User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Dangers of a medical board investigation: How to protect yourself
Cynthia H. Moran, MD, has a medical degree, a passion for treating the elderly, and a desire to work. What she doesn’t have is a job or hopes of getting one anytime soon.
The Houston physician has never been charged with a crime, but she did run afoul of the Texas Medical Board, an experience she said has left her destitute and virtually unemployable in the medical field.
“By the time the board gets through with you, you will be bankrupt and have nothing,” she said.
Dr. Moran has a long, tangled history with the board involving self-prescribing, opioid abuse, depression, and unprofessional conduct. After years of license suspension, drug testing, additional CME, substance abuse treatment, and work restrictions, her supervision by the board ended in 2019, but she has been largely unable to find work as a physician.
“I feel like a felon. I really understand what it’s like to be someone who does their time but then can’t get a job, can’t get an apartment. It’s in your record and there’s nothing you can do about it,” she said.
Although Dr. Moran largely created her own troubles, her experience shows the power state medical licensing boards have when it comes to disciplining physicians.
Reprimands to revocations
Many physicians think of their state medical boards as simply the bodies that issue their medical licenses, but the boards have other functions, including investigating complaints against licensed medical professionals and sometimes disciplining them.
According to 2017 statistics from the Federation of State Medical Boards (the most recent available), state boards took 8,813 actions that year. These included 796 suspensions, 764 probations, 570 surrendered licenses, and 264 revoked licenses.
Boards also can order doctors to enter state-run physician health plans to receive treatment for substance abuse, or they can allow physicians to practice only under the supervision of colleagues.
Although they vary by state, the boards are fundamentally similar. Members are appointed by the governor. A majority of them are physicians, and the remainder are nonmedical professionals. Their investigators, often retired law enforcement officials, have broad powers to collect evidence, including medical records. Their authority is backed by the state attorney general.
Although physicians tend to worry more about being sued for malpractice, a medical board investigation can be more worrisome, said William Sullivan, DO, JD, an ED physician and attorney in Illinois who has represented doctors before that state’s board. Board disciplinary actions outnumber malpractice awards by four to one in that state.
“The gravity of this is something that many physicians don’t understand,” he said.
You can be the subject of anonymous complaints and investigations
Anyone can file a complaint against a physician with a state board. The grievances can be about anything from a crowded waiting room to physician impairment.
Of course, the most trivial complaints (out-of-date magazines in the waiting room) are dismissed out of hand, but boards have the authority to investigate whatever it chooses. The most common investigations center around complaints of impairment, substance abuse, improper prescribing, faulty medical records, mental and physical health problems, and standard of care. Boards also will act if a physician is found guilty of a crime or misconduct unrelated to his or her medical practice.
“There are a lot of ways doctors get into trouble,” said Edward Dauer, MD, a radiologist who served on the Florida board for 11 years.
Investigations often expand beyond their original scope into all aspects of a practice. “Once you’re on their radar, they can find something,” Dr. Sullivan said.
All punitive actions taken by state boards are reported to the Department of Health & Human Services’ National Practitioner Data Bank, which is accessible to all state boards. Sanctioned physicians who set up practice in another state often find that their new home has adopted the sanctions leveled by the original state, something boards can do without conducting their own investigations.
“For doctors, discipline is forever. It never goes off your record,” Dr. Dauer said.
In addition, Medicare, Medicaid, and private insurers can exclude disciplined physicians, which can cripple a practice’s finances. So what can doctors do to avoid problems with the boards?
Don’t do anything wrong
That sounds glib and obvious, but many physicians get into trouble by unwittingly violating state medical regulations regarding such things as CME, insurance requirements, failure to notify the board of address changes, and personal relationships with current or former patients.
“The best advice to avoid these issues is to do a Google search for the Medical Practice Act in the state in which they practice,” said Dr. Sullivan. He noted that doctors should regularly check for changes in regulations.
Keeping on good terms with colleagues and patients also helps, he said, noting that many complaints stem from personal disputes and grievances.
But what if a physician becomes the subject of an investigation? What should they do?
Take any complaint seriously
Too many physicians dismiss investigations initially. “Some people have the wrong idea that if they ignore it, it will go away. It won’t go away,” Dr. Sullivan said.
Whether the initial contact comes through a letter or a visit from a board investigator, it should be treated with urgency. Ohio attorney Beth Collis said one client angrily scrawled one-word answers with a Sharpie on the questionnaire he was mailed – answers he was stuck defending throughout the rest of the investigation. Other doctors have ordered investigators out of their offices – another mistake. Failure to cooperate can result in an immediate license suspension.
“They should be speaking to these investigators like they were talking to a highway patrolman on the side of the road. They hold all the cards,” said Ms. Collis, who specializes in representing professionals before licensing boards.
Some physicians mistakenly assume that because their state board is made up mostly of fellow doctors, they will be able to make a complaint go away with some collegial chat.
Not so. “Medical board members see themselves as protecting the public. They’re very punitive,” Ms. Collis said.
At one time, state boards might have been lax in their supervision of physicians, but that changed in the 1980s when the watchdog group Public Citizen began ranking state medical boards by how effective they were in policing doctors.
Public Citizen used FSMB data on serious disciplinary actions per 1,000 doctors in each state to calculate its rankings, a practice that FSMB called incomplete and a misuse of its statistics. Nonetheless, the annual rankings generated a lot of publicity critical of state boards and might have spurred a tougher approach by regulators.
Public Citizen stopped publishing its annual rankings in 2013 after FSMB ceased supplying the data, but the get-tough approach remains, lawyers said.
About 95% of complaints are dismissed with nothing more serious than a letter to the doctor, but boards don’t hesitate to act when the misconduct is serious, said Dr. Dauer. “I felt it was my obligation to protect the public.”
Don’t try to fix it yourself
Although many complaints are anonymous, doctors can often figure out what or who it involves. Their impulse might be to contact a patient who complained, correct a medical record, or otherwise try to resolve the matter personally.
It’s better to leave things alone, the experts said. Don’t contact a patient. Give the board access to whatever information it asks for, but don’t alter anything, particularly medical records. “That’s how you’re going to get your license revoked,” Dr. Dauer said. He noted that when doctors add notations to records, they must date them.
Hire a lawyer
Many physicians assume they can resolve the complaint easily by explaining themselves to the board or investigators, or they don’t realize their license or practice could be at stake.
They’re better off letting a lawyer speak for them. Attorneys knowledgeable in this realm specialize in representing licensed professionals before regulatory boards and have the greatest knowledge of administrative law and how to negotiate the hearings and procedures.
Typically, a hearing is held before a subcommittee of the board, which can recommend a settlement to the full panel. Cases in which a settlement is not reached can go before the entire board.
Although full hearings can be similar to a trial, there are crucial differences regarding evidentiary rules and other matters, Ms. Collis said. For example, in Ohio, defendant physicians do not get to see the board’s full case against them before the hearing, which can make preparing a defense difficult. And the standard for burden of proof is a preponderance of evidence, as in civil suits, not evidence beyond a reasonable doubt, as in a criminal trial.
Cases that go to full hearings and beyond to appeals in state courts can take years to resolve, and a physician’s license can be suspended for the duration.
Get help before it’s too late
Physicians looking for support and advice can turn to organizations such as the Coalition for Physician Rights, an organization formed in 2018 by Kernan Manion, MD, a former psychiatrist who was forced to deactivate his license after an investigation by the North Carolina medical board.
The Coalition for Physician Rights has advised hundreds of physicians, most of whom he said come to him once they realize they’re in over their heads. “Almost everyone comes in too late,” Dr. Manion said. “They’re sitting ducks. They don’t know how to respond.”
In addition to offering advice and support, the Coalition for Physician Rights lobbies for reform in how boards operate. A number of states, including Oklahoma, have made reforms in recent years.
The appointed boards are too reliant on their administration and staff and usually rubber-stamp disciplinary recommendations, Dr. Manion said. He also criticized the boards’ lack of accountability: “A board operates without external or internal oversight. It is an autonomous entity operating on its own.”
As for Dr. Moran, at age 61, she’s interviewing for physician jobs around the country, refusing to give up medicine.
“What else can I do?” she said. “It’s what I’ve done my entire life. It’s what I went to school for. I don’t know how to do anything else.”
A version of this article originally appeared on Medscape.com.
Cynthia H. Moran, MD, has a medical degree, a passion for treating the elderly, and a desire to work. What she doesn’t have is a job or hopes of getting one anytime soon.
The Houston physician has never been charged with a crime, but she did run afoul of the Texas Medical Board, an experience she said has left her destitute and virtually unemployable in the medical field.
“By the time the board gets through with you, you will be bankrupt and have nothing,” she said.
Dr. Moran has a long, tangled history with the board involving self-prescribing, opioid abuse, depression, and unprofessional conduct. After years of license suspension, drug testing, additional CME, substance abuse treatment, and work restrictions, her supervision by the board ended in 2019, but she has been largely unable to find work as a physician.
“I feel like a felon. I really understand what it’s like to be someone who does their time but then can’t get a job, can’t get an apartment. It’s in your record and there’s nothing you can do about it,” she said.
Although Dr. Moran largely created her own troubles, her experience shows the power state medical licensing boards have when it comes to disciplining physicians.
Reprimands to revocations
Many physicians think of their state medical boards as simply the bodies that issue their medical licenses, but the boards have other functions, including investigating complaints against licensed medical professionals and sometimes disciplining them.
According to 2017 statistics from the Federation of State Medical Boards (the most recent available), state boards took 8,813 actions that year. These included 796 suspensions, 764 probations, 570 surrendered licenses, and 264 revoked licenses.
Boards also can order doctors to enter state-run physician health plans to receive treatment for substance abuse, or they can allow physicians to practice only under the supervision of colleagues.
Although they vary by state, the boards are fundamentally similar. Members are appointed by the governor. A majority of them are physicians, and the remainder are nonmedical professionals. Their investigators, often retired law enforcement officials, have broad powers to collect evidence, including medical records. Their authority is backed by the state attorney general.
Although physicians tend to worry more about being sued for malpractice, a medical board investigation can be more worrisome, said William Sullivan, DO, JD, an ED physician and attorney in Illinois who has represented doctors before that state’s board. Board disciplinary actions outnumber malpractice awards by four to one in that state.
“The gravity of this is something that many physicians don’t understand,” he said.
You can be the subject of anonymous complaints and investigations
Anyone can file a complaint against a physician with a state board. The grievances can be about anything from a crowded waiting room to physician impairment.
Of course, the most trivial complaints (out-of-date magazines in the waiting room) are dismissed out of hand, but boards have the authority to investigate whatever it chooses. The most common investigations center around complaints of impairment, substance abuse, improper prescribing, faulty medical records, mental and physical health problems, and standard of care. Boards also will act if a physician is found guilty of a crime or misconduct unrelated to his or her medical practice.
“There are a lot of ways doctors get into trouble,” said Edward Dauer, MD, a radiologist who served on the Florida board for 11 years.
Investigations often expand beyond their original scope into all aspects of a practice. “Once you’re on their radar, they can find something,” Dr. Sullivan said.
All punitive actions taken by state boards are reported to the Department of Health & Human Services’ National Practitioner Data Bank, which is accessible to all state boards. Sanctioned physicians who set up practice in another state often find that their new home has adopted the sanctions leveled by the original state, something boards can do without conducting their own investigations.
“For doctors, discipline is forever. It never goes off your record,” Dr. Dauer said.
In addition, Medicare, Medicaid, and private insurers can exclude disciplined physicians, which can cripple a practice’s finances. So what can doctors do to avoid problems with the boards?
Don’t do anything wrong
That sounds glib and obvious, but many physicians get into trouble by unwittingly violating state medical regulations regarding such things as CME, insurance requirements, failure to notify the board of address changes, and personal relationships with current or former patients.
“The best advice to avoid these issues is to do a Google search for the Medical Practice Act in the state in which they practice,” said Dr. Sullivan. He noted that doctors should regularly check for changes in regulations.
Keeping on good terms with colleagues and patients also helps, he said, noting that many complaints stem from personal disputes and grievances.
But what if a physician becomes the subject of an investigation? What should they do?
Take any complaint seriously
Too many physicians dismiss investigations initially. “Some people have the wrong idea that if they ignore it, it will go away. It won’t go away,” Dr. Sullivan said.
Whether the initial contact comes through a letter or a visit from a board investigator, it should be treated with urgency. Ohio attorney Beth Collis said one client angrily scrawled one-word answers with a Sharpie on the questionnaire he was mailed – answers he was stuck defending throughout the rest of the investigation. Other doctors have ordered investigators out of their offices – another mistake. Failure to cooperate can result in an immediate license suspension.
“They should be speaking to these investigators like they were talking to a highway patrolman on the side of the road. They hold all the cards,” said Ms. Collis, who specializes in representing professionals before licensing boards.
Some physicians mistakenly assume that because their state board is made up mostly of fellow doctors, they will be able to make a complaint go away with some collegial chat.
Not so. “Medical board members see themselves as protecting the public. They’re very punitive,” Ms. Collis said.
At one time, state boards might have been lax in their supervision of physicians, but that changed in the 1980s when the watchdog group Public Citizen began ranking state medical boards by how effective they were in policing doctors.
Public Citizen used FSMB data on serious disciplinary actions per 1,000 doctors in each state to calculate its rankings, a practice that FSMB called incomplete and a misuse of its statistics. Nonetheless, the annual rankings generated a lot of publicity critical of state boards and might have spurred a tougher approach by regulators.
Public Citizen stopped publishing its annual rankings in 2013 after FSMB ceased supplying the data, but the get-tough approach remains, lawyers said.
About 95% of complaints are dismissed with nothing more serious than a letter to the doctor, but boards don’t hesitate to act when the misconduct is serious, said Dr. Dauer. “I felt it was my obligation to protect the public.”
Don’t try to fix it yourself
Although many complaints are anonymous, doctors can often figure out what or who it involves. Their impulse might be to contact a patient who complained, correct a medical record, or otherwise try to resolve the matter personally.
It’s better to leave things alone, the experts said. Don’t contact a patient. Give the board access to whatever information it asks for, but don’t alter anything, particularly medical records. “That’s how you’re going to get your license revoked,” Dr. Dauer said. He noted that when doctors add notations to records, they must date them.
Hire a lawyer
Many physicians assume they can resolve the complaint easily by explaining themselves to the board or investigators, or they don’t realize their license or practice could be at stake.
They’re better off letting a lawyer speak for them. Attorneys knowledgeable in this realm specialize in representing licensed professionals before regulatory boards and have the greatest knowledge of administrative law and how to negotiate the hearings and procedures.
Typically, a hearing is held before a subcommittee of the board, which can recommend a settlement to the full panel. Cases in which a settlement is not reached can go before the entire board.
Although full hearings can be similar to a trial, there are crucial differences regarding evidentiary rules and other matters, Ms. Collis said. For example, in Ohio, defendant physicians do not get to see the board’s full case against them before the hearing, which can make preparing a defense difficult. And the standard for burden of proof is a preponderance of evidence, as in civil suits, not evidence beyond a reasonable doubt, as in a criminal trial.
Cases that go to full hearings and beyond to appeals in state courts can take years to resolve, and a physician’s license can be suspended for the duration.
Get help before it’s too late
Physicians looking for support and advice can turn to organizations such as the Coalition for Physician Rights, an organization formed in 2018 by Kernan Manion, MD, a former psychiatrist who was forced to deactivate his license after an investigation by the North Carolina medical board.
The Coalition for Physician Rights has advised hundreds of physicians, most of whom he said come to him once they realize they’re in over their heads. “Almost everyone comes in too late,” Dr. Manion said. “They’re sitting ducks. They don’t know how to respond.”
In addition to offering advice and support, the Coalition for Physician Rights lobbies for reform in how boards operate. A number of states, including Oklahoma, have made reforms in recent years.
The appointed boards are too reliant on their administration and staff and usually rubber-stamp disciplinary recommendations, Dr. Manion said. He also criticized the boards’ lack of accountability: “A board operates without external or internal oversight. It is an autonomous entity operating on its own.”
As for Dr. Moran, at age 61, she’s interviewing for physician jobs around the country, refusing to give up medicine.
“What else can I do?” she said. “It’s what I’ve done my entire life. It’s what I went to school for. I don’t know how to do anything else.”
A version of this article originally appeared on Medscape.com.
Cynthia H. Moran, MD, has a medical degree, a passion for treating the elderly, and a desire to work. What she doesn’t have is a job or hopes of getting one anytime soon.
The Houston physician has never been charged with a crime, but she did run afoul of the Texas Medical Board, an experience she said has left her destitute and virtually unemployable in the medical field.
“By the time the board gets through with you, you will be bankrupt and have nothing,” she said.
Dr. Moran has a long, tangled history with the board involving self-prescribing, opioid abuse, depression, and unprofessional conduct. After years of license suspension, drug testing, additional CME, substance abuse treatment, and work restrictions, her supervision by the board ended in 2019, but she has been largely unable to find work as a physician.
“I feel like a felon. I really understand what it’s like to be someone who does their time but then can’t get a job, can’t get an apartment. It’s in your record and there’s nothing you can do about it,” she said.
Although Dr. Moran largely created her own troubles, her experience shows the power state medical licensing boards have when it comes to disciplining physicians.
Reprimands to revocations
Many physicians think of their state medical boards as simply the bodies that issue their medical licenses, but the boards have other functions, including investigating complaints against licensed medical professionals and sometimes disciplining them.
According to 2017 statistics from the Federation of State Medical Boards (the most recent available), state boards took 8,813 actions that year. These included 796 suspensions, 764 probations, 570 surrendered licenses, and 264 revoked licenses.
Boards also can order doctors to enter state-run physician health plans to receive treatment for substance abuse, or they can allow physicians to practice only under the supervision of colleagues.
Although they vary by state, the boards are fundamentally similar. Members are appointed by the governor. A majority of them are physicians, and the remainder are nonmedical professionals. Their investigators, often retired law enforcement officials, have broad powers to collect evidence, including medical records. Their authority is backed by the state attorney general.
Although physicians tend to worry more about being sued for malpractice, a medical board investigation can be more worrisome, said William Sullivan, DO, JD, an ED physician and attorney in Illinois who has represented doctors before that state’s board. Board disciplinary actions outnumber malpractice awards by four to one in that state.
“The gravity of this is something that many physicians don’t understand,” he said.
You can be the subject of anonymous complaints and investigations
Anyone can file a complaint against a physician with a state board. The grievances can be about anything from a crowded waiting room to physician impairment.
Of course, the most trivial complaints (out-of-date magazines in the waiting room) are dismissed out of hand, but boards have the authority to investigate whatever it chooses. The most common investigations center around complaints of impairment, substance abuse, improper prescribing, faulty medical records, mental and physical health problems, and standard of care. Boards also will act if a physician is found guilty of a crime or misconduct unrelated to his or her medical practice.
“There are a lot of ways doctors get into trouble,” said Edward Dauer, MD, a radiologist who served on the Florida board for 11 years.
Investigations often expand beyond their original scope into all aspects of a practice. “Once you’re on their radar, they can find something,” Dr. Sullivan said.
All punitive actions taken by state boards are reported to the Department of Health & Human Services’ National Practitioner Data Bank, which is accessible to all state boards. Sanctioned physicians who set up practice in another state often find that their new home has adopted the sanctions leveled by the original state, something boards can do without conducting their own investigations.
“For doctors, discipline is forever. It never goes off your record,” Dr. Dauer said.
In addition, Medicare, Medicaid, and private insurers can exclude disciplined physicians, which can cripple a practice’s finances. So what can doctors do to avoid problems with the boards?
Don’t do anything wrong
That sounds glib and obvious, but many physicians get into trouble by unwittingly violating state medical regulations regarding such things as CME, insurance requirements, failure to notify the board of address changes, and personal relationships with current or former patients.
“The best advice to avoid these issues is to do a Google search for the Medical Practice Act in the state in which they practice,” said Dr. Sullivan. He noted that doctors should regularly check for changes in regulations.
Keeping on good terms with colleagues and patients also helps, he said, noting that many complaints stem from personal disputes and grievances.
But what if a physician becomes the subject of an investigation? What should they do?
Take any complaint seriously
Too many physicians dismiss investigations initially. “Some people have the wrong idea that if they ignore it, it will go away. It won’t go away,” Dr. Sullivan said.
Whether the initial contact comes through a letter or a visit from a board investigator, it should be treated with urgency. Ohio attorney Beth Collis said one client angrily scrawled one-word answers with a Sharpie on the questionnaire he was mailed – answers he was stuck defending throughout the rest of the investigation. Other doctors have ordered investigators out of their offices – another mistake. Failure to cooperate can result in an immediate license suspension.
“They should be speaking to these investigators like they were talking to a highway patrolman on the side of the road. They hold all the cards,” said Ms. Collis, who specializes in representing professionals before licensing boards.
Some physicians mistakenly assume that because their state board is made up mostly of fellow doctors, they will be able to make a complaint go away with some collegial chat.
Not so. “Medical board members see themselves as protecting the public. They’re very punitive,” Ms. Collis said.
At one time, state boards might have been lax in their supervision of physicians, but that changed in the 1980s when the watchdog group Public Citizen began ranking state medical boards by how effective they were in policing doctors.
Public Citizen used FSMB data on serious disciplinary actions per 1,000 doctors in each state to calculate its rankings, a practice that FSMB called incomplete and a misuse of its statistics. Nonetheless, the annual rankings generated a lot of publicity critical of state boards and might have spurred a tougher approach by regulators.
Public Citizen stopped publishing its annual rankings in 2013 after FSMB ceased supplying the data, but the get-tough approach remains, lawyers said.
About 95% of complaints are dismissed with nothing more serious than a letter to the doctor, but boards don’t hesitate to act when the misconduct is serious, said Dr. Dauer. “I felt it was my obligation to protect the public.”
Don’t try to fix it yourself
Although many complaints are anonymous, doctors can often figure out what or who it involves. Their impulse might be to contact a patient who complained, correct a medical record, or otherwise try to resolve the matter personally.
It’s better to leave things alone, the experts said. Don’t contact a patient. Give the board access to whatever information it asks for, but don’t alter anything, particularly medical records. “That’s how you’re going to get your license revoked,” Dr. Dauer said. He noted that when doctors add notations to records, they must date them.
Hire a lawyer
Many physicians assume they can resolve the complaint easily by explaining themselves to the board or investigators, or they don’t realize their license or practice could be at stake.
They’re better off letting a lawyer speak for them. Attorneys knowledgeable in this realm specialize in representing licensed professionals before regulatory boards and have the greatest knowledge of administrative law and how to negotiate the hearings and procedures.
Typically, a hearing is held before a subcommittee of the board, which can recommend a settlement to the full panel. Cases in which a settlement is not reached can go before the entire board.
Although full hearings can be similar to a trial, there are crucial differences regarding evidentiary rules and other matters, Ms. Collis said. For example, in Ohio, defendant physicians do not get to see the board’s full case against them before the hearing, which can make preparing a defense difficult. And the standard for burden of proof is a preponderance of evidence, as in civil suits, not evidence beyond a reasonable doubt, as in a criminal trial.
Cases that go to full hearings and beyond to appeals in state courts can take years to resolve, and a physician’s license can be suspended for the duration.
Get help before it’s too late
Physicians looking for support and advice can turn to organizations such as the Coalition for Physician Rights, an organization formed in 2018 by Kernan Manion, MD, a former psychiatrist who was forced to deactivate his license after an investigation by the North Carolina medical board.
The Coalition for Physician Rights has advised hundreds of physicians, most of whom he said come to him once they realize they’re in over their heads. “Almost everyone comes in too late,” Dr. Manion said. “They’re sitting ducks. They don’t know how to respond.”
In addition to offering advice and support, the Coalition for Physician Rights lobbies for reform in how boards operate. A number of states, including Oklahoma, have made reforms in recent years.
The appointed boards are too reliant on their administration and staff and usually rubber-stamp disciplinary recommendations, Dr. Manion said. He also criticized the boards’ lack of accountability: “A board operates without external or internal oversight. It is an autonomous entity operating on its own.”
As for Dr. Moran, at age 61, she’s interviewing for physician jobs around the country, refusing to give up medicine.
“What else can I do?” she said. “It’s what I’ve done my entire life. It’s what I went to school for. I don’t know how to do anything else.”
A version of this article originally appeared on Medscape.com.
FDA authorizes baricitinib combo for COVID-19
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
Pfizer’s COVID-19 vaccine 95% effective in final phase 3 results
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
‘Hospital at home’ increases COVID capacity in large study
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
One-third of critical illness survivors emerge from ICU with functional deterioration
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
FROM CRITICAL CARE MEDICINE
Open enrollment 2021: A big start for HealthCare.gov
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.
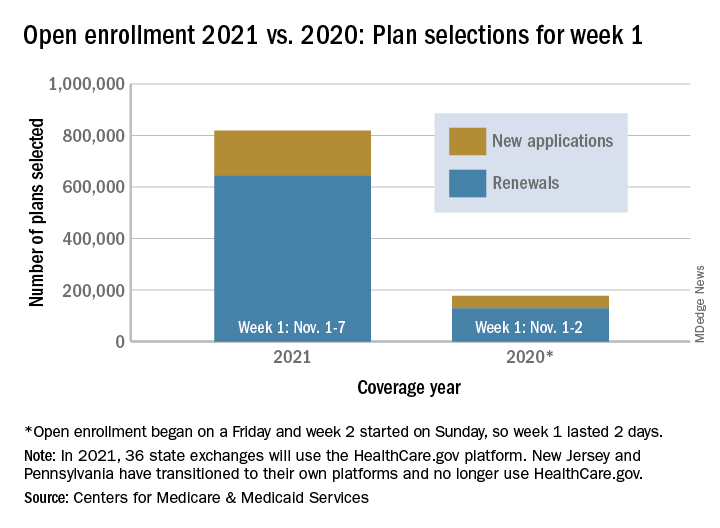
The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.

The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.

The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
AMA creates COVID-19 CPT codes for Pfizer, Moderna vaccines
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
Should our patients really go home for the holidays?
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Biden plan to lower Medicare eligibility age to 60 faces hostility from hospitals
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
What happened to melanoma care during COVID-19 sequestration
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
REPORTING FROM THE CUTANEOUS MALIGNANCIES FORUM