User login
Sexually Transmitted Infections Caused by Mycoplasma genitalium and Neisseria gonorrhoeae: Diagnosis and Treatment
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
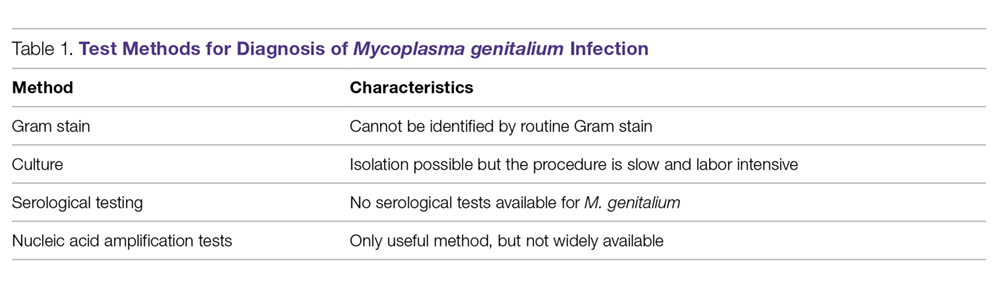
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
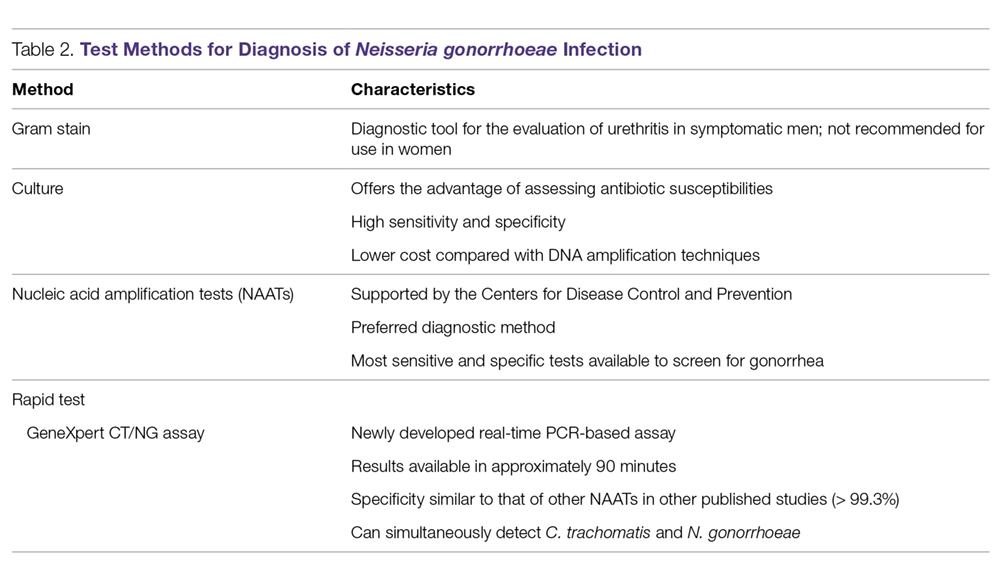
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
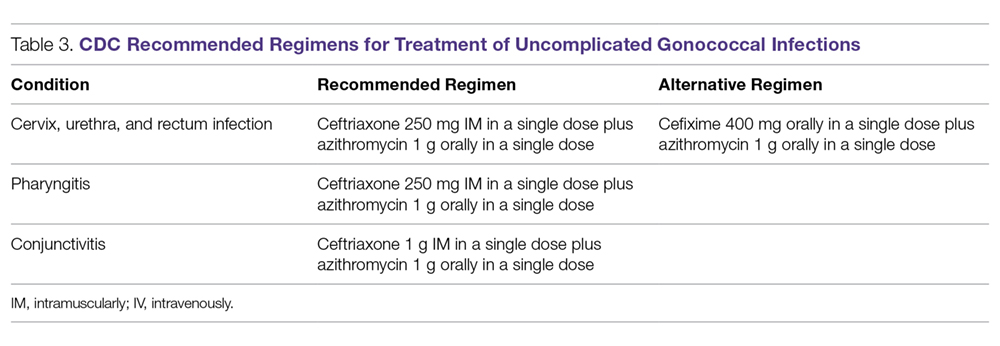
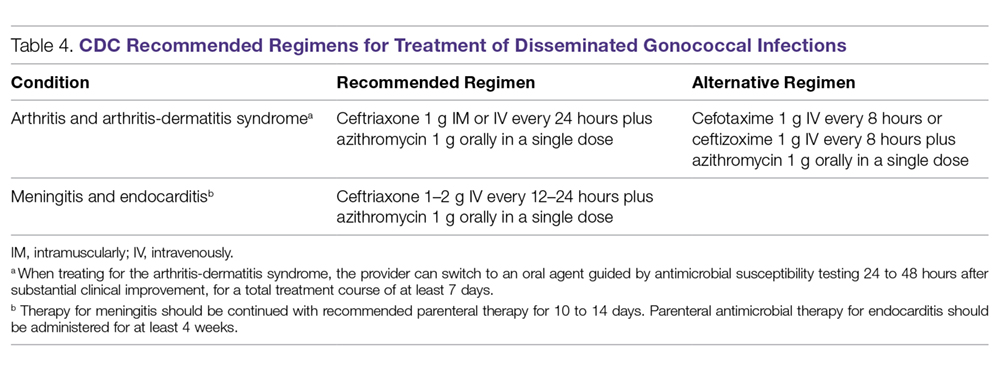
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.

N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.

Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45

In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.

Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.

N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.

Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45

In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.

Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
Screening for Lynch Syndrome Among Patients with Colorectal Cancer: Experiences from a Multihospital Health System
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
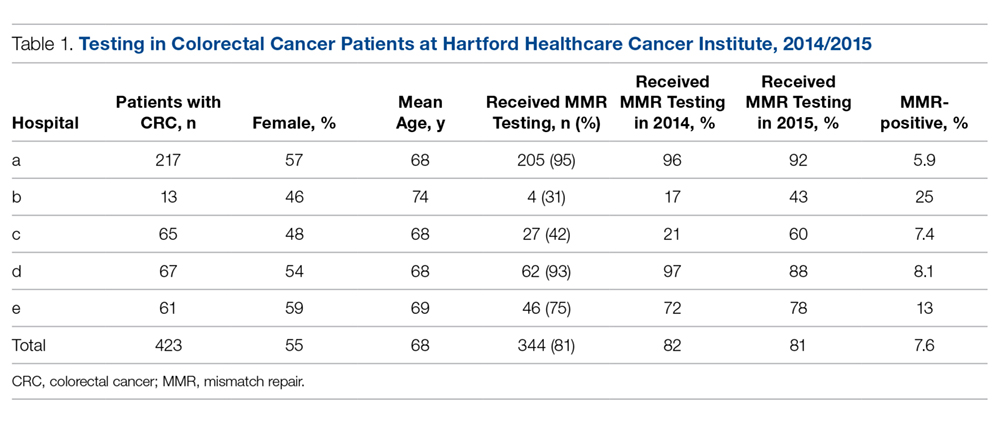
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
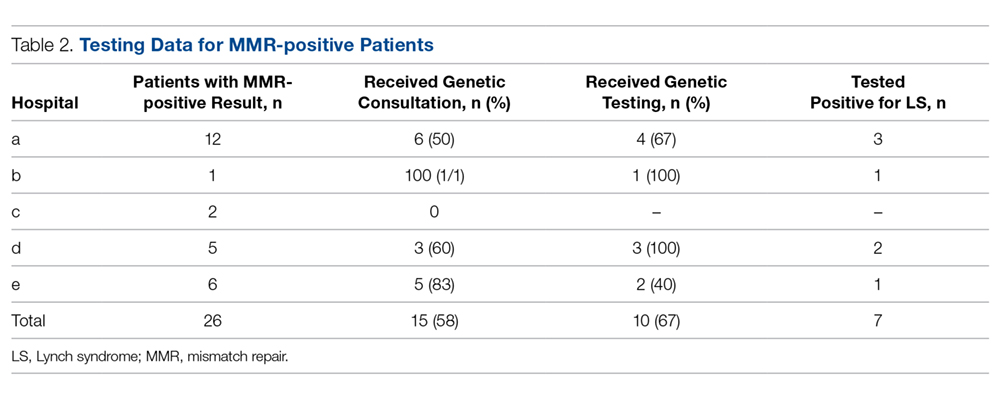
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; [email protected].
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.

Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.

Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).

Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; [email protected].
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.

Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.

Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).

Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; [email protected].
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
Bundled Hospital-at-Home and Transitional Care Program Is Associated with Reduced Rate of Hospital Readmission
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
Effectiveness of Epinephrine in Out-of-Hospital Cardiac Arrest
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
Quality of Life After Treatment of Chronic Total Occlusions with Revascularization versus Optimal Medical Therapy
Study Overview
Objective. To compare the benefit of percutaneous coronary intervention (PCI) plus optimal medical therapy (OMT) versus OMT alone on the health status of patients with chronic total occlusions (CTOs).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 396 patients with at least 1 CTO were assigned to PCI or OMT with a 2:1 randomization ratio.
Main outcome measures. The primary endpoint was the change in health status as assessed by the Seattle Angina Questionnaire (SAQ) between baseline and 12-month follow-up.
Main results. At 12 months, greater improvement of 3 SAQ domains was observed with PCI compared to OMT: angina frequency (5.23, 95% confidence interval [CI], 1.75-8.31, P = 0.0003), physical limitation (P = 0.02), and quality of life (6.62, 95% CI 1.78-11.46, P = 0.0007). More patients in the PCI group than in the OMT group had complete freedom from angina (71.6% vs. 57.8%, P = 0.008). There were no occurrences of periprocedural death or myocardial infarction.
Conclusion. Among patients with stable angina and CTO, PCI leads to significant health status improvement compared with OMT alone.
Commentary
CTOs are present in 15% to 25% of patients undergoing coronary angiogram1 and are associated with increased mortality.2 The benefits of successful CTO intervention observed in multiple large-scale registries include improvement in quality of life, left ventricular function, and survival as well as avoidance of coronary bypass surgery. The main indication for CTO intervention is improvement in quality of life,3 although this has not been confirmed by a randomized controlled trial comparing medical therapy to CTO-PCI.
Previous studies have assessed the health status benefits associated with CTO-PCI.4,5 Most recently, the OPEN CTO study showed significant improvement in health status in 1000 consecutive patients undergoing CTO-PCI in 12 experienced U.S. centers.6 Similarly, in a Canadian registry, revascularization of CTO was associated with greater health status benefit compared to medical therapy alone.4 However, these studies compared CTO-PCI success to failure, rather than to medical therapy.
In this context, Werner and colleagues investigated the value of PCI versus OMT for CTO by performing a well-designed randomized clinical trial in patients with CTO by assessing their health status with the SAQ.7 The SAQ is a 19-item questionnaire with a 4-week recall period that measures 5 domains of health status in patients with coronary artery disease (CAD).8,9 Scores in each domain range from 0 to 100, with higher scores indicating fewer symptoms and better quality of life. The SAQ has undergone extensive reliability and validity testing and is associated with long-term survival and health care utilization among patients with chronic CAD.10,11 At 12 months follow-up, patients who underwent CTO-PCI had greater improvement in SAQ subscales, including angina frequency and quality of life, reaching the pre-specified significance level of 0.01. There was also numerical improvement in physical limitation (P = 0.02)
The strengths of this current study include the randomized design and the careful treatment of non-CTO- PCI lesions before enrollment into the study. These non-CTO lesions were treated before the baseline health status assessment so that the additional health status benefit of non-CTO-PCI would not affect the results. This was one of multiple major limitations of the recently presented DECISION-CTO trial, as the non-CTO lesions were treated after the randomization and baseline assessment, leading to inaccurate comparison between medical therapy and CTO-PCI.12
Another interesting point of the current study is the patient selection. Since the treatment sites included were all expert centers in Europe, many patients who were referred to their institution for CTO-PCI were excluded from the study. For example, among the 1980 patients with screening log, 1381 were excluded because they were referred for CTO-PCI and 122 were excluded because they were “too symptomatic.” This suggests that the population studied were less symptomatic than the overall symptomatic CTO population from previous registries, as evidenced by about 40% of patients having Canadian Cardiovascular Society (CCS) class I/II angina at baseline. In the recent consecutively enrolled OPEN CTO registry, only 26% of patients reported CCS class I/II angina at baseline.6 These observations likely represent biases to the null, and thus one can reasonably speculate that the impact among unselected patients would be greater. Degree of baseline angina has been reported to be a predictor in patients with stable angina.13 Moreover, the degree of health status improvement is significantly larger in patients with refractory angina undergoing CTO- PCI.14
In this study, the success rate of CTO PCI was 83.1% at the initial attempt and 86.6% at the final attempt. The in-hospital complication rate was 2.9%, which included pericardial tamponade, vascular surgical repair, and need for blood transfusion. The success rate and complication rates were consistent with previous observational studies from expert centers.1,6
Applications for Clinical Practice
In patients presenting with stable angina with CTO, the health status improvement is larger with CTO-PCI plus medical therapy compared to medical therapy alone. CTO-PCI should be offered to symptomatic patients in conjunction with OMT.
—Taishi Hirai, MD, and J. Aaron Grantham, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
2. Ramunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:1535-1544.
3. Grantham JA, Marso SP, Spertus J, et al. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2:479-486.
4. Wijeysundera HC, Norris C, Fefer P, et al. Relationship between initial treatment strategy and quality of life in patients with coronary chronic total occlusions. EuroIntervention. 2014;9:1165-1172.
5. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes. 2010;3:284-290.
6. Sapontis J, Salisbury AC, Yeh RW, C et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523-1534.
7. Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484-2993.
8. Spertus JA, Winder JA, Dewhurst TA, et al. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240-1244.
9. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333-341.
10. Mozaffarian D, Bryson CL, Spertus JA, et al. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015-1022.
11. Spertus JA, Jones P, McDonell M, et al. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43-49.
12. Park S. Drug-eluting stent versus optimal medical therapy in patients with coronary chronic total occlusion: DECISION CTO randomized trial. Presented at the American College of Cardiology Annual Scientific Session (ACC 2017), Washington, DC, March 18, 2017.
13. Spertus JA, Salisbury AC, Jones PG, et al. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789-3794.
14. Hirai T, Grantham JA, Gosch K, L et al. Quality of life in patients with refractory angina after chronic total occlusion angioplasty. J Am Coll Cardiol. 2018;72(13 supplement):TCT-79.
Study Overview
Objective. To compare the benefit of percutaneous coronary intervention (PCI) plus optimal medical therapy (OMT) versus OMT alone on the health status of patients with chronic total occlusions (CTOs).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 396 patients with at least 1 CTO were assigned to PCI or OMT with a 2:1 randomization ratio.
Main outcome measures. The primary endpoint was the change in health status as assessed by the Seattle Angina Questionnaire (SAQ) between baseline and 12-month follow-up.
Main results. At 12 months, greater improvement of 3 SAQ domains was observed with PCI compared to OMT: angina frequency (5.23, 95% confidence interval [CI], 1.75-8.31, P = 0.0003), physical limitation (P = 0.02), and quality of life (6.62, 95% CI 1.78-11.46, P = 0.0007). More patients in the PCI group than in the OMT group had complete freedom from angina (71.6% vs. 57.8%, P = 0.008). There were no occurrences of periprocedural death or myocardial infarction.
Conclusion. Among patients with stable angina and CTO, PCI leads to significant health status improvement compared with OMT alone.
Commentary
CTOs are present in 15% to 25% of patients undergoing coronary angiogram1 and are associated with increased mortality.2 The benefits of successful CTO intervention observed in multiple large-scale registries include improvement in quality of life, left ventricular function, and survival as well as avoidance of coronary bypass surgery. The main indication for CTO intervention is improvement in quality of life,3 although this has not been confirmed by a randomized controlled trial comparing medical therapy to CTO-PCI.
Previous studies have assessed the health status benefits associated with CTO-PCI.4,5 Most recently, the OPEN CTO study showed significant improvement in health status in 1000 consecutive patients undergoing CTO-PCI in 12 experienced U.S. centers.6 Similarly, in a Canadian registry, revascularization of CTO was associated with greater health status benefit compared to medical therapy alone.4 However, these studies compared CTO-PCI success to failure, rather than to medical therapy.
In this context, Werner and colleagues investigated the value of PCI versus OMT for CTO by performing a well-designed randomized clinical trial in patients with CTO by assessing their health status with the SAQ.7 The SAQ is a 19-item questionnaire with a 4-week recall period that measures 5 domains of health status in patients with coronary artery disease (CAD).8,9 Scores in each domain range from 0 to 100, with higher scores indicating fewer symptoms and better quality of life. The SAQ has undergone extensive reliability and validity testing and is associated with long-term survival and health care utilization among patients with chronic CAD.10,11 At 12 months follow-up, patients who underwent CTO-PCI had greater improvement in SAQ subscales, including angina frequency and quality of life, reaching the pre-specified significance level of 0.01. There was also numerical improvement in physical limitation (P = 0.02)
The strengths of this current study include the randomized design and the careful treatment of non-CTO- PCI lesions before enrollment into the study. These non-CTO lesions were treated before the baseline health status assessment so that the additional health status benefit of non-CTO-PCI would not affect the results. This was one of multiple major limitations of the recently presented DECISION-CTO trial, as the non-CTO lesions were treated after the randomization and baseline assessment, leading to inaccurate comparison between medical therapy and CTO-PCI.12
Another interesting point of the current study is the patient selection. Since the treatment sites included were all expert centers in Europe, many patients who were referred to their institution for CTO-PCI were excluded from the study. For example, among the 1980 patients with screening log, 1381 were excluded because they were referred for CTO-PCI and 122 were excluded because they were “too symptomatic.” This suggests that the population studied were less symptomatic than the overall symptomatic CTO population from previous registries, as evidenced by about 40% of patients having Canadian Cardiovascular Society (CCS) class I/II angina at baseline. In the recent consecutively enrolled OPEN CTO registry, only 26% of patients reported CCS class I/II angina at baseline.6 These observations likely represent biases to the null, and thus one can reasonably speculate that the impact among unselected patients would be greater. Degree of baseline angina has been reported to be a predictor in patients with stable angina.13 Moreover, the degree of health status improvement is significantly larger in patients with refractory angina undergoing CTO- PCI.14
In this study, the success rate of CTO PCI was 83.1% at the initial attempt and 86.6% at the final attempt. The in-hospital complication rate was 2.9%, which included pericardial tamponade, vascular surgical repair, and need for blood transfusion. The success rate and complication rates were consistent with previous observational studies from expert centers.1,6
Applications for Clinical Practice
In patients presenting with stable angina with CTO, the health status improvement is larger with CTO-PCI plus medical therapy compared to medical therapy alone. CTO-PCI should be offered to symptomatic patients in conjunction with OMT.
—Taishi Hirai, MD, and J. Aaron Grantham, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
Study Overview
Objective. To compare the benefit of percutaneous coronary intervention (PCI) plus optimal medical therapy (OMT) versus OMT alone on the health status of patients with chronic total occlusions (CTOs).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 396 patients with at least 1 CTO were assigned to PCI or OMT with a 2:1 randomization ratio.
Main outcome measures. The primary endpoint was the change in health status as assessed by the Seattle Angina Questionnaire (SAQ) between baseline and 12-month follow-up.
Main results. At 12 months, greater improvement of 3 SAQ domains was observed with PCI compared to OMT: angina frequency (5.23, 95% confidence interval [CI], 1.75-8.31, P = 0.0003), physical limitation (P = 0.02), and quality of life (6.62, 95% CI 1.78-11.46, P = 0.0007). More patients in the PCI group than in the OMT group had complete freedom from angina (71.6% vs. 57.8%, P = 0.008). There were no occurrences of periprocedural death or myocardial infarction.
Conclusion. Among patients with stable angina and CTO, PCI leads to significant health status improvement compared with OMT alone.
Commentary
CTOs are present in 15% to 25% of patients undergoing coronary angiogram1 and are associated with increased mortality.2 The benefits of successful CTO intervention observed in multiple large-scale registries include improvement in quality of life, left ventricular function, and survival as well as avoidance of coronary bypass surgery. The main indication for CTO intervention is improvement in quality of life,3 although this has not been confirmed by a randomized controlled trial comparing medical therapy to CTO-PCI.
Previous studies have assessed the health status benefits associated with CTO-PCI.4,5 Most recently, the OPEN CTO study showed significant improvement in health status in 1000 consecutive patients undergoing CTO-PCI in 12 experienced U.S. centers.6 Similarly, in a Canadian registry, revascularization of CTO was associated with greater health status benefit compared to medical therapy alone.4 However, these studies compared CTO-PCI success to failure, rather than to medical therapy.
In this context, Werner and colleagues investigated the value of PCI versus OMT for CTO by performing a well-designed randomized clinical trial in patients with CTO by assessing their health status with the SAQ.7 The SAQ is a 19-item questionnaire with a 4-week recall period that measures 5 domains of health status in patients with coronary artery disease (CAD).8,9 Scores in each domain range from 0 to 100, with higher scores indicating fewer symptoms and better quality of life. The SAQ has undergone extensive reliability and validity testing and is associated with long-term survival and health care utilization among patients with chronic CAD.10,11 At 12 months follow-up, patients who underwent CTO-PCI had greater improvement in SAQ subscales, including angina frequency and quality of life, reaching the pre-specified significance level of 0.01. There was also numerical improvement in physical limitation (P = 0.02)
The strengths of this current study include the randomized design and the careful treatment of non-CTO- PCI lesions before enrollment into the study. These non-CTO lesions were treated before the baseline health status assessment so that the additional health status benefit of non-CTO-PCI would not affect the results. This was one of multiple major limitations of the recently presented DECISION-CTO trial, as the non-CTO lesions were treated after the randomization and baseline assessment, leading to inaccurate comparison between medical therapy and CTO-PCI.12
Another interesting point of the current study is the patient selection. Since the treatment sites included were all expert centers in Europe, many patients who were referred to their institution for CTO-PCI were excluded from the study. For example, among the 1980 patients with screening log, 1381 were excluded because they were referred for CTO-PCI and 122 were excluded because they were “too symptomatic.” This suggests that the population studied were less symptomatic than the overall symptomatic CTO population from previous registries, as evidenced by about 40% of patients having Canadian Cardiovascular Society (CCS) class I/II angina at baseline. In the recent consecutively enrolled OPEN CTO registry, only 26% of patients reported CCS class I/II angina at baseline.6 These observations likely represent biases to the null, and thus one can reasonably speculate that the impact among unselected patients would be greater. Degree of baseline angina has been reported to be a predictor in patients with stable angina.13 Moreover, the degree of health status improvement is significantly larger in patients with refractory angina undergoing CTO- PCI.14
In this study, the success rate of CTO PCI was 83.1% at the initial attempt and 86.6% at the final attempt. The in-hospital complication rate was 2.9%, which included pericardial tamponade, vascular surgical repair, and need for blood transfusion. The success rate and complication rates were consistent with previous observational studies from expert centers.1,6
Applications for Clinical Practice
In patients presenting with stable angina with CTO, the health status improvement is larger with CTO-PCI plus medical therapy compared to medical therapy alone. CTO-PCI should be offered to symptomatic patients in conjunction with OMT.
—Taishi Hirai, MD, and J. Aaron Grantham, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
2. Ramunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:1535-1544.
3. Grantham JA, Marso SP, Spertus J, et al. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2:479-486.
4. Wijeysundera HC, Norris C, Fefer P, et al. Relationship between initial treatment strategy and quality of life in patients with coronary chronic total occlusions. EuroIntervention. 2014;9:1165-1172.
5. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes. 2010;3:284-290.
6. Sapontis J, Salisbury AC, Yeh RW, C et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523-1534.
7. Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484-2993.
8. Spertus JA, Winder JA, Dewhurst TA, et al. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240-1244.
9. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333-341.
10. Mozaffarian D, Bryson CL, Spertus JA, et al. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015-1022.
11. Spertus JA, Jones P, McDonell M, et al. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43-49.
12. Park S. Drug-eluting stent versus optimal medical therapy in patients with coronary chronic total occlusion: DECISION CTO randomized trial. Presented at the American College of Cardiology Annual Scientific Session (ACC 2017), Washington, DC, March 18, 2017.
13. Spertus JA, Salisbury AC, Jones PG, et al. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789-3794.
14. Hirai T, Grantham JA, Gosch K, L et al. Quality of life in patients with refractory angina after chronic total occlusion angioplasty. J Am Coll Cardiol. 2018;72(13 supplement):TCT-79.
1. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
2. Ramunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:1535-1544.
3. Grantham JA, Marso SP, Spertus J, et al. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2:479-486.
4. Wijeysundera HC, Norris C, Fefer P, et al. Relationship between initial treatment strategy and quality of life in patients with coronary chronic total occlusions. EuroIntervention. 2014;9:1165-1172.
5. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes. 2010;3:284-290.
6. Sapontis J, Salisbury AC, Yeh RW, C et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523-1534.
7. Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484-2993.
8. Spertus JA, Winder JA, Dewhurst TA, et al. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240-1244.
9. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333-341.
10. Mozaffarian D, Bryson CL, Spertus JA, et al. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015-1022.
11. Spertus JA, Jones P, McDonell M, et al. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43-49.
12. Park S. Drug-eluting stent versus optimal medical therapy in patients with coronary chronic total occlusion: DECISION CTO randomized trial. Presented at the American College of Cardiology Annual Scientific Session (ACC 2017), Washington, DC, March 18, 2017.
13. Spertus JA, Salisbury AC, Jones PG, et al. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789-3794.
14. Hirai T, Grantham JA, Gosch K, L et al. Quality of life in patients with refractory angina after chronic total occlusion angioplasty. J Am Coll Cardiol. 2018;72(13 supplement):TCT-79.
Aspirin as CVD prevention in seniors? Think twice
Resources
US Preventive Services Task Force. Final recommendation statement: Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication.
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer.
Published April 2016. Accessed September 14, 2018.
McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. 2018;379:1499-1508.
https://www.nejm.org/doi/full/10.1056/NEJMoa1800722. Accessed November 7, 2018.
McNeil JJ, Nelson MR, Woods JE, et al. Effect of aspirin on all-cause mortality in the healthy elderly. 2018;379:1519-1528.
https://www.nejm.org/doi/full/10.1056/NEJMoa1803955. Accessed November 7, 2018.
Resources
US Preventive Services Task Force. Final recommendation statement: Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication.
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer.
Published April 2016. Accessed September 14, 2018.
McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. 2018;379:1499-1508.
https://www.nejm.org/doi/full/10.1056/NEJMoa1800722. Accessed November 7, 2018.
McNeil JJ, Nelson MR, Woods JE, et al. Effect of aspirin on all-cause mortality in the healthy elderly. 2018;379:1519-1528.
https://www.nejm.org/doi/full/10.1056/NEJMoa1803955. Accessed November 7, 2018.
Resources
US Preventive Services Task Force. Final recommendation statement: Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication.
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer.
Published April 2016. Accessed September 14, 2018.
McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. 2018;379:1499-1508.
https://www.nejm.org/doi/full/10.1056/NEJMoa1800722. Accessed November 7, 2018.
McNeil JJ, Nelson MR, Woods JE, et al. Effect of aspirin on all-cause mortality in the healthy elderly. 2018;379:1519-1528.
https://www.nejm.org/doi/full/10.1056/NEJMoa1803955. Accessed November 7, 2018.
News from the CHEST Board of Regents
In 2013, CHEST began work with the Chinese Ministry of Health and the Chinese Medical Doctor Association to establish the specialty of Pulmonary and Critical Care Medicine in China. CHEST members, among them Drs. Renli Qiao, Jack Buckley, Darcy Marciniuk, Mark Rosen, and Stephanie Levine, helped to establish a curriculum and a board exam and have now seen the first class of fellows complete their training. At our October Board meeting, Dr. Buckley reported at this meeting that the Chinese PCCM program, the first medical subspecialty to be established in China, is prepared to stand on its own, without further support from CHEST. This is a huge accomplishment for both the Chinese Medical Doctor Association and for CHEST, and the Board heartily congratulated everyone who contributed to this impressive project.
Another important function at this October meeting is to approve the Governance Committee’s recommendations for a new slate of board members and a new President-Designate. The board bid farewell to four valued members at the end of their terms: Drs. Robert Aranson (Freeport, ME), Subhakar Kandi (Hyderabad, India), Janet Maurer (Desert Hills, AZ), and Hassan Bencheqroun (San Diego, CA). All contributed immensely to the success of CHEST, and the remaining board members expressed their gratitude. The Board also approved Drs. Vera De Palo (Providence, RI), Neil Freedman (Evanston, IL), Francesco DeBlasio (Napoli, Italy), and Lynn Tanoue (New Haven, CT) as at-large regents, and Dr. Steven Simpson (Kansas City, KS) as the new President-Designate. The Board is committed to ensuring that its makeup be representative of the entirety of our membership base. As CHEST continues to grow internationally and as we gain more members who are women and historically underrepresented minorities, we are dedicated to ensuring that there is no glass ceiling in our organization and that all have the opportunity to contribute to the full extent of their ability. We are, likewise, dedicated to providing mentorship and leadership opportunities for members of groups who are under-represented.
Following the resignation of CHEST’s CEO during the summer, the Chief Operating Officer, Dr. Robert Musacchio, became interim CEO. Dr. Musacchio is a PhD economist who joined CHEST in 2015 after a 35-year stint at the American Medical Association and who has broad and deep experience in the business of running a nonprofit medical organization. He brings an extraordinary skill set in both business and staff development to the role, and we very much look forward to working with him in this new position! Dr. Musacchio gave an update on educational efforts, domestic and international growth in membership, changes in the structure of the professional staff, and the state of our flagship journal, CHEST®.
In 2013, CHEST began work with the Chinese Ministry of Health and the Chinese Medical Doctor Association to establish the specialty of Pulmonary and Critical Care Medicine in China. CHEST members, among them Drs. Renli Qiao, Jack Buckley, Darcy Marciniuk, Mark Rosen, and Stephanie Levine, helped to establish a curriculum and a board exam and have now seen the first class of fellows complete their training. At our October Board meeting, Dr. Buckley reported at this meeting that the Chinese PCCM program, the first medical subspecialty to be established in China, is prepared to stand on its own, without further support from CHEST. This is a huge accomplishment for both the Chinese Medical Doctor Association and for CHEST, and the Board heartily congratulated everyone who contributed to this impressive project.
Another important function at this October meeting is to approve the Governance Committee’s recommendations for a new slate of board members and a new President-Designate. The board bid farewell to four valued members at the end of their terms: Drs. Robert Aranson (Freeport, ME), Subhakar Kandi (Hyderabad, India), Janet Maurer (Desert Hills, AZ), and Hassan Bencheqroun (San Diego, CA). All contributed immensely to the success of CHEST, and the remaining board members expressed their gratitude. The Board also approved Drs. Vera De Palo (Providence, RI), Neil Freedman (Evanston, IL), Francesco DeBlasio (Napoli, Italy), and Lynn Tanoue (New Haven, CT) as at-large regents, and Dr. Steven Simpson (Kansas City, KS) as the new President-Designate. The Board is committed to ensuring that its makeup be representative of the entirety of our membership base. As CHEST continues to grow internationally and as we gain more members who are women and historically underrepresented minorities, we are dedicated to ensuring that there is no glass ceiling in our organization and that all have the opportunity to contribute to the full extent of their ability. We are, likewise, dedicated to providing mentorship and leadership opportunities for members of groups who are under-represented.
Following the resignation of CHEST’s CEO during the summer, the Chief Operating Officer, Dr. Robert Musacchio, became interim CEO. Dr. Musacchio is a PhD economist who joined CHEST in 2015 after a 35-year stint at the American Medical Association and who has broad and deep experience in the business of running a nonprofit medical organization. He brings an extraordinary skill set in both business and staff development to the role, and we very much look forward to working with him in this new position! Dr. Musacchio gave an update on educational efforts, domestic and international growth in membership, changes in the structure of the professional staff, and the state of our flagship journal, CHEST®.
In 2013, CHEST began work with the Chinese Ministry of Health and the Chinese Medical Doctor Association to establish the specialty of Pulmonary and Critical Care Medicine in China. CHEST members, among them Drs. Renli Qiao, Jack Buckley, Darcy Marciniuk, Mark Rosen, and Stephanie Levine, helped to establish a curriculum and a board exam and have now seen the first class of fellows complete their training. At our October Board meeting, Dr. Buckley reported at this meeting that the Chinese PCCM program, the first medical subspecialty to be established in China, is prepared to stand on its own, without further support from CHEST. This is a huge accomplishment for both the Chinese Medical Doctor Association and for CHEST, and the Board heartily congratulated everyone who contributed to this impressive project.
Another important function at this October meeting is to approve the Governance Committee’s recommendations for a new slate of board members and a new President-Designate. The board bid farewell to four valued members at the end of their terms: Drs. Robert Aranson (Freeport, ME), Subhakar Kandi (Hyderabad, India), Janet Maurer (Desert Hills, AZ), and Hassan Bencheqroun (San Diego, CA). All contributed immensely to the success of CHEST, and the remaining board members expressed their gratitude. The Board also approved Drs. Vera De Palo (Providence, RI), Neil Freedman (Evanston, IL), Francesco DeBlasio (Napoli, Italy), and Lynn Tanoue (New Haven, CT) as at-large regents, and Dr. Steven Simpson (Kansas City, KS) as the new President-Designate. The Board is committed to ensuring that its makeup be representative of the entirety of our membership base. As CHEST continues to grow internationally and as we gain more members who are women and historically underrepresented minorities, we are dedicated to ensuring that there is no glass ceiling in our organization and that all have the opportunity to contribute to the full extent of their ability. We are, likewise, dedicated to providing mentorship and leadership opportunities for members of groups who are under-represented.
Following the resignation of CHEST’s CEO during the summer, the Chief Operating Officer, Dr. Robert Musacchio, became interim CEO. Dr. Musacchio is a PhD economist who joined CHEST in 2015 after a 35-year stint at the American Medical Association and who has broad and deep experience in the business of running a nonprofit medical organization. He brings an extraordinary skill set in both business and staff development to the role, and we very much look forward to working with him in this new position! Dr. Musacchio gave an update on educational efforts, domestic and international growth in membership, changes in the structure of the professional staff, and the state of our flagship journal, CHEST®.
NAMDRC update
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices
NAMDRC’s mission statement clearly signals its commitment to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment. Adhering to that commitment presents challenges in the rapidly changing structure of the delivery of health care. For example, 10 years ago, the majority of NAMDRC members were private practitioners/group practices, many with contracts to provide a range of services to institutions. While those agreements varied, the underlying principles were relatively constant – structure your agreements that were mutually beneficial to physician and hospital.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level % E&M codes, but due to strong reaction from man within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
• Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
• Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices
NAMDRC’s mission statement clearly signals its commitment to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment. Adhering to that commitment presents challenges in the rapidly changing structure of the delivery of health care. For example, 10 years ago, the majority of NAMDRC members were private practitioners/group practices, many with contracts to provide a range of services to institutions. While those agreements varied, the underlying principles were relatively constant – structure your agreements that were mutually beneficial to physician and hospital.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level % E&M codes, but due to strong reaction from man within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
• Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
• Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices
NAMDRC’s mission statement clearly signals its commitment to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment. Adhering to that commitment presents challenges in the rapidly changing structure of the delivery of health care. For example, 10 years ago, the majority of NAMDRC members were private practitioners/group practices, many with contracts to provide a range of services to institutions. While those agreements varied, the underlying principles were relatively constant – structure your agreements that were mutually beneficial to physician and hospital.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level % E&M codes, but due to strong reaction from man within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
• Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
• Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
CHEST Foundation support for young career clinicians
As the CHEST Foundation continues to grow, so does our ability to impact the careers of early career clinicians. What began as a small travel grants program for the 2015 winners of the NetWorks Challenge to help offset their trainee members’ travel to CHEST 2015 in Montreal, was quickly identified as opportunity for the CHEST Foundation to deepen their engagement with early career clinicians. The CHEST Foundation travel grants program has grown immensely since then, but the core tenants of the program remain unchanged – to provide excellent trainees, medical students, and all other members of the care team with the fiscal support they need to become successful clinicians and faithfully treat their patients and community. Some of the ways our travel grants are put to good use is to attend the CHEST Annual Meeting and to further engage them as active members of CHEST. In addition to travel grant support to offset the costs of attending the annual meeting, recipients of these competitive grants receive free registration to the meeting; individualized mentorship from a CHEST member who is currently or has been part of CHEST leadership (ie, served on one of the boards, as faculty, on committees, as well as chairs and vice-chairs of the NetWorks); learn best practices for applying for research and community service grants from previous grant winners; invitations to exclusive receptions to network with peers and potential employers; and access to several sessions at the annual meeting intended to strengthen their clinical skill set. All of these programmatic pieces come together to help propel these young leaders’ careers and invest in the future of our discipline as CHEST clinicians.
Due to your overwhelming philanthropic support, CHEST Foundation’s travel grant programs continue to flourish. In 2017, the CHEST Foundation supported
a total of 43 early career clinicians’ travel to attend the CHEST Annual Meeting in Toronto. Through continued donor support, a successful NetWorks Challenge
fundraiser, and an overwhelming number of qualified early career applicants for the travel grants, that number swelled to 72 clinicians for the 2018 CHEST Annual Meeting in San Antonio. In total, the CHEST Foundation dispensed over $70,000 in travel grants for CHEST 2018. We can’t thank you enough for the impact you have made in these early career clinicians’ professional lives, and we urge you to increase your gifts, so we can advance these important professional development opportunities for clinicians by CHEST 2019!
“I’m so thankful to be a recipient of the CHEST travel grant! It enabled me to connect with such a wide array of health-care professionals and learn from my peers. It was wonderful to discover that there are many ways for me as a respiratory therapist to become involved in CHEST! Thank you to all the donors who made these awards a reality!”
- Maya Jenkins, RRT
“As an international medical graduate fellow, I experience challenges spanning from economic (inability to moonlight), professional (scarce funding and sponsorship opportunities, mentorship) to immigration-related difficulties. The CHEST Foundation grant is a superbly structured and implemented opportunity that allowed me a chance to address most of these challenges as I advance in my academic career. The grant itinerary permitted me to network with mentors and, subsequently, resulted in critical leads: A collaborative research project, offers to write letters in support of my visa situation, interest from a journal for one my manuscripts, plans to submit proposals for #CHEST2019, and, most importantly, support from leaders in our field who offered guidance and sponsorship (huge shout out to Dr. Chris Carroll)! I would like to thank the Foundation for awarding this grant as it isn’t just the grant but the slew of opportunities that came along with it that can, and, in my case, catapult fledgling careers in the field of pulmonary and critical care medicine.”
-Viren Kaul, MD
“CHEST education is the cornerstone of pulmonary medicine and delivering world-class health care. CHEST and the CHEST Foundation care about me and the importance of being the best practitioner I can be for my patients. Having impactful conversations with other clinicians, seeing new innovations, and learning through a diverse number of ways while at CHEST 2018 gave me meaningful lessons to apply in my daily practice. The travel grant made this possible!”
- Sarah Brundidge, MSc, RRT
As the CHEST Foundation continues to grow, so does our ability to impact the careers of early career clinicians. What began as a small travel grants program for the 2015 winners of the NetWorks Challenge to help offset their trainee members’ travel to CHEST 2015 in Montreal, was quickly identified as opportunity for the CHEST Foundation to deepen their engagement with early career clinicians. The CHEST Foundation travel grants program has grown immensely since then, but the core tenants of the program remain unchanged – to provide excellent trainees, medical students, and all other members of the care team with the fiscal support they need to become successful clinicians and faithfully treat their patients and community. Some of the ways our travel grants are put to good use is to attend the CHEST Annual Meeting and to further engage them as active members of CHEST. In addition to travel grant support to offset the costs of attending the annual meeting, recipients of these competitive grants receive free registration to the meeting; individualized mentorship from a CHEST member who is currently or has been part of CHEST leadership (ie, served on one of the boards, as faculty, on committees, as well as chairs and vice-chairs of the NetWorks); learn best practices for applying for research and community service grants from previous grant winners; invitations to exclusive receptions to network with peers and potential employers; and access to several sessions at the annual meeting intended to strengthen their clinical skill set. All of these programmatic pieces come together to help propel these young leaders’ careers and invest in the future of our discipline as CHEST clinicians.
Due to your overwhelming philanthropic support, CHEST Foundation’s travel grant programs continue to flourish. In 2017, the CHEST Foundation supported
a total of 43 early career clinicians’ travel to attend the CHEST Annual Meeting in Toronto. Through continued donor support, a successful NetWorks Challenge
fundraiser, and an overwhelming number of qualified early career applicants for the travel grants, that number swelled to 72 clinicians for the 2018 CHEST Annual Meeting in San Antonio. In total, the CHEST Foundation dispensed over $70,000 in travel grants for CHEST 2018. We can’t thank you enough for the impact you have made in these early career clinicians’ professional lives, and we urge you to increase your gifts, so we can advance these important professional development opportunities for clinicians by CHEST 2019!
“I’m so thankful to be a recipient of the CHEST travel grant! It enabled me to connect with such a wide array of health-care professionals and learn from my peers. It was wonderful to discover that there are many ways for me as a respiratory therapist to become involved in CHEST! Thank you to all the donors who made these awards a reality!”
- Maya Jenkins, RRT
“As an international medical graduate fellow, I experience challenges spanning from economic (inability to moonlight), professional (scarce funding and sponsorship opportunities, mentorship) to immigration-related difficulties. The CHEST Foundation grant is a superbly structured and implemented opportunity that allowed me a chance to address most of these challenges as I advance in my academic career. The grant itinerary permitted me to network with mentors and, subsequently, resulted in critical leads: A collaborative research project, offers to write letters in support of my visa situation, interest from a journal for one my manuscripts, plans to submit proposals for #CHEST2019, and, most importantly, support from leaders in our field who offered guidance and sponsorship (huge shout out to Dr. Chris Carroll)! I would like to thank the Foundation for awarding this grant as it isn’t just the grant but the slew of opportunities that came along with it that can, and, in my case, catapult fledgling careers in the field of pulmonary and critical care medicine.”
-Viren Kaul, MD
“CHEST education is the cornerstone of pulmonary medicine and delivering world-class health care. CHEST and the CHEST Foundation care about me and the importance of being the best practitioner I can be for my patients. Having impactful conversations with other clinicians, seeing new innovations, and learning through a diverse number of ways while at CHEST 2018 gave me meaningful lessons to apply in my daily practice. The travel grant made this possible!”
- Sarah Brundidge, MSc, RRT
As the CHEST Foundation continues to grow, so does our ability to impact the careers of early career clinicians. What began as a small travel grants program for the 2015 winners of the NetWorks Challenge to help offset their trainee members’ travel to CHEST 2015 in Montreal, was quickly identified as opportunity for the CHEST Foundation to deepen their engagement with early career clinicians. The CHEST Foundation travel grants program has grown immensely since then, but the core tenants of the program remain unchanged – to provide excellent trainees, medical students, and all other members of the care team with the fiscal support they need to become successful clinicians and faithfully treat their patients and community. Some of the ways our travel grants are put to good use is to attend the CHEST Annual Meeting and to further engage them as active members of CHEST. In addition to travel grant support to offset the costs of attending the annual meeting, recipients of these competitive grants receive free registration to the meeting; individualized mentorship from a CHEST member who is currently or has been part of CHEST leadership (ie, served on one of the boards, as faculty, on committees, as well as chairs and vice-chairs of the NetWorks); learn best practices for applying for research and community service grants from previous grant winners; invitations to exclusive receptions to network with peers and potential employers; and access to several sessions at the annual meeting intended to strengthen their clinical skill set. All of these programmatic pieces come together to help propel these young leaders’ careers and invest in the future of our discipline as CHEST clinicians.
Due to your overwhelming philanthropic support, CHEST Foundation’s travel grant programs continue to flourish. In 2017, the CHEST Foundation supported
a total of 43 early career clinicians’ travel to attend the CHEST Annual Meeting in Toronto. Through continued donor support, a successful NetWorks Challenge
fundraiser, and an overwhelming number of qualified early career applicants for the travel grants, that number swelled to 72 clinicians for the 2018 CHEST Annual Meeting in San Antonio. In total, the CHEST Foundation dispensed over $70,000 in travel grants for CHEST 2018. We can’t thank you enough for the impact you have made in these early career clinicians’ professional lives, and we urge you to increase your gifts, so we can advance these important professional development opportunities for clinicians by CHEST 2019!
“I’m so thankful to be a recipient of the CHEST travel grant! It enabled me to connect with such a wide array of health-care professionals and learn from my peers. It was wonderful to discover that there are many ways for me as a respiratory therapist to become involved in CHEST! Thank you to all the donors who made these awards a reality!”
- Maya Jenkins, RRT
“As an international medical graduate fellow, I experience challenges spanning from economic (inability to moonlight), professional (scarce funding and sponsorship opportunities, mentorship) to immigration-related difficulties. The CHEST Foundation grant is a superbly structured and implemented opportunity that allowed me a chance to address most of these challenges as I advance in my academic career. The grant itinerary permitted me to network with mentors and, subsequently, resulted in critical leads: A collaborative research project, offers to write letters in support of my visa situation, interest from a journal for one my manuscripts, plans to submit proposals for #CHEST2019, and, most importantly, support from leaders in our field who offered guidance and sponsorship (huge shout out to Dr. Chris Carroll)! I would like to thank the Foundation for awarding this grant as it isn’t just the grant but the slew of opportunities that came along with it that can, and, in my case, catapult fledgling careers in the field of pulmonary and critical care medicine.”
-Viren Kaul, MD
“CHEST education is the cornerstone of pulmonary medicine and delivering world-class health care. CHEST and the CHEST Foundation care about me and the importance of being the best practitioner I can be for my patients. Having impactful conversations with other clinicians, seeing new innovations, and learning through a diverse number of ways while at CHEST 2018 gave me meaningful lessons to apply in my daily practice. The travel grant made this possible!”
- Sarah Brundidge, MSc, RRT
Winners all
Everyone who attended CHEST Annual Meeting 2018 is a winner, but we would like to call out the winners participating in CHEST’s special categories of awards and events. Congratulations to all!
ANNUAL CHEST AWARDS
Master FCCP
David Gutterman, MD, Master FCCP
Distinguished Service Award
David Gutterman, MD, Master FCCP
College Medalist Award
Ghada Bourjeily, MD, FCCP
Master Clinician Educator
Lisa Moores, MD, FCCP
Early Career Clinician Educator
Amy Morris, MD, FCCP
Alfred Soffer Award for Editorial Excellence
Jean Rice
Presidential Citation
Darcy Marciniuk, MD, FCCP
Presidential Citation
D. Robert McCaffree, MD, Master FCCP
HONOR LECTURES AND MEMORIAL AWARDS
Edward C. Rosenow III, MD, Master FCCP/Master Teacher Honor Lecture Accelerated Aging in COPD and Its Comorbidities: Novel Therapeutic Targets
Peter Barnes, MD, Master FCCP
The lecture is generously funded by the CHEST Foundation.
Distinguished Scientist Honor Lecture in Cardiopulmonary Physiology
Understanding Diaphragm Performance: The Role of Ultrasound
F. Dennis McCool, MD, FCCP
The lecture is generously funded by the CHEST Foundation.
Presidential Honor Lecture
Asthma: Past, Present, and Future
Jay Peters, MD, FCCP
Thomas L. Petty, MD, Master FCCP Memorial Lecture
Recent Developments in Pulmonary Rehabilitation and Long-Term Oxygen Therapy: Would Tom Petty be Pleased?
Richard Casaburi, MD, PhD, FCCP
The lecture is generously funded by the CHEST Foundation.
Margaret Pfrommer Memorial Lecture in Long-term Mechanical Ventilation
Saving Lives…One Ventilator at a Time - HMV in 2018 and Beyond
Douglas McKim, MD, FCCP
The Margaret Pfrommer Memorial Lecture in Long-term Mechanical Ventilation is generously supported by International Ventilator Users Network of Post-Polio Health International and the CHEST Foundation.
Pasquale Ciaglia Memorial Lecture in Interventional Medicine
Evolution of Endobronchial Ultrasound: From Diagnostics to Therapeutics
Kazuhiro Yasufuku, MD, PhD, FCCP
The lecture is generously funded by the CHEST Foundation.
Roger C. Bone Memorial Lecture in Critical Care
Methylprednisolone in ARDS: A Highly Effective Treatment. How it Works, How to Use it
G. Umberto Meduri, MD
The lecture is generously funded by the CHEST Foundation.
CHEST FOUNDATION GRANT WINNERS
Distinguished Scholar
Robert C. Hyzy, MD, FCCP
Eli Lilly and Company Distinguished Scholar in Critical Care MedicineGrant Title: The Use of Electrical Impedance Tomography to Assess Mechanical Ventilation in Acute Respiratory Distress Syndrome
This grant is made possible due to the philanthropic support from Eli Lilly and Company.
Community Service Grantees
Deborah Haisch, MD
Columbia University Medical Center – New York, NY
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: East African Training Initiative in Pulmonary and Critical Care Medicine
Pamela Garrett, CCRN, MN
Gwinnett Medical Center – Lawrenceville, GA
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: Breathe Better Gwinnett
Phillip Sheridan
Mobile Care Chicago – Chicago, IL
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: Home Environment Education for Children with Asthma
These grants are supported in full by the CHEST Foundation.
Research Grant Winners
Ayodeji Adegunsoye, MD, MS
Research Grant in Pulmonary Fibrosis
Grant Title: Impact of Telomere Length on Pulmonary Fibrosis Clusters Across Diverse Racial Cohorts
Justin Oldham, MD, MS
Research Grant in Pulmonary Fibrosis
Grant Title: Plasma Biomarkers to Predict Outcomes and Treatment Response in Patients with Pulmonary Fibrosis
These grants above are supported by Boehringer Ingelheim Pharmaceuticals, Inc and Genentech.
Jacob Brenner, MD, PhD
Research Grant in Chronic Obstructive Pulmonary Disease
Grant Title: Ambulatory Cuirass Ventilation for Relief of Exertional Dyspnea in Severe COPD Patients
William Zhang, MD
Research Grant in Chronic Obstructive Pulmonary Disease
Grant Title: Pulmonary Iron Overload as a Novel COPD Endotype
These grants above are supported by AstraZeneca LP and Sunovion Pharmaceuticals Inc.
Margaret Bublitz, PhD
CHEST Foundation Research Grant in Women’s Lung Health
Grant Title: Sex as a Predictor of Sleep-Disordered Breathing and Its Consequences in Pregnancy
This grant is supported in full by the CHEST Foundation.
Tim Morris, MD, FCCP
CHEST Foundation Research Grant in Venous Thromboembolism
Grant Title: Long-term Follow-up of Acute Pulmonary Embolism
This grant is supported in full by the CHEST Foundation.
Monica Mukherjee, MD, MPH
CHEST Foundation Research Grant in Pulmonary Arterial Hypertension
Grant Title: Exercise Provocation in the Noninvasive Detection of Occult Right Ventricular Dysfunction and Emerging Pulmonary Hypertension in Systemic Sclerosis
This grant is supported in full by the CHEST Foundation.
Don Sanders, MD, MS
CHEST Foundation Research Grant in Cystic Fibrosis
Grant Title: Whole-genome Shotgun Sequencing of Oropharyngeal Swabs in Infants With CF
This grant is supported by Vertex Pharmaceuticals.
Imran Sulaiman, MD, PhD
CHEST Foundation Research Grant in Nontuberculosis Mycobacteria Diseases
Grant Title: Lower Airway Microbiota Signatures Associated W ith Impaired Immune Response in Non-Tuberculous Mycobacterium
This grant is supported by Insmed.
Samira Shojaee, MD, MPH, FCCP
CHEST Foundation Research Grant in Lung Cancer
Grant Title: Extracellular Vesicle miRNA as a Biomarker in Malignant Pleural Effusion
This grant is supported in full by the CHEST Foundation.
Anna Volerman, MD
CHEST Foundation Research Grant in Severe Asthma
Grant Title: A Randomized Clinical Trial Evaluating the Effectiveness of Virtual Teach-to-Goal(TM) Education versus Brief Intervention for Children with Severe Asthma
This grant is supported by AstraZeneca LP.
ABSTRACT AND CASE REPORT WINNERS
Alfred Soffer Research Award Winners
Clauden Louis, MD: Left ventricular assist devices in Intermacs 1 acute cardiogenic shock patients
Babith J. Mankidy, MBBS, FCCP: Reduction in in-hospital cardiac arrest with early interventions in the emergency department and non-ICU units by a novel approach of rapid response teams and mobile ICU management
Young Investigator Award Winners
Fayez Kheir, MD, MSc: Intrapleural tissue plasminogen activator and deoxyribonuclease therapy vs early medical thoracoscopy for treatment of pleural infection: a randomized clinical trial
Michael Rosman, MD: The utility of end tidal CO2 (ETCO2) monitoring during in-hospital cardiac arrest to predict return of spontaneous circulation
Top 5 Abstract Poster Winners
Neha Agarwal, MD: The 3 wishes project: a feasible intervention to improve end of life care in the ICU at UCLA
Hiroaki Harada, MD: Usefulness of comprehensive preoperative pulmonary rehabilitation program including intensive nutritional support concomitant with physical exercise through an interdisciplinary team approach
Joseph M. Carrington, DO, MHA: Targeting the trans-IL-6 signaling pathway to reduce agriculture organic dust exposure-induced airway inflammation in mice
Yu Kuang Lai, MBBCh: The utility of parametric response mapping in pulmonary graft vs host disease following hematopoietic stem cell transplant
Top Abstract Poster Finalists
Ligia M. Puiu, MD, PhD, FCCP: Association between echocardiographic and lipid parameters to workers in the metalliferous mines
Kush R. Dholakia, MD: Colloids vs crystalloids for postoperative resuscitation in patients undergoing off-pump coronary artery bypass surgery
Kulothungan Gunasekaran, MD, MBBS: Risk of VTE in idiopathic pulmonary fibrosis: a systematic review
Laura B. Sutton, PharmD: Ease and correct use of Ellipta by age in patients with asthma and COPD
Ankur Mogla, MD: To assess the utilization of pulmonary function testing for perioperative respiratory complications in bariatric surgery patients
Ali Ammar: Tracheostomy and admission diagnosis as predictors for an extended length of stay (ELOS)
Charlene Kalani, PharmD: Efficacy and safety of direct oral anticoagulants (DOACS) in morbidly obese patients
Jonghoo Lee, MD: Performances of modified CRB-65 score compared to SIRS and QSOFA as a rapid screening tool for sepsis among infected patients in initial emergency department: a propensity score matching study
Frank J. Trudo, MD, FCCP: Clinical burden of eosinophilic COPD
Elise L. Stephenson, MD: Vitamin C and point of care glucose measurements: a retrospective, observational study
Faisal Siddiqi, MD: Implementation of an early mobility program in the medical ICU
Eileen Harder, MD: Connective tissue disease-associated pulmonary arterial hypertension hospitalizations from 2001-2014
Sophie Korzan, MD: Exhaled nitric oxide and asthma-COPD overlap in patients hospitalized with exacerbations of airway disease: preliminary observations
Andreas Grove, MD: MicroRNA (MIRNA) and biological markers discriminate between normotensive and prehypertensive young men in hypobaric hypoxic environments
Snigdha Nutalapati, MBBS: Large cell neuroendocrine cancer of the lung: SEER 2004-2014 analysis
Anubhav Jain, MBBS: Survival benefit of beta-blockers in patients hospitalized for acute exacerbation of COPD
Case Report Slide Winners
Ze Ying Tan: All that wheezes is not asthma
Jason Lam: Pulmonary mucor mycetoma
Adam Young: Nonresolving pneumonia and cyclic fevers in an immunocompetent patient
Ritu Modi: Histopathological misdiagnosis of pulmonary coccidiodes
Argun Can: A rare inborn error of fatty acid oxidation presenting with severe hyperammonemia in the ICU
Morgan Gilani: A colorful cause of cardiovascular collapse
Katie Jeans: A sweet surprise
Anthony Mattox: Unusual case of interstitial lung disease
Andrew Berglund: Pulmonary light chain deposition disease in a 29-year-old army soldier
Cristia Maysol Morales: A case report of a primary malignant melanoma of anterior mediastinum
Anthony McClafferty: Fibrosing mediastinitis and rheumatoid arthritis: an autoimmune inflammatory connection
Ahmed Munir: HIV with disseminated tularemia: a rare presentation Benjamin Garren: Mycobacterium avium complex mediastinal lymphadenitis in an immunocompetent adolescent with erosion into the airway
Robert Hilton: Obtunded with a chest mass: a case of a rare neurologic paraneoplastic syndrome,
Audra Schwalk: Mucoepidermoid carcinoma: a rare malignancy treated endobronchially
Jessica Riggs: Successful transplantation defies genetics: a case of rapidly-progressive pulmonary fibrosis due to Hermansky-Pudlak syndrome
Meghan Cirulis: Acute vasodilator testing: an opportunity to refine study design and provide precision care in pulmonary hypertension
Patrick Chan: VATS lobectomy for bronchial atresia in an adult
Andrew Mehlman: Multivessel coronary artery aneurysms presenting as myocardial ischemia
Scott Maughan: Diagnosing milliary Mycobacterium bovis from the prostate of an immunocompetent host
Adam Austin: Survived ECMO, death by BLASTO: the first reported fatal case of disseminated blastomycosis in pregnancy
Tie: Donnie Carter: Subclinical polycythemia vera presenting as extensive thrombosis due to massive transfusion, and
Lindsay Hammons: Rare case of Serratia pneumonia causing transient aplastic anemia
Paola Baskin: Novel observations during point-of-care ultrasound (POCUS) in cardiopulmonary resuscitation: a case of ultrasound-guided probe pressure to reduce esophageal insufflation during bag-valve-mask ventilator
David Dennis: Pulmonary alveolar proteinosis presenting as intracerebral nocardiosis
Rakin Choudhury: Severe asthma caused by therapy-resistant asthmatic granulomatosis
Andrew Lytle: Lung adenocarcinoma in a patient with Turcot syndrome
Chelsea Leipold: Case of a granulomatous-lymphocytic interstitial lung disease in a patient with common variable immunodeficiency disorder
Galyna Ivashchuk: Double trouble: ANCA vasculitis with concomitant IGA nephropathy presenting as massive diffuse alveolar hemorrhage and fulminant renal failure
Case Report Poster Winners
Christine Zhou: Role of transbronchial lung cryobiopsy in the diagnosis of adenocarcinoma in situ
Parin Shah: A rare case of Erdheim-Chester disease masquerading as metastatic lung cancer
Avanthika Wynn : A rare asthma mimic
Muhammad S. Ali: Severe pancolitis: a rare adverse effect of nintedanib
Brian Foster: Don’t forget to breathe: a case of hypoxemia after carotid body resection
Kelly Pennington: Intra-cardiac embolization of an inferior vena cava filter resulting in cardiac arrest
George Elkomos-Botros: Acute generalized exanthematous pustulosis presenting as distributive shock with multi-organ failure
Ashley M. Scott: Avian occupational hypersensitivity pneumonitis in a restaurant employee
Andrew Polito: Pulmonary amyloidosis: an unusual presentation of a rare disease
CHEST B-I-N-G-O WINNERS
Stella Ogake, MD
Erin E. Peterson, APRN, CNP
Megan J. Castillo, PA-C
Gretchen R. Winter, MD
Jeanette P. Brown, MD, PhD
Yu Hong Chan, MBBS
Anita Naik, DO
Gary A. Aaronson, DO, FCCP
Allison S. Cowl, MD
Kyle Halligan, MD
Palaniappan Muthappan, MD
Faizullah S. Lokhandwala, MBBS, FCCP
Jamie R. Chua, MD
Francis L. Ervin, MD, FCCP
Robyn Luper
CHEST CHALLENGE WINNER (AND RUNNER’S-UP)
Emory University (First Place)
Mirza Haider Ali, MD
Mohleen Kang, MD
Matthew Schimmel, MD
University of Michigan (Second Place)
Patrick Bradley, MD
Matthew Hensley, MD
Bonnie Wang, MD
Cleveland Clinic (Third Place)
Jorge Mirales-Estrella, MD
Apostolos Perelas, MD
Gretchen Winter, MD
2018 DISTINGUISHED CHEST EDUCATORS
Michael H Ackerman, DNSc
Sandra G Adams, MD, MS, FCCP
Doreen J Addrizzo-Harris, MD, FCCP
Cara Lyn Agerstrand, MD, BS
Jason A Akulian, MD, FCCP
Raed H Alalawi, MD, FCCP
A. Christine Argento, MD, FCCP
Robert Arntfield, MD, FCCP
Alex A Balekian, MD
Meyer S Balter, MD, FCCP
Gisela I Banauch, MD, MS, FCCP
Robert P Baughman, MD, FCCP
David G Bell, MD, FCCP
Michel A Boivin, MD, FCCP
Gabriel T Bosslet, MD, FCCP
Jean Bourbeau, MD, MS, FCCP
Ghada R Bourjeily, MD, FCCP
David L Bowton, MD, FCCM
Jack D Buckley, MD, MPH, FCCP
Marie M Budev, DO, MPH, FCCP
Kristin M Burkart, MD, MS, FCCP
Brian Carlin, MD, FCCP
Christopher L Carroll, MD, FCCP
Roberto F Casal, MD
Kevin M Chan, MD, FCCP
Subani Chandra, MD, FCCP
Ching-Fei Chang, MD
Alexander C Chen, MD
Nancy A Collop, MD, FCCP
Clayton T Cowl, MD, MS, FCCP
Angel O Coz Yataco, MD, FCCP
Gerard J Criner, MD, FCCP
Carolyn M D’Ambrosio, MD, FCCP
Mauricio Danckers, MD, FCCP
Aneesa M Das, MD, FCCP
John Davies, RRT, MA, FCCP
Zachary S DePew, MD, FCCP
Frank C Detterbeck, MD, FCCP
Naresh A. Dewan, MBBS, FCCP
Kevin C Doerschug, MD, MS, FCCP
Meagan Dubosky, RRT-ACCS
Kevin M Dushay, MD, FCCP
Eric S Edell, MD, FCCP
Jean M Elwing, MD, FCCP
William Enfinger
Michael E Ezzie, MD, FCCP
Kevin J Felner, MD, FCCP
Mark E Fenton, MD, MSc, FCCP
Jason Filopei, MD
Neil S Freedman, MD, FCCP
Laura Kathleen Frye, MD
Thomas M Fuhrman, MD, MS, FCCP
John P Gaillard, MD, FCCP
Colin T Gillespie, MD
Yonatan Y Greenstein, MD
Maritza L Groth, MD, FCCP
Keith P Guevarra, DO, FCCP
Jesse B Hall, MD, FCCP
Nicola A Hanania, MD, MBBS, FCCP
D Kyle Hogarth, MD, FCCP
Steven M Hollenberg, MD, FCCP
David W Hsia, MD, FCCP
Candace A Huebert, MD, FCCP
Robert C Hyzy, MD, FCCP
Octavian C Ioachimescu, MD, PhD, FCCP
Richard S Irwin, MD, Master FCCP
Kirk D Jones, MD
Nader Kamangar, MD, MS, FCCP
Carl A Kaplan, MD, FCCP
Brian S Kaufman, MD, FCCP
William F Kelly, MD, FCCP
Marcus P Kennedy, MD, FCCP
Sandhya Khurana, MD, FCCP
James R Klinger, MD, FCCP
Seth J Koenig, MD, FCCP
Lindsey Kreisher, RRT
Karol Kremens, MD, FCCP
Patricia A Kritek, MD, FCCP
Sunita Kumar, MD, MBBS, FCCP
Rudy P Lackner, MD, FCCP
Viera Lakticova, MD
Carla R Lamb, MD, FCCP
Hans J Lee, MD, FCCP
Peter H Lenz, MD, MEd, FCCP
Stephanie M Levine, MD, FCCP
Deborah Jo Levine, MD, MS, FCCP
Andrea Loiselle, MD
Kenneth E Lyn-Kew, MD
Michael S Machuzak, MD, FCCP
Neil R MacIntyre, MD, FCCP
Donald A Mahler, MD, FCCP
Fabien Maldonado, MD, FCCP
Atul Malhotra, MD, FCCP
Darcy D Marciniuk, MD, FCCP
Diego J Maselli Caceres, MD, FCCP
Paul H Mayo, MD, FCCP
Peter J Mazzone, MD, MPH, FCCP
John K McIlwaine, DO, MBA, FCCP
Matthew C Miles, MD, FCCP
Scott Millington, MD
Taro Minami, MD, FCCP
Lisa K Moores, MD, FCCP
Amy E Morris, MD, FCCP
John J Mullon, MD, FCCP
Septimiu D Murgu, MD, FCCP
Mangala Narasimhan, DO, FCCP
Michael S Niederman, MD, FCCP
Alexander S Niven, MD, FCCP
Anne E O’Donnell, MD, FCCP
Erik C Osborn, MD
David E Ost, MD, MPH, FCCP
Ronald J Oudiz, MD, FCCP
Daniel R Ouellette, MD, MS, FCCP
Amit D Parulekar, MD, MS, FCCP
Nicholas J Pastis, MD, FCCP
Nina M Patel, MD, FCCP
Paru S Patrawalla, MD, FCCP
Jay I Peters, MD, FCCP
Barbara A Phillips, MD, MSPH, FCCP
Margaret A Pisani, MD, MS, FCCP
Janos Porszasz, MD, PhD
Whitney S Prince, MD, FCCP
Suhail Raoof, MBBS, Master FCCP
Ruben D Restrepo, RRT, FCCP
Marcos I Restrepo, MD, PhD, FCCP
Otis B Rickman, DO, FCCP
Roy D Ridgeway
Mary Ried, RN, CCRN
Linda Rogers, MD, FCCP
Mark J Rosen, MD, Master FCCP
Bernard J Roth, MD, FCCP
Ashutosh Sachdeva, MBBS, FCCP
Anthony G Saleh, MD, FCCP
Juan F Sanchez, MD, FCCP
Pralay K Sarkar, MBBS, FCCP
Lewis G Satterwhite, MD, BA, FCCP
Gregory A Schmidt, MD, FCCP
Mary Beth Scholand, MD, FCCP
David A Schulman, MD, MPH, FCCP
Brady Scott, RRT, MS, FCCP
Bernardo Selim, MD, FCCP
Curtis N Sessler, MD, FCCP
Rakesh D Shah, MD, FCCP
Ray Wes Shepherd, MD, FCCP
John H Sherner, MD, FCCP
Ariel L Shiloh, MD
Samira Shojaee, MD, FCCP
Marcos Silva Restrepo
Gerard A Silvestri, MD, MS, FCCP
Steven Q Simpson, MD, FCCP
James K Stoller, MD, MS, FCCP
Charlie Strange, MD, FCCP
Mary E Strek, MD, FCCP
William W Stringer, MD, FCCP
Eleanor M Summerhill, MD, FCCP
Maximiliano A Tamae Kakazu, MD, FCCP
Nichole T Tanner, MD, MS, FCCP
Lynn T Tanoue, MD, FCCP
Victor J Test, MD, FCCP
Arthur J Tokarczyk, MD, FCCP
Alain Tremblay, MD, FCCP
Adey Tsegaye, MD, FCCP
Anil Vachani, MD, FCCP
Momen M Wahidi, MD, MBA, FCCP
Keith M Wille, MD, FCCP
Lisa F Wolfe, MD
Richard G Wunderink, MD, FCCP
Lonny B Yarmus, DO, FCCP
Kazuhiro Yasufuku, MD, PhD, FCCP
Gulrukh Zaidi, MD, FCCP
David Zielinski, MD, FCCP
Everyone who attended CHEST Annual Meeting 2018 is a winner, but we would like to call out the winners participating in CHEST’s special categories of awards and events. Congratulations to all!
ANNUAL CHEST AWARDS
Master FCCP
David Gutterman, MD, Master FCCP
Distinguished Service Award
David Gutterman, MD, Master FCCP
College Medalist Award
Ghada Bourjeily, MD, FCCP
Master Clinician Educator
Lisa Moores, MD, FCCP
Early Career Clinician Educator
Amy Morris, MD, FCCP
Alfred Soffer Award for Editorial Excellence
Jean Rice
Presidential Citation
Darcy Marciniuk, MD, FCCP
Presidential Citation
D. Robert McCaffree, MD, Master FCCP
HONOR LECTURES AND MEMORIAL AWARDS
Edward C. Rosenow III, MD, Master FCCP/Master Teacher Honor Lecture Accelerated Aging in COPD and Its Comorbidities: Novel Therapeutic Targets
Peter Barnes, MD, Master FCCP
The lecture is generously funded by the CHEST Foundation.
Distinguished Scientist Honor Lecture in Cardiopulmonary Physiology
Understanding Diaphragm Performance: The Role of Ultrasound
F. Dennis McCool, MD, FCCP
The lecture is generously funded by the CHEST Foundation.
Presidential Honor Lecture
Asthma: Past, Present, and Future
Jay Peters, MD, FCCP
Thomas L. Petty, MD, Master FCCP Memorial Lecture
Recent Developments in Pulmonary Rehabilitation and Long-Term Oxygen Therapy: Would Tom Petty be Pleased?
Richard Casaburi, MD, PhD, FCCP
The lecture is generously funded by the CHEST Foundation.
Margaret Pfrommer Memorial Lecture in Long-term Mechanical Ventilation
Saving Lives…One Ventilator at a Time - HMV in 2018 and Beyond
Douglas McKim, MD, FCCP
The Margaret Pfrommer Memorial Lecture in Long-term Mechanical Ventilation is generously supported by International Ventilator Users Network of Post-Polio Health International and the CHEST Foundation.
Pasquale Ciaglia Memorial Lecture in Interventional Medicine
Evolution of Endobronchial Ultrasound: From Diagnostics to Therapeutics
Kazuhiro Yasufuku, MD, PhD, FCCP
The lecture is generously funded by the CHEST Foundation.
Roger C. Bone Memorial Lecture in Critical Care
Methylprednisolone in ARDS: A Highly Effective Treatment. How it Works, How to Use it
G. Umberto Meduri, MD
The lecture is generously funded by the CHEST Foundation.
CHEST FOUNDATION GRANT WINNERS
Distinguished Scholar
Robert C. Hyzy, MD, FCCP
Eli Lilly and Company Distinguished Scholar in Critical Care MedicineGrant Title: The Use of Electrical Impedance Tomography to Assess Mechanical Ventilation in Acute Respiratory Distress Syndrome
This grant is made possible due to the philanthropic support from Eli Lilly and Company.
Community Service Grantees
Deborah Haisch, MD
Columbia University Medical Center – New York, NY
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: East African Training Initiative in Pulmonary and Critical Care Medicine
Pamela Garrett, CCRN, MN
Gwinnett Medical Center – Lawrenceville, GA
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: Breathe Better Gwinnett
Phillip Sheridan
Mobile Care Chicago – Chicago, IL
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: Home Environment Education for Children with Asthma
These grants are supported in full by the CHEST Foundation.
Research Grant Winners
Ayodeji Adegunsoye, MD, MS
Research Grant in Pulmonary Fibrosis
Grant Title: Impact of Telomere Length on Pulmonary Fibrosis Clusters Across Diverse Racial Cohorts
Justin Oldham, MD, MS
Research Grant in Pulmonary Fibrosis
Grant Title: Plasma Biomarkers to Predict Outcomes and Treatment Response in Patients with Pulmonary Fibrosis
These grants above are supported by Boehringer Ingelheim Pharmaceuticals, Inc and Genentech.
Jacob Brenner, MD, PhD
Research Grant in Chronic Obstructive Pulmonary Disease
Grant Title: Ambulatory Cuirass Ventilation for Relief of Exertional Dyspnea in Severe COPD Patients
William Zhang, MD
Research Grant in Chronic Obstructive Pulmonary Disease
Grant Title: Pulmonary Iron Overload as a Novel COPD Endotype
These grants above are supported by AstraZeneca LP and Sunovion Pharmaceuticals Inc.
Margaret Bublitz, PhD
CHEST Foundation Research Grant in Women’s Lung Health
Grant Title: Sex as a Predictor of Sleep-Disordered Breathing and Its Consequences in Pregnancy
This grant is supported in full by the CHEST Foundation.
Tim Morris, MD, FCCP
CHEST Foundation Research Grant in Venous Thromboembolism
Grant Title: Long-term Follow-up of Acute Pulmonary Embolism
This grant is supported in full by the CHEST Foundation.
Monica Mukherjee, MD, MPH
CHEST Foundation Research Grant in Pulmonary Arterial Hypertension
Grant Title: Exercise Provocation in the Noninvasive Detection of Occult Right Ventricular Dysfunction and Emerging Pulmonary Hypertension in Systemic Sclerosis
This grant is supported in full by the CHEST Foundation.
Don Sanders, MD, MS
CHEST Foundation Research Grant in Cystic Fibrosis
Grant Title: Whole-genome Shotgun Sequencing of Oropharyngeal Swabs in Infants With CF
This grant is supported by Vertex Pharmaceuticals.
Imran Sulaiman, MD, PhD
CHEST Foundation Research Grant in Nontuberculosis Mycobacteria Diseases
Grant Title: Lower Airway Microbiota Signatures Associated W ith Impaired Immune Response in Non-Tuberculous Mycobacterium
This grant is supported by Insmed.
Samira Shojaee, MD, MPH, FCCP
CHEST Foundation Research Grant in Lung Cancer
Grant Title: Extracellular Vesicle miRNA as a Biomarker in Malignant Pleural Effusion
This grant is supported in full by the CHEST Foundation.
Anna Volerman, MD
CHEST Foundation Research Grant in Severe Asthma
Grant Title: A Randomized Clinical Trial Evaluating the Effectiveness of Virtual Teach-to-Goal(TM) Education versus Brief Intervention for Children with Severe Asthma
This grant is supported by AstraZeneca LP.
ABSTRACT AND CASE REPORT WINNERS
Alfred Soffer Research Award Winners
Clauden Louis, MD: Left ventricular assist devices in Intermacs 1 acute cardiogenic shock patients
Babith J. Mankidy, MBBS, FCCP: Reduction in in-hospital cardiac arrest with early interventions in the emergency department and non-ICU units by a novel approach of rapid response teams and mobile ICU management
Young Investigator Award Winners
Fayez Kheir, MD, MSc: Intrapleural tissue plasminogen activator and deoxyribonuclease therapy vs early medical thoracoscopy for treatment of pleural infection: a randomized clinical trial
Michael Rosman, MD: The utility of end tidal CO2 (ETCO2) monitoring during in-hospital cardiac arrest to predict return of spontaneous circulation
Top 5 Abstract Poster Winners
Neha Agarwal, MD: The 3 wishes project: a feasible intervention to improve end of life care in the ICU at UCLA
Hiroaki Harada, MD: Usefulness of comprehensive preoperative pulmonary rehabilitation program including intensive nutritional support concomitant with physical exercise through an interdisciplinary team approach
Joseph M. Carrington, DO, MHA: Targeting the trans-IL-6 signaling pathway to reduce agriculture organic dust exposure-induced airway inflammation in mice
Yu Kuang Lai, MBBCh: The utility of parametric response mapping in pulmonary graft vs host disease following hematopoietic stem cell transplant
Top Abstract Poster Finalists
Ligia M. Puiu, MD, PhD, FCCP: Association between echocardiographic and lipid parameters to workers in the metalliferous mines
Kush R. Dholakia, MD: Colloids vs crystalloids for postoperative resuscitation in patients undergoing off-pump coronary artery bypass surgery
Kulothungan Gunasekaran, MD, MBBS: Risk of VTE in idiopathic pulmonary fibrosis: a systematic review
Laura B. Sutton, PharmD: Ease and correct use of Ellipta by age in patients with asthma and COPD
Ankur Mogla, MD: To assess the utilization of pulmonary function testing for perioperative respiratory complications in bariatric surgery patients
Ali Ammar: Tracheostomy and admission diagnosis as predictors for an extended length of stay (ELOS)
Charlene Kalani, PharmD: Efficacy and safety of direct oral anticoagulants (DOACS) in morbidly obese patients
Jonghoo Lee, MD: Performances of modified CRB-65 score compared to SIRS and QSOFA as a rapid screening tool for sepsis among infected patients in initial emergency department: a propensity score matching study
Frank J. Trudo, MD, FCCP: Clinical burden of eosinophilic COPD
Elise L. Stephenson, MD: Vitamin C and point of care glucose measurements: a retrospective, observational study
Faisal Siddiqi, MD: Implementation of an early mobility program in the medical ICU
Eileen Harder, MD: Connective tissue disease-associated pulmonary arterial hypertension hospitalizations from 2001-2014
Sophie Korzan, MD: Exhaled nitric oxide and asthma-COPD overlap in patients hospitalized with exacerbations of airway disease: preliminary observations
Andreas Grove, MD: MicroRNA (MIRNA) and biological markers discriminate between normotensive and prehypertensive young men in hypobaric hypoxic environments
Snigdha Nutalapati, MBBS: Large cell neuroendocrine cancer of the lung: SEER 2004-2014 analysis
Anubhav Jain, MBBS: Survival benefit of beta-blockers in patients hospitalized for acute exacerbation of COPD
Case Report Slide Winners
Ze Ying Tan: All that wheezes is not asthma
Jason Lam: Pulmonary mucor mycetoma
Adam Young: Nonresolving pneumonia and cyclic fevers in an immunocompetent patient
Ritu Modi: Histopathological misdiagnosis of pulmonary coccidiodes
Argun Can: A rare inborn error of fatty acid oxidation presenting with severe hyperammonemia in the ICU
Morgan Gilani: A colorful cause of cardiovascular collapse
Katie Jeans: A sweet surprise
Anthony Mattox: Unusual case of interstitial lung disease
Andrew Berglund: Pulmonary light chain deposition disease in a 29-year-old army soldier
Cristia Maysol Morales: A case report of a primary malignant melanoma of anterior mediastinum
Anthony McClafferty: Fibrosing mediastinitis and rheumatoid arthritis: an autoimmune inflammatory connection
Ahmed Munir: HIV with disseminated tularemia: a rare presentation Benjamin Garren: Mycobacterium avium complex mediastinal lymphadenitis in an immunocompetent adolescent with erosion into the airway
Robert Hilton: Obtunded with a chest mass: a case of a rare neurologic paraneoplastic syndrome,
Audra Schwalk: Mucoepidermoid carcinoma: a rare malignancy treated endobronchially
Jessica Riggs: Successful transplantation defies genetics: a case of rapidly-progressive pulmonary fibrosis due to Hermansky-Pudlak syndrome
Meghan Cirulis: Acute vasodilator testing: an opportunity to refine study design and provide precision care in pulmonary hypertension
Patrick Chan: VATS lobectomy for bronchial atresia in an adult
Andrew Mehlman: Multivessel coronary artery aneurysms presenting as myocardial ischemia
Scott Maughan: Diagnosing milliary Mycobacterium bovis from the prostate of an immunocompetent host
Adam Austin: Survived ECMO, death by BLASTO: the first reported fatal case of disseminated blastomycosis in pregnancy
Tie: Donnie Carter: Subclinical polycythemia vera presenting as extensive thrombosis due to massive transfusion, and
Lindsay Hammons: Rare case of Serratia pneumonia causing transient aplastic anemia
Paola Baskin: Novel observations during point-of-care ultrasound (POCUS) in cardiopulmonary resuscitation: a case of ultrasound-guided probe pressure to reduce esophageal insufflation during bag-valve-mask ventilator
David Dennis: Pulmonary alveolar proteinosis presenting as intracerebral nocardiosis
Rakin Choudhury: Severe asthma caused by therapy-resistant asthmatic granulomatosis
Andrew Lytle: Lung adenocarcinoma in a patient with Turcot syndrome
Chelsea Leipold: Case of a granulomatous-lymphocytic interstitial lung disease in a patient with common variable immunodeficiency disorder
Galyna Ivashchuk: Double trouble: ANCA vasculitis with concomitant IGA nephropathy presenting as massive diffuse alveolar hemorrhage and fulminant renal failure
Case Report Poster Winners
Christine Zhou: Role of transbronchial lung cryobiopsy in the diagnosis of adenocarcinoma in situ
Parin Shah: A rare case of Erdheim-Chester disease masquerading as metastatic lung cancer
Avanthika Wynn : A rare asthma mimic
Muhammad S. Ali: Severe pancolitis: a rare adverse effect of nintedanib
Brian Foster: Don’t forget to breathe: a case of hypoxemia after carotid body resection
Kelly Pennington: Intra-cardiac embolization of an inferior vena cava filter resulting in cardiac arrest
George Elkomos-Botros: Acute generalized exanthematous pustulosis presenting as distributive shock with multi-organ failure
Ashley M. Scott: Avian occupational hypersensitivity pneumonitis in a restaurant employee
Andrew Polito: Pulmonary amyloidosis: an unusual presentation of a rare disease
CHEST B-I-N-G-O WINNERS
Stella Ogake, MD
Erin E. Peterson, APRN, CNP
Megan J. Castillo, PA-C
Gretchen R. Winter, MD
Jeanette P. Brown, MD, PhD
Yu Hong Chan, MBBS
Anita Naik, DO
Gary A. Aaronson, DO, FCCP
Allison S. Cowl, MD
Kyle Halligan, MD
Palaniappan Muthappan, MD
Faizullah S. Lokhandwala, MBBS, FCCP
Jamie R. Chua, MD
Francis L. Ervin, MD, FCCP
Robyn Luper
CHEST CHALLENGE WINNER (AND RUNNER’S-UP)
Emory University (First Place)
Mirza Haider Ali, MD
Mohleen Kang, MD
Matthew Schimmel, MD
University of Michigan (Second Place)
Patrick Bradley, MD
Matthew Hensley, MD
Bonnie Wang, MD
Cleveland Clinic (Third Place)
Jorge Mirales-Estrella, MD
Apostolos Perelas, MD
Gretchen Winter, MD
2018 DISTINGUISHED CHEST EDUCATORS
Michael H Ackerman, DNSc
Sandra G Adams, MD, MS, FCCP
Doreen J Addrizzo-Harris, MD, FCCP
Cara Lyn Agerstrand, MD, BS
Jason A Akulian, MD, FCCP
Raed H Alalawi, MD, FCCP
A. Christine Argento, MD, FCCP
Robert Arntfield, MD, FCCP
Alex A Balekian, MD
Meyer S Balter, MD, FCCP
Gisela I Banauch, MD, MS, FCCP
Robert P Baughman, MD, FCCP
David G Bell, MD, FCCP
Michel A Boivin, MD, FCCP
Gabriel T Bosslet, MD, FCCP
Jean Bourbeau, MD, MS, FCCP
Ghada R Bourjeily, MD, FCCP
David L Bowton, MD, FCCM
Jack D Buckley, MD, MPH, FCCP
Marie M Budev, DO, MPH, FCCP
Kristin M Burkart, MD, MS, FCCP
Brian Carlin, MD, FCCP
Christopher L Carroll, MD, FCCP
Roberto F Casal, MD
Kevin M Chan, MD, FCCP
Subani Chandra, MD, FCCP
Ching-Fei Chang, MD
Alexander C Chen, MD
Nancy A Collop, MD, FCCP
Clayton T Cowl, MD, MS, FCCP
Angel O Coz Yataco, MD, FCCP
Gerard J Criner, MD, FCCP
Carolyn M D’Ambrosio, MD, FCCP
Mauricio Danckers, MD, FCCP
Aneesa M Das, MD, FCCP
John Davies, RRT, MA, FCCP
Zachary S DePew, MD, FCCP
Frank C Detterbeck, MD, FCCP
Naresh A. Dewan, MBBS, FCCP
Kevin C Doerschug, MD, MS, FCCP
Meagan Dubosky, RRT-ACCS
Kevin M Dushay, MD, FCCP
Eric S Edell, MD, FCCP
Jean M Elwing, MD, FCCP
William Enfinger
Michael E Ezzie, MD, FCCP
Kevin J Felner, MD, FCCP
Mark E Fenton, MD, MSc, FCCP
Jason Filopei, MD
Neil S Freedman, MD, FCCP
Laura Kathleen Frye, MD
Thomas M Fuhrman, MD, MS, FCCP
John P Gaillard, MD, FCCP
Colin T Gillespie, MD
Yonatan Y Greenstein, MD
Maritza L Groth, MD, FCCP
Keith P Guevarra, DO, FCCP
Jesse B Hall, MD, FCCP
Nicola A Hanania, MD, MBBS, FCCP
D Kyle Hogarth, MD, FCCP
Steven M Hollenberg, MD, FCCP
David W Hsia, MD, FCCP
Candace A Huebert, MD, FCCP
Robert C Hyzy, MD, FCCP
Octavian C Ioachimescu, MD, PhD, FCCP
Richard S Irwin, MD, Master FCCP
Kirk D Jones, MD
Nader Kamangar, MD, MS, FCCP
Carl A Kaplan, MD, FCCP
Brian S Kaufman, MD, FCCP
William F Kelly, MD, FCCP
Marcus P Kennedy, MD, FCCP
Sandhya Khurana, MD, FCCP
James R Klinger, MD, FCCP
Seth J Koenig, MD, FCCP
Lindsey Kreisher, RRT
Karol Kremens, MD, FCCP
Patricia A Kritek, MD, FCCP
Sunita Kumar, MD, MBBS, FCCP
Rudy P Lackner, MD, FCCP
Viera Lakticova, MD
Carla R Lamb, MD, FCCP
Hans J Lee, MD, FCCP
Peter H Lenz, MD, MEd, FCCP
Stephanie M Levine, MD, FCCP
Deborah Jo Levine, MD, MS, FCCP
Andrea Loiselle, MD
Kenneth E Lyn-Kew, MD
Michael S Machuzak, MD, FCCP
Neil R MacIntyre, MD, FCCP
Donald A Mahler, MD, FCCP
Fabien Maldonado, MD, FCCP
Atul Malhotra, MD, FCCP
Darcy D Marciniuk, MD, FCCP
Diego J Maselli Caceres, MD, FCCP
Paul H Mayo, MD, FCCP
Peter J Mazzone, MD, MPH, FCCP
John K McIlwaine, DO, MBA, FCCP
Matthew C Miles, MD, FCCP
Scott Millington, MD
Taro Minami, MD, FCCP
Lisa K Moores, MD, FCCP
Amy E Morris, MD, FCCP
John J Mullon, MD, FCCP
Septimiu D Murgu, MD, FCCP
Mangala Narasimhan, DO, FCCP
Michael S Niederman, MD, FCCP
Alexander S Niven, MD, FCCP
Anne E O’Donnell, MD, FCCP
Erik C Osborn, MD
David E Ost, MD, MPH, FCCP
Ronald J Oudiz, MD, FCCP
Daniel R Ouellette, MD, MS, FCCP
Amit D Parulekar, MD, MS, FCCP
Nicholas J Pastis, MD, FCCP
Nina M Patel, MD, FCCP
Paru S Patrawalla, MD, FCCP
Jay I Peters, MD, FCCP
Barbara A Phillips, MD, MSPH, FCCP
Margaret A Pisani, MD, MS, FCCP
Janos Porszasz, MD, PhD
Whitney S Prince, MD, FCCP
Suhail Raoof, MBBS, Master FCCP
Ruben D Restrepo, RRT, FCCP
Marcos I Restrepo, MD, PhD, FCCP
Otis B Rickman, DO, FCCP
Roy D Ridgeway
Mary Ried, RN, CCRN
Linda Rogers, MD, FCCP
Mark J Rosen, MD, Master FCCP
Bernard J Roth, MD, FCCP
Ashutosh Sachdeva, MBBS, FCCP
Anthony G Saleh, MD, FCCP
Juan F Sanchez, MD, FCCP
Pralay K Sarkar, MBBS, FCCP
Lewis G Satterwhite, MD, BA, FCCP
Gregory A Schmidt, MD, FCCP
Mary Beth Scholand, MD, FCCP
David A Schulman, MD, MPH, FCCP
Brady Scott, RRT, MS, FCCP
Bernardo Selim, MD, FCCP
Curtis N Sessler, MD, FCCP
Rakesh D Shah, MD, FCCP
Ray Wes Shepherd, MD, FCCP
John H Sherner, MD, FCCP
Ariel L Shiloh, MD
Samira Shojaee, MD, FCCP
Marcos Silva Restrepo
Gerard A Silvestri, MD, MS, FCCP
Steven Q Simpson, MD, FCCP
James K Stoller, MD, MS, FCCP
Charlie Strange, MD, FCCP
Mary E Strek, MD, FCCP
William W Stringer, MD, FCCP
Eleanor M Summerhill, MD, FCCP
Maximiliano A Tamae Kakazu, MD, FCCP
Nichole T Tanner, MD, MS, FCCP
Lynn T Tanoue, MD, FCCP
Victor J Test, MD, FCCP
Arthur J Tokarczyk, MD, FCCP
Alain Tremblay, MD, FCCP
Adey Tsegaye, MD, FCCP
Anil Vachani, MD, FCCP
Momen M Wahidi, MD, MBA, FCCP
Keith M Wille, MD, FCCP
Lisa F Wolfe, MD
Richard G Wunderink, MD, FCCP
Lonny B Yarmus, DO, FCCP
Kazuhiro Yasufuku, MD, PhD, FCCP
Gulrukh Zaidi, MD, FCCP
David Zielinski, MD, FCCP
Everyone who attended CHEST Annual Meeting 2018 is a winner, but we would like to call out the winners participating in CHEST’s special categories of awards and events. Congratulations to all!
ANNUAL CHEST AWARDS
Master FCCP
David Gutterman, MD, Master FCCP
Distinguished Service Award
David Gutterman, MD, Master FCCP
College Medalist Award
Ghada Bourjeily, MD, FCCP
Master Clinician Educator
Lisa Moores, MD, FCCP
Early Career Clinician Educator
Amy Morris, MD, FCCP
Alfred Soffer Award for Editorial Excellence
Jean Rice
Presidential Citation
Darcy Marciniuk, MD, FCCP
Presidential Citation
D. Robert McCaffree, MD, Master FCCP
HONOR LECTURES AND MEMORIAL AWARDS
Edward C. Rosenow III, MD, Master FCCP/Master Teacher Honor Lecture Accelerated Aging in COPD and Its Comorbidities: Novel Therapeutic Targets
Peter Barnes, MD, Master FCCP
The lecture is generously funded by the CHEST Foundation.
Distinguished Scientist Honor Lecture in Cardiopulmonary Physiology
Understanding Diaphragm Performance: The Role of Ultrasound
F. Dennis McCool, MD, FCCP
The lecture is generously funded by the CHEST Foundation.
Presidential Honor Lecture
Asthma: Past, Present, and Future
Jay Peters, MD, FCCP
Thomas L. Petty, MD, Master FCCP Memorial Lecture
Recent Developments in Pulmonary Rehabilitation and Long-Term Oxygen Therapy: Would Tom Petty be Pleased?
Richard Casaburi, MD, PhD, FCCP
The lecture is generously funded by the CHEST Foundation.
Margaret Pfrommer Memorial Lecture in Long-term Mechanical Ventilation
Saving Lives…One Ventilator at a Time - HMV in 2018 and Beyond
Douglas McKim, MD, FCCP
The Margaret Pfrommer Memorial Lecture in Long-term Mechanical Ventilation is generously supported by International Ventilator Users Network of Post-Polio Health International and the CHEST Foundation.
Pasquale Ciaglia Memorial Lecture in Interventional Medicine
Evolution of Endobronchial Ultrasound: From Diagnostics to Therapeutics
Kazuhiro Yasufuku, MD, PhD, FCCP
The lecture is generously funded by the CHEST Foundation.
Roger C. Bone Memorial Lecture in Critical Care
Methylprednisolone in ARDS: A Highly Effective Treatment. How it Works, How to Use it
G. Umberto Meduri, MD
The lecture is generously funded by the CHEST Foundation.
CHEST FOUNDATION GRANT WINNERS
Distinguished Scholar
Robert C. Hyzy, MD, FCCP
Eli Lilly and Company Distinguished Scholar in Critical Care MedicineGrant Title: The Use of Electrical Impedance Tomography to Assess Mechanical Ventilation in Acute Respiratory Distress Syndrome
This grant is made possible due to the philanthropic support from Eli Lilly and Company.
Community Service Grantees
Deborah Haisch, MD
Columbia University Medical Center – New York, NY
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: East African Training Initiative in Pulmonary and Critical Care Medicine
Pamela Garrett, CCRN, MN
Gwinnett Medical Center – Lawrenceville, GA
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: Breathe Better Gwinnett
Phillip Sheridan
Mobile Care Chicago – Chicago, IL
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Grant Title: Home Environment Education for Children with Asthma
These grants are supported in full by the CHEST Foundation.
Research Grant Winners
Ayodeji Adegunsoye, MD, MS
Research Grant in Pulmonary Fibrosis
Grant Title: Impact of Telomere Length on Pulmonary Fibrosis Clusters Across Diverse Racial Cohorts
Justin Oldham, MD, MS
Research Grant in Pulmonary Fibrosis
Grant Title: Plasma Biomarkers to Predict Outcomes and Treatment Response in Patients with Pulmonary Fibrosis
These grants above are supported by Boehringer Ingelheim Pharmaceuticals, Inc and Genentech.
Jacob Brenner, MD, PhD
Research Grant in Chronic Obstructive Pulmonary Disease
Grant Title: Ambulatory Cuirass Ventilation for Relief of Exertional Dyspnea in Severe COPD Patients
William Zhang, MD
Research Grant in Chronic Obstructive Pulmonary Disease
Grant Title: Pulmonary Iron Overload as a Novel COPD Endotype
These grants above are supported by AstraZeneca LP and Sunovion Pharmaceuticals Inc.
Margaret Bublitz, PhD
CHEST Foundation Research Grant in Women’s Lung Health
Grant Title: Sex as a Predictor of Sleep-Disordered Breathing and Its Consequences in Pregnancy
This grant is supported in full by the CHEST Foundation.
Tim Morris, MD, FCCP
CHEST Foundation Research Grant in Venous Thromboembolism
Grant Title: Long-term Follow-up of Acute Pulmonary Embolism
This grant is supported in full by the CHEST Foundation.
Monica Mukherjee, MD, MPH
CHEST Foundation Research Grant in Pulmonary Arterial Hypertension
Grant Title: Exercise Provocation in the Noninvasive Detection of Occult Right Ventricular Dysfunction and Emerging Pulmonary Hypertension in Systemic Sclerosis
This grant is supported in full by the CHEST Foundation.
Don Sanders, MD, MS
CHEST Foundation Research Grant in Cystic Fibrosis
Grant Title: Whole-genome Shotgun Sequencing of Oropharyngeal Swabs in Infants With CF
This grant is supported by Vertex Pharmaceuticals.
Imran Sulaiman, MD, PhD
CHEST Foundation Research Grant in Nontuberculosis Mycobacteria Diseases
Grant Title: Lower Airway Microbiota Signatures Associated W ith Impaired Immune Response in Non-Tuberculous Mycobacterium
This grant is supported by Insmed.
Samira Shojaee, MD, MPH, FCCP
CHEST Foundation Research Grant in Lung Cancer
Grant Title: Extracellular Vesicle miRNA as a Biomarker in Malignant Pleural Effusion
This grant is supported in full by the CHEST Foundation.
Anna Volerman, MD
CHEST Foundation Research Grant in Severe Asthma
Grant Title: A Randomized Clinical Trial Evaluating the Effectiveness of Virtual Teach-to-Goal(TM) Education versus Brief Intervention for Children with Severe Asthma
This grant is supported by AstraZeneca LP.
ABSTRACT AND CASE REPORT WINNERS
Alfred Soffer Research Award Winners
Clauden Louis, MD: Left ventricular assist devices in Intermacs 1 acute cardiogenic shock patients
Babith J. Mankidy, MBBS, FCCP: Reduction in in-hospital cardiac arrest with early interventions in the emergency department and non-ICU units by a novel approach of rapid response teams and mobile ICU management
Young Investigator Award Winners
Fayez Kheir, MD, MSc: Intrapleural tissue plasminogen activator and deoxyribonuclease therapy vs early medical thoracoscopy for treatment of pleural infection: a randomized clinical trial
Michael Rosman, MD: The utility of end tidal CO2 (ETCO2) monitoring during in-hospital cardiac arrest to predict return of spontaneous circulation
Top 5 Abstract Poster Winners
Neha Agarwal, MD: The 3 wishes project: a feasible intervention to improve end of life care in the ICU at UCLA
Hiroaki Harada, MD: Usefulness of comprehensive preoperative pulmonary rehabilitation program including intensive nutritional support concomitant with physical exercise through an interdisciplinary team approach
Joseph M. Carrington, DO, MHA: Targeting the trans-IL-6 signaling pathway to reduce agriculture organic dust exposure-induced airway inflammation in mice
Yu Kuang Lai, MBBCh: The utility of parametric response mapping in pulmonary graft vs host disease following hematopoietic stem cell transplant
Top Abstract Poster Finalists
Ligia M. Puiu, MD, PhD, FCCP: Association between echocardiographic and lipid parameters to workers in the metalliferous mines
Kush R. Dholakia, MD: Colloids vs crystalloids for postoperative resuscitation in patients undergoing off-pump coronary artery bypass surgery
Kulothungan Gunasekaran, MD, MBBS: Risk of VTE in idiopathic pulmonary fibrosis: a systematic review
Laura B. Sutton, PharmD: Ease and correct use of Ellipta by age in patients with asthma and COPD
Ankur Mogla, MD: To assess the utilization of pulmonary function testing for perioperative respiratory complications in bariatric surgery patients
Ali Ammar: Tracheostomy and admission diagnosis as predictors for an extended length of stay (ELOS)
Charlene Kalani, PharmD: Efficacy and safety of direct oral anticoagulants (DOACS) in morbidly obese patients
Jonghoo Lee, MD: Performances of modified CRB-65 score compared to SIRS and QSOFA as a rapid screening tool for sepsis among infected patients in initial emergency department: a propensity score matching study
Frank J. Trudo, MD, FCCP: Clinical burden of eosinophilic COPD
Elise L. Stephenson, MD: Vitamin C and point of care glucose measurements: a retrospective, observational study
Faisal Siddiqi, MD: Implementation of an early mobility program in the medical ICU
Eileen Harder, MD: Connective tissue disease-associated pulmonary arterial hypertension hospitalizations from 2001-2014
Sophie Korzan, MD: Exhaled nitric oxide and asthma-COPD overlap in patients hospitalized with exacerbations of airway disease: preliminary observations
Andreas Grove, MD: MicroRNA (MIRNA) and biological markers discriminate between normotensive and prehypertensive young men in hypobaric hypoxic environments
Snigdha Nutalapati, MBBS: Large cell neuroendocrine cancer of the lung: SEER 2004-2014 analysis
Anubhav Jain, MBBS: Survival benefit of beta-blockers in patients hospitalized for acute exacerbation of COPD
Case Report Slide Winners
Ze Ying Tan: All that wheezes is not asthma
Jason Lam: Pulmonary mucor mycetoma
Adam Young: Nonresolving pneumonia and cyclic fevers in an immunocompetent patient
Ritu Modi: Histopathological misdiagnosis of pulmonary coccidiodes
Argun Can: A rare inborn error of fatty acid oxidation presenting with severe hyperammonemia in the ICU
Morgan Gilani: A colorful cause of cardiovascular collapse
Katie Jeans: A sweet surprise
Anthony Mattox: Unusual case of interstitial lung disease
Andrew Berglund: Pulmonary light chain deposition disease in a 29-year-old army soldier
Cristia Maysol Morales: A case report of a primary malignant melanoma of anterior mediastinum
Anthony McClafferty: Fibrosing mediastinitis and rheumatoid arthritis: an autoimmune inflammatory connection
Ahmed Munir: HIV with disseminated tularemia: a rare presentation Benjamin Garren: Mycobacterium avium complex mediastinal lymphadenitis in an immunocompetent adolescent with erosion into the airway
Robert Hilton: Obtunded with a chest mass: a case of a rare neurologic paraneoplastic syndrome,
Audra Schwalk: Mucoepidermoid carcinoma: a rare malignancy treated endobronchially
Jessica Riggs: Successful transplantation defies genetics: a case of rapidly-progressive pulmonary fibrosis due to Hermansky-Pudlak syndrome
Meghan Cirulis: Acute vasodilator testing: an opportunity to refine study design and provide precision care in pulmonary hypertension
Patrick Chan: VATS lobectomy for bronchial atresia in an adult
Andrew Mehlman: Multivessel coronary artery aneurysms presenting as myocardial ischemia
Scott Maughan: Diagnosing milliary Mycobacterium bovis from the prostate of an immunocompetent host
Adam Austin: Survived ECMO, death by BLASTO: the first reported fatal case of disseminated blastomycosis in pregnancy
Tie: Donnie Carter: Subclinical polycythemia vera presenting as extensive thrombosis due to massive transfusion, and
Lindsay Hammons: Rare case of Serratia pneumonia causing transient aplastic anemia
Paola Baskin: Novel observations during point-of-care ultrasound (POCUS) in cardiopulmonary resuscitation: a case of ultrasound-guided probe pressure to reduce esophageal insufflation during bag-valve-mask ventilator
David Dennis: Pulmonary alveolar proteinosis presenting as intracerebral nocardiosis
Rakin Choudhury: Severe asthma caused by therapy-resistant asthmatic granulomatosis
Andrew Lytle: Lung adenocarcinoma in a patient with Turcot syndrome
Chelsea Leipold: Case of a granulomatous-lymphocytic interstitial lung disease in a patient with common variable immunodeficiency disorder
Galyna Ivashchuk: Double trouble: ANCA vasculitis with concomitant IGA nephropathy presenting as massive diffuse alveolar hemorrhage and fulminant renal failure
Case Report Poster Winners
Christine Zhou: Role of transbronchial lung cryobiopsy in the diagnosis of adenocarcinoma in situ
Parin Shah: A rare case of Erdheim-Chester disease masquerading as metastatic lung cancer
Avanthika Wynn : A rare asthma mimic
Muhammad S. Ali: Severe pancolitis: a rare adverse effect of nintedanib
Brian Foster: Don’t forget to breathe: a case of hypoxemia after carotid body resection
Kelly Pennington: Intra-cardiac embolization of an inferior vena cava filter resulting in cardiac arrest
George Elkomos-Botros: Acute generalized exanthematous pustulosis presenting as distributive shock with multi-organ failure
Ashley M. Scott: Avian occupational hypersensitivity pneumonitis in a restaurant employee
Andrew Polito: Pulmonary amyloidosis: an unusual presentation of a rare disease
CHEST B-I-N-G-O WINNERS
Stella Ogake, MD
Erin E. Peterson, APRN, CNP
Megan J. Castillo, PA-C
Gretchen R. Winter, MD
Jeanette P. Brown, MD, PhD
Yu Hong Chan, MBBS
Anita Naik, DO
Gary A. Aaronson, DO, FCCP
Allison S. Cowl, MD
Kyle Halligan, MD
Palaniappan Muthappan, MD
Faizullah S. Lokhandwala, MBBS, FCCP
Jamie R. Chua, MD
Francis L. Ervin, MD, FCCP
Robyn Luper
CHEST CHALLENGE WINNER (AND RUNNER’S-UP)
Emory University (First Place)
Mirza Haider Ali, MD
Mohleen Kang, MD
Matthew Schimmel, MD
University of Michigan (Second Place)
Patrick Bradley, MD
Matthew Hensley, MD
Bonnie Wang, MD
Cleveland Clinic (Third Place)
Jorge Mirales-Estrella, MD
Apostolos Perelas, MD
Gretchen Winter, MD
2018 DISTINGUISHED CHEST EDUCATORS
Michael H Ackerman, DNSc
Sandra G Adams, MD, MS, FCCP
Doreen J Addrizzo-Harris, MD, FCCP
Cara Lyn Agerstrand, MD, BS
Jason A Akulian, MD, FCCP
Raed H Alalawi, MD, FCCP
A. Christine Argento, MD, FCCP
Robert Arntfield, MD, FCCP
Alex A Balekian, MD
Meyer S Balter, MD, FCCP
Gisela I Banauch, MD, MS, FCCP
Robert P Baughman, MD, FCCP
David G Bell, MD, FCCP
Michel A Boivin, MD, FCCP
Gabriel T Bosslet, MD, FCCP
Jean Bourbeau, MD, MS, FCCP
Ghada R Bourjeily, MD, FCCP
David L Bowton, MD, FCCM
Jack D Buckley, MD, MPH, FCCP
Marie M Budev, DO, MPH, FCCP
Kristin M Burkart, MD, MS, FCCP
Brian Carlin, MD, FCCP
Christopher L Carroll, MD, FCCP
Roberto F Casal, MD
Kevin M Chan, MD, FCCP
Subani Chandra, MD, FCCP
Ching-Fei Chang, MD
Alexander C Chen, MD
Nancy A Collop, MD, FCCP
Clayton T Cowl, MD, MS, FCCP
Angel O Coz Yataco, MD, FCCP
Gerard J Criner, MD, FCCP
Carolyn M D’Ambrosio, MD, FCCP
Mauricio Danckers, MD, FCCP
Aneesa M Das, MD, FCCP
John Davies, RRT, MA, FCCP
Zachary S DePew, MD, FCCP
Frank C Detterbeck, MD, FCCP
Naresh A. Dewan, MBBS, FCCP
Kevin C Doerschug, MD, MS, FCCP
Meagan Dubosky, RRT-ACCS
Kevin M Dushay, MD, FCCP
Eric S Edell, MD, FCCP
Jean M Elwing, MD, FCCP
William Enfinger
Michael E Ezzie, MD, FCCP
Kevin J Felner, MD, FCCP
Mark E Fenton, MD, MSc, FCCP
Jason Filopei, MD
Neil S Freedman, MD, FCCP
Laura Kathleen Frye, MD
Thomas M Fuhrman, MD, MS, FCCP
John P Gaillard, MD, FCCP
Colin T Gillespie, MD
Yonatan Y Greenstein, MD
Maritza L Groth, MD, FCCP
Keith P Guevarra, DO, FCCP
Jesse B Hall, MD, FCCP
Nicola A Hanania, MD, MBBS, FCCP
D Kyle Hogarth, MD, FCCP
Steven M Hollenberg, MD, FCCP
David W Hsia, MD, FCCP
Candace A Huebert, MD, FCCP
Robert C Hyzy, MD, FCCP
Octavian C Ioachimescu, MD, PhD, FCCP
Richard S Irwin, MD, Master FCCP
Kirk D Jones, MD
Nader Kamangar, MD, MS, FCCP
Carl A Kaplan, MD, FCCP
Brian S Kaufman, MD, FCCP
William F Kelly, MD, FCCP
Marcus P Kennedy, MD, FCCP
Sandhya Khurana, MD, FCCP
James R Klinger, MD, FCCP
Seth J Koenig, MD, FCCP
Lindsey Kreisher, RRT
Karol Kremens, MD, FCCP
Patricia A Kritek, MD, FCCP
Sunita Kumar, MD, MBBS, FCCP
Rudy P Lackner, MD, FCCP
Viera Lakticova, MD
Carla R Lamb, MD, FCCP
Hans J Lee, MD, FCCP
Peter H Lenz, MD, MEd, FCCP
Stephanie M Levine, MD, FCCP
Deborah Jo Levine, MD, MS, FCCP
Andrea Loiselle, MD
Kenneth E Lyn-Kew, MD
Michael S Machuzak, MD, FCCP
Neil R MacIntyre, MD, FCCP
Donald A Mahler, MD, FCCP
Fabien Maldonado, MD, FCCP
Atul Malhotra, MD, FCCP
Darcy D Marciniuk, MD, FCCP
Diego J Maselli Caceres, MD, FCCP
Paul H Mayo, MD, FCCP
Peter J Mazzone, MD, MPH, FCCP
John K McIlwaine, DO, MBA, FCCP
Matthew C Miles, MD, FCCP
Scott Millington, MD
Taro Minami, MD, FCCP
Lisa K Moores, MD, FCCP
Amy E Morris, MD, FCCP
John J Mullon, MD, FCCP
Septimiu D Murgu, MD, FCCP
Mangala Narasimhan, DO, FCCP
Michael S Niederman, MD, FCCP
Alexander S Niven, MD, FCCP
Anne E O’Donnell, MD, FCCP
Erik C Osborn, MD
David E Ost, MD, MPH, FCCP
Ronald J Oudiz, MD, FCCP
Daniel R Ouellette, MD, MS, FCCP
Amit D Parulekar, MD, MS, FCCP
Nicholas J Pastis, MD, FCCP
Nina M Patel, MD, FCCP
Paru S Patrawalla, MD, FCCP
Jay I Peters, MD, FCCP
Barbara A Phillips, MD, MSPH, FCCP
Margaret A Pisani, MD, MS, FCCP
Janos Porszasz, MD, PhD
Whitney S Prince, MD, FCCP
Suhail Raoof, MBBS, Master FCCP
Ruben D Restrepo, RRT, FCCP
Marcos I Restrepo, MD, PhD, FCCP
Otis B Rickman, DO, FCCP
Roy D Ridgeway
Mary Ried, RN, CCRN
Linda Rogers, MD, FCCP
Mark J Rosen, MD, Master FCCP
Bernard J Roth, MD, FCCP
Ashutosh Sachdeva, MBBS, FCCP
Anthony G Saleh, MD, FCCP
Juan F Sanchez, MD, FCCP
Pralay K Sarkar, MBBS, FCCP
Lewis G Satterwhite, MD, BA, FCCP
Gregory A Schmidt, MD, FCCP
Mary Beth Scholand, MD, FCCP
David A Schulman, MD, MPH, FCCP
Brady Scott, RRT, MS, FCCP
Bernardo Selim, MD, FCCP
Curtis N Sessler, MD, FCCP
Rakesh D Shah, MD, FCCP
Ray Wes Shepherd, MD, FCCP
John H Sherner, MD, FCCP
Ariel L Shiloh, MD
Samira Shojaee, MD, FCCP
Marcos Silva Restrepo
Gerard A Silvestri, MD, MS, FCCP
Steven Q Simpson, MD, FCCP
James K Stoller, MD, MS, FCCP
Charlie Strange, MD, FCCP
Mary E Strek, MD, FCCP
William W Stringer, MD, FCCP
Eleanor M Summerhill, MD, FCCP
Maximiliano A Tamae Kakazu, MD, FCCP
Nichole T Tanner, MD, MS, FCCP
Lynn T Tanoue, MD, FCCP
Victor J Test, MD, FCCP
Arthur J Tokarczyk, MD, FCCP
Alain Tremblay, MD, FCCP
Adey Tsegaye, MD, FCCP
Anil Vachani, MD, FCCP
Momen M Wahidi, MD, MBA, FCCP
Keith M Wille, MD, FCCP
Lisa F Wolfe, MD
Richard G Wunderink, MD, FCCP
Lonny B Yarmus, DO, FCCP
Kazuhiro Yasufuku, MD, PhD, FCCP
Gulrukh Zaidi, MD, FCCP
David Zielinski, MD, FCCP
