User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Primary care journals address systemic racism in medicine
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
New return-to-play recommendations for athletes with COVID-19
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Surgery may not be needed with locally advanced rectal cancer
A short course of radiation therapy followed by neoadjuvant chemotherapy resulted in a clinical complete response (CR) in almost half of 90 patients with locally advanced rectal cancer, allowing the majority of responders to skip surgical resection, a retrospective study indicates.
Specifically, at a median follow-up of 16.6 months for living patients, the initial clinical CR rate was 48% overall.
“While we do not have enough follow-up yet to know the late side-effect profile of this regimen, our preliminary results are promising,” Re-I Chin, MD, of Washington University School of Medicine, St. Louis, Missouri, told Medscape Medical News in an email.
The study was presented at the virtual 2020 meeting of the American Society of Radiation Oncology (ASTRO).
“Certainly, longer follow-up will be needed in this study, but none of the observed patients to date has experienced an unsalvageable failure,” said meeting discussant Amol Narang, MD, of Johns Hopkins University, Baltimore, Maryland.
He reminded conference attendees that, despite good evidence supporting equivalency in oncologic outcomes between short-course radiation and long-course chemoradiation, the former is “highly underutilized in the US” with a mere 1% usage rate between 2004 and 2014.
The current study’s short-course treatment approach was compared in this setting to long-course chemoradiation and adjuvant chemotherapy in the RAPIDO trial reported at the 2020 annual meeting of the American Society of Clinical Oncology (ASCO), Narang pointed out.
Short-course patients had a higher rate of pathological complete response (pCR) and a lower rate of treatment failure compared with patients who received long-course chemoradiation and adjuvant chemotherapy; both patient groups underwent total mesorectal excision — which is different from the current analysis. The RAPIDO investigators concluded that the approach featuring the short course “can be considered as a new standard of care.”
Narang said the data collectively “begs the question as to whether the superiority of long-course chemoradiation should really have to be demonstrated to justify its use.”
But Chin highlighted toxicity issues. “Historically, there have been concerns regarding toxicity with short-course radiation therapy since it requires larger doses of radiation given over a shorter period of time,” Chin explained. “But [the short course] is particularly convenient for patients since it saves them more than a month of daily trips to the radiation oncology department.”
Seven local failures
The single-center study involved patients with newly diagnosed, nonmetastatic rectal adenocarcinoma treated with short-course radiation therapy followed by chemotherapy in 2018 and 2019. Nearly all (96%) had locally advanced disease, with either a T3/T4 tumor or node-positive disease. Median tumor size was 4.6 cm.
“Many of the patients in the study had low lying tumors,” Chin reported, with a median distance from the anal verge of 7 cm.
Radiation therapy was delivered in 25 Gy given in five fractions over 5 consecutive days, with the option to boost the dose to 30 Gy or 35 Gy in five fractions if extra-mesorectal lymph nodes were involved. Conventional 3D or intensity-modulated radiation was used and all patients completed treatment.
The median interval between diagnosis of rectal cancer and initiation of radiation therapy was 1.4 months, while the median interval between completion of radiation to initiation of chemotherapy was 2.7 weeks.
The most common chemotherapy regimen was FOLFOX – consisting of leucovorin, fluorouracil (5-FU), and oxaliplatin – or modified FOLFOX. For patients who received six or more cycles of chemotherapy, the median time spent on treatment was 3.9 months.
For those who completed at least six cycles of chemotherapy, the overall clinical CR was 51%, and, for patients with locally advanced disease, the clinical CR rate was 49%. Five of the 43 patients who achieved an initial clinical CR still underwent surgical resection because of patient or physician preference. Among this small group of patients, 4 of the 5 achieved a pCR, and the remaining patient achieved a pathological partial response (pPR).
A total of 42 patients (47% of the group) achieved a partial response following the radiation plus chemotherapy paradigm, and one patient had progressive disease. All underwent surgical resection. One patient did not complete chemotherapy and did not get surgery and subsequently died.
This left 38 patients to be managed nonoperatively. In this nonoperative cohort, 79% of patients continued to have a clinical CR at the end of follow-up. Of the 7 patients with local failure, 6 were salvaged by surgery, one was salvaged by chemotherapy, and all 7 treatment failures had no evidence of disease at last follow-up.
Of the small group of 5 patients who achieved an initial clinical CR following short-course radiation therapy and neoadjuvant chemotherapy, there were no further events in this group, whereas, for patients who achieved only an initial partial response or who had progressive disease, 72% remained event-free.
Approximately half of those who achieved a clinical CR to the treatment regimen had no late gastrointestinal toxicities, and no grade 3 or 4 toxicities were observed. “Surgical resection of tumors — even without a permanent stoma — can result in significantly decreased bowel function, so our goal is to treat patients without surgery and maintain good bowel function,” Chin noted.
“For rectal cancer, both short-course radiation therapy and nonoperative management are emerging treatment paradigms that may be more cost-effective and convenient compared to long-course chemoradiation followed by surgery, [especially since] the COVID-19 pandemic...has spurred changes in clinical practices in radiation oncology,” she said.
Chin has disclosed no relevant financial relationships. Narang reports receiving research support from Boston Scientific.
This article first appeared on Medscape.com.
A short course of radiation therapy followed by neoadjuvant chemotherapy resulted in a clinical complete response (CR) in almost half of 90 patients with locally advanced rectal cancer, allowing the majority of responders to skip surgical resection, a retrospective study indicates.
Specifically, at a median follow-up of 16.6 months for living patients, the initial clinical CR rate was 48% overall.
“While we do not have enough follow-up yet to know the late side-effect profile of this regimen, our preliminary results are promising,” Re-I Chin, MD, of Washington University School of Medicine, St. Louis, Missouri, told Medscape Medical News in an email.
The study was presented at the virtual 2020 meeting of the American Society of Radiation Oncology (ASTRO).
“Certainly, longer follow-up will be needed in this study, but none of the observed patients to date has experienced an unsalvageable failure,” said meeting discussant Amol Narang, MD, of Johns Hopkins University, Baltimore, Maryland.
He reminded conference attendees that, despite good evidence supporting equivalency in oncologic outcomes between short-course radiation and long-course chemoradiation, the former is “highly underutilized in the US” with a mere 1% usage rate between 2004 and 2014.
The current study’s short-course treatment approach was compared in this setting to long-course chemoradiation and adjuvant chemotherapy in the RAPIDO trial reported at the 2020 annual meeting of the American Society of Clinical Oncology (ASCO), Narang pointed out.
Short-course patients had a higher rate of pathological complete response (pCR) and a lower rate of treatment failure compared with patients who received long-course chemoradiation and adjuvant chemotherapy; both patient groups underwent total mesorectal excision — which is different from the current analysis. The RAPIDO investigators concluded that the approach featuring the short course “can be considered as a new standard of care.”
Narang said the data collectively “begs the question as to whether the superiority of long-course chemoradiation should really have to be demonstrated to justify its use.”
But Chin highlighted toxicity issues. “Historically, there have been concerns regarding toxicity with short-course radiation therapy since it requires larger doses of radiation given over a shorter period of time,” Chin explained. “But [the short course] is particularly convenient for patients since it saves them more than a month of daily trips to the radiation oncology department.”
Seven local failures
The single-center study involved patients with newly diagnosed, nonmetastatic rectal adenocarcinoma treated with short-course radiation therapy followed by chemotherapy in 2018 and 2019. Nearly all (96%) had locally advanced disease, with either a T3/T4 tumor or node-positive disease. Median tumor size was 4.6 cm.
“Many of the patients in the study had low lying tumors,” Chin reported, with a median distance from the anal verge of 7 cm.
Radiation therapy was delivered in 25 Gy given in five fractions over 5 consecutive days, with the option to boost the dose to 30 Gy or 35 Gy in five fractions if extra-mesorectal lymph nodes were involved. Conventional 3D or intensity-modulated radiation was used and all patients completed treatment.
The median interval between diagnosis of rectal cancer and initiation of radiation therapy was 1.4 months, while the median interval between completion of radiation to initiation of chemotherapy was 2.7 weeks.
The most common chemotherapy regimen was FOLFOX – consisting of leucovorin, fluorouracil (5-FU), and oxaliplatin – or modified FOLFOX. For patients who received six or more cycles of chemotherapy, the median time spent on treatment was 3.9 months.
For those who completed at least six cycles of chemotherapy, the overall clinical CR was 51%, and, for patients with locally advanced disease, the clinical CR rate was 49%. Five of the 43 patients who achieved an initial clinical CR still underwent surgical resection because of patient or physician preference. Among this small group of patients, 4 of the 5 achieved a pCR, and the remaining patient achieved a pathological partial response (pPR).
A total of 42 patients (47% of the group) achieved a partial response following the radiation plus chemotherapy paradigm, and one patient had progressive disease. All underwent surgical resection. One patient did not complete chemotherapy and did not get surgery and subsequently died.
This left 38 patients to be managed nonoperatively. In this nonoperative cohort, 79% of patients continued to have a clinical CR at the end of follow-up. Of the 7 patients with local failure, 6 were salvaged by surgery, one was salvaged by chemotherapy, and all 7 treatment failures had no evidence of disease at last follow-up.
Of the small group of 5 patients who achieved an initial clinical CR following short-course radiation therapy and neoadjuvant chemotherapy, there were no further events in this group, whereas, for patients who achieved only an initial partial response or who had progressive disease, 72% remained event-free.
Approximately half of those who achieved a clinical CR to the treatment regimen had no late gastrointestinal toxicities, and no grade 3 or 4 toxicities were observed. “Surgical resection of tumors — even without a permanent stoma — can result in significantly decreased bowel function, so our goal is to treat patients without surgery and maintain good bowel function,” Chin noted.
“For rectal cancer, both short-course radiation therapy and nonoperative management are emerging treatment paradigms that may be more cost-effective and convenient compared to long-course chemoradiation followed by surgery, [especially since] the COVID-19 pandemic...has spurred changes in clinical practices in radiation oncology,” she said.
Chin has disclosed no relevant financial relationships. Narang reports receiving research support from Boston Scientific.
This article first appeared on Medscape.com.
A short course of radiation therapy followed by neoadjuvant chemotherapy resulted in a clinical complete response (CR) in almost half of 90 patients with locally advanced rectal cancer, allowing the majority of responders to skip surgical resection, a retrospective study indicates.
Specifically, at a median follow-up of 16.6 months for living patients, the initial clinical CR rate was 48% overall.
“While we do not have enough follow-up yet to know the late side-effect profile of this regimen, our preliminary results are promising,” Re-I Chin, MD, of Washington University School of Medicine, St. Louis, Missouri, told Medscape Medical News in an email.
The study was presented at the virtual 2020 meeting of the American Society of Radiation Oncology (ASTRO).
“Certainly, longer follow-up will be needed in this study, but none of the observed patients to date has experienced an unsalvageable failure,” said meeting discussant Amol Narang, MD, of Johns Hopkins University, Baltimore, Maryland.
He reminded conference attendees that, despite good evidence supporting equivalency in oncologic outcomes between short-course radiation and long-course chemoradiation, the former is “highly underutilized in the US” with a mere 1% usage rate between 2004 and 2014.
The current study’s short-course treatment approach was compared in this setting to long-course chemoradiation and adjuvant chemotherapy in the RAPIDO trial reported at the 2020 annual meeting of the American Society of Clinical Oncology (ASCO), Narang pointed out.
Short-course patients had a higher rate of pathological complete response (pCR) and a lower rate of treatment failure compared with patients who received long-course chemoradiation and adjuvant chemotherapy; both patient groups underwent total mesorectal excision — which is different from the current analysis. The RAPIDO investigators concluded that the approach featuring the short course “can be considered as a new standard of care.”
Narang said the data collectively “begs the question as to whether the superiority of long-course chemoradiation should really have to be demonstrated to justify its use.”
But Chin highlighted toxicity issues. “Historically, there have been concerns regarding toxicity with short-course radiation therapy since it requires larger doses of radiation given over a shorter period of time,” Chin explained. “But [the short course] is particularly convenient for patients since it saves them more than a month of daily trips to the radiation oncology department.”
Seven local failures
The single-center study involved patients with newly diagnosed, nonmetastatic rectal adenocarcinoma treated with short-course radiation therapy followed by chemotherapy in 2018 and 2019. Nearly all (96%) had locally advanced disease, with either a T3/T4 tumor or node-positive disease. Median tumor size was 4.6 cm.
“Many of the patients in the study had low lying tumors,” Chin reported, with a median distance from the anal verge of 7 cm.
Radiation therapy was delivered in 25 Gy given in five fractions over 5 consecutive days, with the option to boost the dose to 30 Gy or 35 Gy in five fractions if extra-mesorectal lymph nodes were involved. Conventional 3D or intensity-modulated radiation was used and all patients completed treatment.
The median interval between diagnosis of rectal cancer and initiation of radiation therapy was 1.4 months, while the median interval between completion of radiation to initiation of chemotherapy was 2.7 weeks.
The most common chemotherapy regimen was FOLFOX – consisting of leucovorin, fluorouracil (5-FU), and oxaliplatin – or modified FOLFOX. For patients who received six or more cycles of chemotherapy, the median time spent on treatment was 3.9 months.
For those who completed at least six cycles of chemotherapy, the overall clinical CR was 51%, and, for patients with locally advanced disease, the clinical CR rate was 49%. Five of the 43 patients who achieved an initial clinical CR still underwent surgical resection because of patient or physician preference. Among this small group of patients, 4 of the 5 achieved a pCR, and the remaining patient achieved a pathological partial response (pPR).
A total of 42 patients (47% of the group) achieved a partial response following the radiation plus chemotherapy paradigm, and one patient had progressive disease. All underwent surgical resection. One patient did not complete chemotherapy and did not get surgery and subsequently died.
This left 38 patients to be managed nonoperatively. In this nonoperative cohort, 79% of patients continued to have a clinical CR at the end of follow-up. Of the 7 patients with local failure, 6 were salvaged by surgery, one was salvaged by chemotherapy, and all 7 treatment failures had no evidence of disease at last follow-up.
Of the small group of 5 patients who achieved an initial clinical CR following short-course radiation therapy and neoadjuvant chemotherapy, there were no further events in this group, whereas, for patients who achieved only an initial partial response or who had progressive disease, 72% remained event-free.
Approximately half of those who achieved a clinical CR to the treatment regimen had no late gastrointestinal toxicities, and no grade 3 or 4 toxicities were observed. “Surgical resection of tumors — even without a permanent stoma — can result in significantly decreased bowel function, so our goal is to treat patients without surgery and maintain good bowel function,” Chin noted.
“For rectal cancer, both short-course radiation therapy and nonoperative management are emerging treatment paradigms that may be more cost-effective and convenient compared to long-course chemoradiation followed by surgery, [especially since] the COVID-19 pandemic...has spurred changes in clinical practices in radiation oncology,” she said.
Chin has disclosed no relevant financial relationships. Narang reports receiving research support from Boston Scientific.
This article first appeared on Medscape.com.
Burnout risk may be exacerbated by COVID crisis
New kinds of job stress multiply in unusual times
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.
New kinds of job stress multiply in unusual times
New kinds of job stress multiply in unusual times
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.
Blood test for Alzheimer’s disease comes to the clinic
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.
How cannabis-based therapeutics could help fight COVID inflammation
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A skin test for Parkinson’s disease diagnosis?
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM MOVEMENT DISORDERS
Twelve medical groups pen letter opposing UHC copay accumulator program
ACR leads outcry against the insurer’s proposed move
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
ACR leads outcry against the insurer’s proposed move
ACR leads outcry against the insurer’s proposed move
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
Survey finds European dermatologists unhappy with pandemic teledermatology experience
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
FROM THE EADV CONGRESS
COVID-19: U.S. sets new weekly high in children
the American Academy of Pediatrics announced Nov. 2.
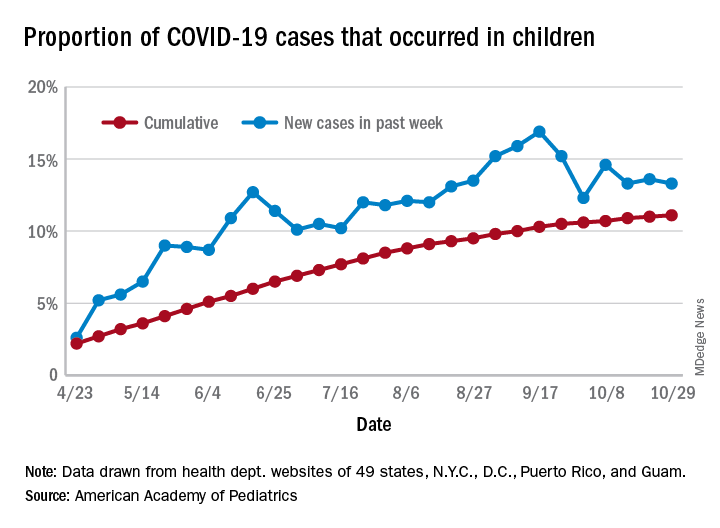
For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.









