User login
A Multi-Membership Approach for Attributing Patient-Level Outcomes to Providers in an Inpatient Setting
From Banner Health Corporation, Phoenix, AZ.
Background: Health care providers are routinely incentivized with pay-for-performance (P4P) metrics to increase the quality of care. In an inpatient setting, P4P models typically measure quality by attributing each patient’s outcome to a single provider even though many providers routinely care for the patient. This study investigates a new attribution approach aiming to distribute each outcome across all providers who provided care.
Methods: The methodology relies on a multi-membership model and is demonstrated in the Banner Health system using 3 clinical outcome measures (length of stay, 30-day readmissions, and mortality) and responses to 3 survey questions that measure a patient’s perception of their care. The new approach is compared to the “standard” method, which attributes each patient to only 1 provider.
Results: When ranking by clinical outcomes, both methods were concordant 72.1% to 82.1% of the time for top-half/bottom-half rankings, with a median percentile difference between 7 and 15. When ranking by survey scores, there was more agreement, with concordance between 84.1% and 86.6% and a median percentile difference between 11 and 13. Last, Pearson correlation coefficients of the paired percentiles ranged from 0.56 to 0.78.
Conclusion: The new approach provides a fairer solution when measuring provider performance.
Keywords: patient attribution; PAMM; PAPR; random effect model; pay for performance.
Providers practicing in hospitals are routinely evaluated based on their performance and, in many cases, are financially incentivized for a better-than-average performance within a pay-for-performance (P4P) model. The use of P4P models is based on the belief that they will “improve, motivate, and enhance providers to pursue aggressively and ultimately achieve the quality performance targets thus decreasing the number of medical errors with less malpractice events.”1 Although P4P models continue to be a movement in health care, they have been challenging to implement.
One concern involves the general quality of implementation, such as defining metrics and targets, setting payout amounts, managing technology and market conditions, and gauging the level of transparency to the provider.2 Another challenge, and the focus of this project, are concerns around measuring performance to avoid perceptions of unfairness. This concern can be minimized if the attribution is handled in a fairer way, by spreading it across all providers who affected the outcome, both in a positive or negative direction.3
To implement these models, the performance of providers needs to be measured and tracked periodically. This requires linking, or attributing, a patient’s outcome to a provider, which is almost always the attending or discharging provider (ie, a single provider).3 In this single-provider attribution approach, one provider will receive all the credit (good or bad) for their respective patients’ outcomes, even though the provider may have seen the patient only a fraction of the time during the hospitalization. Attributing outcomes—for example, length of stay (LOS), readmission rate, mortality rate, net promoter score (NPS)—using this approach reduces the validity of metrics designed to measure provider performance, especially in a rotating provider environment where many providers interact with and care for a patient. For example, the quality of providers’ interpersonal skills and competence were among the strongest determinants of patient satisfaction,4 but it is not credible that this is solely based on the last provider during a hospitalization.
Proportionally distributing the attribution of an outcome has been used successfully in other contexts. Typically, a statistical modeling approach using a multi-membership framework is used because it can handle the sometimes-complicated relationships within the hierarchy. It also allows for auxiliary variables to be introduced, which can help explain and control for exogenous effects.5-7 For example, in the education setting, standardized testing is administered to students at defined years of schooling: at grades 4, 8, and 10, for instance. The progress of students, measured as the academic gains between test years, are proportionally attributed to all the teachers who the student has had between the test years. These partial attributions are combined to evaluate an overall teacher performance.8,9
Although the multi-membership framework has been used in other industries, it has yet to be applied in measuring provider performance. The purpose of this project is to investigate the impact of using a multi-provider approach compared to the standard single-provider approach. The findings may lead to modifications in the way a provider’s performance is measured and, thus, how providers are compensated. A similar study investigated the impact of proportionally distributing patients’ outcomes across all rotating providers using a weighting method based on billing practices to measure the partial impact of each provider.3
This study is different in 2 fundamental ways. First, attribution is weighted based on the number of clinically documented interactions (via clinical notes) between a patient and all rotating providers during the hospitalization. Second, performance is measured via multi-membership models, which can estimate the effect (both positive and negative) that a provider has on an outcome, even when caring for a patient a fraction of the time during the hospitalization.
Methods
Setting
Banner Health is a non-profit, multi-hospital health care system across 6 states in the western United States that is uniquely positioned to study provider quality attribution models. It not only has a large number of providers and serves a broad patient population, but Banner Health also uses an instance of Cerner (Kansas City, MO), an enterprise-level electronic health record (EHR) system that connects all its facilities and allows for advanced analytics across its system.
For this study, we included only general medicine and surgery patients admitted and discharged from the inpatient setting between January 1, 2018, and December 31, 2018, who were between 18 and 89 years old at admission, and who had a LOS between 1 and 14 days. Visit- and patient-level data were collected from Cerner, while outcome data, and corresponding expected outcome data, were obtained from Premier, Inc. (Charlotte, NC) using their CareScience methodologies.10 To measure patient experience, response data were extracted from post-discharge surveys administered by InMoment (Salt Lake City, UT).
Provider Attribution Models
Provider Attribution by Physician of Record (PAPR). In the standard approach, denoted here as the PAPR model, 1 provider—typically the attending or discharging provider, which may be the same person—is attributed to the entire hospitalization. This provider is responsible for the patient’s care, and all patient outcomes are aggregated and attributed to the provider to gauge his or her performance. The PAPR model is the most popular form of attribution across many health care systems and is routinely used for P4P incentives.
In this study, the discharging provider was used when attributing hospitalizations using the PAPR model. Providers responsible for fewer than 12 discharges in the calendar year were excluded. Because of the directness of this type of attribution, the performance of 1 provider does not account for the performance of the other rotating providers during hospitalizations.
Provider Attribution by Multiple Membership (PAMM). In contrast, we introduce another attribution approach here that is designed to assign partial attribution to each provider who cares for the patient during the hospitalization. To aggregate the partial attributions, and possibly control for any exogenous or risk-based factors, a multiple-membership, or multi-member (MM), model is used. The MM model can measure the effect of a provider on an outcome even when the patient-to-provider relationship is complex, such as in a rotating provider environment.8
The purpose of this study is to compare attribution models and to determine whether there are meaningful differences between them. Therefore, for comparison purposes, the same discharging providers using the PAPR approach are eligible for the PAMM approach, so that both attribution models are using the same set of providers. All other providers are excluded because their performance would not be comparable to the PAPR approach.
While there are many ways to document provider-to-patient interactions, 2 methods are available in almost all health care systems. The first method is to link a provider’s billing charges to each patient-day combination. This approach limits the attribution to 1 provider per patient per day because multiple rotating providers cannot charge for the same patient-day combination.3 However, many providers interact with a patient on the same day, so using this approach excludes non-billed provider-to-patient interactions.
The second method, which was used in this study, relies on documented clinical notes within the EHR to determine how attribution is shared. In this approach, attribution is weighted based on the authorship of 3 types of eligible clinical notes: admitting history/physical notes (during admission), progress notes (during subsequent days), and discharge summary notes (during final discharge). This will (likely) result in many providers being linked to a patient on each day, which better reflects the clinical setting (Figure). Recently, clinical notes were used to attribute care of patients in an inpatient setting, and it was found that this approach provides a reliable way of tracking interactions and assigning ownership.11
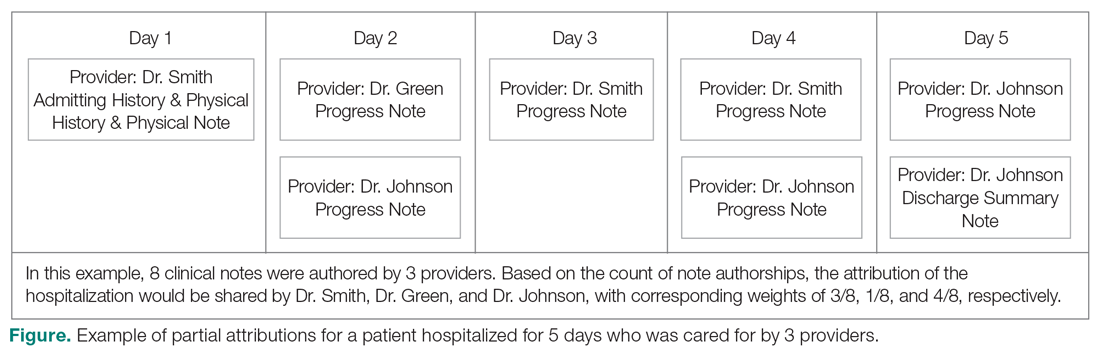
The provider-level attribution weights are based on the share of authorships of eligible note types. Specifically, for each provider j, let aij be the total count of eligible note types for hospitalization i authored by provider j, and let ai be the overall total count of eligible note types for hospitalization i. Then the attribution weight is
(Eq. 1)
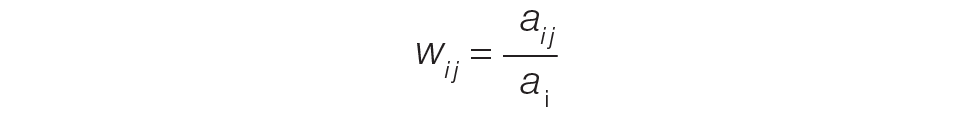
for hospitalization i and provider j. Note that ∑jwij = 1: in other words, the total attribution, summed across all providers, is constrained to be 1 for each hospitalization.
Patient Outcomes
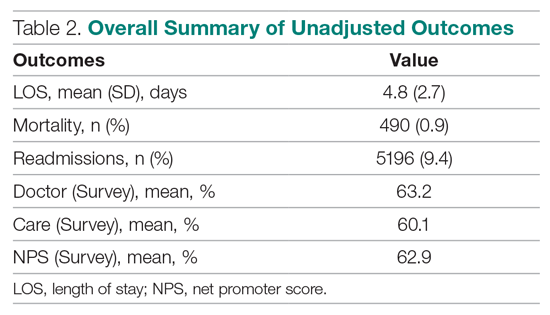
Outcomes were chosen based on their routine use in health care systems as standards when evaluating provider performance. This study included 6 outcomes: inpatient LOS, inpatient mortality, 30-day inpatient readmission, and patient responses from 3 survey questions. These outcomes can be collected without any manual chart reviews, and therefore are viewed as objective outcomes of provider performance.
Each outcome was aggregated for each provider using both attribution methods independently. For the PAPR method, observed-to-expected (OE) indices for LOS, mortality, and readmissions were calculated along with average patient survey scores. For the PAMM method, provider-level random effects from the fitted models were used. In both cases, the calculated measures were used for ranking purposes when determining top (or bottom) providers for each outcome.
Individual Provider Metrics for the PAPR Method
Inpatient LOS Index. Hospital inpatient LOS was measured as the number of days between admission date and discharge date. For each hospital visit, an expected LOS was determined using Premier’s CareScience Analytics (CSA) risk-adjustment methodology.10 The CSA methodology for LOS incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let oij and eij be the observed and expected LOS, respectively, for hospitalization i = 1,…,nj attributed to provider j. Then the inpatient LOS index for provider j is Lj = ∑ioij⁄∑ieij.
Inpatient Mortality Index. Inpatient mortality was defined as the death of the patient during hospitalization. For each hospitalization, an expected mortality probability was determined using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for mortality incorporates a patient’s demographics and comorbidities.
Just as before, let nj be the number of hospitalizations attributed to provider j. Let mij = 1 if the patient died during hospitalization i = 1, … , nj attributed to provider j; mij = 0 otherwise. Let pij(m) be the corresponding expected mortality probability. Then the inpatient mortality index for provider j is Mj = ∑imij⁄∑ipij(m).
30-Day Inpatient Readmission Index. A 30-day inpatient readmission was defined as the event when a patient is discharged and readmits back into the inpatient setting within 30 days. The inclusion criteria defined by the Centers for Medicare and Medicaid Services (CMS) all-cause hospital-wide readmission measure was used and, consequently, planned readmissions were excluded.12 Readmissions could occur at any Banner hospital, including the same hospital. For each hospital visit, an expected readmission probability was derived using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for readmissions incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let rij = 1 if the patient had a readmission following hospitalization i = 1, … , nj attributed to provider j; rij = 0 otherwise. Let pij(r) be the corresponding expected readmission probability. Then the 30-day inpatient readmission index for provider j is Rj = ∑irij ⁄∑ipij(r).
Patient Survey Scores. The satisfaction of the patient’s experience during hospitalization was measured via post-discharge surveys administered by InMoment. Two survey questions were selected because they related directly to a provider’s interaction with the patient: “My interactions with doctors were excellent” (Doctor) and “I received the best possible care” (Care). A third question, “I would recommend this hospital to my family and friends,” was selected as a proxy measure of the overall experience and, in the aggregate, is referred to as the net promoter score (NPS).13,14 The responses were measured on an 11-point Likert scale, ranging from “Strongly Disagree” (0) to “Strongly Agree” (10); “N/A” or missing responses were excluded.
The Likert responses were coded to 3 discrete values as follows: if the value was between 0 and 6, then -1 (ie, detractor); between 7 and 8 (ie, neutral), then 0; otherwise 1 (ie, promoter). Averaging these coded responses results in a patient survey score for each question. Specifically, let nj be the number of hospitalizations attributed to provider j in which the patient responded to the survey question. Let sij ∈{−1, 0, 1} be the coded response linked to hospitalization i = 1, … , nj attributed to provider j. Then the patient experience score for provider j is Sj = ∑isij⁄nj.
Handling Ties in Provider Performance Measures. Because ties can occur in the PAPR approach for all measures, a tie-breaking strategy is needed. For LOS indices, ties are less likely because their numerator is strictly greater than 0, and expected LOS values are typically distinct enough. Indeed, no ties were found in this study for LOS indices. However, mortality and readmission indices can routinely result in ties when the best possible index is achieved, such as 0 deaths or readmissions among attributed hospitalizations. To help differentiate between those indices in the PAPR approach, the total estimated risk (denominator) was utilized as a secondary scoring criterion.
Mortality and readmission metrics were addressed by sorting first by the outcome (mortality index), and second by the denominator (total estimated risk). For example, if provider A has the same mortality rate as provider B, then provider A would be ranked higher if the denominator was larger, indicating a higher risk for mortality.
Similarly, it was very common for providers to have the same overall average rating for a survey question. Therefore, the denominator (number of respondents) was used to break ties. However, the denominator sorting was bidirectional. For example, if the tied score was positive (more promoters than detractors) for providers A and B, then provider A would be ranked higher if the denominator was larger. Conversely, if the tied score between providers A and B was neutral or negative (more detractors than promoters), then provider A would be ranked lower if the denominator was larger.
Individual Provider Metrics for the PAMM Method
For the PAMM method, model-based metrics were derived using a MM model.8 Specifically, let J be the number of rotating providers in a health care system. Let Yi be an outcome of interest from hospitalization i, X1i, …, Xpi be fixed effects or covariates, and ß1, …, ßp be the coefficients for the respective covariates. Then the generalized MM statistical model is
(Eq. 2)
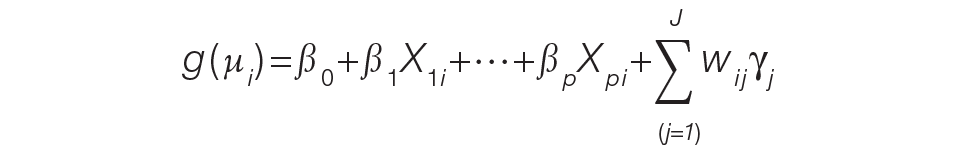
where g(μi ) is a link function between the mean of the outcome, μi, and its linear predictor, ß0, is the marginal intercept, wij represents the attribution weight of provider j on hospitalization i (described in Equation 1), and γj represents the random effect of provider j on the outcome with γj~N(0,σγ2).
For the mortality and readmission binary outcomes, logistic regression was performed using a logit link function, with the corresponding expected probability as the only fixed covariate. The expected probabilities were first converted into odds and then log-transformed before entering the model. For LOS, Poisson regression was performed using a log link function with the log-transformed expected LOS as the only fixed covariate. For coded patient experience responses, an ordered logistic regression was performed using a cumulative logit link function (no fixed effects were added).
MM Model-based Metrics. Each fitted MM model produces a predicted random effect for each provider. The provider-specific random effects can be interpreted as the unobserved influence of each provider on the outcome after controlling for any fixed effect included in the model. Therefore, the provider-specific random effects were used to evaluate the relative provider performance, which is analogous to the individual provider-level metrics used in the PAPR method.
Measuring provider performance using a MM model is more flexible and robust to outliers compared to the standard approach using OE indices or simple averages. First, although not investigated here, the effect of patient-, visit-, provider-, and/or temporal-level covariates can be controlled when evaluating provider performance. For example, a patient’s socioeconomic status, a provider’s workload, and seasonal factors can be added to the MM model. These external factors are not accounted for in OE indices.
Another advantage of using predicted random effects is the concept of “shrinkage.” The process of estimating random effects inherently accounts for small sample sizes (when providers do not treat a large enough sample of patients) and/or when there is a large ratio of patient variance to provider variance (for instance, when patient outcome variability is much higher compared to provider performance variability). In both cases, the estimation of the random effect is pulled ever closer to 0, signaling that the provider performance is closer to the population average. See Henderson15 and Mood16 for further details.
In contrast, OE indices can result in unreliable estimates when a provider has not cared for many patients. This is especially prevalent when the outcome is binary with a low probability of occurring, such as mortality. Indeed, provider-level mortality OE indices are routinely 0 when the patient counts are low, which skews performance rankings unfairly. Finally, OE indices also ignore the magnitude of the variance of an outcome between providers and patients, which can be large.
Comparison Methodology
In this study, we seek to compare the 2 methods of attribution, PAPR and PAMM, to determine whether there are meaningful differences between them when measuring provider performance. Using retrospective data described in the next section, each attribution method was used independently to derive provider-level metrics. To assess relative performance, percentiles were assigned to each provider based on their metric values so that, in the end, there were 2 percentile ranks for each provider for each metric.
Using these paired percentiles, we derived the following measures of concordance, similar to Herzke, Michtalik3: (1) the percent concordance measure—defined as the number of providers who landed in the top half (greater than the median) or bottom half under both attribution models—divided by the total number of providers; (2) the median of the absolute difference in percentiles under both attribution models; and (3) the Pearson correlation coefficient of the paired provider ranks. The first measure is a global measure of concordance between the 2 approaches and would be expected to be 50% by chance. The second measure gauges how an individual provider’s rank is affected by the change in attribution methodologies. The third measure is a statistical measure of linear correlation of the paired percentiles and was not included in the Herzke, Michtalik3 study.
All statistical analyses were performed on SAS (version 9.4; Cary, NC) and the MM models were fitted using PROC GLIMMIX with the EFFECT statement. The Banner Health Institutional Review Board approved this study.
Results
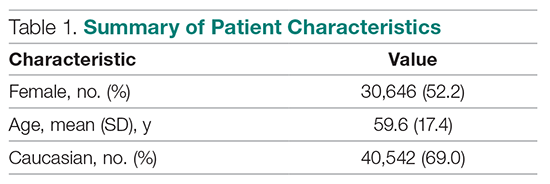
Descriptive Statistics
A total of
Multi-Membership Model Results
Table 3 displays the results after independently fitting MM models to each of the 3 clinical outcomes. Along with a marginal intercept, the only covariate in each model was the corresponding expected value after a transformation. This was added to use the same information that is typically used in OE indices, therefore allowing for a proper comparison between the 2 attribution methods. The provider-level variance represents the between-provider variation and measures the amount of influence providers have on the corresponding outcome after controlling for any covariates in the model. A provider-level variance of 0 would indicate that providers do not have any influence on the outcome. While the mortality and readmission model results can be compared to each other, the LOS model cannot given its different scale and transformation altogether.
The results in Table 3 suggest that each expected value covariate is highly correlated with its corresponding outcome, which is the anticipated conclusion given that they are constructed in this fashion. The estimated provider-level variances indicate that, after including an expected value in the model, providers have less of an influence on a patient’s LOS and likelihood of being readmitted. On the other hand, the results suggest that providers have much more influence on the likelihood of a patient dying in the hospital, even after controlling for an expected mortality covariate.
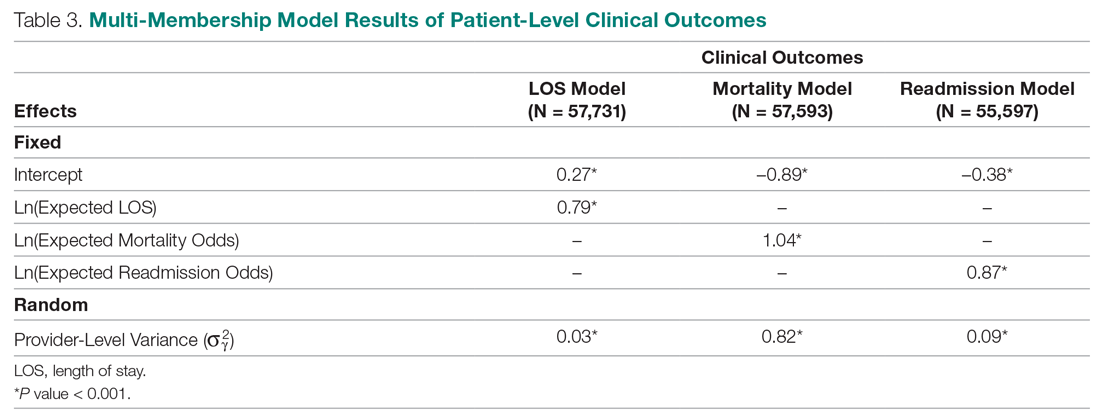
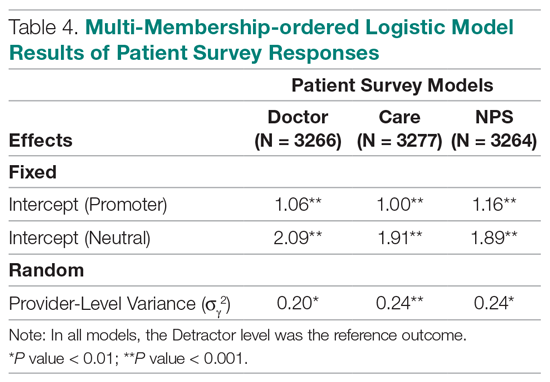
Table 4 shows the results after independently fitting MM-ordered logistic models to each of the 3 survey questions. The similar provider-level variances suggest that providers have the same influence on the patient’s perception of the quality of their interactions with the doctor (Doctor), the quality of the care they received (Care), and their likelihood to recommend a friend or family member to the hospital (NPS).
Comparison Results Between Both Attribution Methods
Table 5 compares the 2 attribution methods when ranking providers based on their performance on each outcome measure. The comparison metrics gauge how well the 2 methods agree overall (percent concordance), agree at the provider level (absolute percentile difference and interquartile range [IQR]), and how the paired percentiles linearly correlate to each other (Pearson correlation coefficient).
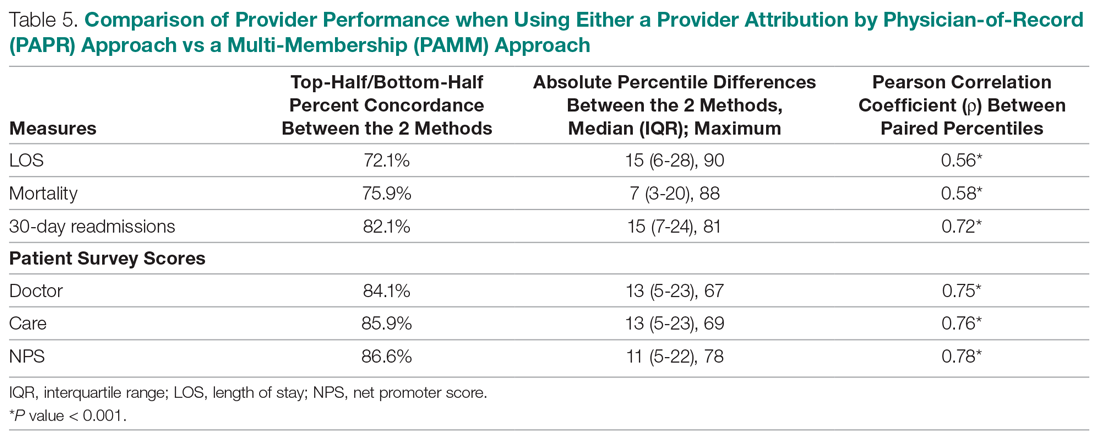
LOS, by a small margin, had the lowest concordance of clinical outcomes (72.1%), followed by mortality (75.9%) and readmissions (82.1%). Generally, the survey scores had higher percent concordance than the clinical outcome measures, with Doctor at 84.1%, Care at 85.9%, and NPS having the highest percent concordance at 86.6%. Given that by chance the percent concordance is expected to be 50%, there was notable discordance, especially with the clinical outcome measures. Using LOS performance as an example, one attribution methodology would rank a provider in the top half or bottom half, while the other attribution methodology would rank the same provider exactly the opposite way about 28% of the time.
The median absolute percentile difference between the 2 methods was more modest (between 7 and 15). Still, there were some providers whose performance ranking was heavily impacted by the attribution methodology that was used. This was especially true when evaluating performance for certain clinical measures, where the attribution method that was used could change the provider performance percentile by up to 90 levels.
The paired percentiles were positively correlated when ranking performance using any of the 6 measures. This suggests that both methodologies assess performance generally in the same direction, irrespective of the methodology and measure. We did not investigate more complex correlation measures and left this for future research.
It should be noted that ties occurred much more frequently with the PAPR method than when using PAMM and therefore required tie-breaking rules to be designed. Given the nature of OE indices, PAPR methodology is especially sensitive to ties whenever the measure includes counting the number of events (for example, mortality and readmissions) and whenever there are many providers with very few attributed patients. On the other hand, using the PAMM method is much more robust against ties given that the summation of all the weighted attributed outcomes will rarely result in ties, even with a nominal set of providers.
Discussion
In this study, the PAMM methodology was introduced and was used to assess relative provider performance on 3 clinical outcome measures and 3 patient survey scores. The new approach aims to distribute each outcome among all providers who provided care for a patient in an inpatient setting. Clinical notes were used to account for patient-to-provider interactions, and fitted MM statistical models were used to compute the effects that each provider had on each outcome. The provider effect was introduced as a random effect, and the set of predicted random effects was used to rank the performance of each provider.
The PAMM approach was compared to the more traditional methodology, PAPR, where each patient is attributed to only 1 provider: the discharging physician in this study. Using this approach, OE indices of clinical outcomes and averages of survey scores were used to rank the performance of each provider. This approach resulted in many ties, which were broken based on the number of hospitalizations, although other tie-breaking methods may be used in practice.
Both methodologies showed modest concordance with each other for the clinical outcomes, but higher concordance for the patient survey scores. This was also true when using the Pearson correlation coefficient to assess agreement. The 1 outcome measure that showed the least concordance and least linear correlation between methods was LOS, which would suggest that LOS performance is more sensitive to the attribution methodology that is used. However, it was the least concordant by a small margin.
Furthermore, although the medians of the absolute percentile differences were small, there were some providers who had large deviations, suggesting that some providers would move from being shown as high-performers to low-performers and vice versa based on the chosen attribution method. We investigated examples of this and determined that the root cause was the difference in effective sample sizes for a provider. For the PAPR method, the effective sample size is simply the number of hospitalizations attributed to the provider. For the PAMM method, the effective sample size is the sum of all non-zero weights across all hospitalizations where the provider cared for a patient. By and large, the PAMM methodology provides more information of the provider effect on an outcome than the PAPR approach because every provider-patient interaction is considered. For example, providers who do not routinely discharge patients, but often care for patients, will have rankings that differ dramatically between the 2 methods.
The PAMM methodology has many statistical advantages that were not fully utilized in this comparative study. For example, we did not include any covariates in the MM models except for the expected value of the outcome, when it was available. Still, it is known that other covariates can impact an outcome as well, such as the patient’s age, socioeconomic indicators, existing chronic conditions, and severity of hospitalization, which can be added to the MM models as fixed effects. In this way, the PAMM approach can control for these other covariates, which are typically outside of the control of providers but typically ignored using OE indices. Therefore, using the PAMM approach would provide a fairer comparison of provider performance.
Using the PAMM method, most providers had a large sample size to assess their performance once all the weighted interactions were included. Still, there were a few who did not care for many patients for a variety of reasons. In these scenarios, MM models “borrow” strength from other providers to produce a more robust predicted provider effect by using a weighted average between the overall population trend and the specific provider outcomes (see Rao and Molina17). As a result, PAMM is a more suitable approach when the sample sizes of patients attributed to providers can be small.
One of the most interesting findings of this study was the relative size of the provider-level variance to the size of the fixed effect in each model (Table 3). Except for mortality, these variances suggest that there is a small difference in performance from one provider to another. However, these should be interpreted as the variance when only 1 provider is involved in the care of a patient. When multiple providers are involved, using basic statistical theory, the overall provider-level variance will be σγ2 ∑wij2 (see Equation 2). For example, the estimated variance among providers for LOS was 0.03 (on a log scale), but, using the scenario in the Figure, the overall provider-level variance for this hospitalization will be 0.03 (0.3752 + 0.1252 + 0.52) = 0.012. Hence, the combined effect of providers on LOS is less than would be expected. Indeed, as more providers are involved with a patient’s care, the more their combined influence on an outcome is diluted.
In this study, the PAMM approach placed an equal weight on all provider-patient interactions via clinical note authorship, but that may not be optimal in some settings. For example, it may make more sense to set a higher weight on the provider who admitted or discharged the patient while placing less (or 0) weight on all other interactions. In the extreme, if the full weight were placed on 1 provider interaction (eg, during discharge, then the MM model would be reduced to a one-way random effects model. The flexibility of weighting interactions is a feature of the PAMM approach, but any weighting framework must be transparent to the providers before implementation.
Conclusion
This study demonstrates that the PAMM approach is a feasible option within a large health care organization. For P4P programs to be successful, providers must be able to trust that their performance will be fairly assessed and that all provider-patient interactions are captured to provide a full comparison amongst their peers. The PAMM methodology is one solution to spread the positive (and negative) outcomes across all providers who cared for a patient and therefore, if implemented, would add trust and fairness when measuring and assessing provider performance.
Acknowledgments: The authors thank Barrie Bradley for his support in the initial stages of this research and Dr. Syed Ismail Jafri for his help and support on the standard approaches of assessing and measuring provider performances.
Corresponding author: Rachel Ginn, MS, Banner Health Corporation, 2901 N. Central Ave., Phoenix, AZ 85012; [email protected].
Financial disclosures: None.
1. Abduljawad A, Al-Assaf AF. Incentives for better performance in health care. Sultan Qaboos Univ Med J. 2011;11:201-206.
2. Milstein R, Schreyoegg J. Pay for performance in the inpatient sector: a review of 34 P4P programs in 14 OECD countries. Health Policy. 2016;120:1125-1140.
3. Herzke CA, Michtalik HJ, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. 2018;13:470-475.
4. Batbaatar E, Dorjdagva J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: a systematic review. Perspect Public Health. 2017;137:89-101.
5. Ballou D, Sanders W, Wright P. Controlling for student background in value-added assessment of teachers. J Educ Behav Stat. 2004;29:37-65.
6. Hill PW, Goldstein H. Multilevel modeling of educational data with cross-classification and missing identification for units. J Educ Behav Stat. 1998;23:117-128.
7. Rasbash J, Browne WJ. Handbook of Multilevel Analysis. Springer; 2007.
8. Brown WJ, Goldstein H, Rasbash J. Multiple membership multiple classification (MMMC) models. Statistical Modeling. 2001;1:103-124.
9. Sanders WL, Horn SP. The Tennessee Value-Added Assessment System (TVAAS)—mixed-model methodology in educational assessment. J Pers Eval Educ. 1994;8:299-311.
10. Kroch EA, Duan M. CareScience Risk Assessment Model: Hospital Performance Measurement. Premier, Inc., 2008. http://www.ahrq.gov/qual/mortality/KrochRisk.htm
11. Schumacher DJ, Wu DTY, Meganathan K, et al. A feasibility study to attribute patients to primary interns on inpatient ward teams using electronic health record data. Acad Med. 2019;94:1376-1383.
12. Simoes J, Krumholz HM, Lin Z. Hospital-level 30-day risk-standardized readmission measure. Centers for Medicare & Medicaid Services, 2018. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Hospital-Wide-All-Cause-Readmission-Updates.zip
13. Krol MW, de Boer D, Delnoij DM, Rademakers JJDJM. The Net Promoter Score: an asset to patient experience surveys? Health Expect. 2015;18:3099-3109.
14. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3:e001570.
15. Henderson CR. Sire evaluation and genetic trends. J Anim Sci. 1973;1973:10-41.
16. Mood AM. Introduction to the Theory of Statistics. McGraw-Hill; 1950:xiii, 433-xiii.
17. Rao JNK, Molina I. Small Area Estimation. Wiley; 2015.
From Banner Health Corporation, Phoenix, AZ.
Background: Health care providers are routinely incentivized with pay-for-performance (P4P) metrics to increase the quality of care. In an inpatient setting, P4P models typically measure quality by attributing each patient’s outcome to a single provider even though many providers routinely care for the patient. This study investigates a new attribution approach aiming to distribute each outcome across all providers who provided care.
Methods: The methodology relies on a multi-membership model and is demonstrated in the Banner Health system using 3 clinical outcome measures (length of stay, 30-day readmissions, and mortality) and responses to 3 survey questions that measure a patient’s perception of their care. The new approach is compared to the “standard” method, which attributes each patient to only 1 provider.
Results: When ranking by clinical outcomes, both methods were concordant 72.1% to 82.1% of the time for top-half/bottom-half rankings, with a median percentile difference between 7 and 15. When ranking by survey scores, there was more agreement, with concordance between 84.1% and 86.6% and a median percentile difference between 11 and 13. Last, Pearson correlation coefficients of the paired percentiles ranged from 0.56 to 0.78.
Conclusion: The new approach provides a fairer solution when measuring provider performance.
Keywords: patient attribution; PAMM; PAPR; random effect model; pay for performance.
Providers practicing in hospitals are routinely evaluated based on their performance and, in many cases, are financially incentivized for a better-than-average performance within a pay-for-performance (P4P) model. The use of P4P models is based on the belief that they will “improve, motivate, and enhance providers to pursue aggressively and ultimately achieve the quality performance targets thus decreasing the number of medical errors with less malpractice events.”1 Although P4P models continue to be a movement in health care, they have been challenging to implement.
One concern involves the general quality of implementation, such as defining metrics and targets, setting payout amounts, managing technology and market conditions, and gauging the level of transparency to the provider.2 Another challenge, and the focus of this project, are concerns around measuring performance to avoid perceptions of unfairness. This concern can be minimized if the attribution is handled in a fairer way, by spreading it across all providers who affected the outcome, both in a positive or negative direction.3
To implement these models, the performance of providers needs to be measured and tracked periodically. This requires linking, or attributing, a patient’s outcome to a provider, which is almost always the attending or discharging provider (ie, a single provider).3 In this single-provider attribution approach, one provider will receive all the credit (good or bad) for their respective patients’ outcomes, even though the provider may have seen the patient only a fraction of the time during the hospitalization. Attributing outcomes—for example, length of stay (LOS), readmission rate, mortality rate, net promoter score (NPS)—using this approach reduces the validity of metrics designed to measure provider performance, especially in a rotating provider environment where many providers interact with and care for a patient. For example, the quality of providers’ interpersonal skills and competence were among the strongest determinants of patient satisfaction,4 but it is not credible that this is solely based on the last provider during a hospitalization.
Proportionally distributing the attribution of an outcome has been used successfully in other contexts. Typically, a statistical modeling approach using a multi-membership framework is used because it can handle the sometimes-complicated relationships within the hierarchy. It also allows for auxiliary variables to be introduced, which can help explain and control for exogenous effects.5-7 For example, in the education setting, standardized testing is administered to students at defined years of schooling: at grades 4, 8, and 10, for instance. The progress of students, measured as the academic gains between test years, are proportionally attributed to all the teachers who the student has had between the test years. These partial attributions are combined to evaluate an overall teacher performance.8,9
Although the multi-membership framework has been used in other industries, it has yet to be applied in measuring provider performance. The purpose of this project is to investigate the impact of using a multi-provider approach compared to the standard single-provider approach. The findings may lead to modifications in the way a provider’s performance is measured and, thus, how providers are compensated. A similar study investigated the impact of proportionally distributing patients’ outcomes across all rotating providers using a weighting method based on billing practices to measure the partial impact of each provider.3
This study is different in 2 fundamental ways. First, attribution is weighted based on the number of clinically documented interactions (via clinical notes) between a patient and all rotating providers during the hospitalization. Second, performance is measured via multi-membership models, which can estimate the effect (both positive and negative) that a provider has on an outcome, even when caring for a patient a fraction of the time during the hospitalization.
Methods
Setting
Banner Health is a non-profit, multi-hospital health care system across 6 states in the western United States that is uniquely positioned to study provider quality attribution models. It not only has a large number of providers and serves a broad patient population, but Banner Health also uses an instance of Cerner (Kansas City, MO), an enterprise-level electronic health record (EHR) system that connects all its facilities and allows for advanced analytics across its system.
For this study, we included only general medicine and surgery patients admitted and discharged from the inpatient setting between January 1, 2018, and December 31, 2018, who were between 18 and 89 years old at admission, and who had a LOS between 1 and 14 days. Visit- and patient-level data were collected from Cerner, while outcome data, and corresponding expected outcome data, were obtained from Premier, Inc. (Charlotte, NC) using their CareScience methodologies.10 To measure patient experience, response data were extracted from post-discharge surveys administered by InMoment (Salt Lake City, UT).
Provider Attribution Models
Provider Attribution by Physician of Record (PAPR). In the standard approach, denoted here as the PAPR model, 1 provider—typically the attending or discharging provider, which may be the same person—is attributed to the entire hospitalization. This provider is responsible for the patient’s care, and all patient outcomes are aggregated and attributed to the provider to gauge his or her performance. The PAPR model is the most popular form of attribution across many health care systems and is routinely used for P4P incentives.
In this study, the discharging provider was used when attributing hospitalizations using the PAPR model. Providers responsible for fewer than 12 discharges in the calendar year were excluded. Because of the directness of this type of attribution, the performance of 1 provider does not account for the performance of the other rotating providers during hospitalizations.
Provider Attribution by Multiple Membership (PAMM). In contrast, we introduce another attribution approach here that is designed to assign partial attribution to each provider who cares for the patient during the hospitalization. To aggregate the partial attributions, and possibly control for any exogenous or risk-based factors, a multiple-membership, or multi-member (MM), model is used. The MM model can measure the effect of a provider on an outcome even when the patient-to-provider relationship is complex, such as in a rotating provider environment.8
The purpose of this study is to compare attribution models and to determine whether there are meaningful differences between them. Therefore, for comparison purposes, the same discharging providers using the PAPR approach are eligible for the PAMM approach, so that both attribution models are using the same set of providers. All other providers are excluded because their performance would not be comparable to the PAPR approach.
While there are many ways to document provider-to-patient interactions, 2 methods are available in almost all health care systems. The first method is to link a provider’s billing charges to each patient-day combination. This approach limits the attribution to 1 provider per patient per day because multiple rotating providers cannot charge for the same patient-day combination.3 However, many providers interact with a patient on the same day, so using this approach excludes non-billed provider-to-patient interactions.
The second method, which was used in this study, relies on documented clinical notes within the EHR to determine how attribution is shared. In this approach, attribution is weighted based on the authorship of 3 types of eligible clinical notes: admitting history/physical notes (during admission), progress notes (during subsequent days), and discharge summary notes (during final discharge). This will (likely) result in many providers being linked to a patient on each day, which better reflects the clinical setting (Figure). Recently, clinical notes were used to attribute care of patients in an inpatient setting, and it was found that this approach provides a reliable way of tracking interactions and assigning ownership.11

The provider-level attribution weights are based on the share of authorships of eligible note types. Specifically, for each provider j, let aij be the total count of eligible note types for hospitalization i authored by provider j, and let ai be the overall total count of eligible note types for hospitalization i. Then the attribution weight is
(Eq. 1)
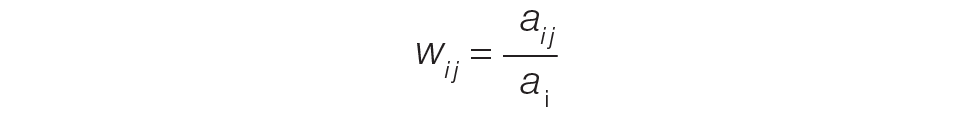
for hospitalization i and provider j. Note that ∑jwij = 1: in other words, the total attribution, summed across all providers, is constrained to be 1 for each hospitalization.
Patient Outcomes
Outcomes were chosen based on their routine use in health care systems as standards when evaluating provider performance. This study included 6 outcomes: inpatient LOS, inpatient mortality, 30-day inpatient readmission, and patient responses from 3 survey questions. These outcomes can be collected without any manual chart reviews, and therefore are viewed as objective outcomes of provider performance.
Each outcome was aggregated for each provider using both attribution methods independently. For the PAPR method, observed-to-expected (OE) indices for LOS, mortality, and readmissions were calculated along with average patient survey scores. For the PAMM method, provider-level random effects from the fitted models were used. In both cases, the calculated measures were used for ranking purposes when determining top (or bottom) providers for each outcome.
Individual Provider Metrics for the PAPR Method
Inpatient LOS Index. Hospital inpatient LOS was measured as the number of days between admission date and discharge date. For each hospital visit, an expected LOS was determined using Premier’s CareScience Analytics (CSA) risk-adjustment methodology.10 The CSA methodology for LOS incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let oij and eij be the observed and expected LOS, respectively, for hospitalization i = 1,…,nj attributed to provider j. Then the inpatient LOS index for provider j is Lj = ∑ioij⁄∑ieij.
Inpatient Mortality Index. Inpatient mortality was defined as the death of the patient during hospitalization. For each hospitalization, an expected mortality probability was determined using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for mortality incorporates a patient’s demographics and comorbidities.
Just as before, let nj be the number of hospitalizations attributed to provider j. Let mij = 1 if the patient died during hospitalization i = 1, … , nj attributed to provider j; mij = 0 otherwise. Let pij(m) be the corresponding expected mortality probability. Then the inpatient mortality index for provider j is Mj = ∑imij⁄∑ipij(m).
30-Day Inpatient Readmission Index. A 30-day inpatient readmission was defined as the event when a patient is discharged and readmits back into the inpatient setting within 30 days. The inclusion criteria defined by the Centers for Medicare and Medicaid Services (CMS) all-cause hospital-wide readmission measure was used and, consequently, planned readmissions were excluded.12 Readmissions could occur at any Banner hospital, including the same hospital. For each hospital visit, an expected readmission probability was derived using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for readmissions incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let rij = 1 if the patient had a readmission following hospitalization i = 1, … , nj attributed to provider j; rij = 0 otherwise. Let pij(r) be the corresponding expected readmission probability. Then the 30-day inpatient readmission index for provider j is Rj = ∑irij ⁄∑ipij(r).
Patient Survey Scores. The satisfaction of the patient’s experience during hospitalization was measured via post-discharge surveys administered by InMoment. Two survey questions were selected because they related directly to a provider’s interaction with the patient: “My interactions with doctors were excellent” (Doctor) and “I received the best possible care” (Care). A third question, “I would recommend this hospital to my family and friends,” was selected as a proxy measure of the overall experience and, in the aggregate, is referred to as the net promoter score (NPS).13,14 The responses were measured on an 11-point Likert scale, ranging from “Strongly Disagree” (0) to “Strongly Agree” (10); “N/A” or missing responses were excluded.
The Likert responses were coded to 3 discrete values as follows: if the value was between 0 and 6, then -1 (ie, detractor); between 7 and 8 (ie, neutral), then 0; otherwise 1 (ie, promoter). Averaging these coded responses results in a patient survey score for each question. Specifically, let nj be the number of hospitalizations attributed to provider j in which the patient responded to the survey question. Let sij ∈{−1, 0, 1} be the coded response linked to hospitalization i = 1, … , nj attributed to provider j. Then the patient experience score for provider j is Sj = ∑isij⁄nj.
Handling Ties in Provider Performance Measures. Because ties can occur in the PAPR approach for all measures, a tie-breaking strategy is needed. For LOS indices, ties are less likely because their numerator is strictly greater than 0, and expected LOS values are typically distinct enough. Indeed, no ties were found in this study for LOS indices. However, mortality and readmission indices can routinely result in ties when the best possible index is achieved, such as 0 deaths or readmissions among attributed hospitalizations. To help differentiate between those indices in the PAPR approach, the total estimated risk (denominator) was utilized as a secondary scoring criterion.
Mortality and readmission metrics were addressed by sorting first by the outcome (mortality index), and second by the denominator (total estimated risk). For example, if provider A has the same mortality rate as provider B, then provider A would be ranked higher if the denominator was larger, indicating a higher risk for mortality.
Similarly, it was very common for providers to have the same overall average rating for a survey question. Therefore, the denominator (number of respondents) was used to break ties. However, the denominator sorting was bidirectional. For example, if the tied score was positive (more promoters than detractors) for providers A and B, then provider A would be ranked higher if the denominator was larger. Conversely, if the tied score between providers A and B was neutral or negative (more detractors than promoters), then provider A would be ranked lower if the denominator was larger.
Individual Provider Metrics for the PAMM Method
For the PAMM method, model-based metrics were derived using a MM model.8 Specifically, let J be the number of rotating providers in a health care system. Let Yi be an outcome of interest from hospitalization i, X1i, …, Xpi be fixed effects or covariates, and ß1, …, ßp be the coefficients for the respective covariates. Then the generalized MM statistical model is
(Eq. 2)
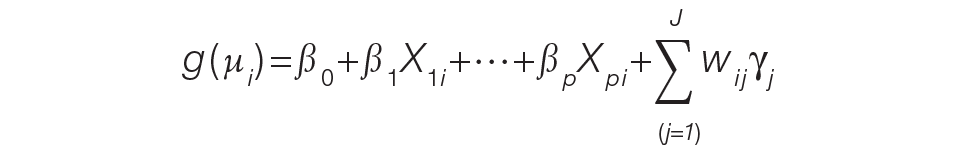
where g(μi ) is a link function between the mean of the outcome, μi, and its linear predictor, ß0, is the marginal intercept, wij represents the attribution weight of provider j on hospitalization i (described in Equation 1), and γj represents the random effect of provider j on the outcome with γj~N(0,σγ2).
For the mortality and readmission binary outcomes, logistic regression was performed using a logit link function, with the corresponding expected probability as the only fixed covariate. The expected probabilities were first converted into odds and then log-transformed before entering the model. For LOS, Poisson regression was performed using a log link function with the log-transformed expected LOS as the only fixed covariate. For coded patient experience responses, an ordered logistic regression was performed using a cumulative logit link function (no fixed effects were added).
MM Model-based Metrics. Each fitted MM model produces a predicted random effect for each provider. The provider-specific random effects can be interpreted as the unobserved influence of each provider on the outcome after controlling for any fixed effect included in the model. Therefore, the provider-specific random effects were used to evaluate the relative provider performance, which is analogous to the individual provider-level metrics used in the PAPR method.
Measuring provider performance using a MM model is more flexible and robust to outliers compared to the standard approach using OE indices or simple averages. First, although not investigated here, the effect of patient-, visit-, provider-, and/or temporal-level covariates can be controlled when evaluating provider performance. For example, a patient’s socioeconomic status, a provider’s workload, and seasonal factors can be added to the MM model. These external factors are not accounted for in OE indices.
Another advantage of using predicted random effects is the concept of “shrinkage.” The process of estimating random effects inherently accounts for small sample sizes (when providers do not treat a large enough sample of patients) and/or when there is a large ratio of patient variance to provider variance (for instance, when patient outcome variability is much higher compared to provider performance variability). In both cases, the estimation of the random effect is pulled ever closer to 0, signaling that the provider performance is closer to the population average. See Henderson15 and Mood16 for further details.
In contrast, OE indices can result in unreliable estimates when a provider has not cared for many patients. This is especially prevalent when the outcome is binary with a low probability of occurring, such as mortality. Indeed, provider-level mortality OE indices are routinely 0 when the patient counts are low, which skews performance rankings unfairly. Finally, OE indices also ignore the magnitude of the variance of an outcome between providers and patients, which can be large.
Comparison Methodology
In this study, we seek to compare the 2 methods of attribution, PAPR and PAMM, to determine whether there are meaningful differences between them when measuring provider performance. Using retrospective data described in the next section, each attribution method was used independently to derive provider-level metrics. To assess relative performance, percentiles were assigned to each provider based on their metric values so that, in the end, there were 2 percentile ranks for each provider for each metric.
Using these paired percentiles, we derived the following measures of concordance, similar to Herzke, Michtalik3: (1) the percent concordance measure—defined as the number of providers who landed in the top half (greater than the median) or bottom half under both attribution models—divided by the total number of providers; (2) the median of the absolute difference in percentiles under both attribution models; and (3) the Pearson correlation coefficient of the paired provider ranks. The first measure is a global measure of concordance between the 2 approaches and would be expected to be 50% by chance. The second measure gauges how an individual provider’s rank is affected by the change in attribution methodologies. The third measure is a statistical measure of linear correlation of the paired percentiles and was not included in the Herzke, Michtalik3 study.
All statistical analyses were performed on SAS (version 9.4; Cary, NC) and the MM models were fitted using PROC GLIMMIX with the EFFECT statement. The Banner Health Institutional Review Board approved this study.
Results
Descriptive Statistics
A total of

Multi-Membership Model Results
Table 3 displays the results after independently fitting MM models to each of the 3 clinical outcomes. Along with a marginal intercept, the only covariate in each model was the corresponding expected value after a transformation. This was added to use the same information that is typically used in OE indices, therefore allowing for a proper comparison between the 2 attribution methods. The provider-level variance represents the between-provider variation and measures the amount of influence providers have on the corresponding outcome after controlling for any covariates in the model. A provider-level variance of 0 would indicate that providers do not have any influence on the outcome. While the mortality and readmission model results can be compared to each other, the LOS model cannot given its different scale and transformation altogether.

The results in Table 3 suggest that each expected value covariate is highly correlated with its corresponding outcome, which is the anticipated conclusion given that they are constructed in this fashion. The estimated provider-level variances indicate that, after including an expected value in the model, providers have less of an influence on a patient’s LOS and likelihood of being readmitted. On the other hand, the results suggest that providers have much more influence on the likelihood of a patient dying in the hospital, even after controlling for an expected mortality covariate.

Table 4 shows the results after independently fitting MM-ordered logistic models to each of the 3 survey questions. The similar provider-level variances suggest that providers have the same influence on the patient’s perception of the quality of their interactions with the doctor (Doctor), the quality of the care they received (Care), and their likelihood to recommend a friend or family member to the hospital (NPS).

Comparison Results Between Both Attribution Methods
Table 5 compares the 2 attribution methods when ranking providers based on their performance on each outcome measure. The comparison metrics gauge how well the 2 methods agree overall (percent concordance), agree at the provider level (absolute percentile difference and interquartile range [IQR]), and how the paired percentiles linearly correlate to each other (Pearson correlation coefficient).

LOS, by a small margin, had the lowest concordance of clinical outcomes (72.1%), followed by mortality (75.9%) and readmissions (82.1%). Generally, the survey scores had higher percent concordance than the clinical outcome measures, with Doctor at 84.1%, Care at 85.9%, and NPS having the highest percent concordance at 86.6%. Given that by chance the percent concordance is expected to be 50%, there was notable discordance, especially with the clinical outcome measures. Using LOS performance as an example, one attribution methodology would rank a provider in the top half or bottom half, while the other attribution methodology would rank the same provider exactly the opposite way about 28% of the time.
The median absolute percentile difference between the 2 methods was more modest (between 7 and 15). Still, there were some providers whose performance ranking was heavily impacted by the attribution methodology that was used. This was especially true when evaluating performance for certain clinical measures, where the attribution method that was used could change the provider performance percentile by up to 90 levels.
The paired percentiles were positively correlated when ranking performance using any of the 6 measures. This suggests that both methodologies assess performance generally in the same direction, irrespective of the methodology and measure. We did not investigate more complex correlation measures and left this for future research.
It should be noted that ties occurred much more frequently with the PAPR method than when using PAMM and therefore required tie-breaking rules to be designed. Given the nature of OE indices, PAPR methodology is especially sensitive to ties whenever the measure includes counting the number of events (for example, mortality and readmissions) and whenever there are many providers with very few attributed patients. On the other hand, using the PAMM method is much more robust against ties given that the summation of all the weighted attributed outcomes will rarely result in ties, even with a nominal set of providers.
Discussion
In this study, the PAMM methodology was introduced and was used to assess relative provider performance on 3 clinical outcome measures and 3 patient survey scores. The new approach aims to distribute each outcome among all providers who provided care for a patient in an inpatient setting. Clinical notes were used to account for patient-to-provider interactions, and fitted MM statistical models were used to compute the effects that each provider had on each outcome. The provider effect was introduced as a random effect, and the set of predicted random effects was used to rank the performance of each provider.
The PAMM approach was compared to the more traditional methodology, PAPR, where each patient is attributed to only 1 provider: the discharging physician in this study. Using this approach, OE indices of clinical outcomes and averages of survey scores were used to rank the performance of each provider. This approach resulted in many ties, which were broken based on the number of hospitalizations, although other tie-breaking methods may be used in practice.
Both methodologies showed modest concordance with each other for the clinical outcomes, but higher concordance for the patient survey scores. This was also true when using the Pearson correlation coefficient to assess agreement. The 1 outcome measure that showed the least concordance and least linear correlation between methods was LOS, which would suggest that LOS performance is more sensitive to the attribution methodology that is used. However, it was the least concordant by a small margin.
Furthermore, although the medians of the absolute percentile differences were small, there were some providers who had large deviations, suggesting that some providers would move from being shown as high-performers to low-performers and vice versa based on the chosen attribution method. We investigated examples of this and determined that the root cause was the difference in effective sample sizes for a provider. For the PAPR method, the effective sample size is simply the number of hospitalizations attributed to the provider. For the PAMM method, the effective sample size is the sum of all non-zero weights across all hospitalizations where the provider cared for a patient. By and large, the PAMM methodology provides more information of the provider effect on an outcome than the PAPR approach because every provider-patient interaction is considered. For example, providers who do not routinely discharge patients, but often care for patients, will have rankings that differ dramatically between the 2 methods.
The PAMM methodology has many statistical advantages that were not fully utilized in this comparative study. For example, we did not include any covariates in the MM models except for the expected value of the outcome, when it was available. Still, it is known that other covariates can impact an outcome as well, such as the patient’s age, socioeconomic indicators, existing chronic conditions, and severity of hospitalization, which can be added to the MM models as fixed effects. In this way, the PAMM approach can control for these other covariates, which are typically outside of the control of providers but typically ignored using OE indices. Therefore, using the PAMM approach would provide a fairer comparison of provider performance.
Using the PAMM method, most providers had a large sample size to assess their performance once all the weighted interactions were included. Still, there were a few who did not care for many patients for a variety of reasons. In these scenarios, MM models “borrow” strength from other providers to produce a more robust predicted provider effect by using a weighted average between the overall population trend and the specific provider outcomes (see Rao and Molina17). As a result, PAMM is a more suitable approach when the sample sizes of patients attributed to providers can be small.
One of the most interesting findings of this study was the relative size of the provider-level variance to the size of the fixed effect in each model (Table 3). Except for mortality, these variances suggest that there is a small difference in performance from one provider to another. However, these should be interpreted as the variance when only 1 provider is involved in the care of a patient. When multiple providers are involved, using basic statistical theory, the overall provider-level variance will be σγ2 ∑wij2 (see Equation 2). For example, the estimated variance among providers for LOS was 0.03 (on a log scale), but, using the scenario in the Figure, the overall provider-level variance for this hospitalization will be 0.03 (0.3752 + 0.1252 + 0.52) = 0.012. Hence, the combined effect of providers on LOS is less than would be expected. Indeed, as more providers are involved with a patient’s care, the more their combined influence on an outcome is diluted.
In this study, the PAMM approach placed an equal weight on all provider-patient interactions via clinical note authorship, but that may not be optimal in some settings. For example, it may make more sense to set a higher weight on the provider who admitted or discharged the patient while placing less (or 0) weight on all other interactions. In the extreme, if the full weight were placed on 1 provider interaction (eg, during discharge, then the MM model would be reduced to a one-way random effects model. The flexibility of weighting interactions is a feature of the PAMM approach, but any weighting framework must be transparent to the providers before implementation.
Conclusion
This study demonstrates that the PAMM approach is a feasible option within a large health care organization. For P4P programs to be successful, providers must be able to trust that their performance will be fairly assessed and that all provider-patient interactions are captured to provide a full comparison amongst their peers. The PAMM methodology is one solution to spread the positive (and negative) outcomes across all providers who cared for a patient and therefore, if implemented, would add trust and fairness when measuring and assessing provider performance.
Acknowledgments: The authors thank Barrie Bradley for his support in the initial stages of this research and Dr. Syed Ismail Jafri for his help and support on the standard approaches of assessing and measuring provider performances.
Corresponding author: Rachel Ginn, MS, Banner Health Corporation, 2901 N. Central Ave., Phoenix, AZ 85012; [email protected].
Financial disclosures: None.
From Banner Health Corporation, Phoenix, AZ.
Background: Health care providers are routinely incentivized with pay-for-performance (P4P) metrics to increase the quality of care. In an inpatient setting, P4P models typically measure quality by attributing each patient’s outcome to a single provider even though many providers routinely care for the patient. This study investigates a new attribution approach aiming to distribute each outcome across all providers who provided care.
Methods: The methodology relies on a multi-membership model and is demonstrated in the Banner Health system using 3 clinical outcome measures (length of stay, 30-day readmissions, and mortality) and responses to 3 survey questions that measure a patient’s perception of their care. The new approach is compared to the “standard” method, which attributes each patient to only 1 provider.
Results: When ranking by clinical outcomes, both methods were concordant 72.1% to 82.1% of the time for top-half/bottom-half rankings, with a median percentile difference between 7 and 15. When ranking by survey scores, there was more agreement, with concordance between 84.1% and 86.6% and a median percentile difference between 11 and 13. Last, Pearson correlation coefficients of the paired percentiles ranged from 0.56 to 0.78.
Conclusion: The new approach provides a fairer solution when measuring provider performance.
Keywords: patient attribution; PAMM; PAPR; random effect model; pay for performance.
Providers practicing in hospitals are routinely evaluated based on their performance and, in many cases, are financially incentivized for a better-than-average performance within a pay-for-performance (P4P) model. The use of P4P models is based on the belief that they will “improve, motivate, and enhance providers to pursue aggressively and ultimately achieve the quality performance targets thus decreasing the number of medical errors with less malpractice events.”1 Although P4P models continue to be a movement in health care, they have been challenging to implement.
One concern involves the general quality of implementation, such as defining metrics and targets, setting payout amounts, managing technology and market conditions, and gauging the level of transparency to the provider.2 Another challenge, and the focus of this project, are concerns around measuring performance to avoid perceptions of unfairness. This concern can be minimized if the attribution is handled in a fairer way, by spreading it across all providers who affected the outcome, both in a positive or negative direction.3
To implement these models, the performance of providers needs to be measured and tracked periodically. This requires linking, or attributing, a patient’s outcome to a provider, which is almost always the attending or discharging provider (ie, a single provider).3 In this single-provider attribution approach, one provider will receive all the credit (good or bad) for their respective patients’ outcomes, even though the provider may have seen the patient only a fraction of the time during the hospitalization. Attributing outcomes—for example, length of stay (LOS), readmission rate, mortality rate, net promoter score (NPS)—using this approach reduces the validity of metrics designed to measure provider performance, especially in a rotating provider environment where many providers interact with and care for a patient. For example, the quality of providers’ interpersonal skills and competence were among the strongest determinants of patient satisfaction,4 but it is not credible that this is solely based on the last provider during a hospitalization.
Proportionally distributing the attribution of an outcome has been used successfully in other contexts. Typically, a statistical modeling approach using a multi-membership framework is used because it can handle the sometimes-complicated relationships within the hierarchy. It also allows for auxiliary variables to be introduced, which can help explain and control for exogenous effects.5-7 For example, in the education setting, standardized testing is administered to students at defined years of schooling: at grades 4, 8, and 10, for instance. The progress of students, measured as the academic gains between test years, are proportionally attributed to all the teachers who the student has had between the test years. These partial attributions are combined to evaluate an overall teacher performance.8,9
Although the multi-membership framework has been used in other industries, it has yet to be applied in measuring provider performance. The purpose of this project is to investigate the impact of using a multi-provider approach compared to the standard single-provider approach. The findings may lead to modifications in the way a provider’s performance is measured and, thus, how providers are compensated. A similar study investigated the impact of proportionally distributing patients’ outcomes across all rotating providers using a weighting method based on billing practices to measure the partial impact of each provider.3
This study is different in 2 fundamental ways. First, attribution is weighted based on the number of clinically documented interactions (via clinical notes) between a patient and all rotating providers during the hospitalization. Second, performance is measured via multi-membership models, which can estimate the effect (both positive and negative) that a provider has on an outcome, even when caring for a patient a fraction of the time during the hospitalization.
Methods
Setting
Banner Health is a non-profit, multi-hospital health care system across 6 states in the western United States that is uniquely positioned to study provider quality attribution models. It not only has a large number of providers and serves a broad patient population, but Banner Health also uses an instance of Cerner (Kansas City, MO), an enterprise-level electronic health record (EHR) system that connects all its facilities and allows for advanced analytics across its system.
For this study, we included only general medicine and surgery patients admitted and discharged from the inpatient setting between January 1, 2018, and December 31, 2018, who were between 18 and 89 years old at admission, and who had a LOS between 1 and 14 days. Visit- and patient-level data were collected from Cerner, while outcome data, and corresponding expected outcome data, were obtained from Premier, Inc. (Charlotte, NC) using their CareScience methodologies.10 To measure patient experience, response data were extracted from post-discharge surveys administered by InMoment (Salt Lake City, UT).
Provider Attribution Models
Provider Attribution by Physician of Record (PAPR). In the standard approach, denoted here as the PAPR model, 1 provider—typically the attending or discharging provider, which may be the same person—is attributed to the entire hospitalization. This provider is responsible for the patient’s care, and all patient outcomes are aggregated and attributed to the provider to gauge his or her performance. The PAPR model is the most popular form of attribution across many health care systems and is routinely used for P4P incentives.
In this study, the discharging provider was used when attributing hospitalizations using the PAPR model. Providers responsible for fewer than 12 discharges in the calendar year were excluded. Because of the directness of this type of attribution, the performance of 1 provider does not account for the performance of the other rotating providers during hospitalizations.
Provider Attribution by Multiple Membership (PAMM). In contrast, we introduce another attribution approach here that is designed to assign partial attribution to each provider who cares for the patient during the hospitalization. To aggregate the partial attributions, and possibly control for any exogenous or risk-based factors, a multiple-membership, or multi-member (MM), model is used. The MM model can measure the effect of a provider on an outcome even when the patient-to-provider relationship is complex, such as in a rotating provider environment.8
The purpose of this study is to compare attribution models and to determine whether there are meaningful differences between them. Therefore, for comparison purposes, the same discharging providers using the PAPR approach are eligible for the PAMM approach, so that both attribution models are using the same set of providers. All other providers are excluded because their performance would not be comparable to the PAPR approach.
While there are many ways to document provider-to-patient interactions, 2 methods are available in almost all health care systems. The first method is to link a provider’s billing charges to each patient-day combination. This approach limits the attribution to 1 provider per patient per day because multiple rotating providers cannot charge for the same patient-day combination.3 However, many providers interact with a patient on the same day, so using this approach excludes non-billed provider-to-patient interactions.
The second method, which was used in this study, relies on documented clinical notes within the EHR to determine how attribution is shared. In this approach, attribution is weighted based on the authorship of 3 types of eligible clinical notes: admitting history/physical notes (during admission), progress notes (during subsequent days), and discharge summary notes (during final discharge). This will (likely) result in many providers being linked to a patient on each day, which better reflects the clinical setting (Figure). Recently, clinical notes were used to attribute care of patients in an inpatient setting, and it was found that this approach provides a reliable way of tracking interactions and assigning ownership.11

The provider-level attribution weights are based on the share of authorships of eligible note types. Specifically, for each provider j, let aij be the total count of eligible note types for hospitalization i authored by provider j, and let ai be the overall total count of eligible note types for hospitalization i. Then the attribution weight is
(Eq. 1)
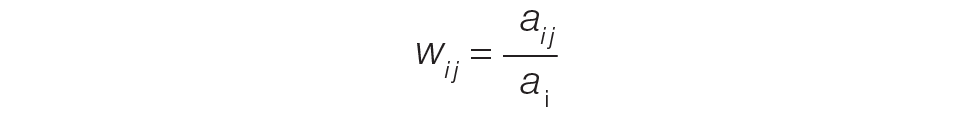
for hospitalization i and provider j. Note that ∑jwij = 1: in other words, the total attribution, summed across all providers, is constrained to be 1 for each hospitalization.
Patient Outcomes
Outcomes were chosen based on their routine use in health care systems as standards when evaluating provider performance. This study included 6 outcomes: inpatient LOS, inpatient mortality, 30-day inpatient readmission, and patient responses from 3 survey questions. These outcomes can be collected without any manual chart reviews, and therefore are viewed as objective outcomes of provider performance.
Each outcome was aggregated for each provider using both attribution methods independently. For the PAPR method, observed-to-expected (OE) indices for LOS, mortality, and readmissions were calculated along with average patient survey scores. For the PAMM method, provider-level random effects from the fitted models were used. In both cases, the calculated measures were used for ranking purposes when determining top (or bottom) providers for each outcome.
Individual Provider Metrics for the PAPR Method
Inpatient LOS Index. Hospital inpatient LOS was measured as the number of days between admission date and discharge date. For each hospital visit, an expected LOS was determined using Premier’s CareScience Analytics (CSA) risk-adjustment methodology.10 The CSA methodology for LOS incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let oij and eij be the observed and expected LOS, respectively, for hospitalization i = 1,…,nj attributed to provider j. Then the inpatient LOS index for provider j is Lj = ∑ioij⁄∑ieij.
Inpatient Mortality Index. Inpatient mortality was defined as the death of the patient during hospitalization. For each hospitalization, an expected mortality probability was determined using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for mortality incorporates a patient’s demographics and comorbidities.
Just as before, let nj be the number of hospitalizations attributed to provider j. Let mij = 1 if the patient died during hospitalization i = 1, … , nj attributed to provider j; mij = 0 otherwise. Let pij(m) be the corresponding expected mortality probability. Then the inpatient mortality index for provider j is Mj = ∑imij⁄∑ipij(m).
30-Day Inpatient Readmission Index. A 30-day inpatient readmission was defined as the event when a patient is discharged and readmits back into the inpatient setting within 30 days. The inclusion criteria defined by the Centers for Medicare and Medicaid Services (CMS) all-cause hospital-wide readmission measure was used and, consequently, planned readmissions were excluded.12 Readmissions could occur at any Banner hospital, including the same hospital. For each hospital visit, an expected readmission probability was derived using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for readmissions incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let rij = 1 if the patient had a readmission following hospitalization i = 1, … , nj attributed to provider j; rij = 0 otherwise. Let pij(r) be the corresponding expected readmission probability. Then the 30-day inpatient readmission index for provider j is Rj = ∑irij ⁄∑ipij(r).
Patient Survey Scores. The satisfaction of the patient’s experience during hospitalization was measured via post-discharge surveys administered by InMoment. Two survey questions were selected because they related directly to a provider’s interaction with the patient: “My interactions with doctors were excellent” (Doctor) and “I received the best possible care” (Care). A third question, “I would recommend this hospital to my family and friends,” was selected as a proxy measure of the overall experience and, in the aggregate, is referred to as the net promoter score (NPS).13,14 The responses were measured on an 11-point Likert scale, ranging from “Strongly Disagree” (0) to “Strongly Agree” (10); “N/A” or missing responses were excluded.
The Likert responses were coded to 3 discrete values as follows: if the value was between 0 and 6, then -1 (ie, detractor); between 7 and 8 (ie, neutral), then 0; otherwise 1 (ie, promoter). Averaging these coded responses results in a patient survey score for each question. Specifically, let nj be the number of hospitalizations attributed to provider j in which the patient responded to the survey question. Let sij ∈{−1, 0, 1} be the coded response linked to hospitalization i = 1, … , nj attributed to provider j. Then the patient experience score for provider j is Sj = ∑isij⁄nj.
Handling Ties in Provider Performance Measures. Because ties can occur in the PAPR approach for all measures, a tie-breaking strategy is needed. For LOS indices, ties are less likely because their numerator is strictly greater than 0, and expected LOS values are typically distinct enough. Indeed, no ties were found in this study for LOS indices. However, mortality and readmission indices can routinely result in ties when the best possible index is achieved, such as 0 deaths or readmissions among attributed hospitalizations. To help differentiate between those indices in the PAPR approach, the total estimated risk (denominator) was utilized as a secondary scoring criterion.
Mortality and readmission metrics were addressed by sorting first by the outcome (mortality index), and second by the denominator (total estimated risk). For example, if provider A has the same mortality rate as provider B, then provider A would be ranked higher if the denominator was larger, indicating a higher risk for mortality.
Similarly, it was very common for providers to have the same overall average rating for a survey question. Therefore, the denominator (number of respondents) was used to break ties. However, the denominator sorting was bidirectional. For example, if the tied score was positive (more promoters than detractors) for providers A and B, then provider A would be ranked higher if the denominator was larger. Conversely, if the tied score between providers A and B was neutral or negative (more detractors than promoters), then provider A would be ranked lower if the denominator was larger.
Individual Provider Metrics for the PAMM Method
For the PAMM method, model-based metrics were derived using a MM model.8 Specifically, let J be the number of rotating providers in a health care system. Let Yi be an outcome of interest from hospitalization i, X1i, …, Xpi be fixed effects or covariates, and ß1, …, ßp be the coefficients for the respective covariates. Then the generalized MM statistical model is
(Eq. 2)
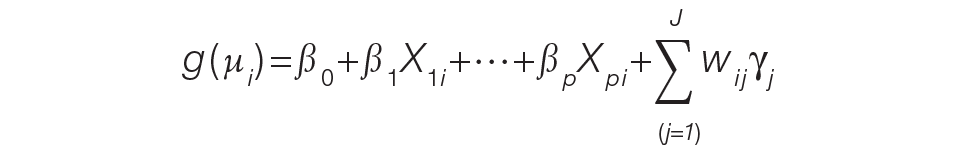
where g(μi ) is a link function between the mean of the outcome, μi, and its linear predictor, ß0, is the marginal intercept, wij represents the attribution weight of provider j on hospitalization i (described in Equation 1), and γj represents the random effect of provider j on the outcome with γj~N(0,σγ2).
For the mortality and readmission binary outcomes, logistic regression was performed using a logit link function, with the corresponding expected probability as the only fixed covariate. The expected probabilities were first converted into odds and then log-transformed before entering the model. For LOS, Poisson regression was performed using a log link function with the log-transformed expected LOS as the only fixed covariate. For coded patient experience responses, an ordered logistic regression was performed using a cumulative logit link function (no fixed effects were added).
MM Model-based Metrics. Each fitted MM model produces a predicted random effect for each provider. The provider-specific random effects can be interpreted as the unobserved influence of each provider on the outcome after controlling for any fixed effect included in the model. Therefore, the provider-specific random effects were used to evaluate the relative provider performance, which is analogous to the individual provider-level metrics used in the PAPR method.
Measuring provider performance using a MM model is more flexible and robust to outliers compared to the standard approach using OE indices or simple averages. First, although not investigated here, the effect of patient-, visit-, provider-, and/or temporal-level covariates can be controlled when evaluating provider performance. For example, a patient’s socioeconomic status, a provider’s workload, and seasonal factors can be added to the MM model. These external factors are not accounted for in OE indices.
Another advantage of using predicted random effects is the concept of “shrinkage.” The process of estimating random effects inherently accounts for small sample sizes (when providers do not treat a large enough sample of patients) and/or when there is a large ratio of patient variance to provider variance (for instance, when patient outcome variability is much higher compared to provider performance variability). In both cases, the estimation of the random effect is pulled ever closer to 0, signaling that the provider performance is closer to the population average. See Henderson15 and Mood16 for further details.
In contrast, OE indices can result in unreliable estimates when a provider has not cared for many patients. This is especially prevalent when the outcome is binary with a low probability of occurring, such as mortality. Indeed, provider-level mortality OE indices are routinely 0 when the patient counts are low, which skews performance rankings unfairly. Finally, OE indices also ignore the magnitude of the variance of an outcome between providers and patients, which can be large.
Comparison Methodology
In this study, we seek to compare the 2 methods of attribution, PAPR and PAMM, to determine whether there are meaningful differences between them when measuring provider performance. Using retrospective data described in the next section, each attribution method was used independently to derive provider-level metrics. To assess relative performance, percentiles were assigned to each provider based on their metric values so that, in the end, there were 2 percentile ranks for each provider for each metric.
Using these paired percentiles, we derived the following measures of concordance, similar to Herzke, Michtalik3: (1) the percent concordance measure—defined as the number of providers who landed in the top half (greater than the median) or bottom half under both attribution models—divided by the total number of providers; (2) the median of the absolute difference in percentiles under both attribution models; and (3) the Pearson correlation coefficient of the paired provider ranks. The first measure is a global measure of concordance between the 2 approaches and would be expected to be 50% by chance. The second measure gauges how an individual provider’s rank is affected by the change in attribution methodologies. The third measure is a statistical measure of linear correlation of the paired percentiles and was not included in the Herzke, Michtalik3 study.
All statistical analyses were performed on SAS (version 9.4; Cary, NC) and the MM models were fitted using PROC GLIMMIX with the EFFECT statement. The Banner Health Institutional Review Board approved this study.
Results
Descriptive Statistics
A total of

Multi-Membership Model Results
Table 3 displays the results after independently fitting MM models to each of the 3 clinical outcomes. Along with a marginal intercept, the only covariate in each model was the corresponding expected value after a transformation. This was added to use the same information that is typically used in OE indices, therefore allowing for a proper comparison between the 2 attribution methods. The provider-level variance represents the between-provider variation and measures the amount of influence providers have on the corresponding outcome after controlling for any covariates in the model. A provider-level variance of 0 would indicate that providers do not have any influence on the outcome. While the mortality and readmission model results can be compared to each other, the LOS model cannot given its different scale and transformation altogether.

The results in Table 3 suggest that each expected value covariate is highly correlated with its corresponding outcome, which is the anticipated conclusion given that they are constructed in this fashion. The estimated provider-level variances indicate that, after including an expected value in the model, providers have less of an influence on a patient’s LOS and likelihood of being readmitted. On the other hand, the results suggest that providers have much more influence on the likelihood of a patient dying in the hospital, even after controlling for an expected mortality covariate.

Table 4 shows the results after independently fitting MM-ordered logistic models to each of the 3 survey questions. The similar provider-level variances suggest that providers have the same influence on the patient’s perception of the quality of their interactions with the doctor (Doctor), the quality of the care they received (Care), and their likelihood to recommend a friend or family member to the hospital (NPS).

Comparison Results Between Both Attribution Methods
Table 5 compares the 2 attribution methods when ranking providers based on their performance on each outcome measure. The comparison metrics gauge how well the 2 methods agree overall (percent concordance), agree at the provider level (absolute percentile difference and interquartile range [IQR]), and how the paired percentiles linearly correlate to each other (Pearson correlation coefficient).

LOS, by a small margin, had the lowest concordance of clinical outcomes (72.1%), followed by mortality (75.9%) and readmissions (82.1%). Generally, the survey scores had higher percent concordance than the clinical outcome measures, with Doctor at 84.1%, Care at 85.9%, and NPS having the highest percent concordance at 86.6%. Given that by chance the percent concordance is expected to be 50%, there was notable discordance, especially with the clinical outcome measures. Using LOS performance as an example, one attribution methodology would rank a provider in the top half or bottom half, while the other attribution methodology would rank the same provider exactly the opposite way about 28% of the time.
The median absolute percentile difference between the 2 methods was more modest (between 7 and 15). Still, there were some providers whose performance ranking was heavily impacted by the attribution methodology that was used. This was especially true when evaluating performance for certain clinical measures, where the attribution method that was used could change the provider performance percentile by up to 90 levels.
The paired percentiles were positively correlated when ranking performance using any of the 6 measures. This suggests that both methodologies assess performance generally in the same direction, irrespective of the methodology and measure. We did not investigate more complex correlation measures and left this for future research.
It should be noted that ties occurred much more frequently with the PAPR method than when using PAMM and therefore required tie-breaking rules to be designed. Given the nature of OE indices, PAPR methodology is especially sensitive to ties whenever the measure includes counting the number of events (for example, mortality and readmissions) and whenever there are many providers with very few attributed patients. On the other hand, using the PAMM method is much more robust against ties given that the summation of all the weighted attributed outcomes will rarely result in ties, even with a nominal set of providers.
Discussion
In this study, the PAMM methodology was introduced and was used to assess relative provider performance on 3 clinical outcome measures and 3 patient survey scores. The new approach aims to distribute each outcome among all providers who provided care for a patient in an inpatient setting. Clinical notes were used to account for patient-to-provider interactions, and fitted MM statistical models were used to compute the effects that each provider had on each outcome. The provider effect was introduced as a random effect, and the set of predicted random effects was used to rank the performance of each provider.
The PAMM approach was compared to the more traditional methodology, PAPR, where each patient is attributed to only 1 provider: the discharging physician in this study. Using this approach, OE indices of clinical outcomes and averages of survey scores were used to rank the performance of each provider. This approach resulted in many ties, which were broken based on the number of hospitalizations, although other tie-breaking methods may be used in practice.
Both methodologies showed modest concordance with each other for the clinical outcomes, but higher concordance for the patient survey scores. This was also true when using the Pearson correlation coefficient to assess agreement. The 1 outcome measure that showed the least concordance and least linear correlation between methods was LOS, which would suggest that LOS performance is more sensitive to the attribution methodology that is used. However, it was the least concordant by a small margin.
Furthermore, although the medians of the absolute percentile differences were small, there were some providers who had large deviations, suggesting that some providers would move from being shown as high-performers to low-performers and vice versa based on the chosen attribution method. We investigated examples of this and determined that the root cause was the difference in effective sample sizes for a provider. For the PAPR method, the effective sample size is simply the number of hospitalizations attributed to the provider. For the PAMM method, the effective sample size is the sum of all non-zero weights across all hospitalizations where the provider cared for a patient. By and large, the PAMM methodology provides more information of the provider effect on an outcome than the PAPR approach because every provider-patient interaction is considered. For example, providers who do not routinely discharge patients, but often care for patients, will have rankings that differ dramatically between the 2 methods.
The PAMM methodology has many statistical advantages that were not fully utilized in this comparative study. For example, we did not include any covariates in the MM models except for the expected value of the outcome, when it was available. Still, it is known that other covariates can impact an outcome as well, such as the patient’s age, socioeconomic indicators, existing chronic conditions, and severity of hospitalization, which can be added to the MM models as fixed effects. In this way, the PAMM approach can control for these other covariates, which are typically outside of the control of providers but typically ignored using OE indices. Therefore, using the PAMM approach would provide a fairer comparison of provider performance.
Using the PAMM method, most providers had a large sample size to assess their performance once all the weighted interactions were included. Still, there were a few who did not care for many patients for a variety of reasons. In these scenarios, MM models “borrow” strength from other providers to produce a more robust predicted provider effect by using a weighted average between the overall population trend and the specific provider outcomes (see Rao and Molina17). As a result, PAMM is a more suitable approach when the sample sizes of patients attributed to providers can be small.
One of the most interesting findings of this study was the relative size of the provider-level variance to the size of the fixed effect in each model (Table 3). Except for mortality, these variances suggest that there is a small difference in performance from one provider to another. However, these should be interpreted as the variance when only 1 provider is involved in the care of a patient. When multiple providers are involved, using basic statistical theory, the overall provider-level variance will be σγ2 ∑wij2 (see Equation 2). For example, the estimated variance among providers for LOS was 0.03 (on a log scale), but, using the scenario in the Figure, the overall provider-level variance for this hospitalization will be 0.03 (0.3752 + 0.1252 + 0.52) = 0.012. Hence, the combined effect of providers on LOS is less than would be expected. Indeed, as more providers are involved with a patient’s care, the more their combined influence on an outcome is diluted.
In this study, the PAMM approach placed an equal weight on all provider-patient interactions via clinical note authorship, but that may not be optimal in some settings. For example, it may make more sense to set a higher weight on the provider who admitted or discharged the patient while placing less (or 0) weight on all other interactions. In the extreme, if the full weight were placed on 1 provider interaction (eg, during discharge, then the MM model would be reduced to a one-way random effects model. The flexibility of weighting interactions is a feature of the PAMM approach, but any weighting framework must be transparent to the providers before implementation.
Conclusion
This study demonstrates that the PAMM approach is a feasible option within a large health care organization. For P4P programs to be successful, providers must be able to trust that their performance will be fairly assessed and that all provider-patient interactions are captured to provide a full comparison amongst their peers. The PAMM methodology is one solution to spread the positive (and negative) outcomes across all providers who cared for a patient and therefore, if implemented, would add trust and fairness when measuring and assessing provider performance.
Acknowledgments: The authors thank Barrie Bradley for his support in the initial stages of this research and Dr. Syed Ismail Jafri for his help and support on the standard approaches of assessing and measuring provider performances.
Corresponding author: Rachel Ginn, MS, Banner Health Corporation, 2901 N. Central Ave., Phoenix, AZ 85012; [email protected].
Financial disclosures: None.
1. Abduljawad A, Al-Assaf AF. Incentives for better performance in health care. Sultan Qaboos Univ Med J. 2011;11:201-206.
2. Milstein R, Schreyoegg J. Pay for performance in the inpatient sector: a review of 34 P4P programs in 14 OECD countries. Health Policy. 2016;120:1125-1140.
3. Herzke CA, Michtalik HJ, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. 2018;13:470-475.
4. Batbaatar E, Dorjdagva J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: a systematic review. Perspect Public Health. 2017;137:89-101.
5. Ballou D, Sanders W, Wright P. Controlling for student background in value-added assessment of teachers. J Educ Behav Stat. 2004;29:37-65.
6. Hill PW, Goldstein H. Multilevel modeling of educational data with cross-classification and missing identification for units. J Educ Behav Stat. 1998;23:117-128.
7. Rasbash J, Browne WJ. Handbook of Multilevel Analysis. Springer; 2007.
8. Brown WJ, Goldstein H, Rasbash J. Multiple membership multiple classification (MMMC) models. Statistical Modeling. 2001;1:103-124.
9. Sanders WL, Horn SP. The Tennessee Value-Added Assessment System (TVAAS)—mixed-model methodology in educational assessment. J Pers Eval Educ. 1994;8:299-311.
10. Kroch EA, Duan M. CareScience Risk Assessment Model: Hospital Performance Measurement. Premier, Inc., 2008. http://www.ahrq.gov/qual/mortality/KrochRisk.htm
11. Schumacher DJ, Wu DTY, Meganathan K, et al. A feasibility study to attribute patients to primary interns on inpatient ward teams using electronic health record data. Acad Med. 2019;94:1376-1383.
12. Simoes J, Krumholz HM, Lin Z. Hospital-level 30-day risk-standardized readmission measure. Centers for Medicare & Medicaid Services, 2018. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Hospital-Wide-All-Cause-Readmission-Updates.zip
13. Krol MW, de Boer D, Delnoij DM, Rademakers JJDJM. The Net Promoter Score: an asset to patient experience surveys? Health Expect. 2015;18:3099-3109.
14. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3:e001570.
15. Henderson CR. Sire evaluation and genetic trends. J Anim Sci. 1973;1973:10-41.
16. Mood AM. Introduction to the Theory of Statistics. McGraw-Hill; 1950:xiii, 433-xiii.
17. Rao JNK, Molina I. Small Area Estimation. Wiley; 2015.
1. Abduljawad A, Al-Assaf AF. Incentives for better performance in health care. Sultan Qaboos Univ Med J. 2011;11:201-206.
2. Milstein R, Schreyoegg J. Pay for performance in the inpatient sector: a review of 34 P4P programs in 14 OECD countries. Health Policy. 2016;120:1125-1140.
3. Herzke CA, Michtalik HJ, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. 2018;13:470-475.
4. Batbaatar E, Dorjdagva J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: a systematic review. Perspect Public Health. 2017;137:89-101.
5. Ballou D, Sanders W, Wright P. Controlling for student background in value-added assessment of teachers. J Educ Behav Stat. 2004;29:37-65.
6. Hill PW, Goldstein H. Multilevel modeling of educational data with cross-classification and missing identification for units. J Educ Behav Stat. 1998;23:117-128.
7. Rasbash J, Browne WJ. Handbook of Multilevel Analysis. Springer; 2007.
8. Brown WJ, Goldstein H, Rasbash J. Multiple membership multiple classification (MMMC) models. Statistical Modeling. 2001;1:103-124.
9. Sanders WL, Horn SP. The Tennessee Value-Added Assessment System (TVAAS)—mixed-model methodology in educational assessment. J Pers Eval Educ. 1994;8:299-311.
10. Kroch EA, Duan M. CareScience Risk Assessment Model: Hospital Performance Measurement. Premier, Inc., 2008. http://www.ahrq.gov/qual/mortality/KrochRisk.htm
11. Schumacher DJ, Wu DTY, Meganathan K, et al. A feasibility study to attribute patients to primary interns on inpatient ward teams using electronic health record data. Acad Med. 2019;94:1376-1383.
12. Simoes J, Krumholz HM, Lin Z. Hospital-level 30-day risk-standardized readmission measure. Centers for Medicare & Medicaid Services, 2018. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Hospital-Wide-All-Cause-Readmission-Updates.zip
13. Krol MW, de Boer D, Delnoij DM, Rademakers JJDJM. The Net Promoter Score: an asset to patient experience surveys? Health Expect. 2015;18:3099-3109.
14. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3:e001570.
15. Henderson CR. Sire evaluation and genetic trends. J Anim Sci. 1973;1973:10-41.
16. Mood AM. Introduction to the Theory of Statistics. McGraw-Hill; 1950:xiii, 433-xiii.
17. Rao JNK, Molina I. Small Area Estimation. Wiley; 2015.
Pharmacists’ Bleed Risk Tool and Treatment Preferences Prior to Initiating Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Cross-Sectional Survey
From Nova Southeastern University College of Pharmacy, Fort Lauderdale, FL.
Abstract
- Objective: To determine pharmacists’ preferences in bleed risk tool (BRT) usage and gastroprotection when bleed risk was lower than or equal to stroke risk in patients with nonvalvular atrial fibrillation and who were candidates for oral anticoagulation therapy (warfarin or direct oral anticoagulants [DOACs]).
- Methods: A survey consisting of 4 domains (demographics, clinical experience, BRT usage, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk) was developed. The anonymous survey was disseminated via REDCap software to members of the American College of Clinical Pharmacy ambulatory care and cardiology Practice-based Research Networks. Descriptive statistics were calculated for all study variables and inferential statistics were employed as necessary.
- Results: Of 165 BRT users, 97% preferred HAS-BLED. When bleed risk was lower than stroke risk, 151 respondents chose either DOACs (65%) or warfarin (35%); 15% added gastroprotection. When bleed risk was equal to stroke risk, 141 respondents chose DOACs (50%), warfarin (45%), or aspirin (5%); 40% added gastroprotection.
- Conclusion: In addition to BRT usage, pharmacists were judicious in their recommendation to add gastroprotection and would consider doing so if there was a specific indication. As more than 80% of extracranial bleeds are gastrointestinal bleeds and most BRTs are nonspecific for predicting these bleeds, randomized, prospective studies stratified by HAS-BLED and stroke risk scores are needed to provide further guidance on the efficacy and safety of oral anticoagulation therapy with or without gastroprotection.
Keywords: NVAF; gastroprotection; proton pump inhibitors; warfarin; oral anticoagulants.
Management of patients with nonvalvular atrial fibrillation (NVAF) with oral anticoagulation therapy (OACT) requires constant attention to maintain a balance between preventing strokes and minimizing bleeds. Several validated bleed risk tools (BRTs) available for use in NVAF patients include HAS-BLED, HEMORR2HAGES, ATRIA, and mOBRI.1,2 A high bleed risk score is not a contraindication to OACT, but, prior to and throughout therapy, bleed risk should be assessed and modifiable risk factors addressed.3 While intraluminal gastrointestinal (GI) bleeds are not considered a critical bleed site, they are a common complication of chronic OACT and can result in hemodynamic compromise and permanent discontinuation of therapy.4,5 In 3233 patients with nonvariceal upper GI bleeds (2005-2016), the adjusted odds ratio of hospital admission, transfusion, and re-bleeding while on OACT (warfarin, heparin, or apixaban) was 3.48, 2.53, and 2.26, respectively.6 Addition of acid-suppressive therapy with a proton pump inhibitor (PPI) or histamine-2 receptor antagonist (H2RA) in NVAF patients at increased risk for upper GI bleeds and receiving OACT may result in fewer bleeds.7,8
Pharmacists play an integral part in managing patients on warfarin,9-11 and data on their role in managing patients receiving direct oral anticoagulants (DOACs) are increasing.12-16 Inpatient pharmacists actively participate in multidisciplinary collaborative teams and use clinical decision-support systems or enhanced monitoring to ensure safe prescribing of high-risk medications.12,15,16 Pharmacist-managed, outpatient-based anticoagulation services in patients on warfarin were associated with lower rates of bleeding and thromboembolic events and lower health care utilization versus routine care.17 However, it is unclear how pharmacists manage patients who are candidates for OACT but who may be at increased risk for upper GI bleeds. Using a US-based survey, the investigators sought to determine pharmacists’ preferences in BRT usage and gastroprotection when bleed risk was lower than or equal to stroke risk.
Methods
This cross-sectional study was conducted after receiving approval by Nova Southeastern University’s Institutional Review Board. The survey consisted of 16 items divided into 4 domains: demographics, clinical experience, use of BRTs, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk (Figure 1). Queries were multiple choice and allowed for free-text input when “Other” was selected. Licensed pharmacists ≥ 18 years of age who routinely provided care to patients with NVAF were eligible to participate in the study. Participants who reported using a BRT (users) completed all study domains, while participants who reported not using a BRT (nonusers) completed domains 1 through 3 only.
An invitation containing the survey link was sent to the American College of Clinical Pharmacy ambulatory care (n = 2237) and cardiology (n = 1318) pharmacists listed in the organization’s Practice-based Research Networks. The survey was administered in the United States between April and June 2016 via Research Electronic Data Capture (REDCap) software, a secure Web application for building and managing online surveys designed to support data collection for research studies.18
Survey responses were downloaded, and data were analyzed using NCSS 2019 Statistical Software, LLC (Kaysville, UT). Descriptive statistics were calculated for all study variables. Demographic and clinical experience data for the group that used a BRT versus the group that did not were compared using Pearson’s chi-square, ANOVA, or the Cochran-Armitage test for trends. Logistic regression with hierarchical forward selection with switching was used to identify predictors of drug selection and use of gastroprotection.
Results
Of 230 respondents who completed the survey (response rate 6.5%), 165 (72%) used a BRT and 65 (28%) did not. No significant differences were found for age, gender, duration in clinical practice, the percentage of time spent in patient care, or practice specialty between users and nonusers (Table). The median age of users was 32 years; 68% were females; the median duration in clinical practice was 6 years; 75% of their time was spent in clinical practice; and clinical settings included ambulatory care, cardiology, and internal medicine. A significant difference was found for practice region between users versus nonusers (P = 0.014). Respondents who managed more than 200 NVAF patients per year used a BRT more often than those who managed fewer than 100 NVAF patients per year (P = 0.001).
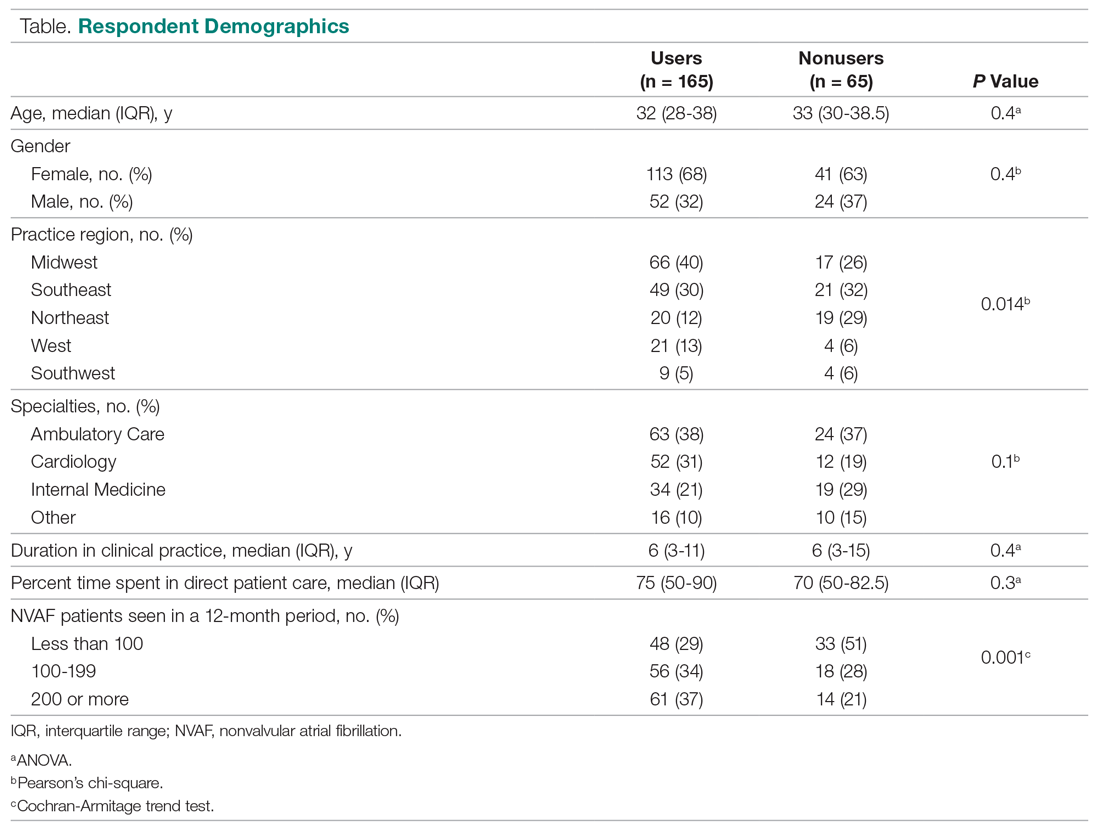
Of those who used a BRT, 97% utilized the HAS-BLED tool (n = 160). The remainder used HEMORR2HAGES (n = 3), ATRIA (n = 1), and mOBRI (n = 1). Reasons for choosing HAS-BLED included “familiarity/ease-of-use,” “preference by institution/clinical team,” and the fact that it was a “validated tool for NVAF.”
When bleed risk was lower than stroke risk, 151 of 165 users (92%) chose a treatment option (Figure 2). Of those, 65% chose a DOAC and 35% chose warfarin. Fourteen respondents chose “other” and explained that they “would initiate OACT after weighing patient factors and preferences.” When a DOAC was selected, 9% (n = 9) chose PPI co-therapy and 4% (n = 4) chose a H2RA. When warfarin was selected, 13% (n = 7) chose PPI co-therapy and 4% (n = 2) chose a H2RA. Respondents who chose gastroprotection did not provide reasons for doing so, but those who did not add it explained that they “would add gastroprotection only if patient is also on an NSAID or has a history of GI bleed” or cited “patient preference.” Specific to warfarin, some respondents would not add gastroprotection, as anticoagulation with warfarin is “easily reversed.”
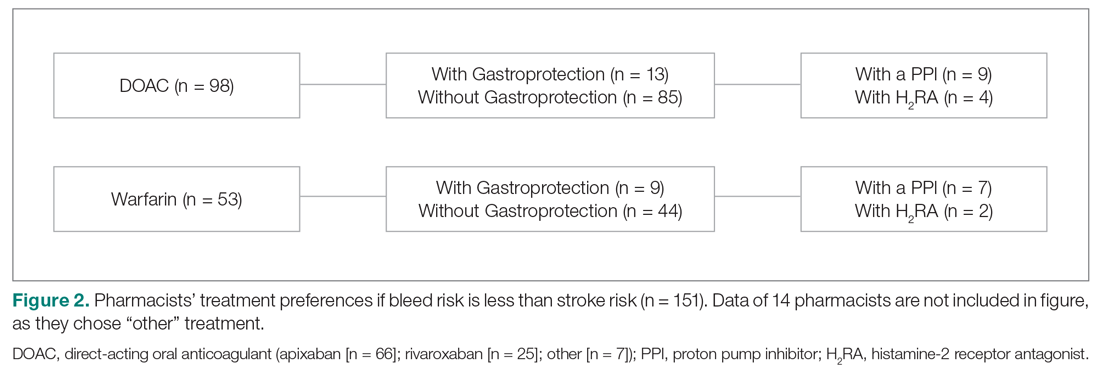
When bleed risk was equal to stroke risk, 141 of 165 users (85%) chose a treatment option (Figure 3). Fifty percent chose DOACs, 45% chose warfarin, and 5% chose aspirin.
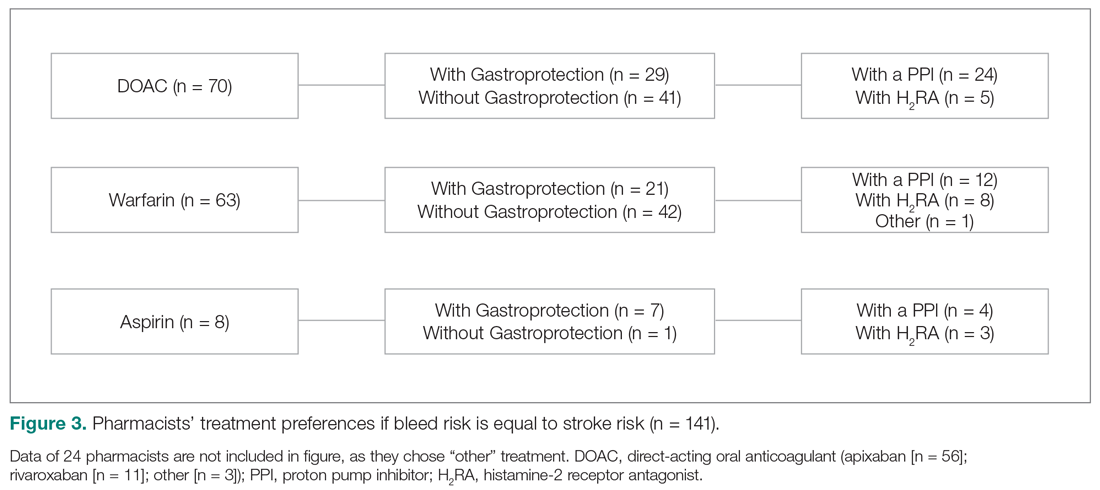
Of respondents who selected either a DOAC or warfarin, 38% (n = 50) also added gastroprotection (Figure 3). When a DOAC was selected, 34% (n = 24) favored PPI co-therapy and 7% (n = 5) chose a H2RA. When warfarin was selected, 19% (n = 12) favored PPI co-therapy, while 13% (n = 8) chose a H2RA. Rationale for choosing gastroprotection, regardless of OACT selection, included “stroke is more devastating, so if patient wants to continue treatment, but knew risks of bleeding were similar, would recommend gastroprotection to help minimize bleeding risk” and “patient-specific consideration.” Rationales for not choosing gastroprotection included “would add gastroprotection only if patient is on dual antiplatelet therapy or has another indication”; “in most patients, stroke risk outweighs bleed risk so no need for gastroprotection unless there is a stated reason”; “would use apixaban as has lowest bleeding rate of all DOACs in clinical trials”; and “gastroprotection has not been shown to be beneficial in large scale trials.”
Eight respondents chose aspirin because it was “easy and relatively low cost.” Twenty-four respondents chose “other” and explained that the choice of OACT depended on patient preference after they had discussed stroke and bleed risk with the patient and/or determined the etiology driving bleed risk.
Discussion
This is the first national survey exploring US pharmacists’ preferences in BRT usage and treatment based on bleed risk. Pharmacists preferred the HAS-BLED tool and considered patient-specific factors and evidence-based data when weighing the risk-benefit of OACT with or without gastroprotective therapy.
Similar to our findings, where three-quarters of pharmacists used a BRT, a recent Medscape/American College of Cardiology (ACC) survey reported that 74% of cardiologists used a BRT (eg, HAS-BLED) always/most of the time or sometimes to assess a patient’s overall risk of bleeding prior to initiating DOAC therapy; 27% never or rarely used a bleed risk score before prescribing DOACs.19 Although reasons for BRT preference were not provided, they may be similar to those reported by our respondents (ie, familiarity/ease-of-use). In both surveys, rationales for not using a BRT were not obtained, but possible reasons include lack of confidence with bleed risk calculators,20 inconsistent implementation of comprehensive assessments (stroke risk, bleed risk, and medication-related issues prior to decision-making),21 and nonspecific guideline recommendations.22
More recently, a network meta-analysis found that HAS-BLED and HEMORR2HAGES had modest but balanced sensitivity (
Although more than 80% of extracranial bleeds are GI bleeds,24 most BRTs are nonspecific for predicting GI bleeds. Indeed, one respondent used a spreadsheet with several BRTs to maximize treatment guidance for patients with multiple risk factors for strokes and bleeds. A comprehensive approach to determining factors that increase bleed risk should be adopted. These factors include age (HAS-BLED, HEMORR2HAGES, mOBRI, ATRIA); anemia (mOBRI, HEMORR2HAGES, ATRIA); hepatic/renal disease (HAS-BLED, HEMORR2HAGES, ATRIA, mOBRI); concomitant medications/alcohol use, including NSAIDs, corticosteroids, and antiplatelet therapy (HAS-BLED, HEMORR2HAGES); bleed history/rebleeding risk (HEMORR2HAGES, HAS-BLED, ATRIA); and GI bleeds (mOBRI).1,2 Additional risk factors for GI bleeds include being a tobacco smoker and/or being infected with Helicobacter pylori. A prospective cohort study that analyzed data from questionnaires completed by 99,359 individuals from the Copenhagen General Population Study reported that the multivariable adjusted hazard ratio for current smokers versus never smokers was 2.20 (95% CI, 1.84-2.62) for GI bleeds.25 Presence of H pylori should be investigated, with a subsequent eradication regimen implemented, as patients with warfarin-associated upper GI bleeds who were H pylori-positive had lower HAS-BLED scores versus those who were negative.26
When bleed risk was lower than stroke risk (eg, HAS-BLED < 3, CHA2DS2VASc ≥ 1), respondents appropriately initiated therapy with an OAC (predominantly apixaban); a small proportion also added gastroprotection. If the patient did not have any other GI bleed risk factors (eg, a previous GI bleed or on chronic antiplatelet or NSAID therapy), the choice of OACT depended on the attributes of each OAC and patient preference.27 Selection of warfarin was appropriate if cost, formulary restrictions, and availability of an inexpensive reversal agent were important concerns to patients and/or their health care providers. Rivaroxaban was selected because of its once-daily dosing and low risk for GI bleeding.
The recently published ARISTOPHANES study provides evidence that apixaban is an appropriate choice in patients with a HAS-BLED score < 3. In this retrospective observational study, more than 70% of patients received standard doses of DOACs (apixaban 5 mg, dabigatran 150 mg, or rivaroxaban 20 mg) and about 20% had a bleeding history, about 30% were on PPIs, less than 25% were on NSAIDs, and about 40% had a HAS-BLED score < 3. The study found that apixaban was more effective (reduced rates of ischemic or hemorrhagic strokes/systemic embolism) and safer (reduced rates of major GI bleed or intracranial bleed) than warfarin.28 Dabigatran and rivaroxaban were also more effective than warfarin for stroke prevention and had a lower risk for major intracranial bleed risk; while the risk of major GI bleed was similar between dabigatran and warfarin, major GI bleed risk was higher for rivaroxaban. When compared with each other, the 3 DOACs were effective at stroke prevention, with apixaban more effective than dabigatran and rivaroxaban; similar efficacy was noted for dabigatran versus rivaroxaban. Apixaban was associated with fewer GI bleeds versus dabigatran and rivaroxaban, but with similar intracranial bleed risks; dabigatran was associated with fewer GI bleeds but similar intracranial bleed risks versus rivaroxaban.28 Efficacy and safety findings from a subgroup analysis based on HAS-BLED scores < 3 and ≥ 3 were generally consistent with the main results.
When bleed risk was equal to stroke risk, the difficulty was determining how OACT in a patient at high stroke risk (CHA2DS2VASc score ≥ 2) and high bleed risk (HAS-BLED score ≥ 3) should be managed.
Another important finding was pharmacists’ uncertainty as to the effectiveness of PPIs in preventing GI bleeds in combination with DOACs. The data are conflicting. A meta-analysis of older studies (2007-2015) showed that PPIs (but not H2RAs) reduced the risk of upper GI bleeds in patients on warfarin but not for dabigatran
Limitations
Limitations of our survey included an overall low response rate,
Conclusion
In addition to applying BRTs in the management of NVAF patients, pharmacists considered patient-specific variables, prescriber preferences, and evidence-based guidance when recommending OACT with or without gastroprotection. To avoid suboptimal patient management, busy pharmacists should be granted time to attend continuing education programs describing optimal OACT selection and formulation of individualized, evidence-based plans to address modifiable risk factors for bleeding, including the appropriate use of gastroprotection. Randomized, prospective, long-term studies stratified by HAS-BLED and CHA2DS2VASc scores are needed to further clarify efficacy, safety, and cost-effectiveness of OACT, with and without PPIs, in patients who may be at risk for upper GI bleeds.
Acknowledgments:
Corresponding author: Devada Singh-Franco, PharmD, CDE, Nova Southeastern University College of Pharmacy, 3200 S University Drive, Fort Lauderdale, FL 33328; [email protected]
Disclosures: None.
Funding: The study was supported by Nova Southeastern University’s Health Professions Division Internal Research Grant.
1. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60:861-867.
2. Chang G, Xie Q, Ma L, et al. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta-analysis. J Thromb Haemost. 2020;18:791-801.
3. Ding WY, Harrison SL, Lane DA, Lip GYH. Considerations when choosing an appropriate bleeding risk assessment tool for patients with atrial fibrillation. J Thromb Haemost. 2020;18:788-790.
4. Lauffenburger JC, Rhoney DH, Farley JF, et al. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560-568.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:594-622.
6. Taha A, McCloskey C, Craigen T, Angerson W. Antiplatelet versus anticoagulant effects in non-variceal upper gastrointestinal bleeding. Gut. 2019;68(suppl 2):A152.
7. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: A population-based study. Gastroenterology. 2015;149:586-595.
8. Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320:2221-2230.
9. Brunetti L, Lee S-M, Doherty N, et al. Impact of warfarin discharge education program on hospital readmission and treatment costs. Int J Clin Pharm. 2018;40:721-729.
10. Hasan SS, Kow CS, Curley LE, et al. Economic evaluation of prescribing conventional and newer oral anticoagulants in older adults. Expert Rev Pharmacoecon Outcomes Res. 2018;18:371-377.
11. Phelps E, Delate T, Witt DM, et al. Effect of increased time in the therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb Res. 2018;163:54-59.
12. Ahuja T, Raco V, Papadopoulos J, Green D. Antithrombotic stewardship: Assessing use of computerized clinical decision support tools to enhance safe prescribing of direct oral anticoagulants in hospitalized patients. J Patient Saf. 2018 Sep 25. [Epub ahead of print]
13. Leef GC, Perino AC, Askari M, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation: Insights from the Veterans Health Administration. J Pharm Pract. 2020;33:647-653.
14. Papastergiou J, Kheir N, Ladova K, et al. Pharmacists’ confidence when providing pharmaceutical care on anticoagulants, a multinational survey. Int J Clin Pharm. 2017;39:1282-1290.
15. Perlman A, Horwitz E, Hirsh-Raccah B, et al. Clinical pharmacist led hospital-wide direct oral anticoagulant stewardship program. Isr J Health Policy Res. 2019;8:19.
16. Uppuluri EM, McComb MN, Shapiro NL. Implementation of a direct oral anticoagulation screening service at a large academic medical center provided by a pharmacist-managed antithrombosis clinic as a method to expand antithrombotic stewardship efforts. J Pharm Pract. 2020;33:271-275.
17. Manzoor BS, Cheng W-H, Lee JC, et al. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: A systematic review. Ann Pharmacother. 2017;51:1122-1137.
18. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
19. Brooks M. AF management: Are clinicians in agreement? Medscape. May 30, 2019. Accessed December 29, 2020. https://www.medscape.com/viewarticle/913386
20. Amroze A, Mazor K, Crawford S, et al. Survey of confidence in use of stroke and bleeding risk calculators, knowledge of anticoagulants, and comfort with prescription of anticoagulation in challenging scenarios: SUPPORT-AF II study. J Thromb Thrombolysis. 2019;48:629-637.
21. Wang Y, Bajorek B. Decision-making around antithrombotics for stroke prevention in atrial fibrillation: the health professionals’ views. Int J Clin Pharm. 2016;38:985-995.
22. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267.
23. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104-132.
24. Anghel L, Sascu R, Trifan A, et al. Non-vitamin K antagonist oral anticoagulants and the gastrointestinal bleeding risk in real-world studies. J Clin Med. 2020;9:1398.
25. Langsted A, Nordestgaard BG. Smoking is associated with increased risk of major bleeding: a prospective cohort study. Thromb Haemost. 2019;119:39-47.
26. Faye AS, Hung KW, Cheng K, et al. HAS-BLED scores underestimate gastrointestinal bleeding risk among those with H. pylori. Am J Gastroenterol. 2019;114:S364.
27. Fawzy AM, Yang W-Y, Lip GY. Safety of direct oral anticoagulants in real-world clinical practice: translating the trials to everyday clinical management. Expert Opin Drug Saf. 2019;18:187-209.
28. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933-2944.
29. Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857.
30. Holster IL, Valkhoff VE, Kuipers EJ, Tjwa E. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145:105-112.
31. Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66:2271-2281.
32. Di Minno A, Spadarella G, Spadarella E, et al. Gastrointestinal bleeding in patients receiving oral anticoagulation: Current treatment and pharmacological perspectives. Thromb Res. 2015;136:1074-1081.
33. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the Concomitant Use of Proton Pump Inhibitors and Thienopyridines: A Focused Update of the ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use. Circulation. 2010;122:2619-2633.
34. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
35. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
36. Bang CS, Joo MK, Kim BW, et al. The role of acid suppressants in the prevention of anticoagulant-related gastrointestinal bleeding: a systematic review and meta-analysis. Gut Liver. 2020;14:57-66.
37. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354-364.
38. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. Int J Mol Sci. 2019;20:5203.
39. Haastrup PF, Thompson W, Sondergaard J, Jarbol DE. Side effects of long-term proton pump inhibitor use: A review. Basic Clin Pharmacol Toxicol. 2018;123:114-121.
40. Wong JM, Maddox TM, Kennedy K, Shaw RE. Comparing major bleeding risk in outpatients with atrial fibrillation or flutter by oral anticoagulant type (from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence Registry). Am J Cardiol. 2020;125:1500-1507.
41. Nagata N, Niikura R, Aoki T, et al. Effect of proton-pump inhibitors on the risk of lower gastrointestinal bleeding associated with NSAIDs, aspirin, clopidogrel, and warfarin. J Gastroenterol. 2015;50:1079-1086.
From Nova Southeastern University College of Pharmacy, Fort Lauderdale, FL.
Abstract
- Objective: To determine pharmacists’ preferences in bleed risk tool (BRT) usage and gastroprotection when bleed risk was lower than or equal to stroke risk in patients with nonvalvular atrial fibrillation and who were candidates for oral anticoagulation therapy (warfarin or direct oral anticoagulants [DOACs]).
- Methods: A survey consisting of 4 domains (demographics, clinical experience, BRT usage, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk) was developed. The anonymous survey was disseminated via REDCap software to members of the American College of Clinical Pharmacy ambulatory care and cardiology Practice-based Research Networks. Descriptive statistics were calculated for all study variables and inferential statistics were employed as necessary.
- Results: Of 165 BRT users, 97% preferred HAS-BLED. When bleed risk was lower than stroke risk, 151 respondents chose either DOACs (65%) or warfarin (35%); 15% added gastroprotection. When bleed risk was equal to stroke risk, 141 respondents chose DOACs (50%), warfarin (45%), or aspirin (5%); 40% added gastroprotection.
- Conclusion: In addition to BRT usage, pharmacists were judicious in their recommendation to add gastroprotection and would consider doing so if there was a specific indication. As more than 80% of extracranial bleeds are gastrointestinal bleeds and most BRTs are nonspecific for predicting these bleeds, randomized, prospective studies stratified by HAS-BLED and stroke risk scores are needed to provide further guidance on the efficacy and safety of oral anticoagulation therapy with or without gastroprotection.
Keywords: NVAF; gastroprotection; proton pump inhibitors; warfarin; oral anticoagulants.
Management of patients with nonvalvular atrial fibrillation (NVAF) with oral anticoagulation therapy (OACT) requires constant attention to maintain a balance between preventing strokes and minimizing bleeds. Several validated bleed risk tools (BRTs) available for use in NVAF patients include HAS-BLED, HEMORR2HAGES, ATRIA, and mOBRI.1,2 A high bleed risk score is not a contraindication to OACT, but, prior to and throughout therapy, bleed risk should be assessed and modifiable risk factors addressed.3 While intraluminal gastrointestinal (GI) bleeds are not considered a critical bleed site, they are a common complication of chronic OACT and can result in hemodynamic compromise and permanent discontinuation of therapy.4,5 In 3233 patients with nonvariceal upper GI bleeds (2005-2016), the adjusted odds ratio of hospital admission, transfusion, and re-bleeding while on OACT (warfarin, heparin, or apixaban) was 3.48, 2.53, and 2.26, respectively.6 Addition of acid-suppressive therapy with a proton pump inhibitor (PPI) or histamine-2 receptor antagonist (H2RA) in NVAF patients at increased risk for upper GI bleeds and receiving OACT may result in fewer bleeds.7,8
Pharmacists play an integral part in managing patients on warfarin,9-11 and data on their role in managing patients receiving direct oral anticoagulants (DOACs) are increasing.12-16 Inpatient pharmacists actively participate in multidisciplinary collaborative teams and use clinical decision-support systems or enhanced monitoring to ensure safe prescribing of high-risk medications.12,15,16 Pharmacist-managed, outpatient-based anticoagulation services in patients on warfarin were associated with lower rates of bleeding and thromboembolic events and lower health care utilization versus routine care.17 However, it is unclear how pharmacists manage patients who are candidates for OACT but who may be at increased risk for upper GI bleeds. Using a US-based survey, the investigators sought to determine pharmacists’ preferences in BRT usage and gastroprotection when bleed risk was lower than or equal to stroke risk.
Methods
This cross-sectional study was conducted after receiving approval by Nova Southeastern University’s Institutional Review Board. The survey consisted of 16 items divided into 4 domains: demographics, clinical experience, use of BRTs, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk (Figure 1). Queries were multiple choice and allowed for free-text input when “Other” was selected. Licensed pharmacists ≥ 18 years of age who routinely provided care to patients with NVAF were eligible to participate in the study. Participants who reported using a BRT (users) completed all study domains, while participants who reported not using a BRT (nonusers) completed domains 1 through 3 only.

An invitation containing the survey link was sent to the American College of Clinical Pharmacy ambulatory care (n = 2237) and cardiology (n = 1318) pharmacists listed in the organization’s Practice-based Research Networks. The survey was administered in the United States between April and June 2016 via Research Electronic Data Capture (REDCap) software, a secure Web application for building and managing online surveys designed to support data collection for research studies.18
Survey responses were downloaded, and data were analyzed using NCSS 2019 Statistical Software, LLC (Kaysville, UT). Descriptive statistics were calculated for all study variables. Demographic and clinical experience data for the group that used a BRT versus the group that did not were compared using Pearson’s chi-square, ANOVA, or the Cochran-Armitage test for trends. Logistic regression with hierarchical forward selection with switching was used to identify predictors of drug selection and use of gastroprotection.
Results
Of 230 respondents who completed the survey (response rate 6.5%), 165 (72%) used a BRT and 65 (28%) did not. No significant differences were found for age, gender, duration in clinical practice, the percentage of time spent in patient care, or practice specialty between users and nonusers (Table). The median age of users was 32 years; 68% were females; the median duration in clinical practice was 6 years; 75% of their time was spent in clinical practice; and clinical settings included ambulatory care, cardiology, and internal medicine. A significant difference was found for practice region between users versus nonusers (P = 0.014). Respondents who managed more than 200 NVAF patients per year used a BRT more often than those who managed fewer than 100 NVAF patients per year (P = 0.001).

Of those who used a BRT, 97% utilized the HAS-BLED tool (n = 160). The remainder used HEMORR2HAGES (n = 3), ATRIA (n = 1), and mOBRI (n = 1). Reasons for choosing HAS-BLED included “familiarity/ease-of-use,” “preference by institution/clinical team,” and the fact that it was a “validated tool for NVAF.”
When bleed risk was lower than stroke risk, 151 of 165 users (92%) chose a treatment option (Figure 2). Of those, 65% chose a DOAC and 35% chose warfarin. Fourteen respondents chose “other” and explained that they “would initiate OACT after weighing patient factors and preferences.” When a DOAC was selected, 9% (n = 9) chose PPI co-therapy and 4% (n = 4) chose a H2RA. When warfarin was selected, 13% (n = 7) chose PPI co-therapy and 4% (n = 2) chose a H2RA. Respondents who chose gastroprotection did not provide reasons for doing so, but those who did not add it explained that they “would add gastroprotection only if patient is also on an NSAID or has a history of GI bleed” or cited “patient preference.” Specific to warfarin, some respondents would not add gastroprotection, as anticoagulation with warfarin is “easily reversed.”

When bleed risk was equal to stroke risk, 141 of 165 users (85%) chose a treatment option (Figure 3). Fifty percent chose DOACs, 45% chose warfarin, and 5% chose aspirin.

Of respondents who selected either a DOAC or warfarin, 38% (n = 50) also added gastroprotection (Figure 3). When a DOAC was selected, 34% (n = 24) favored PPI co-therapy and 7% (n = 5) chose a H2RA. When warfarin was selected, 19% (n = 12) favored PPI co-therapy, while 13% (n = 8) chose a H2RA. Rationale for choosing gastroprotection, regardless of OACT selection, included “stroke is more devastating, so if patient wants to continue treatment, but knew risks of bleeding were similar, would recommend gastroprotection to help minimize bleeding risk” and “patient-specific consideration.” Rationales for not choosing gastroprotection included “would add gastroprotection only if patient is on dual antiplatelet therapy or has another indication”; “in most patients, stroke risk outweighs bleed risk so no need for gastroprotection unless there is a stated reason”; “would use apixaban as has lowest bleeding rate of all DOACs in clinical trials”; and “gastroprotection has not been shown to be beneficial in large scale trials.”
Eight respondents chose aspirin because it was “easy and relatively low cost.” Twenty-four respondents chose “other” and explained that the choice of OACT depended on patient preference after they had discussed stroke and bleed risk with the patient and/or determined the etiology driving bleed risk.
Discussion
This is the first national survey exploring US pharmacists’ preferences in BRT usage and treatment based on bleed risk. Pharmacists preferred the HAS-BLED tool and considered patient-specific factors and evidence-based data when weighing the risk-benefit of OACT with or without gastroprotective therapy.
Similar to our findings, where three-quarters of pharmacists used a BRT, a recent Medscape/American College of Cardiology (ACC) survey reported that 74% of cardiologists used a BRT (eg, HAS-BLED) always/most of the time or sometimes to assess a patient’s overall risk of bleeding prior to initiating DOAC therapy; 27% never or rarely used a bleed risk score before prescribing DOACs.19 Although reasons for BRT preference were not provided, they may be similar to those reported by our respondents (ie, familiarity/ease-of-use). In both surveys, rationales for not using a BRT were not obtained, but possible reasons include lack of confidence with bleed risk calculators,20 inconsistent implementation of comprehensive assessments (stroke risk, bleed risk, and medication-related issues prior to decision-making),21 and nonspecific guideline recommendations.22
More recently, a network meta-analysis found that HAS-BLED and HEMORR2HAGES had modest but balanced sensitivity (
Although more than 80% of extracranial bleeds are GI bleeds,24 most BRTs are nonspecific for predicting GI bleeds. Indeed, one respondent used a spreadsheet with several BRTs to maximize treatment guidance for patients with multiple risk factors for strokes and bleeds. A comprehensive approach to determining factors that increase bleed risk should be adopted. These factors include age (HAS-BLED, HEMORR2HAGES, mOBRI, ATRIA); anemia (mOBRI, HEMORR2HAGES, ATRIA); hepatic/renal disease (HAS-BLED, HEMORR2HAGES, ATRIA, mOBRI); concomitant medications/alcohol use, including NSAIDs, corticosteroids, and antiplatelet therapy (HAS-BLED, HEMORR2HAGES); bleed history/rebleeding risk (HEMORR2HAGES, HAS-BLED, ATRIA); and GI bleeds (mOBRI).1,2 Additional risk factors for GI bleeds include being a tobacco smoker and/or being infected with Helicobacter pylori. A prospective cohort study that analyzed data from questionnaires completed by 99,359 individuals from the Copenhagen General Population Study reported that the multivariable adjusted hazard ratio for current smokers versus never smokers was 2.20 (95% CI, 1.84-2.62) for GI bleeds.25 Presence of H pylori should be investigated, with a subsequent eradication regimen implemented, as patients with warfarin-associated upper GI bleeds who were H pylori-positive had lower HAS-BLED scores versus those who were negative.26
When bleed risk was lower than stroke risk (eg, HAS-BLED < 3, CHA2DS2VASc ≥ 1), respondents appropriately initiated therapy with an OAC (predominantly apixaban); a small proportion also added gastroprotection. If the patient did not have any other GI bleed risk factors (eg, a previous GI bleed or on chronic antiplatelet or NSAID therapy), the choice of OACT depended on the attributes of each OAC and patient preference.27 Selection of warfarin was appropriate if cost, formulary restrictions, and availability of an inexpensive reversal agent were important concerns to patients and/or their health care providers. Rivaroxaban was selected because of its once-daily dosing and low risk for GI bleeding.
The recently published ARISTOPHANES study provides evidence that apixaban is an appropriate choice in patients with a HAS-BLED score < 3. In this retrospective observational study, more than 70% of patients received standard doses of DOACs (apixaban 5 mg, dabigatran 150 mg, or rivaroxaban 20 mg) and about 20% had a bleeding history, about 30% were on PPIs, less than 25% were on NSAIDs, and about 40% had a HAS-BLED score < 3. The study found that apixaban was more effective (reduced rates of ischemic or hemorrhagic strokes/systemic embolism) and safer (reduced rates of major GI bleed or intracranial bleed) than warfarin.28 Dabigatran and rivaroxaban were also more effective than warfarin for stroke prevention and had a lower risk for major intracranial bleed risk; while the risk of major GI bleed was similar between dabigatran and warfarin, major GI bleed risk was higher for rivaroxaban. When compared with each other, the 3 DOACs were effective at stroke prevention, with apixaban more effective than dabigatran and rivaroxaban; similar efficacy was noted for dabigatran versus rivaroxaban. Apixaban was associated with fewer GI bleeds versus dabigatran and rivaroxaban, but with similar intracranial bleed risks; dabigatran was associated with fewer GI bleeds but similar intracranial bleed risks versus rivaroxaban.28 Efficacy and safety findings from a subgroup analysis based on HAS-BLED scores < 3 and ≥ 3 were generally consistent with the main results.
When bleed risk was equal to stroke risk, the difficulty was determining how OACT in a patient at high stroke risk (CHA2DS2VASc score ≥ 2) and high bleed risk (HAS-BLED score ≥ 3) should be managed.
Another important finding was pharmacists’ uncertainty as to the effectiveness of PPIs in preventing GI bleeds in combination with DOACs. The data are conflicting. A meta-analysis of older studies (2007-2015) showed that PPIs (but not H2RAs) reduced the risk of upper GI bleeds in patients on warfarin but not for dabigatran
Limitations
Limitations of our survey included an overall low response rate,
Conclusion
In addition to applying BRTs in the management of NVAF patients, pharmacists considered patient-specific variables, prescriber preferences, and evidence-based guidance when recommending OACT with or without gastroprotection. To avoid suboptimal patient management, busy pharmacists should be granted time to attend continuing education programs describing optimal OACT selection and formulation of individualized, evidence-based plans to address modifiable risk factors for bleeding, including the appropriate use of gastroprotection. Randomized, prospective, long-term studies stratified by HAS-BLED and CHA2DS2VASc scores are needed to further clarify efficacy, safety, and cost-effectiveness of OACT, with and without PPIs, in patients who may be at risk for upper GI bleeds.
Acknowledgments:
Corresponding author: Devada Singh-Franco, PharmD, CDE, Nova Southeastern University College of Pharmacy, 3200 S University Drive, Fort Lauderdale, FL 33328; [email protected]
Disclosures: None.
Funding: The study was supported by Nova Southeastern University’s Health Professions Division Internal Research Grant.
From Nova Southeastern University College of Pharmacy, Fort Lauderdale, FL.
Abstract
- Objective: To determine pharmacists’ preferences in bleed risk tool (BRT) usage and gastroprotection when bleed risk was lower than or equal to stroke risk in patients with nonvalvular atrial fibrillation and who were candidates for oral anticoagulation therapy (warfarin or direct oral anticoagulants [DOACs]).
- Methods: A survey consisting of 4 domains (demographics, clinical experience, BRT usage, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk) was developed. The anonymous survey was disseminated via REDCap software to members of the American College of Clinical Pharmacy ambulatory care and cardiology Practice-based Research Networks. Descriptive statistics were calculated for all study variables and inferential statistics were employed as necessary.
- Results: Of 165 BRT users, 97% preferred HAS-BLED. When bleed risk was lower than stroke risk, 151 respondents chose either DOACs (65%) or warfarin (35%); 15% added gastroprotection. When bleed risk was equal to stroke risk, 141 respondents chose DOACs (50%), warfarin (45%), or aspirin (5%); 40% added gastroprotection.
- Conclusion: In addition to BRT usage, pharmacists were judicious in their recommendation to add gastroprotection and would consider doing so if there was a specific indication. As more than 80% of extracranial bleeds are gastrointestinal bleeds and most BRTs are nonspecific for predicting these bleeds, randomized, prospective studies stratified by HAS-BLED and stroke risk scores are needed to provide further guidance on the efficacy and safety of oral anticoagulation therapy with or without gastroprotection.
Keywords: NVAF; gastroprotection; proton pump inhibitors; warfarin; oral anticoagulants.
Management of patients with nonvalvular atrial fibrillation (NVAF) with oral anticoagulation therapy (OACT) requires constant attention to maintain a balance between preventing strokes and minimizing bleeds. Several validated bleed risk tools (BRTs) available for use in NVAF patients include HAS-BLED, HEMORR2HAGES, ATRIA, and mOBRI.1,2 A high bleed risk score is not a contraindication to OACT, but, prior to and throughout therapy, bleed risk should be assessed and modifiable risk factors addressed.3 While intraluminal gastrointestinal (GI) bleeds are not considered a critical bleed site, they are a common complication of chronic OACT and can result in hemodynamic compromise and permanent discontinuation of therapy.4,5 In 3233 patients with nonvariceal upper GI bleeds (2005-2016), the adjusted odds ratio of hospital admission, transfusion, and re-bleeding while on OACT (warfarin, heparin, or apixaban) was 3.48, 2.53, and 2.26, respectively.6 Addition of acid-suppressive therapy with a proton pump inhibitor (PPI) or histamine-2 receptor antagonist (H2RA) in NVAF patients at increased risk for upper GI bleeds and receiving OACT may result in fewer bleeds.7,8
Pharmacists play an integral part in managing patients on warfarin,9-11 and data on their role in managing patients receiving direct oral anticoagulants (DOACs) are increasing.12-16 Inpatient pharmacists actively participate in multidisciplinary collaborative teams and use clinical decision-support systems or enhanced monitoring to ensure safe prescribing of high-risk medications.12,15,16 Pharmacist-managed, outpatient-based anticoagulation services in patients on warfarin were associated with lower rates of bleeding and thromboembolic events and lower health care utilization versus routine care.17 However, it is unclear how pharmacists manage patients who are candidates for OACT but who may be at increased risk for upper GI bleeds. Using a US-based survey, the investigators sought to determine pharmacists’ preferences in BRT usage and gastroprotection when bleed risk was lower than or equal to stroke risk.
Methods
This cross-sectional study was conducted after receiving approval by Nova Southeastern University’s Institutional Review Board. The survey consisted of 16 items divided into 4 domains: demographics, clinical experience, use of BRTs, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk (Figure 1). Queries were multiple choice and allowed for free-text input when “Other” was selected. Licensed pharmacists ≥ 18 years of age who routinely provided care to patients with NVAF were eligible to participate in the study. Participants who reported using a BRT (users) completed all study domains, while participants who reported not using a BRT (nonusers) completed domains 1 through 3 only.

An invitation containing the survey link was sent to the American College of Clinical Pharmacy ambulatory care (n = 2237) and cardiology (n = 1318) pharmacists listed in the organization’s Practice-based Research Networks. The survey was administered in the United States between April and June 2016 via Research Electronic Data Capture (REDCap) software, a secure Web application for building and managing online surveys designed to support data collection for research studies.18
Survey responses were downloaded, and data were analyzed using NCSS 2019 Statistical Software, LLC (Kaysville, UT). Descriptive statistics were calculated for all study variables. Demographic and clinical experience data for the group that used a BRT versus the group that did not were compared using Pearson’s chi-square, ANOVA, or the Cochran-Armitage test for trends. Logistic regression with hierarchical forward selection with switching was used to identify predictors of drug selection and use of gastroprotection.
Results
Of 230 respondents who completed the survey (response rate 6.5%), 165 (72%) used a BRT and 65 (28%) did not. No significant differences were found for age, gender, duration in clinical practice, the percentage of time spent in patient care, or practice specialty between users and nonusers (Table). The median age of users was 32 years; 68% were females; the median duration in clinical practice was 6 years; 75% of their time was spent in clinical practice; and clinical settings included ambulatory care, cardiology, and internal medicine. A significant difference was found for practice region between users versus nonusers (P = 0.014). Respondents who managed more than 200 NVAF patients per year used a BRT more often than those who managed fewer than 100 NVAF patients per year (P = 0.001).

Of those who used a BRT, 97% utilized the HAS-BLED tool (n = 160). The remainder used HEMORR2HAGES (n = 3), ATRIA (n = 1), and mOBRI (n = 1). Reasons for choosing HAS-BLED included “familiarity/ease-of-use,” “preference by institution/clinical team,” and the fact that it was a “validated tool for NVAF.”
When bleed risk was lower than stroke risk, 151 of 165 users (92%) chose a treatment option (Figure 2). Of those, 65% chose a DOAC and 35% chose warfarin. Fourteen respondents chose “other” and explained that they “would initiate OACT after weighing patient factors and preferences.” When a DOAC was selected, 9% (n = 9) chose PPI co-therapy and 4% (n = 4) chose a H2RA. When warfarin was selected, 13% (n = 7) chose PPI co-therapy and 4% (n = 2) chose a H2RA. Respondents who chose gastroprotection did not provide reasons for doing so, but those who did not add it explained that they “would add gastroprotection only if patient is also on an NSAID or has a history of GI bleed” or cited “patient preference.” Specific to warfarin, some respondents would not add gastroprotection, as anticoagulation with warfarin is “easily reversed.”

When bleed risk was equal to stroke risk, 141 of 165 users (85%) chose a treatment option (Figure 3). Fifty percent chose DOACs, 45% chose warfarin, and 5% chose aspirin.

Of respondents who selected either a DOAC or warfarin, 38% (n = 50) also added gastroprotection (Figure 3). When a DOAC was selected, 34% (n = 24) favored PPI co-therapy and 7% (n = 5) chose a H2RA. When warfarin was selected, 19% (n = 12) favored PPI co-therapy, while 13% (n = 8) chose a H2RA. Rationale for choosing gastroprotection, regardless of OACT selection, included “stroke is more devastating, so if patient wants to continue treatment, but knew risks of bleeding were similar, would recommend gastroprotection to help minimize bleeding risk” and “patient-specific consideration.” Rationales for not choosing gastroprotection included “would add gastroprotection only if patient is on dual antiplatelet therapy or has another indication”; “in most patients, stroke risk outweighs bleed risk so no need for gastroprotection unless there is a stated reason”; “would use apixaban as has lowest bleeding rate of all DOACs in clinical trials”; and “gastroprotection has not been shown to be beneficial in large scale trials.”
Eight respondents chose aspirin because it was “easy and relatively low cost.” Twenty-four respondents chose “other” and explained that the choice of OACT depended on patient preference after they had discussed stroke and bleed risk with the patient and/or determined the etiology driving bleed risk.
Discussion
This is the first national survey exploring US pharmacists’ preferences in BRT usage and treatment based on bleed risk. Pharmacists preferred the HAS-BLED tool and considered patient-specific factors and evidence-based data when weighing the risk-benefit of OACT with or without gastroprotective therapy.
Similar to our findings, where three-quarters of pharmacists used a BRT, a recent Medscape/American College of Cardiology (ACC) survey reported that 74% of cardiologists used a BRT (eg, HAS-BLED) always/most of the time or sometimes to assess a patient’s overall risk of bleeding prior to initiating DOAC therapy; 27% never or rarely used a bleed risk score before prescribing DOACs.19 Although reasons for BRT preference were not provided, they may be similar to those reported by our respondents (ie, familiarity/ease-of-use). In both surveys, rationales for not using a BRT were not obtained, but possible reasons include lack of confidence with bleed risk calculators,20 inconsistent implementation of comprehensive assessments (stroke risk, bleed risk, and medication-related issues prior to decision-making),21 and nonspecific guideline recommendations.22
More recently, a network meta-analysis found that HAS-BLED and HEMORR2HAGES had modest but balanced sensitivity (
Although more than 80% of extracranial bleeds are GI bleeds,24 most BRTs are nonspecific for predicting GI bleeds. Indeed, one respondent used a spreadsheet with several BRTs to maximize treatment guidance for patients with multiple risk factors for strokes and bleeds. A comprehensive approach to determining factors that increase bleed risk should be adopted. These factors include age (HAS-BLED, HEMORR2HAGES, mOBRI, ATRIA); anemia (mOBRI, HEMORR2HAGES, ATRIA); hepatic/renal disease (HAS-BLED, HEMORR2HAGES, ATRIA, mOBRI); concomitant medications/alcohol use, including NSAIDs, corticosteroids, and antiplatelet therapy (HAS-BLED, HEMORR2HAGES); bleed history/rebleeding risk (HEMORR2HAGES, HAS-BLED, ATRIA); and GI bleeds (mOBRI).1,2 Additional risk factors for GI bleeds include being a tobacco smoker and/or being infected with Helicobacter pylori. A prospective cohort study that analyzed data from questionnaires completed by 99,359 individuals from the Copenhagen General Population Study reported that the multivariable adjusted hazard ratio for current smokers versus never smokers was 2.20 (95% CI, 1.84-2.62) for GI bleeds.25 Presence of H pylori should be investigated, with a subsequent eradication regimen implemented, as patients with warfarin-associated upper GI bleeds who were H pylori-positive had lower HAS-BLED scores versus those who were negative.26
When bleed risk was lower than stroke risk (eg, HAS-BLED < 3, CHA2DS2VASc ≥ 1), respondents appropriately initiated therapy with an OAC (predominantly apixaban); a small proportion also added gastroprotection. If the patient did not have any other GI bleed risk factors (eg, a previous GI bleed or on chronic antiplatelet or NSAID therapy), the choice of OACT depended on the attributes of each OAC and patient preference.27 Selection of warfarin was appropriate if cost, formulary restrictions, and availability of an inexpensive reversal agent were important concerns to patients and/or their health care providers. Rivaroxaban was selected because of its once-daily dosing and low risk for GI bleeding.
The recently published ARISTOPHANES study provides evidence that apixaban is an appropriate choice in patients with a HAS-BLED score < 3. In this retrospective observational study, more than 70% of patients received standard doses of DOACs (apixaban 5 mg, dabigatran 150 mg, or rivaroxaban 20 mg) and about 20% had a bleeding history, about 30% were on PPIs, less than 25% were on NSAIDs, and about 40% had a HAS-BLED score < 3. The study found that apixaban was more effective (reduced rates of ischemic or hemorrhagic strokes/systemic embolism) and safer (reduced rates of major GI bleed or intracranial bleed) than warfarin.28 Dabigatran and rivaroxaban were also more effective than warfarin for stroke prevention and had a lower risk for major intracranial bleed risk; while the risk of major GI bleed was similar between dabigatran and warfarin, major GI bleed risk was higher for rivaroxaban. When compared with each other, the 3 DOACs were effective at stroke prevention, with apixaban more effective than dabigatran and rivaroxaban; similar efficacy was noted for dabigatran versus rivaroxaban. Apixaban was associated with fewer GI bleeds versus dabigatran and rivaroxaban, but with similar intracranial bleed risks; dabigatran was associated with fewer GI bleeds but similar intracranial bleed risks versus rivaroxaban.28 Efficacy and safety findings from a subgroup analysis based on HAS-BLED scores < 3 and ≥ 3 were generally consistent with the main results.
When bleed risk was equal to stroke risk, the difficulty was determining how OACT in a patient at high stroke risk (CHA2DS2VASc score ≥ 2) and high bleed risk (HAS-BLED score ≥ 3) should be managed.
Another important finding was pharmacists’ uncertainty as to the effectiveness of PPIs in preventing GI bleeds in combination with DOACs. The data are conflicting. A meta-analysis of older studies (2007-2015) showed that PPIs (but not H2RAs) reduced the risk of upper GI bleeds in patients on warfarin but not for dabigatran
Limitations
Limitations of our survey included an overall low response rate,
Conclusion
In addition to applying BRTs in the management of NVAF patients, pharmacists considered patient-specific variables, prescriber preferences, and evidence-based guidance when recommending OACT with or without gastroprotection. To avoid suboptimal patient management, busy pharmacists should be granted time to attend continuing education programs describing optimal OACT selection and formulation of individualized, evidence-based plans to address modifiable risk factors for bleeding, including the appropriate use of gastroprotection. Randomized, prospective, long-term studies stratified by HAS-BLED and CHA2DS2VASc scores are needed to further clarify efficacy, safety, and cost-effectiveness of OACT, with and without PPIs, in patients who may be at risk for upper GI bleeds.
Acknowledgments:
Corresponding author: Devada Singh-Franco, PharmD, CDE, Nova Southeastern University College of Pharmacy, 3200 S University Drive, Fort Lauderdale, FL 33328; [email protected]
Disclosures: None.
Funding: The study was supported by Nova Southeastern University’s Health Professions Division Internal Research Grant.
1. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60:861-867.
2. Chang G, Xie Q, Ma L, et al. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta-analysis. J Thromb Haemost. 2020;18:791-801.
3. Ding WY, Harrison SL, Lane DA, Lip GYH. Considerations when choosing an appropriate bleeding risk assessment tool for patients with atrial fibrillation. J Thromb Haemost. 2020;18:788-790.
4. Lauffenburger JC, Rhoney DH, Farley JF, et al. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560-568.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:594-622.
6. Taha A, McCloskey C, Craigen T, Angerson W. Antiplatelet versus anticoagulant effects in non-variceal upper gastrointestinal bleeding. Gut. 2019;68(suppl 2):A152.
7. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: A population-based study. Gastroenterology. 2015;149:586-595.
8. Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320:2221-2230.
9. Brunetti L, Lee S-M, Doherty N, et al. Impact of warfarin discharge education program on hospital readmission and treatment costs. Int J Clin Pharm. 2018;40:721-729.
10. Hasan SS, Kow CS, Curley LE, et al. Economic evaluation of prescribing conventional and newer oral anticoagulants in older adults. Expert Rev Pharmacoecon Outcomes Res. 2018;18:371-377.
11. Phelps E, Delate T, Witt DM, et al. Effect of increased time in the therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb Res. 2018;163:54-59.
12. Ahuja T, Raco V, Papadopoulos J, Green D. Antithrombotic stewardship: Assessing use of computerized clinical decision support tools to enhance safe prescribing of direct oral anticoagulants in hospitalized patients. J Patient Saf. 2018 Sep 25. [Epub ahead of print]
13. Leef GC, Perino AC, Askari M, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation: Insights from the Veterans Health Administration. J Pharm Pract. 2020;33:647-653.
14. Papastergiou J, Kheir N, Ladova K, et al. Pharmacists’ confidence when providing pharmaceutical care on anticoagulants, a multinational survey. Int J Clin Pharm. 2017;39:1282-1290.
15. Perlman A, Horwitz E, Hirsh-Raccah B, et al. Clinical pharmacist led hospital-wide direct oral anticoagulant stewardship program. Isr J Health Policy Res. 2019;8:19.
16. Uppuluri EM, McComb MN, Shapiro NL. Implementation of a direct oral anticoagulation screening service at a large academic medical center provided by a pharmacist-managed antithrombosis clinic as a method to expand antithrombotic stewardship efforts. J Pharm Pract. 2020;33:271-275.
17. Manzoor BS, Cheng W-H, Lee JC, et al. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: A systematic review. Ann Pharmacother. 2017;51:1122-1137.
18. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
19. Brooks M. AF management: Are clinicians in agreement? Medscape. May 30, 2019. Accessed December 29, 2020. https://www.medscape.com/viewarticle/913386
20. Amroze A, Mazor K, Crawford S, et al. Survey of confidence in use of stroke and bleeding risk calculators, knowledge of anticoagulants, and comfort with prescription of anticoagulation in challenging scenarios: SUPPORT-AF II study. J Thromb Thrombolysis. 2019;48:629-637.
21. Wang Y, Bajorek B. Decision-making around antithrombotics for stroke prevention in atrial fibrillation: the health professionals’ views. Int J Clin Pharm. 2016;38:985-995.
22. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267.
23. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104-132.
24. Anghel L, Sascu R, Trifan A, et al. Non-vitamin K antagonist oral anticoagulants and the gastrointestinal bleeding risk in real-world studies. J Clin Med. 2020;9:1398.
25. Langsted A, Nordestgaard BG. Smoking is associated with increased risk of major bleeding: a prospective cohort study. Thromb Haemost. 2019;119:39-47.
26. Faye AS, Hung KW, Cheng K, et al. HAS-BLED scores underestimate gastrointestinal bleeding risk among those with H. pylori. Am J Gastroenterol. 2019;114:S364.
27. Fawzy AM, Yang W-Y, Lip GY. Safety of direct oral anticoagulants in real-world clinical practice: translating the trials to everyday clinical management. Expert Opin Drug Saf. 2019;18:187-209.
28. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933-2944.
29. Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857.
30. Holster IL, Valkhoff VE, Kuipers EJ, Tjwa E. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145:105-112.
31. Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66:2271-2281.
32. Di Minno A, Spadarella G, Spadarella E, et al. Gastrointestinal bleeding in patients receiving oral anticoagulation: Current treatment and pharmacological perspectives. Thromb Res. 2015;136:1074-1081.
33. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the Concomitant Use of Proton Pump Inhibitors and Thienopyridines: A Focused Update of the ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use. Circulation. 2010;122:2619-2633.
34. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
35. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
36. Bang CS, Joo MK, Kim BW, et al. The role of acid suppressants in the prevention of anticoagulant-related gastrointestinal bleeding: a systematic review and meta-analysis. Gut Liver. 2020;14:57-66.
37. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354-364.
38. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. Int J Mol Sci. 2019;20:5203.
39. Haastrup PF, Thompson W, Sondergaard J, Jarbol DE. Side effects of long-term proton pump inhibitor use: A review. Basic Clin Pharmacol Toxicol. 2018;123:114-121.
40. Wong JM, Maddox TM, Kennedy K, Shaw RE. Comparing major bleeding risk in outpatients with atrial fibrillation or flutter by oral anticoagulant type (from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence Registry). Am J Cardiol. 2020;125:1500-1507.
41. Nagata N, Niikura R, Aoki T, et al. Effect of proton-pump inhibitors on the risk of lower gastrointestinal bleeding associated with NSAIDs, aspirin, clopidogrel, and warfarin. J Gastroenterol. 2015;50:1079-1086.
1. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60:861-867.
2. Chang G, Xie Q, Ma L, et al. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta-analysis. J Thromb Haemost. 2020;18:791-801.
3. Ding WY, Harrison SL, Lane DA, Lip GYH. Considerations when choosing an appropriate bleeding risk assessment tool for patients with atrial fibrillation. J Thromb Haemost. 2020;18:788-790.
4. Lauffenburger JC, Rhoney DH, Farley JF, et al. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560-568.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:594-622.
6. Taha A, McCloskey C, Craigen T, Angerson W. Antiplatelet versus anticoagulant effects in non-variceal upper gastrointestinal bleeding. Gut. 2019;68(suppl 2):A152.
7. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: A population-based study. Gastroenterology. 2015;149:586-595.
8. Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320:2221-2230.
9. Brunetti L, Lee S-M, Doherty N, et al. Impact of warfarin discharge education program on hospital readmission and treatment costs. Int J Clin Pharm. 2018;40:721-729.
10. Hasan SS, Kow CS, Curley LE, et al. Economic evaluation of prescribing conventional and newer oral anticoagulants in older adults. Expert Rev Pharmacoecon Outcomes Res. 2018;18:371-377.
11. Phelps E, Delate T, Witt DM, et al. Effect of increased time in the therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb Res. 2018;163:54-59.
12. Ahuja T, Raco V, Papadopoulos J, Green D. Antithrombotic stewardship: Assessing use of computerized clinical decision support tools to enhance safe prescribing of direct oral anticoagulants in hospitalized patients. J Patient Saf. 2018 Sep 25. [Epub ahead of print]
13. Leef GC, Perino AC, Askari M, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation: Insights from the Veterans Health Administration. J Pharm Pract. 2020;33:647-653.
14. Papastergiou J, Kheir N, Ladova K, et al. Pharmacists’ confidence when providing pharmaceutical care on anticoagulants, a multinational survey. Int J Clin Pharm. 2017;39:1282-1290.
15. Perlman A, Horwitz E, Hirsh-Raccah B, et al. Clinical pharmacist led hospital-wide direct oral anticoagulant stewardship program. Isr J Health Policy Res. 2019;8:19.
16. Uppuluri EM, McComb MN, Shapiro NL. Implementation of a direct oral anticoagulation screening service at a large academic medical center provided by a pharmacist-managed antithrombosis clinic as a method to expand antithrombotic stewardship efforts. J Pharm Pract. 2020;33:271-275.
17. Manzoor BS, Cheng W-H, Lee JC, et al. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: A systematic review. Ann Pharmacother. 2017;51:1122-1137.
18. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
19. Brooks M. AF management: Are clinicians in agreement? Medscape. May 30, 2019. Accessed December 29, 2020. https://www.medscape.com/viewarticle/913386
20. Amroze A, Mazor K, Crawford S, et al. Survey of confidence in use of stroke and bleeding risk calculators, knowledge of anticoagulants, and comfort with prescription of anticoagulation in challenging scenarios: SUPPORT-AF II study. J Thromb Thrombolysis. 2019;48:629-637.
21. Wang Y, Bajorek B. Decision-making around antithrombotics for stroke prevention in atrial fibrillation: the health professionals’ views. Int J Clin Pharm. 2016;38:985-995.
22. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267.
23. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104-132.
24. Anghel L, Sascu R, Trifan A, et al. Non-vitamin K antagonist oral anticoagulants and the gastrointestinal bleeding risk in real-world studies. J Clin Med. 2020;9:1398.
25. Langsted A, Nordestgaard BG. Smoking is associated with increased risk of major bleeding: a prospective cohort study. Thromb Haemost. 2019;119:39-47.
26. Faye AS, Hung KW, Cheng K, et al. HAS-BLED scores underestimate gastrointestinal bleeding risk among those with H. pylori. Am J Gastroenterol. 2019;114:S364.
27. Fawzy AM, Yang W-Y, Lip GY. Safety of direct oral anticoagulants in real-world clinical practice: translating the trials to everyday clinical management. Expert Opin Drug Saf. 2019;18:187-209.
28. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933-2944.
29. Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857.
30. Holster IL, Valkhoff VE, Kuipers EJ, Tjwa E. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145:105-112.
31. Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66:2271-2281.
32. Di Minno A, Spadarella G, Spadarella E, et al. Gastrointestinal bleeding in patients receiving oral anticoagulation: Current treatment and pharmacological perspectives. Thromb Res. 2015;136:1074-1081.
33. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the Concomitant Use of Proton Pump Inhibitors and Thienopyridines: A Focused Update of the ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use. Circulation. 2010;122:2619-2633.
34. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
35. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
36. Bang CS, Joo MK, Kim BW, et al. The role of acid suppressants in the prevention of anticoagulant-related gastrointestinal bleeding: a systematic review and meta-analysis. Gut Liver. 2020;14:57-66.
37. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354-364.
38. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. Int J Mol Sci. 2019;20:5203.
39. Haastrup PF, Thompson W, Sondergaard J, Jarbol DE. Side effects of long-term proton pump inhibitor use: A review. Basic Clin Pharmacol Toxicol. 2018;123:114-121.
40. Wong JM, Maddox TM, Kennedy K, Shaw RE. Comparing major bleeding risk in outpatients with atrial fibrillation or flutter by oral anticoagulant type (from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence Registry). Am J Cardiol. 2020;125:1500-1507.
41. Nagata N, Niikura R, Aoki T, et al. Effect of proton-pump inhibitors on the risk of lower gastrointestinal bleeding associated with NSAIDs, aspirin, clopidogrel, and warfarin. J Gastroenterol. 2015;50:1079-1086.
Theory of Planned Behavior Provides A Theoretical Explanation For Enhanced Behavior Change With Genetic-Based Lifestyle Interventions
Study Overview
Objective. To determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the Theory of Planned Behavior (TPB), a widely accepted theory used to help predict human lifestyle-related behaviors.
Design. Pragmatic, cluster, randomized controlled trial.
Settings and participants. This study took place at the East Elgin Family Health Team, a primary care clinic in Aylmer, Ontario, Canada. Recruitment occurred between April 2017 and September 2018, with staggered intervention cohorts occurring from May 2017 to September 2019. Participants enrolled in a weight management program at the clinic were invited to participate in the study if they met the following inclusion criteria: body mass index (BMI) ≥25 kg/m2, >18 years of age, English-speaking, willing to undergo genetic testing, having access to a computer with internet at least 1 day per week, and not seeing another health care provider for weight loss advice outside of the study. Exclusion criteria included pregnancy and lactation. All participants provided written informed consent.
Interventions. At baseline, weight management program cohorts (average cohort size was 14 participants) were randomized (1:1) to receive either the standard population-based intervention (Group Lifestyle Balance, or GLB) or a modified GLB intervention, which included the provision of lifestyle genomics (LGx) information and advice (GLB+LGx). Both interventions aimed to assist participants with weight management and healthy lifestyle change, with particular focus on nutrition and physical activity (PA). Interventions were 12 months long, consisting of 23 group-based sessions and 3 one-on-one sessions with a registered dietitian after 3, 6, and 12 months (all sessions were face-to-face). To improve intervention adherence, participants were given reminder calls for their one-on-one appointments and for the start of their program. A sample size was calculated based on the primary outcome indicating that a total of 74 participants were needed (n = 37 per group) for this trial. By September 2019, this sample size was exceeded with 10 randomized groups (n = 140).
The 5 randomized standard GLB groups followed the established GLB program curriculum comprising population-based information and advice while focusing on following a calorie-controlled, moderate-fat (25% of calories) nutrition plan with at least 150 minutes of weekly moderate-intensity PA. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting outlining population-based targets, including acceptable macronutrient distribution ranges for protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
The 5 randomized modified GLB+LGx groups followed a modified GLB program curriculum in which participants were given genetic-based information and advice, which differed from the advice given to the standard GLB group, while focusing on following a calorie-controlled nutrition plan. The nutrition and PA targets were personalized based on their individual genetic variation. For example, participants with the AA variant of FTO (rs9939609) were advised to engage in at least 30 to 60 minutes of PA daily 6 days per week, with muscle-strengthening activities at least 2 days per week, rather than receiving the standard population-based advice to aim for 150 minutes weekly of PA with at least 2 days per week of muscle-strengthening activity. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting, which outlined genetic-based information and advice related to protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
Measures and analysis. Change in the TPB components (attitudes, subjective norms and perceived behavioral control) were measured via a TPB questionnaire at 5 time points: baseline (2-week run-in period), immediately after the first group session (where participants received a summary report of either population-based or genetic-based recommendations depending on group assignment), and after 3-, 6- and 12-month follow-ups. Attitudes, subjective norms, and perceived behavioral control were measured on a Likert scale from 1 through 7. Self-reported measures of actual behavioral control (including annual household income, perceptions about events arising in one’s day-to-day life that suddenly take up one’s free time, perceptions about the frequency of feeling ill or tired, and highest achieved level of education) were collected via survey questions and assessed on a Likert scale of 1 through 7. Stage of change was also measured, based on the Transtheoretical Model, using a Likert scale of 1 through 6.
Linear mixed models were used to conduct within- and between-group analyses using SPSS version 26.0, while controlling for measures of actual behavioral control. All analyses were intention-to-treat by originally assigned groups, with mean value imputation conducted for missing data. A Bonferroni correction for multiple testing was used. For all statistical analyses, the level of significance was set at P < 0.05 and trending towards significance at P = 0.05–0.06.
Main results. Participants consisted of primarily middle-age, middle-income, Caucasian females. Baseline attitudes towards the effectiveness of nutrition and PA for weight management were generally positive, and participants perceived that undergoing genetic testing would assist with weight management. Participants had overall neutral subjective norms related to friends and family consuming a healthy diet and engaging in PA, but perceived that their friends, family, and health care team (HCT) believed it was important for them to achieve their nutrition and PA recommendations. Participants overall also perceived that their HCT believed genetic testing could assist with weight management. Baseline measures of perceived behavioral control were overall neutral, with baseline stage of change between “motivation” and “action” (short-term; <3 months).
In within-group analyses, significant improvements (P < 0.05) in attitudes towards the effectiveness of nutrition and PA recommendations for weight management, subjective norms related to both friends and family consuming a healthy diet, and perceived behavioral control in changing PA/dietary intake and managing weight tended to be short-term in the GLB group and long-term for the GLB+LGx group. In all cases of between-group differences for changes in TPB components, the GLB group exhibited reductions in scores, whereas the GLB+LGx group exhibited increases or improvements. Between-group differences (short-term and long-term) in several measures of subjective norms were observed. For example, after 3 months, significant between-group differences were observed in changes in perception that friends believed LGx would help with weight management (P = 0.024). After 12 months, between-group differences trending towards significance were also observed in changes in perception that family members believed genetic testing would help with weight management (P = 0.05). Significant between-group differences and differences trending towards significance were also observed at 12 months for changes in perception that family believed it was important for the participant to achieve the PA recommendations (P = 0.049) and nutrition recommendations (P = 0.05). Between-group differences trending towards significance were also observed at 3 months in attitudes towards the effectiveness of LGx for weight management (P = 0.06). There were no significant between-group differences observed in changes in perceived behavioral control.
Conclusion. Results from this study support the hypothesis that the TPB can help provide a theoretical explanation for why genetically tailored lifestyle information and advice can lead to improvements in lifestyle behavior change.
Commentary
Because health behaviors are critical in areas such as prevention, treatment, and rehabilitation, it is important to describe and understand what drives these behaviors.1 Theories are important tools in this effort as they aim to explain and predict health behavior and are used in the design and evaluation of interventions.1 The TPB is one of the most widely accepted behavior change theories and posits that attitudes, subjective norms (or social pressures and behaviors), and perceived behavioral control are significant predictors of an individual’s intention to engage in behaviors.2 TPB has been highlighted in the literature as a validated theory for predicting nutrition and PA intentions and resulting behaviors.3,4
Motivating lifestyle behavior change in clinical practice can be challenging, but some studies have demonstrated how providing genetic information and advice (or lifestyle genomics) can help motivate changes in nutrition and PA among patients.5-7 Because this has yet to be explained using the TPB, this study is an important contribution to the literature as it aimed to determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the TPB. Briefly, results from within-group analyses in this study demonstrated that the provision of genetically tailored lifestyle information and advice (via the GLB+LGx intervention) tended to impact antecedents of behavior change, more so over the long-term, while population-based advice (via the standard GLB intervention) tended to impact antecedents of behavior change over the short-term (eg, attitudes towards dietary fat intake, perceptions that friends and family consume a healthy diet, and perceptions about the impact of genetic-based advice for weight management). In addition, between-group differences in subjective norms observed at 12 months suggested that social pressures and norms may be influencing long-term changes in lifestyle habits.
While key strengths of this study include its pragmatic cluster randomized controlled trial design, 12-month intervention duration, and intent-to-treat analyses, there are some study limitations, which are acknowledged by the authors. Generalizability is limited to the demographic characteristics of the study population (ie, middle-aged, middle-income, Caucasian females enrolled in a lifestyle change weight management program). Thus, replication of the study is needed in more diverse study populations and with health-related outcomes beyond weight management. In addition, as the authors indicate, future research should ensure the inclusion of theory-based questionnaires in genetic-based intervention studies assessing lifestyle behavior change to elucidate theory-based mechanisms of change.
Applications for Clinical Practice
Population-based research has consistently indicated that nutrition interventions typically impact short-term dietary changes. Confronting the challenge of long-term adherence to nutrition and PA recommendations requires an understanding of factors impacting long-term motivation and behavior change. With increased attention on and research into genetically tailored lifestyle advice (or lifestyle genomics), it is important for clinical practitioners to be familiar with the evidence supporting these approaches. In addition, this research highlights the need to consider individual factors (attitudes, subjective norms, and perceived behavioral control) that may predict successful change in lifestyle habits when providing nutrition and PA recommendations, whether population-based or genetically tailored.
—Katrina F. Mateo, PhD, MPH
1. Lippke S, Ziegelmann JP. Theory-based health behavior change: Developing, testing, and applying theories for evidence-based interventions. Appl Psychol. 2008;57:698-716.
2. Ajzen I. The Theory of planned behaviour: reactions and reflections. Psychol Health. 2011;26:1113-1127.
3. McDermott MS, Oliver M, Simnadis T, et al. The Theory of Planned Behaviour and dietary patterns: A systematic review and meta-analysis. Prev Med (Baltim). 2015;81:150-156.
4. McEachan RRC, Conner M, Taylor NJ, Lawton RJ. Prospective prediction of health-related behaviours with the theory of planned behaviour: A meta-analysis. Health Psychol Rev. 2011;5:97-144.
5. Hietaranta-Luoma H-L, Tahvonen R, Iso-Touru T, et al A. An intervention study of individual, APOE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2014;7:161-174.
6. Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. DeAngelis MM, ed. PLoS One. 2014;9(11):e112665.
7. Egglestone C, Morris A, O’Brien A. Effect of direct‐to‐consumer genetic tests on health behaviour and anxiety: a survey of consumers and potential consumers. J Genet Couns. 2013;22:565-575.
Study Overview
Objective. To determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the Theory of Planned Behavior (TPB), a widely accepted theory used to help predict human lifestyle-related behaviors.
Design. Pragmatic, cluster, randomized controlled trial.
Settings and participants. This study took place at the East Elgin Family Health Team, a primary care clinic in Aylmer, Ontario, Canada. Recruitment occurred between April 2017 and September 2018, with staggered intervention cohorts occurring from May 2017 to September 2019. Participants enrolled in a weight management program at the clinic were invited to participate in the study if they met the following inclusion criteria: body mass index (BMI) ≥25 kg/m2, >18 years of age, English-speaking, willing to undergo genetic testing, having access to a computer with internet at least 1 day per week, and not seeing another health care provider for weight loss advice outside of the study. Exclusion criteria included pregnancy and lactation. All participants provided written informed consent.
Interventions. At baseline, weight management program cohorts (average cohort size was 14 participants) were randomized (1:1) to receive either the standard population-based intervention (Group Lifestyle Balance, or GLB) or a modified GLB intervention, which included the provision of lifestyle genomics (LGx) information and advice (GLB+LGx). Both interventions aimed to assist participants with weight management and healthy lifestyle change, with particular focus on nutrition and physical activity (PA). Interventions were 12 months long, consisting of 23 group-based sessions and 3 one-on-one sessions with a registered dietitian after 3, 6, and 12 months (all sessions were face-to-face). To improve intervention adherence, participants were given reminder calls for their one-on-one appointments and for the start of their program. A sample size was calculated based on the primary outcome indicating that a total of 74 participants were needed (n = 37 per group) for this trial. By September 2019, this sample size was exceeded with 10 randomized groups (n = 140).
The 5 randomized standard GLB groups followed the established GLB program curriculum comprising population-based information and advice while focusing on following a calorie-controlled, moderate-fat (25% of calories) nutrition plan with at least 150 minutes of weekly moderate-intensity PA. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting outlining population-based targets, including acceptable macronutrient distribution ranges for protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
The 5 randomized modified GLB+LGx groups followed a modified GLB program curriculum in which participants were given genetic-based information and advice, which differed from the advice given to the standard GLB group, while focusing on following a calorie-controlled nutrition plan. The nutrition and PA targets were personalized based on their individual genetic variation. For example, participants with the AA variant of FTO (rs9939609) were advised to engage in at least 30 to 60 minutes of PA daily 6 days per week, with muscle-strengthening activities at least 2 days per week, rather than receiving the standard population-based advice to aim for 150 minutes weekly of PA with at least 2 days per week of muscle-strengthening activity. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting, which outlined genetic-based information and advice related to protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
Measures and analysis. Change in the TPB components (attitudes, subjective norms and perceived behavioral control) were measured via a TPB questionnaire at 5 time points: baseline (2-week run-in period), immediately after the first group session (where participants received a summary report of either population-based or genetic-based recommendations depending on group assignment), and after 3-, 6- and 12-month follow-ups. Attitudes, subjective norms, and perceived behavioral control were measured on a Likert scale from 1 through 7. Self-reported measures of actual behavioral control (including annual household income, perceptions about events arising in one’s day-to-day life that suddenly take up one’s free time, perceptions about the frequency of feeling ill or tired, and highest achieved level of education) were collected via survey questions and assessed on a Likert scale of 1 through 7. Stage of change was also measured, based on the Transtheoretical Model, using a Likert scale of 1 through 6.
Linear mixed models were used to conduct within- and between-group analyses using SPSS version 26.0, while controlling for measures of actual behavioral control. All analyses were intention-to-treat by originally assigned groups, with mean value imputation conducted for missing data. A Bonferroni correction for multiple testing was used. For all statistical analyses, the level of significance was set at P < 0.05 and trending towards significance at P = 0.05–0.06.
Main results. Participants consisted of primarily middle-age, middle-income, Caucasian females. Baseline attitudes towards the effectiveness of nutrition and PA for weight management were generally positive, and participants perceived that undergoing genetic testing would assist with weight management. Participants had overall neutral subjective norms related to friends and family consuming a healthy diet and engaging in PA, but perceived that their friends, family, and health care team (HCT) believed it was important for them to achieve their nutrition and PA recommendations. Participants overall also perceived that their HCT believed genetic testing could assist with weight management. Baseline measures of perceived behavioral control were overall neutral, with baseline stage of change between “motivation” and “action” (short-term; <3 months).
In within-group analyses, significant improvements (P < 0.05) in attitudes towards the effectiveness of nutrition and PA recommendations for weight management, subjective norms related to both friends and family consuming a healthy diet, and perceived behavioral control in changing PA/dietary intake and managing weight tended to be short-term in the GLB group and long-term for the GLB+LGx group. In all cases of between-group differences for changes in TPB components, the GLB group exhibited reductions in scores, whereas the GLB+LGx group exhibited increases or improvements. Between-group differences (short-term and long-term) in several measures of subjective norms were observed. For example, after 3 months, significant between-group differences were observed in changes in perception that friends believed LGx would help with weight management (P = 0.024). After 12 months, between-group differences trending towards significance were also observed in changes in perception that family members believed genetic testing would help with weight management (P = 0.05). Significant between-group differences and differences trending towards significance were also observed at 12 months for changes in perception that family believed it was important for the participant to achieve the PA recommendations (P = 0.049) and nutrition recommendations (P = 0.05). Between-group differences trending towards significance were also observed at 3 months in attitudes towards the effectiveness of LGx for weight management (P = 0.06). There were no significant between-group differences observed in changes in perceived behavioral control.
Conclusion. Results from this study support the hypothesis that the TPB can help provide a theoretical explanation for why genetically tailored lifestyle information and advice can lead to improvements in lifestyle behavior change.
Commentary
Because health behaviors are critical in areas such as prevention, treatment, and rehabilitation, it is important to describe and understand what drives these behaviors.1 Theories are important tools in this effort as they aim to explain and predict health behavior and are used in the design and evaluation of interventions.1 The TPB is one of the most widely accepted behavior change theories and posits that attitudes, subjective norms (or social pressures and behaviors), and perceived behavioral control are significant predictors of an individual’s intention to engage in behaviors.2 TPB has been highlighted in the literature as a validated theory for predicting nutrition and PA intentions and resulting behaviors.3,4
Motivating lifestyle behavior change in clinical practice can be challenging, but some studies have demonstrated how providing genetic information and advice (or lifestyle genomics) can help motivate changes in nutrition and PA among patients.5-7 Because this has yet to be explained using the TPB, this study is an important contribution to the literature as it aimed to determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the TPB. Briefly, results from within-group analyses in this study demonstrated that the provision of genetically tailored lifestyle information and advice (via the GLB+LGx intervention) tended to impact antecedents of behavior change, more so over the long-term, while population-based advice (via the standard GLB intervention) tended to impact antecedents of behavior change over the short-term (eg, attitudes towards dietary fat intake, perceptions that friends and family consume a healthy diet, and perceptions about the impact of genetic-based advice for weight management). In addition, between-group differences in subjective norms observed at 12 months suggested that social pressures and norms may be influencing long-term changes in lifestyle habits.
While key strengths of this study include its pragmatic cluster randomized controlled trial design, 12-month intervention duration, and intent-to-treat analyses, there are some study limitations, which are acknowledged by the authors. Generalizability is limited to the demographic characteristics of the study population (ie, middle-aged, middle-income, Caucasian females enrolled in a lifestyle change weight management program). Thus, replication of the study is needed in more diverse study populations and with health-related outcomes beyond weight management. In addition, as the authors indicate, future research should ensure the inclusion of theory-based questionnaires in genetic-based intervention studies assessing lifestyle behavior change to elucidate theory-based mechanisms of change.
Applications for Clinical Practice
Population-based research has consistently indicated that nutrition interventions typically impact short-term dietary changes. Confronting the challenge of long-term adherence to nutrition and PA recommendations requires an understanding of factors impacting long-term motivation and behavior change. With increased attention on and research into genetically tailored lifestyle advice (or lifestyle genomics), it is important for clinical practitioners to be familiar with the evidence supporting these approaches. In addition, this research highlights the need to consider individual factors (attitudes, subjective norms, and perceived behavioral control) that may predict successful change in lifestyle habits when providing nutrition and PA recommendations, whether population-based or genetically tailored.
—Katrina F. Mateo, PhD, MPH
Study Overview
Objective. To determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the Theory of Planned Behavior (TPB), a widely accepted theory used to help predict human lifestyle-related behaviors.
Design. Pragmatic, cluster, randomized controlled trial.
Settings and participants. This study took place at the East Elgin Family Health Team, a primary care clinic in Aylmer, Ontario, Canada. Recruitment occurred between April 2017 and September 2018, with staggered intervention cohorts occurring from May 2017 to September 2019. Participants enrolled in a weight management program at the clinic were invited to participate in the study if they met the following inclusion criteria: body mass index (BMI) ≥25 kg/m2, >18 years of age, English-speaking, willing to undergo genetic testing, having access to a computer with internet at least 1 day per week, and not seeing another health care provider for weight loss advice outside of the study. Exclusion criteria included pregnancy and lactation. All participants provided written informed consent.
Interventions. At baseline, weight management program cohorts (average cohort size was 14 participants) were randomized (1:1) to receive either the standard population-based intervention (Group Lifestyle Balance, or GLB) or a modified GLB intervention, which included the provision of lifestyle genomics (LGx) information and advice (GLB+LGx). Both interventions aimed to assist participants with weight management and healthy lifestyle change, with particular focus on nutrition and physical activity (PA). Interventions were 12 months long, consisting of 23 group-based sessions and 3 one-on-one sessions with a registered dietitian after 3, 6, and 12 months (all sessions were face-to-face). To improve intervention adherence, participants were given reminder calls for their one-on-one appointments and for the start of their program. A sample size was calculated based on the primary outcome indicating that a total of 74 participants were needed (n = 37 per group) for this trial. By September 2019, this sample size was exceeded with 10 randomized groups (n = 140).
The 5 randomized standard GLB groups followed the established GLB program curriculum comprising population-based information and advice while focusing on following a calorie-controlled, moderate-fat (25% of calories) nutrition plan with at least 150 minutes of weekly moderate-intensity PA. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting outlining population-based targets, including acceptable macronutrient distribution ranges for protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
The 5 randomized modified GLB+LGx groups followed a modified GLB program curriculum in which participants were given genetic-based information and advice, which differed from the advice given to the standard GLB group, while focusing on following a calorie-controlled nutrition plan. The nutrition and PA targets were personalized based on their individual genetic variation. For example, participants with the AA variant of FTO (rs9939609) were advised to engage in at least 30 to 60 minutes of PA daily 6 days per week, with muscle-strengthening activities at least 2 days per week, rather than receiving the standard population-based advice to aim for 150 minutes weekly of PA with at least 2 days per week of muscle-strengthening activity. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting, which outlined genetic-based information and advice related to protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
Measures and analysis. Change in the TPB components (attitudes, subjective norms and perceived behavioral control) were measured via a TPB questionnaire at 5 time points: baseline (2-week run-in period), immediately after the first group session (where participants received a summary report of either population-based or genetic-based recommendations depending on group assignment), and after 3-, 6- and 12-month follow-ups. Attitudes, subjective norms, and perceived behavioral control were measured on a Likert scale from 1 through 7. Self-reported measures of actual behavioral control (including annual household income, perceptions about events arising in one’s day-to-day life that suddenly take up one’s free time, perceptions about the frequency of feeling ill or tired, and highest achieved level of education) were collected via survey questions and assessed on a Likert scale of 1 through 7. Stage of change was also measured, based on the Transtheoretical Model, using a Likert scale of 1 through 6.
Linear mixed models were used to conduct within- and between-group analyses using SPSS version 26.0, while controlling for measures of actual behavioral control. All analyses were intention-to-treat by originally assigned groups, with mean value imputation conducted for missing data. A Bonferroni correction for multiple testing was used. For all statistical analyses, the level of significance was set at P < 0.05 and trending towards significance at P = 0.05–0.06.
Main results. Participants consisted of primarily middle-age, middle-income, Caucasian females. Baseline attitudes towards the effectiveness of nutrition and PA for weight management were generally positive, and participants perceived that undergoing genetic testing would assist with weight management. Participants had overall neutral subjective norms related to friends and family consuming a healthy diet and engaging in PA, but perceived that their friends, family, and health care team (HCT) believed it was important for them to achieve their nutrition and PA recommendations. Participants overall also perceived that their HCT believed genetic testing could assist with weight management. Baseline measures of perceived behavioral control were overall neutral, with baseline stage of change between “motivation” and “action” (short-term; <3 months).
In within-group analyses, significant improvements (P < 0.05) in attitudes towards the effectiveness of nutrition and PA recommendations for weight management, subjective norms related to both friends and family consuming a healthy diet, and perceived behavioral control in changing PA/dietary intake and managing weight tended to be short-term in the GLB group and long-term for the GLB+LGx group. In all cases of between-group differences for changes in TPB components, the GLB group exhibited reductions in scores, whereas the GLB+LGx group exhibited increases or improvements. Between-group differences (short-term and long-term) in several measures of subjective norms were observed. For example, after 3 months, significant between-group differences were observed in changes in perception that friends believed LGx would help with weight management (P = 0.024). After 12 months, between-group differences trending towards significance were also observed in changes in perception that family members believed genetic testing would help with weight management (P = 0.05). Significant between-group differences and differences trending towards significance were also observed at 12 months for changes in perception that family believed it was important for the participant to achieve the PA recommendations (P = 0.049) and nutrition recommendations (P = 0.05). Between-group differences trending towards significance were also observed at 3 months in attitudes towards the effectiveness of LGx for weight management (P = 0.06). There were no significant between-group differences observed in changes in perceived behavioral control.
Conclusion. Results from this study support the hypothesis that the TPB can help provide a theoretical explanation for why genetically tailored lifestyle information and advice can lead to improvements in lifestyle behavior change.
Commentary
Because health behaviors are critical in areas such as prevention, treatment, and rehabilitation, it is important to describe and understand what drives these behaviors.1 Theories are important tools in this effort as they aim to explain and predict health behavior and are used in the design and evaluation of interventions.1 The TPB is one of the most widely accepted behavior change theories and posits that attitudes, subjective norms (or social pressures and behaviors), and perceived behavioral control are significant predictors of an individual’s intention to engage in behaviors.2 TPB has been highlighted in the literature as a validated theory for predicting nutrition and PA intentions and resulting behaviors.3,4
Motivating lifestyle behavior change in clinical practice can be challenging, but some studies have demonstrated how providing genetic information and advice (or lifestyle genomics) can help motivate changes in nutrition and PA among patients.5-7 Because this has yet to be explained using the TPB, this study is an important contribution to the literature as it aimed to determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the TPB. Briefly, results from within-group analyses in this study demonstrated that the provision of genetically tailored lifestyle information and advice (via the GLB+LGx intervention) tended to impact antecedents of behavior change, more so over the long-term, while population-based advice (via the standard GLB intervention) tended to impact antecedents of behavior change over the short-term (eg, attitudes towards dietary fat intake, perceptions that friends and family consume a healthy diet, and perceptions about the impact of genetic-based advice for weight management). In addition, between-group differences in subjective norms observed at 12 months suggested that social pressures and norms may be influencing long-term changes in lifestyle habits.
While key strengths of this study include its pragmatic cluster randomized controlled trial design, 12-month intervention duration, and intent-to-treat analyses, there are some study limitations, which are acknowledged by the authors. Generalizability is limited to the demographic characteristics of the study population (ie, middle-aged, middle-income, Caucasian females enrolled in a lifestyle change weight management program). Thus, replication of the study is needed in more diverse study populations and with health-related outcomes beyond weight management. In addition, as the authors indicate, future research should ensure the inclusion of theory-based questionnaires in genetic-based intervention studies assessing lifestyle behavior change to elucidate theory-based mechanisms of change.
Applications for Clinical Practice
Population-based research has consistently indicated that nutrition interventions typically impact short-term dietary changes. Confronting the challenge of long-term adherence to nutrition and PA recommendations requires an understanding of factors impacting long-term motivation and behavior change. With increased attention on and research into genetically tailored lifestyle advice (or lifestyle genomics), it is important for clinical practitioners to be familiar with the evidence supporting these approaches. In addition, this research highlights the need to consider individual factors (attitudes, subjective norms, and perceived behavioral control) that may predict successful change in lifestyle habits when providing nutrition and PA recommendations, whether population-based or genetically tailored.
—Katrina F. Mateo, PhD, MPH
1. Lippke S, Ziegelmann JP. Theory-based health behavior change: Developing, testing, and applying theories for evidence-based interventions. Appl Psychol. 2008;57:698-716.
2. Ajzen I. The Theory of planned behaviour: reactions and reflections. Psychol Health. 2011;26:1113-1127.
3. McDermott MS, Oliver M, Simnadis T, et al. The Theory of Planned Behaviour and dietary patterns: A systematic review and meta-analysis. Prev Med (Baltim). 2015;81:150-156.
4. McEachan RRC, Conner M, Taylor NJ, Lawton RJ. Prospective prediction of health-related behaviours with the theory of planned behaviour: A meta-analysis. Health Psychol Rev. 2011;5:97-144.
5. Hietaranta-Luoma H-L, Tahvonen R, Iso-Touru T, et al A. An intervention study of individual, APOE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2014;7:161-174.
6. Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. DeAngelis MM, ed. PLoS One. 2014;9(11):e112665.
7. Egglestone C, Morris A, O’Brien A. Effect of direct‐to‐consumer genetic tests on health behaviour and anxiety: a survey of consumers and potential consumers. J Genet Couns. 2013;22:565-575.
1. Lippke S, Ziegelmann JP. Theory-based health behavior change: Developing, testing, and applying theories for evidence-based interventions. Appl Psychol. 2008;57:698-716.
2. Ajzen I. The Theory of planned behaviour: reactions and reflections. Psychol Health. 2011;26:1113-1127.
3. McDermott MS, Oliver M, Simnadis T, et al. The Theory of Planned Behaviour and dietary patterns: A systematic review and meta-analysis. Prev Med (Baltim). 2015;81:150-156.
4. McEachan RRC, Conner M, Taylor NJ, Lawton RJ. Prospective prediction of health-related behaviours with the theory of planned behaviour: A meta-analysis. Health Psychol Rev. 2011;5:97-144.
5. Hietaranta-Luoma H-L, Tahvonen R, Iso-Touru T, et al A. An intervention study of individual, APOE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2014;7:161-174.
6. Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. DeAngelis MM, ed. PLoS One. 2014;9(11):e112665.
7. Egglestone C, Morris A, O’Brien A. Effect of direct‐to‐consumer genetic tests on health behaviour and anxiety: a survey of consumers and potential consumers. J Genet Couns. 2013;22:565-575.
Noninvasive Ventilation Use Among Medicare Beneficiaries at the End of Life
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
Weekly COVID-19 cases in children dropped 22%
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
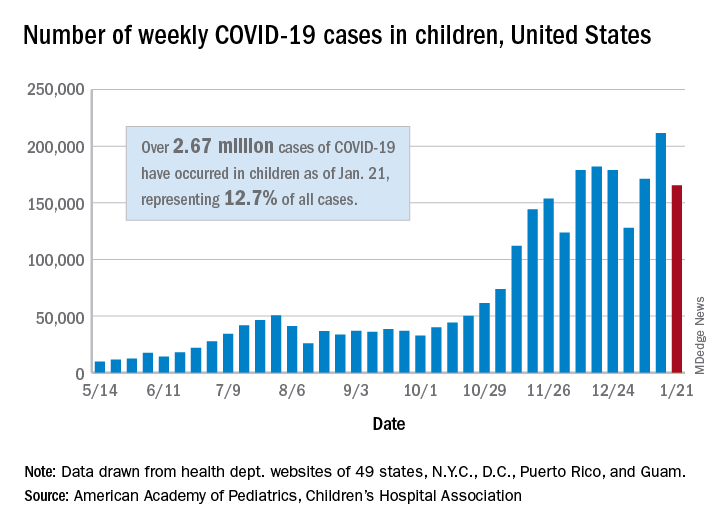
The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
COVID-19 risks linked to medications in IBD
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
FROM THE CROHN’S & COLITIS CONGRESS
Bathing now more widely accepted as an eczema treatment strategy
According to Noreen Heer Nicol, PhD, RN, FNP, frustration still exists for patients, families, and health care providers regarding the lack of consensus that routine bathing is good for patients with atopic dermatitis.
During the Revolutionizing Atopic Dermatitis symposium, she said that conflicting and vague guidelines currently exist on the topic.
“This stems from the fact that we just don’t have good studies,” said Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado at Denver, Aurora. “Particularly, we don’t have randomized, controlled trials on the effects of water and bathing. It’s not just parents that are frustrated, but health care providers are as well.”
In an observational analysis, researchers evaluated results from three online surveys of dermatologists, allergists, and immunologists, and primary care physicians regarding routine bathing frequency recommendations for children with AD. It found that PCPs recommended daily bathing less than 50% of the time, while specialists recommended daily bathing more than 50% of the time.
“It seems like the PCPs have embraced that old dermatology notion when bathing was avoided in patients with AD,” Dr. Nicol said. “This lack of consensus on the basic daily care steps in AD management causes a great deal of confusion amongst patients, families, and young health care providers, in particular,” she added.
She believes that this goes back to a century-long debate about the pros and cons of bathing in AD. “We used to say that bathing will dry the skin out if you take a bath or a shower without immediately applying something like a good moisturizer. That’s where the 3-minute rule came along from the National Eczema Association, meaning that bathing hydrates the stratum corneum if you take a bath or a shower and you immediately apply that good moisturizer within 3 minutes to retain that hydration and keep the barrier intact and flexible.”
Dr. Nicol presented a stepwise management model that she has published many times over the years (see Pediatr Nursing 2020;46[2]:92-8 and J Allergy Clin Immunol Pract 2019;7[1]:1-16).
Step 1 consists of basic care, including skin hydration/bathing, application of a daily moisturizer, avoiding irritants, and identifying and addressing specific triggers. “This is the foundation for every step as you go forward,” she explained. Soak and seal has been a mainstay of treatment at National Jewish Health, she noted. “By that, I mean taking a soaking 10-15 minute bath in warm water daily. Gently pat away excess water. Immediately apply skin medications or moisturizer within 3 minutes. Using a gentle fragrance-free, dye-free cleanser to clean skin is also important. Avoid scrubbing.”
A review article on bathing and associated treatments in AD was published in 2017 and includes 144 references to bathing studies. A separate recommendation known as the “AD Yardstick” published by Dr. Nicol’s colleague at National Jewish Health, Mark Boguniewicz, MD, and coauthors, elaborated on the definition of basic skin care for nonlesional AD. Besides recommending the liberal and frequent application of moisturizers, it suggests management with warm baths or showers using nonsoap cleansers, usually once per day, followed by application of a moisturizer, even on clear areas.
“This is now what people are thinking as the basis of skin care in patients with AD,” Dr. Nicol said. “Warm baths and showers don’t look so controversial anymore. This model nicely lays out what we want people to remember. In the past, many times we just skipped that important step of telling people about bathing.”
In a small 2009 study, researchers conducted a quantitative assessment of combination bathing and moisturizing regimens on skin hydration in AD. They found that bathing followed by application of a moisturizer provides modest hydration benefits, though less than that of simply applying moisturizer alone. “That has not been the case for most of us who are bathing advocates,” Dr. Nicol said. “We believe that there is an additional hydration that’s gained from bathing and moisturizers done properly.”
In an earlier retrospective study of 28 patients referred to a tertiary care center for refractory chronic pruritic eruptions, researchers found that a plain-water 20-minute soak followed by smearing of midstrength corticosteroid ointment led to clearing or dramatic improvement of the lesions (Arch Dermatol 2005;14:1556-9). The authors recommended prospective studies to confirm the findings.
In a separate review of medical literature, researchers explored the role of frequent bathing in the treatment of pediatric AD (Ann Allergy Asthma Immunol 2016;117[1]:9-13). They found that the weight of evidence suggests that the frequent soak and smear bathing is preferred to infrequent bathing in the management of AD. Frequent bathing was defined as bathing at least once a day, while infrequent bathing was defined as bathing less than once a day.
“Bleach baths have received much attention in recent years, and have been endorsed by multiple AD guidelines, though not to the same degree as regular bathing,” Dr. Nicol said. “Right now, you can find almost as much literature for this practice as against it. The populations that seem to value from beach baths the most, however, are those with frequent infections, particularly those who are methicillin resistant. Most people recommend a maximum of two to three times per week but only with an active infection. Care must be taken to avoid additional drying or irritation of the skin from bleach.”
Many bleach bath recipes call for adding one-eighth to one-half of a cup of bleach to a tub full or water.
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly.
According to Noreen Heer Nicol, PhD, RN, FNP, frustration still exists for patients, families, and health care providers regarding the lack of consensus that routine bathing is good for patients with atopic dermatitis.
During the Revolutionizing Atopic Dermatitis symposium, she said that conflicting and vague guidelines currently exist on the topic.
“This stems from the fact that we just don’t have good studies,” said Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado at Denver, Aurora. “Particularly, we don’t have randomized, controlled trials on the effects of water and bathing. It’s not just parents that are frustrated, but health care providers are as well.”
In an observational analysis, researchers evaluated results from three online surveys of dermatologists, allergists, and immunologists, and primary care physicians regarding routine bathing frequency recommendations for children with AD. It found that PCPs recommended daily bathing less than 50% of the time, while specialists recommended daily bathing more than 50% of the time.
“It seems like the PCPs have embraced that old dermatology notion when bathing was avoided in patients with AD,” Dr. Nicol said. “This lack of consensus on the basic daily care steps in AD management causes a great deal of confusion amongst patients, families, and young health care providers, in particular,” she added.
She believes that this goes back to a century-long debate about the pros and cons of bathing in AD. “We used to say that bathing will dry the skin out if you take a bath or a shower without immediately applying something like a good moisturizer. That’s where the 3-minute rule came along from the National Eczema Association, meaning that bathing hydrates the stratum corneum if you take a bath or a shower and you immediately apply that good moisturizer within 3 minutes to retain that hydration and keep the barrier intact and flexible.”
Dr. Nicol presented a stepwise management model that she has published many times over the years (see Pediatr Nursing 2020;46[2]:92-8 and J Allergy Clin Immunol Pract 2019;7[1]:1-16).
Step 1 consists of basic care, including skin hydration/bathing, application of a daily moisturizer, avoiding irritants, and identifying and addressing specific triggers. “This is the foundation for every step as you go forward,” she explained. Soak and seal has been a mainstay of treatment at National Jewish Health, she noted. “By that, I mean taking a soaking 10-15 minute bath in warm water daily. Gently pat away excess water. Immediately apply skin medications or moisturizer within 3 minutes. Using a gentle fragrance-free, dye-free cleanser to clean skin is also important. Avoid scrubbing.”
A review article on bathing and associated treatments in AD was published in 2017 and includes 144 references to bathing studies. A separate recommendation known as the “AD Yardstick” published by Dr. Nicol’s colleague at National Jewish Health, Mark Boguniewicz, MD, and coauthors, elaborated on the definition of basic skin care for nonlesional AD. Besides recommending the liberal and frequent application of moisturizers, it suggests management with warm baths or showers using nonsoap cleansers, usually once per day, followed by application of a moisturizer, even on clear areas.
“This is now what people are thinking as the basis of skin care in patients with AD,” Dr. Nicol said. “Warm baths and showers don’t look so controversial anymore. This model nicely lays out what we want people to remember. In the past, many times we just skipped that important step of telling people about bathing.”
In a small 2009 study, researchers conducted a quantitative assessment of combination bathing and moisturizing regimens on skin hydration in AD. They found that bathing followed by application of a moisturizer provides modest hydration benefits, though less than that of simply applying moisturizer alone. “That has not been the case for most of us who are bathing advocates,” Dr. Nicol said. “We believe that there is an additional hydration that’s gained from bathing and moisturizers done properly.”
In an earlier retrospective study of 28 patients referred to a tertiary care center for refractory chronic pruritic eruptions, researchers found that a plain-water 20-minute soak followed by smearing of midstrength corticosteroid ointment led to clearing or dramatic improvement of the lesions (Arch Dermatol 2005;14:1556-9). The authors recommended prospective studies to confirm the findings.
In a separate review of medical literature, researchers explored the role of frequent bathing in the treatment of pediatric AD (Ann Allergy Asthma Immunol 2016;117[1]:9-13). They found that the weight of evidence suggests that the frequent soak and smear bathing is preferred to infrequent bathing in the management of AD. Frequent bathing was defined as bathing at least once a day, while infrequent bathing was defined as bathing less than once a day.
“Bleach baths have received much attention in recent years, and have been endorsed by multiple AD guidelines, though not to the same degree as regular bathing,” Dr. Nicol said. “Right now, you can find almost as much literature for this practice as against it. The populations that seem to value from beach baths the most, however, are those with frequent infections, particularly those who are methicillin resistant. Most people recommend a maximum of two to three times per week but only with an active infection. Care must be taken to avoid additional drying or irritation of the skin from bleach.”
Many bleach bath recipes call for adding one-eighth to one-half of a cup of bleach to a tub full or water.
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly.
According to Noreen Heer Nicol, PhD, RN, FNP, frustration still exists for patients, families, and health care providers regarding the lack of consensus that routine bathing is good for patients with atopic dermatitis.
During the Revolutionizing Atopic Dermatitis symposium, she said that conflicting and vague guidelines currently exist on the topic.
“This stems from the fact that we just don’t have good studies,” said Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado at Denver, Aurora. “Particularly, we don’t have randomized, controlled trials on the effects of water and bathing. It’s not just parents that are frustrated, but health care providers are as well.”
In an observational analysis, researchers evaluated results from three online surveys of dermatologists, allergists, and immunologists, and primary care physicians regarding routine bathing frequency recommendations for children with AD. It found that PCPs recommended daily bathing less than 50% of the time, while specialists recommended daily bathing more than 50% of the time.
“It seems like the PCPs have embraced that old dermatology notion when bathing was avoided in patients with AD,” Dr. Nicol said. “This lack of consensus on the basic daily care steps in AD management causes a great deal of confusion amongst patients, families, and young health care providers, in particular,” she added.
She believes that this goes back to a century-long debate about the pros and cons of bathing in AD. “We used to say that bathing will dry the skin out if you take a bath or a shower without immediately applying something like a good moisturizer. That’s where the 3-minute rule came along from the National Eczema Association, meaning that bathing hydrates the stratum corneum if you take a bath or a shower and you immediately apply that good moisturizer within 3 minutes to retain that hydration and keep the barrier intact and flexible.”
Dr. Nicol presented a stepwise management model that she has published many times over the years (see Pediatr Nursing 2020;46[2]:92-8 and J Allergy Clin Immunol Pract 2019;7[1]:1-16).
Step 1 consists of basic care, including skin hydration/bathing, application of a daily moisturizer, avoiding irritants, and identifying and addressing specific triggers. “This is the foundation for every step as you go forward,” she explained. Soak and seal has been a mainstay of treatment at National Jewish Health, she noted. “By that, I mean taking a soaking 10-15 minute bath in warm water daily. Gently pat away excess water. Immediately apply skin medications or moisturizer within 3 minutes. Using a gentle fragrance-free, dye-free cleanser to clean skin is also important. Avoid scrubbing.”
A review article on bathing and associated treatments in AD was published in 2017 and includes 144 references to bathing studies. A separate recommendation known as the “AD Yardstick” published by Dr. Nicol’s colleague at National Jewish Health, Mark Boguniewicz, MD, and coauthors, elaborated on the definition of basic skin care for nonlesional AD. Besides recommending the liberal and frequent application of moisturizers, it suggests management with warm baths or showers using nonsoap cleansers, usually once per day, followed by application of a moisturizer, even on clear areas.
“This is now what people are thinking as the basis of skin care in patients with AD,” Dr. Nicol said. “Warm baths and showers don’t look so controversial anymore. This model nicely lays out what we want people to remember. In the past, many times we just skipped that important step of telling people about bathing.”
In a small 2009 study, researchers conducted a quantitative assessment of combination bathing and moisturizing regimens on skin hydration in AD. They found that bathing followed by application of a moisturizer provides modest hydration benefits, though less than that of simply applying moisturizer alone. “That has not been the case for most of us who are bathing advocates,” Dr. Nicol said. “We believe that there is an additional hydration that’s gained from bathing and moisturizers done properly.”
In an earlier retrospective study of 28 patients referred to a tertiary care center for refractory chronic pruritic eruptions, researchers found that a plain-water 20-minute soak followed by smearing of midstrength corticosteroid ointment led to clearing or dramatic improvement of the lesions (Arch Dermatol 2005;14:1556-9). The authors recommended prospective studies to confirm the findings.
In a separate review of medical literature, researchers explored the role of frequent bathing in the treatment of pediatric AD (Ann Allergy Asthma Immunol 2016;117[1]:9-13). They found that the weight of evidence suggests that the frequent soak and smear bathing is preferred to infrequent bathing in the management of AD. Frequent bathing was defined as bathing at least once a day, while infrequent bathing was defined as bathing less than once a day.
“Bleach baths have received much attention in recent years, and have been endorsed by multiple AD guidelines, though not to the same degree as regular bathing,” Dr. Nicol said. “Right now, you can find almost as much literature for this practice as against it. The populations that seem to value from beach baths the most, however, are those with frequent infections, particularly those who are methicillin resistant. Most people recommend a maximum of two to three times per week but only with an active infection. Care must be taken to avoid additional drying or irritation of the skin from bleach.”
Many bleach bath recipes call for adding one-eighth to one-half of a cup of bleach to a tub full or water.
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly.
FROM REVOLUTIONIZING AD 2020
Erythema, Blisters, and Scars on the Elbows, Knees, and Legs
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1
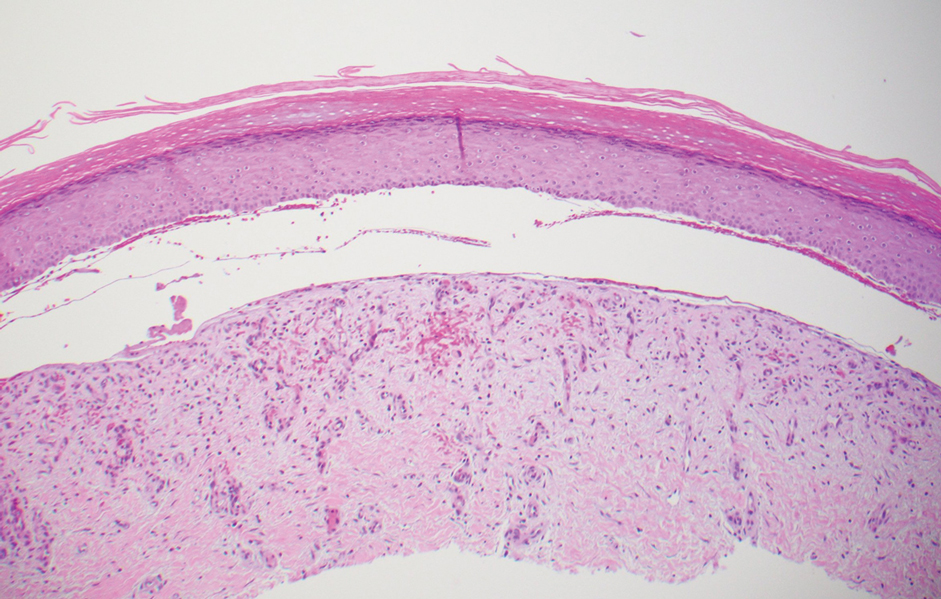
Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1



Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1



Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
A 69-year-old man presented with an asymptomatic rash on the extensor surfaces of 2 years' duration. He reported recurrent blisters that would then scar over. The lesions did not occur in relation to any known trauma. The patient's medical history revealed dialysis-dependent end-stage renal disease secondary to type 2 diabetes mellitus. His medications were noncontributory, and there was no family history of blistering disorders. He had tried triamcinolone cream without any improvement. Physical examination was remarkable for erythematous blisters and bullae with scales and milia on the elbows, knees, and lower legs. The oral mucosa was unremarkable. Shave biopsies of the skin for direct immunofluorescence and salt-split skin studies were obtained.
Hospitalist movers and shakers: January 2021
Daniel Steinberg, MD, SFHM, recently was among 10 medical educators across the county to receive the Accreditation Council for Graduate Medical Education 2021 Parker J. Palmer Courage to Teach Award. Considered the most prestigious award given to graduate medical education program directors, it “recognizes program directors who have fostered innovation and improvement in their residency/fellowship program and served as exemplary role models for residents and fellows.”
Dr. Steinberg was program director for internal medicine residency at Mount Sinai Beth Israel, New York, for 11 years (2009-20) before becoming associate dean for quality and patient safety in graduate medical education in September. He is a professor of medicine and medical education at Icahn School of Medicine at Mount Sinai, New York.
Dr. Steinberg also is a leader within SHM, serving on the education, physicians-in-training, and annual conference committees. He is the course director for SHM Converge 2021.
Ann Sheehy, MD, SFHM, was honored in a virtual ceremony in December 2020 by the University of Wisconsin celebrating Physician Excellence Award winners. She was presented with the Physician Excellence Leadership Award.
Dr. Sheehy is division chief of the division of hospital medicine at the University of Wisconsin–Madison, and chair of the SHM Public Policy Committee.
Donald Schmidt, MD, has been named chief medical officer and vice president of medical affairs at Madonna Rehabilitation Hospitals in Omaha and Lincoln, Neb. He will replace Thomas Stalder, MD, who is retiring. Dr. Schmidt brings 20 years of experience to Madonna Rehabilitation Hospitals, including his most recent post as a hospitalist and medical director of the hospitalist program at Catholic Health Initiatives Health St. Elizabeth (Lincoln, Neb.).
Dr. Schmidt currently serves on the board of directors for OneHealth Nebraska, an independent physicians association.
Ezinne Nwude, MD, recently was presented with the SCP Health Excellence in Leadership Award during the organization’s Medical Leadership Conference. Dr. Nwude is chief of staff and hospitalist at the Medical Center of South Arkansas, El Dorado.
SCP Health coordinates staffing for more than 7,500 providers covering 30 states and is one of the nation’s largest clinical practice management companies. More than 420 medical leaders nationwide were eligible for the award. Dr. Nwude has focused on positive culture and health education since her start at MSCA in 2014. She has been chief of staff since October 2018.
RWJ Barnabas Health (West Orange, N.J.) recently named two new health system leaders from among its hospital medicine ranks, as Christopher Freer, MD, was tabbed as senior vice president for emergency and hospital medicine, and Maninder “Dolly” Abraham, MD, was picked as chief of hospital medicine. The moves were made as RWJBH takes over as the direct employer for Envision Physician Services in Nashville, Tenn.
Dr. Freer was elevated to his new role after spending the past 5 years as RWJBH’s system director for emergency services. He has nearly 3 decades of experience in hospital medicine.
Dr. Abraham comes to his new position after directing the hospitalist program at Saint Barnabas and serving as regional medical director with Envision.
Newman Regional Health (Emporia, Kan.) recently established a partnership with FreeState Healthcare (Wichita, Kan.). FreeState will be responsible for providing hospitalist services to adult inpatients and observation patients at Newman Regional Health during overnights.
Daniel Steinberg, MD, SFHM, recently was among 10 medical educators across the county to receive the Accreditation Council for Graduate Medical Education 2021 Parker J. Palmer Courage to Teach Award. Considered the most prestigious award given to graduate medical education program directors, it “recognizes program directors who have fostered innovation and improvement in their residency/fellowship program and served as exemplary role models for residents and fellows.”
Dr. Steinberg was program director for internal medicine residency at Mount Sinai Beth Israel, New York, for 11 years (2009-20) before becoming associate dean for quality and patient safety in graduate medical education in September. He is a professor of medicine and medical education at Icahn School of Medicine at Mount Sinai, New York.
Dr. Steinberg also is a leader within SHM, serving on the education, physicians-in-training, and annual conference committees. He is the course director for SHM Converge 2021.
Ann Sheehy, MD, SFHM, was honored in a virtual ceremony in December 2020 by the University of Wisconsin celebrating Physician Excellence Award winners. She was presented with the Physician Excellence Leadership Award.
Dr. Sheehy is division chief of the division of hospital medicine at the University of Wisconsin–Madison, and chair of the SHM Public Policy Committee.
Donald Schmidt, MD, has been named chief medical officer and vice president of medical affairs at Madonna Rehabilitation Hospitals in Omaha and Lincoln, Neb. He will replace Thomas Stalder, MD, who is retiring. Dr. Schmidt brings 20 years of experience to Madonna Rehabilitation Hospitals, including his most recent post as a hospitalist and medical director of the hospitalist program at Catholic Health Initiatives Health St. Elizabeth (Lincoln, Neb.).
Dr. Schmidt currently serves on the board of directors for OneHealth Nebraska, an independent physicians association.
Ezinne Nwude, MD, recently was presented with the SCP Health Excellence in Leadership Award during the organization’s Medical Leadership Conference. Dr. Nwude is chief of staff and hospitalist at the Medical Center of South Arkansas, El Dorado.
SCP Health coordinates staffing for more than 7,500 providers covering 30 states and is one of the nation’s largest clinical practice management companies. More than 420 medical leaders nationwide were eligible for the award. Dr. Nwude has focused on positive culture and health education since her start at MSCA in 2014. She has been chief of staff since October 2018.
RWJ Barnabas Health (West Orange, N.J.) recently named two new health system leaders from among its hospital medicine ranks, as Christopher Freer, MD, was tabbed as senior vice president for emergency and hospital medicine, and Maninder “Dolly” Abraham, MD, was picked as chief of hospital medicine. The moves were made as RWJBH takes over as the direct employer for Envision Physician Services in Nashville, Tenn.
Dr. Freer was elevated to his new role after spending the past 5 years as RWJBH’s system director for emergency services. He has nearly 3 decades of experience in hospital medicine.
Dr. Abraham comes to his new position after directing the hospitalist program at Saint Barnabas and serving as regional medical director with Envision.
Newman Regional Health (Emporia, Kan.) recently established a partnership with FreeState Healthcare (Wichita, Kan.). FreeState will be responsible for providing hospitalist services to adult inpatients and observation patients at Newman Regional Health during overnights.
Daniel Steinberg, MD, SFHM, recently was among 10 medical educators across the county to receive the Accreditation Council for Graduate Medical Education 2021 Parker J. Palmer Courage to Teach Award. Considered the most prestigious award given to graduate medical education program directors, it “recognizes program directors who have fostered innovation and improvement in their residency/fellowship program and served as exemplary role models for residents and fellows.”
Dr. Steinberg was program director for internal medicine residency at Mount Sinai Beth Israel, New York, for 11 years (2009-20) before becoming associate dean for quality and patient safety in graduate medical education in September. He is a professor of medicine and medical education at Icahn School of Medicine at Mount Sinai, New York.
Dr. Steinberg also is a leader within SHM, serving on the education, physicians-in-training, and annual conference committees. He is the course director for SHM Converge 2021.
Ann Sheehy, MD, SFHM, was honored in a virtual ceremony in December 2020 by the University of Wisconsin celebrating Physician Excellence Award winners. She was presented with the Physician Excellence Leadership Award.
Dr. Sheehy is division chief of the division of hospital medicine at the University of Wisconsin–Madison, and chair of the SHM Public Policy Committee.
Donald Schmidt, MD, has been named chief medical officer and vice president of medical affairs at Madonna Rehabilitation Hospitals in Omaha and Lincoln, Neb. He will replace Thomas Stalder, MD, who is retiring. Dr. Schmidt brings 20 years of experience to Madonna Rehabilitation Hospitals, including his most recent post as a hospitalist and medical director of the hospitalist program at Catholic Health Initiatives Health St. Elizabeth (Lincoln, Neb.).
Dr. Schmidt currently serves on the board of directors for OneHealth Nebraska, an independent physicians association.
Ezinne Nwude, MD, recently was presented with the SCP Health Excellence in Leadership Award during the organization’s Medical Leadership Conference. Dr. Nwude is chief of staff and hospitalist at the Medical Center of South Arkansas, El Dorado.
SCP Health coordinates staffing for more than 7,500 providers covering 30 states and is one of the nation’s largest clinical practice management companies. More than 420 medical leaders nationwide were eligible for the award. Dr. Nwude has focused on positive culture and health education since her start at MSCA in 2014. She has been chief of staff since October 2018.
RWJ Barnabas Health (West Orange, N.J.) recently named two new health system leaders from among its hospital medicine ranks, as Christopher Freer, MD, was tabbed as senior vice president for emergency and hospital medicine, and Maninder “Dolly” Abraham, MD, was picked as chief of hospital medicine. The moves were made as RWJBH takes over as the direct employer for Envision Physician Services in Nashville, Tenn.
Dr. Freer was elevated to his new role after spending the past 5 years as RWJBH’s system director for emergency services. He has nearly 3 decades of experience in hospital medicine.
Dr. Abraham comes to his new position after directing the hospitalist program at Saint Barnabas and serving as regional medical director with Envision.
Newman Regional Health (Emporia, Kan.) recently established a partnership with FreeState Healthcare (Wichita, Kan.). FreeState will be responsible for providing hospitalist services to adult inpatients and observation patients at Newman Regional Health during overnights.
Oral JAK1 inhibitor shows promise for hidradenitis suppurativa
A (HS) in a pair of small, randomized, phase 2 studies that established proof-of-concept for the novel agent, Afsaneh Alavi, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These favorable clinical findings were buttressed by a proteomic analysis demonstrating dose-dependent reductions in circulating inflammatory mediators, added Dr. Alavi, a dermatologist at the Mayo Clinic in Rochester, Minn.
The investigational oral small molecule, known for now as INCB54707, is 52 times more selective for JAK1 than JAK2.
Both multicenter studies entailed 8 weeks of active treatment with INCB54707 followed by a 4-week safety observation. In one study, 10 patients received 15 mg of the investigational agent once daily in open-label fashion. The other trial randomized 35 patients to the JAK1 inhibitor at 30 mg, 60 mg, or 90 mg per day or placebo. About 70% of participants in the studies had Hurley stage II HS; the rest were stage III.
Safety and tolerability were the primary outcomes in the two studies. One patient in the open-label study dropped out because of a flare of fibromyalgia. In the larger randomized trial, four patients – all in the group assigned to 90 mg/day of the JAK1 inhibitor – developed thrombocytopenia, resulting in temporary discontinuation of treatment for up to 2 weeks. In all four instances, the laboratory abnormality was reversed after temporary interruption of treatment, with no sequelae upon restarting the drug. There were no serious treatment-emergent adverse events in either study.
In the low-dose, open-label study, four of nine completers (44%) experienced a Hidradenitis Suppurativa Clinical Response (HiSCR) at week 8, defined as at least a 50% reduction in inflammatory lesion count with no increase in abscesses or draining fistulae compared to baseline. In the randomized trial, the week-8 HiSCR rate was 57% in placebo-treated controls, 56% in those on 30 mg/day or 60 mg/day of the JAK1 inhibitor, and significantly better at 88% in the group on 90 mg/day.
The rapidity of response to the JAK1 inhibitor was noteworthy. After just 1 week of treatment, an abscess and inflammatory nodule count of zero to two lesions was present in 22% of patients on INCB54707 at 60 mg/day and 29% of those on 90 mg/day, compared with none of the patients on 30 mg/day or placebo. At week 2, an abscess and nodule count of 0-2 was documented in 33% of participants on the JAK1 inhibitor at 30 mg/day, 58% at 60 mg/day, and 50% with 90 mg/day. At week 8, the rates were 57% with placebo, 44% with active treatment at 30 or 60 mg/day, and 63% in patients on 90 mg/day.
A dose-dependent significant improvement in Hidradenitis Suppurativa Quality of Life scores was documented in response to the JAK1 inhibitor.
There is an unmet need for effective therapies for HS, a chronic, extremely painful inflammatory condition with a large negative impact on quality of life. At present, the only Food and Drug Administration–approved medication for HS is the tumor necrosis factor inhibitor, adalimumab (Humira), noted Dr. Alavi. Ongoing studies are evaluating other JAK inhibitors, as well as TNF inhibitors and interleukin-17 and -23 blockers.
She reported receiving research funding from and serving as a consultant to Incyte, the studies’ sponsor, and more than a dozen other pharmaceutical companies.
A (HS) in a pair of small, randomized, phase 2 studies that established proof-of-concept for the novel agent, Afsaneh Alavi, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These favorable clinical findings were buttressed by a proteomic analysis demonstrating dose-dependent reductions in circulating inflammatory mediators, added Dr. Alavi, a dermatologist at the Mayo Clinic in Rochester, Minn.
The investigational oral small molecule, known for now as INCB54707, is 52 times more selective for JAK1 than JAK2.
Both multicenter studies entailed 8 weeks of active treatment with INCB54707 followed by a 4-week safety observation. In one study, 10 patients received 15 mg of the investigational agent once daily in open-label fashion. The other trial randomized 35 patients to the JAK1 inhibitor at 30 mg, 60 mg, or 90 mg per day or placebo. About 70% of participants in the studies had Hurley stage II HS; the rest were stage III.
Safety and tolerability were the primary outcomes in the two studies. One patient in the open-label study dropped out because of a flare of fibromyalgia. In the larger randomized trial, four patients – all in the group assigned to 90 mg/day of the JAK1 inhibitor – developed thrombocytopenia, resulting in temporary discontinuation of treatment for up to 2 weeks. In all four instances, the laboratory abnormality was reversed after temporary interruption of treatment, with no sequelae upon restarting the drug. There were no serious treatment-emergent adverse events in either study.
In the low-dose, open-label study, four of nine completers (44%) experienced a Hidradenitis Suppurativa Clinical Response (HiSCR) at week 8, defined as at least a 50% reduction in inflammatory lesion count with no increase in abscesses or draining fistulae compared to baseline. In the randomized trial, the week-8 HiSCR rate was 57% in placebo-treated controls, 56% in those on 30 mg/day or 60 mg/day of the JAK1 inhibitor, and significantly better at 88% in the group on 90 mg/day.
The rapidity of response to the JAK1 inhibitor was noteworthy. After just 1 week of treatment, an abscess and inflammatory nodule count of zero to two lesions was present in 22% of patients on INCB54707 at 60 mg/day and 29% of those on 90 mg/day, compared with none of the patients on 30 mg/day or placebo. At week 2, an abscess and nodule count of 0-2 was documented in 33% of participants on the JAK1 inhibitor at 30 mg/day, 58% at 60 mg/day, and 50% with 90 mg/day. At week 8, the rates were 57% with placebo, 44% with active treatment at 30 or 60 mg/day, and 63% in patients on 90 mg/day.
A dose-dependent significant improvement in Hidradenitis Suppurativa Quality of Life scores was documented in response to the JAK1 inhibitor.
There is an unmet need for effective therapies for HS, a chronic, extremely painful inflammatory condition with a large negative impact on quality of life. At present, the only Food and Drug Administration–approved medication for HS is the tumor necrosis factor inhibitor, adalimumab (Humira), noted Dr. Alavi. Ongoing studies are evaluating other JAK inhibitors, as well as TNF inhibitors and interleukin-17 and -23 blockers.
She reported receiving research funding from and serving as a consultant to Incyte, the studies’ sponsor, and more than a dozen other pharmaceutical companies.
A (HS) in a pair of small, randomized, phase 2 studies that established proof-of-concept for the novel agent, Afsaneh Alavi, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These favorable clinical findings were buttressed by a proteomic analysis demonstrating dose-dependent reductions in circulating inflammatory mediators, added Dr. Alavi, a dermatologist at the Mayo Clinic in Rochester, Minn.
The investigational oral small molecule, known for now as INCB54707, is 52 times more selective for JAK1 than JAK2.
Both multicenter studies entailed 8 weeks of active treatment with INCB54707 followed by a 4-week safety observation. In one study, 10 patients received 15 mg of the investigational agent once daily in open-label fashion. The other trial randomized 35 patients to the JAK1 inhibitor at 30 mg, 60 mg, or 90 mg per day or placebo. About 70% of participants in the studies had Hurley stage II HS; the rest were stage III.
Safety and tolerability were the primary outcomes in the two studies. One patient in the open-label study dropped out because of a flare of fibromyalgia. In the larger randomized trial, four patients – all in the group assigned to 90 mg/day of the JAK1 inhibitor – developed thrombocytopenia, resulting in temporary discontinuation of treatment for up to 2 weeks. In all four instances, the laboratory abnormality was reversed after temporary interruption of treatment, with no sequelae upon restarting the drug. There were no serious treatment-emergent adverse events in either study.
In the low-dose, open-label study, four of nine completers (44%) experienced a Hidradenitis Suppurativa Clinical Response (HiSCR) at week 8, defined as at least a 50% reduction in inflammatory lesion count with no increase in abscesses or draining fistulae compared to baseline. In the randomized trial, the week-8 HiSCR rate was 57% in placebo-treated controls, 56% in those on 30 mg/day or 60 mg/day of the JAK1 inhibitor, and significantly better at 88% in the group on 90 mg/day.
The rapidity of response to the JAK1 inhibitor was noteworthy. After just 1 week of treatment, an abscess and inflammatory nodule count of zero to two lesions was present in 22% of patients on INCB54707 at 60 mg/day and 29% of those on 90 mg/day, compared with none of the patients on 30 mg/day or placebo. At week 2, an abscess and nodule count of 0-2 was documented in 33% of participants on the JAK1 inhibitor at 30 mg/day, 58% at 60 mg/day, and 50% with 90 mg/day. At week 8, the rates were 57% with placebo, 44% with active treatment at 30 or 60 mg/day, and 63% in patients on 90 mg/day.
A dose-dependent significant improvement in Hidradenitis Suppurativa Quality of Life scores was documented in response to the JAK1 inhibitor.
There is an unmet need for effective therapies for HS, a chronic, extremely painful inflammatory condition with a large negative impact on quality of life. At present, the only Food and Drug Administration–approved medication for HS is the tumor necrosis factor inhibitor, adalimumab (Humira), noted Dr. Alavi. Ongoing studies are evaluating other JAK inhibitors, as well as TNF inhibitors and interleukin-17 and -23 blockers.
She reported receiving research funding from and serving as a consultant to Incyte, the studies’ sponsor, and more than a dozen other pharmaceutical companies.
FROM THE EADV CONGRESS