User login
FDA OKs Pfizer COVID booster for 65 and over, those at high risk
The agency’s move comes as a Centers for Disease Control and Prevention (CDC) panel ended the first day of a 2-day meeting. That panel, the Advisory Committee on Immunization Practices (ACIP), is expected to vote Sept. 23 to instruct doctors on how to administer the boosters.
The FDA officially authorized the vaccine not only for individuals 65 and older, but also for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection.
“After considering the totality of the available scientific evidence and the deliberations of our advisory committee of independent, external experts, the FDA amended the EUA for the Pfizer-BioNTech COVID-19 vaccine to allow for a booster dose in certain populations such as health care workers, teachers and daycare staff, grocery workers and those in homeless shelters or prisons, among others,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release.
The recommendations align with those from an FDA advisory panel Sept. 17.
The agency determined that the benefits of a booster dose outweigh the risks for people now authorized to receive it, according to the news release.
Other questions remain
So, how will this work? That was the main question weighing on the minds of the CDC’s ACIP during their first day of a 2-day meeting where they are expected to make recommendations on booster doses for Americans.
The panel discussed situations the FDA will still need to consider, such as what should be done for Americans who were originally vaccinated with a Moderna or Johnson and Johnson vaccine, but are not covered under the revised EUA, which is only for those people who received Pfizer’s two-dose vaccine regimen.
“That’s going to leave half of the people immunized in this age group having received the vaccine and being told that they’re at risk now for waning immunity and hospitalization unable to get a booster dose,” said committee member Sarah S. Long, MD, a professor of pediatrics at Drexel University College of Medicine in Philadelphia. “So that’s a big public health panic that we would like to avoid.”
Johnson and Johnson recently reported that second doses of its vaccine boosted its efficacy to almost 94% against COVID-19. A new study, published ahead of peer review, suggests that the efficacy of the single-dose Johnson and Johnson shot has fallen to about 78% against symptomatic infection during the Delta surge.
Moderna has applied for permission to market third doses of its vaccine in the United States, but the FDA has given no timeline on when it might make a decision.
Doran Fink, MD, PhD, deputy director of the FDA’s Division of Vaccines and Related Products Applications, a representative advising the committee Sept. 22, said the agency was working as rapidly as possible on Moderna’s submission.
Regarding the question of whether it was OK to mix vaccines, rather than match them, Dr. Fink said there are currently not enough data available to inform that decision.
Those answers are coming, though. John Beigel, MD, associate director of clinical research at the National Institute of Allergy and Infectious Diseases, revealed that the federal government has a study underway to see what happens when the vaccines are mixed with each other.
He said that data from the study would be available later this fall, and would certainly help physicians and other healthcare providers know whether it’s effective or safe to use them interchangeably.
Correlates of immunity
The ACIP left much of its schedule open Sept. 23 to discuss extra Pfizer doses and vote on how they should be used.
Pfizer had originally applied to the FDA for an amendment to its FDA approval, which would have given doctors a freer hand to prescribe third doses as they saw fit, in patients as young as 16.
But the FDA’s Vaccines and Related Biological Products Advisory Committee voted Sept. 17 against granting the amendment. The committee was particularly concerned about the lack of data in teens ages 16 and 17, who have the highest risk for a rare side effect that causes heart inflammation that requires hospital care.
Instead, they recommended — and the FDA agreed per their decision Sept. 22 — that third doses should be given to people at higher risk for severe breakthrough infections because of advanced age or because they work in an occupation that puts them at high risk for exposure.
The CDC panel heard important presentations on new science that is helping to identify the correlates of immunity.
The correlates of immunity are biomarkers that can be measured in blood that help doctors understand how protected a person may be against COVID-19. These markers of immunity are not yet known for the COVID-19 vaccines.
Emerging evidence shows that booster doses of the Pfizer vaccine cause front-line immune defenders — called binding antibodies — to roughly triple soon after a person gets the third shot.
Neutralizing antibodies also jump soon after two vaccine doses, but they fall over time, which is natural. The body doesn’t need these foot soldiers to be on guard all the time, so they go away.
The body retains its memory of how to make them, however, so they can quickly be marshaled again, if needed.
Early studies suggest that antibodies account for about two thirds of a person’s protection against COVID, while the longer-lasting T-cells and B-cells account for about one third.
After the antibody levels fall, it may take a few days to recreate this army. In the meantime, the virus can try to break in. This can cause symptoms, which can make a person feel terrible, but for the most part, vaccinated individuals don’t need hospital care and are nearly always protected from dying — even against the Delta variant.
Those most likely to be at risk for a breakthrough infection are older, because immune function wanes with age.
Essential workers
Essential workers, such as those who work in healthcare, may also benefit from high antibody levels, which can minimize symptoms and help them get back to work more quickly.
Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, said that in her area staffing levels are critical right now.
“I’m actually sitting in one of the deepest red [states] with high rates of COVID. We don’t have enough health care workers currently to take care of the unvaccinated,” she said.
“When we have beds, we are often missing staff, and so the idea of vaccinating health care workers is to be a little bit different than our idea of using vaccines in the general population,” Dr. Talbot said.
Oliver Brooks, MD, chief medical officer of the Watts Healthcare Corporation in Los Angeles, said he was in favor of making a public statement about the temporary nature of the potential recommendations Sept. 23, because they probably won’t cover all who might need a third shot.
“We may want to go on record stating what it is that would allow us to broaden our recommendation or restrict our recommendation,” Dr. Brooks said.
The considerations of who should get an extra dose are not always straightforward.
New modeling by the Harvard TH Chan School of Public Health and the CDC to assist the government’s decisions on boosters had a surprise finding: in nursing homes, it’s more effective to vaccinate healthcare workers than it is to give booster doses to these residents. Nursing homes are at the mercy of community transmission.
In regions with high transmission, it’s easy for a caregiver to bring the virus into a facility — so the models found that the transmission from these workers is a more effective strategy than giving third doses to the already highly vaccinated group of seniors who live in them.
A version of this article first appeared on Medscape.com.
The agency’s move comes as a Centers for Disease Control and Prevention (CDC) panel ended the first day of a 2-day meeting. That panel, the Advisory Committee on Immunization Practices (ACIP), is expected to vote Sept. 23 to instruct doctors on how to administer the boosters.
The FDA officially authorized the vaccine not only for individuals 65 and older, but also for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection.
“After considering the totality of the available scientific evidence and the deliberations of our advisory committee of independent, external experts, the FDA amended the EUA for the Pfizer-BioNTech COVID-19 vaccine to allow for a booster dose in certain populations such as health care workers, teachers and daycare staff, grocery workers and those in homeless shelters or prisons, among others,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release.
The recommendations align with those from an FDA advisory panel Sept. 17.
The agency determined that the benefits of a booster dose outweigh the risks for people now authorized to receive it, according to the news release.
Other questions remain
So, how will this work? That was the main question weighing on the minds of the CDC’s ACIP during their first day of a 2-day meeting where they are expected to make recommendations on booster doses for Americans.
The panel discussed situations the FDA will still need to consider, such as what should be done for Americans who were originally vaccinated with a Moderna or Johnson and Johnson vaccine, but are not covered under the revised EUA, which is only for those people who received Pfizer’s two-dose vaccine regimen.
“That’s going to leave half of the people immunized in this age group having received the vaccine and being told that they’re at risk now for waning immunity and hospitalization unable to get a booster dose,” said committee member Sarah S. Long, MD, a professor of pediatrics at Drexel University College of Medicine in Philadelphia. “So that’s a big public health panic that we would like to avoid.”
Johnson and Johnson recently reported that second doses of its vaccine boosted its efficacy to almost 94% against COVID-19. A new study, published ahead of peer review, suggests that the efficacy of the single-dose Johnson and Johnson shot has fallen to about 78% against symptomatic infection during the Delta surge.
Moderna has applied for permission to market third doses of its vaccine in the United States, but the FDA has given no timeline on when it might make a decision.
Doran Fink, MD, PhD, deputy director of the FDA’s Division of Vaccines and Related Products Applications, a representative advising the committee Sept. 22, said the agency was working as rapidly as possible on Moderna’s submission.
Regarding the question of whether it was OK to mix vaccines, rather than match them, Dr. Fink said there are currently not enough data available to inform that decision.
Those answers are coming, though. John Beigel, MD, associate director of clinical research at the National Institute of Allergy and Infectious Diseases, revealed that the federal government has a study underway to see what happens when the vaccines are mixed with each other.
He said that data from the study would be available later this fall, and would certainly help physicians and other healthcare providers know whether it’s effective or safe to use them interchangeably.
Correlates of immunity
The ACIP left much of its schedule open Sept. 23 to discuss extra Pfizer doses and vote on how they should be used.
Pfizer had originally applied to the FDA for an amendment to its FDA approval, which would have given doctors a freer hand to prescribe third doses as they saw fit, in patients as young as 16.
But the FDA’s Vaccines and Related Biological Products Advisory Committee voted Sept. 17 against granting the amendment. The committee was particularly concerned about the lack of data in teens ages 16 and 17, who have the highest risk for a rare side effect that causes heart inflammation that requires hospital care.
Instead, they recommended — and the FDA agreed per their decision Sept. 22 — that third doses should be given to people at higher risk for severe breakthrough infections because of advanced age or because they work in an occupation that puts them at high risk for exposure.
The CDC panel heard important presentations on new science that is helping to identify the correlates of immunity.
The correlates of immunity are biomarkers that can be measured in blood that help doctors understand how protected a person may be against COVID-19. These markers of immunity are not yet known for the COVID-19 vaccines.
Emerging evidence shows that booster doses of the Pfizer vaccine cause front-line immune defenders — called binding antibodies — to roughly triple soon after a person gets the third shot.
Neutralizing antibodies also jump soon after two vaccine doses, but they fall over time, which is natural. The body doesn’t need these foot soldiers to be on guard all the time, so they go away.
The body retains its memory of how to make them, however, so they can quickly be marshaled again, if needed.
Early studies suggest that antibodies account for about two thirds of a person’s protection against COVID, while the longer-lasting T-cells and B-cells account for about one third.
After the antibody levels fall, it may take a few days to recreate this army. In the meantime, the virus can try to break in. This can cause symptoms, which can make a person feel terrible, but for the most part, vaccinated individuals don’t need hospital care and are nearly always protected from dying — even against the Delta variant.
Those most likely to be at risk for a breakthrough infection are older, because immune function wanes with age.
Essential workers
Essential workers, such as those who work in healthcare, may also benefit from high antibody levels, which can minimize symptoms and help them get back to work more quickly.
Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, said that in her area staffing levels are critical right now.
“I’m actually sitting in one of the deepest red [states] with high rates of COVID. We don’t have enough health care workers currently to take care of the unvaccinated,” she said.
“When we have beds, we are often missing staff, and so the idea of vaccinating health care workers is to be a little bit different than our idea of using vaccines in the general population,” Dr. Talbot said.
Oliver Brooks, MD, chief medical officer of the Watts Healthcare Corporation in Los Angeles, said he was in favor of making a public statement about the temporary nature of the potential recommendations Sept. 23, because they probably won’t cover all who might need a third shot.
“We may want to go on record stating what it is that would allow us to broaden our recommendation or restrict our recommendation,” Dr. Brooks said.
The considerations of who should get an extra dose are not always straightforward.
New modeling by the Harvard TH Chan School of Public Health and the CDC to assist the government’s decisions on boosters had a surprise finding: in nursing homes, it’s more effective to vaccinate healthcare workers than it is to give booster doses to these residents. Nursing homes are at the mercy of community transmission.
In regions with high transmission, it’s easy for a caregiver to bring the virus into a facility — so the models found that the transmission from these workers is a more effective strategy than giving third doses to the already highly vaccinated group of seniors who live in them.
A version of this article first appeared on Medscape.com.
The agency’s move comes as a Centers for Disease Control and Prevention (CDC) panel ended the first day of a 2-day meeting. That panel, the Advisory Committee on Immunization Practices (ACIP), is expected to vote Sept. 23 to instruct doctors on how to administer the boosters.
The FDA officially authorized the vaccine not only for individuals 65 and older, but also for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection.
“After considering the totality of the available scientific evidence and the deliberations of our advisory committee of independent, external experts, the FDA amended the EUA for the Pfizer-BioNTech COVID-19 vaccine to allow for a booster dose in certain populations such as health care workers, teachers and daycare staff, grocery workers and those in homeless shelters or prisons, among others,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release.
The recommendations align with those from an FDA advisory panel Sept. 17.
The agency determined that the benefits of a booster dose outweigh the risks for people now authorized to receive it, according to the news release.
Other questions remain
So, how will this work? That was the main question weighing on the minds of the CDC’s ACIP during their first day of a 2-day meeting where they are expected to make recommendations on booster doses for Americans.
The panel discussed situations the FDA will still need to consider, such as what should be done for Americans who were originally vaccinated with a Moderna or Johnson and Johnson vaccine, but are not covered under the revised EUA, which is only for those people who received Pfizer’s two-dose vaccine regimen.
“That’s going to leave half of the people immunized in this age group having received the vaccine and being told that they’re at risk now for waning immunity and hospitalization unable to get a booster dose,” said committee member Sarah S. Long, MD, a professor of pediatrics at Drexel University College of Medicine in Philadelphia. “So that’s a big public health panic that we would like to avoid.”
Johnson and Johnson recently reported that second doses of its vaccine boosted its efficacy to almost 94% against COVID-19. A new study, published ahead of peer review, suggests that the efficacy of the single-dose Johnson and Johnson shot has fallen to about 78% against symptomatic infection during the Delta surge.
Moderna has applied for permission to market third doses of its vaccine in the United States, but the FDA has given no timeline on when it might make a decision.
Doran Fink, MD, PhD, deputy director of the FDA’s Division of Vaccines and Related Products Applications, a representative advising the committee Sept. 22, said the agency was working as rapidly as possible on Moderna’s submission.
Regarding the question of whether it was OK to mix vaccines, rather than match them, Dr. Fink said there are currently not enough data available to inform that decision.
Those answers are coming, though. John Beigel, MD, associate director of clinical research at the National Institute of Allergy and Infectious Diseases, revealed that the federal government has a study underway to see what happens when the vaccines are mixed with each other.
He said that data from the study would be available later this fall, and would certainly help physicians and other healthcare providers know whether it’s effective or safe to use them interchangeably.
Correlates of immunity
The ACIP left much of its schedule open Sept. 23 to discuss extra Pfizer doses and vote on how they should be used.
Pfizer had originally applied to the FDA for an amendment to its FDA approval, which would have given doctors a freer hand to prescribe third doses as they saw fit, in patients as young as 16.
But the FDA’s Vaccines and Related Biological Products Advisory Committee voted Sept. 17 against granting the amendment. The committee was particularly concerned about the lack of data in teens ages 16 and 17, who have the highest risk for a rare side effect that causes heart inflammation that requires hospital care.
Instead, they recommended — and the FDA agreed per their decision Sept. 22 — that third doses should be given to people at higher risk for severe breakthrough infections because of advanced age or because they work in an occupation that puts them at high risk for exposure.
The CDC panel heard important presentations on new science that is helping to identify the correlates of immunity.
The correlates of immunity are biomarkers that can be measured in blood that help doctors understand how protected a person may be against COVID-19. These markers of immunity are not yet known for the COVID-19 vaccines.
Emerging evidence shows that booster doses of the Pfizer vaccine cause front-line immune defenders — called binding antibodies — to roughly triple soon after a person gets the third shot.
Neutralizing antibodies also jump soon after two vaccine doses, but they fall over time, which is natural. The body doesn’t need these foot soldiers to be on guard all the time, so they go away.
The body retains its memory of how to make them, however, so they can quickly be marshaled again, if needed.
Early studies suggest that antibodies account for about two thirds of a person’s protection against COVID, while the longer-lasting T-cells and B-cells account for about one third.
After the antibody levels fall, it may take a few days to recreate this army. In the meantime, the virus can try to break in. This can cause symptoms, which can make a person feel terrible, but for the most part, vaccinated individuals don’t need hospital care and are nearly always protected from dying — even against the Delta variant.
Those most likely to be at risk for a breakthrough infection are older, because immune function wanes with age.
Essential workers
Essential workers, such as those who work in healthcare, may also benefit from high antibody levels, which can minimize symptoms and help them get back to work more quickly.
Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, said that in her area staffing levels are critical right now.
“I’m actually sitting in one of the deepest red [states] with high rates of COVID. We don’t have enough health care workers currently to take care of the unvaccinated,” she said.
“When we have beds, we are often missing staff, and so the idea of vaccinating health care workers is to be a little bit different than our idea of using vaccines in the general population,” Dr. Talbot said.
Oliver Brooks, MD, chief medical officer of the Watts Healthcare Corporation in Los Angeles, said he was in favor of making a public statement about the temporary nature of the potential recommendations Sept. 23, because they probably won’t cover all who might need a third shot.
“We may want to go on record stating what it is that would allow us to broaden our recommendation or restrict our recommendation,” Dr. Brooks said.
The considerations of who should get an extra dose are not always straightforward.
New modeling by the Harvard TH Chan School of Public Health and the CDC to assist the government’s decisions on boosters had a surprise finding: in nursing homes, it’s more effective to vaccinate healthcare workers than it is to give booster doses to these residents. Nursing homes are at the mercy of community transmission.
In regions with high transmission, it’s easy for a caregiver to bring the virus into a facility — so the models found that the transmission from these workers is a more effective strategy than giving third doses to the already highly vaccinated group of seniors who live in them.
A version of this article first appeared on Medscape.com.
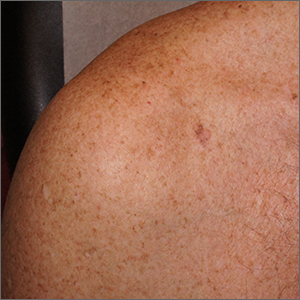
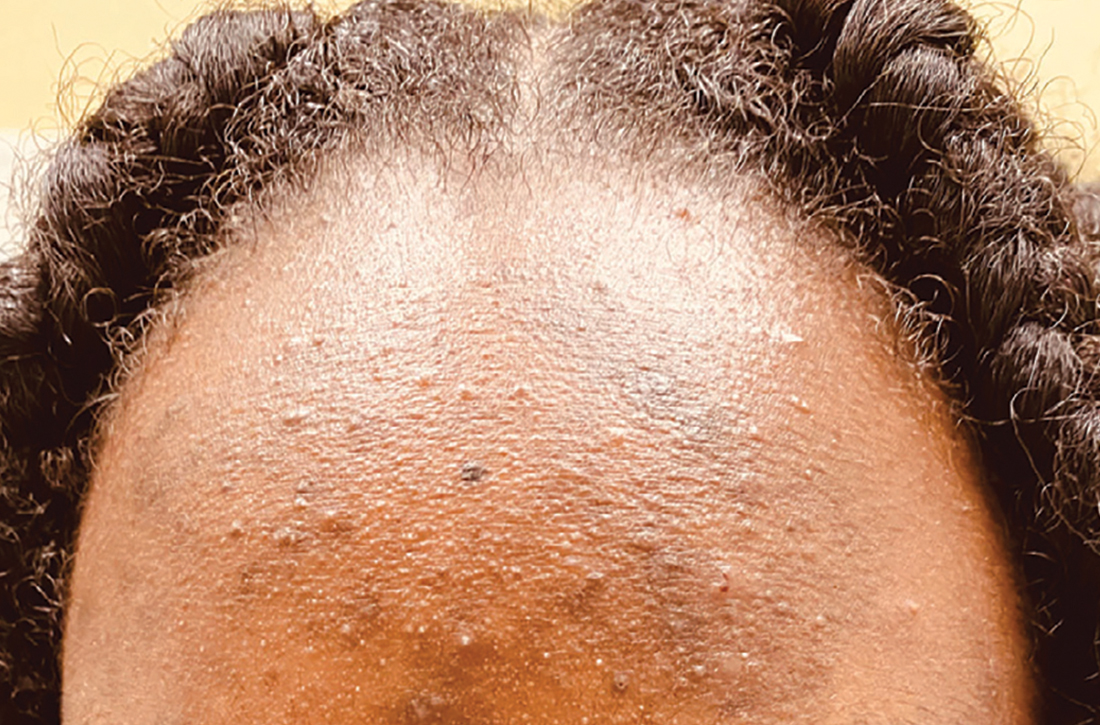
Itchy patch on the clavicle
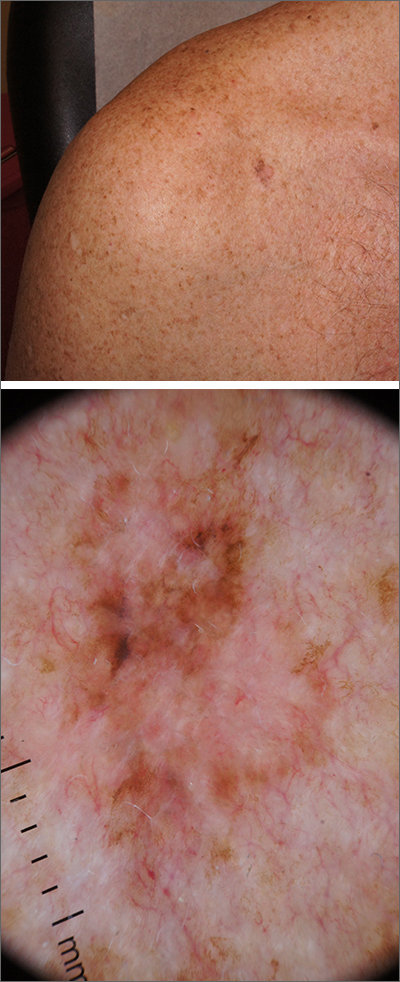
A broad shave biopsy, utilizing dermoscopy to help delineate the edges of the lesion, confirmed the diagnosis of melanoma in situ, superficial spreading type.
From the standpoint of ABCDE criteria (Asymmetry, Border irregularity, Color [varying shades or deep black color], Diameter > 6 mm, or Evolving/changing), this lesion was quite worrisome. Helpful clues on clinical exam included the asymmetry, border irregularity, color variations (brown, pink, and red), size, and history of change. A dermatoscope helped to refine the features of the lesion and highlighted the melanocytic-specific characteristics of its pigment network, streaks, and regression structures (pepper-like gray dots and white streaks).1
A broad shave biopsy allows a very thorough evaluation of such a heterogeneous lesion. A smaller punch biopsy can miss the most worrisome features. That said, a smaller punch biopsy is sometimes necessary in challenging anatomic locations, such as the palm or sole.
Patients given a diagnosis of melanoma in situ should undergo wide local excision with 5-mm margins or 1-cm margins in instances of lentigo maligna, which is a subtype of melanoma in situ with a higher risk of clinically uncertain margins. (A sentinel lymph node biopsy is not recommended for melanoma in situ, as the Breslow depth is 0 mm.)
For this patient, an in-office wide local excision was performed down to the fascia and the skin was repaired in a layered fashion. The plan for this patient was for him to return for skin examinations 3 to 4 times in the first year of diagnosis, followed by twice annually until he was 5 years from his diagnosis. After that, he would undergo annual skin exams.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Ribero S, Moscarella E, Ferrara G, et al. Regression in cutaneous melanoma: a comprehensive review from diagnosis to prognosis. J Eur Acad Dermatol Venereol. 2016;30:2030-2037. doi: 10.1111/jdv.13815

A broad shave biopsy, utilizing dermoscopy to help delineate the edges of the lesion, confirmed the diagnosis of melanoma in situ, superficial spreading type.
From the standpoint of ABCDE criteria (Asymmetry, Border irregularity, Color [varying shades or deep black color], Diameter > 6 mm, or Evolving/changing), this lesion was quite worrisome. Helpful clues on clinical exam included the asymmetry, border irregularity, color variations (brown, pink, and red), size, and history of change. A dermatoscope helped to refine the features of the lesion and highlighted the melanocytic-specific characteristics of its pigment network, streaks, and regression structures (pepper-like gray dots and white streaks).1
A broad shave biopsy allows a very thorough evaluation of such a heterogeneous lesion. A smaller punch biopsy can miss the most worrisome features. That said, a smaller punch biopsy is sometimes necessary in challenging anatomic locations, such as the palm or sole.
Patients given a diagnosis of melanoma in situ should undergo wide local excision with 5-mm margins or 1-cm margins in instances of lentigo maligna, which is a subtype of melanoma in situ with a higher risk of clinically uncertain margins. (A sentinel lymph node biopsy is not recommended for melanoma in situ, as the Breslow depth is 0 mm.)
For this patient, an in-office wide local excision was performed down to the fascia and the skin was repaired in a layered fashion. The plan for this patient was for him to return for skin examinations 3 to 4 times in the first year of diagnosis, followed by twice annually until he was 5 years from his diagnosis. After that, he would undergo annual skin exams.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A broad shave biopsy, utilizing dermoscopy to help delineate the edges of the lesion, confirmed the diagnosis of melanoma in situ, superficial spreading type.
From the standpoint of ABCDE criteria (Asymmetry, Border irregularity, Color [varying shades or deep black color], Diameter > 6 mm, or Evolving/changing), this lesion was quite worrisome. Helpful clues on clinical exam included the asymmetry, border irregularity, color variations (brown, pink, and red), size, and history of change. A dermatoscope helped to refine the features of the lesion and highlighted the melanocytic-specific characteristics of its pigment network, streaks, and regression structures (pepper-like gray dots and white streaks).1
A broad shave biopsy allows a very thorough evaluation of such a heterogeneous lesion. A smaller punch biopsy can miss the most worrisome features. That said, a smaller punch biopsy is sometimes necessary in challenging anatomic locations, such as the palm or sole.
Patients given a diagnosis of melanoma in situ should undergo wide local excision with 5-mm margins or 1-cm margins in instances of lentigo maligna, which is a subtype of melanoma in situ with a higher risk of clinically uncertain margins. (A sentinel lymph node biopsy is not recommended for melanoma in situ, as the Breslow depth is 0 mm.)
For this patient, an in-office wide local excision was performed down to the fascia and the skin was repaired in a layered fashion. The plan for this patient was for him to return for skin examinations 3 to 4 times in the first year of diagnosis, followed by twice annually until he was 5 years from his diagnosis. After that, he would undergo annual skin exams.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Ribero S, Moscarella E, Ferrara G, et al. Regression in cutaneous melanoma: a comprehensive review from diagnosis to prognosis. J Eur Acad Dermatol Venereol. 2016;30:2030-2037. doi: 10.1111/jdv.13815
Ribero S, Moscarella E, Ferrara G, et al. Regression in cutaneous melanoma: a comprehensive review from diagnosis to prognosis. J Eur Acad Dermatol Venereol. 2016;30:2030-2037. doi: 10.1111/jdv.13815
Botulinum Toxin for the Treatment of Intractable Raynaud Phenomenon
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
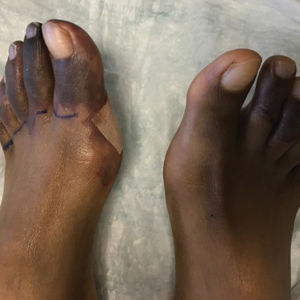
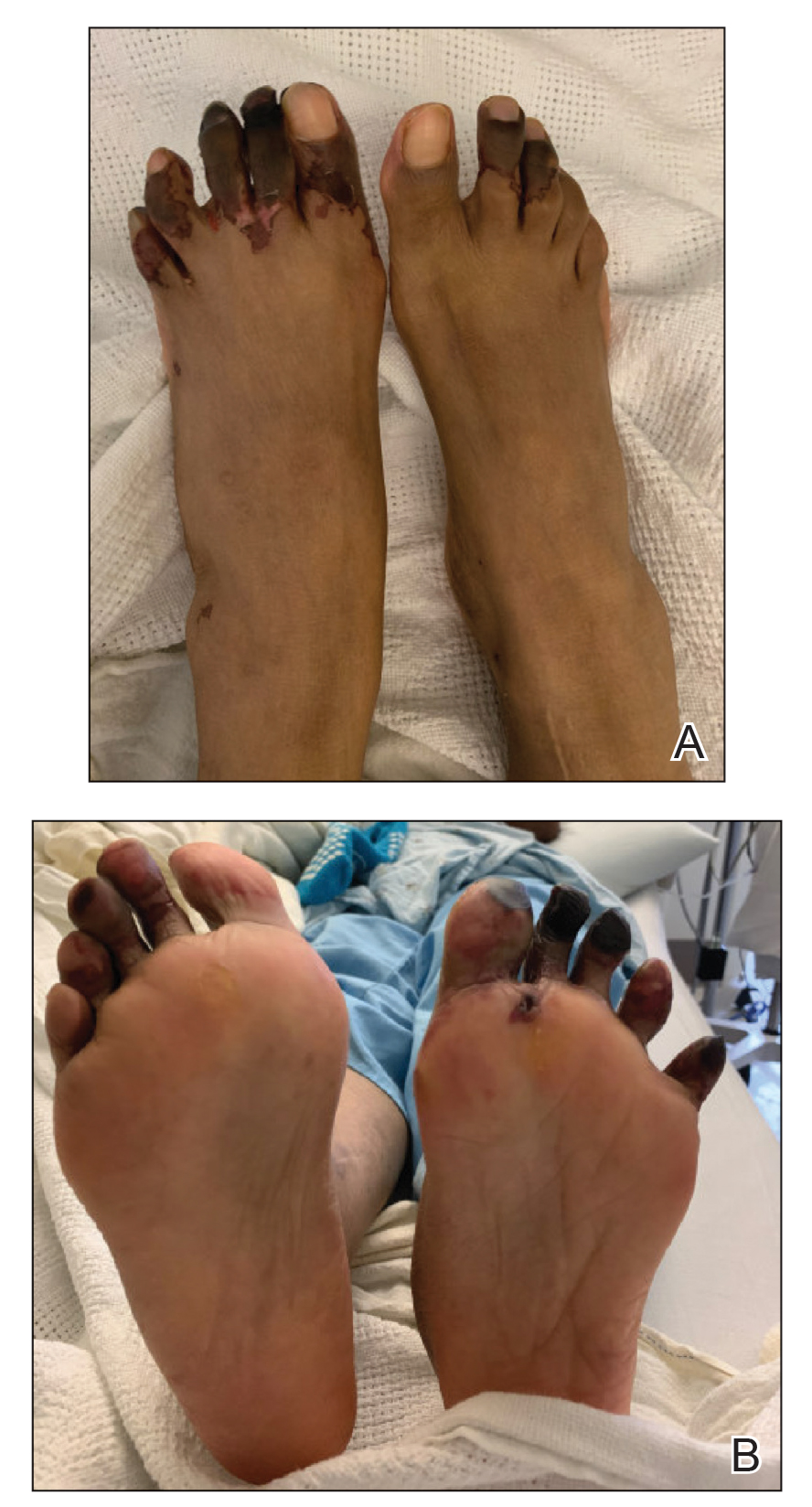
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.
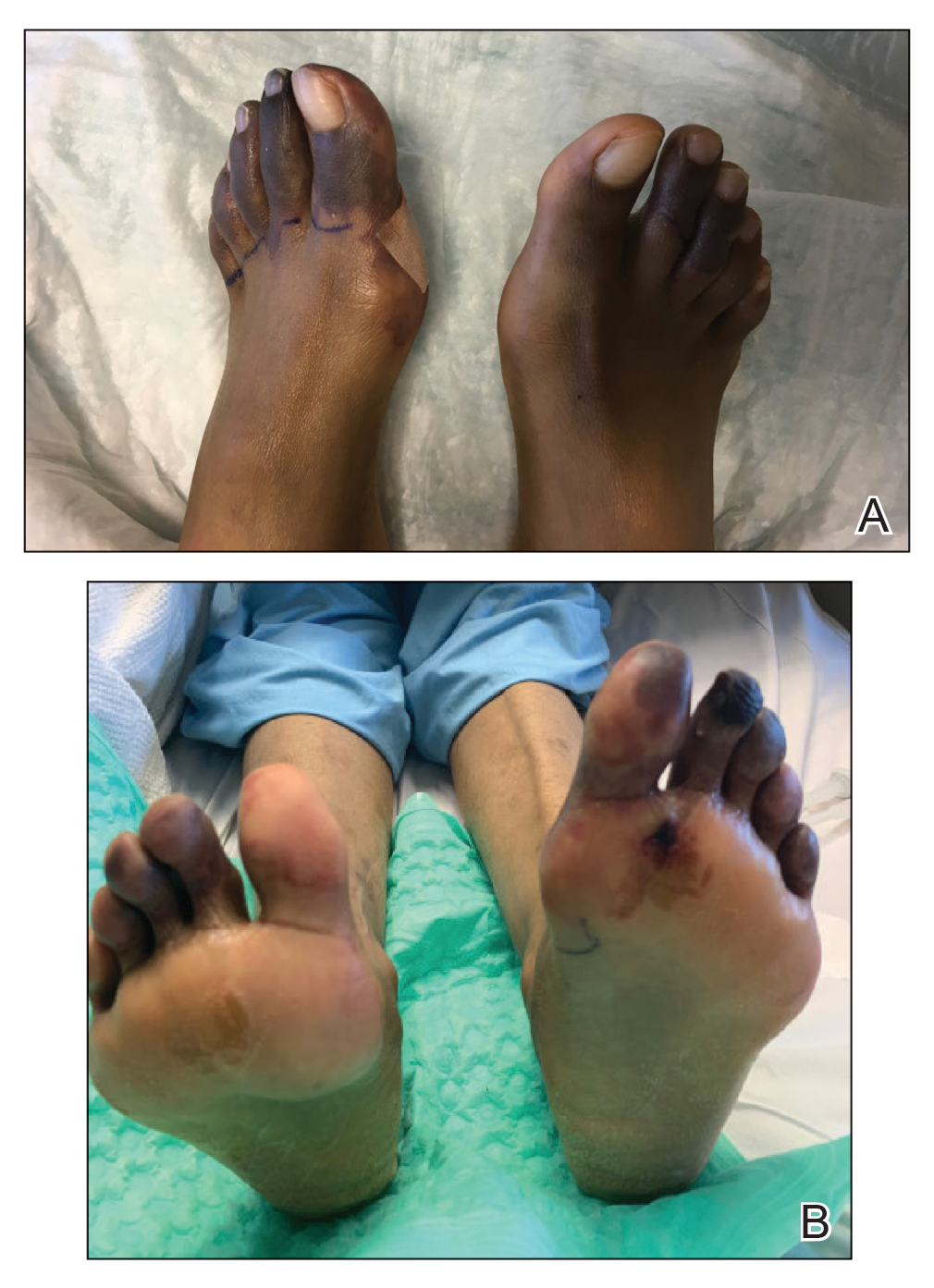
Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).
One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.
Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.

Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).

One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.

Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.

Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).

One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.

Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
Practice Points
- Raynaud phenomenon (RP) is a vascular disorder characterized by episodic vasospasms of the digits often due to cold temperature or stress.
- OnabotulinumtoxinA has been implemented as a treatment of intractable RP after failure with traditional treatments, such as calcium channel blockers, angiotensin receptor blockers, prostaglandins, endothelin receptor blockers, and phosphodiesterase 5 inhibitors.
- A standard technique of delivery of onabotulinumtoxinA involves injection of 5 U/mL into the medial and lateral aspects of each finger at its base (near the metacarpal head) for a total of 50 U per hand or foot.
New finasteride lawsuit brings renewed attention to psychiatric, ED adverse event reports
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
COVID-detecting dogs pilot first airport program
If she identifies a specific scent, she’ll let her handler know simply by sitting down. When this good girl sits, that means Cobra has detected an olfactory signal of the coronavirus, the virus that causes COVID-19.
Cobra, a Belgian Malinois, is one of two canines – her partner is One Betta, a Dutch shepherd – working this checkpoint at Miami International. They are part of a pilot program with the Global Forensic and Justice Center at Florida International University, using the detection dogs as a quick screen for people who have COVID-19.
Their detection rate is high, at more than 98%, and the program has been such a success that it’s being extended for another month at the airport.
If these two dogs continue to accurately detect COVID-19, they and other canines with similar training could be deployed to other places with lots of people coming and going at once, including other airports or even schools. In fact, COVID-sniffing dogs are in use in some university classrooms already.
But building up a big brigade of live animals as disease detectors involves some thorny issues, including where the animals retire once their careers are complete.
“When COVID first arose, we said let’s see if we can train these two dogs on either the virus or the odor of COVID-19,” says Kenneth Furton, PhD, a professor of chemistry and biochemistry, provost, and executive vice president at Florida International University.
His team had completed a study with what he calls “medical detector dogs,” animals that might be able to detect the odor of someone having a seizure. That led them to see how well the animals could detect other kinds of disorders.
Training a dog to sniff out specific odors starts with getting them to understand the task in general. Dr. Furton says that the animals first are trained to grasp that their job is to detect one odor among many. Once the dogs grasp that, they can be trained on just about any specific odor.
In fact, in addition to detecting seizures, dogs reportedly have been able to identify diabetes and even some cancers, such as ovarian cancer.
Dr. Furton says he’s not aware of any previous use of dogs to screen for infectious disease. That may simply be because nothing recently has struck with the global ferocity of COVID, driving humans to turn to their best friends for help.
Cobra and One Betta got their start learning to identify the presence of laurel wilt, a fungus that attacks avocado trees and kills them, costing Florida growers millions. With that expertise under their collars, the two dogs need only a few weeks to get good at detecting other smells assigned to them.
Training the dogs, safely
To train Cobra and One Betta on COVID-19 odors, Dr. Furton’s team first acquired mask samples from people hospitalized with COVID and people who did not have the disease. In battling the viruses, people produce certain chemicals that they exhale every time they breathe. When Dr. Furton and his colleagues compared the exhaled components trapped in the masks, they found differences between masks from people with COVID and those without.
Having confirmed that exhalations can be COVID-specific, the research team trained four dogs – Cobra, One Betta, Hubble, and Max – to detect masks from people with COVID among an assortment of mask choices. Before this step, though, the researchers made sure that any trace of active virus was destroyed by ultraviolet light so that the dogs would not be infected.
Each time the dogs accurately selected a mask from a COVID patient, their reward was access to a favorite toy: A red ball to chew on. Although all four dogs performed very well, yes, they did, Cobra and One Betta showed the most accuracy, outperforming their training colleagues. From their training scores, Cobra ranked first, with 99.45% accuracy. Despite her name, says Dr. Furton, One Betta was “not one better,” coming in second at 98.1%, which is still quite high.
Both dogs are good at their airport screening duties. If one of them sits after sniffing a mask at the checkpoint, the next step is for the mask owner to be tested.
From Aug. 23 to Sept. 8, the two canines screened 1,093 people during 8 working days, alerting on only one case, according to Greg Chin, communications director for the Miami-Dade Aviation Department. That person had tested positive for COVID 2 weeks earlier and was returning to work after quarantine, and their rapid test after the dog alerted was negative.
Dr. Furton says that there are some reports of dogs also alerting before tests can show a positive result, suggesting the dogs’ odor detection can be more precise. They hope to expand their study to see how tight the window of dog-based detection is.
For now, the detector dogs are doing so well that the program has been extended for 30 more days, Mr. Chin says.
As promising as this seems, using dogs for screening carries some logistical and ethical tangles. Training a canine army to deploy for high-volume detection points means that once the work is done, a whole lot of dogs will need a safe place to retire. In addition, the initial training takes several months, says Dr. Furton, whereas if a device were developed for screening, manufacturing could likely be ramped up quickly to meet demand.
The dogs might not need to retire right away, though.
“We envision that they could be redeployed to another type of detection for another infectious disease” if the need arises, Dr. Furton says. But in the end, when working with dogs, he says, there is “a moral connection that you don’t have to deal with using instruments.”
Although the pilot screening at Miami International is the first airport test, the dogs have also done this work in other venues, including at a state emergency operations center in Florida and in some university classrooms, says Dr. Furton.
A version of this article first appeared on WebMD.com.
If she identifies a specific scent, she’ll let her handler know simply by sitting down. When this good girl sits, that means Cobra has detected an olfactory signal of the coronavirus, the virus that causes COVID-19.
Cobra, a Belgian Malinois, is one of two canines – her partner is One Betta, a Dutch shepherd – working this checkpoint at Miami International. They are part of a pilot program with the Global Forensic and Justice Center at Florida International University, using the detection dogs as a quick screen for people who have COVID-19.
Their detection rate is high, at more than 98%, and the program has been such a success that it’s being extended for another month at the airport.
If these two dogs continue to accurately detect COVID-19, they and other canines with similar training could be deployed to other places with lots of people coming and going at once, including other airports or even schools. In fact, COVID-sniffing dogs are in use in some university classrooms already.
But building up a big brigade of live animals as disease detectors involves some thorny issues, including where the animals retire once their careers are complete.
“When COVID first arose, we said let’s see if we can train these two dogs on either the virus or the odor of COVID-19,” says Kenneth Furton, PhD, a professor of chemistry and biochemistry, provost, and executive vice president at Florida International University.
His team had completed a study with what he calls “medical detector dogs,” animals that might be able to detect the odor of someone having a seizure. That led them to see how well the animals could detect other kinds of disorders.
Training a dog to sniff out specific odors starts with getting them to understand the task in general. Dr. Furton says that the animals first are trained to grasp that their job is to detect one odor among many. Once the dogs grasp that, they can be trained on just about any specific odor.
In fact, in addition to detecting seizures, dogs reportedly have been able to identify diabetes and even some cancers, such as ovarian cancer.
Dr. Furton says he’s not aware of any previous use of dogs to screen for infectious disease. That may simply be because nothing recently has struck with the global ferocity of COVID, driving humans to turn to their best friends for help.
Cobra and One Betta got their start learning to identify the presence of laurel wilt, a fungus that attacks avocado trees and kills them, costing Florida growers millions. With that expertise under their collars, the two dogs need only a few weeks to get good at detecting other smells assigned to them.
Training the dogs, safely
To train Cobra and One Betta on COVID-19 odors, Dr. Furton’s team first acquired mask samples from people hospitalized with COVID and people who did not have the disease. In battling the viruses, people produce certain chemicals that they exhale every time they breathe. When Dr. Furton and his colleagues compared the exhaled components trapped in the masks, they found differences between masks from people with COVID and those without.
Having confirmed that exhalations can be COVID-specific, the research team trained four dogs – Cobra, One Betta, Hubble, and Max – to detect masks from people with COVID among an assortment of mask choices. Before this step, though, the researchers made sure that any trace of active virus was destroyed by ultraviolet light so that the dogs would not be infected.
Each time the dogs accurately selected a mask from a COVID patient, their reward was access to a favorite toy: A red ball to chew on. Although all four dogs performed very well, yes, they did, Cobra and One Betta showed the most accuracy, outperforming their training colleagues. From their training scores, Cobra ranked first, with 99.45% accuracy. Despite her name, says Dr. Furton, One Betta was “not one better,” coming in second at 98.1%, which is still quite high.
Both dogs are good at their airport screening duties. If one of them sits after sniffing a mask at the checkpoint, the next step is for the mask owner to be tested.
From Aug. 23 to Sept. 8, the two canines screened 1,093 people during 8 working days, alerting on only one case, according to Greg Chin, communications director for the Miami-Dade Aviation Department. That person had tested positive for COVID 2 weeks earlier and was returning to work after quarantine, and their rapid test after the dog alerted was negative.
Dr. Furton says that there are some reports of dogs also alerting before tests can show a positive result, suggesting the dogs’ odor detection can be more precise. They hope to expand their study to see how tight the window of dog-based detection is.
For now, the detector dogs are doing so well that the program has been extended for 30 more days, Mr. Chin says.
As promising as this seems, using dogs for screening carries some logistical and ethical tangles. Training a canine army to deploy for high-volume detection points means that once the work is done, a whole lot of dogs will need a safe place to retire. In addition, the initial training takes several months, says Dr. Furton, whereas if a device were developed for screening, manufacturing could likely be ramped up quickly to meet demand.
The dogs might not need to retire right away, though.
“We envision that they could be redeployed to another type of detection for another infectious disease” if the need arises, Dr. Furton says. But in the end, when working with dogs, he says, there is “a moral connection that you don’t have to deal with using instruments.”
Although the pilot screening at Miami International is the first airport test, the dogs have also done this work in other venues, including at a state emergency operations center in Florida and in some university classrooms, says Dr. Furton.
A version of this article first appeared on WebMD.com.
If she identifies a specific scent, she’ll let her handler know simply by sitting down. When this good girl sits, that means Cobra has detected an olfactory signal of the coronavirus, the virus that causes COVID-19.
Cobra, a Belgian Malinois, is one of two canines – her partner is One Betta, a Dutch shepherd – working this checkpoint at Miami International. They are part of a pilot program with the Global Forensic and Justice Center at Florida International University, using the detection dogs as a quick screen for people who have COVID-19.
Their detection rate is high, at more than 98%, and the program has been such a success that it’s being extended for another month at the airport.
If these two dogs continue to accurately detect COVID-19, they and other canines with similar training could be deployed to other places with lots of people coming and going at once, including other airports or even schools. In fact, COVID-sniffing dogs are in use in some university classrooms already.
But building up a big brigade of live animals as disease detectors involves some thorny issues, including where the animals retire once their careers are complete.
“When COVID first arose, we said let’s see if we can train these two dogs on either the virus or the odor of COVID-19,” says Kenneth Furton, PhD, a professor of chemistry and biochemistry, provost, and executive vice president at Florida International University.
His team had completed a study with what he calls “medical detector dogs,” animals that might be able to detect the odor of someone having a seizure. That led them to see how well the animals could detect other kinds of disorders.
Training a dog to sniff out specific odors starts with getting them to understand the task in general. Dr. Furton says that the animals first are trained to grasp that their job is to detect one odor among many. Once the dogs grasp that, they can be trained on just about any specific odor.
In fact, in addition to detecting seizures, dogs reportedly have been able to identify diabetes and even some cancers, such as ovarian cancer.
Dr. Furton says he’s not aware of any previous use of dogs to screen for infectious disease. That may simply be because nothing recently has struck with the global ferocity of COVID, driving humans to turn to their best friends for help.
Cobra and One Betta got their start learning to identify the presence of laurel wilt, a fungus that attacks avocado trees and kills them, costing Florida growers millions. With that expertise under their collars, the two dogs need only a few weeks to get good at detecting other smells assigned to them.
Training the dogs, safely
To train Cobra and One Betta on COVID-19 odors, Dr. Furton’s team first acquired mask samples from people hospitalized with COVID and people who did not have the disease. In battling the viruses, people produce certain chemicals that they exhale every time they breathe. When Dr. Furton and his colleagues compared the exhaled components trapped in the masks, they found differences between masks from people with COVID and those without.
Having confirmed that exhalations can be COVID-specific, the research team trained four dogs – Cobra, One Betta, Hubble, and Max – to detect masks from people with COVID among an assortment of mask choices. Before this step, though, the researchers made sure that any trace of active virus was destroyed by ultraviolet light so that the dogs would not be infected.
Each time the dogs accurately selected a mask from a COVID patient, their reward was access to a favorite toy: A red ball to chew on. Although all four dogs performed very well, yes, they did, Cobra and One Betta showed the most accuracy, outperforming their training colleagues. From their training scores, Cobra ranked first, with 99.45% accuracy. Despite her name, says Dr. Furton, One Betta was “not one better,” coming in second at 98.1%, which is still quite high.
Both dogs are good at their airport screening duties. If one of them sits after sniffing a mask at the checkpoint, the next step is for the mask owner to be tested.
From Aug. 23 to Sept. 8, the two canines screened 1,093 people during 8 working days, alerting on only one case, according to Greg Chin, communications director for the Miami-Dade Aviation Department. That person had tested positive for COVID 2 weeks earlier and was returning to work after quarantine, and their rapid test after the dog alerted was negative.
Dr. Furton says that there are some reports of dogs also alerting before tests can show a positive result, suggesting the dogs’ odor detection can be more precise. They hope to expand their study to see how tight the window of dog-based detection is.
For now, the detector dogs are doing so well that the program has been extended for 30 more days, Mr. Chin says.
As promising as this seems, using dogs for screening carries some logistical and ethical tangles. Training a canine army to deploy for high-volume detection points means that once the work is done, a whole lot of dogs will need a safe place to retire. In addition, the initial training takes several months, says Dr. Furton, whereas if a device were developed for screening, manufacturing could likely be ramped up quickly to meet demand.
The dogs might not need to retire right away, though.
“We envision that they could be redeployed to another type of detection for another infectious disease” if the need arises, Dr. Furton says. But in the end, when working with dogs, he says, there is “a moral connection that you don’t have to deal with using instruments.”
Although the pilot screening at Miami International is the first airport test, the dogs have also done this work in other venues, including at a state emergency operations center in Florida and in some university classrooms, says Dr. Furton.
A version of this article first appeared on WebMD.com.
Gut health ‘vitally important’ for mental health
Disturbances in gut microbiota are associated with depletion of anti-inflammatory bacteria and proliferation of proinflammatory bacteria, a pattern tied to several major psychiatric disorders including depression, bipolar disorder (BD), schizophrenia, and anxiety, new research shows.
A meta-analysis of 59 studies, encompassing roughly 2,600 patients with psychiatric conditions, showed a decrease in microbial richness in patients with psychiatric conditions versus controls.
In addition, those with depression, anxiety, BD, and psychosis had a similar set of abnormalities in the microbiota, particularly lower levels of Faecalibacterium and Coprococcus – two types of bacteria that have an anti-inflammatory effect in gut – and higher levels of Eggerthella, a bacterium with proinflammatory effects.
“The wealth of evidence we have summarized clearly demonstrates that the gut microbiota is vitally important to the wider mental health of individuals,” lead author Viktoriya Nikolova, MRes, Centre for Affective Disorders, King’s College London, said in an interview.
“While it is still too early to recommend specific interventions, it’s clear that clinicians need to place a greater awareness of gut health when considering the treatment of certain psychiatric disorders,” she said.
The study was published online Sept. 15, 2021, in JAMA Psychiatry.
Reliable biomarkers
“Evidence of gut microbiota perturbations has accumulated for multiple psychiatric disorders, with microbiota signatures proposed as potential biomarkers,” the authors wrote.
However, “while there is a wealth of evidence to suggest that abnormalities within the composition of the gut microbiota are connected to a number of psychiatric disorders, there haven’t been any attempts to evaluate the specificity of this evidence – that is, if these changes are unique to specific disorders or shared across many,” Ms. Nikolova said.
Previous research in individual disorders has identified “patterns that may be promising biomarker targets,” with the potential to “improve diagnostic accuracy, guide treatment, and assist the monitoring of treatment response,” the authors noted.
“We wanted to see if we could reliably establish biomarkers for individual conditions in an effort to further our understanding of the relationship between mental illness and gut microbiota,” said Ms. Nikolova.
The researchers wanted to “evaluate the specificity and reproducibility of gut microbiota alterations and delineate those with potential to become biomarkers.”
They identified 59 studies (64 case-control comparisons; n = 2,643 patients, 2,336 controls). Most (54.2%) were conducted in East Asia, followed by Westernized populations (40.7%) and Africa (1.7%).
These studies evaluated diversity or abundance of gut microbes in adult populations encompassing an array of psychiatric disorders: major depressive disorder (MDD), BD, psychosis and schizophrenia, eating disorders (anorexia nervosa and bulimia nervosa), anxiety, obsessive-compulsive disorder (OCD), PTSD, and ADHD.
Although studies were similar in exclusion criteria, few attempted to minimize dietary changes or control dietary intake. In addition, use of psychiatric medication also “varied substantially.”
The researchers conducted several analyses, with primary outcomes consisting of “community-level measures of gut microbiota composition (alpha and beta diversity) as well as taxonomic findings at the phylum, family, and genus levels (relative abundance).”
Alpha diversity provides a “summary of the microbial community in individual samples,” which “can be compared across groups to evaluate the role of a particular factor (in this case psychiatric diagnosis) on the richness (number of species) and evenness (how well each species is represented) in the sample.”
Beta diversity, on the other hand, “measures interindividual (between samples) diversity that assesses similarity of communities, compared with the other samples analyzed.”
Control samples consisted of participants without the relevant condition.
Biological overlap?
The alpha-diversity meta-analysis encompassed 34 studies (n = 1,519 patients, 1,429 controls). The researchers found significant decreases in microbial richness in patients, compared with controls (observed species standardized mean difference, −0.26; 95% CI, −0.47 to −0.06; Chao1 SMD, −0.5; 95% CI, −0.79 to −0.21). On the other hand, when they examined each diagnosis separately, they found consistent decreases only in bipolar disorder. There was a small, nonsignificant decrease in phylogenetic diversity between groups.
MDD, psychosis, and schizophrenia were the only conditions in which differences in beta diversity were consistently observed.
“These findings suggest there is reliable evidence for differences in the shared phylogenetic structure in MDD and psychosis and schizophrenia compared with controls,” the authors write.
However, “method of measurement and method of patient classification (symptom vs. diagnosis based) may affect findings,” they added.
When they focused on relative abundance, they found “little evidence” of disorder specificity, but rather a “transdiagnostic pattern of microbiota signatures.”
In particular, depleted levels of Faecalibacterium and Coprococcus and enriched levels of Eggerthella were “consistently shared” between MDD, BD, psychosis and schizophrenia, and anxiety, “suggesting these disorders are characterized by a reduction of anti-inflammatory butyrate-producing bacteria, while proinflammatory genera are enriched.”
“The finding that these perturbations do not appear to be disorder-specific suggests that the microbiota is affected in a similar manner by conditions such as depression, anxiety, bipolar disorder, and psychosis,” said Ms. Nikolova.
“We have seen similar findings from previous meta-analyses of inflammatory marker studies and genetic studies, for example, suggesting that there is a biological overlap between these conditions, which we have now also seen in the microbiota.”
The authors highlighted potential confounders, including study region and medication use.
Conditions such as MDD, psychosis, and schizophrenia were “largely investigated in the East,” while anorexia nervosa and OCD were primarily investigated in the West.
Moreover, comparing results from medication-free studies with those in which 80% or more of patients were taking psychiatric medication showed increases in bacterial families Lactobacillaceae, Klebsiella, Streptococcus, and Megasphaera only in medicated groups, and decreases in Dialister.
In light of these confounders, the findings should be considered “preliminary,” the investigators noted.
Greater standardization needed
Commenting on the study, Emeran Mayer, MD, director of the Oppenheimer Center for Neurobiology of Stress and Resilience at the University of California, Los Angeles, said it is “intriguing to speculate that low-grade immune activation due to reduced production of butyrate may be such a generalized factor affecting microbial composition shared similarly in several brain disorders. However, such a mechanism has not been confirmed in mechanistic studies to date.”
In addition, the study “lumps together a large number of studies and heterogeneous patient populations, with and without centrally acting medication, without adequate dietary history, studied in different ethnic populations, studied with highly variable collection and analysis methods, including highly variable sample and study sizes for different diseases, and using only measures of microbial composition but not function,” cautioned Dr. Mayer, who was not involved in the research.
Future studies “with much greater standardization of subject populations and clinical and biological analyses techniques should be performed to reevaluate the results of the current study and confirm or reject the main hypotheses,” asserted Dr. Mayer, who is also the founding director of the UCLA Brain Gut Microbiome Center.
Ms. Nikolova is funded by a Medical Research Council PhD Studentship. Other sources of funding include the National Institute for Health Research Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. Ms. Nikolova has disclosed no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com .
Disturbances in gut microbiota are associated with depletion of anti-inflammatory bacteria and proliferation of proinflammatory bacteria, a pattern tied to several major psychiatric disorders including depression, bipolar disorder (BD), schizophrenia, and anxiety, new research shows.
A meta-analysis of 59 studies, encompassing roughly 2,600 patients with psychiatric conditions, showed a decrease in microbial richness in patients with psychiatric conditions versus controls.
In addition, those with depression, anxiety, BD, and psychosis had a similar set of abnormalities in the microbiota, particularly lower levels of Faecalibacterium and Coprococcus – two types of bacteria that have an anti-inflammatory effect in gut – and higher levels of Eggerthella, a bacterium with proinflammatory effects.
“The wealth of evidence we have summarized clearly demonstrates that the gut microbiota is vitally important to the wider mental health of individuals,” lead author Viktoriya Nikolova, MRes, Centre for Affective Disorders, King’s College London, said in an interview.
“While it is still too early to recommend specific interventions, it’s clear that clinicians need to place a greater awareness of gut health when considering the treatment of certain psychiatric disorders,” she said.
The study was published online Sept. 15, 2021, in JAMA Psychiatry.
Reliable biomarkers
“Evidence of gut microbiota perturbations has accumulated for multiple psychiatric disorders, with microbiota signatures proposed as potential biomarkers,” the authors wrote.
However, “while there is a wealth of evidence to suggest that abnormalities within the composition of the gut microbiota are connected to a number of psychiatric disorders, there haven’t been any attempts to evaluate the specificity of this evidence – that is, if these changes are unique to specific disorders or shared across many,” Ms. Nikolova said.
Previous research in individual disorders has identified “patterns that may be promising biomarker targets,” with the potential to “improve diagnostic accuracy, guide treatment, and assist the monitoring of treatment response,” the authors noted.
“We wanted to see if we could reliably establish biomarkers for individual conditions in an effort to further our understanding of the relationship between mental illness and gut microbiota,” said Ms. Nikolova.
The researchers wanted to “evaluate the specificity and reproducibility of gut microbiota alterations and delineate those with potential to become biomarkers.”
They identified 59 studies (64 case-control comparisons; n = 2,643 patients, 2,336 controls). Most (54.2%) were conducted in East Asia, followed by Westernized populations (40.7%) and Africa (1.7%).
These studies evaluated diversity or abundance of gut microbes in adult populations encompassing an array of psychiatric disorders: major depressive disorder (MDD), BD, psychosis and schizophrenia, eating disorders (anorexia nervosa and bulimia nervosa), anxiety, obsessive-compulsive disorder (OCD), PTSD, and ADHD.
Although studies were similar in exclusion criteria, few attempted to minimize dietary changes or control dietary intake. In addition, use of psychiatric medication also “varied substantially.”
The researchers conducted several analyses, with primary outcomes consisting of “community-level measures of gut microbiota composition (alpha and beta diversity) as well as taxonomic findings at the phylum, family, and genus levels (relative abundance).”
Alpha diversity provides a “summary of the microbial community in individual samples,” which “can be compared across groups to evaluate the role of a particular factor (in this case psychiatric diagnosis) on the richness (number of species) and evenness (how well each species is represented) in the sample.”
Beta diversity, on the other hand, “measures interindividual (between samples) diversity that assesses similarity of communities, compared with the other samples analyzed.”
Control samples consisted of participants without the relevant condition.
Biological overlap?
The alpha-diversity meta-analysis encompassed 34 studies (n = 1,519 patients, 1,429 controls). The researchers found significant decreases in microbial richness in patients, compared with controls (observed species standardized mean difference, −0.26; 95% CI, −0.47 to −0.06; Chao1 SMD, −0.5; 95% CI, −0.79 to −0.21). On the other hand, when they examined each diagnosis separately, they found consistent decreases only in bipolar disorder. There was a small, nonsignificant decrease in phylogenetic diversity between groups.
MDD, psychosis, and schizophrenia were the only conditions in which differences in beta diversity were consistently observed.
“These findings suggest there is reliable evidence for differences in the shared phylogenetic structure in MDD and psychosis and schizophrenia compared with controls,” the authors write.
However, “method of measurement and method of patient classification (symptom vs. diagnosis based) may affect findings,” they added.
When they focused on relative abundance, they found “little evidence” of disorder specificity, but rather a “transdiagnostic pattern of microbiota signatures.”
In particular, depleted levels of Faecalibacterium and Coprococcus and enriched levels of Eggerthella were “consistently shared” between MDD, BD, psychosis and schizophrenia, and anxiety, “suggesting these disorders are characterized by a reduction of anti-inflammatory butyrate-producing bacteria, while proinflammatory genera are enriched.”
“The finding that these perturbations do not appear to be disorder-specific suggests that the microbiota is affected in a similar manner by conditions such as depression, anxiety, bipolar disorder, and psychosis,” said Ms. Nikolova.
“We have seen similar findings from previous meta-analyses of inflammatory marker studies and genetic studies, for example, suggesting that there is a biological overlap between these conditions, which we have now also seen in the microbiota.”
The authors highlighted potential confounders, including study region and medication use.
Conditions such as MDD, psychosis, and schizophrenia were “largely investigated in the East,” while anorexia nervosa and OCD were primarily investigated in the West.
Moreover, comparing results from medication-free studies with those in which 80% or more of patients were taking psychiatric medication showed increases in bacterial families Lactobacillaceae, Klebsiella, Streptococcus, and Megasphaera only in medicated groups, and decreases in Dialister.
In light of these confounders, the findings should be considered “preliminary,” the investigators noted.
Greater standardization needed
Commenting on the study, Emeran Mayer, MD, director of the Oppenheimer Center for Neurobiology of Stress and Resilience at the University of California, Los Angeles, said it is “intriguing to speculate that low-grade immune activation due to reduced production of butyrate may be such a generalized factor affecting microbial composition shared similarly in several brain disorders. However, such a mechanism has not been confirmed in mechanistic studies to date.”
In addition, the study “lumps together a large number of studies and heterogeneous patient populations, with and without centrally acting medication, without adequate dietary history, studied in different ethnic populations, studied with highly variable collection and analysis methods, including highly variable sample and study sizes for different diseases, and using only measures of microbial composition but not function,” cautioned Dr. Mayer, who was not involved in the research.
Future studies “with much greater standardization of subject populations and clinical and biological analyses techniques should be performed to reevaluate the results of the current study and confirm or reject the main hypotheses,” asserted Dr. Mayer, who is also the founding director of the UCLA Brain Gut Microbiome Center.
Ms. Nikolova is funded by a Medical Research Council PhD Studentship. Other sources of funding include the National Institute for Health Research Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. Ms. Nikolova has disclosed no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com .
Disturbances in gut microbiota are associated with depletion of anti-inflammatory bacteria and proliferation of proinflammatory bacteria, a pattern tied to several major psychiatric disorders including depression, bipolar disorder (BD), schizophrenia, and anxiety, new research shows.
A meta-analysis of 59 studies, encompassing roughly 2,600 patients with psychiatric conditions, showed a decrease in microbial richness in patients with psychiatric conditions versus controls.
In addition, those with depression, anxiety, BD, and psychosis had a similar set of abnormalities in the microbiota, particularly lower levels of Faecalibacterium and Coprococcus – two types of bacteria that have an anti-inflammatory effect in gut – and higher levels of Eggerthella, a bacterium with proinflammatory effects.
“The wealth of evidence we have summarized clearly demonstrates that the gut microbiota is vitally important to the wider mental health of individuals,” lead author Viktoriya Nikolova, MRes, Centre for Affective Disorders, King’s College London, said in an interview.
“While it is still too early to recommend specific interventions, it’s clear that clinicians need to place a greater awareness of gut health when considering the treatment of certain psychiatric disorders,” she said.
The study was published online Sept. 15, 2021, in JAMA Psychiatry.
Reliable biomarkers
“Evidence of gut microbiota perturbations has accumulated for multiple psychiatric disorders, with microbiota signatures proposed as potential biomarkers,” the authors wrote.
However, “while there is a wealth of evidence to suggest that abnormalities within the composition of the gut microbiota are connected to a number of psychiatric disorders, there haven’t been any attempts to evaluate the specificity of this evidence – that is, if these changes are unique to specific disorders or shared across many,” Ms. Nikolova said.
Previous research in individual disorders has identified “patterns that may be promising biomarker targets,” with the potential to “improve diagnostic accuracy, guide treatment, and assist the monitoring of treatment response,” the authors noted.
“We wanted to see if we could reliably establish biomarkers for individual conditions in an effort to further our understanding of the relationship between mental illness and gut microbiota,” said Ms. Nikolova.
The researchers wanted to “evaluate the specificity and reproducibility of gut microbiota alterations and delineate those with potential to become biomarkers.”
They identified 59 studies (64 case-control comparisons; n = 2,643 patients, 2,336 controls). Most (54.2%) were conducted in East Asia, followed by Westernized populations (40.7%) and Africa (1.7%).
These studies evaluated diversity or abundance of gut microbes in adult populations encompassing an array of psychiatric disorders: major depressive disorder (MDD), BD, psychosis and schizophrenia, eating disorders (anorexia nervosa and bulimia nervosa), anxiety, obsessive-compulsive disorder (OCD), PTSD, and ADHD.
Although studies were similar in exclusion criteria, few attempted to minimize dietary changes or control dietary intake. In addition, use of psychiatric medication also “varied substantially.”
The researchers conducted several analyses, with primary outcomes consisting of “community-level measures of gut microbiota composition (alpha and beta diversity) as well as taxonomic findings at the phylum, family, and genus levels (relative abundance).”
Alpha diversity provides a “summary of the microbial community in individual samples,” which “can be compared across groups to evaluate the role of a particular factor (in this case psychiatric diagnosis) on the richness (number of species) and evenness (how well each species is represented) in the sample.”
Beta diversity, on the other hand, “measures interindividual (between samples) diversity that assesses similarity of communities, compared with the other samples analyzed.”
Control samples consisted of participants without the relevant condition.
Biological overlap?
The alpha-diversity meta-analysis encompassed 34 studies (n = 1,519 patients, 1,429 controls). The researchers found significant decreases in microbial richness in patients, compared with controls (observed species standardized mean difference, −0.26; 95% CI, −0.47 to −0.06; Chao1 SMD, −0.5; 95% CI, −0.79 to −0.21). On the other hand, when they examined each diagnosis separately, they found consistent decreases only in bipolar disorder. There was a small, nonsignificant decrease in phylogenetic diversity between groups.
MDD, psychosis, and schizophrenia were the only conditions in which differences in beta diversity were consistently observed.
“These findings suggest there is reliable evidence for differences in the shared phylogenetic structure in MDD and psychosis and schizophrenia compared with controls,” the authors write.
However, “method of measurement and method of patient classification (symptom vs. diagnosis based) may affect findings,” they added.
When they focused on relative abundance, they found “little evidence” of disorder specificity, but rather a “transdiagnostic pattern of microbiota signatures.”
In particular, depleted levels of Faecalibacterium and Coprococcus and enriched levels of Eggerthella were “consistently shared” between MDD, BD, psychosis and schizophrenia, and anxiety, “suggesting these disorders are characterized by a reduction of anti-inflammatory butyrate-producing bacteria, while proinflammatory genera are enriched.”
“The finding that these perturbations do not appear to be disorder-specific suggests that the microbiota is affected in a similar manner by conditions such as depression, anxiety, bipolar disorder, and psychosis,” said Ms. Nikolova.
“We have seen similar findings from previous meta-analyses of inflammatory marker studies and genetic studies, for example, suggesting that there is a biological overlap between these conditions, which we have now also seen in the microbiota.”
The authors highlighted potential confounders, including study region and medication use.
Conditions such as MDD, psychosis, and schizophrenia were “largely investigated in the East,” while anorexia nervosa and OCD were primarily investigated in the West.
Moreover, comparing results from medication-free studies with those in which 80% or more of patients were taking psychiatric medication showed increases in bacterial families Lactobacillaceae, Klebsiella, Streptococcus, and Megasphaera only in medicated groups, and decreases in Dialister.
In light of these confounders, the findings should be considered “preliminary,” the investigators noted.
Greater standardization needed
Commenting on the study, Emeran Mayer, MD, director of the Oppenheimer Center for Neurobiology of Stress and Resilience at the University of California, Los Angeles, said it is “intriguing to speculate that low-grade immune activation due to reduced production of butyrate may be such a generalized factor affecting microbial composition shared similarly in several brain disorders. However, such a mechanism has not been confirmed in mechanistic studies to date.”
In addition, the study “lumps together a large number of studies and heterogeneous patient populations, with and without centrally acting medication, without adequate dietary history, studied in different ethnic populations, studied with highly variable collection and analysis methods, including highly variable sample and study sizes for different diseases, and using only measures of microbial composition but not function,” cautioned Dr. Mayer, who was not involved in the research.
Future studies “with much greater standardization of subject populations and clinical and biological analyses techniques should be performed to reevaluate the results of the current study and confirm or reject the main hypotheses,” asserted Dr. Mayer, who is also the founding director of the UCLA Brain Gut Microbiome Center.
Ms. Nikolova is funded by a Medical Research Council PhD Studentship. Other sources of funding include the National Institute for Health Research Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. Ms. Nikolova has disclosed no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com .
Surge in new-onset tics in adults tied to COVID-19 stress
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Will ‘Dr. Disinformation’ ever face the music?
On Sept. 5, Rashid Buttar, DO, posted on Twitter that COVID-19 “was a planned operation” and shared an article alleging that most people who got the COVID vaccine would be dead by 2025.
Others include testimony in June by Sherri Jane Tenpenny, DO, before Ohio state legislators that the vaccine could cause people to become magnetized. Clips from the hearing went viral on the Internet. On April 9, 2020, Joseph Mercola, DO, posted a video titled “Could hydrogen peroxide treat coronavirus?” which was shared more than 4,600 times. In the video, Dr. Mercola said inhaling hydrogen peroxide through a nebulizer could prevent or cure COVID.
These physicians are identified as members of the “Disinformation Dozen,” a group of top superspreaders of COVID vaccine misinformation on social media, according to a 2021 report by the nonprofit Center for Countering Digital Hate. The report, based on an analysis of antivaccine content on social media platforms, found that 12 people were responsible for 65% of it. The group is composed of physicians, antivaccine activists, and people known for promoting alternative medicine.
The physician voices are of particular concern because their medical credentials lend credence to their unproven, often dangerous pronouncements. All three continue to hold medical licenses and have not faced consequences for their COVID-related statements.
But leaders of professional medical organizations increasingly are calling for that to change and urging medical oversight boards to take more aggressive action.
In July, the Federation of State Medical Boards, the national umbrella organization for the state-based boards, issued a statement making clear that doctors who generate and spread COVID misinformation could be subject to disciplinary action, including the suspension or revocation of their licenses. The American Board of Family Medicine, American Board of Internal Medicine, and American Board of Pediatrics issued a joint statement Sept. 9 in support of the state boards’ position, warning that “such unethical or unprofessional conduct may prompt their respective board to take action that could put their certification at risk.”
And the superspreaders identified by the center’s report are not alone. KHN identified 20 other doctors who have made false or misleading claims about COVID by combing through published fact checks and other news coverage.
For example, at an Indiana school board meeting in August, Dan Stock, MD, claimed the surge in covid cases this summer was due to “antibody mediated viral enhancement” from people receiving covid vaccines. PolitiFact rated his claim “Pants on Fire” false.
Stella Immanuel, MD, a member of a group America’s Frontline Doctors, which has consistently made false statements about COVID, said in a video that went viral in July 2020 that masks weren’t needed because covid could be cured by hydroxychloroquine. Dr. Immanuel’s website currently promotes a set of vitamins, as well as hydroxychloroquine and ivermectin, as COVID treatments.
Two of the doctors mentioned by name in this article responded to requests for comment. Dr. Mercola offered documents to rebut criticisms of his hydrogen peroxide COVID treatment and took issue with the center’s “Disinformation Dozen” report methodology. Dr. Buttar defended his positions, saying via email that “the science is clear and anyone who contests it, has a suspect agenda at best and/or lacks a moral compass.” He also pointed to data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Recording System, considered inconclusive by many experts.
Since the onset of the COVID pandemic, misinformation has been widespread on social media platforms. And many experts blame it for undermining efforts to curb the coronavirus’s spread. A recent poll showed that more than 50% of Americans who won’t get vaccinated cited conspiracy theories as their reasons – for example, saying the vaccines cause infertility or alter DNA.
Some physicians have gained notoriety by embracing COVID-related fringe ideas, quack treatments and falsehoods via social media, conservative talk shows, and even in person with patients. Whether promoting the use of ivermectin, an antiparasitic drug for animals, or a mix of vitamins to treat COVID, doctors’ words can be especially powerful. Public opinion polls consistently show that Americans have high trust in doctors.
“There is a sense of credibility that comes with being a doctor,” said Rachel Moran, PhD, a researcher who studies COVID misinformation at the University of Washington. “There is also a sense they have access to insider info that we don’t. This is a very confusing time, and it can seem that if anyone knows what I should be doing in this situation, it’s a doctor.”
While COVID is a novel and complicated infectious disease, physicians spreading misinformation generally have no particular expertise in infectious diseases. Scott Atlas, MD, who endorsed former President Donald Trump’s unproven statements about the course of the pandemic, is a radiation oncologist.
Traditionally, the responsibility of policing physicians has fallen to state medical boards. Beyond overseeing the licensing process, these panels investigate complaints about doctors and discipline those who engage in unethical, unprofessional or, in extreme cases, criminal activity. Any member of the public can submit a complaint about a physician.
“The boards are relatively slow and weak and it’s a long, slow process to pull somebody’s license,” said Arthur Caplan, PhD, founding head of the department of medical ethics at New York University. “In many states, they have their hands full with doctors who have committed felonies, doctors who are molesting their patients. Keeping an eye on misinformation is somewhat down on the priority list.”
To date, only two doctors have reportedly faced such sanctions. In Oregon, Steven LaTulippe, MD, had his license suspended in December 2020 for refusing to wear a face mask at his clinic and telling patients that masks were ineffective in curbing the spread of COVID, and even dangerous. Thomas Cowan, MD, a San Francisco physician who posted a YouTube video that went viral in March 2020 stating that 5G networks cause COVID, voluntarily surrendered his medical license to California’s medical board in February 2021.
Humayun Chaudhry, DO, president of the Federation of State Medical Boards, however, said it’s possible some doctors could already be the subject of inquiries and investigations, since these actions are not made public until sanctions are handed down.
KHN reached out to the medical and osteopathic boards of all 50 states and the District of Columbia to see if they had received COVID misinformation complaints. Of the 43 that responded, only a handful shared specifics.
During a 1-week period in August, Kansas’ medical board received six such complaints. In all, the state has received 35 complaints against 20 licensees about spreading covid misinformation on social media and in person. Indiana has received about 30 in the past year. South Carolina said it had about 10 since January. Rhode Island didn’t share the number of complaints but said it has taken disciplinary action against one doctor for spreading misinformation, though it hasn’t moved to suspend his license. (The disciplinary measures include a fine, a reprimand on the doctor’s record and a mandate to complete an ethics course.) Five states said they had received only a couple, and 11 states reported receiving no complaints regarding COVID misinformation.
Confidentiality laws in 13 states prevented those boards from sharing information about complaints.
Social media companies have also been slow to take action. Some doctors’ accounts – specifically those among the Disinformation Dozen – have been suspended, but others are still active and posting misinformation.
Imran Ahmed, CEO of the Center for Countering Digital Hate, said social media platforms often don’t consistently apply their rules against spreading misinformation.
“Even when it’s the same companies, Facebook will sometimes take posts down, but Instagram will not,” Mr. Ahmed said, referring to Facebook’s ownership of Instagram. “It goes to show their piecemeal, ineffective approach to enforcing their own rules.”
A Facebook spokesperson said the company has removed over 3,000 accounts, pages and groups for repeatedly violating COVID and vaccine misinformation policies since the beginning of the pandemic. Dr. Buttar’s Facebook and Instagram pages and Tenpenny’s Facebook page have been removed, while Dr. Mercola’s Facebook posts have been demoted, which means fewer people will see them. Dr. Tenpenny and Dr. Mercola still have Instagram accounts.
Part of the challenge may be that these doctors sometimes present scientific opinions that aren’t mainstream but are viewed as potentially valid by some of their colleagues.
“It can be difficult to prove that what is being said is outside the range of scientific and medical consensus,” said Dr. Caplan. “The doctors who were advising Trump – like Scott Atlas – recommended herd immunity. That was far from the consensus of epidemiologists, but you couldn’t get a board to take his license away because it was a fringe opinion.”
Even if these physicians don’t face consequences, it is likely, experts said, that the public health will.
“Medical misinformation doesn’t just result in people making bad personal and community health choices, but it also divides communities and families, leaving an emotional toll,” said Dr. Moran. “Misinformation narratives have real sticking power and impact people’s ability to make safe health choices.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On Sept. 5, Rashid Buttar, DO, posted on Twitter that COVID-19 “was a planned operation” and shared an article alleging that most people who got the COVID vaccine would be dead by 2025.
Others include testimony in June by Sherri Jane Tenpenny, DO, before Ohio state legislators that the vaccine could cause people to become magnetized. Clips from the hearing went viral on the Internet. On April 9, 2020, Joseph Mercola, DO, posted a video titled “Could hydrogen peroxide treat coronavirus?” which was shared more than 4,600 times. In the video, Dr. Mercola said inhaling hydrogen peroxide through a nebulizer could prevent or cure COVID.
These physicians are identified as members of the “Disinformation Dozen,” a group of top superspreaders of COVID vaccine misinformation on social media, according to a 2021 report by the nonprofit Center for Countering Digital Hate. The report, based on an analysis of antivaccine content on social media platforms, found that 12 people were responsible for 65% of it. The group is composed of physicians, antivaccine activists, and people known for promoting alternative medicine.
The physician voices are of particular concern because their medical credentials lend credence to their unproven, often dangerous pronouncements. All three continue to hold medical licenses and have not faced consequences for their COVID-related statements.
But leaders of professional medical organizations increasingly are calling for that to change and urging medical oversight boards to take more aggressive action.
In July, the Federation of State Medical Boards, the national umbrella organization for the state-based boards, issued a statement making clear that doctors who generate and spread COVID misinformation could be subject to disciplinary action, including the suspension or revocation of their licenses. The American Board of Family Medicine, American Board of Internal Medicine, and American Board of Pediatrics issued a joint statement Sept. 9 in support of the state boards’ position, warning that “such unethical or unprofessional conduct may prompt their respective board to take action that could put their certification at risk.”
And the superspreaders identified by the center’s report are not alone. KHN identified 20 other doctors who have made false or misleading claims about COVID by combing through published fact checks and other news coverage.
For example, at an Indiana school board meeting in August, Dan Stock, MD, claimed the surge in covid cases this summer was due to “antibody mediated viral enhancement” from people receiving covid vaccines. PolitiFact rated his claim “Pants on Fire” false.
Stella Immanuel, MD, a member of a group America’s Frontline Doctors, which has consistently made false statements about COVID, said in a video that went viral in July 2020 that masks weren’t needed because covid could be cured by hydroxychloroquine. Dr. Immanuel’s website currently promotes a set of vitamins, as well as hydroxychloroquine and ivermectin, as COVID treatments.
Two of the doctors mentioned by name in this article responded to requests for comment. Dr. Mercola offered documents to rebut criticisms of his hydrogen peroxide COVID treatment and took issue with the center’s “Disinformation Dozen” report methodology. Dr. Buttar defended his positions, saying via email that “the science is clear and anyone who contests it, has a suspect agenda at best and/or lacks a moral compass.” He also pointed to data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Recording System, considered inconclusive by many experts.
Since the onset of the COVID pandemic, misinformation has been widespread on social media platforms. And many experts blame it for undermining efforts to curb the coronavirus’s spread. A recent poll showed that more than 50% of Americans who won’t get vaccinated cited conspiracy theories as their reasons – for example, saying the vaccines cause infertility or alter DNA.
Some physicians have gained notoriety by embracing COVID-related fringe ideas, quack treatments and falsehoods via social media, conservative talk shows, and even in person with patients. Whether promoting the use of ivermectin, an antiparasitic drug for animals, or a mix of vitamins to treat COVID, doctors’ words can be especially powerful. Public opinion polls consistently show that Americans have high trust in doctors.
“There is a sense of credibility that comes with being a doctor,” said Rachel Moran, PhD, a researcher who studies COVID misinformation at the University of Washington. “There is also a sense they have access to insider info that we don’t. This is a very confusing time, and it can seem that if anyone knows what I should be doing in this situation, it’s a doctor.”
While COVID is a novel and complicated infectious disease, physicians spreading misinformation generally have no particular expertise in infectious diseases. Scott Atlas, MD, who endorsed former President Donald Trump’s unproven statements about the course of the pandemic, is a radiation oncologist.
Traditionally, the responsibility of policing physicians has fallen to state medical boards. Beyond overseeing the licensing process, these panels investigate complaints about doctors and discipline those who engage in unethical, unprofessional or, in extreme cases, criminal activity. Any member of the public can submit a complaint about a physician.
“The boards are relatively slow and weak and it’s a long, slow process to pull somebody’s license,” said Arthur Caplan, PhD, founding head of the department of medical ethics at New York University. “In many states, they have their hands full with doctors who have committed felonies, doctors who are molesting their patients. Keeping an eye on misinformation is somewhat down on the priority list.”
To date, only two doctors have reportedly faced such sanctions. In Oregon, Steven LaTulippe, MD, had his license suspended in December 2020 for refusing to wear a face mask at his clinic and telling patients that masks were ineffective in curbing the spread of COVID, and even dangerous. Thomas Cowan, MD, a San Francisco physician who posted a YouTube video that went viral in March 2020 stating that 5G networks cause COVID, voluntarily surrendered his medical license to California’s medical board in February 2021.
Humayun Chaudhry, DO, president of the Federation of State Medical Boards, however, said it’s possible some doctors could already be the subject of inquiries and investigations, since these actions are not made public until sanctions are handed down.
KHN reached out to the medical and osteopathic boards of all 50 states and the District of Columbia to see if they had received COVID misinformation complaints. Of the 43 that responded, only a handful shared specifics.
During a 1-week period in August, Kansas’ medical board received six such complaints. In all, the state has received 35 complaints against 20 licensees about spreading covid misinformation on social media and in person. Indiana has received about 30 in the past year. South Carolina said it had about 10 since January. Rhode Island didn’t share the number of complaints but said it has taken disciplinary action against one doctor for spreading misinformation, though it hasn’t moved to suspend his license. (The disciplinary measures include a fine, a reprimand on the doctor’s record and a mandate to complete an ethics course.) Five states said they had received only a couple, and 11 states reported receiving no complaints regarding COVID misinformation.
Confidentiality laws in 13 states prevented those boards from sharing information about complaints.
Social media companies have also been slow to take action. Some doctors’ accounts – specifically those among the Disinformation Dozen – have been suspended, but others are still active and posting misinformation.
Imran Ahmed, CEO of the Center for Countering Digital Hate, said social media platforms often don’t consistently apply their rules against spreading misinformation.
“Even when it’s the same companies, Facebook will sometimes take posts down, but Instagram will not,” Mr. Ahmed said, referring to Facebook’s ownership of Instagram. “It goes to show their piecemeal, ineffective approach to enforcing their own rules.”
A Facebook spokesperson said the company has removed over 3,000 accounts, pages and groups for repeatedly violating COVID and vaccine misinformation policies since the beginning of the pandemic. Dr. Buttar’s Facebook and Instagram pages and Tenpenny’s Facebook page have been removed, while Dr. Mercola’s Facebook posts have been demoted, which means fewer people will see them. Dr. Tenpenny and Dr. Mercola still have Instagram accounts.
Part of the challenge may be that these doctors sometimes present scientific opinions that aren’t mainstream but are viewed as potentially valid by some of their colleagues.
“It can be difficult to prove that what is being said is outside the range of scientific and medical consensus,” said Dr. Caplan. “The doctors who were advising Trump – like Scott Atlas – recommended herd immunity. That was far from the consensus of epidemiologists, but you couldn’t get a board to take his license away because it was a fringe opinion.”
Even if these physicians don’t face consequences, it is likely, experts said, that the public health will.
“Medical misinformation doesn’t just result in people making bad personal and community health choices, but it also divides communities and families, leaving an emotional toll,” said Dr. Moran. “Misinformation narratives have real sticking power and impact people’s ability to make safe health choices.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On Sept. 5, Rashid Buttar, DO, posted on Twitter that COVID-19 “was a planned operation” and shared an article alleging that most people who got the COVID vaccine would be dead by 2025.
Others include testimony in June by Sherri Jane Tenpenny, DO, before Ohio state legislators that the vaccine could cause people to become magnetized. Clips from the hearing went viral on the Internet. On April 9, 2020, Joseph Mercola, DO, posted a video titled “Could hydrogen peroxide treat coronavirus?” which was shared more than 4,600 times. In the video, Dr. Mercola said inhaling hydrogen peroxide through a nebulizer could prevent or cure COVID.
These physicians are identified as members of the “Disinformation Dozen,” a group of top superspreaders of COVID vaccine misinformation on social media, according to a 2021 report by the nonprofit Center for Countering Digital Hate. The report, based on an analysis of antivaccine content on social media platforms, found that 12 people were responsible for 65% of it. The group is composed of physicians, antivaccine activists, and people known for promoting alternative medicine.
The physician voices are of particular concern because their medical credentials lend credence to their unproven, often dangerous pronouncements. All three continue to hold medical licenses and have not faced consequences for their COVID-related statements.
But leaders of professional medical organizations increasingly are calling for that to change and urging medical oversight boards to take more aggressive action.
In July, the Federation of State Medical Boards, the national umbrella organization for the state-based boards, issued a statement making clear that doctors who generate and spread COVID misinformation could be subject to disciplinary action, including the suspension or revocation of their licenses. The American Board of Family Medicine, American Board of Internal Medicine, and American Board of Pediatrics issued a joint statement Sept. 9 in support of the state boards’ position, warning that “such unethical or unprofessional conduct may prompt their respective board to take action that could put their certification at risk.”
And the superspreaders identified by the center’s report are not alone. KHN identified 20 other doctors who have made false or misleading claims about COVID by combing through published fact checks and other news coverage.
For example, at an Indiana school board meeting in August, Dan Stock, MD, claimed the surge in covid cases this summer was due to “antibody mediated viral enhancement” from people receiving covid vaccines. PolitiFact rated his claim “Pants on Fire” false.
Stella Immanuel, MD, a member of a group America’s Frontline Doctors, which has consistently made false statements about COVID, said in a video that went viral in July 2020 that masks weren’t needed because covid could be cured by hydroxychloroquine. Dr. Immanuel’s website currently promotes a set of vitamins, as well as hydroxychloroquine and ivermectin, as COVID treatments.
Two of the doctors mentioned by name in this article responded to requests for comment. Dr. Mercola offered documents to rebut criticisms of his hydrogen peroxide COVID treatment and took issue with the center’s “Disinformation Dozen” report methodology. Dr. Buttar defended his positions, saying via email that “the science is clear and anyone who contests it, has a suspect agenda at best and/or lacks a moral compass.” He also pointed to data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Recording System, considered inconclusive by many experts.
Since the onset of the COVID pandemic, misinformation has been widespread on social media platforms. And many experts blame it for undermining efforts to curb the coronavirus’s spread. A recent poll showed that more than 50% of Americans who won’t get vaccinated cited conspiracy theories as their reasons – for example, saying the vaccines cause infertility or alter DNA.
Some physicians have gained notoriety by embracing COVID-related fringe ideas, quack treatments and falsehoods via social media, conservative talk shows, and even in person with patients. Whether promoting the use of ivermectin, an antiparasitic drug for animals, or a mix of vitamins to treat COVID, doctors’ words can be especially powerful. Public opinion polls consistently show that Americans have high trust in doctors.
“There is a sense of credibility that comes with being a doctor,” said Rachel Moran, PhD, a researcher who studies COVID misinformation at the University of Washington. “There is also a sense they have access to insider info that we don’t. This is a very confusing time, and it can seem that if anyone knows what I should be doing in this situation, it’s a doctor.”
While COVID is a novel and complicated infectious disease, physicians spreading misinformation generally have no particular expertise in infectious diseases. Scott Atlas, MD, who endorsed former President Donald Trump’s unproven statements about the course of the pandemic, is a radiation oncologist.
Traditionally, the responsibility of policing physicians has fallen to state medical boards. Beyond overseeing the licensing process, these panels investigate complaints about doctors and discipline those who engage in unethical, unprofessional or, in extreme cases, criminal activity. Any member of the public can submit a complaint about a physician.
“The boards are relatively slow and weak and it’s a long, slow process to pull somebody’s license,” said Arthur Caplan, PhD, founding head of the department of medical ethics at New York University. “In many states, they have their hands full with doctors who have committed felonies, doctors who are molesting their patients. Keeping an eye on misinformation is somewhat down on the priority list.”
To date, only two doctors have reportedly faced such sanctions. In Oregon, Steven LaTulippe, MD, had his license suspended in December 2020 for refusing to wear a face mask at his clinic and telling patients that masks were ineffective in curbing the spread of COVID, and even dangerous. Thomas Cowan, MD, a San Francisco physician who posted a YouTube video that went viral in March 2020 stating that 5G networks cause COVID, voluntarily surrendered his medical license to California’s medical board in February 2021.
Humayun Chaudhry, DO, president of the Federation of State Medical Boards, however, said it’s possible some doctors could already be the subject of inquiries and investigations, since these actions are not made public until sanctions are handed down.
KHN reached out to the medical and osteopathic boards of all 50 states and the District of Columbia to see if they had received COVID misinformation complaints. Of the 43 that responded, only a handful shared specifics.
During a 1-week period in August, Kansas’ medical board received six such complaints. In all, the state has received 35 complaints against 20 licensees about spreading covid misinformation on social media and in person. Indiana has received about 30 in the past year. South Carolina said it had about 10 since January. Rhode Island didn’t share the number of complaints but said it has taken disciplinary action against one doctor for spreading misinformation, though it hasn’t moved to suspend his license. (The disciplinary measures include a fine, a reprimand on the doctor’s record and a mandate to complete an ethics course.) Five states said they had received only a couple, and 11 states reported receiving no complaints regarding COVID misinformation.
Confidentiality laws in 13 states prevented those boards from sharing information about complaints.
Social media companies have also been slow to take action. Some doctors’ accounts – specifically those among the Disinformation Dozen – have been suspended, but others are still active and posting misinformation.
Imran Ahmed, CEO of the Center for Countering Digital Hate, said social media platforms often don’t consistently apply their rules against spreading misinformation.
“Even when it’s the same companies, Facebook will sometimes take posts down, but Instagram will not,” Mr. Ahmed said, referring to Facebook’s ownership of Instagram. “It goes to show their piecemeal, ineffective approach to enforcing their own rules.”
A Facebook spokesperson said the company has removed over 3,000 accounts, pages and groups for repeatedly violating COVID and vaccine misinformation policies since the beginning of the pandemic. Dr. Buttar’s Facebook and Instagram pages and Tenpenny’s Facebook page have been removed, while Dr. Mercola’s Facebook posts have been demoted, which means fewer people will see them. Dr. Tenpenny and Dr. Mercola still have Instagram accounts.
Part of the challenge may be that these doctors sometimes present scientific opinions that aren’t mainstream but are viewed as potentially valid by some of their colleagues.
“It can be difficult to prove that what is being said is outside the range of scientific and medical consensus,” said Dr. Caplan. “The doctors who were advising Trump – like Scott Atlas – recommended herd immunity. That was far from the consensus of epidemiologists, but you couldn’t get a board to take his license away because it was a fringe opinion.”
Even if these physicians don’t face consequences, it is likely, experts said, that the public health will.
“Medical misinformation doesn’t just result in people making bad personal and community health choices, but it also divides communities and families, leaving an emotional toll,” said Dr. Moran. “Misinformation narratives have real sticking power and impact people’s ability to make safe health choices.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Watchful waiting in BCC: Which patients can benefit?
Basal cell carcinomas (BCCs), the most common form of skin cancer, are generally slow-growing tumors that occur in older patients.
Given the low rates of metastasis and mortality associated with BCC, some patients do not require treatment. However, there have been no evidence-based recommendations on who may benefit from a watch-and-wait approach.
.
The investigators found that, for older people with low-grade BCCs and limited life expectancy, the risks associated with surgery – bleeding, infection, and wound dehiscence – appeared to outweigh the advantages. According to the authors, these patients “might not live long enough to benefit from treatment.”
This finding mirrors oncologists’ observations regarding low-risk prostate cancer, for which watchful waiting is now the standard of care.
“At present, however, procedure rates [for patients with BCC] increase with age, and many basal cell carcinomas are treated surgically regardless of a patient’s life expectancy,” Eleni Linos, MD, PhD, professor of dermatology at Stanford (Calif.) University, and Mary-Margaret Chren, MD, chair of dermatology at Vanderbilt University, Nashville, Tenn., write in a viewpoint article published in August in JAMA Internal Medicine.
Considering the current treatment patterns for BCC, patients would “benefit from the existence of an evidence-based standard of care that includes active surveillance,” Mackenzie Wehner, MD, assistant professor at MD Anderson Cancer Center, Houston, Tex., writes in an editorial that accompanies the article in JAMA Dermatology.
Insights from the Dutch study
The article in JAMA Dermatology presents a cohort study conducted at Radboud University Medical Center in Nijmegen, the Netherlands. The study included 89 patients who were managed with watchful waiting. The patients received no treatment for at least 3 months following their diagnoses.
The median age of the patients was 83 years. The patients had a total of 280 BCCs. The median initial diameter of the BCCs was 9.5 mm. Just over half of the patients were men, and about half of the BCCs were in the head and neck region.
The median follow-up was 9 months; the maximum follow-up was 6.5 years. Remarkably, the investigators say, more than half the tumors (53.2%) did not grow, and some even shrank. The majority of patients were asymptomatic at presentation, and fewer than 10% developed new symptoms, such as bleeding and itching, during follow-up.
Among the tumors that did grow, 70% were low-risk superficial/nodular tumors, which only increased in size by an estimated 1.06 mm over a year. Thirty percent were higher-risk micronodular/infiltrative tumors, which grew an estimated 4.46 mm over a 12-month period.
About two-thirds of patients eventually chose to have at least one of their BCCs removed after a median of about 7 months. Only three BCCs (2.8%) needed more extensive surgery – reconstructive surgery, rather than primary closure, for instance – than would have been necessary with an earlier excision.
No deaths from BCC were reported in the study.
The investigators tracked the reasons patients opted for watchful waiting. Many understood that their tumors likely would not cause problems in their remaining years. Others prioritized dealing with more pressing health or family problems. Logistics came into play for some, such as not having reliable transportation for hospital visits.
“In patients with [limited life expectancy] and asymptomatic low-risk tumors, [watchful waiting] should be discussed as a potentially appropriate approach,” the investigators, led by Marieke E. C. van Winden, MD, a dermatology resident at Radboud University, conclude.
For patients who wish to pursue a watchful waiting approach, the Dutch team recommends conducting follow-up visits every 3-6 months to see whether patients wish to continue with watchful waiting and to determine whether the risk-to-benefit ratio has shifted.
These recommendations are in line with criteria Dr. Linos and Dr. Chren propose in their viewpoint article in JAMA Internal Medicine. They characterize low-risk BCCs as asymptomatic, smaller than 1 cm in diameter, and located on the trunk or extremities in immunocompetent patients. They note that details regarding active surveillance for BCCs need to be worked out.
“Active surveillance should be studied as a management option because it is supported by the available evidence, congruent with the care of other low-risk cancers, and in accord with principles of shared decision-making,” Dr. Linos and Dr. Chren write.
No funding source was reported. Dr. Wehner, Dr. van Winden, Dr. Linos, and Dr. Chren have disclosed no relevant financial relationships. Two of Dr. van Winden’s coauthors report ties to several companies, including Sanofi Genzyme, AbbVie, Novartis, and Janssen.
A version of this article first appeared on Medscape.com.
Basal cell carcinomas (BCCs), the most common form of skin cancer, are generally slow-growing tumors that occur in older patients.
Given the low rates of metastasis and mortality associated with BCC, some patients do not require treatment. However, there have been no evidence-based recommendations on who may benefit from a watch-and-wait approach.
.
The investigators found that, for older people with low-grade BCCs and limited life expectancy, the risks associated with surgery – bleeding, infection, and wound dehiscence – appeared to outweigh the advantages. According to the authors, these patients “might not live long enough to benefit from treatment.”
This finding mirrors oncologists’ observations regarding low-risk prostate cancer, for which watchful waiting is now the standard of care.
“At present, however, procedure rates [for patients with BCC] increase with age, and many basal cell carcinomas are treated surgically regardless of a patient’s life expectancy,” Eleni Linos, MD, PhD, professor of dermatology at Stanford (Calif.) University, and Mary-Margaret Chren, MD, chair of dermatology at Vanderbilt University, Nashville, Tenn., write in a viewpoint article published in August in JAMA Internal Medicine.
Considering the current treatment patterns for BCC, patients would “benefit from the existence of an evidence-based standard of care that includes active surveillance,” Mackenzie Wehner, MD, assistant professor at MD Anderson Cancer Center, Houston, Tex., writes in an editorial that accompanies the article in JAMA Dermatology.
Insights from the Dutch study
The article in JAMA Dermatology presents a cohort study conducted at Radboud University Medical Center in Nijmegen, the Netherlands. The study included 89 patients who were managed with watchful waiting. The patients received no treatment for at least 3 months following their diagnoses.
The median age of the patients was 83 years. The patients had a total of 280 BCCs. The median initial diameter of the BCCs was 9.5 mm. Just over half of the patients were men, and about half of the BCCs were in the head and neck region.
The median follow-up was 9 months; the maximum follow-up was 6.5 years. Remarkably, the investigators say, more than half the tumors (53.2%) did not grow, and some even shrank. The majority of patients were asymptomatic at presentation, and fewer than 10% developed new symptoms, such as bleeding and itching, during follow-up.
Among the tumors that did grow, 70% were low-risk superficial/nodular tumors, which only increased in size by an estimated 1.06 mm over a year. Thirty percent were higher-risk micronodular/infiltrative tumors, which grew an estimated 4.46 mm over a 12-month period.
About two-thirds of patients eventually chose to have at least one of their BCCs removed after a median of about 7 months. Only three BCCs (2.8%) needed more extensive surgery – reconstructive surgery, rather than primary closure, for instance – than would have been necessary with an earlier excision.
No deaths from BCC were reported in the study.
The investigators tracked the reasons patients opted for watchful waiting. Many understood that their tumors likely would not cause problems in their remaining years. Others prioritized dealing with more pressing health or family problems. Logistics came into play for some, such as not having reliable transportation for hospital visits.
“In patients with [limited life expectancy] and asymptomatic low-risk tumors, [watchful waiting] should be discussed as a potentially appropriate approach,” the investigators, led by Marieke E. C. van Winden, MD, a dermatology resident at Radboud University, conclude.
For patients who wish to pursue a watchful waiting approach, the Dutch team recommends conducting follow-up visits every 3-6 months to see whether patients wish to continue with watchful waiting and to determine whether the risk-to-benefit ratio has shifted.
These recommendations are in line with criteria Dr. Linos and Dr. Chren propose in their viewpoint article in JAMA Internal Medicine. They characterize low-risk BCCs as asymptomatic, smaller than 1 cm in diameter, and located on the trunk or extremities in immunocompetent patients. They note that details regarding active surveillance for BCCs need to be worked out.
“Active surveillance should be studied as a management option because it is supported by the available evidence, congruent with the care of other low-risk cancers, and in accord with principles of shared decision-making,” Dr. Linos and Dr. Chren write.
No funding source was reported. Dr. Wehner, Dr. van Winden, Dr. Linos, and Dr. Chren have disclosed no relevant financial relationships. Two of Dr. van Winden’s coauthors report ties to several companies, including Sanofi Genzyme, AbbVie, Novartis, and Janssen.
A version of this article first appeared on Medscape.com.
Basal cell carcinomas (BCCs), the most common form of skin cancer, are generally slow-growing tumors that occur in older patients.
Given the low rates of metastasis and mortality associated with BCC, some patients do not require treatment. However, there have been no evidence-based recommendations on who may benefit from a watch-and-wait approach.
.
The investigators found that, for older people with low-grade BCCs and limited life expectancy, the risks associated with surgery – bleeding, infection, and wound dehiscence – appeared to outweigh the advantages. According to the authors, these patients “might not live long enough to benefit from treatment.”
This finding mirrors oncologists’ observations regarding low-risk prostate cancer, for which watchful waiting is now the standard of care.
“At present, however, procedure rates [for patients with BCC] increase with age, and many basal cell carcinomas are treated surgically regardless of a patient’s life expectancy,” Eleni Linos, MD, PhD, professor of dermatology at Stanford (Calif.) University, and Mary-Margaret Chren, MD, chair of dermatology at Vanderbilt University, Nashville, Tenn., write in a viewpoint article published in August in JAMA Internal Medicine.
Considering the current treatment patterns for BCC, patients would “benefit from the existence of an evidence-based standard of care that includes active surveillance,” Mackenzie Wehner, MD, assistant professor at MD Anderson Cancer Center, Houston, Tex., writes in an editorial that accompanies the article in JAMA Dermatology.
Insights from the Dutch study
The article in JAMA Dermatology presents a cohort study conducted at Radboud University Medical Center in Nijmegen, the Netherlands. The study included 89 patients who were managed with watchful waiting. The patients received no treatment for at least 3 months following their diagnoses.
The median age of the patients was 83 years. The patients had a total of 280 BCCs. The median initial diameter of the BCCs was 9.5 mm. Just over half of the patients were men, and about half of the BCCs were in the head and neck region.
The median follow-up was 9 months; the maximum follow-up was 6.5 years. Remarkably, the investigators say, more than half the tumors (53.2%) did not grow, and some even shrank. The majority of patients were asymptomatic at presentation, and fewer than 10% developed new symptoms, such as bleeding and itching, during follow-up.
Among the tumors that did grow, 70% were low-risk superficial/nodular tumors, which only increased in size by an estimated 1.06 mm over a year. Thirty percent were higher-risk micronodular/infiltrative tumors, which grew an estimated 4.46 mm over a 12-month period.
About two-thirds of patients eventually chose to have at least one of their BCCs removed after a median of about 7 months. Only three BCCs (2.8%) needed more extensive surgery – reconstructive surgery, rather than primary closure, for instance – than would have been necessary with an earlier excision.
No deaths from BCC were reported in the study.
The investigators tracked the reasons patients opted for watchful waiting. Many understood that their tumors likely would not cause problems in their remaining years. Others prioritized dealing with more pressing health or family problems. Logistics came into play for some, such as not having reliable transportation for hospital visits.
“In patients with [limited life expectancy] and asymptomatic low-risk tumors, [watchful waiting] should be discussed as a potentially appropriate approach,” the investigators, led by Marieke E. C. van Winden, MD, a dermatology resident at Radboud University, conclude.
For patients who wish to pursue a watchful waiting approach, the Dutch team recommends conducting follow-up visits every 3-6 months to see whether patients wish to continue with watchful waiting and to determine whether the risk-to-benefit ratio has shifted.
These recommendations are in line with criteria Dr. Linos and Dr. Chren propose in their viewpoint article in JAMA Internal Medicine. They characterize low-risk BCCs as asymptomatic, smaller than 1 cm in diameter, and located on the trunk or extremities in immunocompetent patients. They note that details regarding active surveillance for BCCs need to be worked out.
“Active surveillance should be studied as a management option because it is supported by the available evidence, congruent with the care of other low-risk cancers, and in accord with principles of shared decision-making,” Dr. Linos and Dr. Chren write.
No funding source was reported. Dr. Wehner, Dr. van Winden, Dr. Linos, and Dr. Chren have disclosed no relevant financial relationships. Two of Dr. van Winden’s coauthors report ties to several companies, including Sanofi Genzyme, AbbVie, Novartis, and Janssen.
A version of this article first appeared on Medscape.com.
Acne vulgaris
THE COMPARISON
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.
Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (FIGURE C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1 Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6
1. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
2. Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
3. Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
4. Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
5. Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
6. Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.
THE COMPARISON
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.

Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (FIGURE C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1 Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6
THE COMPARISON
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.

Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (FIGURE C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1 Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6
1. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
2. Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
3. Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
4. Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
5. Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
6. Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.
1. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
2. Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
3. Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
4. Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
5. Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
6. Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.