User login
Migraine: Combination therapy more effective than either of manual therapies
Key clinical point: A combined manual therapeutic approach that included soft-tissue and articulatory manual therapy techniques was more effective in reducing migraine impact than either technique alone.
Major finding: After 4 weeks of intervention, the improvement in pain intensity was greater with combined soft-tissue and articulatory manual therapy vs only soft-tissue (P < .001) or articulatory (P = .014) manual therapy. Reduction in migraine duration was significant with combined vs soft-tissue therapy (P = .02), with improvements maintained through a 4-week follow-up. No serious side-effects were reported.
Study details: Findings are from a randomized controlled trial including 75 patients with chronic or episodic migraine who were randomly assigned to receive soft-tissue therapy, articulatory therapy, or combination of both manual therapies.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Muñoz-Gómez E et al. Potential add-on effects of manual therapy techniques in migraine patients: A randomised controlled trial. J Clin Med. 2022;11(16):4686 (Aug 11). Doi: 10.3390/jcm11164686
Key clinical point: A combined manual therapeutic approach that included soft-tissue and articulatory manual therapy techniques was more effective in reducing migraine impact than either technique alone.
Major finding: After 4 weeks of intervention, the improvement in pain intensity was greater with combined soft-tissue and articulatory manual therapy vs only soft-tissue (P < .001) or articulatory (P = .014) manual therapy. Reduction in migraine duration was significant with combined vs soft-tissue therapy (P = .02), with improvements maintained through a 4-week follow-up. No serious side-effects were reported.
Study details: Findings are from a randomized controlled trial including 75 patients with chronic or episodic migraine who were randomly assigned to receive soft-tissue therapy, articulatory therapy, or combination of both manual therapies.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Muñoz-Gómez E et al. Potential add-on effects of manual therapy techniques in migraine patients: A randomised controlled trial. J Clin Med. 2022;11(16):4686 (Aug 11). Doi: 10.3390/jcm11164686
Key clinical point: A combined manual therapeutic approach that included soft-tissue and articulatory manual therapy techniques was more effective in reducing migraine impact than either technique alone.
Major finding: After 4 weeks of intervention, the improvement in pain intensity was greater with combined soft-tissue and articulatory manual therapy vs only soft-tissue (P < .001) or articulatory (P = .014) manual therapy. Reduction in migraine duration was significant with combined vs soft-tissue therapy (P = .02), with improvements maintained through a 4-week follow-up. No serious side-effects were reported.
Study details: Findings are from a randomized controlled trial including 75 patients with chronic or episodic migraine who were randomly assigned to receive soft-tissue therapy, articulatory therapy, or combination of both manual therapies.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Muñoz-Gómez E et al. Potential add-on effects of manual therapy techniques in migraine patients: A randomised controlled trial. J Clin Med. 2022;11(16):4686 (Aug 11). Doi: 10.3390/jcm11164686
Lasmiditan effective for acute treatment of perimenstrual migraine
Key clinical point: Treatment of perimenstrual migraine attacks with lasmiditan was associated with early and sustained freedom from migraine-related pain.
Major finding: Women who received 200 mg lasmiditan vs placebo during perimenstrual migraine attacks were more likely to report freedom from migraine-related pain (odds ratio [OR] 6.04; P < .001) and pain relief (OR 2.29; P = .007) at 2 hours and sustained freedom from pain at 24 hours (OR 11.67; P = .001).
Study details: Findings are from a post hoc analysis of the phase 2 MONONOFU and phase 3 CENTURION trials including 303 women with migraine with or without aura who had ≥1 perimenstrual migraine attacks and were randomly assigned to receive lasmiditan (50, 100, or 200 mg) or placebo.
Disclosures: This study was funded by Eli Lilly and Company. Four authors declared being employees and minor stockholders of Eli Lilly and Company. The other authors reported ties with various sources, including Eli Lilly and Company.
Source: MacGregor EA et al. Efficacy of lasmiditan for the acute treatment of perimenstrual migraine. Cephalalgia. 2022 (Aug 18). Doi: 10.1177/03331024221118929
Key clinical point: Treatment of perimenstrual migraine attacks with lasmiditan was associated with early and sustained freedom from migraine-related pain.
Major finding: Women who received 200 mg lasmiditan vs placebo during perimenstrual migraine attacks were more likely to report freedom from migraine-related pain (odds ratio [OR] 6.04; P < .001) and pain relief (OR 2.29; P = .007) at 2 hours and sustained freedom from pain at 24 hours (OR 11.67; P = .001).
Study details: Findings are from a post hoc analysis of the phase 2 MONONOFU and phase 3 CENTURION trials including 303 women with migraine with or without aura who had ≥1 perimenstrual migraine attacks and were randomly assigned to receive lasmiditan (50, 100, or 200 mg) or placebo.
Disclosures: This study was funded by Eli Lilly and Company. Four authors declared being employees and minor stockholders of Eli Lilly and Company. The other authors reported ties with various sources, including Eli Lilly and Company.
Source: MacGregor EA et al. Efficacy of lasmiditan for the acute treatment of perimenstrual migraine. Cephalalgia. 2022 (Aug 18). Doi: 10.1177/03331024221118929
Key clinical point: Treatment of perimenstrual migraine attacks with lasmiditan was associated with early and sustained freedom from migraine-related pain.
Major finding: Women who received 200 mg lasmiditan vs placebo during perimenstrual migraine attacks were more likely to report freedom from migraine-related pain (odds ratio [OR] 6.04; P < .001) and pain relief (OR 2.29; P = .007) at 2 hours and sustained freedom from pain at 24 hours (OR 11.67; P = .001).
Study details: Findings are from a post hoc analysis of the phase 2 MONONOFU and phase 3 CENTURION trials including 303 women with migraine with or without aura who had ≥1 perimenstrual migraine attacks and were randomly assigned to receive lasmiditan (50, 100, or 200 mg) or placebo.
Disclosures: This study was funded by Eli Lilly and Company. Four authors declared being employees and minor stockholders of Eli Lilly and Company. The other authors reported ties with various sources, including Eli Lilly and Company.
Source: MacGregor EA et al. Efficacy of lasmiditan for the acute treatment of perimenstrual migraine. Cephalalgia. 2022 (Aug 18). Doi: 10.1177/03331024221118929
‘Game changer’ semaglutide halves diabetes risk from obesity
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
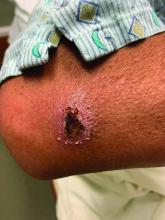
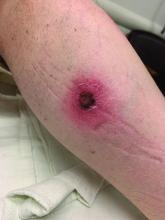
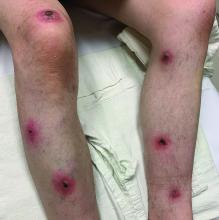
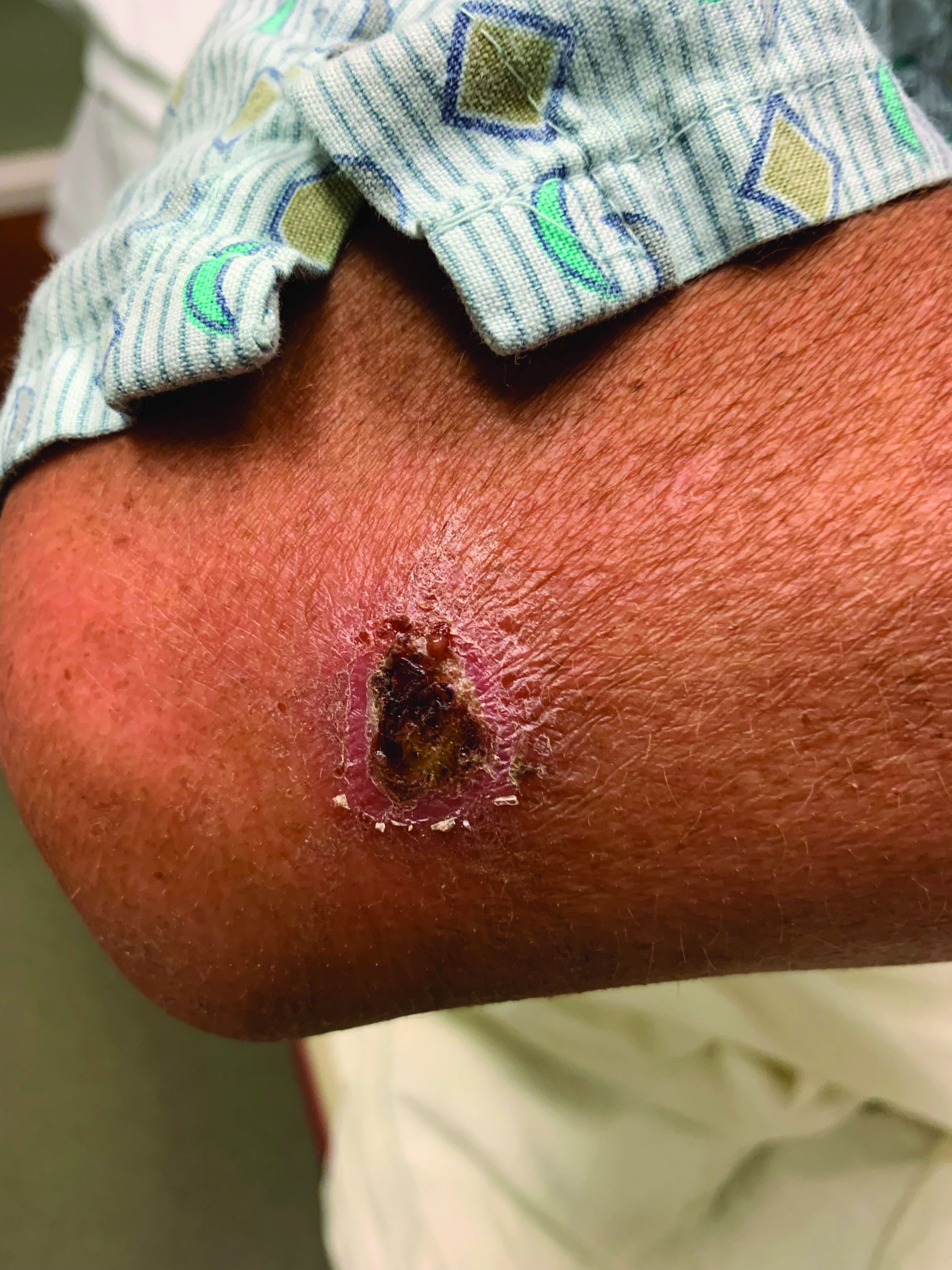
A White male presented with a 1-month history of recurrent, widespread, painful sores
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Family affair: OncBrothers host oncology hangout online
It’s hard out there for a small-town cancer doctor. Just ask Wederson M. Claudino, MD, who serves the town of Paducah in far western Kentucky. The nearest cities with significant numbers of hematologist/oncologists are hours away in cities like St. Louis and Nashville, Tenn., too far to go to talk shop over coffee, drinks, or lunch.
“It’s very challenging in a rural or small community,” he said. “I miss the opportunity to elaborate on a case.”
Now Dr. Claudino and hundreds of colleagues have discovered that useful cancer conversations are just a click away.
Urban and rural oncologists gather there to discuss new research, compare notes about challenging cases, and get to know each other.
“Following their Twitter feed and the comments and discussions make me feel like part of a bigger community,” Dr. Claudino said. And @OncBrothers is indeed a bustling Internet destination: The account’s 4,300 followers include hundreds who participate in discussions and offer perspective.
For instance, the brothers recently posted a poll asking followers how they’d treat a 55-year-old patient with non–small cell lung cancer. Nearly 250 people responded with their preferred approaches, and the survey thread included comments from oncologists from the City of Hope National Medical Center, the University of Florida, UC San Diego, and elsewhere.
In an interview, the Gosain brothers said the Twitter account is an outgrowth of their phone conversations in recent years, as they trained and settled into their early careers as general medical oncologists in smaller communities.
“After our clinic days, we’ll jump on the phone for 30-45 minutes. We’d talk about patients, how he would treat a case, and what I would do,” Rahul said. “We realized that we were living in a bubble, but we also thought that there are a lot more people in the same boat. They might jump at being able to do the same thing.”
Rahul recently became medical director at the new Wilmot Webster Cancer Center in Rochester, N.Y., after working in Corning, a tiny New York town just north of the Pennsylvania border. His brother Rohit is chair of hematology and oncology at the University of Pittsburgh’s Hillman Cancer Center in Jamestown, a small town at the western edge of New York.
“When we initially kicked off the Twitter account in August 2021, we didn’t realize the traction it would get,” Rohit said. “Now we realize that there really is a need for this.”
On an ordinary day, the @OncBrothers account may highlight research presented at a oncology conference, retweet posts by other oncologists about new guidelines or FDA drug approvals, and ask followers to consider how they’d handle a difficult case.
The brothers are especially thrilled when posts spawn discussions that draw voices from leading medical institutions who normally don’t interact much with community oncologists. “You’ll have someone from Sloan Kettering or Dana-Farber saying ‘This is what would do,’ ” Rahul said. “You have the brightest minds pitching in, and we get to learn from them.”
The Gosain brothers were both born in India and immigrated as children to Toronto. They each went to medical school in the Caribbean – for Rohit, it was after a stint as a computer engineer – and they each embraced oncology. “For me, it was about having the right mentors while I was doing my clinical rotations as a medical student and as a resident,” Rahul said. In addition, he said, “this field was moving and is still moving so fast. It really intrigued and excited me and made me want to be at the forefront of it.”
The fast-moving nature of oncology, in fact, was one of the drivers behind the daily conversations between the brothers and the subsequent creation of the @OncBrothers account. “Just last year, in 2021, there were 40 new drugs that were indicated for hematology-oncology,” Rahul said. “To stay on top of that is very, very hard.”
It’s especially difficult to figure out treatment plans when multiple options exist. A 2022 thread on @OncBrothers revealed a wide divergence of opinions about triple therapy in prostate cancer: The 322 respondents to a Twitter poll were sharply divided about the best three-drug combination from these options – docetaxel, daratumumab, abiraterone, androgen deprivation therapy, and alpha-reductase inhibitors.
To make things more challenging, community oncologists often are generalists who treat patients with a wide variety of cancers from prostate and lung to breast and colon. As a result, these oncologists must keep up on developments across the entire cancer field. Rohit highlighted a 2022 thread that polled users about the approach they’d take to another patient with non–small cell lung cancer; 474 people responded. The accompanying discussion emphasized the need for the next-generation sequencing (NGS).
“A significant portion of community oncologists are not even doing NGS testing, which is FDA-approved,” Rohit said. “There’s a huge gap that still exists, and we weren’t even aware of it. We continue to learn from these conversations.”
The brothers contend that there’s a crucial need for education among community oncologists in light of evidence suggesting that some cancer outcomes are worse than those in urban areas.
In fact, Rohit led a 2019 study published in the journal Cancer that found that overall survival in rural patients with neuroendocrine tumors trended toward worse outcomes than in urban patients.
“There are many factors such as financial burden, lack of education, and rural patients not willing to travel to the city,” Rohit said. “We need to be more creative and ask, ‘How can we equip our medical oncologist in rural settings to continue to do better?’ ”
What’s next for the OncBrothers? The Gosains have created a website (www.oncbrothers.com) that highlights their social media work, and they’re exploring options such as podcasts and short videos. “Our goal is to focus on how to continue to keep general medical oncologists up to date, informed, and educated so patients can get the best care close to home,” Rahul said. “We need to do better.”
It’s hard out there for a small-town cancer doctor. Just ask Wederson M. Claudino, MD, who serves the town of Paducah in far western Kentucky. The nearest cities with significant numbers of hematologist/oncologists are hours away in cities like St. Louis and Nashville, Tenn., too far to go to talk shop over coffee, drinks, or lunch.
“It’s very challenging in a rural or small community,” he said. “I miss the opportunity to elaborate on a case.”
Now Dr. Claudino and hundreds of colleagues have discovered that useful cancer conversations are just a click away.
Urban and rural oncologists gather there to discuss new research, compare notes about challenging cases, and get to know each other.
“Following their Twitter feed and the comments and discussions make me feel like part of a bigger community,” Dr. Claudino said. And @OncBrothers is indeed a bustling Internet destination: The account’s 4,300 followers include hundreds who participate in discussions and offer perspective.
For instance, the brothers recently posted a poll asking followers how they’d treat a 55-year-old patient with non–small cell lung cancer. Nearly 250 people responded with their preferred approaches, and the survey thread included comments from oncologists from the City of Hope National Medical Center, the University of Florida, UC San Diego, and elsewhere.
In an interview, the Gosain brothers said the Twitter account is an outgrowth of their phone conversations in recent years, as they trained and settled into their early careers as general medical oncologists in smaller communities.
“After our clinic days, we’ll jump on the phone for 30-45 minutes. We’d talk about patients, how he would treat a case, and what I would do,” Rahul said. “We realized that we were living in a bubble, but we also thought that there are a lot more people in the same boat. They might jump at being able to do the same thing.”
Rahul recently became medical director at the new Wilmot Webster Cancer Center in Rochester, N.Y., after working in Corning, a tiny New York town just north of the Pennsylvania border. His brother Rohit is chair of hematology and oncology at the University of Pittsburgh’s Hillman Cancer Center in Jamestown, a small town at the western edge of New York.
“When we initially kicked off the Twitter account in August 2021, we didn’t realize the traction it would get,” Rohit said. “Now we realize that there really is a need for this.”
On an ordinary day, the @OncBrothers account may highlight research presented at a oncology conference, retweet posts by other oncologists about new guidelines or FDA drug approvals, and ask followers to consider how they’d handle a difficult case.
The brothers are especially thrilled when posts spawn discussions that draw voices from leading medical institutions who normally don’t interact much with community oncologists. “You’ll have someone from Sloan Kettering or Dana-Farber saying ‘This is what would do,’ ” Rahul said. “You have the brightest minds pitching in, and we get to learn from them.”
The Gosain brothers were both born in India and immigrated as children to Toronto. They each went to medical school in the Caribbean – for Rohit, it was after a stint as a computer engineer – and they each embraced oncology. “For me, it was about having the right mentors while I was doing my clinical rotations as a medical student and as a resident,” Rahul said. In addition, he said, “this field was moving and is still moving so fast. It really intrigued and excited me and made me want to be at the forefront of it.”
The fast-moving nature of oncology, in fact, was one of the drivers behind the daily conversations between the brothers and the subsequent creation of the @OncBrothers account. “Just last year, in 2021, there were 40 new drugs that were indicated for hematology-oncology,” Rahul said. “To stay on top of that is very, very hard.”
It’s especially difficult to figure out treatment plans when multiple options exist. A 2022 thread on @OncBrothers revealed a wide divergence of opinions about triple therapy in prostate cancer: The 322 respondents to a Twitter poll were sharply divided about the best three-drug combination from these options – docetaxel, daratumumab, abiraterone, androgen deprivation therapy, and alpha-reductase inhibitors.
To make things more challenging, community oncologists often are generalists who treat patients with a wide variety of cancers from prostate and lung to breast and colon. As a result, these oncologists must keep up on developments across the entire cancer field. Rohit highlighted a 2022 thread that polled users about the approach they’d take to another patient with non–small cell lung cancer; 474 people responded. The accompanying discussion emphasized the need for the next-generation sequencing (NGS).
“A significant portion of community oncologists are not even doing NGS testing, which is FDA-approved,” Rohit said. “There’s a huge gap that still exists, and we weren’t even aware of it. We continue to learn from these conversations.”
The brothers contend that there’s a crucial need for education among community oncologists in light of evidence suggesting that some cancer outcomes are worse than those in urban areas.
In fact, Rohit led a 2019 study published in the journal Cancer that found that overall survival in rural patients with neuroendocrine tumors trended toward worse outcomes than in urban patients.
“There are many factors such as financial burden, lack of education, and rural patients not willing to travel to the city,” Rohit said. “We need to be more creative and ask, ‘How can we equip our medical oncologist in rural settings to continue to do better?’ ”
What’s next for the OncBrothers? The Gosains have created a website (www.oncbrothers.com) that highlights their social media work, and they’re exploring options such as podcasts and short videos. “Our goal is to focus on how to continue to keep general medical oncologists up to date, informed, and educated so patients can get the best care close to home,” Rahul said. “We need to do better.”
It’s hard out there for a small-town cancer doctor. Just ask Wederson M. Claudino, MD, who serves the town of Paducah in far western Kentucky. The nearest cities with significant numbers of hematologist/oncologists are hours away in cities like St. Louis and Nashville, Tenn., too far to go to talk shop over coffee, drinks, or lunch.
“It’s very challenging in a rural or small community,” he said. “I miss the opportunity to elaborate on a case.”
Now Dr. Claudino and hundreds of colleagues have discovered that useful cancer conversations are just a click away.
Urban and rural oncologists gather there to discuss new research, compare notes about challenging cases, and get to know each other.
“Following their Twitter feed and the comments and discussions make me feel like part of a bigger community,” Dr. Claudino said. And @OncBrothers is indeed a bustling Internet destination: The account’s 4,300 followers include hundreds who participate in discussions and offer perspective.
For instance, the brothers recently posted a poll asking followers how they’d treat a 55-year-old patient with non–small cell lung cancer. Nearly 250 people responded with their preferred approaches, and the survey thread included comments from oncologists from the City of Hope National Medical Center, the University of Florida, UC San Diego, and elsewhere.
In an interview, the Gosain brothers said the Twitter account is an outgrowth of their phone conversations in recent years, as they trained and settled into their early careers as general medical oncologists in smaller communities.
“After our clinic days, we’ll jump on the phone for 30-45 minutes. We’d talk about patients, how he would treat a case, and what I would do,” Rahul said. “We realized that we were living in a bubble, but we also thought that there are a lot more people in the same boat. They might jump at being able to do the same thing.”
Rahul recently became medical director at the new Wilmot Webster Cancer Center in Rochester, N.Y., after working in Corning, a tiny New York town just north of the Pennsylvania border. His brother Rohit is chair of hematology and oncology at the University of Pittsburgh’s Hillman Cancer Center in Jamestown, a small town at the western edge of New York.
“When we initially kicked off the Twitter account in August 2021, we didn’t realize the traction it would get,” Rohit said. “Now we realize that there really is a need for this.”
On an ordinary day, the @OncBrothers account may highlight research presented at a oncology conference, retweet posts by other oncologists about new guidelines or FDA drug approvals, and ask followers to consider how they’d handle a difficult case.
The brothers are especially thrilled when posts spawn discussions that draw voices from leading medical institutions who normally don’t interact much with community oncologists. “You’ll have someone from Sloan Kettering or Dana-Farber saying ‘This is what would do,’ ” Rahul said. “You have the brightest minds pitching in, and we get to learn from them.”
The Gosain brothers were both born in India and immigrated as children to Toronto. They each went to medical school in the Caribbean – for Rohit, it was after a stint as a computer engineer – and they each embraced oncology. “For me, it was about having the right mentors while I was doing my clinical rotations as a medical student and as a resident,” Rahul said. In addition, he said, “this field was moving and is still moving so fast. It really intrigued and excited me and made me want to be at the forefront of it.”
The fast-moving nature of oncology, in fact, was one of the drivers behind the daily conversations between the brothers and the subsequent creation of the @OncBrothers account. “Just last year, in 2021, there were 40 new drugs that were indicated for hematology-oncology,” Rahul said. “To stay on top of that is very, very hard.”
It’s especially difficult to figure out treatment plans when multiple options exist. A 2022 thread on @OncBrothers revealed a wide divergence of opinions about triple therapy in prostate cancer: The 322 respondents to a Twitter poll were sharply divided about the best three-drug combination from these options – docetaxel, daratumumab, abiraterone, androgen deprivation therapy, and alpha-reductase inhibitors.
To make things more challenging, community oncologists often are generalists who treat patients with a wide variety of cancers from prostate and lung to breast and colon. As a result, these oncologists must keep up on developments across the entire cancer field. Rohit highlighted a 2022 thread that polled users about the approach they’d take to another patient with non–small cell lung cancer; 474 people responded. The accompanying discussion emphasized the need for the next-generation sequencing (NGS).
“A significant portion of community oncologists are not even doing NGS testing, which is FDA-approved,” Rohit said. “There’s a huge gap that still exists, and we weren’t even aware of it. We continue to learn from these conversations.”
The brothers contend that there’s a crucial need for education among community oncologists in light of evidence suggesting that some cancer outcomes are worse than those in urban areas.
In fact, Rohit led a 2019 study published in the journal Cancer that found that overall survival in rural patients with neuroendocrine tumors trended toward worse outcomes than in urban patients.
“There are many factors such as financial burden, lack of education, and rural patients not willing to travel to the city,” Rohit said. “We need to be more creative and ask, ‘How can we equip our medical oncologist in rural settings to continue to do better?’ ”
What’s next for the OncBrothers? The Gosains have created a website (www.oncbrothers.com) that highlights their social media work, and they’re exploring options such as podcasts and short videos. “Our goal is to focus on how to continue to keep general medical oncologists up to date, informed, and educated so patients can get the best care close to home,” Rahul said. “We need to do better.”
Obesity and intestinal inflammation might influence development of IBS in children
Key clinical point: Pediatric patients with irritable bowel syndrome (IBS) and normal weight had higher levels of fecal calprotectin than those with IBS and obesity, suggesting the role of obesity and intestinal inflammation in the development and manifestations of IBS in children.
Major finding: The mean calprotectin levels were significantly higher in patients with body mass index <85th vs 85th to <95th percentile (P = .028) and ≥95th percentile (P ≥ .025), with the difference being prominent among children aged between 6 and 12 years (P = .029) but not among adolescents aged between 12 and 18 years (P = .139).
Study details: The data come from a retrospective analysis of 277 pediatric patients with IBS.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim JH et al. Association between body mass index and fecal calprotectin levels in children and adolescents with irritable bowel syndrome. Medicine (Baltimore). 2022;101(32):e29968 (Aug 12). Doi: 10.1097/MD.0000000000029968.
Key clinical point: Pediatric patients with irritable bowel syndrome (IBS) and normal weight had higher levels of fecal calprotectin than those with IBS and obesity, suggesting the role of obesity and intestinal inflammation in the development and manifestations of IBS in children.
Major finding: The mean calprotectin levels were significantly higher in patients with body mass index <85th vs 85th to <95th percentile (P = .028) and ≥95th percentile (P ≥ .025), with the difference being prominent among children aged between 6 and 12 years (P = .029) but not among adolescents aged between 12 and 18 years (P = .139).
Study details: The data come from a retrospective analysis of 277 pediatric patients with IBS.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim JH et al. Association between body mass index and fecal calprotectin levels in children and adolescents with irritable bowel syndrome. Medicine (Baltimore). 2022;101(32):e29968 (Aug 12). Doi: 10.1097/MD.0000000000029968.
Key clinical point: Pediatric patients with irritable bowel syndrome (IBS) and normal weight had higher levels of fecal calprotectin than those with IBS and obesity, suggesting the role of obesity and intestinal inflammation in the development and manifestations of IBS in children.
Major finding: The mean calprotectin levels were significantly higher in patients with body mass index <85th vs 85th to <95th percentile (P = .028) and ≥95th percentile (P ≥ .025), with the difference being prominent among children aged between 6 and 12 years (P = .029) but not among adolescents aged between 12 and 18 years (P = .139).
Study details: The data come from a retrospective analysis of 277 pediatric patients with IBS.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim JH et al. Association between body mass index and fecal calprotectin levels in children and adolescents with irritable bowel syndrome. Medicine (Baltimore). 2022;101(32):e29968 (Aug 12). Doi: 10.1097/MD.0000000000029968.
Meta-analysis evaluates association between Helicobacter pylori infection and IBS
Key clinical point: Meta-analysis indicated a relatively higher but nonsignificantly increased risk for Helicobacter pylori infection (HPI) in patients with irritable bowel syndrome (IBS) vs non-IBS participants; however, the association between IBS and HPI could be an underestimation, with a positive association persisting between HPI and diarrhea-type IBS (IBS-D).
Major finding: A nonsignificant positive association was observed between HPI and IBS (odds ratio [OR], 1.03 ;lt; P = .84); however, the association was significant after excluding studies with defined confounding factors (adjusted OR, 1.29; P = .03), indicating an underestimation. The positive association of HPI persisted with IBS-D (OR, 1.54; P = .0003) but not with IBS-C (P = .17) or IBS-M (P = .33).
Study details: Findings are from a systematic review and meta-analysis of 13 studies including 1,403 patients with IBS and 11,770 non-IBS participants.
Disclosures: This research was funded by the National Natural Science Foundation of China and the Natural Science Foundation of Guangdong Province. The authors declared no conflicts of interest.
Source: Wang Z et al. Helicobacterpylori infection-A risk factor for irritable bowel syndrome? An updated systematic review and meta-analysis. Medicina (Kaunas). 2022;58(8):1035 (Aug 2). Doi: 10.3390/medicina58081035.
Key clinical point: Meta-analysis indicated a relatively higher but nonsignificantly increased risk for Helicobacter pylori infection (HPI) in patients with irritable bowel syndrome (IBS) vs non-IBS participants; however, the association between IBS and HPI could be an underestimation, with a positive association persisting between HPI and diarrhea-type IBS (IBS-D).
Major finding: A nonsignificant positive association was observed between HPI and IBS (odds ratio [OR], 1.03 ;lt; P = .84); however, the association was significant after excluding studies with defined confounding factors (adjusted OR, 1.29; P = .03), indicating an underestimation. The positive association of HPI persisted with IBS-D (OR, 1.54; P = .0003) but not with IBS-C (P = .17) or IBS-M (P = .33).
Study details: Findings are from a systematic review and meta-analysis of 13 studies including 1,403 patients with IBS and 11,770 non-IBS participants.
Disclosures: This research was funded by the National Natural Science Foundation of China and the Natural Science Foundation of Guangdong Province. The authors declared no conflicts of interest.
Source: Wang Z et al. Helicobacterpylori infection-A risk factor for irritable bowel syndrome? An updated systematic review and meta-analysis. Medicina (Kaunas). 2022;58(8):1035 (Aug 2). Doi: 10.3390/medicina58081035.
Key clinical point: Meta-analysis indicated a relatively higher but nonsignificantly increased risk for Helicobacter pylori infection (HPI) in patients with irritable bowel syndrome (IBS) vs non-IBS participants; however, the association between IBS and HPI could be an underestimation, with a positive association persisting between HPI and diarrhea-type IBS (IBS-D).
Major finding: A nonsignificant positive association was observed between HPI and IBS (odds ratio [OR], 1.03 ;lt; P = .84); however, the association was significant after excluding studies with defined confounding factors (adjusted OR, 1.29; P = .03), indicating an underestimation. The positive association of HPI persisted with IBS-D (OR, 1.54; P = .0003) but not with IBS-C (P = .17) or IBS-M (P = .33).
Study details: Findings are from a systematic review and meta-analysis of 13 studies including 1,403 patients with IBS and 11,770 non-IBS participants.
Disclosures: This research was funded by the National Natural Science Foundation of China and the Natural Science Foundation of Guangdong Province. The authors declared no conflicts of interest.
Source: Wang Z et al. Helicobacterpylori infection-A risk factor for irritable bowel syndrome? An updated systematic review and meta-analysis. Medicina (Kaunas). 2022;58(8):1035 (Aug 2). Doi: 10.3390/medicina58081035.
IBS prevalence in veterans and its associations with psychological factors
Key clinical point: Irritable bowel syndrome (IBS) is prevalent among veterans and is associated with significant psychological comorbidities.
Major finding: Overall, 28.4% of veterans met Rome IV IBS criteria, and significant associations of IBS with anxiety (adjusted odds ratio [aOR], 3.47; P < .001), depression (aOR, 2.88; P < .001), post-traumatic stress disorder (aOR, 3.09; P < .001), prior infectious enteritis (aOR, 4.44; P < .001), and a history of bowel problems after antibiotics (aOR, 1.84; P = .005) were observed.
Study details: The data come from a cross-sectional survey including 858 veteran respondents (mean age, 53.6 years), of whom 244 had IBS.
Disclosures: The authors declared no conflicts of interest.
Source: Shin A et al. The prevalence, humanistic burden, and healthcare impact of irritable bowel syndrome (IBS) among United States veterans. Clin Gastroenterol Hepatol. 2022 (Aug 11). Doi: 10.1016/j.cgh.2022.08.005.
Key clinical point: Irritable bowel syndrome (IBS) is prevalent among veterans and is associated with significant psychological comorbidities.
Major finding: Overall, 28.4% of veterans met Rome IV IBS criteria, and significant associations of IBS with anxiety (adjusted odds ratio [aOR], 3.47; P < .001), depression (aOR, 2.88; P < .001), post-traumatic stress disorder (aOR, 3.09; P < .001), prior infectious enteritis (aOR, 4.44; P < .001), and a history of bowel problems after antibiotics (aOR, 1.84; P = .005) were observed.
Study details: The data come from a cross-sectional survey including 858 veteran respondents (mean age, 53.6 years), of whom 244 had IBS.
Disclosures: The authors declared no conflicts of interest.
Source: Shin A et al. The prevalence, humanistic burden, and healthcare impact of irritable bowel syndrome (IBS) among United States veterans. Clin Gastroenterol Hepatol. 2022 (Aug 11). Doi: 10.1016/j.cgh.2022.08.005.
Key clinical point: Irritable bowel syndrome (IBS) is prevalent among veterans and is associated with significant psychological comorbidities.
Major finding: Overall, 28.4% of veterans met Rome IV IBS criteria, and significant associations of IBS with anxiety (adjusted odds ratio [aOR], 3.47; P < .001), depression (aOR, 2.88; P < .001), post-traumatic stress disorder (aOR, 3.09; P < .001), prior infectious enteritis (aOR, 4.44; P < .001), and a history of bowel problems after antibiotics (aOR, 1.84; P = .005) were observed.
Study details: The data come from a cross-sectional survey including 858 veteran respondents (mean age, 53.6 years), of whom 244 had IBS.
Disclosures: The authors declared no conflicts of interest.
Source: Shin A et al. The prevalence, humanistic burden, and healthcare impact of irritable bowel syndrome (IBS) among United States veterans. Clin Gastroenterol Hepatol. 2022 (Aug 11). Doi: 10.1016/j.cgh.2022.08.005.
Somatization and celiac disease among primary risk factors for IBS
Key clinical point: Somatization and celiac disease are the primary risk factors associated with irritable bowel syndrome (IBS) in both men and women.
Major finding: The risk for IBS was almost 4-fold higher in men (adjusted odds ratio [aOR] 4.786; 95% CI 4.544-5.041) and women (aOR 5.326; 95% CI 4.863-5.832) experiencing somatization, with the second important influencing factor being celiac disease (men: aOR 4.107; 95% CI 3.132-5.385; women: aOR 3.783; 95% CI 3.310-4.323).
Study details: This study included 31,918 participants who met the Rome III criteria for IBS and completed the Digestive Health Questionnaire.
Disclosures: This study was supported by QW of the National Natural Science Foundation of China and the National Office for Philosophy and Social Sciences. The authors declared no potential conflicts of interest.
Source: Wang K et al. Factors related to irritable bowel syndrome and differences among subtypes: A cross-sectional study in the UK Biobank. Front Pharmacol. 2022;13:905564 (Aug 26). Doi: 10.3389/fphar.2022.905564
Key clinical point: Somatization and celiac disease are the primary risk factors associated with irritable bowel syndrome (IBS) in both men and women.
Major finding: The risk for IBS was almost 4-fold higher in men (adjusted odds ratio [aOR] 4.786; 95% CI 4.544-5.041) and women (aOR 5.326; 95% CI 4.863-5.832) experiencing somatization, with the second important influencing factor being celiac disease (men: aOR 4.107; 95% CI 3.132-5.385; women: aOR 3.783; 95% CI 3.310-4.323).
Study details: This study included 31,918 participants who met the Rome III criteria for IBS and completed the Digestive Health Questionnaire.
Disclosures: This study was supported by QW of the National Natural Science Foundation of China and the National Office for Philosophy and Social Sciences. The authors declared no potential conflicts of interest.
Source: Wang K et al. Factors related to irritable bowel syndrome and differences among subtypes: A cross-sectional study in the UK Biobank. Front Pharmacol. 2022;13:905564 (Aug 26). Doi: 10.3389/fphar.2022.905564
Key clinical point: Somatization and celiac disease are the primary risk factors associated with irritable bowel syndrome (IBS) in both men and women.
Major finding: The risk for IBS was almost 4-fold higher in men (adjusted odds ratio [aOR] 4.786; 95% CI 4.544-5.041) and women (aOR 5.326; 95% CI 4.863-5.832) experiencing somatization, with the second important influencing factor being celiac disease (men: aOR 4.107; 95% CI 3.132-5.385; women: aOR 3.783; 95% CI 3.310-4.323).
Study details: This study included 31,918 participants who met the Rome III criteria for IBS and completed the Digestive Health Questionnaire.
Disclosures: This study was supported by QW of the National Natural Science Foundation of China and the National Office for Philosophy and Social Sciences. The authors declared no potential conflicts of interest.
Source: Wang K et al. Factors related to irritable bowel syndrome and differences among subtypes: A cross-sectional study in the UK Biobank. Front Pharmacol. 2022;13:905564 (Aug 26). Doi: 10.3389/fphar.2022.905564
Supplementation with a multistrain probiotic improves leaky gut in patients with IBS-D
Key clinical point: Supplementation with a multistrain probiotic for 30 days improved intestinal permeability, stool consistency, and health-related quality-of-life (QoL) in a considerable proportion of patients with diarrhea-predominant irritable bowel syndrome (IBS-D).
Major finding: On day 30, the intestinal permeability improved or normalized in 81.5% of patients, with the intestinal permeability decreasing by 3.4 units (P = .0005), the Bristol Stool Scale score decreasing by 0.9 units (P = .0057), and the IBS-QoL total score increasing by 8.0 points (95% CI 3.0-12.9). The multistrain probiotic was well tolerated.
Study details: The data come from a pilot, open-label, prospective, phase 4 study including 30 patients with IBS-D and leaky gut who received 2 capsules of multistrain probiotic daily for 30 days.
Disclosures: This study was sponsored by PiLeJe Laboratoire. SA Abdellah and C Gal declared being employees of PiLeJe Laboratoire. L Laterza and A Gasbarrini reported ties with various sources.
Source: Ait Abdellah S et al. Effect of a multistrain probiotic on leaky gut in patients with diarrhea-predominant irritable bowel syndrome (IBS-D): A pilot study. Dig Dis. 2022 (Aug 25). Doi: 10.1159/000526712
Key clinical point: Supplementation with a multistrain probiotic for 30 days improved intestinal permeability, stool consistency, and health-related quality-of-life (QoL) in a considerable proportion of patients with diarrhea-predominant irritable bowel syndrome (IBS-D).
Major finding: On day 30, the intestinal permeability improved or normalized in 81.5% of patients, with the intestinal permeability decreasing by 3.4 units (P = .0005), the Bristol Stool Scale score decreasing by 0.9 units (P = .0057), and the IBS-QoL total score increasing by 8.0 points (95% CI 3.0-12.9). The multistrain probiotic was well tolerated.
Study details: The data come from a pilot, open-label, prospective, phase 4 study including 30 patients with IBS-D and leaky gut who received 2 capsules of multistrain probiotic daily for 30 days.
Disclosures: This study was sponsored by PiLeJe Laboratoire. SA Abdellah and C Gal declared being employees of PiLeJe Laboratoire. L Laterza and A Gasbarrini reported ties with various sources.
Source: Ait Abdellah S et al. Effect of a multistrain probiotic on leaky gut in patients with diarrhea-predominant irritable bowel syndrome (IBS-D): A pilot study. Dig Dis. 2022 (Aug 25). Doi: 10.1159/000526712
Key clinical point: Supplementation with a multistrain probiotic for 30 days improved intestinal permeability, stool consistency, and health-related quality-of-life (QoL) in a considerable proportion of patients with diarrhea-predominant irritable bowel syndrome (IBS-D).
Major finding: On day 30, the intestinal permeability improved or normalized in 81.5% of patients, with the intestinal permeability decreasing by 3.4 units (P = .0005), the Bristol Stool Scale score decreasing by 0.9 units (P = .0057), and the IBS-QoL total score increasing by 8.0 points (95% CI 3.0-12.9). The multistrain probiotic was well tolerated.
Study details: The data come from a pilot, open-label, prospective, phase 4 study including 30 patients with IBS-D and leaky gut who received 2 capsules of multistrain probiotic daily for 30 days.
Disclosures: This study was sponsored by PiLeJe Laboratoire. SA Abdellah and C Gal declared being employees of PiLeJe Laboratoire. L Laterza and A Gasbarrini reported ties with various sources.
Source: Ait Abdellah S et al. Effect of a multistrain probiotic on leaky gut in patients with diarrhea-predominant irritable bowel syndrome (IBS-D): A pilot study. Dig Dis. 2022 (Aug 25). Doi: 10.1159/000526712