User login
Lobectomy shown safe after concurrent chemo and radiation
Lobectomy can be done safely after concurrent chemotherapy and high-dose radiation in patients with resectable N2-positive stage IIIA non–small cell lung cancer, according to study findings presented by Jessica S. Donington, MD.
Dr. Donington of New York University and her colleagues presented analysis of two prospective trials conducted by NRG Oncology, RTOG 0229 and RTOG 0839 at the AATS Annual Meeting. Both trials’ primary endpoints were mediastinal node sterilization after concurrent chemotherapy and full-dose radiation and those results were previously reported.
Dr. Donington and her fellow investigators specifically examined short-term surgical outcomes, given the significant controversy regarding the safety of resection after full-dose thoracic radiation. In both trials, patients received weekly carboplatin and paclitaxel. Those in the 0229 trial underwent 61.2 Gy of radiation in 34 fractions, while patients in the 0839 trial underwent 60 Gy in 30 fractions. In addition, patients in the 0839 trial were randomized 2:1 to receive weekly panitumumab, an EGFR monoclonal antibody, with their induction therapy.
Surgical expertise was considered essential to this treatment strategy. Therefore, all surgeons were certified by RTOG prior to enrolling patients, and all patients were surgically evaluated before beginning induction therapy to determine resectability and appropriateness for trimodality therapy. Of 125 eligible patients enrolled in the two trials, 93 patients (74%) underwent anatomic resection. A total of 77 patients underwent lobectomy, 8 underwent pneumonectomy, 6 underwent bilobectomy, and 2 patients had sleeve lobectomy.
Medical contraindication and persistent nodal disease found during post-induction invasive staging were the most common reasons patients didn’t undergo resection. Eighty-five of the 93 surgical patients had R0 resections (91%). Surgeons attempted 14 minimally invasive resections (15%), 2 of which uneventfully converted to open resection. Just over one-quarter (28%) of patients suffered greater than Grade 3 adverse events (AEs) related to surgery, the majority of which were pulmonary in nature.
The 30-day mortality rate was 4%, and all four deaths were linked to pulmonary adverse events, including acute respiratory distress syndrome, bronchopleural fistula, pulmonary artery hemorrhage, and respiratory failure. Multivariable analysis for mortality identified the addition of panitumumab and use of an extended resection to be associated with an increased risk for operative mortality.
In the patients undergoing lobectomy, rates for greater than Grade 3 adverse events and 30-day mortality were 26%, and 1.3%, respectively. Those are similar to rates reported for lobectomy without induction therapy in the National Inpatient Sample (NIS) and the STS General Thoracic Surgery Database over the same time period.
These two RTOG trials are the first to prospectively demonstrate that trimodality therapy with full-dose neoadjuvant radiation therapy is safe in a multi-institutional setting, Dr. Donington said.
As her conclusions, she listed that “we have demonstrated the safety of resection following full-dose concurrent CRT in a multi-insitutional setting, that EGFR-AB and T2/T3 stage was associated with decreased nonfatal morbidity, but that extended resection and EGFR-AB were associated with excessive surgical mortality.” Dr. Donington added that the morbidity and mortality for lobectomy compared favorably with large national databases.She pointed out several limitations of the study, which included the relatively small size, and the unexpected toxicity of the EGFR antibody, “which severely limited our ability to assess trimodality therapy.”
She added that “our short-term outcomes tell us nothing about the long-term oncologic benefit.
Dr. David R. Jones, chief of thoracic surgery at the Memorial Sloan-Kettering Cancer Center, New York, was the invited discussant of the paper. “Despite combining these [2] trials, there are still only 93 patients in the analysis and 77 of these had a lobectomy,” Dr. Jones stated. He pointed out that “there was a significant increase in 30- and 90-day mortality in the 16 patients who had more than a straightforward lobectomy,” and asked whether this increased mortality could be due to the use of radiation in the induction therapy.
Dr. Donington replied that there was a wide variation in causes of death, but that “the fact that these patients had induction therapy, be that radiation or chemo, and then went on to this bigger operation, it was overall a difficult thing for them to overcome.” Dr. Donington also agreed with a comment by Dr. Jones that it would be important to follow predicted postoperative diffusion capacity in these patients to potentially help determine the amount of lung to be taken and would be a valuable preoperative consideration.
Dr. Donington reported that two of her coauthors received grant support from NCI and AMGen for the work she was reporting, but that there were no other related disclosures. Dr. Jones reported that he had no disclosures.
Lobectomy can be done safely after concurrent chemotherapy and high-dose radiation in patients with resectable N2-positive stage IIIA non–small cell lung cancer, according to study findings presented by Jessica S. Donington, MD.
Dr. Donington of New York University and her colleagues presented analysis of two prospective trials conducted by NRG Oncology, RTOG 0229 and RTOG 0839 at the AATS Annual Meeting. Both trials’ primary endpoints were mediastinal node sterilization after concurrent chemotherapy and full-dose radiation and those results were previously reported.
Dr. Donington and her fellow investigators specifically examined short-term surgical outcomes, given the significant controversy regarding the safety of resection after full-dose thoracic radiation. In both trials, patients received weekly carboplatin and paclitaxel. Those in the 0229 trial underwent 61.2 Gy of radiation in 34 fractions, while patients in the 0839 trial underwent 60 Gy in 30 fractions. In addition, patients in the 0839 trial were randomized 2:1 to receive weekly panitumumab, an EGFR monoclonal antibody, with their induction therapy.
Surgical expertise was considered essential to this treatment strategy. Therefore, all surgeons were certified by RTOG prior to enrolling patients, and all patients were surgically evaluated before beginning induction therapy to determine resectability and appropriateness for trimodality therapy. Of 125 eligible patients enrolled in the two trials, 93 patients (74%) underwent anatomic resection. A total of 77 patients underwent lobectomy, 8 underwent pneumonectomy, 6 underwent bilobectomy, and 2 patients had sleeve lobectomy.
Medical contraindication and persistent nodal disease found during post-induction invasive staging were the most common reasons patients didn’t undergo resection. Eighty-five of the 93 surgical patients had R0 resections (91%). Surgeons attempted 14 minimally invasive resections (15%), 2 of which uneventfully converted to open resection. Just over one-quarter (28%) of patients suffered greater than Grade 3 adverse events (AEs) related to surgery, the majority of which were pulmonary in nature.
The 30-day mortality rate was 4%, and all four deaths were linked to pulmonary adverse events, including acute respiratory distress syndrome, bronchopleural fistula, pulmonary artery hemorrhage, and respiratory failure. Multivariable analysis for mortality identified the addition of panitumumab and use of an extended resection to be associated with an increased risk for operative mortality.
In the patients undergoing lobectomy, rates for greater than Grade 3 adverse events and 30-day mortality were 26%, and 1.3%, respectively. Those are similar to rates reported for lobectomy without induction therapy in the National Inpatient Sample (NIS) and the STS General Thoracic Surgery Database over the same time period.
These two RTOG trials are the first to prospectively demonstrate that trimodality therapy with full-dose neoadjuvant radiation therapy is safe in a multi-institutional setting, Dr. Donington said.
As her conclusions, she listed that “we have demonstrated the safety of resection following full-dose concurrent CRT in a multi-insitutional setting, that EGFR-AB and T2/T3 stage was associated with decreased nonfatal morbidity, but that extended resection and EGFR-AB were associated with excessive surgical mortality.” Dr. Donington added that the morbidity and mortality for lobectomy compared favorably with large national databases.She pointed out several limitations of the study, which included the relatively small size, and the unexpected toxicity of the EGFR antibody, “which severely limited our ability to assess trimodality therapy.”
She added that “our short-term outcomes tell us nothing about the long-term oncologic benefit.
Dr. David R. Jones, chief of thoracic surgery at the Memorial Sloan-Kettering Cancer Center, New York, was the invited discussant of the paper. “Despite combining these [2] trials, there are still only 93 patients in the analysis and 77 of these had a lobectomy,” Dr. Jones stated. He pointed out that “there was a significant increase in 30- and 90-day mortality in the 16 patients who had more than a straightforward lobectomy,” and asked whether this increased mortality could be due to the use of radiation in the induction therapy.
Dr. Donington replied that there was a wide variation in causes of death, but that “the fact that these patients had induction therapy, be that radiation or chemo, and then went on to this bigger operation, it was overall a difficult thing for them to overcome.” Dr. Donington also agreed with a comment by Dr. Jones that it would be important to follow predicted postoperative diffusion capacity in these patients to potentially help determine the amount of lung to be taken and would be a valuable preoperative consideration.
Dr. Donington reported that two of her coauthors received grant support from NCI and AMGen for the work she was reporting, but that there were no other related disclosures. Dr. Jones reported that he had no disclosures.
Lobectomy can be done safely after concurrent chemotherapy and high-dose radiation in patients with resectable N2-positive stage IIIA non–small cell lung cancer, according to study findings presented by Jessica S. Donington, MD.
Dr. Donington of New York University and her colleagues presented analysis of two prospective trials conducted by NRG Oncology, RTOG 0229 and RTOG 0839 at the AATS Annual Meeting. Both trials’ primary endpoints were mediastinal node sterilization after concurrent chemotherapy and full-dose radiation and those results were previously reported.
Dr. Donington and her fellow investigators specifically examined short-term surgical outcomes, given the significant controversy regarding the safety of resection after full-dose thoracic radiation. In both trials, patients received weekly carboplatin and paclitaxel. Those in the 0229 trial underwent 61.2 Gy of radiation in 34 fractions, while patients in the 0839 trial underwent 60 Gy in 30 fractions. In addition, patients in the 0839 trial were randomized 2:1 to receive weekly panitumumab, an EGFR monoclonal antibody, with their induction therapy.
Surgical expertise was considered essential to this treatment strategy. Therefore, all surgeons were certified by RTOG prior to enrolling patients, and all patients were surgically evaluated before beginning induction therapy to determine resectability and appropriateness for trimodality therapy. Of 125 eligible patients enrolled in the two trials, 93 patients (74%) underwent anatomic resection. A total of 77 patients underwent lobectomy, 8 underwent pneumonectomy, 6 underwent bilobectomy, and 2 patients had sleeve lobectomy.
Medical contraindication and persistent nodal disease found during post-induction invasive staging were the most common reasons patients didn’t undergo resection. Eighty-five of the 93 surgical patients had R0 resections (91%). Surgeons attempted 14 minimally invasive resections (15%), 2 of which uneventfully converted to open resection. Just over one-quarter (28%) of patients suffered greater than Grade 3 adverse events (AEs) related to surgery, the majority of which were pulmonary in nature.
The 30-day mortality rate was 4%, and all four deaths were linked to pulmonary adverse events, including acute respiratory distress syndrome, bronchopleural fistula, pulmonary artery hemorrhage, and respiratory failure. Multivariable analysis for mortality identified the addition of panitumumab and use of an extended resection to be associated with an increased risk for operative mortality.
In the patients undergoing lobectomy, rates for greater than Grade 3 adverse events and 30-day mortality were 26%, and 1.3%, respectively. Those are similar to rates reported for lobectomy without induction therapy in the National Inpatient Sample (NIS) and the STS General Thoracic Surgery Database over the same time period.
These two RTOG trials are the first to prospectively demonstrate that trimodality therapy with full-dose neoadjuvant radiation therapy is safe in a multi-institutional setting, Dr. Donington said.
As her conclusions, she listed that “we have demonstrated the safety of resection following full-dose concurrent CRT in a multi-insitutional setting, that EGFR-AB and T2/T3 stage was associated with decreased nonfatal morbidity, but that extended resection and EGFR-AB were associated with excessive surgical mortality.” Dr. Donington added that the morbidity and mortality for lobectomy compared favorably with large national databases.She pointed out several limitations of the study, which included the relatively small size, and the unexpected toxicity of the EGFR antibody, “which severely limited our ability to assess trimodality therapy.”
She added that “our short-term outcomes tell us nothing about the long-term oncologic benefit.
Dr. David R. Jones, chief of thoracic surgery at the Memorial Sloan-Kettering Cancer Center, New York, was the invited discussant of the paper. “Despite combining these [2] trials, there are still only 93 patients in the analysis and 77 of these had a lobectomy,” Dr. Jones stated. He pointed out that “there was a significant increase in 30- and 90-day mortality in the 16 patients who had more than a straightforward lobectomy,” and asked whether this increased mortality could be due to the use of radiation in the induction therapy.
Dr. Donington replied that there was a wide variation in causes of death, but that “the fact that these patients had induction therapy, be that radiation or chemo, and then went on to this bigger operation, it was overall a difficult thing for them to overcome.” Dr. Donington also agreed with a comment by Dr. Jones that it would be important to follow predicted postoperative diffusion capacity in these patients to potentially help determine the amount of lung to be taken and would be a valuable preoperative consideration.
Dr. Donington reported that two of her coauthors received grant support from NCI and AMGen for the work she was reporting, but that there were no other related disclosures. Dr. Jones reported that he had no disclosures.
FROM THE AMERICAN ASSOCIATION FOR THORACIC SURGERY ANNUAL MEETING
Hospital-led interventions cut pediatric asthma hospitalizations
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
FROM JAMA PEDIATRICS
Key clinical point: A hospital-driven intervention to improve management of asthma in children has achieved significant reductions in asthma-related hospitalizations and emergency department visits and increased medication uptake.
Major finding: A multifactorial intervention to improve asthma management in children was associated with a 41.8% relative reduction in asthma-related hospitalizations and a 42.4% reduction in emergency department visits.
Data source: A hospital-based intervention.
Disclosures: The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
Breastfeeding practices have improved, but some attitudes are concerning
Pediatricians’ recommendations and practices for breastfeeding have become more closely aligned with American Academy of Pediatrics policy since 1995, but attitudes toward breastfeeding show cause for concern, according to a study.
The percentage of surveyed pediatricians who advise exclusive breastfeeding during the first month rose from 66% in 1995 to 75% in 2014 (P less than .05), reported Lori Feldman-Winter, MD, MPH, of Rowan University, Camden, N.J., and her coauthors (Pediatrics. 2017. doi: 10.1542/peds.2017-1229).
Physicians also were less likely to recommend formula supplementation (12% in 1995; 4.5% in 2014; P less than .05).
In addition, pediatricians in 2014 were significantly more likely to report that their affiliated hospitals had applied to be a baby-friendly hospital (56%), compared with results for 1995 (12%) and 2004 (22%) (P less than .05), the investigators said. The physicians also were more likely to report that their practices were more in line with the “Ten Steps to Successful Breastfeeding” policy.
Despite this general trend toward AAP recommendation–compliant practices, many pediatricians doubt the likelihood of breastfeeding success. Although in 1995, 70% of pediatricians reported that almost any mother can be successful at breastfeeding if she keeps trying, only 56% reported the same in 2014 (P less than .05), Dr. Feldman-Winter and her coauthors said. Similarly, only 50% reported that the benefits of breastfeeding outweigh the difficulties in 2014, compared with 70% in 1995 (P less than .05). This may be in part because younger pediatricians reported less confidence in managing common breastfeeding problems and being able to adequately address parents’ questions about breastfeeding; there was a statistically significant difference between pediatricians younger than 45 years and those 45 years and older (P less than .01).
“Pediatricians have demonstrated a modest decline in attitudes about the potential for breastfeeding success,” the investigators wrote. “Lack of resident support for breastfeeding is apparent among many programs and may set the stage for attitudes about breastfeeding for years to come. There are continued opportunities to enhance training in breastfeeding and participate in breastfeeding management and support.”
The study was funded by the American Academy of Pediatrics and the Maternal and Child Health Bureau, Health Resources and Services Administration, and Department of Health and Human Services. None of the authors reported any financial disclosures.
Despite changes in breastfeeding recommendations and practices as well as numerous initiatives from organizations such as the Centers for Disease Control and Prevention and the Department of Health and Human Services, residency training for breastfeeding is not universal.
The study by Feldman et al. shows some concerning trends toward a lack of belief among pediatricians that the benefits of breastfeeding outweigh the difficulties or inconveniences and toward less confidence in managing breastfeeding. They also indicate that baby-friendly hospitals are providing the required breastfeeding education, but this is only a start.
As the trend toward staffing hospitals with pediatric hospitalists increases, it is essential to remember that ambulatory pediatricians also need breastfeeding education because they are responsible for ongoing follow-up care. Maintenance of certification should include breastfeeding as a “core competency” for general pediatricians as well.
Although this analysis shows progress, “the importance of routine integration of breastfeeding into all aspects of medical education cannot be overstated. Breastfeeding education should be as routine in the curriculum as other preventive health strategies, such as immunization.” Hopefully, future studies will show that pediatricians have the skills, attitudes, and confidence necessary to provide competent support to their patients.
Joan Younger Meek, MD, is the associate dean for graduate medical education and a professor at Florida State University in Tallahassee. Her comments were with the Feldman-Winter et al. article in Pediatrics (2017. doi: 10.1542/peds.2017-2509). She reported no relevant financial disclosures or external funding.
Despite changes in breastfeeding recommendations and practices as well as numerous initiatives from organizations such as the Centers for Disease Control and Prevention and the Department of Health and Human Services, residency training for breastfeeding is not universal.
The study by Feldman et al. shows some concerning trends toward a lack of belief among pediatricians that the benefits of breastfeeding outweigh the difficulties or inconveniences and toward less confidence in managing breastfeeding. They also indicate that baby-friendly hospitals are providing the required breastfeeding education, but this is only a start.
As the trend toward staffing hospitals with pediatric hospitalists increases, it is essential to remember that ambulatory pediatricians also need breastfeeding education because they are responsible for ongoing follow-up care. Maintenance of certification should include breastfeeding as a “core competency” for general pediatricians as well.
Although this analysis shows progress, “the importance of routine integration of breastfeeding into all aspects of medical education cannot be overstated. Breastfeeding education should be as routine in the curriculum as other preventive health strategies, such as immunization.” Hopefully, future studies will show that pediatricians have the skills, attitudes, and confidence necessary to provide competent support to their patients.
Joan Younger Meek, MD, is the associate dean for graduate medical education and a professor at Florida State University in Tallahassee. Her comments were with the Feldman-Winter et al. article in Pediatrics (2017. doi: 10.1542/peds.2017-2509). She reported no relevant financial disclosures or external funding.
Despite changes in breastfeeding recommendations and practices as well as numerous initiatives from organizations such as the Centers for Disease Control and Prevention and the Department of Health and Human Services, residency training for breastfeeding is not universal.
The study by Feldman et al. shows some concerning trends toward a lack of belief among pediatricians that the benefits of breastfeeding outweigh the difficulties or inconveniences and toward less confidence in managing breastfeeding. They also indicate that baby-friendly hospitals are providing the required breastfeeding education, but this is only a start.
As the trend toward staffing hospitals with pediatric hospitalists increases, it is essential to remember that ambulatory pediatricians also need breastfeeding education because they are responsible for ongoing follow-up care. Maintenance of certification should include breastfeeding as a “core competency” for general pediatricians as well.
Although this analysis shows progress, “the importance of routine integration of breastfeeding into all aspects of medical education cannot be overstated. Breastfeeding education should be as routine in the curriculum as other preventive health strategies, such as immunization.” Hopefully, future studies will show that pediatricians have the skills, attitudes, and confidence necessary to provide competent support to their patients.
Joan Younger Meek, MD, is the associate dean for graduate medical education and a professor at Florida State University in Tallahassee. Her comments were with the Feldman-Winter et al. article in Pediatrics (2017. doi: 10.1542/peds.2017-2509). She reported no relevant financial disclosures or external funding.
Pediatricians’ recommendations and practices for breastfeeding have become more closely aligned with American Academy of Pediatrics policy since 1995, but attitudes toward breastfeeding show cause for concern, according to a study.
The percentage of surveyed pediatricians who advise exclusive breastfeeding during the first month rose from 66% in 1995 to 75% in 2014 (P less than .05), reported Lori Feldman-Winter, MD, MPH, of Rowan University, Camden, N.J., and her coauthors (Pediatrics. 2017. doi: 10.1542/peds.2017-1229).
Physicians also were less likely to recommend formula supplementation (12% in 1995; 4.5% in 2014; P less than .05).
In addition, pediatricians in 2014 were significantly more likely to report that their affiliated hospitals had applied to be a baby-friendly hospital (56%), compared with results for 1995 (12%) and 2004 (22%) (P less than .05), the investigators said. The physicians also were more likely to report that their practices were more in line with the “Ten Steps to Successful Breastfeeding” policy.
Despite this general trend toward AAP recommendation–compliant practices, many pediatricians doubt the likelihood of breastfeeding success. Although in 1995, 70% of pediatricians reported that almost any mother can be successful at breastfeeding if she keeps trying, only 56% reported the same in 2014 (P less than .05), Dr. Feldman-Winter and her coauthors said. Similarly, only 50% reported that the benefits of breastfeeding outweigh the difficulties in 2014, compared with 70% in 1995 (P less than .05). This may be in part because younger pediatricians reported less confidence in managing common breastfeeding problems and being able to adequately address parents’ questions about breastfeeding; there was a statistically significant difference between pediatricians younger than 45 years and those 45 years and older (P less than .01).
“Pediatricians have demonstrated a modest decline in attitudes about the potential for breastfeeding success,” the investigators wrote. “Lack of resident support for breastfeeding is apparent among many programs and may set the stage for attitudes about breastfeeding for years to come. There are continued opportunities to enhance training in breastfeeding and participate in breastfeeding management and support.”
The study was funded by the American Academy of Pediatrics and the Maternal and Child Health Bureau, Health Resources and Services Administration, and Department of Health and Human Services. None of the authors reported any financial disclosures.
Pediatricians’ recommendations and practices for breastfeeding have become more closely aligned with American Academy of Pediatrics policy since 1995, but attitudes toward breastfeeding show cause for concern, according to a study.
The percentage of surveyed pediatricians who advise exclusive breastfeeding during the first month rose from 66% in 1995 to 75% in 2014 (P less than .05), reported Lori Feldman-Winter, MD, MPH, of Rowan University, Camden, N.J., and her coauthors (Pediatrics. 2017. doi: 10.1542/peds.2017-1229).
Physicians also were less likely to recommend formula supplementation (12% in 1995; 4.5% in 2014; P less than .05).
In addition, pediatricians in 2014 were significantly more likely to report that their affiliated hospitals had applied to be a baby-friendly hospital (56%), compared with results for 1995 (12%) and 2004 (22%) (P less than .05), the investigators said. The physicians also were more likely to report that their practices were more in line with the “Ten Steps to Successful Breastfeeding” policy.
Despite this general trend toward AAP recommendation–compliant practices, many pediatricians doubt the likelihood of breastfeeding success. Although in 1995, 70% of pediatricians reported that almost any mother can be successful at breastfeeding if she keeps trying, only 56% reported the same in 2014 (P less than .05), Dr. Feldman-Winter and her coauthors said. Similarly, only 50% reported that the benefits of breastfeeding outweigh the difficulties in 2014, compared with 70% in 1995 (P less than .05). This may be in part because younger pediatricians reported less confidence in managing common breastfeeding problems and being able to adequately address parents’ questions about breastfeeding; there was a statistically significant difference between pediatricians younger than 45 years and those 45 years and older (P less than .01).
“Pediatricians have demonstrated a modest decline in attitudes about the potential for breastfeeding success,” the investigators wrote. “Lack of resident support for breastfeeding is apparent among many programs and may set the stage for attitudes about breastfeeding for years to come. There are continued opportunities to enhance training in breastfeeding and participate in breastfeeding management and support.”
The study was funded by the American Academy of Pediatrics and the Maternal and Child Health Bureau, Health Resources and Services Administration, and Department of Health and Human Services. None of the authors reported any financial disclosures.
FROM PEDIATRICS
Key clinical point:
Major finding: The percentage of surveyed physicians who advise exclusive breastfeeding during the first month rose from 66% in 1995 to 75% in 2014 (P less than .05).
Data source: An analysis of data from three AAP Periodic Surveys of Fellows.
Disclosures: The study was funded by the American Academy of Pediatrics and the Maternal and Child Health Bureau, Health Resources and Services Administration, and Department of Health and Human Services. None of the authors reported any financial disclosures.
Subtle hearing loss after concussion could impair learning
CHICAGO – Children who experience concussion can develop deficits in auditory processing that could impair long-term academic performance and carry implications for return-to-play strategies, according to a study.
Investigators assessed 40 children aged 8-15 years, half of whom experienced concussion. The postconcussion group of 20 children had slower and smaller neural responses to speech, compared with 20 control children, on the noninvasive frequency-following response measure.
“The ability to hear in noise following concussion is impaired in the pediatric population, based on our results, which suggests it might pose additional challenges for classroom learning,” Ms. Thompson said. The study also suggests that auditory function should be considered part of acute and long-term assessment of children post concussion.
Importantly, all participants in the study had normal hearing. “It’s important to know these are very subtle auditory deficits that only emerge if you’re looking for them,” Ms. Thompson said at the annual meeting of the American Academy of Pediatrics.
Children in the concussion group were recruited from the concussion clinic at the Ann and Robert H. Lurie Children’s Hospital of Chicago. Patients were assessed an average of 27 days post injury, with most still symptomatic. The children in the control group had been treated for musculoskeletal injuries. The concussed and control groups were matched for sex and gender.
“We think that these results have implications beyond the classroom,” Ms. Thompson said. “Auditory deficits might increase risk of reinjury if sports are being played in loud, noisy gymnasiums or crowded soccer fields. So this is important to consider with return-to-play strategies.” She added, “There is hope. Auditory processing is a malleable skill, and it can be a useful target for rehabilitation and recovery.”
Ms. Thompson had no relevant financial disclosures. The study was supported by the Knowles Hearing Center.
CHICAGO – Children who experience concussion can develop deficits in auditory processing that could impair long-term academic performance and carry implications for return-to-play strategies, according to a study.
Investigators assessed 40 children aged 8-15 years, half of whom experienced concussion. The postconcussion group of 20 children had slower and smaller neural responses to speech, compared with 20 control children, on the noninvasive frequency-following response measure.
“The ability to hear in noise following concussion is impaired in the pediatric population, based on our results, which suggests it might pose additional challenges for classroom learning,” Ms. Thompson said. The study also suggests that auditory function should be considered part of acute and long-term assessment of children post concussion.
Importantly, all participants in the study had normal hearing. “It’s important to know these are very subtle auditory deficits that only emerge if you’re looking for them,” Ms. Thompson said at the annual meeting of the American Academy of Pediatrics.
Children in the concussion group were recruited from the concussion clinic at the Ann and Robert H. Lurie Children’s Hospital of Chicago. Patients were assessed an average of 27 days post injury, with most still symptomatic. The children in the control group had been treated for musculoskeletal injuries. The concussed and control groups were matched for sex and gender.
“We think that these results have implications beyond the classroom,” Ms. Thompson said. “Auditory deficits might increase risk of reinjury if sports are being played in loud, noisy gymnasiums or crowded soccer fields. So this is important to consider with return-to-play strategies.” She added, “There is hope. Auditory processing is a malleable skill, and it can be a useful target for rehabilitation and recovery.”
Ms. Thompson had no relevant financial disclosures. The study was supported by the Knowles Hearing Center.
CHICAGO – Children who experience concussion can develop deficits in auditory processing that could impair long-term academic performance and carry implications for return-to-play strategies, according to a study.
Investigators assessed 40 children aged 8-15 years, half of whom experienced concussion. The postconcussion group of 20 children had slower and smaller neural responses to speech, compared with 20 control children, on the noninvasive frequency-following response measure.
“The ability to hear in noise following concussion is impaired in the pediatric population, based on our results, which suggests it might pose additional challenges for classroom learning,” Ms. Thompson said. The study also suggests that auditory function should be considered part of acute and long-term assessment of children post concussion.
Importantly, all participants in the study had normal hearing. “It’s important to know these are very subtle auditory deficits that only emerge if you’re looking for them,” Ms. Thompson said at the annual meeting of the American Academy of Pediatrics.
Children in the concussion group were recruited from the concussion clinic at the Ann and Robert H. Lurie Children’s Hospital of Chicago. Patients were assessed an average of 27 days post injury, with most still symptomatic. The children in the control group had been treated for musculoskeletal injuries. The concussed and control groups were matched for sex and gender.
“We think that these results have implications beyond the classroom,” Ms. Thompson said. “Auditory deficits might increase risk of reinjury if sports are being played in loud, noisy gymnasiums or crowded soccer fields. So this is important to consider with return-to-play strategies.” She added, “There is hope. Auditory processing is a malleable skill, and it can be a useful target for rehabilitation and recovery.”
Ms. Thompson had no relevant financial disclosures. The study was supported by the Knowles Hearing Center.
AT AAP 2017
Key clinical point:
Major finding: Children with concussion symptoms performed significantly poorer on the Hearing in Noise Test, compared with nonconcussed peers (P = .001).
Data source: Study of 40 children in a sports medicine tertiary clinic: half experienced concussion and half served as controls.
Disclosures: Ms. Thompson had no relevant financial disclosures. The study was supported by the Knowles Hearing Center.
Combo vaccines improve compliance
, reported Samantha K. Kurosky of RTI Health Solutions, Research Triangle Park, N.C., and her colleagues.
Data from the 2012 National Immunization Survey was used to assess vaccination completion and compliance in 11,561 children age 24-35 months. Most children had providers who were in private practice (58%); about half of the children were enrolled in Medicaid or the Children’s Health Insurance Program (CHIP).
Completion of the full 4:3:1:3:3:1:4 vaccine series (4 DTaP, 3 inactivated polio vaccine [IPV], 1 MMR, 3 or 4 Haemophilus influenzae type b [Hib], 3 hepatitis B, 1 varicella, and 4 pneumococcal conjugate vaccine) was better among those who received combination vaccines, at 69%, compared with children who received single-antigen vaccine only (50%).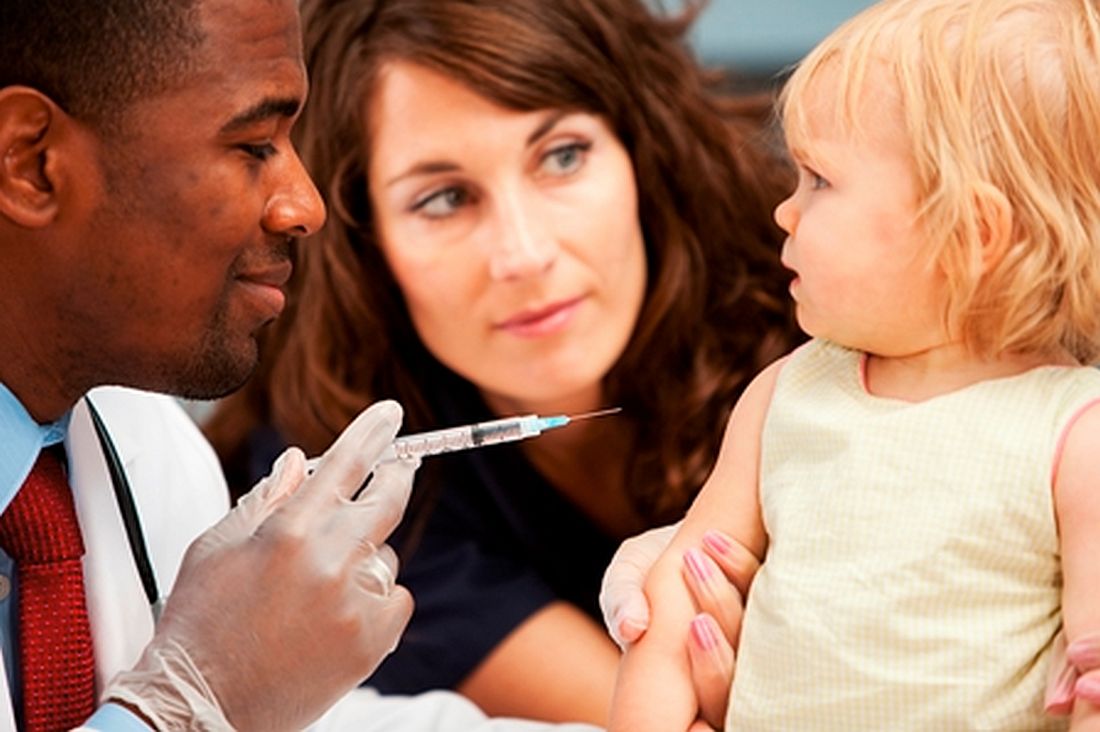
Children receiving combination vaccines also had a significantly higher compliance rate for the 4:3:1:3:3:1:4 series, at 24%, compared with those receiving single-antigen vaccines only, at 13% (P less than .001). Of children who received at least one vaccine by 24 months, 86% received at least one combo vaccine.
Children getting at least one combo vaccine were 2.2 times more likely to get all vaccines on time, and 2.4 times more likely to spend less time undervaccinated (less than 7 months), compared with those receiving single-antigen vaccines only, Ms. Kurosky and her associates said.
Previous studies have found that parents who refuse or intentionally delay vaccines often have higher income and are married mothers with college educations. In this study, parents with these demographics tended to have children who received single-antigen–only vaccines – that is, children who were less likely to complete a full vaccine series or have a high compliance rate, they said.
GlaxoSmithKline funded the research.
Read more at Human Vaccines & Immunotherapeutics (2017 Sep 7. doi: 10.1080/21645515.2017.1362515).
, reported Samantha K. Kurosky of RTI Health Solutions, Research Triangle Park, N.C., and her colleagues.
Data from the 2012 National Immunization Survey was used to assess vaccination completion and compliance in 11,561 children age 24-35 months. Most children had providers who were in private practice (58%); about half of the children were enrolled in Medicaid or the Children’s Health Insurance Program (CHIP).
Completion of the full 4:3:1:3:3:1:4 vaccine series (4 DTaP, 3 inactivated polio vaccine [IPV], 1 MMR, 3 or 4 Haemophilus influenzae type b [Hib], 3 hepatitis B, 1 varicella, and 4 pneumococcal conjugate vaccine) was better among those who received combination vaccines, at 69%, compared with children who received single-antigen vaccine only (50%).
Children receiving combination vaccines also had a significantly higher compliance rate for the 4:3:1:3:3:1:4 series, at 24%, compared with those receiving single-antigen vaccines only, at 13% (P less than .001). Of children who received at least one vaccine by 24 months, 86% received at least one combo vaccine.
Children getting at least one combo vaccine were 2.2 times more likely to get all vaccines on time, and 2.4 times more likely to spend less time undervaccinated (less than 7 months), compared with those receiving single-antigen vaccines only, Ms. Kurosky and her associates said.
Previous studies have found that parents who refuse or intentionally delay vaccines often have higher income and are married mothers with college educations. In this study, parents with these demographics tended to have children who received single-antigen–only vaccines – that is, children who were less likely to complete a full vaccine series or have a high compliance rate, they said.
GlaxoSmithKline funded the research.
Read more at Human Vaccines & Immunotherapeutics (2017 Sep 7. doi: 10.1080/21645515.2017.1362515).
, reported Samantha K. Kurosky of RTI Health Solutions, Research Triangle Park, N.C., and her colleagues.
Data from the 2012 National Immunization Survey was used to assess vaccination completion and compliance in 11,561 children age 24-35 months. Most children had providers who were in private practice (58%); about half of the children were enrolled in Medicaid or the Children’s Health Insurance Program (CHIP).
Completion of the full 4:3:1:3:3:1:4 vaccine series (4 DTaP, 3 inactivated polio vaccine [IPV], 1 MMR, 3 or 4 Haemophilus influenzae type b [Hib], 3 hepatitis B, 1 varicella, and 4 pneumococcal conjugate vaccine) was better among those who received combination vaccines, at 69%, compared with children who received single-antigen vaccine only (50%).
Children receiving combination vaccines also had a significantly higher compliance rate for the 4:3:1:3:3:1:4 series, at 24%, compared with those receiving single-antigen vaccines only, at 13% (P less than .001). Of children who received at least one vaccine by 24 months, 86% received at least one combo vaccine.
Children getting at least one combo vaccine were 2.2 times more likely to get all vaccines on time, and 2.4 times more likely to spend less time undervaccinated (less than 7 months), compared with those receiving single-antigen vaccines only, Ms. Kurosky and her associates said.
Previous studies have found that parents who refuse or intentionally delay vaccines often have higher income and are married mothers with college educations. In this study, parents with these demographics tended to have children who received single-antigen–only vaccines – that is, children who were less likely to complete a full vaccine series or have a high compliance rate, they said.
GlaxoSmithKline funded the research.
Read more at Human Vaccines & Immunotherapeutics (2017 Sep 7. doi: 10.1080/21645515.2017.1362515).
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS
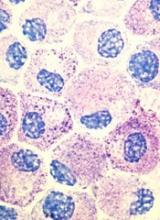
CHMP recommends approval of generic imatinib
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization for Imatinib Teva B.V., a generic of Glivec.
The recommendation is that Imatinib Teva B.V. be approved to treat chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), gastrointestinal stromal tumors (GIST), and dermatofibrosarcoma protuberans (DFSP).
The European Commission typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The European Commission’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
If approved, Imatinib Teva B.V. will be available as capsules and film-coated tablets (100 mg and 400 mg). And it will be authorized:
- As monotherapy for pediatric patients with newly diagnosed, Philadelphia-chromosome-positive (Ph+) CML for whom bone marrow transplant is not considered the first line of treatment.
- As monotherapy for pediatric patients with Ph+ CML in chronic phase after failure of interferon-alpha therapy or in accelerated phase or blast crisis.
- As monotherapy for adults with Ph+ CML in blast crisis.
- Integrated with chemotherapy to treat adult and pediatric patients with newly diagnosed, Ph+ ALL.
- As monotherapy for adults with relapsed or refractory Ph+ ALL.
- As monotherapy for adults with MDS/MPNs associated with platelet-derived growth factor receptor gene re-arrangements.
- As monotherapy for adults with advanced HES and/or CEL with FIP1L1-PDGFRα rearrangement.
- As monotherapy for adults with Kit- (CD117-) positive, unresectable and/or metastatic malignant GISTs.
- For the adjuvant treatment of adults who are at significant risk of relapse following resection of Kit-positive GIST. Patients who have a low or very low risk of recurrence should not receive adjuvant treatment.
- As monotherapy for adults with unresectable DFSP and adults with recurrent and/or metastatic DFSP who are not eligible for surgery.
The CHMP said studies have demonstrated the satisfactory quality of Imatinib Teva B.V. and its bioequivalence to the reference product, Glivec.
In adult and pediatric patients, the effectiveness of imatinib is based on:
- Overall hematologic and cytogenetic response rates and progression-free survival in CML
- Hematologic and cytogenetic response rates in Ph+ ALL and MDS/MPNs
- Hematologic response rates in HES/CEL
- Objective response rates in adults with unresectable and/or metastatic GIST and DFSP
- Recurrence-free survival in adjuvant GIST.
The experience with imatinib in patients with MDS/MPNs associated with PDGFR gene re-arrangements is very limited. There are no controlled trials demonstrating a clinical benefit or increased survival for these diseases. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization for Imatinib Teva B.V., a generic of Glivec.
The recommendation is that Imatinib Teva B.V. be approved to treat chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), gastrointestinal stromal tumors (GIST), and dermatofibrosarcoma protuberans (DFSP).
The European Commission typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The European Commission’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
If approved, Imatinib Teva B.V. will be available as capsules and film-coated tablets (100 mg and 400 mg). And it will be authorized:
- As monotherapy for pediatric patients with newly diagnosed, Philadelphia-chromosome-positive (Ph+) CML for whom bone marrow transplant is not considered the first line of treatment.
- As monotherapy for pediatric patients with Ph+ CML in chronic phase after failure of interferon-alpha therapy or in accelerated phase or blast crisis.
- As monotherapy for adults with Ph+ CML in blast crisis.
- Integrated with chemotherapy to treat adult and pediatric patients with newly diagnosed, Ph+ ALL.
- As monotherapy for adults with relapsed or refractory Ph+ ALL.
- As monotherapy for adults with MDS/MPNs associated with platelet-derived growth factor receptor gene re-arrangements.
- As monotherapy for adults with advanced HES and/or CEL with FIP1L1-PDGFRα rearrangement.
- As monotherapy for adults with Kit- (CD117-) positive, unresectable and/or metastatic malignant GISTs.
- For the adjuvant treatment of adults who are at significant risk of relapse following resection of Kit-positive GIST. Patients who have a low or very low risk of recurrence should not receive adjuvant treatment.
- As monotherapy for adults with unresectable DFSP and adults with recurrent and/or metastatic DFSP who are not eligible for surgery.
The CHMP said studies have demonstrated the satisfactory quality of Imatinib Teva B.V. and its bioequivalence to the reference product, Glivec.
In adult and pediatric patients, the effectiveness of imatinib is based on:
- Overall hematologic and cytogenetic response rates and progression-free survival in CML
- Hematologic and cytogenetic response rates in Ph+ ALL and MDS/MPNs
- Hematologic response rates in HES/CEL
- Objective response rates in adults with unresectable and/or metastatic GIST and DFSP
- Recurrence-free survival in adjuvant GIST.
The experience with imatinib in patients with MDS/MPNs associated with PDGFR gene re-arrangements is very limited. There are no controlled trials demonstrating a clinical benefit or increased survival for these diseases. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization for Imatinib Teva B.V., a generic of Glivec.
The recommendation is that Imatinib Teva B.V. be approved to treat chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), gastrointestinal stromal tumors (GIST), and dermatofibrosarcoma protuberans (DFSP).
The European Commission typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The European Commission’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
If approved, Imatinib Teva B.V. will be available as capsules and film-coated tablets (100 mg and 400 mg). And it will be authorized:
- As monotherapy for pediatric patients with newly diagnosed, Philadelphia-chromosome-positive (Ph+) CML for whom bone marrow transplant is not considered the first line of treatment.
- As monotherapy for pediatric patients with Ph+ CML in chronic phase after failure of interferon-alpha therapy or in accelerated phase or blast crisis.
- As monotherapy for adults with Ph+ CML in blast crisis.
- Integrated with chemotherapy to treat adult and pediatric patients with newly diagnosed, Ph+ ALL.
- As monotherapy for adults with relapsed or refractory Ph+ ALL.
- As monotherapy for adults with MDS/MPNs associated with platelet-derived growth factor receptor gene re-arrangements.
- As monotherapy for adults with advanced HES and/or CEL with FIP1L1-PDGFRα rearrangement.
- As monotherapy for adults with Kit- (CD117-) positive, unresectable and/or metastatic malignant GISTs.
- For the adjuvant treatment of adults who are at significant risk of relapse following resection of Kit-positive GIST. Patients who have a low or very low risk of recurrence should not receive adjuvant treatment.
- As monotherapy for adults with unresectable DFSP and adults with recurrent and/or metastatic DFSP who are not eligible for surgery.
The CHMP said studies have demonstrated the satisfactory quality of Imatinib Teva B.V. and its bioequivalence to the reference product, Glivec.
In adult and pediatric patients, the effectiveness of imatinib is based on:
- Overall hematologic and cytogenetic response rates and progression-free survival in CML
- Hematologic and cytogenetic response rates in Ph+ ALL and MDS/MPNs
- Hematologic response rates in HES/CEL
- Objective response rates in adults with unresectable and/or metastatic GIST and DFSP
- Recurrence-free survival in adjuvant GIST.
The experience with imatinib in patients with MDS/MPNs associated with PDGFR gene re-arrangements is very limited. There are no controlled trials demonstrating a clinical benefit or increased survival for these diseases. ![]()
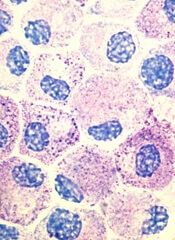
CHMP advocates refusal of application for SM drug
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of the marketing authorization for masitinib (Masipro).
Masitinib is a tyrosine kinase inhibitor being developed by AB Science to treat adults with smoldering or indolent severe systemic mastocytosis (SM).
To support the application for masitinib, AB Science presented data from a study involving 135 SM patients who had severe symptoms, including at least one of the following: itching, hot flashes, depression, and tiredness.
Researchers compared masitinib to placebo in these patients, looking for improvements in any of the symptoms during the first 24 weeks of treatment.
The CHMP said it was concerned about the reliability of the study results because a routine good clinical practice inspection at the study sites revealed serious failings in the way the study had been conducted.
In addition, major changes were made to the study design while the study was underway, which made the results difficult to interpret.
Finally, data on the safety of masitinib were limited. And the CHMP was concerned about side effects, including neutropenia and harmful effects on the skin and liver, which were particularly relevant because masitinib was intended to be used long-term.
Therefore, the CHMP concluded the benefits of masitinib do not appear to outweigh the risks, and the committee recommended the drug be refused marketing authorization.
The CHMP informed AB Science of this negative opinion in May, and the company asked the committee to re-examine its opinion. However, the CHMP ultimately concluded that masitinib should be refused marketing authorization.
AB Science said this decision does not have any consequences for patients in clinical trials or compassionate use programs of masitinib.
The company also said it intends to initiate a confirmatory study in patients with smoldering or indolent severe SM that is unresponsive to optimal symptomatic treatment in order to confirm the results from the first pivotal study. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of the marketing authorization for masitinib (Masipro).
Masitinib is a tyrosine kinase inhibitor being developed by AB Science to treat adults with smoldering or indolent severe systemic mastocytosis (SM).
To support the application for masitinib, AB Science presented data from a study involving 135 SM patients who had severe symptoms, including at least one of the following: itching, hot flashes, depression, and tiredness.
Researchers compared masitinib to placebo in these patients, looking for improvements in any of the symptoms during the first 24 weeks of treatment.
The CHMP said it was concerned about the reliability of the study results because a routine good clinical practice inspection at the study sites revealed serious failings in the way the study had been conducted.
In addition, major changes were made to the study design while the study was underway, which made the results difficult to interpret.
Finally, data on the safety of masitinib were limited. And the CHMP was concerned about side effects, including neutropenia and harmful effects on the skin and liver, which were particularly relevant because masitinib was intended to be used long-term.
Therefore, the CHMP concluded the benefits of masitinib do not appear to outweigh the risks, and the committee recommended the drug be refused marketing authorization.
The CHMP informed AB Science of this negative opinion in May, and the company asked the committee to re-examine its opinion. However, the CHMP ultimately concluded that masitinib should be refused marketing authorization.
AB Science said this decision does not have any consequences for patients in clinical trials or compassionate use programs of masitinib.
The company also said it intends to initiate a confirmatory study in patients with smoldering or indolent severe SM that is unresponsive to optimal symptomatic treatment in order to confirm the results from the first pivotal study. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of the marketing authorization for masitinib (Masipro).
Masitinib is a tyrosine kinase inhibitor being developed by AB Science to treat adults with smoldering or indolent severe systemic mastocytosis (SM).
To support the application for masitinib, AB Science presented data from a study involving 135 SM patients who had severe symptoms, including at least one of the following: itching, hot flashes, depression, and tiredness.
Researchers compared masitinib to placebo in these patients, looking for improvements in any of the symptoms during the first 24 weeks of treatment.
The CHMP said it was concerned about the reliability of the study results because a routine good clinical practice inspection at the study sites revealed serious failings in the way the study had been conducted.
In addition, major changes were made to the study design while the study was underway, which made the results difficult to interpret.
Finally, data on the safety of masitinib were limited. And the CHMP was concerned about side effects, including neutropenia and harmful effects on the skin and liver, which were particularly relevant because masitinib was intended to be used long-term.
Therefore, the CHMP concluded the benefits of masitinib do not appear to outweigh the risks, and the committee recommended the drug be refused marketing authorization.
The CHMP informed AB Science of this negative opinion in May, and the company asked the committee to re-examine its opinion. However, the CHMP ultimately concluded that masitinib should be refused marketing authorization.
AB Science said this decision does not have any consequences for patients in clinical trials or compassionate use programs of masitinib.
The company also said it intends to initiate a confirmatory study in patients with smoldering or indolent severe SM that is unresponsive to optimal symptomatic treatment in order to confirm the results from the first pivotal study. ![]()
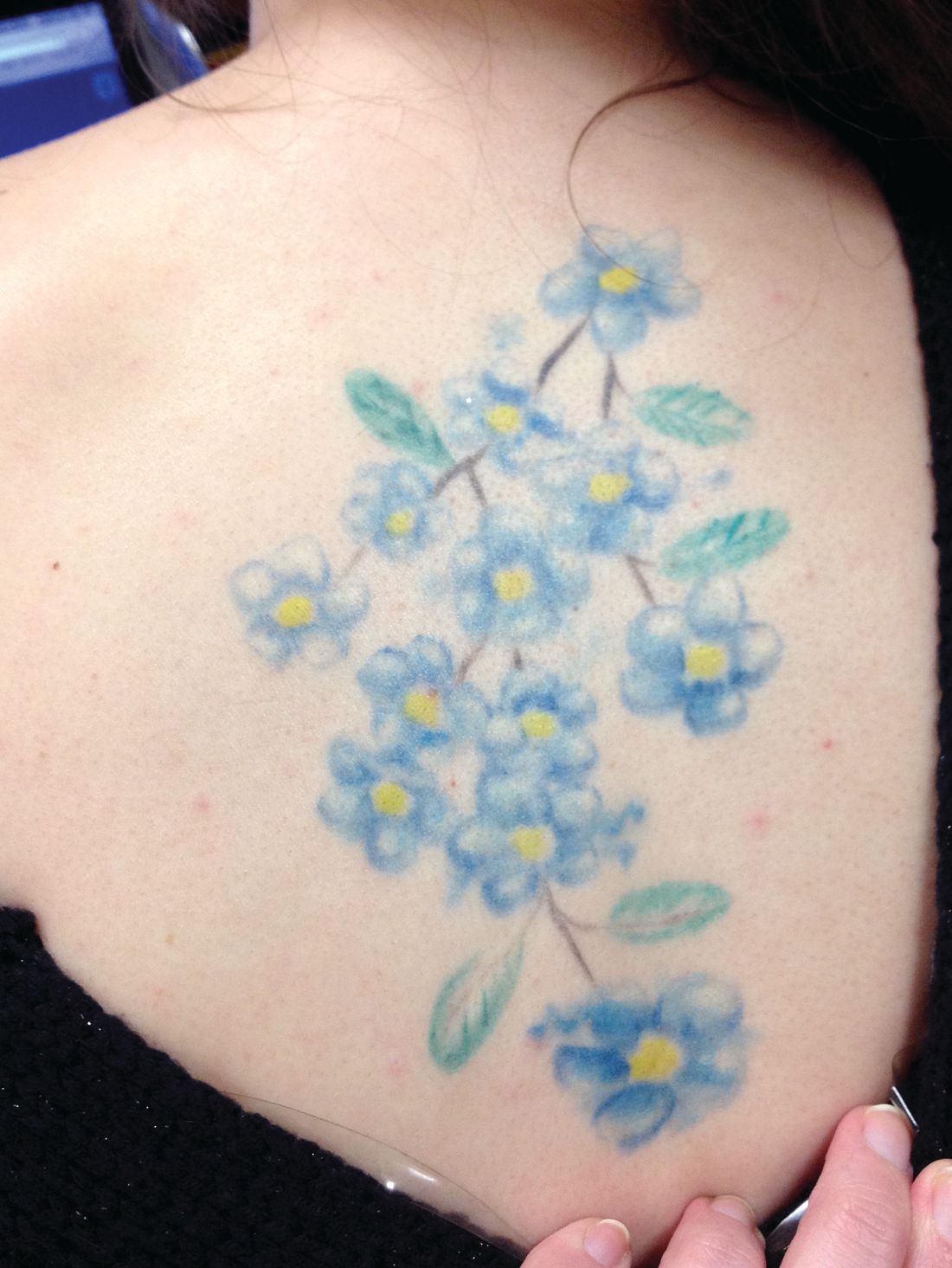
Tattooing and piercing are no longer taboo, but health concerns persist
Educate adolescents about the potential medical complications and social consequences of tattooing and body piercing as their popularity rises, an American Academy of Pediatrics clinical report recommends.
The most common complications post tattooing are bacterial and viral infections, and inflammation at the site of the tattoo. Rarely, more serious complications can arise in the form of endocarditis, gangrene, and amputations. Postprocedure care is important in preventing most complications: “Reputable tattoo parlors and piercing salons should provide a long list of do’s and don’ts on how to care for the area that was worked on, and what signs might indicate a problem,” Cora C. Breuner, MD, chairperson of the AAP Committee on Adolescence and coauthor of the report, said in a press statement. The clinical report was presented at the AAP annual meeting in Chicago and simultaneously published in the journal Pediatrics (2017 Sep 18. doi: 10.1542/peds.2017-1962).
Data concerning adolescent tattooing and piercing vary by source and age, but there is a distinct trend of adolescents getting or having an interest in body modification. In samples of adolescents attending clinics at ages 12-22 years, 10%-23% had tattoos and 27%-42% had body piercing (other than the earlobe); rates were higher among girls vs. boys and among older vs. young adolescents. “Of students with current piercings, high-ear cartilage (53%) was the most common visible piercing, followed by navel (38%), tongue (13%), and nipple and genital (9%) piercings” according to the report.
A concern that many adolescents and young adults may not consider is how tattoos affect society’s perception of tattooed and pierced people. A 2008 study found that 29% of people surveyed thought tattooed people were more likely to engage in deviant behavior; this belief had decreased to 24% by 2012 , according to a Harris Poll.
While society at large may appear more accepting of tattooed individuals, employers may be less open to hiring them. According to an executive career coach, “37% of human resource managers cite tattoos as the third physical attribute likely to limit career potential” with non-ear piercings in the top two barriers to career advancement (Am J Nurs. 2012;112[5]:15). In a 2014 survey of 2,675 people, 76% thought that tattoos and/or piercings had hurt their chances of getting a job, and 39% thought employees with tattoos and/or body piercings reflect poorly on their employers. Also, 42% of those surveyed felt visible tattoos are inappropriate at work, with 55% felt the same about body piercings.
“In most cases, teens just enjoy the look of the tattoo or piercing, but we do advise them to talk any decision over with their parents or another adult first,” David Levine, MD, coauthor of the AAP report, said in a press statement. “They may not realize how expensive it is to remove a tattoo, or how a piercing on your tongue might result in a chipped tooth.”
Laser removal of tattoos can range from $49 to $300 per square inch of treatment area, according to the report.
Some tips from the report
- You should advise adolescent patients to assess sanitary and hygienic practices of the tattoo parlors and tattoo artists, including: “use of new, disposable gloves; removal of the new needle and equipment from a sealed, sterile container; and the use of fresh, unused ink poured into a new, disposable container with each new client.”
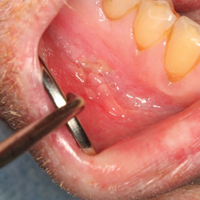
- You should advise adolescents with tattoos to come to the office if there are signs and symptoms of infection .
- Lesions that appear to grow and/or change within a tattoo suggest a neoplasm.
- You should familiarize yourself with local laws and regulations related to tattooing so you can inform patients and parents.
- Counsel adolescents about the implications of visible tattoos on jobs.
- Use antibiotic agents with good coverage against Pseudomonas and Staphylococcus species (such as fluoroquinolones) to treat piercing-associated infections of the auricular cartilage.
- Recommend removing all jewelry during contact sports. If jewelry interferes with mouth guards or protective equipment, it should be removed before play. Have patients remove nipple jewelry prior to breastfeeding.
The authors have no relevant financial disclosures.
Educate adolescents about the potential medical complications and social consequences of tattooing and body piercing as their popularity rises, an American Academy of Pediatrics clinical report recommends.
The most common complications post tattooing are bacterial and viral infections, and inflammation at the site of the tattoo. Rarely, more serious complications can arise in the form of endocarditis, gangrene, and amputations. Postprocedure care is important in preventing most complications: “Reputable tattoo parlors and piercing salons should provide a long list of do’s and don’ts on how to care for the area that was worked on, and what signs might indicate a problem,” Cora C. Breuner, MD, chairperson of the AAP Committee on Adolescence and coauthor of the report, said in a press statement. The clinical report was presented at the AAP annual meeting in Chicago and simultaneously published in the journal Pediatrics (2017 Sep 18. doi: 10.1542/peds.2017-1962).
Data concerning adolescent tattooing and piercing vary by source and age, but there is a distinct trend of adolescents getting or having an interest in body modification. In samples of adolescents attending clinics at ages 12-22 years, 10%-23% had tattoos and 27%-42% had body piercing (other than the earlobe); rates were higher among girls vs. boys and among older vs. young adolescents. “Of students with current piercings, high-ear cartilage (53%) was the most common visible piercing, followed by navel (38%), tongue (13%), and nipple and genital (9%) piercings” according to the report.
A concern that many adolescents and young adults may not consider is how tattoos affect society’s perception of tattooed and pierced people. A 2008 study found that 29% of people surveyed thought tattooed people were more likely to engage in deviant behavior; this belief had decreased to 24% by 2012 , according to a Harris Poll.
While society at large may appear more accepting of tattooed individuals, employers may be less open to hiring them. According to an executive career coach, “37% of human resource managers cite tattoos as the third physical attribute likely to limit career potential” with non-ear piercings in the top two barriers to career advancement (Am J Nurs. 2012;112[5]:15). In a 2014 survey of 2,675 people, 76% thought that tattoos and/or piercings had hurt their chances of getting a job, and 39% thought employees with tattoos and/or body piercings reflect poorly on their employers. Also, 42% of those surveyed felt visible tattoos are inappropriate at work, with 55% felt the same about body piercings.
“In most cases, teens just enjoy the look of the tattoo or piercing, but we do advise them to talk any decision over with their parents or another adult first,” David Levine, MD, coauthor of the AAP report, said in a press statement. “They may not realize how expensive it is to remove a tattoo, or how a piercing on your tongue might result in a chipped tooth.”
Laser removal of tattoos can range from $49 to $300 per square inch of treatment area, according to the report.
Some tips from the report
- You should advise adolescent patients to assess sanitary and hygienic practices of the tattoo parlors and tattoo artists, including: “use of new, disposable gloves; removal of the new needle and equipment from a sealed, sterile container; and the use of fresh, unused ink poured into a new, disposable container with each new client.”
- You should advise adolescents with tattoos to come to the office if there are signs and symptoms of infection .
- Lesions that appear to grow and/or change within a tattoo suggest a neoplasm.
- You should familiarize yourself with local laws and regulations related to tattooing so you can inform patients and parents.
- Counsel adolescents about the implications of visible tattoos on jobs.
- Use antibiotic agents with good coverage against Pseudomonas and Staphylococcus species (such as fluoroquinolones) to treat piercing-associated infections of the auricular cartilage.
- Recommend removing all jewelry during contact sports. If jewelry interferes with mouth guards or protective equipment, it should be removed before play. Have patients remove nipple jewelry prior to breastfeeding.
The authors have no relevant financial disclosures.
Educate adolescents about the potential medical complications and social consequences of tattooing and body piercing as their popularity rises, an American Academy of Pediatrics clinical report recommends.
The most common complications post tattooing are bacterial and viral infections, and inflammation at the site of the tattoo. Rarely, more serious complications can arise in the form of endocarditis, gangrene, and amputations. Postprocedure care is important in preventing most complications: “Reputable tattoo parlors and piercing salons should provide a long list of do’s and don’ts on how to care for the area that was worked on, and what signs might indicate a problem,” Cora C. Breuner, MD, chairperson of the AAP Committee on Adolescence and coauthor of the report, said in a press statement. The clinical report was presented at the AAP annual meeting in Chicago and simultaneously published in the journal Pediatrics (2017 Sep 18. doi: 10.1542/peds.2017-1962).
Data concerning adolescent tattooing and piercing vary by source and age, but there is a distinct trend of adolescents getting or having an interest in body modification. In samples of adolescents attending clinics at ages 12-22 years, 10%-23% had tattoos and 27%-42% had body piercing (other than the earlobe); rates were higher among girls vs. boys and among older vs. young adolescents. “Of students with current piercings, high-ear cartilage (53%) was the most common visible piercing, followed by navel (38%), tongue (13%), and nipple and genital (9%) piercings” according to the report.
A concern that many adolescents and young adults may not consider is how tattoos affect society’s perception of tattooed and pierced people. A 2008 study found that 29% of people surveyed thought tattooed people were more likely to engage in deviant behavior; this belief had decreased to 24% by 2012 , according to a Harris Poll.
While society at large may appear more accepting of tattooed individuals, employers may be less open to hiring them. According to an executive career coach, “37% of human resource managers cite tattoos as the third physical attribute likely to limit career potential” with non-ear piercings in the top two barriers to career advancement (Am J Nurs. 2012;112[5]:15). In a 2014 survey of 2,675 people, 76% thought that tattoos and/or piercings had hurt their chances of getting a job, and 39% thought employees with tattoos and/or body piercings reflect poorly on their employers. Also, 42% of those surveyed felt visible tattoos are inappropriate at work, with 55% felt the same about body piercings.
“In most cases, teens just enjoy the look of the tattoo or piercing, but we do advise them to talk any decision over with their parents or another adult first,” David Levine, MD, coauthor of the AAP report, said in a press statement. “They may not realize how expensive it is to remove a tattoo, or how a piercing on your tongue might result in a chipped tooth.”
Laser removal of tattoos can range from $49 to $300 per square inch of treatment area, according to the report.
Some tips from the report
- You should advise adolescent patients to assess sanitary and hygienic practices of the tattoo parlors and tattoo artists, including: “use of new, disposable gloves; removal of the new needle and equipment from a sealed, sterile container; and the use of fresh, unused ink poured into a new, disposable container with each new client.”
- You should advise adolescents with tattoos to come to the office if there are signs and symptoms of infection .
- Lesions that appear to grow and/or change within a tattoo suggest a neoplasm.
- You should familiarize yourself with local laws and regulations related to tattooing so you can inform patients and parents.
- Counsel adolescents about the implications of visible tattoos on jobs.
- Use antibiotic agents with good coverage against Pseudomonas and Staphylococcus species (such as fluoroquinolones) to treat piercing-associated infections of the auricular cartilage.
- Recommend removing all jewelry during contact sports. If jewelry interferes with mouth guards or protective equipment, it should be removed before play. Have patients remove nipple jewelry prior to breastfeeding.
The authors have no relevant financial disclosures.
FROM AAP 2017
Oral Cues to Disease
1. A 21-year-old man complains of prolonged diarrhea with steady abdominal pain and recent weight loss. He also reports painful knee and hip joints and generalized fatigue. Physical exam reveals fissure-like ulcerations of the mandibular buccal vestibule and localized abdominal pain with fullness in the right lower quadrant.
Diagnosis: Crohn disease can affect the gastrointestinal tract anywhere from the mouth to the anus, and can manifest with oral findings that may not correlate with abdominal symptoms (eg, mucosal cobble stoning, mucosal tags, deep linear ulcerations, gingival hyperplasia, lip fissuring, aphthous ulcers, angular cheilitis). Other features may include diffuse, painless swelling of the lips and mucosal erythema.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
2. Over the past few months, this 43-year-old woman has had multiple severely painful, slow-healing oral ulcers, resulting in the erosion of the lower lip. She reports pain on swallowing and occasional bloody nose after blowing in the morning.
Diagnosis: Vesiculo-bullous lesions in the mouth may be seen with pemphigus vulgaris or bullous pemphigoid. Pemphigus vulgaris, an autoimmune intraepithelial blistering disease, often manifests as flaccid bullae or painful ulcerations in the oral cavity prior to the onset of skin lesions.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
3. A 26-year-old woman presents with fever and fatigue, as well as arthralgia in her hands. Upon examination, she has a malar rash and lichenoid inflammation of the buccal mucosa. She has a family history of autoimmune disorders.
Diagnosis: Oral findings suggestive of systemic or discoid lupus erythematosus may greatly resemble those of oral lichen planus.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
4. This 14-year-old girl is concerned about the multiple ecchymoses and thrombi in her mouth. She feels fatigued and has a fever. Her history includes a tendency to bruise easily, menorrhagia, and a recent infection treated with vancomycin. Physical exam is notable for retinal hemorrhages and jaundice.

Diagnosis: Thrombocytopenia purpura may manifest with oral petechiae, purpura, oral hematomas, or hemorrhagic bullae.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
1. A 21-year-old man complains of prolonged diarrhea with steady abdominal pain and recent weight loss. He also reports painful knee and hip joints and generalized fatigue. Physical exam reveals fissure-like ulcerations of the mandibular buccal vestibule and localized abdominal pain with fullness in the right lower quadrant.

Diagnosis: Crohn disease can affect the gastrointestinal tract anywhere from the mouth to the anus, and can manifest with oral findings that may not correlate with abdominal symptoms (eg, mucosal cobble stoning, mucosal tags, deep linear ulcerations, gingival hyperplasia, lip fissuring, aphthous ulcers, angular cheilitis). Other features may include diffuse, painless swelling of the lips and mucosal erythema.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
2. Over the past few months, this 43-year-old woman has had multiple severely painful, slow-healing oral ulcers, resulting in the erosion of the lower lip. She reports pain on swallowing and occasional bloody nose after blowing in the morning.

Diagnosis: Vesiculo-bullous lesions in the mouth may be seen with pemphigus vulgaris or bullous pemphigoid. Pemphigus vulgaris, an autoimmune intraepithelial blistering disease, often manifests as flaccid bullae or painful ulcerations in the oral cavity prior to the onset of skin lesions.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
3. A 26-year-old woman presents with fever and fatigue, as well as arthralgia in her hands. Upon examination, she has a malar rash and lichenoid inflammation of the buccal mucosa. She has a family history of autoimmune disorders.

Diagnosis: Oral findings suggestive of systemic or discoid lupus erythematosus may greatly resemble those of oral lichen planus.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
4. This 14-year-old girl is concerned about the multiple ecchymoses and thrombi in her mouth. She feels fatigued and has a fever. Her history includes a tendency to bruise easily, menorrhagia, and a recent infection treated with vancomycin. Physical exam is notable for retinal hemorrhages and jaundice.

Diagnosis: Thrombocytopenia purpura may manifest with oral petechiae, purpura, oral hematomas, or hemorrhagic bullae.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
1. A 21-year-old man complains of prolonged diarrhea with steady abdominal pain and recent weight loss. He also reports painful knee and hip joints and generalized fatigue. Physical exam reveals fissure-like ulcerations of the mandibular buccal vestibule and localized abdominal pain with fullness in the right lower quadrant.

Diagnosis: Crohn disease can affect the gastrointestinal tract anywhere from the mouth to the anus, and can manifest with oral findings that may not correlate with abdominal symptoms (eg, mucosal cobble stoning, mucosal tags, deep linear ulcerations, gingival hyperplasia, lip fissuring, aphthous ulcers, angular cheilitis). Other features may include diffuse, painless swelling of the lips and mucosal erythema.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
2. Over the past few months, this 43-year-old woman has had multiple severely painful, slow-healing oral ulcers, resulting in the erosion of the lower lip. She reports pain on swallowing and occasional bloody nose after blowing in the morning.

Diagnosis: Vesiculo-bullous lesions in the mouth may be seen with pemphigus vulgaris or bullous pemphigoid. Pemphigus vulgaris, an autoimmune intraepithelial blistering disease, often manifests as flaccid bullae or painful ulcerations in the oral cavity prior to the onset of skin lesions.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
3. A 26-year-old woman presents with fever and fatigue, as well as arthralgia in her hands. Upon examination, she has a malar rash and lichenoid inflammation of the buccal mucosa. She has a family history of autoimmune disorders.

Diagnosis: Oral findings suggestive of systemic or discoid lupus erythematosus may greatly resemble those of oral lichen planus.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.
4. This 14-year-old girl is concerned about the multiple ecchymoses and thrombi in her mouth. She feels fatigued and has a fever. Her history includes a tendency to bruise easily, menorrhagia, and a recent infection treated with vancomycin. Physical exam is notable for retinal hemorrhages and jaundice.

Diagnosis: Thrombocytopenia purpura may manifest with oral petechiae, purpura, oral hematomas, or hemorrhagic bullae.
For more information, see “Oral lesions you can’t afford to miss.” J Fam Pract. 2015;64(7):392-399.