User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Doctors sound off about future of medical meetings
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
The power and promise of person-generated health data (Part II)
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1
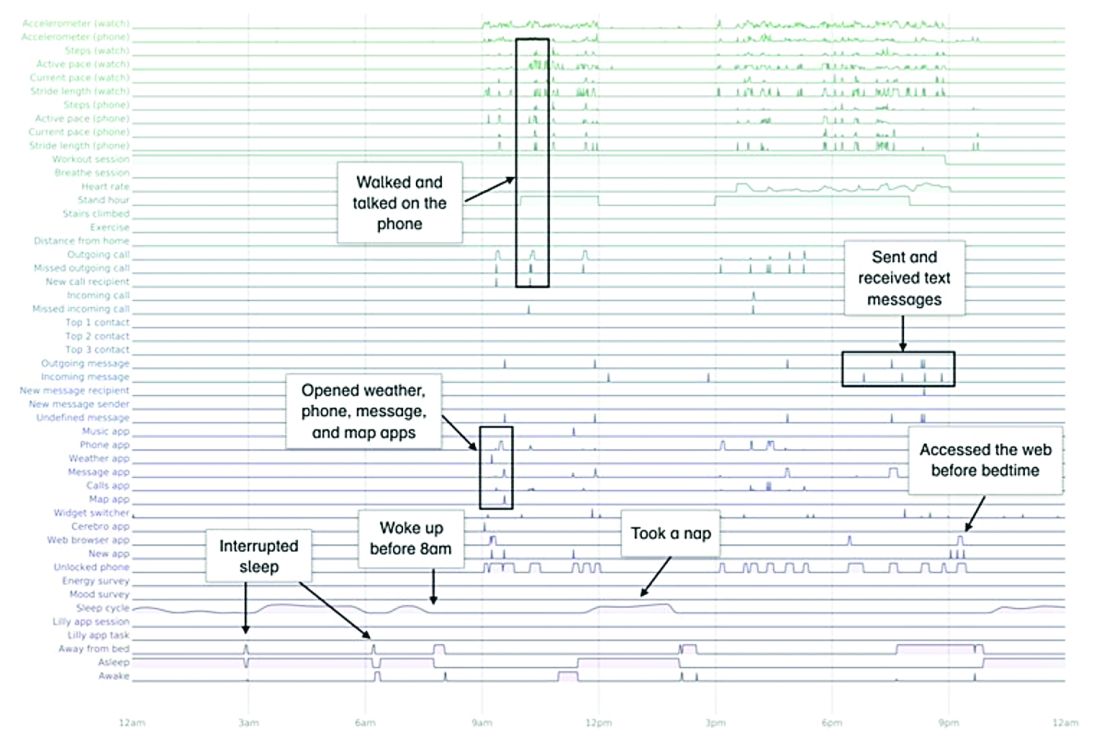
A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
Despite strict controls, some infants born to mothers with COVID-19 appear infected
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
FROM JAMA PEDIATRICS
Wuhan data link COVID-19 with myocardial damage
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
FROM JAMA CARDIOLOGY
HCV screening risk factors in pregnant women need updating
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
FROM OBSTETRICS & GYNECOLOGY
Cabazitaxel Improves Progression-Free and Overall Survival in Metastatic Prostate Cancer After Progression on Abiraterone or Enzalutamide
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
Rheumatologists seek to reassure amid hydroxychloroquine shortage
Physicians and pharmacists are reporting shortages of hydroxychloroquine and chloroquine following President Trump’s promotion of the medications as potential COVID-19 treatments, leaving patients with rheumatic diseases wondering how it will impact their access.
The American Medical Association, the American Pharmacists Association, and the American Society of Health-System Pharmacists, issued a joint statement that strongly opposed prophylactic prescribing of these medications for COVID-19 or stockpiling them in anticipation of use for COVID-19. The concerns over shortages have also prompted the American College of Rheumatology, American Academy of Dermatology, Arthritis Foundation, and Lupus Foundation of America to send a joint statement to the Trump administration and the nation’s governors highlighting critical hydroxychloroquine access issues and asking policymakers to work together with health care providers and patient communities to ensure continued availability of these drugs.
Now
In a Q and A interview, NYU Langone Health rheumatology division director and Lupus Center director Jill P. Buyon, MD, and associate professor of rheumatology, Peter M. Izmirly, MD, noted that, while shortages have been reported across the United States because of large increases in off-label prescribing, many of the drugs’ manufacturers have committed to donating millions of doses and/or stepping up production to meet demand.
Later in this article, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Langone Health, New York, answered questions about a new multicenter study called COLCORONA getting underway to test the anti-inflammatory drug colchicine. The answers in this Q&A have been edited for length and clarity.
Questions about hydroxychloroquine shortage
Q: What is the current situation with hydroxychloroquine in your practice?
A: We have been getting calls from our patients asking about getting refills for hydroxychloroquine. Our group has been calling local pharmacies asking about the availability of hydroxychloroquine, and we are compiling a list of pharmacies in New York with current availabilities to share with patients. We are somewhat limited by our electronic health record system, Epic, which can only send a prescription to one pharmacy, so that has placed some limitations on knowing where it is available. Some pharmacies have not had hydroxychloroquine available, while others have. We have also been encouraging patients to check online and look for mail-order possibilities for 90-day supplies.
Nearly all prescriptions are for generic hydroxychloroquine. Branded hydroxychloroquine (Plaquenil) is much more expensive, and we can run into obstacles with getting it approved by insurers, too.
Q: What are you telling patients who seek to refill their prescription or call with concerns? Is it feasible for patients to stop hydroxychloroquine or cut their dosage if necessary?
A: If someone’s been on hydroxychloroquine and has benefited from its use there’s no reason to come off it at this time, and given the possibility that it may have an effect on COVID-19, that is all the better. But we want to reassure patients that they can get the drug and that it is not difficult to manufacture.
Given the significantly higher risk of disease flare that was first described in lupus patients who discontinued hydroxychloroquine in the Canadian Hydroxychloroquine Study Group’s 1991 randomized, controlled trial, it is not advisable for patients to stop the drug.
Some patients do split their dosage day-to-day if they are taking less than 400 mg daily, such that someone taking 300 mg daily may take two 200-mg tablets one day and just one 200-mg tablet the next day, and so on. To avoid eye toxicity that can occur after years of taking the drug, hydroxychloroquine is generally prescribed based on weight at 5 mg/kg.
The drug also stays in the body for quite a while [often up to 3 months and even longer], so that is helpful for patients to know.
Given the current situation and the possibility of its effectiveness against COVID-19, it is ironic that we are actually trying to recruit older lupus patients who have had long-term stable disease while on hydroxychloroquine to a trial of stopping the drug to reduce the risk of developing the side effect of retinopathy. We want to see if patients can safely withdraw hydroxychloroquine without flaring, so we hope to not run into enrollment difficulties based on the current situation with COVID-19.
Q: How do you view the balance between having enough hydroxychloroquine for patients with lupus or other rheumatic diseases and its use in COVID-19 patients?
A: We want to reassure patients that hydroxychloroquine will be available, and there is no reason to hoard the drug or to worry excessively about being unable to obtain it. Efforts to increase production by Mylan, Teva, Sanofi, Novartis, and other manufacturers of hydroxychloroquine should really help out.
Q: Are there pharmacy restrictions on prescription amounts?
A: This is not universal at this time, but some institutions are cutting back and offering only 1-month supplies.
Colchicine COVID-19 trial underway
Dr. Pillinger, of NYU Langone Health, explored the COLCORONA study of colchicine as a treatment for people infected with COVID-19 and the worry that shortage concerns may arise for it, too.
Q: What is the general availability of colchicine and its susceptibility to shortage?
A: There are two major manufacturers of colchicine in the United States, Takeda and Hikma, who together manufacture the majority of the drug.
The greatest use of colchicine in the United States is for gout, which affects approximately 4 million Americans, but the drug is not used chronically, so a much smaller number of patients are using colchicine at any one time. Colchicine is also used for other inflammatory conditions, primarily calcium pyrophosphate crystal disease and familial Mediterranean fever (FMF is rare in the United States). Cardiologists also regularly prescribe colchicine in pericarditis for short-term use. Physicians may use it off label for other purposes, too.
Overall, the number of patients using colchicine is much larger than that for the use of hydroxychloroquine, for example, suggesting that the immediate risk of shortage could be lower. However, if individuals started using it off label, or prescribing inappropriately for the COVID-19 indication, the supply would rapidly run short.
Q: What other points are there to consider regarding the use of colchicine to treat COVID-19?
A: There is no evidence – zero – that colchicine has any benefit for COVID-19, not even case reports. There is some rationale that it might be beneficial, but that is exactly why the COLCORONA trial would be logical to try.
The COLCORONA trial is exactly the kind of trial that would be needed for assessing colchicine, and it is big enough and happening quickly enough to get an answer. But if people start to use colchicine off label, we may never know the truth.
While colchicine can be used safely in most people, it can be very problematic and requires an experienced doctor’s supervision. Overdoses can be fatal, and colchicine interacts with many drugs, all of which require dose adjustment and some of which must be stopped in order to use colchicine – it isn’t candy. Some of the other drugs being looked at for COVID-19 in fact may interact with colchicine.
Colchicine must also be dose adjusted for kidney disease, and, in some of the COVID-19 patients, kidney function changes rapidly. So again, its use would require expert supervision even if there were evidence for its utility.
The side effects of colchicine, if mis-dosed, can be very unpleasant, including nausea, vomiting, and diarrhea. Even at the apparent right dose, some people will get these side effects, so colchicine has to be something that works to make the risk/benefit ratio worth it.
Some preparations of colchicine are made combined with probenecid, a gout drug. This is even more problematic because probenecid can raise the level of drugs excreted by the kidney and could affect other treatments.
So in sum, what may be a good idea in theory can turn out to be a disastrous idea in practice, and here we have nothing but theory. This is not an agent to use randomly; the studies will be rushed out quickly and hopefully will give us the knowledge to know what to do.
Dr. Izmirly and Dr. Buyon said they have research grants with the National Institutes of Health to study hydroxychloroquine in patients with lupus and in anti–SSA/Ro-positive pregnant women with a previous child with congenital heart block. Dr. Pillinger reports that he has an investigator-initiated grant from Hikma to study colchicine in osteoarthritis.
This article was reformatted on 3/30/2020 for clarity.
Physicians and pharmacists are reporting shortages of hydroxychloroquine and chloroquine following President Trump’s promotion of the medications as potential COVID-19 treatments, leaving patients with rheumatic diseases wondering how it will impact their access.
The American Medical Association, the American Pharmacists Association, and the American Society of Health-System Pharmacists, issued a joint statement that strongly opposed prophylactic prescribing of these medications for COVID-19 or stockpiling them in anticipation of use for COVID-19. The concerns over shortages have also prompted the American College of Rheumatology, American Academy of Dermatology, Arthritis Foundation, and Lupus Foundation of America to send a joint statement to the Trump administration and the nation’s governors highlighting critical hydroxychloroquine access issues and asking policymakers to work together with health care providers and patient communities to ensure continued availability of these drugs.
Now
In a Q and A interview, NYU Langone Health rheumatology division director and Lupus Center director Jill P. Buyon, MD, and associate professor of rheumatology, Peter M. Izmirly, MD, noted that, while shortages have been reported across the United States because of large increases in off-label prescribing, many of the drugs’ manufacturers have committed to donating millions of doses and/or stepping up production to meet demand.
Later in this article, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Langone Health, New York, answered questions about a new multicenter study called COLCORONA getting underway to test the anti-inflammatory drug colchicine. The answers in this Q&A have been edited for length and clarity.
Questions about hydroxychloroquine shortage
Q: What is the current situation with hydroxychloroquine in your practice?
A: We have been getting calls from our patients asking about getting refills for hydroxychloroquine. Our group has been calling local pharmacies asking about the availability of hydroxychloroquine, and we are compiling a list of pharmacies in New York with current availabilities to share with patients. We are somewhat limited by our electronic health record system, Epic, which can only send a prescription to one pharmacy, so that has placed some limitations on knowing where it is available. Some pharmacies have not had hydroxychloroquine available, while others have. We have also been encouraging patients to check online and look for mail-order possibilities for 90-day supplies.
Nearly all prescriptions are for generic hydroxychloroquine. Branded hydroxychloroquine (Plaquenil) is much more expensive, and we can run into obstacles with getting it approved by insurers, too.
Q: What are you telling patients who seek to refill their prescription or call with concerns? Is it feasible for patients to stop hydroxychloroquine or cut their dosage if necessary?
A: If someone’s been on hydroxychloroquine and has benefited from its use there’s no reason to come off it at this time, and given the possibility that it may have an effect on COVID-19, that is all the better. But we want to reassure patients that they can get the drug and that it is not difficult to manufacture.
Given the significantly higher risk of disease flare that was first described in lupus patients who discontinued hydroxychloroquine in the Canadian Hydroxychloroquine Study Group’s 1991 randomized, controlled trial, it is not advisable for patients to stop the drug.
Some patients do split their dosage day-to-day if they are taking less than 400 mg daily, such that someone taking 300 mg daily may take two 200-mg tablets one day and just one 200-mg tablet the next day, and so on. To avoid eye toxicity that can occur after years of taking the drug, hydroxychloroquine is generally prescribed based on weight at 5 mg/kg.
The drug also stays in the body for quite a while [often up to 3 months and even longer], so that is helpful for patients to know.
Given the current situation and the possibility of its effectiveness against COVID-19, it is ironic that we are actually trying to recruit older lupus patients who have had long-term stable disease while on hydroxychloroquine to a trial of stopping the drug to reduce the risk of developing the side effect of retinopathy. We want to see if patients can safely withdraw hydroxychloroquine without flaring, so we hope to not run into enrollment difficulties based on the current situation with COVID-19.
Q: How do you view the balance between having enough hydroxychloroquine for patients with lupus or other rheumatic diseases and its use in COVID-19 patients?
A: We want to reassure patients that hydroxychloroquine will be available, and there is no reason to hoard the drug or to worry excessively about being unable to obtain it. Efforts to increase production by Mylan, Teva, Sanofi, Novartis, and other manufacturers of hydroxychloroquine should really help out.
Q: Are there pharmacy restrictions on prescription amounts?
A: This is not universal at this time, but some institutions are cutting back and offering only 1-month supplies.
Colchicine COVID-19 trial underway
Dr. Pillinger, of NYU Langone Health, explored the COLCORONA study of colchicine as a treatment for people infected with COVID-19 and the worry that shortage concerns may arise for it, too.
Q: What is the general availability of colchicine and its susceptibility to shortage?
A: There are two major manufacturers of colchicine in the United States, Takeda and Hikma, who together manufacture the majority of the drug.
The greatest use of colchicine in the United States is for gout, which affects approximately 4 million Americans, but the drug is not used chronically, so a much smaller number of patients are using colchicine at any one time. Colchicine is also used for other inflammatory conditions, primarily calcium pyrophosphate crystal disease and familial Mediterranean fever (FMF is rare in the United States). Cardiologists also regularly prescribe colchicine in pericarditis for short-term use. Physicians may use it off label for other purposes, too.
Overall, the number of patients using colchicine is much larger than that for the use of hydroxychloroquine, for example, suggesting that the immediate risk of shortage could be lower. However, if individuals started using it off label, or prescribing inappropriately for the COVID-19 indication, the supply would rapidly run short.
Q: What other points are there to consider regarding the use of colchicine to treat COVID-19?
A: There is no evidence – zero – that colchicine has any benefit for COVID-19, not even case reports. There is some rationale that it might be beneficial, but that is exactly why the COLCORONA trial would be logical to try.
The COLCORONA trial is exactly the kind of trial that would be needed for assessing colchicine, and it is big enough and happening quickly enough to get an answer. But if people start to use colchicine off label, we may never know the truth.
While colchicine can be used safely in most people, it can be very problematic and requires an experienced doctor’s supervision. Overdoses can be fatal, and colchicine interacts with many drugs, all of which require dose adjustment and some of which must be stopped in order to use colchicine – it isn’t candy. Some of the other drugs being looked at for COVID-19 in fact may interact with colchicine.
Colchicine must also be dose adjusted for kidney disease, and, in some of the COVID-19 patients, kidney function changes rapidly. So again, its use would require expert supervision even if there were evidence for its utility.
The side effects of colchicine, if mis-dosed, can be very unpleasant, including nausea, vomiting, and diarrhea. Even at the apparent right dose, some people will get these side effects, so colchicine has to be something that works to make the risk/benefit ratio worth it.
Some preparations of colchicine are made combined with probenecid, a gout drug. This is even more problematic because probenecid can raise the level of drugs excreted by the kidney and could affect other treatments.
So in sum, what may be a good idea in theory can turn out to be a disastrous idea in practice, and here we have nothing but theory. This is not an agent to use randomly; the studies will be rushed out quickly and hopefully will give us the knowledge to know what to do.
Dr. Izmirly and Dr. Buyon said they have research grants with the National Institutes of Health to study hydroxychloroquine in patients with lupus and in anti–SSA/Ro-positive pregnant women with a previous child with congenital heart block. Dr. Pillinger reports that he has an investigator-initiated grant from Hikma to study colchicine in osteoarthritis.
This article was reformatted on 3/30/2020 for clarity.
Physicians and pharmacists are reporting shortages of hydroxychloroquine and chloroquine following President Trump’s promotion of the medications as potential COVID-19 treatments, leaving patients with rheumatic diseases wondering how it will impact their access.
The American Medical Association, the American Pharmacists Association, and the American Society of Health-System Pharmacists, issued a joint statement that strongly opposed prophylactic prescribing of these medications for COVID-19 or stockpiling them in anticipation of use for COVID-19. The concerns over shortages have also prompted the American College of Rheumatology, American Academy of Dermatology, Arthritis Foundation, and Lupus Foundation of America to send a joint statement to the Trump administration and the nation’s governors highlighting critical hydroxychloroquine access issues and asking policymakers to work together with health care providers and patient communities to ensure continued availability of these drugs.
Now
In a Q and A interview, NYU Langone Health rheumatology division director and Lupus Center director Jill P. Buyon, MD, and associate professor of rheumatology, Peter M. Izmirly, MD, noted that, while shortages have been reported across the United States because of large increases in off-label prescribing, many of the drugs’ manufacturers have committed to donating millions of doses and/or stepping up production to meet demand.
Later in this article, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Langone Health, New York, answered questions about a new multicenter study called COLCORONA getting underway to test the anti-inflammatory drug colchicine. The answers in this Q&A have been edited for length and clarity.
Questions about hydroxychloroquine shortage
Q: What is the current situation with hydroxychloroquine in your practice?
A: We have been getting calls from our patients asking about getting refills for hydroxychloroquine. Our group has been calling local pharmacies asking about the availability of hydroxychloroquine, and we are compiling a list of pharmacies in New York with current availabilities to share with patients. We are somewhat limited by our electronic health record system, Epic, which can only send a prescription to one pharmacy, so that has placed some limitations on knowing where it is available. Some pharmacies have not had hydroxychloroquine available, while others have. We have also been encouraging patients to check online and look for mail-order possibilities for 90-day supplies.
Nearly all prescriptions are for generic hydroxychloroquine. Branded hydroxychloroquine (Plaquenil) is much more expensive, and we can run into obstacles with getting it approved by insurers, too.
Q: What are you telling patients who seek to refill their prescription or call with concerns? Is it feasible for patients to stop hydroxychloroquine or cut their dosage if necessary?
A: If someone’s been on hydroxychloroquine and has benefited from its use there’s no reason to come off it at this time, and given the possibility that it may have an effect on COVID-19, that is all the better. But we want to reassure patients that they can get the drug and that it is not difficult to manufacture.
Given the significantly higher risk of disease flare that was first described in lupus patients who discontinued hydroxychloroquine in the Canadian Hydroxychloroquine Study Group’s 1991 randomized, controlled trial, it is not advisable for patients to stop the drug.
Some patients do split their dosage day-to-day if they are taking less than 400 mg daily, such that someone taking 300 mg daily may take two 200-mg tablets one day and just one 200-mg tablet the next day, and so on. To avoid eye toxicity that can occur after years of taking the drug, hydroxychloroquine is generally prescribed based on weight at 5 mg/kg.
The drug also stays in the body for quite a while [often up to 3 months and even longer], so that is helpful for patients to know.
Given the current situation and the possibility of its effectiveness against COVID-19, it is ironic that we are actually trying to recruit older lupus patients who have had long-term stable disease while on hydroxychloroquine to a trial of stopping the drug to reduce the risk of developing the side effect of retinopathy. We want to see if patients can safely withdraw hydroxychloroquine without flaring, so we hope to not run into enrollment difficulties based on the current situation with COVID-19.
Q: How do you view the balance between having enough hydroxychloroquine for patients with lupus or other rheumatic diseases and its use in COVID-19 patients?
A: We want to reassure patients that hydroxychloroquine will be available, and there is no reason to hoard the drug or to worry excessively about being unable to obtain it. Efforts to increase production by Mylan, Teva, Sanofi, Novartis, and other manufacturers of hydroxychloroquine should really help out.
Q: Are there pharmacy restrictions on prescription amounts?
A: This is not universal at this time, but some institutions are cutting back and offering only 1-month supplies.
Colchicine COVID-19 trial underway
Dr. Pillinger, of NYU Langone Health, explored the COLCORONA study of colchicine as a treatment for people infected with COVID-19 and the worry that shortage concerns may arise for it, too.
Q: What is the general availability of colchicine and its susceptibility to shortage?
A: There are two major manufacturers of colchicine in the United States, Takeda and Hikma, who together manufacture the majority of the drug.
The greatest use of colchicine in the United States is for gout, which affects approximately 4 million Americans, but the drug is not used chronically, so a much smaller number of patients are using colchicine at any one time. Colchicine is also used for other inflammatory conditions, primarily calcium pyrophosphate crystal disease and familial Mediterranean fever (FMF is rare in the United States). Cardiologists also regularly prescribe colchicine in pericarditis for short-term use. Physicians may use it off label for other purposes, too.
Overall, the number of patients using colchicine is much larger than that for the use of hydroxychloroquine, for example, suggesting that the immediate risk of shortage could be lower. However, if individuals started using it off label, or prescribing inappropriately for the COVID-19 indication, the supply would rapidly run short.
Q: What other points are there to consider regarding the use of colchicine to treat COVID-19?
A: There is no evidence – zero – that colchicine has any benefit for COVID-19, not even case reports. There is some rationale that it might be beneficial, but that is exactly why the COLCORONA trial would be logical to try.
The COLCORONA trial is exactly the kind of trial that would be needed for assessing colchicine, and it is big enough and happening quickly enough to get an answer. But if people start to use colchicine off label, we may never know the truth.
While colchicine can be used safely in most people, it can be very problematic and requires an experienced doctor’s supervision. Overdoses can be fatal, and colchicine interacts with many drugs, all of which require dose adjustment and some of which must be stopped in order to use colchicine – it isn’t candy. Some of the other drugs being looked at for COVID-19 in fact may interact with colchicine.
Colchicine must also be dose adjusted for kidney disease, and, in some of the COVID-19 patients, kidney function changes rapidly. So again, its use would require expert supervision even if there were evidence for its utility.
The side effects of colchicine, if mis-dosed, can be very unpleasant, including nausea, vomiting, and diarrhea. Even at the apparent right dose, some people will get these side effects, so colchicine has to be something that works to make the risk/benefit ratio worth it.
Some preparations of colchicine are made combined with probenecid, a gout drug. This is even more problematic because probenecid can raise the level of drugs excreted by the kidney and could affect other treatments.
So in sum, what may be a good idea in theory can turn out to be a disastrous idea in practice, and here we have nothing but theory. This is not an agent to use randomly; the studies will be rushed out quickly and hopefully will give us the knowledge to know what to do.
Dr. Izmirly and Dr. Buyon said they have research grants with the National Institutes of Health to study hydroxychloroquine in patients with lupus and in anti–SSA/Ro-positive pregnant women with a previous child with congenital heart block. Dr. Pillinger reports that he has an investigator-initiated grant from Hikma to study colchicine in osteoarthritis.
This article was reformatted on 3/30/2020 for clarity.
HIV shortens life expectancy 9 years, healthy life expectancy 16 years
Despite highly effective antiretroviral therapy, HIV still shortens life expectancy by 9 years and healthy life expectancy free of comorbidities 16 years, according to a review of HIV patients and matched controls at Kaiser Permanente facilities in California and the mid-Atlantic states during 2000-2016.
The good news is that starting antiretroviral therapy (ART) when CD4 counts are 500 cells/mm3 or higher closes the mortality gap. People who do so can expect to live into their mid-80s, the same as people without HIV, and the years they can expect to be free of diabetes and cancer is catching up to uninfected people, although the gap for other comorbidities hasn’t changed and the overall comorbidity gap remains 16 years, according to the report, which was presented at the Conference on Retroviruses & Opportunistic Infections
“We were excited about finding no difference in lifespan for people starting ART with high CD4 counts, but we were surprised by how wide the gap was for the number of comorbidity free years. Greater attention to comorbidity prevention is needed,” said study lead Julia Marcus, PhD, an infectious disease epidemiologist and assistant professor of population medicine at Harvard Medical School, Boston.
The team estimated the average number of total and comorbidity-free years of life remaining at age 21 for 39,000 people with HIV who were matched 1:10 with 387,767 uninfected adults by sex, race/ethnicity, year, and medical center.
Overall, adults with HIV could expect to live until they were 77 years old, versus 86 years for people without HIV, during 2014-2016. It’s a large improvement over the 22 year gap during 2000-2003, when the numbers were 59 versus 81 years, respectively, Dr. Marcus reported at the virtual meeting, which was scheduled to be in Boston, but held online this year because of concerns about spreading the COVID-19 virus.
But the overall comorbidity gap didn’t budge during 2000-2016. People with HIV during 2014-2016 could expect to be comorbidity free until age 36 years, versus 52 years for the general population, the same 16-year difference during 2000-2003, when the numbers were age 32 versus age 48 years.
During 2014-2016, liver disease came 24 years sooner with HIV, and chronic kidney disease 17 years, chronic lung disease 16 years, cancer 9 years, and diabetes and cancer both 8 years sooner. Early ART didn’t narrow the gap for most comorbidities. Dr. Marcus didn’t address the reasons for the differences, except to note that “smoking rates were definitely higher among people with HIV.”
The results weren’t broken down by sex, but the majority of subjects, 88%, were men. The mean age was 41 years, and about half were white, with most of the rest either black or Hispanic. Transmission was among men who have sex with men in 70% of the cases, heterosexual sex in 20%, and IV drug accounted for the rest. Almost a third of the subjects started ART with CD4 counts at or above 500 cells/mm3.
Dr. Marcus said the results are likely generalizable to most insured people with HIV, but also that comorbidity screening might be higher in the HIV population, which could have affected the results.
The work was funded by the National Institutes of Health. Dr. Marcus is an adviser for Gilead.
SOURCE: Marcus JL et al. CROI 2020. Abstract 151.
Despite highly effective antiretroviral therapy, HIV still shortens life expectancy by 9 years and healthy life expectancy free of comorbidities 16 years, according to a review of HIV patients and matched controls at Kaiser Permanente facilities in California and the mid-Atlantic states during 2000-2016.
The good news is that starting antiretroviral therapy (ART) when CD4 counts are 500 cells/mm3 or higher closes the mortality gap. People who do so can expect to live into their mid-80s, the same as people without HIV, and the years they can expect to be free of diabetes and cancer is catching up to uninfected people, although the gap for other comorbidities hasn’t changed and the overall comorbidity gap remains 16 years, according to the report, which was presented at the Conference on Retroviruses & Opportunistic Infections
“We were excited about finding no difference in lifespan for people starting ART with high CD4 counts, but we were surprised by how wide the gap was for the number of comorbidity free years. Greater attention to comorbidity prevention is needed,” said study lead Julia Marcus, PhD, an infectious disease epidemiologist and assistant professor of population medicine at Harvard Medical School, Boston.
The team estimated the average number of total and comorbidity-free years of life remaining at age 21 for 39,000 people with HIV who were matched 1:10 with 387,767 uninfected adults by sex, race/ethnicity, year, and medical center.
Overall, adults with HIV could expect to live until they were 77 years old, versus 86 years for people without HIV, during 2014-2016. It’s a large improvement over the 22 year gap during 2000-2003, when the numbers were 59 versus 81 years, respectively, Dr. Marcus reported at the virtual meeting, which was scheduled to be in Boston, but held online this year because of concerns about spreading the COVID-19 virus.
But the overall comorbidity gap didn’t budge during 2000-2016. People with HIV during 2014-2016 could expect to be comorbidity free until age 36 years, versus 52 years for the general population, the same 16-year difference during 2000-2003, when the numbers were age 32 versus age 48 years.
During 2014-2016, liver disease came 24 years sooner with HIV, and chronic kidney disease 17 years, chronic lung disease 16 years, cancer 9 years, and diabetes and cancer both 8 years sooner. Early ART didn’t narrow the gap for most comorbidities. Dr. Marcus didn’t address the reasons for the differences, except to note that “smoking rates were definitely higher among people with HIV.”
The results weren’t broken down by sex, but the majority of subjects, 88%, were men. The mean age was 41 years, and about half were white, with most of the rest either black or Hispanic. Transmission was among men who have sex with men in 70% of the cases, heterosexual sex in 20%, and IV drug accounted for the rest. Almost a third of the subjects started ART with CD4 counts at or above 500 cells/mm3.
Dr. Marcus said the results are likely generalizable to most insured people with HIV, but also that comorbidity screening might be higher in the HIV population, which could have affected the results.
The work was funded by the National Institutes of Health. Dr. Marcus is an adviser for Gilead.
SOURCE: Marcus JL et al. CROI 2020. Abstract 151.
Despite highly effective antiretroviral therapy, HIV still shortens life expectancy by 9 years and healthy life expectancy free of comorbidities 16 years, according to a review of HIV patients and matched controls at Kaiser Permanente facilities in California and the mid-Atlantic states during 2000-2016.
The good news is that starting antiretroviral therapy (ART) when CD4 counts are 500 cells/mm3 or higher closes the mortality gap. People who do so can expect to live into their mid-80s, the same as people without HIV, and the years they can expect to be free of diabetes and cancer is catching up to uninfected people, although the gap for other comorbidities hasn’t changed and the overall comorbidity gap remains 16 years, according to the report, which was presented at the Conference on Retroviruses & Opportunistic Infections
“We were excited about finding no difference in lifespan for people starting ART with high CD4 counts, but we were surprised by how wide the gap was for the number of comorbidity free years. Greater attention to comorbidity prevention is needed,” said study lead Julia Marcus, PhD, an infectious disease epidemiologist and assistant professor of population medicine at Harvard Medical School, Boston.
The team estimated the average number of total and comorbidity-free years of life remaining at age 21 for 39,000 people with HIV who were matched 1:10 with 387,767 uninfected adults by sex, race/ethnicity, year, and medical center.
Overall, adults with HIV could expect to live until they were 77 years old, versus 86 years for people without HIV, during 2014-2016. It’s a large improvement over the 22 year gap during 2000-2003, when the numbers were 59 versus 81 years, respectively, Dr. Marcus reported at the virtual meeting, which was scheduled to be in Boston, but held online this year because of concerns about spreading the COVID-19 virus.
But the overall comorbidity gap didn’t budge during 2000-2016. People with HIV during 2014-2016 could expect to be comorbidity free until age 36 years, versus 52 years for the general population, the same 16-year difference during 2000-2003, when the numbers were age 32 versus age 48 years.
During 2014-2016, liver disease came 24 years sooner with HIV, and chronic kidney disease 17 years, chronic lung disease 16 years, cancer 9 years, and diabetes and cancer both 8 years sooner. Early ART didn’t narrow the gap for most comorbidities. Dr. Marcus didn’t address the reasons for the differences, except to note that “smoking rates were definitely higher among people with HIV.”
The results weren’t broken down by sex, but the majority of subjects, 88%, were men. The mean age was 41 years, and about half were white, with most of the rest either black or Hispanic. Transmission was among men who have sex with men in 70% of the cases, heterosexual sex in 20%, and IV drug accounted for the rest. Almost a third of the subjects started ART with CD4 counts at or above 500 cells/mm3.
Dr. Marcus said the results are likely generalizable to most insured people with HIV, but also that comorbidity screening might be higher in the HIV population, which could have affected the results.
The work was funded by the National Institutes of Health. Dr. Marcus is an adviser for Gilead.
SOURCE: Marcus JL et al. CROI 2020. Abstract 151.
FROM CROI 2020
Physicians pessimistic despite increased COVID-19 test kits
according to a survey.
One positive finding from the physicians who participated in this survey March 19-20 was that the availability of COVID-19 test kits has more than doubled since late February.
Reported access to test kits went from 31% in the first wave of a series of surveys (Jan. 31–Feb. 4), down to 20% in the second (Feb. 26-27), and then jumped to 67% by the third wave (March 19-20), InCrowd reported March 26.
Views on several other COVID-related topics were negative among the majority of responding physicians – all of whom had or were currently treating 20 or more patients with flu-like symptoms at the time of the survey.
“Their frustrations and concerns about their ability to protect themselves while meeting upcoming patient care levels has increased significantly in the last 3 months,” Daniel S. Fitzgerald, CEO and president of InCrowd, said in a written statement.
In the third wave, 78% of respondents were “concerned for the safety of loved ones due to my exposure as a physician to COVID-19” and only 16% believed that their facility was “staffed adequately to treat the influx of patients anticipated in the next 30 days,” InCrowd said.
One primary care physician from California elaborated on the issue of safety equipment: “First, [the CDC] said we need N95 masks and other masks would not protect us. As those are running out then they said just use regular surgical masks. Now they are saying use bandannas and scarves! It’s like they don’t care about the safety of the people who will be treating the ill! We don’t want to bring it home to our families!”
“Overall, morale appears low, with few optimistic about the efficacy of public-private collaboration (21%), their own safety given current PPE [personal protective equipment] supply (13%), and the U.S.’s ability to ‘flatten the curve’ (12%),” InCrowd noted in the report.
The first two waves each had 150 respondents, but the number increased to 263 for wave 3, with similar proportions – about 50% emergency medicine or critical care specialists, 25% pediatricians, and 25% primary care physicians – in all three.
according to a survey.

One positive finding from the physicians who participated in this survey March 19-20 was that the availability of COVID-19 test kits has more than doubled since late February.
Reported access to test kits went from 31% in the first wave of a series of surveys (Jan. 31–Feb. 4), down to 20% in the second (Feb. 26-27), and then jumped to 67% by the third wave (March 19-20), InCrowd reported March 26.
Views on several other COVID-related topics were negative among the majority of responding physicians – all of whom had or were currently treating 20 or more patients with flu-like symptoms at the time of the survey.
“Their frustrations and concerns about their ability to protect themselves while meeting upcoming patient care levels has increased significantly in the last 3 months,” Daniel S. Fitzgerald, CEO and president of InCrowd, said in a written statement.
In the third wave, 78% of respondents were “concerned for the safety of loved ones due to my exposure as a physician to COVID-19” and only 16% believed that their facility was “staffed adequately to treat the influx of patients anticipated in the next 30 days,” InCrowd said.
One primary care physician from California elaborated on the issue of safety equipment: “First, [the CDC] said we need N95 masks and other masks would not protect us. As those are running out then they said just use regular surgical masks. Now they are saying use bandannas and scarves! It’s like they don’t care about the safety of the people who will be treating the ill! We don’t want to bring it home to our families!”
“Overall, morale appears low, with few optimistic about the efficacy of public-private collaboration (21%), their own safety given current PPE [personal protective equipment] supply (13%), and the U.S.’s ability to ‘flatten the curve’ (12%),” InCrowd noted in the report.
The first two waves each had 150 respondents, but the number increased to 263 for wave 3, with similar proportions – about 50% emergency medicine or critical care specialists, 25% pediatricians, and 25% primary care physicians – in all three.
according to a survey.

One positive finding from the physicians who participated in this survey March 19-20 was that the availability of COVID-19 test kits has more than doubled since late February.
Reported access to test kits went from 31% in the first wave of a series of surveys (Jan. 31–Feb. 4), down to 20% in the second (Feb. 26-27), and then jumped to 67% by the third wave (March 19-20), InCrowd reported March 26.
Views on several other COVID-related topics were negative among the majority of responding physicians – all of whom had or were currently treating 20 or more patients with flu-like symptoms at the time of the survey.
“Their frustrations and concerns about their ability to protect themselves while meeting upcoming patient care levels has increased significantly in the last 3 months,” Daniel S. Fitzgerald, CEO and president of InCrowd, said in a written statement.
In the third wave, 78% of respondents were “concerned for the safety of loved ones due to my exposure as a physician to COVID-19” and only 16% believed that their facility was “staffed adequately to treat the influx of patients anticipated in the next 30 days,” InCrowd said.
One primary care physician from California elaborated on the issue of safety equipment: “First, [the CDC] said we need N95 masks and other masks would not protect us. As those are running out then they said just use regular surgical masks. Now they are saying use bandannas and scarves! It’s like they don’t care about the safety of the people who will be treating the ill! We don’t want to bring it home to our families!”
“Overall, morale appears low, with few optimistic about the efficacy of public-private collaboration (21%), their own safety given current PPE [personal protective equipment] supply (13%), and the U.S.’s ability to ‘flatten the curve’ (12%),” InCrowd noted in the report.
The first two waves each had 150 respondents, but the number increased to 263 for wave 3, with similar proportions – about 50% emergency medicine or critical care specialists, 25% pediatricians, and 25% primary care physicians – in all three.
Keep calm: Under 25s with diabetes aren't being hospitalized for COVID-19
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.