User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
ACC expert consensus on post-TAVR arrhythmias
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
Lilly stops antibody trial in hospitalized COVID-19 patients, other trials continue
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
AACE issues ‘cookbook’ algorithm to manage dyslipidemia
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).
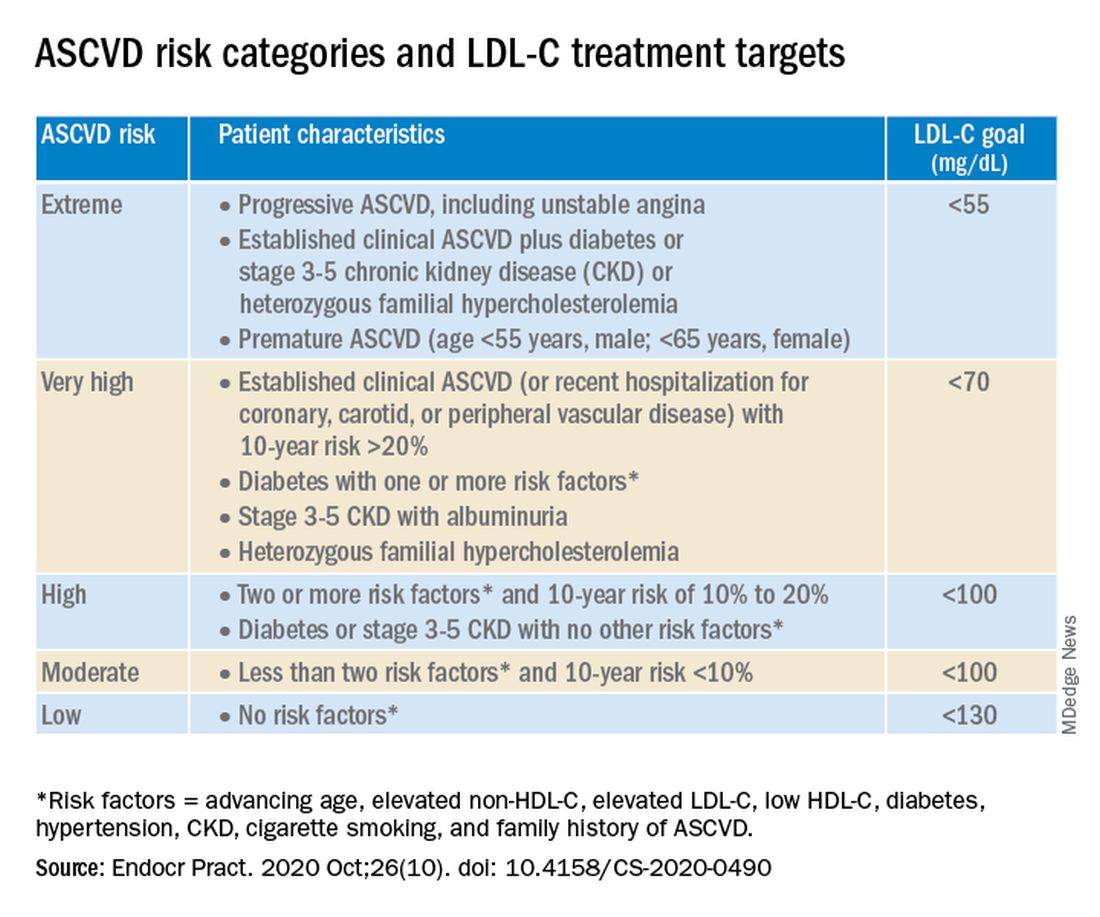
“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).

“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).

“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
ODC1 gene linked to newly described neurodevelopmental disorder
but it may be treated with diet modifications and available therapies, according to the researcher whose group first identified the disorder.
Lance Rodan, MD, of Boston Children’s Hospital and Harvard Medical School, reported on research into ODC1 gain-of-function disorder –named for ornithine decarboxylase 1, the rate-limiting enzyme involved in polyamine synthesis – in the Linda De Meirleir Neurometabolic award lecture at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Dr. Rodan and colleagues first described ODC1 disorder in a multicenter case series.
Dr. Rodan noted that dysregulated polyamine levels are associated with cancer, and that ODC1 is expressed “ubiquitously” throughout the body.
Pathophysiology and phenotypes
In an interview, he described the metabolic process more fully. “GI flora can produce putrescine, which is the polyamine that accumulates in excess in the ODC1 gain-of-function disorder. It is yet to be elucidated if decreasing putrescine production by GI flora and/or reducing dietary sources of putrescine may play a role in the management of this disorder.”
In the De Meirleir lecture, Dr. Rodan described four patients from his group’s published case series, all found to have heterozygous de novo variants in the ODC1 gene, along with a fifth patient reported by Caleb Bupp, MD, and colleagues at Michigan State University, East Lansing.
“There’s a recognizable phenotype to this disorder,” Dr. Rodan said. “These individuals have neurodevelopment abnormalities. They may have behavioral concerns. They have low-tone central hypertonia and macrocephaly.”
One of the most distinctive characteristics of ODC1 disorder is alopecia, he said, “which in almost everybody with this condition involves the eyebrows and eyelashes and in some individuals also involves the scalp hair.”
These patients also have what Dr. Rodan called “a common yet subtle facial gestalt.” That can include hypertelorism, spareness of the eyebrows and eyelashes, and a tubular- shaped nose with a short columella and a short philtrum.
They may also have abnormalities of the nails and cryptorchidism, and typically a prenatal history of polyhydramnios, he said.
MRI findings include prominent perivascular spaces, periventricular cysts, abnormal white matter and corpus callosum abnormalities, he said, adding that the fetal case MRI demonstrated subepidermal cysts, white matter cysts in the temporal pole, deficiency of the falx cerebri and abnormal white-matter signals.
Biochemical features of ODC1 disorder include increased N-acetylputrescine levels with normal spermine and spermidine levels, Dr. Rodan said. He also noted that Dr. Bupp’s group reported increased putrescine in fibroblasts and increased ODC1 protein levels in red blood cells.
Dr. Rodan also described possible molecular mechanisms in ODC1 disorder. One was the location of the ODC1 variants: all were reported closely located to truncating variants in the final exon of the ODC1 gene. This allows truncating proteins to survive, adding to the degradation that results in a net gain-of-function of ODC1 enzyme activity.
With regard to pathophysiology of ODC1 disorder, Dr. Rodan noted that research has implicated chronically elevated putrescine levels in the alopecia, a finding animal models support. “Since putrescine is a precursor for gamma-aminobutyric acid, it’s possible perturbed GABA levels may also be involved,” he said. Abnormal modulation N-methyl-D-aspirate receptors may also be involved, he said.
Another hypothesis purports that potential of elevated levels of toxic aldehydes/H2O2 similar to Snyder-Robinson syndrome, the better known polyamine-related neurometabolic disorder. “Along those lines, maybe there’s also a secondary mitochondrial or lysosomal dysfunction, but this is something that’s still being actively studied,” Dr. Rodan said.
Treatment
Because ODC1 disorder was only first described 2 years ago, research into treatment is nascent. “In terms of management, I think one of the more fundamental questions is whether this is more of a static developmental disorder or whether this actually represents a progressive degenerative disorder,” Dr. Rodan said.
One potential treatment that has been explored, he said, is difluoromethylornithine, a synthetic ODC1 inhibitor already Food and Drug Administration approved for African sleeping sickness and as a topical treatment for hirsutism. It is also the subject of ongoing clinical trials in colon cancer and neuroblastoma. Potential side effects include myelosuppression, seizures and hearing loss.
Dr. Rodan noted that a single-center study reported that difluoromethylornithine in a 3-year-old patient with ODC1 disorder reduced ODC protein activity and putrescine to control levels.
Other potential treatments include the natural ODC1 inhibitors agmatine and turmeric/curcumin, flagyl/rifaximin to decrease putrescine production in the gut, a low-dairy diet to lower putrescine levels, and antioxidants. “There could be a role for antioxidant stress similar to what is seen in Snyder-Robinson syndrome,” Dr. Rodan said.
Based on mouse studies, patients with ODC1 may be at risk of skin cancer, so regular skin checks along with sun protection should be part of management, he said. “This also raises the question of whether there should be surveillance for other types of cancer given the role of polyamine in various types of tumors.”
Dr. Rodan has no relevant financial relationships to disclose.
but it may be treated with diet modifications and available therapies, according to the researcher whose group first identified the disorder.
Lance Rodan, MD, of Boston Children’s Hospital and Harvard Medical School, reported on research into ODC1 gain-of-function disorder –named for ornithine decarboxylase 1, the rate-limiting enzyme involved in polyamine synthesis – in the Linda De Meirleir Neurometabolic award lecture at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Dr. Rodan and colleagues first described ODC1 disorder in a multicenter case series.
Dr. Rodan noted that dysregulated polyamine levels are associated with cancer, and that ODC1 is expressed “ubiquitously” throughout the body.
Pathophysiology and phenotypes
In an interview, he described the metabolic process more fully. “GI flora can produce putrescine, which is the polyamine that accumulates in excess in the ODC1 gain-of-function disorder. It is yet to be elucidated if decreasing putrescine production by GI flora and/or reducing dietary sources of putrescine may play a role in the management of this disorder.”
In the De Meirleir lecture, Dr. Rodan described four patients from his group’s published case series, all found to have heterozygous de novo variants in the ODC1 gene, along with a fifth patient reported by Caleb Bupp, MD, and colleagues at Michigan State University, East Lansing.
“There’s a recognizable phenotype to this disorder,” Dr. Rodan said. “These individuals have neurodevelopment abnormalities. They may have behavioral concerns. They have low-tone central hypertonia and macrocephaly.”
One of the most distinctive characteristics of ODC1 disorder is alopecia, he said, “which in almost everybody with this condition involves the eyebrows and eyelashes and in some individuals also involves the scalp hair.”
These patients also have what Dr. Rodan called “a common yet subtle facial gestalt.” That can include hypertelorism, spareness of the eyebrows and eyelashes, and a tubular- shaped nose with a short columella and a short philtrum.
They may also have abnormalities of the nails and cryptorchidism, and typically a prenatal history of polyhydramnios, he said.
MRI findings include prominent perivascular spaces, periventricular cysts, abnormal white matter and corpus callosum abnormalities, he said, adding that the fetal case MRI demonstrated subepidermal cysts, white matter cysts in the temporal pole, deficiency of the falx cerebri and abnormal white-matter signals.
Biochemical features of ODC1 disorder include increased N-acetylputrescine levels with normal spermine and spermidine levels, Dr. Rodan said. He also noted that Dr. Bupp’s group reported increased putrescine in fibroblasts and increased ODC1 protein levels in red blood cells.
Dr. Rodan also described possible molecular mechanisms in ODC1 disorder. One was the location of the ODC1 variants: all were reported closely located to truncating variants in the final exon of the ODC1 gene. This allows truncating proteins to survive, adding to the degradation that results in a net gain-of-function of ODC1 enzyme activity.
With regard to pathophysiology of ODC1 disorder, Dr. Rodan noted that research has implicated chronically elevated putrescine levels in the alopecia, a finding animal models support. “Since putrescine is a precursor for gamma-aminobutyric acid, it’s possible perturbed GABA levels may also be involved,” he said. Abnormal modulation N-methyl-D-aspirate receptors may also be involved, he said.
Another hypothesis purports that potential of elevated levels of toxic aldehydes/H2O2 similar to Snyder-Robinson syndrome, the better known polyamine-related neurometabolic disorder. “Along those lines, maybe there’s also a secondary mitochondrial or lysosomal dysfunction, but this is something that’s still being actively studied,” Dr. Rodan said.
Treatment
Because ODC1 disorder was only first described 2 years ago, research into treatment is nascent. “In terms of management, I think one of the more fundamental questions is whether this is more of a static developmental disorder or whether this actually represents a progressive degenerative disorder,” Dr. Rodan said.
One potential treatment that has been explored, he said, is difluoromethylornithine, a synthetic ODC1 inhibitor already Food and Drug Administration approved for African sleeping sickness and as a topical treatment for hirsutism. It is also the subject of ongoing clinical trials in colon cancer and neuroblastoma. Potential side effects include myelosuppression, seizures and hearing loss.
Dr. Rodan noted that a single-center study reported that difluoromethylornithine in a 3-year-old patient with ODC1 disorder reduced ODC protein activity and putrescine to control levels.
Other potential treatments include the natural ODC1 inhibitors agmatine and turmeric/curcumin, flagyl/rifaximin to decrease putrescine production in the gut, a low-dairy diet to lower putrescine levels, and antioxidants. “There could be a role for antioxidant stress similar to what is seen in Snyder-Robinson syndrome,” Dr. Rodan said.
Based on mouse studies, patients with ODC1 may be at risk of skin cancer, so regular skin checks along with sun protection should be part of management, he said. “This also raises the question of whether there should be surveillance for other types of cancer given the role of polyamine in various types of tumors.”
Dr. Rodan has no relevant financial relationships to disclose.
but it may be treated with diet modifications and available therapies, according to the researcher whose group first identified the disorder.
Lance Rodan, MD, of Boston Children’s Hospital and Harvard Medical School, reported on research into ODC1 gain-of-function disorder –named for ornithine decarboxylase 1, the rate-limiting enzyme involved in polyamine synthesis – in the Linda De Meirleir Neurometabolic award lecture at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Dr. Rodan and colleagues first described ODC1 disorder in a multicenter case series.
Dr. Rodan noted that dysregulated polyamine levels are associated with cancer, and that ODC1 is expressed “ubiquitously” throughout the body.
Pathophysiology and phenotypes
In an interview, he described the metabolic process more fully. “GI flora can produce putrescine, which is the polyamine that accumulates in excess in the ODC1 gain-of-function disorder. It is yet to be elucidated if decreasing putrescine production by GI flora and/or reducing dietary sources of putrescine may play a role in the management of this disorder.”
In the De Meirleir lecture, Dr. Rodan described four patients from his group’s published case series, all found to have heterozygous de novo variants in the ODC1 gene, along with a fifth patient reported by Caleb Bupp, MD, and colleagues at Michigan State University, East Lansing.
“There’s a recognizable phenotype to this disorder,” Dr. Rodan said. “These individuals have neurodevelopment abnormalities. They may have behavioral concerns. They have low-tone central hypertonia and macrocephaly.”
One of the most distinctive characteristics of ODC1 disorder is alopecia, he said, “which in almost everybody with this condition involves the eyebrows and eyelashes and in some individuals also involves the scalp hair.”
These patients also have what Dr. Rodan called “a common yet subtle facial gestalt.” That can include hypertelorism, spareness of the eyebrows and eyelashes, and a tubular- shaped nose with a short columella and a short philtrum.
They may also have abnormalities of the nails and cryptorchidism, and typically a prenatal history of polyhydramnios, he said.
MRI findings include prominent perivascular spaces, periventricular cysts, abnormal white matter and corpus callosum abnormalities, he said, adding that the fetal case MRI demonstrated subepidermal cysts, white matter cysts in the temporal pole, deficiency of the falx cerebri and abnormal white-matter signals.
Biochemical features of ODC1 disorder include increased N-acetylputrescine levels with normal spermine and spermidine levels, Dr. Rodan said. He also noted that Dr. Bupp’s group reported increased putrescine in fibroblasts and increased ODC1 protein levels in red blood cells.
Dr. Rodan also described possible molecular mechanisms in ODC1 disorder. One was the location of the ODC1 variants: all were reported closely located to truncating variants in the final exon of the ODC1 gene. This allows truncating proteins to survive, adding to the degradation that results in a net gain-of-function of ODC1 enzyme activity.
With regard to pathophysiology of ODC1 disorder, Dr. Rodan noted that research has implicated chronically elevated putrescine levels in the alopecia, a finding animal models support. “Since putrescine is a precursor for gamma-aminobutyric acid, it’s possible perturbed GABA levels may also be involved,” he said. Abnormal modulation N-methyl-D-aspirate receptors may also be involved, he said.
Another hypothesis purports that potential of elevated levels of toxic aldehydes/H2O2 similar to Snyder-Robinson syndrome, the better known polyamine-related neurometabolic disorder. “Along those lines, maybe there’s also a secondary mitochondrial or lysosomal dysfunction, but this is something that’s still being actively studied,” Dr. Rodan said.
Treatment
Because ODC1 disorder was only first described 2 years ago, research into treatment is nascent. “In terms of management, I think one of the more fundamental questions is whether this is more of a static developmental disorder or whether this actually represents a progressive degenerative disorder,” Dr. Rodan said.
One potential treatment that has been explored, he said, is difluoromethylornithine, a synthetic ODC1 inhibitor already Food and Drug Administration approved for African sleeping sickness and as a topical treatment for hirsutism. It is also the subject of ongoing clinical trials in colon cancer and neuroblastoma. Potential side effects include myelosuppression, seizures and hearing loss.
Dr. Rodan noted that a single-center study reported that difluoromethylornithine in a 3-year-old patient with ODC1 disorder reduced ODC protein activity and putrescine to control levels.
Other potential treatments include the natural ODC1 inhibitors agmatine and turmeric/curcumin, flagyl/rifaximin to decrease putrescine production in the gut, a low-dairy diet to lower putrescine levels, and antioxidants. “There could be a role for antioxidant stress similar to what is seen in Snyder-Robinson syndrome,” Dr. Rodan said.
Based on mouse studies, patients with ODC1 may be at risk of skin cancer, so regular skin checks along with sun protection should be part of management, he said. “This also raises the question of whether there should be surveillance for other types of cancer given the role of polyamine in various types of tumors.”
Dr. Rodan has no relevant financial relationships to disclose.
FROM CNS-ICNA 2020
Higher serum omega-3 tied to better outcome after STEMI
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Statins may lower risk of colorectal cancer
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
FROM ACG 2020
Decide ADHD pharmacotherapy based on medication onset, duration of action
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
FROM PSYCHOPHARMACOLOGY UPDATE
Sleep apnea found to impact pain severity in younger adults
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Novel agents hold promise for frontline AML treatment
Novel therapies are poised to dramatically change frontline therapy for acute myeloid leukemia (AML), and they have the potential to replace chemotherapy, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus.
But more work needs to be done, noted Alexander Perl, MD, MS, associate professor at the University of Pennsylvania, Philadelphia. While advances have transformed AML treatment in the relapsed/refractory setting, “we’re just not seeing that substantive improvement” for newly diagnosed patients, he said. “We need to find the disease-modifying drugs that work in the relapsed/refractory setting and move those frontline. That’s where we’re going to see the transformations.”
Research suggests that low-intensity therapy holds tremendous promise, he said, “with the idea that we could make therapy much more tolerable for the vast majority of patients affected by AML, who, as we know, are older patients.”
Dr. Perl highlighted the 2020 VIALE-A study – venetoclax/azacitidine versus azacitidine/placebo – which reported that “in previously untreated patients who were ineligible for intensive chemotherapy, overall survival was longer and the incidence of remission was higher among patients who received azacitidine plus venetoclax than among those who received azacitidine alone.”
Venetoclax promotes apoptosis in leukemia cells, Dr. Perl said. “To a certain extent, you can think of it as putting the rubber to the road in terms of what actually chemotherapy is designed to do, which is to make leukemic blasts apoptose. It does so without DNA damage and with much less toxicity to the patient. Therefore it can be added to any number of regimens – granted, with mild suppression, but with relatively little extramedullary toxicity.”
Dr. Perl noted that the venetoclax arm “showed a higher response rate than azacitidine in pretty much every subgroup that was looked at, whether patients had de novo leukemia, secondary leukemia, multiple mutational complements, various different karyotypes. The response rates on this study are as high as what we often will see with intensive chemotherapy.” He added that “the winning arm on this trial seems to hold up against any low-intensity therapy, and I would argue against many high-intensity therapies in older patients.”
As for other targeted agents, isocitrate dehydrogenase (IDH) inhibitors “are very promising drugs in the relapsed/refractory setting, which is primarily where these drugs are given. In regard to frontline treatment, “data are coming from a very small study, but they’re very encouraging. It’s hard to entirely say that we’re ready to change practice based on this. But it’s very encouraging – the idea that earlier use of a drug-targeting IDH mutation might lead to substantially better outcomes.”
Moving forward, he said, “we could put all of our eggs in one basket and use many active drugs [at] front line. Or we can perhaps be smart about sequencing these drugs one after another, or using more intensive approaches followed by maintenance approaches followed by more intensive approaches.”
This approach is similar to strategies in myeloma patients “who less and less are relying on an autologous transplant for durable control of their disease, and more and more are using low-intensity biologically targeted drugs,” he said.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Perl reported numerous disclosures, including relationships with Daiichi Sankyo, Abbvie, and Astellas.
Novel therapies are poised to dramatically change frontline therapy for acute myeloid leukemia (AML), and they have the potential to replace chemotherapy, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus.
But more work needs to be done, noted Alexander Perl, MD, MS, associate professor at the University of Pennsylvania, Philadelphia. While advances have transformed AML treatment in the relapsed/refractory setting, “we’re just not seeing that substantive improvement” for newly diagnosed patients, he said. “We need to find the disease-modifying drugs that work in the relapsed/refractory setting and move those frontline. That’s where we’re going to see the transformations.”
Research suggests that low-intensity therapy holds tremendous promise, he said, “with the idea that we could make therapy much more tolerable for the vast majority of patients affected by AML, who, as we know, are older patients.”
Dr. Perl highlighted the 2020 VIALE-A study – venetoclax/azacitidine versus azacitidine/placebo – which reported that “in previously untreated patients who were ineligible for intensive chemotherapy, overall survival was longer and the incidence of remission was higher among patients who received azacitidine plus venetoclax than among those who received azacitidine alone.”
Venetoclax promotes apoptosis in leukemia cells, Dr. Perl said. “To a certain extent, you can think of it as putting the rubber to the road in terms of what actually chemotherapy is designed to do, which is to make leukemic blasts apoptose. It does so without DNA damage and with much less toxicity to the patient. Therefore it can be added to any number of regimens – granted, with mild suppression, but with relatively little extramedullary toxicity.”
Dr. Perl noted that the venetoclax arm “showed a higher response rate than azacitidine in pretty much every subgroup that was looked at, whether patients had de novo leukemia, secondary leukemia, multiple mutational complements, various different karyotypes. The response rates on this study are as high as what we often will see with intensive chemotherapy.” He added that “the winning arm on this trial seems to hold up against any low-intensity therapy, and I would argue against many high-intensity therapies in older patients.”
As for other targeted agents, isocitrate dehydrogenase (IDH) inhibitors “are very promising drugs in the relapsed/refractory setting, which is primarily where these drugs are given. In regard to frontline treatment, “data are coming from a very small study, but they’re very encouraging. It’s hard to entirely say that we’re ready to change practice based on this. But it’s very encouraging – the idea that earlier use of a drug-targeting IDH mutation might lead to substantially better outcomes.”
Moving forward, he said, “we could put all of our eggs in one basket and use many active drugs [at] front line. Or we can perhaps be smart about sequencing these drugs one after another, or using more intensive approaches followed by maintenance approaches followed by more intensive approaches.”
This approach is similar to strategies in myeloma patients “who less and less are relying on an autologous transplant for durable control of their disease, and more and more are using low-intensity biologically targeted drugs,” he said.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Perl reported numerous disclosures, including relationships with Daiichi Sankyo, Abbvie, and Astellas.
Novel therapies are poised to dramatically change frontline therapy for acute myeloid leukemia (AML), and they have the potential to replace chemotherapy, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus.
But more work needs to be done, noted Alexander Perl, MD, MS, associate professor at the University of Pennsylvania, Philadelphia. While advances have transformed AML treatment in the relapsed/refractory setting, “we’re just not seeing that substantive improvement” for newly diagnosed patients, he said. “We need to find the disease-modifying drugs that work in the relapsed/refractory setting and move those frontline. That’s where we’re going to see the transformations.”
Research suggests that low-intensity therapy holds tremendous promise, he said, “with the idea that we could make therapy much more tolerable for the vast majority of patients affected by AML, who, as we know, are older patients.”
Dr. Perl highlighted the 2020 VIALE-A study – venetoclax/azacitidine versus azacitidine/placebo – which reported that “in previously untreated patients who were ineligible for intensive chemotherapy, overall survival was longer and the incidence of remission was higher among patients who received azacitidine plus venetoclax than among those who received azacitidine alone.”
Venetoclax promotes apoptosis in leukemia cells, Dr. Perl said. “To a certain extent, you can think of it as putting the rubber to the road in terms of what actually chemotherapy is designed to do, which is to make leukemic blasts apoptose. It does so without DNA damage and with much less toxicity to the patient. Therefore it can be added to any number of regimens – granted, with mild suppression, but with relatively little extramedullary toxicity.”
Dr. Perl noted that the venetoclax arm “showed a higher response rate than azacitidine in pretty much every subgroup that was looked at, whether patients had de novo leukemia, secondary leukemia, multiple mutational complements, various different karyotypes. The response rates on this study are as high as what we often will see with intensive chemotherapy.” He added that “the winning arm on this trial seems to hold up against any low-intensity therapy, and I would argue against many high-intensity therapies in older patients.”
As for other targeted agents, isocitrate dehydrogenase (IDH) inhibitors “are very promising drugs in the relapsed/refractory setting, which is primarily where these drugs are given. In regard to frontline treatment, “data are coming from a very small study, but they’re very encouraging. It’s hard to entirely say that we’re ready to change practice based on this. But it’s very encouraging – the idea that earlier use of a drug-targeting IDH mutation might lead to substantially better outcomes.”
Moving forward, he said, “we could put all of our eggs in one basket and use many active drugs [at] front line. Or we can perhaps be smart about sequencing these drugs one after another, or using more intensive approaches followed by maintenance approaches followed by more intensive approaches.”
This approach is similar to strategies in myeloma patients “who less and less are relying on an autologous transplant for durable control of their disease, and more and more are using low-intensity biologically targeted drugs,” he said.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Perl reported numerous disclosures, including relationships with Daiichi Sankyo, Abbvie, and Astellas.
FROM ALF 2020
CAR T for all R/R DLBCL patients: The jury is still out
Is it time to consider chimeric antigen receptor (CAR) T-cell therapy for all relapsed/refractory diffuse large B-cell lymphoma patients? Maybe not, according to Andrew Zelenetz, MD, PhD.
CAR T-cell therapy has demonstrated activity in relapsed/refractory non-Hodgkin lymphoma (NHL), including diffuse large B-cell lymphoma (DLBCL), transformed indolent NHL, and mantle cell lymphoma, and can provide durable complete responses in a portion of patients with chemorefractory disease, Dr. Zelenetz, chair of the National Comprehensive Cancer Network Lymphoma Guidelines Panel and a specialist in lymphoma at Memorial Sloan Kettering Cancer Center in New York, said at the NCCN Hematologic Malignancies Annual Congress.
In chemosensitive patients, however, its role requires further examination, especially given findings from a recent analysis of patients from the Center for International Blood & Marrow Transplant Research (CIBMTR) registry showing comparable outcomes with high-dose chemotherapy and autologous stem cell rescue for patients with a positron emission testing–positive partial response (PR) after second-line chemotherapy, he said.
Of 249 patients who underwent a first autologous transplant for DLBCL between 2003 and 2018, received front-line rituximab chemotherapy, and had PET– or computed tomography–positive disease prior to transplant, 182 had early chemotherapy failure (within 12 months) and 67 had late chemotherapy failure (at 12 months or later) after therapy, according to findings from the study as reported at ASCO 2020.
The adjusted nonrelapse mortality rates in the early- and late-failure patients, respectively, were not significantly different at 7% and 3% at 1 year, and at 10% and 8% at 5 years. The corresponding progression/relapse rates were 41% and 35% at 1 year and 48% and 57% at 5 years; these were also not significantly different.
The adjusted progression-free survival (PFS) and overall survival (OS) in the groups at 5 years also did not differ significantly (PFS of 41% in both the early- and late-failure groups, and OS of 51% and 63%, respectively).
These outcomes are comparable to those seen with CAR T-cell therapy in refractory DLBCL patients in trials of CAR T-cell products, including the ZUMA-1 study of axicabtagene cyloleucel (Yescarta), which, in a 2019 update, showed survival plateaus of about 40% vs. the 5%-10% expected rate based on pre-CAR-T outcomes data; the JULIET trial of tisagenlecleucel (Kymriah), which showed survival plateaus in the range of 30%-35%; and the recently published TRANSCEND study of the investigational modified CAR-T product, lisocabtagene maraleucel, which also showed survival plateaus “in the range of 40%.”
“So all three agents are showing that CAR T cells represent a new treatment for diffuse large B-cell lymphoma in the relapsed/refractory setting,” Dr. Zelenetz said. “And as a result, [CAR T-cell therapy has] been included in the NCCN guidelines for transformed follicular lymphoma, for transformed marginal zone lymphoma, and for diffuse large B-cell lymphoma, as well as for refractory large B-cell lymphoma.
“But are CAR T cells absolutely required? Generally what we consider these days is that if you’re not in a PET-negative CR prior to high-dose therapy stem cell rescue, you should go on to CAR T cells,” Dr. Zelenetz said.
The analysis based on the CIBMTR registry data, however, suggests there may be other alternatives.
“The bottom line is that nonrelapse mortality was very low. Progression occurred in about half of the patients, but if we look at the overall and progression-free survival curves, there’s a plateau at around 45%,” Dr. Zelenetz said, explaining that the results are “very similar to the results that we’re getting in third-line treatment with CAR T cells, and this is a very similar population [of] PET-positive patients after second-line chemotherapy.”
CAR T-cell therapy can provide a durable CR in a portion of chemorefractory patients, and although there is room for improvement, “this represents a major step forward for these patients,” he said.
However, it’s not clear that CAR T cells are clearly superior to high-dose therapy and stem cell rescue for chemosensitive patients, he added, noting that “additional randomized trials are needed to answer this question, and they are ongoing as we speak.”
Dr. Zelenetz reported clinical research support or data safety monitoring board activity for BeiGene, Genentech, Juno Therapeutics, and MEI Pharma, and scientific advisory board, consulting, or expert witness activity for Celgene Corporation, Curries, Genentech, Gilead Sciences, Janssen Pharmaceutical Products, and several other pharmaceutical and biotechnology companies.
Is it time to consider chimeric antigen receptor (CAR) T-cell therapy for all relapsed/refractory diffuse large B-cell lymphoma patients? Maybe not, according to Andrew Zelenetz, MD, PhD.
CAR T-cell therapy has demonstrated activity in relapsed/refractory non-Hodgkin lymphoma (NHL), including diffuse large B-cell lymphoma (DLBCL), transformed indolent NHL, and mantle cell lymphoma, and can provide durable complete responses in a portion of patients with chemorefractory disease, Dr. Zelenetz, chair of the National Comprehensive Cancer Network Lymphoma Guidelines Panel and a specialist in lymphoma at Memorial Sloan Kettering Cancer Center in New York, said at the NCCN Hematologic Malignancies Annual Congress.
In chemosensitive patients, however, its role requires further examination, especially given findings from a recent analysis of patients from the Center for International Blood & Marrow Transplant Research (CIBMTR) registry showing comparable outcomes with high-dose chemotherapy and autologous stem cell rescue for patients with a positron emission testing–positive partial response (PR) after second-line chemotherapy, he said.
Of 249 patients who underwent a first autologous transplant for DLBCL between 2003 and 2018, received front-line rituximab chemotherapy, and had PET– or computed tomography–positive disease prior to transplant, 182 had early chemotherapy failure (within 12 months) and 67 had late chemotherapy failure (at 12 months or later) after therapy, according to findings from the study as reported at ASCO 2020.
The adjusted nonrelapse mortality rates in the early- and late-failure patients, respectively, were not significantly different at 7% and 3% at 1 year, and at 10% and 8% at 5 years. The corresponding progression/relapse rates were 41% and 35% at 1 year and 48% and 57% at 5 years; these were also not significantly different.
The adjusted progression-free survival (PFS) and overall survival (OS) in the groups at 5 years also did not differ significantly (PFS of 41% in both the early- and late-failure groups, and OS of 51% and 63%, respectively).
These outcomes are comparable to those seen with CAR T-cell therapy in refractory DLBCL patients in trials of CAR T-cell products, including the ZUMA-1 study of axicabtagene cyloleucel (Yescarta), which, in a 2019 update, showed survival plateaus of about 40% vs. the 5%-10% expected rate based on pre-CAR-T outcomes data; the JULIET trial of tisagenlecleucel (Kymriah), which showed survival plateaus in the range of 30%-35%; and the recently published TRANSCEND study of the investigational modified CAR-T product, lisocabtagene maraleucel, which also showed survival plateaus “in the range of 40%.”
“So all three agents are showing that CAR T cells represent a new treatment for diffuse large B-cell lymphoma in the relapsed/refractory setting,” Dr. Zelenetz said. “And as a result, [CAR T-cell therapy has] been included in the NCCN guidelines for transformed follicular lymphoma, for transformed marginal zone lymphoma, and for diffuse large B-cell lymphoma, as well as for refractory large B-cell lymphoma.
“But are CAR T cells absolutely required? Generally what we consider these days is that if you’re not in a PET-negative CR prior to high-dose therapy stem cell rescue, you should go on to CAR T cells,” Dr. Zelenetz said.
The analysis based on the CIBMTR registry data, however, suggests there may be other alternatives.
“The bottom line is that nonrelapse mortality was very low. Progression occurred in about half of the patients, but if we look at the overall and progression-free survival curves, there’s a plateau at around 45%,” Dr. Zelenetz said, explaining that the results are “very similar to the results that we’re getting in third-line treatment with CAR T cells, and this is a very similar population [of] PET-positive patients after second-line chemotherapy.”
CAR T-cell therapy can provide a durable CR in a portion of chemorefractory patients, and although there is room for improvement, “this represents a major step forward for these patients,” he said.
However, it’s not clear that CAR T cells are clearly superior to high-dose therapy and stem cell rescue for chemosensitive patients, he added, noting that “additional randomized trials are needed to answer this question, and they are ongoing as we speak.”
Dr. Zelenetz reported clinical research support or data safety monitoring board activity for BeiGene, Genentech, Juno Therapeutics, and MEI Pharma, and scientific advisory board, consulting, or expert witness activity for Celgene Corporation, Curries, Genentech, Gilead Sciences, Janssen Pharmaceutical Products, and several other pharmaceutical and biotechnology companies.
Is it time to consider chimeric antigen receptor (CAR) T-cell therapy for all relapsed/refractory diffuse large B-cell lymphoma patients? Maybe not, according to Andrew Zelenetz, MD, PhD.
CAR T-cell therapy has demonstrated activity in relapsed/refractory non-Hodgkin lymphoma (NHL), including diffuse large B-cell lymphoma (DLBCL), transformed indolent NHL, and mantle cell lymphoma, and can provide durable complete responses in a portion of patients with chemorefractory disease, Dr. Zelenetz, chair of the National Comprehensive Cancer Network Lymphoma Guidelines Panel and a specialist in lymphoma at Memorial Sloan Kettering Cancer Center in New York, said at the NCCN Hematologic Malignancies Annual Congress.
In chemosensitive patients, however, its role requires further examination, especially given findings from a recent analysis of patients from the Center for International Blood & Marrow Transplant Research (CIBMTR) registry showing comparable outcomes with high-dose chemotherapy and autologous stem cell rescue for patients with a positron emission testing–positive partial response (PR) after second-line chemotherapy, he said.
Of 249 patients who underwent a first autologous transplant for DLBCL between 2003 and 2018, received front-line rituximab chemotherapy, and had PET– or computed tomography–positive disease prior to transplant, 182 had early chemotherapy failure (within 12 months) and 67 had late chemotherapy failure (at 12 months or later) after therapy, according to findings from the study as reported at ASCO 2020.
The adjusted nonrelapse mortality rates in the early- and late-failure patients, respectively, were not significantly different at 7% and 3% at 1 year, and at 10% and 8% at 5 years. The corresponding progression/relapse rates were 41% and 35% at 1 year and 48% and 57% at 5 years; these were also not significantly different.
The adjusted progression-free survival (PFS) and overall survival (OS) in the groups at 5 years also did not differ significantly (PFS of 41% in both the early- and late-failure groups, and OS of 51% and 63%, respectively).
These outcomes are comparable to those seen with CAR T-cell therapy in refractory DLBCL patients in trials of CAR T-cell products, including the ZUMA-1 study of axicabtagene cyloleucel (Yescarta), which, in a 2019 update, showed survival plateaus of about 40% vs. the 5%-10% expected rate based on pre-CAR-T outcomes data; the JULIET trial of tisagenlecleucel (Kymriah), which showed survival plateaus in the range of 30%-35%; and the recently published TRANSCEND study of the investigational modified CAR-T product, lisocabtagene maraleucel, which also showed survival plateaus “in the range of 40%.”
“So all three agents are showing that CAR T cells represent a new treatment for diffuse large B-cell lymphoma in the relapsed/refractory setting,” Dr. Zelenetz said. “And as a result, [CAR T-cell therapy has] been included in the NCCN guidelines for transformed follicular lymphoma, for transformed marginal zone lymphoma, and for diffuse large B-cell lymphoma, as well as for refractory large B-cell lymphoma.
“But are CAR T cells absolutely required? Generally what we consider these days is that if you’re not in a PET-negative CR prior to high-dose therapy stem cell rescue, you should go on to CAR T cells,” Dr. Zelenetz said.
The analysis based on the CIBMTR registry data, however, suggests there may be other alternatives.
“The bottom line is that nonrelapse mortality was very low. Progression occurred in about half of the patients, but if we look at the overall and progression-free survival curves, there’s a plateau at around 45%,” Dr. Zelenetz said, explaining that the results are “very similar to the results that we’re getting in third-line treatment with CAR T cells, and this is a very similar population [of] PET-positive patients after second-line chemotherapy.”
CAR T-cell therapy can provide a durable CR in a portion of chemorefractory patients, and although there is room for improvement, “this represents a major step forward for these patients,” he said.
However, it’s not clear that CAR T cells are clearly superior to high-dose therapy and stem cell rescue for chemosensitive patients, he added, noting that “additional randomized trials are needed to answer this question, and they are ongoing as we speak.”
Dr. Zelenetz reported clinical research support or data safety monitoring board activity for BeiGene, Genentech, Juno Therapeutics, and MEI Pharma, and scientific advisory board, consulting, or expert witness activity for Celgene Corporation, Curries, Genentech, Gilead Sciences, Janssen Pharmaceutical Products, and several other pharmaceutical and biotechnology companies.
FROM NCCN HEMATOLOGIC MALIGNANCIES











