User login
Endoscopic Lifting Agents: AGA Issues New Clinical Practice Update
Published in Clinical Gastroenterology and Hepatology, the commentary reviews available agents and provides clinically relevant commentary on their indications and use — with the caveat that it is not a formal systematic review but rather empirical advice for endoscopists. No formal rating of the quality of evidence or strength of recommendations was performed.
Led by Tobias Zuchelli, MD, a clinical associate professor at Michigan State University and a gastroenterologist at the Henry Ford Health System in Detroit, the expert panel noted that endoscopists are increasingly resecting precancerous lesions and early cancers of the gastrointestinal tract.
“Although new endoscopic procedures have been developed, there had not been much in terms of high-quality guidance on lifting agents,” panelist Amit V. Patel, MD, a professor of medicine at Duke University and director of Endoscopy at Durham Veterans Affairs Medical Center in Durham, North Carolina, told GI & Hepatology News. “With our better understanding and use of techniques, this commentary was timely. It summarizes the available data on the topic and includes our clinical experiences.”
Filling that knowledge gap, the document reviews in detail the timing and methods of agent injection according to procedure type, including the dynamic needle approach, the empirical merits of different agents such as saline (with or without blue contrast) and viscous agents, as well as lift-enhancing assistive devices — for example, the ERBEJET 2 high-pressure water jet, an adjustable hydrosurgical device to facilitate lifting. A chart provides an at-a-glance summary of agents and their pros and cons.
“The feedback from gastroenterologists so far has been quite positive on social media and on GI channels,” Patel said.
Endoscopic resection has evolved from snare polypectomy to endoscopic mucosal resection (EMR) and now, endoscopic submucosal dissection (ESD). The primary benefit of submucosal lifting is the creation of a separating submucosal cushion between the lesion and muscularis propria (MP), which reduces the risk for immediate or delayed perforation of the muscle. Adding a contrast agent also demarcates lesion margins and stains the submucosa, which is fundamental to ESD and allows for assessment of MP injury during EMR.
For decades, homemade solutions were used to lift lesions before removal, with the sentinel agent being normal saline, later mixed with a blue contrast agent, usually indigo carmine or methylene blue. The authors noted that some endoscopists performing ESD start the submucosal injection and incision using a prepackaged viscous solution. “The endoscopist may continue with the viscous fluid or transition to saline or another less expensive solution,” they wrote.
Saline tends to dissipate more quickly than viscous solutions, however. In 2015, the polymer compound SIC-8000 became the first FDA-approved submucosal injection agent. Since then, several other fluids have come on the market, although homemade agents remain available.
Among the update’s recommendations, the fluid selected for EMR should be determined by lesion size, predicted histology, and endoscopist preference. Based on the US Multi-Society Task Force (USMSTF) on Colorectal Cancer, submucosal injection is optional for nonpedunculated colorectal lesions (NPCRLs) of intermediate size (10-19 mm).
Cold snare polypectomy without submucosal injection was later found to be non-inferior to other resection methods utilizing submucosal injection for NPCRLs ≤ 15 mm.
The update noted that the USMSTF considers EMR first-line therapy for most NPCRLs ≥ 20 mm and advocates viscous solutions as preferred, while the use of lifting agents for pedunculated polyps is generally at the discretion of the endoscopist.
For Patel, the main “clinical pearls” in the update are adding a contrast agent to normal saline, using a viscous agent for cold EMR, and manipulating the injection needle first tangentially and then dynamically toward the lumen to maximize separation of the lesion.
In terms of the ideal, an optimal lifting solution would be readily available, inexpensive, and premixed, providing a sustained submucosal cushion. “However, this ideal solution currently does not exist. Injection fluids should, therefore, be selected based on planned resection method, predicted histology, local expertise and preferences, and cost,” the panelists wrote.
Added Patel, “A lot of the agents out there check most of these boxes, but we’re hoping for further development toward the ideal.”
Offering a nonparticipant’s perspective on the overview, Wasseem Skef, MD, a gastroenterologist at UTHealth Houston, found the update very useful. “It always helps to have the literature summarized,” he told GI & Hepatology News. “It’s a pretty balanced review that pulls together the various options but allows people to stick to their preferred practice.”
In his practice, the lifting agent selected depends on the type of resection. “Viscous agents are generally more popular for EMR-type resections,” Skef said. One unanswered question, he noted, is whether adding a hemostatic agent would be superior to a viscous agent alone. “But overall, this is a nice summary of available agents. Gastroenterologists should consider these different options if doing procedures like EMR.”
This review was sponsored by the AGA Institute.
Zuchelli is a consultant for Boston Scientific. Patel consults for Medpace, Renexxion, and Sanofi. Skef reported having no relevant disclosures.
A version of this article appeared on Medscape.com .
Published in Clinical Gastroenterology and Hepatology, the commentary reviews available agents and provides clinically relevant commentary on their indications and use — with the caveat that it is not a formal systematic review but rather empirical advice for endoscopists. No formal rating of the quality of evidence or strength of recommendations was performed.
Led by Tobias Zuchelli, MD, a clinical associate professor at Michigan State University and a gastroenterologist at the Henry Ford Health System in Detroit, the expert panel noted that endoscopists are increasingly resecting precancerous lesions and early cancers of the gastrointestinal tract.
“Although new endoscopic procedures have been developed, there had not been much in terms of high-quality guidance on lifting agents,” panelist Amit V. Patel, MD, a professor of medicine at Duke University and director of Endoscopy at Durham Veterans Affairs Medical Center in Durham, North Carolina, told GI & Hepatology News. “With our better understanding and use of techniques, this commentary was timely. It summarizes the available data on the topic and includes our clinical experiences.”
Filling that knowledge gap, the document reviews in detail the timing and methods of agent injection according to procedure type, including the dynamic needle approach, the empirical merits of different agents such as saline (with or without blue contrast) and viscous agents, as well as lift-enhancing assistive devices — for example, the ERBEJET 2 high-pressure water jet, an adjustable hydrosurgical device to facilitate lifting. A chart provides an at-a-glance summary of agents and their pros and cons.
“The feedback from gastroenterologists so far has been quite positive on social media and on GI channels,” Patel said.
Endoscopic resection has evolved from snare polypectomy to endoscopic mucosal resection (EMR) and now, endoscopic submucosal dissection (ESD). The primary benefit of submucosal lifting is the creation of a separating submucosal cushion between the lesion and muscularis propria (MP), which reduces the risk for immediate or delayed perforation of the muscle. Adding a contrast agent also demarcates lesion margins and stains the submucosa, which is fundamental to ESD and allows for assessment of MP injury during EMR.
For decades, homemade solutions were used to lift lesions before removal, with the sentinel agent being normal saline, later mixed with a blue contrast agent, usually indigo carmine or methylene blue. The authors noted that some endoscopists performing ESD start the submucosal injection and incision using a prepackaged viscous solution. “The endoscopist may continue with the viscous fluid or transition to saline or another less expensive solution,” they wrote.
Saline tends to dissipate more quickly than viscous solutions, however. In 2015, the polymer compound SIC-8000 became the first FDA-approved submucosal injection agent. Since then, several other fluids have come on the market, although homemade agents remain available.
Among the update’s recommendations, the fluid selected for EMR should be determined by lesion size, predicted histology, and endoscopist preference. Based on the US Multi-Society Task Force (USMSTF) on Colorectal Cancer, submucosal injection is optional for nonpedunculated colorectal lesions (NPCRLs) of intermediate size (10-19 mm).
Cold snare polypectomy without submucosal injection was later found to be non-inferior to other resection methods utilizing submucosal injection for NPCRLs ≤ 15 mm.
The update noted that the USMSTF considers EMR first-line therapy for most NPCRLs ≥ 20 mm and advocates viscous solutions as preferred, while the use of lifting agents for pedunculated polyps is generally at the discretion of the endoscopist.
For Patel, the main “clinical pearls” in the update are adding a contrast agent to normal saline, using a viscous agent for cold EMR, and manipulating the injection needle first tangentially and then dynamically toward the lumen to maximize separation of the lesion.
In terms of the ideal, an optimal lifting solution would be readily available, inexpensive, and premixed, providing a sustained submucosal cushion. “However, this ideal solution currently does not exist. Injection fluids should, therefore, be selected based on planned resection method, predicted histology, local expertise and preferences, and cost,” the panelists wrote.
Added Patel, “A lot of the agents out there check most of these boxes, but we’re hoping for further development toward the ideal.”
Offering a nonparticipant’s perspective on the overview, Wasseem Skef, MD, a gastroenterologist at UTHealth Houston, found the update very useful. “It always helps to have the literature summarized,” he told GI & Hepatology News. “It’s a pretty balanced review that pulls together the various options but allows people to stick to their preferred practice.”
In his practice, the lifting agent selected depends on the type of resection. “Viscous agents are generally more popular for EMR-type resections,” Skef said. One unanswered question, he noted, is whether adding a hemostatic agent would be superior to a viscous agent alone. “But overall, this is a nice summary of available agents. Gastroenterologists should consider these different options if doing procedures like EMR.”
This review was sponsored by the AGA Institute.
Zuchelli is a consultant for Boston Scientific. Patel consults for Medpace, Renexxion, and Sanofi. Skef reported having no relevant disclosures.
A version of this article appeared on Medscape.com .
Published in Clinical Gastroenterology and Hepatology, the commentary reviews available agents and provides clinically relevant commentary on their indications and use — with the caveat that it is not a formal systematic review but rather empirical advice for endoscopists. No formal rating of the quality of evidence or strength of recommendations was performed.
Led by Tobias Zuchelli, MD, a clinical associate professor at Michigan State University and a gastroenterologist at the Henry Ford Health System in Detroit, the expert panel noted that endoscopists are increasingly resecting precancerous lesions and early cancers of the gastrointestinal tract.
“Although new endoscopic procedures have been developed, there had not been much in terms of high-quality guidance on lifting agents,” panelist Amit V. Patel, MD, a professor of medicine at Duke University and director of Endoscopy at Durham Veterans Affairs Medical Center in Durham, North Carolina, told GI & Hepatology News. “With our better understanding and use of techniques, this commentary was timely. It summarizes the available data on the topic and includes our clinical experiences.”
Filling that knowledge gap, the document reviews in detail the timing and methods of agent injection according to procedure type, including the dynamic needle approach, the empirical merits of different agents such as saline (with or without blue contrast) and viscous agents, as well as lift-enhancing assistive devices — for example, the ERBEJET 2 high-pressure water jet, an adjustable hydrosurgical device to facilitate lifting. A chart provides an at-a-glance summary of agents and their pros and cons.
“The feedback from gastroenterologists so far has been quite positive on social media and on GI channels,” Patel said.
Endoscopic resection has evolved from snare polypectomy to endoscopic mucosal resection (EMR) and now, endoscopic submucosal dissection (ESD). The primary benefit of submucosal lifting is the creation of a separating submucosal cushion between the lesion and muscularis propria (MP), which reduces the risk for immediate or delayed perforation of the muscle. Adding a contrast agent also demarcates lesion margins and stains the submucosa, which is fundamental to ESD and allows for assessment of MP injury during EMR.
For decades, homemade solutions were used to lift lesions before removal, with the sentinel agent being normal saline, later mixed with a blue contrast agent, usually indigo carmine or methylene blue. The authors noted that some endoscopists performing ESD start the submucosal injection and incision using a prepackaged viscous solution. “The endoscopist may continue with the viscous fluid or transition to saline or another less expensive solution,” they wrote.
Saline tends to dissipate more quickly than viscous solutions, however. In 2015, the polymer compound SIC-8000 became the first FDA-approved submucosal injection agent. Since then, several other fluids have come on the market, although homemade agents remain available.
Among the update’s recommendations, the fluid selected for EMR should be determined by lesion size, predicted histology, and endoscopist preference. Based on the US Multi-Society Task Force (USMSTF) on Colorectal Cancer, submucosal injection is optional for nonpedunculated colorectal lesions (NPCRLs) of intermediate size (10-19 mm).
Cold snare polypectomy without submucosal injection was later found to be non-inferior to other resection methods utilizing submucosal injection for NPCRLs ≤ 15 mm.
The update noted that the USMSTF considers EMR first-line therapy for most NPCRLs ≥ 20 mm and advocates viscous solutions as preferred, while the use of lifting agents for pedunculated polyps is generally at the discretion of the endoscopist.
For Patel, the main “clinical pearls” in the update are adding a contrast agent to normal saline, using a viscous agent for cold EMR, and manipulating the injection needle first tangentially and then dynamically toward the lumen to maximize separation of the lesion.
In terms of the ideal, an optimal lifting solution would be readily available, inexpensive, and premixed, providing a sustained submucosal cushion. “However, this ideal solution currently does not exist. Injection fluids should, therefore, be selected based on planned resection method, predicted histology, local expertise and preferences, and cost,” the panelists wrote.
Added Patel, “A lot of the agents out there check most of these boxes, but we’re hoping for further development toward the ideal.”
Offering a nonparticipant’s perspective on the overview, Wasseem Skef, MD, a gastroenterologist at UTHealth Houston, found the update very useful. “It always helps to have the literature summarized,” he told GI & Hepatology News. “It’s a pretty balanced review that pulls together the various options but allows people to stick to their preferred practice.”
In his practice, the lifting agent selected depends on the type of resection. “Viscous agents are generally more popular for EMR-type resections,” Skef said. One unanswered question, he noted, is whether adding a hemostatic agent would be superior to a viscous agent alone. “But overall, this is a nice summary of available agents. Gastroenterologists should consider these different options if doing procedures like EMR.”
This review was sponsored by the AGA Institute.
Zuchelli is a consultant for Boston Scientific. Patel consults for Medpace, Renexxion, and Sanofi. Skef reported having no relevant disclosures.
A version of this article appeared on Medscape.com .
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
IBS, Chronic Idiopathic Constipation Surged During Pandemic
, with a near doubling of the national rate of IBS over 2 years, a study has found.
The uptick is probably due to not only the direct impact of SARS-CoV-2 infection on the gastrointestinal tract but also to the psychological stress associated with pandemic life, the study team said.
“COVID infection itself can definitely cause gastrointestinal symptoms like diarrhea, nausea, and abdominal pain — and for some people, those symptoms can linger and lead to chronic conditions like IBS,” Christopher V. Almario, MD, MSHPM, lead author and gastroenterologist at Cedars-Sinai Medical Center, Los Angeles, California, told GI & Hepatology News.
“But the stress of living through the pandemic — lockdowns, fear, isolation — also likely played a major role as well in the increased prevalence of digestive disorders. Both the infection itself and the psychological toll of the pandemic can disrupt the gut-brain axis and trigger chronic digestive disorders like IBS,” Almario said.
The study was published in Neurogastroenterology & Motility.
Growing Burden of Gut Disorders
Disorders of gut-brain interaction (DGBIs) are a heterogeneous group of conditions in which gastrointestinal symptoms occur without any detectable structural or biochemical abnormalities in the digestive tract. They include IBS, functional dyspepsia, and chronic idiopathic constipation, among others.
DGBIs are highly prevalent. Research has shown that nearly 40% of people in the US meet Rome IV criteria for at least one DGBI.
Almario and colleagues assessed trends in prevalence of these conditions during the COVID-19 pandemic. Starting in May 2020 through May 2022, they conducted a series of online surveys with more than 160,000 adults aged 18 or older using validated Rome IV diagnostic questionnaires.
Results showed that during the pandemic, IBS prevalence rose from 6.1% in May 2020 to 11.0% by May 2022, an increase of 0.188% per month (adjusted P < .001).
Chronic idiopathic constipation showed a smaller but statistically significant increase, from 6.0% to 6.4% (0.056% per month; adjusted P < .001).
Within the IBS subtypes, mixed-type IBS showed the largest relative increase (0.085% per month), followed by IBS with constipation (0.041% per month) and IBS with diarrhea (0.037% per month).
There were no significant changes in the prevalence of other DGBIs, such as functional bloating, functional diarrhea, or functional dyspepsia, during the study period.
Almario told GI & Hepatology News only about 9% of those surveyed reported a positive COVID test at the time of the surveys, but that figure probably underrepresents actual infections, especially in the early months of the pandemic. “Most of the survey responses came in during the earlier phases of the pandemic, and the percentage reporting a positive test increased over time,” he explained.
Almario also noted that this study did not directly compare digestive disorder rates between infected and uninfected individuals. However, a separate study by the Cedars-Sinai team currently undergoing peer review addresses that question more directly. “That study, along with several other studies, show that having COVID increases the risk of developing conditions like IBS and functional dyspepsia,” Almario said.
Taken together, the findings “underscore the increasing healthcare and economic burden of DGBI in the post-pandemic era, emphasizing the need for targeted efforts to effectively diagnose and manage these complex conditions,” they wrote.
“This will be especially challenging for healthcare systems to address, given the existing shortage of primary care physicians and gastroenterologists — clinicians who primarily manage individuals with DGBI,” they noted.
Support for this study was received from Ironwood Pharmaceuticals and Salix Pharmaceuticals in the form of institutional research grants to Cedars-Sinai. Almario has consulted for Exact Sciences, Greenspace Labs, Owlstone Medical, Salix Pharmaceuticals, and Universal DX.
A version of this article appeared on Medscape.com.
, with a near doubling of the national rate of IBS over 2 years, a study has found.
The uptick is probably due to not only the direct impact of SARS-CoV-2 infection on the gastrointestinal tract but also to the psychological stress associated with pandemic life, the study team said.
“COVID infection itself can definitely cause gastrointestinal symptoms like diarrhea, nausea, and abdominal pain — and for some people, those symptoms can linger and lead to chronic conditions like IBS,” Christopher V. Almario, MD, MSHPM, lead author and gastroenterologist at Cedars-Sinai Medical Center, Los Angeles, California, told GI & Hepatology News.
“But the stress of living through the pandemic — lockdowns, fear, isolation — also likely played a major role as well in the increased prevalence of digestive disorders. Both the infection itself and the psychological toll of the pandemic can disrupt the gut-brain axis and trigger chronic digestive disorders like IBS,” Almario said.
The study was published in Neurogastroenterology & Motility.
Growing Burden of Gut Disorders
Disorders of gut-brain interaction (DGBIs) are a heterogeneous group of conditions in which gastrointestinal symptoms occur without any detectable structural or biochemical abnormalities in the digestive tract. They include IBS, functional dyspepsia, and chronic idiopathic constipation, among others.
DGBIs are highly prevalent. Research has shown that nearly 40% of people in the US meet Rome IV criteria for at least one DGBI.
Almario and colleagues assessed trends in prevalence of these conditions during the COVID-19 pandemic. Starting in May 2020 through May 2022, they conducted a series of online surveys with more than 160,000 adults aged 18 or older using validated Rome IV diagnostic questionnaires.
Results showed that during the pandemic, IBS prevalence rose from 6.1% in May 2020 to 11.0% by May 2022, an increase of 0.188% per month (adjusted P < .001).
Chronic idiopathic constipation showed a smaller but statistically significant increase, from 6.0% to 6.4% (0.056% per month; adjusted P < .001).
Within the IBS subtypes, mixed-type IBS showed the largest relative increase (0.085% per month), followed by IBS with constipation (0.041% per month) and IBS with diarrhea (0.037% per month).
There were no significant changes in the prevalence of other DGBIs, such as functional bloating, functional diarrhea, or functional dyspepsia, during the study period.
Almario told GI & Hepatology News only about 9% of those surveyed reported a positive COVID test at the time of the surveys, but that figure probably underrepresents actual infections, especially in the early months of the pandemic. “Most of the survey responses came in during the earlier phases of the pandemic, and the percentage reporting a positive test increased over time,” he explained.
Almario also noted that this study did not directly compare digestive disorder rates between infected and uninfected individuals. However, a separate study by the Cedars-Sinai team currently undergoing peer review addresses that question more directly. “That study, along with several other studies, show that having COVID increases the risk of developing conditions like IBS and functional dyspepsia,” Almario said.
Taken together, the findings “underscore the increasing healthcare and economic burden of DGBI in the post-pandemic era, emphasizing the need for targeted efforts to effectively diagnose and manage these complex conditions,” they wrote.
“This will be especially challenging for healthcare systems to address, given the existing shortage of primary care physicians and gastroenterologists — clinicians who primarily manage individuals with DGBI,” they noted.
Support for this study was received from Ironwood Pharmaceuticals and Salix Pharmaceuticals in the form of institutional research grants to Cedars-Sinai. Almario has consulted for Exact Sciences, Greenspace Labs, Owlstone Medical, Salix Pharmaceuticals, and Universal DX.
A version of this article appeared on Medscape.com.
, with a near doubling of the national rate of IBS over 2 years, a study has found.
The uptick is probably due to not only the direct impact of SARS-CoV-2 infection on the gastrointestinal tract but also to the psychological stress associated with pandemic life, the study team said.
“COVID infection itself can definitely cause gastrointestinal symptoms like diarrhea, nausea, and abdominal pain — and for some people, those symptoms can linger and lead to chronic conditions like IBS,” Christopher V. Almario, MD, MSHPM, lead author and gastroenterologist at Cedars-Sinai Medical Center, Los Angeles, California, told GI & Hepatology News.
“But the stress of living through the pandemic — lockdowns, fear, isolation — also likely played a major role as well in the increased prevalence of digestive disorders. Both the infection itself and the psychological toll of the pandemic can disrupt the gut-brain axis and trigger chronic digestive disorders like IBS,” Almario said.
The study was published in Neurogastroenterology & Motility.
Growing Burden of Gut Disorders
Disorders of gut-brain interaction (DGBIs) are a heterogeneous group of conditions in which gastrointestinal symptoms occur without any detectable structural or biochemical abnormalities in the digestive tract. They include IBS, functional dyspepsia, and chronic idiopathic constipation, among others.
DGBIs are highly prevalent. Research has shown that nearly 40% of people in the US meet Rome IV criteria for at least one DGBI.
Almario and colleagues assessed trends in prevalence of these conditions during the COVID-19 pandemic. Starting in May 2020 through May 2022, they conducted a series of online surveys with more than 160,000 adults aged 18 or older using validated Rome IV diagnostic questionnaires.
Results showed that during the pandemic, IBS prevalence rose from 6.1% in May 2020 to 11.0% by May 2022, an increase of 0.188% per month (adjusted P < .001).
Chronic idiopathic constipation showed a smaller but statistically significant increase, from 6.0% to 6.4% (0.056% per month; adjusted P < .001).
Within the IBS subtypes, mixed-type IBS showed the largest relative increase (0.085% per month), followed by IBS with constipation (0.041% per month) and IBS with diarrhea (0.037% per month).
There were no significant changes in the prevalence of other DGBIs, such as functional bloating, functional diarrhea, or functional dyspepsia, during the study period.
Almario told GI & Hepatology News only about 9% of those surveyed reported a positive COVID test at the time of the surveys, but that figure probably underrepresents actual infections, especially in the early months of the pandemic. “Most of the survey responses came in during the earlier phases of the pandemic, and the percentage reporting a positive test increased over time,” he explained.
Almario also noted that this study did not directly compare digestive disorder rates between infected and uninfected individuals. However, a separate study by the Cedars-Sinai team currently undergoing peer review addresses that question more directly. “That study, along with several other studies, show that having COVID increases the risk of developing conditions like IBS and functional dyspepsia,” Almario said.
Taken together, the findings “underscore the increasing healthcare and economic burden of DGBI in the post-pandemic era, emphasizing the need for targeted efforts to effectively diagnose and manage these complex conditions,” they wrote.
“This will be especially challenging for healthcare systems to address, given the existing shortage of primary care physicians and gastroenterologists — clinicians who primarily manage individuals with DGBI,” they noted.
Support for this study was received from Ironwood Pharmaceuticals and Salix Pharmaceuticals in the form of institutional research grants to Cedars-Sinai. Almario has consulted for Exact Sciences, Greenspace Labs, Owlstone Medical, Salix Pharmaceuticals, and Universal DX.
A version of this article appeared on Medscape.com.
FDA Issues Early Alert for Medtronic pH-Monitoring Capsules
The notice follows two letters sent in June to customers by the devices’ manufacturer Medtronic and its subsidiary Given Imaging Inc., recommending that customers using certain Bravo CF Capsule Delivery Devices (lot numbers below) for esophageal pH monitoring be removed from all sites of use and sale.
All three of the capsule models listed below are thought to pose a potential risk because the capsules fail to attach to the esophagus’s mucosal wall or to detach from the delivery device as intended owing to a misapplication of adhesive during manufacture. The devices transmit pH data to a recorder attached to the waist of the patient, who interacts with the recorder to indicate symptoms, thereby allowing the physician to compare the symptoms with the occurrence of reflux episodes.
Risks associated with the devices include aspiration/inhalation, perforation of the esophagus, obstruction of the airway, hemorrhage/blood loss/bleeding, laceration of the esophagus, a delay in diagnosis, and foreign bodies remaining in the patient.
Medtronic has reported 33 serious injuries but no deaths associated with the devices.
The lot numbers of the three affected units, which should be identified and quarantined immediately are:
- Bravo CF Capsule Delivery Device, 5-pk, Product Number FGS-0635, Unique Device Identifier-Device Identifier (UDI-DI) 07290101369707
- Bravo CF Capsule Delivery Device 5-pk, FGS-0635, UDI-DI 10613994000009
- Bravo CF Capsule Delivery Device 1-pk, FGS-0636, UDI-DI 07290101369714
These lot identifiers can be found on both the 5-pks’ FGS-0635 outer labels and on the 1-pk FGS-036 individual unit. Customers are advised to return all unused affected products to Medtronic for replacement or credit. In addition, they should pass on this notice to all those who need to be aware within their organizations or to any organizations to which the affected products have been distributed.
They are also advised to check the FDA recall website above for updates as it continues to review information about this potentially high-risk device issue.
Healthcare professionals with concerns or reports of adverse events can contact Medtronic at 800-448-3644 or MedWatch: The FDA Safety Information and Adverse Event Reporting Program.
A version of this article appeared on Medscape.com.
The notice follows two letters sent in June to customers by the devices’ manufacturer Medtronic and its subsidiary Given Imaging Inc., recommending that customers using certain Bravo CF Capsule Delivery Devices (lot numbers below) for esophageal pH monitoring be removed from all sites of use and sale.
All three of the capsule models listed below are thought to pose a potential risk because the capsules fail to attach to the esophagus’s mucosal wall or to detach from the delivery device as intended owing to a misapplication of adhesive during manufacture. The devices transmit pH data to a recorder attached to the waist of the patient, who interacts with the recorder to indicate symptoms, thereby allowing the physician to compare the symptoms with the occurrence of reflux episodes.
Risks associated with the devices include aspiration/inhalation, perforation of the esophagus, obstruction of the airway, hemorrhage/blood loss/bleeding, laceration of the esophagus, a delay in diagnosis, and foreign bodies remaining in the patient.
Medtronic has reported 33 serious injuries but no deaths associated with the devices.
The lot numbers of the three affected units, which should be identified and quarantined immediately are:
- Bravo CF Capsule Delivery Device, 5-pk, Product Number FGS-0635, Unique Device Identifier-Device Identifier (UDI-DI) 07290101369707
- Bravo CF Capsule Delivery Device 5-pk, FGS-0635, UDI-DI 10613994000009
- Bravo CF Capsule Delivery Device 1-pk, FGS-0636, UDI-DI 07290101369714
These lot identifiers can be found on both the 5-pks’ FGS-0635 outer labels and on the 1-pk FGS-036 individual unit. Customers are advised to return all unused affected products to Medtronic for replacement or credit. In addition, they should pass on this notice to all those who need to be aware within their organizations or to any organizations to which the affected products have been distributed.
They are also advised to check the FDA recall website above for updates as it continues to review information about this potentially high-risk device issue.
Healthcare professionals with concerns or reports of adverse events can contact Medtronic at 800-448-3644 or MedWatch: The FDA Safety Information and Adverse Event Reporting Program.
A version of this article appeared on Medscape.com.
The notice follows two letters sent in June to customers by the devices’ manufacturer Medtronic and its subsidiary Given Imaging Inc., recommending that customers using certain Bravo CF Capsule Delivery Devices (lot numbers below) for esophageal pH monitoring be removed from all sites of use and sale.
All three of the capsule models listed below are thought to pose a potential risk because the capsules fail to attach to the esophagus’s mucosal wall or to detach from the delivery device as intended owing to a misapplication of adhesive during manufacture. The devices transmit pH data to a recorder attached to the waist of the patient, who interacts with the recorder to indicate symptoms, thereby allowing the physician to compare the symptoms with the occurrence of reflux episodes.
Risks associated with the devices include aspiration/inhalation, perforation of the esophagus, obstruction of the airway, hemorrhage/blood loss/bleeding, laceration of the esophagus, a delay in diagnosis, and foreign bodies remaining in the patient.
Medtronic has reported 33 serious injuries but no deaths associated with the devices.
The lot numbers of the three affected units, which should be identified and quarantined immediately are:
- Bravo CF Capsule Delivery Device, 5-pk, Product Number FGS-0635, Unique Device Identifier-Device Identifier (UDI-DI) 07290101369707
- Bravo CF Capsule Delivery Device 5-pk, FGS-0635, UDI-DI 10613994000009
- Bravo CF Capsule Delivery Device 1-pk, FGS-0636, UDI-DI 07290101369714
These lot identifiers can be found on both the 5-pks’ FGS-0635 outer labels and on the 1-pk FGS-036 individual unit. Customers are advised to return all unused affected products to Medtronic for replacement or credit. In addition, they should pass on this notice to all those who need to be aware within their organizations or to any organizations to which the affected products have been distributed.
They are also advised to check the FDA recall website above for updates as it continues to review information about this potentially high-risk device issue.
Healthcare professionals with concerns or reports of adverse events can contact Medtronic at 800-448-3644 or MedWatch: The FDA Safety Information and Adverse Event Reporting Program.
A version of this article appeared on Medscape.com.
Help Sustain GI Research
Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
Treatment options for digestive diseases begin with rigorous research, but the limited funding available for physician-scientists to conduct research puts the field at risk of losing talented investigators.
As an AGA member, you have the power to make a difference. By increasing the number of talented women and men doing state-of-the-art research, you can help improve care for all patients suffering from digestive diseases.
Your gift to the AGA Research Foundation will catalyze discovery and career growth for a promising researcher in gastroenterology and hepatology. Please help us fund the next generation of GI researchers by donating today at https://foundation.gastro.org.
Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
Treatment options for digestive diseases begin with rigorous research, but the limited funding available for physician-scientists to conduct research puts the field at risk of losing talented investigators.
As an AGA member, you have the power to make a difference. By increasing the number of talented women and men doing state-of-the-art research, you can help improve care for all patients suffering from digestive diseases.
Your gift to the AGA Research Foundation will catalyze discovery and career growth for a promising researcher in gastroenterology and hepatology. Please help us fund the next generation of GI researchers by donating today at https://foundation.gastro.org.
Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
Treatment options for digestive diseases begin with rigorous research, but the limited funding available for physician-scientists to conduct research puts the field at risk of losing talented investigators.
As an AGA member, you have the power to make a difference. By increasing the number of talented women and men doing state-of-the-art research, you can help improve care for all patients suffering from digestive diseases.
Your gift to the AGA Research Foundation will catalyze discovery and career growth for a promising researcher in gastroenterology and hepatology. Please help us fund the next generation of GI researchers by donating today at https://foundation.gastro.org.
Impact of Rapid Blood Culture Identification on Antibiotic De-escalation at a Veterans Affairs Medical Center
Impact of Rapid Blood Culture Identification on Antibiotic De-escalation at a Veterans Affairs Medical Center
About 530,000 to 628,000 episodes of bloodstream infections (BSI) occur annually in the US.1 Early identification and treatment of bacteremia are essential to improve patient outcomes because it allows for more timely targeted antibiotic therapy.2 Organism identification and susceptibility testing can take 2 to 5 days, prolonging the use of broad-spectrum empiric antibiotics and increasing the risk of adverse events.3,4 The Infectious Disease Society of America recommends the use of rapid diagnostic testing and antimicrobial stewardship programs (ASPs) to improve rates of antibiotic susceptibilities to targeted antibiotics and optimize resource utilization.3 Rapid blood culture identification (BCID) technologies reduce the duration of empiric antibiotics in patients with contaminated blood cultures, resulting in shorter hospital stays and saving money per each patient tested.4
In March 2023, Veteran Health Indiana (VHI) implemented the BioFire FilmArray Blood Culture Identification (BCID2), a BSI panel test that identifies select gram-negative bacteria, gram-positive bacteria, yeast, and antimicrobial resistance genes with an aggregate sensitivity of 99% and a specificity of 99.8%. The BCID2 presents clinically relevant information faster than traditional culture methods, allowing clinicians to make more efficient and educated antibiotic regimen decisions than with previous methods.5
It takes 24 to 48 hours from blood collection for culture incubation, positivity, and gram staining to occur at VHI. If the gram stain is positive, the blood culture is placed on the BioFire BCID2 in addition to traditional culture medium. BioFire BCID2 results are ready in 45 to 60 minutes. Results are uploaded into the electronic health record (EHR) ≤ 2 hours after they are obtained and the primary team is notified if the test is positive for certain critical results. Susceptibility testing of an identified organism typically requires an additional 24 to 48 hours for finalization. VHI Infectious Disease created an evidence-based antibiotic recommendation chart for certain medication(s) and alternate therapies based on the reported organism and its interpreted presence of resistance markers (eg, ceftriaxone for Escherichia coli when extended-spectrum beta lactamases are not detected vs meropenem if extended-spectrum beta lactamases marker are present). These charts optimize the antibiotic regimen while awaiting susceptibility finalizations.
Two previous studies describe the impact of rapid diagnostic testing technology at US Department of Veterans Affairs (VA) medical centers.6,7 In Texas, the ASP reviewed BCID panel results via clinical decision support software for about 1 hour per day.6 A Los Angeles study analyzed the impact of Biofire BCID with an interpretation guide centered on unnecessary vancomycin use and determined that shorter duration of the medication may have been the result of more frequent infectious disease consultation.7
This study assessed the time to optimal antibiotic de-escalation before and after the implementation of BioFire BCID2 with results reviewed by the ASP without active notification or assistance of any clinical decision support technology. The primary objective was to evaluate difference in time to optimal antibiotics from blood culture draw pre- vs postintervention. Secondary objectives included differences in time to organism identification, difference in time on broad-spectrum antibiotics, and difference in time to appropriate antibiotics.
Methods
This quasi-experimental retrospective chart review assessed the impact of BioFire BCID2 use on timely antibiotic de-escalation for patients who experienced a BSI at VHI between March 1, 2022, and October 1, 2023. Microbiology laboratory records identified eligible patients with positive blood cultures within the study time frame. Data were collected from the VHI EHR.
Patients were included if they had a positive bacterial blood culture and received ≥ 1 antibiotic indicated for bacteremia while receiving inpatient care. Patients were excluded if they died prior to blood culture results, transferred out of VHI, left against medical advice, or had untreated contaminants in blood culture results (ie, never received antibiotics aimed at the contaminated culture).
Patient lists were generated for before and after implementation of BioFire BCID2 (pre- and postintervention) using the VHI EHR and microbiology laboratory record system. The pre- and postinterventions groups were different sizes. As a result, a random sampling of the preintervention group was selected and included patients from March 1, 2022, through March 26, 2023. The postintervention group was smaller due to time constraints between initiation of BioFire BCID2 for data collection and included all patients from March 27, 2023, through October 1, 2023.
Optimal antibiotics were defined as escalation from inappropriate therapy to broader agent(s), de-escalation from broad-spectrum therapy to targeted agent(s), discontinuation of therapy due to an organism being identified as a contaminant, or optimization of a regimen to the preferred antimicrobial agent based on evidence-based consensus guidelines. Broad-spectrum antibiotics included: piperacillin/tazobactam, cefepime, ceftazidime, ceftazidime-avibactam, cefiderocol, carbapenems, fluroquinolones, vancomycin, daptomycin, ceftaroline, linezolid, or aztreonam. Appropriate antibiotics were defined as those with activity toward the final identified organism(s).
Deidentified participant data were entered into Microsoft Excel and kept on a secure VA server to complete statistical analyses. Parametric continuous data, such as age, were analyzed using the t-test, while nonparametric continuous data, such as time to optimal antibiotics, were analyzed using the Mann-Whitney U test. Categorical data, like sex and race, were analyzed using either Fisher exact test for small sample sizes or X2 test for a larger sample size. Statistical significance levels was defined as P < .05.
Results
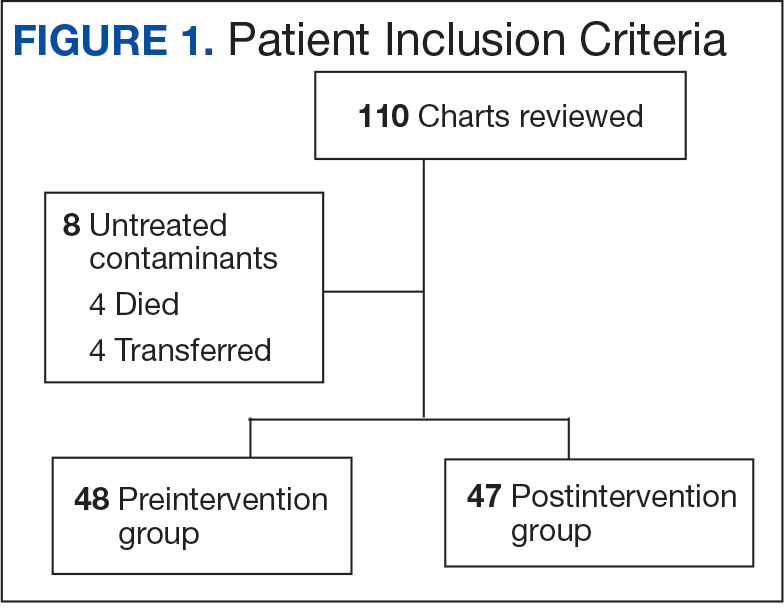
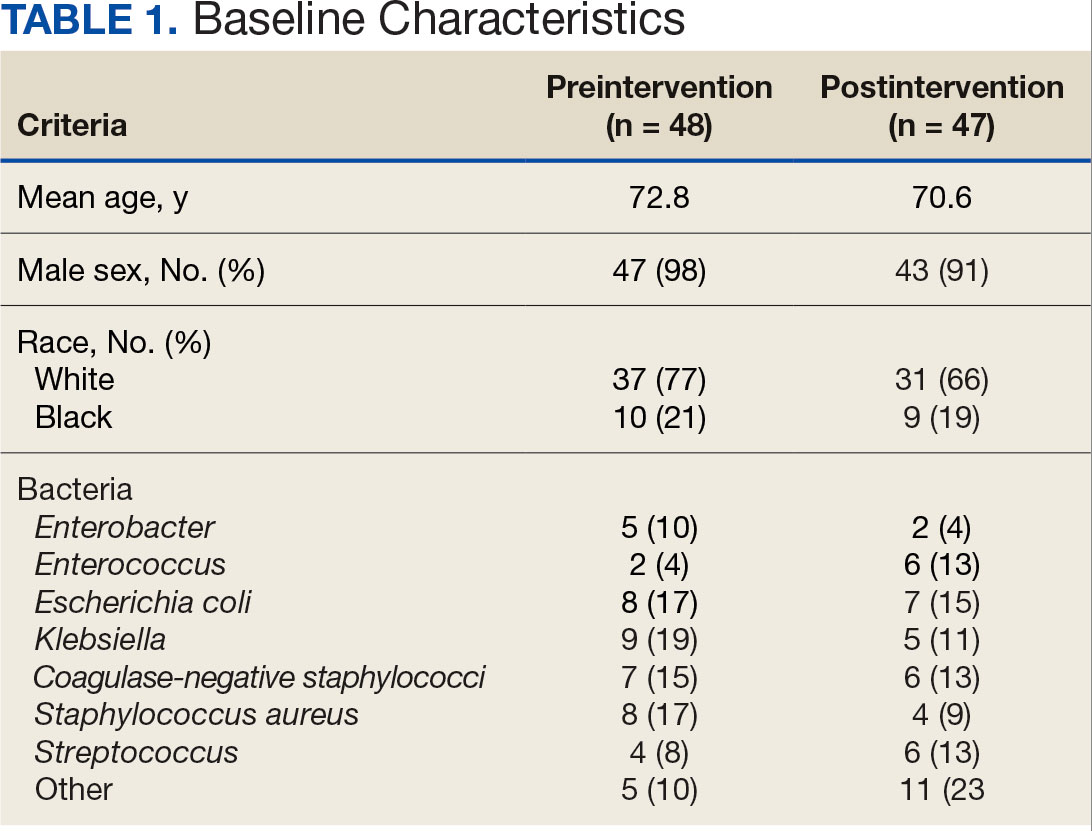
Using patient lists drawn from the EHR and the microbiology laboratory records, 110 electronic charts were randomly selected for review. Fifteen patients were excluded: 8 had untreated contaminants, 4 died, and 3 were transferred out of VHI. Of the 95 patients included, 48 were in the preintervention group and 47 were in the postintervention group (Figure 1).
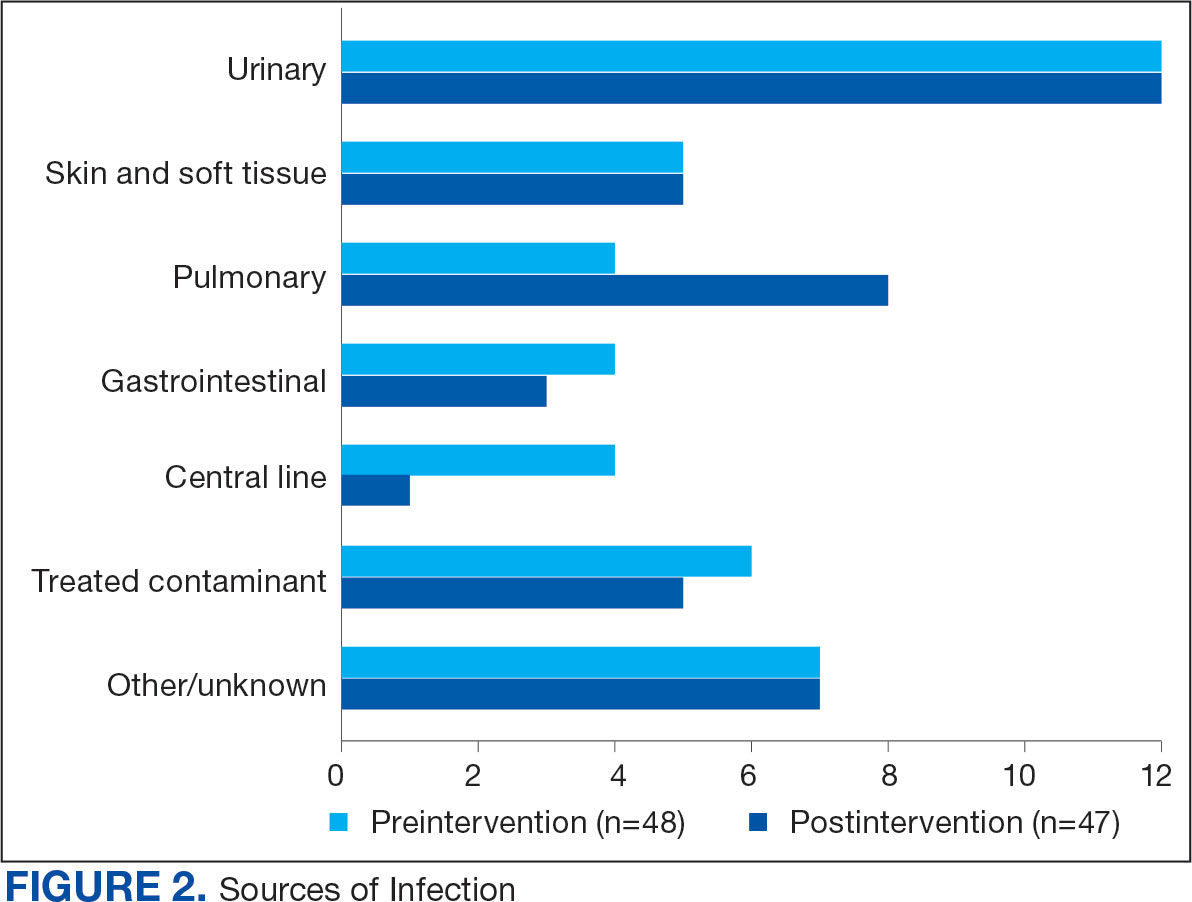
Baseline characteristics were similar between the 2 groups (Table 1). Most patients were White males aged > 70 years in the EHR. The urinary tract was the most common source of infection, impacting 12 patients in each group (Figure 2). Escherichia coli, Klebsiella, Staphylococcus, and Streptococcus were the most common bloodstream isolates identified.
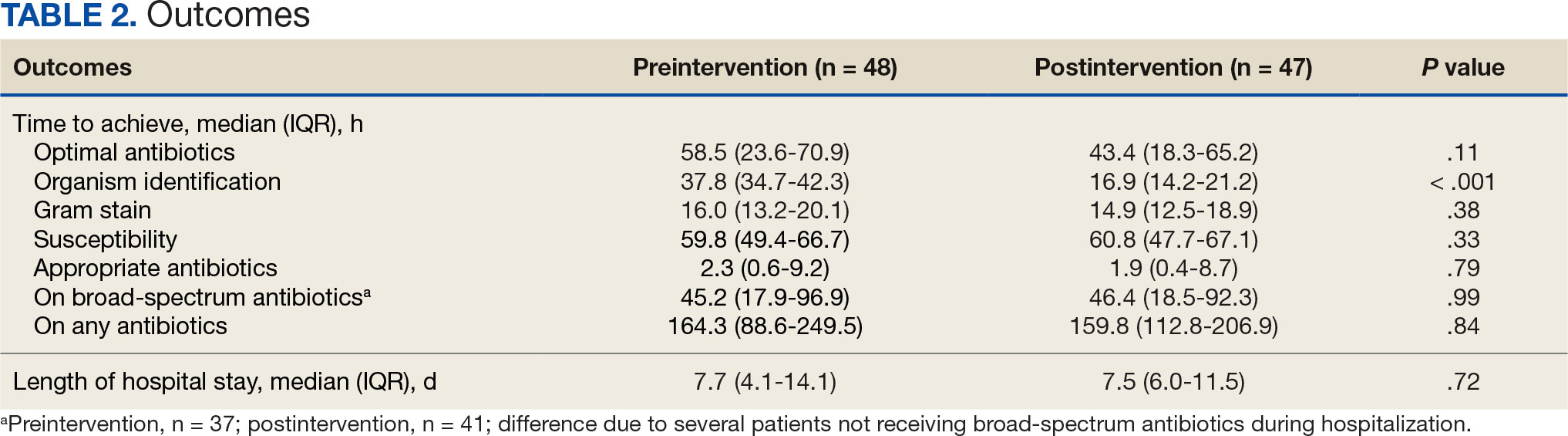
The median time to optimal antibiotics in the preintervention group was 58.5 hours vs 43.4 hours in the postintervention group (P = .11). The median time to organism identification was 37.8 hours in the preintervention group vs 16.9 hours in the postintervention group (P < .001). The median time on broad-spectrum antibiotics was 45.2 hours in the preintervention group vs 46.6 hours in the postintervention group (P = .99). The median time on appropriate antibiotics in the preintervention group was 2.3 hours vs 1.9 hours in the postintervention group (P = .79). Differences in other measured outcomes between the groups were not statistically significant (Table 2).
Although implementation of rapid diagnostic technology reduced the median time to optimal antibiotics, the results were not statistically significant. Shorter time to organism identification in the postintervention group compared to the preintervention group was the lone statistically significant metric (P < .001).
Discussion
A lack of statistical significance in the primary outcome may have been due to nonadherence to facility de-escalation protocols or a suboptimal BioFire BCID2 result notification system. Additionally, use of rapid BCID at VHI may improve over time as clinicians become more familiar with the technology. Gaps in clinical pharmacy coverage during the night shift may have also contributed to delays in antibiotic optimization, particularly if other clinicians are not equipped with the knowledge or training to appropriately deescalate antibiotics based on microorganisms identified. A 2017 study by Donner et al concluded that physician interpretation of BCID results is suboptimal and should be augmented with clinical decision support tools as new technology becomes available.8 Despite the statistically insignificant results of this study, it did highlight potential areas of improvement which can lead to improved patient care.
Previous research has evaluated the impact of rapid BCID technology on antibiotic treatment and clinical outcomes. Chiasson et al found that median time to optimal therapy was 73.8 hours in the pre-BCID arm compared to 34.7 hours in the post- BCID arm (P ≤ .001), emphasizing the importance of combining rapid BCID with clinical decision support tools and pharmacy input.6 Senok et al found that BCID2 implementation led to a significant decrease in median time to culture result, which informed optimal antibiotic therapy and decreased 30-day mortality in the intensive care setting.9 In contrast, the current study did not stratify patients according to medical ward or illness severity even though clinicians may be less likely to de-escalate antibiotic therapy in critically ill patients.
Bae et al reported findings consistent with the current study and concluded that BCID did not affect the clinical outcomes of overall BSIs; however, it contributed to early administration of effective antibiotics in cases of BSIs caused by multidrug-resistant organisms.10 Results of this study were not stratified according to multidrug-resistant organisms because the sample size was too small. The current study also included patients with polymicrobial infections, which may have impacted the results due to a less streamlined approach to antibiotic optimization.
Limitations
This single-center, retrospective study had a small sample size, short time frame, and lacked patient diversity, and therefore may not be generalizable to other health care systems. The sample size was limited by shorter date range and smaller patient list between BioFire BCID2 implementation and data collection, which was used to determine the number of charts selected in each group. Some patients received antibiotics prior to blood cultures being drawn, which may falsely decrease time to optimal/ appropriate antibiotics and falsely increase time on broad spectrum/any antibiotics due to early antibiotic administration. The total number of patients on broad-spectrum antibiotics differed from the total number of patients for other outcomes because several patients never received the defined broad spectrum antibiotics.
Conclusions
When combined with a pre-existing ASP without active notification, the implementation of BioFire BCID2 did not return statistically significant data showing a decrease in time to optimal antibiotics, time to appropriate antibiotics, or time on broad-spectrum antibiotics at VHI. To make this program more successful, pharmacist intervention and clinical decision support tools may be needed.
Additional research is required to determine the optimal integration of antimicrobial stewardship, rapid diagnostic technology, and pharmacy services for maximum benefit. Even though the primary outcome was not statistically significant, the results may be clinically significant from a stewardship perspective. Realigning microbiology workflows to mimic other research, which emphasizes the importance of funneling rapid BCID results through the ASP, may improve outcomes. Future studies may be warranted following the implementation of clinical decision support tools to assess their impact on stewardship practices and patient outcomes.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501- 509. doi:10.1111/1469-0691.12195
- Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis. 2016;84(2):159-164. doi:10.1016/j.diagmicrobio.2015.10.023.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi:10.1093/cid/ciw118
- BIOFIRE® Blood Culture Identification 2 (BCID2) Panel. Biomerierux. Updated 2025. Accessed May 10, 2025. https://www.biofiredx.com/products/the-filmarray-panels/filmarraybcid/
- Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57(9):1237-1245. doi:10.1093/cid/cit498
- Chiasson JM, Smith WJ, Jodlowski TZ, Kouma MA, Cutrell JB. Impact of a rapid blood culture diagnostic panel on time to optimal antimicrobial therapy at a veterans affairs medical center. J Pharm Pract. 2022;35(5):722-729. doi:10.1177/08971900211000686
- Wu S, Watson RL, Graber CJ. 2007. Impact of combining rapid diagnostics with an interpretation guide on vancomycin usage for contaminant blood cultures growing coagulase- negative staphylococci (CoNS). Open Forum Infect Dis. 2019;6(Suppl 2):S674. doi:10.1093/ofid/ofz360.1687
- Donner LM, Campbell WS, Lyden E, Van Schooneveld TC. Assessment of rapid-blood-culture-identification result interpretation and antibiotic prescribing practices. J Clin Microbiol. 2017;55(5):1496-1507. doi:10.1128/JCM.02395-16
- Senok A, Dabal LA, Alfaresi M, et al. Clinical impact of the BIOFIRE blood culture identification 2 panel in adult patients with bloodstream infection: a multicentre observational study in the United Arab Emirates. Diagnostics (Basel). 2023;13(14):2433. doi:10.3390/diagnostics13142433
- Bae JY, Bae J, So MK, Choi HJ, Lee M. The impact of the rapid blood culture identification panel on antibiotic treatment and clinical outcomes in bloodstream infections, particularly those associated with multidrug-resistant micro-organisms. Diagnostics (Basel). 2023;13(23):3504. doi:10.3390/diagnostics13233504
About 530,000 to 628,000 episodes of bloodstream infections (BSI) occur annually in the US.1 Early identification and treatment of bacteremia are essential to improve patient outcomes because it allows for more timely targeted antibiotic therapy.2 Organism identification and susceptibility testing can take 2 to 5 days, prolonging the use of broad-spectrum empiric antibiotics and increasing the risk of adverse events.3,4 The Infectious Disease Society of America recommends the use of rapid diagnostic testing and antimicrobial stewardship programs (ASPs) to improve rates of antibiotic susceptibilities to targeted antibiotics and optimize resource utilization.3 Rapid blood culture identification (BCID) technologies reduce the duration of empiric antibiotics in patients with contaminated blood cultures, resulting in shorter hospital stays and saving money per each patient tested.4
In March 2023, Veteran Health Indiana (VHI) implemented the BioFire FilmArray Blood Culture Identification (BCID2), a BSI panel test that identifies select gram-negative bacteria, gram-positive bacteria, yeast, and antimicrobial resistance genes with an aggregate sensitivity of 99% and a specificity of 99.8%. The BCID2 presents clinically relevant information faster than traditional culture methods, allowing clinicians to make more efficient and educated antibiotic regimen decisions than with previous methods.5
It takes 24 to 48 hours from blood collection for culture incubation, positivity, and gram staining to occur at VHI. If the gram stain is positive, the blood culture is placed on the BioFire BCID2 in addition to traditional culture medium. BioFire BCID2 results are ready in 45 to 60 minutes. Results are uploaded into the electronic health record (EHR) ≤ 2 hours after they are obtained and the primary team is notified if the test is positive for certain critical results. Susceptibility testing of an identified organism typically requires an additional 24 to 48 hours for finalization. VHI Infectious Disease created an evidence-based antibiotic recommendation chart for certain medication(s) and alternate therapies based on the reported organism and its interpreted presence of resistance markers (eg, ceftriaxone for Escherichia coli when extended-spectrum beta lactamases are not detected vs meropenem if extended-spectrum beta lactamases marker are present). These charts optimize the antibiotic regimen while awaiting susceptibility finalizations.
Two previous studies describe the impact of rapid diagnostic testing technology at US Department of Veterans Affairs (VA) medical centers.6,7 In Texas, the ASP reviewed BCID panel results via clinical decision support software for about 1 hour per day.6 A Los Angeles study analyzed the impact of Biofire BCID with an interpretation guide centered on unnecessary vancomycin use and determined that shorter duration of the medication may have been the result of more frequent infectious disease consultation.7
This study assessed the time to optimal antibiotic de-escalation before and after the implementation of BioFire BCID2 with results reviewed by the ASP without active notification or assistance of any clinical decision support technology. The primary objective was to evaluate difference in time to optimal antibiotics from blood culture draw pre- vs postintervention. Secondary objectives included differences in time to organism identification, difference in time on broad-spectrum antibiotics, and difference in time to appropriate antibiotics.
Methods
This quasi-experimental retrospective chart review assessed the impact of BioFire BCID2 use on timely antibiotic de-escalation for patients who experienced a BSI at VHI between March 1, 2022, and October 1, 2023. Microbiology laboratory records identified eligible patients with positive blood cultures within the study time frame. Data were collected from the VHI EHR.
Patients were included if they had a positive bacterial blood culture and received ≥ 1 antibiotic indicated for bacteremia while receiving inpatient care. Patients were excluded if they died prior to blood culture results, transferred out of VHI, left against medical advice, or had untreated contaminants in blood culture results (ie, never received antibiotics aimed at the contaminated culture).
Patient lists were generated for before and after implementation of BioFire BCID2 (pre- and postintervention) using the VHI EHR and microbiology laboratory record system. The pre- and postinterventions groups were different sizes. As a result, a random sampling of the preintervention group was selected and included patients from March 1, 2022, through March 26, 2023. The postintervention group was smaller due to time constraints between initiation of BioFire BCID2 for data collection and included all patients from March 27, 2023, through October 1, 2023.
Optimal antibiotics were defined as escalation from inappropriate therapy to broader agent(s), de-escalation from broad-spectrum therapy to targeted agent(s), discontinuation of therapy due to an organism being identified as a contaminant, or optimization of a regimen to the preferred antimicrobial agent based on evidence-based consensus guidelines. Broad-spectrum antibiotics included: piperacillin/tazobactam, cefepime, ceftazidime, ceftazidime-avibactam, cefiderocol, carbapenems, fluroquinolones, vancomycin, daptomycin, ceftaroline, linezolid, or aztreonam. Appropriate antibiotics were defined as those with activity toward the final identified organism(s).
Deidentified participant data were entered into Microsoft Excel and kept on a secure VA server to complete statistical analyses. Parametric continuous data, such as age, were analyzed using the t-test, while nonparametric continuous data, such as time to optimal antibiotics, were analyzed using the Mann-Whitney U test. Categorical data, like sex and race, were analyzed using either Fisher exact test for small sample sizes or X2 test for a larger sample size. Statistical significance levels was defined as P < .05.
Results
Using patient lists drawn from the EHR and the microbiology laboratory records, 110 electronic charts were randomly selected for review. Fifteen patients were excluded: 8 had untreated contaminants, 4 died, and 3 were transferred out of VHI. Of the 95 patients included, 48 were in the preintervention group and 47 were in the postintervention group (Figure 1).

Baseline characteristics were similar between the 2 groups (Table 1). Most patients were White males aged > 70 years in the EHR. The urinary tract was the most common source of infection, impacting 12 patients in each group (Figure 2). Escherichia coli, Klebsiella, Staphylococcus, and Streptococcus were the most common bloodstream isolates identified.


The median time to optimal antibiotics in the preintervention group was 58.5 hours vs 43.4 hours in the postintervention group (P = .11). The median time to organism identification was 37.8 hours in the preintervention group vs 16.9 hours in the postintervention group (P < .001). The median time on broad-spectrum antibiotics was 45.2 hours in the preintervention group vs 46.6 hours in the postintervention group (P = .99). The median time on appropriate antibiotics in the preintervention group was 2.3 hours vs 1.9 hours in the postintervention group (P = .79). Differences in other measured outcomes between the groups were not statistically significant (Table 2).

Although implementation of rapid diagnostic technology reduced the median time to optimal antibiotics, the results were not statistically significant. Shorter time to organism identification in the postintervention group compared to the preintervention group was the lone statistically significant metric (P < .001).
Discussion
A lack of statistical significance in the primary outcome may have been due to nonadherence to facility de-escalation protocols or a suboptimal BioFire BCID2 result notification system. Additionally, use of rapid BCID at VHI may improve over time as clinicians become more familiar with the technology. Gaps in clinical pharmacy coverage during the night shift may have also contributed to delays in antibiotic optimization, particularly if other clinicians are not equipped with the knowledge or training to appropriately deescalate antibiotics based on microorganisms identified. A 2017 study by Donner et al concluded that physician interpretation of BCID results is suboptimal and should be augmented with clinical decision support tools as new technology becomes available.8 Despite the statistically insignificant results of this study, it did highlight potential areas of improvement which can lead to improved patient care.
Previous research has evaluated the impact of rapid BCID technology on antibiotic treatment and clinical outcomes. Chiasson et al found that median time to optimal therapy was 73.8 hours in the pre-BCID arm compared to 34.7 hours in the post- BCID arm (P ≤ .001), emphasizing the importance of combining rapid BCID with clinical decision support tools and pharmacy input.6 Senok et al found that BCID2 implementation led to a significant decrease in median time to culture result, which informed optimal antibiotic therapy and decreased 30-day mortality in the intensive care setting.9 In contrast, the current study did not stratify patients according to medical ward or illness severity even though clinicians may be less likely to de-escalate antibiotic therapy in critically ill patients.
Bae et al reported findings consistent with the current study and concluded that BCID did not affect the clinical outcomes of overall BSIs; however, it contributed to early administration of effective antibiotics in cases of BSIs caused by multidrug-resistant organisms.10 Results of this study were not stratified according to multidrug-resistant organisms because the sample size was too small. The current study also included patients with polymicrobial infections, which may have impacted the results due to a less streamlined approach to antibiotic optimization.
Limitations
This single-center, retrospective study had a small sample size, short time frame, and lacked patient diversity, and therefore may not be generalizable to other health care systems. The sample size was limited by shorter date range and smaller patient list between BioFire BCID2 implementation and data collection, which was used to determine the number of charts selected in each group. Some patients received antibiotics prior to blood cultures being drawn, which may falsely decrease time to optimal/ appropriate antibiotics and falsely increase time on broad spectrum/any antibiotics due to early antibiotic administration. The total number of patients on broad-spectrum antibiotics differed from the total number of patients for other outcomes because several patients never received the defined broad spectrum antibiotics.
Conclusions
When combined with a pre-existing ASP without active notification, the implementation of BioFire BCID2 did not return statistically significant data showing a decrease in time to optimal antibiotics, time to appropriate antibiotics, or time on broad-spectrum antibiotics at VHI. To make this program more successful, pharmacist intervention and clinical decision support tools may be needed.
Additional research is required to determine the optimal integration of antimicrobial stewardship, rapid diagnostic technology, and pharmacy services for maximum benefit. Even though the primary outcome was not statistically significant, the results may be clinically significant from a stewardship perspective. Realigning microbiology workflows to mimic other research, which emphasizes the importance of funneling rapid BCID results through the ASP, may improve outcomes. Future studies may be warranted following the implementation of clinical decision support tools to assess their impact on stewardship practices and patient outcomes.
About 530,000 to 628,000 episodes of bloodstream infections (BSI) occur annually in the US.1 Early identification and treatment of bacteremia are essential to improve patient outcomes because it allows for more timely targeted antibiotic therapy.2 Organism identification and susceptibility testing can take 2 to 5 days, prolonging the use of broad-spectrum empiric antibiotics and increasing the risk of adverse events.3,4 The Infectious Disease Society of America recommends the use of rapid diagnostic testing and antimicrobial stewardship programs (ASPs) to improve rates of antibiotic susceptibilities to targeted antibiotics and optimize resource utilization.3 Rapid blood culture identification (BCID) technologies reduce the duration of empiric antibiotics in patients with contaminated blood cultures, resulting in shorter hospital stays and saving money per each patient tested.4
In March 2023, Veteran Health Indiana (VHI) implemented the BioFire FilmArray Blood Culture Identification (BCID2), a BSI panel test that identifies select gram-negative bacteria, gram-positive bacteria, yeast, and antimicrobial resistance genes with an aggregate sensitivity of 99% and a specificity of 99.8%. The BCID2 presents clinically relevant information faster than traditional culture methods, allowing clinicians to make more efficient and educated antibiotic regimen decisions than with previous methods.5
It takes 24 to 48 hours from blood collection for culture incubation, positivity, and gram staining to occur at VHI. If the gram stain is positive, the blood culture is placed on the BioFire BCID2 in addition to traditional culture medium. BioFire BCID2 results are ready in 45 to 60 minutes. Results are uploaded into the electronic health record (EHR) ≤ 2 hours after they are obtained and the primary team is notified if the test is positive for certain critical results. Susceptibility testing of an identified organism typically requires an additional 24 to 48 hours for finalization. VHI Infectious Disease created an evidence-based antibiotic recommendation chart for certain medication(s) and alternate therapies based on the reported organism and its interpreted presence of resistance markers (eg, ceftriaxone for Escherichia coli when extended-spectrum beta lactamases are not detected vs meropenem if extended-spectrum beta lactamases marker are present). These charts optimize the antibiotic regimen while awaiting susceptibility finalizations.
Two previous studies describe the impact of rapid diagnostic testing technology at US Department of Veterans Affairs (VA) medical centers.6,7 In Texas, the ASP reviewed BCID panel results via clinical decision support software for about 1 hour per day.6 A Los Angeles study analyzed the impact of Biofire BCID with an interpretation guide centered on unnecessary vancomycin use and determined that shorter duration of the medication may have been the result of more frequent infectious disease consultation.7
This study assessed the time to optimal antibiotic de-escalation before and after the implementation of BioFire BCID2 with results reviewed by the ASP without active notification or assistance of any clinical decision support technology. The primary objective was to evaluate difference in time to optimal antibiotics from blood culture draw pre- vs postintervention. Secondary objectives included differences in time to organism identification, difference in time on broad-spectrum antibiotics, and difference in time to appropriate antibiotics.
Methods
This quasi-experimental retrospective chart review assessed the impact of BioFire BCID2 use on timely antibiotic de-escalation for patients who experienced a BSI at VHI between March 1, 2022, and October 1, 2023. Microbiology laboratory records identified eligible patients with positive blood cultures within the study time frame. Data were collected from the VHI EHR.
Patients were included if they had a positive bacterial blood culture and received ≥ 1 antibiotic indicated for bacteremia while receiving inpatient care. Patients were excluded if they died prior to blood culture results, transferred out of VHI, left against medical advice, or had untreated contaminants in blood culture results (ie, never received antibiotics aimed at the contaminated culture).
Patient lists were generated for before and after implementation of BioFire BCID2 (pre- and postintervention) using the VHI EHR and microbiology laboratory record system. The pre- and postinterventions groups were different sizes. As a result, a random sampling of the preintervention group was selected and included patients from March 1, 2022, through March 26, 2023. The postintervention group was smaller due to time constraints between initiation of BioFire BCID2 for data collection and included all patients from March 27, 2023, through October 1, 2023.
Optimal antibiotics were defined as escalation from inappropriate therapy to broader agent(s), de-escalation from broad-spectrum therapy to targeted agent(s), discontinuation of therapy due to an organism being identified as a contaminant, or optimization of a regimen to the preferred antimicrobial agent based on evidence-based consensus guidelines. Broad-spectrum antibiotics included: piperacillin/tazobactam, cefepime, ceftazidime, ceftazidime-avibactam, cefiderocol, carbapenems, fluroquinolones, vancomycin, daptomycin, ceftaroline, linezolid, or aztreonam. Appropriate antibiotics were defined as those with activity toward the final identified organism(s).
Deidentified participant data were entered into Microsoft Excel and kept on a secure VA server to complete statistical analyses. Parametric continuous data, such as age, were analyzed using the t-test, while nonparametric continuous data, such as time to optimal antibiotics, were analyzed using the Mann-Whitney U test. Categorical data, like sex and race, were analyzed using either Fisher exact test for small sample sizes or X2 test for a larger sample size. Statistical significance levels was defined as P < .05.
Results
Using patient lists drawn from the EHR and the microbiology laboratory records, 110 electronic charts were randomly selected for review. Fifteen patients were excluded: 8 had untreated contaminants, 4 died, and 3 were transferred out of VHI. Of the 95 patients included, 48 were in the preintervention group and 47 were in the postintervention group (Figure 1).

Baseline characteristics were similar between the 2 groups (Table 1). Most patients were White males aged > 70 years in the EHR. The urinary tract was the most common source of infection, impacting 12 patients in each group (Figure 2). Escherichia coli, Klebsiella, Staphylococcus, and Streptococcus were the most common bloodstream isolates identified.


The median time to optimal antibiotics in the preintervention group was 58.5 hours vs 43.4 hours in the postintervention group (P = .11). The median time to organism identification was 37.8 hours in the preintervention group vs 16.9 hours in the postintervention group (P < .001). The median time on broad-spectrum antibiotics was 45.2 hours in the preintervention group vs 46.6 hours in the postintervention group (P = .99). The median time on appropriate antibiotics in the preintervention group was 2.3 hours vs 1.9 hours in the postintervention group (P = .79). Differences in other measured outcomes between the groups were not statistically significant (Table 2).

Although implementation of rapid diagnostic technology reduced the median time to optimal antibiotics, the results were not statistically significant. Shorter time to organism identification in the postintervention group compared to the preintervention group was the lone statistically significant metric (P < .001).
Discussion
A lack of statistical significance in the primary outcome may have been due to nonadherence to facility de-escalation protocols or a suboptimal BioFire BCID2 result notification system. Additionally, use of rapid BCID at VHI may improve over time as clinicians become more familiar with the technology. Gaps in clinical pharmacy coverage during the night shift may have also contributed to delays in antibiotic optimization, particularly if other clinicians are not equipped with the knowledge or training to appropriately deescalate antibiotics based on microorganisms identified. A 2017 study by Donner et al concluded that physician interpretation of BCID results is suboptimal and should be augmented with clinical decision support tools as new technology becomes available.8 Despite the statistically insignificant results of this study, it did highlight potential areas of improvement which can lead to improved patient care.
Previous research has evaluated the impact of rapid BCID technology on antibiotic treatment and clinical outcomes. Chiasson et al found that median time to optimal therapy was 73.8 hours in the pre-BCID arm compared to 34.7 hours in the post- BCID arm (P ≤ .001), emphasizing the importance of combining rapid BCID with clinical decision support tools and pharmacy input.6 Senok et al found that BCID2 implementation led to a significant decrease in median time to culture result, which informed optimal antibiotic therapy and decreased 30-day mortality in the intensive care setting.9 In contrast, the current study did not stratify patients according to medical ward or illness severity even though clinicians may be less likely to de-escalate antibiotic therapy in critically ill patients.
Bae et al reported findings consistent with the current study and concluded that BCID did not affect the clinical outcomes of overall BSIs; however, it contributed to early administration of effective antibiotics in cases of BSIs caused by multidrug-resistant organisms.10 Results of this study were not stratified according to multidrug-resistant organisms because the sample size was too small. The current study also included patients with polymicrobial infections, which may have impacted the results due to a less streamlined approach to antibiotic optimization.
Limitations
This single-center, retrospective study had a small sample size, short time frame, and lacked patient diversity, and therefore may not be generalizable to other health care systems. The sample size was limited by shorter date range and smaller patient list between BioFire BCID2 implementation and data collection, which was used to determine the number of charts selected in each group. Some patients received antibiotics prior to blood cultures being drawn, which may falsely decrease time to optimal/ appropriate antibiotics and falsely increase time on broad spectrum/any antibiotics due to early antibiotic administration. The total number of patients on broad-spectrum antibiotics differed from the total number of patients for other outcomes because several patients never received the defined broad spectrum antibiotics.
Conclusions
When combined with a pre-existing ASP without active notification, the implementation of BioFire BCID2 did not return statistically significant data showing a decrease in time to optimal antibiotics, time to appropriate antibiotics, or time on broad-spectrum antibiotics at VHI. To make this program more successful, pharmacist intervention and clinical decision support tools may be needed.
Additional research is required to determine the optimal integration of antimicrobial stewardship, rapid diagnostic technology, and pharmacy services for maximum benefit. Even though the primary outcome was not statistically significant, the results may be clinically significant from a stewardship perspective. Realigning microbiology workflows to mimic other research, which emphasizes the importance of funneling rapid BCID results through the ASP, may improve outcomes. Future studies may be warranted following the implementation of clinical decision support tools to assess their impact on stewardship practices and patient outcomes.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501- 509. doi:10.1111/1469-0691.12195
- Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis. 2016;84(2):159-164. doi:10.1016/j.diagmicrobio.2015.10.023.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi:10.1093/cid/ciw118
- BIOFIRE® Blood Culture Identification 2 (BCID2) Panel. Biomerierux. Updated 2025. Accessed May 10, 2025. https://www.biofiredx.com/products/the-filmarray-panels/filmarraybcid/
- Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57(9):1237-1245. doi:10.1093/cid/cit498
- Chiasson JM, Smith WJ, Jodlowski TZ, Kouma MA, Cutrell JB. Impact of a rapid blood culture diagnostic panel on time to optimal antimicrobial therapy at a veterans affairs medical center. J Pharm Pract. 2022;35(5):722-729. doi:10.1177/08971900211000686
- Wu S, Watson RL, Graber CJ. 2007. Impact of combining rapid diagnostics with an interpretation guide on vancomycin usage for contaminant blood cultures growing coagulase- negative staphylococci (CoNS). Open Forum Infect Dis. 2019;6(Suppl 2):S674. doi:10.1093/ofid/ofz360.1687
- Donner LM, Campbell WS, Lyden E, Van Schooneveld TC. Assessment of rapid-blood-culture-identification result interpretation and antibiotic prescribing practices. J Clin Microbiol. 2017;55(5):1496-1507. doi:10.1128/JCM.02395-16
- Senok A, Dabal LA, Alfaresi M, et al. Clinical impact of the BIOFIRE blood culture identification 2 panel in adult patients with bloodstream infection: a multicentre observational study in the United Arab Emirates. Diagnostics (Basel). 2023;13(14):2433. doi:10.3390/diagnostics13142433
- Bae JY, Bae J, So MK, Choi HJ, Lee M. The impact of the rapid blood culture identification panel on antibiotic treatment and clinical outcomes in bloodstream infections, particularly those associated with multidrug-resistant micro-organisms. Diagnostics (Basel). 2023;13(23):3504. doi:10.3390/diagnostics13233504
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501- 509. doi:10.1111/1469-0691.12195
- Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis. 2016;84(2):159-164. doi:10.1016/j.diagmicrobio.2015.10.023.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi:10.1093/cid/ciw118
- BIOFIRE® Blood Culture Identification 2 (BCID2) Panel. Biomerierux. Updated 2025. Accessed May 10, 2025. https://www.biofiredx.com/products/the-filmarray-panels/filmarraybcid/
- Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57(9):1237-1245. doi:10.1093/cid/cit498
- Chiasson JM, Smith WJ, Jodlowski TZ, Kouma MA, Cutrell JB. Impact of a rapid blood culture diagnostic panel on time to optimal antimicrobial therapy at a veterans affairs medical center. J Pharm Pract. 2022;35(5):722-729. doi:10.1177/08971900211000686
- Wu S, Watson RL, Graber CJ. 2007. Impact of combining rapid diagnostics with an interpretation guide on vancomycin usage for contaminant blood cultures growing coagulase- negative staphylococci (CoNS). Open Forum Infect Dis. 2019;6(Suppl 2):S674. doi:10.1093/ofid/ofz360.1687
- Donner LM, Campbell WS, Lyden E, Van Schooneveld TC. Assessment of rapid-blood-culture-identification result interpretation and antibiotic prescribing practices. J Clin Microbiol. 2017;55(5):1496-1507. doi:10.1128/JCM.02395-16
- Senok A, Dabal LA, Alfaresi M, et al. Clinical impact of the BIOFIRE blood culture identification 2 panel in adult patients with bloodstream infection: a multicentre observational study in the United Arab Emirates. Diagnostics (Basel). 2023;13(14):2433. doi:10.3390/diagnostics13142433
- Bae JY, Bae J, So MK, Choi HJ, Lee M. The impact of the rapid blood culture identification panel on antibiotic treatment and clinical outcomes in bloodstream infections, particularly those associated with multidrug-resistant micro-organisms. Diagnostics (Basel). 2023;13(23):3504. doi:10.3390/diagnostics13233504
Impact of Rapid Blood Culture Identification on Antibiotic De-escalation at a Veterans Affairs Medical Center
Impact of Rapid Blood Culture Identification on Antibiotic De-escalation at a Veterans Affairs Medical Center
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.
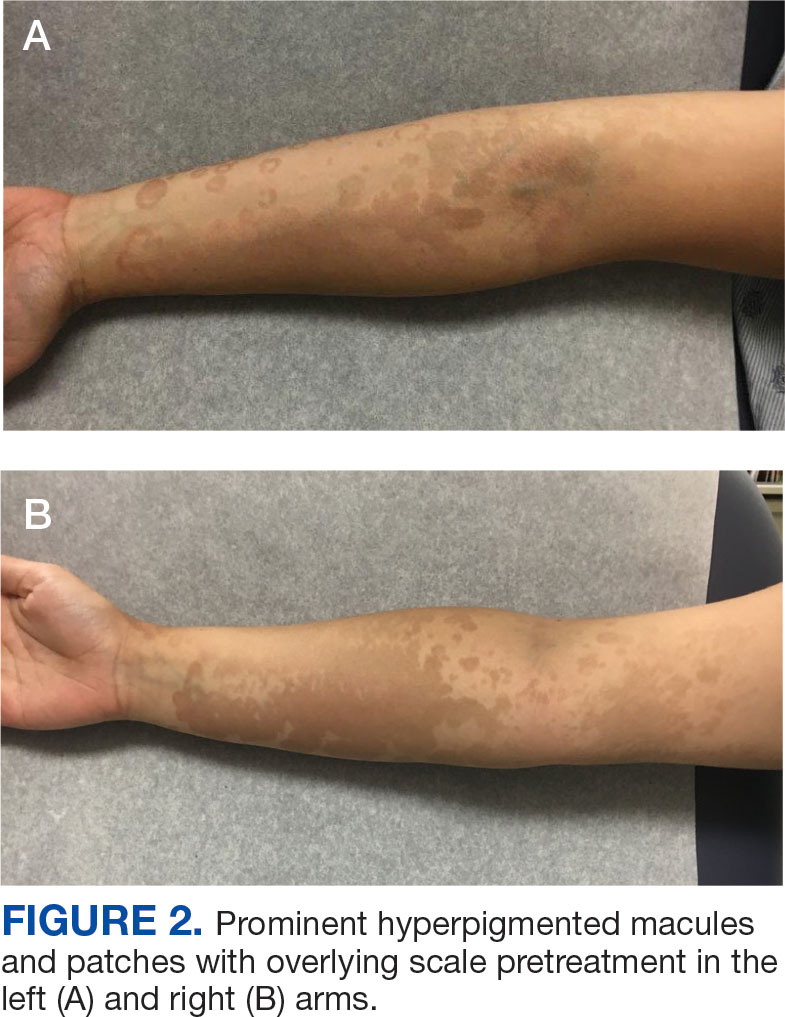
The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.
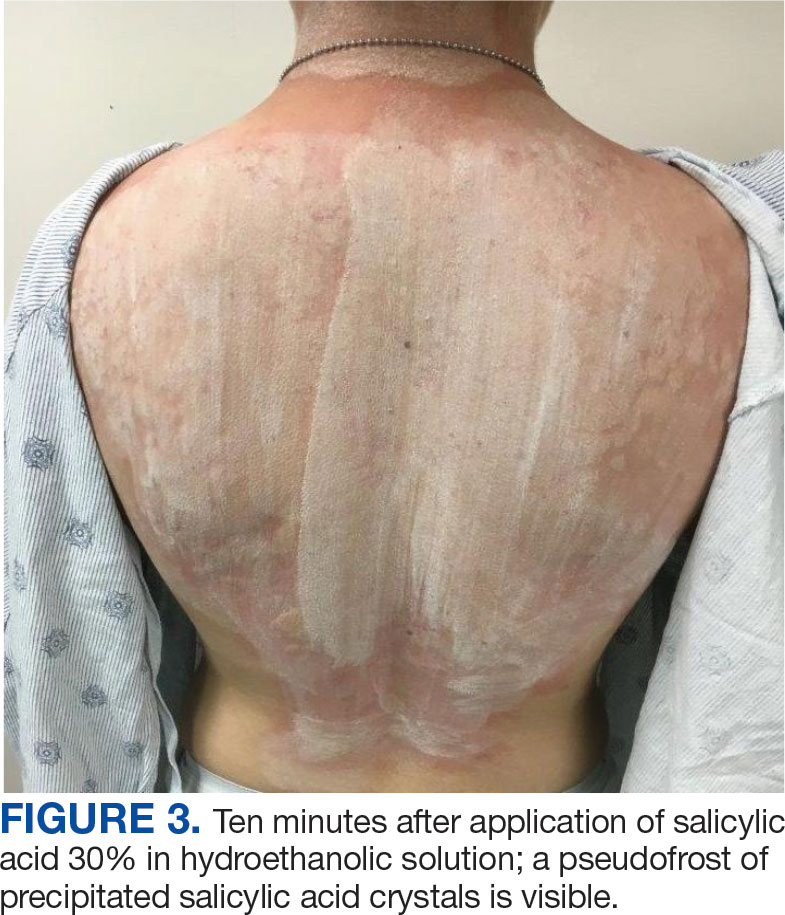
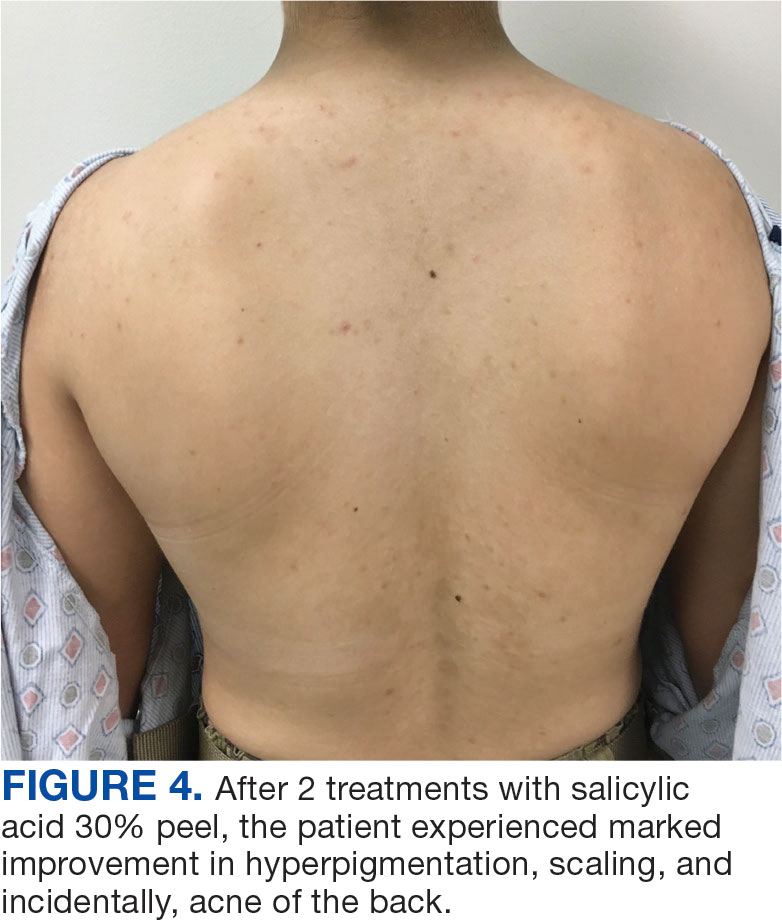
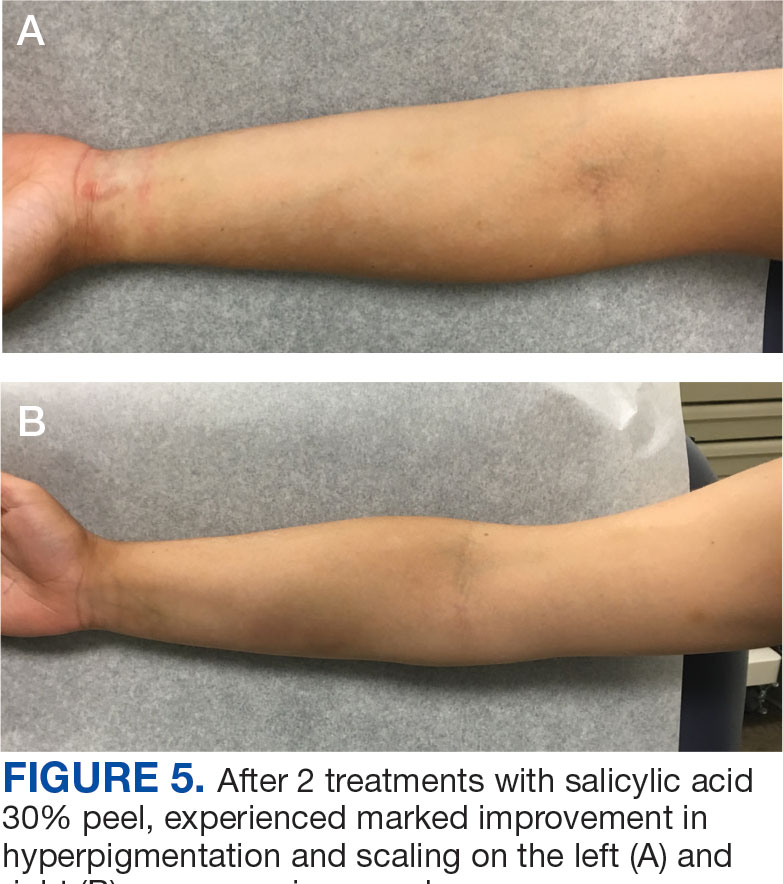
Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12
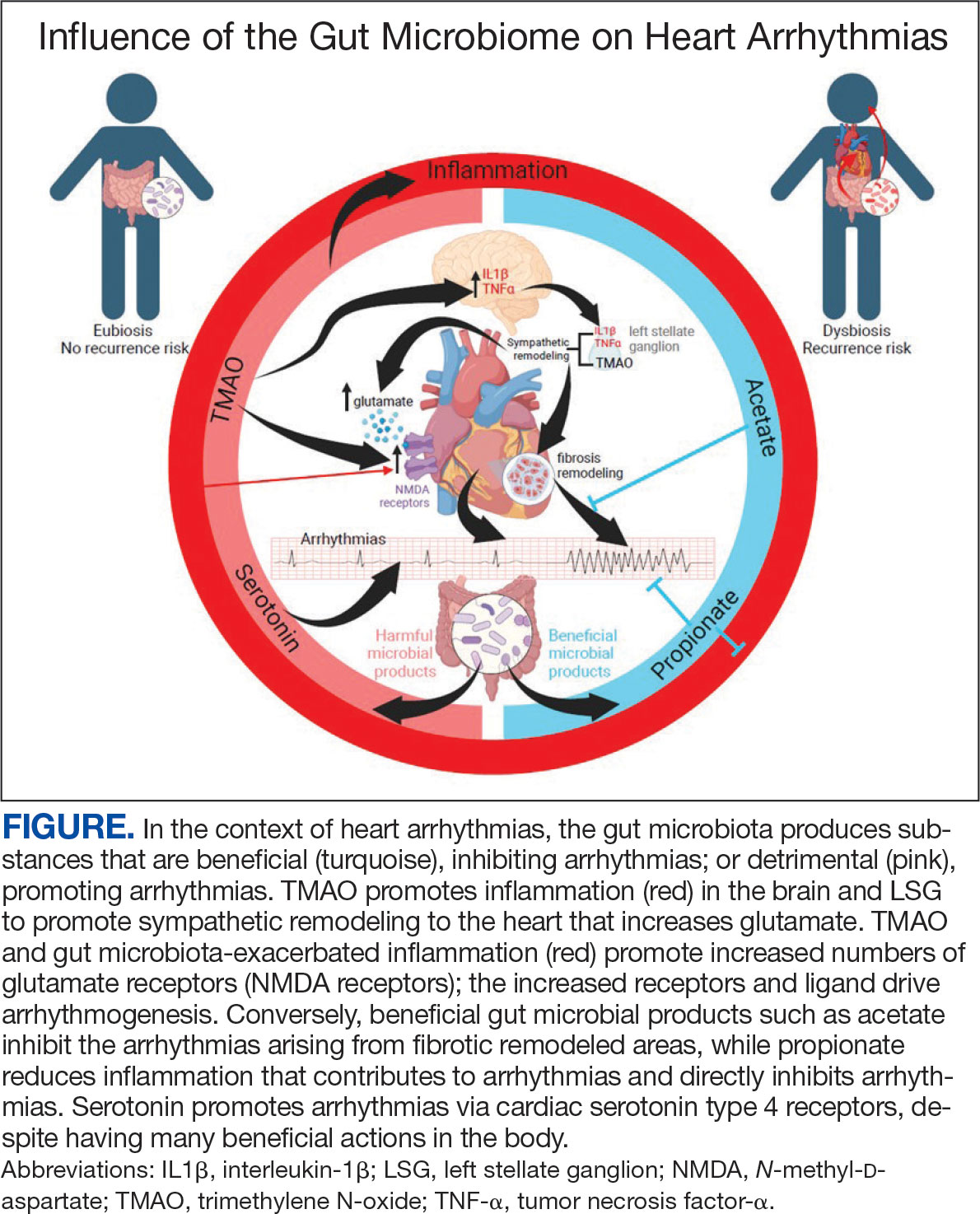
Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).
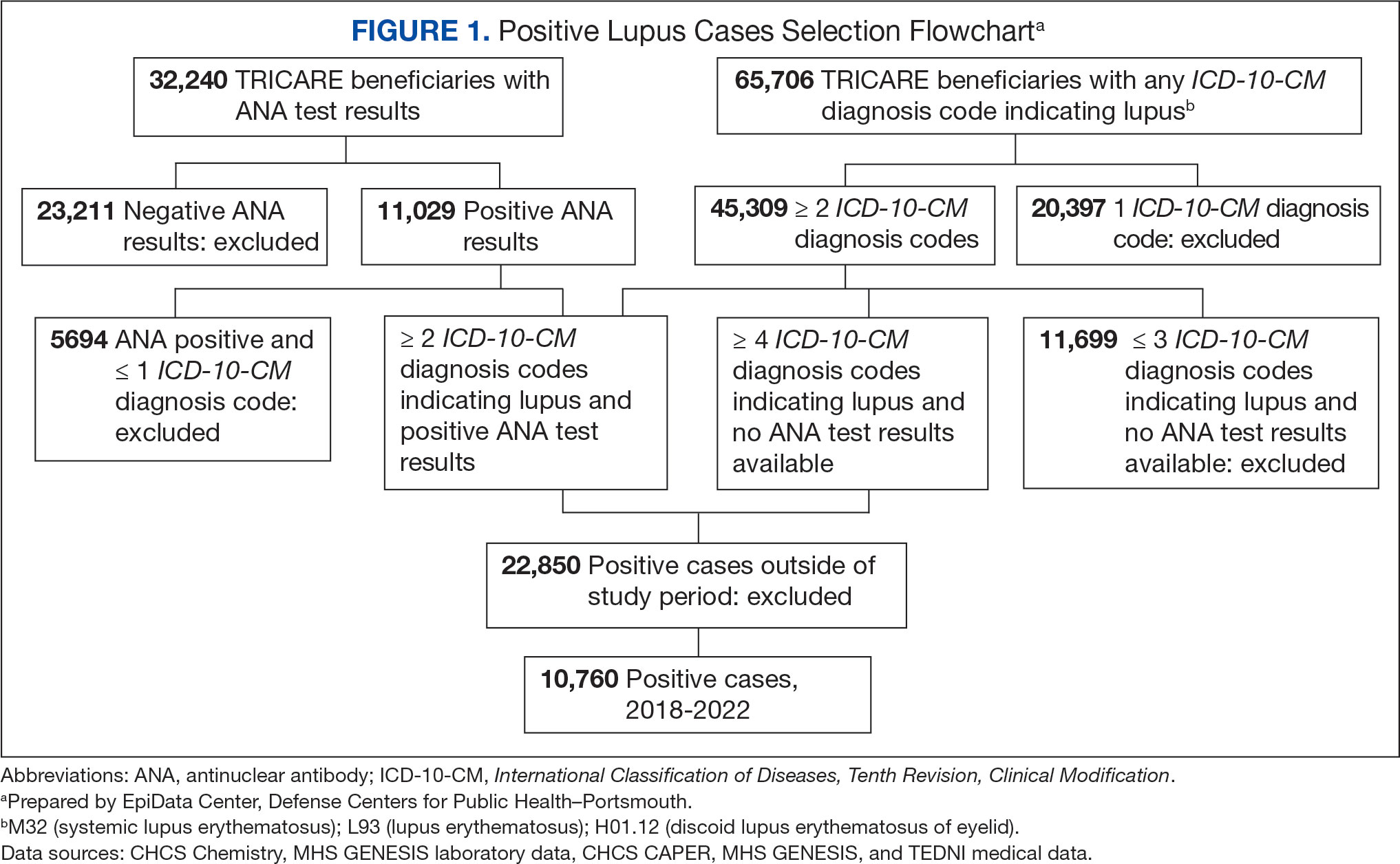
Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.
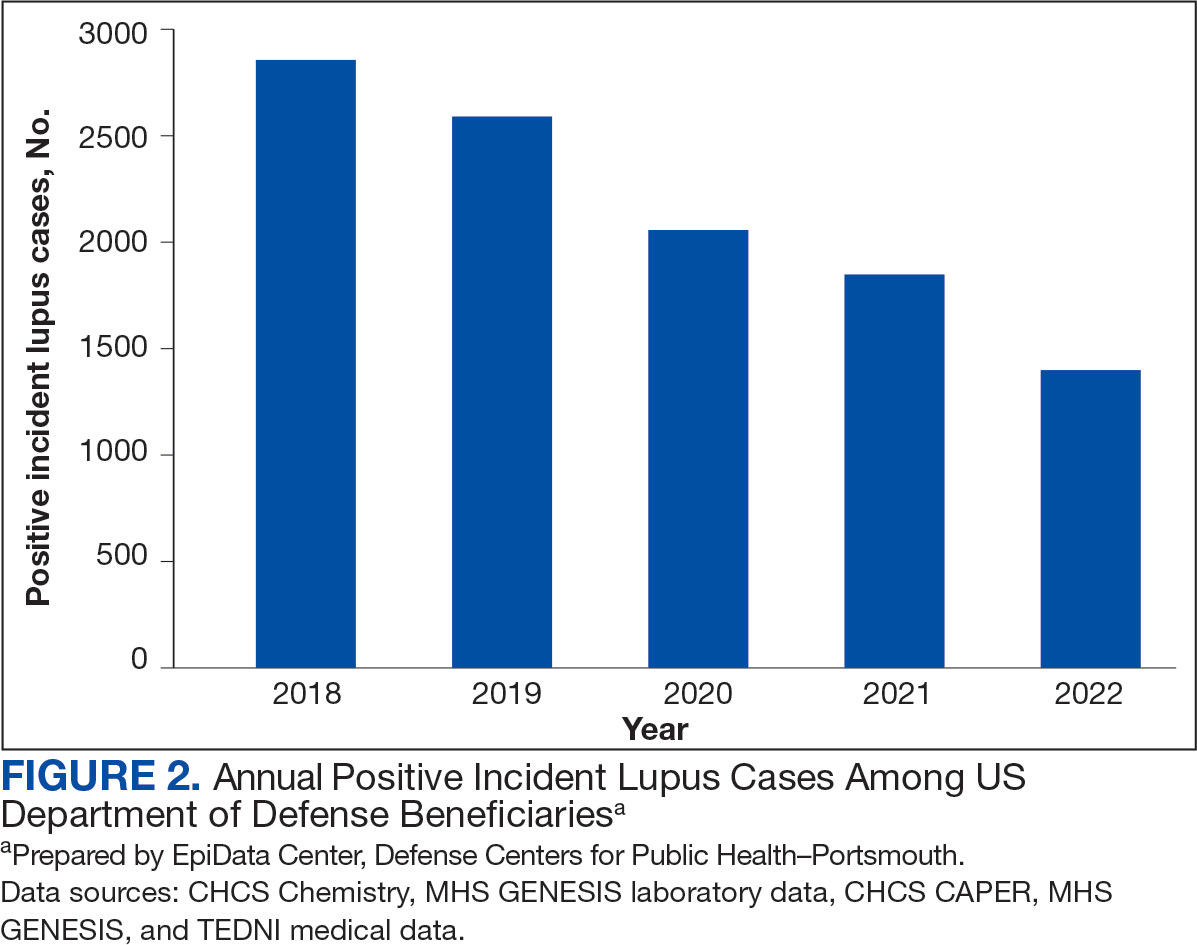
The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.
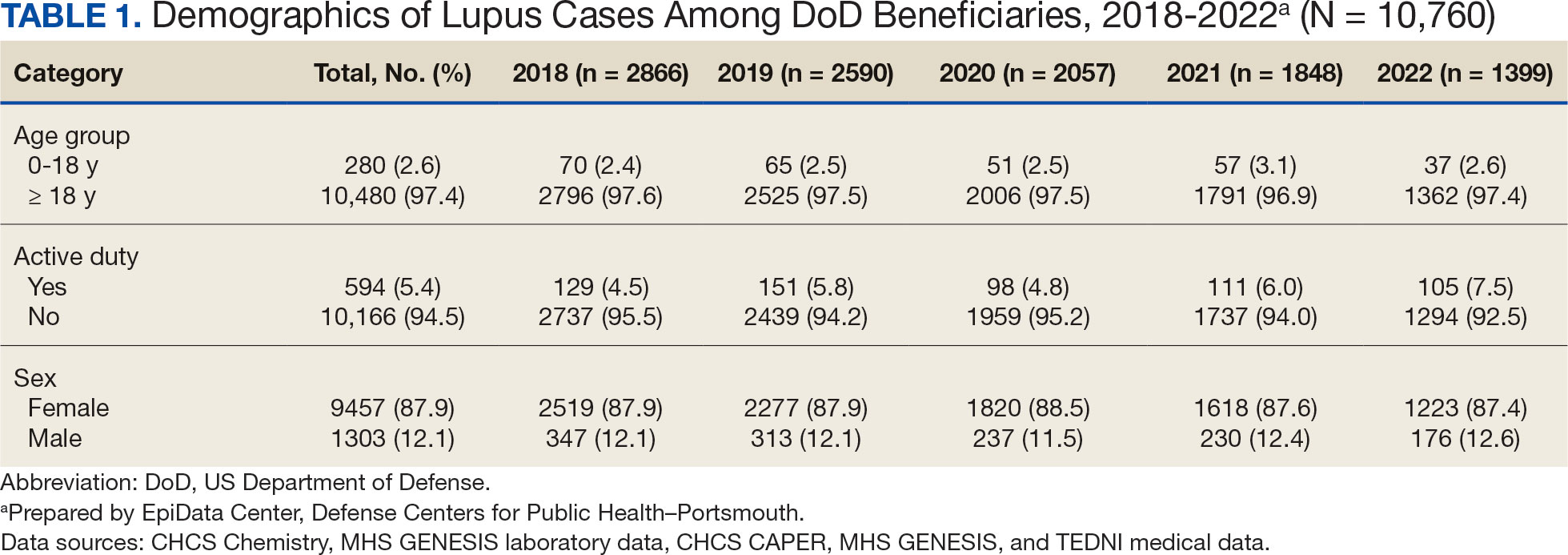
A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.
Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).

Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.

The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.

A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.

Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).

Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.

The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.

A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.

Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
- Izmirly PM, Ferucci ED, Somers EC, et al. Incidence rates of systemic lupus erythematosus in the USA: estimates from a meta-analysis of the Centers for Disease Control and Prevention national lupus registries. Lupus Sci Med. 2021;8(1):e000614. doi:10.1136/lupus-2021-000614
- Centers for Disease Control and Prevention. People with lupus. May 15, 2024. Accessed May 10, 2025. https:// www.cdc.gov/lupus/data-research/index.html
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526-1533. doi:10.1056/nejmoa021933
- Li ZX, Zeng S, Wu HX, Zhou Y. The risk of systemic lupus erythematosus associated with Epstein–Barr virus infection: a systematic review and meta-analysis. Clin Exp Med. 2019;19(1):23-36. doi:10.1007/s10238-018-0535-0
- Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi:10.1126/science.abd4585
- Chang SE, Feng A, Meng W, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):5417. doi:10.1038/s41467-021-25509-3
- Lee SJ, Yoon T, Ha JW, et al. Prevalence, clinical significance, and persistence of autoantibodies in COVID-19. Virol J. 2023;20(1):236. doi:10.1186/s12985-023-02191-z
- Woodruff MC, Ramonell RP, Haddad NS, et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature. 2022;611(7934):139-147. doi:10.1038/s41586-022-05273-0
- Taeschler P, Cervia C, Zurbuchen Y, et al. Autoantibodies in COVID-19 correlate with antiviral humoral responses and distinct immune signatures. Allergy. 2022;77(8):2415-2430. doi:10.1111/all.15302
- Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592 doi:10.3390/cells10123592
- Barnado A, Carroll R, Denny JC, Crofford L. Using IC-10-CM codes to identify patients with systemic lupus erythematosus in the electronic health record [abstract]. Arthritis Rheumatol. 2018;70(suppl 9):abstract 1692. Accessed May 10, 2025. https://acrabstracts.org/abstract/using-icd-10-cm-codes-to-identify-patients-with-systemic-lupus-erythematosus-in-the-electronic-health-record
- Feldman C, Curtis JR, Oates JC, Yazdany J, Izmirly P. Validating claims-based algorithms for a systemic lupus erythematosus diagnosis in Medicare data for informed use of the Lupus Index: a tool for geospatial research. Lupus Sci Med. 2024;11(2):e001329. doi:10.1136/lupus-2024-001329
- Moe SR, Haukeland H, Brunborg C, et al. POS1472: Accuracy of disease-specific ICD-10 code for incident systemic lupus erythematosus; results from a population-based cohort study set in Norway [abstract]. Ann Rheum Dis. 2023;82(suppl 1):1090-1091. doi:10.1136/annrheumdis-2023-eular.1189
- Denagamage P, Mabila SL, McQuistan AA. Trends and disparities in systemic lupus erythematosus incidence among U.S. active component service members, 2000–2022. MSMR. 2023;30(12):2-5.
- Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. EClinicalMedicine. 2023;56:101783. doi:10.1016/j.eclinm.2022.101783
- Tesch F, Ehm F, Vivirito A, et al. Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study. Clin Rheumatol. 2023;42(10):2905- 2914. doi:10.1007/s10067-023-06670-0
- Peng K, Li X, Yang D, et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. EClinicalMedicine. 2023;63:102154. doi:10.1016/j.eclinm.2023.102154
- US Department of Defense. Coronavirus: DOD response. Updated December 20, 2022. Accessed May 10, 2025. https://www.defense.gov/Spotlights/Coronavirus-DOD-Response/
- Elflein J. Number of cumulative cases of COVID-19 in the United States from January 20, 2020 to November 11, 2022, by week. Statista. https://www.statista.com/statistics/1103185/cumulative-coronavirus-covid19-cases-number-us-by-day
- Ye Z, Chen L, Zhong H, Cao L, Fu P, Xu J. Epidemiology and clinical characteristics of Epstein-Barr virus infection among children in Shanghai, China, 2017- 2022. Front Cell Infect Microbiol. 2023;13:1139068. doi:10.3389/fcimb.2023.1139068
- Johnson D, Jiang W. Infectious diseases, autoantibodies, and autoimmunity. J Autoimmun. 2023;137:102962. doi:10.1016/j.jaut.2022.102962
Sclerosing Mesenteritis: What GIs Need to Know About This Rare Disease
AGA has issued an updated pragmatic review on sclerosing mesenteritis (SM). Published in Clinical Gastroenterology and Hepatology, the update evaluates available evidence for diagnosis and treatment and examines opportunities for future research in SM, previously known by such names as misty mesentery, mesenteric panniculitis, and inflammatory pseudotumor.
Led by Mark T. Worthington, MD, AGAF, a professor of medicine in the Division of Gastroenterology and Hepatology at the University of Virginia in Charlottesville, Virginia, an expert AGA panel described SM as an uncommon benign idiopathic autoimmune disease of the mesenteric fat. Although of poorly understood etiology, gastroenterologists need to be prepared to diagnose it.
“CT radiologists increasingly are reporting SM and related lesions, such as misty mesentery,” Worthington told GI & Hepatology News. “We are also seeing new SM cases caused by immune checkpoint inhibitors in cancer treatment, and the oncologists ask us to manage this because it interferes with the treatment of the underlying malignancy. Those are often readily treated because we catch them so early.” Metabolic syndrome and associated conditions increase the risk for SM, as does aging.
The recent changes are intended to help clinicians predict disease activity and the need for other testing or treatment. “For instance, most cases are indolent and do not require aggressive treatment — often no treatment at all — but for those that are aggressive, we want the clinician to be able to identify those and make sure the treatment is appropriate. The aggressive cases may warrant tertiary referral,” Worthington said. “A secondary cancer is a possibility in this condition, so drawing from the SM radiology studies, we try to help the clinician decide who needs other testing, such as PET-CT or biopsy, and who can be monitored.”
As many as 60% of cases are asymptomatic, requiring no treatment. Abdominal pain is the most frequent symptom and its location on clinical examination should correspond to the SM lesion on imaging. Treatment involves anti-inflammatory medications tailored to disease severity and clinical response.
No biopsy is not necessary if the lesion meets three of the five CT criteria reported by B. Coulier and has no features of more aggressive disease or malignancy. Although some have suggested that SM may be a paraneoplastic syndrome, current evidence does not support this. SM needs to be differentiated from other diagnoses such as non-Hodgkin’s lymphoma, peritoneal carcinomatosis, and mesenteric fibromatosis.
“There are now CT guidelines for who actually has SM, who needs a biopsy or a PET-CT to rule-out malignancy, and who doesn’t,” said Worthington. “Radiologists do not always use the Coulier criteria for diagnosis, but often they will with encouragement. From this review, a GI clinician should be able to identify SM on CT.”
Epidemiologically, retrospective CT studies have reported a frequency of 0.6%-1.1%, the panelists noted. And while demographic data are limited, a large early case series reported that SM patients had a mean age of 55 years and more likely to be men and of White race.
Patients with SM do not have a higher prevalence of autoimmunity in general, but may have increased rates of metabolic syndrome, obesity, coronary artery disease, and urolithiasis, the panelists noted.
The update allows room for differences in clinical judgment. “For instance, a longer or more frequent CT surveillance interval can be justified depending on the patient’s findings, and no one should feel locked in by these recommendations,” Worthington said.
Medical Therapy
Although there is no surgical cure, pharmacologic options are many. These include prednisone, tamoxifen, colchicine, azathioprine, thalidomide, cyclophosphamide, and methotrexate, as well as the biologics rituximab, infliximab and ustekinumab. Current corticosteroid-based therapies often require months to achieve a clinical response, however.
Bowel obstruction is managed nonoperatively when feasible, but medically refractory disease may require surgical bypass.
Offering his perspective on the guidance but not involved in its formulation, Gastroenterologist Stephen B. Hanauer, MD, AGAF, a professor of medicine at Northwestern Medicine in Chicago, said, “The most useful component of the practical review is the algorithm for diagnosis and determination when biopsy or follow-up imaging is reasonable in the absence of evidence.” He stressed that the recommendations are pragmatic rather than evidence-based “as there are no controlled trials and the presentation is heterogeneous.”
Hanauer added that none of the recommended treatments have been shown to impact reduction on imaging. “Hence, all of the treatments are empiric without biological or imaging endpoints.”
In his experience, patients with inflammatory features are the best candidates for immune-directed therapies as reduction in inflammatory markers is a potential endpoint, although no therapies have demonstrated an effect on imaging or progression. “As an IBD doctor, I favor steroids and azathioprine or anti-TNF directed therapy, but again, there is no evidence beyond reports of symptomatic improvement.”
Worthington and colleagues agreed that treatment protocols have developed empirically. “Future investigation for symptomatic SM should focus on the nature of the inflammatory response, including causative cytokines and other proinflammatory mediators, the goal being targeted therapy with fewer side effects and a more rapid clinical response,” they wrote.
Currently, said Worthington, the biggest gaps remain in treatment. “Even the best studies are small and anecdotal, and we do not know the cytokine or other proinflammatory mediators.”
A version of this article appeared on Medscape.com.
AGA has issued an updated pragmatic review on sclerosing mesenteritis (SM). Published in Clinical Gastroenterology and Hepatology, the update evaluates available evidence for diagnosis and treatment and examines opportunities for future research in SM, previously known by such names as misty mesentery, mesenteric panniculitis, and inflammatory pseudotumor.
Led by Mark T. Worthington, MD, AGAF, a professor of medicine in the Division of Gastroenterology and Hepatology at the University of Virginia in Charlottesville, Virginia, an expert AGA panel described SM as an uncommon benign idiopathic autoimmune disease of the mesenteric fat. Although of poorly understood etiology, gastroenterologists need to be prepared to diagnose it.
“CT radiologists increasingly are reporting SM and related lesions, such as misty mesentery,” Worthington told GI & Hepatology News. “We are also seeing new SM cases caused by immune checkpoint inhibitors in cancer treatment, and the oncologists ask us to manage this because it interferes with the treatment of the underlying malignancy. Those are often readily treated because we catch them so early.” Metabolic syndrome and associated conditions increase the risk for SM, as does aging.
The recent changes are intended to help clinicians predict disease activity and the need for other testing or treatment. “For instance, most cases are indolent and do not require aggressive treatment — often no treatment at all — but for those that are aggressive, we want the clinician to be able to identify those and make sure the treatment is appropriate. The aggressive cases may warrant tertiary referral,” Worthington said. “A secondary cancer is a possibility in this condition, so drawing from the SM radiology studies, we try to help the clinician decide who needs other testing, such as PET-CT or biopsy, and who can be monitored.”
As many as 60% of cases are asymptomatic, requiring no treatment. Abdominal pain is the most frequent symptom and its location on clinical examination should correspond to the SM lesion on imaging. Treatment involves anti-inflammatory medications tailored to disease severity and clinical response.
No biopsy is not necessary if the lesion meets three of the five CT criteria reported by B. Coulier and has no features of more aggressive disease or malignancy. Although some have suggested that SM may be a paraneoplastic syndrome, current evidence does not support this. SM needs to be differentiated from other diagnoses such as non-Hodgkin’s lymphoma, peritoneal carcinomatosis, and mesenteric fibromatosis.
“There are now CT guidelines for who actually has SM, who needs a biopsy or a PET-CT to rule-out malignancy, and who doesn’t,” said Worthington. “Radiologists do not always use the Coulier criteria for diagnosis, but often they will with encouragement. From this review, a GI clinician should be able to identify SM on CT.”
Epidemiologically, retrospective CT studies have reported a frequency of 0.6%-1.1%, the panelists noted. And while demographic data are limited, a large early case series reported that SM patients had a mean age of 55 years and more likely to be men and of White race.
Patients with SM do not have a higher prevalence of autoimmunity in general, but may have increased rates of metabolic syndrome, obesity, coronary artery disease, and urolithiasis, the panelists noted.
The update allows room for differences in clinical judgment. “For instance, a longer or more frequent CT surveillance interval can be justified depending on the patient’s findings, and no one should feel locked in by these recommendations,” Worthington said.
Medical Therapy
Although there is no surgical cure, pharmacologic options are many. These include prednisone, tamoxifen, colchicine, azathioprine, thalidomide, cyclophosphamide, and methotrexate, as well as the biologics rituximab, infliximab and ustekinumab. Current corticosteroid-based therapies often require months to achieve a clinical response, however.
Bowel obstruction is managed nonoperatively when feasible, but medically refractory disease may require surgical bypass.
Offering his perspective on the guidance but not involved in its formulation, Gastroenterologist Stephen B. Hanauer, MD, AGAF, a professor of medicine at Northwestern Medicine in Chicago, said, “The most useful component of the practical review is the algorithm for diagnosis and determination when biopsy or follow-up imaging is reasonable in the absence of evidence.” He stressed that the recommendations are pragmatic rather than evidence-based “as there are no controlled trials and the presentation is heterogeneous.”
Hanauer added that none of the recommended treatments have been shown to impact reduction on imaging. “Hence, all of the treatments are empiric without biological or imaging endpoints.”
In his experience, patients with inflammatory features are the best candidates for immune-directed therapies as reduction in inflammatory markers is a potential endpoint, although no therapies have demonstrated an effect on imaging or progression. “As an IBD doctor, I favor steroids and azathioprine or anti-TNF directed therapy, but again, there is no evidence beyond reports of symptomatic improvement.”
Worthington and colleagues agreed that treatment protocols have developed empirically. “Future investigation for symptomatic SM should focus on the nature of the inflammatory response, including causative cytokines and other proinflammatory mediators, the goal being targeted therapy with fewer side effects and a more rapid clinical response,” they wrote.
Currently, said Worthington, the biggest gaps remain in treatment. “Even the best studies are small and anecdotal, and we do not know the cytokine or other proinflammatory mediators.”
A version of this article appeared on Medscape.com.
AGA has issued an updated pragmatic review on sclerosing mesenteritis (SM). Published in Clinical Gastroenterology and Hepatology, the update evaluates available evidence for diagnosis and treatment and examines opportunities for future research in SM, previously known by such names as misty mesentery, mesenteric panniculitis, and inflammatory pseudotumor.
Led by Mark T. Worthington, MD, AGAF, a professor of medicine in the Division of Gastroenterology and Hepatology at the University of Virginia in Charlottesville, Virginia, an expert AGA panel described SM as an uncommon benign idiopathic autoimmune disease of the mesenteric fat. Although of poorly understood etiology, gastroenterologists need to be prepared to diagnose it.
“CT radiologists increasingly are reporting SM and related lesions, such as misty mesentery,” Worthington told GI & Hepatology News. “We are also seeing new SM cases caused by immune checkpoint inhibitors in cancer treatment, and the oncologists ask us to manage this because it interferes with the treatment of the underlying malignancy. Those are often readily treated because we catch them so early.” Metabolic syndrome and associated conditions increase the risk for SM, as does aging.
The recent changes are intended to help clinicians predict disease activity and the need for other testing or treatment. “For instance, most cases are indolent and do not require aggressive treatment — often no treatment at all — but for those that are aggressive, we want the clinician to be able to identify those and make sure the treatment is appropriate. The aggressive cases may warrant tertiary referral,” Worthington said. “A secondary cancer is a possibility in this condition, so drawing from the SM radiology studies, we try to help the clinician decide who needs other testing, such as PET-CT or biopsy, and who can be monitored.”
As many as 60% of cases are asymptomatic, requiring no treatment. Abdominal pain is the most frequent symptom and its location on clinical examination should correspond to the SM lesion on imaging. Treatment involves anti-inflammatory medications tailored to disease severity and clinical response.
No biopsy is not necessary if the lesion meets three of the five CT criteria reported by B. Coulier and has no features of more aggressive disease or malignancy. Although some have suggested that SM may be a paraneoplastic syndrome, current evidence does not support this. SM needs to be differentiated from other diagnoses such as non-Hodgkin’s lymphoma, peritoneal carcinomatosis, and mesenteric fibromatosis.
“There are now CT guidelines for who actually has SM, who needs a biopsy or a PET-CT to rule-out malignancy, and who doesn’t,” said Worthington. “Radiologists do not always use the Coulier criteria for diagnosis, but often they will with encouragement. From this review, a GI clinician should be able to identify SM on CT.”
Epidemiologically, retrospective CT studies have reported a frequency of 0.6%-1.1%, the panelists noted. And while demographic data are limited, a large early case series reported that SM patients had a mean age of 55 years and more likely to be men and of White race.
Patients with SM do not have a higher prevalence of autoimmunity in general, but may have increased rates of metabolic syndrome, obesity, coronary artery disease, and urolithiasis, the panelists noted.
The update allows room for differences in clinical judgment. “For instance, a longer or more frequent CT surveillance interval can be justified depending on the patient’s findings, and no one should feel locked in by these recommendations,” Worthington said.
Medical Therapy
Although there is no surgical cure, pharmacologic options are many. These include prednisone, tamoxifen, colchicine, azathioprine, thalidomide, cyclophosphamide, and methotrexate, as well as the biologics rituximab, infliximab and ustekinumab. Current corticosteroid-based therapies often require months to achieve a clinical response, however.
Bowel obstruction is managed nonoperatively when feasible, but medically refractory disease may require surgical bypass.
Offering his perspective on the guidance but not involved in its formulation, Gastroenterologist Stephen B. Hanauer, MD, AGAF, a professor of medicine at Northwestern Medicine in Chicago, said, “The most useful component of the practical review is the algorithm for diagnosis and determination when biopsy or follow-up imaging is reasonable in the absence of evidence.” He stressed that the recommendations are pragmatic rather than evidence-based “as there are no controlled trials and the presentation is heterogeneous.”
Hanauer added that none of the recommended treatments have been shown to impact reduction on imaging. “Hence, all of the treatments are empiric without biological or imaging endpoints.”
In his experience, patients with inflammatory features are the best candidates for immune-directed therapies as reduction in inflammatory markers is a potential endpoint, although no therapies have demonstrated an effect on imaging or progression. “As an IBD doctor, I favor steroids and azathioprine or anti-TNF directed therapy, but again, there is no evidence beyond reports of symptomatic improvement.”
Worthington and colleagues agreed that treatment protocols have developed empirically. “Future investigation for symptomatic SM should focus on the nature of the inflammatory response, including causative cytokines and other proinflammatory mediators, the goal being targeted therapy with fewer side effects and a more rapid clinical response,” they wrote.
Currently, said Worthington, the biggest gaps remain in treatment. “Even the best studies are small and anecdotal, and we do not know the cytokine or other proinflammatory mediators.”
A version of this article appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY