User login
TARE beats systemic therapy for survival benefits in hepatocellular carcinoma with major vascular invasion
Key clinical point: Transarterial radioembolization (TARE) was associated with a significantly higher overall survival compared to systemic therapy in patients with hepatocellular carcinoma with major vascular invasion (HCC-MVI).
Major finding: In a propensity-score matched and landmark-time adjusted analysis, the median overall survival for HCC-MVI patients treated with TARE was 7.1 months, compared to 4.9 months for patients treated with systemic therapy. Target trial emulation of an additional 236 patients with HCC-MVI showed a similar advantage with TARE.
Study details: The data come from 1,514 patients with hepatocellular carcinoma with major vascular invasion (HCC-MVI) identified from the National Cancer Database for the period between 2010 and 2015.
Disclosures: The study was supported by the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. The researchers had no financial conflicts to disclose.
Source: Kwee SA et al. J Vasc Interv Radiol. 2021 Jul 6. doi: 10.1016/j.jvir.2021.07.001.
Key clinical point: Transarterial radioembolization (TARE) was associated with a significantly higher overall survival compared to systemic therapy in patients with hepatocellular carcinoma with major vascular invasion (HCC-MVI).
Major finding: In a propensity-score matched and landmark-time adjusted analysis, the median overall survival for HCC-MVI patients treated with TARE was 7.1 months, compared to 4.9 months for patients treated with systemic therapy. Target trial emulation of an additional 236 patients with HCC-MVI showed a similar advantage with TARE.
Study details: The data come from 1,514 patients with hepatocellular carcinoma with major vascular invasion (HCC-MVI) identified from the National Cancer Database for the period between 2010 and 2015.
Disclosures: The study was supported by the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. The researchers had no financial conflicts to disclose.
Source: Kwee SA et al. J Vasc Interv Radiol. 2021 Jul 6. doi: 10.1016/j.jvir.2021.07.001.
Key clinical point: Transarterial radioembolization (TARE) was associated with a significantly higher overall survival compared to systemic therapy in patients with hepatocellular carcinoma with major vascular invasion (HCC-MVI).
Major finding: In a propensity-score matched and landmark-time adjusted analysis, the median overall survival for HCC-MVI patients treated with TARE was 7.1 months, compared to 4.9 months for patients treated with systemic therapy. Target trial emulation of an additional 236 patients with HCC-MVI showed a similar advantage with TARE.
Study details: The data come from 1,514 patients with hepatocellular carcinoma with major vascular invasion (HCC-MVI) identified from the National Cancer Database for the period between 2010 and 2015.
Disclosures: The study was supported by the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. The researchers had no financial conflicts to disclose.
Source: Kwee SA et al. J Vasc Interv Radiol. 2021 Jul 6. doi: 10.1016/j.jvir.2021.07.001.
TARE extends health-related quality of life in HCC patients versus sorafenib
Key clinical point: Health-related quality of life was preserved longer in HCC patients treated with transarterial radioembolization (TARE) compared to those treated with sorafenib.
Major finding: The median time to deterioration in global health status was 3.9 months in TARE patients, vs 2.6 months in sorafenib patients. TARE patients also showed less deterioration in measures of physical functioning, role functioning, and social functioning compared to sorafenib patients.
Study details: The data come from 285 adults who were participants in a randomized trial of transarterial radioembolization (122 patients) or sorafenib (163 patients) for the treatment of locally advanced or inoperable HCC. Quality of life was assessed from the date of randomization until disease progression or study discontinuation, using the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire.
Disclosures: The study received no outside funding. Lead author Dr. Pereira had no financial conflicts to disclose.
Source: Pereira H et al. Eur J Cancer. 2021 Jul 6. doi: 10.1016/j.ejca.2021.05.032.
Key clinical point: Health-related quality of life was preserved longer in HCC patients treated with transarterial radioembolization (TARE) compared to those treated with sorafenib.
Major finding: The median time to deterioration in global health status was 3.9 months in TARE patients, vs 2.6 months in sorafenib patients. TARE patients also showed less deterioration in measures of physical functioning, role functioning, and social functioning compared to sorafenib patients.
Study details: The data come from 285 adults who were participants in a randomized trial of transarterial radioembolization (122 patients) or sorafenib (163 patients) for the treatment of locally advanced or inoperable HCC. Quality of life was assessed from the date of randomization until disease progression or study discontinuation, using the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire.
Disclosures: The study received no outside funding. Lead author Dr. Pereira had no financial conflicts to disclose.
Source: Pereira H et al. Eur J Cancer. 2021 Jul 6. doi: 10.1016/j.ejca.2021.05.032.
Key clinical point: Health-related quality of life was preserved longer in HCC patients treated with transarterial radioembolization (TARE) compared to those treated with sorafenib.
Major finding: The median time to deterioration in global health status was 3.9 months in TARE patients, vs 2.6 months in sorafenib patients. TARE patients also showed less deterioration in measures of physical functioning, role functioning, and social functioning compared to sorafenib patients.
Study details: The data come from 285 adults who were participants in a randomized trial of transarterial radioembolization (122 patients) or sorafenib (163 patients) for the treatment of locally advanced or inoperable HCC. Quality of life was assessed from the date of randomization until disease progression or study discontinuation, using the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire.
Disclosures: The study received no outside funding. Lead author Dr. Pereira had no financial conflicts to disclose.
Source: Pereira H et al. Eur J Cancer. 2021 Jul 6. doi: 10.1016/j.ejca.2021.05.032.
Diabetes duration linked to increasing heart failure risk
In a multivariable analysis the rate of incident heart failure increased steadily and significantly as diabetes duration increased. Among the 168 study subjects (2% of the total study group) who had diabetes for at least 15 years, the subsequent incidence of heart failure was nearly threefold higher than among the 4,802 subjects (49%) who never had diabetes or prediabetes, reported Justin B. Echouffo-Tcheugui, MD, PhD, and coauthors in an article published in JACC Heart Failure.
People with prediabetes (32% of the study population) had a significant but modest increased rate of incident heart failure that was 16% higher than in control subjects who never developed diabetes. People with diabetes for durations of 0-4.9 years, 5.0-9.9 years, or 10-14.9 years, had steadily increasing relative incident heart failure rates of 29%, 97%, and 210%, respectively, compared with controls, reported Dr. Echouffo-Tcheugui, an endocrinologist at Johns Hopkins Medicine in Baltimore.
Similar rates of HFrEF and HFpEF
Among all 1,841 people in the dataset with diabetes for any length of time each additional 5 years of the disorder linked with a significant, relative 17% increase in the rate of incident heart failure. Incidence of heart failure rose even more sharply with added duration among those with a hemoglobin A1c of 7% or greater, compared with those with better glycemic control. And the rate of incident heart failure with reduced ejection fraction (HFrEF) roughly matched the rate of incident heart failure with preserved ejection fraction (HFpEF).
The study dataset included 9,734 adults enrolled into the Atherosclerosis Risk in Communities (ARIC) study, and during a median follow-up of 22.5 years they had nearly 2,000 episodes of either hospitalization or death secondary to incident heart failure. This included 617 (31%) events involving HFpEF, 495 events (25%) involving HFrEF, and 876 unclassified heart failure events.
The cohort averaged 63 years of age; 58% were women, 23% were Black, and 77% were White (the study design excluded people with other racial and ethnic backgrounds). The study design also excluded people with a history of heart failure or coronary artery disease, as well as those diagnosed with diabetes prior to age 18 resulting in a study group that presumably mostly had type 2 diabetes when diabetes was present. The report provided no data on the specific numbers of patients with type 1 or type 2 diabetes.
“It’s not surprising that a longer duration of diabetes is associated with heart failure, but the etiology remains problematic,” commented Robert H. Eckel, MD, an endocrinologist at the University of Colorado at Denver, Aurora. “The impact of diabetes on incident heart failure is not well know, particularly duration of diabetes,” although disorders often found in patients with diabetes, such as hypertension and diabetic cardiomyopathy, likely have roles in causing heart failure, he said.
Diabetes duration may signal need for an SGLT2 inhibitor
“With emerging novel treatments like the SGLT2 [sodium-glucose cotransporter 2] inhibitors for preventing heart failure hospitalizations and deaths in patients with type 2 diabetes, this is a timely analysis,” Dr. Eckel said in an interview.
“There is no question that with increased duration of type 2 diabetes” the need for an agent from the SGLT2-inhibitor class increases. Although, because of the proven protection these drugs give against heart failure events and progression of chronic kidney disease, treatment with this drug class should start early in patients with type 2 diabetes, he added.
Dr. Echouffo-Tcheugui and his coauthors agreed, citing two important clinical take-aways from their findings:
First, interventions that delay the onset of diabetes may potentially reduce incident heart failure; second, patients with diabetes might benefit from cardioprotective treatments such as SGLT2 inhibitors, the report said.
“Our observations suggest the potential prognostic relevance of diabetes duration in assessing heart failure,” the authors wrote. Integrating diabetes duration into heart failure risk estimation in people with diabetes “could help refine the selection of high-risk individuals who may derive the greatest absolute benefit from aggressive cardioprotective therapies such as SGLT2 inhibitors.”
The analysis also identified several other demographic and clinical factors that influenced the relative effect of diabetes duration. Longer duration was linked with higher rates of incident heart failure in women compared with men, in Blacks compared with Whites, in people younger than 65 compared with older people, in people with an A1c of 7% or higher, and in those with a body mass index of 30 kg/m2 or greater.
The ARIC study and the analyses run by Dr. Echouffo-Tcheugui and his coauthors received no commercial funding. Dr. Echouffo-Tcheugui and Dr. Eckel had no relevant disclosures.
In a multivariable analysis the rate of incident heart failure increased steadily and significantly as diabetes duration increased. Among the 168 study subjects (2% of the total study group) who had diabetes for at least 15 years, the subsequent incidence of heart failure was nearly threefold higher than among the 4,802 subjects (49%) who never had diabetes or prediabetes, reported Justin B. Echouffo-Tcheugui, MD, PhD, and coauthors in an article published in JACC Heart Failure.
People with prediabetes (32% of the study population) had a significant but modest increased rate of incident heart failure that was 16% higher than in control subjects who never developed diabetes. People with diabetes for durations of 0-4.9 years, 5.0-9.9 years, or 10-14.9 years, had steadily increasing relative incident heart failure rates of 29%, 97%, and 210%, respectively, compared with controls, reported Dr. Echouffo-Tcheugui, an endocrinologist at Johns Hopkins Medicine in Baltimore.
Similar rates of HFrEF and HFpEF
Among all 1,841 people in the dataset with diabetes for any length of time each additional 5 years of the disorder linked with a significant, relative 17% increase in the rate of incident heart failure. Incidence of heart failure rose even more sharply with added duration among those with a hemoglobin A1c of 7% or greater, compared with those with better glycemic control. And the rate of incident heart failure with reduced ejection fraction (HFrEF) roughly matched the rate of incident heart failure with preserved ejection fraction (HFpEF).
The study dataset included 9,734 adults enrolled into the Atherosclerosis Risk in Communities (ARIC) study, and during a median follow-up of 22.5 years they had nearly 2,000 episodes of either hospitalization or death secondary to incident heart failure. This included 617 (31%) events involving HFpEF, 495 events (25%) involving HFrEF, and 876 unclassified heart failure events.
The cohort averaged 63 years of age; 58% were women, 23% were Black, and 77% were White (the study design excluded people with other racial and ethnic backgrounds). The study design also excluded people with a history of heart failure or coronary artery disease, as well as those diagnosed with diabetes prior to age 18 resulting in a study group that presumably mostly had type 2 diabetes when diabetes was present. The report provided no data on the specific numbers of patients with type 1 or type 2 diabetes.
“It’s not surprising that a longer duration of diabetes is associated with heart failure, but the etiology remains problematic,” commented Robert H. Eckel, MD, an endocrinologist at the University of Colorado at Denver, Aurora. “The impact of diabetes on incident heart failure is not well know, particularly duration of diabetes,” although disorders often found in patients with diabetes, such as hypertension and diabetic cardiomyopathy, likely have roles in causing heart failure, he said.
Diabetes duration may signal need for an SGLT2 inhibitor
“With emerging novel treatments like the SGLT2 [sodium-glucose cotransporter 2] inhibitors for preventing heart failure hospitalizations and deaths in patients with type 2 diabetes, this is a timely analysis,” Dr. Eckel said in an interview.
“There is no question that with increased duration of type 2 diabetes” the need for an agent from the SGLT2-inhibitor class increases. Although, because of the proven protection these drugs give against heart failure events and progression of chronic kidney disease, treatment with this drug class should start early in patients with type 2 diabetes, he added.
Dr. Echouffo-Tcheugui and his coauthors agreed, citing two important clinical take-aways from their findings:
First, interventions that delay the onset of diabetes may potentially reduce incident heart failure; second, patients with diabetes might benefit from cardioprotective treatments such as SGLT2 inhibitors, the report said.
“Our observations suggest the potential prognostic relevance of diabetes duration in assessing heart failure,” the authors wrote. Integrating diabetes duration into heart failure risk estimation in people with diabetes “could help refine the selection of high-risk individuals who may derive the greatest absolute benefit from aggressive cardioprotective therapies such as SGLT2 inhibitors.”
The analysis also identified several other demographic and clinical factors that influenced the relative effect of diabetes duration. Longer duration was linked with higher rates of incident heart failure in women compared with men, in Blacks compared with Whites, in people younger than 65 compared with older people, in people with an A1c of 7% or higher, and in those with a body mass index of 30 kg/m2 or greater.
The ARIC study and the analyses run by Dr. Echouffo-Tcheugui and his coauthors received no commercial funding. Dr. Echouffo-Tcheugui and Dr. Eckel had no relevant disclosures.
In a multivariable analysis the rate of incident heart failure increased steadily and significantly as diabetes duration increased. Among the 168 study subjects (2% of the total study group) who had diabetes for at least 15 years, the subsequent incidence of heart failure was nearly threefold higher than among the 4,802 subjects (49%) who never had diabetes or prediabetes, reported Justin B. Echouffo-Tcheugui, MD, PhD, and coauthors in an article published in JACC Heart Failure.
People with prediabetes (32% of the study population) had a significant but modest increased rate of incident heart failure that was 16% higher than in control subjects who never developed diabetes. People with diabetes for durations of 0-4.9 years, 5.0-9.9 years, or 10-14.9 years, had steadily increasing relative incident heart failure rates of 29%, 97%, and 210%, respectively, compared with controls, reported Dr. Echouffo-Tcheugui, an endocrinologist at Johns Hopkins Medicine in Baltimore.
Similar rates of HFrEF and HFpEF
Among all 1,841 people in the dataset with diabetes for any length of time each additional 5 years of the disorder linked with a significant, relative 17% increase in the rate of incident heart failure. Incidence of heart failure rose even more sharply with added duration among those with a hemoglobin A1c of 7% or greater, compared with those with better glycemic control. And the rate of incident heart failure with reduced ejection fraction (HFrEF) roughly matched the rate of incident heart failure with preserved ejection fraction (HFpEF).
The study dataset included 9,734 adults enrolled into the Atherosclerosis Risk in Communities (ARIC) study, and during a median follow-up of 22.5 years they had nearly 2,000 episodes of either hospitalization or death secondary to incident heart failure. This included 617 (31%) events involving HFpEF, 495 events (25%) involving HFrEF, and 876 unclassified heart failure events.
The cohort averaged 63 years of age; 58% were women, 23% were Black, and 77% were White (the study design excluded people with other racial and ethnic backgrounds). The study design also excluded people with a history of heart failure or coronary artery disease, as well as those diagnosed with diabetes prior to age 18 resulting in a study group that presumably mostly had type 2 diabetes when diabetes was present. The report provided no data on the specific numbers of patients with type 1 or type 2 diabetes.
“It’s not surprising that a longer duration of diabetes is associated with heart failure, but the etiology remains problematic,” commented Robert H. Eckel, MD, an endocrinologist at the University of Colorado at Denver, Aurora. “The impact of diabetes on incident heart failure is not well know, particularly duration of diabetes,” although disorders often found in patients with diabetes, such as hypertension and diabetic cardiomyopathy, likely have roles in causing heart failure, he said.
Diabetes duration may signal need for an SGLT2 inhibitor
“With emerging novel treatments like the SGLT2 [sodium-glucose cotransporter 2] inhibitors for preventing heart failure hospitalizations and deaths in patients with type 2 diabetes, this is a timely analysis,” Dr. Eckel said in an interview.
“There is no question that with increased duration of type 2 diabetes” the need for an agent from the SGLT2-inhibitor class increases. Although, because of the proven protection these drugs give against heart failure events and progression of chronic kidney disease, treatment with this drug class should start early in patients with type 2 diabetes, he added.
Dr. Echouffo-Tcheugui and his coauthors agreed, citing two important clinical take-aways from their findings:
First, interventions that delay the onset of diabetes may potentially reduce incident heart failure; second, patients with diabetes might benefit from cardioprotective treatments such as SGLT2 inhibitors, the report said.
“Our observations suggest the potential prognostic relevance of diabetes duration in assessing heart failure,” the authors wrote. Integrating diabetes duration into heart failure risk estimation in people with diabetes “could help refine the selection of high-risk individuals who may derive the greatest absolute benefit from aggressive cardioprotective therapies such as SGLT2 inhibitors.”
The analysis also identified several other demographic and clinical factors that influenced the relative effect of diabetes duration. Longer duration was linked with higher rates of incident heart failure in women compared with men, in Blacks compared with Whites, in people younger than 65 compared with older people, in people with an A1c of 7% or higher, and in those with a body mass index of 30 kg/m2 or greater.
The ARIC study and the analyses run by Dr. Echouffo-Tcheugui and his coauthors received no commercial funding. Dr. Echouffo-Tcheugui and Dr. Eckel had no relevant disclosures.
FROM JACC HEART FAILURE
Hand ulceration
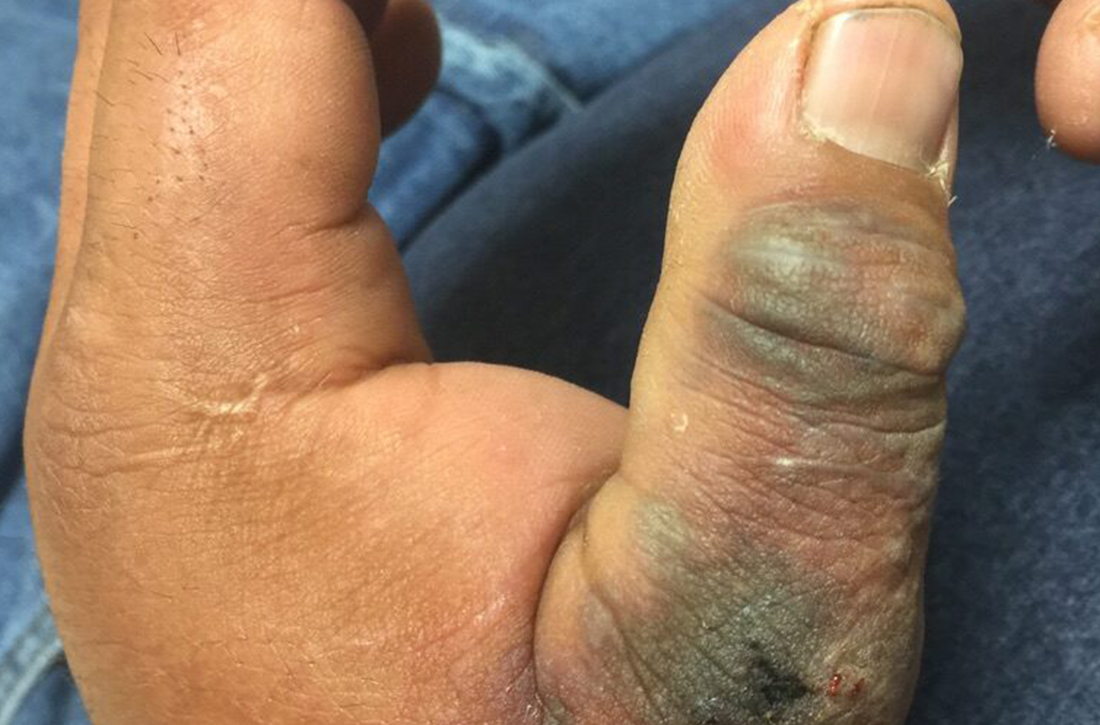
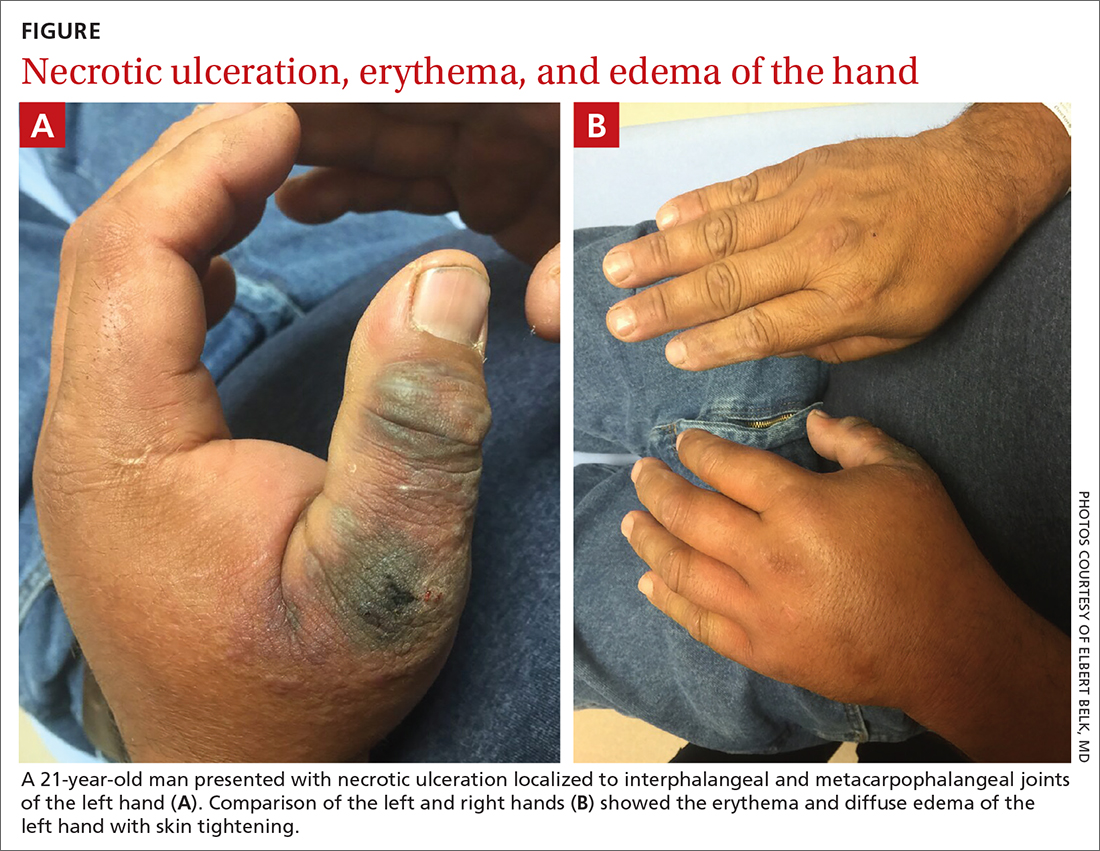
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
Moving patients beyond injury and back to work
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
Electrosurgical hysteroscopy: Principles and expert techniques for optimizing the resectoscope loop
Hysteroscopic mechanical morcellators have gained popularity given their ease of use. Consequently, the resectoscope loop is being used less frequently, which has resulted in less familiarity with this device. The resectoscope loop, however, not only is cost effective but also allows for multiple distinct advantages, such as cold loop dissection of myomas and the ability to obtain electrosurgical hemostasis during operative hysteroscopy.
In this article, we review the basics of electrosurgical principles, compare outcomes associated with monopolar and bipolar resectoscopes, and discuss tips and tricks for optimizing surgical techniques when using the resectoscope loop for hysteroscopic myomectomy.
Evolution of hysteroscopy
The term hysteroscopy comes from the Greek words hystera, for uterus, and skopeo, meaning “to see.” The idea to investigate the uterus dates back to the year 1000 when physicians used a mirror with light to peer into the vaginal vault.
The first known successful hysteroscopy occurred in 1869 when Pantaleoni used an endoscope with a light source to identify uterine polyps in a 60-year-old woman with abnormal uterine bleeding. In 1898, Simon Duplay and Spiro Clado published the first textbook on hysteroscopy in which they described several models of hysteroscopic instruments and techniques.
In the 1950s, Harold Horace Hopkins and Karl Storz modified the shape and length of lenses within the endoscope by substituting longer cylindrical lenses for the old spherical lenses; this permitted improved image brightness and sharpness as well as a smaller diameter of the hysteroscope. Between the 1970s and 1980s, technological improvements allowed for the creation of practical and usable hysteroscopic instruments such as the resectoscope. The resectoscope, originally used in urology for transurethral resection of the prostate, was modified for hysteroscopy by incorporating the use of electrosurgical currents to aid in procedures.
Over the past few decades, continued refinements in technology have improved visualization and surgical techniques. For example, image clarity has been markedly improved, and narrow hysteroscope diameters, as small as 3 to 5 mm, require minimal to no cervical dilation.
Monopolar and bipolar resectoscopes
Electrosurgery is the application of an alternating electrical current to tissue to achieve the clinical effects of surgical cutting or hemostasis via cell vaporization or coagulation. Current runs from the electrosurgical unit (ESU) to the active electrode of the surgical instrument, then goes from the active electrode through the patient’s tissue to the return electrode, and then travels back to the ESU. This flow of current creates an electrical circuit (FIGURE).
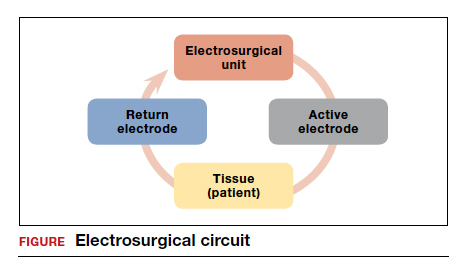
All electrosurgical devices have an active and a return electrode. The difference between monopolar and bipolar resectoscope devices lies in how the resectoscope loop is constructed. Bipolar resectoscope loops house the active and return electrodes on the same tip of the surgical device, which limits how much of the current flows through the patient. Alternatively, monopolar resectoscopes have only the active electrode on the tip of the device and the return electrode is off the surgical field, so the current flows through more of the patient. On monopolar electrosurgical devices, the current runs from the ESU to the active electrode (monopolar loop), which is then applied to tissue to produce the desired tissue effect. The current then travels via a path of least resistance from the surgical field through the patient to the return electrode, which is usually placed on the patient’s thigh, and then back to the ESU. The return electrode is often referred to as the grounding pad.
Continue to: How monopolar energy works...
How monopolar energy works
When first developed, all resectoscopes used monopolar energy. As such, throughout the 1990s, the monopolar resectoscope was the gold standard for performing electrosurgical hysteroscopy. Because the current travels a long distance between the active and the return electrode in a monopolar setup, a hypotonic, nonelectrolyte-rich medium (a poor conductor), such as glycine 1.5%, mannitol 5%, or sorbitol 3%, must be used. If an electrolyte-rich medium, such as normal saline, is used with a monopolar device, the current would be dispersed throughout the medium outside the operative field, causing unwanted tissue effects.
Although nonelectrolyte distension media improve visibility when encountering bleeding, they can be associated with hyponatremia, hyperglycemia, and even lifethreatening cerebral edema. Furthermore, glycine use is contraindicated in patients with renal or hepatic failure since oxidative deamination may cause hyperammonemia. Because of these numerous risk factors, the fluid deficit for hypotonic, nonelectrolyte distension media is limited to 1,000 mL, with a suggested maximum fluid deficit of 750 mL for elderly or fragile patients. Additionally, because the return electrode is off the surgical field in monopolar surgery, there is a risk of current diversion to the cervix, vagina, or vulva because the current travels between the active electrode on the surgical field to the return electrode on the patient’s thigh. The risk of current diversion is greater if there is damage to electrode insulation, loss of contact between the external sheath and the cervix, or direct coupling between the electrode and the surrounding tissue.
Advantages of the bipolar resectoscope
Because of the potential risks associated with the monopolar resectoscope, over the past 25 years the bipolar resectoscope emerged as an alternative due to its numerous benefits (TABLE 1).
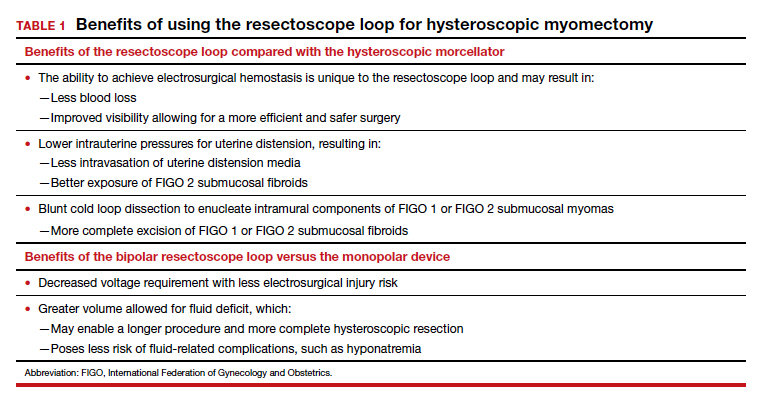
Unlike monopolar resectoscopes, bipolar resectoscopes require an electrolyte-rich distension medium such as 0.9% normal saline or lactated Ringer’s. These isotonic distension media allow a much higher fluid deficit (2,500 mL for healthy patients, 1,500 mL for elderly patients or patients with comorbidities) as the isotonic solution is safer to use. Furthermore, it allows for lower voltage settings and decreased electrical spread compared to the monopolar resectoscope since the current stays between the 2 electrodes. Because isotonic media are miscible with blood, however, a potential drawback is that in cases with bleeding, visibility may be more limited compared to hypotonic distension media.
Evidence on fertility outcomes
Several studies have compared operative and fertility outcomes with the use of monopolar versus bipolar hysteroscopy.
In a randomized controlled trial (RCT) comparing outcomes after hysteroscopy with a monopolar (glycine 1.5%) versus bipolar (0.9% normal saline) 26 French resectoscope loop, Berg and colleagues found that the only significant difference between the 2 groups was that the change in serum sodium pre and postoperatively was greater in the monopolar group despite having a smaller mean fluid deficit (765 mL vs 1,227 mL).1
Similarly, in a study of fertility outcomes after monopolar versus bipolar hysteroscopic myomectomy with use of a 26 French resectoscope Collins knife, Roy and colleagues found no significant differences in postoperative pregnancy rates or successful pregnancy outcomes, operative time, fluid deficit, or improvement in menstrual symptoms.2 However, the monopolar group had a much higher incidence of postoperative hyponatremia (30% vs 0%) that required additional days of hospitalization despite similar fluid deficits of between 600 and 700 mL.2
Similar findings were noted in another RCT that compared operative outcomes between monopolar and bipolar resectoscope usage during metroplasty for infertility, with a postoperative hyponatremia incidence of 17.1% in the monopolar group versus 0% in the bipolar group despite similar fluid deficits.3 Energy type had no effect on reproductive outcomes in either group.3
Continue to: How does the resectoscope compare with mechanical tissue removal systems?...
How does the resectoscope compare with mechanical tissue removal systems?
In 2005, the first hysteroscopic mechanical tissue removal system was introduced in the United States, providing an additional treatment method for such intrauterine masses as fibroids and polyps.
Advantages. Rather than using an electrical current, these tissue removal systems use a rotating blade with suction that is introduced through a specially designed rigid hysteroscopic sheath. As the instrument incises the pathology, the tissue is removed from the intrauterine cavity and collected in a specimen bag inside the fluid management system. This immediate removal of tissue allows for insertion of the device only once during initial entry, decreasing both the risk of perforation and operative times. Furthermore, mechanical tissue removal systems can be used with isotonic media, negating the risks associated with hypotonic media. Currently, the 2 mechanical tissue removal systems available in the United States are the TruClear and the MyoSure hysteroscopic tissue removal systems.
Studies comparing mechanical tissue removal of polyps and myomas with conventional resectoscope resection have found that mechanical tissue removal is associated with reduced operative time, fluid deficit, and number of instrument insertions.4-8 However, studies have found no significant difference in postoperative patient satisfaction.7,9
Additionally, hysteroscopic tissue removal systems have an easier learning curve. Van Dongen and colleagues conducted an RCT to compare resident-in-training comfort levels when learning to use both a mechanical tissue removal system and a traditional resectoscope; they found increased comfort with the hysteroscopic tissue removal system, suggesting greater ease of use.10
Drawbacks. Despite their many benefits, mechanical tissue removal systems have some disadvantages when compared with the resectoscope. First, mechanical tissue removal systems are associated with higher instrument costs. In addition, they have extremely limited ability to achieve hemostasis when encountering blood vessels during resection, resulting in poor visibility especially when resecting large myomas with feeding vessels.
Hysteroscopic mechanical tissue removal systems typically use higher intrauterine pressures for uterine distension compared with the resectoscope, especially when trying to improve visibility in a bloody surgical field. Increasing the intrauterine pressure with the distension media allows for compression of the blood vessels. As a result, however, submucosal fibroids classified as FIGO 2 (International Federation of Gynecology and Obstetrics) may be less visible since the higher intrauterine pressure can compress both blood vessels and submucosal fibroids
Additionally, mechanical tissue removal systems have limited ability to resect the intramural component of FIGO 1 or FIGO 2 submucosal fibroids since the intramural portion is embedded in the myometrium. Use of the resectoscope loop instead allows for a technique called the cold loop dissection, which uses the resectoscope loop to bluntly dissect and enucleate the intramural component of FIGO 1 and FIGO 2 submucosal myomas from the surrounding myometrium without activating the current. This blunt cold loop dissection technique allows for a deeper and more thorough resection. Often, if the pseudocapsule plane is identified, even the intramural component of FIGO 1 or FIGO 2 submucosal fibroids can be resected, enabling complete removal.
Lastly, mechanical tissue removal systems are not always faster than resectoscopes for all pathology. We prefer using the resectoscope for larger myomas (>3 cm) as the resectoscope allows for resection and removal of larger myoma chips, helping to decrease operative times. Given the many benefits of the resectoscope, we argue that the resectoscope loop remains a crucial instrument in operative gynecology and that learners should continue to hone their hysteroscopic skills with both the resectoscope and mechanical tissue removal systems.
Tips and tricks for hysteroscopic myomectomy with the resectoscope loop
In the video below, "Bipolar resectoscope: Optimizing safe myomectomy," we review specific surgical techniques for optimizing outcomes and safety with the resectoscope loop. These include:
- bow-and-arrow technique
- identification of the fibroid anatomy (pseudocapsule plane)
- blunt cold loop dissection
- the push-and-tuck method
- efficient electrosurgical hemostasis (TABLE 2).
Although we use bipolar energy during this resection, the resection technique using the monopolar loop is the same.


The takeaway
The resectoscope loop is a valuable tool that offers gynecologic surgeons a wider range of techniques for myomectomy. It also offers several surgical and clinical advantages. It is important to train residents in the use of both hysteroscopic mechanical tissue removal systems and resectoscope loops. ●
- Berg A, Sandvik L, Langebrekke A, et al. A randomized trial comparing monopolar electrodes using glycine 1.5% with two different types of bipolar electrodes (TCRis, Versapoint) using saline, in hysteroscopic surgery. Fertil Steril. 2009;91:1273- 1278.
- Roy KK, Metta S, Kansal Y, et al. A prospective randomized study comparing unipolar versus bipolar hysteroscopic myomectomy in infertile women. J Hum Reprod Sci. 2017;10:185-193.
- Roy KK, Kansal Y, Subbaiah M, et al. Hysteroscopic septal resection using unipolar resectoscope versus bipolar resectoscope: prospective, randomized study. J Obstet Gynaecol Res. 2015;41:952-956.
- Borg MH, Shehata A. Uterine morcellator versus resectoscopy in the management of heavy menstrual flow in reproductiveage women. J Gyn Res. 2016;2:1-8.
- Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12:62-66.
- Smith PP, Middleton LJ, Connor M, et al. Hysteroscopic morcellation compared with electrical resection of endometrial polyps: a randomized controlled trial. Obstet Gynecol. 2014;123:745-751.
- Vitale SG, Sapia F, Rapisarda AMC, et al. Hysteroscopic morcellation of submucous myomas: a systematic review. Biomed Res Int. 2017;2017:6848250.
- Stoll F, Lecointre L, Meyer N, et al. Randomized study comparing a reusable morcellator with a resectoscope in the hysteroscopic treatment of uterine polyps: the RESMO study. J Minimal Invasive Gyn. 2021;28:801-810.
- Lee MM, Matsuzono T. Hysteroscopic intrauterine morcellation of submucosal fibroids: preliminary results in Hong Kong and comparisons with conventional hysteroscopic monopolar loop resection. Hong Kong Med J. 2016;22:56-61.
- van Dongen H, Emanuel MH, Wolterbeek R, et al. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15:466-471.
Hysteroscopic mechanical morcellators have gained popularity given their ease of use. Consequently, the resectoscope loop is being used less frequently, which has resulted in less familiarity with this device. The resectoscope loop, however, not only is cost effective but also allows for multiple distinct advantages, such as cold loop dissection of myomas and the ability to obtain electrosurgical hemostasis during operative hysteroscopy.
In this article, we review the basics of electrosurgical principles, compare outcomes associated with monopolar and bipolar resectoscopes, and discuss tips and tricks for optimizing surgical techniques when using the resectoscope loop for hysteroscopic myomectomy.
Evolution of hysteroscopy
The term hysteroscopy comes from the Greek words hystera, for uterus, and skopeo, meaning “to see.” The idea to investigate the uterus dates back to the year 1000 when physicians used a mirror with light to peer into the vaginal vault.
The first known successful hysteroscopy occurred in 1869 when Pantaleoni used an endoscope with a light source to identify uterine polyps in a 60-year-old woman with abnormal uterine bleeding. In 1898, Simon Duplay and Spiro Clado published the first textbook on hysteroscopy in which they described several models of hysteroscopic instruments and techniques.
In the 1950s, Harold Horace Hopkins and Karl Storz modified the shape and length of lenses within the endoscope by substituting longer cylindrical lenses for the old spherical lenses; this permitted improved image brightness and sharpness as well as a smaller diameter of the hysteroscope. Between the 1970s and 1980s, technological improvements allowed for the creation of practical and usable hysteroscopic instruments such as the resectoscope. The resectoscope, originally used in urology for transurethral resection of the prostate, was modified for hysteroscopy by incorporating the use of electrosurgical currents to aid in procedures.
Over the past few decades, continued refinements in technology have improved visualization and surgical techniques. For example, image clarity has been markedly improved, and narrow hysteroscope diameters, as small as 3 to 5 mm, require minimal to no cervical dilation.
Monopolar and bipolar resectoscopes
Electrosurgery is the application of an alternating electrical current to tissue to achieve the clinical effects of surgical cutting or hemostasis via cell vaporization or coagulation. Current runs from the electrosurgical unit (ESU) to the active electrode of the surgical instrument, then goes from the active electrode through the patient’s tissue to the return electrode, and then travels back to the ESU. This flow of current creates an electrical circuit (FIGURE).

All electrosurgical devices have an active and a return electrode. The difference between monopolar and bipolar resectoscope devices lies in how the resectoscope loop is constructed. Bipolar resectoscope loops house the active and return electrodes on the same tip of the surgical device, which limits how much of the current flows through the patient. Alternatively, monopolar resectoscopes have only the active electrode on the tip of the device and the return electrode is off the surgical field, so the current flows through more of the patient. On monopolar electrosurgical devices, the current runs from the ESU to the active electrode (monopolar loop), which is then applied to tissue to produce the desired tissue effect. The current then travels via a path of least resistance from the surgical field through the patient to the return electrode, which is usually placed on the patient’s thigh, and then back to the ESU. The return electrode is often referred to as the grounding pad.
Continue to: How monopolar energy works...
How monopolar energy works
When first developed, all resectoscopes used monopolar energy. As such, throughout the 1990s, the monopolar resectoscope was the gold standard for performing electrosurgical hysteroscopy. Because the current travels a long distance between the active and the return electrode in a monopolar setup, a hypotonic, nonelectrolyte-rich medium (a poor conductor), such as glycine 1.5%, mannitol 5%, or sorbitol 3%, must be used. If an electrolyte-rich medium, such as normal saline, is used with a monopolar device, the current would be dispersed throughout the medium outside the operative field, causing unwanted tissue effects.
Although nonelectrolyte distension media improve visibility when encountering bleeding, they can be associated with hyponatremia, hyperglycemia, and even lifethreatening cerebral edema. Furthermore, glycine use is contraindicated in patients with renal or hepatic failure since oxidative deamination may cause hyperammonemia. Because of these numerous risk factors, the fluid deficit for hypotonic, nonelectrolyte distension media is limited to 1,000 mL, with a suggested maximum fluid deficit of 750 mL for elderly or fragile patients. Additionally, because the return electrode is off the surgical field in monopolar surgery, there is a risk of current diversion to the cervix, vagina, or vulva because the current travels between the active electrode on the surgical field to the return electrode on the patient’s thigh. The risk of current diversion is greater if there is damage to electrode insulation, loss of contact between the external sheath and the cervix, or direct coupling between the electrode and the surrounding tissue.
Advantages of the bipolar resectoscope
Because of the potential risks associated with the monopolar resectoscope, over the past 25 years the bipolar resectoscope emerged as an alternative due to its numerous benefits (TABLE 1).

Unlike monopolar resectoscopes, bipolar resectoscopes require an electrolyte-rich distension medium such as 0.9% normal saline or lactated Ringer’s. These isotonic distension media allow a much higher fluid deficit (2,500 mL for healthy patients, 1,500 mL for elderly patients or patients with comorbidities) as the isotonic solution is safer to use. Furthermore, it allows for lower voltage settings and decreased electrical spread compared to the monopolar resectoscope since the current stays between the 2 electrodes. Because isotonic media are miscible with blood, however, a potential drawback is that in cases with bleeding, visibility may be more limited compared to hypotonic distension media.
Evidence on fertility outcomes
Several studies have compared operative and fertility outcomes with the use of monopolar versus bipolar hysteroscopy.
In a randomized controlled trial (RCT) comparing outcomes after hysteroscopy with a monopolar (glycine 1.5%) versus bipolar (0.9% normal saline) 26 French resectoscope loop, Berg and colleagues found that the only significant difference between the 2 groups was that the change in serum sodium pre and postoperatively was greater in the monopolar group despite having a smaller mean fluid deficit (765 mL vs 1,227 mL).1
Similarly, in a study of fertility outcomes after monopolar versus bipolar hysteroscopic myomectomy with use of a 26 French resectoscope Collins knife, Roy and colleagues found no significant differences in postoperative pregnancy rates or successful pregnancy outcomes, operative time, fluid deficit, or improvement in menstrual symptoms.2 However, the monopolar group had a much higher incidence of postoperative hyponatremia (30% vs 0%) that required additional days of hospitalization despite similar fluid deficits of between 600 and 700 mL.2
Similar findings were noted in another RCT that compared operative outcomes between monopolar and bipolar resectoscope usage during metroplasty for infertility, with a postoperative hyponatremia incidence of 17.1% in the monopolar group versus 0% in the bipolar group despite similar fluid deficits.3 Energy type had no effect on reproductive outcomes in either group.3
Continue to: How does the resectoscope compare with mechanical tissue removal systems?...
How does the resectoscope compare with mechanical tissue removal systems?
In 2005, the first hysteroscopic mechanical tissue removal system was introduced in the United States, providing an additional treatment method for such intrauterine masses as fibroids and polyps.
Advantages. Rather than using an electrical current, these tissue removal systems use a rotating blade with suction that is introduced through a specially designed rigid hysteroscopic sheath. As the instrument incises the pathology, the tissue is removed from the intrauterine cavity and collected in a specimen bag inside the fluid management system. This immediate removal of tissue allows for insertion of the device only once during initial entry, decreasing both the risk of perforation and operative times. Furthermore, mechanical tissue removal systems can be used with isotonic media, negating the risks associated with hypotonic media. Currently, the 2 mechanical tissue removal systems available in the United States are the TruClear and the MyoSure hysteroscopic tissue removal systems.
Studies comparing mechanical tissue removal of polyps and myomas with conventional resectoscope resection have found that mechanical tissue removal is associated with reduced operative time, fluid deficit, and number of instrument insertions.4-8 However, studies have found no significant difference in postoperative patient satisfaction.7,9
Additionally, hysteroscopic tissue removal systems have an easier learning curve. Van Dongen and colleagues conducted an RCT to compare resident-in-training comfort levels when learning to use both a mechanical tissue removal system and a traditional resectoscope; they found increased comfort with the hysteroscopic tissue removal system, suggesting greater ease of use.10
Drawbacks. Despite their many benefits, mechanical tissue removal systems have some disadvantages when compared with the resectoscope. First, mechanical tissue removal systems are associated with higher instrument costs. In addition, they have extremely limited ability to achieve hemostasis when encountering blood vessels during resection, resulting in poor visibility especially when resecting large myomas with feeding vessels.
Hysteroscopic mechanical tissue removal systems typically use higher intrauterine pressures for uterine distension compared with the resectoscope, especially when trying to improve visibility in a bloody surgical field. Increasing the intrauterine pressure with the distension media allows for compression of the blood vessels. As a result, however, submucosal fibroids classified as FIGO 2 (International Federation of Gynecology and Obstetrics) may be less visible since the higher intrauterine pressure can compress both blood vessels and submucosal fibroids
Additionally, mechanical tissue removal systems have limited ability to resect the intramural component of FIGO 1 or FIGO 2 submucosal fibroids since the intramural portion is embedded in the myometrium. Use of the resectoscope loop instead allows for a technique called the cold loop dissection, which uses the resectoscope loop to bluntly dissect and enucleate the intramural component of FIGO 1 and FIGO 2 submucosal myomas from the surrounding myometrium without activating the current. This blunt cold loop dissection technique allows for a deeper and more thorough resection. Often, if the pseudocapsule plane is identified, even the intramural component of FIGO 1 or FIGO 2 submucosal fibroids can be resected, enabling complete removal.
Lastly, mechanical tissue removal systems are not always faster than resectoscopes for all pathology. We prefer using the resectoscope for larger myomas (>3 cm) as the resectoscope allows for resection and removal of larger myoma chips, helping to decrease operative times. Given the many benefits of the resectoscope, we argue that the resectoscope loop remains a crucial instrument in operative gynecology and that learners should continue to hone their hysteroscopic skills with both the resectoscope and mechanical tissue removal systems.
Tips and tricks for hysteroscopic myomectomy with the resectoscope loop
In the video below, "Bipolar resectoscope: Optimizing safe myomectomy," we review specific surgical techniques for optimizing outcomes and safety with the resectoscope loop. These include:
- bow-and-arrow technique
- identification of the fibroid anatomy (pseudocapsule plane)
- blunt cold loop dissection
- the push-and-tuck method
- efficient electrosurgical hemostasis (TABLE 2).
Although we use bipolar energy during this resection, the resection technique using the monopolar loop is the same.


The takeaway
The resectoscope loop is a valuable tool that offers gynecologic surgeons a wider range of techniques for myomectomy. It also offers several surgical and clinical advantages. It is important to train residents in the use of both hysteroscopic mechanical tissue removal systems and resectoscope loops. ●
Hysteroscopic mechanical morcellators have gained popularity given their ease of use. Consequently, the resectoscope loop is being used less frequently, which has resulted in less familiarity with this device. The resectoscope loop, however, not only is cost effective but also allows for multiple distinct advantages, such as cold loop dissection of myomas and the ability to obtain electrosurgical hemostasis during operative hysteroscopy.
In this article, we review the basics of electrosurgical principles, compare outcomes associated with monopolar and bipolar resectoscopes, and discuss tips and tricks for optimizing surgical techniques when using the resectoscope loop for hysteroscopic myomectomy.
Evolution of hysteroscopy
The term hysteroscopy comes from the Greek words hystera, for uterus, and skopeo, meaning “to see.” The idea to investigate the uterus dates back to the year 1000 when physicians used a mirror with light to peer into the vaginal vault.
The first known successful hysteroscopy occurred in 1869 when Pantaleoni used an endoscope with a light source to identify uterine polyps in a 60-year-old woman with abnormal uterine bleeding. In 1898, Simon Duplay and Spiro Clado published the first textbook on hysteroscopy in which they described several models of hysteroscopic instruments and techniques.
In the 1950s, Harold Horace Hopkins and Karl Storz modified the shape and length of lenses within the endoscope by substituting longer cylindrical lenses for the old spherical lenses; this permitted improved image brightness and sharpness as well as a smaller diameter of the hysteroscope. Between the 1970s and 1980s, technological improvements allowed for the creation of practical and usable hysteroscopic instruments such as the resectoscope. The resectoscope, originally used in urology for transurethral resection of the prostate, was modified for hysteroscopy by incorporating the use of electrosurgical currents to aid in procedures.
Over the past few decades, continued refinements in technology have improved visualization and surgical techniques. For example, image clarity has been markedly improved, and narrow hysteroscope diameters, as small as 3 to 5 mm, require minimal to no cervical dilation.
Monopolar and bipolar resectoscopes
Electrosurgery is the application of an alternating electrical current to tissue to achieve the clinical effects of surgical cutting or hemostasis via cell vaporization or coagulation. Current runs from the electrosurgical unit (ESU) to the active electrode of the surgical instrument, then goes from the active electrode through the patient’s tissue to the return electrode, and then travels back to the ESU. This flow of current creates an electrical circuit (FIGURE).

All electrosurgical devices have an active and a return electrode. The difference between monopolar and bipolar resectoscope devices lies in how the resectoscope loop is constructed. Bipolar resectoscope loops house the active and return electrodes on the same tip of the surgical device, which limits how much of the current flows through the patient. Alternatively, monopolar resectoscopes have only the active electrode on the tip of the device and the return electrode is off the surgical field, so the current flows through more of the patient. On monopolar electrosurgical devices, the current runs from the ESU to the active electrode (monopolar loop), which is then applied to tissue to produce the desired tissue effect. The current then travels via a path of least resistance from the surgical field through the patient to the return electrode, which is usually placed on the patient’s thigh, and then back to the ESU. The return electrode is often referred to as the grounding pad.
Continue to: How monopolar energy works...
How monopolar energy works
When first developed, all resectoscopes used monopolar energy. As such, throughout the 1990s, the monopolar resectoscope was the gold standard for performing electrosurgical hysteroscopy. Because the current travels a long distance between the active and the return electrode in a monopolar setup, a hypotonic, nonelectrolyte-rich medium (a poor conductor), such as glycine 1.5%, mannitol 5%, or sorbitol 3%, must be used. If an electrolyte-rich medium, such as normal saline, is used with a monopolar device, the current would be dispersed throughout the medium outside the operative field, causing unwanted tissue effects.
Although nonelectrolyte distension media improve visibility when encountering bleeding, they can be associated with hyponatremia, hyperglycemia, and even lifethreatening cerebral edema. Furthermore, glycine use is contraindicated in patients with renal or hepatic failure since oxidative deamination may cause hyperammonemia. Because of these numerous risk factors, the fluid deficit for hypotonic, nonelectrolyte distension media is limited to 1,000 mL, with a suggested maximum fluid deficit of 750 mL for elderly or fragile patients. Additionally, because the return electrode is off the surgical field in monopolar surgery, there is a risk of current diversion to the cervix, vagina, or vulva because the current travels between the active electrode on the surgical field to the return electrode on the patient’s thigh. The risk of current diversion is greater if there is damage to electrode insulation, loss of contact between the external sheath and the cervix, or direct coupling between the electrode and the surrounding tissue.
Advantages of the bipolar resectoscope
Because of the potential risks associated with the monopolar resectoscope, over the past 25 years the bipolar resectoscope emerged as an alternative due to its numerous benefits (TABLE 1).

Unlike monopolar resectoscopes, bipolar resectoscopes require an electrolyte-rich distension medium such as 0.9% normal saline or lactated Ringer’s. These isotonic distension media allow a much higher fluid deficit (2,500 mL for healthy patients, 1,500 mL for elderly patients or patients with comorbidities) as the isotonic solution is safer to use. Furthermore, it allows for lower voltage settings and decreased electrical spread compared to the monopolar resectoscope since the current stays between the 2 electrodes. Because isotonic media are miscible with blood, however, a potential drawback is that in cases with bleeding, visibility may be more limited compared to hypotonic distension media.
Evidence on fertility outcomes
Several studies have compared operative and fertility outcomes with the use of monopolar versus bipolar hysteroscopy.
In a randomized controlled trial (RCT) comparing outcomes after hysteroscopy with a monopolar (glycine 1.5%) versus bipolar (0.9% normal saline) 26 French resectoscope loop, Berg and colleagues found that the only significant difference between the 2 groups was that the change in serum sodium pre and postoperatively was greater in the monopolar group despite having a smaller mean fluid deficit (765 mL vs 1,227 mL).1
Similarly, in a study of fertility outcomes after monopolar versus bipolar hysteroscopic myomectomy with use of a 26 French resectoscope Collins knife, Roy and colleagues found no significant differences in postoperative pregnancy rates or successful pregnancy outcomes, operative time, fluid deficit, or improvement in menstrual symptoms.2 However, the monopolar group had a much higher incidence of postoperative hyponatremia (30% vs 0%) that required additional days of hospitalization despite similar fluid deficits of between 600 and 700 mL.2
Similar findings were noted in another RCT that compared operative outcomes between monopolar and bipolar resectoscope usage during metroplasty for infertility, with a postoperative hyponatremia incidence of 17.1% in the monopolar group versus 0% in the bipolar group despite similar fluid deficits.3 Energy type had no effect on reproductive outcomes in either group.3
Continue to: How does the resectoscope compare with mechanical tissue removal systems?...
How does the resectoscope compare with mechanical tissue removal systems?
In 2005, the first hysteroscopic mechanical tissue removal system was introduced in the United States, providing an additional treatment method for such intrauterine masses as fibroids and polyps.
Advantages. Rather than using an electrical current, these tissue removal systems use a rotating blade with suction that is introduced through a specially designed rigid hysteroscopic sheath. As the instrument incises the pathology, the tissue is removed from the intrauterine cavity and collected in a specimen bag inside the fluid management system. This immediate removal of tissue allows for insertion of the device only once during initial entry, decreasing both the risk of perforation and operative times. Furthermore, mechanical tissue removal systems can be used with isotonic media, negating the risks associated with hypotonic media. Currently, the 2 mechanical tissue removal systems available in the United States are the TruClear and the MyoSure hysteroscopic tissue removal systems.
Studies comparing mechanical tissue removal of polyps and myomas with conventional resectoscope resection have found that mechanical tissue removal is associated with reduced operative time, fluid deficit, and number of instrument insertions.4-8 However, studies have found no significant difference in postoperative patient satisfaction.7,9
Additionally, hysteroscopic tissue removal systems have an easier learning curve. Van Dongen and colleagues conducted an RCT to compare resident-in-training comfort levels when learning to use both a mechanical tissue removal system and a traditional resectoscope; they found increased comfort with the hysteroscopic tissue removal system, suggesting greater ease of use.10
Drawbacks. Despite their many benefits, mechanical tissue removal systems have some disadvantages when compared with the resectoscope. First, mechanical tissue removal systems are associated with higher instrument costs. In addition, they have extremely limited ability to achieve hemostasis when encountering blood vessels during resection, resulting in poor visibility especially when resecting large myomas with feeding vessels.
Hysteroscopic mechanical tissue removal systems typically use higher intrauterine pressures for uterine distension compared with the resectoscope, especially when trying to improve visibility in a bloody surgical field. Increasing the intrauterine pressure with the distension media allows for compression of the blood vessels. As a result, however, submucosal fibroids classified as FIGO 2 (International Federation of Gynecology and Obstetrics) may be less visible since the higher intrauterine pressure can compress both blood vessels and submucosal fibroids
Additionally, mechanical tissue removal systems have limited ability to resect the intramural component of FIGO 1 or FIGO 2 submucosal fibroids since the intramural portion is embedded in the myometrium. Use of the resectoscope loop instead allows for a technique called the cold loop dissection, which uses the resectoscope loop to bluntly dissect and enucleate the intramural component of FIGO 1 and FIGO 2 submucosal myomas from the surrounding myometrium without activating the current. This blunt cold loop dissection technique allows for a deeper and more thorough resection. Often, if the pseudocapsule plane is identified, even the intramural component of FIGO 1 or FIGO 2 submucosal fibroids can be resected, enabling complete removal.
Lastly, mechanical tissue removal systems are not always faster than resectoscopes for all pathology. We prefer using the resectoscope for larger myomas (>3 cm) as the resectoscope allows for resection and removal of larger myoma chips, helping to decrease operative times. Given the many benefits of the resectoscope, we argue that the resectoscope loop remains a crucial instrument in operative gynecology and that learners should continue to hone their hysteroscopic skills with both the resectoscope and mechanical tissue removal systems.
Tips and tricks for hysteroscopic myomectomy with the resectoscope loop
In the video below, "Bipolar resectoscope: Optimizing safe myomectomy," we review specific surgical techniques for optimizing outcomes and safety with the resectoscope loop. These include:
- bow-and-arrow technique
- identification of the fibroid anatomy (pseudocapsule plane)
- blunt cold loop dissection
- the push-and-tuck method
- efficient electrosurgical hemostasis (TABLE 2).
Although we use bipolar energy during this resection, the resection technique using the monopolar loop is the same.


The takeaway
The resectoscope loop is a valuable tool that offers gynecologic surgeons a wider range of techniques for myomectomy. It also offers several surgical and clinical advantages. It is important to train residents in the use of both hysteroscopic mechanical tissue removal systems and resectoscope loops. ●
- Berg A, Sandvik L, Langebrekke A, et al. A randomized trial comparing monopolar electrodes using glycine 1.5% with two different types of bipolar electrodes (TCRis, Versapoint) using saline, in hysteroscopic surgery. Fertil Steril. 2009;91:1273- 1278.
- Roy KK, Metta S, Kansal Y, et al. A prospective randomized study comparing unipolar versus bipolar hysteroscopic myomectomy in infertile women. J Hum Reprod Sci. 2017;10:185-193.
- Roy KK, Kansal Y, Subbaiah M, et al. Hysteroscopic septal resection using unipolar resectoscope versus bipolar resectoscope: prospective, randomized study. J Obstet Gynaecol Res. 2015;41:952-956.
- Borg MH, Shehata A. Uterine morcellator versus resectoscopy in the management of heavy menstrual flow in reproductiveage women. J Gyn Res. 2016;2:1-8.
- Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12:62-66.
- Smith PP, Middleton LJ, Connor M, et al. Hysteroscopic morcellation compared with electrical resection of endometrial polyps: a randomized controlled trial. Obstet Gynecol. 2014;123:745-751.
- Vitale SG, Sapia F, Rapisarda AMC, et al. Hysteroscopic morcellation of submucous myomas: a systematic review. Biomed Res Int. 2017;2017:6848250.
- Stoll F, Lecointre L, Meyer N, et al. Randomized study comparing a reusable morcellator with a resectoscope in the hysteroscopic treatment of uterine polyps: the RESMO study. J Minimal Invasive Gyn. 2021;28:801-810.
- Lee MM, Matsuzono T. Hysteroscopic intrauterine morcellation of submucosal fibroids: preliminary results in Hong Kong and comparisons with conventional hysteroscopic monopolar loop resection. Hong Kong Med J. 2016;22:56-61.
- van Dongen H, Emanuel MH, Wolterbeek R, et al. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15:466-471.
- Berg A, Sandvik L, Langebrekke A, et al. A randomized trial comparing monopolar electrodes using glycine 1.5% with two different types of bipolar electrodes (TCRis, Versapoint) using saline, in hysteroscopic surgery. Fertil Steril. 2009;91:1273- 1278.
- Roy KK, Metta S, Kansal Y, et al. A prospective randomized study comparing unipolar versus bipolar hysteroscopic myomectomy in infertile women. J Hum Reprod Sci. 2017;10:185-193.
- Roy KK, Kansal Y, Subbaiah M, et al. Hysteroscopic septal resection using unipolar resectoscope versus bipolar resectoscope: prospective, randomized study. J Obstet Gynaecol Res. 2015;41:952-956.
- Borg MH, Shehata A. Uterine morcellator versus resectoscopy in the management of heavy menstrual flow in reproductiveage women. J Gyn Res. 2016;2:1-8.
- Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12:62-66.
- Smith PP, Middleton LJ, Connor M, et al. Hysteroscopic morcellation compared with electrical resection of endometrial polyps: a randomized controlled trial. Obstet Gynecol. 2014;123:745-751.
- Vitale SG, Sapia F, Rapisarda AMC, et al. Hysteroscopic morcellation of submucous myomas: a systematic review. Biomed Res Int. 2017;2017:6848250.
- Stoll F, Lecointre L, Meyer N, et al. Randomized study comparing a reusable morcellator with a resectoscope in the hysteroscopic treatment of uterine polyps: the RESMO study. J Minimal Invasive Gyn. 2021;28:801-810.
- Lee MM, Matsuzono T. Hysteroscopic intrauterine morcellation of submucosal fibroids: preliminary results in Hong Kong and comparisons with conventional hysteroscopic monopolar loop resection. Hong Kong Med J. 2016;22:56-61.
- van Dongen H, Emanuel MH, Wolterbeek R, et al. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15:466-471.
Psoriasis
THE COMPARISON
A Elbow and forearm with erythematous, well-demarcated, pink plaques with mild micaceous scale in a 42-year-old White woman.
B Elbow and forearm with violaceous, well-demarcated plaques with micaceous scale and hyperpigmented patches around the active plaques in a 58-year-old Black man.
Epidemiology
Psoriasis prevalence in the United States has been estimated at 3.7%.1-3 If broken down by race or ethnicity, the prevalence of psoriasis varies: 2.5% to 3.7% in White adults1-4; 1.3% to 2% in Black adults1-4; 1.6% in Hispanics/other adults1-3; 1% in children overall; 0.29% in White children1,5; and 0.06% in Black children.1,5
Key clinical features in people with darker skin tones include:
- plaques that may appear more violaceous in color instead of pink or erythematous
- higher body surface area of involvement4 and thicker, more scaly plaques6
- increased likelihood of postinflammatory hyperpigmentation (PIH).
Worth noting
Although individuals of all skin tones may experience the psychosocial impact of psoriasis, quality-of-life measures have been found to be worse in those with skin of color (SOC) compared to White patients. 1,4 This may be due to the lingering PIH and hypopigmentation that occurs even after inflammatory plaques are treated. Of course, lack of access to care contributes to greater disease burden and more devastating psychological impact.
Health disparity highlight
Psoriasis may be underreported and underdiagnosed in individuals with SOC, as factors contributing to health care disparities may play a role, such as access to health care in general,1,7 and access to clinicians proficient in diagnosing cutaneous diseases in SOC may be delayed.8
Biologic medications are used less often in Black patients than in White patients, despite biologic medications being very efficacious for treatment of psoriasis.1,9,10
1. Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
2. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
5. Wu JJ, Black MH, Smith N, et al. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65:957-964.
6. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
7. Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
8. Mundluru SN, Ramalingam ND, Tran HN. Addressing internal medicine residents’ discomfort with basic dermatology in persons of color in the primary care clinic. Am J Med Qual. 2019;34:513-513.
9. Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
10. Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
THE COMPARISON
A Elbow and forearm with erythematous, well-demarcated, pink plaques with mild micaceous scale in a 42-year-old White woman.
B Elbow and forearm with violaceous, well-demarcated plaques with micaceous scale and hyperpigmented patches around the active plaques in a 58-year-old Black man.

Epidemiology
Psoriasis prevalence in the United States has been estimated at 3.7%.1-3 If broken down by race or ethnicity, the prevalence of psoriasis varies: 2.5% to 3.7% in White adults1-4; 1.3% to 2% in Black adults1-4; 1.6% in Hispanics/other adults1-3; 1% in children overall; 0.29% in White children1,5; and 0.06% in Black children.1,5
Key clinical features in people with darker skin tones include:
- plaques that may appear more violaceous in color instead of pink or erythematous
- higher body surface area of involvement4 and thicker, more scaly plaques6
- increased likelihood of postinflammatory hyperpigmentation (PIH).
Worth noting
Although individuals of all skin tones may experience the psychosocial impact of psoriasis, quality-of-life measures have been found to be worse in those with skin of color (SOC) compared to White patients. 1,4 This may be due to the lingering PIH and hypopigmentation that occurs even after inflammatory plaques are treated. Of course, lack of access to care contributes to greater disease burden and more devastating psychological impact.
Health disparity highlight
Psoriasis may be underreported and underdiagnosed in individuals with SOC, as factors contributing to health care disparities may play a role, such as access to health care in general,1,7 and access to clinicians proficient in diagnosing cutaneous diseases in SOC may be delayed.8
Biologic medications are used less often in Black patients than in White patients, despite biologic medications being very efficacious for treatment of psoriasis.1,9,10
THE COMPARISON
A Elbow and forearm with erythematous, well-demarcated, pink plaques with mild micaceous scale in a 42-year-old White woman.
B Elbow and forearm with violaceous, well-demarcated plaques with micaceous scale and hyperpigmented patches around the active plaques in a 58-year-old Black man.

Epidemiology
Psoriasis prevalence in the United States has been estimated at 3.7%.1-3 If broken down by race or ethnicity, the prevalence of psoriasis varies: 2.5% to 3.7% in White adults1-4; 1.3% to 2% in Black adults1-4; 1.6% in Hispanics/other adults1-3; 1% in children overall; 0.29% in White children1,5; and 0.06% in Black children.1,5
Key clinical features in people with darker skin tones include:
- plaques that may appear more violaceous in color instead of pink or erythematous
- higher body surface area of involvement4 and thicker, more scaly plaques6
- increased likelihood of postinflammatory hyperpigmentation (PIH).
Worth noting
Although individuals of all skin tones may experience the psychosocial impact of psoriasis, quality-of-life measures have been found to be worse in those with skin of color (SOC) compared to White patients. 1,4 This may be due to the lingering PIH and hypopigmentation that occurs even after inflammatory plaques are treated. Of course, lack of access to care contributes to greater disease burden and more devastating psychological impact.
Health disparity highlight
Psoriasis may be underreported and underdiagnosed in individuals with SOC, as factors contributing to health care disparities may play a role, such as access to health care in general,1,7 and access to clinicians proficient in diagnosing cutaneous diseases in SOC may be delayed.8
Biologic medications are used less often in Black patients than in White patients, despite biologic medications being very efficacious for treatment of psoriasis.1,9,10
1. Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
2. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
5. Wu JJ, Black MH, Smith N, et al. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65:957-964.
6. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
7. Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
8. Mundluru SN, Ramalingam ND, Tran HN. Addressing internal medicine residents’ discomfort with basic dermatology in persons of color in the primary care clinic. Am J Med Qual. 2019;34:513-513.
9. Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
10. Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
1. Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
2. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
5. Wu JJ, Black MH, Smith N, et al. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65:957-964.
6. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
7. Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
8. Mundluru SN, Ramalingam ND, Tran HN. Addressing internal medicine residents’ discomfort with basic dermatology in persons of color in the primary care clinic. Am J Med Qual. 2019;34:513-513.
9. Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
10. Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis may not be effective
Background: Spontaneous bacterial peritonitis is common and is associated with significant short-term mortality. Antibiotic prophylaxis is the mainstay preventive treatment, but there is concern about development of drug resistance and other adverse events. There is uncertainty regarding relative efficacy and optimal combination of the different available prophylactic treatments.
Study design: 29 randomized clinical trials.
Synopsis: Across 29 randomized clinical trials (total of 3,896 participants) looking at nine different antibiotic regimens for prophylaxis of spontaneous bacterial peritonitis, there was no evidence of differences between any of the antibiotics and no intervention in terms of mortality or serious adverse events, though there was very low certainty of evidence. The authors felt only two small studies were conducted without flaws. There was no difference between any of the antibiotics and no intervention in the proportion of people who developed spontaneous bacterial peritonitis. Overall, 10% of trial participants developed spontaneous bacterial peritonitis and 15% of trial participants died. The lack of effectiveness of across several outcomes may be because of sparse data and selective reporting bias.
Bottom line: Whether antibiotics are effective prophylaxis to prevent spontaneous bacterial peritonitis and which antibiotics should be used is still uncertain; future well-designed studies are needed.
Citation: Komolafe O et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013125. doi: 10.1002/14651858.CD013125.pub2.
Dr. Millard is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Spontaneous bacterial peritonitis is common and is associated with significant short-term mortality. Antibiotic prophylaxis is the mainstay preventive treatment, but there is concern about development of drug resistance and other adverse events. There is uncertainty regarding relative efficacy and optimal combination of the different available prophylactic treatments.
Study design: 29 randomized clinical trials.
Synopsis: Across 29 randomized clinical trials (total of 3,896 participants) looking at nine different antibiotic regimens for prophylaxis of spontaneous bacterial peritonitis, there was no evidence of differences between any of the antibiotics and no intervention in terms of mortality or serious adverse events, though there was very low certainty of evidence. The authors felt only two small studies were conducted without flaws. There was no difference between any of the antibiotics and no intervention in the proportion of people who developed spontaneous bacterial peritonitis. Overall, 10% of trial participants developed spontaneous bacterial peritonitis and 15% of trial participants died. The lack of effectiveness of across several outcomes may be because of sparse data and selective reporting bias.
Bottom line: Whether antibiotics are effective prophylaxis to prevent spontaneous bacterial peritonitis and which antibiotics should be used is still uncertain; future well-designed studies are needed.
Citation: Komolafe O et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013125. doi: 10.1002/14651858.CD013125.pub2.
Dr. Millard is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Spontaneous bacterial peritonitis is common and is associated with significant short-term mortality. Antibiotic prophylaxis is the mainstay preventive treatment, but there is concern about development of drug resistance and other adverse events. There is uncertainty regarding relative efficacy and optimal combination of the different available prophylactic treatments.
Study design: 29 randomized clinical trials.
Synopsis: Across 29 randomized clinical trials (total of 3,896 participants) looking at nine different antibiotic regimens for prophylaxis of spontaneous bacterial peritonitis, there was no evidence of differences between any of the antibiotics and no intervention in terms of mortality or serious adverse events, though there was very low certainty of evidence. The authors felt only two small studies were conducted without flaws. There was no difference between any of the antibiotics and no intervention in the proportion of people who developed spontaneous bacterial peritonitis. Overall, 10% of trial participants developed spontaneous bacterial peritonitis and 15% of trial participants died. The lack of effectiveness of across several outcomes may be because of sparse data and selective reporting bias.
Bottom line: Whether antibiotics are effective prophylaxis to prevent spontaneous bacterial peritonitis and which antibiotics should be used is still uncertain; future well-designed studies are needed.
Citation: Komolafe O et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013125. doi: 10.1002/14651858.CD013125.pub2.
Dr. Millard is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Strong support for causal role of cannabis in schizophrenia
The long-observed association between cannabis use and schizophrenia is likely partially causal in nature, new research shows.
Investigators found a clear increase in the proportion of schizophrenia cases linked to cannabis use disorder over the past 25 years.
“In my view, the association is most likely causative, at least to a large extent,” first author Carsten Hjorthøj, PhD, from the Copenhagen Research Center for Mental Health, Copenhagen University Hospital, told this news organization.
“It is, of course, nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” said Dr. Hjorthøj.
The study was published online July 21 in JAMA Psychiatry.
Far from harmless
The findings are based on Danish national health registry data. The study sample included all people in Denmark born before Dec. 31, 2000 who were aged 16 years or older at some point from Jan. 1, 1972 to Dec. 31, 2016. The data analysis was conducted from August 2020 to April 2021.
Despite some fluctuation, there was a general increase in the population-attributable risk fraction (PARF) for cannabis use disorder with regard to schizophrenia over time, the researchers report. The PARF increased from about 2% in 1995 to about 4% in 2000 and has hovered from 6% to 8% since 2010.
“Although not in itself proof of causality, our study provides evidence of the theory of cannabis being a component cause of schizophrenia,” the investigators write.
The findings are “particularly important with the increasing legalization of cannabis for both medicinal and recreational uses seeming to lead to an increase in the perception of cannabis as relatively harmless and possibly in the uptake of cannabis use, especially among youth,” they add.
“Although psychosis is not the only outcome of interest in terms of cannabis use, our study clearly indicates that cannabis should not be considered harmless,” they conclude.
Cases linked to cannabis underestimated?
In an accompanying editorial, Tyler VanderWeele, PhD, Harvard School of Public Health, Boston, notes that estimates in this study could be conservative as a result of underdiagnosis of cannabis use disorder and because it only examined cannabis use disorder.
“Cannabis use disorder is not responsible for most schizophrenia cases, but it is responsible for a nonnegligible and increasing proportion. This should be considered in discussions regarding legalization and regulation of the use of cannabis,” Dr. VanderWeele writes.
Experts with the Science Media Center, a U.K. nonprofit organization, also weighed in on the results.
Terrie Moffitt, PhD, with King’s College London, said the study “adds important evidence that patients with diagnosed cannabis use disorder are more at risk for psychosis now than they used to be.”
“ However, most cannabis users, even those who are dependent on it, never come in to clinics for treatment. Also, it is known that people who seek treatment tend to have multiple mental health problems, not solely cannabis problems,” Dr. Moffitt commented.
Emir Englund, PhD, also from King’s College London, said the study “strengthens an already well-established association between the two. However, it is unable to shed additional light on whether cannabis causes schizophrenia or not, due to the observational nature of the study.”
“In my opinion, the current scientific view of cannabis use as a ‘component cause’ which interacts with other risk factors to cause schizophrenia but is neither necessary nor sufficient to do so on its own still stands,” Dr. Englund said.
The study was supported by a grant from Lundbeckfonden. The authors have disclosed no relevant financial relationships. Dr. VanderWeele has received grants from the National Cancer Institute and the John Templeton Foundation. Dr. Moffitt and Dr. Englund have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The long-observed association between cannabis use and schizophrenia is likely partially causal in nature, new research shows.
Investigators found a clear increase in the proportion of schizophrenia cases linked to cannabis use disorder over the past 25 years.
“In my view, the association is most likely causative, at least to a large extent,” first author Carsten Hjorthøj, PhD, from the Copenhagen Research Center for Mental Health, Copenhagen University Hospital, told this news organization.
“It is, of course, nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” said Dr. Hjorthøj.
The study was published online July 21 in JAMA Psychiatry.
Far from harmless
The findings are based on Danish national health registry data. The study sample included all people in Denmark born before Dec. 31, 2000 who were aged 16 years or older at some point from Jan. 1, 1972 to Dec. 31, 2016. The data analysis was conducted from August 2020 to April 2021.
Despite some fluctuation, there was a general increase in the population-attributable risk fraction (PARF) for cannabis use disorder with regard to schizophrenia over time, the researchers report. The PARF increased from about 2% in 1995 to about 4% in 2000 and has hovered from 6% to 8% since 2010.
“Although not in itself proof of causality, our study provides evidence of the theory of cannabis being a component cause of schizophrenia,” the investigators write.
The findings are “particularly important with the increasing legalization of cannabis for both medicinal and recreational uses seeming to lead to an increase in the perception of cannabis as relatively harmless and possibly in the uptake of cannabis use, especially among youth,” they add.
“Although psychosis is not the only outcome of interest in terms of cannabis use, our study clearly indicates that cannabis should not be considered harmless,” they conclude.
Cases linked to cannabis underestimated?
In an accompanying editorial, Tyler VanderWeele, PhD, Harvard School of Public Health, Boston, notes that estimates in this study could be conservative as a result of underdiagnosis of cannabis use disorder and because it only examined cannabis use disorder.
“Cannabis use disorder is not responsible for most schizophrenia cases, but it is responsible for a nonnegligible and increasing proportion. This should be considered in discussions regarding legalization and regulation of the use of cannabis,” Dr. VanderWeele writes.
Experts with the Science Media Center, a U.K. nonprofit organization, also weighed in on the results.
Terrie Moffitt, PhD, with King’s College London, said the study “adds important evidence that patients with diagnosed cannabis use disorder are more at risk for psychosis now than they used to be.”
“ However, most cannabis users, even those who are dependent on it, never come in to clinics for treatment. Also, it is known that people who seek treatment tend to have multiple mental health problems, not solely cannabis problems,” Dr. Moffitt commented.
Emir Englund, PhD, also from King’s College London, said the study “strengthens an already well-established association between the two. However, it is unable to shed additional light on whether cannabis causes schizophrenia or not, due to the observational nature of the study.”
“In my opinion, the current scientific view of cannabis use as a ‘component cause’ which interacts with other risk factors to cause schizophrenia but is neither necessary nor sufficient to do so on its own still stands,” Dr. Englund said.
The study was supported by a grant from Lundbeckfonden. The authors have disclosed no relevant financial relationships. Dr. VanderWeele has received grants from the National Cancer Institute and the John Templeton Foundation. Dr. Moffitt and Dr. Englund have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The long-observed association between cannabis use and schizophrenia is likely partially causal in nature, new research shows.
Investigators found a clear increase in the proportion of schizophrenia cases linked to cannabis use disorder over the past 25 years.
“In my view, the association is most likely causative, at least to a large extent,” first author Carsten Hjorthøj, PhD, from the Copenhagen Research Center for Mental Health, Copenhagen University Hospital, told this news organization.
“It is, of course, nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” said Dr. Hjorthøj.
The study was published online July 21 in JAMA Psychiatry.
Far from harmless
The findings are based on Danish national health registry data. The study sample included all people in Denmark born before Dec. 31, 2000 who were aged 16 years or older at some point from Jan. 1, 1972 to Dec. 31, 2016. The data analysis was conducted from August 2020 to April 2021.
Despite some fluctuation, there was a general increase in the population-attributable risk fraction (PARF) for cannabis use disorder with regard to schizophrenia over time, the researchers report. The PARF increased from about 2% in 1995 to about 4% in 2000 and has hovered from 6% to 8% since 2010.
“Although not in itself proof of causality, our study provides evidence of the theory of cannabis being a component cause of schizophrenia,” the investigators write.
The findings are “particularly important with the increasing legalization of cannabis for both medicinal and recreational uses seeming to lead to an increase in the perception of cannabis as relatively harmless and possibly in the uptake of cannabis use, especially among youth,” they add.
“Although psychosis is not the only outcome of interest in terms of cannabis use, our study clearly indicates that cannabis should not be considered harmless,” they conclude.
Cases linked to cannabis underestimated?
In an accompanying editorial, Tyler VanderWeele, PhD, Harvard School of Public Health, Boston, notes that estimates in this study could be conservative as a result of underdiagnosis of cannabis use disorder and because it only examined cannabis use disorder.
“Cannabis use disorder is not responsible for most schizophrenia cases, but it is responsible for a nonnegligible and increasing proportion. This should be considered in discussions regarding legalization and regulation of the use of cannabis,” Dr. VanderWeele writes.
Experts with the Science Media Center, a U.K. nonprofit organization, also weighed in on the results.
Terrie Moffitt, PhD, with King’s College London, said the study “adds important evidence that patients with diagnosed cannabis use disorder are more at risk for psychosis now than they used to be.”
“ However, most cannabis users, even those who are dependent on it, never come in to clinics for treatment. Also, it is known that people who seek treatment tend to have multiple mental health problems, not solely cannabis problems,” Dr. Moffitt commented.
Emir Englund, PhD, also from King’s College London, said the study “strengthens an already well-established association between the two. However, it is unable to shed additional light on whether cannabis causes schizophrenia or not, due to the observational nature of the study.”
“In my opinion, the current scientific view of cannabis use as a ‘component cause’ which interacts with other risk factors to cause schizophrenia but is neither necessary nor sufficient to do so on its own still stands,” Dr. Englund said.
The study was supported by a grant from Lundbeckfonden. The authors have disclosed no relevant financial relationships. Dr. VanderWeele has received grants from the National Cancer Institute and the John Templeton Foundation. Dr. Moffitt and Dr. Englund have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This is not the time to modify a HTN regimen
ILLUSTRATIVE CASE
A 67-year-old man with hypertension that is well controlled on hydrochlorothiazide 25 mg po daily was admitted to the family medicine inpatient service for community-acquired pneumonia requiring antibiotic therapy and oxygen support. Despite improvement in his overall condition, his blood pressure was consistently > 160/90 mm Hg during his hospitalization. He was treated with lisinopril 10 mg po daily in addition to his home medications, which helped achieve recommended blood pressure goals.
Prior to discharge, his blood pressure was noted to be 108/62 mm Hg. He asks if it is necessary to continue this new blood pressure medicine, as his home blood pressure readings had been within the goal set by his primary care physician. Should you continue this new antihypertensive agent at discharge?
Outpatient antihypertensive medication regimens are commonly intensified at hospital discharge in response to transient short-term elevations in blood pressure during inpatient encounters for noncardiac conditions.1,2 This is typically a reflexive response during a hospitalization, despite the unknown long-term, patient-oriented clinical outcomes. These short-term, in-hospital blood pressure elevations may be due to numerous temporary causes, such as stress/anxiety, a pain response, agitation, a medication adverse effect, or volume overload.3
The transition from inpatient to outpatient care is a high-risk period, especially for older adults, as functional status is generally worse at hospital discharge than prehospitalization baseline.4 To compound this problem, adverse drug reactions are a common cause of hospitalization for older adults. Changing blood pressure medications in response to acute physiologic changes during illness may contribute to patient harm. Although observational studies of adverse drug reactions related to blood pressure medications are numerous, researchers have only evaluated adverse drug reactions pertaining to hospital admissions.5-8 This study sought to evaluate the clinical outcomes associated with intensification of antihypertensive regimens at discharge among older adults.
STUDY SUMMARY
Increased risk of readmission, adverse events after intensification at discharge
This retrospective cohort study, which was conducted across multiple
Antihypertensive medication changes at discharge were evaluated using information pulled from VHA pharmacies, combined with clinical data merged from VHA and Medicare claims. Intensification was defined as either adding a new blood pressure medication or a dose increase of more than 20% on a previously prescribed antihypertensive medication. Patients were excluded if they were discharged with a secondary diagnosis that required modifications to a blood pressure medication (such as atrial fibrillation, acute coronary syndrome, or stroke), were hospitalized in the previous 30 days, were admitted from a skilled nursing facility, or received more than 20% of their care (including filling prescriptions) outside the VHA system.
Primary outcomes included hospital readmission or SAEs (falls, syncope, hypotension, serious electrolyte abnormalities, or acute kidney injury) within 30 days or having a cardiovascular event within 1 year of hospital discharge. Secondary outcomes included the change in systolic blood pressure (SBP) within 1 year after discharge. Propensity score matching was used as a balancing factor to create a matched-pairs cohort to compare those receiving blood pressure medication intensification at hospital discharge with those who did not.
Continue to: Intensification of the blood pressure...
Intensification of the blood pressure regimen at hospital discharge was associated with an increased risk in 30-day hospital readmission (hazard ratio [HR] = 1.23; 95% CI, 1.07–1.42; number needed to harm [NNH] = 27) and SAEs (HR = 1.41; 95% CI, 1.06–1.88; NNH = 63). There was no associated reduction in cardiovascular events (HR = 1.18; 95% CI, 0.99–1.40) or change in mean SBP within 1 year after hospital discharge in those who received intensification vs those who did not (mean BP, 134.7 vs 134.4 mm Hg; difference-in-differences estimate = 0.2 mm Hg; 95% CI, −2.0 to 2.4 mm Hg).
WHAT’S NEW
First study on outcomes related to HTN med changes at hospital discharge
This well-designed, retrospective cohort study provides important clinical data to help guide inpatient blood pressure management decisions for patients with noncardiac conditions. No clinical trials up to that time had assessed patient-oriented outcomes when antihypertensive medication regimens were intensified at hospital discharge.
CAVEATS
Study population: Primarily older men with noncardiac conditions
Selected populations benefit from intensive blood pressure control based on specific risk factors and medical conditions. In patients at high risk for cardiovascular disease, without a history of stroke or diabetes, intensive blood pressure control (SBP < 120 mm Hg) improves cardiovascular outcomes and overall survival compared with standard therapy (SBP < 140 mm Hg).9 This retrospective cohort study involved mainly elderly male patients with noncardiac conditions. The study also excluded patients with a secondary diagnosis requiring modifications to an antihypertensive regimen, such as atrial fibrillation, acute coronary syndrome, or cerebrovascular accident. Thus, the findings may not be applicable to these patient populations.
CHALLENGES TO IMPLEMENTATION
Clinicians will need to address individual needs
Physicians have to balance various antihypertensive management strategies, as competing medical specialty society guidelines recommend differing targets for optimal blood pressure control. Given the concern for medicolegal liability and potential harms of therapeutic inertia, inpatient physicians must consider whether hospitalization is the best time to alter medications for long-term outpatient blood pressure control. Finally, the decision to leave blood pressure management to outpatient physicians assumes the patient has a continuity relationship with a primary care medical home.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536.
2. Harris CM, Sridharan A, Landis R, et al. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9:150-153.
3. Aung WM, Menon SV, Materson BJ. Management of hypertension in hospitalized patients. Hosp Pract (1995). 2015;43:101-106.
4. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451-458.
5. Omer HMRB, Hodson J, Pontefract SK, et al. Inpatient falls in older adults: a cohort study of antihypertensive prescribing pre- and post-fall. BMC Geriatr. 2018;18:58.
6. Alhawassi TM, Krass I, Pont LG. Antihypertensive-related adverse drug reactions among older hospitalized adults. Int J Clin Pharm. 2018;40:428-435.
7. Passarelli MCG, Jacob-Filho W, Figueras A. Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging. 2005;22:767-777.
8. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
9. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116. Published correction appears in N Engl J Med. 2017;377:2506.
ILLUSTRATIVE CASE
A 67-year-old man with hypertension that is well controlled on hydrochlorothiazide 25 mg po daily was admitted to the family medicine inpatient service for community-acquired pneumonia requiring antibiotic therapy and oxygen support. Despite improvement in his overall condition, his blood pressure was consistently > 160/90 mm Hg during his hospitalization. He was treated with lisinopril 10 mg po daily in addition to his home medications, which helped achieve recommended blood pressure goals.
Prior to discharge, his blood pressure was noted to be 108/62 mm Hg. He asks if it is necessary to continue this new blood pressure medicine, as his home blood pressure readings had been within the goal set by his primary care physician. Should you continue this new antihypertensive agent at discharge?
Outpatient antihypertensive medication regimens are commonly intensified at hospital discharge in response to transient short-term elevations in blood pressure during inpatient encounters for noncardiac conditions.1,2 This is typically a reflexive response during a hospitalization, despite the unknown long-term, patient-oriented clinical outcomes. These short-term, in-hospital blood pressure elevations may be due to numerous temporary causes, such as stress/anxiety, a pain response, agitation, a medication adverse effect, or volume overload.3
The transition from inpatient to outpatient care is a high-risk period, especially for older adults, as functional status is generally worse at hospital discharge than prehospitalization baseline.4 To compound this problem, adverse drug reactions are a common cause of hospitalization for older adults. Changing blood pressure medications in response to acute physiologic changes during illness may contribute to patient harm. Although observational studies of adverse drug reactions related to blood pressure medications are numerous, researchers have only evaluated adverse drug reactions pertaining to hospital admissions.5-8 This study sought to evaluate the clinical outcomes associated with intensification of antihypertensive regimens at discharge among older adults.
STUDY SUMMARY
Increased risk of readmission, adverse events after intensification at discharge
This retrospective cohort study, which was conducted across multiple
Antihypertensive medication changes at discharge were evaluated using information pulled from VHA pharmacies, combined with clinical data merged from VHA and Medicare claims. Intensification was defined as either adding a new blood pressure medication or a dose increase of more than 20% on a previously prescribed antihypertensive medication. Patients were excluded if they were discharged with a secondary diagnosis that required modifications to a blood pressure medication (such as atrial fibrillation, acute coronary syndrome, or stroke), were hospitalized in the previous 30 days, were admitted from a skilled nursing facility, or received more than 20% of their care (including filling prescriptions) outside the VHA system.
Primary outcomes included hospital readmission or SAEs (falls, syncope, hypotension, serious electrolyte abnormalities, or acute kidney injury) within 30 days or having a cardiovascular event within 1 year of hospital discharge. Secondary outcomes included the change in systolic blood pressure (SBP) within 1 year after discharge. Propensity score matching was used as a balancing factor to create a matched-pairs cohort to compare those receiving blood pressure medication intensification at hospital discharge with those who did not.
Continue to: Intensification of the blood pressure...
Intensification of the blood pressure regimen at hospital discharge was associated with an increased risk in 30-day hospital readmission (hazard ratio [HR] = 1.23; 95% CI, 1.07–1.42; number needed to harm [NNH] = 27) and SAEs (HR = 1.41; 95% CI, 1.06–1.88; NNH = 63). There was no associated reduction in cardiovascular events (HR = 1.18; 95% CI, 0.99–1.40) or change in mean SBP within 1 year after hospital discharge in those who received intensification vs those who did not (mean BP, 134.7 vs 134.4 mm Hg; difference-in-differences estimate = 0.2 mm Hg; 95% CI, −2.0 to 2.4 mm Hg).
WHAT’S NEW
First study on outcomes related to HTN med changes at hospital discharge
This well-designed, retrospective cohort study provides important clinical data to help guide inpatient blood pressure management decisions for patients with noncardiac conditions. No clinical trials up to that time had assessed patient-oriented outcomes when antihypertensive medication regimens were intensified at hospital discharge.
CAVEATS
Study population: Primarily older men with noncardiac conditions
Selected populations benefit from intensive blood pressure control based on specific risk factors and medical conditions. In patients at high risk for cardiovascular disease, without a history of stroke or diabetes, intensive blood pressure control (SBP < 120 mm Hg) improves cardiovascular outcomes and overall survival compared with standard therapy (SBP < 140 mm Hg).9 This retrospective cohort study involved mainly elderly male patients with noncardiac conditions. The study also excluded patients with a secondary diagnosis requiring modifications to an antihypertensive regimen, such as atrial fibrillation, acute coronary syndrome, or cerebrovascular accident. Thus, the findings may not be applicable to these patient populations.
CHALLENGES TO IMPLEMENTATION
Clinicians will need to address individual needs
Physicians have to balance various antihypertensive management strategies, as competing medical specialty society guidelines recommend differing targets for optimal blood pressure control. Given the concern for medicolegal liability and potential harms of therapeutic inertia, inpatient physicians must consider whether hospitalization is the best time to alter medications for long-term outpatient blood pressure control. Finally, the decision to leave blood pressure management to outpatient physicians assumes the patient has a continuity relationship with a primary care medical home.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 67-year-old man with hypertension that is well controlled on hydrochlorothiazide 25 mg po daily was admitted to the family medicine inpatient service for community-acquired pneumonia requiring antibiotic therapy and oxygen support. Despite improvement in his overall condition, his blood pressure was consistently > 160/90 mm Hg during his hospitalization. He was treated with lisinopril 10 mg po daily in addition to his home medications, which helped achieve recommended blood pressure goals.
Prior to discharge, his blood pressure was noted to be 108/62 mm Hg. He asks if it is necessary to continue this new blood pressure medicine, as his home blood pressure readings had been within the goal set by his primary care physician. Should you continue this new antihypertensive agent at discharge?
Outpatient antihypertensive medication regimens are commonly intensified at hospital discharge in response to transient short-term elevations in blood pressure during inpatient encounters for noncardiac conditions.1,2 This is typically a reflexive response during a hospitalization, despite the unknown long-term, patient-oriented clinical outcomes. These short-term, in-hospital blood pressure elevations may be due to numerous temporary causes, such as stress/anxiety, a pain response, agitation, a medication adverse effect, or volume overload.3
The transition from inpatient to outpatient care is a high-risk period, especially for older adults, as functional status is generally worse at hospital discharge than prehospitalization baseline.4 To compound this problem, adverse drug reactions are a common cause of hospitalization for older adults. Changing blood pressure medications in response to acute physiologic changes during illness may contribute to patient harm. Although observational studies of adverse drug reactions related to blood pressure medications are numerous, researchers have only evaluated adverse drug reactions pertaining to hospital admissions.5-8 This study sought to evaluate the clinical outcomes associated with intensification of antihypertensive regimens at discharge among older adults.
STUDY SUMMARY
Increased risk of readmission, adverse events after intensification at discharge
This retrospective cohort study, which was conducted across multiple
Antihypertensive medication changes at discharge were evaluated using information pulled from VHA pharmacies, combined with clinical data merged from VHA and Medicare claims. Intensification was defined as either adding a new blood pressure medication or a dose increase of more than 20% on a previously prescribed antihypertensive medication. Patients were excluded if they were discharged with a secondary diagnosis that required modifications to a blood pressure medication (such as atrial fibrillation, acute coronary syndrome, or stroke), were hospitalized in the previous 30 days, were admitted from a skilled nursing facility, or received more than 20% of their care (including filling prescriptions) outside the VHA system.
Primary outcomes included hospital readmission or SAEs (falls, syncope, hypotension, serious electrolyte abnormalities, or acute kidney injury) within 30 days or having a cardiovascular event within 1 year of hospital discharge. Secondary outcomes included the change in systolic blood pressure (SBP) within 1 year after discharge. Propensity score matching was used as a balancing factor to create a matched-pairs cohort to compare those receiving blood pressure medication intensification at hospital discharge with those who did not.
Continue to: Intensification of the blood pressure...
Intensification of the blood pressure regimen at hospital discharge was associated with an increased risk in 30-day hospital readmission (hazard ratio [HR] = 1.23; 95% CI, 1.07–1.42; number needed to harm [NNH] = 27) and SAEs (HR = 1.41; 95% CI, 1.06–1.88; NNH = 63). There was no associated reduction in cardiovascular events (HR = 1.18; 95% CI, 0.99–1.40) or change in mean SBP within 1 year after hospital discharge in those who received intensification vs those who did not (mean BP, 134.7 vs 134.4 mm Hg; difference-in-differences estimate = 0.2 mm Hg; 95% CI, −2.0 to 2.4 mm Hg).
WHAT’S NEW
First study on outcomes related to HTN med changes at hospital discharge
This well-designed, retrospective cohort study provides important clinical data to help guide inpatient blood pressure management decisions for patients with noncardiac conditions. No clinical trials up to that time had assessed patient-oriented outcomes when antihypertensive medication regimens were intensified at hospital discharge.
CAVEATS
Study population: Primarily older men with noncardiac conditions
Selected populations benefit from intensive blood pressure control based on specific risk factors and medical conditions. In patients at high risk for cardiovascular disease, without a history of stroke or diabetes, intensive blood pressure control (SBP < 120 mm Hg) improves cardiovascular outcomes and overall survival compared with standard therapy (SBP < 140 mm Hg).9 This retrospective cohort study involved mainly elderly male patients with noncardiac conditions. The study also excluded patients with a secondary diagnosis requiring modifications to an antihypertensive regimen, such as atrial fibrillation, acute coronary syndrome, or cerebrovascular accident. Thus, the findings may not be applicable to these patient populations.
CHALLENGES TO IMPLEMENTATION
Clinicians will need to address individual needs
Physicians have to balance various antihypertensive management strategies, as competing medical specialty society guidelines recommend differing targets for optimal blood pressure control. Given the concern for medicolegal liability and potential harms of therapeutic inertia, inpatient physicians must consider whether hospitalization is the best time to alter medications for long-term outpatient blood pressure control. Finally, the decision to leave blood pressure management to outpatient physicians assumes the patient has a continuity relationship with a primary care medical home.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536.
2. Harris CM, Sridharan A, Landis R, et al. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9:150-153.
3. Aung WM, Menon SV, Materson BJ. Management of hypertension in hospitalized patients. Hosp Pract (1995). 2015;43:101-106.
4. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451-458.
5. Omer HMRB, Hodson J, Pontefract SK, et al. Inpatient falls in older adults: a cohort study of antihypertensive prescribing pre- and post-fall. BMC Geriatr. 2018;18:58.
6. Alhawassi TM, Krass I, Pont LG. Antihypertensive-related adverse drug reactions among older hospitalized adults. Int J Clin Pharm. 2018;40:428-435.
7. Passarelli MCG, Jacob-Filho W, Figueras A. Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging. 2005;22:767-777.
8. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
9. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116. Published correction appears in N Engl J Med. 2017;377:2506.
1. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536.
2. Harris CM, Sridharan A, Landis R, et al. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9:150-153.
3. Aung WM, Menon SV, Materson BJ. Management of hypertension in hospitalized patients. Hosp Pract (1995). 2015;43:101-106.
4. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451-458.
5. Omer HMRB, Hodson J, Pontefract SK, et al. Inpatient falls in older adults: a cohort study of antihypertensive prescribing pre- and post-fall. BMC Geriatr. 2018;18:58.
6. Alhawassi TM, Krass I, Pont LG. Antihypertensive-related adverse drug reactions among older hospitalized adults. Int J Clin Pharm. 2018;40:428-435.
7. Passarelli MCG, Jacob-Filho W, Figueras A. Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging. 2005;22:767-777.
8. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
9. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116. Published correction appears in N Engl J Med. 2017;377:2506.
PRACTICE CHANGER
Avoid intensifying antihypertensive medication regimens at hospital discharge in older adults; making such changes increases the risk of serious adverse events (SAEs) and hospital readmission within 30 days without reducing the risk of serious cardiovascular events at 1 year post discharge.
STRENGTH OF RECOMMENDATION
B: Based on a large retrospective cohort study evaluating patient-oriented outcomes.1
Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536.