User login
Cardiovascular Disease and Risk of Hip Fracture
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673.
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673.
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673.
OTC Analgesics Not Associated with Acute Decompensation in Cirrhotic Patients
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Modified IV Acetylcysteine Infusion Reduces Adverse Effects
Clinical question: Does a shorter regimen of IV acetylcysteine reduce adverse effects compared to the standard regimen?
Background: Acetaminophen poisoning is common, and recommended treatment is IV acetylcysteine; however, the standard regimen has many adverse effects, including vomiting and anaphylactoid reactions. Although studies have outlined these side effects, no published trials have compared their frequency to that of a shorter protocol.
Study design: Double-blinded, randomized controlled trial.
Setting: Three acute care hospitals in the United Kingdom.
Synopsis: Of 3,311 patients who presented with acetaminophen overdose, 222 underwent randomization to the standard (duration 20-25 hours) or modified (12 hours) acetylcysteine regimen, with or without pre-treatment with IV ondansetron 4 mg. The primary outcome of vomiting, retching, or need for rescue antiemetic treatment within two hours of acetylcysteine initiation was significantly less frequent in patients who received the shorter regimen, compared to those allocated to the standard regimen.
Specifically, the adjusted odds ratio was 0.26 with the modified regimen (97.5% CI, 0.13-0.52; P<0.0001). The primary outcome was significantly less in patients pre-treated with ondansetron compared to placebo (OR 0.41, 97.5% CI 0.2-0.8; P=0.003). Anaphylactic reactions were significantly reduced with the shorter protocol; no significant difference in hepatotoxicity was noted.
It is reasonable to infer that the shorter acetylcysteine regimen substantially reduces the frequency of vomiting and serious anaphylactoid reactions when compared with the standard schedule; however, hospitalists should note that this study was not powered to assess for non-inferiority of the shorter regimen with regard to prevention of acetaminophen’s hepatotoxic effects. Further studies are needed to confirm the efficacy and safety of the modified regimen before widespread adoption into clinical practice.
Bottom line: A shorter acetylcysteine regimen is associated with decreased occurrence of vomiting and anaphylactoid reactions compared to the standard protocol for treating acetaminophen toxicity. Additional research is needed to assess non-inferiority of this modified regimen for prevention of hepatotoxic effects.
Citation: Bateman DN, Dear JW, Thanacoody HK, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomized controlled trial. Lancet. 2014;383(9918):697-704.
Clinical question: Does a shorter regimen of IV acetylcysteine reduce adverse effects compared to the standard regimen?
Background: Acetaminophen poisoning is common, and recommended treatment is IV acetylcysteine; however, the standard regimen has many adverse effects, including vomiting and anaphylactoid reactions. Although studies have outlined these side effects, no published trials have compared their frequency to that of a shorter protocol.
Study design: Double-blinded, randomized controlled trial.
Setting: Three acute care hospitals in the United Kingdom.
Synopsis: Of 3,311 patients who presented with acetaminophen overdose, 222 underwent randomization to the standard (duration 20-25 hours) or modified (12 hours) acetylcysteine regimen, with or without pre-treatment with IV ondansetron 4 mg. The primary outcome of vomiting, retching, or need for rescue antiemetic treatment within two hours of acetylcysteine initiation was significantly less frequent in patients who received the shorter regimen, compared to those allocated to the standard regimen.
Specifically, the adjusted odds ratio was 0.26 with the modified regimen (97.5% CI, 0.13-0.52; P<0.0001). The primary outcome was significantly less in patients pre-treated with ondansetron compared to placebo (OR 0.41, 97.5% CI 0.2-0.8; P=0.003). Anaphylactic reactions were significantly reduced with the shorter protocol; no significant difference in hepatotoxicity was noted.
It is reasonable to infer that the shorter acetylcysteine regimen substantially reduces the frequency of vomiting and serious anaphylactoid reactions when compared with the standard schedule; however, hospitalists should note that this study was not powered to assess for non-inferiority of the shorter regimen with regard to prevention of acetaminophen’s hepatotoxic effects. Further studies are needed to confirm the efficacy and safety of the modified regimen before widespread adoption into clinical practice.
Bottom line: A shorter acetylcysteine regimen is associated with decreased occurrence of vomiting and anaphylactoid reactions compared to the standard protocol for treating acetaminophen toxicity. Additional research is needed to assess non-inferiority of this modified regimen for prevention of hepatotoxic effects.
Citation: Bateman DN, Dear JW, Thanacoody HK, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomized controlled trial. Lancet. 2014;383(9918):697-704.
Clinical question: Does a shorter regimen of IV acetylcysteine reduce adverse effects compared to the standard regimen?
Background: Acetaminophen poisoning is common, and recommended treatment is IV acetylcysteine; however, the standard regimen has many adverse effects, including vomiting and anaphylactoid reactions. Although studies have outlined these side effects, no published trials have compared their frequency to that of a shorter protocol.
Study design: Double-blinded, randomized controlled trial.
Setting: Three acute care hospitals in the United Kingdom.
Synopsis: Of 3,311 patients who presented with acetaminophen overdose, 222 underwent randomization to the standard (duration 20-25 hours) or modified (12 hours) acetylcysteine regimen, with or without pre-treatment with IV ondansetron 4 mg. The primary outcome of vomiting, retching, or need for rescue antiemetic treatment within two hours of acetylcysteine initiation was significantly less frequent in patients who received the shorter regimen, compared to those allocated to the standard regimen.
Specifically, the adjusted odds ratio was 0.26 with the modified regimen (97.5% CI, 0.13-0.52; P<0.0001). The primary outcome was significantly less in patients pre-treated with ondansetron compared to placebo (OR 0.41, 97.5% CI 0.2-0.8; P=0.003). Anaphylactic reactions were significantly reduced with the shorter protocol; no significant difference in hepatotoxicity was noted.
It is reasonable to infer that the shorter acetylcysteine regimen substantially reduces the frequency of vomiting and serious anaphylactoid reactions when compared with the standard schedule; however, hospitalists should note that this study was not powered to assess for non-inferiority of the shorter regimen with regard to prevention of acetaminophen’s hepatotoxic effects. Further studies are needed to confirm the efficacy and safety of the modified regimen before widespread adoption into clinical practice.
Bottom line: A shorter acetylcysteine regimen is associated with decreased occurrence of vomiting and anaphylactoid reactions compared to the standard protocol for treating acetaminophen toxicity. Additional research is needed to assess non-inferiority of this modified regimen for prevention of hepatotoxic effects.
Citation: Bateman DN, Dear JW, Thanacoody HK, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomized controlled trial. Lancet. 2014;383(9918):697-704.
No Mortality Benefit with Treatment of Single Peripheral Pulmonary Emboli
Clinical question: Does treatment of single peripheral pulmonary emboli impact mortality and rates of post-discharge venous thromboembolism (VTE)?
Background: With the increase in CT pulmonary angiography (CTPA) use the past decade, there has been an increased rate of detection of peripheral filling defects. When confronted with a single peripheral filling defect (SPFD), clinicians face the dilemma of whether treatment is necessary, given the risks associated with anticoagulation.
Study design: Retrospective cohort.
Setting: Community teaching hospital in Norwalk, Conn.
Synopsis: A total of 4,906 CTPAs were screened, revealing 153 scans with an SPFD. Primary analysis included 134 patients 18 years or older. Of these patients, 61 (45.5%) received treatment with anticoagulation (n=51) or IVC filter alone (n=10).
This study revealed no difference in adjusted 90-day mortality between treated and untreated groups. No statistically significant difference was found in the rate of post-discharge VTE within 90 days.
Characteristics associated with treatment for SPFD were patient immobility, previous VTE, and radiology labeling the filling defect as a pulmonary embolus. It is important to note that none of the patients who had a normal second imaging study (e.g. V/Q scan or ultrasound) were treated; therefore, the use of secondary studies could mitigate some of the uncertainty around SPFD management, though this is not recommended in current diagnostic algorithms. Because this is a single-center study with a modest sample size, the comparability of findings to other centers might be limited. Larger studies are needed to help clarify these findings.
Bottom line: Treatment of SPFD was not associated with a difference in mortality or post-discharge VTE within 90 days.
Citation: Green O, Lempel J, Kolodziej A, et al. Treatment of single peripheral pulmonary emboli: patient outcomes and factors associated with decision to treat. J Hosp Med. 2014;9(1):42-47.
Clinical question: Does treatment of single peripheral pulmonary emboli impact mortality and rates of post-discharge venous thromboembolism (VTE)?
Background: With the increase in CT pulmonary angiography (CTPA) use the past decade, there has been an increased rate of detection of peripheral filling defects. When confronted with a single peripheral filling defect (SPFD), clinicians face the dilemma of whether treatment is necessary, given the risks associated with anticoagulation.
Study design: Retrospective cohort.
Setting: Community teaching hospital in Norwalk, Conn.
Synopsis: A total of 4,906 CTPAs were screened, revealing 153 scans with an SPFD. Primary analysis included 134 patients 18 years or older. Of these patients, 61 (45.5%) received treatment with anticoagulation (n=51) or IVC filter alone (n=10).
This study revealed no difference in adjusted 90-day mortality between treated and untreated groups. No statistically significant difference was found in the rate of post-discharge VTE within 90 days.
Characteristics associated with treatment for SPFD were patient immobility, previous VTE, and radiology labeling the filling defect as a pulmonary embolus. It is important to note that none of the patients who had a normal second imaging study (e.g. V/Q scan or ultrasound) were treated; therefore, the use of secondary studies could mitigate some of the uncertainty around SPFD management, though this is not recommended in current diagnostic algorithms. Because this is a single-center study with a modest sample size, the comparability of findings to other centers might be limited. Larger studies are needed to help clarify these findings.
Bottom line: Treatment of SPFD was not associated with a difference in mortality or post-discharge VTE within 90 days.
Citation: Green O, Lempel J, Kolodziej A, et al. Treatment of single peripheral pulmonary emboli: patient outcomes and factors associated with decision to treat. J Hosp Med. 2014;9(1):42-47.
Clinical question: Does treatment of single peripheral pulmonary emboli impact mortality and rates of post-discharge venous thromboembolism (VTE)?
Background: With the increase in CT pulmonary angiography (CTPA) use the past decade, there has been an increased rate of detection of peripheral filling defects. When confronted with a single peripheral filling defect (SPFD), clinicians face the dilemma of whether treatment is necessary, given the risks associated with anticoagulation.
Study design: Retrospective cohort.
Setting: Community teaching hospital in Norwalk, Conn.
Synopsis: A total of 4,906 CTPAs were screened, revealing 153 scans with an SPFD. Primary analysis included 134 patients 18 years or older. Of these patients, 61 (45.5%) received treatment with anticoagulation (n=51) or IVC filter alone (n=10).
This study revealed no difference in adjusted 90-day mortality between treated and untreated groups. No statistically significant difference was found in the rate of post-discharge VTE within 90 days.
Characteristics associated with treatment for SPFD were patient immobility, previous VTE, and radiology labeling the filling defect as a pulmonary embolus. It is important to note that none of the patients who had a normal second imaging study (e.g. V/Q scan or ultrasound) were treated; therefore, the use of secondary studies could mitigate some of the uncertainty around SPFD management, though this is not recommended in current diagnostic algorithms. Because this is a single-center study with a modest sample size, the comparability of findings to other centers might be limited. Larger studies are needed to help clarify these findings.
Bottom line: Treatment of SPFD was not associated with a difference in mortality or post-discharge VTE within 90 days.
Citation: Green O, Lempel J, Kolodziej A, et al. Treatment of single peripheral pulmonary emboli: patient outcomes and factors associated with decision to treat. J Hosp Med. 2014;9(1):42-47.
Healthcare-Associated Infections Continue to Impact the U.S. Healthcare System Financially
Clinical question: What is the estimated cost of healthcare-associated infections (HAI) to the U.S. healthcare system?
Background: In spite of education efforts, HAIs occur frequently and contribute to high healthcare costs in the U.S. This study sought to estimate the costs of HAIs to the U.S. system using statistical analyses of published data.
Study design: Simulations of published data.
Setting: Published studies on five major HAIs.
Synopsis: Monte Carlo simulations based upon published point estimates were used to estimate per-case cost and confidence intervals, with extrapolation to total costs to the U.S. healthcare system. Overall, five major HAIs occur approximately 440,000 times annually and cost the healthcare system an estimated $9.78 billion (range $8.28 to $11.5 billion) in 2009.
Surgical site infections (36.0%) were the most common of the studied HAIs, with increased per-case cost of $20,785, equating to an estimated $3.30 billion annually (33.7% of total HAI costs). Clostridium difficile infection accounted for 30.3% of HAI but only 15.4% of costs ($1.51 billion). Central line-associated bloodstream infections were most costly per case ($45,814), with total costs of $1.85 billion (18.9% of costs). Ventilator-associated pneumonia accounted for $3.09 billion, or 31.7% of total costs. Catheter-associated urinary tract infection only represented 0.3% of total costs, or $27.9 million annually.
The authors suggest that changes in payment reform likely will drive hospitals to further invest in HAI reduction efforts.
Bottom line: HAIs remain frequent and expensive complications of hospitalization, in spite of improvement efforts to date.
Citation: Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046.
Clinical question: What is the estimated cost of healthcare-associated infections (HAI) to the U.S. healthcare system?
Background: In spite of education efforts, HAIs occur frequently and contribute to high healthcare costs in the U.S. This study sought to estimate the costs of HAIs to the U.S. system using statistical analyses of published data.
Study design: Simulations of published data.
Setting: Published studies on five major HAIs.
Synopsis: Monte Carlo simulations based upon published point estimates were used to estimate per-case cost and confidence intervals, with extrapolation to total costs to the U.S. healthcare system. Overall, five major HAIs occur approximately 440,000 times annually and cost the healthcare system an estimated $9.78 billion (range $8.28 to $11.5 billion) in 2009.
Surgical site infections (36.0%) were the most common of the studied HAIs, with increased per-case cost of $20,785, equating to an estimated $3.30 billion annually (33.7% of total HAI costs). Clostridium difficile infection accounted for 30.3% of HAI but only 15.4% of costs ($1.51 billion). Central line-associated bloodstream infections were most costly per case ($45,814), with total costs of $1.85 billion (18.9% of costs). Ventilator-associated pneumonia accounted for $3.09 billion, or 31.7% of total costs. Catheter-associated urinary tract infection only represented 0.3% of total costs, or $27.9 million annually.
The authors suggest that changes in payment reform likely will drive hospitals to further invest in HAI reduction efforts.
Bottom line: HAIs remain frequent and expensive complications of hospitalization, in spite of improvement efforts to date.
Citation: Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046.
Clinical question: What is the estimated cost of healthcare-associated infections (HAI) to the U.S. healthcare system?
Background: In spite of education efforts, HAIs occur frequently and contribute to high healthcare costs in the U.S. This study sought to estimate the costs of HAIs to the U.S. system using statistical analyses of published data.
Study design: Simulations of published data.
Setting: Published studies on five major HAIs.
Synopsis: Monte Carlo simulations based upon published point estimates were used to estimate per-case cost and confidence intervals, with extrapolation to total costs to the U.S. healthcare system. Overall, five major HAIs occur approximately 440,000 times annually and cost the healthcare system an estimated $9.78 billion (range $8.28 to $11.5 billion) in 2009.
Surgical site infections (36.0%) were the most common of the studied HAIs, with increased per-case cost of $20,785, equating to an estimated $3.30 billion annually (33.7% of total HAI costs). Clostridium difficile infection accounted for 30.3% of HAI but only 15.4% of costs ($1.51 billion). Central line-associated bloodstream infections were most costly per case ($45,814), with total costs of $1.85 billion (18.9% of costs). Ventilator-associated pneumonia accounted for $3.09 billion, or 31.7% of total costs. Catheter-associated urinary tract infection only represented 0.3% of total costs, or $27.9 million annually.
The authors suggest that changes in payment reform likely will drive hospitals to further invest in HAI reduction efforts.
Bottom line: HAIs remain frequent and expensive complications of hospitalization, in spite of improvement efforts to date.
Citation: Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046.
Lower Perioperative Mortality with Endovascular Vs. Open Abdominal Aortic Aneurysm Repair
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
Discontinuation of Beta Blockers Increases Risk of Postoperative Myocardial Infarction and Death
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Cancer Guideline for VTE Prophylaxis for Inpatients and Long-Term Treatment With Low-Molecular-Weight Heparin for Acute VTE
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Trauma Patients with Pulmonary Embolism Might Not Have DVT on Imaging of Lower Extremities
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Genetics of Renal Disease: APOL1 Variations
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
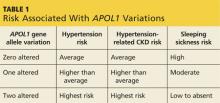
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.