User login
Ten tips for boosting patient communication
This transcript has been edited for clarity.
Here are 10 ways to improve health communication with patients.
No. 1: Be an active listener
The first tip is to be an active listener and help guide the history-taking process by asking for clarification when needed.
Quickly figure out the patient’s chief complaint. Which symptom is the most severe?
Ask them for symptom-modifying factors, such as onset, duration, frequency, and a pain description. Is the abdominal pain sharp or crampy, dull and achy, or pressure-like? What makes the symptoms better or worse?
As many of us were taught in medical school, 80% of the diagnosis is in a patient’s history and description.
No. 2: Ask questions that resonate with patients
What can we do to help elicit an accurate history from the patient when they’re not providing the needed information or being helpful enough?
The easiest way is to ask your patient in a completely different way but one that resonates with them. For instance, ask how the abdominal pain is affecting their quality of life. That will help focus the history taking and encourage the patient to share details.
Does the pain keep them awake at night? Are they able to eat a normal-sized meal? Or are they forced to eat tiny snacks? Is the pain interfering with work or school?
By providing a framework, the patient will be more passionate about sharing the details of their history.
No. 3: Help patients organize their story
Sometimes, patients provide details in a nonchronological order, jumping all over the place.
A super helpful technique is to explain to the patient that you have a story to write for your computer note, and for them to think back to when they first started noticing their abdominal pain or rectal bleeding symptoms. When were the most-severe episodes? How frequent are the episodes? What’s the volume of their rectal bleeding?
If the patient realizes that you’re trying to write a story synopsis, they will provide information in a much more organized way.
No. 4: Determine patient’s language preference
Quickly determine the patient’s language preference. We want patients to feel extremely comfortable.
Whenever possible, use a certified interpreter. Language phone lines, in-person interpreters, and video conferencing are widely available today. It’s worth investing in this technology so that we can provide the best possible care to immigrants and refugees.
Conversely, avoid using family members as interpreters because they may not be adequately trained in medical vocabulary.
No. 5: Use simple language
When providing explanations, use simple language that your patient can understand and identify with.
For example, use analogies like “the heart is a pump” or the diverticula are thin areas of the colon that can bleed if the blood vessel is too close to the surface.
No. 6: Determine level of medical literacy
Determine your patient’s level of medical literacy. Some of our patients did not graduate from high school. Some patients can’t read very well. Therefore, your discharge instructions and handouts should sometimes be written on a third-grade level.
If patients can’t read, write medication instructions with symbols. Draw a sun for medications that are supposed to be taken in the morning and draw a moon if a medication is supposed to be taken at night.
Always very carefully review the instructions with the patient.
No. 7: Check in with the patient
During the visit, frequently check in with the patient to make sure that they understand what you’re asking or what you’re trying to explain to them.
No. 8: Include family member as patient advocate
If the patient is accompanied by a family member, help them serve in the important role as a patient advocate.
If the family member wants to take notes, encourage them because that provides an awesome value.
Sometimes patients can forget clinic and hospital medical conversations, and that family member might be the key to improving your patient’s health.
No. 9: Follow-up with the patient
If your clinic has the capability, follow up with a patient the next day to make sure that they understood everything.
Check to make sure the patient was able to pick up all of the medications that you prescribed.
Check that laboratory tests are arranged or completed.
Check that important procedures, such as esophagogastroduodenoscopy and colonoscopy, and imaging, such as ultrasounds and CTs, are scheduled.
No. 10: Identify barriers to care
Have fun talking with a patient. Find out what they do for a living. Build a rapport. Listen to their stressors in life.
Try to identify any barriers to care or external stressors, like taking care of a sick parent, which might interfere with their scheduling an important diagnostic colonoscopy for rectal bleeding.
Good luck incorporating these communication strategies into your clinic and hospital work. Together, we can help improve the delivery of health care.
Dr. Levy is a gastroenterologist at the University of Chicago. In 2017, Dr. Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Dr. Levy, who recently gave a TEDx Talk about building health education campaigns using music and concerts, organizes Tune It Up: A Concert To Raise Colorectal Cancer Awareness with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Here are 10 ways to improve health communication with patients.
No. 1: Be an active listener
The first tip is to be an active listener and help guide the history-taking process by asking for clarification when needed.
Quickly figure out the patient’s chief complaint. Which symptom is the most severe?
Ask them for symptom-modifying factors, such as onset, duration, frequency, and a pain description. Is the abdominal pain sharp or crampy, dull and achy, or pressure-like? What makes the symptoms better or worse?
As many of us were taught in medical school, 80% of the diagnosis is in a patient’s history and description.
No. 2: Ask questions that resonate with patients
What can we do to help elicit an accurate history from the patient when they’re not providing the needed information or being helpful enough?
The easiest way is to ask your patient in a completely different way but one that resonates with them. For instance, ask how the abdominal pain is affecting their quality of life. That will help focus the history taking and encourage the patient to share details.
Does the pain keep them awake at night? Are they able to eat a normal-sized meal? Or are they forced to eat tiny snacks? Is the pain interfering with work or school?
By providing a framework, the patient will be more passionate about sharing the details of their history.
No. 3: Help patients organize their story
Sometimes, patients provide details in a nonchronological order, jumping all over the place.
A super helpful technique is to explain to the patient that you have a story to write for your computer note, and for them to think back to when they first started noticing their abdominal pain or rectal bleeding symptoms. When were the most-severe episodes? How frequent are the episodes? What’s the volume of their rectal bleeding?
If the patient realizes that you’re trying to write a story synopsis, they will provide information in a much more organized way.
No. 4: Determine patient’s language preference
Quickly determine the patient’s language preference. We want patients to feel extremely comfortable.
Whenever possible, use a certified interpreter. Language phone lines, in-person interpreters, and video conferencing are widely available today. It’s worth investing in this technology so that we can provide the best possible care to immigrants and refugees.
Conversely, avoid using family members as interpreters because they may not be adequately trained in medical vocabulary.
No. 5: Use simple language
When providing explanations, use simple language that your patient can understand and identify with.
For example, use analogies like “the heart is a pump” or the diverticula are thin areas of the colon that can bleed if the blood vessel is too close to the surface.
No. 6: Determine level of medical literacy
Determine your patient’s level of medical literacy. Some of our patients did not graduate from high school. Some patients can’t read very well. Therefore, your discharge instructions and handouts should sometimes be written on a third-grade level.
If patients can’t read, write medication instructions with symbols. Draw a sun for medications that are supposed to be taken in the morning and draw a moon if a medication is supposed to be taken at night.
Always very carefully review the instructions with the patient.
No. 7: Check in with the patient
During the visit, frequently check in with the patient to make sure that they understand what you’re asking or what you’re trying to explain to them.
No. 8: Include family member as patient advocate
If the patient is accompanied by a family member, help them serve in the important role as a patient advocate.
If the family member wants to take notes, encourage them because that provides an awesome value.
Sometimes patients can forget clinic and hospital medical conversations, and that family member might be the key to improving your patient’s health.
No. 9: Follow-up with the patient
If your clinic has the capability, follow up with a patient the next day to make sure that they understood everything.
Check to make sure the patient was able to pick up all of the medications that you prescribed.
Check that laboratory tests are arranged or completed.
Check that important procedures, such as esophagogastroduodenoscopy and colonoscopy, and imaging, such as ultrasounds and CTs, are scheduled.
No. 10: Identify barriers to care
Have fun talking with a patient. Find out what they do for a living. Build a rapport. Listen to their stressors in life.
Try to identify any barriers to care or external stressors, like taking care of a sick parent, which might interfere with their scheduling an important diagnostic colonoscopy for rectal bleeding.
Good luck incorporating these communication strategies into your clinic and hospital work. Together, we can help improve the delivery of health care.
Dr. Levy is a gastroenterologist at the University of Chicago. In 2017, Dr. Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Dr. Levy, who recently gave a TEDx Talk about building health education campaigns using music and concerts, organizes Tune It Up: A Concert To Raise Colorectal Cancer Awareness with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Here are 10 ways to improve health communication with patients.
No. 1: Be an active listener
The first tip is to be an active listener and help guide the history-taking process by asking for clarification when needed.
Quickly figure out the patient’s chief complaint. Which symptom is the most severe?
Ask them for symptom-modifying factors, such as onset, duration, frequency, and a pain description. Is the abdominal pain sharp or crampy, dull and achy, or pressure-like? What makes the symptoms better or worse?
As many of us were taught in medical school, 80% of the diagnosis is in a patient’s history and description.
No. 2: Ask questions that resonate with patients
What can we do to help elicit an accurate history from the patient when they’re not providing the needed information or being helpful enough?
The easiest way is to ask your patient in a completely different way but one that resonates with them. For instance, ask how the abdominal pain is affecting their quality of life. That will help focus the history taking and encourage the patient to share details.
Does the pain keep them awake at night? Are they able to eat a normal-sized meal? Or are they forced to eat tiny snacks? Is the pain interfering with work or school?
By providing a framework, the patient will be more passionate about sharing the details of their history.
No. 3: Help patients organize their story
Sometimes, patients provide details in a nonchronological order, jumping all over the place.
A super helpful technique is to explain to the patient that you have a story to write for your computer note, and for them to think back to when they first started noticing their abdominal pain or rectal bleeding symptoms. When were the most-severe episodes? How frequent are the episodes? What’s the volume of their rectal bleeding?
If the patient realizes that you’re trying to write a story synopsis, they will provide information in a much more organized way.
No. 4: Determine patient’s language preference
Quickly determine the patient’s language preference. We want patients to feel extremely comfortable.
Whenever possible, use a certified interpreter. Language phone lines, in-person interpreters, and video conferencing are widely available today. It’s worth investing in this technology so that we can provide the best possible care to immigrants and refugees.
Conversely, avoid using family members as interpreters because they may not be adequately trained in medical vocabulary.
No. 5: Use simple language
When providing explanations, use simple language that your patient can understand and identify with.
For example, use analogies like “the heart is a pump” or the diverticula are thin areas of the colon that can bleed if the blood vessel is too close to the surface.
No. 6: Determine level of medical literacy
Determine your patient’s level of medical literacy. Some of our patients did not graduate from high school. Some patients can’t read very well. Therefore, your discharge instructions and handouts should sometimes be written on a third-grade level.
If patients can’t read, write medication instructions with symbols. Draw a sun for medications that are supposed to be taken in the morning and draw a moon if a medication is supposed to be taken at night.
Always very carefully review the instructions with the patient.
No. 7: Check in with the patient
During the visit, frequently check in with the patient to make sure that they understand what you’re asking or what you’re trying to explain to them.
No. 8: Include family member as patient advocate
If the patient is accompanied by a family member, help them serve in the important role as a patient advocate.
If the family member wants to take notes, encourage them because that provides an awesome value.
Sometimes patients can forget clinic and hospital medical conversations, and that family member might be the key to improving your patient’s health.
No. 9: Follow-up with the patient
If your clinic has the capability, follow up with a patient the next day to make sure that they understood everything.
Check to make sure the patient was able to pick up all of the medications that you prescribed.
Check that laboratory tests are arranged or completed.
Check that important procedures, such as esophagogastroduodenoscopy and colonoscopy, and imaging, such as ultrasounds and CTs, are scheduled.
No. 10: Identify barriers to care
Have fun talking with a patient. Find out what they do for a living. Build a rapport. Listen to their stressors in life.
Try to identify any barriers to care or external stressors, like taking care of a sick parent, which might interfere with their scheduling an important diagnostic colonoscopy for rectal bleeding.
Good luck incorporating these communication strategies into your clinic and hospital work. Together, we can help improve the delivery of health care.
Dr. Levy is a gastroenterologist at the University of Chicago. In 2017, Dr. Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Dr. Levy, who recently gave a TEDx Talk about building health education campaigns using music and concerts, organizes Tune It Up: A Concert To Raise Colorectal Cancer Awareness with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study evaluating in utero treatment for hypohidrotic ectodermal dysplasia seeks enrollees
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
What factors cause multiple biologic failure in psoriasis?
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Babe Ruth’s unique cane, and why he used it
Babe Ruth was arguably the greatest athlete in American history.
Certainly, there have been, and always will be, many great figures in all sports. But none of them – Michael Jordan or LeBron James or Tom Brady – have ever, probably will never, dominate sports AND society in the way Babe Ruth did.
Ruth wasn’t an angel, nor did he claim to be. But he was a center of American life the way no athlete ever was or will be.
He was a remarkably good baseball player. In an era where home runs were rarities, he hit more than the entire rest of Major League Baseball combined. But he wasn’t just a slugger, he was an excellent play maker, fielder, and pitcher. (He was actually one of the best pitchers of his era, something else mostly forgotten today.)
Ruth retired in 1935. He never entirely left the limelight, with fans showing up even to watch him play golf in celebrity tournaments. In 1939 he spoke on July 4 at Lou Gehrig appreciation day as his former teammate was publicly dying of ALS.
In 1946 Ruth began having trouble swallowing and developed pain over his right eye. He was found to have nasopharyngeal carcinoma spreading down into his skull base and neck.
Even today surgery to remove cancer from that area is tricky. In 1946 it didn’t exist. An experimental treatment of combined radiation and chemotherapy – today standard – was tried, including a new folic acid derivative called teropterin. He improved somewhat – enough that he was an unnamed case study presented at a medical meeting – but had lost 80 pounds. After a brief respite he continued to go downhill. On June 13, 1948, he appeared at Yankee Stadium – the house that Ruth built – for the last time, where he was honored. He had difficulty walking and used a baseball bat as a cane. His pharynx was so damaged his voice could barely be heard. He died 2 months later on Aug. 16, 1948.
This isn’t a sports column, I’m not a sports writer, and this definitely ain’t Sport Illustrated. So why am I writing this?
Because Babe Ruth never knew he had cancer. Was never told he was dying. His family was afraid he’d harm himself if he knew, so his doctors were under strict instructions to keep the bad news from him.
Now, Ruth wasn’t stupid. Wild, unrepentant, hedonistic, and a lot of other things – but not stupid. He certainly must have figured it out with getting radiation, or chemotherapy, or his declining physical status. But none of his doctors or family ever told him he had cancer and was dying (what they did tell him I have no idea).
Let’s look at this as a case history: A 51-year-old male, possessed of all his mental faculties, presents with headaches, dysphonia, and dysphagia. Workup reveals advanced, inoperable, nasopharyngeal cancer. The family is willing to accept treatment, but understands the prognosis is poor. Family members request that, under no circumstances, he be told of the diagnosis or prognosis.
The fact that the patient is probably the biggest celebrity of his era shouldn’t make a difference, but it does.
I’m sure most of us would want to tell the patient. We live in an age of patient autonomy. . But what if the family has concerns that the patient would hurt himself, as Ruth’s family did?
This summer is 75 years since the Babe died. Medicine has changed a lot, but some questions never will.
What would you do?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Babe Ruth was arguably the greatest athlete in American history.
Certainly, there have been, and always will be, many great figures in all sports. But none of them – Michael Jordan or LeBron James or Tom Brady – have ever, probably will never, dominate sports AND society in the way Babe Ruth did.
Ruth wasn’t an angel, nor did he claim to be. But he was a center of American life the way no athlete ever was or will be.
He was a remarkably good baseball player. In an era where home runs were rarities, he hit more than the entire rest of Major League Baseball combined. But he wasn’t just a slugger, he was an excellent play maker, fielder, and pitcher. (He was actually one of the best pitchers of his era, something else mostly forgotten today.)
Ruth retired in 1935. He never entirely left the limelight, with fans showing up even to watch him play golf in celebrity tournaments. In 1939 he spoke on July 4 at Lou Gehrig appreciation day as his former teammate was publicly dying of ALS.
In 1946 Ruth began having trouble swallowing and developed pain over his right eye. He was found to have nasopharyngeal carcinoma spreading down into his skull base and neck.
Even today surgery to remove cancer from that area is tricky. In 1946 it didn’t exist. An experimental treatment of combined radiation and chemotherapy – today standard – was tried, including a new folic acid derivative called teropterin. He improved somewhat – enough that he was an unnamed case study presented at a medical meeting – but had lost 80 pounds. After a brief respite he continued to go downhill. On June 13, 1948, he appeared at Yankee Stadium – the house that Ruth built – for the last time, where he was honored. He had difficulty walking and used a baseball bat as a cane. His pharynx was so damaged his voice could barely be heard. He died 2 months later on Aug. 16, 1948.
This isn’t a sports column, I’m not a sports writer, and this definitely ain’t Sport Illustrated. So why am I writing this?
Because Babe Ruth never knew he had cancer. Was never told he was dying. His family was afraid he’d harm himself if he knew, so his doctors were under strict instructions to keep the bad news from him.
Now, Ruth wasn’t stupid. Wild, unrepentant, hedonistic, and a lot of other things – but not stupid. He certainly must have figured it out with getting radiation, or chemotherapy, or his declining physical status. But none of his doctors or family ever told him he had cancer and was dying (what they did tell him I have no idea).
Let’s look at this as a case history: A 51-year-old male, possessed of all his mental faculties, presents with headaches, dysphonia, and dysphagia. Workup reveals advanced, inoperable, nasopharyngeal cancer. The family is willing to accept treatment, but understands the prognosis is poor. Family members request that, under no circumstances, he be told of the diagnosis or prognosis.
The fact that the patient is probably the biggest celebrity of his era shouldn’t make a difference, but it does.
I’m sure most of us would want to tell the patient. We live in an age of patient autonomy. . But what if the family has concerns that the patient would hurt himself, as Ruth’s family did?
This summer is 75 years since the Babe died. Medicine has changed a lot, but some questions never will.
What would you do?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Babe Ruth was arguably the greatest athlete in American history.
Certainly, there have been, and always will be, many great figures in all sports. But none of them – Michael Jordan or LeBron James or Tom Brady – have ever, probably will never, dominate sports AND society in the way Babe Ruth did.
Ruth wasn’t an angel, nor did he claim to be. But he was a center of American life the way no athlete ever was or will be.
He was a remarkably good baseball player. In an era where home runs were rarities, he hit more than the entire rest of Major League Baseball combined. But he wasn’t just a slugger, he was an excellent play maker, fielder, and pitcher. (He was actually one of the best pitchers of his era, something else mostly forgotten today.)
Ruth retired in 1935. He never entirely left the limelight, with fans showing up even to watch him play golf in celebrity tournaments. In 1939 he spoke on July 4 at Lou Gehrig appreciation day as his former teammate was publicly dying of ALS.
In 1946 Ruth began having trouble swallowing and developed pain over his right eye. He was found to have nasopharyngeal carcinoma spreading down into his skull base and neck.
Even today surgery to remove cancer from that area is tricky. In 1946 it didn’t exist. An experimental treatment of combined radiation and chemotherapy – today standard – was tried, including a new folic acid derivative called teropterin. He improved somewhat – enough that he was an unnamed case study presented at a medical meeting – but had lost 80 pounds. After a brief respite he continued to go downhill. On June 13, 1948, he appeared at Yankee Stadium – the house that Ruth built – for the last time, where he was honored. He had difficulty walking and used a baseball bat as a cane. His pharynx was so damaged his voice could barely be heard. He died 2 months later on Aug. 16, 1948.
This isn’t a sports column, I’m not a sports writer, and this definitely ain’t Sport Illustrated. So why am I writing this?
Because Babe Ruth never knew he had cancer. Was never told he was dying. His family was afraid he’d harm himself if he knew, so his doctors were under strict instructions to keep the bad news from him.
Now, Ruth wasn’t stupid. Wild, unrepentant, hedonistic, and a lot of other things – but not stupid. He certainly must have figured it out with getting radiation, or chemotherapy, or his declining physical status. But none of his doctors or family ever told him he had cancer and was dying (what they did tell him I have no idea).
Let’s look at this as a case history: A 51-year-old male, possessed of all his mental faculties, presents with headaches, dysphonia, and dysphagia. Workup reveals advanced, inoperable, nasopharyngeal cancer. The family is willing to accept treatment, but understands the prognosis is poor. Family members request that, under no circumstances, he be told of the diagnosis or prognosis.
The fact that the patient is probably the biggest celebrity of his era shouldn’t make a difference, but it does.
I’m sure most of us would want to tell the patient. We live in an age of patient autonomy. . But what if the family has concerns that the patient would hurt himself, as Ruth’s family did?
This summer is 75 years since the Babe died. Medicine has changed a lot, but some questions never will.
What would you do?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Oxycodone tied to persistent use only after vaginal delivery
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
The unique approach involved in age-specific concerns surrounding young patients with breast cancer
This transcript has been edited for clarity.
Dr. Partridge:
Olivia, let’s get started. What kinds of things do we need to think about when we’re seeing a young patient in clinic, beyond the usual things we think about for patients with breast cancer?
Dr. Pagani: The idea of selecting age as a determinant of care of young women is because they have specific issues, which are different from older, premenopausal patients but also older patients in general. We need to take care of many things, which can go from their job, family, fertility, and all these things are specific to these women and can impact their treatment, survivorship issues, side effects, and long-term problems. It’s a different world, compared with other patients with breast cancer.
Dr. Partridge: One of the areas that you and I have been very deep in the weeds in is the fertility issues. That’s obviously one of the things that’s pretty age-specific. There are some new data around that that we’re excited about. What do we think about when we think about trying to have a pregnancy or not after a breast cancer diagnosis?
Dr. Pagani: Yeah. I think it’s great times for that because we succeeded in building up a very important trial, which broke a taboo that was there for many, many decades: You had breast cancer so forget your pregnancy desire.
Despite many retrospective data from many groups that suggested pregnancy after breast cancer was not detrimental, there were so many obstacles for these women to address their pregnancy desire. I think we succeeded in explaining and showing in a quite solid way that if you desire a baby after breast cancer, you can try to have him or her.
Dr. Partridge: This was called the POSITIVE trial, with early findings published in the New England Journal of Medicine this past year, which was very exciting. Let’s dig a little deeper into that. Is this relevant for all patients with breast cancer or select patients with breast cancer who want to get pregnant?
Dr. Pagani: The accrual of the trial was open to all patients with stage I-III disease, but the majority of the patients were low risk, which means that the majority were node negative with small tumors. I think, so far, we can say that in low-risk women, pregnancy after breast cancer can be discussed and planned.
Summarizing, I think the evidence is for low-risk patients with early breast cancer. A minority had huge tumors or node-positive disease.
Dr. Partridge: It’s nice to be able to have these data to say a temporary interruption of endocrine therapy – not coming off forever, getting back on – was not associated with any worsening in terms of their breast cancer events in the future, which is great news for the women who are diagnosed when they’re trying to get pregnant and build their families or not having completed their families. It’s been fantastic.
What about for our patients with advanced disease who come in, and we’re treating them more to try and manage the cancer and improve their survival and quality of life, but cure may not be the goal. How do we manage the fertility issues for them?
Dr. Pagani: This is, I think, still an open issue despite overall survival for many women with advanced disease, especially HER2 positive or endocrine responsive; it is improving and it’s getting better and better. There are few women with oligometastatic disease that can be cured.
We are not yet there. At the Advanced Breast Cancer conference, we started to open the door to say that fertility should be discussed with patients with advanced breast cancer as well. We cannot recommend to patients with advanced breast cancer to pursue a pregnancy.
We have no data. For sure, this needs to be taken into account and discussed openly with all the patients who desire to discuss this.
Dr. Partridge: Yes. To help people to either grieve their losses or find alternative ways to build their family, I think, is something that we focus on.
How to optimize the plan of care for young patients
Dr. Partridge: Shifting gears into the psychosocial, we know that our young women of all stages have a harder time adjusting to a breast cancer diagnosis for good reason. It’s not normative at all to be dealing with a lot of the slings and arrows that our young women deal with at the age that they do. How do you manage that in your clinic, Olivia?
Dr. Pagani: Well, I think it’s always tough. One of the problems, which is also true for early breast cancer in general, which I think is common to you as well, is that in our society many women get breast cancer before even having thought of their family planning. That’s many of them in our reality.
In other countries, maybe they have already two to three children. In our countries, they are aged 30-35 years with no children, no stable relationship, and then are faced with all these things, and their pregnancy desire can be blown up because they understand there is no time, especially if they are metastatic. This can be devastating.
We are not very good at that yet. I think we need to develop better tools, better competence, and knowledge to support them to this extent as well.
Dr. Partridge: I know that whether people want kids or not, the diagnosis of breast cancer has financial toxicity and the inconvenience of going through this kind of experience while managing a busy life. Many of our patients, especially our young patients, are trying to develop their careers, to graduate from schools, and to grow a nest egg. They’re not retired yet, on average.
I agree that we have a large amount of work to do. The one thing I try and do is always bring in our social workers and our psychosocial supportive care providers for our young patients; not that I don’t bring them in for everybody that needs them, but our young patients on average seem to need them a little bit more just because it can be just so hard on them from a psychosocial and emotional standpoint, don’t you think?
Dr. Pagani: Yes, I think so. Do you have any specific program going on at Harvard?
Dr. Partridge: We do. We’ve built a program for young women that focuses on their unique and specific needs that capitalizes on groups that are already there. We have a social work department. We just have smoothed the pathway, and we send our young people in there more quickly and have some dedicated support groups and one-to-one interventions where patients can guide other young patients. We’ve built out the supportive care for these young patients and programming.
The other big area we’ve developed that’s not unique to young age but certainly enhanced in our young patients is genetics. We have a big genetic component at our cancer center. The young patients, more so than any other group, need to have the genetic counseling and the genetic testing not only to know about future risks and about their families but also to inform their treatment decisions these days. Do you want to comment on that?
Dr. Pagani: Yes, of course. Genetic counseling, especially for the most common BRCA1 and BRCA2, can change their local treatment (e.g., bilateral mastectomy instead of conservative surgery) but they have also to take care of their ovaries. They need to think of prophylactic oophorectomy, which makes fertility and pregnancy even more complicated. For them, it’s much more complex to address everything.
I think it’s really very complex, and I think we need a better understanding of all the nuances. Sometimes, we really do not consider, as you mentioned, that not every woman desires to have a baby.
The occurrence of breast cancer can wake up a desire that was not conscious but becomes conscious because you feel that you will not be able to do that. With the social support, the psychological support, and support groups – we have a very strong breast cancer support group for younger women — they could face these things. The young women support group was supportive of the POSITIVE trial: they helped to develop and financed a video, which was very helpful to promote POSITIVE.
I think that having a relationship or a network between patients, health professionals, social workers, and psychologists can help everyone, including those who want to become mothers, those who cannot, and those who do not want to.
Dr. Partridge: I think that’s great, Olivia. I think you rounded it out by just shining a light on these issues for our young patients and elevating it to being okay to talk about these issues. I think historically, it’s been: “You’ve got breast cancer, forget about this. We just need to get you to a better survival.”
We’re increasingly recognizing for patients of all ages, but particularly our young patients, that just surviving through breast cancer or cancer in general is not enough. We need to help people live the best and fullest life possible in their survivorship.
Education and communication: Key aspects moving forward
Dr. Pagani: I think another issue we need really to improve is health professional competence and knowledge. After you presented the POSITIVE trial in San Antonio, I had many calls with patients. They told me, “Well, I had this information, but my gynecologist, my oncologist, or my general practitioner still discouraged me.” This is a great barrier.
I think we need to do more to teach the health professionals. Otherwise, what we do is never enough because it will be blocked. They are scared and they do not want to go against their doctors. I think this is a very big conflict.
Dr. Partridge: That’s a really important point, and I appreciate you bringing it up. We as clinicians and educators who are building the research base need to really get it out there.
Dr. Pagani is a professor at the University of Geneva. Dr. Partridge is professor of medicine at Harvard Medical School and vice chair of clinical oncology at Dana-Farber Cancer Institute, both in Boston. Dr. Pagani reported conflicts of interest with PRIME, Roche, Eli Lilly, Novartis, Takeda, Pfizer, and Debiopharm. Dr. Partridge reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. Partridge:
Olivia, let’s get started. What kinds of things do we need to think about when we’re seeing a young patient in clinic, beyond the usual things we think about for patients with breast cancer?
Dr. Pagani: The idea of selecting age as a determinant of care of young women is because they have specific issues, which are different from older, premenopausal patients but also older patients in general. We need to take care of many things, which can go from their job, family, fertility, and all these things are specific to these women and can impact their treatment, survivorship issues, side effects, and long-term problems. It’s a different world, compared with other patients with breast cancer.
Dr. Partridge: One of the areas that you and I have been very deep in the weeds in is the fertility issues. That’s obviously one of the things that’s pretty age-specific. There are some new data around that that we’re excited about. What do we think about when we think about trying to have a pregnancy or not after a breast cancer diagnosis?
Dr. Pagani: Yeah. I think it’s great times for that because we succeeded in building up a very important trial, which broke a taboo that was there for many, many decades: You had breast cancer so forget your pregnancy desire.
Despite many retrospective data from many groups that suggested pregnancy after breast cancer was not detrimental, there were so many obstacles for these women to address their pregnancy desire. I think we succeeded in explaining and showing in a quite solid way that if you desire a baby after breast cancer, you can try to have him or her.
Dr. Partridge: This was called the POSITIVE trial, with early findings published in the New England Journal of Medicine this past year, which was very exciting. Let’s dig a little deeper into that. Is this relevant for all patients with breast cancer or select patients with breast cancer who want to get pregnant?
Dr. Pagani: The accrual of the trial was open to all patients with stage I-III disease, but the majority of the patients were low risk, which means that the majority were node negative with small tumors. I think, so far, we can say that in low-risk women, pregnancy after breast cancer can be discussed and planned.
Summarizing, I think the evidence is for low-risk patients with early breast cancer. A minority had huge tumors or node-positive disease.
Dr. Partridge: It’s nice to be able to have these data to say a temporary interruption of endocrine therapy – not coming off forever, getting back on – was not associated with any worsening in terms of their breast cancer events in the future, which is great news for the women who are diagnosed when they’re trying to get pregnant and build their families or not having completed their families. It’s been fantastic.
What about for our patients with advanced disease who come in, and we’re treating them more to try and manage the cancer and improve their survival and quality of life, but cure may not be the goal. How do we manage the fertility issues for them?
Dr. Pagani: This is, I think, still an open issue despite overall survival for many women with advanced disease, especially HER2 positive or endocrine responsive; it is improving and it’s getting better and better. There are few women with oligometastatic disease that can be cured.
We are not yet there. At the Advanced Breast Cancer conference, we started to open the door to say that fertility should be discussed with patients with advanced breast cancer as well. We cannot recommend to patients with advanced breast cancer to pursue a pregnancy.
We have no data. For sure, this needs to be taken into account and discussed openly with all the patients who desire to discuss this.
Dr. Partridge: Yes. To help people to either grieve their losses or find alternative ways to build their family, I think, is something that we focus on.
How to optimize the plan of care for young patients
Dr. Partridge: Shifting gears into the psychosocial, we know that our young women of all stages have a harder time adjusting to a breast cancer diagnosis for good reason. It’s not normative at all to be dealing with a lot of the slings and arrows that our young women deal with at the age that they do. How do you manage that in your clinic, Olivia?
Dr. Pagani: Well, I think it’s always tough. One of the problems, which is also true for early breast cancer in general, which I think is common to you as well, is that in our society many women get breast cancer before even having thought of their family planning. That’s many of them in our reality.
In other countries, maybe they have already two to three children. In our countries, they are aged 30-35 years with no children, no stable relationship, and then are faced with all these things, and their pregnancy desire can be blown up because they understand there is no time, especially if they are metastatic. This can be devastating.
We are not very good at that yet. I think we need to develop better tools, better competence, and knowledge to support them to this extent as well.
Dr. Partridge: I know that whether people want kids or not, the diagnosis of breast cancer has financial toxicity and the inconvenience of going through this kind of experience while managing a busy life. Many of our patients, especially our young patients, are trying to develop their careers, to graduate from schools, and to grow a nest egg. They’re not retired yet, on average.
I agree that we have a large amount of work to do. The one thing I try and do is always bring in our social workers and our psychosocial supportive care providers for our young patients; not that I don’t bring them in for everybody that needs them, but our young patients on average seem to need them a little bit more just because it can be just so hard on them from a psychosocial and emotional standpoint, don’t you think?
Dr. Pagani: Yes, I think so. Do you have any specific program going on at Harvard?
Dr. Partridge: We do. We’ve built a program for young women that focuses on their unique and specific needs that capitalizes on groups that are already there. We have a social work department. We just have smoothed the pathway, and we send our young people in there more quickly and have some dedicated support groups and one-to-one interventions where patients can guide other young patients. We’ve built out the supportive care for these young patients and programming.
The other big area we’ve developed that’s not unique to young age but certainly enhanced in our young patients is genetics. We have a big genetic component at our cancer center. The young patients, more so than any other group, need to have the genetic counseling and the genetic testing not only to know about future risks and about their families but also to inform their treatment decisions these days. Do you want to comment on that?
Dr. Pagani: Yes, of course. Genetic counseling, especially for the most common BRCA1 and BRCA2, can change their local treatment (e.g., bilateral mastectomy instead of conservative surgery) but they have also to take care of their ovaries. They need to think of prophylactic oophorectomy, which makes fertility and pregnancy even more complicated. For them, it’s much more complex to address everything.
I think it’s really very complex, and I think we need a better understanding of all the nuances. Sometimes, we really do not consider, as you mentioned, that not every woman desires to have a baby.
The occurrence of breast cancer can wake up a desire that was not conscious but becomes conscious because you feel that you will not be able to do that. With the social support, the psychological support, and support groups – we have a very strong breast cancer support group for younger women — they could face these things. The young women support group was supportive of the POSITIVE trial: they helped to develop and financed a video, which was very helpful to promote POSITIVE.
I think that having a relationship or a network between patients, health professionals, social workers, and psychologists can help everyone, including those who want to become mothers, those who cannot, and those who do not want to.
Dr. Partridge: I think that’s great, Olivia. I think you rounded it out by just shining a light on these issues for our young patients and elevating it to being okay to talk about these issues. I think historically, it’s been: “You’ve got breast cancer, forget about this. We just need to get you to a better survival.”
We’re increasingly recognizing for patients of all ages, but particularly our young patients, that just surviving through breast cancer or cancer in general is not enough. We need to help people live the best and fullest life possible in their survivorship.
Education and communication: Key aspects moving forward
Dr. Pagani: I think another issue we need really to improve is health professional competence and knowledge. After you presented the POSITIVE trial in San Antonio, I had many calls with patients. They told me, “Well, I had this information, but my gynecologist, my oncologist, or my general practitioner still discouraged me.” This is a great barrier.
I think we need to do more to teach the health professionals. Otherwise, what we do is never enough because it will be blocked. They are scared and they do not want to go against their doctors. I think this is a very big conflict.
Dr. Partridge: That’s a really important point, and I appreciate you bringing it up. We as clinicians and educators who are building the research base need to really get it out there.
Dr. Pagani is a professor at the University of Geneva. Dr. Partridge is professor of medicine at Harvard Medical School and vice chair of clinical oncology at Dana-Farber Cancer Institute, both in Boston. Dr. Pagani reported conflicts of interest with PRIME, Roche, Eli Lilly, Novartis, Takeda, Pfizer, and Debiopharm. Dr. Partridge reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. Partridge:
Olivia, let’s get started. What kinds of things do we need to think about when we’re seeing a young patient in clinic, beyond the usual things we think about for patients with breast cancer?
Dr. Pagani: The idea of selecting age as a determinant of care of young women is because they have specific issues, which are different from older, premenopausal patients but also older patients in general. We need to take care of many things, which can go from their job, family, fertility, and all these things are specific to these women and can impact their treatment, survivorship issues, side effects, and long-term problems. It’s a different world, compared with other patients with breast cancer.
Dr. Partridge: One of the areas that you and I have been very deep in the weeds in is the fertility issues. That’s obviously one of the things that’s pretty age-specific. There are some new data around that that we’re excited about. What do we think about when we think about trying to have a pregnancy or not after a breast cancer diagnosis?
Dr. Pagani: Yeah. I think it’s great times for that because we succeeded in building up a very important trial, which broke a taboo that was there for many, many decades: You had breast cancer so forget your pregnancy desire.
Despite many retrospective data from many groups that suggested pregnancy after breast cancer was not detrimental, there were so many obstacles for these women to address their pregnancy desire. I think we succeeded in explaining and showing in a quite solid way that if you desire a baby after breast cancer, you can try to have him or her.
Dr. Partridge: This was called the POSITIVE trial, with early findings published in the New England Journal of Medicine this past year, which was very exciting. Let’s dig a little deeper into that. Is this relevant for all patients with breast cancer or select patients with breast cancer who want to get pregnant?
Dr. Pagani: The accrual of the trial was open to all patients with stage I-III disease, but the majority of the patients were low risk, which means that the majority were node negative with small tumors. I think, so far, we can say that in low-risk women, pregnancy after breast cancer can be discussed and planned.
Summarizing, I think the evidence is for low-risk patients with early breast cancer. A minority had huge tumors or node-positive disease.
Dr. Partridge: It’s nice to be able to have these data to say a temporary interruption of endocrine therapy – not coming off forever, getting back on – was not associated with any worsening in terms of their breast cancer events in the future, which is great news for the women who are diagnosed when they’re trying to get pregnant and build their families or not having completed their families. It’s been fantastic.
What about for our patients with advanced disease who come in, and we’re treating them more to try and manage the cancer and improve their survival and quality of life, but cure may not be the goal. How do we manage the fertility issues for them?
Dr. Pagani: This is, I think, still an open issue despite overall survival for many women with advanced disease, especially HER2 positive or endocrine responsive; it is improving and it’s getting better and better. There are few women with oligometastatic disease that can be cured.
We are not yet there. At the Advanced Breast Cancer conference, we started to open the door to say that fertility should be discussed with patients with advanced breast cancer as well. We cannot recommend to patients with advanced breast cancer to pursue a pregnancy.
We have no data. For sure, this needs to be taken into account and discussed openly with all the patients who desire to discuss this.
Dr. Partridge: Yes. To help people to either grieve their losses or find alternative ways to build their family, I think, is something that we focus on.
How to optimize the plan of care for young patients
Dr. Partridge: Shifting gears into the psychosocial, we know that our young women of all stages have a harder time adjusting to a breast cancer diagnosis for good reason. It’s not normative at all to be dealing with a lot of the slings and arrows that our young women deal with at the age that they do. How do you manage that in your clinic, Olivia?
Dr. Pagani: Well, I think it’s always tough. One of the problems, which is also true for early breast cancer in general, which I think is common to you as well, is that in our society many women get breast cancer before even having thought of their family planning. That’s many of them in our reality.
In other countries, maybe they have already two to three children. In our countries, they are aged 30-35 years with no children, no stable relationship, and then are faced with all these things, and their pregnancy desire can be blown up because they understand there is no time, especially if they are metastatic. This can be devastating.
We are not very good at that yet. I think we need to develop better tools, better competence, and knowledge to support them to this extent as well.
Dr. Partridge: I know that whether people want kids or not, the diagnosis of breast cancer has financial toxicity and the inconvenience of going through this kind of experience while managing a busy life. Many of our patients, especially our young patients, are trying to develop their careers, to graduate from schools, and to grow a nest egg. They’re not retired yet, on average.
I agree that we have a large amount of work to do. The one thing I try and do is always bring in our social workers and our psychosocial supportive care providers for our young patients; not that I don’t bring them in for everybody that needs them, but our young patients on average seem to need them a little bit more just because it can be just so hard on them from a psychosocial and emotional standpoint, don’t you think?
Dr. Pagani: Yes, I think so. Do you have any specific program going on at Harvard?
Dr. Partridge: We do. We’ve built a program for young women that focuses on their unique and specific needs that capitalizes on groups that are already there. We have a social work department. We just have smoothed the pathway, and we send our young people in there more quickly and have some dedicated support groups and one-to-one interventions where patients can guide other young patients. We’ve built out the supportive care for these young patients and programming.
The other big area we’ve developed that’s not unique to young age but certainly enhanced in our young patients is genetics. We have a big genetic component at our cancer center. The young patients, more so than any other group, need to have the genetic counseling and the genetic testing not only to know about future risks and about their families but also to inform their treatment decisions these days. Do you want to comment on that?
Dr. Pagani: Yes, of course. Genetic counseling, especially for the most common BRCA1 and BRCA2, can change their local treatment (e.g., bilateral mastectomy instead of conservative surgery) but they have also to take care of their ovaries. They need to think of prophylactic oophorectomy, which makes fertility and pregnancy even more complicated. For them, it’s much more complex to address everything.
I think it’s really very complex, and I think we need a better understanding of all the nuances. Sometimes, we really do not consider, as you mentioned, that not every woman desires to have a baby.
The occurrence of breast cancer can wake up a desire that was not conscious but becomes conscious because you feel that you will not be able to do that. With the social support, the psychological support, and support groups – we have a very strong breast cancer support group for younger women — they could face these things. The young women support group was supportive of the POSITIVE trial: they helped to develop and financed a video, which was very helpful to promote POSITIVE.
I think that having a relationship or a network between patients, health professionals, social workers, and psychologists can help everyone, including those who want to become mothers, those who cannot, and those who do not want to.
Dr. Partridge: I think that’s great, Olivia. I think you rounded it out by just shining a light on these issues for our young patients and elevating it to being okay to talk about these issues. I think historically, it’s been: “You’ve got breast cancer, forget about this. We just need to get you to a better survival.”
We’re increasingly recognizing for patients of all ages, but particularly our young patients, that just surviving through breast cancer or cancer in general is not enough. We need to help people live the best and fullest life possible in their survivorship.
Education and communication: Key aspects moving forward
Dr. Pagani: I think another issue we need really to improve is health professional competence and knowledge. After you presented the POSITIVE trial in San Antonio, I had many calls with patients. They told me, “Well, I had this information, but my gynecologist, my oncologist, or my general practitioner still discouraged me.” This is a great barrier.
I think we need to do more to teach the health professionals. Otherwise, what we do is never enough because it will be blocked. They are scared and they do not want to go against their doctors. I think this is a very big conflict.
Dr. Partridge: That’s a really important point, and I appreciate you bringing it up. We as clinicians and educators who are building the research base need to really get it out there.
Dr. Pagani is a professor at the University of Geneva. Dr. Partridge is professor of medicine at Harvard Medical School and vice chair of clinical oncology at Dana-Farber Cancer Institute, both in Boston. Dr. Pagani reported conflicts of interest with PRIME, Roche, Eli Lilly, Novartis, Takeda, Pfizer, and Debiopharm. Dr. Partridge reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Weekend Botox training: Shortcut to cash or risky business?
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
Even one drink a day tied to increased BP in healthy adults
“A vexing question has been whether usual intake of small amounts of alcohol is associated with a higher level of BP. We identified a continuous, more or less linear association, with no evidence of a threshold for the association,” study coauthor Paul Whelton, MD, of Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
For systolic BP (SBP), “the most important BP risk indicator for CVD [cardiovascular disease], the association was robust, being present in both men and women and in both North America as well as Asia,” Dr. Whelton noted.
Based on the results, “the lower the better, and no consumption even better, as we did not find any indication that human health may benefit from consumption of very small amounts of alcohol,” senior author Marco Vinceti, MD, PhD, of University of Modena and Reggio Emilia University in Italy, told this news organization.
“Clearly, alcohol is not the only or necessarily the main determinant of high blood pressure, and the effects of small intakes of alcohol emerging from our pooled analysis were certainly not biologically as relevant and meaningful as those induced by high intakes,” Dr. Vinceti added.
The study was published online in Hypertension.
The researchers analyzed data from seven large, observational studies conducted in the United States, Korea, and Japan involving 19,548 adults (65% men).
Participants ranged in age from 20 years to the early 70s at baseline and were followed for a median of 5.3 years (range, 4-12 years). None of the participants had previously been diagnosed with hypertension or other CVD, diabetes, liver disease, alcoholism, or binge drinking.
Compared with nondrinkers, SBP was 1.25 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 4.90 mm Hg in adults consuming an average of 48 grams of alcohol per day.
For reference, in the United States, 12 ounces of regular beer, 5 ounces of wine, or a 1.5-ounce shot of distilled spirits contains about 14 grams of alcohol.
Diastolic BP (DBP) was 1.14 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 3.10 mm Hg in those who consumed an average of 48 grams of alcohol per day.
Subgroup analyses by gender showed an almost linear association between baseline alcohol intake and SBP changes in men and women and for DBP in men, while in women, there was an inverted U-shaped association.
No safe level
“From a BP perspective, it’s best to avoid alcohol intake. This is what the WHO [World Health Organization] recommends,” Dr. Whelton said.
“If someone is already drinking alcohol and does not want to stop doing so, minimizing alcohol consumption is desirable; many guidelines recommend not starting to drink alcohol but in those already drinking alcohol, consumption of two or less standard drinks per day for men and one or less standard drinks of alcohol per day for women,” Dr. Whelton noted.
Commenting on the study for this article, Alberto Ascherio, MD, of Harvard T. H. Chan School of Public Health, Boston, said it’s been known for more than 30 years that alcohol intake is associated with increased systolic and diastolic BP. The added value of this new study is a “refinement of the estimate of the dose response.”
Dr. Ascherio noted that “moderate alcohol consumption is associated with a modest increase in risk of cancer, but, in spite of the adverse association with BP, with a potentially beneficial effect on cardiovascular disease.” However, “the causality of the latter association has been questioned, but there is no consensus on this.”
Also weighing in, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York City, said this new study represents “yet another piece of evidence suggesting that there simply is no ‘healthy’ amount of alcohol use in humans.
“Even small amounts of alcohol intake can have negative health effects, as demonstrated in this study,” Dr. Brennan said. “There is still a widely held belief among people that drinking in moderation is good for you. It is becoming more and more clear that this is simply not the case. As health authorities grapple with drinking ‘recommendations,’ additional datasets like these will be helpful.”
The study had no specific funding. Dr. Whelton, Dr. Vinceti, Dr. Ascherio, and Dr. Brennan have no relevant disclosures.
A version of this article first appeared on Medscape.com.
“A vexing question has been whether usual intake of small amounts of alcohol is associated with a higher level of BP. We identified a continuous, more or less linear association, with no evidence of a threshold for the association,” study coauthor Paul Whelton, MD, of Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
For systolic BP (SBP), “the most important BP risk indicator for CVD [cardiovascular disease], the association was robust, being present in both men and women and in both North America as well as Asia,” Dr. Whelton noted.
Based on the results, “the lower the better, and no consumption even better, as we did not find any indication that human health may benefit from consumption of very small amounts of alcohol,” senior author Marco Vinceti, MD, PhD, of University of Modena and Reggio Emilia University in Italy, told this news organization.
“Clearly, alcohol is not the only or necessarily the main determinant of high blood pressure, and the effects of small intakes of alcohol emerging from our pooled analysis were certainly not biologically as relevant and meaningful as those induced by high intakes,” Dr. Vinceti added.
The study was published online in Hypertension.
The researchers analyzed data from seven large, observational studies conducted in the United States, Korea, and Japan involving 19,548 adults (65% men).
Participants ranged in age from 20 years to the early 70s at baseline and were followed for a median of 5.3 years (range, 4-12 years). None of the participants had previously been diagnosed with hypertension or other CVD, diabetes, liver disease, alcoholism, or binge drinking.
Compared with nondrinkers, SBP was 1.25 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 4.90 mm Hg in adults consuming an average of 48 grams of alcohol per day.
For reference, in the United States, 12 ounces of regular beer, 5 ounces of wine, or a 1.5-ounce shot of distilled spirits contains about 14 grams of alcohol.
Diastolic BP (DBP) was 1.14 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 3.10 mm Hg in those who consumed an average of 48 grams of alcohol per day.
Subgroup analyses by gender showed an almost linear association between baseline alcohol intake and SBP changes in men and women and for DBP in men, while in women, there was an inverted U-shaped association.
No safe level
“From a BP perspective, it’s best to avoid alcohol intake. This is what the WHO [World Health Organization] recommends,” Dr. Whelton said.
“If someone is already drinking alcohol and does not want to stop doing so, minimizing alcohol consumption is desirable; many guidelines recommend not starting to drink alcohol but in those already drinking alcohol, consumption of two or less standard drinks per day for men and one or less standard drinks of alcohol per day for women,” Dr. Whelton noted.
Commenting on the study for this article, Alberto Ascherio, MD, of Harvard T. H. Chan School of Public Health, Boston, said it’s been known for more than 30 years that alcohol intake is associated with increased systolic and diastolic BP. The added value of this new study is a “refinement of the estimate of the dose response.”
Dr. Ascherio noted that “moderate alcohol consumption is associated with a modest increase in risk of cancer, but, in spite of the adverse association with BP, with a potentially beneficial effect on cardiovascular disease.” However, “the causality of the latter association has been questioned, but there is no consensus on this.”
Also weighing in, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York City, said this new study represents “yet another piece of evidence suggesting that there simply is no ‘healthy’ amount of alcohol use in humans.
“Even small amounts of alcohol intake can have negative health effects, as demonstrated in this study,” Dr. Brennan said. “There is still a widely held belief among people that drinking in moderation is good for you. It is becoming more and more clear that this is simply not the case. As health authorities grapple with drinking ‘recommendations,’ additional datasets like these will be helpful.”
The study had no specific funding. Dr. Whelton, Dr. Vinceti, Dr. Ascherio, and Dr. Brennan have no relevant disclosures.
A version of this article first appeared on Medscape.com.
“A vexing question has been whether usual intake of small amounts of alcohol is associated with a higher level of BP. We identified a continuous, more or less linear association, with no evidence of a threshold for the association,” study coauthor Paul Whelton, MD, of Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
For systolic BP (SBP), “the most important BP risk indicator for CVD [cardiovascular disease], the association was robust, being present in both men and women and in both North America as well as Asia,” Dr. Whelton noted.
Based on the results, “the lower the better, and no consumption even better, as we did not find any indication that human health may benefit from consumption of very small amounts of alcohol,” senior author Marco Vinceti, MD, PhD, of University of Modena and Reggio Emilia University in Italy, told this news organization.
“Clearly, alcohol is not the only or necessarily the main determinant of high blood pressure, and the effects of small intakes of alcohol emerging from our pooled analysis were certainly not biologically as relevant and meaningful as those induced by high intakes,” Dr. Vinceti added.
The study was published online in Hypertension.
The researchers analyzed data from seven large, observational studies conducted in the United States, Korea, and Japan involving 19,548 adults (65% men).
Participants ranged in age from 20 years to the early 70s at baseline and were followed for a median of 5.3 years (range, 4-12 years). None of the participants had previously been diagnosed with hypertension or other CVD, diabetes, liver disease, alcoholism, or binge drinking.
Compared with nondrinkers, SBP was 1.25 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 4.90 mm Hg in adults consuming an average of 48 grams of alcohol per day.
For reference, in the United States, 12 ounces of regular beer, 5 ounces of wine, or a 1.5-ounce shot of distilled spirits contains about 14 grams of alcohol.
Diastolic BP (DBP) was 1.14 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 3.10 mm Hg in those who consumed an average of 48 grams of alcohol per day.
Subgroup analyses by gender showed an almost linear association between baseline alcohol intake and SBP changes in men and women and for DBP in men, while in women, there was an inverted U-shaped association.
No safe level
“From a BP perspective, it’s best to avoid alcohol intake. This is what the WHO [World Health Organization] recommends,” Dr. Whelton said.
“If someone is already drinking alcohol and does not want to stop doing so, minimizing alcohol consumption is desirable; many guidelines recommend not starting to drink alcohol but in those already drinking alcohol, consumption of two or less standard drinks per day for men and one or less standard drinks of alcohol per day for women,” Dr. Whelton noted.
Commenting on the study for this article, Alberto Ascherio, MD, of Harvard T. H. Chan School of Public Health, Boston, said it’s been known for more than 30 years that alcohol intake is associated with increased systolic and diastolic BP. The added value of this new study is a “refinement of the estimate of the dose response.”
Dr. Ascherio noted that “moderate alcohol consumption is associated with a modest increase in risk of cancer, but, in spite of the adverse association with BP, with a potentially beneficial effect on cardiovascular disease.” However, “the causality of the latter association has been questioned, but there is no consensus on this.”
Also weighing in, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York City, said this new study represents “yet another piece of evidence suggesting that there simply is no ‘healthy’ amount of alcohol use in humans.
“Even small amounts of alcohol intake can have negative health effects, as demonstrated in this study,” Dr. Brennan said. “There is still a widely held belief among people that drinking in moderation is good for you. It is becoming more and more clear that this is simply not the case. As health authorities grapple with drinking ‘recommendations,’ additional datasets like these will be helpful.”
The study had no specific funding. Dr. Whelton, Dr. Vinceti, Dr. Ascherio, and Dr. Brennan have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
Erythematous Plaques on the Dorsal Aspect of the Hand
The Diagnosis: Majocchi Granuloma
Histopathology showed rare follicular-based organisms highlighted by periodic acid–Schiff staining. This finding along with her use of clobetasol ointment on the hands led to a diagnosis of Majocchi granuloma in our patient. Clobetasol and crisaborole ointments were discontinued, and she was started on oral terbinafine 250 mg daily for 4 weeks, which resulted in resolution of the rash.
Majocchi granuloma (also known as nodular granulomatous perifolliculitis) is a perifollicular granulomatous process caused by a dermatophyte infection of the hair follicles. Trichophyton rubrum is the most commonly implicated organism, followed by Trichophyton mentagrophytes and Epidermophyton floccosum, which also cause tinea corporis and tinea pedis.1 This condition most commonly occurs in women aged 20 to 35 years. Risk factors include trauma, occlusion of the hair follicles, immunosuppression, and use of potent topical corticosteroids in patients with tinea.2,3 Immunocompetent patients present with perifollicular papules or pustules with erythematous scaly plaques on the extremities, while immunocompromised patients may have subcutaneous nodules or abscesses on any hair-bearing parts of the body.3
Majocchi granuloma is considered a dermal fungal infection in which the disruption of hair follicles from occlusion or trauma allows fungal organisms and keratinaceous material substrates to be introduced into the dermis. The differential diagnosis is based on the types of presenting lesions. The papules of Majocchi granuloma can resemble folliculitis, acne, or insect bites, while nodules can resemble erythema nodosum or furunculosis.4 Plaques, such as those seen in our patient, can mimic cellulitis and allergic or irritant contact dermatitis.4 Additionally, the plaques may appear annular or figurate, which may resemble erythema gyratum repens or erythema annulare centrifugum.
The diagnosis of Majocchi granuloma often requires fungal culture and biopsy because a potassium hydroxide preparation is unable to distinguish between superficial and invasive dermatophytes.3 Histopathology will show perifollicular granulomatous inflammation. Fungal elements can be detected with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining of the hairs and hair follicles as well as dermal infiltrates.4
Topical corticosteroids should be discontinued. Systemic antifungals are the treatment of choice for Majocchi granuloma, as topical antifungals are not effective against deep fungal infections. Although there are no standard guidelines on duration or dosage, recommended regimens in immunocompetent patients include terbinafine 250 mg/d for 4 weeks; itraconazole pulse therapy consisting of 200 mg twice daily for 1 week with 2 weeks off therapy, then repeat the cycle for a total of 2 to 3 pulses; and griseofulvin 500 mg twice daily for 8 to 12 weeks (Table).3 For immunocompromised patients, combination therapy with more than one antifungal may be necessary.
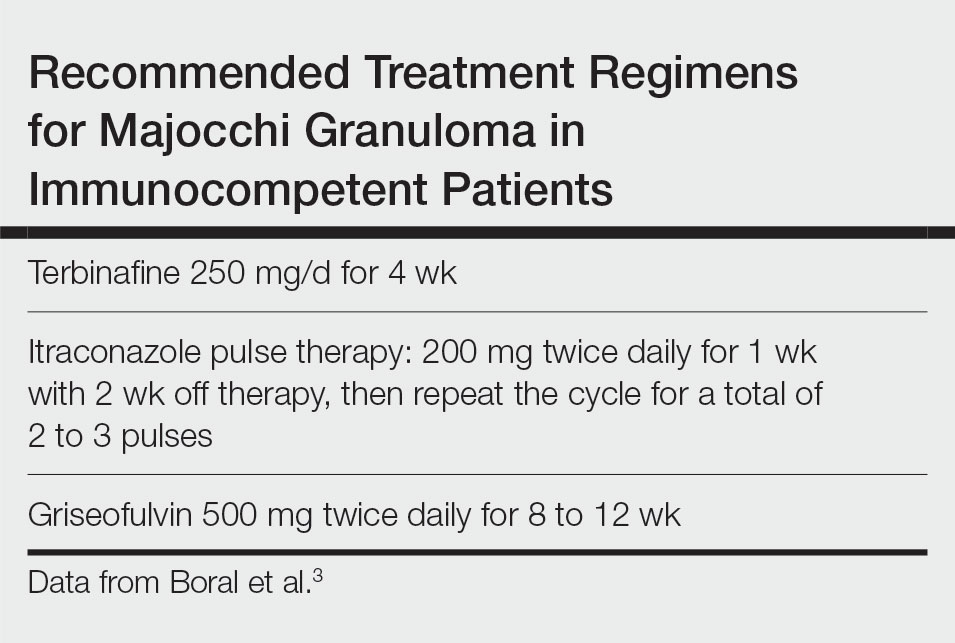
- James WD, Berger T, Elston DM. Diseases resulting from fungi and yeasts. In: James WD, Berger T, Elston D, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Saunders Elsevier; 2016:285-318.
- Li FQ, Lv S, Xia JX. Majocchi’s granuloma after topical corticosteroids therapy. Case Rep Dermatol Med. 2014;2014:507176.
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives. Infect Drug Resist. 2018;11:751-760.
- I˙lkit M, Durdu M, Karakas¸ M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
The Diagnosis: Majocchi Granuloma
Histopathology showed rare follicular-based organisms highlighted by periodic acid–Schiff staining. This finding along with her use of clobetasol ointment on the hands led to a diagnosis of Majocchi granuloma in our patient. Clobetasol and crisaborole ointments were discontinued, and she was started on oral terbinafine 250 mg daily for 4 weeks, which resulted in resolution of the rash.
Majocchi granuloma (also known as nodular granulomatous perifolliculitis) is a perifollicular granulomatous process caused by a dermatophyte infection of the hair follicles. Trichophyton rubrum is the most commonly implicated organism, followed by Trichophyton mentagrophytes and Epidermophyton floccosum, which also cause tinea corporis and tinea pedis.1 This condition most commonly occurs in women aged 20 to 35 years. Risk factors include trauma, occlusion of the hair follicles, immunosuppression, and use of potent topical corticosteroids in patients with tinea.2,3 Immunocompetent patients present with perifollicular papules or pustules with erythematous scaly plaques on the extremities, while immunocompromised patients may have subcutaneous nodules or abscesses on any hair-bearing parts of the body.3
Majocchi granuloma is considered a dermal fungal infection in which the disruption of hair follicles from occlusion or trauma allows fungal organisms and keratinaceous material substrates to be introduced into the dermis. The differential diagnosis is based on the types of presenting lesions. The papules of Majocchi granuloma can resemble folliculitis, acne, or insect bites, while nodules can resemble erythema nodosum or furunculosis.4 Plaques, such as those seen in our patient, can mimic cellulitis and allergic or irritant contact dermatitis.4 Additionally, the plaques may appear annular or figurate, which may resemble erythema gyratum repens or erythema annulare centrifugum.
The diagnosis of Majocchi granuloma often requires fungal culture and biopsy because a potassium hydroxide preparation is unable to distinguish between superficial and invasive dermatophytes.3 Histopathology will show perifollicular granulomatous inflammation. Fungal elements can be detected with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining of the hairs and hair follicles as well as dermal infiltrates.4
Topical corticosteroids should be discontinued. Systemic antifungals are the treatment of choice for Majocchi granuloma, as topical antifungals are not effective against deep fungal infections. Although there are no standard guidelines on duration or dosage, recommended regimens in immunocompetent patients include terbinafine 250 mg/d for 4 weeks; itraconazole pulse therapy consisting of 200 mg twice daily for 1 week with 2 weeks off therapy, then repeat the cycle for a total of 2 to 3 pulses; and griseofulvin 500 mg twice daily for 8 to 12 weeks (Table).3 For immunocompromised patients, combination therapy with more than one antifungal may be necessary.

The Diagnosis: Majocchi Granuloma
Histopathology showed rare follicular-based organisms highlighted by periodic acid–Schiff staining. This finding along with her use of clobetasol ointment on the hands led to a diagnosis of Majocchi granuloma in our patient. Clobetasol and crisaborole ointments were discontinued, and she was started on oral terbinafine 250 mg daily for 4 weeks, which resulted in resolution of the rash.
Majocchi granuloma (also known as nodular granulomatous perifolliculitis) is a perifollicular granulomatous process caused by a dermatophyte infection of the hair follicles. Trichophyton rubrum is the most commonly implicated organism, followed by Trichophyton mentagrophytes and Epidermophyton floccosum, which also cause tinea corporis and tinea pedis.1 This condition most commonly occurs in women aged 20 to 35 years. Risk factors include trauma, occlusion of the hair follicles, immunosuppression, and use of potent topical corticosteroids in patients with tinea.2,3 Immunocompetent patients present with perifollicular papules or pustules with erythematous scaly plaques on the extremities, while immunocompromised patients may have subcutaneous nodules or abscesses on any hair-bearing parts of the body.3
Majocchi granuloma is considered a dermal fungal infection in which the disruption of hair follicles from occlusion or trauma allows fungal organisms and keratinaceous material substrates to be introduced into the dermis. The differential diagnosis is based on the types of presenting lesions. The papules of Majocchi granuloma can resemble folliculitis, acne, or insect bites, while nodules can resemble erythema nodosum or furunculosis.4 Plaques, such as those seen in our patient, can mimic cellulitis and allergic or irritant contact dermatitis.4 Additionally, the plaques may appear annular or figurate, which may resemble erythema gyratum repens or erythema annulare centrifugum.
The diagnosis of Majocchi granuloma often requires fungal culture and biopsy because a potassium hydroxide preparation is unable to distinguish between superficial and invasive dermatophytes.3 Histopathology will show perifollicular granulomatous inflammation. Fungal elements can be detected with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining of the hairs and hair follicles as well as dermal infiltrates.4
Topical corticosteroids should be discontinued. Systemic antifungals are the treatment of choice for Majocchi granuloma, as topical antifungals are not effective against deep fungal infections. Although there are no standard guidelines on duration or dosage, recommended regimens in immunocompetent patients include terbinafine 250 mg/d for 4 weeks; itraconazole pulse therapy consisting of 200 mg twice daily for 1 week with 2 weeks off therapy, then repeat the cycle for a total of 2 to 3 pulses; and griseofulvin 500 mg twice daily for 8 to 12 weeks (Table).3 For immunocompromised patients, combination therapy with more than one antifungal may be necessary.

- James WD, Berger T, Elston DM. Diseases resulting from fungi and yeasts. In: James WD, Berger T, Elston D, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Saunders Elsevier; 2016:285-318.
- Li FQ, Lv S, Xia JX. Majocchi’s granuloma after topical corticosteroids therapy. Case Rep Dermatol Med. 2014;2014:507176.
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives. Infect Drug Resist. 2018;11:751-760.
- I˙lkit M, Durdu M, Karakas¸ M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- James WD, Berger T, Elston DM. Diseases resulting from fungi and yeasts. In: James WD, Berger T, Elston D, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Saunders Elsevier; 2016:285-318.
- Li FQ, Lv S, Xia JX. Majocchi’s granuloma after topical corticosteroids therapy. Case Rep Dermatol Med. 2014;2014:507176.
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives. Infect Drug Resist. 2018;11:751-760.
- I˙lkit M, Durdu M, Karakas¸ M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
A 33-year-old woman presented with an asymptomatic rash on the left hand that was suspected by her primary care physician to be a flare of hand dermatitis. The patient had a history of irritant hand dermatitis diagnosed 2 years prior that was suspected to be secondary to frequent handwashing and was well controlled with clobetasol and crisaborole ointments for 1 year. Four months prior to the current presentation, she developed a flare that was refractory to these topical therapies; treatment with biweekly dupilumab 300 mg was initiated by dermatology, but the rash continued to evolve. A punch biopsy was performed to confirm the diagnosis.

Could your practice be more profitable if you outsource?
Outsourcing certain staff functions in a practice to outside contractors working in remote locations has become commonplace in many medical practices.
Health care outsourcing services, also known as virtual assistants (VAs), were already booming in 2017, when volume grew by 36%. Then, the COVID-19 pandemic in 2020 normalized off-site work, which was a boon to outsourcing providers.
The most popular services being outsourced today by medical practices include billing, scribes, telephone calls to patients, and processing prior authorizations.
“Outsourcing is not for everyone, but I’ve seen it work for many practices,” said Lara Hochman, MD, a practice management consultant in Austin, Tex. She said that practices have used outsourcing to solve problems like high staff turnover, tight budgets, and inefficient use of staff.
When in-house staffing is insufficient or not appropriately aligned with the task, outsourcing can produce big savings, said Teri Deabler, a practice management consultant with the Texas Medical Association.
For example, she said that a client was paying an in-house accountant $80,000 a year. When the accountant retired, she was replaced with a part-time bookkeeper earning $20,000 while her accounting work was outsourced at a cost of $20,000 a year. “The practice’s costs for this service were cut in half,” Ms. Deabler said.
What functions lend themselves to outsourcing?
Clinical services are rarely outsourced by individual practices – although hospitals now outsource numerous clinical services – but virtually any kind of administrative service can be contracted out. Outsourcing used to be limited mainly to billing and off-hours phone services, but today, more services are available, such as scribing, processing prior authorizations, accounting and bookkeeping, human resources (HR) and payroll, interactions with social media, recredentialing, medical transcription, and marketing.
Meanwhile, the original outsourced services have evolved. Billing and collections may now be handled by off-shore VAs, and phone services now deal with a wider variety of tasks, such as answering patients’ questions, scheduling appointments, and making referrals.
Ron Holder, chief operating officer of Medical Group Management Association in Englewood, Colo., said that some outsourcing services can also adjust the amount of work provided based on the customer’s needs. “For instance, an IT outsourcer may allow you to scale up IT support for a new big tech project, such as installing a new electronic health record,” he said.
The outsourced service provider, who might work in another state or another country, is connected to the practice by phone and electronically, and represents the practice when dealing with patients, insurers, or other vendors.
“No one, including patients and your physicians, should know that they are dealing with an outsourced company,” said Mr. Holder. “The work, look, and feel of the outsourced functions should be seamless. Employees at the outsourcer should always identify themselves as the practice, not the outsourcing service.”
Dr. Hochman said that many outsourcing companies dedicate a particular worker to a particular practice and train them to work there. One example of this approach is Provider’s Choice Scribe Services, based in San Antonio. On its website, the company notes that each scribe is paired with a doctor and learns his or her documentation preferences, EMR use, and charting requirements.
What medical practices benefit most from outsourcing?
All kinds and sizes of practices contract with outsourcing firms, but the arrangement is particularly useful for smaller practices, Mr. Holder said. “Larger practices have the economies of scale that allow services to be in-house,” he said, “but smaller practices don’t have that opportunity.”
Dr. Hochman added that outsourcing firms can be hired part-time when the practice doesn’t have enough work for a full-time position. Alternatively, a full-time outsourcing firm can perform two or more separate tasks, such as scribing while handling prior authorizations, she said.
Outsourcing is also useful for new practices, Ms. Deabler said. “A new practice is not earning much money, so it has to have a bare-bones staff,” she said. “Billing, for example, should be contracted out, but it won’t cost that much, because the outsourcer typically charges by volume, and the volume in a new practice is low.”
Meanwhile, Mr. Holder said that the outsourcing of prior authorization work can particularly benefit specialty practices because they typically have a lot of prior authorizations to deal with.
The pros and cons of outsourcing
Experts with experience in outsourcing agree there are both pluses and minuses. “Practices with outsourced workers have less overhead, don’t have to deal with staff turnover, and costs may be lower than for in-house staff,” Ms. Deabler said. “However, you have limited control over outsourced workers and the practice may seem more anonymous to patients, so you need to consider this option very carefully.”
“With outsourcing, you lose control,” said John Machata, MD, a recently retired solo family physician in Wickford, R.I. “You’re trusting someone else to do work that you could do anyway.”
When he briefly considered outsourcing the practice’s billing many years ago, he found that billing companies wouldn’t handle bills that took a lot of work, such as getting in touch with the insurance company and explaining the patient’s situation. “They would only handle the easy bills, which the practice could do anyway,” he said.
However, he does think that answering services may be useful to outsource. “Patients are more inclined to call an anonymous entity than the doctor,” he said. When he gave patients his cell phone number, he said that some patients held off from calling because they didn’t want to bother him.
“Outsourced staff should be less expensive than in-house staff,” said Daniel Shay, an attorney at Gosfield & Associates in Philadelphia. “On the other hand, you are liable for the outsourcer’s mistakes. If your outsourced billing company is upcoding claims, your practice would be on the hook for repayment and penalties.”
Mr. Holder said: “An outsourcer ought to be more efficient at its chosen task because that is what they know how to do. This is a plus at a small practice, where the practice manager may need to do the billing, HR, IT, marketing, some legal work, and accounting,” he said. “No one person can do all of those things well.”
He added, however, “If you choose outsourcing and then decide you don’t like it, it’s difficult to unwind the arrangement. Staff that have been dismissed can’t easily be hired back, so it shouldn’t be an easy decision to make.”
Also, sometimes the staff at offshore outsourcing firms may have accents that are harder for patients to understand, and the offshore staff may not readily understand a U.S. caller. However, Dr. Hochman said that practices often have a chance to interview and select specific persons on the offshore team who best fit their needs.
Offshore outsourcing
Outsourcing firms have been moving abroad, where costs are lower. Typical venues are India and the Philippines because there are larger percentages of people who speak English. Since 2020, demand at offshore medical billing companies has been growing faster than their domestic counterparts, according to a recent analysis.
The difference in price can be substantial. In 2020, the average salary for scribes in India was $500 a month, compared with $2,500 for scribes in the United States.
However, offshore outsourcing is starting to face limitations in some places because of privacy issues, according to David J. Zetter, a practice management consultant in Mechanicsburg, Pa. He pointed to a new Florida law that limits use of offshore vendors because they deal with confidential patient information. The law, which became effective July 1, requires that any protected health information must be maintained in the United States or Canada.
“This will make it very hard for many types of offshore vendors to operate in Florida,” he said. He noted that Florida is the only state with such a restriction, but similar proposals are under consideration in a few other states, such as Texas.
How to select the right company
Mr. Zetter said that the biggest mistake practices make when choosing a company is failing to take enough time to examine their choice. “Quite often, practices don’t validate that companies know what they are doing,” he said. “They get a recommendation and go with it.”
“Choose a company with experience in your specialty,” Mr. Zetter advised. “Speak with the company’s clients, not just the ones the company gives you to speak to. You should ask for the full list of clients and speak to all of them.”
Ms. Deabler said that it’s fairly easy to find respected outsourcing companies. “Colleagues can make recommendations, state and specialty societies can provide lists of preferred vendors, and you can visit vendors’ booths at medical conferences,” she said. She added that it’s also easy to find evaluations of each company. “You can Google the company and come up with all kinds of information about it,” she said.
Mr. Shay said that practices should make sure they understand the terms of the contract with a VA. “Depending on how the contract is worded, you may be stuck with the relationship for many years,” he said. “Before you sign an outsourcing contract, you need to make sure it has a reasonable termination provision.”
Because vetting companies properly can require extensive work, Ms. Deabler said, the work can be given to an experienced practice management consultant. “The consultant can start with a cost-benefit analysis that will show you whether outsourcing would be worthwhile,” she said.
Working with outsource service providers
Mr. Holder said that doctors should keep track of what the outsourcer is doing rather than simply let them do their work. “For example, doctors should understand the billing codes they use most often, such as the five levels of evaluation and management codes, and not just blindly rely on the billing company to code and bill their work correctly,” he noted.
Ms. Deabler said that companies provide monthly reports on their work. “Doctors should be reading these reports and contacting the company if expectations aren’t met,” she said.
Even in the reports, companies can hide problems from untrained eyes, Mr. Holder said. “For example, anyone can meet a metric like days in accounts receivable simply by writing off any charge that isn’t paid after 90 days.”
“You need to be engaged with the outsourcer,” he said. “It’s also a good idea to bring in a consultant to periodically check an outsourcer’s work.”
Will outsourcing expand in the future?
Mr. Holder said that the increasing use of value-based care may require practices to rely more on outsourcing in the future. “For instance, if a practice has a value-based contract that requires providing behavioral health services to patients, it might make sense to outsource that work rather than hire psychologists in-house,” he said.
Practices rarely outsource clinical services, but Mr. Holder said that this may happen in the future: “Now that Medicare is paying less for telehealth, practices have to find a way to provide it without using expensive examining room space,” he said. “Some practices may decide to outsource telehealth instead.”
Mr. Shay said that there are many reasons why outsourcing has a strong future. “It allows you to concentrate on your clinical care, and it is a solution to problems with turnover of in-house staff,” he said. “It can also be more efficient because the service is presumably an expert in areas like billing and collections, which means it may be able to ensure more efficient and faster reimbursements. And if the work is outsourced overseas, you can save money through lower worker salaries.”
A version of this article first appeared on Medscape.com.
Outsourcing certain staff functions in a practice to outside contractors working in remote locations has become commonplace in many medical practices.
Health care outsourcing services, also known as virtual assistants (VAs), were already booming in 2017, when volume grew by 36%. Then, the COVID-19 pandemic in 2020 normalized off-site work, which was a boon to outsourcing providers.
The most popular services being outsourced today by medical practices include billing, scribes, telephone calls to patients, and processing prior authorizations.
“Outsourcing is not for everyone, but I’ve seen it work for many practices,” said Lara Hochman, MD, a practice management consultant in Austin, Tex. She said that practices have used outsourcing to solve problems like high staff turnover, tight budgets, and inefficient use of staff.
When in-house staffing is insufficient or not appropriately aligned with the task, outsourcing can produce big savings, said Teri Deabler, a practice management consultant with the Texas Medical Association.
For example, she said that a client was paying an in-house accountant $80,000 a year. When the accountant retired, she was replaced with a part-time bookkeeper earning $20,000 while her accounting work was outsourced at a cost of $20,000 a year. “The practice’s costs for this service were cut in half,” Ms. Deabler said.
What functions lend themselves to outsourcing?
Clinical services are rarely outsourced by individual practices – although hospitals now outsource numerous clinical services – but virtually any kind of administrative service can be contracted out. Outsourcing used to be limited mainly to billing and off-hours phone services, but today, more services are available, such as scribing, processing prior authorizations, accounting and bookkeeping, human resources (HR) and payroll, interactions with social media, recredentialing, medical transcription, and marketing.
Meanwhile, the original outsourced services have evolved. Billing and collections may now be handled by off-shore VAs, and phone services now deal with a wider variety of tasks, such as answering patients’ questions, scheduling appointments, and making referrals.
Ron Holder, chief operating officer of Medical Group Management Association in Englewood, Colo., said that some outsourcing services can also adjust the amount of work provided based on the customer’s needs. “For instance, an IT outsourcer may allow you to scale up IT support for a new big tech project, such as installing a new electronic health record,” he said.
The outsourced service provider, who might work in another state or another country, is connected to the practice by phone and electronically, and represents the practice when dealing with patients, insurers, or other vendors.
“No one, including patients and your physicians, should know that they are dealing with an outsourced company,” said Mr. Holder. “The work, look, and feel of the outsourced functions should be seamless. Employees at the outsourcer should always identify themselves as the practice, not the outsourcing service.”
Dr. Hochman said that many outsourcing companies dedicate a particular worker to a particular practice and train them to work there. One example of this approach is Provider’s Choice Scribe Services, based in San Antonio. On its website, the company notes that each scribe is paired with a doctor and learns his or her documentation preferences, EMR use, and charting requirements.
What medical practices benefit most from outsourcing?
All kinds and sizes of practices contract with outsourcing firms, but the arrangement is particularly useful for smaller practices, Mr. Holder said. “Larger practices have the economies of scale that allow services to be in-house,” he said, “but smaller practices don’t have that opportunity.”
Dr. Hochman added that outsourcing firms can be hired part-time when the practice doesn’t have enough work for a full-time position. Alternatively, a full-time outsourcing firm can perform two or more separate tasks, such as scribing while handling prior authorizations, she said.
Outsourcing is also useful for new practices, Ms. Deabler said. “A new practice is not earning much money, so it has to have a bare-bones staff,” she said. “Billing, for example, should be contracted out, but it won’t cost that much, because the outsourcer typically charges by volume, and the volume in a new practice is low.”
Meanwhile, Mr. Holder said that the outsourcing of prior authorization work can particularly benefit specialty practices because they typically have a lot of prior authorizations to deal with.
The pros and cons of outsourcing
Experts with experience in outsourcing agree there are both pluses and minuses. “Practices with outsourced workers have less overhead, don’t have to deal with staff turnover, and costs may be lower than for in-house staff,” Ms. Deabler said. “However, you have limited control over outsourced workers and the practice may seem more anonymous to patients, so you need to consider this option very carefully.”
“With outsourcing, you lose control,” said John Machata, MD, a recently retired solo family physician in Wickford, R.I. “You’re trusting someone else to do work that you could do anyway.”
When he briefly considered outsourcing the practice’s billing many years ago, he found that billing companies wouldn’t handle bills that took a lot of work, such as getting in touch with the insurance company and explaining the patient’s situation. “They would only handle the easy bills, which the practice could do anyway,” he said.
However, he does think that answering services may be useful to outsource. “Patients are more inclined to call an anonymous entity than the doctor,” he said. When he gave patients his cell phone number, he said that some patients held off from calling because they didn’t want to bother him.
“Outsourced staff should be less expensive than in-house staff,” said Daniel Shay, an attorney at Gosfield & Associates in Philadelphia. “On the other hand, you are liable for the outsourcer’s mistakes. If your outsourced billing company is upcoding claims, your practice would be on the hook for repayment and penalties.”
Mr. Holder said: “An outsourcer ought to be more efficient at its chosen task because that is what they know how to do. This is a plus at a small practice, where the practice manager may need to do the billing, HR, IT, marketing, some legal work, and accounting,” he said. “No one person can do all of those things well.”
He added, however, “If you choose outsourcing and then decide you don’t like it, it’s difficult to unwind the arrangement. Staff that have been dismissed can’t easily be hired back, so it shouldn’t be an easy decision to make.”
Also, sometimes the staff at offshore outsourcing firms may have accents that are harder for patients to understand, and the offshore staff may not readily understand a U.S. caller. However, Dr. Hochman said that practices often have a chance to interview and select specific persons on the offshore team who best fit their needs.
Offshore outsourcing
Outsourcing firms have been moving abroad, where costs are lower. Typical venues are India and the Philippines because there are larger percentages of people who speak English. Since 2020, demand at offshore medical billing companies has been growing faster than their domestic counterparts, according to a recent analysis.
The difference in price can be substantial. In 2020, the average salary for scribes in India was $500 a month, compared with $2,500 for scribes in the United States.
However, offshore outsourcing is starting to face limitations in some places because of privacy issues, according to David J. Zetter, a practice management consultant in Mechanicsburg, Pa. He pointed to a new Florida law that limits use of offshore vendors because they deal with confidential patient information. The law, which became effective July 1, requires that any protected health information must be maintained in the United States or Canada.
“This will make it very hard for many types of offshore vendors to operate in Florida,” he said. He noted that Florida is the only state with such a restriction, but similar proposals are under consideration in a few other states, such as Texas.
How to select the right company
Mr. Zetter said that the biggest mistake practices make when choosing a company is failing to take enough time to examine their choice. “Quite often, practices don’t validate that companies know what they are doing,” he said. “They get a recommendation and go with it.”
“Choose a company with experience in your specialty,” Mr. Zetter advised. “Speak with the company’s clients, not just the ones the company gives you to speak to. You should ask for the full list of clients and speak to all of them.”
Ms. Deabler said that it’s fairly easy to find respected outsourcing companies. “Colleagues can make recommendations, state and specialty societies can provide lists of preferred vendors, and you can visit vendors’ booths at medical conferences,” she said. She added that it’s also easy to find evaluations of each company. “You can Google the company and come up with all kinds of information about it,” she said.
Mr. Shay said that practices should make sure they understand the terms of the contract with a VA. “Depending on how the contract is worded, you may be stuck with the relationship for many years,” he said. “Before you sign an outsourcing contract, you need to make sure it has a reasonable termination provision.”
Because vetting companies properly can require extensive work, Ms. Deabler said, the work can be given to an experienced practice management consultant. “The consultant can start with a cost-benefit analysis that will show you whether outsourcing would be worthwhile,” she said.
Working with outsource service providers
Mr. Holder said that doctors should keep track of what the outsourcer is doing rather than simply let them do their work. “For example, doctors should understand the billing codes they use most often, such as the five levels of evaluation and management codes, and not just blindly rely on the billing company to code and bill their work correctly,” he noted.
Ms. Deabler said that companies provide monthly reports on their work. “Doctors should be reading these reports and contacting the company if expectations aren’t met,” she said.
Even in the reports, companies can hide problems from untrained eyes, Mr. Holder said. “For example, anyone can meet a metric like days in accounts receivable simply by writing off any charge that isn’t paid after 90 days.”
“You need to be engaged with the outsourcer,” he said. “It’s also a good idea to bring in a consultant to periodically check an outsourcer’s work.”
Will outsourcing expand in the future?
Mr. Holder said that the increasing use of value-based care may require practices to rely more on outsourcing in the future. “For instance, if a practice has a value-based contract that requires providing behavioral health services to patients, it might make sense to outsource that work rather than hire psychologists in-house,” he said.
Practices rarely outsource clinical services, but Mr. Holder said that this may happen in the future: “Now that Medicare is paying less for telehealth, practices have to find a way to provide it without using expensive examining room space,” he said. “Some practices may decide to outsource telehealth instead.”
Mr. Shay said that there are many reasons why outsourcing has a strong future. “It allows you to concentrate on your clinical care, and it is a solution to problems with turnover of in-house staff,” he said. “It can also be more efficient because the service is presumably an expert in areas like billing and collections, which means it may be able to ensure more efficient and faster reimbursements. And if the work is outsourced overseas, you can save money through lower worker salaries.”
A version of this article first appeared on Medscape.com.
Outsourcing certain staff functions in a practice to outside contractors working in remote locations has become commonplace in many medical practices.
Health care outsourcing services, also known as virtual assistants (VAs), were already booming in 2017, when volume grew by 36%. Then, the COVID-19 pandemic in 2020 normalized off-site work, which was a boon to outsourcing providers.
The most popular services being outsourced today by medical practices include billing, scribes, telephone calls to patients, and processing prior authorizations.
“Outsourcing is not for everyone, but I’ve seen it work for many practices,” said Lara Hochman, MD, a practice management consultant in Austin, Tex. She said that practices have used outsourcing to solve problems like high staff turnover, tight budgets, and inefficient use of staff.
When in-house staffing is insufficient or not appropriately aligned with the task, outsourcing can produce big savings, said Teri Deabler, a practice management consultant with the Texas Medical Association.
For example, she said that a client was paying an in-house accountant $80,000 a year. When the accountant retired, she was replaced with a part-time bookkeeper earning $20,000 while her accounting work was outsourced at a cost of $20,000 a year. “The practice’s costs for this service were cut in half,” Ms. Deabler said.
What functions lend themselves to outsourcing?
Clinical services are rarely outsourced by individual practices – although hospitals now outsource numerous clinical services – but virtually any kind of administrative service can be contracted out. Outsourcing used to be limited mainly to billing and off-hours phone services, but today, more services are available, such as scribing, processing prior authorizations, accounting and bookkeeping, human resources (HR) and payroll, interactions with social media, recredentialing, medical transcription, and marketing.
Meanwhile, the original outsourced services have evolved. Billing and collections may now be handled by off-shore VAs, and phone services now deal with a wider variety of tasks, such as answering patients’ questions, scheduling appointments, and making referrals.
Ron Holder, chief operating officer of Medical Group Management Association in Englewood, Colo., said that some outsourcing services can also adjust the amount of work provided based on the customer’s needs. “For instance, an IT outsourcer may allow you to scale up IT support for a new big tech project, such as installing a new electronic health record,” he said.
The outsourced service provider, who might work in another state or another country, is connected to the practice by phone and electronically, and represents the practice when dealing with patients, insurers, or other vendors.
“No one, including patients and your physicians, should know that they are dealing with an outsourced company,” said Mr. Holder. “The work, look, and feel of the outsourced functions should be seamless. Employees at the outsourcer should always identify themselves as the practice, not the outsourcing service.”
Dr. Hochman said that many outsourcing companies dedicate a particular worker to a particular practice and train them to work there. One example of this approach is Provider’s Choice Scribe Services, based in San Antonio. On its website, the company notes that each scribe is paired with a doctor and learns his or her documentation preferences, EMR use, and charting requirements.
What medical practices benefit most from outsourcing?
All kinds and sizes of practices contract with outsourcing firms, but the arrangement is particularly useful for smaller practices, Mr. Holder said. “Larger practices have the economies of scale that allow services to be in-house,” he said, “but smaller practices don’t have that opportunity.”
Dr. Hochman added that outsourcing firms can be hired part-time when the practice doesn’t have enough work for a full-time position. Alternatively, a full-time outsourcing firm can perform two or more separate tasks, such as scribing while handling prior authorizations, she said.
Outsourcing is also useful for new practices, Ms. Deabler said. “A new practice is not earning much money, so it has to have a bare-bones staff,” she said. “Billing, for example, should be contracted out, but it won’t cost that much, because the outsourcer typically charges by volume, and the volume in a new practice is low.”
Meanwhile, Mr. Holder said that the outsourcing of prior authorization work can particularly benefit specialty practices because they typically have a lot of prior authorizations to deal with.
The pros and cons of outsourcing
Experts with experience in outsourcing agree there are both pluses and minuses. “Practices with outsourced workers have less overhead, don’t have to deal with staff turnover, and costs may be lower than for in-house staff,” Ms. Deabler said. “However, you have limited control over outsourced workers and the practice may seem more anonymous to patients, so you need to consider this option very carefully.”
“With outsourcing, you lose control,” said John Machata, MD, a recently retired solo family physician in Wickford, R.I. “You’re trusting someone else to do work that you could do anyway.”
When he briefly considered outsourcing the practice’s billing many years ago, he found that billing companies wouldn’t handle bills that took a lot of work, such as getting in touch with the insurance company and explaining the patient’s situation. “They would only handle the easy bills, which the practice could do anyway,” he said.
However, he does think that answering services may be useful to outsource. “Patients are more inclined to call an anonymous entity than the doctor,” he said. When he gave patients his cell phone number, he said that some patients held off from calling because they didn’t want to bother him.
“Outsourced staff should be less expensive than in-house staff,” said Daniel Shay, an attorney at Gosfield & Associates in Philadelphia. “On the other hand, you are liable for the outsourcer’s mistakes. If your outsourced billing company is upcoding claims, your practice would be on the hook for repayment and penalties.”
Mr. Holder said: “An outsourcer ought to be more efficient at its chosen task because that is what they know how to do. This is a plus at a small practice, where the practice manager may need to do the billing, HR, IT, marketing, some legal work, and accounting,” he said. “No one person can do all of those things well.”
He added, however, “If you choose outsourcing and then decide you don’t like it, it’s difficult to unwind the arrangement. Staff that have been dismissed can’t easily be hired back, so it shouldn’t be an easy decision to make.”
Also, sometimes the staff at offshore outsourcing firms may have accents that are harder for patients to understand, and the offshore staff may not readily understand a U.S. caller. However, Dr. Hochman said that practices often have a chance to interview and select specific persons on the offshore team who best fit their needs.
Offshore outsourcing
Outsourcing firms have been moving abroad, where costs are lower. Typical venues are India and the Philippines because there are larger percentages of people who speak English. Since 2020, demand at offshore medical billing companies has been growing faster than their domestic counterparts, according to a recent analysis.
The difference in price can be substantial. In 2020, the average salary for scribes in India was $500 a month, compared with $2,500 for scribes in the United States.
However, offshore outsourcing is starting to face limitations in some places because of privacy issues, according to David J. Zetter, a practice management consultant in Mechanicsburg, Pa. He pointed to a new Florida law that limits use of offshore vendors because they deal with confidential patient information. The law, which became effective July 1, requires that any protected health information must be maintained in the United States or Canada.
“This will make it very hard for many types of offshore vendors to operate in Florida,” he said. He noted that Florida is the only state with such a restriction, but similar proposals are under consideration in a few other states, such as Texas.
How to select the right company
Mr. Zetter said that the biggest mistake practices make when choosing a company is failing to take enough time to examine their choice. “Quite often, practices don’t validate that companies know what they are doing,” he said. “They get a recommendation and go with it.”
“Choose a company with experience in your specialty,” Mr. Zetter advised. “Speak with the company’s clients, not just the ones the company gives you to speak to. You should ask for the full list of clients and speak to all of them.”
Ms. Deabler said that it’s fairly easy to find respected outsourcing companies. “Colleagues can make recommendations, state and specialty societies can provide lists of preferred vendors, and you can visit vendors’ booths at medical conferences,” she said. She added that it’s also easy to find evaluations of each company. “You can Google the company and come up with all kinds of information about it,” she said.
Mr. Shay said that practices should make sure they understand the terms of the contract with a VA. “Depending on how the contract is worded, you may be stuck with the relationship for many years,” he said. “Before you sign an outsourcing contract, you need to make sure it has a reasonable termination provision.”
Because vetting companies properly can require extensive work, Ms. Deabler said, the work can be given to an experienced practice management consultant. “The consultant can start with a cost-benefit analysis that will show you whether outsourcing would be worthwhile,” she said.
Working with outsource service providers
Mr. Holder said that doctors should keep track of what the outsourcer is doing rather than simply let them do their work. “For example, doctors should understand the billing codes they use most often, such as the five levels of evaluation and management codes, and not just blindly rely on the billing company to code and bill their work correctly,” he noted.
Ms. Deabler said that companies provide monthly reports on their work. “Doctors should be reading these reports and contacting the company if expectations aren’t met,” she said.
Even in the reports, companies can hide problems from untrained eyes, Mr. Holder said. “For example, anyone can meet a metric like days in accounts receivable simply by writing off any charge that isn’t paid after 90 days.”
“You need to be engaged with the outsourcer,” he said. “It’s also a good idea to bring in a consultant to periodically check an outsourcer’s work.”
Will outsourcing expand in the future?
Mr. Holder said that the increasing use of value-based care may require practices to rely more on outsourcing in the future. “For instance, if a practice has a value-based contract that requires providing behavioral health services to patients, it might make sense to outsource that work rather than hire psychologists in-house,” he said.
Practices rarely outsource clinical services, but Mr. Holder said that this may happen in the future: “Now that Medicare is paying less for telehealth, practices have to find a way to provide it without using expensive examining room space,” he said. “Some practices may decide to outsource telehealth instead.”
Mr. Shay said that there are many reasons why outsourcing has a strong future. “It allows you to concentrate on your clinical care, and it is a solution to problems with turnover of in-house staff,” he said. “It can also be more efficient because the service is presumably an expert in areas like billing and collections, which means it may be able to ensure more efficient and faster reimbursements. And if the work is outsourced overseas, you can save money through lower worker salaries.”
A version of this article first appeared on Medscape.com.