User login
Fungal infection can mimic lung cancer metastases
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
REPORTING FROM CHEST 2021
Advanced rectal cancer: Delaying surgery not advisable in patients not responding to preoperative CRT
Key clinical point: Delaying surgery after completing neoadjuvant chemoradiotherapy (CRT) could be a risk factor for worse outcomes in patients with locally advanced rectal cancer with poor or no pathological response to preoperative CRT.
Major finding: A longer vs. shorter waiting period between surgery and end of CRT was associated with worse overall survival (5 years: 67.6% vs 80.3%; 10 years: 40.1% vs 57.8%; P < .001) and disease-free survival (5 years: 59.6% vs 72.0%; 10 years: 36.2% vs 53.9%; P < .001).
Study details: Findings are from a retrospective analysis of 1,064 patients who underwent CRT and surgery for locally advanced rectal cancer and showed partial or no pathological response to neoadjuvant CRT. Wait time between CRT completion and colorectal surgery categorized patients into shorter (8 weeks or less; n=579) or longer (greater than 8 weeks; n=485) interval groups.
Disclosures: The study did not declare any source of funding. No conflict of interests was reported.
Source: Deidda S et al. JAMA Surg. 2021 Sep 29. doi: 10.1001/jamasurg.2021.4566.
Key clinical point: Delaying surgery after completing neoadjuvant chemoradiotherapy (CRT) could be a risk factor for worse outcomes in patients with locally advanced rectal cancer with poor or no pathological response to preoperative CRT.
Major finding: A longer vs. shorter waiting period between surgery and end of CRT was associated with worse overall survival (5 years: 67.6% vs 80.3%; 10 years: 40.1% vs 57.8%; P < .001) and disease-free survival (5 years: 59.6% vs 72.0%; 10 years: 36.2% vs 53.9%; P < .001).
Study details: Findings are from a retrospective analysis of 1,064 patients who underwent CRT and surgery for locally advanced rectal cancer and showed partial or no pathological response to neoadjuvant CRT. Wait time between CRT completion and colorectal surgery categorized patients into shorter (8 weeks or less; n=579) or longer (greater than 8 weeks; n=485) interval groups.
Disclosures: The study did not declare any source of funding. No conflict of interests was reported.
Source: Deidda S et al. JAMA Surg. 2021 Sep 29. doi: 10.1001/jamasurg.2021.4566.
Key clinical point: Delaying surgery after completing neoadjuvant chemoradiotherapy (CRT) could be a risk factor for worse outcomes in patients with locally advanced rectal cancer with poor or no pathological response to preoperative CRT.
Major finding: A longer vs. shorter waiting period between surgery and end of CRT was associated with worse overall survival (5 years: 67.6% vs 80.3%; 10 years: 40.1% vs 57.8%; P < .001) and disease-free survival (5 years: 59.6% vs 72.0%; 10 years: 36.2% vs 53.9%; P < .001).
Study details: Findings are from a retrospective analysis of 1,064 patients who underwent CRT and surgery for locally advanced rectal cancer and showed partial or no pathological response to neoadjuvant CRT. Wait time between CRT completion and colorectal surgery categorized patients into shorter (8 weeks or less; n=579) or longer (greater than 8 weeks; n=485) interval groups.
Disclosures: The study did not declare any source of funding. No conflict of interests was reported.
Source: Deidda S et al. JAMA Surg. 2021 Sep 29. doi: 10.1001/jamasurg.2021.4566.
Resection of asymptomatic primary tumor worsens outcomes in nonresectable metastatic CRC
Key clinical point: Primary tumor resection (PTR) of asymptomatic tumor followed by systemic therapy is associated with a significantly higher 60-day mortality than systemic therapy alone in patients with nonresectable metastatic colorectal cancer (mCRC).
Major finding: Deaths within 60 days of randomization were significantly higher in the PTR vs systemic therapy alone (11% vs 3%; P = .03) arm with elevated levels of lactate dehydrogenase (P = .046), neutrophils (P = .04), aspartate aminotransferase (P < .001), and alanine aminotransferase (P = .002) being associated with higher 60-day mortality in the PTR arm.
Study details: CAIRO4 is a randomized phase 3 trial including 198 patients with nonresectable mCRC and asymptomatic primary tumor randomly assigned to systemic therapy alone or PTR followed by systemic therapy with palliative intent.
Disclosures: The study was funded by Dutch Cancer Society and Hoffmann-La Roche. Dr. DEW van der Kruijssen, Dr. GR Vink, Dr. JHW de Wilt, and Dr. M Koopman reported receiving grants and/or nonfinancial support from various sources including Hoffmann-La Roche Ltd.
Source: van der Kruijssen DEW et al. JAMA Surg. 2021 Oct 6. doi: 10.1001/jamasurg.2021.4992.
Key clinical point: Primary tumor resection (PTR) of asymptomatic tumor followed by systemic therapy is associated with a significantly higher 60-day mortality than systemic therapy alone in patients with nonresectable metastatic colorectal cancer (mCRC).
Major finding: Deaths within 60 days of randomization were significantly higher in the PTR vs systemic therapy alone (11% vs 3%; P = .03) arm with elevated levels of lactate dehydrogenase (P = .046), neutrophils (P = .04), aspartate aminotransferase (P < .001), and alanine aminotransferase (P = .002) being associated with higher 60-day mortality in the PTR arm.
Study details: CAIRO4 is a randomized phase 3 trial including 198 patients with nonresectable mCRC and asymptomatic primary tumor randomly assigned to systemic therapy alone or PTR followed by systemic therapy with palliative intent.
Disclosures: The study was funded by Dutch Cancer Society and Hoffmann-La Roche. Dr. DEW van der Kruijssen, Dr. GR Vink, Dr. JHW de Wilt, and Dr. M Koopman reported receiving grants and/or nonfinancial support from various sources including Hoffmann-La Roche Ltd.
Source: van der Kruijssen DEW et al. JAMA Surg. 2021 Oct 6. doi: 10.1001/jamasurg.2021.4992.
Key clinical point: Primary tumor resection (PTR) of asymptomatic tumor followed by systemic therapy is associated with a significantly higher 60-day mortality than systemic therapy alone in patients with nonresectable metastatic colorectal cancer (mCRC).
Major finding: Deaths within 60 days of randomization were significantly higher in the PTR vs systemic therapy alone (11% vs 3%; P = .03) arm with elevated levels of lactate dehydrogenase (P = .046), neutrophils (P = .04), aspartate aminotransferase (P < .001), and alanine aminotransferase (P = .002) being associated with higher 60-day mortality in the PTR arm.
Study details: CAIRO4 is a randomized phase 3 trial including 198 patients with nonresectable mCRC and asymptomatic primary tumor randomly assigned to systemic therapy alone or PTR followed by systemic therapy with palliative intent.
Disclosures: The study was funded by Dutch Cancer Society and Hoffmann-La Roche. Dr. DEW van der Kruijssen, Dr. GR Vink, Dr. JHW de Wilt, and Dr. M Koopman reported receiving grants and/or nonfinancial support from various sources including Hoffmann-La Roche Ltd.
Source: van der Kruijssen DEW et al. JAMA Surg. 2021 Oct 6. doi: 10.1001/jamasurg.2021.4992.
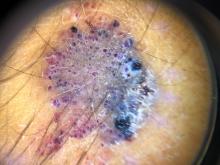
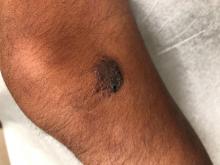
Teen boy’s knee lesion has changed
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A 14-year-old male was referred to our pediatric dermatology clinic for evaluation of a lesion on the left knee that appeared at 1 year of age. The lesion has been growing with him and was not symptomatic until 6 months prior to the consultation, when it started bleeding and feeling wet.
He has a history of attention-deficit/hyperactivity disorder managed with dextroamphetamine-amphetamine. The changes noted on the knee lesion seem to occur at the same time that his ADHD medication was started.
On physical exam he had a violaceous circular plaque on the left knee.
On dermoscopy the lesion showed multiple dilated red and violaceous lacunae and whitish blue hue.
Post-COVID-19 syndrome for a year after illness is common
Key clinical point: Persistence of COVID-19-related symptoms for 1 year after the onset of illness is common, even in some individuals with initial mild disease.
Major finding: Overall, 40.7% of participants continued to report 1 or more COVID-19-related symptoms at 12 months after illness onset. 16.4%, 49.5%, and 52.5% of patients with mild, moderate, and severe/critical COVID-19 had 1 or more COVID-19-related symptoms at 12 months after illness onset
Study details: The data come from a Dutch prospective cohort study (RECoVERED) involving 342 patients with COVID-19.
Disclosures: This study was supported by the Netherlands Organisation for Health Research and Development (ZonMw) and the Public Health Service of Amsterdam. A Boyd received a grant from ANRS and participated in the Data Safety Monitoring Board or Advisory Board for ZonMw for another study. G de Bree served as a paid member of the scientific advisory board of ExeVir. The remaining authors declared no conflict of interests.
Source: Wynberg E et al. Clin Infect Dis. 2021 Sep 2. doi: 10.1093/cid/ciab759.
Key clinical point: Persistence of COVID-19-related symptoms for 1 year after the onset of illness is common, even in some individuals with initial mild disease.
Major finding: Overall, 40.7% of participants continued to report 1 or more COVID-19-related symptoms at 12 months after illness onset. 16.4%, 49.5%, and 52.5% of patients with mild, moderate, and severe/critical COVID-19 had 1 or more COVID-19-related symptoms at 12 months after illness onset
Study details: The data come from a Dutch prospective cohort study (RECoVERED) involving 342 patients with COVID-19.
Disclosures: This study was supported by the Netherlands Organisation for Health Research and Development (ZonMw) and the Public Health Service of Amsterdam. A Boyd received a grant from ANRS and participated in the Data Safety Monitoring Board or Advisory Board for ZonMw for another study. G de Bree served as a paid member of the scientific advisory board of ExeVir. The remaining authors declared no conflict of interests.
Source: Wynberg E et al. Clin Infect Dis. 2021 Sep 2. doi: 10.1093/cid/ciab759.
Key clinical point: Persistence of COVID-19-related symptoms for 1 year after the onset of illness is common, even in some individuals with initial mild disease.
Major finding: Overall, 40.7% of participants continued to report 1 or more COVID-19-related symptoms at 12 months after illness onset. 16.4%, 49.5%, and 52.5% of patients with mild, moderate, and severe/critical COVID-19 had 1 or more COVID-19-related symptoms at 12 months after illness onset
Study details: The data come from a Dutch prospective cohort study (RECoVERED) involving 342 patients with COVID-19.
Disclosures: This study was supported by the Netherlands Organisation for Health Research and Development (ZonMw) and the Public Health Service of Amsterdam. A Boyd received a grant from ANRS and participated in the Data Safety Monitoring Board or Advisory Board for ZonMw for another study. G de Bree served as a paid member of the scientific advisory board of ExeVir. The remaining authors declared no conflict of interests.
Source: Wynberg E et al. Clin Infect Dis. 2021 Sep 2. doi: 10.1093/cid/ciab759.
COVID-19: CPAP has no advantage over conventional oxygen therapy
Key clinical point: Continuous positive airway pressure (CPAP) therapy in patients with severe COVID-19 who are not likely to benefit from invasive mechanical ventilation (IMV) does not confer a survival advantage over oxygen alone.
Major finding: The overall 30-day mortality rate was 75.6% in the oxygen group vs 77.7% in the CPAP group (Pearson’s Chi-square, P = .8).
Study details: The data come from a retrospective multicentre cohort study involving 479 patients with COVID-19 ineligible for IMV from 7 UK hospitals. Patients given CPAP were compared with those receiving oxygen therapy.
Disclosures: L Pearmain is supported by the Medical Research Council, and TW Felton is supported by the National Institute for Health Research Manchester Biomedical Research Centre. AB reported relationships with Fisher and Paykel and Sanofi Genzyme. The remaining authors declared no conflict of interests.
Source: Bradley P et al. EClinicalMedicine. 2021 Sep 8. doi: 10.1016/j.eclinm.2021.101122.
Key clinical point: Continuous positive airway pressure (CPAP) therapy in patients with severe COVID-19 who are not likely to benefit from invasive mechanical ventilation (IMV) does not confer a survival advantage over oxygen alone.
Major finding: The overall 30-day mortality rate was 75.6% in the oxygen group vs 77.7% in the CPAP group (Pearson’s Chi-square, P = .8).
Study details: The data come from a retrospective multicentre cohort study involving 479 patients with COVID-19 ineligible for IMV from 7 UK hospitals. Patients given CPAP were compared with those receiving oxygen therapy.
Disclosures: L Pearmain is supported by the Medical Research Council, and TW Felton is supported by the National Institute for Health Research Manchester Biomedical Research Centre. AB reported relationships with Fisher and Paykel and Sanofi Genzyme. The remaining authors declared no conflict of interests.
Source: Bradley P et al. EClinicalMedicine. 2021 Sep 8. doi: 10.1016/j.eclinm.2021.101122.
Key clinical point: Continuous positive airway pressure (CPAP) therapy in patients with severe COVID-19 who are not likely to benefit from invasive mechanical ventilation (IMV) does not confer a survival advantage over oxygen alone.
Major finding: The overall 30-day mortality rate was 75.6% in the oxygen group vs 77.7% in the CPAP group (Pearson’s Chi-square, P = .8).
Study details: The data come from a retrospective multicentre cohort study involving 479 patients with COVID-19 ineligible for IMV from 7 UK hospitals. Patients given CPAP were compared with those receiving oxygen therapy.
Disclosures: L Pearmain is supported by the Medical Research Council, and TW Felton is supported by the National Institute for Health Research Manchester Biomedical Research Centre. AB reported relationships with Fisher and Paykel and Sanofi Genzyme. The remaining authors declared no conflict of interests.
Source: Bradley P et al. EClinicalMedicine. 2021 Sep 8. doi: 10.1016/j.eclinm.2021.101122.
Does smoking influence COVID-19 outcomes?
Key clinical point: Smokers have a significantly higher risk for COVID-19 related hospital admission and mortality than nonsmokers.
Major finding: Current smokers had an 80% higher risk for hospitalization than never-smokers (odds ratio, 1.80; 95% confidence interval, 1.26-2.29). Smokers who smoked more than 20 cigarettes a day had a 6.11-fold higher risk of dying from COVID-19 than never-smokers.
Study details: The data come from an observational study of 421,469 individuals from the UK Biobank.
Disclosures: The study was supported by individual grants to several authors. PS Tan, CAC Coupland, MR Munafò, J Hippisley-Cox, and A von Ende reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Clift AK et al. Thorax. 2021 Sep 27. doi: 10.1136/thoraxjnl-2021-217080.
Key clinical point: Smokers have a significantly higher risk for COVID-19 related hospital admission and mortality than nonsmokers.
Major finding: Current smokers had an 80% higher risk for hospitalization than never-smokers (odds ratio, 1.80; 95% confidence interval, 1.26-2.29). Smokers who smoked more than 20 cigarettes a day had a 6.11-fold higher risk of dying from COVID-19 than never-smokers.
Study details: The data come from an observational study of 421,469 individuals from the UK Biobank.
Disclosures: The study was supported by individual grants to several authors. PS Tan, CAC Coupland, MR Munafò, J Hippisley-Cox, and A von Ende reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Clift AK et al. Thorax. 2021 Sep 27. doi: 10.1136/thoraxjnl-2021-217080.
Key clinical point: Smokers have a significantly higher risk for COVID-19 related hospital admission and mortality than nonsmokers.
Major finding: Current smokers had an 80% higher risk for hospitalization than never-smokers (odds ratio, 1.80; 95% confidence interval, 1.26-2.29). Smokers who smoked more than 20 cigarettes a day had a 6.11-fold higher risk of dying from COVID-19 than never-smokers.
Study details: The data come from an observational study of 421,469 individuals from the UK Biobank.
Disclosures: The study was supported by individual grants to several authors. PS Tan, CAC Coupland, MR Munafò, J Hippisley-Cox, and A von Ende reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Clift AK et al. Thorax. 2021 Sep 27. doi: 10.1136/thoraxjnl-2021-217080.
Anti-CD20-treated multiple sclerosis patients show robust response to COVID-19 vaccines
Key clinical point: Patients with multiple sclerosis (MS) receiving anti-CD20 (aCD20) treatment demonstrate a robust T-cell response to an mRNA COVID-19 vaccines.
Major finding: Thirty days following the second dose of an mRNA COVID-19 vaccine, 89% of patients with MS had antispike antibodies, and 50% had mounted anti-receptor-binding domain responses.
Study details: A study assessed antibody and T-cell responses in 20 patients with MS receiving aCD20 treatment and 10 healthy controls.
Disclosures: The study was supported by individual grants to several authors. SE Hensley, EJ Wherry, A Sette, ET Luning Prak, D Jacobs, and A Bar-Or reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Apostolidis SA et al. Nat Med. 2021 Sep 14. doi: 10.1038/s41591-021-01507-2.
Key clinical point: Patients with multiple sclerosis (MS) receiving anti-CD20 (aCD20) treatment demonstrate a robust T-cell response to an mRNA COVID-19 vaccines.
Major finding: Thirty days following the second dose of an mRNA COVID-19 vaccine, 89% of patients with MS had antispike antibodies, and 50% had mounted anti-receptor-binding domain responses.
Study details: A study assessed antibody and T-cell responses in 20 patients with MS receiving aCD20 treatment and 10 healthy controls.
Disclosures: The study was supported by individual grants to several authors. SE Hensley, EJ Wherry, A Sette, ET Luning Prak, D Jacobs, and A Bar-Or reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Apostolidis SA et al. Nat Med. 2021 Sep 14. doi: 10.1038/s41591-021-01507-2.
Key clinical point: Patients with multiple sclerosis (MS) receiving anti-CD20 (aCD20) treatment demonstrate a robust T-cell response to an mRNA COVID-19 vaccines.
Major finding: Thirty days following the second dose of an mRNA COVID-19 vaccine, 89% of patients with MS had antispike antibodies, and 50% had mounted anti-receptor-binding domain responses.
Study details: A study assessed antibody and T-cell responses in 20 patients with MS receiving aCD20 treatment and 10 healthy controls.
Disclosures: The study was supported by individual grants to several authors. SE Hensley, EJ Wherry, A Sette, ET Luning Prak, D Jacobs, and A Bar-Or reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Apostolidis SA et al. Nat Med. 2021 Sep 14. doi: 10.1038/s41591-021-01507-2.
Study identifies 3 behaviors that increase COVID-19 risk
Key clinical point: A study has identified at least 3 modifiable behaviors that may increase the personal risk for COVID-19.
Major finding: Higher number of nonhousehold contacts (odds ratio [OR], 1.10 per 10 contacts; P = .024), attending events having at least 10 people (OR, 1.26 per 10 events; P = .007), and visiting restaurants (OR, 1.95 per 10 visits; P less than .001) were associated with a significantly increased risk for incident COVID-19.
Study details: The data come from a prospective cohort study of 28,575 individuals across 99 countries.
Disclosures: The study was supported by grants from the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering to GM Marcus, J Olgin, and M Pletcher. The authors declared no competing interests.
Source: Lin A et al. BMJ Open. 2021 Sep 21. doi: 10.1136/bmjopen-2021-052025.
Key clinical point: A study has identified at least 3 modifiable behaviors that may increase the personal risk for COVID-19.
Major finding: Higher number of nonhousehold contacts (odds ratio [OR], 1.10 per 10 contacts; P = .024), attending events having at least 10 people (OR, 1.26 per 10 events; P = .007), and visiting restaurants (OR, 1.95 per 10 visits; P less than .001) were associated with a significantly increased risk for incident COVID-19.
Study details: The data come from a prospective cohort study of 28,575 individuals across 99 countries.
Disclosures: The study was supported by grants from the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering to GM Marcus, J Olgin, and M Pletcher. The authors declared no competing interests.
Source: Lin A et al. BMJ Open. 2021 Sep 21. doi: 10.1136/bmjopen-2021-052025.
Key clinical point: A study has identified at least 3 modifiable behaviors that may increase the personal risk for COVID-19.
Major finding: Higher number of nonhousehold contacts (odds ratio [OR], 1.10 per 10 contacts; P = .024), attending events having at least 10 people (OR, 1.26 per 10 events; P = .007), and visiting restaurants (OR, 1.95 per 10 visits; P less than .001) were associated with a significantly increased risk for incident COVID-19.
Study details: The data come from a prospective cohort study of 28,575 individuals across 99 countries.
Disclosures: The study was supported by grants from the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering to GM Marcus, J Olgin, and M Pletcher. The authors declared no competing interests.
Source: Lin A et al. BMJ Open. 2021 Sep 21. doi: 10.1136/bmjopen-2021-052025.
COVID-19: Influence of household members' immunity on nonimmune individuals
Key clinical point: The risk of contracting COVID-19 among nonimmune family members decreases as the number of immune family members increases.
Major finding: An inverse dose-response association was seen between the number of immune members in each family and the risk for incident COVID-19 in nonimmune family members. Families with 1 immune member had a 45%-61% lower risk for COVID-19, whereas families with 4 immune members had a 97% lower risk.
Study details: The data come from an analysis of 1,789,728 individuals from 814,806 families, consisting of 2-5 members each.
Disclosures: Information on funding was not available. The authors declared no competing interests.
Source: Nordström P et al. JAMA Intern Med. 2021 Oct 11. doi: 10.1001/jamainternmed.2021.5814.
Key clinical point: The risk of contracting COVID-19 among nonimmune family members decreases as the number of immune family members increases.
Major finding: An inverse dose-response association was seen between the number of immune members in each family and the risk for incident COVID-19 in nonimmune family members. Families with 1 immune member had a 45%-61% lower risk for COVID-19, whereas families with 4 immune members had a 97% lower risk.
Study details: The data come from an analysis of 1,789,728 individuals from 814,806 families, consisting of 2-5 members each.
Disclosures: Information on funding was not available. The authors declared no competing interests.
Source: Nordström P et al. JAMA Intern Med. 2021 Oct 11. doi: 10.1001/jamainternmed.2021.5814.
Key clinical point: The risk of contracting COVID-19 among nonimmune family members decreases as the number of immune family members increases.
Major finding: An inverse dose-response association was seen between the number of immune members in each family and the risk for incident COVID-19 in nonimmune family members. Families with 1 immune member had a 45%-61% lower risk for COVID-19, whereas families with 4 immune members had a 97% lower risk.
Study details: The data come from an analysis of 1,789,728 individuals from 814,806 families, consisting of 2-5 members each.
Disclosures: Information on funding was not available. The authors declared no competing interests.
Source: Nordström P et al. JAMA Intern Med. 2021 Oct 11. doi: 10.1001/jamainternmed.2021.5814.