User login
Association of Weekend Admission and Weekend Discharge with Length of Stay and 30-Day Readmission in Children’s Hospitals
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
© 2018 Society of Hospital Medicine
Identifying Observation Stays in Medicare Data: Policy Implications of a Definition
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
© 2019 Society of Hospital Medicine
SPRINT MIND with extension planned
Also today, medical ethics and economics, PTSD after traumatic brain injury may be predicted by race and ethnicity, and aspirin for primary cardiovascular prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, medical ethics and economics, PTSD after traumatic brain injury may be predicted by race and ethnicity, and aspirin for primary cardiovascular prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, medical ethics and economics, PTSD after traumatic brain injury may be predicted by race and ethnicity, and aspirin for primary cardiovascular prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
February 2019 Prostate Cancer
Click here to access February 2019 Prostate Cancer
Table of Contents
- Prostate Cancer Surveillance After Radiation Therapy in a National Delivery System
- Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing
- Primary Urethral Carcinoma With Nodal Metastasis
- Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate
- Research News: Prostate Cancer
- Management of Patients With Treatment-Resistant Metastatic Prostate Cancer

Click here to access February 2019 Prostate Cancer
Table of Contents
- Prostate Cancer Surveillance After Radiation Therapy in a National Delivery System
- Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing
- Primary Urethral Carcinoma With Nodal Metastasis
- Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate
- Research News: Prostate Cancer
- Management of Patients With Treatment-Resistant Metastatic Prostate Cancer

Click here to access February 2019 Prostate Cancer
Table of Contents
- Prostate Cancer Surveillance After Radiation Therapy in a National Delivery System
- Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing
- Primary Urethral Carcinoma With Nodal Metastasis
- Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate
- Research News: Prostate Cancer
- Management of Patients With Treatment-Resistant Metastatic Prostate Cancer

2019 Directory of VA and DoD Facilities
AGA Clinical Practice Update: Functional gastrointestinal symptoms in patients with inflammatory bowel disease
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30
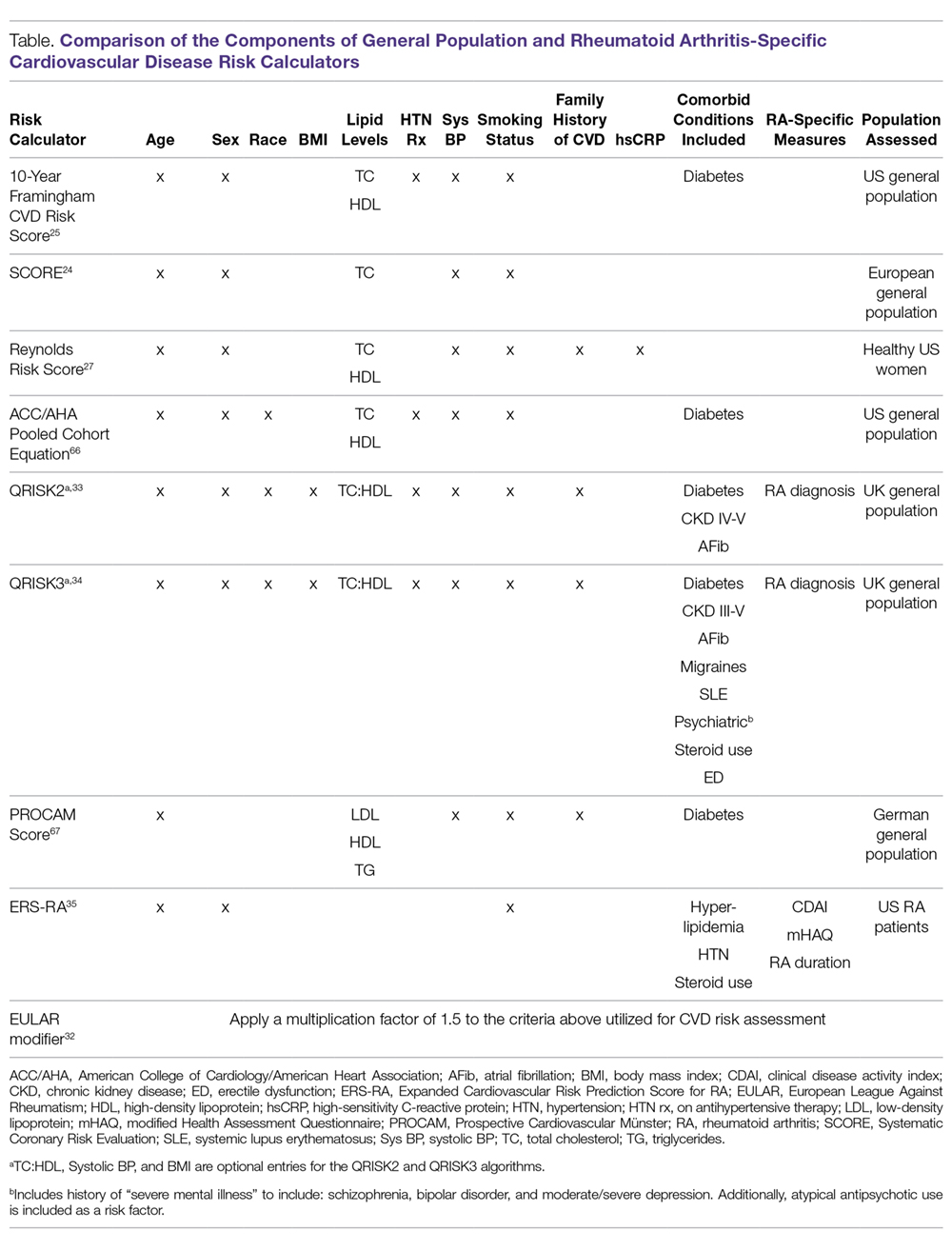
Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30

Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30

Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
Gastric Electric Stimulation for Refractory Gastroparesis
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.
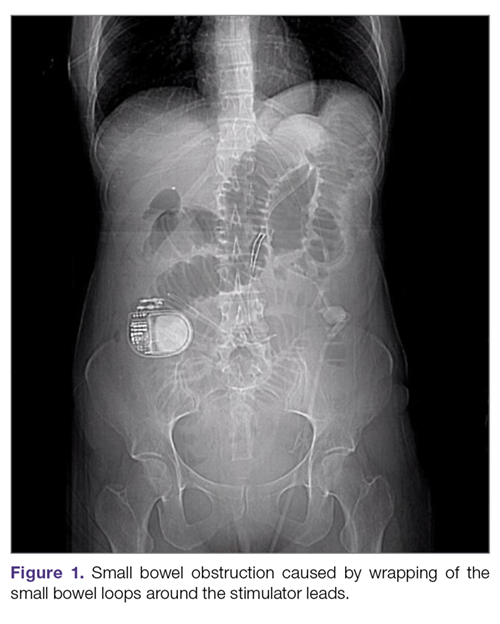
Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.
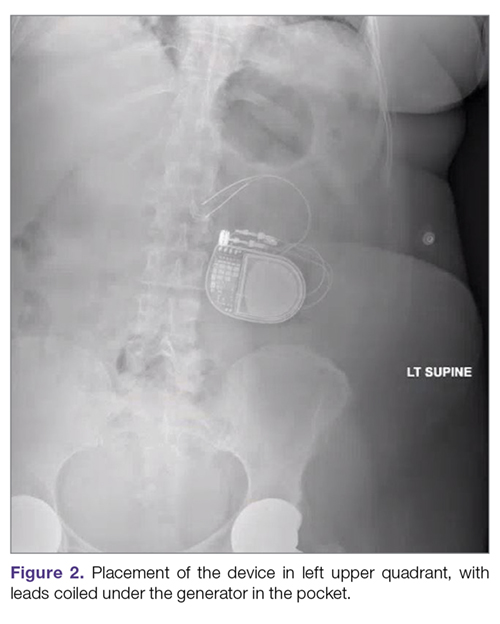
Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.
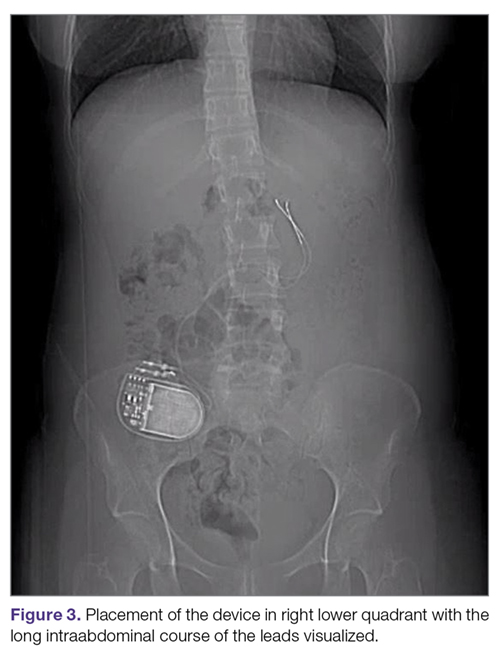
Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.
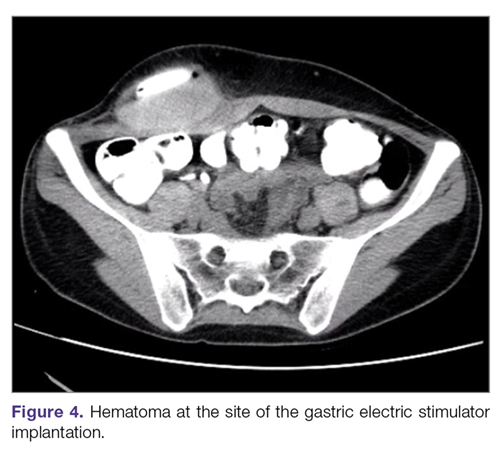
Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29
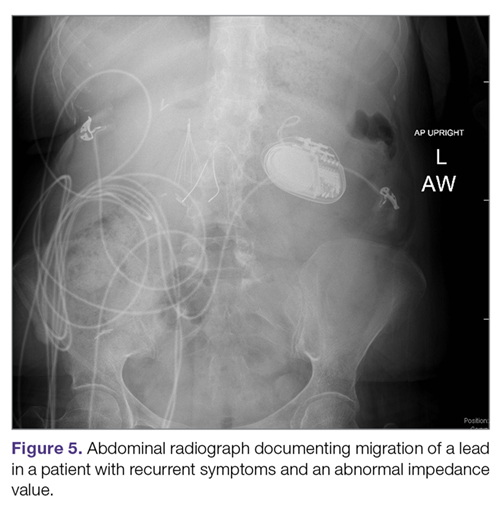
Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38
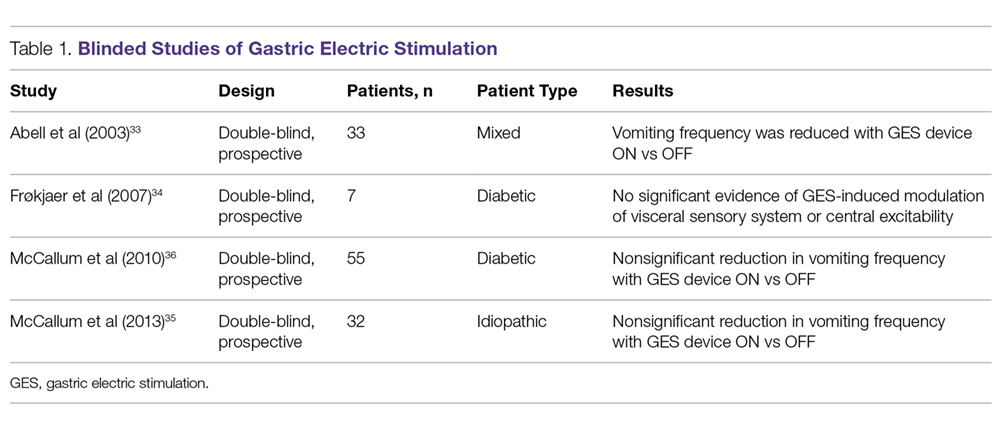
In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.
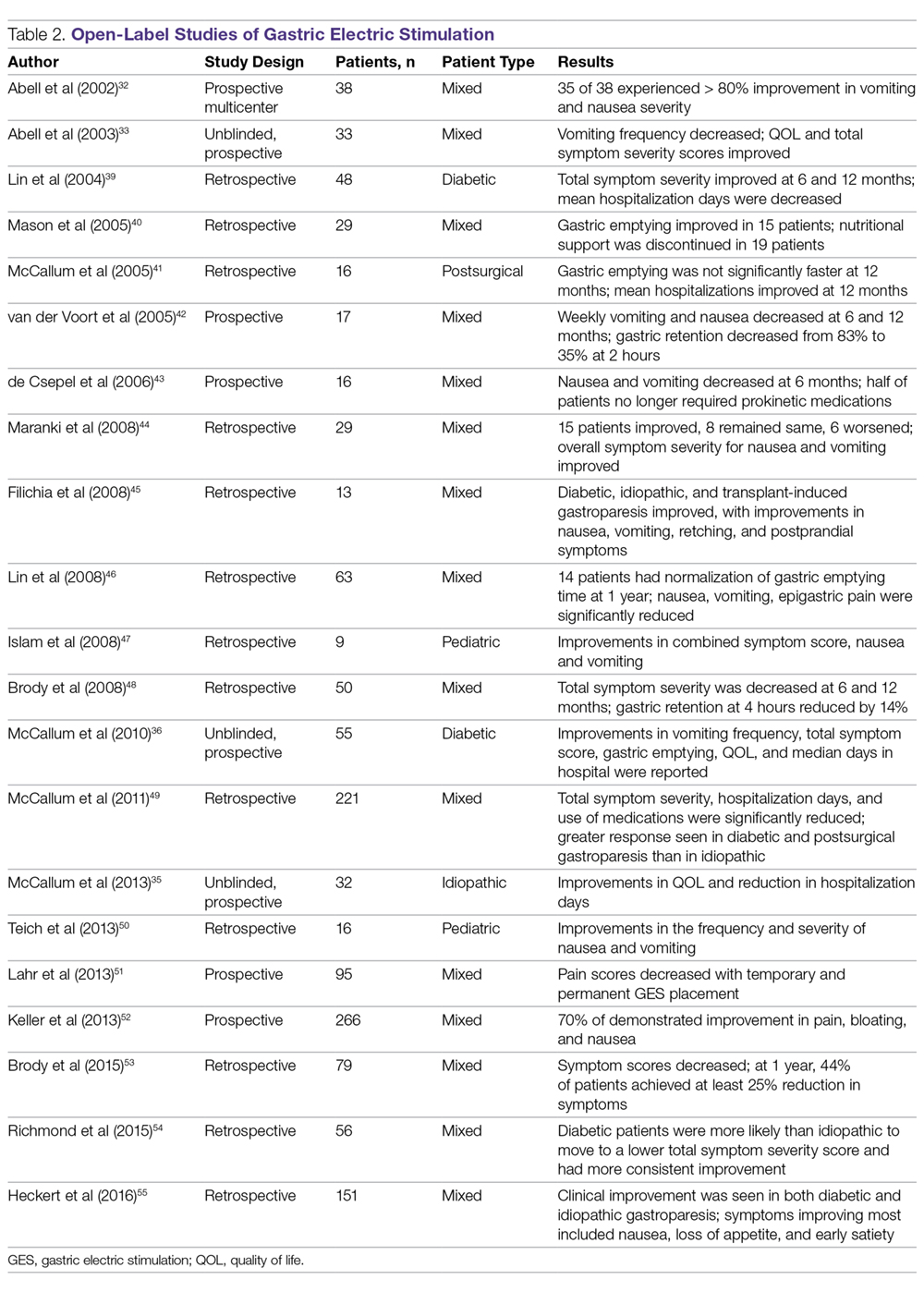
In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.

Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.

Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.

Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.

Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29

Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38

In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.

In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.

Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.

Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.

Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.

Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29

Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38

In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.

In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
Reducing Rates of Perioperative Deep Vein Thrombosis and Pulmonary Emboli in Hip and Knee Arthroplasty Patients: A Quality Improvement Project
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.

From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.
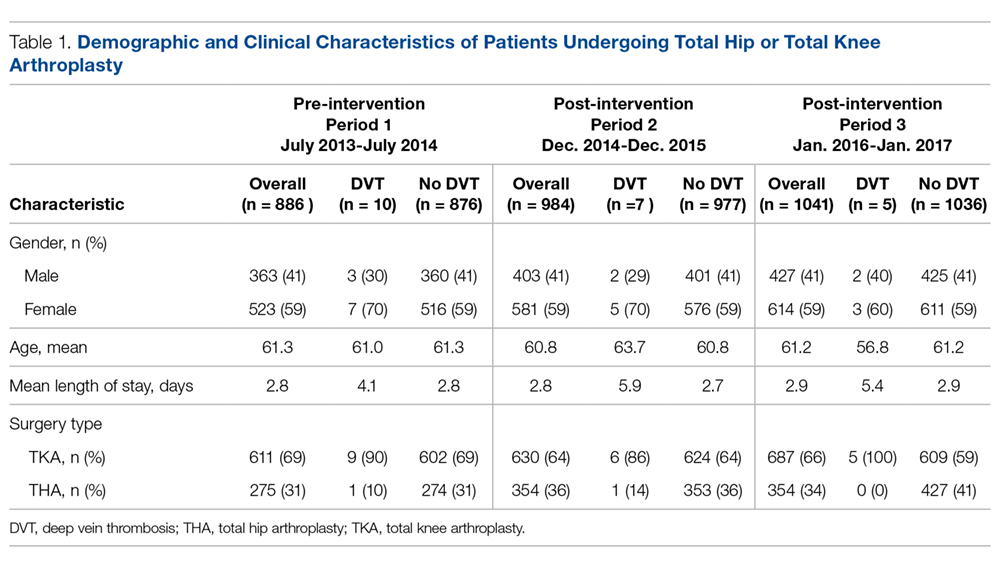
Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.
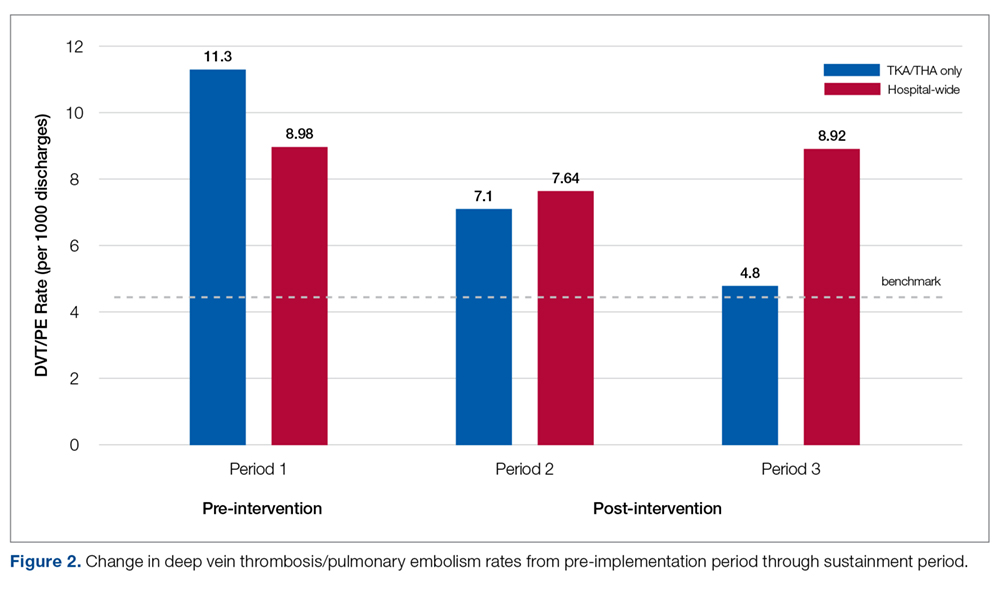
Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.
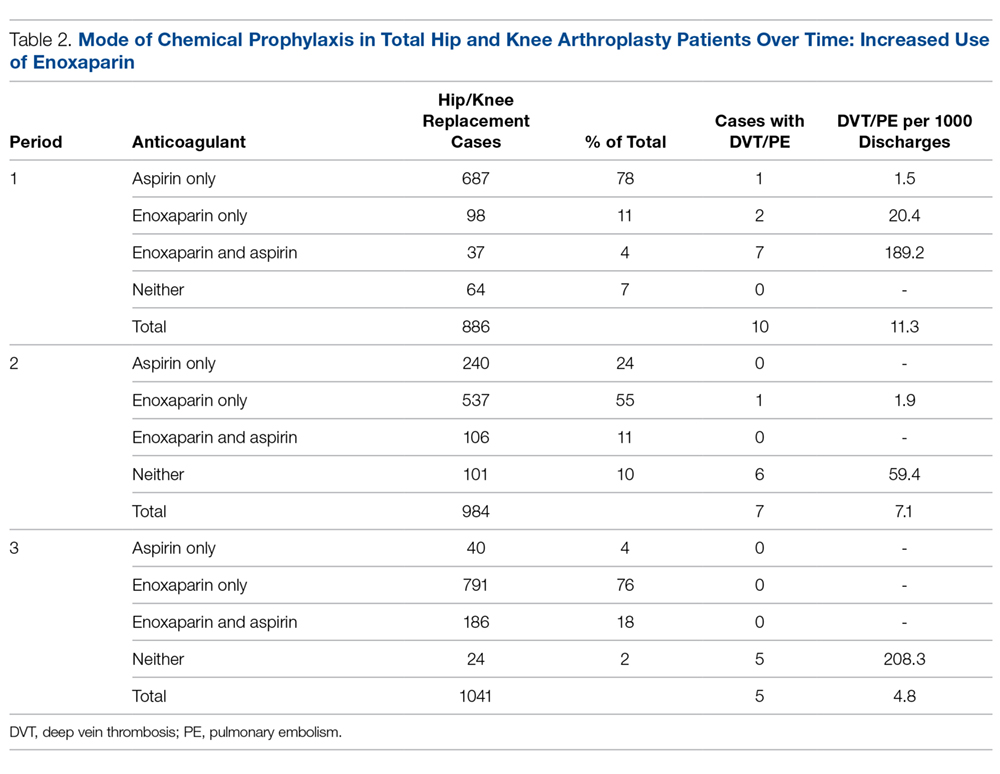
Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.

From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.

Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.

Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.

Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.

From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.

Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.

Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.

Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
Procalcitonin, Will It Guide Us?
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.