User login
QI boosts adherence to protocol-based care for elevated blood lead levels
according to current guidelines, reported Courtney M. Brown, MD, and associates at Cincinnati Children’s Hospital and the University of Cincinnati.
The study protocol, undertaken at Cincinnati Children’s Hospital Medical Center’s pediatric primary care center, consisted of ordering multivitamins with iron and follow-up lead testing, educating families about identifying and reducing sources of lead exposure, and referring to a specialty environmental health clinic when indicated. Quality improvement and a real-time decision support program called Epic SmartPhrase was used to increase provider adherence. Results from patients aged 9-27 months who were seen at the hospital from February 2016 to June 2018 were included, according to the researchers. Their findings were published in Pediatrics.
Over the study period, 634 elevated blood lead levels (BLLs) were recorded. Between February 2016 – when the protocol was distributed – and May 2017 – when Epic SmartPhrase was introduced – a mean of 5% of cases received protocol-based care. After introduction of Epic Smartphase, the rate of adherence to protocol increased to 90%, which was maintained for the rest of the study.
“A reliable system for responding to BLLs is critical for optimizing outcomes for individuals, as well as activating public health systems to reduce environmental lead sources. Using tools within the EHR, we increased provider adherence with published guidelines. Our Epic SmartPhrase could be easily reproduced by other practices using EHRs. Similar strategies could be applied for standardizing the response to other laboratory tests,” the investigators wrote. “This type of intervention could ensure that screenings of all kinds trigger meaningful interventions.”
The study was supported by the Cincinnati Children’s Hospital Medical Center through the All Children Thrive community health initiative; the study authors had no relevant financial disclosures.
SOURCE: Brown CM et al. Pediatrics. 2019 May 9. doi: 10.1542/peds.2018-3085.
according to current guidelines, reported Courtney M. Brown, MD, and associates at Cincinnati Children’s Hospital and the University of Cincinnati.
The study protocol, undertaken at Cincinnati Children’s Hospital Medical Center’s pediatric primary care center, consisted of ordering multivitamins with iron and follow-up lead testing, educating families about identifying and reducing sources of lead exposure, and referring to a specialty environmental health clinic when indicated. Quality improvement and a real-time decision support program called Epic SmartPhrase was used to increase provider adherence. Results from patients aged 9-27 months who were seen at the hospital from February 2016 to June 2018 were included, according to the researchers. Their findings were published in Pediatrics.
Over the study period, 634 elevated blood lead levels (BLLs) were recorded. Between February 2016 – when the protocol was distributed – and May 2017 – when Epic SmartPhrase was introduced – a mean of 5% of cases received protocol-based care. After introduction of Epic Smartphase, the rate of adherence to protocol increased to 90%, which was maintained for the rest of the study.
“A reliable system for responding to BLLs is critical for optimizing outcomes for individuals, as well as activating public health systems to reduce environmental lead sources. Using tools within the EHR, we increased provider adherence with published guidelines. Our Epic SmartPhrase could be easily reproduced by other practices using EHRs. Similar strategies could be applied for standardizing the response to other laboratory tests,” the investigators wrote. “This type of intervention could ensure that screenings of all kinds trigger meaningful interventions.”
The study was supported by the Cincinnati Children’s Hospital Medical Center through the All Children Thrive community health initiative; the study authors had no relevant financial disclosures.
SOURCE: Brown CM et al. Pediatrics. 2019 May 9. doi: 10.1542/peds.2018-3085.
according to current guidelines, reported Courtney M. Brown, MD, and associates at Cincinnati Children’s Hospital and the University of Cincinnati.
The study protocol, undertaken at Cincinnati Children’s Hospital Medical Center’s pediatric primary care center, consisted of ordering multivitamins with iron and follow-up lead testing, educating families about identifying and reducing sources of lead exposure, and referring to a specialty environmental health clinic when indicated. Quality improvement and a real-time decision support program called Epic SmartPhrase was used to increase provider adherence. Results from patients aged 9-27 months who were seen at the hospital from February 2016 to June 2018 were included, according to the researchers. Their findings were published in Pediatrics.
Over the study period, 634 elevated blood lead levels (BLLs) were recorded. Between February 2016 – when the protocol was distributed – and May 2017 – when Epic SmartPhrase was introduced – a mean of 5% of cases received protocol-based care. After introduction of Epic Smartphase, the rate of adherence to protocol increased to 90%, which was maintained for the rest of the study.
“A reliable system for responding to BLLs is critical for optimizing outcomes for individuals, as well as activating public health systems to reduce environmental lead sources. Using tools within the EHR, we increased provider adherence with published guidelines. Our Epic SmartPhrase could be easily reproduced by other practices using EHRs. Similar strategies could be applied for standardizing the response to other laboratory tests,” the investigators wrote. “This type of intervention could ensure that screenings of all kinds trigger meaningful interventions.”
The study was supported by the Cincinnati Children’s Hospital Medical Center through the All Children Thrive community health initiative; the study authors had no relevant financial disclosures.
SOURCE: Brown CM et al. Pediatrics. 2019 May 9. doi: 10.1542/peds.2018-3085.
FROM PEDIATRICS
Study finds lower quality of life for patients with hemophilia A
Real‐world data suggests that patients with hemophilia A with inhibitors had lower health-related quality of life (HRQOL) while receiving standard therapy, according to an international study.
“The objective of this analysis was to characterize disease‐specific HRQOL, overall health status and the effect of bleeding on health status,” wrote Johnny Mahlangu, MD, of the University of the Witwatersrand in Johannesburg, South Africa, and colleagues. The study was published in Haemophilia.
The researchers conducted a prospective, noninterventional study of 103 patients aged 12 years and older with hemophilia A who resided in several different countries, including Australia, Japan, South Africa, and the United States, among others.
The majority of participants (n = 75) received episodic treatment at study enrollment, while others (n = 28) received prophylactic-based therapy. Patients were treated with standard therapy, based on local institutional practice.
HRQOL outcome data were collected in adult and adolescent participants using the Haemophilia Quality of Life Questionnaire for Adults and the Haemophilia‐specific Quality of Life Questionnaire for Children Short Form. Other validated instruments were used to measure additional health‐related outcomes.
After analysis, the researchers found that HRQOL scores revealed impaired quality of life in adult and adolescent participants treated with both episodic and prophylactic regimens. The mean scores in the majority of HRQOL domains showed impairments occurring on average “sometimes” to “often,” the researchers reported.
Adults had highest scores, correlated with greatest impairments, in sports and leisure. Similarly, adolescents reported greatest impairment in the sports and school domain.
“These health‐related outcomes may result from a combination of poor bleed control and treatment burden,” the researchers wrote. “Compliance with prophylactic treatment was low, likely reflecting the high burden associated with standard therapies.”
The researchers acknowledged a key limitation of the study was participant dropout. As a result, some time-related data may be incomplete.
“These [data] demonstrate that patients with hemophilia A with inhibitors have impaired HRQOL, despite standard treatment, and that more effective treatment options are needed,” the researchers concluded.
The study was funded by F. Hoffmann-La Roche. The authors reported financial affiliations with Baxalta, Bayer, CSL Behring, Kaketsuken, Novo Nordisk, Pfizer, and several others.
SOURCE: Mahlangu J et al. Haemophilia. 2019 Apr 24. doi: 10.1111/hae.13731.
Real‐world data suggests that patients with hemophilia A with inhibitors had lower health-related quality of life (HRQOL) while receiving standard therapy, according to an international study.
“The objective of this analysis was to characterize disease‐specific HRQOL, overall health status and the effect of bleeding on health status,” wrote Johnny Mahlangu, MD, of the University of the Witwatersrand in Johannesburg, South Africa, and colleagues. The study was published in Haemophilia.
The researchers conducted a prospective, noninterventional study of 103 patients aged 12 years and older with hemophilia A who resided in several different countries, including Australia, Japan, South Africa, and the United States, among others.
The majority of participants (n = 75) received episodic treatment at study enrollment, while others (n = 28) received prophylactic-based therapy. Patients were treated with standard therapy, based on local institutional practice.
HRQOL outcome data were collected in adult and adolescent participants using the Haemophilia Quality of Life Questionnaire for Adults and the Haemophilia‐specific Quality of Life Questionnaire for Children Short Form. Other validated instruments were used to measure additional health‐related outcomes.
After analysis, the researchers found that HRQOL scores revealed impaired quality of life in adult and adolescent participants treated with both episodic and prophylactic regimens. The mean scores in the majority of HRQOL domains showed impairments occurring on average “sometimes” to “often,” the researchers reported.
Adults had highest scores, correlated with greatest impairments, in sports and leisure. Similarly, adolescents reported greatest impairment in the sports and school domain.
“These health‐related outcomes may result from a combination of poor bleed control and treatment burden,” the researchers wrote. “Compliance with prophylactic treatment was low, likely reflecting the high burden associated with standard therapies.”
The researchers acknowledged a key limitation of the study was participant dropout. As a result, some time-related data may be incomplete.
“These [data] demonstrate that patients with hemophilia A with inhibitors have impaired HRQOL, despite standard treatment, and that more effective treatment options are needed,” the researchers concluded.
The study was funded by F. Hoffmann-La Roche. The authors reported financial affiliations with Baxalta, Bayer, CSL Behring, Kaketsuken, Novo Nordisk, Pfizer, and several others.
SOURCE: Mahlangu J et al. Haemophilia. 2019 Apr 24. doi: 10.1111/hae.13731.
Real‐world data suggests that patients with hemophilia A with inhibitors had lower health-related quality of life (HRQOL) while receiving standard therapy, according to an international study.
“The objective of this analysis was to characterize disease‐specific HRQOL, overall health status and the effect of bleeding on health status,” wrote Johnny Mahlangu, MD, of the University of the Witwatersrand in Johannesburg, South Africa, and colleagues. The study was published in Haemophilia.
The researchers conducted a prospective, noninterventional study of 103 patients aged 12 years and older with hemophilia A who resided in several different countries, including Australia, Japan, South Africa, and the United States, among others.
The majority of participants (n = 75) received episodic treatment at study enrollment, while others (n = 28) received prophylactic-based therapy. Patients were treated with standard therapy, based on local institutional practice.
HRQOL outcome data were collected in adult and adolescent participants using the Haemophilia Quality of Life Questionnaire for Adults and the Haemophilia‐specific Quality of Life Questionnaire for Children Short Form. Other validated instruments were used to measure additional health‐related outcomes.
After analysis, the researchers found that HRQOL scores revealed impaired quality of life in adult and adolescent participants treated with both episodic and prophylactic regimens. The mean scores in the majority of HRQOL domains showed impairments occurring on average “sometimes” to “often,” the researchers reported.
Adults had highest scores, correlated with greatest impairments, in sports and leisure. Similarly, adolescents reported greatest impairment in the sports and school domain.
“These health‐related outcomes may result from a combination of poor bleed control and treatment burden,” the researchers wrote. “Compliance with prophylactic treatment was low, likely reflecting the high burden associated with standard therapies.”
The researchers acknowledged a key limitation of the study was participant dropout. As a result, some time-related data may be incomplete.
“These [data] demonstrate that patients with hemophilia A with inhibitors have impaired HRQOL, despite standard treatment, and that more effective treatment options are needed,” the researchers concluded.
The study was funded by F. Hoffmann-La Roche. The authors reported financial affiliations with Baxalta, Bayer, CSL Behring, Kaketsuken, Novo Nordisk, Pfizer, and several others.
SOURCE: Mahlangu J et al. Haemophilia. 2019 Apr 24. doi: 10.1111/hae.13731.
FROM HAEMOPHILIA
Unrecognized focal stenosis after angiographically successful PCI
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
REPORTING FROM ACC 19
Can an antisense oligonucleotide benefit patients with SOD1-ALS?
PHILADELPHIA – In patients with amyotrophic lateral sclerosis (ALS) caused by gain-of-toxic function mutations in the SOD1 gene, according to phase 1/2 trial results presented at the annual meeting of the American Academy of Neurology. Exploratory analyses suggest that the drug, known as tofersen (also known as BIIB067), may lessen declines in function, respiratory function, and strength. The treatment generally was safe and well tolerated, researchers said.
Most ALS cases are sporadic, but about 10% are genetic, of which approximately 20% are caused by SOD1 mutations. “Although SOD1-ALS disease progression is heterogeneous, the underlying pathophysiology, attributable to mutant SOD1 toxicity, is thought to be consistent across SOD1 mutation types,” said Timothy M. Miller, MD, PhD, of Washington University, St. Louis, and colleagues. “As such, effective reduction of SOD1 protein, irrespective of mutation, has the potential to alter the disease course of people with SOD1-ALS.”
Tofersen is an antisense oligonucleotide ribonuclease H1-mediated inhibitor of SOD1 messenger RNA. To study its safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy in patients with SOD1-ALS, investigators conducted a double-blind study. The investigators randomized 50 patients with ALS with a SOD1 mutation to 20 mg (n = 10), 40 mg (n = 9), 60 mg (n = 9), or 100 mg (n = 10) of tofersen or placebo (n = 12).
Patients received tofersen by intrathecal bolus over 1-3 minutes. They received a loading regimen on days 1, 15, and 29 and maintenance dosing on days 57 and 85. After the dosing period, patients completed a 12-week follow-up period.
Adverse events
All patients received at least one dose of the study treatment, and 48 received all treatments. Three patients died during the study. One patient in the 20-mg group died of pulmonary embolism, and one patient in both the 60-mg group and placebo group died of respiratory failure. Investigators considered the deaths secondary to ALS or other conditions and not drug related.
Most adverse events were mild or moderate. The most common adverse events among tofersen-treated patients were headache (n = 16), procedural pain (n = 14), and post–lumbar puncture syndrome (n = 13). Five patients who received tofersen and two who received placebo experienced serious adverse events; no serious adverse events occurred in the 100-mg dose group.
“A reduction from baseline in CSF SOD1 concentrations was observed in the tofersen 40-, 60-, and 100-mg cohorts with the maximal reduction observed in the 100 mg–treated group [37% vs. no reduction in the placebo group; P less than 0.002] at day 85,” the investigators reported.
Possible efficacy
In exploratory analyses, the 100-mg dose slowed decline on the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ASLFRS-R), compared with placebo. Mean change in ASLFRS-R from baseline to day 85 was –1.1 in patients who received 100 mg of tofersen, compared with –5.3 for patients who received placebo. Declines on measures of respiratory function and muscle strength also slowed. “Across clinical measures, separation from placebo was most apparent in participants with fast progressing disease,” the researchers said.
“Lower concentrations of the protein in the spinal fluid suggest that there were also lower concentrations in the brain and spinal cord,” Dr. Miller said in a news release. “Such reductions could lead to preservation of motor neurons and slow progression of the disease, but more study is needed to examine this further.”
The study was sponsored by Biogen, which is developing tofersen. Some of the study authors are Biogen employees. Dr. Miller is on Biogen’s medical advisory board and receives clinical research support from Biogen. In addition, he consults, has licensing agreements with, and is a principal investigator for other companies.
PHILADELPHIA – In patients with amyotrophic lateral sclerosis (ALS) caused by gain-of-toxic function mutations in the SOD1 gene, according to phase 1/2 trial results presented at the annual meeting of the American Academy of Neurology. Exploratory analyses suggest that the drug, known as tofersen (also known as BIIB067), may lessen declines in function, respiratory function, and strength. The treatment generally was safe and well tolerated, researchers said.
Most ALS cases are sporadic, but about 10% are genetic, of which approximately 20% are caused by SOD1 mutations. “Although SOD1-ALS disease progression is heterogeneous, the underlying pathophysiology, attributable to mutant SOD1 toxicity, is thought to be consistent across SOD1 mutation types,” said Timothy M. Miller, MD, PhD, of Washington University, St. Louis, and colleagues. “As such, effective reduction of SOD1 protein, irrespective of mutation, has the potential to alter the disease course of people with SOD1-ALS.”
Tofersen is an antisense oligonucleotide ribonuclease H1-mediated inhibitor of SOD1 messenger RNA. To study its safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy in patients with SOD1-ALS, investigators conducted a double-blind study. The investigators randomized 50 patients with ALS with a SOD1 mutation to 20 mg (n = 10), 40 mg (n = 9), 60 mg (n = 9), or 100 mg (n = 10) of tofersen or placebo (n = 12).
Patients received tofersen by intrathecal bolus over 1-3 minutes. They received a loading regimen on days 1, 15, and 29 and maintenance dosing on days 57 and 85. After the dosing period, patients completed a 12-week follow-up period.
Adverse events
All patients received at least one dose of the study treatment, and 48 received all treatments. Three patients died during the study. One patient in the 20-mg group died of pulmonary embolism, and one patient in both the 60-mg group and placebo group died of respiratory failure. Investigators considered the deaths secondary to ALS or other conditions and not drug related.
Most adverse events were mild or moderate. The most common adverse events among tofersen-treated patients were headache (n = 16), procedural pain (n = 14), and post–lumbar puncture syndrome (n = 13). Five patients who received tofersen and two who received placebo experienced serious adverse events; no serious adverse events occurred in the 100-mg dose group.
“A reduction from baseline in CSF SOD1 concentrations was observed in the tofersen 40-, 60-, and 100-mg cohorts with the maximal reduction observed in the 100 mg–treated group [37% vs. no reduction in the placebo group; P less than 0.002] at day 85,” the investigators reported.
Possible efficacy
In exploratory analyses, the 100-mg dose slowed decline on the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ASLFRS-R), compared with placebo. Mean change in ASLFRS-R from baseline to day 85 was –1.1 in patients who received 100 mg of tofersen, compared with –5.3 for patients who received placebo. Declines on measures of respiratory function and muscle strength also slowed. “Across clinical measures, separation from placebo was most apparent in participants with fast progressing disease,” the researchers said.
“Lower concentrations of the protein in the spinal fluid suggest that there were also lower concentrations in the brain and spinal cord,” Dr. Miller said in a news release. “Such reductions could lead to preservation of motor neurons and slow progression of the disease, but more study is needed to examine this further.”
The study was sponsored by Biogen, which is developing tofersen. Some of the study authors are Biogen employees. Dr. Miller is on Biogen’s medical advisory board and receives clinical research support from Biogen. In addition, he consults, has licensing agreements with, and is a principal investigator for other companies.
PHILADELPHIA – In patients with amyotrophic lateral sclerosis (ALS) caused by gain-of-toxic function mutations in the SOD1 gene, according to phase 1/2 trial results presented at the annual meeting of the American Academy of Neurology. Exploratory analyses suggest that the drug, known as tofersen (also known as BIIB067), may lessen declines in function, respiratory function, and strength. The treatment generally was safe and well tolerated, researchers said.
Most ALS cases are sporadic, but about 10% are genetic, of which approximately 20% are caused by SOD1 mutations. “Although SOD1-ALS disease progression is heterogeneous, the underlying pathophysiology, attributable to mutant SOD1 toxicity, is thought to be consistent across SOD1 mutation types,” said Timothy M. Miller, MD, PhD, of Washington University, St. Louis, and colleagues. “As such, effective reduction of SOD1 protein, irrespective of mutation, has the potential to alter the disease course of people with SOD1-ALS.”
Tofersen is an antisense oligonucleotide ribonuclease H1-mediated inhibitor of SOD1 messenger RNA. To study its safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy in patients with SOD1-ALS, investigators conducted a double-blind study. The investigators randomized 50 patients with ALS with a SOD1 mutation to 20 mg (n = 10), 40 mg (n = 9), 60 mg (n = 9), or 100 mg (n = 10) of tofersen or placebo (n = 12).
Patients received tofersen by intrathecal bolus over 1-3 minutes. They received a loading regimen on days 1, 15, and 29 and maintenance dosing on days 57 and 85. After the dosing period, patients completed a 12-week follow-up period.
Adverse events
All patients received at least one dose of the study treatment, and 48 received all treatments. Three patients died during the study. One patient in the 20-mg group died of pulmonary embolism, and one patient in both the 60-mg group and placebo group died of respiratory failure. Investigators considered the deaths secondary to ALS or other conditions and not drug related.
Most adverse events were mild or moderate. The most common adverse events among tofersen-treated patients were headache (n = 16), procedural pain (n = 14), and post–lumbar puncture syndrome (n = 13). Five patients who received tofersen and two who received placebo experienced serious adverse events; no serious adverse events occurred in the 100-mg dose group.
“A reduction from baseline in CSF SOD1 concentrations was observed in the tofersen 40-, 60-, and 100-mg cohorts with the maximal reduction observed in the 100 mg–treated group [37% vs. no reduction in the placebo group; P less than 0.002] at day 85,” the investigators reported.
Possible efficacy
In exploratory analyses, the 100-mg dose slowed decline on the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ASLFRS-R), compared with placebo. Mean change in ASLFRS-R from baseline to day 85 was –1.1 in patients who received 100 mg of tofersen, compared with –5.3 for patients who received placebo. Declines on measures of respiratory function and muscle strength also slowed. “Across clinical measures, separation from placebo was most apparent in participants with fast progressing disease,” the researchers said.
“Lower concentrations of the protein in the spinal fluid suggest that there were also lower concentrations in the brain and spinal cord,” Dr. Miller said in a news release. “Such reductions could lead to preservation of motor neurons and slow progression of the disease, but more study is needed to examine this further.”
The study was sponsored by Biogen, which is developing tofersen. Some of the study authors are Biogen employees. Dr. Miller is on Biogen’s medical advisory board and receives clinical research support from Biogen. In addition, he consults, has licensing agreements with, and is a principal investigator for other companies.
REPORTING FROM AAN 2019
Experts discuss what’s new in migraine treatment
PHILADELPHIA – At the annual meeting of the American Academy of Neurology, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, sat down with Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., to discuss in a video some of the new data presented at the meeting regarding the CGRP monoclonal antibodies, the small molecule receptor antagonists (gepants), and what Dr. Tepper described as “a real shift in paradigm and a watershed moment in migraine.”
The three gepants that are farthest along in clinical trials are ubrogepant, rimegepant, and atogepant. “Reassuring data” was presented, Dr. Tepper said, regarding liver toxicity, which has been a concern with earlier generations of the gepants. The Food and Drug Administration had mandated a close look at liver function with the use of these drugs, which are metabolized in the liver, and, to date, no safety signals have emerged.
The three CGRP monoclonal antibodies that are currently on the market are erenumab (Aimovig), fremanezumab (Ajovy), and galcanezumab (Emgality). Data from numerous open-label extension studies were presented. In general, it seems that “the monoclonal antibodies accumulate greater efficacy over time,” Dr. Tepper said. No safety concerns have emerged from 5 years of clinical trial data. With 250,000 patients on these drugs worldwide, that is “very reassuring,” Dr. Tepper said.
New data also show that the majority of patients with chronic migraine who are taking monoclonal antibodies convert from chronic migraine to episodic migraine. Additionally, new data show that use of monoclonal antibodies “dramatically reduce all migraine medication use,” Dr. Tepper said.
PHILADELPHIA – At the annual meeting of the American Academy of Neurology, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, sat down with Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., to discuss in a video some of the new data presented at the meeting regarding the CGRP monoclonal antibodies, the small molecule receptor antagonists (gepants), and what Dr. Tepper described as “a real shift in paradigm and a watershed moment in migraine.”
The three gepants that are farthest along in clinical trials are ubrogepant, rimegepant, and atogepant. “Reassuring data” was presented, Dr. Tepper said, regarding liver toxicity, which has been a concern with earlier generations of the gepants. The Food and Drug Administration had mandated a close look at liver function with the use of these drugs, which are metabolized in the liver, and, to date, no safety signals have emerged.
The three CGRP monoclonal antibodies that are currently on the market are erenumab (Aimovig), fremanezumab (Ajovy), and galcanezumab (Emgality). Data from numerous open-label extension studies were presented. In general, it seems that “the monoclonal antibodies accumulate greater efficacy over time,” Dr. Tepper said. No safety concerns have emerged from 5 years of clinical trial data. With 250,000 patients on these drugs worldwide, that is “very reassuring,” Dr. Tepper said.
New data also show that the majority of patients with chronic migraine who are taking monoclonal antibodies convert from chronic migraine to episodic migraine. Additionally, new data show that use of monoclonal antibodies “dramatically reduce all migraine medication use,” Dr. Tepper said.
PHILADELPHIA – At the annual meeting of the American Academy of Neurology, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, sat down with Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., to discuss in a video some of the new data presented at the meeting regarding the CGRP monoclonal antibodies, the small molecule receptor antagonists (gepants), and what Dr. Tepper described as “a real shift in paradigm and a watershed moment in migraine.”
The three gepants that are farthest along in clinical trials are ubrogepant, rimegepant, and atogepant. “Reassuring data” was presented, Dr. Tepper said, regarding liver toxicity, which has been a concern with earlier generations of the gepants. The Food and Drug Administration had mandated a close look at liver function with the use of these drugs, which are metabolized in the liver, and, to date, no safety signals have emerged.
The three CGRP monoclonal antibodies that are currently on the market are erenumab (Aimovig), fremanezumab (Ajovy), and galcanezumab (Emgality). Data from numerous open-label extension studies were presented. In general, it seems that “the monoclonal antibodies accumulate greater efficacy over time,” Dr. Tepper said. No safety concerns have emerged from 5 years of clinical trial data. With 250,000 patients on these drugs worldwide, that is “very reassuring,” Dr. Tepper said.
New data also show that the majority of patients with chronic migraine who are taking monoclonal antibodies convert from chronic migraine to episodic migraine. Additionally, new data show that use of monoclonal antibodies “dramatically reduce all migraine medication use,” Dr. Tepper said.
EXPERT ANALYSIS FROM AAN 2019
Appendectomy linked to increased risk of subsequent Parkinson’s
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
REPORTING FROM DDW 2019
Key clinical point: Appendectomy appears to increase the risk of Parkinson’s disease.
Major finding: The overall relative risk of developing Parkinson’s disease in patients after appendectomy was 3.19 (95% CI, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure.
Study details: A population-based study of more than 62 million medical records from a national database.
Disclosures: The researchers reported having no financial disclosures.
Source: Sheriff MZ et al. DDW 2019, Abstract 739.
A Literally Massive Problem
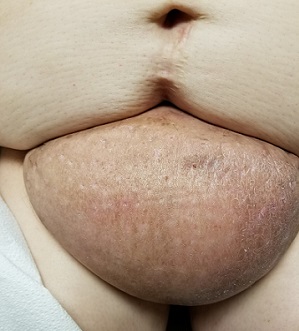
A 60-year-old woman presents to dermatology with a longstanding complaint of a tender, irritated mass hanging from her lower abdomen. The patient says it started as a large fold in her lower abdomen but over the years has grown and become more pendulous. It is now large enough to interfere with normal activity, including walking.
Numerous providers, dermatologists included, have rendered diagnoses—most recently, hidradenitis suppurativa. The antibiotics prescribed for that diagnosis have not helped, however. Similarly, cultures performed on samples from draining sores on the mass’s posterior have failed to illuminate the situation, showing only mixed normal flora.
The patient’s primary care provider referred her to surgery for consideration of removal, or at least reduction, of the mass. The surgeon offered a presumptive diagnosis of elephantiasis nostras verrucosa of the pannus and agreed to perform the surgery. But the patient’s primary care provider requested a second opinion from dermatology.
EXAMINATION
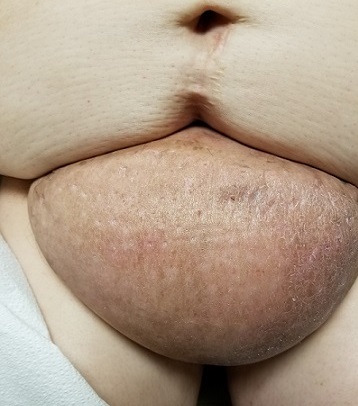
The edematous, pendulous mass is the size of a soccer ball and hangs down from its abdominal base. When the patient stands, the mass stretches almost to the level of her knees. The anterior surface is edematous but otherwise normal in appearance. The intertriginous surfaces of the lesion look entirely different, with multiple small, draining puncta and a few open comedones.
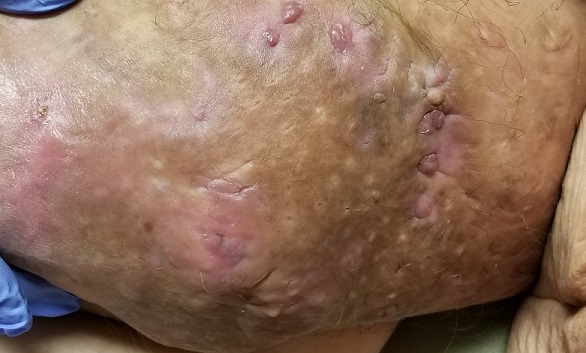
The body of the mass is quite indurated but is neither hot nor tender. There are no comedones or cysts in other intertriginous locations, as might be seen in hidradenitis.
What’s the diagnosis?
DISCUSSION
This case involves a rare entity: vast lymphedema of the pannus leading to the formation of a pendulous mass so large that it filled the space between the patient’s legs, causing pain and discomfort. These findings are analogous to those seen in advanced venous insufficiency. Both manifestations share a name: elephantiasis nostras verrucosa. (Neither has anything to do with the more notorious cases of filarial elephantiasis seen in tropical locations.)
Elephantiasis nostras verrucosa of the lower extremities involves striking skin changes: edema, along with extreme thickening, papularity, and roughness of the skin. These typically manifest downward from just below the knee. The condition represents the effects of late-stage chronic venous insufficiency, often worsened by obesity and a sedentary lifestyle. Other causes of dependent lymphedema, such as congestive heart failure, can also contribute to the problem.
This same pathophysiologic process can affect other areas as well—including the pannus, as seen in this case. Since I had only ever encountered this problem in legs, I did a literature search. I found several references, all of which indicated that surgical removal (panniculectomy) was the best treatment. I could not find any information on the success rate of this surgery, but I did refer the patient back to the surgeon, who had made the correct diagnosis.
TAKE-HOME LEARNING POINTS
- Elephantiasis nostras verrucosa (ENV) is a consequence of uncontrolled venous insufficiency that commonly manifests on lower extremities.
- ENV is a distinctly rare (though not unknown) problem when areas other than legs are affected.
- This patient’s condition is, in my opinion, beyond the reach of medical treatment. But in milder cases, approaches such as weight loss and use of diuretics have been tried with mixed success.
- The best treatment appears to be surgical removal, which is not without potential complications: risk for infection, pain, prolonged recovery time, and wound dehiscence; these issues were discussed thoroughly with the case patient.
A 60-year-old woman presents to dermatology with a longstanding complaint of a tender, irritated mass hanging from her lower abdomen. The patient says it started as a large fold in her lower abdomen but over the years has grown and become more pendulous. It is now large enough to interfere with normal activity, including walking.
Numerous providers, dermatologists included, have rendered diagnoses—most recently, hidradenitis suppurativa. The antibiotics prescribed for that diagnosis have not helped, however. Similarly, cultures performed on samples from draining sores on the mass’s posterior have failed to illuminate the situation, showing only mixed normal flora.
The patient’s primary care provider referred her to surgery for consideration of removal, or at least reduction, of the mass. The surgeon offered a presumptive diagnosis of elephantiasis nostras verrucosa of the pannus and agreed to perform the surgery. But the patient’s primary care provider requested a second opinion from dermatology.

EXAMINATION
The edematous, pendulous mass is the size of a soccer ball and hangs down from its abdominal base. When the patient stands, the mass stretches almost to the level of her knees. The anterior surface is edematous but otherwise normal in appearance. The intertriginous surfaces of the lesion look entirely different, with multiple small, draining puncta and a few open comedones.

The body of the mass is quite indurated but is neither hot nor tender. There are no comedones or cysts in other intertriginous locations, as might be seen in hidradenitis.
What’s the diagnosis?
DISCUSSION
This case involves a rare entity: vast lymphedema of the pannus leading to the formation of a pendulous mass so large that it filled the space between the patient’s legs, causing pain and discomfort. These findings are analogous to those seen in advanced venous insufficiency. Both manifestations share a name: elephantiasis nostras verrucosa. (Neither has anything to do with the more notorious cases of filarial elephantiasis seen in tropical locations.)
Elephantiasis nostras verrucosa of the lower extremities involves striking skin changes: edema, along with extreme thickening, papularity, and roughness of the skin. These typically manifest downward from just below the knee. The condition represents the effects of late-stage chronic venous insufficiency, often worsened by obesity and a sedentary lifestyle. Other causes of dependent lymphedema, such as congestive heart failure, can also contribute to the problem.
This same pathophysiologic process can affect other areas as well—including the pannus, as seen in this case. Since I had only ever encountered this problem in legs, I did a literature search. I found several references, all of which indicated that surgical removal (panniculectomy) was the best treatment. I could not find any information on the success rate of this surgery, but I did refer the patient back to the surgeon, who had made the correct diagnosis.
TAKE-HOME LEARNING POINTS
- Elephantiasis nostras verrucosa (ENV) is a consequence of uncontrolled venous insufficiency that commonly manifests on lower extremities.
- ENV is a distinctly rare (though not unknown) problem when areas other than legs are affected.
- This patient’s condition is, in my opinion, beyond the reach of medical treatment. But in milder cases, approaches such as weight loss and use of diuretics have been tried with mixed success.
- The best treatment appears to be surgical removal, which is not without potential complications: risk for infection, pain, prolonged recovery time, and wound dehiscence; these issues were discussed thoroughly with the case patient.
A 60-year-old woman presents to dermatology with a longstanding complaint of a tender, irritated mass hanging from her lower abdomen. The patient says it started as a large fold in her lower abdomen but over the years has grown and become more pendulous. It is now large enough to interfere with normal activity, including walking.
Numerous providers, dermatologists included, have rendered diagnoses—most recently, hidradenitis suppurativa. The antibiotics prescribed for that diagnosis have not helped, however. Similarly, cultures performed on samples from draining sores on the mass’s posterior have failed to illuminate the situation, showing only mixed normal flora.
The patient’s primary care provider referred her to surgery for consideration of removal, or at least reduction, of the mass. The surgeon offered a presumptive diagnosis of elephantiasis nostras verrucosa of the pannus and agreed to perform the surgery. But the patient’s primary care provider requested a second opinion from dermatology.

EXAMINATION
The edematous, pendulous mass is the size of a soccer ball and hangs down from its abdominal base. When the patient stands, the mass stretches almost to the level of her knees. The anterior surface is edematous but otherwise normal in appearance. The intertriginous surfaces of the lesion look entirely different, with multiple small, draining puncta and a few open comedones.

The body of the mass is quite indurated but is neither hot nor tender. There are no comedones or cysts in other intertriginous locations, as might be seen in hidradenitis.
What’s the diagnosis?
DISCUSSION
This case involves a rare entity: vast lymphedema of the pannus leading to the formation of a pendulous mass so large that it filled the space between the patient’s legs, causing pain and discomfort. These findings are analogous to those seen in advanced venous insufficiency. Both manifestations share a name: elephantiasis nostras verrucosa. (Neither has anything to do with the more notorious cases of filarial elephantiasis seen in tropical locations.)
Elephantiasis nostras verrucosa of the lower extremities involves striking skin changes: edema, along with extreme thickening, papularity, and roughness of the skin. These typically manifest downward from just below the knee. The condition represents the effects of late-stage chronic venous insufficiency, often worsened by obesity and a sedentary lifestyle. Other causes of dependent lymphedema, such as congestive heart failure, can also contribute to the problem.
This same pathophysiologic process can affect other areas as well—including the pannus, as seen in this case. Since I had only ever encountered this problem in legs, I did a literature search. I found several references, all of which indicated that surgical removal (panniculectomy) was the best treatment. I could not find any information on the success rate of this surgery, but I did refer the patient back to the surgeon, who had made the correct diagnosis.
TAKE-HOME LEARNING POINTS
- Elephantiasis nostras verrucosa (ENV) is a consequence of uncontrolled venous insufficiency that commonly manifests on lower extremities.
- ENV is a distinctly rare (though not unknown) problem when areas other than legs are affected.
- This patient’s condition is, in my opinion, beyond the reach of medical treatment. But in milder cases, approaches such as weight loss and use of diuretics have been tried with mixed success.
- The best treatment appears to be surgical removal, which is not without potential complications: risk for infection, pain, prolonged recovery time, and wound dehiscence; these issues were discussed thoroughly with the case patient.
Widespread hyperpigmented plaques
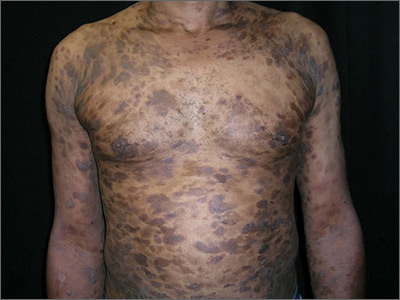
The differential diagnosis included psoriasis, drug eruption, and a cutaneous T-cell lymphoma.
A drug eruption could have been due to an over-the-counter medication or supplement, so the lack of improvement from stopping the antihypertensive medication did not rule out this diagnosis. Psoriasis does not always show erythema in persons of color, but these plaques were not typical of psoriasis. (There also were some flat patches that were even less typical of psoriasis.)
The FP performed a 4-mm punch biopsy on one of the hyperpigmented plaques on the abdomen. A 4-mm punch biopsy is generally an ideal method for determining the cause of an unknown skin rash, and it is usually better to choose a lesion on the upper body rather than below the waist if the rash is widespread. (See the Watch & Learn video on “Punch biopsy.”)
The FP also prescribed a 1-pound tub of 0.1% triamcinolone ointment for symptomatic relief as this could help any of the possible diagnoses being considered. The pathology report came back as mycosis fungoides, the most common type of cutaneous T-cell lymphoma.
The patient was sent to Hematology/Oncology for further evaluation and treatment. Mycosis fungoides can have both patches and plaques and frequently involves the trunk more than the extremities (which was the situation in this case). It is important to consider uncommon diagnoses like this in the differential when the initial diagnosis does not appear to be responding to treatment or there is something atypical about the presentation of an expected diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The differential diagnosis included psoriasis, drug eruption, and a cutaneous T-cell lymphoma.
A drug eruption could have been due to an over-the-counter medication or supplement, so the lack of improvement from stopping the antihypertensive medication did not rule out this diagnosis. Psoriasis does not always show erythema in persons of color, but these plaques were not typical of psoriasis. (There also were some flat patches that were even less typical of psoriasis.)
The FP performed a 4-mm punch biopsy on one of the hyperpigmented plaques on the abdomen. A 4-mm punch biopsy is generally an ideal method for determining the cause of an unknown skin rash, and it is usually better to choose a lesion on the upper body rather than below the waist if the rash is widespread. (See the Watch & Learn video on “Punch biopsy.”)
The FP also prescribed a 1-pound tub of 0.1% triamcinolone ointment for symptomatic relief as this could help any of the possible diagnoses being considered. The pathology report came back as mycosis fungoides, the most common type of cutaneous T-cell lymphoma.
The patient was sent to Hematology/Oncology for further evaluation and treatment. Mycosis fungoides can have both patches and plaques and frequently involves the trunk more than the extremities (which was the situation in this case). It is important to consider uncommon diagnoses like this in the differential when the initial diagnosis does not appear to be responding to treatment or there is something atypical about the presentation of an expected diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The differential diagnosis included psoriasis, drug eruption, and a cutaneous T-cell lymphoma.
A drug eruption could have been due to an over-the-counter medication or supplement, so the lack of improvement from stopping the antihypertensive medication did not rule out this diagnosis. Psoriasis does not always show erythema in persons of color, but these plaques were not typical of psoriasis. (There also were some flat patches that were even less typical of psoriasis.)
The FP performed a 4-mm punch biopsy on one of the hyperpigmented plaques on the abdomen. A 4-mm punch biopsy is generally an ideal method for determining the cause of an unknown skin rash, and it is usually better to choose a lesion on the upper body rather than below the waist if the rash is widespread. (See the Watch & Learn video on “Punch biopsy.”)
The FP also prescribed a 1-pound tub of 0.1% triamcinolone ointment for symptomatic relief as this could help any of the possible diagnoses being considered. The pathology report came back as mycosis fungoides, the most common type of cutaneous T-cell lymphoma.
The patient was sent to Hematology/Oncology for further evaluation and treatment. Mycosis fungoides can have both patches and plaques and frequently involves the trunk more than the extremities (which was the situation in this case). It is important to consider uncommon diagnoses like this in the differential when the initial diagnosis does not appear to be responding to treatment or there is something atypical about the presentation of an expected diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
LC screening. microRNAs. Impulse oscillometry. PH definition change. LC & women
Interventional Chest/Diagnostic Procedures
Complications and economic burden of diagnostic procedures for lung abnormalities in the community setting
The influential National Lung Screening Trial (NLST) reported a 20% reduction in lung cancer-related deaths using low dose CT scan when compared with plain chest radiography (Aberle et al. N Engl J Med. 2011;365[5]:395). Many medical societies responded by recommending screening individuals at high-risk for lung cancer, and community-based lung cancer screening programs were developed across the US. A concerning feature of the study was the rate (23.3%) of false-positive findings after three rounds of screening and the potential for complications secondary to diagnostic invasive procedures.
Using a 2008-2013 cohort of community inpatient and outpatient practice settings, Hou and colleagues searched administrative databases for procedure and diagnostic codes used in the NLST (Hou et al. JAMA Intern Med. 2019;179[3]:324). The study team created an age-matched control cohort that did not have an invasive procedure and used the difference in complications rates as an indicator of a procedure-related complication. Additionally, they estimated 1-year medical costs associated with complications. More than 340,000 patients were included in the study, and the overall complication rate was far higher than what was reported in the NLST. This difference was more pronounced in the older group in the study cohort (23.8% vs 8.5%). The associated economic burden of complications was substantial, and cost more than the initial procedure itself.
Although this was not a lung cancer screening cohort and used an administrative database, some valuable lessons can be offered from this study. First, complication rates of procedures like those performed in the NLST are likely to be higher in low-volume centers. Second, in order to minimize procedures, associated complications, and costs, we should be cognizant of the diagnostic limitations of each type of intervention when evaluating patients with lung nodules, wisely choosing the correct procedure for the correct patient after multidisciplinary discussion. We should seek to minimize biopsies of lesions that are likely benign. Third, it is evident that more research is needed regarding this topic. The ideal study would need to include both academic and community-based lung cancer screening programs, and, prospectively, analyze the diagnostic yield and complication rates, as well as downstream costs. Finally, the results of this study call all of us to properly follow the lung cancer screening guidelines and reconcile them with our common sense when evaluating a patient with a screen-detected nodule. Injudicious testing invites unnecessary complications, increases the cost of care, and diverts resources from those more likely to benefit from appropriate interventions.
Jose Cardenas-Garcia, MD, FCCP
Steering Committee Member
Douglas Arenberg, MD, FCCP
NetWork Member
Pediatric Chest Medicine
microRNAs: A New Biomarker
Biomarkers are essential tools in a clinician’s armamentarium. Biomarkers have multiple uses being indicators of a pathologic or physiologic process. One promising biomarker, now studied across multiple disorders, is microRNA (miRNA).
miRNAs are short (18–22 nucleotide) regulatory RNAs that bind mRNAs and decrease protein translation. miRNAs are generally co-transcribed with neighboring genes or co-transcribed within a cluster of miRNAs (a polycistronic cluster). Over 2,000 miRNAs are listed on miRBase (http://www.mirbase.org/), considered the central repository.
Function and biomarker utility of miRNAs are specific to the cells in which they are expressed. miRNAs isolated from circulating plasma exosomes have been shown to be stable over time, which is key in establishing their utility (Sanz-Rubio, et al. Sci Rep. 2018;8[1]:10306).
miRNAs have been credited with the function of micromanaging the circadian clock and sleep homeostasis in virtually all living organisms (Goodwin, et al. Cell Rep. 2018;23[13]:3776; Mehta, et al. J Mol Biol. 2013;425[19]:3609).
Preliminary work has identified dysregulated miRNAs in patients with obstructive sleep apnea (Li, et al. Medicine (Baltimore). 2017;96[34]:e7917). Exosomal miRNA has been shown to predict and protect against severe bronchopulmonary dysplasia (Lal, et al. JCI Insight. 2018;3[5]. pii: 93994).
Circadian miRNAs in salivary samples were found to have “altered” expression in autistic children with disordered sleep relative to peers with typical sleep (Hicks, et al. PLoS One. 2018;13[7]:e0198288). Collection from salivary samples facilitates multiple timed collection feasible at home and has multiple benefits.
Work on miRNAs, though preliminary, appears promising in providing a much-needed new perspective on pathophysiology and treatment in many disease processes.
Harish Rao, MD
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation
Using impulse oscillometry in clinical practice
Impulse oscillometry (iOS) is an effort-independent test that requires minimal cooperation from the patient. It provides measures of respiratory mechanics during normal tidal breathing, including resistance (R), reactance (X), and impedance (Z) (Oostveen E, et al. Eur Respir J. 2003;22[6]:1026).
Airway R is largely, but not entirely, determined by cross-sectional area (Poiseuille’s Law). X is a surrogate for lung elastance, which is the inverse of compliance. Z is the combination of R and X and isn’t used clinically.
There are several benefits to using iOS, as opposed to or in conjunction with standard spirometry. First, iOS yields respiratory function measurements for patients, like the elderly and young children, who cannot provide acceptable and reproducible spirometry (Pezzoli L, et al. Age Ageing. 2003;32[1]:43). Second, it provides a real-world assessment of lung function because R and X values are obtained during tidal breathing. Humans don’t use the forced maneuvers needed for spirometry during normal daily activities, which weakens the correlation of FEV1 with respiratory symptoms. Forced maneuvers also create artifacts from gas compression and cause small airway closure, which limits inferences made from standard spirometry (Brusasco V, et al. Eur Respir J. 2005;26[5]:948). Lastly, R and X provide information not available from spirometry, and iOS is particularly sensitive for detecting small airway dysfunction (Berger K, et al. Chest. 2015;148[5]:1131).
Clinical and disease-specific indications for iOS are still being established. As discussed above, iOS is appropriate for any patient unable to perform spirometry. As new inhalers designed to deliver medication to the distal airways become available, subtle abnormalities detected via iOS will provide a target for specific therapies (Lipworth B. Ann Allergy Asthma Immunol. 2013;110[4]:233). iOS shows significant promise as a noninvasive assessment for supraglottic diseases, like vocal cord dysfunction, and can quantify changes over time following invasive intervention to relieve upper airway obstruction (Bikov A, et al. Chest. 2015;148[3]:731; Horan T, et al. Chest. 2001:120[1]:69). As their comfort level with interpretation improves, pulmonologists will find iOS is an important tool for disease diagnosis and treatment.
Aaron Holley, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
Hemodynamic definition of pulmonary hypertension changed
Many patients worldwide went to bed February 26, 2018, with normal pulmonary pressures and woke up the next morning with pulmonary hypertension (PH). That day, experts met at the World Symposium on PH in Nice, France, and changed the definition of resting PH from a mean pulmonary artery pressure (mPAP) of greater than or equal to 25 mm Hg to a mPAP greater 20 mm Hg (Simmoneau, et al. Eur Respir J. 2019;53:1801913). The First World Health Organization symposium on PH in 1973 established the 25 mm Hg cutoff to distinguish primary PH from what was then considered less severe forms of PH. This definition, acknowledged as arbitrary and conservative at the time, has persisted due to a paucity of data establishing a definitively abnormal mPAP threshold.
Two contemporary findings provide justification for the definition change: (1)Normal mPAP is 14 ± 3.3 mm Hg in healthy subjects (Kovacs, et al. Eur Respir J. 2009;34[4]:888). (2) Patients with mPAP greater than 20 mm Hg suffer worse outcomes compared with control subjects (Maron, et al. Circulation. 2016;133[13]:1240).
Preserving the other hemodynamic criteria for group 1 PH, pulmonary artery wedge pressure less than or equal to 15 mm Hg and pulmonary vascular resistance greater than or equal to 3 Wood units, experts also recommend applying the new definition to all pre-capillary PH, including groups 3, 4, and applicable group 5 diagnoses.
Importantly, new guidelines do not recommend treating PH patients with mPAP 21-24 mm Hg: “A change in the hemodynamic definition of PH due to [pulmonary vascular diseases] does not imply treating these additional patients, but highlights the importance of close monitoring in this population.”
John Kingrey, MD
Steering Committee Member
Thoracic Oncology
Lung Cancer and Women
While the overall incidence of lung cancer (LC) has decreased among both men and women, the decline among men has been steeper compared with women. Further, in women born in the 1950s to 1960s, the incidence has actually increased and cannot be fully explained by sex differences in smoking behavior (Jemal, et al. N Engl J Med. 2018;378:1999). Data suggest that women may be more susceptible to the harmful effects of tobacco and that the biology of LC may be different in women. In addition, LC in nonsmokers is more likely to occur in women.
LC is the leading cause of cancer death in both women and men worldwide, but the dramatic rise in the mortality rate from LC in women was qualified as a “full blown epidemic” in the Surgeon General’s 2001 Women and Smoking report.
The benefits of lung cancer screening (LCS) in the National Lung Screening Trial (NLST) were higher in women than in men and significantly greater in the subset of women (16%) that entered the Nelson trial – reduction in 10-year LC mortality of 61% vs. 26% in men (De Koning, et al. J Thorac Oncol. 2018;13[10]: suppl S185. Abstract PL02.05). A retrospective review of patients diagnosed with LC between 2005 and 2011 showed that only 37% of women vs. 50% of men met LCS criteria (Wang, et al. JAMA 2015;313[8]:853).
Lung cancer needs to be recognized as an important women’s health issue, and there is need for continued attention to sex differences in LC risk, LCS criteria, and outcomes.
Anne Gonzalez, MD, FCCP
Steering Committee Member
Interventional Chest/Diagnostic Procedures
Complications and economic burden of diagnostic procedures for lung abnormalities in the community setting
The influential National Lung Screening Trial (NLST) reported a 20% reduction in lung cancer-related deaths using low dose CT scan when compared with plain chest radiography (Aberle et al. N Engl J Med. 2011;365[5]:395). Many medical societies responded by recommending screening individuals at high-risk for lung cancer, and community-based lung cancer screening programs were developed across the US. A concerning feature of the study was the rate (23.3%) of false-positive findings after three rounds of screening and the potential for complications secondary to diagnostic invasive procedures.
Using a 2008-2013 cohort of community inpatient and outpatient practice settings, Hou and colleagues searched administrative databases for procedure and diagnostic codes used in the NLST (Hou et al. JAMA Intern Med. 2019;179[3]:324). The study team created an age-matched control cohort that did not have an invasive procedure and used the difference in complications rates as an indicator of a procedure-related complication. Additionally, they estimated 1-year medical costs associated with complications. More than 340,000 patients were included in the study, and the overall complication rate was far higher than what was reported in the NLST. This difference was more pronounced in the older group in the study cohort (23.8% vs 8.5%). The associated economic burden of complications was substantial, and cost more than the initial procedure itself.
Although this was not a lung cancer screening cohort and used an administrative database, some valuable lessons can be offered from this study. First, complication rates of procedures like those performed in the NLST are likely to be higher in low-volume centers. Second, in order to minimize procedures, associated complications, and costs, we should be cognizant of the diagnostic limitations of each type of intervention when evaluating patients with lung nodules, wisely choosing the correct procedure for the correct patient after multidisciplinary discussion. We should seek to minimize biopsies of lesions that are likely benign. Third, it is evident that more research is needed regarding this topic. The ideal study would need to include both academic and community-based lung cancer screening programs, and, prospectively, analyze the diagnostic yield and complication rates, as well as downstream costs. Finally, the results of this study call all of us to properly follow the lung cancer screening guidelines and reconcile them with our common sense when evaluating a patient with a screen-detected nodule. Injudicious testing invites unnecessary complications, increases the cost of care, and diverts resources from those more likely to benefit from appropriate interventions.
Jose Cardenas-Garcia, MD, FCCP
Steering Committee Member
Douglas Arenberg, MD, FCCP
NetWork Member
Pediatric Chest Medicine
microRNAs: A New Biomarker
Biomarkers are essential tools in a clinician’s armamentarium. Biomarkers have multiple uses being indicators of a pathologic or physiologic process. One promising biomarker, now studied across multiple disorders, is microRNA (miRNA).
miRNAs are short (18–22 nucleotide) regulatory RNAs that bind mRNAs and decrease protein translation. miRNAs are generally co-transcribed with neighboring genes or co-transcribed within a cluster of miRNAs (a polycistronic cluster). Over 2,000 miRNAs are listed on miRBase (http://www.mirbase.org/), considered the central repository.
Function and biomarker utility of miRNAs are specific to the cells in which they are expressed. miRNAs isolated from circulating plasma exosomes have been shown to be stable over time, which is key in establishing their utility (Sanz-Rubio, et al. Sci Rep. 2018;8[1]:10306).
miRNAs have been credited with the function of micromanaging the circadian clock and sleep homeostasis in virtually all living organisms (Goodwin, et al. Cell Rep. 2018;23[13]:3776; Mehta, et al. J Mol Biol. 2013;425[19]:3609).
Preliminary work has identified dysregulated miRNAs in patients with obstructive sleep apnea (Li, et al. Medicine (Baltimore). 2017;96[34]:e7917). Exosomal miRNA has been shown to predict and protect against severe bronchopulmonary dysplasia (Lal, et al. JCI Insight. 2018;3[5]. pii: 93994).
Circadian miRNAs in salivary samples were found to have “altered” expression in autistic children with disordered sleep relative to peers with typical sleep (Hicks, et al. PLoS One. 2018;13[7]:e0198288). Collection from salivary samples facilitates multiple timed collection feasible at home and has multiple benefits.
Work on miRNAs, though preliminary, appears promising in providing a much-needed new perspective on pathophysiology and treatment in many disease processes.
Harish Rao, MD
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation
Using impulse oscillometry in clinical practice
Impulse oscillometry (iOS) is an effort-independent test that requires minimal cooperation from the patient. It provides measures of respiratory mechanics during normal tidal breathing, including resistance (R), reactance (X), and impedance (Z) (Oostveen E, et al. Eur Respir J. 2003;22[6]:1026).
Airway R is largely, but not entirely, determined by cross-sectional area (Poiseuille’s Law). X is a surrogate for lung elastance, which is the inverse of compliance. Z is the combination of R and X and isn’t used clinically.
There are several benefits to using iOS, as opposed to or in conjunction with standard spirometry. First, iOS yields respiratory function measurements for patients, like the elderly and young children, who cannot provide acceptable and reproducible spirometry (Pezzoli L, et al. Age Ageing. 2003;32[1]:43). Second, it provides a real-world assessment of lung function because R and X values are obtained during tidal breathing. Humans don’t use the forced maneuvers needed for spirometry during normal daily activities, which weakens the correlation of FEV1 with respiratory symptoms. Forced maneuvers also create artifacts from gas compression and cause small airway closure, which limits inferences made from standard spirometry (Brusasco V, et al. Eur Respir J. 2005;26[5]:948). Lastly, R and X provide information not available from spirometry, and iOS is particularly sensitive for detecting small airway dysfunction (Berger K, et al. Chest. 2015;148[5]:1131).
Clinical and disease-specific indications for iOS are still being established. As discussed above, iOS is appropriate for any patient unable to perform spirometry. As new inhalers designed to deliver medication to the distal airways become available, subtle abnormalities detected via iOS will provide a target for specific therapies (Lipworth B. Ann Allergy Asthma Immunol. 2013;110[4]:233). iOS shows significant promise as a noninvasive assessment for supraglottic diseases, like vocal cord dysfunction, and can quantify changes over time following invasive intervention to relieve upper airway obstruction (Bikov A, et al. Chest. 2015;148[3]:731; Horan T, et al. Chest. 2001:120[1]:69). As their comfort level with interpretation improves, pulmonologists will find iOS is an important tool for disease diagnosis and treatment.
Aaron Holley, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
Hemodynamic definition of pulmonary hypertension changed
Many patients worldwide went to bed February 26, 2018, with normal pulmonary pressures and woke up the next morning with pulmonary hypertension (PH). That day, experts met at the World Symposium on PH in Nice, France, and changed the definition of resting PH from a mean pulmonary artery pressure (mPAP) of greater than or equal to 25 mm Hg to a mPAP greater 20 mm Hg (Simmoneau, et al. Eur Respir J. 2019;53:1801913). The First World Health Organization symposium on PH in 1973 established the 25 mm Hg cutoff to distinguish primary PH from what was then considered less severe forms of PH. This definition, acknowledged as arbitrary and conservative at the time, has persisted due to a paucity of data establishing a definitively abnormal mPAP threshold.
Two contemporary findings provide justification for the definition change: (1)Normal mPAP is 14 ± 3.3 mm Hg in healthy subjects (Kovacs, et al. Eur Respir J. 2009;34[4]:888). (2) Patients with mPAP greater than 20 mm Hg suffer worse outcomes compared with control subjects (Maron, et al. Circulation. 2016;133[13]:1240).
Preserving the other hemodynamic criteria for group 1 PH, pulmonary artery wedge pressure less than or equal to 15 mm Hg and pulmonary vascular resistance greater than or equal to 3 Wood units, experts also recommend applying the new definition to all pre-capillary PH, including groups 3, 4, and applicable group 5 diagnoses.
Importantly, new guidelines do not recommend treating PH patients with mPAP 21-24 mm Hg: “A change in the hemodynamic definition of PH due to [pulmonary vascular diseases] does not imply treating these additional patients, but highlights the importance of close monitoring in this population.”
John Kingrey, MD
Steering Committee Member
Thoracic Oncology
Lung Cancer and Women
While the overall incidence of lung cancer (LC) has decreased among both men and women, the decline among men has been steeper compared with women. Further, in women born in the 1950s to 1960s, the incidence has actually increased and cannot be fully explained by sex differences in smoking behavior (Jemal, et al. N Engl J Med. 2018;378:1999). Data suggest that women may be more susceptible to the harmful effects of tobacco and that the biology of LC may be different in women. In addition, LC in nonsmokers is more likely to occur in women.
LC is the leading cause of cancer death in both women and men worldwide, but the dramatic rise in the mortality rate from LC in women was qualified as a “full blown epidemic” in the Surgeon General’s 2001 Women and Smoking report.
The benefits of lung cancer screening (LCS) in the National Lung Screening Trial (NLST) were higher in women than in men and significantly greater in the subset of women (16%) that entered the Nelson trial – reduction in 10-year LC mortality of 61% vs. 26% in men (De Koning, et al. J Thorac Oncol. 2018;13[10]: suppl S185. Abstract PL02.05). A retrospective review of patients diagnosed with LC between 2005 and 2011 showed that only 37% of women vs. 50% of men met LCS criteria (Wang, et al. JAMA 2015;313[8]:853).
Lung cancer needs to be recognized as an important women’s health issue, and there is need for continued attention to sex differences in LC risk, LCS criteria, and outcomes.
Anne Gonzalez, MD, FCCP
Steering Committee Member
Interventional Chest/Diagnostic Procedures
Complications and economic burden of diagnostic procedures for lung abnormalities in the community setting
The influential National Lung Screening Trial (NLST) reported a 20% reduction in lung cancer-related deaths using low dose CT scan when compared with plain chest radiography (Aberle et al. N Engl J Med. 2011;365[5]:395). Many medical societies responded by recommending screening individuals at high-risk for lung cancer, and community-based lung cancer screening programs were developed across the US. A concerning feature of the study was the rate (23.3%) of false-positive findings after three rounds of screening and the potential for complications secondary to diagnostic invasive procedures.
Using a 2008-2013 cohort of community inpatient and outpatient practice settings, Hou and colleagues searched administrative databases for procedure and diagnostic codes used in the NLST (Hou et al. JAMA Intern Med. 2019;179[3]:324). The study team created an age-matched control cohort that did not have an invasive procedure and used the difference in complications rates as an indicator of a procedure-related complication. Additionally, they estimated 1-year medical costs associated with complications. More than 340,000 patients were included in the study, and the overall complication rate was far higher than what was reported in the NLST. This difference was more pronounced in the older group in the study cohort (23.8% vs 8.5%). The associated economic burden of complications was substantial, and cost more than the initial procedure itself.
Although this was not a lung cancer screening cohort and used an administrative database, some valuable lessons can be offered from this study. First, complication rates of procedures like those performed in the NLST are likely to be higher in low-volume centers. Second, in order to minimize procedures, associated complications, and costs, we should be cognizant of the diagnostic limitations of each type of intervention when evaluating patients with lung nodules, wisely choosing the correct procedure for the correct patient after multidisciplinary discussion. We should seek to minimize biopsies of lesions that are likely benign. Third, it is evident that more research is needed regarding this topic. The ideal study would need to include both academic and community-based lung cancer screening programs, and, prospectively, analyze the diagnostic yield and complication rates, as well as downstream costs. Finally, the results of this study call all of us to properly follow the lung cancer screening guidelines and reconcile them with our common sense when evaluating a patient with a screen-detected nodule. Injudicious testing invites unnecessary complications, increases the cost of care, and diverts resources from those more likely to benefit from appropriate interventions.
Jose Cardenas-Garcia, MD, FCCP
Steering Committee Member
Douglas Arenberg, MD, FCCP
NetWork Member
Pediatric Chest Medicine
microRNAs: A New Biomarker
Biomarkers are essential tools in a clinician’s armamentarium. Biomarkers have multiple uses being indicators of a pathologic or physiologic process. One promising biomarker, now studied across multiple disorders, is microRNA (miRNA).
miRNAs are short (18–22 nucleotide) regulatory RNAs that bind mRNAs and decrease protein translation. miRNAs are generally co-transcribed with neighboring genes or co-transcribed within a cluster of miRNAs (a polycistronic cluster). Over 2,000 miRNAs are listed on miRBase (http://www.mirbase.org/), considered the central repository.
Function and biomarker utility of miRNAs are specific to the cells in which they are expressed. miRNAs isolated from circulating plasma exosomes have been shown to be stable over time, which is key in establishing their utility (Sanz-Rubio, et al. Sci Rep. 2018;8[1]:10306).
miRNAs have been credited with the function of micromanaging the circadian clock and sleep homeostasis in virtually all living organisms (Goodwin, et al. Cell Rep. 2018;23[13]:3776; Mehta, et al. J Mol Biol. 2013;425[19]:3609).
Preliminary work has identified dysregulated miRNAs in patients with obstructive sleep apnea (Li, et al. Medicine (Baltimore). 2017;96[34]:e7917). Exosomal miRNA has been shown to predict and protect against severe bronchopulmonary dysplasia (Lal, et al. JCI Insight. 2018;3[5]. pii: 93994).
Circadian miRNAs in salivary samples were found to have “altered” expression in autistic children with disordered sleep relative to peers with typical sleep (Hicks, et al. PLoS One. 2018;13[7]:e0198288). Collection from salivary samples facilitates multiple timed collection feasible at home and has multiple benefits.
Work on miRNAs, though preliminary, appears promising in providing a much-needed new perspective on pathophysiology and treatment in many disease processes.
Harish Rao, MD
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation
Using impulse oscillometry in clinical practice
Impulse oscillometry (iOS) is an effort-independent test that requires minimal cooperation from the patient. It provides measures of respiratory mechanics during normal tidal breathing, including resistance (R), reactance (X), and impedance (Z) (Oostveen E, et al. Eur Respir J. 2003;22[6]:1026).
Airway R is largely, but not entirely, determined by cross-sectional area (Poiseuille’s Law). X is a surrogate for lung elastance, which is the inverse of compliance. Z is the combination of R and X and isn’t used clinically.
There are several benefits to using iOS, as opposed to or in conjunction with standard spirometry. First, iOS yields respiratory function measurements for patients, like the elderly and young children, who cannot provide acceptable and reproducible spirometry (Pezzoli L, et al. Age Ageing. 2003;32[1]:43). Second, it provides a real-world assessment of lung function because R and X values are obtained during tidal breathing. Humans don’t use the forced maneuvers needed for spirometry during normal daily activities, which weakens the correlation of FEV1 with respiratory symptoms. Forced maneuvers also create artifacts from gas compression and cause small airway closure, which limits inferences made from standard spirometry (Brusasco V, et al. Eur Respir J. 2005;26[5]:948). Lastly, R and X provide information not available from spirometry, and iOS is particularly sensitive for detecting small airway dysfunction (Berger K, et al. Chest. 2015;148[5]:1131).
Clinical and disease-specific indications for iOS are still being established. As discussed above, iOS is appropriate for any patient unable to perform spirometry. As new inhalers designed to deliver medication to the distal airways become available, subtle abnormalities detected via iOS will provide a target for specific therapies (Lipworth B. Ann Allergy Asthma Immunol. 2013;110[4]:233). iOS shows significant promise as a noninvasive assessment for supraglottic diseases, like vocal cord dysfunction, and can quantify changes over time following invasive intervention to relieve upper airway obstruction (Bikov A, et al. Chest. 2015;148[3]:731; Horan T, et al. Chest. 2001:120[1]:69). As their comfort level with interpretation improves, pulmonologists will find iOS is an important tool for disease diagnosis and treatment.
Aaron Holley, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
Hemodynamic definition of pulmonary hypertension changed
Many patients worldwide went to bed February 26, 2018, with normal pulmonary pressures and woke up the next morning with pulmonary hypertension (PH). That day, experts met at the World Symposium on PH in Nice, France, and changed the definition of resting PH from a mean pulmonary artery pressure (mPAP) of greater than or equal to 25 mm Hg to a mPAP greater 20 mm Hg (Simmoneau, et al. Eur Respir J. 2019;53:1801913). The First World Health Organization symposium on PH in 1973 established the 25 mm Hg cutoff to distinguish primary PH from what was then considered less severe forms of PH. This definition, acknowledged as arbitrary and conservative at the time, has persisted due to a paucity of data establishing a definitively abnormal mPAP threshold.
Two contemporary findings provide justification for the definition change: (1)Normal mPAP is 14 ± 3.3 mm Hg in healthy subjects (Kovacs, et al. Eur Respir J. 2009;34[4]:888). (2) Patients with mPAP greater than 20 mm Hg suffer worse outcomes compared with control subjects (Maron, et al. Circulation. 2016;133[13]:1240).
Preserving the other hemodynamic criteria for group 1 PH, pulmonary artery wedge pressure less than or equal to 15 mm Hg and pulmonary vascular resistance greater than or equal to 3 Wood units, experts also recommend applying the new definition to all pre-capillary PH, including groups 3, 4, and applicable group 5 diagnoses.
Importantly, new guidelines do not recommend treating PH patients with mPAP 21-24 mm Hg: “A change in the hemodynamic definition of PH due to [pulmonary vascular diseases] does not imply treating these additional patients, but highlights the importance of close monitoring in this population.”
John Kingrey, MD
Steering Committee Member
Thoracic Oncology
Lung Cancer and Women
While the overall incidence of lung cancer (LC) has decreased among both men and women, the decline among men has been steeper compared with women. Further, in women born in the 1950s to 1960s, the incidence has actually increased and cannot be fully explained by sex differences in smoking behavior (Jemal, et al. N Engl J Med. 2018;378:1999). Data suggest that women may be more susceptible to the harmful effects of tobacco and that the biology of LC may be different in women. In addition, LC in nonsmokers is more likely to occur in women.
LC is the leading cause of cancer death in both women and men worldwide, but the dramatic rise in the mortality rate from LC in women was qualified as a “full blown epidemic” in the Surgeon General’s 2001 Women and Smoking report.
The benefits of lung cancer screening (LCS) in the National Lung Screening Trial (NLST) were higher in women than in men and significantly greater in the subset of women (16%) that entered the Nelson trial – reduction in 10-year LC mortality of 61% vs. 26% in men (De Koning, et al. J Thorac Oncol. 2018;13[10]: suppl S185. Abstract PL02.05). A retrospective review of patients diagnosed with LC between 2005 and 2011 showed that only 37% of women vs. 50% of men met LCS criteria (Wang, et al. JAMA 2015;313[8]:853).
Lung cancer needs to be recognized as an important women’s health issue, and there is need for continued attention to sex differences in LC risk, LCS criteria, and outcomes.
Anne Gonzalez, MD, FCCP
Steering Committee Member
The burgeoning role of sleep-related chronic hypoxia in long-term outcomes
Clinicians are well aware of the acute effects of hypoxemia when encountered in conditions such as pulmonary embolism, pulmonary edema, COPD exacerbation, and others, whereas effects of chronic hypoxemia, such as pulmonary hypertension and polycythemia, are more difficult to recognize. Chronic hypoxemia is frequent in chronic lung diseases, such as COPD, but how it leads to increased mortality in severe COPD is unknown (NHLBI Working Group for LTOT in COPD. Am J Respir Crit Care Med. 2006;174:373). Chronic hypoxemia following high altitude exposure tends to have more unpredictable effects. Chronic hypoxemia, greater than that expected for the altitude of residence, is encountered frequently in high altitude dwellers. Here it has been implicated in the pathophysiology of chronic mountain sickness (Villafeurte and Corante. High Alt Med Biol. 2016;17[2]:61) and low birth weights (Maatta J, et al. Sci Rep. 2018;8[1]:13583), even though high altitude residence has been linked to better cardiovascular outcomes and reduced cancer-related deaths (Burstcher M. Aging Dis. 2013;5[4]:274). Chronic hypoxia effects at high altitude may, therefore, be variegated depending on a number of factors that include organ-system-specific effects, severity of chronic hypoxia, and a propensity to disease determined by genetic background and generations of residence.
Such diverse effects of chronic sleep-related hypoxemia are also being reported with obstructive sleep apnea (OSA). While sleep can result in sustained drops in ventilation and consequent hypoxemia similar to what is seen in COPD, OSA is typified by a form of sleep-related hypoxemia in a pattern termed as chronic intermittent hypoxia (CIH). CIH is characterized by rapid fluctuations in oxygen saturations (Figure 1) that is virtually pathognomonic of sleep apnea either from recurrent upper airway obstructions (as in OSA) or pauses in respiratory generator firing (as in central sleep apnea). OSA-driven CIH has received most attention, given its purported role in in the causation of the wide range of pathologic conditions associated with OSA. Outcomes from cross-sectional and longitudinal studies have correlated time spent below 90% or recurrent oxygen desaturations to a number of OSA-related outcomes such as cardiovascular disease, diabetes, and cognitive dysfunction (Dewan et al. Chest. 2015;147[1]:266). While these effects of OSA-related intermittent hypoxemia occur over long periods of time, as with other forms of chronic hypoxia, some effects, such as hypertension, are demonstrable in animal models after much shorter durations of sleep-related intermittent hypoxia exposure. As seen with other forms of chronic hypoxemia, an opposing beneficial effect has also been demonstrated on the size of myocardial infarct during acute coronary events and from mild OSA-related mortality in elderly subjects (Javaheri et al. J Am Coll Cardiol. 2017;69[7]:841).
Given how common sleep-related hypoxemia and OSA are, it is important to understand the implications of different patterns of sleep-related hypoxemia that a vast segment of the population experiences on a nightly basis. A number of factors may determine chronic outcomes with sleep-related hypoxemia that include the pattern of sleep-related hypoxemia (chronic sustained hypoxemia associated with sleep-related hypoventilation vs chronic intermittent hypoxemia of OSA), degree of hypoxemia, presence of underlying disease, and hitherto undescribed individual factors. While a correlation between hypoxemic burden secondary to sleep-disordered breathing and cardiovascular outcomes has been shown (Azabarzin A, et al. Eur Heart J. 2018 Oct 30), CPAP interventional studies that address OSA-related CIH have shown mixed results for prevention of cardiovascular disease (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919). It has also been difficult to draw upon results of oxygen supplementation in other forms of hypoxemia, such as COPD, when specifically targeted to addressing the hypoxemia seen only at night or with exercise (LOTT Research Group. N Engl J Med. 2016;375:1617 ). To complicate this further, high altitude residence (that may result in similar levels of sleep-related hypoxemia) is not associated with any differences in life-expectancy but may provide a reduction in cardiovascular outcomes (Ezzati, et al. J Epidemiol Community Health. 2012;66[7]:e17).
How do we reconcile such disparate effects of chronic hypoxemia? Part of difference may be in the pattern of chronic intermittent hypoxemia noted with OSA characterized not only by rapid drops in oxygen but also rapid reoxygenation events secondary to arousals terminating an apnea – these reoxygenation events have been attributed to the increased oxidant stress demonstrable in multiple tissues. While chronic hypoxia itself may cause increased oxidant stress, such effects seen with sustained forms of hypoxia, such as sleep-related hypoventilation or high altitude residence, may be more gradual resulting in lesser degrees of tissue effects and regulation of antioxidant defenses with sustained exposure. Herein lies the importance of understanding physiologic and biological effects stemming from chronic hypoxia to explain its variegated effects on different organ systems. In this regard, the role of carotid body, a structure with unique vascular supply and with the ability to respond to minor changes in oxygen saturation as is seen in patients with OSA is key to the causation of hypertension associated with OSA (Shell et al. Curr Hyperten Rep. 2016;18[3]:19). Carotid body activation by intermittent hypoxia and long-term sensory facilitation drives the elevated sympathetic activity and consequent increases in blood pressure that can be improved by supplemental oxygen (Turnbull CD, et al. Am J Respir Crit Care Med. 2019;199[2]:211).
While carotid body responses are key to the pathophysiology of OSA, every organ in the body (in fact, every cell within the body) has the ability to sense and respond to hypoxia. This ability to sense oxygen tensions is ingrained in every cell by virtue of oxygen’s critical role in the genesis of life and evolution. These cellular responses to hypoxia are mediated by hypoxia-inducible factors (HIFs), isoforms of which include the more ubiquitous HIF-1 found in all parenchymal cells and HIF-2 found in specialized erythropoietin-producing cells of the kidney and the pulmonary circulation (the polycythemia and pulmonary vasoconstrictive responses from hypoxia are mediated through HIF-2 ). HIFs mediate the transcription of hundreds of genes, and they have been implicated in the pathobiology of a wide range of phenomena, from cancer to atherosclerotic vascular disease, metabolic syndrome, neurodegenerative disorders, pulmonary hypertension, and nonalcoholic fatty liver disease (Prabhakar and Semenza. Physiol Rev. 2012;92[3]:967). While HIF activation is an attractive target for examining the effects of chronic hypoxia of high altitude and sleep-disordered breathing, HIF activation varies from tissue to tissue and interacts with a number of other cellular systems in leading to differential effects. The short half-life of HIF proteins make them difficult to detect in tissues, so a number of secondary HIF-effects has been measured with mixed results depending on animal model utilized, pattern and degree of hypoxia studied, and the target effect measured. Comparative effects of intermittent vs sustained hypoxemia need to be systematically studied in different organ systems in different species, given the differing oxygen thresholds of individual cells due to unique blood flows and variations in the system of co-factors and prolyl hydroxylases that regulate the activation of HIFs. While the thrust of the work has been centered on HIF-related effects and the role of NF-kB-driven inflammation seen in OSA, there is substantial evidence to the role of oxidant stress that may be directly related to reoxygenation events occurring with CIH (Lavie L. Sleep Med Rev. 2015;20:27).
For life that has been intricately involved with oxygen from its genesis, it is not unreasonable to expect adaptations of cells, organs, and the whole individual to a wide range of oxygen tensions. Attempts to understand the import of sleep-disordered breathing has led to a need to unravel the implications of OSA-related chronic intermittent hypoxia and sleep-hypoventilation. This has led to a resurgence of interest in hypoxia-related research. Whether such chronic sleep-related sustained and intermittent hypoxemia is a harbinger of chronic disease is still not fully clear. A number of challenges exist with the understanding of these chronic hypoxia effects that include the long time needed for disease occurrence, its differential effects on organ systems, the role of hypoxia vs reoxygenation injury, importance of local blood flow, etc. Understanding these pathways will be crucial in prognosticating the role of sleep-related hypoxemia, the recognition of which has become part and parcel of routine management in sleep medicine.
Dr. Sundar is Medical Director, Sleep-Wake Center, Clinical Professor, Pulmonary, Critical Care & Sleep Medicine, Department of Medicine, University of Utah, Salt Lake City, Utah.
Clinicians are well aware of the acute effects of hypoxemia when encountered in conditions such as pulmonary embolism, pulmonary edema, COPD exacerbation, and others, whereas effects of chronic hypoxemia, such as pulmonary hypertension and polycythemia, are more difficult to recognize. Chronic hypoxemia is frequent in chronic lung diseases, such as COPD, but how it leads to increased mortality in severe COPD is unknown (NHLBI Working Group for LTOT in COPD. Am J Respir Crit Care Med. 2006;174:373). Chronic hypoxemia following high altitude exposure tends to have more unpredictable effects. Chronic hypoxemia, greater than that expected for the altitude of residence, is encountered frequently in high altitude dwellers. Here it has been implicated in the pathophysiology of chronic mountain sickness (Villafeurte and Corante. High Alt Med Biol. 2016;17[2]:61) and low birth weights (Maatta J, et al. Sci Rep. 2018;8[1]:13583), even though high altitude residence has been linked to better cardiovascular outcomes and reduced cancer-related deaths (Burstcher M. Aging Dis. 2013;5[4]:274). Chronic hypoxia effects at high altitude may, therefore, be variegated depending on a number of factors that include organ-system-specific effects, severity of chronic hypoxia, and a propensity to disease determined by genetic background and generations of residence.
Such diverse effects of chronic sleep-related hypoxemia are also being reported with obstructive sleep apnea (OSA). While sleep can result in sustained drops in ventilation and consequent hypoxemia similar to what is seen in COPD, OSA is typified by a form of sleep-related hypoxemia in a pattern termed as chronic intermittent hypoxia (CIH). CIH is characterized by rapid fluctuations in oxygen saturations (Figure 1) that is virtually pathognomonic of sleep apnea either from recurrent upper airway obstructions (as in OSA) or pauses in respiratory generator firing (as in central sleep apnea). OSA-driven CIH has received most attention, given its purported role in in the causation of the wide range of pathologic conditions associated with OSA. Outcomes from cross-sectional and longitudinal studies have correlated time spent below 90% or recurrent oxygen desaturations to a number of OSA-related outcomes such as cardiovascular disease, diabetes, and cognitive dysfunction (Dewan et al. Chest. 2015;147[1]:266). While these effects of OSA-related intermittent hypoxemia occur over long periods of time, as with other forms of chronic hypoxia, some effects, such as hypertension, are demonstrable in animal models after much shorter durations of sleep-related intermittent hypoxia exposure. As seen with other forms of chronic hypoxemia, an opposing beneficial effect has also been demonstrated on the size of myocardial infarct during acute coronary events and from mild OSA-related mortality in elderly subjects (Javaheri et al. J Am Coll Cardiol. 2017;69[7]:841).
Given how common sleep-related hypoxemia and OSA are, it is important to understand the implications of different patterns of sleep-related hypoxemia that a vast segment of the population experiences on a nightly basis. A number of factors may determine chronic outcomes with sleep-related hypoxemia that include the pattern of sleep-related hypoxemia (chronic sustained hypoxemia associated with sleep-related hypoventilation vs chronic intermittent hypoxemia of OSA), degree of hypoxemia, presence of underlying disease, and hitherto undescribed individual factors. While a correlation between hypoxemic burden secondary to sleep-disordered breathing and cardiovascular outcomes has been shown (Azabarzin A, et al. Eur Heart J. 2018 Oct 30), CPAP interventional studies that address OSA-related CIH have shown mixed results for prevention of cardiovascular disease (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919). It has also been difficult to draw upon results of oxygen supplementation in other forms of hypoxemia, such as COPD, when specifically targeted to addressing the hypoxemia seen only at night or with exercise (LOTT Research Group. N Engl J Med. 2016;375:1617 ). To complicate this further, high altitude residence (that may result in similar levels of sleep-related hypoxemia) is not associated with any differences in life-expectancy but may provide a reduction in cardiovascular outcomes (Ezzati, et al. J Epidemiol Community Health. 2012;66[7]:e17).
How do we reconcile such disparate effects of chronic hypoxemia? Part of difference may be in the pattern of chronic intermittent hypoxemia noted with OSA characterized not only by rapid drops in oxygen but also rapid reoxygenation events secondary to arousals terminating an apnea – these reoxygenation events have been attributed to the increased oxidant stress demonstrable in multiple tissues. While chronic hypoxia itself may cause increased oxidant stress, such effects seen with sustained forms of hypoxia, such as sleep-related hypoventilation or high altitude residence, may be more gradual resulting in lesser degrees of tissue effects and regulation of antioxidant defenses with sustained exposure. Herein lies the importance of understanding physiologic and biological effects stemming from chronic hypoxia to explain its variegated effects on different organ systems. In this regard, the role of carotid body, a structure with unique vascular supply and with the ability to respond to minor changes in oxygen saturation as is seen in patients with OSA is key to the causation of hypertension associated with OSA (Shell et al. Curr Hyperten Rep. 2016;18[3]:19). Carotid body activation by intermittent hypoxia and long-term sensory facilitation drives the elevated sympathetic activity and consequent increases in blood pressure that can be improved by supplemental oxygen (Turnbull CD, et al. Am J Respir Crit Care Med. 2019;199[2]:211).
While carotid body responses are key to the pathophysiology of OSA, every organ in the body (in fact, every cell within the body) has the ability to sense and respond to hypoxia. This ability to sense oxygen tensions is ingrained in every cell by virtue of oxygen’s critical role in the genesis of life and evolution. These cellular responses to hypoxia are mediated by hypoxia-inducible factors (HIFs), isoforms of which include the more ubiquitous HIF-1 found in all parenchymal cells and HIF-2 found in specialized erythropoietin-producing cells of the kidney and the pulmonary circulation (the polycythemia and pulmonary vasoconstrictive responses from hypoxia are mediated through HIF-2 ). HIFs mediate the transcription of hundreds of genes, and they have been implicated in the pathobiology of a wide range of phenomena, from cancer to atherosclerotic vascular disease, metabolic syndrome, neurodegenerative disorders, pulmonary hypertension, and nonalcoholic fatty liver disease (Prabhakar and Semenza. Physiol Rev. 2012;92[3]:967). While HIF activation is an attractive target for examining the effects of chronic hypoxia of high altitude and sleep-disordered breathing, HIF activation varies from tissue to tissue and interacts with a number of other cellular systems in leading to differential effects. The short half-life of HIF proteins make them difficult to detect in tissues, so a number of secondary HIF-effects has been measured with mixed results depending on animal model utilized, pattern and degree of hypoxia studied, and the target effect measured. Comparative effects of intermittent vs sustained hypoxemia need to be systematically studied in different organ systems in different species, given the differing oxygen thresholds of individual cells due to unique blood flows and variations in the system of co-factors and prolyl hydroxylases that regulate the activation of HIFs. While the thrust of the work has been centered on HIF-related effects and the role of NF-kB-driven inflammation seen in OSA, there is substantial evidence to the role of oxidant stress that may be directly related to reoxygenation events occurring with CIH (Lavie L. Sleep Med Rev. 2015;20:27).
For life that has been intricately involved with oxygen from its genesis, it is not unreasonable to expect adaptations of cells, organs, and the whole individual to a wide range of oxygen tensions. Attempts to understand the import of sleep-disordered breathing has led to a need to unravel the implications of OSA-related chronic intermittent hypoxia and sleep-hypoventilation. This has led to a resurgence of interest in hypoxia-related research. Whether such chronic sleep-related sustained and intermittent hypoxemia is a harbinger of chronic disease is still not fully clear. A number of challenges exist with the understanding of these chronic hypoxia effects that include the long time needed for disease occurrence, its differential effects on organ systems, the role of hypoxia vs reoxygenation injury, importance of local blood flow, etc. Understanding these pathways will be crucial in prognosticating the role of sleep-related hypoxemia, the recognition of which has become part and parcel of routine management in sleep medicine.
Dr. Sundar is Medical Director, Sleep-Wake Center, Clinical Professor, Pulmonary, Critical Care & Sleep Medicine, Department of Medicine, University of Utah, Salt Lake City, Utah.
Clinicians are well aware of the acute effects of hypoxemia when encountered in conditions such as pulmonary embolism, pulmonary edema, COPD exacerbation, and others, whereas effects of chronic hypoxemia, such as pulmonary hypertension and polycythemia, are more difficult to recognize. Chronic hypoxemia is frequent in chronic lung diseases, such as COPD, but how it leads to increased mortality in severe COPD is unknown (NHLBI Working Group for LTOT in COPD. Am J Respir Crit Care Med. 2006;174:373). Chronic hypoxemia following high altitude exposure tends to have more unpredictable effects. Chronic hypoxemia, greater than that expected for the altitude of residence, is encountered frequently in high altitude dwellers. Here it has been implicated in the pathophysiology of chronic mountain sickness (Villafeurte and Corante. High Alt Med Biol. 2016;17[2]:61) and low birth weights (Maatta J, et al. Sci Rep. 2018;8[1]:13583), even though high altitude residence has been linked to better cardiovascular outcomes and reduced cancer-related deaths (Burstcher M. Aging Dis. 2013;5[4]:274). Chronic hypoxia effects at high altitude may, therefore, be variegated depending on a number of factors that include organ-system-specific effects, severity of chronic hypoxia, and a propensity to disease determined by genetic background and generations of residence.
Such diverse effects of chronic sleep-related hypoxemia are also being reported with obstructive sleep apnea (OSA). While sleep can result in sustained drops in ventilation and consequent hypoxemia similar to what is seen in COPD, OSA is typified by a form of sleep-related hypoxemia in a pattern termed as chronic intermittent hypoxia (CIH). CIH is characterized by rapid fluctuations in oxygen saturations (Figure 1) that is virtually pathognomonic of sleep apnea either from recurrent upper airway obstructions (as in OSA) or pauses in respiratory generator firing (as in central sleep apnea). OSA-driven CIH has received most attention, given its purported role in in the causation of the wide range of pathologic conditions associated with OSA. Outcomes from cross-sectional and longitudinal studies have correlated time spent below 90% or recurrent oxygen desaturations to a number of OSA-related outcomes such as cardiovascular disease, diabetes, and cognitive dysfunction (Dewan et al. Chest. 2015;147[1]:266). While these effects of OSA-related intermittent hypoxemia occur over long periods of time, as with other forms of chronic hypoxia, some effects, such as hypertension, are demonstrable in animal models after much shorter durations of sleep-related intermittent hypoxia exposure. As seen with other forms of chronic hypoxemia, an opposing beneficial effect has also been demonstrated on the size of myocardial infarct during acute coronary events and from mild OSA-related mortality in elderly subjects (Javaheri et al. J Am Coll Cardiol. 2017;69[7]:841).
Given how common sleep-related hypoxemia and OSA are, it is important to understand the implications of different patterns of sleep-related hypoxemia that a vast segment of the population experiences on a nightly basis. A number of factors may determine chronic outcomes with sleep-related hypoxemia that include the pattern of sleep-related hypoxemia (chronic sustained hypoxemia associated with sleep-related hypoventilation vs chronic intermittent hypoxemia of OSA), degree of hypoxemia, presence of underlying disease, and hitherto undescribed individual factors. While a correlation between hypoxemic burden secondary to sleep-disordered breathing and cardiovascular outcomes has been shown (Azabarzin A, et al. Eur Heart J. 2018 Oct 30), CPAP interventional studies that address OSA-related CIH have shown mixed results for prevention of cardiovascular disease (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919). It has also been difficult to draw upon results of oxygen supplementation in other forms of hypoxemia, such as COPD, when specifically targeted to addressing the hypoxemia seen only at night or with exercise (LOTT Research Group. N Engl J Med. 2016;375:1617 ). To complicate this further, high altitude residence (that may result in similar levels of sleep-related hypoxemia) is not associated with any differences in life-expectancy but may provide a reduction in cardiovascular outcomes (Ezzati, et al. J Epidemiol Community Health. 2012;66[7]:e17).
How do we reconcile such disparate effects of chronic hypoxemia? Part of difference may be in the pattern of chronic intermittent hypoxemia noted with OSA characterized not only by rapid drops in oxygen but also rapid reoxygenation events secondary to arousals terminating an apnea – these reoxygenation events have been attributed to the increased oxidant stress demonstrable in multiple tissues. While chronic hypoxia itself may cause increased oxidant stress, such effects seen with sustained forms of hypoxia, such as sleep-related hypoventilation or high altitude residence, may be more gradual resulting in lesser degrees of tissue effects and regulation of antioxidant defenses with sustained exposure. Herein lies the importance of understanding physiologic and biological effects stemming from chronic hypoxia to explain its variegated effects on different organ systems. In this regard, the role of carotid body, a structure with unique vascular supply and with the ability to respond to minor changes in oxygen saturation as is seen in patients with OSA is key to the causation of hypertension associated with OSA (Shell et al. Curr Hyperten Rep. 2016;18[3]:19). Carotid body activation by intermittent hypoxia and long-term sensory facilitation drives the elevated sympathetic activity and consequent increases in blood pressure that can be improved by supplemental oxygen (Turnbull CD, et al. Am J Respir Crit Care Med. 2019;199[2]:211).
While carotid body responses are key to the pathophysiology of OSA, every organ in the body (in fact, every cell within the body) has the ability to sense and respond to hypoxia. This ability to sense oxygen tensions is ingrained in every cell by virtue of oxygen’s critical role in the genesis of life and evolution. These cellular responses to hypoxia are mediated by hypoxia-inducible factors (HIFs), isoforms of which include the more ubiquitous HIF-1 found in all parenchymal cells and HIF-2 found in specialized erythropoietin-producing cells of the kidney and the pulmonary circulation (the polycythemia and pulmonary vasoconstrictive responses from hypoxia are mediated through HIF-2 ). HIFs mediate the transcription of hundreds of genes, and they have been implicated in the pathobiology of a wide range of phenomena, from cancer to atherosclerotic vascular disease, metabolic syndrome, neurodegenerative disorders, pulmonary hypertension, and nonalcoholic fatty liver disease (Prabhakar and Semenza. Physiol Rev. 2012;92[3]:967). While HIF activation is an attractive target for examining the effects of chronic hypoxia of high altitude and sleep-disordered breathing, HIF activation varies from tissue to tissue and interacts with a number of other cellular systems in leading to differential effects. The short half-life of HIF proteins make them difficult to detect in tissues, so a number of secondary HIF-effects has been measured with mixed results depending on animal model utilized, pattern and degree of hypoxia studied, and the target effect measured. Comparative effects of intermittent vs sustained hypoxemia need to be systematically studied in different organ systems in different species, given the differing oxygen thresholds of individual cells due to unique blood flows and variations in the system of co-factors and prolyl hydroxylases that regulate the activation of HIFs. While the thrust of the work has been centered on HIF-related effects and the role of NF-kB-driven inflammation seen in OSA, there is substantial evidence to the role of oxidant stress that may be directly related to reoxygenation events occurring with CIH (Lavie L. Sleep Med Rev. 2015;20:27).
For life that has been intricately involved with oxygen from its genesis, it is not unreasonable to expect adaptations of cells, organs, and the whole individual to a wide range of oxygen tensions. Attempts to understand the import of sleep-disordered breathing has led to a need to unravel the implications of OSA-related chronic intermittent hypoxia and sleep-hypoventilation. This has led to a resurgence of interest in hypoxia-related research. Whether such chronic sleep-related sustained and intermittent hypoxemia is a harbinger of chronic disease is still not fully clear. A number of challenges exist with the understanding of these chronic hypoxia effects that include the long time needed for disease occurrence, its differential effects on organ systems, the role of hypoxia vs reoxygenation injury, importance of local blood flow, etc. Understanding these pathways will be crucial in prognosticating the role of sleep-related hypoxemia, the recognition of which has become part and parcel of routine management in sleep medicine.
Dr. Sundar is Medical Director, Sleep-Wake Center, Clinical Professor, Pulmonary, Critical Care & Sleep Medicine, Department of Medicine, University of Utah, Salt Lake City, Utah.