User login
Royal Australasian College of Surgeons partners with ACS for Annual Scientific Congress
The Royal Australasian College of Surgeons (RACS), along with the American College of Surgeons (ACS), will host the 87th Annual Scientific Congress, May 7–11 at the International Convention Centre in Sydney, Australia.
The theme of the 2018 Scientific Congress, Reflecting on What Really Matters, explores the challenges of providing quality patient care within complex health care systems—a universal situation familiar to U.S. surgeons.
The ACS has partnered in the planning of this program, and many U.S. surgeons will be featured as speakers throughout the week. Additionally, an ACS panel will take place the morning of Thursday, May 10. The ACS also will be involved in other Annual Scientific Congress activities, including the following:
• ACS Lecture, The Surgical Patient in the ICU—Insights into Survivorship, by Mayur B. Patel, MD, MPH, FACS, a neurosurgeon and surgical intensivist, from Nashville, TN
• Region 16 meeting for ACS Fellows from Australia and New Zealand, the U.S., and other Pacific countries
Among the ACS leaders attending the Congress are Barbara L. Bass, MD, FACS, FRCS(Hon),
ACS President; Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of
Research and Optimal Patient Care; Ronald V. Maier, MD, FACS, ACS President-Elect; M.
Margaret (Peggy) Knudson, MD, FACS, Medical Director, Military Health System Strategi
Partnership American College of Surgeons; and Tyler G. Hughes, MD, FACS, Co-Editor, ACS
Surgery News, and Editor-in-Chief, ACS Communities.
For more information on the conference and to register, visit the RACS 87th Annual Scientific Congress website at https://asc.surgeons.org/.
The Royal Australasian College of Surgeons (RACS), along with the American College of Surgeons (ACS), will host the 87th Annual Scientific Congress, May 7–11 at the International Convention Centre in Sydney, Australia.
The theme of the 2018 Scientific Congress, Reflecting on What Really Matters, explores the challenges of providing quality patient care within complex health care systems—a universal situation familiar to U.S. surgeons.
The ACS has partnered in the planning of this program, and many U.S. surgeons will be featured as speakers throughout the week. Additionally, an ACS panel will take place the morning of Thursday, May 10. The ACS also will be involved in other Annual Scientific Congress activities, including the following:
• ACS Lecture, The Surgical Patient in the ICU—Insights into Survivorship, by Mayur B. Patel, MD, MPH, FACS, a neurosurgeon and surgical intensivist, from Nashville, TN
• Region 16 meeting for ACS Fellows from Australia and New Zealand, the U.S., and other Pacific countries
Among the ACS leaders attending the Congress are Barbara L. Bass, MD, FACS, FRCS(Hon),
ACS President; Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of
Research and Optimal Patient Care; Ronald V. Maier, MD, FACS, ACS President-Elect; M.
Margaret (Peggy) Knudson, MD, FACS, Medical Director, Military Health System Strategi
Partnership American College of Surgeons; and Tyler G. Hughes, MD, FACS, Co-Editor, ACS
Surgery News, and Editor-in-Chief, ACS Communities.
For more information on the conference and to register, visit the RACS 87th Annual Scientific Congress website at https://asc.surgeons.org/.
The Royal Australasian College of Surgeons (RACS), along with the American College of Surgeons (ACS), will host the 87th Annual Scientific Congress, May 7–11 at the International Convention Centre in Sydney, Australia.
The theme of the 2018 Scientific Congress, Reflecting on What Really Matters, explores the challenges of providing quality patient care within complex health care systems—a universal situation familiar to U.S. surgeons.
The ACS has partnered in the planning of this program, and many U.S. surgeons will be featured as speakers throughout the week. Additionally, an ACS panel will take place the morning of Thursday, May 10. The ACS also will be involved in other Annual Scientific Congress activities, including the following:
• ACS Lecture, The Surgical Patient in the ICU—Insights into Survivorship, by Mayur B. Patel, MD, MPH, FACS, a neurosurgeon and surgical intensivist, from Nashville, TN
• Region 16 meeting for ACS Fellows from Australia and New Zealand, the U.S., and other Pacific countries
Among the ACS leaders attending the Congress are Barbara L. Bass, MD, FACS, FRCS(Hon),
ACS President; Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of
Research and Optimal Patient Care; Ronald V. Maier, MD, FACS, ACS President-Elect; M.
Margaret (Peggy) Knudson, MD, FACS, Medical Director, Military Health System Strategi
Partnership American College of Surgeons; and Tyler G. Hughes, MD, FACS, Co-Editor, ACS
Surgery News, and Editor-in-Chief, ACS Communities.
For more information on the conference and to register, visit the RACS 87th Annual Scientific Congress website at https://asc.surgeons.org/.
ACS releases 2018 update to the Physicians as Assistants at Surgery report
The American College of Surgeons (ACS), in collaboration with 15 other national specialty surgical organizations, has recently published the eighth edition of the Physicians as Assistants at Surgery report, a study first undertaken in 1994. The 2018 report reflects the most recent clinical practices and provides guidance on how often an operation might require a physician to assist at surgery. The report is available on the ACS website at www.facs.org/~/media/files/advocacy/pubs/2018_pas.ashx.
Using the American Medical Association’s Current Procedural Terminology (CPT) codes from the 2018 manual, each participating organization reviewed new or revised codes since 2016 and any other codes of interest that are applicable to their specialty and indicated whether the operation requires a physician as an assistant with the following frequency: almost always, almost never, or some of the time. The 2018 report adds 93 codes that the CPT Editorial Panel has approved since the last report was issued in 2016. In addition, the 2018 report updates 384 revised codes and deletes 48 codes that are no longer in CPT.
The ACS maintains that a physician who assists with an operation should be trained to participate in and actively assist the surgeon in safely completing the operation. When a surgeon is unavailable to serve as an assistant, a qualified surgical resident or other qualified health care professional, such as a nurse practitioner or physician assistant with experience in assisting, may participate in operations, according to the ACS Statements on Principles (available at www.facs.org/about-acs/statements/stonprin).
Organizations that collaborated with the ACS to conduct the study include the American Academy of Ophthalmology, the American Academy of Orthopaedic Surgeons, the American Academy of Otolaryngology–Head and Neck Surgery, the American Association of Neurological Surgeons, the American Pediatric Surgical Association, the American Society of Colon and Rectal Surgeons, the American Society of Plastic Surgeons, the American Society of Transplant Surgeons, the American Urological Association, the Congress of Neurological Surgeons, the Society for Surgical Oncology, the Society for Vascular Surgery, the Society of American Gastrointestinal Endoscopic Surgeons, the American College of Obstetricians and Gynecologists, and the Society of Thoracic Surgeons.
The American College of Surgeons (ACS), in collaboration with 15 other national specialty surgical organizations, has recently published the eighth edition of the Physicians as Assistants at Surgery report, a study first undertaken in 1994. The 2018 report reflects the most recent clinical practices and provides guidance on how often an operation might require a physician to assist at surgery. The report is available on the ACS website at www.facs.org/~/media/files/advocacy/pubs/2018_pas.ashx.
Using the American Medical Association’s Current Procedural Terminology (CPT) codes from the 2018 manual, each participating organization reviewed new or revised codes since 2016 and any other codes of interest that are applicable to their specialty and indicated whether the operation requires a physician as an assistant with the following frequency: almost always, almost never, or some of the time. The 2018 report adds 93 codes that the CPT Editorial Panel has approved since the last report was issued in 2016. In addition, the 2018 report updates 384 revised codes and deletes 48 codes that are no longer in CPT.
The ACS maintains that a physician who assists with an operation should be trained to participate in and actively assist the surgeon in safely completing the operation. When a surgeon is unavailable to serve as an assistant, a qualified surgical resident or other qualified health care professional, such as a nurse practitioner or physician assistant with experience in assisting, may participate in operations, according to the ACS Statements on Principles (available at www.facs.org/about-acs/statements/stonprin).
Organizations that collaborated with the ACS to conduct the study include the American Academy of Ophthalmology, the American Academy of Orthopaedic Surgeons, the American Academy of Otolaryngology–Head and Neck Surgery, the American Association of Neurological Surgeons, the American Pediatric Surgical Association, the American Society of Colon and Rectal Surgeons, the American Society of Plastic Surgeons, the American Society of Transplant Surgeons, the American Urological Association, the Congress of Neurological Surgeons, the Society for Surgical Oncology, the Society for Vascular Surgery, the Society of American Gastrointestinal Endoscopic Surgeons, the American College of Obstetricians and Gynecologists, and the Society of Thoracic Surgeons.
The American College of Surgeons (ACS), in collaboration with 15 other national specialty surgical organizations, has recently published the eighth edition of the Physicians as Assistants at Surgery report, a study first undertaken in 1994. The 2018 report reflects the most recent clinical practices and provides guidance on how often an operation might require a physician to assist at surgery. The report is available on the ACS website at www.facs.org/~/media/files/advocacy/pubs/2018_pas.ashx.
Using the American Medical Association’s Current Procedural Terminology (CPT) codes from the 2018 manual, each participating organization reviewed new or revised codes since 2016 and any other codes of interest that are applicable to their specialty and indicated whether the operation requires a physician as an assistant with the following frequency: almost always, almost never, or some of the time. The 2018 report adds 93 codes that the CPT Editorial Panel has approved since the last report was issued in 2016. In addition, the 2018 report updates 384 revised codes and deletes 48 codes that are no longer in CPT.
The ACS maintains that a physician who assists with an operation should be trained to participate in and actively assist the surgeon in safely completing the operation. When a surgeon is unavailable to serve as an assistant, a qualified surgical resident or other qualified health care professional, such as a nurse practitioner or physician assistant with experience in assisting, may participate in operations, according to the ACS Statements on Principles (available at www.facs.org/about-acs/statements/stonprin).
Organizations that collaborated with the ACS to conduct the study include the American Academy of Ophthalmology, the American Academy of Orthopaedic Surgeons, the American Academy of Otolaryngology–Head and Neck Surgery, the American Association of Neurological Surgeons, the American Pediatric Surgical Association, the American Society of Colon and Rectal Surgeons, the American Society of Plastic Surgeons, the American Society of Transplant Surgeons, the American Urological Association, the Congress of Neurological Surgeons, the Society for Surgical Oncology, the Society for Vascular Surgery, the Society of American Gastrointestinal Endoscopic Surgeons, the American College of Obstetricians and Gynecologists, and the Society of Thoracic Surgeons.
Applications for ACS Academy of Master Surgeon Educators are now being accepted –
The American College of Surgeons (ACS) Academy of Master Surgeon Educators, a new College enterprise that will advance the science and implementation of education across all surgical specialties, is now accepting applications for Membership and Associate Membership. Applications are due May 14, 2018.
You could be considered for membership in the Academy through two avenues:
• You may apply directly.
• You may be nominated by a colleague and then complete the application.
Background
In October 2014, the American College of Surgeons (ACS) Board of Regents approved a proposal from the ACS Division of Education to establish the ACS Academy of Master Surgeon Educators. A Steering Committee was appointed to create a model for the Academy, which articulated the desired outcomes, defined standards and criteria for membership, and developed the process for application. The ACS Steering Committee for the Academy of Master Surgeon Educators is co-chaired by ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), and Ajit K. Sachdeva, MD, FACS, FRCSC, Director, ACS Division of Education. Other members include Sir Murray Brennan, MD, FACS, ACS Distinguished Service Award recipient; Haile Debas, MD, FACS, founding executive director, Global Health Sciences, University of California, San Francisco; David B. Hoyt, MD, FACS, ACS Executive Director; L. Scott Levin, MD, FACS, ACS Regent; Leigh Neumayer, MD, FACS, Chair, ACS Board of Regents; and Carlos Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), ACS Past-President.
The Academy formally launched at the ACS Clinical Congress 2017 in San Diego, CA, and was received enthusiastically.
Purposes of the Academy
The goals of this unique Academy are to define megatrends in surgical education, steer advances in this field, and underscore the critical importance of surgical education in the changing milieu of health care. The Academy will meet these goals by recognizing and assembling a cadre of master surgeon educators of national and international renown who will support cutting-edge surgical education and provide mentorship to the next generation of surgeon educators.
Members of the Academy will be selected through a rigorous peer-review process, and induction will be a high honor in the field of surgical education. Members of the Academy will be expected to engage in activities to address the aforementioned goals. Membership in the Academy will be open to Master Surgeon Educators from across the surgical specialties.
Three categories of membership will be available: Member, Associate Member, and Affiliate Member. Applications for Membership and Associate Membership in the Academy are now being accepted. You are invited to apply or nominate a colleague for membership via the ACS website at facs.org/acsacademy.
The ACS is truly excited about this seminal endeavor, which will impact the profession of surgery for generations to come.
The American College of Surgeons (ACS) Academy of Master Surgeon Educators, a new College enterprise that will advance the science and implementation of education across all surgical specialties, is now accepting applications for Membership and Associate Membership. Applications are due May 14, 2018.
You could be considered for membership in the Academy through two avenues:
• You may apply directly.
• You may be nominated by a colleague and then complete the application.
Background
In October 2014, the American College of Surgeons (ACS) Board of Regents approved a proposal from the ACS Division of Education to establish the ACS Academy of Master Surgeon Educators. A Steering Committee was appointed to create a model for the Academy, which articulated the desired outcomes, defined standards and criteria for membership, and developed the process for application. The ACS Steering Committee for the Academy of Master Surgeon Educators is co-chaired by ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), and Ajit K. Sachdeva, MD, FACS, FRCSC, Director, ACS Division of Education. Other members include Sir Murray Brennan, MD, FACS, ACS Distinguished Service Award recipient; Haile Debas, MD, FACS, founding executive director, Global Health Sciences, University of California, San Francisco; David B. Hoyt, MD, FACS, ACS Executive Director; L. Scott Levin, MD, FACS, ACS Regent; Leigh Neumayer, MD, FACS, Chair, ACS Board of Regents; and Carlos Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), ACS Past-President.
The Academy formally launched at the ACS Clinical Congress 2017 in San Diego, CA, and was received enthusiastically.
Purposes of the Academy
The goals of this unique Academy are to define megatrends in surgical education, steer advances in this field, and underscore the critical importance of surgical education in the changing milieu of health care. The Academy will meet these goals by recognizing and assembling a cadre of master surgeon educators of national and international renown who will support cutting-edge surgical education and provide mentorship to the next generation of surgeon educators.
Members of the Academy will be selected through a rigorous peer-review process, and induction will be a high honor in the field of surgical education. Members of the Academy will be expected to engage in activities to address the aforementioned goals. Membership in the Academy will be open to Master Surgeon Educators from across the surgical specialties.
Three categories of membership will be available: Member, Associate Member, and Affiliate Member. Applications for Membership and Associate Membership in the Academy are now being accepted. You are invited to apply or nominate a colleague for membership via the ACS website at facs.org/acsacademy.
The ACS is truly excited about this seminal endeavor, which will impact the profession of surgery for generations to come.
The American College of Surgeons (ACS) Academy of Master Surgeon Educators, a new College enterprise that will advance the science and implementation of education across all surgical specialties, is now accepting applications for Membership and Associate Membership. Applications are due May 14, 2018.
You could be considered for membership in the Academy through two avenues:
• You may apply directly.
• You may be nominated by a colleague and then complete the application.
Background
In October 2014, the American College of Surgeons (ACS) Board of Regents approved a proposal from the ACS Division of Education to establish the ACS Academy of Master Surgeon Educators. A Steering Committee was appointed to create a model for the Academy, which articulated the desired outcomes, defined standards and criteria for membership, and developed the process for application. The ACS Steering Committee for the Academy of Master Surgeon Educators is co-chaired by ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), and Ajit K. Sachdeva, MD, FACS, FRCSC, Director, ACS Division of Education. Other members include Sir Murray Brennan, MD, FACS, ACS Distinguished Service Award recipient; Haile Debas, MD, FACS, founding executive director, Global Health Sciences, University of California, San Francisco; David B. Hoyt, MD, FACS, ACS Executive Director; L. Scott Levin, MD, FACS, ACS Regent; Leigh Neumayer, MD, FACS, Chair, ACS Board of Regents; and Carlos Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), ACS Past-President.
The Academy formally launched at the ACS Clinical Congress 2017 in San Diego, CA, and was received enthusiastically.
Purposes of the Academy
The goals of this unique Academy are to define megatrends in surgical education, steer advances in this field, and underscore the critical importance of surgical education in the changing milieu of health care. The Academy will meet these goals by recognizing and assembling a cadre of master surgeon educators of national and international renown who will support cutting-edge surgical education and provide mentorship to the next generation of surgeon educators.
Members of the Academy will be selected through a rigorous peer-review process, and induction will be a high honor in the field of surgical education. Members of the Academy will be expected to engage in activities to address the aforementioned goals. Membership in the Academy will be open to Master Surgeon Educators from across the surgical specialties.
Three categories of membership will be available: Member, Associate Member, and Affiliate Member. Applications for Membership and Associate Membership in the Academy are now being accepted. You are invited to apply or nominate a colleague for membership via the ACS website at facs.org/acsacademy.
The ACS is truly excited about this seminal endeavor, which will impact the profession of surgery for generations to come.
ACS WiSC seeks ACS Fellows to serve as new members
The mission of the WiSC is to enable women surgeons of all ages, specialties, and practice types to develop their individual potential as professionals; promote an environment that fosters inclusion, respect, and success; develop, encourage, and advance women surgeons as leaders; and provide a forum and networking opportunities to enhance women’s surgical career satisfaction.
Surgeons interested in advancing the role of women in the ACS and encouraging and mentoring women in surgery should apply. Nominations are open to both men and women, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Read the full eligibility requirements and how to apply on the ACS website at facs.org/about-acs/governance/acs-committees/women-in-surgery-committee/wisc-call. Eligible candidates will be selected and notified by the committee in June, and will be invited to attend the October 22 meeting of the WiSC, held in conjunction with Clinical Congress 2018 in Boston, MA. Travel reimbursement will not be provided.
Apply online at www.surveymonkey.com/r/2018WiSCMbrApp. Applications are due May 31, 2018. Questions can be directed to Connie Bura at [email protected].
The mission of the WiSC is to enable women surgeons of all ages, specialties, and practice types to develop their individual potential as professionals; promote an environment that fosters inclusion, respect, and success; develop, encourage, and advance women surgeons as leaders; and provide a forum and networking opportunities to enhance women’s surgical career satisfaction.
Surgeons interested in advancing the role of women in the ACS and encouraging and mentoring women in surgery should apply. Nominations are open to both men and women, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Read the full eligibility requirements and how to apply on the ACS website at facs.org/about-acs/governance/acs-committees/women-in-surgery-committee/wisc-call. Eligible candidates will be selected and notified by the committee in June, and will be invited to attend the October 22 meeting of the WiSC, held in conjunction with Clinical Congress 2018 in Boston, MA. Travel reimbursement will not be provided.
Apply online at www.surveymonkey.com/r/2018WiSCMbrApp. Applications are due May 31, 2018. Questions can be directed to Connie Bura at [email protected].
The mission of the WiSC is to enable women surgeons of all ages, specialties, and practice types to develop their individual potential as professionals; promote an environment that fosters inclusion, respect, and success; develop, encourage, and advance women surgeons as leaders; and provide a forum and networking opportunities to enhance women’s surgical career satisfaction.
Surgeons interested in advancing the role of women in the ACS and encouraging and mentoring women in surgery should apply. Nominations are open to both men and women, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Read the full eligibility requirements and how to apply on the ACS website at facs.org/about-acs/governance/acs-committees/women-in-surgery-committee/wisc-call. Eligible candidates will be selected and notified by the committee in June, and will be invited to attend the October 22 meeting of the WiSC, held in conjunction with Clinical Congress 2018 in Boston, MA. Travel reimbursement will not be provided.
Apply online at www.surveymonkey.com/r/2018WiSCMbrApp. Applications are due May 31, 2018. Questions can be directed to Connie Bura at [email protected].
Disproportionately low U.S. research funding targets gynecologic cancers
NEW ORLEANS – The National Cancer Institute is woefully underfunding gynecologic cancer research, compared with several other cancer types, when the money the institute is spending annually is factored by the incidence and lethal impact of each cancer using U.S. data from 2007 to 2014.
That period featured “systematic and pervasive underfunding of gynecologic cancers in relation to other cancer sites,” Ryan J. Spencer, MD, said at the annual meeting of the Society of Gynecologic Oncology. The trends over the period he studied worsened with time and pose the risk that progress in gynecologic cancers – uterine, cervical, and ovarian – will “lag behind” other cancers’ progress in prevention, treatment, and improved survival, said Dr. Spencer, a gynecologic oncologist at the University of Wisconsin–Madison.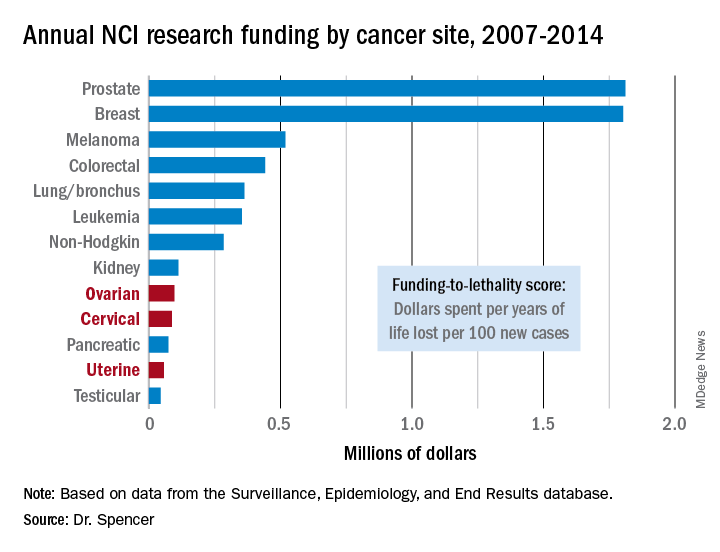
Additional time trend analyses showed that the annual funding-to-lethality score for each of the three gynecologic cancers declined during the period studied.
“We must do everything we can to reverse these trends,” Dr. Spencer concluded.
SOURCE: Spencer R et al. SGO 2018, Abstract 3.
The data reported by Dr. Spencer and his associates are very sobering. They present an elegant analysis that documents a lag and decline in funding for gynecologic cancers that factors in the lethality of various cancers. By several other measures as well, funding for research into gynecologic cancers has been slipping in recent years. During 2011-2016, we saw a 90% drop in enrollment into U.S. clinical trials for gynecologic cancers, and from a peak in 2012-2016 the total number of trials for gynecologic cancers fell by more than two-thirds.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no disclosures. She made these comments as designated discussant for the report.
The data reported by Dr. Spencer and his associates are very sobering. They present an elegant analysis that documents a lag and decline in funding for gynecologic cancers that factors in the lethality of various cancers. By several other measures as well, funding for research into gynecologic cancers has been slipping in recent years. During 2011-2016, we saw a 90% drop in enrollment into U.S. clinical trials for gynecologic cancers, and from a peak in 2012-2016 the total number of trials for gynecologic cancers fell by more than two-thirds.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no disclosures. She made these comments as designated discussant for the report.
The data reported by Dr. Spencer and his associates are very sobering. They present an elegant analysis that documents a lag and decline in funding for gynecologic cancers that factors in the lethality of various cancers. By several other measures as well, funding for research into gynecologic cancers has been slipping in recent years. During 2011-2016, we saw a 90% drop in enrollment into U.S. clinical trials for gynecologic cancers, and from a peak in 2012-2016 the total number of trials for gynecologic cancers fell by more than two-thirds.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no disclosures. She made these comments as designated discussant for the report.
NEW ORLEANS – The National Cancer Institute is woefully underfunding gynecologic cancer research, compared with several other cancer types, when the money the institute is spending annually is factored by the incidence and lethal impact of each cancer using U.S. data from 2007 to 2014.
That period featured “systematic and pervasive underfunding of gynecologic cancers in relation to other cancer sites,” Ryan J. Spencer, MD, said at the annual meeting of the Society of Gynecologic Oncology. The trends over the period he studied worsened with time and pose the risk that progress in gynecologic cancers – uterine, cervical, and ovarian – will “lag behind” other cancers’ progress in prevention, treatment, and improved survival, said Dr. Spencer, a gynecologic oncologist at the University of Wisconsin–Madison.
Additional time trend analyses showed that the annual funding-to-lethality score for each of the three gynecologic cancers declined during the period studied.
“We must do everything we can to reverse these trends,” Dr. Spencer concluded.
SOURCE: Spencer R et al. SGO 2018, Abstract 3.
NEW ORLEANS – The National Cancer Institute is woefully underfunding gynecologic cancer research, compared with several other cancer types, when the money the institute is spending annually is factored by the incidence and lethal impact of each cancer using U.S. data from 2007 to 2014.
That period featured “systematic and pervasive underfunding of gynecologic cancers in relation to other cancer sites,” Ryan J. Spencer, MD, said at the annual meeting of the Society of Gynecologic Oncology. The trends over the period he studied worsened with time and pose the risk that progress in gynecologic cancers – uterine, cervical, and ovarian – will “lag behind” other cancers’ progress in prevention, treatment, and improved survival, said Dr. Spencer, a gynecologic oncologist at the University of Wisconsin–Madison.
Additional time trend analyses showed that the annual funding-to-lethality score for each of the three gynecologic cancers declined during the period studied.
“We must do everything we can to reverse these trends,” Dr. Spencer concluded.
SOURCE: Spencer R et al. SGO 2018, Abstract 3.
REPORTING FROM SGO 2018
Key clinical point: The National Cancer Institute underfunds gynecologic cancer research.
Major finding: Ovarian cancer research funding averaged $97,000 per year of life lost per 100 new cases, compared with $1.8 million for both breast and prostate cancer.
Study details: A review of U.S. data collected by the National Cancer Institute during 2007-2014.
Disclosures: Dr. Spencer had no disclosures.
Source: Spencer R et al. SGO 2018, Abstract 3.
Hyaluronic acid filler preferred for infraorbital hollowing
Most patients who responded to surveys reported being satisfied after off-label treatment with Juvéderm Voluma XC hyaluronic acid filler for infraorbital hollowing, a study finds.
Adverse effects were reported in 12% of patients.
The treatment’s “high patient satisfaction profile and a similar safety profile among other soft-tissue fillers make it an excellent adjunct in the plastic surgeon’s armamentarium,” reported Michael B. Hall, MD, and his associates at their private, ambulatory facial plastic and reconstructive surgery practice in Austin, Texas, in JAMA Facial Plastic Surgery.
According to the researchers, the Food and Drug Administration has not approved any soft-tissue fillers for the periorbital complex. At their practice, Dr. Hall and his associates treat infraorbital hollows with Juvéderm Voluma XC, which is approved by the FDA for certain types of cheek augmentation. Other studies have examined Belotero or Restylane as treatments for building volume in the periorbital area, the authors wrote, but research into cosmetic injections of Juvéderm Voluma XC is lacking.
For the new study, the researchers retrospectively analyzed the cases of 101 patients (aged 32-75 years, with average age of 54 years; 89% female; 54% Fitzpatrick skin type II; racial breakdown not reported) who were electively treated with the filler for infraorbital hollowing in 2016 and 2017. The patients received an average 1 mL of the treatment gel.
The patients were photographed and answered surveys, and they were evaluated using the Allergan Infraorbital Hollows Scale. Follow-up time averaged 12 months.
A total of 18 patients (18%) required touch-up within 3 months, and 2 required multiple touch-ups. A total of 12 subjects (12%) had adverse effects (including 3 who had more than one), which included bruising (10%), contour irregularities (2%), edema (3%) and Tyndall effect (1%). Hyaluronidase was required in 3 patients (3%), and 24 patients sought further treatment after 3 months.
The researchers sent two satisfaction surveys to the participants. A total of 41% responded to the FACE-Q Satisfaction With Eyes survey, and 42% responded to the FACE-Q Satisfaction With Decision survey.
Depending on the question, 70%-85% of the respondents to the Satisfaction With Eyes survey said they were “definitely” or “somewhat” satisfied with the treatment outcome.
The highest levels of dissatisfaction came in response to a questions about whether the subjects felt their eyes looked alert (not tired) or youthful. The highest levels of satisfaction were in response to questions about whether the subjects were happy with the shape, attractiveness, and openness of their eyes.
Depending on the question, 73%-85% of the subjects who took the Satisfaction With Decision survey reported that they “definitely” or “somewhat” agree with positive statements about the treatment. While differences were small, they agreed the most with a statement saying the procedure was “worth the time and effort.”
No external funding or remuneration was received. The study authors reported no relevant disclosures.
SOURCE: Hall MB et al. JAMA Facial Plast Surg. 2018 Apr 5. doi:10.1001/jamafacial.2018.0230.
Most patients who responded to surveys reported being satisfied after off-label treatment with Juvéderm Voluma XC hyaluronic acid filler for infraorbital hollowing, a study finds.
Adverse effects were reported in 12% of patients.
The treatment’s “high patient satisfaction profile and a similar safety profile among other soft-tissue fillers make it an excellent adjunct in the plastic surgeon’s armamentarium,” reported Michael B. Hall, MD, and his associates at their private, ambulatory facial plastic and reconstructive surgery practice in Austin, Texas, in JAMA Facial Plastic Surgery.
According to the researchers, the Food and Drug Administration has not approved any soft-tissue fillers for the periorbital complex. At their practice, Dr. Hall and his associates treat infraorbital hollows with Juvéderm Voluma XC, which is approved by the FDA for certain types of cheek augmentation. Other studies have examined Belotero or Restylane as treatments for building volume in the periorbital area, the authors wrote, but research into cosmetic injections of Juvéderm Voluma XC is lacking.
For the new study, the researchers retrospectively analyzed the cases of 101 patients (aged 32-75 years, with average age of 54 years; 89% female; 54% Fitzpatrick skin type II; racial breakdown not reported) who were electively treated with the filler for infraorbital hollowing in 2016 and 2017. The patients received an average 1 mL of the treatment gel.
The patients were photographed and answered surveys, and they were evaluated using the Allergan Infraorbital Hollows Scale. Follow-up time averaged 12 months.
A total of 18 patients (18%) required touch-up within 3 months, and 2 required multiple touch-ups. A total of 12 subjects (12%) had adverse effects (including 3 who had more than one), which included bruising (10%), contour irregularities (2%), edema (3%) and Tyndall effect (1%). Hyaluronidase was required in 3 patients (3%), and 24 patients sought further treatment after 3 months.
The researchers sent two satisfaction surveys to the participants. A total of 41% responded to the FACE-Q Satisfaction With Eyes survey, and 42% responded to the FACE-Q Satisfaction With Decision survey.
Depending on the question, 70%-85% of the respondents to the Satisfaction With Eyes survey said they were “definitely” or “somewhat” satisfied with the treatment outcome.
The highest levels of dissatisfaction came in response to a questions about whether the subjects felt their eyes looked alert (not tired) or youthful. The highest levels of satisfaction were in response to questions about whether the subjects were happy with the shape, attractiveness, and openness of their eyes.
Depending on the question, 73%-85% of the subjects who took the Satisfaction With Decision survey reported that they “definitely” or “somewhat” agree with positive statements about the treatment. While differences were small, they agreed the most with a statement saying the procedure was “worth the time and effort.”
No external funding or remuneration was received. The study authors reported no relevant disclosures.
SOURCE: Hall MB et al. JAMA Facial Plast Surg. 2018 Apr 5. doi:10.1001/jamafacial.2018.0230.
Most patients who responded to surveys reported being satisfied after off-label treatment with Juvéderm Voluma XC hyaluronic acid filler for infraorbital hollowing, a study finds.
Adverse effects were reported in 12% of patients.
The treatment’s “high patient satisfaction profile and a similar safety profile among other soft-tissue fillers make it an excellent adjunct in the plastic surgeon’s armamentarium,” reported Michael B. Hall, MD, and his associates at their private, ambulatory facial plastic and reconstructive surgery practice in Austin, Texas, in JAMA Facial Plastic Surgery.
According to the researchers, the Food and Drug Administration has not approved any soft-tissue fillers for the periorbital complex. At their practice, Dr. Hall and his associates treat infraorbital hollows with Juvéderm Voluma XC, which is approved by the FDA for certain types of cheek augmentation. Other studies have examined Belotero or Restylane as treatments for building volume in the periorbital area, the authors wrote, but research into cosmetic injections of Juvéderm Voluma XC is lacking.
For the new study, the researchers retrospectively analyzed the cases of 101 patients (aged 32-75 years, with average age of 54 years; 89% female; 54% Fitzpatrick skin type II; racial breakdown not reported) who were electively treated with the filler for infraorbital hollowing in 2016 and 2017. The patients received an average 1 mL of the treatment gel.
The patients were photographed and answered surveys, and they were evaluated using the Allergan Infraorbital Hollows Scale. Follow-up time averaged 12 months.
A total of 18 patients (18%) required touch-up within 3 months, and 2 required multiple touch-ups. A total of 12 subjects (12%) had adverse effects (including 3 who had more than one), which included bruising (10%), contour irregularities (2%), edema (3%) and Tyndall effect (1%). Hyaluronidase was required in 3 patients (3%), and 24 patients sought further treatment after 3 months.
The researchers sent two satisfaction surveys to the participants. A total of 41% responded to the FACE-Q Satisfaction With Eyes survey, and 42% responded to the FACE-Q Satisfaction With Decision survey.
Depending on the question, 70%-85% of the respondents to the Satisfaction With Eyes survey said they were “definitely” or “somewhat” satisfied with the treatment outcome.
The highest levels of dissatisfaction came in response to a questions about whether the subjects felt their eyes looked alert (not tired) or youthful. The highest levels of satisfaction were in response to questions about whether the subjects were happy with the shape, attractiveness, and openness of their eyes.
Depending on the question, 73%-85% of the subjects who took the Satisfaction With Decision survey reported that they “definitely” or “somewhat” agree with positive statements about the treatment. While differences were small, they agreed the most with a statement saying the procedure was “worth the time and effort.”
No external funding or remuneration was received. The study authors reported no relevant disclosures.
SOURCE: Hall MB et al. JAMA Facial Plast Surg. 2018 Apr 5. doi:10.1001/jamafacial.2018.0230.
FROM JAMA FACIAL PLASTIC SURGERY
Key clinical point:
Major finding: Adverse effects occurred at a rate of 12%, and most who responded to surveys reported satisfaction postprocedure (70%-85%).
Study details: A retrospective observational study of 101 patients.
Disclosures: No external funding or remuneration was received. The study authors reported no relevant disclosures.
Source: Hall MB et al. JAMA Facial Plast Surg. 2018 Apr 5. doi:10.1001/jamafacial.2018.0230.
Cancer clinics continue to close and consolidate
and increased costs in the process, according to the Community Oncology Alliance.
From 2008 to 2018, a total of 1,653 oncology clinics and/or practices were affected in the following ways: 423 clinics closed, 658 practices were acquired by hospitals, 168 practices merged or were acquired by a corporate entity, 359 practices reported that they were struggling financially, and 45 practices sent patients elsewhere for chemotherapy, the COA said its 2018 Practice Impact Report.
“The shifting and shrinking community cancer care system reduces access to cancer care, inflates spending at the more expensive hospital setting, and is a disservice to patients, their caregivers, and support networks,” Jeff Vacirca, MD, president of COA said in a written statement.
Crunching the numbers another way shows that 13.8 practices a month have closed, been acquired by hospitals, or undergone mergers since 2008. The number of community clinics that have closed increased by 11.3% since the 2016 Practice Impact Report, and consolidations with hospitals rose by 8%. Practices with financial struggles were down by 7.9% from 2016, “which is proportional to the number of practices that have been acquired or moved into the hospital setting,” the COA said in the 2018 report.
“No one can look at [recent] trends and say that there hasn’t been a clear and negative dismantling of our cancer care system over the last decade,” Ted Okon, executive director of COA, said in the statement accompanying the report. “This situation is the direct result of the misguided 340B [drug discount program] and [the 2013] sequester cut allowed to take place by our elected officials in Washington. The pressures of these misguided public policies have been a one-two punch, pushing and pulling community oncology practices to close, consolidate, or be acquired by hospitals, all at the expense of patients.”
and increased costs in the process, according to the Community Oncology Alliance.
From 2008 to 2018, a total of 1,653 oncology clinics and/or practices were affected in the following ways: 423 clinics closed, 658 practices were acquired by hospitals, 168 practices merged or were acquired by a corporate entity, 359 practices reported that they were struggling financially, and 45 practices sent patients elsewhere for chemotherapy, the COA said its 2018 Practice Impact Report.
“The shifting and shrinking community cancer care system reduces access to cancer care, inflates spending at the more expensive hospital setting, and is a disservice to patients, their caregivers, and support networks,” Jeff Vacirca, MD, president of COA said in a written statement.
Crunching the numbers another way shows that 13.8 practices a month have closed, been acquired by hospitals, or undergone mergers since 2008. The number of community clinics that have closed increased by 11.3% since the 2016 Practice Impact Report, and consolidations with hospitals rose by 8%. Practices with financial struggles were down by 7.9% from 2016, “which is proportional to the number of practices that have been acquired or moved into the hospital setting,” the COA said in the 2018 report.
“No one can look at [recent] trends and say that there hasn’t been a clear and negative dismantling of our cancer care system over the last decade,” Ted Okon, executive director of COA, said in the statement accompanying the report. “This situation is the direct result of the misguided 340B [drug discount program] and [the 2013] sequester cut allowed to take place by our elected officials in Washington. The pressures of these misguided public policies have been a one-two punch, pushing and pulling community oncology practices to close, consolidate, or be acquired by hospitals, all at the expense of patients.”
and increased costs in the process, according to the Community Oncology Alliance.
From 2008 to 2018, a total of 1,653 oncology clinics and/or practices were affected in the following ways: 423 clinics closed, 658 practices were acquired by hospitals, 168 practices merged or were acquired by a corporate entity, 359 practices reported that they were struggling financially, and 45 practices sent patients elsewhere for chemotherapy, the COA said its 2018 Practice Impact Report.
“The shifting and shrinking community cancer care system reduces access to cancer care, inflates spending at the more expensive hospital setting, and is a disservice to patients, their caregivers, and support networks,” Jeff Vacirca, MD, president of COA said in a written statement.
Crunching the numbers another way shows that 13.8 practices a month have closed, been acquired by hospitals, or undergone mergers since 2008. The number of community clinics that have closed increased by 11.3% since the 2016 Practice Impact Report, and consolidations with hospitals rose by 8%. Practices with financial struggles were down by 7.9% from 2016, “which is proportional to the number of practices that have been acquired or moved into the hospital setting,” the COA said in the 2018 report.
“No one can look at [recent] trends and say that there hasn’t been a clear and negative dismantling of our cancer care system over the last decade,” Ted Okon, executive director of COA, said in the statement accompanying the report. “This situation is the direct result of the misguided 340B [drug discount program] and [the 2013] sequester cut allowed to take place by our elected officials in Washington. The pressures of these misguided public policies have been a one-two punch, pushing and pulling community oncology practices to close, consolidate, or be acquired by hospitals, all at the expense of patients.”
More Older Patients Should be Included in Epilepsy Drug Trials
Too few older adults are included in antiepileptic drug trials according to a systematic review and meta-analysis that included 184 studies.
- In 1991 to 1992, the mean age of patients included in clinical trials was 27 years, which had increased to about 42 years in 2015 to 2016.
- In 83 studies (44%), inclusion criteria included a maximum age limit.
- The requirement for limiting a participant’s maximum age did not decline significantly over time between the two time periods (r = 0.072, P=.816).
- The only disease-related exclusion criteria for entry into a clinical trial for antiepileptic drugs that was linked to a drop in the average age of enrolled patients were neurological conditions other than epilepsy.
Desmarais P, Miville C, Milán-Tomás A, et al. Age representation in antiepileptic drug trials: a systematic review and meta-analysis. Epilepsy Res. 2018;142:9-15.
Too few older adults are included in antiepileptic drug trials according to a systematic review and meta-analysis that included 184 studies.
- In 1991 to 1992, the mean age of patients included in clinical trials was 27 years, which had increased to about 42 years in 2015 to 2016.
- In 83 studies (44%), inclusion criteria included a maximum age limit.
- The requirement for limiting a participant’s maximum age did not decline significantly over time between the two time periods (r = 0.072, P=.816).
- The only disease-related exclusion criteria for entry into a clinical trial for antiepileptic drugs that was linked to a drop in the average age of enrolled patients were neurological conditions other than epilepsy.
Desmarais P, Miville C, Milán-Tomás A, et al. Age representation in antiepileptic drug trials: a systematic review and meta-analysis. Epilepsy Res. 2018;142:9-15.
Too few older adults are included in antiepileptic drug trials according to a systematic review and meta-analysis that included 184 studies.
- In 1991 to 1992, the mean age of patients included in clinical trials was 27 years, which had increased to about 42 years in 2015 to 2016.
- In 83 studies (44%), inclusion criteria included a maximum age limit.
- The requirement for limiting a participant’s maximum age did not decline significantly over time between the two time periods (r = 0.072, P=.816).
- The only disease-related exclusion criteria for entry into a clinical trial for antiepileptic drugs that was linked to a drop in the average age of enrolled patients were neurological conditions other than epilepsy.
Desmarais P, Miville C, Milán-Tomás A, et al. Age representation in antiepileptic drug trials: a systematic review and meta-analysis. Epilepsy Res. 2018;142:9-15.
New Definitions for NORSE and FIRES
An international team of experts has published standardized definitions of several seizure-related disorders, including new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related disorders.
- NORSE is described as a clinical presentation rather than a specific diagnosis.
- NORSE occurs in a patient who is not experiencing active epilepsy or any other preexisting neurological disorder.
- In NORSE, the patient does not have a clear acute or active structural, toxic or metabolic cause of their condition.
- The expert group defined FIRES as a subtype of NORSE that involves a prior febrile infection that started between 2 weeks and 24 hours before the refractory status epilepticus began.
- The experts also offered standardized definitions for infantile hemiconvulsion-hemiplegia and epilepsy syndrome (IHHE) and for prolonged, refractory and super-refractory status epilepticus.
Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739-744.
An international team of experts has published standardized definitions of several seizure-related disorders, including new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related disorders.
- NORSE is described as a clinical presentation rather than a specific diagnosis.
- NORSE occurs in a patient who is not experiencing active epilepsy or any other preexisting neurological disorder.
- In NORSE, the patient does not have a clear acute or active structural, toxic or metabolic cause of their condition.
- The expert group defined FIRES as a subtype of NORSE that involves a prior febrile infection that started between 2 weeks and 24 hours before the refractory status epilepticus began.
- The experts also offered standardized definitions for infantile hemiconvulsion-hemiplegia and epilepsy syndrome (IHHE) and for prolonged, refractory and super-refractory status epilepticus.
Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739-744.
An international team of experts has published standardized definitions of several seizure-related disorders, including new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related disorders.
- NORSE is described as a clinical presentation rather than a specific diagnosis.
- NORSE occurs in a patient who is not experiencing active epilepsy or any other preexisting neurological disorder.
- In NORSE, the patient does not have a clear acute or active structural, toxic or metabolic cause of their condition.
- The expert group defined FIRES as a subtype of NORSE that involves a prior febrile infection that started between 2 weeks and 24 hours before the refractory status epilepticus began.
- The experts also offered standardized definitions for infantile hemiconvulsion-hemiplegia and epilepsy syndrome (IHHE) and for prolonged, refractory and super-refractory status epilepticus.
Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739-744.
Understanding and Managing Sunflower Syndrome
Sunflower syndrome, a type of reflex seizure usually accompanied by eyelid myoclonia, can be managed with valproate or polypharmacy suggests a recent study of affected patients managed by the Department of Neurology, Division of Child Neurology, Stanford University School of Medicine.
- Sunflower syndrome, a photosensitive epilepsy, is triggered when patients wave their hands in front of the sun.
- A review of 13 cases, occurring mostly in girls with an average age of onset of 5.5 years, found that many of these patients had intellectual, attentional, or academic problems.
- Most patients had eyelid myoclonia, with or without absence seizures, while six also had spontaneous seizures.
- Nine children received valproate, of which 66% experienced significant improvement or stopped having seizures.
- No patients taking levetiracetam or lamotrigine monotherapy saw their seizures resolve, but 3 patients did improve on polypharmacy.
Baumer FM, Porter BE. Clinical and electrographic features of sunflower syndrome. Epilepsy Res. 2018;142:58-63.
Sunflower syndrome, a type of reflex seizure usually accompanied by eyelid myoclonia, can be managed with valproate or polypharmacy suggests a recent study of affected patients managed by the Department of Neurology, Division of Child Neurology, Stanford University School of Medicine.
- Sunflower syndrome, a photosensitive epilepsy, is triggered when patients wave their hands in front of the sun.
- A review of 13 cases, occurring mostly in girls with an average age of onset of 5.5 years, found that many of these patients had intellectual, attentional, or academic problems.
- Most patients had eyelid myoclonia, with or without absence seizures, while six also had spontaneous seizures.
- Nine children received valproate, of which 66% experienced significant improvement or stopped having seizures.
- No patients taking levetiracetam or lamotrigine monotherapy saw their seizures resolve, but 3 patients did improve on polypharmacy.
Baumer FM, Porter BE. Clinical and electrographic features of sunflower syndrome. Epilepsy Res. 2018;142:58-63.
Sunflower syndrome, a type of reflex seizure usually accompanied by eyelid myoclonia, can be managed with valproate or polypharmacy suggests a recent study of affected patients managed by the Department of Neurology, Division of Child Neurology, Stanford University School of Medicine.
- Sunflower syndrome, a photosensitive epilepsy, is triggered when patients wave their hands in front of the sun.
- A review of 13 cases, occurring mostly in girls with an average age of onset of 5.5 years, found that many of these patients had intellectual, attentional, or academic problems.
- Most patients had eyelid myoclonia, with or without absence seizures, while six also had spontaneous seizures.
- Nine children received valproate, of which 66% experienced significant improvement or stopped having seizures.
- No patients taking levetiracetam or lamotrigine monotherapy saw their seizures resolve, but 3 patients did improve on polypharmacy.
Baumer FM, Porter BE. Clinical and electrographic features of sunflower syndrome. Epilepsy Res. 2018;142:58-63.




