User login
Intimate partner violence: Screen others, besides heterosexual women
We were happy to learn in “Time to routinely screen for intimate partner violence?” (PURLs. J Fam Pract. 2013;62:90-92) that the US Preventive Services Task Force (USPSTF) agrees with the Institute of Medicine (IOM) that all women of childbearing age should be screened for intimate partner violence (IPV).1 Although the USPSTF recommendation comes 2 years after that of the IOM, it is truly better late than never.
Two populations with known IPV issues require special consideration: lesbian, gay, bisexual, transgender (LGBT) patients and heterosexual men. The rate of IPV is higher in the LGBT population than in heterosexual men and women cohabitating with their partners.2 Despite high rates of IPV within the LGBT population, women in this group frequently are overlooked for IPV screening.2
We must remember to screen men in heterosexual relationships, as well. In 2000, the National Violence Against Women survey found that 7% of men reported having experienced IPV in their lifetime.2 Given this data, we believe that all patients ages 14 years and older—regardless of gender or sexual orientation—should be screened for IPV. This would be a much-needed step towards addressing a major public health problem.
Barbara McMillan-Persaud, MD
Kyra P. Clark, MD
Riba Kelsey-Harris, MD
Folashade Omole, MD, FAAFP
Atlanta, Ga
1. Screening for intimate partner violence and abuse of elderly and vulnerable adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed September 16, 2013.
2. Artd KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
We were happy to learn in “Time to routinely screen for intimate partner violence?” (PURLs. J Fam Pract. 2013;62:90-92) that the US Preventive Services Task Force (USPSTF) agrees with the Institute of Medicine (IOM) that all women of childbearing age should be screened for intimate partner violence (IPV).1 Although the USPSTF recommendation comes 2 years after that of the IOM, it is truly better late than never.
Two populations with known IPV issues require special consideration: lesbian, gay, bisexual, transgender (LGBT) patients and heterosexual men. The rate of IPV is higher in the LGBT population than in heterosexual men and women cohabitating with their partners.2 Despite high rates of IPV within the LGBT population, women in this group frequently are overlooked for IPV screening.2
We must remember to screen men in heterosexual relationships, as well. In 2000, the National Violence Against Women survey found that 7% of men reported having experienced IPV in their lifetime.2 Given this data, we believe that all patients ages 14 years and older—regardless of gender or sexual orientation—should be screened for IPV. This would be a much-needed step towards addressing a major public health problem.
Barbara McMillan-Persaud, MD
Kyra P. Clark, MD
Riba Kelsey-Harris, MD
Folashade Omole, MD, FAAFP
Atlanta, Ga
We were happy to learn in “Time to routinely screen for intimate partner violence?” (PURLs. J Fam Pract. 2013;62:90-92) that the US Preventive Services Task Force (USPSTF) agrees with the Institute of Medicine (IOM) that all women of childbearing age should be screened for intimate partner violence (IPV).1 Although the USPSTF recommendation comes 2 years after that of the IOM, it is truly better late than never.
Two populations with known IPV issues require special consideration: lesbian, gay, bisexual, transgender (LGBT) patients and heterosexual men. The rate of IPV is higher in the LGBT population than in heterosexual men and women cohabitating with their partners.2 Despite high rates of IPV within the LGBT population, women in this group frequently are overlooked for IPV screening.2
We must remember to screen men in heterosexual relationships, as well. In 2000, the National Violence Against Women survey found that 7% of men reported having experienced IPV in their lifetime.2 Given this data, we believe that all patients ages 14 years and older—regardless of gender or sexual orientation—should be screened for IPV. This would be a much-needed step towards addressing a major public health problem.
Barbara McMillan-Persaud, MD
Kyra P. Clark, MD
Riba Kelsey-Harris, MD
Folashade Omole, MD, FAAFP
Atlanta, Ga
1. Screening for intimate partner violence and abuse of elderly and vulnerable adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed September 16, 2013.
2. Artd KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
1. Screening for intimate partner violence and abuse of elderly and vulnerable adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed September 16, 2013.
2. Artd KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
Persistent fever, left-sided neck pain, night sweats—Dx?
THE CASE
A previously healthy 35-year-old man with a one-week history of left-sided neck pain and fever as high as 104°F sought care at our emergency department. He was given a diagnosis of viral pharyngitis and discharged. He returned the next day and indicated that he was now experiencing drenching night sweats and weakness.
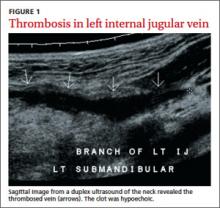
The patient was anxious, but not distressed. His temperature was 100.1°F; blood pressure, 113/65 mm Hg; heart rate, 150 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 95% on room air. Head and neck examination revealed bilateral cervical lymphadenopathy with pronounced tenderness on the left side of his neck. Oral exam revealed dry mucous membranes, halitosis, and bilateral tonsillar enlargement without exudate. The cardiopulmonary exam was within normal limits. Lab tests showed a white blood cell (WBC) count of 5.9 x 109/L. An ultrasound of the neck revealed thrombosis in the left submandibular branch of the left internal jugular vein (IJV) (FIGURE 1).
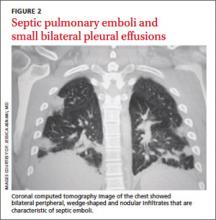
The next day, the patient remained febrile (102.8°F) and developed rigors, diarrhea, pleuritic chest pain, and an elevated WBC count (14.5). A blood culture grew gramnegative rods. The patient was started on piperacillin/tazobactam, and doxycycline was added to treat possible tick-borne infections. Computed tomography (CT) scans of the chest showed the presence of septic pulmonary emboli and small bilateral pleural effusions (FIGURE 2).
THE DIAGNOSIS
We made a diagnosis of Lemierre’s syndrome because our patient met all 4 criteria for the condition:1,2
• a recent oropharyngeal infection
• clinical or radiological evidence of IJV thrombosis
• isolation of anaerobic pathogens (mainly Fusobacterium necrophorum)
• evidence of at least one septic focus, most commonly in the lungs.
We changed the patient’s antibiotic therapy to intravenous (IV) meropenem. His WBC and fever improved, and on Day 10 he was discharged to complete a 28-day course of IV meropenem via a peripherally inserted central catheter.
DISCUSSION
Lemierre’s—A “forgotten” condition that’s making a comeback
In 1936, French microbiologist Andrew Lemierre formally characterized the syndrome in a review of 20 patients who had sepsis, metastatic pulmonary lesions, and isolation of Bacillus funduliformis (now known as F necrophorum).1,2 Other organisms that have been identified in this syndrome include Fusobacterium nucleatum, Candida, Staphylococcus, and Streptococcus.2
Before the antibiotic era, Lemierre’s syndrome was common and often fatal. But with the introduction of penicillin in the 1940s, the incidence of the syndrome dropped, and it eventually became known as “the forgotten disease.”2 Since the 1990s, however, there has been a marked resurgence of Lemierre’s syndrome.3 The incidence of Lemierre’s syndrome today is 0.6 to 2.3 cases per 1 million people per year, with a mortality rate of up to 18%.3,4
This resurgence of Lemierre’s syndrome has been linked to the restricted use of antibiotics for throat infections.3 (One study found the number of prescriptions written for antibiotics decreased by 23% from 1992 to 2000.5) Other factors cited for the increased incidence of Lemierre’s syndrome include improved identification of anaerobic organisms, more effective blood culture methods, and an increased awareness of this syndrome among clinical microbiologists.6
Diagnosis requires a high degree of suspicion
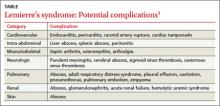
Lemierre’s syndrome typically occurs in healthy young adults. Pharyngitis is the most common initial symptom, occurring in 87% of patients.2 This is followed by a fever (102.2°F - 105.8°F) usually 4 to 5 days after the onset of sore throat.3 Other common symptoms include chills, dysphagia, dyspnea, chest pain, hemoptysis, cervical neck discomfort, arthralgia, malaise, and night sweats.2 Following suppurative thrombophlebitis of the IJV, infection spreads to other organ systems. Pulmonary involvement is the most common site (97% of cases).3 Other complications of this syndrome are listed in the TABLE.3
The differential includes mononucleosis
The differential diagnosis encompasses several common illnesses, including mononucleosis, Group A streptococcal pharyngitis, and peritonsillar abscess. However, while patients with these conditions might have a fever and an elevated WBC count, they typically would not have the pleuritic chest pain that is characteristic of Lemierre’s syndrome. In addition, while patients with peritonsillar abscess would have tonsillar exudates, patients with Lemierre’s syndrome would not likely have them.
Influenza is also part of the differential, although focal neck pain usually isn’t a finding in patients who have the flu.
Once other common illnesses have been ruled out, it’s important to have a high index of suspicion for Lemierre’s syndrome because the oropharyngeal infection may resolve by the time of presentation, and there may be few findings on physical exam.7 Therefore, suspect Lemierre’s if a patient comes in with neck pain and/or pleuritic chest pain and has a recent history of oropharyngeal infection and fever.
CT scan of the neck and chest with contrast is the optimal diagnostic modality because it allows physicians to visualize the IJV8 and detect pulmonary emboli.9 Doppler ultrasound also can be used to diagnose IJV thrombosis. Ultrasound findings would reveal an echogenic focus within a dilated IJV or a complex mass of cystic and solid components.10
Prompt antibiotic treatment is essential
Patients with Lemierre’s syndrome require prompt and appropriate antimicrobial therapy. Researchers have reported mortality rates of 25% among patients who received delayed antibiotic therapy, compared with rates of up to 18% with prompt therapy.3 Metronidazole is the most commonly prescribed antibiotic.8 When combined with ceftriaxone, it provides coverage for both F necrophorum and streptococci, a common copathogen. Monotherapy with a carbapenem antibiotic, clindamycin, ampicillin/sulbactam, or antipseudomonal penicillin also are appropriate options.5 Antimicrobial treatment for 3 to 6 weeks is recommended because relapses have been noted in patients treated for less than 2 weeks.11
Anticoagulation is controversial.2 Proponents of anticoagulation to treat Lemierre’s syndrome believe it may prevent formation of septic emboli and could expedite recovery.4,12 Others believe that clots associated with Lemierre’s syndrome dissolve on their own and that anticoagulation may increase the likelihood of septic emboli.13 Many case reports, including this one, have demonstrated that complete recovery is possible without anticoagulation.10,13-15 Anticoagulation therapy can be considered for patients with Lemierre’s syndrome in the absence of any contraindications such as gastrointestinal or intracranial bleeding.
THE TAKEAWAY
Suspect Lemierre’s syndrome when a patient complains of neck pain, high fever, rigors, dry cough, and pleuritic chest pain and mentions a sore throat that he or she had in the pretceding 7 days. Diagnosis can be confirmed by radiological findings and blood cultures positive for F. necrophorum. Patients with Lemierre’s syndrome should be promptly treated with antibiotics; evidence for anticoagulation is inconclusive.
1. Golpe R, Marin B, Alonso M. Lemierre’s syndrome (necrobacillosis). Postgrad Med J. 1999;75:141-144.
2. Wright WF, Shiner CN, Ribes JA. Lemierre syndrome. South Med J. 2012;105:283-288.
3. Riordan T, Wilson M. Lemierre’s syndrome: more than a historical curiosa. Postgrad Med J. 2004;80:328-334.
4. Ridgway JM, Parikh DA, Wright R, et al. Lemierre syndrome: a pediatric case series and review of literature. Am J Otolaryngol. 2010;31:38-45.
5. McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992–2000. Emerg Infect Dis. 2003;9:432-437.
6. Hagelskjaer Kristensen L, Prag J. Human necrobacillosis, with emphasis on Lemierre’s syndrome. Clin Infect Dis. 2000;31:524-532.
7. Kupalli K, Livorsi D, Talati N, et al. Lemierre’s syndrome due to fusobacterium necrophorum. Lancet Infect Dis. 2012;12:808-815.
8. Armstrong AW, Spooner K, Sanders JW. Lemierre’s syndrome. Curr Infect Dis Rep. 2000;2:168-173.
9. Screaton NJ, Ravenel JG, Lehner PJ, et al. Lemierre syndrome: forgotten but not extinct--report of four cases. Radiology. 1999;213:369-374.
10. Chirinos JA, Lichtstein DM, Garcia J, et al. The evolution of Lemierre syndrome: report of 2 cases and review of the literature. Medicine (Baltimore). 2002;81:458-465.
11. Karkos PD, Asrani S, Karkos CD, et al. Lemierre syndrome: a systematic review. Laryngoscope. 2009;119:1552-1559.
12. Phan T, So TY. Use of anticoagulation therapy for jugular vein thrombus in pediatric patients with Lemierre’s syndrome. Int J Clin Pharm. 2012;34:818-821.
13. O’Brien WT, Cohen RA. Lemierre’ syndrome. Applied Radiology. 2011;40:37-38.
14. Vandenberg SJ, Hartig GK. Lemierre’s syndrome. Otolaryngol Head Neck Surg. 1998;119:516-518.
15. Goldhagen J, Alford BA, Prewitt LH, et al. Suppurative thrombophlebitis of the internal jugular vein: report of three cases and review of the pediatric literature. Pediatr Infect Dis J. 1988;7:410-414.
THE CASE
A previously healthy 35-year-old man with a one-week history of left-sided neck pain and fever as high as 104°F sought care at our emergency department. He was given a diagnosis of viral pharyngitis and discharged. He returned the next day and indicated that he was now experiencing drenching night sweats and weakness.
The patient was anxious, but not distressed. His temperature was 100.1°F; blood pressure, 113/65 mm Hg; heart rate, 150 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 95% on room air. Head and neck examination revealed bilateral cervical lymphadenopathy with pronounced tenderness on the left side of his neck. Oral exam revealed dry mucous membranes, halitosis, and bilateral tonsillar enlargement without exudate. The cardiopulmonary exam was within normal limits. Lab tests showed a white blood cell (WBC) count of 5.9 x 109/L. An ultrasound of the neck revealed thrombosis in the left submandibular branch of the left internal jugular vein (IJV) (FIGURE 1).
The next day, the patient remained febrile (102.8°F) and developed rigors, diarrhea, pleuritic chest pain, and an elevated WBC count (14.5). A blood culture grew gramnegative rods. The patient was started on piperacillin/tazobactam, and doxycycline was added to treat possible tick-borne infections. Computed tomography (CT) scans of the chest showed the presence of septic pulmonary emboli and small bilateral pleural effusions (FIGURE 2).
THE DIAGNOSIS
We made a diagnosis of Lemierre’s syndrome because our patient met all 4 criteria for the condition:1,2
• a recent oropharyngeal infection
• clinical or radiological evidence of IJV thrombosis
• isolation of anaerobic pathogens (mainly Fusobacterium necrophorum)
• evidence of at least one septic focus, most commonly in the lungs.
We changed the patient’s antibiotic therapy to intravenous (IV) meropenem. His WBC and fever improved, and on Day 10 he was discharged to complete a 28-day course of IV meropenem via a peripherally inserted central catheter.
DISCUSSION
Lemierre’s—A “forgotten” condition that’s making a comeback
In 1936, French microbiologist Andrew Lemierre formally characterized the syndrome in a review of 20 patients who had sepsis, metastatic pulmonary lesions, and isolation of Bacillus funduliformis (now known as F necrophorum).1,2 Other organisms that have been identified in this syndrome include Fusobacterium nucleatum, Candida, Staphylococcus, and Streptococcus.2
Before the antibiotic era, Lemierre’s syndrome was common and often fatal. But with the introduction of penicillin in the 1940s, the incidence of the syndrome dropped, and it eventually became known as “the forgotten disease.”2 Since the 1990s, however, there has been a marked resurgence of Lemierre’s syndrome.3 The incidence of Lemierre’s syndrome today is 0.6 to 2.3 cases per 1 million people per year, with a mortality rate of up to 18%.3,4
This resurgence of Lemierre’s syndrome has been linked to the restricted use of antibiotics for throat infections.3 (One study found the number of prescriptions written for antibiotics decreased by 23% from 1992 to 2000.5) Other factors cited for the increased incidence of Lemierre’s syndrome include improved identification of anaerobic organisms, more effective blood culture methods, and an increased awareness of this syndrome among clinical microbiologists.6
Diagnosis requires a high degree of suspicion
Lemierre’s syndrome typically occurs in healthy young adults. Pharyngitis is the most common initial symptom, occurring in 87% of patients.2 This is followed by a fever (102.2°F - 105.8°F) usually 4 to 5 days after the onset of sore throat.3 Other common symptoms include chills, dysphagia, dyspnea, chest pain, hemoptysis, cervical neck discomfort, arthralgia, malaise, and night sweats.2 Following suppurative thrombophlebitis of the IJV, infection spreads to other organ systems. Pulmonary involvement is the most common site (97% of cases).3 Other complications of this syndrome are listed in the TABLE.3
The differential includes mononucleosis
The differential diagnosis encompasses several common illnesses, including mononucleosis, Group A streptococcal pharyngitis, and peritonsillar abscess. However, while patients with these conditions might have a fever and an elevated WBC count, they typically would not have the pleuritic chest pain that is characteristic of Lemierre’s syndrome. In addition, while patients with peritonsillar abscess would have tonsillar exudates, patients with Lemierre’s syndrome would not likely have them.
Influenza is also part of the differential, although focal neck pain usually isn’t a finding in patients who have the flu.
Once other common illnesses have been ruled out, it’s important to have a high index of suspicion for Lemierre’s syndrome because the oropharyngeal infection may resolve by the time of presentation, and there may be few findings on physical exam.7 Therefore, suspect Lemierre’s if a patient comes in with neck pain and/or pleuritic chest pain and has a recent history of oropharyngeal infection and fever.
CT scan of the neck and chest with contrast is the optimal diagnostic modality because it allows physicians to visualize the IJV8 and detect pulmonary emboli.9 Doppler ultrasound also can be used to diagnose IJV thrombosis. Ultrasound findings would reveal an echogenic focus within a dilated IJV or a complex mass of cystic and solid components.10
Prompt antibiotic treatment is essential
Patients with Lemierre’s syndrome require prompt and appropriate antimicrobial therapy. Researchers have reported mortality rates of 25% among patients who received delayed antibiotic therapy, compared with rates of up to 18% with prompt therapy.3 Metronidazole is the most commonly prescribed antibiotic.8 When combined with ceftriaxone, it provides coverage for both F necrophorum and streptococci, a common copathogen. Monotherapy with a carbapenem antibiotic, clindamycin, ampicillin/sulbactam, or antipseudomonal penicillin also are appropriate options.5 Antimicrobial treatment for 3 to 6 weeks is recommended because relapses have been noted in patients treated for less than 2 weeks.11
Anticoagulation is controversial.2 Proponents of anticoagulation to treat Lemierre’s syndrome believe it may prevent formation of septic emboli and could expedite recovery.4,12 Others believe that clots associated with Lemierre’s syndrome dissolve on their own and that anticoagulation may increase the likelihood of septic emboli.13 Many case reports, including this one, have demonstrated that complete recovery is possible without anticoagulation.10,13-15 Anticoagulation therapy can be considered for patients with Lemierre’s syndrome in the absence of any contraindications such as gastrointestinal or intracranial bleeding.
THE TAKEAWAY
Suspect Lemierre’s syndrome when a patient complains of neck pain, high fever, rigors, dry cough, and pleuritic chest pain and mentions a sore throat that he or she had in the pretceding 7 days. Diagnosis can be confirmed by radiological findings and blood cultures positive for F. necrophorum. Patients with Lemierre’s syndrome should be promptly treated with antibiotics; evidence for anticoagulation is inconclusive.
THE CASE
A previously healthy 35-year-old man with a one-week history of left-sided neck pain and fever as high as 104°F sought care at our emergency department. He was given a diagnosis of viral pharyngitis and discharged. He returned the next day and indicated that he was now experiencing drenching night sweats and weakness.
The patient was anxious, but not distressed. His temperature was 100.1°F; blood pressure, 113/65 mm Hg; heart rate, 150 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 95% on room air. Head and neck examination revealed bilateral cervical lymphadenopathy with pronounced tenderness on the left side of his neck. Oral exam revealed dry mucous membranes, halitosis, and bilateral tonsillar enlargement without exudate. The cardiopulmonary exam was within normal limits. Lab tests showed a white blood cell (WBC) count of 5.9 x 109/L. An ultrasound of the neck revealed thrombosis in the left submandibular branch of the left internal jugular vein (IJV) (FIGURE 1).
The next day, the patient remained febrile (102.8°F) and developed rigors, diarrhea, pleuritic chest pain, and an elevated WBC count (14.5). A blood culture grew gramnegative rods. The patient was started on piperacillin/tazobactam, and doxycycline was added to treat possible tick-borne infections. Computed tomography (CT) scans of the chest showed the presence of septic pulmonary emboli and small bilateral pleural effusions (FIGURE 2).
THE DIAGNOSIS
We made a diagnosis of Lemierre’s syndrome because our patient met all 4 criteria for the condition:1,2
• a recent oropharyngeal infection
• clinical or radiological evidence of IJV thrombosis
• isolation of anaerobic pathogens (mainly Fusobacterium necrophorum)
• evidence of at least one septic focus, most commonly in the lungs.
We changed the patient’s antibiotic therapy to intravenous (IV) meropenem. His WBC and fever improved, and on Day 10 he was discharged to complete a 28-day course of IV meropenem via a peripherally inserted central catheter.
DISCUSSION
Lemierre’s—A “forgotten” condition that’s making a comeback
In 1936, French microbiologist Andrew Lemierre formally characterized the syndrome in a review of 20 patients who had sepsis, metastatic pulmonary lesions, and isolation of Bacillus funduliformis (now known as F necrophorum).1,2 Other organisms that have been identified in this syndrome include Fusobacterium nucleatum, Candida, Staphylococcus, and Streptococcus.2
Before the antibiotic era, Lemierre’s syndrome was common and often fatal. But with the introduction of penicillin in the 1940s, the incidence of the syndrome dropped, and it eventually became known as “the forgotten disease.”2 Since the 1990s, however, there has been a marked resurgence of Lemierre’s syndrome.3 The incidence of Lemierre’s syndrome today is 0.6 to 2.3 cases per 1 million people per year, with a mortality rate of up to 18%.3,4
This resurgence of Lemierre’s syndrome has been linked to the restricted use of antibiotics for throat infections.3 (One study found the number of prescriptions written for antibiotics decreased by 23% from 1992 to 2000.5) Other factors cited for the increased incidence of Lemierre’s syndrome include improved identification of anaerobic organisms, more effective blood culture methods, and an increased awareness of this syndrome among clinical microbiologists.6
Diagnosis requires a high degree of suspicion
Lemierre’s syndrome typically occurs in healthy young adults. Pharyngitis is the most common initial symptom, occurring in 87% of patients.2 This is followed by a fever (102.2°F - 105.8°F) usually 4 to 5 days after the onset of sore throat.3 Other common symptoms include chills, dysphagia, dyspnea, chest pain, hemoptysis, cervical neck discomfort, arthralgia, malaise, and night sweats.2 Following suppurative thrombophlebitis of the IJV, infection spreads to other organ systems. Pulmonary involvement is the most common site (97% of cases).3 Other complications of this syndrome are listed in the TABLE.3
The differential includes mononucleosis
The differential diagnosis encompasses several common illnesses, including mononucleosis, Group A streptococcal pharyngitis, and peritonsillar abscess. However, while patients with these conditions might have a fever and an elevated WBC count, they typically would not have the pleuritic chest pain that is characteristic of Lemierre’s syndrome. In addition, while patients with peritonsillar abscess would have tonsillar exudates, patients with Lemierre’s syndrome would not likely have them.
Influenza is also part of the differential, although focal neck pain usually isn’t a finding in patients who have the flu.
Once other common illnesses have been ruled out, it’s important to have a high index of suspicion for Lemierre’s syndrome because the oropharyngeal infection may resolve by the time of presentation, and there may be few findings on physical exam.7 Therefore, suspect Lemierre’s if a patient comes in with neck pain and/or pleuritic chest pain and has a recent history of oropharyngeal infection and fever.
CT scan of the neck and chest with contrast is the optimal diagnostic modality because it allows physicians to visualize the IJV8 and detect pulmonary emboli.9 Doppler ultrasound also can be used to diagnose IJV thrombosis. Ultrasound findings would reveal an echogenic focus within a dilated IJV or a complex mass of cystic and solid components.10
Prompt antibiotic treatment is essential
Patients with Lemierre’s syndrome require prompt and appropriate antimicrobial therapy. Researchers have reported mortality rates of 25% among patients who received delayed antibiotic therapy, compared with rates of up to 18% with prompt therapy.3 Metronidazole is the most commonly prescribed antibiotic.8 When combined with ceftriaxone, it provides coverage for both F necrophorum and streptococci, a common copathogen. Monotherapy with a carbapenem antibiotic, clindamycin, ampicillin/sulbactam, or antipseudomonal penicillin also are appropriate options.5 Antimicrobial treatment for 3 to 6 weeks is recommended because relapses have been noted in patients treated for less than 2 weeks.11
Anticoagulation is controversial.2 Proponents of anticoagulation to treat Lemierre’s syndrome believe it may prevent formation of septic emboli and could expedite recovery.4,12 Others believe that clots associated with Lemierre’s syndrome dissolve on their own and that anticoagulation may increase the likelihood of septic emboli.13 Many case reports, including this one, have demonstrated that complete recovery is possible without anticoagulation.10,13-15 Anticoagulation therapy can be considered for patients with Lemierre’s syndrome in the absence of any contraindications such as gastrointestinal or intracranial bleeding.
THE TAKEAWAY
Suspect Lemierre’s syndrome when a patient complains of neck pain, high fever, rigors, dry cough, and pleuritic chest pain and mentions a sore throat that he or she had in the pretceding 7 days. Diagnosis can be confirmed by radiological findings and blood cultures positive for F. necrophorum. Patients with Lemierre’s syndrome should be promptly treated with antibiotics; evidence for anticoagulation is inconclusive.
1. Golpe R, Marin B, Alonso M. Lemierre’s syndrome (necrobacillosis). Postgrad Med J. 1999;75:141-144.
2. Wright WF, Shiner CN, Ribes JA. Lemierre syndrome. South Med J. 2012;105:283-288.
3. Riordan T, Wilson M. Lemierre’s syndrome: more than a historical curiosa. Postgrad Med J. 2004;80:328-334.
4. Ridgway JM, Parikh DA, Wright R, et al. Lemierre syndrome: a pediatric case series and review of literature. Am J Otolaryngol. 2010;31:38-45.
5. McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992–2000. Emerg Infect Dis. 2003;9:432-437.
6. Hagelskjaer Kristensen L, Prag J. Human necrobacillosis, with emphasis on Lemierre’s syndrome. Clin Infect Dis. 2000;31:524-532.
7. Kupalli K, Livorsi D, Talati N, et al. Lemierre’s syndrome due to fusobacterium necrophorum. Lancet Infect Dis. 2012;12:808-815.
8. Armstrong AW, Spooner K, Sanders JW. Lemierre’s syndrome. Curr Infect Dis Rep. 2000;2:168-173.
9. Screaton NJ, Ravenel JG, Lehner PJ, et al. Lemierre syndrome: forgotten but not extinct--report of four cases. Radiology. 1999;213:369-374.
10. Chirinos JA, Lichtstein DM, Garcia J, et al. The evolution of Lemierre syndrome: report of 2 cases and review of the literature. Medicine (Baltimore). 2002;81:458-465.
11. Karkos PD, Asrani S, Karkos CD, et al. Lemierre syndrome: a systematic review. Laryngoscope. 2009;119:1552-1559.
12. Phan T, So TY. Use of anticoagulation therapy for jugular vein thrombus in pediatric patients with Lemierre’s syndrome. Int J Clin Pharm. 2012;34:818-821.
13. O’Brien WT, Cohen RA. Lemierre’ syndrome. Applied Radiology. 2011;40:37-38.
14. Vandenberg SJ, Hartig GK. Lemierre’s syndrome. Otolaryngol Head Neck Surg. 1998;119:516-518.
15. Goldhagen J, Alford BA, Prewitt LH, et al. Suppurative thrombophlebitis of the internal jugular vein: report of three cases and review of the pediatric literature. Pediatr Infect Dis J. 1988;7:410-414.
1. Golpe R, Marin B, Alonso M. Lemierre’s syndrome (necrobacillosis). Postgrad Med J. 1999;75:141-144.
2. Wright WF, Shiner CN, Ribes JA. Lemierre syndrome. South Med J. 2012;105:283-288.
3. Riordan T, Wilson M. Lemierre’s syndrome: more than a historical curiosa. Postgrad Med J. 2004;80:328-334.
4. Ridgway JM, Parikh DA, Wright R, et al. Lemierre syndrome: a pediatric case series and review of literature. Am J Otolaryngol. 2010;31:38-45.
5. McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992–2000. Emerg Infect Dis. 2003;9:432-437.
6. Hagelskjaer Kristensen L, Prag J. Human necrobacillosis, with emphasis on Lemierre’s syndrome. Clin Infect Dis. 2000;31:524-532.
7. Kupalli K, Livorsi D, Talati N, et al. Lemierre’s syndrome due to fusobacterium necrophorum. Lancet Infect Dis. 2012;12:808-815.
8. Armstrong AW, Spooner K, Sanders JW. Lemierre’s syndrome. Curr Infect Dis Rep. 2000;2:168-173.
9. Screaton NJ, Ravenel JG, Lehner PJ, et al. Lemierre syndrome: forgotten but not extinct--report of four cases. Radiology. 1999;213:369-374.
10. Chirinos JA, Lichtstein DM, Garcia J, et al. The evolution of Lemierre syndrome: report of 2 cases and review of the literature. Medicine (Baltimore). 2002;81:458-465.
11. Karkos PD, Asrani S, Karkos CD, et al. Lemierre syndrome: a systematic review. Laryngoscope. 2009;119:1552-1559.
12. Phan T, So TY. Use of anticoagulation therapy for jugular vein thrombus in pediatric patients with Lemierre’s syndrome. Int J Clin Pharm. 2012;34:818-821.
13. O’Brien WT, Cohen RA. Lemierre’ syndrome. Applied Radiology. 2011;40:37-38.
14. Vandenberg SJ, Hartig GK. Lemierre’s syndrome. Otolaryngol Head Neck Surg. 1998;119:516-518.
15. Goldhagen J, Alford BA, Prewitt LH, et al. Suppurative thrombophlebitis of the internal jugular vein: report of three cases and review of the pediatric literature. Pediatr Infect Dis J. 1988;7:410-414.
What you should know about patients who bring a list to their office visit
ABSTRACT
Purpose Little is known about patients who present a written list during a medical consultation. In this preliminary study, we sought to examine and characterize patients who use a prepared list.
Methods The design was an open observational case-controlled study that took place at 2 urban primary care clinics. We enrolled patients consecutively as they arrived with a written list for consultation. Consecutive patients presenting without a list served as the control group. Physician interviews and completed questionnaires provided demographic and medical characteristics of this group and explanations for list preparation.
Results Fifty-four patients presented with a list and were compared with controls. Statistically, patients arriving with a list were significantly more likely to be older and retired, and less likely to be salaried workers or housewives. These patients had more chronic diseases and consumed more long-term medications. They had a greater number of doctor visits in the past year compared with controls, and perceived an increase in memory loss. There were no differences between the groups in terms of psychiatric disease or personality disorders.
Conclusions Aside from certain demographic and health characteristics, patients who use written lists do not differ substantially from those who don’t. They have no discernible ill intention, and the list serves as a memory aid to make the most of the visit.
Nonverbal communication is a significant part of the physician-patient encounter, in part revealing clues to underlying attitudes and emotions or indicating whether one agrees or disagrees with expressed statements.1 Nonverbal communication exhibited by both doctor and patient strongly influences how each participant perceives the encounter and helps determine how the physician-patient relationship will develop.2-4
Patients, for example, are affected by the amount of physician eye contact and computer use. Less eye contact and greater attention to the computer tend to lower patients’ opinions of the consultation.1,5 These and other behaviors may contribute to the finding that 30% to 80% of patients feel their expectations are not met in routine primary care visits.6
Physicians, despite attempts to remain nonjudgmental, can be affected by a patient’s demeanor on entering the consulting room. Subtle prejudices may be evoked by age, gender, ethnicity, manner of dress, tone of voice, mannerisms, cell phone interruptions, and the like.7,8 Negative reactions can create barriers to good communication. Awareness of them may be the first step to preventing or removing hindrances to meaningful dialogue.9
One aspect of a patient’s presentation that may be viewed negatively is possession of a list. But this need not be the case. The list, if viewed as a patient-initiated agenda, can lead to a gratifying encounter for both patient and physician. In fact, there is reason to believe that a list representing a set agenda at the start of a visit may enhance patient satisfaction without increasing visit length.10 In fact, the Agency for Healthcare Research and Quality (AHRQ) advises patients to “Write down your questions before your visit. List the most important ones first to make sure they get asked and answered.”11
To learn more about the people who arrive at a consultation with a written list, we conducted a study at 2 clinics in Clalit Health Services—Southern District (CHS-SD), which we designed to focus on answering the following questions:
1. Do patients with lists have a unique sociodemographic profile?
2. Do they present with specific medical ailments but have a high frequency of psychiatric disorders?
3. What are the underlying motives leading to list use?
METHODS
Design
This was an open observational case-controlled study, approved by the institutional review board and the clinical research board of the Department of Family Medicine, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel.
Setting
We conducted our study at 2 urban primary care clinics serving a population of 7000 people of diverse ages, 10% of whom are recent immigrants.
Selection of participants
We consecutively recruited patients who carried a list to use during the consultation. After obtaining patients’ informed consent to participate in the study, we asked them to spend the time necessary to disclose requested information. We excluded those who were not fluent in the language of their physicians.
Intervention
Family physicians at the participating clinics distributed a questionnaire to patients arriving with a written list, then conducted guided interviews. We defined “list use” as the patient’s choice to refer to a list as an agenda for that visit, whether to remind one’s self to cover all complaints, to accurately describe symptoms, to request medication prescriptions, or to ask about test results.
First, through interview or questionnaire, we gathered standard sociodemographic data. Second, we focused on general health issues, chronic medical disorders, psychiatric disorders, chronic medication consumption, and number of visits. We derived this information from computerized medical records. Third, through the questionnaire, we inquired about the reason patients used a list, and asked patients to subjectively rate their memory and give the general reasons for their visit.
The control group consisted of patients recruited consecutively at arbitrary points in time until its size matched that of the study group. These patients volunteered information to the same line of inquiry. Some members of the groups chose to complete the questionnaires in writing without the physician’s assistance.
Statistical analysis
We processed results using SPSS software. We applied the x2 test for statistical interpretation and set statistical significance at P<.05.
RESULTS
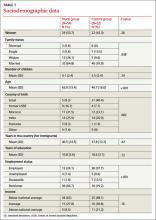
Twenty-five men and twenty-nine women ages 21 to 82 years of age comprised the group of patients presenting with a list. All patients who met inclusion criteria agreed to cooperate. TABLE 1 summarizes the sociodemographic data. The control group consisted of 30 men and 22 women ages 20 to 86 years of age.
There was no statistically meaningful difference in gender ratio between the groups. In the study and control groups, respectively, the average number of children of each subject was 4.1 and 3.5, years of formal education were 10.8 and 10.6, and years since immigration were 40.5 and 37.8 (P=.42). Marital status and average household income were also similar in both groups.
Statistically significant findings with the study group were relatively older ages and likelihood to be pensioners. The study group included fewer employed individuals and fewer housewives (P<.001). They also were more likely to have more than 3 chronic diseases (48.1% vs. 9.6%; P<.001) and took more long-term medications, including benzodiazepines (TABLE 2). They had a greater number of doctor visits in the past year compared with controls and reported a perceived increase in memory loss (TABLE 3). There were no significant differences between the groups in psychiatric or personality disorders, as determined by surveying patients’ electronic records.
The reasons most commonly given for using a list indicated a desire to completely satisfy the objectives of the visit. Most of the individuals decided to prepare a list on their own initiative without persuasion from any external source.
DISCUSSION
In an survey of 216 family physicians and internists at the University of Wisconsin, >60% of respondents said their patients bring in lists very often or sometimes.11 This figure seems much higher than would be found in our country (Israel). However, the practice is certainly common; although the actual frequency is unknown.
Other published observations about list use. Middleton et al12 studied the effects of planned use of agenda forms, completed by patients and handed to physicians at the outset of a primary care visit. The written agenda significantly increased the number of problems identified in each consultation. Patient satisfaction increased and deepened the doctor-patient relationship. However, the duration of consultations also increased.
A commentary by Schrager et al11 acknowledges that lists are dreaded by some physicians. Particularly if the expectation is for a patient to present with a single complaint, the appearance of a list may be an unwelcome surprise, suggesting a collection of separate complaints. And compulsive and somatizing patients can raise a series of overwhelming issues that encumber a short visit. But the commentary points out that, in general, fear of a list is unfounded, and that acceptance without prejudging can lead to a constructive outcome.
A number of researchers have examined the relationship between negative physician attitudes and certain patient attributes such as sociodemographic characteristics or a persistent emotional component to their ailments.13 Katz14 reported that patients who generated the most frustration were those who demanded a cure, those who added unrelated complaints at the end of the visit, malingerers, and those who refused to accept responsibility for their own maladies. List users, we believe, should not be lumped in with this group automatically.
In our study, patients with lists did request more frequent consultations. However, this correlated closely with a heavier burden of disease. Using patient-centered communication to set the agenda for the visit and address the entirety of patients’ concerns has been shown to improve not only patient satisfaction but also adherence to treatment recommendations.15,16 One way for patients to become more involved in their care is to bring a list of questions to each visit, as advised by AHRQ.
What our study revealed about list users. Our results show that those who made use of a list were older than the ones who did not, more likely to be pensioners, and less likely to be employed or be housewives. They had more chronic diseases, were receiving more medications including benzodiazepines, and had a significantly higher rate of medical appointments than did the controls. Psychiatric diagnoses were no more common among the list users, and reasons given for list use were congruent with aging and an increased burden of disease and medication. We could not discern the exact contribution of each independent factor of advanced age or disease burden. This would be an interesting issue to address in more elaborate research, as would be the actual frequency of list use.
Limitations of our study. The weaknesses of our study include its questionable generalizability and the possibility that a number of list bearers may not have been recruited due to time constraints on patients or physicians. Randomization could have been improved if we had selected the controls consecutively after selecting the study patients, and not at a separate time. We did not time the length of the consultations, something that should be done in future studies.
The fact that the study group consumed more benzodiazepine medications may hint that its members suffer from greater levels of anxiety or depression. Nevertheless, we assumed that such conditions were likely of modest intensity since they were not included in the medical records.
Large-scale research could yield far more trustworthy results by adjusting for age, country of origin, and disease burden.
CORRESPONDENCE
Sody Naimer, MD, Department of Family Medicine, Faculty of Health Sciences, Ben-Gurion University of the Negev, POB 653, Beer-Sheva 84105, Israel; [email protected]
Acknowledgement
The authors thank Dr. Joseph Herman and Mrs. Barbra Schipper for their assistance in preparing this manuscript.
1. Silverman J, Kinnersley P. Doctors’ non-verbal behaviour in consultations: look at the patient before you look at the computer. Br J Gen Pract. 2010;60:76-78.
2. Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
3. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67:315-318.
4. Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. Mechanisms and outcomes. J Gen Intern Med. 2006;21 suppl 1:S28-S34.
5. Marcinowicz L, Konstantynowicz J, Godlewski C. Patients’ perceptions of GP non-verbal communication: a qualitative study. Br J Gen Pract. 2010;60:83-87.
6. Kravitz RL. Patients’ expectations for medical care: an expanded formulation based on review of the literature. Med Care Res Rev. 1996;53:3-27.
7. Hooper EM, Comstock LM, Goodwin JM, et al. Patient characteristics that influence physician behavior. Med Care. 1982;20:630-638.
8. Naimer SA, Biton A. A pilot study of behaviour and impact of cellular telephone ringing interrupting the medical consultation. Isr J Fam Pr. 2002;19:35-39.
9. Quill TE. Recognizing and adjusting to barriers in doctor-patient communication. Ann Intern Med. 1989;111:51-57.
10. Brock DM, Mauksch LB, Witteborn S, et al. Effectiveness of intensive physician training in upfront agenda setting. J Gen Intern Med. 2011;26:1317-1323.
11. Schrager S, Gaard S. What should you do when your patient brings a list? Fam Pract Manag. 2009;16:23-27.
12. Middleton JF, McKinley RK, Gillies CL. Effect of patient completed agenda forms and doctors’ education about the agenda on the outcome of consultations: randomised controlled trial. BMJ. 2006;332:1238-1242.
13. Aaker E, Knudsen A, Wynn R, et al. General practitioners’ reactions to non-compliant patients. Scand J Prim Health Care. 2001;19:103-106.
14. Katz RC. “Difficult patients” as family physicians perceive them. Psychol Rep. 1996;79:539-544.
15. Epstein RM, Mauksch L, Carroll J, et al. Have you really addressed your patient’s concerns? Fam Pract Manag. 2008;15:35-40.
16. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296:2848-2851.
ABSTRACT
Purpose Little is known about patients who present a written list during a medical consultation. In this preliminary study, we sought to examine and characterize patients who use a prepared list.
Methods The design was an open observational case-controlled study that took place at 2 urban primary care clinics. We enrolled patients consecutively as they arrived with a written list for consultation. Consecutive patients presenting without a list served as the control group. Physician interviews and completed questionnaires provided demographic and medical characteristics of this group and explanations for list preparation.
Results Fifty-four patients presented with a list and were compared with controls. Statistically, patients arriving with a list were significantly more likely to be older and retired, and less likely to be salaried workers or housewives. These patients had more chronic diseases and consumed more long-term medications. They had a greater number of doctor visits in the past year compared with controls, and perceived an increase in memory loss. There were no differences between the groups in terms of psychiatric disease or personality disorders.
Conclusions Aside from certain demographic and health characteristics, patients who use written lists do not differ substantially from those who don’t. They have no discernible ill intention, and the list serves as a memory aid to make the most of the visit.
Nonverbal communication is a significant part of the physician-patient encounter, in part revealing clues to underlying attitudes and emotions or indicating whether one agrees or disagrees with expressed statements.1 Nonverbal communication exhibited by both doctor and patient strongly influences how each participant perceives the encounter and helps determine how the physician-patient relationship will develop.2-4
Patients, for example, are affected by the amount of physician eye contact and computer use. Less eye contact and greater attention to the computer tend to lower patients’ opinions of the consultation.1,5 These and other behaviors may contribute to the finding that 30% to 80% of patients feel their expectations are not met in routine primary care visits.6
Physicians, despite attempts to remain nonjudgmental, can be affected by a patient’s demeanor on entering the consulting room. Subtle prejudices may be evoked by age, gender, ethnicity, manner of dress, tone of voice, mannerisms, cell phone interruptions, and the like.7,8 Negative reactions can create barriers to good communication. Awareness of them may be the first step to preventing or removing hindrances to meaningful dialogue.9
One aspect of a patient’s presentation that may be viewed negatively is possession of a list. But this need not be the case. The list, if viewed as a patient-initiated agenda, can lead to a gratifying encounter for both patient and physician. In fact, there is reason to believe that a list representing a set agenda at the start of a visit may enhance patient satisfaction without increasing visit length.10 In fact, the Agency for Healthcare Research and Quality (AHRQ) advises patients to “Write down your questions before your visit. List the most important ones first to make sure they get asked and answered.”11
To learn more about the people who arrive at a consultation with a written list, we conducted a study at 2 clinics in Clalit Health Services—Southern District (CHS-SD), which we designed to focus on answering the following questions:
1. Do patients with lists have a unique sociodemographic profile?
2. Do they present with specific medical ailments but have a high frequency of psychiatric disorders?
3. What are the underlying motives leading to list use?
METHODS
Design
This was an open observational case-controlled study, approved by the institutional review board and the clinical research board of the Department of Family Medicine, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel.
Setting
We conducted our study at 2 urban primary care clinics serving a population of 7000 people of diverse ages, 10% of whom are recent immigrants.
Selection of participants
We consecutively recruited patients who carried a list to use during the consultation. After obtaining patients’ informed consent to participate in the study, we asked them to spend the time necessary to disclose requested information. We excluded those who were not fluent in the language of their physicians.
Intervention
Family physicians at the participating clinics distributed a questionnaire to patients arriving with a written list, then conducted guided interviews. We defined “list use” as the patient’s choice to refer to a list as an agenda for that visit, whether to remind one’s self to cover all complaints, to accurately describe symptoms, to request medication prescriptions, or to ask about test results.
First, through interview or questionnaire, we gathered standard sociodemographic data. Second, we focused on general health issues, chronic medical disorders, psychiatric disorders, chronic medication consumption, and number of visits. We derived this information from computerized medical records. Third, through the questionnaire, we inquired about the reason patients used a list, and asked patients to subjectively rate their memory and give the general reasons for their visit.
The control group consisted of patients recruited consecutively at arbitrary points in time until its size matched that of the study group. These patients volunteered information to the same line of inquiry. Some members of the groups chose to complete the questionnaires in writing without the physician’s assistance.
Statistical analysis
We processed results using SPSS software. We applied the x2 test for statistical interpretation and set statistical significance at P<.05.
RESULTS
Twenty-five men and twenty-nine women ages 21 to 82 years of age comprised the group of patients presenting with a list. All patients who met inclusion criteria agreed to cooperate. TABLE 1 summarizes the sociodemographic data. The control group consisted of 30 men and 22 women ages 20 to 86 years of age.
There was no statistically meaningful difference in gender ratio between the groups. In the study and control groups, respectively, the average number of children of each subject was 4.1 and 3.5, years of formal education were 10.8 and 10.6, and years since immigration were 40.5 and 37.8 (P=.42). Marital status and average household income were also similar in both groups.
Statistically significant findings with the study group were relatively older ages and likelihood to be pensioners. The study group included fewer employed individuals and fewer housewives (P<.001). They also were more likely to have more than 3 chronic diseases (48.1% vs. 9.6%; P<.001) and took more long-term medications, including benzodiazepines (TABLE 2). They had a greater number of doctor visits in the past year compared with controls and reported a perceived increase in memory loss (TABLE 3). There were no significant differences between the groups in psychiatric or personality disorders, as determined by surveying patients’ electronic records.
The reasons most commonly given for using a list indicated a desire to completely satisfy the objectives of the visit. Most of the individuals decided to prepare a list on their own initiative without persuasion from any external source.
DISCUSSION
In an survey of 216 family physicians and internists at the University of Wisconsin, >60% of respondents said their patients bring in lists very often or sometimes.11 This figure seems much higher than would be found in our country (Israel). However, the practice is certainly common; although the actual frequency is unknown.
Other published observations about list use. Middleton et al12 studied the effects of planned use of agenda forms, completed by patients and handed to physicians at the outset of a primary care visit. The written agenda significantly increased the number of problems identified in each consultation. Patient satisfaction increased and deepened the doctor-patient relationship. However, the duration of consultations also increased.
A commentary by Schrager et al11 acknowledges that lists are dreaded by some physicians. Particularly if the expectation is for a patient to present with a single complaint, the appearance of a list may be an unwelcome surprise, suggesting a collection of separate complaints. And compulsive and somatizing patients can raise a series of overwhelming issues that encumber a short visit. But the commentary points out that, in general, fear of a list is unfounded, and that acceptance without prejudging can lead to a constructive outcome.
A number of researchers have examined the relationship between negative physician attitudes and certain patient attributes such as sociodemographic characteristics or a persistent emotional component to their ailments.13 Katz14 reported that patients who generated the most frustration were those who demanded a cure, those who added unrelated complaints at the end of the visit, malingerers, and those who refused to accept responsibility for their own maladies. List users, we believe, should not be lumped in with this group automatically.
In our study, patients with lists did request more frequent consultations. However, this correlated closely with a heavier burden of disease. Using patient-centered communication to set the agenda for the visit and address the entirety of patients’ concerns has been shown to improve not only patient satisfaction but also adherence to treatment recommendations.15,16 One way for patients to become more involved in their care is to bring a list of questions to each visit, as advised by AHRQ.
What our study revealed about list users. Our results show that those who made use of a list were older than the ones who did not, more likely to be pensioners, and less likely to be employed or be housewives. They had more chronic diseases, were receiving more medications including benzodiazepines, and had a significantly higher rate of medical appointments than did the controls. Psychiatric diagnoses were no more common among the list users, and reasons given for list use were congruent with aging and an increased burden of disease and medication. We could not discern the exact contribution of each independent factor of advanced age or disease burden. This would be an interesting issue to address in more elaborate research, as would be the actual frequency of list use.
Limitations of our study. The weaknesses of our study include its questionable generalizability and the possibility that a number of list bearers may not have been recruited due to time constraints on patients or physicians. Randomization could have been improved if we had selected the controls consecutively after selecting the study patients, and not at a separate time. We did not time the length of the consultations, something that should be done in future studies.
The fact that the study group consumed more benzodiazepine medications may hint that its members suffer from greater levels of anxiety or depression. Nevertheless, we assumed that such conditions were likely of modest intensity since they were not included in the medical records.
Large-scale research could yield far more trustworthy results by adjusting for age, country of origin, and disease burden.
CORRESPONDENCE
Sody Naimer, MD, Department of Family Medicine, Faculty of Health Sciences, Ben-Gurion University of the Negev, POB 653, Beer-Sheva 84105, Israel; [email protected]
Acknowledgement
The authors thank Dr. Joseph Herman and Mrs. Barbra Schipper for their assistance in preparing this manuscript.
ABSTRACT
Purpose Little is known about patients who present a written list during a medical consultation. In this preliminary study, we sought to examine and characterize patients who use a prepared list.
Methods The design was an open observational case-controlled study that took place at 2 urban primary care clinics. We enrolled patients consecutively as they arrived with a written list for consultation. Consecutive patients presenting without a list served as the control group. Physician interviews and completed questionnaires provided demographic and medical characteristics of this group and explanations for list preparation.
Results Fifty-four patients presented with a list and were compared with controls. Statistically, patients arriving with a list were significantly more likely to be older and retired, and less likely to be salaried workers or housewives. These patients had more chronic diseases and consumed more long-term medications. They had a greater number of doctor visits in the past year compared with controls, and perceived an increase in memory loss. There were no differences between the groups in terms of psychiatric disease or personality disorders.
Conclusions Aside from certain demographic and health characteristics, patients who use written lists do not differ substantially from those who don’t. They have no discernible ill intention, and the list serves as a memory aid to make the most of the visit.
Nonverbal communication is a significant part of the physician-patient encounter, in part revealing clues to underlying attitudes and emotions or indicating whether one agrees or disagrees with expressed statements.1 Nonverbal communication exhibited by both doctor and patient strongly influences how each participant perceives the encounter and helps determine how the physician-patient relationship will develop.2-4
Patients, for example, are affected by the amount of physician eye contact and computer use. Less eye contact and greater attention to the computer tend to lower patients’ opinions of the consultation.1,5 These and other behaviors may contribute to the finding that 30% to 80% of patients feel their expectations are not met in routine primary care visits.6
Physicians, despite attempts to remain nonjudgmental, can be affected by a patient’s demeanor on entering the consulting room. Subtle prejudices may be evoked by age, gender, ethnicity, manner of dress, tone of voice, mannerisms, cell phone interruptions, and the like.7,8 Negative reactions can create barriers to good communication. Awareness of them may be the first step to preventing or removing hindrances to meaningful dialogue.9
One aspect of a patient’s presentation that may be viewed negatively is possession of a list. But this need not be the case. The list, if viewed as a patient-initiated agenda, can lead to a gratifying encounter for both patient and physician. In fact, there is reason to believe that a list representing a set agenda at the start of a visit may enhance patient satisfaction without increasing visit length.10 In fact, the Agency for Healthcare Research and Quality (AHRQ) advises patients to “Write down your questions before your visit. List the most important ones first to make sure they get asked and answered.”11
To learn more about the people who arrive at a consultation with a written list, we conducted a study at 2 clinics in Clalit Health Services—Southern District (CHS-SD), which we designed to focus on answering the following questions:
1. Do patients with lists have a unique sociodemographic profile?
2. Do they present with specific medical ailments but have a high frequency of psychiatric disorders?
3. What are the underlying motives leading to list use?
METHODS
Design
This was an open observational case-controlled study, approved by the institutional review board and the clinical research board of the Department of Family Medicine, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel.
Setting
We conducted our study at 2 urban primary care clinics serving a population of 7000 people of diverse ages, 10% of whom are recent immigrants.
Selection of participants
We consecutively recruited patients who carried a list to use during the consultation. After obtaining patients’ informed consent to participate in the study, we asked them to spend the time necessary to disclose requested information. We excluded those who were not fluent in the language of their physicians.
Intervention
Family physicians at the participating clinics distributed a questionnaire to patients arriving with a written list, then conducted guided interviews. We defined “list use” as the patient’s choice to refer to a list as an agenda for that visit, whether to remind one’s self to cover all complaints, to accurately describe symptoms, to request medication prescriptions, or to ask about test results.
First, through interview or questionnaire, we gathered standard sociodemographic data. Second, we focused on general health issues, chronic medical disorders, psychiatric disorders, chronic medication consumption, and number of visits. We derived this information from computerized medical records. Third, through the questionnaire, we inquired about the reason patients used a list, and asked patients to subjectively rate their memory and give the general reasons for their visit.
The control group consisted of patients recruited consecutively at arbitrary points in time until its size matched that of the study group. These patients volunteered information to the same line of inquiry. Some members of the groups chose to complete the questionnaires in writing without the physician’s assistance.
Statistical analysis
We processed results using SPSS software. We applied the x2 test for statistical interpretation and set statistical significance at P<.05.
RESULTS
Twenty-five men and twenty-nine women ages 21 to 82 years of age comprised the group of patients presenting with a list. All patients who met inclusion criteria agreed to cooperate. TABLE 1 summarizes the sociodemographic data. The control group consisted of 30 men and 22 women ages 20 to 86 years of age.
There was no statistically meaningful difference in gender ratio between the groups. In the study and control groups, respectively, the average number of children of each subject was 4.1 and 3.5, years of formal education were 10.8 and 10.6, and years since immigration were 40.5 and 37.8 (P=.42). Marital status and average household income were also similar in both groups.
Statistically significant findings with the study group were relatively older ages and likelihood to be pensioners. The study group included fewer employed individuals and fewer housewives (P<.001). They also were more likely to have more than 3 chronic diseases (48.1% vs. 9.6%; P<.001) and took more long-term medications, including benzodiazepines (TABLE 2). They had a greater number of doctor visits in the past year compared with controls and reported a perceived increase in memory loss (TABLE 3). There were no significant differences between the groups in psychiatric or personality disorders, as determined by surveying patients’ electronic records.
The reasons most commonly given for using a list indicated a desire to completely satisfy the objectives of the visit. Most of the individuals decided to prepare a list on their own initiative without persuasion from any external source.
DISCUSSION
In an survey of 216 family physicians and internists at the University of Wisconsin, >60% of respondents said their patients bring in lists very often or sometimes.11 This figure seems much higher than would be found in our country (Israel). However, the practice is certainly common; although the actual frequency is unknown.
Other published observations about list use. Middleton et al12 studied the effects of planned use of agenda forms, completed by patients and handed to physicians at the outset of a primary care visit. The written agenda significantly increased the number of problems identified in each consultation. Patient satisfaction increased and deepened the doctor-patient relationship. However, the duration of consultations also increased.
A commentary by Schrager et al11 acknowledges that lists are dreaded by some physicians. Particularly if the expectation is for a patient to present with a single complaint, the appearance of a list may be an unwelcome surprise, suggesting a collection of separate complaints. And compulsive and somatizing patients can raise a series of overwhelming issues that encumber a short visit. But the commentary points out that, in general, fear of a list is unfounded, and that acceptance without prejudging can lead to a constructive outcome.
A number of researchers have examined the relationship between negative physician attitudes and certain patient attributes such as sociodemographic characteristics or a persistent emotional component to their ailments.13 Katz14 reported that patients who generated the most frustration were those who demanded a cure, those who added unrelated complaints at the end of the visit, malingerers, and those who refused to accept responsibility for their own maladies. List users, we believe, should not be lumped in with this group automatically.
In our study, patients with lists did request more frequent consultations. However, this correlated closely with a heavier burden of disease. Using patient-centered communication to set the agenda for the visit and address the entirety of patients’ concerns has been shown to improve not only patient satisfaction but also adherence to treatment recommendations.15,16 One way for patients to become more involved in their care is to bring a list of questions to each visit, as advised by AHRQ.
What our study revealed about list users. Our results show that those who made use of a list were older than the ones who did not, more likely to be pensioners, and less likely to be employed or be housewives. They had more chronic diseases, were receiving more medications including benzodiazepines, and had a significantly higher rate of medical appointments than did the controls. Psychiatric diagnoses were no more common among the list users, and reasons given for list use were congruent with aging and an increased burden of disease and medication. We could not discern the exact contribution of each independent factor of advanced age or disease burden. This would be an interesting issue to address in more elaborate research, as would be the actual frequency of list use.
Limitations of our study. The weaknesses of our study include its questionable generalizability and the possibility that a number of list bearers may not have been recruited due to time constraints on patients or physicians. Randomization could have been improved if we had selected the controls consecutively after selecting the study patients, and not at a separate time. We did not time the length of the consultations, something that should be done in future studies.
The fact that the study group consumed more benzodiazepine medications may hint that its members suffer from greater levels of anxiety or depression. Nevertheless, we assumed that such conditions were likely of modest intensity since they were not included in the medical records.
Large-scale research could yield far more trustworthy results by adjusting for age, country of origin, and disease burden.
CORRESPONDENCE
Sody Naimer, MD, Department of Family Medicine, Faculty of Health Sciences, Ben-Gurion University of the Negev, POB 653, Beer-Sheva 84105, Israel; [email protected]
Acknowledgement
The authors thank Dr. Joseph Herman and Mrs. Barbra Schipper for their assistance in preparing this manuscript.
1. Silverman J, Kinnersley P. Doctors’ non-verbal behaviour in consultations: look at the patient before you look at the computer. Br J Gen Pract. 2010;60:76-78.
2. Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
3. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67:315-318.
4. Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. Mechanisms and outcomes. J Gen Intern Med. 2006;21 suppl 1:S28-S34.
5. Marcinowicz L, Konstantynowicz J, Godlewski C. Patients’ perceptions of GP non-verbal communication: a qualitative study. Br J Gen Pract. 2010;60:83-87.
6. Kravitz RL. Patients’ expectations for medical care: an expanded formulation based on review of the literature. Med Care Res Rev. 1996;53:3-27.
7. Hooper EM, Comstock LM, Goodwin JM, et al. Patient characteristics that influence physician behavior. Med Care. 1982;20:630-638.
8. Naimer SA, Biton A. A pilot study of behaviour and impact of cellular telephone ringing interrupting the medical consultation. Isr J Fam Pr. 2002;19:35-39.
9. Quill TE. Recognizing and adjusting to barriers in doctor-patient communication. Ann Intern Med. 1989;111:51-57.
10. Brock DM, Mauksch LB, Witteborn S, et al. Effectiveness of intensive physician training in upfront agenda setting. J Gen Intern Med. 2011;26:1317-1323.
11. Schrager S, Gaard S. What should you do when your patient brings a list? Fam Pract Manag. 2009;16:23-27.
12. Middleton JF, McKinley RK, Gillies CL. Effect of patient completed agenda forms and doctors’ education about the agenda on the outcome of consultations: randomised controlled trial. BMJ. 2006;332:1238-1242.
13. Aaker E, Knudsen A, Wynn R, et al. General practitioners’ reactions to non-compliant patients. Scand J Prim Health Care. 2001;19:103-106.
14. Katz RC. “Difficult patients” as family physicians perceive them. Psychol Rep. 1996;79:539-544.
15. Epstein RM, Mauksch L, Carroll J, et al. Have you really addressed your patient’s concerns? Fam Pract Manag. 2008;15:35-40.
16. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296:2848-2851.
1. Silverman J, Kinnersley P. Doctors’ non-verbal behaviour in consultations: look at the patient before you look at the computer. Br J Gen Pract. 2010;60:76-78.
2. Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
3. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67:315-318.
4. Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. Mechanisms and outcomes. J Gen Intern Med. 2006;21 suppl 1:S28-S34.
5. Marcinowicz L, Konstantynowicz J, Godlewski C. Patients’ perceptions of GP non-verbal communication: a qualitative study. Br J Gen Pract. 2010;60:83-87.
6. Kravitz RL. Patients’ expectations for medical care: an expanded formulation based on review of the literature. Med Care Res Rev. 1996;53:3-27.
7. Hooper EM, Comstock LM, Goodwin JM, et al. Patient characteristics that influence physician behavior. Med Care. 1982;20:630-638.
8. Naimer SA, Biton A. A pilot study of behaviour and impact of cellular telephone ringing interrupting the medical consultation. Isr J Fam Pr. 2002;19:35-39.
9. Quill TE. Recognizing and adjusting to barriers in doctor-patient communication. Ann Intern Med. 1989;111:51-57.
10. Brock DM, Mauksch LB, Witteborn S, et al. Effectiveness of intensive physician training in upfront agenda setting. J Gen Intern Med. 2011;26:1317-1323.
11. Schrager S, Gaard S. What should you do when your patient brings a list? Fam Pract Manag. 2009;16:23-27.
12. Middleton JF, McKinley RK, Gillies CL. Effect of patient completed agenda forms and doctors’ education about the agenda on the outcome of consultations: randomised controlled trial. BMJ. 2006;332:1238-1242.
13. Aaker E, Knudsen A, Wynn R, et al. General practitioners’ reactions to non-compliant patients. Scand J Prim Health Care. 2001;19:103-106.
14. Katz RC. “Difficult patients” as family physicians perceive them. Psychol Rep. 1996;79:539-544.
15. Epstein RM, Mauksch L, Carroll J, et al. Have you really addressed your patient’s concerns? Fam Pract Manag. 2008;15:35-40.
16. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296:2848-2851.
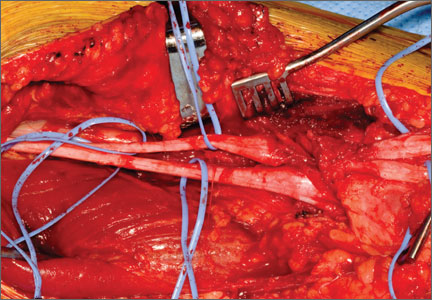
Failed First Metatarsophalangeal Arthroplasty Salvaged by Hamstring Interposition Arthroplasty: Metallic Debris From Grommets
joint, arthroplasty, implant, metallic debris, grommets, titanium, synovitis, foot
joint, arthroplasty, implant, metallic debris, grommets, titanium, synovitis, foot
joint, arthroplasty, implant, metallic debris, grommets, titanium, synovitis, foot
New and Noteworthy Information—April 2014
Little evidence suggests that most complementary or alternative medicine therapies treat the symptoms of multiple sclerosis (MS), according to an American Academy of Neurology guideline published March 25 in Neurology. Oral cannabis and oral medical marijuana spray, however, may ease patients’ reported symptoms of spasticity, pain related to spasticity, and frequent urination in MS. Not enough evidence is available to show whether smoking marijuana helps treat MS symptoms, according to the guideline. The authors concluded that magnetic therapy is probably effective for fatigue and probably ineffective for depression. Fish oil is probably ineffective for relapses, disability, fatigue, MRI lesions, and quality of life, according to the guideline. In addition, evidence indicates that ginkgo biloba is ineffective for cognition and possibly effective for fatigue, said the authors.
People who develop diabetes and high blood pressure in middle age are more likely to have brain cell loss and problems with memory and thinking skills than people who never have diabetes or high blood pressure or who develop them in old age, according to a study published online ahead of print March 19 in Neurology. Investigators evaluated the thinking and memory skills of 1,437 people (average age, 80), conducted brain scans, and reviewed participants’ medical records to determine whether the latter had been diagnosed with diabetes or high blood pressure in middle age or later. Midlife diabetes was associated with subcortical infarctions, reduced hippocampal volume, reduced whole brain volume, and prevalent mild cognitive impairment. Midlife hypertension was associated with infarctions and white matter hyperintensity volume.
Each 15-minute decrease in treatment delay may provide a patient an average equivalent of one month of additional disability-free life, according to a study published online ahead of print March 13 in Stroke. Researchers examined observational prospective data for 2,258 consecutive stroke patients treated with IV thrombolysis to determine distributions of age, sex, stroke severity, onset-to-treatment times, and three-month modified Rankin Scale score in daily clinical practice. The investigators found that for every one-minute reduction in onset-to-treatment time, patients gained an average 1.8 days of healthy life. Although all patients benefited from faster treatment, younger patients with longer life expectancies gained a little more than older patients. Women gained slightly more than men throughout their longer lifetimes. The awareness of the importance of speed could promote practice change, said the authors.
The FDA has approved extended-release Qudexy XR (topiramate) capsules as initial monotherapy in patients 10 or older with partial-onset seizures or primary generalized tonic-clonic seizures. The drug also received approval as adjunctive therapy in patients age 2 or older with partial-onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox–Gastaut syndrome. In a randomized, double-blind, placebo-controlled study, the drug demonstrated favorable safety and tolerability in patients with refractory partial-onset seizures. The extended-release formulation was associated with a significantly greater median percent reduction from baseline in seizure frequency, compared with placebo (39.5% vs 21.7%) after 11 weeks of treatment. Upsher-Smith Laboratories, headquartered in Maple Grove, Minnesota, manufactures the drug and expects it to be available during the second quarter of 2014.
The FDA has approved the Cefaly medical device as a preventive treatment for migraine headaches. The product is the first transcutaneous electrical nerve stimulation device specifically authorized for use before the onset of pain. The product is a small, portable, battery-powered unit resembling a plastic headband worn across the forehead once per day for 20 minutes. The device applies an electric current to the skin and underlying tissues to stimulate branches of the trigeminal nerve. In a study including 67 participants, patients who used Cefaly had significantly fewer days with migraines per month and used less migraine attack medication, compared with patients who used a placebo device. STX-Med, which is headquartered in Herstal, Liege, Belgium, manufactures the device, which is indicated for patients 18 and older.
Children with autism who are fed infant formula containing soy protein rather than milk protein may have a higher rate of seizures, according to research published March 12 in PLOS One. Researchers analyzed medical record data for 1,949 children with autism, including information on infant formula use, seizure incidence, the specific type of seizure exhibited, and IQ. Soy-based formula was given in 17.5% of the study population. About 13% of the subjects were female. The researchers found a 2.6-fold higher rate of febrile seizures (4.2% vs 1.6%), a 2.1-fold higher rate of epilepsy comorbidity (3.6% vs 1.7%), and a fourfold higher rate of simple partial seizures (1.2% vs 0.3%) in the children with autism who were fed soy-based formula. No statistically significant associations were found with other outcomes.
For patients with Alzheimer’s disease, levels of markers of neuronal injury in the spinal fluid may decrease as symptoms of memory loss and mental decline appear, according to research published March 5 in Science Translational Medicine. Investigators studied data from the Dominantly Inherited Alzheimer’s Network, which includes participants from families with genetic mutations that cause rare inherited forms of Alzheimer’s disease. The group examined levels of tau, p-tau, and visinin-like protein-1 (VILIP-1). Asymptomatic mutation carriers had elevated concentrations of CSF tau, p-tau181, and VILIP-1 10 to 20 years before their estimated age at symptom onset and before cognitive deficits were detected. The concentrations of CSF biomarkers of neuronal injury or death decreased after their estimated age at symptom onset, suggesting a slowing of acute neurodegenerative processes with symptomatic disease progression.
Men with poor cardiovascular fitness or a low IQ at age 18 are more likely to develop dementia before age 60, investigators reported online ahead of print March 6 in Brain. The researchers conducted a population-based cohort study of more than 1.1 million Swedish male conscripts (age 18) who underwent conscription exams between 1968 and 2005. Participants were followed for as long as 42 years. In fully adjusted models, low cardiovascular fitness and cognitive performance at age 18 were associated with increased risk for future early-onset dementia and mild cognitive impairment, compared with high cardiovascular fitness and cognitive performance. Poor performance on cardiovascular fitness and cognitive tests was associated with a greater-than-sevenfold and a greater than eightfold increased risk of early-onset dementia and early-onset mild cognitive impairment, respectively.
A test that detects low levels of prion protein in the blood may accurately screen for infection with the agent responsible for variant Creutzfeldt-Jakob disease (vCJD), according to a study published online ahead of print March 3 in JAMA Neurology. Researchers performed the test on samples from national blood collection and prion disease centers in the US and the UK. The samples were taken from healthy donors, patients with nonprion neurodegenerative disease, patients in whom a prion disease diagnosis was likely, and patients with confirmed vCJD. The assay’s specificity was confirmed as 100% in a healthy UK cohort. No potentially cross-reactive blood samples from patients with nonprion neurodegenerative diseases tested positive. Two patients with sporadic CJD tested positive. The authors’ previous sensitivity estimate was reconfirmed but not refined.
The FDA has approved Neuraceq (florbetaben F18 injection) for PET imaging of the brain to estimate beta-amyloid neuritic plaque density in adults with cognitive impairment who are being evaluated for Alzheimer’s disease and other causes of cognitive decline. The approval is based on safety data from 872 patients who participated in global clinical trials, and on three studies that examined images from adults with a range of cognitive function. Images were analyzed from 82 subjects with postmortem confirmation of the presence or absence of beta-amyloid neuritic plaques. Correlation of the visual PET interpretation with histopathology in these 82 brains demonstrated that Neuraceq (Piramal Imaging; Boston) accurately detects moderate to frequent beta-amyloid neuritic plaques in the brain.
A combination of human umbilical cord blood cells (hUCBs) and granulocyte colony stimulating factor (G-CSF) may provide more benefit for patients with traumatic brain injury (TBI) than either therapy alone, according to research published March 12 in PLOS One. Adult rats underwent moderate TBI and, seven days later, were treated with saline alone, G-CSF and saline, hUCB and saline, or hUCB and G-CSF. The rats treated with saline exhibited widespread neuroinflammation, impaired endogenous neurogenesis, and severe hippocampal cell loss. hUCB monotherapy suppressed neuroinflammation, nearly normalized neurogenesis, and reduced hippocampal cell loss, compared with saline alone. G-CSF monotherapy produced partial and short-lived benefits characterized by low levels of neuroinflammation, modest neurogenesis, and moderate reduction of hippocampal cell loss. Combined therapy robustly dampened neuroinflammation, enhanced endogenous neurogenesis, and reduced hippocampal cell loss.
The ability to learn new information may be significantly poorer among patients with Parkinson’s disease than among healthy individuals, according to research published online ahead of print February 24 in Movement Disorders. Investigators examined 27 patients with Parkinson’s disease without dementia and 27 age-, gender-, and education-matched healthy controls with a neuropsychologic test battery designed to assess new learning and memory. The researchers found a significant difference in the groups’ ability to learn a list of 10 semantically related words. Once the groups were equated on learning abilities, the investigators found no significant difference between patients with Parkinson’s disease and controls in recall or recognition of the newly learned material. The memory deficit in nondemented patients with Parkinson’s disease largely results from a deficit in learning new information, said the authors.
Chronic sleep loss may lead to irreversible physical damage to and loss of brain cells, according to research published March 19 in the Journal of Neuroscience. Investigators examined mice following periods of normal rest, short wakefulness, or extended wakefulness to model shift workers’ typical sleep patterns. In response to short-term sleep loss, locus coeruleus neurons upregulated the sirtuin type 3 (SirT3) protein, which protects neurons from metabolic injury. After several days of shift worker sleep patterns, locus coeruleus neurons in the mice had reduced SirT3 and increased cell death. In addition, oxidative stress and acetylation of mitochondrial proteins increased. The mice lost 25% of their locus coeruleus neurons. “This is the first report that sleep loss can actually result in a loss of neurons,” said the authors.
—Erik Greb
Little evidence suggests that most complementary or alternative medicine therapies treat the symptoms of multiple sclerosis (MS), according to an American Academy of Neurology guideline published March 25 in Neurology. Oral cannabis and oral medical marijuana spray, however, may ease patients’ reported symptoms of spasticity, pain related to spasticity, and frequent urination in MS. Not enough evidence is available to show whether smoking marijuana helps treat MS symptoms, according to the guideline. The authors concluded that magnetic therapy is probably effective for fatigue and probably ineffective for depression. Fish oil is probably ineffective for relapses, disability, fatigue, MRI lesions, and quality of life, according to the guideline. In addition, evidence indicates that ginkgo biloba is ineffective for cognition and possibly effective for fatigue, said the authors.
People who develop diabetes and high blood pressure in middle age are more likely to have brain cell loss and problems with memory and thinking skills than people who never have diabetes or high blood pressure or who develop them in old age, according to a study published online ahead of print March 19 in Neurology. Investigators evaluated the thinking and memory skills of 1,437 people (average age, 80), conducted brain scans, and reviewed participants’ medical records to determine whether the latter had been diagnosed with diabetes or high blood pressure in middle age or later. Midlife diabetes was associated with subcortical infarctions, reduced hippocampal volume, reduced whole brain volume, and prevalent mild cognitive impairment. Midlife hypertension was associated with infarctions and white matter hyperintensity volume.
Each 15-minute decrease in treatment delay may provide a patient an average equivalent of one month of additional disability-free life, according to a study published online ahead of print March 13 in Stroke. Researchers examined observational prospective data for 2,258 consecutive stroke patients treated with IV thrombolysis to determine distributions of age, sex, stroke severity, onset-to-treatment times, and three-month modified Rankin Scale score in daily clinical practice. The investigators found that for every one-minute reduction in onset-to-treatment time, patients gained an average 1.8 days of healthy life. Although all patients benefited from faster treatment, younger patients with longer life expectancies gained a little more than older patients. Women gained slightly more than men throughout their longer lifetimes. The awareness of the importance of speed could promote practice change, said the authors.
The FDA has approved extended-release Qudexy XR (topiramate) capsules as initial monotherapy in patients 10 or older with partial-onset seizures or primary generalized tonic-clonic seizures. The drug also received approval as adjunctive therapy in patients age 2 or older with partial-onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox–Gastaut syndrome. In a randomized, double-blind, placebo-controlled study, the drug demonstrated favorable safety and tolerability in patients with refractory partial-onset seizures. The extended-release formulation was associated with a significantly greater median percent reduction from baseline in seizure frequency, compared with placebo (39.5% vs 21.7%) after 11 weeks of treatment. Upsher-Smith Laboratories, headquartered in Maple Grove, Minnesota, manufactures the drug and expects it to be available during the second quarter of 2014.
The FDA has approved the Cefaly medical device as a preventive treatment for migraine headaches. The product is the first transcutaneous electrical nerve stimulation device specifically authorized for use before the onset of pain. The product is a small, portable, battery-powered unit resembling a plastic headband worn across the forehead once per day for 20 minutes. The device applies an electric current to the skin and underlying tissues to stimulate branches of the trigeminal nerve. In a study including 67 participants, patients who used Cefaly had significantly fewer days with migraines per month and used less migraine attack medication, compared with patients who used a placebo device. STX-Med, which is headquartered in Herstal, Liege, Belgium, manufactures the device, which is indicated for patients 18 and older.
Children with autism who are fed infant formula containing soy protein rather than milk protein may have a higher rate of seizures, according to research published March 12 in PLOS One. Researchers analyzed medical record data for 1,949 children with autism, including information on infant formula use, seizure incidence, the specific type of seizure exhibited, and IQ. Soy-based formula was given in 17.5% of the study population. About 13% of the subjects were female. The researchers found a 2.6-fold higher rate of febrile seizures (4.2% vs 1.6%), a 2.1-fold higher rate of epilepsy comorbidity (3.6% vs 1.7%), and a fourfold higher rate of simple partial seizures (1.2% vs 0.3%) in the children with autism who were fed soy-based formula. No statistically significant associations were found with other outcomes.
For patients with Alzheimer’s disease, levels of markers of neuronal injury in the spinal fluid may decrease as symptoms of memory loss and mental decline appear, according to research published March 5 in Science Translational Medicine. Investigators studied data from the Dominantly Inherited Alzheimer’s Network, which includes participants from families with genetic mutations that cause rare inherited forms of Alzheimer’s disease. The group examined levels of tau, p-tau, and visinin-like protein-1 (VILIP-1). Asymptomatic mutation carriers had elevated concentrations of CSF tau, p-tau181, and VILIP-1 10 to 20 years before their estimated age at symptom onset and before cognitive deficits were detected. The concentrations of CSF biomarkers of neuronal injury or death decreased after their estimated age at symptom onset, suggesting a slowing of acute neurodegenerative processes with symptomatic disease progression.
Men with poor cardiovascular fitness or a low IQ at age 18 are more likely to develop dementia before age 60, investigators reported online ahead of print March 6 in Brain. The researchers conducted a population-based cohort study of more than 1.1 million Swedish male conscripts (age 18) who underwent conscription exams between 1968 and 2005. Participants were followed for as long as 42 years. In fully adjusted models, low cardiovascular fitness and cognitive performance at age 18 were associated with increased risk for future early-onset dementia and mild cognitive impairment, compared with high cardiovascular fitness and cognitive performance. Poor performance on cardiovascular fitness and cognitive tests was associated with a greater-than-sevenfold and a greater than eightfold increased risk of early-onset dementia and early-onset mild cognitive impairment, respectively.
A test that detects low levels of prion protein in the blood may accurately screen for infection with the agent responsible for variant Creutzfeldt-Jakob disease (vCJD), according to a study published online ahead of print March 3 in JAMA Neurology. Researchers performed the test on samples from national blood collection and prion disease centers in the US and the UK. The samples were taken from healthy donors, patients with nonprion neurodegenerative disease, patients in whom a prion disease diagnosis was likely, and patients with confirmed vCJD. The assay’s specificity was confirmed as 100% in a healthy UK cohort. No potentially cross-reactive blood samples from patients with nonprion neurodegenerative diseases tested positive. Two patients with sporadic CJD tested positive. The authors’ previous sensitivity estimate was reconfirmed but not refined.
The FDA has approved Neuraceq (florbetaben F18 injection) for PET imaging of the brain to estimate beta-amyloid neuritic plaque density in adults with cognitive impairment who are being evaluated for Alzheimer’s disease and other causes of cognitive decline. The approval is based on safety data from 872 patients who participated in global clinical trials, and on three studies that examined images from adults with a range of cognitive function. Images were analyzed from 82 subjects with postmortem confirmation of the presence or absence of beta-amyloid neuritic plaques. Correlation of the visual PET interpretation with histopathology in these 82 brains demonstrated that Neuraceq (Piramal Imaging; Boston) accurately detects moderate to frequent beta-amyloid neuritic plaques in the brain.
A combination of human umbilical cord blood cells (hUCBs) and granulocyte colony stimulating factor (G-CSF) may provide more benefit for patients with traumatic brain injury (TBI) than either therapy alone, according to research published March 12 in PLOS One. Adult rats underwent moderate TBI and, seven days later, were treated with saline alone, G-CSF and saline, hUCB and saline, or hUCB and G-CSF. The rats treated with saline exhibited widespread neuroinflammation, impaired endogenous neurogenesis, and severe hippocampal cell loss. hUCB monotherapy suppressed neuroinflammation, nearly normalized neurogenesis, and reduced hippocampal cell loss, compared with saline alone. G-CSF monotherapy produced partial and short-lived benefits characterized by low levels of neuroinflammation, modest neurogenesis, and moderate reduction of hippocampal cell loss. Combined therapy robustly dampened neuroinflammation, enhanced endogenous neurogenesis, and reduced hippocampal cell loss.
The ability to learn new information may be significantly poorer among patients with Parkinson’s disease than among healthy individuals, according to research published online ahead of print February 24 in Movement Disorders. Investigators examined 27 patients with Parkinson’s disease without dementia and 27 age-, gender-, and education-matched healthy controls with a neuropsychologic test battery designed to assess new learning and memory. The researchers found a significant difference in the groups’ ability to learn a list of 10 semantically related words. Once the groups were equated on learning abilities, the investigators found no significant difference between patients with Parkinson’s disease and controls in recall or recognition of the newly learned material. The memory deficit in nondemented patients with Parkinson’s disease largely results from a deficit in learning new information, said the authors.
Chronic sleep loss may lead to irreversible physical damage to and loss of brain cells, according to research published March 19 in the Journal of Neuroscience. Investigators examined mice following periods of normal rest, short wakefulness, or extended wakefulness to model shift workers’ typical sleep patterns. In response to short-term sleep loss, locus coeruleus neurons upregulated the sirtuin type 3 (SirT3) protein, which protects neurons from metabolic injury. After several days of shift worker sleep patterns, locus coeruleus neurons in the mice had reduced SirT3 and increased cell death. In addition, oxidative stress and acetylation of mitochondrial proteins increased. The mice lost 25% of their locus coeruleus neurons. “This is the first report that sleep loss can actually result in a loss of neurons,” said the authors.
—Erik Greb
Little evidence suggests that most complementary or alternative medicine therapies treat the symptoms of multiple sclerosis (MS), according to an American Academy of Neurology guideline published March 25 in Neurology. Oral cannabis and oral medical marijuana spray, however, may ease patients’ reported symptoms of spasticity, pain related to spasticity, and frequent urination in MS. Not enough evidence is available to show whether smoking marijuana helps treat MS symptoms, according to the guideline. The authors concluded that magnetic therapy is probably effective for fatigue and probably ineffective for depression. Fish oil is probably ineffective for relapses, disability, fatigue, MRI lesions, and quality of life, according to the guideline. In addition, evidence indicates that ginkgo biloba is ineffective for cognition and possibly effective for fatigue, said the authors.
People who develop diabetes and high blood pressure in middle age are more likely to have brain cell loss and problems with memory and thinking skills than people who never have diabetes or high blood pressure or who develop them in old age, according to a study published online ahead of print March 19 in Neurology. Investigators evaluated the thinking and memory skills of 1,437 people (average age, 80), conducted brain scans, and reviewed participants’ medical records to determine whether the latter had been diagnosed with diabetes or high blood pressure in middle age or later. Midlife diabetes was associated with subcortical infarctions, reduced hippocampal volume, reduced whole brain volume, and prevalent mild cognitive impairment. Midlife hypertension was associated with infarctions and white matter hyperintensity volume.
Each 15-minute decrease in treatment delay may provide a patient an average equivalent of one month of additional disability-free life, according to a study published online ahead of print March 13 in Stroke. Researchers examined observational prospective data for 2,258 consecutive stroke patients treated with IV thrombolysis to determine distributions of age, sex, stroke severity, onset-to-treatment times, and three-month modified Rankin Scale score in daily clinical practice. The investigators found that for every one-minute reduction in onset-to-treatment time, patients gained an average 1.8 days of healthy life. Although all patients benefited from faster treatment, younger patients with longer life expectancies gained a little more than older patients. Women gained slightly more than men throughout their longer lifetimes. The awareness of the importance of speed could promote practice change, said the authors.
The FDA has approved extended-release Qudexy XR (topiramate) capsules as initial monotherapy in patients 10 or older with partial-onset seizures or primary generalized tonic-clonic seizures. The drug also received approval as adjunctive therapy in patients age 2 or older with partial-onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox–Gastaut syndrome. In a randomized, double-blind, placebo-controlled study, the drug demonstrated favorable safety and tolerability in patients with refractory partial-onset seizures. The extended-release formulation was associated with a significantly greater median percent reduction from baseline in seizure frequency, compared with placebo (39.5% vs 21.7%) after 11 weeks of treatment. Upsher-Smith Laboratories, headquartered in Maple Grove, Minnesota, manufactures the drug and expects it to be available during the second quarter of 2014.
The FDA has approved the Cefaly medical device as a preventive treatment for migraine headaches. The product is the first transcutaneous electrical nerve stimulation device specifically authorized for use before the onset of pain. The product is a small, portable, battery-powered unit resembling a plastic headband worn across the forehead once per day for 20 minutes. The device applies an electric current to the skin and underlying tissues to stimulate branches of the trigeminal nerve. In a study including 67 participants, patients who used Cefaly had significantly fewer days with migraines per month and used less migraine attack medication, compared with patients who used a placebo device. STX-Med, which is headquartered in Herstal, Liege, Belgium, manufactures the device, which is indicated for patients 18 and older.
Children with autism who are fed infant formula containing soy protein rather than milk protein may have a higher rate of seizures, according to research published March 12 in PLOS One. Researchers analyzed medical record data for 1,949 children with autism, including information on infant formula use, seizure incidence, the specific type of seizure exhibited, and IQ. Soy-based formula was given in 17.5% of the study population. About 13% of the subjects were female. The researchers found a 2.6-fold higher rate of febrile seizures (4.2% vs 1.6%), a 2.1-fold higher rate of epilepsy comorbidity (3.6% vs 1.7%), and a fourfold higher rate of simple partial seizures (1.2% vs 0.3%) in the children with autism who were fed soy-based formula. No statistically significant associations were found with other outcomes.
For patients with Alzheimer’s disease, levels of markers of neuronal injury in the spinal fluid may decrease as symptoms of memory loss and mental decline appear, according to research published March 5 in Science Translational Medicine. Investigators studied data from the Dominantly Inherited Alzheimer’s Network, which includes participants from families with genetic mutations that cause rare inherited forms of Alzheimer’s disease. The group examined levels of tau, p-tau, and visinin-like protein-1 (VILIP-1). Asymptomatic mutation carriers had elevated concentrations of CSF tau, p-tau181, and VILIP-1 10 to 20 years before their estimated age at symptom onset and before cognitive deficits were detected. The concentrations of CSF biomarkers of neuronal injury or death decreased after their estimated age at symptom onset, suggesting a slowing of acute neurodegenerative processes with symptomatic disease progression.
Men with poor cardiovascular fitness or a low IQ at age 18 are more likely to develop dementia before age 60, investigators reported online ahead of print March 6 in Brain. The researchers conducted a population-based cohort study of more than 1.1 million Swedish male conscripts (age 18) who underwent conscription exams between 1968 and 2005. Participants were followed for as long as 42 years. In fully adjusted models, low cardiovascular fitness and cognitive performance at age 18 were associated with increased risk for future early-onset dementia and mild cognitive impairment, compared with high cardiovascular fitness and cognitive performance. Poor performance on cardiovascular fitness and cognitive tests was associated with a greater-than-sevenfold and a greater than eightfold increased risk of early-onset dementia and early-onset mild cognitive impairment, respectively.
A test that detects low levels of prion protein in the blood may accurately screen for infection with the agent responsible for variant Creutzfeldt-Jakob disease (vCJD), according to a study published online ahead of print March 3 in JAMA Neurology. Researchers performed the test on samples from national blood collection and prion disease centers in the US and the UK. The samples were taken from healthy donors, patients with nonprion neurodegenerative disease, patients in whom a prion disease diagnosis was likely, and patients with confirmed vCJD. The assay’s specificity was confirmed as 100% in a healthy UK cohort. No potentially cross-reactive blood samples from patients with nonprion neurodegenerative diseases tested positive. Two patients with sporadic CJD tested positive. The authors’ previous sensitivity estimate was reconfirmed but not refined.
The FDA has approved Neuraceq (florbetaben F18 injection) for PET imaging of the brain to estimate beta-amyloid neuritic plaque density in adults with cognitive impairment who are being evaluated for Alzheimer’s disease and other causes of cognitive decline. The approval is based on safety data from 872 patients who participated in global clinical trials, and on three studies that examined images from adults with a range of cognitive function. Images were analyzed from 82 subjects with postmortem confirmation of the presence or absence of beta-amyloid neuritic plaques. Correlation of the visual PET interpretation with histopathology in these 82 brains demonstrated that Neuraceq (Piramal Imaging; Boston) accurately detects moderate to frequent beta-amyloid neuritic plaques in the brain.
A combination of human umbilical cord blood cells (hUCBs) and granulocyte colony stimulating factor (G-CSF) may provide more benefit for patients with traumatic brain injury (TBI) than either therapy alone, according to research published March 12 in PLOS One. Adult rats underwent moderate TBI and, seven days later, were treated with saline alone, G-CSF and saline, hUCB and saline, or hUCB and G-CSF. The rats treated with saline exhibited widespread neuroinflammation, impaired endogenous neurogenesis, and severe hippocampal cell loss. hUCB monotherapy suppressed neuroinflammation, nearly normalized neurogenesis, and reduced hippocampal cell loss, compared with saline alone. G-CSF monotherapy produced partial and short-lived benefits characterized by low levels of neuroinflammation, modest neurogenesis, and moderate reduction of hippocampal cell loss. Combined therapy robustly dampened neuroinflammation, enhanced endogenous neurogenesis, and reduced hippocampal cell loss.
The ability to learn new information may be significantly poorer among patients with Parkinson’s disease than among healthy individuals, according to research published online ahead of print February 24 in Movement Disorders. Investigators examined 27 patients with Parkinson’s disease without dementia and 27 age-, gender-, and education-matched healthy controls with a neuropsychologic test battery designed to assess new learning and memory. The researchers found a significant difference in the groups’ ability to learn a list of 10 semantically related words. Once the groups were equated on learning abilities, the investigators found no significant difference between patients with Parkinson’s disease and controls in recall or recognition of the newly learned material. The memory deficit in nondemented patients with Parkinson’s disease largely results from a deficit in learning new information, said the authors.
Chronic sleep loss may lead to irreversible physical damage to and loss of brain cells, according to research published March 19 in the Journal of Neuroscience. Investigators examined mice following periods of normal rest, short wakefulness, or extended wakefulness to model shift workers’ typical sleep patterns. In response to short-term sleep loss, locus coeruleus neurons upregulated the sirtuin type 3 (SirT3) protein, which protects neurons from metabolic injury. After several days of shift worker sleep patterns, locus coeruleus neurons in the mice had reduced SirT3 and increased cell death. In addition, oxidative stress and acetylation of mitochondrial proteins increased. The mice lost 25% of their locus coeruleus neurons. “This is the first report that sleep loss can actually result in a loss of neurons,” said the authors.
—Erik Greb
Iatrogenic Transection of the Peroneal and Partial Transection of the Tibial Nerve During Arthroscopic Lateral Meniscal Debridement and Removal of Osteochondral Fragment
Atypical Presentation of Soft-Tissue Mass With Gonococcal Infection in the Hand
Effect of Capsulotomy on Hip Stability—A Consideration During Hip Arthroscopy
Hookahs vs cigarettes: What to tell patients
How to discuss sex with elderly patients
› Keep in mind that elderly patients may want to discuss matters of sexuality but can also be embarrassed, fearful, or reluctant to do so with a younger caregiver. C
› Consider making a patient’s sexual history part of your general health screening, perhaps using the PLISSIT model for facilitating discussion. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sexuality is a central aspect of being human. It encompasses sex, gender identities and roles, sexual orientation, pleasure, eroticism, and intimacy, and is a major contributor to an individual’s quality of life and sense of wellbeing.1,2 Positive sexual relationships and behaviors are integral to maintaining good health and general well-being later in life, as well.2,3 Cynthia Graber, a reporter with Scientific American, reported that sex is a key reason retirees have a happy life.4
While there is a decline in sexual activity with age, a great number of men and women continue to engage in vaginal or anal intercourse, oral sex, and masturbation into the eighth and ninth decades of life.2,5 In a survey conducted among married men and women, about 90% of respondents between the ages of 60 and 64 and almost 30% of those older than age 80 said they were still sexually active.2 Another study reported that 62% of men and 30% of women 80 to 102 years of age were still sexually active.6 However, sexuality is rarely discussed with the elderly, and most physicians are unsure about how to handle such conversations.7
The baby boomer population is aging in the United States and elsewhere. By 2030, 20% of the US population will be ≥65 years old, and 4% (3 million) will be lesbian, gay, bisexual, transgender, and queer (LGBTQ) elderly adults.3,8 Given the impact of sex on maintaining quality of life, it is important for health care providers to be comfortable discussing sexuality with the elderly.9
Barriers to discussing sexuality
Physician barriers
Primary care physicians typically are the first point of contact for elderly adults experiencing health problems, including sexual dysfunction. According to the American Psychological Association, sex is not discussed enough with the elderly. Most physicians do not address sexual health proactively, and rarely do they include a sexual history as part of general health screening in the elderly.2,10,11 Inadequate training of physicians in sexual health is likely a contributing factor.5 Physicians also often feel discomfort when discussing such matters with patients of the opposite sex.12 (For a suggested approach to these conversations, see “Discussing sexuality with elderly patients: Getting beyond ‘don’t ask, don’t tell,” below.) With the increasing number of LGBTQ elderly adults, physicians should not assume their patients have any particular sexual behavior or orientation. This will help elderly LGBTQ patients feel more comfortable discussing their sexual health needs.8
The PLISSIT model, developed in 1976 by clinical psychologist Dr. Jack Annon, can facilitate a discussion of sexuality with elderly patients.11,13 First, the healthcare provider seeks permission (P) to discuss sexuality with the patient. After permission is given, the provider can share limited information (LI) about sexual issues that affect the older adult. Next, the provider may offer specific suggestions (SS) to improve sexual health or resolve problems. Finally, referral for intensive therapy (IT) may be needed for someone whose sexual dysfunction goes beyond the scope of the health care provider’s expertise. In 2000, open-ended questions were added to the PLISSIT model to more effectively guide an assessment of sexuality in older adults13,14:
• Can you tell me how you express your sexuality?
• What concerns or questions do you have about fulfilling your continuing sexual needs?
• In what ways has your sexual relationship with your partner changed as you have aged?
Many physicians have only a vague understanding of the sexual needs of the elderly, and some may even consider sexuality among elderly people a taboo.5 The reality is that elderly adults need to be touched, held, and feel loved, and this does not diminish with age.15-17 Unfortunately, many healthcare professionals have a mindset of, “I don’t want to think about my parents having sex, let alone my grandparents.” It is critical that physicians address intimacy needs as part of a medical assessment of the elderly.
Loss of physical and emotional intimacy is profound and often ignored as a source of suffering for the elderly. Most elderly patients want to discuss sexual issues with their physician, according to the Global Study of Sexual Attitudes among men and women ages 40 to 80 years.18 Surprisingly, even geriatricians often fail to take a sexual history of their patients. In one study, only 57% of 120 geriatricians surveyed routinely took a sexual history, even though 97% of them believed that patients with sexual problems should be managed further.1
Patient barriers
Even given a desire to discuss sexual concerns with their health care provider, elderly patients can be reluctant due to embarrassment or a fear of sexuality. Others may hesitate because their caregiver is younger than they or is of the opposite sex.19,20 The attitude of a medical professional has a powerful impact on the sexual attitudes and behaviors of elderly patients, and on their level of comfort in discussing sexual issues.21 Elderly patients do not usually complain to their physicians about sexual dysfunctions; 92% of men and 96% of women who reported at least one sexual problem in a survey had not sought help at all.18
Addressing issues in sexual dysfunction
Though sexual desires and needs may not decline with age, sexual function might, for any number of reasons.1,2,7 Many chronic diseases are known to interfere with sexual function (TABLE).2 Polypharmacy can lead to physical challenges, cognitive changes, and impaired sexual arousal, especially in men.3 However, the reason cited most often for absence of sexual activity is lack of a partner or a willing partner.2 Unfortunately as one ages, the chance of finding a partner diminishes. Hence the need to discuss alternative expressions of sexuality that may not require a partner.3 Many elderly individuals enjoy masturbation as a form of sexual expression.
Men and women have different sexual problems, but they are all treatable. For instance, with normal aging, levels of testosterone in men and estrogen in women decrease.5,15 Despite the number of sexual health dysfunctions, only 14% of men and 1% of women use medications to treat them.2,5 With men who have erectile dysfunction, discuss possible testosterone replacement or medication. For women with postmenopausal (atrophic) vaginitis, estrogen therapy or a lubricant (for those with contraindication to estrogen therapy) can improve sexual function. Anorgasmia and low libido are other concerns for postmenopausal women, and may warrant gynecologic referral.
For elderly adults moving into assisted living or a nursing home, the transition can signal the end of a sexual life.16,22 There is limited opportunity for men and women in residential settings to engage in sexual activity, in part due to a lack of privacy.23 The nursing home is still a home, and facility staff should provide opportunities for privacy and intimacy. In a study conducted in a residential setting, more than 25% of those ages 65 to 85 reported an active sex life, while 90% of those surveyed had sexual thoughts and fantasies.22 Of course, many elderly adults enter residential settings without a partner. They should be allowed to engage in sexual activities if they can understand, consent to, and form a relationship. Sexual needs remain even in those with dementia. But cognitive impairment frequently manifests as inappropriate sexual behavior. A study of cognitively impaired older adults revealed that 1.8% had displayed sexually inappropriate verbal or physical behavior.24 In these situations, a behavior medicine specialist can be of great help.
Health risks of sexual activity in the elderly
In 2011, the Centers for Disease Control and Prevention reported that 5% of new human immunodeficiency virus (HIV) cases occurred in those ≥55 years, and almost 2% of new diagnoses were in the those ≥65 years.25 Sexually active elderly individuals are at risk for acquiring HIV, in part because they do not consider themselves to be at risk for sexually transmitted diseases (STDs).26 They also might not have received education about the importance of condom use.11,26 In addition, prescribing erectile dysfunction medications for men and hormone replacement therapy for women might have played a part in increasing STDs among the elderly, particularly Chlamydia and HIV.27 The long-term effects of STDs left untreated can easily be mistaken for other symptoms or diseases of aging, which further underscores the importance of discussing sexuality with elderly patients.
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; [email protected]
1. Balami JS. Are geriatricians guilty of failure to take a sexual history? J Clin Gerontol Geriatr. 2011;2:17-20.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
3. Bradford A, Meston CM. Senior sexual health: The effects of aging on sexuality. In: VandeCreek L, Petersen FL, Bley JW, eds. Innovations in Clinical Practice: Focus on Sexual Health. Sarasota, FL: Professional Resource Press; 2007:35-45.
4. Graber C. Sex keeps elderly happier in marriage. Scientific American.
Available at: http://www.scientificamerican.com/podcast/episode/sex-keeps-elderly-happier-in-marria-11-11-29. Accessed March 26, 2014.
5. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
6. Tobin JM, Harindra V. Attendance by older patients at a genitourinary medicine clinic. Sex Transm Infect. 2001;77:289-291.
7. Bauer M, McAuliffe L, Nay R. Sexuality, health care and the older person: an overview of the literature. Int J Older People Nurs. 2007;2:63-68.
8. Wallace SP, Cochran SD, Durazo EM, et al. The health of aging lesbian, gay and bisexual adults in California. Policy Brief UCLA Cent Health Policy Res. 2011;(PB2011-2):1-8.
9. Henry J, McNab W. Forever young: a health promotion focus on sexuality and aging. Gerontol Geriatr Education. 2003;23:57-74.
10. Gott M, Hinchliff S, Galena E. General practitioner attitudes to discussing sexual health issues with older people. Soc Sci Med. 2004;58:2093-2103.
11. Nusbaum MR, Hamilton CD. The proactive sexual health history. Am Fam Physician. 2002;66:1705-1712.
12. Burd ID, Nevadunsky N, Bachmann G. Impact of physician gender on sexual history taking in a multispecialty practice. J Sex Med. 2006;3:194-200.
13. Kazer MW. Sexuality Assessment for Older Adults. Hartford Institute for Geriatric Nursing Web site. Available at: http://consultgerirn.org/uploads/File/trythis/try_this_10.pdf. Updated 2012. Accessed March 14, 2014.
14. Wallace MA. Assessment of sexual health in older adults. Am J Nursing. 2012;108:52-60.
15. Sexuality in later life. National Institute on Aging Web site. Available at: http://www.nia.nih.gov/health/publication/sexualitylater-life. Updated March 11, 2014. Accessed March 21, 2014.
16. Hajjar RR, Kamel HK. Sexuality in the nursing home, part 1: attitudes and barriers to sexual expression. J Am Med Dir Assoc. 2004;5(2 suppl):S42-S47.
17. Bildtgård T. The sexuality of elderly people on film—visual limitations. J Aging Identity. 2000;5:169-183.
18. Moreira ED Jr, Brock G, Glasser DB, et al; GSSAB Investigators’ Group. Help-seeking behaviour for sexual problems: the global study of sexual attitudes and behaviors. Int J Clin Pract. 2005;59:6-16.
19. Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract. 2003;20:690-695.
20. Politi MC, Clark MA, Armstrong G, et al. Patient-provider communication about sexual health among unmarried middle-aged and older women. J Gen Intern Med. 2009;24:511-516.
21. Bouman W, Arcelus J, Benbow S. Nottingham study of sexuality & ageing (NoSSA I). Attitudes regarding sexuality and older people: a review of the literature. Sex Relationship Ther. 2006;21:149-161.
22. Low LPL, Lui MHL, Lee DTF, et al. Promoting awareness of sexuality of older people in residential care. Electronic J Human Sexuality. 2005;8:8-16.
23. Rheaume C, Mitty E. Sexuality and intimacy in older adults. Geriatr Nurs. 2008;29:342-349.
24. Nagaratnam N, Gayagay G Jr. Hypersexuality in nursing care facilities—a descriptive study. Arch Gerontol Geriatr. 2002;35:195-203.
25. HIV among older Americans. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/risk/age/olderamericans/. Updated December 23, 2013. Accessed February 28, 2014.
26. Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453-472.
27. Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
› Keep in mind that elderly patients may want to discuss matters of sexuality but can also be embarrassed, fearful, or reluctant to do so with a younger caregiver. C
› Consider making a patient’s sexual history part of your general health screening, perhaps using the PLISSIT model for facilitating discussion. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sexuality is a central aspect of being human. It encompasses sex, gender identities and roles, sexual orientation, pleasure, eroticism, and intimacy, and is a major contributor to an individual’s quality of life and sense of wellbeing.1,2 Positive sexual relationships and behaviors are integral to maintaining good health and general well-being later in life, as well.2,3 Cynthia Graber, a reporter with Scientific American, reported that sex is a key reason retirees have a happy life.4
While there is a decline in sexual activity with age, a great number of men and women continue to engage in vaginal or anal intercourse, oral sex, and masturbation into the eighth and ninth decades of life.2,5 In a survey conducted among married men and women, about 90% of respondents between the ages of 60 and 64 and almost 30% of those older than age 80 said they were still sexually active.2 Another study reported that 62% of men and 30% of women 80 to 102 years of age were still sexually active.6 However, sexuality is rarely discussed with the elderly, and most physicians are unsure about how to handle such conversations.7
The baby boomer population is aging in the United States and elsewhere. By 2030, 20% of the US population will be ≥65 years old, and 4% (3 million) will be lesbian, gay, bisexual, transgender, and queer (LGBTQ) elderly adults.3,8 Given the impact of sex on maintaining quality of life, it is important for health care providers to be comfortable discussing sexuality with the elderly.9
Barriers to discussing sexuality
Physician barriers
Primary care physicians typically are the first point of contact for elderly adults experiencing health problems, including sexual dysfunction. According to the American Psychological Association, sex is not discussed enough with the elderly. Most physicians do not address sexual health proactively, and rarely do they include a sexual history as part of general health screening in the elderly.2,10,11 Inadequate training of physicians in sexual health is likely a contributing factor.5 Physicians also often feel discomfort when discussing such matters with patients of the opposite sex.12 (For a suggested approach to these conversations, see “Discussing sexuality with elderly patients: Getting beyond ‘don’t ask, don’t tell,” below.) With the increasing number of LGBTQ elderly adults, physicians should not assume their patients have any particular sexual behavior or orientation. This will help elderly LGBTQ patients feel more comfortable discussing their sexual health needs.8
The PLISSIT model, developed in 1976 by clinical psychologist Dr. Jack Annon, can facilitate a discussion of sexuality with elderly patients.11,13 First, the healthcare provider seeks permission (P) to discuss sexuality with the patient. After permission is given, the provider can share limited information (LI) about sexual issues that affect the older adult. Next, the provider may offer specific suggestions (SS) to improve sexual health or resolve problems. Finally, referral for intensive therapy (IT) may be needed for someone whose sexual dysfunction goes beyond the scope of the health care provider’s expertise. In 2000, open-ended questions were added to the PLISSIT model to more effectively guide an assessment of sexuality in older adults13,14:
• Can you tell me how you express your sexuality?
• What concerns or questions do you have about fulfilling your continuing sexual needs?
• In what ways has your sexual relationship with your partner changed as you have aged?
Many physicians have only a vague understanding of the sexual needs of the elderly, and some may even consider sexuality among elderly people a taboo.5 The reality is that elderly adults need to be touched, held, and feel loved, and this does not diminish with age.15-17 Unfortunately, many healthcare professionals have a mindset of, “I don’t want to think about my parents having sex, let alone my grandparents.” It is critical that physicians address intimacy needs as part of a medical assessment of the elderly.
Loss of physical and emotional intimacy is profound and often ignored as a source of suffering for the elderly. Most elderly patients want to discuss sexual issues with their physician, according to the Global Study of Sexual Attitudes among men and women ages 40 to 80 years.18 Surprisingly, even geriatricians often fail to take a sexual history of their patients. In one study, only 57% of 120 geriatricians surveyed routinely took a sexual history, even though 97% of them believed that patients with sexual problems should be managed further.1
Patient barriers
Even given a desire to discuss sexual concerns with their health care provider, elderly patients can be reluctant due to embarrassment or a fear of sexuality. Others may hesitate because their caregiver is younger than they or is of the opposite sex.19,20 The attitude of a medical professional has a powerful impact on the sexual attitudes and behaviors of elderly patients, and on their level of comfort in discussing sexual issues.21 Elderly patients do not usually complain to their physicians about sexual dysfunctions; 92% of men and 96% of women who reported at least one sexual problem in a survey had not sought help at all.18
Addressing issues in sexual dysfunction
Though sexual desires and needs may not decline with age, sexual function might, for any number of reasons.1,2,7 Many chronic diseases are known to interfere with sexual function (TABLE).2 Polypharmacy can lead to physical challenges, cognitive changes, and impaired sexual arousal, especially in men.3 However, the reason cited most often for absence of sexual activity is lack of a partner or a willing partner.2 Unfortunately as one ages, the chance of finding a partner diminishes. Hence the need to discuss alternative expressions of sexuality that may not require a partner.3 Many elderly individuals enjoy masturbation as a form of sexual expression.
Men and women have different sexual problems, but they are all treatable. For instance, with normal aging, levels of testosterone in men and estrogen in women decrease.5,15 Despite the number of sexual health dysfunctions, only 14% of men and 1% of women use medications to treat them.2,5 With men who have erectile dysfunction, discuss possible testosterone replacement or medication. For women with postmenopausal (atrophic) vaginitis, estrogen therapy or a lubricant (for those with contraindication to estrogen therapy) can improve sexual function. Anorgasmia and low libido are other concerns for postmenopausal women, and may warrant gynecologic referral.
For elderly adults moving into assisted living or a nursing home, the transition can signal the end of a sexual life.16,22 There is limited opportunity for men and women in residential settings to engage in sexual activity, in part due to a lack of privacy.23 The nursing home is still a home, and facility staff should provide opportunities for privacy and intimacy. In a study conducted in a residential setting, more than 25% of those ages 65 to 85 reported an active sex life, while 90% of those surveyed had sexual thoughts and fantasies.22 Of course, many elderly adults enter residential settings without a partner. They should be allowed to engage in sexual activities if they can understand, consent to, and form a relationship. Sexual needs remain even in those with dementia. But cognitive impairment frequently manifests as inappropriate sexual behavior. A study of cognitively impaired older adults revealed that 1.8% had displayed sexually inappropriate verbal or physical behavior.24 In these situations, a behavior medicine specialist can be of great help.
Health risks of sexual activity in the elderly
In 2011, the Centers for Disease Control and Prevention reported that 5% of new human immunodeficiency virus (HIV) cases occurred in those ≥55 years, and almost 2% of new diagnoses were in the those ≥65 years.25 Sexually active elderly individuals are at risk for acquiring HIV, in part because they do not consider themselves to be at risk for sexually transmitted diseases (STDs).26 They also might not have received education about the importance of condom use.11,26 In addition, prescribing erectile dysfunction medications for men and hormone replacement therapy for women might have played a part in increasing STDs among the elderly, particularly Chlamydia and HIV.27 The long-term effects of STDs left untreated can easily be mistaken for other symptoms or diseases of aging, which further underscores the importance of discussing sexuality with elderly patients.
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; [email protected]
› Keep in mind that elderly patients may want to discuss matters of sexuality but can also be embarrassed, fearful, or reluctant to do so with a younger caregiver. C
› Consider making a patient’s sexual history part of your general health screening, perhaps using the PLISSIT model for facilitating discussion. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sexuality is a central aspect of being human. It encompasses sex, gender identities and roles, sexual orientation, pleasure, eroticism, and intimacy, and is a major contributor to an individual’s quality of life and sense of wellbeing.1,2 Positive sexual relationships and behaviors are integral to maintaining good health and general well-being later in life, as well.2,3 Cynthia Graber, a reporter with Scientific American, reported that sex is a key reason retirees have a happy life.4
While there is a decline in sexual activity with age, a great number of men and women continue to engage in vaginal or anal intercourse, oral sex, and masturbation into the eighth and ninth decades of life.2,5 In a survey conducted among married men and women, about 90% of respondents between the ages of 60 and 64 and almost 30% of those older than age 80 said they were still sexually active.2 Another study reported that 62% of men and 30% of women 80 to 102 years of age were still sexually active.6 However, sexuality is rarely discussed with the elderly, and most physicians are unsure about how to handle such conversations.7
The baby boomer population is aging in the United States and elsewhere. By 2030, 20% of the US population will be ≥65 years old, and 4% (3 million) will be lesbian, gay, bisexual, transgender, and queer (LGBTQ) elderly adults.3,8 Given the impact of sex on maintaining quality of life, it is important for health care providers to be comfortable discussing sexuality with the elderly.9
Barriers to discussing sexuality
Physician barriers
Primary care physicians typically are the first point of contact for elderly adults experiencing health problems, including sexual dysfunction. According to the American Psychological Association, sex is not discussed enough with the elderly. Most physicians do not address sexual health proactively, and rarely do they include a sexual history as part of general health screening in the elderly.2,10,11 Inadequate training of physicians in sexual health is likely a contributing factor.5 Physicians also often feel discomfort when discussing such matters with patients of the opposite sex.12 (For a suggested approach to these conversations, see “Discussing sexuality with elderly patients: Getting beyond ‘don’t ask, don’t tell,” below.) With the increasing number of LGBTQ elderly adults, physicians should not assume their patients have any particular sexual behavior or orientation. This will help elderly LGBTQ patients feel more comfortable discussing their sexual health needs.8
The PLISSIT model, developed in 1976 by clinical psychologist Dr. Jack Annon, can facilitate a discussion of sexuality with elderly patients.11,13 First, the healthcare provider seeks permission (P) to discuss sexuality with the patient. After permission is given, the provider can share limited information (LI) about sexual issues that affect the older adult. Next, the provider may offer specific suggestions (SS) to improve sexual health or resolve problems. Finally, referral for intensive therapy (IT) may be needed for someone whose sexual dysfunction goes beyond the scope of the health care provider’s expertise. In 2000, open-ended questions were added to the PLISSIT model to more effectively guide an assessment of sexuality in older adults13,14:
• Can you tell me how you express your sexuality?
• What concerns or questions do you have about fulfilling your continuing sexual needs?
• In what ways has your sexual relationship with your partner changed as you have aged?
Many physicians have only a vague understanding of the sexual needs of the elderly, and some may even consider sexuality among elderly people a taboo.5 The reality is that elderly adults need to be touched, held, and feel loved, and this does not diminish with age.15-17 Unfortunately, many healthcare professionals have a mindset of, “I don’t want to think about my parents having sex, let alone my grandparents.” It is critical that physicians address intimacy needs as part of a medical assessment of the elderly.
Loss of physical and emotional intimacy is profound and often ignored as a source of suffering for the elderly. Most elderly patients want to discuss sexual issues with their physician, according to the Global Study of Sexual Attitudes among men and women ages 40 to 80 years.18 Surprisingly, even geriatricians often fail to take a sexual history of their patients. In one study, only 57% of 120 geriatricians surveyed routinely took a sexual history, even though 97% of them believed that patients with sexual problems should be managed further.1
Patient barriers
Even given a desire to discuss sexual concerns with their health care provider, elderly patients can be reluctant due to embarrassment or a fear of sexuality. Others may hesitate because their caregiver is younger than they or is of the opposite sex.19,20 The attitude of a medical professional has a powerful impact on the sexual attitudes and behaviors of elderly patients, and on their level of comfort in discussing sexual issues.21 Elderly patients do not usually complain to their physicians about sexual dysfunctions; 92% of men and 96% of women who reported at least one sexual problem in a survey had not sought help at all.18
Addressing issues in sexual dysfunction
Though sexual desires and needs may not decline with age, sexual function might, for any number of reasons.1,2,7 Many chronic diseases are known to interfere with sexual function (TABLE).2 Polypharmacy can lead to physical challenges, cognitive changes, and impaired sexual arousal, especially in men.3 However, the reason cited most often for absence of sexual activity is lack of a partner or a willing partner.2 Unfortunately as one ages, the chance of finding a partner diminishes. Hence the need to discuss alternative expressions of sexuality that may not require a partner.3 Many elderly individuals enjoy masturbation as a form of sexual expression.
Men and women have different sexual problems, but they are all treatable. For instance, with normal aging, levels of testosterone in men and estrogen in women decrease.5,15 Despite the number of sexual health dysfunctions, only 14% of men and 1% of women use medications to treat them.2,5 With men who have erectile dysfunction, discuss possible testosterone replacement or medication. For women with postmenopausal (atrophic) vaginitis, estrogen therapy or a lubricant (for those with contraindication to estrogen therapy) can improve sexual function. Anorgasmia and low libido are other concerns for postmenopausal women, and may warrant gynecologic referral.
For elderly adults moving into assisted living or a nursing home, the transition can signal the end of a sexual life.16,22 There is limited opportunity for men and women in residential settings to engage in sexual activity, in part due to a lack of privacy.23 The nursing home is still a home, and facility staff should provide opportunities for privacy and intimacy. In a study conducted in a residential setting, more than 25% of those ages 65 to 85 reported an active sex life, while 90% of those surveyed had sexual thoughts and fantasies.22 Of course, many elderly adults enter residential settings without a partner. They should be allowed to engage in sexual activities if they can understand, consent to, and form a relationship. Sexual needs remain even in those with dementia. But cognitive impairment frequently manifests as inappropriate sexual behavior. A study of cognitively impaired older adults revealed that 1.8% had displayed sexually inappropriate verbal or physical behavior.24 In these situations, a behavior medicine specialist can be of great help.
Health risks of sexual activity in the elderly
In 2011, the Centers for Disease Control and Prevention reported that 5% of new human immunodeficiency virus (HIV) cases occurred in those ≥55 years, and almost 2% of new diagnoses were in the those ≥65 years.25 Sexually active elderly individuals are at risk for acquiring HIV, in part because they do not consider themselves to be at risk for sexually transmitted diseases (STDs).26 They also might not have received education about the importance of condom use.11,26 In addition, prescribing erectile dysfunction medications for men and hormone replacement therapy for women might have played a part in increasing STDs among the elderly, particularly Chlamydia and HIV.27 The long-term effects of STDs left untreated can easily be mistaken for other symptoms or diseases of aging, which further underscores the importance of discussing sexuality with elderly patients.
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; [email protected]
1. Balami JS. Are geriatricians guilty of failure to take a sexual history? J Clin Gerontol Geriatr. 2011;2:17-20.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
3. Bradford A, Meston CM. Senior sexual health: The effects of aging on sexuality. In: VandeCreek L, Petersen FL, Bley JW, eds. Innovations in Clinical Practice: Focus on Sexual Health. Sarasota, FL: Professional Resource Press; 2007:35-45.
4. Graber C. Sex keeps elderly happier in marriage. Scientific American.
Available at: http://www.scientificamerican.com/podcast/episode/sex-keeps-elderly-happier-in-marria-11-11-29. Accessed March 26, 2014.
5. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
6. Tobin JM, Harindra V. Attendance by older patients at a genitourinary medicine clinic. Sex Transm Infect. 2001;77:289-291.
7. Bauer M, McAuliffe L, Nay R. Sexuality, health care and the older person: an overview of the literature. Int J Older People Nurs. 2007;2:63-68.
8. Wallace SP, Cochran SD, Durazo EM, et al. The health of aging lesbian, gay and bisexual adults in California. Policy Brief UCLA Cent Health Policy Res. 2011;(PB2011-2):1-8.
9. Henry J, McNab W. Forever young: a health promotion focus on sexuality and aging. Gerontol Geriatr Education. 2003;23:57-74.
10. Gott M, Hinchliff S, Galena E. General practitioner attitudes to discussing sexual health issues with older people. Soc Sci Med. 2004;58:2093-2103.
11. Nusbaum MR, Hamilton CD. The proactive sexual health history. Am Fam Physician. 2002;66:1705-1712.
12. Burd ID, Nevadunsky N, Bachmann G. Impact of physician gender on sexual history taking in a multispecialty practice. J Sex Med. 2006;3:194-200.
13. Kazer MW. Sexuality Assessment for Older Adults. Hartford Institute for Geriatric Nursing Web site. Available at: http://consultgerirn.org/uploads/File/trythis/try_this_10.pdf. Updated 2012. Accessed March 14, 2014.
14. Wallace MA. Assessment of sexual health in older adults. Am J Nursing. 2012;108:52-60.
15. Sexuality in later life. National Institute on Aging Web site. Available at: http://www.nia.nih.gov/health/publication/sexualitylater-life. Updated March 11, 2014. Accessed March 21, 2014.
16. Hajjar RR, Kamel HK. Sexuality in the nursing home, part 1: attitudes and barriers to sexual expression. J Am Med Dir Assoc. 2004;5(2 suppl):S42-S47.
17. Bildtgård T. The sexuality of elderly people on film—visual limitations. J Aging Identity. 2000;5:169-183.
18. Moreira ED Jr, Brock G, Glasser DB, et al; GSSAB Investigators’ Group. Help-seeking behaviour for sexual problems: the global study of sexual attitudes and behaviors. Int J Clin Pract. 2005;59:6-16.
19. Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract. 2003;20:690-695.
20. Politi MC, Clark MA, Armstrong G, et al. Patient-provider communication about sexual health among unmarried middle-aged and older women. J Gen Intern Med. 2009;24:511-516.
21. Bouman W, Arcelus J, Benbow S. Nottingham study of sexuality & ageing (NoSSA I). Attitudes regarding sexuality and older people: a review of the literature. Sex Relationship Ther. 2006;21:149-161.
22. Low LPL, Lui MHL, Lee DTF, et al. Promoting awareness of sexuality of older people in residential care. Electronic J Human Sexuality. 2005;8:8-16.
23. Rheaume C, Mitty E. Sexuality and intimacy in older adults. Geriatr Nurs. 2008;29:342-349.
24. Nagaratnam N, Gayagay G Jr. Hypersexuality in nursing care facilities—a descriptive study. Arch Gerontol Geriatr. 2002;35:195-203.
25. HIV among older Americans. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/risk/age/olderamericans/. Updated December 23, 2013. Accessed February 28, 2014.
26. Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453-472.
27. Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
1. Balami JS. Are geriatricians guilty of failure to take a sexual history? J Clin Gerontol Geriatr. 2011;2:17-20.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
3. Bradford A, Meston CM. Senior sexual health: The effects of aging on sexuality. In: VandeCreek L, Petersen FL, Bley JW, eds. Innovations in Clinical Practice: Focus on Sexual Health. Sarasota, FL: Professional Resource Press; 2007:35-45.
4. Graber C. Sex keeps elderly happier in marriage. Scientific American.
Available at: http://www.scientificamerican.com/podcast/episode/sex-keeps-elderly-happier-in-marria-11-11-29. Accessed March 26, 2014.
5. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
6. Tobin JM, Harindra V. Attendance by older patients at a genitourinary medicine clinic. Sex Transm Infect. 2001;77:289-291.
7. Bauer M, McAuliffe L, Nay R. Sexuality, health care and the older person: an overview of the literature. Int J Older People Nurs. 2007;2:63-68.
8. Wallace SP, Cochran SD, Durazo EM, et al. The health of aging lesbian, gay and bisexual adults in California. Policy Brief UCLA Cent Health Policy Res. 2011;(PB2011-2):1-8.
9. Henry J, McNab W. Forever young: a health promotion focus on sexuality and aging. Gerontol Geriatr Education. 2003;23:57-74.
10. Gott M, Hinchliff S, Galena E. General practitioner attitudes to discussing sexual health issues with older people. Soc Sci Med. 2004;58:2093-2103.
11. Nusbaum MR, Hamilton CD. The proactive sexual health history. Am Fam Physician. 2002;66:1705-1712.
12. Burd ID, Nevadunsky N, Bachmann G. Impact of physician gender on sexual history taking in a multispecialty practice. J Sex Med. 2006;3:194-200.
13. Kazer MW. Sexuality Assessment for Older Adults. Hartford Institute for Geriatric Nursing Web site. Available at: http://consultgerirn.org/uploads/File/trythis/try_this_10.pdf. Updated 2012. Accessed March 14, 2014.
14. Wallace MA. Assessment of sexual health in older adults. Am J Nursing. 2012;108:52-60.
15. Sexuality in later life. National Institute on Aging Web site. Available at: http://www.nia.nih.gov/health/publication/sexualitylater-life. Updated March 11, 2014. Accessed March 21, 2014.
16. Hajjar RR, Kamel HK. Sexuality in the nursing home, part 1: attitudes and barriers to sexual expression. J Am Med Dir Assoc. 2004;5(2 suppl):S42-S47.
17. Bildtgård T. The sexuality of elderly people on film—visual limitations. J Aging Identity. 2000;5:169-183.
18. Moreira ED Jr, Brock G, Glasser DB, et al; GSSAB Investigators’ Group. Help-seeking behaviour for sexual problems: the global study of sexual attitudes and behaviors. Int J Clin Pract. 2005;59:6-16.
19. Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract. 2003;20:690-695.
20. Politi MC, Clark MA, Armstrong G, et al. Patient-provider communication about sexual health among unmarried middle-aged and older women. J Gen Intern Med. 2009;24:511-516.
21. Bouman W, Arcelus J, Benbow S. Nottingham study of sexuality & ageing (NoSSA I). Attitudes regarding sexuality and older people: a review of the literature. Sex Relationship Ther. 2006;21:149-161.
22. Low LPL, Lui MHL, Lee DTF, et al. Promoting awareness of sexuality of older people in residential care. Electronic J Human Sexuality. 2005;8:8-16.
23. Rheaume C, Mitty E. Sexuality and intimacy in older adults. Geriatr Nurs. 2008;29:342-349.
24. Nagaratnam N, Gayagay G Jr. Hypersexuality in nursing care facilities—a descriptive study. Arch Gerontol Geriatr. 2002;35:195-203.
25. HIV among older Americans. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/risk/age/olderamericans/. Updated December 23, 2013. Accessed February 28, 2014.
26. Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453-472.
27. Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.