User login
Recent psoriasis pathophysiology insights carry treatment implications
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
CAR-T in children branching out to solid tumors
Although the only pediatric indication for chimeric antigen receptor T-cell therapy currently approved by the Food and Drug Administration is B-lineage acute lymphoblastic leukemia (ALL) that is refractory to at least two frontline induction attempts or is in second or later relapse, clinical trials of CAR-T therapy for pediatric solid tumors are also currently in progress, said Gregory Yanik, MD, from the CS Mott Children’s Hospital at the University of Michigan, Ann Arbor, at the Transplant & Cellular Therapies Meetings.
In his presentation, Dr. Yanik discussed progress in solid tumor studies as well as some issues involving the current use of CAR-T therapy for ALL.
Solid tumor studies
Malignancies such as sarcomas, brain tumors, and neuroblastomas pose unique challenges, “In contrast to hematologic malignancies, the protein we’re targeting may not be present on the cell surface of all the tumor cells. There are lower-expression profiles, and this is a problem. In fact, many people have postulated that with CAR-T for pediatric solid tumors we’ll have to do repeated cycles, almost like we do with chemotherapy,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
There are currently 14 studies of CAR-T for central nervous system tumors in children, targeting either epidermal growth factor receptor (EGFR) in glioblastoma multiforme and high-grade gliomas, HER2 in a variety of CNS tumors, the GD2 antigen on pontine gliomas, and the checkpoint molecular B7H3 in medulloblastomas and pontine gliomas.
“In sarcomas in kids there are currently 12 trials in progress. Most of the targeting epitopes are targeting either HER2 or GD2. Repetitive CAR-T infusions are being used in several of these trials in sarcomas.
For neuroblastomas there are currently 13 studies in progress, nearly all of which target GD2. Some of the trials include combining CAR-T with immune checkpoint inhibitors or C7R, an engineered cytokine driver designed to prevent T-cell exhaustion.
In addition, several trials of tumor pulsed dendritic cell vaccines are underway for treatment of children with Wilms tumor, Dr. Yanik noted.
Unresolved procedural questions
It’s still early days in CAR-T therapy, and there are several still unanswered questions regarding optimal therapy for and management of patients undergoing CAR-T procedures, Dr. Yanik said.
For example, the optimal time to collect T cells during apheresis is still unclear, he said. Collecting prior to reinduction therapy raises the risk of transducing leukemic cells, while collecting after reinduction may result in inadequate quantity or quality of cells. Regardless of when cells are collected, apheresis should be performed only when the absolute lymphocyte count is above 500/mcL or the CD3 count is above 150/mcL at the time of apheresis.
In the case tisagenlecleucel (Kymriah), his center typically collects 1x109 CD3 cells regardless of age or weight.
The number of CAR T-cells infused also appears to matter, as responses are improved at CAR-T doses above 1.5x106/kg, while risk for higher-grade cytokine release syndrome (CRS) occurs at higher infusion doses.
Blinatumomab or inotuzumab?
Along with CAR-T, two other agents, the bispecific T-cell engager blinatumomab (Blincyto) and the antibody conjugate inotuzumab ozogamicin (Besponsa) are also approved for the treatment of patients with relapsed/refractory B-cell ALL.
Like CAR-T therapy, the primary toxicities associated with blinatumomab are CRS and neurologic adverse events, whereas at inotuzumab is largely associated with hematologic and hepatic toxicities.
The logistics of therapy differ widely, with a 28-day infusion required for blinatumomab, compared with weekly dosing of inotuzumab, and the multiple visits for apheresis and infusion required for CAR-T.
Blinatumomab is approved for both children and adults with relapsed/refractory ALL, but inotuzumab is approved only for adults, and CAR-T with tisagenlecleucel is approved only for children in this indication.
CD-19 expression
There is evidence to suggest that CD19 expression prior to CAR-T has an effect on outcomes, Dr. Yanik said.
“Does blinatumomab pre–CAR-T impact outcome? The answer is probably yes,” he said.
He referred to a study by investigators at the Children’s Hospital of Philadelphia showing that, “if you’re giving blinatumomab prior to CAR-T therapy, you’re potentially reducing the cell-surface expression of CD19 on your leukemic blasts, and now while you’re bringing these patients in for CAR-T therapy, you’re getting a much higher population of dim CD19 expressers, and this is associated with a higher relapse rate and lower remission rate.”
Predicting relapse
Dr. Yanik referred to a study, currently unpublished, which will show that next-generation sequencing (NGS) is more sensitive than flow cytometry for detection of minimal residual disease (MRD), and that MRD analysis of marrow was more sensitive than analysis of peripheral blood.
“Poor outcomes were seen post CAR-T for patients who were in morphologic remission on day 28 or day 100, but had positive MRD. This especially held true if it was next-gen sequencing MRD-positive at day 100, for which relapse rates were over 95%,” he said.
The absence of B-cells is a surrogate marker for the persistence of CAR-T, and conversely, the recovery of CD19-positive B cells may be a predictor for relapse, especially if the B-cell recovery occurs within the first 6 months following CAR-T infusion.
Transplant after CAR-T?
Bone marrow transplant after CAR-T is recommend for patients with high risk of relapse, including those with B-cell recovery within the first 6 months after CAR-T, patients with MRD positivity at days 28 or 100, and patients with mixed lineage leukemia.
“Should we transplant good-risk patients, meaning, if you have NGS-MRD negative patients, is there a role for transplant? You have to look at the risk versus benefit there. These patients may have a cure rate that’s in the 80%-plus range, could we potentially optimize that even more if we consolidate them with an allo[geneic] transplant,” Dr. Yank said.
Move CAR-T up front?
A Children’s Oncology Group study is currently examining whether giving CAR-T therapy to patients with MRD of 0.01% or greater following first consolidation could result in lower tumor burden, fewer relapse, and less CRS with CAR-T.
Dr. Yanik reported that he had no conflicts of interest to disclose.
Although the only pediatric indication for chimeric antigen receptor T-cell therapy currently approved by the Food and Drug Administration is B-lineage acute lymphoblastic leukemia (ALL) that is refractory to at least two frontline induction attempts or is in second or later relapse, clinical trials of CAR-T therapy for pediatric solid tumors are also currently in progress, said Gregory Yanik, MD, from the CS Mott Children’s Hospital at the University of Michigan, Ann Arbor, at the Transplant & Cellular Therapies Meetings.
In his presentation, Dr. Yanik discussed progress in solid tumor studies as well as some issues involving the current use of CAR-T therapy for ALL.
Solid tumor studies
Malignancies such as sarcomas, brain tumors, and neuroblastomas pose unique challenges, “In contrast to hematologic malignancies, the protein we’re targeting may not be present on the cell surface of all the tumor cells. There are lower-expression profiles, and this is a problem. In fact, many people have postulated that with CAR-T for pediatric solid tumors we’ll have to do repeated cycles, almost like we do with chemotherapy,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
There are currently 14 studies of CAR-T for central nervous system tumors in children, targeting either epidermal growth factor receptor (EGFR) in glioblastoma multiforme and high-grade gliomas, HER2 in a variety of CNS tumors, the GD2 antigen on pontine gliomas, and the checkpoint molecular B7H3 in medulloblastomas and pontine gliomas.
“In sarcomas in kids there are currently 12 trials in progress. Most of the targeting epitopes are targeting either HER2 or GD2. Repetitive CAR-T infusions are being used in several of these trials in sarcomas.
For neuroblastomas there are currently 13 studies in progress, nearly all of which target GD2. Some of the trials include combining CAR-T with immune checkpoint inhibitors or C7R, an engineered cytokine driver designed to prevent T-cell exhaustion.
In addition, several trials of tumor pulsed dendritic cell vaccines are underway for treatment of children with Wilms tumor, Dr. Yanik noted.
Unresolved procedural questions
It’s still early days in CAR-T therapy, and there are several still unanswered questions regarding optimal therapy for and management of patients undergoing CAR-T procedures, Dr. Yanik said.
For example, the optimal time to collect T cells during apheresis is still unclear, he said. Collecting prior to reinduction therapy raises the risk of transducing leukemic cells, while collecting after reinduction may result in inadequate quantity or quality of cells. Regardless of when cells are collected, apheresis should be performed only when the absolute lymphocyte count is above 500/mcL or the CD3 count is above 150/mcL at the time of apheresis.
In the case tisagenlecleucel (Kymriah), his center typically collects 1x109 CD3 cells regardless of age or weight.
The number of CAR T-cells infused also appears to matter, as responses are improved at CAR-T doses above 1.5x106/kg, while risk for higher-grade cytokine release syndrome (CRS) occurs at higher infusion doses.
Blinatumomab or inotuzumab?
Along with CAR-T, two other agents, the bispecific T-cell engager blinatumomab (Blincyto) and the antibody conjugate inotuzumab ozogamicin (Besponsa) are also approved for the treatment of patients with relapsed/refractory B-cell ALL.
Like CAR-T therapy, the primary toxicities associated with blinatumomab are CRS and neurologic adverse events, whereas at inotuzumab is largely associated with hematologic and hepatic toxicities.
The logistics of therapy differ widely, with a 28-day infusion required for blinatumomab, compared with weekly dosing of inotuzumab, and the multiple visits for apheresis and infusion required for CAR-T.
Blinatumomab is approved for both children and adults with relapsed/refractory ALL, but inotuzumab is approved only for adults, and CAR-T with tisagenlecleucel is approved only for children in this indication.
CD-19 expression
There is evidence to suggest that CD19 expression prior to CAR-T has an effect on outcomes, Dr. Yanik said.
“Does blinatumomab pre–CAR-T impact outcome? The answer is probably yes,” he said.
He referred to a study by investigators at the Children’s Hospital of Philadelphia showing that, “if you’re giving blinatumomab prior to CAR-T therapy, you’re potentially reducing the cell-surface expression of CD19 on your leukemic blasts, and now while you’re bringing these patients in for CAR-T therapy, you’re getting a much higher population of dim CD19 expressers, and this is associated with a higher relapse rate and lower remission rate.”
Predicting relapse
Dr. Yanik referred to a study, currently unpublished, which will show that next-generation sequencing (NGS) is more sensitive than flow cytometry for detection of minimal residual disease (MRD), and that MRD analysis of marrow was more sensitive than analysis of peripheral blood.
“Poor outcomes were seen post CAR-T for patients who were in morphologic remission on day 28 or day 100, but had positive MRD. This especially held true if it was next-gen sequencing MRD-positive at day 100, for which relapse rates were over 95%,” he said.
The absence of B-cells is a surrogate marker for the persistence of CAR-T, and conversely, the recovery of CD19-positive B cells may be a predictor for relapse, especially if the B-cell recovery occurs within the first 6 months following CAR-T infusion.
Transplant after CAR-T?
Bone marrow transplant after CAR-T is recommend for patients with high risk of relapse, including those with B-cell recovery within the first 6 months after CAR-T, patients with MRD positivity at days 28 or 100, and patients with mixed lineage leukemia.
“Should we transplant good-risk patients, meaning, if you have NGS-MRD negative patients, is there a role for transplant? You have to look at the risk versus benefit there. These patients may have a cure rate that’s in the 80%-plus range, could we potentially optimize that even more if we consolidate them with an allo[geneic] transplant,” Dr. Yank said.
Move CAR-T up front?
A Children’s Oncology Group study is currently examining whether giving CAR-T therapy to patients with MRD of 0.01% or greater following first consolidation could result in lower tumor burden, fewer relapse, and less CRS with CAR-T.
Dr. Yanik reported that he had no conflicts of interest to disclose.
Although the only pediatric indication for chimeric antigen receptor T-cell therapy currently approved by the Food and Drug Administration is B-lineage acute lymphoblastic leukemia (ALL) that is refractory to at least two frontline induction attempts or is in second or later relapse, clinical trials of CAR-T therapy for pediatric solid tumors are also currently in progress, said Gregory Yanik, MD, from the CS Mott Children’s Hospital at the University of Michigan, Ann Arbor, at the Transplant & Cellular Therapies Meetings.
In his presentation, Dr. Yanik discussed progress in solid tumor studies as well as some issues involving the current use of CAR-T therapy for ALL.
Solid tumor studies
Malignancies such as sarcomas, brain tumors, and neuroblastomas pose unique challenges, “In contrast to hematologic malignancies, the protein we’re targeting may not be present on the cell surface of all the tumor cells. There are lower-expression profiles, and this is a problem. In fact, many people have postulated that with CAR-T for pediatric solid tumors we’ll have to do repeated cycles, almost like we do with chemotherapy,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
There are currently 14 studies of CAR-T for central nervous system tumors in children, targeting either epidermal growth factor receptor (EGFR) in glioblastoma multiforme and high-grade gliomas, HER2 in a variety of CNS tumors, the GD2 antigen on pontine gliomas, and the checkpoint molecular B7H3 in medulloblastomas and pontine gliomas.
“In sarcomas in kids there are currently 12 trials in progress. Most of the targeting epitopes are targeting either HER2 or GD2. Repetitive CAR-T infusions are being used in several of these trials in sarcomas.
For neuroblastomas there are currently 13 studies in progress, nearly all of which target GD2. Some of the trials include combining CAR-T with immune checkpoint inhibitors or C7R, an engineered cytokine driver designed to prevent T-cell exhaustion.
In addition, several trials of tumor pulsed dendritic cell vaccines are underway for treatment of children with Wilms tumor, Dr. Yanik noted.
Unresolved procedural questions
It’s still early days in CAR-T therapy, and there are several still unanswered questions regarding optimal therapy for and management of patients undergoing CAR-T procedures, Dr. Yanik said.
For example, the optimal time to collect T cells during apheresis is still unclear, he said. Collecting prior to reinduction therapy raises the risk of transducing leukemic cells, while collecting after reinduction may result in inadequate quantity or quality of cells. Regardless of when cells are collected, apheresis should be performed only when the absolute lymphocyte count is above 500/mcL or the CD3 count is above 150/mcL at the time of apheresis.
In the case tisagenlecleucel (Kymriah), his center typically collects 1x109 CD3 cells regardless of age or weight.
The number of CAR T-cells infused also appears to matter, as responses are improved at CAR-T doses above 1.5x106/kg, while risk for higher-grade cytokine release syndrome (CRS) occurs at higher infusion doses.
Blinatumomab or inotuzumab?
Along with CAR-T, two other agents, the bispecific T-cell engager blinatumomab (Blincyto) and the antibody conjugate inotuzumab ozogamicin (Besponsa) are also approved for the treatment of patients with relapsed/refractory B-cell ALL.
Like CAR-T therapy, the primary toxicities associated with blinatumomab are CRS and neurologic adverse events, whereas at inotuzumab is largely associated with hematologic and hepatic toxicities.
The logistics of therapy differ widely, with a 28-day infusion required for blinatumomab, compared with weekly dosing of inotuzumab, and the multiple visits for apheresis and infusion required for CAR-T.
Blinatumomab is approved for both children and adults with relapsed/refractory ALL, but inotuzumab is approved only for adults, and CAR-T with tisagenlecleucel is approved only for children in this indication.
CD-19 expression
There is evidence to suggest that CD19 expression prior to CAR-T has an effect on outcomes, Dr. Yanik said.
“Does blinatumomab pre–CAR-T impact outcome? The answer is probably yes,” he said.
He referred to a study by investigators at the Children’s Hospital of Philadelphia showing that, “if you’re giving blinatumomab prior to CAR-T therapy, you’re potentially reducing the cell-surface expression of CD19 on your leukemic blasts, and now while you’re bringing these patients in for CAR-T therapy, you’re getting a much higher population of dim CD19 expressers, and this is associated with a higher relapse rate and lower remission rate.”
Predicting relapse
Dr. Yanik referred to a study, currently unpublished, which will show that next-generation sequencing (NGS) is more sensitive than flow cytometry for detection of minimal residual disease (MRD), and that MRD analysis of marrow was more sensitive than analysis of peripheral blood.
“Poor outcomes were seen post CAR-T for patients who were in morphologic remission on day 28 or day 100, but had positive MRD. This especially held true if it was next-gen sequencing MRD-positive at day 100, for which relapse rates were over 95%,” he said.
The absence of B-cells is a surrogate marker for the persistence of CAR-T, and conversely, the recovery of CD19-positive B cells may be a predictor for relapse, especially if the B-cell recovery occurs within the first 6 months following CAR-T infusion.
Transplant after CAR-T?
Bone marrow transplant after CAR-T is recommend for patients with high risk of relapse, including those with B-cell recovery within the first 6 months after CAR-T, patients with MRD positivity at days 28 or 100, and patients with mixed lineage leukemia.
“Should we transplant good-risk patients, meaning, if you have NGS-MRD negative patients, is there a role for transplant? You have to look at the risk versus benefit there. These patients may have a cure rate that’s in the 80%-plus range, could we potentially optimize that even more if we consolidate them with an allo[geneic] transplant,” Dr. Yank said.
Move CAR-T up front?
A Children’s Oncology Group study is currently examining whether giving CAR-T therapy to patients with MRD of 0.01% or greater following first consolidation could result in lower tumor burden, fewer relapse, and less CRS with CAR-T.
Dr. Yanik reported that he had no conflicts of interest to disclose.
FROM TCT 2021
Roundtable discussion: The Pluripotent Hospitalist
In honor of National Hospitalist Day, the Society of Hospital Medicine and the Explore the Space podcast are teaming up to bring you a roundtable discussion, featuring a diverse group of hospitalists from all stages in their careers, on Thursday, March 4, at 7 p.m. ET / 4 p.m. PT.
Registration is required. Sign up here.
Hosted by Mark Shapiro, MD, hospitalist and founder, producer, and host of Explore the Space, the roundtable will include:
- Gurpreet Dhaliwal, MD, a clinician-educator and professor of medicine at the University of California, San Francisco. He studies, writes, and speaks about how doctors think – how they make diagnoses, how they develop diagnostic expertise, and what motivates them to improve their practice and the systems in which they work.
- Anika Kumar, MD, FHM, a clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, and a pediatric hospitalist at Cleveland Clinic Children’s. She also serves as the pediatric editor of the Hospitalist, SHM’s monthly news magazine.
- Maylyn S. Martinez, MD, a clinician-researcher and clinical associate at the University of Chicago. Her research focuses on hospital-associated disability and she recently authored a perspectives piece in the Journal of Hospital Medicine with her mentor, Vineet Arora, MD, MHM, on why the COVID-19 pandemic might exacerbate this problem.
- Ndidi Unaka, MD, MEd, an associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. Dr. Unaka has served as the associate program director of the pediatric residency program since 2011. She is also the medical director of an inpatient unit that serves as the primary home.
For more information about SHM, please visit hospitalmedicine.org. To learn more about Explore the Space, please visit explorethespaceshow.com.
Register now.
In honor of National Hospitalist Day, the Society of Hospital Medicine and the Explore the Space podcast are teaming up to bring you a roundtable discussion, featuring a diverse group of hospitalists from all stages in their careers, on Thursday, March 4, at 7 p.m. ET / 4 p.m. PT.
Registration is required. Sign up here.
Hosted by Mark Shapiro, MD, hospitalist and founder, producer, and host of Explore the Space, the roundtable will include:
- Gurpreet Dhaliwal, MD, a clinician-educator and professor of medicine at the University of California, San Francisco. He studies, writes, and speaks about how doctors think – how they make diagnoses, how they develop diagnostic expertise, and what motivates them to improve their practice and the systems in which they work.
- Anika Kumar, MD, FHM, a clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, and a pediatric hospitalist at Cleveland Clinic Children’s. She also serves as the pediatric editor of the Hospitalist, SHM’s monthly news magazine.
- Maylyn S. Martinez, MD, a clinician-researcher and clinical associate at the University of Chicago. Her research focuses on hospital-associated disability and she recently authored a perspectives piece in the Journal of Hospital Medicine with her mentor, Vineet Arora, MD, MHM, on why the COVID-19 pandemic might exacerbate this problem.
- Ndidi Unaka, MD, MEd, an associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. Dr. Unaka has served as the associate program director of the pediatric residency program since 2011. She is also the medical director of an inpatient unit that serves as the primary home.
For more information about SHM, please visit hospitalmedicine.org. To learn more about Explore the Space, please visit explorethespaceshow.com.
Register now.
In honor of National Hospitalist Day, the Society of Hospital Medicine and the Explore the Space podcast are teaming up to bring you a roundtable discussion, featuring a diverse group of hospitalists from all stages in their careers, on Thursday, March 4, at 7 p.m. ET / 4 p.m. PT.
Registration is required. Sign up here.
Hosted by Mark Shapiro, MD, hospitalist and founder, producer, and host of Explore the Space, the roundtable will include:
- Gurpreet Dhaliwal, MD, a clinician-educator and professor of medicine at the University of California, San Francisco. He studies, writes, and speaks about how doctors think – how they make diagnoses, how they develop diagnostic expertise, and what motivates them to improve their practice and the systems in which they work.
- Anika Kumar, MD, FHM, a clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, and a pediatric hospitalist at Cleveland Clinic Children’s. She also serves as the pediatric editor of the Hospitalist, SHM’s monthly news magazine.
- Maylyn S. Martinez, MD, a clinician-researcher and clinical associate at the University of Chicago. Her research focuses on hospital-associated disability and she recently authored a perspectives piece in the Journal of Hospital Medicine with her mentor, Vineet Arora, MD, MHM, on why the COVID-19 pandemic might exacerbate this problem.
- Ndidi Unaka, MD, MEd, an associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. Dr. Unaka has served as the associate program director of the pediatric residency program since 2011. She is also the medical director of an inpatient unit that serves as the primary home.
For more information about SHM, please visit hospitalmedicine.org. To learn more about Explore the Space, please visit explorethespaceshow.com.
Register now.
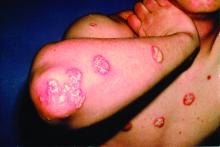
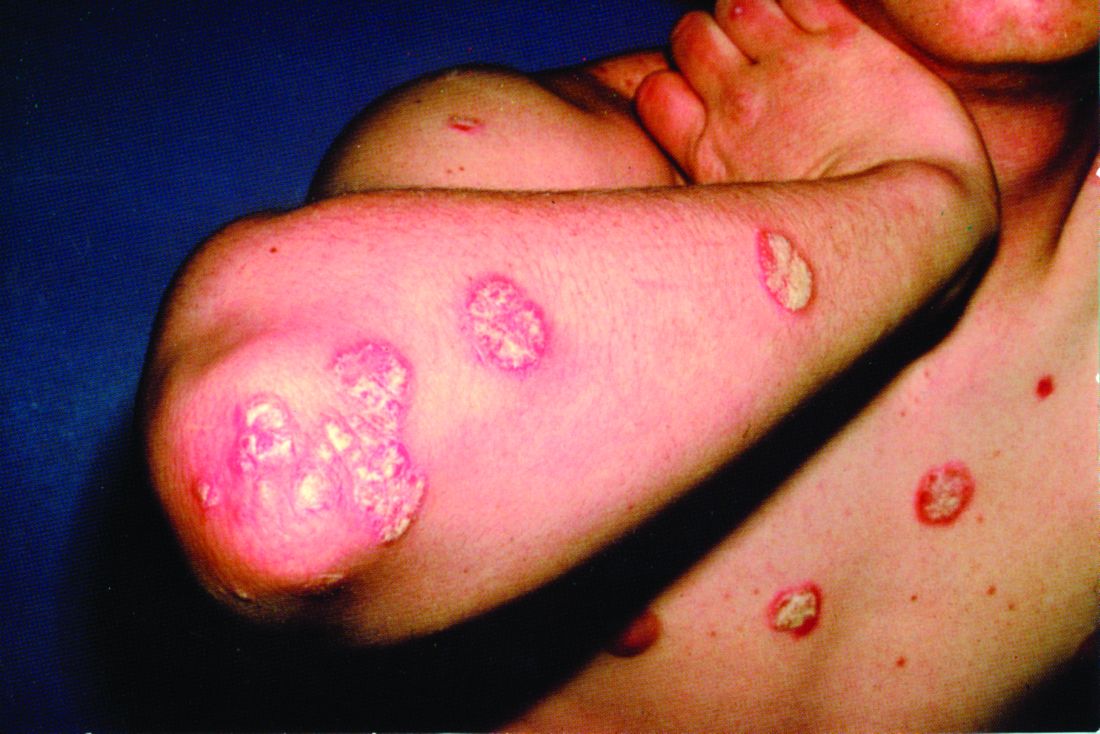
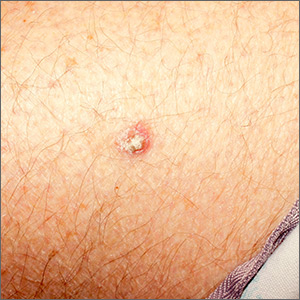
Rough lesion on the thigh
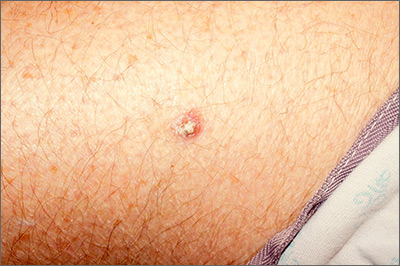
A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037

A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037
Acute care of migraine and cluster headaches: Mainstay treatments and emerging strategies
Acute migraine headache attacks
A recent review in the Journal of Neuro-Ophthalmology by Konstantinos Spingos and colleagues (including me as the senior author) details typical and new treatments for migraine. We all know about the longstanding options, including the 7 triptans and ergots, as well as over-the-counter analgesics, which can be combined with caffeine, nonsteroidal anti-inflammatory drugs; and many use the 2 categories of medication that I no longer use for migraine, butalbital-containing medications, and opioids.
Now 2 gepants are available—small molecule calcitonin gene-related peptide (CGRP) receptor antagonists. These medications are thought to be a useful alternative for those in whom triptans do not work or are relatively contraindicated due to coronary and cerebrovascular problems and other cardiac risk factors like obesity, smoking, lack of exercise, high cholesterol, and diabetes. Ubrogepant was approved by the FDA in 2019, and rimegepant soon followed in 2020.
- Ubrogepant: In the ACHIEVE trials, approximately 1 in 5 participants who received the 50 mg dose were pain-free at 2 hours. Moreover, nearly 40% of individuals who received it said their worst migraine symptom was resolved at 2 hours. Pain relief at 2 hours was 59%
- Rimegepant: Like ubrogepant, about 20% of trial participants who received the 75 mg melt tablet dose of rimegepant were pain-free at 2 hours. Thirty-seven percent reported that their worst migraine symptom was gone at 2 hours. Patients began to return to normal functioning in 15 minutes.
In addition to gepants, there is 1 ditan approved, which stimulates 5-HT1F receptors. Lasmiditan is the first medication in this class to be FDA-approved. It, too, is considered an alternative in patients in whom triptans are ineffective or when patients should not take a vasoconstrictor. In the most recent phase 3 study, the percentage of individuals who received lasmiditan and were pain-free at 2 hours were 28% (50 mg), 31% (100 mg) and 39% (200 mg). Relief from the migraine sufferers’ most bothersome symptom at 2 hours occurred in 41%, 44%, and 49% of patients, respectively. Lasmiditan is a Class V controlled substance. It has 18% dizziness in clinical trials. After administration, patients should not drive for 8 hours, and it should only be used once in a 24-hour period.
Non-pharmaceutical treatment options for acute migraine include nerve stimulation using electrical and magnetic stimulation devices, and behavioral approaches such as biofeedback training and mindfulness. The Nerivio device for the upper arm is controlled by a smart phone app and seems to work as well as a triptan in some patients with almost no adverse events. Just approved in February is the Relivion device which is worn like a tiara on the head and stimulates the frontal branches of the trigeminal nerve as well as the 2 occipital nerves in the back of the head.
Acute care of cluster headache attacks
In 2011, Ashkenazi and Schwedt published a comprehensive table in Headache outlining the treatment options for acute cluster headache. More recently, a review in CNS Drugs by Brandt and colleagues presented the choices with level 1 evidence for efficacy. They include:
- Sumatriptan, 6 mg subcutaneous injection, or 20 mg nasal spray
- Zolmitriptan, 5 or 10 mg nasal spray
- Oxygen, 100%, 7 to 12 liters per minute via a mask over the nose and mouth
The authors recommend subcutaneous sumatriptan 6 mg and/or high-flow oxygen at 9- to 12- liters per minute for 15 minutes. Subcutaneous sumatriptan, they note, has been shown to achieve pain relief within 15 minutes in 75% of patients who receive it. Moreover, one-third report pain freedom. Oxygen’s efficacy has long been established, and relief comes with no adverse events. As for mask type, though no significant differences have been observed in studies, patients appear to express a preference for the demand valve oxygen type, which allows a high flow rate and is dependent on the user’s breathing rate.
Lidocaine intranasally has been found to be effective when triptans or oxygen do not work, according to a review in The Lancet Neurology by Hoffman and May. The medication is dripped or sprayed into the ipsilateral nostril at a concentration of between 4% and 10%. Pain relief is typically achieved within 10 minutes. This review also reports efficacy with percutaneous vagus nerve stimulation with the gammaCore device and neurostimulation of the sphenopalatine ganglion, though the mechanisms of these approaches are poorly understood.
Evolving therapies for acute cluster headache include the aforementioned CGRP receptor-antagonists. Additionally, intranasal ketamine hydrochloride is under investigation in an open-label, proof-of-concept study; and a zolmitriptan patch is being evaluated in a double-blind, placebo-controlled trial.
Attacks of migraine occur in 12% of the adult population, 3 times more in women than men and are painful and debilitating. Cluster attacks are even more painful and occur in about 0.1% of the population, somewhat more in men. Both types of headache have a variety of effective treatment as detailed above.
Acute migraine headache attacks
A recent review in the Journal of Neuro-Ophthalmology by Konstantinos Spingos and colleagues (including me as the senior author) details typical and new treatments for migraine. We all know about the longstanding options, including the 7 triptans and ergots, as well as over-the-counter analgesics, which can be combined with caffeine, nonsteroidal anti-inflammatory drugs; and many use the 2 categories of medication that I no longer use for migraine, butalbital-containing medications, and opioids.
Now 2 gepants are available—small molecule calcitonin gene-related peptide (CGRP) receptor antagonists. These medications are thought to be a useful alternative for those in whom triptans do not work or are relatively contraindicated due to coronary and cerebrovascular problems and other cardiac risk factors like obesity, smoking, lack of exercise, high cholesterol, and diabetes. Ubrogepant was approved by the FDA in 2019, and rimegepant soon followed in 2020.
- Ubrogepant: In the ACHIEVE trials, approximately 1 in 5 participants who received the 50 mg dose were pain-free at 2 hours. Moreover, nearly 40% of individuals who received it said their worst migraine symptom was resolved at 2 hours. Pain relief at 2 hours was 59%
- Rimegepant: Like ubrogepant, about 20% of trial participants who received the 75 mg melt tablet dose of rimegepant were pain-free at 2 hours. Thirty-seven percent reported that their worst migraine symptom was gone at 2 hours. Patients began to return to normal functioning in 15 minutes.
In addition to gepants, there is 1 ditan approved, which stimulates 5-HT1F receptors. Lasmiditan is the first medication in this class to be FDA-approved. It, too, is considered an alternative in patients in whom triptans are ineffective or when patients should not take a vasoconstrictor. In the most recent phase 3 study, the percentage of individuals who received lasmiditan and were pain-free at 2 hours were 28% (50 mg), 31% (100 mg) and 39% (200 mg). Relief from the migraine sufferers’ most bothersome symptom at 2 hours occurred in 41%, 44%, and 49% of patients, respectively. Lasmiditan is a Class V controlled substance. It has 18% dizziness in clinical trials. After administration, patients should not drive for 8 hours, and it should only be used once in a 24-hour period.
Non-pharmaceutical treatment options for acute migraine include nerve stimulation using electrical and magnetic stimulation devices, and behavioral approaches such as biofeedback training and mindfulness. The Nerivio device for the upper arm is controlled by a smart phone app and seems to work as well as a triptan in some patients with almost no adverse events. Just approved in February is the Relivion device which is worn like a tiara on the head and stimulates the frontal branches of the trigeminal nerve as well as the 2 occipital nerves in the back of the head.
Acute care of cluster headache attacks
In 2011, Ashkenazi and Schwedt published a comprehensive table in Headache outlining the treatment options for acute cluster headache. More recently, a review in CNS Drugs by Brandt and colleagues presented the choices with level 1 evidence for efficacy. They include:
- Sumatriptan, 6 mg subcutaneous injection, or 20 mg nasal spray
- Zolmitriptan, 5 or 10 mg nasal spray
- Oxygen, 100%, 7 to 12 liters per minute via a mask over the nose and mouth
The authors recommend subcutaneous sumatriptan 6 mg and/or high-flow oxygen at 9- to 12- liters per minute for 15 minutes. Subcutaneous sumatriptan, they note, has been shown to achieve pain relief within 15 minutes in 75% of patients who receive it. Moreover, one-third report pain freedom. Oxygen’s efficacy has long been established, and relief comes with no adverse events. As for mask type, though no significant differences have been observed in studies, patients appear to express a preference for the demand valve oxygen type, which allows a high flow rate and is dependent on the user’s breathing rate.
Lidocaine intranasally has been found to be effective when triptans or oxygen do not work, according to a review in The Lancet Neurology by Hoffman and May. The medication is dripped or sprayed into the ipsilateral nostril at a concentration of between 4% and 10%. Pain relief is typically achieved within 10 minutes. This review also reports efficacy with percutaneous vagus nerve stimulation with the gammaCore device and neurostimulation of the sphenopalatine ganglion, though the mechanisms of these approaches are poorly understood.
Evolving therapies for acute cluster headache include the aforementioned CGRP receptor-antagonists. Additionally, intranasal ketamine hydrochloride is under investigation in an open-label, proof-of-concept study; and a zolmitriptan patch is being evaluated in a double-blind, placebo-controlled trial.
Attacks of migraine occur in 12% of the adult population, 3 times more in women than men and are painful and debilitating. Cluster attacks are even more painful and occur in about 0.1% of the population, somewhat more in men. Both types of headache have a variety of effective treatment as detailed above.
Acute migraine headache attacks
A recent review in the Journal of Neuro-Ophthalmology by Konstantinos Spingos and colleagues (including me as the senior author) details typical and new treatments for migraine. We all know about the longstanding options, including the 7 triptans and ergots, as well as over-the-counter analgesics, which can be combined with caffeine, nonsteroidal anti-inflammatory drugs; and many use the 2 categories of medication that I no longer use for migraine, butalbital-containing medications, and opioids.
Now 2 gepants are available—small molecule calcitonin gene-related peptide (CGRP) receptor antagonists. These medications are thought to be a useful alternative for those in whom triptans do not work or are relatively contraindicated due to coronary and cerebrovascular problems and other cardiac risk factors like obesity, smoking, lack of exercise, high cholesterol, and diabetes. Ubrogepant was approved by the FDA in 2019, and rimegepant soon followed in 2020.
- Ubrogepant: In the ACHIEVE trials, approximately 1 in 5 participants who received the 50 mg dose were pain-free at 2 hours. Moreover, nearly 40% of individuals who received it said their worst migraine symptom was resolved at 2 hours. Pain relief at 2 hours was 59%
- Rimegepant: Like ubrogepant, about 20% of trial participants who received the 75 mg melt tablet dose of rimegepant were pain-free at 2 hours. Thirty-seven percent reported that their worst migraine symptom was gone at 2 hours. Patients began to return to normal functioning in 15 minutes.
In addition to gepants, there is 1 ditan approved, which stimulates 5-HT1F receptors. Lasmiditan is the first medication in this class to be FDA-approved. It, too, is considered an alternative in patients in whom triptans are ineffective or when patients should not take a vasoconstrictor. In the most recent phase 3 study, the percentage of individuals who received lasmiditan and were pain-free at 2 hours were 28% (50 mg), 31% (100 mg) and 39% (200 mg). Relief from the migraine sufferers’ most bothersome symptom at 2 hours occurred in 41%, 44%, and 49% of patients, respectively. Lasmiditan is a Class V controlled substance. It has 18% dizziness in clinical trials. After administration, patients should not drive for 8 hours, and it should only be used once in a 24-hour period.
Non-pharmaceutical treatment options for acute migraine include nerve stimulation using electrical and magnetic stimulation devices, and behavioral approaches such as biofeedback training and mindfulness. The Nerivio device for the upper arm is controlled by a smart phone app and seems to work as well as a triptan in some patients with almost no adverse events. Just approved in February is the Relivion device which is worn like a tiara on the head and stimulates the frontal branches of the trigeminal nerve as well as the 2 occipital nerves in the back of the head.
Acute care of cluster headache attacks
In 2011, Ashkenazi and Schwedt published a comprehensive table in Headache outlining the treatment options for acute cluster headache. More recently, a review in CNS Drugs by Brandt and colleagues presented the choices with level 1 evidence for efficacy. They include:
- Sumatriptan, 6 mg subcutaneous injection, or 20 mg nasal spray
- Zolmitriptan, 5 or 10 mg nasal spray
- Oxygen, 100%, 7 to 12 liters per minute via a mask over the nose and mouth
The authors recommend subcutaneous sumatriptan 6 mg and/or high-flow oxygen at 9- to 12- liters per minute for 15 minutes. Subcutaneous sumatriptan, they note, has been shown to achieve pain relief within 15 minutes in 75% of patients who receive it. Moreover, one-third report pain freedom. Oxygen’s efficacy has long been established, and relief comes with no adverse events. As for mask type, though no significant differences have been observed in studies, patients appear to express a preference for the demand valve oxygen type, which allows a high flow rate and is dependent on the user’s breathing rate.
Lidocaine intranasally has been found to be effective when triptans or oxygen do not work, according to a review in The Lancet Neurology by Hoffman and May. The medication is dripped or sprayed into the ipsilateral nostril at a concentration of between 4% and 10%. Pain relief is typically achieved within 10 minutes. This review also reports efficacy with percutaneous vagus nerve stimulation with the gammaCore device and neurostimulation of the sphenopalatine ganglion, though the mechanisms of these approaches are poorly understood.
Evolving therapies for acute cluster headache include the aforementioned CGRP receptor-antagonists. Additionally, intranasal ketamine hydrochloride is under investigation in an open-label, proof-of-concept study; and a zolmitriptan patch is being evaluated in a double-blind, placebo-controlled trial.
Attacks of migraine occur in 12% of the adult population, 3 times more in women than men and are painful and debilitating. Cluster attacks are even more painful and occur in about 0.1% of the population, somewhat more in men. Both types of headache have a variety of effective treatment as detailed above.
The psychiatrist’s role in navigating a toxic news cycle
This transcript has been edited for clarity.
I’m Dr. Gregory Scott Brown, an Austin (Tex.)-based psychiatrist and an affiliate faculty member at the University of Texas Dell Medical School.
Recently, a patient of mine told me that, because of the political environment we find ourselves in, he’s avoiding conversations with some of his longtime friends. Because of this, he’s feeling even more isolated than before.
We’re all coming off the heels of a tough year, and many of us expected that when we entered 2021 we’d quickly turn the page and life would get a whole lot easier. Since the reality is that we’re still dealing with deep divisions, social injustices, and the politicization of evidence-based medicine, emotions are naturally running high.
In listening to my patients over the past few weeks, there’s definitely a sense of optimism about coronavirus vaccines and getting back to life as usual. But there’s also a lingering sense of uncertainty and fear, especially when it comes to the possibility of saying the wrong thing, offending our friends, or just having conversations with people we may disagree with. I’m hearing concerns from my patients that 2020 exposed a dormant hatred that was brewing in the underbelly of our society, in our politics, and in our institutions. Patients are telling me that these concerns are making them anxious and some are avoiding interacting with people they may disagree with altogether because they’re afraid of the difficult conversations that may follow.
Since, like many of my patients as well as many of you, I follow the news, including stories of COVID-related deaths, economic hardships, peaceful protests gone bad, and political vitriol, I’ve had to remind myself about the importance of intentional kindness for effective communication and for supporting mental health.
I was reminded of the paper “Hate in the Counter-Transference” by the well-known pediatrician and psychoanalyst Donald Winnicott. He focuses on how to manage and sort through the strong emotions that may be experienced even during an encounter between a therapist and a patient. Although some of what he has to say doesn’t translate well to modern times, his recommendation that we acknowledge and try to normalize some of our feelings does. And this is how I’ve been starting a conversation with my patients – just by normalizing things a bit.
The past year or so has brought with it a range of intense emotions, including frustration, exhaustion, and some degree of sadness for most of us. When we’re self-aware about our feelings, we can make sense of them early on so that they don’t evolve into maladaptive ones like unhinged anger or hatred. My patients and I actively discuss how our feelings don’t have to get in the way of our ability to live and to interact with each other as we’d like to.
Considering the basic tenets of kindness is a good place to start. I recently spoke with Kelli Harding, MD, a psychiatrist and author of The Rabbit Effect: Live Longer, Happier, and Healthier with the Groundbreaking Science of Kindness. She pointed to research suggesting that kindness can benefit multiple areas of our health, from reducing cardiovascular events to improving mood and anxiety. In her book, she notes that
I tell my patients that we can’t always change our environment, but we can definitely change how we respond to it. This doesn’t mean it’s always an easy process, but there are things we can do. First, we have to acknowledge that some degree of conflict or cordial disagreement is inevitable and it doesn’t have to disrupt our mood.
An interesting study on conflict management pointed out that healthy conflict is actually beneficial in some cases. For instance, in the work environment, conflict can help with team development and better group decision-making. But it’s rigid personality differences, poor communication, emotional stress, and lack of candor that may contribute to so-called high-tension events, and this is where conflict can go awry. These are the areas that we can all focus on improving, not only for performance benefits but for our overall health and well-being as well.
The authors also recommend an active style of engagement as a technique to manage conflict, but in a way that feels both natural and safe. Other authors agree that so-called engaged coping is associated with a higher sense of control and overall improved psychological well-being. What this means is that avoidance may be necessary in the short term, but over time it may lead to more emotional stress and anxiety.
Overcoming the tendency to avoid requires both motivation and self-awareness. We need to know about patterns in our own behavior and how the behavior of others can push our buttons and spiral a healthy disagreement into a heated argument.
I like to recommend the hunger, angry, lonely, tired (HALT) model, which is often used as a self-care gauge in addiction treatment and to reduce medication errors. But I think it’s also a useful way to assess personal readiness for having a difficult conversation. I tell my patients to ask themselves in this moment: “Am I hungry, angry, lonely, or tired?” And if they are, perhaps it’s not the best time for the conversation.
Because 2020 brought with it a new set of challenges, it also forced many of us to focus on things that we just didn’t pay as much attention to before, including checking in on our feelings and the feelings of those around us. It also taught us to pay attention to the way and manner in which we communicate. I think that kindness is much easier to carry into difficult conversations if we approach them with a sense of curiosity before judgment. Ultimately, kindness is one of the best tools for balancing the intense emotions that many of us are feeling right now.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Gregory Scott Brown, an Austin (Tex.)-based psychiatrist and an affiliate faculty member at the University of Texas Dell Medical School.
Recently, a patient of mine told me that, because of the political environment we find ourselves in, he’s avoiding conversations with some of his longtime friends. Because of this, he’s feeling even more isolated than before.
We’re all coming off the heels of a tough year, and many of us expected that when we entered 2021 we’d quickly turn the page and life would get a whole lot easier. Since the reality is that we’re still dealing with deep divisions, social injustices, and the politicization of evidence-based medicine, emotions are naturally running high.
In listening to my patients over the past few weeks, there’s definitely a sense of optimism about coronavirus vaccines and getting back to life as usual. But there’s also a lingering sense of uncertainty and fear, especially when it comes to the possibility of saying the wrong thing, offending our friends, or just having conversations with people we may disagree with. I’m hearing concerns from my patients that 2020 exposed a dormant hatred that was brewing in the underbelly of our society, in our politics, and in our institutions. Patients are telling me that these concerns are making them anxious and some are avoiding interacting with people they may disagree with altogether because they’re afraid of the difficult conversations that may follow.
Since, like many of my patients as well as many of you, I follow the news, including stories of COVID-related deaths, economic hardships, peaceful protests gone bad, and political vitriol, I’ve had to remind myself about the importance of intentional kindness for effective communication and for supporting mental health.
I was reminded of the paper “Hate in the Counter-Transference” by the well-known pediatrician and psychoanalyst Donald Winnicott. He focuses on how to manage and sort through the strong emotions that may be experienced even during an encounter between a therapist and a patient. Although some of what he has to say doesn’t translate well to modern times, his recommendation that we acknowledge and try to normalize some of our feelings does. And this is how I’ve been starting a conversation with my patients – just by normalizing things a bit.
The past year or so has brought with it a range of intense emotions, including frustration, exhaustion, and some degree of sadness for most of us. When we’re self-aware about our feelings, we can make sense of them early on so that they don’t evolve into maladaptive ones like unhinged anger or hatred. My patients and I actively discuss how our feelings don’t have to get in the way of our ability to live and to interact with each other as we’d like to.
Considering the basic tenets of kindness is a good place to start. I recently spoke with Kelli Harding, MD, a psychiatrist and author of The Rabbit Effect: Live Longer, Happier, and Healthier with the Groundbreaking Science of Kindness. She pointed to research suggesting that kindness can benefit multiple areas of our health, from reducing cardiovascular events to improving mood and anxiety. In her book, she notes that
I tell my patients that we can’t always change our environment, but we can definitely change how we respond to it. This doesn’t mean it’s always an easy process, but there are things we can do. First, we have to acknowledge that some degree of conflict or cordial disagreement is inevitable and it doesn’t have to disrupt our mood.
An interesting study on conflict management pointed out that healthy conflict is actually beneficial in some cases. For instance, in the work environment, conflict can help with team development and better group decision-making. But it’s rigid personality differences, poor communication, emotional stress, and lack of candor that may contribute to so-called high-tension events, and this is where conflict can go awry. These are the areas that we can all focus on improving, not only for performance benefits but for our overall health and well-being as well.
The authors also recommend an active style of engagement as a technique to manage conflict, but in a way that feels both natural and safe. Other authors agree that so-called engaged coping is associated with a higher sense of control and overall improved psychological well-being. What this means is that avoidance may be necessary in the short term, but over time it may lead to more emotional stress and anxiety.
Overcoming the tendency to avoid requires both motivation and self-awareness. We need to know about patterns in our own behavior and how the behavior of others can push our buttons and spiral a healthy disagreement into a heated argument.
I like to recommend the hunger, angry, lonely, tired (HALT) model, which is often used as a self-care gauge in addiction treatment and to reduce medication errors. But I think it’s also a useful way to assess personal readiness for having a difficult conversation. I tell my patients to ask themselves in this moment: “Am I hungry, angry, lonely, or tired?” And if they are, perhaps it’s not the best time for the conversation.
Because 2020 brought with it a new set of challenges, it also forced many of us to focus on things that we just didn’t pay as much attention to before, including checking in on our feelings and the feelings of those around us. It also taught us to pay attention to the way and manner in which we communicate. I think that kindness is much easier to carry into difficult conversations if we approach them with a sense of curiosity before judgment. Ultimately, kindness is one of the best tools for balancing the intense emotions that many of us are feeling right now.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Gregory Scott Brown, an Austin (Tex.)-based psychiatrist and an affiliate faculty member at the University of Texas Dell Medical School.
Recently, a patient of mine told me that, because of the political environment we find ourselves in, he’s avoiding conversations with some of his longtime friends. Because of this, he’s feeling even more isolated than before.
We’re all coming off the heels of a tough year, and many of us expected that when we entered 2021 we’d quickly turn the page and life would get a whole lot easier. Since the reality is that we’re still dealing with deep divisions, social injustices, and the politicization of evidence-based medicine, emotions are naturally running high.
In listening to my patients over the past few weeks, there’s definitely a sense of optimism about coronavirus vaccines and getting back to life as usual. But there’s also a lingering sense of uncertainty and fear, especially when it comes to the possibility of saying the wrong thing, offending our friends, or just having conversations with people we may disagree with. I’m hearing concerns from my patients that 2020 exposed a dormant hatred that was brewing in the underbelly of our society, in our politics, and in our institutions. Patients are telling me that these concerns are making them anxious and some are avoiding interacting with people they may disagree with altogether because they’re afraid of the difficult conversations that may follow.
Since, like many of my patients as well as many of you, I follow the news, including stories of COVID-related deaths, economic hardships, peaceful protests gone bad, and political vitriol, I’ve had to remind myself about the importance of intentional kindness for effective communication and for supporting mental health.
I was reminded of the paper “Hate in the Counter-Transference” by the well-known pediatrician and psychoanalyst Donald Winnicott. He focuses on how to manage and sort through the strong emotions that may be experienced even during an encounter between a therapist and a patient. Although some of what he has to say doesn’t translate well to modern times, his recommendation that we acknowledge and try to normalize some of our feelings does. And this is how I’ve been starting a conversation with my patients – just by normalizing things a bit.
The past year or so has brought with it a range of intense emotions, including frustration, exhaustion, and some degree of sadness for most of us. When we’re self-aware about our feelings, we can make sense of them early on so that they don’t evolve into maladaptive ones like unhinged anger or hatred. My patients and I actively discuss how our feelings don’t have to get in the way of our ability to live and to interact with each other as we’d like to.
Considering the basic tenets of kindness is a good place to start. I recently spoke with Kelli Harding, MD, a psychiatrist and author of The Rabbit Effect: Live Longer, Happier, and Healthier with the Groundbreaking Science of Kindness. She pointed to research suggesting that kindness can benefit multiple areas of our health, from reducing cardiovascular events to improving mood and anxiety. In her book, she notes that
I tell my patients that we can’t always change our environment, but we can definitely change how we respond to it. This doesn’t mean it’s always an easy process, but there are things we can do. First, we have to acknowledge that some degree of conflict or cordial disagreement is inevitable and it doesn’t have to disrupt our mood.
An interesting study on conflict management pointed out that healthy conflict is actually beneficial in some cases. For instance, in the work environment, conflict can help with team development and better group decision-making. But it’s rigid personality differences, poor communication, emotional stress, and lack of candor that may contribute to so-called high-tension events, and this is where conflict can go awry. These are the areas that we can all focus on improving, not only for performance benefits but for our overall health and well-being as well.
The authors also recommend an active style of engagement as a technique to manage conflict, but in a way that feels both natural and safe. Other authors agree that so-called engaged coping is associated with a higher sense of control and overall improved psychological well-being. What this means is that avoidance may be necessary in the short term, but over time it may lead to more emotional stress and anxiety.
Overcoming the tendency to avoid requires both motivation and self-awareness. We need to know about patterns in our own behavior and how the behavior of others can push our buttons and spiral a healthy disagreement into a heated argument.
I like to recommend the hunger, angry, lonely, tired (HALT) model, which is often used as a self-care gauge in addiction treatment and to reduce medication errors. But I think it’s also a useful way to assess personal readiness for having a difficult conversation. I tell my patients to ask themselves in this moment: “Am I hungry, angry, lonely, or tired?” And if they are, perhaps it’s not the best time for the conversation.
Because 2020 brought with it a new set of challenges, it also forced many of us to focus on things that we just didn’t pay as much attention to before, including checking in on our feelings and the feelings of those around us. It also taught us to pay attention to the way and manner in which we communicate. I think that kindness is much easier to carry into difficult conversations if we approach them with a sense of curiosity before judgment. Ultimately, kindness is one of the best tools for balancing the intense emotions that many of us are feeling right now.
A version of this article first appeared on Medscape.com.
No vascular benefit of testosterone over exercise in aging men
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).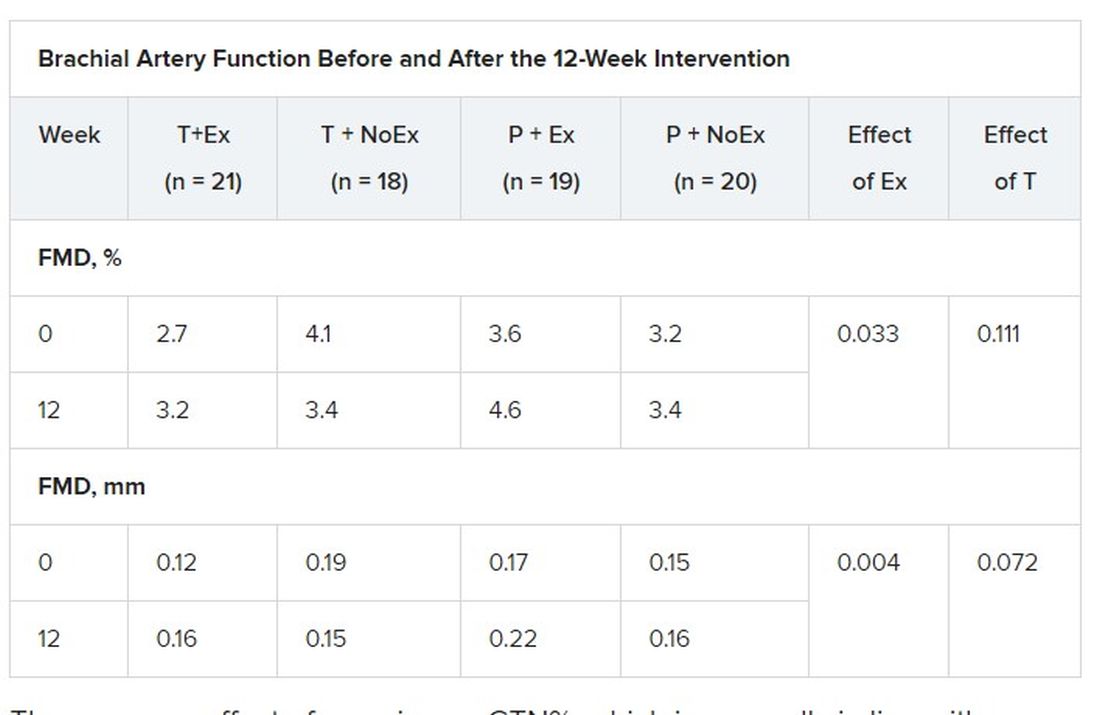
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
‘Landmark’ schizophrenia drug in the wings?
A novel therapy that combines a muscarinic receptor agonist with an anticholinergic agent is associated with a greater reduction in psychosis symptoms, compared with placebo, new research shows.
In a randomized, phase 2 trial composed of nearly 200 participants, xanomeline-trospium (KarXT) was generally well tolerated and had none of the common side effects linked to current antipsychotics, including weight gain and extrapyramidal symptoms such as dystonia, parkinsonism, and tardive dyskinesia.
“The results showing robust therapeutic efficacy of a non–dopamine targeting antipsychotic drug is an important milestone in the advance of the therapeutics of schizophrenia and other psychotic disorders,” coinvestigator Jeffrey A. Lieberman, MD, professor and chairman in the department of psychiatry, Columbia University, New York, said in an interview.
If approved, the new agent will be a “landmark” drug, Dr. Lieberman added.
The study was published in the Feb. 25, 2021, issue of the New England Journal of Medicine.
Long journey
The journey to develop an effective schizophrenia drug that reduces psychosis symptoms without onerous side effects has been a long one full of excitement and disappointment.
First-generation antipsychotics, dating back to the 1950s, targeted the postsynaptic dopamine-2 (D2) receptor. At the time, it was a “breakthrough” similar in scope to insulin for diabetes or antibiotics for infections, said Dr. Lieberman.
That was followed by development of numerous “me too” drugs with the same mechanism of action. However, these drugs had significant side effects, especially neurologic adverse events such as parkinsonism.
In 1989, second-generation antipsychotics were introduced, beginning with clozapine. They still targeted the D2 receptor but were “kinder and gentler,” Dr. Lieberman noted. “They didn’t bind to [the receptor] with such affinity that it shut things down completely, so had fewer neurologic side effects.”
However, these agents had other adverse consequences, such as weight gain and other metabolic effects including hyperglycemia and hyperlipidemia.
Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
“The pharmaceutical industry, biotech industry, and academic psychiatric community have been desperately trying to find novel strategies for antipsychotic drug development and asking, ‘Is D2 the only holy grail or are there other ways of treating psychotic symptoms of schizophrenia?’ ” Dr. Lieberman said.
Enter KarXT – a novel combination of xanomeline with trospium.
An ‘ingenious’ combination
Xanomeline, an oral muscarinic cholinergic receptor agonist, does not have direct effects on the dopamine receptor. Evidence suggests the muscarinic cholinergic system is involved in the pathophysiology of schizophrenia.
However, there may be dose-dependent adverse events with the medication, such as nausea, vomiting, diarrhea, sweating, and hypersalivation from stimulation of peripheral muscarinic cholinergic receptors.
That’s where trospium chloride, an oral panmuscarinic receptor antagonist approved for treating overactive bladder, comes in. It does not reach detectable levels in the cerebrospinal fluid and should avoid adverse central nervous system effects.
Dr. Lieberman said the idea of the drug combination is “ingenious.”
The new phase 2, multisite study included adult patients with a validated diagnosis of schizophrenia who were hospitalized with an acute exacerbation of psychosis, and who were free of antipsychotic medication for at least 2 weeks.
Participants were required to have a baseline Positive and Negative Syndrome Scale (PANSS) total score of 80 points or more.
In addition to seven positive symptom items, including delusions, hallucinations, and conceptual disorganization, the PANSS has seven negative symptom items. These include restricted emotional expression, paucity of speech, and diminished interest, social drive, and activity. Each item is scored from 1 to 7, with higher scores indicating more severe symptoms.
Patients also had to have a score on the Clinical Global Impression–Severity (CGI-S) scale of 4 or higher. Scores on the CGI-S range from 1 to 7, with higher scores indicating greater severity of illness.
The modified intention-to-treat analysis included patients randomly assigned to receive oral xanomeline-trospium (n = 83) or placebo (n = 87).
The dosing schedule was flexible, starting with 50 mg of xanomeline and 20 mg of trospium twice daily. The schedule increased to a maximum of 125 mg of xanomeline and 30 mg of trospium twice daily, with the option of lowering the dose if there were unacceptable side effects.
Mean scores at baseline for the treatment and placebo groups were 97.7 versus 96.6 for the PANSS total score, 26.4 versus 26.3 for the positive subscore, 22.6 versus 22.8 for the negative subscore, and 5.0 versus 4.9 in the CGI-S scale.
‘Impressively robust’ effect size
The primary endpoint was change in the PANSS total score at 5 weeks. Results showed a change of –17.4 points in the treatment group and –5.9 points in the placebo group (least-squares mean difference, –11.6 points; 95% confidence interval, –16.1 to –7.1; P < .001).
The effect size, which was almost 0.8 (0.75), was “impressively robust,” said Dr. Lieberman, adding that a moderate effect size in this patient population might be in the order of 0.4 or 0.5.
“That gives hope that this drug may not just be as effective as other antipsychotics, albeit acting in a novel way and in a way that has a less of side effect burden, but that it may actually have some elements of superior efficacy,” he said.
There were significant benefits on some secondary outcomes, including change in the PANSS positive symptom subscore (–5.6 points in the treatment group vs. –2.4 points in the placebo group; least-squares mean difference, –3.2 points; 95% CI, –4.8 to –1.7; P < .001).
The active treatment also came out on top for CGI-S scores (P < .001), and PANSS negative symptom subscore (P < .001).
Because participants were hospitalized with an acute exacerbation of positive symptoms at time of study, it is difficult to determine “definitive efficacy” for negative symptoms, Dr. Lieberman noted. Negative symptoms may have improved simply because positive symptoms got better, he said.
Although the study included adults only, “there is nothing in the KarXT clinical profile that suggests it would be problematic for younger people,” Dr. Lieberman noted. This could include teenagers with first-episode psychosis.
Safety profile
Adverse events (AEs) were reported in 54% of the treatment group and 43% of the placebo group. AEs that were more common in the active treatment group included constipation (17% vs. 3%), nausea (17% vs. 4%), dry mouth (9% vs. 1%), dyspepsia (9% vs. 4%), and vomiting (9% vs. 4%). All AEs were rated as mild or moderate in severity and none resulted in discontinuation of treatment.
Rates of nausea, vomiting, and dry mouth were highest early in the trial and lower at the end, whereas constipation remained constant throughout the study.
Persistent constipation could affect the drug’s “utility” in elderly patients with cognitive issues but may be more of a “minor nuisance,” compared with other antipsychotics for those with schizophrenia, said Dr. Lieberman. He added that constipation might be mitigated with an over-the-counter treatment such as Metamucil. Importantly, there was no difference between groups in extrapyramidal symptoms.
In addition, participants receiving the active treatment did not have greater weight gain, which was about 3% versus 4% in the placebo group. The mean change in weight was 1.5 kg (3.3 lb) and 1.1 kg (2.4 lb), respectively.
Dr. Lieberman praised the manufacturer for undertaking the study.
“In an era when Big Pharma has retreated to a considerable degree from psychotropic drug development, it’s commendable that some companies have stayed the course and are succeeding in drug development,” he said.
Exciting mechanism
Commenting on the findings in an interview, Thomas Sedlak, MD, PhD, director of the Schizophrenia and Psychosis Consult Clinic and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, called some aspects of the study “exciting.”
What’s especially important is that the novel agent “acts by a different mechanism than what we have been using for maybe 60 years,” said Dr. Sedlak, who was not involved with the research.
“There has been a lot of research in the last few decades into drugs that might act by alternate mechanisms of action, but nothing’s really gotten to market for schizophrenia,” he noted.
Another interesting aspect of the study is that it examined a combination drug that aims to maximize effects on the brain while minimizing periphery effects, Dr. Sedlak said.
In addition, he called the PANSS results “respectable.”
“It’s interesting that you get a big change in PANSS total score, but it’s not as big in the positive symptom score as you might have expected, and it’s not a huge drop in negative symptoms either,” said Dr. Sedlak.
It’s possible, he said, that the drug is targeting PANSS elements not captured in the main positive or negative symptom items; for example, anxiety, depression, guilt, attention, impulse control, disorientation, and judgment.
Dr. Sedlak noted that, while the new results are exciting, the enthusiasm may not be long-lasting. “When a new drug comes out, there’s often a lot of excitement about it, but once you start using it, expectations may deflate..
“More flexible ratios” of the two components of the drug might be useful to treat individual patients, he added.
In addition, using the components by themselves might be preferable for some clinicians. “I think a lot of physicians prefer using two separate drugs so they can alter the dose of each one independently and not be stuck with the ratio the manufacturer is providing,” Dr. Sedlak said.
However, he acknowledged that studying different choices of ratios would require much larger trials.
The study was supported by Karuna Therapeutics and the Wellcome Trust. Dr. Lieberman reported serving on an advisory board for Karuna Therapeutics and Intra-Cellular Therapies. Dr. Sedlak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel therapy that combines a muscarinic receptor agonist with an anticholinergic agent is associated with a greater reduction in psychosis symptoms, compared with placebo, new research shows.
In a randomized, phase 2 trial composed of nearly 200 participants, xanomeline-trospium (KarXT) was generally well tolerated and had none of the common side effects linked to current antipsychotics, including weight gain and extrapyramidal symptoms such as dystonia, parkinsonism, and tardive dyskinesia.
“The results showing robust therapeutic efficacy of a non–dopamine targeting antipsychotic drug is an important milestone in the advance of the therapeutics of schizophrenia and other psychotic disorders,” coinvestigator Jeffrey A. Lieberman, MD, professor and chairman in the department of psychiatry, Columbia University, New York, said in an interview.
If approved, the new agent will be a “landmark” drug, Dr. Lieberman added.
The study was published in the Feb. 25, 2021, issue of the New England Journal of Medicine.
Long journey
The journey to develop an effective schizophrenia drug that reduces psychosis symptoms without onerous side effects has been a long one full of excitement and disappointment.
First-generation antipsychotics, dating back to the 1950s, targeted the postsynaptic dopamine-2 (D2) receptor. At the time, it was a “breakthrough” similar in scope to insulin for diabetes or antibiotics for infections, said Dr. Lieberman.
That was followed by development of numerous “me too” drugs with the same mechanism of action. However, these drugs had significant side effects, especially neurologic adverse events such as parkinsonism.
In 1989, second-generation antipsychotics were introduced, beginning with clozapine. They still targeted the D2 receptor but were “kinder and gentler,” Dr. Lieberman noted. “They didn’t bind to [the receptor] with such affinity that it shut things down completely, so had fewer neurologic side effects.”
However, these agents had other adverse consequences, such as weight gain and other metabolic effects including hyperglycemia and hyperlipidemia.
Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
“The pharmaceutical industry, biotech industry, and academic psychiatric community have been desperately trying to find novel strategies for antipsychotic drug development and asking, ‘Is D2 the only holy grail or are there other ways of treating psychotic symptoms of schizophrenia?’ ” Dr. Lieberman said.
Enter KarXT – a novel combination of xanomeline with trospium.
An ‘ingenious’ combination
Xanomeline, an oral muscarinic cholinergic receptor agonist, does not have direct effects on the dopamine receptor. Evidence suggests the muscarinic cholinergic system is involved in the pathophysiology of schizophrenia.
However, there may be dose-dependent adverse events with the medication, such as nausea, vomiting, diarrhea, sweating, and hypersalivation from stimulation of peripheral muscarinic cholinergic receptors.
That’s where trospium chloride, an oral panmuscarinic receptor antagonist approved for treating overactive bladder, comes in. It does not reach detectable levels in the cerebrospinal fluid and should avoid adverse central nervous system effects.
Dr. Lieberman said the idea of the drug combination is “ingenious.”
The new phase 2, multisite study included adult patients with a validated diagnosis of schizophrenia who were hospitalized with an acute exacerbation of psychosis, and who were free of antipsychotic medication for at least 2 weeks.
Participants were required to have a baseline Positive and Negative Syndrome Scale (PANSS) total score of 80 points or more.
In addition to seven positive symptom items, including delusions, hallucinations, and conceptual disorganization, the PANSS has seven negative symptom items. These include restricted emotional expression, paucity of speech, and diminished interest, social drive, and activity. Each item is scored from 1 to 7, with higher scores indicating more severe symptoms.
Patients also had to have a score on the Clinical Global Impression–Severity (CGI-S) scale of 4 or higher. Scores on the CGI-S range from 1 to 7, with higher scores indicating greater severity of illness.
The modified intention-to-treat analysis included patients randomly assigned to receive oral xanomeline-trospium (n = 83) or placebo (n = 87).
The dosing schedule was flexible, starting with 50 mg of xanomeline and 20 mg of trospium twice daily. The schedule increased to a maximum of 125 mg of xanomeline and 30 mg of trospium twice daily, with the option of lowering the dose if there were unacceptable side effects.
Mean scores at baseline for the treatment and placebo groups were 97.7 versus 96.6 for the PANSS total score, 26.4 versus 26.3 for the positive subscore, 22.6 versus 22.8 for the negative subscore, and 5.0 versus 4.9 in the CGI-S scale.
‘Impressively robust’ effect size
The primary endpoint was change in the PANSS total score at 5 weeks. Results showed a change of –17.4 points in the treatment group and –5.9 points in the placebo group (least-squares mean difference, –11.6 points; 95% confidence interval, –16.1 to –7.1; P < .001).
The effect size, which was almost 0.8 (0.75), was “impressively robust,” said Dr. Lieberman, adding that a moderate effect size in this patient population might be in the order of 0.4 or 0.5.
“That gives hope that this drug may not just be as effective as other antipsychotics, albeit acting in a novel way and in a way that has a less of side effect burden, but that it may actually have some elements of superior efficacy,” he said.
There were significant benefits on some secondary outcomes, including change in the PANSS positive symptom subscore (–5.6 points in the treatment group vs. –2.4 points in the placebo group; least-squares mean difference, –3.2 points; 95% CI, –4.8 to –1.7; P < .001).
The active treatment also came out on top for CGI-S scores (P < .001), and PANSS negative symptom subscore (P < .001).
Because participants were hospitalized with an acute exacerbation of positive symptoms at time of study, it is difficult to determine “definitive efficacy” for negative symptoms, Dr. Lieberman noted. Negative symptoms may have improved simply because positive symptoms got better, he said.
Although the study included adults only, “there is nothing in the KarXT clinical profile that suggests it would be problematic for younger people,” Dr. Lieberman noted. This could include teenagers with first-episode psychosis.
Safety profile
Adverse events (AEs) were reported in 54% of the treatment group and 43% of the placebo group. AEs that were more common in the active treatment group included constipation (17% vs. 3%), nausea (17% vs. 4%), dry mouth (9% vs. 1%), dyspepsia (9% vs. 4%), and vomiting (9% vs. 4%). All AEs were rated as mild or moderate in severity and none resulted in discontinuation of treatment.
Rates of nausea, vomiting, and dry mouth were highest early in the trial and lower at the end, whereas constipation remained constant throughout the study.
Persistent constipation could affect the drug’s “utility” in elderly patients with cognitive issues but may be more of a “minor nuisance,” compared with other antipsychotics for those with schizophrenia, said Dr. Lieberman. He added that constipation might be mitigated with an over-the-counter treatment such as Metamucil. Importantly, there was no difference between groups in extrapyramidal symptoms.
In addition, participants receiving the active treatment did not have greater weight gain, which was about 3% versus 4% in the placebo group. The mean change in weight was 1.5 kg (3.3 lb) and 1.1 kg (2.4 lb), respectively.
Dr. Lieberman praised the manufacturer for undertaking the study.
“In an era when Big Pharma has retreated to a considerable degree from psychotropic drug development, it’s commendable that some companies have stayed the course and are succeeding in drug development,” he said.
Exciting mechanism
Commenting on the findings in an interview, Thomas Sedlak, MD, PhD, director of the Schizophrenia and Psychosis Consult Clinic and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, called some aspects of the study “exciting.”
What’s especially important is that the novel agent “acts by a different mechanism than what we have been using for maybe 60 years,” said Dr. Sedlak, who was not involved with the research.
“There has been a lot of research in the last few decades into drugs that might act by alternate mechanisms of action, but nothing’s really gotten to market for schizophrenia,” he noted.
Another interesting aspect of the study is that it examined a combination drug that aims to maximize effects on the brain while minimizing periphery effects, Dr. Sedlak said.
In addition, he called the PANSS results “respectable.”
“It’s interesting that you get a big change in PANSS total score, but it’s not as big in the positive symptom score as you might have expected, and it’s not a huge drop in negative symptoms either,” said Dr. Sedlak.
It’s possible, he said, that the drug is targeting PANSS elements not captured in the main positive or negative symptom items; for example, anxiety, depression, guilt, attention, impulse control, disorientation, and judgment.
Dr. Sedlak noted that, while the new results are exciting, the enthusiasm may not be long-lasting. “When a new drug comes out, there’s often a lot of excitement about it, but once you start using it, expectations may deflate..
“More flexible ratios” of the two components of the drug might be useful to treat individual patients, he added.
In addition, using the components by themselves might be preferable for some clinicians. “I think a lot of physicians prefer using two separate drugs so they can alter the dose of each one independently and not be stuck with the ratio the manufacturer is providing,” Dr. Sedlak said.
However, he acknowledged that studying different choices of ratios would require much larger trials.
The study was supported by Karuna Therapeutics and the Wellcome Trust. Dr. Lieberman reported serving on an advisory board for Karuna Therapeutics and Intra-Cellular Therapies. Dr. Sedlak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel therapy that combines a muscarinic receptor agonist with an anticholinergic agent is associated with a greater reduction in psychosis symptoms, compared with placebo, new research shows.
In a randomized, phase 2 trial composed of nearly 200 participants, xanomeline-trospium (KarXT) was generally well tolerated and had none of the common side effects linked to current antipsychotics, including weight gain and extrapyramidal symptoms such as dystonia, parkinsonism, and tardive dyskinesia.
“The results showing robust therapeutic efficacy of a non–dopamine targeting antipsychotic drug is an important milestone in the advance of the therapeutics of schizophrenia and other psychotic disorders,” coinvestigator Jeffrey A. Lieberman, MD, professor and chairman in the department of psychiatry, Columbia University, New York, said in an interview.
If approved, the new agent will be a “landmark” drug, Dr. Lieberman added.
The study was published in the Feb. 25, 2021, issue of the New England Journal of Medicine.
Long journey
The journey to develop an effective schizophrenia drug that reduces psychosis symptoms without onerous side effects has been a long one full of excitement and disappointment.
First-generation antipsychotics, dating back to the 1950s, targeted the postsynaptic dopamine-2 (D2) receptor. At the time, it was a “breakthrough” similar in scope to insulin for diabetes or antibiotics for infections, said Dr. Lieberman.
That was followed by development of numerous “me too” drugs with the same mechanism of action. However, these drugs had significant side effects, especially neurologic adverse events such as parkinsonism.
In 1989, second-generation antipsychotics were introduced, beginning with clozapine. They still targeted the D2 receptor but were “kinder and gentler,” Dr. Lieberman noted. “They didn’t bind to [the receptor] with such affinity that it shut things down completely, so had fewer neurologic side effects.”
However, these agents had other adverse consequences, such as weight gain and other metabolic effects including hyperglycemia and hyperlipidemia.
Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
“The pharmaceutical industry, biotech industry, and academic psychiatric community have been desperately trying to find novel strategies for antipsychotic drug development and asking, ‘Is D2 the only holy grail or are there other ways of treating psychotic symptoms of schizophrenia?’ ” Dr. Lieberman said.
Enter KarXT – a novel combination of xanomeline with trospium.
An ‘ingenious’ combination
Xanomeline, an oral muscarinic cholinergic receptor agonist, does not have direct effects on the dopamine receptor. Evidence suggests the muscarinic cholinergic system is involved in the pathophysiology of schizophrenia.
However, there may be dose-dependent adverse events with the medication, such as nausea, vomiting, diarrhea, sweating, and hypersalivation from stimulation of peripheral muscarinic cholinergic receptors.
That’s where trospium chloride, an oral panmuscarinic receptor antagonist approved for treating overactive bladder, comes in. It does not reach detectable levels in the cerebrospinal fluid and should avoid adverse central nervous system effects.
Dr. Lieberman said the idea of the drug combination is “ingenious.”
The new phase 2, multisite study included adult patients with a validated diagnosis of schizophrenia who were hospitalized with an acute exacerbation of psychosis, and who were free of antipsychotic medication for at least 2 weeks.
Participants were required to have a baseline Positive and Negative Syndrome Scale (PANSS) total score of 80 points or more.
In addition to seven positive symptom items, including delusions, hallucinations, and conceptual disorganization, the PANSS has seven negative symptom items. These include restricted emotional expression, paucity of speech, and diminished interest, social drive, and activity. Each item is scored from 1 to 7, with higher scores indicating more severe symptoms.
Patients also had to have a score on the Clinical Global Impression–Severity (CGI-S) scale of 4 or higher. Scores on the CGI-S range from 1 to 7, with higher scores indicating greater severity of illness.
The modified intention-to-treat analysis included patients randomly assigned to receive oral xanomeline-trospium (n = 83) or placebo (n = 87).
The dosing schedule was flexible, starting with 50 mg of xanomeline and 20 mg of trospium twice daily. The schedule increased to a maximum of 125 mg of xanomeline and 30 mg of trospium twice daily, with the option of lowering the dose if there were unacceptable side effects.
Mean scores at baseline for the treatment and placebo groups were 97.7 versus 96.6 for the PANSS total score, 26.4 versus 26.3 for the positive subscore, 22.6 versus 22.8 for the negative subscore, and 5.0 versus 4.9 in the CGI-S scale.
‘Impressively robust’ effect size
The primary endpoint was change in the PANSS total score at 5 weeks. Results showed a change of –17.4 points in the treatment group and –5.9 points in the placebo group (least-squares mean difference, –11.6 points; 95% confidence interval, –16.1 to –7.1; P < .001).
The effect size, which was almost 0.8 (0.75), was “impressively robust,” said Dr. Lieberman, adding that a moderate effect size in this patient population might be in the order of 0.4 or 0.5.
“That gives hope that this drug may not just be as effective as other antipsychotics, albeit acting in a novel way and in a way that has a less of side effect burden, but that it may actually have some elements of superior efficacy,” he said.
There were significant benefits on some secondary outcomes, including change in the PANSS positive symptom subscore (–5.6 points in the treatment group vs. –2.4 points in the placebo group; least-squares mean difference, –3.2 points; 95% CI, –4.8 to –1.7; P < .001).
The active treatment also came out on top for CGI-S scores (P < .001), and PANSS negative symptom subscore (P < .001).
Because participants were hospitalized with an acute exacerbation of positive symptoms at time of study, it is difficult to determine “definitive efficacy” for negative symptoms, Dr. Lieberman noted. Negative symptoms may have improved simply because positive symptoms got better, he said.
Although the study included adults only, “there is nothing in the KarXT clinical profile that suggests it would be problematic for younger people,” Dr. Lieberman noted. This could include teenagers with first-episode psychosis.
Safety profile
Adverse events (AEs) were reported in 54% of the treatment group and 43% of the placebo group. AEs that were more common in the active treatment group included constipation (17% vs. 3%), nausea (17% vs. 4%), dry mouth (9% vs. 1%), dyspepsia (9% vs. 4%), and vomiting (9% vs. 4%). All AEs were rated as mild or moderate in severity and none resulted in discontinuation of treatment.
Rates of nausea, vomiting, and dry mouth were highest early in the trial and lower at the end, whereas constipation remained constant throughout the study.
Persistent constipation could affect the drug’s “utility” in elderly patients with cognitive issues but may be more of a “minor nuisance,” compared with other antipsychotics for those with schizophrenia, said Dr. Lieberman. He added that constipation might be mitigated with an over-the-counter treatment such as Metamucil. Importantly, there was no difference between groups in extrapyramidal symptoms.
In addition, participants receiving the active treatment did not have greater weight gain, which was about 3% versus 4% in the placebo group. The mean change in weight was 1.5 kg (3.3 lb) and 1.1 kg (2.4 lb), respectively.
Dr. Lieberman praised the manufacturer for undertaking the study.
“In an era when Big Pharma has retreated to a considerable degree from psychotropic drug development, it’s commendable that some companies have stayed the course and are succeeding in drug development,” he said.
Exciting mechanism
Commenting on the findings in an interview, Thomas Sedlak, MD, PhD, director of the Schizophrenia and Psychosis Consult Clinic and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, called some aspects of the study “exciting.”
What’s especially important is that the novel agent “acts by a different mechanism than what we have been using for maybe 60 years,” said Dr. Sedlak, who was not involved with the research.
“There has been a lot of research in the last few decades into drugs that might act by alternate mechanisms of action, but nothing’s really gotten to market for schizophrenia,” he noted.
Another interesting aspect of the study is that it examined a combination drug that aims to maximize effects on the brain while minimizing periphery effects, Dr. Sedlak said.
In addition, he called the PANSS results “respectable.”
“It’s interesting that you get a big change in PANSS total score, but it’s not as big in the positive symptom score as you might have expected, and it’s not a huge drop in negative symptoms either,” said Dr. Sedlak.
It’s possible, he said, that the drug is targeting PANSS elements not captured in the main positive or negative symptom items; for example, anxiety, depression, guilt, attention, impulse control, disorientation, and judgment.
Dr. Sedlak noted that, while the new results are exciting, the enthusiasm may not be long-lasting. “When a new drug comes out, there’s often a lot of excitement about it, but once you start using it, expectations may deflate..
“More flexible ratios” of the two components of the drug might be useful to treat individual patients, he added.
In addition, using the components by themselves might be preferable for some clinicians. “I think a lot of physicians prefer using two separate drugs so they can alter the dose of each one independently and not be stuck with the ratio the manufacturer is providing,” Dr. Sedlak said.
However, he acknowledged that studying different choices of ratios would require much larger trials.
The study was supported by Karuna Therapeutics and the Wellcome Trust. Dr. Lieberman reported serving on an advisory board for Karuna Therapeutics and Intra-Cellular Therapies. Dr. Sedlak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
RA experts highlight key developments over the past year
A massive Swedish cohort study suggesting that biologic agents reduce the known excess risk of lymphoma in patients with rheumatoid arthritis was hailed as one of the high points of the last year by speakers at the 2021 Rheumatology Winter Clinical Symposium.
The speakers gave additional shout-outs regarding other key developments in RA during the pandemic year, including two huge studies showing that even low-dose corticosteroids at 5 mg/day or less carry the price of increased risk of cardiovascular disease and serious infections; Swedish data showing that RA disease activity can be used for venous thromboembolism (VTE) risk stratification; evidence from the venerable BeST study showing at 17 years of follow-up that treat-to-target strategies didn’t prevent an increased mortality risk of RA compared to the general population; and a signal that artificial intelligence using machine learning will become useful in clinical practice sooner than many realize.
Also, the speakers offered kudos to the investigators in the SEAM-RA trial, whose results they expect to be practice changing. In addition, they praised the SELECT-CHOICE trial for providing insight into the relative safety and efficacy of upadacitinib (Rinvoq) and abatacept (Orencia) as second- or third-line therapy in RA. And they warned their colleagues of rocky upcoming months for tofacitinib (Xeljanz) as the Food and Drug Administration reviews concerning new data from a long-term postmarketing safety trial.
Swedes show biologics may reduce excess lymphoma risk in RA
Decades ago, Swedish investigators harnessed their nation’s comprehensive linked health registries to demonstrate that patients with RA are inherently at greater risk for developing lymphoma. Now the Swedes have used the registries to identify a strong signal that this excess risk of lymphoma is reduced in RA patients treated with biologic drugs.
The study included 16,392 RA patients who initiated treatment with a biologic during the study period, 55,253 who were biologic-naive, and 229,047 age- and sex-matched controls from the general population. During the years 2006-2016, there was an adjusted 31% reduction in the risk of incident lymphoma in RA patients who started a first biologic agent, compared with those who did not, and a 54% reduction in lymphoma in biologic-treated patients, compared with those who switched from one conventional synthetic disease-modifying antirheumatic drug (DMARD) to another.
Moreover, while RA patients with 0-2 years of accumulated time on biologics were at a statistically significant 3.42-fold increased risk of lymphoma, compared with the matched general population, that risk shrunk with longer exposure to these medications, dropping to a nonsignificant 1.53-fold increase with 3-4 years of biologic exposure and a 1.04-fold risk with 5 years or more. The lymphoma risks didn’t vary significantly among the TNF inhibitors, nor with the use of TNF inhibitors versus other biologics.
“I think this is really quite encouraging,” said John J. Cush, MD, professor of internal medicine at the University of Texas, Dallas. “If the lymphoma risk was related to inflammation, I think you’d see results like this. It really does speak to the fact that control of inflammation over time does lessen one’s risk of cancer.”
Arthur Kavanaugh, MD, observed that patients with RA continue to ask their rheumatologists if going on a TNF inhibitor will increase their risk of lymphoma, so these Swedish data are going to be useful in conversations in the clinic.
“I thought this study was very exciting,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and director of RWCS. “Maybe we can make the case now that the longer you’re on biologic therapies and the more recently you’re being treated, the better you do in terms of lymphoma risk.”
Beware underappreciated hazards of very-low-dose glucocorticoids
The increased risk of osteoporotic fractures associated with corticosteroid therapy gets tons of attention. But the past year brought two cautionary studies that newly identified significantly increased risks of cardiovascular events and serious infection as well, even at doses of less than 5 mg/day, which have traditionally been believed safe long term.
Investigators at the University of Leeds (England) conducted a population-based cohort study in which they used the U.K. Clinical Practice Research Datalink to analyze the oral corticosteroid dose-dependent cardiovascular risk in 25,324 patients with RA and 62,470 with five other immune-mediated inflammatory diseases: inflammatory bowel disease, giant cell arteritis, polymyalgia rheumatica, systemic lupus erythematosus, and vasculitis. None had known cardiovascular disease at baseline. The analysis accounted for changes in oral steroid dose over time and for the use of potentially confounding medications.
Over a median 5 years of follow-up, a strong steroid dose-dependent risk of acute MI, heart failure, atrial fibrillation, cerebrovascular disease, and abdominal aortic aneurysm was evident in patients with any of the six of the immune-mediated inflammatory diseases. The 1-year adjusted cumulative risk of major adverse cardiovascular events jumped from 1.4% during periods of nonuse to 8.9% at a daily dose of 25 mg or more. Notably, when patients were on oral steroids at doses less than 5 mg/day, their risk of one or more of these major adverse cardiovascular events was 74% greater than when off steroids.
A separate red flag regarding low-dose glucocorticoids was raised by a retrospective cohort study of the 1-year risk of hospitalization for serious infection in 172,041 RA patients in the Medicare claims database for the years 2005-2015. After 6 months of stable use of DMARDs, 47.1% of those patients were on corticosteroids. Their 1-year cumulative incidence of severe infection resulting in hospitalization was 11% if their dose was 5 mg/day or less, 14.4% at >5 to 10 mg/day, and 17.7% at >10 mg/day, all significantly greater than the 8.6% rate in patients not on steroids. The same pattern held true when the investigators separately scrutinized 44,118 RA patients in the Optum Clinformatics Data Mart database for the years 2006-2015.
“This is a really important paper telling us what you’ve always known, which is that there’s a dose-related increase in serious infections in people on steroids. Everybody knows that, but usually people think of 10 mg/day and above,” Dr. Cush said. “This is a little bit sobering for those of you who think, ‘Well, I’m doing OK using 2.5 or 4 mg/day.’ In this study, even at doses of 5 mg/day or less, there’s a significant increase in serious hospitalizable infections.”
Taken together, the message of these two huge studies is clear, he added: “The idea is we probably shouldn’t be using steroids.”
Given the large array of therapies now available to address various aspects of the inflammatory and immune responses in RA, Dr. Kavanaugh asked, why do so many rheumatologists still maintain their patients on low-dose steroids?
”Because when we find something that works, we stick to it,” Dr. Cush replied.
RA disease activity predicts VTE risk
The risk of VTE climbs with RA disease activity such that patients with high disease activity – defined using the erythrocyte sedimentation rate-based Disease Activity Score in 28 joints (DAS28-ESR) – have double the VTE risk of those in disease remission, according to a Swedish nationwide cohort study using data from the Swedish Rheumatology Quality Register. The investigators concluded that RA disease activity can be used in clinical practice as an additional tool for VTE risk stratification, alongside such established risk factors as immobilization, age, surgery, and comorbid conditions.
The study included 46,316 patients with RA who collectively experienced 2,241 VTE events within 1 year after one of their collective 322,601 visits to their rheumatologist. The overall cumulative 1-year incidence of VTE in the RA population was 0.71%, versus 0.36% in a control group composed of 215,843 randomly selected age- and sex-matched individuals drawn from the general Swedish population. That translates to an adjusted 1.88-fold increased risk in the RA group.
The VTE incidence was 0.52% in the year following a rheumatologist visit at which RA patients were found to be in DAS28-ESR remission. The adjusted risk for VTE climbed significantly in stepwise fashion with increasing disease activity: 12% greater risk with DAS28-ESR low disease activity, 48% greater risk with moderate disease activity, and 2.03-fold greater risk with high disease activity.
SEAM-RA shows which drug to withdraw to maintain remission gained on combo therapy
The SEAM-RA trial presented at the 2020 annual meeting of the American College of Rheumatology (ACR) and published shortly afterward in Arthritis & Rheumatology provides guidance on how to best implement a drug tapering strategy in patients who have achieved sustained remission on combination therapy with etanercept (Enbrel) and methotrexate. The purpose of such a strategy is to reduce patients’ medication burden and exposure to safety concerns inherent in continuing therapy. SEAM-RA included 371 RA patients in sustained disease remission during 24 weeks of open-label treatment with etanercept plus methotrexate. At that point, they were randomized 2:2:1 to 48 weeks of double-blind methotrexate monotherapy with etanercept withdrawal, etanercept monotherapy, or continued combination therapy. Those who experienced disease worsening on double-blind monotherapy were eligible for combination rescue therapy with both drugs.
The primary endpoint was maintenance of remission without disease worsening at week 48. This was accomplished in 52.9% of the combination therapy group, with a similar 49.5% success rate in patients on etanercept monotherapy, both significantly better than the 28.7% rate with methotrexate monotherapy. The inference is that biologic monotherapy might be an advantageous strategy for maintenance after achieving prolonged remission on combination therapy.
“This study could impact guidelines,” Dr. Cush predicted.
Among the key study findings, in his view, was that disease worsening occurred early following the switch to methotrexate monotherapy: typically within the first 2-4 weeks. And combination rescue therapy worked: Almost 75% of patients were able to recapture disease remission regardless of which drug they’d been on as monotherapy, and almost 90% of patients on rescue therapy achieved a low-disease-activity state.
Still, just over half of patients were able to maintain long-term remission after downshifting to etanercept monotherapy, Dr. Kavanaugh noted. “The bedeviling thing is we don’t know which patients can do this,” he said.
This was made abundantly clear in PREDICTRA, a phase 4, double-blind study in which 122 RA patients in sustained remission on 40 mg of adalimumab (Humira) every 2 weeks were randomized 5:1 to adalimumab taper or withdrawal for 36 weeks. Overall, 36% of the taper group and 45% of those who halted the biologic flared within 36 weeks. Nothing on an extensive list of candidate baseline predictors predicted flare in either group.
”I think the interesting thing here is that nothing predicted it, not drug levels, antidrug antibodies, not even MRI evidence of inflammation while in remission. The lack of predictors of who’s going to do well off therapy makes us crazy,” Dr. Kavanaugh said. Also noteworthy was that when full-dose, open-label adalimumab was reinstituted as rescue therapy in patients who flared, only half of them regained remission during the following 4 months, he observed.
Dr. Cush didn’t mince words regarding his view of the concept underlying PREDICTRA.
“Why are we even talking about this? We spend our whole lives trying to get these patients into remission, we’re heavily invested in combination therapy, we’re very proud of our great successes, and now we want to find data to condone, ‘Let’s get off drug therapy?’ ” he argued.
“There are untold consequences to this,” the rheumatologist continued. “There are cardiovascular consequences, and we’ve seen the x-ray studies that show that patients may be doing clinically better but still have x-ray worsening because you’ve withdrawn the TNF inhibitor. I think this is all nonsense, and I’m totally against it.”
Making a SELECT-CHOICE in refractory RA
In patients whose RA has proved refractory to one or more biologics, opting for the oral Janus kinase inhibitor upadacitinib as next-line therapy is significantly more effective than turning to the T-cell costimulation modulator abatacept, but at the cost of a doubled risk of severe adverse events, according to the findings of the phase 3, double-blind, 24-week, randomized SELECT-CHOICE trial.
In this 612-patient study, the week-12 clinical remission rate as defined by a C-reactive protein (CRP)-based DAS28 below 2.6 was 30% in the upadacitinib arm, compared to 13.3% with abatacept. At week 24, the remission rates were 46% and 31%, respectively. However, the severe adverse event rate was 6.3% in the upadacitinib group, including one death, one stroke, and two cases of VTE, compared to 3.2% with abatacept.
“Maybe this is a win for upadacitinib, but abatacept is a win when it comes to safety,” Dr. Cush said. “So the question is, when you’re making your second or third change in a biologic DMARD, are you going for safety or are you going for efficacy?”
Make way for machine learning
Studies of machine learning and artificial intelligence aimed at developing a more individualized treatment approach are popping up with increasing frequency at the major rheumatology meetings.
“This is really, I think, the future because for most of us, in most of our choices, as great as all of us are in managing RA, it is a crystal ball and a coin toss as to whether our next therapy is going to work,” Dr. Cush said.
He highlighted a study presented at the 2020 ACR annual meeting as an example of how machine learning can generate better evidence for selecting therapies. An international team of investigators used artificial intelligence to develop a simple rule to predict an RA patient’s likely response to the interleukin-6 receptor inhibitor sarilumab (Kevzara). The researchers developed a list of 42 candidate predictors of an ACR20 response, including demographics, biomarkers, and disease activity scores, then applied machine learning to data from the 1,197-patient, phase 3 MOBILITY trial of sarilumab versus placebo in order to generate a predictive rule of better outcomes.
Out of the 42 candidate predictive values, the best predictive rule turned out to be the combination of the presence of anti-citrullinated protein antibodies and a baseline CRP level greater than 12.3 mg/L. This rule was then put to the test using data from four phase 3 clinical trials in which 34%-51% of RA patients randomized to sarilumab were rule-positive. The rule-positive patients had worse baseline prognostic factors and more severe RA. Yet rule-positive patients had a better outcome than that of those who were rule-negative. For example, a week-24 ACR70 response was achieved in 34% of rule-positive patients, nearly fivefold greater than the 7% rate in the rule-negative group.
Treat-to-target strategies don’t tame excess mortality in RA
At a mean of 17 years of follow-up in the Dutch BeST study – a pioneering treat-to-target study in RA – patients with early RA who were assigned to one of four treat-to-target strategies for 10 years showed excess mortality compared to the general age- and sex-matched Dutch population, Johanna M. Maassen, MD, reported at the 2020 annual meeting of the European Alliance of Associations for Rheumatology (EULAR). Indeed, mortality was 37% higher in the RA patients than in the comparator population, with no significant differences in survival curves evident between the four tight-control strategies.
“This raises the question of whether survival in patients not strictly treated to target would have been even lower. Are we not doing as well as we think we’re doing?” Dr. Kavanaugh said.
“This is very sobering. It tells us we really do need to be better at managing RA. And I would say based on this data we need to be better at managing RA really early to get better outcomes really late,” said Dr. Cush. “The damage that led to cardiovascular risk could have been incurred earlier on and then carried forward when you look out to 15-20 years.”
More trouble for tofacitinib
Tofacitinib faces an uncertain future following Pfizer’s late-January press release announcing that the JAK inhibitor failed to meet noninferiority compared to anti-TNF therapy in terms of adjudicated major adverse cardiovascular events (MACE) and adjudicated malignancies in an FDA-mandated postmarketing safety study. The study, known as ORAL Surveillance, or Study A3921133 – ‘1133’ for short – randomized 4,362 RA patients aged 50 years or older with at least one additional cardiovascular risk factor to tofacitinib at 5 or 10 mg twice daily or to a TNF inhibitor.
The Pfizer press release was soon followed by an FDA safety warning and announcement that the agency is reviewing the full study data before making any final recommendations. The FDA already requires black box warnings for tofacitinib regarding increased risks of blood clots and death, but only at the 10-mg twice-daily dose. In the ORAL Surveillance study, however, the primary endpoint was noninferiority of the combined tofacitinib doses, and there was no evidence of a difference in endpoints between the 5- and 10-mg twice-daily doses.
Only the topline results released in the Pfizer press release are publicly available so far. The combined tofacitinib doses were associated with a 1.33-fold greater risk of adjudicated MACE and a 1.48-fold increase in adjudicated malignancies other than nonmelanoma skin cancer, compared to adalimumab or etanercept.
The jury is still out pending release of the full study data, in Dr. Kavanaugh’s view.
“When I get to look at the data, I want to see who are the people who had the events, their characteristics,” he said.
Alexis R. Ogdie-Beatty, MD, said the study’s topline results “make me a little nervous.
“The effect size is pretty large. It’s in the 1.4 range, which means there might be something there for real,” said Dr. Ogdie-Beatty, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
In contrast, Eric M. Ruderman, MD, said he’s not concerned “just yet” about the results.
“Frankly, the FDA was just plain wrong in their press release because the confidence intervals for the MACE events crossed 1.0 meaningfully [0.91, 1.94], so there’s nothing there. And the confidence intervals hit 1.0 for the malignancies,” observed Dr. Ruderman, professor of medicine at Northwestern University, Chicago.
This was a nearly 4,400-patient study, and given that it couldn’t convincingly show a safety risk, such risk – if it even exists – would seem to be tiny, he added.
“It’s probably not going to change the way we prescribe these drugs. So I don’t see that trial taking away from the big run for JAK inhibitors we’re going to see in the next year,” the rheumatologist said.
The speakers reported having financial relationships with numerous pharmaceutical companies.
A massive Swedish cohort study suggesting that biologic agents reduce the known excess risk of lymphoma in patients with rheumatoid arthritis was hailed as one of the high points of the last year by speakers at the 2021 Rheumatology Winter Clinical Symposium.
The speakers gave additional shout-outs regarding other key developments in RA during the pandemic year, including two huge studies showing that even low-dose corticosteroids at 5 mg/day or less carry the price of increased risk of cardiovascular disease and serious infections; Swedish data showing that RA disease activity can be used for venous thromboembolism (VTE) risk stratification; evidence from the venerable BeST study showing at 17 years of follow-up that treat-to-target strategies didn’t prevent an increased mortality risk of RA compared to the general population; and a signal that artificial intelligence using machine learning will become useful in clinical practice sooner than many realize.
Also, the speakers offered kudos to the investigators in the SEAM-RA trial, whose results they expect to be practice changing. In addition, they praised the SELECT-CHOICE trial for providing insight into the relative safety and efficacy of upadacitinib (Rinvoq) and abatacept (Orencia) as second- or third-line therapy in RA. And they warned their colleagues of rocky upcoming months for tofacitinib (Xeljanz) as the Food and Drug Administration reviews concerning new data from a long-term postmarketing safety trial.
Swedes show biologics may reduce excess lymphoma risk in RA
Decades ago, Swedish investigators harnessed their nation’s comprehensive linked health registries to demonstrate that patients with RA are inherently at greater risk for developing lymphoma. Now the Swedes have used the registries to identify a strong signal that this excess risk of lymphoma is reduced in RA patients treated with biologic drugs.
The study included 16,392 RA patients who initiated treatment with a biologic during the study period, 55,253 who were biologic-naive, and 229,047 age- and sex-matched controls from the general population. During the years 2006-2016, there was an adjusted 31% reduction in the risk of incident lymphoma in RA patients who started a first biologic agent, compared with those who did not, and a 54% reduction in lymphoma in biologic-treated patients, compared with those who switched from one conventional synthetic disease-modifying antirheumatic drug (DMARD) to another.
Moreover, while RA patients with 0-2 years of accumulated time on biologics were at a statistically significant 3.42-fold increased risk of lymphoma, compared with the matched general population, that risk shrunk with longer exposure to these medications, dropping to a nonsignificant 1.53-fold increase with 3-4 years of biologic exposure and a 1.04-fold risk with 5 years or more. The lymphoma risks didn’t vary significantly among the TNF inhibitors, nor with the use of TNF inhibitors versus other biologics.
“I think this is really quite encouraging,” said John J. Cush, MD, professor of internal medicine at the University of Texas, Dallas. “If the lymphoma risk was related to inflammation, I think you’d see results like this. It really does speak to the fact that control of inflammation over time does lessen one’s risk of cancer.”
Arthur Kavanaugh, MD, observed that patients with RA continue to ask their rheumatologists if going on a TNF inhibitor will increase their risk of lymphoma, so these Swedish data are going to be useful in conversations in the clinic.
“I thought this study was very exciting,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and director of RWCS. “Maybe we can make the case now that the longer you’re on biologic therapies and the more recently you’re being treated, the better you do in terms of lymphoma risk.”
Beware underappreciated hazards of very-low-dose glucocorticoids
The increased risk of osteoporotic fractures associated with corticosteroid therapy gets tons of attention. But the past year brought two cautionary studies that newly identified significantly increased risks of cardiovascular events and serious infection as well, even at doses of less than 5 mg/day, which have traditionally been believed safe long term.
Investigators at the University of Leeds (England) conducted a population-based cohort study in which they used the U.K. Clinical Practice Research Datalink to analyze the oral corticosteroid dose-dependent cardiovascular risk in 25,324 patients with RA and 62,470 with five other immune-mediated inflammatory diseases: inflammatory bowel disease, giant cell arteritis, polymyalgia rheumatica, systemic lupus erythematosus, and vasculitis. None had known cardiovascular disease at baseline. The analysis accounted for changes in oral steroid dose over time and for the use of potentially confounding medications.
Over a median 5 years of follow-up, a strong steroid dose-dependent risk of acute MI, heart failure, atrial fibrillation, cerebrovascular disease, and abdominal aortic aneurysm was evident in patients with any of the six of the immune-mediated inflammatory diseases. The 1-year adjusted cumulative risk of major adverse cardiovascular events jumped from 1.4% during periods of nonuse to 8.9% at a daily dose of 25 mg or more. Notably, when patients were on oral steroids at doses less than 5 mg/day, their risk of one or more of these major adverse cardiovascular events was 74% greater than when off steroids.
A separate red flag regarding low-dose glucocorticoids was raised by a retrospective cohort study of the 1-year risk of hospitalization for serious infection in 172,041 RA patients in the Medicare claims database for the years 2005-2015. After 6 months of stable use of DMARDs, 47.1% of those patients were on corticosteroids. Their 1-year cumulative incidence of severe infection resulting in hospitalization was 11% if their dose was 5 mg/day or less, 14.4% at >5 to 10 mg/day, and 17.7% at >10 mg/day, all significantly greater than the 8.6% rate in patients not on steroids. The same pattern held true when the investigators separately scrutinized 44,118 RA patients in the Optum Clinformatics Data Mart database for the years 2006-2015.
“This is a really important paper telling us what you’ve always known, which is that there’s a dose-related increase in serious infections in people on steroids. Everybody knows that, but usually people think of 10 mg/day and above,” Dr. Cush said. “This is a little bit sobering for those of you who think, ‘Well, I’m doing OK using 2.5 or 4 mg/day.’ In this study, even at doses of 5 mg/day or less, there’s a significant increase in serious hospitalizable infections.”
Taken together, the message of these two huge studies is clear, he added: “The idea is we probably shouldn’t be using steroids.”
Given the large array of therapies now available to address various aspects of the inflammatory and immune responses in RA, Dr. Kavanaugh asked, why do so many rheumatologists still maintain their patients on low-dose steroids?
”Because when we find something that works, we stick to it,” Dr. Cush replied.
RA disease activity predicts VTE risk
The risk of VTE climbs with RA disease activity such that patients with high disease activity – defined using the erythrocyte sedimentation rate-based Disease Activity Score in 28 joints (DAS28-ESR) – have double the VTE risk of those in disease remission, according to a Swedish nationwide cohort study using data from the Swedish Rheumatology Quality Register. The investigators concluded that RA disease activity can be used in clinical practice as an additional tool for VTE risk stratification, alongside such established risk factors as immobilization, age, surgery, and comorbid conditions.
The study included 46,316 patients with RA who collectively experienced 2,241 VTE events within 1 year after one of their collective 322,601 visits to their rheumatologist. The overall cumulative 1-year incidence of VTE in the RA population was 0.71%, versus 0.36% in a control group composed of 215,843 randomly selected age- and sex-matched individuals drawn from the general Swedish population. That translates to an adjusted 1.88-fold increased risk in the RA group.
The VTE incidence was 0.52% in the year following a rheumatologist visit at which RA patients were found to be in DAS28-ESR remission. The adjusted risk for VTE climbed significantly in stepwise fashion with increasing disease activity: 12% greater risk with DAS28-ESR low disease activity, 48% greater risk with moderate disease activity, and 2.03-fold greater risk with high disease activity.
SEAM-RA shows which drug to withdraw to maintain remission gained on combo therapy
The SEAM-RA trial presented at the 2020 annual meeting of the American College of Rheumatology (ACR) and published shortly afterward in Arthritis & Rheumatology provides guidance on how to best implement a drug tapering strategy in patients who have achieved sustained remission on combination therapy with etanercept (Enbrel) and methotrexate. The purpose of such a strategy is to reduce patients’ medication burden and exposure to safety concerns inherent in continuing therapy. SEAM-RA included 371 RA patients in sustained disease remission during 24 weeks of open-label treatment with etanercept plus methotrexate. At that point, they were randomized 2:2:1 to 48 weeks of double-blind methotrexate monotherapy with etanercept withdrawal, etanercept monotherapy, or continued combination therapy. Those who experienced disease worsening on double-blind monotherapy were eligible for combination rescue therapy with both drugs.
The primary endpoint was maintenance of remission without disease worsening at week 48. This was accomplished in 52.9% of the combination therapy group, with a similar 49.5% success rate in patients on etanercept monotherapy, both significantly better than the 28.7% rate with methotrexate monotherapy. The inference is that biologic monotherapy might be an advantageous strategy for maintenance after achieving prolonged remission on combination therapy.
“This study could impact guidelines,” Dr. Cush predicted.
Among the key study findings, in his view, was that disease worsening occurred early following the switch to methotrexate monotherapy: typically within the first 2-4 weeks. And combination rescue therapy worked: Almost 75% of patients were able to recapture disease remission regardless of which drug they’d been on as monotherapy, and almost 90% of patients on rescue therapy achieved a low-disease-activity state.
Still, just over half of patients were able to maintain long-term remission after downshifting to etanercept monotherapy, Dr. Kavanaugh noted. “The bedeviling thing is we don’t know which patients can do this,” he said.
This was made abundantly clear in PREDICTRA, a phase 4, double-blind study in which 122 RA patients in sustained remission on 40 mg of adalimumab (Humira) every 2 weeks were randomized 5:1 to adalimumab taper or withdrawal for 36 weeks. Overall, 36% of the taper group and 45% of those who halted the biologic flared within 36 weeks. Nothing on an extensive list of candidate baseline predictors predicted flare in either group.
”I think the interesting thing here is that nothing predicted it, not drug levels, antidrug antibodies, not even MRI evidence of inflammation while in remission. The lack of predictors of who’s going to do well off therapy makes us crazy,” Dr. Kavanaugh said. Also noteworthy was that when full-dose, open-label adalimumab was reinstituted as rescue therapy in patients who flared, only half of them regained remission during the following 4 months, he observed.
Dr. Cush didn’t mince words regarding his view of the concept underlying PREDICTRA.
“Why are we even talking about this? We spend our whole lives trying to get these patients into remission, we’re heavily invested in combination therapy, we’re very proud of our great successes, and now we want to find data to condone, ‘Let’s get off drug therapy?’ ” he argued.
“There are untold consequences to this,” the rheumatologist continued. “There are cardiovascular consequences, and we’ve seen the x-ray studies that show that patients may be doing clinically better but still have x-ray worsening because you’ve withdrawn the TNF inhibitor. I think this is all nonsense, and I’m totally against it.”
Making a SELECT-CHOICE in refractory RA
In patients whose RA has proved refractory to one or more biologics, opting for the oral Janus kinase inhibitor upadacitinib as next-line therapy is significantly more effective than turning to the T-cell costimulation modulator abatacept, but at the cost of a doubled risk of severe adverse events, according to the findings of the phase 3, double-blind, 24-week, randomized SELECT-CHOICE trial.
In this 612-patient study, the week-12 clinical remission rate as defined by a C-reactive protein (CRP)-based DAS28 below 2.6 was 30% in the upadacitinib arm, compared to 13.3% with abatacept. At week 24, the remission rates were 46% and 31%, respectively. However, the severe adverse event rate was 6.3% in the upadacitinib group, including one death, one stroke, and two cases of VTE, compared to 3.2% with abatacept.
“Maybe this is a win for upadacitinib, but abatacept is a win when it comes to safety,” Dr. Cush said. “So the question is, when you’re making your second or third change in a biologic DMARD, are you going for safety or are you going for efficacy?”
Make way for machine learning
Studies of machine learning and artificial intelligence aimed at developing a more individualized treatment approach are popping up with increasing frequency at the major rheumatology meetings.
“This is really, I think, the future because for most of us, in most of our choices, as great as all of us are in managing RA, it is a crystal ball and a coin toss as to whether our next therapy is going to work,” Dr. Cush said.
He highlighted a study presented at the 2020 ACR annual meeting as an example of how machine learning can generate better evidence for selecting therapies. An international team of investigators used artificial intelligence to develop a simple rule to predict an RA patient’s likely response to the interleukin-6 receptor inhibitor sarilumab (Kevzara). The researchers developed a list of 42 candidate predictors of an ACR20 response, including demographics, biomarkers, and disease activity scores, then applied machine learning to data from the 1,197-patient, phase 3 MOBILITY trial of sarilumab versus placebo in order to generate a predictive rule of better outcomes.
Out of the 42 candidate predictive values, the best predictive rule turned out to be the combination of the presence of anti-citrullinated protein antibodies and a baseline CRP level greater than 12.3 mg/L. This rule was then put to the test using data from four phase 3 clinical trials in which 34%-51% of RA patients randomized to sarilumab were rule-positive. The rule-positive patients had worse baseline prognostic factors and more severe RA. Yet rule-positive patients had a better outcome than that of those who were rule-negative. For example, a week-24 ACR70 response was achieved in 34% of rule-positive patients, nearly fivefold greater than the 7% rate in the rule-negative group.
Treat-to-target strategies don’t tame excess mortality in RA
At a mean of 17 years of follow-up in the Dutch BeST study – a pioneering treat-to-target study in RA – patients with early RA who were assigned to one of four treat-to-target strategies for 10 years showed excess mortality compared to the general age- and sex-matched Dutch population, Johanna M. Maassen, MD, reported at the 2020 annual meeting of the European Alliance of Associations for Rheumatology (EULAR). Indeed, mortality was 37% higher in the RA patients than in the comparator population, with no significant differences in survival curves evident between the four tight-control strategies.
“This raises the question of whether survival in patients not strictly treated to target would have been even lower. Are we not doing as well as we think we’re doing?” Dr. Kavanaugh said.
“This is very sobering. It tells us we really do need to be better at managing RA. And I would say based on this data we need to be better at managing RA really early to get better outcomes really late,” said Dr. Cush. “The damage that led to cardiovascular risk could have been incurred earlier on and then carried forward when you look out to 15-20 years.”
More trouble for tofacitinib
Tofacitinib faces an uncertain future following Pfizer’s late-January press release announcing that the JAK inhibitor failed to meet noninferiority compared to anti-TNF therapy in terms of adjudicated major adverse cardiovascular events (MACE) and adjudicated malignancies in an FDA-mandated postmarketing safety study. The study, known as ORAL Surveillance, or Study A3921133 – ‘1133’ for short – randomized 4,362 RA patients aged 50 years or older with at least one additional cardiovascular risk factor to tofacitinib at 5 or 10 mg twice daily or to a TNF inhibitor.
The Pfizer press release was soon followed by an FDA safety warning and announcement that the agency is reviewing the full study data before making any final recommendations. The FDA already requires black box warnings for tofacitinib regarding increased risks of blood clots and death, but only at the 10-mg twice-daily dose. In the ORAL Surveillance study, however, the primary endpoint was noninferiority of the combined tofacitinib doses, and there was no evidence of a difference in endpoints between the 5- and 10-mg twice-daily doses.
Only the topline results released in the Pfizer press release are publicly available so far. The combined tofacitinib doses were associated with a 1.33-fold greater risk of adjudicated MACE and a 1.48-fold increase in adjudicated malignancies other than nonmelanoma skin cancer, compared to adalimumab or etanercept.
The jury is still out pending release of the full study data, in Dr. Kavanaugh’s view.
“When I get to look at the data, I want to see who are the people who had the events, their characteristics,” he said.
Alexis R. Ogdie-Beatty, MD, said the study’s topline results “make me a little nervous.
“The effect size is pretty large. It’s in the 1.4 range, which means there might be something there for real,” said Dr. Ogdie-Beatty, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
In contrast, Eric M. Ruderman, MD, said he’s not concerned “just yet” about the results.
“Frankly, the FDA was just plain wrong in their press release because the confidence intervals for the MACE events crossed 1.0 meaningfully [0.91, 1.94], so there’s nothing there. And the confidence intervals hit 1.0 for the malignancies,” observed Dr. Ruderman, professor of medicine at Northwestern University, Chicago.
This was a nearly 4,400-patient study, and given that it couldn’t convincingly show a safety risk, such risk – if it even exists – would seem to be tiny, he added.
“It’s probably not going to change the way we prescribe these drugs. So I don’t see that trial taking away from the big run for JAK inhibitors we’re going to see in the next year,” the rheumatologist said.
The speakers reported having financial relationships with numerous pharmaceutical companies.
A massive Swedish cohort study suggesting that biologic agents reduce the known excess risk of lymphoma in patients with rheumatoid arthritis was hailed as one of the high points of the last year by speakers at the 2021 Rheumatology Winter Clinical Symposium.
The speakers gave additional shout-outs regarding other key developments in RA during the pandemic year, including two huge studies showing that even low-dose corticosteroids at 5 mg/day or less carry the price of increased risk of cardiovascular disease and serious infections; Swedish data showing that RA disease activity can be used for venous thromboembolism (VTE) risk stratification; evidence from the venerable BeST study showing at 17 years of follow-up that treat-to-target strategies didn’t prevent an increased mortality risk of RA compared to the general population; and a signal that artificial intelligence using machine learning will become useful in clinical practice sooner than many realize.
Also, the speakers offered kudos to the investigators in the SEAM-RA trial, whose results they expect to be practice changing. In addition, they praised the SELECT-CHOICE trial for providing insight into the relative safety and efficacy of upadacitinib (Rinvoq) and abatacept (Orencia) as second- or third-line therapy in RA. And they warned their colleagues of rocky upcoming months for tofacitinib (Xeljanz) as the Food and Drug Administration reviews concerning new data from a long-term postmarketing safety trial.
Swedes show biologics may reduce excess lymphoma risk in RA
Decades ago, Swedish investigators harnessed their nation’s comprehensive linked health registries to demonstrate that patients with RA are inherently at greater risk for developing lymphoma. Now the Swedes have used the registries to identify a strong signal that this excess risk of lymphoma is reduced in RA patients treated with biologic drugs.
The study included 16,392 RA patients who initiated treatment with a biologic during the study period, 55,253 who were biologic-naive, and 229,047 age- and sex-matched controls from the general population. During the years 2006-2016, there was an adjusted 31% reduction in the risk of incident lymphoma in RA patients who started a first biologic agent, compared with those who did not, and a 54% reduction in lymphoma in biologic-treated patients, compared with those who switched from one conventional synthetic disease-modifying antirheumatic drug (DMARD) to another.
Moreover, while RA patients with 0-2 years of accumulated time on biologics were at a statistically significant 3.42-fold increased risk of lymphoma, compared with the matched general population, that risk shrunk with longer exposure to these medications, dropping to a nonsignificant 1.53-fold increase with 3-4 years of biologic exposure and a 1.04-fold risk with 5 years or more. The lymphoma risks didn’t vary significantly among the TNF inhibitors, nor with the use of TNF inhibitors versus other biologics.
“I think this is really quite encouraging,” said John J. Cush, MD, professor of internal medicine at the University of Texas, Dallas. “If the lymphoma risk was related to inflammation, I think you’d see results like this. It really does speak to the fact that control of inflammation over time does lessen one’s risk of cancer.”
Arthur Kavanaugh, MD, observed that patients with RA continue to ask their rheumatologists if going on a TNF inhibitor will increase their risk of lymphoma, so these Swedish data are going to be useful in conversations in the clinic.
“I thought this study was very exciting,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and director of RWCS. “Maybe we can make the case now that the longer you’re on biologic therapies and the more recently you’re being treated, the better you do in terms of lymphoma risk.”
Beware underappreciated hazards of very-low-dose glucocorticoids
The increased risk of osteoporotic fractures associated with corticosteroid therapy gets tons of attention. But the past year brought two cautionary studies that newly identified significantly increased risks of cardiovascular events and serious infection as well, even at doses of less than 5 mg/day, which have traditionally been believed safe long term.
Investigators at the University of Leeds (England) conducted a population-based cohort study in which they used the U.K. Clinical Practice Research Datalink to analyze the oral corticosteroid dose-dependent cardiovascular risk in 25,324 patients with RA and 62,470 with five other immune-mediated inflammatory diseases: inflammatory bowel disease, giant cell arteritis, polymyalgia rheumatica, systemic lupus erythematosus, and vasculitis. None had known cardiovascular disease at baseline. The analysis accounted for changes in oral steroid dose over time and for the use of potentially confounding medications.
Over a median 5 years of follow-up, a strong steroid dose-dependent risk of acute MI, heart failure, atrial fibrillation, cerebrovascular disease, and abdominal aortic aneurysm was evident in patients with any of the six of the immune-mediated inflammatory diseases. The 1-year adjusted cumulative risk of major adverse cardiovascular events jumped from 1.4% during periods of nonuse to 8.9% at a daily dose of 25 mg or more. Notably, when patients were on oral steroids at doses less than 5 mg/day, their risk of one or more of these major adverse cardiovascular events was 74% greater than when off steroids.
A separate red flag regarding low-dose glucocorticoids was raised by a retrospective cohort study of the 1-year risk of hospitalization for serious infection in 172,041 RA patients in the Medicare claims database for the years 2005-2015. After 6 months of stable use of DMARDs, 47.1% of those patients were on corticosteroids. Their 1-year cumulative incidence of severe infection resulting in hospitalization was 11% if their dose was 5 mg/day or less, 14.4% at >5 to 10 mg/day, and 17.7% at >10 mg/day, all significantly greater than the 8.6% rate in patients not on steroids. The same pattern held true when the investigators separately scrutinized 44,118 RA patients in the Optum Clinformatics Data Mart database for the years 2006-2015.
“This is a really important paper telling us what you’ve always known, which is that there’s a dose-related increase in serious infections in people on steroids. Everybody knows that, but usually people think of 10 mg/day and above,” Dr. Cush said. “This is a little bit sobering for those of you who think, ‘Well, I’m doing OK using 2.5 or 4 mg/day.’ In this study, even at doses of 5 mg/day or less, there’s a significant increase in serious hospitalizable infections.”
Taken together, the message of these two huge studies is clear, he added: “The idea is we probably shouldn’t be using steroids.”
Given the large array of therapies now available to address various aspects of the inflammatory and immune responses in RA, Dr. Kavanaugh asked, why do so many rheumatologists still maintain their patients on low-dose steroids?
”Because when we find something that works, we stick to it,” Dr. Cush replied.
RA disease activity predicts VTE risk
The risk of VTE climbs with RA disease activity such that patients with high disease activity – defined using the erythrocyte sedimentation rate-based Disease Activity Score in 28 joints (DAS28-ESR) – have double the VTE risk of those in disease remission, according to a Swedish nationwide cohort study using data from the Swedish Rheumatology Quality Register. The investigators concluded that RA disease activity can be used in clinical practice as an additional tool for VTE risk stratification, alongside such established risk factors as immobilization, age, surgery, and comorbid conditions.
The study included 46,316 patients with RA who collectively experienced 2,241 VTE events within 1 year after one of their collective 322,601 visits to their rheumatologist. The overall cumulative 1-year incidence of VTE in the RA population was 0.71%, versus 0.36% in a control group composed of 215,843 randomly selected age- and sex-matched individuals drawn from the general Swedish population. That translates to an adjusted 1.88-fold increased risk in the RA group.
The VTE incidence was 0.52% in the year following a rheumatologist visit at which RA patients were found to be in DAS28-ESR remission. The adjusted risk for VTE climbed significantly in stepwise fashion with increasing disease activity: 12% greater risk with DAS28-ESR low disease activity, 48% greater risk with moderate disease activity, and 2.03-fold greater risk with high disease activity.
SEAM-RA shows which drug to withdraw to maintain remission gained on combo therapy
The SEAM-RA trial presented at the 2020 annual meeting of the American College of Rheumatology (ACR) and published shortly afterward in Arthritis & Rheumatology provides guidance on how to best implement a drug tapering strategy in patients who have achieved sustained remission on combination therapy with etanercept (Enbrel) and methotrexate. The purpose of such a strategy is to reduce patients’ medication burden and exposure to safety concerns inherent in continuing therapy. SEAM-RA included 371 RA patients in sustained disease remission during 24 weeks of open-label treatment with etanercept plus methotrexate. At that point, they were randomized 2:2:1 to 48 weeks of double-blind methotrexate monotherapy with etanercept withdrawal, etanercept monotherapy, or continued combination therapy. Those who experienced disease worsening on double-blind monotherapy were eligible for combination rescue therapy with both drugs.
The primary endpoint was maintenance of remission without disease worsening at week 48. This was accomplished in 52.9% of the combination therapy group, with a similar 49.5% success rate in patients on etanercept monotherapy, both significantly better than the 28.7% rate with methotrexate monotherapy. The inference is that biologic monotherapy might be an advantageous strategy for maintenance after achieving prolonged remission on combination therapy.
“This study could impact guidelines,” Dr. Cush predicted.
Among the key study findings, in his view, was that disease worsening occurred early following the switch to methotrexate monotherapy: typically within the first 2-4 weeks. And combination rescue therapy worked: Almost 75% of patients were able to recapture disease remission regardless of which drug they’d been on as monotherapy, and almost 90% of patients on rescue therapy achieved a low-disease-activity state.
Still, just over half of patients were able to maintain long-term remission after downshifting to etanercept monotherapy, Dr. Kavanaugh noted. “The bedeviling thing is we don’t know which patients can do this,” he said.
This was made abundantly clear in PREDICTRA, a phase 4, double-blind study in which 122 RA patients in sustained remission on 40 mg of adalimumab (Humira) every 2 weeks were randomized 5:1 to adalimumab taper or withdrawal for 36 weeks. Overall, 36% of the taper group and 45% of those who halted the biologic flared within 36 weeks. Nothing on an extensive list of candidate baseline predictors predicted flare in either group.
”I think the interesting thing here is that nothing predicted it, not drug levels, antidrug antibodies, not even MRI evidence of inflammation while in remission. The lack of predictors of who’s going to do well off therapy makes us crazy,” Dr. Kavanaugh said. Also noteworthy was that when full-dose, open-label adalimumab was reinstituted as rescue therapy in patients who flared, only half of them regained remission during the following 4 months, he observed.
Dr. Cush didn’t mince words regarding his view of the concept underlying PREDICTRA.
“Why are we even talking about this? We spend our whole lives trying to get these patients into remission, we’re heavily invested in combination therapy, we’re very proud of our great successes, and now we want to find data to condone, ‘Let’s get off drug therapy?’ ” he argued.
“There are untold consequences to this,” the rheumatologist continued. “There are cardiovascular consequences, and we’ve seen the x-ray studies that show that patients may be doing clinically better but still have x-ray worsening because you’ve withdrawn the TNF inhibitor. I think this is all nonsense, and I’m totally against it.”
Making a SELECT-CHOICE in refractory RA
In patients whose RA has proved refractory to one or more biologics, opting for the oral Janus kinase inhibitor upadacitinib as next-line therapy is significantly more effective than turning to the T-cell costimulation modulator abatacept, but at the cost of a doubled risk of severe adverse events, according to the findings of the phase 3, double-blind, 24-week, randomized SELECT-CHOICE trial.
In this 612-patient study, the week-12 clinical remission rate as defined by a C-reactive protein (CRP)-based DAS28 below 2.6 was 30% in the upadacitinib arm, compared to 13.3% with abatacept. At week 24, the remission rates were 46% and 31%, respectively. However, the severe adverse event rate was 6.3% in the upadacitinib group, including one death, one stroke, and two cases of VTE, compared to 3.2% with abatacept.
“Maybe this is a win for upadacitinib, but abatacept is a win when it comes to safety,” Dr. Cush said. “So the question is, when you’re making your second or third change in a biologic DMARD, are you going for safety or are you going for efficacy?”
Make way for machine learning
Studies of machine learning and artificial intelligence aimed at developing a more individualized treatment approach are popping up with increasing frequency at the major rheumatology meetings.
“This is really, I think, the future because for most of us, in most of our choices, as great as all of us are in managing RA, it is a crystal ball and a coin toss as to whether our next therapy is going to work,” Dr. Cush said.
He highlighted a study presented at the 2020 ACR annual meeting as an example of how machine learning can generate better evidence for selecting therapies. An international team of investigators used artificial intelligence to develop a simple rule to predict an RA patient’s likely response to the interleukin-6 receptor inhibitor sarilumab (Kevzara). The researchers developed a list of 42 candidate predictors of an ACR20 response, including demographics, biomarkers, and disease activity scores, then applied machine learning to data from the 1,197-patient, phase 3 MOBILITY trial of sarilumab versus placebo in order to generate a predictive rule of better outcomes.
Out of the 42 candidate predictive values, the best predictive rule turned out to be the combination of the presence of anti-citrullinated protein antibodies and a baseline CRP level greater than 12.3 mg/L. This rule was then put to the test using data from four phase 3 clinical trials in which 34%-51% of RA patients randomized to sarilumab were rule-positive. The rule-positive patients had worse baseline prognostic factors and more severe RA. Yet rule-positive patients had a better outcome than that of those who were rule-negative. For example, a week-24 ACR70 response was achieved in 34% of rule-positive patients, nearly fivefold greater than the 7% rate in the rule-negative group.
Treat-to-target strategies don’t tame excess mortality in RA
At a mean of 17 years of follow-up in the Dutch BeST study – a pioneering treat-to-target study in RA – patients with early RA who were assigned to one of four treat-to-target strategies for 10 years showed excess mortality compared to the general age- and sex-matched Dutch population, Johanna M. Maassen, MD, reported at the 2020 annual meeting of the European Alliance of Associations for Rheumatology (EULAR). Indeed, mortality was 37% higher in the RA patients than in the comparator population, with no significant differences in survival curves evident between the four tight-control strategies.
“This raises the question of whether survival in patients not strictly treated to target would have been even lower. Are we not doing as well as we think we’re doing?” Dr. Kavanaugh said.
“This is very sobering. It tells us we really do need to be better at managing RA. And I would say based on this data we need to be better at managing RA really early to get better outcomes really late,” said Dr. Cush. “The damage that led to cardiovascular risk could have been incurred earlier on and then carried forward when you look out to 15-20 years.”
More trouble for tofacitinib
Tofacitinib faces an uncertain future following Pfizer’s late-January press release announcing that the JAK inhibitor failed to meet noninferiority compared to anti-TNF therapy in terms of adjudicated major adverse cardiovascular events (MACE) and adjudicated malignancies in an FDA-mandated postmarketing safety study. The study, known as ORAL Surveillance, or Study A3921133 – ‘1133’ for short – randomized 4,362 RA patients aged 50 years or older with at least one additional cardiovascular risk factor to tofacitinib at 5 or 10 mg twice daily or to a TNF inhibitor.
The Pfizer press release was soon followed by an FDA safety warning and announcement that the agency is reviewing the full study data before making any final recommendations. The FDA already requires black box warnings for tofacitinib regarding increased risks of blood clots and death, but only at the 10-mg twice-daily dose. In the ORAL Surveillance study, however, the primary endpoint was noninferiority of the combined tofacitinib doses, and there was no evidence of a difference in endpoints between the 5- and 10-mg twice-daily doses.
Only the topline results released in the Pfizer press release are publicly available so far. The combined tofacitinib doses were associated with a 1.33-fold greater risk of adjudicated MACE and a 1.48-fold increase in adjudicated malignancies other than nonmelanoma skin cancer, compared to adalimumab or etanercept.
The jury is still out pending release of the full study data, in Dr. Kavanaugh’s view.
“When I get to look at the data, I want to see who are the people who had the events, their characteristics,” he said.
Alexis R. Ogdie-Beatty, MD, said the study’s topline results “make me a little nervous.
“The effect size is pretty large. It’s in the 1.4 range, which means there might be something there for real,” said Dr. Ogdie-Beatty, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
In contrast, Eric M. Ruderman, MD, said he’s not concerned “just yet” about the results.
“Frankly, the FDA was just plain wrong in their press release because the confidence intervals for the MACE events crossed 1.0 meaningfully [0.91, 1.94], so there’s nothing there. And the confidence intervals hit 1.0 for the malignancies,” observed Dr. Ruderman, professor of medicine at Northwestern University, Chicago.
This was a nearly 4,400-patient study, and given that it couldn’t convincingly show a safety risk, such risk – if it even exists – would seem to be tiny, he added.
“It’s probably not going to change the way we prescribe these drugs. So I don’t see that trial taking away from the big run for JAK inhibitors we’re going to see in the next year,” the rheumatologist said.
The speakers reported having financial relationships with numerous pharmaceutical companies.
FROM RWCS 2021
Inpatient telemedicine can help address hospitalist pain points
COVID-19 has increased confidence in the technology
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.
Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)
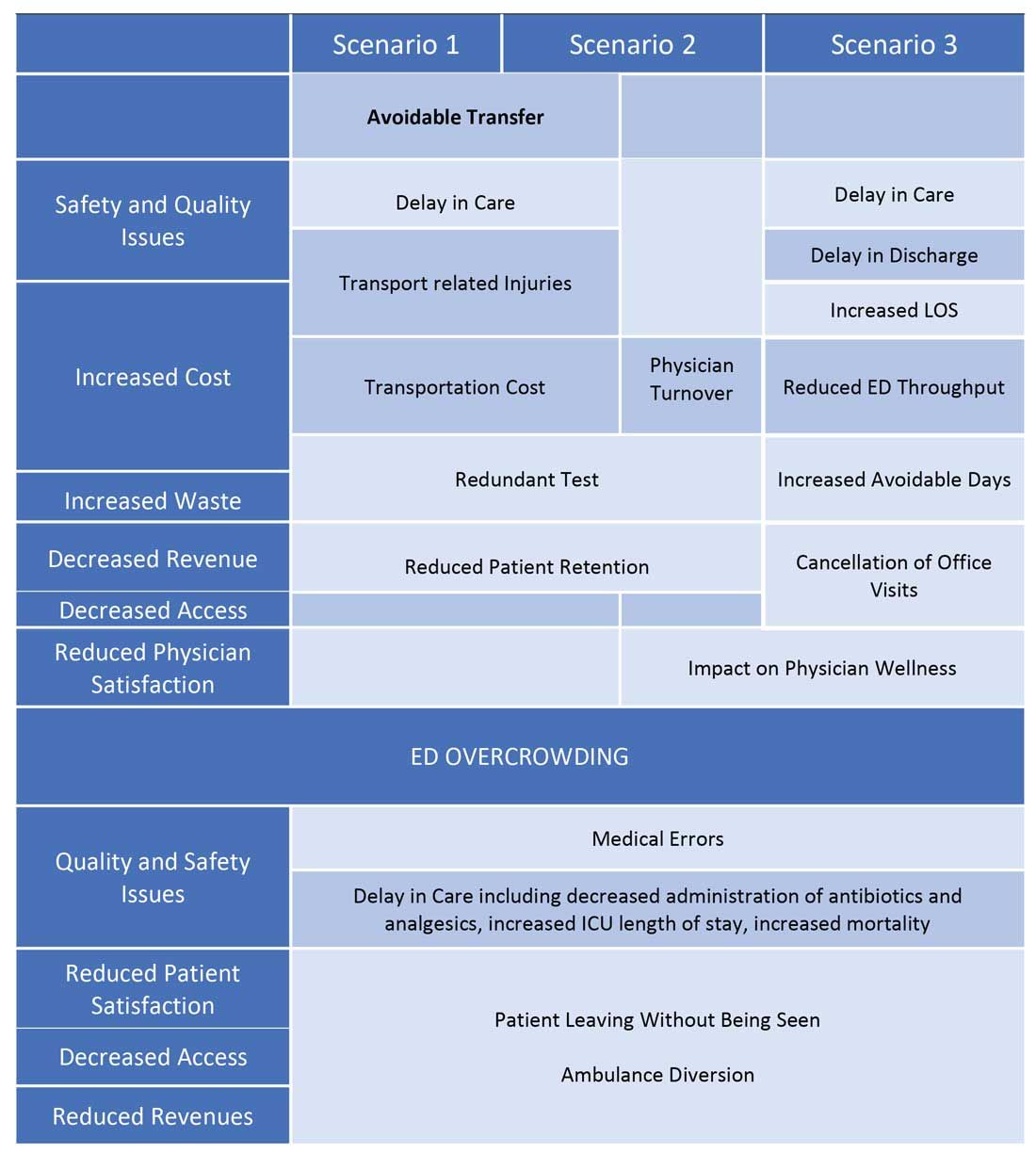
Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)
To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.
COVID-19 has increased confidence in the technology
COVID-19 has increased confidence in the technology
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.

Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)

Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)

To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.

Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)

Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)

To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.