User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
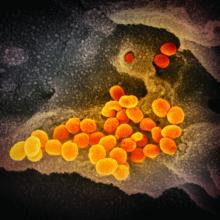
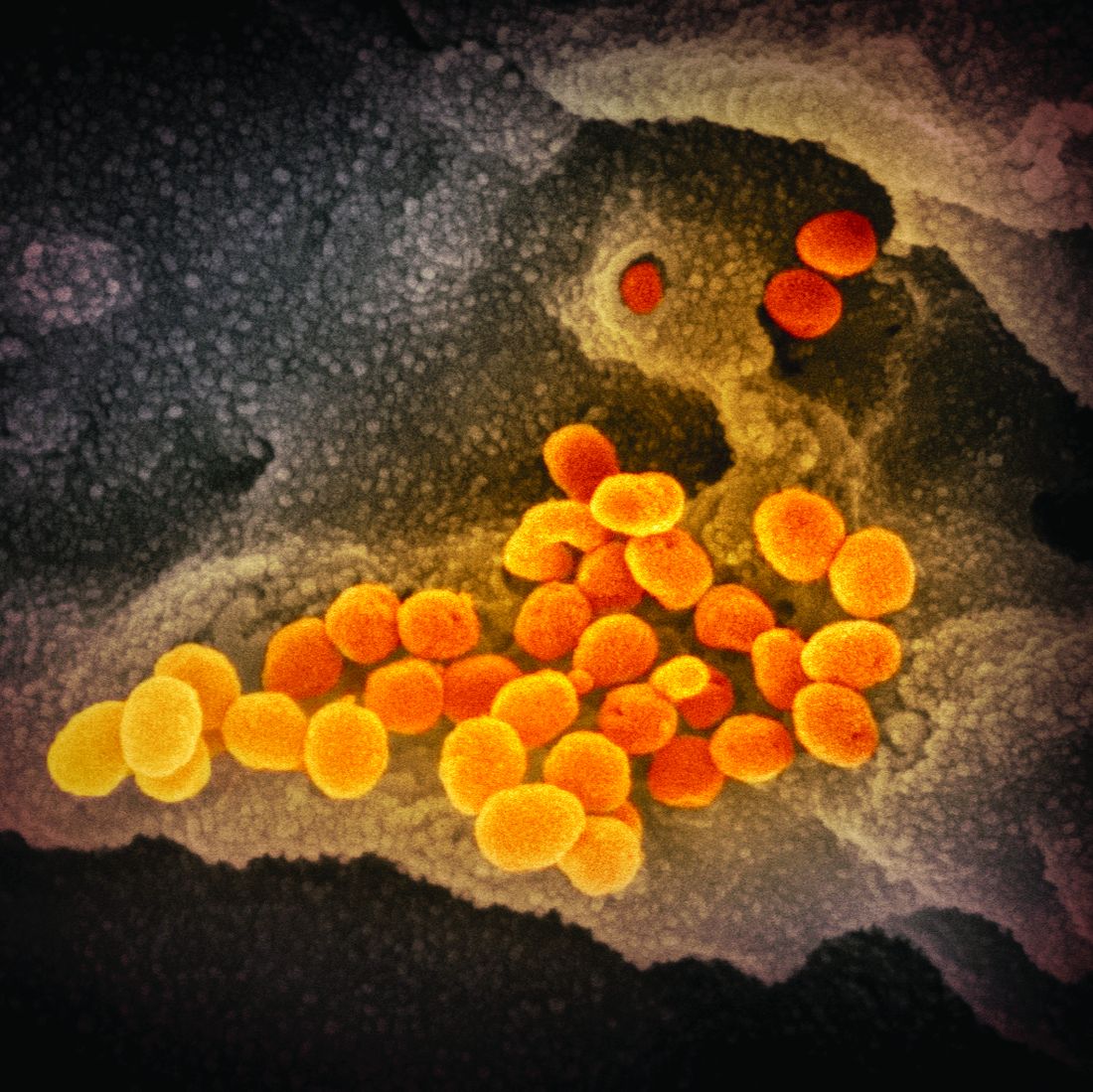
CDC expects eventual community spread of coronavirus in U.S.
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
USPSTF again deems evidence insufficient to recommend cognitive impairment screening in older adults
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
FROM JAMA
Genetic risk score may flag post-GDM incidence of type 2 disease
Women who had gestational diabetes mellitus had an increased risk for later type 2 diabetes if they carried certain genetic risk factors for the disease, according to a new analysis in BMJ Open Diabetes Research & Care of data from two independent populations.
A higher genetic risk score (GRS) had a modest association with developing type 2 diabetes, but a healthier diet may mitigate this risk, as Mengying Li, PhD, and her colleagues found for participants in the Nurses’ Health Study and members of the Danish National Birth Cohort who developed gestational diabetes mellitus (GDM).
Of 1,884 white women with a history of GDM in the Nurses’ Health Study II (NHSII), 446 (23.7%) went on to develop type 2 diabetes, and of the 550 women who had GDM in the Danish National Birth Cohort (DNBC), 155 (28.2%) developed the disease. The researchers calculated a GRS for type 2 diabetes for the full cohort. Genome-wide association studies completed in European populations were used to identify 59 single-nucleotide polymorphisms (SNPs) associated with the disease.
Dr. Li, an epidemiologist and postdoctoral researcher at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md., and her coauthors found that women whose GRS was in the highest quartile had a relative risk of 1.19 for type 2 diabetes. The relative risks for the three lower quartiles were 1.25, 0.97, and 1.00, respectively (P value for trend = .02). For each increase of five risk alleles in the GRS, NHSII participants had a 7% increased risk for type 2 diabetes, and DNBC participants saw a 9% increased risk.
Comparing these findings with other studies looking at genetic risk and type 2 diabetes in the general population, Dr. Li and her coauthors noted that the increase in relative risk for type 2 disease with increase in GRS was actually slightly weaker in the GDM cohort they studied. “The smaller effect size among women with GDM likely reflects an already higher baseline genetic risk for [type 2 diabetes] than the general population, as we have demonstrated,” they explained.
Though 11 individual SNPs had a significant individual association with the risk for type 2 diabetes initially, that association disappeared after correction for a false-discovery rate. Dr. Li and her coinvestigators conducted a sensitivity analysis that included only 42 SNPs that were later definitively associated with type 2 disease and they saw essentially unchanged results.
The researchers also investigated how dietary quality affected the GRS–type 2 diabetes association by dichotomizing self-reported diet quality in both cohorts into healthier diet quality and less healthy diet quality. They found a tighter association between GRS and type 2 diabetes for women with diet quality below the median, whereas women with higher diet quality did not have such a strong association between GRS and type 2 disease. The researchers wrote that there was “suggestive evidence that a healthful diet might mitigate the excessive risk of T2D [type 2 diabetes] related to greater genetic susceptibility, which supports public health efforts of encouraging a healthful diet” for diabetes prevention in this high-risk population.
Patients in the NHSII were followed for a mean 21.3 years, and those in the DNBC were followed for a mean 12.7 years. Mean age at index pregnancy was 30.5 years for the NHSII cohort and 31.7 for the DNBC cohort. In the NHSII cohort, just 8.4% of participants reported smoking before pregnancy, compared with 26.4% of those in the DNBC cohort. The NHSII cohort participants, wrote Dr. Li and her coauthors, “were also less likely to have a family history of diabetes, less likely to smoke, and be leaner than women in the DNBC.”
Dr. Li and her coauthors noted that, “despite being the largest genetic study by far on [type 2 diabetes] among women with GDM, our study may not be sufficiently powered to examine the associations of individual T2D SNPs in relation to the risk of developing T2D.” Another limitation was that for the Danish cohort, information about diet was drawn from a one-time questionnaire administered between 9 and 16 years after the index pregnancy, so full data about dietary quality over time was not available. Also of note is that the study included only white participants, limiting generalizability to women of color. The authors called for expanding this research into more racially diverse populations.
The study was supported by the National Institutes of Health. The authors reported that they had no conflicts of interest.
SOURCE: Li M et al. BMJ Open Diab Res Care. 2020 Feb 13. doi: 10.1136/bmjdrc-2019-000850.
Women who had gestational diabetes mellitus had an increased risk for later type 2 diabetes if they carried certain genetic risk factors for the disease, according to a new analysis in BMJ Open Diabetes Research & Care of data from two independent populations.
A higher genetic risk score (GRS) had a modest association with developing type 2 diabetes, but a healthier diet may mitigate this risk, as Mengying Li, PhD, and her colleagues found for participants in the Nurses’ Health Study and members of the Danish National Birth Cohort who developed gestational diabetes mellitus (GDM).
Of 1,884 white women with a history of GDM in the Nurses’ Health Study II (NHSII), 446 (23.7%) went on to develop type 2 diabetes, and of the 550 women who had GDM in the Danish National Birth Cohort (DNBC), 155 (28.2%) developed the disease. The researchers calculated a GRS for type 2 diabetes for the full cohort. Genome-wide association studies completed in European populations were used to identify 59 single-nucleotide polymorphisms (SNPs) associated with the disease.
Dr. Li, an epidemiologist and postdoctoral researcher at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md., and her coauthors found that women whose GRS was in the highest quartile had a relative risk of 1.19 for type 2 diabetes. The relative risks for the three lower quartiles were 1.25, 0.97, and 1.00, respectively (P value for trend = .02). For each increase of five risk alleles in the GRS, NHSII participants had a 7% increased risk for type 2 diabetes, and DNBC participants saw a 9% increased risk.
Comparing these findings with other studies looking at genetic risk and type 2 diabetes in the general population, Dr. Li and her coauthors noted that the increase in relative risk for type 2 disease with increase in GRS was actually slightly weaker in the GDM cohort they studied. “The smaller effect size among women with GDM likely reflects an already higher baseline genetic risk for [type 2 diabetes] than the general population, as we have demonstrated,” they explained.
Though 11 individual SNPs had a significant individual association with the risk for type 2 diabetes initially, that association disappeared after correction for a false-discovery rate. Dr. Li and her coinvestigators conducted a sensitivity analysis that included only 42 SNPs that were later definitively associated with type 2 disease and they saw essentially unchanged results.
The researchers also investigated how dietary quality affected the GRS–type 2 diabetes association by dichotomizing self-reported diet quality in both cohorts into healthier diet quality and less healthy diet quality. They found a tighter association between GRS and type 2 diabetes for women with diet quality below the median, whereas women with higher diet quality did not have such a strong association between GRS and type 2 disease. The researchers wrote that there was “suggestive evidence that a healthful diet might mitigate the excessive risk of T2D [type 2 diabetes] related to greater genetic susceptibility, which supports public health efforts of encouraging a healthful diet” for diabetes prevention in this high-risk population.
Patients in the NHSII were followed for a mean 21.3 years, and those in the DNBC were followed for a mean 12.7 years. Mean age at index pregnancy was 30.5 years for the NHSII cohort and 31.7 for the DNBC cohort. In the NHSII cohort, just 8.4% of participants reported smoking before pregnancy, compared with 26.4% of those in the DNBC cohort. The NHSII cohort participants, wrote Dr. Li and her coauthors, “were also less likely to have a family history of diabetes, less likely to smoke, and be leaner than women in the DNBC.”
Dr. Li and her coauthors noted that, “despite being the largest genetic study by far on [type 2 diabetes] among women with GDM, our study may not be sufficiently powered to examine the associations of individual T2D SNPs in relation to the risk of developing T2D.” Another limitation was that for the Danish cohort, information about diet was drawn from a one-time questionnaire administered between 9 and 16 years after the index pregnancy, so full data about dietary quality over time was not available. Also of note is that the study included only white participants, limiting generalizability to women of color. The authors called for expanding this research into more racially diverse populations.
The study was supported by the National Institutes of Health. The authors reported that they had no conflicts of interest.
SOURCE: Li M et al. BMJ Open Diab Res Care. 2020 Feb 13. doi: 10.1136/bmjdrc-2019-000850.
Women who had gestational diabetes mellitus had an increased risk for later type 2 diabetes if they carried certain genetic risk factors for the disease, according to a new analysis in BMJ Open Diabetes Research & Care of data from two independent populations.
A higher genetic risk score (GRS) had a modest association with developing type 2 diabetes, but a healthier diet may mitigate this risk, as Mengying Li, PhD, and her colleagues found for participants in the Nurses’ Health Study and members of the Danish National Birth Cohort who developed gestational diabetes mellitus (GDM).
Of 1,884 white women with a history of GDM in the Nurses’ Health Study II (NHSII), 446 (23.7%) went on to develop type 2 diabetes, and of the 550 women who had GDM in the Danish National Birth Cohort (DNBC), 155 (28.2%) developed the disease. The researchers calculated a GRS for type 2 diabetes for the full cohort. Genome-wide association studies completed in European populations were used to identify 59 single-nucleotide polymorphisms (SNPs) associated with the disease.
Dr. Li, an epidemiologist and postdoctoral researcher at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md., and her coauthors found that women whose GRS was in the highest quartile had a relative risk of 1.19 for type 2 diabetes. The relative risks for the three lower quartiles were 1.25, 0.97, and 1.00, respectively (P value for trend = .02). For each increase of five risk alleles in the GRS, NHSII participants had a 7% increased risk for type 2 diabetes, and DNBC participants saw a 9% increased risk.
Comparing these findings with other studies looking at genetic risk and type 2 diabetes in the general population, Dr. Li and her coauthors noted that the increase in relative risk for type 2 disease with increase in GRS was actually slightly weaker in the GDM cohort they studied. “The smaller effect size among women with GDM likely reflects an already higher baseline genetic risk for [type 2 diabetes] than the general population, as we have demonstrated,” they explained.
Though 11 individual SNPs had a significant individual association with the risk for type 2 diabetes initially, that association disappeared after correction for a false-discovery rate. Dr. Li and her coinvestigators conducted a sensitivity analysis that included only 42 SNPs that were later definitively associated with type 2 disease and they saw essentially unchanged results.
The researchers also investigated how dietary quality affected the GRS–type 2 diabetes association by dichotomizing self-reported diet quality in both cohorts into healthier diet quality and less healthy diet quality. They found a tighter association between GRS and type 2 diabetes for women with diet quality below the median, whereas women with higher diet quality did not have such a strong association between GRS and type 2 disease. The researchers wrote that there was “suggestive evidence that a healthful diet might mitigate the excessive risk of T2D [type 2 diabetes] related to greater genetic susceptibility, which supports public health efforts of encouraging a healthful diet” for diabetes prevention in this high-risk population.
Patients in the NHSII were followed for a mean 21.3 years, and those in the DNBC were followed for a mean 12.7 years. Mean age at index pregnancy was 30.5 years for the NHSII cohort and 31.7 for the DNBC cohort. In the NHSII cohort, just 8.4% of participants reported smoking before pregnancy, compared with 26.4% of those in the DNBC cohort. The NHSII cohort participants, wrote Dr. Li and her coauthors, “were also less likely to have a family history of diabetes, less likely to smoke, and be leaner than women in the DNBC.”
Dr. Li and her coauthors noted that, “despite being the largest genetic study by far on [type 2 diabetes] among women with GDM, our study may not be sufficiently powered to examine the associations of individual T2D SNPs in relation to the risk of developing T2D.” Another limitation was that for the Danish cohort, information about diet was drawn from a one-time questionnaire administered between 9 and 16 years after the index pregnancy, so full data about dietary quality over time was not available. Also of note is that the study included only white participants, limiting generalizability to women of color. The authors called for expanding this research into more racially diverse populations.
The study was supported by the National Institutes of Health. The authors reported that they had no conflicts of interest.
SOURCE: Li M et al. BMJ Open Diab Res Care. 2020 Feb 13. doi: 10.1136/bmjdrc-2019-000850.
FROM BMJ OPEN DIABETES RESEARCH & CARE
Medicaid expansion linked to more early cancer diagnoses
Cancer patients in states that opted to expand Medicaid insurance coverage under the Affordable Care Act saw a slightly better rate of early diagnosis, compared with patients in states that refused expansion, according to a new study. However, time to treatment was similar in states that opted for expansion and states that did not.
Samuel U. Takvorian, MD, of the University of Pennsylvania, Philadelphia, and colleagues reported these results in JAMA Network Open.
The researchers used the National Cancer Database to examine the changes in health insurance coverage and cancer health outcomes in nonelderly patients following implementation of the Affordable Care Act in January 2014. The investigators identified records for 925,543 patients who had new-onset breast (59%), colon (15%), or non–small cell lung (27%) cancer between 2011 and 2016. The patients’ mean age was 55 years (range, 40-64 years), 79% were women, 14% were black, and 6% were Hispanic.
The researchers looked at insurance status, cancer stage at diagnosis, and treatment initiation within 30 and 90 days of diagnosis. The cohort was equally divided between residents of Medicaid expansion states (48%) and nonexpansion states (52%).
Using a statistical technique that mimics a controlled experiment, the investigators found the percentage of uninsured patients decreased more in the expansion states (adjusted difference-in-differences, −0.7 percentage points; 95% confidence interval, −1.2 to −0.3; P = .001), compared with nonexpansion states. Expansion states also had a greater increase in early-stage cancer diagnoses (adjusted DID, 0.8; 95% CI 0.3-1.2; P = .001) and a greater decrease in advanced-stage cancer diagnoses (adjusted DID, −0.5; 95% CI, −0.9 to −0.2; P = .003).
Among the 848,329 patients who underwent cancer treatment within a year of diagnosis, the percentage initiating treatment within 30 days declined from 52.7% before to 48% after Medicaid expansion in states opting in (unadjusted DID, −4.7; percentage points, 95% CI; −5.1 to −4.5). States that did not expand their Medicaid programs, meanwhile, saw the share decline from 56.9% to 51.5% in the same time period (adjusted DID, −5.4; 95% CI, −5.6 to −5.1). There was no statistically significant difference in timely treatment associated with Medicaid expansion (adjusted DID, 0.6; 95% CI, −0.2 to 1.4; P = .14).
The researchers speculated that the lack of significant between-group differences in time to treatment, despite an improvement in early-stage diagnoses associated with Medicaid expansion, could reflect a cancer care system strained by a surge in insured patients, overall increases in cancer prevalence and complexity of care, a shortage of workers, or a mixture of factors.
In a related editorial, Sue Fu, MD, of Stanford (Calif.) University, and colleagues wrote that, while the findings of increased early diagnosis seen in the study are promising, the time to treatment results are “puzzling” and deserve further consideration.
Time to treatment is important in cancer, as longer times are associated with increased mortality, Dr. Fu and colleagues noted. Slowing times to cancer treatment is a systemic problem in the United States that has been documented since the mid-2000s. Paradoxically, expanded insurance coverage could contribute to increasing time to treatment even after timely diagnosis by adding administrative burdens leading to longer wait times. “Newly insured and underinsured individuals may be particularly vulnerable to this,” the editorialists wrote.
Dr. Takvorian and colleagues noted as weaknesses of their study its observational design, a limited range of ages and cancers included, and an inability to adjust for state-level effects.
This study was funded by the National Cancer Institute and the Agency for Health Research and Quality. The authors of the study and the editorial disclosed no relevant conflicts of interest.
SOURCES: Takvorian SU et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921653; Fu S et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921690.
Cancer patients in states that opted to expand Medicaid insurance coverage under the Affordable Care Act saw a slightly better rate of early diagnosis, compared with patients in states that refused expansion, according to a new study. However, time to treatment was similar in states that opted for expansion and states that did not.
Samuel U. Takvorian, MD, of the University of Pennsylvania, Philadelphia, and colleagues reported these results in JAMA Network Open.
The researchers used the National Cancer Database to examine the changes in health insurance coverage and cancer health outcomes in nonelderly patients following implementation of the Affordable Care Act in January 2014. The investigators identified records for 925,543 patients who had new-onset breast (59%), colon (15%), or non–small cell lung (27%) cancer between 2011 and 2016. The patients’ mean age was 55 years (range, 40-64 years), 79% were women, 14% were black, and 6% were Hispanic.
The researchers looked at insurance status, cancer stage at diagnosis, and treatment initiation within 30 and 90 days of diagnosis. The cohort was equally divided between residents of Medicaid expansion states (48%) and nonexpansion states (52%).
Using a statistical technique that mimics a controlled experiment, the investigators found the percentage of uninsured patients decreased more in the expansion states (adjusted difference-in-differences, −0.7 percentage points; 95% confidence interval, −1.2 to −0.3; P = .001), compared with nonexpansion states. Expansion states also had a greater increase in early-stage cancer diagnoses (adjusted DID, 0.8; 95% CI 0.3-1.2; P = .001) and a greater decrease in advanced-stage cancer diagnoses (adjusted DID, −0.5; 95% CI, −0.9 to −0.2; P = .003).
Among the 848,329 patients who underwent cancer treatment within a year of diagnosis, the percentage initiating treatment within 30 days declined from 52.7% before to 48% after Medicaid expansion in states opting in (unadjusted DID, −4.7; percentage points, 95% CI; −5.1 to −4.5). States that did not expand their Medicaid programs, meanwhile, saw the share decline from 56.9% to 51.5% in the same time period (adjusted DID, −5.4; 95% CI, −5.6 to −5.1). There was no statistically significant difference in timely treatment associated with Medicaid expansion (adjusted DID, 0.6; 95% CI, −0.2 to 1.4; P = .14).
The researchers speculated that the lack of significant between-group differences in time to treatment, despite an improvement in early-stage diagnoses associated with Medicaid expansion, could reflect a cancer care system strained by a surge in insured patients, overall increases in cancer prevalence and complexity of care, a shortage of workers, or a mixture of factors.
In a related editorial, Sue Fu, MD, of Stanford (Calif.) University, and colleagues wrote that, while the findings of increased early diagnosis seen in the study are promising, the time to treatment results are “puzzling” and deserve further consideration.
Time to treatment is important in cancer, as longer times are associated with increased mortality, Dr. Fu and colleagues noted. Slowing times to cancer treatment is a systemic problem in the United States that has been documented since the mid-2000s. Paradoxically, expanded insurance coverage could contribute to increasing time to treatment even after timely diagnosis by adding administrative burdens leading to longer wait times. “Newly insured and underinsured individuals may be particularly vulnerable to this,” the editorialists wrote.
Dr. Takvorian and colleagues noted as weaknesses of their study its observational design, a limited range of ages and cancers included, and an inability to adjust for state-level effects.
This study was funded by the National Cancer Institute and the Agency for Health Research and Quality. The authors of the study and the editorial disclosed no relevant conflicts of interest.
SOURCES: Takvorian SU et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921653; Fu S et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921690.
Cancer patients in states that opted to expand Medicaid insurance coverage under the Affordable Care Act saw a slightly better rate of early diagnosis, compared with patients in states that refused expansion, according to a new study. However, time to treatment was similar in states that opted for expansion and states that did not.
Samuel U. Takvorian, MD, of the University of Pennsylvania, Philadelphia, and colleagues reported these results in JAMA Network Open.
The researchers used the National Cancer Database to examine the changes in health insurance coverage and cancer health outcomes in nonelderly patients following implementation of the Affordable Care Act in January 2014. The investigators identified records for 925,543 patients who had new-onset breast (59%), colon (15%), or non–small cell lung (27%) cancer between 2011 and 2016. The patients’ mean age was 55 years (range, 40-64 years), 79% were women, 14% were black, and 6% were Hispanic.
The researchers looked at insurance status, cancer stage at diagnosis, and treatment initiation within 30 and 90 days of diagnosis. The cohort was equally divided between residents of Medicaid expansion states (48%) and nonexpansion states (52%).
Using a statistical technique that mimics a controlled experiment, the investigators found the percentage of uninsured patients decreased more in the expansion states (adjusted difference-in-differences, −0.7 percentage points; 95% confidence interval, −1.2 to −0.3; P = .001), compared with nonexpansion states. Expansion states also had a greater increase in early-stage cancer diagnoses (adjusted DID, 0.8; 95% CI 0.3-1.2; P = .001) and a greater decrease in advanced-stage cancer diagnoses (adjusted DID, −0.5; 95% CI, −0.9 to −0.2; P = .003).
Among the 848,329 patients who underwent cancer treatment within a year of diagnosis, the percentage initiating treatment within 30 days declined from 52.7% before to 48% after Medicaid expansion in states opting in (unadjusted DID, −4.7; percentage points, 95% CI; −5.1 to −4.5). States that did not expand their Medicaid programs, meanwhile, saw the share decline from 56.9% to 51.5% in the same time period (adjusted DID, −5.4; 95% CI, −5.6 to −5.1). There was no statistically significant difference in timely treatment associated with Medicaid expansion (adjusted DID, 0.6; 95% CI, −0.2 to 1.4; P = .14).
The researchers speculated that the lack of significant between-group differences in time to treatment, despite an improvement in early-stage diagnoses associated with Medicaid expansion, could reflect a cancer care system strained by a surge in insured patients, overall increases in cancer prevalence and complexity of care, a shortage of workers, or a mixture of factors.
In a related editorial, Sue Fu, MD, of Stanford (Calif.) University, and colleagues wrote that, while the findings of increased early diagnosis seen in the study are promising, the time to treatment results are “puzzling” and deserve further consideration.
Time to treatment is important in cancer, as longer times are associated with increased mortality, Dr. Fu and colleagues noted. Slowing times to cancer treatment is a systemic problem in the United States that has been documented since the mid-2000s. Paradoxically, expanded insurance coverage could contribute to increasing time to treatment even after timely diagnosis by adding administrative burdens leading to longer wait times. “Newly insured and underinsured individuals may be particularly vulnerable to this,” the editorialists wrote.
Dr. Takvorian and colleagues noted as weaknesses of their study its observational design, a limited range of ages and cancers included, and an inability to adjust for state-level effects.
This study was funded by the National Cancer Institute and the Agency for Health Research and Quality. The authors of the study and the editorial disclosed no relevant conflicts of interest.
SOURCES: Takvorian SU et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921653; Fu S et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921690.
FROM JAMA NETWORK OPEN
FDA, FTC uniting to promote biosimilars
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
FDA approves first IV migraine prevention drug
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
FROM MEDSCAPE.COM
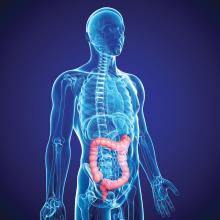
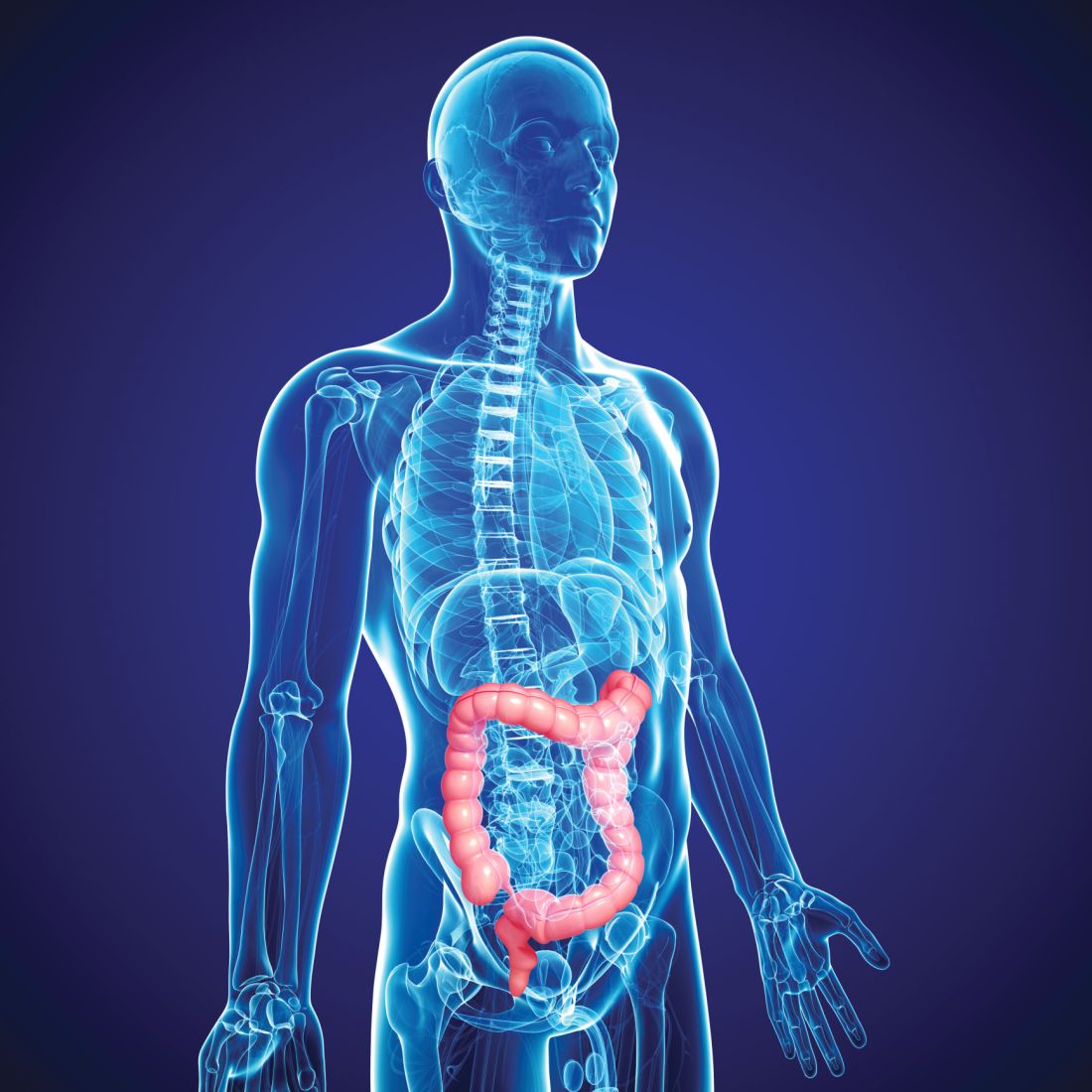
Should routine colon cancer screening start at 45, not 50?
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM
Guidance defines vaping-related respiratory syndrome
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
REPORTING FROM CCC49
Medicare beneficiaries get few home health visits after ICU stay
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
REPORTING FROM CCC49
Opioid use disorder up in sepsis hospitalizations
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
REPORTING FROM CCC49