence as a valid brief measure of the most important symptoms of advanced bladder cancer, as rated by both patients and oncology clinical experts. The NFBlSI-18 should have greater acceptability to regulatory authorities than previously developed questionnaires.
*Click on the PDF icon at the top of this introduction to read the full article.
Nice to meet
Recently, hospitalists around the country gathered together at Hospital Medicine 2013 to gain new and practical clinical insights we can use to optimize the medical care we provide to our patients, imbibe new research on the horizon, master clinical care guidelines, and sometimes, just relax and enjoy meeting new colleagues from around the country – naturally, comparing notes on how their practices stack up to our own. You could even pick up a book or two geared to hospitalists. I bought "Clinical Care Conundrums: Challenging Diagnoses in Hospital Medicine" and "Becoming a Consummate Clinician: What Every Student, House Office, and Hospital Practitioner Needs to Know." Learning just doesn’t get any better than this.
Information abounded, challenges were issued, and I think most of us learned how much we really need to learn more about. I believe we were thoroughly challenged to look at our current practice style, incorporate our new knowledge, and take our clinical acumen to the next level.
The first challenge began when I had to roll out of bed around 5 a.m. to prepare to make the hour-long drive down I-95 to the conference site at National Harbor, Md., to attend a 7:40 a.m. lecture: "Pain Management for the Hospitalist." I have not a single regret. It was well worth the bleary ride. I think many hospitalists share my concerns about overmedicating patients on the one hand, and being on guard for true drug seekers on the other.
My main takeaway was that when a patient is truly in pain, narcotic pain medication can be titrated up much more quickly than most of us currently feel comfortable with.
The lecture was presented by Dr. Eric Roeland of the University of Carolina, San Diego, who said that he sometimes doubles narcotic analgesics every 10 minutes under certain circumstances. Some participants were shocked (including me). He orders a dose of pain medication and then checks on the patient around the time of CMax (maximum concentration of the drug). For IV pain medications, this is approximately 10 minutes, for SC/IM it is 30 minutes, and for PO/PR it is 60 minutes. If his patient has not gotten pain relief by CMax, he orders twice the dose and checks back in again at the next CMax. If the patient still has no relief, Dr. Roeland will order four times the initial dose.
He stated that since sedation comes before respiratory depression, he feels comfortable increasing narcotics rapidly in patients who are "alert and playing Atari" or are otherwise highly functional. (I didn’t know Atari was still on the market.)
Click here for the presentation slides from this lecture and here for others.
Hope to see you next year when Hospital Medicine meets in Las Vegas!
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a mobile app for iOS.
Recently, hospitalists around the country gathered together at Hospital Medicine 2013 to gain new and practical clinical insights we can use to optimize the medical care we provide to our patients, imbibe new research on the horizon, master clinical care guidelines, and sometimes, just relax and enjoy meeting new colleagues from around the country – naturally, comparing notes on how their practices stack up to our own. You could even pick up a book or two geared to hospitalists. I bought "Clinical Care Conundrums: Challenging Diagnoses in Hospital Medicine" and "Becoming a Consummate Clinician: What Every Student, House Office, and Hospital Practitioner Needs to Know." Learning just doesn’t get any better than this.
Information abounded, challenges were issued, and I think most of us learned how much we really need to learn more about. I believe we were thoroughly challenged to look at our current practice style, incorporate our new knowledge, and take our clinical acumen to the next level.
The first challenge began when I had to roll out of bed around 5 a.m. to prepare to make the hour-long drive down I-95 to the conference site at National Harbor, Md., to attend a 7:40 a.m. lecture: "Pain Management for the Hospitalist." I have not a single regret. It was well worth the bleary ride. I think many hospitalists share my concerns about overmedicating patients on the one hand, and being on guard for true drug seekers on the other.
My main takeaway was that when a patient is truly in pain, narcotic pain medication can be titrated up much more quickly than most of us currently feel comfortable with.
The lecture was presented by Dr. Eric Roeland of the University of Carolina, San Diego, who said that he sometimes doubles narcotic analgesics every 10 minutes under certain circumstances. Some participants were shocked (including me). He orders a dose of pain medication and then checks on the patient around the time of CMax (maximum concentration of the drug). For IV pain medications, this is approximately 10 minutes, for SC/IM it is 30 minutes, and for PO/PR it is 60 minutes. If his patient has not gotten pain relief by CMax, he orders twice the dose and checks back in again at the next CMax. If the patient still has no relief, Dr. Roeland will order four times the initial dose.
He stated that since sedation comes before respiratory depression, he feels comfortable increasing narcotics rapidly in patients who are "alert and playing Atari" or are otherwise highly functional. (I didn’t know Atari was still on the market.)
Click here for the presentation slides from this lecture and here for others.
Hope to see you next year when Hospital Medicine meets in Las Vegas!
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a mobile app for iOS.
Recently, hospitalists around the country gathered together at Hospital Medicine 2013 to gain new and practical clinical insights we can use to optimize the medical care we provide to our patients, imbibe new research on the horizon, master clinical care guidelines, and sometimes, just relax and enjoy meeting new colleagues from around the country – naturally, comparing notes on how their practices stack up to our own. You could even pick up a book or two geared to hospitalists. I bought "Clinical Care Conundrums: Challenging Diagnoses in Hospital Medicine" and "Becoming a Consummate Clinician: What Every Student, House Office, and Hospital Practitioner Needs to Know." Learning just doesn’t get any better than this.
Information abounded, challenges were issued, and I think most of us learned how much we really need to learn more about. I believe we were thoroughly challenged to look at our current practice style, incorporate our new knowledge, and take our clinical acumen to the next level.
The first challenge began when I had to roll out of bed around 5 a.m. to prepare to make the hour-long drive down I-95 to the conference site at National Harbor, Md., to attend a 7:40 a.m. lecture: "Pain Management for the Hospitalist." I have not a single regret. It was well worth the bleary ride. I think many hospitalists share my concerns about overmedicating patients on the one hand, and being on guard for true drug seekers on the other.
My main takeaway was that when a patient is truly in pain, narcotic pain medication can be titrated up much more quickly than most of us currently feel comfortable with.
The lecture was presented by Dr. Eric Roeland of the University of Carolina, San Diego, who said that he sometimes doubles narcotic analgesics every 10 minutes under certain circumstances. Some participants were shocked (including me). He orders a dose of pain medication and then checks on the patient around the time of CMax (maximum concentration of the drug). For IV pain medications, this is approximately 10 minutes, for SC/IM it is 30 minutes, and for PO/PR it is 60 minutes. If his patient has not gotten pain relief by CMax, he orders twice the dose and checks back in again at the next CMax. If the patient still has no relief, Dr. Roeland will order four times the initial dose.
He stated that since sedation comes before respiratory depression, he feels comfortable increasing narcotics rapidly in patients who are "alert and playing Atari" or are otherwise highly functional. (I didn’t know Atari was still on the market.)
Click here for the presentation slides from this lecture and here for others.
Hope to see you next year when Hospital Medicine meets in Las Vegas!
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a mobile app for iOS.
Study supports laws requiring cyclists to wear helmets
WASHINGTON – Mandatory bicycle helmet laws are associated with a lower incidence of fatalities among youth cyclists involved in motor vehicle collisions, a national analysis has shown.
States with laws that require bicyclists to wear helmets had only 84% of the deaths per population that those without helmet laws had, after adjustment for other potential confounding factors, Dr. William P. Meehan III reported at the annual meeting of the Pediatric Academic Societies.
"Twenty-nine states still do not have [laws requiring cyclists to wear helmets], and we think they should be considered," said Dr. Meehan, director of the Micheli Center for Sports Injury Prevention and the Sports Concussion Clinic in the division of sports medicine at Boston Children’s Hospital.
Approximately 900 people die each year in bicycle crashes, three-quarters of them from traumatic brain injuries. On a local level, previous studies have shown that the laws result in increased helmet usage and decreased risk of injury and death in bicycle–motor vehicle collisions.
To assess the effect nationally, Dr. Meehan and his colleagues analyzed data on U.S. bicyclists younger than 16 years of age who died between January 1999 and December 2009. They used the Fatality Analysis Reporting System (FARS) to compare death rates in states with and without mandatory helmet laws. FARS is run by the National Highway Traffic Safety Administration and collects data on all motor vehicle accidents that result in the death of someone involved in a collision.
Over the study period, 1,612 bicyclists under the age of 16 died in a collision with a motor vehicle. The mean unadjusted rate of fatalities was 2 per million in states with helmet laws, versus 2.5 per million in states without the laws. The overall unadjusted incidence rate ratio was 0.83.
After adjustment for three factors that could potentially influence the rate of fatality in bicycle–motor vehicle collisions – elderly driver licensure laws, legal blood alcohol limits (lower than .08% versus .08% or higher), and household income, states with mandatory helmet laws continued to be associated with lower rates of fatalities, with an adjusted incidence rate ratio of 0.84, reported Dr. Meehan, also of the department of pediatrics at Harvard Medical School, Boston.
Because the FARS database includes only collisions that resulted in fatalities – and not other severe injuries – there’s "potentially much greater benefit in helmet legislation" than is reflected by the study findings, he noted.
The American Academy of Pediatrics supports legislation that requires all cyclists to use helmets.
State laws requiring the use of bicycle helmets cover populations up to varying ages, but almost all cover children up to 16 years of age.
At the start of the study period, 16 states had bicycle helmet laws. By the end, in 2009, 19 states and the District of Columbia had helmet laws. (At least two of the additional laws were enacted in the earlier part of the study period). Helmet mandates have also been established by some local municipalities throughout the country, but the analysis covered only states.
The study was funded in part by the Micheli Center for Sports Injury Prevention and the National Institutes of Health. Dr. Meehan reported that he had no relevant financial disclosures.
WASHINGTON – Mandatory bicycle helmet laws are associated with a lower incidence of fatalities among youth cyclists involved in motor vehicle collisions, a national analysis has shown.
States with laws that require bicyclists to wear helmets had only 84% of the deaths per population that those without helmet laws had, after adjustment for other potential confounding factors, Dr. William P. Meehan III reported at the annual meeting of the Pediatric Academic Societies.
"Twenty-nine states still do not have [laws requiring cyclists to wear helmets], and we think they should be considered," said Dr. Meehan, director of the Micheli Center for Sports Injury Prevention and the Sports Concussion Clinic in the division of sports medicine at Boston Children’s Hospital.
Approximately 900 people die each year in bicycle crashes, three-quarters of them from traumatic brain injuries. On a local level, previous studies have shown that the laws result in increased helmet usage and decreased risk of injury and death in bicycle–motor vehicle collisions.
To assess the effect nationally, Dr. Meehan and his colleagues analyzed data on U.S. bicyclists younger than 16 years of age who died between January 1999 and December 2009. They used the Fatality Analysis Reporting System (FARS) to compare death rates in states with and without mandatory helmet laws. FARS is run by the National Highway Traffic Safety Administration and collects data on all motor vehicle accidents that result in the death of someone involved in a collision.
Over the study period, 1,612 bicyclists under the age of 16 died in a collision with a motor vehicle. The mean unadjusted rate of fatalities was 2 per million in states with helmet laws, versus 2.5 per million in states without the laws. The overall unadjusted incidence rate ratio was 0.83.
After adjustment for three factors that could potentially influence the rate of fatality in bicycle–motor vehicle collisions – elderly driver licensure laws, legal blood alcohol limits (lower than .08% versus .08% or higher), and household income, states with mandatory helmet laws continued to be associated with lower rates of fatalities, with an adjusted incidence rate ratio of 0.84, reported Dr. Meehan, also of the department of pediatrics at Harvard Medical School, Boston.
Because the FARS database includes only collisions that resulted in fatalities – and not other severe injuries – there’s "potentially much greater benefit in helmet legislation" than is reflected by the study findings, he noted.
The American Academy of Pediatrics supports legislation that requires all cyclists to use helmets.
State laws requiring the use of bicycle helmets cover populations up to varying ages, but almost all cover children up to 16 years of age.
At the start of the study period, 16 states had bicycle helmet laws. By the end, in 2009, 19 states and the District of Columbia had helmet laws. (At least two of the additional laws were enacted in the earlier part of the study period). Helmet mandates have also been established by some local municipalities throughout the country, but the analysis covered only states.
The study was funded in part by the Micheli Center for Sports Injury Prevention and the National Institutes of Health. Dr. Meehan reported that he had no relevant financial disclosures.
WASHINGTON – Mandatory bicycle helmet laws are associated with a lower incidence of fatalities among youth cyclists involved in motor vehicle collisions, a national analysis has shown.
States with laws that require bicyclists to wear helmets had only 84% of the deaths per population that those without helmet laws had, after adjustment for other potential confounding factors, Dr. William P. Meehan III reported at the annual meeting of the Pediatric Academic Societies.
"Twenty-nine states still do not have [laws requiring cyclists to wear helmets], and we think they should be considered," said Dr. Meehan, director of the Micheli Center for Sports Injury Prevention and the Sports Concussion Clinic in the division of sports medicine at Boston Children’s Hospital.
Approximately 900 people die each year in bicycle crashes, three-quarters of them from traumatic brain injuries. On a local level, previous studies have shown that the laws result in increased helmet usage and decreased risk of injury and death in bicycle–motor vehicle collisions.
To assess the effect nationally, Dr. Meehan and his colleagues analyzed data on U.S. bicyclists younger than 16 years of age who died between January 1999 and December 2009. They used the Fatality Analysis Reporting System (FARS) to compare death rates in states with and without mandatory helmet laws. FARS is run by the National Highway Traffic Safety Administration and collects data on all motor vehicle accidents that result in the death of someone involved in a collision.
Over the study period, 1,612 bicyclists under the age of 16 died in a collision with a motor vehicle. The mean unadjusted rate of fatalities was 2 per million in states with helmet laws, versus 2.5 per million in states without the laws. The overall unadjusted incidence rate ratio was 0.83.
After adjustment for three factors that could potentially influence the rate of fatality in bicycle–motor vehicle collisions – elderly driver licensure laws, legal blood alcohol limits (lower than .08% versus .08% or higher), and household income, states with mandatory helmet laws continued to be associated with lower rates of fatalities, with an adjusted incidence rate ratio of 0.84, reported Dr. Meehan, also of the department of pediatrics at Harvard Medical School, Boston.
Because the FARS database includes only collisions that resulted in fatalities – and not other severe injuries – there’s "potentially much greater benefit in helmet legislation" than is reflected by the study findings, he noted.
The American Academy of Pediatrics supports legislation that requires all cyclists to use helmets.
State laws requiring the use of bicycle helmets cover populations up to varying ages, but almost all cover children up to 16 years of age.
At the start of the study period, 16 states had bicycle helmet laws. By the end, in 2009, 19 states and the District of Columbia had helmet laws. (At least two of the additional laws were enacted in the earlier part of the study period). Helmet mandates have also been established by some local municipalities throughout the country, but the analysis covered only states.
The study was funded in part by the Micheli Center for Sports Injury Prevention and the National Institutes of Health. Dr. Meehan reported that he had no relevant financial disclosures.
AT THE PAS ANNUAL MEETING
Major finding: States with mandatory bicycle helmet laws had only 84% of the deaths per population that states without helmet laws had, after adjustment for potential confounding factors.
Data source: A cross-sectional study of data from the national Fatality Analysis Reporting System.
Disclosures: Dr. Meehan reported that he had no relevant financial disclosures.
Palliative care training and associations with burnout in oncology fellows
ABSTRACT
Background Burnout among physicians can lead to decreased career satisfaction, physical and emotional exhaustion, and increased medical errors. In oncologists, high exposure to fatal illness is associated with burnout.
Methods The Maslach Burnout Inventory, measuring Emotional Exhaustion (EE), Depersonalization (DP), and Personal Accomplishment (PA), was administered to second-year US oncology fellows. Bivariate and multivariate analyses explored associations between burnout and fellow demographics, attitudes, and educational experiences.
Results A total of 254 fellows out of 402 eligible US fellows responded (63.2%) and 24.2% reported high EE, 30.0% reported high DP, and 26.8% reported low PA. Over half of the fellows reported burnout in at least one domain. Lower EE scores were associated with the fellows’ perceptions of having received better teaching, explicit teaching about certain end-of-life topics, and receipt of direct observation of goals-of-care discussions. Fellows who reported better overall teaching quality and more frequent observation of their skills had less depersonalization. Fellows who felt a responsibility to help patients at the end of life to prepare for death had higher PA.
Limitations This survey relies on the fellows’ self-reported perceptions without an objective measure for validation. Factors associated with burnout may not be causal. The number of analyses performed raises the concern for Type I errors; therefore, a stringent P value (.01) was used.
Conclusions Burnout is prevalent during oncology training. Higher-quality teaching is associated with less burnout among fellows. Fellowship programs should recognize the prevalence of burnout among oncology fellows as well as components of training that may protect against burnout.
*For a PDF of the full article, click on the link to the left of this introduction.
Life, Communication
ABSTRACT
Background Burnout among physicians can lead to decreased career satisfaction, physical and emotional exhaustion, and increased medical errors. In oncologists, high exposure to fatal illness is associated with burnout.
Methods The Maslach Burnout Inventory, measuring Emotional Exhaustion (EE), Depersonalization (DP), and Personal Accomplishment (PA), was administered to second-year US oncology fellows. Bivariate and multivariate analyses explored associations between burnout and fellow demographics, attitudes, and educational experiences.
Results A total of 254 fellows out of 402 eligible US fellows responded (63.2%) and 24.2% reported high EE, 30.0% reported high DP, and 26.8% reported low PA. Over half of the fellows reported burnout in at least one domain. Lower EE scores were associated with the fellows’ perceptions of having received better teaching, explicit teaching about certain end-of-life topics, and receipt of direct observation of goals-of-care discussions. Fellows who reported better overall teaching quality and more frequent observation of their skills had less depersonalization. Fellows who felt a responsibility to help patients at the end of life to prepare for death had higher PA.
Limitations This survey relies on the fellows’ self-reported perceptions without an objective measure for validation. Factors associated with burnout may not be causal. The number of analyses performed raises the concern for Type I errors; therefore, a stringent P value (.01) was used.
Conclusions Burnout is prevalent during oncology training. Higher-quality teaching is associated with less burnout among fellows. Fellowship programs should recognize the prevalence of burnout among oncology fellows as well as components of training that may protect against burnout.
*For a PDF of the full article, click on the link to the left of this introduction.
ABSTRACT
Background Burnout among physicians can lead to decreased career satisfaction, physical and emotional exhaustion, and increased medical errors. In oncologists, high exposure to fatal illness is associated with burnout.
Methods The Maslach Burnout Inventory, measuring Emotional Exhaustion (EE), Depersonalization (DP), and Personal Accomplishment (PA), was administered to second-year US oncology fellows. Bivariate and multivariate analyses explored associations between burnout and fellow demographics, attitudes, and educational experiences.
Results A total of 254 fellows out of 402 eligible US fellows responded (63.2%) and 24.2% reported high EE, 30.0% reported high DP, and 26.8% reported low PA. Over half of the fellows reported burnout in at least one domain. Lower EE scores were associated with the fellows’ perceptions of having received better teaching, explicit teaching about certain end-of-life topics, and receipt of direct observation of goals-of-care discussions. Fellows who reported better overall teaching quality and more frequent observation of their skills had less depersonalization. Fellows who felt a responsibility to help patients at the end of life to prepare for death had higher PA.
Limitations This survey relies on the fellows’ self-reported perceptions without an objective measure for validation. Factors associated with burnout may not be causal. The number of analyses performed raises the concern for Type I errors; therefore, a stringent P value (.01) was used.
Conclusions Burnout is prevalent during oncology training. Higher-quality teaching is associated with less burnout among fellows. Fellowship programs should recognize the prevalence of burnout among oncology fellows as well as components of training that may protect against burnout.
*For a PDF of the full article, click on the link to the left of this introduction.
Life, Communication
Life, Communication