User login
Patient Preferences for Physician Attire: A Multicenter Study in Japan
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
© 2020 Society of Hospital Medicine
Portable Ultrasound Device Usage and Learning Outcomes Among Internal Medicine Trainees: A Parallel-Group Randomized Trial
Point-of-care ultrasonography (POCUS) can transform healthcare delivery through its diagnostic and therapeutic expediency.1 POCUS has been shown to bolster diagnostic accuracy, reduce procedural complications, decrease inpatient length of stay, and improve patient satisfaction by encouraging the physician to be present at the bedside.2-8
POCUS has become widespread across a variety of clinical settings as more investigations have demonstrated its positive impact on patient care.1,9-12 This includes the use of POCUS by trainees, who are now utilizing this technology as part of their assessments of patients.13,14 However, trainees may be performing these examinations with minimal oversight, and outside of emergency medicine, there are few guidelines on how to effectively teach POCUS or measure competency.13,14 While POCUS is rapidly becoming a part of inpatient care, teaching physicians may have little experience in ultrasound or the expertise to adequately supervise trainees.14 There is a growing need to study what trainees can learn and how this knowledge is acquired.
Previous investigations have demonstrated that inexperienced users can be taught to use POCUS to identify a variety of pathological states.2,3,15-23 Most of these curricula used a single lecture series as their pedagogical vehicle, and they variably included junior medical trainees. More importantly, the investigations did not explore whether personal access to handheld ultrasound devices (HUDs) improved learning. In theory, improved access to POCUS devices increases opportunities for authentic and deliberate practice, which may be needed to improve trainee skill with POCUS beyond the classroom setting.14
This study aimed to address several ongoing gaps in knowledge related to learning POCUS. First, we hypothesized that personal HUD access would improve trainees’ POCUS-related knowledge and interpretive ability as a result of increased practice opportunities. Second, we hypothesized that trainees who receive personal access to HUDs would be more likely to perform POCUS examinations and feel more confident in their interpretations. Finally, we hypothesized that repeated exposure to POCUS-related lectures would result in greater improvements in knowledge as compared with a single lecture series.
METHODS
Participants and Setting
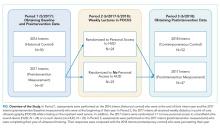
The 2017 intern class (n = 47) at an academic internal medicine residency program participated in the study. Control data were obtained from the 2016 intern class (historical control; n = 50) and the 2018 intern class (contemporaneous control; n = 52). The Stanford University Institutional Review Board approved this study.
Study Design
The 2017 intern class (n = 47) received POCUS didactics from June 2017 to June 2018. To evaluate if increased access to HUDs improved learning outcomes, the 2017 interns were randomized 1:1 to receive their own personal HUD that could be used for patient care and/or self-directed learning (n = 24) vs no-HUD (n = 23; Figure). Learning outcomes were assessed over the course of 1 year (see “Outcomes” below) and were compared with the 2016 and 2018 controls. The 2016 intern class had completed a year of training but had not received formalized POCUS didactics (historical control), whereas the 2018 intern class was assessed at the beginning of their year (contemporaneous control; Figure). In order to make comparisons based on intern experience, baseline data for the 2017 intern class were compared with the 2018 intern class, whereas end-of-study data for 2017 interns were compared with 2016 interns.
Outcomes
The primary outcome was the difference in assessment scores at the end of the study period between interns randomized to receive a HUD and those who were not. Secondary outcomes included differences in HUD usage rates, lecture attendance, and assessment scores. To assess whether repeated lecture exposure resulted in greater amounts of learning, this study evaluated for assessment score improvements after each lecture block. Finally, trainee attitudes toward POCUS and their confidence in their interpretative ability were measured at the beginning and end of the study period.
Curriculum Implementation
The lectures were administered as once-weekly didactics of 1-hour duration to interns rotating on the inpatient wards rotation. This rotation is 4 weeks long, and each intern will experience the rotation two to four times per year. Each lecture contained two parts: (1) 20-30 minutes of didactics via Microsoft PowerPointTM and (2) 30-40 minutes of supervised practice using HUDs on standardized patients. Four lectures were given each month: (1) introduction to POCUS and ultrasound physics, (2) thoracic/lung ultrasound, (3) echocardiography, and (4) abdominal POCUS. The lectures consisted of contrasting cases of normal/abnormal videos and clinical vignettes. These four lectures were repeated each month as new interns rotated on service. Some interns experienced the same content multiple times, which was intentional in order to assess their rates of learning over time. Lecture contents were based on previously published guidelines and expert consensus for teaching POCUS in internal medicine.13, 24-26 Content from the Accreditation Council for Graduate Medical Education (ACGME) and the American College of Emergency Physicians (ACEP) was also incorporated because these organizations had published relevant guidelines for teaching POCUS.13,26 Further development of the lectures occurred through review of previously described POCUS-relevant curricula.27-32
Handheld Ultrasound Devices
This study used the Philips LumifyTM, a United States Food and Drug Administration–approved device. Interns randomized to HUDs received their own device at the start of the rotation. It was at their discretion to use the device outside of the course. All devices were approved for patient use and were encrypted in compliance with our information security office. For privacy reasons, any saved patient images were not reviewed by the researchers. Interns were encouraged to share their findings with supervising physicians during rounds, but actual oversight was not measured. Interns not randomized to HUDs could access a single community device that was shared among all residents and fellows in the hospital. Interns reported the average number of POCUS examinations performed each week via a survey sent during the last week of the rotation.
Assessment Design and Implementation
Assessments evaluating trainee knowledge were administered before, during, and after the study period (Figure). For the 2017 cohort, assessments were also administered at the start and end of the ward month to track knowledge acquisition. Assessment contents were selected from POCUS guidelines for internal medicine and adaptation of the ACGME and ACEP guidelines.13,24,26 Additional content was obtained from major society POCUS tutorials and deidentified images collected by the study authors.13,24,33 In keeping with previously described methodology, the images were shown for approximately 12 seconds, followed by five additional seconds to allow the learner to answer the question.32 Final assessment contents were determined by the authors using the Delphi method.34 A sample assessment can be found in the Appendix Material.
Surveys
Surveys were administered alongside the assessments to the 2016-2018 intern classes. These surveys assessed trainee attitudes toward POCUS and were based on previously validated assessments.27,28,30 Attitudes were measured using 5-point Likert scales.
Statistical Analysis
For the primary outcome, we performed generalized binomial mixed-effect regressions using the survey periods, randomization group, and the interaction of the two as independent variables after adjusting for attendance and controlling of intra-intern correlations. The bivariate unadjusted analysis was performed to display the distribution of overall correctness on the assessments. Wilcoxon signed rank test was used to determine score significance for dependent score variables (R-Statistical Programming Language, Vienna, Austria).
RESULTS
Baseline Characteristics
There were 149 interns who participated in this study (Figure). Assessment/survey completion rates were as follows: 2016 control: 68.0%; 2017 preintervention: 97.9%; 2017 postintervention: 89.4%; and 2018 control: 100%. The 2017 interns reported similar amounts of prior POCUS exposure in medical school (Table 1).
Primary Outcome: Assessment Scores (HUD vs no HUD)
There were no significant differences in assessment scores at the end of the study between interns randomized to personal HUD access vs those to no-HUD access (Table 1). HUD interns reported performing POCUS assessments on patients a mean 6.8 (standard deviation [SD] 2.2) times per week vs 6.4 (SD 2.9) times per week in the no-HUD arm (P = .66). The mean lecture attendance was 75.0% and did not significantly differ between the HUD arms (Table 1).
Secondary Outcomes
Impact of Repeating Lectures
The 2017 interns demonstrated significant increases in preblock vs postblock assessment scores after first-time exposure to the lectures (median preblock score 0.61 [interquartile range (IQR), 0.53-0.70] vs postblock score 0.81 [IQR, 0.72-0.86]; P < .001; Table 2). However, intern performance on the preblock vs postblock assessments after second-time exposure to the curriculum failed to improve (median second preblock score 0.78 [IQR, 0.69-0.83] vs postblock score 0.81 [IQR, 0.64-0.89]; P = .94). Intern performance on individual domains of knowledge for each block is listed in Appendix Table 1.
Intervention Performance vs Controls
The 2016 historical control had significantly higher scores compared with the 2017 preintervention group (P < .001; Appendix Table 2). The year-long lecture series resulted in significant increases in median scores for the 2017 group (median preintervention score 0.55 [0.41-0.61] vs median postintervention score 0.84 [0.71-0.90]; P = .006; Appendix Table 1). At the end of the study, the 2017 postintervention scores were significantly higher across multiple knowledge domains compared with the 2016 historical control (Appendix Table 2).
Survey Results
Notably, the 2017 intern class at the end of the intervention did not have significantly different assessment scores for several disease-specific domains, compared with the 2016 control (Appendix Table 2). Nonetheless, the 2017 intern class reported higher levels of confidence in these same domains despite similar scores (Supplementary Figure). The HUD group seldomly cited a lack of confidence in their abilities as a barrier to performing POCUS examinations (17.6%), compared with the no-HUD group (50.0%), despite nearly identical assessment scores between the two groups (Table 1).
DISCUSSION
Previous guidelines have recommended increased HUD access for learners,13,24,35,36 but there have been few investigations that have evaluated the impact of such access on learning POCUS. One previous investigation found that hospitalists who carried HUDs were more likely to identify heart failure on bedside examination.37 In contrast, our study found no improvement in interpretative ability when randomizing interns to carry HUDs for patient care. Notably, interns did not perform more POCUS examinations when given HUDs. We offer several explanations for this finding. First, time-motion studies have demonstrated that internal medicine interns spend less than 15% of their time toward direct patient care.38 It is possible that the demands of being an intern impeded their ability to perform more POCUS examinations on their patients, regardless of HUD access. Alternatively, the interns randomized to no personal access may have used the community device more frequently as a result of the lecture series. Given the cost of HUDs, further studies are needed to assess the degree to which HUD access will improve trainee interpretive ability, especially as more training programs consider the creation of ultrasound curricula.10,11,24,39,40
This study was unique because it followed interns over a year-long course that repeated the same material to assess rates of learning with repeated exposure. Learners improved their scores after the first, but not second, block. Furthermore, the median scores were nearly identical between the first postblock assessment and second preblock assessment (0.81 vs 0.78), suggesting that knowledge was retained between blocks. Together, these findings suggest there may be limitations of traditional lectures that use standardized patient models for practice. Supplementary pedagogies, such as in-the-moment feedback with actual patients, may be needed to promote mastery.14,35
Despite no formal curriculum, the 2016 intern class (historical control) had learned POCUS to some degree based on their higher assessment scores compared with the 2017 intern class during the preintervention period. Such learning may be informal, and yet, trainees may feel confident in making clinical decisions without formalized training, accreditation, or oversight. As suggested by this study, adding regular didactics or giving trainees HUDs may not immediately solve this issue. For assessment items in which the 2017 interns did not significantly differ from the controls, they nonetheless reported higher confidence in their abilities. Similarly, interns randomized to HUDs less frequently cited a lack of confidence in their abilities, despite similar scores to the no-HUD group. Such confidence may be incongruent with their actual knowledge or ability to safely use POCUS. This phenomenon of misplaced confidence is known as the Dunning–Kruger effect, and it may be common with ultrasound learning.41 While confidence can be part of a holistic definition of competency,14 these results raise the concern that trainees may have difficulty assessing their own competency level with POCUS.35
There are several limitations to this study. It was performed at a single institution with limited sample size. It examined only intern physicians because of funding constraints, which limits the generalizability of these findings among medical trainees. Technical ability assessments (including obtaining and interpreting images) were not included. We were unable to track the timing or location of the devices’ usage, and the interns’ self-reported usage rates may be subject to recall bias. To our knowledge, there were no significant lapses in device availability/functionality. Intern physicians in the HUD arm did not receive formal feedback on personally acquired patient images, which may have limited the intervention’s impact.
In conclusion, internal medicine interns who received personal HUDs were not better at recognizing normal/abnormal findings on image assessments, and they did not report performing more POCUS examinations. Since the minority of a trainee’s time is spent toward direct patient care, offering trainees HUDs without substantial guidance may not be enough to promote mastery. Notably, trainees who received HUDs felt more confident in their abilities, despite no objective increase in their actual skill. Finally, interns who received POCUS-related lectures experienced significant benefit upon first exposure to the material, while repeated exposures did not improve performance. Future investigations should stringently track trainee POCUS usage rates with HUDs and assess whether image acquisition ability improves as a result of personal access.
1. Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
2. Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509-1511. https://doi.org/10.1016/j.ajem.2013.07.006.
3. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. https://doi.org/10.1016/j.echo.2011.07.013.
4. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
5. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: Report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. https://doi.org/10.1186/1477-7819-12-139.
6. Testa A, Francesconi A, Giannuzzi R, Berardi S, Sbraccia P. Economic analysis of bedside ultrasonography (US) implementation in an Internal Medicine department. Intern Emerg Med. 2015;10(8):1015-1024. https://doi.org/10.1007/s11739-015-1320-7.
7. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. https://doi.org/10.1016/j.jemermed.2013.05.044.
8. Park YH, Jung RB, Lee YG, et al. Does the use of bedside ultrasonography reduce emergency department length of stay for patients with renal colic? A pilot study. Clin Exp Emerg Med. 2016;3(4):197-203. https://doi.org/10.15441/ceem.15.109.
9. Glomb N, D’Amico B, Rus M, Chen C. Point-of-care ultrasound in resource-limited settings. Clin Pediatr Emerg Med. 2015;16(4):256-261. https://doi.org/10.1016/j.cpem.2015.10.001.
10. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
11. Hall JWW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: A CERA study. Fam Med. 2015;47(9):706-711.
12. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: A national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. https://doi.org/10.4300/JGME-D-12-00215.1.
13. Stolz LA, Stolz U, Fields JM, et al. Emergency medicine resident assessment of the emergency ultrasound milestones and current training recommendations. Acad Emerg Med. 2017;24(3):353-361. https://doi.org/10.1111/acem.13113.
14. Kumar, A., Jensen, T., Kugler, J. Evaluation of trainee competency with point-of-care ultrasonography (POCUS): A conceptual framework and review of existing assessments. J Gen Intern Med. 2019;34(6):1025-1031. https://doi.org/10.1007/s11606-019-04945-4.
15. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part ii: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. https://doi.org/10.1097/CCM.0000000000001847.
16. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96(7):1002-1006. https://doi.org/10.1016/j.amjcard.2005.05.060.
17. Ceriani E, Cogliati C. Update on bedside ultrasound diagnosis of pericardial effusion. Intern Emerg Med. 2016;11(3):477-480. https://doi.org/10.1007/s11739-015-1372-8.
18. Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225-1230. https://doi.org/10.1016/j.echo.2010.10.005.
19. Keil-Ríos D, Terrazas-Solís H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. https://doi.org/10.1007/s11739-016-1406-x.
20. Riddell J, Case A, Wopat R, et al. Sensitivity of emergency bedside ultrasound to detect hydronephrosis in patients with computed tomography–proven stones. West J Emerg Med. 2014;15(1):96-100. https://doi.org/10.5811/westjem.2013.9.15874.
21. Dalziel PJ, Noble VE. Bedside ultrasound and the assessment of renal colic: A review. Emerg Med J. 2013;30(1):3-8. https://doi.org/10.1136/emermed-2012-201375.
22. Whitson MR, Mayo PH. Ultrasonography in the emergency department. Crit Care. 2016;20(1):227. https://doi.org/10.1186/s13054-016-1399-x.
23. Kumar A, Liu G, Chi J, Kugler J. The role of technology in the bedside encounter. Med Clin North Am. 2018;102(3):443-451. https://doi.org/10.1016/j.mcna.2017.12.006.
24. Ma IWY, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: Consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. https://doi.org/10.1007/s11606-017-4071-5.
15. Sabath BF, Singh G. Point-of-care ultrasonography as a training milestone for internal medicine residents: The time is now. J Community Hosp Intern Med Perspect. 2016;6(5):33094. https://doi.org/10.3402/jchimp.v6.33094.
26. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
27. Ramsingh D, Rinehart J, Kain Z, et al. Impact assessment of perioperative point-of-care ultrasound training on anesthesiology residents. Anesthesiology. 2015;123(3):670-682. https://doi.org/10.1097/ALN.0000000000000776.
28. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. https://doi.org/10.1186/1472-6920-11-75.
29. Townsend NT, Kendall J, Barnett C, Robinson T. An effective curriculum for focused assessment diagnostic echocardiography: Establishing the learning curve in surgical residents. J Surg Educ. 2016;73(2):190-196. https://doi.org/10.1016/j.jsurg.2015.10.009.
30. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7(1):18. https://doi.org/10.1186/s13089-015-0035-3.
31. Skalski JH, Elrashidi M, Reed DA, McDonald FS, Bhagra A. Using standardized patients to teach point-of-care ultrasound–guided physical examination skills to internal medicine residents. J Grad Med Educ. 2015;7(1):95-97. https://doi.org/10.4300/JGME-D-14-00178.1.
32. Chisholm CB, Dodge WR, Balise RR, Williams SR, Gharahbaghian L, Beraud A-S. Focused cardiac ultrasound training: How much is enough? J Emerg Med. 2013;44(4):818-822. https://doi.org/10.1016/j.jemermed.2012.07.092.
33. Schmidt GA, Schraufnagel D. Introduction to ATS seminars: Intensive care ultrasound. Ann Am Thorac Soc. 2013;10(5):538-539. https://doi.org/10.1513/AnnalsATS.201306-203ED.
34. Skaarup SH, Laursen CB, Bjerrum AS, Hilberg O. Objective and structured assessment of lung ultrasound competence. A multispecialty Delphi consensus and construct validity study. Ann Am Thorac Soc. 2017;14(4):555-560. https://doi.org/10.1513/AnnalsATS.201611-894OC.
35. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
36. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part i: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
37. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed by hospitalists: Does it improve the cardiac physical examination? Am J Med. 2009;122(1):35-41. https://doi.org/10.1016/j.amjmed.2008.07.022.
38. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018;378(16):1494-1508. https://doi.org/10.1056/NEJMoa1800965.
39. Baltarowich OH, Di Salvo DN, Scoutt LM, et al. National ultrasound curriculum for medical students. Ultrasound Q. 2014;30(1):13-19. https://doi.org/10.1097/RUQ.0000000000000066.
40. Beal EW, Sigmond BR, Sage-Silski L, Lahey S, Nguyen V, Bahner DP. Point-of-care ultrasound in general surgery residency training: A proposal for milestones in graduate medical education ultrasound. J Ultrasound Med. 2017;36(12):2577-2584. https://doi.org/10.1002/jum.14298.
41. Kruger J, Dunning D. Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Pers Soc Psychol. 1999;77(6):1121-1134. https://doi.org/10.1037//0022-3514.77.6.1121.
Point-of-care ultrasonography (POCUS) can transform healthcare delivery through its diagnostic and therapeutic expediency.1 POCUS has been shown to bolster diagnostic accuracy, reduce procedural complications, decrease inpatient length of stay, and improve patient satisfaction by encouraging the physician to be present at the bedside.2-8
POCUS has become widespread across a variety of clinical settings as more investigations have demonstrated its positive impact on patient care.1,9-12 This includes the use of POCUS by trainees, who are now utilizing this technology as part of their assessments of patients.13,14 However, trainees may be performing these examinations with minimal oversight, and outside of emergency medicine, there are few guidelines on how to effectively teach POCUS or measure competency.13,14 While POCUS is rapidly becoming a part of inpatient care, teaching physicians may have little experience in ultrasound or the expertise to adequately supervise trainees.14 There is a growing need to study what trainees can learn and how this knowledge is acquired.
Previous investigations have demonstrated that inexperienced users can be taught to use POCUS to identify a variety of pathological states.2,3,15-23 Most of these curricula used a single lecture series as their pedagogical vehicle, and they variably included junior medical trainees. More importantly, the investigations did not explore whether personal access to handheld ultrasound devices (HUDs) improved learning. In theory, improved access to POCUS devices increases opportunities for authentic and deliberate practice, which may be needed to improve trainee skill with POCUS beyond the classroom setting.14
This study aimed to address several ongoing gaps in knowledge related to learning POCUS. First, we hypothesized that personal HUD access would improve trainees’ POCUS-related knowledge and interpretive ability as a result of increased practice opportunities. Second, we hypothesized that trainees who receive personal access to HUDs would be more likely to perform POCUS examinations and feel more confident in their interpretations. Finally, we hypothesized that repeated exposure to POCUS-related lectures would result in greater improvements in knowledge as compared with a single lecture series.
METHODS
Participants and Setting
The 2017 intern class (n = 47) at an academic internal medicine residency program participated in the study. Control data were obtained from the 2016 intern class (historical control; n = 50) and the 2018 intern class (contemporaneous control; n = 52). The Stanford University Institutional Review Board approved this study.
Study Design
The 2017 intern class (n = 47) received POCUS didactics from June 2017 to June 2018. To evaluate if increased access to HUDs improved learning outcomes, the 2017 interns were randomized 1:1 to receive their own personal HUD that could be used for patient care and/or self-directed learning (n = 24) vs no-HUD (n = 23; Figure). Learning outcomes were assessed over the course of 1 year (see “Outcomes” below) and were compared with the 2016 and 2018 controls. The 2016 intern class had completed a year of training but had not received formalized POCUS didactics (historical control), whereas the 2018 intern class was assessed at the beginning of their year (contemporaneous control; Figure). In order to make comparisons based on intern experience, baseline data for the 2017 intern class were compared with the 2018 intern class, whereas end-of-study data for 2017 interns were compared with 2016 interns.
Outcomes
The primary outcome was the difference in assessment scores at the end of the study period between interns randomized to receive a HUD and those who were not. Secondary outcomes included differences in HUD usage rates, lecture attendance, and assessment scores. To assess whether repeated lecture exposure resulted in greater amounts of learning, this study evaluated for assessment score improvements after each lecture block. Finally, trainee attitudes toward POCUS and their confidence in their interpretative ability were measured at the beginning and end of the study period.
Curriculum Implementation
The lectures were administered as once-weekly didactics of 1-hour duration to interns rotating on the inpatient wards rotation. This rotation is 4 weeks long, and each intern will experience the rotation two to four times per year. Each lecture contained two parts: (1) 20-30 minutes of didactics via Microsoft PowerPointTM and (2) 30-40 minutes of supervised practice using HUDs on standardized patients. Four lectures were given each month: (1) introduction to POCUS and ultrasound physics, (2) thoracic/lung ultrasound, (3) echocardiography, and (4) abdominal POCUS. The lectures consisted of contrasting cases of normal/abnormal videos and clinical vignettes. These four lectures were repeated each month as new interns rotated on service. Some interns experienced the same content multiple times, which was intentional in order to assess their rates of learning over time. Lecture contents were based on previously published guidelines and expert consensus for teaching POCUS in internal medicine.13, 24-26 Content from the Accreditation Council for Graduate Medical Education (ACGME) and the American College of Emergency Physicians (ACEP) was also incorporated because these organizations had published relevant guidelines for teaching POCUS.13,26 Further development of the lectures occurred through review of previously described POCUS-relevant curricula.27-32
Handheld Ultrasound Devices
This study used the Philips LumifyTM, a United States Food and Drug Administration–approved device. Interns randomized to HUDs received their own device at the start of the rotation. It was at their discretion to use the device outside of the course. All devices were approved for patient use and were encrypted in compliance with our information security office. For privacy reasons, any saved patient images were not reviewed by the researchers. Interns were encouraged to share their findings with supervising physicians during rounds, but actual oversight was not measured. Interns not randomized to HUDs could access a single community device that was shared among all residents and fellows in the hospital. Interns reported the average number of POCUS examinations performed each week via a survey sent during the last week of the rotation.
Assessment Design and Implementation
Assessments evaluating trainee knowledge were administered before, during, and after the study period (Figure). For the 2017 cohort, assessments were also administered at the start and end of the ward month to track knowledge acquisition. Assessment contents were selected from POCUS guidelines for internal medicine and adaptation of the ACGME and ACEP guidelines.13,24,26 Additional content was obtained from major society POCUS tutorials and deidentified images collected by the study authors.13,24,33 In keeping with previously described methodology, the images were shown for approximately 12 seconds, followed by five additional seconds to allow the learner to answer the question.32 Final assessment contents were determined by the authors using the Delphi method.34 A sample assessment can be found in the Appendix Material.
Surveys
Surveys were administered alongside the assessments to the 2016-2018 intern classes. These surveys assessed trainee attitudes toward POCUS and were based on previously validated assessments.27,28,30 Attitudes were measured using 5-point Likert scales.
Statistical Analysis
For the primary outcome, we performed generalized binomial mixed-effect regressions using the survey periods, randomization group, and the interaction of the two as independent variables after adjusting for attendance and controlling of intra-intern correlations. The bivariate unadjusted analysis was performed to display the distribution of overall correctness on the assessments. Wilcoxon signed rank test was used to determine score significance for dependent score variables (R-Statistical Programming Language, Vienna, Austria).
RESULTS
Baseline Characteristics
There were 149 interns who participated in this study (Figure). Assessment/survey completion rates were as follows: 2016 control: 68.0%; 2017 preintervention: 97.9%; 2017 postintervention: 89.4%; and 2018 control: 100%. The 2017 interns reported similar amounts of prior POCUS exposure in medical school (Table 1).
Primary Outcome: Assessment Scores (HUD vs no HUD)
There were no significant differences in assessment scores at the end of the study between interns randomized to personal HUD access vs those to no-HUD access (Table 1). HUD interns reported performing POCUS assessments on patients a mean 6.8 (standard deviation [SD] 2.2) times per week vs 6.4 (SD 2.9) times per week in the no-HUD arm (P = .66). The mean lecture attendance was 75.0% and did not significantly differ between the HUD arms (Table 1).
Secondary Outcomes
Impact of Repeating Lectures
The 2017 interns demonstrated significant increases in preblock vs postblock assessment scores after first-time exposure to the lectures (median preblock score 0.61 [interquartile range (IQR), 0.53-0.70] vs postblock score 0.81 [IQR, 0.72-0.86]; P < .001; Table 2). However, intern performance on the preblock vs postblock assessments after second-time exposure to the curriculum failed to improve (median second preblock score 0.78 [IQR, 0.69-0.83] vs postblock score 0.81 [IQR, 0.64-0.89]; P = .94). Intern performance on individual domains of knowledge for each block is listed in Appendix Table 1.
Intervention Performance vs Controls
The 2016 historical control had significantly higher scores compared with the 2017 preintervention group (P < .001; Appendix Table 2). The year-long lecture series resulted in significant increases in median scores for the 2017 group (median preintervention score 0.55 [0.41-0.61] vs median postintervention score 0.84 [0.71-0.90]; P = .006; Appendix Table 1). At the end of the study, the 2017 postintervention scores were significantly higher across multiple knowledge domains compared with the 2016 historical control (Appendix Table 2).
Survey Results
Notably, the 2017 intern class at the end of the intervention did not have significantly different assessment scores for several disease-specific domains, compared with the 2016 control (Appendix Table 2). Nonetheless, the 2017 intern class reported higher levels of confidence in these same domains despite similar scores (Supplementary Figure). The HUD group seldomly cited a lack of confidence in their abilities as a barrier to performing POCUS examinations (17.6%), compared with the no-HUD group (50.0%), despite nearly identical assessment scores between the two groups (Table 1).
DISCUSSION
Previous guidelines have recommended increased HUD access for learners,13,24,35,36 but there have been few investigations that have evaluated the impact of such access on learning POCUS. One previous investigation found that hospitalists who carried HUDs were more likely to identify heart failure on bedside examination.37 In contrast, our study found no improvement in interpretative ability when randomizing interns to carry HUDs for patient care. Notably, interns did not perform more POCUS examinations when given HUDs. We offer several explanations for this finding. First, time-motion studies have demonstrated that internal medicine interns spend less than 15% of their time toward direct patient care.38 It is possible that the demands of being an intern impeded their ability to perform more POCUS examinations on their patients, regardless of HUD access. Alternatively, the interns randomized to no personal access may have used the community device more frequently as a result of the lecture series. Given the cost of HUDs, further studies are needed to assess the degree to which HUD access will improve trainee interpretive ability, especially as more training programs consider the creation of ultrasound curricula.10,11,24,39,40
This study was unique because it followed interns over a year-long course that repeated the same material to assess rates of learning with repeated exposure. Learners improved their scores after the first, but not second, block. Furthermore, the median scores were nearly identical between the first postblock assessment and second preblock assessment (0.81 vs 0.78), suggesting that knowledge was retained between blocks. Together, these findings suggest there may be limitations of traditional lectures that use standardized patient models for practice. Supplementary pedagogies, such as in-the-moment feedback with actual patients, may be needed to promote mastery.14,35
Despite no formal curriculum, the 2016 intern class (historical control) had learned POCUS to some degree based on their higher assessment scores compared with the 2017 intern class during the preintervention period. Such learning may be informal, and yet, trainees may feel confident in making clinical decisions without formalized training, accreditation, or oversight. As suggested by this study, adding regular didactics or giving trainees HUDs may not immediately solve this issue. For assessment items in which the 2017 interns did not significantly differ from the controls, they nonetheless reported higher confidence in their abilities. Similarly, interns randomized to HUDs less frequently cited a lack of confidence in their abilities, despite similar scores to the no-HUD group. Such confidence may be incongruent with their actual knowledge or ability to safely use POCUS. This phenomenon of misplaced confidence is known as the Dunning–Kruger effect, and it may be common with ultrasound learning.41 While confidence can be part of a holistic definition of competency,14 these results raise the concern that trainees may have difficulty assessing their own competency level with POCUS.35
There are several limitations to this study. It was performed at a single institution with limited sample size. It examined only intern physicians because of funding constraints, which limits the generalizability of these findings among medical trainees. Technical ability assessments (including obtaining and interpreting images) were not included. We were unable to track the timing or location of the devices’ usage, and the interns’ self-reported usage rates may be subject to recall bias. To our knowledge, there were no significant lapses in device availability/functionality. Intern physicians in the HUD arm did not receive formal feedback on personally acquired patient images, which may have limited the intervention’s impact.
In conclusion, internal medicine interns who received personal HUDs were not better at recognizing normal/abnormal findings on image assessments, and they did not report performing more POCUS examinations. Since the minority of a trainee’s time is spent toward direct patient care, offering trainees HUDs without substantial guidance may not be enough to promote mastery. Notably, trainees who received HUDs felt more confident in their abilities, despite no objective increase in their actual skill. Finally, interns who received POCUS-related lectures experienced significant benefit upon first exposure to the material, while repeated exposures did not improve performance. Future investigations should stringently track trainee POCUS usage rates with HUDs and assess whether image acquisition ability improves as a result of personal access.
Point-of-care ultrasonography (POCUS) can transform healthcare delivery through its diagnostic and therapeutic expediency.1 POCUS has been shown to bolster diagnostic accuracy, reduce procedural complications, decrease inpatient length of stay, and improve patient satisfaction by encouraging the physician to be present at the bedside.2-8
POCUS has become widespread across a variety of clinical settings as more investigations have demonstrated its positive impact on patient care.1,9-12 This includes the use of POCUS by trainees, who are now utilizing this technology as part of their assessments of patients.13,14 However, trainees may be performing these examinations with minimal oversight, and outside of emergency medicine, there are few guidelines on how to effectively teach POCUS or measure competency.13,14 While POCUS is rapidly becoming a part of inpatient care, teaching physicians may have little experience in ultrasound or the expertise to adequately supervise trainees.14 There is a growing need to study what trainees can learn and how this knowledge is acquired.
Previous investigations have demonstrated that inexperienced users can be taught to use POCUS to identify a variety of pathological states.2,3,15-23 Most of these curricula used a single lecture series as their pedagogical vehicle, and they variably included junior medical trainees. More importantly, the investigations did not explore whether personal access to handheld ultrasound devices (HUDs) improved learning. In theory, improved access to POCUS devices increases opportunities for authentic and deliberate practice, which may be needed to improve trainee skill with POCUS beyond the classroom setting.14
This study aimed to address several ongoing gaps in knowledge related to learning POCUS. First, we hypothesized that personal HUD access would improve trainees’ POCUS-related knowledge and interpretive ability as a result of increased practice opportunities. Second, we hypothesized that trainees who receive personal access to HUDs would be more likely to perform POCUS examinations and feel more confident in their interpretations. Finally, we hypothesized that repeated exposure to POCUS-related lectures would result in greater improvements in knowledge as compared with a single lecture series.
METHODS
Participants and Setting
The 2017 intern class (n = 47) at an academic internal medicine residency program participated in the study. Control data were obtained from the 2016 intern class (historical control; n = 50) and the 2018 intern class (contemporaneous control; n = 52). The Stanford University Institutional Review Board approved this study.
Study Design
The 2017 intern class (n = 47) received POCUS didactics from June 2017 to June 2018. To evaluate if increased access to HUDs improved learning outcomes, the 2017 interns were randomized 1:1 to receive their own personal HUD that could be used for patient care and/or self-directed learning (n = 24) vs no-HUD (n = 23; Figure). Learning outcomes were assessed over the course of 1 year (see “Outcomes” below) and were compared with the 2016 and 2018 controls. The 2016 intern class had completed a year of training but had not received formalized POCUS didactics (historical control), whereas the 2018 intern class was assessed at the beginning of their year (contemporaneous control; Figure). In order to make comparisons based on intern experience, baseline data for the 2017 intern class were compared with the 2018 intern class, whereas end-of-study data for 2017 interns were compared with 2016 interns.
Outcomes
The primary outcome was the difference in assessment scores at the end of the study period between interns randomized to receive a HUD and those who were not. Secondary outcomes included differences in HUD usage rates, lecture attendance, and assessment scores. To assess whether repeated lecture exposure resulted in greater amounts of learning, this study evaluated for assessment score improvements after each lecture block. Finally, trainee attitudes toward POCUS and their confidence in their interpretative ability were measured at the beginning and end of the study period.
Curriculum Implementation
The lectures were administered as once-weekly didactics of 1-hour duration to interns rotating on the inpatient wards rotation. This rotation is 4 weeks long, and each intern will experience the rotation two to four times per year. Each lecture contained two parts: (1) 20-30 minutes of didactics via Microsoft PowerPointTM and (2) 30-40 minutes of supervised practice using HUDs on standardized patients. Four lectures were given each month: (1) introduction to POCUS and ultrasound physics, (2) thoracic/lung ultrasound, (3) echocardiography, and (4) abdominal POCUS. The lectures consisted of contrasting cases of normal/abnormal videos and clinical vignettes. These four lectures were repeated each month as new interns rotated on service. Some interns experienced the same content multiple times, which was intentional in order to assess their rates of learning over time. Lecture contents were based on previously published guidelines and expert consensus for teaching POCUS in internal medicine.13, 24-26 Content from the Accreditation Council for Graduate Medical Education (ACGME) and the American College of Emergency Physicians (ACEP) was also incorporated because these organizations had published relevant guidelines for teaching POCUS.13,26 Further development of the lectures occurred through review of previously described POCUS-relevant curricula.27-32
Handheld Ultrasound Devices
This study used the Philips LumifyTM, a United States Food and Drug Administration–approved device. Interns randomized to HUDs received their own device at the start of the rotation. It was at their discretion to use the device outside of the course. All devices were approved for patient use and were encrypted in compliance with our information security office. For privacy reasons, any saved patient images were not reviewed by the researchers. Interns were encouraged to share their findings with supervising physicians during rounds, but actual oversight was not measured. Interns not randomized to HUDs could access a single community device that was shared among all residents and fellows in the hospital. Interns reported the average number of POCUS examinations performed each week via a survey sent during the last week of the rotation.
Assessment Design and Implementation
Assessments evaluating trainee knowledge were administered before, during, and after the study period (Figure). For the 2017 cohort, assessments were also administered at the start and end of the ward month to track knowledge acquisition. Assessment contents were selected from POCUS guidelines for internal medicine and adaptation of the ACGME and ACEP guidelines.13,24,26 Additional content was obtained from major society POCUS tutorials and deidentified images collected by the study authors.13,24,33 In keeping with previously described methodology, the images were shown for approximately 12 seconds, followed by five additional seconds to allow the learner to answer the question.32 Final assessment contents were determined by the authors using the Delphi method.34 A sample assessment can be found in the Appendix Material.
Surveys
Surveys were administered alongside the assessments to the 2016-2018 intern classes. These surveys assessed trainee attitudes toward POCUS and were based on previously validated assessments.27,28,30 Attitudes were measured using 5-point Likert scales.
Statistical Analysis
For the primary outcome, we performed generalized binomial mixed-effect regressions using the survey periods, randomization group, and the interaction of the two as independent variables after adjusting for attendance and controlling of intra-intern correlations. The bivariate unadjusted analysis was performed to display the distribution of overall correctness on the assessments. Wilcoxon signed rank test was used to determine score significance for dependent score variables (R-Statistical Programming Language, Vienna, Austria).
RESULTS
Baseline Characteristics
There were 149 interns who participated in this study (Figure). Assessment/survey completion rates were as follows: 2016 control: 68.0%; 2017 preintervention: 97.9%; 2017 postintervention: 89.4%; and 2018 control: 100%. The 2017 interns reported similar amounts of prior POCUS exposure in medical school (Table 1).
Primary Outcome: Assessment Scores (HUD vs no HUD)
There were no significant differences in assessment scores at the end of the study between interns randomized to personal HUD access vs those to no-HUD access (Table 1). HUD interns reported performing POCUS assessments on patients a mean 6.8 (standard deviation [SD] 2.2) times per week vs 6.4 (SD 2.9) times per week in the no-HUD arm (P = .66). The mean lecture attendance was 75.0% and did not significantly differ between the HUD arms (Table 1).
Secondary Outcomes
Impact of Repeating Lectures
The 2017 interns demonstrated significant increases in preblock vs postblock assessment scores after first-time exposure to the lectures (median preblock score 0.61 [interquartile range (IQR), 0.53-0.70] vs postblock score 0.81 [IQR, 0.72-0.86]; P < .001; Table 2). However, intern performance on the preblock vs postblock assessments after second-time exposure to the curriculum failed to improve (median second preblock score 0.78 [IQR, 0.69-0.83] vs postblock score 0.81 [IQR, 0.64-0.89]; P = .94). Intern performance on individual domains of knowledge for each block is listed in Appendix Table 1.
Intervention Performance vs Controls
The 2016 historical control had significantly higher scores compared with the 2017 preintervention group (P < .001; Appendix Table 2). The year-long lecture series resulted in significant increases in median scores for the 2017 group (median preintervention score 0.55 [0.41-0.61] vs median postintervention score 0.84 [0.71-0.90]; P = .006; Appendix Table 1). At the end of the study, the 2017 postintervention scores were significantly higher across multiple knowledge domains compared with the 2016 historical control (Appendix Table 2).
Survey Results
Notably, the 2017 intern class at the end of the intervention did not have significantly different assessment scores for several disease-specific domains, compared with the 2016 control (Appendix Table 2). Nonetheless, the 2017 intern class reported higher levels of confidence in these same domains despite similar scores (Supplementary Figure). The HUD group seldomly cited a lack of confidence in their abilities as a barrier to performing POCUS examinations (17.6%), compared with the no-HUD group (50.0%), despite nearly identical assessment scores between the two groups (Table 1).
DISCUSSION
Previous guidelines have recommended increased HUD access for learners,13,24,35,36 but there have been few investigations that have evaluated the impact of such access on learning POCUS. One previous investigation found that hospitalists who carried HUDs were more likely to identify heart failure on bedside examination.37 In contrast, our study found no improvement in interpretative ability when randomizing interns to carry HUDs for patient care. Notably, interns did not perform more POCUS examinations when given HUDs. We offer several explanations for this finding. First, time-motion studies have demonstrated that internal medicine interns spend less than 15% of their time toward direct patient care.38 It is possible that the demands of being an intern impeded their ability to perform more POCUS examinations on their patients, regardless of HUD access. Alternatively, the interns randomized to no personal access may have used the community device more frequently as a result of the lecture series. Given the cost of HUDs, further studies are needed to assess the degree to which HUD access will improve trainee interpretive ability, especially as more training programs consider the creation of ultrasound curricula.10,11,24,39,40
This study was unique because it followed interns over a year-long course that repeated the same material to assess rates of learning with repeated exposure. Learners improved their scores after the first, but not second, block. Furthermore, the median scores were nearly identical between the first postblock assessment and second preblock assessment (0.81 vs 0.78), suggesting that knowledge was retained between blocks. Together, these findings suggest there may be limitations of traditional lectures that use standardized patient models for practice. Supplementary pedagogies, such as in-the-moment feedback with actual patients, may be needed to promote mastery.14,35
Despite no formal curriculum, the 2016 intern class (historical control) had learned POCUS to some degree based on their higher assessment scores compared with the 2017 intern class during the preintervention period. Such learning may be informal, and yet, trainees may feel confident in making clinical decisions without formalized training, accreditation, or oversight. As suggested by this study, adding regular didactics or giving trainees HUDs may not immediately solve this issue. For assessment items in which the 2017 interns did not significantly differ from the controls, they nonetheless reported higher confidence in their abilities. Similarly, interns randomized to HUDs less frequently cited a lack of confidence in their abilities, despite similar scores to the no-HUD group. Such confidence may be incongruent with their actual knowledge or ability to safely use POCUS. This phenomenon of misplaced confidence is known as the Dunning–Kruger effect, and it may be common with ultrasound learning.41 While confidence can be part of a holistic definition of competency,14 these results raise the concern that trainees may have difficulty assessing their own competency level with POCUS.35
There are several limitations to this study. It was performed at a single institution with limited sample size. It examined only intern physicians because of funding constraints, which limits the generalizability of these findings among medical trainees. Technical ability assessments (including obtaining and interpreting images) were not included. We were unable to track the timing or location of the devices’ usage, and the interns’ self-reported usage rates may be subject to recall bias. To our knowledge, there were no significant lapses in device availability/functionality. Intern physicians in the HUD arm did not receive formal feedback on personally acquired patient images, which may have limited the intervention’s impact.
In conclusion, internal medicine interns who received personal HUDs were not better at recognizing normal/abnormal findings on image assessments, and they did not report performing more POCUS examinations. Since the minority of a trainee’s time is spent toward direct patient care, offering trainees HUDs without substantial guidance may not be enough to promote mastery. Notably, trainees who received HUDs felt more confident in their abilities, despite no objective increase in their actual skill. Finally, interns who received POCUS-related lectures experienced significant benefit upon first exposure to the material, while repeated exposures did not improve performance. Future investigations should stringently track trainee POCUS usage rates with HUDs and assess whether image acquisition ability improves as a result of personal access.
1. Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
2. Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509-1511. https://doi.org/10.1016/j.ajem.2013.07.006.
3. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. https://doi.org/10.1016/j.echo.2011.07.013.
4. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
5. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: Report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. https://doi.org/10.1186/1477-7819-12-139.
6. Testa A, Francesconi A, Giannuzzi R, Berardi S, Sbraccia P. Economic analysis of bedside ultrasonography (US) implementation in an Internal Medicine department. Intern Emerg Med. 2015;10(8):1015-1024. https://doi.org/10.1007/s11739-015-1320-7.
7. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. https://doi.org/10.1016/j.jemermed.2013.05.044.
8. Park YH, Jung RB, Lee YG, et al. Does the use of bedside ultrasonography reduce emergency department length of stay for patients with renal colic? A pilot study. Clin Exp Emerg Med. 2016;3(4):197-203. https://doi.org/10.15441/ceem.15.109.
9. Glomb N, D’Amico B, Rus M, Chen C. Point-of-care ultrasound in resource-limited settings. Clin Pediatr Emerg Med. 2015;16(4):256-261. https://doi.org/10.1016/j.cpem.2015.10.001.
10. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
11. Hall JWW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: A CERA study. Fam Med. 2015;47(9):706-711.
12. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: A national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. https://doi.org/10.4300/JGME-D-12-00215.1.
13. Stolz LA, Stolz U, Fields JM, et al. Emergency medicine resident assessment of the emergency ultrasound milestones and current training recommendations. Acad Emerg Med. 2017;24(3):353-361. https://doi.org/10.1111/acem.13113.
14. Kumar, A., Jensen, T., Kugler, J. Evaluation of trainee competency with point-of-care ultrasonography (POCUS): A conceptual framework and review of existing assessments. J Gen Intern Med. 2019;34(6):1025-1031. https://doi.org/10.1007/s11606-019-04945-4.
15. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part ii: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. https://doi.org/10.1097/CCM.0000000000001847.
16. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96(7):1002-1006. https://doi.org/10.1016/j.amjcard.2005.05.060.
17. Ceriani E, Cogliati C. Update on bedside ultrasound diagnosis of pericardial effusion. Intern Emerg Med. 2016;11(3):477-480. https://doi.org/10.1007/s11739-015-1372-8.
18. Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225-1230. https://doi.org/10.1016/j.echo.2010.10.005.
19. Keil-Ríos D, Terrazas-Solís H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. https://doi.org/10.1007/s11739-016-1406-x.
20. Riddell J, Case A, Wopat R, et al. Sensitivity of emergency bedside ultrasound to detect hydronephrosis in patients with computed tomography–proven stones. West J Emerg Med. 2014;15(1):96-100. https://doi.org/10.5811/westjem.2013.9.15874.
21. Dalziel PJ, Noble VE. Bedside ultrasound and the assessment of renal colic: A review. Emerg Med J. 2013;30(1):3-8. https://doi.org/10.1136/emermed-2012-201375.
22. Whitson MR, Mayo PH. Ultrasonography in the emergency department. Crit Care. 2016;20(1):227. https://doi.org/10.1186/s13054-016-1399-x.
23. Kumar A, Liu G, Chi J, Kugler J. The role of technology in the bedside encounter. Med Clin North Am. 2018;102(3):443-451. https://doi.org/10.1016/j.mcna.2017.12.006.
24. Ma IWY, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: Consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. https://doi.org/10.1007/s11606-017-4071-5.
15. Sabath BF, Singh G. Point-of-care ultrasonography as a training milestone for internal medicine residents: The time is now. J Community Hosp Intern Med Perspect. 2016;6(5):33094. https://doi.org/10.3402/jchimp.v6.33094.
26. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
27. Ramsingh D, Rinehart J, Kain Z, et al. Impact assessment of perioperative point-of-care ultrasound training on anesthesiology residents. Anesthesiology. 2015;123(3):670-682. https://doi.org/10.1097/ALN.0000000000000776.
28. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. https://doi.org/10.1186/1472-6920-11-75.
29. Townsend NT, Kendall J, Barnett C, Robinson T. An effective curriculum for focused assessment diagnostic echocardiography: Establishing the learning curve in surgical residents. J Surg Educ. 2016;73(2):190-196. https://doi.org/10.1016/j.jsurg.2015.10.009.
30. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7(1):18. https://doi.org/10.1186/s13089-015-0035-3.
31. Skalski JH, Elrashidi M, Reed DA, McDonald FS, Bhagra A. Using standardized patients to teach point-of-care ultrasound–guided physical examination skills to internal medicine residents. J Grad Med Educ. 2015;7(1):95-97. https://doi.org/10.4300/JGME-D-14-00178.1.
32. Chisholm CB, Dodge WR, Balise RR, Williams SR, Gharahbaghian L, Beraud A-S. Focused cardiac ultrasound training: How much is enough? J Emerg Med. 2013;44(4):818-822. https://doi.org/10.1016/j.jemermed.2012.07.092.
33. Schmidt GA, Schraufnagel D. Introduction to ATS seminars: Intensive care ultrasound. Ann Am Thorac Soc. 2013;10(5):538-539. https://doi.org/10.1513/AnnalsATS.201306-203ED.
34. Skaarup SH, Laursen CB, Bjerrum AS, Hilberg O. Objective and structured assessment of lung ultrasound competence. A multispecialty Delphi consensus and construct validity study. Ann Am Thorac Soc. 2017;14(4):555-560. https://doi.org/10.1513/AnnalsATS.201611-894OC.
35. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
36. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part i: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
37. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed by hospitalists: Does it improve the cardiac physical examination? Am J Med. 2009;122(1):35-41. https://doi.org/10.1016/j.amjmed.2008.07.022.
38. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018;378(16):1494-1508. https://doi.org/10.1056/NEJMoa1800965.
39. Baltarowich OH, Di Salvo DN, Scoutt LM, et al. National ultrasound curriculum for medical students. Ultrasound Q. 2014;30(1):13-19. https://doi.org/10.1097/RUQ.0000000000000066.
40. Beal EW, Sigmond BR, Sage-Silski L, Lahey S, Nguyen V, Bahner DP. Point-of-care ultrasound in general surgery residency training: A proposal for milestones in graduate medical education ultrasound. J Ultrasound Med. 2017;36(12):2577-2584. https://doi.org/10.1002/jum.14298.
41. Kruger J, Dunning D. Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Pers Soc Psychol. 1999;77(6):1121-1134. https://doi.org/10.1037//0022-3514.77.6.1121.
1. Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
2. Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509-1511. https://doi.org/10.1016/j.ajem.2013.07.006.
3. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. https://doi.org/10.1016/j.echo.2011.07.013.
4. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
5. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: Report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. https://doi.org/10.1186/1477-7819-12-139.
6. Testa A, Francesconi A, Giannuzzi R, Berardi S, Sbraccia P. Economic analysis of bedside ultrasonography (US) implementation in an Internal Medicine department. Intern Emerg Med. 2015;10(8):1015-1024. https://doi.org/10.1007/s11739-015-1320-7.
7. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. https://doi.org/10.1016/j.jemermed.2013.05.044.
8. Park YH, Jung RB, Lee YG, et al. Does the use of bedside ultrasonography reduce emergency department length of stay for patients with renal colic? A pilot study. Clin Exp Emerg Med. 2016;3(4):197-203. https://doi.org/10.15441/ceem.15.109.
9. Glomb N, D’Amico B, Rus M, Chen C. Point-of-care ultrasound in resource-limited settings. Clin Pediatr Emerg Med. 2015;16(4):256-261. https://doi.org/10.1016/j.cpem.2015.10.001.
10. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
11. Hall JWW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: A CERA study. Fam Med. 2015;47(9):706-711.
12. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: A national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. https://doi.org/10.4300/JGME-D-12-00215.1.
13. Stolz LA, Stolz U, Fields JM, et al. Emergency medicine resident assessment of the emergency ultrasound milestones and current training recommendations. Acad Emerg Med. 2017;24(3):353-361. https://doi.org/10.1111/acem.13113.
14. Kumar, A., Jensen, T., Kugler, J. Evaluation of trainee competency with point-of-care ultrasonography (POCUS): A conceptual framework and review of existing assessments. J Gen Intern Med. 2019;34(6):1025-1031. https://doi.org/10.1007/s11606-019-04945-4.
15. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part ii: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. https://doi.org/10.1097/CCM.0000000000001847.
16. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96(7):1002-1006. https://doi.org/10.1016/j.amjcard.2005.05.060.
17. Ceriani E, Cogliati C. Update on bedside ultrasound diagnosis of pericardial effusion. Intern Emerg Med. 2016;11(3):477-480. https://doi.org/10.1007/s11739-015-1372-8.
18. Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225-1230. https://doi.org/10.1016/j.echo.2010.10.005.
19. Keil-Ríos D, Terrazas-Solís H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. https://doi.org/10.1007/s11739-016-1406-x.
20. Riddell J, Case A, Wopat R, et al. Sensitivity of emergency bedside ultrasound to detect hydronephrosis in patients with computed tomography–proven stones. West J Emerg Med. 2014;15(1):96-100. https://doi.org/10.5811/westjem.2013.9.15874.
21. Dalziel PJ, Noble VE. Bedside ultrasound and the assessment of renal colic: A review. Emerg Med J. 2013;30(1):3-8. https://doi.org/10.1136/emermed-2012-201375.
22. Whitson MR, Mayo PH. Ultrasonography in the emergency department. Crit Care. 2016;20(1):227. https://doi.org/10.1186/s13054-016-1399-x.
23. Kumar A, Liu G, Chi J, Kugler J. The role of technology in the bedside encounter. Med Clin North Am. 2018;102(3):443-451. https://doi.org/10.1016/j.mcna.2017.12.006.
24. Ma IWY, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: Consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. https://doi.org/10.1007/s11606-017-4071-5.
15. Sabath BF, Singh G. Point-of-care ultrasonography as a training milestone for internal medicine residents: The time is now. J Community Hosp Intern Med Perspect. 2016;6(5):33094. https://doi.org/10.3402/jchimp.v6.33094.
26. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
27. Ramsingh D, Rinehart J, Kain Z, et al. Impact assessment of perioperative point-of-care ultrasound training on anesthesiology residents. Anesthesiology. 2015;123(3):670-682. https://doi.org/10.1097/ALN.0000000000000776.
28. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. https://doi.org/10.1186/1472-6920-11-75.
29. Townsend NT, Kendall J, Barnett C, Robinson T. An effective curriculum for focused assessment diagnostic echocardiography: Establishing the learning curve in surgical residents. J Surg Educ. 2016;73(2):190-196. https://doi.org/10.1016/j.jsurg.2015.10.009.
30. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7(1):18. https://doi.org/10.1186/s13089-015-0035-3.
31. Skalski JH, Elrashidi M, Reed DA, McDonald FS, Bhagra A. Using standardized patients to teach point-of-care ultrasound–guided physical examination skills to internal medicine residents. J Grad Med Educ. 2015;7(1):95-97. https://doi.org/10.4300/JGME-D-14-00178.1.
32. Chisholm CB, Dodge WR, Balise RR, Williams SR, Gharahbaghian L, Beraud A-S. Focused cardiac ultrasound training: How much is enough? J Emerg Med. 2013;44(4):818-822. https://doi.org/10.1016/j.jemermed.2012.07.092.
33. Schmidt GA, Schraufnagel D. Introduction to ATS seminars: Intensive care ultrasound. Ann Am Thorac Soc. 2013;10(5):538-539. https://doi.org/10.1513/AnnalsATS.201306-203ED.
34. Skaarup SH, Laursen CB, Bjerrum AS, Hilberg O. Objective and structured assessment of lung ultrasound competence. A multispecialty Delphi consensus and construct validity study. Ann Am Thorac Soc. 2017;14(4):555-560. https://doi.org/10.1513/AnnalsATS.201611-894OC.
35. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
36. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part i: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
37. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed by hospitalists: Does it improve the cardiac physical examination? Am J Med. 2009;122(1):35-41. https://doi.org/10.1016/j.amjmed.2008.07.022.
38. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018;378(16):1494-1508. https://doi.org/10.1056/NEJMoa1800965.
39. Baltarowich OH, Di Salvo DN, Scoutt LM, et al. National ultrasound curriculum for medical students. Ultrasound Q. 2014;30(1):13-19. https://doi.org/10.1097/RUQ.0000000000000066.
40. Beal EW, Sigmond BR, Sage-Silski L, Lahey S, Nguyen V, Bahner DP. Point-of-care ultrasound in general surgery residency training: A proposal for milestones in graduate medical education ultrasound. J Ultrasound Med. 2017;36(12):2577-2584. https://doi.org/10.1002/jum.14298.
41. Kruger J, Dunning D. Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Pers Soc Psychol. 1999;77(6):1121-1134. https://doi.org/10.1037//0022-3514.77.6.1121.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Card Flipping Rounds

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 32-year-old man with a history of polysubstance use disorder is hospitalized with endocarditis. The senior resident on the inpatient medical team suggests that the team “card flip” on this patient, citing a large number of patients on the team census, time constraints, and concerns that his substance use history will make bedside rounds uncomfortable.
BACKGROUND
“Rounds” is an inpatient care model in which teams of practitioners assess patients, determine care plans, and communicate with patients, families, and other healthcare professionals.1 One form of rounds is bedside rounding (BSR) through which an entire patient presentation occurs at the bedside, analogous to family-centered rounds common in pediatric inpatient care.2 This style of rounding is distinct from “walk rounding” that involves presentations occurring separately from a patient followed by a brief team bedside encounter. BSR is also different from “card flipping” or “table rounding” that involves presentations of a case separately without a team-patient encounter. The frequency of BSR at academic institutions has markedly decreased across the United States, and the time spent at the bedside is only a small fraction of rounding time.3
WHY YOU MIGHT THINK CARD FLIPPING IS HELPFUL
There are several reasons to employ strategies such as card-flipping or walk-rounding for discussing patient care away from the bedside. These BSR risks can be organized into patient harm, inefficiency, and risks to healthcare professional training.
First, BSR may result in patient harm. For example, discussing private health information in a semiprivate room may not only be uncomfortable for patients but may also violate patient privacy.4 Care teams are often large in number and rounding at the bedside can simultaneously trigger anxiety among patients, cause confusion about plans, or result in lack of clarity on the role of each provider.4 Furthermore, delivering bad news during BSR, or discussing sensitive topics such as substance use, psychiatric illness, or concerns of malingering behavior, may be difficult and uncomfortable.4,5 Additionally, some potential diagnoses, such as cancer or human immunodeficiency virus, even if unlikely, could induce panic among patients when they hear them being discussed.5 Trainees may also lose situational awareness because they focus on the agenda of bedside rounds and fail to respond to patients’ emotional needs.6
Efficiency is another reason to avoid BSR. The systemic factors of changing hospital demographics, such as short length of stay and increasing patient volumes, generate a substantial administrative burden on trainees.7 Modern trainees are also constrained by work hour restrictions, engagement with mandatory curricula, and other professional development opportunities. Furthermore, changes in a medical work environment cause trainees to rely heavily on electronic health records, which forces them to be at a computer instead of in a patient’s room.8 This confluence of factors results in substantial time pressure, and BSR is perceived as an inefficient use of time.9
The impact on education and trainee development is another concern of BSR. Rounding away from a patient ensures a safe environment for learners to interpret data and articulate clinical reasoning without the risk of embarrassment in front of a patient. This time outside a patient room also allows the team to have a shared mental model so that communication is aligned when a patient encounter does occur. Card flipping may result in improved trainee autonomy because the constant presence of attending supervision, particularly in front of patients, can risk undermining resident leadership and patient trust.9
WHY WE SHOULD RETURN TO THE BEDSIDE
The cited reasons for provider hesitancy to BSR, including possible patient harm and inefficiency, may be mostly related to individual perceptions and have recently been questioned.10,11
Several studies have suggested that bedside rounds may be better for patients’ experience over traditional walk-rounding or card-flipping models. In these studies, patients signal a preference for bedside rounds and suggest that discussing sensitive issues or concerning differential diagnoses during BSR may not be as concerning as physicians worry.11 For example, one randomized trial found that 87% of patients are untroubled by bedside discussions,12 and another trial revealed no difference between rounding models in emotional distress to patients or families.11 Patients and families also report higher levels of clarity from physicians, and they cited significantly improved levels of understanding their illness10 and test results.9 Furthermore, patients describe that physicians spend about twice as much time on their care when BSR is used.12 In many related studies, patients report a preference for BSR as a rounding strategy.2,11-13 For example, one study found that 99% of patients prefer BSR.13 Another study showed that 85% of families request to be part of bedside family-centered rounds over traditional walk rounding.2
Rounding away from a patient via card flipping or walk rounding seems more efficient, but this idea may be illusory. Although these strategies may seem faster, the lack of communication and coordination between team members and the patient may cause inefficiencies and delays in care throughout the day.14 For example, one study has demonstrated that family-centered bedside rounds are about 20% longer than walk rounding, but everyone involved, including housestaff, felt it was more efficient and saved time later in the day.2 Additionally, a study comparing BSR with walk rounding13 found no difference in time spent per patient, and another study has shown similar results in terms of family-centered rounds.15 Both studies have reported a similar amount of time spent per patient.
Physicians should return to BSR not only to improve patient experience but also to develop the clinical skills of trainees. The direct observation of trainees with patients allows high-level impactful clinical feedback and provides a basis for calibrating how much autonomy to allow.16 Trainees also indicate that teaching is more impactful during BSR than during walk rounding or card flipping, and clinical skill training during BSR is superior to a discussion in a conference room or a hallway context.2,3,15,17,18 One study has even suggested that the education of bedside rounds may help improve clinical skills in comparison with traditional models.18
The lack of BSR during medical school and residency training results in a deleterious cycle. Trainees become less proficient and less comfortable with BSR skills and therefore graduate as faculty members who are unskilled or uncomfortable insisting on BSR. As such, the cycle continues. As a result and as the traditional cornerstone of clinical training and inpatient care, BSR is recommended as standard practice by some professional organizations.19
WHAT WE SHOULD DO INSTEAD
Developing buy-in is an important first step for engaging in BSR. We recommend starting by demonstrating the value of BSR to overcome initial team or trainee hesitancy. Regardless of systems established to improve the efficiency of BSR, it is our experience that learners hesitantly engage if they do not understand the value of a given activity. We also urge attendings to demonstrate value by articulating how BSR fits in a patient-centered approach to emphasize the evidence-based positive impacts of BSR on patients.9 Beyond reviewing the benefits, faculty should set an expectation that the team will carry out BSR.9 Doing so sets an informal curriculum showing that BSR is important and sets the standard of care, which allows an inpatient team to adapt early in a rotation.
Next, faculty should ensure that BSR remains efficient.9 We believe that efficiency starts by setting expectations with patients. Patient expectations can be set by an attending or a supervising resident and should include a preview about how each encounter will progress, who will be in the room, how large the team will be, and what their role is during the encounter. Patients should be invited to be part of the discussion, offered an opportunity to opt out, and informed that questions arising from or clarifications needed following encounters can be addressed later within the day or after BSR. Nurses should be invited to actively participate during patient presentations. Each bedside encounter should be kept brief and standardized.20,21 To maximize efficiency, we also believe that roles should be delegated ahead of time and positioning in the room should be deliberate.22 Team members should know who is speaking when and in what order, who is accessing the electronic health record, and who will be examining the patient. Ideally, goals should be set ahead of time and tailored to each individual encounter. Finally, ensure everyone is on the same page by huddling briefly before each encounter to establish goals and roles and huddle afterward to debrief for learning and teamwork calibration.
In order to mitigate the learner’s anxiety about presenting in front of patients, build a partnership with the trainee, and time should be allotted to establish a safe learning environment.9 Sustain a supportive learning environment by providing positive feedback to learners in front of patients and teams. Faculty members should demonstrate how to bedside round effectively by leading initial encounters and generate momentum by selecting initial patient encounters that are most likely to succeed.23 Checklists can also be useful cognitive aids to facilitate an encounter and manage the cognitive load of learners.24 Ultimately, hesitancies can be overcome with experience.
Faculty members should ensure that bedside encounters are educationally valuable for an entire team.9 This initiative starts by preparing ahead of time, which allows the mental energy during encounters to be directly observed by learners in action.16 Preparation also allows the presentation to focus more on clinical reasoning rather than data gathering.20 Faculty members should also consider ways to foster resident autonomy and establish the role of a supervising resident as the team leader. Positioning in the room is critical22; we suggest that faculty members should position themselves near the head of the bed, out of a patient’s direct eyesight. In this way, they can observe how individual team members and the team as a whole interact with patients. The supervising resident should be at the foot of the bed, central to the team and the focal point of a patient’s view. The presenting intern or student should be seated near the head of the bed and opposite the supervising attending. Clinical teaching should also be kept short and pertinent to the patient, and questions should be phrased as “how” or “why” rather than “what” to reduce the risk of “wrong” answers in front of patients and the team.
WHEN IS CARD FLIPPING APPROPRIATE?
We believe that bedside rounds are most consistent with patient-centered inpatient care and should be considered the first-line approach. We also acknowledge that it is not always possible to bedside round on every patient on an inpatient census. For example, at an average of 13-15 minutes per patient,2,13 a census of 16 patients can take up to 4 hours to round. This timeline is not always feasible given the timing of training program didactics, interprofessional or case management rounds, and pressure for early discharges. Similar to all aspects of medicine, many approaches have been established to provide patient care, and context is important. Therefore, card flipping and walk rounding are beneficial to patients in some instances. For example, consider BSR for new, sick, or undifferentiated patients or when the history or exam findings need clarification; walk rounding or card flipping is suitable for patients with clear plans in place or when an encounter will be too disruptive to the rounding flow.21 Census size and individual patient or family concerns should dictate the style of rounding; in most situations, BSR may be equally efficient because it offers significant benefits to patients and families.
RECOMMENDATIONS
- Expectations should be set early with both trainees and patients. Patients should be informed that the team can come back later for more in-depth discussions.
- Trainees should be taught evidence-based approaches supporting the value of bedside rounds for patients.
- Faculty should consider leading initial encounters to demonstrate how to bedside round and to model behaviors.
- Positive feedback should be provided in front of patients and the team to build confidence.
- Encounters should be kept brief and efficient.
- A sufficient space for resident autonomy should be ensured through deliberate positioning, delegation of responsibilities, and huddling before and after encounters.
- Bedside rounds should be educationally worthwhile.
CONCLUSION
BSR is a traditional cornerstone of clinical training and inpatient care. Teaching at the bedside has many established benefits, such as connecting with patients and families, affording educators a valuable opportunity to assess learners and role model, and solidifying medical content by integrating teaching with clinical care. Concerns about bedside rounding may be based more on conjecture than on available evidence and can be overcome with deliberate education and proper planning. We propose several recommendations to successfully implement efficient, patient-centered, and educationally valuable bedside rounds.
For this (and most) patient(s), we recommend BSR. If this BSR is the first encounter, we suggest that the team should start with a more straightforward patient and come back to the new admission after the team has a chance to practice with other patients.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
2. Muething SE, Kotagal UR, Schoettker PJ, del Rey JG, DeWitt TG. Family-centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119(4):829-832. https://doi.org/10.1542/peds.2006-2528.
3. Ngo TL, Blankenburg R, Yu CE. Teaching at the bedside: strategies for optimizing education on patient and family centered rounds. Pediatr Clin North Am. 2019;66(4):881-889. https://doi.org/10.1016/j.pcl.2019.03.012.
4. Berkwitt A, Grossman M. A Qualitative analysis of pediatric patient attitudes regarding family-centered rounds. Hosp Pediatr. 2015;5(7):357. https://doi.org/10.1542/hpeds.2014-0198.
5. Rabinowitz R, Farnan J, Hulland O, et al. Rounds today: a qualitative study of internal medicine and pediatrics resident perceptions. J Grad Med Educ. 2016;8(4):523-531. https://doi.org/10.4300/JGME-D-15-00106.1.
6. Pingree EW, Freed JA, Riviello ED, et al. A tale of two rounds: managing conflict during the worst of times in family-centered rounds. Hosp Pediatr. 2019;9(7):563-565. https://doi.org/10.1542/hpeds.2019-0047.
7. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148.
8. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
9. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326-334. https://doi.org/10.1097/ACM.0000000000000100.
10. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. https://doi.org/10.1097/00001888-200309000-00023.
11. Landry M-A, Lafrenaye S, Roy M-C, Cyr C. A randomized, controlled trial of bedside versus conference-room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275-280. https://doi.org/10.1542/peds.2007-0107.
12. Lehmann LS, Brancati FL, Chen M-C, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1156. https://doi.org/10.1056/NEJM199704173361606.
13. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. https://doi.org/10.1007/s11606-010-1344-7.
14. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. https://doi.org/10.2147/JMDH.S72633.
15. Kelly MM, Xie A, Li Y, et al. System factors influencing the use of a family-centered rounds checklist. Pediatr Qual Saf. 2019;4(4):e196. https://doi.org/10.1097/pq9.0000000000000196.
16. Kogan JR, Hatala R, Hauer KE, Holmboe E. Guidelines: The do’s, don’ts and don’t knows of direct observation of clinical skills in medical education. Perspect Med Educ. 2017;6(5):286-305. https://doi.org/10.1007/s40037-017-0376-7.
17. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. https://doi.org/10.1097/ACM.0b013e3181637f3e.
18. Heckmann JG, Bleh C, Dütsch M, Lang CJG, Neundörfer B. Does improved problem-based teaching influence students’ knowledge at the end of their neurology elective? An observational study of 40 students. J Neurol. 2003;250(12):1464-1468. https://doi.org/10.1007/s00415-003-0255-5.
19. Committee on hospital care and institute for patient and family centered care. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
20. Dhaliwal G, Hauer KE. The oral patient presentation in the era of night float admissions. JAMA. 2013;310(21):2247. https://doi.org/10.1001/jama.2013.282322.
21. Wiese JG. Teaching in the Hospital. Philadelphia, PA: ACP PRess; 2010. https://books.google.co.uk/books?hl=en&lr=&id=qquGWP4d2Q4C&oi=fnd&pg=PR13&dq=Wiese+J.+2010.+ACP+Teaching+Medicine+Series:+Teaching+in+the+Hospital.+Philadelphia,+PA:+ACP+Press&ots=JSRFojkBSn&sig=c33tapsL9DzV9nuFhENA6eObISA#v=onepage&q=bedside round&f=fals. Accessed November 29, 2019.
22. Lopez M, Vaks Y, Wilson M, et al. Impacting satisfaction, learning, and efficiency through structured interdisciplinary rounding in a pediatric intensive care unit. Pediatr Qual Saf. 2019;4(3):e176. https://doi.org/10.1097/pq9.0000000000000176.
23. Benbassat J. Role modeling in medical education: the importance of a reflective imitation. Acad Med. 2014;89(4):550-554. https://doi.org/10.1097/ACM.0000000000000189.
24. Cox ED, Jacobsohn GC, Rajamanickam VP, et al. A family-centered rounds checklist, family engagement, and patient safety: a randomized trial. Pediatrics. 2017;139(5):e20161688. https://doi.org/10.1542/peds.2016-1688.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 32-year-old man with a history of polysubstance use disorder is hospitalized with endocarditis. The senior resident on the inpatient medical team suggests that the team “card flip” on this patient, citing a large number of patients on the team census, time constraints, and concerns that his substance use history will make bedside rounds uncomfortable.
BACKGROUND
“Rounds” is an inpatient care model in which teams of practitioners assess patients, determine care plans, and communicate with patients, families, and other healthcare professionals.1 One form of rounds is bedside rounding (BSR) through which an entire patient presentation occurs at the bedside, analogous to family-centered rounds common in pediatric inpatient care.2 This style of rounding is distinct from “walk rounding” that involves presentations occurring separately from a patient followed by a brief team bedside encounter. BSR is also different from “card flipping” or “table rounding” that involves presentations of a case separately without a team-patient encounter. The frequency of BSR at academic institutions has markedly decreased across the United States, and the time spent at the bedside is only a small fraction of rounding time.3
WHY YOU MIGHT THINK CARD FLIPPING IS HELPFUL
There are several reasons to employ strategies such as card-flipping or walk-rounding for discussing patient care away from the bedside. These BSR risks can be organized into patient harm, inefficiency, and risks to healthcare professional training.
First, BSR may result in patient harm. For example, discussing private health information in a semiprivate room may not only be uncomfortable for patients but may also violate patient privacy.4 Care teams are often large in number and rounding at the bedside can simultaneously trigger anxiety among patients, cause confusion about plans, or result in lack of clarity on the role of each provider.4 Furthermore, delivering bad news during BSR, or discussing sensitive topics such as substance use, psychiatric illness, or concerns of malingering behavior, may be difficult and uncomfortable.4,5 Additionally, some potential diagnoses, such as cancer or human immunodeficiency virus, even if unlikely, could induce panic among patients when they hear them being discussed.5 Trainees may also lose situational awareness because they focus on the agenda of bedside rounds and fail to respond to patients’ emotional needs.6
Efficiency is another reason to avoid BSR. The systemic factors of changing hospital demographics, such as short length of stay and increasing patient volumes, generate a substantial administrative burden on trainees.7 Modern trainees are also constrained by work hour restrictions, engagement with mandatory curricula, and other professional development opportunities. Furthermore, changes in a medical work environment cause trainees to rely heavily on electronic health records, which forces them to be at a computer instead of in a patient’s room.8 This confluence of factors results in substantial time pressure, and BSR is perceived as an inefficient use of time.9
The impact on education and trainee development is another concern of BSR. Rounding away from a patient ensures a safe environment for learners to interpret data and articulate clinical reasoning without the risk of embarrassment in front of a patient. This time outside a patient room also allows the team to have a shared mental model so that communication is aligned when a patient encounter does occur. Card flipping may result in improved trainee autonomy because the constant presence of attending supervision, particularly in front of patients, can risk undermining resident leadership and patient trust.9
WHY WE SHOULD RETURN TO THE BEDSIDE
The cited reasons for provider hesitancy to BSR, including possible patient harm and inefficiency, may be mostly related to individual perceptions and have recently been questioned.10,11
Several studies have suggested that bedside rounds may be better for patients’ experience over traditional walk-rounding or card-flipping models. In these studies, patients signal a preference for bedside rounds and suggest that discussing sensitive issues or concerning differential diagnoses during BSR may not be as concerning as physicians worry.11 For example, one randomized trial found that 87% of patients are untroubled by bedside discussions,12 and another trial revealed no difference between rounding models in emotional distress to patients or families.11 Patients and families also report higher levels of clarity from physicians, and they cited significantly improved levels of understanding their illness10 and test results.9 Furthermore, patients describe that physicians spend about twice as much time on their care when BSR is used.12 In many related studies, patients report a preference for BSR as a rounding strategy.2,11-13 For example, one study found that 99% of patients prefer BSR.13 Another study showed that 85% of families request to be part of bedside family-centered rounds over traditional walk rounding.2
Rounding away from a patient via card flipping or walk rounding seems more efficient, but this idea may be illusory. Although these strategies may seem faster, the lack of communication and coordination between team members and the patient may cause inefficiencies and delays in care throughout the day.14 For example, one study has demonstrated that family-centered bedside rounds are about 20% longer than walk rounding, but everyone involved, including housestaff, felt it was more efficient and saved time later in the day.2 Additionally, a study comparing BSR with walk rounding13 found no difference in time spent per patient, and another study has shown similar results in terms of family-centered rounds.15 Both studies have reported a similar amount of time spent per patient.
Physicians should return to BSR not only to improve patient experience but also to develop the clinical skills of trainees. The direct observation of trainees with patients allows high-level impactful clinical feedback and provides a basis for calibrating how much autonomy to allow.16 Trainees also indicate that teaching is more impactful during BSR than during walk rounding or card flipping, and clinical skill training during BSR is superior to a discussion in a conference room or a hallway context.2,3,15,17,18 One study has even suggested that the education of bedside rounds may help improve clinical skills in comparison with traditional models.18
The lack of BSR during medical school and residency training results in a deleterious cycle. Trainees become less proficient and less comfortable with BSR skills and therefore graduate as faculty members who are unskilled or uncomfortable insisting on BSR. As such, the cycle continues. As a result and as the traditional cornerstone of clinical training and inpatient care, BSR is recommended as standard practice by some professional organizations.19
WHAT WE SHOULD DO INSTEAD
Developing buy-in is an important first step for engaging in BSR. We recommend starting by demonstrating the value of BSR to overcome initial team or trainee hesitancy. Regardless of systems established to improve the efficiency of BSR, it is our experience that learners hesitantly engage if they do not understand the value of a given activity. We also urge attendings to demonstrate value by articulating how BSR fits in a patient-centered approach to emphasize the evidence-based positive impacts of BSR on patients.9 Beyond reviewing the benefits, faculty should set an expectation that the team will carry out BSR.9 Doing so sets an informal curriculum showing that BSR is important and sets the standard of care, which allows an inpatient team to adapt early in a rotation.
Next, faculty should ensure that BSR remains efficient.9 We believe that efficiency starts by setting expectations with patients. Patient expectations can be set by an attending or a supervising resident and should include a preview about how each encounter will progress, who will be in the room, how large the team will be, and what their role is during the encounter. Patients should be invited to be part of the discussion, offered an opportunity to opt out, and informed that questions arising from or clarifications needed following encounters can be addressed later within the day or after BSR. Nurses should be invited to actively participate during patient presentations. Each bedside encounter should be kept brief and standardized.20,21 To maximize efficiency, we also believe that roles should be delegated ahead of time and positioning in the room should be deliberate.22 Team members should know who is speaking when and in what order, who is accessing the electronic health record, and who will be examining the patient. Ideally, goals should be set ahead of time and tailored to each individual encounter. Finally, ensure everyone is on the same page by huddling briefly before each encounter to establish goals and roles and huddle afterward to debrief for learning and teamwork calibration.
In order to mitigate the learner’s anxiety about presenting in front of patients, build a partnership with the trainee, and time should be allotted to establish a safe learning environment.9 Sustain a supportive learning environment by providing positive feedback to learners in front of patients and teams. Faculty members should demonstrate how to bedside round effectively by leading initial encounters and generate momentum by selecting initial patient encounters that are most likely to succeed.23 Checklists can also be useful cognitive aids to facilitate an encounter and manage the cognitive load of learners.24 Ultimately, hesitancies can be overcome with experience.
Faculty members should ensure that bedside encounters are educationally valuable for an entire team.9 This initiative starts by preparing ahead of time, which allows the mental energy during encounters to be directly observed by learners in action.16 Preparation also allows the presentation to focus more on clinical reasoning rather than data gathering.20 Faculty members should also consider ways to foster resident autonomy and establish the role of a supervising resident as the team leader. Positioning in the room is critical22; we suggest that faculty members should position themselves near the head of the bed, out of a patient’s direct eyesight. In this way, they can observe how individual team members and the team as a whole interact with patients. The supervising resident should be at the foot of the bed, central to the team and the focal point of a patient’s view. The presenting intern or student should be seated near the head of the bed and opposite the supervising attending. Clinical teaching should also be kept short and pertinent to the patient, and questions should be phrased as “how” or “why” rather than “what” to reduce the risk of “wrong” answers in front of patients and the team.
WHEN IS CARD FLIPPING APPROPRIATE?
We believe that bedside rounds are most consistent with patient-centered inpatient care and should be considered the first-line approach. We also acknowledge that it is not always possible to bedside round on every patient on an inpatient census. For example, at an average of 13-15 minutes per patient,2,13 a census of 16 patients can take up to 4 hours to round. This timeline is not always feasible given the timing of training program didactics, interprofessional or case management rounds, and pressure for early discharges. Similar to all aspects of medicine, many approaches have been established to provide patient care, and context is important. Therefore, card flipping and walk rounding are beneficial to patients in some instances. For example, consider BSR for new, sick, or undifferentiated patients or when the history or exam findings need clarification; walk rounding or card flipping is suitable for patients with clear plans in place or when an encounter will be too disruptive to the rounding flow.21 Census size and individual patient or family concerns should dictate the style of rounding; in most situations, BSR may be equally efficient because it offers significant benefits to patients and families.
RECOMMENDATIONS
- Expectations should be set early with both trainees and patients. Patients should be informed that the team can come back later for more in-depth discussions.
- Trainees should be taught evidence-based approaches supporting the value of bedside rounds for patients.
- Faculty should consider leading initial encounters to demonstrate how to bedside round and to model behaviors.
- Positive feedback should be provided in front of patients and the team to build confidence.
- Encounters should be kept brief and efficient.
- A sufficient space for resident autonomy should be ensured through deliberate positioning, delegation of responsibilities, and huddling before and after encounters.
- Bedside rounds should be educationally worthwhile.
CONCLUSION
BSR is a traditional cornerstone of clinical training and inpatient care. Teaching at the bedside has many established benefits, such as connecting with patients and families, affording educators a valuable opportunity to assess learners and role model, and solidifying medical content by integrating teaching with clinical care. Concerns about bedside rounding may be based more on conjecture than on available evidence and can be overcome with deliberate education and proper planning. We propose several recommendations to successfully implement efficient, patient-centered, and educationally valuable bedside rounds.
For this (and most) patient(s), we recommend BSR. If this BSR is the first encounter, we suggest that the team should start with a more straightforward patient and come back to the new admission after the team has a chance to practice with other patients.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 32-year-old man with a history of polysubstance use disorder is hospitalized with endocarditis. The senior resident on the inpatient medical team suggests that the team “card flip” on this patient, citing a large number of patients on the team census, time constraints, and concerns that his substance use history will make bedside rounds uncomfortable.
BACKGROUND
“Rounds” is an inpatient care model in which teams of practitioners assess patients, determine care plans, and communicate with patients, families, and other healthcare professionals.1 One form of rounds is bedside rounding (BSR) through which an entire patient presentation occurs at the bedside, analogous to family-centered rounds common in pediatric inpatient care.2 This style of rounding is distinct from “walk rounding” that involves presentations occurring separately from a patient followed by a brief team bedside encounter. BSR is also different from “card flipping” or “table rounding” that involves presentations of a case separately without a team-patient encounter. The frequency of BSR at academic institutions has markedly decreased across the United States, and the time spent at the bedside is only a small fraction of rounding time.3
WHY YOU MIGHT THINK CARD FLIPPING IS HELPFUL
There are several reasons to employ strategies such as card-flipping or walk-rounding for discussing patient care away from the bedside. These BSR risks can be organized into patient harm, inefficiency, and risks to healthcare professional training.
First, BSR may result in patient harm. For example, discussing private health information in a semiprivate room may not only be uncomfortable for patients but may also violate patient privacy.4 Care teams are often large in number and rounding at the bedside can simultaneously trigger anxiety among patients, cause confusion about plans, or result in lack of clarity on the role of each provider.4 Furthermore, delivering bad news during BSR, or discussing sensitive topics such as substance use, psychiatric illness, or concerns of malingering behavior, may be difficult and uncomfortable.4,5 Additionally, some potential diagnoses, such as cancer or human immunodeficiency virus, even if unlikely, could induce panic among patients when they hear them being discussed.5 Trainees may also lose situational awareness because they focus on the agenda of bedside rounds and fail to respond to patients’ emotional needs.6
Efficiency is another reason to avoid BSR. The systemic factors of changing hospital demographics, such as short length of stay and increasing patient volumes, generate a substantial administrative burden on trainees.7 Modern trainees are also constrained by work hour restrictions, engagement with mandatory curricula, and other professional development opportunities. Furthermore, changes in a medical work environment cause trainees to rely heavily on electronic health records, which forces them to be at a computer instead of in a patient’s room.8 This confluence of factors results in substantial time pressure, and BSR is perceived as an inefficient use of time.9
The impact on education and trainee development is another concern of BSR. Rounding away from a patient ensures a safe environment for learners to interpret data and articulate clinical reasoning without the risk of embarrassment in front of a patient. This time outside a patient room also allows the team to have a shared mental model so that communication is aligned when a patient encounter does occur. Card flipping may result in improved trainee autonomy because the constant presence of attending supervision, particularly in front of patients, can risk undermining resident leadership and patient trust.9
WHY WE SHOULD RETURN TO THE BEDSIDE
The cited reasons for provider hesitancy to BSR, including possible patient harm and inefficiency, may be mostly related to individual perceptions and have recently been questioned.10,11
Several studies have suggested that bedside rounds may be better for patients’ experience over traditional walk-rounding or card-flipping models. In these studies, patients signal a preference for bedside rounds and suggest that discussing sensitive issues or concerning differential diagnoses during BSR may not be as concerning as physicians worry.11 For example, one randomized trial found that 87% of patients are untroubled by bedside discussions,12 and another trial revealed no difference between rounding models in emotional distress to patients or families.11 Patients and families also report higher levels of clarity from physicians, and they cited significantly improved levels of understanding their illness10 and test results.9 Furthermore, patients describe that physicians spend about twice as much time on their care when BSR is used.12 In many related studies, patients report a preference for BSR as a rounding strategy.2,11-13 For example, one study found that 99% of patients prefer BSR.13 Another study showed that 85% of families request to be part of bedside family-centered rounds over traditional walk rounding.2
Rounding away from a patient via card flipping or walk rounding seems more efficient, but this idea may be illusory. Although these strategies may seem faster, the lack of communication and coordination between team members and the patient may cause inefficiencies and delays in care throughout the day.14 For example, one study has demonstrated that family-centered bedside rounds are about 20% longer than walk rounding, but everyone involved, including housestaff, felt it was more efficient and saved time later in the day.2 Additionally, a study comparing BSR with walk rounding13 found no difference in time spent per patient, and another study has shown similar results in terms of family-centered rounds.15 Both studies have reported a similar amount of time spent per patient.
Physicians should return to BSR not only to improve patient experience but also to develop the clinical skills of trainees. The direct observation of trainees with patients allows high-level impactful clinical feedback and provides a basis for calibrating how much autonomy to allow.16 Trainees also indicate that teaching is more impactful during BSR than during walk rounding or card flipping, and clinical skill training during BSR is superior to a discussion in a conference room or a hallway context.2,3,15,17,18 One study has even suggested that the education of bedside rounds may help improve clinical skills in comparison with traditional models.18
The lack of BSR during medical school and residency training results in a deleterious cycle. Trainees become less proficient and less comfortable with BSR skills and therefore graduate as faculty members who are unskilled or uncomfortable insisting on BSR. As such, the cycle continues. As a result and as the traditional cornerstone of clinical training and inpatient care, BSR is recommended as standard practice by some professional organizations.19
WHAT WE SHOULD DO INSTEAD
Developing buy-in is an important first step for engaging in BSR. We recommend starting by demonstrating the value of BSR to overcome initial team or trainee hesitancy. Regardless of systems established to improve the efficiency of BSR, it is our experience that learners hesitantly engage if they do not understand the value of a given activity. We also urge attendings to demonstrate value by articulating how BSR fits in a patient-centered approach to emphasize the evidence-based positive impacts of BSR on patients.9 Beyond reviewing the benefits, faculty should set an expectation that the team will carry out BSR.9 Doing so sets an informal curriculum showing that BSR is important and sets the standard of care, which allows an inpatient team to adapt early in a rotation.
Next, faculty should ensure that BSR remains efficient.9 We believe that efficiency starts by setting expectations with patients. Patient expectations can be set by an attending or a supervising resident and should include a preview about how each encounter will progress, who will be in the room, how large the team will be, and what their role is during the encounter. Patients should be invited to be part of the discussion, offered an opportunity to opt out, and informed that questions arising from or clarifications needed following encounters can be addressed later within the day or after BSR. Nurses should be invited to actively participate during patient presentations. Each bedside encounter should be kept brief and standardized.20,21 To maximize efficiency, we also believe that roles should be delegated ahead of time and positioning in the room should be deliberate.22 Team members should know who is speaking when and in what order, who is accessing the electronic health record, and who will be examining the patient. Ideally, goals should be set ahead of time and tailored to each individual encounter. Finally, ensure everyone is on the same page by huddling briefly before each encounter to establish goals and roles and huddle afterward to debrief for learning and teamwork calibration.
In order to mitigate the learner’s anxiety about presenting in front of patients, build a partnership with the trainee, and time should be allotted to establish a safe learning environment.9 Sustain a supportive learning environment by providing positive feedback to learners in front of patients and teams. Faculty members should demonstrate how to bedside round effectively by leading initial encounters and generate momentum by selecting initial patient encounters that are most likely to succeed.23 Checklists can also be useful cognitive aids to facilitate an encounter and manage the cognitive load of learners.24 Ultimately, hesitancies can be overcome with experience.
Faculty members should ensure that bedside encounters are educationally valuable for an entire team.9 This initiative starts by preparing ahead of time, which allows the mental energy during encounters to be directly observed by learners in action.16 Preparation also allows the presentation to focus more on clinical reasoning rather than data gathering.20 Faculty members should also consider ways to foster resident autonomy and establish the role of a supervising resident as the team leader. Positioning in the room is critical22; we suggest that faculty members should position themselves near the head of the bed, out of a patient’s direct eyesight. In this way, they can observe how individual team members and the team as a whole interact with patients. The supervising resident should be at the foot of the bed, central to the team and the focal point of a patient’s view. The presenting intern or student should be seated near the head of the bed and opposite the supervising attending. Clinical teaching should also be kept short and pertinent to the patient, and questions should be phrased as “how” or “why” rather than “what” to reduce the risk of “wrong” answers in front of patients and the team.
WHEN IS CARD FLIPPING APPROPRIATE?
We believe that bedside rounds are most consistent with patient-centered inpatient care and should be considered the first-line approach. We also acknowledge that it is not always possible to bedside round on every patient on an inpatient census. For example, at an average of 13-15 minutes per patient,2,13 a census of 16 patients can take up to 4 hours to round. This timeline is not always feasible given the timing of training program didactics, interprofessional or case management rounds, and pressure for early discharges. Similar to all aspects of medicine, many approaches have been established to provide patient care, and context is important. Therefore, card flipping and walk rounding are beneficial to patients in some instances. For example, consider BSR for new, sick, or undifferentiated patients or when the history or exam findings need clarification; walk rounding or card flipping is suitable for patients with clear plans in place or when an encounter will be too disruptive to the rounding flow.21 Census size and individual patient or family concerns should dictate the style of rounding; in most situations, BSR may be equally efficient because it offers significant benefits to patients and families.
RECOMMENDATIONS
- Expectations should be set early with both trainees and patients. Patients should be informed that the team can come back later for more in-depth discussions.
- Trainees should be taught evidence-based approaches supporting the value of bedside rounds for patients.
- Faculty should consider leading initial encounters to demonstrate how to bedside round and to model behaviors.
- Positive feedback should be provided in front of patients and the team to build confidence.
- Encounters should be kept brief and efficient.
- A sufficient space for resident autonomy should be ensured through deliberate positioning, delegation of responsibilities, and huddling before and after encounters.
- Bedside rounds should be educationally worthwhile.
CONCLUSION
BSR is a traditional cornerstone of clinical training and inpatient care. Teaching at the bedside has many established benefits, such as connecting with patients and families, affording educators a valuable opportunity to assess learners and role model, and solidifying medical content by integrating teaching with clinical care. Concerns about bedside rounding may be based more on conjecture than on available evidence and can be overcome with deliberate education and proper planning. We propose several recommendations to successfully implement efficient, patient-centered, and educationally valuable bedside rounds.
For this (and most) patient(s), we recommend BSR. If this BSR is the first encounter, we suggest that the team should start with a more straightforward patient and come back to the new admission after the team has a chance to practice with other patients.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
2. Muething SE, Kotagal UR, Schoettker PJ, del Rey JG, DeWitt TG. Family-centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119(4):829-832. https://doi.org/10.1542/peds.2006-2528.
3. Ngo TL, Blankenburg R, Yu CE. Teaching at the bedside: strategies for optimizing education on patient and family centered rounds. Pediatr Clin North Am. 2019;66(4):881-889. https://doi.org/10.1016/j.pcl.2019.03.012.
4. Berkwitt A, Grossman M. A Qualitative analysis of pediatric patient attitudes regarding family-centered rounds. Hosp Pediatr. 2015;5(7):357. https://doi.org/10.1542/hpeds.2014-0198.
5. Rabinowitz R, Farnan J, Hulland O, et al. Rounds today: a qualitative study of internal medicine and pediatrics resident perceptions. J Grad Med Educ. 2016;8(4):523-531. https://doi.org/10.4300/JGME-D-15-00106.1.
6. Pingree EW, Freed JA, Riviello ED, et al. A tale of two rounds: managing conflict during the worst of times in family-centered rounds. Hosp Pediatr. 2019;9(7):563-565. https://doi.org/10.1542/hpeds.2019-0047.
7. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148.
8. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
9. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326-334. https://doi.org/10.1097/ACM.0000000000000100.
10. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. https://doi.org/10.1097/00001888-200309000-00023.
11. Landry M-A, Lafrenaye S, Roy M-C, Cyr C. A randomized, controlled trial of bedside versus conference-room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275-280. https://doi.org/10.1542/peds.2007-0107.
12. Lehmann LS, Brancati FL, Chen M-C, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1156. https://doi.org/10.1056/NEJM199704173361606.
13. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. https://doi.org/10.1007/s11606-010-1344-7.
14. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. https://doi.org/10.2147/JMDH.S72633.
15. Kelly MM, Xie A, Li Y, et al. System factors influencing the use of a family-centered rounds checklist. Pediatr Qual Saf. 2019;4(4):e196. https://doi.org/10.1097/pq9.0000000000000196.
16. Kogan JR, Hatala R, Hauer KE, Holmboe E. Guidelines: The do’s, don’ts and don’t knows of direct observation of clinical skills in medical education. Perspect Med Educ. 2017;6(5):286-305. https://doi.org/10.1007/s40037-017-0376-7.
17. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. https://doi.org/10.1097/ACM.0b013e3181637f3e.
18. Heckmann JG, Bleh C, Dütsch M, Lang CJG, Neundörfer B. Does improved problem-based teaching influence students’ knowledge at the end of their neurology elective? An observational study of 40 students. J Neurol. 2003;250(12):1464-1468. https://doi.org/10.1007/s00415-003-0255-5.
19. Committee on hospital care and institute for patient and family centered care. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
20. Dhaliwal G, Hauer KE. The oral patient presentation in the era of night float admissions. JAMA. 2013;310(21):2247. https://doi.org/10.1001/jama.2013.282322.
21. Wiese JG. Teaching in the Hospital. Philadelphia, PA: ACP PRess; 2010. https://books.google.co.uk/books?hl=en&lr=&id=qquGWP4d2Q4C&oi=fnd&pg=PR13&dq=Wiese+J.+2010.+ACP+Teaching+Medicine+Series:+Teaching+in+the+Hospital.+Philadelphia,+PA:+ACP+Press&ots=JSRFojkBSn&sig=c33tapsL9DzV9nuFhENA6eObISA#v=onepage&q=bedside round&f=fals. Accessed November 29, 2019.
22. Lopez M, Vaks Y, Wilson M, et al. Impacting satisfaction, learning, and efficiency through structured interdisciplinary rounding in a pediatric intensive care unit. Pediatr Qual Saf. 2019;4(3):e176. https://doi.org/10.1097/pq9.0000000000000176.
23. Benbassat J. Role modeling in medical education: the importance of a reflective imitation. Acad Med. 2014;89(4):550-554. https://doi.org/10.1097/ACM.0000000000000189.
24. Cox ED, Jacobsohn GC, Rajamanickam VP, et al. A family-centered rounds checklist, family engagement, and patient safety: a randomized trial. Pediatrics. 2017;139(5):e20161688. https://doi.org/10.1542/peds.2016-1688.
1. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
2. Muething SE, Kotagal UR, Schoettker PJ, del Rey JG, DeWitt TG. Family-centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119(4):829-832. https://doi.org/10.1542/peds.2006-2528.
3. Ngo TL, Blankenburg R, Yu CE. Teaching at the bedside: strategies for optimizing education on patient and family centered rounds. Pediatr Clin North Am. 2019;66(4):881-889. https://doi.org/10.1016/j.pcl.2019.03.012.
4. Berkwitt A, Grossman M. A Qualitative analysis of pediatric patient attitudes regarding family-centered rounds. Hosp Pediatr. 2015;5(7):357. https://doi.org/10.1542/hpeds.2014-0198.
5. Rabinowitz R, Farnan J, Hulland O, et al. Rounds today: a qualitative study of internal medicine and pediatrics resident perceptions. J Grad Med Educ. 2016;8(4):523-531. https://doi.org/10.4300/JGME-D-15-00106.1.
6. Pingree EW, Freed JA, Riviello ED, et al. A tale of two rounds: managing conflict during the worst of times in family-centered rounds. Hosp Pediatr. 2019;9(7):563-565. https://doi.org/10.1542/hpeds.2019-0047.
7. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148.
8. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
9. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326-334. https://doi.org/10.1097/ACM.0000000000000100.
10. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. https://doi.org/10.1097/00001888-200309000-00023.
11. Landry M-A, Lafrenaye S, Roy M-C, Cyr C. A randomized, controlled trial of bedside versus conference-room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275-280. https://doi.org/10.1542/peds.2007-0107.
12. Lehmann LS, Brancati FL, Chen M-C, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1156. https://doi.org/10.1056/NEJM199704173361606.
13. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. https://doi.org/10.1007/s11606-010-1344-7.
14. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. https://doi.org/10.2147/JMDH.S72633.
15. Kelly MM, Xie A, Li Y, et al. System factors influencing the use of a family-centered rounds checklist. Pediatr Qual Saf. 2019;4(4):e196. https://doi.org/10.1097/pq9.0000000000000196.
16. Kogan JR, Hatala R, Hauer KE, Holmboe E. Guidelines: The do’s, don’ts and don’t knows of direct observation of clinical skills in medical education. Perspect Med Educ. 2017;6(5):286-305. https://doi.org/10.1007/s40037-017-0376-7.
17. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. https://doi.org/10.1097/ACM.0b013e3181637f3e.
18. Heckmann JG, Bleh C, Dütsch M, Lang CJG, Neundörfer B. Does improved problem-based teaching influence students’ knowledge at the end of their neurology elective? An observational study of 40 students. J Neurol. 2003;250(12):1464-1468. https://doi.org/10.1007/s00415-003-0255-5.
19. Committee on hospital care and institute for patient and family centered care. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
20. Dhaliwal G, Hauer KE. The oral patient presentation in the era of night float admissions. JAMA. 2013;310(21):2247. https://doi.org/10.1001/jama.2013.282322.
21. Wiese JG. Teaching in the Hospital. Philadelphia, PA: ACP PRess; 2010. https://books.google.co.uk/books?hl=en&lr=&id=qquGWP4d2Q4C&oi=fnd&pg=PR13&dq=Wiese+J.+2010.+ACP+Teaching+Medicine+Series:+Teaching+in+the+Hospital.+Philadelphia,+PA:+ACP+Press&ots=JSRFojkBSn&sig=c33tapsL9DzV9nuFhENA6eObISA#v=onepage&q=bedside round&f=fals. Accessed November 29, 2019.
22. Lopez M, Vaks Y, Wilson M, et al. Impacting satisfaction, learning, and efficiency through structured interdisciplinary rounding in a pediatric intensive care unit. Pediatr Qual Saf. 2019;4(3):e176. https://doi.org/10.1097/pq9.0000000000000176.
23. Benbassat J. Role modeling in medical education: the importance of a reflective imitation. Acad Med. 2014;89(4):550-554. https://doi.org/10.1097/ACM.0000000000000189.
24. Cox ED, Jacobsohn GC, Rajamanickam VP, et al. A family-centered rounds checklist, family engagement, and patient safety: a randomized trial. Pediatrics. 2017;139(5):e20161688. https://doi.org/10.1542/peds.2016-1688.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routinely Prescribing Transfusion Premedication To Prevent Acute Transfusion Reactions

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routine Thyroid-Stimulating Hormone Testing in the Hospital

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.
© 2020 Society of Hospital Medicine
Hindsight Is 20/20
A 38-year-old woman presented to her primary care clinic with 3 weeks of progressive numbness and tingling sensation, which began in both hands and then progressed to involv
As with all neurological complaints, localization of the process will often inform a more specific differential diagnosis. If both sensory and motor findings are present, both central and peripheral nerve processes deserve consideration. The onset of paresthesia in the hands, rapid progression to the trunk, and unilateral leg weakness would be inconsistent with a length-dependent peripheral neuropathy. The distribution of complaints and the sacral sparing suggests a myelopathic process involving the cervical region rather than a cauda equina or conus lesions. In an otherwise healthy person of this age and gender, an inflammatory demyelinating disease affecting the cord including multiple sclerosis (MS) would be a strong consideration, although metabolic, vascular, infectious, compressive, or neoplastic disease of the spinal cord could also present with similar subacute onset and pattern of deficits.
Her medical history included morbid obesity, dry eyes, depression, iron deficiency anemia requiring recurrent intravenous replenishment, and abnormal uterine bleeding. Her surgical history included gastric band placement 7 years earlier with removal 5 years later due to persistent gastroesophageal reflux disease, dysphagia, nausea, and vomiting. The gastric band removal was complicated by chronic abdominal pain. Her medications consisted of duloxetine, intermittent iron infusions, artificial tears, loratadine, and pregabalin. She was sexually active with her husband. She consumed alcohol occasionally but did not smoke tobacco or use illicit drugs.
On exam, her temperature was 36.6°C (97.8°F), blood pressure 132/84 mm Hg, and heart rate 85 beats per minute. Body mass index was 39.5 kg/m2. The cardiac, pulmonary, and skin examinations were normal. The abdomen was soft with diffuse tenderness to palpation without rebound or guarding. Examination of cranial nerves 2-12 was normal. Cognition, strength, proprioception, deep tendon reflexes, and light touch were all normal. Her gait was normal, and the Romberg test was negative.
The normal neurologic exam is reassuring but imperfectly sensitive and does not eliminate the possibility of underlying neuropathology. Bariatric surgery may result in an array of nutritional deficiencies such as vitamin E, B12, and copper, which can cause myelopathy and/or neuropathy. However, these abnormalities occur less frequently with gastric banding procedures. If her dry eyes are part of the sicca syndrome, an underlying autoimmune diathesis may be present. Her unexplained chronic abdominal pain prompts considering nonmenstrual causes of iron deficiency anemia, such as celiac disease. Bariatric surgery may contribute to iron deficiency through impaired iron absorption. Her stable weight and lack of diarrhea argue against Crohn’s or celiac disease. Iron deficiency predisposes individuals to pica, most commonly described with ice chip ingestion. If lead pica had occurred, abdominal and neurological symptoms could result. Nevertheless, the abdominal pain is nonspecific, and its occurrence after gastric band removal makes its link to her neurologic syndrome unclear. An initial evaluation would include basic metabolic panel, complete blood count with differential, erythrocyte sedimentation rate, C-reactive protein (CRP), thyroid-stimulating hormone, vitamin B12, and copper levels.
A basic metabolic panel was normal. The white cell count was 5,710 per cubic millimeter, hemoglobin level 12.2 g per deciliter, mean corpuscular volume 85.2 fl, and platelet count 279,000 per cubic millimeter. The serum ferritin level was 18 ng per milliliter (normal range, 13-150), iron 28 µg per deciliter (normal range, 50-170), total iron-binding capacity 364 µg per deciliter (normal range, 250-450), and iron saturation 8% (normal range, 20-55). The vitamin B12 level was 621 pg per milliliter (normal range, 232-1,245) and thyroid-stimulating hormone level 1.87 units per milliliter (normal range, 0.50-4.50). Electrolyte and aminotransferase levels were within normal limits. CRP was 1.0 mg per deciliter (normal range, <0.5) and erythrocyte sedimentation rate 33 millimeters per hour (normal range, 4-25). Hepatitis C and HIV antibodies were nonreactive.
The ongoing iron deficiency despite parenteral iron replacement raises the question of ongoing gastrointestinal or genitourinary blood loss. While the level of vitamin B12 in the serum may be misleadingly normal with cobalamin deficiency, a methylmalonic acid level is indicated to evaluate whether tissue stores are depleted. Copper levels are warranted given the prior bariatric surgery. The mild elevations of inflammatory markers are nonspecific but reduce the likelihood of a highly inflammatory process to account for the neurological and abdominal symptoms.
At her 3-month follow-up visit, she noted that the paresthesia had improved and was now limited to her bilateral lower extremities. During the same clinic visit, she experienced a 45-minute episode of ascending left upper extremity numbness. Her physical examination revealed normal strength and reflexes. She had diminished response to pinprick in both legs to the knees and in both hands to the wrists. Vibration sense was diminished in the bilateral lower extremities.
A glycosylated hemoglobin (HbA1c) level was 6.2%. Methylmalonic acid was 69 nmol per liter (normal range, 45-325). Antibodies to Borrelia burgdorferi and Treponema pallidum were absent. Impaired glucose metabolism was the leading diagnosis for her polyneuropathy, and it was recommended that she undergo an oral glucose tolerance test. Electromyography was not performed.
The neurological symptoms are now chronic, and importantly, the patient has developed sensory deficits on neurological examination, suggesting worsening of the underlying process. While the paresthesia is now limited to a “stocking/glove” distribution consistent with distal sensory polyneuropathy, there should still be a concern for spinal cord pathology given that the HbA1c level of 6.2 would not explain her initial distribution of symptoms. Myelopathy may mimic peripheral nerve disease if, for example, there is involvement of the dorsal columns leading to sensory deficits of vibration and proprioception. Additionally, the transient episode of upper extremity numbness raises the question of sensory nerve root involvement (ie, sensory radiculopathy). Unexplained abdominal pain could possibly represent the involvement of other nerve roots innervating the abdominal wall. The patient’s episode of focal arm numbness recalls the lancinating radicular pain of tabes dorsalis; however, the negative specific treponemal antibody test excludes neurosyphilis.
The differential diagnosis going forward will be strongly conditioned by the localization of the neurological lesion(s). To differentiate between myelopathy, radiculopathy, and peripheral neuropathy, I would perform nerve conduction studies, magnetic resonance imaging (MRI) of the spinal cord, and cerebrospinal fluid analysis.
The patient began taking a multivitamin, and after weeks her paresthesia had resolved. One month later, she developed an intermittent, throbbing left-sided headache and pain behind the left eye that was worsened with ocular movement. She then noted decreased visual acuity in her left eye that progressed the following month. She denied photophobia, flashers, or floaters.
In the emergency department, visual acuity was 20/25 in her right eye; in the left eye she was only able to count fingers. Extraocular movements of both eyes were normal as was her right pupillary reflex. Red desaturation and a relative afferent papillary defect were present in the left eye. Fundoscopic exam demonstrated left optic disc swelling. The remainder of her cranial nerves were normal. She had pronation of the left upper extremity and mild right finger-to-nose dysmetria. Muscle tone, strength, sensation, and deep tendon reflexes were normal.
The improvement in the sensory symptoms was unlikely to be related to the nutritional intervention and provides a clue to an underlying waxing and waning illness. That interpretation is supported by the subsequent development of new visual symptoms and signs, which point to optic nerve pathology. Optic neuropathy has a broad differential diagnosis that includes ischemic, metabolic, toxic, and compressive causes. Eye pain, swelling of the optic disc, and prominent impairment of color vision all point to the more specific syndrome of optic neuritis caused by infections (including both Treponema pallidum and Borrelia species), systemic autoimmune diseases (systemic lupus erythematosus or Sjogren’s syndrome), and central nervous system (CNS) demyelinating diseases. Of these, inflammatory demyelinating processes would be the likeliest explanation of intermittent and improving neurologic findings.
With relapsing symptoms and findings that are separate in distribution and time, two diagnoses become most likely, and both of these are most often diagnosed in young women. MS is common, and optic neuritis occurs in more than 50% of patients over the course of illness. Neuromyelitis optica spectrum disorder (NMOSD) is a rare condition that can exist in isolation or be associated with other autoimmune illnesses. While these entities are difficult to differentiate clinically, neuroimaging that demonstrates extensive intracerebral demyelinating lesions and cerebrospinal fluid with oligoclonal bands favor MS, whereas extensive, predominant spinal cord involvement is suggestive of NMOSD. Approximately 70% of NMO patients harbor an antibody directed against the aquaporin-4 channel, and these antibodies are not seen in patients with MS. A milder NMO-like disorder has also been associated with antimyelin oligodendrocyte antibodies (MOG).
Testing for antinuclear antibodies, anti–double-stranded DNA, anti-Ro (SSA), and anti-La (SSB) antibodies was negative. The level of C3 was 162 mg per deciliter (normal range 81-157) and C4 38 (normal range 13-39). T-spot testing for latent tuberculosis was negative.
There is no serological evidence of active systemic lupus erythematosus or Sjogren’s syndrome. The pretest probability of CNS tuberculosis was low in light of her presenting complaints, relatively protracted course, and overall clinical stability without antituberculous therapy. Tests for latent tuberculosis infection have significant limitations of both sensitivity and specificity for the diagnosis of active disease.
Optical coherence tomography showed optic disc edema in the left eye only. MRI of the head with contrast revealed abnormal signal intensity involving the posterior aspect of the pons, right middle cerebellar peduncle, anterior left temporal lobe, bilateral periventricular white matter, subcortical white matter of the frontal lobes bilaterally, and medulla with abnormal signal and enhancement of the left optic nerve (Figure, Panel A). MRI of the cervical and thoracic spine demonstrated multifocal demyelinating lesions at C3, C4, C7, T4, T5, T7, and T8 (Figure, Panel B). The lesions were not longitudinally extensive. There was no significant postcontrast enhancement to suggest active demyelination.
The cerebrospinal fluid analysis revealed glucose of 105 mg per deciliter and a total protein of 26.1 mg per deciliter. In the fourth tube, there were 20 red cells per cubic and four white cells with a differential of 62% neutrophils, 35% lymphocytes, and 3% monocytes. Epstein-Barr and herpes simplex virus DNA were negative. A Venereal Disease Research Laboratory test was negative. Multiple oligoclonal IgG bands were identified only in the cerebrospinal fluid. Aquaporin-4 IgG and MOG antibodies were negative.
In addition to the expected finding of enhancement of the optic nerve, MRI demonstrated numerous multifocal white matter lesions throughout the cerebrum, brainstem, and spinal cord. Many of the lesions were in “silent” areas, which is not directly attributable to specific symptoms, but several did correlate with the subtler deficits of weakness and dysmetria that were noted on examination. Although such lesions may be seen with a diverse group of systemic diseases including adrenal leukodystrophy, sarcoidosis, Behcet’s, cerebral lupus, and vasculitis, primary CNS inflammatory demyelinating diseases are much more likely. The extensive distribution of demyelination argues against NMOSD. The negative aquaporin-4 and MOG assays support this conclusion. Not all multifocal CNS demyelination is caused by MS and can be seen in posterior reversible encephalopathy syndrome, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, and adult polyglucosan body disease. Osmotic demyelination is increasingly being recognized as a process that can be more widespread rather than just being limited to the pons. Viral infections of the CNS such as the JC virus (PML) may also provoke multifocal demyelination. Acute disseminated encephalomyelitis is most often seen during childhood, usually after vaccination or after an infectious prodrome. The tempo of the progression of these other diseases tends to be much more rapid than this woman’s course, and often, the neurological deficits are more profound and debilitating. The clinical presentation of sensory-predominant myelopathy, followed by optic neuritis, absence of systemic inflammatory signs or laboratory markers, exclusion of other relevant diseases, multifocal white matter lesions on imaging, minimal pleocytosis, and presence of oligoclonal bands in cerebrospinal fluid, all point to a diagnosis of relapsing-remitting MS.
The patient was diagnosed with MS. She was admitted to the neurology service and treated with 1,000 mg IV methylprednisolone for 3 days with a prompt improvement in her vision. She was started on natalizumab without a relapse of symptoms over the past year.
COMMENTARY
Multiple sclerosis is a chronic demyelinating disease of the CNS.1 The diagnosis of MS has classically been based upon compatible clinical and radiographic evidence of pathology that is disseminated in space and time. Patients typically present with an initial clinically isolated syndrome—involving changes in vision, sensation, strength, mobility, or cognition—for which there is radiographic evidence of demyelination.2 A diagnosis of clinically definite MS is then often made based on a subsequent relapse of symptoms.3
An interval from initial symptoms has been central to the diagnosis of MS (“lesions disseminated in time”). However, recent evidence questions this diagnostic paradigm, and a more rapid diagnosis of MS has been recommended. This recommendation is reflected in the updated McDonald criteria, according to which, if a clinical presentation is supported by the presence of oligoclonal bands in the cerebrospinal fluid, a diagnosis can be made on the basis of radiographic evidence of dissemination of disease in space, without evidence of dissemination in time.4 The importance of such early diagnosis has been supported by numerous studies that have demonstrated improved clinical outcomes with early therapy.5-7
Despite the McDonald criteria, delays in definitive diagnosis are common in MS. Patients with MS in Spain were found to experience a 2-year delay from the first onset of symptoms to diagnosis.8 In this cohort, patients exhibited delays in presenting to a healthcare provider, as well as delays in diagnosis with an average time from seeing an initial provider to diagnosis of 6 months. When patients who were referred for a demyelinating episode were surveyed, over a third reported a prior suggestive event.9 The time from the first suggestive episode to referral to a neurologist for a recognized demyelinating event was 46 months. Other studies have shown that delays in diagnosis are especially common in younger patients, those with primary progressive MS, and those with comorbid disease.10,11
Misapplication of an MS diagnosis also occurs frequently. In one case series, such misapplication was found most often in cases involving migraine, fibromyalgia, psychogenic disorders, and NMOSD.12 NMOSD is distinguished from MS by the presence of typical brain and spine findings on MRI.13 Antibodies to aquaporin-4 are highly specific and moderately sensitive for the disease.14 It is important to distinguish NMOSD from MS as certain disease-modifying drugs used for MS might actually exacerbate NMOSD.15 A lesion that traverses over three or more contiguous vertebral segments with predominant involvement of central gray matter (ie, longitudinally extensive transverse myelitis) on MRI is the most distinct finding of NMOSD. In contrast, similar to our patient, short and often multiple lesions are demonstrated on spinal cord MRI in patients with MS. Sensitive and specific findings of brain MRI in patients with MS include the presence of lateral ventricle and inferior temporal lobe lesion, Dawson’s fingers, central vein sign, or an S-shaped U-fiber lesion. In NMOSD, brain MRI might reveal periependymal lesions surrounding the ventricular system.
This case highlights the diagnostic challenges related to presentations of a waxing and waning neurological process. At the time of the second evaluation, the presentation was interpreted as a length-dependent polyneuropathy due to glucose intolerance. Our patient’s relatively normal HbA1c, subacute onset of neuropathic symptoms (ie, <4 weeks), sensory and motor complaints, and onset in the upper extremities suggested an alternative diagnosis to prediabetes. Once the patient presented with optic neuritis, the cause of the initial symptoms was obvious, but then, hindsight is 20/20.
TEACHING POINTS
- Early treatment of MS results in improved clinical outcomes.
- Delays in the definitive diagnosis of MS are common, especially in younger patients, those with primary progressive MS, and those with comorbid disease.
- If a clinical presentation is supported by the presence of oligoclonal bands in the cerebrospinal fluid, a diagnosis of MS can be made on the basis of radiographic evidence of dissemination of disease in space, without evidence of dissemination in time.
Acknowledgments
The authors wish to thank Rabih Geha, MD, and Gurpreet Dhaliwal, MD, for providing feedback on an earlier version of this manuscript.
1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169-180. https://doi.org/10.1056/NEJMra140148.
2. Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389(10076):1336-1346. https://doi.org/10.1016/S0140-6736(16)30959-X.
3. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636. https://doi.org/10.1016/S0140-6736(18)30481-1.
4. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. https://doi.org/10.1016/S1474-4422(17)30470-2.
5. Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389(10076):1347-1356. https://doi.org/10.1016/S0140-6736(16)32388-1.
6. Freedman MS, Comi G, De Stefano N, et al. Moving toward earlier treatment of multiple sclerosis: Findings from a decade of clinical trials and implications for clinical practice. Mult Scler Relat Disord. 2014;3(2):147-155. https://doi.org/10.1016/j.msard.2013.07.001.
7. Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76(5):536-541. https://doi.org/10.1001/jamaneurol.2018.4905.
8. Fernández O, Fernández V, Arbizu T, et al. Characteristics of multiple sclerosis at onset and delay of diagnosis and treatment in Spain (the Novo Study). J Neurol. 257(9):1500-1507. https://doi.org/10.1007/s00415-010-5560-1.
9. Gout O, Lebrun-Frenay C, Labauge P, et al. Prior suggestive symptoms in one-third of patients consulting for a “first” demyelinating event. J Neurol Neurosurg Psychiatry 2011;82(3):323-325. https://doi.org/10.1136/jnnp.2008.166421.
10. Kingwell E, Leung A, Roger E, et al. Factors associated with delay to medical recognition in two Canadian multiple sclerosis cohorts. J Neurol Sci. 2010(1-2);292:57-62. https://doi.org/10.1016/j.jns.2010.02.007.
11. Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72(2):117-124. https://doi.org/10.1212/01.wnl.0000333252.78173.5f.
12. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87(13):1393-1399. https://doi.org/10.1212/WNL.0000000000003152.
13. Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: An international update. Neurology. 2015;84(11):1165-1173. https://doi.org/10.1212/WNL.0000000000001367.
14. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. https://doi.org/10.1212/WNL.0000000000001729.
15. Jacob A, Hutchinson M, Elsone L, et al. Does natalizumab therapy worsen neuromyelitis optica? Neurology. 2012;79(10):1065-1066. https://doi.org/10.1212/WNL.0b013e31826845fe.
A 38-year-old woman presented to her primary care clinic with 3 weeks of progressive numbness and tingling sensation, which began in both hands and then progressed to involv
As with all neurological complaints, localization of the process will often inform a more specific differential diagnosis. If both sensory and motor findings are present, both central and peripheral nerve processes deserve consideration. The onset of paresthesia in the hands, rapid progression to the trunk, and unilateral leg weakness would be inconsistent with a length-dependent peripheral neuropathy. The distribution of complaints and the sacral sparing suggests a myelopathic process involving the cervical region rather than a cauda equina or conus lesions. In an otherwise healthy person of this age and gender, an inflammatory demyelinating disease affecting the cord including multiple sclerosis (MS) would be a strong consideration, although metabolic, vascular, infectious, compressive, or neoplastic disease of the spinal cord could also present with similar subacute onset and pattern of deficits.
Her medical history included morbid obesity, dry eyes, depression, iron deficiency anemia requiring recurrent intravenous replenishment, and abnormal uterine bleeding. Her surgical history included gastric band placement 7 years earlier with removal 5 years later due to persistent gastroesophageal reflux disease, dysphagia, nausea, and vomiting. The gastric band removal was complicated by chronic abdominal pain. Her medications consisted of duloxetine, intermittent iron infusions, artificial tears, loratadine, and pregabalin. She was sexually active with her husband. She consumed alcohol occasionally but did not smoke tobacco or use illicit drugs.
On exam, her temperature was 36.6°C (97.8°F), blood pressure 132/84 mm Hg, and heart rate 85 beats per minute. Body mass index was 39.5 kg/m2. The cardiac, pulmonary, and skin examinations were normal. The abdomen was soft with diffuse tenderness to palpation without rebound or guarding. Examination of cranial nerves 2-12 was normal. Cognition, strength, proprioception, deep tendon reflexes, and light touch were all normal. Her gait was normal, and the Romberg test was negative.
The normal neurologic exam is reassuring but imperfectly sensitive and does not eliminate the possibility of underlying neuropathology. Bariatric surgery may result in an array of nutritional deficiencies such as vitamin E, B12, and copper, which can cause myelopathy and/or neuropathy. However, these abnormalities occur less frequently with gastric banding procedures. If her dry eyes are part of the sicca syndrome, an underlying autoimmune diathesis may be present. Her unexplained chronic abdominal pain prompts considering nonmenstrual causes of iron deficiency anemia, such as celiac disease. Bariatric surgery may contribute to iron deficiency through impaired iron absorption. Her stable weight and lack of diarrhea argue against Crohn’s or celiac disease. Iron deficiency predisposes individuals to pica, most commonly described with ice chip ingestion. If lead pica had occurred, abdominal and neurological symptoms could result. Nevertheless, the abdominal pain is nonspecific, and its occurrence after gastric band removal makes its link to her neurologic syndrome unclear. An initial evaluation would include basic metabolic panel, complete blood count with differential, erythrocyte sedimentation rate, C-reactive protein (CRP), thyroid-stimulating hormone, vitamin B12, and copper levels.
A basic metabolic panel was normal. The white cell count was 5,710 per cubic millimeter, hemoglobin level 12.2 g per deciliter, mean corpuscular volume 85.2 fl, and platelet count 279,000 per cubic millimeter. The serum ferritin level was 18 ng per milliliter (normal range, 13-150), iron 28 µg per deciliter (normal range, 50-170), total iron-binding capacity 364 µg per deciliter (normal range, 250-450), and iron saturation 8% (normal range, 20-55). The vitamin B12 level was 621 pg per milliliter (normal range, 232-1,245) and thyroid-stimulating hormone level 1.87 units per milliliter (normal range, 0.50-4.50). Electrolyte and aminotransferase levels were within normal limits. CRP was 1.0 mg per deciliter (normal range, <0.5) and erythrocyte sedimentation rate 33 millimeters per hour (normal range, 4-25). Hepatitis C and HIV antibodies were nonreactive.
The ongoing iron deficiency despite parenteral iron replacement raises the question of ongoing gastrointestinal or genitourinary blood loss. While the level of vitamin B12 in the serum may be misleadingly normal with cobalamin deficiency, a methylmalonic acid level is indicated to evaluate whether tissue stores are depleted. Copper levels are warranted given the prior bariatric surgery. The mild elevations of inflammatory markers are nonspecific but reduce the likelihood of a highly inflammatory process to account for the neurological and abdominal symptoms.
At her 3-month follow-up visit, she noted that the paresthesia had improved and was now limited to her bilateral lower extremities. During the same clinic visit, she experienced a 45-minute episode of ascending left upper extremity numbness. Her physical examination revealed normal strength and reflexes. She had diminished response to pinprick in both legs to the knees and in both hands to the wrists. Vibration sense was diminished in the bilateral lower extremities.
A glycosylated hemoglobin (HbA1c) level was 6.2%. Methylmalonic acid was 69 nmol per liter (normal range, 45-325). Antibodies to Borrelia burgdorferi and Treponema pallidum were absent. Impaired glucose metabolism was the leading diagnosis for her polyneuropathy, and it was recommended that she undergo an oral glucose tolerance test. Electromyography was not performed.
The neurological symptoms are now chronic, and importantly, the patient has developed sensory deficits on neurological examination, suggesting worsening of the underlying process. While the paresthesia is now limited to a “stocking/glove” distribution consistent with distal sensory polyneuropathy, there should still be a concern for spinal cord pathology given that the HbA1c level of 6.2 would not explain her initial distribution of symptoms. Myelopathy may mimic peripheral nerve disease if, for example, there is involvement of the dorsal columns leading to sensory deficits of vibration and proprioception. Additionally, the transient episode of upper extremity numbness raises the question of sensory nerve root involvement (ie, sensory radiculopathy). Unexplained abdominal pain could possibly represent the involvement of other nerve roots innervating the abdominal wall. The patient’s episode of focal arm numbness recalls the lancinating radicular pain of tabes dorsalis; however, the negative specific treponemal antibody test excludes neurosyphilis.
The differential diagnosis going forward will be strongly conditioned by the localization of the neurological lesion(s). To differentiate between myelopathy, radiculopathy, and peripheral neuropathy, I would perform nerve conduction studies, magnetic resonance imaging (MRI) of the spinal cord, and cerebrospinal fluid analysis.
The patient began taking a multivitamin, and after weeks her paresthesia had resolved. One month later, she developed an intermittent, throbbing left-sided headache and pain behind the left eye that was worsened with ocular movement. She then noted decreased visual acuity in her left eye that progressed the following month. She denied photophobia, flashers, or floaters.
In the emergency department, visual acuity was 20/25 in her right eye; in the left eye she was only able to count fingers. Extraocular movements of both eyes were normal as was her right pupillary reflex. Red desaturation and a relative afferent papillary defect were present in the left eye. Fundoscopic exam demonstrated left optic disc swelling. The remainder of her cranial nerves were normal. She had pronation of the left upper extremity and mild right finger-to-nose dysmetria. Muscle tone, strength, sensation, and deep tendon reflexes were normal.
The improvement in the sensory symptoms was unlikely to be related to the nutritional intervention and provides a clue to an underlying waxing and waning illness. That interpretation is supported by the subsequent development of new visual symptoms and signs, which point to optic nerve pathology. Optic neuropathy has a broad differential diagnosis that includes ischemic, metabolic, toxic, and compressive causes. Eye pain, swelling of the optic disc, and prominent impairment of color vision all point to the more specific syndrome of optic neuritis caused by infections (including both Treponema pallidum and Borrelia species), systemic autoimmune diseases (systemic lupus erythematosus or Sjogren’s syndrome), and central nervous system (CNS) demyelinating diseases. Of these, inflammatory demyelinating processes would be the likeliest explanation of intermittent and improving neurologic findings.
With relapsing symptoms and findings that are separate in distribution and time, two diagnoses become most likely, and both of these are most often diagnosed in young women. MS is common, and optic neuritis occurs in more than 50% of patients over the course of illness. Neuromyelitis optica spectrum disorder (NMOSD) is a rare condition that can exist in isolation or be associated with other autoimmune illnesses. While these entities are difficult to differentiate clinically, neuroimaging that demonstrates extensive intracerebral demyelinating lesions and cerebrospinal fluid with oligoclonal bands favor MS, whereas extensive, predominant spinal cord involvement is suggestive of NMOSD. Approximately 70% of NMO patients harbor an antibody directed against the aquaporin-4 channel, and these antibodies are not seen in patients with MS. A milder NMO-like disorder has also been associated with antimyelin oligodendrocyte antibodies (MOG).
Testing for antinuclear antibodies, anti–double-stranded DNA, anti-Ro (SSA), and anti-La (SSB) antibodies was negative. The level of C3 was 162 mg per deciliter (normal range 81-157) and C4 38 (normal range 13-39). T-spot testing for latent tuberculosis was negative.
There is no serological evidence of active systemic lupus erythematosus or Sjogren’s syndrome. The pretest probability of CNS tuberculosis was low in light of her presenting complaints, relatively protracted course, and overall clinical stability without antituberculous therapy. Tests for latent tuberculosis infection have significant limitations of both sensitivity and specificity for the diagnosis of active disease.
Optical coherence tomography showed optic disc edema in the left eye only. MRI of the head with contrast revealed abnormal signal intensity involving the posterior aspect of the pons, right middle cerebellar peduncle, anterior left temporal lobe, bilateral periventricular white matter, subcortical white matter of the frontal lobes bilaterally, and medulla with abnormal signal and enhancement of the left optic nerve (Figure, Panel A). MRI of the cervical and thoracic spine demonstrated multifocal demyelinating lesions at C3, C4, C7, T4, T5, T7, and T8 (Figure, Panel B). The lesions were not longitudinally extensive. There was no significant postcontrast enhancement to suggest active demyelination.
The cerebrospinal fluid analysis revealed glucose of 105 mg per deciliter and a total protein of 26.1 mg per deciliter. In the fourth tube, there were 20 red cells per cubic and four white cells with a differential of 62% neutrophils, 35% lymphocytes, and 3% monocytes. Epstein-Barr and herpes simplex virus DNA were negative. A Venereal Disease Research Laboratory test was negative. Multiple oligoclonal IgG bands were identified only in the cerebrospinal fluid. Aquaporin-4 IgG and MOG antibodies were negative.
In addition to the expected finding of enhancement of the optic nerve, MRI demonstrated numerous multifocal white matter lesions throughout the cerebrum, brainstem, and spinal cord. Many of the lesions were in “silent” areas, which is not directly attributable to specific symptoms, but several did correlate with the subtler deficits of weakness and dysmetria that were noted on examination. Although such lesions may be seen with a diverse group of systemic diseases including adrenal leukodystrophy, sarcoidosis, Behcet’s, cerebral lupus, and vasculitis, primary CNS inflammatory demyelinating diseases are much more likely. The extensive distribution of demyelination argues against NMOSD. The negative aquaporin-4 and MOG assays support this conclusion. Not all multifocal CNS demyelination is caused by MS and can be seen in posterior reversible encephalopathy syndrome, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, and adult polyglucosan body disease. Osmotic demyelination is increasingly being recognized as a process that can be more widespread rather than just being limited to the pons. Viral infections of the CNS such as the JC virus (PML) may also provoke multifocal demyelination. Acute disseminated encephalomyelitis is most often seen during childhood, usually after vaccination or after an infectious prodrome. The tempo of the progression of these other diseases tends to be much more rapid than this woman’s course, and often, the neurological deficits are more profound and debilitating. The clinical presentation of sensory-predominant myelopathy, followed by optic neuritis, absence of systemic inflammatory signs or laboratory markers, exclusion of other relevant diseases, multifocal white matter lesions on imaging, minimal pleocytosis, and presence of oligoclonal bands in cerebrospinal fluid, all point to a diagnosis of relapsing-remitting MS.
The patient was diagnosed with MS. She was admitted to the neurology service and treated with 1,000 mg IV methylprednisolone for 3 days with a prompt improvement in her vision. She was started on natalizumab without a relapse of symptoms over the past year.
COMMENTARY
Multiple sclerosis is a chronic demyelinating disease of the CNS.1 The diagnosis of MS has classically been based upon compatible clinical and radiographic evidence of pathology that is disseminated in space and time. Patients typically present with an initial clinically isolated syndrome—involving changes in vision, sensation, strength, mobility, or cognition—for which there is radiographic evidence of demyelination.2 A diagnosis of clinically definite MS is then often made based on a subsequent relapse of symptoms.3
An interval from initial symptoms has been central to the diagnosis of MS (“lesions disseminated in time”). However, recent evidence questions this diagnostic paradigm, and a more rapid diagnosis of MS has been recommended. This recommendation is reflected in the updated McDonald criteria, according to which, if a clinical presentation is supported by the presence of oligoclonal bands in the cerebrospinal fluid, a diagnosis can be made on the basis of radiographic evidence of dissemination of disease in space, without evidence of dissemination in time.4 The importance of such early diagnosis has been supported by numerous studies that have demonstrated improved clinical outcomes with early therapy.5-7
Despite the McDonald criteria, delays in definitive diagnosis are common in MS. Patients with MS in Spain were found to experience a 2-year delay from the first onset of symptoms to diagnosis.8 In this cohort, patients exhibited delays in presenting to a healthcare provider, as well as delays in diagnosis with an average time from seeing an initial provider to diagnosis of 6 months. When patients who were referred for a demyelinating episode were surveyed, over a third reported a prior suggestive event.9 The time from the first suggestive episode to referral to a neurologist for a recognized demyelinating event was 46 months. Other studies have shown that delays in diagnosis are especially common in younger patients, those with primary progressive MS, and those with comorbid disease.10,11
Misapplication of an MS diagnosis also occurs frequently. In one case series, such misapplication was found most often in cases involving migraine, fibromyalgia, psychogenic disorders, and NMOSD.12 NMOSD is distinguished from MS by the presence of typical brain and spine findings on MRI.13 Antibodies to aquaporin-4 are highly specific and moderately sensitive for the disease.14 It is important to distinguish NMOSD from MS as certain disease-modifying drugs used for MS might actually exacerbate NMOSD.15 A lesion that traverses over three or more contiguous vertebral segments with predominant involvement of central gray matter (ie, longitudinally extensive transverse myelitis) on MRI is the most distinct finding of NMOSD. In contrast, similar to our patient, short and often multiple lesions are demonstrated on spinal cord MRI in patients with MS. Sensitive and specific findings of brain MRI in patients with MS include the presence of lateral ventricle and inferior temporal lobe lesion, Dawson’s fingers, central vein sign, or an S-shaped U-fiber lesion. In NMOSD, brain MRI might reveal periependymal lesions surrounding the ventricular system.
This case highlights the diagnostic challenges related to presentations of a waxing and waning neurological process. At the time of the second evaluation, the presentation was interpreted as a length-dependent polyneuropathy due to glucose intolerance. Our patient’s relatively normal HbA1c, subacute onset of neuropathic symptoms (ie, <4 weeks), sensory and motor complaints, and onset in the upper extremities suggested an alternative diagnosis to prediabetes. Once the patient presented with optic neuritis, the cause of the initial symptoms was obvious, but then, hindsight is 20/20.
TEACHING POINTS
- Early treatment of MS results in improved clinical outcomes.
- Delays in the definitive diagnosis of MS are common, especially in younger patients, those with primary progressive MS, and those with comorbid disease.
- If a clinical presentation is supported by the presence of oligoclonal bands in the cerebrospinal fluid, a diagnosis of MS can be made on the basis of radiographic evidence of dissemination of disease in space, without evidence of dissemination in time.
Acknowledgments
The authors wish to thank Rabih Geha, MD, and Gurpreet Dhaliwal, MD, for providing feedback on an earlier version of this manuscript.
A 38-year-old woman presented to her primary care clinic with 3 weeks of progressive numbness and tingling sensation, which began in both hands and then progressed to involv
As with all neurological complaints, localization of the process will often inform a more specific differential diagnosis. If both sensory and motor findings are present, both central and peripheral nerve processes deserve consideration. The onset of paresthesia in the hands, rapid progression to the trunk, and unilateral leg weakness would be inconsistent with a length-dependent peripheral neuropathy. The distribution of complaints and the sacral sparing suggests a myelopathic process involving the cervical region rather than a cauda equina or conus lesions. In an otherwise healthy person of this age and gender, an inflammatory demyelinating disease affecting the cord including multiple sclerosis (MS) would be a strong consideration, although metabolic, vascular, infectious, compressive, or neoplastic disease of the spinal cord could also present with similar subacute onset and pattern of deficits.
Her medical history included morbid obesity, dry eyes, depression, iron deficiency anemia requiring recurrent intravenous replenishment, and abnormal uterine bleeding. Her surgical history included gastric band placement 7 years earlier with removal 5 years later due to persistent gastroesophageal reflux disease, dysphagia, nausea, and vomiting. The gastric band removal was complicated by chronic abdominal pain. Her medications consisted of duloxetine, intermittent iron infusions, artificial tears, loratadine, and pregabalin. She was sexually active with her husband. She consumed alcohol occasionally but did not smoke tobacco or use illicit drugs.
On exam, her temperature was 36.6°C (97.8°F), blood pressure 132/84 mm Hg, and heart rate 85 beats per minute. Body mass index was 39.5 kg/m2. The cardiac, pulmonary, and skin examinations were normal. The abdomen was soft with diffuse tenderness to palpation without rebound or guarding. Examination of cranial nerves 2-12 was normal. Cognition, strength, proprioception, deep tendon reflexes, and light touch were all normal. Her gait was normal, and the Romberg test was negative.
The normal neurologic exam is reassuring but imperfectly sensitive and does not eliminate the possibility of underlying neuropathology. Bariatric surgery may result in an array of nutritional deficiencies such as vitamin E, B12, and copper, which can cause myelopathy and/or neuropathy. However, these abnormalities occur less frequently with gastric banding procedures. If her dry eyes are part of the sicca syndrome, an underlying autoimmune diathesis may be present. Her unexplained chronic abdominal pain prompts considering nonmenstrual causes of iron deficiency anemia, such as celiac disease. Bariatric surgery may contribute to iron deficiency through impaired iron absorption. Her stable weight and lack of diarrhea argue against Crohn’s or celiac disease. Iron deficiency predisposes individuals to pica, most commonly described with ice chip ingestion. If lead pica had occurred, abdominal and neurological symptoms could result. Nevertheless, the abdominal pain is nonspecific, and its occurrence after gastric band removal makes its link to her neurologic syndrome unclear. An initial evaluation would include basic metabolic panel, complete blood count with differential, erythrocyte sedimentation rate, C-reactive protein (CRP), thyroid-stimulating hormone, vitamin B12, and copper levels.
A basic metabolic panel was normal. The white cell count was 5,710 per cubic millimeter, hemoglobin level 12.2 g per deciliter, mean corpuscular volume 85.2 fl, and platelet count 279,000 per cubic millimeter. The serum ferritin level was 18 ng per milliliter (normal range, 13-150), iron 28 µg per deciliter (normal range, 50-170), total iron-binding capacity 364 µg per deciliter (normal range, 250-450), and iron saturation 8% (normal range, 20-55). The vitamin B12 level was 621 pg per milliliter (normal range, 232-1,245) and thyroid-stimulating hormone level 1.87 units per milliliter (normal range, 0.50-4.50). Electrolyte and aminotransferase levels were within normal limits. CRP was 1.0 mg per deciliter (normal range, <0.5) and erythrocyte sedimentation rate 33 millimeters per hour (normal range, 4-25). Hepatitis C and HIV antibodies were nonreactive.
The ongoing iron deficiency despite parenteral iron replacement raises the question of ongoing gastrointestinal or genitourinary blood loss. While the level of vitamin B12 in the serum may be misleadingly normal with cobalamin deficiency, a methylmalonic acid level is indicated to evaluate whether tissue stores are depleted. Copper levels are warranted given the prior bariatric surgery. The mild elevations of inflammatory markers are nonspecific but reduce the likelihood of a highly inflammatory process to account for the neurological and abdominal symptoms.
At her 3-month follow-up visit, she noted that the paresthesia had improved and was now limited to her bilateral lower extremities. During the same clinic visit, she experienced a 45-minute episode of ascending left upper extremity numbness. Her physical examination revealed normal strength and reflexes. She had diminished response to pinprick in both legs to the knees and in both hands to the wrists. Vibration sense was diminished in the bilateral lower extremities.
A glycosylated hemoglobin (HbA1c) level was 6.2%. Methylmalonic acid was 69 nmol per liter (normal range, 45-325). Antibodies to Borrelia burgdorferi and Treponema pallidum were absent. Impaired glucose metabolism was the leading diagnosis for her polyneuropathy, and it was recommended that she undergo an oral glucose tolerance test. Electromyography was not performed.
The neurological symptoms are now chronic, and importantly, the patient has developed sensory deficits on neurological examination, suggesting worsening of the underlying process. While the paresthesia is now limited to a “stocking/glove” distribution consistent with distal sensory polyneuropathy, there should still be a concern for spinal cord pathology given that the HbA1c level of 6.2 would not explain her initial distribution of symptoms. Myelopathy may mimic peripheral nerve disease if, for example, there is involvement of the dorsal columns leading to sensory deficits of vibration and proprioception. Additionally, the transient episode of upper extremity numbness raises the question of sensory nerve root involvement (ie, sensory radiculopathy). Unexplained abdominal pain could possibly represent the involvement of other nerve roots innervating the abdominal wall. The patient’s episode of focal arm numbness recalls the lancinating radicular pain of tabes dorsalis; however, the negative specific treponemal antibody test excludes neurosyphilis.
The differential diagnosis going forward will be strongly conditioned by the localization of the neurological lesion(s). To differentiate between myelopathy, radiculopathy, and peripheral neuropathy, I would perform nerve conduction studies, magnetic resonance imaging (MRI) of the spinal cord, and cerebrospinal fluid analysis.
The patient began taking a multivitamin, and after weeks her paresthesia had resolved. One month later, she developed an intermittent, throbbing left-sided headache and pain behind the left eye that was worsened with ocular movement. She then noted decreased visual acuity in her left eye that progressed the following month. She denied photophobia, flashers, or floaters.
In the emergency department, visual acuity was 20/25 in her right eye; in the left eye she was only able to count fingers. Extraocular movements of both eyes were normal as was her right pupillary reflex. Red desaturation and a relative afferent papillary defect were present in the left eye. Fundoscopic exam demonstrated left optic disc swelling. The remainder of her cranial nerves were normal. She had pronation of the left upper extremity and mild right finger-to-nose dysmetria. Muscle tone, strength, sensation, and deep tendon reflexes were normal.
The improvement in the sensory symptoms was unlikely to be related to the nutritional intervention and provides a clue to an underlying waxing and waning illness. That interpretation is supported by the subsequent development of new visual symptoms and signs, which point to optic nerve pathology. Optic neuropathy has a broad differential diagnosis that includes ischemic, metabolic, toxic, and compressive causes. Eye pain, swelling of the optic disc, and prominent impairment of color vision all point to the more specific syndrome of optic neuritis caused by infections (including both Treponema pallidum and Borrelia species), systemic autoimmune diseases (systemic lupus erythematosus or Sjogren’s syndrome), and central nervous system (CNS) demyelinating diseases. Of these, inflammatory demyelinating processes would be the likeliest explanation of intermittent and improving neurologic findings.
With relapsing symptoms and findings that are separate in distribution and time, two diagnoses become most likely, and both of these are most often diagnosed in young women. MS is common, and optic neuritis occurs in more than 50% of patients over the course of illness. Neuromyelitis optica spectrum disorder (NMOSD) is a rare condition that can exist in isolation or be associated with other autoimmune illnesses. While these entities are difficult to differentiate clinically, neuroimaging that demonstrates extensive intracerebral demyelinating lesions and cerebrospinal fluid with oligoclonal bands favor MS, whereas extensive, predominant spinal cord involvement is suggestive of NMOSD. Approximately 70% of NMO patients harbor an antibody directed against the aquaporin-4 channel, and these antibodies are not seen in patients with MS. A milder NMO-like disorder has also been associated with antimyelin oligodendrocyte antibodies (MOG).
Testing for antinuclear antibodies, anti–double-stranded DNA, anti-Ro (SSA), and anti-La (SSB) antibodies was negative. The level of C3 was 162 mg per deciliter (normal range 81-157) and C4 38 (normal range 13-39). T-spot testing for latent tuberculosis was negative.
There is no serological evidence of active systemic lupus erythematosus or Sjogren’s syndrome. The pretest probability of CNS tuberculosis was low in light of her presenting complaints, relatively protracted course, and overall clinical stability without antituberculous therapy. Tests for latent tuberculosis infection have significant limitations of both sensitivity and specificity for the diagnosis of active disease.
Optical coherence tomography showed optic disc edema in the left eye only. MRI of the head with contrast revealed abnormal signal intensity involving the posterior aspect of the pons, right middle cerebellar peduncle, anterior left temporal lobe, bilateral periventricular white matter, subcortical white matter of the frontal lobes bilaterally, and medulla with abnormal signal and enhancement of the left optic nerve (Figure, Panel A). MRI of the cervical and thoracic spine demonstrated multifocal demyelinating lesions at C3, C4, C7, T4, T5, T7, and T8 (Figure, Panel B). The lesions were not longitudinally extensive. There was no significant postcontrast enhancement to suggest active demyelination.
The cerebrospinal fluid analysis revealed glucose of 105 mg per deciliter and a total protein of 26.1 mg per deciliter. In the fourth tube, there were 20 red cells per cubic and four white cells with a differential of 62% neutrophils, 35% lymphocytes, and 3% monocytes. Epstein-Barr and herpes simplex virus DNA were negative. A Venereal Disease Research Laboratory test was negative. Multiple oligoclonal IgG bands were identified only in the cerebrospinal fluid. Aquaporin-4 IgG and MOG antibodies were negative.
In addition to the expected finding of enhancement of the optic nerve, MRI demonstrated numerous multifocal white matter lesions throughout the cerebrum, brainstem, and spinal cord. Many of the lesions were in “silent” areas, which is not directly attributable to specific symptoms, but several did correlate with the subtler deficits of weakness and dysmetria that were noted on examination. Although such lesions may be seen with a diverse group of systemic diseases including adrenal leukodystrophy, sarcoidosis, Behcet’s, cerebral lupus, and vasculitis, primary CNS inflammatory demyelinating diseases are much more likely. The extensive distribution of demyelination argues against NMOSD. The negative aquaporin-4 and MOG assays support this conclusion. Not all multifocal CNS demyelination is caused by MS and can be seen in posterior reversible encephalopathy syndrome, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, and adult polyglucosan body disease. Osmotic demyelination is increasingly being recognized as a process that can be more widespread rather than just being limited to the pons. Viral infections of the CNS such as the JC virus (PML) may also provoke multifocal demyelination. Acute disseminated encephalomyelitis is most often seen during childhood, usually after vaccination or after an infectious prodrome. The tempo of the progression of these other diseases tends to be much more rapid than this woman’s course, and often, the neurological deficits are more profound and debilitating. The clinical presentation of sensory-predominant myelopathy, followed by optic neuritis, absence of systemic inflammatory signs or laboratory markers, exclusion of other relevant diseases, multifocal white matter lesions on imaging, minimal pleocytosis, and presence of oligoclonal bands in cerebrospinal fluid, all point to a diagnosis of relapsing-remitting MS.
The patient was diagnosed with MS. She was admitted to the neurology service and treated with 1,000 mg IV methylprednisolone for 3 days with a prompt improvement in her vision. She was started on natalizumab without a relapse of symptoms over the past year.
COMMENTARY
Multiple sclerosis is a chronic demyelinating disease of the CNS.1 The diagnosis of MS has classically been based upon compatible clinical and radiographic evidence of pathology that is disseminated in space and time. Patients typically present with an initial clinically isolated syndrome—involving changes in vision, sensation, strength, mobility, or cognition—for which there is radiographic evidence of demyelination.2 A diagnosis of clinically definite MS is then often made based on a subsequent relapse of symptoms.3
An interval from initial symptoms has been central to the diagnosis of MS (“lesions disseminated in time”). However, recent evidence questions this diagnostic paradigm, and a more rapid diagnosis of MS has been recommended. This recommendation is reflected in the updated McDonald criteria, according to which, if a clinical presentation is supported by the presence of oligoclonal bands in the cerebrospinal fluid, a diagnosis can be made on the basis of radiographic evidence of dissemination of disease in space, without evidence of dissemination in time.4 The importance of such early diagnosis has been supported by numerous studies that have demonstrated improved clinical outcomes with early therapy.5-7
Despite the McDonald criteria, delays in definitive diagnosis are common in MS. Patients with MS in Spain were found to experience a 2-year delay from the first onset of symptoms to diagnosis.8 In this cohort, patients exhibited delays in presenting to a healthcare provider, as well as delays in diagnosis with an average time from seeing an initial provider to diagnosis of 6 months. When patients who were referred for a demyelinating episode were surveyed, over a third reported a prior suggestive event.9 The time from the first suggestive episode to referral to a neurologist for a recognized demyelinating event was 46 months. Other studies have shown that delays in diagnosis are especially common in younger patients, those with primary progressive MS, and those with comorbid disease.10,11
Misapplication of an MS diagnosis also occurs frequently. In one case series, such misapplication was found most often in cases involving migraine, fibromyalgia, psychogenic disorders, and NMOSD.12 NMOSD is distinguished from MS by the presence of typical brain and spine findings on MRI.13 Antibodies to aquaporin-4 are highly specific and moderately sensitive for the disease.14 It is important to distinguish NMOSD from MS as certain disease-modifying drugs used for MS might actually exacerbate NMOSD.15 A lesion that traverses over three or more contiguous vertebral segments with predominant involvement of central gray matter (ie, longitudinally extensive transverse myelitis) on MRI is the most distinct finding of NMOSD. In contrast, similar to our patient, short and often multiple lesions are demonstrated on spinal cord MRI in patients with MS. Sensitive and specific findings of brain MRI in patients with MS include the presence of lateral ventricle and inferior temporal lobe lesion, Dawson’s fingers, central vein sign, or an S-shaped U-fiber lesion. In NMOSD, brain MRI might reveal periependymal lesions surrounding the ventricular system.
This case highlights the diagnostic challenges related to presentations of a waxing and waning neurological process. At the time of the second evaluation, the presentation was interpreted as a length-dependent polyneuropathy due to glucose intolerance. Our patient’s relatively normal HbA1c, subacute onset of neuropathic symptoms (ie, <4 weeks), sensory and motor complaints, and onset in the upper extremities suggested an alternative diagnosis to prediabetes. Once the patient presented with optic neuritis, the cause of the initial symptoms was obvious, but then, hindsight is 20/20.
TEACHING POINTS
- Early treatment of MS results in improved clinical outcomes.
- Delays in the definitive diagnosis of MS are common, especially in younger patients, those with primary progressive MS, and those with comorbid disease.
- If a clinical presentation is supported by the presence of oligoclonal bands in the cerebrospinal fluid, a diagnosis of MS can be made on the basis of radiographic evidence of dissemination of disease in space, without evidence of dissemination in time.
Acknowledgments
The authors wish to thank Rabih Geha, MD, and Gurpreet Dhaliwal, MD, for providing feedback on an earlier version of this manuscript.
1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169-180. https://doi.org/10.1056/NEJMra140148.
2. Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389(10076):1336-1346. https://doi.org/10.1016/S0140-6736(16)30959-X.
3. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636. https://doi.org/10.1016/S0140-6736(18)30481-1.
4. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. https://doi.org/10.1016/S1474-4422(17)30470-2.
5. Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389(10076):1347-1356. https://doi.org/10.1016/S0140-6736(16)32388-1.
6. Freedman MS, Comi G, De Stefano N, et al. Moving toward earlier treatment of multiple sclerosis: Findings from a decade of clinical trials and implications for clinical practice. Mult Scler Relat Disord. 2014;3(2):147-155. https://doi.org/10.1016/j.msard.2013.07.001.
7. Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76(5):536-541. https://doi.org/10.1001/jamaneurol.2018.4905.
8. Fernández O, Fernández V, Arbizu T, et al. Characteristics of multiple sclerosis at onset and delay of diagnosis and treatment in Spain (the Novo Study). J Neurol. 257(9):1500-1507. https://doi.org/10.1007/s00415-010-5560-1.
9. Gout O, Lebrun-Frenay C, Labauge P, et al. Prior suggestive symptoms in one-third of patients consulting for a “first” demyelinating event. J Neurol Neurosurg Psychiatry 2011;82(3):323-325. https://doi.org/10.1136/jnnp.2008.166421.
10. Kingwell E, Leung A, Roger E, et al. Factors associated with delay to medical recognition in two Canadian multiple sclerosis cohorts. J Neurol Sci. 2010(1-2);292:57-62. https://doi.org/10.1016/j.jns.2010.02.007.
11. Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72(2):117-124. https://doi.org/10.1212/01.wnl.0000333252.78173.5f.
12. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87(13):1393-1399. https://doi.org/10.1212/WNL.0000000000003152.
13. Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: An international update. Neurology. 2015;84(11):1165-1173. https://doi.org/10.1212/WNL.0000000000001367.
14. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. https://doi.org/10.1212/WNL.0000000000001729.
15. Jacob A, Hutchinson M, Elsone L, et al. Does natalizumab therapy worsen neuromyelitis optica? Neurology. 2012;79(10):1065-1066. https://doi.org/10.1212/WNL.0b013e31826845fe.
1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169-180. https://doi.org/10.1056/NEJMra140148.
2. Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389(10076):1336-1346. https://doi.org/10.1016/S0140-6736(16)30959-X.
3. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636. https://doi.org/10.1016/S0140-6736(18)30481-1.
4. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. https://doi.org/10.1016/S1474-4422(17)30470-2.
5. Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389(10076):1347-1356. https://doi.org/10.1016/S0140-6736(16)32388-1.
6. Freedman MS, Comi G, De Stefano N, et al. Moving toward earlier treatment of multiple sclerosis: Findings from a decade of clinical trials and implications for clinical practice. Mult Scler Relat Disord. 2014;3(2):147-155. https://doi.org/10.1016/j.msard.2013.07.001.
7. Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76(5):536-541. https://doi.org/10.1001/jamaneurol.2018.4905.
8. Fernández O, Fernández V, Arbizu T, et al. Characteristics of multiple sclerosis at onset and delay of diagnosis and treatment in Spain (the Novo Study). J Neurol. 257(9):1500-1507. https://doi.org/10.1007/s00415-010-5560-1.
9. Gout O, Lebrun-Frenay C, Labauge P, et al. Prior suggestive symptoms in one-third of patients consulting for a “first” demyelinating event. J Neurol Neurosurg Psychiatry 2011;82(3):323-325. https://doi.org/10.1136/jnnp.2008.166421.
10. Kingwell E, Leung A, Roger E, et al. Factors associated with delay to medical recognition in two Canadian multiple sclerosis cohorts. J Neurol Sci. 2010(1-2);292:57-62. https://doi.org/10.1016/j.jns.2010.02.007.
11. Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72(2):117-124. https://doi.org/10.1212/01.wnl.0000333252.78173.5f.
12. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87(13):1393-1399. https://doi.org/10.1212/WNL.0000000000003152.
13. Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: An international update. Neurology. 2015;84(11):1165-1173. https://doi.org/10.1212/WNL.0000000000001367.
14. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. https://doi.org/10.1212/WNL.0000000000001729.
15. Jacob A, Hutchinson M, Elsone L, et al. Does natalizumab therapy worsen neuromyelitis optica? Neurology. 2012;79(10):1065-1066. https://doi.org/10.1212/WNL.0b013e31826845fe.
© 2020 Society of Hospital Medicine
Surgical Comanagement by Hospitalists: Continued Improvement Over 5 Years
In surgical comanagement (SCM), surgeons and hospitalists share responsibility of care for surgical patients. While SCM has been increasingly utilized, many of the reported models are a modification of the consultation model, in which a group of rotating hospitalists, internists, or geriatricians care for the surgical patients, often after medical complications may have occured.1-4
In August 2012, we implemented SCM in Orthopedic and Neurosurgery services at our institution.5 This model is unique because the same Internal Medicine hospitalists are dedicated year round to the same surgical service. SCM hospitalists see patients on their assigned surgical service only; they do not see patients on the Internal Medicine service. After the first year of implementing SCM, we conducted a propensity score–weighted study with 17,057 discharges in the pre-SCM group (January 2009 to July 2012) and 5,533 discharges in the post-SCM group (September 2012 to September 2013).5 In this study, SCM was associated with a decrease in medical complications, length of stay (LOS), medical consultations, 30-day readmissions, and cost.5
Since SCM requires ongoing investment by institutions, we now report a follow-up study to explore if there were continued improvements in patient outcomes with SCM. In this study, we evaluate if there was a decrease in medical complications, LOS, number of medical consultations, rapid response team calls, and code blues and an increase in patient satisfaction with SCM in Orthopedic and Neurosurgery services between 2012 and 2018.
METHODS
We included 26,380 discharges from Orthopedic and Neurosurgery services between September 1, 2012, and June 30, 2018, at our academic medical center. We excluded patients discharged in August 2012 as we transitioned to the SCM model. Our Institutional Review Board exempted this study from further review.
SCM Structure
SCM structure was detailed in a prior article.5 We have 3.0 clinical full-time equivalents on the Orthopedic surgery SCM service and 1.2 on the Neurosurgery SCM service. On weekdays, during the day (8
During the day, SCM hospitalists receive the first call for medical issues. After 5
SCM hospitalists screen the entire patient list on their assigned surgery service each day. After screening the patient list, SCM hospitalists formally see select patients with preventable or active medical conditions and write notes on the patient’s chart. There are no set criteria to determine which patients would be seen by SCM. This is because surgeries can decompensate stable medical conditions or new unexpected medical complications may occur. Additionally, in our prior study, we reported that SCM reduced medical complications and LOS regardless of age or patient acuity.5
Outcomes
Our primary outcome was proportion of patients with ≥1 medical complication (sepsis, pneumonia, urinary tract infection, delirium, acute kidney injury, atrial fibrillation, or ileus). Our secondary outcomes included mean LOS, proportion of patients with ≥2 medical consultations, rapid response team calls, code blues, and top-box patient satisfaction score. Though cost is an important consideration in implementing SCM, limited financial data were available. However, since LOS is a key component in calculating direct costs,6 we estimated the cost savings per discharge using mean direct cost per day and the difference in mean LOS between pre- and post-SCM groups.5
We defined medical complications using International Classification of Disease (ICD) Codes 9 or 10 that were coded as “not present on admission” (Appendix 1). We used Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey for three questions for patient satisfaction: Did doctors treat with courtesy and respect, listen carefully, and explain things in a way you could understand?
Statistical Analysis
We used regression analysis to assess trends in patient characteristics by year (Appendix 2). Logistic regression with logit link was used to assess the yearly change in our binary outcomes (proportion of patients with ≥1 medical complication, those with ≥2 medical consultations, rapid response team calls, code blue, and top-box patient satisfaction score) and reported odds ratios. Gamma regression with identity link was performed for our continuous outcome (LOS). Beta coefficient was reported to estimate the yearly change in LOS under their original scales. Age, primary insurance, race, Charlson comorbidity score, general or regional anesthesia, surgical service, and duration of surgery were adjusted in the regression analyses for outcomes. SAS 9.4 was used for analysis.
RESULTS
Patient characteristics are shown in Table 1. Overall, 62.8% patients were discharged from Orthopedic surgery service, 72.5% patients underwent elective surgery, and 88.8% received general anesthesia. Between 2012 and 2018, there was a significant increase in the median age of patients (from 60 years to 63 years), mean Charlson comorbidity score increased from 1.07 to 1.46, and median case mix index, a measure of patient acuity, increased from 2.10 to 2.36 (Appendix 2).
Comparing pre-SCM unadjusted rates reported in our prior study (January 2009 to July 2012) to post-SCM (September 2012 to June 2018; Appendix 3), patients with ≥1 medical complication decreased from 10.1% to 6.1%, LOS (mean ± standard deviation) changed from 5.4 ± 2.2 days to 4.6 ± 5.8 days, patients with ≥2 medical consultations decreased from 19.4% to 9.2%, rapid response team calls changed from 1% to 0.9%, code blues changed from 0.3% to 0.2%, and patients with top-box patient satisfaction score increased from 86.4% to 94.2%.5
In the adjusted analysis from 2012 to 2018, the odds of patients with ≥1 medical complication decreased by 3.8% per year (P = .01), estimated LOS decreased by 0.3 days per year (P < .0001), and the odds of rapid response team calls decreased by 12.2% per year (P = .001; Table 2). Changes over time in the odds of patients with ≥2 medical consultations, code blues, or top-box patient satisfaction score were not statistically significant (Table 2). Based on the LOS reduction pre- to post-SCM, there were estimated average direct cost savings of $3,424 per discharge between 2012 and 2018.
DISCUSSION
Since the implementation of SCM on Orthopedic and Neurosurgery services at our institution, there was a decrease in medical complications, LOS, and rapid response team calls. To our knowledge, this is one of the largest studies evaluating the benefits of SCM over 5.8 years. Similar to our prior studies on this SCM model of care,5,7 other studies have reported a decrease in medical complications,8-10 LOS,11-13 and cost of care14 with SCM.
While the changes in the unadjusted rates of outcomes over the years appeared to be small, while our patient population became older and sicker, there were significant changes in several of our outcomes in the adjusted analysis. We believe that SCM hospitalists have developed a skill set and understanding of these surgical patients over time and can manage more medically complex patients without an increase in medical complications or LOS. We attribute this to our unique SCM model in which the same hospitalists stay year round on the same surgical service. SCM hospitalists have built trusting relationships with the surgical team with greater involvement in decision making, care planning, and patient selection. With minimal turnover in the SCM group and with ongoing learning, SCM hospitalists can anticipate fluid or pain medication requirements after specific surgeries and the surgery-specific medical complications. SCM hospitalists are available on the patient units to provide timely intervention in case of medical deterioration; answer any questions from patients, families, or nursing while the surgical teams may be in the operating room; and coordinate with other medical consultants or outpatient providers as needed.
This study has several limitations. This is a single-center study at an academic institution, limited to two surgical services. We did not have a control group and multiple hospital-wide interventions may have affected these outcomes. This is an observational study in which unobserved variables may bias the results. We used ICD codes to identify medical complications, which relies on the quality of physician documentation. While our response rate of 21.1% for HCAHPS was comparable to the national average of 26.7%, it may not reliably represent our patient population.15 Lastly, we had limited financial data.
CONCLUSION
With the move toward value-based payment and increasing medical complexity of surgical patients, SCM by hospitalists may deliver high-quality care.
1. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. https://doi.org/10.1001/archinternmed.2010.432
2. Ruiz ME, Merino RÁ, Rodríguez R, Sánchez GM, Alonso A, Barbero M. Effect of comanagement with internal medicine on hospital stay of patients admitted to the service of otolaryngology. Acta Otorrinolaringol Esp. 2015;66(5):264-268. https://doi.org/10.1016/j.otorri.2014.09.010.
3. Tadros RO, Faries PL, Malik R, et al. The effect of a hospitalist comanagement service on vascular surgery inpatients. J Vasc Surg. 2015;61(6):1550-1555. https://doi.org/10.1016/j.jvs.2015.01.006
4. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: A propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Polverejan E, Gardiner JC, Bradley CJ, Holmes-Rovner M, Rovner D. Estimating mean hospital cost as a function of length of stay and patient characteristics. Health Econ. 2003;12(11):935-947. https://doi.org/10.1002/hec.774
7. Rohatgi N, Wei PH, Grujic O, Ahuja N. Surgical Comanagement by hospitalists in colorectal surgery. J Am Coll Surg. 2018;227(4):404-410. https://doi.org/10.1016/j.jamcollsurg.2018.06.011
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: An economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4.
10. Iberti CT, Briones A, Gabriel E, Dunn AS. Hospitalist-vascular surgery comanagement: Effects on complications and mortality. Hosp Pract. 2016;44(5):233-236. https://doi.org/10.1080/21548331.2016.1259543.
11. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service--A literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. https://doi.org/10.1007/s00198-010-1396-x.
12. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
13. Duplantier NL, Briski DC, Luce LT, Meyer MS, Ochsner JL, Chimento GF. The effects of a hospitalist comanagement model for joint arthroplasty patients in a teaching facility. J Arthroplasty. 2016;31(3):567-572. https://doi.org/10.1016/j.arth.2015.10.010.
14. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. https://doi.org/10.4065/81.1.28.
15. Godden E, Paseka A, Gnida J, Inguanzo J. The impact of response rate on Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) dimension scores. Patient Exp J. 2019;6(1):105-114. https://doi.org/10.35680/2372-0247.1357.
In surgical comanagement (SCM), surgeons and hospitalists share responsibility of care for surgical patients. While SCM has been increasingly utilized, many of the reported models are a modification of the consultation model, in which a group of rotating hospitalists, internists, or geriatricians care for the surgical patients, often after medical complications may have occured.1-4
In August 2012, we implemented SCM in Orthopedic and Neurosurgery services at our institution.5 This model is unique because the same Internal Medicine hospitalists are dedicated year round to the same surgical service. SCM hospitalists see patients on their assigned surgical service only; they do not see patients on the Internal Medicine service. After the first year of implementing SCM, we conducted a propensity score–weighted study with 17,057 discharges in the pre-SCM group (January 2009 to July 2012) and 5,533 discharges in the post-SCM group (September 2012 to September 2013).5 In this study, SCM was associated with a decrease in medical complications, length of stay (LOS), medical consultations, 30-day readmissions, and cost.5
Since SCM requires ongoing investment by institutions, we now report a follow-up study to explore if there were continued improvements in patient outcomes with SCM. In this study, we evaluate if there was a decrease in medical complications, LOS, number of medical consultations, rapid response team calls, and code blues and an increase in patient satisfaction with SCM in Orthopedic and Neurosurgery services between 2012 and 2018.
METHODS
We included 26,380 discharges from Orthopedic and Neurosurgery services between September 1, 2012, and June 30, 2018, at our academic medical center. We excluded patients discharged in August 2012 as we transitioned to the SCM model. Our Institutional Review Board exempted this study from further review.
SCM Structure
SCM structure was detailed in a prior article.5 We have 3.0 clinical full-time equivalents on the Orthopedic surgery SCM service and 1.2 on the Neurosurgery SCM service. On weekdays, during the day (8
During the day, SCM hospitalists receive the first call for medical issues. After 5
SCM hospitalists screen the entire patient list on their assigned surgery service each day. After screening the patient list, SCM hospitalists formally see select patients with preventable or active medical conditions and write notes on the patient’s chart. There are no set criteria to determine which patients would be seen by SCM. This is because surgeries can decompensate stable medical conditions or new unexpected medical complications may occur. Additionally, in our prior study, we reported that SCM reduced medical complications and LOS regardless of age or patient acuity.5
Outcomes
Our primary outcome was proportion of patients with ≥1 medical complication (sepsis, pneumonia, urinary tract infection, delirium, acute kidney injury, atrial fibrillation, or ileus). Our secondary outcomes included mean LOS, proportion of patients with ≥2 medical consultations, rapid response team calls, code blues, and top-box patient satisfaction score. Though cost is an important consideration in implementing SCM, limited financial data were available. However, since LOS is a key component in calculating direct costs,6 we estimated the cost savings per discharge using mean direct cost per day and the difference in mean LOS between pre- and post-SCM groups.5
We defined medical complications using International Classification of Disease (ICD) Codes 9 or 10 that were coded as “not present on admission” (Appendix 1). We used Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey for three questions for patient satisfaction: Did doctors treat with courtesy and respect, listen carefully, and explain things in a way you could understand?
Statistical Analysis
We used regression analysis to assess trends in patient characteristics by year (Appendix 2). Logistic regression with logit link was used to assess the yearly change in our binary outcomes (proportion of patients with ≥1 medical complication, those with ≥2 medical consultations, rapid response team calls, code blue, and top-box patient satisfaction score) and reported odds ratios. Gamma regression with identity link was performed for our continuous outcome (LOS). Beta coefficient was reported to estimate the yearly change in LOS under their original scales. Age, primary insurance, race, Charlson comorbidity score, general or regional anesthesia, surgical service, and duration of surgery were adjusted in the regression analyses for outcomes. SAS 9.4 was used for analysis.
RESULTS
Patient characteristics are shown in Table 1. Overall, 62.8% patients were discharged from Orthopedic surgery service, 72.5% patients underwent elective surgery, and 88.8% received general anesthesia. Between 2012 and 2018, there was a significant increase in the median age of patients (from 60 years to 63 years), mean Charlson comorbidity score increased from 1.07 to 1.46, and median case mix index, a measure of patient acuity, increased from 2.10 to 2.36 (Appendix 2).
Comparing pre-SCM unadjusted rates reported in our prior study (January 2009 to July 2012) to post-SCM (September 2012 to June 2018; Appendix 3), patients with ≥1 medical complication decreased from 10.1% to 6.1%, LOS (mean ± standard deviation) changed from 5.4 ± 2.2 days to 4.6 ± 5.8 days, patients with ≥2 medical consultations decreased from 19.4% to 9.2%, rapid response team calls changed from 1% to 0.9%, code blues changed from 0.3% to 0.2%, and patients with top-box patient satisfaction score increased from 86.4% to 94.2%.5
In the adjusted analysis from 2012 to 2018, the odds of patients with ≥1 medical complication decreased by 3.8% per year (P = .01), estimated LOS decreased by 0.3 days per year (P < .0001), and the odds of rapid response team calls decreased by 12.2% per year (P = .001; Table 2). Changes over time in the odds of patients with ≥2 medical consultations, code blues, or top-box patient satisfaction score were not statistically significant (Table 2). Based on the LOS reduction pre- to post-SCM, there were estimated average direct cost savings of $3,424 per discharge between 2012 and 2018.
DISCUSSION
Since the implementation of SCM on Orthopedic and Neurosurgery services at our institution, there was a decrease in medical complications, LOS, and rapid response team calls. To our knowledge, this is one of the largest studies evaluating the benefits of SCM over 5.8 years. Similar to our prior studies on this SCM model of care,5,7 other studies have reported a decrease in medical complications,8-10 LOS,11-13 and cost of care14 with SCM.
While the changes in the unadjusted rates of outcomes over the years appeared to be small, while our patient population became older and sicker, there were significant changes in several of our outcomes in the adjusted analysis. We believe that SCM hospitalists have developed a skill set and understanding of these surgical patients over time and can manage more medically complex patients without an increase in medical complications or LOS. We attribute this to our unique SCM model in which the same hospitalists stay year round on the same surgical service. SCM hospitalists have built trusting relationships with the surgical team with greater involvement in decision making, care planning, and patient selection. With minimal turnover in the SCM group and with ongoing learning, SCM hospitalists can anticipate fluid or pain medication requirements after specific surgeries and the surgery-specific medical complications. SCM hospitalists are available on the patient units to provide timely intervention in case of medical deterioration; answer any questions from patients, families, or nursing while the surgical teams may be in the operating room; and coordinate with other medical consultants or outpatient providers as needed.
This study has several limitations. This is a single-center study at an academic institution, limited to two surgical services. We did not have a control group and multiple hospital-wide interventions may have affected these outcomes. This is an observational study in which unobserved variables may bias the results. We used ICD codes to identify medical complications, which relies on the quality of physician documentation. While our response rate of 21.1% for HCAHPS was comparable to the national average of 26.7%, it may not reliably represent our patient population.15 Lastly, we had limited financial data.
CONCLUSION
With the move toward value-based payment and increasing medical complexity of surgical patients, SCM by hospitalists may deliver high-quality care.
In surgical comanagement (SCM), surgeons and hospitalists share responsibility of care for surgical patients. While SCM has been increasingly utilized, many of the reported models are a modification of the consultation model, in which a group of rotating hospitalists, internists, or geriatricians care for the surgical patients, often after medical complications may have occured.1-4
In August 2012, we implemented SCM in Orthopedic and Neurosurgery services at our institution.5 This model is unique because the same Internal Medicine hospitalists are dedicated year round to the same surgical service. SCM hospitalists see patients on their assigned surgical service only; they do not see patients on the Internal Medicine service. After the first year of implementing SCM, we conducted a propensity score–weighted study with 17,057 discharges in the pre-SCM group (January 2009 to July 2012) and 5,533 discharges in the post-SCM group (September 2012 to September 2013).5 In this study, SCM was associated with a decrease in medical complications, length of stay (LOS), medical consultations, 30-day readmissions, and cost.5
Since SCM requires ongoing investment by institutions, we now report a follow-up study to explore if there were continued improvements in patient outcomes with SCM. In this study, we evaluate if there was a decrease in medical complications, LOS, number of medical consultations, rapid response team calls, and code blues and an increase in patient satisfaction with SCM in Orthopedic and Neurosurgery services between 2012 and 2018.
METHODS
We included 26,380 discharges from Orthopedic and Neurosurgery services between September 1, 2012, and June 30, 2018, at our academic medical center. We excluded patients discharged in August 2012 as we transitioned to the SCM model. Our Institutional Review Board exempted this study from further review.
SCM Structure
SCM structure was detailed in a prior article.5 We have 3.0 clinical full-time equivalents on the Orthopedic surgery SCM service and 1.2 on the Neurosurgery SCM service. On weekdays, during the day (8
During the day, SCM hospitalists receive the first call for medical issues. After 5
SCM hospitalists screen the entire patient list on their assigned surgery service each day. After screening the patient list, SCM hospitalists formally see select patients with preventable or active medical conditions and write notes on the patient’s chart. There are no set criteria to determine which patients would be seen by SCM. This is because surgeries can decompensate stable medical conditions or new unexpected medical complications may occur. Additionally, in our prior study, we reported that SCM reduced medical complications and LOS regardless of age or patient acuity.5
Outcomes
Our primary outcome was proportion of patients with ≥1 medical complication (sepsis, pneumonia, urinary tract infection, delirium, acute kidney injury, atrial fibrillation, or ileus). Our secondary outcomes included mean LOS, proportion of patients with ≥2 medical consultations, rapid response team calls, code blues, and top-box patient satisfaction score. Though cost is an important consideration in implementing SCM, limited financial data were available. However, since LOS is a key component in calculating direct costs,6 we estimated the cost savings per discharge using mean direct cost per day and the difference in mean LOS between pre- and post-SCM groups.5
We defined medical complications using International Classification of Disease (ICD) Codes 9 or 10 that were coded as “not present on admission” (Appendix 1). We used Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey for three questions for patient satisfaction: Did doctors treat with courtesy and respect, listen carefully, and explain things in a way you could understand?
Statistical Analysis
We used regression analysis to assess trends in patient characteristics by year (Appendix 2). Logistic regression with logit link was used to assess the yearly change in our binary outcomes (proportion of patients with ≥1 medical complication, those with ≥2 medical consultations, rapid response team calls, code blue, and top-box patient satisfaction score) and reported odds ratios. Gamma regression with identity link was performed for our continuous outcome (LOS). Beta coefficient was reported to estimate the yearly change in LOS under their original scales. Age, primary insurance, race, Charlson comorbidity score, general or regional anesthesia, surgical service, and duration of surgery were adjusted in the regression analyses for outcomes. SAS 9.4 was used for analysis.
RESULTS
Patient characteristics are shown in Table 1. Overall, 62.8% patients were discharged from Orthopedic surgery service, 72.5% patients underwent elective surgery, and 88.8% received general anesthesia. Between 2012 and 2018, there was a significant increase in the median age of patients (from 60 years to 63 years), mean Charlson comorbidity score increased from 1.07 to 1.46, and median case mix index, a measure of patient acuity, increased from 2.10 to 2.36 (Appendix 2).
Comparing pre-SCM unadjusted rates reported in our prior study (January 2009 to July 2012) to post-SCM (September 2012 to June 2018; Appendix 3), patients with ≥1 medical complication decreased from 10.1% to 6.1%, LOS (mean ± standard deviation) changed from 5.4 ± 2.2 days to 4.6 ± 5.8 days, patients with ≥2 medical consultations decreased from 19.4% to 9.2%, rapid response team calls changed from 1% to 0.9%, code blues changed from 0.3% to 0.2%, and patients with top-box patient satisfaction score increased from 86.4% to 94.2%.5
In the adjusted analysis from 2012 to 2018, the odds of patients with ≥1 medical complication decreased by 3.8% per year (P = .01), estimated LOS decreased by 0.3 days per year (P < .0001), and the odds of rapid response team calls decreased by 12.2% per year (P = .001; Table 2). Changes over time in the odds of patients with ≥2 medical consultations, code blues, or top-box patient satisfaction score were not statistically significant (Table 2). Based on the LOS reduction pre- to post-SCM, there were estimated average direct cost savings of $3,424 per discharge between 2012 and 2018.
DISCUSSION
Since the implementation of SCM on Orthopedic and Neurosurgery services at our institution, there was a decrease in medical complications, LOS, and rapid response team calls. To our knowledge, this is one of the largest studies evaluating the benefits of SCM over 5.8 years. Similar to our prior studies on this SCM model of care,5,7 other studies have reported a decrease in medical complications,8-10 LOS,11-13 and cost of care14 with SCM.
While the changes in the unadjusted rates of outcomes over the years appeared to be small, while our patient population became older and sicker, there were significant changes in several of our outcomes in the adjusted analysis. We believe that SCM hospitalists have developed a skill set and understanding of these surgical patients over time and can manage more medically complex patients without an increase in medical complications or LOS. We attribute this to our unique SCM model in which the same hospitalists stay year round on the same surgical service. SCM hospitalists have built trusting relationships with the surgical team with greater involvement in decision making, care planning, and patient selection. With minimal turnover in the SCM group and with ongoing learning, SCM hospitalists can anticipate fluid or pain medication requirements after specific surgeries and the surgery-specific medical complications. SCM hospitalists are available on the patient units to provide timely intervention in case of medical deterioration; answer any questions from patients, families, or nursing while the surgical teams may be in the operating room; and coordinate with other medical consultants or outpatient providers as needed.
This study has several limitations. This is a single-center study at an academic institution, limited to two surgical services. We did not have a control group and multiple hospital-wide interventions may have affected these outcomes. This is an observational study in which unobserved variables may bias the results. We used ICD codes to identify medical complications, which relies on the quality of physician documentation. While our response rate of 21.1% for HCAHPS was comparable to the national average of 26.7%, it may not reliably represent our patient population.15 Lastly, we had limited financial data.
CONCLUSION
With the move toward value-based payment and increasing medical complexity of surgical patients, SCM by hospitalists may deliver high-quality care.
1. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. https://doi.org/10.1001/archinternmed.2010.432
2. Ruiz ME, Merino RÁ, Rodríguez R, Sánchez GM, Alonso A, Barbero M. Effect of comanagement with internal medicine on hospital stay of patients admitted to the service of otolaryngology. Acta Otorrinolaringol Esp. 2015;66(5):264-268. https://doi.org/10.1016/j.otorri.2014.09.010.
3. Tadros RO, Faries PL, Malik R, et al. The effect of a hospitalist comanagement service on vascular surgery inpatients. J Vasc Surg. 2015;61(6):1550-1555. https://doi.org/10.1016/j.jvs.2015.01.006
4. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: A propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Polverejan E, Gardiner JC, Bradley CJ, Holmes-Rovner M, Rovner D. Estimating mean hospital cost as a function of length of stay and patient characteristics. Health Econ. 2003;12(11):935-947. https://doi.org/10.1002/hec.774
7. Rohatgi N, Wei PH, Grujic O, Ahuja N. Surgical Comanagement by hospitalists in colorectal surgery. J Am Coll Surg. 2018;227(4):404-410. https://doi.org/10.1016/j.jamcollsurg.2018.06.011
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: An economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4.
10. Iberti CT, Briones A, Gabriel E, Dunn AS. Hospitalist-vascular surgery comanagement: Effects on complications and mortality. Hosp Pract. 2016;44(5):233-236. https://doi.org/10.1080/21548331.2016.1259543.
11. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service--A literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. https://doi.org/10.1007/s00198-010-1396-x.
12. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
13. Duplantier NL, Briski DC, Luce LT, Meyer MS, Ochsner JL, Chimento GF. The effects of a hospitalist comanagement model for joint arthroplasty patients in a teaching facility. J Arthroplasty. 2016;31(3):567-572. https://doi.org/10.1016/j.arth.2015.10.010.
14. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. https://doi.org/10.4065/81.1.28.
15. Godden E, Paseka A, Gnida J, Inguanzo J. The impact of response rate on Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) dimension scores. Patient Exp J. 2019;6(1):105-114. https://doi.org/10.35680/2372-0247.1357.
1. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. https://doi.org/10.1001/archinternmed.2010.432
2. Ruiz ME, Merino RÁ, Rodríguez R, Sánchez GM, Alonso A, Barbero M. Effect of comanagement with internal medicine on hospital stay of patients admitted to the service of otolaryngology. Acta Otorrinolaringol Esp. 2015;66(5):264-268. https://doi.org/10.1016/j.otorri.2014.09.010.
3. Tadros RO, Faries PL, Malik R, et al. The effect of a hospitalist comanagement service on vascular surgery inpatients. J Vasc Surg. 2015;61(6):1550-1555. https://doi.org/10.1016/j.jvs.2015.01.006
4. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: A propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Polverejan E, Gardiner JC, Bradley CJ, Holmes-Rovner M, Rovner D. Estimating mean hospital cost as a function of length of stay and patient characteristics. Health Econ. 2003;12(11):935-947. https://doi.org/10.1002/hec.774
7. Rohatgi N, Wei PH, Grujic O, Ahuja N. Surgical Comanagement by hospitalists in colorectal surgery. J Am Coll Surg. 2018;227(4):404-410. https://doi.org/10.1016/j.jamcollsurg.2018.06.011
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: An economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4.
10. Iberti CT, Briones A, Gabriel E, Dunn AS. Hospitalist-vascular surgery comanagement: Effects on complications and mortality. Hosp Pract. 2016;44(5):233-236. https://doi.org/10.1080/21548331.2016.1259543.
11. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service--A literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. https://doi.org/10.1007/s00198-010-1396-x.
12. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
13. Duplantier NL, Briski DC, Luce LT, Meyer MS, Ochsner JL, Chimento GF. The effects of a hospitalist comanagement model for joint arthroplasty patients in a teaching facility. J Arthroplasty. 2016;31(3):567-572. https://doi.org/10.1016/j.arth.2015.10.010.
14. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. https://doi.org/10.4065/81.1.28.
15. Godden E, Paseka A, Gnida J, Inguanzo J. The impact of response rate on Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) dimension scores. Patient Exp J. 2019;6(1):105-114. https://doi.org/10.35680/2372-0247.1357.
© 2020 Society of Hospital Medicine
Describing Variability of Inpatient Consultation Practices: Physician, Patient, and Admission Factors
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
© 2020 Society of Hospital Medicine
The Hospital Readmissions Reduction Program and COPD: More Answers, More Questions
Many provisions of the Affordable Care Act (ACA) have served to support the hospitalized patient. The expansion of Medicaid and the creation of state and federal insurance exchanges for the individual insurance market both significantly lessened the financial burden of hospital care for millions of Americans. Other aspects have proven more controversial, as many of the ACA’s health policy interventions linked to cost and quality in new ways, implementing untested concepts derived from healthcare services research on a national scale.
The Hospital Readmissions Reduction Program (HRRP) was no exception. Based on early research examining readmissions,1 the ACA included a mandate for the Centers for Medicare and Medicaid Services (CMS) to establish the HRRP. Beginning in Fiscal Year 2013, the HRRP reduced payments for excessive, 30-day, risk-standardized readmissions covering six conditions and procedures. As the third leading cause of 30-day readmissions, chronic obstructive pulmonary disease (COPD) was included in the list of designated HRRP conditions.
This inclusion of COPD in HRRP was not without controversy; analysis of Medicare data from before the ACA’s implementation demonstrated that only half of all readmissions for acute exacerbations of COPD were respiratory-related and only a third were directly related to COPD.2 Unsurprisingly, the high proportion of readmissions due to non-COPD-related causes is considered to be one of the leading factors for the failure of COPD readmission reduction programs to find significant reductions in readmissions.3 In this month’s issue of the Journal of Hospital Medicine, Buhr and colleagues explore differential readmission diagnoses following acute exacerbations of COPD using a validated, national, all-payer database.4
Like many analyses of payer datasets, this study has several limitations. First, although a large area of the US was included, the data did not include all US states. Further, as the study used multiple cross-sectional data using pooling techniques, it was not truly a longitudinal study. It was additionally limited to 10 months out of the calendar year, missing December and January, which have a high seasonal prevalence of viral respiratory illness. Finally, due to the nature of the data, COPD diagnoses were identified through administrative data known to be highly unreliable for fully capturing admissions for acute exacerbation of COPD.
Despite these limitations, the analysis by Buhr and colleagues provides additional value. They found an overall readmission rate of 17%, with just under half (7.69%) due to recurrent COPD. Patients with COPD-related readmissions were younger, had a higher proportion with Medicaid as the payer, were more frequently discharged home without services, had a shorter length of stay, and had fewer comorbidities.
Most critically, Buhr and colleagues—with a multipayer database—confirmed what researchers found in uni-payer5 and site-specific6 datasets: over half of readmissions are due to diagnoses other than COPD or respiratory-related causes. Patients readmitted due to other, unrelated diagnoses had a higher mean Elixhauser Comorbidity Index score along with higher rates of congestive heart failure and renal failure. To the practicing hospitalist, this finding supports what our internal clinical voice tells us: sicker patients are readmitted more often and more frequently with conditions unrelated to their index admission diagnosis.
The reaffirmation of the finding that the majority of readmissions are due to nonrespiratory-related causes suggests that perhaps we have a different problem than physicians and policymakers originally thought when adding COPD to the HRRP. Many COPD patients suffer from a polychronic disease, requiring a more holistic approach rather than a traditional, disease-driven, siloed approach focused solely on improving COPD-related care. It may also be true that for other subpopulations of patients with COPD, additional in-hospital and transition of care interventions are required to address patients’ multimorbidity and social determinants of health.
As physicians on the front lines of the readmitted patient, hospitalists are uniquely situated to see the challenges of populations with increasing disease complexity and disease combinations.7 The HRRP policy remains controversial. This is due in large part to recent work suggesting that while the HRRP may have helped reduce readmissions, its implementation may have driven the unintended consequence of increased mortality.8 Thus, our profession faces an existential challenge to traditional care delivery models targeting diseases. What has not been well parsed by the hospital industry or policymakers is what to do about it.
Readmission of the multimorbid patient, coupled with the challenges of the HRRP, focuses our attention on the need to transition care delivery to a model that is better suited to our patients’ needs: mass-customized, mass-produced service delivery. As physicians, we know that care delivery must be oriented around patients who have many diseases and unique life circumstances. It is our profession’s greatest challenge to collaborate with researchers and administrators to help do this with scale.
The authors thank Mary Akel for her assistance with manuscript submission.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalization among patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
2. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
3. Press VG, Au DH, Bourbeau J, Dransfield MT, Gershon AS, Krishnan JA, et al. An American thoracic society workshop report: reducing COPD hospital readmissions. Ann Am Thorac Soc. 2019;16(2):161-170. https://doi.org/10.1513/AnnalsATS.201811-755WS.
4. Buhr R, Jackson N, Kominski G, Ong M, Mangione C. Factors associated with differential readmission diagnoses following acute exacerbations of COPD. J Hosp Med. 2020;15(4):252-253. https://doi.org/10.12788/jhm.3367.
5. Sharif R, Parekh TM, Pierson KS, Kuo Y-F, Sharma G. Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Annals ATS. 2014;11(5):685-694. https://doi.org/10.1513/AnnalsATS.201310-358OC.
6. Glaser JB, El-Haddad H. Exploring novel Medicare readmission risk variables in chronic obstructive pulmonary disease patients at high risk of readmission within 30 days of hospital discharge. Ann Am Thorac Soc. 2015;12(9):1288-1293. https://doi.org/10.1513/AnnalsATS.201504-228OC.
7. Sorace J, Wong HH, Worrall C, Kelman J, Saneinejad S, MaCurdy T. The complexity of disease combinations in the Medicare population. Popul Health Manag. 2011;14(4):161-166. https://doi.org/10.1089/pop.2010.0044
8. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232.
Many provisions of the Affordable Care Act (ACA) have served to support the hospitalized patient. The expansion of Medicaid and the creation of state and federal insurance exchanges for the individual insurance market both significantly lessened the financial burden of hospital care for millions of Americans. Other aspects have proven more controversial, as many of the ACA’s health policy interventions linked to cost and quality in new ways, implementing untested concepts derived from healthcare services research on a national scale.
The Hospital Readmissions Reduction Program (HRRP) was no exception. Based on early research examining readmissions,1 the ACA included a mandate for the Centers for Medicare and Medicaid Services (CMS) to establish the HRRP. Beginning in Fiscal Year 2013, the HRRP reduced payments for excessive, 30-day, risk-standardized readmissions covering six conditions and procedures. As the third leading cause of 30-day readmissions, chronic obstructive pulmonary disease (COPD) was included in the list of designated HRRP conditions.
This inclusion of COPD in HRRP was not without controversy; analysis of Medicare data from before the ACA’s implementation demonstrated that only half of all readmissions for acute exacerbations of COPD were respiratory-related and only a third were directly related to COPD.2 Unsurprisingly, the high proportion of readmissions due to non-COPD-related causes is considered to be one of the leading factors for the failure of COPD readmission reduction programs to find significant reductions in readmissions.3 In this month’s issue of the Journal of Hospital Medicine, Buhr and colleagues explore differential readmission diagnoses following acute exacerbations of COPD using a validated, national, all-payer database.4
Like many analyses of payer datasets, this study has several limitations. First, although a large area of the US was included, the data did not include all US states. Further, as the study used multiple cross-sectional data using pooling techniques, it was not truly a longitudinal study. It was additionally limited to 10 months out of the calendar year, missing December and January, which have a high seasonal prevalence of viral respiratory illness. Finally, due to the nature of the data, COPD diagnoses were identified through administrative data known to be highly unreliable for fully capturing admissions for acute exacerbation of COPD.
Despite these limitations, the analysis by Buhr and colleagues provides additional value. They found an overall readmission rate of 17%, with just under half (7.69%) due to recurrent COPD. Patients with COPD-related readmissions were younger, had a higher proportion with Medicaid as the payer, were more frequently discharged home without services, had a shorter length of stay, and had fewer comorbidities.
Most critically, Buhr and colleagues—with a multipayer database—confirmed what researchers found in uni-payer5 and site-specific6 datasets: over half of readmissions are due to diagnoses other than COPD or respiratory-related causes. Patients readmitted due to other, unrelated diagnoses had a higher mean Elixhauser Comorbidity Index score along with higher rates of congestive heart failure and renal failure. To the practicing hospitalist, this finding supports what our internal clinical voice tells us: sicker patients are readmitted more often and more frequently with conditions unrelated to their index admission diagnosis.
The reaffirmation of the finding that the majority of readmissions are due to nonrespiratory-related causes suggests that perhaps we have a different problem than physicians and policymakers originally thought when adding COPD to the HRRP. Many COPD patients suffer from a polychronic disease, requiring a more holistic approach rather than a traditional, disease-driven, siloed approach focused solely on improving COPD-related care. It may also be true that for other subpopulations of patients with COPD, additional in-hospital and transition of care interventions are required to address patients’ multimorbidity and social determinants of health.
As physicians on the front lines of the readmitted patient, hospitalists are uniquely situated to see the challenges of populations with increasing disease complexity and disease combinations.7 The HRRP policy remains controversial. This is due in large part to recent work suggesting that while the HRRP may have helped reduce readmissions, its implementation may have driven the unintended consequence of increased mortality.8 Thus, our profession faces an existential challenge to traditional care delivery models targeting diseases. What has not been well parsed by the hospital industry or policymakers is what to do about it.
Readmission of the multimorbid patient, coupled with the challenges of the HRRP, focuses our attention on the need to transition care delivery to a model that is better suited to our patients’ needs: mass-customized, mass-produced service delivery. As physicians, we know that care delivery must be oriented around patients who have many diseases and unique life circumstances. It is our profession’s greatest challenge to collaborate with researchers and administrators to help do this with scale.
The authors thank Mary Akel for her assistance with manuscript submission.
Many provisions of the Affordable Care Act (ACA) have served to support the hospitalized patient. The expansion of Medicaid and the creation of state and federal insurance exchanges for the individual insurance market both significantly lessened the financial burden of hospital care for millions of Americans. Other aspects have proven more controversial, as many of the ACA’s health policy interventions linked to cost and quality in new ways, implementing untested concepts derived from healthcare services research on a national scale.
The Hospital Readmissions Reduction Program (HRRP) was no exception. Based on early research examining readmissions,1 the ACA included a mandate for the Centers for Medicare and Medicaid Services (CMS) to establish the HRRP. Beginning in Fiscal Year 2013, the HRRP reduced payments for excessive, 30-day, risk-standardized readmissions covering six conditions and procedures. As the third leading cause of 30-day readmissions, chronic obstructive pulmonary disease (COPD) was included in the list of designated HRRP conditions.
This inclusion of COPD in HRRP was not without controversy; analysis of Medicare data from before the ACA’s implementation demonstrated that only half of all readmissions for acute exacerbations of COPD were respiratory-related and only a third were directly related to COPD.2 Unsurprisingly, the high proportion of readmissions due to non-COPD-related causes is considered to be one of the leading factors for the failure of COPD readmission reduction programs to find significant reductions in readmissions.3 In this month’s issue of the Journal of Hospital Medicine, Buhr and colleagues explore differential readmission diagnoses following acute exacerbations of COPD using a validated, national, all-payer database.4
Like many analyses of payer datasets, this study has several limitations. First, although a large area of the US was included, the data did not include all US states. Further, as the study used multiple cross-sectional data using pooling techniques, it was not truly a longitudinal study. It was additionally limited to 10 months out of the calendar year, missing December and January, which have a high seasonal prevalence of viral respiratory illness. Finally, due to the nature of the data, COPD diagnoses were identified through administrative data known to be highly unreliable for fully capturing admissions for acute exacerbation of COPD.
Despite these limitations, the analysis by Buhr and colleagues provides additional value. They found an overall readmission rate of 17%, with just under half (7.69%) due to recurrent COPD. Patients with COPD-related readmissions were younger, had a higher proportion with Medicaid as the payer, were more frequently discharged home without services, had a shorter length of stay, and had fewer comorbidities.
Most critically, Buhr and colleagues—with a multipayer database—confirmed what researchers found in uni-payer5 and site-specific6 datasets: over half of readmissions are due to diagnoses other than COPD or respiratory-related causes. Patients readmitted due to other, unrelated diagnoses had a higher mean Elixhauser Comorbidity Index score along with higher rates of congestive heart failure and renal failure. To the practicing hospitalist, this finding supports what our internal clinical voice tells us: sicker patients are readmitted more often and more frequently with conditions unrelated to their index admission diagnosis.
The reaffirmation of the finding that the majority of readmissions are due to nonrespiratory-related causes suggests that perhaps we have a different problem than physicians and policymakers originally thought when adding COPD to the HRRP. Many COPD patients suffer from a polychronic disease, requiring a more holistic approach rather than a traditional, disease-driven, siloed approach focused solely on improving COPD-related care. It may also be true that for other subpopulations of patients with COPD, additional in-hospital and transition of care interventions are required to address patients’ multimorbidity and social determinants of health.
As physicians on the front lines of the readmitted patient, hospitalists are uniquely situated to see the challenges of populations with increasing disease complexity and disease combinations.7 The HRRP policy remains controversial. This is due in large part to recent work suggesting that while the HRRP may have helped reduce readmissions, its implementation may have driven the unintended consequence of increased mortality.8 Thus, our profession faces an existential challenge to traditional care delivery models targeting diseases. What has not been well parsed by the hospital industry or policymakers is what to do about it.
Readmission of the multimorbid patient, coupled with the challenges of the HRRP, focuses our attention on the need to transition care delivery to a model that is better suited to our patients’ needs: mass-customized, mass-produced service delivery. As physicians, we know that care delivery must be oriented around patients who have many diseases and unique life circumstances. It is our profession’s greatest challenge to collaborate with researchers and administrators to help do this with scale.
The authors thank Mary Akel for her assistance with manuscript submission.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalization among patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
2. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
3. Press VG, Au DH, Bourbeau J, Dransfield MT, Gershon AS, Krishnan JA, et al. An American thoracic society workshop report: reducing COPD hospital readmissions. Ann Am Thorac Soc. 2019;16(2):161-170. https://doi.org/10.1513/AnnalsATS.201811-755WS.
4. Buhr R, Jackson N, Kominski G, Ong M, Mangione C. Factors associated with differential readmission diagnoses following acute exacerbations of COPD. J Hosp Med. 2020;15(4):252-253. https://doi.org/10.12788/jhm.3367.
5. Sharif R, Parekh TM, Pierson KS, Kuo Y-F, Sharma G. Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Annals ATS. 2014;11(5):685-694. https://doi.org/10.1513/AnnalsATS.201310-358OC.
6. Glaser JB, El-Haddad H. Exploring novel Medicare readmission risk variables in chronic obstructive pulmonary disease patients at high risk of readmission within 30 days of hospital discharge. Ann Am Thorac Soc. 2015;12(9):1288-1293. https://doi.org/10.1513/AnnalsATS.201504-228OC.
7. Sorace J, Wong HH, Worrall C, Kelman J, Saneinejad S, MaCurdy T. The complexity of disease combinations in the Medicare population. Popul Health Manag. 2011;14(4):161-166. https://doi.org/10.1089/pop.2010.0044
8. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalization among patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
2. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
3. Press VG, Au DH, Bourbeau J, Dransfield MT, Gershon AS, Krishnan JA, et al. An American thoracic society workshop report: reducing COPD hospital readmissions. Ann Am Thorac Soc. 2019;16(2):161-170. https://doi.org/10.1513/AnnalsATS.201811-755WS.
4. Buhr R, Jackson N, Kominski G, Ong M, Mangione C. Factors associated with differential readmission diagnoses following acute exacerbations of COPD. J Hosp Med. 2020;15(4):252-253. https://doi.org/10.12788/jhm.3367.
5. Sharif R, Parekh TM, Pierson KS, Kuo Y-F, Sharma G. Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Annals ATS. 2014;11(5):685-694. https://doi.org/10.1513/AnnalsATS.201310-358OC.
6. Glaser JB, El-Haddad H. Exploring novel Medicare readmission risk variables in chronic obstructive pulmonary disease patients at high risk of readmission within 30 days of hospital discharge. Ann Am Thorac Soc. 2015;12(9):1288-1293. https://doi.org/10.1513/AnnalsATS.201504-228OC.
7. Sorace J, Wong HH, Worrall C, Kelman J, Saneinejad S, MaCurdy T. The complexity of disease combinations in the Medicare population. Popul Health Manag. 2011;14(4):161-166. https://doi.org/10.1089/pop.2010.0044
8. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232.
© 2020 Society of Hospital Medicine
Hypofractionated radiotherapy for prostate cancer stands the test of time
SAN FRANCISCO – an update of the CHHiP trial shows.
The 3,216 men in the phase 3 trial had node-negative T1b-T3a prostate cancer and were evenly assigned to a conventional regimen of 74 Gy delivered in 37 fractions, a hypofractionated regimen of 60 Gy in 20 fractions, or a hypofractionated regimen of 57 Gy in 19 fractions. All regimens were delivered with intensity-modulated techniques.
The trial’s 5-year results, previously reported, showed noninferiority of the 60-Gy regimen, compared with the 74-Gy regimen on risk of biochemical or clinical failure (hazard ratio, 0.84), prompting recommendation of the former as a new standard of care for localized prostate cancer (Lancet Oncol. 2016;17:1047-60). Noninferiority could not be established for the 57-Gy regimen.
The 8-year results were essentially the same, confirming noninferiority of the 60-Gy regimen (HR, 0.85) but not the 57-Gy regimen. Meanwhile, bowel and bladder toxicity continued to be low across regimens.
David P. Dearnaley, MB BCh, MD, of the Royal Marsden NHS Foundation Trust, London, reported the 8-year results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Study details
At a median follow-up of 9.3 years, the 8-year rate of freedom from biochemical failure (defined by Phoenix consensus guidelines) or clinical failure (cancer recurrence) was 80.6% with 74 Gy, 83.7% with 60 Gy, and 78.5% with 57 Gy, Dr. Dearnaley reported.
Analyses confirmed noninferiority of the 60-Gy regimen (HR, 0.85; 95% confidence interval, 0.72-1.01; P = .11), but not the 57-Gy regimen (HR, 1.17; 95% CI, 1.00-1.36; P = .10), as the upper bound of the confidence interval crossed the predefined 1.21 boundary for noninferiority.
In an unplanned analysis, the pattern among men younger than 75 years was similar to that in the entire trial population. But among men 75 years of age and older, the 57-Gy arm is actually outperforming the 74-Gy arm (HR, 0.77).
The three regimens yielded a similarly high rate of freedom from metastases, at about 95% in each arm. The 60-Gy regimen had an edge in overall survival relative to the 74-Gy regimen (88.6% vs. 85.9%; HR, 0.84) that is hard to explain, according to Dr. Dearnaley.
“Because there is an 8:1 ratio of non–prostate cancer deaths to prostate cancer deaths, you would have to postulate something other than prostate cancer being affected by the radiotherapy fractionation,” he said. “The answers on a postcard, because I can’t think of one.”
On central pathology review, nearly a fifth of evaluated trial patients had high-risk disease. “I know everybody wants to know about high-risk patients, but I’d rather take the trial results as a whole and look to see if there is any heterogeneity between those groups rather than perform a specific high-risk subgroup analysis,” Dr. Dearnaley said, expressing concern about performing too many subgroup analyses.
That said, older patients on the trial tended to have higher risk. “It does seem hypofractionation was particularly useful in those patients,” he noted. “Now, whether that’s anything to do with their pathology or whether it’s due to their age per se, I really don’t know.”
There were no differences between groups on rates of Radiation Therapy Oncology Group toxicity at 5 years, with grade 2 or worse bowel toxicity and bladder toxicity each seen in about 2% of patients.
There were no significant differences in rates of patient-reported “moderate or big” bowel bother (roughly 5%-8%) and urinary bother (roughly 7%-9%). For all regimens, bowel and urinary symptoms remained stable from 2-5 years.
Reassuring for practice
These updated findings “support the continued use of 60 Gy in 20 fractions as the standard of care,” Dr. Dearnaley said.
When the math is run to permit comparison, efficacy findings of the CHHiP trial show “amazing agreement” with those of the similar multinational PROFIT trial, he noted (J Clin Oncol. 2017 Jun 10;35(17):1884-90).
The absolute advantage in the failure-free rate of 3.1% and the overall survival rate of 2.7% for the 60-Gy regimen in CHHiP generated interest among symposium attendees about its possible superiority. “I think the 60 Gy is marginally more effective than the 74 Gy,” Dr. Dearnaley said, but he acknowledged that there are no statistics to prove that.
“This CHHiP update is fantastic,” said session cochair Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston. “It is very reassuring that the initial results the investigators presented several years ago still hold up in the long term. It’s even more reassuring for the use of hypofractionation, and it’s great to know that we can use it across the age spectrum and it works well.”
This trial is the only noninferiority hypofractionation trial in prostate cancer that includes a sizable share of patients at high risk for poor outcomes, a population for whom efficacy of this strategy is of particular interest, Dr. Nguyen noted.
“That’s always been a question,” he said. “The majority of the data from the noninferiority trials is for the low- and intermediate-risk patients. So it really would be interesting to learn whatever we can about high-risk patients from this trial.”
The trial was funded by Cancer Research UK, Department of Health (UK), and the National Institute for Health Research Cancer Research Network. Dr. Dearnaley and Dr. Nguyen disclosed relationships with a range of pharmaceutical companies.
SOURCE: Dearnaley DP et al. GUCS 2020. Abstract 325.
SAN FRANCISCO – an update of the CHHiP trial shows.
The 3,216 men in the phase 3 trial had node-negative T1b-T3a prostate cancer and were evenly assigned to a conventional regimen of 74 Gy delivered in 37 fractions, a hypofractionated regimen of 60 Gy in 20 fractions, or a hypofractionated regimen of 57 Gy in 19 fractions. All regimens were delivered with intensity-modulated techniques.
The trial’s 5-year results, previously reported, showed noninferiority of the 60-Gy regimen, compared with the 74-Gy regimen on risk of biochemical or clinical failure (hazard ratio, 0.84), prompting recommendation of the former as a new standard of care for localized prostate cancer (Lancet Oncol. 2016;17:1047-60). Noninferiority could not be established for the 57-Gy regimen.
The 8-year results were essentially the same, confirming noninferiority of the 60-Gy regimen (HR, 0.85) but not the 57-Gy regimen. Meanwhile, bowel and bladder toxicity continued to be low across regimens.
David P. Dearnaley, MB BCh, MD, of the Royal Marsden NHS Foundation Trust, London, reported the 8-year results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Study details
At a median follow-up of 9.3 years, the 8-year rate of freedom from biochemical failure (defined by Phoenix consensus guidelines) or clinical failure (cancer recurrence) was 80.6% with 74 Gy, 83.7% with 60 Gy, and 78.5% with 57 Gy, Dr. Dearnaley reported.
Analyses confirmed noninferiority of the 60-Gy regimen (HR, 0.85; 95% confidence interval, 0.72-1.01; P = .11), but not the 57-Gy regimen (HR, 1.17; 95% CI, 1.00-1.36; P = .10), as the upper bound of the confidence interval crossed the predefined 1.21 boundary for noninferiority.
In an unplanned analysis, the pattern among men younger than 75 years was similar to that in the entire trial population. But among men 75 years of age and older, the 57-Gy arm is actually outperforming the 74-Gy arm (HR, 0.77).
The three regimens yielded a similarly high rate of freedom from metastases, at about 95% in each arm. The 60-Gy regimen had an edge in overall survival relative to the 74-Gy regimen (88.6% vs. 85.9%; HR, 0.84) that is hard to explain, according to Dr. Dearnaley.
“Because there is an 8:1 ratio of non–prostate cancer deaths to prostate cancer deaths, you would have to postulate something other than prostate cancer being affected by the radiotherapy fractionation,” he said. “The answers on a postcard, because I can’t think of one.”
On central pathology review, nearly a fifth of evaluated trial patients had high-risk disease. “I know everybody wants to know about high-risk patients, but I’d rather take the trial results as a whole and look to see if there is any heterogeneity between those groups rather than perform a specific high-risk subgroup analysis,” Dr. Dearnaley said, expressing concern about performing too many subgroup analyses.
That said, older patients on the trial tended to have higher risk. “It does seem hypofractionation was particularly useful in those patients,” he noted. “Now, whether that’s anything to do with their pathology or whether it’s due to their age per se, I really don’t know.”
There were no differences between groups on rates of Radiation Therapy Oncology Group toxicity at 5 years, with grade 2 or worse bowel toxicity and bladder toxicity each seen in about 2% of patients.
There were no significant differences in rates of patient-reported “moderate or big” bowel bother (roughly 5%-8%) and urinary bother (roughly 7%-9%). For all regimens, bowel and urinary symptoms remained stable from 2-5 years.
Reassuring for practice
These updated findings “support the continued use of 60 Gy in 20 fractions as the standard of care,” Dr. Dearnaley said.
When the math is run to permit comparison, efficacy findings of the CHHiP trial show “amazing agreement” with those of the similar multinational PROFIT trial, he noted (J Clin Oncol. 2017 Jun 10;35(17):1884-90).
The absolute advantage in the failure-free rate of 3.1% and the overall survival rate of 2.7% for the 60-Gy regimen in CHHiP generated interest among symposium attendees about its possible superiority. “I think the 60 Gy is marginally more effective than the 74 Gy,” Dr. Dearnaley said, but he acknowledged that there are no statistics to prove that.
“This CHHiP update is fantastic,” said session cochair Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston. “It is very reassuring that the initial results the investigators presented several years ago still hold up in the long term. It’s even more reassuring for the use of hypofractionation, and it’s great to know that we can use it across the age spectrum and it works well.”
This trial is the only noninferiority hypofractionation trial in prostate cancer that includes a sizable share of patients at high risk for poor outcomes, a population for whom efficacy of this strategy is of particular interest, Dr. Nguyen noted.
“That’s always been a question,” he said. “The majority of the data from the noninferiority trials is for the low- and intermediate-risk patients. So it really would be interesting to learn whatever we can about high-risk patients from this trial.”
The trial was funded by Cancer Research UK, Department of Health (UK), and the National Institute for Health Research Cancer Research Network. Dr. Dearnaley and Dr. Nguyen disclosed relationships with a range of pharmaceutical companies.
SOURCE: Dearnaley DP et al. GUCS 2020. Abstract 325.
SAN FRANCISCO – an update of the CHHiP trial shows.
The 3,216 men in the phase 3 trial had node-negative T1b-T3a prostate cancer and were evenly assigned to a conventional regimen of 74 Gy delivered in 37 fractions, a hypofractionated regimen of 60 Gy in 20 fractions, or a hypofractionated regimen of 57 Gy in 19 fractions. All regimens were delivered with intensity-modulated techniques.
The trial’s 5-year results, previously reported, showed noninferiority of the 60-Gy regimen, compared with the 74-Gy regimen on risk of biochemical or clinical failure (hazard ratio, 0.84), prompting recommendation of the former as a new standard of care for localized prostate cancer (Lancet Oncol. 2016;17:1047-60). Noninferiority could not be established for the 57-Gy regimen.
The 8-year results were essentially the same, confirming noninferiority of the 60-Gy regimen (HR, 0.85) but not the 57-Gy regimen. Meanwhile, bowel and bladder toxicity continued to be low across regimens.
David P. Dearnaley, MB BCh, MD, of the Royal Marsden NHS Foundation Trust, London, reported the 8-year results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Study details
At a median follow-up of 9.3 years, the 8-year rate of freedom from biochemical failure (defined by Phoenix consensus guidelines) or clinical failure (cancer recurrence) was 80.6% with 74 Gy, 83.7% with 60 Gy, and 78.5% with 57 Gy, Dr. Dearnaley reported.
Analyses confirmed noninferiority of the 60-Gy regimen (HR, 0.85; 95% confidence interval, 0.72-1.01; P = .11), but not the 57-Gy regimen (HR, 1.17; 95% CI, 1.00-1.36; P = .10), as the upper bound of the confidence interval crossed the predefined 1.21 boundary for noninferiority.
In an unplanned analysis, the pattern among men younger than 75 years was similar to that in the entire trial population. But among men 75 years of age and older, the 57-Gy arm is actually outperforming the 74-Gy arm (HR, 0.77).
The three regimens yielded a similarly high rate of freedom from metastases, at about 95% in each arm. The 60-Gy regimen had an edge in overall survival relative to the 74-Gy regimen (88.6% vs. 85.9%; HR, 0.84) that is hard to explain, according to Dr. Dearnaley.
“Because there is an 8:1 ratio of non–prostate cancer deaths to prostate cancer deaths, you would have to postulate something other than prostate cancer being affected by the radiotherapy fractionation,” he said. “The answers on a postcard, because I can’t think of one.”
On central pathology review, nearly a fifth of evaluated trial patients had high-risk disease. “I know everybody wants to know about high-risk patients, but I’d rather take the trial results as a whole and look to see if there is any heterogeneity between those groups rather than perform a specific high-risk subgroup analysis,” Dr. Dearnaley said, expressing concern about performing too many subgroup analyses.
That said, older patients on the trial tended to have higher risk. “It does seem hypofractionation was particularly useful in those patients,” he noted. “Now, whether that’s anything to do with their pathology or whether it’s due to their age per se, I really don’t know.”
There were no differences between groups on rates of Radiation Therapy Oncology Group toxicity at 5 years, with grade 2 or worse bowel toxicity and bladder toxicity each seen in about 2% of patients.
There were no significant differences in rates of patient-reported “moderate or big” bowel bother (roughly 5%-8%) and urinary bother (roughly 7%-9%). For all regimens, bowel and urinary symptoms remained stable from 2-5 years.
Reassuring for practice
These updated findings “support the continued use of 60 Gy in 20 fractions as the standard of care,” Dr. Dearnaley said.
When the math is run to permit comparison, efficacy findings of the CHHiP trial show “amazing agreement” with those of the similar multinational PROFIT trial, he noted (J Clin Oncol. 2017 Jun 10;35(17):1884-90).
The absolute advantage in the failure-free rate of 3.1% and the overall survival rate of 2.7% for the 60-Gy regimen in CHHiP generated interest among symposium attendees about its possible superiority. “I think the 60 Gy is marginally more effective than the 74 Gy,” Dr. Dearnaley said, but he acknowledged that there are no statistics to prove that.
“This CHHiP update is fantastic,” said session cochair Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston. “It is very reassuring that the initial results the investigators presented several years ago still hold up in the long term. It’s even more reassuring for the use of hypofractionation, and it’s great to know that we can use it across the age spectrum and it works well.”
This trial is the only noninferiority hypofractionation trial in prostate cancer that includes a sizable share of patients at high risk for poor outcomes, a population for whom efficacy of this strategy is of particular interest, Dr. Nguyen noted.
“That’s always been a question,” he said. “The majority of the data from the noninferiority trials is for the low- and intermediate-risk patients. So it really would be interesting to learn whatever we can about high-risk patients from this trial.”
The trial was funded by Cancer Research UK, Department of Health (UK), and the National Institute for Health Research Cancer Research Network. Dr. Dearnaley and Dr. Nguyen disclosed relationships with a range of pharmaceutical companies.
SOURCE: Dearnaley DP et al. GUCS 2020. Abstract 325.
REPORTING FROM GUCS 2020