User login
Biomaterial stops bleeding, doesn’t rely on thrombosis

Photo courtesy of USDA
Bioengineers have developed an injectable material that may provide a better way to stop bleeding in injured patients, even those taking anticoagulants and individuals with coagulopathy.
The so-called shear-thinning biomaterial (STB) is composed of gelatin and silicate nanoplatelet hydrogel.
It can be injected through a catheter or needle to occlude blood vessels.
The STB demonstrated efficacy in vitro and in experiments with mice and pigs.
“This work is an example of how bioengineering can help address the challenges that clinicians and patients face,” said Ali Khademhosseini, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“Our work thus far has been in the lab, but we are on a translational path to bring this new biomaterial for embolization to the clinic to improve patient care.”
Dr Khademhosseini and his colleagues described their work with STB in Science Translational Medicine.
The researchers noted that trauma or injury often leads to excessive bleeding that can result in death.
Embolic materials, such as metallic coils or liquid embolic agents, are commonly used to block injured blood vessels and stem bleeding, but these materials can cause complications such as coil migration or breakthrough bleeding.
Because these materials rely on intrinsic thrombosis, they are often ineffective in patients with severe bleeding disorders or those on anticoagulation therapy.
In search of a safer and more effective alternative, Dr Khademhosseini and his colleagues developed their STB.
The STB can be flowed into a blood vessel using a catheter, but the material is able to maintain its shape once inside the vessel, obstructing the vessel without relying on the formation of a blood clot.
In vitro, the STB performed just as well as metallic coils and was able to withstand high pressures without fragmenting or being dislodged in explant vessels.
The STB was even effective in stemming anticoagulated blood flow in vitro, suggesting that it could potentially be used in patients with bleeding disorders or those on anticoagulants.
The STB successfully blocked arteries and veins in mice and pigs, forming an impenetrable cast of the vessels that remained in place for up to 24 days.
The researchers said some of the beneficial properties of the STB include its ability to withstand pressure within the blood vessel, remain at the site of injection, and naturally degrade over time.
In addition, the STB attracted cells to migrate and deposit themselves at the site of the STB, helping to block the vessel.
The researchers noted that the individual component materials that make up the STB have been previously used in humans, which may mean a quicker path to regulatory approval. ![]()

Photo courtesy of USDA
Bioengineers have developed an injectable material that may provide a better way to stop bleeding in injured patients, even those taking anticoagulants and individuals with coagulopathy.
The so-called shear-thinning biomaterial (STB) is composed of gelatin and silicate nanoplatelet hydrogel.
It can be injected through a catheter or needle to occlude blood vessels.
The STB demonstrated efficacy in vitro and in experiments with mice and pigs.
“This work is an example of how bioengineering can help address the challenges that clinicians and patients face,” said Ali Khademhosseini, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“Our work thus far has been in the lab, but we are on a translational path to bring this new biomaterial for embolization to the clinic to improve patient care.”
Dr Khademhosseini and his colleagues described their work with STB in Science Translational Medicine.
The researchers noted that trauma or injury often leads to excessive bleeding that can result in death.
Embolic materials, such as metallic coils or liquid embolic agents, are commonly used to block injured blood vessels and stem bleeding, but these materials can cause complications such as coil migration or breakthrough bleeding.
Because these materials rely on intrinsic thrombosis, they are often ineffective in patients with severe bleeding disorders or those on anticoagulation therapy.
In search of a safer and more effective alternative, Dr Khademhosseini and his colleagues developed their STB.
The STB can be flowed into a blood vessel using a catheter, but the material is able to maintain its shape once inside the vessel, obstructing the vessel without relying on the formation of a blood clot.
In vitro, the STB performed just as well as metallic coils and was able to withstand high pressures without fragmenting or being dislodged in explant vessels.
The STB was even effective in stemming anticoagulated blood flow in vitro, suggesting that it could potentially be used in patients with bleeding disorders or those on anticoagulants.
The STB successfully blocked arteries and veins in mice and pigs, forming an impenetrable cast of the vessels that remained in place for up to 24 days.
The researchers said some of the beneficial properties of the STB include its ability to withstand pressure within the blood vessel, remain at the site of injection, and naturally degrade over time.
In addition, the STB attracted cells to migrate and deposit themselves at the site of the STB, helping to block the vessel.
The researchers noted that the individual component materials that make up the STB have been previously used in humans, which may mean a quicker path to regulatory approval. ![]()

Photo courtesy of USDA
Bioengineers have developed an injectable material that may provide a better way to stop bleeding in injured patients, even those taking anticoagulants and individuals with coagulopathy.
The so-called shear-thinning biomaterial (STB) is composed of gelatin and silicate nanoplatelet hydrogel.
It can be injected through a catheter or needle to occlude blood vessels.
The STB demonstrated efficacy in vitro and in experiments with mice and pigs.
“This work is an example of how bioengineering can help address the challenges that clinicians and patients face,” said Ali Khademhosseini, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“Our work thus far has been in the lab, but we are on a translational path to bring this new biomaterial for embolization to the clinic to improve patient care.”
Dr Khademhosseini and his colleagues described their work with STB in Science Translational Medicine.
The researchers noted that trauma or injury often leads to excessive bleeding that can result in death.
Embolic materials, such as metallic coils or liquid embolic agents, are commonly used to block injured blood vessels and stem bleeding, but these materials can cause complications such as coil migration or breakthrough bleeding.
Because these materials rely on intrinsic thrombosis, they are often ineffective in patients with severe bleeding disorders or those on anticoagulation therapy.
In search of a safer and more effective alternative, Dr Khademhosseini and his colleagues developed their STB.
The STB can be flowed into a blood vessel using a catheter, but the material is able to maintain its shape once inside the vessel, obstructing the vessel without relying on the formation of a blood clot.
In vitro, the STB performed just as well as metallic coils and was able to withstand high pressures without fragmenting or being dislodged in explant vessels.
The STB was even effective in stemming anticoagulated blood flow in vitro, suggesting that it could potentially be used in patients with bleeding disorders or those on anticoagulants.
The STB successfully blocked arteries and veins in mice and pigs, forming an impenetrable cast of the vessels that remained in place for up to 24 days.
The researchers said some of the beneficial properties of the STB include its ability to withstand pressure within the blood vessel, remain at the site of injection, and naturally degrade over time.
In addition, the STB attracted cells to migrate and deposit themselves at the site of the STB, helping to block the vessel.
The researchers noted that the individual component materials that make up the STB have been previously used in humans, which may mean a quicker path to regulatory approval. ![]()
Device provides long-lasting drug delivery

with 6 arms that can be folded
and encased in a capsule.
Photo by Melanie Gonick/MIT
A new device can provide long-term, controlled drug release, according to research published in Science Translational Medicine.
Researchers tested this device—a 6-armed structure encased in a capsule—by loading it with ivermectin, an antiparasitic drug that disrupts malaria transmission by killing infected mosquitoes.
When administered to pigs, the device safely stayed in the stomach, slowly releasing ivermectin for up to 14 days.
Researchers believe this type of lasting drug delivery could help bolster the success of malaria elimination campaigns, which rely on treatment adherence and cost-effective approaches that reach large, rural populations.
The team also believes this device could be used to treat a range of other diseases, particularly those in which treatment adherence may be an issue.
“We want to make it as easy as possible for people to take their medications over a sustained period of time,” said study author C. Giovanni Traverso, MB, BChir, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge.
“When patients have to remember to take a drug every day or multiple times a day, we start to see less and less adherence to the regimen. Being able to swallow a capsule once a week or once a month could change the way we think about delivering medications.”
This research has led to the launch of Lyndra, a company that is developing this technology with a focus on diseases for which patients would benefit the most from sustained drug delivery. This includes neuropsychiatric disorders, HIV, diabetes, and epilepsy.
Designing, testing the device
To provide long-term drug delivery, the researchers designed a star-shaped device with 6 arms that can be folded inward and encased in a smooth capsule.
Drug molecules are loaded into the arms, which are made of a rigid polymer called polycaprolactone. Each arm is attached to a rubber-like core by a linker that is designed to eventually break down.
After the capsule is swallowed, stomach acid dissolves the outer layer, allowing the 6-armed device to unfold.
Once the device expands, it is large enough to stay in the stomach and resist the forces that would normally push an object further down the digestive tract. However, it is not large enough to cause any harmful blockage of the digestive tract.
The drug is gradually released over a period of several days. After that, the linkers that join the arms to the core of the device dissolve, allowing the arms to break off. The pieces are small enough that they can pass harmlessly through the digestive tract.
In pigs, the device slowly released ivermectin for up to 14 days without causing injury to the stomach or obstructing the passage of food, before breaking apart and passing safely out of the body.
Modeling the impact
The researchers used mathematical modeling to predict the potential impact of this drug delivery method.
The data suggested that if the device were used to deliver ivermectin, it could increase the efficacy of mass drug administration campaigns designed to fight malaria.
“What we showed is that we stand to significantly amplify the effect of those campaigns,” Dr Traverso said. “The introduction of this kind of system could have a substantial impact on the fight against malaria and transform clinical care in general by ensuring patients receive their medication.”
Potential applications
“Until now, oral drugs would almost never last for more than a day,” said study author Robert Langer, ScD, of MIT.
“This really opens the door to ultra-long-lasting oral systems, which could have an effect on all kinds of diseases, such as Alzheimer’s or mental health disorders. There are a lot of exciting things this could someday enable.”
“This is a platform into which you can incorporate any drug,” added Mousa Jafari, PhD, of MIT. “This can be used with any drug that requires frequent dosing. We can replace that dosing with a single administration.”
This type of delivery could also help researchers run better clinical trials by making it easier for patients to take the drugs, said Shiyi Zhang, PhD, of MIT.
“It may help doctors and the pharma industry to better evaluate the efficacy of certain drugs because, currently, a lot of patients in clinical trials have serious medication adherence problems that will mislead the clinical studies,” he said. ![]()

with 6 arms that can be folded
and encased in a capsule.
Photo by Melanie Gonick/MIT
A new device can provide long-term, controlled drug release, according to research published in Science Translational Medicine.
Researchers tested this device—a 6-armed structure encased in a capsule—by loading it with ivermectin, an antiparasitic drug that disrupts malaria transmission by killing infected mosquitoes.
When administered to pigs, the device safely stayed in the stomach, slowly releasing ivermectin for up to 14 days.
Researchers believe this type of lasting drug delivery could help bolster the success of malaria elimination campaigns, which rely on treatment adherence and cost-effective approaches that reach large, rural populations.
The team also believes this device could be used to treat a range of other diseases, particularly those in which treatment adherence may be an issue.
“We want to make it as easy as possible for people to take their medications over a sustained period of time,” said study author C. Giovanni Traverso, MB, BChir, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge.
“When patients have to remember to take a drug every day or multiple times a day, we start to see less and less adherence to the regimen. Being able to swallow a capsule once a week or once a month could change the way we think about delivering medications.”
This research has led to the launch of Lyndra, a company that is developing this technology with a focus on diseases for which patients would benefit the most from sustained drug delivery. This includes neuropsychiatric disorders, HIV, diabetes, and epilepsy.
Designing, testing the device
To provide long-term drug delivery, the researchers designed a star-shaped device with 6 arms that can be folded inward and encased in a smooth capsule.
Drug molecules are loaded into the arms, which are made of a rigid polymer called polycaprolactone. Each arm is attached to a rubber-like core by a linker that is designed to eventually break down.
After the capsule is swallowed, stomach acid dissolves the outer layer, allowing the 6-armed device to unfold.
Once the device expands, it is large enough to stay in the stomach and resist the forces that would normally push an object further down the digestive tract. However, it is not large enough to cause any harmful blockage of the digestive tract.
The drug is gradually released over a period of several days. After that, the linkers that join the arms to the core of the device dissolve, allowing the arms to break off. The pieces are small enough that they can pass harmlessly through the digestive tract.
In pigs, the device slowly released ivermectin for up to 14 days without causing injury to the stomach or obstructing the passage of food, before breaking apart and passing safely out of the body.
Modeling the impact
The researchers used mathematical modeling to predict the potential impact of this drug delivery method.
The data suggested that if the device were used to deliver ivermectin, it could increase the efficacy of mass drug administration campaigns designed to fight malaria.
“What we showed is that we stand to significantly amplify the effect of those campaigns,” Dr Traverso said. “The introduction of this kind of system could have a substantial impact on the fight against malaria and transform clinical care in general by ensuring patients receive their medication.”
Potential applications
“Until now, oral drugs would almost never last for more than a day,” said study author Robert Langer, ScD, of MIT.
“This really opens the door to ultra-long-lasting oral systems, which could have an effect on all kinds of diseases, such as Alzheimer’s or mental health disorders. There are a lot of exciting things this could someday enable.”
“This is a platform into which you can incorporate any drug,” added Mousa Jafari, PhD, of MIT. “This can be used with any drug that requires frequent dosing. We can replace that dosing with a single administration.”
This type of delivery could also help researchers run better clinical trials by making it easier for patients to take the drugs, said Shiyi Zhang, PhD, of MIT.
“It may help doctors and the pharma industry to better evaluate the efficacy of certain drugs because, currently, a lot of patients in clinical trials have serious medication adherence problems that will mislead the clinical studies,” he said. ![]()

with 6 arms that can be folded
and encased in a capsule.
Photo by Melanie Gonick/MIT
A new device can provide long-term, controlled drug release, according to research published in Science Translational Medicine.
Researchers tested this device—a 6-armed structure encased in a capsule—by loading it with ivermectin, an antiparasitic drug that disrupts malaria transmission by killing infected mosquitoes.
When administered to pigs, the device safely stayed in the stomach, slowly releasing ivermectin for up to 14 days.
Researchers believe this type of lasting drug delivery could help bolster the success of malaria elimination campaigns, which rely on treatment adherence and cost-effective approaches that reach large, rural populations.
The team also believes this device could be used to treat a range of other diseases, particularly those in which treatment adherence may be an issue.
“We want to make it as easy as possible for people to take their medications over a sustained period of time,” said study author C. Giovanni Traverso, MB, BChir, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge.
“When patients have to remember to take a drug every day or multiple times a day, we start to see less and less adherence to the regimen. Being able to swallow a capsule once a week or once a month could change the way we think about delivering medications.”
This research has led to the launch of Lyndra, a company that is developing this technology with a focus on diseases for which patients would benefit the most from sustained drug delivery. This includes neuropsychiatric disorders, HIV, diabetes, and epilepsy.
Designing, testing the device
To provide long-term drug delivery, the researchers designed a star-shaped device with 6 arms that can be folded inward and encased in a smooth capsule.
Drug molecules are loaded into the arms, which are made of a rigid polymer called polycaprolactone. Each arm is attached to a rubber-like core by a linker that is designed to eventually break down.
After the capsule is swallowed, stomach acid dissolves the outer layer, allowing the 6-armed device to unfold.
Once the device expands, it is large enough to stay in the stomach and resist the forces that would normally push an object further down the digestive tract. However, it is not large enough to cause any harmful blockage of the digestive tract.
The drug is gradually released over a period of several days. After that, the linkers that join the arms to the core of the device dissolve, allowing the arms to break off. The pieces are small enough that they can pass harmlessly through the digestive tract.
In pigs, the device slowly released ivermectin for up to 14 days without causing injury to the stomach or obstructing the passage of food, before breaking apart and passing safely out of the body.
Modeling the impact
The researchers used mathematical modeling to predict the potential impact of this drug delivery method.
The data suggested that if the device were used to deliver ivermectin, it could increase the efficacy of mass drug administration campaigns designed to fight malaria.
“What we showed is that we stand to significantly amplify the effect of those campaigns,” Dr Traverso said. “The introduction of this kind of system could have a substantial impact on the fight against malaria and transform clinical care in general by ensuring patients receive their medication.”
Potential applications
“Until now, oral drugs would almost never last for more than a day,” said study author Robert Langer, ScD, of MIT.
“This really opens the door to ultra-long-lasting oral systems, which could have an effect on all kinds of diseases, such as Alzheimer’s or mental health disorders. There are a lot of exciting things this could someday enable.”
“This is a platform into which you can incorporate any drug,” added Mousa Jafari, PhD, of MIT. “This can be used with any drug that requires frequent dosing. We can replace that dosing with a single administration.”
This type of delivery could also help researchers run better clinical trials by making it easier for patients to take the drugs, said Shiyi Zhang, PhD, of MIT.
“It may help doctors and the pharma industry to better evaluate the efficacy of certain drugs because, currently, a lot of patients in clinical trials have serious medication adherence problems that will mislead the clinical studies,” he said. ![]()
Compound could treat leukemia, other cancers
and Patrick Harran with
a model of DZ-2384
Photo courtesy of
Reed Hutchinson/UCLA
A new compound holds promise for treating leukemia and other cancers, according to researchers.
The compound, DZ-2384, is a synthetic version of diazonamide A, a toxin isolated from a marine animal.
DZ-2384 is a microtubule-targeting agent (MTA). It binds to and alters the stability of microtubules, disrupting the normal function of the mitotic spindle and arresting cell-cycle progression at mitosis, ultimately leading to cell death.
However, researchers found that DZ-2384 behaves somewhat differently from other MTAs.
They reported these findings in Science Translational Medicine. The research was supported, in part, by Diazon Pharmaceuticals Inc.
The researchers tested DZ-2384 in mouse models of various cancers, including adult Philadelphia chromosome–negative acute lymphocytic leukemia.
In animals receiving DZ-2384, tumors shrank as much as or more than when a conventional MTA was used, but with much less toxicity at effective doses.
In particular, animals receiving DZ-2384 had markedly less peripheral neuropathy than those that received docetaxel, a conventional MTA. Peripheral neuropathy is one of the chief side effects of MTAs and can prompt treatment discontinuation.
“We have good reason to expect that human clinical trials of DZ-2384 will show that, at doses effective for treating a person’s cancer, there will be much less risk of the peripheral neuropathy that can force clinicians to stop treatment,” said study author Patrick Harran, PhD, of the University of California, Los Angeles.
Dr Harran believes clinical trials of DZ-2384 could begin within 2 years.
How this study began
Dr Harran began his work with diazonamides as a fundamental chemistry research problem.
In 1991, a group of researchers described diazonamides A and B, which they had isolated from the marine animal Diazona chinensis.
But Dr Harran and his colleagues found the described structure of diazonamide A was incorrect. In 2001, the team published a study that corrected the chemical structure of diazonamide A, and, 2 years later, they had synthesized the true structure in their lab.
Next, they began studying what the molecule might be doing to stop cells from dividing. In 2007, they discovered that the synthetic diazonamides they had produced minimized undesirable toxic effects that are commonly associated with chemotherapy.
Specifically, the compounds had an unusually large therapeutic window. In experiment after experiment, Dr Harran said, the researchers found that synthetic diazonamides’ therapeutic window was at least 10 times larger than that of traditional MTAs.
A key finding
In 2015, researchers prepared a form of DZ-2384 that was engineered with a molecular-scale “tracking device” so they could monitor its activity and better understand how it worked.
That helped the team confirm what they had come to suspect: that the compound binds to tubulin, which is a building block of mitotic spindles and a common target of MTAs.
Armed with this information, the researchers used X-ray crystallography to determine the structure of DZ-2384 bound to tubulin.
Their work offers a possible explanation for how DZ-2384 could disrupt dynamic tubulin polymers during cell division but spare those polymers in resting cells like neurons in the peripheral nervous system.
And that is what appears to allow the compound to attack growing cancer cells while minimizing damage to healthy cells. ![]()

and Patrick Harran with
a model of DZ-2384
Photo courtesy of
Reed Hutchinson/UCLA
A new compound holds promise for treating leukemia and other cancers, according to researchers.
The compound, DZ-2384, is a synthetic version of diazonamide A, a toxin isolated from a marine animal.
DZ-2384 is a microtubule-targeting agent (MTA). It binds to and alters the stability of microtubules, disrupting the normal function of the mitotic spindle and arresting cell-cycle progression at mitosis, ultimately leading to cell death.
However, researchers found that DZ-2384 behaves somewhat differently from other MTAs.
They reported these findings in Science Translational Medicine. The research was supported, in part, by Diazon Pharmaceuticals Inc.
The researchers tested DZ-2384 in mouse models of various cancers, including adult Philadelphia chromosome–negative acute lymphocytic leukemia.
In animals receiving DZ-2384, tumors shrank as much as or more than when a conventional MTA was used, but with much less toxicity at effective doses.
In particular, animals receiving DZ-2384 had markedly less peripheral neuropathy than those that received docetaxel, a conventional MTA. Peripheral neuropathy is one of the chief side effects of MTAs and can prompt treatment discontinuation.
“We have good reason to expect that human clinical trials of DZ-2384 will show that, at doses effective for treating a person’s cancer, there will be much less risk of the peripheral neuropathy that can force clinicians to stop treatment,” said study author Patrick Harran, PhD, of the University of California, Los Angeles.
Dr Harran believes clinical trials of DZ-2384 could begin within 2 years.
How this study began
Dr Harran began his work with diazonamides as a fundamental chemistry research problem.
In 1991, a group of researchers described diazonamides A and B, which they had isolated from the marine animal Diazona chinensis.
But Dr Harran and his colleagues found the described structure of diazonamide A was incorrect. In 2001, the team published a study that corrected the chemical structure of diazonamide A, and, 2 years later, they had synthesized the true structure in their lab.
Next, they began studying what the molecule might be doing to stop cells from dividing. In 2007, they discovered that the synthetic diazonamides they had produced minimized undesirable toxic effects that are commonly associated with chemotherapy.
Specifically, the compounds had an unusually large therapeutic window. In experiment after experiment, Dr Harran said, the researchers found that synthetic diazonamides’ therapeutic window was at least 10 times larger than that of traditional MTAs.
A key finding
In 2015, researchers prepared a form of DZ-2384 that was engineered with a molecular-scale “tracking device” so they could monitor its activity and better understand how it worked.
That helped the team confirm what they had come to suspect: that the compound binds to tubulin, which is a building block of mitotic spindles and a common target of MTAs.
Armed with this information, the researchers used X-ray crystallography to determine the structure of DZ-2384 bound to tubulin.
Their work offers a possible explanation for how DZ-2384 could disrupt dynamic tubulin polymers during cell division but spare those polymers in resting cells like neurons in the peripheral nervous system.
And that is what appears to allow the compound to attack growing cancer cells while minimizing damage to healthy cells. ![]()

and Patrick Harran with
a model of DZ-2384
Photo courtesy of
Reed Hutchinson/UCLA
A new compound holds promise for treating leukemia and other cancers, according to researchers.
The compound, DZ-2384, is a synthetic version of diazonamide A, a toxin isolated from a marine animal.
DZ-2384 is a microtubule-targeting agent (MTA). It binds to and alters the stability of microtubules, disrupting the normal function of the mitotic spindle and arresting cell-cycle progression at mitosis, ultimately leading to cell death.
However, researchers found that DZ-2384 behaves somewhat differently from other MTAs.
They reported these findings in Science Translational Medicine. The research was supported, in part, by Diazon Pharmaceuticals Inc.
The researchers tested DZ-2384 in mouse models of various cancers, including adult Philadelphia chromosome–negative acute lymphocytic leukemia.
In animals receiving DZ-2384, tumors shrank as much as or more than when a conventional MTA was used, but with much less toxicity at effective doses.
In particular, animals receiving DZ-2384 had markedly less peripheral neuropathy than those that received docetaxel, a conventional MTA. Peripheral neuropathy is one of the chief side effects of MTAs and can prompt treatment discontinuation.
“We have good reason to expect that human clinical trials of DZ-2384 will show that, at doses effective for treating a person’s cancer, there will be much less risk of the peripheral neuropathy that can force clinicians to stop treatment,” said study author Patrick Harran, PhD, of the University of California, Los Angeles.
Dr Harran believes clinical trials of DZ-2384 could begin within 2 years.
How this study began
Dr Harran began his work with diazonamides as a fundamental chemistry research problem.
In 1991, a group of researchers described diazonamides A and B, which they had isolated from the marine animal Diazona chinensis.
But Dr Harran and his colleagues found the described structure of diazonamide A was incorrect. In 2001, the team published a study that corrected the chemical structure of diazonamide A, and, 2 years later, they had synthesized the true structure in their lab.
Next, they began studying what the molecule might be doing to stop cells from dividing. In 2007, they discovered that the synthetic diazonamides they had produced minimized undesirable toxic effects that are commonly associated with chemotherapy.
Specifically, the compounds had an unusually large therapeutic window. In experiment after experiment, Dr Harran said, the researchers found that synthetic diazonamides’ therapeutic window was at least 10 times larger than that of traditional MTAs.
A key finding
In 2015, researchers prepared a form of DZ-2384 that was engineered with a molecular-scale “tracking device” so they could monitor its activity and better understand how it worked.
That helped the team confirm what they had come to suspect: that the compound binds to tubulin, which is a building block of mitotic spindles and a common target of MTAs.
Armed with this information, the researchers used X-ray crystallography to determine the structure of DZ-2384 bound to tubulin.
Their work offers a possible explanation for how DZ-2384 could disrupt dynamic tubulin polymers during cell division but spare those polymers in resting cells like neurons in the peripheral nervous system.
And that is what appears to allow the compound to attack growing cancer cells while minimizing damage to healthy cells. ![]()
Anticoagulant therapies appear comparable
Photo from Business Wire
NEW ORLEANS—Two types of anticoagulant therapy produce comparable outcomes for patients undergoing percutaneous coronary intervention (PCI), according to a new study.
The study was designed to determine which of 2 treatment methods—heparin combined with a short-term (less than 6 hours) infusion of tirofiban, or short-term periprocedural bivalirudin—was more effective.
Results showed that the 1-year risk of death, myocardial infarction, and urgent target vessel revascularization (UTVR) was not significantly different between the 2 treatment methods.
The incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding at 30 days was also similar between the treatment groups.
Results of this study were presented at the American Heart Association Scientific Sessions (abstract 15074). The study was funded by an unrestricted grant from the Medicure Corporation.
“Bivalirudin has been considered the gold standard for reducing bleeding during percutaneous coronary intervention, but our study shows heparin plus short-term tirofiban is just as good and possibly better,” said study investigator J. Brent Muhlestein, MD, of Intermountain Medical Center Heart Institute in Salt Lake City, Utah.
“The results certainly justify a randomized clinical trial to explore identified trends.”
Dr Muhlestein and his colleagues studied data on patients who underwent PCI between January 2013 and December 2015.
Of the 857 patients enrolled in the study, 402 received heparin plus short-term tirofiban treatment, and 455 received bivalirudin. The patients were between the ages of 51 and 78.
Results
At 30 days, the incidence of TIMI major bleeding was 1.2% for patients treated with heparin and tirofiban and 3.1% for bivalirudin-treated patients (P=0.10).
Also at 30 days, the incidence of death was 0.7% in the heparin/tirofiban group and 1.9% in the bivalirudin group (P=0.23). The incidence of myocardial infarction was 0.5% and 0.7%, respectively (P=1.00). And the incidence of UTVR was 0% and 0.7%, respectively (P=0.25).
At 1 year, the incidence of death was 3.4% for patients treated with heparin and tirofiban and 5.5% for bivalirudin-treated patients (P=0.42).
The incidence of myocardial infarction at 1 year was 2.9% and 3.0%, respectively (P=1.00). And the incidence of UTVR was 2.0% and 1.5%, respectively (P=0.67).
In multivariable analysis, the odds ratio (OR) for 30-day TIMI major bleeding (heparin/tirofiban vs bivalirudin) was 0.41 (P=0.11).
The OR for death at 1 year was 0.50 (P=0.33). The OR for non-fatal myocardial infarction at 1 year was 1.09 (P=0.91). And the OR for UTVR at 1 year was 1.23 (P=0.84). ![]()

Photo from Business Wire
NEW ORLEANS—Two types of anticoagulant therapy produce comparable outcomes for patients undergoing percutaneous coronary intervention (PCI), according to a new study.
The study was designed to determine which of 2 treatment methods—heparin combined with a short-term (less than 6 hours) infusion of tirofiban, or short-term periprocedural bivalirudin—was more effective.
Results showed that the 1-year risk of death, myocardial infarction, and urgent target vessel revascularization (UTVR) was not significantly different between the 2 treatment methods.
The incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding at 30 days was also similar between the treatment groups.
Results of this study were presented at the American Heart Association Scientific Sessions (abstract 15074). The study was funded by an unrestricted grant from the Medicure Corporation.
“Bivalirudin has been considered the gold standard for reducing bleeding during percutaneous coronary intervention, but our study shows heparin plus short-term tirofiban is just as good and possibly better,” said study investigator J. Brent Muhlestein, MD, of Intermountain Medical Center Heart Institute in Salt Lake City, Utah.
“The results certainly justify a randomized clinical trial to explore identified trends.”
Dr Muhlestein and his colleagues studied data on patients who underwent PCI between January 2013 and December 2015.
Of the 857 patients enrolled in the study, 402 received heparin plus short-term tirofiban treatment, and 455 received bivalirudin. The patients were between the ages of 51 and 78.
Results
At 30 days, the incidence of TIMI major bleeding was 1.2% for patients treated with heparin and tirofiban and 3.1% for bivalirudin-treated patients (P=0.10).
Also at 30 days, the incidence of death was 0.7% in the heparin/tirofiban group and 1.9% in the bivalirudin group (P=0.23). The incidence of myocardial infarction was 0.5% and 0.7%, respectively (P=1.00). And the incidence of UTVR was 0% and 0.7%, respectively (P=0.25).
At 1 year, the incidence of death was 3.4% for patients treated with heparin and tirofiban and 5.5% for bivalirudin-treated patients (P=0.42).
The incidence of myocardial infarction at 1 year was 2.9% and 3.0%, respectively (P=1.00). And the incidence of UTVR was 2.0% and 1.5%, respectively (P=0.67).
In multivariable analysis, the odds ratio (OR) for 30-day TIMI major bleeding (heparin/tirofiban vs bivalirudin) was 0.41 (P=0.11).
The OR for death at 1 year was 0.50 (P=0.33). The OR for non-fatal myocardial infarction at 1 year was 1.09 (P=0.91). And the OR for UTVR at 1 year was 1.23 (P=0.84). ![]()

Photo from Business Wire
NEW ORLEANS—Two types of anticoagulant therapy produce comparable outcomes for patients undergoing percutaneous coronary intervention (PCI), according to a new study.
The study was designed to determine which of 2 treatment methods—heparin combined with a short-term (less than 6 hours) infusion of tirofiban, or short-term periprocedural bivalirudin—was more effective.
Results showed that the 1-year risk of death, myocardial infarction, and urgent target vessel revascularization (UTVR) was not significantly different between the 2 treatment methods.
The incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding at 30 days was also similar between the treatment groups.
Results of this study were presented at the American Heart Association Scientific Sessions (abstract 15074). The study was funded by an unrestricted grant from the Medicure Corporation.
“Bivalirudin has been considered the gold standard for reducing bleeding during percutaneous coronary intervention, but our study shows heparin plus short-term tirofiban is just as good and possibly better,” said study investigator J. Brent Muhlestein, MD, of Intermountain Medical Center Heart Institute in Salt Lake City, Utah.
“The results certainly justify a randomized clinical trial to explore identified trends.”
Dr Muhlestein and his colleagues studied data on patients who underwent PCI between January 2013 and December 2015.
Of the 857 patients enrolled in the study, 402 received heparin plus short-term tirofiban treatment, and 455 received bivalirudin. The patients were between the ages of 51 and 78.
Results
At 30 days, the incidence of TIMI major bleeding was 1.2% for patients treated with heparin and tirofiban and 3.1% for bivalirudin-treated patients (P=0.10).
Also at 30 days, the incidence of death was 0.7% in the heparin/tirofiban group and 1.9% in the bivalirudin group (P=0.23). The incidence of myocardial infarction was 0.5% and 0.7%, respectively (P=1.00). And the incidence of UTVR was 0% and 0.7%, respectively (P=0.25).
At 1 year, the incidence of death was 3.4% for patients treated with heparin and tirofiban and 5.5% for bivalirudin-treated patients (P=0.42).
The incidence of myocardial infarction at 1 year was 2.9% and 3.0%, respectively (P=1.00). And the incidence of UTVR was 2.0% and 1.5%, respectively (P=0.67).
In multivariable analysis, the odds ratio (OR) for 30-day TIMI major bleeding (heparin/tirofiban vs bivalirudin) was 0.41 (P=0.11).
The OR for death at 1 year was 0.50 (P=0.33). The OR for non-fatal myocardial infarction at 1 year was 1.09 (P=0.91). And the OR for UTVR at 1 year was 1.23 (P=0.84). ![]()
‘I Don’t Know How to Get Exercise’
Much research has been devoted to the importance of exercise and finding out how to get people to exercise more. Yet > 1 in 4 American adults over the age of 50 do not engage in physical activity, according to a CDC report. Women are more likely to be inactive than men (30% vs 26%), and inactivity is highest in the South (30%) and Midwest (28%), although the Northeast (27%) and West (23%) followed closely.
Related: Walk the Talk: VA Mental Health Care Professionals’ Role in Promoting Physical Activity
A study of 110 women living in rural southern Illinois may help clarify the reasons for inactivity. The women, divided into 4 age groups, participated in focus groups held in various community settings across 7 counties.
The women talked about engaging in physical activity to relieve chronic pain, manage illness, improve mental health, and feel more energetic. However, they also talked about the barriers to being physically active, such as not knowing how to fit exercise into their lives.
Related: Exercise Lowers Risk of Some Cancers
When the researchers analyzed responses by age, they found that young women did not describe exercise as an activity to do with other women. For them, work, household, and family commitments competed with social activities, including those associated with physical activity. Older women, on the other hand, might be more inclined to spend time in activity-related groups, such as walking groups, to spend time with others. But all of the women over the age of 30 described physical and mental health challenges that interfered with being active.
Younger women and mothers also were more likely to be interested in spaces for outdoor recreation, where they could be active with their children. Older women rarely discussed outdoor resources; perhaps because they had more flexible schedules that allowed for structured groups and more affordable options due to senior discounts, the researchers suggest.
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
By understanding the multifactorial reasons for why women aren’t getting enough exercise, the researchers speculate that it might be possible to develop interventions that reduce the barriers and “capitalize on what motivates women at different times in their lives.”
Much research has been devoted to the importance of exercise and finding out how to get people to exercise more. Yet > 1 in 4 American adults over the age of 50 do not engage in physical activity, according to a CDC report. Women are more likely to be inactive than men (30% vs 26%), and inactivity is highest in the South (30%) and Midwest (28%), although the Northeast (27%) and West (23%) followed closely.
Related: Walk the Talk: VA Mental Health Care Professionals’ Role in Promoting Physical Activity
A study of 110 women living in rural southern Illinois may help clarify the reasons for inactivity. The women, divided into 4 age groups, participated in focus groups held in various community settings across 7 counties.
The women talked about engaging in physical activity to relieve chronic pain, manage illness, improve mental health, and feel more energetic. However, they also talked about the barriers to being physically active, such as not knowing how to fit exercise into their lives.
Related: Exercise Lowers Risk of Some Cancers
When the researchers analyzed responses by age, they found that young women did not describe exercise as an activity to do with other women. For them, work, household, and family commitments competed with social activities, including those associated with physical activity. Older women, on the other hand, might be more inclined to spend time in activity-related groups, such as walking groups, to spend time with others. But all of the women over the age of 30 described physical and mental health challenges that interfered with being active.
Younger women and mothers also were more likely to be interested in spaces for outdoor recreation, where they could be active with their children. Older women rarely discussed outdoor resources; perhaps because they had more flexible schedules that allowed for structured groups and more affordable options due to senior discounts, the researchers suggest.
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
By understanding the multifactorial reasons for why women aren’t getting enough exercise, the researchers speculate that it might be possible to develop interventions that reduce the barriers and “capitalize on what motivates women at different times in their lives.”
Much research has been devoted to the importance of exercise and finding out how to get people to exercise more. Yet > 1 in 4 American adults over the age of 50 do not engage in physical activity, according to a CDC report. Women are more likely to be inactive than men (30% vs 26%), and inactivity is highest in the South (30%) and Midwest (28%), although the Northeast (27%) and West (23%) followed closely.
Related: Walk the Talk: VA Mental Health Care Professionals’ Role in Promoting Physical Activity
A study of 110 women living in rural southern Illinois may help clarify the reasons for inactivity. The women, divided into 4 age groups, participated in focus groups held in various community settings across 7 counties.
The women talked about engaging in physical activity to relieve chronic pain, manage illness, improve mental health, and feel more energetic. However, they also talked about the barriers to being physically active, such as not knowing how to fit exercise into their lives.
Related: Exercise Lowers Risk of Some Cancers
When the researchers analyzed responses by age, they found that young women did not describe exercise as an activity to do with other women. For them, work, household, and family commitments competed with social activities, including those associated with physical activity. Older women, on the other hand, might be more inclined to spend time in activity-related groups, such as walking groups, to spend time with others. But all of the women over the age of 30 described physical and mental health challenges that interfered with being active.
Younger women and mothers also were more likely to be interested in spaces for outdoor recreation, where they could be active with their children. Older women rarely discussed outdoor resources; perhaps because they had more flexible schedules that allowed for structured groups and more affordable options due to senior discounts, the researchers suggest.
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
By understanding the multifactorial reasons for why women aren’t getting enough exercise, the researchers speculate that it might be possible to develop interventions that reduce the barriers and “capitalize on what motivates women at different times in their lives.”
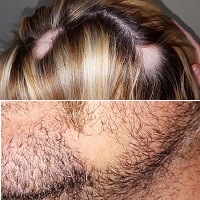
His & Hers Hair Loss
A 29-year-old woman and her 40-year-old husband present together for evaluation of similar problems: One month ago, they both experienced sudden hair loss—the wife in two spots on her scalp, the husband in his beard. In both cases, the hair came out suddenly, without any symptoms. Neither has had this problem before. Both patients are otherwise healthy, but they admit to being under a great deal of stress in the weeks prior to onset of the condition.
Before consulting dermatology, they were seen in an urgent care clinic, where they were diagnosed with “ringworm.” Twice-daily application of nystatin cream was prescribed—to no good effect.
EXAMINATION
On each side of the woman’s parietal scalp is a round, completely hairless patch with exceptionally well-defined margins. The sites are otherwise unremarkable, free of redness, edema, or scaling. Each lesion measures about 3.5 cm in diameter. No lymph nodes are palpable in the adjacent neck.
On the man’s left jawline is a solitary, round, 4-cm, hairless patch. It is also free of edema, erythema, or epidermal disturbance of any kind. There are no palpable lymph nodes in the drainage area.
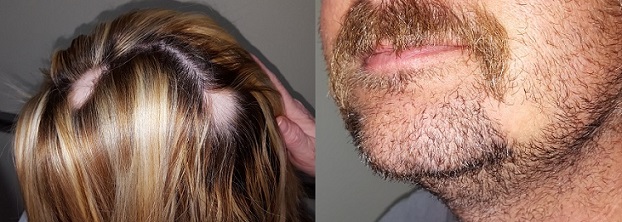
What is the diagnosis?
DISCUSSION
Sudden, asymptomatic, complete, well-marginated hair loss in any hair-bearing area is likely to be alopecia areata (AA). AA is an autoimmune process in which hair follicles are immobilized and unable to grow new hair until the process stops, which usually happens within weeks to months.
Because stress and AA have a suspected correlation, the patients’ histories of recent stress corroborate the diagnosis. Other features that bolster this impression are the lack of epidermal disturbance (eg, scaling, redness, edema) or palpable nodes in the area. The unusual factor in this case was the simultaneous appearance of the condition in husband and wife—for which I have no good explanation.
There are many items in the differential for localized hair loss, including discoid lupus, lichen planopilaris, and tinea capitis. However, these almost always manifest with frank inflammation characterized by epidermal disturbance.
Treatments such as intralesional steroid injection can promote modest hair growth but are generally ineffective for long-term recovery; unless the procedure is repeated, the hair will continue to fall out. These patients were offered treatment but declined, and they recovered in a short period of time.
TAKE-HOME LEARNING POINTS
• Alopecia areata (AA) typically displays as acute, asymptomatic, complete hair loss in one or more hair-bearing locations, usually in a round, well-defined configuration.
• AA is an autoimmune process that immobilizes hair follicles, causing them to fall out and not be replaced until the process ceases. This can take months, and the condition does not respond well to treatment.
• AA can affect the scalp, beard, brows, or even genital hair.
• The differential for localized hair loss includes tinea capitis, discoid lupus, lichen planopilaris, and lues; however, these all involve disruption of the overlying skin with scaling, redness, or edema.
A 29-year-old woman and her 40-year-old husband present together for evaluation of similar problems: One month ago, they both experienced sudden hair loss—the wife in two spots on her scalp, the husband in his beard. In both cases, the hair came out suddenly, without any symptoms. Neither has had this problem before. Both patients are otherwise healthy, but they admit to being under a great deal of stress in the weeks prior to onset of the condition.
Before consulting dermatology, they were seen in an urgent care clinic, where they were diagnosed with “ringworm.” Twice-daily application of nystatin cream was prescribed—to no good effect.
EXAMINATION
On each side of the woman’s parietal scalp is a round, completely hairless patch with exceptionally well-defined margins. The sites are otherwise unremarkable, free of redness, edema, or scaling. Each lesion measures about 3.5 cm in diameter. No lymph nodes are palpable in the adjacent neck.
On the man’s left jawline is a solitary, round, 4-cm, hairless patch. It is also free of edema, erythema, or epidermal disturbance of any kind. There are no palpable lymph nodes in the drainage area.

What is the diagnosis?
DISCUSSION
Sudden, asymptomatic, complete, well-marginated hair loss in any hair-bearing area is likely to be alopecia areata (AA). AA is an autoimmune process in which hair follicles are immobilized and unable to grow new hair until the process stops, which usually happens within weeks to months.
Because stress and AA have a suspected correlation, the patients’ histories of recent stress corroborate the diagnosis. Other features that bolster this impression are the lack of epidermal disturbance (eg, scaling, redness, edema) or palpable nodes in the area. The unusual factor in this case was the simultaneous appearance of the condition in husband and wife—for which I have no good explanation.
There are many items in the differential for localized hair loss, including discoid lupus, lichen planopilaris, and tinea capitis. However, these almost always manifest with frank inflammation characterized by epidermal disturbance.
Treatments such as intralesional steroid injection can promote modest hair growth but are generally ineffective for long-term recovery; unless the procedure is repeated, the hair will continue to fall out. These patients were offered treatment but declined, and they recovered in a short period of time.
TAKE-HOME LEARNING POINTS
• Alopecia areata (AA) typically displays as acute, asymptomatic, complete hair loss in one or more hair-bearing locations, usually in a round, well-defined configuration.
• AA is an autoimmune process that immobilizes hair follicles, causing them to fall out and not be replaced until the process ceases. This can take months, and the condition does not respond well to treatment.
• AA can affect the scalp, beard, brows, or even genital hair.
• The differential for localized hair loss includes tinea capitis, discoid lupus, lichen planopilaris, and lues; however, these all involve disruption of the overlying skin with scaling, redness, or edema.
A 29-year-old woman and her 40-year-old husband present together for evaluation of similar problems: One month ago, they both experienced sudden hair loss—the wife in two spots on her scalp, the husband in his beard. In both cases, the hair came out suddenly, without any symptoms. Neither has had this problem before. Both patients are otherwise healthy, but they admit to being under a great deal of stress in the weeks prior to onset of the condition.
Before consulting dermatology, they were seen in an urgent care clinic, where they were diagnosed with “ringworm.” Twice-daily application of nystatin cream was prescribed—to no good effect.
EXAMINATION
On each side of the woman’s parietal scalp is a round, completely hairless patch with exceptionally well-defined margins. The sites are otherwise unremarkable, free of redness, edema, or scaling. Each lesion measures about 3.5 cm in diameter. No lymph nodes are palpable in the adjacent neck.
On the man’s left jawline is a solitary, round, 4-cm, hairless patch. It is also free of edema, erythema, or epidermal disturbance of any kind. There are no palpable lymph nodes in the drainage area.

What is the diagnosis?
DISCUSSION
Sudden, asymptomatic, complete, well-marginated hair loss in any hair-bearing area is likely to be alopecia areata (AA). AA is an autoimmune process in which hair follicles are immobilized and unable to grow new hair until the process stops, which usually happens within weeks to months.
Because stress and AA have a suspected correlation, the patients’ histories of recent stress corroborate the diagnosis. Other features that bolster this impression are the lack of epidermal disturbance (eg, scaling, redness, edema) or palpable nodes in the area. The unusual factor in this case was the simultaneous appearance of the condition in husband and wife—for which I have no good explanation.
There are many items in the differential for localized hair loss, including discoid lupus, lichen planopilaris, and tinea capitis. However, these almost always manifest with frank inflammation characterized by epidermal disturbance.
Treatments such as intralesional steroid injection can promote modest hair growth but are generally ineffective for long-term recovery; unless the procedure is repeated, the hair will continue to fall out. These patients were offered treatment but declined, and they recovered in a short period of time.
TAKE-HOME LEARNING POINTS
• Alopecia areata (AA) typically displays as acute, asymptomatic, complete hair loss in one or more hair-bearing locations, usually in a round, well-defined configuration.
• AA is an autoimmune process that immobilizes hair follicles, causing them to fall out and not be replaced until the process ceases. This can take months, and the condition does not respond well to treatment.
• AA can affect the scalp, beard, brows, or even genital hair.
• The differential for localized hair loss includes tinea capitis, discoid lupus, lichen planopilaris, and lues; however, these all involve disruption of the overlying skin with scaling, redness, or edema.
“Jock itch” or something else?
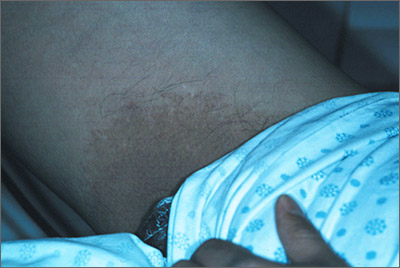
The family physician (FP) agreed that this could be a tinea cruris infection. He performed a potassium hydroxide (KOH) preparation, but did not see any hyphae or fungal elements. (See video on how to perform a KOH preparation here.)
He told the patient that there was no evidence of fungus under the microscope, and took out his Woods lamp (ultraviolet light) to check for erythrasma. The involved area fluoresced a coral red, confirming the diagnosis of erythrasma. Erythrasma is a bacterial infection caused by Corynebacterium minutissimum.
Treatment options include topical erythromycin, topical clindamycin, oral erythromycin, or oral clarithromycin. The patient decided to take oral erythromycin and the FP prescribed 250 mg twice a day for 2 weeks. At a follow-up visit one month later, the rash had completely resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The family physician (FP) agreed that this could be a tinea cruris infection. He performed a potassium hydroxide (KOH) preparation, but did not see any hyphae or fungal elements. (See video on how to perform a KOH preparation here.)
He told the patient that there was no evidence of fungus under the microscope, and took out his Woods lamp (ultraviolet light) to check for erythrasma. The involved area fluoresced a coral red, confirming the diagnosis of erythrasma. Erythrasma is a bacterial infection caused by Corynebacterium minutissimum.
Treatment options include topical erythromycin, topical clindamycin, oral erythromycin, or oral clarithromycin. The patient decided to take oral erythromycin and the FP prescribed 250 mg twice a day for 2 weeks. At a follow-up visit one month later, the rash had completely resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The family physician (FP) agreed that this could be a tinea cruris infection. He performed a potassium hydroxide (KOH) preparation, but did not see any hyphae or fungal elements. (See video on how to perform a KOH preparation here.)
He told the patient that there was no evidence of fungus under the microscope, and took out his Woods lamp (ultraviolet light) to check for erythrasma. The involved area fluoresced a coral red, confirming the diagnosis of erythrasma. Erythrasma is a bacterial infection caused by Corynebacterium minutissimum.
Treatment options include topical erythromycin, topical clindamycin, oral erythromycin, or oral clarithromycin. The patient decided to take oral erythromycin and the FP prescribed 250 mg twice a day for 2 weeks. At a follow-up visit one month later, the rash had completely resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
STUDY: More children have access to care, insurance
Children’s access to pediatricians has improved since 2000 with markedly more patients carrying health insurance, according to a new study.
An analysis showed the rate of uninsured children dropped from 12% in 2000 to 5% in 2014, while children’s access rose for physician visits and regular care.
Kandyce Larson, PhD, of the American Academy of Pediatrics and her colleagues reviewed trends for 178,038 children in the Centers for Disease Control & Prevention’ National Health Interview Survey from 2000 to 2014. Researchers examined statistics for health insurance and trends across five access indicators: well-child visits, doctor office visits, dental visits, usual source of care, and unmet health needs. Results showed the uninsured rate declined by more than 50% from 12% of children in 2000 to 5% in 2014. Findings showed an accompanying increase in public health insurance coverage (19% to 39%), while private coverage decreased (69% to 56%) during the same time period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2176).
Uninsured rates for minority and poor children showed the largest decreases, the study found. The uninsured rate for Hispanic children decreased from 26% in 2000 to 9% in 2014, while uninsured black children dropped from 12% to 3%, according to the study. Significant declines in the uninsured rate also were seen for children in poor families (22% to 6%) and near-poor families (21% to 9%).
Health care access improved across all five indicators. Rates for no well-child visit declined from 29% in 2000 to 16% in 2014, while no doctor office visit went from 13% to 9%, and no dental visit decreased from 30% to 21%. No usual source of care dropped from 7% to 4%, and unmet health care needs decreased from 8% to 6%. The results amounted to an additional 9 million children receiving a well-child visit in 2014, compared with 2000. Improvements in access were generally greater for black and Hispanic children and those in poor and near-poor families, according to the study.
“It’s critical for our nation’s future health that we provide children at all income levels access to quality health care,” he said in a statement.
The rise in children who have a usual source of care and are undergoing regular well-child visits is especially heartening, added pediatrician Andrew D. Racine, MD, PhD, coauthor of the study and a member of the AAP Committee on Child Health Financing.
“When children see providers who know their medical history and can monitor their physical and socio-emotional development, they are more likely to have better overall health, be up to date on immunizations, perform better in school, and receive care in the most cost-effective way,” he said in a statement. Dr. Racine is a professor of clinical pediatrics at Albert Einstein College of Medicine, Bronx, N.Y.
[email protected]
On Twitter @legal_med
The findings of the current study have important implications for future child health care policy decisions.
First, children should have coverage, and strong efforts must be made to reach the 5.3% of children who remain uninsured. Second, the reauthorization and continued funding of the Children’s Health Insurance Plan, due to terminate in 2019, is essential and should be addressed sooner rather than later.
In addition, while this study focuses attention on health care access measures, advocates and policy makers need to address having meaningful health outcomes. Because health care only contributes 10% to 20% to maximizing population health, a new child health policy should focus on addressing the social determinants of health and the reduction of behaviors that compromise health, such as smoking, excessive alcohol intake, substance abuse, and poor nutrition.
Stephen Berman, MD, is a professor of pediatrics at the University of Colorado at Denver, holds an endowed chair in academic general pediatrics at the Children’s Hospital Colorado, and is the director of the Center for Global Health in the Colorado School of Public Health, all in Aurora. These remarks are excerpted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2823). He reported having no relevant financial disclosures.
The findings of the current study have important implications for future child health care policy decisions.
First, children should have coverage, and strong efforts must be made to reach the 5.3% of children who remain uninsured. Second, the reauthorization and continued funding of the Children’s Health Insurance Plan, due to terminate in 2019, is essential and should be addressed sooner rather than later.
In addition, while this study focuses attention on health care access measures, advocates and policy makers need to address having meaningful health outcomes. Because health care only contributes 10% to 20% to maximizing population health, a new child health policy should focus on addressing the social determinants of health and the reduction of behaviors that compromise health, such as smoking, excessive alcohol intake, substance abuse, and poor nutrition.
Stephen Berman, MD, is a professor of pediatrics at the University of Colorado at Denver, holds an endowed chair in academic general pediatrics at the Children’s Hospital Colorado, and is the director of the Center for Global Health in the Colorado School of Public Health, all in Aurora. These remarks are excerpted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2823). He reported having no relevant financial disclosures.
The findings of the current study have important implications for future child health care policy decisions.
First, children should have coverage, and strong efforts must be made to reach the 5.3% of children who remain uninsured. Second, the reauthorization and continued funding of the Children’s Health Insurance Plan, due to terminate in 2019, is essential and should be addressed sooner rather than later.
In addition, while this study focuses attention on health care access measures, advocates and policy makers need to address having meaningful health outcomes. Because health care only contributes 10% to 20% to maximizing population health, a new child health policy should focus on addressing the social determinants of health and the reduction of behaviors that compromise health, such as smoking, excessive alcohol intake, substance abuse, and poor nutrition.
Stephen Berman, MD, is a professor of pediatrics at the University of Colorado at Denver, holds an endowed chair in academic general pediatrics at the Children’s Hospital Colorado, and is the director of the Center for Global Health in the Colorado School of Public Health, all in Aurora. These remarks are excerpted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2823). He reported having no relevant financial disclosures.
Children’s access to pediatricians has improved since 2000 with markedly more patients carrying health insurance, according to a new study.
An analysis showed the rate of uninsured children dropped from 12% in 2000 to 5% in 2014, while children’s access rose for physician visits and regular care.
Kandyce Larson, PhD, of the American Academy of Pediatrics and her colleagues reviewed trends for 178,038 children in the Centers for Disease Control & Prevention’ National Health Interview Survey from 2000 to 2014. Researchers examined statistics for health insurance and trends across five access indicators: well-child visits, doctor office visits, dental visits, usual source of care, and unmet health needs. Results showed the uninsured rate declined by more than 50% from 12% of children in 2000 to 5% in 2014. Findings showed an accompanying increase in public health insurance coverage (19% to 39%), while private coverage decreased (69% to 56%) during the same time period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2176).
Uninsured rates for minority and poor children showed the largest decreases, the study found. The uninsured rate for Hispanic children decreased from 26% in 2000 to 9% in 2014, while uninsured black children dropped from 12% to 3%, according to the study. Significant declines in the uninsured rate also were seen for children in poor families (22% to 6%) and near-poor families (21% to 9%).
Health care access improved across all five indicators. Rates for no well-child visit declined from 29% in 2000 to 16% in 2014, while no doctor office visit went from 13% to 9%, and no dental visit decreased from 30% to 21%. No usual source of care dropped from 7% to 4%, and unmet health care needs decreased from 8% to 6%. The results amounted to an additional 9 million children receiving a well-child visit in 2014, compared with 2000. Improvements in access were generally greater for black and Hispanic children and those in poor and near-poor families, according to the study.
“It’s critical for our nation’s future health that we provide children at all income levels access to quality health care,” he said in a statement.
The rise in children who have a usual source of care and are undergoing regular well-child visits is especially heartening, added pediatrician Andrew D. Racine, MD, PhD, coauthor of the study and a member of the AAP Committee on Child Health Financing.
“When children see providers who know their medical history and can monitor their physical and socio-emotional development, they are more likely to have better overall health, be up to date on immunizations, perform better in school, and receive care in the most cost-effective way,” he said in a statement. Dr. Racine is a professor of clinical pediatrics at Albert Einstein College of Medicine, Bronx, N.Y.
[email protected]
On Twitter @legal_med
Children’s access to pediatricians has improved since 2000 with markedly more patients carrying health insurance, according to a new study.
An analysis showed the rate of uninsured children dropped from 12% in 2000 to 5% in 2014, while children’s access rose for physician visits and regular care.
Kandyce Larson, PhD, of the American Academy of Pediatrics and her colleagues reviewed trends for 178,038 children in the Centers for Disease Control & Prevention’ National Health Interview Survey from 2000 to 2014. Researchers examined statistics for health insurance and trends across five access indicators: well-child visits, doctor office visits, dental visits, usual source of care, and unmet health needs. Results showed the uninsured rate declined by more than 50% from 12% of children in 2000 to 5% in 2014. Findings showed an accompanying increase in public health insurance coverage (19% to 39%), while private coverage decreased (69% to 56%) during the same time period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2176).
Uninsured rates for minority and poor children showed the largest decreases, the study found. The uninsured rate for Hispanic children decreased from 26% in 2000 to 9% in 2014, while uninsured black children dropped from 12% to 3%, according to the study. Significant declines in the uninsured rate also were seen for children in poor families (22% to 6%) and near-poor families (21% to 9%).
Health care access improved across all five indicators. Rates for no well-child visit declined from 29% in 2000 to 16% in 2014, while no doctor office visit went from 13% to 9%, and no dental visit decreased from 30% to 21%. No usual source of care dropped from 7% to 4%, and unmet health care needs decreased from 8% to 6%. The results amounted to an additional 9 million children receiving a well-child visit in 2014, compared with 2000. Improvements in access were generally greater for black and Hispanic children and those in poor and near-poor families, according to the study.
“It’s critical for our nation’s future health that we provide children at all income levels access to quality health care,” he said in a statement.
The rise in children who have a usual source of care and are undergoing regular well-child visits is especially heartening, added pediatrician Andrew D. Racine, MD, PhD, coauthor of the study and a member of the AAP Committee on Child Health Financing.
“When children see providers who know their medical history and can monitor their physical and socio-emotional development, they are more likely to have better overall health, be up to date on immunizations, perform better in school, and receive care in the most cost-effective way,” he said in a statement. Dr. Racine is a professor of clinical pediatrics at Albert Einstein College of Medicine, Bronx, N.Y.
[email protected]
On Twitter @legal_med
Key clinical point:
Major finding: The rate of uninsured children dropped from 12% in 2000 to 5% in 2014.
Data source: Study of survey data of 178,038 children.
Disclosures: The authors have indicated they have no relevant financial disclosures.
Palbociclib extends PFS in advanced breast cancer
Adding the CDK inhibitor palbociclib to standard endocrine therapy (letrozole) extended progression-free survival in a manufacturer-sponsored phase III trial of advanced ER-positive, HER2-negative breast cancer, investigators reported in the New England Journal of Medicine.
Compared with placebo, palbociclib significantly improved progression-free survival regardless of patient age, patient performance status, site of metastasis, prior use of chemotherapy, prior use of endocrine therapy, length of the disease-free interval before progression, or the cancer’s histologic subtype. “The median progression-free survival of 24.8 months in [our study] is longer than that seen in other phase III studies involving women with advanced breast cancer. Whether this progression-free survival will result in longer overall survival is uncertain until further follow-up is completed,” wrote Richard S. Finn, MD, of the University of California, Los Angeles, and his associates.
The primary endpoint – time to disease progression or death – was 24.8 months with active treatment, compared with 14.5 months with placebo, for a hazard ratio of 0.58. The rate of disease progression or death was 43.7% in the palbociclib group, compared with 61.7% in the placebo group. In addition, the rate of objective tumor response was 42.1% with palbociclib, compared with 34.7% with placebo, they reported.
“Data on overall survival were immature at the time of this analysis of the primary end point, and the final overall survival analysis will be performed when a total of 390 deaths occur, per protocol and in agreement with regulatory agencies,” Dr. Finn and his associates said (N Engl J Med. 2016 Nov. 17. doi: 10.1056/NEJMoa1607303). As expected, the rate of hematologic adverse events was high with palbociclib. “Although the incidence of neutropenia of any grade in the palbobiclib/letrozole group was 79.5% in the current study, the incidence of febrile neutropenia was lower than 2%. In addition, the rate of permanent treatment discontinuation associated with an adverse event did not differ significantly between the two study groups, although dose reductions were more common with palbociclib than with placebo,” they added.
Myelotoxic effects have been managed successfully with appropriate supportive care and dose reductions. At the time of this analysis, 199 patients (44.8%) in the active-treatment group were still taking palbociclib and letrozole, the investigators said.
This study was supported by Pfizer. Dr. Finn reported receiving grants, personal fees, and other support from Pfizer, Bayer, Novartis, and Bristol-Myers Squibb. His associates reported ties to numerous industry sources.
Inhibition of CDK4 and CDK6, in combination with antiestrogens, is clearly the new standard of treatment for advanced ER-positive breast cancer.
Antonio C. Wolff, MD, is professor of oncology at Johns Hopkins University’s Sidney Kimmel Comprehensive Cancer Center, Baltimore, and executive officer of the Translational Breast Cancer Research Consortium. He reported ties to Pfizer. Dr. Wolff made these remarks in an editorial accompanying Dr. Finn’s report (N Engl J Med. 2016 Nov 17. doi: 10.1056/NEJMe1611926).
Inhibition of CDK4 and CDK6, in combination with antiestrogens, is clearly the new standard of treatment for advanced ER-positive breast cancer.
Antonio C. Wolff, MD, is professor of oncology at Johns Hopkins University’s Sidney Kimmel Comprehensive Cancer Center, Baltimore, and executive officer of the Translational Breast Cancer Research Consortium. He reported ties to Pfizer. Dr. Wolff made these remarks in an editorial accompanying Dr. Finn’s report (N Engl J Med. 2016 Nov 17. doi: 10.1056/NEJMe1611926).
Inhibition of CDK4 and CDK6, in combination with antiestrogens, is clearly the new standard of treatment for advanced ER-positive breast cancer.
Antonio C. Wolff, MD, is professor of oncology at Johns Hopkins University’s Sidney Kimmel Comprehensive Cancer Center, Baltimore, and executive officer of the Translational Breast Cancer Research Consortium. He reported ties to Pfizer. Dr. Wolff made these remarks in an editorial accompanying Dr. Finn’s report (N Engl J Med. 2016 Nov 17. doi: 10.1056/NEJMe1611926).
Adding the CDK inhibitor palbociclib to standard endocrine therapy (letrozole) extended progression-free survival in a manufacturer-sponsored phase III trial of advanced ER-positive, HER2-negative breast cancer, investigators reported in the New England Journal of Medicine.
Compared with placebo, palbociclib significantly improved progression-free survival regardless of patient age, patient performance status, site of metastasis, prior use of chemotherapy, prior use of endocrine therapy, length of the disease-free interval before progression, or the cancer’s histologic subtype. “The median progression-free survival of 24.8 months in [our study] is longer than that seen in other phase III studies involving women with advanced breast cancer. Whether this progression-free survival will result in longer overall survival is uncertain until further follow-up is completed,” wrote Richard S. Finn, MD, of the University of California, Los Angeles, and his associates.
The primary endpoint – time to disease progression or death – was 24.8 months with active treatment, compared with 14.5 months with placebo, for a hazard ratio of 0.58. The rate of disease progression or death was 43.7% in the palbociclib group, compared with 61.7% in the placebo group. In addition, the rate of objective tumor response was 42.1% with palbociclib, compared with 34.7% with placebo, they reported.
“Data on overall survival were immature at the time of this analysis of the primary end point, and the final overall survival analysis will be performed when a total of 390 deaths occur, per protocol and in agreement with regulatory agencies,” Dr. Finn and his associates said (N Engl J Med. 2016 Nov. 17. doi: 10.1056/NEJMoa1607303). As expected, the rate of hematologic adverse events was high with palbociclib. “Although the incidence of neutropenia of any grade in the palbobiclib/letrozole group was 79.5% in the current study, the incidence of febrile neutropenia was lower than 2%. In addition, the rate of permanent treatment discontinuation associated with an adverse event did not differ significantly between the two study groups, although dose reductions were more common with palbociclib than with placebo,” they added.
Myelotoxic effects have been managed successfully with appropriate supportive care and dose reductions. At the time of this analysis, 199 patients (44.8%) in the active-treatment group were still taking palbociclib and letrozole, the investigators said.
This study was supported by Pfizer. Dr. Finn reported receiving grants, personal fees, and other support from Pfizer, Bayer, Novartis, and Bristol-Myers Squibb. His associates reported ties to numerous industry sources.
Adding the CDK inhibitor palbociclib to standard endocrine therapy (letrozole) extended progression-free survival in a manufacturer-sponsored phase III trial of advanced ER-positive, HER2-negative breast cancer, investigators reported in the New England Journal of Medicine.
Compared with placebo, palbociclib significantly improved progression-free survival regardless of patient age, patient performance status, site of metastasis, prior use of chemotherapy, prior use of endocrine therapy, length of the disease-free interval before progression, or the cancer’s histologic subtype. “The median progression-free survival of 24.8 months in [our study] is longer than that seen in other phase III studies involving women with advanced breast cancer. Whether this progression-free survival will result in longer overall survival is uncertain until further follow-up is completed,” wrote Richard S. Finn, MD, of the University of California, Los Angeles, and his associates.
The primary endpoint – time to disease progression or death – was 24.8 months with active treatment, compared with 14.5 months with placebo, for a hazard ratio of 0.58. The rate of disease progression or death was 43.7% in the palbociclib group, compared with 61.7% in the placebo group. In addition, the rate of objective tumor response was 42.1% with palbociclib, compared with 34.7% with placebo, they reported.
“Data on overall survival were immature at the time of this analysis of the primary end point, and the final overall survival analysis will be performed when a total of 390 deaths occur, per protocol and in agreement with regulatory agencies,” Dr. Finn and his associates said (N Engl J Med. 2016 Nov. 17. doi: 10.1056/NEJMoa1607303). As expected, the rate of hematologic adverse events was high with palbociclib. “Although the incidence of neutropenia of any grade in the palbobiclib/letrozole group was 79.5% in the current study, the incidence of febrile neutropenia was lower than 2%. In addition, the rate of permanent treatment discontinuation associated with an adverse event did not differ significantly between the two study groups, although dose reductions were more common with palbociclib than with placebo,” they added.
Myelotoxic effects have been managed successfully with appropriate supportive care and dose reductions. At the time of this analysis, 199 patients (44.8%) in the active-treatment group were still taking palbociclib and letrozole, the investigators said.
This study was supported by Pfizer. Dr. Finn reported receiving grants, personal fees, and other support from Pfizer, Bayer, Novartis, and Bristol-Myers Squibb. His associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The primary endpoint – time to disease progression or death – was 24.8 months with active treatment, compared with 14.5 months with placebo, for an hazard ratio of 0.58.
Data source: An international, randomized, double-blind phase III clinical trial involving 666 women.
Disclosures: This study was supported by Pfizer. Dr. Finn reported receiving grants, personal fees, and other support from Pfizer, Bayer, Novartis, and Bristol-Myers Squibb. His associates reported ties to numerous industry sources.
Ustekinumab leads to high 6-week clinical response and 44-week remission rates
Intravenous induction of the monoclonal antibody ustekinumab induced significantly higher 6-week clinical response rates in patients with moderately to severely active Crohn’s disease, compared with those who received placebo.
In addition, among those who had achieved clinical response to induction therapy, subcutaneous administration of ustekinumab led to high 44-week remission rates.
All three trials were global, multisite, double-blind, placebo-controlled studies involving adults who had Crohn’s Disease Activity Index (CDAI) scores ranging from 220 to 450 out of 600, with higher scores indicating more severe disease.
“At week 0, patients in both induction trials were randomly assigned, in a 1:1:1 ratio, to receive a single intravenous infusion of 130 mg of ustekinumab, a weight-range–based dose that approximated 6 mg of ustekinumab per kilogram of body weight, or placebo,” Dr. Feagan, Dr. Sandborn, and their associates wrote.
A total of 741 patients participated in UNITI-1, while 628 patients participated in the UNITI-2 trial. Baseline and disease characteristics were similar among all groups, according to the researchers.
In UNITI-1, the percentage of patients who achieved the study’s primary endpoint of 6-week clinical response (defined as a 100-point decrease from baseline CDAI score or total CDAI score less than 150) was significantly higher in the groups that received ustekinumab at a dose of either 130 mg or 6 mg/kg (34.3% and 33.7%, respectively) than in the placebo group (21.5%).
The absolute difference between the 130-mg ustekinumab group and the placebo group was 12.8 percentage points (95% confidence interval, 5.0-20.7; P = .002), and the absolute difference between the 6-mg/kg ustekinumab dose and placebo was 12.3 percentage points (95% CI, 4.5-20.1; P = .003), the investigators reported.
Similarly, in UNITI-2, the percentages of patients who achieved 6-week clinical response also were significantly higher in the groups that received ustekinumab at a dose of either 130 mg or 6-mg/kg (51.7% and 55.5%, respectively), compared with the placebo group (28.7%).
The absolute difference between the 130-mg ustekinumab dose and placebo was 23.0 percentage points (95% CI, 13.8-32.1; P less than .001). Between the 6-mg/kg ustekinumab dose and placebo, the absolute difference was 26.8 percentage points (95% CI, 17.7-35.9; P less than .001).
“In the induction trials, both doses of ustekinumab were associated with greater reductions in and normalization of serum [C-reactive protein] levels than was placebo. The differences between ustekinumab and placebo were nominally significant and were observed as early as week 3 and persisted through week 8. Similar effects were observed for fecal calprotectin levels at week 6,” the investigators summarized.
In the maintenance trial IM-UNITI, patients from UNITI-1 and UNITI-2 (n = 1,281) who had a response to ustekinumab induction therapy at week 8 were randomly assigned, in a 1:1:1 ratio, to receive subcutaneous injections of 90 mg of ustekinumab every 8 weeks, 90 mg of ustekinumab every 12 weeks, or placebo through week 40.
In general, participants who received maintenance therapy with ustekinumab had significantly higher 44-week remission rates, compared with those who received placebo (53.1% for 8-week group, 48.8% for 12-week group, 35.9% for placebo).
“The rate of remission at week 44 was significantly higher among patients who entered maintenance in remission and who received treatment every 8 weeks – but not those who received treatment every 12 weeks – than among those who received placebo,” Dr. Feagan, Dr. Sandborn and their associates added.
Janssen Research and Development supported this study. Dr. Feagan, Dr. Sandborn, and 24 other investigators reported receiving financial compensation from various pharmaceutical companies, including Janssen. Nine of the investigators are employees of Janssen.
[email protected]
On Twitter @jessnicolecraig
Intravenous induction of the monoclonal antibody ustekinumab induced significantly higher 6-week clinical response rates in patients with moderately to severely active Crohn’s disease, compared with those who received placebo.
In addition, among those who had achieved clinical response to induction therapy, subcutaneous administration of ustekinumab led to high 44-week remission rates.
All three trials were global, multisite, double-blind, placebo-controlled studies involving adults who had Crohn’s Disease Activity Index (CDAI) scores ranging from 220 to 450 out of 600, with higher scores indicating more severe disease.
“At week 0, patients in both induction trials were randomly assigned, in a 1:1:1 ratio, to receive a single intravenous infusion of 130 mg of ustekinumab, a weight-range–based dose that approximated 6 mg of ustekinumab per kilogram of body weight, or placebo,” Dr. Feagan, Dr. Sandborn, and their associates wrote.
A total of 741 patients participated in UNITI-1, while 628 patients participated in the UNITI-2 trial. Baseline and disease characteristics were similar among all groups, according to the researchers.
In UNITI-1, the percentage of patients who achieved the study’s primary endpoint of 6-week clinical response (defined as a 100-point decrease from baseline CDAI score or total CDAI score less than 150) was significantly higher in the groups that received ustekinumab at a dose of either 130 mg or 6 mg/kg (34.3% and 33.7%, respectively) than in the placebo group (21.5%).
The absolute difference between the 130-mg ustekinumab group and the placebo group was 12.8 percentage points (95% confidence interval, 5.0-20.7; P = .002), and the absolute difference between the 6-mg/kg ustekinumab dose and placebo was 12.3 percentage points (95% CI, 4.5-20.1; P = .003), the investigators reported.
Similarly, in UNITI-2, the percentages of patients who achieved 6-week clinical response also were significantly higher in the groups that received ustekinumab at a dose of either 130 mg or 6-mg/kg (51.7% and 55.5%, respectively), compared with the placebo group (28.7%).
The absolute difference between the 130-mg ustekinumab dose and placebo was 23.0 percentage points (95% CI, 13.8-32.1; P less than .001). Between the 6-mg/kg ustekinumab dose and placebo, the absolute difference was 26.8 percentage points (95% CI, 17.7-35.9; P less than .001).
“In the induction trials, both doses of ustekinumab were associated with greater reductions in and normalization of serum [C-reactive protein] levels than was placebo. The differences between ustekinumab and placebo were nominally significant and were observed as early as week 3 and persisted through week 8. Similar effects were observed for fecal calprotectin levels at week 6,” the investigators summarized.
In the maintenance trial IM-UNITI, patients from UNITI-1 and UNITI-2 (n = 1,281) who had a response to ustekinumab induction therapy at week 8 were randomly assigned, in a 1:1:1 ratio, to receive subcutaneous injections of 90 mg of ustekinumab every 8 weeks, 90 mg of ustekinumab every 12 weeks, or placebo through week 40.
In general, participants who received maintenance therapy with ustekinumab had significantly higher 44-week remission rates, compared with those who received placebo (53.1% for 8-week group, 48.8% for 12-week group, 35.9% for placebo).
“The rate of remission at week 44 was significantly higher among patients who entered maintenance in remission and who received treatment every 8 weeks – but not those who received treatment every 12 weeks – than among those who received placebo,” Dr. Feagan, Dr. Sandborn and their associates added.
Janssen Research and Development supported this study. Dr. Feagan, Dr. Sandborn, and 24 other investigators reported receiving financial compensation from various pharmaceutical companies, including Janssen. Nine of the investigators are employees of Janssen.
[email protected]
On Twitter @jessnicolecraig
Intravenous induction of the monoclonal antibody ustekinumab induced significantly higher 6-week clinical response rates in patients with moderately to severely active Crohn’s disease, compared with those who received placebo.
In addition, among those who had achieved clinical response to induction therapy, subcutaneous administration of ustekinumab led to high 44-week remission rates.
All three trials were global, multisite, double-blind, placebo-controlled studies involving adults who had Crohn’s Disease Activity Index (CDAI) scores ranging from 220 to 450 out of 600, with higher scores indicating more severe disease.
“At week 0, patients in both induction trials were randomly assigned, in a 1:1:1 ratio, to receive a single intravenous infusion of 130 mg of ustekinumab, a weight-range–based dose that approximated 6 mg of ustekinumab per kilogram of body weight, or placebo,” Dr. Feagan, Dr. Sandborn, and their associates wrote.
A total of 741 patients participated in UNITI-1, while 628 patients participated in the UNITI-2 trial. Baseline and disease characteristics were similar among all groups, according to the researchers.
In UNITI-1, the percentage of patients who achieved the study’s primary endpoint of 6-week clinical response (defined as a 100-point decrease from baseline CDAI score or total CDAI score less than 150) was significantly higher in the groups that received ustekinumab at a dose of either 130 mg or 6 mg/kg (34.3% and 33.7%, respectively) than in the placebo group (21.5%).
The absolute difference between the 130-mg ustekinumab group and the placebo group was 12.8 percentage points (95% confidence interval, 5.0-20.7; P = .002), and the absolute difference between the 6-mg/kg ustekinumab dose and placebo was 12.3 percentage points (95% CI, 4.5-20.1; P = .003), the investigators reported.
Similarly, in UNITI-2, the percentages of patients who achieved 6-week clinical response also were significantly higher in the groups that received ustekinumab at a dose of either 130 mg or 6-mg/kg (51.7% and 55.5%, respectively), compared with the placebo group (28.7%).
The absolute difference between the 130-mg ustekinumab dose and placebo was 23.0 percentage points (95% CI, 13.8-32.1; P less than .001). Between the 6-mg/kg ustekinumab dose and placebo, the absolute difference was 26.8 percentage points (95% CI, 17.7-35.9; P less than .001).
“In the induction trials, both doses of ustekinumab were associated with greater reductions in and normalization of serum [C-reactive protein] levels than was placebo. The differences between ustekinumab and placebo were nominally significant and were observed as early as week 3 and persisted through week 8. Similar effects were observed for fecal calprotectin levels at week 6,” the investigators summarized.
In the maintenance trial IM-UNITI, patients from UNITI-1 and UNITI-2 (n = 1,281) who had a response to ustekinumab induction therapy at week 8 were randomly assigned, in a 1:1:1 ratio, to receive subcutaneous injections of 90 mg of ustekinumab every 8 weeks, 90 mg of ustekinumab every 12 weeks, or placebo through week 40.
In general, participants who received maintenance therapy with ustekinumab had significantly higher 44-week remission rates, compared with those who received placebo (53.1% for 8-week group, 48.8% for 12-week group, 35.9% for placebo).
“The rate of remission at week 44 was significantly higher among patients who entered maintenance in remission and who received treatment every 8 weeks – but not those who received treatment every 12 weeks – than among those who received placebo,” Dr. Feagan, Dr. Sandborn and their associates added.
Janssen Research and Development supported this study. Dr. Feagan, Dr. Sandborn, and 24 other investigators reported receiving financial compensation from various pharmaceutical companies, including Janssen. Nine of the investigators are employees of Janssen.
[email protected]
On Twitter @jessnicolecraig
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: 53.1% of patients who received ustekinumab every 8 weeks achieved 44-week remission, compared with 35.9% for placebo.
Data source: Three multisite, double-blind, placebo-controlled studies.
Disclosures: Janssen Research and Development supported this study. Dr. Feagan, Dr. Sandborn, and 24 other investigators reported receiving financial compensation from various pharmaceutical companies, including Janssen. Nine of the investigators are employees of Janssen.