User login
Baricitinib a promising treatment option for difficult-to-treat atopic dermatitis in daily practice
Key clinical point: Baricitinib could serve as an effective treatment option for patients with difficult-to-treat moderate-to-severe atopic dermatitis (AD), including those unresponsive to dupilumab treatment; however, a high discontinuation rate indicates its rather heterogenous efficacy.
Major finding: At week 16, the mean Eczema Area and Severity Index score and numerical rating scale-pruritis significantly decreased from 18.3 to 11.1 (P < .0001) and from 6.6 to 5.3 (P < .0001), respectively. The most frequent adverse events (AE) were nausea (11.8%), urinary tract infection (9.8%), and herpes simplex infections (7.8%). Baricitinib treatment was discontinued by 43.2% of patients due to ineffectiveness or AE.
Study details: This multicenter prospective observational study included 51 adult patients with moderate-to-severe AD from the BioDay registry who received baricitinib over 16 weeks.
Disclosures: This study did not report a source of funding. Some authors declared serving as speakers, consultants, advisory board members, or investigators for various organizations.
Source: Boesjes CM et al. Daily practice experience of baricitinib treatment for patients with difficult-to-treat atopic dermatitis: Results from the BioDay registry. Acta Derm Venereol. 2022;102:adv00820 (Nov 24). Doi: 10.2340/actadv.v102.3978
Key clinical point: Baricitinib could serve as an effective treatment option for patients with difficult-to-treat moderate-to-severe atopic dermatitis (AD), including those unresponsive to dupilumab treatment; however, a high discontinuation rate indicates its rather heterogenous efficacy.
Major finding: At week 16, the mean Eczema Area and Severity Index score and numerical rating scale-pruritis significantly decreased from 18.3 to 11.1 (P < .0001) and from 6.6 to 5.3 (P < .0001), respectively. The most frequent adverse events (AE) were nausea (11.8%), urinary tract infection (9.8%), and herpes simplex infections (7.8%). Baricitinib treatment was discontinued by 43.2% of patients due to ineffectiveness or AE.
Study details: This multicenter prospective observational study included 51 adult patients with moderate-to-severe AD from the BioDay registry who received baricitinib over 16 weeks.
Disclosures: This study did not report a source of funding. Some authors declared serving as speakers, consultants, advisory board members, or investigators for various organizations.
Source: Boesjes CM et al. Daily practice experience of baricitinib treatment for patients with difficult-to-treat atopic dermatitis: Results from the BioDay registry. Acta Derm Venereol. 2022;102:adv00820 (Nov 24). Doi: 10.2340/actadv.v102.3978
Key clinical point: Baricitinib could serve as an effective treatment option for patients with difficult-to-treat moderate-to-severe atopic dermatitis (AD), including those unresponsive to dupilumab treatment; however, a high discontinuation rate indicates its rather heterogenous efficacy.
Major finding: At week 16, the mean Eczema Area and Severity Index score and numerical rating scale-pruritis significantly decreased from 18.3 to 11.1 (P < .0001) and from 6.6 to 5.3 (P < .0001), respectively. The most frequent adverse events (AE) were nausea (11.8%), urinary tract infection (9.8%), and herpes simplex infections (7.8%). Baricitinib treatment was discontinued by 43.2% of patients due to ineffectiveness or AE.
Study details: This multicenter prospective observational study included 51 adult patients with moderate-to-severe AD from the BioDay registry who received baricitinib over 16 weeks.
Disclosures: This study did not report a source of funding. Some authors declared serving as speakers, consultants, advisory board members, or investigators for various organizations.
Source: Boesjes CM et al. Daily practice experience of baricitinib treatment for patients with difficult-to-treat atopic dermatitis: Results from the BioDay registry. Acta Derm Venereol. 2022;102:adv00820 (Nov 24). Doi: 10.2340/actadv.v102.3978
Incidental skin finding
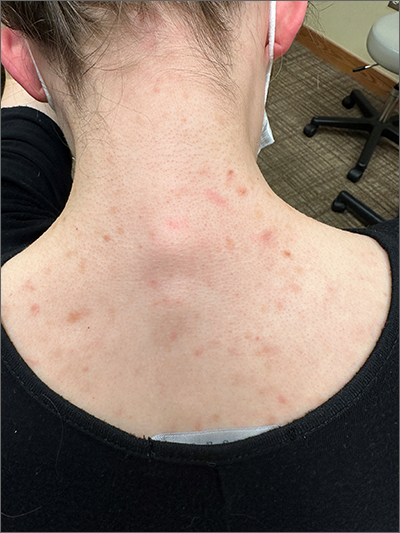
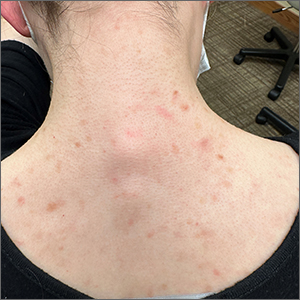
This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034

This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034
First exposure to general anesthesia not a risk factor for atopic dermatitis in the pediatric population
Key clinical point: The first exposure of pediatric individuals to general anesthesia (GA) is not associated with an increased or decreased risk of developing atopic dermatitis (AD).
Major finding: Multivariate analysis revealed that individuals who were exposed vs not exposed to GA did not have an increased or decreased risk of developing AD (adjusted hazard ratio 1.03; P = .701).
Study details: This retrospective cohort study included pediatric individuals aged ≤18 years who were (n = 7,681) or were not (n = 38,405; control individuals) exposed to GA.
Disclosures: This study was funded by a 2020 Amorepacific (South Korea) grant. The authors declared no conflicts of interest.
Source: Kim DC et al. No association between first exposure to general anaesthesia and atopic dermatitis in the paediatric population. Acta Derm Venereol. 2022;102:adv00813 (Nov 14). Doi: 10.2340/actadv.v102.2738
Key clinical point: The first exposure of pediatric individuals to general anesthesia (GA) is not associated with an increased or decreased risk of developing atopic dermatitis (AD).
Major finding: Multivariate analysis revealed that individuals who were exposed vs not exposed to GA did not have an increased or decreased risk of developing AD (adjusted hazard ratio 1.03; P = .701).
Study details: This retrospective cohort study included pediatric individuals aged ≤18 years who were (n = 7,681) or were not (n = 38,405; control individuals) exposed to GA.
Disclosures: This study was funded by a 2020 Amorepacific (South Korea) grant. The authors declared no conflicts of interest.
Source: Kim DC et al. No association between first exposure to general anaesthesia and atopic dermatitis in the paediatric population. Acta Derm Venereol. 2022;102:adv00813 (Nov 14). Doi: 10.2340/actadv.v102.2738
Key clinical point: The first exposure of pediatric individuals to general anesthesia (GA) is not associated with an increased or decreased risk of developing atopic dermatitis (AD).
Major finding: Multivariate analysis revealed that individuals who were exposed vs not exposed to GA did not have an increased or decreased risk of developing AD (adjusted hazard ratio 1.03; P = .701).
Study details: This retrospective cohort study included pediatric individuals aged ≤18 years who were (n = 7,681) or were not (n = 38,405; control individuals) exposed to GA.
Disclosures: This study was funded by a 2020 Amorepacific (South Korea) grant. The authors declared no conflicts of interest.
Source: Kim DC et al. No association between first exposure to general anaesthesia and atopic dermatitis in the paediatric population. Acta Derm Venereol. 2022;102:adv00813 (Nov 14). Doi: 10.2340/actadv.v102.2738
High treatment flexibility with baricitinib in moderate-to-severe atopic dermatitis
Key clinical point: Downtitrated baricitinib treatment was efficacious in most patients with moderate-to-severe atopic dermatitis (AD) through 16 weeks; clinically relevant efficacy was observed in most patients who were readministered the original dose due to downtitration or treatment withdrawal-related efficacy loss.
Major finding: In the 4-mg and 2-mg cohorts, 61% and 71% of patients maintained a validated Investigator’s Global Assessment for AD (vIGA-AD) score of 0/1/2 at downtitration week 16 and 80%/85%/88% and 90%/56%/86% of patients who switched to original dose in the continuous dosing/downtitration/treatment withdrawal group re-achieved vIGA-AD 0/1/2, respectively.
Study details: This BREEZE-AD3 trial substudy included 526 patients with moderate-to-severe AD treated with 4/2 mg baricitinib at trial entry who achieved vIGA-AD 0/1/2 at week 52, with each cohort being re-assigned to continuous dosing, downtitration, or treatment withdrawal.
Disclosures: This study was sponsored by Eli Lilly and Company under license from Incyte Corporation. Some authors reported ties with various sources, including Eli Lilly and Incyte. Three authors declared being current or former employees and shareholders of Eli Lilly.
Source: Reich K et al. Efficacy of downtitration or treatment withdrawal compared to continuous dosing after successful treatment with baricitinib in patients with moderate-to-severe atopic dermatitis in a randomised substudy from the long-term extension study, BREEZE-AD3. Br J Dermatol. 2022 (Nov 17). Doi: 10.1093/bjd/ljac057
Key clinical point: Downtitrated baricitinib treatment was efficacious in most patients with moderate-to-severe atopic dermatitis (AD) through 16 weeks; clinically relevant efficacy was observed in most patients who were readministered the original dose due to downtitration or treatment withdrawal-related efficacy loss.
Major finding: In the 4-mg and 2-mg cohorts, 61% and 71% of patients maintained a validated Investigator’s Global Assessment for AD (vIGA-AD) score of 0/1/2 at downtitration week 16 and 80%/85%/88% and 90%/56%/86% of patients who switched to original dose in the continuous dosing/downtitration/treatment withdrawal group re-achieved vIGA-AD 0/1/2, respectively.
Study details: This BREEZE-AD3 trial substudy included 526 patients with moderate-to-severe AD treated with 4/2 mg baricitinib at trial entry who achieved vIGA-AD 0/1/2 at week 52, with each cohort being re-assigned to continuous dosing, downtitration, or treatment withdrawal.
Disclosures: This study was sponsored by Eli Lilly and Company under license from Incyte Corporation. Some authors reported ties with various sources, including Eli Lilly and Incyte. Three authors declared being current or former employees and shareholders of Eli Lilly.
Source: Reich K et al. Efficacy of downtitration or treatment withdrawal compared to continuous dosing after successful treatment with baricitinib in patients with moderate-to-severe atopic dermatitis in a randomised substudy from the long-term extension study, BREEZE-AD3. Br J Dermatol. 2022 (Nov 17). Doi: 10.1093/bjd/ljac057
Key clinical point: Downtitrated baricitinib treatment was efficacious in most patients with moderate-to-severe atopic dermatitis (AD) through 16 weeks; clinically relevant efficacy was observed in most patients who were readministered the original dose due to downtitration or treatment withdrawal-related efficacy loss.
Major finding: In the 4-mg and 2-mg cohorts, 61% and 71% of patients maintained a validated Investigator’s Global Assessment for AD (vIGA-AD) score of 0/1/2 at downtitration week 16 and 80%/85%/88% and 90%/56%/86% of patients who switched to original dose in the continuous dosing/downtitration/treatment withdrawal group re-achieved vIGA-AD 0/1/2, respectively.
Study details: This BREEZE-AD3 trial substudy included 526 patients with moderate-to-severe AD treated with 4/2 mg baricitinib at trial entry who achieved vIGA-AD 0/1/2 at week 52, with each cohort being re-assigned to continuous dosing, downtitration, or treatment withdrawal.
Disclosures: This study was sponsored by Eli Lilly and Company under license from Incyte Corporation. Some authors reported ties with various sources, including Eli Lilly and Incyte. Three authors declared being current or former employees and shareholders of Eli Lilly.
Source: Reich K et al. Efficacy of downtitration or treatment withdrawal compared to continuous dosing after successful treatment with baricitinib in patients with moderate-to-severe atopic dermatitis in a randomised substudy from the long-term extension study, BREEZE-AD3. Br J Dermatol. 2022 (Nov 17). Doi: 10.1093/bjd/ljac057
Dupilumab is clinically effective and safe for treating pediatric atopic dermatitis in daily practice
Key clinical point: Dupilumab decreases disease severity, disease-associated symptoms, and severity-associated serum biomarker levels in pediatric patients with atopic dermatitis (AD) in daily practice.
Major finding: At week 28, 49.2% of patients achieved Eczema Area and Severity Index-75; 84.7%, 45.3%, and 77.4% achieved a ≥4-point improvement in the Patient-Oriented Eczema Measure, Numeric Rating Scale (NRS)-pruritus, and NRS-pain scores, respectively; 36.1% scored clear or almost clear on the Investigator’s Global Assessment. The levels of severity-associated markers soluble IL-2-receptor alpha (P < .05), periostin (P < .05), thymus- and activation-regulated chemokine (P < .005), and pulmonary- and activation-regulated chemokine (P < .005) were significantly reduced at week 4. Conjunctivitis (16.4%) and headache (6.6%) were the most common side effects.
Study details: This multicenter prospective observational study included 61 patients (children ≥6 to <12 years; adolescents ≥12 to <18 years) from the BioDay registry with moderate-to-severe AD who received dupilumab for 28 weeks.
Disclosures: The BioDay registry is sponsored by Sanofi/Regeneron and others. Some authors reported ties with various sources, including Sanofi.
Source: Kamphuis E, Boesjes CM et al. Dupilumab in daily practice for the treatment of pediatric atopic dermatitis: 28-week clinical and biomarker results from the BioDay registry. Pediatr Allergy Immunol. 2022;13(12):e13887 (Dec 5). Doi: 10.1111/pai.13887
Key clinical point: Dupilumab decreases disease severity, disease-associated symptoms, and severity-associated serum biomarker levels in pediatric patients with atopic dermatitis (AD) in daily practice.
Major finding: At week 28, 49.2% of patients achieved Eczema Area and Severity Index-75; 84.7%, 45.3%, and 77.4% achieved a ≥4-point improvement in the Patient-Oriented Eczema Measure, Numeric Rating Scale (NRS)-pruritus, and NRS-pain scores, respectively; 36.1% scored clear or almost clear on the Investigator’s Global Assessment. The levels of severity-associated markers soluble IL-2-receptor alpha (P < .05), periostin (P < .05), thymus- and activation-regulated chemokine (P < .005), and pulmonary- and activation-regulated chemokine (P < .005) were significantly reduced at week 4. Conjunctivitis (16.4%) and headache (6.6%) were the most common side effects.
Study details: This multicenter prospective observational study included 61 patients (children ≥6 to <12 years; adolescents ≥12 to <18 years) from the BioDay registry with moderate-to-severe AD who received dupilumab for 28 weeks.
Disclosures: The BioDay registry is sponsored by Sanofi/Regeneron and others. Some authors reported ties with various sources, including Sanofi.
Source: Kamphuis E, Boesjes CM et al. Dupilumab in daily practice for the treatment of pediatric atopic dermatitis: 28-week clinical and biomarker results from the BioDay registry. Pediatr Allergy Immunol. 2022;13(12):e13887 (Dec 5). Doi: 10.1111/pai.13887
Key clinical point: Dupilumab decreases disease severity, disease-associated symptoms, and severity-associated serum biomarker levels in pediatric patients with atopic dermatitis (AD) in daily practice.
Major finding: At week 28, 49.2% of patients achieved Eczema Area and Severity Index-75; 84.7%, 45.3%, and 77.4% achieved a ≥4-point improvement in the Patient-Oriented Eczema Measure, Numeric Rating Scale (NRS)-pruritus, and NRS-pain scores, respectively; 36.1% scored clear or almost clear on the Investigator’s Global Assessment. The levels of severity-associated markers soluble IL-2-receptor alpha (P < .05), periostin (P < .05), thymus- and activation-regulated chemokine (P < .005), and pulmonary- and activation-regulated chemokine (P < .005) were significantly reduced at week 4. Conjunctivitis (16.4%) and headache (6.6%) were the most common side effects.
Study details: This multicenter prospective observational study included 61 patients (children ≥6 to <12 years; adolescents ≥12 to <18 years) from the BioDay registry with moderate-to-severe AD who received dupilumab for 28 weeks.
Disclosures: The BioDay registry is sponsored by Sanofi/Regeneron and others. Some authors reported ties with various sources, including Sanofi.
Source: Kamphuis E, Boesjes CM et al. Dupilumab in daily practice for the treatment of pediatric atopic dermatitis: 28-week clinical and biomarker results from the BioDay registry. Pediatr Allergy Immunol. 2022;13(12):e13887 (Dec 5). Doi: 10.1111/pai.13887
Early emollient application: An effective strategy for atopic dermatitis prevention in infants
Key clinical point: Early application of emollients can effectively prevent atopic dermatitis (AD) in infants, with emollient emulsion seeming an optimal treatment option in infancy compared with creams or mixed emollients.
Major finding: The incidence of AD was significantly lower in high-risk infants receiving early emollients vs standard care (risk ratio 0.64; 95% CI 0.47-0.88), with surface under the cumulative ranking curve analysis revealing emollient emulsion (82.6%) as the optimal treatment for AD prevention in infants, followed by mixed emollient (77.4%) and emollient cream (21.9%).
Study details: This was a systematic review and network meta-analysis of 11 randomized controlled trials including 3483 infants without AD who received either prophylactic emollients (cream, emulsion, or mixed types) or standard care.
Disclosures: This study was supported by the Key Research and Development Project of Xinjiang Uygur Autonomous Region, China. The authors declared no conflicts of interest.
Source: Liang J, Hu F et al. Systematic review and network meta‐analysis of different types of emollient for the prevention of atopic dermatitis in infants. J Eur Acad Dermatol Venereol. 2022 (Nov 23). Doi: 10.1111/jdv.18688
Key clinical point: Early application of emollients can effectively prevent atopic dermatitis (AD) in infants, with emollient emulsion seeming an optimal treatment option in infancy compared with creams or mixed emollients.
Major finding: The incidence of AD was significantly lower in high-risk infants receiving early emollients vs standard care (risk ratio 0.64; 95% CI 0.47-0.88), with surface under the cumulative ranking curve analysis revealing emollient emulsion (82.6%) as the optimal treatment for AD prevention in infants, followed by mixed emollient (77.4%) and emollient cream (21.9%).
Study details: This was a systematic review and network meta-analysis of 11 randomized controlled trials including 3483 infants without AD who received either prophylactic emollients (cream, emulsion, or mixed types) or standard care.
Disclosures: This study was supported by the Key Research and Development Project of Xinjiang Uygur Autonomous Region, China. The authors declared no conflicts of interest.
Source: Liang J, Hu F et al. Systematic review and network meta‐analysis of different types of emollient for the prevention of atopic dermatitis in infants. J Eur Acad Dermatol Venereol. 2022 (Nov 23). Doi: 10.1111/jdv.18688
Key clinical point: Early application of emollients can effectively prevent atopic dermatitis (AD) in infants, with emollient emulsion seeming an optimal treatment option in infancy compared with creams or mixed emollients.
Major finding: The incidence of AD was significantly lower in high-risk infants receiving early emollients vs standard care (risk ratio 0.64; 95% CI 0.47-0.88), with surface under the cumulative ranking curve analysis revealing emollient emulsion (82.6%) as the optimal treatment for AD prevention in infants, followed by mixed emollient (77.4%) and emollient cream (21.9%).
Study details: This was a systematic review and network meta-analysis of 11 randomized controlled trials including 3483 infants without AD who received either prophylactic emollients (cream, emulsion, or mixed types) or standard care.
Disclosures: This study was supported by the Key Research and Development Project of Xinjiang Uygur Autonomous Region, China. The authors declared no conflicts of interest.
Source: Liang J, Hu F et al. Systematic review and network meta‐analysis of different types of emollient for the prevention of atopic dermatitis in infants. J Eur Acad Dermatol Venereol. 2022 (Nov 23). Doi: 10.1111/jdv.18688
Phase 3 studies confirm long-term disease control with ruxolitinib cream in atopic dermatitis
Key clinical point: Ruxolitinib cream demonstrated effective disease control and was well tolerated in patients with atopic dermatitis (AD) during 44 weeks of as-needed treatment.
Major finding: At week 52, 74.1%-77.8% of patients had an Investigator’s Global Assessment score of 0/1, with the mean affected body surface area being 1.4%-1.8%. Treatment-related adverse events were reported in 8.7%/7.4% of patients on 0.75%/1.5% ruxolitinib and in 2.0%/6.1% of those who switched from vehicle to 0.75%/1.5% ruxolitinib, respectively.
Study details: This study analyzed pooled data from two phase 3 studies, TRuE-AD1 and TRuE-AD2, including 1249 patients aged ≥12 years with AD who were randomly assigned to receive 0.75% or 1.5% ruxolitinib cream or vehicle for 8 weeks; thereafter, the vehicle group patients were re-assigned to receive either strength ruxolitinib cream for 44 weeks.
Disclosures: This study was funded by Incyte Corporation, U.S. Some authors reported ties with various sources, including Incyte. Four authors declared being current or former employees and shareholders of Incyte.
Source: Papp K et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: Results from two phase 3 studies. J Am Acad Dermatol. 2022 (Nov 25). Doi: 10.1016/j.jaad.2022.09.060
Key clinical point: Ruxolitinib cream demonstrated effective disease control and was well tolerated in patients with atopic dermatitis (AD) during 44 weeks of as-needed treatment.
Major finding: At week 52, 74.1%-77.8% of patients had an Investigator’s Global Assessment score of 0/1, with the mean affected body surface area being 1.4%-1.8%. Treatment-related adverse events were reported in 8.7%/7.4% of patients on 0.75%/1.5% ruxolitinib and in 2.0%/6.1% of those who switched from vehicle to 0.75%/1.5% ruxolitinib, respectively.
Study details: This study analyzed pooled data from two phase 3 studies, TRuE-AD1 and TRuE-AD2, including 1249 patients aged ≥12 years with AD who were randomly assigned to receive 0.75% or 1.5% ruxolitinib cream or vehicle for 8 weeks; thereafter, the vehicle group patients were re-assigned to receive either strength ruxolitinib cream for 44 weeks.
Disclosures: This study was funded by Incyte Corporation, U.S. Some authors reported ties with various sources, including Incyte. Four authors declared being current or former employees and shareholders of Incyte.
Source: Papp K et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: Results from two phase 3 studies. J Am Acad Dermatol. 2022 (Nov 25). Doi: 10.1016/j.jaad.2022.09.060
Key clinical point: Ruxolitinib cream demonstrated effective disease control and was well tolerated in patients with atopic dermatitis (AD) during 44 weeks of as-needed treatment.
Major finding: At week 52, 74.1%-77.8% of patients had an Investigator’s Global Assessment score of 0/1, with the mean affected body surface area being 1.4%-1.8%. Treatment-related adverse events were reported in 8.7%/7.4% of patients on 0.75%/1.5% ruxolitinib and in 2.0%/6.1% of those who switched from vehicle to 0.75%/1.5% ruxolitinib, respectively.
Study details: This study analyzed pooled data from two phase 3 studies, TRuE-AD1 and TRuE-AD2, including 1249 patients aged ≥12 years with AD who were randomly assigned to receive 0.75% or 1.5% ruxolitinib cream or vehicle for 8 weeks; thereafter, the vehicle group patients were re-assigned to receive either strength ruxolitinib cream for 44 weeks.
Disclosures: This study was funded by Incyte Corporation, U.S. Some authors reported ties with various sources, including Incyte. Four authors declared being current or former employees and shareholders of Incyte.
Source: Papp K et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: Results from two phase 3 studies. J Am Acad Dermatol. 2022 (Nov 25). Doi: 10.1016/j.jaad.2022.09.060
Tralokinumab improves microbial dysbiosis in lesional skin in AD
Key clinical point: Specific targeting of interleukin-13 alone with tralokinumab improved microbial diversity and reduced Staphylococcus aureus abundance in the lesional skin in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The 16-week tralokinumab treatment reduced S. aureus abundance by 20.7-fold (P < .0001), whereas placebo led to a non-statistically significant reduction. Tralokinumab also led to a significant increase in microbial diversity as early as week 8 (P < .001) and also at week 16 (P < .05).
Study details: The data come from the phase 3 ECZTRA 1 trial including 802 patients with moderate-to-severe AD who were randomly assigned to receive 300 mg subcutaneous tralokinumab or placebo.
Disclosures: This study and the ECZTRA 1 trial were funded by LEO Pharma A/S. Some authors declared serving as speakers, consultants, investigators, scientific advisors, or clinical study investigators or receiving institutional research grants from various sources, including LEO Pharma.
Source: Beck LA et al. Tralokinumab treatment improves the skin microbiota by increasing the microbial diversity in adults with moderate-to-severe atopic dermatitis: Analysis of microbial diversity in ECZTRA 1, a randomized controlled trial. J Am Acad Dermatol. 2022 (Dec 2). Doi: 10.1016/j.jaad.2022.11.047
Key clinical point: Specific targeting of interleukin-13 alone with tralokinumab improved microbial diversity and reduced Staphylococcus aureus abundance in the lesional skin in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The 16-week tralokinumab treatment reduced S. aureus abundance by 20.7-fold (P < .0001), whereas placebo led to a non-statistically significant reduction. Tralokinumab also led to a significant increase in microbial diversity as early as week 8 (P < .001) and also at week 16 (P < .05).
Study details: The data come from the phase 3 ECZTRA 1 trial including 802 patients with moderate-to-severe AD who were randomly assigned to receive 300 mg subcutaneous tralokinumab or placebo.
Disclosures: This study and the ECZTRA 1 trial were funded by LEO Pharma A/S. Some authors declared serving as speakers, consultants, investigators, scientific advisors, or clinical study investigators or receiving institutional research grants from various sources, including LEO Pharma.
Source: Beck LA et al. Tralokinumab treatment improves the skin microbiota by increasing the microbial diversity in adults with moderate-to-severe atopic dermatitis: Analysis of microbial diversity in ECZTRA 1, a randomized controlled trial. J Am Acad Dermatol. 2022 (Dec 2). Doi: 10.1016/j.jaad.2022.11.047
Key clinical point: Specific targeting of interleukin-13 alone with tralokinumab improved microbial diversity and reduced Staphylococcus aureus abundance in the lesional skin in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The 16-week tralokinumab treatment reduced S. aureus abundance by 20.7-fold (P < .0001), whereas placebo led to a non-statistically significant reduction. Tralokinumab also led to a significant increase in microbial diversity as early as week 8 (P < .001) and also at week 16 (P < .05).
Study details: The data come from the phase 3 ECZTRA 1 trial including 802 patients with moderate-to-severe AD who were randomly assigned to receive 300 mg subcutaneous tralokinumab or placebo.
Disclosures: This study and the ECZTRA 1 trial were funded by LEO Pharma A/S. Some authors declared serving as speakers, consultants, investigators, scientific advisors, or clinical study investigators or receiving institutional research grants from various sources, including LEO Pharma.
Source: Beck LA et al. Tralokinumab treatment improves the skin microbiota by increasing the microbial diversity in adults with moderate-to-severe atopic dermatitis: Analysis of microbial diversity in ECZTRA 1, a randomized controlled trial. J Am Acad Dermatol. 2022 (Dec 2). Doi: 10.1016/j.jaad.2022.11.047
Tralokinumab improves microbial dysbiosis in lesional skin in AD
Key clinical point: Specific targeting of interleukin-13 alone with tralokinumab improved microbial diversity and reduced Staphylococcus aureus abundance in the lesional skin in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The 16-week tralokinumab treatment reduced S. aureus abundance by 20.7-fold (P < .0001), whereas placebo led to a non-statistically significant reduction. Tralokinumab also led to a significant increase in microbial diversity as early as week 8 (P < .001) and also at week 16 (P < .05).
Study details: The data come from the phase 3 ECZTRA 1 trial including 802 patients with moderate-to-severe AD who were randomly assigned to receive 300 mg subcutaneous tralokinumab or placebo.
Disclosures: This study and the ECZTRA 1 trial were funded by LEO Pharma A/S. Some authors declared serving as speakers, consultants, investigators, scientific advisors, or clinical study investigators or receiving institutional research grants from various sources, including LEO Pharma.
Source: Beck LA et al. Tralokinumab treatment improves the skin microbiota by increasing the microbial diversity in adults with moderate-to-severe atopic dermatitis: Analysis of microbial diversity in ECZTRA 1, a randomized controlled trial. J Am Acad Dermatol. 2022 (Dec 2). Doi: 10.1016/j.jaad.2022.11.047
Key clinical point: Specific targeting of interleukin-13 alone with tralokinumab improved microbial diversity and reduced Staphylococcus aureus abundance in the lesional skin in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The 16-week tralokinumab treatment reduced S. aureus abundance by 20.7-fold (P < .0001), whereas placebo led to a non-statistically significant reduction. Tralokinumab also led to a significant increase in microbial diversity as early as week 8 (P < .001) and also at week 16 (P < .05).
Study details: The data come from the phase 3 ECZTRA 1 trial including 802 patients with moderate-to-severe AD who were randomly assigned to receive 300 mg subcutaneous tralokinumab or placebo.
Disclosures: This study and the ECZTRA 1 trial were funded by LEO Pharma A/S. Some authors declared serving as speakers, consultants, investigators, scientific advisors, or clinical study investigators or receiving institutional research grants from various sources, including LEO Pharma.
Source: Beck LA et al. Tralokinumab treatment improves the skin microbiota by increasing the microbial diversity in adults with moderate-to-severe atopic dermatitis: Analysis of microbial diversity in ECZTRA 1, a randomized controlled trial. J Am Acad Dermatol. 2022 (Dec 2). Doi: 10.1016/j.jaad.2022.11.047
Key clinical point: Specific targeting of interleukin-13 alone with tralokinumab improved microbial diversity and reduced Staphylococcus aureus abundance in the lesional skin in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The 16-week tralokinumab treatment reduced S. aureus abundance by 20.7-fold (P < .0001), whereas placebo led to a non-statistically significant reduction. Tralokinumab also led to a significant increase in microbial diversity as early as week 8 (P < .001) and also at week 16 (P < .05).
Study details: The data come from the phase 3 ECZTRA 1 trial including 802 patients with moderate-to-severe AD who were randomly assigned to receive 300 mg subcutaneous tralokinumab or placebo.
Disclosures: This study and the ECZTRA 1 trial were funded by LEO Pharma A/S. Some authors declared serving as speakers, consultants, investigators, scientific advisors, or clinical study investigators or receiving institutional research grants from various sources, including LEO Pharma.
Source: Beck LA et al. Tralokinumab treatment improves the skin microbiota by increasing the microbial diversity in adults with moderate-to-severe atopic dermatitis: Analysis of microbial diversity in ECZTRA 1, a randomized controlled trial. J Am Acad Dermatol. 2022 (Dec 2). Doi: 10.1016/j.jaad.2022.11.047
Meta-analysis supports safe use of topical calcineurin inhibitors in AD
Key clinical point: An exposure to topical calcineurin inhibitors did not increase the risk for cancer in patients with atopic dermatitis (AD), with findings being similar among infants, children, and adults.
Major finding: Compared with no exposure, topical calcineurin inhibitor exposure was not associated with an increased risk for cancer (odds ratio [OR] 1.03; 95% credible interval [CrI] 0.94-1.11), with neither pimecrolimus (OR 1.05; 95% CrI 0.94-1.15) nor tacrolimus (OR 0.99; 95% CrI 0.89-1.09) use revealing any association with increased cancer risk, across all age groups.
Study details: This was a systematic review and meta-analysis of 110 unique studies (52 randomized controlled trials and 69 nonrandomized studies) including 3.4 million patients with AD followed-up for a mean of 11 months.
Disclosures: This study was funded by the American Academy of Allergy, Asthma, and Immunology and the American College of Allergy, Asthma, and Immunology. L Schneider declared receiving consulting fees and payments to her institutions from, serving on data safety monitoring and advisory boards for, and holding stock or stock options in various sources.
Source: Devasenapathy N et al for the AAAAI/ACAAI Joint Task Force on Practice Parameters for Atopic Dermatitis Guideline Development Group. Cancer risk with topical calcineurin inhibitors, pimecrolimus and tacrolimus, for atopic dermatitis: A systematic review and meta-analysis. Lancet Child Adolesc Health. 2022 (Nov 9). Doi: 10.1016/S2352-4642(22)00283-8
Key clinical point: An exposure to topical calcineurin inhibitors did not increase the risk for cancer in patients with atopic dermatitis (AD), with findings being similar among infants, children, and adults.
Major finding: Compared with no exposure, topical calcineurin inhibitor exposure was not associated with an increased risk for cancer (odds ratio [OR] 1.03; 95% credible interval [CrI] 0.94-1.11), with neither pimecrolimus (OR 1.05; 95% CrI 0.94-1.15) nor tacrolimus (OR 0.99; 95% CrI 0.89-1.09) use revealing any association with increased cancer risk, across all age groups.
Study details: This was a systematic review and meta-analysis of 110 unique studies (52 randomized controlled trials and 69 nonrandomized studies) including 3.4 million patients with AD followed-up for a mean of 11 months.
Disclosures: This study was funded by the American Academy of Allergy, Asthma, and Immunology and the American College of Allergy, Asthma, and Immunology. L Schneider declared receiving consulting fees and payments to her institutions from, serving on data safety monitoring and advisory boards for, and holding stock or stock options in various sources.
Source: Devasenapathy N et al for the AAAAI/ACAAI Joint Task Force on Practice Parameters for Atopic Dermatitis Guideline Development Group. Cancer risk with topical calcineurin inhibitors, pimecrolimus and tacrolimus, for atopic dermatitis: A systematic review and meta-analysis. Lancet Child Adolesc Health. 2022 (Nov 9). Doi: 10.1016/S2352-4642(22)00283-8
Key clinical point: An exposure to topical calcineurin inhibitors did not increase the risk for cancer in patients with atopic dermatitis (AD), with findings being similar among infants, children, and adults.
Major finding: Compared with no exposure, topical calcineurin inhibitor exposure was not associated with an increased risk for cancer (odds ratio [OR] 1.03; 95% credible interval [CrI] 0.94-1.11), with neither pimecrolimus (OR 1.05; 95% CrI 0.94-1.15) nor tacrolimus (OR 0.99; 95% CrI 0.89-1.09) use revealing any association with increased cancer risk, across all age groups.
Study details: This was a systematic review and meta-analysis of 110 unique studies (52 randomized controlled trials and 69 nonrandomized studies) including 3.4 million patients with AD followed-up for a mean of 11 months.
Disclosures: This study was funded by the American Academy of Allergy, Asthma, and Immunology and the American College of Allergy, Asthma, and Immunology. L Schneider declared receiving consulting fees and payments to her institutions from, serving on data safety monitoring and advisory boards for, and holding stock or stock options in various sources.
Source: Devasenapathy N et al for the AAAAI/ACAAI Joint Task Force on Practice Parameters for Atopic Dermatitis Guideline Development Group. Cancer risk with topical calcineurin inhibitors, pimecrolimus and tacrolimus, for atopic dermatitis: A systematic review and meta-analysis. Lancet Child Adolesc Health. 2022 (Nov 9). Doi: 10.1016/S2352-4642(22)00283-8