User login
PsA: Golimumab effective under long-term real-life clinical setting
Key clinical point: Golimumab was effective for both musculoskeletal and cutaneous manifestations along with good drug persistence in patients with moderate-to-severe psoriatic arthritis (PsA) and concomitant psoriasis in a long-term real-life clinical setting.
Major finding: Disease activity in PsA score (P < .0001) and psoriasis activity and severity index score (P < .01) improved significantly after 6, 12, 24, 36, and 48 months of treatment. The retention rate of golimumab was 82.8%, 73.4%, 62.0%, and 54.4% at 6, 12, 24, and 48 months, respectively. The major reasons for drug discontinuation were primary/secondary inefficacy.
Study details: Findings are from a retrospective observational study including 105 patients with moderate-to-severe PsA and concomitant psoriasis with high disease activity and elevated prevalence of comorbidities and who started treatment with golimumab.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chimenti MS et al. Clin Rheumatol. 2021 Aug 19. doi: 10.1007/s10067-021-05874-6.
Key clinical point: Golimumab was effective for both musculoskeletal and cutaneous manifestations along with good drug persistence in patients with moderate-to-severe psoriatic arthritis (PsA) and concomitant psoriasis in a long-term real-life clinical setting.
Major finding: Disease activity in PsA score (P < .0001) and psoriasis activity and severity index score (P < .01) improved significantly after 6, 12, 24, 36, and 48 months of treatment. The retention rate of golimumab was 82.8%, 73.4%, 62.0%, and 54.4% at 6, 12, 24, and 48 months, respectively. The major reasons for drug discontinuation were primary/secondary inefficacy.
Study details: Findings are from a retrospective observational study including 105 patients with moderate-to-severe PsA and concomitant psoriasis with high disease activity and elevated prevalence of comorbidities and who started treatment with golimumab.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chimenti MS et al. Clin Rheumatol. 2021 Aug 19. doi: 10.1007/s10067-021-05874-6.
Key clinical point: Golimumab was effective for both musculoskeletal and cutaneous manifestations along with good drug persistence in patients with moderate-to-severe psoriatic arthritis (PsA) and concomitant psoriasis in a long-term real-life clinical setting.
Major finding: Disease activity in PsA score (P < .0001) and psoriasis activity and severity index score (P < .01) improved significantly after 6, 12, 24, 36, and 48 months of treatment. The retention rate of golimumab was 82.8%, 73.4%, 62.0%, and 54.4% at 6, 12, 24, and 48 months, respectively. The major reasons for drug discontinuation were primary/secondary inefficacy.
Study details: Findings are from a retrospective observational study including 105 patients with moderate-to-severe PsA and concomitant psoriasis with high disease activity and elevated prevalence of comorbidities and who started treatment with golimumab.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chimenti MS et al. Clin Rheumatol. 2021 Aug 19. doi: 10.1007/s10067-021-05874-6.
Rates of relevant counseling/education lower at dermatology vs. primary care PsA outpatient visits
Key clinical point: The chances of counseling or education for modifiable lifestyle risk factors were rare during psoriatic arthritis (PsA) or psoriasis outpatient visits, with rates being even lower among dermatologists compared to nondermatologists.
Major finding: Overall, low rates of counseling were observed for any modifiable lifestyle risk factor (11.1%; 95% CI 7.9%-15.3%), tobacco (4.8%; 95% CI 2.8%-8.0%), and obesity (2.8%; 95% CI 1.7%-4.5%). Moreover, counseling rates for any modifiable risk factor were lower for dermatologists compared to nondermatologists visits (0.9% vs. 22.6%; P < .001).
Study details: This study used the National Ambulatory Medical Care Survey (2002-2016) and the National Hospital Ambulatory Medical Care Survey (2002-2011) conducted in the United States to assess the frequency of education/counseling for modifiable risk factors in an estimated 41.8 million psoriasis or PsA outpatient visits.
Disclosures: Dr. Barbieri is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and receives partial support from Pfizer. The authors declared no conflicts of interest.
Source: Taylor MT et al. J Am Acad Dermatol. 2021 Aug 24. doi: 10.1016/j.jaad.2021.08.034.
Key clinical point: The chances of counseling or education for modifiable lifestyle risk factors were rare during psoriatic arthritis (PsA) or psoriasis outpatient visits, with rates being even lower among dermatologists compared to nondermatologists.
Major finding: Overall, low rates of counseling were observed for any modifiable lifestyle risk factor (11.1%; 95% CI 7.9%-15.3%), tobacco (4.8%; 95% CI 2.8%-8.0%), and obesity (2.8%; 95% CI 1.7%-4.5%). Moreover, counseling rates for any modifiable risk factor were lower for dermatologists compared to nondermatologists visits (0.9% vs. 22.6%; P < .001).
Study details: This study used the National Ambulatory Medical Care Survey (2002-2016) and the National Hospital Ambulatory Medical Care Survey (2002-2011) conducted in the United States to assess the frequency of education/counseling for modifiable risk factors in an estimated 41.8 million psoriasis or PsA outpatient visits.
Disclosures: Dr. Barbieri is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and receives partial support from Pfizer. The authors declared no conflicts of interest.
Source: Taylor MT et al. J Am Acad Dermatol. 2021 Aug 24. doi: 10.1016/j.jaad.2021.08.034.
Key clinical point: The chances of counseling or education for modifiable lifestyle risk factors were rare during psoriatic arthritis (PsA) or psoriasis outpatient visits, with rates being even lower among dermatologists compared to nondermatologists.
Major finding: Overall, low rates of counseling were observed for any modifiable lifestyle risk factor (11.1%; 95% CI 7.9%-15.3%), tobacco (4.8%; 95% CI 2.8%-8.0%), and obesity (2.8%; 95% CI 1.7%-4.5%). Moreover, counseling rates for any modifiable risk factor were lower for dermatologists compared to nondermatologists visits (0.9% vs. 22.6%; P < .001).
Study details: This study used the National Ambulatory Medical Care Survey (2002-2016) and the National Hospital Ambulatory Medical Care Survey (2002-2011) conducted in the United States to assess the frequency of education/counseling for modifiable risk factors in an estimated 41.8 million psoriasis or PsA outpatient visits.
Disclosures: Dr. Barbieri is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and receives partial support from Pfizer. The authors declared no conflicts of interest.
Source: Taylor MT et al. J Am Acad Dermatol. 2021 Aug 24. doi: 10.1016/j.jaad.2021.08.034.
Affected body surface area predicts risk of PsA in patients with psoriasis
Key clinical point: Patients with psoriasis with higher vs. lower affected body surface area (BSA) were at an increased risk of developing psoriatic arthritis (PsA).
Major finding: During a mean follow-up of 4.2 years, the incidence of PsA was 5.4 cases per 1,000 person years. Compared with BSA < 3%, BSA > 10% (hazard ratio [HR] 2.01; 95% CI 1.29-3.13) and BSA = 3%-10% (HR 1.44; 95% CI 1.02-2.03) were associated with incident PsA.
Study details: Findings are from a prospective, population-based cohort study including 9,056 patients with at least 1 code for psoriasis (mild to severe) and 90,547 matched general population controls.
Disclosures: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Some authors declared serving as consultant or co-patent holder or receiving grants, honoraria, or payments for medical education from several sources.
Source: Ogdie A et al. Rheumatology. 2021 Sep 11. doi: 10.1093/rheumatology/keab622.
Key clinical point: Patients with psoriasis with higher vs. lower affected body surface area (BSA) were at an increased risk of developing psoriatic arthritis (PsA).
Major finding: During a mean follow-up of 4.2 years, the incidence of PsA was 5.4 cases per 1,000 person years. Compared with BSA < 3%, BSA > 10% (hazard ratio [HR] 2.01; 95% CI 1.29-3.13) and BSA = 3%-10% (HR 1.44; 95% CI 1.02-2.03) were associated with incident PsA.
Study details: Findings are from a prospective, population-based cohort study including 9,056 patients with at least 1 code for psoriasis (mild to severe) and 90,547 matched general population controls.
Disclosures: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Some authors declared serving as consultant or co-patent holder or receiving grants, honoraria, or payments for medical education from several sources.
Source: Ogdie A et al. Rheumatology. 2021 Sep 11. doi: 10.1093/rheumatology/keab622.
Key clinical point: Patients with psoriasis with higher vs. lower affected body surface area (BSA) were at an increased risk of developing psoriatic arthritis (PsA).
Major finding: During a mean follow-up of 4.2 years, the incidence of PsA was 5.4 cases per 1,000 person years. Compared with BSA < 3%, BSA > 10% (hazard ratio [HR] 2.01; 95% CI 1.29-3.13) and BSA = 3%-10% (HR 1.44; 95% CI 1.02-2.03) were associated with incident PsA.
Study details: Findings are from a prospective, population-based cohort study including 9,056 patients with at least 1 code for psoriasis (mild to severe) and 90,547 matched general population controls.
Disclosures: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Some authors declared serving as consultant or co-patent holder or receiving grants, honoraria, or payments for medical education from several sources.
Source: Ogdie A et al. Rheumatology. 2021 Sep 11. doi: 10.1093/rheumatology/keab622.
Risk of developing PsA significantly lower in psoriasis patients treated with biologics
Key clinical point: Treating skin manifestations with biologics significantly reduced the risk of developing psoriatic arthritis (PsA) in patients with psoriasis.
Major finding: The risk of developing PsA was significantly higher in patients who did not receive treatment with biologics vs. the biological treatment group (adjusted hazard ratio 1.39; 95% CI 1.03-1.87).
Study details: Findings are from a retrospective cohort including 1,326 patients with psoriasis without PsA, of which 663 patients received biological treatment and 663 patients did not receive biological treatment.
Disclosures: This study did not report any external funding. Dr. Pavlovsky declared serving as investigator, advisor, consultant, and invited lecturer for various sources.
Source: Rosenthal YS et al. Arthritis Rheumatol. 2021 Aug 23. doi: 10.1002/art.41946.
Key clinical point: Treating skin manifestations with biologics significantly reduced the risk of developing psoriatic arthritis (PsA) in patients with psoriasis.
Major finding: The risk of developing PsA was significantly higher in patients who did not receive treatment with biologics vs. the biological treatment group (adjusted hazard ratio 1.39; 95% CI 1.03-1.87).
Study details: Findings are from a retrospective cohort including 1,326 patients with psoriasis without PsA, of which 663 patients received biological treatment and 663 patients did not receive biological treatment.
Disclosures: This study did not report any external funding. Dr. Pavlovsky declared serving as investigator, advisor, consultant, and invited lecturer for various sources.
Source: Rosenthal YS et al. Arthritis Rheumatol. 2021 Aug 23. doi: 10.1002/art.41946.
Key clinical point: Treating skin manifestations with biologics significantly reduced the risk of developing psoriatic arthritis (PsA) in patients with psoriasis.
Major finding: The risk of developing PsA was significantly higher in patients who did not receive treatment with biologics vs. the biological treatment group (adjusted hazard ratio 1.39; 95% CI 1.03-1.87).
Study details: Findings are from a retrospective cohort including 1,326 patients with psoriasis without PsA, of which 663 patients received biological treatment and 663 patients did not receive biological treatment.
Disclosures: This study did not report any external funding. Dr. Pavlovsky declared serving as investigator, advisor, consultant, and invited lecturer for various sources.
Source: Rosenthal YS et al. Arthritis Rheumatol. 2021 Aug 23. doi: 10.1002/art.41946.
Pelvic floor dysfunction imaging: New guidelines provide recommendations
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA issues proposed order for over-the-counter sunscreens
Federal efforts to improve the quality, safety, and efficacy of over-the-counter sunscreens took a step forward today with the release of two orders aimed at updating regulatory requirements for most sunscreen products in the United States.
“We see it as a key public health priority and our regulatory obligation to make sure that marketed sunscreen products offer protection from the sun’s effects and that they deliver on those promises to consumers,” Theresa Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said during a media briefing.
When the Coronavirus Aid, Relief, and Economic Security (CARES) Act was passed in 2020, the FDA was in the middle of amending a sunscreen monograph through the previous rule-making process, and the agency had issued a proposed rule for sunscreens in February of 2019. The CARES Act provided the FDA with new authority related to OTC drugs including sunscreens.
It also established a deemed final order for sunscreens, which set the current requirements for OTC sunscreen products marketed without an application. The deemed final order, released on Sept. 24, “essentially preserves the pre-CARES Act status quo marketing conditions for these sunscreens,” Dr. Michele explained. “Before the CARES Act was passed, sunscreens were marketed according to nearly identical terms that were described in an FDA enforcement discretion policy. For this reason, the agency believes that most sunscreens on the market today are already in compliance with this order.”
The CARES Act also required the FDA to issue a proposed order by Sept. 27 to amend and revise the deemed final order. Dr. Michele described the proposed order, which was released on Sept. 24, as “a vehicle to effectively transition our ongoing consideration of the appropriate requirements for OTC sunscreens marketed without approved applications from the previous rule-making process to this new order process. The provisions in today’s proposed order are therefore substantively the same as those described in the FDA’s 2019 proposed rule on sunscreens. With this proposed order, we’re proposing new requirements to improve the quality, safety, and efficacy of sunscreens that Americans use every day.”
The order proposes to update the generally recognized as safe (GRASE) status for the 16 active ingredients listed in the deemed final order. It also proposes that dosage forms that are GRASE for use as sunscreens include oils, lotions, creams, gels, butters, pastes, ointments, and sticks, and proposes GRASE status for spray sunscreens, subject to testing and labeling requirements.
Adam Friedman, MD, FAAD, professor and chair of dermatology at George Washington University, Washington, emphasized that photoprotection “is important for everyone, regardless of skin tone,” in an interview. “Broad-spectrum sunscreens with an SPF of 15 and higher play an important role in this. This should not be lost amidst the proposed order.”
Changes between the deemed and proposed order that he highlighted include a maximum SPF of 60+ (though up to 80 might be allowed) and that zinc oxide and titanium dioxide are GRASE. “The FDA did not say that nanoparticle formulations of these, which are easier to use, are not GRASE; they are asking for community input,” he said.
Other changes between the deemed and proposed order are that PABA and trolamine salicylate are not GRASE and that broad-spectrum testing will be mandatory. In addition, Dr. Friedman said, “sprays will be considered for GRASE so long as properly tested, labeling should be clearer (and a warning will be applied to those sunscreens not shown to prevent all the bad stuff with UVR [ultraviolet radiation]), and bug spray–sunscreen combos are a no-go.”
The FDA will consider comments on the proposed order submitted during a 45-day public comment period before issuing a revised final order. “As part of this process, we’ll consider all timely comments submitted both in response to the February 2019 proposed rule and to the current proposed order,” Dr. Michele said.
Dr. Friedman reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is also a speaker for Regeneron, Sanofi Genzyme, Abbvie, LRP, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
Federal efforts to improve the quality, safety, and efficacy of over-the-counter sunscreens took a step forward today with the release of two orders aimed at updating regulatory requirements for most sunscreen products in the United States.
“We see it as a key public health priority and our regulatory obligation to make sure that marketed sunscreen products offer protection from the sun’s effects and that they deliver on those promises to consumers,” Theresa Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said during a media briefing.
When the Coronavirus Aid, Relief, and Economic Security (CARES) Act was passed in 2020, the FDA was in the middle of amending a sunscreen monograph through the previous rule-making process, and the agency had issued a proposed rule for sunscreens in February of 2019. The CARES Act provided the FDA with new authority related to OTC drugs including sunscreens.
It also established a deemed final order for sunscreens, which set the current requirements for OTC sunscreen products marketed without an application. The deemed final order, released on Sept. 24, “essentially preserves the pre-CARES Act status quo marketing conditions for these sunscreens,” Dr. Michele explained. “Before the CARES Act was passed, sunscreens were marketed according to nearly identical terms that were described in an FDA enforcement discretion policy. For this reason, the agency believes that most sunscreens on the market today are already in compliance with this order.”
The CARES Act also required the FDA to issue a proposed order by Sept. 27 to amend and revise the deemed final order. Dr. Michele described the proposed order, which was released on Sept. 24, as “a vehicle to effectively transition our ongoing consideration of the appropriate requirements for OTC sunscreens marketed without approved applications from the previous rule-making process to this new order process. The provisions in today’s proposed order are therefore substantively the same as those described in the FDA’s 2019 proposed rule on sunscreens. With this proposed order, we’re proposing new requirements to improve the quality, safety, and efficacy of sunscreens that Americans use every day.”
The order proposes to update the generally recognized as safe (GRASE) status for the 16 active ingredients listed in the deemed final order. It also proposes that dosage forms that are GRASE for use as sunscreens include oils, lotions, creams, gels, butters, pastes, ointments, and sticks, and proposes GRASE status for spray sunscreens, subject to testing and labeling requirements.
Adam Friedman, MD, FAAD, professor and chair of dermatology at George Washington University, Washington, emphasized that photoprotection “is important for everyone, regardless of skin tone,” in an interview. “Broad-spectrum sunscreens with an SPF of 15 and higher play an important role in this. This should not be lost amidst the proposed order.”
Changes between the deemed and proposed order that he highlighted include a maximum SPF of 60+ (though up to 80 might be allowed) and that zinc oxide and titanium dioxide are GRASE. “The FDA did not say that nanoparticle formulations of these, which are easier to use, are not GRASE; they are asking for community input,” he said.
Other changes between the deemed and proposed order are that PABA and trolamine salicylate are not GRASE and that broad-spectrum testing will be mandatory. In addition, Dr. Friedman said, “sprays will be considered for GRASE so long as properly tested, labeling should be clearer (and a warning will be applied to those sunscreens not shown to prevent all the bad stuff with UVR [ultraviolet radiation]), and bug spray–sunscreen combos are a no-go.”
The FDA will consider comments on the proposed order submitted during a 45-day public comment period before issuing a revised final order. “As part of this process, we’ll consider all timely comments submitted both in response to the February 2019 proposed rule and to the current proposed order,” Dr. Michele said.
Dr. Friedman reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is also a speaker for Regeneron, Sanofi Genzyme, Abbvie, LRP, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
Federal efforts to improve the quality, safety, and efficacy of over-the-counter sunscreens took a step forward today with the release of two orders aimed at updating regulatory requirements for most sunscreen products in the United States.
“We see it as a key public health priority and our regulatory obligation to make sure that marketed sunscreen products offer protection from the sun’s effects and that they deliver on those promises to consumers,” Theresa Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said during a media briefing.
When the Coronavirus Aid, Relief, and Economic Security (CARES) Act was passed in 2020, the FDA was in the middle of amending a sunscreen monograph through the previous rule-making process, and the agency had issued a proposed rule for sunscreens in February of 2019. The CARES Act provided the FDA with new authority related to OTC drugs including sunscreens.
It also established a deemed final order for sunscreens, which set the current requirements for OTC sunscreen products marketed without an application. The deemed final order, released on Sept. 24, “essentially preserves the pre-CARES Act status quo marketing conditions for these sunscreens,” Dr. Michele explained. “Before the CARES Act was passed, sunscreens were marketed according to nearly identical terms that were described in an FDA enforcement discretion policy. For this reason, the agency believes that most sunscreens on the market today are already in compliance with this order.”
The CARES Act also required the FDA to issue a proposed order by Sept. 27 to amend and revise the deemed final order. Dr. Michele described the proposed order, which was released on Sept. 24, as “a vehicle to effectively transition our ongoing consideration of the appropriate requirements for OTC sunscreens marketed without approved applications from the previous rule-making process to this new order process. The provisions in today’s proposed order are therefore substantively the same as those described in the FDA’s 2019 proposed rule on sunscreens. With this proposed order, we’re proposing new requirements to improve the quality, safety, and efficacy of sunscreens that Americans use every day.”
The order proposes to update the generally recognized as safe (GRASE) status for the 16 active ingredients listed in the deemed final order. It also proposes that dosage forms that are GRASE for use as sunscreens include oils, lotions, creams, gels, butters, pastes, ointments, and sticks, and proposes GRASE status for spray sunscreens, subject to testing and labeling requirements.
Adam Friedman, MD, FAAD, professor and chair of dermatology at George Washington University, Washington, emphasized that photoprotection “is important for everyone, regardless of skin tone,” in an interview. “Broad-spectrum sunscreens with an SPF of 15 and higher play an important role in this. This should not be lost amidst the proposed order.”
Changes between the deemed and proposed order that he highlighted include a maximum SPF of 60+ (though up to 80 might be allowed) and that zinc oxide and titanium dioxide are GRASE. “The FDA did not say that nanoparticle formulations of these, which are easier to use, are not GRASE; they are asking for community input,” he said.
Other changes between the deemed and proposed order are that PABA and trolamine salicylate are not GRASE and that broad-spectrum testing will be mandatory. In addition, Dr. Friedman said, “sprays will be considered for GRASE so long as properly tested, labeling should be clearer (and a warning will be applied to those sunscreens not shown to prevent all the bad stuff with UVR [ultraviolet radiation]), and bug spray–sunscreen combos are a no-go.”
The FDA will consider comments on the proposed order submitted during a 45-day public comment period before issuing a revised final order. “As part of this process, we’ll consider all timely comments submitted both in response to the February 2019 proposed rule and to the current proposed order,” Dr. Michele said.
Dr. Friedman reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is also a speaker for Regeneron, Sanofi Genzyme, Abbvie, LRP, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
Doctors preyed on homeless for slip-and-fall schemes; more
a story in the New York Daily News, among other news outlets, indicates.
In the latest iteration of this age-old practice, a team of attorneys and doctors allegedly recruited more than 400 New York City inhabitants, many of whom were homeless or addicted to drugs, over the course of 5 years to participate in their scam.
These people, who were often desperate for money, were coached to claim they’d tripped and fallen over one of the many obstacles pedestrians encounter in the city — sidewalk cracks, potholes, open or protruding cellar doors, and the like. The participant would be cajoled into signing his or her name to a fraudulent suit. Over the years, the scam netted the bosses more than $31 million from city businesses and their insurance companies.
In some cases, to add authenticity to the swindle, the scammers convinced the so-called victims to undergo an operation, promising them up to $1,500 to go under the knife.
Accused by the U.S. Attorney’s Office of masterminding the slip-and-fall operation were two attorneys, George Constantine and Marc Elefant, and a pair of doctors, Andrew Dowd, MD, an orthopedic surgeon, and Sady Ribeiro, MD, a pain management specialist and surgeon.
According to the charges, Mr. Constantine and Mr. Elefant filed lawsuits — either together or separately, though the report isn’t clear — on behalf of hundreds of people who took part in the scheme. Dr. Dowd and Dr. Ribeiro also profited handsomely, according to authorities. Dr. Dowd, they say, earned roughly $9,500 per surgery. Dr. Ribeiro is accused of treating nearly 200 participants during the con and of often paying kickbacks to them to drum up additional referrals.
If convicted on all charges, including mail and wire fraud, each of the defendants could face up to 20 years in prison.
This isn’t the first time such a scheme has been hatched and carried out in New York City.
In May 2020, three men were sentenced to prison for their involvement in a similar slip-and-fall operation that took place between 2013 and 2018. (Their sentencing followed an earlier conviction for conspiracy to commit mail and wire fraud.)
Like the most recent scheme, the one that began in 2013 preyed on the hopeless. “The whole essence of this conspiracy is to find the down-and-out, find the desperate, find the homeless,” the sentencing judge said at the time. “No person who has a job and education and can support his or her family even minimally is going to say, ‘Oh, I’ll undergo unnecessary back surgery for a thousand dollars.’ These people were vulnerable and desperate.”
Sales reps in the OR — helpful or harmful?
Although medical device makers insist that the practice of placing sales reps in operating rooms (ORs) has proven beneficial to surgeons over the years, others claim it has contributed to unwanted outcomes, according to a report published in Kaiser Health News.
In May 2018, for instance, Cristina Martinez underwent what was supposed to be a routine spinal implant procedure. In the course of the operation, her Houston-based spine surgeon discovered that the implant he was about to insert in her back was larger than the one he had intended to use. Under different circumstances, the device maker’s sales representative who was present in the OR that day might have been able to supply the smaller implant. But the rep didn’t have one available, so the surgeon proceeded with the operation using the larger plastic disk.
Four days later, in another procedure, the surgeon removed that disk and replaced it with one that was the correct size. Ms. Martinez has claimed that she awakened from the second procedure in pain, the result of nerve damage that she says led to loss of feeling in her left leg.
In a suit against the doctor, the device maker, and its distributor and sales reps, Ms. Martinez alleges that her injuries were the direct result of their negligence in not having the proper disk available during the initial operation.
The defendants have each denied any wrongdoing.
The accused spine surgeon submitted to the court his operating notes, which reportedly say that he had depended on a company distributor and its sales reps to provide “all lengths available” of the implant. In another filing, he contends that the “small area of leg numbness experienced by Ms. Martinez was a known complication of the first surgery...and was not the result of any alleged negligence.”
The device maker also denies any wrongdoing. In its filings, it claims that sales reps initially ordered a sterile kit that included implants ranging from 50 mm to 55 mm in length. The kit, says the manufacturer, was duly shipped to Houston, but the surgeon replaced the original implant with a 40-mm version during Ms. Martinez’ reoperation.
A trial is scheduled for this November.
But was the Houston incident typical?
Device makers argue that having sales reps in the OR makes sense: Well trained, they offer surgeons vital technical guidance in the use of products that are often complex.
Kaiser Health News says its investigation found otherwise. This practice and others “have been blamed for contributing to serious patient harm in thousands of medical malpractice, product liability, and whistleblower lawsuits filed over the past decade,” it reports.
The list of allegations is long: Some patients say they were injured because sales reps sold or delivered either the wrong-size implant or a defective one. Others say that in their communications with doctors, device makers were shown to have been less than truthful about the safety and durability of their products.
Currently, more than 28,000 suits have been consolidated into six multi-district federal cases. Most involve patients who claim injury after receiving hip implants. Some of the procedures required painful reoperations.
Other court actions cite device makers for other misdeeds, including keeping federal regulators in the dark about potentially dangerous product defects and plying surgeons with millions of dollars in illegal kickbacks.
Device makers have denied these and other allegations. Many of their cases have been settled confidentially.
This high-risk specialty found a way to lower its claim rates
Ob.gyns. who undergo training that simulates team interactions during a high-acuity clinical case face fewer malpractice claims afterward, according to a study published in August in Obstetrics and Gynecology.
Researchers examined the claim rates of 292 ob.gyns. who had undergone one or more such training simulations from 2002 to 2019. To gauge the effect of simulation training on the rate of medical malpractice claims, the researchers compared pretraining rates with posttraining rates. Because participants were insured by the same carrier, the team had access to the relevant claims data as well as the doctors’ durations of coverage.
The investigators assessed claim rates for the period 2002 to 2019 (the full study period), as well as rates for 2-year and 1-year participation.
Researchers found a nonsignificant drop in claim rates for doctors in the 1-year group. The drop was greater for those in the 2-year group; it decreased from 9.2 to 5.4 claims per 100 physician coverage years. (A coverage year is defined as 12 months of indemnity protection.)
For doctors who took part for the entire study period — and were therefore more likely to be among the nearly 20% of doctors who attended three or more training sessions — postsimulation claim rates dropped significantly, from 11.2 to 5.7 per 100 physician coverage years. (Attendance in more than one simulation session correlated with a greater reduction in claim rates.)
“We observed a significant reduction in malpractice claim rates after simulation training,” the researchers concluded. “Wider use of simulation training within obstetrics and gynecology should be considered.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
a story in the New York Daily News, among other news outlets, indicates.
In the latest iteration of this age-old practice, a team of attorneys and doctors allegedly recruited more than 400 New York City inhabitants, many of whom were homeless or addicted to drugs, over the course of 5 years to participate in their scam.
These people, who were often desperate for money, were coached to claim they’d tripped and fallen over one of the many obstacles pedestrians encounter in the city — sidewalk cracks, potholes, open or protruding cellar doors, and the like. The participant would be cajoled into signing his or her name to a fraudulent suit. Over the years, the scam netted the bosses more than $31 million from city businesses and their insurance companies.
In some cases, to add authenticity to the swindle, the scammers convinced the so-called victims to undergo an operation, promising them up to $1,500 to go under the knife.
Accused by the U.S. Attorney’s Office of masterminding the slip-and-fall operation were two attorneys, George Constantine and Marc Elefant, and a pair of doctors, Andrew Dowd, MD, an orthopedic surgeon, and Sady Ribeiro, MD, a pain management specialist and surgeon.
According to the charges, Mr. Constantine and Mr. Elefant filed lawsuits — either together or separately, though the report isn’t clear — on behalf of hundreds of people who took part in the scheme. Dr. Dowd and Dr. Ribeiro also profited handsomely, according to authorities. Dr. Dowd, they say, earned roughly $9,500 per surgery. Dr. Ribeiro is accused of treating nearly 200 participants during the con and of often paying kickbacks to them to drum up additional referrals.
If convicted on all charges, including mail and wire fraud, each of the defendants could face up to 20 years in prison.
This isn’t the first time such a scheme has been hatched and carried out in New York City.
In May 2020, three men were sentenced to prison for their involvement in a similar slip-and-fall operation that took place between 2013 and 2018. (Their sentencing followed an earlier conviction for conspiracy to commit mail and wire fraud.)
Like the most recent scheme, the one that began in 2013 preyed on the hopeless. “The whole essence of this conspiracy is to find the down-and-out, find the desperate, find the homeless,” the sentencing judge said at the time. “No person who has a job and education and can support his or her family even minimally is going to say, ‘Oh, I’ll undergo unnecessary back surgery for a thousand dollars.’ These people were vulnerable and desperate.”
Sales reps in the OR — helpful or harmful?
Although medical device makers insist that the practice of placing sales reps in operating rooms (ORs) has proven beneficial to surgeons over the years, others claim it has contributed to unwanted outcomes, according to a report published in Kaiser Health News.
In May 2018, for instance, Cristina Martinez underwent what was supposed to be a routine spinal implant procedure. In the course of the operation, her Houston-based spine surgeon discovered that the implant he was about to insert in her back was larger than the one he had intended to use. Under different circumstances, the device maker’s sales representative who was present in the OR that day might have been able to supply the smaller implant. But the rep didn’t have one available, so the surgeon proceeded with the operation using the larger plastic disk.
Four days later, in another procedure, the surgeon removed that disk and replaced it with one that was the correct size. Ms. Martinez has claimed that she awakened from the second procedure in pain, the result of nerve damage that she says led to loss of feeling in her left leg.
In a suit against the doctor, the device maker, and its distributor and sales reps, Ms. Martinez alleges that her injuries were the direct result of their negligence in not having the proper disk available during the initial operation.
The defendants have each denied any wrongdoing.
The accused spine surgeon submitted to the court his operating notes, which reportedly say that he had depended on a company distributor and its sales reps to provide “all lengths available” of the implant. In another filing, he contends that the “small area of leg numbness experienced by Ms. Martinez was a known complication of the first surgery...and was not the result of any alleged negligence.”
The device maker also denies any wrongdoing. In its filings, it claims that sales reps initially ordered a sterile kit that included implants ranging from 50 mm to 55 mm in length. The kit, says the manufacturer, was duly shipped to Houston, but the surgeon replaced the original implant with a 40-mm version during Ms. Martinez’ reoperation.
A trial is scheduled for this November.
But was the Houston incident typical?
Device makers argue that having sales reps in the OR makes sense: Well trained, they offer surgeons vital technical guidance in the use of products that are often complex.
Kaiser Health News says its investigation found otherwise. This practice and others “have been blamed for contributing to serious patient harm in thousands of medical malpractice, product liability, and whistleblower lawsuits filed over the past decade,” it reports.
The list of allegations is long: Some patients say they were injured because sales reps sold or delivered either the wrong-size implant or a defective one. Others say that in their communications with doctors, device makers were shown to have been less than truthful about the safety and durability of their products.
Currently, more than 28,000 suits have been consolidated into six multi-district federal cases. Most involve patients who claim injury after receiving hip implants. Some of the procedures required painful reoperations.
Other court actions cite device makers for other misdeeds, including keeping federal regulators in the dark about potentially dangerous product defects and plying surgeons with millions of dollars in illegal kickbacks.
Device makers have denied these and other allegations. Many of their cases have been settled confidentially.
This high-risk specialty found a way to lower its claim rates
Ob.gyns. who undergo training that simulates team interactions during a high-acuity clinical case face fewer malpractice claims afterward, according to a study published in August in Obstetrics and Gynecology.
Researchers examined the claim rates of 292 ob.gyns. who had undergone one or more such training simulations from 2002 to 2019. To gauge the effect of simulation training on the rate of medical malpractice claims, the researchers compared pretraining rates with posttraining rates. Because participants were insured by the same carrier, the team had access to the relevant claims data as well as the doctors’ durations of coverage.
The investigators assessed claim rates for the period 2002 to 2019 (the full study period), as well as rates for 2-year and 1-year participation.
Researchers found a nonsignificant drop in claim rates for doctors in the 1-year group. The drop was greater for those in the 2-year group; it decreased from 9.2 to 5.4 claims per 100 physician coverage years. (A coverage year is defined as 12 months of indemnity protection.)
For doctors who took part for the entire study period — and were therefore more likely to be among the nearly 20% of doctors who attended three or more training sessions — postsimulation claim rates dropped significantly, from 11.2 to 5.7 per 100 physician coverage years. (Attendance in more than one simulation session correlated with a greater reduction in claim rates.)
“We observed a significant reduction in malpractice claim rates after simulation training,” the researchers concluded. “Wider use of simulation training within obstetrics and gynecology should be considered.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
a story in the New York Daily News, among other news outlets, indicates.
In the latest iteration of this age-old practice, a team of attorneys and doctors allegedly recruited more than 400 New York City inhabitants, many of whom were homeless or addicted to drugs, over the course of 5 years to participate in their scam.
These people, who were often desperate for money, were coached to claim they’d tripped and fallen over one of the many obstacles pedestrians encounter in the city — sidewalk cracks, potholes, open or protruding cellar doors, and the like. The participant would be cajoled into signing his or her name to a fraudulent suit. Over the years, the scam netted the bosses more than $31 million from city businesses and their insurance companies.
In some cases, to add authenticity to the swindle, the scammers convinced the so-called victims to undergo an operation, promising them up to $1,500 to go under the knife.
Accused by the U.S. Attorney’s Office of masterminding the slip-and-fall operation were two attorneys, George Constantine and Marc Elefant, and a pair of doctors, Andrew Dowd, MD, an orthopedic surgeon, and Sady Ribeiro, MD, a pain management specialist and surgeon.
According to the charges, Mr. Constantine and Mr. Elefant filed lawsuits — either together or separately, though the report isn’t clear — on behalf of hundreds of people who took part in the scheme. Dr. Dowd and Dr. Ribeiro also profited handsomely, according to authorities. Dr. Dowd, they say, earned roughly $9,500 per surgery. Dr. Ribeiro is accused of treating nearly 200 participants during the con and of often paying kickbacks to them to drum up additional referrals.
If convicted on all charges, including mail and wire fraud, each of the defendants could face up to 20 years in prison.
This isn’t the first time such a scheme has been hatched and carried out in New York City.
In May 2020, three men were sentenced to prison for their involvement in a similar slip-and-fall operation that took place between 2013 and 2018. (Their sentencing followed an earlier conviction for conspiracy to commit mail and wire fraud.)
Like the most recent scheme, the one that began in 2013 preyed on the hopeless. “The whole essence of this conspiracy is to find the down-and-out, find the desperate, find the homeless,” the sentencing judge said at the time. “No person who has a job and education and can support his or her family even minimally is going to say, ‘Oh, I’ll undergo unnecessary back surgery for a thousand dollars.’ These people were vulnerable and desperate.”
Sales reps in the OR — helpful or harmful?
Although medical device makers insist that the practice of placing sales reps in operating rooms (ORs) has proven beneficial to surgeons over the years, others claim it has contributed to unwanted outcomes, according to a report published in Kaiser Health News.
In May 2018, for instance, Cristina Martinez underwent what was supposed to be a routine spinal implant procedure. In the course of the operation, her Houston-based spine surgeon discovered that the implant he was about to insert in her back was larger than the one he had intended to use. Under different circumstances, the device maker’s sales representative who was present in the OR that day might have been able to supply the smaller implant. But the rep didn’t have one available, so the surgeon proceeded with the operation using the larger plastic disk.
Four days later, in another procedure, the surgeon removed that disk and replaced it with one that was the correct size. Ms. Martinez has claimed that she awakened from the second procedure in pain, the result of nerve damage that she says led to loss of feeling in her left leg.
In a suit against the doctor, the device maker, and its distributor and sales reps, Ms. Martinez alleges that her injuries were the direct result of their negligence in not having the proper disk available during the initial operation.
The defendants have each denied any wrongdoing.
The accused spine surgeon submitted to the court his operating notes, which reportedly say that he had depended on a company distributor and its sales reps to provide “all lengths available” of the implant. In another filing, he contends that the “small area of leg numbness experienced by Ms. Martinez was a known complication of the first surgery...and was not the result of any alleged negligence.”
The device maker also denies any wrongdoing. In its filings, it claims that sales reps initially ordered a sterile kit that included implants ranging from 50 mm to 55 mm in length. The kit, says the manufacturer, was duly shipped to Houston, but the surgeon replaced the original implant with a 40-mm version during Ms. Martinez’ reoperation.
A trial is scheduled for this November.
But was the Houston incident typical?
Device makers argue that having sales reps in the OR makes sense: Well trained, they offer surgeons vital technical guidance in the use of products that are often complex.
Kaiser Health News says its investigation found otherwise. This practice and others “have been blamed for contributing to serious patient harm in thousands of medical malpractice, product liability, and whistleblower lawsuits filed over the past decade,” it reports.
The list of allegations is long: Some patients say they were injured because sales reps sold or delivered either the wrong-size implant or a defective one. Others say that in their communications with doctors, device makers were shown to have been less than truthful about the safety and durability of their products.
Currently, more than 28,000 suits have been consolidated into six multi-district federal cases. Most involve patients who claim injury after receiving hip implants. Some of the procedures required painful reoperations.
Other court actions cite device makers for other misdeeds, including keeping federal regulators in the dark about potentially dangerous product defects and plying surgeons with millions of dollars in illegal kickbacks.
Device makers have denied these and other allegations. Many of their cases have been settled confidentially.
This high-risk specialty found a way to lower its claim rates
Ob.gyns. who undergo training that simulates team interactions during a high-acuity clinical case face fewer malpractice claims afterward, according to a study published in August in Obstetrics and Gynecology.
Researchers examined the claim rates of 292 ob.gyns. who had undergone one or more such training simulations from 2002 to 2019. To gauge the effect of simulation training on the rate of medical malpractice claims, the researchers compared pretraining rates with posttraining rates. Because participants were insured by the same carrier, the team had access to the relevant claims data as well as the doctors’ durations of coverage.
The investigators assessed claim rates for the period 2002 to 2019 (the full study period), as well as rates for 2-year and 1-year participation.
Researchers found a nonsignificant drop in claim rates for doctors in the 1-year group. The drop was greater for those in the 2-year group; it decreased from 9.2 to 5.4 claims per 100 physician coverage years. (A coverage year is defined as 12 months of indemnity protection.)
For doctors who took part for the entire study period — and were therefore more likely to be among the nearly 20% of doctors who attended three or more training sessions — postsimulation claim rates dropped significantly, from 11.2 to 5.7 per 100 physician coverage years. (Attendance in more than one simulation session correlated with a greater reduction in claim rates.)
“We observed a significant reduction in malpractice claim rates after simulation training,” the researchers concluded. “Wider use of simulation training within obstetrics and gynecology should be considered.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
ACOG amicus brief supports case against Mississippi abortion ban
The American College of Obstetricians and Gynecologists (ACOG), took a prominent stand in the battle over abortion legislation by filing an amicus brief to the United States Supreme Court in the case of Dobbs v. Jackson Women’s Health Organization, according to a statement from ACOG issued on Sept. 21.
The case, filed by Thomas E. Dobbs, MD, state health officer of the Mississippi Department of Health, and others, appeals the decision by the U.S. Court of Appeals for the Fifth Circuit to throw out Mississippi’s law banning abortion after 15 weeks of pregnancy.*
ACOG’s amicus brief, which was signed by 24 additional medical organizations, including the American Medical Association, “represents an unprecedented level of support from a diverse group of physicians, nurses, and other health care professionals, which demonstrates the concrete medical consensus of opposition to abortion restriction legislation such as the law at the heart of Dobbs v. Jackson,” according to the ACOG statement.
The brief explains how the ban goes against not only the ability of health professionals to provide safe and essential care, but also goes against scientific evidence and medical ethics. “By preventing clinicians from providing patients with necessary medical care, the ban represents gross interference in the patient-clinician relationship,” according to the ACOG brief.
Potential implications if the ban is upheld include health risks to pregnant women at or near 15 weeks’ gestation, who might be forced to travel out of state, attempt self-induced abortion, or carry a pregnancy to term, according to the brief.
“Each of these outcomes increases the likelihood of negative consequences to a woman’s physical and psychological health that could be avoided if care were available,” according to the brief.
The brief also emphasizes that the ban will have a disproportionate effect on women who are already at risk for being medically underserved and who make up a majority of women seeking abortion: women of color, women in rural areas, and women with limited financial resources.
“This law is an example of harmful legislative interference into the practice of medicine,” said ACOG President J. Martin Tucker, MD, FACOG, on behalf of ACOG, in the statement.
“The outcome of this case could overturn decades of legal precedent that safeguards safe, legal abortion before viability, and the consequences of this case have national implications,” said Maureen G. Phipps, MD, MPH, CEO of ACOG, in an interview, as reported by ACOG press person Kate Connors.
“If the court does not strike down this law, clinicians in states across the country may face similar restrictions in their ability to provide necessary, evidence-based medical care,” Dr. Phipps explained. “If states are allowed to create new laws that further restrict abortion access, patients and families across the country will suffer,” she said.
“We hope that the Supreme Court will respond to the arguments of our brief and to the remarkable medical consensus represented by 25 organization signing the brief,” Dr. Phipps said. “We will continue educating and working through the judicial system in support of our patients’ access to evidence-based care and in opposition to legislative interference in the practice of medicine,” she emphasized.
Other medical organizations that signed the brief in support of the case against the Mississippi abortion ban included the American Academy of Pediatrics, the American Association of Family Physicians, the American College of Nurse Midwives, the American College of Physicians, the American Psychological Association, the American Society for Reproductive Medicine, the Association of Women’s Health, Obstetric and Neonatal Nurses, the American Medical Women’s Association, the Council of University Chairs of Obstetrics and Gynecology, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, the Society of OB/GYN Hospitalists, and the Society of Family Planning.
*This story was updated on 10/7/2021.
The American College of Obstetricians and Gynecologists (ACOG), took a prominent stand in the battle over abortion legislation by filing an amicus brief to the United States Supreme Court in the case of Dobbs v. Jackson Women’s Health Organization, according to a statement from ACOG issued on Sept. 21.
The case, filed by Thomas E. Dobbs, MD, state health officer of the Mississippi Department of Health, and others, appeals the decision by the U.S. Court of Appeals for the Fifth Circuit to throw out Mississippi’s law banning abortion after 15 weeks of pregnancy.*
ACOG’s amicus brief, which was signed by 24 additional medical organizations, including the American Medical Association, “represents an unprecedented level of support from a diverse group of physicians, nurses, and other health care professionals, which demonstrates the concrete medical consensus of opposition to abortion restriction legislation such as the law at the heart of Dobbs v. Jackson,” according to the ACOG statement.
The brief explains how the ban goes against not only the ability of health professionals to provide safe and essential care, but also goes against scientific evidence and medical ethics. “By preventing clinicians from providing patients with necessary medical care, the ban represents gross interference in the patient-clinician relationship,” according to the ACOG brief.
Potential implications if the ban is upheld include health risks to pregnant women at or near 15 weeks’ gestation, who might be forced to travel out of state, attempt self-induced abortion, or carry a pregnancy to term, according to the brief.
“Each of these outcomes increases the likelihood of negative consequences to a woman’s physical and psychological health that could be avoided if care were available,” according to the brief.
The brief also emphasizes that the ban will have a disproportionate effect on women who are already at risk for being medically underserved and who make up a majority of women seeking abortion: women of color, women in rural areas, and women with limited financial resources.
“This law is an example of harmful legislative interference into the practice of medicine,” said ACOG President J. Martin Tucker, MD, FACOG, on behalf of ACOG, in the statement.
“The outcome of this case could overturn decades of legal precedent that safeguards safe, legal abortion before viability, and the consequences of this case have national implications,” said Maureen G. Phipps, MD, MPH, CEO of ACOG, in an interview, as reported by ACOG press person Kate Connors.
“If the court does not strike down this law, clinicians in states across the country may face similar restrictions in their ability to provide necessary, evidence-based medical care,” Dr. Phipps explained. “If states are allowed to create new laws that further restrict abortion access, patients and families across the country will suffer,” she said.
“We hope that the Supreme Court will respond to the arguments of our brief and to the remarkable medical consensus represented by 25 organization signing the brief,” Dr. Phipps said. “We will continue educating and working through the judicial system in support of our patients’ access to evidence-based care and in opposition to legislative interference in the practice of medicine,” she emphasized.
Other medical organizations that signed the brief in support of the case against the Mississippi abortion ban included the American Academy of Pediatrics, the American Association of Family Physicians, the American College of Nurse Midwives, the American College of Physicians, the American Psychological Association, the American Society for Reproductive Medicine, the Association of Women’s Health, Obstetric and Neonatal Nurses, the American Medical Women’s Association, the Council of University Chairs of Obstetrics and Gynecology, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, the Society of OB/GYN Hospitalists, and the Society of Family Planning.
*This story was updated on 10/7/2021.
The American College of Obstetricians and Gynecologists (ACOG), took a prominent stand in the battle over abortion legislation by filing an amicus brief to the United States Supreme Court in the case of Dobbs v. Jackson Women’s Health Organization, according to a statement from ACOG issued on Sept. 21.
The case, filed by Thomas E. Dobbs, MD, state health officer of the Mississippi Department of Health, and others, appeals the decision by the U.S. Court of Appeals for the Fifth Circuit to throw out Mississippi’s law banning abortion after 15 weeks of pregnancy.*
ACOG’s amicus brief, which was signed by 24 additional medical organizations, including the American Medical Association, “represents an unprecedented level of support from a diverse group of physicians, nurses, and other health care professionals, which demonstrates the concrete medical consensus of opposition to abortion restriction legislation such as the law at the heart of Dobbs v. Jackson,” according to the ACOG statement.
The brief explains how the ban goes against not only the ability of health professionals to provide safe and essential care, but also goes against scientific evidence and medical ethics. “By preventing clinicians from providing patients with necessary medical care, the ban represents gross interference in the patient-clinician relationship,” according to the ACOG brief.
Potential implications if the ban is upheld include health risks to pregnant women at or near 15 weeks’ gestation, who might be forced to travel out of state, attempt self-induced abortion, or carry a pregnancy to term, according to the brief.
“Each of these outcomes increases the likelihood of negative consequences to a woman’s physical and psychological health that could be avoided if care were available,” according to the brief.
The brief also emphasizes that the ban will have a disproportionate effect on women who are already at risk for being medically underserved and who make up a majority of women seeking abortion: women of color, women in rural areas, and women with limited financial resources.
“This law is an example of harmful legislative interference into the practice of medicine,” said ACOG President J. Martin Tucker, MD, FACOG, on behalf of ACOG, in the statement.
“The outcome of this case could overturn decades of legal precedent that safeguards safe, legal abortion before viability, and the consequences of this case have national implications,” said Maureen G. Phipps, MD, MPH, CEO of ACOG, in an interview, as reported by ACOG press person Kate Connors.
“If the court does not strike down this law, clinicians in states across the country may face similar restrictions in their ability to provide necessary, evidence-based medical care,” Dr. Phipps explained. “If states are allowed to create new laws that further restrict abortion access, patients and families across the country will suffer,” she said.
“We hope that the Supreme Court will respond to the arguments of our brief and to the remarkable medical consensus represented by 25 organization signing the brief,” Dr. Phipps said. “We will continue educating and working through the judicial system in support of our patients’ access to evidence-based care and in opposition to legislative interference in the practice of medicine,” she emphasized.
Other medical organizations that signed the brief in support of the case against the Mississippi abortion ban included the American Academy of Pediatrics, the American Association of Family Physicians, the American College of Nurse Midwives, the American College of Physicians, the American Psychological Association, the American Society for Reproductive Medicine, the Association of Women’s Health, Obstetric and Neonatal Nurses, the American Medical Women’s Association, the Council of University Chairs of Obstetrics and Gynecology, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, the Society of OB/GYN Hospitalists, and the Society of Family Planning.
*This story was updated on 10/7/2021.
New fellowship, no problem
Using growth mindset to tackle fellowship in a new program
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
Using growth mindset to tackle fellowship in a new program
Using growth mindset to tackle fellowship in a new program
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
Mobile Integrated Health: Reducing Chronic Obstructive Pulmonary Disease Hospitalizations Through Novel Outpatient Care Initiatives
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.
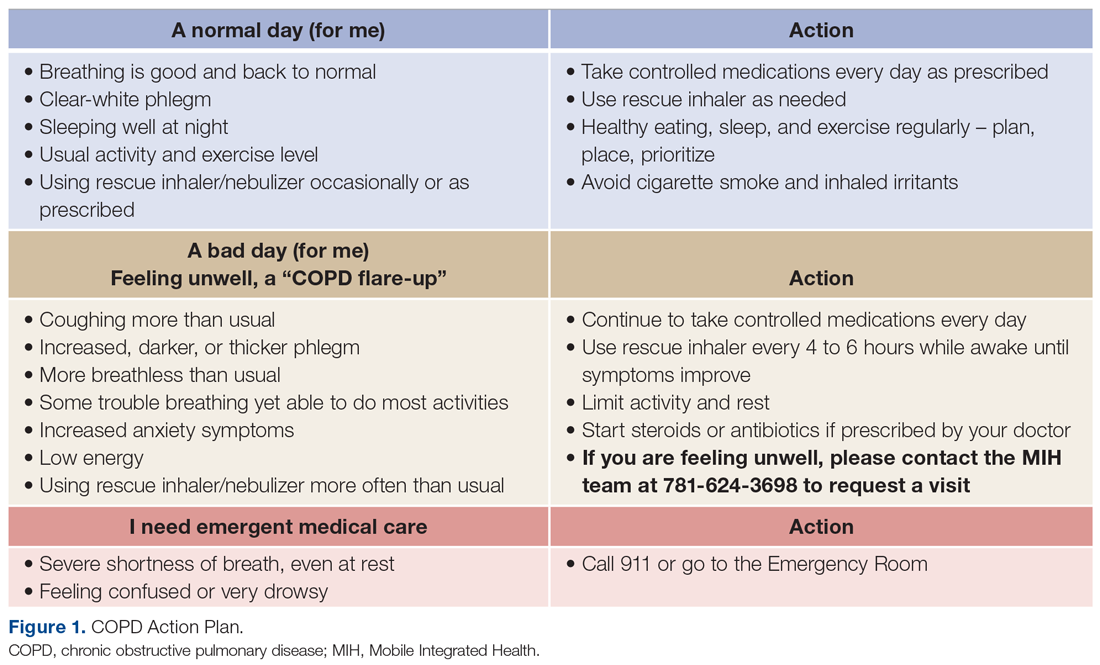
The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.
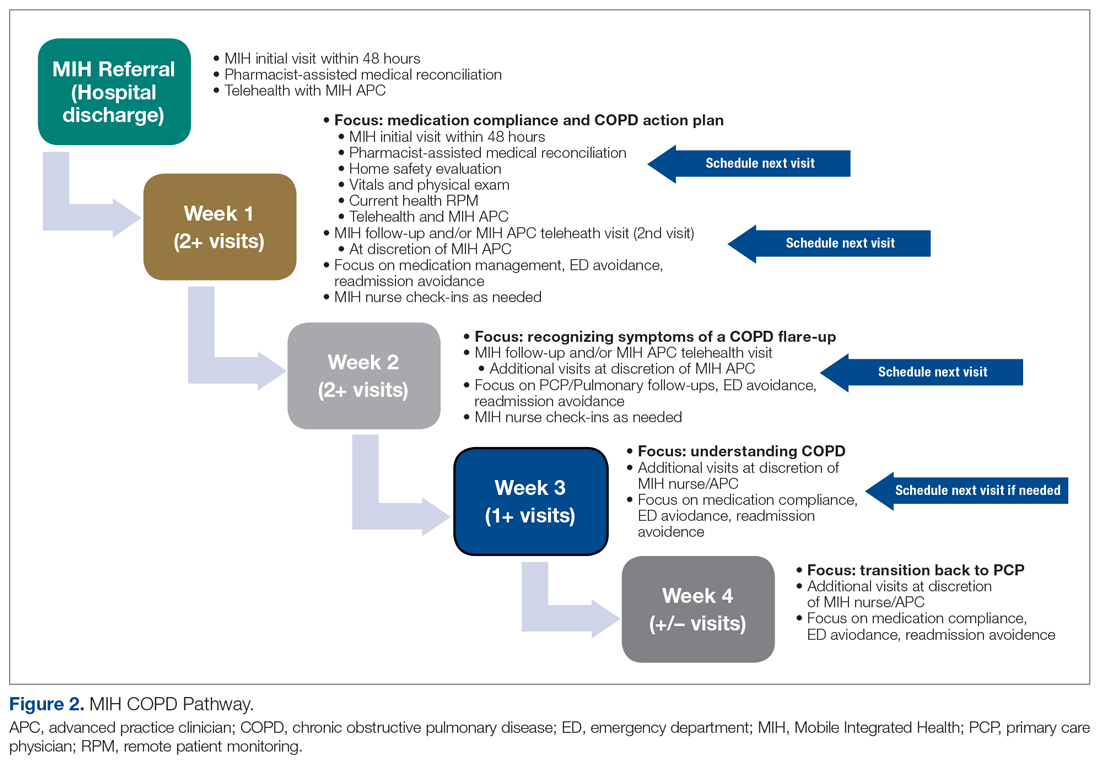
Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).
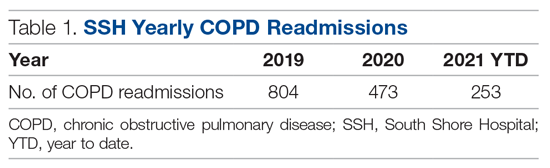
Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.
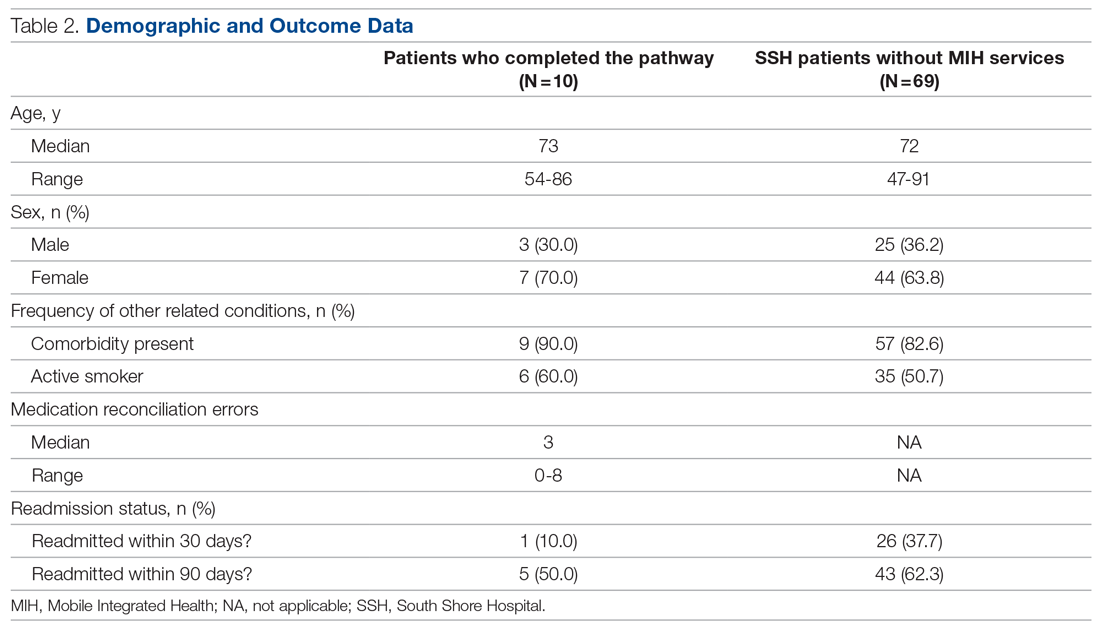
Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.

The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.

Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).

Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.

Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; [email protected].
Financial disclosures: None.
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.

The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.

Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).

Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.

Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232



