User login
Children with autism show distinct brain features related to motor impairment
Previous research suggests that individuals with ASD overlap in motor impairment with those with DCD. But these two conditions may differ significantly in some areas, as children with ASD tend to show weaker skills in social motor tasks such as imitation, wrote Emil Kilroy, PhD, of the University of Southern California, Los Angeles, and colleagues.
The neurobiological basis of autism remains unknown, despite many research efforts, in part because of the heterogeneity of the disease, said corresponding author Lisa Aziz-Zadeh, PhD, also of the University of Southern California, in an interview.
Comorbidity with other disorders is a strong contributing factor to heterogeneity, and approximately 80% of autistic individuals have motor impairments and meet criteria for a diagnosis of DCD, said Dr. Aziz-Zadeh. “Controlling for other comorbidities, such as developmental coordination disorder, when trying to understand the neural basis of autism is important, so that we can understand which neural circuits are related to [core symptoms of autism] and which ones are related to motor impairments that are comorbid with autism, but not necessarily part of the core symptomology,” she explained. “We focused on white matter pathways here because many researchers now think the underlying basis of autism, besides genetics, is brain connectivity differences.”
In their study published in Scientific Reports, the researchers reviewed data from whole-brain correlational tractography for 22 individuals with autism spectrum disorder, 16 with developmental coordination disorder, and 21 normally developing individuals, who served as the control group. The mean age of the participants was approximately 11 years; the age range was 8-17 years.
Overall, patterns of brain diffusion (movement of fluid, mainly water molecules, in the brain) were significantly different in ASD children, compared with typically developing children.
The ASD group showed significantly reduced diffusivity in the bilateral fronto-parietal cingulum and the left parolfactory cingulum. This finding reflects previous studies suggesting an association between brain patterns in the cingulum area and ASD. But the current study is “the first to identify the fronto-parietal and the parolfactory portions of the cingulum as well as the anterior caudal u-fibers as specific to core ASD symptomatology and not related to motor-related comorbidity,” the researchers wrote.
Differences in brain diffusivity were associated with worse performance on motor skills and behavioral measures for children with ASD and children with DCD, compared with controls.
Motor development was assessed using the Total Movement Assessment Battery for Children-2 (MABC-2) and the Florida Apraxia Battery modified for children (FAB-M). The MABC-2 is among the most common tools for measuring motor skills and identifying clinically relevant motor deficits in children and teens aged 3-16 years. The test includes three subtest scores (manual dexterity, gross-motor aiming and catching, and balance) and a total score. Scores are based on a child’s best performance on each component, and higher scores indicate better functioning. In the new study, The MABC-2 total scores averaged 10.57 for controls, compared with 5.76 in the ASD group, and 4.31 in the DCD group.
Children with ASD differed from the other groups in social measures. Social skills were measured using several tools, including the Social Responsivity Scale (SRS Total), which is a parent-completed survey that includes a total score designed to reflect the severity of social deficits in ASD. It is divided into five subscales for parents to assess a child’s social skill impairment: social awareness, social cognition, social communication, social motivation, and mannerisms. Scores for the SRS are calculated in T-scores, in which a score of 50 represents the mean. T-scores of 59 and below are generally not associated with ASD, and patients with these scores are considered to have low to no symptomatology. Scores on the SRS Total in the new study were 45.95, 77.45, and 55.81 for the controls, ASD group, and DCD group, respectively.
Results should raise awareness
“The results were largely predicted in our hypotheses – that we would find specific white matter pathways in autism that would differ from [what we saw in typically developing patients and those with DCD], and that diffusivity in ASD would be related to socioemotional differences,” Dr. Aziz-Zadeh said, in an interview.
“What was surprising was that some pathways that had previously been thought to be different in autism were also compromised in DCD, indicating that they were common to motor deficits which both groups shared, not to core autism symptomology,” she noted.
A message for clinicians from the study is that a dual diagnosis of DCD is often missing in ASD practice, said Dr. Aziz-Zadeh. “Given that approximately 80% of children with ASD have DCD, testing for DCD and addressing potential motor issues should be more common practice,” she said.
Dr. Aziz-Zadeh and colleagues are now investigating relationships between the brain, behavior, and the gut microbiome. “We think that understanding autism from a full-body perspective, examining interactions between the brain and the body, will be an important step in this field,” she emphasized.
The study was limited by several factors, including the small sample size, the use of only right-handed participants, and the use of self-reports by children and parents, the researchers noted. Additionally, they noted that white matter develops at different rates in different age groups, and future studies might consider age as a factor, as well as further behavioral assessments, they said.
Small sample size limits conclusions
“Understanding the neuroanatomic differences that may contribute to the core symptoms of ASD is a very important goal for the field, particularly how they relate to other comorbid symptoms and neurodevelopmental disorders,” said Michael Gandal, MD, of the department of psychiatry at the University of Pennsylvania, Philadelphia, and a member of the Lifespan Brain Institute at the Children’s Hospital of Philadelphia, in an interview.
“While this study provides some clues into how structural connectivity may relate to motor coordination in ASD, it will be important to replicate these findings in a much larger sample before we can really appreciate how robust these findings are and how well they generalize to the broader ASD population,” Dr. Gandal emphasized.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Gandal had no financial conflicts to disclose.
Previous research suggests that individuals with ASD overlap in motor impairment with those with DCD. But these two conditions may differ significantly in some areas, as children with ASD tend to show weaker skills in social motor tasks such as imitation, wrote Emil Kilroy, PhD, of the University of Southern California, Los Angeles, and colleagues.
The neurobiological basis of autism remains unknown, despite many research efforts, in part because of the heterogeneity of the disease, said corresponding author Lisa Aziz-Zadeh, PhD, also of the University of Southern California, in an interview.
Comorbidity with other disorders is a strong contributing factor to heterogeneity, and approximately 80% of autistic individuals have motor impairments and meet criteria for a diagnosis of DCD, said Dr. Aziz-Zadeh. “Controlling for other comorbidities, such as developmental coordination disorder, when trying to understand the neural basis of autism is important, so that we can understand which neural circuits are related to [core symptoms of autism] and which ones are related to motor impairments that are comorbid with autism, but not necessarily part of the core symptomology,” she explained. “We focused on white matter pathways here because many researchers now think the underlying basis of autism, besides genetics, is brain connectivity differences.”
In their study published in Scientific Reports, the researchers reviewed data from whole-brain correlational tractography for 22 individuals with autism spectrum disorder, 16 with developmental coordination disorder, and 21 normally developing individuals, who served as the control group. The mean age of the participants was approximately 11 years; the age range was 8-17 years.
Overall, patterns of brain diffusion (movement of fluid, mainly water molecules, in the brain) were significantly different in ASD children, compared with typically developing children.
The ASD group showed significantly reduced diffusivity in the bilateral fronto-parietal cingulum and the left parolfactory cingulum. This finding reflects previous studies suggesting an association between brain patterns in the cingulum area and ASD. But the current study is “the first to identify the fronto-parietal and the parolfactory portions of the cingulum as well as the anterior caudal u-fibers as specific to core ASD symptomatology and not related to motor-related comorbidity,” the researchers wrote.
Differences in brain diffusivity were associated with worse performance on motor skills and behavioral measures for children with ASD and children with DCD, compared with controls.
Motor development was assessed using the Total Movement Assessment Battery for Children-2 (MABC-2) and the Florida Apraxia Battery modified for children (FAB-M). The MABC-2 is among the most common tools for measuring motor skills and identifying clinically relevant motor deficits in children and teens aged 3-16 years. The test includes three subtest scores (manual dexterity, gross-motor aiming and catching, and balance) and a total score. Scores are based on a child’s best performance on each component, and higher scores indicate better functioning. In the new study, The MABC-2 total scores averaged 10.57 for controls, compared with 5.76 in the ASD group, and 4.31 in the DCD group.
Children with ASD differed from the other groups in social measures. Social skills were measured using several tools, including the Social Responsivity Scale (SRS Total), which is a parent-completed survey that includes a total score designed to reflect the severity of social deficits in ASD. It is divided into five subscales for parents to assess a child’s social skill impairment: social awareness, social cognition, social communication, social motivation, and mannerisms. Scores for the SRS are calculated in T-scores, in which a score of 50 represents the mean. T-scores of 59 and below are generally not associated with ASD, and patients with these scores are considered to have low to no symptomatology. Scores on the SRS Total in the new study were 45.95, 77.45, and 55.81 for the controls, ASD group, and DCD group, respectively.
Results should raise awareness
“The results were largely predicted in our hypotheses – that we would find specific white matter pathways in autism that would differ from [what we saw in typically developing patients and those with DCD], and that diffusivity in ASD would be related to socioemotional differences,” Dr. Aziz-Zadeh said, in an interview.
“What was surprising was that some pathways that had previously been thought to be different in autism were also compromised in DCD, indicating that they were common to motor deficits which both groups shared, not to core autism symptomology,” she noted.
A message for clinicians from the study is that a dual diagnosis of DCD is often missing in ASD practice, said Dr. Aziz-Zadeh. “Given that approximately 80% of children with ASD have DCD, testing for DCD and addressing potential motor issues should be more common practice,” she said.
Dr. Aziz-Zadeh and colleagues are now investigating relationships between the brain, behavior, and the gut microbiome. “We think that understanding autism from a full-body perspective, examining interactions between the brain and the body, will be an important step in this field,” she emphasized.
The study was limited by several factors, including the small sample size, the use of only right-handed participants, and the use of self-reports by children and parents, the researchers noted. Additionally, they noted that white matter develops at different rates in different age groups, and future studies might consider age as a factor, as well as further behavioral assessments, they said.
Small sample size limits conclusions
“Understanding the neuroanatomic differences that may contribute to the core symptoms of ASD is a very important goal for the field, particularly how they relate to other comorbid symptoms and neurodevelopmental disorders,” said Michael Gandal, MD, of the department of psychiatry at the University of Pennsylvania, Philadelphia, and a member of the Lifespan Brain Institute at the Children’s Hospital of Philadelphia, in an interview.
“While this study provides some clues into how structural connectivity may relate to motor coordination in ASD, it will be important to replicate these findings in a much larger sample before we can really appreciate how robust these findings are and how well they generalize to the broader ASD population,” Dr. Gandal emphasized.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Gandal had no financial conflicts to disclose.
Previous research suggests that individuals with ASD overlap in motor impairment with those with DCD. But these two conditions may differ significantly in some areas, as children with ASD tend to show weaker skills in social motor tasks such as imitation, wrote Emil Kilroy, PhD, of the University of Southern California, Los Angeles, and colleagues.
The neurobiological basis of autism remains unknown, despite many research efforts, in part because of the heterogeneity of the disease, said corresponding author Lisa Aziz-Zadeh, PhD, also of the University of Southern California, in an interview.
Comorbidity with other disorders is a strong contributing factor to heterogeneity, and approximately 80% of autistic individuals have motor impairments and meet criteria for a diagnosis of DCD, said Dr. Aziz-Zadeh. “Controlling for other comorbidities, such as developmental coordination disorder, when trying to understand the neural basis of autism is important, so that we can understand which neural circuits are related to [core symptoms of autism] and which ones are related to motor impairments that are comorbid with autism, but not necessarily part of the core symptomology,” she explained. “We focused on white matter pathways here because many researchers now think the underlying basis of autism, besides genetics, is brain connectivity differences.”
In their study published in Scientific Reports, the researchers reviewed data from whole-brain correlational tractography for 22 individuals with autism spectrum disorder, 16 with developmental coordination disorder, and 21 normally developing individuals, who served as the control group. The mean age of the participants was approximately 11 years; the age range was 8-17 years.
Overall, patterns of brain diffusion (movement of fluid, mainly water molecules, in the brain) were significantly different in ASD children, compared with typically developing children.
The ASD group showed significantly reduced diffusivity in the bilateral fronto-parietal cingulum and the left parolfactory cingulum. This finding reflects previous studies suggesting an association between brain patterns in the cingulum area and ASD. But the current study is “the first to identify the fronto-parietal and the parolfactory portions of the cingulum as well as the anterior caudal u-fibers as specific to core ASD symptomatology and not related to motor-related comorbidity,” the researchers wrote.
Differences in brain diffusivity were associated with worse performance on motor skills and behavioral measures for children with ASD and children with DCD, compared with controls.
Motor development was assessed using the Total Movement Assessment Battery for Children-2 (MABC-2) and the Florida Apraxia Battery modified for children (FAB-M). The MABC-2 is among the most common tools for measuring motor skills and identifying clinically relevant motor deficits in children and teens aged 3-16 years. The test includes three subtest scores (manual dexterity, gross-motor aiming and catching, and balance) and a total score. Scores are based on a child’s best performance on each component, and higher scores indicate better functioning. In the new study, The MABC-2 total scores averaged 10.57 for controls, compared with 5.76 in the ASD group, and 4.31 in the DCD group.
Children with ASD differed from the other groups in social measures. Social skills were measured using several tools, including the Social Responsivity Scale (SRS Total), which is a parent-completed survey that includes a total score designed to reflect the severity of social deficits in ASD. It is divided into five subscales for parents to assess a child’s social skill impairment: social awareness, social cognition, social communication, social motivation, and mannerisms. Scores for the SRS are calculated in T-scores, in which a score of 50 represents the mean. T-scores of 59 and below are generally not associated with ASD, and patients with these scores are considered to have low to no symptomatology. Scores on the SRS Total in the new study were 45.95, 77.45, and 55.81 for the controls, ASD group, and DCD group, respectively.
Results should raise awareness
“The results were largely predicted in our hypotheses – that we would find specific white matter pathways in autism that would differ from [what we saw in typically developing patients and those with DCD], and that diffusivity in ASD would be related to socioemotional differences,” Dr. Aziz-Zadeh said, in an interview.
“What was surprising was that some pathways that had previously been thought to be different in autism were also compromised in DCD, indicating that they were common to motor deficits which both groups shared, not to core autism symptomology,” she noted.
A message for clinicians from the study is that a dual diagnosis of DCD is often missing in ASD practice, said Dr. Aziz-Zadeh. “Given that approximately 80% of children with ASD have DCD, testing for DCD and addressing potential motor issues should be more common practice,” she said.
Dr. Aziz-Zadeh and colleagues are now investigating relationships between the brain, behavior, and the gut microbiome. “We think that understanding autism from a full-body perspective, examining interactions between the brain and the body, will be an important step in this field,” she emphasized.
The study was limited by several factors, including the small sample size, the use of only right-handed participants, and the use of self-reports by children and parents, the researchers noted. Additionally, they noted that white matter develops at different rates in different age groups, and future studies might consider age as a factor, as well as further behavioral assessments, they said.
Small sample size limits conclusions
“Understanding the neuroanatomic differences that may contribute to the core symptoms of ASD is a very important goal for the field, particularly how they relate to other comorbid symptoms and neurodevelopmental disorders,” said Michael Gandal, MD, of the department of psychiatry at the University of Pennsylvania, Philadelphia, and a member of the Lifespan Brain Institute at the Children’s Hospital of Philadelphia, in an interview.
“While this study provides some clues into how structural connectivity may relate to motor coordination in ASD, it will be important to replicate these findings in a much larger sample before we can really appreciate how robust these findings are and how well they generalize to the broader ASD population,” Dr. Gandal emphasized.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Gandal had no financial conflicts to disclose.
FROM SCIENTIFIC REPORTS
Could intermittent fasting improve GERD symptoms?
, suggests a small U.S. study.
Twenty-five individuals with suspected GERD symptoms underwent 96-hour pH monitoring. They were asked to follow their normal diet for the first 48 hours; for the second 48 hours, they were asked to switch to a 16-hour fast, which was followed by an 8-hour eating window.
Just over a third of participants were fully compliant with the 16:8 intermittent fasting. But those who followed the regimen experienced a mild reduction in mean acid exposure time and self-reported GERD symptoms scores.
The research was published online by the Journal of Clinical Gastroenterology.
Costly condition
The prevalence of GERD in the United States is estimated at 18%-28%. Annual costs of the condition are more than $18 billion per year, largely through pharmacologic therapies and diagnostic testing, write lead author Yan Jiang, MD, division of gastrointestinal and liver D-diseases, Keck Medicine of University of Southern California, Los Angeles, and colleagues.
Proton pump inhibitor (PPI) therapy is one of the most prescribed classes of medications in the United States, the authors write. But concerns over the long-term safety of the drugs, as well as the fact that half of patients report breakthrough GERD symptoms, have generated interest in non-PPI treatments among patients and providers.
The role of diet in the management of GERD, however, remains poorly understood, despite the fact that obesity and weight gain have been linked to reflux.
The authors note that intermittent fasting has shown benefits in coronary artery disease, inflammatory disorders, obesity, and diabetes. Proposed mechanisms include anti-inflammatory effects, weight loss, and alterations in hormone secretion.
Intervention test in a 96-hour clinical evaluation for GERD
To investigate the effects of intermittent fasting in GERD, the researchers screened patients referred to the Stanford University gastrointestinal clinic for diagnostic 96-hour ambulatory wireless pH monitoring of suspected acid reflux symptoms.
They excluded patients younger than 18 years, pregnant women, those with insulin-dependent diabetes, and those who had used PPIs within the previous 7 days. There were other exclusion criteria as well.
The study was completed by 25 participants. The mean age of the patients was 43.5 years; 52% were women. Just under half (44%) were White, and the mean body mass index was 25.8 kg/m2.
For the first 48 hours of the pH monitoring, the patients followed their baseline diet. For the second 48 hours, they were asked to follow an intermittent fasting regimen.
In that regimen, during a 24-hour period, there was an 8-hour caloric intake window and no caloric intake during the other 16 consecutive hours. Participants who fasted for at least 15 hours, as indicated on a self-report food log, were considered successful.
Only 36% of participants were fully adherent to the fasting regimen; 84% were partially compliant, defined as following the regimen for at least 1 of the 2 days of intermittent fasting.
On intermittent fasting days, the mean acid exposure time was 3.5%, compared with 4.3% on the baseline diet. The team calculated that adhering to the 16:8 intermittent fasting regimen reduced the mean acid exposure time by 0.64%.
Intermittent fasting was also associated with a reduction in total GERD symptom scores, at 9.9 following day 4 versus 14.3 following day 2. There were reductions in heartburn symptoms scores of 2.6 and in regurgitation scores of 1.8.
When the researchers compared individuals who were compliant with intermittent fasting with those who were only partially compliant, they found that there was still an improvement in GERD symptoms, with a reduction in scores of 3.2.
More acid, bigger benefits
There could be several explanations for the findings, Dr. Jiang said in an interview.
In the short-term study, fewer meals during intermittent fasting and more hours between the last meal and bedtime can help with the supine symptoms of GERD, Dr. Jiang said.
Over the longer term, he added, previous studies have suggested that fasting-induced alterations in inflammatory cytokines or cells could be a contributory mechanism, “but it’s not something that we can glean from our study.”
Participants with elevated acid exposure at baseline and who were more likely to have GERD diagnosed by the pH monitoring seemed to experience the greatest benefit from intermittent fasting, Dr. Jiang pointed out.
“This study looked at all comers with GERD symptoms,” he said. “But if you were to do another study with people with proven GERD, they might experience a bigger impact with intermittent fasting.”
Dr. Jiang added, “If a patient is willing to do intermittent fasting, and certainly if they have other reasons [for doing so], I think it doesn’t hurt, and it might actually help them a little bit in their current symptoms.”
Larger scale, longer follow-up studies needed
Luigi Bonavina, MD, department of biomedical sciences for health, University of Milan, IRCCS Policlinico San Donato, Italy, said in an interview that it was a “nice, original study.”
It is “noteworthy that only one previous study explored the effect of Ramadan on GERD symptoms and found a small improvement of GERD symptoms,” Dr. Bonavina said. “Unfortunately, the magnitude of effect [in the current study] was not as one may have expected, due to small sample size and low compliance with intermittent fasting.”
Although the effect was “mild compared to that seen with PPIs,” it would “be interesting to see whether the results of this pilot, proof-of-concept study can be confirmed on a larger scale with longer follow-up to prove that reflux symptoms will not worsen over time,” he said.
“Intermittent fasting may be recommended, especially in overweight-obese patients with GERD symptoms who are poor responders to gastric acid inhibitors,” Dr. Bonavina added. “Reduction of inflammation, reduction of meal intake, and going to bed with an empty stomach may also work in patients with GERD.”
No funding for the study has been declared. The authors and Dr. Bonavina report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a small U.S. study.
Twenty-five individuals with suspected GERD symptoms underwent 96-hour pH monitoring. They were asked to follow their normal diet for the first 48 hours; for the second 48 hours, they were asked to switch to a 16-hour fast, which was followed by an 8-hour eating window.
Just over a third of participants were fully compliant with the 16:8 intermittent fasting. But those who followed the regimen experienced a mild reduction in mean acid exposure time and self-reported GERD symptoms scores.
The research was published online by the Journal of Clinical Gastroenterology.
Costly condition
The prevalence of GERD in the United States is estimated at 18%-28%. Annual costs of the condition are more than $18 billion per year, largely through pharmacologic therapies and diagnostic testing, write lead author Yan Jiang, MD, division of gastrointestinal and liver D-diseases, Keck Medicine of University of Southern California, Los Angeles, and colleagues.
Proton pump inhibitor (PPI) therapy is one of the most prescribed classes of medications in the United States, the authors write. But concerns over the long-term safety of the drugs, as well as the fact that half of patients report breakthrough GERD symptoms, have generated interest in non-PPI treatments among patients and providers.
The role of diet in the management of GERD, however, remains poorly understood, despite the fact that obesity and weight gain have been linked to reflux.
The authors note that intermittent fasting has shown benefits in coronary artery disease, inflammatory disorders, obesity, and diabetes. Proposed mechanisms include anti-inflammatory effects, weight loss, and alterations in hormone secretion.
Intervention test in a 96-hour clinical evaluation for GERD
To investigate the effects of intermittent fasting in GERD, the researchers screened patients referred to the Stanford University gastrointestinal clinic for diagnostic 96-hour ambulatory wireless pH monitoring of suspected acid reflux symptoms.
They excluded patients younger than 18 years, pregnant women, those with insulin-dependent diabetes, and those who had used PPIs within the previous 7 days. There were other exclusion criteria as well.
The study was completed by 25 participants. The mean age of the patients was 43.5 years; 52% were women. Just under half (44%) were White, and the mean body mass index was 25.8 kg/m2.
For the first 48 hours of the pH monitoring, the patients followed their baseline diet. For the second 48 hours, they were asked to follow an intermittent fasting regimen.
In that regimen, during a 24-hour period, there was an 8-hour caloric intake window and no caloric intake during the other 16 consecutive hours. Participants who fasted for at least 15 hours, as indicated on a self-report food log, were considered successful.
Only 36% of participants were fully adherent to the fasting regimen; 84% were partially compliant, defined as following the regimen for at least 1 of the 2 days of intermittent fasting.
On intermittent fasting days, the mean acid exposure time was 3.5%, compared with 4.3% on the baseline diet. The team calculated that adhering to the 16:8 intermittent fasting regimen reduced the mean acid exposure time by 0.64%.
Intermittent fasting was also associated with a reduction in total GERD symptom scores, at 9.9 following day 4 versus 14.3 following day 2. There were reductions in heartburn symptoms scores of 2.6 and in regurgitation scores of 1.8.
When the researchers compared individuals who were compliant with intermittent fasting with those who were only partially compliant, they found that there was still an improvement in GERD symptoms, with a reduction in scores of 3.2.
More acid, bigger benefits
There could be several explanations for the findings, Dr. Jiang said in an interview.
In the short-term study, fewer meals during intermittent fasting and more hours between the last meal and bedtime can help with the supine symptoms of GERD, Dr. Jiang said.
Over the longer term, he added, previous studies have suggested that fasting-induced alterations in inflammatory cytokines or cells could be a contributory mechanism, “but it’s not something that we can glean from our study.”
Participants with elevated acid exposure at baseline and who were more likely to have GERD diagnosed by the pH monitoring seemed to experience the greatest benefit from intermittent fasting, Dr. Jiang pointed out.
“This study looked at all comers with GERD symptoms,” he said. “But if you were to do another study with people with proven GERD, they might experience a bigger impact with intermittent fasting.”
Dr. Jiang added, “If a patient is willing to do intermittent fasting, and certainly if they have other reasons [for doing so], I think it doesn’t hurt, and it might actually help them a little bit in their current symptoms.”
Larger scale, longer follow-up studies needed
Luigi Bonavina, MD, department of biomedical sciences for health, University of Milan, IRCCS Policlinico San Donato, Italy, said in an interview that it was a “nice, original study.”
It is “noteworthy that only one previous study explored the effect of Ramadan on GERD symptoms and found a small improvement of GERD symptoms,” Dr. Bonavina said. “Unfortunately, the magnitude of effect [in the current study] was not as one may have expected, due to small sample size and low compliance with intermittent fasting.”
Although the effect was “mild compared to that seen with PPIs,” it would “be interesting to see whether the results of this pilot, proof-of-concept study can be confirmed on a larger scale with longer follow-up to prove that reflux symptoms will not worsen over time,” he said.
“Intermittent fasting may be recommended, especially in overweight-obese patients with GERD symptoms who are poor responders to gastric acid inhibitors,” Dr. Bonavina added. “Reduction of inflammation, reduction of meal intake, and going to bed with an empty stomach may also work in patients with GERD.”
No funding for the study has been declared. The authors and Dr. Bonavina report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a small U.S. study.
Twenty-five individuals with suspected GERD symptoms underwent 96-hour pH monitoring. They were asked to follow their normal diet for the first 48 hours; for the second 48 hours, they were asked to switch to a 16-hour fast, which was followed by an 8-hour eating window.
Just over a third of participants were fully compliant with the 16:8 intermittent fasting. But those who followed the regimen experienced a mild reduction in mean acid exposure time and self-reported GERD symptoms scores.
The research was published online by the Journal of Clinical Gastroenterology.
Costly condition
The prevalence of GERD in the United States is estimated at 18%-28%. Annual costs of the condition are more than $18 billion per year, largely through pharmacologic therapies and diagnostic testing, write lead author Yan Jiang, MD, division of gastrointestinal and liver D-diseases, Keck Medicine of University of Southern California, Los Angeles, and colleagues.
Proton pump inhibitor (PPI) therapy is one of the most prescribed classes of medications in the United States, the authors write. But concerns over the long-term safety of the drugs, as well as the fact that half of patients report breakthrough GERD symptoms, have generated interest in non-PPI treatments among patients and providers.
The role of diet in the management of GERD, however, remains poorly understood, despite the fact that obesity and weight gain have been linked to reflux.
The authors note that intermittent fasting has shown benefits in coronary artery disease, inflammatory disorders, obesity, and diabetes. Proposed mechanisms include anti-inflammatory effects, weight loss, and alterations in hormone secretion.
Intervention test in a 96-hour clinical evaluation for GERD
To investigate the effects of intermittent fasting in GERD, the researchers screened patients referred to the Stanford University gastrointestinal clinic for diagnostic 96-hour ambulatory wireless pH monitoring of suspected acid reflux symptoms.
They excluded patients younger than 18 years, pregnant women, those with insulin-dependent diabetes, and those who had used PPIs within the previous 7 days. There were other exclusion criteria as well.
The study was completed by 25 participants. The mean age of the patients was 43.5 years; 52% were women. Just under half (44%) were White, and the mean body mass index was 25.8 kg/m2.
For the first 48 hours of the pH monitoring, the patients followed their baseline diet. For the second 48 hours, they were asked to follow an intermittent fasting regimen.
In that regimen, during a 24-hour period, there was an 8-hour caloric intake window and no caloric intake during the other 16 consecutive hours. Participants who fasted for at least 15 hours, as indicated on a self-report food log, were considered successful.
Only 36% of participants were fully adherent to the fasting regimen; 84% were partially compliant, defined as following the regimen for at least 1 of the 2 days of intermittent fasting.
On intermittent fasting days, the mean acid exposure time was 3.5%, compared with 4.3% on the baseline diet. The team calculated that adhering to the 16:8 intermittent fasting regimen reduced the mean acid exposure time by 0.64%.
Intermittent fasting was also associated with a reduction in total GERD symptom scores, at 9.9 following day 4 versus 14.3 following day 2. There were reductions in heartburn symptoms scores of 2.6 and in regurgitation scores of 1.8.
When the researchers compared individuals who were compliant with intermittent fasting with those who were only partially compliant, they found that there was still an improvement in GERD symptoms, with a reduction in scores of 3.2.
More acid, bigger benefits
There could be several explanations for the findings, Dr. Jiang said in an interview.
In the short-term study, fewer meals during intermittent fasting and more hours between the last meal and bedtime can help with the supine symptoms of GERD, Dr. Jiang said.
Over the longer term, he added, previous studies have suggested that fasting-induced alterations in inflammatory cytokines or cells could be a contributory mechanism, “but it’s not something that we can glean from our study.”
Participants with elevated acid exposure at baseline and who were more likely to have GERD diagnosed by the pH monitoring seemed to experience the greatest benefit from intermittent fasting, Dr. Jiang pointed out.
“This study looked at all comers with GERD symptoms,” he said. “But if you were to do another study with people with proven GERD, they might experience a bigger impact with intermittent fasting.”
Dr. Jiang added, “If a patient is willing to do intermittent fasting, and certainly if they have other reasons [for doing so], I think it doesn’t hurt, and it might actually help them a little bit in their current symptoms.”
Larger scale, longer follow-up studies needed
Luigi Bonavina, MD, department of biomedical sciences for health, University of Milan, IRCCS Policlinico San Donato, Italy, said in an interview that it was a “nice, original study.”
It is “noteworthy that only one previous study explored the effect of Ramadan on GERD symptoms and found a small improvement of GERD symptoms,” Dr. Bonavina said. “Unfortunately, the magnitude of effect [in the current study] was not as one may have expected, due to small sample size and low compliance with intermittent fasting.”
Although the effect was “mild compared to that seen with PPIs,” it would “be interesting to see whether the results of this pilot, proof-of-concept study can be confirmed on a larger scale with longer follow-up to prove that reflux symptoms will not worsen over time,” he said.
“Intermittent fasting may be recommended, especially in overweight-obese patients with GERD symptoms who are poor responders to gastric acid inhibitors,” Dr. Bonavina added. “Reduction of inflammation, reduction of meal intake, and going to bed with an empty stomach may also work in patients with GERD.”
No funding for the study has been declared. The authors and Dr. Bonavina report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL GASTROENTEROLOGY
At-home births rose during the pandemic, CDC reports
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
Diffuse Papular Eruption With Erosions and Ulcerations
The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
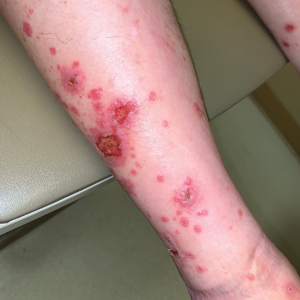
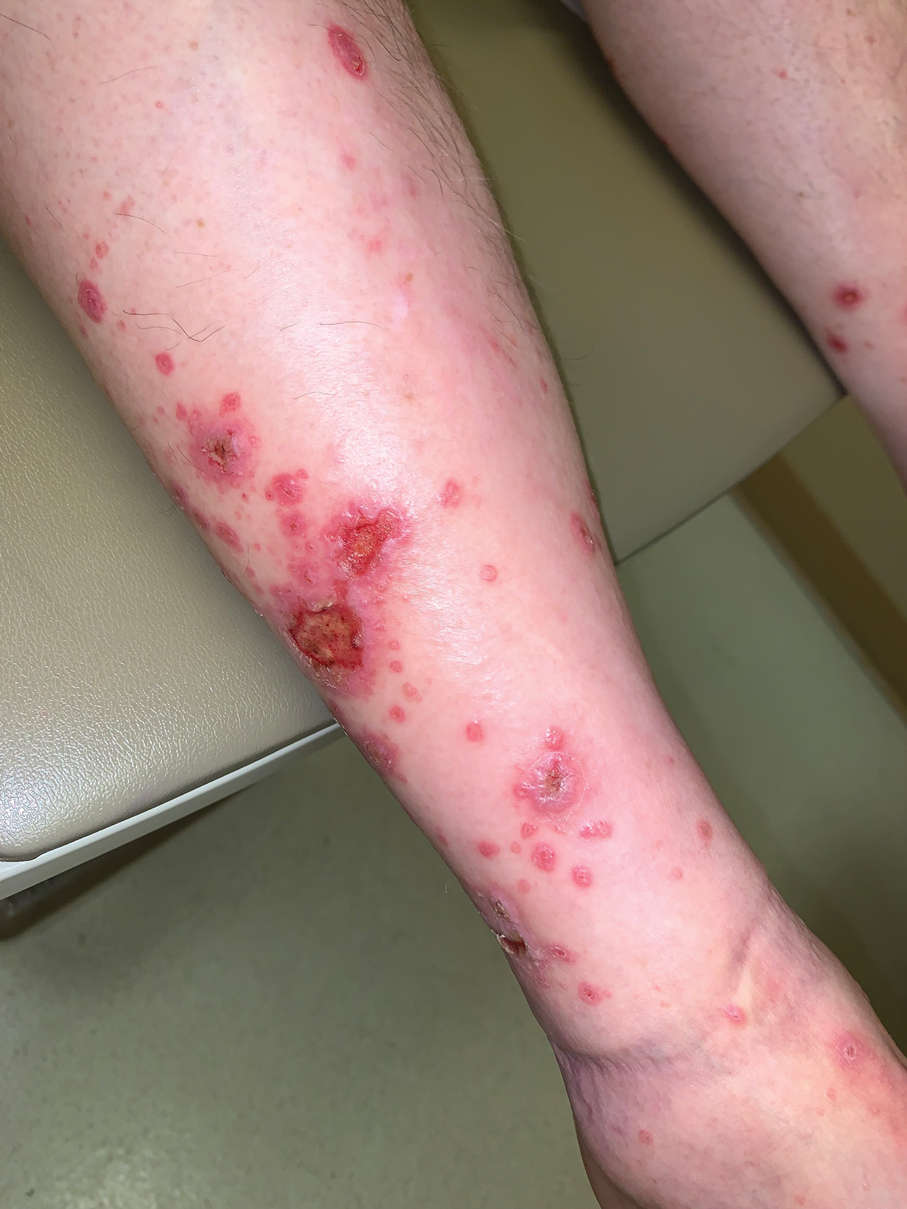
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
A 70-year-old man presented with a painful, pruritic, diffuse eruption on the trunk, legs, and arms of 2 months’ duration. He had a history of stage IV pleomorphic cell sarcoma of the retroperitoneum and was started on pembrolizumab therapy 6 weeks prior to the eruption. Physical examination revealed violaceous papules and plaques with shiny reticulated scaling as well as multiple scattered eroded papules and shallow ulcerations. The oral mucosa and genitals were spared. The patient endorsed blisters followed by open sores that were both itchy and painful. He denied self-infliction. Both the patient and his wife denied scratching. Two biopsies for direct immunofluorescence and histopathology were performed.
Poor NAFLD outcomes with increased VCTE-measured liver stiffness
according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Although previous retrospective studies have suggested that increased liver stiffness, as measured by VCTE (FibroScan), is associated with increases in liver-related events, there is a paucity of prospective data, reported Samer Gawrieh, MD, from Indiana University, Carmel and Indianapolis. VCTE is a noninvasive measure of cirrhosis progression.
In their prospective cohort study of patients representing the entire spectrum of NAFLD, the progression to LSM-defined cirrhosis was independently associated with the risk for a composite clinical outcome of death, decompensation, hepatocellular carcinoma, or a Model for End Stage Liver Disease (MELD) score of greater than 15, he said.
Their findings show that “progression to LSM-defined cirrhosis by VCTE is strongly associated with poor clinical outcomes,” Dr. Gawrieh said.
Study findings
Investigators looked at prospective data on 894 patients with biopsy-proven NAFLD in the Nonalcoholic Steatohepatitis (NASH) Clinical Research Network database. The sample included patients with a minimum of two LSM readings taken from 2014 through 2022.
They defined LSM-defined cirrhosis as reaching LSM of greater than 14.9 kPa (90% specificity cutoff) among patients without cirrhosis on the baseline VCTE (a 90% sensitivity cutoff of LSM less than 12.1 kPa).
They also performed a histology-based subanalysis, including data only from those patients who had LSM within 6 months of a liver biopsy.
The median patient age was 60 years, 37% were male, and 80.9% were White and 11.5% were Hispanic/Latino. The median body mass index (BMI) was 32.
Out of all the patients, 119 (13.3%) had progression to LSM-defined cirrhosis.
At a median follow-up of 3.69 years for the 775 patients without LSM progression, 79 (10.2%) had one or more of the events in the composite clinical outcome.
In contrast, after a median 5.48 years of follow-up, 31 of the 119 patients with progression (26.1%) had one or more of the composite events (P < .0001).
The median rates of progression to LSM-defined cirrhosis in the overall cohort were 2% at 1 year, 11% at 3 years, and 16% at 5 years.
Researchers found a correlation between progression to LSM-defined cirrhosis and baseline histological fibrosis stage on biopsy, with a rate of 7% among those with no baseline fibrosis, 9% each for patients with stage I A-C or stage II fibrosis, 24% of those with baseline bridging fibrosis, and 25% of those with baseline cirrhosis.
A comparison of the time to a composite clinical outcome event between patients with progression to LSM-defined cirrhosis and those without progression showed that LSM-defined progression was associated with near doubling in risk, with a hazard ratio of 1.84 (P = .0039).
In a multivariate Cox regression analysis controlling for age, sex, race, BMI, diabetes status, and baseline LSM, only LSM-defined progression (HR, 1.93; P < .01) and age (HR, 1.03; P < .01) were significant predictors.
Dr. Gawrieh noted that while age was a statistically significant factor, it was only weakly associated.
“These data suggest that development of cirrhosis LSM criteria is a promising surrogate for clinical outcomes in patients with NAFLD,” Dr. Gawrieh concluded.
Progression definition questioned
Following the presentation, Nezam Afdhal, MD, chief of the division of gastroenterology, hepatology, and nutrition at Beth Israel Deaconess Hospital in Boston, questioned how 25% of patients who had biopsy-proven cirrhosis could progress to LSM-defined cirrhosis.
Dr. Gawrieh said that, according to inclusion criteria, the patients could not have LSM-defined cirrhosis with the sensitivity cutoff of 12.1 kPa, and that of the 10 patients with baseline cirrhosis in the cohort, all had LSM of less than 12.1 kPa. However, he admitted that because those 10 patients were technically not progressors to cirrhosis, they should have been removed from the analysis for clinical outcomes.
Mark Hartman, MD, a clinical researcher at Eli Lilly and Company in Indianapolis, said the study is valuable but noted that those patients who progressed tended to have higher LSM at baseline as well as a higher [fibrosis-4 score].
Dr. Gawrieh added that the investigators are exploring variables that might explain progression to cirrhosis among patients without high baseline liver stiffness, such as alcohol use or drug-induced liver injury.
The study was supported by the National Institutes of Health and the NASH Clinical Research Network institutions. Dr. Gawrieh disclosed research grants from NIH, Zydus, Viking, and Sonic Incytes, and consulting for TransMedics and Pfizer. Dr. Afdhal and Dr. Hartman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Although previous retrospective studies have suggested that increased liver stiffness, as measured by VCTE (FibroScan), is associated with increases in liver-related events, there is a paucity of prospective data, reported Samer Gawrieh, MD, from Indiana University, Carmel and Indianapolis. VCTE is a noninvasive measure of cirrhosis progression.
In their prospective cohort study of patients representing the entire spectrum of NAFLD, the progression to LSM-defined cirrhosis was independently associated with the risk for a composite clinical outcome of death, decompensation, hepatocellular carcinoma, or a Model for End Stage Liver Disease (MELD) score of greater than 15, he said.
Their findings show that “progression to LSM-defined cirrhosis by VCTE is strongly associated with poor clinical outcomes,” Dr. Gawrieh said.
Study findings
Investigators looked at prospective data on 894 patients with biopsy-proven NAFLD in the Nonalcoholic Steatohepatitis (NASH) Clinical Research Network database. The sample included patients with a minimum of two LSM readings taken from 2014 through 2022.
They defined LSM-defined cirrhosis as reaching LSM of greater than 14.9 kPa (90% specificity cutoff) among patients without cirrhosis on the baseline VCTE (a 90% sensitivity cutoff of LSM less than 12.1 kPa).
They also performed a histology-based subanalysis, including data only from those patients who had LSM within 6 months of a liver biopsy.
The median patient age was 60 years, 37% were male, and 80.9% were White and 11.5% were Hispanic/Latino. The median body mass index (BMI) was 32.
Out of all the patients, 119 (13.3%) had progression to LSM-defined cirrhosis.
At a median follow-up of 3.69 years for the 775 patients without LSM progression, 79 (10.2%) had one or more of the events in the composite clinical outcome.
In contrast, after a median 5.48 years of follow-up, 31 of the 119 patients with progression (26.1%) had one or more of the composite events (P < .0001).
The median rates of progression to LSM-defined cirrhosis in the overall cohort were 2% at 1 year, 11% at 3 years, and 16% at 5 years.
Researchers found a correlation between progression to LSM-defined cirrhosis and baseline histological fibrosis stage on biopsy, with a rate of 7% among those with no baseline fibrosis, 9% each for patients with stage I A-C or stage II fibrosis, 24% of those with baseline bridging fibrosis, and 25% of those with baseline cirrhosis.
A comparison of the time to a composite clinical outcome event between patients with progression to LSM-defined cirrhosis and those without progression showed that LSM-defined progression was associated with near doubling in risk, with a hazard ratio of 1.84 (P = .0039).
In a multivariate Cox regression analysis controlling for age, sex, race, BMI, diabetes status, and baseline LSM, only LSM-defined progression (HR, 1.93; P < .01) and age (HR, 1.03; P < .01) were significant predictors.
Dr. Gawrieh noted that while age was a statistically significant factor, it was only weakly associated.
“These data suggest that development of cirrhosis LSM criteria is a promising surrogate for clinical outcomes in patients with NAFLD,” Dr. Gawrieh concluded.
Progression definition questioned
Following the presentation, Nezam Afdhal, MD, chief of the division of gastroenterology, hepatology, and nutrition at Beth Israel Deaconess Hospital in Boston, questioned how 25% of patients who had biopsy-proven cirrhosis could progress to LSM-defined cirrhosis.
Dr. Gawrieh said that, according to inclusion criteria, the patients could not have LSM-defined cirrhosis with the sensitivity cutoff of 12.1 kPa, and that of the 10 patients with baseline cirrhosis in the cohort, all had LSM of less than 12.1 kPa. However, he admitted that because those 10 patients were technically not progressors to cirrhosis, they should have been removed from the analysis for clinical outcomes.
Mark Hartman, MD, a clinical researcher at Eli Lilly and Company in Indianapolis, said the study is valuable but noted that those patients who progressed tended to have higher LSM at baseline as well as a higher [fibrosis-4 score].
Dr. Gawrieh added that the investigators are exploring variables that might explain progression to cirrhosis among patients without high baseline liver stiffness, such as alcohol use or drug-induced liver injury.
The study was supported by the National Institutes of Health and the NASH Clinical Research Network institutions. Dr. Gawrieh disclosed research grants from NIH, Zydus, Viking, and Sonic Incytes, and consulting for TransMedics and Pfizer. Dr. Afdhal and Dr. Hartman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Although previous retrospective studies have suggested that increased liver stiffness, as measured by VCTE (FibroScan), is associated with increases in liver-related events, there is a paucity of prospective data, reported Samer Gawrieh, MD, from Indiana University, Carmel and Indianapolis. VCTE is a noninvasive measure of cirrhosis progression.
In their prospective cohort study of patients representing the entire spectrum of NAFLD, the progression to LSM-defined cirrhosis was independently associated with the risk for a composite clinical outcome of death, decompensation, hepatocellular carcinoma, or a Model for End Stage Liver Disease (MELD) score of greater than 15, he said.
Their findings show that “progression to LSM-defined cirrhosis by VCTE is strongly associated with poor clinical outcomes,” Dr. Gawrieh said.
Study findings
Investigators looked at prospective data on 894 patients with biopsy-proven NAFLD in the Nonalcoholic Steatohepatitis (NASH) Clinical Research Network database. The sample included patients with a minimum of two LSM readings taken from 2014 through 2022.
They defined LSM-defined cirrhosis as reaching LSM of greater than 14.9 kPa (90% specificity cutoff) among patients without cirrhosis on the baseline VCTE (a 90% sensitivity cutoff of LSM less than 12.1 kPa).
They also performed a histology-based subanalysis, including data only from those patients who had LSM within 6 months of a liver biopsy.
The median patient age was 60 years, 37% were male, and 80.9% were White and 11.5% were Hispanic/Latino. The median body mass index (BMI) was 32.
Out of all the patients, 119 (13.3%) had progression to LSM-defined cirrhosis.
At a median follow-up of 3.69 years for the 775 patients without LSM progression, 79 (10.2%) had one or more of the events in the composite clinical outcome.
In contrast, after a median 5.48 years of follow-up, 31 of the 119 patients with progression (26.1%) had one or more of the composite events (P < .0001).
The median rates of progression to LSM-defined cirrhosis in the overall cohort were 2% at 1 year, 11% at 3 years, and 16% at 5 years.
Researchers found a correlation between progression to LSM-defined cirrhosis and baseline histological fibrosis stage on biopsy, with a rate of 7% among those with no baseline fibrosis, 9% each for patients with stage I A-C or stage II fibrosis, 24% of those with baseline bridging fibrosis, and 25% of those with baseline cirrhosis.
A comparison of the time to a composite clinical outcome event between patients with progression to LSM-defined cirrhosis and those without progression showed that LSM-defined progression was associated with near doubling in risk, with a hazard ratio of 1.84 (P = .0039).
In a multivariate Cox regression analysis controlling for age, sex, race, BMI, diabetes status, and baseline LSM, only LSM-defined progression (HR, 1.93; P < .01) and age (HR, 1.03; P < .01) were significant predictors.
Dr. Gawrieh noted that while age was a statistically significant factor, it was only weakly associated.
“These data suggest that development of cirrhosis LSM criteria is a promising surrogate for clinical outcomes in patients with NAFLD,” Dr. Gawrieh concluded.
Progression definition questioned
Following the presentation, Nezam Afdhal, MD, chief of the division of gastroenterology, hepatology, and nutrition at Beth Israel Deaconess Hospital in Boston, questioned how 25% of patients who had biopsy-proven cirrhosis could progress to LSM-defined cirrhosis.
Dr. Gawrieh said that, according to inclusion criteria, the patients could not have LSM-defined cirrhosis with the sensitivity cutoff of 12.1 kPa, and that of the 10 patients with baseline cirrhosis in the cohort, all had LSM of less than 12.1 kPa. However, he admitted that because those 10 patients were technically not progressors to cirrhosis, they should have been removed from the analysis for clinical outcomes.
Mark Hartman, MD, a clinical researcher at Eli Lilly and Company in Indianapolis, said the study is valuable but noted that those patients who progressed tended to have higher LSM at baseline as well as a higher [fibrosis-4 score].
Dr. Gawrieh added that the investigators are exploring variables that might explain progression to cirrhosis among patients without high baseline liver stiffness, such as alcohol use or drug-induced liver injury.
The study was supported by the National Institutes of Health and the NASH Clinical Research Network institutions. Dr. Gawrieh disclosed research grants from NIH, Zydus, Viking, and Sonic Incytes, and consulting for TransMedics and Pfizer. Dr. Afdhal and Dr. Hartman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
Long-term behavioral follow-up of children exposed to mood stabilizers and antidepressants: A look forward
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
How accurate is transcutaneous bilirubin testing in newborns with darker skin tones?
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
EVIDENCE-BASED ANSWER:
Fairly accurate. Photometric transcutaneous bilirubin (TcB) testing may overestimate total serum bilirubin (TSB) in neonates with darker skin tones by a mean of 0.68 to > 2 mg/dL (strength of recommendation [SOR]: C, diagnostic cohort studies with differing reference standards).
Overall, TcB meters retain acceptable accuracy in infants of all skin tones across a range of bilirubin levels, despite being more likely to underestimate lighter skin tones and overestimate darker ones (SOR: C, diagnostic cohort studies with differing reference standards). It is unclear if the higher readings prompt an increase in blood draws or otherwise alter care.
Positive sounds during REM sleep may help nightmares
For people with clinically diagnosed “nightmare disorder,” learning to redirect disturbing dreams to more positive ones is usually the return ticket to sleep.
But for nearly one-third of people, that method – called imagery rehearsal therapy – isn’t effective.
A new study shows that listening to positive sounds while sleeping reduces the frequency of nightmares.
“This is a promising development. It does appear that adding a well-timed sound during REM sleep augments the effect of image rehearsal therapy ... which is a standard and perhaps one of the most effective nonpharmacologic therapies at this time,” said Timothy Morgenthaler, MD, in an interview with CNN.
Dr. Morgenthaler, who was not involved in this latest study, is lead author of the American Academy of Sleep Medicine’s current guidelines on nightmares.
For the new research, nightmares were defined as “the experience of strong negative emotions occurring usually during REM sleep. They involve images and thoughts of aggression, interpersonal conflict, and failure, and emotions like fear, anger, and sadness.” in social, occupational, or other important areas of functioning.”
Left untreated, nightmare disorder can persist for decades, the authors said.
The study, conducted in Switzerland, enrolled 36 participants with nightmare disorder. All 36 participated in a daytime lesson of imagery rehearsal therapy that taught them to redirect their nightmares to positive dreams. Participants were taught to recall a nightmare, change the negative story line toward a more positive one, and then rehearse the so-called “rewritten dream” during the day.
Half of the participants also had a special sound played while they practiced reimagining their new positive dreams. At night for the following 2 weeks while they slept, the sound was played during their REM cycles.
Those who heard the sound reported significantly fewer nightmares.
“This difference displayed a medium to large effect size and was sustainable at the 3-month follow-up,” the authors reported.
They did note that both groups showed improvement, likely because the lesson to reimagine nightmares into positive dreams is known to be effective. However, the authors allowed that other factors may have contributed in ways their study design could not control.
“The result should be replicated,” Dr. Morgenthaler said. “But I was a bit excited at this new possibility.”
A version of this article first appeared on WebMD.com.
For people with clinically diagnosed “nightmare disorder,” learning to redirect disturbing dreams to more positive ones is usually the return ticket to sleep.
But for nearly one-third of people, that method – called imagery rehearsal therapy – isn’t effective.
A new study shows that listening to positive sounds while sleeping reduces the frequency of nightmares.
“This is a promising development. It does appear that adding a well-timed sound during REM sleep augments the effect of image rehearsal therapy ... which is a standard and perhaps one of the most effective nonpharmacologic therapies at this time,” said Timothy Morgenthaler, MD, in an interview with CNN.
Dr. Morgenthaler, who was not involved in this latest study, is lead author of the American Academy of Sleep Medicine’s current guidelines on nightmares.
For the new research, nightmares were defined as “the experience of strong negative emotions occurring usually during REM sleep. They involve images and thoughts of aggression, interpersonal conflict, and failure, and emotions like fear, anger, and sadness.” in social, occupational, or other important areas of functioning.”
Left untreated, nightmare disorder can persist for decades, the authors said.
The study, conducted in Switzerland, enrolled 36 participants with nightmare disorder. All 36 participated in a daytime lesson of imagery rehearsal therapy that taught them to redirect their nightmares to positive dreams. Participants were taught to recall a nightmare, change the negative story line toward a more positive one, and then rehearse the so-called “rewritten dream” during the day.
Half of the participants also had a special sound played while they practiced reimagining their new positive dreams. At night for the following 2 weeks while they slept, the sound was played during their REM cycles.
Those who heard the sound reported significantly fewer nightmares.
“This difference displayed a medium to large effect size and was sustainable at the 3-month follow-up,” the authors reported.
They did note that both groups showed improvement, likely because the lesson to reimagine nightmares into positive dreams is known to be effective. However, the authors allowed that other factors may have contributed in ways their study design could not control.
“The result should be replicated,” Dr. Morgenthaler said. “But I was a bit excited at this new possibility.”
A version of this article first appeared on WebMD.com.
For people with clinically diagnosed “nightmare disorder,” learning to redirect disturbing dreams to more positive ones is usually the return ticket to sleep.
But for nearly one-third of people, that method – called imagery rehearsal therapy – isn’t effective.
A new study shows that listening to positive sounds while sleeping reduces the frequency of nightmares.
“This is a promising development. It does appear that adding a well-timed sound during REM sleep augments the effect of image rehearsal therapy ... which is a standard and perhaps one of the most effective nonpharmacologic therapies at this time,” said Timothy Morgenthaler, MD, in an interview with CNN.
Dr. Morgenthaler, who was not involved in this latest study, is lead author of the American Academy of Sleep Medicine’s current guidelines on nightmares.
For the new research, nightmares were defined as “the experience of strong negative emotions occurring usually during REM sleep. They involve images and thoughts of aggression, interpersonal conflict, and failure, and emotions like fear, anger, and sadness.” in social, occupational, or other important areas of functioning.”
Left untreated, nightmare disorder can persist for decades, the authors said.
The study, conducted in Switzerland, enrolled 36 participants with nightmare disorder. All 36 participated in a daytime lesson of imagery rehearsal therapy that taught them to redirect their nightmares to positive dreams. Participants were taught to recall a nightmare, change the negative story line toward a more positive one, and then rehearse the so-called “rewritten dream” during the day.
Half of the participants also had a special sound played while they practiced reimagining their new positive dreams. At night for the following 2 weeks while they slept, the sound was played during their REM cycles.
Those who heard the sound reported significantly fewer nightmares.
“This difference displayed a medium to large effect size and was sustainable at the 3-month follow-up,” the authors reported.
They did note that both groups showed improvement, likely because the lesson to reimagine nightmares into positive dreams is known to be effective. However, the authors allowed that other factors may have contributed in ways their study design could not control.
“The result should be replicated,” Dr. Morgenthaler said. “But I was a bit excited at this new possibility.”
A version of this article first appeared on WebMD.com.
FROM CURRENT BIOLOGY
26-year-old woman • nausea and vomiting • currently breastfeeding • ketogenic diet • Dx?
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; [email protected]
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; [email protected]
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; [email protected]
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
An FP’s guide to identifying—and treating—postpartum depression
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; [email protected]
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; [email protected]
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; [email protected]
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4