User login
Diagnosing endometriosis: Is laparoscopy the gold standard?
This CME supplement to OBG Management provides readers with an understanding of the following topics:
- The relationship between chronic pelvic pain and endometriosis
- The limitations of laparocopy in the diagnosis of endometriosis
- The emerging role of imaging in the diagnosis of endometriosis
Click Here to read the full supplement and access a posttest and evaluation for CME credit.
This CME supplement to OBG Management provides readers with an understanding of the following topics:
- The relationship between chronic pelvic pain and endometriosis
- The limitations of laparocopy in the diagnosis of endometriosis
- The emerging role of imaging in the diagnosis of endometriosis
Click Here to read the full supplement and access a posttest and evaluation for CME credit.
This CME supplement to OBG Management provides readers with an understanding of the following topics:
- The relationship between chronic pelvic pain and endometriosis
- The limitations of laparocopy in the diagnosis of endometriosis
- The emerging role of imaging in the diagnosis of endometriosis
Click Here to read the full supplement and access a posttest and evaluation for CME credit.
Anxiety can impact patient satisfaction after GERD surgery
BALTIMORE – according to a study from the Ohio State University presented at the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons.
Carla Holcomb, MD, a minimally invasive surgery/bariatric fellow at the Ohio State’s Wexner Medical Center, Columbus, reported the upshot of the study findings. “Preoperative counseling is especially important regarding postoperative expectations in patients with anxiety,” she said.
The retrospective study evaluated 271 patients who had laparoscopic Nissen fundoplication (LNF) during 2011-2016 at the medical center, comparing outcomes in patients who were on serotonin-modulating medication for depression (n = 103), benzodiazepines for anxiety (n = 44), or neither (n = 124). The researchers evaluated a number of metrics – DeMeester score of esophageal acid exposure, pre- and postoperative health-related quality of life, and postoperative antacid use and need for endoscopic dilation – across all cohorts. While some scores among the anxiety cohort trended higher (DeMeester score of 43 vs. 38 for the no-anxiety patients) they were not statistically significant, Dr. Holcomb noted. Patients taking antidepressants reported similar subjective outcomes and satisfaction rates to those not taking antidepressants.
However, when patients were queried about their overall satisfaction after laparoscopic Nissen fundoplication 77%-87% in the no-depression, depression, and no-anxiety groups reported they were satisfied, while only 37% of those in the anxiety group did so. That is based on a response rate of 53% to a telephone inquiry 15 months after LNF.
“The patients who had anxiety looked vastly different from the rest of the population,” said Dr. Holcomb. “Patients taking antidepressants reported similar objective outcomes and high satisfaction rates, [compared with] patients not taking antidepressants after LNF, and although LNF does improve gastroesophageal reflux disease symptoms in patients taking anxiolytics, they rarely achieve satisfaction in long-term follow-up.”
Among the study limitations Dr. Holcomb acknowledged were the 53% long-term response rate and not knowing if an anatomical reason may explain the higher health-related quality of life scores in the anxiety group – 7 vs. 4 in the no-anxiety group – at long-term follow-up, although the overall score was low at 5.
Dr. Holcomb had no relevant financial disclosures.
SOURCE: Holcomb CN et al. SAGES 2019, Session SS04.
BALTIMORE – according to a study from the Ohio State University presented at the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons.
Carla Holcomb, MD, a minimally invasive surgery/bariatric fellow at the Ohio State’s Wexner Medical Center, Columbus, reported the upshot of the study findings. “Preoperative counseling is especially important regarding postoperative expectations in patients with anxiety,” she said.
The retrospective study evaluated 271 patients who had laparoscopic Nissen fundoplication (LNF) during 2011-2016 at the medical center, comparing outcomes in patients who were on serotonin-modulating medication for depression (n = 103), benzodiazepines for anxiety (n = 44), or neither (n = 124). The researchers evaluated a number of metrics – DeMeester score of esophageal acid exposure, pre- and postoperative health-related quality of life, and postoperative antacid use and need for endoscopic dilation – across all cohorts. While some scores among the anxiety cohort trended higher (DeMeester score of 43 vs. 38 for the no-anxiety patients) they were not statistically significant, Dr. Holcomb noted. Patients taking antidepressants reported similar subjective outcomes and satisfaction rates to those not taking antidepressants.
However, when patients were queried about their overall satisfaction after laparoscopic Nissen fundoplication 77%-87% in the no-depression, depression, and no-anxiety groups reported they were satisfied, while only 37% of those in the anxiety group did so. That is based on a response rate of 53% to a telephone inquiry 15 months after LNF.
“The patients who had anxiety looked vastly different from the rest of the population,” said Dr. Holcomb. “Patients taking antidepressants reported similar objective outcomes and high satisfaction rates, [compared with] patients not taking antidepressants after LNF, and although LNF does improve gastroesophageal reflux disease symptoms in patients taking anxiolytics, they rarely achieve satisfaction in long-term follow-up.”
Among the study limitations Dr. Holcomb acknowledged were the 53% long-term response rate and not knowing if an anatomical reason may explain the higher health-related quality of life scores in the anxiety group – 7 vs. 4 in the no-anxiety group – at long-term follow-up, although the overall score was low at 5.
Dr. Holcomb had no relevant financial disclosures.
SOURCE: Holcomb CN et al. SAGES 2019, Session SS04.
BALTIMORE – according to a study from the Ohio State University presented at the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons.
Carla Holcomb, MD, a minimally invasive surgery/bariatric fellow at the Ohio State’s Wexner Medical Center, Columbus, reported the upshot of the study findings. “Preoperative counseling is especially important regarding postoperative expectations in patients with anxiety,” she said.
The retrospective study evaluated 271 patients who had laparoscopic Nissen fundoplication (LNF) during 2011-2016 at the medical center, comparing outcomes in patients who were on serotonin-modulating medication for depression (n = 103), benzodiazepines for anxiety (n = 44), or neither (n = 124). The researchers evaluated a number of metrics – DeMeester score of esophageal acid exposure, pre- and postoperative health-related quality of life, and postoperative antacid use and need for endoscopic dilation – across all cohorts. While some scores among the anxiety cohort trended higher (DeMeester score of 43 vs. 38 for the no-anxiety patients) they were not statistically significant, Dr. Holcomb noted. Patients taking antidepressants reported similar subjective outcomes and satisfaction rates to those not taking antidepressants.
However, when patients were queried about their overall satisfaction after laparoscopic Nissen fundoplication 77%-87% in the no-depression, depression, and no-anxiety groups reported they were satisfied, while only 37% of those in the anxiety group did so. That is based on a response rate of 53% to a telephone inquiry 15 months after LNF.
“The patients who had anxiety looked vastly different from the rest of the population,” said Dr. Holcomb. “Patients taking antidepressants reported similar objective outcomes and high satisfaction rates, [compared with] patients not taking antidepressants after LNF, and although LNF does improve gastroesophageal reflux disease symptoms in patients taking anxiolytics, they rarely achieve satisfaction in long-term follow-up.”
Among the study limitations Dr. Holcomb acknowledged were the 53% long-term response rate and not knowing if an anatomical reason may explain the higher health-related quality of life scores in the anxiety group – 7 vs. 4 in the no-anxiety group – at long-term follow-up, although the overall score was low at 5.
Dr. Holcomb had no relevant financial disclosures.
SOURCE: Holcomb CN et al. SAGES 2019, Session SS04.
REPORTING FROM SAGES 2019
Key clinical point: Patients on anxiolytics for anxiety would benefit from counseling before laparoscopic Nissen fundoplication.
Major finding: Fewer than 40% of patients with anxiety reported satisfaction after LNF despite vast improvement in reflux symptoms.
Study details: Retrospective cohort, single-center study with a prospectively maintained database of 271 patients who had laparoscopic Nissen fundoplication during 2011-2016.
Disclosures: Dr. Holcomb had no financial relationships to disclose
Source: Holcomb CN et al. SAGES 2019, Session SS04.
Gout Drug May Help in Metabolic Syndrome
Colchicine inhibits the formation of the Nod-like Receptor Family Pyrin Domain Containing 3 (NLRP3) inflammasome, a key component in the obesity-associated inflammatory cascade. In a retrospective study, long-term colchicine treatment had glycemic benefit in patients with gout. Other research has suggested that suppressing NLRP3 could improve peripheral insulin resistance as well as β-cell insulin production. However, no randomized controlled trial had yet investigated colchicine’s long-term effects on glucose metabolism in adults with obesity and metabolic syndrome (MetS).
The NIH researchers enrolled 40 adults to receive either colchicine or placebo; 37 completed the 3-month study. Adherence was high in both groups.
Colchicine significantly reduced multiple markers of obesity-associated inflammation, including high sensitivity C-reactive protein and erythrocyte sedimentation rate. The colchicine group also had moderate but statistically significant reductions in white blood cell count, monocytes, neutrophils, and platelets, without significant effects on lymphocyte count.
Although colchicine’s effects on the primary outcome of insulin sensitivity were not significant, some of the secondary outcomes related to glucose homeostasis—eg, insulin resistance and fasting insulin—suggest colchicine treatment may improve hepatic insulin sensitivity. Moreover, the researchers say, a trend toward improvement in disposition index suggests that the drug might potentially delay the onset of diabetes in people at risk.
While some small, short-term studies had suggested that colchicine might worsen metabolic variables by inhibiting insulin secretion, other recent retrospective studies found long-term colchicine use did not negatively affect insulin secretion or glycemic control. In this study, similarly, the researchers say, chronic colchicine use did not impair first-phase insulin response or insulin sensitivity, and other markers of metabolic health, such as hemoglobin A1c and cholesterol, were not significantly changed. However, the researchers acknowledge that their study may have been too small to confirm those differences, and say larger studies are warranted.
Colchicine inhibits the formation of the Nod-like Receptor Family Pyrin Domain Containing 3 (NLRP3) inflammasome, a key component in the obesity-associated inflammatory cascade. In a retrospective study, long-term colchicine treatment had glycemic benefit in patients with gout. Other research has suggested that suppressing NLRP3 could improve peripheral insulin resistance as well as β-cell insulin production. However, no randomized controlled trial had yet investigated colchicine’s long-term effects on glucose metabolism in adults with obesity and metabolic syndrome (MetS).
The NIH researchers enrolled 40 adults to receive either colchicine or placebo; 37 completed the 3-month study. Adherence was high in both groups.
Colchicine significantly reduced multiple markers of obesity-associated inflammation, including high sensitivity C-reactive protein and erythrocyte sedimentation rate. The colchicine group also had moderate but statistically significant reductions in white blood cell count, monocytes, neutrophils, and platelets, without significant effects on lymphocyte count.
Although colchicine’s effects on the primary outcome of insulin sensitivity were not significant, some of the secondary outcomes related to glucose homeostasis—eg, insulin resistance and fasting insulin—suggest colchicine treatment may improve hepatic insulin sensitivity. Moreover, the researchers say, a trend toward improvement in disposition index suggests that the drug might potentially delay the onset of diabetes in people at risk.
While some small, short-term studies had suggested that colchicine might worsen metabolic variables by inhibiting insulin secretion, other recent retrospective studies found long-term colchicine use did not negatively affect insulin secretion or glycemic control. In this study, similarly, the researchers say, chronic colchicine use did not impair first-phase insulin response or insulin sensitivity, and other markers of metabolic health, such as hemoglobin A1c and cholesterol, were not significantly changed. However, the researchers acknowledge that their study may have been too small to confirm those differences, and say larger studies are warranted.
Colchicine inhibits the formation of the Nod-like Receptor Family Pyrin Domain Containing 3 (NLRP3) inflammasome, a key component in the obesity-associated inflammatory cascade. In a retrospective study, long-term colchicine treatment had glycemic benefit in patients with gout. Other research has suggested that suppressing NLRP3 could improve peripheral insulin resistance as well as β-cell insulin production. However, no randomized controlled trial had yet investigated colchicine’s long-term effects on glucose metabolism in adults with obesity and metabolic syndrome (MetS).
The NIH researchers enrolled 40 adults to receive either colchicine or placebo; 37 completed the 3-month study. Adherence was high in both groups.
Colchicine significantly reduced multiple markers of obesity-associated inflammation, including high sensitivity C-reactive protein and erythrocyte sedimentation rate. The colchicine group also had moderate but statistically significant reductions in white blood cell count, monocytes, neutrophils, and platelets, without significant effects on lymphocyte count.
Although colchicine’s effects on the primary outcome of insulin sensitivity were not significant, some of the secondary outcomes related to glucose homeostasis—eg, insulin resistance and fasting insulin—suggest colchicine treatment may improve hepatic insulin sensitivity. Moreover, the researchers say, a trend toward improvement in disposition index suggests that the drug might potentially delay the onset of diabetes in people at risk.
While some small, short-term studies had suggested that colchicine might worsen metabolic variables by inhibiting insulin secretion, other recent retrospective studies found long-term colchicine use did not negatively affect insulin secretion or glycemic control. In this study, similarly, the researchers say, chronic colchicine use did not impair first-phase insulin response or insulin sensitivity, and other markers of metabolic health, such as hemoglobin A1c and cholesterol, were not significantly changed. However, the researchers acknowledge that their study may have been too small to confirm those differences, and say larger studies are warranted.
2018 at a glance: Recently approved therapies in oncology
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.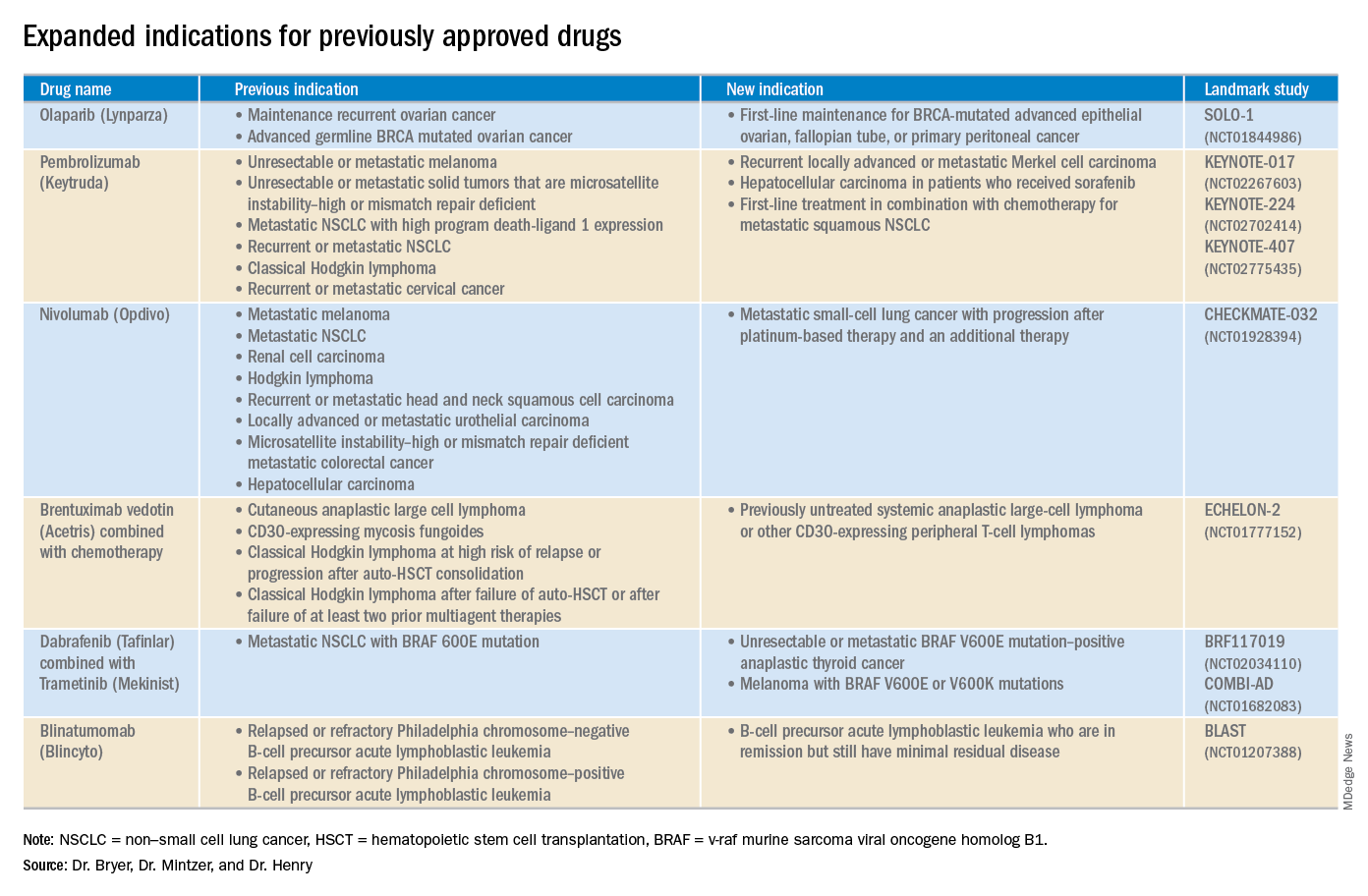
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.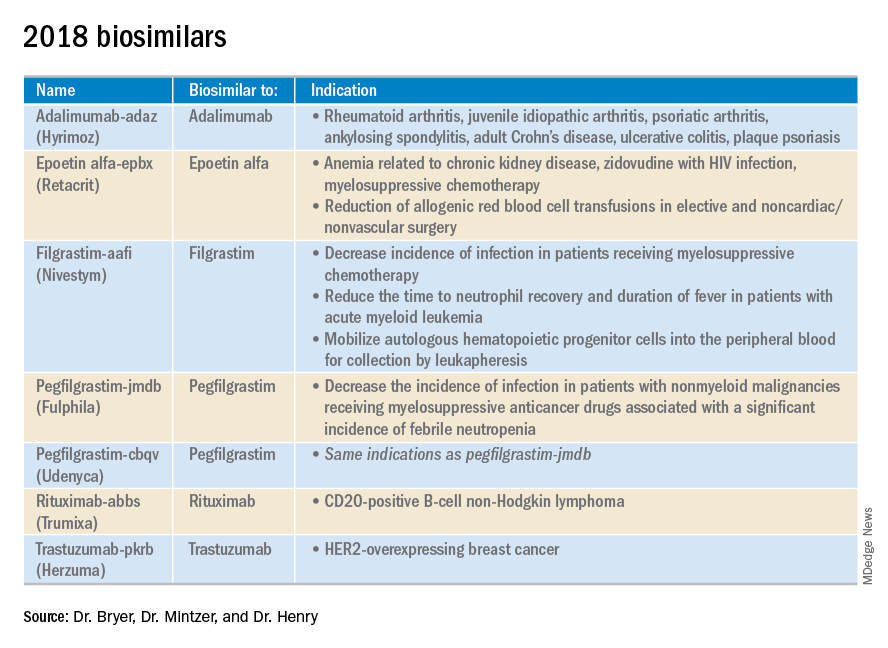
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.
Busiest week yet brings 2019 measles total to 555 cases
according to the Centers for Disease Control and Prevention.
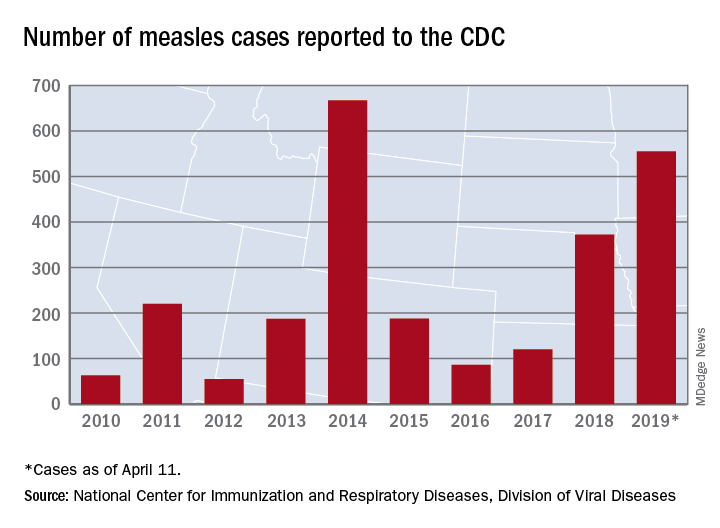
The 90 measles cases reported during the week ending April 11 mark the third consecutive weekly high for 2019, topping the 78 recorded during the week of April 4 and the 73 reported during the week of March 28. Meanwhile, this year’s total trails only the 667 cases reported in 2014 for the highest in the postelimination era, the CDC said April 15.
New York reported 26 new cases in Brooklyn’s Williamsburg neighborhood last week, which puts the borough at 227 for the year, with another two occurring in the Flushing section of Queens. A public health emergency declared on April 9 covers several zip codes in Williamsburg and requires unvaccinated individuals who may have been exposed to measles to receive “the measles-mumps-rubella vaccine in order to protect others in the community and help curtail the ongoing outbreak,” the city’s health department said in a written statement.
Maryland became the 20th state to report a measles case this year, and the state’s department of health said it was notifying those in the vicinity of a medical office building in Pikesville about possible exposure on April 2.
The recent outbreak in Michigan’s Oakland County did not result in any new patients over the last week and remains at 38 cases, with the state reporting one additional case in Wayne County. More recent reports of a case in Washtenaw County and another in Oakland County were reversed after additional testing, the state health department reported.
according to the Centers for Disease Control and Prevention.

The 90 measles cases reported during the week ending April 11 mark the third consecutive weekly high for 2019, topping the 78 recorded during the week of April 4 and the 73 reported during the week of March 28. Meanwhile, this year’s total trails only the 667 cases reported in 2014 for the highest in the postelimination era, the CDC said April 15.
New York reported 26 new cases in Brooklyn’s Williamsburg neighborhood last week, which puts the borough at 227 for the year, with another two occurring in the Flushing section of Queens. A public health emergency declared on April 9 covers several zip codes in Williamsburg and requires unvaccinated individuals who may have been exposed to measles to receive “the measles-mumps-rubella vaccine in order to protect others in the community and help curtail the ongoing outbreak,” the city’s health department said in a written statement.
Maryland became the 20th state to report a measles case this year, and the state’s department of health said it was notifying those in the vicinity of a medical office building in Pikesville about possible exposure on April 2.
The recent outbreak in Michigan’s Oakland County did not result in any new patients over the last week and remains at 38 cases, with the state reporting one additional case in Wayne County. More recent reports of a case in Washtenaw County and another in Oakland County were reversed after additional testing, the state health department reported.
according to the Centers for Disease Control and Prevention.

The 90 measles cases reported during the week ending April 11 mark the third consecutive weekly high for 2019, topping the 78 recorded during the week of April 4 and the 73 reported during the week of March 28. Meanwhile, this year’s total trails only the 667 cases reported in 2014 for the highest in the postelimination era, the CDC said April 15.
New York reported 26 new cases in Brooklyn’s Williamsburg neighborhood last week, which puts the borough at 227 for the year, with another two occurring in the Flushing section of Queens. A public health emergency declared on April 9 covers several zip codes in Williamsburg and requires unvaccinated individuals who may have been exposed to measles to receive “the measles-mumps-rubella vaccine in order to protect others in the community and help curtail the ongoing outbreak,” the city’s health department said in a written statement.
Maryland became the 20th state to report a measles case this year, and the state’s department of health said it was notifying those in the vicinity of a medical office building in Pikesville about possible exposure on April 2.
The recent outbreak in Michigan’s Oakland County did not result in any new patients over the last week and remains at 38 cases, with the state reporting one additional case in Wayne County. More recent reports of a case in Washtenaw County and another in Oakland County were reversed after additional testing, the state health department reported.
The ARRIVE trial: Women’s desideratum versus logistical concerns
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
Afatinib shows safety and efficacy among elderly
GENEVA – Afatinib appears safe and effective for elderly patients with EGFR-positive non–small cell lung cancer (NSCLC), according to investigators.
A retrospective analysis of the phase 3 GIDEON trial showed similar objective responses, disease control rates, and progression-free survival rates among elderly patients, compared with younger patients, reported lead author Wolfgang M. Brückl, MD, of Universitätsklinik der Paracelsus Medizinischen Privatuniversität in Nürnberg, Germany, and his colleagues. These findings were presented in a poster at the European Lung Cancer Congress.
“Elderly patients are often underrepresented in clinical trials,” the investigators wrote, “which can lead to uncertainties regarding optimal treatment of such patients in routine clinical practice. The GIDEON noninterventional study enrolled a high proportion of patients aged 70 years or older, providing an opportunity to study the real-world use of afatinib in older individuals.”
The GIDEON study involved 160 patients with EGFR-positive NSCLC who were treated at 49 centers in Germany between 2014 and 2016. From this total, 151 patients were available for interim analysis, and 67 patients (44%) were at least 70-years old. Among this elderly group, about one out of five patients (22%) had brain metastases and Eastern Cooperative Oncology Group (ECOG) performance status scores were typically 1 (45%) or 0 (42%).
Compared with younger patients, elderly patients were more likely to receive a lower starting dose of afatinib (62% vs. 83%), which usually entailed a decrease from 40 mg to 30 mg. Thereafter, dose reduction rates were similar between age groups, with 58% of younger patients requiring lower doses and 55% of elderly patients requiring reduced doses. Adverse events were comparable across age groups, although a fraction more of the patients 70 years or older discontinued treatment because of serious adverse drug reactions (12% vs. 7%).
Efficacy results were also comparable between age groups. Overall response rates were slightly higher among elderly patients than younger patients, with a 70-year age threshold (78% vs. 70%); disease control rate was marginally higher among the elderly (93% vs. 89%); and elderly patients had a slightly better 12-month PFS rate (62.2% vs. 49.1%).
“Data from the GIDEON noninterventional study provide important information on the routine clinical use of afatinib in elderly patients,” the investigators concluded.
The study was funded by Boehringer Ingelheim. The investigators did not report conflicts of interest.
SOURCE: Brückl WM et al. ELCC 2019. Abstract 125P.
GENEVA – Afatinib appears safe and effective for elderly patients with EGFR-positive non–small cell lung cancer (NSCLC), according to investigators.
A retrospective analysis of the phase 3 GIDEON trial showed similar objective responses, disease control rates, and progression-free survival rates among elderly patients, compared with younger patients, reported lead author Wolfgang M. Brückl, MD, of Universitätsklinik der Paracelsus Medizinischen Privatuniversität in Nürnberg, Germany, and his colleagues. These findings were presented in a poster at the European Lung Cancer Congress.
“Elderly patients are often underrepresented in clinical trials,” the investigators wrote, “which can lead to uncertainties regarding optimal treatment of such patients in routine clinical practice. The GIDEON noninterventional study enrolled a high proportion of patients aged 70 years or older, providing an opportunity to study the real-world use of afatinib in older individuals.”
The GIDEON study involved 160 patients with EGFR-positive NSCLC who were treated at 49 centers in Germany between 2014 and 2016. From this total, 151 patients were available for interim analysis, and 67 patients (44%) were at least 70-years old. Among this elderly group, about one out of five patients (22%) had brain metastases and Eastern Cooperative Oncology Group (ECOG) performance status scores were typically 1 (45%) or 0 (42%).
Compared with younger patients, elderly patients were more likely to receive a lower starting dose of afatinib (62% vs. 83%), which usually entailed a decrease from 40 mg to 30 mg. Thereafter, dose reduction rates were similar between age groups, with 58% of younger patients requiring lower doses and 55% of elderly patients requiring reduced doses. Adverse events were comparable across age groups, although a fraction more of the patients 70 years or older discontinued treatment because of serious adverse drug reactions (12% vs. 7%).
Efficacy results were also comparable between age groups. Overall response rates were slightly higher among elderly patients than younger patients, with a 70-year age threshold (78% vs. 70%); disease control rate was marginally higher among the elderly (93% vs. 89%); and elderly patients had a slightly better 12-month PFS rate (62.2% vs. 49.1%).
“Data from the GIDEON noninterventional study provide important information on the routine clinical use of afatinib in elderly patients,” the investigators concluded.
The study was funded by Boehringer Ingelheim. The investigators did not report conflicts of interest.
SOURCE: Brückl WM et al. ELCC 2019. Abstract 125P.
GENEVA – Afatinib appears safe and effective for elderly patients with EGFR-positive non–small cell lung cancer (NSCLC), according to investigators.
A retrospective analysis of the phase 3 GIDEON trial showed similar objective responses, disease control rates, and progression-free survival rates among elderly patients, compared with younger patients, reported lead author Wolfgang M. Brückl, MD, of Universitätsklinik der Paracelsus Medizinischen Privatuniversität in Nürnberg, Germany, and his colleagues. These findings were presented in a poster at the European Lung Cancer Congress.
“Elderly patients are often underrepresented in clinical trials,” the investigators wrote, “which can lead to uncertainties regarding optimal treatment of such patients in routine clinical practice. The GIDEON noninterventional study enrolled a high proportion of patients aged 70 years or older, providing an opportunity to study the real-world use of afatinib in older individuals.”
The GIDEON study involved 160 patients with EGFR-positive NSCLC who were treated at 49 centers in Germany between 2014 and 2016. From this total, 151 patients were available for interim analysis, and 67 patients (44%) were at least 70-years old. Among this elderly group, about one out of five patients (22%) had brain metastases and Eastern Cooperative Oncology Group (ECOG) performance status scores were typically 1 (45%) or 0 (42%).
Compared with younger patients, elderly patients were more likely to receive a lower starting dose of afatinib (62% vs. 83%), which usually entailed a decrease from 40 mg to 30 mg. Thereafter, dose reduction rates were similar between age groups, with 58% of younger patients requiring lower doses and 55% of elderly patients requiring reduced doses. Adverse events were comparable across age groups, although a fraction more of the patients 70 years or older discontinued treatment because of serious adverse drug reactions (12% vs. 7%).
Efficacy results were also comparable between age groups. Overall response rates were slightly higher among elderly patients than younger patients, with a 70-year age threshold (78% vs. 70%); disease control rate was marginally higher among the elderly (93% vs. 89%); and elderly patients had a slightly better 12-month PFS rate (62.2% vs. 49.1%).
“Data from the GIDEON noninterventional study provide important information on the routine clinical use of afatinib in elderly patients,” the investigators concluded.
The study was funded by Boehringer Ingelheim. The investigators did not report conflicts of interest.
SOURCE: Brückl WM et al. ELCC 2019. Abstract 125P.
REPORTING FROM ELCC 2019
Symptomatic former NFL players may have tau deposition consistent with CTE
according to research published online ahead of print April 10 in the New England Journal of Medicine. The distribution of tau in the players’ brains appears to be similar to that in persons with chronic traumatic encephalopathy (CTE).
CTE is a neurodegenerative disease that has been associated with a history of repetitive head impacts, such as those withstood in contact sports. The basis for the neuropathological diagnosis of CTE is a distinct pattern of tau deposition with minimal deposition of amyloid-beta. Paired helical filament tau aggregates are first observed in the frontal, temporal, and parietal cortices. They later spread throughout the cerebral cortex, medial temporal lobe, diencephalon, and brainstem. CTE is diagnosed only through post mortem neuropathological examinations.
To examine whether tau and amyloid deposition can be detected in the brains of living people at risk for CTE, Robert A. Stern, PhD, and his colleagues studied living former NFL players and asymptomatic controls with flortaucipir PET (to detect tau) and 18F-florbetapir PET (to detect amyloid-beta). Dr. Stern is director of clinical research at the CTE Center at Boston University. Eligible former players were male, aged 40-69 years, had played football in the NFL for at least 2 years, had had at least 12 years of total tackle football experience, and reported cognitive, behavioral, and mood symptoms through telephone screening. Eligible controls were male, aged 40-69 years, and had no cognitive symptoms or history of traumatic brain injury.
All subjects underwent flortaucipir PET, florbetapir PET, and T1-weighted volumetric MRI of the head. Dr. Stern and his colleagues used automated image-analysis algorithms to compare the regional tau standardized uptake value ratio (SUVR) between the two patient groups and to evaluate potential associations between that ratio and symptom severity or years of football play.
The investigators included 26 former players and 31 controls in their analysis. The group of former players had a higher percentage of black participants and a lower mean Mini-Mental State Examination score, compared with controls. The mean flortaucipir SUVR was higher among former players than among controls in the bilateral superior frontal (1.09 vs. 0.98), bilateral medial temporal (1.23 vs. 1.12), and left parietal (1.12 vs. 1.01) regions. Dr. Stern and his colleagues found no association between tau deposition in those regions and results on cognitive and neuropsychiatric tests. In a post hoc analysis, they calculated the correlation coefficients in the three brain regions between the SUVRs and years of play to be 0.58 in the bilateral superior frontal region, 0.45 in the bilateral medial temporal region, and 0.50 in the left parietal region. Mean cortical:cerebellar florbetapir SUVRs did not differ significantly between groups.
“These findings suggest that the cognitive difficulties reported by the former players were not related to Alzheimer’s disease amyloid-beta deposition,” said the authors. The study may have been insufficiently powered to detect associations between flortaucipir uptake and the clinical measures, they added. Also, paired helical filament tau pathology alone may not be associated with the former players’ neuropsychiatric symptoms and cognitive impairment. “Although this study showed between-group differences in flortaucipir PET measurements, our analyses do not pertain to detection of tau pathology in individual participants,” the authors concluded.
The study was supported by an investigator-initiated grant from Avid Radiopharmaceuticals. The National Institutes of Health, the state of Arizona, and the U.S. Department of Defense also supported the study.
SOURCE: Stern RA et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1900757 (Epub ahead of print).
The study by Stern et al offers valuable information, but the relationships between various features of chronic traumatic encephalopathy (CTE) still are not well understood, said Allan H. Ropper, MD, executive vice chair of neurology at Harvard Medical School in Boston, in an accompanying editorial (N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1903746). The risk of CTE associated with a long period of playing football does not correspond with the number, severity, or serial occurrence of concussions, he observed. In addition, “individual factors such as the player’s size, head-to-neck configuration, style of play, and position, as well as biologic attributes, may influence the deposition of tau.” Because of the absence of an association between neuropsychological test results and tau deposition, neurologists can draw few conclusions based on the presence of neuropsychological abnormalities in athletes who are at risk for CTE, said Dr. Ropper.
“As with Alzheimer’s disease, the CTE field is in a phase of fumbling with circumstantial evidence for a connection between tau deposition and a clinical syndrome. ... The report in this issue certainly does strengthen the case that tau is the offender early in CTE, but other links remain to be clarified,” he concluded.
Dr. Ropper reported no relevant conflicts of interest. He is deputy editor of the New England Journal of Medicine.
The study by Stern et al offers valuable information, but the relationships between various features of chronic traumatic encephalopathy (CTE) still are not well understood, said Allan H. Ropper, MD, executive vice chair of neurology at Harvard Medical School in Boston, in an accompanying editorial (N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1903746). The risk of CTE associated with a long period of playing football does not correspond with the number, severity, or serial occurrence of concussions, he observed. In addition, “individual factors such as the player’s size, head-to-neck configuration, style of play, and position, as well as biologic attributes, may influence the deposition of tau.” Because of the absence of an association between neuropsychological test results and tau deposition, neurologists can draw few conclusions based on the presence of neuropsychological abnormalities in athletes who are at risk for CTE, said Dr. Ropper.
“As with Alzheimer’s disease, the CTE field is in a phase of fumbling with circumstantial evidence for a connection between tau deposition and a clinical syndrome. ... The report in this issue certainly does strengthen the case that tau is the offender early in CTE, but other links remain to be clarified,” he concluded.
Dr. Ropper reported no relevant conflicts of interest. He is deputy editor of the New England Journal of Medicine.
The study by Stern et al offers valuable information, but the relationships between various features of chronic traumatic encephalopathy (CTE) still are not well understood, said Allan H. Ropper, MD, executive vice chair of neurology at Harvard Medical School in Boston, in an accompanying editorial (N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1903746). The risk of CTE associated with a long period of playing football does not correspond with the number, severity, or serial occurrence of concussions, he observed. In addition, “individual factors such as the player’s size, head-to-neck configuration, style of play, and position, as well as biologic attributes, may influence the deposition of tau.” Because of the absence of an association between neuropsychological test results and tau deposition, neurologists can draw few conclusions based on the presence of neuropsychological abnormalities in athletes who are at risk for CTE, said Dr. Ropper.
“As with Alzheimer’s disease, the CTE field is in a phase of fumbling with circumstantial evidence for a connection between tau deposition and a clinical syndrome. ... The report in this issue certainly does strengthen the case that tau is the offender early in CTE, but other links remain to be clarified,” he concluded.
Dr. Ropper reported no relevant conflicts of interest. He is deputy editor of the New England Journal of Medicine.
according to research published online ahead of print April 10 in the New England Journal of Medicine. The distribution of tau in the players’ brains appears to be similar to that in persons with chronic traumatic encephalopathy (CTE).
CTE is a neurodegenerative disease that has been associated with a history of repetitive head impacts, such as those withstood in contact sports. The basis for the neuropathological diagnosis of CTE is a distinct pattern of tau deposition with minimal deposition of amyloid-beta. Paired helical filament tau aggregates are first observed in the frontal, temporal, and parietal cortices. They later spread throughout the cerebral cortex, medial temporal lobe, diencephalon, and brainstem. CTE is diagnosed only through post mortem neuropathological examinations.
To examine whether tau and amyloid deposition can be detected in the brains of living people at risk for CTE, Robert A. Stern, PhD, and his colleagues studied living former NFL players and asymptomatic controls with flortaucipir PET (to detect tau) and 18F-florbetapir PET (to detect amyloid-beta). Dr. Stern is director of clinical research at the CTE Center at Boston University. Eligible former players were male, aged 40-69 years, had played football in the NFL for at least 2 years, had had at least 12 years of total tackle football experience, and reported cognitive, behavioral, and mood symptoms through telephone screening. Eligible controls were male, aged 40-69 years, and had no cognitive symptoms or history of traumatic brain injury.
All subjects underwent flortaucipir PET, florbetapir PET, and T1-weighted volumetric MRI of the head. Dr. Stern and his colleagues used automated image-analysis algorithms to compare the regional tau standardized uptake value ratio (SUVR) between the two patient groups and to evaluate potential associations between that ratio and symptom severity or years of football play.
The investigators included 26 former players and 31 controls in their analysis. The group of former players had a higher percentage of black participants and a lower mean Mini-Mental State Examination score, compared with controls. The mean flortaucipir SUVR was higher among former players than among controls in the bilateral superior frontal (1.09 vs. 0.98), bilateral medial temporal (1.23 vs. 1.12), and left parietal (1.12 vs. 1.01) regions. Dr. Stern and his colleagues found no association between tau deposition in those regions and results on cognitive and neuropsychiatric tests. In a post hoc analysis, they calculated the correlation coefficients in the three brain regions between the SUVRs and years of play to be 0.58 in the bilateral superior frontal region, 0.45 in the bilateral medial temporal region, and 0.50 in the left parietal region. Mean cortical:cerebellar florbetapir SUVRs did not differ significantly between groups.
“These findings suggest that the cognitive difficulties reported by the former players were not related to Alzheimer’s disease amyloid-beta deposition,” said the authors. The study may have been insufficiently powered to detect associations between flortaucipir uptake and the clinical measures, they added. Also, paired helical filament tau pathology alone may not be associated with the former players’ neuropsychiatric symptoms and cognitive impairment. “Although this study showed between-group differences in flortaucipir PET measurements, our analyses do not pertain to detection of tau pathology in individual participants,” the authors concluded.
The study was supported by an investigator-initiated grant from Avid Radiopharmaceuticals. The National Institutes of Health, the state of Arizona, and the U.S. Department of Defense also supported the study.
SOURCE: Stern RA et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1900757 (Epub ahead of print).
according to research published online ahead of print April 10 in the New England Journal of Medicine. The distribution of tau in the players’ brains appears to be similar to that in persons with chronic traumatic encephalopathy (CTE).
CTE is a neurodegenerative disease that has been associated with a history of repetitive head impacts, such as those withstood in contact sports. The basis for the neuropathological diagnosis of CTE is a distinct pattern of tau deposition with minimal deposition of amyloid-beta. Paired helical filament tau aggregates are first observed in the frontal, temporal, and parietal cortices. They later spread throughout the cerebral cortex, medial temporal lobe, diencephalon, and brainstem. CTE is diagnosed only through post mortem neuropathological examinations.
To examine whether tau and amyloid deposition can be detected in the brains of living people at risk for CTE, Robert A. Stern, PhD, and his colleagues studied living former NFL players and asymptomatic controls with flortaucipir PET (to detect tau) and 18F-florbetapir PET (to detect amyloid-beta). Dr. Stern is director of clinical research at the CTE Center at Boston University. Eligible former players were male, aged 40-69 years, had played football in the NFL for at least 2 years, had had at least 12 years of total tackle football experience, and reported cognitive, behavioral, and mood symptoms through telephone screening. Eligible controls were male, aged 40-69 years, and had no cognitive symptoms or history of traumatic brain injury.
All subjects underwent flortaucipir PET, florbetapir PET, and T1-weighted volumetric MRI of the head. Dr. Stern and his colleagues used automated image-analysis algorithms to compare the regional tau standardized uptake value ratio (SUVR) between the two patient groups and to evaluate potential associations between that ratio and symptom severity or years of football play.
The investigators included 26 former players and 31 controls in their analysis. The group of former players had a higher percentage of black participants and a lower mean Mini-Mental State Examination score, compared with controls. The mean flortaucipir SUVR was higher among former players than among controls in the bilateral superior frontal (1.09 vs. 0.98), bilateral medial temporal (1.23 vs. 1.12), and left parietal (1.12 vs. 1.01) regions. Dr. Stern and his colleagues found no association between tau deposition in those regions and results on cognitive and neuropsychiatric tests. In a post hoc analysis, they calculated the correlation coefficients in the three brain regions between the SUVRs and years of play to be 0.58 in the bilateral superior frontal region, 0.45 in the bilateral medial temporal region, and 0.50 in the left parietal region. Mean cortical:cerebellar florbetapir SUVRs did not differ significantly between groups.
“These findings suggest that the cognitive difficulties reported by the former players were not related to Alzheimer’s disease amyloid-beta deposition,” said the authors. The study may have been insufficiently powered to detect associations between flortaucipir uptake and the clinical measures, they added. Also, paired helical filament tau pathology alone may not be associated with the former players’ neuropsychiatric symptoms and cognitive impairment. “Although this study showed between-group differences in flortaucipir PET measurements, our analyses do not pertain to detection of tau pathology in individual participants,” the authors concluded.
The study was supported by an investigator-initiated grant from Avid Radiopharmaceuticals. The National Institutes of Health, the state of Arizona, and the U.S. Department of Defense also supported the study.
SOURCE: Stern RA et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1900757 (Epub ahead of print).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
MicroRNA-375 may be key to fibrolamellar carcinoma
Up-regulation of microRNA-375 may be a future therapeutic strategy for patients with fibrolamellar carcinoma (FLC), according to investigators.
Analysis of primary FLC tumors showed that microRNA-375 was the most abnormal microRNA, down-regulated 27-fold, reported lead author Timothy A. Dinh, MD, of the University of North Carolina at Chapel Hill and his colleagues. Overexpression of microRNA-375 in an FLC cell line suppressed cell migration and proliferation, hinting at therapeutic potential.
“Overall, our results show that miR-375 [microRNA-375] functions as a tumor suppressor in FLC and points toward future therapies based on miR-375 mimics that may provide a viable option for patients,” the investigators wrote in a Cellular and Molecular Gastroenterology and Hepatology.
FLC is an uncommon liver cancer in adolescents and young adults. Currently, surgery is the only effective treatment; unfortunately, many patients have metastatic disease at the time of diagnosis, disallowing surgical cure.
“The lack of knowledge of underlying disease mechanisms has hindered our understanding of this cancer and the development of novel therapeutics for FLC patients,” the investigators wrote.
Previous research has shown that almost all patients with FLC (80%-100%) have a heterozygous deletion mutation on chromosome 19. However, it is not a loss of genetic information that incites neoplasia; instead, the deletion causes a fusion of genes DNAJB1 and PRKACA. This fusion is capable of triggering liver tumors, a phenomenon confirmed through mouse models. The present study built on these findings, along with recent awareness that several microRNAs are dysregulated in FLC, compared with normal liver tissue.
First, the investigators performed small RNA-sequencing in six primary FLC tumors from The Cancer Genome Atlas (TCGA). They found that 30 microRNAs were up-regulated and 46 microRNAs were down-regulated. Among these, microRNA-375 was the most significantly down-regulated, at 27-fold (P = .009). To confirm these findings, the same process was repeated in 18 independent samples, with the same result.
The investigators explained that, in addition to magnitude of down-regulation, microRNA-375 deserved attention for at least three other reasons: It is down-regulated in numerous cancer types, it directly targets known oncogenes, and it is suppressed by the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling axis, which is overactive in FLC.
Further testing confirmed that microRNA-375 was consistently more down-regulated in samples of FLC, by up to 20-fold, than it was in nonmalignant liver tissue. To confirm that loss of microRNA-375 expression occurred in FLC tumor cells instead of other cell types, such as stromal cells, a patient-derived xenograft of FLC was compared with liver lineage cells, including adult hepatocytes, hepatoblasts, hepatic stem cells, and biliary tree stem cells. Again, microRNA-375 was down-regulated most in the FLC cells. Additional comparisons within the TCGA showed that microRNA-375 was more down-regulated in FLC than 21 out of 22 other tumor types (second only to melanoma).
“Taken together with our findings from primary tumor tissue, our results strongly suggest that miR-375 may function as a tumor suppressor in FLC,” the investigators wrote.
Having confirmed the ubiquity of microRNA-375 down-regulation in FLC, the investigators turned to the relationship between the DNAJB1-PRKACA fusion and microRNA-375. Using two methods – gene deletion with CRISPR/Cas9 and transposon injection – the investigators found that creating the DNAJB1-PRKACA fusion in cells of mice was sufficient to suppress microRNA-375 expression, which supports a downstream relationship.
Finally, the investigators showed that treating an FLC cell line with an microRNA-375 mimic suppressed the Hippo signaling pathway, including connective tissue growth factor (CTGF) and yes-associated protein 1 (YAP1). These events translated to reduced cellular activity, which suggests that up-regulating microRNA-375 could, indeed, control FLC.
“Importantly, introduction of a miR-375 mimic significantly reduced colony formation, EdU incorporation, and migration, indicative of reduced survival, proliferation, and metastatic potential, respectively,” the investigators wrote.
“With RNA-based therapies showing increasing promise, miR-375–based therapies merit future consideration for FLC therapeutics,” they concluded.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Alcohol Abuse and Alcoholism, and the Fibrolamellar Cancer Foundation. The investigators declared no conflicts of interest.
SOURCE: Dinh TA et al. Cell Mol Gastroenterol Hepatol. 2019 Feb 11. doi: 10.1016/j.jcmgh.2019.01.008.
For several decades, fibrolamellar carcinoma was the enigmatic liver cancer. Neither etiology nor molecular causes were known. The breakthrough came when tumor sequencing identified a hitherto undescribed fusion gene in 15 out of 15 patients analyzed: A small portion of the heat shock protein DNAJB1 was fused to the catalytic subunit of protein kinase A (PKA, or PRKACA), which retained full kinase activity.
Underscoring the significance of this finding, the DNAJB1-PRKACA fusion gene was shown to be sufficient to elicit tumors similar to human fibrolamellar carcinoma when engineered in mice. The absence of conspicuous codriver genes makes DNAJB1-PRKACA a primary candidate for therapeutic target. However, PKA inhibitors would be problematic in the clinic because of the vital physiological functions of PKA. Consequently, the hunt is on to decipher the oncogenic signaling pathways emanating from DNAJB1-PRKACA with the hope to identify alternative targets among its downstream mediators.
In this work, the Sethupathy lab performed a thorough study on abnormally regulated microRNAs in fibrolamellar carcinoma tumors. Intriguingly, they identified several microRNAs controlled by DNAJB1-PRKACA that have oncogenic or tumor suppressor function in other cancers. In particular, the tumor suppressor microRNA-375 was massively down-regulated by DNAJB1-PRKACA. Furthermore, introducing a microRNA-375 mimic in fibrolamellar cancer cells suppressed proliferation and motility. Important studies like this open up new avenues aiming to manipulate cancer microRNAs as alternative or complementary approaches for targeting DNAJB1-PRKACA signaling in the highly fatal fibrolamellar carcinoma.
Morten Frödin, MSc, PhD, is an associate professor and group leader of the Biotech Research and Innovation Centre, University of Copenhagen.
For several decades, fibrolamellar carcinoma was the enigmatic liver cancer. Neither etiology nor molecular causes were known. The breakthrough came when tumor sequencing identified a hitherto undescribed fusion gene in 15 out of 15 patients analyzed: A small portion of the heat shock protein DNAJB1 was fused to the catalytic subunit of protein kinase A (PKA, or PRKACA), which retained full kinase activity.
Underscoring the significance of this finding, the DNAJB1-PRKACA fusion gene was shown to be sufficient to elicit tumors similar to human fibrolamellar carcinoma when engineered in mice. The absence of conspicuous codriver genes makes DNAJB1-PRKACA a primary candidate for therapeutic target. However, PKA inhibitors would be problematic in the clinic because of the vital physiological functions of PKA. Consequently, the hunt is on to decipher the oncogenic signaling pathways emanating from DNAJB1-PRKACA with the hope to identify alternative targets among its downstream mediators.
In this work, the Sethupathy lab performed a thorough study on abnormally regulated microRNAs in fibrolamellar carcinoma tumors. Intriguingly, they identified several microRNAs controlled by DNAJB1-PRKACA that have oncogenic or tumor suppressor function in other cancers. In particular, the tumor suppressor microRNA-375 was massively down-regulated by DNAJB1-PRKACA. Furthermore, introducing a microRNA-375 mimic in fibrolamellar cancer cells suppressed proliferation and motility. Important studies like this open up new avenues aiming to manipulate cancer microRNAs as alternative or complementary approaches for targeting DNAJB1-PRKACA signaling in the highly fatal fibrolamellar carcinoma.
Morten Frödin, MSc, PhD, is an associate professor and group leader of the Biotech Research and Innovation Centre, University of Copenhagen.
For several decades, fibrolamellar carcinoma was the enigmatic liver cancer. Neither etiology nor molecular causes were known. The breakthrough came when tumor sequencing identified a hitherto undescribed fusion gene in 15 out of 15 patients analyzed: A small portion of the heat shock protein DNAJB1 was fused to the catalytic subunit of protein kinase A (PKA, or PRKACA), which retained full kinase activity.
Underscoring the significance of this finding, the DNAJB1-PRKACA fusion gene was shown to be sufficient to elicit tumors similar to human fibrolamellar carcinoma when engineered in mice. The absence of conspicuous codriver genes makes DNAJB1-PRKACA a primary candidate for therapeutic target. However, PKA inhibitors would be problematic in the clinic because of the vital physiological functions of PKA. Consequently, the hunt is on to decipher the oncogenic signaling pathways emanating from DNAJB1-PRKACA with the hope to identify alternative targets among its downstream mediators.
In this work, the Sethupathy lab performed a thorough study on abnormally regulated microRNAs in fibrolamellar carcinoma tumors. Intriguingly, they identified several microRNAs controlled by DNAJB1-PRKACA that have oncogenic or tumor suppressor function in other cancers. In particular, the tumor suppressor microRNA-375 was massively down-regulated by DNAJB1-PRKACA. Furthermore, introducing a microRNA-375 mimic in fibrolamellar cancer cells suppressed proliferation and motility. Important studies like this open up new avenues aiming to manipulate cancer microRNAs as alternative or complementary approaches for targeting DNAJB1-PRKACA signaling in the highly fatal fibrolamellar carcinoma.
Morten Frödin, MSc, PhD, is an associate professor and group leader of the Biotech Research and Innovation Centre, University of Copenhagen.
Up-regulation of microRNA-375 may be a future therapeutic strategy for patients with fibrolamellar carcinoma (FLC), according to investigators.
Analysis of primary FLC tumors showed that microRNA-375 was the most abnormal microRNA, down-regulated 27-fold, reported lead author Timothy A. Dinh, MD, of the University of North Carolina at Chapel Hill and his colleagues. Overexpression of microRNA-375 in an FLC cell line suppressed cell migration and proliferation, hinting at therapeutic potential.
“Overall, our results show that miR-375 [microRNA-375] functions as a tumor suppressor in FLC and points toward future therapies based on miR-375 mimics that may provide a viable option for patients,” the investigators wrote in a Cellular and Molecular Gastroenterology and Hepatology.
FLC is an uncommon liver cancer in adolescents and young adults. Currently, surgery is the only effective treatment; unfortunately, many patients have metastatic disease at the time of diagnosis, disallowing surgical cure.
“The lack of knowledge of underlying disease mechanisms has hindered our understanding of this cancer and the development of novel therapeutics for FLC patients,” the investigators wrote.
Previous research has shown that almost all patients with FLC (80%-100%) have a heterozygous deletion mutation on chromosome 19. However, it is not a loss of genetic information that incites neoplasia; instead, the deletion causes a fusion of genes DNAJB1 and PRKACA. This fusion is capable of triggering liver tumors, a phenomenon confirmed through mouse models. The present study built on these findings, along with recent awareness that several microRNAs are dysregulated in FLC, compared with normal liver tissue.
First, the investigators performed small RNA-sequencing in six primary FLC tumors from The Cancer Genome Atlas (TCGA). They found that 30 microRNAs were up-regulated and 46 microRNAs were down-regulated. Among these, microRNA-375 was the most significantly down-regulated, at 27-fold (P = .009). To confirm these findings, the same process was repeated in 18 independent samples, with the same result.
The investigators explained that, in addition to magnitude of down-regulation, microRNA-375 deserved attention for at least three other reasons: It is down-regulated in numerous cancer types, it directly targets known oncogenes, and it is suppressed by the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling axis, which is overactive in FLC.
Further testing confirmed that microRNA-375 was consistently more down-regulated in samples of FLC, by up to 20-fold, than it was in nonmalignant liver tissue. To confirm that loss of microRNA-375 expression occurred in FLC tumor cells instead of other cell types, such as stromal cells, a patient-derived xenograft of FLC was compared with liver lineage cells, including adult hepatocytes, hepatoblasts, hepatic stem cells, and biliary tree stem cells. Again, microRNA-375 was down-regulated most in the FLC cells. Additional comparisons within the TCGA showed that microRNA-375 was more down-regulated in FLC than 21 out of 22 other tumor types (second only to melanoma).
“Taken together with our findings from primary tumor tissue, our results strongly suggest that miR-375 may function as a tumor suppressor in FLC,” the investigators wrote.
Having confirmed the ubiquity of microRNA-375 down-regulation in FLC, the investigators turned to the relationship between the DNAJB1-PRKACA fusion and microRNA-375. Using two methods – gene deletion with CRISPR/Cas9 and transposon injection – the investigators found that creating the DNAJB1-PRKACA fusion in cells of mice was sufficient to suppress microRNA-375 expression, which supports a downstream relationship.
Finally, the investigators showed that treating an FLC cell line with an microRNA-375 mimic suppressed the Hippo signaling pathway, including connective tissue growth factor (CTGF) and yes-associated protein 1 (YAP1). These events translated to reduced cellular activity, which suggests that up-regulating microRNA-375 could, indeed, control FLC.
“Importantly, introduction of a miR-375 mimic significantly reduced colony formation, EdU incorporation, and migration, indicative of reduced survival, proliferation, and metastatic potential, respectively,” the investigators wrote.
“With RNA-based therapies showing increasing promise, miR-375–based therapies merit future consideration for FLC therapeutics,” they concluded.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Alcohol Abuse and Alcoholism, and the Fibrolamellar Cancer Foundation. The investigators declared no conflicts of interest.
SOURCE: Dinh TA et al. Cell Mol Gastroenterol Hepatol. 2019 Feb 11. doi: 10.1016/j.jcmgh.2019.01.008.
Up-regulation of microRNA-375 may be a future therapeutic strategy for patients with fibrolamellar carcinoma (FLC), according to investigators.
Analysis of primary FLC tumors showed that microRNA-375 was the most abnormal microRNA, down-regulated 27-fold, reported lead author Timothy A. Dinh, MD, of the University of North Carolina at Chapel Hill and his colleagues. Overexpression of microRNA-375 in an FLC cell line suppressed cell migration and proliferation, hinting at therapeutic potential.
“Overall, our results show that miR-375 [microRNA-375] functions as a tumor suppressor in FLC and points toward future therapies based on miR-375 mimics that may provide a viable option for patients,” the investigators wrote in a Cellular and Molecular Gastroenterology and Hepatology.
FLC is an uncommon liver cancer in adolescents and young adults. Currently, surgery is the only effective treatment; unfortunately, many patients have metastatic disease at the time of diagnosis, disallowing surgical cure.
“The lack of knowledge of underlying disease mechanisms has hindered our understanding of this cancer and the development of novel therapeutics for FLC patients,” the investigators wrote.
Previous research has shown that almost all patients with FLC (80%-100%) have a heterozygous deletion mutation on chromosome 19. However, it is not a loss of genetic information that incites neoplasia; instead, the deletion causes a fusion of genes DNAJB1 and PRKACA. This fusion is capable of triggering liver tumors, a phenomenon confirmed through mouse models. The present study built on these findings, along with recent awareness that several microRNAs are dysregulated in FLC, compared with normal liver tissue.
First, the investigators performed small RNA-sequencing in six primary FLC tumors from The Cancer Genome Atlas (TCGA). They found that 30 microRNAs were up-regulated and 46 microRNAs were down-regulated. Among these, microRNA-375 was the most significantly down-regulated, at 27-fold (P = .009). To confirm these findings, the same process was repeated in 18 independent samples, with the same result.
The investigators explained that, in addition to magnitude of down-regulation, microRNA-375 deserved attention for at least three other reasons: It is down-regulated in numerous cancer types, it directly targets known oncogenes, and it is suppressed by the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling axis, which is overactive in FLC.
Further testing confirmed that microRNA-375 was consistently more down-regulated in samples of FLC, by up to 20-fold, than it was in nonmalignant liver tissue. To confirm that loss of microRNA-375 expression occurred in FLC tumor cells instead of other cell types, such as stromal cells, a patient-derived xenograft of FLC was compared with liver lineage cells, including adult hepatocytes, hepatoblasts, hepatic stem cells, and biliary tree stem cells. Again, microRNA-375 was down-regulated most in the FLC cells. Additional comparisons within the TCGA showed that microRNA-375 was more down-regulated in FLC than 21 out of 22 other tumor types (second only to melanoma).
“Taken together with our findings from primary tumor tissue, our results strongly suggest that miR-375 may function as a tumor suppressor in FLC,” the investigators wrote.
Having confirmed the ubiquity of microRNA-375 down-regulation in FLC, the investigators turned to the relationship between the DNAJB1-PRKACA fusion and microRNA-375. Using two methods – gene deletion with CRISPR/Cas9 and transposon injection – the investigators found that creating the DNAJB1-PRKACA fusion in cells of mice was sufficient to suppress microRNA-375 expression, which supports a downstream relationship.
Finally, the investigators showed that treating an FLC cell line with an microRNA-375 mimic suppressed the Hippo signaling pathway, including connective tissue growth factor (CTGF) and yes-associated protein 1 (YAP1). These events translated to reduced cellular activity, which suggests that up-regulating microRNA-375 could, indeed, control FLC.
“Importantly, introduction of a miR-375 mimic significantly reduced colony formation, EdU incorporation, and migration, indicative of reduced survival, proliferation, and metastatic potential, respectively,” the investigators wrote.
“With RNA-based therapies showing increasing promise, miR-375–based therapies merit future consideration for FLC therapeutics,” they concluded.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Alcohol Abuse and Alcoholism, and the Fibrolamellar Cancer Foundation. The investigators declared no conflicts of interest.
SOURCE: Dinh TA et al. Cell Mol Gastroenterol Hepatol. 2019 Feb 11. doi: 10.1016/j.jcmgh.2019.01.008.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Immunotherapy overtaking breast cancer treatment landscape
Combination treatment strategies that include immunotherapeutic agents are expected to become the future of breast cancer treatment, according to a review.
“There is tremendous interest in using immunotherapy to treat breast cancer, as evidenced by the more than 290 clinical trials ongoing,” wrote Sylvia Adams, MD, MS, of New York University, along with her colleagues. The report is in JAMA Oncology.
“It is anticipated that combination therapy strategies will be the way forward for immunotherapy in breast cancer, with an improved understanding of tumor, microenvironment, and host factors informing treatment combination decisions, they said.
Dr. Adams and her colleagues searched major databases for clinical trials investigating the use of immunotherapy in both early-stage and metastatic breast cancer.
After the search, the team found that immune checkpoint blockade (ICB) agents were the most studied type of immunotherapy in breast cancer today.
In addition, Dr. Adams and her colleagues reported that when UCB agents were used as monotherapy in patients with breast cancer, objective responses have been seen, especially when given in the initial stages of treatment. “For responding patients, those responses are durable,” they added.
Recent findings have indicated that combining immune checkpoint blockade agents with chemotherapy may be useful in early breast cancer as neoadjuvant therapy.
“Combination trials were more common than single-agent studies, with the most commonly combined modalities being chemotherapy or targeted therapy,” the researchers wrote.
The review is limited by the rapid advancement of the current treatment landscape. As a result, additional data may now be available beyond the date of publication.
“Thoughtful study design incorporating appropriate end points and correlative studies will be critical in identifying optimal strategies for enhancing the immune response against breast tumors,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Amgen, Celgene, Genentech, Eli Lilly, Ipsen, Novartis, Pfizer, and several others.
SOURCE: Adams S et al. JAMA Oncol. 2019 Apr 11. doi: 10.1001/jamaoncol.2018.7147.
Combination treatment strategies that include immunotherapeutic agents are expected to become the future of breast cancer treatment, according to a review.
“There is tremendous interest in using immunotherapy to treat breast cancer, as evidenced by the more than 290 clinical trials ongoing,” wrote Sylvia Adams, MD, MS, of New York University, along with her colleagues. The report is in JAMA Oncology.
“It is anticipated that combination therapy strategies will be the way forward for immunotherapy in breast cancer, with an improved understanding of tumor, microenvironment, and host factors informing treatment combination decisions, they said.
Dr. Adams and her colleagues searched major databases for clinical trials investigating the use of immunotherapy in both early-stage and metastatic breast cancer.
After the search, the team found that immune checkpoint blockade (ICB) agents were the most studied type of immunotherapy in breast cancer today.
In addition, Dr. Adams and her colleagues reported that when UCB agents were used as monotherapy in patients with breast cancer, objective responses have been seen, especially when given in the initial stages of treatment. “For responding patients, those responses are durable,” they added.
Recent findings have indicated that combining immune checkpoint blockade agents with chemotherapy may be useful in early breast cancer as neoadjuvant therapy.
“Combination trials were more common than single-agent studies, with the most commonly combined modalities being chemotherapy or targeted therapy,” the researchers wrote.
The review is limited by the rapid advancement of the current treatment landscape. As a result, additional data may now be available beyond the date of publication.
“Thoughtful study design incorporating appropriate end points and correlative studies will be critical in identifying optimal strategies for enhancing the immune response against breast tumors,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Amgen, Celgene, Genentech, Eli Lilly, Ipsen, Novartis, Pfizer, and several others.
SOURCE: Adams S et al. JAMA Oncol. 2019 Apr 11. doi: 10.1001/jamaoncol.2018.7147.
Combination treatment strategies that include immunotherapeutic agents are expected to become the future of breast cancer treatment, according to a review.
“There is tremendous interest in using immunotherapy to treat breast cancer, as evidenced by the more than 290 clinical trials ongoing,” wrote Sylvia Adams, MD, MS, of New York University, along with her colleagues. The report is in JAMA Oncology.
“It is anticipated that combination therapy strategies will be the way forward for immunotherapy in breast cancer, with an improved understanding of tumor, microenvironment, and host factors informing treatment combination decisions, they said.
Dr. Adams and her colleagues searched major databases for clinical trials investigating the use of immunotherapy in both early-stage and metastatic breast cancer.
After the search, the team found that immune checkpoint blockade (ICB) agents were the most studied type of immunotherapy in breast cancer today.
In addition, Dr. Adams and her colleagues reported that when UCB agents were used as monotherapy in patients with breast cancer, objective responses have been seen, especially when given in the initial stages of treatment. “For responding patients, those responses are durable,” they added.
Recent findings have indicated that combining immune checkpoint blockade agents with chemotherapy may be useful in early breast cancer as neoadjuvant therapy.
“Combination trials were more common than single-agent studies, with the most commonly combined modalities being chemotherapy or targeted therapy,” the researchers wrote.
The review is limited by the rapid advancement of the current treatment landscape. As a result, additional data may now be available beyond the date of publication.
“Thoughtful study design incorporating appropriate end points and correlative studies will be critical in identifying optimal strategies for enhancing the immune response against breast tumors,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Amgen, Celgene, Genentech, Eli Lilly, Ipsen, Novartis, Pfizer, and several others.
SOURCE: Adams S et al. JAMA Oncol. 2019 Apr 11. doi: 10.1001/jamaoncol.2018.7147.
FROM JAMA ONCOLOGY