User login
Role of Diet in Treating Skin Conditions



Reviews of Self-Instructional Materials
An Integrated System for the Recording and Retrieval of Medical Data in a Primary Care Setting: Part 2: Classification of Diseases
Reviews of Self-Instructional Materials
Poor response to statins hikes risk of cardiovascular events
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
FROM HEART
An Integrated System for the Recording and Retrieval of Medical Data in a Primary Care Setting: Part 5: Implications of Filing Charts by Area of Residence
The hospital outpatient facility may soon lose its secret sauce
If it’s happening in Cincinnati, it is happening everywhere. All the young doctors are being hired by hospital systems at better pay than private practices can afford. When I asked the CEO at one Cincinnati hospital about the trend, he explained: “We like to have referrals available for our primary care physicians.” Sounds nice, but I don’t believe it.
Hospital outpatient facilities have been reaping the benefits of site-of-service differential payments for years. Under the current Centers for Medicare/Medicaid Services payment scales, identical services are reimbursed at extraordinarily higher rates – differentials amounting to, on average, approximately 360% of Medicare’s payment for the same mix of services when they are performed in a physician’s office – if they are delivered at off-campus hospital outpatient departments rather than independent doctors’ offices. Technically, these outpatient departments can be an office that has been bought by the hospital and proximity is not an issue. Many off-campus hospital outpatient departments in my area are as far away as 35 miles, some in strip malls no less!
That situation may soon change, though. The CMS has proposed eliminating the site-of-service differentials for hospital outpatient services. The proposal is being aggressively opposed by hospital lobbyists and has even inspired lawsuits because it would blow the lid off the extraordinary payment differential available to hospital outpatient departments (“Proposed site-neutral payment policy sets the stage for battle royale between CMS, hospitals,” Modern Healthcare, July 26, 2018).
It’s a change that’s long overdue.
In the period from 2001 to 2017, Medicare Part B payments to physicians increased only 6%, while Medicare’s index of inflation measuring the cost of running a medical practice increased 30%. After adjustment for inflation in practice costs, physician pay has declined 19%, thus failing to match increases in office overhead costs. In that same 17-year period, Medicare hospital payments increased roughly 50%, including average annual increases of 2.6% for inpatient services and 2.5% per year for outpatient services. Hospitals have thus received payment increases more than eightfold greater than payment adjustments to physicians in the period from 2001 to 2017!
I think we have found the secret sauce!
Obviously, some of this largesse was spread over the recruitment of physicians, buying offices, and creating of secret sauce clinics. Hospital purchases of private offices and physician employment at hospitals soared to nearly 33%.
But much went to the hospital’s bottom line.
Hospitals have enjoyed 28 annual year-over-year increases in payments for services rendered in hospital outpatient facilities.
Many of these hospital systems claim to make no extra money using the hospital outpatient system. If so, eliminating the site-of-service differential will not affect them. We will see.
I think the elimination of the site-of-service differential will have profound impacts on office medicine. While the AMA is asking that the savings (several billion over 10 years) be funneled back into the office setting to correct past underpayments, just the correction of the distortion will benefit office practice. The recruitment of new physicians by hospitals, and the practice-buying binge, appear to have subsided. Expect many of these satellites to close, and their employed physicians, young and old, to be coming back into the job market. Expect Medicare beneficiaries to pay lower copays and deductibles.
Corrections of distortions like the site-of-service differential empower patients and independent physicians. Thank the AMA for exposing the unfairness and allowing the CMS to act. You may not know it, but the American Medical Association puts together a tremendous – some would say overwhelming – amount of research together on topics of importance to physicians. Some of this is fascinating. I direct you to the AMA’s Report 4 of the Council on Medical Service (I-18)
This report lays it all out, and explains what has happened.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
If it’s happening in Cincinnati, it is happening everywhere. All the young doctors are being hired by hospital systems at better pay than private practices can afford. When I asked the CEO at one Cincinnati hospital about the trend, he explained: “We like to have referrals available for our primary care physicians.” Sounds nice, but I don’t believe it.
Hospital outpatient facilities have been reaping the benefits of site-of-service differential payments for years. Under the current Centers for Medicare/Medicaid Services payment scales, identical services are reimbursed at extraordinarily higher rates – differentials amounting to, on average, approximately 360% of Medicare’s payment for the same mix of services when they are performed in a physician’s office – if they are delivered at off-campus hospital outpatient departments rather than independent doctors’ offices. Technically, these outpatient departments can be an office that has been bought by the hospital and proximity is not an issue. Many off-campus hospital outpatient departments in my area are as far away as 35 miles, some in strip malls no less!
That situation may soon change, though. The CMS has proposed eliminating the site-of-service differentials for hospital outpatient services. The proposal is being aggressively opposed by hospital lobbyists and has even inspired lawsuits because it would blow the lid off the extraordinary payment differential available to hospital outpatient departments (“Proposed site-neutral payment policy sets the stage for battle royale between CMS, hospitals,” Modern Healthcare, July 26, 2018).
It’s a change that’s long overdue.
In the period from 2001 to 2017, Medicare Part B payments to physicians increased only 6%, while Medicare’s index of inflation measuring the cost of running a medical practice increased 30%. After adjustment for inflation in practice costs, physician pay has declined 19%, thus failing to match increases in office overhead costs. In that same 17-year period, Medicare hospital payments increased roughly 50%, including average annual increases of 2.6% for inpatient services and 2.5% per year for outpatient services. Hospitals have thus received payment increases more than eightfold greater than payment adjustments to physicians in the period from 2001 to 2017!
I think we have found the secret sauce!
Obviously, some of this largesse was spread over the recruitment of physicians, buying offices, and creating of secret sauce clinics. Hospital purchases of private offices and physician employment at hospitals soared to nearly 33%.
But much went to the hospital’s bottom line.
Hospitals have enjoyed 28 annual year-over-year increases in payments for services rendered in hospital outpatient facilities.
Many of these hospital systems claim to make no extra money using the hospital outpatient system. If so, eliminating the site-of-service differential will not affect them. We will see.
I think the elimination of the site-of-service differential will have profound impacts on office medicine. While the AMA is asking that the savings (several billion over 10 years) be funneled back into the office setting to correct past underpayments, just the correction of the distortion will benefit office practice. The recruitment of new physicians by hospitals, and the practice-buying binge, appear to have subsided. Expect many of these satellites to close, and their employed physicians, young and old, to be coming back into the job market. Expect Medicare beneficiaries to pay lower copays and deductibles.
Corrections of distortions like the site-of-service differential empower patients and independent physicians. Thank the AMA for exposing the unfairness and allowing the CMS to act. You may not know it, but the American Medical Association puts together a tremendous – some would say overwhelming – amount of research together on topics of importance to physicians. Some of this is fascinating. I direct you to the AMA’s Report 4 of the Council on Medical Service (I-18)
This report lays it all out, and explains what has happened.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
If it’s happening in Cincinnati, it is happening everywhere. All the young doctors are being hired by hospital systems at better pay than private practices can afford. When I asked the CEO at one Cincinnati hospital about the trend, he explained: “We like to have referrals available for our primary care physicians.” Sounds nice, but I don’t believe it.
Hospital outpatient facilities have been reaping the benefits of site-of-service differential payments for years. Under the current Centers for Medicare/Medicaid Services payment scales, identical services are reimbursed at extraordinarily higher rates – differentials amounting to, on average, approximately 360% of Medicare’s payment for the same mix of services when they are performed in a physician’s office – if they are delivered at off-campus hospital outpatient departments rather than independent doctors’ offices. Technically, these outpatient departments can be an office that has been bought by the hospital and proximity is not an issue. Many off-campus hospital outpatient departments in my area are as far away as 35 miles, some in strip malls no less!
That situation may soon change, though. The CMS has proposed eliminating the site-of-service differentials for hospital outpatient services. The proposal is being aggressively opposed by hospital lobbyists and has even inspired lawsuits because it would blow the lid off the extraordinary payment differential available to hospital outpatient departments (“Proposed site-neutral payment policy sets the stage for battle royale between CMS, hospitals,” Modern Healthcare, July 26, 2018).
It’s a change that’s long overdue.
In the period from 2001 to 2017, Medicare Part B payments to physicians increased only 6%, while Medicare’s index of inflation measuring the cost of running a medical practice increased 30%. After adjustment for inflation in practice costs, physician pay has declined 19%, thus failing to match increases in office overhead costs. In that same 17-year period, Medicare hospital payments increased roughly 50%, including average annual increases of 2.6% for inpatient services and 2.5% per year for outpatient services. Hospitals have thus received payment increases more than eightfold greater than payment adjustments to physicians in the period from 2001 to 2017!
I think we have found the secret sauce!
Obviously, some of this largesse was spread over the recruitment of physicians, buying offices, and creating of secret sauce clinics. Hospital purchases of private offices and physician employment at hospitals soared to nearly 33%.
But much went to the hospital’s bottom line.
Hospitals have enjoyed 28 annual year-over-year increases in payments for services rendered in hospital outpatient facilities.
Many of these hospital systems claim to make no extra money using the hospital outpatient system. If so, eliminating the site-of-service differential will not affect them. We will see.
I think the elimination of the site-of-service differential will have profound impacts on office medicine. While the AMA is asking that the savings (several billion over 10 years) be funneled back into the office setting to correct past underpayments, just the correction of the distortion will benefit office practice. The recruitment of new physicians by hospitals, and the practice-buying binge, appear to have subsided. Expect many of these satellites to close, and their employed physicians, young and old, to be coming back into the job market. Expect Medicare beneficiaries to pay lower copays and deductibles.
Corrections of distortions like the site-of-service differential empower patients and independent physicians. Thank the AMA for exposing the unfairness and allowing the CMS to act. You may not know it, but the American Medical Association puts together a tremendous – some would say overwhelming – amount of research together on topics of importance to physicians. Some of this is fascinating. I direct you to the AMA’s Report 4 of the Council on Medical Service (I-18)
This report lays it all out, and explains what has happened.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Childhood-onset SLE rate doubles in children born in winter
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
SAN FRANCISCO – Simone Appenzeller, MD, PhD, reported at an international congress on systemic lupus erythematosus.
She presented a cross-sectional study of 760 consecutive SLE patients seen in a university lupus clinic and 700 healthy controls. Ninety-eight of the lupus patients had childhood-onset disease that began no later than age 16 years, while 662 had adult-onset SLE.
Southeastern Brazil, where the study was conducted, features a mostly subtropical climate with relatively mild winters. Because it is in the southern hemisphere, winter lasts from June 21 to September 21, while spring runs from September 22 to December 20.
Forty-six percent of subjects with childhood-onset SLE were born in winter, 17% in spring, another 17% in summer, and just over 19% in autumn, according to Dr. Appenzeller, a rheumatologist at the University of Campinas (Brazil).
In contrast, the occurrence of adult-onset SLE showed no variation by birth month or season.
The birth disparity between childhood- and adult-onset SLE was greatest in August, the depth of Brazilian winter, when 15.3% of all subjects with childhood-onset SLE were born, compared with 9% of adult-onset SLE patients and 8% of healthy controls.
The explanation for the increased likelihood of patients with childhood-onset SLE to be born in the winter months probably involves a gene-environment interaction, Dr. Appenzeller said. The most likely environmental factors are low seasonal maternal vitamin D levels and/or exposure to winter respiratory infections. The existence of a genetic component to the birth month disparity is suggested by another study by the Brazilian investigators in which they determined that the prevalence of SLE in both the first- and second-degree relatives of individuals with childhood-onset SLE was significantly higher than in patients with adult-onset disease.
Dr. Appenzeller reported having no financial conflicts regarding her study, funded by the Brazilian National Council for Scientific and Technological Development and other noncommercial research organizations.
REPORTING FROM LUPUS 2019
One percent better
Hurray! . Of course, I might be overplaying my excitement here: EMR updates are emotionally closest to a trip to the dentist – usually turn out fine, but hardly worth circling on the calendar.
Like a good dental cleaning however, these updates can be beneficial. Although uncomfortable, EMR redesigned menus and shortcuts can help – once you’re used to them. It typically doesn’t take long for most of us to learn the “improvements” so we’re at least not worse off. It is also a good time to update customized features. I try to use updates as an opportunity to refresh SmartPhrases, update order preferences, or rearrange my desktop to be more efficient.
Given how much my quality of life depends upon my EMR skills, it’s a shame I don’t make an effort to work on it more often. Really, why wait to make improvements just once a year? Why not get better every day?
This idea of continuous improvement is a popular meme in the self-improvement community right now. Instead of working on goals or adjusting your routine episodically, set an intention to get better, just a little, daily. Sometimes it’s described as the 1% model. The idea is that improving your habits or work flow by 1% each day will yield compound benefits with time. It is an aggregation of marginal gains with a surprising payout. For example, daily 1% improvements would mean you are 37 times more effective by the end of a year. Now, I don’t believe this mathematical model is necessarily accurate or even necessary. But the concept that a little development done daily yields lasting improvement seems to be true.
The corollary, that if you got a little worse each day, you’d be much worse off at the end of a year, is also reasonable. That’s how most health problems set in: continuous and insidious aggregation of bad choices. Why then not use that same principle for good instead?
The Japanese thought of this idea a generation ago. Applied to manufacturing, they called it Kaizen, “continuous improvement.” It was a managerial principle that reminded people to look for opportunities to improve, just a little, wherever they were in the process and to do so each day. It led to remarkable reductions in waste and became a key to their economic success.
Opportunities for relentless improvement abound in our work too. For example, when you write an order or work up a diagnosis, rather than just enter it, you might save it as a panel. When you find yourself using the same word or phrase, save it to your dictionary to pull it up with minimal keystrokes. When you research a difficult disease you’ve not seen lately, save the diagnostic questions as a template so you can pull it up in real time the next time it walks in. When you set up your procedure tray, place items so they can be picked up efficiently and moved out of the way quickly. No matter how good your setup or template is today, you can find a tiny improvement that would make it a little better tomorrow. And you’ll reap gains from that day forward.
I don’t expect this EMR update will have much impact on my quality of life. It will however be a reminder that like flossing, improvements are best done daily. You and your dentist will thank me someday.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Hurray! . Of course, I might be overplaying my excitement here: EMR updates are emotionally closest to a trip to the dentist – usually turn out fine, but hardly worth circling on the calendar.
Like a good dental cleaning however, these updates can be beneficial. Although uncomfortable, EMR redesigned menus and shortcuts can help – once you’re used to them. It typically doesn’t take long for most of us to learn the “improvements” so we’re at least not worse off. It is also a good time to update customized features. I try to use updates as an opportunity to refresh SmartPhrases, update order preferences, or rearrange my desktop to be more efficient.
Given how much my quality of life depends upon my EMR skills, it’s a shame I don’t make an effort to work on it more often. Really, why wait to make improvements just once a year? Why not get better every day?
This idea of continuous improvement is a popular meme in the self-improvement community right now. Instead of working on goals or adjusting your routine episodically, set an intention to get better, just a little, daily. Sometimes it’s described as the 1% model. The idea is that improving your habits or work flow by 1% each day will yield compound benefits with time. It is an aggregation of marginal gains with a surprising payout. For example, daily 1% improvements would mean you are 37 times more effective by the end of a year. Now, I don’t believe this mathematical model is necessarily accurate or even necessary. But the concept that a little development done daily yields lasting improvement seems to be true.
The corollary, that if you got a little worse each day, you’d be much worse off at the end of a year, is also reasonable. That’s how most health problems set in: continuous and insidious aggregation of bad choices. Why then not use that same principle for good instead?
The Japanese thought of this idea a generation ago. Applied to manufacturing, they called it Kaizen, “continuous improvement.” It was a managerial principle that reminded people to look for opportunities to improve, just a little, wherever they were in the process and to do so each day. It led to remarkable reductions in waste and became a key to their economic success.
Opportunities for relentless improvement abound in our work too. For example, when you write an order or work up a diagnosis, rather than just enter it, you might save it as a panel. When you find yourself using the same word or phrase, save it to your dictionary to pull it up with minimal keystrokes. When you research a difficult disease you’ve not seen lately, save the diagnostic questions as a template so you can pull it up in real time the next time it walks in. When you set up your procedure tray, place items so they can be picked up efficiently and moved out of the way quickly. No matter how good your setup or template is today, you can find a tiny improvement that would make it a little better tomorrow. And you’ll reap gains from that day forward.
I don’t expect this EMR update will have much impact on my quality of life. It will however be a reminder that like flossing, improvements are best done daily. You and your dentist will thank me someday.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Hurray! . Of course, I might be overplaying my excitement here: EMR updates are emotionally closest to a trip to the dentist – usually turn out fine, but hardly worth circling on the calendar.
Like a good dental cleaning however, these updates can be beneficial. Although uncomfortable, EMR redesigned menus and shortcuts can help – once you’re used to them. It typically doesn’t take long for most of us to learn the “improvements” so we’re at least not worse off. It is also a good time to update customized features. I try to use updates as an opportunity to refresh SmartPhrases, update order preferences, or rearrange my desktop to be more efficient.
Given how much my quality of life depends upon my EMR skills, it’s a shame I don’t make an effort to work on it more often. Really, why wait to make improvements just once a year? Why not get better every day?
This idea of continuous improvement is a popular meme in the self-improvement community right now. Instead of working on goals or adjusting your routine episodically, set an intention to get better, just a little, daily. Sometimes it’s described as the 1% model. The idea is that improving your habits or work flow by 1% each day will yield compound benefits with time. It is an aggregation of marginal gains with a surprising payout. For example, daily 1% improvements would mean you are 37 times more effective by the end of a year. Now, I don’t believe this mathematical model is necessarily accurate or even necessary. But the concept that a little development done daily yields lasting improvement seems to be true.
The corollary, that if you got a little worse each day, you’d be much worse off at the end of a year, is also reasonable. That’s how most health problems set in: continuous and insidious aggregation of bad choices. Why then not use that same principle for good instead?
The Japanese thought of this idea a generation ago. Applied to manufacturing, they called it Kaizen, “continuous improvement.” It was a managerial principle that reminded people to look for opportunities to improve, just a little, wherever they were in the process and to do so each day. It led to remarkable reductions in waste and became a key to their economic success.
Opportunities for relentless improvement abound in our work too. For example, when you write an order or work up a diagnosis, rather than just enter it, you might save it as a panel. When you find yourself using the same word or phrase, save it to your dictionary to pull it up with minimal keystrokes. When you research a difficult disease you’ve not seen lately, save the diagnostic questions as a template so you can pull it up in real time the next time it walks in. When you set up your procedure tray, place items so they can be picked up efficiently and moved out of the way quickly. No matter how good your setup or template is today, you can find a tiny improvement that would make it a little better tomorrow. And you’ll reap gains from that day forward.
I don’t expect this EMR update will have much impact on my quality of life. It will however be a reminder that like flossing, improvements are best done daily. You and your dentist will thank me someday.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
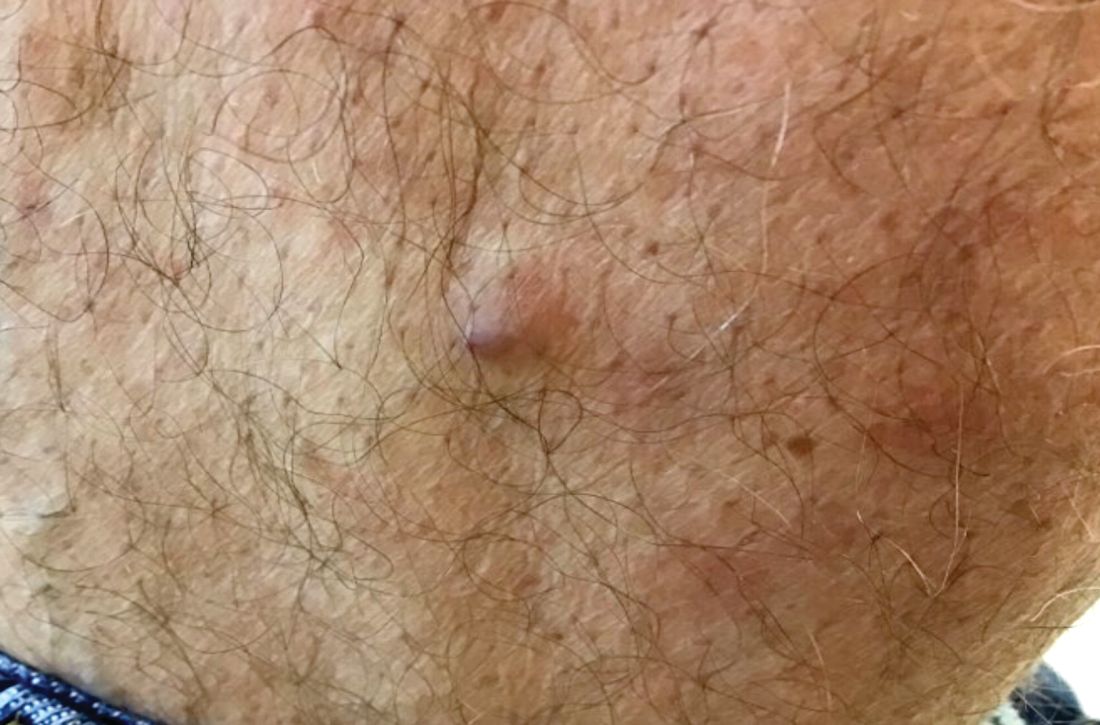
A 60-year-old white male presented with a painful nodule on the right lateral thigh that had been present for years
Benign tumors consisting of glomus cells may be subdivided into two types: glomus tumors and glomuvenous malformations or glomangiomas. Glomus cells are modified smooth muscle cells that normally line the Sucquet-Hoyer canal, an arteriovenous fistula that is involved in temperature regulation in the digits.
. In women, lesions more frequently occur on the fingers (especially nail beds). Glomus tumors are firm subcutaneous nodules, often skin colored or bluish in color. Subungual tumors tend to appear bluish under the nail plate. Lesions are extremely tender or painful, with worse pain upon palpation. Occasionally, nontender lesions can be seen.
In children, multiple nontender lesions are called glomangiomas or glomuvenous malformations. They may be sporadic or can be inherited in an autosomal dominant fashion due to a mutation in glomulin on chromosome 1p21-p22. Multiple lesions may be scattered or grouped, often in a segmental distribution. Congenital lesions tend to be large, blue-purple in color with a cobblestone appearance. They are more superficial than venous malformations.
Histologically, a proliferation of blood vessels surrounded by glomus cells is seen. Glomus cells appear as monotonous cells with a dense, round nucleus and abundant pink cytoplasm. Glomus cells can also be appreciated single-filing through pale stroma, resembling strings of black pearls. Glomus cells stain positive for smooth muscle actin and vimentin.
The painful tumor differential diagnosis has been described in the literature by the mnemonic “LEND AN EGG:” leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomus tumor, and granular cell tumor.
The malignant counterpart is glomangiosarcoma, which is a rare tumor. These lesions are often large and deeply located on the extremities. Histologically, sarcomatous areas are mixed with areas of benign glomus tumor.
Surgical excision is the treatment of choice for solitary glomus tumors to provide pain relief. Subungual tumors are more challenging due to their small size, but may be excised as well. Glomuvenous malformations may require different treatment modalities, such as surgery and laser, due to their larger size.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at www.mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Benign tumors consisting of glomus cells may be subdivided into two types: glomus tumors and glomuvenous malformations or glomangiomas. Glomus cells are modified smooth muscle cells that normally line the Sucquet-Hoyer canal, an arteriovenous fistula that is involved in temperature regulation in the digits.
. In women, lesions more frequently occur on the fingers (especially nail beds). Glomus tumors are firm subcutaneous nodules, often skin colored or bluish in color. Subungual tumors tend to appear bluish under the nail plate. Lesions are extremely tender or painful, with worse pain upon palpation. Occasionally, nontender lesions can be seen.
In children, multiple nontender lesions are called glomangiomas or glomuvenous malformations. They may be sporadic or can be inherited in an autosomal dominant fashion due to a mutation in glomulin on chromosome 1p21-p22. Multiple lesions may be scattered or grouped, often in a segmental distribution. Congenital lesions tend to be large, blue-purple in color with a cobblestone appearance. They are more superficial than venous malformations.
Histologically, a proliferation of blood vessels surrounded by glomus cells is seen. Glomus cells appear as monotonous cells with a dense, round nucleus and abundant pink cytoplasm. Glomus cells can also be appreciated single-filing through pale stroma, resembling strings of black pearls. Glomus cells stain positive for smooth muscle actin and vimentin.
The painful tumor differential diagnosis has been described in the literature by the mnemonic “LEND AN EGG:” leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomus tumor, and granular cell tumor.
The malignant counterpart is glomangiosarcoma, which is a rare tumor. These lesions are often large and deeply located on the extremities. Histologically, sarcomatous areas are mixed with areas of benign glomus tumor.
Surgical excision is the treatment of choice for solitary glomus tumors to provide pain relief. Subungual tumors are more challenging due to their small size, but may be excised as well. Glomuvenous malformations may require different treatment modalities, such as surgery and laser, due to their larger size.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at www.mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Benign tumors consisting of glomus cells may be subdivided into two types: glomus tumors and glomuvenous malformations or glomangiomas. Glomus cells are modified smooth muscle cells that normally line the Sucquet-Hoyer canal, an arteriovenous fistula that is involved in temperature regulation in the digits.
. In women, lesions more frequently occur on the fingers (especially nail beds). Glomus tumors are firm subcutaneous nodules, often skin colored or bluish in color. Subungual tumors tend to appear bluish under the nail plate. Lesions are extremely tender or painful, with worse pain upon palpation. Occasionally, nontender lesions can be seen.
In children, multiple nontender lesions are called glomangiomas or glomuvenous malformations. They may be sporadic or can be inherited in an autosomal dominant fashion due to a mutation in glomulin on chromosome 1p21-p22. Multiple lesions may be scattered or grouped, often in a segmental distribution. Congenital lesions tend to be large, blue-purple in color with a cobblestone appearance. They are more superficial than venous malformations.
Histologically, a proliferation of blood vessels surrounded by glomus cells is seen. Glomus cells appear as monotonous cells with a dense, round nucleus and abundant pink cytoplasm. Glomus cells can also be appreciated single-filing through pale stroma, resembling strings of black pearls. Glomus cells stain positive for smooth muscle actin and vimentin.
The painful tumor differential diagnosis has been described in the literature by the mnemonic “LEND AN EGG:” leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomus tumor, and granular cell tumor.
The malignant counterpart is glomangiosarcoma, which is a rare tumor. These lesions are often large and deeply located on the extremities. Histologically, sarcomatous areas are mixed with areas of benign glomus tumor.
Surgical excision is the treatment of choice for solitary glomus tumors to provide pain relief. Subungual tumors are more challenging due to their small size, but may be excised as well. Glomuvenous malformations may require different treatment modalities, such as surgery and laser, due to their larger size.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at www.mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
A 60-year-old white male presented with a painful nodule on the right lateral thigh that had been present for years. It has slowly been increasing in size over time.