User login
ICYMI: Dabigatran no better than aspirin for recurrent stroke prevention
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
ICYMI: Alpelisib/fulvestrant combo boosts PFS in advanced breast cancer
Patients with PIK3CA-mutated, hormone receptor–positive, HER2-negative advanced breast cancer who had previously undergone endocrine therapy experienced longer progression-free survival when receiving alpelisib and fulvestrant, compared with placebo and fulvestrant (11.0 vs. 5.7 months; hazard ratio, 0.65; 95% confidence interval, 0.50-0.85; P less than .001), according to a randomized, phase 3 trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813904).
We first reported on the results of this trial when they were presented at the European Society for Medical Oncology Congress. Find our coverage at the link below.
Patients with PIK3CA-mutated, hormone receptor–positive, HER2-negative advanced breast cancer who had previously undergone endocrine therapy experienced longer progression-free survival when receiving alpelisib and fulvestrant, compared with placebo and fulvestrant (11.0 vs. 5.7 months; hazard ratio, 0.65; 95% confidence interval, 0.50-0.85; P less than .001), according to a randomized, phase 3 trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813904).
We first reported on the results of this trial when they were presented at the European Society for Medical Oncology Congress. Find our coverage at the link below.
Patients with PIK3CA-mutated, hormone receptor–positive, HER2-negative advanced breast cancer who had previously undergone endocrine therapy experienced longer progression-free survival when receiving alpelisib and fulvestrant, compared with placebo and fulvestrant (11.0 vs. 5.7 months; hazard ratio, 0.65; 95% confidence interval, 0.50-0.85; P less than .001), according to a randomized, phase 3 trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813904).
We first reported on the results of this trial when they were presented at the European Society for Medical Oncology Congress. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Lonely elderly patients suffer worse health outcomes
WASHINGTON – More lonely elderly patients suffered from health symptoms and received very aggressive end of life care than nonlonely elderly patients, according to a study presented at the annual meeting of the Society of General Internal Medicine.
“Loneliness and social isolation are very common problems, especially in older Americans, and inflict about 30%-40% of older Americans. But while we know that this may have implications for their quality [of] life and may actually lead to premature death, we know very little about the end of life experience,” said Nauzley Abedini, MD, MSc, a hospitalist in internal medicine at the University of Michigan, Ann Arbor.
The study sought to determine the association between loneliness and end of life experience as measured by symptom burden, intensity of care, and advance care planning in adults. The pooled cohort study used data from the Health and Retirement Study (HRS) to analyze older Americans (aged 50 years or more) who died between 2004 and 2014. Investigators conducted postmortem “exit interviews” with the next of kin after each participant’s death. There were 2,896 participants included in the survey. Of these participants, 34% (942) were lonely; the remaining 1,954 of elderly adults were classified as nonlonely.
Loneliness was defined using the three-item Revised University of California, Los Angeles, Loneliness Scale score from a decedent’s last HRS interview prior to death. These items included feeling left out, feeling isolated, and lacking companionship. Investigators used this data to create a loneliness variable on previously established cutpoints for “lonely” and “nonlonely” participants. The data was used from the most recent survey prior to death.
Results showed more lonely older adults suffered from health symptoms in the last year of life, compared with nonlonely older adults (69.1% vs. 59.5%; odds ratio, 1.52; 95% confidence interval, 1.30-1.78). These symptoms included being troubled by pain, having difficulty breathing, experiencing severe fatigue, and having periodic confusion.
Patients with loneliness associated with intensity of health care at the end of life were more likely to die in a nursing home than at home, compared with nonlonely adults (OR, 1.68; 95% CI, 1.25-2.27). The lonely patients also were more likely to use life support during their last 2 years of life (OR, 1.41; 95% CI, 1.16-1.70).
“For clinicians, we need to identify end of life as an additional vulnerable time for people who are lonely. Currently, most of our interventions in terms of screening for loneliness are in the outpatient setting, but I would argue that working in hospitals, hospices, nursing homes, and community organizations, where these folks are living and dying, would be useful places to screen for this,” Dr. Abedini said.
The authors had no disclosures.
WASHINGTON – More lonely elderly patients suffered from health symptoms and received very aggressive end of life care than nonlonely elderly patients, according to a study presented at the annual meeting of the Society of General Internal Medicine.
“Loneliness and social isolation are very common problems, especially in older Americans, and inflict about 30%-40% of older Americans. But while we know that this may have implications for their quality [of] life and may actually lead to premature death, we know very little about the end of life experience,” said Nauzley Abedini, MD, MSc, a hospitalist in internal medicine at the University of Michigan, Ann Arbor.
The study sought to determine the association between loneliness and end of life experience as measured by symptom burden, intensity of care, and advance care planning in adults. The pooled cohort study used data from the Health and Retirement Study (HRS) to analyze older Americans (aged 50 years or more) who died between 2004 and 2014. Investigators conducted postmortem “exit interviews” with the next of kin after each participant’s death. There were 2,896 participants included in the survey. Of these participants, 34% (942) were lonely; the remaining 1,954 of elderly adults were classified as nonlonely.
Loneliness was defined using the three-item Revised University of California, Los Angeles, Loneliness Scale score from a decedent’s last HRS interview prior to death. These items included feeling left out, feeling isolated, and lacking companionship. Investigators used this data to create a loneliness variable on previously established cutpoints for “lonely” and “nonlonely” participants. The data was used from the most recent survey prior to death.
Results showed more lonely older adults suffered from health symptoms in the last year of life, compared with nonlonely older adults (69.1% vs. 59.5%; odds ratio, 1.52; 95% confidence interval, 1.30-1.78). These symptoms included being troubled by pain, having difficulty breathing, experiencing severe fatigue, and having periodic confusion.
Patients with loneliness associated with intensity of health care at the end of life were more likely to die in a nursing home than at home, compared with nonlonely adults (OR, 1.68; 95% CI, 1.25-2.27). The lonely patients also were more likely to use life support during their last 2 years of life (OR, 1.41; 95% CI, 1.16-1.70).
“For clinicians, we need to identify end of life as an additional vulnerable time for people who are lonely. Currently, most of our interventions in terms of screening for loneliness are in the outpatient setting, but I would argue that working in hospitals, hospices, nursing homes, and community organizations, where these folks are living and dying, would be useful places to screen for this,” Dr. Abedini said.
The authors had no disclosures.
WASHINGTON – More lonely elderly patients suffered from health symptoms and received very aggressive end of life care than nonlonely elderly patients, according to a study presented at the annual meeting of the Society of General Internal Medicine.
“Loneliness and social isolation are very common problems, especially in older Americans, and inflict about 30%-40% of older Americans. But while we know that this may have implications for their quality [of] life and may actually lead to premature death, we know very little about the end of life experience,” said Nauzley Abedini, MD, MSc, a hospitalist in internal medicine at the University of Michigan, Ann Arbor.
The study sought to determine the association between loneliness and end of life experience as measured by symptom burden, intensity of care, and advance care planning in adults. The pooled cohort study used data from the Health and Retirement Study (HRS) to analyze older Americans (aged 50 years or more) who died between 2004 and 2014. Investigators conducted postmortem “exit interviews” with the next of kin after each participant’s death. There were 2,896 participants included in the survey. Of these participants, 34% (942) were lonely; the remaining 1,954 of elderly adults were classified as nonlonely.
Loneliness was defined using the three-item Revised University of California, Los Angeles, Loneliness Scale score from a decedent’s last HRS interview prior to death. These items included feeling left out, feeling isolated, and lacking companionship. Investigators used this data to create a loneliness variable on previously established cutpoints for “lonely” and “nonlonely” participants. The data was used from the most recent survey prior to death.
Results showed more lonely older adults suffered from health symptoms in the last year of life, compared with nonlonely older adults (69.1% vs. 59.5%; odds ratio, 1.52; 95% confidence interval, 1.30-1.78). These symptoms included being troubled by pain, having difficulty breathing, experiencing severe fatigue, and having periodic confusion.
Patients with loneliness associated with intensity of health care at the end of life were more likely to die in a nursing home than at home, compared with nonlonely adults (OR, 1.68; 95% CI, 1.25-2.27). The lonely patients also were more likely to use life support during their last 2 years of life (OR, 1.41; 95% CI, 1.16-1.70).
“For clinicians, we need to identify end of life as an additional vulnerable time for people who are lonely. Currently, most of our interventions in terms of screening for loneliness are in the outpatient setting, but I would argue that working in hospitals, hospices, nursing homes, and community organizations, where these folks are living and dying, would be useful places to screen for this,” Dr. Abedini said.
The authors had no disclosures.
REPORTING FROM SGIM 2019
TVEC may improve response rates in nonmetastatic TNBC
ATLANTA – Adding intratumoral talimogene laherparepvec (TVEC) to neoadjuvant chemotherapy appears to improve response rates in patients with nonmetastatic triple-negative breast cancer, according to findings from a phase 1 trial.
The pathologic complete response rate (pCR) in nine patients aged 18-70 years with stage T2-T3NO-2 disease who were enrolled in the single-center trial was 55%, whereas the expected rate with neoadjuvant chemotherapy alone was 30%, Hatem Soliman, MD, reported at the annual meeting of the American Association for Cancer Research.
“Even in the patients with residual disease, they had almost complete obliteration of their tumors, as well,” said Dr. Soliman of Moffitt Cancer Center, Tampa, Fla., adding that preliminary analyses of T cell subtypes indicated that CD45RO+ cells, which are associated with activated memory effector phenotype cells, were found in higher percentages in tumor specimens from patients who had pCR vs. those who did not.
“This could be an emerging marker that could be associated with pCR from TVEC,” he said, stressing, however, that “this is preliminary data [that] needs to be borne out in subsequent studies.”
Of the nine participants, six had stage II disease and three had stage III disease, and tumor size was greater than 5 cm in 2 patients, and 2-5 cm in 7 patients. Three received 106 plaque-forming units (PFUs) x 5 injections (dose level 1), and 6 received 106 PFUs for the first injection then 108 PFUs for 4 additional injections. Patients also received neoadjuvant paclitaxel followed by doxorubicin/cyclophosphamide chemotherapy.
They then went on to surgery and were evaluated for pCR.
No dose-limiting toxicities occurred, therefore the maximum-tolerated dose was dose level 2, Dr. Soliman noted.
The most common toxicities due to TVEC were fevers, chills, and injection site reactions, and “these are considered expected side effects of TVEC administration and were manageable,” he said.
Two serious adverse events occurred in the trial: a pulmonary embolism and a postoperative case of severe bradycardia thought to be a vasovagal episode; there were no deaths or any sequelae from these adverse events and they completely resolved.
One latent genital wild type herpes simplex 1 virus (HSV1) reactivation event occurred and was treated with a topical agent.
Early-stage triple-negative breast cancer is associated with a higher risk of early relapse, but attaining a pCR after neoadjuvant chemotherapy is associated with improved prognosis, Dr. Soliman said.
“Historically, standard regimens used for triple-negative breast cancer include an anthracycline and a taxane, and the rates of pCR in those patients hover around 30%,” he said. “Others have looked at the relationship of increased tumor infiltrating lymphocytes (TILs] in triple-negative breast cancers and other breast cancers, and found that those tumors that did have a higher de novo TIL infiltrate, either at the beginning of treatment or acquired during treatment, seemed to have a higher rate of pCR and also seemed to have improved prognosis.”
This suggested that these TIL infiltrates may be both a predictive and prognostic biologic marker for the biology of the disease, he noted.
Further, promising prior research on the role of oncolytic viruses as a potential treatment modality for cancer led to interest in their use during neoadjuvant chemotherapy “with the idea that we could potentially improve pathologic complete response rates both through direct tumor cell lysis of treated tumors, but also through recruitment of a robust antitumor immune response caused by the viruses’ mechanism of action,” he said, adding that “this could be beneficial, particularly in those immunologically ‘cold’ tumors.”
This led to the incorporation of TVEC, a genetically engineered oncolytic HSV1 currently approved for the treatment of melanoma, in this neoadjuvant trial. TVEC preferentially lyses tumor cells over normal tissue to release tumor associated antigens, produces granulocyte-macrophage colony-stimulating factor to activate dendritic cells, and stimulates T cells to provoke an adaptive immune response, he explained.
“[This] then can not only potentially attack tumor cells at the primary site, but then may potentially spread around the body to improve host surveillance and try to eradicate micrometastatic disease, as well,” he said.
The current findings show that adding TVEC to neoadjuvant chemotherapy is feasible at the full Food and Drug Administration–approved dose and has manageable toxicity, he concluded, noting that “a phase 2 single-arm trial of this regimen is ongoing, and we are actively accruing patients to further evaluate the efficacy signal and formally test the hypothesis along with immune correlates.”
Dr. Soliman reported relationships with Eli Lilly, Pfizer, Celgene, AstraZeneca, PUMA, Novartis, Eisai, and Amgen.
SOURCE: Soliman H et al. AACR 2019, Abstract CT040.
ATLANTA – Adding intratumoral talimogene laherparepvec (TVEC) to neoadjuvant chemotherapy appears to improve response rates in patients with nonmetastatic triple-negative breast cancer, according to findings from a phase 1 trial.
The pathologic complete response rate (pCR) in nine patients aged 18-70 years with stage T2-T3NO-2 disease who were enrolled in the single-center trial was 55%, whereas the expected rate with neoadjuvant chemotherapy alone was 30%, Hatem Soliman, MD, reported at the annual meeting of the American Association for Cancer Research.
“Even in the patients with residual disease, they had almost complete obliteration of their tumors, as well,” said Dr. Soliman of Moffitt Cancer Center, Tampa, Fla., adding that preliminary analyses of T cell subtypes indicated that CD45RO+ cells, which are associated with activated memory effector phenotype cells, were found in higher percentages in tumor specimens from patients who had pCR vs. those who did not.
“This could be an emerging marker that could be associated with pCR from TVEC,” he said, stressing, however, that “this is preliminary data [that] needs to be borne out in subsequent studies.”
Of the nine participants, six had stage II disease and three had stage III disease, and tumor size was greater than 5 cm in 2 patients, and 2-5 cm in 7 patients. Three received 106 plaque-forming units (PFUs) x 5 injections (dose level 1), and 6 received 106 PFUs for the first injection then 108 PFUs for 4 additional injections. Patients also received neoadjuvant paclitaxel followed by doxorubicin/cyclophosphamide chemotherapy.
They then went on to surgery and were evaluated for pCR.
No dose-limiting toxicities occurred, therefore the maximum-tolerated dose was dose level 2, Dr. Soliman noted.
The most common toxicities due to TVEC were fevers, chills, and injection site reactions, and “these are considered expected side effects of TVEC administration and were manageable,” he said.
Two serious adverse events occurred in the trial: a pulmonary embolism and a postoperative case of severe bradycardia thought to be a vasovagal episode; there were no deaths or any sequelae from these adverse events and they completely resolved.
One latent genital wild type herpes simplex 1 virus (HSV1) reactivation event occurred and was treated with a topical agent.
Early-stage triple-negative breast cancer is associated with a higher risk of early relapse, but attaining a pCR after neoadjuvant chemotherapy is associated with improved prognosis, Dr. Soliman said.
“Historically, standard regimens used for triple-negative breast cancer include an anthracycline and a taxane, and the rates of pCR in those patients hover around 30%,” he said. “Others have looked at the relationship of increased tumor infiltrating lymphocytes (TILs] in triple-negative breast cancers and other breast cancers, and found that those tumors that did have a higher de novo TIL infiltrate, either at the beginning of treatment or acquired during treatment, seemed to have a higher rate of pCR and also seemed to have improved prognosis.”
This suggested that these TIL infiltrates may be both a predictive and prognostic biologic marker for the biology of the disease, he noted.
Further, promising prior research on the role of oncolytic viruses as a potential treatment modality for cancer led to interest in their use during neoadjuvant chemotherapy “with the idea that we could potentially improve pathologic complete response rates both through direct tumor cell lysis of treated tumors, but also through recruitment of a robust antitumor immune response caused by the viruses’ mechanism of action,” he said, adding that “this could be beneficial, particularly in those immunologically ‘cold’ tumors.”
This led to the incorporation of TVEC, a genetically engineered oncolytic HSV1 currently approved for the treatment of melanoma, in this neoadjuvant trial. TVEC preferentially lyses tumor cells over normal tissue to release tumor associated antigens, produces granulocyte-macrophage colony-stimulating factor to activate dendritic cells, and stimulates T cells to provoke an adaptive immune response, he explained.
“[This] then can not only potentially attack tumor cells at the primary site, but then may potentially spread around the body to improve host surveillance and try to eradicate micrometastatic disease, as well,” he said.
The current findings show that adding TVEC to neoadjuvant chemotherapy is feasible at the full Food and Drug Administration–approved dose and has manageable toxicity, he concluded, noting that “a phase 2 single-arm trial of this regimen is ongoing, and we are actively accruing patients to further evaluate the efficacy signal and formally test the hypothesis along with immune correlates.”
Dr. Soliman reported relationships with Eli Lilly, Pfizer, Celgene, AstraZeneca, PUMA, Novartis, Eisai, and Amgen.
SOURCE: Soliman H et al. AACR 2019, Abstract CT040.
ATLANTA – Adding intratumoral talimogene laherparepvec (TVEC) to neoadjuvant chemotherapy appears to improve response rates in patients with nonmetastatic triple-negative breast cancer, according to findings from a phase 1 trial.
The pathologic complete response rate (pCR) in nine patients aged 18-70 years with stage T2-T3NO-2 disease who were enrolled in the single-center trial was 55%, whereas the expected rate with neoadjuvant chemotherapy alone was 30%, Hatem Soliman, MD, reported at the annual meeting of the American Association for Cancer Research.
“Even in the patients with residual disease, they had almost complete obliteration of their tumors, as well,” said Dr. Soliman of Moffitt Cancer Center, Tampa, Fla., adding that preliminary analyses of T cell subtypes indicated that CD45RO+ cells, which are associated with activated memory effector phenotype cells, were found in higher percentages in tumor specimens from patients who had pCR vs. those who did not.
“This could be an emerging marker that could be associated with pCR from TVEC,” he said, stressing, however, that “this is preliminary data [that] needs to be borne out in subsequent studies.”
Of the nine participants, six had stage II disease and three had stage III disease, and tumor size was greater than 5 cm in 2 patients, and 2-5 cm in 7 patients. Three received 106 plaque-forming units (PFUs) x 5 injections (dose level 1), and 6 received 106 PFUs for the first injection then 108 PFUs for 4 additional injections. Patients also received neoadjuvant paclitaxel followed by doxorubicin/cyclophosphamide chemotherapy.
They then went on to surgery and were evaluated for pCR.
No dose-limiting toxicities occurred, therefore the maximum-tolerated dose was dose level 2, Dr. Soliman noted.
The most common toxicities due to TVEC were fevers, chills, and injection site reactions, and “these are considered expected side effects of TVEC administration and were manageable,” he said.
Two serious adverse events occurred in the trial: a pulmonary embolism and a postoperative case of severe bradycardia thought to be a vasovagal episode; there were no deaths or any sequelae from these adverse events and they completely resolved.
One latent genital wild type herpes simplex 1 virus (HSV1) reactivation event occurred and was treated with a topical agent.
Early-stage triple-negative breast cancer is associated with a higher risk of early relapse, but attaining a pCR after neoadjuvant chemotherapy is associated with improved prognosis, Dr. Soliman said.
“Historically, standard regimens used for triple-negative breast cancer include an anthracycline and a taxane, and the rates of pCR in those patients hover around 30%,” he said. “Others have looked at the relationship of increased tumor infiltrating lymphocytes (TILs] in triple-negative breast cancers and other breast cancers, and found that those tumors that did have a higher de novo TIL infiltrate, either at the beginning of treatment or acquired during treatment, seemed to have a higher rate of pCR and also seemed to have improved prognosis.”
This suggested that these TIL infiltrates may be both a predictive and prognostic biologic marker for the biology of the disease, he noted.
Further, promising prior research on the role of oncolytic viruses as a potential treatment modality for cancer led to interest in their use during neoadjuvant chemotherapy “with the idea that we could potentially improve pathologic complete response rates both through direct tumor cell lysis of treated tumors, but also through recruitment of a robust antitumor immune response caused by the viruses’ mechanism of action,” he said, adding that “this could be beneficial, particularly in those immunologically ‘cold’ tumors.”
This led to the incorporation of TVEC, a genetically engineered oncolytic HSV1 currently approved for the treatment of melanoma, in this neoadjuvant trial. TVEC preferentially lyses tumor cells over normal tissue to release tumor associated antigens, produces granulocyte-macrophage colony-stimulating factor to activate dendritic cells, and stimulates T cells to provoke an adaptive immune response, he explained.
“[This] then can not only potentially attack tumor cells at the primary site, but then may potentially spread around the body to improve host surveillance and try to eradicate micrometastatic disease, as well,” he said.
The current findings show that adding TVEC to neoadjuvant chemotherapy is feasible at the full Food and Drug Administration–approved dose and has manageable toxicity, he concluded, noting that “a phase 2 single-arm trial of this regimen is ongoing, and we are actively accruing patients to further evaluate the efficacy signal and formally test the hypothesis along with immune correlates.”
Dr. Soliman reported relationships with Eli Lilly, Pfizer, Celgene, AstraZeneca, PUMA, Novartis, Eisai, and Amgen.
SOURCE: Soliman H et al. AACR 2019, Abstract CT040.
REPORTING FROM AACR 2019
Key clinical point: Adding TVEC to neoadjuvant chemotherapy for nonmetastatic TNBC appears to improve pCR response rates.
Major finding: The pCR was 55% with TVEC compared with an expected rate of 30% without TVEC.
Study details: A phase 1 study of 9 patients.
Disclosures: Dr. Soliman reported relationships with Eli Lilly, Pfizer, Celgene, AstraZeneca, PUMA, Novartis, Eisai, and Amgen.
Source: Soliman H et al. AACR 2019, Abstract CT040.
Pexidartinib gets ODAC nod for tenosynovial giant cell tumor treatment
The Oncologic Drugs Advisory Committee (ODAC) of the Food and Drug Administration voted to support approval of the small molecule kinase inhibitor pexidartinib for the treatment of adults with symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and is not amenable to improvement with surgery.
The drug was favored by a 12-3 margin (no abstentions), with the majority of panel members agreeing that it offers clinical benefits that outweigh significant risk for elevated liver enzymes and small but real potential for serious or even fatal liver injury.
The FDA usually follows the recommendation of advisory committees in deciding final approval. Daiichi Sankyo plans to market the drug under the trade name Turalio.
Final approval and marketing of the drug will hinge on a mandatory Risk Evaluation and Mitigation Strategy that will require certification of prescribers, patient participation in education about the need for frequent liver function testing and the signs and symptoms of liver injury, and distribution of the drug only to certified pharmacies.
Both the ODAC panel members and pexidartinib’s manufacturer, Daiichi Sankyo, agreed that the drug is effective, but opinions about the degree of clinical benefit and the risk-benefit ratio differed.
TGCT is a rare, nonmalignant, and nonlethal tumor of the synovium, bursae, or tendon sheath that can be locally aggressive, and for some patients completely disabling. Surgery is the primary mode of treatment, but less than 10% of patients have disease that is not amenable to resection; for these patients treatment options are limited, because there are no approved systemic therapies for the disease.
ENLIVEN
Evidence submitted to support the application comes from the phase 3 ENLIVEN trial, in which patients with TGCT not amenable to surgery were randomly assigned to receive pexidartinib or placebo. The trial was designed to enroll 126 patients to provide 90% power to detect a difference in objective response rate at a two-sided alpha level of 0.05, assuming an overall response rate of 10% with placebo, and 35% with pexidartinib.
The actual trial enrollment, however, fell a little short, with a total of 120 patients randomized.
The ORR at week 25 as assessed by blinded independent central reviewers, the primary endpoint, was 39% for the 61 patients in the pexidartinib group, compared with 0% for the 59 patients in the placebo group (P less than .0001).
There were also statistically significant improvements in the pexidartinib arm at week 25 in the secondary endpoints of mean change in range of motion (ROM), ORR by tumor-volume score at week 25, mean change from baseline in the Patient-Reported Outcomes Measurement Information physical function scale, and mean change in the Worst Stiffness numeric rating scale item. There were no significant differences between the groups for worst pain, however.
The FDA briefing document notes that “interpretation of the results of the secondary endpoints should be viewed with caution as there was a high proportion of missing data at week 25 for ROM, physical function, and worst stiffness (27%, 43% 43%, respectively); the proportion of patients with missing data was similar across study arms.”
Risk and benefits
The major issues before the ODAC included the validity of clinical outcome assessment given the large chunks of missing data and the major adverse event of liver injury, with a majority of patients on pexidartinib experiencing transaminase elevations. The potential for liver injury may be exacerbated by chronic use of the drug or by drugs used to treat comorbidities such as diabetes or cardiovascular disease, several panel members noted.
During the clinical development program for the drug, 2 of 768 patients who received the drug developed irreversible liver injury leading to liver transplant in 1 patient and death in the other.
Many of the panelists who voted to support approval did so with some reluctance about the adverse events. For example, Karin Anton Calis, PharmD, of the National Institutes of Health voted yes because of the efficacy of the drug in a disabling condition.
“Hopefully it will be used in a restricted system so that there can be adequate monitoring, but I’m still concerned about those patients that may have this unpredictable liver toxicity, “ he said.
Victor M. Villalobos MD, PhD, from the University of Colorado at Denver, Aurora, also voted yes.
“This is an ultra-rare disease with no good therapies available to patients and it can be highly morbid, and I feel that getting real-world data on how we can use this drug in a safe and effective manner will be really important to the academic community going forward,” he said.
Other panel members who also voted to support approval expressed concerns about the hepatotoxicity, but noted that the drug has the potential to change lives, as attested by TCGT patients who spoke during the public comment portion of the meeting.
However, panelist Doris Strader, MD, from the University of Vermont, Burlington, said that she voted no because, while she was sensitive to the potential benefits of the drug for some patients, “I was concerned about the missing data and was not convinced that there was clinically meaningful benefit. Likewise, while I understand that the hepatic injury is not liver failure, I am concerned that this may be persistent for a long time, and I worry that there is not enough to suggest that there is going to be rigorous monitoring of patients over their lifetimes.”
The Oncologic Drugs Advisory Committee (ODAC) of the Food and Drug Administration voted to support approval of the small molecule kinase inhibitor pexidartinib for the treatment of adults with symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and is not amenable to improvement with surgery.
The drug was favored by a 12-3 margin (no abstentions), with the majority of panel members agreeing that it offers clinical benefits that outweigh significant risk for elevated liver enzymes and small but real potential for serious or even fatal liver injury.
The FDA usually follows the recommendation of advisory committees in deciding final approval. Daiichi Sankyo plans to market the drug under the trade name Turalio.
Final approval and marketing of the drug will hinge on a mandatory Risk Evaluation and Mitigation Strategy that will require certification of prescribers, patient participation in education about the need for frequent liver function testing and the signs and symptoms of liver injury, and distribution of the drug only to certified pharmacies.
Both the ODAC panel members and pexidartinib’s manufacturer, Daiichi Sankyo, agreed that the drug is effective, but opinions about the degree of clinical benefit and the risk-benefit ratio differed.
TGCT is a rare, nonmalignant, and nonlethal tumor of the synovium, bursae, or tendon sheath that can be locally aggressive, and for some patients completely disabling. Surgery is the primary mode of treatment, but less than 10% of patients have disease that is not amenable to resection; for these patients treatment options are limited, because there are no approved systemic therapies for the disease.
ENLIVEN
Evidence submitted to support the application comes from the phase 3 ENLIVEN trial, in which patients with TGCT not amenable to surgery were randomly assigned to receive pexidartinib or placebo. The trial was designed to enroll 126 patients to provide 90% power to detect a difference in objective response rate at a two-sided alpha level of 0.05, assuming an overall response rate of 10% with placebo, and 35% with pexidartinib.
The actual trial enrollment, however, fell a little short, with a total of 120 patients randomized.
The ORR at week 25 as assessed by blinded independent central reviewers, the primary endpoint, was 39% for the 61 patients in the pexidartinib group, compared with 0% for the 59 patients in the placebo group (P less than .0001).
There were also statistically significant improvements in the pexidartinib arm at week 25 in the secondary endpoints of mean change in range of motion (ROM), ORR by tumor-volume score at week 25, mean change from baseline in the Patient-Reported Outcomes Measurement Information physical function scale, and mean change in the Worst Stiffness numeric rating scale item. There were no significant differences between the groups for worst pain, however.
The FDA briefing document notes that “interpretation of the results of the secondary endpoints should be viewed with caution as there was a high proportion of missing data at week 25 for ROM, physical function, and worst stiffness (27%, 43% 43%, respectively); the proportion of patients with missing data was similar across study arms.”
Risk and benefits
The major issues before the ODAC included the validity of clinical outcome assessment given the large chunks of missing data and the major adverse event of liver injury, with a majority of patients on pexidartinib experiencing transaminase elevations. The potential for liver injury may be exacerbated by chronic use of the drug or by drugs used to treat comorbidities such as diabetes or cardiovascular disease, several panel members noted.
During the clinical development program for the drug, 2 of 768 patients who received the drug developed irreversible liver injury leading to liver transplant in 1 patient and death in the other.
Many of the panelists who voted to support approval did so with some reluctance about the adverse events. For example, Karin Anton Calis, PharmD, of the National Institutes of Health voted yes because of the efficacy of the drug in a disabling condition.
“Hopefully it will be used in a restricted system so that there can be adequate monitoring, but I’m still concerned about those patients that may have this unpredictable liver toxicity, “ he said.
Victor M. Villalobos MD, PhD, from the University of Colorado at Denver, Aurora, also voted yes.
“This is an ultra-rare disease with no good therapies available to patients and it can be highly morbid, and I feel that getting real-world data on how we can use this drug in a safe and effective manner will be really important to the academic community going forward,” he said.
Other panel members who also voted to support approval expressed concerns about the hepatotoxicity, but noted that the drug has the potential to change lives, as attested by TCGT patients who spoke during the public comment portion of the meeting.
However, panelist Doris Strader, MD, from the University of Vermont, Burlington, said that she voted no because, while she was sensitive to the potential benefits of the drug for some patients, “I was concerned about the missing data and was not convinced that there was clinically meaningful benefit. Likewise, while I understand that the hepatic injury is not liver failure, I am concerned that this may be persistent for a long time, and I worry that there is not enough to suggest that there is going to be rigorous monitoring of patients over their lifetimes.”
The Oncologic Drugs Advisory Committee (ODAC) of the Food and Drug Administration voted to support approval of the small molecule kinase inhibitor pexidartinib for the treatment of adults with symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and is not amenable to improvement with surgery.
The drug was favored by a 12-3 margin (no abstentions), with the majority of panel members agreeing that it offers clinical benefits that outweigh significant risk for elevated liver enzymes and small but real potential for serious or even fatal liver injury.
The FDA usually follows the recommendation of advisory committees in deciding final approval. Daiichi Sankyo plans to market the drug under the trade name Turalio.
Final approval and marketing of the drug will hinge on a mandatory Risk Evaluation and Mitigation Strategy that will require certification of prescribers, patient participation in education about the need for frequent liver function testing and the signs and symptoms of liver injury, and distribution of the drug only to certified pharmacies.
Both the ODAC panel members and pexidartinib’s manufacturer, Daiichi Sankyo, agreed that the drug is effective, but opinions about the degree of clinical benefit and the risk-benefit ratio differed.
TGCT is a rare, nonmalignant, and nonlethal tumor of the synovium, bursae, or tendon sheath that can be locally aggressive, and for some patients completely disabling. Surgery is the primary mode of treatment, but less than 10% of patients have disease that is not amenable to resection; for these patients treatment options are limited, because there are no approved systemic therapies for the disease.
ENLIVEN
Evidence submitted to support the application comes from the phase 3 ENLIVEN trial, in which patients with TGCT not amenable to surgery were randomly assigned to receive pexidartinib or placebo. The trial was designed to enroll 126 patients to provide 90% power to detect a difference in objective response rate at a two-sided alpha level of 0.05, assuming an overall response rate of 10% with placebo, and 35% with pexidartinib.
The actual trial enrollment, however, fell a little short, with a total of 120 patients randomized.
The ORR at week 25 as assessed by blinded independent central reviewers, the primary endpoint, was 39% for the 61 patients in the pexidartinib group, compared with 0% for the 59 patients in the placebo group (P less than .0001).
There were also statistically significant improvements in the pexidartinib arm at week 25 in the secondary endpoints of mean change in range of motion (ROM), ORR by tumor-volume score at week 25, mean change from baseline in the Patient-Reported Outcomes Measurement Information physical function scale, and mean change in the Worst Stiffness numeric rating scale item. There were no significant differences between the groups for worst pain, however.
The FDA briefing document notes that “interpretation of the results of the secondary endpoints should be viewed with caution as there was a high proportion of missing data at week 25 for ROM, physical function, and worst stiffness (27%, 43% 43%, respectively); the proportion of patients with missing data was similar across study arms.”
Risk and benefits
The major issues before the ODAC included the validity of clinical outcome assessment given the large chunks of missing data and the major adverse event of liver injury, with a majority of patients on pexidartinib experiencing transaminase elevations. The potential for liver injury may be exacerbated by chronic use of the drug or by drugs used to treat comorbidities such as diabetes or cardiovascular disease, several panel members noted.
During the clinical development program for the drug, 2 of 768 patients who received the drug developed irreversible liver injury leading to liver transplant in 1 patient and death in the other.
Many of the panelists who voted to support approval did so with some reluctance about the adverse events. For example, Karin Anton Calis, PharmD, of the National Institutes of Health voted yes because of the efficacy of the drug in a disabling condition.
“Hopefully it will be used in a restricted system so that there can be adequate monitoring, but I’m still concerned about those patients that may have this unpredictable liver toxicity, “ he said.
Victor M. Villalobos MD, PhD, from the University of Colorado at Denver, Aurora, also voted yes.
“This is an ultra-rare disease with no good therapies available to patients and it can be highly morbid, and I feel that getting real-world data on how we can use this drug in a safe and effective manner will be really important to the academic community going forward,” he said.
Other panel members who also voted to support approval expressed concerns about the hepatotoxicity, but noted that the drug has the potential to change lives, as attested by TCGT patients who spoke during the public comment portion of the meeting.
However, panelist Doris Strader, MD, from the University of Vermont, Burlington, said that she voted no because, while she was sensitive to the potential benefits of the drug for some patients, “I was concerned about the missing data and was not convinced that there was clinically meaningful benefit. Likewise, while I understand that the hepatic injury is not liver failure, I am concerned that this may be persistent for a long time, and I worry that there is not enough to suggest that there is going to be rigorous monitoring of patients over their lifetimes.”
Immunotherapy Induces Improvements in PML
Key clinical point: Adoptive transfer of donor-derived T cells represents a potentially life-saving strategy for patients with progressive multifocal leukoencephalopathy.
Major finding: Seven of 12 patients stabilized and, in some cases, experienced significant neurological improvement.
Study details: A pilot study including 12 patients with refractory PML.
Disclosures: The study was funded by NINDS. The investigators disclosed no conflicts related to the study.
Citation: Cortese I et al. AAN 2019, Abstract Plen01.002.
Key clinical point: Adoptive transfer of donor-derived T cells represents a potentially life-saving strategy for patients with progressive multifocal leukoencephalopathy.
Major finding: Seven of 12 patients stabilized and, in some cases, experienced significant neurological improvement.
Study details: A pilot study including 12 patients with refractory PML.
Disclosures: The study was funded by NINDS. The investigators disclosed no conflicts related to the study.
Citation: Cortese I et al. AAN 2019, Abstract Plen01.002.
Key clinical point: Adoptive transfer of donor-derived T cells represents a potentially life-saving strategy for patients with progressive multifocal leukoencephalopathy.
Major finding: Seven of 12 patients stabilized and, in some cases, experienced significant neurological improvement.
Study details: A pilot study including 12 patients with refractory PML.
Disclosures: The study was funded by NINDS. The investigators disclosed no conflicts related to the study.
Citation: Cortese I et al. AAN 2019, Abstract Plen01.002.
Criterion Based on the Central Vein Sign Distinguishes Between MS and Mimics
Key clinical point: Applying a criterion of three lesions with the central vein sign distinguishes between multiple sclerosis and its mimics.
Major finding: The criterion has a sensitivity and specificity of 61.9% and 89.0%, respectively.
Study details: A multicenter study of 606 participants with clinically isolated syndrome, multiple sclerosis, and multiple sclerosis mimics.
Disclosures: Dr. Sinnecker reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Actelion.
Citation: Sinnecker T et al. AAN 2019, Abstract S6.002.
Key clinical point: Applying a criterion of three lesions with the central vein sign distinguishes between multiple sclerosis and its mimics.
Major finding: The criterion has a sensitivity and specificity of 61.9% and 89.0%, respectively.
Study details: A multicenter study of 606 participants with clinically isolated syndrome, multiple sclerosis, and multiple sclerosis mimics.
Disclosures: Dr. Sinnecker reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Actelion.
Citation: Sinnecker T et al. AAN 2019, Abstract S6.002.
Key clinical point: Applying a criterion of three lesions with the central vein sign distinguishes between multiple sclerosis and its mimics.
Major finding: The criterion has a sensitivity and specificity of 61.9% and 89.0%, respectively.
Study details: A multicenter study of 606 participants with clinically isolated syndrome, multiple sclerosis, and multiple sclerosis mimics.
Disclosures: Dr. Sinnecker reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Actelion.
Citation: Sinnecker T et al. AAN 2019, Abstract S6.002.
Multiple Sclerosis May Not Flare Up After Pregnancy
Key clinical point: Women with MS may be able to have children, breastfeed, and resume treatment without experiencing an increased risk of relapse during the postpartum period.
Major finding: Patients’ annualized relapse rate was 0.39 pre-pregnancy, 0.07-0.14 during pregnancy, 0.27 in the first 3 months postpartum, and 0.37 at 4-6 months postpartum.
Study details: An analysis of prospectively collected data from 466 pregnancies among 375 women with MS between 2008 and 2016.
Disclosures: The study was supported by the National Multiple Sclerosis Society. The researchers had no disclosures.
Citation: Langer-Gould A et al. AAN 2019, Abstract S6.007.
Key clinical point: Women with MS may be able to have children, breastfeed, and resume treatment without experiencing an increased risk of relapse during the postpartum period.
Major finding: Patients’ annualized relapse rate was 0.39 pre-pregnancy, 0.07-0.14 during pregnancy, 0.27 in the first 3 months postpartum, and 0.37 at 4-6 months postpartum.
Study details: An analysis of prospectively collected data from 466 pregnancies among 375 women with MS between 2008 and 2016.
Disclosures: The study was supported by the National Multiple Sclerosis Society. The researchers had no disclosures.
Citation: Langer-Gould A et al. AAN 2019, Abstract S6.007.
Key clinical point: Women with MS may be able to have children, breastfeed, and resume treatment without experiencing an increased risk of relapse during the postpartum period.
Major finding: Patients’ annualized relapse rate was 0.39 pre-pregnancy, 0.07-0.14 during pregnancy, 0.27 in the first 3 months postpartum, and 0.37 at 4-6 months postpartum.
Study details: An analysis of prospectively collected data from 466 pregnancies among 375 women with MS between 2008 and 2016.
Disclosures: The study was supported by the National Multiple Sclerosis Society. The researchers had no disclosures.
Citation: Langer-Gould A et al. AAN 2019, Abstract S6.007.
Pancreatic cancers often contained targetable mutations
researchers reported in Gastroenterology.
“We identified mutations in genes that could contribute to progression of intraductal papillary mucinous neoplasms into malignancies. These alterations might be used as biomarkers for early detection,” wrote Aatur D. Singhi, MD, PhD, of the University of Pittsburgh and associates.
The most common genomic mutations in pancreatic ductal adenocarcinoma (PDAC) involve KRAS, TP53, CDKN2A, and SMAD4, none of which can be treated by currently approved targeted agents. But PDACs are genomically very heterogeneous and contain low levels (less than 5%) of many other mutations, the researchers noted. In small studies, these low-prevalence mutations included kinase gene amplifications and rearrangements, which may be useful as treatment targets or predictive biomarkers of response.
To further characterize PDAC mutations and their relative penetrance, the researchers performed targeted genomic profile analyses of 3,594 PDAC tumor specimens from an international cohort. The tests included capture-based targeted genomic profiling of up to 315 cancer-linked genes and the intron regions of 28 genes that are rearranged in cancer cells. The researchers classified genomic alterations based on published signaling pathways, including receptor tyrosine kinase/ras/mitogen-activated protein kinase (RTK/ras/MAPK) activation, DNA damage repair, cell cycle control, transforming growth factor beta signaling, histone modification, switching/sucrose nonfermenting complex, phosphoinositide 3-kinase/mammalian target of rapamycin signaling, Wnt/beta-catenin pathway, RNA splicing, Notch pathway, angiogenesis, and hedgehog signaling. In addition, they analyzed tumor mutation burden in 1,021 samples and microsatellite instability status in 2,563 samples.
In all, the samples contained 19,120 genomic alterations of 317 genes. A total of 608 (17%) specimens harbored mutations considered actionable targets. These involved either the RTK/ras/MAPK signaling or DNA damage repair pathways. As expected, KRAS mutations were most common, but their penetrance (88%) was lower than in prior studies. This might be because the current study covered both resectable and nonresectable PDACs, while past studies tended to focus on resected PDACs only, the researchers said. Importantly, the 12% of KRAS wild-type PDACs often harbored other potentially targetable alterations of genes in the RTK/ras/MAPK pathway, such as kinase fusion, amplification, missense mutations, and intragenic-in-frame deletions.
A total of 81% of samples contained alterations of TP53 or other genes involved in DNA damage repair. Reflecting prior studies, the penetrance of individual DNA damage repair mutations was low – usually less than 5%. Most germline mutations involved the BRCA-FANC DNA repair pathway, and these may be targetable with agents such as poly (ADP-ribose) polymerase inhibitors and DNA strand-damaging, platinum-based cytotoxic regimens, the investigators wrote.
Among 3,117 samples with clinicopathologic data, 51% were from the primary tumor and the rest were from distal metastases of the liver, lung, nonregional lymph nodes, peritoneum, omentum, and other sites. Not surprisingly, BRCA1 and BRCA2 mutations were more common in younger patients, but KRAS, SMAD4, DNMT3A, and AP mutations were more common in older patients, and mutational frequencies also varied by sex, primary versus metastatic tumor status, and site of metastasis.
“The complex genomic heterogeneity in otherwise histologically similar PDACs suggests a one-size-fits-all approach to treating patients will not be successful,” they concluded. “Targeted genomic profiling of known genomic alterations across multiple tumor types highlights potentially targetable and predictive biomarkers for treatment in a significant subset of PDACs.”
Funders included the Pancreatic Cancer Action Network, the National Pancreas Foundation, and the Sky Foundation. Dr. Singhi and two coinvestigators reported receiving honoraria from Foundation Medicine, and seven other coinvestigators reported employment by and stock ownership in Foundation Medicine. No other disclosures were reported.
SOURCE: Singhi AD et al. Gastroenterology. 2019 Mar 2. doi: 10.1053/j.gastro.2019.02.03.
researchers reported in Gastroenterology.
“We identified mutations in genes that could contribute to progression of intraductal papillary mucinous neoplasms into malignancies. These alterations might be used as biomarkers for early detection,” wrote Aatur D. Singhi, MD, PhD, of the University of Pittsburgh and associates.
The most common genomic mutations in pancreatic ductal adenocarcinoma (PDAC) involve KRAS, TP53, CDKN2A, and SMAD4, none of which can be treated by currently approved targeted agents. But PDACs are genomically very heterogeneous and contain low levels (less than 5%) of many other mutations, the researchers noted. In small studies, these low-prevalence mutations included kinase gene amplifications and rearrangements, which may be useful as treatment targets or predictive biomarkers of response.
To further characterize PDAC mutations and their relative penetrance, the researchers performed targeted genomic profile analyses of 3,594 PDAC tumor specimens from an international cohort. The tests included capture-based targeted genomic profiling of up to 315 cancer-linked genes and the intron regions of 28 genes that are rearranged in cancer cells. The researchers classified genomic alterations based on published signaling pathways, including receptor tyrosine kinase/ras/mitogen-activated protein kinase (RTK/ras/MAPK) activation, DNA damage repair, cell cycle control, transforming growth factor beta signaling, histone modification, switching/sucrose nonfermenting complex, phosphoinositide 3-kinase/mammalian target of rapamycin signaling, Wnt/beta-catenin pathway, RNA splicing, Notch pathway, angiogenesis, and hedgehog signaling. In addition, they analyzed tumor mutation burden in 1,021 samples and microsatellite instability status in 2,563 samples.
In all, the samples contained 19,120 genomic alterations of 317 genes. A total of 608 (17%) specimens harbored mutations considered actionable targets. These involved either the RTK/ras/MAPK signaling or DNA damage repair pathways. As expected, KRAS mutations were most common, but their penetrance (88%) was lower than in prior studies. This might be because the current study covered both resectable and nonresectable PDACs, while past studies tended to focus on resected PDACs only, the researchers said. Importantly, the 12% of KRAS wild-type PDACs often harbored other potentially targetable alterations of genes in the RTK/ras/MAPK pathway, such as kinase fusion, amplification, missense mutations, and intragenic-in-frame deletions.
A total of 81% of samples contained alterations of TP53 or other genes involved in DNA damage repair. Reflecting prior studies, the penetrance of individual DNA damage repair mutations was low – usually less than 5%. Most germline mutations involved the BRCA-FANC DNA repair pathway, and these may be targetable with agents such as poly (ADP-ribose) polymerase inhibitors and DNA strand-damaging, platinum-based cytotoxic regimens, the investigators wrote.
Among 3,117 samples with clinicopathologic data, 51% were from the primary tumor and the rest were from distal metastases of the liver, lung, nonregional lymph nodes, peritoneum, omentum, and other sites. Not surprisingly, BRCA1 and BRCA2 mutations were more common in younger patients, but KRAS, SMAD4, DNMT3A, and AP mutations were more common in older patients, and mutational frequencies also varied by sex, primary versus metastatic tumor status, and site of metastasis.
“The complex genomic heterogeneity in otherwise histologically similar PDACs suggests a one-size-fits-all approach to treating patients will not be successful,” they concluded. “Targeted genomic profiling of known genomic alterations across multiple tumor types highlights potentially targetable and predictive biomarkers for treatment in a significant subset of PDACs.”
Funders included the Pancreatic Cancer Action Network, the National Pancreas Foundation, and the Sky Foundation. Dr. Singhi and two coinvestigators reported receiving honoraria from Foundation Medicine, and seven other coinvestigators reported employment by and stock ownership in Foundation Medicine. No other disclosures were reported.
SOURCE: Singhi AD et al. Gastroenterology. 2019 Mar 2. doi: 10.1053/j.gastro.2019.02.03.
researchers reported in Gastroenterology.
“We identified mutations in genes that could contribute to progression of intraductal papillary mucinous neoplasms into malignancies. These alterations might be used as biomarkers for early detection,” wrote Aatur D. Singhi, MD, PhD, of the University of Pittsburgh and associates.
The most common genomic mutations in pancreatic ductal adenocarcinoma (PDAC) involve KRAS, TP53, CDKN2A, and SMAD4, none of which can be treated by currently approved targeted agents. But PDACs are genomically very heterogeneous and contain low levels (less than 5%) of many other mutations, the researchers noted. In small studies, these low-prevalence mutations included kinase gene amplifications and rearrangements, which may be useful as treatment targets or predictive biomarkers of response.
To further characterize PDAC mutations and their relative penetrance, the researchers performed targeted genomic profile analyses of 3,594 PDAC tumor specimens from an international cohort. The tests included capture-based targeted genomic profiling of up to 315 cancer-linked genes and the intron regions of 28 genes that are rearranged in cancer cells. The researchers classified genomic alterations based on published signaling pathways, including receptor tyrosine kinase/ras/mitogen-activated protein kinase (RTK/ras/MAPK) activation, DNA damage repair, cell cycle control, transforming growth factor beta signaling, histone modification, switching/sucrose nonfermenting complex, phosphoinositide 3-kinase/mammalian target of rapamycin signaling, Wnt/beta-catenin pathway, RNA splicing, Notch pathway, angiogenesis, and hedgehog signaling. In addition, they analyzed tumor mutation burden in 1,021 samples and microsatellite instability status in 2,563 samples.
In all, the samples contained 19,120 genomic alterations of 317 genes. A total of 608 (17%) specimens harbored mutations considered actionable targets. These involved either the RTK/ras/MAPK signaling or DNA damage repair pathways. As expected, KRAS mutations were most common, but their penetrance (88%) was lower than in prior studies. This might be because the current study covered both resectable and nonresectable PDACs, while past studies tended to focus on resected PDACs only, the researchers said. Importantly, the 12% of KRAS wild-type PDACs often harbored other potentially targetable alterations of genes in the RTK/ras/MAPK pathway, such as kinase fusion, amplification, missense mutations, and intragenic-in-frame deletions.
A total of 81% of samples contained alterations of TP53 or other genes involved in DNA damage repair. Reflecting prior studies, the penetrance of individual DNA damage repair mutations was low – usually less than 5%. Most germline mutations involved the BRCA-FANC DNA repair pathway, and these may be targetable with agents such as poly (ADP-ribose) polymerase inhibitors and DNA strand-damaging, platinum-based cytotoxic regimens, the investigators wrote.
Among 3,117 samples with clinicopathologic data, 51% were from the primary tumor and the rest were from distal metastases of the liver, lung, nonregional lymph nodes, peritoneum, omentum, and other sites. Not surprisingly, BRCA1 and BRCA2 mutations were more common in younger patients, but KRAS, SMAD4, DNMT3A, and AP mutations were more common in older patients, and mutational frequencies also varied by sex, primary versus metastatic tumor status, and site of metastasis.
“The complex genomic heterogeneity in otherwise histologically similar PDACs suggests a one-size-fits-all approach to treating patients will not be successful,” they concluded. “Targeted genomic profiling of known genomic alterations across multiple tumor types highlights potentially targetable and predictive biomarkers for treatment in a significant subset of PDACs.”
Funders included the Pancreatic Cancer Action Network, the National Pancreas Foundation, and the Sky Foundation. Dr. Singhi and two coinvestigators reported receiving honoraria from Foundation Medicine, and seven other coinvestigators reported employment by and stock ownership in Foundation Medicine. No other disclosures were reported.
SOURCE: Singhi AD et al. Gastroenterology. 2019 Mar 2. doi: 10.1053/j.gastro.2019.02.03.
FROM GASTROENTEROLOGY
Young children with neuromuscular disease are vulnerable to respiratory viruses
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.
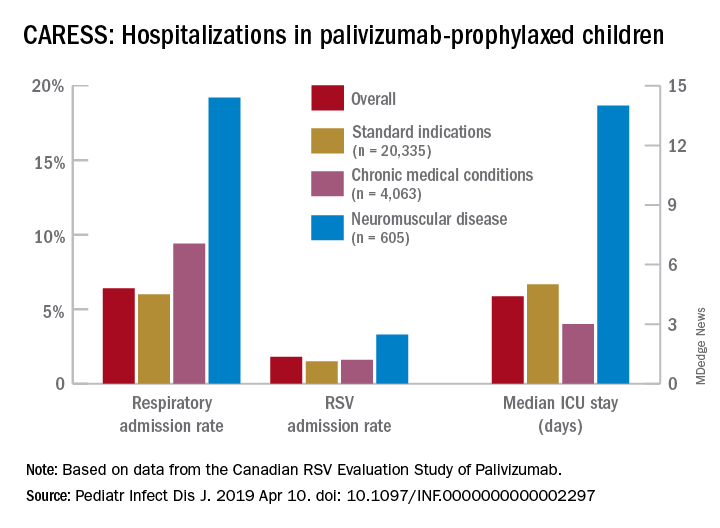
RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
This highlights the need for new vaccines
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.



