User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Baseline gene expression predicts TKI response in CML
Baseline gene expression in patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitor (TKI) therapy in the phase 3 ENESTnd trial differentiated those who achieved a good response from those with a poor response at 5 years in an exploratory analysis.
The investigators developed gene-expression models based on RNA sequencing of whole blood samples collected prior to treatment with nilotinib or imatinib in study participants who completed at least 5 years of therapy, including both good responders – those who achieved a major molecular response (MMR), defined as BCR-ABL1IS (a gene sequence found in an abnormal chromosome 22) less than 0.01% by 12 months and sustained deep molecular response (DMR) by 5 years, and poor responders – those without MMR by 12 months or with BCR-ABL1IS greater than 10% at 3 months.
A model based on the comparison of gene signatures from 47 patients who achieved a molecular response of 4.5 (MR4.5) on the International Scale (BCR-ABL1S less than 0.00032%), compared with 23 patients with a poor response, best predicted 5-year responder status (area under the receiver operating characteristic curve, 0.76), Jerald P. Radich, MD, reported during the Society of Hematologic Oncology virtual meeting.
“For this kind of work, that’s really quite good,” said Dr. Radich of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle.
Notably, the differences in patient responses observed by 12 months in ENESTnd persisted for up to 10 years, he said.
The findings have potential implications for drug development and facilitation of DMR in patients on TKI therapy – a prerequisite for attempting treatment-free remission, he said.
Dr. Radich and colleagues assessed 24 clinical factors – such as Sokal risk score, TKI therapy type, age, and sex – according to responder status, and applied penalized regression to the clinical variables, to expression of 13,575 genes, and to a combination of the clinical variables and gene expression.
Clinical variables didn’t predict response in the trial, and including the clinical variables in the gene-expression model in the exploratory analysis did not improve it’s performance (AUC, 0.75). However, both the MR4.5 plus clinical variables model and the MR4.5-only model outperformed the clinical variables–only model (AUC, 0.59), he noted, adding: “So gene expression seems to be highly correlated with response.”
Of note, 458 genes were differentially expressed; those found in responders were most often associated with immune response, whereas those in poor responders were more likely to be associated with drug catabolism, WNT signaling, and cell cycle.
This suggests that good responders, compared with poor responders, have an activated immune system that is better able to engage after TKI therapy is administered to “cull through the heard, so to speak,” Dr. Radich said.
The findings were validated in an independent dataset of 19 good responders and 25 poor responders (AUC, 0.67 for the MR4.5 vs. poor-responder model).
A comparison of the expression of immune cell marker genes in good responders and poor responders further showed that T cells – particularly CD8 T cells, B cells, natural killer cells, and aggregate cytotoxic lymphocytes were expressed at significantly higher levels in good responders.
This was true in both the ENESTnd cohort and the validation dataset, he said.
The ENESTnd study is a randomized, open-label study comparing nilotinib and imatinib in adults with newly diagnosed Philadelphia chromosome–positive chronic-phase CML. A 5-year study update published in 2016 showed that 54% and 52% of patients in nilotinib 300- and 400-mg twice-daily arms, respectively, achieved MR4.5, compared with 31% of those in an imatinib 400-mg once-daily arm. In the current exploratory analysis, the gene expression model differentiated between good and poor responders regardless of the TKI used, Dr. Radich said.
The findings are of note because achieving sustained deep molecular response is necessary before CML patients can attempt treatment-free remission and because biomarkers for predicting DMR have been lacking, he explained.
“These findings could really be used, potentially, for a couple of things: One is to predict response, and that could drive patient goals, expectations, and maybe drug choice,” he said.
The findings could also be used to inform clinical trials to investigate how to best treat poor responders to improve their response, he added.
“I think there’s a lot of work to be done and a lot things to chew over, and we’re hoping that we’ll have more to talk to you about in the future,” he said.
The study was sponsored by Novartis. Dr. Radich is a paid consultant for Genentech, Cepheid, Bristol-Myers Squibb, Takeda, and Novartis.
SOURCE: Radich JP et al. SOHO 2020, Abstract CML-109.
Baseline gene expression in patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitor (TKI) therapy in the phase 3 ENESTnd trial differentiated those who achieved a good response from those with a poor response at 5 years in an exploratory analysis.
The investigators developed gene-expression models based on RNA sequencing of whole blood samples collected prior to treatment with nilotinib or imatinib in study participants who completed at least 5 years of therapy, including both good responders – those who achieved a major molecular response (MMR), defined as BCR-ABL1IS (a gene sequence found in an abnormal chromosome 22) less than 0.01% by 12 months and sustained deep molecular response (DMR) by 5 years, and poor responders – those without MMR by 12 months or with BCR-ABL1IS greater than 10% at 3 months.
A model based on the comparison of gene signatures from 47 patients who achieved a molecular response of 4.5 (MR4.5) on the International Scale (BCR-ABL1S less than 0.00032%), compared with 23 patients with a poor response, best predicted 5-year responder status (area under the receiver operating characteristic curve, 0.76), Jerald P. Radich, MD, reported during the Society of Hematologic Oncology virtual meeting.
“For this kind of work, that’s really quite good,” said Dr. Radich of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle.
Notably, the differences in patient responses observed by 12 months in ENESTnd persisted for up to 10 years, he said.
The findings have potential implications for drug development and facilitation of DMR in patients on TKI therapy – a prerequisite for attempting treatment-free remission, he said.
Dr. Radich and colleagues assessed 24 clinical factors – such as Sokal risk score, TKI therapy type, age, and sex – according to responder status, and applied penalized regression to the clinical variables, to expression of 13,575 genes, and to a combination of the clinical variables and gene expression.
Clinical variables didn’t predict response in the trial, and including the clinical variables in the gene-expression model in the exploratory analysis did not improve it’s performance (AUC, 0.75). However, both the MR4.5 plus clinical variables model and the MR4.5-only model outperformed the clinical variables–only model (AUC, 0.59), he noted, adding: “So gene expression seems to be highly correlated with response.”
Of note, 458 genes were differentially expressed; those found in responders were most often associated with immune response, whereas those in poor responders were more likely to be associated with drug catabolism, WNT signaling, and cell cycle.
This suggests that good responders, compared with poor responders, have an activated immune system that is better able to engage after TKI therapy is administered to “cull through the heard, so to speak,” Dr. Radich said.
The findings were validated in an independent dataset of 19 good responders and 25 poor responders (AUC, 0.67 for the MR4.5 vs. poor-responder model).
A comparison of the expression of immune cell marker genes in good responders and poor responders further showed that T cells – particularly CD8 T cells, B cells, natural killer cells, and aggregate cytotoxic lymphocytes were expressed at significantly higher levels in good responders.
This was true in both the ENESTnd cohort and the validation dataset, he said.
The ENESTnd study is a randomized, open-label study comparing nilotinib and imatinib in adults with newly diagnosed Philadelphia chromosome–positive chronic-phase CML. A 5-year study update published in 2016 showed that 54% and 52% of patients in nilotinib 300- and 400-mg twice-daily arms, respectively, achieved MR4.5, compared with 31% of those in an imatinib 400-mg once-daily arm. In the current exploratory analysis, the gene expression model differentiated between good and poor responders regardless of the TKI used, Dr. Radich said.
The findings are of note because achieving sustained deep molecular response is necessary before CML patients can attempt treatment-free remission and because biomarkers for predicting DMR have been lacking, he explained.
“These findings could really be used, potentially, for a couple of things: One is to predict response, and that could drive patient goals, expectations, and maybe drug choice,” he said.
The findings could also be used to inform clinical trials to investigate how to best treat poor responders to improve their response, he added.
“I think there’s a lot of work to be done and a lot things to chew over, and we’re hoping that we’ll have more to talk to you about in the future,” he said.
The study was sponsored by Novartis. Dr. Radich is a paid consultant for Genentech, Cepheid, Bristol-Myers Squibb, Takeda, and Novartis.
SOURCE: Radich JP et al. SOHO 2020, Abstract CML-109.
Baseline gene expression in patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitor (TKI) therapy in the phase 3 ENESTnd trial differentiated those who achieved a good response from those with a poor response at 5 years in an exploratory analysis.
The investigators developed gene-expression models based on RNA sequencing of whole blood samples collected prior to treatment with nilotinib or imatinib in study participants who completed at least 5 years of therapy, including both good responders – those who achieved a major molecular response (MMR), defined as BCR-ABL1IS (a gene sequence found in an abnormal chromosome 22) less than 0.01% by 12 months and sustained deep molecular response (DMR) by 5 years, and poor responders – those without MMR by 12 months or with BCR-ABL1IS greater than 10% at 3 months.
A model based on the comparison of gene signatures from 47 patients who achieved a molecular response of 4.5 (MR4.5) on the International Scale (BCR-ABL1S less than 0.00032%), compared with 23 patients with a poor response, best predicted 5-year responder status (area under the receiver operating characteristic curve, 0.76), Jerald P. Radich, MD, reported during the Society of Hematologic Oncology virtual meeting.
“For this kind of work, that’s really quite good,” said Dr. Radich of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle.
Notably, the differences in patient responses observed by 12 months in ENESTnd persisted for up to 10 years, he said.
The findings have potential implications for drug development and facilitation of DMR in patients on TKI therapy – a prerequisite for attempting treatment-free remission, he said.
Dr. Radich and colleagues assessed 24 clinical factors – such as Sokal risk score, TKI therapy type, age, and sex – according to responder status, and applied penalized regression to the clinical variables, to expression of 13,575 genes, and to a combination of the clinical variables and gene expression.
Clinical variables didn’t predict response in the trial, and including the clinical variables in the gene-expression model in the exploratory analysis did not improve it’s performance (AUC, 0.75). However, both the MR4.5 plus clinical variables model and the MR4.5-only model outperformed the clinical variables–only model (AUC, 0.59), he noted, adding: “So gene expression seems to be highly correlated with response.”
Of note, 458 genes were differentially expressed; those found in responders were most often associated with immune response, whereas those in poor responders were more likely to be associated with drug catabolism, WNT signaling, and cell cycle.
This suggests that good responders, compared with poor responders, have an activated immune system that is better able to engage after TKI therapy is administered to “cull through the heard, so to speak,” Dr. Radich said.
The findings were validated in an independent dataset of 19 good responders and 25 poor responders (AUC, 0.67 for the MR4.5 vs. poor-responder model).
A comparison of the expression of immune cell marker genes in good responders and poor responders further showed that T cells – particularly CD8 T cells, B cells, natural killer cells, and aggregate cytotoxic lymphocytes were expressed at significantly higher levels in good responders.
This was true in both the ENESTnd cohort and the validation dataset, he said.
The ENESTnd study is a randomized, open-label study comparing nilotinib and imatinib in adults with newly diagnosed Philadelphia chromosome–positive chronic-phase CML. A 5-year study update published in 2016 showed that 54% and 52% of patients in nilotinib 300- and 400-mg twice-daily arms, respectively, achieved MR4.5, compared with 31% of those in an imatinib 400-mg once-daily arm. In the current exploratory analysis, the gene expression model differentiated between good and poor responders regardless of the TKI used, Dr. Radich said.
The findings are of note because achieving sustained deep molecular response is necessary before CML patients can attempt treatment-free remission and because biomarkers for predicting DMR have been lacking, he explained.
“These findings could really be used, potentially, for a couple of things: One is to predict response, and that could drive patient goals, expectations, and maybe drug choice,” he said.
The findings could also be used to inform clinical trials to investigate how to best treat poor responders to improve their response, he added.
“I think there’s a lot of work to be done and a lot things to chew over, and we’re hoping that we’ll have more to talk to you about in the future,” he said.
The study was sponsored by Novartis. Dr. Radich is a paid consultant for Genentech, Cepheid, Bristol-Myers Squibb, Takeda, and Novartis.
SOURCE: Radich JP et al. SOHO 2020, Abstract CML-109.
FROM SOHO 2020
COVID-19 and Blood Clots: Inside the Battle to Save Patients
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
Tough to tell COVID from smoke inhalation symptoms — And flu season’s coming
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
COVID-19 outcomes no worse in patients on TNF inhibitors or methotrexate
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
For lower-risk MDS, treat ‘what bugs patients most’
Clinicians who treat patients with lower-risk myelodysplastic syndrome should focus on “what bugs patients most,” with therapeutic goals reflecting and respecting the patients’ goals, a specialist in MDS recommended.
“There’s an uncomfortable truth in treating lower-risk MDS: No treatment that we have has ever been demonstrated in a prospective trial to prolong survival in lower-risk MDS, so in the end, what we’re doing is trying to improve transfusion needs and to improve quality of life,” said Michael A. Sekeres, MD, MS, from the Cleveland Clinic.
Dr. Sekeres described optimal therapy for patients with lower-risk MDS in an online presentation during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
He acknowledged that the definition of MDS as “a heterogeneous clonal hematopoietic disorder derived from an abnormal multipotent progenitor cell, characterized by a hyperproliferative bone marrow, dysplasia of the cellular elements, and ineffective hematopoiesis” can be confusing even for hematologists well versed in the disorder.
An easier-to-grasp explanation, he said, is that “MDS is considered a cancer, and like other cancers it has a clonal origin, involves the abnormal growth of cells that exceeds the growth of other cells around them and don’t know when to stop growing, and it takes over normal tissue, so that the normal tissues – in this case the hematopoietic precursors in the bone marrow – don’t function normally, resulting in cytopenias.”
‘Mild displeasure syndrome’
Approximately 95% of patients with MDS have a discrete genetic abnormality, but only one driver mutation, in the gene SF3B1, is considered to be a lower-risk abnormality, with a more favorable prognosis.
Treatment options for patients with lower-risk MDS, defined as an International Prognostic Scoring System score of 1 or less, or a Revised IPSS score of 3.5 or less, will depend on the patients’ transfusion needs and quality of life.
Patients with no transfusion requirements and a generally good quality of life may be followed by observation alone, with blood counts every 1 to 6 months depending on clinical presentation.
“We have some folks coming in who really don’t have very bad blood counts and have a good quality of life,” Dr. Sekeres said. “Those folks we would consider to have a very good risk type of MDS, which one of my patients referred to once as ‘mild displeasure syndrome.’ It was a displeasure to him to have to fight the traffic to come into Cleveland to see me every month, or 2 months, or 6 months, but beyond that we didn’t have to treat his MDS.”
Isolated cytopenias
Patients with isolated anemia, with hemoglobin less than 10 g/dL and/or transfusion dependence, and who are symptomatic should be started on an erythopoiesis-stimulating agent (ESA), either recombinant humanized erythropoietin or darbepoetin, or the erythroid-maturing agent luspatercept (Reblozyl).
The probability of a response to ESAs in this populations ranges from about 15% to 35%, with patients who have low baseline serum erythropoietin and no or few transfusions most likely to respond.
“On the other hand, patients who come into our clinic who are already dependent on red blood cell transfusions and have a sky-high [erythropoietin] level in the hundreds or even thousands have a very low likelihood of responding to exogenously administered ESAs,” he said.
Patients with no response to ESAs or luspatercept or a loss of response suggestive of disease progression should undergo repeat bone marrow biopsy. Patients who develop deletion 5q should be started on lenalidomide (Revlimid). In these patients, next-generation sequencing may also reveal targetable abnormalities.
For patients with isolated thrombocytopenia, thrombopoietin agonists such as romiplostim or eltrombopag may help to reduce platelet transfusion requirements and clinically significant bleeding events, but these agents come with a very important caveat: in addition to promoting platelet production, thrombopoietin receptor agonists can promote the growth of blasts, which could in turn promote the transformation of MDS to acute myeloid leukemia.
“This is an off-label use of romiplostim for the treatment of MDS with thrombocytopenia, and this drug should never, never, never be given to a patient who has excess blasts at baseline MDS; the same is true of its cousin eltrombopag.” Dr. Sekeres said.
Multlineage dysplasia
Patients with multilineage dysplasia can have good responses to hypomethylating agents, either azacitidine 75 mg/m2 IV or subcutaneously for 3 days every 4 weeks, or decitabine 20 mg/m2 IV for 3 days every 4 weeks.
“Another approach to treating patients with multilineage dysplasia is to consider the use of antithymocyte globulin; in other words, treat these patients as if they have aplastic anemia, because there are some types of MDS in which immune-mediated destruction of bone marrow plays a role,” Dr. Sekeres said.
“This is particularly appealing in patients who have a hyperplastic marrow, or those who have other autoimmune conditions that are going on that may indicate a broader autoimmune process that’s involved in the bone marrow,” he added.
Patients treated with antithymocyte globulin require hospitalization with discharge on steroids for 1 month to prevent serum sickness in response to the treatment, and maintenance on low-dose cyclosporine.
“In MDS, unfortunately, our understanding of the biology of the disease far exceeds what we can do about it, but we’re starting to catch up,” Dr. Sekeres said.
No funding source for the presentation was disclosed. Dr. Sekeres disclosed serving on advisory boards for Celegene/Bristol-Myers Squibb, Takeda/Millenium, and Pfizer.
Clinicians who treat patients with lower-risk myelodysplastic syndrome should focus on “what bugs patients most,” with therapeutic goals reflecting and respecting the patients’ goals, a specialist in MDS recommended.
“There’s an uncomfortable truth in treating lower-risk MDS: No treatment that we have has ever been demonstrated in a prospective trial to prolong survival in lower-risk MDS, so in the end, what we’re doing is trying to improve transfusion needs and to improve quality of life,” said Michael A. Sekeres, MD, MS, from the Cleveland Clinic.
Dr. Sekeres described optimal therapy for patients with lower-risk MDS in an online presentation during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
He acknowledged that the definition of MDS as “a heterogeneous clonal hematopoietic disorder derived from an abnormal multipotent progenitor cell, characterized by a hyperproliferative bone marrow, dysplasia of the cellular elements, and ineffective hematopoiesis” can be confusing even for hematologists well versed in the disorder.
An easier-to-grasp explanation, he said, is that “MDS is considered a cancer, and like other cancers it has a clonal origin, involves the abnormal growth of cells that exceeds the growth of other cells around them and don’t know when to stop growing, and it takes over normal tissue, so that the normal tissues – in this case the hematopoietic precursors in the bone marrow – don’t function normally, resulting in cytopenias.”
‘Mild displeasure syndrome’
Approximately 95% of patients with MDS have a discrete genetic abnormality, but only one driver mutation, in the gene SF3B1, is considered to be a lower-risk abnormality, with a more favorable prognosis.
Treatment options for patients with lower-risk MDS, defined as an International Prognostic Scoring System score of 1 or less, or a Revised IPSS score of 3.5 or less, will depend on the patients’ transfusion needs and quality of life.
Patients with no transfusion requirements and a generally good quality of life may be followed by observation alone, with blood counts every 1 to 6 months depending on clinical presentation.
“We have some folks coming in who really don’t have very bad blood counts and have a good quality of life,” Dr. Sekeres said. “Those folks we would consider to have a very good risk type of MDS, which one of my patients referred to once as ‘mild displeasure syndrome.’ It was a displeasure to him to have to fight the traffic to come into Cleveland to see me every month, or 2 months, or 6 months, but beyond that we didn’t have to treat his MDS.”
Isolated cytopenias
Patients with isolated anemia, with hemoglobin less than 10 g/dL and/or transfusion dependence, and who are symptomatic should be started on an erythopoiesis-stimulating agent (ESA), either recombinant humanized erythropoietin or darbepoetin, or the erythroid-maturing agent luspatercept (Reblozyl).
The probability of a response to ESAs in this populations ranges from about 15% to 35%, with patients who have low baseline serum erythropoietin and no or few transfusions most likely to respond.
“On the other hand, patients who come into our clinic who are already dependent on red blood cell transfusions and have a sky-high [erythropoietin] level in the hundreds or even thousands have a very low likelihood of responding to exogenously administered ESAs,” he said.
Patients with no response to ESAs or luspatercept or a loss of response suggestive of disease progression should undergo repeat bone marrow biopsy. Patients who develop deletion 5q should be started on lenalidomide (Revlimid). In these patients, next-generation sequencing may also reveal targetable abnormalities.
For patients with isolated thrombocytopenia, thrombopoietin agonists such as romiplostim or eltrombopag may help to reduce platelet transfusion requirements and clinically significant bleeding events, but these agents come with a very important caveat: in addition to promoting platelet production, thrombopoietin receptor agonists can promote the growth of blasts, which could in turn promote the transformation of MDS to acute myeloid leukemia.
“This is an off-label use of romiplostim for the treatment of MDS with thrombocytopenia, and this drug should never, never, never be given to a patient who has excess blasts at baseline MDS; the same is true of its cousin eltrombopag.” Dr. Sekeres said.
Multlineage dysplasia
Patients with multilineage dysplasia can have good responses to hypomethylating agents, either azacitidine 75 mg/m2 IV or subcutaneously for 3 days every 4 weeks, or decitabine 20 mg/m2 IV for 3 days every 4 weeks.
“Another approach to treating patients with multilineage dysplasia is to consider the use of antithymocyte globulin; in other words, treat these patients as if they have aplastic anemia, because there are some types of MDS in which immune-mediated destruction of bone marrow plays a role,” Dr. Sekeres said.
“This is particularly appealing in patients who have a hyperplastic marrow, or those who have other autoimmune conditions that are going on that may indicate a broader autoimmune process that’s involved in the bone marrow,” he added.
Patients treated with antithymocyte globulin require hospitalization with discharge on steroids for 1 month to prevent serum sickness in response to the treatment, and maintenance on low-dose cyclosporine.
“In MDS, unfortunately, our understanding of the biology of the disease far exceeds what we can do about it, but we’re starting to catch up,” Dr. Sekeres said.
No funding source for the presentation was disclosed. Dr. Sekeres disclosed serving on advisory boards for Celegene/Bristol-Myers Squibb, Takeda/Millenium, and Pfizer.
Clinicians who treat patients with lower-risk myelodysplastic syndrome should focus on “what bugs patients most,” with therapeutic goals reflecting and respecting the patients’ goals, a specialist in MDS recommended.
“There’s an uncomfortable truth in treating lower-risk MDS: No treatment that we have has ever been demonstrated in a prospective trial to prolong survival in lower-risk MDS, so in the end, what we’re doing is trying to improve transfusion needs and to improve quality of life,” said Michael A. Sekeres, MD, MS, from the Cleveland Clinic.
Dr. Sekeres described optimal therapy for patients with lower-risk MDS in an online presentation during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
He acknowledged that the definition of MDS as “a heterogeneous clonal hematopoietic disorder derived from an abnormal multipotent progenitor cell, characterized by a hyperproliferative bone marrow, dysplasia of the cellular elements, and ineffective hematopoiesis” can be confusing even for hematologists well versed in the disorder.
An easier-to-grasp explanation, he said, is that “MDS is considered a cancer, and like other cancers it has a clonal origin, involves the abnormal growth of cells that exceeds the growth of other cells around them and don’t know when to stop growing, and it takes over normal tissue, so that the normal tissues – in this case the hematopoietic precursors in the bone marrow – don’t function normally, resulting in cytopenias.”
‘Mild displeasure syndrome’
Approximately 95% of patients with MDS have a discrete genetic abnormality, but only one driver mutation, in the gene SF3B1, is considered to be a lower-risk abnormality, with a more favorable prognosis.
Treatment options for patients with lower-risk MDS, defined as an International Prognostic Scoring System score of 1 or less, or a Revised IPSS score of 3.5 or less, will depend on the patients’ transfusion needs and quality of life.
Patients with no transfusion requirements and a generally good quality of life may be followed by observation alone, with blood counts every 1 to 6 months depending on clinical presentation.
“We have some folks coming in who really don’t have very bad blood counts and have a good quality of life,” Dr. Sekeres said. “Those folks we would consider to have a very good risk type of MDS, which one of my patients referred to once as ‘mild displeasure syndrome.’ It was a displeasure to him to have to fight the traffic to come into Cleveland to see me every month, or 2 months, or 6 months, but beyond that we didn’t have to treat his MDS.”
Isolated cytopenias
Patients with isolated anemia, with hemoglobin less than 10 g/dL and/or transfusion dependence, and who are symptomatic should be started on an erythopoiesis-stimulating agent (ESA), either recombinant humanized erythropoietin or darbepoetin, or the erythroid-maturing agent luspatercept (Reblozyl).
The probability of a response to ESAs in this populations ranges from about 15% to 35%, with patients who have low baseline serum erythropoietin and no or few transfusions most likely to respond.
“On the other hand, patients who come into our clinic who are already dependent on red blood cell transfusions and have a sky-high [erythropoietin] level in the hundreds or even thousands have a very low likelihood of responding to exogenously administered ESAs,” he said.
Patients with no response to ESAs or luspatercept or a loss of response suggestive of disease progression should undergo repeat bone marrow biopsy. Patients who develop deletion 5q should be started on lenalidomide (Revlimid). In these patients, next-generation sequencing may also reveal targetable abnormalities.
For patients with isolated thrombocytopenia, thrombopoietin agonists such as romiplostim or eltrombopag may help to reduce platelet transfusion requirements and clinically significant bleeding events, but these agents come with a very important caveat: in addition to promoting platelet production, thrombopoietin receptor agonists can promote the growth of blasts, which could in turn promote the transformation of MDS to acute myeloid leukemia.
“This is an off-label use of romiplostim for the treatment of MDS with thrombocytopenia, and this drug should never, never, never be given to a patient who has excess blasts at baseline MDS; the same is true of its cousin eltrombopag.” Dr. Sekeres said.
Multlineage dysplasia
Patients with multilineage dysplasia can have good responses to hypomethylating agents, either azacitidine 75 mg/m2 IV or subcutaneously for 3 days every 4 weeks, or decitabine 20 mg/m2 IV for 3 days every 4 weeks.
“Another approach to treating patients with multilineage dysplasia is to consider the use of antithymocyte globulin; in other words, treat these patients as if they have aplastic anemia, because there are some types of MDS in which immune-mediated destruction of bone marrow plays a role,” Dr. Sekeres said.
“This is particularly appealing in patients who have a hyperplastic marrow, or those who have other autoimmune conditions that are going on that may indicate a broader autoimmune process that’s involved in the bone marrow,” he added.
Patients treated with antithymocyte globulin require hospitalization with discharge on steroids for 1 month to prevent serum sickness in response to the treatment, and maintenance on low-dose cyclosporine.
“In MDS, unfortunately, our understanding of the biology of the disease far exceeds what we can do about it, but we’re starting to catch up,” Dr. Sekeres said.
No funding source for the presentation was disclosed. Dr. Sekeres disclosed serving on advisory boards for Celegene/Bristol-Myers Squibb, Takeda/Millenium, and Pfizer.
FROM ASH HEMATOLOGIC MALIGNANCIES 2020
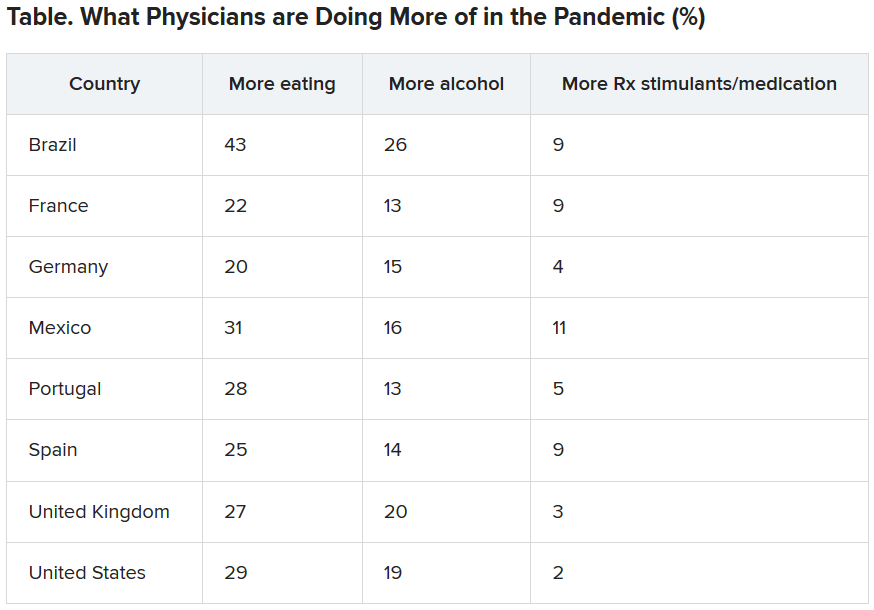
Physician income drops, burnout spikes globally in pandemic
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
The march of immunotherapy continues at ESMO 2020
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FROM ESMO 2020
Reworked OxyContin fails to cut overall opioid abuse, FDA panel says
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
Mega vitamin D harms bone in women, not men, without osteoporosis
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.
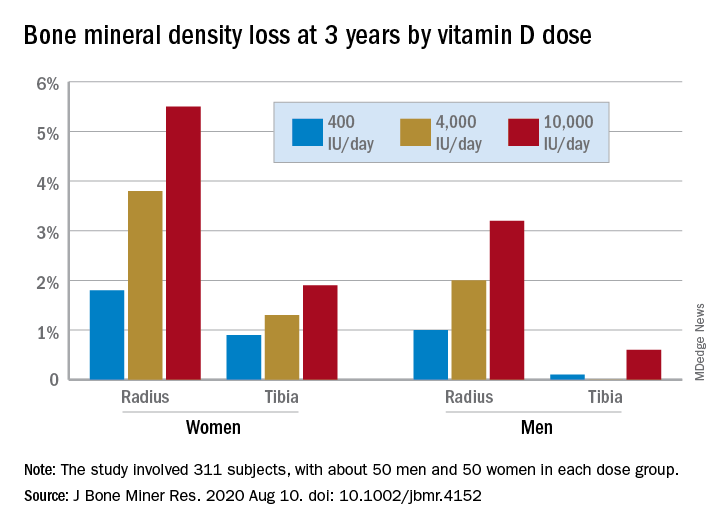
In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.

In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.

In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
Infectious COVID-19 can persist in gut for weeks
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.