User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
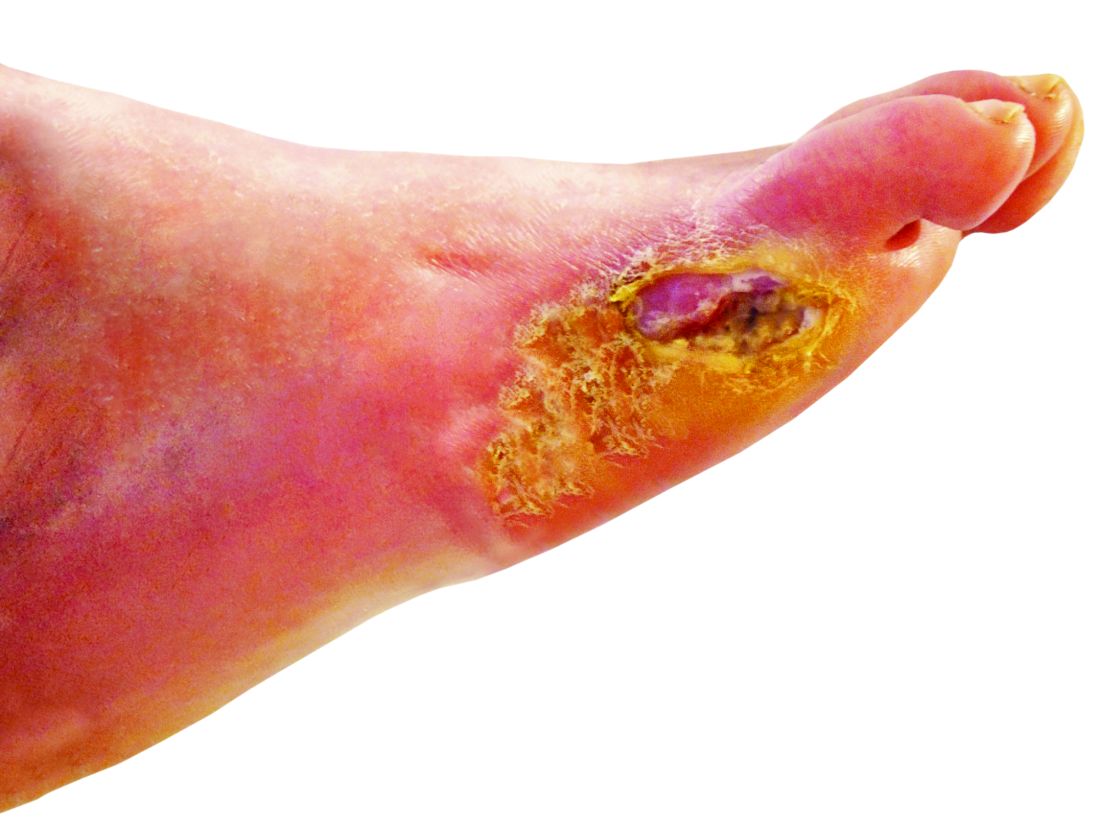
Models stratify hysterectomy risk with benign conditions
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
FROM CANADIAN MEDICAL ASSOCIATION JOURNAL
Why private practice will always survive: Seven doctors who left employment tell why
Employed physicians are often torn. Many relish the steady salary and ability to focus on being a physician rather than handle administrative duties, but they bemoan their employers’ rules and their lack of input into key decisions. And thus, many doctors are leaving employment to start a private practice. For this article,
Leaving employment is ‘an invigorating time’
On Sept. 9, Aaron Przybysz, MD, gave notice to his employer, a large academic medical center in Southern California, that he would be leaving to start a private practice.
“It’s an invigorating time,” said Dr. Przybysz, 41, an anesthesiologist and pain management physician who plans to open his new pain management practice on Dec. 1 in Orange County. He has picked out the space he will rent but has not yet hired his staff.
“I’ve been serious about doing this for at least a year,” Dr. Przybysz said. “What held me back is the concern that my business could fail. But even if that happens, what’s the worst that could occur? I’d have to find a new job as an employed physician.
“I feel comfortable with the business side of medicine,” he said. His father was an executive in the automotive industry and his father-in-law is an entrepreneur in construction and housing.
“One of the biggest reasons for moving to private practice is making sure I don’t miss my kids’ activities,” he said, referring to his children, ages 9 and 7. Recently, he said, “I had to spend the whole weekend on call in the hospital. I came home and had to sleep most of the next day.
“I love the people that I have been working with and I’ve learned and matured as a physician during that time,” he said. “But it was time to move on.”
The desire to be in charge
In Medscape’s recent Employed Physicians Report, doctors said they enjoy the steady salary and ability to focus on patients, which comes with being employed.
Other physicians feel differently. John Machata, MD, a solo family physician in the village of Wickford, R.I., 20 miles south of Providence, chose private practice because “I have total control,” he said. “I make decisions that I couldn’t have made as an employed physician, such as closing my practice to new patients.”
He can also decide on his work hours. “I see patients for 35 hours, 4 days a week and then I have a 3-day weekend.” In a large organization, “the focus is on revenue,” said Dr. Machata. “They’re always measuring your productivity. If you are slower, you won’t make enough money for them.”
When he worked for a large group practice about a decade ago, “I felt burnt out every day,” he said. “I had to see patients every 10 minutes, with no breaks for anything in between. Within a month I was devising my exit strategy.”
Dr. Machata maintains long appointments – 25 minutes for a typical follow-up visit and 55 minutes for an annual check-in – but he still earns above the state average for primary care doctors. “I have no nurse or front-office staff, which means I can save $125,000 to $150,000 a year,” he said.
In 2018, for the first time, employed physicians outnumbered self-employed physicians, according to a survey by the American Medical Association (AMA). By the end of 2021, more than half (52.1%) of U.S. physicians were employed by hospitals or health systems.
Yet the negatives of employment have begun to turn some physicians back toward private practice. Many physicians who were employed by a hospital or a large practice have become disillusioned and want to return to private practice.
His practice is the ‘best of both worlds’
Adam Bruggeman, MD, a 42-year-old spine surgeon who is CEO of Texas Spine Care Center, a solo practice in San Antonio, said he has “the best of both worlds.”
“As a solo physician, I have total control over how I practice,” he said. “But I also have access to value-based contracts and the data and staff needed to implement them.
“You need a lot of administrative overhead to take on these contracts, which a private practice normally doesn’t have,” he said. But Dr. Bruggeman gets this work done through a clinically integrated network (CIN) of private practices, Spinalytics of Texas, where he is chief medical director.
The CIN represents 150 musculoskeletal care providers and provides access to bundled networks, such as a total joint bundle with Blue Cross Blue Shield of Texas, as well as fee-for-service contracts.
He is also building a new ambulatory surgery center (ASC) that is scheduled to open in January. There, he plans to perform total joint and spine surgeries at a lower cost than at the hospital, which will be useful for value-based contracts through the CIN.
Dr. Bruggeman said it would be hard to run his private practice without the CIN and the ASC. “Private practice has changed,” he said. “The days of hanging up a shingle and immediately being successful are gone. You’ve got to be smart about business to run a successful practice.”
He started his practice while the pandemic raged
Joe Greene, MD, 42, an orthopedic surgeon in Louisville, Ky., had to open his hip and knee surgery practice when the COVID-19 pandemic was raging a year and a half ago, but that did not stop him.
“Federal financing of small bank loans completely stopped, but that only amounted to a small delay because our bank took care of it,” said Dr. Greene, who codirects his new practice with another orthopedic surgeon.
Even during the pandemic, “we could be very nimble,” he said. “For instance, when we want to institute new technology or a new patient-centric educational platform, we can do it immediately rather than going through an approval process at a health system.”
The partners, both ex-employees of a health system, also have an ASC, which allows them better control over their surgery schedules. “At a hospital, you can be bumped from the schedule by other surgeries, and you can’t be as productive as an ASC in the number of surgeries per day,” he said.
Dr. Greene attributes the practice’s success to long and careful planning. “We had to learn about business,” he said. “We did 3 to 4 years of research to find the right business model and implement it.”
As they were considering the new practice, a survey of patients showed that more than 75% chose them by word-of-mouth – because they specialize in complex and revision surgeries – rather than through referrals within their health system. This meant they could survive without their employer.
Planning for the new practice took up all his free time, but now he can relax and spend time with his three daughters, ages 12, 10, and 8. Dr. Greene currently coaches two of their teams. “We’re loving it,” he said.
Colleagues want to know how he did it
Clinton Sheets, MD, an ophthalmologist in Hudson and Clearwater, Fla., went solo in 2019 after being in a group practice for 11 years. Since he opened up, “I get phone calls from colleagues all the time, asking me about how I did it,” he said. “At least two of them followed in my footsteps.
“I tell them it’s very doable,” he said. “If you have the motivation, you can do it. Depending on your competence, you can outsource as much or as little as you want. Some management companies can do almost all of the nonclinical work for you.
“Smaller practices can streamline processes because they have a flatter organizational structure and have fewer issues with administrative bloat than larger organizations,” he said.
“Technology hinders and helps a private practice,” he said. On the one hand, he had to buy a lot of expensive equipment that otherwise would be shared by a group of doctors. On the other hand, using the cloud makes it possible to easily store practice management software and the electronic health record.
He’s opening a private practice while staying employed
In December, Dev Basu, MD, a hospitalist in Baltimore, plans to start his own private practice, seeing patients in skilled nursing homes, while still working as a nocturnist in a large health care system.
He said his employer has been supportive of his plans. “My work will not directly compete with them and it will benefit them by serving patients discharged from their hospitals,” said Dr. Basu, 38. He added, however, that he will be allowed to work only at certain facilities, and these will be subject to annual review.
“The financial risk of the new practice is low, because I haven’t had to invest much,” he said. “I won’t have a staff or an office.” He plans to maintain his full schedule as an employee, working 12 nights a month, because it will give him a great deal of time to do the new work.
“I also have no particular interest in running a business,” he said. “I come from a family of doctors, professors, and teachers who never ran a business. But I’m willing to learn so that I can practice medicine the way I want to.
“The ability to set my own schedule and deal with patients in the way I think is best is very important to me,” he said.
Private practitioners don’t have to face ‘moral distress’
One thing private practitioners typically don’t have to contend with is “moral distress,” which occurs when you have to follow institutional concerns on how much time you can spend with a patient or on the need to keep referrals in-house, according to Marie T. Brown, MD.
Dr. Brown is an internist who ran a small private practice in Oak Park, Ill., and is now the physician lead for the American Medical Association’s STEPS Forward program, which provides strategies on how to improve a medical practice.
“In my private practice, I could control the time I spent with each patient,” she said. “I also had control over my schedule. If I didn’t have the time, I just took a lower income, but that was okay.”
Dr. Brown said it is a myth that employment offers a better work-life balance. “Young physicians who take employment for this reason may find that they’re not allowed to drop off and pick up their children from school at a certain time. But you can do that in a private practice.”
She said it’s not that hard to run a practice. “Young physicians don’t think they could run a practice because they don’t have any business skills,” she said. “Yes, you do need some management skills, and you have to devote time to management. But you don’t need to have special expertise. You can outsource much of the work.”
A growing trend?
David J. Zetter, a consultant in Mechanicsburg, Pa., who helps doctors set up private practices, sees more interest in this in the past 5 years. “The overwhelming trend used to be private practices being bought up by hospitals and other entities,” he said. “Now we’re seeing the pendulum swing in the opposite direction.
“Generally, these doctors are fed up with being employed at a large organization,” he added. “Recently I got a call from a doctor who had never thought about running his own business, but he’s had it with being an employed physician.”
Switching to private practice is scary for a lot of them, but the alternative is worse. “A podiatrist I’m working with tells me she is scared to death about setting up a private practice, but she’s doing it because she doesn’t want to be employed anymore,” Mr. Zetter said.
A version of this article first appeared on Medscape.com.
Employed physicians are often torn. Many relish the steady salary and ability to focus on being a physician rather than handle administrative duties, but they bemoan their employers’ rules and their lack of input into key decisions. And thus, many doctors are leaving employment to start a private practice. For this article,
Leaving employment is ‘an invigorating time’
On Sept. 9, Aaron Przybysz, MD, gave notice to his employer, a large academic medical center in Southern California, that he would be leaving to start a private practice.
“It’s an invigorating time,” said Dr. Przybysz, 41, an anesthesiologist and pain management physician who plans to open his new pain management practice on Dec. 1 in Orange County. He has picked out the space he will rent but has not yet hired his staff.
“I’ve been serious about doing this for at least a year,” Dr. Przybysz said. “What held me back is the concern that my business could fail. But even if that happens, what’s the worst that could occur? I’d have to find a new job as an employed physician.
“I feel comfortable with the business side of medicine,” he said. His father was an executive in the automotive industry and his father-in-law is an entrepreneur in construction and housing.
“One of the biggest reasons for moving to private practice is making sure I don’t miss my kids’ activities,” he said, referring to his children, ages 9 and 7. Recently, he said, “I had to spend the whole weekend on call in the hospital. I came home and had to sleep most of the next day.
“I love the people that I have been working with and I’ve learned and matured as a physician during that time,” he said. “But it was time to move on.”
The desire to be in charge
In Medscape’s recent Employed Physicians Report, doctors said they enjoy the steady salary and ability to focus on patients, which comes with being employed.
Other physicians feel differently. John Machata, MD, a solo family physician in the village of Wickford, R.I., 20 miles south of Providence, chose private practice because “I have total control,” he said. “I make decisions that I couldn’t have made as an employed physician, such as closing my practice to new patients.”
He can also decide on his work hours. “I see patients for 35 hours, 4 days a week and then I have a 3-day weekend.” In a large organization, “the focus is on revenue,” said Dr. Machata. “They’re always measuring your productivity. If you are slower, you won’t make enough money for them.”
When he worked for a large group practice about a decade ago, “I felt burnt out every day,” he said. “I had to see patients every 10 minutes, with no breaks for anything in between. Within a month I was devising my exit strategy.”
Dr. Machata maintains long appointments – 25 minutes for a typical follow-up visit and 55 minutes for an annual check-in – but he still earns above the state average for primary care doctors. “I have no nurse or front-office staff, which means I can save $125,000 to $150,000 a year,” he said.
In 2018, for the first time, employed physicians outnumbered self-employed physicians, according to a survey by the American Medical Association (AMA). By the end of 2021, more than half (52.1%) of U.S. physicians were employed by hospitals or health systems.
Yet the negatives of employment have begun to turn some physicians back toward private practice. Many physicians who were employed by a hospital or a large practice have become disillusioned and want to return to private practice.
His practice is the ‘best of both worlds’
Adam Bruggeman, MD, a 42-year-old spine surgeon who is CEO of Texas Spine Care Center, a solo practice in San Antonio, said he has “the best of both worlds.”
“As a solo physician, I have total control over how I practice,” he said. “But I also have access to value-based contracts and the data and staff needed to implement them.
“You need a lot of administrative overhead to take on these contracts, which a private practice normally doesn’t have,” he said. But Dr. Bruggeman gets this work done through a clinically integrated network (CIN) of private practices, Spinalytics of Texas, where he is chief medical director.
The CIN represents 150 musculoskeletal care providers and provides access to bundled networks, such as a total joint bundle with Blue Cross Blue Shield of Texas, as well as fee-for-service contracts.
He is also building a new ambulatory surgery center (ASC) that is scheduled to open in January. There, he plans to perform total joint and spine surgeries at a lower cost than at the hospital, which will be useful for value-based contracts through the CIN.
Dr. Bruggeman said it would be hard to run his private practice without the CIN and the ASC. “Private practice has changed,” he said. “The days of hanging up a shingle and immediately being successful are gone. You’ve got to be smart about business to run a successful practice.”
He started his practice while the pandemic raged
Joe Greene, MD, 42, an orthopedic surgeon in Louisville, Ky., had to open his hip and knee surgery practice when the COVID-19 pandemic was raging a year and a half ago, but that did not stop him.
“Federal financing of small bank loans completely stopped, but that only amounted to a small delay because our bank took care of it,” said Dr. Greene, who codirects his new practice with another orthopedic surgeon.
Even during the pandemic, “we could be very nimble,” he said. “For instance, when we want to institute new technology or a new patient-centric educational platform, we can do it immediately rather than going through an approval process at a health system.”
The partners, both ex-employees of a health system, also have an ASC, which allows them better control over their surgery schedules. “At a hospital, you can be bumped from the schedule by other surgeries, and you can’t be as productive as an ASC in the number of surgeries per day,” he said.
Dr. Greene attributes the practice’s success to long and careful planning. “We had to learn about business,” he said. “We did 3 to 4 years of research to find the right business model and implement it.”
As they were considering the new practice, a survey of patients showed that more than 75% chose them by word-of-mouth – because they specialize in complex and revision surgeries – rather than through referrals within their health system. This meant they could survive without their employer.
Planning for the new practice took up all his free time, but now he can relax and spend time with his three daughters, ages 12, 10, and 8. Dr. Greene currently coaches two of their teams. “We’re loving it,” he said.
Colleagues want to know how he did it
Clinton Sheets, MD, an ophthalmologist in Hudson and Clearwater, Fla., went solo in 2019 after being in a group practice for 11 years. Since he opened up, “I get phone calls from colleagues all the time, asking me about how I did it,” he said. “At least two of them followed in my footsteps.
“I tell them it’s very doable,” he said. “If you have the motivation, you can do it. Depending on your competence, you can outsource as much or as little as you want. Some management companies can do almost all of the nonclinical work for you.
“Smaller practices can streamline processes because they have a flatter organizational structure and have fewer issues with administrative bloat than larger organizations,” he said.
“Technology hinders and helps a private practice,” he said. On the one hand, he had to buy a lot of expensive equipment that otherwise would be shared by a group of doctors. On the other hand, using the cloud makes it possible to easily store practice management software and the electronic health record.
He’s opening a private practice while staying employed
In December, Dev Basu, MD, a hospitalist in Baltimore, plans to start his own private practice, seeing patients in skilled nursing homes, while still working as a nocturnist in a large health care system.
He said his employer has been supportive of his plans. “My work will not directly compete with them and it will benefit them by serving patients discharged from their hospitals,” said Dr. Basu, 38. He added, however, that he will be allowed to work only at certain facilities, and these will be subject to annual review.
“The financial risk of the new practice is low, because I haven’t had to invest much,” he said. “I won’t have a staff or an office.” He plans to maintain his full schedule as an employee, working 12 nights a month, because it will give him a great deal of time to do the new work.
“I also have no particular interest in running a business,” he said. “I come from a family of doctors, professors, and teachers who never ran a business. But I’m willing to learn so that I can practice medicine the way I want to.
“The ability to set my own schedule and deal with patients in the way I think is best is very important to me,” he said.
Private practitioners don’t have to face ‘moral distress’
One thing private practitioners typically don’t have to contend with is “moral distress,” which occurs when you have to follow institutional concerns on how much time you can spend with a patient or on the need to keep referrals in-house, according to Marie T. Brown, MD.
Dr. Brown is an internist who ran a small private practice in Oak Park, Ill., and is now the physician lead for the American Medical Association’s STEPS Forward program, which provides strategies on how to improve a medical practice.
“In my private practice, I could control the time I spent with each patient,” she said. “I also had control over my schedule. If I didn’t have the time, I just took a lower income, but that was okay.”
Dr. Brown said it is a myth that employment offers a better work-life balance. “Young physicians who take employment for this reason may find that they’re not allowed to drop off and pick up their children from school at a certain time. But you can do that in a private practice.”
She said it’s not that hard to run a practice. “Young physicians don’t think they could run a practice because they don’t have any business skills,” she said. “Yes, you do need some management skills, and you have to devote time to management. But you don’t need to have special expertise. You can outsource much of the work.”
A growing trend?
David J. Zetter, a consultant in Mechanicsburg, Pa., who helps doctors set up private practices, sees more interest in this in the past 5 years. “The overwhelming trend used to be private practices being bought up by hospitals and other entities,” he said. “Now we’re seeing the pendulum swing in the opposite direction.
“Generally, these doctors are fed up with being employed at a large organization,” he added. “Recently I got a call from a doctor who had never thought about running his own business, but he’s had it with being an employed physician.”
Switching to private practice is scary for a lot of them, but the alternative is worse. “A podiatrist I’m working with tells me she is scared to death about setting up a private practice, but she’s doing it because she doesn’t want to be employed anymore,” Mr. Zetter said.
A version of this article first appeared on Medscape.com.
Employed physicians are often torn. Many relish the steady salary and ability to focus on being a physician rather than handle administrative duties, but they bemoan their employers’ rules and their lack of input into key decisions. And thus, many doctors are leaving employment to start a private practice. For this article,
Leaving employment is ‘an invigorating time’
On Sept. 9, Aaron Przybysz, MD, gave notice to his employer, a large academic medical center in Southern California, that he would be leaving to start a private practice.
“It’s an invigorating time,” said Dr. Przybysz, 41, an anesthesiologist and pain management physician who plans to open his new pain management practice on Dec. 1 in Orange County. He has picked out the space he will rent but has not yet hired his staff.
“I’ve been serious about doing this for at least a year,” Dr. Przybysz said. “What held me back is the concern that my business could fail. But even if that happens, what’s the worst that could occur? I’d have to find a new job as an employed physician.
“I feel comfortable with the business side of medicine,” he said. His father was an executive in the automotive industry and his father-in-law is an entrepreneur in construction and housing.
“One of the biggest reasons for moving to private practice is making sure I don’t miss my kids’ activities,” he said, referring to his children, ages 9 and 7. Recently, he said, “I had to spend the whole weekend on call in the hospital. I came home and had to sleep most of the next day.
“I love the people that I have been working with and I’ve learned and matured as a physician during that time,” he said. “But it was time to move on.”
The desire to be in charge
In Medscape’s recent Employed Physicians Report, doctors said they enjoy the steady salary and ability to focus on patients, which comes with being employed.
Other physicians feel differently. John Machata, MD, a solo family physician in the village of Wickford, R.I., 20 miles south of Providence, chose private practice because “I have total control,” he said. “I make decisions that I couldn’t have made as an employed physician, such as closing my practice to new patients.”
He can also decide on his work hours. “I see patients for 35 hours, 4 days a week and then I have a 3-day weekend.” In a large organization, “the focus is on revenue,” said Dr. Machata. “They’re always measuring your productivity. If you are slower, you won’t make enough money for them.”
When he worked for a large group practice about a decade ago, “I felt burnt out every day,” he said. “I had to see patients every 10 minutes, with no breaks for anything in between. Within a month I was devising my exit strategy.”
Dr. Machata maintains long appointments – 25 minutes for a typical follow-up visit and 55 minutes for an annual check-in – but he still earns above the state average for primary care doctors. “I have no nurse or front-office staff, which means I can save $125,000 to $150,000 a year,” he said.
In 2018, for the first time, employed physicians outnumbered self-employed physicians, according to a survey by the American Medical Association (AMA). By the end of 2021, more than half (52.1%) of U.S. physicians were employed by hospitals or health systems.
Yet the negatives of employment have begun to turn some physicians back toward private practice. Many physicians who were employed by a hospital or a large practice have become disillusioned and want to return to private practice.
His practice is the ‘best of both worlds’
Adam Bruggeman, MD, a 42-year-old spine surgeon who is CEO of Texas Spine Care Center, a solo practice in San Antonio, said he has “the best of both worlds.”
“As a solo physician, I have total control over how I practice,” he said. “But I also have access to value-based contracts and the data and staff needed to implement them.
“You need a lot of administrative overhead to take on these contracts, which a private practice normally doesn’t have,” he said. But Dr. Bruggeman gets this work done through a clinically integrated network (CIN) of private practices, Spinalytics of Texas, where he is chief medical director.
The CIN represents 150 musculoskeletal care providers and provides access to bundled networks, such as a total joint bundle with Blue Cross Blue Shield of Texas, as well as fee-for-service contracts.
He is also building a new ambulatory surgery center (ASC) that is scheduled to open in January. There, he plans to perform total joint and spine surgeries at a lower cost than at the hospital, which will be useful for value-based contracts through the CIN.
Dr. Bruggeman said it would be hard to run his private practice without the CIN and the ASC. “Private practice has changed,” he said. “The days of hanging up a shingle and immediately being successful are gone. You’ve got to be smart about business to run a successful practice.”
He started his practice while the pandemic raged
Joe Greene, MD, 42, an orthopedic surgeon in Louisville, Ky., had to open his hip and knee surgery practice when the COVID-19 pandemic was raging a year and a half ago, but that did not stop him.
“Federal financing of small bank loans completely stopped, but that only amounted to a small delay because our bank took care of it,” said Dr. Greene, who codirects his new practice with another orthopedic surgeon.
Even during the pandemic, “we could be very nimble,” he said. “For instance, when we want to institute new technology or a new patient-centric educational platform, we can do it immediately rather than going through an approval process at a health system.”
The partners, both ex-employees of a health system, also have an ASC, which allows them better control over their surgery schedules. “At a hospital, you can be bumped from the schedule by other surgeries, and you can’t be as productive as an ASC in the number of surgeries per day,” he said.
Dr. Greene attributes the practice’s success to long and careful planning. “We had to learn about business,” he said. “We did 3 to 4 years of research to find the right business model and implement it.”
As they were considering the new practice, a survey of patients showed that more than 75% chose them by word-of-mouth – because they specialize in complex and revision surgeries – rather than through referrals within their health system. This meant they could survive without their employer.
Planning for the new practice took up all his free time, but now he can relax and spend time with his three daughters, ages 12, 10, and 8. Dr. Greene currently coaches two of their teams. “We’re loving it,” he said.
Colleagues want to know how he did it
Clinton Sheets, MD, an ophthalmologist in Hudson and Clearwater, Fla., went solo in 2019 after being in a group practice for 11 years. Since he opened up, “I get phone calls from colleagues all the time, asking me about how I did it,” he said. “At least two of them followed in my footsteps.
“I tell them it’s very doable,” he said. “If you have the motivation, you can do it. Depending on your competence, you can outsource as much or as little as you want. Some management companies can do almost all of the nonclinical work for you.
“Smaller practices can streamline processes because they have a flatter organizational structure and have fewer issues with administrative bloat than larger organizations,” he said.
“Technology hinders and helps a private practice,” he said. On the one hand, he had to buy a lot of expensive equipment that otherwise would be shared by a group of doctors. On the other hand, using the cloud makes it possible to easily store practice management software and the electronic health record.
He’s opening a private practice while staying employed
In December, Dev Basu, MD, a hospitalist in Baltimore, plans to start his own private practice, seeing patients in skilled nursing homes, while still working as a nocturnist in a large health care system.
He said his employer has been supportive of his plans. “My work will not directly compete with them and it will benefit them by serving patients discharged from their hospitals,” said Dr. Basu, 38. He added, however, that he will be allowed to work only at certain facilities, and these will be subject to annual review.
“The financial risk of the new practice is low, because I haven’t had to invest much,” he said. “I won’t have a staff or an office.” He plans to maintain his full schedule as an employee, working 12 nights a month, because it will give him a great deal of time to do the new work.
“I also have no particular interest in running a business,” he said. “I come from a family of doctors, professors, and teachers who never ran a business. But I’m willing to learn so that I can practice medicine the way I want to.
“The ability to set my own schedule and deal with patients in the way I think is best is very important to me,” he said.
Private practitioners don’t have to face ‘moral distress’
One thing private practitioners typically don’t have to contend with is “moral distress,” which occurs when you have to follow institutional concerns on how much time you can spend with a patient or on the need to keep referrals in-house, according to Marie T. Brown, MD.
Dr. Brown is an internist who ran a small private practice in Oak Park, Ill., and is now the physician lead for the American Medical Association’s STEPS Forward program, which provides strategies on how to improve a medical practice.
“In my private practice, I could control the time I spent with each patient,” she said. “I also had control over my schedule. If I didn’t have the time, I just took a lower income, but that was okay.”
Dr. Brown said it is a myth that employment offers a better work-life balance. “Young physicians who take employment for this reason may find that they’re not allowed to drop off and pick up their children from school at a certain time. But you can do that in a private practice.”
She said it’s not that hard to run a practice. “Young physicians don’t think they could run a practice because they don’t have any business skills,” she said. “Yes, you do need some management skills, and you have to devote time to management. But you don’t need to have special expertise. You can outsource much of the work.”
A growing trend?
David J. Zetter, a consultant in Mechanicsburg, Pa., who helps doctors set up private practices, sees more interest in this in the past 5 years. “The overwhelming trend used to be private practices being bought up by hospitals and other entities,” he said. “Now we’re seeing the pendulum swing in the opposite direction.
“Generally, these doctors are fed up with being employed at a large organization,” he added. “Recently I got a call from a doctor who had never thought about running his own business, but he’s had it with being an employed physician.”
Switching to private practice is scary for a lot of them, but the alternative is worse. “A podiatrist I’m working with tells me she is scared to death about setting up a private practice, but she’s doing it because she doesn’t want to be employed anymore,” Mr. Zetter said.
A version of this article first appeared on Medscape.com.
Meet our newest genetically engineered frenemy, herpes
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Gender-affirming mastectomy boosts image and quality of life in gender-diverse youth
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
FROM JAMA PEDIATRICS
Postpartum sexual enjoyment: Does mode of delivery matter?
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
Clinic and doc must pay millions after faulty hernia surgery
reports a story in the Bowling Green Daily News.
On May 31, 2013, Alice Duff went to Graves-Gilbert Clinic, a multispecialty group whose main campus is in Bowling Green, for hernia surgery. Her surgeon for the procedure at a nearby facility was Tage Haase, MD, a member of the clinic.
Originally, Mrs. Duff was expected to remain in the facility for 23 hours following the procedure. But her recovery didn’t proceed as expected, and that initial period was extended by roughly 3 days. During this time, Ms. Duff’s husband, Lloyd, allegedly requested multiple times that doctors order a CT scan for his wife.
Ten days after the procedure, and with Ms. Duff out of the hospital, the Duffs successfully urged their family physician to order a CT scan. It showed large amounts of free air in Alice’s abdomen, a condition that’s known as pneumoperitoneum.
On June 10, 2013, Mrs. Duff underwent a second surgery, during which doctors discovered that she had sustained a perforated bowel during the first procedure. As a result, her bowel contents had spilled into her abdomen, causing an infection that required an extended hospital stay and five additional surgeries. The infection also led to retinal damage that has left her legally blind.
In their claim against Graves-Gilbert and Dr. Haase, the Duffs argued that Dr. Haase and an assistant doctor who was not named in the suit had failed to meet the standard of care. Specifically, argued the Duffs, because bowel perforation is a known complication in hernia surgeries, Dr. Haase was negligent in not diagnosing and treating Ms. Duff’s problem earlier.
For their part, Graves-Gilbert and Dr. Haase maintained that there was no indication before the second surgery that Mrs. Duff was demonstrating symptoms that necessitated a follow-up procedure. Dr. Haase further argued that Mrs. Duff’s bowel perforation was probably caused by the sawblade-like effect of the suture material he had used to close her incision.
The jury didn’t see it this way, however. It awarded approximately $1.3 million to Mrs. Duff for past medical expenses, plus another $12 million for pain and suffering. Her husband, Lloyd, received an additional $8 million in damages.
The attorney representing the clinic and Dr. Haase has vowed to pursue “all available remedies to have the verdict vacated and the case set for a new trial.”
Case hinged on proper use of a ‘power morcellator’
A claim for punitive damages has been thrown out against a New Jersey doctor and the hospital he’s affiliated with, in a ruling that could help to clarify the standards for such damages in medical malpractice cases, according to a story first reported on NorthJersey.com.
In October 2014, Howard H. Jones, MD, director of minimally invasive gynecologic surgery at The Valley Hospital, in Ridgewood, N.J., treated a patient from nearby Nyack, N.Y., for uterine fibroid tumors. As part of that treatment at the hospital, Dr. Jones used a “laparoscopic power morcellator,” which during a myomectomy procedure cuts, or morcellates, fibroid tumors into pieces small enough to be removed through an incision that’s generally 2 cm or fewer.
While use of the device offers doctors an alternative to open surgery and its longer recovery times, it also risks spreading previously undiagnosed cancer cells throughout the abdomen, thereby shortening a patient’s life. Because of this risk, which has given rise to a number of malpractice cases around the country, the Food and Drug Administration issued a draft guidance in the spring of 2022 for the use of power morcellators.
One recommendation is that physicians employ a “compatible” containment system to catch morcellated tissue, including any with cancer cells. Another is that the device be used selectively, which is to say on patients who have a minimal cancer risk and who have been informed of the procedure’s possible side effects beforehand.
Within a month of the procedure, the patient was diagnosed with metastatic leiomyosarcoma and died in September 2015. Following her death, her sister, the executor of her estate, sued both The Valley Hospital and Dr. Jones. In her suit, the sister argued that the defendants knew, or should have known, the risks involved in using a power morcellator because of both an earlier (2014) FDA “safety communication” discouraging the use of the device and the death of another of Dr. Jones’ patients following a similar procedure the year before.
The suit further alleged that, even after the FDA had issued its safety caution, the hospital had used the device on 37 other patients, “without informing them of the [FDA] letter or obtaining their informed consent to use the device.”
In light of these alleged lapses, Mirian Rivera, the patient’s sister, sought both compensatory and punitive damages. Historically, punitive damages have been limited to the small number of med-mal cases where a doctor or hospital has been found to have acted with actual malice or “wanton and willful disregard.”
Both Valley and Dr. Jones strongly disagreed with Ms. Rivera’s claim, arguing that prior to the procedure Dr. Jones had in fact met with the patient several times and had conducted the proper cancer-detecting tests. Moreover, the defendants emphasized, the request for punitive damages in the absence of actual malice or other factors would almost certainly establish a dangerous legal precedent. Several industry groups – including the American Medical Association, the Medical Society of New Jersey, and the New Jersey Hospital Association – agreed and filed friend-of-court briefs in support of Valley Hospital and Dr. Jones.
But two lower courts refused to dismiss the plaintiff’s claims for punitive damages. That’s when attorneys for Valley and Dr. Jones appealed to the New Jersey Supreme Court. (Claims against the device manufacturer, a German company, had already been resolved.)
Ruling unanimously, the high court sided with the defendants: “As a matter of law, the evidence presented, even affording plaintiffs all favorable inferences, does not establish that defendants’ acts or omissions were motivated by actual malice or accompanied by wanton and willful disregard for the patient’s health and safety.”
The court also found that Valley had in fact reviewed hospital policy and drafted a patient-consent form after the release of the 2014 FDA safety communication on power morcellators. (The consent form had not been adopted before the surgery in question, however.)
The suit will now go back to Superior Court in Bergen County, New Jersey; unless a settlement is reached beforehand, the jury will weigh claims of negligence and compensatory damages.
At press time, no trial date had been set.
Will med-mal cases get tougher to defend in this state?
Late in August, the Pennsylvania Supreme Court reversed its own longstanding rule that required that medical malpractice cases be filed in the county where the alleged injury occurred, as an Associated Press story on NBCPhiladelphia.com, among other news sites, reports.
More than 2 decades ago, in response to what was then seen as a crisis in the med-mal system, the state legislature overwhelmingly passed MCARE (the Medical Care Availability and Reduction of Error Fund), which among other things restricted the venue of medical suits. The legislation was signed into law in March 2002 by then-Gov. Mark Schweiker.
The following year, the state’s high court adopted a similar venue rule.
Over the years, doctor and hospital groups have been big supporters of the rule, arguing that any attempt to shift cases back to allegedly more plaintiff-friendly courts in Philadelphia and other cities would likely retrigger a crisis of higher med-mal premiums, doctor flight, and worse health care.
But a 2020 report by Pennsylvania’s nonpartisan Legislative Budget and Finance Committee took issue with these conclusions. It said that, following a national trend, the cost of medical professional liability insurance had fallen in the state since 2007. The report concluded that nothing in the available data supports the “conclusion that changes in the availability, cost, and affordability of medical professional liability insurance are the result of changes in Pennsylvania law.”
A more recent report by the high court’s Civil Procedural Rules Committee reached a similar conclusion, noting that med-mal cases should be subject to the same rules as any other type of civil litigation. A majority of the high court agreed.
Predictably, this decision sits well with patient groups and officials representing trial attorneys in the Keystone State.
“Cases should be heard before 12 jurors that do not have a connection to a hospital or surgical center that is often times the largest employer in the county,” said Kila Baldwin, president of the Pennsylvania Association for Justice. “The new rule levels the playing field and will improve access to justice for all Pennsylvanians.”
Doctors, hospitals, and other health care providers, however, have predicted a “ruinous path” ahead.
A version of this article first appeared on Medscape.com.
reports a story in the Bowling Green Daily News.
On May 31, 2013, Alice Duff went to Graves-Gilbert Clinic, a multispecialty group whose main campus is in Bowling Green, for hernia surgery. Her surgeon for the procedure at a nearby facility was Tage Haase, MD, a member of the clinic.
Originally, Mrs. Duff was expected to remain in the facility for 23 hours following the procedure. But her recovery didn’t proceed as expected, and that initial period was extended by roughly 3 days. During this time, Ms. Duff’s husband, Lloyd, allegedly requested multiple times that doctors order a CT scan for his wife.
Ten days after the procedure, and with Ms. Duff out of the hospital, the Duffs successfully urged their family physician to order a CT scan. It showed large amounts of free air in Alice’s abdomen, a condition that’s known as pneumoperitoneum.
On June 10, 2013, Mrs. Duff underwent a second surgery, during which doctors discovered that she had sustained a perforated bowel during the first procedure. As a result, her bowel contents had spilled into her abdomen, causing an infection that required an extended hospital stay and five additional surgeries. The infection also led to retinal damage that has left her legally blind.
In their claim against Graves-Gilbert and Dr. Haase, the Duffs argued that Dr. Haase and an assistant doctor who was not named in the suit had failed to meet the standard of care. Specifically, argued the Duffs, because bowel perforation is a known complication in hernia surgeries, Dr. Haase was negligent in not diagnosing and treating Ms. Duff’s problem earlier.
For their part, Graves-Gilbert and Dr. Haase maintained that there was no indication before the second surgery that Mrs. Duff was demonstrating symptoms that necessitated a follow-up procedure. Dr. Haase further argued that Mrs. Duff’s bowel perforation was probably caused by the sawblade-like effect of the suture material he had used to close her incision.
The jury didn’t see it this way, however. It awarded approximately $1.3 million to Mrs. Duff for past medical expenses, plus another $12 million for pain and suffering. Her husband, Lloyd, received an additional $8 million in damages.
The attorney representing the clinic and Dr. Haase has vowed to pursue “all available remedies to have the verdict vacated and the case set for a new trial.”
Case hinged on proper use of a ‘power morcellator’
A claim for punitive damages has been thrown out against a New Jersey doctor and the hospital he’s affiliated with, in a ruling that could help to clarify the standards for such damages in medical malpractice cases, according to a story first reported on NorthJersey.com.
In October 2014, Howard H. Jones, MD, director of minimally invasive gynecologic surgery at The Valley Hospital, in Ridgewood, N.J., treated a patient from nearby Nyack, N.Y., for uterine fibroid tumors. As part of that treatment at the hospital, Dr. Jones used a “laparoscopic power morcellator,” which during a myomectomy procedure cuts, or morcellates, fibroid tumors into pieces small enough to be removed through an incision that’s generally 2 cm or fewer.
While use of the device offers doctors an alternative to open surgery and its longer recovery times, it also risks spreading previously undiagnosed cancer cells throughout the abdomen, thereby shortening a patient’s life. Because of this risk, which has given rise to a number of malpractice cases around the country, the Food and Drug Administration issued a draft guidance in the spring of 2022 for the use of power morcellators.
One recommendation is that physicians employ a “compatible” containment system to catch morcellated tissue, including any with cancer cells. Another is that the device be used selectively, which is to say on patients who have a minimal cancer risk and who have been informed of the procedure’s possible side effects beforehand.
Within a month of the procedure, the patient was diagnosed with metastatic leiomyosarcoma and died in September 2015. Following her death, her sister, the executor of her estate, sued both The Valley Hospital and Dr. Jones. In her suit, the sister argued that the defendants knew, or should have known, the risks involved in using a power morcellator because of both an earlier (2014) FDA “safety communication” discouraging the use of the device and the death of another of Dr. Jones’ patients following a similar procedure the year before.
The suit further alleged that, even after the FDA had issued its safety caution, the hospital had used the device on 37 other patients, “without informing them of the [FDA] letter or obtaining their informed consent to use the device.”
In light of these alleged lapses, Mirian Rivera, the patient’s sister, sought both compensatory and punitive damages. Historically, punitive damages have been limited to the small number of med-mal cases where a doctor or hospital has been found to have acted with actual malice or “wanton and willful disregard.”
Both Valley and Dr. Jones strongly disagreed with Ms. Rivera’s claim, arguing that prior to the procedure Dr. Jones had in fact met with the patient several times and had conducted the proper cancer-detecting tests. Moreover, the defendants emphasized, the request for punitive damages in the absence of actual malice or other factors would almost certainly establish a dangerous legal precedent. Several industry groups – including the American Medical Association, the Medical Society of New Jersey, and the New Jersey Hospital Association – agreed and filed friend-of-court briefs in support of Valley Hospital and Dr. Jones.
But two lower courts refused to dismiss the plaintiff’s claims for punitive damages. That’s when attorneys for Valley and Dr. Jones appealed to the New Jersey Supreme Court. (Claims against the device manufacturer, a German company, had already been resolved.)
Ruling unanimously, the high court sided with the defendants: “As a matter of law, the evidence presented, even affording plaintiffs all favorable inferences, does not establish that defendants’ acts or omissions were motivated by actual malice or accompanied by wanton and willful disregard for the patient’s health and safety.”
The court also found that Valley had in fact reviewed hospital policy and drafted a patient-consent form after the release of the 2014 FDA safety communication on power morcellators. (The consent form had not been adopted before the surgery in question, however.)
The suit will now go back to Superior Court in Bergen County, New Jersey; unless a settlement is reached beforehand, the jury will weigh claims of negligence and compensatory damages.
At press time, no trial date had been set.
Will med-mal cases get tougher to defend in this state?
Late in August, the Pennsylvania Supreme Court reversed its own longstanding rule that required that medical malpractice cases be filed in the county where the alleged injury occurred, as an Associated Press story on NBCPhiladelphia.com, among other news sites, reports.
More than 2 decades ago, in response to what was then seen as a crisis in the med-mal system, the state legislature overwhelmingly passed MCARE (the Medical Care Availability and Reduction of Error Fund), which among other things restricted the venue of medical suits. The legislation was signed into law in March 2002 by then-Gov. Mark Schweiker.
The following year, the state’s high court adopted a similar venue rule.
Over the years, doctor and hospital groups have been big supporters of the rule, arguing that any attempt to shift cases back to allegedly more plaintiff-friendly courts in Philadelphia and other cities would likely retrigger a crisis of higher med-mal premiums, doctor flight, and worse health care.
But a 2020 report by Pennsylvania’s nonpartisan Legislative Budget and Finance Committee took issue with these conclusions. It said that, following a national trend, the cost of medical professional liability insurance had fallen in the state since 2007. The report concluded that nothing in the available data supports the “conclusion that changes in the availability, cost, and affordability of medical professional liability insurance are the result of changes in Pennsylvania law.”
A more recent report by the high court’s Civil Procedural Rules Committee reached a similar conclusion, noting that med-mal cases should be subject to the same rules as any other type of civil litigation. A majority of the high court agreed.
Predictably, this decision sits well with patient groups and officials representing trial attorneys in the Keystone State.
“Cases should be heard before 12 jurors that do not have a connection to a hospital or surgical center that is often times the largest employer in the county,” said Kila Baldwin, president of the Pennsylvania Association for Justice. “The new rule levels the playing field and will improve access to justice for all Pennsylvanians.”
Doctors, hospitals, and other health care providers, however, have predicted a “ruinous path” ahead.
A version of this article first appeared on Medscape.com.
reports a story in the Bowling Green Daily News.
On May 31, 2013, Alice Duff went to Graves-Gilbert Clinic, a multispecialty group whose main campus is in Bowling Green, for hernia surgery. Her surgeon for the procedure at a nearby facility was Tage Haase, MD, a member of the clinic.
Originally, Mrs. Duff was expected to remain in the facility for 23 hours following the procedure. But her recovery didn’t proceed as expected, and that initial period was extended by roughly 3 days. During this time, Ms. Duff’s husband, Lloyd, allegedly requested multiple times that doctors order a CT scan for his wife.
Ten days after the procedure, and with Ms. Duff out of the hospital, the Duffs successfully urged their family physician to order a CT scan. It showed large amounts of free air in Alice’s abdomen, a condition that’s known as pneumoperitoneum.
On June 10, 2013, Mrs. Duff underwent a second surgery, during which doctors discovered that she had sustained a perforated bowel during the first procedure. As a result, her bowel contents had spilled into her abdomen, causing an infection that required an extended hospital stay and five additional surgeries. The infection also led to retinal damage that has left her legally blind.
In their claim against Graves-Gilbert and Dr. Haase, the Duffs argued that Dr. Haase and an assistant doctor who was not named in the suit had failed to meet the standard of care. Specifically, argued the Duffs, because bowel perforation is a known complication in hernia surgeries, Dr. Haase was negligent in not diagnosing and treating Ms. Duff’s problem earlier.
For their part, Graves-Gilbert and Dr. Haase maintained that there was no indication before the second surgery that Mrs. Duff was demonstrating symptoms that necessitated a follow-up procedure. Dr. Haase further argued that Mrs. Duff’s bowel perforation was probably caused by the sawblade-like effect of the suture material he had used to close her incision.
The jury didn’t see it this way, however. It awarded approximately $1.3 million to Mrs. Duff for past medical expenses, plus another $12 million for pain and suffering. Her husband, Lloyd, received an additional $8 million in damages.
The attorney representing the clinic and Dr. Haase has vowed to pursue “all available remedies to have the verdict vacated and the case set for a new trial.”
Case hinged on proper use of a ‘power morcellator’
A claim for punitive damages has been thrown out against a New Jersey doctor and the hospital he’s affiliated with, in a ruling that could help to clarify the standards for such damages in medical malpractice cases, according to a story first reported on NorthJersey.com.
In October 2014, Howard H. Jones, MD, director of minimally invasive gynecologic surgery at The Valley Hospital, in Ridgewood, N.J., treated a patient from nearby Nyack, N.Y., for uterine fibroid tumors. As part of that treatment at the hospital, Dr. Jones used a “laparoscopic power morcellator,” which during a myomectomy procedure cuts, or morcellates, fibroid tumors into pieces small enough to be removed through an incision that’s generally 2 cm or fewer.
While use of the device offers doctors an alternative to open surgery and its longer recovery times, it also risks spreading previously undiagnosed cancer cells throughout the abdomen, thereby shortening a patient’s life. Because of this risk, which has given rise to a number of malpractice cases around the country, the Food and Drug Administration issued a draft guidance in the spring of 2022 for the use of power morcellators.
One recommendation is that physicians employ a “compatible” containment system to catch morcellated tissue, including any with cancer cells. Another is that the device be used selectively, which is to say on patients who have a minimal cancer risk and who have been informed of the procedure’s possible side effects beforehand.
Within a month of the procedure, the patient was diagnosed with metastatic leiomyosarcoma and died in September 2015. Following her death, her sister, the executor of her estate, sued both The Valley Hospital and Dr. Jones. In her suit, the sister argued that the defendants knew, or should have known, the risks involved in using a power morcellator because of both an earlier (2014) FDA “safety communication” discouraging the use of the device and the death of another of Dr. Jones’ patients following a similar procedure the year before.
The suit further alleged that, even after the FDA had issued its safety caution, the hospital had used the device on 37 other patients, “without informing them of the [FDA] letter or obtaining their informed consent to use the device.”
In light of these alleged lapses, Mirian Rivera, the patient’s sister, sought both compensatory and punitive damages. Historically, punitive damages have been limited to the small number of med-mal cases where a doctor or hospital has been found to have acted with actual malice or “wanton and willful disregard.”
Both Valley and Dr. Jones strongly disagreed with Ms. Rivera’s claim, arguing that prior to the procedure Dr. Jones had in fact met with the patient several times and had conducted the proper cancer-detecting tests. Moreover, the defendants emphasized, the request for punitive damages in the absence of actual malice or other factors would almost certainly establish a dangerous legal precedent. Several industry groups – including the American Medical Association, the Medical Society of New Jersey, and the New Jersey Hospital Association – agreed and filed friend-of-court briefs in support of Valley Hospital and Dr. Jones.
But two lower courts refused to dismiss the plaintiff’s claims for punitive damages. That’s when attorneys for Valley and Dr. Jones appealed to the New Jersey Supreme Court. (Claims against the device manufacturer, a German company, had already been resolved.)
Ruling unanimously, the high court sided with the defendants: “As a matter of law, the evidence presented, even affording plaintiffs all favorable inferences, does not establish that defendants’ acts or omissions were motivated by actual malice or accompanied by wanton and willful disregard for the patient’s health and safety.”
The court also found that Valley had in fact reviewed hospital policy and drafted a patient-consent form after the release of the 2014 FDA safety communication on power morcellators. (The consent form had not been adopted before the surgery in question, however.)
The suit will now go back to Superior Court in Bergen County, New Jersey; unless a settlement is reached beforehand, the jury will weigh claims of negligence and compensatory damages.
At press time, no trial date had been set.
Will med-mal cases get tougher to defend in this state?
Late in August, the Pennsylvania Supreme Court reversed its own longstanding rule that required that medical malpractice cases be filed in the county where the alleged injury occurred, as an Associated Press story on NBCPhiladelphia.com, among other news sites, reports.
More than 2 decades ago, in response to what was then seen as a crisis in the med-mal system, the state legislature overwhelmingly passed MCARE (the Medical Care Availability and Reduction of Error Fund), which among other things restricted the venue of medical suits. The legislation was signed into law in March 2002 by then-Gov. Mark Schweiker.
The following year, the state’s high court adopted a similar venue rule.
Over the years, doctor and hospital groups have been big supporters of the rule, arguing that any attempt to shift cases back to allegedly more plaintiff-friendly courts in Philadelphia and other cities would likely retrigger a crisis of higher med-mal premiums, doctor flight, and worse health care.
But a 2020 report by Pennsylvania’s nonpartisan Legislative Budget and Finance Committee took issue with these conclusions. It said that, following a national trend, the cost of medical professional liability insurance had fallen in the state since 2007. The report concluded that nothing in the available data supports the “conclusion that changes in the availability, cost, and affordability of medical professional liability insurance are the result of changes in Pennsylvania law.”
A more recent report by the high court’s Civil Procedural Rules Committee reached a similar conclusion, noting that med-mal cases should be subject to the same rules as any other type of civil litigation. A majority of the high court agreed.
Predictably, this decision sits well with patient groups and officials representing trial attorneys in the Keystone State.
“Cases should be heard before 12 jurors that do not have a connection to a hospital or surgical center that is often times the largest employer in the county,” said Kila Baldwin, president of the Pennsylvania Association for Justice. “The new rule levels the playing field and will improve access to justice for all Pennsylvanians.”
Doctors, hospitals, and other health care providers, however, have predicted a “ruinous path” ahead.
A version of this article first appeared on Medscape.com.
Amulet, Watchman 2.5 LAAO outcomes neck and neck at 3 years
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Early bird gets the worm, night owl gets the diabetes
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Angiography in patients with prior CABG does better when planned with CT
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
AT TCT 2022
‘Amazing’ data for cheap beta-blocker gel for diabetic foot ulcers
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EASD 2022