User login
Telehealth consults for vascular surgery reimbursed at par with office visits
NEW YORK – Telehealth should be embraced by vascular surgeons for their own self-interest independent of the evidence that it is well accepted and more convenient for patients, according to an update on an evolution that is already underway.
“One of the great advantages of telehealth is the efficacy of time for the clinician,” John W. Hallett, MD, professor of vascular surgery at the Medical University of South Carolina, Charleston, said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
This efficiency is purchased with no loss of revenue, he added. He said that many clinicians are unaware of the opportunity this affords.
“Almost every payer reimburses telehealth visit at the same rate as that of an office visit,” Dr. Hallett explained. The only additional step is adding a “GT” modifier when billing Medicare or a “95” modifier when billing private payers.
Telemedicine is not a new concept. Published studies date back decades, but this interaction is increasingly understood to be the future. Along with an increasing array of sensors employing smartphone technology to allow physicians remote access to vital signs and other clinical data, patient attitudes have changed.
“Patients like telemedicine. It is convenient for them,” said Dr. Hallett, who noted that many providers are recognizing telemedicine as a potential marketing tool.
“On my way in from the airport yesterday, there was an advertisement for telemedicine from NYU on the television in the cab,” said Dr. Hallett, referring to the New York University health system.
The data supporting the benefits of telemedicine even include studies undertaken in vascular surgery patients. In one recent retrospective study cited by Dr. Hallett, substantial time and travel costs were saved for every vascular surgery consult conducted by telemedicine rather than in an office visit (Paquette S et al. Ann Vasc Surg. 2019;59:167-172).
“There was no difference in the rate of complications, and 94% of the patients considered the telehealth consultation adequate,” Dr. Hallett said.
He said there is urgency for vascular surgeons to pursue telemedicine. With the number of individuals over the age of 65 growing by thousands in the United States every day, there will be increasing pressure on the relatively fixed pool of vascular surgeons to improve their efficiency.
In addition, telemedicine is coming whether vascular surgeons like it or not.
“Patients are becoming more interested in looking at an app on their smartphone than coming to the office,” said Tony S. Das, MD, an interventional cardiologist who practices in Dallas. Dr. Das also spoke about the value of telemedicine for the vascular and cardiovascular surgeon at the VIETHsymposium.
In his overview, Dr. Das spoke about telehealth in the context of the estimated $12 billion dollars that will be spent on digital health in vascular medicine by 2021. The growth in digital health in vascular medicine is a reflection of a global change in clinical care. According to Dr. Das, there were more than 600 vendors of wearable sensors to monitor disease and health at a recent consumer electronics convention.
“This technology is here to stay,” said Dr. Das, who, appropriately, was not present at the symposium but delivered his presentation remotely.
Both the Centers for Medicare and Medicaid Services and the Food and Drug Administration have digital health action plans, according to Dr. Das. The CMS has already developed reimbursement codes to pay for remote monitoring services and more are expected.
Calling this type of telehealth “untethered vascular care,” Dr. Das agreed with Dr. Hallett that an evolution is coming whether vascular surgeons choose to get on board now or are forced to take action later.
SOURCE: VIETHsymposium
NEW YORK – Telehealth should be embraced by vascular surgeons for their own self-interest independent of the evidence that it is well accepted and more convenient for patients, according to an update on an evolution that is already underway.
“One of the great advantages of telehealth is the efficacy of time for the clinician,” John W. Hallett, MD, professor of vascular surgery at the Medical University of South Carolina, Charleston, said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
This efficiency is purchased with no loss of revenue, he added. He said that many clinicians are unaware of the opportunity this affords.
“Almost every payer reimburses telehealth visit at the same rate as that of an office visit,” Dr. Hallett explained. The only additional step is adding a “GT” modifier when billing Medicare or a “95” modifier when billing private payers.
Telemedicine is not a new concept. Published studies date back decades, but this interaction is increasingly understood to be the future. Along with an increasing array of sensors employing smartphone technology to allow physicians remote access to vital signs and other clinical data, patient attitudes have changed.
“Patients like telemedicine. It is convenient for them,” said Dr. Hallett, who noted that many providers are recognizing telemedicine as a potential marketing tool.
“On my way in from the airport yesterday, there was an advertisement for telemedicine from NYU on the television in the cab,” said Dr. Hallett, referring to the New York University health system.
The data supporting the benefits of telemedicine even include studies undertaken in vascular surgery patients. In one recent retrospective study cited by Dr. Hallett, substantial time and travel costs were saved for every vascular surgery consult conducted by telemedicine rather than in an office visit (Paquette S et al. Ann Vasc Surg. 2019;59:167-172).
“There was no difference in the rate of complications, and 94% of the patients considered the telehealth consultation adequate,” Dr. Hallett said.
He said there is urgency for vascular surgeons to pursue telemedicine. With the number of individuals over the age of 65 growing by thousands in the United States every day, there will be increasing pressure on the relatively fixed pool of vascular surgeons to improve their efficiency.
In addition, telemedicine is coming whether vascular surgeons like it or not.
“Patients are becoming more interested in looking at an app on their smartphone than coming to the office,” said Tony S. Das, MD, an interventional cardiologist who practices in Dallas. Dr. Das also spoke about the value of telemedicine for the vascular and cardiovascular surgeon at the VIETHsymposium.
In his overview, Dr. Das spoke about telehealth in the context of the estimated $12 billion dollars that will be spent on digital health in vascular medicine by 2021. The growth in digital health in vascular medicine is a reflection of a global change in clinical care. According to Dr. Das, there were more than 600 vendors of wearable sensors to monitor disease and health at a recent consumer electronics convention.
“This technology is here to stay,” said Dr. Das, who, appropriately, was not present at the symposium but delivered his presentation remotely.
Both the Centers for Medicare and Medicaid Services and the Food and Drug Administration have digital health action plans, according to Dr. Das. The CMS has already developed reimbursement codes to pay for remote monitoring services and more are expected.
Calling this type of telehealth “untethered vascular care,” Dr. Das agreed with Dr. Hallett that an evolution is coming whether vascular surgeons choose to get on board now or are forced to take action later.
SOURCE: VIETHsymposium
NEW YORK – Telehealth should be embraced by vascular surgeons for their own self-interest independent of the evidence that it is well accepted and more convenient for patients, according to an update on an evolution that is already underway.
“One of the great advantages of telehealth is the efficacy of time for the clinician,” John W. Hallett, MD, professor of vascular surgery at the Medical University of South Carolina, Charleston, said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
This efficiency is purchased with no loss of revenue, he added. He said that many clinicians are unaware of the opportunity this affords.
“Almost every payer reimburses telehealth visit at the same rate as that of an office visit,” Dr. Hallett explained. The only additional step is adding a “GT” modifier when billing Medicare or a “95” modifier when billing private payers.
Telemedicine is not a new concept. Published studies date back decades, but this interaction is increasingly understood to be the future. Along with an increasing array of sensors employing smartphone technology to allow physicians remote access to vital signs and other clinical data, patient attitudes have changed.
“Patients like telemedicine. It is convenient for them,” said Dr. Hallett, who noted that many providers are recognizing telemedicine as a potential marketing tool.
“On my way in from the airport yesterday, there was an advertisement for telemedicine from NYU on the television in the cab,” said Dr. Hallett, referring to the New York University health system.
The data supporting the benefits of telemedicine even include studies undertaken in vascular surgery patients. In one recent retrospective study cited by Dr. Hallett, substantial time and travel costs were saved for every vascular surgery consult conducted by telemedicine rather than in an office visit (Paquette S et al. Ann Vasc Surg. 2019;59:167-172).
“There was no difference in the rate of complications, and 94% of the patients considered the telehealth consultation adequate,” Dr. Hallett said.
He said there is urgency for vascular surgeons to pursue telemedicine. With the number of individuals over the age of 65 growing by thousands in the United States every day, there will be increasing pressure on the relatively fixed pool of vascular surgeons to improve their efficiency.
In addition, telemedicine is coming whether vascular surgeons like it or not.
“Patients are becoming more interested in looking at an app on their smartphone than coming to the office,” said Tony S. Das, MD, an interventional cardiologist who practices in Dallas. Dr. Das also spoke about the value of telemedicine for the vascular and cardiovascular surgeon at the VIETHsymposium.
In his overview, Dr. Das spoke about telehealth in the context of the estimated $12 billion dollars that will be spent on digital health in vascular medicine by 2021. The growth in digital health in vascular medicine is a reflection of a global change in clinical care. According to Dr. Das, there were more than 600 vendors of wearable sensors to monitor disease and health at a recent consumer electronics convention.
“This technology is here to stay,” said Dr. Das, who, appropriately, was not present at the symposium but delivered his presentation remotely.
Both the Centers for Medicare and Medicaid Services and the Food and Drug Administration have digital health action plans, according to Dr. Das. The CMS has already developed reimbursement codes to pay for remote monitoring services and more are expected.
Calling this type of telehealth “untethered vascular care,” Dr. Das agreed with Dr. Hallett that an evolution is coming whether vascular surgeons choose to get on board now or are forced to take action later.
SOURCE: VIETHsymposium
REPORTING FROM VIETH SYMPOSIUM
Depression linked to persistent opioid use after hysterectomy
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
REPORTING FROM THE AAGL GLOBAL CONGRESS
An alarming number of bipolar disorder diagnoses or something else?
During a particularly busy day in my inpatient and outpatient practice, I realized that nearly every one of the patients had been given the diagnosis of bipolar disorder at one point or another. The interesting thing is this wasn’t an unusual day.
Nearly all of my patients and their family members have been given the diagnosis of bipolar disorder. Because prevalence of bipolar affective disorders is a little over 2%, this seemed a little odd. Could there be an epidemic of bipolar disorder in the area? Should someone sound the alarm on this unique cluster and get Julia Roberts ready? Unfortunately, the story behind this mystery is a little less sexy but nevertheless interesting.
When I probe more into what symptoms might have led to the diagnosis of bipolar disorder, I most often get some sort of answer about being easily angered (“I’m fine 1 minute and the next minute I’m yelling at my mom”) or mood changing from 1 minute to the next. Rarely do they tell me about sleeping less, increased energy, change in mood (elation, anger, irritability), increase in activity level, and increased pleasurable though dangerous activities all happening around the same time(s). So what is going on?
Beginning in the 1990s, a debate about the phenotypic presentation of pediatric bipolar disorder polarized the field. It was theorized that mania could present with severe nonepisodic irritability with extended periods of very rapid mood cycling within the day as opposed to discrete episodic mood cycles in children and adolescents. With this broader conceptualization in the United States, the rate of bipolar diagnosis increased by over 40 times in less than a decade.1 Similarly, the use of mood stabilizers and atypical antipsychotics in children also rose substantially.2
To help assess if severe nonepisodic irritability belongs in the spectrum of bipolar disorders, the National Institutes of Mental Health proposed a syndrome called “Severe Mood Dysregulation” or SMD, to promote the study of children with this phenotype. In longitudinal studies, Stringaris et al. compared rates of manic episodes in youth with SMD versus bipolar disorder over 2 years and found only one youth (1%) with SMD who presented with manic, hypomanic, or mixed episodes, compared with 58 (62%) with bipolar disorder.3 Leibenluft et al.showed that chronic irritability during early adolescence predicted ADHD at late adolescence and major depressive disorder in early adulthood whereas episodic irritability predicted mania.4 Twenty-year follow-up of the same sample showed chronic irritability in adolescence predicted dysthymia, generalized anxiety disorders, and major depressive disorder.5 Other longitudinal studies essentially have shown the same results.6
At this point, the question of whether chronic irritability is a part of the bipolar spectrum disorder is largely resolved – 7 The diagnosis emphasizes the episodic nature of the illness, and that irritability would wax and wane with other manic symptoms such as changes in energy and sleep. And the ultrarapid mood changes (mood changes within the day) appear to describe mood fluctuations within a manic episode as opposed to each change being a separate episode.
So, most likely, my patients were caught in a time of uncertainty before data were able to clarify their phenotype.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Biol Psychiatry. 2007 Jul 15;62(2):107–14.
2. JAMA Psychiatry. 2015 Sep;72(9):859-60.
3. J Am Acad Child Adolesc Psychiatry. 2010 Apr;49(4):397-405.
4. J Child Adolesc Psychopharmacol 2006;16(4):456-66.
5. Am J Psychiatry. 2009 Sep;166(9):1048-54.
6. Biol Psychiatry. 2006 Nov 1;60(9):991-7.
7. Bipolar Disord. 2017 Nov;19(7):524-43.
During a particularly busy day in my inpatient and outpatient practice, I realized that nearly every one of the patients had been given the diagnosis of bipolar disorder at one point or another. The interesting thing is this wasn’t an unusual day.
Nearly all of my patients and their family members have been given the diagnosis of bipolar disorder. Because prevalence of bipolar affective disorders is a little over 2%, this seemed a little odd. Could there be an epidemic of bipolar disorder in the area? Should someone sound the alarm on this unique cluster and get Julia Roberts ready? Unfortunately, the story behind this mystery is a little less sexy but nevertheless interesting.
When I probe more into what symptoms might have led to the diagnosis of bipolar disorder, I most often get some sort of answer about being easily angered (“I’m fine 1 minute and the next minute I’m yelling at my mom”) or mood changing from 1 minute to the next. Rarely do they tell me about sleeping less, increased energy, change in mood (elation, anger, irritability), increase in activity level, and increased pleasurable though dangerous activities all happening around the same time(s). So what is going on?
Beginning in the 1990s, a debate about the phenotypic presentation of pediatric bipolar disorder polarized the field. It was theorized that mania could present with severe nonepisodic irritability with extended periods of very rapid mood cycling within the day as opposed to discrete episodic mood cycles in children and adolescents. With this broader conceptualization in the United States, the rate of bipolar diagnosis increased by over 40 times in less than a decade.1 Similarly, the use of mood stabilizers and atypical antipsychotics in children also rose substantially.2
To help assess if severe nonepisodic irritability belongs in the spectrum of bipolar disorders, the National Institutes of Mental Health proposed a syndrome called “Severe Mood Dysregulation” or SMD, to promote the study of children with this phenotype. In longitudinal studies, Stringaris et al. compared rates of manic episodes in youth with SMD versus bipolar disorder over 2 years and found only one youth (1%) with SMD who presented with manic, hypomanic, or mixed episodes, compared with 58 (62%) with bipolar disorder.3 Leibenluft et al.showed that chronic irritability during early adolescence predicted ADHD at late adolescence and major depressive disorder in early adulthood whereas episodic irritability predicted mania.4 Twenty-year follow-up of the same sample showed chronic irritability in adolescence predicted dysthymia, generalized anxiety disorders, and major depressive disorder.5 Other longitudinal studies essentially have shown the same results.6
At this point, the question of whether chronic irritability is a part of the bipolar spectrum disorder is largely resolved – 7 The diagnosis emphasizes the episodic nature of the illness, and that irritability would wax and wane with other manic symptoms such as changes in energy and sleep. And the ultrarapid mood changes (mood changes within the day) appear to describe mood fluctuations within a manic episode as opposed to each change being a separate episode.
So, most likely, my patients were caught in a time of uncertainty before data were able to clarify their phenotype.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Biol Psychiatry. 2007 Jul 15;62(2):107–14.
2. JAMA Psychiatry. 2015 Sep;72(9):859-60.
3. J Am Acad Child Adolesc Psychiatry. 2010 Apr;49(4):397-405.
4. J Child Adolesc Psychopharmacol 2006;16(4):456-66.
5. Am J Psychiatry. 2009 Sep;166(9):1048-54.
6. Biol Psychiatry. 2006 Nov 1;60(9):991-7.
7. Bipolar Disord. 2017 Nov;19(7):524-43.
During a particularly busy day in my inpatient and outpatient practice, I realized that nearly every one of the patients had been given the diagnosis of bipolar disorder at one point or another. The interesting thing is this wasn’t an unusual day.
Nearly all of my patients and their family members have been given the diagnosis of bipolar disorder. Because prevalence of bipolar affective disorders is a little over 2%, this seemed a little odd. Could there be an epidemic of bipolar disorder in the area? Should someone sound the alarm on this unique cluster and get Julia Roberts ready? Unfortunately, the story behind this mystery is a little less sexy but nevertheless interesting.
When I probe more into what symptoms might have led to the diagnosis of bipolar disorder, I most often get some sort of answer about being easily angered (“I’m fine 1 minute and the next minute I’m yelling at my mom”) or mood changing from 1 minute to the next. Rarely do they tell me about sleeping less, increased energy, change in mood (elation, anger, irritability), increase in activity level, and increased pleasurable though dangerous activities all happening around the same time(s). So what is going on?
Beginning in the 1990s, a debate about the phenotypic presentation of pediatric bipolar disorder polarized the field. It was theorized that mania could present with severe nonepisodic irritability with extended periods of very rapid mood cycling within the day as opposed to discrete episodic mood cycles in children and adolescents. With this broader conceptualization in the United States, the rate of bipolar diagnosis increased by over 40 times in less than a decade.1 Similarly, the use of mood stabilizers and atypical antipsychotics in children also rose substantially.2
To help assess if severe nonepisodic irritability belongs in the spectrum of bipolar disorders, the National Institutes of Mental Health proposed a syndrome called “Severe Mood Dysregulation” or SMD, to promote the study of children with this phenotype. In longitudinal studies, Stringaris et al. compared rates of manic episodes in youth with SMD versus bipolar disorder over 2 years and found only one youth (1%) with SMD who presented with manic, hypomanic, or mixed episodes, compared with 58 (62%) with bipolar disorder.3 Leibenluft et al.showed that chronic irritability during early adolescence predicted ADHD at late adolescence and major depressive disorder in early adulthood whereas episodic irritability predicted mania.4 Twenty-year follow-up of the same sample showed chronic irritability in adolescence predicted dysthymia, generalized anxiety disorders, and major depressive disorder.5 Other longitudinal studies essentially have shown the same results.6
At this point, the question of whether chronic irritability is a part of the bipolar spectrum disorder is largely resolved – 7 The diagnosis emphasizes the episodic nature of the illness, and that irritability would wax and wane with other manic symptoms such as changes in energy and sleep. And the ultrarapid mood changes (mood changes within the day) appear to describe mood fluctuations within a manic episode as opposed to each change being a separate episode.
So, most likely, my patients were caught in a time of uncertainty before data were able to clarify their phenotype.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Biol Psychiatry. 2007 Jul 15;62(2):107–14.
2. JAMA Psychiatry. 2015 Sep;72(9):859-60.
3. J Am Acad Child Adolesc Psychiatry. 2010 Apr;49(4):397-405.
4. J Child Adolesc Psychopharmacol 2006;16(4):456-66.
5. Am J Psychiatry. 2009 Sep;166(9):1048-54.
6. Biol Psychiatry. 2006 Nov 1;60(9):991-7.
7. Bipolar Disord. 2017 Nov;19(7):524-43.
Proposed RESPONSE Act targets potential shooters
As I’m writing, my Twitter feed announces yet another public shooting, this one at a Walmart in Oklahoma. It’s a problem that gets worse as it gets more attention and the argument over how to approach the issue of mass shootings still continues down two separate and distinct pathways: Is this the result of too-easy access to firearms or is it one of untreated mental illness?
Sen. John Cornyn (R-Tex.) spoke on the Senate floor on Oct. 23, 2019, about new legislation he is cosponsoring in the aftermath of two mass shootings in Texas this past August. The Restoring, Enhancing, Strengthening, and Promoting Our Nation’s Safety Efforts Act of 2019 (S. 2690), or the RESPONSE Act, is designed to “reduce mass violence, strengthen mental health collaboration in communities, improve school safety, and for other purposes.” Sen. Cornyn notes that in the aftermath of those shootings he met with his constituents and he heard a common refrain: Please do something.
“Unfortunately, there is no quick fix, no simple answer, instead we are left to look at the factors that led to these attacks and to try to do something to prevent the sequence of events from playing out again in the future,” Sen. Cornyn said.
“While mental illness is not the prevailing cause of mass violence, enhanced mental health resources are critical to saving lives,” he said, adding that most gun deaths are from suicide. In his speech, he outlined the issues it would address – and despite his statement that mental illness is not the cause of mass violence – he went on to elaborate on the issues that the bill would address.
“First, this legislation takes aim at unlicensed firearms dealers who are breaking the law,” he said. This legislation would create a task force to prosecute those who buy and sell firearms through unlicensed dealers, and he notes that one of the Texas shooters was denied a gun by a licensed firearms dealer before purchasing one from an unlicensed dealer. That Sen. Cornyn’s proposed legislation would not create any new gun legislation is not a surprise: he has an A+ rating from the National Rifle Association and his website’s fun facts include the statement: “Sen. Cornyn owns several firearms and hunts as often as he can.”
The rest of the RESPONSE Act takes aim at those who have or might have psychiatric disorders or a tendency toward violence. Sen. Cornyn noted that the act would expand assisted outpatient treatment (AOT, or outpatient civil commitment). He referenced this as a way for families to get care for their loved ones in the community rather than in a hospital and did not allude to the involuntary nature of the treatment.
Marvin Swartz, MD, is professor of psychiatry at Duke University, Durham, N.C., and lead investigator on outcome studies following the implementation of outpatient civil commitment legislation.
“AOT may be justified in improving treatment adherence and service provision,” Dr. Swartz noted, “but there is no direct line to serious violence. The violence we documented as reduced were mainly minor acts of interpersonal violence – pushing and shoving – what we call minor acts of violence. There is no evidence that AOT is a remedy to serious acts of violence – mass shootings included.”
In addition, Sen. Cornyn noted there would be expanded crisis intervention teams and increased coordination between mental health providers and law enforcement. Furthermore, the bill would make schools safer by identifying students whose behavior indicated a threat of violence and providing those students with the services they need. This would be done “by promoting best practices within our schools and promoting Internet safety.”
Finally, Sen. Cornyn talked about using social media as a means to identify those who might be a danger. “Because so often these shooters advertise on social media ... this legislation includes provisions to [ensure] that law enforcement can receive timely information about threats made online.”
The bill already has garnered both support and opposition. It has been supported by the National Council for Behavioral Health, the National Alliance on Mental Illness (NAMI), and the Treatment Advocacy Center. Those opposed to the legislation include the National Disability Rights Network, the American Association of People with Disabilities, the National Council on Independent Living, the Disability Rights Education & Defense Fund, the Bazelon Center for Mental Health Law, and the Autistic Self Advocacy Network. The American Psychiatric Association has not made a statement on the proposed legislation as of this writing.
The National Council for Behavioral Health posted an endorsement on its website. It notes: “The RESPONSE Act authorizes up to $10 million of existing funds in the Department of Justice for partnership between law enforcement and mental health providers to increase access to long-acting medically assisted treatment. Additionally, it requires the Department of Health and Human Services (HHS) to develop and disseminate guidance for states to fund mental health programs and crisis intervention teams through Medicaid as well as to issue a report to Congress on best practices to expand the mental health workforce. These provisions aim to divert more individuals from incarceration and will create more opportunities for community-based treatment and recovery.”
There is no question that psychiatric treatment for those with mental illness is underfunded and often inaccessible. But while it is true that some individuals become violent when they are ill, most do not, and targeting those one in five Americans who suffer from a psychiatric disorder each year in an effort to identify, then thwart, the rare mass murderer among us makes no sense.
Acts of mass violence remain rare. In 2018, the year we had a record-breaking number of mass shootings, there were 12 mass murders in the United States, according to the criteria used by Mother Jones, and 27 active shooter incidents using the FBI’s criteria. Approximately half of all mass shooters showed signs of mental illness prior to the shooting and of those, some had never come to the attention of mental health professionals in a way that would have predicted violence. While linking mass violence to mental illness may seem reasonable, the numbers just don’t make sense and targeting this presumed link between mental illness and mass violence is stigmatizing.
The text of the RESPONSE Act reveals proposed legislation that is perhaps more thoughtful than Sen. Cornyn’s speech suggested; the bill starts with funding services for those with psychiatric disorders who are being released from the correctional system, a population that may be at higher risk for acts of violence. The funding for outpatient civil commitment is worded in such a way that it is hard to know exactly what is required. The bill starts by mandating that each state must use 10% of the funding it gets from this bill for court-ordered treatment (AOT), but then lists alternative ways states may use that 10%, including “otherwise support evidence-based programs that address the needs of eligible patients.” In all, the proposed legislation is long and complex and attempts to address issues related to terrorism, the Internet, mental health, and the educational system. It’s an ambitious use of $10 million a year for our entire country.
At a time when mental health care is desperately underfunded and many are unable to access treatment, it is tempting to endorse any legislation that improves funding. But does it serve society to endorse legislation that suggests psychiatrists can prevent mass shootings? Does that ultimately serve our patients?
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
As I’m writing, my Twitter feed announces yet another public shooting, this one at a Walmart in Oklahoma. It’s a problem that gets worse as it gets more attention and the argument over how to approach the issue of mass shootings still continues down two separate and distinct pathways: Is this the result of too-easy access to firearms or is it one of untreated mental illness?
Sen. John Cornyn (R-Tex.) spoke on the Senate floor on Oct. 23, 2019, about new legislation he is cosponsoring in the aftermath of two mass shootings in Texas this past August. The Restoring, Enhancing, Strengthening, and Promoting Our Nation’s Safety Efforts Act of 2019 (S. 2690), or the RESPONSE Act, is designed to “reduce mass violence, strengthen mental health collaboration in communities, improve school safety, and for other purposes.” Sen. Cornyn notes that in the aftermath of those shootings he met with his constituents and he heard a common refrain: Please do something.
“Unfortunately, there is no quick fix, no simple answer, instead we are left to look at the factors that led to these attacks and to try to do something to prevent the sequence of events from playing out again in the future,” Sen. Cornyn said.
“While mental illness is not the prevailing cause of mass violence, enhanced mental health resources are critical to saving lives,” he said, adding that most gun deaths are from suicide. In his speech, he outlined the issues it would address – and despite his statement that mental illness is not the cause of mass violence – he went on to elaborate on the issues that the bill would address.
“First, this legislation takes aim at unlicensed firearms dealers who are breaking the law,” he said. This legislation would create a task force to prosecute those who buy and sell firearms through unlicensed dealers, and he notes that one of the Texas shooters was denied a gun by a licensed firearms dealer before purchasing one from an unlicensed dealer. That Sen. Cornyn’s proposed legislation would not create any new gun legislation is not a surprise: he has an A+ rating from the National Rifle Association and his website’s fun facts include the statement: “Sen. Cornyn owns several firearms and hunts as often as he can.”
The rest of the RESPONSE Act takes aim at those who have or might have psychiatric disorders or a tendency toward violence. Sen. Cornyn noted that the act would expand assisted outpatient treatment (AOT, or outpatient civil commitment). He referenced this as a way for families to get care for their loved ones in the community rather than in a hospital and did not allude to the involuntary nature of the treatment.
Marvin Swartz, MD, is professor of psychiatry at Duke University, Durham, N.C., and lead investigator on outcome studies following the implementation of outpatient civil commitment legislation.
“AOT may be justified in improving treatment adherence and service provision,” Dr. Swartz noted, “but there is no direct line to serious violence. The violence we documented as reduced were mainly minor acts of interpersonal violence – pushing and shoving – what we call minor acts of violence. There is no evidence that AOT is a remedy to serious acts of violence – mass shootings included.”
In addition, Sen. Cornyn noted there would be expanded crisis intervention teams and increased coordination between mental health providers and law enforcement. Furthermore, the bill would make schools safer by identifying students whose behavior indicated a threat of violence and providing those students with the services they need. This would be done “by promoting best practices within our schools and promoting Internet safety.”
Finally, Sen. Cornyn talked about using social media as a means to identify those who might be a danger. “Because so often these shooters advertise on social media ... this legislation includes provisions to [ensure] that law enforcement can receive timely information about threats made online.”
The bill already has garnered both support and opposition. It has been supported by the National Council for Behavioral Health, the National Alliance on Mental Illness (NAMI), and the Treatment Advocacy Center. Those opposed to the legislation include the National Disability Rights Network, the American Association of People with Disabilities, the National Council on Independent Living, the Disability Rights Education & Defense Fund, the Bazelon Center for Mental Health Law, and the Autistic Self Advocacy Network. The American Psychiatric Association has not made a statement on the proposed legislation as of this writing.
The National Council for Behavioral Health posted an endorsement on its website. It notes: “The RESPONSE Act authorizes up to $10 million of existing funds in the Department of Justice for partnership between law enforcement and mental health providers to increase access to long-acting medically assisted treatment. Additionally, it requires the Department of Health and Human Services (HHS) to develop and disseminate guidance for states to fund mental health programs and crisis intervention teams through Medicaid as well as to issue a report to Congress on best practices to expand the mental health workforce. These provisions aim to divert more individuals from incarceration and will create more opportunities for community-based treatment and recovery.”
There is no question that psychiatric treatment for those with mental illness is underfunded and often inaccessible. But while it is true that some individuals become violent when they are ill, most do not, and targeting those one in five Americans who suffer from a psychiatric disorder each year in an effort to identify, then thwart, the rare mass murderer among us makes no sense.
Acts of mass violence remain rare. In 2018, the year we had a record-breaking number of mass shootings, there were 12 mass murders in the United States, according to the criteria used by Mother Jones, and 27 active shooter incidents using the FBI’s criteria. Approximately half of all mass shooters showed signs of mental illness prior to the shooting and of those, some had never come to the attention of mental health professionals in a way that would have predicted violence. While linking mass violence to mental illness may seem reasonable, the numbers just don’t make sense and targeting this presumed link between mental illness and mass violence is stigmatizing.
The text of the RESPONSE Act reveals proposed legislation that is perhaps more thoughtful than Sen. Cornyn’s speech suggested; the bill starts with funding services for those with psychiatric disorders who are being released from the correctional system, a population that may be at higher risk for acts of violence. The funding for outpatient civil commitment is worded in such a way that it is hard to know exactly what is required. The bill starts by mandating that each state must use 10% of the funding it gets from this bill for court-ordered treatment (AOT), but then lists alternative ways states may use that 10%, including “otherwise support evidence-based programs that address the needs of eligible patients.” In all, the proposed legislation is long and complex and attempts to address issues related to terrorism, the Internet, mental health, and the educational system. It’s an ambitious use of $10 million a year for our entire country.
At a time when mental health care is desperately underfunded and many are unable to access treatment, it is tempting to endorse any legislation that improves funding. But does it serve society to endorse legislation that suggests psychiatrists can prevent mass shootings? Does that ultimately serve our patients?
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
As I’m writing, my Twitter feed announces yet another public shooting, this one at a Walmart in Oklahoma. It’s a problem that gets worse as it gets more attention and the argument over how to approach the issue of mass shootings still continues down two separate and distinct pathways: Is this the result of too-easy access to firearms or is it one of untreated mental illness?
Sen. John Cornyn (R-Tex.) spoke on the Senate floor on Oct. 23, 2019, about new legislation he is cosponsoring in the aftermath of two mass shootings in Texas this past August. The Restoring, Enhancing, Strengthening, and Promoting Our Nation’s Safety Efforts Act of 2019 (S. 2690), or the RESPONSE Act, is designed to “reduce mass violence, strengthen mental health collaboration in communities, improve school safety, and for other purposes.” Sen. Cornyn notes that in the aftermath of those shootings he met with his constituents and he heard a common refrain: Please do something.
“Unfortunately, there is no quick fix, no simple answer, instead we are left to look at the factors that led to these attacks and to try to do something to prevent the sequence of events from playing out again in the future,” Sen. Cornyn said.
“While mental illness is not the prevailing cause of mass violence, enhanced mental health resources are critical to saving lives,” he said, adding that most gun deaths are from suicide. In his speech, he outlined the issues it would address – and despite his statement that mental illness is not the cause of mass violence – he went on to elaborate on the issues that the bill would address.
“First, this legislation takes aim at unlicensed firearms dealers who are breaking the law,” he said. This legislation would create a task force to prosecute those who buy and sell firearms through unlicensed dealers, and he notes that one of the Texas shooters was denied a gun by a licensed firearms dealer before purchasing one from an unlicensed dealer. That Sen. Cornyn’s proposed legislation would not create any new gun legislation is not a surprise: he has an A+ rating from the National Rifle Association and his website’s fun facts include the statement: “Sen. Cornyn owns several firearms and hunts as often as he can.”
The rest of the RESPONSE Act takes aim at those who have or might have psychiatric disorders or a tendency toward violence. Sen. Cornyn noted that the act would expand assisted outpatient treatment (AOT, or outpatient civil commitment). He referenced this as a way for families to get care for their loved ones in the community rather than in a hospital and did not allude to the involuntary nature of the treatment.
Marvin Swartz, MD, is professor of psychiatry at Duke University, Durham, N.C., and lead investigator on outcome studies following the implementation of outpatient civil commitment legislation.
“AOT may be justified in improving treatment adherence and service provision,” Dr. Swartz noted, “but there is no direct line to serious violence. The violence we documented as reduced were mainly minor acts of interpersonal violence – pushing and shoving – what we call minor acts of violence. There is no evidence that AOT is a remedy to serious acts of violence – mass shootings included.”
In addition, Sen. Cornyn noted there would be expanded crisis intervention teams and increased coordination between mental health providers and law enforcement. Furthermore, the bill would make schools safer by identifying students whose behavior indicated a threat of violence and providing those students with the services they need. This would be done “by promoting best practices within our schools and promoting Internet safety.”
Finally, Sen. Cornyn talked about using social media as a means to identify those who might be a danger. “Because so often these shooters advertise on social media ... this legislation includes provisions to [ensure] that law enforcement can receive timely information about threats made online.”
The bill already has garnered both support and opposition. It has been supported by the National Council for Behavioral Health, the National Alliance on Mental Illness (NAMI), and the Treatment Advocacy Center. Those opposed to the legislation include the National Disability Rights Network, the American Association of People with Disabilities, the National Council on Independent Living, the Disability Rights Education & Defense Fund, the Bazelon Center for Mental Health Law, and the Autistic Self Advocacy Network. The American Psychiatric Association has not made a statement on the proposed legislation as of this writing.
The National Council for Behavioral Health posted an endorsement on its website. It notes: “The RESPONSE Act authorizes up to $10 million of existing funds in the Department of Justice for partnership between law enforcement and mental health providers to increase access to long-acting medically assisted treatment. Additionally, it requires the Department of Health and Human Services (HHS) to develop and disseminate guidance for states to fund mental health programs and crisis intervention teams through Medicaid as well as to issue a report to Congress on best practices to expand the mental health workforce. These provisions aim to divert more individuals from incarceration and will create more opportunities for community-based treatment and recovery.”
There is no question that psychiatric treatment for those with mental illness is underfunded and often inaccessible. But while it is true that some individuals become violent when they are ill, most do not, and targeting those one in five Americans who suffer from a psychiatric disorder each year in an effort to identify, then thwart, the rare mass murderer among us makes no sense.
Acts of mass violence remain rare. In 2018, the year we had a record-breaking number of mass shootings, there were 12 mass murders in the United States, according to the criteria used by Mother Jones, and 27 active shooter incidents using the FBI’s criteria. Approximately half of all mass shooters showed signs of mental illness prior to the shooting and of those, some had never come to the attention of mental health professionals in a way that would have predicted violence. While linking mass violence to mental illness may seem reasonable, the numbers just don’t make sense and targeting this presumed link between mental illness and mass violence is stigmatizing.
The text of the RESPONSE Act reveals proposed legislation that is perhaps more thoughtful than Sen. Cornyn’s speech suggested; the bill starts with funding services for those with psychiatric disorders who are being released from the correctional system, a population that may be at higher risk for acts of violence. The funding for outpatient civil commitment is worded in such a way that it is hard to know exactly what is required. The bill starts by mandating that each state must use 10% of the funding it gets from this bill for court-ordered treatment (AOT), but then lists alternative ways states may use that 10%, including “otherwise support evidence-based programs that address the needs of eligible patients.” In all, the proposed legislation is long and complex and attempts to address issues related to terrorism, the Internet, mental health, and the educational system. It’s an ambitious use of $10 million a year for our entire country.
At a time when mental health care is desperately underfunded and many are unable to access treatment, it is tempting to endorse any legislation that improves funding. But does it serve society to endorse legislation that suggests psychiatrists can prevent mass shootings? Does that ultimately serve our patients?
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
Papulonecrotic Tuberculid Secondary to Mycobacterium avium Complex
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
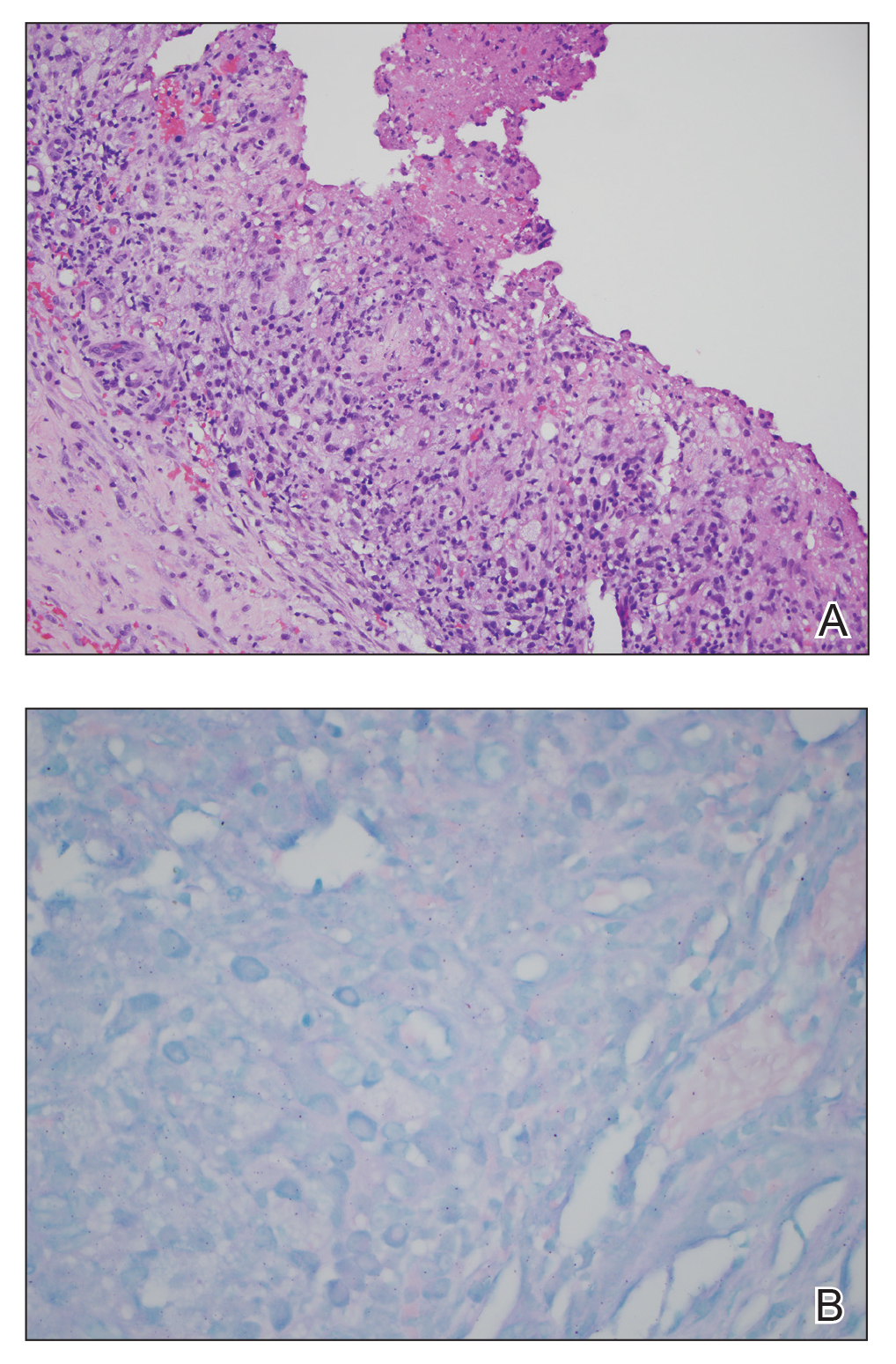
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
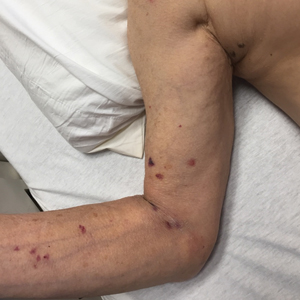
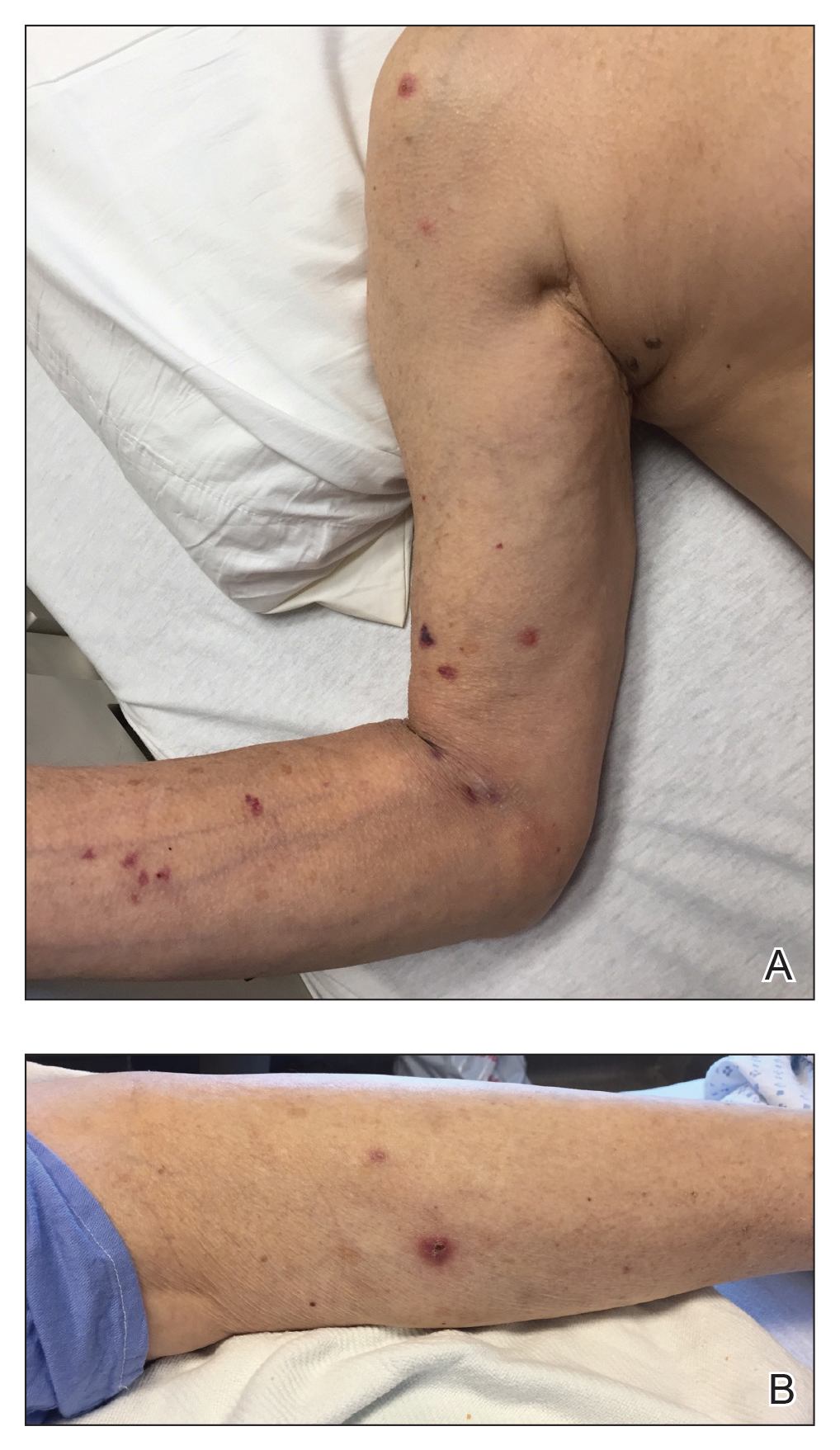
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.

During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.

Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.

During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.

Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
Practice Points
- Papulonecrotic tuberculid (PNT) is a hypersensitivity reaction that presents with reddish papules with central necrosis on the extremities.
- Early PNT histopathology shows leukocytoclastic vasculitis. Later lesions demonstrate granulomatous inflammation on histopathology.
- Mycobacterium avium is difficult to culture; therefore, if you suspect it, we recommend polymerase chain reaction genotyping for bacterial classification.
Melanoma incidence drops in younger age groups
, according to results of a population-based registry study of 988,103 cases of invasive melanoma.
These data are observational, “and thus cannot conclusively determine the cause of this statistically and clinically significant decrease,” wrote Kelly G. Paulson, MD, PhD, of the Fred Hutchinson Cancer Research Center, Seattle, and colleagues. However, they added, “a likely explanation for the reduced melanoma incidence in adolescents and young adults is success at increased UV exposure protection. These data provide an impetus to further improve multimodal efforts aimed at reducing the burden of melanoma and encourage ongoing UV exposure protection efforts throughout the lifetime of individuals.”
Public health measures to promote sun-protective behaviors including sunscreen use, protective clothing, and seeking shade were initiated in the United States in the late 1990s and early 2000s, but the public health impact remains unknown, they noted in the study, published in JAMA Dermatology.
For the study, they reviewed data from the National Program of Cancer Registries – Surveillance Epidemiology and End Results combined database for the years 2001-2015. Overall, the incidence of invasive melanoma among people of all ages in the United States increased from 50,272 cases in 2001 to 83,362 in 2015. However, in 2015 only 67 cases were reported in children younger than 10 years, 251 in adolescents aged 10-19 years, and 1,973 in young adults (aged 20-29 years).
Between 2006 and 2015, the annual percentage change in melanoma incidence decreased by 4.4% for male adolescents, 5.4% for female adolescents, 3.7% for male young adults, and 3.6% for female young adults; these changes were statistically significant. The trends in incidence was similar when the population was limited to non-Hispanic whites, considered a high-risk group for melanoma.
By contrast, melanoma incidence increased by an annual percentage change of 1.8% for both men and women aged 40 years and older during the same period of time. Young adult women had a greater incidence of melanoma compared with young adult men (about twofold greater), but older men had a greater incidence of melanoma compared with older women, the researchers said.
The findings were limited by a lack of data about potential confounders, such as skin pigmentation, UV light exposure, sunburn history, sunscreen use, sun avoidance, protective clothing, and tanning bed use; and the absence of information kept the researchers from estimating an association between increased sun-protective behaviors and decreased incidence of melanoma.
“However, this change in behavior remains a plausible explanation for decreased melanoma rates in adolescent and young adult populations,” and the data support continued strategies to promote UV protection throughout life, they said.
The study was supported in part by the National Institutes of Health, the Fred Hutchinson Cancer Research Center Integrated Immunotherapy Research Core, and a Society for Immunotherapy of Cancer–Merck fellowship. Dr. Paulson disclosed grants from the Society for Immunotherapy of Cancer–Merck, bluebird biosciences, EMD Serono; she also disclosed an issued and licensed patent for a Merkel cell carcinoma T cell receptor.
SOURCE: Paulson KG et al. JAMA Dermatol. 2019. Nov 13. doi: 10.1001/jamadermatol.2019.3353.
, according to results of a population-based registry study of 988,103 cases of invasive melanoma.
These data are observational, “and thus cannot conclusively determine the cause of this statistically and clinically significant decrease,” wrote Kelly G. Paulson, MD, PhD, of the Fred Hutchinson Cancer Research Center, Seattle, and colleagues. However, they added, “a likely explanation for the reduced melanoma incidence in adolescents and young adults is success at increased UV exposure protection. These data provide an impetus to further improve multimodal efforts aimed at reducing the burden of melanoma and encourage ongoing UV exposure protection efforts throughout the lifetime of individuals.”
Public health measures to promote sun-protective behaviors including sunscreen use, protective clothing, and seeking shade were initiated in the United States in the late 1990s and early 2000s, but the public health impact remains unknown, they noted in the study, published in JAMA Dermatology.
For the study, they reviewed data from the National Program of Cancer Registries – Surveillance Epidemiology and End Results combined database for the years 2001-2015. Overall, the incidence of invasive melanoma among people of all ages in the United States increased from 50,272 cases in 2001 to 83,362 in 2015. However, in 2015 only 67 cases were reported in children younger than 10 years, 251 in adolescents aged 10-19 years, and 1,973 in young adults (aged 20-29 years).
Between 2006 and 2015, the annual percentage change in melanoma incidence decreased by 4.4% for male adolescents, 5.4% for female adolescents, 3.7% for male young adults, and 3.6% for female young adults; these changes were statistically significant. The trends in incidence was similar when the population was limited to non-Hispanic whites, considered a high-risk group for melanoma.
By contrast, melanoma incidence increased by an annual percentage change of 1.8% for both men and women aged 40 years and older during the same period of time. Young adult women had a greater incidence of melanoma compared with young adult men (about twofold greater), but older men had a greater incidence of melanoma compared with older women, the researchers said.
The findings were limited by a lack of data about potential confounders, such as skin pigmentation, UV light exposure, sunburn history, sunscreen use, sun avoidance, protective clothing, and tanning bed use; and the absence of information kept the researchers from estimating an association between increased sun-protective behaviors and decreased incidence of melanoma.
“However, this change in behavior remains a plausible explanation for decreased melanoma rates in adolescent and young adult populations,” and the data support continued strategies to promote UV protection throughout life, they said.
The study was supported in part by the National Institutes of Health, the Fred Hutchinson Cancer Research Center Integrated Immunotherapy Research Core, and a Society for Immunotherapy of Cancer–Merck fellowship. Dr. Paulson disclosed grants from the Society for Immunotherapy of Cancer–Merck, bluebird biosciences, EMD Serono; she also disclosed an issued and licensed patent for a Merkel cell carcinoma T cell receptor.
SOURCE: Paulson KG et al. JAMA Dermatol. 2019. Nov 13. doi: 10.1001/jamadermatol.2019.3353.
, according to results of a population-based registry study of 988,103 cases of invasive melanoma.
These data are observational, “and thus cannot conclusively determine the cause of this statistically and clinically significant decrease,” wrote Kelly G. Paulson, MD, PhD, of the Fred Hutchinson Cancer Research Center, Seattle, and colleagues. However, they added, “a likely explanation for the reduced melanoma incidence in adolescents and young adults is success at increased UV exposure protection. These data provide an impetus to further improve multimodal efforts aimed at reducing the burden of melanoma and encourage ongoing UV exposure protection efforts throughout the lifetime of individuals.”
Public health measures to promote sun-protective behaviors including sunscreen use, protective clothing, and seeking shade were initiated in the United States in the late 1990s and early 2000s, but the public health impact remains unknown, they noted in the study, published in JAMA Dermatology.
For the study, they reviewed data from the National Program of Cancer Registries – Surveillance Epidemiology and End Results combined database for the years 2001-2015. Overall, the incidence of invasive melanoma among people of all ages in the United States increased from 50,272 cases in 2001 to 83,362 in 2015. However, in 2015 only 67 cases were reported in children younger than 10 years, 251 in adolescents aged 10-19 years, and 1,973 in young adults (aged 20-29 years).
Between 2006 and 2015, the annual percentage change in melanoma incidence decreased by 4.4% for male adolescents, 5.4% for female adolescents, 3.7% for male young adults, and 3.6% for female young adults; these changes were statistically significant. The trends in incidence was similar when the population was limited to non-Hispanic whites, considered a high-risk group for melanoma.
By contrast, melanoma incidence increased by an annual percentage change of 1.8% for both men and women aged 40 years and older during the same period of time. Young adult women had a greater incidence of melanoma compared with young adult men (about twofold greater), but older men had a greater incidence of melanoma compared with older women, the researchers said.
The findings were limited by a lack of data about potential confounders, such as skin pigmentation, UV light exposure, sunburn history, sunscreen use, sun avoidance, protective clothing, and tanning bed use; and the absence of information kept the researchers from estimating an association between increased sun-protective behaviors and decreased incidence of melanoma.
“However, this change in behavior remains a plausible explanation for decreased melanoma rates in adolescent and young adult populations,” and the data support continued strategies to promote UV protection throughout life, they said.
The study was supported in part by the National Institutes of Health, the Fred Hutchinson Cancer Research Center Integrated Immunotherapy Research Core, and a Society for Immunotherapy of Cancer–Merck fellowship. Dr. Paulson disclosed grants from the Society for Immunotherapy of Cancer–Merck, bluebird biosciences, EMD Serono; she also disclosed an issued and licensed patent for a Merkel cell carcinoma T cell receptor.
SOURCE: Paulson KG et al. JAMA Dermatol. 2019. Nov 13. doi: 10.1001/jamadermatol.2019.3353.
FROM JAMA DERMATOLOGY
Advances in digital otoscopy help improve AOM diagnoses
NEW ORLEANS – The incidence of acute otitis media has decreased by 25% to 35% in the past decade, thanks largely to the widespread and near universal use of the pneumococcal conjugate vaccine, according to Ellen R. Wald, MD.
“To a smaller degree, it is also attributable to the use of influenza vaccine, and to the use of more stringent diagnostic criteria,” Dr. Wald, who chairs the department of pediatrics at the University of Wisconsin, Madison, said at the annual meeting of the American Academy of Pediatrics. “The fact that we are decreasing the number of episodes of otitis media in children in the first year of life means that we’re going to have fewer otitis-prone children and therefore less of a need for tympanostomy tubes, either as a solution to the problem of recurrence of acute otitis media (AOM) or for the problem of persistent effusion.”
said Dr. Wald, pediatrician-in-chief at the American Family Children’s Hospital in Madison. She noted that OME is a nonbacterial inflammatory state that usually resolves spontaneously. It tends to occur before or after AOM, and often without ever progressing to AOM. “Its principal importance is as a cause of hearing loss and as a confounder in the diagnosis AOM,” she explained. “Because it is a nonbacterial process, antibiotics are not indicated in the management of OME. In contrast, children with AOM have a bacterial infection that will benefit from the use of antimicrobials.”*
Middle ear effusion is common to both OME and AOM, she continued. To discriminate between the two conditions, clinicians must look for signs of acute inflammation of the tympanic membrane, “which we expect to see in AOM,” she said. “The most powerful sign of inflammation of the tympanic membrane is distinct fullness or bulging of the tympanic membrane on exam.”
Dr. Wald advises clinicians to be as systematic as possible when conducting the otoscopic exam, by looking at color and classifying it as pink, gray, white, yellow, red, amber, or blue, and by documenting the position as neutral, retracted, full, or bulging. “When we gauge how light passes through the tympanic membrane, we judge it as translucent, opaque, or partially opaque, and mobility as normal, decreased, or absent,” she added. “When we find decreased or absent mobility of the tympanic membrane, it tells us that we have fluid in the middle ear, but it does not discriminate between AOM and OME.”
Advances in digital otoscopy are helping pediatricians to improve their diagnostic skills. An early device, the iPhone otoscope by CellScope, uses an iOS smartphone to capture images and videos of the external ear canal and eardrum. “The image is pretty much the same as that seen through the eye of a hand-held otoscope,” Dr. Wald said. “The problem with this particular design is that the speculum is kind of large. It does still require the removal of cerumen, and the smartphone is kind of awkward to use as a handle during an otoscopic exam.”
A new digital otoscope called Wispr was unveiled at the AAP meeting. First developed at the University of Wisconsin and now marketed by WiscMed, Wispr delivers high-resolution views of the eardrum in even small or partially obstructed ear canals with one-button image and video capture. WiscMed was founded by Jim Berbee, MD, MBA, an engineer turned emergency medicine physician.
“One of the advantages of this particular model is that it handles a lot more like a usual otoscope and can be attached to the rechargeable handles that are commercially available,” Dr. Wald said. “It has an extremely tiny speculum. Within the head, there is even a smaller camera that allows the photographs to be taken. Because the speculum is so tiny, it allows the device to sometimes avoid the presence of cerumen, or sometimes go through it and still obtain an image.”
Priced at $1,500, the Wispr also features a built-in USB port for computer download of captures images and video. “This way, multiple observers can look at the uploaded image and have an opportunity to view it at greater length,” she said. “Our hope is that the availability of digital otoscopy in the office setting may improve our diagnostic skills and therefore lead to more judicious use of antimicrobials. This remains to be seen. Prospective studies need to be done, but it’s an exciting development,” Dr. Wald said.
She reported having no financial disclosures.
*This article was updated 12/13/2019
NEW ORLEANS – The incidence of acute otitis media has decreased by 25% to 35% in the past decade, thanks largely to the widespread and near universal use of the pneumococcal conjugate vaccine, according to Ellen R. Wald, MD.
“To a smaller degree, it is also attributable to the use of influenza vaccine, and to the use of more stringent diagnostic criteria,” Dr. Wald, who chairs the department of pediatrics at the University of Wisconsin, Madison, said at the annual meeting of the American Academy of Pediatrics. “The fact that we are decreasing the number of episodes of otitis media in children in the first year of life means that we’re going to have fewer otitis-prone children and therefore less of a need for tympanostomy tubes, either as a solution to the problem of recurrence of acute otitis media (AOM) or for the problem of persistent effusion.”
said Dr. Wald, pediatrician-in-chief at the American Family Children’s Hospital in Madison. She noted that OME is a nonbacterial inflammatory state that usually resolves spontaneously. It tends to occur before or after AOM, and often without ever progressing to AOM. “Its principal importance is as a cause of hearing loss and as a confounder in the diagnosis AOM,” she explained. “Because it is a nonbacterial process, antibiotics are not indicated in the management of OME. In contrast, children with AOM have a bacterial infection that will benefit from the use of antimicrobials.”*
Middle ear effusion is common to both OME and AOM, she continued. To discriminate between the two conditions, clinicians must look for signs of acute inflammation of the tympanic membrane, “which we expect to see in AOM,” she said. “The most powerful sign of inflammation of the tympanic membrane is distinct fullness or bulging of the tympanic membrane on exam.”
Dr. Wald advises clinicians to be as systematic as possible when conducting the otoscopic exam, by looking at color and classifying it as pink, gray, white, yellow, red, amber, or blue, and by documenting the position as neutral, retracted, full, or bulging. “When we gauge how light passes through the tympanic membrane, we judge it as translucent, opaque, or partially opaque, and mobility as normal, decreased, or absent,” she added. “When we find decreased or absent mobility of the tympanic membrane, it tells us that we have fluid in the middle ear, but it does not discriminate between AOM and OME.”
Advances in digital otoscopy are helping pediatricians to improve their diagnostic skills. An early device, the iPhone otoscope by CellScope, uses an iOS smartphone to capture images and videos of the external ear canal and eardrum. “The image is pretty much the same as that seen through the eye of a hand-held otoscope,” Dr. Wald said. “The problem with this particular design is that the speculum is kind of large. It does still require the removal of cerumen, and the smartphone is kind of awkward to use as a handle during an otoscopic exam.”
A new digital otoscope called Wispr was unveiled at the AAP meeting. First developed at the University of Wisconsin and now marketed by WiscMed, Wispr delivers high-resolution views of the eardrum in even small or partially obstructed ear canals with one-button image and video capture. WiscMed was founded by Jim Berbee, MD, MBA, an engineer turned emergency medicine physician.
“One of the advantages of this particular model is that it handles a lot more like a usual otoscope and can be attached to the rechargeable handles that are commercially available,” Dr. Wald said. “It has an extremely tiny speculum. Within the head, there is even a smaller camera that allows the photographs to be taken. Because the speculum is so tiny, it allows the device to sometimes avoid the presence of cerumen, or sometimes go through it and still obtain an image.”
Priced at $1,500, the Wispr also features a built-in USB port for computer download of captures images and video. “This way, multiple observers can look at the uploaded image and have an opportunity to view it at greater length,” she said. “Our hope is that the availability of digital otoscopy in the office setting may improve our diagnostic skills and therefore lead to more judicious use of antimicrobials. This remains to be seen. Prospective studies need to be done, but it’s an exciting development,” Dr. Wald said.
She reported having no financial disclosures.
*This article was updated 12/13/2019
NEW ORLEANS – The incidence of acute otitis media has decreased by 25% to 35% in the past decade, thanks largely to the widespread and near universal use of the pneumococcal conjugate vaccine, according to Ellen R. Wald, MD.
“To a smaller degree, it is also attributable to the use of influenza vaccine, and to the use of more stringent diagnostic criteria,” Dr. Wald, who chairs the department of pediatrics at the University of Wisconsin, Madison, said at the annual meeting of the American Academy of Pediatrics. “The fact that we are decreasing the number of episodes of otitis media in children in the first year of life means that we’re going to have fewer otitis-prone children and therefore less of a need for tympanostomy tubes, either as a solution to the problem of recurrence of acute otitis media (AOM) or for the problem of persistent effusion.”
said Dr. Wald, pediatrician-in-chief at the American Family Children’s Hospital in Madison. She noted that OME is a nonbacterial inflammatory state that usually resolves spontaneously. It tends to occur before or after AOM, and often without ever progressing to AOM. “Its principal importance is as a cause of hearing loss and as a confounder in the diagnosis AOM,” she explained. “Because it is a nonbacterial process, antibiotics are not indicated in the management of OME. In contrast, children with AOM have a bacterial infection that will benefit from the use of antimicrobials.”*
Middle ear effusion is common to both OME and AOM, she continued. To discriminate between the two conditions, clinicians must look for signs of acute inflammation of the tympanic membrane, “which we expect to see in AOM,” she said. “The most powerful sign of inflammation of the tympanic membrane is distinct fullness or bulging of the tympanic membrane on exam.”
Dr. Wald advises clinicians to be as systematic as possible when conducting the otoscopic exam, by looking at color and classifying it as pink, gray, white, yellow, red, amber, or blue, and by documenting the position as neutral, retracted, full, or bulging. “When we gauge how light passes through the tympanic membrane, we judge it as translucent, opaque, or partially opaque, and mobility as normal, decreased, or absent,” she added. “When we find decreased or absent mobility of the tympanic membrane, it tells us that we have fluid in the middle ear, but it does not discriminate between AOM and OME.”
Advances in digital otoscopy are helping pediatricians to improve their diagnostic skills. An early device, the iPhone otoscope by CellScope, uses an iOS smartphone to capture images and videos of the external ear canal and eardrum. “The image is pretty much the same as that seen through the eye of a hand-held otoscope,” Dr. Wald said. “The problem with this particular design is that the speculum is kind of large. It does still require the removal of cerumen, and the smartphone is kind of awkward to use as a handle during an otoscopic exam.”
A new digital otoscope called Wispr was unveiled at the AAP meeting. First developed at the University of Wisconsin and now marketed by WiscMed, Wispr delivers high-resolution views of the eardrum in even small or partially obstructed ear canals with one-button image and video capture. WiscMed was founded by Jim Berbee, MD, MBA, an engineer turned emergency medicine physician.
“One of the advantages of this particular model is that it handles a lot more like a usual otoscope and can be attached to the rechargeable handles that are commercially available,” Dr. Wald said. “It has an extremely tiny speculum. Within the head, there is even a smaller camera that allows the photographs to be taken. Because the speculum is so tiny, it allows the device to sometimes avoid the presence of cerumen, or sometimes go through it and still obtain an image.”
Priced at $1,500, the Wispr also features a built-in USB port for computer download of captures images and video. “This way, multiple observers can look at the uploaded image and have an opportunity to view it at greater length,” she said. “Our hope is that the availability of digital otoscopy in the office setting may improve our diagnostic skills and therefore lead to more judicious use of antimicrobials. This remains to be seen. Prospective studies need to be done, but it’s an exciting development,” Dr. Wald said.
She reported having no financial disclosures.
*This article was updated 12/13/2019
EXPERT ANALYSIS AT AAP 19
ED-based HCV screening found feasible, linkage low
BOSTON – ED-based screening is a feasible method of detecting hepatitis C virus (HCV) in high-risk populations, but linkage to care remains low, according to investigators.
An HCV screening program involving three Seattle hospitals and more than 4,000 patients showed that linkage to care was lowest among patients who were younger, homeless, or used injection drugs, reported lead author Charles S. Landis, MD, PhD, of the University of Washington, Seattle.
“In the U.S., rates of acute HCV infections are increasing in younger patients and in areas disproportionally affected by the opiate epidemic,” Dr. Landis said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In order to achieve a goal of elimination, HCV screening, appropriate linkage to care, and treatment will need to be directed toward younger, marginalized, and underserved populations.”
Dr. Landis explained that EDs are suitable for HCV screening because users of emergency services are disproportionately affected by HCV, compared with patients in primary and specialty care settings. Despite this, linkage to care remains historically higher in primary and specialty care settings at approximately 70%, compared with 30% via the ED, Dr. Landis said.
Historically, EDs have been resistant to HCV screening programs, Dr. Landis said, but with the model used in the present study, which relied upon a full-time staff member in each ED who was employed by the infectious disease or hepatology department, no ED resources were needed.
Participants were willing adults who had reliable contact information. Patients were excluded if they were non–English speaking, incarcerated, enrolled or expected to enroll in another clinical study which excludes coenrollment, planned to move out of the region in the next 6 months, admitted to the ED with an acute life-threatening illness, or admitted to the ED for sexual assault. The program had three objectives: Screening, linkage to care, and treatment, all of which were coordinated by the aforementioned case manager.
To date, 4,182 patients have been screened, 936 have been enrolled, 95 have tested positive for HCV RNA, 32 have been linked with care, and 19 have been treated.
“So you can see, a lot of squeeze for a just a little bit of juice here,” Dr. Landis said, referring to the relatively low number of treated patients, compared with how many were screened.
The prevalence of HCV infection based on RNA testing was 2%, though one hospital had a rate of 5%. “This [prevalence] compares to, but is maybe slightly less than, the prevalence seen in others studies based in the emergency department,” Dr. Landis said. “The thought is, not all emergency departments are equal in terms of the patient population that they serve.”
Data analysis showed that the overall linkage to care was 36%. “This is still suboptimal, from my perspective,” Dr. Landis said, “but it does compare with several other ED-based studies.”
A closer look at the data showed that linkage was not uniform across the population. Among patients with homes, linkage to care was 59%, compared with 20% for patients who were homeless (P = .02).
“Ultimately, we need to tailor our approaches for linking homeless patients differently than patients who are not homeless,” Dr. Landis said.
Patients who reported no injection-drug use had a linkage to care of 50%, which was numerically higher than the rate of 20% among users of injection drugs; this difference was not statistically significant, which Dr. Landis attributed to insufficient population size. Similarly, younger patients showed numerical trends toward lower linkage to care.
“Future work will attempt to optimize linkage to care strategies based on patient demographic factors, such as active injection drug use or homelessness,” Dr. Landis said.
During discussion, a conference attendee from the United States expressed skepticism of the program’s merits.
“I may be a glass-half-empty person, but is it worth all this effort?” the attendee asked. “In all honesty, you treated a few dozen [patients] for 180,000 visits [per year]. I’m really not sure it’s worth those efforts, and I’m wondering if those efforts could be placed in different areas, especially for a higher yield.”
“Point well taken,” Dr. Landis said. “I think that was the purpose of the study, to see if the emergency department is a place to screen and link patients to care, and we’re trying to optimize that. Remember, there were 4,000 patients, but for many of those, it took literally a minute to screen them.”
An attendee from Australia offered a slightly more positive take on the findings, followed by a suggestion to improve linkage in marginalized populations.
“I’m not sure I’d be pessimistic,” the attendee said. “I think you ought to be commended for getting that number of people to link, because it is very difficult when we are looking at linking people from a hospital-based setting who actually live in the community and suffer from homelessness and mental health issues and incarceration and a whole range of other things. ... Maybe we need to change our idea of having these centralized silos where people are referred, and go out into the community, much like [tuberculosis] clinics used to do, and track people down.”
The study was funded by Gilead. The investigators disclosed additional relationships with HighTide Therapeutics, Intercept, AbbVie, and others.
SOURCE: Landis CS et al. The Liver Meeting 2019, Abstract 168.
BOSTON – ED-based screening is a feasible method of detecting hepatitis C virus (HCV) in high-risk populations, but linkage to care remains low, according to investigators.
An HCV screening program involving three Seattle hospitals and more than 4,000 patients showed that linkage to care was lowest among patients who were younger, homeless, or used injection drugs, reported lead author Charles S. Landis, MD, PhD, of the University of Washington, Seattle.
“In the U.S., rates of acute HCV infections are increasing in younger patients and in areas disproportionally affected by the opiate epidemic,” Dr. Landis said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In order to achieve a goal of elimination, HCV screening, appropriate linkage to care, and treatment will need to be directed toward younger, marginalized, and underserved populations.”
Dr. Landis explained that EDs are suitable for HCV screening because users of emergency services are disproportionately affected by HCV, compared with patients in primary and specialty care settings. Despite this, linkage to care remains historically higher in primary and specialty care settings at approximately 70%, compared with 30% via the ED, Dr. Landis said.
Historically, EDs have been resistant to HCV screening programs, Dr. Landis said, but with the model used in the present study, which relied upon a full-time staff member in each ED who was employed by the infectious disease or hepatology department, no ED resources were needed.
Participants were willing adults who had reliable contact information. Patients were excluded if they were non–English speaking, incarcerated, enrolled or expected to enroll in another clinical study which excludes coenrollment, planned to move out of the region in the next 6 months, admitted to the ED with an acute life-threatening illness, or admitted to the ED for sexual assault. The program had three objectives: Screening, linkage to care, and treatment, all of which were coordinated by the aforementioned case manager.
To date, 4,182 patients have been screened, 936 have been enrolled, 95 have tested positive for HCV RNA, 32 have been linked with care, and 19 have been treated.
“So you can see, a lot of squeeze for a just a little bit of juice here,” Dr. Landis said, referring to the relatively low number of treated patients, compared with how many were screened.
The prevalence of HCV infection based on RNA testing was 2%, though one hospital had a rate of 5%. “This [prevalence] compares to, but is maybe slightly less than, the prevalence seen in others studies based in the emergency department,” Dr. Landis said. “The thought is, not all emergency departments are equal in terms of the patient population that they serve.”
Data analysis showed that the overall linkage to care was 36%. “This is still suboptimal, from my perspective,” Dr. Landis said, “but it does compare with several other ED-based studies.”
A closer look at the data showed that linkage was not uniform across the population. Among patients with homes, linkage to care was 59%, compared with 20% for patients who were homeless (P = .02).
“Ultimately, we need to tailor our approaches for linking homeless patients differently than patients who are not homeless,” Dr. Landis said.
Patients who reported no injection-drug use had a linkage to care of 50%, which was numerically higher than the rate of 20% among users of injection drugs; this difference was not statistically significant, which Dr. Landis attributed to insufficient population size. Similarly, younger patients showed numerical trends toward lower linkage to care.
“Future work will attempt to optimize linkage to care strategies based on patient demographic factors, such as active injection drug use or homelessness,” Dr. Landis said.
During discussion, a conference attendee from the United States expressed skepticism of the program’s merits.
“I may be a glass-half-empty person, but is it worth all this effort?” the attendee asked. “In all honesty, you treated a few dozen [patients] for 180,000 visits [per year]. I’m really not sure it’s worth those efforts, and I’m wondering if those efforts could be placed in different areas, especially for a higher yield.”
“Point well taken,” Dr. Landis said. “I think that was the purpose of the study, to see if the emergency department is a place to screen and link patients to care, and we’re trying to optimize that. Remember, there were 4,000 patients, but for many of those, it took literally a minute to screen them.”
An attendee from Australia offered a slightly more positive take on the findings, followed by a suggestion to improve linkage in marginalized populations.
“I’m not sure I’d be pessimistic,” the attendee said. “I think you ought to be commended for getting that number of people to link, because it is very difficult when we are looking at linking people from a hospital-based setting who actually live in the community and suffer from homelessness and mental health issues and incarceration and a whole range of other things. ... Maybe we need to change our idea of having these centralized silos where people are referred, and go out into the community, much like [tuberculosis] clinics used to do, and track people down.”
The study was funded by Gilead. The investigators disclosed additional relationships with HighTide Therapeutics, Intercept, AbbVie, and others.
SOURCE: Landis CS et al. The Liver Meeting 2019, Abstract 168.
BOSTON – ED-based screening is a feasible method of detecting hepatitis C virus (HCV) in high-risk populations, but linkage to care remains low, according to investigators.
An HCV screening program involving three Seattle hospitals and more than 4,000 patients showed that linkage to care was lowest among patients who were younger, homeless, or used injection drugs, reported lead author Charles S. Landis, MD, PhD, of the University of Washington, Seattle.
“In the U.S., rates of acute HCV infections are increasing in younger patients and in areas disproportionally affected by the opiate epidemic,” Dr. Landis said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In order to achieve a goal of elimination, HCV screening, appropriate linkage to care, and treatment will need to be directed toward younger, marginalized, and underserved populations.”
Dr. Landis explained that EDs are suitable for HCV screening because users of emergency services are disproportionately affected by HCV, compared with patients in primary and specialty care settings. Despite this, linkage to care remains historically higher in primary and specialty care settings at approximately 70%, compared with 30% via the ED, Dr. Landis said.
Historically, EDs have been resistant to HCV screening programs, Dr. Landis said, but with the model used in the present study, which relied upon a full-time staff member in each ED who was employed by the infectious disease or hepatology department, no ED resources were needed.
Participants were willing adults who had reliable contact information. Patients were excluded if they were non–English speaking, incarcerated, enrolled or expected to enroll in another clinical study which excludes coenrollment, planned to move out of the region in the next 6 months, admitted to the ED with an acute life-threatening illness, or admitted to the ED for sexual assault. The program had three objectives: Screening, linkage to care, and treatment, all of which were coordinated by the aforementioned case manager.
To date, 4,182 patients have been screened, 936 have been enrolled, 95 have tested positive for HCV RNA, 32 have been linked with care, and 19 have been treated.
“So you can see, a lot of squeeze for a just a little bit of juice here,” Dr. Landis said, referring to the relatively low number of treated patients, compared with how many were screened.
The prevalence of HCV infection based on RNA testing was 2%, though one hospital had a rate of 5%. “This [prevalence] compares to, but is maybe slightly less than, the prevalence seen in others studies based in the emergency department,” Dr. Landis said. “The thought is, not all emergency departments are equal in terms of the patient population that they serve.”
Data analysis showed that the overall linkage to care was 36%. “This is still suboptimal, from my perspective,” Dr. Landis said, “but it does compare with several other ED-based studies.”
A closer look at the data showed that linkage was not uniform across the population. Among patients with homes, linkage to care was 59%, compared with 20% for patients who were homeless (P = .02).
“Ultimately, we need to tailor our approaches for linking homeless patients differently than patients who are not homeless,” Dr. Landis said.
Patients who reported no injection-drug use had a linkage to care of 50%, which was numerically higher than the rate of 20% among users of injection drugs; this difference was not statistically significant, which Dr. Landis attributed to insufficient population size. Similarly, younger patients showed numerical trends toward lower linkage to care.
“Future work will attempt to optimize linkage to care strategies based on patient demographic factors, such as active injection drug use or homelessness,” Dr. Landis said.
During discussion, a conference attendee from the United States expressed skepticism of the program’s merits.
“I may be a glass-half-empty person, but is it worth all this effort?” the attendee asked. “In all honesty, you treated a few dozen [patients] for 180,000 visits [per year]. I’m really not sure it’s worth those efforts, and I’m wondering if those efforts could be placed in different areas, especially for a higher yield.”
“Point well taken,” Dr. Landis said. “I think that was the purpose of the study, to see if the emergency department is a place to screen and link patients to care, and we’re trying to optimize that. Remember, there were 4,000 patients, but for many of those, it took literally a minute to screen them.”
An attendee from Australia offered a slightly more positive take on the findings, followed by a suggestion to improve linkage in marginalized populations.
“I’m not sure I’d be pessimistic,” the attendee said. “I think you ought to be commended for getting that number of people to link, because it is very difficult when we are looking at linking people from a hospital-based setting who actually live in the community and suffer from homelessness and mental health issues and incarceration and a whole range of other things. ... Maybe we need to change our idea of having these centralized silos where people are referred, and go out into the community, much like [tuberculosis] clinics used to do, and track people down.”
The study was funded by Gilead. The investigators disclosed additional relationships with HighTide Therapeutics, Intercept, AbbVie, and others.
SOURCE: Landis CS et al. The Liver Meeting 2019, Abstract 168.
REPORTING FROM THE LIVER MEETING 2019
Frontline ibrutinib saves money over chemoimmunotherapy
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Pulmonary embolism treatment teams adopted widely for complex disease
NEW YORK – Seven years after the formation of the first pulmonary embolism response team (PERT), more than 100 institutions have joined the PERT Consortium, which was created to guide care and research for this thrombotic complication, according to a status report at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“Why are PERTs needed? Pulmonary embolism patients are like snowflakes. No two are the same,” explained Richard Channick, MD, director of the pulmonary vascular disease program, University of California, Los Angeles.
Patient variability is an issue because algorithms for pulmonary embolism (PE) often differ at the point of diagnosis, such as the emergency department or intensive are unit, according to Dr. Channick, who was present when the first PERT was created in 2012 at Massachusetts General Hospital (MGH) in Boston. In addition, treatment algorithms can seem complex at a time when patients are deteriorating quickly.
“The treatment algorithms always say consider this or consider that, and then you get a recommendation with a 2B grade of evidence. So what do you do?” Dr. Channick asked, “This has really been crying for an organized approach.”
PERTs were created to fill this need. In most centers, PERTs are organized to respond to a diagnosis of PE wherever it occurs in the hospital. The goal is rapid activation of a team of experts who can reach a single consensus recommendation.
At MGH and UCLA, a similar relatively simple scheme has been created to guide physicians on how to activate the PERT and which situations make this appropriate.
“A big part of the PERT value has been our ability to conduct a real-time virtual consultation where we leverage online technology to look at images together in order to agree on a strategy,” Dr. Channick explained.
Although frequently asked what specialists are needed for an effective PERT, Dr. Channick said it depends on institutional structures, the types of specialists available, and, in some cases, the specific characteristics of the patient. In many situations, a pulmonary vascular specialist and an interventional radiologist might be sufficient. In others, team members might include some combination of an interventional cardiologist, a cardiac surgeon, and a hematologist.
It is also appropriate to include clinicians likely to participate in care following acute treatment of the PE. “One of the most critical values to PERT is the ability to systematically follow patients” after the PE is treated, Dr. Channick said.
So far, there are no data to confirm patients managed with PERT achieve better outcomes than those who are not. Reductions in mortality, length of stay, and costs are reasonably anticipated and might eventually be demonstrated, but Dr. Channick said that PERTs already have value.
“I think the efficiency of care is important,”he said. He called PERT a “one-stop shopping” approach to ensuring that multiple strategies are considered systematically.
There are many anecdotal examples of the benefits of shared decision-making for PE treatment. In one, a pulmonary specialist in a PERT team narrowly averted a planned thrombolysis in a patient diagnosed with PE who was actually found to have severe pulmonary fibrosis, according to Dr. Channick.
Not least important, the shared decision-making of a PERT could relieve the burden of difficult choices in complex situations. Bad outcomes in PE can be unavoidable even with optimal therapy.
“To me personally, a very important benefit of being part of a PERT is the feeling that we are all in it together,” Dr. Channick said. “Patients can go from being pretty stable to being dead very quickly.”
The PERT Consortium has sponsored an annual meeting on PE since 2015. It also maintains an ongoing registry for PE data from member institutions. These data are expected to have increasing value for comparing the impact of patient characteristics, treatment strategies, and other variables on outcomes.
For clinicians who are uncertain whether the PE incidence at their institution justifies a PERT, Dr. Channick had some advice. “If you build it, they will clot,” he said, meaning that due to the frequency of PE, a PERT will generally have plenty of work once created.
SOURCE: VEITHSYMPOSIUM
NEW YORK – Seven years after the formation of the first pulmonary embolism response team (PERT), more than 100 institutions have joined the PERT Consortium, which was created to guide care and research for this thrombotic complication, according to a status report at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“Why are PERTs needed? Pulmonary embolism patients are like snowflakes. No two are the same,” explained Richard Channick, MD, director of the pulmonary vascular disease program, University of California, Los Angeles.
Patient variability is an issue because algorithms for pulmonary embolism (PE) often differ at the point of diagnosis, such as the emergency department or intensive are unit, according to Dr. Channick, who was present when the first PERT was created in 2012 at Massachusetts General Hospital (MGH) in Boston. In addition, treatment algorithms can seem complex at a time when patients are deteriorating quickly.
“The treatment algorithms always say consider this or consider that, and then you get a recommendation with a 2B grade of evidence. So what do you do?” Dr. Channick asked, “This has really been crying for an organized approach.”
PERTs were created to fill this need. In most centers, PERTs are organized to respond to a diagnosis of PE wherever it occurs in the hospital. The goal is rapid activation of a team of experts who can reach a single consensus recommendation.
At MGH and UCLA, a similar relatively simple scheme has been created to guide physicians on how to activate the PERT and which situations make this appropriate.
“A big part of the PERT value has been our ability to conduct a real-time virtual consultation where we leverage online technology to look at images together in order to agree on a strategy,” Dr. Channick explained.
Although frequently asked what specialists are needed for an effective PERT, Dr. Channick said it depends on institutional structures, the types of specialists available, and, in some cases, the specific characteristics of the patient. In many situations, a pulmonary vascular specialist and an interventional radiologist might be sufficient. In others, team members might include some combination of an interventional cardiologist, a cardiac surgeon, and a hematologist.
It is also appropriate to include clinicians likely to participate in care following acute treatment of the PE. “One of the most critical values to PERT is the ability to systematically follow patients” after the PE is treated, Dr. Channick said.
So far, there are no data to confirm patients managed with PERT achieve better outcomes than those who are not. Reductions in mortality, length of stay, and costs are reasonably anticipated and might eventually be demonstrated, but Dr. Channick said that PERTs already have value.
“I think the efficiency of care is important,”he said. He called PERT a “one-stop shopping” approach to ensuring that multiple strategies are considered systematically.
There are many anecdotal examples of the benefits of shared decision-making for PE treatment. In one, a pulmonary specialist in a PERT team narrowly averted a planned thrombolysis in a patient diagnosed with PE who was actually found to have severe pulmonary fibrosis, according to Dr. Channick.
Not least important, the shared decision-making of a PERT could relieve the burden of difficult choices in complex situations. Bad outcomes in PE can be unavoidable even with optimal therapy.
“To me personally, a very important benefit of being part of a PERT is the feeling that we are all in it together,” Dr. Channick said. “Patients can go from being pretty stable to being dead very quickly.”
The PERT Consortium has sponsored an annual meeting on PE since 2015. It also maintains an ongoing registry for PE data from member institutions. These data are expected to have increasing value for comparing the impact of patient characteristics, treatment strategies, and other variables on outcomes.
For clinicians who are uncertain whether the PE incidence at their institution justifies a PERT, Dr. Channick had some advice. “If you build it, they will clot,” he said, meaning that due to the frequency of PE, a PERT will generally have plenty of work once created.
SOURCE: VEITHSYMPOSIUM
NEW YORK – Seven years after the formation of the first pulmonary embolism response team (PERT), more than 100 institutions have joined the PERT Consortium, which was created to guide care and research for this thrombotic complication, according to a status report at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“Why are PERTs needed? Pulmonary embolism patients are like snowflakes. No two are the same,” explained Richard Channick, MD, director of the pulmonary vascular disease program, University of California, Los Angeles.
Patient variability is an issue because algorithms for pulmonary embolism (PE) often differ at the point of diagnosis, such as the emergency department or intensive are unit, according to Dr. Channick, who was present when the first PERT was created in 2012 at Massachusetts General Hospital (MGH) in Boston. In addition, treatment algorithms can seem complex at a time when patients are deteriorating quickly.
“The treatment algorithms always say consider this or consider that, and then you get a recommendation with a 2B grade of evidence. So what do you do?” Dr. Channick asked, “This has really been crying for an organized approach.”
PERTs were created to fill this need. In most centers, PERTs are organized to respond to a diagnosis of PE wherever it occurs in the hospital. The goal is rapid activation of a team of experts who can reach a single consensus recommendation.
At MGH and UCLA, a similar relatively simple scheme has been created to guide physicians on how to activate the PERT and which situations make this appropriate.
“A big part of the PERT value has been our ability to conduct a real-time virtual consultation where we leverage online technology to look at images together in order to agree on a strategy,” Dr. Channick explained.
Although frequently asked what specialists are needed for an effective PERT, Dr. Channick said it depends on institutional structures, the types of specialists available, and, in some cases, the specific characteristics of the patient. In many situations, a pulmonary vascular specialist and an interventional radiologist might be sufficient. In others, team members might include some combination of an interventional cardiologist, a cardiac surgeon, and a hematologist.
It is also appropriate to include clinicians likely to participate in care following acute treatment of the PE. “One of the most critical values to PERT is the ability to systematically follow patients” after the PE is treated, Dr. Channick said.
So far, there are no data to confirm patients managed with PERT achieve better outcomes than those who are not. Reductions in mortality, length of stay, and costs are reasonably anticipated and might eventually be demonstrated, but Dr. Channick said that PERTs already have value.
“I think the efficiency of care is important,”he said. He called PERT a “one-stop shopping” approach to ensuring that multiple strategies are considered systematically.
There are many anecdotal examples of the benefits of shared decision-making for PE treatment. In one, a pulmonary specialist in a PERT team narrowly averted a planned thrombolysis in a patient diagnosed with PE who was actually found to have severe pulmonary fibrosis, according to Dr. Channick.
Not least important, the shared decision-making of a PERT could relieve the burden of difficult choices in complex situations. Bad outcomes in PE can be unavoidable even with optimal therapy.
“To me personally, a very important benefit of being part of a PERT is the feeling that we are all in it together,” Dr. Channick said. “Patients can go from being pretty stable to being dead very quickly.”
The PERT Consortium has sponsored an annual meeting on PE since 2015. It also maintains an ongoing registry for PE data from member institutions. These data are expected to have increasing value for comparing the impact of patient characteristics, treatment strategies, and other variables on outcomes.
For clinicians who are uncertain whether the PE incidence at their institution justifies a PERT, Dr. Channick had some advice. “If you build it, they will clot,” he said, meaning that due to the frequency of PE, a PERT will generally have plenty of work once created.
SOURCE: VEITHSYMPOSIUM
REPORTING FROM THE VEITHSYMPOSIUM