User login
Key to MGUS and myeloma may lie in Iceland
NEW ORLEANS – Everyone aged 40 years and older on the island nation of Iceland is being screened for monoclonal gammopathy of undetermined significance, smoldering myeloma, and full-blown multiple myeloma in an unprecedented project to identify the malignancy’s genetic roots, Joseph Mikhael, MD, said at the annual meeting of the American College of Physicians.
This effort to decode the underlying genetics of multiple myeloma is enormously facilitated by the fact that the DNA sequencing of the entire Icelandic population is already known, and everyone’s blood samples are stored in the national health care system.
The International Myeloma Foundation is funding the Icelandic project, called iStopMM (Iceland Screens Treats or Prevents Multiple Myeloma).
The results of iStopMM could be far reaching, in part because the findings will show whether screening an asymptomatic general population for MGUS – as for example, all American adults – is worthwhile.
In the interim, it’s important for primary care physicians to recognize when it is and isn’t appropriate to order a serum protein electrophoresis (SPE) study, on which the diagnosis of MGUS hinges. It’s also essential to recognize the difference between smoldering and full-blown multiple myeloma, because the distinction has implications for patient monitoring and treatment, added Dr. Mikhael, a hematologist at the City of Hope Cancer Center in Duarte, Calif.
Multiple myeloma accounts for 1% of all cancers and 10% of hematologic malignancies. MGUS is an obligate precursor of multiple myeloma. But MGUS is common, and therein lies a challenge for physicians – as well as a major source of anxiety for many MGUS-positive patients.
In a 12-year-old study, MGUS had a 3% prevalence in the general U.S. population older than age 50 years, and a greater than 5% prevalence after age 70. However, Dr. Mikhael thinks that more refined testing will show the true figure to be close to 10%. Thus, the great majority of patients with MGUS will never develop multiple myeloma.
Pending the potentially practice-changing outcome of iStopMM, Dr. Mikhael said SPE shouldn’t be ordered routinely in an asymptomatic patient, even one with a positive family history for multiple myeloma. The annual cost of monitoring the roughly 540,000 U.S. patients who now carry a diagnosis of MGUS – typically established as an incidental finding by primary care physicians while doing a work-up for another reason – is at least $110 million. And there’s no point in adding to that burden until the benefit of mass screening has been established.
An SPE is appropriate, however, in an older patient with unexplained anemia, known low immunoglobulin levels, unexplained renal insufficiency or neuropathy, or osteopenia or osteoporosis inconsistent with the patient’s age or gender – provided the patient doesn’t have a coexisting plasma cell dyscrasia or B-cell lymphoproliferative disorder, which would throw off the prognostic value of the test results, he continued.
When ordering an SPE to rule in/out MGUS, it’s essential to also order serum free light-chain testing, because it provides key prognostic information.
A landmark study led by investigators at the Mayo Clinic demonstrated that the risk of progression of MGUS to multiple myeloma or a related disorder is independently predicted by three key factors: a high serum M-protein spike of 15 g/L or more on the SPE; the presence of non-IgG MGUS; and an abnormal serum free light-chain ratio of less than 0.26 or more than 1.65. In this study, the 20-year risk of malignant transformation of MGUS ranged from 58% if all three risk factors were present, to just 5% if none were (Blood. 2005 Aug 1;106[3]:812-7).
CRAB vs. SLiM CRAB
Myeloma, smoldering myeloma, and MGUS were redefined a few years ago to reflect differences in prognosis. MGUS still requires the presence of a serum monoclonal protein in a concentration of 3 g/dL or less, less than 10% plasmacytosis in the bone marrow, asymptomatic status, and absence of end-organ damage as traditionally defined in the acronym CRAB (calcium elevation, renal insufficiency, anemia, or bony disease).
If CRAB is present in a patient with at least 10% plasma cells in bone marrow, that is by definition multiple myeloma warranting treatment. Smoldering myeloma requires at least 10% plasmacytosis in bone marrow and absence of the CRAB criteria. However, in a significant change, ultra–high-risk smoldering melanoma, defined by the acronym SLiM CRAB, is now considered active myeloma and should be treated (Lancet Oncol. 2014 Nov;15[12]:e538-48).
“Traditionally, we waited until CRAB [to define myeloma],” Dr. Mikhael explained. “But if you’re running toward a cliff, I don’t have to wait until you’re falling off to know you’re in trouble.”
The SLiM half of SLiM CRAB consists of 60% or more plasmacytosis in bone marrow, light chains in a kappa-to-lambda or lambda-to-kappa ratio of greater than 100, and MRI showing one or more focal lesions. If a patient is SLiM, with or without CRAB, that is now considered active myeloma warranting treatment.
Not all MGUS needs a bone marrow biopsy
A bone marrow biopsy and skeletal survey via whole-body CT or conventional radiographs can be deferred in patients with low-risk MGUS and no bony symptoms. Using the Mayo Clinic risk stratification model, low risk is defined as a serum M protein of 1.5 g/dL or less on SPE, an IgG isotype, and a normal free light-chain ratio.
The lifetime risk of progression in patients with MGUS who meet all three criteria is only about 2%. They can be followed at 6 months with an SPE, free light-chain testing, a CBC, and serum calcium and creatinine, then annually thereafter.
“For those who aren’t in this low-risk category, we actually do need to do a bone marrow test,” according to Dr. Mikhael. “Then, based on that, if they have malignancy, send them to a myeloma geek like me or to another hematologist. And if they don’t have a malignancy, they can be followed at 6 months and then subsequently at least every year.”
Dr. Mikhael has received research grants from AbbVie, Celgene, and Sanofi.
NEW ORLEANS – Everyone aged 40 years and older on the island nation of Iceland is being screened for monoclonal gammopathy of undetermined significance, smoldering myeloma, and full-blown multiple myeloma in an unprecedented project to identify the malignancy’s genetic roots, Joseph Mikhael, MD, said at the annual meeting of the American College of Physicians.
This effort to decode the underlying genetics of multiple myeloma is enormously facilitated by the fact that the DNA sequencing of the entire Icelandic population is already known, and everyone’s blood samples are stored in the national health care system.
The International Myeloma Foundation is funding the Icelandic project, called iStopMM (Iceland Screens Treats or Prevents Multiple Myeloma).
The results of iStopMM could be far reaching, in part because the findings will show whether screening an asymptomatic general population for MGUS – as for example, all American adults – is worthwhile.
In the interim, it’s important for primary care physicians to recognize when it is and isn’t appropriate to order a serum protein electrophoresis (SPE) study, on which the diagnosis of MGUS hinges. It’s also essential to recognize the difference between smoldering and full-blown multiple myeloma, because the distinction has implications for patient monitoring and treatment, added Dr. Mikhael, a hematologist at the City of Hope Cancer Center in Duarte, Calif.
Multiple myeloma accounts for 1% of all cancers and 10% of hematologic malignancies. MGUS is an obligate precursor of multiple myeloma. But MGUS is common, and therein lies a challenge for physicians – as well as a major source of anxiety for many MGUS-positive patients.
In a 12-year-old study, MGUS had a 3% prevalence in the general U.S. population older than age 50 years, and a greater than 5% prevalence after age 70. However, Dr. Mikhael thinks that more refined testing will show the true figure to be close to 10%. Thus, the great majority of patients with MGUS will never develop multiple myeloma.
Pending the potentially practice-changing outcome of iStopMM, Dr. Mikhael said SPE shouldn’t be ordered routinely in an asymptomatic patient, even one with a positive family history for multiple myeloma. The annual cost of monitoring the roughly 540,000 U.S. patients who now carry a diagnosis of MGUS – typically established as an incidental finding by primary care physicians while doing a work-up for another reason – is at least $110 million. And there’s no point in adding to that burden until the benefit of mass screening has been established.
An SPE is appropriate, however, in an older patient with unexplained anemia, known low immunoglobulin levels, unexplained renal insufficiency or neuropathy, or osteopenia or osteoporosis inconsistent with the patient’s age or gender – provided the patient doesn’t have a coexisting plasma cell dyscrasia or B-cell lymphoproliferative disorder, which would throw off the prognostic value of the test results, he continued.
When ordering an SPE to rule in/out MGUS, it’s essential to also order serum free light-chain testing, because it provides key prognostic information.
A landmark study led by investigators at the Mayo Clinic demonstrated that the risk of progression of MGUS to multiple myeloma or a related disorder is independently predicted by three key factors: a high serum M-protein spike of 15 g/L or more on the SPE; the presence of non-IgG MGUS; and an abnormal serum free light-chain ratio of less than 0.26 or more than 1.65. In this study, the 20-year risk of malignant transformation of MGUS ranged from 58% if all three risk factors were present, to just 5% if none were (Blood. 2005 Aug 1;106[3]:812-7).
CRAB vs. SLiM CRAB
Myeloma, smoldering myeloma, and MGUS were redefined a few years ago to reflect differences in prognosis. MGUS still requires the presence of a serum monoclonal protein in a concentration of 3 g/dL or less, less than 10% plasmacytosis in the bone marrow, asymptomatic status, and absence of end-organ damage as traditionally defined in the acronym CRAB (calcium elevation, renal insufficiency, anemia, or bony disease).
If CRAB is present in a patient with at least 10% plasma cells in bone marrow, that is by definition multiple myeloma warranting treatment. Smoldering myeloma requires at least 10% plasmacytosis in bone marrow and absence of the CRAB criteria. However, in a significant change, ultra–high-risk smoldering melanoma, defined by the acronym SLiM CRAB, is now considered active myeloma and should be treated (Lancet Oncol. 2014 Nov;15[12]:e538-48).
“Traditionally, we waited until CRAB [to define myeloma],” Dr. Mikhael explained. “But if you’re running toward a cliff, I don’t have to wait until you’re falling off to know you’re in trouble.”
The SLiM half of SLiM CRAB consists of 60% or more plasmacytosis in bone marrow, light chains in a kappa-to-lambda or lambda-to-kappa ratio of greater than 100, and MRI showing one or more focal lesions. If a patient is SLiM, with or without CRAB, that is now considered active myeloma warranting treatment.
Not all MGUS needs a bone marrow biopsy
A bone marrow biopsy and skeletal survey via whole-body CT or conventional radiographs can be deferred in patients with low-risk MGUS and no bony symptoms. Using the Mayo Clinic risk stratification model, low risk is defined as a serum M protein of 1.5 g/dL or less on SPE, an IgG isotype, and a normal free light-chain ratio.
The lifetime risk of progression in patients with MGUS who meet all three criteria is only about 2%. They can be followed at 6 months with an SPE, free light-chain testing, a CBC, and serum calcium and creatinine, then annually thereafter.
“For those who aren’t in this low-risk category, we actually do need to do a bone marrow test,” according to Dr. Mikhael. “Then, based on that, if they have malignancy, send them to a myeloma geek like me or to another hematologist. And if they don’t have a malignancy, they can be followed at 6 months and then subsequently at least every year.”
Dr. Mikhael has received research grants from AbbVie, Celgene, and Sanofi.
NEW ORLEANS – Everyone aged 40 years and older on the island nation of Iceland is being screened for monoclonal gammopathy of undetermined significance, smoldering myeloma, and full-blown multiple myeloma in an unprecedented project to identify the malignancy’s genetic roots, Joseph Mikhael, MD, said at the annual meeting of the American College of Physicians.
This effort to decode the underlying genetics of multiple myeloma is enormously facilitated by the fact that the DNA sequencing of the entire Icelandic population is already known, and everyone’s blood samples are stored in the national health care system.
The International Myeloma Foundation is funding the Icelandic project, called iStopMM (Iceland Screens Treats or Prevents Multiple Myeloma).
The results of iStopMM could be far reaching, in part because the findings will show whether screening an asymptomatic general population for MGUS – as for example, all American adults – is worthwhile.
In the interim, it’s important for primary care physicians to recognize when it is and isn’t appropriate to order a serum protein electrophoresis (SPE) study, on which the diagnosis of MGUS hinges. It’s also essential to recognize the difference between smoldering and full-blown multiple myeloma, because the distinction has implications for patient monitoring and treatment, added Dr. Mikhael, a hematologist at the City of Hope Cancer Center in Duarte, Calif.
Multiple myeloma accounts for 1% of all cancers and 10% of hematologic malignancies. MGUS is an obligate precursor of multiple myeloma. But MGUS is common, and therein lies a challenge for physicians – as well as a major source of anxiety for many MGUS-positive patients.
In a 12-year-old study, MGUS had a 3% prevalence in the general U.S. population older than age 50 years, and a greater than 5% prevalence after age 70. However, Dr. Mikhael thinks that more refined testing will show the true figure to be close to 10%. Thus, the great majority of patients with MGUS will never develop multiple myeloma.
Pending the potentially practice-changing outcome of iStopMM, Dr. Mikhael said SPE shouldn’t be ordered routinely in an asymptomatic patient, even one with a positive family history for multiple myeloma. The annual cost of monitoring the roughly 540,000 U.S. patients who now carry a diagnosis of MGUS – typically established as an incidental finding by primary care physicians while doing a work-up for another reason – is at least $110 million. And there’s no point in adding to that burden until the benefit of mass screening has been established.
An SPE is appropriate, however, in an older patient with unexplained anemia, known low immunoglobulin levels, unexplained renal insufficiency or neuropathy, or osteopenia or osteoporosis inconsistent with the patient’s age or gender – provided the patient doesn’t have a coexisting plasma cell dyscrasia or B-cell lymphoproliferative disorder, which would throw off the prognostic value of the test results, he continued.
When ordering an SPE to rule in/out MGUS, it’s essential to also order serum free light-chain testing, because it provides key prognostic information.
A landmark study led by investigators at the Mayo Clinic demonstrated that the risk of progression of MGUS to multiple myeloma or a related disorder is independently predicted by three key factors: a high serum M-protein spike of 15 g/L or more on the SPE; the presence of non-IgG MGUS; and an abnormal serum free light-chain ratio of less than 0.26 or more than 1.65. In this study, the 20-year risk of malignant transformation of MGUS ranged from 58% if all three risk factors were present, to just 5% if none were (Blood. 2005 Aug 1;106[3]:812-7).
CRAB vs. SLiM CRAB
Myeloma, smoldering myeloma, and MGUS were redefined a few years ago to reflect differences in prognosis. MGUS still requires the presence of a serum monoclonal protein in a concentration of 3 g/dL or less, less than 10% plasmacytosis in the bone marrow, asymptomatic status, and absence of end-organ damage as traditionally defined in the acronym CRAB (calcium elevation, renal insufficiency, anemia, or bony disease).
If CRAB is present in a patient with at least 10% plasma cells in bone marrow, that is by definition multiple myeloma warranting treatment. Smoldering myeloma requires at least 10% plasmacytosis in bone marrow and absence of the CRAB criteria. However, in a significant change, ultra–high-risk smoldering melanoma, defined by the acronym SLiM CRAB, is now considered active myeloma and should be treated (Lancet Oncol. 2014 Nov;15[12]:e538-48).
“Traditionally, we waited until CRAB [to define myeloma],” Dr. Mikhael explained. “But if you’re running toward a cliff, I don’t have to wait until you’re falling off to know you’re in trouble.”
The SLiM half of SLiM CRAB consists of 60% or more plasmacytosis in bone marrow, light chains in a kappa-to-lambda or lambda-to-kappa ratio of greater than 100, and MRI showing one or more focal lesions. If a patient is SLiM, with or without CRAB, that is now considered active myeloma warranting treatment.
Not all MGUS needs a bone marrow biopsy
A bone marrow biopsy and skeletal survey via whole-body CT or conventional radiographs can be deferred in patients with low-risk MGUS and no bony symptoms. Using the Mayo Clinic risk stratification model, low risk is defined as a serum M protein of 1.5 g/dL or less on SPE, an IgG isotype, and a normal free light-chain ratio.
The lifetime risk of progression in patients with MGUS who meet all three criteria is only about 2%. They can be followed at 6 months with an SPE, free light-chain testing, a CBC, and serum calcium and creatinine, then annually thereafter.
“For those who aren’t in this low-risk category, we actually do need to do a bone marrow test,” according to Dr. Mikhael. “Then, based on that, if they have malignancy, send them to a myeloma geek like me or to another hematologist. And if they don’t have a malignancy, they can be followed at 6 months and then subsequently at least every year.”
Dr. Mikhael has received research grants from AbbVie, Celgene, and Sanofi.
EXPERT ANALYSIS FROM ACP INTERNAL MEDICINE
Noninvasive cardiac testing to rule out acute coronary syndromes provides no benefit in low-risk chest pain patients
Background: The 2014 American College of Cardiology/American Heart Association clinical guideline includes a recommendation for noninvasive testing (exercise test or coronary commuted tomographic angiography [CCTA]) in patients with chest pain but no evidence of ischemia. The ROMICAT-II (Rule Out Myocardial Ischemia/Infarction by Computer Assisted Tomography) trial randomized 1,000 ED patients with chest pain to undergo CCTA or usual care with unknown benefits and risks of this testing recommendation. The question of whether this testing may be omitted is addressed in secondary analysis.
Study design: Retrospective analysis of the ROMICAT-II trial data.
Setting: Emergency department.
Synopsis: As compared with 882 patients who underwent noninvasive testing, 118 patients in the ROMICAT-II usual care group who did not undergo noninvasive testing had shorter lengths of stay (20.3 vs. 27.9 hours; P less than .001), lower rates of diagnostic testing (P less than .001), angiography (2% vs. 11%; P less than .001), and lower costs ($2,261.50 vs. $2,584.30). There was no difference in percutaneous coronary intervention (2% vs. 5%; P = .15), coronary artery bypass surgery (0% vs. 1%; P = .61), major adverse cardiac events (MACE; 2% vs. 1%; P = .24), or return ED visits (5.8% vs. 2.8%; P = .08) during the 28-day follow-up period. These findings suggest noninvasive cardiac testing may be omitted in low- to intermediate-risk patients presenting with chest pain. However, this study was not designed or powered to address this question and patients were not randomized. These data may support hospitalists who choose not to order noninvasive testing in ED patients with chest pain.
Bottom line: In this secondary analysis of ROMICAT-II clinical trial data, patients who underwent clinical evaluation without noninvasive testing had shorter length of stay, less diagnostic testing, lower radiation exposure, and reduced costs with no difference in missed diagnosis of acute coronary syndromes, development of MACE, or return ED visits.
Citation: Reinhardt SW et al. Noninvasive cardiac testing vs. clinical evaluation alone in acute chest pain: A secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018 Feb 1;178(2):212-9.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: The 2014 American College of Cardiology/American Heart Association clinical guideline includes a recommendation for noninvasive testing (exercise test or coronary commuted tomographic angiography [CCTA]) in patients with chest pain but no evidence of ischemia. The ROMICAT-II (Rule Out Myocardial Ischemia/Infarction by Computer Assisted Tomography) trial randomized 1,000 ED patients with chest pain to undergo CCTA or usual care with unknown benefits and risks of this testing recommendation. The question of whether this testing may be omitted is addressed in secondary analysis.
Study design: Retrospective analysis of the ROMICAT-II trial data.
Setting: Emergency department.
Synopsis: As compared with 882 patients who underwent noninvasive testing, 118 patients in the ROMICAT-II usual care group who did not undergo noninvasive testing had shorter lengths of stay (20.3 vs. 27.9 hours; P less than .001), lower rates of diagnostic testing (P less than .001), angiography (2% vs. 11%; P less than .001), and lower costs ($2,261.50 vs. $2,584.30). There was no difference in percutaneous coronary intervention (2% vs. 5%; P = .15), coronary artery bypass surgery (0% vs. 1%; P = .61), major adverse cardiac events (MACE; 2% vs. 1%; P = .24), or return ED visits (5.8% vs. 2.8%; P = .08) during the 28-day follow-up period. These findings suggest noninvasive cardiac testing may be omitted in low- to intermediate-risk patients presenting with chest pain. However, this study was not designed or powered to address this question and patients were not randomized. These data may support hospitalists who choose not to order noninvasive testing in ED patients with chest pain.
Bottom line: In this secondary analysis of ROMICAT-II clinical trial data, patients who underwent clinical evaluation without noninvasive testing had shorter length of stay, less diagnostic testing, lower radiation exposure, and reduced costs with no difference in missed diagnosis of acute coronary syndromes, development of MACE, or return ED visits.
Citation: Reinhardt SW et al. Noninvasive cardiac testing vs. clinical evaluation alone in acute chest pain: A secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018 Feb 1;178(2):212-9.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: The 2014 American College of Cardiology/American Heart Association clinical guideline includes a recommendation for noninvasive testing (exercise test or coronary commuted tomographic angiography [CCTA]) in patients with chest pain but no evidence of ischemia. The ROMICAT-II (Rule Out Myocardial Ischemia/Infarction by Computer Assisted Tomography) trial randomized 1,000 ED patients with chest pain to undergo CCTA or usual care with unknown benefits and risks of this testing recommendation. The question of whether this testing may be omitted is addressed in secondary analysis.
Study design: Retrospective analysis of the ROMICAT-II trial data.
Setting: Emergency department.
Synopsis: As compared with 882 patients who underwent noninvasive testing, 118 patients in the ROMICAT-II usual care group who did not undergo noninvasive testing had shorter lengths of stay (20.3 vs. 27.9 hours; P less than .001), lower rates of diagnostic testing (P less than .001), angiography (2% vs. 11%; P less than .001), and lower costs ($2,261.50 vs. $2,584.30). There was no difference in percutaneous coronary intervention (2% vs. 5%; P = .15), coronary artery bypass surgery (0% vs. 1%; P = .61), major adverse cardiac events (MACE; 2% vs. 1%; P = .24), or return ED visits (5.8% vs. 2.8%; P = .08) during the 28-day follow-up period. These findings suggest noninvasive cardiac testing may be omitted in low- to intermediate-risk patients presenting with chest pain. However, this study was not designed or powered to address this question and patients were not randomized. These data may support hospitalists who choose not to order noninvasive testing in ED patients with chest pain.
Bottom line: In this secondary analysis of ROMICAT-II clinical trial data, patients who underwent clinical evaluation without noninvasive testing had shorter length of stay, less diagnostic testing, lower radiation exposure, and reduced costs with no difference in missed diagnosis of acute coronary syndromes, development of MACE, or return ED visits.
Citation: Reinhardt SW et al. Noninvasive cardiac testing vs. clinical evaluation alone in acute chest pain: A secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018 Feb 1;178(2):212-9.
Dr. Moulder is assistant professor, University of Virginia Health System.
Medicare sets outpatient CAR T-cell therapy rates
Officials at the Centers for Medicare & Medicaid Services announced reimbursement rates for outpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, settling on a fee that is roughly the wholesale acquisition cost plus 6%.
The agency will pay $395,380 to those who administer axicabtagene ciloleucel (Yescarta) on an outpatient basis and $500,839 for outpatient use of tisagenlecleucel (Kymriah), a similar CAR T-cell therapy for cancer patients. The two drugs have list prices of $373,000 and $475,000, respectively.
Although CMS set the Medicare Part B copayment for axicabtagene ciloleucel at $79,076, the agency later clarified that out-of-pocket expenses for Medicare patients are capped at around $1,340 in 2018 – the amount of the inpatient hospital deductible.
The Medicare rate is a first step to being able to use and get paid for CAR T-cell therapies, said Richard T. Maziarz, MD, a bone marrow transplantation and blood cancer specialist at the Oregon Health and Science University Knight Cancer Institute in Portland. However, the rate is not as straight forward as it sounds, he pointed out.
“This is big news,” Dr. Maziarz said in an interview. “This allows us to start to deliver these therapies, but there is a risk: If we give this in the outpatient setting and someone ends up needing hospitalization, we may end up not being reimbursed for the drug product.”
That’s because of CMS’s 3-day payment window rule, Dr. Maziarz explained: If medical treatment is provided in an outpatient setting and the patient needs inpatient care within 72 hours, all payments prior to that 72-hour window become part of the inpatient stay, according to the rule. Inpatient reimbursement varies depending on treatment, previous patient comorbidities, and complications during their stay. Analysts say that inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted so doctors can monitor them for serious side effects.
“If I have a drug that costs me $373,000, what happens if I admit the patient?” Dr. Maziarz said. “I don’t get $373,000; I get between $8,000 and $18,000. So if we give this and someone gets sick in 48 hours, then we may be at risk for losing.”
On the inpatient side, Medicare payment for CAR T-cell therapy is currently bundled into the payment for the inpatient stay, rather than being paid separately. The drug manufacturers – Gilead Sciences and Novartis AG – have requested that CMS set a separate “new technology add-on payment,” but the agency has not yet issued a decision.
Further advancements are expected for CAR T-cell therapies in 2018, said Cai Xuan, PhD, senior analyst in oncology and hematology for GlobalData, a data analytics and commercial intelligence firm.
For starters, pharmaceutical companies are now working toward next-generation CAR T-cell therapies that can be mass produced, Ms. Xuan noted. At a recent American Association for Cancer Research meeting, for example, the biopharmaceutical company Cellectis presented early clinical data in pediatric B-cell acute lymphoblastic leukemia for its off-the-shelf CAR T-cell candidate UCART19. Additionally, CRISPR Therapeutics presented preclinical data for one of its off-the-shelf CAR T-cell candidates for multiple myeloma, and the company announced it would apply for approval to start human trials by the end of 2018.
“The trend for 2018 is very much focusing on how to eliminate some of the profitability issues with first-generation CAR Ts because companies realize that manufacturing individualized treatments for each patient is not an ideal business model,” Dr. Xuan said in an interview.
More market competition is also in the forecast, particularly from smaller companies, Dr. Xuan said.
“What we are likely to see in the future is larger companies acquiring smaller ones once their CAR T technology has matured to a certain point,” she said. “We have seen it with the Gilead-Kite acquisition, as well as Celgene’s acquisition of Juno Therapeutics, and this trend will continue as long as smaller companies are able to develop proprietary next-generation CAR T technologies.”
Cost and accessibility remain key concerns with the therapies, especially for the Medicare population. Cost estimates have put the cost of CAR T-cell therapies as high as $1.5 million per patient.
“There remain unanswered questions about value and cost in older adults,” said Walid F. Gellad, MD, codirector for the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh. “There are many life-saving treatments in the medical system that cost much less than this therapy. Presumably, its cost will go down as the indications expand and the experience with creating the CAR T cells improves. At least, one would hope.”
“In the longer term, there’s obviously a lot of people looking at how [the treatments] can be made more accessible,” she said in an interview. “These are the first generation CAR T [products], and I think there’ll be lots of refinements – both to make them more effective and safer, but also eventually with trying to use a third party product – to bring the cost of goods down.”
Other lingering unknowns about CAR T-cell therapies include how many patients in real-world clinical practice will have serious side effects, compared with those in trials, and the long-term recurrence rates after therapy use, Dr. Gellad noted. A recent paper by Dr. Gellad in the New England Journal of Medicine proposes that government payers reimburse only the cost of manufacturing and some predetermined mark-up for such therapies until confirmatory trials demonstrate clinical benefit (N Engl J Med. 2017;376[21]:2001-04).
Time will tell how reimbursement plays out during clinical practice, but one thing is for certain: The current CAR T-cell therapies are only the beginning, Dr. Maziarz said.
“Genetically-engineered cell products are going to explode over the next decade,” he said. “This is not the end of the line, this is the starting point.”
Dr. Maziarz has received consulting fees from Novartis, Juno Therapeutics, and Kite Pharma. Dr. Heslop has received consulting fees from Novartis, has conducted research for Cell Medica and holds intellectual property rights/patents from Cell Medica, and has ownership interest in ViraCyte and Marker Therapeutics. Dr. Gellad reports grants from Express Scripts.
Officials at the Centers for Medicare & Medicaid Services announced reimbursement rates for outpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, settling on a fee that is roughly the wholesale acquisition cost plus 6%.
The agency will pay $395,380 to those who administer axicabtagene ciloleucel (Yescarta) on an outpatient basis and $500,839 for outpatient use of tisagenlecleucel (Kymriah), a similar CAR T-cell therapy for cancer patients. The two drugs have list prices of $373,000 and $475,000, respectively.
Although CMS set the Medicare Part B copayment for axicabtagene ciloleucel at $79,076, the agency later clarified that out-of-pocket expenses for Medicare patients are capped at around $1,340 in 2018 – the amount of the inpatient hospital deductible.
The Medicare rate is a first step to being able to use and get paid for CAR T-cell therapies, said Richard T. Maziarz, MD, a bone marrow transplantation and blood cancer specialist at the Oregon Health and Science University Knight Cancer Institute in Portland. However, the rate is not as straight forward as it sounds, he pointed out.
“This is big news,” Dr. Maziarz said in an interview. “This allows us to start to deliver these therapies, but there is a risk: If we give this in the outpatient setting and someone ends up needing hospitalization, we may end up not being reimbursed for the drug product.”
That’s because of CMS’s 3-day payment window rule, Dr. Maziarz explained: If medical treatment is provided in an outpatient setting and the patient needs inpatient care within 72 hours, all payments prior to that 72-hour window become part of the inpatient stay, according to the rule. Inpatient reimbursement varies depending on treatment, previous patient comorbidities, and complications during their stay. Analysts say that inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted so doctors can monitor them for serious side effects.
“If I have a drug that costs me $373,000, what happens if I admit the patient?” Dr. Maziarz said. “I don’t get $373,000; I get between $8,000 and $18,000. So if we give this and someone gets sick in 48 hours, then we may be at risk for losing.”
On the inpatient side, Medicare payment for CAR T-cell therapy is currently bundled into the payment for the inpatient stay, rather than being paid separately. The drug manufacturers – Gilead Sciences and Novartis AG – have requested that CMS set a separate “new technology add-on payment,” but the agency has not yet issued a decision.
Further advancements are expected for CAR T-cell therapies in 2018, said Cai Xuan, PhD, senior analyst in oncology and hematology for GlobalData, a data analytics and commercial intelligence firm.
For starters, pharmaceutical companies are now working toward next-generation CAR T-cell therapies that can be mass produced, Ms. Xuan noted. At a recent American Association for Cancer Research meeting, for example, the biopharmaceutical company Cellectis presented early clinical data in pediatric B-cell acute lymphoblastic leukemia for its off-the-shelf CAR T-cell candidate UCART19. Additionally, CRISPR Therapeutics presented preclinical data for one of its off-the-shelf CAR T-cell candidates for multiple myeloma, and the company announced it would apply for approval to start human trials by the end of 2018.
“The trend for 2018 is very much focusing on how to eliminate some of the profitability issues with first-generation CAR Ts because companies realize that manufacturing individualized treatments for each patient is not an ideal business model,” Dr. Xuan said in an interview.
More market competition is also in the forecast, particularly from smaller companies, Dr. Xuan said.
“What we are likely to see in the future is larger companies acquiring smaller ones once their CAR T technology has matured to a certain point,” she said. “We have seen it with the Gilead-Kite acquisition, as well as Celgene’s acquisition of Juno Therapeutics, and this trend will continue as long as smaller companies are able to develop proprietary next-generation CAR T technologies.”
Cost and accessibility remain key concerns with the therapies, especially for the Medicare population. Cost estimates have put the cost of CAR T-cell therapies as high as $1.5 million per patient.
“There remain unanswered questions about value and cost in older adults,” said Walid F. Gellad, MD, codirector for the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh. “There are many life-saving treatments in the medical system that cost much less than this therapy. Presumably, its cost will go down as the indications expand and the experience with creating the CAR T cells improves. At least, one would hope.”
“In the longer term, there’s obviously a lot of people looking at how [the treatments] can be made more accessible,” she said in an interview. “These are the first generation CAR T [products], and I think there’ll be lots of refinements – both to make them more effective and safer, but also eventually with trying to use a third party product – to bring the cost of goods down.”
Other lingering unknowns about CAR T-cell therapies include how many patients in real-world clinical practice will have serious side effects, compared with those in trials, and the long-term recurrence rates after therapy use, Dr. Gellad noted. A recent paper by Dr. Gellad in the New England Journal of Medicine proposes that government payers reimburse only the cost of manufacturing and some predetermined mark-up for such therapies until confirmatory trials demonstrate clinical benefit (N Engl J Med. 2017;376[21]:2001-04).
Time will tell how reimbursement plays out during clinical practice, but one thing is for certain: The current CAR T-cell therapies are only the beginning, Dr. Maziarz said.
“Genetically-engineered cell products are going to explode over the next decade,” he said. “This is not the end of the line, this is the starting point.”
Dr. Maziarz has received consulting fees from Novartis, Juno Therapeutics, and Kite Pharma. Dr. Heslop has received consulting fees from Novartis, has conducted research for Cell Medica and holds intellectual property rights/patents from Cell Medica, and has ownership interest in ViraCyte and Marker Therapeutics. Dr. Gellad reports grants from Express Scripts.
Officials at the Centers for Medicare & Medicaid Services announced reimbursement rates for outpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, settling on a fee that is roughly the wholesale acquisition cost plus 6%.
The agency will pay $395,380 to those who administer axicabtagene ciloleucel (Yescarta) on an outpatient basis and $500,839 for outpatient use of tisagenlecleucel (Kymriah), a similar CAR T-cell therapy for cancer patients. The two drugs have list prices of $373,000 and $475,000, respectively.
Although CMS set the Medicare Part B copayment for axicabtagene ciloleucel at $79,076, the agency later clarified that out-of-pocket expenses for Medicare patients are capped at around $1,340 in 2018 – the amount of the inpatient hospital deductible.
The Medicare rate is a first step to being able to use and get paid for CAR T-cell therapies, said Richard T. Maziarz, MD, a bone marrow transplantation and blood cancer specialist at the Oregon Health and Science University Knight Cancer Institute in Portland. However, the rate is not as straight forward as it sounds, he pointed out.
“This is big news,” Dr. Maziarz said in an interview. “This allows us to start to deliver these therapies, but there is a risk: If we give this in the outpatient setting and someone ends up needing hospitalization, we may end up not being reimbursed for the drug product.”
That’s because of CMS’s 3-day payment window rule, Dr. Maziarz explained: If medical treatment is provided in an outpatient setting and the patient needs inpatient care within 72 hours, all payments prior to that 72-hour window become part of the inpatient stay, according to the rule. Inpatient reimbursement varies depending on treatment, previous patient comorbidities, and complications during their stay. Analysts say that inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted so doctors can monitor them for serious side effects.
“If I have a drug that costs me $373,000, what happens if I admit the patient?” Dr. Maziarz said. “I don’t get $373,000; I get between $8,000 and $18,000. So if we give this and someone gets sick in 48 hours, then we may be at risk for losing.”
On the inpatient side, Medicare payment for CAR T-cell therapy is currently bundled into the payment for the inpatient stay, rather than being paid separately. The drug manufacturers – Gilead Sciences and Novartis AG – have requested that CMS set a separate “new technology add-on payment,” but the agency has not yet issued a decision.
Further advancements are expected for CAR T-cell therapies in 2018, said Cai Xuan, PhD, senior analyst in oncology and hematology for GlobalData, a data analytics and commercial intelligence firm.
For starters, pharmaceutical companies are now working toward next-generation CAR T-cell therapies that can be mass produced, Ms. Xuan noted. At a recent American Association for Cancer Research meeting, for example, the biopharmaceutical company Cellectis presented early clinical data in pediatric B-cell acute lymphoblastic leukemia for its off-the-shelf CAR T-cell candidate UCART19. Additionally, CRISPR Therapeutics presented preclinical data for one of its off-the-shelf CAR T-cell candidates for multiple myeloma, and the company announced it would apply for approval to start human trials by the end of 2018.
“The trend for 2018 is very much focusing on how to eliminate some of the profitability issues with first-generation CAR Ts because companies realize that manufacturing individualized treatments for each patient is not an ideal business model,” Dr. Xuan said in an interview.
More market competition is also in the forecast, particularly from smaller companies, Dr. Xuan said.
“What we are likely to see in the future is larger companies acquiring smaller ones once their CAR T technology has matured to a certain point,” she said. “We have seen it with the Gilead-Kite acquisition, as well as Celgene’s acquisition of Juno Therapeutics, and this trend will continue as long as smaller companies are able to develop proprietary next-generation CAR T technologies.”
Cost and accessibility remain key concerns with the therapies, especially for the Medicare population. Cost estimates have put the cost of CAR T-cell therapies as high as $1.5 million per patient.
“There remain unanswered questions about value and cost in older adults,” said Walid F. Gellad, MD, codirector for the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh. “There are many life-saving treatments in the medical system that cost much less than this therapy. Presumably, its cost will go down as the indications expand and the experience with creating the CAR T cells improves. At least, one would hope.”
“In the longer term, there’s obviously a lot of people looking at how [the treatments] can be made more accessible,” she said in an interview. “These are the first generation CAR T [products], and I think there’ll be lots of refinements – both to make them more effective and safer, but also eventually with trying to use a third party product – to bring the cost of goods down.”
Other lingering unknowns about CAR T-cell therapies include how many patients in real-world clinical practice will have serious side effects, compared with those in trials, and the long-term recurrence rates after therapy use, Dr. Gellad noted. A recent paper by Dr. Gellad in the New England Journal of Medicine proposes that government payers reimburse only the cost of manufacturing and some predetermined mark-up for such therapies until confirmatory trials demonstrate clinical benefit (N Engl J Med. 2017;376[21]:2001-04).
Time will tell how reimbursement plays out during clinical practice, but one thing is for certain: The current CAR T-cell therapies are only the beginning, Dr. Maziarz said.
“Genetically-engineered cell products are going to explode over the next decade,” he said. “This is not the end of the line, this is the starting point.”
Dr. Maziarz has received consulting fees from Novartis, Juno Therapeutics, and Kite Pharma. Dr. Heslop has received consulting fees from Novartis, has conducted research for Cell Medica and holds intellectual property rights/patents from Cell Medica, and has ownership interest in ViraCyte and Marker Therapeutics. Dr. Gellad reports grants from Express Scripts.
Female physicians face enduring wage gap
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.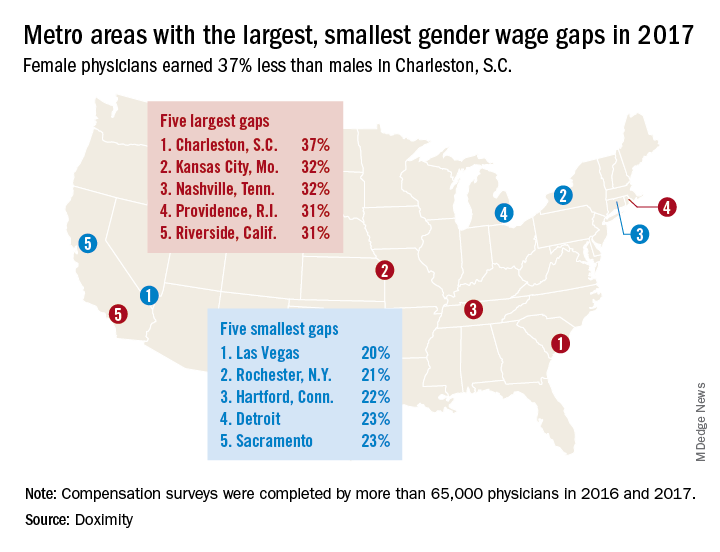
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Rapid Development of Life-Threatening Emamectin Benzoate Poisoning
Emamectin benzoate (EB) is a semisynthetic derivative of avermectin that has acaricidal, nematicidal, and insecticidal action. Avermectin analogs are natural products from soil fungi (Streptomyces avermitilis).1 Emamectin benzoate was initially developed to eradicate lepidopteran larvae, particularly armyworms, and is registered in the United States and Japan for use on vegetable crops.2-4 In addition to its agricultural use, EB also has antiparasitic effects on sea lice (Lepeophtheirus salmonis) that affect Atlantic salmon, and has been registered for use in several countries since 1999.5-7 Although a few studies have evaluated the toxic effects of avermectin on humans, there is a paucity of information regarding human toxicity associated with EB.7 This case report describes rapid deterioration of a patient following ingestion of EB.
Case
A 75-year-old man presented to the ED 20 minutes after intentionally ingesting an agricultural insecticide. Upon presentation, the patient stated that he drank a whole bottle (100 mL) of insecticide after consuming alcohol, but denied coingestion of other toxic substances or any medications. The patient provided the empty bottle upon presentation, and the ingested product was identified as Affirm, an insecticide containing 2.15% EB as the active ingredient.
The patient’s medical history was significant for major depressive disorder, for which he was on alprazolam, donepezil, paroxetine, and quetiapine. The patient stated that he also suffered from chronic back pain, noting that he only took analgesics intermittently as needed.
On examination, the patient was alert and oriented to time and place. Initially, he did not experience any physical discomfort. His vital signs were: blood pressure (BP), 126/74 mm Hg; pulse rate, 67 beats/minute; respiratory rate, mildly tachypneic at 23 breaths/minute; and temperature, 97.9°F. Oxygen saturation was 96% on room air.
Ocular examination revealed both pupils to be equally round, 3 mm in diameter, and reactive to light. Examination of the oropharynx was normal and without signs of mucosal injury. The lung sounds were clear bilaterally, and the heart was a regular rate and rhythm and without murmur. The patient’s abdomen was soft and nontender. No deficits, such as ataxia, dysarthria, or tremor were found on the neurological examination.
Prompt gastric lavage via a nasogastric tube was performed, and activated charcoal was administered. Laboratory evaluation was significant for the following: white blood cell count, 22.77 x 109/L with 78% neutrophils and 16% lymphocytes; sodium, 138 mEq/L; potassium, 3.1 mEq/L; chloride, 109 mEq/L; blood urea nitrogen, 19 mg/dL; and creatinine, 0.7 mg/dL. Arterial blood gas (ABG) results revealed a pH, 7.37; partial pressure of carbon dioxide, 25 mm Hg; partial pressure of oxygen, 93 mm Hg; bicarbonate, 14.5 mEq/L; base excess, –8.9 mEq/L; and an oxygen saturation, 97%. Serum creatine kinase (CK), CK-MB and troponin levels were both within normal range. Lactic acid, serum osmolality, and serum ethanol levels were not obtained. The patient’s electrocardiogram (ECG) and chest radiograph findings were normal.
Approximately 1 hour after presentation, the patient complained of an epigastric burning sensation and continued to exhibit mild tachypnea. A subsequent ABG test revealed progressive metabolic acidosis (Table). Although the patient was given a total of 800 mL of normal saline intravenously (IV) upon arrival at the ED, his total urinary output was less than 100 mL 7 hours afterward. Attempts to increase urinary output with IV furosemide were ineffective.
Along with the progressive metabolic acidosis, the patient became hypotensive, and did not respond to IV fluid resuscitation. A norepinephrine infusion was started to improve BP, but this was likewise ineffective. Serial ECGs did not show any specific abnormalities such as dysrhythmia or ischemia.
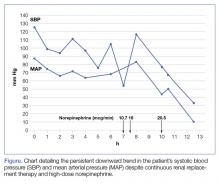
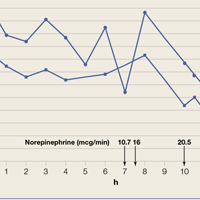
The patient was admitted to the intensive care unit approximately 10.5 hours after presentation where he received continuous renal replacement therapy (CRRT) to correct the severe metabolic acidosis and poor circulation. Metabolic acidosis persisted despite CRRT, and the patient remained hypotensive even after receiving high-dose IV norepinephrine (Figure).
About 12.5 hours after his presentation to the ED, the patient began to vomit profusely and went into cardiac arrest. The cardiac monitor demonstrated pulseless ventricular tachycardia. Aggressive resuscitative efforts were initiated, but failed to restore spontaneous circulation.
Discussion
As an avermectin analog, EB interacts with γ-aminobutyric acid (GABA) receptors and enhances membrane chloride permeability.8 In mammals, GABA-containing neurons and receptors are found in the central nervous system (CNS), but not in the peripheral nervous system. In cases of high-dose avermectin ingestion in humans, CNS toxicity, including agitation and depressed mental status, have been reported, as well as death resulting from respiratory failure.9
With respect to human EB toxicity, there is only one other documented case in the literature by Yen and Lin.7 In their case, the authors report on a patient who ingested 100 mL of Proclaim, which contained 2.15% EB diluted with 400 mL of tap water.7 They note that the patient in their case presented with mild confusion and gastrointestinal (GI) symptoms of nausea, vomiting, and cramping discomfort. Following laboratory and radiological investigation, the patient was found to have aspiration pneumonitis and admitted to the inpatient hospital. On hospital day 2, the patient’s GI symptoms abated and he became alert and oriented. He was discharged 1 week from initial presentation and experienced no sequelae.
In our case, the patient ingested 100 mL of 2.15% EB without dilution. He also experienced GI symptoms, but did not have any CNS depression. The metabolic acidosis rapidly worsened, and could not be corrected, even with intensive therapy. This rapid life-threatening course has not previously been reported with avermectin or EB poisoning. In the avermectin poisoning cases in the literature, seven out of 19 patients (37%) exhibited severe effects, such as hypotension, coma, and aspiration with respiratory failure.9 Six of the seven patients experienced a full recovery; the remaining patient died 18 days after ingestion from multiple organ failure.
The reason for our patient’s rapid progression to metabolic acidosis and progressive deterioration (hypotension and hypoxemia) is not clear. One possible theory is that the solvents or other additives aside from EB in the ingested insecticide might make EB more toxic. In our case, the patient’s rapid deterioration alone or asphyxia by vomitus might have been the cause of the cardiac arrest. Future reports and studies about EB toxicity in humans are warranted to investigate the pathogenesis of toxicity and appropriate treatment.
Conclusion
This is the first report of a human death caused by EB poisoning; the patient experienced severe metabolic acidosis without CNS depression, ultimately leading to death. Emergency physicians should be aware of the possibility of rapid deterioration in patients who present after ingestion of EB and related substances.
1. Lasota JA, Dybas RA. Avermectins, a novel class of compounds: implications for use in arthropod pest control. Annu Rev Entomol. 1991;36:91-117. doi:10.1146/annurev.en.36.010191.000515.
2. Kuo JN, Buday C, van Aggelen G, Ikonomou MG, Pasternak J. Acute toxicity of emamectin benzoate and its desmethyl metabolite to Eohaustorius estuarius. Environ Toxicol Chem. 2010;29(8):1816-1820. doi:10.1002/etc.209.
3. Takai K, Soejima T, Suzuki T, et al. Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag Sci. 2001;57(5):463-466. doi:10.1002/ps.30.
4. Chukwudebe AC, Beavers JB, Jaber M, Wislocki PG. Toxicity of emamectin benzoate to mallard duck and northern bobwhite quail. Environ Toxicol and Chem. 1998;17(6):1118-1123. doi:10.1002/etc.5620170619.
5. Armstrong R, MacPhee D, Katz T, Endris R. A field efficacy evaluation of emamectin benzoate for the control of sea lice on Atlantic salmon. Can Vet J. 2000;41(8):607-612.
6. Ramstad A, Colquhoun DJ, Nordmo R, Sutherland IH, Simmons R. Field trials in Norway with SLICE (0.2% emamectin benzoate) for the oral treatment of sea lice infestation in farmed Atlantic salmon Salmo salar. Dis Aquat Organ. 2002;21;50(1):29-33. doi:10.3354/dao050029.
7. Yen TH, Lin JL. Acute poisoning with emamectin benzoate. J Toxicol Clin Toxicol. 2004;42(5):657-661.
8. Campbell WC, Fisher MH, Stapley EO, Albers-Schönberg G, Jacob TA. Ivermectin: a potent new antiparasitic agent. Science. 1983; 221(4613):823-838.
9. Chung K, Yang CC, Wu ML, Deng JF, Tsai WJ. Agricultural avermectins: an uncommon but potentially fatal cause of pesticide poisoning. Ann Emerg Med. 1999;34(1):51-57.
Emamectin benzoate (EB) is a semisynthetic derivative of avermectin that has acaricidal, nematicidal, and insecticidal action. Avermectin analogs are natural products from soil fungi (Streptomyces avermitilis).1 Emamectin benzoate was initially developed to eradicate lepidopteran larvae, particularly armyworms, and is registered in the United States and Japan for use on vegetable crops.2-4 In addition to its agricultural use, EB also has antiparasitic effects on sea lice (Lepeophtheirus salmonis) that affect Atlantic salmon, and has been registered for use in several countries since 1999.5-7 Although a few studies have evaluated the toxic effects of avermectin on humans, there is a paucity of information regarding human toxicity associated with EB.7 This case report describes rapid deterioration of a patient following ingestion of EB.
Case
A 75-year-old man presented to the ED 20 minutes after intentionally ingesting an agricultural insecticide. Upon presentation, the patient stated that he drank a whole bottle (100 mL) of insecticide after consuming alcohol, but denied coingestion of other toxic substances or any medications. The patient provided the empty bottle upon presentation, and the ingested product was identified as Affirm, an insecticide containing 2.15% EB as the active ingredient.
The patient’s medical history was significant for major depressive disorder, for which he was on alprazolam, donepezil, paroxetine, and quetiapine. The patient stated that he also suffered from chronic back pain, noting that he only took analgesics intermittently as needed.
On examination, the patient was alert and oriented to time and place. Initially, he did not experience any physical discomfort. His vital signs were: blood pressure (BP), 126/74 mm Hg; pulse rate, 67 beats/minute; respiratory rate, mildly tachypneic at 23 breaths/minute; and temperature, 97.9°F. Oxygen saturation was 96% on room air.
Ocular examination revealed both pupils to be equally round, 3 mm in diameter, and reactive to light. Examination of the oropharynx was normal and without signs of mucosal injury. The lung sounds were clear bilaterally, and the heart was a regular rate and rhythm and without murmur. The patient’s abdomen was soft and nontender. No deficits, such as ataxia, dysarthria, or tremor were found on the neurological examination.
Prompt gastric lavage via a nasogastric tube was performed, and activated charcoal was administered. Laboratory evaluation was significant for the following: white blood cell count, 22.77 x 109/L with 78% neutrophils and 16% lymphocytes; sodium, 138 mEq/L; potassium, 3.1 mEq/L; chloride, 109 mEq/L; blood urea nitrogen, 19 mg/dL; and creatinine, 0.7 mg/dL. Arterial blood gas (ABG) results revealed a pH, 7.37; partial pressure of carbon dioxide, 25 mm Hg; partial pressure of oxygen, 93 mm Hg; bicarbonate, 14.5 mEq/L; base excess, –8.9 mEq/L; and an oxygen saturation, 97%. Serum creatine kinase (CK), CK-MB and troponin levels were both within normal range. Lactic acid, serum osmolality, and serum ethanol levels were not obtained. The patient’s electrocardiogram (ECG) and chest radiograph findings were normal.
Approximately 1 hour after presentation, the patient complained of an epigastric burning sensation and continued to exhibit mild tachypnea. A subsequent ABG test revealed progressive metabolic acidosis (Table). Although the patient was given a total of 800 mL of normal saline intravenously (IV) upon arrival at the ED, his total urinary output was less than 100 mL 7 hours afterward. Attempts to increase urinary output with IV furosemide were ineffective.
Along with the progressive metabolic acidosis, the patient became hypotensive, and did not respond to IV fluid resuscitation. A norepinephrine infusion was started to improve BP, but this was likewise ineffective. Serial ECGs did not show any specific abnormalities such as dysrhythmia or ischemia.
The patient was admitted to the intensive care unit approximately 10.5 hours after presentation where he received continuous renal replacement therapy (CRRT) to correct the severe metabolic acidosis and poor circulation. Metabolic acidosis persisted despite CRRT, and the patient remained hypotensive even after receiving high-dose IV norepinephrine (Figure).
About 12.5 hours after his presentation to the ED, the patient began to vomit profusely and went into cardiac arrest. The cardiac monitor demonstrated pulseless ventricular tachycardia. Aggressive resuscitative efforts were initiated, but failed to restore spontaneous circulation.
Discussion
As an avermectin analog, EB interacts with γ-aminobutyric acid (GABA) receptors and enhances membrane chloride permeability.8 In mammals, GABA-containing neurons and receptors are found in the central nervous system (CNS), but not in the peripheral nervous system. In cases of high-dose avermectin ingestion in humans, CNS toxicity, including agitation and depressed mental status, have been reported, as well as death resulting from respiratory failure.9
With respect to human EB toxicity, there is only one other documented case in the literature by Yen and Lin.7 In their case, the authors report on a patient who ingested 100 mL of Proclaim, which contained 2.15% EB diluted with 400 mL of tap water.7 They note that the patient in their case presented with mild confusion and gastrointestinal (GI) symptoms of nausea, vomiting, and cramping discomfort. Following laboratory and radiological investigation, the patient was found to have aspiration pneumonitis and admitted to the inpatient hospital. On hospital day 2, the patient’s GI symptoms abated and he became alert and oriented. He was discharged 1 week from initial presentation and experienced no sequelae.
In our case, the patient ingested 100 mL of 2.15% EB without dilution. He also experienced GI symptoms, but did not have any CNS depression. The metabolic acidosis rapidly worsened, and could not be corrected, even with intensive therapy. This rapid life-threatening course has not previously been reported with avermectin or EB poisoning. In the avermectin poisoning cases in the literature, seven out of 19 patients (37%) exhibited severe effects, such as hypotension, coma, and aspiration with respiratory failure.9 Six of the seven patients experienced a full recovery; the remaining patient died 18 days after ingestion from multiple organ failure.
The reason for our patient’s rapid progression to metabolic acidosis and progressive deterioration (hypotension and hypoxemia) is not clear. One possible theory is that the solvents or other additives aside from EB in the ingested insecticide might make EB more toxic. In our case, the patient’s rapid deterioration alone or asphyxia by vomitus might have been the cause of the cardiac arrest. Future reports and studies about EB toxicity in humans are warranted to investigate the pathogenesis of toxicity and appropriate treatment.
Conclusion
This is the first report of a human death caused by EB poisoning; the patient experienced severe metabolic acidosis without CNS depression, ultimately leading to death. Emergency physicians should be aware of the possibility of rapid deterioration in patients who present after ingestion of EB and related substances.
Emamectin benzoate (EB) is a semisynthetic derivative of avermectin that has acaricidal, nematicidal, and insecticidal action. Avermectin analogs are natural products from soil fungi (Streptomyces avermitilis).1 Emamectin benzoate was initially developed to eradicate lepidopteran larvae, particularly armyworms, and is registered in the United States and Japan for use on vegetable crops.2-4 In addition to its agricultural use, EB also has antiparasitic effects on sea lice (Lepeophtheirus salmonis) that affect Atlantic salmon, and has been registered for use in several countries since 1999.5-7 Although a few studies have evaluated the toxic effects of avermectin on humans, there is a paucity of information regarding human toxicity associated with EB.7 This case report describes rapid deterioration of a patient following ingestion of EB.
Case
A 75-year-old man presented to the ED 20 minutes after intentionally ingesting an agricultural insecticide. Upon presentation, the patient stated that he drank a whole bottle (100 mL) of insecticide after consuming alcohol, but denied coingestion of other toxic substances or any medications. The patient provided the empty bottle upon presentation, and the ingested product was identified as Affirm, an insecticide containing 2.15% EB as the active ingredient.
The patient’s medical history was significant for major depressive disorder, for which he was on alprazolam, donepezil, paroxetine, and quetiapine. The patient stated that he also suffered from chronic back pain, noting that he only took analgesics intermittently as needed.
On examination, the patient was alert and oriented to time and place. Initially, he did not experience any physical discomfort. His vital signs were: blood pressure (BP), 126/74 mm Hg; pulse rate, 67 beats/minute; respiratory rate, mildly tachypneic at 23 breaths/minute; and temperature, 97.9°F. Oxygen saturation was 96% on room air.
Ocular examination revealed both pupils to be equally round, 3 mm in diameter, and reactive to light. Examination of the oropharynx was normal and without signs of mucosal injury. The lung sounds were clear bilaterally, and the heart was a regular rate and rhythm and without murmur. The patient’s abdomen was soft and nontender. No deficits, such as ataxia, dysarthria, or tremor were found on the neurological examination.
Prompt gastric lavage via a nasogastric tube was performed, and activated charcoal was administered. Laboratory evaluation was significant for the following: white blood cell count, 22.77 x 109/L with 78% neutrophils and 16% lymphocytes; sodium, 138 mEq/L; potassium, 3.1 mEq/L; chloride, 109 mEq/L; blood urea nitrogen, 19 mg/dL; and creatinine, 0.7 mg/dL. Arterial blood gas (ABG) results revealed a pH, 7.37; partial pressure of carbon dioxide, 25 mm Hg; partial pressure of oxygen, 93 mm Hg; bicarbonate, 14.5 mEq/L; base excess, –8.9 mEq/L; and an oxygen saturation, 97%. Serum creatine kinase (CK), CK-MB and troponin levels were both within normal range. Lactic acid, serum osmolality, and serum ethanol levels were not obtained. The patient’s electrocardiogram (ECG) and chest radiograph findings were normal.
Approximately 1 hour after presentation, the patient complained of an epigastric burning sensation and continued to exhibit mild tachypnea. A subsequent ABG test revealed progressive metabolic acidosis (Table). Although the patient was given a total of 800 mL of normal saline intravenously (IV) upon arrival at the ED, his total urinary output was less than 100 mL 7 hours afterward. Attempts to increase urinary output with IV furosemide were ineffective.
Along with the progressive metabolic acidosis, the patient became hypotensive, and did not respond to IV fluid resuscitation. A norepinephrine infusion was started to improve BP, but this was likewise ineffective. Serial ECGs did not show any specific abnormalities such as dysrhythmia or ischemia.
The patient was admitted to the intensive care unit approximately 10.5 hours after presentation where he received continuous renal replacement therapy (CRRT) to correct the severe metabolic acidosis and poor circulation. Metabolic acidosis persisted despite CRRT, and the patient remained hypotensive even after receiving high-dose IV norepinephrine (Figure).
About 12.5 hours after his presentation to the ED, the patient began to vomit profusely and went into cardiac arrest. The cardiac monitor demonstrated pulseless ventricular tachycardia. Aggressive resuscitative efforts were initiated, but failed to restore spontaneous circulation.
Discussion
As an avermectin analog, EB interacts with γ-aminobutyric acid (GABA) receptors and enhances membrane chloride permeability.8 In mammals, GABA-containing neurons and receptors are found in the central nervous system (CNS), but not in the peripheral nervous system. In cases of high-dose avermectin ingestion in humans, CNS toxicity, including agitation and depressed mental status, have been reported, as well as death resulting from respiratory failure.9
With respect to human EB toxicity, there is only one other documented case in the literature by Yen and Lin.7 In their case, the authors report on a patient who ingested 100 mL of Proclaim, which contained 2.15% EB diluted with 400 mL of tap water.7 They note that the patient in their case presented with mild confusion and gastrointestinal (GI) symptoms of nausea, vomiting, and cramping discomfort. Following laboratory and radiological investigation, the patient was found to have aspiration pneumonitis and admitted to the inpatient hospital. On hospital day 2, the patient’s GI symptoms abated and he became alert and oriented. He was discharged 1 week from initial presentation and experienced no sequelae.
In our case, the patient ingested 100 mL of 2.15% EB without dilution. He also experienced GI symptoms, but did not have any CNS depression. The metabolic acidosis rapidly worsened, and could not be corrected, even with intensive therapy. This rapid life-threatening course has not previously been reported with avermectin or EB poisoning. In the avermectin poisoning cases in the literature, seven out of 19 patients (37%) exhibited severe effects, such as hypotension, coma, and aspiration with respiratory failure.9 Six of the seven patients experienced a full recovery; the remaining patient died 18 days after ingestion from multiple organ failure.
The reason for our patient’s rapid progression to metabolic acidosis and progressive deterioration (hypotension and hypoxemia) is not clear. One possible theory is that the solvents or other additives aside from EB in the ingested insecticide might make EB more toxic. In our case, the patient’s rapid deterioration alone or asphyxia by vomitus might have been the cause of the cardiac arrest. Future reports and studies about EB toxicity in humans are warranted to investigate the pathogenesis of toxicity and appropriate treatment.
Conclusion
This is the first report of a human death caused by EB poisoning; the patient experienced severe metabolic acidosis without CNS depression, ultimately leading to death. Emergency physicians should be aware of the possibility of rapid deterioration in patients who present after ingestion of EB and related substances.
1. Lasota JA, Dybas RA. Avermectins, a novel class of compounds: implications for use in arthropod pest control. Annu Rev Entomol. 1991;36:91-117. doi:10.1146/annurev.en.36.010191.000515.
2. Kuo JN, Buday C, van Aggelen G, Ikonomou MG, Pasternak J. Acute toxicity of emamectin benzoate and its desmethyl metabolite to Eohaustorius estuarius. Environ Toxicol Chem. 2010;29(8):1816-1820. doi:10.1002/etc.209.
3. Takai K, Soejima T, Suzuki T, et al. Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag Sci. 2001;57(5):463-466. doi:10.1002/ps.30.
4. Chukwudebe AC, Beavers JB, Jaber M, Wislocki PG. Toxicity of emamectin benzoate to mallard duck and northern bobwhite quail. Environ Toxicol and Chem. 1998;17(6):1118-1123. doi:10.1002/etc.5620170619.
5. Armstrong R, MacPhee D, Katz T, Endris R. A field efficacy evaluation of emamectin benzoate for the control of sea lice on Atlantic salmon. Can Vet J. 2000;41(8):607-612.
6. Ramstad A, Colquhoun DJ, Nordmo R, Sutherland IH, Simmons R. Field trials in Norway with SLICE (0.2% emamectin benzoate) for the oral treatment of sea lice infestation in farmed Atlantic salmon Salmo salar. Dis Aquat Organ. 2002;21;50(1):29-33. doi:10.3354/dao050029.
7. Yen TH, Lin JL. Acute poisoning with emamectin benzoate. J Toxicol Clin Toxicol. 2004;42(5):657-661.
8. Campbell WC, Fisher MH, Stapley EO, Albers-Schönberg G, Jacob TA. Ivermectin: a potent new antiparasitic agent. Science. 1983; 221(4613):823-838.
9. Chung K, Yang CC, Wu ML, Deng JF, Tsai WJ. Agricultural avermectins: an uncommon but potentially fatal cause of pesticide poisoning. Ann Emerg Med. 1999;34(1):51-57.
1. Lasota JA, Dybas RA. Avermectins, a novel class of compounds: implications for use in arthropod pest control. Annu Rev Entomol. 1991;36:91-117. doi:10.1146/annurev.en.36.010191.000515.
2. Kuo JN, Buday C, van Aggelen G, Ikonomou MG, Pasternak J. Acute toxicity of emamectin benzoate and its desmethyl metabolite to Eohaustorius estuarius. Environ Toxicol Chem. 2010;29(8):1816-1820. doi:10.1002/etc.209.
3. Takai K, Soejima T, Suzuki T, et al. Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag Sci. 2001;57(5):463-466. doi:10.1002/ps.30.
4. Chukwudebe AC, Beavers JB, Jaber M, Wislocki PG. Toxicity of emamectin benzoate to mallard duck and northern bobwhite quail. Environ Toxicol and Chem. 1998;17(6):1118-1123. doi:10.1002/etc.5620170619.
5. Armstrong R, MacPhee D, Katz T, Endris R. A field efficacy evaluation of emamectin benzoate for the control of sea lice on Atlantic salmon. Can Vet J. 2000;41(8):607-612.
6. Ramstad A, Colquhoun DJ, Nordmo R, Sutherland IH, Simmons R. Field trials in Norway with SLICE (0.2% emamectin benzoate) for the oral treatment of sea lice infestation in farmed Atlantic salmon Salmo salar. Dis Aquat Organ. 2002;21;50(1):29-33. doi:10.3354/dao050029.
7. Yen TH, Lin JL. Acute poisoning with emamectin benzoate. J Toxicol Clin Toxicol. 2004;42(5):657-661.
8. Campbell WC, Fisher MH, Stapley EO, Albers-Schönberg G, Jacob TA. Ivermectin: a potent new antiparasitic agent. Science. 1983; 221(4613):823-838.
9. Chung K, Yang CC, Wu ML, Deng JF, Tsai WJ. Agricultural avermectins: an uncommon but potentially fatal cause of pesticide poisoning. Ann Emerg Med. 1999;34(1):51-57.
‘Rapid autopsy’ programs seek clues to cancer within hours of death
After Keith Beck died of bile duct cancer last year, family members said more than 900 people showed up to pay respects to the popular athletic director at the University of Findlay in northwestern Ohio.
Many were former students who recalled acts of kindness during Mr. Beck’s nearly 30-year career: $20 given to a kid who was broke, textbooks bought for a student whose parents were going through bankruptcy, a spot cleared to sleep on Mr. Beck’s living room floor.
But few knew about Mr. Beck’s final gesture of generosity. The 59-year-old had agreed to a “rapid autopsy,” a procedure conducted within hours of his death on March 28, 2017, so that scientists could learn as much as possible from the cancer that killed him.
“He was 100% for it,” recalled his ex-wife, Nancy Beck, 63, who cared for Mr. Beck at the end of his life. “It wasn’t the easiest thing to do, but it was important.”
Mr. Beck donated his body to a rapid-autopsy research study at the Ohio State University, part of a small but growing effort by more than a dozen medical centers nationwide. The idea is to obtain tumor tissue immediately after death – before it has a chance to degrade. Scientists say such samples are the key to understanding the genetics of cancers that spread through the body, thwarting efforts to cure them.
“People are recognizing that cancer is more heterogeneous than we realize,” said Dr. Sameek Roychowdhury, a medical scientist at OSU’s Comprehensive Cancer Center. “Different parts of your body may have different cancer cells, even though they originated from the same cancer.”
In Mr. Beck’s case, results from the rapid autopsy showed he had developed a mutation that caused the experimental drug he was taking, known as an FGFR inhibitor, to stop working. Dr. Roychowdhury and colleagues plan to report on Mr. Beck’s case in an upcoming paper.
“This is helping us shape how we develop this new drug,” Dr. Roychowdhury said. “How can we make a better drug? Or can we make a better drug combination?”
Rapid-autopsy technology has been available for decades. Researchers at the University of Washington in Seattle have been using the technique to study prostate cancer since 1991. Scientists at the University of Nebraska Medical Center launched a now-robust program in 2000.
But only in recent years have more hospitals been launching and expanding programs, said Dr. Jody Hooper, director of the Legacy Gift Rapid Autopsy Program at Johns Hopkins Medicine in Baltimore. At last count, there were 14 similar programs in the U.S.
Funding for them varies, Dr. Hooper said, but typically they’re supported by a mix of cancer program resources, grants, and researcher fees.
Scientists recognize the value of examining tissue from multiple sites soon after death and obtaining larger samples than they could while a patient was living. Cancer cells can be retrieved during such autopsies and kept alive, allowing researchers to experiment with ways to treat – or kill – them.
“It’s the power of sampling over the entire body at the same time,” said Dr. Hooper, who conducts about one rapid autopsy a month, often providing tissue for up to a half-dozen researchers interested in different questions.
Most programs focus on cancer, but efforts are underway to expand the practice, possibly to shed light on virus reservoirs in HIV patients, for instance.
Speed is essential to preserve RNA and DNA, the building blocks of cells, which can degrade quickly after death. It’s best to obtain specimens of living cells within 6 hours of death and other tissue within 12 hours, Dr. Hooper said.
The need for speed is also what makes such autopsies challenging. Families must consent to the procedure, often while freshly grieving their loved one’s death. And the logistics surrounding retrieving a body, conducting an autopsy, and then returning the body for a funeral are often complicated. Traffic is unpredictable and “one time, there was a blizzard,” Dr. Hooper said.
Dr. Roychowdhury said he and one of his clinical fellows are on call at all times.
“The patients have our cellphone numbers, as well as the next of kin,” he said.
Broaching the subject with patients and families requires tact and compassion. Most patients are enrolled in clinical trials and learn about the autopsies from their doctors or pathologists such as Dr. Hooper. Many are willing, even eager, to cooperate, she said.
“These are mostly patients with metastatic cancer,” she said. “They’ve made their peace with the outcome long before.”
For some, the rapid autopsy is simply the final phase of the clinical trial.
“They want to do something not only for themselves, but also to help others,” Dr. Roychowdhury said.
That’s how Linda Boyed, 52, of Lewis Center, Ohio, sees it. Like Mr. Beck, she has bile duct cancer and is enrolled in a trial to treat it. The drugs are working now, but Ms. Boyed said she has agreed to a rapid autopsy after death so scientists can learn from her when they’re no longer effective.
“I have a strong Christian faith,” she said. “I believe we’re put on this Earth to help each other.”
Because the rapid autopsies are paid through program funds and grants, there’s no cost to the families. Bodies are returned within a day and in a condition that doesn’t affect funeral plans.
“My emphasis is that it was all done with dignity and respect,” said Nancy Beck. “We felt honored to be able to do this.”
Performing the autopsy after treating a patient in life is an honor for doctors, too, Dr. Roychowdhury said.
“This was once a living, breathing person that came into my office every other week,” he said. “The thing I want to think about each day is that they’ve given so much so that others can benefit.
“Everyone has something to teach us after death.”
KHN’s coverage of end-of-life and serious illness issues is supported in part by the Gordon and Betty Moore Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
After Keith Beck died of bile duct cancer last year, family members said more than 900 people showed up to pay respects to the popular athletic director at the University of Findlay in northwestern Ohio.
Many were former students who recalled acts of kindness during Mr. Beck’s nearly 30-year career: $20 given to a kid who was broke, textbooks bought for a student whose parents were going through bankruptcy, a spot cleared to sleep on Mr. Beck’s living room floor.
But few knew about Mr. Beck’s final gesture of generosity. The 59-year-old had agreed to a “rapid autopsy,” a procedure conducted within hours of his death on March 28, 2017, so that scientists could learn as much as possible from the cancer that killed him.
“He was 100% for it,” recalled his ex-wife, Nancy Beck, 63, who cared for Mr. Beck at the end of his life. “It wasn’t the easiest thing to do, but it was important.”
Mr. Beck donated his body to a rapid-autopsy research study at the Ohio State University, part of a small but growing effort by more than a dozen medical centers nationwide. The idea is to obtain tumor tissue immediately after death – before it has a chance to degrade. Scientists say such samples are the key to understanding the genetics of cancers that spread through the body, thwarting efforts to cure them.
“People are recognizing that cancer is more heterogeneous than we realize,” said Dr. Sameek Roychowdhury, a medical scientist at OSU’s Comprehensive Cancer Center. “Different parts of your body may have different cancer cells, even though they originated from the same cancer.”
In Mr. Beck’s case, results from the rapid autopsy showed he had developed a mutation that caused the experimental drug he was taking, known as an FGFR inhibitor, to stop working. Dr. Roychowdhury and colleagues plan to report on Mr. Beck’s case in an upcoming paper.
“This is helping us shape how we develop this new drug,” Dr. Roychowdhury said. “How can we make a better drug? Or can we make a better drug combination?”
Rapid-autopsy technology has been available for decades. Researchers at the University of Washington in Seattle have been using the technique to study prostate cancer since 1991. Scientists at the University of Nebraska Medical Center launched a now-robust program in 2000.
But only in recent years have more hospitals been launching and expanding programs, said Dr. Jody Hooper, director of the Legacy Gift Rapid Autopsy Program at Johns Hopkins Medicine in Baltimore. At last count, there were 14 similar programs in the U.S.
Funding for them varies, Dr. Hooper said, but typically they’re supported by a mix of cancer program resources, grants, and researcher fees.
Scientists recognize the value of examining tissue from multiple sites soon after death and obtaining larger samples than they could while a patient was living. Cancer cells can be retrieved during such autopsies and kept alive, allowing researchers to experiment with ways to treat – or kill – them.
“It’s the power of sampling over the entire body at the same time,” said Dr. Hooper, who conducts about one rapid autopsy a month, often providing tissue for up to a half-dozen researchers interested in different questions.
Most programs focus on cancer, but efforts are underway to expand the practice, possibly to shed light on virus reservoirs in HIV patients, for instance.
Speed is essential to preserve RNA and DNA, the building blocks of cells, which can degrade quickly after death. It’s best to obtain specimens of living cells within 6 hours of death and other tissue within 12 hours, Dr. Hooper said.
The need for speed is also what makes such autopsies challenging. Families must consent to the procedure, often while freshly grieving their loved one’s death. And the logistics surrounding retrieving a body, conducting an autopsy, and then returning the body for a funeral are often complicated. Traffic is unpredictable and “one time, there was a blizzard,” Dr. Hooper said.
Dr. Roychowdhury said he and one of his clinical fellows are on call at all times.
“The patients have our cellphone numbers, as well as the next of kin,” he said.
Broaching the subject with patients and families requires tact and compassion. Most patients are enrolled in clinical trials and learn about the autopsies from their doctors or pathologists such as Dr. Hooper. Many are willing, even eager, to cooperate, she said.
“These are mostly patients with metastatic cancer,” she said. “They’ve made their peace with the outcome long before.”
For some, the rapid autopsy is simply the final phase of the clinical trial.
“They want to do something not only for themselves, but also to help others,” Dr. Roychowdhury said.
That’s how Linda Boyed, 52, of Lewis Center, Ohio, sees it. Like Mr. Beck, she has bile duct cancer and is enrolled in a trial to treat it. The drugs are working now, but Ms. Boyed said she has agreed to a rapid autopsy after death so scientists can learn from her when they’re no longer effective.
“I have a strong Christian faith,” she said. “I believe we’re put on this Earth to help each other.”
Because the rapid autopsies are paid through program funds and grants, there’s no cost to the families. Bodies are returned within a day and in a condition that doesn’t affect funeral plans.
“My emphasis is that it was all done with dignity and respect,” said Nancy Beck. “We felt honored to be able to do this.”
Performing the autopsy after treating a patient in life is an honor for doctors, too, Dr. Roychowdhury said.
“This was once a living, breathing person that came into my office every other week,” he said. “The thing I want to think about each day is that they’ve given so much so that others can benefit.
“Everyone has something to teach us after death.”
KHN’s coverage of end-of-life and serious illness issues is supported in part by the Gordon and Betty Moore Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
After Keith Beck died of bile duct cancer last year, family members said more than 900 people showed up to pay respects to the popular athletic director at the University of Findlay in northwestern Ohio.
Many were former students who recalled acts of kindness during Mr. Beck’s nearly 30-year career: $20 given to a kid who was broke, textbooks bought for a student whose parents were going through bankruptcy, a spot cleared to sleep on Mr. Beck’s living room floor.
But few knew about Mr. Beck’s final gesture of generosity. The 59-year-old had agreed to a “rapid autopsy,” a procedure conducted within hours of his death on March 28, 2017, so that scientists could learn as much as possible from the cancer that killed him.
“He was 100% for it,” recalled his ex-wife, Nancy Beck, 63, who cared for Mr. Beck at the end of his life. “It wasn’t the easiest thing to do, but it was important.”
Mr. Beck donated his body to a rapid-autopsy research study at the Ohio State University, part of a small but growing effort by more than a dozen medical centers nationwide. The idea is to obtain tumor tissue immediately after death – before it has a chance to degrade. Scientists say such samples are the key to understanding the genetics of cancers that spread through the body, thwarting efforts to cure them.
“People are recognizing that cancer is more heterogeneous than we realize,” said Dr. Sameek Roychowdhury, a medical scientist at OSU’s Comprehensive Cancer Center. “Different parts of your body may have different cancer cells, even though they originated from the same cancer.”
In Mr. Beck’s case, results from the rapid autopsy showed he had developed a mutation that caused the experimental drug he was taking, known as an FGFR inhibitor, to stop working. Dr. Roychowdhury and colleagues plan to report on Mr. Beck’s case in an upcoming paper.
“This is helping us shape how we develop this new drug,” Dr. Roychowdhury said. “How can we make a better drug? Or can we make a better drug combination?”
Rapid-autopsy technology has been available for decades. Researchers at the University of Washington in Seattle have been using the technique to study prostate cancer since 1991. Scientists at the University of Nebraska Medical Center launched a now-robust program in 2000.
But only in recent years have more hospitals been launching and expanding programs, said Dr. Jody Hooper, director of the Legacy Gift Rapid Autopsy Program at Johns Hopkins Medicine in Baltimore. At last count, there were 14 similar programs in the U.S.
Funding for them varies, Dr. Hooper said, but typically they’re supported by a mix of cancer program resources, grants, and researcher fees.
Scientists recognize the value of examining tissue from multiple sites soon after death and obtaining larger samples than they could while a patient was living. Cancer cells can be retrieved during such autopsies and kept alive, allowing researchers to experiment with ways to treat – or kill – them.
“It’s the power of sampling over the entire body at the same time,” said Dr. Hooper, who conducts about one rapid autopsy a month, often providing tissue for up to a half-dozen researchers interested in different questions.
Most programs focus on cancer, but efforts are underway to expand the practice, possibly to shed light on virus reservoirs in HIV patients, for instance.
Speed is essential to preserve RNA and DNA, the building blocks of cells, which can degrade quickly after death. It’s best to obtain specimens of living cells within 6 hours of death and other tissue within 12 hours, Dr. Hooper said.
The need for speed is also what makes such autopsies challenging. Families must consent to the procedure, often while freshly grieving their loved one’s death. And the logistics surrounding retrieving a body, conducting an autopsy, and then returning the body for a funeral are often complicated. Traffic is unpredictable and “one time, there was a blizzard,” Dr. Hooper said.
Dr. Roychowdhury said he and one of his clinical fellows are on call at all times.
“The patients have our cellphone numbers, as well as the next of kin,” he said.
Broaching the subject with patients and families requires tact and compassion. Most patients are enrolled in clinical trials and learn about the autopsies from their doctors or pathologists such as Dr. Hooper. Many are willing, even eager, to cooperate, she said.
“These are mostly patients with metastatic cancer,” she said. “They’ve made their peace with the outcome long before.”
For some, the rapid autopsy is simply the final phase of the clinical trial.
“They want to do something not only for themselves, but also to help others,” Dr. Roychowdhury said.
That’s how Linda Boyed, 52, of Lewis Center, Ohio, sees it. Like Mr. Beck, she has bile duct cancer and is enrolled in a trial to treat it. The drugs are working now, but Ms. Boyed said she has agreed to a rapid autopsy after death so scientists can learn from her when they’re no longer effective.
“I have a strong Christian faith,” she said. “I believe we’re put on this Earth to help each other.”
Because the rapid autopsies are paid through program funds and grants, there’s no cost to the families. Bodies are returned within a day and in a condition that doesn’t affect funeral plans.
“My emphasis is that it was all done with dignity and respect,” said Nancy Beck. “We felt honored to be able to do this.”
Performing the autopsy after treating a patient in life is an honor for doctors, too, Dr. Roychowdhury said.
“This was once a living, breathing person that came into my office every other week,” he said. “The thing I want to think about each day is that they’ve given so much so that others can benefit.
“Everyone has something to teach us after death.”
KHN’s coverage of end-of-life and serious illness issues is supported in part by the Gordon and Betty Moore Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Intralymphatic Histiocytosis Treated With Intralesional Triamcinolone Acetonide and Pressure Bandage
Intralymphatic histiocytosis was first described in 1994.1 To date, at least 70 cases have been reported in the English-language literature, the majority being associated with systemic or local inflammatory conditions such as rheumatoid arthritis (RA), malignancy, and metal prostheses. The remaining cases arose independent of any detectable disease process.2 The clinical lesion localizes to areas around surgical scars or inflamed joints and generally presents with erythematous livedoid papules and plaques. Because of its rarity, pathologists and clinicians may be unfamiliar with this entity, leading to delayed or missed diagnoses.
Although the pathogenesis of intralymphatic histiocytosis remains unclear, it may be related to dysregulated immune signaling. The condition follows a chronic, relapsing-remitting course that has shown variable response to topical and systemic treatments. We present a rare case of intralymphatic histiocytosis associated with joint replacement/metal prosthesis3-14 that was responsive to a novel treatment with intralesional steroid injection and pressure bandage.
Case Report
An 89-year-old woman presented with a relapsing and remitting rash on the right calf and popliteal fossa of 11 months’ duration. It was becoming more painful over time and recently began to hurt when walking. Her medical history was remarkable for deep vein thromboses of the bilateral legs, Factor V Leiden deficiency, osteoarthritis, and a popliteal (Baker) cyst on the right leg that ruptured 22 months prior to presentation. Her surgical history included bilateral knee replacements (10 years and 2 years prior to the current presentation for the right and left knees, respectively). Her international normalized ratio (2.0) was therapeutic on warfarin.
Initially, swelling, pain, and redness developed in the right calf, and recurrent right-leg deep venous thrombosis was ruled out by Doppler ultrasound. The findings were considered to be secondary to inflammation from a popliteal cyst. Symptoms persisted despite application of warm compresses, leg elevation, and compression stockings. Treatment with doxycycline prescribed by the patient’s primary care physician 9 months prior for presumed cellulitis produced little improvement. Physical examination revealed a well-healed vertical scar on the right calf from an incisional biopsy within an 8-cm, tender, erythematous, indurated, sclerotic plaque with erythematous streaks radiating from the center of the plaque (Figure 1). There also was red-brown, indurated discoloration on the right shin.
Fine-needle aspiration of the lesion revealed red blood cells and histiocytes. Laboratory studies showed an elevated erythrocyte sedimentation rate of 74 mm/h (reference range, 0–30 mm/h) and a C-reactive protein level of 39 mg/L (reference range, 0–10 mg/L). An incisional biopsy including the muscular fascia showed dense dermal fibrosis with chronic inflammation and scarring. A dermatopathologist (G. A. S.) reviewed the case and confirmed variable fibrosis and chronic inflammation associated with edema in the dermis and epidermal acanthosis. Inspection of vessels in the mid to upper dermis in one area revealed stellate, thin-walled, vascular structures that contained bland epithelioid cells lining the lumen as well as packed within the vessels. The epithelioid cells did not show atypia or mitotic figures, and they did not show intracytoplasmic vacuoles (Figure 2). Immunocytochemical staining for D2-40 was strongly positive in cells lining the vessels, consistent with lymphatics (Figure 3). CD68 immunohistochemistry for histiocytes stained the cells within the lymphatics (Figure 4). A diagnosis of intralymphatic histiocytosis was made.
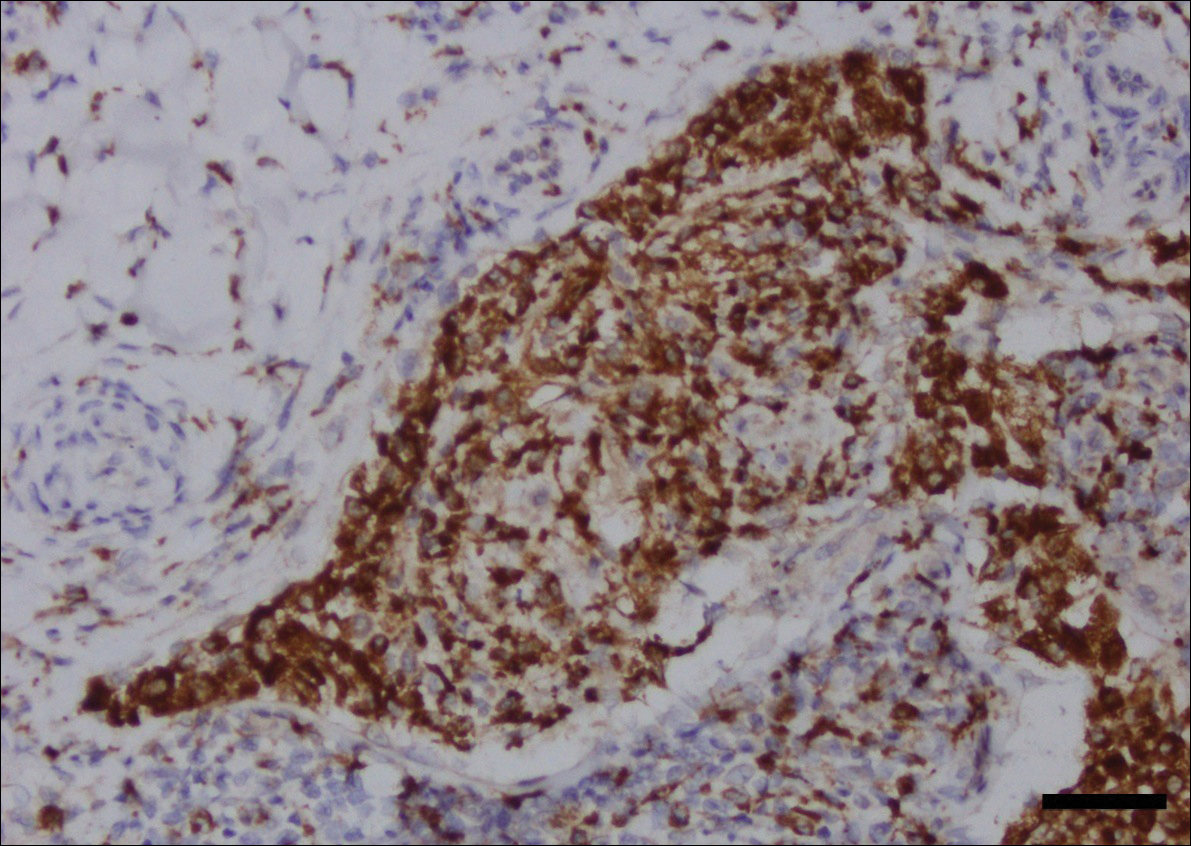
Intralesional triamcinolone acetonide 10 mg/cc×1.6 cc was injected into the plaque once monthly for 2 consecutive months, and daily compression with a pressure bandage of the right lower leg was initiated. Four months after the first treatment with this regimen, the plaque was smaller and no longer sclerotic or painful, and the erythema was markedly reduced (Figure 5). Clinical and symptomatic improvement continued at 1-year follow-up.
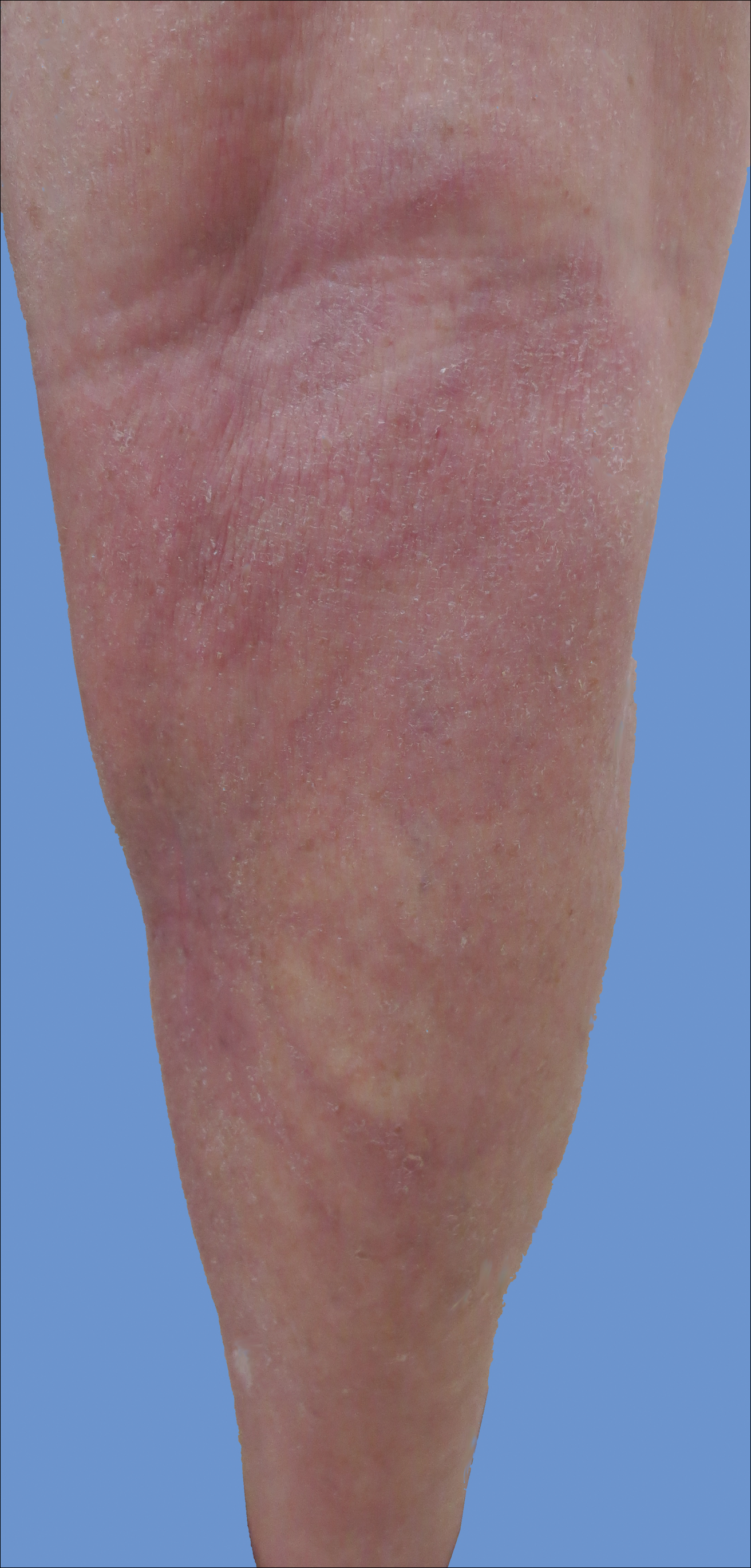
Comment
Intralymphatic histiocytosis is a rare cutaneous disorder defined histologically by histiocytes within the lumina of lymphatics. In addition to the current case, our review of PubMed articles indexed for MEDLINE using the search term intralymphatic histiocytosis yielded more than 70 total cases. The condition has a slight female predominance and typically is seen in individuals over the age of 60 years (age range, 16–89 years).12 Many cases are associated with RA/elevated rheumatoid factor.2,4,8,15-30 At least 9 cases of intralymphatic histiocytosis were associated with premalignant or malignant conditions (ie, adenocarcinoma of the breasts, lungs, and colon; Merkel cell carcinoma; melanoma; melanoma in situ; Mullerian carcinoma, gammopathy).4,15,31-34 Primary disease, defined as occurring in patients who are otherwise healthy, was noted in at least 10 cases.1,2,4,12,35,36 Finally, intralymphatic histiocytosis was identified in areas adjacent to metal implants and joint replacements or exploration in approximately 15 cases (including the current case).3-14,29,37
The condition presents with papules, plaques, and nodules in the setting of characteristic livedoid discoloration; however, some patients present with nonspecific nodules or plaques. Lesions may be symptomatic (eg, pruritic, tender) or asymptomatic. The histologic features of intralymphatic histiocytosis are distinctive but may be focal, as in our case, and the diagnosis is easily missed. The histologic differential diagnosis includes diseases in which intravascular accumulations of cells may be seen, including intravascular B-cell lymphoma, which can be excluded with stains that detect B cells (CD20/CD79a), and reactive angioendotheliomatosis, a benign proliferation of endothelial cells, which may be excluded with stains against endothelial markers (CD31/CD34). The course typically is chronic, and treatment with topical steroids,3,9,15,22,26 cyclophosphamide,15 local radiation,1 thalidomide,35 pentoxifylline,7 and RA medications (eg, prednisolone, methotrexate, nonsteroidal anti-inflammatory drugs, hydroxychloroquine) generally are ineffective.2,16,20,25 Symptoms may improve with joint replacement,4 excision of the involved lesion, treatment of an associated malignancy/infection,33,36,38,39 nonsteroidal anti-inflammatory drugs, intra-articular steroid injection,18 amoxicillin and aspirin,19 infliximab,25 pressure bandage application,26 steroid-containing adhesive application,18 arthrocentesis,3,27 oral pentoxifylline,21 tacrolimus,29 CO2 laser,40 prednisolone,41 and tocilizumab.28 Treatment of associated RA is beneficial in rare cases.2,15,20,25,26
The pathogenesis of intralymphatic histiocytosis has not been elucidated with certainty but may represent an abnormal proliferative response of histiocytes and vessels in response to chronic systemic or local inflammation. Lymphangiectasis caused by lymphatic obstruction secondary to trauma, surgical manipulation, or chronic inflammation can promote lymphostasis and slowed clearance of antigens producing an accumulation of histiocytes and subsequent local immunologic reactions, thus an “immunocompromised district” is formed.42 It also is thought that rheumatic or prosthetic joints produce inflammatory mediator–rich (namely tumor necrosis factor α) synovial fluid that drains and collects within the dilated lymphatics, creating a nidus for histiocytes.1,5 In one case, treatment with an anti–tumor necrosis factor antibody (infliximab) improved the skin presentation and rheumatoid joint pain.25 Bakr et al2 noted an association with increased intralymphatic macrophage HLA-DR expression. This T-cell surface receptor typically is upregulated in cases of chronic antigen stimulation and autoimmune conditions.
Conclusion
Our patient had a history of a joint prosthesis and a popliteal cyst, which could have altered lymphatic drainage promoting abnormal immune cell trafficking contributing to the development of intralymphatic histiocytosis. The response to intralesional steroids supports this pathogenic hypothesis. Specifically, direct injection of the area suppressed the immune dysregulation, while compression lessened the degree of lymphostasis. In light of previously reported cases of intralymphatic histiocytosis in association with metal implants,3-9 we suggest that the condition should be considered in patients with chronic painful livedoid nodules or plaques around an affected joint, even in the absence of RA. The dermatopathologist should be warned to search carefully for the subtle but distinctive histologic features of the disease that confirm the diagnosis. Treatment with intralesional triamcinolone acetonide with an overlying pressure wrap has minimal side effects and can work quickly with sustained benefits.
- O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
- Bakr F, Webber N, Fassihi H, et al. Primary and secondary intralymphatic histiocytosis [published online January 17, 2014]. J Am Acad Dermatol. 2014;70:927-933.
- Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants [published online November 10, 2007]. Br J Dermatol. 2008;158:402-404.
- Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. a clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
- Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
- Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants [published online March 6, 2011]. J Cutan Pathol. 2011;38:534-535.
- de Unamuno Bustos B, García Rabasco A, Ballester Sánchez R, et al. Erythematous indurated plaque on the right upper limb. intralymphatic histiocytosis (IH) associated with orthopedic metal implant. Int J Dermatol. 2013;52:547-549.
- Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
- Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
- Bidier M, Hamsch C, Kutzner H, et al. Two cases of intralymphatic histiocytosis following hip replacement [published online June 9, 2015]. J Dtsch Dermatol Ges. 2015;13:700-702.
- Darling MD, Akin R, Tarbox MB, et al. Intralymphatic histiocytosis overlying hip implantation treated with pentoxifilline. J Biol Regul Homeost Agents. 2015;29(1 suppl):117-121.
- Demirkesen C, Kran T, Leblebici C, et al. Intravascular/intralymphatic histiocytosis: a report of 3 cases. Am J Dermatopathol. 2015;37:783-789.
- Gómez-Sánchez ME, Azaña-Defez JM, Martínez-Martínez ML, et al. Intralymphatic histiocytosis: a report of 2 cases. Actas Dermosifiliogr. 2018;109:E1-E5.
- Haitz KA, Chapman MS, Seidel GD. Intralymphatic histiocytosis associated with an orthopedic metal implant. Cutis. 2016;97:E12-E14.
- Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
- Pruim B, Strutton G, Congdon S, et al. Cutaneous histiocytic lymphangitis: an unusual manifestation of rheumatoid arthritis. Australas J Dermatol. 2000;41:101-105.
- Magro CM, Crowson AN. The spectrum of cutaneous lesions in rheumatoid arthritis: a clinical and pathological study of 43 patients. J Cutan Pathol. 2003;30:1-10.
- Takiwaki H, Adachi A, Kohno H, et al. Intravascular or intralymphatic histiocytosis associated with rheumatoid arthritis: a report of 4 cases.J Am Acad Dermatol. 2004;50:585-590.
- Mensing CH, Krengel S, Tronnier M, et al. Reactive angioendotheliomatosis: is it “intravascular histiocytosis”? J Eur Acad Dermatol Venereol. 2005;19:216-219.
- Okazaki A, Asada H, Niizeki H, et al. Intravascular histiocytosis associated with rheumatoid arthritis: report of a case with lymphatic endothelial proliferation. Br J Dermatol. 2005;152:1385-1387.
- Catalina-Fernández I, Alvárez AC, Martin FC, et al. Cutaneous intralymphatic histiocytosis associated with rheumatoid arthritis: report of a case and review of the literature. Am J Dermatopathol. 2007;29:165-168.
- Nishie W, Sawamura D, Iitoyo M, et al. Intravascular histiocytosis associated with rheumatoid arthritis. Dermatology. 2008;217:144-145.
- Okamoto N, Tanioka M, Yamamoto T, et al. Intralymphatic histiocytosis associated with rheumatoid arthritis. Clin Exp Dermatol. 2008;33:516-518.
- Huang H-Y, Liang C-W, Hu S-L, et al. Cutaneous intravascular histiocytosis associated with rheumatoid arthritis: a case report and review of the literature. Clin Exp Dermatol. 2009;34:E302-E303.
- Sakaguchi M, Nagai H, Tsuji G, et al. Effectiveness of infliximab for intralymphatic histiocytosis with rheumatoid arthritis. Arch Dermatol. 2011;147:131-133.
- Washio K, Nakata K, Nakamura A, et al. Pressure bandage as an effective treatment for intralymphatic histiocytosis associated with rheumatoid arthritis. Dermatology. 2011;223:20-24.
- Kaneko T, Takeuchi S, Nakano H, et al. Intralymphatic histiocytosis with rheumatoid arthritis: possible association with the joint involvement. Case Reports Clin Med. 2014;3:149-152.
- Nakajima T, Kawabata D, Nakabo S, et al. Successful treatment with tocilizumab in a case of intralymphatic histiocytosis associated with rheumatoid arthritis. Intern Med. 2014;53:2255-2258.
- Tsujiwaki M, Hata H, Miyauchi T, et al. Warty intralymphatic histiocytosis successfully treated with topical tacrolimus. J Eur Acad Dermatol Venereol. 2015;29:2267-2269.
- Tanaka M, Funasaka Y, Tsuruta K, et al. Intralymphatic histiocytosis with massive interstitial granulomatous foci in a patient with rheumatoid arthritis. Ann Dermatol. 2017;29:237-238.
- Cornejo KM, Cosar EF, O’Donnell P. Cutaneous intralymphatic histiocytosis associated with lung adenocarcinoma. Am J Dermatopathol. 2016;38:568-570.
- Tran TAN, Tran Q, Carlson JA. Intralymphatic histiocytosis of the appendix and fallopian tube associated with primary peritoneal high-grade, poorly differentiated adenocarcinoma of Müllerian origin. Int J Surg Pathol. 2017;25:357-364.
- Echeverría-García B, Botella-Estrada R, Requena C, et al. Intralymphatic histiocytosis and cancer of the colon [in Spanish]. Actas Dermosifiliogr. 2010;101:257-262.
- Ergen EN, Zwerner JP. Cover image: intralymphatic histiocytosis with giant blanching violaceous plaques. Br J Dermatol. 2017;177:325-326.
- Wang Y, Yang H, Tu P. Upper facial swelling: an uncommon manifestation of intralymphatic histiocytosis. Eur J Dermatol. 2012;22:814-815.
- Rhee D-Y, Lee D-W, Chang S-E, et al. Intravascular histiocytosis without rheumatoid arthritis. J Dermatol. 2008;35:691-693.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Asagoe K, Torigoe R, Ofuji R, et al. Reactive intravascular histiocytosis associated with tonsillitis. Br J Dermatol. 2006;154:560-563.
- Pouryazdanparast P, Yu L, Dalton VK, et al. Intravascular histiocytosis presenting with extensive vulvar necrosis. J Cutan Pathol. 2009;(36 suppl 1):1-7.
- Reznitsky M, Daugaard S, Charabi BW. Two rare cases of laryngeal intralymphatic histiocytosis. Eur Arch Otorhinolaryngol. 2016;273:783-788.
- Fujimoto N, Nakanishi G, Manabe T, et al. Intralymphatic histiocytosis comprises M2 macrophages in superficial dermal lymphatics with or without smooth muscles. J Cutan Pathol. 2016;43:898-902.
- Piccolo V, Ruocco E, Russo T, et al. A possible relationship between metal implant-induced intralymphatic histiocytosis and the concept of the immunocompromised district. Int J Dermatol. 2014;53:E365.
Intralymphatic histiocytosis was first described in 1994.1 To date, at least 70 cases have been reported in the English-language literature, the majority being associated with systemic or local inflammatory conditions such as rheumatoid arthritis (RA), malignancy, and metal prostheses. The remaining cases arose independent of any detectable disease process.2 The clinical lesion localizes to areas around surgical scars or inflamed joints and generally presents with erythematous livedoid papules and plaques. Because of its rarity, pathologists and clinicians may be unfamiliar with this entity, leading to delayed or missed diagnoses.
Although the pathogenesis of intralymphatic histiocytosis remains unclear, it may be related to dysregulated immune signaling. The condition follows a chronic, relapsing-remitting course that has shown variable response to topical and systemic treatments. We present a rare case of intralymphatic histiocytosis associated with joint replacement/metal prosthesis3-14 that was responsive to a novel treatment with intralesional steroid injection and pressure bandage.
Case Report
An 89-year-old woman presented with a relapsing and remitting rash on the right calf and popliteal fossa of 11 months’ duration. It was becoming more painful over time and recently began to hurt when walking. Her medical history was remarkable for deep vein thromboses of the bilateral legs, Factor V Leiden deficiency, osteoarthritis, and a popliteal (Baker) cyst on the right leg that ruptured 22 months prior to presentation. Her surgical history included bilateral knee replacements (10 years and 2 years prior to the current presentation for the right and left knees, respectively). Her international normalized ratio (2.0) was therapeutic on warfarin.
Initially, swelling, pain, and redness developed in the right calf, and recurrent right-leg deep venous thrombosis was ruled out by Doppler ultrasound. The findings were considered to be secondary to inflammation from a popliteal cyst. Symptoms persisted despite application of warm compresses, leg elevation, and compression stockings. Treatment with doxycycline prescribed by the patient’s primary care physician 9 months prior for presumed cellulitis produced little improvement. Physical examination revealed a well-healed vertical scar on the right calf from an incisional biopsy within an 8-cm, tender, erythematous, indurated, sclerotic plaque with erythematous streaks radiating from the center of the plaque (Figure 1). There also was red-brown, indurated discoloration on the right shin.

Fine-needle aspiration of the lesion revealed red blood cells and histiocytes. Laboratory studies showed an elevated erythrocyte sedimentation rate of 74 mm/h (reference range, 0–30 mm/h) and a C-reactive protein level of 39 mg/L (reference range, 0–10 mg/L). An incisional biopsy including the muscular fascia showed dense dermal fibrosis with chronic inflammation and scarring. A dermatopathologist (G. A. S.) reviewed the case and confirmed variable fibrosis and chronic inflammation associated with edema in the dermis and epidermal acanthosis. Inspection of vessels in the mid to upper dermis in one area revealed stellate, thin-walled, vascular structures that contained bland epithelioid cells lining the lumen as well as packed within the vessels. The epithelioid cells did not show atypia or mitotic figures, and they did not show intracytoplasmic vacuoles (Figure 2). Immunocytochemical staining for D2-40 was strongly positive in cells lining the vessels, consistent with lymphatics (Figure 3). CD68 immunohistochemistry for histiocytes stained the cells within the lymphatics (Figure 4). A diagnosis of intralymphatic histiocytosis was made.



Intralesional triamcinolone acetonide 10 mg/cc×1.6 cc was injected into the plaque once monthly for 2 consecutive months, and daily compression with a pressure bandage of the right lower leg was initiated. Four months after the first treatment with this regimen, the plaque was smaller and no longer sclerotic or painful, and the erythema was markedly reduced (Figure 5). Clinical and symptomatic improvement continued at 1-year follow-up.

Comment
Intralymphatic histiocytosis is a rare cutaneous disorder defined histologically by histiocytes within the lumina of lymphatics. In addition to the current case, our review of PubMed articles indexed for MEDLINE using the search term intralymphatic histiocytosis yielded more than 70 total cases. The condition has a slight female predominance and typically is seen in individuals over the age of 60 years (age range, 16–89 years).12 Many cases are associated with RA/elevated rheumatoid factor.2,4,8,15-30 At least 9 cases of intralymphatic histiocytosis were associated with premalignant or malignant conditions (ie, adenocarcinoma of the breasts, lungs, and colon; Merkel cell carcinoma; melanoma; melanoma in situ; Mullerian carcinoma, gammopathy).4,15,31-34 Primary disease, defined as occurring in patients who are otherwise healthy, was noted in at least 10 cases.1,2,4,12,35,36 Finally, intralymphatic histiocytosis was identified in areas adjacent to metal implants and joint replacements or exploration in approximately 15 cases (including the current case).3-14,29,37
The condition presents with papules, plaques, and nodules in the setting of characteristic livedoid discoloration; however, some patients present with nonspecific nodules or plaques. Lesions may be symptomatic (eg, pruritic, tender) or asymptomatic. The histologic features of intralymphatic histiocytosis are distinctive but may be focal, as in our case, and the diagnosis is easily missed. The histologic differential diagnosis includes diseases in which intravascular accumulations of cells may be seen, including intravascular B-cell lymphoma, which can be excluded with stains that detect B cells (CD20/CD79a), and reactive angioendotheliomatosis, a benign proliferation of endothelial cells, which may be excluded with stains against endothelial markers (CD31/CD34). The course typically is chronic, and treatment with topical steroids,3,9,15,22,26 cyclophosphamide,15 local radiation,1 thalidomide,35 pentoxifylline,7 and RA medications (eg, prednisolone, methotrexate, nonsteroidal anti-inflammatory drugs, hydroxychloroquine) generally are ineffective.2,16,20,25 Symptoms may improve with joint replacement,4 excision of the involved lesion, treatment of an associated malignancy/infection,33,36,38,39 nonsteroidal anti-inflammatory drugs, intra-articular steroid injection,18 amoxicillin and aspirin,19 infliximab,25 pressure bandage application,26 steroid-containing adhesive application,18 arthrocentesis,3,27 oral pentoxifylline,21 tacrolimus,29 CO2 laser,40 prednisolone,41 and tocilizumab.28 Treatment of associated RA is beneficial in rare cases.2,15,20,25,26
The pathogenesis of intralymphatic histiocytosis has not been elucidated with certainty but may represent an abnormal proliferative response of histiocytes and vessels in response to chronic systemic or local inflammation. Lymphangiectasis caused by lymphatic obstruction secondary to trauma, surgical manipulation, or chronic inflammation can promote lymphostasis and slowed clearance of antigens producing an accumulation of histiocytes and subsequent local immunologic reactions, thus an “immunocompromised district” is formed.42 It also is thought that rheumatic or prosthetic joints produce inflammatory mediator–rich (namely tumor necrosis factor α) synovial fluid that drains and collects within the dilated lymphatics, creating a nidus for histiocytes.1,5 In one case, treatment with an anti–tumor necrosis factor antibody (infliximab) improved the skin presentation and rheumatoid joint pain.25 Bakr et al2 noted an association with increased intralymphatic macrophage HLA-DR expression. This T-cell surface receptor typically is upregulated in cases of chronic antigen stimulation and autoimmune conditions.
Conclusion
Our patient had a history of a joint prosthesis and a popliteal cyst, which could have altered lymphatic drainage promoting abnormal immune cell trafficking contributing to the development of intralymphatic histiocytosis. The response to intralesional steroids supports this pathogenic hypothesis. Specifically, direct injection of the area suppressed the immune dysregulation, while compression lessened the degree of lymphostasis. In light of previously reported cases of intralymphatic histiocytosis in association with metal implants,3-9 we suggest that the condition should be considered in patients with chronic painful livedoid nodules or plaques around an affected joint, even in the absence of RA. The dermatopathologist should be warned to search carefully for the subtle but distinctive histologic features of the disease that confirm the diagnosis. Treatment with intralesional triamcinolone acetonide with an overlying pressure wrap has minimal side effects and can work quickly with sustained benefits.
Intralymphatic histiocytosis was first described in 1994.1 To date, at least 70 cases have been reported in the English-language literature, the majority being associated with systemic or local inflammatory conditions such as rheumatoid arthritis (RA), malignancy, and metal prostheses. The remaining cases arose independent of any detectable disease process.2 The clinical lesion localizes to areas around surgical scars or inflamed joints and generally presents with erythematous livedoid papules and plaques. Because of its rarity, pathologists and clinicians may be unfamiliar with this entity, leading to delayed or missed diagnoses.
Although the pathogenesis of intralymphatic histiocytosis remains unclear, it may be related to dysregulated immune signaling. The condition follows a chronic, relapsing-remitting course that has shown variable response to topical and systemic treatments. We present a rare case of intralymphatic histiocytosis associated with joint replacement/metal prosthesis3-14 that was responsive to a novel treatment with intralesional steroid injection and pressure bandage.
Case Report
An 89-year-old woman presented with a relapsing and remitting rash on the right calf and popliteal fossa of 11 months’ duration. It was becoming more painful over time and recently began to hurt when walking. Her medical history was remarkable for deep vein thromboses of the bilateral legs, Factor V Leiden deficiency, osteoarthritis, and a popliteal (Baker) cyst on the right leg that ruptured 22 months prior to presentation. Her surgical history included bilateral knee replacements (10 years and 2 years prior to the current presentation for the right and left knees, respectively). Her international normalized ratio (2.0) was therapeutic on warfarin.
Initially, swelling, pain, and redness developed in the right calf, and recurrent right-leg deep venous thrombosis was ruled out by Doppler ultrasound. The findings were considered to be secondary to inflammation from a popliteal cyst. Symptoms persisted despite application of warm compresses, leg elevation, and compression stockings. Treatment with doxycycline prescribed by the patient’s primary care physician 9 months prior for presumed cellulitis produced little improvement. Physical examination revealed a well-healed vertical scar on the right calf from an incisional biopsy within an 8-cm, tender, erythematous, indurated, sclerotic plaque with erythematous streaks radiating from the center of the plaque (Figure 1). There also was red-brown, indurated discoloration on the right shin.

Fine-needle aspiration of the lesion revealed red blood cells and histiocytes. Laboratory studies showed an elevated erythrocyte sedimentation rate of 74 mm/h (reference range, 0–30 mm/h) and a C-reactive protein level of 39 mg/L (reference range, 0–10 mg/L). An incisional biopsy including the muscular fascia showed dense dermal fibrosis with chronic inflammation and scarring. A dermatopathologist (G. A. S.) reviewed the case and confirmed variable fibrosis and chronic inflammation associated with edema in the dermis and epidermal acanthosis. Inspection of vessels in the mid to upper dermis in one area revealed stellate, thin-walled, vascular structures that contained bland epithelioid cells lining the lumen as well as packed within the vessels. The epithelioid cells did not show atypia or mitotic figures, and they did not show intracytoplasmic vacuoles (Figure 2). Immunocytochemical staining for D2-40 was strongly positive in cells lining the vessels, consistent with lymphatics (Figure 3). CD68 immunohistochemistry for histiocytes stained the cells within the lymphatics (Figure 4). A diagnosis of intralymphatic histiocytosis was made.



Intralesional triamcinolone acetonide 10 mg/cc×1.6 cc was injected into the plaque once monthly for 2 consecutive months, and daily compression with a pressure bandage of the right lower leg was initiated. Four months after the first treatment with this regimen, the plaque was smaller and no longer sclerotic or painful, and the erythema was markedly reduced (Figure 5). Clinical and symptomatic improvement continued at 1-year follow-up.

Comment
Intralymphatic histiocytosis is a rare cutaneous disorder defined histologically by histiocytes within the lumina of lymphatics. In addition to the current case, our review of PubMed articles indexed for MEDLINE using the search term intralymphatic histiocytosis yielded more than 70 total cases. The condition has a slight female predominance and typically is seen in individuals over the age of 60 years (age range, 16–89 years).12 Many cases are associated with RA/elevated rheumatoid factor.2,4,8,15-30 At least 9 cases of intralymphatic histiocytosis were associated with premalignant or malignant conditions (ie, adenocarcinoma of the breasts, lungs, and colon; Merkel cell carcinoma; melanoma; melanoma in situ; Mullerian carcinoma, gammopathy).4,15,31-34 Primary disease, defined as occurring in patients who are otherwise healthy, was noted in at least 10 cases.1,2,4,12,35,36 Finally, intralymphatic histiocytosis was identified in areas adjacent to metal implants and joint replacements or exploration in approximately 15 cases (including the current case).3-14,29,37
The condition presents with papules, plaques, and nodules in the setting of characteristic livedoid discoloration; however, some patients present with nonspecific nodules or plaques. Lesions may be symptomatic (eg, pruritic, tender) or asymptomatic. The histologic features of intralymphatic histiocytosis are distinctive but may be focal, as in our case, and the diagnosis is easily missed. The histologic differential diagnosis includes diseases in which intravascular accumulations of cells may be seen, including intravascular B-cell lymphoma, which can be excluded with stains that detect B cells (CD20/CD79a), and reactive angioendotheliomatosis, a benign proliferation of endothelial cells, which may be excluded with stains against endothelial markers (CD31/CD34). The course typically is chronic, and treatment with topical steroids,3,9,15,22,26 cyclophosphamide,15 local radiation,1 thalidomide,35 pentoxifylline,7 and RA medications (eg, prednisolone, methotrexate, nonsteroidal anti-inflammatory drugs, hydroxychloroquine) generally are ineffective.2,16,20,25 Symptoms may improve with joint replacement,4 excision of the involved lesion, treatment of an associated malignancy/infection,33,36,38,39 nonsteroidal anti-inflammatory drugs, intra-articular steroid injection,18 amoxicillin and aspirin,19 infliximab,25 pressure bandage application,26 steroid-containing adhesive application,18 arthrocentesis,3,27 oral pentoxifylline,21 tacrolimus,29 CO2 laser,40 prednisolone,41 and tocilizumab.28 Treatment of associated RA is beneficial in rare cases.2,15,20,25,26
The pathogenesis of intralymphatic histiocytosis has not been elucidated with certainty but may represent an abnormal proliferative response of histiocytes and vessels in response to chronic systemic or local inflammation. Lymphangiectasis caused by lymphatic obstruction secondary to trauma, surgical manipulation, or chronic inflammation can promote lymphostasis and slowed clearance of antigens producing an accumulation of histiocytes and subsequent local immunologic reactions, thus an “immunocompromised district” is formed.42 It also is thought that rheumatic or prosthetic joints produce inflammatory mediator–rich (namely tumor necrosis factor α) synovial fluid that drains and collects within the dilated lymphatics, creating a nidus for histiocytes.1,5 In one case, treatment with an anti–tumor necrosis factor antibody (infliximab) improved the skin presentation and rheumatoid joint pain.25 Bakr et al2 noted an association with increased intralymphatic macrophage HLA-DR expression. This T-cell surface receptor typically is upregulated in cases of chronic antigen stimulation and autoimmune conditions.
Conclusion
Our patient had a history of a joint prosthesis and a popliteal cyst, which could have altered lymphatic drainage promoting abnormal immune cell trafficking contributing to the development of intralymphatic histiocytosis. The response to intralesional steroids supports this pathogenic hypothesis. Specifically, direct injection of the area suppressed the immune dysregulation, while compression lessened the degree of lymphostasis. In light of previously reported cases of intralymphatic histiocytosis in association with metal implants,3-9 we suggest that the condition should be considered in patients with chronic painful livedoid nodules or plaques around an affected joint, even in the absence of RA. The dermatopathologist should be warned to search carefully for the subtle but distinctive histologic features of the disease that confirm the diagnosis. Treatment with intralesional triamcinolone acetonide with an overlying pressure wrap has minimal side effects and can work quickly with sustained benefits.
- O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
- Bakr F, Webber N, Fassihi H, et al. Primary and secondary intralymphatic histiocytosis [published online January 17, 2014]. J Am Acad Dermatol. 2014;70:927-933.
- Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants [published online November 10, 2007]. Br J Dermatol. 2008;158:402-404.
- Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. a clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
- Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
- Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants [published online March 6, 2011]. J Cutan Pathol. 2011;38:534-535.
- de Unamuno Bustos B, García Rabasco A, Ballester Sánchez R, et al. Erythematous indurated plaque on the right upper limb. intralymphatic histiocytosis (IH) associated with orthopedic metal implant. Int J Dermatol. 2013;52:547-549.
- Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
- Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
- Bidier M, Hamsch C, Kutzner H, et al. Two cases of intralymphatic histiocytosis following hip replacement [published online June 9, 2015]. J Dtsch Dermatol Ges. 2015;13:700-702.
- Darling MD, Akin R, Tarbox MB, et al. Intralymphatic histiocytosis overlying hip implantation treated with pentoxifilline. J Biol Regul Homeost Agents. 2015;29(1 suppl):117-121.
- Demirkesen C, Kran T, Leblebici C, et al. Intravascular/intralymphatic histiocytosis: a report of 3 cases. Am J Dermatopathol. 2015;37:783-789.
- Gómez-Sánchez ME, Azaña-Defez JM, Martínez-Martínez ML, et al. Intralymphatic histiocytosis: a report of 2 cases. Actas Dermosifiliogr. 2018;109:E1-E5.
- Haitz KA, Chapman MS, Seidel GD. Intralymphatic histiocytosis associated with an orthopedic metal implant. Cutis. 2016;97:E12-E14.
- Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
- Pruim B, Strutton G, Congdon S, et al. Cutaneous histiocytic lymphangitis: an unusual manifestation of rheumatoid arthritis. Australas J Dermatol. 2000;41:101-105.
- Magro CM, Crowson AN. The spectrum of cutaneous lesions in rheumatoid arthritis: a clinical and pathological study of 43 patients. J Cutan Pathol. 2003;30:1-10.
- Takiwaki H, Adachi A, Kohno H, et al. Intravascular or intralymphatic histiocytosis associated with rheumatoid arthritis: a report of 4 cases.J Am Acad Dermatol. 2004;50:585-590.
- Mensing CH, Krengel S, Tronnier M, et al. Reactive angioendotheliomatosis: is it “intravascular histiocytosis”? J Eur Acad Dermatol Venereol. 2005;19:216-219.
- Okazaki A, Asada H, Niizeki H, et al. Intravascular histiocytosis associated with rheumatoid arthritis: report of a case with lymphatic endothelial proliferation. Br J Dermatol. 2005;152:1385-1387.
- Catalina-Fernández I, Alvárez AC, Martin FC, et al. Cutaneous intralymphatic histiocytosis associated with rheumatoid arthritis: report of a case and review of the literature. Am J Dermatopathol. 2007;29:165-168.
- Nishie W, Sawamura D, Iitoyo M, et al. Intravascular histiocytosis associated with rheumatoid arthritis. Dermatology. 2008;217:144-145.
- Okamoto N, Tanioka M, Yamamoto T, et al. Intralymphatic histiocytosis associated with rheumatoid arthritis. Clin Exp Dermatol. 2008;33:516-518.
- Huang H-Y, Liang C-W, Hu S-L, et al. Cutaneous intravascular histiocytosis associated with rheumatoid arthritis: a case report and review of the literature. Clin Exp Dermatol. 2009;34:E302-E303.
- Sakaguchi M, Nagai H, Tsuji G, et al. Effectiveness of infliximab for intralymphatic histiocytosis with rheumatoid arthritis. Arch Dermatol. 2011;147:131-133.
- Washio K, Nakata K, Nakamura A, et al. Pressure bandage as an effective treatment for intralymphatic histiocytosis associated with rheumatoid arthritis. Dermatology. 2011;223:20-24.
- Kaneko T, Takeuchi S, Nakano H, et al. Intralymphatic histiocytosis with rheumatoid arthritis: possible association with the joint involvement. Case Reports Clin Med. 2014;3:149-152.
- Nakajima T, Kawabata D, Nakabo S, et al. Successful treatment with tocilizumab in a case of intralymphatic histiocytosis associated with rheumatoid arthritis. Intern Med. 2014;53:2255-2258.
- Tsujiwaki M, Hata H, Miyauchi T, et al. Warty intralymphatic histiocytosis successfully treated with topical tacrolimus. J Eur Acad Dermatol Venereol. 2015;29:2267-2269.
- Tanaka M, Funasaka Y, Tsuruta K, et al. Intralymphatic histiocytosis with massive interstitial granulomatous foci in a patient with rheumatoid arthritis. Ann Dermatol. 2017;29:237-238.
- Cornejo KM, Cosar EF, O’Donnell P. Cutaneous intralymphatic histiocytosis associated with lung adenocarcinoma. Am J Dermatopathol. 2016;38:568-570.
- Tran TAN, Tran Q, Carlson JA. Intralymphatic histiocytosis of the appendix and fallopian tube associated with primary peritoneal high-grade, poorly differentiated adenocarcinoma of Müllerian origin. Int J Surg Pathol. 2017;25:357-364.
- Echeverría-García B, Botella-Estrada R, Requena C, et al. Intralymphatic histiocytosis and cancer of the colon [in Spanish]. Actas Dermosifiliogr. 2010;101:257-262.
- Ergen EN, Zwerner JP. Cover image: intralymphatic histiocytosis with giant blanching violaceous plaques. Br J Dermatol. 2017;177:325-326.
- Wang Y, Yang H, Tu P. Upper facial swelling: an uncommon manifestation of intralymphatic histiocytosis. Eur J Dermatol. 2012;22:814-815.
- Rhee D-Y, Lee D-W, Chang S-E, et al. Intravascular histiocytosis without rheumatoid arthritis. J Dermatol. 2008;35:691-693.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Asagoe K, Torigoe R, Ofuji R, et al. Reactive intravascular histiocytosis associated with tonsillitis. Br J Dermatol. 2006;154:560-563.
- Pouryazdanparast P, Yu L, Dalton VK, et al. Intravascular histiocytosis presenting with extensive vulvar necrosis. J Cutan Pathol. 2009;(36 suppl 1):1-7.
- Reznitsky M, Daugaard S, Charabi BW. Two rare cases of laryngeal intralymphatic histiocytosis. Eur Arch Otorhinolaryngol. 2016;273:783-788.
- Fujimoto N, Nakanishi G, Manabe T, et al. Intralymphatic histiocytosis comprises M2 macrophages in superficial dermal lymphatics with or without smooth muscles. J Cutan Pathol. 2016;43:898-902.
- Piccolo V, Ruocco E, Russo T, et al. A possible relationship between metal implant-induced intralymphatic histiocytosis and the concept of the immunocompromised district. Int J Dermatol. 2014;53:E365.
- O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
- Bakr F, Webber N, Fassihi H, et al. Primary and secondary intralymphatic histiocytosis [published online January 17, 2014]. J Am Acad Dermatol. 2014;70:927-933.
- Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants [published online November 10, 2007]. Br J Dermatol. 2008;158:402-404.
- Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. a clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
- Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
- Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants [published online March 6, 2011]. J Cutan Pathol. 2011;38:534-535.
- de Unamuno Bustos B, García Rabasco A, Ballester Sánchez R, et al. Erythematous indurated plaque on the right upper limb. intralymphatic histiocytosis (IH) associated with orthopedic metal implant. Int J Dermatol. 2013;52:547-549.
- Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
- Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
- Bidier M, Hamsch C, Kutzner H, et al. Two cases of intralymphatic histiocytosis following hip replacement [published online June 9, 2015]. J Dtsch Dermatol Ges. 2015;13:700-702.
- Darling MD, Akin R, Tarbox MB, et al. Intralymphatic histiocytosis overlying hip implantation treated with pentoxifilline. J Biol Regul Homeost Agents. 2015;29(1 suppl):117-121.
- Demirkesen C, Kran T, Leblebici C, et al. Intravascular/intralymphatic histiocytosis: a report of 3 cases. Am J Dermatopathol. 2015;37:783-789.
- Gómez-Sánchez ME, Azaña-Defez JM, Martínez-Martínez ML, et al. Intralymphatic histiocytosis: a report of 2 cases. Actas Dermosifiliogr. 2018;109:E1-E5.
- Haitz KA, Chapman MS, Seidel GD. Intralymphatic histiocytosis associated with an orthopedic metal implant. Cutis. 2016;97:E12-E14.
- Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
- Pruim B, Strutton G, Congdon S, et al. Cutaneous histiocytic lymphangitis: an unusual manifestation of rheumatoid arthritis. Australas J Dermatol. 2000;41:101-105.
- Magro CM, Crowson AN. The spectrum of cutaneous lesions in rheumatoid arthritis: a clinical and pathological study of 43 patients. J Cutan Pathol. 2003;30:1-10.
- Takiwaki H, Adachi A, Kohno H, et al. Intravascular or intralymphatic histiocytosis associated with rheumatoid arthritis: a report of 4 cases.J Am Acad Dermatol. 2004;50:585-590.
- Mensing CH, Krengel S, Tronnier M, et al. Reactive angioendotheliomatosis: is it “intravascular histiocytosis”? J Eur Acad Dermatol Venereol. 2005;19:216-219.
- Okazaki A, Asada H, Niizeki H, et al. Intravascular histiocytosis associated with rheumatoid arthritis: report of a case with lymphatic endothelial proliferation. Br J Dermatol. 2005;152:1385-1387.
- Catalina-Fernández I, Alvárez AC, Martin FC, et al. Cutaneous intralymphatic histiocytosis associated with rheumatoid arthritis: report of a case and review of the literature. Am J Dermatopathol. 2007;29:165-168.
- Nishie W, Sawamura D, Iitoyo M, et al. Intravascular histiocytosis associated with rheumatoid arthritis. Dermatology. 2008;217:144-145.
- Okamoto N, Tanioka M, Yamamoto T, et al. Intralymphatic histiocytosis associated with rheumatoid arthritis. Clin Exp Dermatol. 2008;33:516-518.
- Huang H-Y, Liang C-W, Hu S-L, et al. Cutaneous intravascular histiocytosis associated with rheumatoid arthritis: a case report and review of the literature. Clin Exp Dermatol. 2009;34:E302-E303.
- Sakaguchi M, Nagai H, Tsuji G, et al. Effectiveness of infliximab for intralymphatic histiocytosis with rheumatoid arthritis. Arch Dermatol. 2011;147:131-133.
- Washio K, Nakata K, Nakamura A, et al. Pressure bandage as an effective treatment for intralymphatic histiocytosis associated with rheumatoid arthritis. Dermatology. 2011;223:20-24.
- Kaneko T, Takeuchi S, Nakano H, et al. Intralymphatic histiocytosis with rheumatoid arthritis: possible association with the joint involvement. Case Reports Clin Med. 2014;3:149-152.
- Nakajima T, Kawabata D, Nakabo S, et al. Successful treatment with tocilizumab in a case of intralymphatic histiocytosis associated with rheumatoid arthritis. Intern Med. 2014;53:2255-2258.
- Tsujiwaki M, Hata H, Miyauchi T, et al. Warty intralymphatic histiocytosis successfully treated with topical tacrolimus. J Eur Acad Dermatol Venereol. 2015;29:2267-2269.
- Tanaka M, Funasaka Y, Tsuruta K, et al. Intralymphatic histiocytosis with massive interstitial granulomatous foci in a patient with rheumatoid arthritis. Ann Dermatol. 2017;29:237-238.
- Cornejo KM, Cosar EF, O’Donnell P. Cutaneous intralymphatic histiocytosis associated with lung adenocarcinoma. Am J Dermatopathol. 2016;38:568-570.
- Tran TAN, Tran Q, Carlson JA. Intralymphatic histiocytosis of the appendix and fallopian tube associated with primary peritoneal high-grade, poorly differentiated adenocarcinoma of Müllerian origin. Int J Surg Pathol. 2017;25:357-364.
- Echeverría-García B, Botella-Estrada R, Requena C, et al. Intralymphatic histiocytosis and cancer of the colon [in Spanish]. Actas Dermosifiliogr. 2010;101:257-262.
- Ergen EN, Zwerner JP. Cover image: intralymphatic histiocytosis with giant blanching violaceous plaques. Br J Dermatol. 2017;177:325-326.
- Wang Y, Yang H, Tu P. Upper facial swelling: an uncommon manifestation of intralymphatic histiocytosis. Eur J Dermatol. 2012;22:814-815.
- Rhee D-Y, Lee D-W, Chang S-E, et al. Intravascular histiocytosis without rheumatoid arthritis. J Dermatol. 2008;35:691-693.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Asagoe K, Torigoe R, Ofuji R, et al. Reactive intravascular histiocytosis associated with tonsillitis. Br J Dermatol. 2006;154:560-563.
- Pouryazdanparast P, Yu L, Dalton VK, et al. Intravascular histiocytosis presenting with extensive vulvar necrosis. J Cutan Pathol. 2009;(36 suppl 1):1-7.
- Reznitsky M, Daugaard S, Charabi BW. Two rare cases of laryngeal intralymphatic histiocytosis. Eur Arch Otorhinolaryngol. 2016;273:783-788.
- Fujimoto N, Nakanishi G, Manabe T, et al. Intralymphatic histiocytosis comprises M2 macrophages in superficial dermal lymphatics with or without smooth muscles. J Cutan Pathol. 2016;43:898-902.
- Piccolo V, Ruocco E, Russo T, et al. A possible relationship between metal implant-induced intralymphatic histiocytosis and the concept of the immunocompromised district. Int J Dermatol. 2014;53:E365.
Practice Points
- Intralymphatic histiocytosis is a rare disorder often associated with rheumatic arthritis and joint prostheses.
- The diagnosis is made by histopathology as well as D2-40 and CD68 immunostaining.
- While there is no gold standard of treatment for intralymphatic histiocytosis, intralesional triamcinolone proved efficacious in this case with prolonged results.
Foundations for financial security: Get out of student debt and on the fast track to financial prosperity
Approaching the concept of retirement savings is particularly unique for medical professionals: Balancing a tremendously demanding career with family life and personal time allows few to have the luxury of extra time to address financial planning. Many in the field have higher priorities than saving for retirement on their minds, which compounds the issue.
According to the Association of American Medical Colleges, 76% of medical students will have student debt by the time they graduate. Among those students, the average debt is a staggering $190,000. The average American couple has only about $163,000 in savings by the time they are about 60 years old, so coming out of school, the average doctor will have more in debt than most have saved in their lifetimes. This means that many doctors can’t really start saving significantly until the latter half of their careers. With that in mind, consider these tips to help you on your journey to financial security.
Pay off debt or start saving for retirement?
When it comes to the decision of investing toward retirement or paying off debt more aggressively, there is really only one question that needs to be answered: “Can I make more investing than the loan is costing me?” Given the fact that Direct Graduate Plus Loans are now sitting at about 7% interest rates, an investment would have to make more than 7% per year to make sense. While we can look back at the historical performance of the stock market over time, it is pretty safe to say that in this scenario paying off the student loans as aggressively as possible is the best choice. The reason being is that the loans have a guaranteed cost of 7% per year in accrued interest, whereas an investment is never fully guaranteed to grow.
Make no mistake: High-interest debt is a financial dead weight; the longer it sits, the more it will attempt to sink you financially. A general rule of thumb is that the higher the interest rate on the loan, the more aggressively it should be paid off. Once the high interest loans are taken care of, saving for retirement can reenter the equation.
The company match
That being said, there is one caveat to this rule that you should strongly consider if the opportunity exists: the 401(k) or 403(b) company match. If you work in a position that offers a match on retirement plan contributions, taking advantage of this could substantially benefit you. In a typical safe harbor retirement plan, you will see something like a 3.5% company match for a 6% salary contribution. While there is no one formula that applies to every situation, an opportunity such as this shouldn’t be passed up.
Saving for retirement can be difficult enough. Why not take advantage of a situation in which you are getting free money? You should think about contributing enough to get the maximum match and putting the rest toward student debt. If you are unsure about your particular course of action, I’d suggest speaking to your financial professional to assist in coming up with a suitable game plan.
Roth deferral option
While many folks understand the general ins and outs of how retirement plans work, they fail to realize that there are actually two different types of contributions that can be made in most 401(k)s and 403(b)s: traditional deferrals and Roth deferrals. A traditional deferral is the standard pretax contribution option that lets you skip paying taxes now. Instead, you get taxed at your normal income tax bracket when the money is withdrawn in retirement. This is the option that most people use.
Roth deferrals are posttax contributions. Every dollar contributed gets taxed as ordinary income. Why would one do this, you ask? While there are a variety of different benefits, the primary advantage is that you never have to pay taxes on this money again. To reiterate, you pay taxes on this money now and never have to pay any taxes on it again. This can be extraordinarily helpful in retirement because it gives you the flexibility to choose between taxable and tax-free income.
While you can contribute to personal Roth IRAs to the tune of $5,500 a year if you are under 50 years old and $6,500 if you are 50 years and older, high income earners can be hampered by income limits. For instance, if you make over $135,000 a year as a single person or over $199,000 as a married couple, you are ineligible to make Roth IRA contributions. However, a benefit to Roth contributions in your company 401(k) or 403(b) plan is that these income limits don’t apply. Regardless of your level of income, you can make Roth deferrals in company sponsored retirement plans that allow them.
This strategy is best fit for, but not limited to, those who are earlier on in their careers and can reasonably expect to make much more in the future than they do now. Younger investors have the benefit of time: The more time an investment has to grow, the more it should be worth later on. Also, younger professionals are probably going to be paying the least amount of taxes early in their careers. While not all retirement plans allow for Roth deferrals, if the option is available, why not get taxes out of the way while it’s still relatively cheaper to do so?
More aggressive strategies for those who need to “catch up” on retirement savings
Because many in the medical field have burdensome student loans, saving for retirement is often something that is pushed off by necessity. That being said, there are different ways to start saving more aggressively the closer you get toward retirement.
Catch up contributions. 401(k)s, 403(b)s, and IRAs all have built in “catch up” contributions that allow those aged 50 or older to save more. For instance, up to age 49 years, the maximum annual contribution in a 401(k) or 403(b) is $18,500 for 2018. At age 50 years, you are allowed to add an additional $6000 “catch up” contribution for a total of $24,500 per year. Likewise, IRAs allow for an additional $1000 per year contribution at age 50 years for a $6500 total yearly contribution.
Spousal IRAs. If you have a nonworking spouse, you may be able to contribute to an IRA on his or her behalf. To be eligible for a spousal IRA contribution, you must be married, file a joint income tax return, and have an earned income of at least what is being contributed to the IRAs. This would allow an additional $5,500 to $6,500 in retirement savings per year depending on your spouse’s age.
Simplified Employee Pension IRAs. For those who are self-employed, it could be worthwhile to look into opening up a Simplified Employee Pension (SEP) IRA. These types of retirement plans are similar to traditional IRAs except that they can only be opened up by an employer. The benefit of a SEP IRA is that it allows for a maximum pretax contribution of up to $54,000 or 25% of your total income, whichever is less.
Cash balance plans. For very-high-earning business owners or sole proprietors, saving $24,500 a year pretax in a 401(k) isn’t necessarily going to move the needle all that much. However, there is a plan available that may help tremendously. The cash balance plan is a little known hybrid retirement plan that allows high-earning practices and business owners to put away a serious amount of money in a short amount of time. For instance, an optimally set up cash balance plan would allow a 59 year old to save up to $278,000 in qualified pretax dollars in a single year. Undoubtedly, such plans are one of the most effective and efficient ways to save money for retirement for those who qualify.
Check out the maximum contributions limits of some retirement strategies below based on your age group.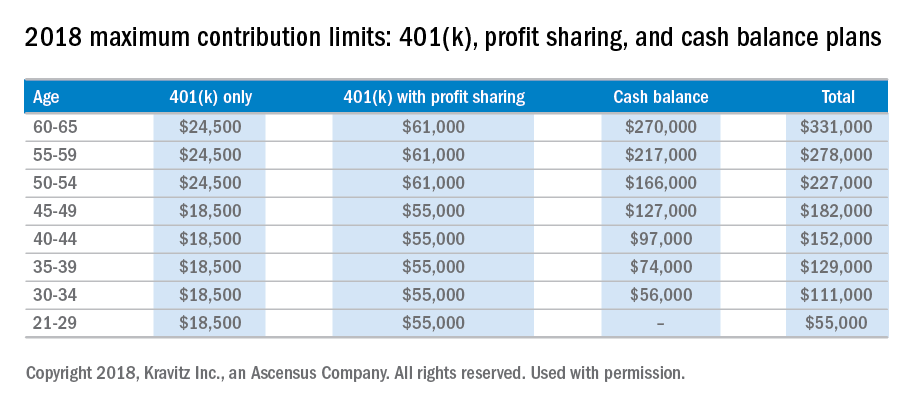
It’s important to realize that it is time that makes money, not timing. Establishing a smart investment plan early in your career will pay huge dividends and save mountains of heartache in your future. If you are unsure about which retirement plan works best for you, I’d recommend speaking to a qualified financial professional to assist you in the process. When it comes to planning for retirement, sooner is always better than later. The financial sacrifices of starting early are never as great as most young professionals fear.
Disclaimer:
This material has been prepared for educational purposes only and is not intended to provide, and should not be relied on for tax, legal, accounting advice, or financial advice. You should consult your own tax, legal, accounting, and financial advisors before engaging in any transaction. Securities offered through Capitol Securities Management Member FINRA, SIPC
Mr. Bellamah is a registered investment advisor with BNB Wealth Management http://www.bnbinc.com/.
Approaching the concept of retirement savings is particularly unique for medical professionals: Balancing a tremendously demanding career with family life and personal time allows few to have the luxury of extra time to address financial planning. Many in the field have higher priorities than saving for retirement on their minds, which compounds the issue.
According to the Association of American Medical Colleges, 76% of medical students will have student debt by the time they graduate. Among those students, the average debt is a staggering $190,000. The average American couple has only about $163,000 in savings by the time they are about 60 years old, so coming out of school, the average doctor will have more in debt than most have saved in their lifetimes. This means that many doctors can’t really start saving significantly until the latter half of their careers. With that in mind, consider these tips to help you on your journey to financial security.
Pay off debt or start saving for retirement?
When it comes to the decision of investing toward retirement or paying off debt more aggressively, there is really only one question that needs to be answered: “Can I make more investing than the loan is costing me?” Given the fact that Direct Graduate Plus Loans are now sitting at about 7% interest rates, an investment would have to make more than 7% per year to make sense. While we can look back at the historical performance of the stock market over time, it is pretty safe to say that in this scenario paying off the student loans as aggressively as possible is the best choice. The reason being is that the loans have a guaranteed cost of 7% per year in accrued interest, whereas an investment is never fully guaranteed to grow.
Make no mistake: High-interest debt is a financial dead weight; the longer it sits, the more it will attempt to sink you financially. A general rule of thumb is that the higher the interest rate on the loan, the more aggressively it should be paid off. Once the high interest loans are taken care of, saving for retirement can reenter the equation.
The company match
That being said, there is one caveat to this rule that you should strongly consider if the opportunity exists: the 401(k) or 403(b) company match. If you work in a position that offers a match on retirement plan contributions, taking advantage of this could substantially benefit you. In a typical safe harbor retirement plan, you will see something like a 3.5% company match for a 6% salary contribution. While there is no one formula that applies to every situation, an opportunity such as this shouldn’t be passed up.
Saving for retirement can be difficult enough. Why not take advantage of a situation in which you are getting free money? You should think about contributing enough to get the maximum match and putting the rest toward student debt. If you are unsure about your particular course of action, I’d suggest speaking to your financial professional to assist in coming up with a suitable game plan.
Roth deferral option
While many folks understand the general ins and outs of how retirement plans work, they fail to realize that there are actually two different types of contributions that can be made in most 401(k)s and 403(b)s: traditional deferrals and Roth deferrals. A traditional deferral is the standard pretax contribution option that lets you skip paying taxes now. Instead, you get taxed at your normal income tax bracket when the money is withdrawn in retirement. This is the option that most people use.
Roth deferrals are posttax contributions. Every dollar contributed gets taxed as ordinary income. Why would one do this, you ask? While there are a variety of different benefits, the primary advantage is that you never have to pay taxes on this money again. To reiterate, you pay taxes on this money now and never have to pay any taxes on it again. This can be extraordinarily helpful in retirement because it gives you the flexibility to choose between taxable and tax-free income.
While you can contribute to personal Roth IRAs to the tune of $5,500 a year if you are under 50 years old and $6,500 if you are 50 years and older, high income earners can be hampered by income limits. For instance, if you make over $135,000 a year as a single person or over $199,000 as a married couple, you are ineligible to make Roth IRA contributions. However, a benefit to Roth contributions in your company 401(k) or 403(b) plan is that these income limits don’t apply. Regardless of your level of income, you can make Roth deferrals in company sponsored retirement plans that allow them.
This strategy is best fit for, but not limited to, those who are earlier on in their careers and can reasonably expect to make much more in the future than they do now. Younger investors have the benefit of time: The more time an investment has to grow, the more it should be worth later on. Also, younger professionals are probably going to be paying the least amount of taxes early in their careers. While not all retirement plans allow for Roth deferrals, if the option is available, why not get taxes out of the way while it’s still relatively cheaper to do so?
More aggressive strategies for those who need to “catch up” on retirement savings
Because many in the medical field have burdensome student loans, saving for retirement is often something that is pushed off by necessity. That being said, there are different ways to start saving more aggressively the closer you get toward retirement.
Catch up contributions. 401(k)s, 403(b)s, and IRAs all have built in “catch up” contributions that allow those aged 50 or older to save more. For instance, up to age 49 years, the maximum annual contribution in a 401(k) or 403(b) is $18,500 for 2018. At age 50 years, you are allowed to add an additional $6000 “catch up” contribution for a total of $24,500 per year. Likewise, IRAs allow for an additional $1000 per year contribution at age 50 years for a $6500 total yearly contribution.
Spousal IRAs. If you have a nonworking spouse, you may be able to contribute to an IRA on his or her behalf. To be eligible for a spousal IRA contribution, you must be married, file a joint income tax return, and have an earned income of at least what is being contributed to the IRAs. This would allow an additional $5,500 to $6,500 in retirement savings per year depending on your spouse’s age.
Simplified Employee Pension IRAs. For those who are self-employed, it could be worthwhile to look into opening up a Simplified Employee Pension (SEP) IRA. These types of retirement plans are similar to traditional IRAs except that they can only be opened up by an employer. The benefit of a SEP IRA is that it allows for a maximum pretax contribution of up to $54,000 or 25% of your total income, whichever is less.
Cash balance plans. For very-high-earning business owners or sole proprietors, saving $24,500 a year pretax in a 401(k) isn’t necessarily going to move the needle all that much. However, there is a plan available that may help tremendously. The cash balance plan is a little known hybrid retirement plan that allows high-earning practices and business owners to put away a serious amount of money in a short amount of time. For instance, an optimally set up cash balance plan would allow a 59 year old to save up to $278,000 in qualified pretax dollars in a single year. Undoubtedly, such plans are one of the most effective and efficient ways to save money for retirement for those who qualify.
Check out the maximum contributions limits of some retirement strategies below based on your age group.
It’s important to realize that it is time that makes money, not timing. Establishing a smart investment plan early in your career will pay huge dividends and save mountains of heartache in your future. If you are unsure about which retirement plan works best for you, I’d recommend speaking to a qualified financial professional to assist you in the process. When it comes to planning for retirement, sooner is always better than later. The financial sacrifices of starting early are never as great as most young professionals fear.
Disclaimer:
This material has been prepared for educational purposes only and is not intended to provide, and should not be relied on for tax, legal, accounting advice, or financial advice. You should consult your own tax, legal, accounting, and financial advisors before engaging in any transaction. Securities offered through Capitol Securities Management Member FINRA, SIPC
Mr. Bellamah is a registered investment advisor with BNB Wealth Management http://www.bnbinc.com/.
Approaching the concept of retirement savings is particularly unique for medical professionals: Balancing a tremendously demanding career with family life and personal time allows few to have the luxury of extra time to address financial planning. Many in the field have higher priorities than saving for retirement on their minds, which compounds the issue.
According to the Association of American Medical Colleges, 76% of medical students will have student debt by the time they graduate. Among those students, the average debt is a staggering $190,000. The average American couple has only about $163,000 in savings by the time they are about 60 years old, so coming out of school, the average doctor will have more in debt than most have saved in their lifetimes. This means that many doctors can’t really start saving significantly until the latter half of their careers. With that in mind, consider these tips to help you on your journey to financial security.
Pay off debt or start saving for retirement?
When it comes to the decision of investing toward retirement or paying off debt more aggressively, there is really only one question that needs to be answered: “Can I make more investing than the loan is costing me?” Given the fact that Direct Graduate Plus Loans are now sitting at about 7% interest rates, an investment would have to make more than 7% per year to make sense. While we can look back at the historical performance of the stock market over time, it is pretty safe to say that in this scenario paying off the student loans as aggressively as possible is the best choice. The reason being is that the loans have a guaranteed cost of 7% per year in accrued interest, whereas an investment is never fully guaranteed to grow.
Make no mistake: High-interest debt is a financial dead weight; the longer it sits, the more it will attempt to sink you financially. A general rule of thumb is that the higher the interest rate on the loan, the more aggressively it should be paid off. Once the high interest loans are taken care of, saving for retirement can reenter the equation.
The company match
That being said, there is one caveat to this rule that you should strongly consider if the opportunity exists: the 401(k) or 403(b) company match. If you work in a position that offers a match on retirement plan contributions, taking advantage of this could substantially benefit you. In a typical safe harbor retirement plan, you will see something like a 3.5% company match for a 6% salary contribution. While there is no one formula that applies to every situation, an opportunity such as this shouldn’t be passed up.
Saving for retirement can be difficult enough. Why not take advantage of a situation in which you are getting free money? You should think about contributing enough to get the maximum match and putting the rest toward student debt. If you are unsure about your particular course of action, I’d suggest speaking to your financial professional to assist in coming up with a suitable game plan.
Roth deferral option
While many folks understand the general ins and outs of how retirement plans work, they fail to realize that there are actually two different types of contributions that can be made in most 401(k)s and 403(b)s: traditional deferrals and Roth deferrals. A traditional deferral is the standard pretax contribution option that lets you skip paying taxes now. Instead, you get taxed at your normal income tax bracket when the money is withdrawn in retirement. This is the option that most people use.
Roth deferrals are posttax contributions. Every dollar contributed gets taxed as ordinary income. Why would one do this, you ask? While there are a variety of different benefits, the primary advantage is that you never have to pay taxes on this money again. To reiterate, you pay taxes on this money now and never have to pay any taxes on it again. This can be extraordinarily helpful in retirement because it gives you the flexibility to choose between taxable and tax-free income.
While you can contribute to personal Roth IRAs to the tune of $5,500 a year if you are under 50 years old and $6,500 if you are 50 years and older, high income earners can be hampered by income limits. For instance, if you make over $135,000 a year as a single person or over $199,000 as a married couple, you are ineligible to make Roth IRA contributions. However, a benefit to Roth contributions in your company 401(k) or 403(b) plan is that these income limits don’t apply. Regardless of your level of income, you can make Roth deferrals in company sponsored retirement plans that allow them.
This strategy is best fit for, but not limited to, those who are earlier on in their careers and can reasonably expect to make much more in the future than they do now. Younger investors have the benefit of time: The more time an investment has to grow, the more it should be worth later on. Also, younger professionals are probably going to be paying the least amount of taxes early in their careers. While not all retirement plans allow for Roth deferrals, if the option is available, why not get taxes out of the way while it’s still relatively cheaper to do so?
More aggressive strategies for those who need to “catch up” on retirement savings
Because many in the medical field have burdensome student loans, saving for retirement is often something that is pushed off by necessity. That being said, there are different ways to start saving more aggressively the closer you get toward retirement.
Catch up contributions. 401(k)s, 403(b)s, and IRAs all have built in “catch up” contributions that allow those aged 50 or older to save more. For instance, up to age 49 years, the maximum annual contribution in a 401(k) or 403(b) is $18,500 for 2018. At age 50 years, you are allowed to add an additional $6000 “catch up” contribution for a total of $24,500 per year. Likewise, IRAs allow for an additional $1000 per year contribution at age 50 years for a $6500 total yearly contribution.
Spousal IRAs. If you have a nonworking spouse, you may be able to contribute to an IRA on his or her behalf. To be eligible for a spousal IRA contribution, you must be married, file a joint income tax return, and have an earned income of at least what is being contributed to the IRAs. This would allow an additional $5,500 to $6,500 in retirement savings per year depending on your spouse’s age.
Simplified Employee Pension IRAs. For those who are self-employed, it could be worthwhile to look into opening up a Simplified Employee Pension (SEP) IRA. These types of retirement plans are similar to traditional IRAs except that they can only be opened up by an employer. The benefit of a SEP IRA is that it allows for a maximum pretax contribution of up to $54,000 or 25% of your total income, whichever is less.
Cash balance plans. For very-high-earning business owners or sole proprietors, saving $24,500 a year pretax in a 401(k) isn’t necessarily going to move the needle all that much. However, there is a plan available that may help tremendously. The cash balance plan is a little known hybrid retirement plan that allows high-earning practices and business owners to put away a serious amount of money in a short amount of time. For instance, an optimally set up cash balance plan would allow a 59 year old to save up to $278,000 in qualified pretax dollars in a single year. Undoubtedly, such plans are one of the most effective and efficient ways to save money for retirement for those who qualify.
Check out the maximum contributions limits of some retirement strategies below based on your age group.
It’s important to realize that it is time that makes money, not timing. Establishing a smart investment plan early in your career will pay huge dividends and save mountains of heartache in your future. If you are unsure about which retirement plan works best for you, I’d recommend speaking to a qualified financial professional to assist you in the process. When it comes to planning for retirement, sooner is always better than later. The financial sacrifices of starting early are never as great as most young professionals fear.
Disclaimer:
This material has been prepared for educational purposes only and is not intended to provide, and should not be relied on for tax, legal, accounting advice, or financial advice. You should consult your own tax, legal, accounting, and financial advisors before engaging in any transaction. Securities offered through Capitol Securities Management Member FINRA, SIPC
Mr. Bellamah is a registered investment advisor with BNB Wealth Management http://www.bnbinc.com/.
Time to scrap LMWH for prevention of placenta-mediated pregnancy complications?
SAN DIEGO – Low molecular weight heparin does not appear to reduce the risk of recurrent placenta-mediated pregnancy complications in women with prior such complications, according to Marc Rodger, MD.
“It’s time to put the needles away for pregnant patients,” he said at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The pathophysiology of placenta-mediated pregnancy complications includes placental thrombosis. Thrombophilias predispose to the development of thrombosis in slow-flow circulation of the placenta. “It’s possible that the etiology mix of placental-mediated pregnancy complications includes thrombophilias, and by extension, that anticoagulants would prevent these complications,” said Dr. Rodger, a senior scientist at the hospital and professor at the University of Ottawa.
In a study from 1999, researchers demonstrated that patients with pregnancy-mediated placental complications were 8.2 times more likely to develop thrombophilia, compared with controls (N Engl J Med. 1999;340:9-13). “But as with positive initial case-control studies, subsequent work downplayed this association,” Dr. Rodger said. “Now, we’re at a point where we recognize that thrombophilias are weakly associated with recurrent early loss, late pregnancy loss, and severe preeclampsia ([odds ratio] of about 1.5-2.0 for all associations), while thrombophilias are not associated with nonsevere preeclampsia and small for gestational age.”
Currently, low-molecular-weight heparin (LMWH) is the preferred pharmacoprophylaxis in pregnancy. Unfractionated heparin, meanwhile, requires b.i.d. or t.i.d. injections, and has a 10-fold higher risk of heparin-induced thrombocytopenia and a greater than 10-fold higher risk of osteoporotic fracture. Warfarin is teratogenic antepartum and inconvenient postpartum, while direct oral anticoagulants cross the placenta and enter breast milk.
Downsides of LMWH include the burden of self-injections and costs of over $10,000 per antepartum period, Dr. Rodger said. Common side effects include minor bleeding and elevated liver function tests, and it complicates regional anesthetic options at term. Uncommon side effects include major bleeding, skin reactions, and postpartum wound complications, while rare but serious complications include heparin-induced thrombocytopenia and osteoporotic fractures.
He offered a hypothetical case. A 32-year-old woman with prior severe preeclampsia who delivered at 32 weeks asks you, “Should I be treated with LMWH in my next pregnancy?” What should you tell her? To answer this question, Dr. Rodger and his associates conducted a study-level meta-analysis of six randomized controlled trials that included 848 pregnant women with prior placenta-mediated pregnancy complications (Blood. 2014;123[6]:822-8). The primary objective was to determine the effect of LMWH in preventing placenta-mediated pregnancy complications in women with prior late placenta-mediated pregnancy complications. This included patients with or without thrombophilia who were treated with or without LMWH. The primary outcome was a composite of preeclampsia, birth of an SGA newborn, placental abruption, or pregnancy loss greater than 20 weeks. Overall, 67 (18.7%) of 358 of women being given prophylactic LMWH had recurrent severe placenta-mediated pregnancy complications, compared with 127 (42.9%) of 296 women with no LMWH (relative risk reduction, 0.52; P = .01, indicating moderate heterogeneity). They identified similar relative risk reductions with LMWH for individual outcomes, including any preeclampsia, severe preeclampsia, SGA below the 10th percentile, SGA below the 5th percentile, preterm delivery less than 37 weeks, and preterm delivery less than 34 weeks with minimal heterogeneity. They concluded that LMWH “may be a promising therapy for recurrent, especially severe, placenta-mediated pregnancy complications, but further research is required.”
At the meeting, Dr. Rodger noted that the positive studies in the analysis were single-center trials, “which are generally acknowledged to be of a lesser methodologic quality, and the majority of patients in these single-center trials are from a small area in the south of France. Multicenter trials don’t show an effect, so is it single-centeredness or is it something else? The other feature that’s distinct is that the positive trials recruited patients with prior severe complications only, while the negative trials included patients with nonsevere complications. So maybe LMWH works in patients who have a very strong phenotype that have had very bad prior complications. We can’t tease that out with a study-level meta-analysis because we’re getting average effects over heterogeneous groups of patients.”
To expand on the study-level meta-analysis, Dr. Rodger and his associates conducted a systematic review and individual patient data meta-analysis of eight randomized trials of 963 patients conducted between 2000 and 2013 of LMWH to prevent recurrent placenta-mediated pregnancy complications (Lancet. 2016;388:2629-41). “In this approach you get individual patient data from the trials, and you create a new randomized, controlled data set,” he explained. “That way we could tease out the patients who have had the prior severe complications and whether their mild or severe outcomes are being prevented or not.”
The study’s composite primary outcome was one or more of the following: early-onset or severe preeclampsia, SGA newborn below the 5th percentile, late pregnancy loss (over 20 weeks), or placental abruption. Dr. Rodger and his associates found that LMWH did not significantly reduce the risk of recurrent placenta-mediated pregnancy complications, compared with patients who did not receive LMWH (14% vs. 22%, respectively; P = .09). In subgroup analyses, however, LMWH in multicenter trials reduced the primary outcome in women with previous abruption (P = .006) but not in any of the other subgroups of previous complications. “There were small numbers of patients in this subgroup, though, so I would use caution,” Dr. Rodger said. Two recent randomized, controlled trials from separate investigators further support the overall null findings of the individual patient data meta-analysis (Obstet Gynecol. 2016;128[5]:1053-63 and Am J Obstet Gynecol. 2017 Mar;216[3]:296.e1-296.e14).
Revisiting the hypothetical case of a 32-year-old woman with prior severe preeclampsia who delivered at 32 weeks, Dr. Rodger said that he would “definitely not” recommend LMWH during her next pregnancy.
He acknowledged limitations of the systematic review, including the limited numbers of patients in subgroups and the large differences between single-center and multicenter trials. “We still can’t explain this, and it remains an open question that bugs me,” he said. “This has been seen in many disease areas. Empirically, single-centeredness leans toward positivity.”
He called for more research in women with recurrent pregnancy loss. Dr. Rodger reported having no financial disclosures.
SAN DIEGO – Low molecular weight heparin does not appear to reduce the risk of recurrent placenta-mediated pregnancy complications in women with prior such complications, according to Marc Rodger, MD.
“It’s time to put the needles away for pregnant patients,” he said at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The pathophysiology of placenta-mediated pregnancy complications includes placental thrombosis. Thrombophilias predispose to the development of thrombosis in slow-flow circulation of the placenta. “It’s possible that the etiology mix of placental-mediated pregnancy complications includes thrombophilias, and by extension, that anticoagulants would prevent these complications,” said Dr. Rodger, a senior scientist at the hospital and professor at the University of Ottawa.
In a study from 1999, researchers demonstrated that patients with pregnancy-mediated placental complications were 8.2 times more likely to develop thrombophilia, compared with controls (N Engl J Med. 1999;340:9-13). “But as with positive initial case-control studies, subsequent work downplayed this association,” Dr. Rodger said. “Now, we’re at a point where we recognize that thrombophilias are weakly associated with recurrent early loss, late pregnancy loss, and severe preeclampsia ([odds ratio] of about 1.5-2.0 for all associations), while thrombophilias are not associated with nonsevere preeclampsia and small for gestational age.”
Currently, low-molecular-weight heparin (LMWH) is the preferred pharmacoprophylaxis in pregnancy. Unfractionated heparin, meanwhile, requires b.i.d. or t.i.d. injections, and has a 10-fold higher risk of heparin-induced thrombocytopenia and a greater than 10-fold higher risk of osteoporotic fracture. Warfarin is teratogenic antepartum and inconvenient postpartum, while direct oral anticoagulants cross the placenta and enter breast milk.
Downsides of LMWH include the burden of self-injections and costs of over $10,000 per antepartum period, Dr. Rodger said. Common side effects include minor bleeding and elevated liver function tests, and it complicates regional anesthetic options at term. Uncommon side effects include major bleeding, skin reactions, and postpartum wound complications, while rare but serious complications include heparin-induced thrombocytopenia and osteoporotic fractures.
He offered a hypothetical case. A 32-year-old woman with prior severe preeclampsia who delivered at 32 weeks asks you, “Should I be treated with LMWH in my next pregnancy?” What should you tell her? To answer this question, Dr. Rodger and his associates conducted a study-level meta-analysis of six randomized controlled trials that included 848 pregnant women with prior placenta-mediated pregnancy complications (Blood. 2014;123[6]:822-8). The primary objective was to determine the effect of LMWH in preventing placenta-mediated pregnancy complications in women with prior late placenta-mediated pregnancy complications. This included patients with or without thrombophilia who were treated with or without LMWH. The primary outcome was a composite of preeclampsia, birth of an SGA newborn, placental abruption, or pregnancy loss greater than 20 weeks. Overall, 67 (18.7%) of 358 of women being given prophylactic LMWH had recurrent severe placenta-mediated pregnancy complications, compared with 127 (42.9%) of 296 women with no LMWH (relative risk reduction, 0.52; P = .01, indicating moderate heterogeneity). They identified similar relative risk reductions with LMWH for individual outcomes, including any preeclampsia, severe preeclampsia, SGA below the 10th percentile, SGA below the 5th percentile, preterm delivery less than 37 weeks, and preterm delivery less than 34 weeks with minimal heterogeneity. They concluded that LMWH “may be a promising therapy for recurrent, especially severe, placenta-mediated pregnancy complications, but further research is required.”
At the meeting, Dr. Rodger noted that the positive studies in the analysis were single-center trials, “which are generally acknowledged to be of a lesser methodologic quality, and the majority of patients in these single-center trials are from a small area in the south of France. Multicenter trials don’t show an effect, so is it single-centeredness or is it something else? The other feature that’s distinct is that the positive trials recruited patients with prior severe complications only, while the negative trials included patients with nonsevere complications. So maybe LMWH works in patients who have a very strong phenotype that have had very bad prior complications. We can’t tease that out with a study-level meta-analysis because we’re getting average effects over heterogeneous groups of patients.”
To expand on the study-level meta-analysis, Dr. Rodger and his associates conducted a systematic review and individual patient data meta-analysis of eight randomized trials of 963 patients conducted between 2000 and 2013 of LMWH to prevent recurrent placenta-mediated pregnancy complications (Lancet. 2016;388:2629-41). “In this approach you get individual patient data from the trials, and you create a new randomized, controlled data set,” he explained. “That way we could tease out the patients who have had the prior severe complications and whether their mild or severe outcomes are being prevented or not.”
The study’s composite primary outcome was one or more of the following: early-onset or severe preeclampsia, SGA newborn below the 5th percentile, late pregnancy loss (over 20 weeks), or placental abruption. Dr. Rodger and his associates found that LMWH did not significantly reduce the risk of recurrent placenta-mediated pregnancy complications, compared with patients who did not receive LMWH (14% vs. 22%, respectively; P = .09). In subgroup analyses, however, LMWH in multicenter trials reduced the primary outcome in women with previous abruption (P = .006) but not in any of the other subgroups of previous complications. “There were small numbers of patients in this subgroup, though, so I would use caution,” Dr. Rodger said. Two recent randomized, controlled trials from separate investigators further support the overall null findings of the individual patient data meta-analysis (Obstet Gynecol. 2016;128[5]:1053-63 and Am J Obstet Gynecol. 2017 Mar;216[3]:296.e1-296.e14).
Revisiting the hypothetical case of a 32-year-old woman with prior severe preeclampsia who delivered at 32 weeks, Dr. Rodger said that he would “definitely not” recommend LMWH during her next pregnancy.
He acknowledged limitations of the systematic review, including the limited numbers of patients in subgroups and the large differences between single-center and multicenter trials. “We still can’t explain this, and it remains an open question that bugs me,” he said. “This has been seen in many disease areas. Empirically, single-centeredness leans toward positivity.”
He called for more research in women with recurrent pregnancy loss. Dr. Rodger reported having no financial disclosures.
SAN DIEGO – Low molecular weight heparin does not appear to reduce the risk of recurrent placenta-mediated pregnancy complications in women with prior such complications, according to Marc Rodger, MD.
“It’s time to put the needles away for pregnant patients,” he said at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The pathophysiology of placenta-mediated pregnancy complications includes placental thrombosis. Thrombophilias predispose to the development of thrombosis in slow-flow circulation of the placenta. “It’s possible that the etiology mix of placental-mediated pregnancy complications includes thrombophilias, and by extension, that anticoagulants would prevent these complications,” said Dr. Rodger, a senior scientist at the hospital and professor at the University of Ottawa.
In a study from 1999, researchers demonstrated that patients with pregnancy-mediated placental complications were 8.2 times more likely to develop thrombophilia, compared with controls (N Engl J Med. 1999;340:9-13). “But as with positive initial case-control studies, subsequent work downplayed this association,” Dr. Rodger said. “Now, we’re at a point where we recognize that thrombophilias are weakly associated with recurrent early loss, late pregnancy loss, and severe preeclampsia ([odds ratio] of about 1.5-2.0 for all associations), while thrombophilias are not associated with nonsevere preeclampsia and small for gestational age.”
Currently, low-molecular-weight heparin (LMWH) is the preferred pharmacoprophylaxis in pregnancy. Unfractionated heparin, meanwhile, requires b.i.d. or t.i.d. injections, and has a 10-fold higher risk of heparin-induced thrombocytopenia and a greater than 10-fold higher risk of osteoporotic fracture. Warfarin is teratogenic antepartum and inconvenient postpartum, while direct oral anticoagulants cross the placenta and enter breast milk.
Downsides of LMWH include the burden of self-injections and costs of over $10,000 per antepartum period, Dr. Rodger said. Common side effects include minor bleeding and elevated liver function tests, and it complicates regional anesthetic options at term. Uncommon side effects include major bleeding, skin reactions, and postpartum wound complications, while rare but serious complications include heparin-induced thrombocytopenia and osteoporotic fractures.
He offered a hypothetical case. A 32-year-old woman with prior severe preeclampsia who delivered at 32 weeks asks you, “Should I be treated with LMWH in my next pregnancy?” What should you tell her? To answer this question, Dr. Rodger and his associates conducted a study-level meta-analysis of six randomized controlled trials that included 848 pregnant women with prior placenta-mediated pregnancy complications (Blood. 2014;123[6]:822-8). The primary objective was to determine the effect of LMWH in preventing placenta-mediated pregnancy complications in women with prior late placenta-mediated pregnancy complications. This included patients with or without thrombophilia who were treated with or without LMWH. The primary outcome was a composite of preeclampsia, birth of an SGA newborn, placental abruption, or pregnancy loss greater than 20 weeks. Overall, 67 (18.7%) of 358 of women being given prophylactic LMWH had recurrent severe placenta-mediated pregnancy complications, compared with 127 (42.9%) of 296 women with no LMWH (relative risk reduction, 0.52; P = .01, indicating moderate heterogeneity). They identified similar relative risk reductions with LMWH for individual outcomes, including any preeclampsia, severe preeclampsia, SGA below the 10th percentile, SGA below the 5th percentile, preterm delivery less than 37 weeks, and preterm delivery less than 34 weeks with minimal heterogeneity. They concluded that LMWH “may be a promising therapy for recurrent, especially severe, placenta-mediated pregnancy complications, but further research is required.”
At the meeting, Dr. Rodger noted that the positive studies in the analysis were single-center trials, “which are generally acknowledged to be of a lesser methodologic quality, and the majority of patients in these single-center trials are from a small area in the south of France. Multicenter trials don’t show an effect, so is it single-centeredness or is it something else? The other feature that’s distinct is that the positive trials recruited patients with prior severe complications only, while the negative trials included patients with nonsevere complications. So maybe LMWH works in patients who have a very strong phenotype that have had very bad prior complications. We can’t tease that out with a study-level meta-analysis because we’re getting average effects over heterogeneous groups of patients.”
To expand on the study-level meta-analysis, Dr. Rodger and his associates conducted a systematic review and individual patient data meta-analysis of eight randomized trials of 963 patients conducted between 2000 and 2013 of LMWH to prevent recurrent placenta-mediated pregnancy complications (Lancet. 2016;388:2629-41). “In this approach you get individual patient data from the trials, and you create a new randomized, controlled data set,” he explained. “That way we could tease out the patients who have had the prior severe complications and whether their mild or severe outcomes are being prevented or not.”
The study’s composite primary outcome was one or more of the following: early-onset or severe preeclampsia, SGA newborn below the 5th percentile, late pregnancy loss (over 20 weeks), or placental abruption. Dr. Rodger and his associates found that LMWH did not significantly reduce the risk of recurrent placenta-mediated pregnancy complications, compared with patients who did not receive LMWH (14% vs. 22%, respectively; P = .09). In subgroup analyses, however, LMWH in multicenter trials reduced the primary outcome in women with previous abruption (P = .006) but not in any of the other subgroups of previous complications. “There were small numbers of patients in this subgroup, though, so I would use caution,” Dr. Rodger said. Two recent randomized, controlled trials from separate investigators further support the overall null findings of the individual patient data meta-analysis (Obstet Gynecol. 2016;128[5]:1053-63 and Am J Obstet Gynecol. 2017 Mar;216[3]:296.e1-296.e14).
Revisiting the hypothetical case of a 32-year-old woman with prior severe preeclampsia who delivered at 32 weeks, Dr. Rodger said that he would “definitely not” recommend LMWH during her next pregnancy.
He acknowledged limitations of the systematic review, including the limited numbers of patients in subgroups and the large differences between single-center and multicenter trials. “We still can’t explain this, and it remains an open question that bugs me,” he said. “This has been seen in many disease areas. Empirically, single-centeredness leans toward positivity.”
He called for more research in women with recurrent pregnancy loss. Dr. Rodger reported having no financial disclosures.
REPORTING FROM THSNA 2018
Why do you need to know about bipolar disorder?
Diagnosing and initiating treatment for bipolar disorders in children deserve the skills of a child psychiatrist. So why do we really need to know about these complicated conditions as pediatricians?
Although the prevalence of bipolar disorder is about 4% of the adult population, first presentation of significant symptoms usually occurs in adolescence (1% prevalence) with many affected adults recalling symptoms in childhood. To be called a disorder, symptoms have to significantly impact daily functioning. That means these children and their families – and often their schoolmates and teachers – are struggling, whether a diagnosis has been made or not. While bipolar disorder is clearly heritable (emerging in 10% of children of affected adults), environmental stresses such as trauma, family discord, life transitions, academic pressure, illness, puberty, and even lack of sleep can bring out a cycle of symptoms. There also is evidence that earlier treatment may reduce symptoms long term. These factors mean that we need to be vigilant for signs that behavior or mood problems actually are symptoms of bipolar disorder and take action.
While uncommon, medical conditions such as epilepsy, hyperthyroidism, multiple sclerosis, strokes, tumors, and infections are in the differential we are best positioned to consider. Prescribed medications such as steroids, Singulair, Accutane and, of course, illicit drugs such as cocaine and misused amphetamines also can cause severe mood changes. The psychiatric differential includes depression, ADHD, oppositional defiant disorder or conduct disorder, disruptive mood dysregulation disorder, generalized anxiety disorder, autism, substance use, and personality disorder.
The first thing you need to understand is the signs of mania, the feature that distinguishes depression (unipolar) from manic-depressive disorder (now called bipolar disorder). Walking into the chaos of a pediatric office, one might think all the patients have mania! In fact, it is now a common joke for ordinary people say their erratic or silly behavior is caused by “being bipolar.” As for all mental disorders, milder forms of the same behaviors that constitute mania may happen to any of us at times: elation often mixed with irritability, unrealistic beliefs about one’s abilities, racing thoughts or speech, trouble concentrating, acting with poor judgment for age, having a decreased need for sleep, and inappropriate sexual behaviors. To be considered mania, though, the child must have five of these behaviors with one being elation or irritability; have behavior distinctly different from usual behavior, and have behaviors that last at least 7 days or require hospitalization. Having the child or parent complete the Mood Disorders Questionnaire or the parent version of the Young Mania Rating Scale can help, but is not definitive. When mania has occurred and there is also a period of depression lasting 2 weeks, the condition is called Bipolar I. If the mania symptoms do not meet criteria or only irritability is present in distinct periods (hypomania), Bipolar II is diagnosed. In children there can be more rapid cycling than in adults, even four to five cycles per day, as well as briefer (1-2 days) or more persistent periods. Mania may manifest as irritability, aggression, rages, or inconsolability. Children with either Bipolar I or II (not otherwise specified or NOS) should be referred to a mental health specialist.
In addition to cueing the need for a referral, recognizing bipolar disorder is critical because some medications we may ordinarily feel comfortable prescribing, notably antidepressants and stimulants, can activate mania if given in the absence of mood stabilizing medications. While such activation can be reversible, the associated behaviors may be difficult to endure for everyone, and frighten parents and children so they won’t try needed treatments in the future. We must be vigilant for signs of bipolar in all children presenting with symptoms of ADHD, because more than 50% of children with bipolar disorder have comorbid ADHD, often as the presenting sign. Children with only ADHD, in contrast, do not have reduced sleep needs, hypersexuality, hallucinations, or suicidality. Similarly, depression is common, and a condition that we should diagnose and treat. About 20% of depressed children will go on to bipolar disorder. When starting medication for either ADHD or depression, we need to advise families of the signs of activation and monitor them closely, not only to optimize treatment for the condition we believe is present, but also for the possibility that our treatment could make them worse. We also need to advise that suicide is always a risk of bipolar disorder (33%-44% attempt if untreated).
You often may be asked to refill medications prescribed by specialists for a child’s bipolar disorder. It is important to become familiar with both the common and rare but dangerous side effects. Patients taking lithium need regular blood levels taken for dose adjustments as well as monitoring for renal, thyroid, and parathyroid insults. Atypical antipsychotics can raise lipid levels and prolactin, as well as cause diabetes, rapid weight gain, and tardive dyskinesia. Although neuroleptic malignant syndrome is a rare and reversible side effect, if undetected it can be fatal. Antiepileptic mood stabilizers can cause low platelets or white counts, and the potentially fatal Stevens-Johnson syndrome presenting as rash. Hydration is especially important, as well as watching for hyperthermia and hypothermia as temperature regulation is affected by these medicines. Drug interactions are common and must be anticipated, such as NSAIDs increase lithium levels; OTC cold and allergy medications increase sedation; and caffeine and smoking reduce effects of atypical antipsychotics.
You are crucial for communicating the expected recurring cyclic nature of episodes of depression and mania or hypomania, and the need for maintenance medication. Recovery from an episode is common (81%) within 2.5 years, but 62% recur within the next 1.5 years. Vigilance is needed, especially from parents, because the affected child is unlikely to be able to tell when a new cycle is starting. Establishing healthy routines of eating, sleep, and exercise, and learning stress-reduction strategies as well as avoiding pregnancy, smoking, and drugs (there is a sixfold risk of abuse) is important to lessen risk of recurrence, buffer stress, and optimize outcomes. We need to counsel or refer to help families learn to manage behaviors using transition reminders, positive reinforcers rather than punishment, and sometimes reduced expectations. We may be the best ones to educate schools and request emotion-based Individualized Education Program (IEP) and 504 accommodations. Cognitive impairment during cycles of bipolar disorder may require reduced workload, extended time, or breaks for support. It can be helpful to reflect on the high energy, creativity, and innovative thinking found in many people with bipolar disorder, including Van Gogh (artist), Sir Isaac Newton (scientist), Ted Turner (founder of CNN), and Mariah Carey (singer)! Children need our honesty and help accepting this chronic disorder with optimism about their futures, which, with good medical management, can be bright.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Diagnosing and initiating treatment for bipolar disorders in children deserve the skills of a child psychiatrist. So why do we really need to know about these complicated conditions as pediatricians?
Although the prevalence of bipolar disorder is about 4% of the adult population, first presentation of significant symptoms usually occurs in adolescence (1% prevalence) with many affected adults recalling symptoms in childhood. To be called a disorder, symptoms have to significantly impact daily functioning. That means these children and their families – and often their schoolmates and teachers – are struggling, whether a diagnosis has been made or not. While bipolar disorder is clearly heritable (emerging in 10% of children of affected adults), environmental stresses such as trauma, family discord, life transitions, academic pressure, illness, puberty, and even lack of sleep can bring out a cycle of symptoms. There also is evidence that earlier treatment may reduce symptoms long term. These factors mean that we need to be vigilant for signs that behavior or mood problems actually are symptoms of bipolar disorder and take action.
While uncommon, medical conditions such as epilepsy, hyperthyroidism, multiple sclerosis, strokes, tumors, and infections are in the differential we are best positioned to consider. Prescribed medications such as steroids, Singulair, Accutane and, of course, illicit drugs such as cocaine and misused amphetamines also can cause severe mood changes. The psychiatric differential includes depression, ADHD, oppositional defiant disorder or conduct disorder, disruptive mood dysregulation disorder, generalized anxiety disorder, autism, substance use, and personality disorder.
The first thing you need to understand is the signs of mania, the feature that distinguishes depression (unipolar) from manic-depressive disorder (now called bipolar disorder). Walking into the chaos of a pediatric office, one might think all the patients have mania! In fact, it is now a common joke for ordinary people say their erratic or silly behavior is caused by “being bipolar.” As for all mental disorders, milder forms of the same behaviors that constitute mania may happen to any of us at times: elation often mixed with irritability, unrealistic beliefs about one’s abilities, racing thoughts or speech, trouble concentrating, acting with poor judgment for age, having a decreased need for sleep, and inappropriate sexual behaviors. To be considered mania, though, the child must have five of these behaviors with one being elation or irritability; have behavior distinctly different from usual behavior, and have behaviors that last at least 7 days or require hospitalization. Having the child or parent complete the Mood Disorders Questionnaire or the parent version of the Young Mania Rating Scale can help, but is not definitive. When mania has occurred and there is also a period of depression lasting 2 weeks, the condition is called Bipolar I. If the mania symptoms do not meet criteria or only irritability is present in distinct periods (hypomania), Bipolar II is diagnosed. In children there can be more rapid cycling than in adults, even four to five cycles per day, as well as briefer (1-2 days) or more persistent periods. Mania may manifest as irritability, aggression, rages, or inconsolability. Children with either Bipolar I or II (not otherwise specified or NOS) should be referred to a mental health specialist.
In addition to cueing the need for a referral, recognizing bipolar disorder is critical because some medications we may ordinarily feel comfortable prescribing, notably antidepressants and stimulants, can activate mania if given in the absence of mood stabilizing medications. While such activation can be reversible, the associated behaviors may be difficult to endure for everyone, and frighten parents and children so they won’t try needed treatments in the future. We must be vigilant for signs of bipolar in all children presenting with symptoms of ADHD, because more than 50% of children with bipolar disorder have comorbid ADHD, often as the presenting sign. Children with only ADHD, in contrast, do not have reduced sleep needs, hypersexuality, hallucinations, or suicidality. Similarly, depression is common, and a condition that we should diagnose and treat. About 20% of depressed children will go on to bipolar disorder. When starting medication for either ADHD or depression, we need to advise families of the signs of activation and monitor them closely, not only to optimize treatment for the condition we believe is present, but also for the possibility that our treatment could make them worse. We also need to advise that suicide is always a risk of bipolar disorder (33%-44% attempt if untreated).
You often may be asked to refill medications prescribed by specialists for a child’s bipolar disorder. It is important to become familiar with both the common and rare but dangerous side effects. Patients taking lithium need regular blood levels taken for dose adjustments as well as monitoring for renal, thyroid, and parathyroid insults. Atypical antipsychotics can raise lipid levels and prolactin, as well as cause diabetes, rapid weight gain, and tardive dyskinesia. Although neuroleptic malignant syndrome is a rare and reversible side effect, if undetected it can be fatal. Antiepileptic mood stabilizers can cause low platelets or white counts, and the potentially fatal Stevens-Johnson syndrome presenting as rash. Hydration is especially important, as well as watching for hyperthermia and hypothermia as temperature regulation is affected by these medicines. Drug interactions are common and must be anticipated, such as NSAIDs increase lithium levels; OTC cold and allergy medications increase sedation; and caffeine and smoking reduce effects of atypical antipsychotics.
You are crucial for communicating the expected recurring cyclic nature of episodes of depression and mania or hypomania, and the need for maintenance medication. Recovery from an episode is common (81%) within 2.5 years, but 62% recur within the next 1.5 years. Vigilance is needed, especially from parents, because the affected child is unlikely to be able to tell when a new cycle is starting. Establishing healthy routines of eating, sleep, and exercise, and learning stress-reduction strategies as well as avoiding pregnancy, smoking, and drugs (there is a sixfold risk of abuse) is important to lessen risk of recurrence, buffer stress, and optimize outcomes. We need to counsel or refer to help families learn to manage behaviors using transition reminders, positive reinforcers rather than punishment, and sometimes reduced expectations. We may be the best ones to educate schools and request emotion-based Individualized Education Program (IEP) and 504 accommodations. Cognitive impairment during cycles of bipolar disorder may require reduced workload, extended time, or breaks for support. It can be helpful to reflect on the high energy, creativity, and innovative thinking found in many people with bipolar disorder, including Van Gogh (artist), Sir Isaac Newton (scientist), Ted Turner (founder of CNN), and Mariah Carey (singer)! Children need our honesty and help accepting this chronic disorder with optimism about their futures, which, with good medical management, can be bright.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Diagnosing and initiating treatment for bipolar disorders in children deserve the skills of a child psychiatrist. So why do we really need to know about these complicated conditions as pediatricians?
Although the prevalence of bipolar disorder is about 4% of the adult population, first presentation of significant symptoms usually occurs in adolescence (1% prevalence) with many affected adults recalling symptoms in childhood. To be called a disorder, symptoms have to significantly impact daily functioning. That means these children and their families – and often their schoolmates and teachers – are struggling, whether a diagnosis has been made or not. While bipolar disorder is clearly heritable (emerging in 10% of children of affected adults), environmental stresses such as trauma, family discord, life transitions, academic pressure, illness, puberty, and even lack of sleep can bring out a cycle of symptoms. There also is evidence that earlier treatment may reduce symptoms long term. These factors mean that we need to be vigilant for signs that behavior or mood problems actually are symptoms of bipolar disorder and take action.
While uncommon, medical conditions such as epilepsy, hyperthyroidism, multiple sclerosis, strokes, tumors, and infections are in the differential we are best positioned to consider. Prescribed medications such as steroids, Singulair, Accutane and, of course, illicit drugs such as cocaine and misused amphetamines also can cause severe mood changes. The psychiatric differential includes depression, ADHD, oppositional defiant disorder or conduct disorder, disruptive mood dysregulation disorder, generalized anxiety disorder, autism, substance use, and personality disorder.
The first thing you need to understand is the signs of mania, the feature that distinguishes depression (unipolar) from manic-depressive disorder (now called bipolar disorder). Walking into the chaos of a pediatric office, one might think all the patients have mania! In fact, it is now a common joke for ordinary people say their erratic or silly behavior is caused by “being bipolar.” As for all mental disorders, milder forms of the same behaviors that constitute mania may happen to any of us at times: elation often mixed with irritability, unrealistic beliefs about one’s abilities, racing thoughts or speech, trouble concentrating, acting with poor judgment for age, having a decreased need for sleep, and inappropriate sexual behaviors. To be considered mania, though, the child must have five of these behaviors with one being elation or irritability; have behavior distinctly different from usual behavior, and have behaviors that last at least 7 days or require hospitalization. Having the child or parent complete the Mood Disorders Questionnaire or the parent version of the Young Mania Rating Scale can help, but is not definitive. When mania has occurred and there is also a period of depression lasting 2 weeks, the condition is called Bipolar I. If the mania symptoms do not meet criteria or only irritability is present in distinct periods (hypomania), Bipolar II is diagnosed. In children there can be more rapid cycling than in adults, even four to five cycles per day, as well as briefer (1-2 days) or more persistent periods. Mania may manifest as irritability, aggression, rages, or inconsolability. Children with either Bipolar I or II (not otherwise specified or NOS) should be referred to a mental health specialist.
In addition to cueing the need for a referral, recognizing bipolar disorder is critical because some medications we may ordinarily feel comfortable prescribing, notably antidepressants and stimulants, can activate mania if given in the absence of mood stabilizing medications. While such activation can be reversible, the associated behaviors may be difficult to endure for everyone, and frighten parents and children so they won’t try needed treatments in the future. We must be vigilant for signs of bipolar in all children presenting with symptoms of ADHD, because more than 50% of children with bipolar disorder have comorbid ADHD, often as the presenting sign. Children with only ADHD, in contrast, do not have reduced sleep needs, hypersexuality, hallucinations, or suicidality. Similarly, depression is common, and a condition that we should diagnose and treat. About 20% of depressed children will go on to bipolar disorder. When starting medication for either ADHD or depression, we need to advise families of the signs of activation and monitor them closely, not only to optimize treatment for the condition we believe is present, but also for the possibility that our treatment could make them worse. We also need to advise that suicide is always a risk of bipolar disorder (33%-44% attempt if untreated).
You often may be asked to refill medications prescribed by specialists for a child’s bipolar disorder. It is important to become familiar with both the common and rare but dangerous side effects. Patients taking lithium need regular blood levels taken for dose adjustments as well as monitoring for renal, thyroid, and parathyroid insults. Atypical antipsychotics can raise lipid levels and prolactin, as well as cause diabetes, rapid weight gain, and tardive dyskinesia. Although neuroleptic malignant syndrome is a rare and reversible side effect, if undetected it can be fatal. Antiepileptic mood stabilizers can cause low platelets or white counts, and the potentially fatal Stevens-Johnson syndrome presenting as rash. Hydration is especially important, as well as watching for hyperthermia and hypothermia as temperature regulation is affected by these medicines. Drug interactions are common and must be anticipated, such as NSAIDs increase lithium levels; OTC cold and allergy medications increase sedation; and caffeine and smoking reduce effects of atypical antipsychotics.
You are crucial for communicating the expected recurring cyclic nature of episodes of depression and mania or hypomania, and the need for maintenance medication. Recovery from an episode is common (81%) within 2.5 years, but 62% recur within the next 1.5 years. Vigilance is needed, especially from parents, because the affected child is unlikely to be able to tell when a new cycle is starting. Establishing healthy routines of eating, sleep, and exercise, and learning stress-reduction strategies as well as avoiding pregnancy, smoking, and drugs (there is a sixfold risk of abuse) is important to lessen risk of recurrence, buffer stress, and optimize outcomes. We need to counsel or refer to help families learn to manage behaviors using transition reminders, positive reinforcers rather than punishment, and sometimes reduced expectations. We may be the best ones to educate schools and request emotion-based Individualized Education Program (IEP) and 504 accommodations. Cognitive impairment during cycles of bipolar disorder may require reduced workload, extended time, or breaks for support. It can be helpful to reflect on the high energy, creativity, and innovative thinking found in many people with bipolar disorder, including Van Gogh (artist), Sir Isaac Newton (scientist), Ted Turner (founder of CNN), and Mariah Carey (singer)! Children need our honesty and help accepting this chronic disorder with optimism about their futures, which, with good medical management, can be bright.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].