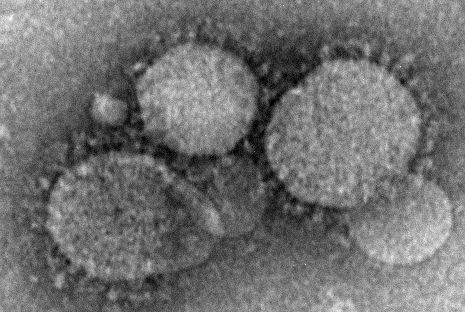User login
The Official Newspaper of the American Association for Thoracic Surgery
SAVR for radiation-induced aortic stenosis has high late mortality
ROME – Radiation-induced aortic stenosis is associated with markedly worse long-term outcome after surgical aortic valve replacement than when the operation is performed in patients without a history of radiotherapy, Milind Y. Desai, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, the Society of Thoracic Surgeons (STS) score isn’t good at risk-stratifying patients with radiation-induced aortic stenosis who are under consideration for surgical aortic valve replacement (SAVR).
Radiation-induced heart disease is a late complication of thoracic radiotherapy. It’s particularly common in patients who got radiation for lymphomas or breast cancer. It can affect any cardiac structure, including the myocardium, pericardium, valves, coronary arteries, and the conduction system.
Aortic stenosis is the most common valvular manifestation, present in roughly 80% of patients with radiation-induced heart disease. At the Cleveland Clinic, the average time from radiotherapy to development of radiation-induced aortic stenosis (RIAS) is about 20 years. The condition is characterized by thickening of the junction between the base of the anterior mitral leaflet and aortic root, known as the aortomitral curtain, Dr. Desai explained.
He presented a retrospective observational cohort study involving 172 patients who underwent SAVR for RIAS and an equal number of SAVR patients with no such history. The groups were matched by age, sex, aortic valve area, and type and timing of SAVR. Of note, the group with RIAS had a mean preoperative STS score of 11, and the control group averaged a similar score of 10.
The key finding: During a mean follow-up of 6 years, the all-cause mortality rate was a hefty 48% in patients with RIAS, compared with just 7% in matched controls. Only about 5% of deaths in the group with RIAS were from recurrent malignancy. The low figure is not surprising given the average 20-year lag between radiotherapy and development of radiation-induced heart disease.
“In our experience, most of these patients develop a recurrent pleural effusion and nasty cardiopulmonary issues that result in their death,” according to Dr. Desai.
In a multivariate Cox proportional hazards analysis, a history of chest radiation therapy was by far the strongest predictor of all-cause mortality, conferring an 8.5-fold increase in risk.
The only other statistically significant predictor of mortality during follow-up in multivariate analysis was a high STS score, with an associated weak albeit statistically significant 1.15-fold increased risk. A total of 30 of 78 (39%) RIAS patients with an STS score below 4 died during follow-up, compared with none of 91 controls.
Thirty-four of 92 (37%) RIAS patients under age 65 died during follow-up, whereas none of 83 control SAVR patients did so.
Having coronary artery bypass surgery or other cardiac surgery at the time of SAVR was not associated with significantly increased risk of mortality compared with solo SAVR.
In-hospital outcomes were consistently worse after SAVR in the RIAS group. Half of the RIAS patients experienced in-hospital atrial fibrillation and 29% developed persistent atrial fibrillation, compared with 30% and 24% of controls. About 22% of RIAS patients were readmitted within 3 months after surgery, as were only 8% of controls. In-hospital mortality occurred in 2% of SAVR patients with RIAS; none of the matched controls did.
Dr. Desai reported having no financial interests relative to this study.
ROME – Radiation-induced aortic stenosis is associated with markedly worse long-term outcome after surgical aortic valve replacement than when the operation is performed in patients without a history of radiotherapy, Milind Y. Desai, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, the Society of Thoracic Surgeons (STS) score isn’t good at risk-stratifying patients with radiation-induced aortic stenosis who are under consideration for surgical aortic valve replacement (SAVR).
Radiation-induced heart disease is a late complication of thoracic radiotherapy. It’s particularly common in patients who got radiation for lymphomas or breast cancer. It can affect any cardiac structure, including the myocardium, pericardium, valves, coronary arteries, and the conduction system.
Aortic stenosis is the most common valvular manifestation, present in roughly 80% of patients with radiation-induced heart disease. At the Cleveland Clinic, the average time from radiotherapy to development of radiation-induced aortic stenosis (RIAS) is about 20 years. The condition is characterized by thickening of the junction between the base of the anterior mitral leaflet and aortic root, known as the aortomitral curtain, Dr. Desai explained.
He presented a retrospective observational cohort study involving 172 patients who underwent SAVR for RIAS and an equal number of SAVR patients with no such history. The groups were matched by age, sex, aortic valve area, and type and timing of SAVR. Of note, the group with RIAS had a mean preoperative STS score of 11, and the control group averaged a similar score of 10.
The key finding: During a mean follow-up of 6 years, the all-cause mortality rate was a hefty 48% in patients with RIAS, compared with just 7% in matched controls. Only about 5% of deaths in the group with RIAS were from recurrent malignancy. The low figure is not surprising given the average 20-year lag between radiotherapy and development of radiation-induced heart disease.
“In our experience, most of these patients develop a recurrent pleural effusion and nasty cardiopulmonary issues that result in their death,” according to Dr. Desai.
In a multivariate Cox proportional hazards analysis, a history of chest radiation therapy was by far the strongest predictor of all-cause mortality, conferring an 8.5-fold increase in risk.
The only other statistically significant predictor of mortality during follow-up in multivariate analysis was a high STS score, with an associated weak albeit statistically significant 1.15-fold increased risk. A total of 30 of 78 (39%) RIAS patients with an STS score below 4 died during follow-up, compared with none of 91 controls.
Thirty-four of 92 (37%) RIAS patients under age 65 died during follow-up, whereas none of 83 control SAVR patients did so.
Having coronary artery bypass surgery or other cardiac surgery at the time of SAVR was not associated with significantly increased risk of mortality compared with solo SAVR.
In-hospital outcomes were consistently worse after SAVR in the RIAS group. Half of the RIAS patients experienced in-hospital atrial fibrillation and 29% developed persistent atrial fibrillation, compared with 30% and 24% of controls. About 22% of RIAS patients were readmitted within 3 months after surgery, as were only 8% of controls. In-hospital mortality occurred in 2% of SAVR patients with RIAS; none of the matched controls did.
Dr. Desai reported having no financial interests relative to this study.
ROME – Radiation-induced aortic stenosis is associated with markedly worse long-term outcome after surgical aortic valve replacement than when the operation is performed in patients without a history of radiotherapy, Milind Y. Desai, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, the Society of Thoracic Surgeons (STS) score isn’t good at risk-stratifying patients with radiation-induced aortic stenosis who are under consideration for surgical aortic valve replacement (SAVR).
Radiation-induced heart disease is a late complication of thoracic radiotherapy. It’s particularly common in patients who got radiation for lymphomas or breast cancer. It can affect any cardiac structure, including the myocardium, pericardium, valves, coronary arteries, and the conduction system.
Aortic stenosis is the most common valvular manifestation, present in roughly 80% of patients with radiation-induced heart disease. At the Cleveland Clinic, the average time from radiotherapy to development of radiation-induced aortic stenosis (RIAS) is about 20 years. The condition is characterized by thickening of the junction between the base of the anterior mitral leaflet and aortic root, known as the aortomitral curtain, Dr. Desai explained.
He presented a retrospective observational cohort study involving 172 patients who underwent SAVR for RIAS and an equal number of SAVR patients with no such history. The groups were matched by age, sex, aortic valve area, and type and timing of SAVR. Of note, the group with RIAS had a mean preoperative STS score of 11, and the control group averaged a similar score of 10.
The key finding: During a mean follow-up of 6 years, the all-cause mortality rate was a hefty 48% in patients with RIAS, compared with just 7% in matched controls. Only about 5% of deaths in the group with RIAS were from recurrent malignancy. The low figure is not surprising given the average 20-year lag between radiotherapy and development of radiation-induced heart disease.
“In our experience, most of these patients develop a recurrent pleural effusion and nasty cardiopulmonary issues that result in their death,” according to Dr. Desai.
In a multivariate Cox proportional hazards analysis, a history of chest radiation therapy was by far the strongest predictor of all-cause mortality, conferring an 8.5-fold increase in risk.
The only other statistically significant predictor of mortality during follow-up in multivariate analysis was a high STS score, with an associated weak albeit statistically significant 1.15-fold increased risk. A total of 30 of 78 (39%) RIAS patients with an STS score below 4 died during follow-up, compared with none of 91 controls.
Thirty-four of 92 (37%) RIAS patients under age 65 died during follow-up, whereas none of 83 control SAVR patients did so.
Having coronary artery bypass surgery or other cardiac surgery at the time of SAVR was not associated with significantly increased risk of mortality compared with solo SAVR.
In-hospital outcomes were consistently worse after SAVR in the RIAS group. Half of the RIAS patients experienced in-hospital atrial fibrillation and 29% developed persistent atrial fibrillation, compared with 30% and 24% of controls. About 22% of RIAS patients were readmitted within 3 months after surgery, as were only 8% of controls. In-hospital mortality occurred in 2% of SAVR patients with RIAS; none of the matched controls did.
Dr. Desai reported having no financial interests relative to this study.
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: All-cause mortality occurred in 48% of 172 patients with radiation-induced severe aortic stenosis during a mean follow-up of 6 years after surgical aortic valve replacement, compared with just 7% of matched controls.
Data source: This was a retrospective observational study involving 172 closely matched pairs of surgical aortic valve replacement patients.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study.
Beta-blockers curb death risk in patients with primary prevention ICD
ROME – Beta-blocker therapy reduces the risks of all-cause mortality as well as cardiac death in patients with a left ventricular ejection fraction below 35% who get an implantable cardioverter-defibrillator for primary prevention, Laurent Fauchier, MD, PhD, reported at the annual congress of the European Society of Cardiology.
Some physicians have recently urged reconsideration of current guidelines recommending routine use of beta-blockers for prevention of cardiovascular events in certain groups of patients with coronary artery disease, including those with chronic heart failure who have received an ICD for primary prevention of sudden death. And indeed it’s true that the now–relatively old randomized trials of ICDs for primary prevention in patients with chronic heart failure don’t provide any real evidence that beta-blockers reduce mortality in this setting. In fact, the guideline recommendation for beta-blockade has been based upon expert opinion. This was the impetus for Dr. Fauchier and coinvestigators to conduct a large retrospective observational study in a contemporary cohort of heart failure patients who received an ICD for primary prevention during a recent 10-year period at the 12 largest centers in France.
Fifteen percent of the 3,975 French ICD recipients did not receive a beta-blocker. They differed from those who did in that they were on average 2 years older, had an absolute 5% lower ejection fraction, and were more likely to also receive cardiac resynchronization therapy. Propensity score matching based on these and 19 other baseline characteristics enabled investigators to assemble a cohort of 541 closely matched patient pairs, explained Dr. Fauchier, professor of cardiology at Francois Rabelais University in Tours, France.
During a mean follow-up of 3.2 years, the risk of all-cause mortality in ICD recipients not on a beta-blocker was 34% higher than in those who were. Moreover, their risk of cardiac death was 50% greater.
In contrast, beta-blocker therapy had no effect on the risks of sudden death or of appropriate or inappropriate shocks.
The finding that beta-blocker therapy doesn’t prevent sudden death in patients with an ICD for primary prevention has not previously been reported. However, it makes sense. The device prevents such events so effectively that a beta-blocker adds nothing further in that regard, according to Dr. Fauchier.
“Beta-blockers should continue to be used widely, as currently recommended, for heart failure in the specific setting of patients with prophylactic ICD implantation. You do not have the benefit for prevention of sudden death, but you still have all the benefit from preventing cardiac death,” the electrophysiologist concluded.
This study was supported by French governmental research grants. Dr. Fauchier reported serving as a consultant to Bayer, Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
ROME – Beta-blocker therapy reduces the risks of all-cause mortality as well as cardiac death in patients with a left ventricular ejection fraction below 35% who get an implantable cardioverter-defibrillator for primary prevention, Laurent Fauchier, MD, PhD, reported at the annual congress of the European Society of Cardiology.
Some physicians have recently urged reconsideration of current guidelines recommending routine use of beta-blockers for prevention of cardiovascular events in certain groups of patients with coronary artery disease, including those with chronic heart failure who have received an ICD for primary prevention of sudden death. And indeed it’s true that the now–relatively old randomized trials of ICDs for primary prevention in patients with chronic heart failure don’t provide any real evidence that beta-blockers reduce mortality in this setting. In fact, the guideline recommendation for beta-blockade has been based upon expert opinion. This was the impetus for Dr. Fauchier and coinvestigators to conduct a large retrospective observational study in a contemporary cohort of heart failure patients who received an ICD for primary prevention during a recent 10-year period at the 12 largest centers in France.
Fifteen percent of the 3,975 French ICD recipients did not receive a beta-blocker. They differed from those who did in that they were on average 2 years older, had an absolute 5% lower ejection fraction, and were more likely to also receive cardiac resynchronization therapy. Propensity score matching based on these and 19 other baseline characteristics enabled investigators to assemble a cohort of 541 closely matched patient pairs, explained Dr. Fauchier, professor of cardiology at Francois Rabelais University in Tours, France.
During a mean follow-up of 3.2 years, the risk of all-cause mortality in ICD recipients not on a beta-blocker was 34% higher than in those who were. Moreover, their risk of cardiac death was 50% greater.
In contrast, beta-blocker therapy had no effect on the risks of sudden death or of appropriate or inappropriate shocks.
The finding that beta-blocker therapy doesn’t prevent sudden death in patients with an ICD for primary prevention has not previously been reported. However, it makes sense. The device prevents such events so effectively that a beta-blocker adds nothing further in that regard, according to Dr. Fauchier.
“Beta-blockers should continue to be used widely, as currently recommended, for heart failure in the specific setting of patients with prophylactic ICD implantation. You do not have the benefit for prevention of sudden death, but you still have all the benefit from preventing cardiac death,” the electrophysiologist concluded.
This study was supported by French governmental research grants. Dr. Fauchier reported serving as a consultant to Bayer, Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
ROME – Beta-blocker therapy reduces the risks of all-cause mortality as well as cardiac death in patients with a left ventricular ejection fraction below 35% who get an implantable cardioverter-defibrillator for primary prevention, Laurent Fauchier, MD, PhD, reported at the annual congress of the European Society of Cardiology.
Some physicians have recently urged reconsideration of current guidelines recommending routine use of beta-blockers for prevention of cardiovascular events in certain groups of patients with coronary artery disease, including those with chronic heart failure who have received an ICD for primary prevention of sudden death. And indeed it’s true that the now–relatively old randomized trials of ICDs for primary prevention in patients with chronic heart failure don’t provide any real evidence that beta-blockers reduce mortality in this setting. In fact, the guideline recommendation for beta-blockade has been based upon expert opinion. This was the impetus for Dr. Fauchier and coinvestigators to conduct a large retrospective observational study in a contemporary cohort of heart failure patients who received an ICD for primary prevention during a recent 10-year period at the 12 largest centers in France.
Fifteen percent of the 3,975 French ICD recipients did not receive a beta-blocker. They differed from those who did in that they were on average 2 years older, had an absolute 5% lower ejection fraction, and were more likely to also receive cardiac resynchronization therapy. Propensity score matching based on these and 19 other baseline characteristics enabled investigators to assemble a cohort of 541 closely matched patient pairs, explained Dr. Fauchier, professor of cardiology at Francois Rabelais University in Tours, France.
During a mean follow-up of 3.2 years, the risk of all-cause mortality in ICD recipients not on a beta-blocker was 34% higher than in those who were. Moreover, their risk of cardiac death was 50% greater.
In contrast, beta-blocker therapy had no effect on the risks of sudden death or of appropriate or inappropriate shocks.
The finding that beta-blocker therapy doesn’t prevent sudden death in patients with an ICD for primary prevention has not previously been reported. However, it makes sense. The device prevents such events so effectively that a beta-blocker adds nothing further in that regard, according to Dr. Fauchier.
“Beta-blockers should continue to be used widely, as currently recommended, for heart failure in the specific setting of patients with prophylactic ICD implantation. You do not have the benefit for prevention of sudden death, but you still have all the benefit from preventing cardiac death,” the electrophysiologist concluded.
This study was supported by French governmental research grants. Dr. Fauchier reported serving as a consultant to Bayer, Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: Patients with heart failure with reduced ejection fraction who received an ICD for primary prevention and were not on a beta-blocker were at an adjusted 50% increased risk for cardiac death and 34% increased risk for all-cause mortality during 3.2 years of follow-up, but they were at no increased risk for sudden death.
Data source: A retrospective observational study of all of the nearly 4,000 patients who received a primary prevention ICD at the 12 largest French centers during a recent 10-year period.
Disclosures: This study was supported by French governmental research funds. The presenter reported serving as a consultant to Bayer, Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
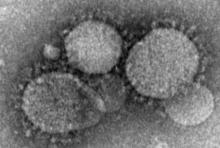
Health care workers at risk for mild MERS-CoV infections
Health care workers directly caring for patients with Middle East respiratory syndrome coronavirus (MERS-CoV) are more highly predisposed to contracting the virus, but in a milder form than that of their patients, thus making it difficult to diagnose and treat.
In a study published in Emerging Infectious Diseases, health care professionals (HCP) from the King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia, were examined to determine their likelihood for getting MERS-CoV based on their proximity to patients who already had it.
“Healthcare settings are important amplifiers of transmission,” explained the investigators, led by Basem M. Alraddadi, MD. “Current MERS-CoV infection control recommendations are based on experience with other viruses rather than on a complete understanding of the epidemiology of MERS-CoV transmission.”
Dr. Alraddadi and his coinvestigators identified 363 HCP, all of whom would be placed into one of three cohorts based on the department in which they worked most extensively: the Medical Intensive Care Unit (MICU), the emergency department (ED), and the neurology unit. A total of 292 HCP were ultimately enrolled in the study: 131 in MICU, 127 in ED, and 34 in neurology. After 9 subjects were excluded because of unavailability of serum specimens, 128 MICU, 122 ED, and 33 neurology unit workers remained.
While none of the neurology unit workers contracted the virus, 15 MICU workers (11.7%) and 5 ED workers (4.1%) did, for a total of 20 out of the 250 subjects in those two cohorts (8%). Radiology technicians were the most susceptible, as 5 of 17 (29.4%) got the virus, followed by 13 of 138 nurses (9.4%), 1 of 31 respiratory therapists (3.2%), and 1 of 41 physicians (2.4%).
“HCP who reported always covering their nose and mouth with either a medical mask or N95 respirator had lower risk for infection than did HCP reporting not always or never doing so, [while] those who reported always using N95 respirators for direct patient contact were less likely to be seropositive, a trend that approached statistical significance (P = .07),” the authors noted.
The most frequent symptoms reported by those surveyed were muscle pain, fevers, headaches, dry cough, and shortness of breath. In the 20-case HCP sample, however, 12 subjects (60%) only had mild illness while 3 (15%) were asymptomatic, making it very hard to diagnose and treat their infection. Three subjects (15%) had severe illness, while another two (10%) had moderate illness, meaning they were admitted to hospital but did not require any mechanical ventilation.
“Our study did not identify strong associations with underlying chronic illnesses, most likely because the prevalence of such conditions was low ([less than] 10%) in this population, [but] HCPs with a history of smoking had a risk for infection almost 3 times that of nonsmokers,” the authors wrote (Emerg Infect Dis. 2016 Nov. doi: 10.3201/eid2211.160920).
The Ministry of Health of Saudi Arabia and the Centers for Disease Control and Prevention funded the study. Dr. Alraddadi and his coauthors did not report any disclosures.
Health care workers directly caring for patients with Middle East respiratory syndrome coronavirus (MERS-CoV) are more highly predisposed to contracting the virus, but in a milder form than that of their patients, thus making it difficult to diagnose and treat.
In a study published in Emerging Infectious Diseases, health care professionals (HCP) from the King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia, were examined to determine their likelihood for getting MERS-CoV based on their proximity to patients who already had it.
“Healthcare settings are important amplifiers of transmission,” explained the investigators, led by Basem M. Alraddadi, MD. “Current MERS-CoV infection control recommendations are based on experience with other viruses rather than on a complete understanding of the epidemiology of MERS-CoV transmission.”
Dr. Alraddadi and his coinvestigators identified 363 HCP, all of whom would be placed into one of three cohorts based on the department in which they worked most extensively: the Medical Intensive Care Unit (MICU), the emergency department (ED), and the neurology unit. A total of 292 HCP were ultimately enrolled in the study: 131 in MICU, 127 in ED, and 34 in neurology. After 9 subjects were excluded because of unavailability of serum specimens, 128 MICU, 122 ED, and 33 neurology unit workers remained.
While none of the neurology unit workers contracted the virus, 15 MICU workers (11.7%) and 5 ED workers (4.1%) did, for a total of 20 out of the 250 subjects in those two cohorts (8%). Radiology technicians were the most susceptible, as 5 of 17 (29.4%) got the virus, followed by 13 of 138 nurses (9.4%), 1 of 31 respiratory therapists (3.2%), and 1 of 41 physicians (2.4%).
“HCP who reported always covering their nose and mouth with either a medical mask or N95 respirator had lower risk for infection than did HCP reporting not always or never doing so, [while] those who reported always using N95 respirators for direct patient contact were less likely to be seropositive, a trend that approached statistical significance (P = .07),” the authors noted.
The most frequent symptoms reported by those surveyed were muscle pain, fevers, headaches, dry cough, and shortness of breath. In the 20-case HCP sample, however, 12 subjects (60%) only had mild illness while 3 (15%) were asymptomatic, making it very hard to diagnose and treat their infection. Three subjects (15%) had severe illness, while another two (10%) had moderate illness, meaning they were admitted to hospital but did not require any mechanical ventilation.
“Our study did not identify strong associations with underlying chronic illnesses, most likely because the prevalence of such conditions was low ([less than] 10%) in this population, [but] HCPs with a history of smoking had a risk for infection almost 3 times that of nonsmokers,” the authors wrote (Emerg Infect Dis. 2016 Nov. doi: 10.3201/eid2211.160920).
The Ministry of Health of Saudi Arabia and the Centers for Disease Control and Prevention funded the study. Dr. Alraddadi and his coauthors did not report any disclosures.
Health care workers directly caring for patients with Middle East respiratory syndrome coronavirus (MERS-CoV) are more highly predisposed to contracting the virus, but in a milder form than that of their patients, thus making it difficult to diagnose and treat.
In a study published in Emerging Infectious Diseases, health care professionals (HCP) from the King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia, were examined to determine their likelihood for getting MERS-CoV based on their proximity to patients who already had it.
“Healthcare settings are important amplifiers of transmission,” explained the investigators, led by Basem M. Alraddadi, MD. “Current MERS-CoV infection control recommendations are based on experience with other viruses rather than on a complete understanding of the epidemiology of MERS-CoV transmission.”
Dr. Alraddadi and his coinvestigators identified 363 HCP, all of whom would be placed into one of three cohorts based on the department in which they worked most extensively: the Medical Intensive Care Unit (MICU), the emergency department (ED), and the neurology unit. A total of 292 HCP were ultimately enrolled in the study: 131 in MICU, 127 in ED, and 34 in neurology. After 9 subjects were excluded because of unavailability of serum specimens, 128 MICU, 122 ED, and 33 neurology unit workers remained.
While none of the neurology unit workers contracted the virus, 15 MICU workers (11.7%) and 5 ED workers (4.1%) did, for a total of 20 out of the 250 subjects in those two cohorts (8%). Radiology technicians were the most susceptible, as 5 of 17 (29.4%) got the virus, followed by 13 of 138 nurses (9.4%), 1 of 31 respiratory therapists (3.2%), and 1 of 41 physicians (2.4%).
“HCP who reported always covering their nose and mouth with either a medical mask or N95 respirator had lower risk for infection than did HCP reporting not always or never doing so, [while] those who reported always using N95 respirators for direct patient contact were less likely to be seropositive, a trend that approached statistical significance (P = .07),” the authors noted.
The most frequent symptoms reported by those surveyed were muscle pain, fevers, headaches, dry cough, and shortness of breath. In the 20-case HCP sample, however, 12 subjects (60%) only had mild illness while 3 (15%) were asymptomatic, making it very hard to diagnose and treat their infection. Three subjects (15%) had severe illness, while another two (10%) had moderate illness, meaning they were admitted to hospital but did not require any mechanical ventilation.
“Our study did not identify strong associations with underlying chronic illnesses, most likely because the prevalence of such conditions was low ([less than] 10%) in this population, [but] HCPs with a history of smoking had a risk for infection almost 3 times that of nonsmokers,” the authors wrote (Emerg Infect Dis. 2016 Nov. doi: 10.3201/eid2211.160920).
The Ministry of Health of Saudi Arabia and the Centers for Disease Control and Prevention funded the study. Dr. Alraddadi and his coauthors did not report any disclosures.
Key clinical point:
Major finding: Among workers who actually treated MERS-CoV patients, 20 out of 250 (8%) contracted the virus, while none of the clerical staff or patient transporters did.
Data source: Retrospective, single-center study of 363 health care personnel during May-June 2014.
Disclosures: The Ministry of Health of Saudi Arabia and the Centers for Disease Control and Prevention funded the study. The authors reported no financial disclosures.
Pembrolizumab ‘new standard of care’ in advanced PD-L1-rich NSCLC
COPENHAGEN – Chalk up another one for immunotherapy: the PD-1 checkpoint inhibitor pembrolizumab cut the risk of disease progression or death in half among select patients with non–small cell lung cancer (NSCLC), compared with standard platinum doublet chemotherapy, in the first-line setting.
Among 305 patients with non–small cell lung cancers with 50% or greater expression of the programmed death ligand 1 (PD-L1), median progression-free survival (PFS) for patients treated with pembrolizumab (Keytruda) was 10.3 months, compared with 6 months for patients assigned to receive platinum-based chemotherapy at the investigators discretion (hazard ratio, 0.50, P less than .001), reported Martin Reck, MD, from the department of thoracic oncology at the Lung Clinic Grosshansdorf, in Germany.
Results of the KEYNOTE-024 study were also published online in the New England Journal of Medicine (2016 Oct 9. doi: 10.1056/NEJMoa1606774). Approximately 23%-30% of patients with advanced non–small cell lung cancers have tumors that express PD-L1 on the membrane of at least 50% of tumor cells, making them attractive targets for pembrolizumab, which is a monoclonal antibody directed against programmed death 1 (PD-1). Pembrolizumab disengages the brake on the immune system caused by the interaction of receptor PD-1 with the PD-L1 and PD-L2 ligands.
The study was conducted to compare upfront pembrolizumab with platinum-based chemotherapy in patients with newly diagnosed advanced NSCLC that did not carry targetable EGFR-activating mutations or ALK translocations.
A total of 305 patients from 16 countries with untreated stage IV NSCLC, good performance status, and tumors with a 50% or greater expression of PD-L1 were enrolled and randomized to either pembrolizumab 200 mg intravenously every 3 weeks for up to 2 years. Or four to six cycles of platinum-doublet chemotherapy at the investigator’s discretion. The combinations included carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel.
At a median follow-up of 11.2 months, 48.1% of patients assigned to pembrolizumab were still on treatment, as were 10% of those assigned to standard chemotherapy.
As noted before, PFS, the primary endpoint, was significantly better with pembrolizumab, as was the secondary endpoint of overall survival at 6 months. In all, 80% of patients treated with pembrolizumab were still alive at 6 months, compared with 72% of patients on chemotherapy (HR, 0.60; P = .005).
The confirmed response rate was also higher in the pembrolizumab arm, at 44.8% vs. 27.8%(P = .0011), and the median duration of response was longer (not reached vs. 6.3 months). There were six complete responses in the pembrolizumab arm.
Pembrolizumab also demonstrated a generally more favorable safety profile, with adverse events of any grade occurring in 73.4% of patients, compared with 90% of those treated with chemotherapy.
Grade 3 or 4 adverse events and treatment-related deaths were also lower in the pembrolizumab arm, at 26.6% vs. 53.3%.
Jean-Charles Soria, MD, chair of drug development at Gustave Roussy Cancer Center in Paris, the invited discussant, noted that the “45% objective response rate in first-line non–small cell lung cancer is unheard of, and is achieved with a monotherapy.”
“Pembrolizumab clearly leads to a higher objective response, a longer duration of response, a lower frequency of adverse events, better PFS, better OS, compared to chemotherapy.”
“We have, probably, a new standard of care” for patients with high PD-L1 expression and no targetable mutations,” he said.
COPENHAGEN – Chalk up another one for immunotherapy: the PD-1 checkpoint inhibitor pembrolizumab cut the risk of disease progression or death in half among select patients with non–small cell lung cancer (NSCLC), compared with standard platinum doublet chemotherapy, in the first-line setting.
Among 305 patients with non–small cell lung cancers with 50% or greater expression of the programmed death ligand 1 (PD-L1), median progression-free survival (PFS) for patients treated with pembrolizumab (Keytruda) was 10.3 months, compared with 6 months for patients assigned to receive platinum-based chemotherapy at the investigators discretion (hazard ratio, 0.50, P less than .001), reported Martin Reck, MD, from the department of thoracic oncology at the Lung Clinic Grosshansdorf, in Germany.
Results of the KEYNOTE-024 study were also published online in the New England Journal of Medicine (2016 Oct 9. doi: 10.1056/NEJMoa1606774). Approximately 23%-30% of patients with advanced non–small cell lung cancers have tumors that express PD-L1 on the membrane of at least 50% of tumor cells, making them attractive targets for pembrolizumab, which is a monoclonal antibody directed against programmed death 1 (PD-1). Pembrolizumab disengages the brake on the immune system caused by the interaction of receptor PD-1 with the PD-L1 and PD-L2 ligands.
The study was conducted to compare upfront pembrolizumab with platinum-based chemotherapy in patients with newly diagnosed advanced NSCLC that did not carry targetable EGFR-activating mutations or ALK translocations.
A total of 305 patients from 16 countries with untreated stage IV NSCLC, good performance status, and tumors with a 50% or greater expression of PD-L1 were enrolled and randomized to either pembrolizumab 200 mg intravenously every 3 weeks for up to 2 years. Or four to six cycles of platinum-doublet chemotherapy at the investigator’s discretion. The combinations included carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel.
At a median follow-up of 11.2 months, 48.1% of patients assigned to pembrolizumab were still on treatment, as were 10% of those assigned to standard chemotherapy.
As noted before, PFS, the primary endpoint, was significantly better with pembrolizumab, as was the secondary endpoint of overall survival at 6 months. In all, 80% of patients treated with pembrolizumab were still alive at 6 months, compared with 72% of patients on chemotherapy (HR, 0.60; P = .005).
The confirmed response rate was also higher in the pembrolizumab arm, at 44.8% vs. 27.8%(P = .0011), and the median duration of response was longer (not reached vs. 6.3 months). There were six complete responses in the pembrolizumab arm.
Pembrolizumab also demonstrated a generally more favorable safety profile, with adverse events of any grade occurring in 73.4% of patients, compared with 90% of those treated with chemotherapy.
Grade 3 or 4 adverse events and treatment-related deaths were also lower in the pembrolizumab arm, at 26.6% vs. 53.3%.
Jean-Charles Soria, MD, chair of drug development at Gustave Roussy Cancer Center in Paris, the invited discussant, noted that the “45% objective response rate in first-line non–small cell lung cancer is unheard of, and is achieved with a monotherapy.”
“Pembrolizumab clearly leads to a higher objective response, a longer duration of response, a lower frequency of adverse events, better PFS, better OS, compared to chemotherapy.”
“We have, probably, a new standard of care” for patients with high PD-L1 expression and no targetable mutations,” he said.
COPENHAGEN – Chalk up another one for immunotherapy: the PD-1 checkpoint inhibitor pembrolizumab cut the risk of disease progression or death in half among select patients with non–small cell lung cancer (NSCLC), compared with standard platinum doublet chemotherapy, in the first-line setting.
Among 305 patients with non–small cell lung cancers with 50% or greater expression of the programmed death ligand 1 (PD-L1), median progression-free survival (PFS) for patients treated with pembrolizumab (Keytruda) was 10.3 months, compared with 6 months for patients assigned to receive platinum-based chemotherapy at the investigators discretion (hazard ratio, 0.50, P less than .001), reported Martin Reck, MD, from the department of thoracic oncology at the Lung Clinic Grosshansdorf, in Germany.
Results of the KEYNOTE-024 study were also published online in the New England Journal of Medicine (2016 Oct 9. doi: 10.1056/NEJMoa1606774). Approximately 23%-30% of patients with advanced non–small cell lung cancers have tumors that express PD-L1 on the membrane of at least 50% of tumor cells, making them attractive targets for pembrolizumab, which is a monoclonal antibody directed against programmed death 1 (PD-1). Pembrolizumab disengages the brake on the immune system caused by the interaction of receptor PD-1 with the PD-L1 and PD-L2 ligands.
The study was conducted to compare upfront pembrolizumab with platinum-based chemotherapy in patients with newly diagnosed advanced NSCLC that did not carry targetable EGFR-activating mutations or ALK translocations.
A total of 305 patients from 16 countries with untreated stage IV NSCLC, good performance status, and tumors with a 50% or greater expression of PD-L1 were enrolled and randomized to either pembrolizumab 200 mg intravenously every 3 weeks for up to 2 years. Or four to six cycles of platinum-doublet chemotherapy at the investigator’s discretion. The combinations included carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel.
At a median follow-up of 11.2 months, 48.1% of patients assigned to pembrolizumab were still on treatment, as were 10% of those assigned to standard chemotherapy.
As noted before, PFS, the primary endpoint, was significantly better with pembrolizumab, as was the secondary endpoint of overall survival at 6 months. In all, 80% of patients treated with pembrolizumab were still alive at 6 months, compared with 72% of patients on chemotherapy (HR, 0.60; P = .005).
The confirmed response rate was also higher in the pembrolizumab arm, at 44.8% vs. 27.8%(P = .0011), and the median duration of response was longer (not reached vs. 6.3 months). There were six complete responses in the pembrolizumab arm.
Pembrolizumab also demonstrated a generally more favorable safety profile, with adverse events of any grade occurring in 73.4% of patients, compared with 90% of those treated with chemotherapy.
Grade 3 or 4 adverse events and treatment-related deaths were also lower in the pembrolizumab arm, at 26.6% vs. 53.3%.
Jean-Charles Soria, MD, chair of drug development at Gustave Roussy Cancer Center in Paris, the invited discussant, noted that the “45% objective response rate in first-line non–small cell lung cancer is unheard of, and is achieved with a monotherapy.”
“Pembrolizumab clearly leads to a higher objective response, a longer duration of response, a lower frequency of adverse events, better PFS, better OS, compared to chemotherapy.”
“We have, probably, a new standard of care” for patients with high PD-L1 expression and no targetable mutations,” he said.
AT ESMO 2016
Key clinical point: Pembrolizumab was superior to chemotherapy in stage IV NSCLC with PD-L1 expression of 50% or more.
Major finding: The hazard ratio for progression-free survival was 0.50 for pembrolizumab vs. platinum-based chemotherapy.
Data source: Randomized phase III trial in 305 patients with untreated stage IV NSCLC with 50% or more of tumor cells expressing PD-L1
Disclosures: The study was sponsored by Merck, Sharp & Dohme. Dr. Reck and Dr. Soria disclosed financial relationships (consulting/honoraria, research funding, etc.) with several companies, but not Merck.
MACRA final rule exempts many more doctors
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
More restrictive hemoglobin threshold recommended for transfusion
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
FROM JAMA
Key clinical point: A restrictive threshold for red blood cell transfusion, in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL, is now recommended for most patients.
Major finding: A more restrictive threshold for red blood cell transfusion is not associated with an increased risk of mortality or other adverse outcomes from transfusion.
Data source: Updated guidelines from the AABB (formerly known as the American Association of Blood Banks).
Disclosures: Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies including CSL and Fresenius Kabi, but no other conflicts of interest were declared.
Patients with stage 1 NSCLC more likely to die of other causes in short term
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients with resected stage 1 non-small cell lung cancer are more likely to die of other causes in the short term. Major finding: The non–cancer-specific cumulative incidence of death was higher than CID from lung cancer for up to 1.5 years after resection.
Data source: A single-center competing risk analysis of 2,186 patients who underwent curative-intent resection for stage 1 non–small cell lung cancer.
Disclosures: Senior author Prasad Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
Lung cryobiopsies could reduce need for surgical biopsy
LONDON – The vast majority of surgical lung biopsies currently used to diagnose interstitial lung diseases (ILDs) could be avoided, suggests research presented at the annual congress of the European Respiratory Society.
During an oral presentation, Benjamin Bondue, MD, of Hopital Erasme, Brussels, presented the preliminary results of a Belgian prospective study evaluating the role of transbronchial lung cryobiopsies in 24 patients with undefined ILD treated at three participating centers.
Cryobiopsies were found to have a diagnostic yield of 79%, meaning that patients might be able to avoid undergoing a more invasive surgical removal of tissue in many cases. Compared with surgical biopsy, cryobiopsies offered the potential advantage of lower morbidity and shorter hospitalization time, Dr. Bondue said. He reported that patients needed to stay in hospital just 1.2 days after the procedure in the study.
“Our data also show that there is some benefit of surgical lung biopsy after cryobiopsy if we identify an NSIP [nonspecific interstitial pneumonia] pattern or idiopathic conditions, or if we cannot obtain a clear pathological diagnosis,” he reported. Acknowledging the study was small and conducted in a single center, he said the use of cryobiopsies following surgical biopsy might be worth further study.
Transbronchial lung cryobiopsy is a relatively new technique that uses a cryoprobe inserted down through a bronchoscope about 1-2 cm from the thoracic wall. Once in place, the probe is cooled for between 3 and 6 seconds, lung tissue freezes to the probe, and the probe and bronchoscope are removed together. This method allows for larger samples of tissue to be taken than does traditional transbronchial biopsy, which involves using large forceps to obtain tissue samples (Respirology. 2014;19:645-54).
In the Belgian study, Dr. Bondue noted that a Fogarty balloon was used to control any bleeding and that four transbronchial lung cryobiopsies were obtained from two different segments of the same lobe of a patient’s lungs. All biopsies were then analyzed by an expert pathologist in ILDs, and reviewed by two other expert pathologists when needed. The mean sample size obtained was 16 mm2.
The patients included in the study had undergone chest X-ray and had inconclusive findings in the majority (84%) of cases. They then had the option to undergo cryobiopsy or surgical lung biopsy, with the latter performed following discussion among a multidisciplinary team’s members.
Following cryobiopsy, 16 of the 24 patients – who were a mean age of 62 years, and over half of whom were past (56%) or current (12%) smokers – were diagnosed with a specific pattern of ILD not due to NSIP. Of the 16 cases, 6 were due to hypersensitive pneumonitis, 4 were due to interstitial pulmonary fibrosis, and 2 were due to sarcoidosis. The other four cases included patients with one of the following conditions: adenocarcinoma, desquamative interstitial pneumonia, eosinophilic pneumonia, and amyloidosis.
Six of the 24 cases were defined as NSIP, with 2 reclassified as definite and 1 as probable hypersensitive pneumonitis, after discussion within the multidisciplinary team.
Five patients – three who had been diagnosed with NSIP and two who had been given no pathological diagnosis after cryobiopsy – underwent surgical lung biopsy. Of these, following the surgical biopsies, only one patient was considered to have NSIP and the other four were eventually diagnosed with interstitial pulmonary fibrosis.
In terms of safety, five patients experienced pneumothorax, two patients required chest drainage, two needed simple aspiration and one underwent observation. In the majority of cases, patients experienced mild bleeding, with only one patient having experienced severe bleeding. During this study, none of the participants experienced significant chest pain, acute exacerbations, or infections, and none of them died.
Dr. Bondue has received research grants and fees for consulting from Boehringer Ingelheim and Roche.
LONDON – The vast majority of surgical lung biopsies currently used to diagnose interstitial lung diseases (ILDs) could be avoided, suggests research presented at the annual congress of the European Respiratory Society.
During an oral presentation, Benjamin Bondue, MD, of Hopital Erasme, Brussels, presented the preliminary results of a Belgian prospective study evaluating the role of transbronchial lung cryobiopsies in 24 patients with undefined ILD treated at three participating centers.
Cryobiopsies were found to have a diagnostic yield of 79%, meaning that patients might be able to avoid undergoing a more invasive surgical removal of tissue in many cases. Compared with surgical biopsy, cryobiopsies offered the potential advantage of lower morbidity and shorter hospitalization time, Dr. Bondue said. He reported that patients needed to stay in hospital just 1.2 days after the procedure in the study.
“Our data also show that there is some benefit of surgical lung biopsy after cryobiopsy if we identify an NSIP [nonspecific interstitial pneumonia] pattern or idiopathic conditions, or if we cannot obtain a clear pathological diagnosis,” he reported. Acknowledging the study was small and conducted in a single center, he said the use of cryobiopsies following surgical biopsy might be worth further study.
Transbronchial lung cryobiopsy is a relatively new technique that uses a cryoprobe inserted down through a bronchoscope about 1-2 cm from the thoracic wall. Once in place, the probe is cooled for between 3 and 6 seconds, lung tissue freezes to the probe, and the probe and bronchoscope are removed together. This method allows for larger samples of tissue to be taken than does traditional transbronchial biopsy, which involves using large forceps to obtain tissue samples (Respirology. 2014;19:645-54).
In the Belgian study, Dr. Bondue noted that a Fogarty balloon was used to control any bleeding and that four transbronchial lung cryobiopsies were obtained from two different segments of the same lobe of a patient’s lungs. All biopsies were then analyzed by an expert pathologist in ILDs, and reviewed by two other expert pathologists when needed. The mean sample size obtained was 16 mm2.
The patients included in the study had undergone chest X-ray and had inconclusive findings in the majority (84%) of cases. They then had the option to undergo cryobiopsy or surgical lung biopsy, with the latter performed following discussion among a multidisciplinary team’s members.
Following cryobiopsy, 16 of the 24 patients – who were a mean age of 62 years, and over half of whom were past (56%) or current (12%) smokers – were diagnosed with a specific pattern of ILD not due to NSIP. Of the 16 cases, 6 were due to hypersensitive pneumonitis, 4 were due to interstitial pulmonary fibrosis, and 2 were due to sarcoidosis. The other four cases included patients with one of the following conditions: adenocarcinoma, desquamative interstitial pneumonia, eosinophilic pneumonia, and amyloidosis.
Six of the 24 cases were defined as NSIP, with 2 reclassified as definite and 1 as probable hypersensitive pneumonitis, after discussion within the multidisciplinary team.
Five patients – three who had been diagnosed with NSIP and two who had been given no pathological diagnosis after cryobiopsy – underwent surgical lung biopsy. Of these, following the surgical biopsies, only one patient was considered to have NSIP and the other four were eventually diagnosed with interstitial pulmonary fibrosis.
In terms of safety, five patients experienced pneumothorax, two patients required chest drainage, two needed simple aspiration and one underwent observation. In the majority of cases, patients experienced mild bleeding, with only one patient having experienced severe bleeding. During this study, none of the participants experienced significant chest pain, acute exacerbations, or infections, and none of them died.
Dr. Bondue has received research grants and fees for consulting from Boehringer Ingelheim and Roche.
LONDON – The vast majority of surgical lung biopsies currently used to diagnose interstitial lung diseases (ILDs) could be avoided, suggests research presented at the annual congress of the European Respiratory Society.
During an oral presentation, Benjamin Bondue, MD, of Hopital Erasme, Brussels, presented the preliminary results of a Belgian prospective study evaluating the role of transbronchial lung cryobiopsies in 24 patients with undefined ILD treated at three participating centers.
Cryobiopsies were found to have a diagnostic yield of 79%, meaning that patients might be able to avoid undergoing a more invasive surgical removal of tissue in many cases. Compared with surgical biopsy, cryobiopsies offered the potential advantage of lower morbidity and shorter hospitalization time, Dr. Bondue said. He reported that patients needed to stay in hospital just 1.2 days after the procedure in the study.
“Our data also show that there is some benefit of surgical lung biopsy after cryobiopsy if we identify an NSIP [nonspecific interstitial pneumonia] pattern or idiopathic conditions, or if we cannot obtain a clear pathological diagnosis,” he reported. Acknowledging the study was small and conducted in a single center, he said the use of cryobiopsies following surgical biopsy might be worth further study.
Transbronchial lung cryobiopsy is a relatively new technique that uses a cryoprobe inserted down through a bronchoscope about 1-2 cm from the thoracic wall. Once in place, the probe is cooled for between 3 and 6 seconds, lung tissue freezes to the probe, and the probe and bronchoscope are removed together. This method allows for larger samples of tissue to be taken than does traditional transbronchial biopsy, which involves using large forceps to obtain tissue samples (Respirology. 2014;19:645-54).
In the Belgian study, Dr. Bondue noted that a Fogarty balloon was used to control any bleeding and that four transbronchial lung cryobiopsies were obtained from two different segments of the same lobe of a patient’s lungs. All biopsies were then analyzed by an expert pathologist in ILDs, and reviewed by two other expert pathologists when needed. The mean sample size obtained was 16 mm2.
The patients included in the study had undergone chest X-ray and had inconclusive findings in the majority (84%) of cases. They then had the option to undergo cryobiopsy or surgical lung biopsy, with the latter performed following discussion among a multidisciplinary team’s members.
Following cryobiopsy, 16 of the 24 patients – who were a mean age of 62 years, and over half of whom were past (56%) or current (12%) smokers – were diagnosed with a specific pattern of ILD not due to NSIP. Of the 16 cases, 6 were due to hypersensitive pneumonitis, 4 were due to interstitial pulmonary fibrosis, and 2 were due to sarcoidosis. The other four cases included patients with one of the following conditions: adenocarcinoma, desquamative interstitial pneumonia, eosinophilic pneumonia, and amyloidosis.
Six of the 24 cases were defined as NSIP, with 2 reclassified as definite and 1 as probable hypersensitive pneumonitis, after discussion within the multidisciplinary team.
Five patients – three who had been diagnosed with NSIP and two who had been given no pathological diagnosis after cryobiopsy – underwent surgical lung biopsy. Of these, following the surgical biopsies, only one patient was considered to have NSIP and the other four were eventually diagnosed with interstitial pulmonary fibrosis.
In terms of safety, five patients experienced pneumothorax, two patients required chest drainage, two needed simple aspiration and one underwent observation. In the majority of cases, patients experienced mild bleeding, with only one patient having experienced severe bleeding. During this study, none of the participants experienced significant chest pain, acute exacerbations, or infections, and none of them died.
Dr. Bondue has received research grants and fees for consulting from Boehringer Ingelheim and Roche.
AT THE ERS CONGRESS 2016 LONDON
Key clinical point: Transbronchial lung cryobiopsies are useful for the diagnosis of interstitial lung diseases and could help avoid surgical lung biopsies.
Major finding: Transbronchial lung cryobiopsy had a diagnostic yield of 79%.
Data source: Single-center study of 24 patients with interstitial lung diseases who underwent transbronchial lung cryobiopsies, surgical lung biopsies, or both.
Disclosures: Dr. Bondue has received research grants and fees for consulting from Boehringer Ingelheim and Roche.
Almost half of health providers will see PQRS pay cut
About half of all doctors who participate in the Physician Quality Reporting System (PQRS) soon will learn that their Medicare pay will be cut by up to 2% in 2017.
The Centers for Medicare & Medicaid Services has completed its assessments for reporting year 2015 and has begun notifying physicians that a 2.0% negative payment adjustment is forthcoming for those who did not satisfactorily report PQRS quality measures or who failed to satisfactorily participate in a qualified clinical data registry.
Doctors have just 2 months to challenge findings that they believe were made in error to spare themselves the 2017 cut, according to a CMS announcement.
If doctors believe their 2017 PQRS pay cut is erroneous, they can submit an informal review request by 11:59 p.m. EST on Nov. 30. CMS will investigate the merits of all review requests and issue a decision within 90 days. All requests for informal review must be submitted via a Web-based tool on the quality reporting communication support page. There are no hardship exemptions for the PQRS pay cuts.
In addition, some 2015 PQRS performance scores will be publicly reported on the Physician Compare website. CMS is hosting two sessions in October to provide further information about such public reporting.
In 2015, approximately 1.15 million professionals were eligible and able to participate in PQRS; just over half (624,077 or 54%) of eligible professionals successfully submitted data. The rest – about 528,000, or 46% – will see a pay cut in 2017, according to CMS.
Last year, slightly more health providers – 558,885 eligible professionals – were subject to the 2016 PQRS pay cut based on their 2014 reporting experience.
Walter J. Gorski, director of regulatory affairs for the American College of Physicians, said the college will be monitoring the notifications as they go out to physicians to assess impacts at the individual practice level and overall.
“In past years, the PQRS experience reports have shown a low level of participation in the program and, thus, a large number of physicians being subject to these negative adjustments,” Mr. Gorski said in an interview. “This low level of overall participation has long been a concern of ours and something we have recommended that CMS work to mitigate by simplifying reporting – something that we are pushing strongly for as the program is rolled into the new MIPS pathway [Merit-Based Incentive Payment System] within the [Quality Payment Program].”
The new Quality Payment Program will replace both PQRS and the Value Modifier program, as well as the separate payment adjustments under the Medicare EHR Incentive Program. The streamlined program will have reduced quality reporting requirements and a flexible design that allows eligible clinicians to pick the pace of participation during the first year, according to CMS.
“The newly announced ‘pick your pace’ approach for the first year of MIPS reporting will give physicians an opportunity to get their feet wet with quality reporting in a way that will protect them from future negative adjustments,” Mr. Gorski said.
[email protected]
On Twitter @legal_med
About half of all doctors who participate in the Physician Quality Reporting System (PQRS) soon will learn that their Medicare pay will be cut by up to 2% in 2017.
The Centers for Medicare & Medicaid Services has completed its assessments for reporting year 2015 and has begun notifying physicians that a 2.0% negative payment adjustment is forthcoming for those who did not satisfactorily report PQRS quality measures or who failed to satisfactorily participate in a qualified clinical data registry.
Doctors have just 2 months to challenge findings that they believe were made in error to spare themselves the 2017 cut, according to a CMS announcement.
If doctors believe their 2017 PQRS pay cut is erroneous, they can submit an informal review request by 11:59 p.m. EST on Nov. 30. CMS will investigate the merits of all review requests and issue a decision within 90 days. All requests for informal review must be submitted via a Web-based tool on the quality reporting communication support page. There are no hardship exemptions for the PQRS pay cuts.
In addition, some 2015 PQRS performance scores will be publicly reported on the Physician Compare website. CMS is hosting two sessions in October to provide further information about such public reporting.
In 2015, approximately 1.15 million professionals were eligible and able to participate in PQRS; just over half (624,077 or 54%) of eligible professionals successfully submitted data. The rest – about 528,000, or 46% – will see a pay cut in 2017, according to CMS.
Last year, slightly more health providers – 558,885 eligible professionals – were subject to the 2016 PQRS pay cut based on their 2014 reporting experience.
Walter J. Gorski, director of regulatory affairs for the American College of Physicians, said the college will be monitoring the notifications as they go out to physicians to assess impacts at the individual practice level and overall.
“In past years, the PQRS experience reports have shown a low level of participation in the program and, thus, a large number of physicians being subject to these negative adjustments,” Mr. Gorski said in an interview. “This low level of overall participation has long been a concern of ours and something we have recommended that CMS work to mitigate by simplifying reporting – something that we are pushing strongly for as the program is rolled into the new MIPS pathway [Merit-Based Incentive Payment System] within the [Quality Payment Program].”
The new Quality Payment Program will replace both PQRS and the Value Modifier program, as well as the separate payment adjustments under the Medicare EHR Incentive Program. The streamlined program will have reduced quality reporting requirements and a flexible design that allows eligible clinicians to pick the pace of participation during the first year, according to CMS.
“The newly announced ‘pick your pace’ approach for the first year of MIPS reporting will give physicians an opportunity to get their feet wet with quality reporting in a way that will protect them from future negative adjustments,” Mr. Gorski said.
[email protected]
On Twitter @legal_med
About half of all doctors who participate in the Physician Quality Reporting System (PQRS) soon will learn that their Medicare pay will be cut by up to 2% in 2017.
The Centers for Medicare & Medicaid Services has completed its assessments for reporting year 2015 and has begun notifying physicians that a 2.0% negative payment adjustment is forthcoming for those who did not satisfactorily report PQRS quality measures or who failed to satisfactorily participate in a qualified clinical data registry.
Doctors have just 2 months to challenge findings that they believe were made in error to spare themselves the 2017 cut, according to a CMS announcement.
If doctors believe their 2017 PQRS pay cut is erroneous, they can submit an informal review request by 11:59 p.m. EST on Nov. 30. CMS will investigate the merits of all review requests and issue a decision within 90 days. All requests for informal review must be submitted via a Web-based tool on the quality reporting communication support page. There are no hardship exemptions for the PQRS pay cuts.
In addition, some 2015 PQRS performance scores will be publicly reported on the Physician Compare website. CMS is hosting two sessions in October to provide further information about such public reporting.
In 2015, approximately 1.15 million professionals were eligible and able to participate in PQRS; just over half (624,077 or 54%) of eligible professionals successfully submitted data. The rest – about 528,000, or 46% – will see a pay cut in 2017, according to CMS.
Last year, slightly more health providers – 558,885 eligible professionals – were subject to the 2016 PQRS pay cut based on their 2014 reporting experience.
Walter J. Gorski, director of regulatory affairs for the American College of Physicians, said the college will be monitoring the notifications as they go out to physicians to assess impacts at the individual practice level and overall.
“In past years, the PQRS experience reports have shown a low level of participation in the program and, thus, a large number of physicians being subject to these negative adjustments,” Mr. Gorski said in an interview. “This low level of overall participation has long been a concern of ours and something we have recommended that CMS work to mitigate by simplifying reporting – something that we are pushing strongly for as the program is rolled into the new MIPS pathway [Merit-Based Incentive Payment System] within the [Quality Payment Program].”
The new Quality Payment Program will replace both PQRS and the Value Modifier program, as well as the separate payment adjustments under the Medicare EHR Incentive Program. The streamlined program will have reduced quality reporting requirements and a flexible design that allows eligible clinicians to pick the pace of participation during the first year, according to CMS.
“The newly announced ‘pick your pace’ approach for the first year of MIPS reporting will give physicians an opportunity to get their feet wet with quality reporting in a way that will protect them from future negative adjustments,” Mr. Gorski said.
[email protected]
On Twitter @legal_med
Steroids could reduce death rate for TB patients with acute respiratory failure
Tuberculosis patients admitted to intensive care units with acute respiratory failure had significantly better survival at 90 days after treatment with corticosteroids and anti-TB drugs, compared with patients not treated with the steroids, according to a retrospective study.
An adjusted inverse probability of treatment weighted analysis using propensity scores revealed corticosteroid use to be independently associated with a significantly reduced 90-day mortality rate (OR = 0.47; 95% CI, 0.22-0.98). This statistical approach was used because it reduces selection bias and other potential confounding factors in a way that a multivariate analysis cannot, wrote Ji Young Yang, MD, of Busan (South Korea) Paik Hospital and Inje University College of Medicine in Busan.
Mortality rates were similar between the steroid-treated and non–steroid-treated groups (48.6% and 50%, respectively), and unadjusted 90-day mortality risk was not affected by steroid administration (odds ratio, 0.94; 95% CI, 0.46-1.92; P = .875), reported Dr. Yang and colleagues (Clin Infect Dis. 2016 Sep 8. doi: 10.1093/cid/ciw616).
The study involved the examination of records of 124 patients (mean age 62, 64% men) admitted to a single center over a 25-year period ending in 2014. Of these, 56.5% received corticosteroids, and 49.2% of the cohort died within 90 days.
The investigators acknowledged that their study was limited by various factors, including its small size, its use of data from a single center, and its lack of a standardized approach to steroid treatment.
“Further prospective randomized controlled trials will therefore be necessary to clarify the role of steroids in the management of these patients,” they wrote in their analysis. However, Dr. Yang and colleagues argued, in acute respiratory failure – a rare but dangerous complication in TB – “corticosteroids represent an attractive option because they can suppress cytokine expression and are effective in managing the inflammatory complications of extrapulmonary tuberculosis. Moreover, corticosteroids have been recently been shown to reduce mortality or treatment failure in patients with tuberculosis or severe pneumonia.”
Robert C. Hyzy, MD, director of the critical care medicine unit at the University of Michigan, Ann Arbor, said the findings “should be considered hypothesis generating.
“Clinicians should wait for prospective validation of this observation before considering the use of corticosteroids in hospitalized patients with tuberculosis,” he added.
Dr. Yang and colleagues disclosed no conflicts of interest or outside funding for their study.
Tuberculosis patients admitted to intensive care units with acute respiratory failure had significantly better survival at 90 days after treatment with corticosteroids and anti-TB drugs, compared with patients not treated with the steroids, according to a retrospective study.
An adjusted inverse probability of treatment weighted analysis using propensity scores revealed corticosteroid use to be independently associated with a significantly reduced 90-day mortality rate (OR = 0.47; 95% CI, 0.22-0.98). This statistical approach was used because it reduces selection bias and other potential confounding factors in a way that a multivariate analysis cannot, wrote Ji Young Yang, MD, of Busan (South Korea) Paik Hospital and Inje University College of Medicine in Busan.
Mortality rates were similar between the steroid-treated and non–steroid-treated groups (48.6% and 50%, respectively), and unadjusted 90-day mortality risk was not affected by steroid administration (odds ratio, 0.94; 95% CI, 0.46-1.92; P = .875), reported Dr. Yang and colleagues (Clin Infect Dis. 2016 Sep 8. doi: 10.1093/cid/ciw616).
The study involved the examination of records of 124 patients (mean age 62, 64% men) admitted to a single center over a 25-year period ending in 2014. Of these, 56.5% received corticosteroids, and 49.2% of the cohort died within 90 days.
The investigators acknowledged that their study was limited by various factors, including its small size, its use of data from a single center, and its lack of a standardized approach to steroid treatment.
“Further prospective randomized controlled trials will therefore be necessary to clarify the role of steroids in the management of these patients,” they wrote in their analysis. However, Dr. Yang and colleagues argued, in acute respiratory failure – a rare but dangerous complication in TB – “corticosteroids represent an attractive option because they can suppress cytokine expression and are effective in managing the inflammatory complications of extrapulmonary tuberculosis. Moreover, corticosteroids have been recently been shown to reduce mortality or treatment failure in patients with tuberculosis or severe pneumonia.”
Robert C. Hyzy, MD, director of the critical care medicine unit at the University of Michigan, Ann Arbor, said the findings “should be considered hypothesis generating.
“Clinicians should wait for prospective validation of this observation before considering the use of corticosteroids in hospitalized patients with tuberculosis,” he added.
Dr. Yang and colleagues disclosed no conflicts of interest or outside funding for their study.
Tuberculosis patients admitted to intensive care units with acute respiratory failure had significantly better survival at 90 days after treatment with corticosteroids and anti-TB drugs, compared with patients not treated with the steroids, according to a retrospective study.
An adjusted inverse probability of treatment weighted analysis using propensity scores revealed corticosteroid use to be independently associated with a significantly reduced 90-day mortality rate (OR = 0.47; 95% CI, 0.22-0.98). This statistical approach was used because it reduces selection bias and other potential confounding factors in a way that a multivariate analysis cannot, wrote Ji Young Yang, MD, of Busan (South Korea) Paik Hospital and Inje University College of Medicine in Busan.
Mortality rates were similar between the steroid-treated and non–steroid-treated groups (48.6% and 50%, respectively), and unadjusted 90-day mortality risk was not affected by steroid administration (odds ratio, 0.94; 95% CI, 0.46-1.92; P = .875), reported Dr. Yang and colleagues (Clin Infect Dis. 2016 Sep 8. doi: 10.1093/cid/ciw616).
The study involved the examination of records of 124 patients (mean age 62, 64% men) admitted to a single center over a 25-year period ending in 2014. Of these, 56.5% received corticosteroids, and 49.2% of the cohort died within 90 days.
The investigators acknowledged that their study was limited by various factors, including its small size, its use of data from a single center, and its lack of a standardized approach to steroid treatment.
“Further prospective randomized controlled trials will therefore be necessary to clarify the role of steroids in the management of these patients,” they wrote in their analysis. However, Dr. Yang and colleagues argued, in acute respiratory failure – a rare but dangerous complication in TB – “corticosteroids represent an attractive option because they can suppress cytokine expression and are effective in managing the inflammatory complications of extrapulmonary tuberculosis. Moreover, corticosteroids have been recently been shown to reduce mortality or treatment failure in patients with tuberculosis or severe pneumonia.”
Robert C. Hyzy, MD, director of the critical care medicine unit at the University of Michigan, Ann Arbor, said the findings “should be considered hypothesis generating.
“Clinicians should wait for prospective validation of this observation before considering the use of corticosteroids in hospitalized patients with tuberculosis,” he added.
Dr. Yang and colleagues disclosed no conflicts of interest or outside funding for their study.
Key clinical point: Corticosteroids used in combination with anti-TB treatment appeared to lower 90-day mortality in TB patients with ARF.
Major finding: Reduced 90-day mortality was associated with corticosteroid use (odds ratio, 0.47; 95% CI, 0.22-0.98; P = .049).
Data source: A retrospective cohort study of 124 patients admitted to intensive care units with TB and ARF in a single Korean center from 1989 to 2014.
Disclosures: The investigators reported no outside funding or conflicts of interest.