User login
FDA issues revised warning for adverse effects associated with canagliflozin
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
Carnosine
A powerful endogenous antioxidant found most abundantly in mammalian tissues, especially brain and skeletal muscle tissue, carnosine is a dipeptide of alanine and histidine.1,2,3,4,5.
Carnosine was first isolated in 1900 by the Russian scientist Gulewitsch as a substance extracted from muscle tissue.6,4. L-carnosine (beta-alanyl-L-histidine) is the synthetic version identical to the natural form alpha-alanyl-L-histidine.7 Carnosine has long been reputed to confer immunomodulating, wound healing, antiglycating, and antineoplastic effects.2 Several reports have shown that carnosine can accelerate the healing of surface skin wounds and burns.4,8
Wound healing
An early study by Nagai et al. in 1986 on carnosine in wound healing showed that rats treated locally with carnosine exhibited greater tensile skin strength at an incision site after hydrocortisone had been administered to hinder healing. The investigators concluded that carnosine bolsters wound healing by stimulating early effusion by histamine and of collagen biosynthesis by beta-alanine. They also found that the compound significantly augmented granulation inhibited by cortisone, mitomycin C, 5-fluorouracil, and bleomycin.9
Studies by Fitzpatrick and Fisher in the early 1980s revealed that carnosine acts as a histidine reserve in relation to histamine production during trauma, suggesting a role for carnosine in wound healing.10,11
In 2012, Ansurudeen et al. examined the effects of carnosine in wound healing in a diabetic mouse model. Carnosine was applied locally and injected daily, yielding significant amelioration in wound healing, with analysis revealing elevated expression of growth factors and cytokines implicated in wound healing. The investigators also observed that carnosine supported cell viability in the presence of high glucose in human dermal fibroblasts and microvascular endothelial cells in vitro.2
Other findings with implications for cutaneous therapy
In 2006, Babizhayev reported that the L-carnosine-related peptidomimetic N-acetylcarnosine (N-acetyl-beta-alanyl-L-histidine) can act as a timed-release (carrier) stable version of L-carnosine in cosmetic preparations, including lubricants.6 Babizhayev et al. have since claimed that they have developed a technology using imidazole-containing dipeptide-based compounds (including L-carnosine and derivatives) that enhances protein hydration in photoaged skin.12,13,14
A double-blind comparative study conducted by Dieamant et al. in 2008 in 124 volunteers with sensitive skin aimed to evaluate the therapeutic potential of the combination of the antioxidant L-carnosine and neuromodulatory Rhodiola rosea. For 28 days, the groups of 62 received twice-daily applications of the 1% combination formulation or placebo. Skin barrier function (reduction of transepidermal water loss) improved in the treatment group, and favorable subjective responses regarding skin dryness were reported. Discomfort after the stinging test was also reduced. In vitro results showed that the release of proopiomelanocortin peptides was spurred by treatment, with the elevated levels of neuropeptides and cytokines produced by keratinocytes exposed to UV radiation returning to normal.15
Two years later, Renner et al. showed that carnosine hindered tumor growth in vivo in an NIH3T3-HER2/neu mouse model. They contended that this naturally occurring dipeptide warrants increased consideration and study for its potential as an anticancer agent.16
In 2012, Federici et al. conducted a randomized, evaluator-blinded, controlled comparative trial over 1month to assess the efficacy of twice-daily topical urea 5% with arginine and carnosine (Ureadin Rx) as compared with twice-daily application of a glycerol-based emollient topical product (Dexeryl) in treating xerosis in 40 type 2 diabetes patients (40-75 years of age). Use of the carnosine-containing formulation yielded significantly greater hydration and an 89% decline in Dryness Areas Severity Index (DASI) scores, compared with baseline. The DASI score after 4 weeks of treatment was much lower in the treatment group than the control group. The Visual Analog Scale (VAS) score was also significantly higher in the Ureadin group than the Dexeryl group. The investigators concluded that the topical application of a urea 5%, arginine, and carnosine cream enhances skin hydration and relieves dryness in type 2 diabetic patients in comparison with a control glycerol-based emollient formulation.17
Antiaging potential
In 1993, Reeve et al. showed that dietary or topically applied carnosine potentiated the contact hypersensitivity reaction in hairless mice and prevented the systemic inhibition of this reaction after dorsal skin exposure to UVB. Carnosine was found to also prevent the systemic suppression provoked by the topical application of a lotion containing cis-urocanic acid.3
Carnosine was a key active ingredient in antiaging products evaluated by Kaczvinsky et al. in 2009 in two double-blind, randomized, controlled, split-face studies. The researchers used the Fast Optical in vivo Topometry of Human Skin (FOITS) technique to measure changes in periorbital wrinkles in the two studies in women between the ages of 30 and 70 years old (study 1, n = 42; study 2, n = 35). They reported that 4 weeks of treatment with the test products, which contained niacinamide, the peptides Pal-KT and Pal-KTTKS, and carnosine, ameliorated periorbital skin, enhancing smoothness and diminishing larger wrinkle depth.18
In 2012, Babizhayev et al. conducted a 4-month randomized, double-blind, controlled study with 42 subjects to evaluate the effects on skin aging of oral nonhydrolyzed carnosine (Can-C Plus formulation). Skin parameters exhibited a consistent and significant improvement during 3 months of supplementation in the treatment group, compared with the placebo group, with overall skin appearance enhanced and fine lines diminished based on visual inspection. There were no reports of adverse effects. The investigators concluded that supplementation with nonhydrolyzed carnosine or carcinine in patented oral formulations has potential as an agent for antiaging purposes.19
Two years later, Emanuele et al. conducted an experimental double-blind irradiation study to compare a complex novel topical product (TPF50) consisting of three active ingredients (traditional physical sunscreens, SPF 50; a liposome-encapsulated DNA repair enzymes complex – photolyase, endonuclease, and 8-oxoguanine glycosylase [OGG1]; and a robust antioxidant complex containing carnosine, arazine, and ergothionine) to available DNA repair and antioxidant and growth factor topical products. They found that the new topical agent was the most effective product in reducing three molecular markers (cyclobutane pyrimidine dimers, protein carbonylation, and 8-oxo-7,8-dihydro-2’-deoxyguanosine) in human skin biopsies. The researchers concluded that the carnosine-containing formulation enhances the genomic and proteomic integrity of skin cells after continual UV exposure, suggesting its potential efficacy in lowering the risk of UV-induced cutaneous aging and nonmelanoma skin cancer.20
Conclusion
Carnosine is an intriguing compound with well-documented antioxidant and wound healing activity. While more research is necessary to determine its wider applications in dermatology, recent work in formulating topical products to impart antiaging effects appears to show promise.
References
1. Nutr. Res. Pract. 2011;5:421-8.
2. Amino Acids 2012;43:127-34.
4. Mol. Aspects Med. 1992;13:379-444.
5. Am. J. Ther. 2012;19:e69-89.
7. J. Cosmet. Dermatol. 2004;3:26-34.
8. Nihon Yakurigaku Zasshi. 1992;100:165-72.
12. Int. J. Cosmet. Sci. 2011;33:1-16.
13. Crit. Rev. Ther. Drug Carrier Syst. 2011;28:203-53.
14. Crit. Rev. Ther. Drug Carrier Syst. 2010;27:85-154.
15. J. Cosmet. Dermatol. 2008;7:112-9.
18. J. Cosmet. Dermatol. 2009;8:228-33.
19. J. Dermatolog. Treat. 2012;23:345-84.
20. J. Drugs Dermatol. 2014;13:309-14.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
A powerful endogenous antioxidant found most abundantly in mammalian tissues, especially brain and skeletal muscle tissue, carnosine is a dipeptide of alanine and histidine.1,2,3,4,5.
Carnosine was first isolated in 1900 by the Russian scientist Gulewitsch as a substance extracted from muscle tissue.6,4. L-carnosine (beta-alanyl-L-histidine) is the synthetic version identical to the natural form alpha-alanyl-L-histidine.7 Carnosine has long been reputed to confer immunomodulating, wound healing, antiglycating, and antineoplastic effects.2 Several reports have shown that carnosine can accelerate the healing of surface skin wounds and burns.4,8
Wound healing
An early study by Nagai et al. in 1986 on carnosine in wound healing showed that rats treated locally with carnosine exhibited greater tensile skin strength at an incision site after hydrocortisone had been administered to hinder healing. The investigators concluded that carnosine bolsters wound healing by stimulating early effusion by histamine and of collagen biosynthesis by beta-alanine. They also found that the compound significantly augmented granulation inhibited by cortisone, mitomycin C, 5-fluorouracil, and bleomycin.9
Studies by Fitzpatrick and Fisher in the early 1980s revealed that carnosine acts as a histidine reserve in relation to histamine production during trauma, suggesting a role for carnosine in wound healing.10,11
In 2012, Ansurudeen et al. examined the effects of carnosine in wound healing in a diabetic mouse model. Carnosine was applied locally and injected daily, yielding significant amelioration in wound healing, with analysis revealing elevated expression of growth factors and cytokines implicated in wound healing. The investigators also observed that carnosine supported cell viability in the presence of high glucose in human dermal fibroblasts and microvascular endothelial cells in vitro.2
Other findings with implications for cutaneous therapy
In 2006, Babizhayev reported that the L-carnosine-related peptidomimetic N-acetylcarnosine (N-acetyl-beta-alanyl-L-histidine) can act as a timed-release (carrier) stable version of L-carnosine in cosmetic preparations, including lubricants.6 Babizhayev et al. have since claimed that they have developed a technology using imidazole-containing dipeptide-based compounds (including L-carnosine and derivatives) that enhances protein hydration in photoaged skin.12,13,14
A double-blind comparative study conducted by Dieamant et al. in 2008 in 124 volunteers with sensitive skin aimed to evaluate the therapeutic potential of the combination of the antioxidant L-carnosine and neuromodulatory Rhodiola rosea. For 28 days, the groups of 62 received twice-daily applications of the 1% combination formulation or placebo. Skin barrier function (reduction of transepidermal water loss) improved in the treatment group, and favorable subjective responses regarding skin dryness were reported. Discomfort after the stinging test was also reduced. In vitro results showed that the release of proopiomelanocortin peptides was spurred by treatment, with the elevated levels of neuropeptides and cytokines produced by keratinocytes exposed to UV radiation returning to normal.15
Two years later, Renner et al. showed that carnosine hindered tumor growth in vivo in an NIH3T3-HER2/neu mouse model. They contended that this naturally occurring dipeptide warrants increased consideration and study for its potential as an anticancer agent.16
In 2012, Federici et al. conducted a randomized, evaluator-blinded, controlled comparative trial over 1month to assess the efficacy of twice-daily topical urea 5% with arginine and carnosine (Ureadin Rx) as compared with twice-daily application of a glycerol-based emollient topical product (Dexeryl) in treating xerosis in 40 type 2 diabetes patients (40-75 years of age). Use of the carnosine-containing formulation yielded significantly greater hydration and an 89% decline in Dryness Areas Severity Index (DASI) scores, compared with baseline. The DASI score after 4 weeks of treatment was much lower in the treatment group than the control group. The Visual Analog Scale (VAS) score was also significantly higher in the Ureadin group than the Dexeryl group. The investigators concluded that the topical application of a urea 5%, arginine, and carnosine cream enhances skin hydration and relieves dryness in type 2 diabetic patients in comparison with a control glycerol-based emollient formulation.17
Antiaging potential
In 1993, Reeve et al. showed that dietary or topically applied carnosine potentiated the contact hypersensitivity reaction in hairless mice and prevented the systemic inhibition of this reaction after dorsal skin exposure to UVB. Carnosine was found to also prevent the systemic suppression provoked by the topical application of a lotion containing cis-urocanic acid.3
Carnosine was a key active ingredient in antiaging products evaluated by Kaczvinsky et al. in 2009 in two double-blind, randomized, controlled, split-face studies. The researchers used the Fast Optical in vivo Topometry of Human Skin (FOITS) technique to measure changes in periorbital wrinkles in the two studies in women between the ages of 30 and 70 years old (study 1, n = 42; study 2, n = 35). They reported that 4 weeks of treatment with the test products, which contained niacinamide, the peptides Pal-KT and Pal-KTTKS, and carnosine, ameliorated periorbital skin, enhancing smoothness and diminishing larger wrinkle depth.18
In 2012, Babizhayev et al. conducted a 4-month randomized, double-blind, controlled study with 42 subjects to evaluate the effects on skin aging of oral nonhydrolyzed carnosine (Can-C Plus formulation). Skin parameters exhibited a consistent and significant improvement during 3 months of supplementation in the treatment group, compared with the placebo group, with overall skin appearance enhanced and fine lines diminished based on visual inspection. There were no reports of adverse effects. The investigators concluded that supplementation with nonhydrolyzed carnosine or carcinine in patented oral formulations has potential as an agent for antiaging purposes.19
Two years later, Emanuele et al. conducted an experimental double-blind irradiation study to compare a complex novel topical product (TPF50) consisting of three active ingredients (traditional physical sunscreens, SPF 50; a liposome-encapsulated DNA repair enzymes complex – photolyase, endonuclease, and 8-oxoguanine glycosylase [OGG1]; and a robust antioxidant complex containing carnosine, arazine, and ergothionine) to available DNA repair and antioxidant and growth factor topical products. They found that the new topical agent was the most effective product in reducing three molecular markers (cyclobutane pyrimidine dimers, protein carbonylation, and 8-oxo-7,8-dihydro-2’-deoxyguanosine) in human skin biopsies. The researchers concluded that the carnosine-containing formulation enhances the genomic and proteomic integrity of skin cells after continual UV exposure, suggesting its potential efficacy in lowering the risk of UV-induced cutaneous aging and nonmelanoma skin cancer.20
Conclusion
Carnosine is an intriguing compound with well-documented antioxidant and wound healing activity. While more research is necessary to determine its wider applications in dermatology, recent work in formulating topical products to impart antiaging effects appears to show promise.
References
1. Nutr. Res. Pract. 2011;5:421-8.
2. Amino Acids 2012;43:127-34.
4. Mol. Aspects Med. 1992;13:379-444.
5. Am. J. Ther. 2012;19:e69-89.
7. J. Cosmet. Dermatol. 2004;3:26-34.
8. Nihon Yakurigaku Zasshi. 1992;100:165-72.
12. Int. J. Cosmet. Sci. 2011;33:1-16.
13. Crit. Rev. Ther. Drug Carrier Syst. 2011;28:203-53.
14. Crit. Rev. Ther. Drug Carrier Syst. 2010;27:85-154.
15. J. Cosmet. Dermatol. 2008;7:112-9.
18. J. Cosmet. Dermatol. 2009;8:228-33.
19. J. Dermatolog. Treat. 2012;23:345-84.
20. J. Drugs Dermatol. 2014;13:309-14.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
A powerful endogenous antioxidant found most abundantly in mammalian tissues, especially brain and skeletal muscle tissue, carnosine is a dipeptide of alanine and histidine.1,2,3,4,5.
Carnosine was first isolated in 1900 by the Russian scientist Gulewitsch as a substance extracted from muscle tissue.6,4. L-carnosine (beta-alanyl-L-histidine) is the synthetic version identical to the natural form alpha-alanyl-L-histidine.7 Carnosine has long been reputed to confer immunomodulating, wound healing, antiglycating, and antineoplastic effects.2 Several reports have shown that carnosine can accelerate the healing of surface skin wounds and burns.4,8
Wound healing
An early study by Nagai et al. in 1986 on carnosine in wound healing showed that rats treated locally with carnosine exhibited greater tensile skin strength at an incision site after hydrocortisone had been administered to hinder healing. The investigators concluded that carnosine bolsters wound healing by stimulating early effusion by histamine and of collagen biosynthesis by beta-alanine. They also found that the compound significantly augmented granulation inhibited by cortisone, mitomycin C, 5-fluorouracil, and bleomycin.9
Studies by Fitzpatrick and Fisher in the early 1980s revealed that carnosine acts as a histidine reserve in relation to histamine production during trauma, suggesting a role for carnosine in wound healing.10,11
In 2012, Ansurudeen et al. examined the effects of carnosine in wound healing in a diabetic mouse model. Carnosine was applied locally and injected daily, yielding significant amelioration in wound healing, with analysis revealing elevated expression of growth factors and cytokines implicated in wound healing. The investigators also observed that carnosine supported cell viability in the presence of high glucose in human dermal fibroblasts and microvascular endothelial cells in vitro.2
Other findings with implications for cutaneous therapy
In 2006, Babizhayev reported that the L-carnosine-related peptidomimetic N-acetylcarnosine (N-acetyl-beta-alanyl-L-histidine) can act as a timed-release (carrier) stable version of L-carnosine in cosmetic preparations, including lubricants.6 Babizhayev et al. have since claimed that they have developed a technology using imidazole-containing dipeptide-based compounds (including L-carnosine and derivatives) that enhances protein hydration in photoaged skin.12,13,14
A double-blind comparative study conducted by Dieamant et al. in 2008 in 124 volunteers with sensitive skin aimed to evaluate the therapeutic potential of the combination of the antioxidant L-carnosine and neuromodulatory Rhodiola rosea. For 28 days, the groups of 62 received twice-daily applications of the 1% combination formulation or placebo. Skin barrier function (reduction of transepidermal water loss) improved in the treatment group, and favorable subjective responses regarding skin dryness were reported. Discomfort after the stinging test was also reduced. In vitro results showed that the release of proopiomelanocortin peptides was spurred by treatment, with the elevated levels of neuropeptides and cytokines produced by keratinocytes exposed to UV radiation returning to normal.15
Two years later, Renner et al. showed that carnosine hindered tumor growth in vivo in an NIH3T3-HER2/neu mouse model. They contended that this naturally occurring dipeptide warrants increased consideration and study for its potential as an anticancer agent.16
In 2012, Federici et al. conducted a randomized, evaluator-blinded, controlled comparative trial over 1month to assess the efficacy of twice-daily topical urea 5% with arginine and carnosine (Ureadin Rx) as compared with twice-daily application of a glycerol-based emollient topical product (Dexeryl) in treating xerosis in 40 type 2 diabetes patients (40-75 years of age). Use of the carnosine-containing formulation yielded significantly greater hydration and an 89% decline in Dryness Areas Severity Index (DASI) scores, compared with baseline. The DASI score after 4 weeks of treatment was much lower in the treatment group than the control group. The Visual Analog Scale (VAS) score was also significantly higher in the Ureadin group than the Dexeryl group. The investigators concluded that the topical application of a urea 5%, arginine, and carnosine cream enhances skin hydration and relieves dryness in type 2 diabetic patients in comparison with a control glycerol-based emollient formulation.17
Antiaging potential
In 1993, Reeve et al. showed that dietary or topically applied carnosine potentiated the contact hypersensitivity reaction in hairless mice and prevented the systemic inhibition of this reaction after dorsal skin exposure to UVB. Carnosine was found to also prevent the systemic suppression provoked by the topical application of a lotion containing cis-urocanic acid.3
Carnosine was a key active ingredient in antiaging products evaluated by Kaczvinsky et al. in 2009 in two double-blind, randomized, controlled, split-face studies. The researchers used the Fast Optical in vivo Topometry of Human Skin (FOITS) technique to measure changes in periorbital wrinkles in the two studies in women between the ages of 30 and 70 years old (study 1, n = 42; study 2, n = 35). They reported that 4 weeks of treatment with the test products, which contained niacinamide, the peptides Pal-KT and Pal-KTTKS, and carnosine, ameliorated periorbital skin, enhancing smoothness and diminishing larger wrinkle depth.18
In 2012, Babizhayev et al. conducted a 4-month randomized, double-blind, controlled study with 42 subjects to evaluate the effects on skin aging of oral nonhydrolyzed carnosine (Can-C Plus formulation). Skin parameters exhibited a consistent and significant improvement during 3 months of supplementation in the treatment group, compared with the placebo group, with overall skin appearance enhanced and fine lines diminished based on visual inspection. There were no reports of adverse effects. The investigators concluded that supplementation with nonhydrolyzed carnosine or carcinine in patented oral formulations has potential as an agent for antiaging purposes.19
Two years later, Emanuele et al. conducted an experimental double-blind irradiation study to compare a complex novel topical product (TPF50) consisting of three active ingredients (traditional physical sunscreens, SPF 50; a liposome-encapsulated DNA repair enzymes complex – photolyase, endonuclease, and 8-oxoguanine glycosylase [OGG1]; and a robust antioxidant complex containing carnosine, arazine, and ergothionine) to available DNA repair and antioxidant and growth factor topical products. They found that the new topical agent was the most effective product in reducing three molecular markers (cyclobutane pyrimidine dimers, protein carbonylation, and 8-oxo-7,8-dihydro-2’-deoxyguanosine) in human skin biopsies. The researchers concluded that the carnosine-containing formulation enhances the genomic and proteomic integrity of skin cells after continual UV exposure, suggesting its potential efficacy in lowering the risk of UV-induced cutaneous aging and nonmelanoma skin cancer.20
Conclusion
Carnosine is an intriguing compound with well-documented antioxidant and wound healing activity. While more research is necessary to determine its wider applications in dermatology, recent work in formulating topical products to impart antiaging effects appears to show promise.
References
1. Nutr. Res. Pract. 2011;5:421-8.
2. Amino Acids 2012;43:127-34.
4. Mol. Aspects Med. 1992;13:379-444.
5. Am. J. Ther. 2012;19:e69-89.
7. J. Cosmet. Dermatol. 2004;3:26-34.
8. Nihon Yakurigaku Zasshi. 1992;100:165-72.
12. Int. J. Cosmet. Sci. 2011;33:1-16.
13. Crit. Rev. Ther. Drug Carrier Syst. 2011;28:203-53.
14. Crit. Rev. Ther. Drug Carrier Syst. 2010;27:85-154.
15. J. Cosmet. Dermatol. 2008;7:112-9.
18. J. Cosmet. Dermatol. 2009;8:228-33.
19. J. Dermatolog. Treat. 2012;23:345-84.
20. J. Drugs Dermatol. 2014;13:309-14.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
So much sugar in long-term care
The prevalence of diabetes increases as patients age and gain weight. More than one-third of nursing home residents have diabetes. The overall treatment goals for elderly patients with diabetes are similar to those for younger patients, but somehow the stakes feel higher. Polypharmacy, decreased activity, shifting dietary patterns, hyperglycemia, fears of hypoglycemia leading to falls, worsening comorbid conditions, and hospitalization pose great challenges to ideal management.
Because of these concerns, caution is raised about the use of insulin or oral agents that cause hypoglycemia in frail older adults in long-term care facilities. But these agents are used, and perhaps we understand little about their comparative risks.
Dr. Francisco J. Pasquel of Emory University, Atlanta, and his colleagues conducted a randomized clinical trial evaluating the comparative safety and effectiveness of basal insulin or an oral antidiabetic drug (OAD) for 26 weeks (BMJ Open Diab Res Care. 2015;3:e000104).
A total of 150 patients, average age 79 years, with a blood glucose level greater than 180 mg/dL or a hemoglobin A1c greater than 7.5%, treated with diet or an oral agent, were randomized to either 0.1 U/kg per day of glargine or continuation of oral agents (metformin, insulin secretagogues, thiazolidinediones, or DPP-4 inhibitors). Glargine was adjusted based on blood sugar readings.
In the OAD group, 16% of patients were treated with metformin plus sulfonylurea, 27% with a sulfonylurea alone, and 8% with sulfonylurea and a DPP-4 inhibitor.
There were 62 hypoglycemic events in the OAD group and 43 in the basal insulin group (P = .4). Overall, daily blood glucose levels did not differ between the groups. Rates of cardiovascular events, renal failure, infection, falls, emergency department visits, hospital admissions, and mortality were similar between the two groups.
Although these data are somewhat reassuring, power may have been an issue, and a larger sample size may have resulted in detection of more hypoglycemic events in the OAD group. On the other hand, the data are balanced and resonate with previous data showing that in older adults with diabetes (about 74 years of age), intensive glycemic control is associated with an increased risk of falls in insulin users but not in those treated with OADs. The goal of the current study was not intensive glycemic control.
So, for patients in a long-term care facility, metformin and the DPP-4 inhibitors will be weight neutral without risk of hypoglycemia. Therefore, these may be the “go-to” drugs if the DPP-4 inhibitors are affordable and you do not have a long way to go for control (DPP-4 inhibitors tend to be relatively mild agents, lowering HbA1c by 0.6%). If a sulfonylurea must be used, glipizide should be chosen, because it has a shorter half-life.
Balance all of this with appropriate HbA1c goals for your patient adjusted for medical comorbidity, goals of care, and life expectancy.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
The prevalence of diabetes increases as patients age and gain weight. More than one-third of nursing home residents have diabetes. The overall treatment goals for elderly patients with diabetes are similar to those for younger patients, but somehow the stakes feel higher. Polypharmacy, decreased activity, shifting dietary patterns, hyperglycemia, fears of hypoglycemia leading to falls, worsening comorbid conditions, and hospitalization pose great challenges to ideal management.
Because of these concerns, caution is raised about the use of insulin or oral agents that cause hypoglycemia in frail older adults in long-term care facilities. But these agents are used, and perhaps we understand little about their comparative risks.
Dr. Francisco J. Pasquel of Emory University, Atlanta, and his colleagues conducted a randomized clinical trial evaluating the comparative safety and effectiveness of basal insulin or an oral antidiabetic drug (OAD) for 26 weeks (BMJ Open Diab Res Care. 2015;3:e000104).
A total of 150 patients, average age 79 years, with a blood glucose level greater than 180 mg/dL or a hemoglobin A1c greater than 7.5%, treated with diet or an oral agent, were randomized to either 0.1 U/kg per day of glargine or continuation of oral agents (metformin, insulin secretagogues, thiazolidinediones, or DPP-4 inhibitors). Glargine was adjusted based on blood sugar readings.
In the OAD group, 16% of patients were treated with metformin plus sulfonylurea, 27% with a sulfonylurea alone, and 8% with sulfonylurea and a DPP-4 inhibitor.
There were 62 hypoglycemic events in the OAD group and 43 in the basal insulin group (P = .4). Overall, daily blood glucose levels did not differ between the groups. Rates of cardiovascular events, renal failure, infection, falls, emergency department visits, hospital admissions, and mortality were similar between the two groups.
Although these data are somewhat reassuring, power may have been an issue, and a larger sample size may have resulted in detection of more hypoglycemic events in the OAD group. On the other hand, the data are balanced and resonate with previous data showing that in older adults with diabetes (about 74 years of age), intensive glycemic control is associated with an increased risk of falls in insulin users but not in those treated with OADs. The goal of the current study was not intensive glycemic control.
So, for patients in a long-term care facility, metformin and the DPP-4 inhibitors will be weight neutral without risk of hypoglycemia. Therefore, these may be the “go-to” drugs if the DPP-4 inhibitors are affordable and you do not have a long way to go for control (DPP-4 inhibitors tend to be relatively mild agents, lowering HbA1c by 0.6%). If a sulfonylurea must be used, glipizide should be chosen, because it has a shorter half-life.
Balance all of this with appropriate HbA1c goals for your patient adjusted for medical comorbidity, goals of care, and life expectancy.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
The prevalence of diabetes increases as patients age and gain weight. More than one-third of nursing home residents have diabetes. The overall treatment goals for elderly patients with diabetes are similar to those for younger patients, but somehow the stakes feel higher. Polypharmacy, decreased activity, shifting dietary patterns, hyperglycemia, fears of hypoglycemia leading to falls, worsening comorbid conditions, and hospitalization pose great challenges to ideal management.
Because of these concerns, caution is raised about the use of insulin or oral agents that cause hypoglycemia in frail older adults in long-term care facilities. But these agents are used, and perhaps we understand little about their comparative risks.
Dr. Francisco J. Pasquel of Emory University, Atlanta, and his colleagues conducted a randomized clinical trial evaluating the comparative safety and effectiveness of basal insulin or an oral antidiabetic drug (OAD) for 26 weeks (BMJ Open Diab Res Care. 2015;3:e000104).
A total of 150 patients, average age 79 years, with a blood glucose level greater than 180 mg/dL or a hemoglobin A1c greater than 7.5%, treated with diet or an oral agent, were randomized to either 0.1 U/kg per day of glargine or continuation of oral agents (metformin, insulin secretagogues, thiazolidinediones, or DPP-4 inhibitors). Glargine was adjusted based on blood sugar readings.
In the OAD group, 16% of patients were treated with metformin plus sulfonylurea, 27% with a sulfonylurea alone, and 8% with sulfonylurea and a DPP-4 inhibitor.
There were 62 hypoglycemic events in the OAD group and 43 in the basal insulin group (P = .4). Overall, daily blood glucose levels did not differ between the groups. Rates of cardiovascular events, renal failure, infection, falls, emergency department visits, hospital admissions, and mortality were similar between the two groups.
Although these data are somewhat reassuring, power may have been an issue, and a larger sample size may have resulted in detection of more hypoglycemic events in the OAD group. On the other hand, the data are balanced and resonate with previous data showing that in older adults with diabetes (about 74 years of age), intensive glycemic control is associated with an increased risk of falls in insulin users but not in those treated with OADs. The goal of the current study was not intensive glycemic control.
So, for patients in a long-term care facility, metformin and the DPP-4 inhibitors will be weight neutral without risk of hypoglycemia. Therefore, these may be the “go-to” drugs if the DPP-4 inhibitors are affordable and you do not have a long way to go for control (DPP-4 inhibitors tend to be relatively mild agents, lowering HbA1c by 0.6%). If a sulfonylurea must be used, glipizide should be chosen, because it has a shorter half-life.
Balance all of this with appropriate HbA1c goals for your patient adjusted for medical comorbidity, goals of care, and life expectancy.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Psoriasis and acne worse in winter, milder in summer
Psoriasis and acne appear to be susceptible to seasonal variations of clearing and worsening, with an analysis revealing both conditions maintained a significant trend of summer clearing and winter worsening, according to a research letter published in the Journal of the American Academy of Dermatology.
Using Physician’s Global Assessment scales for psoriasis and acne, Dr. Vanessa Lindsay Pascoe and Dr. Alexandra Boer Kimball, both of Massachusetts General Hospital, Boston, collected data from 5,468 psoriasis patients and 9,301 acne patients between June 2011 and May 2014 in the New England area. Among the psoriasis patient group, 16% were seen in the summer, 25% in the fall, 31% in the winter, and 28% in the spring. The trend was similar for acne patients, with 18% seen in the summer, 25% in the fall, 28% in the winter, and 29% in the spring. There were no significant seasonal differences in age or sex for either group.
The percentage of psoriasis patients with clear/almost clear disease was highest in the summer at 20.4%, while the percentage of patients with moderate/severe disease was highest in the winter at 40.5%. For acne, the percentage of patients with clear/almost clear disease was highest in the fall at 17.5%, and the percentage of patients with moderate/severe disease was highest in the winter at 45.9%. Fewer psoriasis and acne patients presented to the clinic in the summer, which the researchers suggested could be due to disease improvement.
“Although the climate of the Northeastern United States may not generalize to regions with less seasonal variation, providers may consider seasonal adjustment of acne plans as they have traditionally done for psoriasis,” the authors wrote. “For example, they may wait until after winter to taper a systemic antibiotic for acne, just as some providers may wait until spring to change systemic psoriasis treatments.”
Read the full article in the Journal of the American Academy of Dermatology.
Psoriasis and acne appear to be susceptible to seasonal variations of clearing and worsening, with an analysis revealing both conditions maintained a significant trend of summer clearing and winter worsening, according to a research letter published in the Journal of the American Academy of Dermatology.
Using Physician’s Global Assessment scales for psoriasis and acne, Dr. Vanessa Lindsay Pascoe and Dr. Alexandra Boer Kimball, both of Massachusetts General Hospital, Boston, collected data from 5,468 psoriasis patients and 9,301 acne patients between June 2011 and May 2014 in the New England area. Among the psoriasis patient group, 16% were seen in the summer, 25% in the fall, 31% in the winter, and 28% in the spring. The trend was similar for acne patients, with 18% seen in the summer, 25% in the fall, 28% in the winter, and 29% in the spring. There were no significant seasonal differences in age or sex for either group.
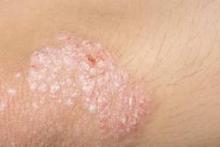
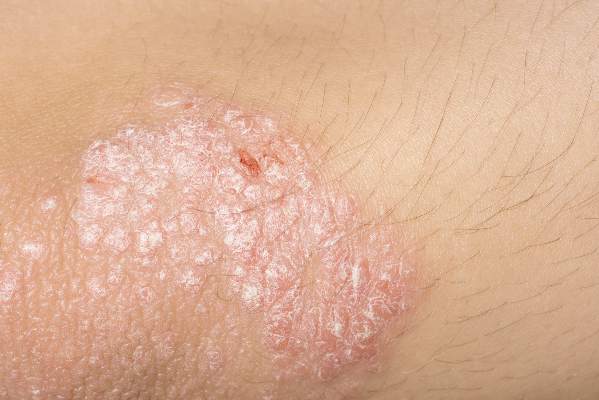
The percentage of psoriasis patients with clear/almost clear disease was highest in the summer at 20.4%, while the percentage of patients with moderate/severe disease was highest in the winter at 40.5%. For acne, the percentage of patients with clear/almost clear disease was highest in the fall at 17.5%, and the percentage of patients with moderate/severe disease was highest in the winter at 45.9%. Fewer psoriasis and acne patients presented to the clinic in the summer, which the researchers suggested could be due to disease improvement.
“Although the climate of the Northeastern United States may not generalize to regions with less seasonal variation, providers may consider seasonal adjustment of acne plans as they have traditionally done for psoriasis,” the authors wrote. “For example, they may wait until after winter to taper a systemic antibiotic for acne, just as some providers may wait until spring to change systemic psoriasis treatments.”
Read the full article in the Journal of the American Academy of Dermatology.
Psoriasis and acne appear to be susceptible to seasonal variations of clearing and worsening, with an analysis revealing both conditions maintained a significant trend of summer clearing and winter worsening, according to a research letter published in the Journal of the American Academy of Dermatology.
Using Physician’s Global Assessment scales for psoriasis and acne, Dr. Vanessa Lindsay Pascoe and Dr. Alexandra Boer Kimball, both of Massachusetts General Hospital, Boston, collected data from 5,468 psoriasis patients and 9,301 acne patients between June 2011 and May 2014 in the New England area. Among the psoriasis patient group, 16% were seen in the summer, 25% in the fall, 31% in the winter, and 28% in the spring. The trend was similar for acne patients, with 18% seen in the summer, 25% in the fall, 28% in the winter, and 29% in the spring. There were no significant seasonal differences in age or sex for either group.
The percentage of psoriasis patients with clear/almost clear disease was highest in the summer at 20.4%, while the percentage of patients with moderate/severe disease was highest in the winter at 40.5%. For acne, the percentage of patients with clear/almost clear disease was highest in the fall at 17.5%, and the percentage of patients with moderate/severe disease was highest in the winter at 45.9%. Fewer psoriasis and acne patients presented to the clinic in the summer, which the researchers suggested could be due to disease improvement.
“Although the climate of the Northeastern United States may not generalize to regions with less seasonal variation, providers may consider seasonal adjustment of acne plans as they have traditionally done for psoriasis,” the authors wrote. “For example, they may wait until after winter to taper a systemic antibiotic for acne, just as some providers may wait until spring to change systemic psoriasis treatments.”
Read the full article in the Journal of the American Academy of Dermatology.
AHA begins Cryptogenic Stroke Initiative
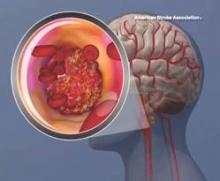
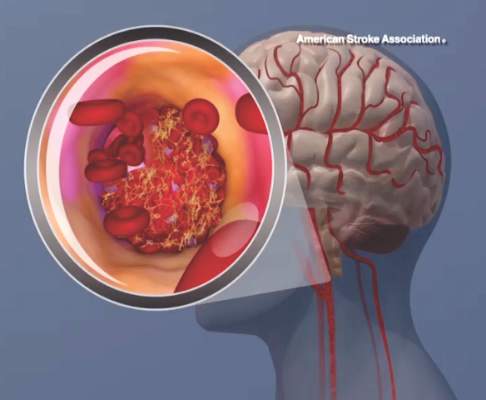
Cryptogenic stroke patients and their caregivers recently surveyed by the American Heart Association/American Stroke Association said that they experience several negative consequences of having an undetermined cause of their stroke, and only one-fifth received information about cryptogenic stroke at the time of diagnosis.
This lack of information prompted the organization to start the Cryptogenic Stroke Initiative. The initiative will inform cryptogenic stroke patients and help “them to work with their healthcare team to prevent a second stroke,” Dr. Mary Ann Bauman, chair of the American Stroke Association Advisory Committee, said in an announcement.
This month, the American Heart Association/American Stroke Association, with support from Medtronic, published a free informational document. This Patient Guide to Cryptogenic Stroke is the first of several guides that the American Stroke Association plans to create for cryptogenic stroke patients. This edition of the guide includes “information on cryptogenic stroke diagnosis, what happens during a stroke, secondary prevention information, questions [for patients to ask their doctor, and] support resources,” the Association said.
In the organizations’ survey, which included 309 cryptogenic stroke patients and caregivers, more than 50% reported anxiety and frustration when the cause of stroke is undetermined. Of the 20% of patients and caregivers who received information about cryptogenic stroke at the time of diagnosis, the information was verbally communicated 75% of the time.
Cryptogenic stroke patients and their caregivers recently surveyed by the American Heart Association/American Stroke Association said that they experience several negative consequences of having an undetermined cause of their stroke, and only one-fifth received information about cryptogenic stroke at the time of diagnosis.
This lack of information prompted the organization to start the Cryptogenic Stroke Initiative. The initiative will inform cryptogenic stroke patients and help “them to work with their healthcare team to prevent a second stroke,” Dr. Mary Ann Bauman, chair of the American Stroke Association Advisory Committee, said in an announcement.
This month, the American Heart Association/American Stroke Association, with support from Medtronic, published a free informational document. This Patient Guide to Cryptogenic Stroke is the first of several guides that the American Stroke Association plans to create for cryptogenic stroke patients. This edition of the guide includes “information on cryptogenic stroke diagnosis, what happens during a stroke, secondary prevention information, questions [for patients to ask their doctor, and] support resources,” the Association said.
In the organizations’ survey, which included 309 cryptogenic stroke patients and caregivers, more than 50% reported anxiety and frustration when the cause of stroke is undetermined. Of the 20% of patients and caregivers who received information about cryptogenic stroke at the time of diagnosis, the information was verbally communicated 75% of the time.
Cryptogenic stroke patients and their caregivers recently surveyed by the American Heart Association/American Stroke Association said that they experience several negative consequences of having an undetermined cause of their stroke, and only one-fifth received information about cryptogenic stroke at the time of diagnosis.
This lack of information prompted the organization to start the Cryptogenic Stroke Initiative. The initiative will inform cryptogenic stroke patients and help “them to work with their healthcare team to prevent a second stroke,” Dr. Mary Ann Bauman, chair of the American Stroke Association Advisory Committee, said in an announcement.
This month, the American Heart Association/American Stroke Association, with support from Medtronic, published a free informational document. This Patient Guide to Cryptogenic Stroke is the first of several guides that the American Stroke Association plans to create for cryptogenic stroke patients. This edition of the guide includes “information on cryptogenic stroke diagnosis, what happens during a stroke, secondary prevention information, questions [for patients to ask their doctor, and] support resources,” the Association said.
In the organizations’ survey, which included 309 cryptogenic stroke patients and caregivers, more than 50% reported anxiety and frustration when the cause of stroke is undetermined. Of the 20% of patients and caregivers who received information about cryptogenic stroke at the time of diagnosis, the information was verbally communicated 75% of the time.
Link Found Between Agent Orange Exposure and Multiple Myeloma
There was a 2.4-fold increased risk for monoclonal gammopathy of undetermined significance (MGUS), a precursor to multiple myeloma (MM), for Air Force veterans exposed to Agent Orange, according to a study reported in JAMA Oncology. Already, veterans who develop MM and were exposed to Agent Orange during military service are eligible to receive benefits, but the study further highlights the relationship.
Related: Management of Myeloma and Its Precursor Syndromes
The Agent Orange used during aerial spray missions of herbicides in the Vietnam War contained 2, 4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), as well as human carcinogen 2,3,7,8-tetrachlorodibenzo-p-doxin in variable amounts. After obtaining the laboratory data from 958 serum samples from Air Force personnel, the Air Force Health Studies questionnaire, and results from the physical exam from all participants, researchers were able to compare their findings with control group veterans.
Related: Nephrotic Syndrome Is a Marker for Occult Cancer
The researchers created 2 test groups from Air Force veterans. The first were 777 participants of Operation Ranch Hand, who conducted aerial herbicidal missions from 1962 to 1971, and the second group was made up of 1,174 participants, who had similar duties but did not participate in the missions.
The risk of MGUS was more pronounced in veterans aged > 70 years (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.27-4.44; P = .007). Among veterans aged > 70 years, there was not a significant increase in risk (OR, 1.4%; 95% CI, 0.55-3.63; P = .63). The crude prevalence of overall MGUS was 7.1% (34 of 479) in the exposed veterans, compared with 3.1% (15 of 479) in the comparison group.
Source
Landgren O, Shim YK, Michalek J, et al. JAMA Oncol. [Published online ahead of print September 3, 2015.]
doi: 10.1001/jamaoncol.2015.2938.
There was a 2.4-fold increased risk for monoclonal gammopathy of undetermined significance (MGUS), a precursor to multiple myeloma (MM), for Air Force veterans exposed to Agent Orange, according to a study reported in JAMA Oncology. Already, veterans who develop MM and were exposed to Agent Orange during military service are eligible to receive benefits, but the study further highlights the relationship.
Related: Management of Myeloma and Its Precursor Syndromes
The Agent Orange used during aerial spray missions of herbicides in the Vietnam War contained 2, 4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), as well as human carcinogen 2,3,7,8-tetrachlorodibenzo-p-doxin in variable amounts. After obtaining the laboratory data from 958 serum samples from Air Force personnel, the Air Force Health Studies questionnaire, and results from the physical exam from all participants, researchers were able to compare their findings with control group veterans.
Related: Nephrotic Syndrome Is a Marker for Occult Cancer
The researchers created 2 test groups from Air Force veterans. The first were 777 participants of Operation Ranch Hand, who conducted aerial herbicidal missions from 1962 to 1971, and the second group was made up of 1,174 participants, who had similar duties but did not participate in the missions.
The risk of MGUS was more pronounced in veterans aged > 70 years (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.27-4.44; P = .007). Among veterans aged > 70 years, there was not a significant increase in risk (OR, 1.4%; 95% CI, 0.55-3.63; P = .63). The crude prevalence of overall MGUS was 7.1% (34 of 479) in the exposed veterans, compared with 3.1% (15 of 479) in the comparison group.
Source
Landgren O, Shim YK, Michalek J, et al. JAMA Oncol. [Published online ahead of print September 3, 2015.]
doi: 10.1001/jamaoncol.2015.2938.
There was a 2.4-fold increased risk for monoclonal gammopathy of undetermined significance (MGUS), a precursor to multiple myeloma (MM), for Air Force veterans exposed to Agent Orange, according to a study reported in JAMA Oncology. Already, veterans who develop MM and were exposed to Agent Orange during military service are eligible to receive benefits, but the study further highlights the relationship.
Related: Management of Myeloma and Its Precursor Syndromes
The Agent Orange used during aerial spray missions of herbicides in the Vietnam War contained 2, 4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), as well as human carcinogen 2,3,7,8-tetrachlorodibenzo-p-doxin in variable amounts. After obtaining the laboratory data from 958 serum samples from Air Force personnel, the Air Force Health Studies questionnaire, and results from the physical exam from all participants, researchers were able to compare their findings with control group veterans.
Related: Nephrotic Syndrome Is a Marker for Occult Cancer
The researchers created 2 test groups from Air Force veterans. The first were 777 participants of Operation Ranch Hand, who conducted aerial herbicidal missions from 1962 to 1971, and the second group was made up of 1,174 participants, who had similar duties but did not participate in the missions.
The risk of MGUS was more pronounced in veterans aged > 70 years (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.27-4.44; P = .007). Among veterans aged > 70 years, there was not a significant increase in risk (OR, 1.4%; 95% CI, 0.55-3.63; P = .63). The crude prevalence of overall MGUS was 7.1% (34 of 479) in the exposed veterans, compared with 3.1% (15 of 479) in the comparison group.
Source
Landgren O, Shim YK, Michalek J, et al. JAMA Oncol. [Published online ahead of print September 3, 2015.]
doi: 10.1001/jamaoncol.2015.2938.
Patchouli
Pogostemon cablin, known in the West as patchouli or guang huo-xiang in China, is a long-time staple in traditional Chinese medicine for various indications, particularly gastrointestinal and skin disorders1.
Patchouli oil, which contains several mono- and sesquiterpenoids, alkaloids, and flavonoids, is thought to possess significant anti-inflammatory and antioxidant qualities2.In fact, it is reputed to impart antiviral, antioxidant, anti-inflammatory, and analgesic effects, and is also known to protect intestinal barrier function3. Peng et al. have found that patchouli oil exerts significant antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA)4.
After a comprehensive 2013 review, Chen et al. deemed P. cablin to have potential clinical benefits as an effective adaptogenic herbal treatment3. It is thought to have some antiacne properties as well1. Further, P. cablin is among the Top 10 most-often-used traditional Chinese medicine prescriptions for skin care and appearance1.
In Brazil, China, Indonesia, and Malaysia, P. cablin is cultivated for its essential oil, which plays an important role in the perfume industry. Patchouli essential oil, featured in perfumes, soaps, cosmetics, and as incense, is used by aromatherapists for its calming and reviving effects. The essential oil has also been shown to impart antioxidant activity5.
In 2014, Lin et al. studied the protective effects of P. cablin essential oil against ultraviolet (UV)-induced skin photoaging in mice. The researchers applied patchouli oil for 2 hours before UV exposure to the dorsal depilated skin of mice. They found that patchouli oil doses of 6 mg/mouse and 9 mg/mouse significantly suppressed skin wrinkle formation, mitigated skin elasticity impairment, and augmented collagen content (21.9% and 26.3%, respectively). The same doses also yielded significant reductions in epidermal thickness and malondialdehyde content, and blocked the disruption of collagen and elastic fibers. Patchouli oil treatment also resulted in the up-regulation of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, and catalase. The investigators concluded that patchouli oil, perhaps due to its antioxidant characteristics, and sesquiterpene constituents in particular, was effective in preventing photoaging in mice, and warrants attention as a potential agent to hinder photoaging in humans1.
Feng et al. also investigated the effects of topically applied patchouli alcohol on UV-induced photoaging in mice that year. For 9 weeks, investigators applied patchouli oil solution or a vehicle to the depilated dorsal skin of 6-week-old mice. They found that patchouli oil significantly hastened the recovery of UV-induced skin lesions, which they ascribed to the antioxidant and anti-inflammatory activity of the agent and its down-regulation of the expression of matrix metalloproteinase (MMP)-1 and MMP-32.
Antimicrobial and mosquito repellent activity
In a 2005 study by Trongtokit et al. of the mosquito-repellent activity of 38 essential oils at three concentrations (10%, 50%, or undiluted) against the mosquito Aedes aegypti under laboratory conditions using human volunteers, undiluted P. cablin oil was one of four [along with Cymbopogon nardus (citronella), Syzygium aromaticum (clove), and Zanthoxylum limonella (Thai name: makaen)] undiluted oils to yield an effect, 2 hours of full repellency. The investigators then tested the same concentrations of these oils for repellency against Culex quinquefasciatus (the Southern house mosquito) and Anopheles dirus (the mosquito considered to be a vector of malaria in Asian forested zones. The undiluted oils provided the greatest protection, with clove oil rendering the most durable repellency6.
Photoaging
Wu et al. determined the acaricidal activity of compounds extracted from patchouli oil against the house dust mite (Dermatophagoides farinae) in 2012. They isolated 2-(1,3-dihydroxy-but-2-enylidene)-6-methyl-3-oxo-heptanoic acid (DHEMH), the hydrolysate of pogostone, and 15 other constituents in patchouli oil, ultimately ascertaining that DHEMH and patchouli oil itself were the most toxic substances to D. farinae. The investigators concluded that patchouli oil and DHEMH warrant consideration and more study for their acaricidal potential as environmentally friendly, effective, and simple fumigant alternatives to chemical agents7.
In 2013, Yang et al. used molecular docking technology to evaluate the antibacterial activity of patchouli oil in vitro. They identified 26 compounds in patchouli oil displaying antibacterial activity, with pogostone and (-)-patchouli alcohol exhibiting the strongest activity8. Later that year, Yang et al. used the same technology to establish that Herba pogostemonis oil exhibited potent antibacterial effects, especially the constituents pogostone and (-)-Herba pogostemonis alcohol9. Raharjo and Fatchiyah also used molecular docking tools and Chimera 1.7s viewer software in a virtual screening of compounds from patchouli oil, concluding that alpha-patchouli alcohol is a potential inhibitor of the cyclo-oxygenase (COX)-1 enzyme. This is notable given the pivotal role of COX-1 in the inflammatory response10.
The next year, Peng et al. isolated one of the primary constituents of patchouli oil, pogostone, and assessed its antibacterial activity in vitro and in vivo. They found that pogostone suppressed both gram-negative and gram-positive bacteria in vitro. The researchers noted that pogostone was active against some drug-resistant bacteria (such as MRSA). Via intraperitoneal injection, pogostone displayed antibacterial activity in male and female Kunming mice against Escherichia coli and MRSA. At concentrations of 50 and 100 mg/kg, 90% of the mice infected with E. coli were protected; 60% of the mice at 25 mg/kg were protected. For mice with MRSA, 60% were protected at a dose of 100 mg/kg and 50% at a dose of 50 mg/kg. The investigators concluded that pogostone is a viable antibacterial agent for clinical use4.
Transdermal delivery
A 2008 study by Luo et al. showed that patchouli oil was among three volatile oils that improved the skin penetration of the flavonoids baicalin11. It was less effective than several compounds, including clove oil, camphor, menthol, and oleic acid, as a transdermal enhancer in a subsequent study by Zheng et al.12.
Conclusion
Patchouli oil continues to be used today in traditional Chinese medicine. In the West, the established literature on Pogostemon cablin is thin, but what has emerged recently, particularly studies on the protection against photoaging in mice, supports the continued investigation of this ancient herb to determine its potential role in dermatologic practice. As it is, much more research is necessary.
References
1. J Ethnopharmacol. 2014;154(2):408-18.
2. Eur J Pharm Sci. 2014;63:113-23.
3. Expert Opin Investig Drugs. 2013;22(2):245-57.
4. Chin Med J. (Engl) 2014;127(23):4001-5.
5. J Agric Food Chem. 2007;55(5):1737-42
6. Phytother Res. 2005;19(4):303-9.
7. Chem Pharm Bull (Tokyo). 2012;60(2):178-82.
8. Iran J Pharm Res. 2013 Summer;12(3):307-16.
9. Pak J Pharm Sci. 2013;26(6):1173-9.
10. Bioinformation 2013;9(6):321-4.
11. Zhong Yao Cai. 2008;31(11):1721-4
12. Zhongguo Zhong Yao Za Zhi. 2009;34(20):2599-603.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Pogostemon cablin, known in the West as patchouli or guang huo-xiang in China, is a long-time staple in traditional Chinese medicine for various indications, particularly gastrointestinal and skin disorders1.
Patchouli oil, which contains several mono- and sesquiterpenoids, alkaloids, and flavonoids, is thought to possess significant anti-inflammatory and antioxidant qualities2.In fact, it is reputed to impart antiviral, antioxidant, anti-inflammatory, and analgesic effects, and is also known to protect intestinal barrier function3. Peng et al. have found that patchouli oil exerts significant antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA)4.
After a comprehensive 2013 review, Chen et al. deemed P. cablin to have potential clinical benefits as an effective adaptogenic herbal treatment3. It is thought to have some antiacne properties as well1. Further, P. cablin is among the Top 10 most-often-used traditional Chinese medicine prescriptions for skin care and appearance1.
In Brazil, China, Indonesia, and Malaysia, P. cablin is cultivated for its essential oil, which plays an important role in the perfume industry. Patchouli essential oil, featured in perfumes, soaps, cosmetics, and as incense, is used by aromatherapists for its calming and reviving effects. The essential oil has also been shown to impart antioxidant activity5.
In 2014, Lin et al. studied the protective effects of P. cablin essential oil against ultraviolet (UV)-induced skin photoaging in mice. The researchers applied patchouli oil for 2 hours before UV exposure to the dorsal depilated skin of mice. They found that patchouli oil doses of 6 mg/mouse and 9 mg/mouse significantly suppressed skin wrinkle formation, mitigated skin elasticity impairment, and augmented collagen content (21.9% and 26.3%, respectively). The same doses also yielded significant reductions in epidermal thickness and malondialdehyde content, and blocked the disruption of collagen and elastic fibers. Patchouli oil treatment also resulted in the up-regulation of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, and catalase. The investigators concluded that patchouli oil, perhaps due to its antioxidant characteristics, and sesquiterpene constituents in particular, was effective in preventing photoaging in mice, and warrants attention as a potential agent to hinder photoaging in humans1.
Feng et al. also investigated the effects of topically applied patchouli alcohol on UV-induced photoaging in mice that year. For 9 weeks, investigators applied patchouli oil solution or a vehicle to the depilated dorsal skin of 6-week-old mice. They found that patchouli oil significantly hastened the recovery of UV-induced skin lesions, which they ascribed to the antioxidant and anti-inflammatory activity of the agent and its down-regulation of the expression of matrix metalloproteinase (MMP)-1 and MMP-32.
Antimicrobial and mosquito repellent activity
In a 2005 study by Trongtokit et al. of the mosquito-repellent activity of 38 essential oils at three concentrations (10%, 50%, or undiluted) against the mosquito Aedes aegypti under laboratory conditions using human volunteers, undiluted P. cablin oil was one of four [along with Cymbopogon nardus (citronella), Syzygium aromaticum (clove), and Zanthoxylum limonella (Thai name: makaen)] undiluted oils to yield an effect, 2 hours of full repellency. The investigators then tested the same concentrations of these oils for repellency against Culex quinquefasciatus (the Southern house mosquito) and Anopheles dirus (the mosquito considered to be a vector of malaria in Asian forested zones. The undiluted oils provided the greatest protection, with clove oil rendering the most durable repellency6.
Photoaging
Wu et al. determined the acaricidal activity of compounds extracted from patchouli oil against the house dust mite (Dermatophagoides farinae) in 2012. They isolated 2-(1,3-dihydroxy-but-2-enylidene)-6-methyl-3-oxo-heptanoic acid (DHEMH), the hydrolysate of pogostone, and 15 other constituents in patchouli oil, ultimately ascertaining that DHEMH and patchouli oil itself were the most toxic substances to D. farinae. The investigators concluded that patchouli oil and DHEMH warrant consideration and more study for their acaricidal potential as environmentally friendly, effective, and simple fumigant alternatives to chemical agents7.
In 2013, Yang et al. used molecular docking technology to evaluate the antibacterial activity of patchouli oil in vitro. They identified 26 compounds in patchouli oil displaying antibacterial activity, with pogostone and (-)-patchouli alcohol exhibiting the strongest activity8. Later that year, Yang et al. used the same technology to establish that Herba pogostemonis oil exhibited potent antibacterial effects, especially the constituents pogostone and (-)-Herba pogostemonis alcohol9. Raharjo and Fatchiyah also used molecular docking tools and Chimera 1.7s viewer software in a virtual screening of compounds from patchouli oil, concluding that alpha-patchouli alcohol is a potential inhibitor of the cyclo-oxygenase (COX)-1 enzyme. This is notable given the pivotal role of COX-1 in the inflammatory response10.
The next year, Peng et al. isolated one of the primary constituents of patchouli oil, pogostone, and assessed its antibacterial activity in vitro and in vivo. They found that pogostone suppressed both gram-negative and gram-positive bacteria in vitro. The researchers noted that pogostone was active against some drug-resistant bacteria (such as MRSA). Via intraperitoneal injection, pogostone displayed antibacterial activity in male and female Kunming mice against Escherichia coli and MRSA. At concentrations of 50 and 100 mg/kg, 90% of the mice infected with E. coli were protected; 60% of the mice at 25 mg/kg were protected. For mice with MRSA, 60% were protected at a dose of 100 mg/kg and 50% at a dose of 50 mg/kg. The investigators concluded that pogostone is a viable antibacterial agent for clinical use4.
Transdermal delivery
A 2008 study by Luo et al. showed that patchouli oil was among three volatile oils that improved the skin penetration of the flavonoids baicalin11. It was less effective than several compounds, including clove oil, camphor, menthol, and oleic acid, as a transdermal enhancer in a subsequent study by Zheng et al.12.
Conclusion
Patchouli oil continues to be used today in traditional Chinese medicine. In the West, the established literature on Pogostemon cablin is thin, but what has emerged recently, particularly studies on the protection against photoaging in mice, supports the continued investigation of this ancient herb to determine its potential role in dermatologic practice. As it is, much more research is necessary.
References
1. J Ethnopharmacol. 2014;154(2):408-18.
2. Eur J Pharm Sci. 2014;63:113-23.
3. Expert Opin Investig Drugs. 2013;22(2):245-57.
4. Chin Med J. (Engl) 2014;127(23):4001-5.
5. J Agric Food Chem. 2007;55(5):1737-42
6. Phytother Res. 2005;19(4):303-9.
7. Chem Pharm Bull (Tokyo). 2012;60(2):178-82.
8. Iran J Pharm Res. 2013 Summer;12(3):307-16.
9. Pak J Pharm Sci. 2013;26(6):1173-9.
10. Bioinformation 2013;9(6):321-4.
11. Zhong Yao Cai. 2008;31(11):1721-4
12. Zhongguo Zhong Yao Za Zhi. 2009;34(20):2599-603.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Pogostemon cablin, known in the West as patchouli or guang huo-xiang in China, is a long-time staple in traditional Chinese medicine for various indications, particularly gastrointestinal and skin disorders1.
Patchouli oil, which contains several mono- and sesquiterpenoids, alkaloids, and flavonoids, is thought to possess significant anti-inflammatory and antioxidant qualities2.In fact, it is reputed to impart antiviral, antioxidant, anti-inflammatory, and analgesic effects, and is also known to protect intestinal barrier function3. Peng et al. have found that patchouli oil exerts significant antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA)4.
After a comprehensive 2013 review, Chen et al. deemed P. cablin to have potential clinical benefits as an effective adaptogenic herbal treatment3. It is thought to have some antiacne properties as well1. Further, P. cablin is among the Top 10 most-often-used traditional Chinese medicine prescriptions for skin care and appearance1.
In Brazil, China, Indonesia, and Malaysia, P. cablin is cultivated for its essential oil, which plays an important role in the perfume industry. Patchouli essential oil, featured in perfumes, soaps, cosmetics, and as incense, is used by aromatherapists for its calming and reviving effects. The essential oil has also been shown to impart antioxidant activity5.
In 2014, Lin et al. studied the protective effects of P. cablin essential oil against ultraviolet (UV)-induced skin photoaging in mice. The researchers applied patchouli oil for 2 hours before UV exposure to the dorsal depilated skin of mice. They found that patchouli oil doses of 6 mg/mouse and 9 mg/mouse significantly suppressed skin wrinkle formation, mitigated skin elasticity impairment, and augmented collagen content (21.9% and 26.3%, respectively). The same doses also yielded significant reductions in epidermal thickness and malondialdehyde content, and blocked the disruption of collagen and elastic fibers. Patchouli oil treatment also resulted in the up-regulation of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, and catalase. The investigators concluded that patchouli oil, perhaps due to its antioxidant characteristics, and sesquiterpene constituents in particular, was effective in preventing photoaging in mice, and warrants attention as a potential agent to hinder photoaging in humans1.
Feng et al. also investigated the effects of topically applied patchouli alcohol on UV-induced photoaging in mice that year. For 9 weeks, investigators applied patchouli oil solution or a vehicle to the depilated dorsal skin of 6-week-old mice. They found that patchouli oil significantly hastened the recovery of UV-induced skin lesions, which they ascribed to the antioxidant and anti-inflammatory activity of the agent and its down-regulation of the expression of matrix metalloproteinase (MMP)-1 and MMP-32.
Antimicrobial and mosquito repellent activity
In a 2005 study by Trongtokit et al. of the mosquito-repellent activity of 38 essential oils at three concentrations (10%, 50%, or undiluted) against the mosquito Aedes aegypti under laboratory conditions using human volunteers, undiluted P. cablin oil was one of four [along with Cymbopogon nardus (citronella), Syzygium aromaticum (clove), and Zanthoxylum limonella (Thai name: makaen)] undiluted oils to yield an effect, 2 hours of full repellency. The investigators then tested the same concentrations of these oils for repellency against Culex quinquefasciatus (the Southern house mosquito) and Anopheles dirus (the mosquito considered to be a vector of malaria in Asian forested zones. The undiluted oils provided the greatest protection, with clove oil rendering the most durable repellency6.
Photoaging
Wu et al. determined the acaricidal activity of compounds extracted from patchouli oil against the house dust mite (Dermatophagoides farinae) in 2012. They isolated 2-(1,3-dihydroxy-but-2-enylidene)-6-methyl-3-oxo-heptanoic acid (DHEMH), the hydrolysate of pogostone, and 15 other constituents in patchouli oil, ultimately ascertaining that DHEMH and patchouli oil itself were the most toxic substances to D. farinae. The investigators concluded that patchouli oil and DHEMH warrant consideration and more study for their acaricidal potential as environmentally friendly, effective, and simple fumigant alternatives to chemical agents7.
In 2013, Yang et al. used molecular docking technology to evaluate the antibacterial activity of patchouli oil in vitro. They identified 26 compounds in patchouli oil displaying antibacterial activity, with pogostone and (-)-patchouli alcohol exhibiting the strongest activity8. Later that year, Yang et al. used the same technology to establish that Herba pogostemonis oil exhibited potent antibacterial effects, especially the constituents pogostone and (-)-Herba pogostemonis alcohol9. Raharjo and Fatchiyah also used molecular docking tools and Chimera 1.7s viewer software in a virtual screening of compounds from patchouli oil, concluding that alpha-patchouli alcohol is a potential inhibitor of the cyclo-oxygenase (COX)-1 enzyme. This is notable given the pivotal role of COX-1 in the inflammatory response10.
The next year, Peng et al. isolated one of the primary constituents of patchouli oil, pogostone, and assessed its antibacterial activity in vitro and in vivo. They found that pogostone suppressed both gram-negative and gram-positive bacteria in vitro. The researchers noted that pogostone was active against some drug-resistant bacteria (such as MRSA). Via intraperitoneal injection, pogostone displayed antibacterial activity in male and female Kunming mice against Escherichia coli and MRSA. At concentrations of 50 and 100 mg/kg, 90% of the mice infected with E. coli were protected; 60% of the mice at 25 mg/kg were protected. For mice with MRSA, 60% were protected at a dose of 100 mg/kg and 50% at a dose of 50 mg/kg. The investigators concluded that pogostone is a viable antibacterial agent for clinical use4.
Transdermal delivery
A 2008 study by Luo et al. showed that patchouli oil was among three volatile oils that improved the skin penetration of the flavonoids baicalin11. It was less effective than several compounds, including clove oil, camphor, menthol, and oleic acid, as a transdermal enhancer in a subsequent study by Zheng et al.12.
Conclusion
Patchouli oil continues to be used today in traditional Chinese medicine. In the West, the established literature on Pogostemon cablin is thin, but what has emerged recently, particularly studies on the protection against photoaging in mice, supports the continued investigation of this ancient herb to determine its potential role in dermatologic practice. As it is, much more research is necessary.
References
1. J Ethnopharmacol. 2014;154(2):408-18.
2. Eur J Pharm Sci. 2014;63:113-23.
3. Expert Opin Investig Drugs. 2013;22(2):245-57.
4. Chin Med J. (Engl) 2014;127(23):4001-5.
5. J Agric Food Chem. 2007;55(5):1737-42
6. Phytother Res. 2005;19(4):303-9.
7. Chem Pharm Bull (Tokyo). 2012;60(2):178-82.
8. Iran J Pharm Res. 2013 Summer;12(3):307-16.
9. Pak J Pharm Sci. 2013;26(6):1173-9.
10. Bioinformation 2013;9(6):321-4.
11. Zhong Yao Cai. 2008;31(11):1721-4
12. Zhongguo Zhong Yao Za Zhi. 2009;34(20):2599-603.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Targeting STAT3 to prevent relapse in AML

Compounds that target a novel binding site on STAT3 may one day be used to prevent relapse in acute myeloid leukemia (AML), according to researchers.
STAT3 is known to interfere with chemotherapy and is thought to play a role in many cases of AML relapse.
But preclinical research suggests a compound known as MM-206 can disrupt STAT3’s disease-promoting effects by targeting a previously unknown ligand-binding site on the protein.
Zachary Ball, PhD, of Rice University in Houston, Texas, and his colleagues described this research in Angewandte Chemie.
The team first discovered that the coiled–coil domain (CCD) is a novel ligand-binding site on STAT3. Then, they identified a naphthalene sulfonamide compound, known as C188, that can target CCD and inhibit STAT3.
The researchers tested C188 and compounds synthesized from it—C188-9, C188-9-Rh2, and MM-206—in vitro and in vivo. Only MM-206 proved effective in vivo.
“Our main advance, from a medicinal perspective, is that this compound also works in a mouse model,” Dr Ball said. “All the other compounds worked in cells, but, in mice, they weren’t potent enough or stable enough.”
MM-206 inhibited STAT3 phosphorylation and induced apoptosis in 3 different AML cell lines. The compound also prompted apoptosis in primary tumor cells from pediatric AML patients.
Among mice engrafted with luciferase-expressing MV4-11 AML cells, those that received MM-206 exhibited slower disease progression than untreated mice.
When the mice received 4 weeks of treatment with MM-206, they had significantly fewer tumor cells in their bone marrow and lived significantly longer than control mice (P=0.019 at 10 weeks).
Follow-up studies should lead to improved versions of MM-206, according to Dr Ball.
“The discovery raises new questions about STAT3 biology and points the way to future anticancer approaches,” he said, “including combination therapies of coiled-coil STAT3 inhibitors in tandem with other agents.” ![]()

Compounds that target a novel binding site on STAT3 may one day be used to prevent relapse in acute myeloid leukemia (AML), according to researchers.
STAT3 is known to interfere with chemotherapy and is thought to play a role in many cases of AML relapse.
But preclinical research suggests a compound known as MM-206 can disrupt STAT3’s disease-promoting effects by targeting a previously unknown ligand-binding site on the protein.
Zachary Ball, PhD, of Rice University in Houston, Texas, and his colleagues described this research in Angewandte Chemie.
The team first discovered that the coiled–coil domain (CCD) is a novel ligand-binding site on STAT3. Then, they identified a naphthalene sulfonamide compound, known as C188, that can target CCD and inhibit STAT3.
The researchers tested C188 and compounds synthesized from it—C188-9, C188-9-Rh2, and MM-206—in vitro and in vivo. Only MM-206 proved effective in vivo.
“Our main advance, from a medicinal perspective, is that this compound also works in a mouse model,” Dr Ball said. “All the other compounds worked in cells, but, in mice, they weren’t potent enough or stable enough.”
MM-206 inhibited STAT3 phosphorylation and induced apoptosis in 3 different AML cell lines. The compound also prompted apoptosis in primary tumor cells from pediatric AML patients.
Among mice engrafted with luciferase-expressing MV4-11 AML cells, those that received MM-206 exhibited slower disease progression than untreated mice.
When the mice received 4 weeks of treatment with MM-206, they had significantly fewer tumor cells in their bone marrow and lived significantly longer than control mice (P=0.019 at 10 weeks).
Follow-up studies should lead to improved versions of MM-206, according to Dr Ball.
“The discovery raises new questions about STAT3 biology and points the way to future anticancer approaches,” he said, “including combination therapies of coiled-coil STAT3 inhibitors in tandem with other agents.” ![]()

Compounds that target a novel binding site on STAT3 may one day be used to prevent relapse in acute myeloid leukemia (AML), according to researchers.
STAT3 is known to interfere with chemotherapy and is thought to play a role in many cases of AML relapse.
But preclinical research suggests a compound known as MM-206 can disrupt STAT3’s disease-promoting effects by targeting a previously unknown ligand-binding site on the protein.
Zachary Ball, PhD, of Rice University in Houston, Texas, and his colleagues described this research in Angewandte Chemie.
The team first discovered that the coiled–coil domain (CCD) is a novel ligand-binding site on STAT3. Then, they identified a naphthalene sulfonamide compound, known as C188, that can target CCD and inhibit STAT3.
The researchers tested C188 and compounds synthesized from it—C188-9, C188-9-Rh2, and MM-206—in vitro and in vivo. Only MM-206 proved effective in vivo.
“Our main advance, from a medicinal perspective, is that this compound also works in a mouse model,” Dr Ball said. “All the other compounds worked in cells, but, in mice, they weren’t potent enough or stable enough.”
MM-206 inhibited STAT3 phosphorylation and induced apoptosis in 3 different AML cell lines. The compound also prompted apoptosis in primary tumor cells from pediatric AML patients.
Among mice engrafted with luciferase-expressing MV4-11 AML cells, those that received MM-206 exhibited slower disease progression than untreated mice.
When the mice received 4 weeks of treatment with MM-206, they had significantly fewer tumor cells in their bone marrow and lived significantly longer than control mice (P=0.019 at 10 weeks).
Follow-up studies should lead to improved versions of MM-206, according to Dr Ball.
“The discovery raises new questions about STAT3 biology and points the way to future anticancer approaches,” he said, “including combination therapies of coiled-coil STAT3 inhibitors in tandem with other agents.” ![]()
Combination induces remission in advanced MM

Photo from Penn Medicine
Investigators have described a 3-pronged treatment approach that induced sustained remission in a patient with advanced multiple myeloma (MM).
The treatment combined chemotherapy, autologous stem cell transplant, and the chimeric antigen receptor T-cell therapy CTL019.
The patient, who had received 9 prior lines of therapy, responded to this combination despite the fact that 99.95% of her neoplastic plasma cells did not express CD19.
“There was some skepticism about whether a CD19-directed therapy would work in this disease, since nearly all of these patients’ cancerous plasma cells do not express CD19,” said study author Edward Stadtmauer, MD, of the University of Pennsylvania in Philadelphia.
“Since there was data showing that the possible stem cells can be CD19-positive, our hypothesis was that we may be able to devise a therapy targeted at early precursors of those cells.”
Dr Stadtmauer and his colleagues described this case, which is part of a larger trial, in NEJM.
Prior to receiving the combination therapy, the MM patient had received 9 different treatment regimens in the 5 years since her diagnosis (at age 43).
The patient had received regimens containing lenalidomide, bortezomib, dexamethasone, carfilzomib, pomalidomide, vorinostat, clarithromycin, and elotuzumab. She also received a previous autologous stem cell transplant, which had only controlled her disease for a few months.
For this study, the patient received high-dose melphalan (140 mg/m2), reinfusion of autologous stem cells (≥2.1×106 cells per kg of body weight), and a CTL019 infusion 12 days later.
Response and adverse effects
The patient experienced transplant-related side effects prior to receiving CTL019, including grade 4 neutropenia and thrombocytopenia, grade 2 nausea and anorexia, neutropenic fever, and Staphylococcus aureus bacteremia. By day 100 after transplant, all transplant-related adverse effects had resolved.
The patient had hypogammaglobulinemia before transplant that persisted at day 100 after transplant and was attributed to the effects of CTL019 on normal B cells and plasma cells. There were no other adverse events attributable to CTL019.
On day 130 after transplant, the patient began to receive lenalidomide maintenance at 5 mg daily (an optional part of the protocol). Her dose was later reduced to 5 mg twice weekly because of gastrointestinal side effects.
At day 130 after CTL019 infusion, tests revealed no evidence of disease. The patient—who was the first to be treated as part of this trial—remains in remission more than 12 months after receiving this therapy.
“We couldn’t be more pleased with this patient’s response,” said study author Alfred Garfall, MD, of the University of Pennsylvania.
“We believe her CTL019 cells made the difference, since we would not have expected such a durable remission with a transplant alone, considering the very transient response this patient had to her first transplant several years ago.”
Dr Garfall and his colleagues also reported on the trial’s overall progress. Of the 10 patients who have received this combination therapy to date, 6 remain progression-free, although 2 patients have been treated very recently.
The additional CTL019-attributable side effects have been grade 1 cytokine release syndrome (n=1) and grade 3 enterocolitis due to autologous graft-vs-host disease (n=1).
This trial is sponsored by Novartis, the company developing CTL019, and others. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis. ![]()

Photo from Penn Medicine
Investigators have described a 3-pronged treatment approach that induced sustained remission in a patient with advanced multiple myeloma (MM).
The treatment combined chemotherapy, autologous stem cell transplant, and the chimeric antigen receptor T-cell therapy CTL019.
The patient, who had received 9 prior lines of therapy, responded to this combination despite the fact that 99.95% of her neoplastic plasma cells did not express CD19.
“There was some skepticism about whether a CD19-directed therapy would work in this disease, since nearly all of these patients’ cancerous plasma cells do not express CD19,” said study author Edward Stadtmauer, MD, of the University of Pennsylvania in Philadelphia.
“Since there was data showing that the possible stem cells can be CD19-positive, our hypothesis was that we may be able to devise a therapy targeted at early precursors of those cells.”
Dr Stadtmauer and his colleagues described this case, which is part of a larger trial, in NEJM.
Prior to receiving the combination therapy, the MM patient had received 9 different treatment regimens in the 5 years since her diagnosis (at age 43).
The patient had received regimens containing lenalidomide, bortezomib, dexamethasone, carfilzomib, pomalidomide, vorinostat, clarithromycin, and elotuzumab. She also received a previous autologous stem cell transplant, which had only controlled her disease for a few months.
For this study, the patient received high-dose melphalan (140 mg/m2), reinfusion of autologous stem cells (≥2.1×106 cells per kg of body weight), and a CTL019 infusion 12 days later.
Response and adverse effects
The patient experienced transplant-related side effects prior to receiving CTL019, including grade 4 neutropenia and thrombocytopenia, grade 2 nausea and anorexia, neutropenic fever, and Staphylococcus aureus bacteremia. By day 100 after transplant, all transplant-related adverse effects had resolved.
The patient had hypogammaglobulinemia before transplant that persisted at day 100 after transplant and was attributed to the effects of CTL019 on normal B cells and plasma cells. There were no other adverse events attributable to CTL019.
On day 130 after transplant, the patient began to receive lenalidomide maintenance at 5 mg daily (an optional part of the protocol). Her dose was later reduced to 5 mg twice weekly because of gastrointestinal side effects.
At day 130 after CTL019 infusion, tests revealed no evidence of disease. The patient—who was the first to be treated as part of this trial—remains in remission more than 12 months after receiving this therapy.
“We couldn’t be more pleased with this patient’s response,” said study author Alfred Garfall, MD, of the University of Pennsylvania.
“We believe her CTL019 cells made the difference, since we would not have expected such a durable remission with a transplant alone, considering the very transient response this patient had to her first transplant several years ago.”
Dr Garfall and his colleagues also reported on the trial’s overall progress. Of the 10 patients who have received this combination therapy to date, 6 remain progression-free, although 2 patients have been treated very recently.
The additional CTL019-attributable side effects have been grade 1 cytokine release syndrome (n=1) and grade 3 enterocolitis due to autologous graft-vs-host disease (n=1).
This trial is sponsored by Novartis, the company developing CTL019, and others. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis. ![]()

Photo from Penn Medicine
Investigators have described a 3-pronged treatment approach that induced sustained remission in a patient with advanced multiple myeloma (MM).
The treatment combined chemotherapy, autologous stem cell transplant, and the chimeric antigen receptor T-cell therapy CTL019.
The patient, who had received 9 prior lines of therapy, responded to this combination despite the fact that 99.95% of her neoplastic plasma cells did not express CD19.
“There was some skepticism about whether a CD19-directed therapy would work in this disease, since nearly all of these patients’ cancerous plasma cells do not express CD19,” said study author Edward Stadtmauer, MD, of the University of Pennsylvania in Philadelphia.
“Since there was data showing that the possible stem cells can be CD19-positive, our hypothesis was that we may be able to devise a therapy targeted at early precursors of those cells.”
Dr Stadtmauer and his colleagues described this case, which is part of a larger trial, in NEJM.
Prior to receiving the combination therapy, the MM patient had received 9 different treatment regimens in the 5 years since her diagnosis (at age 43).
The patient had received regimens containing lenalidomide, bortezomib, dexamethasone, carfilzomib, pomalidomide, vorinostat, clarithromycin, and elotuzumab. She also received a previous autologous stem cell transplant, which had only controlled her disease for a few months.
For this study, the patient received high-dose melphalan (140 mg/m2), reinfusion of autologous stem cells (≥2.1×106 cells per kg of body weight), and a CTL019 infusion 12 days later.
Response and adverse effects
The patient experienced transplant-related side effects prior to receiving CTL019, including grade 4 neutropenia and thrombocytopenia, grade 2 nausea and anorexia, neutropenic fever, and Staphylococcus aureus bacteremia. By day 100 after transplant, all transplant-related adverse effects had resolved.
The patient had hypogammaglobulinemia before transplant that persisted at day 100 after transplant and was attributed to the effects of CTL019 on normal B cells and plasma cells. There were no other adverse events attributable to CTL019.
On day 130 after transplant, the patient began to receive lenalidomide maintenance at 5 mg daily (an optional part of the protocol). Her dose was later reduced to 5 mg twice weekly because of gastrointestinal side effects.
At day 130 after CTL019 infusion, tests revealed no evidence of disease. The patient—who was the first to be treated as part of this trial—remains in remission more than 12 months after receiving this therapy.
“We couldn’t be more pleased with this patient’s response,” said study author Alfred Garfall, MD, of the University of Pennsylvania.
“We believe her CTL019 cells made the difference, since we would not have expected such a durable remission with a transplant alone, considering the very transient response this patient had to her first transplant several years ago.”
Dr Garfall and his colleagues also reported on the trial’s overall progress. Of the 10 patients who have received this combination therapy to date, 6 remain progression-free, although 2 patients have been treated very recently.
The additional CTL019-attributable side effects have been grade 1 cytokine release syndrome (n=1) and grade 3 enterocolitis due to autologous graft-vs-host disease (n=1).
This trial is sponsored by Novartis, the company developing CTL019, and others. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis. ![]()
Groups crowd-source cancer research

Photo by Rhoda Baer
In an attempt to crowd-source cancer research, a pair of Canadian organizations made a small molecule they developed freely available to researchers.
The goal was to fast-track the testing of new treatment strategies and facilitate sharing of the results.
Researchers have already completed preclinical studies of this molecule, a WDR5 inhibitor called OICR-9429, showing that it is active against breast cancer and acute myeloid leukemia (AML).
The organizations that developed OICR-9429 are the Ontario Institute for Cancer Research (OICR) and the Structural Genomics Consortium (SGC).
“In the time that it would normally take to negotiate a legal agreement to provide OICR-9429 to other research teams, we have received results back from our collaborators showing that it can kill 2 different types of cancer cells,” said Cheryl Arrowsmith, PhD, of SGC Toronto in Ontario, Canada.
“Opening our chemistry capabilities to the world’s scientists allowed us to crowd-source and accelerate the research.”
A study published in Nature Chemical Biology suggested that WDR5 is a therapeutic target for a subtype of AML, and OICR-9429 could therefore be used to treat the disease.
The researchers noted that mutations in C/EBPα occur in about 9% of AML cases and lead to the expression of a 30-kDa dominant negative isoform (C/EBPα-p30).
Their experiments revealed that C/EBPα p30 preferentially interacts with WDR5. So it was no surprise that OICR-9429 inhibited proliferation and induced differentiation in p30-expressing AML cells.
In a study published in Nature, OICR-9429 inhibited cancer cell growth in a panel of breast cancer cell lines driven by mutated forms of p53.
Researchers said this work has implications beyond breast cancer because p53 is mutated in at least half of all cancers and is dysregulated in others.
“It is remarkable how quickly our research results were translated into discoveries by the groups around the world,” said Rima Al-awar, PhD, of OICR in Toronto.
“We are looking forward to seeing more research conducted with OICR-9429 and showing that this new approach to early stage drug discovery has significant advantages.”
The SGC and OICR teams said they are continuing their collaboration to identify additional molecules to advance cancer drug discovery. ![]()

Photo by Rhoda Baer
In an attempt to crowd-source cancer research, a pair of Canadian organizations made a small molecule they developed freely available to researchers.
The goal was to fast-track the testing of new treatment strategies and facilitate sharing of the results.
Researchers have already completed preclinical studies of this molecule, a WDR5 inhibitor called OICR-9429, showing that it is active against breast cancer and acute myeloid leukemia (AML).
The organizations that developed OICR-9429 are the Ontario Institute for Cancer Research (OICR) and the Structural Genomics Consortium (SGC).
“In the time that it would normally take to negotiate a legal agreement to provide OICR-9429 to other research teams, we have received results back from our collaborators showing that it can kill 2 different types of cancer cells,” said Cheryl Arrowsmith, PhD, of SGC Toronto in Ontario, Canada.
“Opening our chemistry capabilities to the world’s scientists allowed us to crowd-source and accelerate the research.”
A study published in Nature Chemical Biology suggested that WDR5 is a therapeutic target for a subtype of AML, and OICR-9429 could therefore be used to treat the disease.
The researchers noted that mutations in C/EBPα occur in about 9% of AML cases and lead to the expression of a 30-kDa dominant negative isoform (C/EBPα-p30).
Their experiments revealed that C/EBPα p30 preferentially interacts with WDR5. So it was no surprise that OICR-9429 inhibited proliferation and induced differentiation in p30-expressing AML cells.
In a study published in Nature, OICR-9429 inhibited cancer cell growth in a panel of breast cancer cell lines driven by mutated forms of p53.
Researchers said this work has implications beyond breast cancer because p53 is mutated in at least half of all cancers and is dysregulated in others.
“It is remarkable how quickly our research results were translated into discoveries by the groups around the world,” said Rima Al-awar, PhD, of OICR in Toronto.
“We are looking forward to seeing more research conducted with OICR-9429 and showing that this new approach to early stage drug discovery has significant advantages.”
The SGC and OICR teams said they are continuing their collaboration to identify additional molecules to advance cancer drug discovery. ![]()

Photo by Rhoda Baer
In an attempt to crowd-source cancer research, a pair of Canadian organizations made a small molecule they developed freely available to researchers.
The goal was to fast-track the testing of new treatment strategies and facilitate sharing of the results.
Researchers have already completed preclinical studies of this molecule, a WDR5 inhibitor called OICR-9429, showing that it is active against breast cancer and acute myeloid leukemia (AML).
The organizations that developed OICR-9429 are the Ontario Institute for Cancer Research (OICR) and the Structural Genomics Consortium (SGC).
“In the time that it would normally take to negotiate a legal agreement to provide OICR-9429 to other research teams, we have received results back from our collaborators showing that it can kill 2 different types of cancer cells,” said Cheryl Arrowsmith, PhD, of SGC Toronto in Ontario, Canada.
“Opening our chemistry capabilities to the world’s scientists allowed us to crowd-source and accelerate the research.”
A study published in Nature Chemical Biology suggested that WDR5 is a therapeutic target for a subtype of AML, and OICR-9429 could therefore be used to treat the disease.
The researchers noted that mutations in C/EBPα occur in about 9% of AML cases and lead to the expression of a 30-kDa dominant negative isoform (C/EBPα-p30).
Their experiments revealed that C/EBPα p30 preferentially interacts with WDR5. So it was no surprise that OICR-9429 inhibited proliferation and induced differentiation in p30-expressing AML cells.
In a study published in Nature, OICR-9429 inhibited cancer cell growth in a panel of breast cancer cell lines driven by mutated forms of p53.
Researchers said this work has implications beyond breast cancer because p53 is mutated in at least half of all cancers and is dysregulated in others.
“It is remarkable how quickly our research results were translated into discoveries by the groups around the world,” said Rima Al-awar, PhD, of OICR in Toronto.
“We are looking forward to seeing more research conducted with OICR-9429 and showing that this new approach to early stage drug discovery has significant advantages.”
The SGC and OICR teams said they are continuing their collaboration to identify additional molecules to advance cancer drug discovery. ![]()