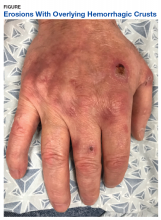
User login
Expert: Eliminating HCV ‘sounds ambitious, but I think it’s possible’
LAS VEGAS – Between 2010 and 2017, the proportion of newly diagnosed cases of acute hepatitis C virus infection rose threefold, driven largely by the concomitant opioid epidemic.
That makes efforts to screen, diagnose, and cure high-risk populations more important than ever, Stevan A. Gonzalez, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
About 70% of HCV cases are related to injection drug use,” said Dr. Gonzalez, medical director of liver transplantation at the Baylor Simmons Transplant Institute at the Baylor Scott & White All Saints Medical Center in Fort Worth, Tex. “This is affecting whites as much as blacks and Hispanics, females as much as males, and in nonurban areas as much as in urban areas.”
Data from the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration indicate that during 2004-2014, the number of acute HCV cases among those aged 18-29 years increased 400%, and the use of injection opioids rose 600%.
At the same time, the number of HCV cases among those aged 30-39 years increased 325%, and the use of injection opioids rose 83%.
“We’re starting to see a pattern overlapping between HCV exposure and opioid injection,” Dr. Gonzalez said. Other high-risk populations include homeless and incarcerated individuals.
More than 70 million people worldwide have chronic HCV infection, Dr. Gonzalez noted, with possibly as many as 5 million cases in the United States. It remains the nation’s most common blood-borne infection.
Chronic disease develops in up to 85% of people who are exposed, infection is asymptomatic, and HCV remains one of the leading indications for liver transplantation and causes of liver cancer.
From a geographic standpoint, the prevalence of HCV in young adults is eclipsing that of Baby Boomers in several states in the Appalachian region and in Northeast, which have long been trouble spots for opioid use disorder (Gastroenterol. 2018 May;154[6]:1850-1).
Surprising exposure risk
The primary risk of transmission is through contaminated blood and the exposure through needles.
“It really doesn’t matter whether it’s a needle that has a small amount of dead space where a little bit of blood can remain or needles that have a larger amount of blood,” Dr. Gonzalez said.
“I’ve had patients who come to me and say, ‘I can’t believe I have HCV. It’s impossible. I always use my own needles. They’re always brand new; I’ve never shared with anybody,’” he continued.
“This is where education and awareness is so critical, because it’s not just the needles,” Dr. Gonzalez explained. “HCV can survive on inanimate objects. For example, on a tabletop surface or a water container, HCV can remain viable up to 3 weeks. In a syringe, 2 months. For that reason, HCV can also be transmitted through crack pipes and nasal drug use, where the prevalence can be up to 35%.”
The duration of a person’s HCV infection drives the transmission.
“That’s important to think about, because people who have chronic hepatitis C are infectious until they’re treated,” Dr. Gonzalez said. “If they don’t know that they have hepatitis C, they continue to transmit the virus to others.”
One study found that half of people living with HCV are unaware of their infection (PLoS One. 2014 Jul 2;9[7]:e101554). According to Dr. Gonzalez, forthcoming guidelines from the U.S. Preventive Services Task Force are expected to recommend a one-time screening for HCV infection in all adults aged 18-79 years, a Grade B recommendation. “That’s a big deal,” he said. (The draft recommendations are available here.)
HCV infection disproportionately affects individuals in correctional institutions. In fact, an estimated one in three inmates in the United States has chronic HCV.
“This is sort of a forgotten population with a lot of substance use and mental illness,” Dr. Gonzalez said. “Injection drug use in that setting is the most common risk factor: It’s about 60% in terms of the risk of transmission within correctional settings. HCV-associated liver disease has now surpassed HIV as a cause of death within correctional settings.”
Weighing treatment options
The most common oral regimens for chronic HCV include sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir. They achieve cure in 93%-100% of cases.
“HCV can be cured; it can be eradicated from the body long term,” Dr. Gonzalez said. “The choice of regimen, treatment duration, and use of ribavirin depends on the presence/absence of cirrhosis, prior treatment experience, and the genotype.”
All six forms of the HCV genotype can be treated with oral medication, he added, and methadone, bupropion, and naloxone are safe to use during therapy.
Reinfection following HCV treatment occurs infrequently. Dr. Gonzalez cited a randomized, controlled trial presented as an abstract at the 2018 annual meeting of the American Association for the Study of Liver Diseases. That study’s researchers found that – among 199 patients on opioid-replacement therapy who were receiving direct-acting antiviral therapy, in whom greater than 50% were actively using drugs – the rate of reinfection at 3 years was 1.8 reinfections/100 person-years.
“That’s lower than people expect,” Dr. Gonzalez said.
How to boost screening
Electronic health record systems can be used as an important tool to increase HCV screening in health care settings.
In 2017, researchers published an analysis of three randomized trials carried out at three separate primary care settings to improve screening for HCV: repeated mailings, an EHR best practice alert (BPA), and patient solicitation (Hepatology 2017 Jan;65[1]:44-53). They evaluated HCV antibody testing, diagnosis, and costs for each of the interventions, compared with standard-of-care testing.
The investigators found that the BPA intervention had the lowest incremental cost per completed test – $24 with fixed start-up costs, including technical design and development of the BPA system; $3 without fixed start-up costs. The BPA intervention also had the lowest incremental cost per new case identified.
Other efforts to expand access to screening and treatment are underway.
In 2019, Louisiana health officials negotiated a one-time fee for unlimited access for 5 years to sofosbuvir/velpatasvir (Epclusa) to treat the estimated 30,000 patients on Louisiana Medicaid and in that state’s department of corrections who have HCV.
“The goal is 90% cure; the burden is on the state health department to screen, diagnose, and dispense medication,” Dr. Gonzalez said.
Also in 2019, the state of Washington used an open bidding process to negotiate access to glecaprevir/pibrentasvir (Mavyret) for the state’s Medicaid population who have HCV.
“Those states are setting the pace,” Dr. Gonzalez said. “They are showing examples of how we can start implementing a process to treat these vulnerable populations.”
Meanwhile, the World Health Organization set a goal of eliminating viral hepatitis as a major public health threat by 2030.
“That sounds ambitious, but I think it’s possible,” Dr. Gonzalez said. “It’s important to address these high-risk populations: the incarcerated, people who use drugs, and the homeless, because those are the groups that have a high prevalence of HCV – mainly through injection drug use.
“If we don’t address that population, and we only target the general population, we’re going to have a continual source of transmission,” Dr. Gonzalez warned. “In that case, we would never be able to achieve elimination.”
Dr. Gonzalez disclosed that he is a member of the speakers bureau for AbbVie and Salix.
LAS VEGAS – Between 2010 and 2017, the proportion of newly diagnosed cases of acute hepatitis C virus infection rose threefold, driven largely by the concomitant opioid epidemic.
That makes efforts to screen, diagnose, and cure high-risk populations more important than ever, Stevan A. Gonzalez, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
About 70% of HCV cases are related to injection drug use,” said Dr. Gonzalez, medical director of liver transplantation at the Baylor Simmons Transplant Institute at the Baylor Scott & White All Saints Medical Center in Fort Worth, Tex. “This is affecting whites as much as blacks and Hispanics, females as much as males, and in nonurban areas as much as in urban areas.”
Data from the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration indicate that during 2004-2014, the number of acute HCV cases among those aged 18-29 years increased 400%, and the use of injection opioids rose 600%.
At the same time, the number of HCV cases among those aged 30-39 years increased 325%, and the use of injection opioids rose 83%.
“We’re starting to see a pattern overlapping between HCV exposure and opioid injection,” Dr. Gonzalez said. Other high-risk populations include homeless and incarcerated individuals.
More than 70 million people worldwide have chronic HCV infection, Dr. Gonzalez noted, with possibly as many as 5 million cases in the United States. It remains the nation’s most common blood-borne infection.
Chronic disease develops in up to 85% of people who are exposed, infection is asymptomatic, and HCV remains one of the leading indications for liver transplantation and causes of liver cancer.
From a geographic standpoint, the prevalence of HCV in young adults is eclipsing that of Baby Boomers in several states in the Appalachian region and in Northeast, which have long been trouble spots for opioid use disorder (Gastroenterol. 2018 May;154[6]:1850-1).
Surprising exposure risk
The primary risk of transmission is through contaminated blood and the exposure through needles.
“It really doesn’t matter whether it’s a needle that has a small amount of dead space where a little bit of blood can remain or needles that have a larger amount of blood,” Dr. Gonzalez said.
“I’ve had patients who come to me and say, ‘I can’t believe I have HCV. It’s impossible. I always use my own needles. They’re always brand new; I’ve never shared with anybody,’” he continued.
“This is where education and awareness is so critical, because it’s not just the needles,” Dr. Gonzalez explained. “HCV can survive on inanimate objects. For example, on a tabletop surface or a water container, HCV can remain viable up to 3 weeks. In a syringe, 2 months. For that reason, HCV can also be transmitted through crack pipes and nasal drug use, where the prevalence can be up to 35%.”
The duration of a person’s HCV infection drives the transmission.
“That’s important to think about, because people who have chronic hepatitis C are infectious until they’re treated,” Dr. Gonzalez said. “If they don’t know that they have hepatitis C, they continue to transmit the virus to others.”
One study found that half of people living with HCV are unaware of their infection (PLoS One. 2014 Jul 2;9[7]:e101554). According to Dr. Gonzalez, forthcoming guidelines from the U.S. Preventive Services Task Force are expected to recommend a one-time screening for HCV infection in all adults aged 18-79 years, a Grade B recommendation. “That’s a big deal,” he said. (The draft recommendations are available here.)
HCV infection disproportionately affects individuals in correctional institutions. In fact, an estimated one in three inmates in the United States has chronic HCV.
“This is sort of a forgotten population with a lot of substance use and mental illness,” Dr. Gonzalez said. “Injection drug use in that setting is the most common risk factor: It’s about 60% in terms of the risk of transmission within correctional settings. HCV-associated liver disease has now surpassed HIV as a cause of death within correctional settings.”
Weighing treatment options
The most common oral regimens for chronic HCV include sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir. They achieve cure in 93%-100% of cases.
“HCV can be cured; it can be eradicated from the body long term,” Dr. Gonzalez said. “The choice of regimen, treatment duration, and use of ribavirin depends on the presence/absence of cirrhosis, prior treatment experience, and the genotype.”
All six forms of the HCV genotype can be treated with oral medication, he added, and methadone, bupropion, and naloxone are safe to use during therapy.
Reinfection following HCV treatment occurs infrequently. Dr. Gonzalez cited a randomized, controlled trial presented as an abstract at the 2018 annual meeting of the American Association for the Study of Liver Diseases. That study’s researchers found that – among 199 patients on opioid-replacement therapy who were receiving direct-acting antiviral therapy, in whom greater than 50% were actively using drugs – the rate of reinfection at 3 years was 1.8 reinfections/100 person-years.
“That’s lower than people expect,” Dr. Gonzalez said.
How to boost screening
Electronic health record systems can be used as an important tool to increase HCV screening in health care settings.
In 2017, researchers published an analysis of three randomized trials carried out at three separate primary care settings to improve screening for HCV: repeated mailings, an EHR best practice alert (BPA), and patient solicitation (Hepatology 2017 Jan;65[1]:44-53). They evaluated HCV antibody testing, diagnosis, and costs for each of the interventions, compared with standard-of-care testing.
The investigators found that the BPA intervention had the lowest incremental cost per completed test – $24 with fixed start-up costs, including technical design and development of the BPA system; $3 without fixed start-up costs. The BPA intervention also had the lowest incremental cost per new case identified.
Other efforts to expand access to screening and treatment are underway.
In 2019, Louisiana health officials negotiated a one-time fee for unlimited access for 5 years to sofosbuvir/velpatasvir (Epclusa) to treat the estimated 30,000 patients on Louisiana Medicaid and in that state’s department of corrections who have HCV.
“The goal is 90% cure; the burden is on the state health department to screen, diagnose, and dispense medication,” Dr. Gonzalez said.
Also in 2019, the state of Washington used an open bidding process to negotiate access to glecaprevir/pibrentasvir (Mavyret) for the state’s Medicaid population who have HCV.
“Those states are setting the pace,” Dr. Gonzalez said. “They are showing examples of how we can start implementing a process to treat these vulnerable populations.”
Meanwhile, the World Health Organization set a goal of eliminating viral hepatitis as a major public health threat by 2030.
“That sounds ambitious, but I think it’s possible,” Dr. Gonzalez said. “It’s important to address these high-risk populations: the incarcerated, people who use drugs, and the homeless, because those are the groups that have a high prevalence of HCV – mainly through injection drug use.
“If we don’t address that population, and we only target the general population, we’re going to have a continual source of transmission,” Dr. Gonzalez warned. “In that case, we would never be able to achieve elimination.”
Dr. Gonzalez disclosed that he is a member of the speakers bureau for AbbVie and Salix.
LAS VEGAS – Between 2010 and 2017, the proportion of newly diagnosed cases of acute hepatitis C virus infection rose threefold, driven largely by the concomitant opioid epidemic.
That makes efforts to screen, diagnose, and cure high-risk populations more important than ever, Stevan A. Gonzalez, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
About 70% of HCV cases are related to injection drug use,” said Dr. Gonzalez, medical director of liver transplantation at the Baylor Simmons Transplant Institute at the Baylor Scott & White All Saints Medical Center in Fort Worth, Tex. “This is affecting whites as much as blacks and Hispanics, females as much as males, and in nonurban areas as much as in urban areas.”
Data from the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration indicate that during 2004-2014, the number of acute HCV cases among those aged 18-29 years increased 400%, and the use of injection opioids rose 600%.
At the same time, the number of HCV cases among those aged 30-39 years increased 325%, and the use of injection opioids rose 83%.
“We’re starting to see a pattern overlapping between HCV exposure and opioid injection,” Dr. Gonzalez said. Other high-risk populations include homeless and incarcerated individuals.
More than 70 million people worldwide have chronic HCV infection, Dr. Gonzalez noted, with possibly as many as 5 million cases in the United States. It remains the nation’s most common blood-borne infection.
Chronic disease develops in up to 85% of people who are exposed, infection is asymptomatic, and HCV remains one of the leading indications for liver transplantation and causes of liver cancer.
From a geographic standpoint, the prevalence of HCV in young adults is eclipsing that of Baby Boomers in several states in the Appalachian region and in Northeast, which have long been trouble spots for opioid use disorder (Gastroenterol. 2018 May;154[6]:1850-1).
Surprising exposure risk
The primary risk of transmission is through contaminated blood and the exposure through needles.
“It really doesn’t matter whether it’s a needle that has a small amount of dead space where a little bit of blood can remain or needles that have a larger amount of blood,” Dr. Gonzalez said.
“I’ve had patients who come to me and say, ‘I can’t believe I have HCV. It’s impossible. I always use my own needles. They’re always brand new; I’ve never shared with anybody,’” he continued.
“This is where education and awareness is so critical, because it’s not just the needles,” Dr. Gonzalez explained. “HCV can survive on inanimate objects. For example, on a tabletop surface or a water container, HCV can remain viable up to 3 weeks. In a syringe, 2 months. For that reason, HCV can also be transmitted through crack pipes and nasal drug use, where the prevalence can be up to 35%.”
The duration of a person’s HCV infection drives the transmission.
“That’s important to think about, because people who have chronic hepatitis C are infectious until they’re treated,” Dr. Gonzalez said. “If they don’t know that they have hepatitis C, they continue to transmit the virus to others.”
One study found that half of people living with HCV are unaware of their infection (PLoS One. 2014 Jul 2;9[7]:e101554). According to Dr. Gonzalez, forthcoming guidelines from the U.S. Preventive Services Task Force are expected to recommend a one-time screening for HCV infection in all adults aged 18-79 years, a Grade B recommendation. “That’s a big deal,” he said. (The draft recommendations are available here.)
HCV infection disproportionately affects individuals in correctional institutions. In fact, an estimated one in three inmates in the United States has chronic HCV.
“This is sort of a forgotten population with a lot of substance use and mental illness,” Dr. Gonzalez said. “Injection drug use in that setting is the most common risk factor: It’s about 60% in terms of the risk of transmission within correctional settings. HCV-associated liver disease has now surpassed HIV as a cause of death within correctional settings.”
Weighing treatment options
The most common oral regimens for chronic HCV include sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir. They achieve cure in 93%-100% of cases.
“HCV can be cured; it can be eradicated from the body long term,” Dr. Gonzalez said. “The choice of regimen, treatment duration, and use of ribavirin depends on the presence/absence of cirrhosis, prior treatment experience, and the genotype.”
All six forms of the HCV genotype can be treated with oral medication, he added, and methadone, bupropion, and naloxone are safe to use during therapy.
Reinfection following HCV treatment occurs infrequently. Dr. Gonzalez cited a randomized, controlled trial presented as an abstract at the 2018 annual meeting of the American Association for the Study of Liver Diseases. That study’s researchers found that – among 199 patients on opioid-replacement therapy who were receiving direct-acting antiviral therapy, in whom greater than 50% were actively using drugs – the rate of reinfection at 3 years was 1.8 reinfections/100 person-years.
“That’s lower than people expect,” Dr. Gonzalez said.
How to boost screening
Electronic health record systems can be used as an important tool to increase HCV screening in health care settings.
In 2017, researchers published an analysis of three randomized trials carried out at three separate primary care settings to improve screening for HCV: repeated mailings, an EHR best practice alert (BPA), and patient solicitation (Hepatology 2017 Jan;65[1]:44-53). They evaluated HCV antibody testing, diagnosis, and costs for each of the interventions, compared with standard-of-care testing.
The investigators found that the BPA intervention had the lowest incremental cost per completed test – $24 with fixed start-up costs, including technical design and development of the BPA system; $3 without fixed start-up costs. The BPA intervention also had the lowest incremental cost per new case identified.
Other efforts to expand access to screening and treatment are underway.
In 2019, Louisiana health officials negotiated a one-time fee for unlimited access for 5 years to sofosbuvir/velpatasvir (Epclusa) to treat the estimated 30,000 patients on Louisiana Medicaid and in that state’s department of corrections who have HCV.
“The goal is 90% cure; the burden is on the state health department to screen, diagnose, and dispense medication,” Dr. Gonzalez said.
Also in 2019, the state of Washington used an open bidding process to negotiate access to glecaprevir/pibrentasvir (Mavyret) for the state’s Medicaid population who have HCV.
“Those states are setting the pace,” Dr. Gonzalez said. “They are showing examples of how we can start implementing a process to treat these vulnerable populations.”
Meanwhile, the World Health Organization set a goal of eliminating viral hepatitis as a major public health threat by 2030.
“That sounds ambitious, but I think it’s possible,” Dr. Gonzalez said. “It’s important to address these high-risk populations: the incarcerated, people who use drugs, and the homeless, because those are the groups that have a high prevalence of HCV – mainly through injection drug use.
“If we don’t address that population, and we only target the general population, we’re going to have a continual source of transmission,” Dr. Gonzalez warned. “In that case, we would never be able to achieve elimination.”
Dr. Gonzalez disclosed that he is a member of the speakers bureau for AbbVie and Salix.
REPORTING FROM NPA 2020
Among all physicians, internists near bottom for workplace happiness
Physicians in internal medicine struggle to find happiness both in and outside the workplace, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

Only 22% of internists reported that they were very happy at work, with only neurologists reporting a worse at-work happiness rate, according to the Medscape report.
The news wasn’t much better when it came to happiness outside the office, as only 48% of internists reported they were very happy. Once again, neurologists had the lowest happiness rate outside the office, at 44%.
The rate of burnout among internists, at 43%, was similar to that of physicians overall at 41%; 14% of internists reported that they were both burned out and depressed. The most common contributing factors to burnout for internists were having too many bureaucratic tasks (62%), receiving a lack of respect from colleagues (34%), and spending too many hours at work (33%).
Internists dealt with burnout by isolating themselves from others (47%), talking with family/friends (45%), and exercising (41%). In addition, only 41% of internists took 3-4 weeks’ vacation, less than the 44% for physicians overall, and 43% took less than 3 weeks of vacation.
About 14% of internists said that they’d contemplated suicide, and 1% reported that they’d attempted it; 80% said they’d never thought about suicide. Only 16% said that they were seeking or planning to seek professional help for symptoms of burnout or depression, and 64% said they weren’t planning on seeking help and had never done so in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Physicians in internal medicine struggle to find happiness both in and outside the workplace, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

Only 22% of internists reported that they were very happy at work, with only neurologists reporting a worse at-work happiness rate, according to the Medscape report.
The news wasn’t much better when it came to happiness outside the office, as only 48% of internists reported they were very happy. Once again, neurologists had the lowest happiness rate outside the office, at 44%.
The rate of burnout among internists, at 43%, was similar to that of physicians overall at 41%; 14% of internists reported that they were both burned out and depressed. The most common contributing factors to burnout for internists were having too many bureaucratic tasks (62%), receiving a lack of respect from colleagues (34%), and spending too many hours at work (33%).
Internists dealt with burnout by isolating themselves from others (47%), talking with family/friends (45%), and exercising (41%). In addition, only 41% of internists took 3-4 weeks’ vacation, less than the 44% for physicians overall, and 43% took less than 3 weeks of vacation.
About 14% of internists said that they’d contemplated suicide, and 1% reported that they’d attempted it; 80% said they’d never thought about suicide. Only 16% said that they were seeking or planning to seek professional help for symptoms of burnout or depression, and 64% said they weren’t planning on seeking help and had never done so in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Physicians in internal medicine struggle to find happiness both in and outside the workplace, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

Only 22% of internists reported that they were very happy at work, with only neurologists reporting a worse at-work happiness rate, according to the Medscape report.
The news wasn’t much better when it came to happiness outside the office, as only 48% of internists reported they were very happy. Once again, neurologists had the lowest happiness rate outside the office, at 44%.
The rate of burnout among internists, at 43%, was similar to that of physicians overall at 41%; 14% of internists reported that they were both burned out and depressed. The most common contributing factors to burnout for internists were having too many bureaucratic tasks (62%), receiving a lack of respect from colleagues (34%), and spending too many hours at work (33%).
Internists dealt with burnout by isolating themselves from others (47%), talking with family/friends (45%), and exercising (41%). In addition, only 41% of internists took 3-4 weeks’ vacation, less than the 44% for physicians overall, and 43% took less than 3 weeks of vacation.
About 14% of internists said that they’d contemplated suicide, and 1% reported that they’d attempted it; 80% said they’d never thought about suicide. Only 16% said that they were seeking or planning to seek professional help for symptoms of burnout or depression, and 64% said they weren’t planning on seeking help and had never done so in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Antibiotic resistance rises among pneumococcus strains in kids
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
FROM CLINICAL INFECTIOUS DISEASES
Incomplete MS relapse recovery predicted greater long-term disability
WEST PALM BEACH, FLA. – Failure to recover completely from early relapses in multiple sclerosis (MS) is significantly associated with higher long-term disability, according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Incomplete recovery thus should be given more consideration when evaluating research and clinical practice outcomes, the study investigators cautioned.
“We found that the recovery from early relapses is an important predictor of future disability,” first author Marinos G. Sotiropoulos, MD, of the department of neurology, Brigham and Women’s Hospital, Boston, said in an interview. “It should be incorporated in future predictive models of disease severity and clinical trials, [and] it could be useful in clinical decision making as well.”
Incomplete recovery from relapses is known to be linked to disability progression and to the likelihood of transitioning to secondary progressive MS. Research on its role in longer-term outcomes is lacking, however.
To investigate the effect of incomplete relapse recovery in the first 3 years of MS on rates of disability at 10 years, Dr. Sotiropoulos and colleagues evaluated data on 360 patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation in Multiple Sclerosis at Brigham and Women’s Hospital) study. CLIMB is a natural history study spanning 20 years, with more than 2,000 patients.
Patients were included if at least 8.5 years had passed since their first documented symptom, if they were at least 18 years at their first visit to the Partners MS Center, if that visit occurred within 1 year of their first symptom, and if they had a diagnosis of relapsing-remitting MS or secondary progressive MS.
Among the 308 patients included in the study, 74% were female and 89% were white, with a mean age at the first symptom of 35.9 years.
A total of 403 early attacks from those 308 patients were included in the study. Half of the attacks (50.4%) were followed by incomplete recovery after 6 months, defined specifically as an increase in the Expanded Disability Status Scale (EDSS) scores from baseline to at least 6 months after the onset of the attack.
As of their 10-year visit, 27.3% of patients had a normal examination, defined as EDSS 0, and 64.1% had no significant disability (EDSS less than 2). The mean EDSS at 10 years was 1.52.
Patients’ recovery index, defined as the percentage of early attacks that recovered completely, was significantly associated with 10-year EDSS scores (P less than .001).
Patient age at first symptom was also a significant predictor of 10-year disability (P less than .004). Factors that were significantly associated with incomplete relapse recovery were the duration of time from first symptom (P less than .001) and moderate severity of the relapse (P = .029).
With the type of drug treatment likely representing an important factor in whether a patient has incomplete relapse recovery, the issue should be the subject of further research, Dr. Sotiropoulos said.
“This is something that is important to look at because none of the clinical trials for the drugs we currently have looked at relapse recovery as an outcome,” he explained.
“There have been some post hoc analyses [that] have shown that some of the new medications can improve recovery from relapses, but there is a lot to look into now that we know relapse recovery is an important clinical parameter,” he said. “We have to factor in the treatment effect in preventing residual disability after relapses.”
The findings suggest that “patients with incomplete early recovery might be considered for highly effective disease-modifying therapy,” added senior author Tanuja Chitnis, MD, also of the department of neurology at Brigham and Women’s Hospital. “We are now analyzing the biological mechanisms associated with relapse recovery.”
The authors of a recent study that echoes the importance of relapse recovery call it “the forgotten variable in multiple sclerosis clinical trials.” In that study, the researchers found an increased likelihood of a benign disease course among patients who received immediate disease-modifying therapy (DMT) initiation after failing to have a good recovery from an initial relapse (Neurol Neuroimmunol Neuroinflamm. 2019 Dec 17;7[2]).
“Some clinicians may choose to hold off DMTs because the patient may not have high disease activity levels,” Burcu Zeydan, MD, a coauthor of that study and an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic, Rochester, Minn., said in an interview.
“What these studies add is that, if a patient is a poor recoverer despite not having highly active disease, that patient should be considered for immediate treatment initiation,” she said. “Otherwise, there is the possibility of a next relapse, which may not happen often. But when it happens, it may lead to more residual deficit with additional disability burden.”
The CLIMB study received funding from Mallinckrodt and the National MS Society Nancy Davis Center Without Walls. Dr. Sotiropoulos has received research support from Mallinckrodt. Dr. Chitnis has served on advisory boards for Biogen, Novartis, and Sanofi-Genzyme, and she has received research support from the Department of Defense, National MS Society, Guthy-Jackson Charitable Foundation, Novartis, Octave, Serono, and Verily. Dr. Zeydan had no disclosures to report.
SOURCE: Sotiropoulos MG et al. ACTRIMS Forum 2020. Abstract LB 317.
WEST PALM BEACH, FLA. – Failure to recover completely from early relapses in multiple sclerosis (MS) is significantly associated with higher long-term disability, according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Incomplete recovery thus should be given more consideration when evaluating research and clinical practice outcomes, the study investigators cautioned.
“We found that the recovery from early relapses is an important predictor of future disability,” first author Marinos G. Sotiropoulos, MD, of the department of neurology, Brigham and Women’s Hospital, Boston, said in an interview. “It should be incorporated in future predictive models of disease severity and clinical trials, [and] it could be useful in clinical decision making as well.”
Incomplete recovery from relapses is known to be linked to disability progression and to the likelihood of transitioning to secondary progressive MS. Research on its role in longer-term outcomes is lacking, however.
To investigate the effect of incomplete relapse recovery in the first 3 years of MS on rates of disability at 10 years, Dr. Sotiropoulos and colleagues evaluated data on 360 patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation in Multiple Sclerosis at Brigham and Women’s Hospital) study. CLIMB is a natural history study spanning 20 years, with more than 2,000 patients.
Patients were included if at least 8.5 years had passed since their first documented symptom, if they were at least 18 years at their first visit to the Partners MS Center, if that visit occurred within 1 year of their first symptom, and if they had a diagnosis of relapsing-remitting MS or secondary progressive MS.
Among the 308 patients included in the study, 74% were female and 89% were white, with a mean age at the first symptom of 35.9 years.
A total of 403 early attacks from those 308 patients were included in the study. Half of the attacks (50.4%) were followed by incomplete recovery after 6 months, defined specifically as an increase in the Expanded Disability Status Scale (EDSS) scores from baseline to at least 6 months after the onset of the attack.
As of their 10-year visit, 27.3% of patients had a normal examination, defined as EDSS 0, and 64.1% had no significant disability (EDSS less than 2). The mean EDSS at 10 years was 1.52.
Patients’ recovery index, defined as the percentage of early attacks that recovered completely, was significantly associated with 10-year EDSS scores (P less than .001).
Patient age at first symptom was also a significant predictor of 10-year disability (P less than .004). Factors that were significantly associated with incomplete relapse recovery were the duration of time from first symptom (P less than .001) and moderate severity of the relapse (P = .029).
With the type of drug treatment likely representing an important factor in whether a patient has incomplete relapse recovery, the issue should be the subject of further research, Dr. Sotiropoulos said.
“This is something that is important to look at because none of the clinical trials for the drugs we currently have looked at relapse recovery as an outcome,” he explained.
“There have been some post hoc analyses [that] have shown that some of the new medications can improve recovery from relapses, but there is a lot to look into now that we know relapse recovery is an important clinical parameter,” he said. “We have to factor in the treatment effect in preventing residual disability after relapses.”
The findings suggest that “patients with incomplete early recovery might be considered for highly effective disease-modifying therapy,” added senior author Tanuja Chitnis, MD, also of the department of neurology at Brigham and Women’s Hospital. “We are now analyzing the biological mechanisms associated with relapse recovery.”
The authors of a recent study that echoes the importance of relapse recovery call it “the forgotten variable in multiple sclerosis clinical trials.” In that study, the researchers found an increased likelihood of a benign disease course among patients who received immediate disease-modifying therapy (DMT) initiation after failing to have a good recovery from an initial relapse (Neurol Neuroimmunol Neuroinflamm. 2019 Dec 17;7[2]).
“Some clinicians may choose to hold off DMTs because the patient may not have high disease activity levels,” Burcu Zeydan, MD, a coauthor of that study and an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic, Rochester, Minn., said in an interview.
“What these studies add is that, if a patient is a poor recoverer despite not having highly active disease, that patient should be considered for immediate treatment initiation,” she said. “Otherwise, there is the possibility of a next relapse, which may not happen often. But when it happens, it may lead to more residual deficit with additional disability burden.”
The CLIMB study received funding from Mallinckrodt and the National MS Society Nancy Davis Center Without Walls. Dr. Sotiropoulos has received research support from Mallinckrodt. Dr. Chitnis has served on advisory boards for Biogen, Novartis, and Sanofi-Genzyme, and she has received research support from the Department of Defense, National MS Society, Guthy-Jackson Charitable Foundation, Novartis, Octave, Serono, and Verily. Dr. Zeydan had no disclosures to report.
SOURCE: Sotiropoulos MG et al. ACTRIMS Forum 2020. Abstract LB 317.
WEST PALM BEACH, FLA. – Failure to recover completely from early relapses in multiple sclerosis (MS) is significantly associated with higher long-term disability, according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Incomplete recovery thus should be given more consideration when evaluating research and clinical practice outcomes, the study investigators cautioned.
“We found that the recovery from early relapses is an important predictor of future disability,” first author Marinos G. Sotiropoulos, MD, of the department of neurology, Brigham and Women’s Hospital, Boston, said in an interview. “It should be incorporated in future predictive models of disease severity and clinical trials, [and] it could be useful in clinical decision making as well.”
Incomplete recovery from relapses is known to be linked to disability progression and to the likelihood of transitioning to secondary progressive MS. Research on its role in longer-term outcomes is lacking, however.
To investigate the effect of incomplete relapse recovery in the first 3 years of MS on rates of disability at 10 years, Dr. Sotiropoulos and colleagues evaluated data on 360 patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation in Multiple Sclerosis at Brigham and Women’s Hospital) study. CLIMB is a natural history study spanning 20 years, with more than 2,000 patients.
Patients were included if at least 8.5 years had passed since their first documented symptom, if they were at least 18 years at their first visit to the Partners MS Center, if that visit occurred within 1 year of their first symptom, and if they had a diagnosis of relapsing-remitting MS or secondary progressive MS.
Among the 308 patients included in the study, 74% were female and 89% were white, with a mean age at the first symptom of 35.9 years.
A total of 403 early attacks from those 308 patients were included in the study. Half of the attacks (50.4%) were followed by incomplete recovery after 6 months, defined specifically as an increase in the Expanded Disability Status Scale (EDSS) scores from baseline to at least 6 months after the onset of the attack.
As of their 10-year visit, 27.3% of patients had a normal examination, defined as EDSS 0, and 64.1% had no significant disability (EDSS less than 2). The mean EDSS at 10 years was 1.52.
Patients’ recovery index, defined as the percentage of early attacks that recovered completely, was significantly associated with 10-year EDSS scores (P less than .001).
Patient age at first symptom was also a significant predictor of 10-year disability (P less than .004). Factors that were significantly associated with incomplete relapse recovery were the duration of time from first symptom (P less than .001) and moderate severity of the relapse (P = .029).
With the type of drug treatment likely representing an important factor in whether a patient has incomplete relapse recovery, the issue should be the subject of further research, Dr. Sotiropoulos said.
“This is something that is important to look at because none of the clinical trials for the drugs we currently have looked at relapse recovery as an outcome,” he explained.
“There have been some post hoc analyses [that] have shown that some of the new medications can improve recovery from relapses, but there is a lot to look into now that we know relapse recovery is an important clinical parameter,” he said. “We have to factor in the treatment effect in preventing residual disability after relapses.”
The findings suggest that “patients with incomplete early recovery might be considered for highly effective disease-modifying therapy,” added senior author Tanuja Chitnis, MD, also of the department of neurology at Brigham and Women’s Hospital. “We are now analyzing the biological mechanisms associated with relapse recovery.”
The authors of a recent study that echoes the importance of relapse recovery call it “the forgotten variable in multiple sclerosis clinical trials.” In that study, the researchers found an increased likelihood of a benign disease course among patients who received immediate disease-modifying therapy (DMT) initiation after failing to have a good recovery from an initial relapse (Neurol Neuroimmunol Neuroinflamm. 2019 Dec 17;7[2]).
“Some clinicians may choose to hold off DMTs because the patient may not have high disease activity levels,” Burcu Zeydan, MD, a coauthor of that study and an assistant professor of radiology in the Center of MS and Autoimmune Neurology at the Mayo Clinic, Rochester, Minn., said in an interview.
“What these studies add is that, if a patient is a poor recoverer despite not having highly active disease, that patient should be considered for immediate treatment initiation,” she said. “Otherwise, there is the possibility of a next relapse, which may not happen often. But when it happens, it may lead to more residual deficit with additional disability burden.”
The CLIMB study received funding from Mallinckrodt and the National MS Society Nancy Davis Center Without Walls. Dr. Sotiropoulos has received research support from Mallinckrodt. Dr. Chitnis has served on advisory boards for Biogen, Novartis, and Sanofi-Genzyme, and she has received research support from the Department of Defense, National MS Society, Guthy-Jackson Charitable Foundation, Novartis, Octave, Serono, and Verily. Dr. Zeydan had no disclosures to report.
SOURCE: Sotiropoulos MG et al. ACTRIMS Forum 2020. Abstract LB 317.
REPORTING FROM ACTRIMS FORUM 2020
Leadership & Professional Development: Cultivating Habits for the Hospitalist
“We are what we repeatedly do. Excellence, then, is not an act, but a habit.”
—Will Durant
We are a collection of our habits—the routine, repetitive, subconscious behaviors we perform on a daily basis. Some of these behaviors are positive, others less so. Habits allow us to perform tasks automatically, without the need for active decision making. Amidst a constantly changing clinical environment, cultivating consistent habits can improve our adherence to best practices and free cognitive effort toward more challenging diagnostic or therapeutic tasks.
Establishing habits requires practice and intentionality. First, identify those habits that are desirable in your personal and professional life. Next, find a method to develop the habit. Then, hold yourself accountable as you work to embed the habit. Simple? Not quite.
In “The Power of Habit,” author Charles Duhigg introduces habit loops as a way to successfully develop this practice.1 Habit loops—sequences comprising a cue, routine, and reward—are integral to developing routines that support professional and personal aspects of hospitalist life. Consider a hospitalist seeking to develop a prerounds routine to increase efficiency and limit missed patient information. First, the clinician should identify a cue to start the routine, such as sitting down to log in at a specific workstation. Second, a sequence of actions is “chunked” into a consistent order, such as a review of vital signs, clinical notes, and patient labs. After the routine is completed, the clinician should finish with a reward, such as a cup of coffee after rounds. Want to set up a habit for ensuring learning goals are set with trainees at the beginning of every block? Set a calendar reminder for this on the first day, standardize how you communicate goals, and reward yourself with a team lunch at the end of the rotation. What if it’s a busy first day on service? Doesn’t matter. As Clay Christensen notes in “How Will You Measure Your Life?,” making one commitment to a habit is easier than deciding whether or not to engage in the routine every time new circumstances arise.2 The intentionality that comes with this act ensures consistency in the practice.
As a busy hospitalist, establishing habits for personal and professional development requires cues and rewards. For example, do you want to cement a habit of reading the latest journal articles or carving out time each day to reflect on your work? Then cultivate the routine by creating a cue, such as a dashboard on a wall to visualize how many articles you’ve read this week or whether you’ve paused to reflect on your rotation. Reinforce the routine by creating a reward: a walk outside, time with family, or another activity you enjoy. Pair the same reward with the same routine to strengthen the habit loop.
A few additional tips for cultivating habits: it is useful to pair an existing reliable habit, or “anchor habit,” with a new one, such as a short meditation after brushing your teeth.3 Doing so reinforces behaviors in a positive way. You may use the same principles to lose unwanted habits (eg, checking e-mail excessively) by removing cues, such as turning off notifications or using airplane mode and rewarding yourself when you see the behavior through.
Habits are larger than behaviors; they can impact your personal and professional life in important ways. By actively creating habits that align with your long-term priorities, you can create a safety net if and when change arrives. Understanding the psychology of habits and employing cues and rewards effectively can lead hospitalists to create positive routines that improve their clinical practice and personal lives.
1. Duhigg C. The Power of Habit: Why We Do What We Do in Life and Business. Random House; 2012.
2. Christensen CM. How Will You Measure Your Life? (Harvard Business Review Classics). Harvard Business Review Press; 2017.
3. Fogg B. Tiny Habits w/Dr. BJ Fogg-Behavior Change: Tiny Habits; 2011.
“We are what we repeatedly do. Excellence, then, is not an act, but a habit.”
—Will Durant
We are a collection of our habits—the routine, repetitive, subconscious behaviors we perform on a daily basis. Some of these behaviors are positive, others less so. Habits allow us to perform tasks automatically, without the need for active decision making. Amidst a constantly changing clinical environment, cultivating consistent habits can improve our adherence to best practices and free cognitive effort toward more challenging diagnostic or therapeutic tasks.
Establishing habits requires practice and intentionality. First, identify those habits that are desirable in your personal and professional life. Next, find a method to develop the habit. Then, hold yourself accountable as you work to embed the habit. Simple? Not quite.
In “The Power of Habit,” author Charles Duhigg introduces habit loops as a way to successfully develop this practice.1 Habit loops—sequences comprising a cue, routine, and reward—are integral to developing routines that support professional and personal aspects of hospitalist life. Consider a hospitalist seeking to develop a prerounds routine to increase efficiency and limit missed patient information. First, the clinician should identify a cue to start the routine, such as sitting down to log in at a specific workstation. Second, a sequence of actions is “chunked” into a consistent order, such as a review of vital signs, clinical notes, and patient labs. After the routine is completed, the clinician should finish with a reward, such as a cup of coffee after rounds. Want to set up a habit for ensuring learning goals are set with trainees at the beginning of every block? Set a calendar reminder for this on the first day, standardize how you communicate goals, and reward yourself with a team lunch at the end of the rotation. What if it’s a busy first day on service? Doesn’t matter. As Clay Christensen notes in “How Will You Measure Your Life?,” making one commitment to a habit is easier than deciding whether or not to engage in the routine every time new circumstances arise.2 The intentionality that comes with this act ensures consistency in the practice.
As a busy hospitalist, establishing habits for personal and professional development requires cues and rewards. For example, do you want to cement a habit of reading the latest journal articles or carving out time each day to reflect on your work? Then cultivate the routine by creating a cue, such as a dashboard on a wall to visualize how many articles you’ve read this week or whether you’ve paused to reflect on your rotation. Reinforce the routine by creating a reward: a walk outside, time with family, or another activity you enjoy. Pair the same reward with the same routine to strengthen the habit loop.
A few additional tips for cultivating habits: it is useful to pair an existing reliable habit, or “anchor habit,” with a new one, such as a short meditation after brushing your teeth.3 Doing so reinforces behaviors in a positive way. You may use the same principles to lose unwanted habits (eg, checking e-mail excessively) by removing cues, such as turning off notifications or using airplane mode and rewarding yourself when you see the behavior through.
Habits are larger than behaviors; they can impact your personal and professional life in important ways. By actively creating habits that align with your long-term priorities, you can create a safety net if and when change arrives. Understanding the psychology of habits and employing cues and rewards effectively can lead hospitalists to create positive routines that improve their clinical practice and personal lives.
“We are what we repeatedly do. Excellence, then, is not an act, but a habit.”
—Will Durant
We are a collection of our habits—the routine, repetitive, subconscious behaviors we perform on a daily basis. Some of these behaviors are positive, others less so. Habits allow us to perform tasks automatically, without the need for active decision making. Amidst a constantly changing clinical environment, cultivating consistent habits can improve our adherence to best practices and free cognitive effort toward more challenging diagnostic or therapeutic tasks.
Establishing habits requires practice and intentionality. First, identify those habits that are desirable in your personal and professional life. Next, find a method to develop the habit. Then, hold yourself accountable as you work to embed the habit. Simple? Not quite.
In “The Power of Habit,” author Charles Duhigg introduces habit loops as a way to successfully develop this practice.1 Habit loops—sequences comprising a cue, routine, and reward—are integral to developing routines that support professional and personal aspects of hospitalist life. Consider a hospitalist seeking to develop a prerounds routine to increase efficiency and limit missed patient information. First, the clinician should identify a cue to start the routine, such as sitting down to log in at a specific workstation. Second, a sequence of actions is “chunked” into a consistent order, such as a review of vital signs, clinical notes, and patient labs. After the routine is completed, the clinician should finish with a reward, such as a cup of coffee after rounds. Want to set up a habit for ensuring learning goals are set with trainees at the beginning of every block? Set a calendar reminder for this on the first day, standardize how you communicate goals, and reward yourself with a team lunch at the end of the rotation. What if it’s a busy first day on service? Doesn’t matter. As Clay Christensen notes in “How Will You Measure Your Life?,” making one commitment to a habit is easier than deciding whether or not to engage in the routine every time new circumstances arise.2 The intentionality that comes with this act ensures consistency in the practice.
As a busy hospitalist, establishing habits for personal and professional development requires cues and rewards. For example, do you want to cement a habit of reading the latest journal articles or carving out time each day to reflect on your work? Then cultivate the routine by creating a cue, such as a dashboard on a wall to visualize how many articles you’ve read this week or whether you’ve paused to reflect on your rotation. Reinforce the routine by creating a reward: a walk outside, time with family, or another activity you enjoy. Pair the same reward with the same routine to strengthen the habit loop.
A few additional tips for cultivating habits: it is useful to pair an existing reliable habit, or “anchor habit,” with a new one, such as a short meditation after brushing your teeth.3 Doing so reinforces behaviors in a positive way. You may use the same principles to lose unwanted habits (eg, checking e-mail excessively) by removing cues, such as turning off notifications or using airplane mode and rewarding yourself when you see the behavior through.
Habits are larger than behaviors; they can impact your personal and professional life in important ways. By actively creating habits that align with your long-term priorities, you can create a safety net if and when change arrives. Understanding the psychology of habits and employing cues and rewards effectively can lead hospitalists to create positive routines that improve their clinical practice and personal lives.
1. Duhigg C. The Power of Habit: Why We Do What We Do in Life and Business. Random House; 2012.
2. Christensen CM. How Will You Measure Your Life? (Harvard Business Review Classics). Harvard Business Review Press; 2017.
3. Fogg B. Tiny Habits w/Dr. BJ Fogg-Behavior Change: Tiny Habits; 2011.
1. Duhigg C. The Power of Habit: Why We Do What We Do in Life and Business. Random House; 2012.
2. Christensen CM. How Will You Measure Your Life? (Harvard Business Review Classics). Harvard Business Review Press; 2017.
3. Fogg B. Tiny Habits w/Dr. BJ Fogg-Behavior Change: Tiny Habits; 2011.
© 2020 Society of Hospital Medicine
Fulfilling the Potential of Point-of-Care Ultrasound in Hospital Medicine
The enthusiasm surrounding point-of-care ultrasound (POCUS) is clear and well founded. POCUS is a powerful tool that produces valuable diagnostic information for common and important clinical problems faced by hospitalists, such as pneumonia, soft-tissue infections,1 and myriad other applications. It can inform the evaluation and management of complex clinical problems such as dyspnea.2 Beyond its diagnostic potential, POCUS is well known to improve common procedures performed by adult and pediatric hospitalists by improving success rates and decreasing complications.
Excitement surrounding this technology continues to grow among hospitalists, leading to a proliferation of high-quality educational programs over the last 5 years. Most notable among these offerings has been the more comprehensive training available through the Society of Hospital Medicine (SHM) certificate-based pathway, though many other strong options exist, including institution-based curricula, such as the HealthPartners CHAMP program,3 and pediatric-focused programs. Growth in training is also occurring among medical students and residents. As of a 2012 survey, the majority (51%) of US medical schools had begun to weave ultrasound into their curricula,4 and this growth is also occurring in internal medicine and pediatric residency programs.5
Given the high potential for this technology and the growth in interest, it is an excellent time to pause and review some of the challenges faced by practitioners, hospitalist groups, and educators seeking to optimize POCUS implementation. A deliberate approach to POCUS education, the development of shared standards for high-quality use, and an ongoing dedication to develop specialty-specific practices will largely determine how much of this potential is fulfilled.
The largest challenge is likely to be educational. Educating clinicians to be able to integrate POCUS into practice is a complex, multistep process requiring not only an adequate core of didactic training and access to machines, but also the structured opportunity to develop rudimentary hands-on skills. Such initial training should be followed by continued practice and feedback as developing POCUS users progress toward independent practice. The study by Kumar et al.6 reaffirms that brief didactic lectures and access to machines are necessary, but they are clearly insufficient for learners to be able to use POCUS independently for a wide variety of applications. Their intervention also contrasts markedly with the 20 hours of didactics and 150 supervised scans recommended by the American College of Emergency Physicians prior to independent use for a core of six applications.7
Shared standards for education, use, and oversight will be crucial to fulfilling the potential of POCUS within hospital medicine. Our belief is that much can be learned from the thoughtful approach taken during the development of POCUS as a mainstream tool in emergency medicine in the early 2000s. In this approach, emergency physicians determined a sufficient and achievable standard of training for core POCUS applications, which was widely adopted. Based on completion of this training, physicians who were required to complete credentialing from their hospitals were widely able to achieve it, without any need for external certification. Emergency medicine guidelines further mandated the documentation of examinations and the creation of an exam report, features that improve clinical communication and facilitate quality improvement. Quality assurance processes that reviewed images and clinician interpretations were established as mandatory, which they should be in hospital medicine. Evidence was produced as to which exams physicians could do reliably with this focused training and which they could not. In the context of these thoughtful constructs, lawsuits have been noted to be exceedingly rare; and when they do occur, they have typically been for the failure to use POCUS rather than the converse.8
While many of these precepts deserve replication, others should also be modified to reflect changes in technology, medical education, and medical practice over the last 20 years and to improve upon this base of success. For example, with POCUS training now appearing in many medical school and residency curricula, training paradigms for both residents and attendings will need to accommodate a wider range of incoming skills. Emphasis should continue to be shifted toward competency-based assessments and entrustment and away from a fixed training time or exam number threshold. Important financial aspects have also changed. The cost of practical machines has dropped considerably, and medicine is shifting away from a fee-for-service model. While it remains appropriate that physicians may bill for POCUS examinations, it is likely that improved diagnosis, improved throughput, and a reduction in complications will yield greater value and should be the emphasis of cost/value discussions.9 Finally, while hospitals may impose credentialing, this process can also create a burden not present for most other noninvasive skills and may deter appropriate use. If this approach is chosen by a hospital, requirements should ideally remain modest, and as these skills become more widespread, POCUS should ultimately be built into board examinations and core credentialing.9
Thoughtful and concerted effort will be required by hospitalist leaders, educational innovators, and professional societies in developing POCUS to best serve hospitalists and their patients. This work has already begun. For example, in 2019 SHM offered a position statement outlining important aspects such as current evidence-based applications, training pathways, quality assurance, and program management.10 These recommendations should guide both adult and pediatric hospitalists. The Alliance for Academic Internal Medicine offered a similar position statement for resident training.11 Interest groups are growing in numerous professional societies, which will facilitate collaboration and promote propagation of best practices. High-quality educational tools are continuing to be developed by numerous organizations.
While further development is needed to add the detail, granularity, and practical tools that educational and practice leaders need to assure that POCUS achieves its potential in hospital medicine, the foundation for POCUS use within the specialty is being thoughtfully constructed. As this process proceeds, it will be vital to continue to learn from our emergency medicine colleagues, who have already met similar challenges, while at the same time be able to develop a modern POCUS model optimized for hospital medicine workflow, training, and patient care.
1. Kinnear B, Kelleher M, Chorny V. Clinical practice update: Point-of-care ultrasound for the pediatric hospitalist. J Hosp Med. 2019;15(3):170-172. https://doi.org/10.12788/jhm.3325.
2. Kelleher M, Kinnear B, Olson A. Clinical progress note: Point-of-care ultrasound in the evaluation of the dyspneic adult. J Hosp Med. 2020;15(3):173-175. https://doi.org/10.12788/jhm.3340.
3. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) Ultrasound Program. J Hosp Med. 2018;13(8):544-550. https://doi.org/10.12788/jhm.2938.
4. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: Results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
5. Reaume M, Siuba M, Wagner M, Woodwyk A, Melgar TA. Prevalence and Scope of point-of-care ultrasound education in internal medicine, pediatric, and medicine-pediatric residency programs in the United States. J Ultrasound Med. 2019;38(6):1433-1439. https://doi.org/10.1002/jum.14821.
6. Kumar A, Weng Y, Wang L, et al. Portable ultrasound device usage and learning outcomes among internal medicine trainees: a parallel-group randomized trial. J Hosp Med. 2020;15(3):154-159. https://doi.org/10.12788/jhm.3351.
7. Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
8. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16(1):1-4. https://doi.org/10.5811/westjem.2014.11.23592.
9, Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of Point-of-Care Ultrasound Competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812
10. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-Care Ultrasound for hospitalists: A position statement of the society of hospital medicine. J Hosp Med. 2019;14. https://doi.org/10.12788/jhm.3079.
11. LoPresti CM, Jensen TP, Dversdal RK, Astiz DJ. Point-of-Care Ultrasound for Internal Medicine Residency Training: A position statement from the Alliance of Academic Internal Medicine. Am J Med. 2019 Nov;132(11):1356-1360. https://doi.org/10.1016/j.amjmed.2019.07.019.
The enthusiasm surrounding point-of-care ultrasound (POCUS) is clear and well founded. POCUS is a powerful tool that produces valuable diagnostic information for common and important clinical problems faced by hospitalists, such as pneumonia, soft-tissue infections,1 and myriad other applications. It can inform the evaluation and management of complex clinical problems such as dyspnea.2 Beyond its diagnostic potential, POCUS is well known to improve common procedures performed by adult and pediatric hospitalists by improving success rates and decreasing complications.
Excitement surrounding this technology continues to grow among hospitalists, leading to a proliferation of high-quality educational programs over the last 5 years. Most notable among these offerings has been the more comprehensive training available through the Society of Hospital Medicine (SHM) certificate-based pathway, though many other strong options exist, including institution-based curricula, such as the HealthPartners CHAMP program,3 and pediatric-focused programs. Growth in training is also occurring among medical students and residents. As of a 2012 survey, the majority (51%) of US medical schools had begun to weave ultrasound into their curricula,4 and this growth is also occurring in internal medicine and pediatric residency programs.5
Given the high potential for this technology and the growth in interest, it is an excellent time to pause and review some of the challenges faced by practitioners, hospitalist groups, and educators seeking to optimize POCUS implementation. A deliberate approach to POCUS education, the development of shared standards for high-quality use, and an ongoing dedication to develop specialty-specific practices will largely determine how much of this potential is fulfilled.
The largest challenge is likely to be educational. Educating clinicians to be able to integrate POCUS into practice is a complex, multistep process requiring not only an adequate core of didactic training and access to machines, but also the structured opportunity to develop rudimentary hands-on skills. Such initial training should be followed by continued practice and feedback as developing POCUS users progress toward independent practice. The study by Kumar et al.6 reaffirms that brief didactic lectures and access to machines are necessary, but they are clearly insufficient for learners to be able to use POCUS independently for a wide variety of applications. Their intervention also contrasts markedly with the 20 hours of didactics and 150 supervised scans recommended by the American College of Emergency Physicians prior to independent use for a core of six applications.7
Shared standards for education, use, and oversight will be crucial to fulfilling the potential of POCUS within hospital medicine. Our belief is that much can be learned from the thoughtful approach taken during the development of POCUS as a mainstream tool in emergency medicine in the early 2000s. In this approach, emergency physicians determined a sufficient and achievable standard of training for core POCUS applications, which was widely adopted. Based on completion of this training, physicians who were required to complete credentialing from their hospitals were widely able to achieve it, without any need for external certification. Emergency medicine guidelines further mandated the documentation of examinations and the creation of an exam report, features that improve clinical communication and facilitate quality improvement. Quality assurance processes that reviewed images and clinician interpretations were established as mandatory, which they should be in hospital medicine. Evidence was produced as to which exams physicians could do reliably with this focused training and which they could not. In the context of these thoughtful constructs, lawsuits have been noted to be exceedingly rare; and when they do occur, they have typically been for the failure to use POCUS rather than the converse.8
While many of these precepts deserve replication, others should also be modified to reflect changes in technology, medical education, and medical practice over the last 20 years and to improve upon this base of success. For example, with POCUS training now appearing in many medical school and residency curricula, training paradigms for both residents and attendings will need to accommodate a wider range of incoming skills. Emphasis should continue to be shifted toward competency-based assessments and entrustment and away from a fixed training time or exam number threshold. Important financial aspects have also changed. The cost of practical machines has dropped considerably, and medicine is shifting away from a fee-for-service model. While it remains appropriate that physicians may bill for POCUS examinations, it is likely that improved diagnosis, improved throughput, and a reduction in complications will yield greater value and should be the emphasis of cost/value discussions.9 Finally, while hospitals may impose credentialing, this process can also create a burden not present for most other noninvasive skills and may deter appropriate use. If this approach is chosen by a hospital, requirements should ideally remain modest, and as these skills become more widespread, POCUS should ultimately be built into board examinations and core credentialing.9
Thoughtful and concerted effort will be required by hospitalist leaders, educational innovators, and professional societies in developing POCUS to best serve hospitalists and their patients. This work has already begun. For example, in 2019 SHM offered a position statement outlining important aspects such as current evidence-based applications, training pathways, quality assurance, and program management.10 These recommendations should guide both adult and pediatric hospitalists. The Alliance for Academic Internal Medicine offered a similar position statement for resident training.11 Interest groups are growing in numerous professional societies, which will facilitate collaboration and promote propagation of best practices. High-quality educational tools are continuing to be developed by numerous organizations.
While further development is needed to add the detail, granularity, and practical tools that educational and practice leaders need to assure that POCUS achieves its potential in hospital medicine, the foundation for POCUS use within the specialty is being thoughtfully constructed. As this process proceeds, it will be vital to continue to learn from our emergency medicine colleagues, who have already met similar challenges, while at the same time be able to develop a modern POCUS model optimized for hospital medicine workflow, training, and patient care.
The enthusiasm surrounding point-of-care ultrasound (POCUS) is clear and well founded. POCUS is a powerful tool that produces valuable diagnostic information for common and important clinical problems faced by hospitalists, such as pneumonia, soft-tissue infections,1 and myriad other applications. It can inform the evaluation and management of complex clinical problems such as dyspnea.2 Beyond its diagnostic potential, POCUS is well known to improve common procedures performed by adult and pediatric hospitalists by improving success rates and decreasing complications.
Excitement surrounding this technology continues to grow among hospitalists, leading to a proliferation of high-quality educational programs over the last 5 years. Most notable among these offerings has been the more comprehensive training available through the Society of Hospital Medicine (SHM) certificate-based pathway, though many other strong options exist, including institution-based curricula, such as the HealthPartners CHAMP program,3 and pediatric-focused programs. Growth in training is also occurring among medical students and residents. As of a 2012 survey, the majority (51%) of US medical schools had begun to weave ultrasound into their curricula,4 and this growth is also occurring in internal medicine and pediatric residency programs.5
Given the high potential for this technology and the growth in interest, it is an excellent time to pause and review some of the challenges faced by practitioners, hospitalist groups, and educators seeking to optimize POCUS implementation. A deliberate approach to POCUS education, the development of shared standards for high-quality use, and an ongoing dedication to develop specialty-specific practices will largely determine how much of this potential is fulfilled.
The largest challenge is likely to be educational. Educating clinicians to be able to integrate POCUS into practice is a complex, multistep process requiring not only an adequate core of didactic training and access to machines, but also the structured opportunity to develop rudimentary hands-on skills. Such initial training should be followed by continued practice and feedback as developing POCUS users progress toward independent practice. The study by Kumar et al.6 reaffirms that brief didactic lectures and access to machines are necessary, but they are clearly insufficient for learners to be able to use POCUS independently for a wide variety of applications. Their intervention also contrasts markedly with the 20 hours of didactics and 150 supervised scans recommended by the American College of Emergency Physicians prior to independent use for a core of six applications.7
Shared standards for education, use, and oversight will be crucial to fulfilling the potential of POCUS within hospital medicine. Our belief is that much can be learned from the thoughtful approach taken during the development of POCUS as a mainstream tool in emergency medicine in the early 2000s. In this approach, emergency physicians determined a sufficient and achievable standard of training for core POCUS applications, which was widely adopted. Based on completion of this training, physicians who were required to complete credentialing from their hospitals were widely able to achieve it, without any need for external certification. Emergency medicine guidelines further mandated the documentation of examinations and the creation of an exam report, features that improve clinical communication and facilitate quality improvement. Quality assurance processes that reviewed images and clinician interpretations were established as mandatory, which they should be in hospital medicine. Evidence was produced as to which exams physicians could do reliably with this focused training and which they could not. In the context of these thoughtful constructs, lawsuits have been noted to be exceedingly rare; and when they do occur, they have typically been for the failure to use POCUS rather than the converse.8
While many of these precepts deserve replication, others should also be modified to reflect changes in technology, medical education, and medical practice over the last 20 years and to improve upon this base of success. For example, with POCUS training now appearing in many medical school and residency curricula, training paradigms for both residents and attendings will need to accommodate a wider range of incoming skills. Emphasis should continue to be shifted toward competency-based assessments and entrustment and away from a fixed training time or exam number threshold. Important financial aspects have also changed. The cost of practical machines has dropped considerably, and medicine is shifting away from a fee-for-service model. While it remains appropriate that physicians may bill for POCUS examinations, it is likely that improved diagnosis, improved throughput, and a reduction in complications will yield greater value and should be the emphasis of cost/value discussions.9 Finally, while hospitals may impose credentialing, this process can also create a burden not present for most other noninvasive skills and may deter appropriate use. If this approach is chosen by a hospital, requirements should ideally remain modest, and as these skills become more widespread, POCUS should ultimately be built into board examinations and core credentialing.9
Thoughtful and concerted effort will be required by hospitalist leaders, educational innovators, and professional societies in developing POCUS to best serve hospitalists and their patients. This work has already begun. For example, in 2019 SHM offered a position statement outlining important aspects such as current evidence-based applications, training pathways, quality assurance, and program management.10 These recommendations should guide both adult and pediatric hospitalists. The Alliance for Academic Internal Medicine offered a similar position statement for resident training.11 Interest groups are growing in numerous professional societies, which will facilitate collaboration and promote propagation of best practices. High-quality educational tools are continuing to be developed by numerous organizations.
While further development is needed to add the detail, granularity, and practical tools that educational and practice leaders need to assure that POCUS achieves its potential in hospital medicine, the foundation for POCUS use within the specialty is being thoughtfully constructed. As this process proceeds, it will be vital to continue to learn from our emergency medicine colleagues, who have already met similar challenges, while at the same time be able to develop a modern POCUS model optimized for hospital medicine workflow, training, and patient care.
1. Kinnear B, Kelleher M, Chorny V. Clinical practice update: Point-of-care ultrasound for the pediatric hospitalist. J Hosp Med. 2019;15(3):170-172. https://doi.org/10.12788/jhm.3325.
2. Kelleher M, Kinnear B, Olson A. Clinical progress note: Point-of-care ultrasound in the evaluation of the dyspneic adult. J Hosp Med. 2020;15(3):173-175. https://doi.org/10.12788/jhm.3340.
3. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) Ultrasound Program. J Hosp Med. 2018;13(8):544-550. https://doi.org/10.12788/jhm.2938.
4. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: Results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
5. Reaume M, Siuba M, Wagner M, Woodwyk A, Melgar TA. Prevalence and Scope of point-of-care ultrasound education in internal medicine, pediatric, and medicine-pediatric residency programs in the United States. J Ultrasound Med. 2019;38(6):1433-1439. https://doi.org/10.1002/jum.14821.
6. Kumar A, Weng Y, Wang L, et al. Portable ultrasound device usage and learning outcomes among internal medicine trainees: a parallel-group randomized trial. J Hosp Med. 2020;15(3):154-159. https://doi.org/10.12788/jhm.3351.
7. Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
8. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16(1):1-4. https://doi.org/10.5811/westjem.2014.11.23592.
9, Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of Point-of-Care Ultrasound Competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812
10. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-Care Ultrasound for hospitalists: A position statement of the society of hospital medicine. J Hosp Med. 2019;14. https://doi.org/10.12788/jhm.3079.
11. LoPresti CM, Jensen TP, Dversdal RK, Astiz DJ. Point-of-Care Ultrasound for Internal Medicine Residency Training: A position statement from the Alliance of Academic Internal Medicine. Am J Med. 2019 Nov;132(11):1356-1360. https://doi.org/10.1016/j.amjmed.2019.07.019.
1. Kinnear B, Kelleher M, Chorny V. Clinical practice update: Point-of-care ultrasound for the pediatric hospitalist. J Hosp Med. 2019;15(3):170-172. https://doi.org/10.12788/jhm.3325.
2. Kelleher M, Kinnear B, Olson A. Clinical progress note: Point-of-care ultrasound in the evaluation of the dyspneic adult. J Hosp Med. 2020;15(3):173-175. https://doi.org/10.12788/jhm.3340.
3. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) Ultrasound Program. J Hosp Med. 2018;13(8):544-550. https://doi.org/10.12788/jhm.2938.
4. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: Results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
5. Reaume M, Siuba M, Wagner M, Woodwyk A, Melgar TA. Prevalence and Scope of point-of-care ultrasound education in internal medicine, pediatric, and medicine-pediatric residency programs in the United States. J Ultrasound Med. 2019;38(6):1433-1439. https://doi.org/10.1002/jum.14821.
6. Kumar A, Weng Y, Wang L, et al. Portable ultrasound device usage and learning outcomes among internal medicine trainees: a parallel-group randomized trial. J Hosp Med. 2020;15(3):154-159. https://doi.org/10.12788/jhm.3351.
7. Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
8. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16(1):1-4. https://doi.org/10.5811/westjem.2014.11.23592.
9, Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of Point-of-Care Ultrasound Competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812
10. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-Care Ultrasound for hospitalists: A position statement of the society of hospital medicine. J Hosp Med. 2019;14. https://doi.org/10.12788/jhm.3079.
11. LoPresti CM, Jensen TP, Dversdal RK, Astiz DJ. Point-of-Care Ultrasound for Internal Medicine Residency Training: A position statement from the Alliance of Academic Internal Medicine. Am J Med. 2019 Nov;132(11):1356-1360. https://doi.org/10.1016/j.amjmed.2019.07.019.
© 2020 Society of Hospital Medicine
MISSION Possible, but Incomplete: Pairing Better Access with Better Transitions in Veteran Care
What childhood game better captures communication exchange than “telephone”: as whispers pass from ear to ear, the original message degrades or transforms entirely. In complex healthcare systems, a more perilous version of “telephone” emerges, distinct from the well-worn metaphor: the signal never arrives at all. The primary care provider never even knew the patient was in the hospital; the discharge summary was never received; the patient cannot remember important details; and key medications are missing. In this edition of the Journal, Roman Ayele et al.1 used qualitative methods to explore this transitional black box between community hospitals and Veterans’ Affairs (VA) primary care clinics, illuminating how signal fragmentation may render the increasing use of care services outside the VA system as inversely proportionate to quality.
To understand why, a small amount of historical context is necessary. The VA has increasingly focused on expanding healthcare options to its nine million veterans. On June 6, 2019, the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act was passed to consolidate existing programs and lower barriers for Veterans to seek care in non-VA urgent care and subspecialty settings.2 Though this act is not specifically focused on access to community hospitals, patients seeking urgent and subspecialty care are likely to be increasingly hospitalized outside of the VA due to geographic factors affecting point-of-care decisions. Concurrent with this expansion of options is the planned replacement of the VA’s legacy electronic health record, VistA.3 Both transformations indicate the need for the VA to be watchful and to intensify its focus on safe, effective exchanges of information.
Against this backdrop, Ayele et al.3 use stakeholder interviews with veterans and both non-VA and VA clinicians to identify the current lack of standardized practices for transitions of veteran care from community hospitals to VA primary care in Eastern Colorado. The themes most linked to care fragmentation included difficulty in identifying veterans and notifying VA primary care of hospital discharges, transferring medical records, making follow-up appointments, and coordinating prescribing with VA pharmacies. Participants identified incomplete or delayed information exchanges that were further complicated by the inability to confirm transmission across systems. A patchwork of postacute care solutions failed to prevent wasteful, low-value transitional care, including unscheduled primary care walk-ins and ED visits for medication refills. Participants arrived at a simple common solution: develop a clinically trained “VA liaison” to work at the interface between VA primary care and non-VA community hospitals so as to provide a single point of contact to coordinate these transitions. In short, to have someone to pick up the phone.
The strengths of this qualitative study lie in its insights into the current gaps in care transitions through the eyes of key stakeholders. By engaging patients and providers in imagining system changes that are actionable in the near- (clinical VA liaisons) and longer-term (pharmacy and EHR integration), Ayele et al. have provided a helpful starting place in studying and improving the interface between VA and non-VA care. Stakeholders emphasized the importance of a clear access point so that outside providers can easily notify VA clinics, arrange follow-ups, and streamline physician prescribing to avoid dangerous and costly delays in care.4 Though similar issues have been illuminated in prior work on care fragmentation,4 perspective in context is a fundamental strength of qualitative research, and further highlights the urgency of this period in veteran care.
There is the old adage: “if you have seen one VA, you have seen one VA”. This is arguably reflected in how each VA medical center is situated in a different regional and local healthcare delivery context, despite a common national infrastructure. The authors acknowledge limited generalizability but provide a framework for reproducing such work in regional VA systems. A national model for transitioning patients from regional community partners to VA primary care would require further testing, and to be a credible system-wide investment, would necessitate meaningful measurement across multiple sites. Given recent evidence of strong internal VA performance compared to the private sector,5 it is time for the VA to intensify focus on external care transitions. Given its history and continued commitment to funding innovation,6 the VA ought to be up to the task. Yet, as VA hospitalists, we know only too well that the system is increasingly under pressure to apply constrained resources inside and outside its own walls. Sending business elsewhere might not only fail at improving care but also weaken the fragile care delivery infrastructure.7
The idea that access and continuity may be in conflict raises an ethical question in modern practice and shared decision-making: how do we advise patients navigating complicated and imperfect health systems to understand the choices they are making and the risks they are taking when they spread care across systems? How are access and convenience weighed against the troubled movement of information across systems? How great is the risk if their care teams do not hear the same message? Knowing that increased fragmentation disproportionately affects the marginalized and vulnerable, especially those with complex chronic care needs,8 should we advise certain patients to stay in place within a single system?
As hospitalists, we are implied players in this dangerous version of the telephone game at a fascinating time in healthcare. Unlike when we advise patients on the risks and benefits of treatment, we have little evidence to guide our patients on when to stay put and when to leave to get care outside the system, inviting the risk of lost signals, garbled messages, and worst of all, frustrating, duplicative, unsafe care. As we strive for incremental improvements toward sweeping transformations in healthcare, we may for a few more years have to remind each other—and our students—of the incredible value of one more phone call: to make sure the intended message was
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Ayele RA, Lawrence E, McCreight M, et al. Perspectives of clinicians, staff, and veterans in transitioning veterans from non-VA hospitals to primary care in a single VA healthcare system. J Hosp Med. 2020;15(3):133-139. https://doi.org/10.12788/jhm.3320.
2. US Department of Veterans Affairs: VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018. https://missionact.va.gov/ at https://www.congress.gov/115/bills/s2372/BILLS-115s2372enr.pdf. Accessed October 31, 2019.
3. US Department of Veterans Affairs: VA EHR Modernization. ehrm.va.gov. Accessed October 31, 2019.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. https://doi.org/10.1001/jamainternmed.2019.2788.
5. Weeks WB, West AN. Veterans Health Administration hospitals outperform non–Veterans health administration hospitals in most health care markets. Ann Intern Med. 2018;170(6):426-428. https://doi.org/10.7326/M18-1540.
6. US Department of Veterans Affairs: VA Innovation Center. https://www.innovation.va.gov/. Accessed October 31, 2019.
7. Shulkin, DL. Implications for veterans’ Health Care: the danger becomes clearer [published online ahead of print July 22, 2019. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2019.2996.
8. Englander H, Michaels L, Chan B, Kansagara D. The care transitions innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. https://doi.org/10.1007/s11606-014-2903-0.
What childhood game better captures communication exchange than “telephone”: as whispers pass from ear to ear, the original message degrades or transforms entirely. In complex healthcare systems, a more perilous version of “telephone” emerges, distinct from the well-worn metaphor: the signal never arrives at all. The primary care provider never even knew the patient was in the hospital; the discharge summary was never received; the patient cannot remember important details; and key medications are missing. In this edition of the Journal, Roman Ayele et al.1 used qualitative methods to explore this transitional black box between community hospitals and Veterans’ Affairs (VA) primary care clinics, illuminating how signal fragmentation may render the increasing use of care services outside the VA system as inversely proportionate to quality.
To understand why, a small amount of historical context is necessary. The VA has increasingly focused on expanding healthcare options to its nine million veterans. On June 6, 2019, the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act was passed to consolidate existing programs and lower barriers for Veterans to seek care in non-VA urgent care and subspecialty settings.2 Though this act is not specifically focused on access to community hospitals, patients seeking urgent and subspecialty care are likely to be increasingly hospitalized outside of the VA due to geographic factors affecting point-of-care decisions. Concurrent with this expansion of options is the planned replacement of the VA’s legacy electronic health record, VistA.3 Both transformations indicate the need for the VA to be watchful and to intensify its focus on safe, effective exchanges of information.
Against this backdrop, Ayele et al.3 use stakeholder interviews with veterans and both non-VA and VA clinicians to identify the current lack of standardized practices for transitions of veteran care from community hospitals to VA primary care in Eastern Colorado. The themes most linked to care fragmentation included difficulty in identifying veterans and notifying VA primary care of hospital discharges, transferring medical records, making follow-up appointments, and coordinating prescribing with VA pharmacies. Participants identified incomplete or delayed information exchanges that were further complicated by the inability to confirm transmission across systems. A patchwork of postacute care solutions failed to prevent wasteful, low-value transitional care, including unscheduled primary care walk-ins and ED visits for medication refills. Participants arrived at a simple common solution: develop a clinically trained “VA liaison” to work at the interface between VA primary care and non-VA community hospitals so as to provide a single point of contact to coordinate these transitions. In short, to have someone to pick up the phone.
The strengths of this qualitative study lie in its insights into the current gaps in care transitions through the eyes of key stakeholders. By engaging patients and providers in imagining system changes that are actionable in the near- (clinical VA liaisons) and longer-term (pharmacy and EHR integration), Ayele et al. have provided a helpful starting place in studying and improving the interface between VA and non-VA care. Stakeholders emphasized the importance of a clear access point so that outside providers can easily notify VA clinics, arrange follow-ups, and streamline physician prescribing to avoid dangerous and costly delays in care.4 Though similar issues have been illuminated in prior work on care fragmentation,4 perspective in context is a fundamental strength of qualitative research, and further highlights the urgency of this period in veteran care.
There is the old adage: “if you have seen one VA, you have seen one VA”. This is arguably reflected in how each VA medical center is situated in a different regional and local healthcare delivery context, despite a common national infrastructure. The authors acknowledge limited generalizability but provide a framework for reproducing such work in regional VA systems. A national model for transitioning patients from regional community partners to VA primary care would require further testing, and to be a credible system-wide investment, would necessitate meaningful measurement across multiple sites. Given recent evidence of strong internal VA performance compared to the private sector,5 it is time for the VA to intensify focus on external care transitions. Given its history and continued commitment to funding innovation,6 the VA ought to be up to the task. Yet, as VA hospitalists, we know only too well that the system is increasingly under pressure to apply constrained resources inside and outside its own walls. Sending business elsewhere might not only fail at improving care but also weaken the fragile care delivery infrastructure.7
The idea that access and continuity may be in conflict raises an ethical question in modern practice and shared decision-making: how do we advise patients navigating complicated and imperfect health systems to understand the choices they are making and the risks they are taking when they spread care across systems? How are access and convenience weighed against the troubled movement of information across systems? How great is the risk if their care teams do not hear the same message? Knowing that increased fragmentation disproportionately affects the marginalized and vulnerable, especially those with complex chronic care needs,8 should we advise certain patients to stay in place within a single system?
As hospitalists, we are implied players in this dangerous version of the telephone game at a fascinating time in healthcare. Unlike when we advise patients on the risks and benefits of treatment, we have little evidence to guide our patients on when to stay put and when to leave to get care outside the system, inviting the risk of lost signals, garbled messages, and worst of all, frustrating, duplicative, unsafe care. As we strive for incremental improvements toward sweeping transformations in healthcare, we may for a few more years have to remind each other—and our students—of the incredible value of one more phone call: to make sure the intended message was
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
What childhood game better captures communication exchange than “telephone”: as whispers pass from ear to ear, the original message degrades or transforms entirely. In complex healthcare systems, a more perilous version of “telephone” emerges, distinct from the well-worn metaphor: the signal never arrives at all. The primary care provider never even knew the patient was in the hospital; the discharge summary was never received; the patient cannot remember important details; and key medications are missing. In this edition of the Journal, Roman Ayele et al.1 used qualitative methods to explore this transitional black box between community hospitals and Veterans’ Affairs (VA) primary care clinics, illuminating how signal fragmentation may render the increasing use of care services outside the VA system as inversely proportionate to quality.
To understand why, a small amount of historical context is necessary. The VA has increasingly focused on expanding healthcare options to its nine million veterans. On June 6, 2019, the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act was passed to consolidate existing programs and lower barriers for Veterans to seek care in non-VA urgent care and subspecialty settings.2 Though this act is not specifically focused on access to community hospitals, patients seeking urgent and subspecialty care are likely to be increasingly hospitalized outside of the VA due to geographic factors affecting point-of-care decisions. Concurrent with this expansion of options is the planned replacement of the VA’s legacy electronic health record, VistA.3 Both transformations indicate the need for the VA to be watchful and to intensify its focus on safe, effective exchanges of information.
Against this backdrop, Ayele et al.3 use stakeholder interviews with veterans and both non-VA and VA clinicians to identify the current lack of standardized practices for transitions of veteran care from community hospitals to VA primary care in Eastern Colorado. The themes most linked to care fragmentation included difficulty in identifying veterans and notifying VA primary care of hospital discharges, transferring medical records, making follow-up appointments, and coordinating prescribing with VA pharmacies. Participants identified incomplete or delayed information exchanges that were further complicated by the inability to confirm transmission across systems. A patchwork of postacute care solutions failed to prevent wasteful, low-value transitional care, including unscheduled primary care walk-ins and ED visits for medication refills. Participants arrived at a simple common solution: develop a clinically trained “VA liaison” to work at the interface between VA primary care and non-VA community hospitals so as to provide a single point of contact to coordinate these transitions. In short, to have someone to pick up the phone.
The strengths of this qualitative study lie in its insights into the current gaps in care transitions through the eyes of key stakeholders. By engaging patients and providers in imagining system changes that are actionable in the near- (clinical VA liaisons) and longer-term (pharmacy and EHR integration), Ayele et al. have provided a helpful starting place in studying and improving the interface between VA and non-VA care. Stakeholders emphasized the importance of a clear access point so that outside providers can easily notify VA clinics, arrange follow-ups, and streamline physician prescribing to avoid dangerous and costly delays in care.4 Though similar issues have been illuminated in prior work on care fragmentation,4 perspective in context is a fundamental strength of qualitative research, and further highlights the urgency of this period in veteran care.
There is the old adage: “if you have seen one VA, you have seen one VA”. This is arguably reflected in how each VA medical center is situated in a different regional and local healthcare delivery context, despite a common national infrastructure. The authors acknowledge limited generalizability but provide a framework for reproducing such work in regional VA systems. A national model for transitioning patients from regional community partners to VA primary care would require further testing, and to be a credible system-wide investment, would necessitate meaningful measurement across multiple sites. Given recent evidence of strong internal VA performance compared to the private sector,5 it is time for the VA to intensify focus on external care transitions. Given its history and continued commitment to funding innovation,6 the VA ought to be up to the task. Yet, as VA hospitalists, we know only too well that the system is increasingly under pressure to apply constrained resources inside and outside its own walls. Sending business elsewhere might not only fail at improving care but also weaken the fragile care delivery infrastructure.7
The idea that access and continuity may be in conflict raises an ethical question in modern practice and shared decision-making: how do we advise patients navigating complicated and imperfect health systems to understand the choices they are making and the risks they are taking when they spread care across systems? How are access and convenience weighed against the troubled movement of information across systems? How great is the risk if their care teams do not hear the same message? Knowing that increased fragmentation disproportionately affects the marginalized and vulnerable, especially those with complex chronic care needs,8 should we advise certain patients to stay in place within a single system?
As hospitalists, we are implied players in this dangerous version of the telephone game at a fascinating time in healthcare. Unlike when we advise patients on the risks and benefits of treatment, we have little evidence to guide our patients on when to stay put and when to leave to get care outside the system, inviting the risk of lost signals, garbled messages, and worst of all, frustrating, duplicative, unsafe care. As we strive for incremental improvements toward sweeping transformations in healthcare, we may for a few more years have to remind each other—and our students—of the incredible value of one more phone call: to make sure the intended message was
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Ayele RA, Lawrence E, McCreight M, et al. Perspectives of clinicians, staff, and veterans in transitioning veterans from non-VA hospitals to primary care in a single VA healthcare system. J Hosp Med. 2020;15(3):133-139. https://doi.org/10.12788/jhm.3320.
2. US Department of Veterans Affairs: VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018. https://missionact.va.gov/ at https://www.congress.gov/115/bills/s2372/BILLS-115s2372enr.pdf. Accessed October 31, 2019.
3. US Department of Veterans Affairs: VA EHR Modernization. ehrm.va.gov. Accessed October 31, 2019.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. https://doi.org/10.1001/jamainternmed.2019.2788.
5. Weeks WB, West AN. Veterans Health Administration hospitals outperform non–Veterans health administration hospitals in most health care markets. Ann Intern Med. 2018;170(6):426-428. https://doi.org/10.7326/M18-1540.
6. US Department of Veterans Affairs: VA Innovation Center. https://www.innovation.va.gov/. Accessed October 31, 2019.
7. Shulkin, DL. Implications for veterans’ Health Care: the danger becomes clearer [published online ahead of print July 22, 2019. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2019.2996.
8. Englander H, Michaels L, Chan B, Kansagara D. The care transitions innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. https://doi.org/10.1007/s11606-014-2903-0.
1. Ayele RA, Lawrence E, McCreight M, et al. Perspectives of clinicians, staff, and veterans in transitioning veterans from non-VA hospitals to primary care in a single VA healthcare system. J Hosp Med. 2020;15(3):133-139. https://doi.org/10.12788/jhm.3320.
2. US Department of Veterans Affairs: VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018. https://missionact.va.gov/ at https://www.congress.gov/115/bills/s2372/BILLS-115s2372enr.pdf. Accessed October 31, 2019.
3. US Department of Veterans Affairs: VA EHR Modernization. ehrm.va.gov. Accessed October 31, 2019.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. https://doi.org/10.1001/jamainternmed.2019.2788.
5. Weeks WB, West AN. Veterans Health Administration hospitals outperform non–Veterans health administration hospitals in most health care markets. Ann Intern Med. 2018;170(6):426-428. https://doi.org/10.7326/M18-1540.
6. US Department of Veterans Affairs: VA Innovation Center. https://www.innovation.va.gov/. Accessed October 31, 2019.
7. Shulkin, DL. Implications for veterans’ Health Care: the danger becomes clearer [published online ahead of print July 22, 2019. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2019.2996.
8. Englander H, Michaels L, Chan B, Kansagara D. The care transitions innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. https://doi.org/10.1007/s11606-014-2903-0.
© 2020 Society of Hospital Medicine
Blistering Disease During the Treatment of Chronic Hepatitis C With Ledipasvir/Sofosbuvir (FULL)
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.
Contrasting qSOFA and SIRS Criteria for Early Sepsis Identification in a Veteran Population (FULL)
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.