User login
Clinical Edge Journal Scan Commentary: CML November 2021
In the new era of the COVID-19 pandemic, patients and physicians still struggle to see how different hematologic conditions may be affected by this viral infection. Although it has been well reported that COVID-19 may not be as lethal in chronic myeloid leukemia (CML) as other malignancies, patients still may be at risk of bad outcomes. A recent publication by Breccia et al1 collected retrospective information on more than 8000 CML patients followed at different institutions in Italy up to January 2021. The authors recorded 217 patients (2.5%) who were SARS-CO-V2 (COVID-19) positive. More than half of the patients had concomitant comorbidities. Almost 80% were quarantined while the rest required hospitalization, although only 3.6 required intensive care unit care. Twelve patients died, which represents 0.13% of the whole cohort. The main predisposing factors were age > 65 years and cardiovascular disorders, similar to that the general population. Most of patients continue tyrosine kinase inhibitor (TKI) therapy during the infection.
While the introduction of TKI for the treatment of CML make the number of allogenic transplants decrease significantly due the high mortality in comparison with TKI therapy, it is still an option for certain patients who failed multiple TKI treatments or progressed to more advanced phases of the disease. Although it has already been described that the introduction of imatinib did not affect outcomes for patients that require this therapeutic option, there was not much data about the effect of second generation TKIs. In a publication by Masouridi-Levrat S et al2 the authors examine the effect of second generation TKIs in a prospective non-interventional study performed by the European Group for Blood and Marrow Transplantation on 383 consecutive CML patients previously treated with dasatinib or nilotinib undergoing allogeneic hematopoietic stem cell transplantation (allo-HCT) from 2009 to 2013. Less than 40% of patients received the transplant in the chronic phase while the rest were in accelerated or blast phase. With a median follow-up of 37 months, 8% of patients developed either primary or secondary graft failure, 34% acute graft-versus-host disease (GvHD), and 60% chronic GvHD. The non-relapse mortality was 18% and 24% at 12 months and at 5 years, respectively. Relapse incidence was 36%, overall survival 56%, and relapse-free survival 40% at 5 years. All these data showed the feasibility of this procedure in patients treated with second generation TKIs with similar post-transplant complications in TKI naive patients or patients treated with imatinib.
Patients under therapy with TKIs may frequently present with elevations of creatine kinase (CK), thought to be in some cases related with the classical associations with muscle and joint pain that is also a common side effect. However the long run effect on treatment outcomes has not been well studied. Bankar A et al.3 recently reported on the relation between CK elevations and overall survival (OS) and event free survival (EFS). Interestingly CK elevations secondary to first or second generation TKIs were associated with a better OS and EFS. As expected, high Sokal score patients had a worse OS and EFS.
References
- Breccia M et al. COVID-19 infection in chronic myeloid leukaemia after one year of the pandemic in Italy. A Campus CML report. Br J Haematol. 2021 Oct 11.
- Masouridi-Levrat S et al. Outcomes and toxicity of allogeneic hematopoietic cell transplantation in chronic myeloid leukemia patients previously treated with second-generation tyrosine kinase inhibitors: a prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT. Bone Marrow Transplant. 2021 Oct 1.
- Bankar A, Lipton JH. Association of creatine kinase elevation with clinical outcomes in chronic myeloid leukemia: a retrospective cohort study Leuk Lymphoma. 2021 Sep 8.
In the new era of the COVID-19 pandemic, patients and physicians still struggle to see how different hematologic conditions may be affected by this viral infection. Although it has been well reported that COVID-19 may not be as lethal in chronic myeloid leukemia (CML) as other malignancies, patients still may be at risk of bad outcomes. A recent publication by Breccia et al1 collected retrospective information on more than 8000 CML patients followed at different institutions in Italy up to January 2021. The authors recorded 217 patients (2.5%) who were SARS-CO-V2 (COVID-19) positive. More than half of the patients had concomitant comorbidities. Almost 80% were quarantined while the rest required hospitalization, although only 3.6 required intensive care unit care. Twelve patients died, which represents 0.13% of the whole cohort. The main predisposing factors were age > 65 years and cardiovascular disorders, similar to that the general population. Most of patients continue tyrosine kinase inhibitor (TKI) therapy during the infection.
While the introduction of TKI for the treatment of CML make the number of allogenic transplants decrease significantly due the high mortality in comparison with TKI therapy, it is still an option for certain patients who failed multiple TKI treatments or progressed to more advanced phases of the disease. Although it has already been described that the introduction of imatinib did not affect outcomes for patients that require this therapeutic option, there was not much data about the effect of second generation TKIs. In a publication by Masouridi-Levrat S et al2 the authors examine the effect of second generation TKIs in a prospective non-interventional study performed by the European Group for Blood and Marrow Transplantation on 383 consecutive CML patients previously treated with dasatinib or nilotinib undergoing allogeneic hematopoietic stem cell transplantation (allo-HCT) from 2009 to 2013. Less than 40% of patients received the transplant in the chronic phase while the rest were in accelerated or blast phase. With a median follow-up of 37 months, 8% of patients developed either primary or secondary graft failure, 34% acute graft-versus-host disease (GvHD), and 60% chronic GvHD. The non-relapse mortality was 18% and 24% at 12 months and at 5 years, respectively. Relapse incidence was 36%, overall survival 56%, and relapse-free survival 40% at 5 years. All these data showed the feasibility of this procedure in patients treated with second generation TKIs with similar post-transplant complications in TKI naive patients or patients treated with imatinib.
Patients under therapy with TKIs may frequently present with elevations of creatine kinase (CK), thought to be in some cases related with the classical associations with muscle and joint pain that is also a common side effect. However the long run effect on treatment outcomes has not been well studied. Bankar A et al.3 recently reported on the relation between CK elevations and overall survival (OS) and event free survival (EFS). Interestingly CK elevations secondary to first or second generation TKIs were associated with a better OS and EFS. As expected, high Sokal score patients had a worse OS and EFS.
References
- Breccia M et al. COVID-19 infection in chronic myeloid leukaemia after one year of the pandemic in Italy. A Campus CML report. Br J Haematol. 2021 Oct 11.
- Masouridi-Levrat S et al. Outcomes and toxicity of allogeneic hematopoietic cell transplantation in chronic myeloid leukemia patients previously treated with second-generation tyrosine kinase inhibitors: a prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT. Bone Marrow Transplant. 2021 Oct 1.
- Bankar A, Lipton JH. Association of creatine kinase elevation with clinical outcomes in chronic myeloid leukemia: a retrospective cohort study Leuk Lymphoma. 2021 Sep 8.
In the new era of the COVID-19 pandemic, patients and physicians still struggle to see how different hematologic conditions may be affected by this viral infection. Although it has been well reported that COVID-19 may not be as lethal in chronic myeloid leukemia (CML) as other malignancies, patients still may be at risk of bad outcomes. A recent publication by Breccia et al1 collected retrospective information on more than 8000 CML patients followed at different institutions in Italy up to January 2021. The authors recorded 217 patients (2.5%) who were SARS-CO-V2 (COVID-19) positive. More than half of the patients had concomitant comorbidities. Almost 80% were quarantined while the rest required hospitalization, although only 3.6 required intensive care unit care. Twelve patients died, which represents 0.13% of the whole cohort. The main predisposing factors were age > 65 years and cardiovascular disorders, similar to that the general population. Most of patients continue tyrosine kinase inhibitor (TKI) therapy during the infection.
While the introduction of TKI for the treatment of CML make the number of allogenic transplants decrease significantly due the high mortality in comparison with TKI therapy, it is still an option for certain patients who failed multiple TKI treatments or progressed to more advanced phases of the disease. Although it has already been described that the introduction of imatinib did not affect outcomes for patients that require this therapeutic option, there was not much data about the effect of second generation TKIs. In a publication by Masouridi-Levrat S et al2 the authors examine the effect of second generation TKIs in a prospective non-interventional study performed by the European Group for Blood and Marrow Transplantation on 383 consecutive CML patients previously treated with dasatinib or nilotinib undergoing allogeneic hematopoietic stem cell transplantation (allo-HCT) from 2009 to 2013. Less than 40% of patients received the transplant in the chronic phase while the rest were in accelerated or blast phase. With a median follow-up of 37 months, 8% of patients developed either primary or secondary graft failure, 34% acute graft-versus-host disease (GvHD), and 60% chronic GvHD. The non-relapse mortality was 18% and 24% at 12 months and at 5 years, respectively. Relapse incidence was 36%, overall survival 56%, and relapse-free survival 40% at 5 years. All these data showed the feasibility of this procedure in patients treated with second generation TKIs with similar post-transplant complications in TKI naive patients or patients treated with imatinib.
Patients under therapy with TKIs may frequently present with elevations of creatine kinase (CK), thought to be in some cases related with the classical associations with muscle and joint pain that is also a common side effect. However the long run effect on treatment outcomes has not been well studied. Bankar A et al.3 recently reported on the relation between CK elevations and overall survival (OS) and event free survival (EFS). Interestingly CK elevations secondary to first or second generation TKIs were associated with a better OS and EFS. As expected, high Sokal score patients had a worse OS and EFS.
References
- Breccia M et al. COVID-19 infection in chronic myeloid leukaemia after one year of the pandemic in Italy. A Campus CML report. Br J Haematol. 2021 Oct 11.
- Masouridi-Levrat S et al. Outcomes and toxicity of allogeneic hematopoietic cell transplantation in chronic myeloid leukemia patients previously treated with second-generation tyrosine kinase inhibitors: a prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT. Bone Marrow Transplant. 2021 Oct 1.
- Bankar A, Lipton JH. Association of creatine kinase elevation with clinical outcomes in chronic myeloid leukemia: a retrospective cohort study Leuk Lymphoma. 2021 Sep 8.
Serotonin-mediated anxiety: How to recognize and treat it
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
Dealing with a difficult boss: A ‘bossectomy’ is rarely the cure
Ms. D is a 48-year-old administrative assistant and married mother of 2 teenagers with a history of adjustment disorder with mixed anxiety and depressed mood. She presents with increasing anxiety, poor sleep, irritability, and occasional feelings of hopelessness in the context of feeling stuck in a “dead-end job.” She describes her main issue as having an uncaring boss with unrealistic expectations. Clearly exasperated, she tells you, “If only I could get rid of my boss, everything would be just fine.”
Ms. D’s situation is common. When confronted with overbearing and demanding supervisors, the natural inclination for some employees is to flee. Symptoms of burnout (eg, emotional exhaustion, depersonalization, and decreased personal accomplishment) often occur, sometimes with more serious symptoms of adjustment disorder or even major depressive disorder or generalized anxiety disorder. To help patients such as Ms. D who are experiencing difficulties with their boss, you can use a simple approach aimed at helping them make the decision to stay at the job or leave for other opportunities, while supporting them along the way.
Clarify, then support and explore
A critical addition to the typical evaluation is a full social history, including prior employment and formative relationships, that may inform current workplace dynamics. Does the patient have a pattern of similar circumstances, or is this unusual for her? How does she view the supervisor-employee relationship, and how do power differentials, potential job loss, and subsequent financial impacts further amplify emotional friction?
Once the dynamics are clarified, support and validate her emotional reaction before exploring potential cognitive distortions and her own contributions to the relationship dysfunction. If her tendency is to lash out in anger, she could fan the flames and risk being fired. If her tendency is to cower or freeze, you can help to gradually empower her. Regardless of relationship dynamics, be careful not to medicalize what may simply be a difficult situation.1 Perhaps she is a perfectionist and minimizes her supervisor’s behaviors that affirm her work and value as a person. In such cases, you can use cognitive-behavioral therapy techniques to help her consider different points of view and nuance. Rarely are people all good or all bad.
Perhaps her perceptions are accurate, and her boss really is a jerk. If this is the case, she likely feels unfairly and helplessly persecuted. She may be suffering from demoralization, or feelings of impotence, isolation, and despair in which her self-esteem is damaged and she feels rejected because of her failure to meet her boss’s and her own expectations.2 In cases of demoralization, oddly enough, hospice literature lends some tools to help her. The Table3 provides some common terms associated with demoralization and discussion points you can use to help her move toward “remoralization.”
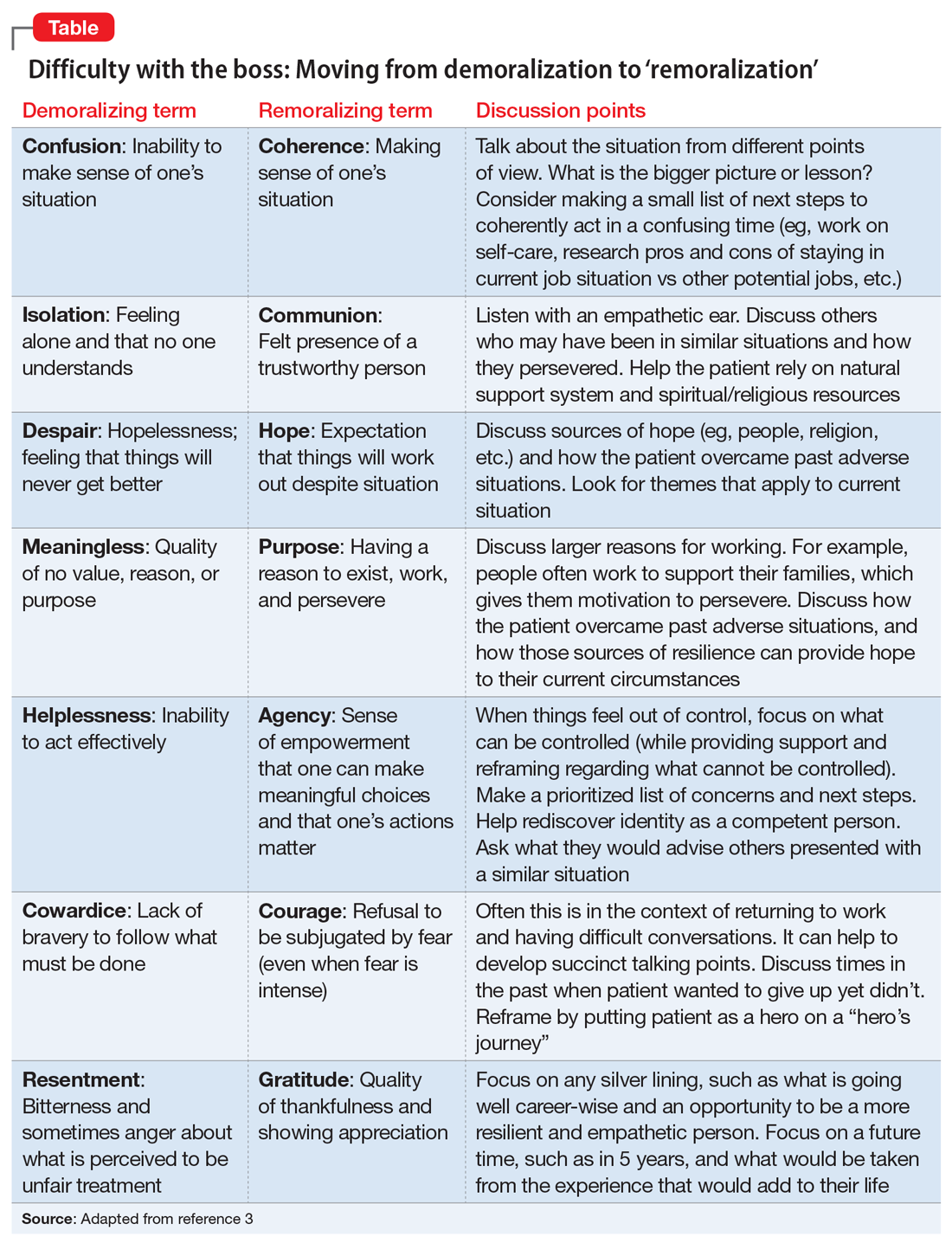
Regardless of the full story, it’s common for people to externalize uncomfortable emotions and attribute symptoms to an external cause. Help her develop self-efficacy by realizing she is in control of how she responds to her emotions. Have her focus on her role in the relationship with her supervisor, looking for common ground and brainstorming practical solutions. Ultimately you can help her realize that she always has choices about whether to stay at the job or look for work elsewhere. Your role is to support her regardless of her decision.
CASE CONTINUED
Over several visits, Ms. D begins to view the relationship with her supervisor in a different light. She has a conversation with him about what she needs to do personally and what she needs professionally from him to be successful at work. Her supervisor acknowledges he has been demanding and could be more supportive. Together they vow to communicate more clearly and regularly assess progress, including celebrating clear victories. Ms. D ultimately decides to stay at the job, and her symptoms resolve without a “bossectomy.”
1. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-e131.
2. Frank JD. Psychotherapy: the restoration of morale. Am J Psychiatry. 1974;131(3):271-274.
3. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116.
Ms. D is a 48-year-old administrative assistant and married mother of 2 teenagers with a history of adjustment disorder with mixed anxiety and depressed mood. She presents with increasing anxiety, poor sleep, irritability, and occasional feelings of hopelessness in the context of feeling stuck in a “dead-end job.” She describes her main issue as having an uncaring boss with unrealistic expectations. Clearly exasperated, she tells you, “If only I could get rid of my boss, everything would be just fine.”
Ms. D’s situation is common. When confronted with overbearing and demanding supervisors, the natural inclination for some employees is to flee. Symptoms of burnout (eg, emotional exhaustion, depersonalization, and decreased personal accomplishment) often occur, sometimes with more serious symptoms of adjustment disorder or even major depressive disorder or generalized anxiety disorder. To help patients such as Ms. D who are experiencing difficulties with their boss, you can use a simple approach aimed at helping them make the decision to stay at the job or leave for other opportunities, while supporting them along the way.
Clarify, then support and explore
A critical addition to the typical evaluation is a full social history, including prior employment and formative relationships, that may inform current workplace dynamics. Does the patient have a pattern of similar circumstances, or is this unusual for her? How does she view the supervisor-employee relationship, and how do power differentials, potential job loss, and subsequent financial impacts further amplify emotional friction?
Once the dynamics are clarified, support and validate her emotional reaction before exploring potential cognitive distortions and her own contributions to the relationship dysfunction. If her tendency is to lash out in anger, she could fan the flames and risk being fired. If her tendency is to cower or freeze, you can help to gradually empower her. Regardless of relationship dynamics, be careful not to medicalize what may simply be a difficult situation.1 Perhaps she is a perfectionist and minimizes her supervisor’s behaviors that affirm her work and value as a person. In such cases, you can use cognitive-behavioral therapy techniques to help her consider different points of view and nuance. Rarely are people all good or all bad.
Perhaps her perceptions are accurate, and her boss really is a jerk. If this is the case, she likely feels unfairly and helplessly persecuted. She may be suffering from demoralization, or feelings of impotence, isolation, and despair in which her self-esteem is damaged and she feels rejected because of her failure to meet her boss’s and her own expectations.2 In cases of demoralization, oddly enough, hospice literature lends some tools to help her. The Table3 provides some common terms associated with demoralization and discussion points you can use to help her move toward “remoralization.”

Regardless of the full story, it’s common for people to externalize uncomfortable emotions and attribute symptoms to an external cause. Help her develop self-efficacy by realizing she is in control of how she responds to her emotions. Have her focus on her role in the relationship with her supervisor, looking for common ground and brainstorming practical solutions. Ultimately you can help her realize that she always has choices about whether to stay at the job or look for work elsewhere. Your role is to support her regardless of her decision.
CASE CONTINUED
Over several visits, Ms. D begins to view the relationship with her supervisor in a different light. She has a conversation with him about what she needs to do personally and what she needs professionally from him to be successful at work. Her supervisor acknowledges he has been demanding and could be more supportive. Together they vow to communicate more clearly and regularly assess progress, including celebrating clear victories. Ms. D ultimately decides to stay at the job, and her symptoms resolve without a “bossectomy.”
Ms. D is a 48-year-old administrative assistant and married mother of 2 teenagers with a history of adjustment disorder with mixed anxiety and depressed mood. She presents with increasing anxiety, poor sleep, irritability, and occasional feelings of hopelessness in the context of feeling stuck in a “dead-end job.” She describes her main issue as having an uncaring boss with unrealistic expectations. Clearly exasperated, she tells you, “If only I could get rid of my boss, everything would be just fine.”
Ms. D’s situation is common. When confronted with overbearing and demanding supervisors, the natural inclination for some employees is to flee. Symptoms of burnout (eg, emotional exhaustion, depersonalization, and decreased personal accomplishment) often occur, sometimes with more serious symptoms of adjustment disorder or even major depressive disorder or generalized anxiety disorder. To help patients such as Ms. D who are experiencing difficulties with their boss, you can use a simple approach aimed at helping them make the decision to stay at the job or leave for other opportunities, while supporting them along the way.
Clarify, then support and explore
A critical addition to the typical evaluation is a full social history, including prior employment and formative relationships, that may inform current workplace dynamics. Does the patient have a pattern of similar circumstances, or is this unusual for her? How does she view the supervisor-employee relationship, and how do power differentials, potential job loss, and subsequent financial impacts further amplify emotional friction?
Once the dynamics are clarified, support and validate her emotional reaction before exploring potential cognitive distortions and her own contributions to the relationship dysfunction. If her tendency is to lash out in anger, she could fan the flames and risk being fired. If her tendency is to cower or freeze, you can help to gradually empower her. Regardless of relationship dynamics, be careful not to medicalize what may simply be a difficult situation.1 Perhaps she is a perfectionist and minimizes her supervisor’s behaviors that affirm her work and value as a person. In such cases, you can use cognitive-behavioral therapy techniques to help her consider different points of view and nuance. Rarely are people all good or all bad.
Perhaps her perceptions are accurate, and her boss really is a jerk. If this is the case, she likely feels unfairly and helplessly persecuted. She may be suffering from demoralization, or feelings of impotence, isolation, and despair in which her self-esteem is damaged and she feels rejected because of her failure to meet her boss’s and her own expectations.2 In cases of demoralization, oddly enough, hospice literature lends some tools to help her. The Table3 provides some common terms associated with demoralization and discussion points you can use to help her move toward “remoralization.”

Regardless of the full story, it’s common for people to externalize uncomfortable emotions and attribute symptoms to an external cause. Help her develop self-efficacy by realizing she is in control of how she responds to her emotions. Have her focus on her role in the relationship with her supervisor, looking for common ground and brainstorming practical solutions. Ultimately you can help her realize that she always has choices about whether to stay at the job or look for work elsewhere. Your role is to support her regardless of her decision.
CASE CONTINUED
Over several visits, Ms. D begins to view the relationship with her supervisor in a different light. She has a conversation with him about what she needs to do personally and what she needs professionally from him to be successful at work. Her supervisor acknowledges he has been demanding and could be more supportive. Together they vow to communicate more clearly and regularly assess progress, including celebrating clear victories. Ms. D ultimately decides to stay at the job, and her symptoms resolve without a “bossectomy.”
1. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-e131.
2. Frank JD. Psychotherapy: the restoration of morale. Am J Psychiatry. 1974;131(3):271-274.
3. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116.
1. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-e131.
2. Frank JD. Psychotherapy: the restoration of morale. Am J Psychiatry. 1974;131(3):271-274.
3. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116.
The impact of modifiable risk factors such as diet and obesity in Pediatric MS patients
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
Clinical Edge Journal Scan Commentary: Uterine Fibroids November 2021
Mahalingam et al in the Journal of Maternal-Fetal & Neonatal Medicine reported the risk of preterm birth among women with uterine fibroids who underwent myomectomy versus those who did not prior to pregnancy. In this retrospective cohort study, the team evaluated 290 women with a viable intrauterine pregnancy and history of uterine fibroids and compared two groups: 70 with history of a prior myomectomy and 220 who did not undergo myomectomy and had at least 1 fibroid of size 5 cm or more detected at less than 21 weeks’ gestation. The team found that women who underwent prior myomectomy versus those who did not were more likely to deliver preterm < 37 weeks gestation (35% vs 21%; P = .02) and deliver a mean 1.4 weeks earlier (36.3±3.6 vs 37.7±3.7 weeks gestation; P = .02). Patients with history of myomectomy had a higher C-section rate (88% vs 53%, P < 0.001). However, when the authors controlled for late preterm pre-labor C-sections recommended by physicians in the myomectomy cohort (n=5), the difference in preterm birth was not significant between the groups.
Lee et al reported that MRI can potentially predict the benefit of GnRH-agonist treatment prior to for large fibroids. In this retrospective analysis published in Acta Radiologica, 30 patients with large uterine fibroids received GnRH agonist prior to uterine artery embolization (UAE) with MRI evaluation before and after treatment. Indications for GnRH-agonist treatment (monthly 3.75 mg leuprolide acetate injections) included intramural or subserosal fibroids > 10 cm in diameter or pedunculated submucosal fibroids > 8 cm, as well as contrast enhancement observed on T1-weighted (T1W) images. Mean maximum fibroid diameter was 11.1 + 1.9 cm and mean number of GnRH-agonist injections received was 2.8. Signal intensity (SI) of the predominant fibroid on T2-weighted (T2W) images was referenced to the SI of the rectus abdominus muscle (F/R). For predicting a volume reduction rate of the large fibroid of >50%, the optimal cut-off value of F/R was 2.58 (sensitivity 80%, specificity 80%). Likewise, large fibroids with a volume rate reduction of <30% had an optimal cut-off volume of 1.69 (sensitivity 100%, specificity 70%). From a clinical perspective, both surgeons and radiologists could use SI of the predominant fibroid on T2W to predict response to GnRH agonist pretreatment.
Mahalingam et al in the Journal of Maternal-Fetal & Neonatal Medicine reported the risk of preterm birth among women with uterine fibroids who underwent myomectomy versus those who did not prior to pregnancy. In this retrospective cohort study, the team evaluated 290 women with a viable intrauterine pregnancy and history of uterine fibroids and compared two groups: 70 with history of a prior myomectomy and 220 who did not undergo myomectomy and had at least 1 fibroid of size 5 cm or more detected at less than 21 weeks’ gestation. The team found that women who underwent prior myomectomy versus those who did not were more likely to deliver preterm < 37 weeks gestation (35% vs 21%; P = .02) and deliver a mean 1.4 weeks earlier (36.3±3.6 vs 37.7±3.7 weeks gestation; P = .02). Patients with history of myomectomy had a higher C-section rate (88% vs 53%, P < 0.001). However, when the authors controlled for late preterm pre-labor C-sections recommended by physicians in the myomectomy cohort (n=5), the difference in preterm birth was not significant between the groups.
Lee et al reported that MRI can potentially predict the benefit of GnRH-agonist treatment prior to for large fibroids. In this retrospective analysis published in Acta Radiologica, 30 patients with large uterine fibroids received GnRH agonist prior to uterine artery embolization (UAE) with MRI evaluation before and after treatment. Indications for GnRH-agonist treatment (monthly 3.75 mg leuprolide acetate injections) included intramural or subserosal fibroids > 10 cm in diameter or pedunculated submucosal fibroids > 8 cm, as well as contrast enhancement observed on T1-weighted (T1W) images. Mean maximum fibroid diameter was 11.1 + 1.9 cm and mean number of GnRH-agonist injections received was 2.8. Signal intensity (SI) of the predominant fibroid on T2-weighted (T2W) images was referenced to the SI of the rectus abdominus muscle (F/R). For predicting a volume reduction rate of the large fibroid of >50%, the optimal cut-off value of F/R was 2.58 (sensitivity 80%, specificity 80%). Likewise, large fibroids with a volume rate reduction of <30% had an optimal cut-off volume of 1.69 (sensitivity 100%, specificity 70%). From a clinical perspective, both surgeons and radiologists could use SI of the predominant fibroid on T2W to predict response to GnRH agonist pretreatment.
Mahalingam et al in the Journal of Maternal-Fetal & Neonatal Medicine reported the risk of preterm birth among women with uterine fibroids who underwent myomectomy versus those who did not prior to pregnancy. In this retrospective cohort study, the team evaluated 290 women with a viable intrauterine pregnancy and history of uterine fibroids and compared two groups: 70 with history of a prior myomectomy and 220 who did not undergo myomectomy and had at least 1 fibroid of size 5 cm or more detected at less than 21 weeks’ gestation. The team found that women who underwent prior myomectomy versus those who did not were more likely to deliver preterm < 37 weeks gestation (35% vs 21%; P = .02) and deliver a mean 1.4 weeks earlier (36.3±3.6 vs 37.7±3.7 weeks gestation; P = .02). Patients with history of myomectomy had a higher C-section rate (88% vs 53%, P < 0.001). However, when the authors controlled for late preterm pre-labor C-sections recommended by physicians in the myomectomy cohort (n=5), the difference in preterm birth was not significant between the groups.
Lee et al reported that MRI can potentially predict the benefit of GnRH-agonist treatment prior to for large fibroids. In this retrospective analysis published in Acta Radiologica, 30 patients with large uterine fibroids received GnRH agonist prior to uterine artery embolization (UAE) with MRI evaluation before and after treatment. Indications for GnRH-agonist treatment (monthly 3.75 mg leuprolide acetate injections) included intramural or subserosal fibroids > 10 cm in diameter or pedunculated submucosal fibroids > 8 cm, as well as contrast enhancement observed on T1-weighted (T1W) images. Mean maximum fibroid diameter was 11.1 + 1.9 cm and mean number of GnRH-agonist injections received was 2.8. Signal intensity (SI) of the predominant fibroid on T2-weighted (T2W) images was referenced to the SI of the rectus abdominus muscle (F/R). For predicting a volume reduction rate of the large fibroid of >50%, the optimal cut-off value of F/R was 2.58 (sensitivity 80%, specificity 80%). Likewise, large fibroids with a volume rate reduction of <30% had an optimal cut-off volume of 1.69 (sensitivity 100%, specificity 70%). From a clinical perspective, both surgeons and radiologists could use SI of the predominant fibroid on T2W to predict response to GnRH agonist pretreatment.
Clinical Edge Journal Scan Commentary: Psoriasis November 2021
Biologic therapy is generally reserved for patients with more moderate-to-severe psoriasis, often defined as BSA ≥10% or PASI of ≥10. Notably, these criteria are all clinician-performed. The Dermatology Life Quality Index (DLQI) measures the impact of the disease on the patient with a score of 0-5, 6-10, and 11-30 indicating mild, moderate, and severe disease, respectively. In a cross-sectional, observational study, 72.4% of psoriasis patients who qualified for systemic therapy initiation based on PASI and/or BSA had a DLQI of less than 10. Conversely, 10.4% of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI and/or BSA. This study highlights the complementary value of considering both clinician and patient determinants of disease severity when choosing a psoriasis therapy (Barbieri JS et al.)
With so many therapeutic options, it is increasingly common for patients to switch from one biologic to another. Many factors that go into the decision to switch therapies. A retrospective study of 115 adult patients with psoriasis found that the primary factor driving switching was lack of sufficient efficacy in treating skin disease. Having concomitant psoriatic arthritis increased the likelihood of switching by 2.69-fold (Akdogan N et al.). These findings suggest that shared decision making with dermatology, rheumatology, and the patient may help in choosing a treatment plan that best addresses both skin and joints.
One consideration when counseling patients about their likelihood of response to a second or third biologic is that in several clinical trials, biologic-naïve patients tend to have higher PASI responses to biologics than do heavier and biologic-experienced patients. A recent retrospective study found that receiving one or more biologic receiving was associated with a lower likelihood of achieving a PASI75 response to guselkumab (Hung YT et al.). However, in a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis receiving risankizumab, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively) (Gerdes S et al.). While both studies were small and no firm conclusions or comparisons can be drawn, real-world data from large multicenter studies that evaluate multiple therapies may guide us in choosing the best therapy for challenging patients who are often underrepresented in clinical trials. Understanding how switching within vs. between classes (TNF, IL-17, and IL-23 inhibitors) impacts efficacy will also help in making choices about biologic switches.
Biologic therapy is generally reserved for patients with more moderate-to-severe psoriasis, often defined as BSA ≥10% or PASI of ≥10. Notably, these criteria are all clinician-performed. The Dermatology Life Quality Index (DLQI) measures the impact of the disease on the patient with a score of 0-5, 6-10, and 11-30 indicating mild, moderate, and severe disease, respectively. In a cross-sectional, observational study, 72.4% of psoriasis patients who qualified for systemic therapy initiation based on PASI and/or BSA had a DLQI of less than 10. Conversely, 10.4% of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI and/or BSA. This study highlights the complementary value of considering both clinician and patient determinants of disease severity when choosing a psoriasis therapy (Barbieri JS et al.)
With so many therapeutic options, it is increasingly common for patients to switch from one biologic to another. Many factors that go into the decision to switch therapies. A retrospective study of 115 adult patients with psoriasis found that the primary factor driving switching was lack of sufficient efficacy in treating skin disease. Having concomitant psoriatic arthritis increased the likelihood of switching by 2.69-fold (Akdogan N et al.). These findings suggest that shared decision making with dermatology, rheumatology, and the patient may help in choosing a treatment plan that best addresses both skin and joints.
One consideration when counseling patients about their likelihood of response to a second or third biologic is that in several clinical trials, biologic-naïve patients tend to have higher PASI responses to biologics than do heavier and biologic-experienced patients. A recent retrospective study found that receiving one or more biologic receiving was associated with a lower likelihood of achieving a PASI75 response to guselkumab (Hung YT et al.). However, in a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis receiving risankizumab, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively) (Gerdes S et al.). While both studies were small and no firm conclusions or comparisons can be drawn, real-world data from large multicenter studies that evaluate multiple therapies may guide us in choosing the best therapy for challenging patients who are often underrepresented in clinical trials. Understanding how switching within vs. between classes (TNF, IL-17, and IL-23 inhibitors) impacts efficacy will also help in making choices about biologic switches.
Biologic therapy is generally reserved for patients with more moderate-to-severe psoriasis, often defined as BSA ≥10% or PASI of ≥10. Notably, these criteria are all clinician-performed. The Dermatology Life Quality Index (DLQI) measures the impact of the disease on the patient with a score of 0-5, 6-10, and 11-30 indicating mild, moderate, and severe disease, respectively. In a cross-sectional, observational study, 72.4% of psoriasis patients who qualified for systemic therapy initiation based on PASI and/or BSA had a DLQI of less than 10. Conversely, 10.4% of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI and/or BSA. This study highlights the complementary value of considering both clinician and patient determinants of disease severity when choosing a psoriasis therapy (Barbieri JS et al.)
With so many therapeutic options, it is increasingly common for patients to switch from one biologic to another. Many factors that go into the decision to switch therapies. A retrospective study of 115 adult patients with psoriasis found that the primary factor driving switching was lack of sufficient efficacy in treating skin disease. Having concomitant psoriatic arthritis increased the likelihood of switching by 2.69-fold (Akdogan N et al.). These findings suggest that shared decision making with dermatology, rheumatology, and the patient may help in choosing a treatment plan that best addresses both skin and joints.
One consideration when counseling patients about their likelihood of response to a second or third biologic is that in several clinical trials, biologic-naïve patients tend to have higher PASI responses to biologics than do heavier and biologic-experienced patients. A recent retrospective study found that receiving one or more biologic receiving was associated with a lower likelihood of achieving a PASI75 response to guselkumab (Hung YT et al.). However, in a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis receiving risankizumab, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively) (Gerdes S et al.). While both studies were small and no firm conclusions or comparisons can be drawn, real-world data from large multicenter studies that evaluate multiple therapies may guide us in choosing the best therapy for challenging patients who are often underrepresented in clinical trials. Understanding how switching within vs. between classes (TNF, IL-17, and IL-23 inhibitors) impacts efficacy will also help in making choices about biologic switches.
Woman presents with weight loss and nausea
It is likely that the polypoid appearance of the colonic lining is a result of chronic inflammation of longstanding Crohn disease with an ileocolonic manifestation. Crohn disease is an idiopathic, chronic inflammatory bowel disease characterized by cycles of relapse and remission. These asymptomatic periods can last for several months up to a few years, as reported by the patient in this case. Up to 50% of cases of Crohn disease are characterized by ileocolitis, or inflammation of the ileum and the colon. Although postinflammatory polyps are a cancer risk factor for inflammatory bowel disease and pseudopolyps are associated with severe disease, their appearance is not necessarily a poor prognostic factor.
When assessing ongoing disease activity in ileocolonic Crohn disease or ulcerative colitis, colonoscopy represents the first-line approach. Endoscopic visualization and biopsy are critical components of the diagnosis. Alternatively, cross-sectional imaging can be used to assess disease phenotype. In addition, plain radiography or a CT scan of the abdomen can identify bowel obstruction and scanning of the pelvis can detect any intra-abdominal abscesses. Ulcerative colitis looms large in the differential diagnosis. Although weight loss, perineal disease, fistulae, and obstruction are common in Crohn disease, they are uncommon or rare in ulcerative colitis, although bleeding is observed much more frequently in ulcerative colitis.
Treatment of Crohn disease is based on the severity, location, and subtype (inflammatory, stricturing, or penetrating). There is also now a focus on determining which patients are at risk for a more severe disease course and may require earlier and more aggressive therapies. Crohn disease is primarily managed through the introduction of early immunosuppressive or combination therapy with biologic agents in high-risk patients, as well as complementary diet modification. Although most patients will ultimately undergo surgery, there is no curative approach, unlike in ulcerative colitis.
In its clinical care pathway, the American Gastroenterological Association supports a top-down approach to therapy for adult patients with moderate to severe luminal Crohn disease (defining moderate to severe disease as having a Crohn Disease Activity Index score of 220 or higher, or having a high risk of complications). This approach supports the early use of biologic agents, with or without immunomodulators, over a stepwise strategy. The patient’s response to this new regimen should be determined in the 12-week period after the initiation of therapy. Endoscopy or transmural responses to therapy should be assessed after 6 months.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships.
It is likely that the polypoid appearance of the colonic lining is a result of chronic inflammation of longstanding Crohn disease with an ileocolonic manifestation. Crohn disease is an idiopathic, chronic inflammatory bowel disease characterized by cycles of relapse and remission. These asymptomatic periods can last for several months up to a few years, as reported by the patient in this case. Up to 50% of cases of Crohn disease are characterized by ileocolitis, or inflammation of the ileum and the colon. Although postinflammatory polyps are a cancer risk factor for inflammatory bowel disease and pseudopolyps are associated with severe disease, their appearance is not necessarily a poor prognostic factor.
When assessing ongoing disease activity in ileocolonic Crohn disease or ulcerative colitis, colonoscopy represents the first-line approach. Endoscopic visualization and biopsy are critical components of the diagnosis. Alternatively, cross-sectional imaging can be used to assess disease phenotype. In addition, plain radiography or a CT scan of the abdomen can identify bowel obstruction and scanning of the pelvis can detect any intra-abdominal abscesses. Ulcerative colitis looms large in the differential diagnosis. Although weight loss, perineal disease, fistulae, and obstruction are common in Crohn disease, they are uncommon or rare in ulcerative colitis, although bleeding is observed much more frequently in ulcerative colitis.
Treatment of Crohn disease is based on the severity, location, and subtype (inflammatory, stricturing, or penetrating). There is also now a focus on determining which patients are at risk for a more severe disease course and may require earlier and more aggressive therapies. Crohn disease is primarily managed through the introduction of early immunosuppressive or combination therapy with biologic agents in high-risk patients, as well as complementary diet modification. Although most patients will ultimately undergo surgery, there is no curative approach, unlike in ulcerative colitis.
In its clinical care pathway, the American Gastroenterological Association supports a top-down approach to therapy for adult patients with moderate to severe luminal Crohn disease (defining moderate to severe disease as having a Crohn Disease Activity Index score of 220 or higher, or having a high risk of complications). This approach supports the early use of biologic agents, with or without immunomodulators, over a stepwise strategy. The patient’s response to this new regimen should be determined in the 12-week period after the initiation of therapy. Endoscopy or transmural responses to therapy should be assessed after 6 months.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships.
It is likely that the polypoid appearance of the colonic lining is a result of chronic inflammation of longstanding Crohn disease with an ileocolonic manifestation. Crohn disease is an idiopathic, chronic inflammatory bowel disease characterized by cycles of relapse and remission. These asymptomatic periods can last for several months up to a few years, as reported by the patient in this case. Up to 50% of cases of Crohn disease are characterized by ileocolitis, or inflammation of the ileum and the colon. Although postinflammatory polyps are a cancer risk factor for inflammatory bowel disease and pseudopolyps are associated with severe disease, their appearance is not necessarily a poor prognostic factor.
When assessing ongoing disease activity in ileocolonic Crohn disease or ulcerative colitis, colonoscopy represents the first-line approach. Endoscopic visualization and biopsy are critical components of the diagnosis. Alternatively, cross-sectional imaging can be used to assess disease phenotype. In addition, plain radiography or a CT scan of the abdomen can identify bowel obstruction and scanning of the pelvis can detect any intra-abdominal abscesses. Ulcerative colitis looms large in the differential diagnosis. Although weight loss, perineal disease, fistulae, and obstruction are common in Crohn disease, they are uncommon or rare in ulcerative colitis, although bleeding is observed much more frequently in ulcerative colitis.
Treatment of Crohn disease is based on the severity, location, and subtype (inflammatory, stricturing, or penetrating). There is also now a focus on determining which patients are at risk for a more severe disease course and may require earlier and more aggressive therapies. Crohn disease is primarily managed through the introduction of early immunosuppressive or combination therapy with biologic agents in high-risk patients, as well as complementary diet modification. Although most patients will ultimately undergo surgery, there is no curative approach, unlike in ulcerative colitis.
In its clinical care pathway, the American Gastroenterological Association supports a top-down approach to therapy for adult patients with moderate to severe luminal Crohn disease (defining moderate to severe disease as having a Crohn Disease Activity Index score of 220 or higher, or having a high risk of complications). This approach supports the early use of biologic agents, with or without immunomodulators, over a stepwise strategy. The patient’s response to this new regimen should be determined in the 12-week period after the initiation of therapy. Endoscopy or transmural responses to therapy should be assessed after 6 months.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships.
A 42-year-old woman presents with pain in her right abdomen, nausea, and diarrhea. She reports a weight loss of about 12 lb in the past several weeks because of a disinterest in food, which typically exacerbates her symptoms. She explains that she has been experiencing mounting stress at work and abdominal cramping and fatigue. Her family medical history is significant for pancreatic cancer and multiple sclerosis. She has not experienced any significant medical events in the past few years. Endoscopy shows polypoid appearance of the colonic lining.
Patient with severe lower abdominal pain
The differential diagnosis of inflammatory bowel disease (IBD) in older patients is complicated by comorbid conditions such as infectious colitis, segmental colitis associated with diverticular disease, nonsteroidal anti-inflammatory drug-induced intestinal injury, and ischemia, each of which can mimic the intestinal inflammation characteristic of IBD.
Ulcerative colitis is one of the two major types of IBD, along with Crohn disease. Unlike Crohn disease, which can affect any part of the gastrointestinal tract, ulcerative colitis characteristically causes inflammation in the large bowel (see image).
Acute, severe ulcerative colitis (ie, > six bloody bowel movements per day, with one of the following: temperature > 38 °C [100.4 °F], hemoglobin level < 10.5 g/dL, heart rate > 90 beats/min, erythrocyte sedimentation rate > 30 mm/hr, or C-reactive protein level > 30 mg/dL) requires hospitalization and treatment with intravenous high-dose corticosteroids (hydrocortisone 400 mg/day or methylprednisolone 60 mg/day).
The diagnosis of ulcerative colitis is best made with endoscopy and mucosal biopsy for histopathologic analysis. Characteristic findings are abnormal erythematous mucosa, with or without ulceration, extending from the rectum to a part or all of the colon; and uniform inflammation without intervening areas of normal mucosa (skip lesions tend to be characteristic of Crohn disease). Contact bleeding may also be observed, with mucus identified in the lumen of the bowel.
The bowel wall in a patient with ulcerative colitis is thin or of normal thickness, but edema, the accumulation of fat, and hypertrophy of the muscle layer may give the impression of a thickened bowel wall. The disease is largely confined to the mucosa and, to a lesser extent, the submucosa.
Laboratory studies are helpful to exclude other diagnoses and assess the patient's nutritional status, but serologic markers can help in the differential diagnosis of IBD. Radiographic imaging has an important role in the workup of patients with suspected IBD and in the differentiation of ulcerative colitis from Crohn disease by demonstrating fistulae or the presence of small bowel disease seen only in Crohn disease.
Much work in the past decade has focused on the development of serologic markers for inflammatory bowel disease. pANCA and anti–Saccharomyces cerevisiae antibodies (ASCA) have been the most intensely studied. The World Gastroenterology Organization states that ulcerative colitis is more likely when the test results are positive for pANCA and negative for ASCA antigen; however, the pANCA test result may be positive in patients with Crohn disease, and this may complicate obtaining a diagnosis in an otherwise uncomplicated colitis.
According to the American Gastroenterological Association, drug classes for the long-term management of moderate to severe ulcerative colitis include tumor necrosis factor-alpha antagonists, anti-integrin agent (vedolizumab), Janus kinase inhibitor (tofacitinib), interleukin-12/23 antagonist (ustekinumab), and immunomodulators (thiopurines, methotrexate). In general, most drugs that are initiated for the induction of remission are continued as maintenance therapy if they are effective.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships.
The differential diagnosis of inflammatory bowel disease (IBD) in older patients is complicated by comorbid conditions such as infectious colitis, segmental colitis associated with diverticular disease, nonsteroidal anti-inflammatory drug-induced intestinal injury, and ischemia, each of which can mimic the intestinal inflammation characteristic of IBD.
Ulcerative colitis is one of the two major types of IBD, along with Crohn disease. Unlike Crohn disease, which can affect any part of the gastrointestinal tract, ulcerative colitis characteristically causes inflammation in the large bowel (see image).
Acute, severe ulcerative colitis (ie, > six bloody bowel movements per day, with one of the following: temperature > 38 °C [100.4 °F], hemoglobin level < 10.5 g/dL, heart rate > 90 beats/min, erythrocyte sedimentation rate > 30 mm/hr, or C-reactive protein level > 30 mg/dL) requires hospitalization and treatment with intravenous high-dose corticosteroids (hydrocortisone 400 mg/day or methylprednisolone 60 mg/day).
The diagnosis of ulcerative colitis is best made with endoscopy and mucosal biopsy for histopathologic analysis. Characteristic findings are abnormal erythematous mucosa, with or without ulceration, extending from the rectum to a part or all of the colon; and uniform inflammation without intervening areas of normal mucosa (skip lesions tend to be characteristic of Crohn disease). Contact bleeding may also be observed, with mucus identified in the lumen of the bowel.
The bowel wall in a patient with ulcerative colitis is thin or of normal thickness, but edema, the accumulation of fat, and hypertrophy of the muscle layer may give the impression of a thickened bowel wall. The disease is largely confined to the mucosa and, to a lesser extent, the submucosa.
Laboratory studies are helpful to exclude other diagnoses and assess the patient's nutritional status, but serologic markers can help in the differential diagnosis of IBD. Radiographic imaging has an important role in the workup of patients with suspected IBD and in the differentiation of ulcerative colitis from Crohn disease by demonstrating fistulae or the presence of small bowel disease seen only in Crohn disease.
Much work in the past decade has focused on the development of serologic markers for inflammatory bowel disease. pANCA and anti–Saccharomyces cerevisiae antibodies (ASCA) have been the most intensely studied. The World Gastroenterology Organization states that ulcerative colitis is more likely when the test results are positive for pANCA and negative for ASCA antigen; however, the pANCA test result may be positive in patients with Crohn disease, and this may complicate obtaining a diagnosis in an otherwise uncomplicated colitis.
According to the American Gastroenterological Association, drug classes for the long-term management of moderate to severe ulcerative colitis include tumor necrosis factor-alpha antagonists, anti-integrin agent (vedolizumab), Janus kinase inhibitor (tofacitinib), interleukin-12/23 antagonist (ustekinumab), and immunomodulators (thiopurines, methotrexate). In general, most drugs that are initiated for the induction of remission are continued as maintenance therapy if they are effective.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships.
The differential diagnosis of inflammatory bowel disease (IBD) in older patients is complicated by comorbid conditions such as infectious colitis, segmental colitis associated with diverticular disease, nonsteroidal anti-inflammatory drug-induced intestinal injury, and ischemia, each of which can mimic the intestinal inflammation characteristic of IBD.
Ulcerative colitis is one of the two major types of IBD, along with Crohn disease. Unlike Crohn disease, which can affect any part of the gastrointestinal tract, ulcerative colitis characteristically causes inflammation in the large bowel (see image).
Acute, severe ulcerative colitis (ie, > six bloody bowel movements per day, with one of the following: temperature > 38 °C [100.4 °F], hemoglobin level < 10.5 g/dL, heart rate > 90 beats/min, erythrocyte sedimentation rate > 30 mm/hr, or C-reactive protein level > 30 mg/dL) requires hospitalization and treatment with intravenous high-dose corticosteroids (hydrocortisone 400 mg/day or methylprednisolone 60 mg/day).
The diagnosis of ulcerative colitis is best made with endoscopy and mucosal biopsy for histopathologic analysis. Characteristic findings are abnormal erythematous mucosa, with or without ulceration, extending from the rectum to a part or all of the colon; and uniform inflammation without intervening areas of normal mucosa (skip lesions tend to be characteristic of Crohn disease). Contact bleeding may also be observed, with mucus identified in the lumen of the bowel.
The bowel wall in a patient with ulcerative colitis is thin or of normal thickness, but edema, the accumulation of fat, and hypertrophy of the muscle layer may give the impression of a thickened bowel wall. The disease is largely confined to the mucosa and, to a lesser extent, the submucosa.
Laboratory studies are helpful to exclude other diagnoses and assess the patient's nutritional status, but serologic markers can help in the differential diagnosis of IBD. Radiographic imaging has an important role in the workup of patients with suspected IBD and in the differentiation of ulcerative colitis from Crohn disease by demonstrating fistulae or the presence of small bowel disease seen only in Crohn disease.
Much work in the past decade has focused on the development of serologic markers for inflammatory bowel disease. pANCA and anti–Saccharomyces cerevisiae antibodies (ASCA) have been the most intensely studied. The World Gastroenterology Organization states that ulcerative colitis is more likely when the test results are positive for pANCA and negative for ASCA antigen; however, the pANCA test result may be positive in patients with Crohn disease, and this may complicate obtaining a diagnosis in an otherwise uncomplicated colitis.
According to the American Gastroenterological Association, drug classes for the long-term management of moderate to severe ulcerative colitis include tumor necrosis factor-alpha antagonists, anti-integrin agent (vedolizumab), Janus kinase inhibitor (tofacitinib), interleukin-12/23 antagonist (ustekinumab), and immunomodulators (thiopurines, methotrexate). In general, most drugs that are initiated for the induction of remission are continued as maintenance therapy if they are effective.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships.
A 74-year-old woman presents with severe lower abdominal pain and dehydration. She also reports bloody diarrhea of 2 weeks' duration and an unintentional 10-lb weight loss. She reports six to seven bloody stools per day. Dietary alterations and loperamide have not helped. She has a temperature of 101.2 °F.
Physical examination reveals tenderness at the site of the left lower quadrant of her abdomen without rebound tenderness or guarding. Bowel sounds are active. She is found to have a purulent rectal discharge. Stool culture results for the most common pathogens are negative. She has hypoalbuminemia (2.5 g/dL), and her test result is positive for perinuclear antineutrophil cytoplasmic antibodies (pANCA). Her serum carcinoembryonic antigen test result is negative. Her C-reactive protein level is 32 mg/dL.
She is admitted to the hospital and receives intravenous fluids. She undergoes a colonoscopy, which reveals inflammation and visible ulcers in the mucosa through the length of the large bowel.
Clinical Edge Journal Scan Commentary: COVID-19 November 2021
Similarly, Lin et al report the results of an international study of adults from 99 countries, which utilized longitudinal mobile based surveys to examine risk factors associated with SARS-CoV-2 infection. The mobile surveys captured baseline characteristics and behaviors of participants, and data was reported out for the study period of March to October 2020 (before vaccines were available). Adjusting for demographics, education level, a proxy for occupational risk, as well as medical comorbidities, authors found that greater number of non-household contacts, attending events with 10 or more individuals and restaurant visits predicted higher risk of SARS-CoV-2. Alternatively, older age was associated with lower risk, likely because of the protective behaviors undertaken by many in the older age group.
Lastly, the RECoVERED Study (Wynberg, at al), based in Netherlands, followed recently diagnosed laboratory confirmed SARS-CoV-2 patients for a year, initially with three in person visits (where disease severity was determined based on vital signs and level of care needed) within the first month of illness and then monthly online surveys. Authors utilized a survey examining severity of 18 symptoms based on the World Health Organization Case Report Form. Authors found 86.7% of those with initial severe disease [95% confidence interval {CI} = 76.5–92.7%]), 63.8% of those moderate disease 63.8% [95% CI = 54.8–71.5%], and 30.7% of those with mild disease [95% CI = 21.1–40.9%] had at least one persistent symptom at 12 weeks. Fatigue was the most common symptom reported at 12 weeks overall, but among those with moderate and severe disease, dyspnea and myalgia also persisted frequently. After one years of follow up, about one-fifth still had one persistent symptom. Over half of those with initial severe disease reported symptom persistence (52.5% [95% CI = 38.0–65.1%]). In a multivariable Cox proportional hazard model, female sex and higher BMI were associated with slower recovery. One limitation of the study was that was no control group recruited.
Similarly, Lin et al report the results of an international study of adults from 99 countries, which utilized longitudinal mobile based surveys to examine risk factors associated with SARS-CoV-2 infection. The mobile surveys captured baseline characteristics and behaviors of participants, and data was reported out for the study period of March to October 2020 (before vaccines were available). Adjusting for demographics, education level, a proxy for occupational risk, as well as medical comorbidities, authors found that greater number of non-household contacts, attending events with 10 or more individuals and restaurant visits predicted higher risk of SARS-CoV-2. Alternatively, older age was associated with lower risk, likely because of the protective behaviors undertaken by many in the older age group.
Lastly, the RECoVERED Study (Wynberg, at al), based in Netherlands, followed recently diagnosed laboratory confirmed SARS-CoV-2 patients for a year, initially with three in person visits (where disease severity was determined based on vital signs and level of care needed) within the first month of illness and then monthly online surveys. Authors utilized a survey examining severity of 18 symptoms based on the World Health Organization Case Report Form. Authors found 86.7% of those with initial severe disease [95% confidence interval {CI} = 76.5–92.7%]), 63.8% of those moderate disease 63.8% [95% CI = 54.8–71.5%], and 30.7% of those with mild disease [95% CI = 21.1–40.9%] had at least one persistent symptom at 12 weeks. Fatigue was the most common symptom reported at 12 weeks overall, but among those with moderate and severe disease, dyspnea and myalgia also persisted frequently. After one years of follow up, about one-fifth still had one persistent symptom. Over half of those with initial severe disease reported symptom persistence (52.5% [95% CI = 38.0–65.1%]). In a multivariable Cox proportional hazard model, female sex and higher BMI were associated with slower recovery. One limitation of the study was that was no control group recruited.
Similarly, Lin et al report the results of an international study of adults from 99 countries, which utilized longitudinal mobile based surveys to examine risk factors associated with SARS-CoV-2 infection. The mobile surveys captured baseline characteristics and behaviors of participants, and data was reported out for the study period of March to October 2020 (before vaccines were available). Adjusting for demographics, education level, a proxy for occupational risk, as well as medical comorbidities, authors found that greater number of non-household contacts, attending events with 10 or more individuals and restaurant visits predicted higher risk of SARS-CoV-2. Alternatively, older age was associated with lower risk, likely because of the protective behaviors undertaken by many in the older age group.
Lastly, the RECoVERED Study (Wynberg, at al), based in Netherlands, followed recently diagnosed laboratory confirmed SARS-CoV-2 patients for a year, initially with three in person visits (where disease severity was determined based on vital signs and level of care needed) within the first month of illness and then monthly online surveys. Authors utilized a survey examining severity of 18 symptoms based on the World Health Organization Case Report Form. Authors found 86.7% of those with initial severe disease [95% confidence interval {CI} = 76.5–92.7%]), 63.8% of those moderate disease 63.8% [95% CI = 54.8–71.5%], and 30.7% of those with mild disease [95% CI = 21.1–40.9%] had at least one persistent symptom at 12 weeks. Fatigue was the most common symptom reported at 12 weeks overall, but among those with moderate and severe disease, dyspnea and myalgia also persisted frequently. After one years of follow up, about one-fifth still had one persistent symptom. Over half of those with initial severe disease reported symptom persistence (52.5% [95% CI = 38.0–65.1%]). In a multivariable Cox proportional hazard model, female sex and higher BMI were associated with slower recovery. One limitation of the study was that was no control group recruited.
ERs are swamped with seriously ill patients, although many don’t have COVID
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.