User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Is COVID-19 leading to a mental illness pandemic?
People living through this crisis are experiencing trauma
We are in the midst of an epidemic and possibly pandemic of anxiety and distress. The worry that folks have about themselves, families, finances, and work is overwhelming for millions.
I speak with people who report periods of racing thoughts jumping back in time and thinking of roads not taken. They also talk about their thoughts jumping forward with life plans of what they’ll do to change their lives in the future – if they survive COVID-19.
that is well-controlled with care (and even without care). Those people are suffering even more. Meanwhile, people with obsessive-compulsive disorder that had been under control appear to have worsened with the added stress.
Social distancing has disrupted our everyday routines. For many, there is no work, no spending time with people we care about, no going to movies or shows, no doing discretionary shopping, no going to school. Parents with children at home report frustration about balancing working from home with completing home-schooling packets. Physicians on the front lines of this unprecedented time report not having the proper protective equipment and worrying about the possibility of exposing their families to SARS-CoV-2.
We hear stories about the illness and even deaths of some young and middle-aged people with no underlying conditions, not to mention the loss of older adults. People are bursting into tears, and becoming easily frustrated and angry. Add in nightmares, ongoing anxiety states, insomnia, and decreased concentration.
We are seeing news reports of people stocking up on guns and ammunition and a case of one taking – and dying from – nonpharmaceutical grade chloroquine in an effort to prevent COVID-19.
I spoke with Juliana Tseng, PsyD, a clinical psychologist based in New York, and she said that the hype, half-truths, and false information from some outlets in the popular media are making things worse. Dr. Tseng added that the lack of coordination among local, state, and federal governments also is increasing fear and alienation.
As I see this period in time, my first thoughts are that we are witnessing a national epidemic of trauma. Specifically, what we have here is a clinical picture of PTSD.
PTSD is defined clearly as a traumatic disorder with a real or perceived fracture with life. Isolation (which we are creating as a way to “flatten the curve” or slow the spread of COVID-19), although that strategy is in our best personal and public health interests, is both painful and stressful. Frustration, flashbacks of past life experiences plus flashbacks of being ill are reported in people I’ve spoken with. Avoidance, even though it is planned in this instance, is part of the PTSD complex.
What can we as mental health professionals do to help alleviate this suffering?
First, of course, we must listen to the scientific experts and the data – and tell people to do the same. Most experts will say that COVID-19 is a mild or moderate illness for the vast majority of people. We also must encourage people to observe precautions outlined by the Centers for Disease Control and Prevention, such as distancing from people, hand washing, and avoiding those who are ill. Explain to people that, currently, there is no vaccine to prevent COVID-19. Treatment is mainly supportive, and some medication trials are being explored. However, we can empower people by helping them to develop skills aimed at increasing the ability to relax and focus on more positive aspects of life to break the chain of the stress and tension of anxiety as well as control the PTSD.
For more than 40 years, I have helped people master relaxation techniques and guided imagery. When taught properly, people are able to use these techniques on their own.
To begin, I teach people how to relax, using a simple three-point method:
- Get comfortable in a nice chair, and slowly count from one to three. At the count of one, do one thing: “roll your eyes up to the top of your head.”
- At the count of two, do two things, “close your lids on your eyes and take a deep breath.”
- At three, exhale slowly, relax your eyes, and concentrate on a restful feeling of floating.
- Do this for about 30 seconds to a minute.
- Count backward, from three to two to one and open your eyes.
The person will notice how nice and restful they will feel.
After that exercise, get the person to move to the graduate level and go beyond just relaxation. In the following exercise, people can go into a relaxed state by imagining a movie screen. Tell the person to do two things:
1. Look at the imagined movie screen and project on it any pleasant scene you wish; this is your screen. You will feel yourself becoming more and more relaxed. The person can do this one, two, three or whatever times a day. The exercise can last 1 minute or 5.
2. Incorporate the 1, 2, 3 relaxation described earlier, allowing yourself to float into this restful state and go to your movie screen. Now, on the screen, imagine a thick line down the center, and on the left side, project your worries and anxieties and fears. The idea is to see but not experience them. Then shift to the ride side of the screen, and again, visualize any pleasant scene you wish. Again, do this for 1 minute or 5 minutes, whatever works.
You will notice that the pleasant scene on the right will overcome the anxiety scene on the left, in that pleasantness, in most instances, overcomes anxiety. For many, these techniques have proved very useful – whether the problem is anxiety or fear – or both. In my experience, these techniques are a good beginning for controlling PTSD and successfully treating it.
We are in the midst of what could be the biggest public health crisis that America has faced since the 1918 pandemic, also known as the Spanish flu. The lockdowns, quarantines, and the myriad of other disruptions can lead to alienation. In fact, it would be strange for us not to experience strong emotions under these extreme conditions. Life will get better! In the meantime, let’s encourage people to hope, pray, and use relaxation techniques and guided imagery approaches to help control anxiety, worry, stress, and issues related to PTSD. These approaches can give our minds and bodies periods of relaxation and recovery, and ultimately, they can calm our minds.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
People living through this crisis are experiencing trauma
People living through this crisis are experiencing trauma
We are in the midst of an epidemic and possibly pandemic of anxiety and distress. The worry that folks have about themselves, families, finances, and work is overwhelming for millions.
I speak with people who report periods of racing thoughts jumping back in time and thinking of roads not taken. They also talk about their thoughts jumping forward with life plans of what they’ll do to change their lives in the future – if they survive COVID-19.
that is well-controlled with care (and even without care). Those people are suffering even more. Meanwhile, people with obsessive-compulsive disorder that had been under control appear to have worsened with the added stress.
Social distancing has disrupted our everyday routines. For many, there is no work, no spending time with people we care about, no going to movies or shows, no doing discretionary shopping, no going to school. Parents with children at home report frustration about balancing working from home with completing home-schooling packets. Physicians on the front lines of this unprecedented time report not having the proper protective equipment and worrying about the possibility of exposing their families to SARS-CoV-2.
We hear stories about the illness and even deaths of some young and middle-aged people with no underlying conditions, not to mention the loss of older adults. People are bursting into tears, and becoming easily frustrated and angry. Add in nightmares, ongoing anxiety states, insomnia, and decreased concentration.
We are seeing news reports of people stocking up on guns and ammunition and a case of one taking – and dying from – nonpharmaceutical grade chloroquine in an effort to prevent COVID-19.
I spoke with Juliana Tseng, PsyD, a clinical psychologist based in New York, and she said that the hype, half-truths, and false information from some outlets in the popular media are making things worse. Dr. Tseng added that the lack of coordination among local, state, and federal governments also is increasing fear and alienation.
As I see this period in time, my first thoughts are that we are witnessing a national epidemic of trauma. Specifically, what we have here is a clinical picture of PTSD.
PTSD is defined clearly as a traumatic disorder with a real or perceived fracture with life. Isolation (which we are creating as a way to “flatten the curve” or slow the spread of COVID-19), although that strategy is in our best personal and public health interests, is both painful and stressful. Frustration, flashbacks of past life experiences plus flashbacks of being ill are reported in people I’ve spoken with. Avoidance, even though it is planned in this instance, is part of the PTSD complex.
What can we as mental health professionals do to help alleviate this suffering?
First, of course, we must listen to the scientific experts and the data – and tell people to do the same. Most experts will say that COVID-19 is a mild or moderate illness for the vast majority of people. We also must encourage people to observe precautions outlined by the Centers for Disease Control and Prevention, such as distancing from people, hand washing, and avoiding those who are ill. Explain to people that, currently, there is no vaccine to prevent COVID-19. Treatment is mainly supportive, and some medication trials are being explored. However, we can empower people by helping them to develop skills aimed at increasing the ability to relax and focus on more positive aspects of life to break the chain of the stress and tension of anxiety as well as control the PTSD.
For more than 40 years, I have helped people master relaxation techniques and guided imagery. When taught properly, people are able to use these techniques on their own.
To begin, I teach people how to relax, using a simple three-point method:
- Get comfortable in a nice chair, and slowly count from one to three. At the count of one, do one thing: “roll your eyes up to the top of your head.”
- At the count of two, do two things, “close your lids on your eyes and take a deep breath.”
- At three, exhale slowly, relax your eyes, and concentrate on a restful feeling of floating.
- Do this for about 30 seconds to a minute.
- Count backward, from three to two to one and open your eyes.
The person will notice how nice and restful they will feel.
After that exercise, get the person to move to the graduate level and go beyond just relaxation. In the following exercise, people can go into a relaxed state by imagining a movie screen. Tell the person to do two things:
1. Look at the imagined movie screen and project on it any pleasant scene you wish; this is your screen. You will feel yourself becoming more and more relaxed. The person can do this one, two, three or whatever times a day. The exercise can last 1 minute or 5.
2. Incorporate the 1, 2, 3 relaxation described earlier, allowing yourself to float into this restful state and go to your movie screen. Now, on the screen, imagine a thick line down the center, and on the left side, project your worries and anxieties and fears. The idea is to see but not experience them. Then shift to the ride side of the screen, and again, visualize any pleasant scene you wish. Again, do this for 1 minute or 5 minutes, whatever works.
You will notice that the pleasant scene on the right will overcome the anxiety scene on the left, in that pleasantness, in most instances, overcomes anxiety. For many, these techniques have proved very useful – whether the problem is anxiety or fear – or both. In my experience, these techniques are a good beginning for controlling PTSD and successfully treating it.
We are in the midst of what could be the biggest public health crisis that America has faced since the 1918 pandemic, also known as the Spanish flu. The lockdowns, quarantines, and the myriad of other disruptions can lead to alienation. In fact, it would be strange for us not to experience strong emotions under these extreme conditions. Life will get better! In the meantime, let’s encourage people to hope, pray, and use relaxation techniques and guided imagery approaches to help control anxiety, worry, stress, and issues related to PTSD. These approaches can give our minds and bodies periods of relaxation and recovery, and ultimately, they can calm our minds.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
We are in the midst of an epidemic and possibly pandemic of anxiety and distress. The worry that folks have about themselves, families, finances, and work is overwhelming for millions.
I speak with people who report periods of racing thoughts jumping back in time and thinking of roads not taken. They also talk about their thoughts jumping forward with life plans of what they’ll do to change their lives in the future – if they survive COVID-19.
that is well-controlled with care (and even without care). Those people are suffering even more. Meanwhile, people with obsessive-compulsive disorder that had been under control appear to have worsened with the added stress.
Social distancing has disrupted our everyday routines. For many, there is no work, no spending time with people we care about, no going to movies or shows, no doing discretionary shopping, no going to school. Parents with children at home report frustration about balancing working from home with completing home-schooling packets. Physicians on the front lines of this unprecedented time report not having the proper protective equipment and worrying about the possibility of exposing their families to SARS-CoV-2.
We hear stories about the illness and even deaths of some young and middle-aged people with no underlying conditions, not to mention the loss of older adults. People are bursting into tears, and becoming easily frustrated and angry. Add in nightmares, ongoing anxiety states, insomnia, and decreased concentration.
We are seeing news reports of people stocking up on guns and ammunition and a case of one taking – and dying from – nonpharmaceutical grade chloroquine in an effort to prevent COVID-19.
I spoke with Juliana Tseng, PsyD, a clinical psychologist based in New York, and she said that the hype, half-truths, and false information from some outlets in the popular media are making things worse. Dr. Tseng added that the lack of coordination among local, state, and federal governments also is increasing fear and alienation.
As I see this period in time, my first thoughts are that we are witnessing a national epidemic of trauma. Specifically, what we have here is a clinical picture of PTSD.
PTSD is defined clearly as a traumatic disorder with a real or perceived fracture with life. Isolation (which we are creating as a way to “flatten the curve” or slow the spread of COVID-19), although that strategy is in our best personal and public health interests, is both painful and stressful. Frustration, flashbacks of past life experiences plus flashbacks of being ill are reported in people I’ve spoken with. Avoidance, even though it is planned in this instance, is part of the PTSD complex.
What can we as mental health professionals do to help alleviate this suffering?
First, of course, we must listen to the scientific experts and the data – and tell people to do the same. Most experts will say that COVID-19 is a mild or moderate illness for the vast majority of people. We also must encourage people to observe precautions outlined by the Centers for Disease Control and Prevention, such as distancing from people, hand washing, and avoiding those who are ill. Explain to people that, currently, there is no vaccine to prevent COVID-19. Treatment is mainly supportive, and some medication trials are being explored. However, we can empower people by helping them to develop skills aimed at increasing the ability to relax and focus on more positive aspects of life to break the chain of the stress and tension of anxiety as well as control the PTSD.
For more than 40 years, I have helped people master relaxation techniques and guided imagery. When taught properly, people are able to use these techniques on their own.
To begin, I teach people how to relax, using a simple three-point method:
- Get comfortable in a nice chair, and slowly count from one to three. At the count of one, do one thing: “roll your eyes up to the top of your head.”
- At the count of two, do two things, “close your lids on your eyes and take a deep breath.”
- At three, exhale slowly, relax your eyes, and concentrate on a restful feeling of floating.
- Do this for about 30 seconds to a minute.
- Count backward, from three to two to one and open your eyes.
The person will notice how nice and restful they will feel.
After that exercise, get the person to move to the graduate level and go beyond just relaxation. In the following exercise, people can go into a relaxed state by imagining a movie screen. Tell the person to do two things:
1. Look at the imagined movie screen and project on it any pleasant scene you wish; this is your screen. You will feel yourself becoming more and more relaxed. The person can do this one, two, three or whatever times a day. The exercise can last 1 minute or 5.
2. Incorporate the 1, 2, 3 relaxation described earlier, allowing yourself to float into this restful state and go to your movie screen. Now, on the screen, imagine a thick line down the center, and on the left side, project your worries and anxieties and fears. The idea is to see but not experience them. Then shift to the ride side of the screen, and again, visualize any pleasant scene you wish. Again, do this for 1 minute or 5 minutes, whatever works.
You will notice that the pleasant scene on the right will overcome the anxiety scene on the left, in that pleasantness, in most instances, overcomes anxiety. For many, these techniques have proved very useful – whether the problem is anxiety or fear – or both. In my experience, these techniques are a good beginning for controlling PTSD and successfully treating it.
We are in the midst of what could be the biggest public health crisis that America has faced since the 1918 pandemic, also known as the Spanish flu. The lockdowns, quarantines, and the myriad of other disruptions can lead to alienation. In fact, it would be strange for us not to experience strong emotions under these extreme conditions. Life will get better! In the meantime, let’s encourage people to hope, pray, and use relaxation techniques and guided imagery approaches to help control anxiety, worry, stress, and issues related to PTSD. These approaches can give our minds and bodies periods of relaxation and recovery, and ultimately, they can calm our minds.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Study finds spironolactone doesn’t boost breast cancer recurrence
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
Dr. Douglas Paauw reflects on practicing in the COVID-19 world
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at [email protected].
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at [email protected].
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at [email protected].
Due to the COVID-19 pandemic, the AAN urges feds to further expand telehealth benefits
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
‘Larger-than-life’ physician Stephen Schwartz dies of COVID-19 at 78
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
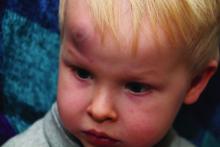
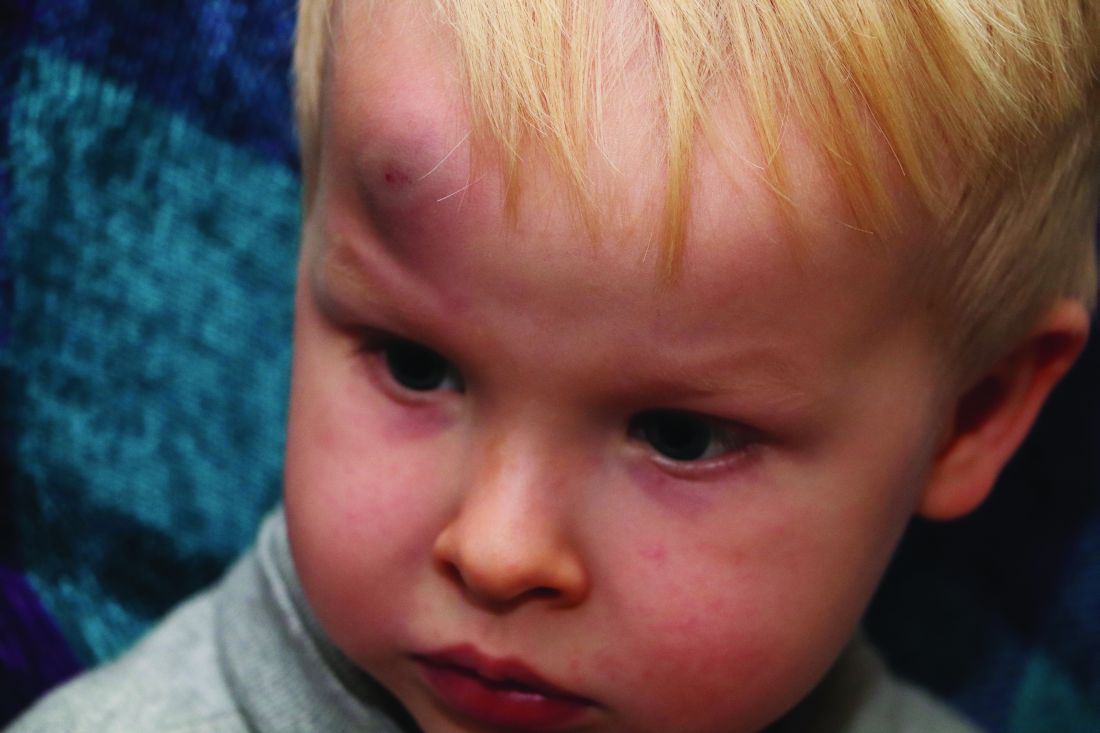
AAP adds specifics to policy on abusive head trauma
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
FROM PEDIATRICS
Week-old COVID-19 urology guidelines already outdated
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
FDA to allow alternative respiratory devices to treat COVID-19
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
Stronger links forged between RA and asthma, COPD
Asthma and chronic obstructive pulmonary disease were both linked to an increased risk of rheumatoid arthritis in a recent large, prospective cohort study, researchers have reported, which adds to a growing body of evidence that airway inflammation is implicated in the development of this disease.
RA risk was increased by about 50% among asthma patients, even when excluding those who had ever smoked, according to the study’s results, which were based on more than 200,000 women in the Nurses’ Health Study I and II.
Risk of RA nearly doubled among those with chronic obstructive pulmonary disease (COPD), with an even stronger association seen in older ever-smokers, according to authors of the study.
The findings not only strengthen the case for the potential role of obstructive lung diseases in RA development, according to the study’s authors, but also suggest that health care providers need to lower the bar for evaluation of patients with lung diseases and inflammatory joint symptoms.
“If these patients develop arthralgias, then the clinicians taking care of them should have a low threshold to consider RA, and perhaps refer, or check these patients with a diagnostic test for RA,” said researcher Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston.
What’s perhaps not as clear now is whether screening obstructive lung disease patients in the absence of early RA signs would be warranted: “I don’t know if we’re quite at the point where we would need to screen these patients if they’re not symptomatic,” Dr. Sparks said in an interview.
The study by Dr. Sparks and colleagues is, by far, not the first study to implicate asthma or other lung conditions in RA development. However, most previous studies are retrospective, and interpretation of the findings has been subject to limitations such as inadequate power to detect an increased risk or lack of adjustment for important confounding factors, such as smoking.
As such, the study by Dr. Sparks and colleagues is believed to be the first-ever prospective study to evaluate asthma and COPD as risk factors for RA, study authors reported in Arthritis & Rheumatology.
Researchers were able to identify 1,060 incident RA cases that developed in 15,148 women with asthma and 3,573 with COPD in the study with more than 4 million person-years of follow-up.
The association between asthma and increased RA risk was seen not only for the asthma population as a whole (hazard ratio, 1.53; 95% confidence interval, 1.24-1.88), but also for the subset of women who had never smoked, to a similar degree (HR, 1.53; 95% CI, 1.14-2.05), the report shows.
COPD’s association with RA risk was apparent overall (HR, 1.89; 95% CI, 1.31-2.75) and even more so in the subgroup of ever-smokers 55 years of age and older (HR, 2.20; 95% CI, 1.38-3.51), the data further show.
Findings of studies looking at the inflammation of airways and other mucosal sites are “critically important to understand” when it comes to trying to prevent RA, said Kevin Deane, MD, of the University of Colorado at Denver at Aurora.
“If we indeed are trying to prevent rheumatoid arthritis in terms of the joint disease, we may need to look at these mucosal sites in individuals who don’t yet have joint disease as potential sites to target for prevention, or at least areas to study to understand how prevention may work,” said Dr. Deane, principal investigator on the National Institutes of Health–funded Strategy for the Prevention of RA (StopRA) trial.
With that in mind, it’s conceivable targeting a lung process might prevent joint disease in a patient with asthma or airway inflammation and blood markers that indicate a risk of RA, Dr. Deane said in an interview.
Blood markers of RA have been evaluated in some recent studies, with findings that provide further evidence of a link between lung diseases and RA, and vice versa.
In particular, anti–citrullinated protein antibodies (ACPA) are clearly central to RA pathogenesis. And while asthma is increasingly linked to RA risk, there have been relatively little data on any potential links between ACPA and asthma.
That research gap led to a case-control study of the Nurses’ Health Study I and II (on which Dr. Sparks was senior author) showing that asthma was strongly linked to elevated ACPA in blood drawn from patients prior to a diagnosis of RA.
Results, published last year in Arthritis Research & Therapy, showed a significant association between asthma and pre-RA ACPA elevation (odds ratio, 3.57; 95% CI, 1.58-8.04), after adjustment for smoking and other potentially confounding factors. Investigators said the findings provided evidence that chronic mucosal airway inflammation is a factor in the development of ACPA and in the pathogenesis of RA.
In a follow-up study published more recently in Arthritis Care & Research, investigators showed that, among women in the Nurses Health Study I and II, pre-RA ACPA elevation was linked to increased risk of COPD, compared with controls (HR, 3.04; 95% CI, 1.33-7.00), while the risk for development of asthma was similar in women with or without elevated pre-RA ACPA.
That study was in part an attempt to establish a “timeline” related to antibodies, lung diseases, and RA onset, Dr. Sparks said in the interview.
“We think that probably the asthma is more important in developing the antibody, but that once you have the antibody, if you didn’t have asthma by then, you’re unlikely to develop it,” he said. “So asthma seems to be something that could happen before the antibody production, whereas COPD seems to happen after – but ACPA seems to be the common link in both of these obstructive lung diseases.”
The study in Arthritis & Rheumatology linking asthma and COPD to risk of incident RA was supported by the National Institutes of Health. Dr. Sparks reported grant support from Amgen and Bristol Myers Squibb and consulting fees from Inova and Optum. Coauthors provided disclosures related to GlaxoSmithKline, AstraZeneca, Merck, Neutrolis, and Genentech.
SOURCE: Ford JA et al. Arthritis Rheumatol. 2020 Mar 4. doi: 10.1002/art.41194.
Asthma and chronic obstructive pulmonary disease were both linked to an increased risk of rheumatoid arthritis in a recent large, prospective cohort study, researchers have reported, which adds to a growing body of evidence that airway inflammation is implicated in the development of this disease.
RA risk was increased by about 50% among asthma patients, even when excluding those who had ever smoked, according to the study’s results, which were based on more than 200,000 women in the Nurses’ Health Study I and II.
Risk of RA nearly doubled among those with chronic obstructive pulmonary disease (COPD), with an even stronger association seen in older ever-smokers, according to authors of the study.
The findings not only strengthen the case for the potential role of obstructive lung diseases in RA development, according to the study’s authors, but also suggest that health care providers need to lower the bar for evaluation of patients with lung diseases and inflammatory joint symptoms.
“If these patients develop arthralgias, then the clinicians taking care of them should have a low threshold to consider RA, and perhaps refer, or check these patients with a diagnostic test for RA,” said researcher Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston.
What’s perhaps not as clear now is whether screening obstructive lung disease patients in the absence of early RA signs would be warranted: “I don’t know if we’re quite at the point where we would need to screen these patients if they’re not symptomatic,” Dr. Sparks said in an interview.
The study by Dr. Sparks and colleagues is, by far, not the first study to implicate asthma or other lung conditions in RA development. However, most previous studies are retrospective, and interpretation of the findings has been subject to limitations such as inadequate power to detect an increased risk or lack of adjustment for important confounding factors, such as smoking.
As such, the study by Dr. Sparks and colleagues is believed to be the first-ever prospective study to evaluate asthma and COPD as risk factors for RA, study authors reported in Arthritis & Rheumatology.
Researchers were able to identify 1,060 incident RA cases that developed in 15,148 women with asthma and 3,573 with COPD in the study with more than 4 million person-years of follow-up.
The association between asthma and increased RA risk was seen not only for the asthma population as a whole (hazard ratio, 1.53; 95% confidence interval, 1.24-1.88), but also for the subset of women who had never smoked, to a similar degree (HR, 1.53; 95% CI, 1.14-2.05), the report shows.
COPD’s association with RA risk was apparent overall (HR, 1.89; 95% CI, 1.31-2.75) and even more so in the subgroup of ever-smokers 55 years of age and older (HR, 2.20; 95% CI, 1.38-3.51), the data further show.
Findings of studies looking at the inflammation of airways and other mucosal sites are “critically important to understand” when it comes to trying to prevent RA, said Kevin Deane, MD, of the University of Colorado at Denver at Aurora.
“If we indeed are trying to prevent rheumatoid arthritis in terms of the joint disease, we may need to look at these mucosal sites in individuals who don’t yet have joint disease as potential sites to target for prevention, or at least areas to study to understand how prevention may work,” said Dr. Deane, principal investigator on the National Institutes of Health–funded Strategy for the Prevention of RA (StopRA) trial.
With that in mind, it’s conceivable targeting a lung process might prevent joint disease in a patient with asthma or airway inflammation and blood markers that indicate a risk of RA, Dr. Deane said in an interview.
Blood markers of RA have been evaluated in some recent studies, with findings that provide further evidence of a link between lung diseases and RA, and vice versa.
In particular, anti–citrullinated protein antibodies (ACPA) are clearly central to RA pathogenesis. And while asthma is increasingly linked to RA risk, there have been relatively little data on any potential links between ACPA and asthma.
That research gap led to a case-control study of the Nurses’ Health Study I and II (on which Dr. Sparks was senior author) showing that asthma was strongly linked to elevated ACPA in blood drawn from patients prior to a diagnosis of RA.
Results, published last year in Arthritis Research & Therapy, showed a significant association between asthma and pre-RA ACPA elevation (odds ratio, 3.57; 95% CI, 1.58-8.04), after adjustment for smoking and other potentially confounding factors. Investigators said the findings provided evidence that chronic mucosal airway inflammation is a factor in the development of ACPA and in the pathogenesis of RA.
In a follow-up study published more recently in Arthritis Care & Research, investigators showed that, among women in the Nurses Health Study I and II, pre-RA ACPA elevation was linked to increased risk of COPD, compared with controls (HR, 3.04; 95% CI, 1.33-7.00), while the risk for development of asthma was similar in women with or without elevated pre-RA ACPA.
That study was in part an attempt to establish a “timeline” related to antibodies, lung diseases, and RA onset, Dr. Sparks said in the interview.
“We think that probably the asthma is more important in developing the antibody, but that once you have the antibody, if you didn’t have asthma by then, you’re unlikely to develop it,” he said. “So asthma seems to be something that could happen before the antibody production, whereas COPD seems to happen after – but ACPA seems to be the common link in both of these obstructive lung diseases.”
The study in Arthritis & Rheumatology linking asthma and COPD to risk of incident RA was supported by the National Institutes of Health. Dr. Sparks reported grant support from Amgen and Bristol Myers Squibb and consulting fees from Inova and Optum. Coauthors provided disclosures related to GlaxoSmithKline, AstraZeneca, Merck, Neutrolis, and Genentech.
SOURCE: Ford JA et al. Arthritis Rheumatol. 2020 Mar 4. doi: 10.1002/art.41194.
Asthma and chronic obstructive pulmonary disease were both linked to an increased risk of rheumatoid arthritis in a recent large, prospective cohort study, researchers have reported, which adds to a growing body of evidence that airway inflammation is implicated in the development of this disease.
RA risk was increased by about 50% among asthma patients, even when excluding those who had ever smoked, according to the study’s results, which were based on more than 200,000 women in the Nurses’ Health Study I and II.
Risk of RA nearly doubled among those with chronic obstructive pulmonary disease (COPD), with an even stronger association seen in older ever-smokers, according to authors of the study.
The findings not only strengthen the case for the potential role of obstructive lung diseases in RA development, according to the study’s authors, but also suggest that health care providers need to lower the bar for evaluation of patients with lung diseases and inflammatory joint symptoms.
“If these patients develop arthralgias, then the clinicians taking care of them should have a low threshold to consider RA, and perhaps refer, or check these patients with a diagnostic test for RA,” said researcher Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston.
What’s perhaps not as clear now is whether screening obstructive lung disease patients in the absence of early RA signs would be warranted: “I don’t know if we’re quite at the point where we would need to screen these patients if they’re not symptomatic,” Dr. Sparks said in an interview.
The study by Dr. Sparks and colleagues is, by far, not the first study to implicate asthma or other lung conditions in RA development. However, most previous studies are retrospective, and interpretation of the findings has been subject to limitations such as inadequate power to detect an increased risk or lack of adjustment for important confounding factors, such as smoking.
As such, the study by Dr. Sparks and colleagues is believed to be the first-ever prospective study to evaluate asthma and COPD as risk factors for RA, study authors reported in Arthritis & Rheumatology.
Researchers were able to identify 1,060 incident RA cases that developed in 15,148 women with asthma and 3,573 with COPD in the study with more than 4 million person-years of follow-up.
The association between asthma and increased RA risk was seen not only for the asthma population as a whole (hazard ratio, 1.53; 95% confidence interval, 1.24-1.88), but also for the subset of women who had never smoked, to a similar degree (HR, 1.53; 95% CI, 1.14-2.05), the report shows.
COPD’s association with RA risk was apparent overall (HR, 1.89; 95% CI, 1.31-2.75) and even more so in the subgroup of ever-smokers 55 years of age and older (HR, 2.20; 95% CI, 1.38-3.51), the data further show.
Findings of studies looking at the inflammation of airways and other mucosal sites are “critically important to understand” when it comes to trying to prevent RA, said Kevin Deane, MD, of the University of Colorado at Denver at Aurora.
“If we indeed are trying to prevent rheumatoid arthritis in terms of the joint disease, we may need to look at these mucosal sites in individuals who don’t yet have joint disease as potential sites to target for prevention, or at least areas to study to understand how prevention may work,” said Dr. Deane, principal investigator on the National Institutes of Health–funded Strategy for the Prevention of RA (StopRA) trial.
With that in mind, it’s conceivable targeting a lung process might prevent joint disease in a patient with asthma or airway inflammation and blood markers that indicate a risk of RA, Dr. Deane said in an interview.
Blood markers of RA have been evaluated in some recent studies, with findings that provide further evidence of a link between lung diseases and RA, and vice versa.
In particular, anti–citrullinated protein antibodies (ACPA) are clearly central to RA pathogenesis. And while asthma is increasingly linked to RA risk, there have been relatively little data on any potential links between ACPA and asthma.
That research gap led to a case-control study of the Nurses’ Health Study I and II (on which Dr. Sparks was senior author) showing that asthma was strongly linked to elevated ACPA in blood drawn from patients prior to a diagnosis of RA.
Results, published last year in Arthritis Research & Therapy, showed a significant association between asthma and pre-RA ACPA elevation (odds ratio, 3.57; 95% CI, 1.58-8.04), after adjustment for smoking and other potentially confounding factors. Investigators said the findings provided evidence that chronic mucosal airway inflammation is a factor in the development of ACPA and in the pathogenesis of RA.
In a follow-up study published more recently in Arthritis Care & Research, investigators showed that, among women in the Nurses Health Study I and II, pre-RA ACPA elevation was linked to increased risk of COPD, compared with controls (HR, 3.04; 95% CI, 1.33-7.00), while the risk for development of asthma was similar in women with or without elevated pre-RA ACPA.
That study was in part an attempt to establish a “timeline” related to antibodies, lung diseases, and RA onset, Dr. Sparks said in the interview.
“We think that probably the asthma is more important in developing the antibody, but that once you have the antibody, if you didn’t have asthma by then, you’re unlikely to develop it,” he said. “So asthma seems to be something that could happen before the antibody production, whereas COPD seems to happen after – but ACPA seems to be the common link in both of these obstructive lung diseases.”
The study in Arthritis & Rheumatology linking asthma and COPD to risk of incident RA was supported by the National Institutes of Health. Dr. Sparks reported grant support from Amgen and Bristol Myers Squibb and consulting fees from Inova and Optum. Coauthors provided disclosures related to GlaxoSmithKline, AstraZeneca, Merck, Neutrolis, and Genentech.
SOURCE: Ford JA et al. Arthritis Rheumatol. 2020 Mar 4. doi: 10.1002/art.41194.
FROM ARTHRITIS & RHEUMATOLOGY
Tribes Outperform Federal Government in COVID-19 Response
Several days ago, Rodney Bordeaux, president of the Rosebud Sioux Tribe in South Dakota, sent a strongly worded SOS to the directors of the World Health Organization and the Pan American Health Organization about COVID-19, saying, “We have approximately 30,000 tribal members living in south central South Dakota with access to fewer than 200 beds within our reservation.”
Not only were beds woefully inadequate to the needs of potential COVID-19 victims, but tests to find out who might need the beds also were lacking. “We believe that some kits have been sent to the states,” Bordeaux wrote, “but it is the states that have been determining who gets a test and who does not.”
In Michigan, Aaron Payment, chair of the Sault Ste. Marie Tribe of Chippewa Indians, told the Native America Calling radio show, “We’re the largest tribe east of the Mississippi, and we have two test kits.”
The “chronically underfunded” Indian Health Service (IHS) was underprepared for handling virus response, Melissa Riley, PhD, executive director of Indigenous Women Rising, charged in a March 24 opinion piece in Rewire News. “If IHS can barely keep up with broken bones and preventive care,” she wrote, “what makes our people across the country think IHS can handle the outbreak of COVID-19?”
The Centers for Disease Control and Prevention (CDC) does not break down data on cases according to race or ethnicity, but according to the IHS website, 42 people in the agency’s jurisdiction had tested positive for COVID-19 as of Mar. 24. Of those, 29 were in Navajo Country. By the evening of that day, according to Native News Online, the number of Navajos testing positive had risen to 49. Given the often-invisible spread of the virus, many more are likely to be infected.
The IHS website directs visitors to visit CDC pages for more information. However, these pages do not provide information “in a culturally literate and responsive manner,” Riley says, that explain ways to stay indoors, nor do they offer contacts for indigenous people—despite the fact, she adds, that on the West Coast they were among the first to contract the virus and to reach out with questions.
For its part, the IHS has said it “continues to work closely with our tribal partners to coordinate a comprehensive public health response to COVID-19,” holding weekly conference calls with tribal and urban Indian health organization leaders to “provide updates, answer questions, and hear concerns.” It also is in constant contact with the White House and the CDC, IHS says. IHS facilities “generally” have access to testing for individuals who may have COVID-19, the website says: However, “there are nationwide shortages of materials that may temporarily affect the availability of COVID-19 testing at a particular location.” Tribes, the website recommends, should first follow their usual process for ordering supplies. If they can’t access supplies, they should contact their IHS Area Office, which can access supplies through the IH National Supply Service Center.
Bordeaux, Payment, and Riley are not alone in their criticisms and concerns. Native Americans and Alaska Natives were hit disproportionately during the 2009 H1N1 influenza pandemic: The death rate was 4 times higher than in all other racial and ethnic groups combined. The NIH says AI/ANs are particularly vulnerable to epidemic infections, due to poverty, underlying chronic illnesses (including asthma), and delayed access to care.
Tribes began taking steps early on to protect their members, even before the federal and state governments began requiring such measures. Lummi Nation leaders, in the Pacific Northwest, for instance, began preparing when the virus first appeared in Wuhan in late 2019, according to an article in The Guardian, and declared a state of emergency on March 3—10 days before President Trump did.
The tribe has been “beefing up” emergency plans, reorganizing services, and gathering medical supplies. It also approved $1 million for emergency response, including repurposing a community fitness center into a field hospital. “We quickly recognized the need to make sacrifices for the greater good, in order to protect our people and the wider community,” Dr. Dakotah Lane, medical director of the tribal health service, said in the Guardian interview.
On March 17, the Navajo Nation shut down its 4 casinos after an Arizona tribe member was diagnosed with the virus. President Jonathan Nez says the tribe stands to lose $3 million to $5 million in revenue. But “[t]he health and well-being of our Navajo people is of utmost importance and not just profit,” Nez said in a Navajo Times interview.
In the meantime, bending to pressure from Rep. Deb Haaland (D-NM) and “a handful” of other lawmakers, according to an article in The Guardian, Congress designated $40 million for tribal health and Urban Indian Health organizations as part of the emergency federal relief legislation.
While the states received the emergency funds immediately, the CDC disburses the money to tribes, who have yet to receive any. Haaland, the first Native American woman elected to Congress, says the tribes needed the money “yesterday.”
Several days ago, Rodney Bordeaux, president of the Rosebud Sioux Tribe in South Dakota, sent a strongly worded SOS to the directors of the World Health Organization and the Pan American Health Organization about COVID-19, saying, “We have approximately 30,000 tribal members living in south central South Dakota with access to fewer than 200 beds within our reservation.”
Not only were beds woefully inadequate to the needs of potential COVID-19 victims, but tests to find out who might need the beds also were lacking. “We believe that some kits have been sent to the states,” Bordeaux wrote, “but it is the states that have been determining who gets a test and who does not.”
In Michigan, Aaron Payment, chair of the Sault Ste. Marie Tribe of Chippewa Indians, told the Native America Calling radio show, “We’re the largest tribe east of the Mississippi, and we have two test kits.”
The “chronically underfunded” Indian Health Service (IHS) was underprepared for handling virus response, Melissa Riley, PhD, executive director of Indigenous Women Rising, charged in a March 24 opinion piece in Rewire News. “If IHS can barely keep up with broken bones and preventive care,” she wrote, “what makes our people across the country think IHS can handle the outbreak of COVID-19?”
The Centers for Disease Control and Prevention (CDC) does not break down data on cases according to race or ethnicity, but according to the IHS website, 42 people in the agency’s jurisdiction had tested positive for COVID-19 as of Mar. 24. Of those, 29 were in Navajo Country. By the evening of that day, according to Native News Online, the number of Navajos testing positive had risen to 49. Given the often-invisible spread of the virus, many more are likely to be infected.
The IHS website directs visitors to visit CDC pages for more information. However, these pages do not provide information “in a culturally literate and responsive manner,” Riley says, that explain ways to stay indoors, nor do they offer contacts for indigenous people—despite the fact, she adds, that on the West Coast they were among the first to contract the virus and to reach out with questions.
For its part, the IHS has said it “continues to work closely with our tribal partners to coordinate a comprehensive public health response to COVID-19,” holding weekly conference calls with tribal and urban Indian health organization leaders to “provide updates, answer questions, and hear concerns.” It also is in constant contact with the White House and the CDC, IHS says. IHS facilities “generally” have access to testing for individuals who may have COVID-19, the website says: However, “there are nationwide shortages of materials that may temporarily affect the availability of COVID-19 testing at a particular location.” Tribes, the website recommends, should first follow their usual process for ordering supplies. If they can’t access supplies, they should contact their IHS Area Office, which can access supplies through the IH National Supply Service Center.
Bordeaux, Payment, and Riley are not alone in their criticisms and concerns. Native Americans and Alaska Natives were hit disproportionately during the 2009 H1N1 influenza pandemic: The death rate was 4 times higher than in all other racial and ethnic groups combined. The NIH says AI/ANs are particularly vulnerable to epidemic infections, due to poverty, underlying chronic illnesses (including asthma), and delayed access to care.
Tribes began taking steps early on to protect their members, even before the federal and state governments began requiring such measures. Lummi Nation leaders, in the Pacific Northwest, for instance, began preparing when the virus first appeared in Wuhan in late 2019, according to an article in The Guardian, and declared a state of emergency on March 3—10 days before President Trump did.
The tribe has been “beefing up” emergency plans, reorganizing services, and gathering medical supplies. It also approved $1 million for emergency response, including repurposing a community fitness center into a field hospital. “We quickly recognized the need to make sacrifices for the greater good, in order to protect our people and the wider community,” Dr. Dakotah Lane, medical director of the tribal health service, said in the Guardian interview.
On March 17, the Navajo Nation shut down its 4 casinos after an Arizona tribe member was diagnosed with the virus. President Jonathan Nez says the tribe stands to lose $3 million to $5 million in revenue. But “[t]he health and well-being of our Navajo people is of utmost importance and not just profit,” Nez said in a Navajo Times interview.
In the meantime, bending to pressure from Rep. Deb Haaland (D-NM) and “a handful” of other lawmakers, according to an article in The Guardian, Congress designated $40 million for tribal health and Urban Indian Health organizations as part of the emergency federal relief legislation.
While the states received the emergency funds immediately, the CDC disburses the money to tribes, who have yet to receive any. Haaland, the first Native American woman elected to Congress, says the tribes needed the money “yesterday.”
Several days ago, Rodney Bordeaux, president of the Rosebud Sioux Tribe in South Dakota, sent a strongly worded SOS to the directors of the World Health Organization and the Pan American Health Organization about COVID-19, saying, “We have approximately 30,000 tribal members living in south central South Dakota with access to fewer than 200 beds within our reservation.”
Not only were beds woefully inadequate to the needs of potential COVID-19 victims, but tests to find out who might need the beds also were lacking. “We believe that some kits have been sent to the states,” Bordeaux wrote, “but it is the states that have been determining who gets a test and who does not.”
In Michigan, Aaron Payment, chair of the Sault Ste. Marie Tribe of Chippewa Indians, told the Native America Calling radio show, “We’re the largest tribe east of the Mississippi, and we have two test kits.”
The “chronically underfunded” Indian Health Service (IHS) was underprepared for handling virus response, Melissa Riley, PhD, executive director of Indigenous Women Rising, charged in a March 24 opinion piece in Rewire News. “If IHS can barely keep up with broken bones and preventive care,” she wrote, “what makes our people across the country think IHS can handle the outbreak of COVID-19?”
The Centers for Disease Control and Prevention (CDC) does not break down data on cases according to race or ethnicity, but according to the IHS website, 42 people in the agency’s jurisdiction had tested positive for COVID-19 as of Mar. 24. Of those, 29 were in Navajo Country. By the evening of that day, according to Native News Online, the number of Navajos testing positive had risen to 49. Given the often-invisible spread of the virus, many more are likely to be infected.
The IHS website directs visitors to visit CDC pages for more information. However, these pages do not provide information “in a culturally literate and responsive manner,” Riley says, that explain ways to stay indoors, nor do they offer contacts for indigenous people—despite the fact, she adds, that on the West Coast they were among the first to contract the virus and to reach out with questions.
For its part, the IHS has said it “continues to work closely with our tribal partners to coordinate a comprehensive public health response to COVID-19,” holding weekly conference calls with tribal and urban Indian health organization leaders to “provide updates, answer questions, and hear concerns.” It also is in constant contact with the White House and the CDC, IHS says. IHS facilities “generally” have access to testing for individuals who may have COVID-19, the website says: However, “there are nationwide shortages of materials that may temporarily affect the availability of COVID-19 testing at a particular location.” Tribes, the website recommends, should first follow their usual process for ordering supplies. If they can’t access supplies, they should contact their IHS Area Office, which can access supplies through the IH National Supply Service Center.
Bordeaux, Payment, and Riley are not alone in their criticisms and concerns. Native Americans and Alaska Natives were hit disproportionately during the 2009 H1N1 influenza pandemic: The death rate was 4 times higher than in all other racial and ethnic groups combined. The NIH says AI/ANs are particularly vulnerable to epidemic infections, due to poverty, underlying chronic illnesses (including asthma), and delayed access to care.
Tribes began taking steps early on to protect their members, even before the federal and state governments began requiring such measures. Lummi Nation leaders, in the Pacific Northwest, for instance, began preparing when the virus first appeared in Wuhan in late 2019, according to an article in The Guardian, and declared a state of emergency on March 3—10 days before President Trump did.
The tribe has been “beefing up” emergency plans, reorganizing services, and gathering medical supplies. It also approved $1 million for emergency response, including repurposing a community fitness center into a field hospital. “We quickly recognized the need to make sacrifices for the greater good, in order to protect our people and the wider community,” Dr. Dakotah Lane, medical director of the tribal health service, said in the Guardian interview.
On March 17, the Navajo Nation shut down its 4 casinos after an Arizona tribe member was diagnosed with the virus. President Jonathan Nez says the tribe stands to lose $3 million to $5 million in revenue. But “[t]he health and well-being of our Navajo people is of utmost importance and not just profit,” Nez said in a Navajo Times interview.
In the meantime, bending to pressure from Rep. Deb Haaland (D-NM) and “a handful” of other lawmakers, according to an article in The Guardian, Congress designated $40 million for tribal health and Urban Indian Health organizations as part of the emergency federal relief legislation.
While the states received the emergency funds immediately, the CDC disburses the money to tribes, who have yet to receive any. Haaland, the first Native American woman elected to Congress, says the tribes needed the money “yesterday.”