User login
Buprenorphine linked with lower risk for neonatal harms than methadone
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
FROM NEW ENGLAND JOURNAL OF MEDICINE
U.S. flu activity already at mid-season levels
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
Yellow Nodule on the Scalp
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4
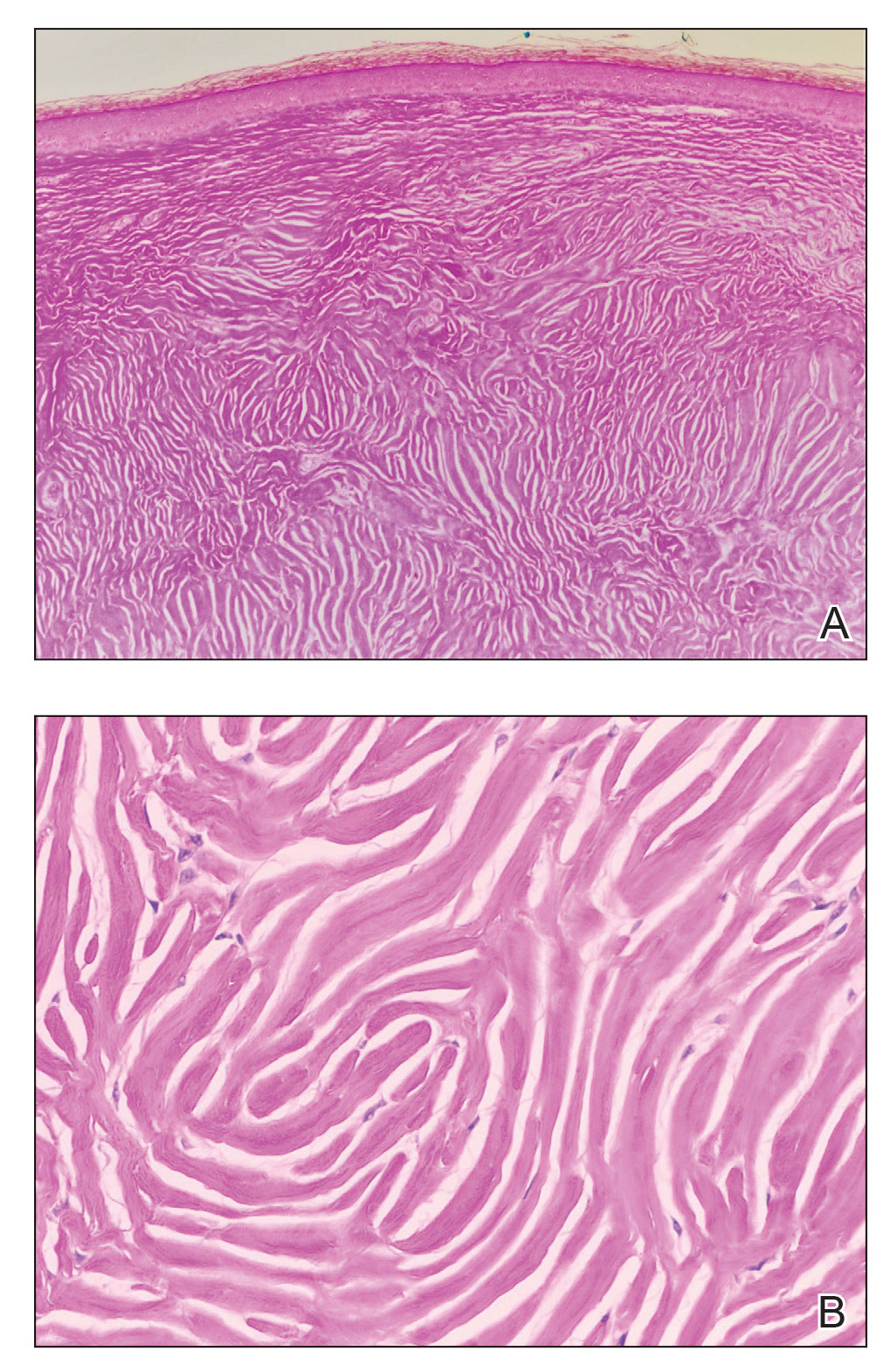
The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
A 45-year-old woman was referred to dermatology by a primary care physician for evaluation of a raised skin lesion on the scalp. She was otherwise healthy. The lesion had been present for many years but recently grew in size. The patient reported that the lesion was subject to recurrent physical trauma and she wanted it removed. Physical examination revealed a 6×6-mm, domeshaped, yellow nodule on the left inferior parietal scalp. There were no similar lesions located elsewhere on the body. A shave removal was performed and sent for histopathologic evaluation.

DEI advances in dermatology unremarkable to date, studies find
suggest.
To evaluate diversity and career goals of graduating allopathic medical students pursuing careers in dermatology, corresponding author Matthew Mansh, MD, of the department of dermatology at the University of Minnesota, Minneapolis, and colleagues drew from the 2016-2019 Association of American Medical Colleges Graduation Questionnaire for their study. The main outcome measures were the proportion of female students, students from racial and ethnic groups underrepresented in medicine (URM), and sexual minority (SM) students pursuing dermatology versus those pursuing other specialties, as well as the proportions and multivariable adjusted odds of intended career goals between students pursuing dermatology and those pursuing other specialties, and by sex, race, and ethnicity, and sexual orientation among students pursuing dermatology.
Of the 58,077 graduating students, 49% were women, 15% were URM, and 6% were SM. The researchers found that women pursuing dermatology were significantly less likely than women pursuing other specialties to identify as URM (11.6% vs. 17.2%; P < .001) or SM (1.9% vs. 5.7%; P < .001).
In multivariable-adjusted analyses of all students, those pursuing dermatology compared with other specialties had decreased odds of intending to care for underserved populations (18.3% vs. 34%; adjusted odd ratio, 0.40; P < .001), practice in underserved areas (12.7% vs. 25.9%; aOR, 0.40; P < .001), and practice public health (17% vs. 30.2%; aOR, 0.44; P < .001). The odds for pursuing research in their careers was greater among those pursuing dermatology (64.7% vs. 51.7%; aOR, 1.76; P < .001).
“Addressing health inequities and improving care for underserved patients is the responsibility of all dermatologists, and efforts are needed to increase diversity and interest in careers focused on underserved care among trainees in the dermatology workforce pipeline,” the authors concluded. They acknowledged certain limitations of the analysis, including lack of data delineating sex, sex assigned at birth, and gender identity, and lack of intersectional analyses between multiple minority identities and multiple career goals. “Importantly, diversity factors and their relationship to underserved care is likely multidimensional, and many students pursuing dermatology identified with multiple minority identities, highlighting the need for future studies focused on intersectionality,” they wrote.
Trends over 15 years
In a separate study, Jazzmin C. Williams, a medical student at the University of California, San Francisco, and coauthors drew from an Association of American Medical Colleges report of trainees’ and applicants’ self-reported race and ethnicity by specialty from 2005 to 2020 to evaluate diversity trends over the 15-year period. They found that Black and Latinx trainees were underrepresented in all specialties, but even more so in dermatology (mean annual rate ratios of 0.32 and 0.14, respectively), compared with those in primary care (mean annual RRs of 0.54 and 0.23) and those in specialty care (mean annual RRs of 0.39 and 0.18).
In other findings, the annual representation of Black trainees remained unchanged in dermatology between 2005 and 2020, but down-trended for primary (P < .001) and specialty care (P = .001). At the same time, representation of Latinx trainees remained unchanged in dermatology and specialty care but increased in primary care (P < .001). Finally, Black and Latinx race and ethnicity comprised a lower mean proportion of matriculating dermatology trainees (postgraduate year-2s) compared with annual dermatology applicants (4.01% vs. 5.97%, respectively, and 2.06% vs. 6.37% among Latinx; P < .001 for all associations).
“Much of these disparities can be attributed to the leaky pipeline – the disproportionate, stepwise reduction in racial and ethnic minority representation along the path to medicine,” the authors wrote. “This leaky pipeline is the direct result of structural racism, which includes, but is not limited to, historical and contemporary economic disinvestment from majority-minority schools, kindergarten through grade 12.” They concluded by stating that “dermatologists must intervene throughout the educational pipeline, including residency selection and mentorship, to effectively increase diversity.”
Solutions to address diversity
In an editorial accompanying the two studies published in the same issue of JAMA Dermatology, Ellen N. Pritchett, MD, MPH, of the department of dermatology at Howard University, Washington, and Andrew J. Park, MD, MBA, and Rebecca Vasquez, MD, of the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, offered several solutions to address diversity in the dermatology work force. They include:
Go beyond individual bias in recruitment. “A residency selection framework that meaningfully incorporates diversity, equity, and inclusion (DEI) will require more than strategies that address individual bias,” they wrote. “Departmental recruitment committees must become familiar with systems that serve to perpetuate individual bias, like institutional racism or practices that disproportionately favor non-URM versus URM individuals.”
Challenge the myth of meritocracy. “The inaccurate notion of meritocracy – that success purely derives from individual effort has become the foundation of residency selection,” the authors wrote. “Unfortunately, this view ignores the inequitably distributed sociostructural resources that limit the rewards of individual effort.”
Avoid tokenism in retention strategies. Tokenism, which they defined as “a symbolic addition of members from a marginalized group to give the impression of social inclusiveness and diversity without meaningful incorporation of DEI in the policies, processes, and culture,” can lead to depression, burnout, and attrition, they wrote. They advise leaders of dermatology departments to “review their residency selection framework to ensure that it allows for meaningful representation, inclusion, and equity among trainees and faculty to better support URM individuals at all levels.”
Omar N. Qutub, MD, a Portland, Ore.–based dermatologist who was asked to comment on the studies, characterized the findings by Dr. Mansh and colleagues as sobering. “It appears that there is work to do as far as improving diversity in the dermatology workforce that will likely benefit greatly from an honest and steadfast approach to equitable application standards as well as mentorship during all stages of the application process,” such as medical school and residency, said Dr. Qutub, who is the director of equity, diversity, and inclusion of the ODAC Dermatology, Aesthetic & Surgical Conference. “With a focused attempt, we are likely to matriculate more racial minorities into our residency programs, maximizing patient outcomes.”
As for the study by Ms. Williams and colleagues, he told this news organization that efforts toward recruiting URM students as well as sexual minority students “is likely to not only improve health inequities in underserved areas, but will also enrich the specialty as a whole, allowing for better understanding of our diverse patient population and [for us to] to deliver quality care more readily for people and in areas where the focus has often been limited.”
In an interview, Chesahna Kindred, MD, a Columbia, Md.–based dermatologist and immediate past chair of the National Medical Association dermatology section, pointed out that the number of Black physicians in the United States has increased by only 4% in the last 120 years. The study by Dr. Mansh and colleagues, she commented, “underscores what I’ve recognized in the last couple of years: Where are the Black male dermatologists? NMA Derm started recruiting this demographic aggressively about a year ago and started the Black Men in Derm events. Black male members of NMA Derm travel to the Student National Medical Association and NMA conference and hold a panel to expose Black male students into dermatology. This article provides the numbers needed to measure how successful this and other programs are to closing the equity gap.”
Ms. Williams reported having no financial disclosures. Dr. Mansh reported receiving grants from National Institute of Environmental Health Sciences outside the submitted work. Dr. Pritchett and colleagues reported having no relevant financial disclosures, as did Dr. Qutub and Dr. Kindred.
suggest.
To evaluate diversity and career goals of graduating allopathic medical students pursuing careers in dermatology, corresponding author Matthew Mansh, MD, of the department of dermatology at the University of Minnesota, Minneapolis, and colleagues drew from the 2016-2019 Association of American Medical Colleges Graduation Questionnaire for their study. The main outcome measures were the proportion of female students, students from racial and ethnic groups underrepresented in medicine (URM), and sexual minority (SM) students pursuing dermatology versus those pursuing other specialties, as well as the proportions and multivariable adjusted odds of intended career goals between students pursuing dermatology and those pursuing other specialties, and by sex, race, and ethnicity, and sexual orientation among students pursuing dermatology.
Of the 58,077 graduating students, 49% were women, 15% were URM, and 6% were SM. The researchers found that women pursuing dermatology were significantly less likely than women pursuing other specialties to identify as URM (11.6% vs. 17.2%; P < .001) or SM (1.9% vs. 5.7%; P < .001).
In multivariable-adjusted analyses of all students, those pursuing dermatology compared with other specialties had decreased odds of intending to care for underserved populations (18.3% vs. 34%; adjusted odd ratio, 0.40; P < .001), practice in underserved areas (12.7% vs. 25.9%; aOR, 0.40; P < .001), and practice public health (17% vs. 30.2%; aOR, 0.44; P < .001). The odds for pursuing research in their careers was greater among those pursuing dermatology (64.7% vs. 51.7%; aOR, 1.76; P < .001).
“Addressing health inequities and improving care for underserved patients is the responsibility of all dermatologists, and efforts are needed to increase diversity and interest in careers focused on underserved care among trainees in the dermatology workforce pipeline,” the authors concluded. They acknowledged certain limitations of the analysis, including lack of data delineating sex, sex assigned at birth, and gender identity, and lack of intersectional analyses between multiple minority identities and multiple career goals. “Importantly, diversity factors and their relationship to underserved care is likely multidimensional, and many students pursuing dermatology identified with multiple minority identities, highlighting the need for future studies focused on intersectionality,” they wrote.
Trends over 15 years
In a separate study, Jazzmin C. Williams, a medical student at the University of California, San Francisco, and coauthors drew from an Association of American Medical Colleges report of trainees’ and applicants’ self-reported race and ethnicity by specialty from 2005 to 2020 to evaluate diversity trends over the 15-year period. They found that Black and Latinx trainees were underrepresented in all specialties, but even more so in dermatology (mean annual rate ratios of 0.32 and 0.14, respectively), compared with those in primary care (mean annual RRs of 0.54 and 0.23) and those in specialty care (mean annual RRs of 0.39 and 0.18).
In other findings, the annual representation of Black trainees remained unchanged in dermatology between 2005 and 2020, but down-trended for primary (P < .001) and specialty care (P = .001). At the same time, representation of Latinx trainees remained unchanged in dermatology and specialty care but increased in primary care (P < .001). Finally, Black and Latinx race and ethnicity comprised a lower mean proportion of matriculating dermatology trainees (postgraduate year-2s) compared with annual dermatology applicants (4.01% vs. 5.97%, respectively, and 2.06% vs. 6.37% among Latinx; P < .001 for all associations).
“Much of these disparities can be attributed to the leaky pipeline – the disproportionate, stepwise reduction in racial and ethnic minority representation along the path to medicine,” the authors wrote. “This leaky pipeline is the direct result of structural racism, which includes, but is not limited to, historical and contemporary economic disinvestment from majority-minority schools, kindergarten through grade 12.” They concluded by stating that “dermatologists must intervene throughout the educational pipeline, including residency selection and mentorship, to effectively increase diversity.”
Solutions to address diversity
In an editorial accompanying the two studies published in the same issue of JAMA Dermatology, Ellen N. Pritchett, MD, MPH, of the department of dermatology at Howard University, Washington, and Andrew J. Park, MD, MBA, and Rebecca Vasquez, MD, of the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, offered several solutions to address diversity in the dermatology work force. They include:
Go beyond individual bias in recruitment. “A residency selection framework that meaningfully incorporates diversity, equity, and inclusion (DEI) will require more than strategies that address individual bias,” they wrote. “Departmental recruitment committees must become familiar with systems that serve to perpetuate individual bias, like institutional racism or practices that disproportionately favor non-URM versus URM individuals.”
Challenge the myth of meritocracy. “The inaccurate notion of meritocracy – that success purely derives from individual effort has become the foundation of residency selection,” the authors wrote. “Unfortunately, this view ignores the inequitably distributed sociostructural resources that limit the rewards of individual effort.”
Avoid tokenism in retention strategies. Tokenism, which they defined as “a symbolic addition of members from a marginalized group to give the impression of social inclusiveness and diversity without meaningful incorporation of DEI in the policies, processes, and culture,” can lead to depression, burnout, and attrition, they wrote. They advise leaders of dermatology departments to “review their residency selection framework to ensure that it allows for meaningful representation, inclusion, and equity among trainees and faculty to better support URM individuals at all levels.”
Omar N. Qutub, MD, a Portland, Ore.–based dermatologist who was asked to comment on the studies, characterized the findings by Dr. Mansh and colleagues as sobering. “It appears that there is work to do as far as improving diversity in the dermatology workforce that will likely benefit greatly from an honest and steadfast approach to equitable application standards as well as mentorship during all stages of the application process,” such as medical school and residency, said Dr. Qutub, who is the director of equity, diversity, and inclusion of the ODAC Dermatology, Aesthetic & Surgical Conference. “With a focused attempt, we are likely to matriculate more racial minorities into our residency programs, maximizing patient outcomes.”
As for the study by Ms. Williams and colleagues, he told this news organization that efforts toward recruiting URM students as well as sexual minority students “is likely to not only improve health inequities in underserved areas, but will also enrich the specialty as a whole, allowing for better understanding of our diverse patient population and [for us to] to deliver quality care more readily for people and in areas where the focus has often been limited.”
In an interview, Chesahna Kindred, MD, a Columbia, Md.–based dermatologist and immediate past chair of the National Medical Association dermatology section, pointed out that the number of Black physicians in the United States has increased by only 4% in the last 120 years. The study by Dr. Mansh and colleagues, she commented, “underscores what I’ve recognized in the last couple of years: Where are the Black male dermatologists? NMA Derm started recruiting this demographic aggressively about a year ago and started the Black Men in Derm events. Black male members of NMA Derm travel to the Student National Medical Association and NMA conference and hold a panel to expose Black male students into dermatology. This article provides the numbers needed to measure how successful this and other programs are to closing the equity gap.”
Ms. Williams reported having no financial disclosures. Dr. Mansh reported receiving grants from National Institute of Environmental Health Sciences outside the submitted work. Dr. Pritchett and colleagues reported having no relevant financial disclosures, as did Dr. Qutub and Dr. Kindred.
suggest.
To evaluate diversity and career goals of graduating allopathic medical students pursuing careers in dermatology, corresponding author Matthew Mansh, MD, of the department of dermatology at the University of Minnesota, Minneapolis, and colleagues drew from the 2016-2019 Association of American Medical Colleges Graduation Questionnaire for their study. The main outcome measures were the proportion of female students, students from racial and ethnic groups underrepresented in medicine (URM), and sexual minority (SM) students pursuing dermatology versus those pursuing other specialties, as well as the proportions and multivariable adjusted odds of intended career goals between students pursuing dermatology and those pursuing other specialties, and by sex, race, and ethnicity, and sexual orientation among students pursuing dermatology.
Of the 58,077 graduating students, 49% were women, 15% were URM, and 6% were SM. The researchers found that women pursuing dermatology were significantly less likely than women pursuing other specialties to identify as URM (11.6% vs. 17.2%; P < .001) or SM (1.9% vs. 5.7%; P < .001).
In multivariable-adjusted analyses of all students, those pursuing dermatology compared with other specialties had decreased odds of intending to care for underserved populations (18.3% vs. 34%; adjusted odd ratio, 0.40; P < .001), practice in underserved areas (12.7% vs. 25.9%; aOR, 0.40; P < .001), and practice public health (17% vs. 30.2%; aOR, 0.44; P < .001). The odds for pursuing research in their careers was greater among those pursuing dermatology (64.7% vs. 51.7%; aOR, 1.76; P < .001).
“Addressing health inequities and improving care for underserved patients is the responsibility of all dermatologists, and efforts are needed to increase diversity and interest in careers focused on underserved care among trainees in the dermatology workforce pipeline,” the authors concluded. They acknowledged certain limitations of the analysis, including lack of data delineating sex, sex assigned at birth, and gender identity, and lack of intersectional analyses between multiple minority identities and multiple career goals. “Importantly, diversity factors and their relationship to underserved care is likely multidimensional, and many students pursuing dermatology identified with multiple minority identities, highlighting the need for future studies focused on intersectionality,” they wrote.
Trends over 15 years
In a separate study, Jazzmin C. Williams, a medical student at the University of California, San Francisco, and coauthors drew from an Association of American Medical Colleges report of trainees’ and applicants’ self-reported race and ethnicity by specialty from 2005 to 2020 to evaluate diversity trends over the 15-year period. They found that Black and Latinx trainees were underrepresented in all specialties, but even more so in dermatology (mean annual rate ratios of 0.32 and 0.14, respectively), compared with those in primary care (mean annual RRs of 0.54 and 0.23) and those in specialty care (mean annual RRs of 0.39 and 0.18).
In other findings, the annual representation of Black trainees remained unchanged in dermatology between 2005 and 2020, but down-trended for primary (P < .001) and specialty care (P = .001). At the same time, representation of Latinx trainees remained unchanged in dermatology and specialty care but increased in primary care (P < .001). Finally, Black and Latinx race and ethnicity comprised a lower mean proportion of matriculating dermatology trainees (postgraduate year-2s) compared with annual dermatology applicants (4.01% vs. 5.97%, respectively, and 2.06% vs. 6.37% among Latinx; P < .001 for all associations).
“Much of these disparities can be attributed to the leaky pipeline – the disproportionate, stepwise reduction in racial and ethnic minority representation along the path to medicine,” the authors wrote. “This leaky pipeline is the direct result of structural racism, which includes, but is not limited to, historical and contemporary economic disinvestment from majority-minority schools, kindergarten through grade 12.” They concluded by stating that “dermatologists must intervene throughout the educational pipeline, including residency selection and mentorship, to effectively increase diversity.”
Solutions to address diversity
In an editorial accompanying the two studies published in the same issue of JAMA Dermatology, Ellen N. Pritchett, MD, MPH, of the department of dermatology at Howard University, Washington, and Andrew J. Park, MD, MBA, and Rebecca Vasquez, MD, of the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, offered several solutions to address diversity in the dermatology work force. They include:
Go beyond individual bias in recruitment. “A residency selection framework that meaningfully incorporates diversity, equity, and inclusion (DEI) will require more than strategies that address individual bias,” they wrote. “Departmental recruitment committees must become familiar with systems that serve to perpetuate individual bias, like institutional racism or practices that disproportionately favor non-URM versus URM individuals.”
Challenge the myth of meritocracy. “The inaccurate notion of meritocracy – that success purely derives from individual effort has become the foundation of residency selection,” the authors wrote. “Unfortunately, this view ignores the inequitably distributed sociostructural resources that limit the rewards of individual effort.”
Avoid tokenism in retention strategies. Tokenism, which they defined as “a symbolic addition of members from a marginalized group to give the impression of social inclusiveness and diversity without meaningful incorporation of DEI in the policies, processes, and culture,” can lead to depression, burnout, and attrition, they wrote. They advise leaders of dermatology departments to “review their residency selection framework to ensure that it allows for meaningful representation, inclusion, and equity among trainees and faculty to better support URM individuals at all levels.”
Omar N. Qutub, MD, a Portland, Ore.–based dermatologist who was asked to comment on the studies, characterized the findings by Dr. Mansh and colleagues as sobering. “It appears that there is work to do as far as improving diversity in the dermatology workforce that will likely benefit greatly from an honest and steadfast approach to equitable application standards as well as mentorship during all stages of the application process,” such as medical school and residency, said Dr. Qutub, who is the director of equity, diversity, and inclusion of the ODAC Dermatology, Aesthetic & Surgical Conference. “With a focused attempt, we are likely to matriculate more racial minorities into our residency programs, maximizing patient outcomes.”
As for the study by Ms. Williams and colleagues, he told this news organization that efforts toward recruiting URM students as well as sexual minority students “is likely to not only improve health inequities in underserved areas, but will also enrich the specialty as a whole, allowing for better understanding of our diverse patient population and [for us to] to deliver quality care more readily for people and in areas where the focus has often been limited.”
In an interview, Chesahna Kindred, MD, a Columbia, Md.–based dermatologist and immediate past chair of the National Medical Association dermatology section, pointed out that the number of Black physicians in the United States has increased by only 4% in the last 120 years. The study by Dr. Mansh and colleagues, she commented, “underscores what I’ve recognized in the last couple of years: Where are the Black male dermatologists? NMA Derm started recruiting this demographic aggressively about a year ago and started the Black Men in Derm events. Black male members of NMA Derm travel to the Student National Medical Association and NMA conference and hold a panel to expose Black male students into dermatology. This article provides the numbers needed to measure how successful this and other programs are to closing the equity gap.”
Ms. Williams reported having no financial disclosures. Dr. Mansh reported receiving grants from National Institute of Environmental Health Sciences outside the submitted work. Dr. Pritchett and colleagues reported having no relevant financial disclosures, as did Dr. Qutub and Dr. Kindred.
FROM JAMA DERMATOLOGY
Your patients are rotting their teeth with vaping
Primary care physicians, and especially pediatricians, should consider telling their patients about the long-term oral health problems associated with vaping.
A new study found that patients who use vapes were at a higher risk of developing tooth decay and periodontal disease.
Vapes were introduced to the U.S. market in 2006 as an alternative to conventional cigarettes and have become widely popular among youth. According to a 2022 survey from the U.S. Centers for Disease Control and Prevention, 2.55 million middle and high school students in this country reported using the devices in the previous 30 days.
The new study, published in the Journal of the American Dental Association, expands on an initial case series published in 2020 of patients who reported use of vapes and who had severe dental decay. Karina Irusa, BDS, assistant professor of comprehensive care at Tufts University, Boston, and lead author of the case series, wanted to investigate whether her initial findings would apply to a large population of vape users.
For the new study, Dr. Irusa and colleagues collected data on 13,216 patients aged 16-40 who attended Tufts dental clinics between 2019 and 2021. All patients had received a diagnosis of tooth decay, had a tooth decay risk assessment on record, and had answered “yes” or “no” to use of vapes in a health history questionnaire.
Patients had records on file of varying types of dental lesions, cavities filled within the previous 3 years, heavy plaque on teeth, inadequate brushing and flushing, and a self-report of recreational drug use and frequent snacking. If patients had these factors on their file, they were at high risk of developing decay that leads to cavities.
The study found that 79% of patients who responded “yes” to being a current user of vapes were at high risk for dental decay, compared with 60% of those who did not report using the devices.
Materials in the vaping liquids further cause an inflammatory response that disrupts an individual’s internal microbiome, according to numerous studies.
“All the ingredients of vaping are surely a recipe for overgrowth of cavities causing bacteria,” said Jennifer Genuardi, MD, an internist and pediatrician at federally qualified community health center Urban Health Plan, in New York, who was not involved in the study.
Dr. Irusa said information on patient’s vaping habits should be included in routine dental and medical history questionnaires as part of their overall electronic health record.
“Decay in its severe form not only affects one’s ability to eat but affects facial aesthetics and self-esteem as well,” Dr. Irusa said.
Dr. Genuardi called the findings unsurprising.
“We are learning daily more and more about the dangers of vaping,” Dr. Genuardi said. “There’s a focus of today’s research on the effect of actions on our microbiome and the subsequent effects on our health.”
Dr. Genuardi also said many of her teenage patients do not enjoy dental visits or having cavities filled, which could serve as a useful deterrent to vaping for a demographic that has been targeted with marketing from vape manufacturers.
“Cavity formation and the experience of having cavities filled is an experience teens can identify with, so this to me seems like perhaps an even more effective angle to try to curb this unhealthy behavior of vaping,” Dr. Genuardi said.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Primary care physicians, and especially pediatricians, should consider telling their patients about the long-term oral health problems associated with vaping.
A new study found that patients who use vapes were at a higher risk of developing tooth decay and periodontal disease.
Vapes were introduced to the U.S. market in 2006 as an alternative to conventional cigarettes and have become widely popular among youth. According to a 2022 survey from the U.S. Centers for Disease Control and Prevention, 2.55 million middle and high school students in this country reported using the devices in the previous 30 days.
The new study, published in the Journal of the American Dental Association, expands on an initial case series published in 2020 of patients who reported use of vapes and who had severe dental decay. Karina Irusa, BDS, assistant professor of comprehensive care at Tufts University, Boston, and lead author of the case series, wanted to investigate whether her initial findings would apply to a large population of vape users.
For the new study, Dr. Irusa and colleagues collected data on 13,216 patients aged 16-40 who attended Tufts dental clinics between 2019 and 2021. All patients had received a diagnosis of tooth decay, had a tooth decay risk assessment on record, and had answered “yes” or “no” to use of vapes in a health history questionnaire.
Patients had records on file of varying types of dental lesions, cavities filled within the previous 3 years, heavy plaque on teeth, inadequate brushing and flushing, and a self-report of recreational drug use and frequent snacking. If patients had these factors on their file, they were at high risk of developing decay that leads to cavities.
The study found that 79% of patients who responded “yes” to being a current user of vapes were at high risk for dental decay, compared with 60% of those who did not report using the devices.
Materials in the vaping liquids further cause an inflammatory response that disrupts an individual’s internal microbiome, according to numerous studies.
“All the ingredients of vaping are surely a recipe for overgrowth of cavities causing bacteria,” said Jennifer Genuardi, MD, an internist and pediatrician at federally qualified community health center Urban Health Plan, in New York, who was not involved in the study.
Dr. Irusa said information on patient’s vaping habits should be included in routine dental and medical history questionnaires as part of their overall electronic health record.
“Decay in its severe form not only affects one’s ability to eat but affects facial aesthetics and self-esteem as well,” Dr. Irusa said.
Dr. Genuardi called the findings unsurprising.
“We are learning daily more and more about the dangers of vaping,” Dr. Genuardi said. “There’s a focus of today’s research on the effect of actions on our microbiome and the subsequent effects on our health.”
Dr. Genuardi also said many of her teenage patients do not enjoy dental visits or having cavities filled, which could serve as a useful deterrent to vaping for a demographic that has been targeted with marketing from vape manufacturers.
“Cavity formation and the experience of having cavities filled is an experience teens can identify with, so this to me seems like perhaps an even more effective angle to try to curb this unhealthy behavior of vaping,” Dr. Genuardi said.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Primary care physicians, and especially pediatricians, should consider telling their patients about the long-term oral health problems associated with vaping.
A new study found that patients who use vapes were at a higher risk of developing tooth decay and periodontal disease.
Vapes were introduced to the U.S. market in 2006 as an alternative to conventional cigarettes and have become widely popular among youth. According to a 2022 survey from the U.S. Centers for Disease Control and Prevention, 2.55 million middle and high school students in this country reported using the devices in the previous 30 days.
The new study, published in the Journal of the American Dental Association, expands on an initial case series published in 2020 of patients who reported use of vapes and who had severe dental decay. Karina Irusa, BDS, assistant professor of comprehensive care at Tufts University, Boston, and lead author of the case series, wanted to investigate whether her initial findings would apply to a large population of vape users.
For the new study, Dr. Irusa and colleagues collected data on 13,216 patients aged 16-40 who attended Tufts dental clinics between 2019 and 2021. All patients had received a diagnosis of tooth decay, had a tooth decay risk assessment on record, and had answered “yes” or “no” to use of vapes in a health history questionnaire.
Patients had records on file of varying types of dental lesions, cavities filled within the previous 3 years, heavy plaque on teeth, inadequate brushing and flushing, and a self-report of recreational drug use and frequent snacking. If patients had these factors on their file, they were at high risk of developing decay that leads to cavities.
The study found that 79% of patients who responded “yes” to being a current user of vapes were at high risk for dental decay, compared with 60% of those who did not report using the devices.
Materials in the vaping liquids further cause an inflammatory response that disrupts an individual’s internal microbiome, according to numerous studies.
“All the ingredients of vaping are surely a recipe for overgrowth of cavities causing bacteria,” said Jennifer Genuardi, MD, an internist and pediatrician at federally qualified community health center Urban Health Plan, in New York, who was not involved in the study.
Dr. Irusa said information on patient’s vaping habits should be included in routine dental and medical history questionnaires as part of their overall electronic health record.
“Decay in its severe form not only affects one’s ability to eat but affects facial aesthetics and self-esteem as well,” Dr. Irusa said.
Dr. Genuardi called the findings unsurprising.
“We are learning daily more and more about the dangers of vaping,” Dr. Genuardi said. “There’s a focus of today’s research on the effect of actions on our microbiome and the subsequent effects on our health.”
Dr. Genuardi also said many of her teenage patients do not enjoy dental visits or having cavities filled, which could serve as a useful deterrent to vaping for a demographic that has been targeted with marketing from vape manufacturers.
“Cavity formation and the experience of having cavities filled is an experience teens can identify with, so this to me seems like perhaps an even more effective angle to try to curb this unhealthy behavior of vaping,” Dr. Genuardi said.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN DENTAL ASSOCIATION
Women need not wait to conceive after miscarriage, abortion
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of health care use after bariatric surgery in teens
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.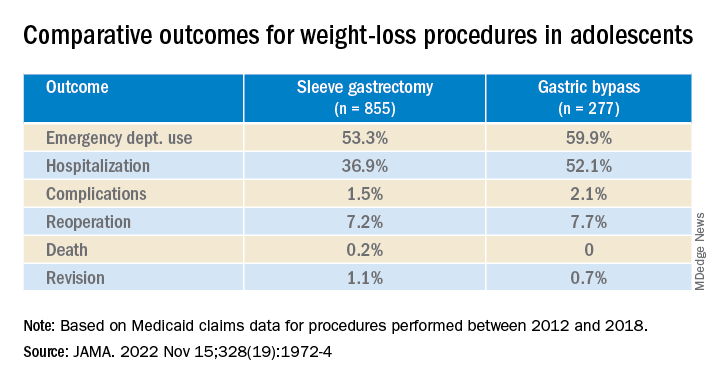
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Rituximab raises remission rate in granulomatosis with polyangiitis
More patients with granulomatosis with polyangiitis (GPA) were in remission at 6 months if they had received rituximab (Rituxan) rather than cyclophosphamide (Cytoxan) as induction therapy, according to a target trial emulation performed by the French Vasculitis Study Group (FVSG).
Remission, which was defined as a score of zero on the validated Birmingham Vasculitis Activity Score (BVAS) and use of no more than 10 mg of prednisone a day, was documented in 73.1% of rituximab-treated patients and 40.1% of cyclophosphamide-treated patients.
Similar rates of remission were observed regardless of whether patients were newly diagnosed with GPA (76.1% vs. 41.6%) or had been recently treated for relapsing disease (75.2% vs. 44.5%), FVSG researchers reported in JAMA Network Open.
This research “may inform clinical decision-making regarding the choice of remission-inducing regimen for patients with GPA,” the researchers suggested.
Practice already shifting to rituximab
“The results are largely in line with previous perceptions,” David R.W. Jayne, MD, honorary consultant and director of the vasculitis and lupus service at Addenbrooke’s Hospital in Cambridge, England, observed in an emailed comment.
“The difference is a bit bigger [in favor of rituximab] than I would have expected,” said Dr. Jayne, who is also professor of clinical autoimmunity at the University of Cambridge. He noted that clinical practice was already moving toward using rituximab in place of cyclophosphamide.
Rituximab gained a European license to treat antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAV) just over a decade ago. Since then, it has slowly started to replace the use of cyclophosphamide and glucocorticoids, which were the preferred method up until then.
Part of the reason for this shift is the toxicity associated with cyclophosphamide, although that’s not to say that rituximab is free from safety concerns, Dr. Jayne said.
“Toxicity issues with rituximab, especially secondary immunodeficiency, more severe COVID, and blocking vaccine responses, are becoming bigger issues for day-to-day practice,” he noted. Nonetheless, “the introduction of rituximab has been a revolution in AAV treatment. It has encouraged pharma investment in the disease, such as recent approval of avacopan [Tavneos], and it is helping patients.”
Why simulate and not perform a randomized trial?
There were several reasons for performing the current evaluation, study coauthor Benjamin Terrier, MD, PhD, said in an interview.
“The pivotal study, published in 2010, that led to the approval of rituximab was a noninferiority study in comparison with cyclophosphamide, but it included patients with both GPA, granulomatosis with polyangiitis, and MPA, microscopic polyangiitis,” explained Dr. Terrier, professor of internal medicine at the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital in Paris.
“A post hoc analysis showed that, in the patients with a PR3 [proteinase 3]-ANCA and in the patients with relapsing disease, rituximab was superior,” he added.
“So, there was some data which suggest that rituximab could be differentially effective between the different subgroups of patients. So that’s why we wanted to answer this question.”
Since it is not always feasible to do a randomized trial, particularly when it concerns a rare disease such as GPA, Dr. Terrier and associates decided to perform a target trial emulation using data from the FVSG registry, which collates information from 32 hospitals in France. Such studies are gaining in popularity and have been shown to provide a very good level of evidence, he said.
Data collections and secondary endpoint results
The researchers obtained data on 194 patients in the registry who were treated for GPA between April 2008 and April 2018. The majority (85.1%) of patients included were newly diagnosed with GPA, and 56.7% were men. The mean age of patients was 54 years.
Information on the PR3-ANCA status of patients was available for 182 patients, and this showed that the majority (80.8%) were positive for this autoantibody.
A weighted analysis was undertaken to iron out any differences in baseline characteristics, such as the fact that more patients had been treated with at least one dose of cyclophosphamide than rituximab (133 vs. 61).
The primary outcome was remission at 6 months, but a key secondary endpoint was the percentage of patients with a BVAS score of zero at this time point. This turned out to be similar among the rituximab- and cyclophosphamide-treated patients (85.5% vs. 82.6%, respectively).
Another secondary endpoint looked at the retention rate without failure at 24 months, with fewer postinclusion treatment failures seen with rituximab than with cyclophosphamide (7 vs. 51 patients, respectively). Most treatment failures were caused by relapses (7 vs. 33).
In terms of safety, the researchers said they found “no increased toxicity signal” for rituximab over cyclophosphamide. In fact, more severe adverse events were noted in the latter group.
Take-home messages
While of course there are limitations, considering the earlier data and the current results, “we probably have enough data to consider that, in the vast majority of GPA patients, in PR3-ANCA patients, rituximab is probably the best option,” Dr. Terrier said.
There are still patients for whom there isn’t a definitive answer on which drug may be best, such as those with severe disease who were not included in the trials or represent few patients in the registry. For them, it is “still a case-by-case discussion, and I think we have to decide really, with caution,” Dr. Terrier said.
What this study also shows is that emulated trials are possible, he added. “I think it shows that we could have some answers to other questions by emulating trials in this rare disease.”
The FVSG registry has received funding from the European Union’s Horizon 2020 research and innovation program. Dr. Terrier reported receiving personal fees from Vifor Pharma Group, GlaxoSmithKline, and AstraZeneca during the conduct of the study. Dr. Jayne has received lecture fees and a research grant from Roche/Genentech.
More patients with granulomatosis with polyangiitis (GPA) were in remission at 6 months if they had received rituximab (Rituxan) rather than cyclophosphamide (Cytoxan) as induction therapy, according to a target trial emulation performed by the French Vasculitis Study Group (FVSG).
Remission, which was defined as a score of zero on the validated Birmingham Vasculitis Activity Score (BVAS) and use of no more than 10 mg of prednisone a day, was documented in 73.1% of rituximab-treated patients and 40.1% of cyclophosphamide-treated patients.
Similar rates of remission were observed regardless of whether patients were newly diagnosed with GPA (76.1% vs. 41.6%) or had been recently treated for relapsing disease (75.2% vs. 44.5%), FVSG researchers reported in JAMA Network Open.
This research “may inform clinical decision-making regarding the choice of remission-inducing regimen for patients with GPA,” the researchers suggested.
Practice already shifting to rituximab
“The results are largely in line with previous perceptions,” David R.W. Jayne, MD, honorary consultant and director of the vasculitis and lupus service at Addenbrooke’s Hospital in Cambridge, England, observed in an emailed comment.
“The difference is a bit bigger [in favor of rituximab] than I would have expected,” said Dr. Jayne, who is also professor of clinical autoimmunity at the University of Cambridge. He noted that clinical practice was already moving toward using rituximab in place of cyclophosphamide.
Rituximab gained a European license to treat antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAV) just over a decade ago. Since then, it has slowly started to replace the use of cyclophosphamide and glucocorticoids, which were the preferred method up until then.
Part of the reason for this shift is the toxicity associated with cyclophosphamide, although that’s not to say that rituximab is free from safety concerns, Dr. Jayne said.
“Toxicity issues with rituximab, especially secondary immunodeficiency, more severe COVID, and blocking vaccine responses, are becoming bigger issues for day-to-day practice,” he noted. Nonetheless, “the introduction of rituximab has been a revolution in AAV treatment. It has encouraged pharma investment in the disease, such as recent approval of avacopan [Tavneos], and it is helping patients.”
Why simulate and not perform a randomized trial?
There were several reasons for performing the current evaluation, study coauthor Benjamin Terrier, MD, PhD, said in an interview.
“The pivotal study, published in 2010, that led to the approval of rituximab was a noninferiority study in comparison with cyclophosphamide, but it included patients with both GPA, granulomatosis with polyangiitis, and MPA, microscopic polyangiitis,” explained Dr. Terrier, professor of internal medicine at the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital in Paris.
“A post hoc analysis showed that, in the patients with a PR3 [proteinase 3]-ANCA and in the patients with relapsing disease, rituximab was superior,” he added.
“So, there was some data which suggest that rituximab could be differentially effective between the different subgroups of patients. So that’s why we wanted to answer this question.”
Since it is not always feasible to do a randomized trial, particularly when it concerns a rare disease such as GPA, Dr. Terrier and associates decided to perform a target trial emulation using data from the FVSG registry, which collates information from 32 hospitals in France. Such studies are gaining in popularity and have been shown to provide a very good level of evidence, he said.
Data collections and secondary endpoint results
The researchers obtained data on 194 patients in the registry who were treated for GPA between April 2008 and April 2018. The majority (85.1%) of patients included were newly diagnosed with GPA, and 56.7% were men. The mean age of patients was 54 years.
Information on the PR3-ANCA status of patients was available for 182 patients, and this showed that the majority (80.8%) were positive for this autoantibody.
A weighted analysis was undertaken to iron out any differences in baseline characteristics, such as the fact that more patients had been treated with at least one dose of cyclophosphamide than rituximab (133 vs. 61).
The primary outcome was remission at 6 months, but a key secondary endpoint was the percentage of patients with a BVAS score of zero at this time point. This turned out to be similar among the rituximab- and cyclophosphamide-treated patients (85.5% vs. 82.6%, respectively).
Another secondary endpoint looked at the retention rate without failure at 24 months, with fewer postinclusion treatment failures seen with rituximab than with cyclophosphamide (7 vs. 51 patients, respectively). Most treatment failures were caused by relapses (7 vs. 33).
In terms of safety, the researchers said they found “no increased toxicity signal” for rituximab over cyclophosphamide. In fact, more severe adverse events were noted in the latter group.
Take-home messages
While of course there are limitations, considering the earlier data and the current results, “we probably have enough data to consider that, in the vast majority of GPA patients, in PR3-ANCA patients, rituximab is probably the best option,” Dr. Terrier said.
There are still patients for whom there isn’t a definitive answer on which drug may be best, such as those with severe disease who were not included in the trials or represent few patients in the registry. For them, it is “still a case-by-case discussion, and I think we have to decide really, with caution,” Dr. Terrier said.
What this study also shows is that emulated trials are possible, he added. “I think it shows that we could have some answers to other questions by emulating trials in this rare disease.”
The FVSG registry has received funding from the European Union’s Horizon 2020 research and innovation program. Dr. Terrier reported receiving personal fees from Vifor Pharma Group, GlaxoSmithKline, and AstraZeneca during the conduct of the study. Dr. Jayne has received lecture fees and a research grant from Roche/Genentech.
More patients with granulomatosis with polyangiitis (GPA) were in remission at 6 months if they had received rituximab (Rituxan) rather than cyclophosphamide (Cytoxan) as induction therapy, according to a target trial emulation performed by the French Vasculitis Study Group (FVSG).
Remission, which was defined as a score of zero on the validated Birmingham Vasculitis Activity Score (BVAS) and use of no more than 10 mg of prednisone a day, was documented in 73.1% of rituximab-treated patients and 40.1% of cyclophosphamide-treated patients.
Similar rates of remission were observed regardless of whether patients were newly diagnosed with GPA (76.1% vs. 41.6%) or had been recently treated for relapsing disease (75.2% vs. 44.5%), FVSG researchers reported in JAMA Network Open.
This research “may inform clinical decision-making regarding the choice of remission-inducing regimen for patients with GPA,” the researchers suggested.
Practice already shifting to rituximab
“The results are largely in line with previous perceptions,” David R.W. Jayne, MD, honorary consultant and director of the vasculitis and lupus service at Addenbrooke’s Hospital in Cambridge, England, observed in an emailed comment.
“The difference is a bit bigger [in favor of rituximab] than I would have expected,” said Dr. Jayne, who is also professor of clinical autoimmunity at the University of Cambridge. He noted that clinical practice was already moving toward using rituximab in place of cyclophosphamide.
Rituximab gained a European license to treat antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAV) just over a decade ago. Since then, it has slowly started to replace the use of cyclophosphamide and glucocorticoids, which were the preferred method up until then.
Part of the reason for this shift is the toxicity associated with cyclophosphamide, although that’s not to say that rituximab is free from safety concerns, Dr. Jayne said.
“Toxicity issues with rituximab, especially secondary immunodeficiency, more severe COVID, and blocking vaccine responses, are becoming bigger issues for day-to-day practice,” he noted. Nonetheless, “the introduction of rituximab has been a revolution in AAV treatment. It has encouraged pharma investment in the disease, such as recent approval of avacopan [Tavneos], and it is helping patients.”
Why simulate and not perform a randomized trial?
There were several reasons for performing the current evaluation, study coauthor Benjamin Terrier, MD, PhD, said in an interview.
“The pivotal study, published in 2010, that led to the approval of rituximab was a noninferiority study in comparison with cyclophosphamide, but it included patients with both GPA, granulomatosis with polyangiitis, and MPA, microscopic polyangiitis,” explained Dr. Terrier, professor of internal medicine at the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital in Paris.
“A post hoc analysis showed that, in the patients with a PR3 [proteinase 3]-ANCA and in the patients with relapsing disease, rituximab was superior,” he added.
“So, there was some data which suggest that rituximab could be differentially effective between the different subgroups of patients. So that’s why we wanted to answer this question.”
Since it is not always feasible to do a randomized trial, particularly when it concerns a rare disease such as GPA, Dr. Terrier and associates decided to perform a target trial emulation using data from the FVSG registry, which collates information from 32 hospitals in France. Such studies are gaining in popularity and have been shown to provide a very good level of evidence, he said.
Data collections and secondary endpoint results
The researchers obtained data on 194 patients in the registry who were treated for GPA between April 2008 and April 2018. The majority (85.1%) of patients included were newly diagnosed with GPA, and 56.7% were men. The mean age of patients was 54 years.
Information on the PR3-ANCA status of patients was available for 182 patients, and this showed that the majority (80.8%) were positive for this autoantibody.
A weighted analysis was undertaken to iron out any differences in baseline characteristics, such as the fact that more patients had been treated with at least one dose of cyclophosphamide than rituximab (133 vs. 61).
The primary outcome was remission at 6 months, but a key secondary endpoint was the percentage of patients with a BVAS score of zero at this time point. This turned out to be similar among the rituximab- and cyclophosphamide-treated patients (85.5% vs. 82.6%, respectively).
Another secondary endpoint looked at the retention rate without failure at 24 months, with fewer postinclusion treatment failures seen with rituximab than with cyclophosphamide (7 vs. 51 patients, respectively). Most treatment failures were caused by relapses (7 vs. 33).
In terms of safety, the researchers said they found “no increased toxicity signal” for rituximab over cyclophosphamide. In fact, more severe adverse events were noted in the latter group.
Take-home messages
While of course there are limitations, considering the earlier data and the current results, “we probably have enough data to consider that, in the vast majority of GPA patients, in PR3-ANCA patients, rituximab is probably the best option,” Dr. Terrier said.
There are still patients for whom there isn’t a definitive answer on which drug may be best, such as those with severe disease who were not included in the trials or represent few patients in the registry. For them, it is “still a case-by-case discussion, and I think we have to decide really, with caution,” Dr. Terrier said.
What this study also shows is that emulated trials are possible, he added. “I think it shows that we could have some answers to other questions by emulating trials in this rare disease.”
The FVSG registry has received funding from the European Union’s Horizon 2020 research and innovation program. Dr. Terrier reported receiving personal fees from Vifor Pharma Group, GlaxoSmithKline, and AstraZeneca during the conduct of the study. Dr. Jayne has received lecture fees and a research grant from Roche/Genentech.
FROM JAMA NETWORK OPEN
Commentary: COVID vaccines and combination therapy in RA, December 2022
Several studies have addressed the efficacy of COVID vaccines in patients with autoimmune and rheumatic diseases owing to the concern for possible reduced vaccine immunogenicity in patients who are immunocompromised or taking immunosuppressive medications. With the availability of additional booster doses for the COVID vaccines, the effect of immunosuppressive medications on the humoral response to mRNA vaccines has been of increased interest in terms of counseling patients on how to manage their medications and vaccine timing. Prior studies have suggested that holding methotrexate after COVID vaccine administration improves antibody response to the COVID vaccine. Stahl and colleagues performed a retrospective study to look at vaccine response to a third (booster) dose in patients with rheumatoid arthritis (RA) over 65 years of age (vs those under 65 years of age) and found that patients over 65 receiving methotrexate had lower levels of neutralizing antibodies than did those receiving other treatments for RA, whereas no differences were seen with different treatments among patients under 65. Although COVID vaccine guidance continues to evolve, this finding raises the possibility of a need to tailor guidance in older patients with RA. However, the finding is far from conclusive given the small number of patients in the study.
In addition to COVID vaccine efficacy, the possibility of vaccine-related adverse effects has been a topic of concern in the rheumatology community, especially regarding the potential for a flare of autoimmune diseases. Anxiety regarding adverse effects may also exacerbate COVID vaccine hesitancy among some people. Naveen and colleagues performed an online international cross-sectional survey study regarding rheumatic diseases and 7-day adverse events. Over 9000 people completed the survey, about half of whom had autoimmune diseases (including nonrheumatic autoimmune diseases). Roughly three quarters of patients with RA reported adverse effects, with differences seen in frequencies of events between the different vaccine manufacturers. However, the majority of these adverse effects were minor (such as fatigue, headache, and body ache), without substantial differences between those with inactive and those with active RA.
The treat-to-target strategy is well-accepted in treatment of RA and pursuit of improved long-term disease outcomes. Hartman and colleagues evaluated the effect of using a treat-to-target strategy starting with the combination therapy with RA-light (COBRA-light) protocol (with initial methotrexate and tapering prednisolone) in early RA. Patients who were deemed at high risk for worsening RA (n = 150) received COBRA-light, whereas those in the low-risk category (n = 40) received methotrexate monotherapy. At 13 weeks, nonresponders were randomized to intensification or continuation of their regimens, with a primary endpoint of European Alliance of Associations for Rheumatology (EULAR) response and secondary endpoint of Disease Activity Score (DAS44). After 13 weeks, 73% of patients in the high-risk category achieved the targets of EULAR good response and DAS44 < 1.6, whereas after 26 weeks, 80% of patients who received intensified therapy and 44% of those who continued their regimens reached the target, though these numbers were small. Overall, the strategy appears to be successful in treatment of early RA in patients at high risk for disease progression, but it does lead to increased use of higher chronic glucocorticoid doses. In addition, the small numbers of patients in the low-risk category, as well as their less aggressive treatment, does not allow for a nuanced analysis of the best initial treatment in this group of patients.
Finally, a cohort study by Takanashi and colleagues evaluated the rates of seropositivity for rheumatoid factor (RF) and anti–cyclic citrullinated peptide (anti-CCP) in patients diagnosed with RA and its association with demographic categories. Seropositivity was associated with smoking and family history of RA, as expected. Among the 1685 patients, 83% of whom were women, older age at RA diagnosis was associated with seronegativity for RF and CCP in women but not in men. The decline in seropositivity with age among women cannot be further evaluated with the limited information in this small study and may have to do with other factors, including erosive or inflammatory osteoarthritis.
Several studies have addressed the efficacy of COVID vaccines in patients with autoimmune and rheumatic diseases owing to the concern for possible reduced vaccine immunogenicity in patients who are immunocompromised or taking immunosuppressive medications. With the availability of additional booster doses for the COVID vaccines, the effect of immunosuppressive medications on the humoral response to mRNA vaccines has been of increased interest in terms of counseling patients on how to manage their medications and vaccine timing. Prior studies have suggested that holding methotrexate after COVID vaccine administration improves antibody response to the COVID vaccine. Stahl and colleagues performed a retrospective study to look at vaccine response to a third (booster) dose in patients with rheumatoid arthritis (RA) over 65 years of age (vs those under 65 years of age) and found that patients over 65 receiving methotrexate had lower levels of neutralizing antibodies than did those receiving other treatments for RA, whereas no differences were seen with different treatments among patients under 65. Although COVID vaccine guidance continues to evolve, this finding raises the possibility of a need to tailor guidance in older patients with RA. However, the finding is far from conclusive given the small number of patients in the study.
In addition to COVID vaccine efficacy, the possibility of vaccine-related adverse effects has been a topic of concern in the rheumatology community, especially regarding the potential for a flare of autoimmune diseases. Anxiety regarding adverse effects may also exacerbate COVID vaccine hesitancy among some people. Naveen and colleagues performed an online international cross-sectional survey study regarding rheumatic diseases and 7-day adverse events. Over 9000 people completed the survey, about half of whom had autoimmune diseases (including nonrheumatic autoimmune diseases). Roughly three quarters of patients with RA reported adverse effects, with differences seen in frequencies of events between the different vaccine manufacturers. However, the majority of these adverse effects were minor (such as fatigue, headache, and body ache), without substantial differences between those with inactive and those with active RA.
The treat-to-target strategy is well-accepted in treatment of RA and pursuit of improved long-term disease outcomes. Hartman and colleagues evaluated the effect of using a treat-to-target strategy starting with the combination therapy with RA-light (COBRA-light) protocol (with initial methotrexate and tapering prednisolone) in early RA. Patients who were deemed at high risk for worsening RA (n = 150) received COBRA-light, whereas those in the low-risk category (n = 40) received methotrexate monotherapy. At 13 weeks, nonresponders were randomized to intensification or continuation of their regimens, with a primary endpoint of European Alliance of Associations for Rheumatology (EULAR) response and secondary endpoint of Disease Activity Score (DAS44). After 13 weeks, 73% of patients in the high-risk category achieved the targets of EULAR good response and DAS44 < 1.6, whereas after 26 weeks, 80% of patients who received intensified therapy and 44% of those who continued their regimens reached the target, though these numbers were small. Overall, the strategy appears to be successful in treatment of early RA in patients at high risk for disease progression, but it does lead to increased use of higher chronic glucocorticoid doses. In addition, the small numbers of patients in the low-risk category, as well as their less aggressive treatment, does not allow for a nuanced analysis of the best initial treatment in this group of patients.
Finally, a cohort study by Takanashi and colleagues evaluated the rates of seropositivity for rheumatoid factor (RF) and anti–cyclic citrullinated peptide (anti-CCP) in patients diagnosed with RA and its association with demographic categories. Seropositivity was associated with smoking and family history of RA, as expected. Among the 1685 patients, 83% of whom were women, older age at RA diagnosis was associated with seronegativity for RF and CCP in women but not in men. The decline in seropositivity with age among women cannot be further evaluated with the limited information in this small study and may have to do with other factors, including erosive or inflammatory osteoarthritis.
Several studies have addressed the efficacy of COVID vaccines in patients with autoimmune and rheumatic diseases owing to the concern for possible reduced vaccine immunogenicity in patients who are immunocompromised or taking immunosuppressive medications. With the availability of additional booster doses for the COVID vaccines, the effect of immunosuppressive medications on the humoral response to mRNA vaccines has been of increased interest in terms of counseling patients on how to manage their medications and vaccine timing. Prior studies have suggested that holding methotrexate after COVID vaccine administration improves antibody response to the COVID vaccine. Stahl and colleagues performed a retrospective study to look at vaccine response to a third (booster) dose in patients with rheumatoid arthritis (RA) over 65 years of age (vs those under 65 years of age) and found that patients over 65 receiving methotrexate had lower levels of neutralizing antibodies than did those receiving other treatments for RA, whereas no differences were seen with different treatments among patients under 65. Although COVID vaccine guidance continues to evolve, this finding raises the possibility of a need to tailor guidance in older patients with RA. However, the finding is far from conclusive given the small number of patients in the study.
In addition to COVID vaccine efficacy, the possibility of vaccine-related adverse effects has been a topic of concern in the rheumatology community, especially regarding the potential for a flare of autoimmune diseases. Anxiety regarding adverse effects may also exacerbate COVID vaccine hesitancy among some people. Naveen and colleagues performed an online international cross-sectional survey study regarding rheumatic diseases and 7-day adverse events. Over 9000 people completed the survey, about half of whom had autoimmune diseases (including nonrheumatic autoimmune diseases). Roughly three quarters of patients with RA reported adverse effects, with differences seen in frequencies of events between the different vaccine manufacturers. However, the majority of these adverse effects were minor (such as fatigue, headache, and body ache), without substantial differences between those with inactive and those with active RA.
The treat-to-target strategy is well-accepted in treatment of RA and pursuit of improved long-term disease outcomes. Hartman and colleagues evaluated the effect of using a treat-to-target strategy starting with the combination therapy with RA-light (COBRA-light) protocol (with initial methotrexate and tapering prednisolone) in early RA. Patients who were deemed at high risk for worsening RA (n = 150) received COBRA-light, whereas those in the low-risk category (n = 40) received methotrexate monotherapy. At 13 weeks, nonresponders were randomized to intensification or continuation of their regimens, with a primary endpoint of European Alliance of Associations for Rheumatology (EULAR) response and secondary endpoint of Disease Activity Score (DAS44). After 13 weeks, 73% of patients in the high-risk category achieved the targets of EULAR good response and DAS44 < 1.6, whereas after 26 weeks, 80% of patients who received intensified therapy and 44% of those who continued their regimens reached the target, though these numbers were small. Overall, the strategy appears to be successful in treatment of early RA in patients at high risk for disease progression, but it does lead to increased use of higher chronic glucocorticoid doses. In addition, the small numbers of patients in the low-risk category, as well as their less aggressive treatment, does not allow for a nuanced analysis of the best initial treatment in this group of patients.
Finally, a cohort study by Takanashi and colleagues evaluated the rates of seropositivity for rheumatoid factor (RF) and anti–cyclic citrullinated peptide (anti-CCP) in patients diagnosed with RA and its association with demographic categories. Seropositivity was associated with smoking and family history of RA, as expected. Among the 1685 patients, 83% of whom were women, older age at RA diagnosis was associated with seronegativity for RF and CCP in women but not in men. The decline in seropositivity with age among women cannot be further evaluated with the limited information in this small study and may have to do with other factors, including erosive or inflammatory osteoarthritis.
Commentary: HER2-Positive EGA, Immunotherapy With Chemoradiation, and Lymph Node Metastasis in GC, December 2022
A study by Hofheinz and colleagues evaluated whether targeting the human epidermal growth factor receptor 2 (HER2) pathway can improve outcomes in early-stage esophagogastric adenocarcinoma (EGA).1 About 15%-20% of EGA overexpress HER2. In the metastatic setting, anti-HER2 therapies have an established role. Trastuzumab in combination with chemotherapy has been part of standard treatment for these tumors for over a decade and now immunotherapy, based on the ongoing KEYNOTE-811 study, has been proven effective as well.2,3 However, prior attempts to effectively target HER2 in the early stage have not been successful.4
A phase 2 trial conducted by the AIO EGA Study Group evaluated the addition of trastuzumab and pertuzumab to FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) chemotherapy in resectable HER2-positive EGA. The trial closed early, before planned full accrual, when the results of the JACOB trial, which evaluated the addition of pertuzumab in the metastatic setting, came out as negative.5 However, the results are still worth discussing here. A total of 81 patients were enrolled in this study (40 in the experimental arm and 41 in the chemotherapy-only control arm). Pathologic complete response was significantly improved with the addition of anti-HER2 therapy (35% vs 12%; P = .02). The rates of R0 resection and surgical complications were similar. Median disease-free survival was not reached in the experimental arm (26 months in the control arm). This study suggests that evaluation of anti-HER2 agents in combination with chemotherapy is warranted. Given the promising results of the KEYNOTE-811 study, future studies should consider incorporative immunotherapy as well.
The Neo-PLANET phase 2 study by Tang and colleagues evaluated the addition of camrelizumab (anti-PD1 antibody) to concurrent chemoradiation in the treatment of advanced EGA.6 The 36 patients enrolled in this study received induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiation with the same chemotherapy backbone. Camrelizumab was added from the time of all chemotherapy initiation. The pathologic complete response observed (33.3%) compared favorably to historical references. No new safety signals were identified. Current standards for resectable EGA include adjuvant nivolumab in patients who have residual disease at the time of resection post chemoradiation. This study demonstrated that the addition of immunotherapy earlier in the treatment course is safe and possibly efficacious. The prospective randomized phase 2/3 ECOG-ACRIN 2174 study will evaluate the addition of nivolumab to chemoradiation, as well as the addition of ipilimumab to nivolumab in the adjuvant setting, and will answer the question regarding the benefits of immunotherapy used earlier in the course of disease in a prospective randomized manner (NCT03604991).
The study by de Jongh and colleagues evaluated the pattern of lymph node metastasis after neoadjuvant chemotherapy with relation to the location of the primary gastric tumor. Tumors from 212 patients who were previously enrolled in the Dutch LOGICA trial comparing laparoscopy vs open D2 gastrectomy were included in this analysis. Although the primary tumor location (proximal vs distal) was associated with a higher frequency of metastasis to certain lymph node groups, this relationship was not exclusive. As such, the extent of lymphadenectomy should not depend on the primary location of the tumor and is not affected by neoadjuvant chemotherapy.
Additional References
1. Hofheinz R-D, Merx K, Haag GM, et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2–positive resectable esophagogastric adenocarcinoma: A randomized phase II trial of the AIO EGA Study Group. J Clin Oncol. 2022;40:3750-3761. Doi: 10.1200/JCO.22.00380
2. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. Doi: 10.1038/s41586-021-04161-3
3. Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. Doi: 10.1016/S0140-6736(10)61121-X
4. Safran HP, Winter K, Hilson D, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2022;23:259-269. Doi: 10.1016/S1470-2045(21)00718-X
5. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018:19:1372-1384. Doi: 10.1016/S1470-2045(18)30481-9
6. Tang Z, Wang Y, Liu D, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022;13:6807. Doi: 10.1038/s41467-022-34403-5
A study by Hofheinz and colleagues evaluated whether targeting the human epidermal growth factor receptor 2 (HER2) pathway can improve outcomes in early-stage esophagogastric adenocarcinoma (EGA).1 About 15%-20% of EGA overexpress HER2. In the metastatic setting, anti-HER2 therapies have an established role. Trastuzumab in combination with chemotherapy has been part of standard treatment for these tumors for over a decade and now immunotherapy, based on the ongoing KEYNOTE-811 study, has been proven effective as well.2,3 However, prior attempts to effectively target HER2 in the early stage have not been successful.4
A phase 2 trial conducted by the AIO EGA Study Group evaluated the addition of trastuzumab and pertuzumab to FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) chemotherapy in resectable HER2-positive EGA. The trial closed early, before planned full accrual, when the results of the JACOB trial, which evaluated the addition of pertuzumab in the metastatic setting, came out as negative.5 However, the results are still worth discussing here. A total of 81 patients were enrolled in this study (40 in the experimental arm and 41 in the chemotherapy-only control arm). Pathologic complete response was significantly improved with the addition of anti-HER2 therapy (35% vs 12%; P = .02). The rates of R0 resection and surgical complications were similar. Median disease-free survival was not reached in the experimental arm (26 months in the control arm). This study suggests that evaluation of anti-HER2 agents in combination with chemotherapy is warranted. Given the promising results of the KEYNOTE-811 study, future studies should consider incorporative immunotherapy as well.
The Neo-PLANET phase 2 study by Tang and colleagues evaluated the addition of camrelizumab (anti-PD1 antibody) to concurrent chemoradiation in the treatment of advanced EGA.6 The 36 patients enrolled in this study received induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiation with the same chemotherapy backbone. Camrelizumab was added from the time of all chemotherapy initiation. The pathologic complete response observed (33.3%) compared favorably to historical references. No new safety signals were identified. Current standards for resectable EGA include adjuvant nivolumab in patients who have residual disease at the time of resection post chemoradiation. This study demonstrated that the addition of immunotherapy earlier in the treatment course is safe and possibly efficacious. The prospective randomized phase 2/3 ECOG-ACRIN 2174 study will evaluate the addition of nivolumab to chemoradiation, as well as the addition of ipilimumab to nivolumab in the adjuvant setting, and will answer the question regarding the benefits of immunotherapy used earlier in the course of disease in a prospective randomized manner (NCT03604991).
The study by de Jongh and colleagues evaluated the pattern of lymph node metastasis after neoadjuvant chemotherapy with relation to the location of the primary gastric tumor. Tumors from 212 patients who were previously enrolled in the Dutch LOGICA trial comparing laparoscopy vs open D2 gastrectomy were included in this analysis. Although the primary tumor location (proximal vs distal) was associated with a higher frequency of metastasis to certain lymph node groups, this relationship was not exclusive. As such, the extent of lymphadenectomy should not depend on the primary location of the tumor and is not affected by neoadjuvant chemotherapy.
Additional References
1. Hofheinz R-D, Merx K, Haag GM, et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2–positive resectable esophagogastric adenocarcinoma: A randomized phase II trial of the AIO EGA Study Group. J Clin Oncol. 2022;40:3750-3761. Doi: 10.1200/JCO.22.00380
2. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. Doi: 10.1038/s41586-021-04161-3
3. Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. Doi: 10.1016/S0140-6736(10)61121-X
4. Safran HP, Winter K, Hilson D, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2022;23:259-269. Doi: 10.1016/S1470-2045(21)00718-X
5. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018:19:1372-1384. Doi: 10.1016/S1470-2045(18)30481-9
6. Tang Z, Wang Y, Liu D, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022;13:6807. Doi: 10.1038/s41467-022-34403-5
A study by Hofheinz and colleagues evaluated whether targeting the human epidermal growth factor receptor 2 (HER2) pathway can improve outcomes in early-stage esophagogastric adenocarcinoma (EGA).1 About 15%-20% of EGA overexpress HER2. In the metastatic setting, anti-HER2 therapies have an established role. Trastuzumab in combination with chemotherapy has been part of standard treatment for these tumors for over a decade and now immunotherapy, based on the ongoing KEYNOTE-811 study, has been proven effective as well.2,3 However, prior attempts to effectively target HER2 in the early stage have not been successful.4
A phase 2 trial conducted by the AIO EGA Study Group evaluated the addition of trastuzumab and pertuzumab to FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) chemotherapy in resectable HER2-positive EGA. The trial closed early, before planned full accrual, when the results of the JACOB trial, which evaluated the addition of pertuzumab in the metastatic setting, came out as negative.5 However, the results are still worth discussing here. A total of 81 patients were enrolled in this study (40 in the experimental arm and 41 in the chemotherapy-only control arm). Pathologic complete response was significantly improved with the addition of anti-HER2 therapy (35% vs 12%; P = .02). The rates of R0 resection and surgical complications were similar. Median disease-free survival was not reached in the experimental arm (26 months in the control arm). This study suggests that evaluation of anti-HER2 agents in combination with chemotherapy is warranted. Given the promising results of the KEYNOTE-811 study, future studies should consider incorporative immunotherapy as well.
The Neo-PLANET phase 2 study by Tang and colleagues evaluated the addition of camrelizumab (anti-PD1 antibody) to concurrent chemoradiation in the treatment of advanced EGA.6 The 36 patients enrolled in this study received induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiation with the same chemotherapy backbone. Camrelizumab was added from the time of all chemotherapy initiation. The pathologic complete response observed (33.3%) compared favorably to historical references. No new safety signals were identified. Current standards for resectable EGA include adjuvant nivolumab in patients who have residual disease at the time of resection post chemoradiation. This study demonstrated that the addition of immunotherapy earlier in the treatment course is safe and possibly efficacious. The prospective randomized phase 2/3 ECOG-ACRIN 2174 study will evaluate the addition of nivolumab to chemoradiation, as well as the addition of ipilimumab to nivolumab in the adjuvant setting, and will answer the question regarding the benefits of immunotherapy used earlier in the course of disease in a prospective randomized manner (NCT03604991).
The study by de Jongh and colleagues evaluated the pattern of lymph node metastasis after neoadjuvant chemotherapy with relation to the location of the primary gastric tumor. Tumors from 212 patients who were previously enrolled in the Dutch LOGICA trial comparing laparoscopy vs open D2 gastrectomy were included in this analysis. Although the primary tumor location (proximal vs distal) was associated with a higher frequency of metastasis to certain lymph node groups, this relationship was not exclusive. As such, the extent of lymphadenectomy should not depend on the primary location of the tumor and is not affected by neoadjuvant chemotherapy.
Additional References
1. Hofheinz R-D, Merx K, Haag GM, et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2–positive resectable esophagogastric adenocarcinoma: A randomized phase II trial of the AIO EGA Study Group. J Clin Oncol. 2022;40:3750-3761. Doi: 10.1200/JCO.22.00380
2. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. Doi: 10.1038/s41586-021-04161-3
3. Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. Doi: 10.1016/S0140-6736(10)61121-X
4. Safran HP, Winter K, Hilson D, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2022;23:259-269. Doi: 10.1016/S1470-2045(21)00718-X
5. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018:19:1372-1384. Doi: 10.1016/S1470-2045(18)30481-9
6. Tang Z, Wang Y, Liu D, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022;13:6807. Doi: 10.1038/s41467-022-34403-5










