User login
Neuroimaging in psychiatry: Potentials and pitfalls
Advances in neuroimaging over the past 25 years have allowed for an increasingly sophisticated understanding of the structural and functional brain abnormalities associated with psychiatric disease.1 It has been postulated that a better understanding of aberrant brain circuitry in psychiatric illness will be critical for transforming the diagnosis and treatment of these illnesses.2 In fact, in 2008, the National Institute of Mental Health launched the Research Domain Criteria project to reformulate psychiatric diagnosis based on biologic underpinnings.3
In the midst of these scientific advances and the increased availability of neuroimaging, some private clinics have begun to offer routine brain scans as part of a comprehensive psychiatric evaluation.4-7 These clinics suggest that single-photon emission computed tomography (SPECT) of the brain can provide objective, reliable psychiatric diagnoses. Unfortunately, using SPECT for psychiatric diagnosis lacks empirical support and carries risks, including exposing patients to radioisotopes and detracting from empirically validated treatments.8 Nonetheless, given the current diagnostic challenges in psychiatry, it is understandable that patients, parents, and clinicians alike have reported high receptivity to the use of neuroimaging for psychiatric diagnosis and treatment planning.9
While neuroimaging is central to the search for improved understanding of the biologic foundations of mental illness, progress in identifying biomarkers has been disappointing. There are currently no neuroimaging biomarkers that can reliably distinguish patients from controls, and no empirical evidence supports the use of neuroimaging in diagnosing psychiatric conditions.10 The current standard of clinical care is to use neuroimaging to diagnose neurologic diseases that are masquerading as psychiatric disorders. However, given the rapid advances and availability of this technology, determining if and when neuroimaging is clinically indicated will likely soon become increasingly complex. Prior to the widespread availability of this technology, it is worth considering the potential advantages and pitfalls to the adoption of neuroimaging in psychiatry. In this article, we:
- outline arguments that support the use of neuroimaging in psychiatry, and some of the limitations
- discuss special considerations for patients with first-episode psychosis (FEP) and forensic psychiatry
- suggest guidelines for best-practice models based on the current evidence.
Advantages of widespread use of neuroimaging in psychiatry
Currently, neuroimaging is used in psychiatry to rule out neurologic disorders such as seizures, tumors, or infectious illness that might be causing psychiatric symptoms. If neuroimaging were routinely used for this purpose, one theoretical advantage would be increased neurologic diagnostic accuracy. Furthermore, increased adoption of neuroimaging may eventually help broaden the phenotype of neurologic disorders. In other words, psychiatric symptoms may be more common in neurologic disorders than we currently recognize. A second advantage might be that early and definitive exclusion of a structural neurologic disorder may help patients and families more readily accept a psychiatric diagnosis and appropriate treatment.
In the future, if biomarkers of psychiatric illness are discerned, using neuroimaging for diagnosis, assessment, and treatment planning may help increase objectivity and reduce the stigma associated with mental illness. Currently, psychiatric diagnoses are based on emotional and behavioral self-report and clinical observations. It is not uncommon for patients to receive different diagnoses and even conflicting recommendations from different clinicians. Tools that aid objective diagnosis will likely improve the reliability of the diagnosis and help in assessing treatment response. Also, concrete biomarkers that respond to treatment may help align psychiatric disorders with other medical illnesses, thereby decreasing stigma.
Cautions against routine neuroimaging
There are several potential pitfalls to the routine use of neuroimaging in psychiatry. First, clinical psychiatry is centered on clinical acumen and the doctor–patient relationship. Many psychiatric clinicians are not accustomed to using lab measures or tests to support the diagnostic process or treatment planning. Psychiatrists may be resistant to technologies that threaten clinical acumen, the power of the therapeutic relationship, and the value of getting to know patients over time.11 Overreliance on neuroimaging for psychiatric diagnosis also carries the risk of becoming overly reductionistic. This approach may overemphasize the biologic aspects of mental illness, while excluding social and psychological factors that may be responsive to treatment.
Second, the widespread use of neuroimaging is likely to result in many incidental findings. This is especially relevant because abnormality does not establish causality. Incidental findings may cause unnecessary anxiety for patients and families, particularly if there are minimal treatment options.
Continue to: Third, it remains unclear...
Third, it remains unclear whether widespread neuroimaging in psychiatry will be cost-effective. Unless imaging results are tied to effective treatments, neuroimaging is unlikely to result in cost savings. Presently, patients who can afford out-of-pocket care might be able to access neuroimaging. If neuroimaging were shown to improve clinical outcomes but remains costly, this unequal distribution of resources would create an ethical quandary.
Finally, neuroimaging is complex and almost certainly not as objective as one might hope. Interpreting images will require specialized knowledge and skills that are beyond those of currently certified general psychiatrists.12 Because there is a great deal of overlap in brain anomalies across psychiatric illnesses, it is unclear whether using neuroimaging for diagnostic purposes will eclipse a thorough clinical assessment. For example, the amygdala and insula show activation across a range of anxiety disorders. Abnormal amygdala activation has also been reported in depression, bipolar disorder, schizophrenia, and psychopathy.13
In addition, psychiatric comorbidity is common. It is unclear how much neuroimaging will add diagnostically when a patient presents with multiple psychiatric disorders. Comorbidity of psychiatric and neurologic disorders also is common. A neurologic illness that is detectable by structural neuroimaging does not necessarily exclude the presence of a psychiatric disorder. This poses yet another challenge to developing reliable, valid neuroimaging techniques for clinical use.
Areas of controversy
First-episode psychosis. Current practice guidelines for neuroimaging in patients with FEP are inconsistent. The Canadian Choosing Wisely Guidelines recommend against routinely ordering neuroimaging in first-episode psychoses in the absence of signs or symptoms that suggest intracranial pathology.14 Similarly, the American Psychiatric Association’s Practice Guideline for the Treatment of Patients with Schizophrenia recommends ordering neuroimaging in patients for whom the clinical picture is unclear or when examination reveals abnormal findings.15 In contrast, the Australian Clinical Guidelines for Early Psychosis recommend that all patients with FEP receive brain MRI.16 Freudenreich et al17 describe 2 philosophies regarding the initial medical workup of FEP: (1) a comprehensive medical workup requires extensive testing, and (2) in their natural histories, most illnesses eventually declare themselves.
Despite this inconsistency, the overall evidence does not seem to support routine brain imaging for patients with FEP in the absence of neurologic or cognitive impairment. A systematic review of 16 studies assessing the clinical utility of structural neuroimaging in FEP found that there was “insufficient evidence to suggest that brain imaging should be routinely ordered for patients presenting with first-episode psychosis without associated neurological or cognitive impairment.”18
Continue to: Forensic psychiatry
Forensic psychiatry. Two academic disciplines—neuroethics and neurolaw—attempt to study how medications and neuroimaging could impact forensic psychiatry.19 And in this golden age of neuroscience, psychiatrists specializing in forensics may be increasingly asked to opine on brain scans. This requires specific thoughtfulness and attention because forensic psychiatrists must “distinguish neuroscience from neuro-nonsense.”20 These specialists will need to consider the Daubert standard, which resulted from the 1993 case Daubert v Merrell Dow Pharmaceuticals, Inc.21 In this case, the US Supreme Court ruled that evidence must be “‘generally accepted’ as reliable in the relevant scientific community” to be admissible. According to the Daubert standard, “evidentiary reliability” is based on scientific validity.21
How should we use neuroimaging?
While neuroimaging is a quickly evolving research tool, empirical support for its clinical use remains limited. The hope is that future neuroimaging research will yield biomarker profiles for mental illness, identification of risk factors, and predictors of vulnerability and treatment response, which will allow for more targeted treatments.1
The current standard of clinical care for using neuroimaging in psychiatry is to diagnose neurologic diseases. Although there are no consensus guidelines for when to order imaging, it is reasonable to consider imaging when a patient has22:
- abrupt onset of symptoms
- change in level of consciousness
- deficits in neurologic or cognitive examination
- a history of head trauma (with loss of consciousness), whole-brain radiation, neurologic comorbidities, or cancer
- late onset of symptoms (age >50)
- atypical presentation of psychiatric illness.
1. Silbersweig DA, Rauch SL. Neuroimaging in psychiatry: a quarter century of progress. Harv Rev Psychiatry. 2017;25(5):195-197.
2. Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303(19):1970-1971.
3. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988-989.
4. Cyranoski D. Neuroscience: thought experiment. Nature. 2011;469:148-149.
5. Amen Clinics. https://www.amenclinics.com/. Accessed October 22, 2019.
6. Pathfinder Brain SPECT Imaging. https://pathfinder.md/. Accessed October 22, 2019.
7. DrSpectScan. http://www.drspectscan.org/. Accessed October 22, 2019.
8. Adinoff B, Devous M. Scientifically unfounded claims in diagnosing and treating patients. Am J Psychiatry. 2010;167(5):598.
9. Borgelt EL, Buchman DZ, Illes J. Neuroimaging in mental health care: voices in translation. Front Hum Neurosci. 2012;6:293.
10. Linden DEJ. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73(1):8-22.
11. Macqueen GM. Will there be a role for neuroimaging in clinical psychiatry? J Psychiatry Neurosci. 2010;35(5):291-293.
12. Boyce AC. Neuroimaging in psychiatry: evaluating the ethical consequences for patient care. Bioethics. 2009;23(6):349-359.
13. Farah MJ, Gillihan SJ. Diagnostic brain imaging in psychiatry: current uses and future prospects. Virtual Mentor. 2012;14(6):464-471.
14. Canadian Academy of Child and Adolescent Psychiatry, et al. Thirteen things physicians and patients should question. Choosing Wisely Canada. https://choosingwiselycanada.org/wp-content/uploads/2017/02/Psychiatry.pdf. Updated June 2017. Accessed October 22, 2019.
15. Lehman AF, Lieberman JA, Dixon LB, et al; Work Group on Schizophrenia. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
16. Australian Clinical Guidelines for Early Psychosis. 2nd edition. The National Centre of Excellence in Youth Mental Health. https://www.orygen.org.au/Campus/Expert-Network/Resources/Free/Clinical-Practice/Australian-Clinical-Guidelines-for-Early-Psychosis/Australian-Clinical-Guidelines-for-Early-Psychosis.aspx?ext=. Updated 2016. Accessed October 22, 2019.
17. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
18. Forbes M, Stefler D, Velakoulis D, et al. The clinical utility of structural neuroimaging in first-episode psychosis: a systematic review. Aust N Z J Psychiatry. 2019:000486741984803. doi: 10.1177/0004867419848035.
19. Aggarwal N. Neuroimaging, culture, and forensic psychiatry. J Am Acad Psychiatry Law. 2009;37(2):239-244
20. Choi O. What neuroscience can and cannot answer. J Am Acad Psychiatry Law. 2017;45(3):278-285.
21. Daubert v Merrell Dow Pharmaceuticals, Inc. 509 US 579 (1993).
22. Camprodon JA, Stern TA. Selecting neuroimaging techniques: a review for the clinician. Prim Care Companion CNS Disord. 2013;15(4):PCC.12f01490. doi: 10.4088/PCC.12f01490.
Advances in neuroimaging over the past 25 years have allowed for an increasingly sophisticated understanding of the structural and functional brain abnormalities associated with psychiatric disease.1 It has been postulated that a better understanding of aberrant brain circuitry in psychiatric illness will be critical for transforming the diagnosis and treatment of these illnesses.2 In fact, in 2008, the National Institute of Mental Health launched the Research Domain Criteria project to reformulate psychiatric diagnosis based on biologic underpinnings.3
In the midst of these scientific advances and the increased availability of neuroimaging, some private clinics have begun to offer routine brain scans as part of a comprehensive psychiatric evaluation.4-7 These clinics suggest that single-photon emission computed tomography (SPECT) of the brain can provide objective, reliable psychiatric diagnoses. Unfortunately, using SPECT for psychiatric diagnosis lacks empirical support and carries risks, including exposing patients to radioisotopes and detracting from empirically validated treatments.8 Nonetheless, given the current diagnostic challenges in psychiatry, it is understandable that patients, parents, and clinicians alike have reported high receptivity to the use of neuroimaging for psychiatric diagnosis and treatment planning.9
While neuroimaging is central to the search for improved understanding of the biologic foundations of mental illness, progress in identifying biomarkers has been disappointing. There are currently no neuroimaging biomarkers that can reliably distinguish patients from controls, and no empirical evidence supports the use of neuroimaging in diagnosing psychiatric conditions.10 The current standard of clinical care is to use neuroimaging to diagnose neurologic diseases that are masquerading as psychiatric disorders. However, given the rapid advances and availability of this technology, determining if and when neuroimaging is clinically indicated will likely soon become increasingly complex. Prior to the widespread availability of this technology, it is worth considering the potential advantages and pitfalls to the adoption of neuroimaging in psychiatry. In this article, we:
- outline arguments that support the use of neuroimaging in psychiatry, and some of the limitations
- discuss special considerations for patients with first-episode psychosis (FEP) and forensic psychiatry
- suggest guidelines for best-practice models based on the current evidence.
Advantages of widespread use of neuroimaging in psychiatry
Currently, neuroimaging is used in psychiatry to rule out neurologic disorders such as seizures, tumors, or infectious illness that might be causing psychiatric symptoms. If neuroimaging were routinely used for this purpose, one theoretical advantage would be increased neurologic diagnostic accuracy. Furthermore, increased adoption of neuroimaging may eventually help broaden the phenotype of neurologic disorders. In other words, psychiatric symptoms may be more common in neurologic disorders than we currently recognize. A second advantage might be that early and definitive exclusion of a structural neurologic disorder may help patients and families more readily accept a psychiatric diagnosis and appropriate treatment.
In the future, if biomarkers of psychiatric illness are discerned, using neuroimaging for diagnosis, assessment, and treatment planning may help increase objectivity and reduce the stigma associated with mental illness. Currently, psychiatric diagnoses are based on emotional and behavioral self-report and clinical observations. It is not uncommon for patients to receive different diagnoses and even conflicting recommendations from different clinicians. Tools that aid objective diagnosis will likely improve the reliability of the diagnosis and help in assessing treatment response. Also, concrete biomarkers that respond to treatment may help align psychiatric disorders with other medical illnesses, thereby decreasing stigma.
Cautions against routine neuroimaging
There are several potential pitfalls to the routine use of neuroimaging in psychiatry. First, clinical psychiatry is centered on clinical acumen and the doctor–patient relationship. Many psychiatric clinicians are not accustomed to using lab measures or tests to support the diagnostic process or treatment planning. Psychiatrists may be resistant to technologies that threaten clinical acumen, the power of the therapeutic relationship, and the value of getting to know patients over time.11 Overreliance on neuroimaging for psychiatric diagnosis also carries the risk of becoming overly reductionistic. This approach may overemphasize the biologic aspects of mental illness, while excluding social and psychological factors that may be responsive to treatment.
Second, the widespread use of neuroimaging is likely to result in many incidental findings. This is especially relevant because abnormality does not establish causality. Incidental findings may cause unnecessary anxiety for patients and families, particularly if there are minimal treatment options.
Continue to: Third, it remains unclear...
Third, it remains unclear whether widespread neuroimaging in psychiatry will be cost-effective. Unless imaging results are tied to effective treatments, neuroimaging is unlikely to result in cost savings. Presently, patients who can afford out-of-pocket care might be able to access neuroimaging. If neuroimaging were shown to improve clinical outcomes but remains costly, this unequal distribution of resources would create an ethical quandary.
Finally, neuroimaging is complex and almost certainly not as objective as one might hope. Interpreting images will require specialized knowledge and skills that are beyond those of currently certified general psychiatrists.12 Because there is a great deal of overlap in brain anomalies across psychiatric illnesses, it is unclear whether using neuroimaging for diagnostic purposes will eclipse a thorough clinical assessment. For example, the amygdala and insula show activation across a range of anxiety disorders. Abnormal amygdala activation has also been reported in depression, bipolar disorder, schizophrenia, and psychopathy.13
In addition, psychiatric comorbidity is common. It is unclear how much neuroimaging will add diagnostically when a patient presents with multiple psychiatric disorders. Comorbidity of psychiatric and neurologic disorders also is common. A neurologic illness that is detectable by structural neuroimaging does not necessarily exclude the presence of a psychiatric disorder. This poses yet another challenge to developing reliable, valid neuroimaging techniques for clinical use.
Areas of controversy
First-episode psychosis. Current practice guidelines for neuroimaging in patients with FEP are inconsistent. The Canadian Choosing Wisely Guidelines recommend against routinely ordering neuroimaging in first-episode psychoses in the absence of signs or symptoms that suggest intracranial pathology.14 Similarly, the American Psychiatric Association’s Practice Guideline for the Treatment of Patients with Schizophrenia recommends ordering neuroimaging in patients for whom the clinical picture is unclear or when examination reveals abnormal findings.15 In contrast, the Australian Clinical Guidelines for Early Psychosis recommend that all patients with FEP receive brain MRI.16 Freudenreich et al17 describe 2 philosophies regarding the initial medical workup of FEP: (1) a comprehensive medical workup requires extensive testing, and (2) in their natural histories, most illnesses eventually declare themselves.
Despite this inconsistency, the overall evidence does not seem to support routine brain imaging for patients with FEP in the absence of neurologic or cognitive impairment. A systematic review of 16 studies assessing the clinical utility of structural neuroimaging in FEP found that there was “insufficient evidence to suggest that brain imaging should be routinely ordered for patients presenting with first-episode psychosis without associated neurological or cognitive impairment.”18
Continue to: Forensic psychiatry
Forensic psychiatry. Two academic disciplines—neuroethics and neurolaw—attempt to study how medications and neuroimaging could impact forensic psychiatry.19 And in this golden age of neuroscience, psychiatrists specializing in forensics may be increasingly asked to opine on brain scans. This requires specific thoughtfulness and attention because forensic psychiatrists must “distinguish neuroscience from neuro-nonsense.”20 These specialists will need to consider the Daubert standard, which resulted from the 1993 case Daubert v Merrell Dow Pharmaceuticals, Inc.21 In this case, the US Supreme Court ruled that evidence must be “‘generally accepted’ as reliable in the relevant scientific community” to be admissible. According to the Daubert standard, “evidentiary reliability” is based on scientific validity.21
How should we use neuroimaging?
While neuroimaging is a quickly evolving research tool, empirical support for its clinical use remains limited. The hope is that future neuroimaging research will yield biomarker profiles for mental illness, identification of risk factors, and predictors of vulnerability and treatment response, which will allow for more targeted treatments.1
The current standard of clinical care for using neuroimaging in psychiatry is to diagnose neurologic diseases. Although there are no consensus guidelines for when to order imaging, it is reasonable to consider imaging when a patient has22:
- abrupt onset of symptoms
- change in level of consciousness
- deficits in neurologic or cognitive examination
- a history of head trauma (with loss of consciousness), whole-brain radiation, neurologic comorbidities, or cancer
- late onset of symptoms (age >50)
- atypical presentation of psychiatric illness.
Advances in neuroimaging over the past 25 years have allowed for an increasingly sophisticated understanding of the structural and functional brain abnormalities associated with psychiatric disease.1 It has been postulated that a better understanding of aberrant brain circuitry in psychiatric illness will be critical for transforming the diagnosis and treatment of these illnesses.2 In fact, in 2008, the National Institute of Mental Health launched the Research Domain Criteria project to reformulate psychiatric diagnosis based on biologic underpinnings.3
In the midst of these scientific advances and the increased availability of neuroimaging, some private clinics have begun to offer routine brain scans as part of a comprehensive psychiatric evaluation.4-7 These clinics suggest that single-photon emission computed tomography (SPECT) of the brain can provide objective, reliable psychiatric diagnoses. Unfortunately, using SPECT for psychiatric diagnosis lacks empirical support and carries risks, including exposing patients to radioisotopes and detracting from empirically validated treatments.8 Nonetheless, given the current diagnostic challenges in psychiatry, it is understandable that patients, parents, and clinicians alike have reported high receptivity to the use of neuroimaging for psychiatric diagnosis and treatment planning.9
While neuroimaging is central to the search for improved understanding of the biologic foundations of mental illness, progress in identifying biomarkers has been disappointing. There are currently no neuroimaging biomarkers that can reliably distinguish patients from controls, and no empirical evidence supports the use of neuroimaging in diagnosing psychiatric conditions.10 The current standard of clinical care is to use neuroimaging to diagnose neurologic diseases that are masquerading as psychiatric disorders. However, given the rapid advances and availability of this technology, determining if and when neuroimaging is clinically indicated will likely soon become increasingly complex. Prior to the widespread availability of this technology, it is worth considering the potential advantages and pitfalls to the adoption of neuroimaging in psychiatry. In this article, we:
- outline arguments that support the use of neuroimaging in psychiatry, and some of the limitations
- discuss special considerations for patients with first-episode psychosis (FEP) and forensic psychiatry
- suggest guidelines for best-practice models based on the current evidence.
Advantages of widespread use of neuroimaging in psychiatry
Currently, neuroimaging is used in psychiatry to rule out neurologic disorders such as seizures, tumors, or infectious illness that might be causing psychiatric symptoms. If neuroimaging were routinely used for this purpose, one theoretical advantage would be increased neurologic diagnostic accuracy. Furthermore, increased adoption of neuroimaging may eventually help broaden the phenotype of neurologic disorders. In other words, psychiatric symptoms may be more common in neurologic disorders than we currently recognize. A second advantage might be that early and definitive exclusion of a structural neurologic disorder may help patients and families more readily accept a psychiatric diagnosis and appropriate treatment.
In the future, if biomarkers of psychiatric illness are discerned, using neuroimaging for diagnosis, assessment, and treatment planning may help increase objectivity and reduce the stigma associated with mental illness. Currently, psychiatric diagnoses are based on emotional and behavioral self-report and clinical observations. It is not uncommon for patients to receive different diagnoses and even conflicting recommendations from different clinicians. Tools that aid objective diagnosis will likely improve the reliability of the diagnosis and help in assessing treatment response. Also, concrete biomarkers that respond to treatment may help align psychiatric disorders with other medical illnesses, thereby decreasing stigma.
Cautions against routine neuroimaging
There are several potential pitfalls to the routine use of neuroimaging in psychiatry. First, clinical psychiatry is centered on clinical acumen and the doctor–patient relationship. Many psychiatric clinicians are not accustomed to using lab measures or tests to support the diagnostic process or treatment planning. Psychiatrists may be resistant to technologies that threaten clinical acumen, the power of the therapeutic relationship, and the value of getting to know patients over time.11 Overreliance on neuroimaging for psychiatric diagnosis also carries the risk of becoming overly reductionistic. This approach may overemphasize the biologic aspects of mental illness, while excluding social and psychological factors that may be responsive to treatment.
Second, the widespread use of neuroimaging is likely to result in many incidental findings. This is especially relevant because abnormality does not establish causality. Incidental findings may cause unnecessary anxiety for patients and families, particularly if there are minimal treatment options.
Continue to: Third, it remains unclear...
Third, it remains unclear whether widespread neuroimaging in psychiatry will be cost-effective. Unless imaging results are tied to effective treatments, neuroimaging is unlikely to result in cost savings. Presently, patients who can afford out-of-pocket care might be able to access neuroimaging. If neuroimaging were shown to improve clinical outcomes but remains costly, this unequal distribution of resources would create an ethical quandary.
Finally, neuroimaging is complex and almost certainly not as objective as one might hope. Interpreting images will require specialized knowledge and skills that are beyond those of currently certified general psychiatrists.12 Because there is a great deal of overlap in brain anomalies across psychiatric illnesses, it is unclear whether using neuroimaging for diagnostic purposes will eclipse a thorough clinical assessment. For example, the amygdala and insula show activation across a range of anxiety disorders. Abnormal amygdala activation has also been reported in depression, bipolar disorder, schizophrenia, and psychopathy.13
In addition, psychiatric comorbidity is common. It is unclear how much neuroimaging will add diagnostically when a patient presents with multiple psychiatric disorders. Comorbidity of psychiatric and neurologic disorders also is common. A neurologic illness that is detectable by structural neuroimaging does not necessarily exclude the presence of a psychiatric disorder. This poses yet another challenge to developing reliable, valid neuroimaging techniques for clinical use.
Areas of controversy
First-episode psychosis. Current practice guidelines for neuroimaging in patients with FEP are inconsistent. The Canadian Choosing Wisely Guidelines recommend against routinely ordering neuroimaging in first-episode psychoses in the absence of signs or symptoms that suggest intracranial pathology.14 Similarly, the American Psychiatric Association’s Practice Guideline for the Treatment of Patients with Schizophrenia recommends ordering neuroimaging in patients for whom the clinical picture is unclear or when examination reveals abnormal findings.15 In contrast, the Australian Clinical Guidelines for Early Psychosis recommend that all patients with FEP receive brain MRI.16 Freudenreich et al17 describe 2 philosophies regarding the initial medical workup of FEP: (1) a comprehensive medical workup requires extensive testing, and (2) in their natural histories, most illnesses eventually declare themselves.
Despite this inconsistency, the overall evidence does not seem to support routine brain imaging for patients with FEP in the absence of neurologic or cognitive impairment. A systematic review of 16 studies assessing the clinical utility of structural neuroimaging in FEP found that there was “insufficient evidence to suggest that brain imaging should be routinely ordered for patients presenting with first-episode psychosis without associated neurological or cognitive impairment.”18
Continue to: Forensic psychiatry
Forensic psychiatry. Two academic disciplines—neuroethics and neurolaw—attempt to study how medications and neuroimaging could impact forensic psychiatry.19 And in this golden age of neuroscience, psychiatrists specializing in forensics may be increasingly asked to opine on brain scans. This requires specific thoughtfulness and attention because forensic psychiatrists must “distinguish neuroscience from neuro-nonsense.”20 These specialists will need to consider the Daubert standard, which resulted from the 1993 case Daubert v Merrell Dow Pharmaceuticals, Inc.21 In this case, the US Supreme Court ruled that evidence must be “‘generally accepted’ as reliable in the relevant scientific community” to be admissible. According to the Daubert standard, “evidentiary reliability” is based on scientific validity.21
How should we use neuroimaging?
While neuroimaging is a quickly evolving research tool, empirical support for its clinical use remains limited. The hope is that future neuroimaging research will yield biomarker profiles for mental illness, identification of risk factors, and predictors of vulnerability and treatment response, which will allow for more targeted treatments.1
The current standard of clinical care for using neuroimaging in psychiatry is to diagnose neurologic diseases. Although there are no consensus guidelines for when to order imaging, it is reasonable to consider imaging when a patient has22:
- abrupt onset of symptoms
- change in level of consciousness
- deficits in neurologic or cognitive examination
- a history of head trauma (with loss of consciousness), whole-brain radiation, neurologic comorbidities, or cancer
- late onset of symptoms (age >50)
- atypical presentation of psychiatric illness.
1. Silbersweig DA, Rauch SL. Neuroimaging in psychiatry: a quarter century of progress. Harv Rev Psychiatry. 2017;25(5):195-197.
2. Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303(19):1970-1971.
3. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988-989.
4. Cyranoski D. Neuroscience: thought experiment. Nature. 2011;469:148-149.
5. Amen Clinics. https://www.amenclinics.com/. Accessed October 22, 2019.
6. Pathfinder Brain SPECT Imaging. https://pathfinder.md/. Accessed October 22, 2019.
7. DrSpectScan. http://www.drspectscan.org/. Accessed October 22, 2019.
8. Adinoff B, Devous M. Scientifically unfounded claims in diagnosing and treating patients. Am J Psychiatry. 2010;167(5):598.
9. Borgelt EL, Buchman DZ, Illes J. Neuroimaging in mental health care: voices in translation. Front Hum Neurosci. 2012;6:293.
10. Linden DEJ. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73(1):8-22.
11. Macqueen GM. Will there be a role for neuroimaging in clinical psychiatry? J Psychiatry Neurosci. 2010;35(5):291-293.
12. Boyce AC. Neuroimaging in psychiatry: evaluating the ethical consequences for patient care. Bioethics. 2009;23(6):349-359.
13. Farah MJ, Gillihan SJ. Diagnostic brain imaging in psychiatry: current uses and future prospects. Virtual Mentor. 2012;14(6):464-471.
14. Canadian Academy of Child and Adolescent Psychiatry, et al. Thirteen things physicians and patients should question. Choosing Wisely Canada. https://choosingwiselycanada.org/wp-content/uploads/2017/02/Psychiatry.pdf. Updated June 2017. Accessed October 22, 2019.
15. Lehman AF, Lieberman JA, Dixon LB, et al; Work Group on Schizophrenia. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
16. Australian Clinical Guidelines for Early Psychosis. 2nd edition. The National Centre of Excellence in Youth Mental Health. https://www.orygen.org.au/Campus/Expert-Network/Resources/Free/Clinical-Practice/Australian-Clinical-Guidelines-for-Early-Psychosis/Australian-Clinical-Guidelines-for-Early-Psychosis.aspx?ext=. Updated 2016. Accessed October 22, 2019.
17. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
18. Forbes M, Stefler D, Velakoulis D, et al. The clinical utility of structural neuroimaging in first-episode psychosis: a systematic review. Aust N Z J Psychiatry. 2019:000486741984803. doi: 10.1177/0004867419848035.
19. Aggarwal N. Neuroimaging, culture, and forensic psychiatry. J Am Acad Psychiatry Law. 2009;37(2):239-244
20. Choi O. What neuroscience can and cannot answer. J Am Acad Psychiatry Law. 2017;45(3):278-285.
21. Daubert v Merrell Dow Pharmaceuticals, Inc. 509 US 579 (1993).
22. Camprodon JA, Stern TA. Selecting neuroimaging techniques: a review for the clinician. Prim Care Companion CNS Disord. 2013;15(4):PCC.12f01490. doi: 10.4088/PCC.12f01490.
1. Silbersweig DA, Rauch SL. Neuroimaging in psychiatry: a quarter century of progress. Harv Rev Psychiatry. 2017;25(5):195-197.
2. Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303(19):1970-1971.
3. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988-989.
4. Cyranoski D. Neuroscience: thought experiment. Nature. 2011;469:148-149.
5. Amen Clinics. https://www.amenclinics.com/. Accessed October 22, 2019.
6. Pathfinder Brain SPECT Imaging. https://pathfinder.md/. Accessed October 22, 2019.
7. DrSpectScan. http://www.drspectscan.org/. Accessed October 22, 2019.
8. Adinoff B, Devous M. Scientifically unfounded claims in diagnosing and treating patients. Am J Psychiatry. 2010;167(5):598.
9. Borgelt EL, Buchman DZ, Illes J. Neuroimaging in mental health care: voices in translation. Front Hum Neurosci. 2012;6:293.
10. Linden DEJ. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73(1):8-22.
11. Macqueen GM. Will there be a role for neuroimaging in clinical psychiatry? J Psychiatry Neurosci. 2010;35(5):291-293.
12. Boyce AC. Neuroimaging in psychiatry: evaluating the ethical consequences for patient care. Bioethics. 2009;23(6):349-359.
13. Farah MJ, Gillihan SJ. Diagnostic brain imaging in psychiatry: current uses and future prospects. Virtual Mentor. 2012;14(6):464-471.
14. Canadian Academy of Child and Adolescent Psychiatry, et al. Thirteen things physicians and patients should question. Choosing Wisely Canada. https://choosingwiselycanada.org/wp-content/uploads/2017/02/Psychiatry.pdf. Updated June 2017. Accessed October 22, 2019.
15. Lehman AF, Lieberman JA, Dixon LB, et al; Work Group on Schizophrenia. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
16. Australian Clinical Guidelines for Early Psychosis. 2nd edition. The National Centre of Excellence in Youth Mental Health. https://www.orygen.org.au/Campus/Expert-Network/Resources/Free/Clinical-Practice/Australian-Clinical-Guidelines-for-Early-Psychosis/Australian-Clinical-Guidelines-for-Early-Psychosis.aspx?ext=. Updated 2016. Accessed October 22, 2019.
17. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
18. Forbes M, Stefler D, Velakoulis D, et al. The clinical utility of structural neuroimaging in first-episode psychosis: a systematic review. Aust N Z J Psychiatry. 2019:000486741984803. doi: 10.1177/0004867419848035.
19. Aggarwal N. Neuroimaging, culture, and forensic psychiatry. J Am Acad Psychiatry Law. 2009;37(2):239-244
20. Choi O. What neuroscience can and cannot answer. J Am Acad Psychiatry Law. 2017;45(3):278-285.
21. Daubert v Merrell Dow Pharmaceuticals, Inc. 509 US 579 (1993).
22. Camprodon JA, Stern TA. Selecting neuroimaging techniques: a review for the clinician. Prim Care Companion CNS Disord. 2013;15(4):PCC.12f01490. doi: 10.4088/PCC.12f01490.
Seeing snakes that aren’t there
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
In-flight psychiatric emergencies: What you should know
Although they are rare, in-flight psychiatric emergencies occur because of large numbers of passengers, nonstop flights over longer distances, delayed flights, cramped cabins, and/or alcohol consumption.1,2 Psychiatric symptoms and substance intoxication/withdrawal each represent up to
When a passenger requires medical or psychiatric treatment, the flight crew often requests aid from any trained medical professionals who are on board to augment their capabilities and resources (eg, the flight crew’s training, ground-based medical support).1 In the United States, off-duty medical professionals are not legally required to assist during an in-flight medical emergency.1 The Aviation Medical Assistance Act of 1998 protects passengers who provide medical assistance from liability, except in cases of gross negligence or willful misconduct.1,3 Flights outside of the United States are governed by a complex combination of public and private international laws.1 Here I suggest how to initiate care during in-flight psychiatric emergencies, and offer therapeutic options to employ for a passenger who is exhibiting psychiatric symptoms.
What to do first
Before volunteering to assist in a mental health emergency, consider your capabilities and limitations. Do not volunteer if you are under the influence of alcohol, illicit substances, or any medications (prescription or over-the-counter) that could affect your judgment.
Inform the flight crew that you are a mental health clinician, and outline your current clinical expertise. While the flight crew obtains the medical emergency kit, work to establish rapport with the passenger to identify the psychiatric problem and help de-escalate the situation. Initiate care by1:
- eliciting a psychiatric history
- inquiring about any use of alcohol, illicit substances, or other mood-altering substances (eg, type, amount, and time of use)
- identifying any use of psychotropic medications (eg, doses, last dose taken, and if these agents are on the aircraft).
The Federal Aviation Administration has minimum requirements for the contents of medical emergency kits aboard US airlines.1,4 However, they are not required to contain antipsychotics, naloxone, or benzodiazepines.1,4 Although you may have limited medical resources at your disposal, you can still help passengers in the following ways1:
Monitor vital signs and mental status changes, identify signs and symptoms of intoxication or withdrawal, and assess for respiratory distress. Provide reassurance to the passenger if appropriate.1
Administer naloxone (if available) for suspected opioid ingestion.1 Antiemetics, which are available in these medical kits, can be used if needed. Encourage passengers to remain hydrated and use oxygen as needed.
Continue to: If verbal de-escalation is ineffective...
If verbal de-escalation is ineffective, consider administering a benzodiazepine or antipsychotic (if available).1 If the passenger is combative, refer to the flight crew for the airline’s security protocols, which may include restraining the passenger or diverting the aircraft. Safety takes priority over attempts at medical management.
If the passenger has respiratory distress, instruct the flight crew to contact ground-based medical support for additional recommendations.1
A challenging situation
Ultimately, the pilot coordinates with the flight dispatcher to manage all operational decisions for the aircraft and is responsible for decisions regarding flight diversion.1 In-flight medical volunteers, the flight crew, and ground-based medical experts can offer recommendations for care.1 Cruising at altitudes of 30,000 to 40,000 feet with limited medical equipment, often hours away from the closest medical facility, will create unfamiliar challenges for any medical professional who volunteers for in-flight psychiatric emergencies.1
1. Martin-Gill C, Doyle TJ, Yealy DM. In-flight medical emergencies: a review. JAMA. 2018;320(24):2580-2590.
2. Naouri D, Lapostolle F, Rondet C, et al. Prevention of medical events during air travel: a narrative review. Am J Med. 2016;129(9):1000.e1-e6.
3. Aviation Medical Assistance Act of 1998, 49 USC §44701, 105th Cong, Public Law 170 (1998).
4. Federal Aviation Administration. FAA Advisory circular No 121-33B: emergency medical equipment. https://www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-33B.pdf. Published January 12, 2006. Accessed November 14, 2019.
Although they are rare, in-flight psychiatric emergencies occur because of large numbers of passengers, nonstop flights over longer distances, delayed flights, cramped cabins, and/or alcohol consumption.1,2 Psychiatric symptoms and substance intoxication/withdrawal each represent up to
When a passenger requires medical or psychiatric treatment, the flight crew often requests aid from any trained medical professionals who are on board to augment their capabilities and resources (eg, the flight crew’s training, ground-based medical support).1 In the United States, off-duty medical professionals are not legally required to assist during an in-flight medical emergency.1 The Aviation Medical Assistance Act of 1998 protects passengers who provide medical assistance from liability, except in cases of gross negligence or willful misconduct.1,3 Flights outside of the United States are governed by a complex combination of public and private international laws.1 Here I suggest how to initiate care during in-flight psychiatric emergencies, and offer therapeutic options to employ for a passenger who is exhibiting psychiatric symptoms.
What to do first
Before volunteering to assist in a mental health emergency, consider your capabilities and limitations. Do not volunteer if you are under the influence of alcohol, illicit substances, or any medications (prescription or over-the-counter) that could affect your judgment.
Inform the flight crew that you are a mental health clinician, and outline your current clinical expertise. While the flight crew obtains the medical emergency kit, work to establish rapport with the passenger to identify the psychiatric problem and help de-escalate the situation. Initiate care by1:
- eliciting a psychiatric history
- inquiring about any use of alcohol, illicit substances, or other mood-altering substances (eg, type, amount, and time of use)
- identifying any use of psychotropic medications (eg, doses, last dose taken, and if these agents are on the aircraft).
The Federal Aviation Administration has minimum requirements for the contents of medical emergency kits aboard US airlines.1,4 However, they are not required to contain antipsychotics, naloxone, or benzodiazepines.1,4 Although you may have limited medical resources at your disposal, you can still help passengers in the following ways1:
Monitor vital signs and mental status changes, identify signs and symptoms of intoxication or withdrawal, and assess for respiratory distress. Provide reassurance to the passenger if appropriate.1
Administer naloxone (if available) for suspected opioid ingestion.1 Antiemetics, which are available in these medical kits, can be used if needed. Encourage passengers to remain hydrated and use oxygen as needed.
Continue to: If verbal de-escalation is ineffective...
If verbal de-escalation is ineffective, consider administering a benzodiazepine or antipsychotic (if available).1 If the passenger is combative, refer to the flight crew for the airline’s security protocols, which may include restraining the passenger or diverting the aircraft. Safety takes priority over attempts at medical management.
If the passenger has respiratory distress, instruct the flight crew to contact ground-based medical support for additional recommendations.1
A challenging situation
Ultimately, the pilot coordinates with the flight dispatcher to manage all operational decisions for the aircraft and is responsible for decisions regarding flight diversion.1 In-flight medical volunteers, the flight crew, and ground-based medical experts can offer recommendations for care.1 Cruising at altitudes of 30,000 to 40,000 feet with limited medical equipment, often hours away from the closest medical facility, will create unfamiliar challenges for any medical professional who volunteers for in-flight psychiatric emergencies.1
Although they are rare, in-flight psychiatric emergencies occur because of large numbers of passengers, nonstop flights over longer distances, delayed flights, cramped cabins, and/or alcohol consumption.1,2 Psychiatric symptoms and substance intoxication/withdrawal each represent up to
When a passenger requires medical or psychiatric treatment, the flight crew often requests aid from any trained medical professionals who are on board to augment their capabilities and resources (eg, the flight crew’s training, ground-based medical support).1 In the United States, off-duty medical professionals are not legally required to assist during an in-flight medical emergency.1 The Aviation Medical Assistance Act of 1998 protects passengers who provide medical assistance from liability, except in cases of gross negligence or willful misconduct.1,3 Flights outside of the United States are governed by a complex combination of public and private international laws.1 Here I suggest how to initiate care during in-flight psychiatric emergencies, and offer therapeutic options to employ for a passenger who is exhibiting psychiatric symptoms.
What to do first
Before volunteering to assist in a mental health emergency, consider your capabilities and limitations. Do not volunteer if you are under the influence of alcohol, illicit substances, or any medications (prescription or over-the-counter) that could affect your judgment.
Inform the flight crew that you are a mental health clinician, and outline your current clinical expertise. While the flight crew obtains the medical emergency kit, work to establish rapport with the passenger to identify the psychiatric problem and help de-escalate the situation. Initiate care by1:
- eliciting a psychiatric history
- inquiring about any use of alcohol, illicit substances, or other mood-altering substances (eg, type, amount, and time of use)
- identifying any use of psychotropic medications (eg, doses, last dose taken, and if these agents are on the aircraft).
The Federal Aviation Administration has minimum requirements for the contents of medical emergency kits aboard US airlines.1,4 However, they are not required to contain antipsychotics, naloxone, or benzodiazepines.1,4 Although you may have limited medical resources at your disposal, you can still help passengers in the following ways1:
Monitor vital signs and mental status changes, identify signs and symptoms of intoxication or withdrawal, and assess for respiratory distress. Provide reassurance to the passenger if appropriate.1
Administer naloxone (if available) for suspected opioid ingestion.1 Antiemetics, which are available in these medical kits, can be used if needed. Encourage passengers to remain hydrated and use oxygen as needed.
Continue to: If verbal de-escalation is ineffective...
If verbal de-escalation is ineffective, consider administering a benzodiazepine or antipsychotic (if available).1 If the passenger is combative, refer to the flight crew for the airline’s security protocols, which may include restraining the passenger or diverting the aircraft. Safety takes priority over attempts at medical management.
If the passenger has respiratory distress, instruct the flight crew to contact ground-based medical support for additional recommendations.1
A challenging situation
Ultimately, the pilot coordinates with the flight dispatcher to manage all operational decisions for the aircraft and is responsible for decisions regarding flight diversion.1 In-flight medical volunteers, the flight crew, and ground-based medical experts can offer recommendations for care.1 Cruising at altitudes of 30,000 to 40,000 feet with limited medical equipment, often hours away from the closest medical facility, will create unfamiliar challenges for any medical professional who volunteers for in-flight psychiatric emergencies.1
1. Martin-Gill C, Doyle TJ, Yealy DM. In-flight medical emergencies: a review. JAMA. 2018;320(24):2580-2590.
2. Naouri D, Lapostolle F, Rondet C, et al. Prevention of medical events during air travel: a narrative review. Am J Med. 2016;129(9):1000.e1-e6.
3. Aviation Medical Assistance Act of 1998, 49 USC §44701, 105th Cong, Public Law 170 (1998).
4. Federal Aviation Administration. FAA Advisory circular No 121-33B: emergency medical equipment. https://www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-33B.pdf. Published January 12, 2006. Accessed November 14, 2019.
1. Martin-Gill C, Doyle TJ, Yealy DM. In-flight medical emergencies: a review. JAMA. 2018;320(24):2580-2590.
2. Naouri D, Lapostolle F, Rondet C, et al. Prevention of medical events during air travel: a narrative review. Am J Med. 2016;129(9):1000.e1-e6.
3. Aviation Medical Assistance Act of 1998, 49 USC §44701, 105th Cong, Public Law 170 (1998).
4. Federal Aviation Administration. FAA Advisory circular No 121-33B: emergency medical equipment. https://www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-33B.pdf. Published January 12, 2006. Accessed November 14, 2019.
My vision as a candidate for APA President-Elect
Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation click here.
I have been informed by the American Psychiatric Association (APA) Nominating Committee that I am a candidate for the position of APA President-Elect. I am honored to be nominated along with 2 other esteemed psychiatrists, David C. Henderson, MD, and Vivian B. Pender, MD.
You have all known me for many years as Editor-in-Chief of this journal, and probably have read many of my 150 editorials in which I frequently discussed and commented on not only the challenges that face psychiatry, but also the great promise and bright future of our evolving clinical neuroscience medical specialty. You can access all of these at MDedge.com/psychiatry/editor.
In this pre-election editorial, I would like to tell you about my qualifications as a candidate for this critical national psychiatry leadership role. Most of you are APA members who will have the opportunity to vote for the candidate of your choice from January 2 to 31, 2020. I hope that you will support my candidacy after learning about my long-standing involvement within the APA governance, as well as my 3 decades of academic leadership experience and productivity. You also know where I stand on the issues from my writings in
APA involvement
- President, Missouri Psychiatric Physicians Association District Branch (2017-2018)
- President, Cincinnati Psychiatric Society (2007-2009)
- President, Ohio Psychiatric Physicians Foundation (2008-2013)
- Editor, Ohio Psychiatric Physicians Association (OPPA) Newsletter (Insight Matters) (2003-2008)
- Executive Council, OPPA (2003-2013)
- APA Council on Research (1993-2000)
- APA Committee on Research in Psychiatric Treatments (1992-1995)
- APA Task Force on Schizophrenia (1998-1999)
- President, Ohio Psychiatric Association Education and Research Foundation (1987-1994)
Academic track record
- Served as Chief of Psychiatry, VA Medical Center, Iowa City, Iowa for 6 years; Chair, Department of Psychiatry, The Ohio State University for 12 years; Chair, Department of Psychiatry, Saint Louis University for 6 years; and Associate Dean, University of Cincinnati for 4 years
- Published >700 articles, 570 abstracts, and 14 books
- Recruited and developed dozens of faculty members; supervised and mentored hundreds of residents, many of whom became medical directors, department chairs, and/or distinguished clinicians
- Received numerous awards and recognitions for clinical, teaching, and research excellence
- Serve as Editor for 3 journals (Current Psychiatry, Schizophrenia Research, and Biomarkers in Neuropsychiatry)
Statement of vision and priorities
I am very optimistic about the future of psychiatry. The breakthroughs and advances in neuroscience all bolster the scientific basis of psychiatric disorders, and will lead to many novel treatments in the future. Psychiatry is a medical specialty that is now much more integrated into the “big tent” of medicine. Psychiatrists are physicians, and I believe the name of our association must reflect that. I was successful in changing the names of 2 district branches to include “physicians” (Ohio Psychiatric Physicians Association and Missouri Psychiatric Physicians Association). If elected, I will propose to the Board of Trustees and the APA members that we change our name to the American Psychiatric Physicians Association, which will emphasize our medical identity within mental health. In its 175-year history, the APA has experienced 2 previous name changes.
I believe the strengths of the APA far exceed its weaknesses, and its opportunities outnumber its threats. However, the following perennial challenges must be forcefully addressed by all of us:
- The pernicious and discriminatory dogma of stigma must be shattered for the sake of patients, their families, their psychiatrists, and the profession.
- Pre-authorization is essentially the insurance companies practicing medicine without a license when, without ever actually examining the patient, they tell physicians what they should or should not prescribe. That’s felonious!
- Competent and safe prescribing is the culmination of extensive medical training (approximately 14,000 hours) and psychologists do not qualify.
- Board certification fees must be reduced, and recertification (Maintenance of Certification) must be simpler and less onerous.
- Effective parity laws must have teeth, not just words!
- Patient care, not computer care! Electronic health records must be more user-friendly and less time-consuming.
- Patients with psychiatric illness who have relapsed must be surrounded by compassionate medical professionals in a hospital setting, not by armed guards in a jail or prison.
- The shortage of psychiatrists can be remedied if the government funds additional residency slots as it did in the 1960s and 1970s. The number of applicants for psychiatric training is rapidly rising, but the number of residency slots has not changed for decades. Approximately 100 US medical school graduates did not match last year, along with >1,000 international medical graduate applicants.
- Lawyers have clients; psychiatrists have patients (as do cardiologists, neurologists, and oncologists). The term “clients” de-medicalizes psychiatric disorders and does not evoke public support or compassion.
- Psychotherapy is in fact a neurobiologic treatment that repairs the mind via neuroplasticity and synaptogenesis. It should get the same respect as pharmacotherapy.
- Untether psychiatric reimbursement from “time”! Psychiatric assessment and treatment are medical procedures. Excising depression, psychosis, panic attacks, or suicidal urges are to the mind what surgery is to the body.
- Clinical psychiatrists have much to offer for medical advances. Their observations generate hypotheses, and if these are published as a case report or letter to the editor, researchers can conduct hypothesis-testing and discover new treatments thanks to astute clinicians.
- The FDA should allow clinical trials to investigate treatments of symptoms, not (often heterogenous) DSM diagnoses. This will enable “off-label use” of medication, which often is necessary.
Continue to: Annual dues
Annual dues. The APA is a great organization that should continue to re-invent itself and re-engineer its procedures and business practices to generate additional revenue streams that could help reduce its annual dues. I know many members who complain about the APA dues, and former members who dropped out because of what they consider to be high dues.
Public education. The APA must intensify public education across all media platforms. This will help dispel myths, eliminate stigma, enforce parity, and portray psychiatry as a medical and scientific discipline. We have a great story to tell about how neurologic circuitry generates the mind and its mental functions, and the neurobiologic foundations of psychiatric brain disorders.
The APA should advocate for (and perhaps organize) an annual mental health check-up (online) in children, adolescents, adults, and the elderly for early detection and intervention.
Collaborative care. We should have close relationships with obstetricians to help prevent neurodevelopmental pathology due to perinatal complications as well as to manage depression in women in the pre- and postpartum phases. Collaborative care with pediatricians, family physicians, internists, and neurologists is necessary to integrate physical and mental health care for our patients, many of whom have multiple medical comorbidities and premature mortality.
Lobbying. The APA must intensify its lobbying to address the unacceptably high rate of suicide, addiction-related deaths, posttraumatic stress disorder due to trauma in children and adults, threats to mental health due to climate change and pollution, refugee mental health, stressful political zeitgeist, and the woefully high rate of uninsured or under-insured individuals.
Continue to: Industry
Industry. There are many significant unmet treatment needs in psychiatry. Approximately 82% of DSM disorders do not have any FDA-approved medication. The APA should constructively engage the pharmaceutical industry (the only entity that develops medications for our patients!) to do more research and development of therapies for conditions with no approved treatments, and to explore new mechanisms of action for more effective or tolerable psychiatric medications. Importantly, the APA should urge major pharmaceutical companies not to abandon neuropsychiatric disorders because they afflict tens of millions of US citizens and are the top causes of long-term disabilities.
Journals. The APA should consider rebranding its journals as “JAPA,” similar to JAMA, which will widen its influence and generate revenue to fund various priorities.
Telepsychiatry. And why can’t the APA create a national telepsychiatry network to meet the needs of underserved populations who have very little access to psychiatric care as in many rural areas? Private companies have filled that space, but the APA and its members can do it better, and this can become a benefit of membership.
Brain bank. Finally, the APA should consider establishing a “Brain Bank” of various psychiatric subspecialties to consult and advise the military, college administrators, corporations, and government agencies about strategies and tactics to solve many problems that arise from overt or covert psychiatric illnesses among their employees, staff, students, or constituents.
The APA cannot solve all societal problems, but it has the moral authority and clinical/scientific depth and gravitas to create an agenda of solutions and to partner with many other stakeholders to achieve mutual societal health goals.
Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation click here.
I have been informed by the American Psychiatric Association (APA) Nominating Committee that I am a candidate for the position of APA President-Elect. I am honored to be nominated along with 2 other esteemed psychiatrists, David C. Henderson, MD, and Vivian B. Pender, MD.
You have all known me for many years as Editor-in-Chief of this journal, and probably have read many of my 150 editorials in which I frequently discussed and commented on not only the challenges that face psychiatry, but also the great promise and bright future of our evolving clinical neuroscience medical specialty. You can access all of these at MDedge.com/psychiatry/editor.
In this pre-election editorial, I would like to tell you about my qualifications as a candidate for this critical national psychiatry leadership role. Most of you are APA members who will have the opportunity to vote for the candidate of your choice from January 2 to 31, 2020. I hope that you will support my candidacy after learning about my long-standing involvement within the APA governance, as well as my 3 decades of academic leadership experience and productivity. You also know where I stand on the issues from my writings in
APA involvement
- President, Missouri Psychiatric Physicians Association District Branch (2017-2018)
- President, Cincinnati Psychiatric Society (2007-2009)
- President, Ohio Psychiatric Physicians Foundation (2008-2013)
- Editor, Ohio Psychiatric Physicians Association (OPPA) Newsletter (Insight Matters) (2003-2008)
- Executive Council, OPPA (2003-2013)
- APA Council on Research (1993-2000)
- APA Committee on Research in Psychiatric Treatments (1992-1995)
- APA Task Force on Schizophrenia (1998-1999)
- President, Ohio Psychiatric Association Education and Research Foundation (1987-1994)
Academic track record
- Served as Chief of Psychiatry, VA Medical Center, Iowa City, Iowa for 6 years; Chair, Department of Psychiatry, The Ohio State University for 12 years; Chair, Department of Psychiatry, Saint Louis University for 6 years; and Associate Dean, University of Cincinnati for 4 years
- Published >700 articles, 570 abstracts, and 14 books
- Recruited and developed dozens of faculty members; supervised and mentored hundreds of residents, many of whom became medical directors, department chairs, and/or distinguished clinicians
- Received numerous awards and recognitions for clinical, teaching, and research excellence
- Serve as Editor for 3 journals (Current Psychiatry, Schizophrenia Research, and Biomarkers in Neuropsychiatry)
Statement of vision and priorities
I am very optimistic about the future of psychiatry. The breakthroughs and advances in neuroscience all bolster the scientific basis of psychiatric disorders, and will lead to many novel treatments in the future. Psychiatry is a medical specialty that is now much more integrated into the “big tent” of medicine. Psychiatrists are physicians, and I believe the name of our association must reflect that. I was successful in changing the names of 2 district branches to include “physicians” (Ohio Psychiatric Physicians Association and Missouri Psychiatric Physicians Association). If elected, I will propose to the Board of Trustees and the APA members that we change our name to the American Psychiatric Physicians Association, which will emphasize our medical identity within mental health. In its 175-year history, the APA has experienced 2 previous name changes.
I believe the strengths of the APA far exceed its weaknesses, and its opportunities outnumber its threats. However, the following perennial challenges must be forcefully addressed by all of us:
- The pernicious and discriminatory dogma of stigma must be shattered for the sake of patients, their families, their psychiatrists, and the profession.
- Pre-authorization is essentially the insurance companies practicing medicine without a license when, without ever actually examining the patient, they tell physicians what they should or should not prescribe. That’s felonious!
- Competent and safe prescribing is the culmination of extensive medical training (approximately 14,000 hours) and psychologists do not qualify.
- Board certification fees must be reduced, and recertification (Maintenance of Certification) must be simpler and less onerous.
- Effective parity laws must have teeth, not just words!
- Patient care, not computer care! Electronic health records must be more user-friendly and less time-consuming.
- Patients with psychiatric illness who have relapsed must be surrounded by compassionate medical professionals in a hospital setting, not by armed guards in a jail or prison.
- The shortage of psychiatrists can be remedied if the government funds additional residency slots as it did in the 1960s and 1970s. The number of applicants for psychiatric training is rapidly rising, but the number of residency slots has not changed for decades. Approximately 100 US medical school graduates did not match last year, along with >1,000 international medical graduate applicants.
- Lawyers have clients; psychiatrists have patients (as do cardiologists, neurologists, and oncologists). The term “clients” de-medicalizes psychiatric disorders and does not evoke public support or compassion.
- Psychotherapy is in fact a neurobiologic treatment that repairs the mind via neuroplasticity and synaptogenesis. It should get the same respect as pharmacotherapy.
- Untether psychiatric reimbursement from “time”! Psychiatric assessment and treatment are medical procedures. Excising depression, psychosis, panic attacks, or suicidal urges are to the mind what surgery is to the body.
- Clinical psychiatrists have much to offer for medical advances. Their observations generate hypotheses, and if these are published as a case report or letter to the editor, researchers can conduct hypothesis-testing and discover new treatments thanks to astute clinicians.
- The FDA should allow clinical trials to investigate treatments of symptoms, not (often heterogenous) DSM diagnoses. This will enable “off-label use” of medication, which often is necessary.
Continue to: Annual dues
Annual dues. The APA is a great organization that should continue to re-invent itself and re-engineer its procedures and business practices to generate additional revenue streams that could help reduce its annual dues. I know many members who complain about the APA dues, and former members who dropped out because of what they consider to be high dues.
Public education. The APA must intensify public education across all media platforms. This will help dispel myths, eliminate stigma, enforce parity, and portray psychiatry as a medical and scientific discipline. We have a great story to tell about how neurologic circuitry generates the mind and its mental functions, and the neurobiologic foundations of psychiatric brain disorders.
The APA should advocate for (and perhaps organize) an annual mental health check-up (online) in children, adolescents, adults, and the elderly for early detection and intervention.
Collaborative care. We should have close relationships with obstetricians to help prevent neurodevelopmental pathology due to perinatal complications as well as to manage depression in women in the pre- and postpartum phases. Collaborative care with pediatricians, family physicians, internists, and neurologists is necessary to integrate physical and mental health care for our patients, many of whom have multiple medical comorbidities and premature mortality.
Lobbying. The APA must intensify its lobbying to address the unacceptably high rate of suicide, addiction-related deaths, posttraumatic stress disorder due to trauma in children and adults, threats to mental health due to climate change and pollution, refugee mental health, stressful political zeitgeist, and the woefully high rate of uninsured or under-insured individuals.
Continue to: Industry
Industry. There are many significant unmet treatment needs in psychiatry. Approximately 82% of DSM disorders do not have any FDA-approved medication. The APA should constructively engage the pharmaceutical industry (the only entity that develops medications for our patients!) to do more research and development of therapies for conditions with no approved treatments, and to explore new mechanisms of action for more effective or tolerable psychiatric medications. Importantly, the APA should urge major pharmaceutical companies not to abandon neuropsychiatric disorders because they afflict tens of millions of US citizens and are the top causes of long-term disabilities.
Journals. The APA should consider rebranding its journals as “JAPA,” similar to JAMA, which will widen its influence and generate revenue to fund various priorities.
Telepsychiatry. And why can’t the APA create a national telepsychiatry network to meet the needs of underserved populations who have very little access to psychiatric care as in many rural areas? Private companies have filled that space, but the APA and its members can do it better, and this can become a benefit of membership.
Brain bank. Finally, the APA should consider establishing a “Brain Bank” of various psychiatric subspecialties to consult and advise the military, college administrators, corporations, and government agencies about strategies and tactics to solve many problems that arise from overt or covert psychiatric illnesses among their employees, staff, students, or constituents.
The APA cannot solve all societal problems, but it has the moral authority and clinical/scientific depth and gravitas to create an agenda of solutions and to partner with many other stakeholders to achieve mutual societal health goals.
Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation click here.
I have been informed by the American Psychiatric Association (APA) Nominating Committee that I am a candidate for the position of APA President-Elect. I am honored to be nominated along with 2 other esteemed psychiatrists, David C. Henderson, MD, and Vivian B. Pender, MD.
You have all known me for many years as Editor-in-Chief of this journal, and probably have read many of my 150 editorials in which I frequently discussed and commented on not only the challenges that face psychiatry, but also the great promise and bright future of our evolving clinical neuroscience medical specialty. You can access all of these at MDedge.com/psychiatry/editor.
In this pre-election editorial, I would like to tell you about my qualifications as a candidate for this critical national psychiatry leadership role. Most of you are APA members who will have the opportunity to vote for the candidate of your choice from January 2 to 31, 2020. I hope that you will support my candidacy after learning about my long-standing involvement within the APA governance, as well as my 3 decades of academic leadership experience and productivity. You also know where I stand on the issues from my writings in
APA involvement
- President, Missouri Psychiatric Physicians Association District Branch (2017-2018)
- President, Cincinnati Psychiatric Society (2007-2009)
- President, Ohio Psychiatric Physicians Foundation (2008-2013)
- Editor, Ohio Psychiatric Physicians Association (OPPA) Newsletter (Insight Matters) (2003-2008)
- Executive Council, OPPA (2003-2013)
- APA Council on Research (1993-2000)
- APA Committee on Research in Psychiatric Treatments (1992-1995)
- APA Task Force on Schizophrenia (1998-1999)
- President, Ohio Psychiatric Association Education and Research Foundation (1987-1994)
Academic track record
- Served as Chief of Psychiatry, VA Medical Center, Iowa City, Iowa for 6 years; Chair, Department of Psychiatry, The Ohio State University for 12 years; Chair, Department of Psychiatry, Saint Louis University for 6 years; and Associate Dean, University of Cincinnati for 4 years
- Published >700 articles, 570 abstracts, and 14 books
- Recruited and developed dozens of faculty members; supervised and mentored hundreds of residents, many of whom became medical directors, department chairs, and/or distinguished clinicians
- Received numerous awards and recognitions for clinical, teaching, and research excellence
- Serve as Editor for 3 journals (Current Psychiatry, Schizophrenia Research, and Biomarkers in Neuropsychiatry)
Statement of vision and priorities
I am very optimistic about the future of psychiatry. The breakthroughs and advances in neuroscience all bolster the scientific basis of psychiatric disorders, and will lead to many novel treatments in the future. Psychiatry is a medical specialty that is now much more integrated into the “big tent” of medicine. Psychiatrists are physicians, and I believe the name of our association must reflect that. I was successful in changing the names of 2 district branches to include “physicians” (Ohio Psychiatric Physicians Association and Missouri Psychiatric Physicians Association). If elected, I will propose to the Board of Trustees and the APA members that we change our name to the American Psychiatric Physicians Association, which will emphasize our medical identity within mental health. In its 175-year history, the APA has experienced 2 previous name changes.
I believe the strengths of the APA far exceed its weaknesses, and its opportunities outnumber its threats. However, the following perennial challenges must be forcefully addressed by all of us:
- The pernicious and discriminatory dogma of stigma must be shattered for the sake of patients, their families, their psychiatrists, and the profession.
- Pre-authorization is essentially the insurance companies practicing medicine without a license when, without ever actually examining the patient, they tell physicians what they should or should not prescribe. That’s felonious!
- Competent and safe prescribing is the culmination of extensive medical training (approximately 14,000 hours) and psychologists do not qualify.
- Board certification fees must be reduced, and recertification (Maintenance of Certification) must be simpler and less onerous.
- Effective parity laws must have teeth, not just words!
- Patient care, not computer care! Electronic health records must be more user-friendly and less time-consuming.
- Patients with psychiatric illness who have relapsed must be surrounded by compassionate medical professionals in a hospital setting, not by armed guards in a jail or prison.
- The shortage of psychiatrists can be remedied if the government funds additional residency slots as it did in the 1960s and 1970s. The number of applicants for psychiatric training is rapidly rising, but the number of residency slots has not changed for decades. Approximately 100 US medical school graduates did not match last year, along with >1,000 international medical graduate applicants.
- Lawyers have clients; psychiatrists have patients (as do cardiologists, neurologists, and oncologists). The term “clients” de-medicalizes psychiatric disorders and does not evoke public support or compassion.
- Psychotherapy is in fact a neurobiologic treatment that repairs the mind via neuroplasticity and synaptogenesis. It should get the same respect as pharmacotherapy.
- Untether psychiatric reimbursement from “time”! Psychiatric assessment and treatment are medical procedures. Excising depression, psychosis, panic attacks, or suicidal urges are to the mind what surgery is to the body.
- Clinical psychiatrists have much to offer for medical advances. Their observations generate hypotheses, and if these are published as a case report or letter to the editor, researchers can conduct hypothesis-testing and discover new treatments thanks to astute clinicians.
- The FDA should allow clinical trials to investigate treatments of symptoms, not (often heterogenous) DSM diagnoses. This will enable “off-label use” of medication, which often is necessary.
Continue to: Annual dues
Annual dues. The APA is a great organization that should continue to re-invent itself and re-engineer its procedures and business practices to generate additional revenue streams that could help reduce its annual dues. I know many members who complain about the APA dues, and former members who dropped out because of what they consider to be high dues.
Public education. The APA must intensify public education across all media platforms. This will help dispel myths, eliminate stigma, enforce parity, and portray psychiatry as a medical and scientific discipline. We have a great story to tell about how neurologic circuitry generates the mind and its mental functions, and the neurobiologic foundations of psychiatric brain disorders.
The APA should advocate for (and perhaps organize) an annual mental health check-up (online) in children, adolescents, adults, and the elderly for early detection and intervention.
Collaborative care. We should have close relationships with obstetricians to help prevent neurodevelopmental pathology due to perinatal complications as well as to manage depression in women in the pre- and postpartum phases. Collaborative care with pediatricians, family physicians, internists, and neurologists is necessary to integrate physical and mental health care for our patients, many of whom have multiple medical comorbidities and premature mortality.
Lobbying. The APA must intensify its lobbying to address the unacceptably high rate of suicide, addiction-related deaths, posttraumatic stress disorder due to trauma in children and adults, threats to mental health due to climate change and pollution, refugee mental health, stressful political zeitgeist, and the woefully high rate of uninsured or under-insured individuals.
Continue to: Industry
Industry. There are many significant unmet treatment needs in psychiatry. Approximately 82% of DSM disorders do not have any FDA-approved medication. The APA should constructively engage the pharmaceutical industry (the only entity that develops medications for our patients!) to do more research and development of therapies for conditions with no approved treatments, and to explore new mechanisms of action for more effective or tolerable psychiatric medications. Importantly, the APA should urge major pharmaceutical companies not to abandon neuropsychiatric disorders because they afflict tens of millions of US citizens and are the top causes of long-term disabilities.
Journals. The APA should consider rebranding its journals as “JAPA,” similar to JAMA, which will widen its influence and generate revenue to fund various priorities.
Telepsychiatry. And why can’t the APA create a national telepsychiatry network to meet the needs of underserved populations who have very little access to psychiatric care as in many rural areas? Private companies have filled that space, but the APA and its members can do it better, and this can become a benefit of membership.
Brain bank. Finally, the APA should consider establishing a “Brain Bank” of various psychiatric subspecialties to consult and advise the military, college administrators, corporations, and government agencies about strategies and tactics to solve many problems that arise from overt or covert psychiatric illnesses among their employees, staff, students, or constituents.
The APA cannot solve all societal problems, but it has the moral authority and clinical/scientific depth and gravitas to create an agenda of solutions and to partner with many other stakeholders to achieve mutual societal health goals.
Feigning alcohol withdrawal symptoms can render the CIWA-Ar scale useless
The Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) scale is a well-established protocol that attempts to measure the degree of alcohol and benzodiazepine withdrawal. The CIWA-Ar scale measures 10 domains and indexes the severity of withdrawal on a scale from 0 to 67; scores >8 are generally considered to be indicative of at least mild-to-moderate withdrawal, and scores >20 represent significant withdrawal.1 Despite its common use in many medical settings, the CIWA-Ar scale has been impugned as a less-than-reliable index of true alcohol withdrawal2 and has the potential for misuse among ordering physicians.3 In this case report, I describe a malingering patient who intentionally and successfully feigned symptoms of alcohol withdrawal, which demonstrates that the purposeful reproduction of symptoms measured by the CIWA-Ar scale can render the protocol clinically useless.
CASE REPORT
Mr. G, a 63-year-old African-American man, was admitted to the general medical floor with a chief complaint of alcohol withdrawal. He had a history of alcohol use disorder, severe, and unspecified depression. He said he had been drinking a gallon of wine plus “a fifth” of vodka every day for the past 1.5 months. More than 1 year ago, he had been admitted for alcohol withdrawal with subsequent delirium tremens, but he denied having any other psychiatric history.
In the emergency department, Mr. G was given IV lorazepam, 6 mg total, for alcohol withdrawal. He was reported to be “scoring” on the CIWA-Ar scale with apparently uncontrollable tremulousness, visual hallucinations, and confusion. His vitals were within normal limits, his mean corpuscular volume and lipase level were within normal limits, and the rest of his presentation was largely unremarkable.
Once admitted to the general medical floor, he continued to receive benzodiazepines for what was documented as severe alcohol withdrawal. When clinical staff were not in the room, the patient was observed to be resting comfortably without tremulousness. When the patient was seen by the psychiatry consultation service, he produced full body tremulousness with marked shoulder and hip thrusting. His account of how much he had been drinking contradicted the amount he reported to other teams in the hospital. When the consulting psychiatrist appeared unimpressed by his full body jerking, the patient abruptly pointed to the corner of the room and yelled “What is that?” when nothing was there. When the primary medical team suggested to the patient that his vitals were within normal limits and he did not appear to be in true alcohol withdrawal, the patient escalated the degree of his full body jerking.
Over the next few days, the patient routinely would tell clinical staff “I’m having DTs.” He also specifically requested lorazepam. After consultation, the medical and psychiatry teams determined the patient was feigning symptoms of alcohol withdrawal. The lorazepam was discontinued, and the patient was discharged home with outpatient psychiatric follow-up.
Limitations of the CIWA-Ar scale
The CIWA-Ar scale is intended to guide the need for medications, such as benzodiazepines, to help mitigate symptoms of alcohol withdrawal. Symptom-triggered benzodiazepine treatment has been shown to be superior to fixed-schedule dosing.4 However, symptom-triggered treatment is problematic in the setting of feigned symptoms.
When psychiatrists and nurses calculate a CIWA-Ar score, they rely on both subjective accounts of a patient’s withdrawal severity as well as objective signs, such as vitals and a physical examination. Many of the elements included in the CIWA-Ar scale can be easily feigned (Table). Feigned alcohol withdrawal may fall into 2 categories: (1) the false reporting of subjective symptoms, and (2) the false portrayal of objective signs.
Continue to: The false reporting...
The false reporting of subjective symptoms can include the reported presence of nausea or vomiting, anxiety, tactile hallucinations, auditory hallucinations, headache or head fullness, and visual hallucinations. The false portrayal of objective signs can include the feigning of tremulousness, agitation, and confusion (eg, incorrectly answering orienting questions). In both categories, the simple presence of these signs or symptoms, whether falsely reported or falsely portrayed, would cause the patient to “score” on the CIWA-Ar scale.
Thus, the need to effectively rule out feigned symptoms is essential because inappropriate dosing of benzodiazepines can be dangerous, costly, and utilize limited hospital resources that could otherwise be diverted to a patient with a true medical or psychiatric illness. In these instances, it is crucial to pay close attention to vital signs because these are more reliable indices of withdrawal. A patient’s ability to purposefully feign symptoms of alcohol withdrawal highlights the limitations of the CIWA-Ar scale as a validated measure of alcohol withdrawal, and renders it effectively useless in the setting of either malingering or factitious disorder.
Resnick5 describes malingering as either pure malingering, partial malingering, or false imputation. Pure malingering refers to the feigning of a nonexistent disorder or illness. Partial malingering refers to the exaggeration of symptoms that are present, but to a lesser degree. False imputation refers to the attribution of symptoms from a separate disorder to one the patient knows is unrelated (eg, attributing chronic low back pain from a prior sports injury to a recent motor vehicle accident). In Mr. G’s case, he had multiple prior admissions for true, non-feigned alcohol withdrawal with subsequent delirium tremens. His knowledge of the signs and symptoms of alcohol withdrawal therefore helped him make calculated efforts to manipulate clinical staff in his quest to obtain benzodiazepines. Whether this was pure or partial malingering remained unclear because Mr. G’s true level of withdrawal could not be adequately assessed.
Potentially serious consequences
The CIWA-Ar scale is among the most widely used scales to determine the level of alcohol withdrawal and need for subsequent benzodiazepine treatment. However, its effective use is limited because it relies on subjective symptoms and objective signs that can be easily feigned or manipulated. In the setting of malingering or factitious disorder, when a patient is feigning symptoms of alcohol withdrawal, the CIWA-Ar scale may be rendered clinically useless. This can lead to dangerous iatrogenic adverse effects, lengthy and nontherapeutic hospital stays, and an increasing financial burden on health care systems.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knight E, Lappalainen L. Clinical Institute Withdrawal Assessment for Alcohol–Revised might be an unreliable tool in the management of alcohol withdrawal. Can Fam Physician. 2017;63(9):691-695.
3. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
4. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121.
5. Resnick PJ. The detection of malingered mental illness. Behav Sci Law. 1984;2(1):20-38.
The Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) scale is a well-established protocol that attempts to measure the degree of alcohol and benzodiazepine withdrawal. The CIWA-Ar scale measures 10 domains and indexes the severity of withdrawal on a scale from 0 to 67; scores >8 are generally considered to be indicative of at least mild-to-moderate withdrawal, and scores >20 represent significant withdrawal.1 Despite its common use in many medical settings, the CIWA-Ar scale has been impugned as a less-than-reliable index of true alcohol withdrawal2 and has the potential for misuse among ordering physicians.3 In this case report, I describe a malingering patient who intentionally and successfully feigned symptoms of alcohol withdrawal, which demonstrates that the purposeful reproduction of symptoms measured by the CIWA-Ar scale can render the protocol clinically useless.
CASE REPORT
Mr. G, a 63-year-old African-American man, was admitted to the general medical floor with a chief complaint of alcohol withdrawal. He had a history of alcohol use disorder, severe, and unspecified depression. He said he had been drinking a gallon of wine plus “a fifth” of vodka every day for the past 1.5 months. More than 1 year ago, he had been admitted for alcohol withdrawal with subsequent delirium tremens, but he denied having any other psychiatric history.
In the emergency department, Mr. G was given IV lorazepam, 6 mg total, for alcohol withdrawal. He was reported to be “scoring” on the CIWA-Ar scale with apparently uncontrollable tremulousness, visual hallucinations, and confusion. His vitals were within normal limits, his mean corpuscular volume and lipase level were within normal limits, and the rest of his presentation was largely unremarkable.
Once admitted to the general medical floor, he continued to receive benzodiazepines for what was documented as severe alcohol withdrawal. When clinical staff were not in the room, the patient was observed to be resting comfortably without tremulousness. When the patient was seen by the psychiatry consultation service, he produced full body tremulousness with marked shoulder and hip thrusting. His account of how much he had been drinking contradicted the amount he reported to other teams in the hospital. When the consulting psychiatrist appeared unimpressed by his full body jerking, the patient abruptly pointed to the corner of the room and yelled “What is that?” when nothing was there. When the primary medical team suggested to the patient that his vitals were within normal limits and he did not appear to be in true alcohol withdrawal, the patient escalated the degree of his full body jerking.
Over the next few days, the patient routinely would tell clinical staff “I’m having DTs.” He also specifically requested lorazepam. After consultation, the medical and psychiatry teams determined the patient was feigning symptoms of alcohol withdrawal. The lorazepam was discontinued, and the patient was discharged home with outpatient psychiatric follow-up.
Limitations of the CIWA-Ar scale
The CIWA-Ar scale is intended to guide the need for medications, such as benzodiazepines, to help mitigate symptoms of alcohol withdrawal. Symptom-triggered benzodiazepine treatment has been shown to be superior to fixed-schedule dosing.4 However, symptom-triggered treatment is problematic in the setting of feigned symptoms.
When psychiatrists and nurses calculate a CIWA-Ar score, they rely on both subjective accounts of a patient’s withdrawal severity as well as objective signs, such as vitals and a physical examination. Many of the elements included in the CIWA-Ar scale can be easily feigned (Table). Feigned alcohol withdrawal may fall into 2 categories: (1) the false reporting of subjective symptoms, and (2) the false portrayal of objective signs.
Continue to: The false reporting...
The false reporting of subjective symptoms can include the reported presence of nausea or vomiting, anxiety, tactile hallucinations, auditory hallucinations, headache or head fullness, and visual hallucinations. The false portrayal of objective signs can include the feigning of tremulousness, agitation, and confusion (eg, incorrectly answering orienting questions). In both categories, the simple presence of these signs or symptoms, whether falsely reported or falsely portrayed, would cause the patient to “score” on the CIWA-Ar scale.
Thus, the need to effectively rule out feigned symptoms is essential because inappropriate dosing of benzodiazepines can be dangerous, costly, and utilize limited hospital resources that could otherwise be diverted to a patient with a true medical or psychiatric illness. In these instances, it is crucial to pay close attention to vital signs because these are more reliable indices of withdrawal. A patient’s ability to purposefully feign symptoms of alcohol withdrawal highlights the limitations of the CIWA-Ar scale as a validated measure of alcohol withdrawal, and renders it effectively useless in the setting of either malingering or factitious disorder.
Resnick5 describes malingering as either pure malingering, partial malingering, or false imputation. Pure malingering refers to the feigning of a nonexistent disorder or illness. Partial malingering refers to the exaggeration of symptoms that are present, but to a lesser degree. False imputation refers to the attribution of symptoms from a separate disorder to one the patient knows is unrelated (eg, attributing chronic low back pain from a prior sports injury to a recent motor vehicle accident). In Mr. G’s case, he had multiple prior admissions for true, non-feigned alcohol withdrawal with subsequent delirium tremens. His knowledge of the signs and symptoms of alcohol withdrawal therefore helped him make calculated efforts to manipulate clinical staff in his quest to obtain benzodiazepines. Whether this was pure or partial malingering remained unclear because Mr. G’s true level of withdrawal could not be adequately assessed.
Potentially serious consequences
The CIWA-Ar scale is among the most widely used scales to determine the level of alcohol withdrawal and need for subsequent benzodiazepine treatment. However, its effective use is limited because it relies on subjective symptoms and objective signs that can be easily feigned or manipulated. In the setting of malingering or factitious disorder, when a patient is feigning symptoms of alcohol withdrawal, the CIWA-Ar scale may be rendered clinically useless. This can lead to dangerous iatrogenic adverse effects, lengthy and nontherapeutic hospital stays, and an increasing financial burden on health care systems.
The Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) scale is a well-established protocol that attempts to measure the degree of alcohol and benzodiazepine withdrawal. The CIWA-Ar scale measures 10 domains and indexes the severity of withdrawal on a scale from 0 to 67; scores >8 are generally considered to be indicative of at least mild-to-moderate withdrawal, and scores >20 represent significant withdrawal.1 Despite its common use in many medical settings, the CIWA-Ar scale has been impugned as a less-than-reliable index of true alcohol withdrawal2 and has the potential for misuse among ordering physicians.3 In this case report, I describe a malingering patient who intentionally and successfully feigned symptoms of alcohol withdrawal, which demonstrates that the purposeful reproduction of symptoms measured by the CIWA-Ar scale can render the protocol clinically useless.
CASE REPORT
Mr. G, a 63-year-old African-American man, was admitted to the general medical floor with a chief complaint of alcohol withdrawal. He had a history of alcohol use disorder, severe, and unspecified depression. He said he had been drinking a gallon of wine plus “a fifth” of vodka every day for the past 1.5 months. More than 1 year ago, he had been admitted for alcohol withdrawal with subsequent delirium tremens, but he denied having any other psychiatric history.
In the emergency department, Mr. G was given IV lorazepam, 6 mg total, for alcohol withdrawal. He was reported to be “scoring” on the CIWA-Ar scale with apparently uncontrollable tremulousness, visual hallucinations, and confusion. His vitals were within normal limits, his mean corpuscular volume and lipase level were within normal limits, and the rest of his presentation was largely unremarkable.
Once admitted to the general medical floor, he continued to receive benzodiazepines for what was documented as severe alcohol withdrawal. When clinical staff were not in the room, the patient was observed to be resting comfortably without tremulousness. When the patient was seen by the psychiatry consultation service, he produced full body tremulousness with marked shoulder and hip thrusting. His account of how much he had been drinking contradicted the amount he reported to other teams in the hospital. When the consulting psychiatrist appeared unimpressed by his full body jerking, the patient abruptly pointed to the corner of the room and yelled “What is that?” when nothing was there. When the primary medical team suggested to the patient that his vitals were within normal limits and he did not appear to be in true alcohol withdrawal, the patient escalated the degree of his full body jerking.
Over the next few days, the patient routinely would tell clinical staff “I’m having DTs.” He also specifically requested lorazepam. After consultation, the medical and psychiatry teams determined the patient was feigning symptoms of alcohol withdrawal. The lorazepam was discontinued, and the patient was discharged home with outpatient psychiatric follow-up.
Limitations of the CIWA-Ar scale
The CIWA-Ar scale is intended to guide the need for medications, such as benzodiazepines, to help mitigate symptoms of alcohol withdrawal. Symptom-triggered benzodiazepine treatment has been shown to be superior to fixed-schedule dosing.4 However, symptom-triggered treatment is problematic in the setting of feigned symptoms.
When psychiatrists and nurses calculate a CIWA-Ar score, they rely on both subjective accounts of a patient’s withdrawal severity as well as objective signs, such as vitals and a physical examination. Many of the elements included in the CIWA-Ar scale can be easily feigned (Table). Feigned alcohol withdrawal may fall into 2 categories: (1) the false reporting of subjective symptoms, and (2) the false portrayal of objective signs.
Continue to: The false reporting...
The false reporting of subjective symptoms can include the reported presence of nausea or vomiting, anxiety, tactile hallucinations, auditory hallucinations, headache or head fullness, and visual hallucinations. The false portrayal of objective signs can include the feigning of tremulousness, agitation, and confusion (eg, incorrectly answering orienting questions). In both categories, the simple presence of these signs or symptoms, whether falsely reported or falsely portrayed, would cause the patient to “score” on the CIWA-Ar scale.
Thus, the need to effectively rule out feigned symptoms is essential because inappropriate dosing of benzodiazepines can be dangerous, costly, and utilize limited hospital resources that could otherwise be diverted to a patient with a true medical or psychiatric illness. In these instances, it is crucial to pay close attention to vital signs because these are more reliable indices of withdrawal. A patient’s ability to purposefully feign symptoms of alcohol withdrawal highlights the limitations of the CIWA-Ar scale as a validated measure of alcohol withdrawal, and renders it effectively useless in the setting of either malingering or factitious disorder.
Resnick5 describes malingering as either pure malingering, partial malingering, or false imputation. Pure malingering refers to the feigning of a nonexistent disorder or illness. Partial malingering refers to the exaggeration of symptoms that are present, but to a lesser degree. False imputation refers to the attribution of symptoms from a separate disorder to one the patient knows is unrelated (eg, attributing chronic low back pain from a prior sports injury to a recent motor vehicle accident). In Mr. G’s case, he had multiple prior admissions for true, non-feigned alcohol withdrawal with subsequent delirium tremens. His knowledge of the signs and symptoms of alcohol withdrawal therefore helped him make calculated efforts to manipulate clinical staff in his quest to obtain benzodiazepines. Whether this was pure or partial malingering remained unclear because Mr. G’s true level of withdrawal could not be adequately assessed.
Potentially serious consequences
The CIWA-Ar scale is among the most widely used scales to determine the level of alcohol withdrawal and need for subsequent benzodiazepine treatment. However, its effective use is limited because it relies on subjective symptoms and objective signs that can be easily feigned or manipulated. In the setting of malingering or factitious disorder, when a patient is feigning symptoms of alcohol withdrawal, the CIWA-Ar scale may be rendered clinically useless. This can lead to dangerous iatrogenic adverse effects, lengthy and nontherapeutic hospital stays, and an increasing financial burden on health care systems.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knight E, Lappalainen L. Clinical Institute Withdrawal Assessment for Alcohol–Revised might be an unreliable tool in the management of alcohol withdrawal. Can Fam Physician. 2017;63(9):691-695.
3. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
4. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121.
5. Resnick PJ. The detection of malingered mental illness. Behav Sci Law. 1984;2(1):20-38.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knight E, Lappalainen L. Clinical Institute Withdrawal Assessment for Alcohol–Revised might be an unreliable tool in the management of alcohol withdrawal. Can Fam Physician. 2017;63(9):691-695.
3. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
4. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121.
5. Resnick PJ. The detection of malingered mental illness. Behav Sci Law. 1984;2(1):20-38.
Black-box warnings: How they can improve your clinical practice
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).
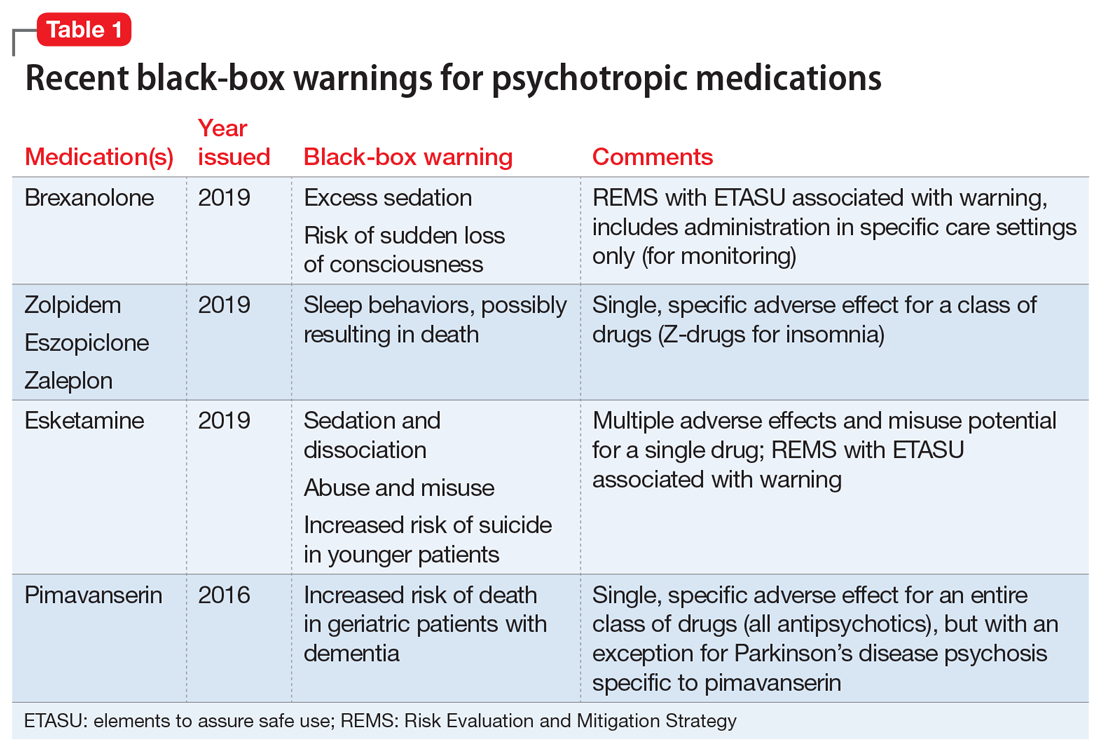
In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5
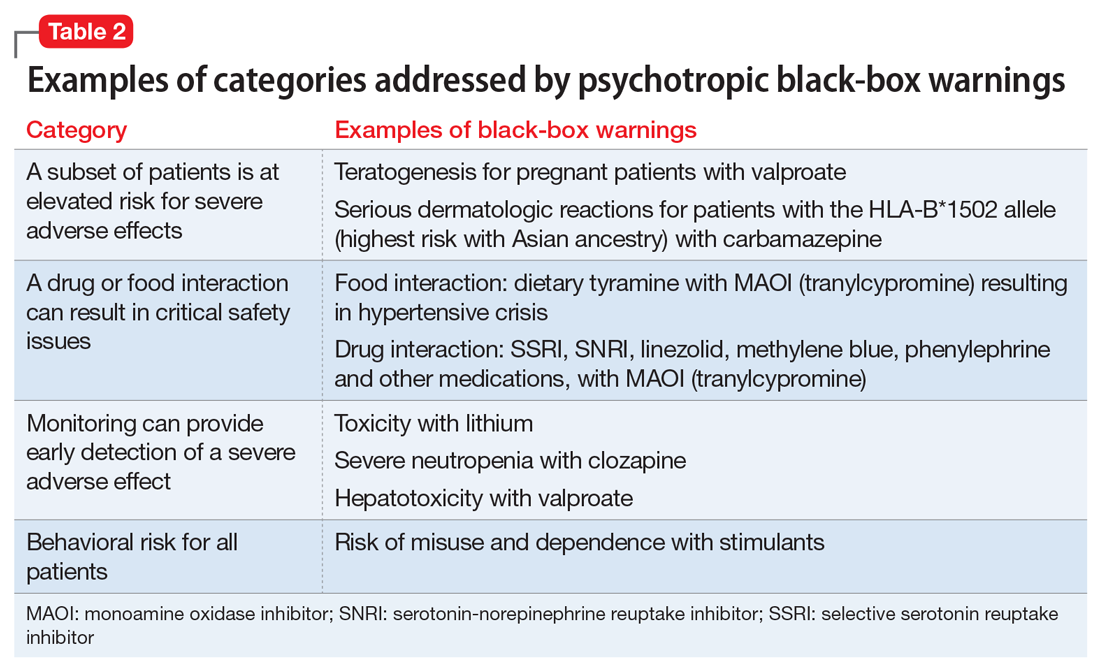
Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
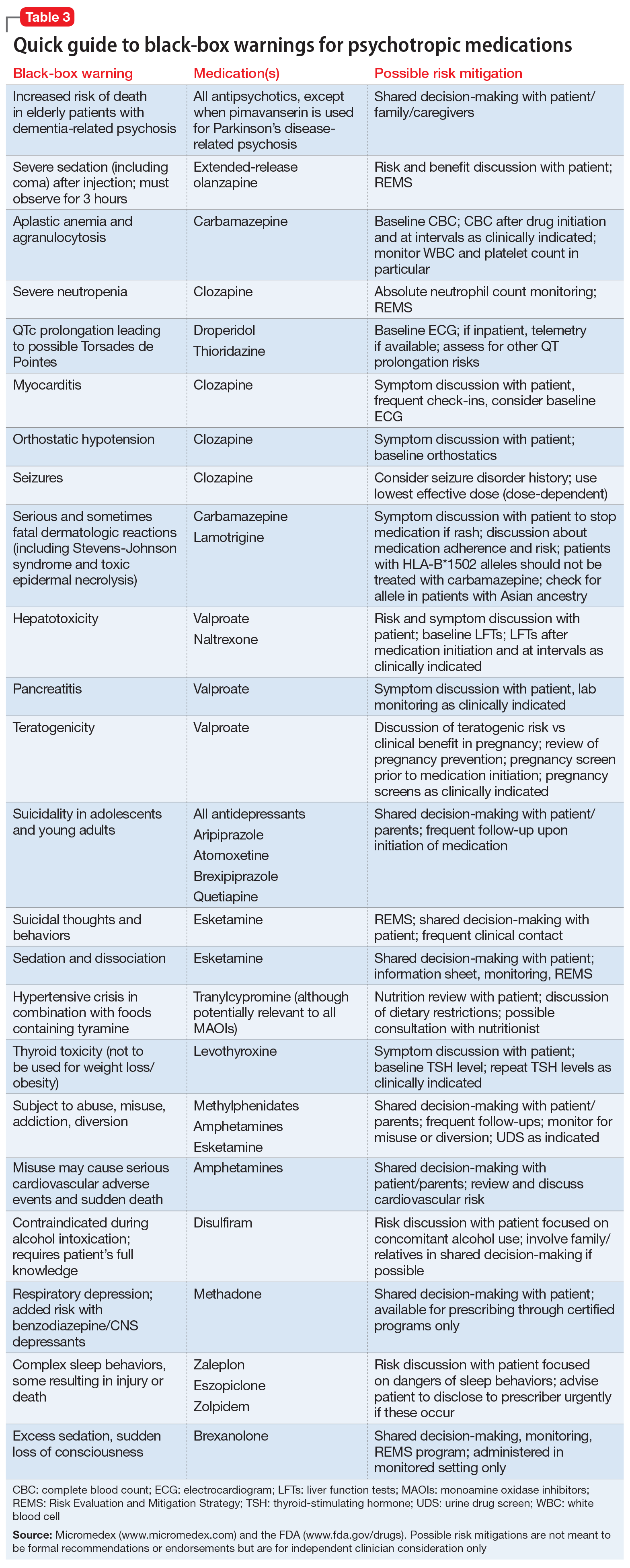
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).

In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5

Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.

The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).

In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5

Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.

The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
The psychiatrist’s role in liver transplantation
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2
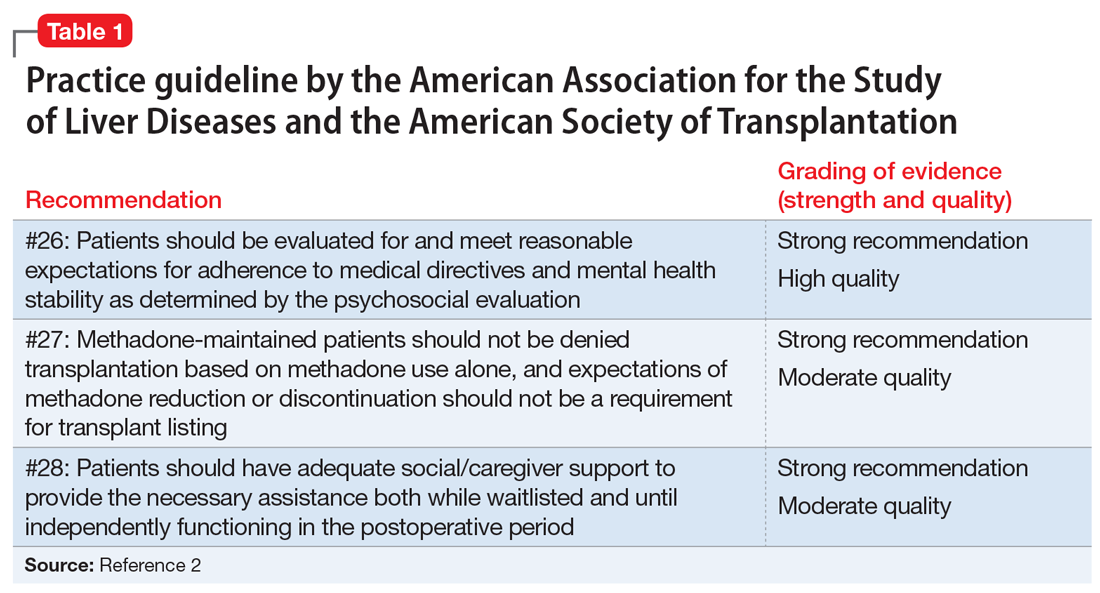
Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9
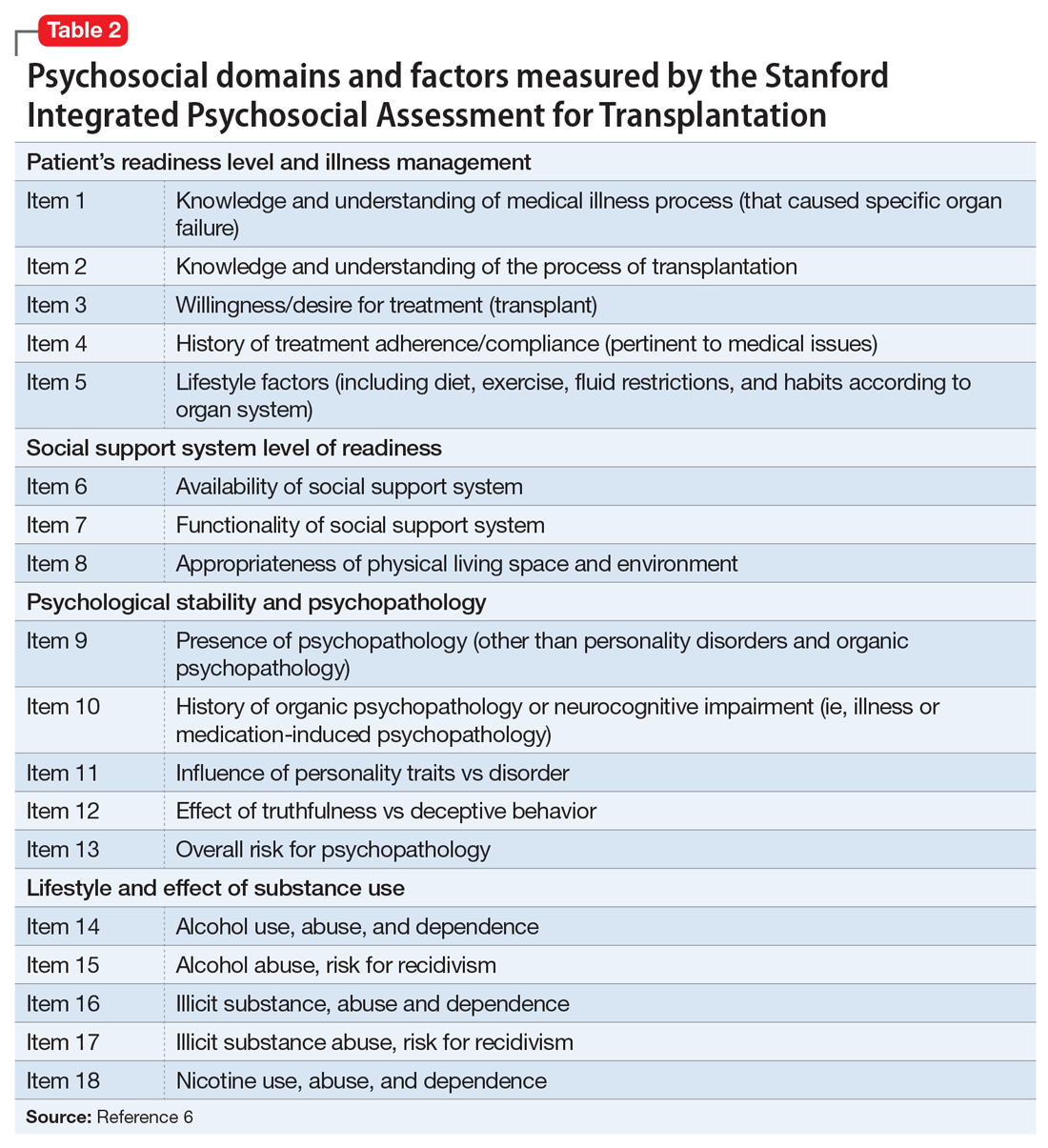
A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2

Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9

A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2

Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9

A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
2019 Peer Reviewers
Aparna Atluru, MD
Stanford Medicine
Anjan Bhattacharyya, MD
Saint Louis University
Caroline Bonham, MD
The University of New Mexico
Catherine Crone, MD
Inova Health System
Sheila Dowd, PhD
Rush Medical College
Ahmed Z. Elmaadawi, MD
Indiana University School of Medicine
Donald Gilbert, MD, MS
Cincinnati Children’s Hospital Medical Center
Mark Gold, MD
Washington University in St. Louis
Elana Harris, MD, PhD
Cincinnati Children’s Hospital
Susan Hatters-Friedman, MD
Case Western Reserve University
Faisal Islam, MD, MBA
Greenvale, New York
Kaustubh G. Joshi, MD
University of South Carolina School of Medicine
Rita Khoury, MD
Saint George Hospital University Medical Center
Suneeta Kumari, MD, MPH
Howard University Hospital
Michelle Magid, MD
Austin PsychCare PA
Michael Maksimowski, MD
Wayne State University
Jose Maldonado, MD
Stanford University
Thomas W. Meeks, MD
Portland VA Medical Center
John Miller, MD
University of South Florida
Armando Morera-Fumero, MD, PhD
Universidad de La Laguna
Mary K. Morreale, MD
Wayne State University
Philip Muskin, MD
Columbia University College of Physicians and Surgeons
Katharine Nelson, MD
University of Minnesota
Carol North, MD
University of Texas Southwestern Medical Center at Dallas
Douglas Opler, MD
Rutgers University School of Medicine
Joseph Pierre, MD
University of California, Los Angeles
Jerrold Pollak, PhD, ABN, ABPP
Seacoast Mental Health Center
Edwin Raffi, MD, MPH
Massachusetts General Hospital Center for Women’s Mental Health
Y. Pritham Raj, MD
Oregon Health and Science University
Jeffrey Rakofsky, MD
Emory University School of Medicine
Laura Ramsey, PhD
Cincinnati Children’s Hospital Medical Center
Erica Rapp, MD
University of Colorado School of Medicine
Abhishek Reddy, MD
The University of Alabama at Birmingham
Eduardo Rueda Vasquez, MD
Williamsport, Pennsylvania
Stephen Saklad, PharmD, BCPP
The University of Texas at Austin
Lauren Schwarz, PhD, ABPP-CN
Saint Louis University School of Medicine
Andreas Sidiropoulos, MD
University of Michigan
Shirshendu Sinha, MD
Mayo Clinic Health System and Mayo Clinic
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Jeffrey Sung, MD
University of Washington
Thida Thant, MD
University of Colorado at Denver
Adele Viguera, MD
Cleveland Clinic Lerner College of Medicine
Aparna Atluru, MD
Stanford Medicine
Anjan Bhattacharyya, MD
Saint Louis University
Caroline Bonham, MD
The University of New Mexico
Catherine Crone, MD
Inova Health System
Sheila Dowd, PhD
Rush Medical College
Ahmed Z. Elmaadawi, MD
Indiana University School of Medicine
Donald Gilbert, MD, MS
Cincinnati Children’s Hospital Medical Center
Mark Gold, MD
Washington University in St. Louis
Elana Harris, MD, PhD
Cincinnati Children’s Hospital
Susan Hatters-Friedman, MD
Case Western Reserve University
Faisal Islam, MD, MBA
Greenvale, New York
Kaustubh G. Joshi, MD
University of South Carolina School of Medicine
Rita Khoury, MD
Saint George Hospital University Medical Center
Suneeta Kumari, MD, MPH
Howard University Hospital
Michelle Magid, MD
Austin PsychCare PA
Michael Maksimowski, MD
Wayne State University
Jose Maldonado, MD
Stanford University
Thomas W. Meeks, MD
Portland VA Medical Center
John Miller, MD
University of South Florida
Armando Morera-Fumero, MD, PhD
Universidad de La Laguna
Mary K. Morreale, MD
Wayne State University
Philip Muskin, MD
Columbia University College of Physicians and Surgeons
Katharine Nelson, MD
University of Minnesota
Carol North, MD
University of Texas Southwestern Medical Center at Dallas
Douglas Opler, MD
Rutgers University School of Medicine
Joseph Pierre, MD
University of California, Los Angeles
Jerrold Pollak, PhD, ABN, ABPP
Seacoast Mental Health Center
Edwin Raffi, MD, MPH
Massachusetts General Hospital Center for Women’s Mental Health
Y. Pritham Raj, MD
Oregon Health and Science University
Jeffrey Rakofsky, MD
Emory University School of Medicine
Laura Ramsey, PhD
Cincinnati Children’s Hospital Medical Center
Erica Rapp, MD
University of Colorado School of Medicine
Abhishek Reddy, MD
The University of Alabama at Birmingham
Eduardo Rueda Vasquez, MD
Williamsport, Pennsylvania
Stephen Saklad, PharmD, BCPP
The University of Texas at Austin
Lauren Schwarz, PhD, ABPP-CN
Saint Louis University School of Medicine
Andreas Sidiropoulos, MD
University of Michigan
Shirshendu Sinha, MD
Mayo Clinic Health System and Mayo Clinic
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Jeffrey Sung, MD
University of Washington
Thida Thant, MD
University of Colorado at Denver
Adele Viguera, MD
Cleveland Clinic Lerner College of Medicine
Aparna Atluru, MD
Stanford Medicine
Anjan Bhattacharyya, MD
Saint Louis University
Caroline Bonham, MD
The University of New Mexico
Catherine Crone, MD
Inova Health System
Sheila Dowd, PhD
Rush Medical College
Ahmed Z. Elmaadawi, MD
Indiana University School of Medicine
Donald Gilbert, MD, MS
Cincinnati Children’s Hospital Medical Center
Mark Gold, MD
Washington University in St. Louis
Elana Harris, MD, PhD
Cincinnati Children’s Hospital
Susan Hatters-Friedman, MD
Case Western Reserve University
Faisal Islam, MD, MBA
Greenvale, New York
Kaustubh G. Joshi, MD
University of South Carolina School of Medicine
Rita Khoury, MD
Saint George Hospital University Medical Center
Suneeta Kumari, MD, MPH
Howard University Hospital
Michelle Magid, MD
Austin PsychCare PA
Michael Maksimowski, MD
Wayne State University
Jose Maldonado, MD
Stanford University
Thomas W. Meeks, MD
Portland VA Medical Center
John Miller, MD
University of South Florida
Armando Morera-Fumero, MD, PhD
Universidad de La Laguna
Mary K. Morreale, MD
Wayne State University
Philip Muskin, MD
Columbia University College of Physicians and Surgeons
Katharine Nelson, MD
University of Minnesota
Carol North, MD
University of Texas Southwestern Medical Center at Dallas
Douglas Opler, MD
Rutgers University School of Medicine
Joseph Pierre, MD
University of California, Los Angeles
Jerrold Pollak, PhD, ABN, ABPP
Seacoast Mental Health Center
Edwin Raffi, MD, MPH
Massachusetts General Hospital Center for Women’s Mental Health
Y. Pritham Raj, MD
Oregon Health and Science University
Jeffrey Rakofsky, MD
Emory University School of Medicine
Laura Ramsey, PhD
Cincinnati Children’s Hospital Medical Center
Erica Rapp, MD
University of Colorado School of Medicine
Abhishek Reddy, MD
The University of Alabama at Birmingham
Eduardo Rueda Vasquez, MD
Williamsport, Pennsylvania
Stephen Saklad, PharmD, BCPP
The University of Texas at Austin
Lauren Schwarz, PhD, ABPP-CN
Saint Louis University School of Medicine
Andreas Sidiropoulos, MD
University of Michigan
Shirshendu Sinha, MD
Mayo Clinic Health System and Mayo Clinic
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Jeffrey Sung, MD
University of Washington
Thida Thant, MD
University of Colorado at Denver
Adele Viguera, MD
Cleveland Clinic Lerner College of Medicine
Resignation
Along with resigning as chairman of the department of hematology and medical oncology at the Cleveland Clinic (Reunion), I am also resigning as editor in chief of Hematology News. In contrast to the drawn out process of choosing the next department chairman, however, I was in the enviable position of being able to hand pick my successor as editor in chief. I am proud to announce that Ifeyinwa (Ify) Osunkwo, MD, MPH, will be the new editor in chief of Hematology News. Dr. Osunkwo’s new perspective and energy will guide the further development of Hematology News for the benefit of our readers.
As editor in chief, I have had the opportunity to write essays for Hematology News that reflect my experience as a leader in an academic medical department. By doing so, I was trying to summarize some of what I learned along my career path. In my final essay, I want to direct some of these nuggets of wisdom directly to aspiring leaders who are closer to the beginning of their career journey than I am.
My junior colleagues are very interested in developing their careers to maximize opportunities in leadership, and I have coached many to try to understand that the path to leadership is not always straight, may be difficult, and does not always end comfortably. While the goal may seem to be in one direction, the path may lead to another. That is what has happened to me.
I did not seek to be Chairman. The opportunity came to me while I was busy doing other things. As I expressed in an earlier editorial (Seeking the chair), those who are diligent about their work without actively trying to rise through the leadership hierarchy are the ones who seem to rise more often.
Ambition is overrated. The ambitious find it harder to accept failure, and some degree of failure is likely. In his book “Falling Upward: A Spirituality for the Two Halves of Life,” Father Richard Rohr suggests that failure is required in order to mature from someone whose life centers on self to someone whose self centers on life.
Junior faculty tend to focus on self. They try to excel at whatever they attempt as they always have. Whether that is teaching, performing research, or treating patients, they try to be the absolute best teacher, researcher, or practitioner they can be. Many try to do all three well. Rare are those who can perfectly balance all three endeavors. Tension results, both at work and at home. Here is where failure often happens. The student disappoints, the paper is rejected, the grant isn’t funded, the patient relapses, and the family wishes you were home more. This confluence of difficulties challenges our concept of self. Maybe we aren’t perfect after all. Perhaps for the first time, failure looms.
In my experience, the usual solution to the possibility of failure is a desire to reduce patient care responsibilities. Academic faculty cherish their protected time and usually look for ways to increase it rather than to balance it (Professional time). Academic careers require thick CVs, not satisfied patients. A talk on leukemia at a major conference is more valued than talking to a patient about their leukemia. The cognitive dissonance between what we think is important and what is actually important challenges our personal sense of identity. The resulting burnout represents the necessary failure required to then mature spiritually and reprioritize our ambitions.
On some level, then, the path most of us are on is the time-honored – but painful – journey that must be traveled in order to attain peace.
I also recommend planning a career path with quality work, not a future title, as the goal. Quality work implies measurable objectives. For teachers, work could be measured by teaching scores and student accomplishments. For researchers, work could be measured by published papers, grants received, and invited lectures. For practitioners, work could be measured by outcomes, particularly patient-reported outcomes. Once work is measured, continuous improvements can be made and tracked. Highly reliable teachers, researchers, and practitioners who value quality work will be rewarded both personally and professionally (Defining high reliability).
There is a difference, however, between trying to be the best and trying to improve. The former implies competition with someone else, while the latter involves only one person. Competition can be motivating, but can also undermine interpersonal relationships while causing unhealthy behaviors like overworking and sleep deprivation. If the position sought requires selfish and destructive behaviors, it is not a position worth seeking (Rat race).
By doing quality work – not just more work – leadership positions will inevitably follow. Once a position is obtained, the work increases because a leader is now responsible for others. There are some easy-to-learn tools that can help with that responsibility. I find them very useful for helping colleagues work through interpersonal struggles and resource issues (Leadership hacks: The drama triangle; Leadership hacks: Structural tension).
Success as a leader is harder to measure, but many institutions employ engagement surveys similar to job satisfaction surveys. Leadership scores are generally accurate reflections of leader effectiveness, as are 360-degree surveys of those who work with you. Of course, being a leader also means holding those in your charge accountable for their behaviors (The white wall; Full disclosure). Leadership is no place for someone unwilling to hold crucial and difficult conversations with colleagues.
Success, of course, begets success and additional leadership roles are offered to successful leaders. Meanwhile, the work you started in order to get to the leadership position will probably need to be scaled back as excellence in teaching, research, patient care, and leadership is daunting, difficult to manage, and threatens work-life balance. The ability to say “no” is a valuable skill to learn as leadership roles increase.
Even though none of us work alone, academic medicine generally rewards only the individual. Yet, the camaraderie developed over time working together helps balance work and life roles. To advance as a leader, learning to work in a team is a critical ability. There is a science behind teamwork and aspiring leaders should acquaint themselves with it (Successful teams). While you may be rewarded as an individual, your success will be dependent on your ability to work on a team.
Finally, at least for clinicians, our obligation to our patients largely supersedes all our other commitments. Knowing the most, or being the most technically gifted, is not what patients value. They value empathy and relationships. We need to develop care designed for them, not us (Timed perfectly). We need to communicate with them on their terms, not ours (Pathologic superstition). We must walk with patients on their path, not ours. A patient-centered approach to care and career can take you far. Good luck on your journey.
Dr. Kalaycio is the outgoing editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Along with resigning as chairman of the department of hematology and medical oncology at the Cleveland Clinic (Reunion), I am also resigning as editor in chief of Hematology News. In contrast to the drawn out process of choosing the next department chairman, however, I was in the enviable position of being able to hand pick my successor as editor in chief. I am proud to announce that Ifeyinwa (Ify) Osunkwo, MD, MPH, will be the new editor in chief of Hematology News. Dr. Osunkwo’s new perspective and energy will guide the further development of Hematology News for the benefit of our readers.
As editor in chief, I have had the opportunity to write essays for Hematology News that reflect my experience as a leader in an academic medical department. By doing so, I was trying to summarize some of what I learned along my career path. In my final essay, I want to direct some of these nuggets of wisdom directly to aspiring leaders who are closer to the beginning of their career journey than I am.
My junior colleagues are very interested in developing their careers to maximize opportunities in leadership, and I have coached many to try to understand that the path to leadership is not always straight, may be difficult, and does not always end comfortably. While the goal may seem to be in one direction, the path may lead to another. That is what has happened to me.
I did not seek to be Chairman. The opportunity came to me while I was busy doing other things. As I expressed in an earlier editorial (Seeking the chair), those who are diligent about their work without actively trying to rise through the leadership hierarchy are the ones who seem to rise more often.
Ambition is overrated. The ambitious find it harder to accept failure, and some degree of failure is likely. In his book “Falling Upward: A Spirituality for the Two Halves of Life,” Father Richard Rohr suggests that failure is required in order to mature from someone whose life centers on self to someone whose self centers on life.
Junior faculty tend to focus on self. They try to excel at whatever they attempt as they always have. Whether that is teaching, performing research, or treating patients, they try to be the absolute best teacher, researcher, or practitioner they can be. Many try to do all three well. Rare are those who can perfectly balance all three endeavors. Tension results, both at work and at home. Here is where failure often happens. The student disappoints, the paper is rejected, the grant isn’t funded, the patient relapses, and the family wishes you were home more. This confluence of difficulties challenges our concept of self. Maybe we aren’t perfect after all. Perhaps for the first time, failure looms.
In my experience, the usual solution to the possibility of failure is a desire to reduce patient care responsibilities. Academic faculty cherish their protected time and usually look for ways to increase it rather than to balance it (Professional time). Academic careers require thick CVs, not satisfied patients. A talk on leukemia at a major conference is more valued than talking to a patient about their leukemia. The cognitive dissonance between what we think is important and what is actually important challenges our personal sense of identity. The resulting burnout represents the necessary failure required to then mature spiritually and reprioritize our ambitions.
On some level, then, the path most of us are on is the time-honored – but painful – journey that must be traveled in order to attain peace.
I also recommend planning a career path with quality work, not a future title, as the goal. Quality work implies measurable objectives. For teachers, work could be measured by teaching scores and student accomplishments. For researchers, work could be measured by published papers, grants received, and invited lectures. For practitioners, work could be measured by outcomes, particularly patient-reported outcomes. Once work is measured, continuous improvements can be made and tracked. Highly reliable teachers, researchers, and practitioners who value quality work will be rewarded both personally and professionally (Defining high reliability).
There is a difference, however, between trying to be the best and trying to improve. The former implies competition with someone else, while the latter involves only one person. Competition can be motivating, but can also undermine interpersonal relationships while causing unhealthy behaviors like overworking and sleep deprivation. If the position sought requires selfish and destructive behaviors, it is not a position worth seeking (Rat race).
By doing quality work – not just more work – leadership positions will inevitably follow. Once a position is obtained, the work increases because a leader is now responsible for others. There are some easy-to-learn tools that can help with that responsibility. I find them very useful for helping colleagues work through interpersonal struggles and resource issues (Leadership hacks: The drama triangle; Leadership hacks: Structural tension).
Success as a leader is harder to measure, but many institutions employ engagement surveys similar to job satisfaction surveys. Leadership scores are generally accurate reflections of leader effectiveness, as are 360-degree surveys of those who work with you. Of course, being a leader also means holding those in your charge accountable for their behaviors (The white wall; Full disclosure). Leadership is no place for someone unwilling to hold crucial and difficult conversations with colleagues.
Success, of course, begets success and additional leadership roles are offered to successful leaders. Meanwhile, the work you started in order to get to the leadership position will probably need to be scaled back as excellence in teaching, research, patient care, and leadership is daunting, difficult to manage, and threatens work-life balance. The ability to say “no” is a valuable skill to learn as leadership roles increase.
Even though none of us work alone, academic medicine generally rewards only the individual. Yet, the camaraderie developed over time working together helps balance work and life roles. To advance as a leader, learning to work in a team is a critical ability. There is a science behind teamwork and aspiring leaders should acquaint themselves with it (Successful teams). While you may be rewarded as an individual, your success will be dependent on your ability to work on a team.
Finally, at least for clinicians, our obligation to our patients largely supersedes all our other commitments. Knowing the most, or being the most technically gifted, is not what patients value. They value empathy and relationships. We need to develop care designed for them, not us (Timed perfectly). We need to communicate with them on their terms, not ours (Pathologic superstition). We must walk with patients on their path, not ours. A patient-centered approach to care and career can take you far. Good luck on your journey.
Dr. Kalaycio is the outgoing editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Along with resigning as chairman of the department of hematology and medical oncology at the Cleveland Clinic (Reunion), I am also resigning as editor in chief of Hematology News. In contrast to the drawn out process of choosing the next department chairman, however, I was in the enviable position of being able to hand pick my successor as editor in chief. I am proud to announce that Ifeyinwa (Ify) Osunkwo, MD, MPH, will be the new editor in chief of Hematology News. Dr. Osunkwo’s new perspective and energy will guide the further development of Hematology News for the benefit of our readers.
As editor in chief, I have had the opportunity to write essays for Hematology News that reflect my experience as a leader in an academic medical department. By doing so, I was trying to summarize some of what I learned along my career path. In my final essay, I want to direct some of these nuggets of wisdom directly to aspiring leaders who are closer to the beginning of their career journey than I am.
My junior colleagues are very interested in developing their careers to maximize opportunities in leadership, and I have coached many to try to understand that the path to leadership is not always straight, may be difficult, and does not always end comfortably. While the goal may seem to be in one direction, the path may lead to another. That is what has happened to me.
I did not seek to be Chairman. The opportunity came to me while I was busy doing other things. As I expressed in an earlier editorial (Seeking the chair), those who are diligent about their work without actively trying to rise through the leadership hierarchy are the ones who seem to rise more often.
Ambition is overrated. The ambitious find it harder to accept failure, and some degree of failure is likely. In his book “Falling Upward: A Spirituality for the Two Halves of Life,” Father Richard Rohr suggests that failure is required in order to mature from someone whose life centers on self to someone whose self centers on life.
Junior faculty tend to focus on self. They try to excel at whatever they attempt as they always have. Whether that is teaching, performing research, or treating patients, they try to be the absolute best teacher, researcher, or practitioner they can be. Many try to do all three well. Rare are those who can perfectly balance all three endeavors. Tension results, both at work and at home. Here is where failure often happens. The student disappoints, the paper is rejected, the grant isn’t funded, the patient relapses, and the family wishes you were home more. This confluence of difficulties challenges our concept of self. Maybe we aren’t perfect after all. Perhaps for the first time, failure looms.
In my experience, the usual solution to the possibility of failure is a desire to reduce patient care responsibilities. Academic faculty cherish their protected time and usually look for ways to increase it rather than to balance it (Professional time). Academic careers require thick CVs, not satisfied patients. A talk on leukemia at a major conference is more valued than talking to a patient about their leukemia. The cognitive dissonance between what we think is important and what is actually important challenges our personal sense of identity. The resulting burnout represents the necessary failure required to then mature spiritually and reprioritize our ambitions.
On some level, then, the path most of us are on is the time-honored – but painful – journey that must be traveled in order to attain peace.
I also recommend planning a career path with quality work, not a future title, as the goal. Quality work implies measurable objectives. For teachers, work could be measured by teaching scores and student accomplishments. For researchers, work could be measured by published papers, grants received, and invited lectures. For practitioners, work could be measured by outcomes, particularly patient-reported outcomes. Once work is measured, continuous improvements can be made and tracked. Highly reliable teachers, researchers, and practitioners who value quality work will be rewarded both personally and professionally (Defining high reliability).
There is a difference, however, between trying to be the best and trying to improve. The former implies competition with someone else, while the latter involves only one person. Competition can be motivating, but can also undermine interpersonal relationships while causing unhealthy behaviors like overworking and sleep deprivation. If the position sought requires selfish and destructive behaviors, it is not a position worth seeking (Rat race).
By doing quality work – not just more work – leadership positions will inevitably follow. Once a position is obtained, the work increases because a leader is now responsible for others. There are some easy-to-learn tools that can help with that responsibility. I find them very useful for helping colleagues work through interpersonal struggles and resource issues (Leadership hacks: The drama triangle; Leadership hacks: Structural tension).
Success as a leader is harder to measure, but many institutions employ engagement surveys similar to job satisfaction surveys. Leadership scores are generally accurate reflections of leader effectiveness, as are 360-degree surveys of those who work with you. Of course, being a leader also means holding those in your charge accountable for their behaviors (The white wall; Full disclosure). Leadership is no place for someone unwilling to hold crucial and difficult conversations with colleagues.
Success, of course, begets success and additional leadership roles are offered to successful leaders. Meanwhile, the work you started in order to get to the leadership position will probably need to be scaled back as excellence in teaching, research, patient care, and leadership is daunting, difficult to manage, and threatens work-life balance. The ability to say “no” is a valuable skill to learn as leadership roles increase.
Even though none of us work alone, academic medicine generally rewards only the individual. Yet, the camaraderie developed over time working together helps balance work and life roles. To advance as a leader, learning to work in a team is a critical ability. There is a science behind teamwork and aspiring leaders should acquaint themselves with it (Successful teams). While you may be rewarded as an individual, your success will be dependent on your ability to work on a team.
Finally, at least for clinicians, our obligation to our patients largely supersedes all our other commitments. Knowing the most, or being the most technically gifted, is not what patients value. They value empathy and relationships. We need to develop care designed for them, not us (Timed perfectly). We need to communicate with them on their terms, not ours (Pathologic superstition). We must walk with patients on their path, not ours. A patient-centered approach to care and career can take you far. Good luck on your journey.
Dr. Kalaycio is the outgoing editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
AGA releases clinical practice update for pancreatic necrosis
The American Gastroenterological Association recently issued a clinical practice update for the management of pancreatic necrosis, including 15 recommendations based on a comprehensive literature review and the experiences of leading experts.
Recommendations range from the general, such as the need for a multidisciplinary approach, to the specific, such as the superiority of metal over plastic stents for endoscopic transmural drainage.
The expert review, which was conducted by lead author Todd H. Baron, MD, of the University of North Carolina in Chapel Hill and three other colleagues, was vetted by the AGA Institute Clinical Practice Updates Committee (CPUC) and the AGA Governing Board. In addition, the update underwent external peer review prior to publication in Gastroenterology.
In the update, the authors outlined the clinical landscape for pancreatic necrosis, including challenges posed by complex cases and a mortality rate as high as 30%.
“Successful management of these patients requires expert multidisciplinary care by gastroenterologists, surgeons, interventional radiologists, and specialists in critical care medicine, infectious disease, and nutrition,” the investigators wrote.
They went on to explain how management has evolved over the past 10 years.
“Whereby major surgical intervention and debridement was once the mainstay of therapy for patients with symptomatic necrotic collections, a minimally invasive approach focusing on percutaneous drainage and/or endoscopic drainage or debridement is now favored,” they wrote. They added that debridement is still generally agreed to be the best choice for cases of infected necrosis or patients with sterile necrosis “marked by abdominal pain, nausea, vomiting, and nutritional failure or with associated complications including gastrointestinal luminal obstruction, biliary obstruction, recurrent acute pancreatitis, fistulas, or persistent systemic inflammatory response syndrome (SIRS).”
Other elements of care, however, remain debated, the investigators noted, which has led to variations in multiple aspects of care, such as interventional timing, intravenous fluids, antibiotics, and nutrition. Within this framework, the present practice update is aimed at offering “concise best practice advice for the optimal management of patients with this highly morbid condition.”
Among these pieces of advice, the authors emphasized that routine prophylactic antibiotics and/or antifungals to prevent infected necrosis are unsupported by multiple clinical trials. When infection is suspected, the update recommends broad spectrum intravenous antibiotics, noting that, in most cases, it is unnecessary to perform CT-guided fine-needle aspiration for cultures and gram stain.
Regarding nutrition, the update recommends against “pancreatic rest”; instead, it calls for early oral intake and, if this is not possible, then initiation of total enteral nutrition. Although the authors deemed multiple routes of enteral feeding acceptable, they favored nasogastric or nasoduodenal tubes, when appropriate, because of ease of placement and maintenance. For prolonged total enteral nutrition or patients unable to tolerate nasoenteric feeding, the authors recommended endoscopic feeding tube placement with a percutaneous endoscopic gastrostomy tube for those who can tolerate gastric feeding or a percutaneous endoscopic jejunostomy tube for those who cannot or have a high risk of aspiration.
As described above, the update recommends debridement for cases of infected pancreatic necrosis. Ideally, this should be performed at least 4 weeks after onset, and avoided altogether within the first 2 weeks, because of associated risks of morbidity and mortality; instead, during this acute phase, percutaneous drainage may be considered.
For walled-off pancreatic necrosis, the authors recommended transmural drainage via endoscopic therapy because this mitigates risk of pancreatocutaneous fistula. Percutaneous drainage may be considered in addition to, or in absence of, endoscopic drainage, depending on clinical status.
The remainder of the update covers decisions related to stents, other minimally invasive techniques, open operative debridement, and disconnected left pancreatic remnants, along with discussions of key supporting clinical trials.
The investigators disclosed relationships with Cook Endoscopy, Boston Scientific, Olympus, and others.
SOURCE: Baron TH et al. Gastroenterology. 2019 Aug 31. doi: 10.1053/j.gastro.2019.07.064.
Review the Gastroenterology clinical guidelines collection for AGA Institute statements detailing preferred approaches to specific medical problems or issues based on the most current available data at https://www.gastrojournal.
The American Gastroenterological Association recently issued a clinical practice update for the management of pancreatic necrosis, including 15 recommendations based on a comprehensive literature review and the experiences of leading experts.
Recommendations range from the general, such as the need for a multidisciplinary approach, to the specific, such as the superiority of metal over plastic stents for endoscopic transmural drainage.
The expert review, which was conducted by lead author Todd H. Baron, MD, of the University of North Carolina in Chapel Hill and three other colleagues, was vetted by the AGA Institute Clinical Practice Updates Committee (CPUC) and the AGA Governing Board. In addition, the update underwent external peer review prior to publication in Gastroenterology.
In the update, the authors outlined the clinical landscape for pancreatic necrosis, including challenges posed by complex cases and a mortality rate as high as 30%.
“Successful management of these patients requires expert multidisciplinary care by gastroenterologists, surgeons, interventional radiologists, and specialists in critical care medicine, infectious disease, and nutrition,” the investigators wrote.
They went on to explain how management has evolved over the past 10 years.
“Whereby major surgical intervention and debridement was once the mainstay of therapy for patients with symptomatic necrotic collections, a minimally invasive approach focusing on percutaneous drainage and/or endoscopic drainage or debridement is now favored,” they wrote. They added that debridement is still generally agreed to be the best choice for cases of infected necrosis or patients with sterile necrosis “marked by abdominal pain, nausea, vomiting, and nutritional failure or with associated complications including gastrointestinal luminal obstruction, biliary obstruction, recurrent acute pancreatitis, fistulas, or persistent systemic inflammatory response syndrome (SIRS).”
Other elements of care, however, remain debated, the investigators noted, which has led to variations in multiple aspects of care, such as interventional timing, intravenous fluids, antibiotics, and nutrition. Within this framework, the present practice update is aimed at offering “concise best practice advice for the optimal management of patients with this highly morbid condition.”
Among these pieces of advice, the authors emphasized that routine prophylactic antibiotics and/or antifungals to prevent infected necrosis are unsupported by multiple clinical trials. When infection is suspected, the update recommends broad spectrum intravenous antibiotics, noting that, in most cases, it is unnecessary to perform CT-guided fine-needle aspiration for cultures and gram stain.
Regarding nutrition, the update recommends against “pancreatic rest”; instead, it calls for early oral intake and, if this is not possible, then initiation of total enteral nutrition. Although the authors deemed multiple routes of enteral feeding acceptable, they favored nasogastric or nasoduodenal tubes, when appropriate, because of ease of placement and maintenance. For prolonged total enteral nutrition or patients unable to tolerate nasoenteric feeding, the authors recommended endoscopic feeding tube placement with a percutaneous endoscopic gastrostomy tube for those who can tolerate gastric feeding or a percutaneous endoscopic jejunostomy tube for those who cannot or have a high risk of aspiration.
As described above, the update recommends debridement for cases of infected pancreatic necrosis. Ideally, this should be performed at least 4 weeks after onset, and avoided altogether within the first 2 weeks, because of associated risks of morbidity and mortality; instead, during this acute phase, percutaneous drainage may be considered.
For walled-off pancreatic necrosis, the authors recommended transmural drainage via endoscopic therapy because this mitigates risk of pancreatocutaneous fistula. Percutaneous drainage may be considered in addition to, or in absence of, endoscopic drainage, depending on clinical status.
The remainder of the update covers decisions related to stents, other minimally invasive techniques, open operative debridement, and disconnected left pancreatic remnants, along with discussions of key supporting clinical trials.
The investigators disclosed relationships with Cook Endoscopy, Boston Scientific, Olympus, and others.
SOURCE: Baron TH et al. Gastroenterology. 2019 Aug 31. doi: 10.1053/j.gastro.2019.07.064.
Review the Gastroenterology clinical guidelines collection for AGA Institute statements detailing preferred approaches to specific medical problems or issues based on the most current available data at https://www.gastrojournal.
The American Gastroenterological Association recently issued a clinical practice update for the management of pancreatic necrosis, including 15 recommendations based on a comprehensive literature review and the experiences of leading experts.
Recommendations range from the general, such as the need for a multidisciplinary approach, to the specific, such as the superiority of metal over plastic stents for endoscopic transmural drainage.
The expert review, which was conducted by lead author Todd H. Baron, MD, of the University of North Carolina in Chapel Hill and three other colleagues, was vetted by the AGA Institute Clinical Practice Updates Committee (CPUC) and the AGA Governing Board. In addition, the update underwent external peer review prior to publication in Gastroenterology.
In the update, the authors outlined the clinical landscape for pancreatic necrosis, including challenges posed by complex cases and a mortality rate as high as 30%.
“Successful management of these patients requires expert multidisciplinary care by gastroenterologists, surgeons, interventional radiologists, and specialists in critical care medicine, infectious disease, and nutrition,” the investigators wrote.
They went on to explain how management has evolved over the past 10 years.
“Whereby major surgical intervention and debridement was once the mainstay of therapy for patients with symptomatic necrotic collections, a minimally invasive approach focusing on percutaneous drainage and/or endoscopic drainage or debridement is now favored,” they wrote. They added that debridement is still generally agreed to be the best choice for cases of infected necrosis or patients with sterile necrosis “marked by abdominal pain, nausea, vomiting, and nutritional failure or with associated complications including gastrointestinal luminal obstruction, biliary obstruction, recurrent acute pancreatitis, fistulas, or persistent systemic inflammatory response syndrome (SIRS).”
Other elements of care, however, remain debated, the investigators noted, which has led to variations in multiple aspects of care, such as interventional timing, intravenous fluids, antibiotics, and nutrition. Within this framework, the present practice update is aimed at offering “concise best practice advice for the optimal management of patients with this highly morbid condition.”
Among these pieces of advice, the authors emphasized that routine prophylactic antibiotics and/or antifungals to prevent infected necrosis are unsupported by multiple clinical trials. When infection is suspected, the update recommends broad spectrum intravenous antibiotics, noting that, in most cases, it is unnecessary to perform CT-guided fine-needle aspiration for cultures and gram stain.
Regarding nutrition, the update recommends against “pancreatic rest”; instead, it calls for early oral intake and, if this is not possible, then initiation of total enteral nutrition. Although the authors deemed multiple routes of enteral feeding acceptable, they favored nasogastric or nasoduodenal tubes, when appropriate, because of ease of placement and maintenance. For prolonged total enteral nutrition or patients unable to tolerate nasoenteric feeding, the authors recommended endoscopic feeding tube placement with a percutaneous endoscopic gastrostomy tube for those who can tolerate gastric feeding or a percutaneous endoscopic jejunostomy tube for those who cannot or have a high risk of aspiration.
As described above, the update recommends debridement for cases of infected pancreatic necrosis. Ideally, this should be performed at least 4 weeks after onset, and avoided altogether within the first 2 weeks, because of associated risks of morbidity and mortality; instead, during this acute phase, percutaneous drainage may be considered.
For walled-off pancreatic necrosis, the authors recommended transmural drainage via endoscopic therapy because this mitigates risk of pancreatocutaneous fistula. Percutaneous drainage may be considered in addition to, or in absence of, endoscopic drainage, depending on clinical status.
The remainder of the update covers decisions related to stents, other minimally invasive techniques, open operative debridement, and disconnected left pancreatic remnants, along with discussions of key supporting clinical trials.
The investigators disclosed relationships with Cook Endoscopy, Boston Scientific, Olympus, and others.
SOURCE: Baron TH et al. Gastroenterology. 2019 Aug 31. doi: 10.1053/j.gastro.2019.07.064.
Review the Gastroenterology clinical guidelines collection for AGA Institute statements detailing preferred approaches to specific medical problems or issues based on the most current available data at https://www.gastrojournal.
FROM GASTROENTEROLOGY